Abstract
Background:
Laboratory testing practices are an important part of sexually transmitted disease (STD) diagnosis and control. The goal of this article is to describe the volume and types of STD tests performed in public health laboratories (PHLs) in the United States in 2016.
Methods:
A total of 105 state and local PHLs who were members of the Association of Public Health Laboratories were invited to participate in a survey between May and August 2017. This web-based survey included questions on types of tests offered for different types of STDs, the number of tests offered in 2016, and number of samples that tested positive for each STD.
Results:
Eighty-one (77.1%) of 105 member PHLs responded. Overall in 2016, 2,242,728 Chlamydia trachomatis tests, 2,298,596 Neisseria gonorrhoeae (GC) tests, 1,235,037 Treponema pallidum (syphilis) tests, 177,571 Trichomonas vaginalis tests, 37,101 herpes simplex virus tests, and 8707 human papillomavirus tests were performed in 2016 in surveyed laboratories. Sixty-seven (82.7%) of PHLs offered C. trachomatis and GC nucleic acid amplification testing. Ninety percent of laboratories performed syphilis testing, 42% performed T. vaginalis testing, and 28.4% performed herpes simplex virus type–specific nucleic acid amplification testing. Few laboratories tested for human papillomavirus.
Conclusions:
This survey collected important information on PHL STD laboratory testing practices. Capacity for important reference laboratory testing, such as GC culture and antimicrobial susceptibility, are needed to support STD programs. Public health laboratories play a key role in STD screening, surveillance, and prevention by offering test methods that are not available at commercial laboratories. Funding constraints affect the resources available for STD screening and surveillance, and thus, it is important to continue to monitor testing practices and the capacity of PHLs to test for STDs.
Sexually transmitted diseases are on the rise in the United States. The Centers for Disease Control and Prevention (CDC) estimates that, in 2017, chlamydia rates increased at 6.89%, gonorrhea rates increased at 18.58%, syphilis rates increased at 10.17%, and congenital syphilis rates increased at 43.66% from 2016.1 Sexually transmitted diseases (STDs) cause adverse reproductive health outcomes and increase risk of HIV infection. Many STDs are asymptomatic or have nonspecific symptoms requiring laboratory detection for diagnosis.2–4 Laboratory technologies and practices to detect STDs are continuously evolving with significant changes over the years, such as the increased need to test extragenital specimens and the recent Food and Drug Administration clearance of these specimen types, the use of reverse algorithm syphilis screening including using automated treponemal assays and/or point-of-care assays as the initial test, and a range of new and improved test technologies on the market. In addition, STD testing guidelines continue to be updated. For example, in 2002, the CDC recommended that repeat testing be routinely performed after a positive nucleic acid amplification test (NAAT) for Chlamydia trachomatis (CT)/Neisseria gonorrhoeae (GC) to improve the positive predictive value of a NAAT test.5 However, studies examining the utility of routine repeat testing of positive specimens demonstrated >90% concurrence with the initial test for CT/GC; therefore, routine additional testing after a positive NAAT result for CT/GC is no longer recommended by CDC.5
In addition, the spread of antimicrobial-resistant sexually transmitted pathogens has led to an increasing need for antimicrobial susceptibility testing. In 2007, emergence of fluoroquinolone-resistant GC in the United States prompted the CDC to cease recommending fluoroquinolones for treatment of GC, leaving only cephalosporins as the sole remaining class of antimicrobials available for treatment of gonorrhea in the United States.6 Antimicrobial resistance to GC treatment regimens is a continuing problem in the United States and worldwide, and the ability to test for GC antimicrobial resistance is of increasing importance.6 Unlike other bacteria, GC is treated empirically, but with the rise of antimicrobial-resistant GC, the risk of treatment failure increases, which is why it is important to be able to test for antimicrobial-resistant GC. However, although many PHLs have the capability to perform these types of tests, many lack the capacity (i.e., lack sufficient money for testing). Billing and reimbursement policies limit the types of STD tests PHLs can be reimbursed for, which can significantly limit their capacity for conducting STD testing to support STD clinics. Over the years, there was a move toward more NAAT and less culture testing, combined with a 40% decrease in federal STD funding since 2003.7 Thus, for many years, GC susceptibility testing was not a prioritized function by PHLs or CDC. Testing for GC treatment failure is a relatively rare event and unlikely to be tested for in the private sector, so PHLs play a vital role in this area. Given the continued spread of antimicrobial-resistant GC, the concern over detecting a treatment failure, and emergence of other antimicrobial-resistant STD pathogens, such as Mycoplasma genitalium, it is important to monitor the PHL testing practices and capacity in this area.
A few states, such as California, routinely survey their public and private laboratories for STD testing procedures and volume, and there have been some surveys of commercial laboratories.8 However, testing practices may differ between commercial laboratories and PHLs, such as use of different testing methods and specimen types. No recent surveys have described STD testing practices among PHLs at the national level. The Association of Public Health Laboratories (APHL) surveyed local and state public health laboratories (PHLs) across the United States about the type and volume of tests being used to detect STDs. Between this survey conducted in 2017 and the previous APHL survey conducted in 2011, the CDC published important updates to changes in laboratory testing guidelines for CT/GC.5 The purposes of this analysis are to describe the types and volume of laboratory tests for CT, GC, Treponema pallidum (syphilis), Trichomonas vaginalis (TV), herpes simplex virus (HSV), and human papillomavirus (HPV) conducted in PHLs in the United States in 2016 and to examine their alignment with current CDC laboratory testing guidelines.
METHODS
Laboratory Survey
A total of 105 state and local PHLs who are members of APHL were invited to participate in a survey between May and August 2017. To be eligible to participate, PHLs had to be members of the APHL. The APHL fielded its survey using a 35-question online tool created by the APHL STD Subcommittee and administered through Qualtrics, a web-based survey instrument. The APHL fielded the survey to all 105 member-institutions, including 54 state and 51 local PHLs. This web-based survey included questions on types of tests offered for different types of STD pathogens, the number of tests offered in 2016, and number of samples that tested positive for each STD pathogen. The survey also included questions on antimicrobial susceptibility testing.
An e-mail was sent to the laboratory directors and included a direct link to a web-based survey with a unique user ID and password to access the survey. Laboratories were asked to complete the survey in 4 weeks. Follow-up reminders were sent via e-mail to nonresponders 1 week before the planned survey closure (June 30th). The deadline was then extended to July 14th via an e-mail notification. Laboratories that had not completed the survey by July 14th were then contacted individually until the end of August, at which point the survey was closed.
Data Analysis
Descriptive statistics were calculated for all survey items to determine the number and percent of PHL that reported performing laboratory tests for each of the STD pathogens listed in the survey. All analyses were conducted in SPSS version 25.
RESULTS
Response Rate
The survey was completed by 81 of 105 PHLs, for an overall response rate of 77.1%. Of the 81 PHLs, 51 (63.0%) were state PHLs and 30 (37.0%) were local PHLs. Of the state PHLs, 51 of 54 completed the survey, for a response rate of 94.4%; of the local PHLs, 30 of 51 completed the survey, for a response rate of 58.8%. The responding laboratories were located in 51 states and territories and the District of Columbia (Fig. 1).
Figure 1.
Map of state and local public health laboratories that completed the survey, 2016. *State PHLs that completed the survey are shaded in blue; local PHLs that completed the survey are shaded in red; state PHLs that did not complete the survey are shaded in gray.
Testing Summary
Overall in 2016, 2,242,728 CT tests, 2,298,596 GC tests, 1,235,037 syphilis tests, 177,571 TV tests, 37,101 HSV tests, and 8707 HPV tests were performed in surveyed laboratories (Table 1). Most PHLs offered testing for syphilis (90.1%), GC (88.9%), and CT (84.0%). Approximately half offered testing for HSV and 42.0% offered testing for TV. Few offered testing for HPV (3.7%).
TABLE 1.
Proportion of Total Tests Volume by Pathogen, 2016 (n = 5,999,740)
| Type of Pathogen | Total (n = 5,999,740), n (%) |
|---|---|
| Treponema pallidum (syphilis) | 1,235,037 (20.6) |
| GC | 2,298,596 (38.3) |
| CT | 2,242,728 (37.4) |
| TV | 177,571 (3.0) |
| HSV | 37,101 (0.6) |
| HPV | 8707 (0.1) |
CT and GC Testing Practices
On average, laboratories received 32,981 specimens for CT testing during the year 2016, with a range of 66 to 208,737 specimens received (median, 20,536; interquartile range [IQR], 8616–48,047). Of all specimens received, 9.75% tested positive on average, with a range of 0 to 40.91% positive (median, 9.27%; IQR, 7.54%–10.46%). The number of GC specimens tested by any method in 2016 was 31,925 on average, with a range of 2 to 209,175 (median, 18,928; IQR, 3570–48,084). The average percent of GC specimens testing positive was 7.79%, with a range of 0 to 100% (median, 3.64%; IQR, 2.05%–5.53%).
Of the 81 responding PHLs, 68 (84.0%) performed at least one method of CT testing in-house and 72 (88.9%) performed at least one method of GC testing in-house. Nucleic acid amplification testing was the most widely reported method of CT testing (n = 67 [82.7%]; 43 state, 24 local), although a few PHLs also reported conducting CT culture (n = 9 [11.1%]; 5 state, 4 local) or direct fluorescent antibody tests (n = 6 [7.4%]; 1 state, 5 local). For GC, 67 (82.7%; 43 state, 24 local) offered NAAT, 52 (64.2%; 33 state, 19 local) offered culture, and 38 (46.9%; 18 state, 20 local) offered Gram stain.
The APTIMA Combo 2 test for CT/NG (Hologic, San Diego, CA) was most commonly reported as the primary test used to detect CT (n = 45 [55.6%]) and GC (n = 44 [54.3%]; Table 2).
TABLE 2.
Primary Testing Method Used for CT and GC by PHLs Offering In-house Testing
| Test | CT (n = 68), n (%) |
GC (n = 72), n (%) |
|---|---|---|
| APTIMA Combo 2 for CT/NG (Tigris, Panther, or DTS; Hologic) | 45 (66.2) | 44 (61.1) |
| APTIMA GC (Tigris, Panther, or DTS; Hologic) | — | 10 (13.9) |
| APTIMA CT (Tigris, Panther, or DTS) (Hologic) | 8 (11.8) | — |
| RealTime CT/NG (Abbott) | 1 (1.5) | 1 (1.4) |
| ProbeTec ET CT/GC Amplified DNA Assays (BD) | 3 (4.4) | 2 (2.8) |
| ProbeTec CT Qx Amplified DNA Assay (for Viper; BD) | 6 (8.8) | — |
| ProbeTec GC Qx Amplified DNA Assay (for Viper; BD) | — | 6 (8.3) |
| GeneXpert CT/NG (Cepheid) | 3 (4.4) | 3 (4.2) |
| Cobas 4800 CT/NG (Roche) | 1 (1.5) | 1 (1.4) |
| Laboratory-developed polymerase chain reaction | 1 (1.5) | — |
| Culture | — | 5 (6.9) |
Public health laboratory testing practices were mostly aligned with CDC guidelines. Of the 67 PHLs that conducted NAAT for CT, 71.6% (n = 48) did not conduct repeat testing on positive CT NAAT specimens, 17.9% (n = 12) occasionally conducted repeat testing on positive specimens, and 10.4% (n = 7) routinely conducted repeat testing. Of the 67 PHLs that conducted GC NAAT, 68.7% (n = 46) did not conduct repeat testing on positive GC NAAT specimens, 20.9% occasionally conducted repeat testing on positive specimens, and 10.4% routinely conducted repeat testing. Reasons cited for repeat testing included quality recheck, equipment failure or laboratory error, test of cure, indeterminate results, or possible cross-contamination or specimen mix-up.
For CT NAAT, most PHLs accepted urine (80.2%), male urethral swabs (75.3%), endocervical swabs (74.1%), vaginal swabs (69.1%), and rectal swabs (58.0%; Table 3). For GC NAAT, most PHLs accepted urine (79.0%), male urethral swabs (75.3%), endocervical swabs (75.3%), vaginal swabs (69.1%), and rectal swabs (59.3%). χ2 Tests revealed no statistically significant differences between state and local PHLs in acceptance of specimen types. Urine was the most common specimen type received for men (69.1%) and women (54.3%) for CT NAAT and for men (70.4%) and women (53.1%) for GC NAAT (Fig. 2). Twelve PHLs (14.8%) reported receiving specimens specifically for lymphogranuloma venereum testing. Of the 22 laboratories (27.2%) that reported performing antimicrobial susceptibility testing for GC, more than half (n = 12 [54.5%]) reported using disc diffusion or Etest to test for GC antimicrobial susceptibility. Of the 22 laboratories that had the capacity to test for GC susceptibility, 77.3% (n = 17) could test for resistance to ceftriaxone, 72.7% (n = 16) to cefixime, and 68.2% (n = 15) to azithromycin.
TABLE 3.
Number of Laboratories That Accept the Following Sample Types for CT and GC NAATs (n = 81)
| Type of Sample | CT, n (%) | GC, n (%) |
|---|---|---|
| Anorectal/rectal swab | 47 (58.0) | 48 (59.3) |
| State PHLs/local PHLs | 29/18 | 30/18 |
| Endocervical swab | 60 (74.1) | 61 (75.3) |
| State PHLs/local PHLs | 37/23 | 38/23 |
| Endocervical specimen in liquid Papanicolaou collector | 4 (4.9) | 5 (6.2) |
| State PHLs/local PHLs | 4/0 | 5/0 |
| Male urethral swab | 61 (75.3) | 61 (75.3) |
| State PHLs/local PHLs | 37/24 | 37/24 |
| Ocular/conjunctival swab | 3 (3.7) | 3 (3.7) |
| State PHLs/local PHLs | 2/1 | 1/2 |
| Oropharyngeal/throat swab | 40 (49.4) | 42 (51.9) |
| State PHLs/local PHLs | 24/16 | 27/15 |
| Serum | 0 (0.0) | 1 (1.2) |
| State PHLs/local PHLs | 0/0 | 0/1 |
| Urine | 65 (80.2) | 64 (79.0) |
| State PHLs/local PHLs | 41/24 | 40/24 |
| Vaginal swab | 56 (69.1) | 56 (69.1) |
| State PHLs/local PHLs | 37/19 | 37/19 |
| Does not conduct NAATs | 14 (17.3) | 14 (17.3) |
| State PHLs/local PHLs | 8/6 | 8/6 |
Figure 2.
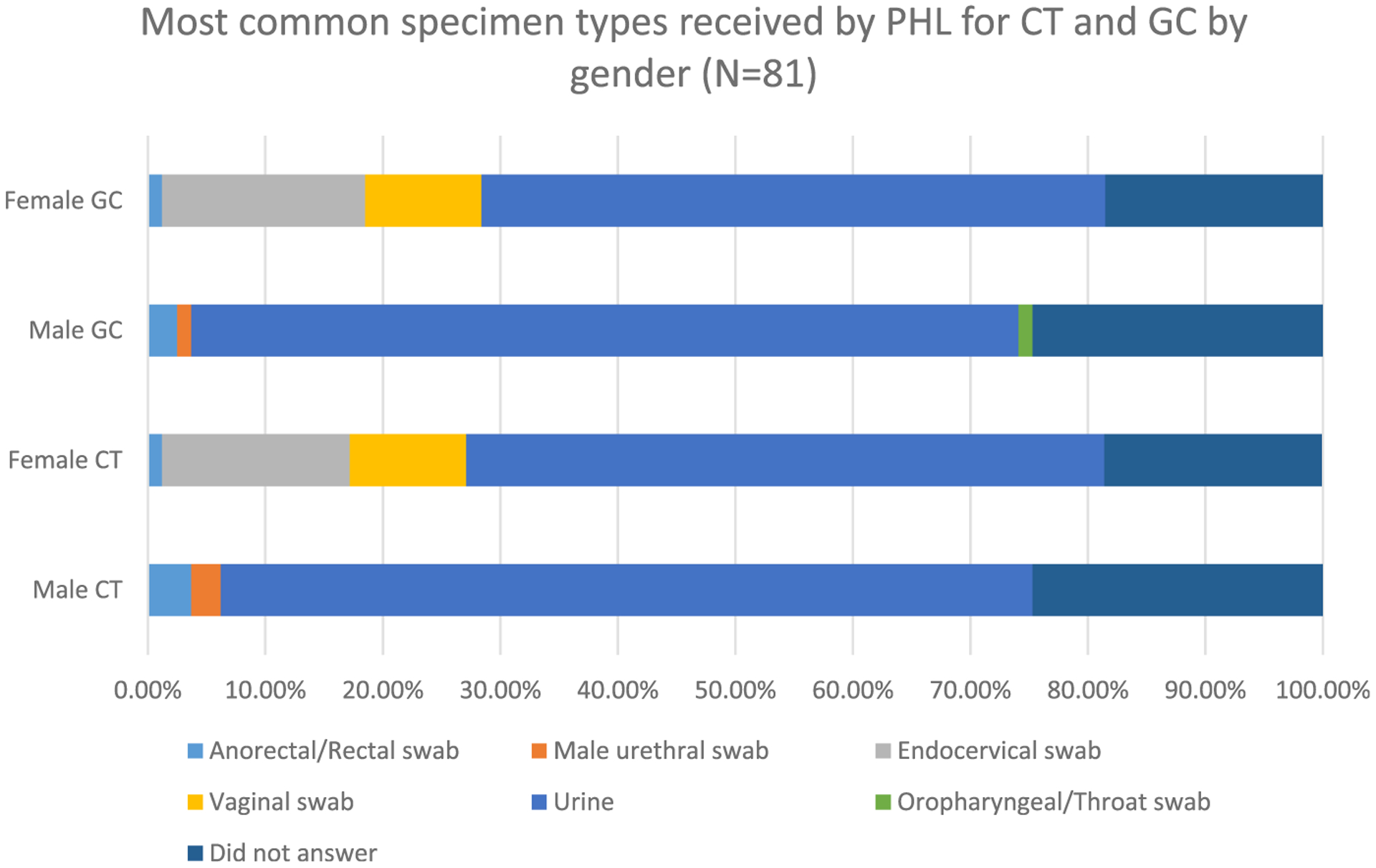
Most common specimen types received by PHL for CT and GC by sex (n = 81).
T. pallidum (Syphilis) Testing
The average number of syphilis specimens received for testing in a PHL was 16,918, with a range of 1 to 149,023 (median, 6663; IQR, 2083–21,933). On average, 9.92% of specimens tested positive for syphilis, with a range of 0 to 53.48% (median, 5.45%; IQR, 3.19%–11.75%).
Of the 81 PHLs, 73 (90.1%) reported conducting any type of syphilis test in-house. Most laboratories (n = 59 [72.8%]) reported conducting rapid plasma reagin (RPR) qualitative testing for syphilis in-house, followed by 67.9% (n = 55) offering RPR quantitative testing, 66.7% (n = 54) offering T. pallidum particle agglutination, and 42.0% (n = 34) offering Venereal Disease Research Laboratory testing (Table 4).
TABLE 4.
Types of Syphilis Tests Offered In-house (n = 81)
| Type of Syphilis Test | In-house, n (%) |
|---|---|
| Dark-field microscopy | 9 (11.1) |
| RPR quantitative | 55 (67.9) |
| RPR qualitative | 59 (72.8) |
| Direct fluorescent antibody | 1 (1.2) |
| Western blot | 2 (2.5) |
| Venereal Disease Research Laboratory | 34 (42.0) |
| Unheated serum reagin | 2 (2.5) |
| Unheated serum test | 0 (0.0) |
| EIA, CIA, MBIA | 21 (25.9) |
| NAAT | 2 (2.5) |
| Fluorescent treponemal antibody absorption | 15 (18.5) |
| T. pallidum particle agglutination | 54 (66.7) |
| CLIA-waived point-of-care test/rapid diagnostic kit | 4 (4.9) |
CIA indicates chemiluminescence immunoassay; CLIA, Clinical Laboratory Improvement Amendments; EIA, enzyme immunoassay; MBIA, microbead immunoassay.
When asked about syphilis testing algorithms for serum/plasma/whole blood samples, most (n = 58 [79.5%]) reported using a traditional algorithm (i.e., starting with a nontreponemal assay) as the first test in the algorithm. For those PHLs performing the traditional algorithm (i.e., starting with a nontreponemal assay as the first test in the testing algorithm), greater than 40% (n = 24) used the Macro-Vue RPR Card Test (BD, Franklin Lakes, NJ), and greater than 25% (n = 16) used the AsiManager AT/ASI RPR Card Test (Arlington Scientific, Springville, UT). Of the 15 PHLs (20.5%) that reported using a nontraditional algorithm (i.e., starting with a treponemal assay as the first test in the testing algorithm), 46.7% (n = 7) used the Trinity CAPTIA Syphilis-G assay (Ireland), 20.0% (n = 3) used the Bio Rad BioPlex 2200 Syphilis IgG assay (Hercules, CA), 13.3% (n = 2) used the Diasorin LIAI-SON Treponema assay (Saluggia, Italy), and 20.0% (n = 3) used other assays.
Of the laboratories that tested for syphilis, only more than a third (n = 26 [35.6%]) reported having an alternative protocol for samples with a history of syphilis. More than a third (36.2% [n = 21]) of PHLs using traditional algorithms had an alternative protocol, and 33.3% (n = 5) of PHLs using nontraditional algorithms had an alternative protocol. Seventeen PHLs (23.3%) reported having been asked to test a sample to determine if the cause of a chancre was syphilis or herpes.
TV Testing
On average, 5223 specimens were collected for TV testing, with a range of 0 to 97,709 (median, 679; IQR, 119–3135). An average of 10.77% of specimens tested positive for TV, with a range of 0 to 60% testing positive (median, 8.74%; IQR, 4.34%–14.77%).
Of the 81 responding PHLs, 34 (42.0%) offered TV testing. T. vaginalis testing was more likely to be offered by local PHLs than by state PHLs. Seventy-three percent (n = 22) of all local PHLs offered TV testing, whereas only 23.5% (n = 12) of state PHLs offered testing. The most common types of TV tests used for in-house testing were Wet Mount (n = 23 [28.4%]) and NAAT (n = 17 [21.0%]).
HSV Testing
The average number of HSV specimens collected was 863, with a range of 18 to 9926 (median, 402; IQR, 200–851). An average of 37.62% of specimens tested positive for HSV, with a range of 8.7% to 63.0% (median, 38.63%; IQR, 28.53%–46.55%).
Of the 81 PHLs, 43 (53.1%) offered any type of HSV testing in-house. Fifty percent (n = 15) of all local PHLs offered HSV testing, and 54.9% (n = 28) of state PHLs offered HSV testing. The most common types of HSV tests for in-house testing were culture (n = 30 [37.0%]), culture with typing (n = 25 [30.9%]), NAAT type–specific testing (n = 23 [28.4%]), direct fluorescent antibody testing (n = 14 [17.3%]), HSV-2 serology type specific (n = 14 [17.3%]), HSV-1 serology type specific (n = 12 [14.8%]), and HSV serology non–type specific (n = 3 [3.7%]). Of the 30 PHLs that used culture, 70.0% used standard cell culture, followed by 26.7% using shell vials. Of the 23 laboratories that used HSV NAAT, 39.1% used a laboratory developed NAAT, followed by 17.4% using the AmpliVue HSV 1 + 2 Assay (Quidel, San Diego, CA). Of the 14 PHLs that conducted HSV-2 serology, 57.1% used the HerpeSelect 2 ELISA IgG HSV-2 test (Focus, Cypress, CA), and of the 12 PHLs that conducted HSV-1 serology, 50.0% used the HerpeSelect 1 ELISA IgG HSV-1 test (Focus).
HPV Testing
An average of 2902 HPV specimens were collected, with a range of 500 to 4372 (median, 3835; IQR, 500–3835). An average of 17.8% of specimens tested positive for HPV, with a range of 10.0% to 23.0% (median, 20.2%; IQR, 10.0%–23.0%).
Very few laboratories (all of them state PHLs) offered HPV testing (n = 3 [3.7%]). It may be that few PHLs offer HPV testing because HPV is not a notifiable STD and HPV testing is normally associated with clinical care versus public health.
Plans to Add or Drop STD Testing Services
More than a third (34.6%) of laboratories said that they planned to add additional STD testing services within the next 12 months (from summer 2017 when the survey was conducted), 40.7% said that they had no plans to add services, and 24.7% said that they were unsure. Most frequent services that laboratories planned to add were any TV testing (35.7%), treponemal syphilis assays (28.6%), any HSV testing (25.0%), any M. genitalium testing (25.0%), and GC antimicrobial susceptibility testing (17.9%; Table 5).
TABLE 5.
Plans to Add the Following STD Tests in 12 Months (n = 28)
| Type of Test | n | % |
|---|---|---|
| NAAT for Detection of CT | 1 | 3.6 |
| Any lymphogranuloma venereum testing | 1 | 3.6 |
| Other chlamydia testing* | 2 | 7.1 |
| NAAT for detection of GC | 1 | 3.6 |
| Gonorrhea culture | 1 | 3.6 |
| GC antimicrobial susceptibility testing | 5 | 17.9 |
| Other gonorrhea testing† | 3 | 10.7 |
| Treponemal syphilis assays (e.g., enzyme immunoassay, rapid) | 8 | 28.6 |
| Other syphilis testing‡ | 4 | 14.3 |
| Any Trichomonas testing | 10 | 35.7 |
| Any herpes testing | 7 | 25.0 |
| Any HPV testing | 2 | 7.1 |
| Any Mycoplasma genitalium testing | 7 | 25.0 |
| Any next-generation sequencing methods for STDs | 1 | 3.6 |
| Any other STDs | 1 | 3.6 |
Includes CT NAAT for extragenital specimens (rectal and oropharyngeal).
Includes GC NAAT for extragenital specimens (rectal and oropharyngeal).
Includes rapid syphilis test for patients with bad veins, nontreponemal quantitative testing and treponemal IgG.
Six laboratories (7.4%) said that they planned to eliminate STD testing services within the next 12 months, whereas 70 (86.4%) said that they did not plan to eliminate services, and 5 (6.2%) said that they were unsure.
DISCUSSION
The results of this laboratory survey show the volume of STD tests conducted by APHL member laboratories in 2016. Comparing with the 2016 CDC STD surveillance report,9 PHLs in this survey identified more than 12.0% of total CT cases (204,546 positive CT test results, 1,598,354 CT cases reported) and 16.8% of total GC cases (78,851 positive GC test results, 468,514 total GC cases reported). Each positive test result at the PHL does not necessarily represent one case, but PHLs detect a fair number of CT and GC cases based on the number of positive test results. For syphilis, 27,814 cases were reported to CDC,1 and PHLs reported 92,293 positive syphilis test results in 2016. Because syphilis testing follows an algorithm, multiple positive test results are required to support a diagnosis of syphilis. However, based on CT and GC, one can conclude that PHLs detect a significant proportion of repeated syphilis cases. Public health laboratories are very likely to report positive cases to public health given their close proximity working directly with CDC and the health departments. However, compliance on reporting practices in the private sector is not as well understood.
In addition, results reflect that PHLs are quick to adapt to changing guidelines and technology in testing for STDs in the United States. The percentage of laboratories that reported using NAATs for CT were less in 2016 (82.7%) than in 2004 (87%), whereas the percentage of PHLs reporting using NAATs for GC was higher in 2016 (82.7%) than in 2004 (78.7%).10 The overall percentage of PHLs that reported performing GC culture was also less in 2016 (64.2%) than in 2004 (77.8%).10 These differences may be due to the fact that previously it was possible to order individual NAATs for CT and GC testing, whereas now CT/GC NAATs are primarily ordered together. It is possible that in 2004 laboratories started using CT NAAT before GC NAAT because it was cheaper than CT culture. However, it should also be noted that the laboratories who participated in the 2004 CDC survey were not necessarily the same laboratories who participated in this survey, so the changes do not necessarily reflect changes within the same laboratories. Although the CDC updated their testing guidelines in 2014, many PHLs are still not aligned with CDC guidelines on preferred specimen types to be used in NAAT for CTand GC. The CDC recommends first-catch urine as the optimal specimen type for men and vaginal swabs for women for CT and GC NAATs.5 Most laboratories reported urine as the most commonly tested specimen for both men and women for CT/GC NAAT. Although this is in line with CDC guidelines for testing male specimens, less than 10% received vaginal swabs (the CDC recommended optimal specimen type) for women as their most common type. Because PHLs can only test the specimens they receive, these findings may point to a broader issue of lack of uptake of new testing and treatment guidelines by clinicians and health care providers. Additional research is needed to determine the underlying reasons why clinicians and health care providers are not adhering to new testing and treatment guidelines and to identify ways to increase uptake of new guidelines.
Among the PHLs surveyed in 2017, only a quarter reported testing for GC antimicrobial resistance in 2016. However, we may see an increase in the capacity of PHLs to test for GC susceptibility in the coming years. In 2014, the White House developed the national strategy to “Combat Antibiotic-Resistant Bacteria.”11 In 2016, when CDC received federal funding to “Combat Antibiotic-Resistant Bacteria,” a number of new initiatives were created (e.g., regional testing for GC antibiotic resistance through the Antibiotic Resistance Laboratory Network) to reinvigorate GC culture and susceptibility testing in PHLs and prevent the spread of antibiotic-resistant GC.11 As these initiatives are expanded and implemented, there will likely be an increase in the number of PHLs conducting GC susceptibility testing and in the total volume of susceptibility tests. Several of the laboratories we surveyed planned to add GC antimicrobial susceptibility testing to their services. Of the 28 laboratories that planned to add STD services in the next year (2018), nearly 18% said that they planned to add GC antimicrobial susceptibility testing, which indicates a growing awareness of this problem and a possible response to additional funds available for GC susceptibility testing.
Most PHLs continued to perform syphilis testing. Public health laboratory capacity to provide access to syphilis testing is very important, particularly given their critical role in providing follow-up nontreponemal and confirmatory testing for treponemal positive persons, especially those identified in outbreak responses. The percent of laboratories that reported performing quantitative RPR tests increased from 15% in 2004 to 67.9% in 2016.10 Most PHLs used a traditional testing algorithm. However, only a third of PHL using a traditional or nontraditional testing algorithm reported having an alternative protocol for samples with a history of syphilis. Particularly for PHLs using nontraditional testing algorithms, alternative protocols need to be put into place to avoid performing treponemal testing on individuals who have past infections.
We also found a significant increase in testing for TV in PHLs. In 2004, only 4 of the PHLs surveyed performed TV testing compared with 34 laboratories in 2016.10 In addition, of the 28 laboratories planning to add STD services in the next year, more than a third of PHLs said that they planned to add TV testing. Although not a notifiable STD, TV is more prevalent than CT, GC, and syphilis combined and results in significant adverse health outcomes, including increased risk of HIV acquisition and transmission and preterm birth.12,13 The increase in laboratory testing of TV may reflect the increased recognition of the importance of this neglected pathogen. This increase may also be due to the increased availability of other assays for TV besides wet mount, including bundling with CT/GC and other assays. In 2013, there were only 3 nonwet mount assays that were Food and Drug Administration cleared for TV,14 but in 2016, that number increased to 8.15
The proportion of overall tests that were HSV tests in 2016 was only 0.6%. Low numbers of HSV tests are likely due to the fact that HSV is not a notifiable pathogen, there are no HSV screening guidelines, the use of HSV serology and culture is limited to symptomatic persons, and HSV is generally considered a clinical care issue, not a public health issue. Data from the National Health and Nutrition Examination Survey show a strong declining trend in both HSV-1 and HSV-2 seroprevalence from 1999 to 2016,16 indicating less of a need for HSV testing. The CDC recommends that HSV should be confirmed by type-specific laboratory testing and states that cell culture and polymerase chain reaction are the preferred HSV testing methods.17 Nucleic acid amplification testing methods, including polymerase chain reaction assays for HSV DNA, are more sensitive and should be used where available.17 In Europe, HSV DNA detection is now considered the criterion standard for diagnosis.18 Compared with cell culture, it is both more sensitive and specific. Thus, in Europe, cell culture is only recommended to determine antiviral sensitivity. Although US testing recommendations have not yet followed European testing guidelines, we found that PHLs were moving away from HSV culture and shifting toward HSV NAAT. There was a large increase in the percentage of PHL that performed NAAT type-specific testing (28.4% in 2016 vs. 6.1% in 2004) and a reduction in the PHLs that used cultures (37% in 2016 compared with 45.6% in 2004).10
There has been no increase in HPV testing from 2004 to 2016. Only 3 PHLs reported performing HPV testing in 2004 and in 2016. Human papillomavirus testing is a useful option along with a Papanicolaou test for screening women 30 years and older. It may be that few PHLs offer HPV testing because HPV is not a notifiable STD and HPV testing is normally associated with clinical care versus public health.
Appropriate testing practices for STDs are critical to efforts to control and prevent these diseases. Testing patterns can also affect our ability to monitor trends in the prevalence of STDs. For example, the use of more sensitive but more expensive NAATs for the detection of CT and GC could affect the number of tests that public screening programs are able to fund. On the other hand, the use of self-collected samples for CT/GC NAATs provides a potential mechanism that is patient directed. Although most self-collection occurs in the context of health care settings, self-collection can also be conducted through at-home test kits, which may provide an opportunity to increase screening among high-risk groups who do not access routine care in health care settings. Future surveys are needed to continue to monitor and evaluate STD testing in PHLs and to identify changes in testing patterns. Testing practices could affect the resources available for STD screening and surveillance, and thus, it is important to monitor these practices and the capacity of PHLs to appropriately test for STDs.
Acknowledgments:
The authors would like to thank the staff members at the public health laboratories that completed this survey.
Conflict of Interest and Sources of Funding:
A.D. is supported by the National Institute on Drug Abuse (K01DA044853) for career development. She also has received consulting fees from the Association of Public Health Laboratories. For the remaining authors, no conflicts of interest were declared. This publication was supported by Cooperative Agreement No. 5NU60OE000103, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
REFERENCES
- 1.Centers for Disease Control and Prevention. STDs in the United States, 2017. Available at: https://www.cdc.gov/nchhstp/newsroom/2018/2017-STD-surveillance-report.html. Accessed June 13, 2019.
- 2.Pack RP, Diclemente RJ, Hook EW 3rd, et al. High prevalence of asymptomatic STDs in incarcerated minority male youth: A case for screening. Sex Transm Dis 2000; 27:175–177. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg W STD screening in asymptomatic women. Patient Care 2004; 15:30–32. [Google Scholar]
- 4.Mayer KH, Bush T, Henry K, et al. Ongoing sexually transmitted disease acquisition and risk-taking behavior among US HIV-infected patients in primary care: Implications for prevention interventions. Sex Transm Dis 2012; 39:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63:1–19. [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines: gonococcal infections. Available at: https://www.cdc.gov/std/tg2015/gonorrhea.htm. Accessed 2015.
- 7.George C STDs hit record high in U.S., mirroring upward trend in Houston. TMC News; 2018. [Google Scholar]
- 8.Association of Public Health Laboratories. 2013 Chlamydia and Gonorrhea Clinical Laboratory Practices Survey. Silver Spring, MD, 2018. Available at: https://www.aphl.org/aboutAPHL/publications/Documents/ID-2018Aug-2013-CTGC-Clin-Lab-Practices-Survey-Summary-Data-Report.pdf. Accessed 2018. [Google Scholar]
- 9.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance, 2016. Available at: https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf. Accessed 2016.
- 10.Dicker LW, Mosure DJ, Steece R, et al. Testing for sexually transmitted diseases in U.S. public health laboratories in 2004. Sex Transm Dis 2007; 34:41–46. [DOI] [PubMed] [Google Scholar]
- 11.Combating the Threat of Antibiotic-Resistant Gonorrhea. 2018. Available at: https://www.cdc.gov/std/gonorrhea/arg/carb.htm. Accessed August 25, 2019.
- 12.Van Der Pol B Trichomonas vaginalis infection: The most prevalent nonviral sexually transmitted infection receives the least public health attention. Clin Infect Dis 2007; 44:23–25. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections. Geneva, Switzerland: WHO, 2008. [Google Scholar]
- 14.Association of Public Health Laboratories. Advances in laboratory detection of Trichomonas vaginalis. 2013. Available at: https://www.aphl.org/AboutAPHL/publications/Documents/ID_2013August_Advances-in-Laboratory-Detection-of-Trichomonas-vaginalis.pdf. Accessed 2013.
- 15.Association of Public Health Laboratories. Advances in laboratory detection of Trichomonas vaginalis 2016; Available at: https://www.aphl.org/aboutAPHL/publications/Documents/ID_2016November-Laboratory-Detection-of-Trichomonas-update.pdf. Accessed December 24, 2019.
- 16.Chemaitelly H, Nagelkerke N, Omori R, et al. Characterizing herpes simplex virus type 1 and type 2 seroprevalence declines and epidemiological association in the United States. PLoS One 2019; 14:e0214151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. STD Treatment Guidelines: Genital HSV Infections. Atlanta, GA: CDC, 2015: Available at: https://www.cdc.gov/std/tg2015/herpes.htm. [Google Scholar]
- 18.Patel R, Kennedy O, Clarke E, et al. 2017 European guidelines for the management of genital herpes. Int J STD AIDS 2017; 28:1366–1379. [DOI] [PubMed] [Google Scholar]


