Abstract
Background:
The notion of obesity as an addictive process is controversial. However, studies show that between 5.9 and 30.7% of adolescents with obesity report food or eating addiction. Few weight management interventions have tested techniques based on addiction medicine principles.
Methods:
This multi-center randomized control trial (RCT) is designed to test the effectiveness of a mobile health (mHealth) weight-loss intervention based on addiction principles, such as withdrawal and tolerance, in a sample of 180 adolescents (ages 14–18) recruited from four pediatric weight management clinics in Southern California. Akin to a Multiphase Optimization Strategy (MOST) design evaluating multicomponent behavioral interventions, we will compare the combination of an app + phone coaching (App+Coach) to app alone (App) and in-clinic multi-disciplinary (Clinic) intervention arms.
The primary outcome is mean change in zBMI and %BMIp95 over 18 months. We hypothesize that youth who receive App+Coach will have a greater reduction in body weight over the 18-month study period at a lower cost than standard of care models. Secondary outcomes include adherence to treatment regimen, intervention satisfaction, effect of the intervention on metabolic factors and activity level. We will also explore potential moderators of intervention effectiveness such as addictive eating habits, self-regulation and executive functioning.
Conclusions:
New and creative approaches are needed to address pediatric obesity. If successful, this RCT may provide an innovative and cost-effective mHealth approach, based on addiction methods, for weight loss among adolescents with overweight and obesity.
Keywords: Obesity, Pediatrics, Mobile health, Weight loss, Eating addiction, Coaching
1. Introduction
The 2007 American Academy of Pediatrics (AAP) consensus guideline recommends comprehensive, multi-disciplinary, family-based interventions for the treatment of pediatric obesity. [1] These clinical interventions require intensive management with a team of multi-disciplinary providers, addressing diet, physical activity, and behavior changes over a series of frequent visits, and 26 contact hours, making them difficult to implement on a large scale. [2] Although these approaches have been shown to be modestly effective in well-resourced outpatient clinics, few have shown long term efficacy and [3] given the prevalence of childhood obesity it is not practical to deliver this kind of intensive intervention in a healthcare setting to all children. [4] Advances in mobile health technologies (mHealth) offer an opportunity to reach and engage adolescents who may not have access to specialized outpatient clinics and to deliver interventions in participants’ natural environment. [5–8] Interestingly, few mHealth weight loss interventions are targeted for adolescents specifically [9]. Thus, mHealth interventions may be a more cost-effective and generalizable alternative to in-person pediatric weight management delivered in outpatient settings. [5,10]
Pretlow et al. examined the effectiveness of a mHealth weight-loss intervention to address obesity in adolescents. [11] The interactive app, supplemented by personalized phone-coaching, utilizes principles and strategies such as craving management, withdrawal control and tolerance prevention rooted in the addiction literature. To date, this mHealth intervention has been tested in two pilot studies. [11,12] The first study involved a self-selected cohort of 47 obese adolescents and young adults (12–21 years of age). Findings indicated that the intervention was well-received in the self-selected sample and [11,13] resulted in a significant decrease in BMI percentile upon completion of the 12 week intervention. [11] The second pilot study was conducted in adolescents referred to a tertiary care weight management clinic [12]. A total of 50 adolescents were recruited; 18 self-selected to participate in the app intervention. Retrospective data from age-matched contemporary youth who completed a multidisciplinary weight management intervention were utilized as control. There was a statistically significant difference in zBMI between the app and control upon completion of the intervention (p = 0.03). App participants had higher retention (100% vs. 37%) and lower total cost per patient ($855.00 vs. $1428.00) than the control. These pilot study results suggest that an interactive addiction-based, mHealth weight loss intervention, with telephone coaching, targeted for adolescents, reduced zBMI better than in-clinic controls and was a cost-effective, timely and labor-saving method for adolescent weight management [12].
The present study builds on these pilot studies to test the larger-scale and sustained impact of an interactive mHealth addiction-based, weight loss intervention, with personalized coaching on a larger participant population. Specifically, the proposed multi-site randomized controlled trial (RCT) tests the efficacy of an interactive addiction mHealth weight-loss intervention with personalized phone-coaching (App+Coach) compared to: 1) interactive addiction model based mHealth weight-loss intervention alone (App) or 2) multidisciplinary in-clinic weight management program (Clinic). Furthermore, obtaining insurance coverage for mHealth interventions for pediatric obesity can be difficult; therefore another key aim of the study is to conduct an economic analysis (costs, cost-savings and non-monetary benefits) of delivering App+Coach compared to Clinic. We hypothesize that App +Coach will achieve greater BMI status reduction at a lower cost than Clinic. If our hypothesis is correct, the analysis will provide a strong argument to payers for reimbursement of mHealth interventions administered in a clinical setting.
2. Methods
2.1. Overview of study design
The present study is a three-arm multi-center (n = 180) randomized controlled trial of an addiction based mHealth weight loss intervention plus personalized coaching (App+Coach) compared to: 1) addiction based mHealth weight loss intervention only (App) and 2) multidisciplinary in-clinic weight management program (Clinic).
2.2. Participant recruitment and eligibility criteria
Study procedures were approved by the Children’s Hospital Los Angeles (CHLA) Institutional Review Board and are in accordance with the Helsinki Declaration of 1975, as revised in 2008. The study will be reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement for randomized trials of nonpharmacological treatments and is registered with ClinicalTrials.gov (NCT03500835).
Center specific clinical research coordinators (CRC) will introduce the study to the youth/family member dyad recruited at that site, over the phone and then will provide additional written information via mail or email. Youth interested in participating will be scheduled for a face to face visit in which written informed consent will be obtained (Fig. 2). Eligible participants are adolescents, ages 14–18 years with BMI ≥85th percentile for age and sex with at least one family member willing to participate in the six-month intervention followed by 12-month maintenance period (Fig. 1). Youth will be excluded if they: are currently participating in an alternative weight loss intervention; have a diagnosis of uncontrolled hypertension; have poorly controlled psychiatric illness and/or severe developmental delay in which they are unable to autonomously interact with the interventions; or are unable to read English.
Fig. 2.
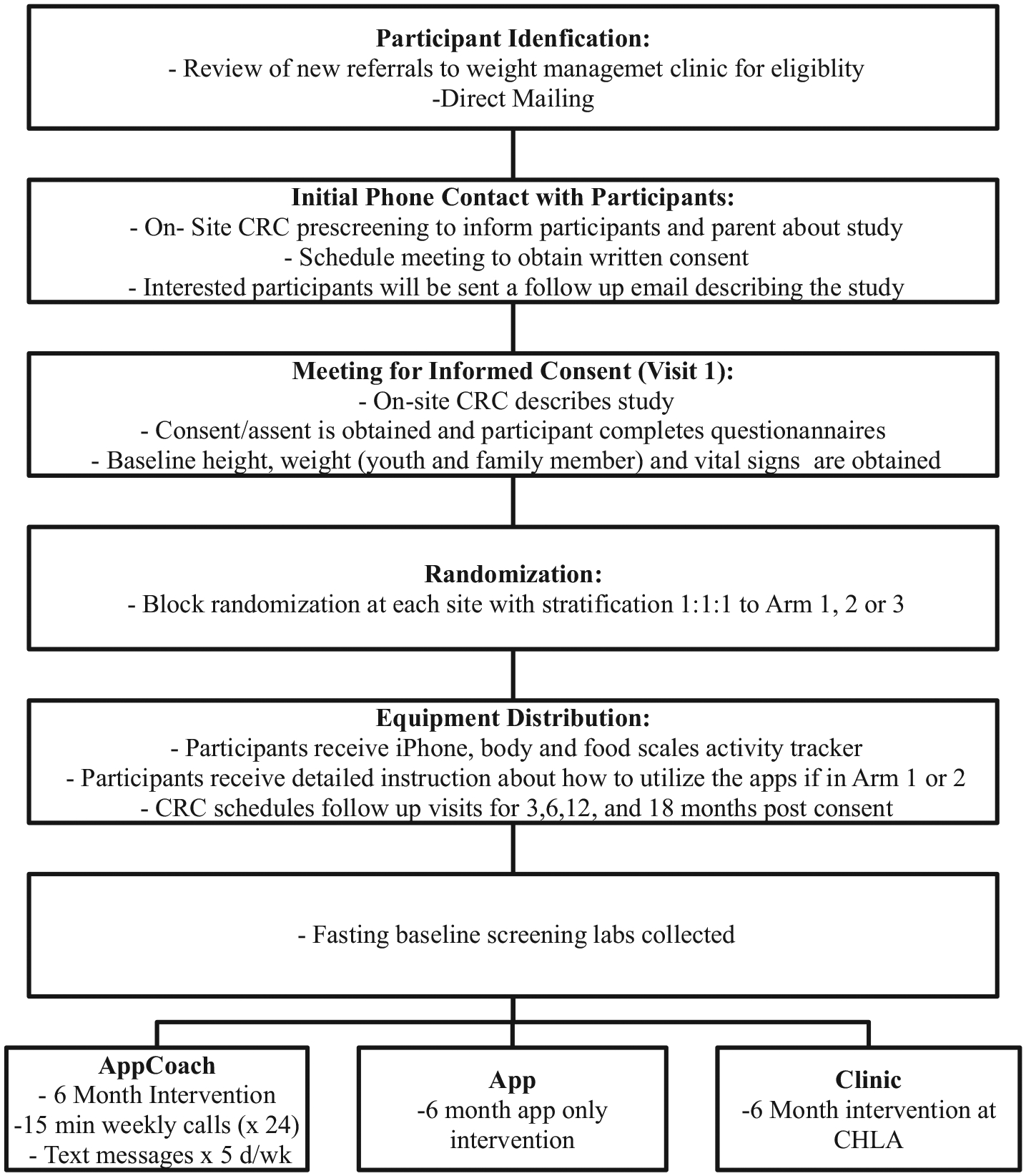
Recruitment flow.
Fig. 1.
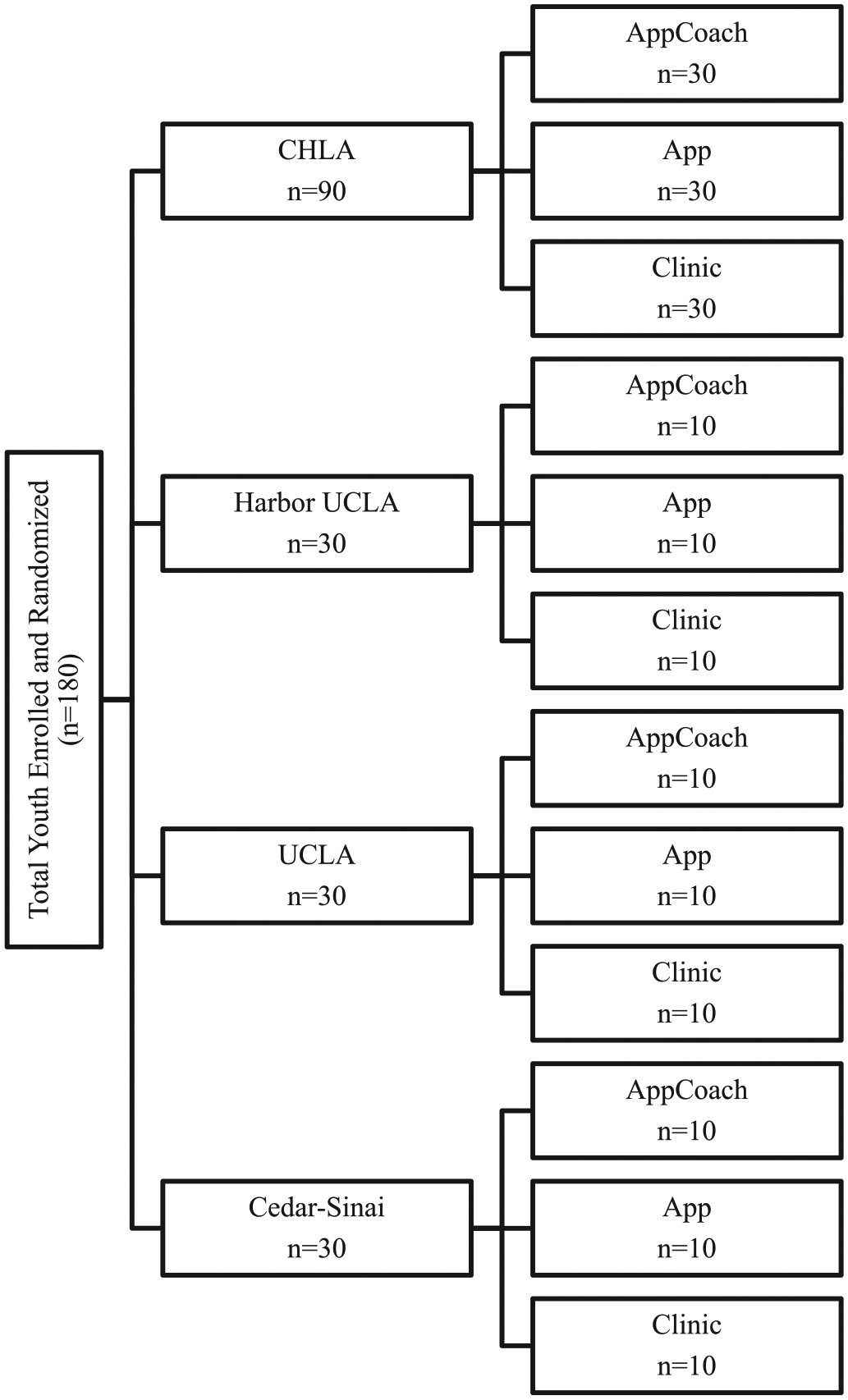
Enrollment flow.
Participants will be recruited from: 1) new referrals to four inter-disciplinary weight management clinics (CHLA, Los Angeles Biomedical Research Institute at Harbor-UCLA (LA Biomed), Mattel Children’s Hospital of the University of California Los Angeles FIT Clinic, and Cedars Sinai) and 2) direct mailing campaign. A direct mailing campaign will be utilized to send 10,000 recruitment letters to families with adolescents’ ages 14–18 years across 40 neighborhoods in LA County.
2.2.1. Trial center and care provider recruitment and eligibility criteria
All participating trial sites will fulfill the following eligibility criteria: 1) have an independent weight management clinic that cares for adolescents, 2) the principle investigator and all the clinical research coordinators/staff at the site have understood the study protocol, 3) the site is within mobile phone range. A trial site will be ineligible if: 1) no participants are registered in 6 months or 2) the hosting site judges the center to be inappropriate to recruit participants.
2.2.2. Randomization
Randomization will be at the level of the youth utilizing block randomization to ensure the groups are balanced in terms of number of subjects and the distribution of potential confounding variables. Youth (n = 180) will be randomly assigned to one of the intervention arms. Investigators and study staff will be blinded to block size.
2.3. Intervention components
Table 1 outlines the components of the three intervention arms.
Table 1.
Intervention arm components.
| App + Coach | App Alone | Clinic | |
|---|---|---|---|
| Staged withdrawal | x | x | |
| Abstinence | x | x | |
| Motivational interviewing | x | x | x |
| Craving control | x | x | |
| Eliminate specific foods from diet | x | x | |
| Eliminate snacking between meals | x | x | |
| Decrease portion size consumed at meals | x | x | x |
| Limit sugar intake | x | ||
| Increase consumption of fruits and non-starchy vegetables | x | ||
| Limit sugar sweetened beverages | x | ||
| Increase daily amount of physical activity | x | ||
| Personalized coaching | x | x | |
| Self-monitoring of weight and target behaviors | x | x | x |
| Financial incentive | x | x | x |
| Parental involvement | x |
2.3.1. App
The specific details of the App intervention have been described by Pretlow et al. and Vidmar et al. [11] [12] The App intervention is founded on three addiction-based principles: divide-and-conquer approach; staged withdrawal/abstinence; and behavioral addiction body focused repetitive behavior intervention methods. [14,15] Specifically, the intervention targets three addictive eating behaviors: 1) staged withdrawal from participant identified problem foods; 2) staged withdrawal from daytime and nighttime snacking between meals; and 3) withdrawal from excessive amounts of food consumed at meals. [16] The curriculum also addresses self-monitoring of weight and targeted behaviors to support habit-formation of eating behaviors [17,18].
The app will be implemented with an iPhone®, which will be securely integrated with a network-server for real-time data access and storage [11]. Problem foods will be defined as specific foods for which participants feel they have cravings, cannot resist when immediately available, and cannot stop eating when started. Participants will sequentially withdrawal from two self-selected problem foods at a time, with the goals of total abstinence from the food for a minimum of 10 days in a row, and craving resolution [2].
During the second phase, which will overlap partially with the first, participants will eliminate snacking by choosing time periods to avoid snacking (i.e. morning, afternoon, evening or nighttime). Each phase is of variable length within the 6 month intervention delivery period. Once the participant has abstained from snacking during their chosen time interval for 10 days, the participant will then choose additional time intervals to abstain from snacking, with the overall goal of abstinence from snacking for the entire day. In the third phase, excessive food amounts at meals will be targeted. Participants will weigh all food items served at meals, and an algorithm in the app will gradually reduce each participant’s weighed food amounts to result in one-pound body weight loss per week. Participants will take photos of meals via the app, before and after the amounts are decreased, to allow for external confirmation of amount reduction. In addition, participants will have access to the app home page, which includes various addiction model strategies, including: motivation, distraction ideas, and coping skills techniques [11,12]. Body focused repetitive behavior treatment methods will be applied in parallel with the staged withdrawal methods.
2.3.2. Coach
Three coaches will implement the study. Coaches will be required to have undergraduate training, complete a structured training program, be compassionate and empathetic with good interpersonal and communication skills. Coaches will come from a variety of backgrounds and professional experiences. Coaches are not required to have achieved or maintained significant weight loss themselves as previous research on coaching interventions suggests that characteristics such as communication skills and empathy are more crucial to success in this role. Coaches will be full time employees at the host institution.
Interactions between coach and participants will be of three types: face-to-face visits, phone calls and text messages (Fig. 3). Each participant in App+Coach will interact with the coach for six months via text messages five days per week, weekly phone calls (15-min duration) and two one-hour face-to-face visits. Participants will keep an electronic log in the app of their progress through the intervention; this log will be reviewed with the coach weekly during the phone interactions. The face-to-face visits will be qualitative interviews (of approximately 60 min duration), which will occur at Visit 2 and 3. The semi-structured interviews will include questions designed to elicit both positive and negative impacts on weight management and identify barriers such as emotional eating, poor coping skills for life stressors and social challenges. The interviews will be broadly structured around key domains of life before the intervention, life during the intervention, and anticipated future life (Fig. 4).
Fig. 3.
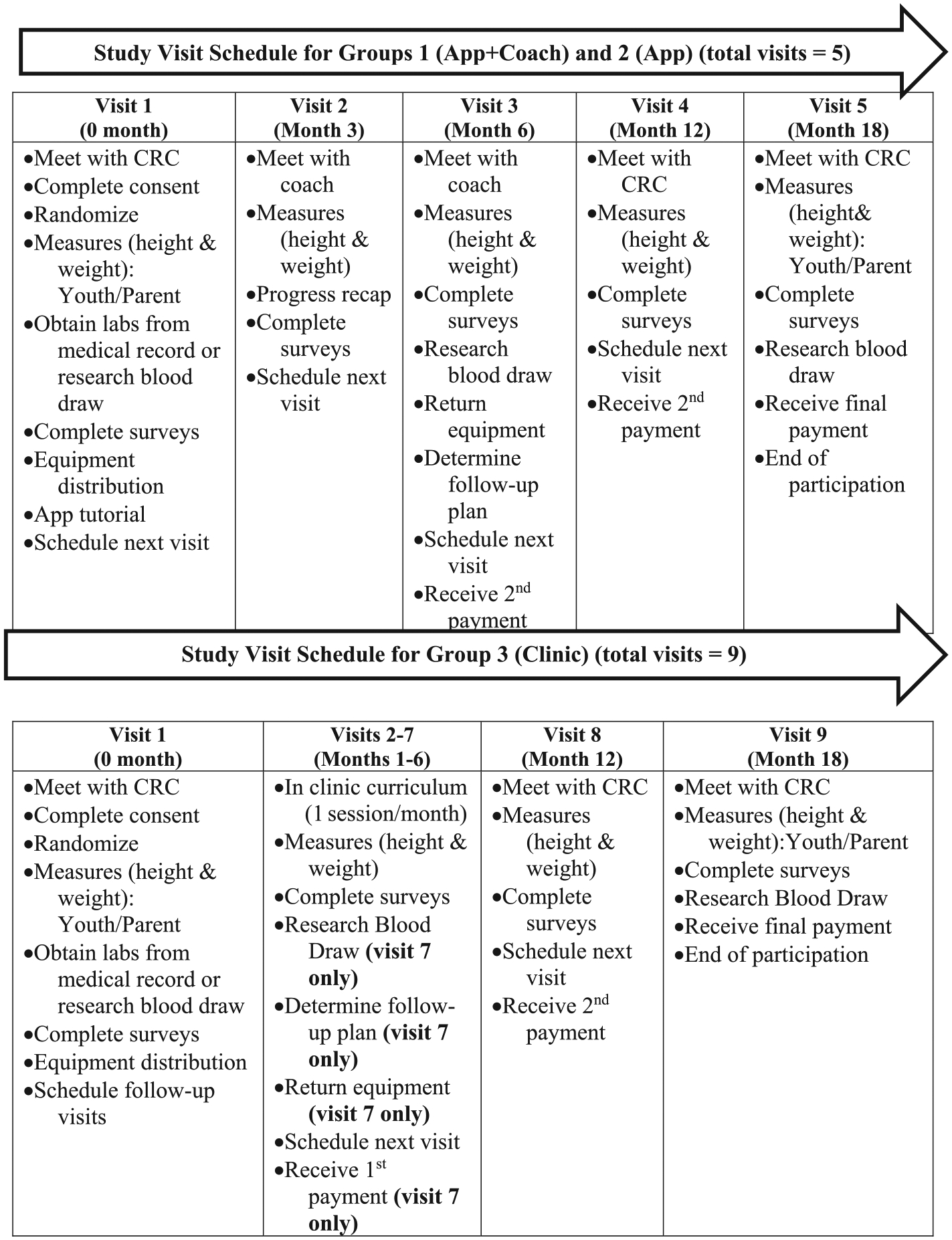
Study visit outline by intervention arm.
Fig. 4.
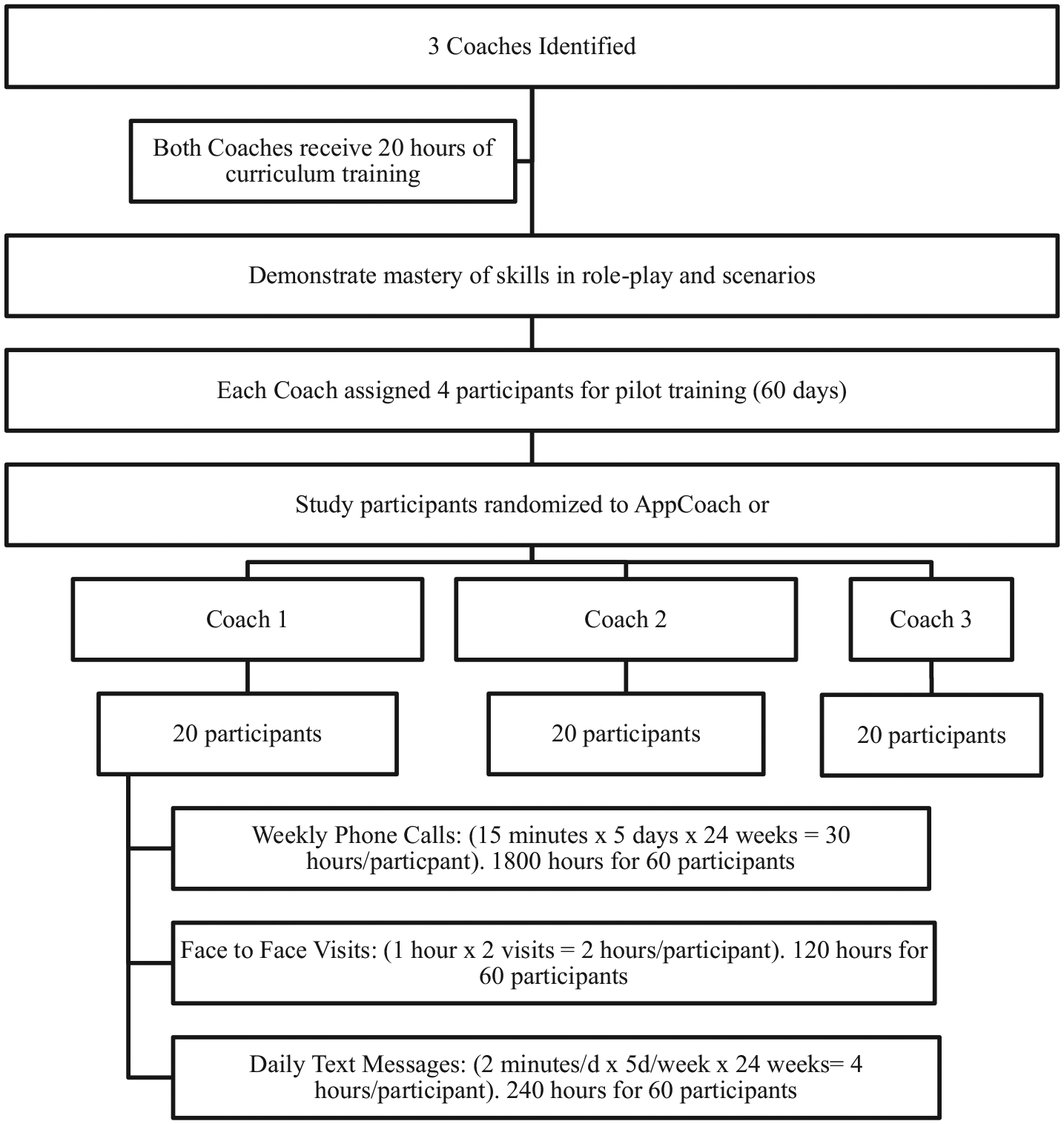
Coach training paradigm.
The majority of the participant-coach interactions will take place through phone calls. Each coach will have a cell phone used solely for the purpose of this study, so that participants may directly contact their coach as needed regarding any questions or concerns that the participant may have. Phone calls and text messages will be utilized for appointment reminders, scheduling weekly phone meetings, providing emotional support, and following up on items discussed in a prior visit or phone call [14]. All interactions will be documented in a database created for the study, including date, time, type, and duration of contact, topics discussed, and any relevant notes. The coach will utilize a motivational interviewing paradigm to interact with the participants, and an electronic system within the app database to document each encounter. All phone conversations will be recorded and transcription of the conversation will be completed every month on 10 participants from each intervention group to assess intervention fidelity.
The coaches delivering the curriculum will receive 20 h of training over a 4-week period from the principal investigator, app creator, and behavioral psychologist. The training will cover: 1) the importance, rationale and education about the theory of food addiction, body focused repetitive behaviors and the proposed intervention, 2) the concept of behavior change, patient centered approach and motivational-interviewing techniques, and 3) practical advice specific to each stage of the intervention and ways to assist the youth with common barriers and triggers. The coaches will also tailor the delivery of information and the setting of goals to the participant’s readiness for change. Coaching curriculum will cover active listening, non-judgmental communications, motivational interviewing techniques, and creating self-management goals. In addition, the two coaches will be required to demonstrate understanding of the app intervention, mastery of coaching skills through simulated role-plays and observations of coaching sessions. Each coach will be required to complete at least two role-plays and have two observed sessions to be approved.
Once the coaches complete the training and demonstrate mastery of the skills, they will be assigned participants via the stratified randomization of enrolled patients to the intervention arms. Each coach will be responsible for mentoring 20 participants each from App+Coach. Each coach will make initial contact (via HIPAA secure video conferencing) with the participants at the time of enrollment to describe his or her role and to discuss participant identified goals.
2.3.3. App + Coach
Participants in App+Coach will complete the 6-month app intervention as described above. The coach will utilize a motivational interviewing paradigm to interact with the participants, [4,12] and an electronic system within the app database to document each encounter. All phone conversations will be recorded and transcription will be completed every month on 10 participants from each intervention group to assess intervention fidelity.
2.3.4. Clinic: multi-disciplinary in-clinic monthly intervention-adapted kids N fitness [15,16] curriculum
Kids N Fitness© (KNF) was developed at Children’s Hospital of Los Angeles in 2000 by a team of pediatric endocrinologists, registered dietitians, social workers, psychologists, and physical and occupational therapists in response to the escalating obesity epidemic [15,16]. The family centered program has been delivered to predominantly low-income Latino families in California for over eighteen years, helping participants reduce their rate of weight gain as they learn how to make healthy lifestyle changes that can be sustained over time. KNF is an evidence-based, weight management program that empowers families to make healthy lifestyle changes by providing digestible education, awareness, motivation, and support to navigate the many obstacles in their environment [15]. Each class consists of interactive nutrition education, physical activity, and family seminars. Over 1800 families have participated in the curriculum over the past 10 years. In an unpublished afterschool pilot, children at four schools (n = 164) who participated in KNF significantly decreased their BMI z-score during the program, and continued to significantly decrease over the following three months after the program ended. At follow-up measurement during the next school year the average BMI z-score was lower than baseline.
For the current study, the KNF curriculum has been adapted from a six session weekly group class to individual curriculum administered monthly for six months by a team of multidisciplinary providers delivering the different components. The clinic team consists of a health educator, physicians (MD), registered dietitian (RD), physical therapist (PT) and psychologist, who will assess participants in a clinic setting. Adaptation included modifications for a clinical setting, identifying and training multidisciplinary providers, creating structured opportunities specific to the adolescent age group.
The six session program will offer monthly 90 min clinic visits that focus on interactive nutrition education for the entire family, physical activity, and family support sessions. Goal setting, self-monitoring, healthy snacks, and health promoting incentives are central components. Youth in the Clinic arm will interact with trained specialists where they are introduced to a variety of fun, engaging and effective physical games and activities that can be done indoors without expensive equipment and/or physical coordination. Family members participate in facilitated discussions to share and learn strategies to support their children in making healthy changes, overcome personal and environmental barriers, and role model healthy behavior. Nutrition lessons cover topics such as the food groups, label reading, grocery shopping tips, fiber and sugar, and mindful eating. Sessions will conclude with a healthy snack prepared with locally available ingredients (Table 1). Youth and one family member are expected to attend monthly clinic visits for six visits. Changes in behavior for the entire family are strongly encouraged. Youth randomized to the in-clinic control arm will complete the intervention at Children’s Hospital of Los Angeles, implemented by one study team. The physician and health educators are part of the research team and the RD, PT and psychologist are specific to the Clinic arm intervention.
2.4. Measurements
Clinical research coordinators will conduct all assessments (Table 2) at baseline, 3,6,12 and 18 months post consent. All data will be collected and stored in REDcap.
Table 2.
Assessment time points and key measures.
| 0 | 3 | 6 | 12 | 18 | |
|---|---|---|---|---|---|
| Covariates | |||||
| aDemographic Information | x | ||||
| Youth’s Perceived Stress Score | x | x | x | x | x |
| Family Member’s BMI | x | x | |||
| Primary Outcomes | |||||
| BMI Status Change- Height, Weight, zBMI, %BMIp95 | x | x | x | x | x |
| Secondary Outcomes | |||||
| Adherence- | x | x | x | x | x |
| Satisfaction- Satisfaction Survey | x | x | x | x | x |
| Physical Activity- PAQ | x | x | x | x | x |
| Fasting Lab Tests- | x | x | x | ||
| Cost Analysis- aHealth Care Encounters | x | x | x | x | x |
| Mediators | |||||
| Addictive eating qualities- YFAS and FCQ | x | x | x | x | x |
| Self-Regulation- ASRI | x | x | x | x | x |
| Executive Function- BRIEF 2 | x | x | x | x | x |
The following measures are completed by the participating family member.
2.4.1. Primary outcomes
2.4.1.1. Participant’s weight change.
Height and weight will be assessed at in-person visits with the on-site CRC at baseline, 3, 6 (intervention completion), 12 and 18 months (weight maintenance measures) for all participants. Height will be measured using a Quick Medical stadiometer, accurate to 0.1 cm (Quick Medical, Issaquah, WA). Weight will be measured on a self-calibrating Mobile Stand Digital Scale, accurate to 0.1 kg. Participants will wear minimal clothing during the height and weight measurements. BMI will be calculated as kilograms per meter squared and zBMI and percent over the 95th percentile (%BMIp95) will be determined utilizing the CDC growth charts.
2.4.2. Secondary outcomes
2.4.2.1. Adherence and retention.
Data relating to participant interaction with the app and the coach and attendance with scheduled research visits will be tracked remotely and stored on HIPAA-compliant central servers. Data will be reported on the three key components specific to the underlying addiction model implemented in the intervention: “problem foods” withdrawal; snacking elimination; and food amount reductions at meals [11]. We will also use strategies to increase retention, including: incentives for participation and adherence; frequent contacts with participants to maintain engagement and foster open communication; email and text reminders about face-to-face visits; and recording contact information of relatives or friends to be able to reach participants.
2.4.2.2. Change in metabolic parameters.
The following laboratory samples will be obtained after an 8-h overnight fast at baseline, 6 and 18 months: plasma glucose (FPG), lipid profile, Alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and hemoglobin A1c (HbA1c). FPG, total cholesterol, HDL cholesterol, and TG will be measured via Vitros 960 colorimetric assay, and LDL cholesterol will be calculated. Fasting insulin level will be obtained and the homeostatic model assessment of insulin resistance will be calculated. Hemoglobin A1c will be measured using a DCA 2000 (Bayer Corporation, Elkhart, IN).
2.4.2.3. Physical activity.
To evaluate the effect of the intervention arms on amount of physical activity performed by the participant, each participant will complete the physical activity questionnaire and recall their activity level over the last 7 days at each study visit.
2.4.2.4. Cost analysis.
All three intervention arms’ (App, App+Coach and Clinic) net impact on direct healthcare costs will be calculated during the study period, and BMI status outcomes will be utilized to numerically estimate future (longer-term) healthcare costs, both direct and indirect. The net-health benefits will be measured in quality of life years (QALY), during the study period and future QALY health benefits will be estimated via computational models. Those results will be used to calculate incremental cost-effectiveness ratios (ICERs) for App +Coach, to inform both reimbursement decisions by healthcare payers, and system-level policy decisions.
2.4.3. Covariates
2.4.3.1. Demographics and medical history.
At baseline, we will assess participant demographics, completed by the family member, including family member’s age, family member and child’s race/ethnicity, household composition, socioeconomic status (education, income), as well as family and child medical history. In addition, each family member will report on the frequency of health encounters for each youth over the past 12 months and assess the frequency in which the youth and family member eat outside the home. These variables will be entered as covariates in the analysis.
2.4.3.2. Family member’s weight.
One family member’s weight and height will be measured at baseline and 18 months using a Quick Medical stadiometer, accurate to 0.1 cm (Quick Medical, Issaquah, WA). Weight will be measured on a self-calibrating Mobile Stand Digital Scale, accurate to 0.1 kg. BMI will be calculated as kilograms per meter squared (BMI = kg/m2).
2.4.4. Moderators
2.4.4.1. Addictive eating behaviors.
Qualities of eating addiction will be measured using the Yale Food Addiction Scale for children (YFAS-c) [17,18]. The YFAS-c is a validated measure of addictive-like eating behavior based on the Diagnostic and Statistical Manual of Mental Disorder V diagnostic criteria for substance dependence [17]. The scale consists of 25 questions that focus on the seven diagnostic criteria for substance dependence [17]. Participants reporting three or more symptoms and clinically significant impairment were considered to have met the criteria for addictive eating habit [19]. The YFAS-c has shown good test-retest reliability [17,20]. The YFAS-c will be used to assess change in addictive eating qualities over time, by assessing pre and post intervention scores compared between arms. In addition, the Food Cravings Questionnaire (FCQ) will be utilized to evaluate baseline food craving control and the effect of the intervention arms on FCQ score [21,22]. Scores on the FCQ-T have been found to be positively associated with eating pathology, body mass index (BMI), low dieting success and increases in state food craving during cognitive tasks involving appealing food stimuli and therefore will be a useful metric to elucidate the efficacy of the app intervention [22].
2.4.4.2. Executive function and self-regulation.
The Behavior Rating Inventory of Executive Function (BRIEF) will be utilized to evaluate baseline executive function and the effect of the intervention on executive function (EF) [23]. The BRIEF provides theoretically and empirically derived clinical scales that measure aspects of executive function [24]. The clinical scales form broad indices of behavior and cognition and an overall score, the global executive composite (GEC) [23,25]. Furthermore, the Adolescent Self-Regulatory Inventory (ASRI) will be utilized to evaluate the degree to which adolescents are able to activate, monitor, maintain, inhibit, and adapt their emotions, thoughts, attention, and behavior [26,27]. These two metrics will be utilized to investigate baseline predictors of treatment success to better target youth for more individualized treatment plans.
2.5. Analytic plan and power analysis
2.5.1. Power and sample size estimates
The power analysis is prepared for comparing (1) the difference in the mean change of zBMI and %BMIp95 between baseline, 6 months, and 18 months across App+Coach, App, and Clinic, (2) the difference in zBMI and %BMIp95 mean change between App+Coach and Clinic, and (3) the difference in zBMI and %BMIp95 mean change between App +Coach and App. Review of the literature revealed that the majority of studies utilize the change in zBMI to evaluate the effectiveness of weight management interventions in adolescents. However, Freedman et al. and the CDC have proposed utilizing alternative metrics for weight change such as %BMI95 which may be more strongly associated with change in body size in adolescents. [28,29] Therefore, both metrics were considered in the power calculation to further explore their use as outcome measures in youth with obesity and severe obesity.
This study will enroll 180 patients and equally allocate into 3 groups. All these calculations utilize an estimate of standard deviation of change based on the conservation of correlation of 0.5 between pre and post measurements within each group, based on Analysis of Variance (ANOVA) with two-sided type I error of 5%. For the first study aim, the difference in the mean change of zBMI and %BMIp95 between baseline, 6 months, and 18 months across App+Coach, App, and Clinic, this study has at least 83% power to detect the effect size of 0.244 with the variance of the group means of 0.0003 for the mean change of zBMI and at least 89% power to detect the effect size of 0.263 with the variance of the group means of 2.903 for the mean change of %BMIp95. For the second study aim, the difference in zBMI and %BMIp95 mean change between App+Coach and Clinic, based on two sample t-test with two-sided type I error of 5%, this study has 88% power to detect an effect size of 0.580 which corresponds to the mean difference of pre and post change in zBMI at least 0.040 standard deviation between the two groups and a 92% power to detect an effect size of 0.625 which corresponds to the mean difference of pre and post change in %BMIp95 at least 4.08% between the two groups. For the third study aim, the difference in zBMI and %BMIp95 mean change between App+Coach and App, based on two sample t-test with two-sided type I error of 5%, this study has 71% power to detect an effect size of 0.464 which corresponds to the mean difference of pre and post change in zBMI at least 0.032 standard deviation between the two groups and a 68% power to detect an effect size of 0.452 which corresponds to the mean difference of pre and post change in %BMIp95 at least 2.80% between the two comparison groups. Assuming 20% dropout rate, this study will achieve the desired study power of 80% for study aim 1 and 2. (nQuery + nTerim 4.0 and Stata/SE 15.1).
2.5.2. Statistical analysis
The primary objective of this analysis will be comparing the BMI status (i.e. zBMI and %BMIp95) change in youth between baseline, the end of the intervention (6 months), and one year follow-up (18 months) using intention to treat (ITT) and per protocol approaches. Successful completion of the intervention will be defined as follows, based on group: 1) App+Coach and App subjects are expected to attend the assigned study visits, complete all three components of the app intervention and for App+Coach attend weekly phone meetings for the 6 month intervention period; 2) Clinic subjects are expected to attend all 6 intervention visits and the assigned study visits. Per protocol BMI status change will be compared between baseline to 6 months as well as to 18 months across three intervention arms by using repeated measure ANOVA to appropriately account for the repeated measure. Then, multivariable linear regression, using mixed-effects model, will be utilized to further examine the effect of the possible factors on BMI status change accounting for demographic characteristics and other measured covariates. Besides that, moderator effect of race/ethnicity will also be examined by including the interaction terms between race and treatment arms into the model. The statistical significance will be set with two-sided test at 5% level throughout the analysis.
Furthermore, a cost-effectiveness analysis will be completed as described above. QALYs will be constructed by combining information on all observed morbidity and mortality events with utility scores (preference-weighted health-related quality of life) derived from the SF-12v2 (converted to SF-6D) and EQ-5D. These comparisons will be conducted using standard regression analysis techniques estimating both the model parameters and goodness of fit.
The model for future health effects will leverage the extensive previous work on obesity simulation already completed for the Schaeffer Center’s Future Americans Model, based on the Future Elderly Model. [28] The specific mathematical approach will employ a dynamic microsimulation (a variant of Markoff-type models). Non-parametric bootstrapping methods will be used to derive confidence intervals around the cost-effectiveness ratios for the 12-month timeframe cost utility analysis (CUA). The mean and variance of the ratios will allow estimate confidence intervals around these ratios to be determined. For the longer timeframe CUA, both univariate and multivariate (probabilistic) sensitivity analyses will be conducted to further bound the ICER estimates. The probabilistic sensitivity analysis will assume an appropriate range and distribution of values for each individual model parameter, reflecting the level of uncertainty for that parameter. Multiple repeated model runs will allow a probability distribution to be constructed that reflects the uncertainty in the overall ICER estimate.
3. Discussion
This study is both clinically and theoretically innovative as the notion of overeating as an addictive process in adolescents is still controversial [30,31]. First, to our knowledge, this study is the first RCT to explore the efficacy of a weight-loss intervention premised on addiction principles delivered via mobile health. This study will contribute to the development of effective mobile health weight management interventions for use in adolescents with obesity. This extended intervention and follow-up (i.e., six months of intervention + one year of follow-up), will provide important information about the maintenance of this intervention. This initiative represents a unique opportunity to increase access to obesity services for adolescent youth with obesity by utilizing a mobile health model.
Second, it will aim to produce preliminary data relating to the use of mobile health interventions for adolescent obesity with coaching compared to mHealth intervention alone to further elucidate the role of the mHealth interventions alone in clinical models for pediatric obesity. However these finding will have to be evaluated and confirmed in a larger trial. Stand-alone mHealth interventions may provide a platform to administer weight loss interventions to adolescents on a larger scale and to provide a more frequent feedback than standard clinical models. The use of artificial intelligence in mHealth interventions allows for interventions to have a personalized component and removes the requirement for personalized coaching. mHealth Interventions in the form of smartphone apps have the potential to be a labor efficient, effective, low-cost program that can be implemented throughout clinical settings to augment pediatric interventions for weight management [32].
Third, this research represents an opportunity to further investigate the addictive model theory in the treatment of adolescents with obesity and to provide formative data to further explain the functionality of this cognitive theory in the obesity treatment paradigm. By exploring the interventions effect on addictive eating behaviors, withdrawal, food cravings, executive function, and self-regulation we aim to develop the behavioral themes underlying this model to better understand the treatment construct to promote the development and distribution of weight management interventions with a specific focus on addictive eating for the pediatric population.
Finally, the current consensus guideline recommendations for the clinical management of pediatric obesity recommend clinic-based, family-centered, multi-disciplinary weight management interventions administered over 26 contact hours. These programs are labor intensive, costly, difficult to implement on a large scale and often only result in modest BMI status reduction. With the growing obesity epidemic in pediatric patients the lack of sustainability of such programs will become even more challenging. Therefore detailed cost analyses of alternative treatment strategies are required. The cost analysis component of this study will provide the data required to make an argument to payers regarding the cost saving potentials of mHealth interventions for pediatric weight management and potentially result in the creation of a reimbursement schema to be utilized in clinical settings.
There are also a number of potential challenges and considerations related to this work. First, because this is the first RCT to assess an addiction model based mHealth intervention we considered making the inclusion criteria more stringent to increase homogeneity of our sample. However, the researchers felt that as this is a pragmatic study design, all youth should be encouraged to participate regardless of any underlying medical complexity or chronic illness as the targeted behaviors are relevant and beneficial for all youth with obesity. Improvement in BMI status may lead to improved control of underlying medical disease and improve morbidity and mortality.
Secondly, the study cannot be adequately powered to analyze the difference in outcome between App+Coach and App alone. The study is powered to assess the primary outcome of interest which is the comparison of App+Coach versus Clinic on BMI status reduction and therefore the evaluation of the app intervention with and without coaching will be utilized to inform future implementation research.
Third, we recognize that the planned Clinic group curriculum provides less contact hours than the clinical practice guidelines recommend (~25 h/6 month intervention) [2,33]. However, our review of the clinical practices of all sites involved in the study revealed that the average contact hours administered was between 8 and 10 contact hours per 6 month intervention period. Therefore, the Clinic arm reflects what is actually delivered in real life clinical practice in stage 2 and 3 pediatric weight management programs in the Los Angeles area, and thus is an accurate metric of what payers are being asked to reimburse for the patient population in this location.
Finally, the proposed study design does not include a group of age-matched adolescents who do not receive a weight management intervention. This type of control group sample is difficult to identify and follow longitudinally. However, the current literature of pediatric obesity interventions and the natural trajectory for youth with obesity, suggests that the weight velocity of many youth not receiving any intervention continues to increase. [2]
4. Conclusions
Given the dearth of intervention options for obese children, the high likelihood that obese children will grow into obese adults, and our lack of understanding as to how best to personalize obesity interventions to optimize outcomes, positive results from this study will have important implications for the management of youth with obesity. Not only does the proposed delivery modality remove many barriers (e.g., transportation, missed school days) that impact access and attrition in conventional, outpatient obesity interventions, it also strategically addresses underlying addictive eating behaviors to better support habit-formation in youth in their day-to-day environment.
Acknowledgements
We gratefully thank our collaborators at UCLA, Harbor-UCLA (LABIO Med) and Cedar-Sinai Medical center. This project was supported by Children’s Hospital Los Angeles Biostatistics Core and NIH/NCRR SC-CTSI Grant Number UL1 TR000130. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We thank Jonathan Corbett for assistance and design of the app component.
Funding source
This project was supported by a grant from eHealth International, Inc.
Financial disclosure
Dr. Pretlow is the CEO of eHealth International, Inc. and owner and developer of the app used in the study. The additional authors (Vidmar, Salvy, Mittelman, Wee, Fink, Fox and Raymond) have no financial relationships or conflict of interest relevant to this article to disclose.
Abbreviations:
- mHealth
Mobile health technologies
- BMI
Body mass index
- zBMI
Body mass index Z-score
- %BMIp95
Percent over the 95th percentile
- fMRI
Functional Magnetic Resonance Imaging
- ALT
Alanine aminotransferase
- YFAS-c
Yale Food Addiction Scale for Children
- coef
Coefficient
- CI
Confidence Interval
- QALY
Quality-adjusted life years
- CAU
Cost analysis unit
Footnotes
Clinical Trial Registration
ClinicalTrials.gov identifier: NCT03500835
References
- [1].Barlow SE, Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report, Pediatrics 120 (Suppl. 4) (2007) S164–S192. [DOI] [PubMed] [Google Scholar]
- [2].Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, Yanovski JA, Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline, J. Clin. Endocrinol. Metab 102 (3) (2017) 709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reinehr T, Long-term effects of adolescent obesity: time to act, Nat. Rev. Endocrinol 14 (3) (2017) 183–188. [DOI] [PubMed] [Google Scholar]
- [4].Rancourt D, Jensen CD, Duraccio KM, Evans EW, Wing RR, Jelalian E, Successful weight loss initiation and maintenance among adolescents with overweight and obesity: does age matter? Clin Obes 8 (3) (2018) 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Majeed-Ariss R, Baildam E, Campbell M, Chieng A, Fallon D, Hall A, McDonagh JE, Stones SR, Thomson W, Swallow V, Apps and adolescents: a systematic review of adolescents’ use of mobile phone and tablet apps that support personal management of their chronic or long-term physical conditions, J. Med. Internet Res 17 (12) (2015) e287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pagoto S, Schneider K, Jojic M, DeBiasse M, Mann D, Evidence-based strategies in weight-loss mobile apps, Am. J. Prev. Med 45 (5) (2013) 576–582. [DOI] [PubMed] [Google Scholar]
- [7].Covolo L, Ceretti E, Moneda M, Castaldi S, Gelatti U, Does evidence support the use of mobile phone apps as a driver for promoting healthy lifestyles from a public health perspective? A systematic review of Randomized Control Trials, Patient Educ. Couns 100 (12) (2017) 2231–2243. [DOI] [PubMed] [Google Scholar]
- [8].Rose T, Barker M, Maria Jacob C, Morrison L, Lawrence W, Strommer S, Vogel C, Woods-Townsend K, Farrell D, Inskip H, Baird J, A systematic review of digital interventions for improving the diet and physical activity behaviors of adolescents, J. Adolesc. Health 61 (6) (2017) 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Deshpande S, Rigby MJ, Blair M, The presence of eHealth support for childhood obesity guidance, Stud. Health Technol. Inform 247 (2018) 945–949. [PubMed] [Google Scholar]
- [10].Darling KE, Sato AF, Systematic review and meta-analysis examining the effectiveness of mobile health technologies in using self-monitoring for pediatric weight management, Child Obes 13 (5) (2017) 347–355. [DOI] [PubMed] [Google Scholar]
- [11].Pretlow RA, Stock CM, Allison S, Roeger L, Treatment of child/adolescent obesity using the addiction model: a smartphone app pilot study, Child Obes 11 (3) (2015) 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vidmar AP, Pretlow R, Borzutzky C, Wee CP, Fox DS, Fink C, Mittelman SD, An addiction model-based mobile health weight loss intervention in adolescents with obesity, Pediatr. Obes 24 (2) (2018) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pretlow RA, Addiction to highly pleasurable food as a cause of the childhood obesity epidemic: a qualitative internet study, Eat. Disord 19 (4) (2011) 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nguyen B, Shrewsbury VA, O’Connor J, Steinbeck KS, Hill AJ, Shah S, Kohn MR, Torvaldsen S, Baur LA, Two-year outcomes of an adjunctive telephone coaching and electronic contact intervention for adolescent weight-loss maintenance: the Loozit randomized controlled trial, Int. J. Obes 37 (3) (2013) 468–472. [DOI] [PubMed] [Google Scholar]
- [15].Monzavi R, Dreimane D, Geffner ME, Braun S, Conrad B, Klier M, Kaufman FR, Improvement in risk factors for metabolic syndrome and insulin resistance in overweight youth who are treated with lifestyle intervention, Pediatrics 117 (6) (2006) e1111–e1118. [DOI] [PubMed] [Google Scholar]
- [16].Dreimane D, Safani D, MacKenzie M, Halvorson M, Braun S, Conrad B, Kaufman F, Feasibility of a hospital-based, family-centered intervention to reduce weight gain in overweight children and adolescents, Diabetes Res. Clin. Pract 75 (2) (2007) 159–168. [DOI] [PubMed] [Google Scholar]
- [17].Schulte EM, Gearhardt AN, Development of the modified yale food addiction scale version 2.0, Eur. Eat. Disord. Rev 25 (4) (2017) 302–308. [DOI] [PubMed] [Google Scholar]
- [18].Gearhardt AN, Roberto CA, Seamans MJ, Corbin WR, Brownell KD, Preliminary validation of the yale food addiction scale for children, Eat. Behav 14 (4) (2013) 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gearhardt AN, Corbin WR, Brownell KD, Preliminary validation of the Yale Food Addiction Scale, Appetite 52 (2) (2009) 430–436. [DOI] [PubMed] [Google Scholar]
- [20].Burrows T, Skinner J, Joyner MA, Palmieri J, Vaughan K, Gearhardt AN, Food addiction in children: Associations with obesity, parental food addiction and feeding practices, Eat. Behav 26 (2017) 114–120. [DOI] [PubMed] [Google Scholar]
- [21].Joyner MA, Gearhardt AN, White MA, Food craving as a mediator between addictive-like eating and problematic eating outcomes, Eat. Behav 19 (2015) 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nijs IM, Franken IH, Muris P, The modified trait and state food-cravings questionnaires: development and validation of a general index of food craving, Appetite 49 (1) (2007) 38–46. [DOI] [PubMed] [Google Scholar]
- [23].Baron IS, Behavior rating inventory of executive function, Child Neuropsychol 6 (3) (2000) 235–238. [DOI] [PubMed] [Google Scholar]
- [24].Roth RM, Erdodi LA, McCulloch LJ, Isquith PK, Much ado about norming: the behavior rating inventory of executive function, Child Neuropsychol 21 (2) (2015) 225–233. [DOI] [PubMed] [Google Scholar]
- [25].Rouel M, Raman J, Hay P, Smith E, Validation of the behaviour rating inventory of executive function - Adult Version (BRIEF-A) in the obese with and without binge eating disorder, Eat. Behav 23 (2016) 58–65. [DOI] [PubMed] [Google Scholar]
- [26].Moilanen K, The Adolescent Self-Regulatory Inventory: The Development and Validation of a Questionnaire of Short-Term and Long-Term Self-Regulation, (2007).
- [27].De Vet E, De Ridder D, Stok M, Brunso K, Baban A, Gaspar T, Assessing self-regulation strategies: development and validation of the tempest self-regulation questionnaire for eating (TESQ-E) in adolescents, Int. J. Behav. Nutr. Phys. Act 11 (2014) 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Freedman DS, Butte NF, Taveras EM, Goodman AB, Ogden CL, Blanck HM, The limitations of transforming very high body mass indexes into z-scores among 8.7 million 2-to 4-year-old children, J. Pediatr 188 (2017) 50–56.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Freedman DS, Butte NF, Taveras EM, Lundeen EA, Blanck HM, Goodman AB, Ogden CL, BMI z-scores are a poor indicator of adiposity among 2-to 19-year-olds with very high BMIs, NHANES 1999–2000 to 2013–2014, Obesity (Silver Spring) 25 (4) (2017) 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tompkins CL, Laurent J, Brock DW, Food addiction: a barrier for effective weight management for obese adolescents, Child Obes 13 (6) (2017) 462–469. [DOI] [PubMed] [Google Scholar]
- [31].Carter A, Hendrikse J, Lee N, Yucel M, Verdejo-Garcia A, Andrews Z, Hall W, The neurobiology of “Food Addiction” and its implications for obesity treatment and policy, Annu. Rev. Nutr 36 (2016) 105–128. [DOI] [PubMed] [Google Scholar]
- [32].Dutton GR, Lewis CE, Cherrington A, Pisu M, Richman J, Turner T, Phillips JM, A weight loss intervention delivered by peer coaches in primary care: Rationale and study design of the PROMISE trial, Contemp. Clin. Trials 72 (2018) 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].August GP, Caprio S, Fennoy I, Freemark M, Kaufman FR, Lustig RH, Silverstein JH, Speiser PW, Styne DM, Montori VM, Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion, J. Clin. Endocrinol. Metab 93 (12) (2008) 4576–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]


