In diabetic patients with nonproliferative diabetic retinopathy, macular edema, and visually significant cataract, combined treatment with phacoemulsification and the dexamethasone intravitreal implant (Ozurdex) was found to be effective, safe, and superior to standard phacoemulsification alone considering both functional and tomographic parameters.
Key words: cataract, dexamethasone implant, diabetic retinopathy, macular edema, phacoemulsification, surgery, visual acuity
Abstract
Purpose:
To compare functional and anatomical results of combined phacoemulsification and dexamethasone intravitreal implant (Ozurdex; DEX-I) with standard phacoemulsification in diabetic patients with cataract.
Methods:
Retrospective, comparative, cohort study. Patients with nonproliferative diabetic retinopathy, macular edema, and cataract, treated routinely at the Eye Clinic, Azienda Ospedaliero Universitaria Policlinico, Bari, Italy with phacoemulsification associated with DEX-I (n = 23; Phaco-Dex) or standard phacoemulsification (n = 23; Phaco-alone). Best-correct visual acuity, central subfield thickness, and intraocular pressure were assessed at baseline and monthly for 3 months after surgery, and t-test was used to assess change from baseline. A multilevel regression model with an unstructured correlation-type matrix to account for repeated data measures was used for statistical analysis in and between groups.
Results:
With Phaco-Dex, best-correct visual acuity increased significantly from the first month (P = 0.0005 vs. baseline) and remained stable at the following visits; central subfield thickness decreased significantly from Month 2 (P = 0.049 and P = 0.04 vs. baseline, respectively); at each timepoint, central subfield thickness was significantly lower in the Phaco-Dex group versus Phaco-alone. Intraocular pressure increased significantly during follow-up (P = 0.001 at Month 3 vs. baseline) but remained within the normal range. In the Phaco-alone group, best-correct visual acuity, and intraocular pressure did not show any significant changes after surgery, whereas central subfield thickness increased from Month 2 (P = 0.05 vs. baseline).
Conclusion:
In diabetic patients with macular edema and visually significant cataract, combined treatment with phacoemulsification and DEX-I seemed to be effective, safe, and superior to standard phacoemulsification considering both functional and tomographic parameters.
Diabetes mellitus is a systemic disease that involves several organs, including the eyes, and among eye complications, cataract is a major cause of visual impairment. Indeed, the prevalence of cataract in diabetic patients is 5 times higher than in the nondiabetic population,1–3 and in cataract patients below the age of 40, the prevalence of diabetes that is 15 to 25 times higher than that in the general population has been reported.4
Hyperglycemia-induced elevation of intracellular glucose levels generates chronic oxidative stress through the flux of glucose through the polyol pathway, leading to the release of proinflammatory cytokines, recruitment of leukocytes, loss of endothelial cells, breakdown of tight junctions, and an increase in vascular endothelial growth factor.5 All of these phenomena result in a significant increase in cell permeability, with the consequent formation of edema within the macula.5
The effect of cataract surgery on the progression of retinopathy is not fully defined, although cataract surgery is associated with an increased risk of postsurgical edema or worsening of the preexisting edema due to postsurgical inflammation that is increased by preexisting diabetic retinopathy 6,7: Indeed, it has been reported that 22% of diabetic patients will develop macular edema (ME).8 For this reason, treatment with nonsteroidal antiinflammatory drugs and corticosteroids is recommended to counteract postsurgical inflammation.9,10 Corticosteroids act by inhibiting the production of prostaglandins, preventing the release of proinflammatory cytokines, chemokines, and metalloproteinases, and promote the release of antiinflammatory factors.11
Treatment with corticosteroids must guarantee constant levels of the drug in the eye: considering that the half-life of dexamethasone is 3 hours to 6 hours in aqueous humor, frequent administrations are needed, and this can be a significant burden for patients, leading to poor compliance and adherence to therapy.11
Dexamethasone intravitreal implant (DEX-I; Ozurdex; Allergan Inc, Irvine, CA) is a sustained release intravitreal rod-shaped (6 mm) implant containing 700 µg of dexamethasone. Dexamethasone intravitreal implant has been approved by the Food and Drug Administration and the European Medicines Agency for the treatment of ME based on the results of two randomized, controlled trials on 1,048 patients with diabetic macular edema (DME), which demonstrated a significant improvement in best-corrected visual acuity (BCVA) and a reduction in central retina thickness with the implant compared with sham treatment.12
The aim of the present study was to compare functional and anatomical results of combined phacoemulsification plus the DEX-I with the standard phacoemulsification approach in a cohort of diabetic patients with cataract and DME.
Patients and Methods
Study Design and Objectives
We conducted a retrospective, comparative, cohort study on 46 patients affected by nonproliferative diabetic retinopathy and with any degree of ME and cataract. The study was reviewed and approved by the ethics committee. Our center conducts approximately 7,000 cataract surgeries per year and more than 6,000 intravitreal injections. During the interval from January 2018 to May 2019, 23 patients were treated with phacoemulsification associated with DEX-I (Phaco-Dex) and matched with 23 consecutive subjects treated with phacoemulsification according to the standard procedure (Phaco-alone). Inclusion criteria were diabetes, clinically significant cataract requiring surgery, HbA1c ≤9% (75 mmol/mol), nonproliferative diabetic retinopathy, and ME (nontractional DME, cystoid pattern, and retinal detachment pattern). Patients with proliferative diabetic retinopathy previously treated with laser photocoagulation were also included. Exclusion criteria included treatment of DME with corticosteroid in the 6 months before surgery; untreated proliferative diabetic retinopathy; a history of ocular hypertension or glaucoma; concomitant conditions that could worsen ME.
The objectives of the study were to compare the effect of DEX-I added to standard phacoemulsification with standard phacoemulsification only in terms of variation of BCVA.
Surgical Procedure
All patients underwent uneventful phacoemulsification with a hydrophilic acrylic intraocular lens implanted within the capsular bag using a 2.4-mm clear cornea tunnel following the axis of corneal astigmatism and dispersive ophthalmic viscoelastic device. In Group 1, intravitreal DEX-I was administered via pars plana directly in the inferotemporal quadrant at the end of cataract surgery.
Assessments
All patients were assessed at baseline and monthly for 3 months after surgery. The baseline visit included the collection of demographic and anamnestic data, and HbA1c levels. During the visit, intraocular pressure (IOP) and BCVA were measured with a Goldmann tonometer and a standardized Early Treatment Diabetic Retinopathy Study protocol, respectively; Early Treatment Diabetic Retinopathy Study values were converted to the logarithm of the minimum angle of resolution for statistical analysis; central subfield thickness (CST) was assessed with spectral-domain optical coherence tomography (SD-OCT; CIRRUS, Carl Zeiss, Jena, Germany). Central subfield thickness, also known as foveal thickness, was defined as the average thickness of the macula in the central 1-mm Early Treatment Diabetic Retinopathy Study grid. The same parameters were evaluated during follow-up visits. Intraocular pressure measurement and all intraoperatory and postoperatory adverse events were recorded for safety evaluation.
Statistical Analysis
All statistical analyses were performed using the software package SAS version 9.1 or higher. We used multilevel regression models with an unstructured correlation-type matrix to account for repeated data measures and compare the difference (delta of delta) between groups during the entire follow-up period (interaction P values) and at separate time points (contrast P values).
Results
Demographic and Baseline Data
Baseline characteristics are reported in Table 1. The study enrolled 46 patients, 23 with Phaco-Dex and 23 with Phaco-alone. There were 7 female patients in both groups. The mean age was 71.9 ± 7.5 years in the Phaco-Dex group and 74 ± 5.4 in the Phaco-alone group; in the Phaco-Dex group, 2 patients had type 1 diabetes. Time from diagnosis of diabetes was similar for the 2 groups, 20.56 years and 19.7 years, respectively. No difference in HbA1c was observed between groups. Nine patients in the Phaco-Dex group were under treatment with intravitreal ranibizumab compared with 11 with Phaco-alone. There were no significant baseline differences between the two groups regarding IOP, BCVA, and mean CST.
Table 1.
Baseline Characteristics of Patients
| Group 1: Phacoemulsification-DEX (n = 23) | Group 2: Phacoemulsification-Alone (n = 23) | P | |
| Age, years | 72 ± 7.5 | 74 ± 5 | 0.3 |
| Type 2 diabetes mellitus | 21 (91.3) | 23 (100.0) | 0.4 |
| Type 1 diabetes mellitus | 2 (8.7) | 0 (0.0) | 0.2 |
| Glycated hemoglobin (%) | 7.5 ± 1 | 8 ± 1.6 | 0.2 |
| Years from diagnosis of diabetes | 20.6 ± 9 | 19.7 ± 7 | 0.9 |
| Hypertension under treatment | 16 (69.6) | 15 (65.2) | 0.3 |
| On treatment with ranibizumab | 9 (39.1) | 11 (47.8) | 0.4 |
| IOP, mmHg | 13.5 ± 2.5 | 14.2 ± 2 | 0.3 |
| BCVA, Snellen | 20/100 | 20/80 | 0.12 |
| CST, microns | 344 ± 76 | 345 ± 82 | 0.9 |
Unless otherwise indicated, values are mean ± SD or no. (%).
Follow-Up
Results and between-group comparisons during follow-up are reported in Figures 1–3 and in Tables 2–4. During the 3-month follow-up, there were no drop-outs in the Phaco-Dex group, whereas in the Phaco-alone group, one patient at the first month and four patients at the second month dropped out because of worsening of ME and BCVA; these patients underwent intravitreal rescue therapy with DEX-I.
Fig. 1.
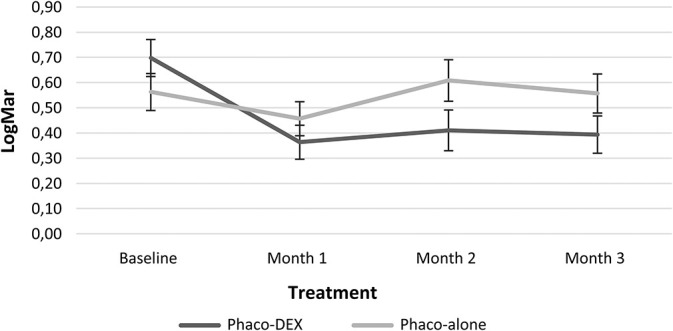
Change in BCVA over time. Phaco, phacoemulsification.
Fig. 3.
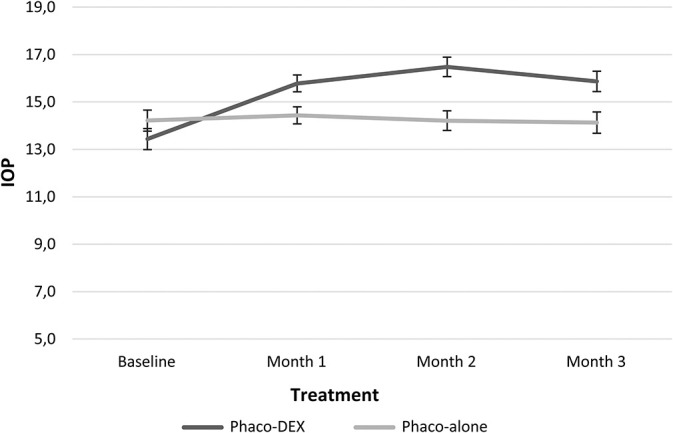
Change in IOP over time. Phaco, phacoemulsification.
Table 2.
P Values for Between-Groups Comparisons Over the Entire Follow-Up Period (Interaction P Values) and at Separate Time Points (Contrast P Values)
| BCVA | CST | IOP | |||
| Interaction | P | Interaction | P | Interaction | P |
| Time* treatment | 0.12 | Time* treatment | <0.0001 | Time* treatment | <0.0001 |
| BCVA | CST | IOP | |||
| Contrasts | P | Contrasts | P | Contrasts | P |
| Baseline vs. month 1 | 0.04 | Baseline vs. month 1 | <0.0001 | Baseline vs. month 1 | 0.0001 |
| Baseline vs. month 2 | 0.04 | Baseline vs. month 2 | <0.0001 | Baseline vs. month 2 | <0.0001 |
| Baseline vs. month 3 | 0.18 | Baseline vs. month 3 | <0.0001 | Baseline vs. month 3 | 0.003 |
| Month 1 vs. month 2 | 0.33 | Month 1 vs. month 2 | 0.0005 | Month 1 vs. month 2 | 0.014 |
| Month 1 vs. month 3 | 0.76 | Month 1 vs. month 3 | <0.0001 | Month 1 vs. month 3 | 0.40 |
| Month 2 vs. month 3 | 0.59 | Month 2 vs. month 3 | <0.0001 | Month 2 vs. month 3 | 0.19 |
Table 4.
Change in Mean IOP Over Time
| IOP, mmHg | Group 1: Phacoemulsification-DEX (n = 23) | Group 2: Phacoemulsification-Alone (n = 23) | ||||||
| Baseline | 1 Month | 2 Months | 3 Months | Baseline | 1 Month | 2 Months* | 3 Months† | |
| 13.4 ± 2.4 | 15.8 ± 1.7 | 16.5 ± 2.3 | 15.9 ± 2.5 | 14.2 ± 1.8 | 14.4 ± 1.7 | 14.2 ± 1.6 | 14.2 ± 1.4 | |
| Mean difference between groups | −0.8 | 1.4 | 2.3 | 1.7 | ||||
| 95% confidence interval | −2.0 to 0.5 | 0.4 to 2.3 | 1.1 to 3.4 | 0.5 to 3.0 | ||||
Values are mean ± SD.
n = 22 at 2 months.
n = 18 at 3 months in the phacoemulsification alone group.
Fig. 2.
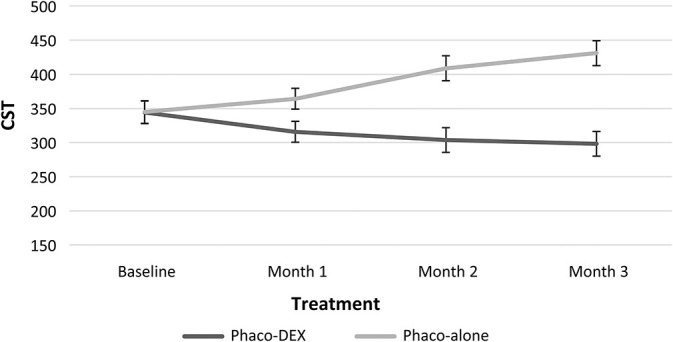
Change in CST over time. Phaco, phacoemulsification.
Best-Corrected Visual Acuity
In the Phaco-Dex group, mean BCVA increased significantly from 20/100 at baseline to 20/50 at Month 1 (P = 0.0005), to 20/50 (P = 0.005) at Month 2, and 20/50 (P = 0.005) at Month 3. After the increase at 1 month, BCVA remained stable and no difference was recorded between each visit and the previous one. In the Phaco-alone group, mean BCVA was 20/80 at baseline, 20/63 at Month 1 (P = 0.35 vs. baseline), 20/80 at Month 2 (P = 0.86 vs. baseline), and 20/80 at Month 3 (P = 0.86 vs. baseline). Comparing the variations of the 2 groups, significant differences were found at Month 1 versus baseline and Month 2 versus baseline (Table 2). After the second month, patients needing rescue therapy were excluded from the analysis, and thus comparison was between the groups of patients treated successfully, with no significant difference at successive timepoints.
Central Subfield Thickness
In the Phaco-Dex group, mean CST showed a nonsignificant decrease from baseline to Month 1, whereas they decreased significantly at Months 2 and 3 (Table 3). Central subfield thickness increased significantly at Month 2 (from 344.8 ± 82 µm at baseline to 400.8 ± 103 µm, P = 0.05). Comparing the variations of the two groups, significant differences were found over the entire follow-up period and at all separate timepoints (Table 2).
Table 3.
Change in Mean Central Subfield Thickness Over Time
| CST, Microns | Group 1: Phacoemulsification-DEX (n = 23) | Group 2: Phacoemulsification-Alone (n = 23) | ||||||
| Baseline | 1 Month | 2 Months | 3 Months | Baseline | 1 Month | 2 Months* | 3 Months† | |
| 344.3 ± 76.1 | 315.8 ± 61.0 | 303.7 ± 58.1 | 298.2 ± 56.5 | 344.8 ± 82.3 | 364.1 ± 84.3 | 400.8± 103.5 | 399.2 ± 101.3 | |
| Mean difference between groups | −0.6 | −48.4 | −105.1 | −132.8 | ||||
| 95% confidence interval | −46.4 to 45.2 | −90.9 to −5.9 | −155.5 to 54.7 | −183.5 to −82.0 | ||||
Values are mean ± SD.
n = 22 at 2 months.
n = 18 at 3 months in the phacoemulsification alone group.
Intraocular Pressure
During the study, IOP increased significantly in the Phaco-Dex group and was higher than in the Phaco-alone group at each timepoint, but remained within the normal range (Table 4). Comparing the variations of the 2 groups, significant differences were found at Months 1, 2, and 3 versus baseline and Month 1 versus Month 2 (Table 2). At Month 2, IOP was 20 mmHg in 3 patients and 21 mmHg in one patient.
In the Phaco-alone group, one patient dropped out at Month 1 because of a serious BCVA reduction from 20/200 to 20/660, and a CST increase from 388 µm to 501 µm; at Month 2, 4 patients dropped out for the same reasons (BCVA reduction from 20/40–20/200 in 3 patients, and from 20/63–20/660 in one patient; CST increased from 257 to 475 µm, from 432 to 530 µm, from 467 to 535 µm, and from 406 to 505 µm, respectively). All these five patients were subsequently treated with DEX-I.
Of note, no cases of infectious endophthalmitis were observed during the study.
Discussion
Patients with diabetes who undergo cataract surgery have a high risk of development or worsening of DME: a review on real-world data on 4,850 eyes reported that the onset of ME has a peak 3 months to 6 months after cataract surgery, and that the risk is higher for patients with preexisting ME.13 For this reason, intravitreal implants of steroids are recommended to counteract local inflammation and decrease the release of proinflammatory factors such as prostaglandins and cytokines.2,10,11 One limitation of steroid treatment is the short half-life of dexamethasone (<4 hours) within the aqueous humor.14 To overcome this, in recent years, intravitreal, slow-release implants of corticosteroids have been developed, which can improve both the efficacy and adherence compared with other local therapies.11,15
Dexamethasone implant is a biodegradable intravitreal implant, with a diameter of approximately 0.46 mm and a length of 6 mm, containing 700 µg of dexamethasone that is indicated for the treatment of adult patients with visual impairment due to DME, ME following either branch retinal vein occlusion or central retinal vein occlusion, and inflammation of the posterior segment of the eye presenting as noninfectious uveitis.16 Many studies have been conducted on the efficacy and safety of the DEX-I implant for the treatment12,17–26 and prevention of ME after cataract surgery.27–29
In the registrative trial (MEAD study) on the treatment of patients with DME, BCVA was significantly improved by DEX-I compared with placebo.12 Dexamethasone implant administered to patients with DME also led to a significantly greater decrease in central macular thickness (CMT) and a similar improvement in BCVA compared with bevacizumab, with a lower number of injections. Dexamethasone implant has also shown similar results when administered immediately after cataract surgery: in a small retrospective study on 24 eyes with DME or RVO, the implant combined with phacoemulsification was reported to be effective and safe, with a significant improvement in visual acuity (from Snellen 20/200–20/66, P = 0.003) and CMT (from 530.2 ± 218.9 µm to 300.7 ± 78.1 µm, P = 0.000).30 Substantial stability of CMT was achieved (from 241.1 µm to 248 µm, P = 0.15) and there was a significant improvement in BCVA (0.37–0.12, P < 0.001) at 3 months.
Another small prospective comparative study on 18 eyes reported the results of DEX-I implant before cataract surgery in patients with DME, compared with surgery alone. Patients treated with the implant had significantly higher increases in VA, and CMT declined by 18.22 µm at 24 weeks compared with preoperative values; in patients with surgery alone, no decrease in CMT was observed. Moreover, 77.8% of eyes in the control group required rescue therapy with triamcinolone.27 In our experience, we prefer to insert the implant after phacoemulsification, because of better control of the implant and possible intraoperative complications. In the prospective study by Panozzo et al,29 19 eyes in 19 patients with type 2 diabetes with DME and cataract underwent standard phacoemulsification and intraocular lens implantation, with DEX-I given at the end of surgery. In these patients, improvements in BCVA were seen at 1 week, and the beneficial effects lasted for at least 3 months. In another study on 16 patients with diabetes and coexisting cataract and DME who received combined phacoemulsification and DEX-I, significant decreases were seen in both central retinal thickness and BCVA, which lasted for at least 3 months.28 Both these studies thus suggest that DEX-I combined with cataract surgery may be a valid approach in patients with cataract and DME when considering morphologic and functional outcomes.
In our study, the use of DEX-I after cataract surgery led to a significant, fast, and durable reduction in CST (from 344 ± 76 µm to 298 ± 56, P = 0.02); in patients treated with phacoemulsification alone, we observed no significant decrease in ME, with a CST from 345 ± 82 µm to 399 ± 101 µm (P = 0.06 for both); at each timepoint, CST was significantly lower in the Phaco-Dex group; visual acuity improved only in the DEX-I group, whereas no difference from baseline was seen in the Phaco-alone group at each follow-up timepoint. Finally, we did not note any temporal differences between groups.
An increase in IOP was observed in the Phaco-Dex group, but the values always remained within normal limits. In all previous studies, no safety issues were observed with the use of DEX-I; in some cases, an increase of IOP occurred, but was rarely above the normal range30 and was easily controlled with topical treatments.25
The good anatomical results observed herein are in agreement with previous studies and suggest that DEX-I is a promising and well-tolerated treatment to prevent postsurgical increases or ex novo development of ME in patients with diabetes. The main limitation of our study is its retrospective, single-center design and the relatively small number of patients; the short follow-up does not allow observation of possible recurrences of ME. Moreover, another limitation of the study is the absence of adjustments for multiplicity and as such all analyses should be regarded as exploratory.
Conclusion
In diabetic patients with ME and visually significant cataract, combined treatment with phacoemulsification and DEX-I is effective, safe, and may be favorable over standard phacoemulsification considering both functional and tomographic parameters. Further prospective studies with a larger number of patients and longer follow-up are warranted.
Acknowledgments
Writing and editorial assistance was provided to the authors by Health Publishing and Service and funded by Allergan SpA Italy at the request of the investigator. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Footnotes
None of the authors has any financial/conflicting interests to disclose.
References
- 1.Badhania M. A review: cataract, a common ocular complication in diabetes. Int J Pharmacol Res 2016;6:189–194. [Google Scholar]
- 2.Javadi MA, Zarei-Ghanavati S. Cataracts in diabetic patients: a review article. J Ophthalmic Vis Res 2008;3:52–65. [PMC free article] [PubMed] [Google Scholar]
- 3.Pollreisz A, Schmidt-Erfurth U. Diabetic cataract-pathogenesis, epidemiology and treatment. J Ophthalmol 2010;2010:608751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernth-Petersen P, Bach E. Epidemiologic aspects of cataract surgery. III: frequencies of diabetes and glaucoma in a cataract population. Acta Ophthalmol (Copenh) 1983;61:406–416. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Zeng H, Bao S, et al. Diabetic macular edema: new concepts in patho-physiology and treatment. Cell Biosci 2014;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American diabetes association. Diabetes Care 2017;40:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowler JG, Hykin PG, Hamilton AM. Phacoemulsification versus extracapsular cataract extraction in patients with diabetes. Ophthalmology 2000;107:457–462. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Equi R, Bressler NM. Analysis of macular edema after cataract surgery in patients with diabetes using optical coherence tomography. Ophthalmology 2007;114:881–889. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg PB, Havnaer A, Oetting TA, Garcia-Ferrer FJ. Cataract surgery practice patterns in the United States Veterans Health Administration. J Cataract Refract Surg 2012;38:705–709. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care: the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 2018;125:1608–1622. [DOI] [PubMed] [Google Scholar]
- 11.Shah TJ, Conway MD, Peyman GA. Intracameral dexamethasone injection in the treatment of cataract surgery induced inflammation: design, development, and place in therapy. Clin Ophthalmol 2018;12:2223–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer DS, Yoon YH, Belfort R, Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014;121:1904–1914. [DOI] [PubMed] [Google Scholar]
- 13.Denniston AK, Chakravarthy U, Zhu H, et al. The UK Diabetic Retinopathy Electronic Medical Record (UK DR EMR) users group, report 2: real-world data for the impact of cataract surgery on diabetic macular oedema. Br J Ophthalmol 2017;101:1673–1678. [DOI] [PubMed] [Google Scholar]
- 14.Kwak HW, D'Amico DJ. Evaluation of the retinal toxicity and pharmacokinetics of dexamethasone after intravitreal injection. Arch Ophthalmol 1992;110:259–266. [DOI] [PubMed] [Google Scholar]
- 15.Mehta H, Gillies M, Fraser-Bell S. Perspective on the role of ozurdex (dexamethasone intravitreal implant) in the management of diabetic macular oedema. Ther Adv Chronic Dis 2015;6:234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozurdex. Summary of product characteristics. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/ozurdex#product-information-section. [Google Scholar]
- 17.Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina (Philadelphia, Pa) 2011;31:915–923. [DOI] [PubMed] [Google Scholar]
- 18.Callanan DG, Gupta S, Boyer DS, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology 2013;120:1843–1851. [DOI] [PubMed] [Google Scholar]
- 19.Callanan DG, Loewenstein A, Patel SS, et al. A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 2017;255:463–473. [DOI] [PubMed] [Google Scholar]
- 20.Chatziralli I, Theodossiadis P, Parikakis E, et al. Dexamethasone intravitreal implant in diabetic macular edema: real-life data from a prospective study and predictive factors for visual outcome. Diabetes Ther 2017;8:1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser-Bell S, Lim LL, Campain A, et al. Bevacizumab or dexamethasone implants for DME: 2-year results (the BEVORDEX study). Ophthalmology 2016;123:1399–1401. [DOI] [PubMed] [Google Scholar]
- 22.Hunter RS, Lobo AM. Dexamethasone intravitreal implant for the treatment of noninfectious uveitis. Clin Ophthalmol 2011;5:1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra SK, Gupta A, Patyal S, et al. Intravitreal dexamethasone implant versus triamcinolone acetonide for macular oedema of central retinal vein occlusion: quantifying efficacy and safety. Int J Retina Vitreous 2018;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poornachandra B, Kumar VBM, Jayadev C, et al. Immortal ozurdex: a 10-month follow-up of an intralenticular implant. Indian J Ophthalmol 2017;65:255–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scaramuzzi M, Querques G, Spina CL, et al. Repeated intravitreal dexamethasone implant (ozurdex) for diabetic macular edema. Retina (Philadelphia, Pa) 2015;35:1216–1222. [DOI] [PubMed] [Google Scholar]
- 26.Tservakis I, Koutsandrea C, Papaconstantinou D, et al. Safety and efficacy of dexamethasone intravitreal implant (Ozurdex) for the treatment of persistent macular edema secondary to retinal vein occlusion in eyes previously treated with anti-vascular endothelial growth factors. Curr Drug Saf 2015;10:145–151. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal A, Gupta V, Ram J, Gupta A. Dexamethasone intravitreal implant during phacoemulsification. Ophthalmology 2013;120:211–215. [DOI] [PubMed] [Google Scholar]
- 28.Furino C, Boscia F, Niro A, et al. Combined phacoemulsification and intravitreal dexamethasone implant (Ozurdex(R)) in diabetic patients with coexisting cataract and diabetic macular edema. J Ophthalmol 2017;2017:4896036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panozzo GA, Gusson E, Panozzo G, Dalla Mura G. Dexamethasone intravitreal implant at the time of cataract surgery in eyes with diabetic macular edema. Eur J Ophthalmol 2017;27:433–437. [DOI] [PubMed] [Google Scholar]
- 30.Calvo P, Ferreras A, Al Adel F, et al. Effect of an intravitreal dexamethasone implant on diabetic macular edema after cataract surgery. Retina (Philadelphia, Pa) 2018;38:490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]


