Abstract
Objective
To determine the association between dysregulated central pain processing and treatment response in rheumatoid arthritis (RA).
Methods
One-hundred and eighty-two participants with active RA were followed for 12 weeks after initiating a disease-modifying anti-rheumatic drug (DMARD). To assess central pain processing, participants underwent quantitative sensory testing (QST), including assessment of pressure pain detection thresholds (PPTs) at the trapezius muscles, temporal summation (TS), and conditioned pain modulation (CPM). QST measures were categorized as high vs. low central dysregulation. The association between baseline central dysregulation and treatment response, defined by the European League Against Rheumatism (EULAR) response criteria, was assessed using multiple logistic regression adjusted for demographics, RA-related variables, and psychosocial variables.
Results
Fewer participants with high CPM dysregulation achieved a good EULAR response than those with low CPM dysregulation (22.5% vs. 40.3%, p = 0.01). A similar trend, though not statistically significant, was noted when central dysregulation was assessed with PPT and TS. The adjusted odds ratios (ORs) for the association between high central dysregulation and good EULAR response were: PPTs: 0.59 (95% CI: 0.28 – 1.23), TS: 0.60 (0.27 – 1.34), and CPM: 0.40 (0.19 – 0.83). In a model examining the combined effects of dysregulated TS and CPM, dysregulation of both measures was associated with a lower odds of good EULAR response (OR 0.23, CI: 0.07 – 0.73).
Conclusion
Low CPM was significantly associated with lower odds of good EULAR response, suggesting that inefficient descending inhibitory mechanisms may be a potential treatment target for further study.
Introduction
Despite the availability of potent disease-modifying anti-rheumatic drugs (DMARDs), less than half of patients with rheumatoid arthritis (RA) achieve low disease activity, measured by composite disease activity indices, after 6 months of therapy (1). While composite disease activity measures, such as the Disease Activity Score (DAS28), are considered surrogates of peripheral joint inflammation, they include subjective components such as the tender joint count (TJC) and patient global assessment (PtGA), which may be influenced by non-inflammatory factors. Our group and others have shown that TJC and PtGA are associated with dysregulated central pain processing, termed “pain centralization” (2, 3).
Pain centralization is characterized by widespread pain due to increased responsiveness of central nervous system (CNS) nociceptive neurons to normal or subthreshold afferent input (4). Pain centralization has been assessed using quantitative sensory testing (QST) in a variety of musculoskeletal conditions, including fibromyalgia, osteoarthritis (OA) and RA (5). The most commonly used QST modality is the assessment of pressure pain detection thresholds (PPTs), which detect overall sensitivity to pressure. Low PPTs at extra-articular sites (e.g. trapezius muscles) are thought to reflect abnormalities in central pain processing (6). Specific abnormalities in central pain processing may be detected by increased temporal summation (TS), which is associated with enhanced facilitation of pain, and decreased conditioned pain modulation (CPM), which is associated with diminished inhibition of pain (7, 8).
Several studies have noted QST abnormalities in patients with RA. For instance, RA patients have lower PPTs, reflecting higher pain sensitivity, than healthy controls (9, 10). Additional studies have reported facilitated TS and inefficient CPM in RA patients compared to healthy controls (10, 11). However, these studies were cross-sectional, and the longitudinal relationship between pain centralization and response to therapy in RA is largely unknown. The objective of this study was to assess baseline pain centralization as a predictor of a good European League Against Rheumatism (EULAR) response to DMARD therapy in a prospective, longitudinal cohort of patients with active RA. We hypothesized that participants with high central dysregulation at baseline would have a lower odds of achieving good EULAR response.
Participants and Methods
Study population
This study is a longitudinal analysis of participants from the Central Pain in Rheumatoid Arthritis (CPIRA) cohort. CPIRA is a multicenter, prospective, observational study of participants with active RA necessitating DMARD initiation or change (12). Participants were recruited from 5 US academic medical centers from January 2014 to July 2017. Inclusion criteria were diagnosis of RA based on the 2010 American College of Rheumatology (ACR)/EULAR criteria and starting or switching a DMARD due to active RA. Exclusion criteria were: 1) use of centrally acting pain medications (e.g., amitriptyline, gabapentin, or duloxetine) within 3 months of enrollment, 2) >10 mg of prednisone or its equivalent, 3) chronic opioid use or any opioid use within 24 hours of study date, 4) systemic autoimmune disease other than RA, 5) severe Raynaud’s disease requiring pharmacologic treatment, 6) severe peripheral vascular disease manifested by claudication or ischemic rest pain, or 7) peripheral neuropathy. Participants were ineligible for this longitudinal analysis if they: 1) failed to start a DMARD within 1 month of the baseline visit, 2) stopped taking the new DMARD within 6 weeks of initiation, 3) had no follow up visit to assess outcome measures, or 4) were missing baseline covariates or QST measurements. The choice of DMARD was left to the discretion of the physician as we assumed that the effect of DMARDs on dysregulated pain processing would be equivalent across DMARDs.
Quantitative Sensory Testing
All participants underwent QST assessment prior to DMARD initiation or change and approximately 3 months after starting the new DMARD, as described previously (2). In brief, all assessors underwent a 1-day training session on the use of QST. Assessments of interrater reliability were performed. We calculated a two way mixed single score intraclass correlation (ICC) (3,1) to assess reproducibility of QST between assessors (13). ICCs were between 0.71 and 0.90 for PPT and TS, respectively. The ICC for CPM was 0.45. Per Cicchetti, ICCs of 0.40–0.59 are fair, 0.60–0.74 are good, and 0.75–1.00 are excellent (14).
PPTs
A Wagner Force 10 FDX algometer was used to assess PPTs at the bilateral trapezius muscles. The algometer probe was placed on the center of the trapezius muscle, and force was applied at 0.5 kpf/second until pain was reported. Three trials were performed per side. The PPT was defined as the mean pressure at which pain was reported. The trapezius was chosen as the site for assessment of PPTs for the following reasons: 1) the trapezius is a commonly used site for PPTs in the pain literature allowing for comparisons to other studies, 2) normal values for PPTs have been reported at the trapezius, and 3) the trapezius is distant from joints commonly affected by RA, minimizing confounding effects of peripheral sensitization from active joint inflammation (15, 16). Low PPTs were considered indicative of pain centralization.
TS
Participants were tested with 6 flat tipped probes with weights ranging from 8 mN to 256 mN. Probes of increasing weight were tested on the participant’s dorsal forearm until a pain score of 30 to 40 out of 100 was produced. The probe generating a pain score between 30 and 40 was used for further testing; if no such pain rating was achieved, then the highest weighted probe was used. The selected probe was tapped 10 times on the dorsal forearm for 0.5 seconds per tap. The participant was asked to rate his/her pain on a 0 to 100 scale at taps 1, 5, and 10. TS was calculated by subtracting the pain score at the first tap from the pain score at the tenth tap. The mean TS was calculated by taking the average of 3 trials. The resulting value was divided by 10 to normalize to a standardized 0–10 pain scale. Higher TS values were indicative of higher levels of pain centralization.
CPM
CPM was assessed using a painful conditioning stimulus to activate the descending inhibitory pain pathways and a test stimulus to assess pain sensitivity. The conditioning stimulus was produced by inserting the participant’s right hand into a 5–7° C water bath. The test stimulus was pressure produced by an algometer placed at the center of the contralateral trapezius. PPTs were measured immediately prior to hand submersion in the cold water bath and after 20 seconds of cold water submersion. The ratio of the first PPT to the second PPT was calculated. Inefficient (lower) CPM was considered indicative of pain centralization.
Assessment of Clinical Variables
Baseline clinical variables were assessed at the initial study visit prior to DMARD initiation or change. These variables included age, sex, race, body mass index (BMI), education, and RA disease duration. Comorbidity was assessed via a modified version of the Charlson comorbidity score (17). Symptoms of depression and sleep disturbance were assessed by the Patient-Reported Outcomes Measurement Information System (PROMIS®) computerized assessment tests (CATs). Serum from the baseline visit was analyzed for C-reactive protein (CRP), rheumatoid factor (RF), and anti-cyclic citrullinated peptide antibody (anti-CCP) at a single laboratory. Serum for CRP measurement was additionally obtained at the 12-week visit. Patient global assessment (PtGA), 28 tender joint count (TJC), and 28 swollen joint count (SJC) were obtained by a trained assessor at the initial study visit and at the 12-week study visit following DMARD initiation or change. The 28 joint count Disease Activity Score (DAS28-CRP) was calculated as previously described (18).
Statistical Analysis
The primary outcome was good response, defined by the EULAR response criteria, at the 12-week visit (19). A good EULAR response was defined as a DAS28-CRP of ≤ 3.2 at the 12-week follow up visit and a change in DAS28-CRP from baseline of > 1.2.
The primary predictors were PPTs at the trapezius, TS, and CPM. To avoid assumptions of linearity and to address differences in pain sensitivity between males and females, the QST measures were categorized into sex-specific tertiles (Supplemental Table 1). For ease of interpretation, each QST measure was further grouped into a low dysregulation group (defined as the least centrally-dysregulated tertile) and a high dysregulation group (defined as the middle and most centrally-dysregulated tertile). For PPTs and CPM, the low dysregulation group was tertile 3 (highest values of PPTs and CPM), and the high dysregulation group included both tertiles 1 and 2 (lower values of PPTs and CPM). For TS, the low dysregulation group was tertile 1 (lowest values of TS), and the high dysregulation group included both tertiles 2 and 3 (higher values of TS) (Supplemental Table 1). We chose this cut-point because, participants with TS in the 2nd and 3rd tertiles reported greater pain severity than participants with TS in the 1st tertile in a prior study (12). Similar results were found for PPTs. For consistency, we chose this categorization strategy for all QST modalities, including CPM, in the current study.
The secondary predictor was a combined measure of TS and CPM. The purpose of the secondary analysis was to assess how different combinations of altered central mechanisms of pain processing would affect DMARD response. We grouped participants into four combinations: 1) low TS and low CPM dysregulation (referent group), 2) predominantly TS dysregulation (high TS and low CPM dysregulation), 3) predominantly CPM dysregulation (low TS and high CPM dysregulation, and 4) high TS and high CPM dysregulation. These combinations were chosen a priori. We chose to include only TS and CPM in these analyses, as they are considered measures of specific pain processing mechanisms. TS is considered primarily a measure of spinal facilitation of pain, and CPM is considered primarily a measure descending pain modulation (20–22). We did not include PPTs in the secondary analysis, as PPTs are general measures of hyperalgesia, which do not imply specific mechanisms of dysregulated pain processing; rather, altered PPTs could be the result of both peripheral and central mechanisms.
The percentage of participants achieving a good EULAR response in each group was calculated. Associations between baseline QST measures and a good EULAR response were examined using multiple logistic regression. All adjusted models included age, sex, race and education. In addition, BMI, PROMIS depression, and PROMIS sleep disturbance were included as covariates due to previously reported associations with pain (11, 23). Seropositivity (defined as presence of either RF of ≥ 14 IU/mL or anti-CCP ≥ 17 U/mL), RA disease duration, and the modified Charlson comorbidity score were also included in the models based on clinical experience suggesting possible relationships with pain. A site variable was used to account for potential population differences among study sites.
Unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the association between high central dysregulation and good EULAR response were calculated. Unadjusted and adjusted ORs were additionally calculated for the combined TS and CPM measure (with the low TS and low CPM group as referent). Trend tests were conducted to evaluate the linear trend in the degree of central dysregulation defined by the combined TS and CPM measure. For the trend test analysis, we hypothesized that the odds of good EULAR response would decrease with increasing number of dysregulated mechanisms. We ranked TS below CPM because it has been suggested that enhanced spinal facilitation of pain signaling (measured by TS) may be reversible upon resolution of peripheral noxious input (e.g., joint inflammation in RA) (24). In contrast, altered baseline descending inhibition of pain is not thought to be reversible. To explore the relationship between central pain dysregulation and specific components of treatment response, a post hoc analysis was performed comparing mean changes in TJC, SJC, PtGA and CRP between participants with high and low dysregulation in CPM. Statistical testing used a nominal α=0.05. All analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Two hundred and thirty-seven participants were eligible for the longitudinal analysis. Of these, 55 participants were excluded from the final analysis due to missing data in the primary outcome or baseline variables. Participants excluded for missing data had similar baseline characteristics compared to the included cohort (Supplemental Table 2).
One hundred and eighty-two participants with complete data were included in the final analysis (Table 1). The mean age was 55.2 years, and 83.0% of participants were female. The mean RA disease duration was 10.1 years, and the mean DAS28-CRP was 4.3. After 12 weeks, 28.6% of participants had a good EULAR response while 32.4% had a moderate EULAR response, and 39.0% had no EULAR response.
Table 1:
Clinical Characteristics (n = 182 patients)
| Characteristic | % or Mean (SD) |
|---|---|
| Age, years | 55.2 (14.4) |
| Women, % | 83.0% |
| Caucasian, % | 76.9% |
| BMI, kg/m2 | 28.5 (7.0) |
| Some college or higher, % | 75.3% |
| Seropositive, % | 70.3% |
| Disease duration, years | 10.1 (12.5) |
| PROMIS Depression1 | 50.3 (9.2) |
| PROMIS Sleep Disturbance1 | 54.7 (8.9) |
| Charlson Comorbidity Score | 1.3 (1.0) |
| DAS28-CRP | 4.3 (1.3) |
| PtGA | 4.2 (2.4) |
| TJC | 10.9 (8.6) |
| SJC | 5.3 (5.3) |
| CRP | 7.7 (12.2) |
Abbreviations: BMI = Body mass index, PROMIS = Patient-Reported Outcomes Management System, DAS28-CRP = Disease activity score in 28 joints using CRP, PtGA = Patient global assessment, TJC = Tender joint count, SJC = Swollen joint count, CRP = C-reactive protein.
Reported as t-scores
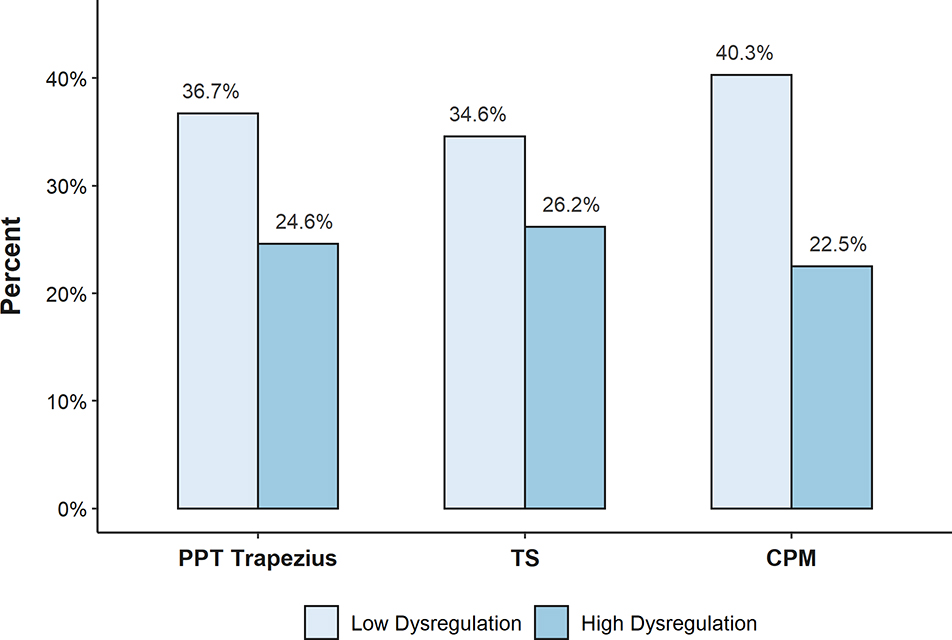
Fewer participants with high CPM dysregulation achieved a good EULAR response compared to participants with low CPM dysregulation (22.5% vs. 40.3%, p = 0.01) (Figure 1). A similar trend was observed when high central dysregulation was assessed via PPTs or TS, but statistical significance was not reached. (PPTs: 24.6% vs. 36.7%, p = 0.09; TS: 26.2% vs. 34.6%, p = 0.25). The adjusted OR for achieving good EULAR response was statistically significant for participants with high dysregulation of CPM compared to low dysregulation (CPM: 0.40, 95% CI: 0.19 to 0.83) (Table 2). The same trends were seen for PPTs and TS but were not statistically significant (PPT: 0.59, 95% CI 0.28 to 1.23; TS: 0.60, 95% CI 0.27 to 1.34). To better understand which components drove the association between CPM and EULAR response, we calculated mean 12-week changes in the DAS28-CRP components in the high vs. low CPM dysregulation groups in an exploratory analysis. The mean (SD) decrease in TJC was 3.52 (6.14) among those with high CPM dysregulation and 6.29 (7.25) among those with low CPM dysregulation (Supplementary Table 3).
Figure 1.
Percent of participants achieving a good EULAR response by QST measures
Table 2:
Odds ratios (ORs) for good EULAR response by QST measure
| QST Measure | Unadjusted OR (95% CI) | Adjusted1 OR (95% CI) |
|---|---|---|
| PPT (high dysregulation) | 0.56 (0.29, 1.10) | 0.59 (0.28, 1.23) |
| TS (high dysregulation) | 0.67 (0.33, 1.34) | 0.60 (0.27, 1.34) |
| CPM (high dysregulation) | 0.43 (0.22, 0.83) | 0.40 (0.19, 0.83) |
Abbreviations: EULAR: European League Against Rheumatism, QST: Quantitative sensory testing, OR: Odds ratio, CI: Confidence interval, PPT: Pressure pain detection threshold, TS: Temporal summation, CPM: Conditioned pain modulation
Adjusted for age, sex, race, BMI, education, seropositivity, disease duration, PROMIS sleep disturbance, PROMIS depression, and modified Charlson comorbidity score.
Bolded values are statistically significant
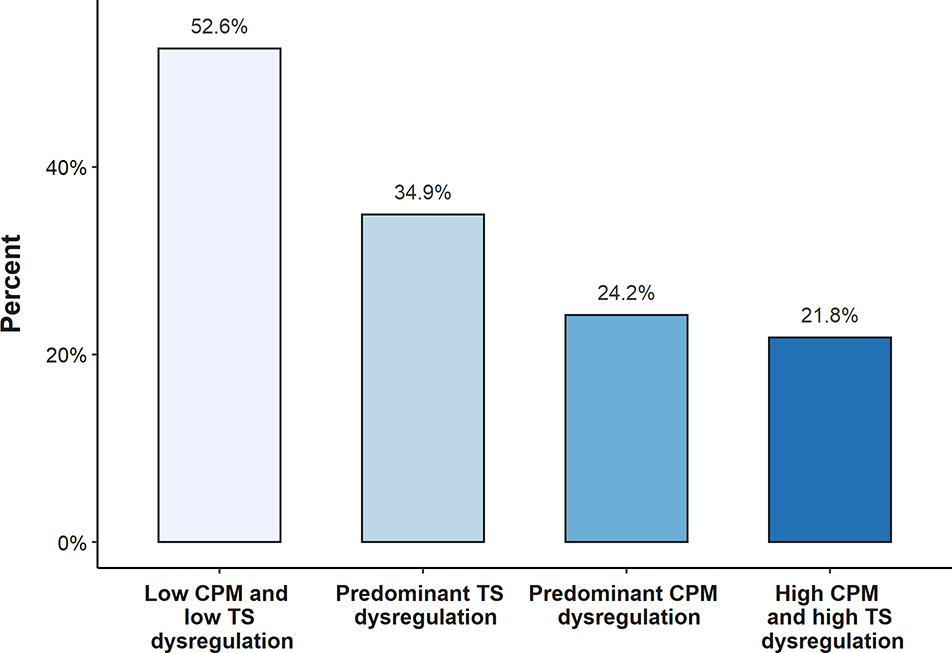
In a secondary analysis, we assessed the percentage of participants who achieved good EULAR response with different combinations of dysregulation in pain facilitaton (TS) and pain inhibition (CPM). The percentage of participants who achieved a good EULAR response in each category was: 52.6% with low TS and low CPM dysregulation, 34.9% with predominant TS dysregulation, 24.2% with predominant CPM dysregulation, and 21.8% with high TS and high CPM dysregulation (Figure 2). The adjusted OR for good EULAR response decreased with increasing number of categories of central dysregulation (p for trend = 0.007) (Table 3). When each category was compared to the referent (low TS and low CPM dysregulation), the only statistically significant association was between the group with both TS and CPM dysregulation and a good EULAR response (OR 0.23; 95% CI 0.07 to 0.73).
Figure 2.
Percent of participants achieving a good EULAR response by TS and CPM dysregulation groups
Table 3:
Odds ratios for good EULAR response by TS and CPM central dysregulation group
| TS and CPM Central Dysregulation Group | |||||
|---|---|---|---|---|---|
| Low TS and low CPM dysregulation | Predominantly TS dysregulation | Predominantly CPM dysregulation | High TS and high CPM dysregulation | ||
| Reference | OR (95% CI) | OR (95% CI) | OR (95% CI) | p-value (trend) | |
| Unadjusted | 1.00 | 0.48 (0.16, 1.45) | 0.29 (0.09, 0.96) | 0.25 (0.09, 0.71) | 0.0072 |
| Adjusted1 | 1.00 | 0.49 (0.14, 1.67) | 0.31 (0.08, 1.13) | 0.23 (0.07, 0.73) | 0.0077 |
Abbreviations: EULAR: European League Against Rheumatism, QST: Quantitative sensory testing, OR: Odds ratio, CI: Confidence interval, PPT: Pressure pain detection threshold, TS: Temporal summation, CPM: Conditioned pain modulation
Adjusted for age, sex, race, BMI, education, seropositivity, disease duration, PROMIS sleep disturbance, PROMIS depression, and modified Charlson comorbidity score.
Bolded values are statistically significant
Discussion
The objective of this longitudinal prospective study was to assess pain centralization as a predictor of response to DMARD therapy in participants with active RA. In the adjusted analysis, high dysregulation in CPM (alone and in combination with high dysregulation in TS) was identified as a significant predictor of lower odds of good EULAR response. These results suggest that pain centralization may play a role in DMARD response in patients with active RA. The specific mechanisms of this finding may include inefficient descending endogenous analgesia, for which low CPM is a surrogate marker (22, 25).
To our knowledge, this study is the first to report that inefficient CPM is associated with a lower odds of treatment response in patients with a systemic inflammatory condition, RA. Similar observations have been reported in non-inflammatory pain conditions, such as OA. For example, in a study of 42 patients with knee OA, less efficient CPM was associated with less pain improvement in response to treatment with ibuprofen and acetaminophen (26). Similarly, in a study of 35 patients with knee OA, inefficient CPM predicted decreased response to diclofenac gel (27), and in a study of 14 patients with knee OA, inefficient CPM before total knee arthroplasty (TKA) predicted less improvement in pain six months after TKA (28).
The observation that CPM in response to an experimental stimulus predicts decreased response to peripherally-directed treatments may be explained in multiple ways. One explanation is that impairments in inhibitory pain processing are uncoupled from peripheral nociceptive input, resulting in continued pain despite resolution of peripheral nociceptive input. An alternative explanation is that patients with low baseline CPM have already fully activated their inhibitory pain pathways due to existing pain from their clinical condition(s) and are thus unable to mount an additional inhibitory response to the experimental stimulus. Both explanations are consistent with the results of our exploratory analysis, which indicate that the association between low CPM and poor DMARD response likely reflects less improvement in pain sensitization (measured by TJC), as opposed to less improvement in inflammation (measured by SJC and CRP).
In contrast, we did not observe a statistically significant association between PPTs and good EULAR response. In other painful conditions, the relationship between PPTs and treatment response has been conflicting (29). Wylde et al. reported that low preoperative PPTs were associated with persistent pain following TKA (30). However, the magnitude of this finding was small, and other studies failed to find a relationship between PPTs and pain following joint replacement (31). It is possible that we did not see a statistically significant relationship between PPTs and treatment response because PPTs reflect overall dysregulation of pain processing, which could be the result of a mixture of altered mechanisms (including both inefficient pain inhibition and enhanced pain facilitation).
Similarly, we did not see a statistically significant association between TS and EULAR response. Consistent with our findings, Christensen et al. did not find a statistically significant association between TS and change in DAS28 at 4 months following DMARD initiation (32), and, the association between TS and treatment response has been inconsistent in studies of other musculoskeletal conditions. In a systematic review by Georgopoulos et al., pooled data from 4 studies indicated that TS predicted pain in musculoskeletal conditions with an unadjusted correlation coefficient of 0.37, but the association was not statistically significant in adjusted analyses (33). In contrast, a subsequent study reported that preoperative facilitated TS was associated with pain following TKA in analyses adjusted for Kellgren and Lawrence score, warm detection threshold, and heat pain threshold (34).
A potential explanation for the differences in results seen across QST modalities may be that suppression of peripheral inflammation affects specific mechanisms of central pain processing differently. For instance, TS has been hypothesized to be a “bottom-up” process, which arises from ongoing noxious peripheral stimuli (24, 35). If an ongoing noxious peripheral stimulus is required to maintain pain centralization, we would expect resolution of pain following treatment of inflammation. Thus, we would not expect an association between TS and treatment response. In contrast, descending inhibition (CPM) may not resolve following resolution of noxious input, making baseline dysregulated CPM a more likely predictor of poor DMARD response. Therefore, some QST modalities may be associated with poor treatment response, while others are not. It is also possible that the association between specific QST modalities and treatment response differs depending on the duration of the initial peripheral noxious stimulus. For example, the effects of heightened TS may be reversible if the duration of the initial noxious peripheral stimulus is limited but become irreversible with time. Future studies are needed to further elucidate the role of TS and CPM in the development and maintenance of chronic pain.
These results have important clinical implications. These findings indicate a role for pain centralization in DMARD response in RA. For a subgroup of patients, remission, as defined by composite disease activity markers, may not be possible to achieve without addressing pain centralization. Furthermore, pain centralization may involve multiple pathways such as the ascending pain pathways as indicated by TS and the descending endogenous analgesia pathway as indicated by CPM. Here, we provide evidence for inefficient endogenous analgesia as a mechanism of pain centralization leading to inadequate EULAR response in RA. Clinicians should consider therapy targeting this pathway, such as serotonin-norepinephrine reuptake inhibitors (SNRIs). For instance, Yarnitsky et al. reported that inefficient CPM predicted a good response to the SNRI duloxetine in patients with painful diabetic neuropathy (36). In contrast, in a previous study, our group did not observe any differences in pain scores between RA patients when treated with SNRI milnacipran vs. placebo (37). However, these patients were not stratified by CPM levels. Future studies, examining the use of SNRIs in RA patients with and without efficient CPM would be useful in determining whether CPM may be utilized to identify individuals who are likely to respond to an SNRI as an adjunctive treatment for pain in RA.
Our study has several strengths. CPIRA is the largest cohort with comprehensive QST data, including PPTs, TS, and CPM, in RA. Our QST protocols were adapted from protocols commonly used in the literature, enabling comparison across previous studies. The use of TS and CPM in addition to PPTs allowed for interrogation of specific mechanisms of pain centralization. Additionally, our study was comprehensive in the collection of clinical variables which enabled us to account for important potential confounders, such as depression, sleep disturbance, and medical comorbidities.
This study included a few important limitations. First was missing data. However, comparison of the demographic data from the included and excluded patients revealed no meaningful differences. Second, interpretation of QST categories as dysregulated may be limited as we did not include a control group of healthy individuals for comparison. Third, while DAS28-CRP response contains an objective marker (CRP), we did not use more sensitive methods of detecting synovitis such as ultrasound or magnetic resonance imaging. Fourth, the measurement properties of current CPM paradigms are imperfect, as evidenced by the lower ICC compared to other QST measures, and it is certainly possible that the chosen method of analysis may influence results. Future studies may focus on finding improved methods of studying descending modulation of pain. For instance, better results may be achieved using imaging of the descending pathways such as functional MRI or MR spectroscopy. Lastly, given that this was not a randomized, controlled trial, confounding by unmeasured factors may have impacted the observed relationships.
In summary, this study provides evidence that pain centralization is associated with inadequate EULAR response. Specifically, we report a potential role for the endogenous descending analgesic pathways (reflected by inefficient CPM in response to a noxious stimulus) in poor treatment response in patients with RA. Clinicians should consider pain centralization as a possible reason for the perception of persistent disease activity in RA. Patients with abnormalities in their CNS pain processing pathways may be candidates for treatment with cognitive behavioral therapy or centrally acting agents. Recognition of pain centralization as a contributor to disease presentation could facilitate optimizing a patient’s medication regimen without escalating DMARD therapy, which is accompanied by inherent risks.
Supplementary Material
Acknowledgments
Sources of Funding:
This work is supported by the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants P30-AR072579, P30-AR070254, K24-AR070892, RO1-AR064850). Andrew C. Heisler MD is supported in part by Grant Number T32 AR007611-13 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Financial Disclosures:
Yvonne C. Lee, MD, MMSc reports advisory board membership for Eli Lilly (unpaid), stock ownership in Cigna, and a research grant from Pfizer. Marcy B. Bolster MD reports advisory board membership for Gilead Sciences (< $10,000) and stock ownership in Johnson and Johnson. Daniel J. Clauw MD has received consultant fees from Pfizer Inc (> $10,000) and Aptinyx, Daiichi Sankyo, Eli Lilly, Intec Pharma, Samumed, Theravance, Tonix, Zynerba (< $10,000), and expert testimony for Eli Lily, Pfizer Inc, Nix Patterson LLP, Williams & Connolly LLP.
References
- 1.Uhlig T, Lie E, Norvang V, Lexberg AS, Rodevand E, Kroll F, et al. Achievement of Remission and Low Disease Activity Definitions in Patients with Rheumatoid Arthritis in Clinical Practice: Results from the NOR-DMARD Study. J Rheumatol. 2016;43(4):716–23. [DOI] [PubMed] [Google Scholar]
- 2.Lee YC, Bingham CO 3rd, Edwards RR, Marder W, Phillips K, Bolster MB, et al. Association Between Pain Sensitization and Disease Activity in Patients With Rheumatoid Arthritis: A Cross-Sectional Study . Arthritis Care Res (Hoboken). 2018;70(2):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McWilliams DF, Kiely PDW, Young A, Joharatnam N, Wilson D, Walsh DA. Interpretation of DAS28 and its components in the assessment of inflammatory and non-inflammatory aspects of rheumatoid arthritis. BMC Rheumatology. 2018;2(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeser JD, Treede RD. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008;137(3):473–7. [DOI] [PubMed] [Google Scholar]
- 5.Pavlakovic G, Petzke F. The role of quantitative sensory testing in the evaluation of musculoskeletal pain conditions. Curr Rheumatol Rep. 2010;12(6):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suokas AK, Walsh DA, McWilliams DF, Condon L, Moreton B, Wylde V, et al. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20(10):1075–85. [DOI] [PubMed] [Google Scholar]
- 7.Boyden SD, Hossain IN, Wohlfahrt A, Lee YC. Non-inflammatory Causes of Pain in Patients with Rheumatoid Arthritis . Curr Rheumatol Rep. 2016;18(6):30. [DOI] [PubMed] [Google Scholar]
- 8.Staud R The important role of CNS facilitation and inhibition for chronic pain. Int J Clin Rheumtol. 2013;8(6):639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerecz-Simon EM, Tunks ER, Heale JA, Kean WF, Buchanan WW. Measurement of pain threshold in patients with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and healthy controls. Clin Rheumatol. 1989;8(4):467–74. [DOI] [PubMed] [Google Scholar]
- 10.Vladimirova N, Jespersen A, Bartels EM, Christensen AW, Bliddal H, Danneskiold-Samsoe B. Pain Sensitisation in Women with Active Rheumatoid Arthritis: A Comparative Cross-Sectional Study . Arthritis. 2015;2015:434109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YC, Lu B, Edwards RR, Wasan AD, Nassikas NJ, Clauw DJ, et al. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum. 2013;65(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heisler AC, Song J, Dunlop DD, Wohlfahrt A, Bingham CO, 3rd,, Bolster MB, et al. Association of Pain Centralization and Patient-Reported Pain in Active Rheumatoid Arthritis . Arthritis Care Res (Hoboken). 2019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychological Methods. 1996;1(1):30–46. [Google Scholar]
- 14.Cicchetti DV. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology . Psychological Assessment. 1994;6(4):284. [Google Scholar]
- 15.Fischer AA. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain. 1987;30(1):115–26. [DOI] [PubMed] [Google Scholar]
- 16.Lee YC, Chibnik LB, Lu B, Wasan AD, Edwards RR, Fossel AH, et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2009;11(5):R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. [DOI] [PubMed] [Google Scholar]
- 18.How to Calculate the DAS28. [cited February 26, 2019]; Available from: https://www.das28.nl/das28/en/difference-between-the-das-and-das28/how-to-measure-the-das28/how-to-calculate-the-das28.html
- 19.Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am. 2009;35(4):745–57, vii-viii. [DOI] [PubMed] [Google Scholar]
- 20.Staud R Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother. 2012;12(5):577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91(1–2):165–75. [DOI] [PubMed] [Google Scholar]
- 22.Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care. 2015;9(2):131–7. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Sandberg ME, Saevarsdottir S, Klareskog L, Alfredsson L, Bengtsson C. Higher education is associated with a better rheumatoid arthritis outcome concerning for pain and function but not disease activity: results from the EIRA cohort and Swedish rheumatology register. Arthritis Res Ther. 2015;17:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harte SE, Harris RE, Clauw DJ. The neurobiology of central sensitization. Journal of Applied Biobehavioral Research. 2018;23(2):e12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: between pro- and antinociception. Pain. 2014;155(4):663–5. [DOI] [PubMed] [Google Scholar]
- 26.Petersen KK, Simonsen O, Olesen AE, Morch CD, Arendt-Nielsen L. Pain inhibitory mechanisms and response to weak analgesics in patients with knee osteoarthritis. Eur J Pain. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Edwards RR, Dolman AJ, Martel MO, Finan PH, Lazaridou A, Cornelius M, et al. Variability in conditioned pain modulation predicts response to NSAID treatment in patients with knee osteoarthritis. BMC Musculoskelet Disord. 2016;17:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaegter HB, Handberg G, Emmeluth C, Graven-Nielsen T. Preoperative Hypoalgesia After Cold Pressor Test and Aerobic Exercise is Associated With Pain Relief 6 Months After Total Knee Replacement. Clin J Pain 2017;33(6):475–84. [DOI] [PubMed] [Google Scholar]
- 29.Sangesland A, Storen C, Vaegter HB. Are preoperative experimental pain assessments correlated with clinical pain outcomes after surgery? A systematic review. Scand J Pain. 2017;15:44–52. [DOI] [PubMed] [Google Scholar]
- 30.Wylde V, Sayers A, Odutola A, Gooberman-Hill R, Dieppe P, Blom AW. Central sensitization as a determinant of patients’ benefit from total hip and knee replacement. Eur J Pain. 2017;21(2):357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haghverdian BA, Wright DJ, Schwarzkopf R. Pressure Pain Threshold as a Predictor of Acute Postoperative Pain Following Total Joint Arthroplasty . Surg Technol Int. 2016;29:320–7. [PubMed] [Google Scholar]
- 32.Christensen AW, Rifbjerg-Madsen S, Christensen R, Dreyer L, Boesen M, Ellegaard K, et al. Ultrasound Doppler but not temporal summation of pain predicts DAS28 response in rheumatoid arthritis: a prospective cohort study. Rheumatology (Oxford). 2016;55(6):1091–8. [DOI] [PubMed] [Google Scholar]
- 33.Georgopoulos V, Akin-Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. Pain. 2019;160(9):1920–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen KK, Simonsen O, Laursen MB, Arendt-Nielsen L. The Role of Preoperative Radiologic Severity, Sensory Testing, and Temporal Summation on Chronic Postoperative Pain Following Total Knee Arthroplasty. Clin J Pain. 2018;34(3):193–7. [DOI] [PubMed] [Google Scholar]
- 35.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153(6):1193–8. [DOI] [PubMed] [Google Scholar]
- 37.Lee YC, Massarotti E, Edwards RR, Lu B, Liu C, Lo Y, et al. Effect of Milnacipran on Pain in Patients with Rheumatoid Arthritis with Widespread Pain: A Randomized Blinded Crossover Trial. J Rheumatol. 2016;43(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.