Supplemental Digital Content is available in the text.
Background.
Galectin-3 may play a causal role in kidney inflammation and fibrosis, which may also be involved in the development of kidney graft failure. With novel galectin-3-targeted pharmacological therapies increasingly coming available, we aimed to investigate whether galectin-3 is associated with risk of late graft failure in kidney transplant recipients (KTR).
Methods.
We studied adult KTR who participated in TransplantLines Insulin Resistance and Inflammation Biobank and Cohort Study, recruited in a university setting (2001–2003). Follow-up was performed for a median of 9.5 (interquartile range, 6.2–10.2) years. Overall and stratified (Pinteraction < 0.05) multivariable-adjusted Cox proportional-hazards regression analyses were performed to study the association of galectin-3 with risk of graft failure (restart of dialysis or retransplantation).
Results.
Among 561 KTR (age 52 ± 12 y; 54% males), baseline median galectin-3 was 21.1 (interquartile range, 17.0–27.2) ng/mL. During follow-up, 72 KTR developed graft failure (13, 18, and 44 events over increasing tertiles of galectin-3). Independent of adjustment for donor, recipient, and transplant characteristics, galectin-3-associated with increased risk of graft failure (hazard ratios [HR] per 1 SD change, 2.12; 95% confidence interval [CI], 1.63-2.75; P < 0.001), particularly among KTR with systolic blood pressure ≥140 mmHg (HR, 2.29; 95% CI, 1.80-2.92; P < 0.001; Pinteraction = 0.01) or smoking history (HR, 2.56; 95% CI, 1.95-3.37; P < 0.001; Pinteraction = 0.03). Similarly, patients in the highest tertile of galectin-3 were consistently at increased risk of graft failure.
Conclusions.
Serum galectin-3 levels are elevated in KTR, and independently associated with increased risk of late graft failure. Whether galectin-3-targeted therapies may represent novel opportunities to decrease the long-standing high burden of late graft failure in stable KTR warrants further studies.
INTRODUCTION
Galectin-3 is a β-galactoside-binding lectin involved in an array of biological processes, for example, acute and chronic inflammatory responses, and causally associated with kidney inflammation and kidney tissue fibrosis.1-8 Cross-sectional epidemiological data first showed that circulating levels of galectin-3 inversely relate with renal function.9,10 More recently, O’Seaghdha et al11 provided the first clinical evidence that prospectively linked galectin-3 with kidney function decline and incident chronic kidney disease in the general population. Following evidence further supported a strong and independent longitudinal association between galectin-3 and both incident and progression of native chronic kidney disease.12,13
Particularly postkidney transplantation, kidney fibrogenesis was shown to be dependent on the expression and secretion of galectin-3.5 With novel galectin-3-targeted pharmacological therapies increasingly becoming available,4,6-8 there is a need for clinical data to prospectively investigate the potential association of galectin-3 with adverse clinical outcomes across the full spectrum of chronic kidney disease patients, especially in kidney transplant recipients (KTR) setting as it may potentially offer novel interventional strategies to decrease the long-standing high burden of late graft failure. The current study aimed to (i) determine circulating galectin-3 levels in a large cohort of extensively phenotyped KTR, (ii) characterize clinical and laboratory determinants of galectin-3, (iii) investigate whether circulating galectin-3 levels are independently associated with risk of late graft failure, and (iv) identify subgroups of KTR at particularly higher risk according to literature-based prespecified potential effect modifiers.
MATERIALS AND METHODS
Study Design and Population
All adult KTR without known or apparent systemic illnesses (ie, malignancies, opportunistic infections) and with a functioning graft ≥1 year, visiting the outpatient clinic of the University Medical Center Groningen (The Netherlands) between August 2001 and July 2003, were invited to participate in this prospective cohort study. A total of 606 of 847 KTR signed informed consent. Patients missing galectin-3 measurements (n = 45) were excluded from the analyses, resulting in 561 KTR, of whom data are presented here (Figure S1, SDC, http://links.lww.com/TP/B960). The study protocol was approved by the Institutional Review Board (Medical Ethical Committee 01/039). The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.” The cohort study is registered at clinicaltrials.gov (TransplantLines Insulin Resistance and Inflammation Biobank and Cohort Study, number NCT03272854). Full details on the study design have been previously reported.14
Ascertainment of Graft Failure
Graft failure was defined as end-stage kidney disease requiring dialysis therapy or retransplantation. The cause of graft failure was obtained from patient records and was reviewed by a blinded nephrologist, as previously described. Chronic allograft dysfunction was defined clinically as gradual decline of renal function with or without progressive proteinuria.15 Follow-up was performed for a median of 9.5 (interquartile range [IQR], 6.2–10.2) years. Collection of these data are ensured by the continuous surveillance system of the outpatient clinic of our university hospital, in which patients visit the outpatient clinic with declining frequency, in accordance with the guidelines of the American Society of Transplantation.16
Data Collection
The current study is a post hoc analysis using samples and data of the TransplantLines Insulin Resistance Biobank and Cohort Study (registered at clinicaltrials.gov with number NCT032727854). For building this cohort study with an underlying biobank, all baseline medical history and medication use were extracted from the Groningen Kidney Transplant Database at inclusion. Cardiovascular history was considered positive if participants had a previous myocardial infarction, transient ischemic attack, or cerebrovascular accident. Lifestyle, smoking status, and alcohol use were obtained using a self-report questionnaire at time of inclusion. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.17 The measurements of all clinical and laboratory parameters have been previously described in detail.14 For baseline laboratory phenotyping, including galectin-3 measurements, blood samples were drawn in the morning after 8–12 hours overnight fasting at inclusion. Galectin-3 levels were determined in serum samples (BG Medicine, Inc, Waltham, MA), as described elsewhere.18 Plasma and urine creatinine concentrations were determined using a modified version of the Jaffé method (MEGA AU510; Merck Diagnostica). Class I and class II antihuman leukocyte antigen antibodies (HLAab) were measured by ELISA (LATM20×5, 1 Lambda, Canoga Park, CA) as previously reported.19
Histopathologic Analysis of Kidney Biopsies
To perform histopathological analyses of kidney biopsies, we requested from our biobank all available kidney biopsies performed more than a year after kidney transplantation, and within a time-frame ranging between 1 year before baseline assessment and 1 year after baseline assessment of patients. Formalin-fixed and paraffin-embedded archival kidney biopsies were used for evaluation. Slides were scanned on a Philips Intellisite scanner and representative images were evaluated. Periodic acid–Schiff and galectin-3 stains were performed on consecutive slides and evaluated by an experienced renal pathologist, who was blind to clinical and laboratory patients’ characteristics. A biopsy was scored positive for fibrosis if atrophic tubules were clearly separated by stromal tissue. Inflammation was assessed according to area percentages of mononuclear inflammation of involved cortex. Galectin-3 (mouse monoclonal antibody 9C4, Roche) was stained on an automated IHC platform (Roche Ventana BenchMark Ultra), using standard diagnostic procedures (CC1 pretreatment for 32 min, Ultraview detection).
Serial Measurement of Galectin-3 Levels in a Sample Population of the New TransplantLines Cohort and Biobank Study
Additionally, to investigate galectin-3 levels over time, we requested serial serum samples (3 mo, 6 mo, 1 y, and 2 y postkidney transplantation) from 19 consecutive KTR enrolled between February 2016 and May 2017 in the ongoing TransplantLines Prospective Cohort and Biobank Study.20 For this substudy, galectin-3 levels were determined in serum samples using the ARCHITECT galectin-3 assay (Abbott, Chicago, IL), as described elsewhere.21 The range of values is between 4.0 and 114.0 ng/mL with an intraassay coefficient of variation (CV) of 3.4% and an interassay CV of 4.1%.21
Statistical Analyses
Data were analyzed using IBM SPSS software version 23.0 (SPSS Inc., Chicago, IL), STATA 14.2 (STATA Corp., College Station, TX), GraphPad Prism 7.02 software (GraphPad Software Inc., San Diego, CA), and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Data are expressed as mean ± SD for normally distributed variables and as median (IQR) for skewed variables. Categorical data are expressed as n (percentage). In all analyses, a 2-sided P < 0.05 was considered significant.
Age, sex, and eGFR-adjusted linear regression analyses were performed to examine the association of baseline characteristics with circulating galectin-3 levels.22 Standard β coefficients represent the difference (in standard deviations) in galectin-3 per 1 SD increment in continuous characteristics or for categorical characteristics the difference (in SDs) in galectin-3 compared with the implied reference group. To study in an integrated manner which baseline variables were independently associated with and were determinants of circulating galectin-3, we performed forward selection of baseline characteristics according to preceding multivariable linear regression analyses (P for inclusion <0.2), followed by stepwise backwards multivariable linear regression analyses (P for exclusion <0.05). Residuals were checked for normality and a natural log-transformation was applied when appropriate.
Prospective Analyses
The prospective association of galectin-3 with risk of graft failure during follow-up was examined by means of univariable Cox proportional-hazards regression analyses and by means of multivariable Cox proportional-hazards regression analyses with time-dependent covariates to calculate hazard ratios (HR) and 95% confidence intervals (CI). In these analyses, the competing risk of death was taken into account by performing analyses according to the proportional cause-specific hazards model approach, which allows estimation of regression parameters that directly quantify HR among those individuals who are actually at risk of developing the event of interest,23-25 which needs to be distinguished from the subdistribution hazards model approach (proposed by Fine and Gray),26 in which subjects who experience a competing event (ie, death) remain in the risk set, although they are in fact no longer at risk of the event of interest (ie, graft failure). Effect estimates were calculated per 1 SD increment of galectin-3 concentration, and per change over tertiles of galectin-3 concentration, with tertile 1 as reference. Schoenfeld residuals were calculated to assess whether proportionality assumptions were satisfied. Collinearity was tested by calculating a variance inflation factor score. A variance inflation factor <5 indicates no evidence for collinearity.
We first performed unadjusted Cox regression analyses, followed by multivariable models in which we performed adjustment for potential confounders, without the intention of comparing predictive strength. Multivariable-adjusted model 1 was adjusted for eGFR, whereas model 2 was adjusted for established demographic, clinical, and laboratory risk factors of late graft failure (donor age, recipient age, body mass index, dialysis vintage, type of transplant, and time since transplantation, in addition to eGFR). Model 2 was then considered the primary multivariable model upon which additional adjustments were performed, with adjustment for immunosuppressive therapy, circulating anti-HLA class I antibodies, circulating anti-HLA class II antibodies, and inflammatory parameters (acute rejection treatment, use of proliferator inhibitor, high-sensitivity C-reactive protein [hs-CRP], and soluble vascular cell adhesion molecule 1) in model 3; diabetes and glucose homeostasis (history of diabetes, glycated hemoglobin, and homeostasis model assessment of insulin resistance) in model 4; traditional cardiovascular risk factors (systolic blood pressure, use of antihypertensive medication, smoking status, triglycerides, high-density lipoprotein cholesterol) in model 5; and, N-terminal pro b-type natriuretic peptide and high-sensitive troponin T in model 6. Because creatinine trajectories parallel the development of graft failure and should therefore not be considered as a potential confounder, we did not perform adjustment for creatinine trajectories in multivariable-adjusted analyses. It should also be realized that the current study is pathogenic in nature. The pathogenic field of epidemiology aims at understanding a certain pathway of disease to allow for treatment or prevention. This needs to be separated from the prediction field of epidemiology, which aims at predicting the risk of an outcome according to a model of statistically significant predictors, which not necessarily represent causal associations.27
Power calculations showed that the minimum detectable HR based on an assumption of 90% power and 2-sided α significance of 0.05 was 1.46. Potential effect-modification of the effect of galectin-3 on graft failure by age, sex, body mass index, eGFR, proteinuria, systolic blood pressure, and smoking status were tested by fitting models containing both main effects and their cross-product terms with galectin-3. Pinteraction < 0.05 was considered to indicate significant effect modification. Subsequently, stratified prospective analyses were performed by subgroups of patients according to significant effect modifiers. For these analyses, cut-off points of originally continuous variables were determined in such a way to concede clinically meaningful strata.
Sensitivity Analyses
In sensitivity analyses, we examined the robustness of our primary findings by means of Cox regression analyses with adjustment for eGFR according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine-cystatin C equation (instead of the standard CKD-EPI equation),28 thus taking cystatin C into account for the estimation of kidney function.
Serial Analyses of Galectin-3 Levels in a Sample Population of the New TransplantLines Cohort and Biobank Study
The intraindividual CV for galectin-3 levels in KTR of the TransplantLines Cohort and Biobank Study was calculated using the formula CV = (SD/mean) × 100, in which SD is the standard deviation and mean is the mean value for galectin-3 concentrations as measured in follow-up samples taken at 3 months, 6 months, 1 year, and 2 years posttransplantation. Next, box plots were used to illustrate medians (IQR) of galectin-3 levels during follow-up visits. Finally, significance of potential change during follow-up visits was tested using the Kruskal-Wallis test and the Friedman test for paired analyses.
RESULTS
Baseline Characteristics
A total of 561 adult KTR (age 52 ± 12 y) were included. Median galectin-3 concentration was 21.1 (IQR, 17.0–27.2) ng/mL. A history of cytomegalovirus disease was present in 112 (19%) KTR. Class I HLAab were absent in 485 (87%), borderline in 19 (3%), and positive in 50 (9%) KTR. Class II HLAab were absent in 489 (87%), borderline in 9 (2%), and positive in 56 (10%). Additional baseline characteristics are summarized in Table 1. Galectin-3 levels were independently and directly associated with systemic and vascular inflammatory biomarkers (hs-CRP; and, soluble vascular cell adhesion molecule 1, sVCAM-1), body mass index, triglycerides, time since transplantation, and high-sensitive troponin T. Whereas, galectin-3 inversely associated with eGFR.
TABLE 1.
Baseline characteristics of 561 kidney transplant recipients and associations of these characteristics with circulating galectin-3
| Galectin-3 (ln) | |||
|---|---|---|---|
| Baseline characteristics | All patients | †Linear regression | ‡Stepwise backwards linear regression |
| Std. β | Std. β | ||
| Kidney transplant recipients, n (%) | 561 (100) | – | – |
| Galectin-3, ng/mL, median (IQR) | 21.1 (17.0–27.2) | – | – |
| Demographics and anthropometrics | |||
| Age, y, mean (SD) | 52 (12) | 0.10*** | ~ |
| Sex, male, n (%) | 304 (54) | –0.04 | |
| Body surface area, m2, mean (SD) | 1.89 (0.19) | 0.09** | ~ |
| Body mass index, kg/m2, mean (SD) | 26.0 (4.3) | 0.16*** | 0.11*** |
| Kidney graft function | |||
| eGFR, mL/min/1.73 m2, mean (SD) | 47 (16) | –0.58*** | –0.47*** |
| Proteinuria ≥0.5 g/24 h, n (%)a | 162 (29) | 0.03 | |
| Cardiovascular history and lifestyle | |||
| History of cardiovascular disease, n (%)b | 71 (13) | 0.01 | |
| NTpro-BNP, pg/mL, median (IQR) | 305 (131–672) | 0.13*** | ~ |
| High-sensitive troponin T, µg/L, median (IQR) | 0.014 (0.008–0.025) | 0.26*** | 0.15*** |
| Systolic blood pressure, mm Hg, mean (SD) | 153 (23) | 0.04 | |
| Diastolic blood pressure, mm Hg, mean (SD) | 90 (10) | 0.02 | |
| Use of antihypertensives, n (%) | 491 (88) | 0.04 | |
| Use of ACE-inhibitors or ARBs, n (%) | 195 (35) | 0.05* | |
| Use of β-blockers, n (%) | 347 (62) | –0.004 | |
| Use of calcium-antagonists, n (%) | 216 (39) | 0.001 | |
| Current or former-smoker, n (%) | 362 (65) | –0.01 | |
| Alcohol use, none, n (%)c | 272 (49) | – | |
| 1–7 units/wk, n (%) | 201 (36) | –0.06* | |
| >7 units/wk, n (%) | 79 (14) | –0.03 | |
| Diabetes and glucose homeostasis | |||
| Diabetes mellitus, n (%) | 101 (18) | 0.09*** | ~ |
| HbA1c, %, mean (SD)d | 6.5 (1.1) | 0.07* | ~ |
| HOMA-IR, score, median (IQR) | 2.3 (1.6–3.6) | 0.12*** | ~ |
| Laboratory parameters | |||
| Hemoglobin, g/dL, mean (SD)a | 8.5 (0.9) | –0.03 | |
| Lipids | |||
| Total cholesterol, mmol/L, mean (SD) | 5.6 (1.1) | 0.02 | |
| HDL cholesterol, mmol/L, mean (SD) | 1.1 (0.3) | –0.10*** | ~ |
| LDL cholesterol, mmol/L, mean (SD) | 3.5 (1.0) | –0.06* | ~ |
| Triglycerides, mmol/L, median (IQR) | 1.92 (1.41–2.65) | 0.21*** | 0.12*** |
| Use of statins, n (%) | 281 (50) | 0.02 | |
| Systemic and vascular inflammation | |||
| hs-CRP, mg/L, median (IQR) | 2.1 (0.8–5.1) | 0.15*** | 0.09** |
| sVCAM-1, ng/mL, median (IQR) | 768 (967–1200) | 0.19*** | 0.15*** |
| Kidney transplant and immunosuppressive therapy | |||
| Dialysis vintage, mo, median (IQR) | 27 (13–48) | 0.08** | ~ |
| Time since transplantation, y, median (IQR) | 6.1 (2.7–11.7) | 0.06* | 0.10*** |
| Donor characteristics | |||
| Donor type (living), n (%) | 78 (14) | –0.10*** | ~ |
| Donor age, y, median (IQR) | 37 (15) | –0.07* | ~ |
| Donor sex, male, n (%)e | 304 (54) | 0.06* | ~ |
| Use of calcineurin inhibitor, n (%) | 438 (78) | –0.02 | |
| Use of proliferation inhibitor, n (%)b | 414 (74) | –0.08** | ~ |
| Acute rejection treatment, high doses of steroids, n (%) | 173 (31) | 0.05* | ~ |
| Prednisolone, mg/d, median (IQR)f | 10.0 (7.5–10.0) | 0.03 | |
*P < 0.2; **P < 0.05; ***P < 0.01.
Linear regression analyses, †adjusted for age, sex, and eGFR.
Std. β coefficients represent the difference (in SDs) in galectin-3 per 1 SD increment in continuous characteristics or for categorical characteristics the difference (in SDs) in galectin-3 compared with the implied reference group.
‡Stepwise backwards linear regression analyses; for inclusion we performed forward selection of baseline characteristics according to preceding multivariable linear regression analyses (P value for inclusion <0.2); for exclusion P value were set at 0.05. ~Excluded from the final model.
Data available in a559, b557, c552, d560, e558, and f554.
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; HbA1c, hemoglobin A1C; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; NTpro-BNP, N-terminal pro b-type natriuretic peptide; SVCAM-1, soluble vascular cell adhesion molecule 1.
Galectin-3 and Risk of Late Graft Failure
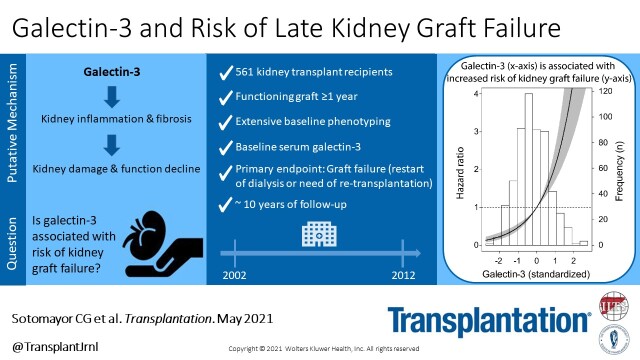
During a median of 9.5 (IQR, 6.2–10.2) years of follow-up, 172 (31%) KTR died and 72 (13%) patients developed graft failure. Causes of death were most largely due to cardiovascular disease, malignancy, and infection, with 73 (42%), 40 (23%), and 24 (14%), respectively. The main reason for graft failure was chronic transplant dysfunction in 55 (73%) cases. Other causes for graft failure were acute rejection in 7 (10%) cases, relapse of original kidney disease in 2 (3%), and a remaining group of unspecified causes in 8 (11%) patients. In unadjusted and multivariable-adjusted Cox regression analyses, we found an independent and direct association between galectin-3 levels and risk of graft failure, both in analyses with galectin-3 as a continuous variable (HR, 2.12; 95% CI, 1.63-2.75; P < 0.001; model 2) and as a categorical variable (with, eg, tertile 3 versus tertile 1: HR, 4.07; 95% CI, 1.96-8.48; P < 0.001; model 2; Table 2). The association of galectin-3 with risk of graft failure using Cox regression analyses with mean concentration of galectin-3 as reference and in relation to the histogram of galectin-3 is visualized in Figure 1.
TABLE 2.
Association of circulating galectin-3 with graft failure in 561 kidney transplant recipients
| Models | Galectin-3 | ||||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Ln, per 1 SD | ||
| Ref. | HR (95% CI) | HR (95% CI) | HR (95% CI) | P | |
| Unadjusted | 1.00 | 1.51 (0.72-3.16) | 4.97 (2.62-9.45) | 2.11 (1.71-2.61) | <0.001 |
| Model 1 | 1.00 | 1.22 (0.58-2.58) | 2.90 (1.44-5.85) | 1.77 (1.38-2.27) | <0.001 |
| Model 2 | 1.00 | 1.53 (0.71-3.27) | 4.07 (1.96-8.48) | 2.12 (1.63-2.75) | <0.001 |
| Model 3 | 1.00 | 1.28 (0.59-2.78) | 3.30 (1.56-6.98) | 2.07 (1.56-2.75) | <0.001 |
| Model 4 | 1.00 | 1.50 (0.70-3.23) | 3.79 (1.81-7.95) | 2.06 (1.58-2.69) | <0.001 |
| Model 5 | 1.00 | 1.48 (0.69-3.17) | 3.73 (1.76-7.91) | 2.13 (1.60-2.48) | <0.001 |
| Model 6 | 1.00 | 1.60 (0.75-3.44) | 3.77 (1.79-7.96) | 1.97 (1.50-2.58) | <0.001 |
Cox proportional-hazards regression analyses were performed to assess the association of galectin-3 with graft failure (n = 72). Multivariable model 1 was adjusted for eGFR. Model 2 was adjusted for eGFR, donor age, recipient age, body mass index, dialysis vintage, type of transplant, and time since transplantation. In each following model, adjustments were performed additive to adjustments performed in model 2. These included adjustment for immunosuppressive therapy, circulating anti-HLA class I antibodies, circulating anti-HLA class II antibodies, and inflammatory parameters (acute rejection treatment, use of proliferator inhibitor, high-sensitivity C-reactive protein, and soluble vascular cell adhesion molecule 1) in model 3; diabetes and glucose homeostasis (history of diabetes, glycated hemoglobin, and homeostasis model assessment of insulin resistance) in model 4; traditional cardiovascular risk factors (systolic blood pressure, use of antihypertensive medication, smoking status, triglycerides, high-density lipoprotein cholesterol) in model 5; and, N-terminal pro b-type natriuretic peptide and high-sensitive troponin T in model 6.
CI, confidence intervals; eGFR, estimated glomerular filtration rate; HR, hazard ratios.
FIGURE 1.
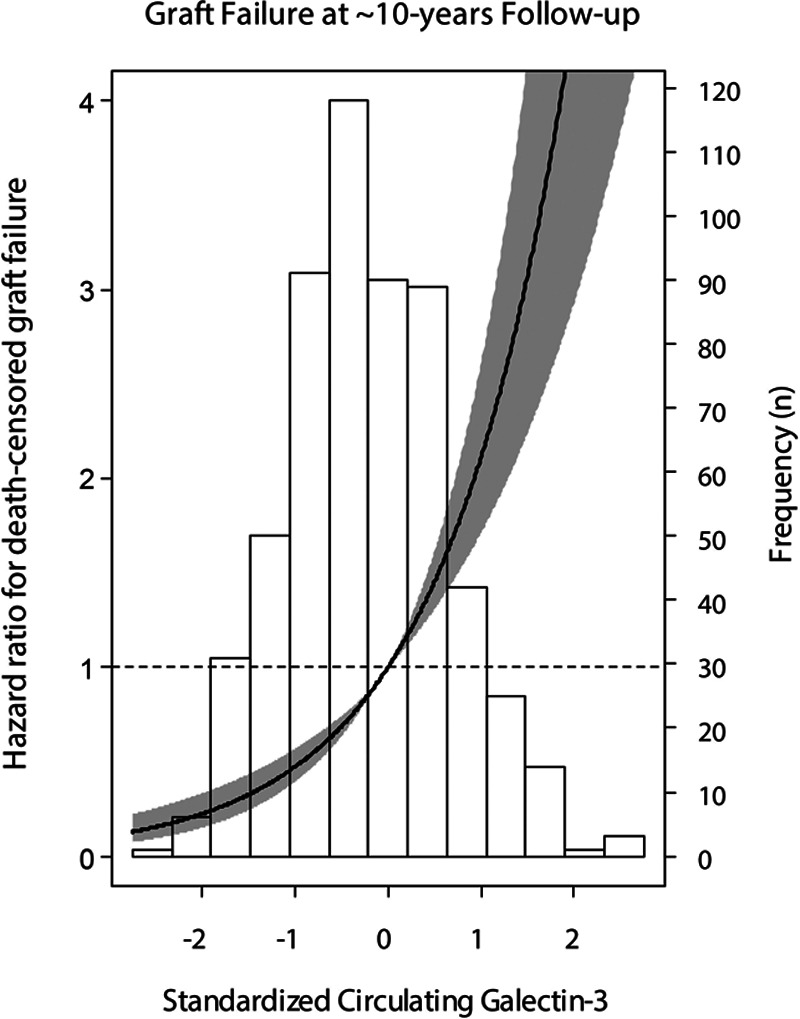
Association of standardized circulating galectin-3 with risk of kidney graft failure. Data were fitted by Cox proportional-hazards regression using mean galectin-3 (21.1 ng/mL) as reference value. The black line represents the hazard ratio and the grey area represents the 95% confidence interval.
Effect-Modification and Stratified Analyses
We observed no effect-modification of the association between galectin-3 and risk of graft failure by age, sex, body mass index, eGFR, or proteinuria (Pinteraction > 0.05 for all), whereas significant effect-modification was observed by systolic blood pressure, and smoking status (Pinteraction < 0.05 for all; Table S1, SDC, http://links.lww.com/TP/B960). In subsequent stratified analyses, we found that the association of galectin-3 with risk of graft failure was particularly strong in the subgroup of patients with systolic blood pressure ≥140 mm Hg (HR, 2.29; 95% CI, 1.80-2.92; P < 0.001), or smoking history (HR, 2.56; 95% CI, 1.95-3.37; P < 0.001). Stratified prospective analyses of the association of galectin-3 with risk of graft failure can be visualized in Figure 2.
FIGURE 2.
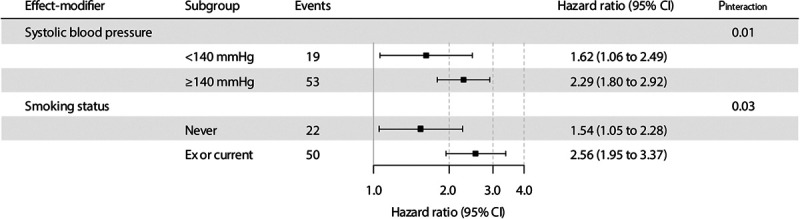
Stratified prospective analyses of the association of galectin-3 with risk of kidney graft failure. Pinteraction was calculated by fitting models which contain both main effects (as continuous variable for systolic blood pressure, and as dichotomized variable for smoking status) and their cross-product term. Hazard ratios (95% CI) are calculate per 1 SD increment in circulating galectin-3. CI, confidence intervals.
Sensitivity Analyses
The association of galectin-3 with risk of graft failure remained materially unchanged in sensitivity analyses with adjustment for eGFR alternatively estimated according to the CKD-EPI creatinine-cystatin C equation (Table 3).
TABLE 3.
Sensitivity analyses; association of circulating galectin-3 with graft failure, with adjustment for eGFR calculated according to the CKD-EPI creatinine-cystatin C equation
| Models | Galectin-3 | ||||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Ln, per 1 SD | ||
| Ref. | HR (95% CI) | HR (95% CI) | HR (95% CI) | P | |
| Crude | 1.00 | 1.51 (0.72-3.16) | 4.97 (2.62-9.45) | 2.11 (1.71-2.61) | <0.001 |
| Model 1 | 1.00 | 1.14 (0.54-2.41) | 2.70 (1.32-5.52) | 1.75 (1.36-2.25) | <0.001 |
| Model 2 | 1.00 | 1.42 (0.66-3.06) | 3.76 (1.77-8.00) | 2.10 (1.60-2.74) | <0.001 |
| Model 3 | 1.00 | 1.20 (0.55-2.62) | 3.15 (1.47-6.75) | 2.07 (1.55-2.76) | <0.001 |
| Model 4 | 1.00 | 1.40 (0.65-3.03) | 3.52 (1.65-7.53) | 2.04 (1.55-2.69) | <0.001 |
| Model 5 | 1.00 | 1.40 (0.65-3.03) | 3.56 (1.65-7.69) | 2.14 (1.60-2.87) | <0.001 |
| Model 6 | 1.00 | 1.54 (0.71-3.33) | 3.67 (1.71-7.88) | 1.98 (1.50-2.61) | <0.001 |
Cox proportional-hazards regression analyses were performed to assess the association of galectin-3 with graft failure (n=72). Multivariable model 1 was adjusted for eGFR. Model 2 was adjusted for eGFR, donor age, recipient age, body mass index, dialysis vintage, type of transplant, and time since transplantation,. In each following model, adjustments were performed additive to adjustments performed in model 2. These included adjustment for immunosuppressive therapy, circulating anti-HLA class I antibodies, circulating anti-HLA class II antibodies, and inflammatory parameters (acute rejection treatment, use of proliferator inhibitor, high-sensitivity C-reactive protein, and soluble vascular cell adhesion molecule 1) in model 3; diabetes and glucose homeostasis (history of diabetes, glycated hemoglobin, and homeostasis model assessment of insulin resistance) in model 4; traditional cardiovascular risk factors (systolic blood pressure, use of antihypertensive medication, smoking status, triglycerides, high-density lipoprotein cholesterol) in model 5; and, N-terminal pro b-type natriuretic peptide and high-sensitive troponin T in model 6.
CI, confidence intervals; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; HR, hazard ratios
Histopathologic Analysis of Kidney Biopsies
Kidney biopsies were available for 17 KTR (mean age, 49 ± 11 y old; eGFR, 33 ± 12 mL/min/1.73 m2), all of which were performed per clinical indication. The biopsies were taken at a median of 4.2 (IQR, 1.9–6.6) years posttransplantation. Three of the biopsies were compatible with rejection (2 acute interstitial rejections and 1 chronic rejection). Galectin-3 staining was observed in 5% (IQR, 4–12.5) of the cortical tissue area, mainly in flattened/cuboidal tubular epithelial cells that directly surround atrophic areas (Figure 3A and B). Biopsy-proven fibrosis associated with atrophy was present in 9 (53%) cases. Inflammation was present in a median (IQR) of 20% (5–45) of the cortical area (minimum 0%, maximum 60%, mean [SD] 23% [21]), usually within areas of tubular atrophy. In linear regression analyses, we found that galectin-3 was significantly associated with kidney fibrosis (standard β = 0.69; P = 0.03) but not with kidney inflammation (standard β = –0.50; P = 0.10). When subjects that had rejection were excluded, biopsy-proven fibrosis was present in 6 (43%) cases, inflammation was present in 15% (IQR, 5–33) of the cortical area, and associations of galectin-3 with fibrosis (standard β = 0.61; P = 0.10), and inflammation (standard β = –0.45; P = 0.21) became slightly weaker and lost significance in case of fibrosis.
FIGURE 3.
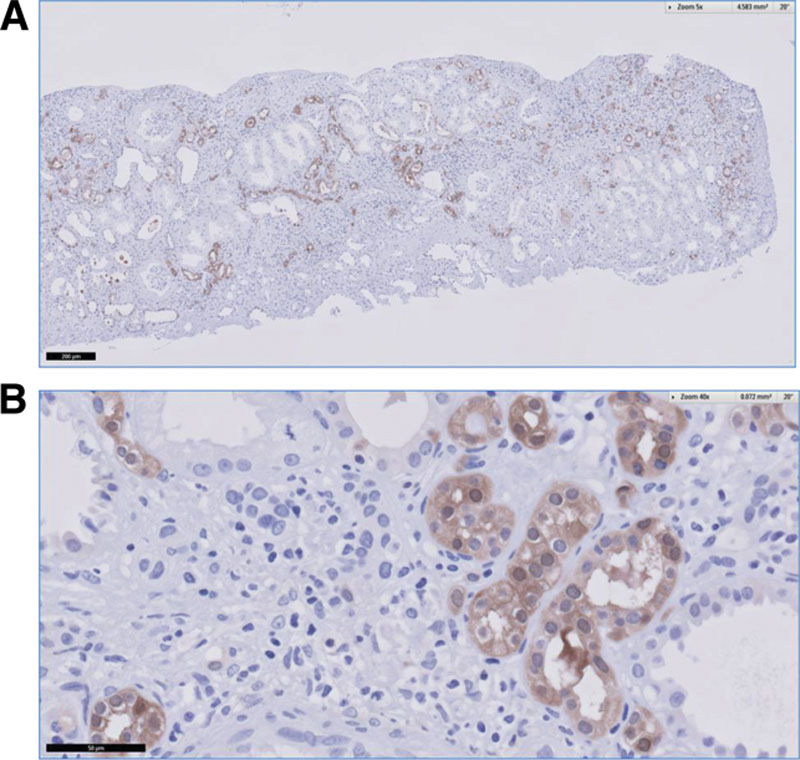
Representative histopathological sample of immunohistochemical expression of galectin-3 showing positive staining mainly in flattened/cuboidal tubular epithelial cells that directly surround atrophic areas in the renal cortex (magnification ×50 [A], and ×400 [B]).
Serial Galectin-3 in a Sample Population of the New TransplantLines Cohort and Biobank Study
Figure S2, SDC, http://links.lww.com/TP/B960, shows box plots with medians (IQR) of galetin-3 levels in 19 KTR (mean age, 50 ± 13 y old; eGFR 50 ± 17 mL/min/1.73 m2) from the TransplantLines Prospective Cohort and Biobank Study,20 at different follow-up visits posttransplant. We found that median (IQR) galectin-3 levels were 16.6 (13.0–21.2), 15.4 (12.3–21.0), 15.8 (14.0–19.6), 16.7 (15.2–20.0) µg/L, at 3 months, 6 months, 1 year, and 2 years posttransplantation, respectively. Median (IQR) intraindividual CV was 3.7% (2.2–5.7%). We did not find signs of a significant change in galectin-3 levels over time (P = 0.93).
DISCUSSION
In stable KTR, galectin-3 levels are increased,10-12,29 and independently associated with meaningful elevated risk of late graft failure, as depicted by an over 2-fold higher risk at approximately 10 years of follow-up. Of note, this finding was independent of adjustment for donor, recipient, and transplant characteristics, including eGFR. Furthermore, in agreement with mechanisms that contribute to the progression of chronic kidney disease, and in line with recent evidence,7,12,30,31 this study provides relevant clinical data of an interaction between galectin-3 and hypertension. In association with higher galectin-3 levels, KTR with high-systolic blood pressure (≥140 mm Hg) or smoking history are at particularly high risk of late graft failure.
In favor of the complex interplay between chronic low-grade inflammatory status of KTR32,33 and progressive kidney disease, galectin-3 has been previously shown to be associated with kidney inflammation, fibrosis, and degenerative histologic changes associated with impaired kidney function.1-13 An elegant study by Lobry et al2 demonstrated that galectin-3 is involved in the recruitment of macrophages, and that over-expression of galectin-3 interacts with the proinflammatory cytokine monocyte-chemoattractant protein-1, ultimately leading to an increase of inflammatory biomarkers. In the particular postkidney transplantation setting, in vivo evidence showed that tubular atrophy and interstitial fibrosis are dependent on expression and secretion of galectin-3.5 These data strongly support the role of galectin-3 in the pathological mechanisms leading to progression of chronic kidney disease, its deleterious sequelae, and ultimately, graft failure. Our results are in agreement with recent community-based clinical studies that prospectively related galectin-3 with kidney function decline and incidence of chronic kidney disease,11-13 and complement the study of galectin-3 in relation to adverse long-term outcomes by extending those findings, for the first time, to the clinical setting of stable KTR.
Over the last decades, maintenance immunosuppressive therapy led to significant improvement in 1-year kidney graft survival, whereas progress in long-term risk management has strongly lagged behind.16,34 This underscores that future advances in the field of renal transplantation are expected from the amelioration of long-term graft attrition.34 Current therapeutic strategies aim to minimize chronic allograft injury, for example, interstitial fibrosis and tubular atrophy, but complete understanding of involved factors and underlying mechanisms driving this process remain elusive. Likewise do, consequently, specific treatments. Galectin-3 inhibition has been proposed among the next generation of therapeutics of chronic kidney disease.35,36 Both natural and pharmacological galectin-3 inhibitors have been studied in various settings. Galectin-3 function was inhibited, and reduced fibrosis was demonstrated, with administration of modified citrus pectin in a model of experimental acute kidney injury.4,37,38 Galectin-3 inhibition by N-acetyllactosamine may improve glomerular filtration function and tubular regeneration, as shown by attenuated myofibroblast activation and reduced proteinuria in a model of rats with hypertensive end-organ damage and increased galectin-3 levels.7 In a different setting, intravenous human administration of the galectin-3 ligand, pectin-derived GCS-100, was shown to be well tolerated.39 More recently, a novel galectin-3 inhibitor (HH1-1) was developed and evaluated in a different clinical setting, however, further evaluation is needed before clinical uptake.40 Of note, galectin-3 is thought to play a general pan-organ role in fibrosis,1 which demands thoughtful interventional strategies given the unpredictability of potential unwanted side effects of an otherwise protective systemic galectin-3-targeted therapy. Organ- and cell-specific potential therapeutics may be required.41 The profibrotic signaling axis between macrophages—which are thought to be the major kidney tissue source of galectin-3—and activated fibroblasts, gives the basis and holds the plea for the development of novel approaches to appropriately direct therapeutic interventions through targeted inhibition of local tissue macrophage–derived galectin-3 expression and secretion.41
Another observation of the current study is the consistent effect-modification by 2 atherosclerotic risk factors, that is, systolic blood pressure and smoking history. The intimate interaction between galectin-3 and systolic blood pressure has been reported before.7,12,30 Our findings are consistent with recent epidemiological observations by Rebholz et al,12 and in agreement with evidence that supports a key contributory role of galectin-3 to vascular fibrosis, arterial stiffness, and atherosclerotic plaque progression through amplification of proinflammatory molecules.31,42,43 Particularly in the context of preexisting vascular damage, these evidence may further support the link between elevated levels of galectin-3 postkidney transplantation and increased risk of late graft failure.
A strength of the current study is that we detached the association between galectin-3 and graft failure from the most thoroughly studied role of galectin-3 in cardiovascular disease, by performing adjustment for cardiovascular history, risk factors, and biomarkers (eg, N-terminal pro b-type natriuretic peptide and high-sensitive troponin T). Independent of cardiovascular covariates, the trend of most recent observations seems to increasingly and strongly position galectin-3 as a biomarker in kidney disease (reviewed by Filipe et al44). Of note, the US Food and Drug Administration approved a galectin-3 commercial assay, and it counts with class IIb indication for additive risk stratification in patients with established heart failure in guidelines of the American Heart Association/American College of Cardiology Foundation Heart Failure.45 However, the cardiac contribution to circulating (systemic) levels of galectin-3 is very minor. Galectin-3 has been reported much more abundant in kidney tissue and fat mass.46 These data seem to justify most recent discussion on the evolving role of galectin-3 as cardiac biomarker and may support its role in novel mechanisms of cardiac-renal interaction, with galectin-3 being involved in shared pathological pathways between kidney injury, impaired renal clearance and progressive cardiovascular tissue fibrosis.22,31,47-50 Such interaction becomes of encompassing epidemiological relevance in stable KTR by taking into account the leading role of cardiovascular disease among major causes of premature mortality in postkidney transplantation.16,34
Other strengths of the current study are its long-term prospective nature design, with performance of extensive phenotyping of a large cohort of stable KTR. Finally, the robustness of our findings over continuous and categorical analyses underline the graded nature of the association between galectin-3 levels and risk of late graft failure in stable KTR. Although the long-term prospective analyses were performed using a single baseline measurement, the current study additionally provides longitudinal data on galectin-3 in a subsample of KTR of the newly generated and ongoing TransplantLines Prospective Cohort and Biobank Study,20 which supports the notion that galectin-3 levels are relatively stable over time, with a low intraindividual CV. It should also be realized that most epidemiologic studies use a single baseline measurement for studying the association of variables with outcomes, which adversely affects the strength and significance of the association of these variables with outcomes.51,52 Thus, if intraindividual variability of variables is taken into account, this results in strengthening of associations that also existed for single measurements of these variables.51,52 Our study also has limitations that warrant consideration in the interpretations of our results. Due to its observational design, we acknowledge that the current study does not allow hard conclusions on causality and reversed causation or residual confounding may occur. Next, our study population consisted predominantly of Caucasian people of the northern part of The Netherlands, which calls for prudence to extrapolate our results to different populations with regard to ethnicity and differences caused by local epidemiology. It should also be noted that we had no data on history of BK nephropathy, presence of donor-specific antibodies or biopsy data on acute rejection events that occurred between transplantation and baseline measurement, which did not allow for presentation of data on type of rejection (T cell– or antibody-mediated) or grade of rejection according to Banff classification. We also had no data on compliance for use of immunosuppressive drugs. By design, this long-term study included many KTR treated with less potent immunosuppressive regimens than contemporary counterparts, which may likely explain the relatively high rate of history of acute rejection episodes. This underscores that longitudinal studies, like our study, with their baseline in the past by design, should be seen as a way to provide evidence for potential involvement of a potential causal factor that, as galectin-3 in this case, precedes performance of targeted pharmacological therapies, and that intervention studies in patients with contemporary immunosuppression therapies are required as next step. Future observational or intervention studies could also investigate whether galectin-3-targeted pharmacological therapies could be of particular benefit after a history of either T cell– or antibody-mediated rejection or both. Finally, to the best of our knowledge, reference values for galectin-3 are currently not established. Given the current findings, standardized assays for galectin-3 with reference values are warranted.
In conclusion, in stable KTR, galectin-3 levels are elevated and independently associated with an over than 2-fold higher risk of graft failure at approximately 10 years of follow-up, particularly in patients with higher systolic blood pressure or smoking history. Further studies are warranted to evaluate whether galectin-3-targeted therapy may represent a novel opportunity to decrease the long-standing high burden of late graft failure in stable KTR.
Supplementary Material
Footnotes
This study is based on data of the TransplantLines Insulin Resistance and Inflammation (TxL-IRI) cohort (Clinicaltrials.gov identifier: NCT03272854), which was funded by the Dutch Kidney Foundation (grant C00.1877). C.A.t.V.-K. is supported by a personal grant from the Dutch Kidney Foundation (Kolff grant 17OKG02). C.G.S. is supported by a personal grant from CONICYT (F 72190118).
The authors declare no conflicts of interest.
C.G.S. was involved in research design, acquired the data, performed analyses and interpretation of the data, drafted the article, and created the figures. C.A.t.V.-K. was involved in data analysis and contributed to the final adjustments to the article after revising it critically for intellectual content. A.D. acquired the data and contributed to the final adjustments to the article after revising it critically for intellectual content. M.v.L. was involved in data analysis and contributed to the final adjustments to the article after revising it critically for intellectual content. R.A.P. was involved in research design and contributed to the final adjustments to the article after revising it critically for intellectual content. A.P. acquired the data and contributed to the final adjustments to the article after revising it critically for intellectual content. R.O.B.G. acquired the data and contributed to the final adjustments to the article after revising it critically for intellectual content. I.M.N. was involved in data analysis and contributed to the final adjustments to the article after revising it critically for intellectual content. R.H.J.A.S. contributed to data interpretation and to the final adjustments to the article after revising it critically for intellectual content. M.H.d.B. contributed to data interpretation and to the final adjustments to the article after revising it critically for intellectual content. S.P.B. contributed to data interpretation and to the final adjustments to the article after revising it critically for intellectual content. R.R. was involved in research design, data interpretation, and contributed to the final adjustments to the article after revising it critically for intellectual content. G.J.N. was involved in research design, data interpretation, and contributed to the final adjustments to the article after revising it critically for intellectual content. R.A.d.B. was involved in research design, data interpretation, and contributed to the final adjustments to the article after revising it critically for intellectual content. S.J.L.B. initiated the study, was involved in research design, data interpretation, and contributed to the final adjustments to the article after revising it critically for intellectual content.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobry T, Miller R, Nevo N, et al. Interaction between galectin-3 and cystinosin uncovers a pathogenic role of inflammation in kidney involvement of cystinosis. Kidney Int. 2019;96:350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langman CB. Oh cystinosin: let me count the ways! Kidney Int. 2019;96:275–277. [DOI] [PubMed] [Google Scholar]
- 4.Kolatsi-Joannou M, Price KL, Winyard PJ, et al. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011;6:e18683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang Z, Mackinnon A, Marson LP, et al. Basic and experimental research tubular atrophy and interstitial fibrosis after renal transplantation is dependent on galectin-3. Transplantation. 2012;93:477–484. doi:10.1097/TP.0b013e318242f40a [DOI] [PubMed] [Google Scholar]
- 6.Calvier L, Martinez-Martinez E, Miana M, et al. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015;3:59–67. [DOI] [PubMed] [Google Scholar]
- 7.Frenay AR, Yu L, van der Velde AR, et al. Pharmacological inhibition of galectin-3 protects against hypertensive nephropathy. Am J Physiol Renal Physiol. 2015;308:F500–F509. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Martinez E, Ibarrola J, Calvier L, et al. Galectin-3 blockade reduces renal fibrosis in two normotensive experimental models of renal damage. PLoS One. 2016;11:e0166272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang WH, Shrestha K, Shao Z, et al. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol. 2011;108:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer RA, van Veldhuisen DJ, Gansevoort RT, et al. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med. 2012;272:55–64. [DOI] [PubMed] [Google Scholar]
- 11.O’Seaghdha CM, Hwang SH, Ho JE, et al. Elevated galectin-3 precedes the development of CKD. Clin J Am Soc Nephrol. 2004;15:1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebholz CM, Selvin E, Liang M, et al. Plasma galectin-3 levels are associated with the risk of incident chronic kidney disease. Kidney Int. 2018;93:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alam ML, Katz R, Bellovich KA, et al. Soluble ST2 and galectin-3 and progression of CKD. Kidney Int Rep. 2019;4:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annema W, Dikkers A, de Boer JF, et al. HDL cholesterol efflux predicts graft failure in renal transplant recipients. J Am Soc Nephrol. 2016;27:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenga MF, Kieneker LM, Soedamah-Muthu SS, et al. Urinary potassium excretion, renal ammoniagenesis, and risk of graft failure and mortality in renal transplant recipients. Am J Clin Nutr. 2016;104:1703–1711. [DOI] [PubMed] [Google Scholar]
- 16.Kasiske BL, Vazquez MA, Harmon WE, et al. Recommendations for the outpatient surveillance of renal transplant recipients. American Society of Transplantation. J Am Soc Nephrol. 2000;11Suppl 15:S1–86. [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christenson RH, Duh SH, Wu AH, et al. Multi-center determination of galectin-3 assay performance characteristics: Anatomy of a novel assay for use in heart failure. Clin Biochem. 2010;437-8683–690. [DOI] [PubMed] [Google Scholar]
- 19.van Timmeren MM, Lems SP, Hepkema BG, et al. Anti-human leukocyte antigen antibodies and development of graft failure after renal transplantation. Transplantation. 2009;88:1399–1400. [DOI] [PubMed] [Google Scholar]
- 20.Eisenga MF, Gomes-Neto AW, Van Londen M, et al. Rationale and design of Transplant Lines: a prospective cohort study and biobank of solid organ transplant recipients. BMJ Open. 2018;8:24502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijers WC, van der Velde AR, de Boer RA. The ARCHITECT galectin-3 assay: comparison with other automated and manual assays for the measurement of circulating galectin-3 levels in heart failure. Expert Rev Mol Diagn. 2014;14:257–266. [DOI] [PubMed] [Google Scholar]
- 22.Zamora E, Lupón J, de Antonio M, et al. Renal function largely influences galectin-3 prognostic value in heart failure. Int J Cardiol. 2014;177:171–177. [DOI] [PubMed] [Google Scholar]
- 23.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen PK, Geskus RB, de Witte T, et al. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noordzij M, Leffondré K, van Stralen KJ, et al. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28:2670–2677. [DOI] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 27.Van Diepen M, Ramspek CL, Jager KJ, et al. Prediction versus aetiology: common pitfalls and how to avoid them. Nephrol Dial Transplant. 2017;32:ii1–ii5. [DOI] [PubMed] [Google Scholar]
- 28.Inker LA, Schmid CH, Tighiouart H, et al. ; CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan R, Liu X, Wang J, et al. Alternations of galectin levels after renal transplantation. Clin Biochem. 2014;47:83–88. [DOI] [PubMed] [Google Scholar]
- 30.van der Velde AR, Meijers WC, van den Heuvel ER, et al. Determinants of temporal changes in galectin-3 level in the general population: data of PREVEND. Int J Cardiol. 2016;222:385–390. [DOI] [PubMed] [Google Scholar]
- 31.Calvier L, Miana M, Reboul P, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33:67–75. [DOI] [PubMed] [Google Scholar]
- 32.Winkelmayer WC, Lorenz M, Kramar R, et al. C-reactive protein and body mass index independently predict mortality in kidney transplant recipients. Am J Transplant. 2004;4:1148–1154. [DOI] [PubMed] [Google Scholar]
- 33.Abedini S, Holme I, März W, et al. ; ALERT study group. Inflammation in renal transplantation. Clin J Am Soc Nephrol. 2009;4:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11:450–462. [DOI] [PubMed] [Google Scholar]
- 35.Breyer MD, Susztak K. The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov. 2016;15:568–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suthahar N, Meijers WC, Silljé HHW, et al. Galectin-3 activation and inhibition in heart failure and cardiovascular disease: an update. Theranostics. 2018;8:593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inohara H, Raz A.Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj J. 1994;11:527–532. [DOI] [PubMed] [Google Scholar]
- 38.Liu HY, Huang ZL, Yang GH, et al. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J Gastroenterol. 2008;14:7386–7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demotte N, Wieërs G, Van Der Smissen P, et al. A galectin-3 ligand corrects the impaired function of human CD4 and CD8 tumor-infiltrating lymphocytes and favors tumor rejection in mice. Cancer Res. 2010;70:7476–7488. [DOI] [PubMed] [Google Scholar]
- 40.Yao Y, Zhou L, Liao W, et al. HH1-1, a novel galectin-3 inhibitor, exerts anti-pancreatic cancer activity by blocking galectin-3/EGFR/AKT/FOXO3 signaling pathway. Carbohydr Polym. 2019;204:111–123. [DOI] [PubMed] [Google Scholar]
- 41.Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–171. [DOI] [PubMed] [Google Scholar]
- 42.Papaspyridonos M, McNeill E, de Bono JP, et al. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler Thromb Vasc Biol. 2008;28:433–440. [DOI] [PubMed] [Google Scholar]
- 43.Nachtigal M, Al-Assaad Z, Mayer EP, et al. Galectin-3 expression in human atherosclerotic lesions. Am J Pathol. 1998;152:1199–1208. [PMC free article] [PubMed] [Google Scholar]
- 44.Filipe MD, Meijers WC, Rogier van der Velde A, et al. Galectin-3 and heart failure: prognosis, prediction & clinical utility. Clinica Chimica Acta. 2015;443:48–56. [DOI] [PubMed] [Google Scholar]
- 45.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 46.Du W, Piek A, Schouten EM, et al. Plasma levels of heart failure biomarkers are primarily a reflection of extracardiac production. Theranostics. 2018;8:4155–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erkilet G, Özpeker C, Böthig D, et al. The biomarker plasma galectin-3 in advanced heart failure and survival with mechanical circulatory support devices. J Heart Lung Transplant. 2013;32:221–230. [DOI] [PubMed] [Google Scholar]
- 48.deFilippi CR, Christenson RH. Evolving role of galectin-3 as a cardiac biomarker. Heart failure with preserved ejection fraction and renal function, important pieces of the puzzle. JACC: Heart Failure. 2015;3:253–256. [DOI] [PubMed] [Google Scholar]
- 49.Meijers WC, van der Velde AR, Ruifrok WP, et al. Renal handling of galectin-3 in the general population, chronic heart failure, and hemodialysis. J Am Heart Assoc. 2014;3:e000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gopal DM, Kommineni M, Ayalon N, et al. Background—galectin-3. Relationship of plasma galectin-3 to renal function in patients with heart failure: effects of clinical status, pathophysiology of heart failure, and presence or absence of heart failure. J Am Heart Assoc. 2012;e000760:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koenig W, Sund M, Fröhlich M, et al. Refinement of the association of serum C-reactive protein concentration and coronary heart disease risk by correction for within-subject variation over time: the MONICA Augsburg studies, 1984 and 1987. Am J Epidemiol. 2003;158:357–364. [DOI] [PubMed] [Google Scholar]
- 52.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.