Abstract
Purpose of Review
The mechanical integrity of bone is determined by the direct measurement of bone mechanical properties. This article presents an overview of the current, most common, and new and upcoming experimental approaches for the mechanical characterization of bone. The key outcome variables of mechanical testing, as well as interpretations of the results in the context of bone structure and biology are also discussed.
Recent Findings
Quasi-static tests are the most commonly used for determining the resistance to structural failure by a single load at the organ (whole bone) level. The resistance to crack initiation or growth by fracture toughness testing and fatigue loading offers additional and more direct characterization of tissue material properties. Non-traditional indentation techniques and in situ testing are being increasingly used to probe the material properties of bone ultrastructure.
Summary
Destructive ex vivo testing or clinical surrogate measures are considered to be the gold standard for estimating fracture risk. The type of mechanical test used for a particular investigation depends on the length scale of interest, where the outcome variables are influenced by the interrelationship between bone structure and composition. Advancement in the sensitivity of mechanical characterization techniques to detect changes in bone at the levels subjected to modifications by aging, disease, and/or pharmaceutical treatment is required. As such, a number of techniques are now available to aid our understanding of the factors that contribute to fracture risk.
Keywords: Strength, Indentation, Toughness, Bone fracture, Mechanical test
Introduction
The mechanical characterization of bone is critical for the direct assessment of its load-bearing capacity and functionality which is affected by aging, disease, and pharmacological intervention [1, 2]. While bone fracture is a complex biomechanical event resulting primarily from the inability of bone to resist excessive loads and strains [3], reducing fracture risk is a major clinical concern. The clinical estimation of fracture risk is based on the amount of areal bone mineral density (aBMD) and is measured by dual-energy X-ray absorptiometry (DEXA) [4]. This non-invasive and commonly used diagnostic screening tool for the evaluation of bone health in response to treatment, is sensitive to bone loss but explains only ~ 60% of the observed variation in bone strength [1, 3, 5]. As such, aBMD is a surrogate measure of bone strength and excludes the contribution of certain properties (i.e., material architecture and composition) to fracture resistance [6]. Several recent studies have therefore highlighted the limits of DEXA and strength-based fracture predictions especially in conditions where bone tissue and the size of bone tissue are compromised [7, 8]. Consequently, research focus has shifted to understanding and developing new surrogate markers for predicting clinical fracture risk in cadaveric and preclinical models [9–11].
The type of mechanical tests chosen for characterizing bone’s mechanical competence and propensity to fracture are based on the hierarchical level or length scale of interest where genetic or epigenetic modifications are expected. More specifically, selecting a length scale helps to isolate the origin of potential factors that are clinically relevant for the assessment of fracture risk at or below the length scale of interest [3]. For example, at the macroscale or whole organ level, whole bone size and shape have a larger influence on the mechanical outcome variables compared with heterogeneity and compositional flaws. Bulk tissue testing allows for determining the contribution of each tissue type, i.e., cortical or cancellous, and the effects of microarchitecture and bone volume fraction (BV/TV) [12]. Tissue level material properties depend on the arrangement, composition, and organization of bone microstructure in particular osteons and trabeculae and not on the bulk structure [13, 14]. For example, changes in bone extracellular matrix (ECM) nano-structure, composition, or crosslinking, and the ionic interaction with mineral are best determined from a tissue level rather than a bulk-level strength test [15–17]. Mechanical tests at the nanoscale provide the contribution of the mineralized collagen fibrils, extrafibrillar mineral, and non-collagenous proteins (NCPs) but are independent of tissue-level microarchitecture.
The resistance of bone to fracture encompasses several factors collectively termed bone quality [3]. Bone mechanical properties are some of the quantitative measures of bone quality that can predict fracture risk [18]. This review summarizes the current and most commonly used experimental approaches for characterizing the mechanical properties of bone. The techniques are divided based on four (though not fixed) hierarchical levels (Fig. 1; Table 1): whole bone, tissue, microscale, and nanoscale testing. For each technique, the outcome variables, interpretation of the results, and important new findings are detailed. It is noteworthy that mechanical testing at each level represents the sum total of changes occurring at and below that level. At a particular level, the test may not be very sensitive of changes at scales that are lower than the length scale of interest.
Fig. 1.
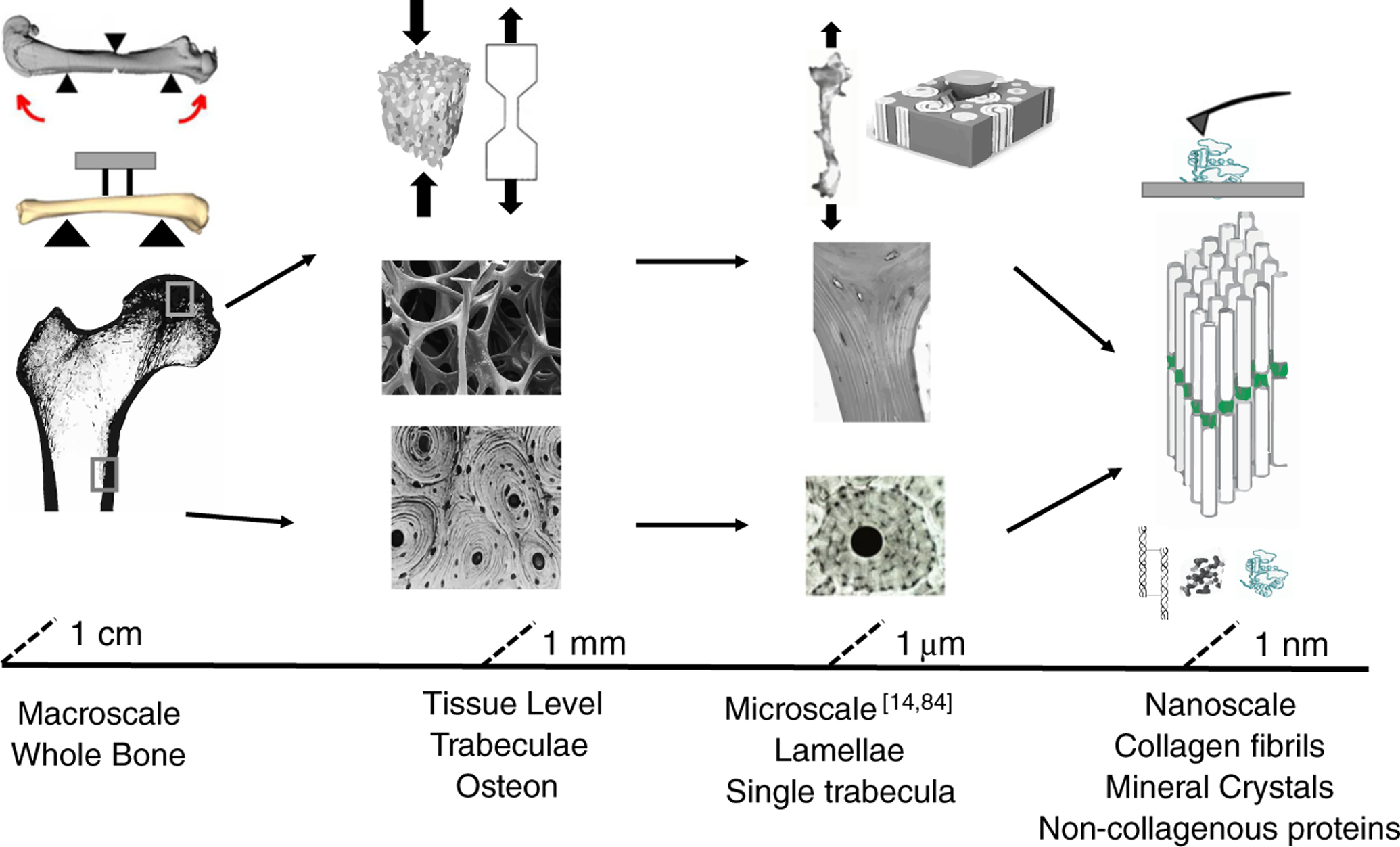
Schematic of bone structural hierarchy with the commonly used mechanical characterization technique at each length scale. Single trabeculae [14] and scratch testing [84] were adapted with permission from Elsevier
Table 1.
Summary of mechanical characterization techniques with outcome variables based on length scale and type of specimen
| Length scale | Type of specimen | Mechanical testing mode | Outcome variables |
|---|---|---|---|
| Whole bone | Long bones, vertebra | Bending, tension, compression, torsion, compression | Extrinsic mechanical properties, i.e., structural stiffness, max load, work to fracture, fracture load |
| Tissue | Cortical beam, dumbbell, dog bone, single-edged notched specimen (SEB), cancellous cores | Tension, compression, torsion, bending fatigue, compression (static or cyclic) | Intrinsic mechanical properties, i.e., Young’s modulus, yield, fatigue and ultimate strength, fracture toughness |
| Micro | Cortical bone cubes up to 5 mm thick, cortical or trabecular bone slices | Microscratch, microindentation, reference point indentation | Material level properties i.e. fracture toughness, hardness, modulus (elastic and reduced), elastic work, total work, total indentation distance, indentation distance increase |
| Nano | Cortical or trabecular bone specimen 2–3 mm thick, cortical or trabecular bone slices | Nanoindentation, in situ testing (tension, compression, bending) | Material level properties, i.e., hardness, modulus, fibril strain, mineral stress |
Whole Bone Testing
The mechanical behavior of whole bone is typically assessed in axial (compression or tension), bending, or torsional loading modes, at either monotonically or cyclically loading rates [3, 12]. Monotonic or quasi-static loading is a single load to failure test and measures the mechanical response of bone during elastic deformation and failure [18]. In cyclic loading, failure occurs gradually at strains much lower than in a monotonic test [20]. Single load to failure test is the simplest and commonly used approach for determining the mechanical properties of bone, although in vivo bones are likely to fail due to loading at high strain rates or by fatigue [20]. Whole bone bending test is particularly useful in determining the mechanical properties of cortical bone of small animals. In contrast, compression test is commonly used for the biomechanical assessment of vertebral trabecular bone [21, 22]. The mechanical properties from whole bone testing are influenced by bone geometry, composition (bone mineral, collagen, non-collagenous proteins, and water), and the random distribution of microstructural defects.
Bending Test
The mechanical properties of long bones of small animals is typically assessed under bending [22, 23]. Tension and compression occurs on opposite surfaces of the bone and testing can be done in 3- or 4-point loading. Three-point bending is advantageous because of its simplicity [23]. The bone sits on two support points, and a downward load is applied at the mid-diaphysis by an upper (loading) point. Fracture occurs generally at or near the loading point due to shear stress [24]. In 4-point bending, there are two loading fixtures which produces uniform moment, pure bending, and zero transverse stresses between the loading points [21]. As such, the bone fractures at its weakest location in this region due to normal loads (i.e., tension) [23]. The upper support fixtures must apply equal loads which can be difficult to achieve with rodent bones due to irregularity in specimen shape [25, 26]. Both methods are widely used to quantify the mechanical strength of rodent bones [26, 27] and have been reviewed in detail elsewhere [21, 23, 25].
In whole bone bending, as the monotonically increasing load is applied to the specimen, deformations are produced and both are simultaneously recorded in a load-displacement curve (Fig. 2). The curve is divided into two regions corresponding to the transition from elastic to irreversible (plastic) deformation and characterizes the extrinsic mechanical properties of cortical bone. Other structural properties from whole bone testing include yield load, ultimate load, and work to fracture (toughness). By measuring the cross-sectional geometry of the specimen, and using engineering beam theory, the intrinsic mechanical properties such as Young’s modulus, ultimate strain, and strength can be calculated [23]. These properties refer to the material and not the structure. It should be noted that the equations assume simple/ideal geometry, linear-elastic, and homogenous material [18] which is not the case for long bones. Nevertheless, such methods have successfully demonstrated differences between genetic deficient, treatment, and control groups [28•, 29•, 30].
Fig. 2.
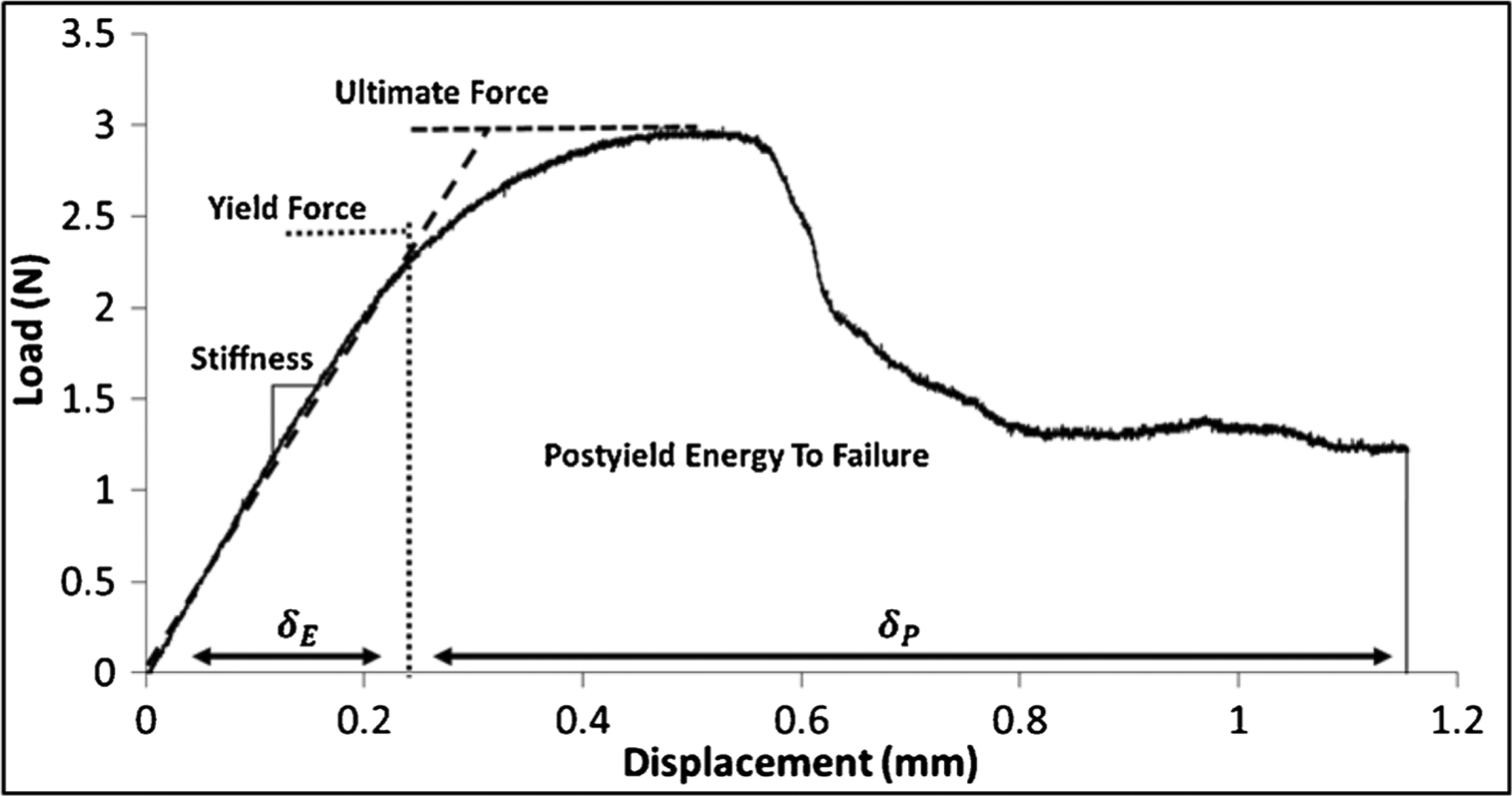
Representative load-displacement curve generated from a mouse radius 3-point bending test. The post-yield energy to failure is defined as the area under the curve after the yield point. Elastic and plastic deformations are indicated by δE and δP, respectively
Preclinical animal models offer the opportunity to genetically alter bone phenotype and material composition to achieve a naturally occurring disease state [31]. The impact on skeletal load-bearing capacity can therefore be investigated in a controlled manner. For example, in mouse models of severe osteogenesis imperfecta (OI), femoral cortical thickness, cortical area, and cross-sectional moment of inertia were significantly reduced compared with wild-type (WT) controls [32, 33]. These cortical bone parameters were associated with reduction in bone mechanical properties particularly stiffness, ultimate load, and strength which were assessed during bending test to failure [32, 33]. OI mice were treated with sclerostin antibody which improved femoral cortical bone parameters and restored the mechanical properties to similar levels as WTs [34].
Disruption of the gut microbiota with antibiotics and the effect on bone mechanical properties was examined in WT mice and an inbred mouse strain with a developmental altered gut microbiome (TLR5KO) [29•]. Antibody treatment resulted in decreased cortical area and cortical thickness with increased marrow area in both genotypes compared with their respective non-treated controls. Reduced peak bending moment and ultimate strength were observed in the treated groups compared with non-treated controls. However, the reduction in whole bone bending strength could not be fully explained by bone geometry, suggesting that alterations in the gut microbiome impairs bone tissue material properties.
Altogether, the results of these studies demonstrate that whole bone mechanical properties are influenced by adjustments in bone mass and geometry as well as material properties. Therapeutic interventions should therefore not only rescue bone morphology but also target key compositional contributors to bone strength. Toughness (energy absorbed) and ductility are also important bone material properties that should be considered since the quality of bone matrix will affect its ability to endure plastic strain and resist catastrophic failure. Furthermore, whole bone testing may be insufficient to capture the effects of treatment on bone tissue. In such a case, tissue level material properties should be assessed with other techniques described in the next sections.
Compression Test
Uniaxial compression testing is performed on whole vertebra because it more closely simulates the loading conditions in vivo [21]. The cranial and caudal endplates and spinous processes are removed to create parallel surfaces for even distribution of load within the vertebral body [35]. The specimen is loaded until failure (defined as initial decrease in load after maximum load) and the structural properties obtained are similar to bending tests. In some cases, the load and deformation variables are normalized by approximating the shape of the specimen as a cylinder in order to calculate mechanical properties at the apparent level such as Young’s modulus and strength. Thus, the extrinsic mechanical properties at this scale are primarily affected by apparent density, the product of tissue density and BV/TV [36], and the size and distribution of trabecular struts [37].
Let us consider the case of chronic kidney disease metabolic bone disorder (CKD-MD) which is associated with an increased risk of vertebral fractures [38, 39]. Biomechanical tests of rodent bones with CKD-MD are the most appropriate to evaluate the effectiveness of therapeutics and predict fracture risk occurring at the vertebral level [40]. In a study by Newman et al., L4 vertebra of CKD rats was subjected to uniaxial compression. CKD rats exhibited lower stiffness, ultimate strength, and toughness compared with normal littermates (NL) [41]. These measures can be attributed to the reduction in thickness of the BV/TV caused by the disease. Antisclerostin antibody combined with calcium-enhanced ultimate load, energy to failure, modulus, and ultimate stress of CKD rats compared with NL due to improvements in vertebral morphology.
Fracture Toughness Test
In contrast with strength-based testing methods discussed previously, the resistance of cortical bone to fracture can be more accurately assessed using a fracture mechanics approach. This technique precracks the sample by introducing a sharp notch into the mid-diaphysis of long bones primarily the femur [42]. The specimen is loaded to failure in 3 pt bending with the crack located on the surface that experiences tension during testing. Precracking of the material represents a dominant flaw (such as a preexisting microcrack) from which fracture will initiate under mechanical loading and cause catastrophic failure. The energy required to fracture the material is termed fracture toughness. By measuring the notch angle and cross-sectional geometry, the individual contributions from crack-initiation and crack-growth can be calculated [43]. More specifically, initiation toughness reports the inherent toughness of the material to the initiation of a crack, while propagation toughness measures the resistance of the material to the propagation of the initiated crack. Fracture toughness is independent of bone geometry, any preexisting flaw-like defect, and requires a much smaller number of samples than conventional strength tests of whole bone [31].
Osteocalcin (OC) and osteopontin (OPN) are major NCPs in bone matrix with biological and mechanical functions [10]. Removal of both OC and OPN in Oc−/−Opn−/− mice showed significant increase in cortical bone geometry, particularly area and thickness, compared with WTs [44•]. However, whole bone stiffness and strength were similar between the two groups. This discrepancy where strength does not follow geometrical changes, can be explained by loss of bone tissue material properties. In particular, material level fracture toughness test showed lower propagation toughness with the loss of OC and/or OPN indicating an increased propensity of bone to fracture in the absence of these NCPs [45].
The Zucker Diabetic Sprague Dawley (ZDSD) rat is a polygenic model of obesity, hyperglycemia, and type-II diabetes [46]. The femoral mid-diaphysis of ZDSD rats showed increased thickness, area, and mineral density as the diseased progressed from 16 to 29 weeks [47]. However, bending strength and work to fracture were not different. To further investigate the impact of disease progression on fracture resistance, the contralateral limb was loaded in notched 3-pt bending. In contrast with strength tests, initiation and propagation toughness were significantly decreased with duration of hyperglycemia [47]. Similarly, a study by Berman et al. found no differences in the mechanical properties of cortical bone between with Raloxifene-treated and control OI mice. However, the fracture toughness at maximum load was significantly improved with treatment due to effects on hydration and indicated that a higher load is required to propagate an existing crack [32]. These results suggest that fracture toughness testing is sensitive to changes in bone matrix independent of bone geometry. It also highlights the need to perform both strength and fracture toughness-based testing as certain conditions significantly alter bone material and increases its propensity to fracture that is not captured by conventional strength test.
Mesoscale Cortical and Cancellous Bone Testing
The material properties of cortical and trabecular bone are distinguished at this scale by machining regularly shaped specimens from the respective tissue type (Fig. 1). The specimens are then typically loaded to failure in compression, tension, torsion, or bending. Using mathematical and engineering models specific to the shape of the specimen, the material properties of bone at the tissue level can be obtained. This method of testing is independent of macroscopic bone geometry but includes the contribution of porosity, microarchitecture, apparent density, and BV/TV.
Tissue Level Strength Test
Let us consider a study by Mirzaali et al. where the mechanical properties of cortical bone were assessed using dumbbell-shaped specimen from the femoral diaphysis of cadavers to understand the structure-mechanical relationships that contribute to skeletal fragility in the elderly [48]. Quasi-static tests were performed in torsion and uniaxial tension and compression in displacement control along the cylindrical main axis of the specimens. The outcome variables included elastic modulus, yield and ultimate strain, yield and ultimate stress, and ultimate work. It was found that irrespective of loading mode (torsion or axial), elastic modulus, yield, and ultimate stress were significantly correlated to porosity. Macroscopic stiffness and strength were also correlated with BV/TV. However, the dependency of mechanical properties on osteon diameter, osteon number, and cement line density could not be identified. These microstructural features are important in crack propagation and toughening mechanisms. As such, fracture mechanics experiments described below would be more suitable for investigating their contribution to bone material properties.
Tissue Level Fracture Toughness Test
For determination of bone material properties by fracture toughness tests in a recent study by Katsamenis et al., cortical bone from human femora were machined into single-edge-notched specimens [49]. The beams were loaded to failure in 3-pt bending, and the crack propagation was monitored. In older donors (low toughness), the crack propagated in a more direct fashion without deflection at the presence of osteons. Whereas, in younger donors (high toughness) multiple in-and out-of-plane deflections were observed. In a similar study, resistance to crack initiation and propagation was higher in bone specimens with a larger osteonal area fraction [50]. After adjustment of age, it was found that vascular porosity and mineralization heterogeneity were strong predictors of initiation toughness and energy dissipated during fracture which is an estimation of the contribution of plastic deformation to overall toughness [42]. These studies suggest that the microstructural features contribute to increased fracture resistance and should be accounted for when evaluating skeletal fragility.
More recently, the fracture toughness of cortical bone was assessed using smaller specimens including the arc-shaped tension specimens (Fig. 3). Transverse cross-sections 1 mm thick from the mid-diaphysis of rabbit ulnae with a gap on the cranial side were loaded in tension until failure [51•]. The advantages of this technique include increased sample size power via repeated measures, decrease number of animals, and site-specific measurements. In this method, cracks propagate in the radial compared with longitudinal or transverse directions. As such, it is difficult to compare fracture toughness measurements with previously published techniques. The coefficient of variation in the measured values of fracture toughness compared favorably to the variation observed for small animals using whole bone [42, 51•]. The fracture toughness of deproteinized arc-shaped specimens were significantly lower than both control and bisphosphonate-treated specimens. These results suggest that this technique is also sensitive in detecting changes to bone matrix quality and fracture resistance. However, the geometry and variability in cortical thickness of arc-shaped specimens increase the likelihood of asymmetric loading at the notch. Such variations should be carefully evaluated in the bones of interest prior to employing this technique.
Fig. 3.
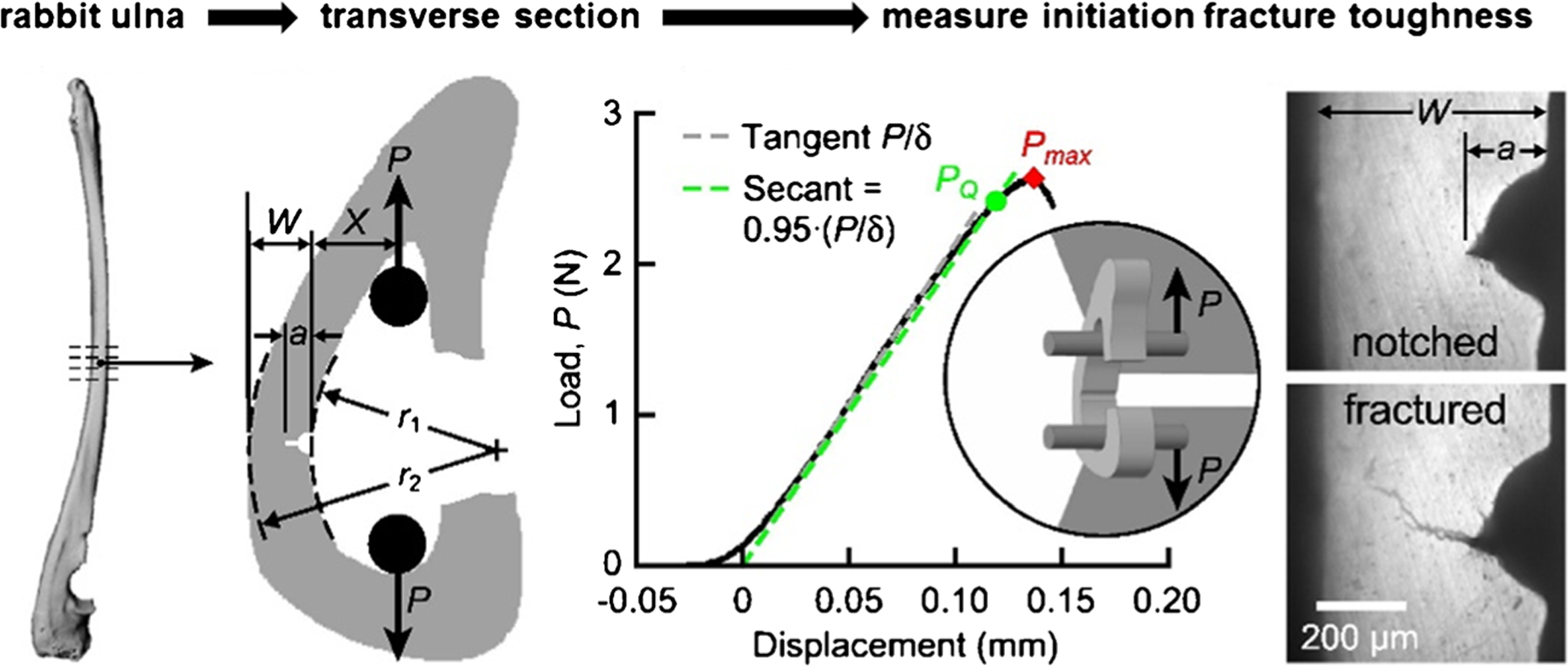
Schematic of arc-shaped tension specimen from the mid-diaphysis of rabbit ulnae. Micro-CT images were used to measure cross-sectional geometry. The measurement of notch depth, cortical width, as well as verification of radial crack propagation at the notch tip was done using optical micrographs. A custom fixture was used to load specimens via two hardened steel pins. The representative load-displacement curve shows the conditional load, PQ, and maximum load (Pmax), used for the calculation of fracture toughness. The radial fracture toughness was measured in multiple replicates per bone. Adopted from Hunckler et al. [51•] with permission obtained from Elsevier
Cyclic Loading
As mentioned earlier in this review, bones are subjected to cyclic loading in vivo with failure occurring at stresses well below the yield point. To evaluate the response of bone in such condition, fatigue tests are often conducted wherein whole bones [52] and machined bone specimens [53] are loaded to different levels of stress or strain, and the number of cycles required to break the specimen are determined. Stress or strain values are then plotted against the number of cycles required for failure, and fatigue strength of bone is estimated from the resulting S-N (stress or strain vs. number of cycle) curve. Similar to the monotonic tests, cyclic loading can be applied in tension, compression, bending, or torsion. Physiological loading however involves simultaneous application of all these loading modes. Vashishth et al. showed that fatigue life of bone is reduced by up to ten times when both axial and torsional modes are applied as fracture initiates easily under shear (torsion) and propagates under tension [54]. Multiaxial tests have shown that with aging, bone loses its ability to resist complex load and fail more easily than younger bone (Fig. 4) [55]. Due to recent reports on occurrence of atypical femoral fractures during normal physiological activities in select patients on long-term antiresorptive therapy, there has been a greater focus on evaluating bone’s cyclic response to pharmaceutical therapies [56•] or towards the role of material heterogeneity in failure of cancellous bone [57•]. Furthermore, similar to monotonic tests, cyclic tests can also employ fracture mechanics to determine the impact of material level changes on crack initiation and propagation [58, 59] but, unlike engineering materials, such advanced methods have been less commonly used for bone and represent an interesting approach to mechanistically investigate occurrences fatigue fractures.
Fig. 4.
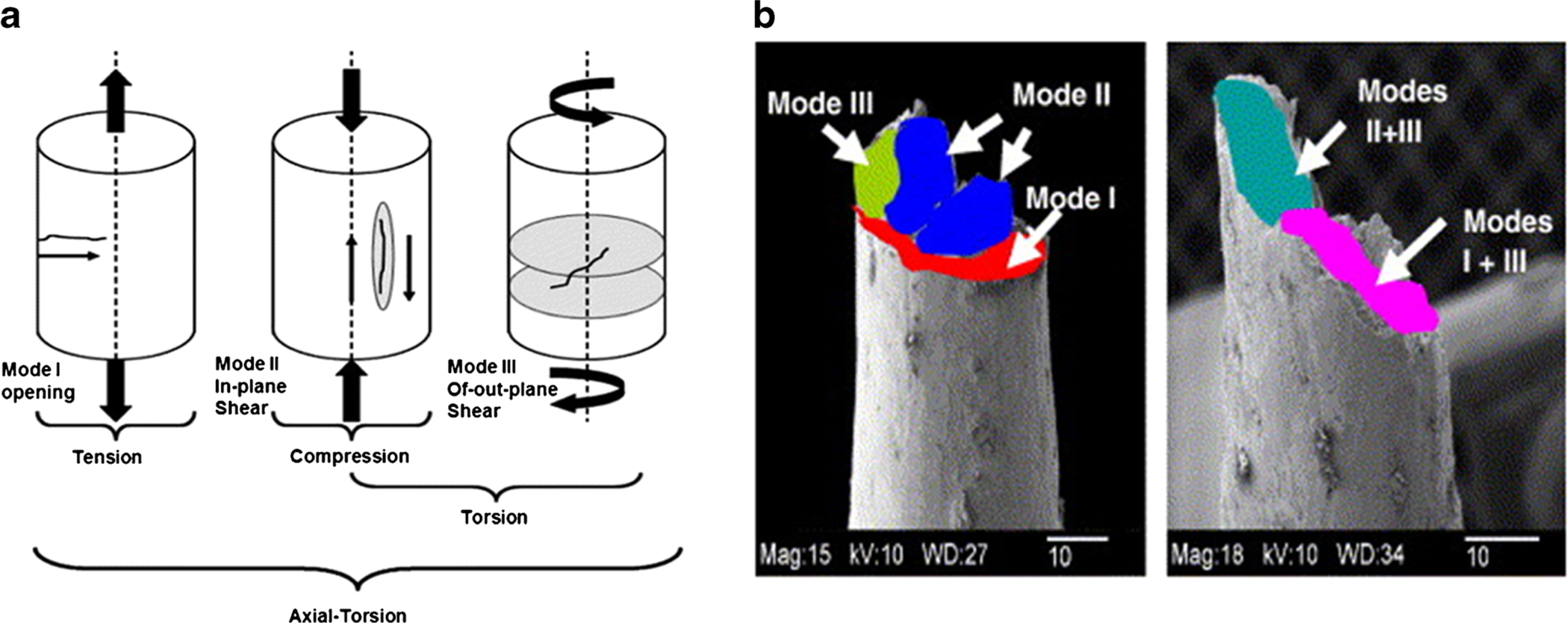
Different combinations of physiological loading cause crack initiation and or propagation in modes I, II, and III shown in (a), and b fracture surface of a young male donor undergoing different modes of failure (left) and old male donor undergoing combined modes of failure (right). Adapted from George and Vashishth [55] with permission from Elsevier
Microscale Testing
The mechanical behavior of bone at the level of osteons and individual trabeculae can be assessed using microindentation (Fig. 1) [6]. Here, a parallel bone slice from cortical or trabecular tissue is prepared with thickness at least one order of magnitude higher, and surface roughness one order of magnitude lower, than the planned indentation depth [60]. The sample is then subjected to a static load with an indenter tip, and the area of contact with resulting impression made by the tip is estimated optically. The axial force and tip displacement are recorded simultaneously. The mechanical outcomes for single indentation includes hardness and elastic modulus at length scales of 5–200 μm [61]. Hardness is defined as the force per contact area, and elastic modulus is the slope of the loading part of the indentation curve [60, 62]. Both of these are preyield properties; however, yield properties can also be extracted using inverse methods [63]. Tissue hydration, surface roughness, loading rate, and tip morphology are among the several factors that must be carefully controlled because they influence the variability in outcome measures. Alternative approaches to indentation testing at the microscale involve testing of single trabeculae under bending [64] and regularly shaped specimens from cortical bone at lamellar [65] and osteonal levels [66], or at osteonal-interstitial interface (cement lines) [67].
Reference Point Indentation
Reference point indentation (RPI) is a type of microindentation technique designed for clinical and preclinical use. Compared with traditional indentation technology, RPI consists of a reference probe which sits on the surface of the bone and remains stationary during testing, and an inner test probe which moves relative to the reference probe and indents the sample [68]. There are currently two main devices: OsteoProbe which utilizes a single impact indent and BioDent which performs cyclic indentation (both extensively reviewed including recently by Allen and colleagues [69]). The outcome variable of OsteoProbe is bone material strength index (BMSi). It is important to note that BMSi does not measure strength but rather indentation distance and how the indentation depth compares with a plastic calibration phantom [70]. The deeper the test probe penetrates the bone, the lower the BMSi value. Tissue toughness is the closest analog to BMSi because it assesses cracking of bone matrix due to separation of the mineralized collagen fibrils. In a study of post-menopausal women with T2D, BMSi was significantly reduced and was lower in patients with the longest duration of the disease [71]. Indentation of the tibia revealed that in patients with fragility fractures and low bone mass, BMSi was lower compared with patients with no history or radiological evidence of a fracture [72]. The low BMSi values were also independent of whether or not the patient sustained a vertebral fracture [72].
In contrast with impact RPI, cyclic RPI provides additional measurements as the material yields [61]. Total indentation distance (TID) and indentation distance increase (IDI) are the main mechanical outcomes [73]. However, compared with traditional testing, these outcomes are insufficient to describe the mechanical behavior of bone. For example, the overlap in IDI values from the mid-diaphysis of different mouse strains made it difficult to detect differences between strains with RPI, even though significant differences were found based on fracture toughness test [74]. Techniques used to embrittle bone tissue and reduce toughness had opposite effects on TID and IDI values contrary with what was predicted [75].
Nanoscale Testing
The most eminent structures of bone at the nanoscale are the collagen fibers infiltrated with hydroxyapatite mineral crystals and fused together with NCPs (Fig. 1) [37, 76]. Mechanical properties of the basic building blocks of bone are difficult to ascertain due to size limitations, but their contribution to bone mechanical competence are inferred from nanoindentation or extrapolated from in situ testing.
Nanoindentation
Nanoindentation (NI) uses the same principle as microindentation where a tip is loaded and unloaded from the material. It is known as depth-sensing indentation because it can assess indentation depths of 100 nm and yields spatial resolution of 1 μm in bone tissue [77]. NI characterizes the mechanical behavior of bone structure primarily within lamellar and interlamellar regions. It is also used to decouple the mechanical properties of cortical bone in the transverse and longitudinal directions at the microscale revealing the effects of anisotropy on these measurements [19, 78]. When NI is used in conjunction with a scanning probe (atomic force microscopy (AFM)), control over indentation location and surface morphology can be achieved [79]. Analysis of the force-displacement curve provides mechanical outcomes of hardness and indentation modulus. Reduced modulus is also calculated as the slope of the unloading portion of the curve [80].
Application of NI on sclerotic regions of the femoral head in patients with osteonecrosis revealed that the elastic modulus and hardness of single trabeculae were significantly greater compared with healthy regions [81]. However, the mechanical properties of any single trabeculae in the necrotic regions did not differ significantly even between fractured or non-fractured bone. These results suggested that collapse of the femoral head and mechanical degradation was not due to changes in micromechanical properties but rather changes in bone macrostructure. Similarly, induction of osteonecrosis of the femoral head in piglets caused significant increase in mineral composition, but the elastic modulus and hardness of the trabecular bone were similar to healthy controls [82]. Nanomechanical properties were only significantly different with treatment of Ibandronate. As such, indentation properties can also be sensitive to pharmacological interventions at this level.
Scratch Test
In situ toughness (total energy dissipation until failure) and fracture toughness of bone can be quantified using a scratch test [83]. In this technique, a hard diamond probe is pulled across the surface of bone with a linearly increasing vertical force (Fig. 5). The penetration depth, scratch force (vertical and horizontal), as well as the acoustic waves released during scratching are recorded [84]. An optical microscope is used for determining the desired areas for scratching. Although the specimen must be thin with low surface features, the available surface area must be large enough to create scratches of different lengths. In situ toughness is a function of the scratch force, average width of the scratch groove, and the residual scratch depth [85]. Fracture toughness is calculated as a function of the scratch force and the shape function of the probe. Using this method, fracture toughness values were similar to the values obtained from other toughness testing techniques [84]. Toughening mechanisms such as crack deflection and crack bridging were also observed. Scratch testing is capable of detecting changes in bone matrix fracture resistance due to removal of water and NCPs [85] where water functions as a plasticizer in bone with the presence of proteoglycans and contributes to its toughness.
Fig. 5.
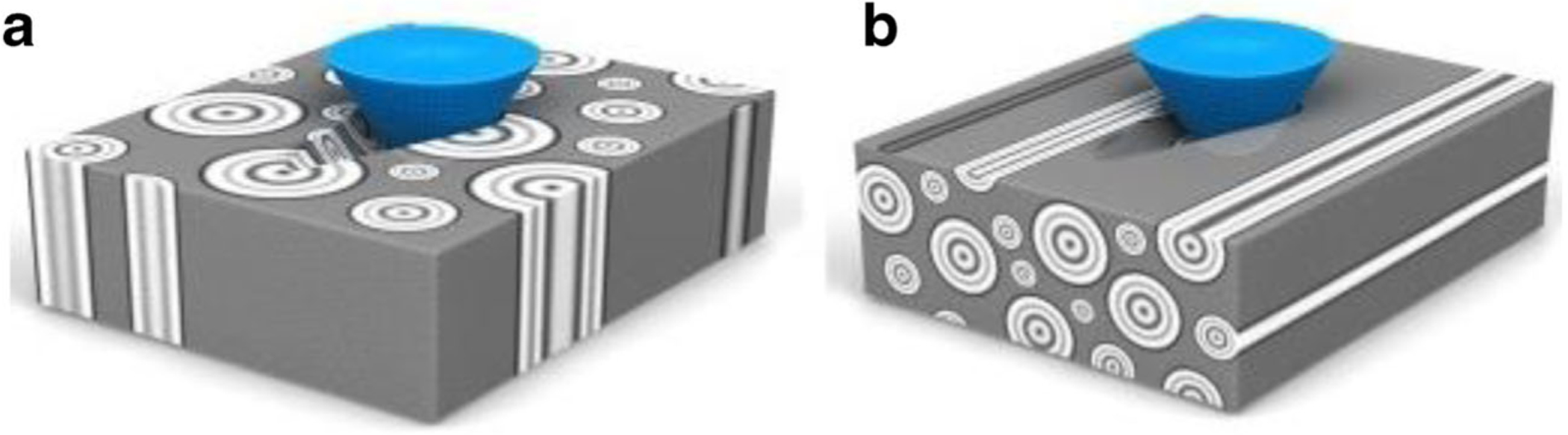
Schematic of scratch test on a longitudinal-transverse and b short-longitudinal cortical bone specimens. These specimens were chosen in order to create fracture surfaces and measure toughness perpendicular and parallel to the long axis of bone respectively. Taken from Kataruka et al. [84] with permission from Elsevier
In Situ Mechanical Testing
Mechanical testing can be performed in tension, compression, or bending in situ [86, 87] with various experimental tools such as X-ray diffractometers, micro-X-ray computed tomography systems, and electron and optical microscopes [88]. This method of testing allows for characterizing specific deformation and failure mechanisms in real time which are often inferred in traditional testing after the experimental data is collected. The mechanical properties at the fibrillar-length scale particularly mineral and mineralized fibril strains are assessed via uniaxial microtensile testing of bone slices. The macroscopic tissue strain is measured from imaging the change in displacement of markers placed directly on the bone surface [87]. By partitioning this strain, the individual fibril and mineral strains can be obtained from small-angle X-ray scattering (SAXS) and wide-angle X-ray diffraction (WAXD), respectively [86]. Axial diffraction patterns of the mineralized collagen fibril are produced due to its 67 nm periodicity. Fibril strain is measured as percent changes in the peak positions of the diffraction patterns from SAXS data [89]. Similarly, the mineral strain is obtained from WAXD as percentage shifts in the 0002 HA peak based on the hexagonal close-packed crystal structure of apatite mineral. Fibril and mineral strains are used to estimate fibril and mineral stresses respectively along the longitudinal axis of the bone.
Using this approach, it was shown that at high strain rates similar to loading experienced during walking, running, or high energy traumatic events, the fibril strains in cortical bone were reduced compared with low strain rates [90]. The results found were independent of age, disease (osteoporosis), and treatment (bisphosphonate) status. Lower fibril strains indicate reduced plasticity or fibril sliding and increased susceptibility to fracture particularly in the osteoporotic population. Samuel et al. demonstrated the influence of hydration on the ultrastructural mechanical properties of bone which was also dependent on loading modes [91]. Cylindrical and dog-bone-shaped specimens of femoral cortical bone were loaded in compression and tension respectively until failure, and X-ray scattering recorded concurrently. The mineral crystal stresses were much larger in dry state than in wet in both tensile and compressive loading, indicating a water-mediated load transfer. Fibril stresses were approximately two orders of magnitude lower than the crystals, implying that the collagen phase is negligible to load bearing at this scale.
Summary
The resistance of bone to fracture involves direct mechanical testing. While this approach includes destructive testing which is infeasible for in vivo clinical applications, these tests are useful and offer certain advantages since bone fracture is inherently a mechanical event [18]. The outcome variables from mechanical testing are influenced by the amount, distribution, and composition of bone tissue, as well as the biophysical interactions between the primary constituents [37]. As such, current research methodologies not only quantify mechanical properties but also use additional characterization techniques to identify the mechanism(s) of failure.
Preclinical animal models are the most appropriate to evaluate changes in whole bone mechanics due to disease, pharmacological agents, or genetic deficiency. Stiffness, maximum load, and strength are likely to explain the changes observed in bone mass and geometry. However, if perturbations affect bone matrix composition to a greater extent than bone morphology, the effects on bone material properties need to be determined through tissue level mechanical testing. Toughness, hardness, and fracture resistance may reflect the modifications occurring to bone ultrastructure.
Current methods used for determining the mechanical properties of bone in vivo have poor correlations to fracture risk. It is still unclear how in vivo indentation properties relate to material heterogeneity, porosity, and toughening mechanisms, all of which influences resistance to fracture. It is important to note that only four levels of bone hierarchy were reviewed and techniques at the highest level are sparser compared with lower length scales. By advancing current and emerging technologies, our understanding of how changes in the different structural levels of bone contribute to fracture risk can be improved.
Acknowledgements
This work was supported by the National Institutes of Health AR49635 and AG20618 and TL1 TR001434 and the National Science Foundation CMMI 1363526.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Osterhoff G, Morgan EF, Shefelbine SJ, Karim L, McNamara LM, Augat P. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47:S11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly E Methods for assessing bone quality: a review. Clin Orthop Relat Res. 2011;469(8):2128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez CJ, Keaveny TM. A biomechanical perspective on bone quality. Bone. 2006;39(6):1173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry. JAMA. 2002;288(15):1889–97. [DOI] [PubMed] [Google Scholar]
- 5.Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(3):13–8. [DOI] [PubMed] [Google Scholar]
- 6.Hunt HB, Donnelly E. Bone quality assessment techniques: geometric, compositional, and mechanical characterization from macroscale to nanoscale. Clin Rev Bone Miner Metab. 2016;14(3):133–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sibai T, Morgan EF, Einhorn TA. Anabolic agents and bone quality. Clin Orthop Relat Res. 2011;469(8):2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi T, Sugimoto T. Bone metabolism and fracture risk in type 2 diabetes mellitus [review]. Endocr J. 2011;58(8):613–24. [DOI] [PubMed] [Google Scholar]
- 9.Voide R, van Lenthe G, Müller R. Bone morphometry strongly predicts cortical bone stiffness and strength, but not toughness, in inbred mouse models of high and low bone mass. J Bone Miner Res. 2008;23(8):1194–203. [DOI] [PubMed] [Google Scholar]
- 10.Morgan S, Poundarik AA, Vashishth D. Do non-collagenous proteins affect skeletal mechanical properties? Calcif Tissue Int. 2015;97(3):281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fajardo RJ, Karim L, Calley VI, Bouxsein ML. A review of rodent models of type 2 diabetic skeletal fragility. J Bone Miner Res. 2014;29(5):1025–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole JH, van der Meulen MCH. Whole bone mechanics and bone quality. Clin Orthop Relat Res. 2011;469(8):2139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Meulen MC, Jepsen KJ, Mikić B. Understanding bone strength: size isn’t everything. Bone. 2001;29(2):101–4. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee A, van der Ham F, et al. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone. 2005;37(6):825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair AK, Gautieri A, Chang S-W, Buehler MJ. Molecular mechanics of mineralized collagen fibrils in bone. Nat Commun. 2013;4: 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poundarik A, Diab T. Dilatational band formation in bone. Proc Natl Acad Sci. 2012;109(47):19178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansma PK, Fantner GE, Kindt JH, Thurner PJ, Schitter G, Turner PJ, et al. Sacrificial bonds in the interfibrillar matrix of bone. J Musculoskelet Neuronal Interact. 2005;5(4):313–5. [PubMed] [Google Scholar]
- 18.Fyhrie DP, Christiansen BA. Bone material properties and skeletal fragility. Calcif Tissue Int. 2015;97(3):213–28. [DOI] [PubMed] [Google Scholar]
- 19.Fan Z, Swadener JG, Rho JY, Roy ME, Pharr GM. Anisotropic properties of human tibial cortical bone as measured by nanoindentation. J Orthop Res. 2002;20(4):806–10. [DOI] [PubMed] [Google Scholar]
- 20.Currey JD. The structure and mechanics of bone. J Mater Sci. 2012;47(1):41–54. [Google Scholar]
- 21.Turner C, Burr D. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14(4):595–608. [DOI] [PubMed] [Google Scholar]
- 22.Goodyear SR, Aspden RM. Mechanical properties of bone ex vivo. In: Helfrich MH, Ralston SH, editors. Bone research protocols. Totowa: Humana Press; 2012. p. 555–71. [DOI] [PubMed] [Google Scholar]
- 23.Jepsen KJ, Silva MJ, Vashishth D, Guo XE, van der Meulen MC. Establishing biomechanical mechanisms in mouse models: practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J Bone Miner Res. 2015;30(6):951–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner CH, Burr DB. Experimental techniques for bone mechanics. In: Cowin SC, editor. Bone Mechanics handbook, vol. 2; 2001. p. 7–1. [Google Scholar]
- 25.Sharir A, Barak MM, Shahar R. Whole bone mechanics and mechanical testing. Vet J. 2008;177(1):8–17. [DOI] [PubMed] [Google Scholar]
- 26.Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res. 1999;14(12):2159–66. [DOI] [PubMed] [Google Scholar]
- 27.Jepsen KJ, Hu B, Tommasini SM, Courtland H-W, Price C, Terranova CJ, et al. Genetic randomization reveals functional relationships among morphologic and tissue-quality traits that contribute to bone strength and fragility. Mamm Genome. 2007;18(6–7): 492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace JM, Golcuk K, Morris MD, Kohn DH. Inbred strain-specific effects of exercise in wild type and Biglycan deficient mice. Ann Biomed Eng. 2010;38(4):1607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Guss JD, Horsfield MW, Fontenele FF, Sandoval TN, Luna M, Apoorva F, et al. Alterations to the gut microbiome impair bone strength and tissue material properties. J Bone Miner Res. 2017;32(6):1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]; The results of this study suggests that tissue material properties may also be impaired and contribute to fracture risk in patients with conditions associated with an altered microbiome.
- 30.Sinder BP, Salemi JD, Ominsky MS, Caird MS, Marini JC, Kozloff KM. Rapidly growing Brtl/+ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment. Bone. 2015;71:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vashishth D Small animal bone biomechanics. Bone. 2008;43(5): 794–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berman AG, Wallace JM, Bart ZR, Allen MR. Raloxifene reduces skeletal fractures in an animal model of osteogenesis imperfecta. Matrix Biol. 2016;52–54:19–28. [DOI] [PubMed] [Google Scholar]
- 33.Bi X, Grafe I, Ding H, Flores R, Munivez E, Jiang MM, et al. Correlations between bone mechanical properties and bone composition parameters in mouse models of dominant and recessive osteogenesis imperfecta and the response to anti-TGF-β treatment. J Bone Miner Res. 2017;32(2):347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grafe I, Alexander S, Yang T, Lietman C, Homan EP, Munivez E, et al. Sclerostin antibody treatment improves the bone phenotype of Crtap−/− mice, a model of recessive osteogenesis imperfecta. J Bone Miner Res. 2016;31(5):1030–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert J, Lamothe JM, Zernicke RF, Auer RN, Reimer RA. Dietary restriction does not adversely affect bone geometry and mechanics in rapidly growing male Wistar rats. Pediatr Res. 2005;57(2):227–31. [DOI] [PubMed] [Google Scholar]
- 36.Oftadeh R, Perez-Viloria M, Villa-Camacho JC, Vaziri A, Biomechanics NA. Mechanobiology of trabecular bone: a review. J Biomech Eng. 2015;137(1):108021–1080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rho JY, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20(2):92–102. [DOI] [PubMed] [Google Scholar]
- 38.Jamal SA, West SL, Miller PD. Fracture risk assessment in patients with chronic kidney disease. Osteoporos Int. 2012;23(4):1191–8. [DOI] [PubMed] [Google Scholar]
- 39.LL KEE, T BC, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167(2):133–9. [DOI] [PubMed] [Google Scholar]
- 40.Oksztulska-Kolanek E, Znorko B, Michałowska M, Pawlak K. The biomechanical testing for the assessment of bone quality in an experimental model of chronic kidney disease. Nephron. 2016;132(1):51–8. [DOI] [PubMed] [Google Scholar]
- 41.Newman CL, Chen NX, Smith E, Smith M, Brown D, Moe SM, et al. Compromised vertebral structural and mechanical properties associated with progressive kidney disease and the effects of traditional pharmacological interventions. Bone. 2015;77:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritchie R, Koester K, Ionova S, Yao W. Measurement of the toughness of bone: a tutorial with special reference to small animal studies. Bone. 2008;43(5):798–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vashishth D Rising crack-growth-resistance behavior in cortical bone: implications for toughness measurements. J Biomech. 2004;37(6):943–6. [DOI] [PubMed] [Google Scholar]
- 44•.Bailey S, Karsenty G, Gundberg C, Vashishth D. Osteocalcin and osteopontin influence bone morphology and mechanical properties. Ann N Y Acad Sci. 2017;1409(1):79–6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that bone strength is maintained in the absence of osteocalcin and osteopontin due to morphological adaptation.
- 45.Poundarik A, Diab T, Sroga G, Ural A, Boskey A, Gundberg C, et al. Dilatational band formation in bone. Proc Natl Acad Sci. 2012;109(47):19178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinwald S, Peterson RG, Allen MR, Burr DB. Skeletal changes associated with the onset of type 2 diabetes in the ZDF and ZDSD rodent models. Am J Physiol Endocrinol Metab. 2009;296(4): E765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Creecy A, Uppuganti S, Merkel AR, O’Neal D, Makowski AJ, Granke M, et al. Changes in the fracture resistance of bone with the progression of type 2 diabetes in the ZDSD rat. Calcif Tissue Int. 2016;99(3):289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirzaali MJ, Schwiedrzik JJ, Thaiwichai S, Best JP, Michler J, Zysset PK, et al. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone. 2016;93:196–211. [DOI] [PubMed] [Google Scholar]
- 49.Katsamenis OL, Jenkins T, Thurner PJ. Toughness and damage susceptibility in human cortical bone is proportional to mechanical inhomogeneity at the osteonal-level. Bone. 2015;76:158–68. [DOI] [PubMed] [Google Scholar]
- 50.Granke M, Makowski AJ, Uppuganti S, Nyman JS. Prevalent role of porosity and osteonal area over mineralization heterogeneity in the fracture toughness of human cortical bone. J Biomech. 2016;49(13): 2748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Hunckler MD, Chu ED, Baumann AP, Curtis TE, Ravosa MJ, Allen MR, et al. The fracture toughness of small animal cortical bone measured using arc-shaped tension specimens: effects of bisphosphonate and deproteinization treatments. Bone. 2017;105:67–74. [DOI] [PubMed] [Google Scholar]; In this study, arc-shaped tension specimens were created as a novel technique for measuring cortical bone fracture toughness at multiple locations in small animals.
- 52.Silva MJ, Touhey DC. Bone formation after damaging in vivo fatigue loading results in recovery of whole-bone monotonic strength and increased fatigue life. J Orthop Res. 2007;25(2):252–61. [DOI] [PubMed] [Google Scholar]
- 53.George WT, Vashishth D. Damage mechanisms and failure modes of cortical bone under components of physiological loading. J Orthop Res. 2005;23(5):1047–53. [DOI] [PubMed] [Google Scholar]
- 54.Vashishth D, Tanner KE, Bonfield W. Fatigue of cortical bone under combined axial-torsional loading. J Orthop Res. 2001;19(3):414–20. [DOI] [PubMed] [Google Scholar]
- 55.George W, Vashishth D. Susceptibility of aging human bone to mixed-mode fracture increases bone fragility. Bone. 2006;38(1): 105–11. [DOI] [PubMed] [Google Scholar]
- 56•.Bajaj D, Geissler JR, Allen MR, Burr DB, Fritton JC. The resistance of cortical bone tissue to failure under cyclic loading is reduced with alendronate. Bone. 2014;64:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports that cyclic mechanical properties of cortical bone are reduced due to alterations of bone structure with bisphonate treatment.
- 57•.Torres AM, Matheny JB, Keaveny TM, Taylor D, Rimnac CM, Hernandez CJ. Material heterogeneity in cancellous bone promotes deformation recovery after mechanical failure. Proc Natl Acad Sci. 2016;113(11):2892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports that stress concentrations at the strut surfaces of cancellous bone are a result of reduced accumulation of advanced glycation end-products.
- 58.Akkus O, Rimnac CM. Cortical bone tissue resists fatigue fracture by deceleration and arrest of microcrack growth. J Biomech. 2001;34(6):757–64. [DOI] [PubMed] [Google Scholar]
- 59.Vashishth D, Tanner KE, Behiri JC, Bonfield W. Failure of osteons under differently applied loads. Trans Orthop Res Soc. 1994;19:429. [Google Scholar]
- 60.Zysset PK. Indentation of bone tissue: a short review. Osteoporos Int. 2009;20(6):1049–55. [DOI] [PubMed] [Google Scholar]
- 61.Nyman JS, Granke M, Singleton RC, Pharr GM. Tissue-level mechanical properties of bone contributing to fracture risk. Curr Osteoporos Rep. 2016;14(4):138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shuman DJ, Costa ALM, Andrade MS. Calculating the elastic modulus from nanoindentation and microindentation reload curves. Mater Charact. 2007;58(4):380–9. [Google Scholar]
- 63.Wolfram U, Schwiedrzik J. Post-yield and failure properties of cortical bone. BoneKEy Reports. 2016;5:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40(4):1144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang S, Allen M, Phipps R, Burr D, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20(6):887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ascenzi M-G, Di Comite M, Mitov P, Michael Kabo J. Hysteretic pinching of human secondary osteons subjected to torsion. J Biomech. 2007. January;40(12):2619–27. [DOI] [PubMed] [Google Scholar]
- 67.Guo XE, He MY, Goldstein SA. Understanding cement line interface in bone tissue: a linear fracture mechanics approach. ASME-Publications-BED. 1995;29:303. [Google Scholar]
- 68.Hansma P, Turner P, Drake B, Yurtsev E, Proctor A, Mathews P, et al. The bone diagnostic instrument II: indentation distance increase. Rev Sci Instrum. 2008;79(6):64303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allen MR, McNerny EMB, Organ JM, Wallace JM. True gold or pyrite: a review of reference point indentation for assessing bone mechanical properties in vivo. J Bone Miner Res. 2015;30(9):1539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jepsen KJ, Schlecht SH. Biomechanical mechanisms: resolving the apparent conundrum of why individuals with type II diabetes show increased fracture incidence despite having normal BMD. J Bone Miner Res. 2014;29(4):784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furst JR, Bandeira LC, Fan W-W, Agarwal S, Nishiyama KK, McMahon DJ, et al. Advanced glycation endproducts and bone material strength in type 2 diabetes. J Clin Endocrinol Metab. 2016;101(6):2502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malgo F, Hamdy NAT, Papapoulos SE, Appelman-Dijkstra NM. Bone material strength index as measured by impact microindentation is low in patients with fractures irrespective of fracture site. Osteoporos Int. 2017;28(8):2433–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gallant MA, Brown DM, Organ JM, Allen MR, Burr DB. Reference-point indentation correlates with bone toughness assessed using whole-bone traditional mechanical testing. Bone. 2013;53(1):301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carriero A, Bruse JL, Oldknow KJ, Millán JL, Farquharson C, Shefelbine SJ. Reference point indentation is not indicative of whole mouse bone measures of stress intensity fracture toughness. Bone. 2014;69:174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krege JB, Aref MW, McNerny E, Wallace JM, Organ JM, Allen MR. Reference point indentation is insufficient for detecting alterations in traditional mechanical properties of bone under common experimental conditions. Bone. 2016;87:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boskey AL, Robey PG. The composition of bone. Prim Metab Bone Dis Disord Miner Metab. 8th ed. 2013;49–58. [Google Scholar]
- 77.Zysset PK, Edward Guo X, Edward Hoffler C, Moore KE, Goldstein SA. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J Biomech. 1999;32(10):1005–12. [DOI] [PubMed] [Google Scholar]
- 78.Casanova M, Balmelli A, Carnelli D, Courty D, Schneider P, Müller R. Nanoindentation analysis of the micromechanical anisotropy in mouse cortical bone. R Soc Open Sci. 2017;4(2):160971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hengsberger S, Kulik A, Zysset PK. A combined atomic force microscopy and nanoindentation technique to investigate the elastic properties of bone structural units. Eur Cells Mater. 2001;1:12–7. [DOI] [PubMed] [Google Scholar]
- 80.Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7(6):1564–83. [Google Scholar]
- 81.Wang C, Wang Y, Meng H, Gou W, Yuan X, Xu X, et al. Microstructure and Nanomechanical properties of single trabecular bone in different regions of osteonecrosis of the femoral head. J Nanosci Nanotechnol. 16:2264–9. [DOI] [PubMed] [Google Scholar]
- 82.Aruwajoye OO, Aswath PB, Kim HKW. Material properties of bone in the femoral head treated with ibandronate and BMP-2 following ischemic osteonecrosis. J Orthop Res. 2017;35(7):1453–60. [DOI] [PubMed] [Google Scholar]
- 83.Islam A, Neil Dong X, Wang X. Mechanistic modeling of a nanoscratch test for determination of in situ toughness of bone. J Mech Behav Biomed Mater. 2012;5(1):156–64. [DOI] [PubMed] [Google Scholar]
- 84.Kataruka A, Mendu K, Okeoghene O, Puthuvelil J, Akono AT. Microscopic assessment of bone toughness using scratch tests. Bone Reports. 2017;6:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Xu H, Huang Y, Gu S, Jiang JX. Coupling effect of water and proteoglycans on the in situ toughness of bone. J Bone Miner Res. 2016;31(5):1026–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zimmermann EA, Gludovatz B, Schaible E, Busse B, Ritchie RO. Fracture resistance of human cortical bone across multiple length-scales at physiological strain rates. Biomaterials. 2014;35(21):5472–81. [DOI] [PubMed] [Google Scholar]
- 87.Gupta HS, Seto J, Wagermaier W, Zaslansky P, Boesecke P, Fratzl P. Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc Natl Acad Sci U S A. 2006;103(47):17741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rudolf C, Boesl B, Agarwal A. In situ mechanical testing techniques for real-time materials deformation characterization. JOM. 2016;68(1):136–42. [Google Scholar]
- 89.Gupta HS, Krauss S, Kerschnitzki M, Karunaratne A, Dunlop JWC, Barber AH, et al. Intrafibrillar plasticity through mineral/collagen sliding is the dominant mechanism for the extreme toughness of antler bone. J Mech Behav Biomed Mater. 2013;28:366–82. [DOI] [PubMed] [Google Scholar]
- 90.Zimmermann EA, Schaible E, Gludovatz B, Schmidt FN, Riedel C, Krause M, et al. Intrinsic mechanical behavior of femoral cortical bone in young, osteoporotic and bisphosphonate-treated individuals in low- and high energy fracture conditions. Sci Rep. 2016;6:21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Samuel J, Park J-S, Almer J, Wang X. Effect of water on nanomechanics of bone is different between tension and compression. J Mech Behav Biomed Mater. 2016;57:128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]


