Supplemental Digital Content is Available in the Text.
BACKGROUND
Crosslinked hyaluronic acid (HA)-based soft tissue fillers possess unique viscoelastic properties intended to match specific product indications. Manufacturing has an impact on HA chain integrity and on filler properties.
OBJECTIVE
This study introduces 2 new rheological parameters to evaluate the macroscopic characteristics of fillers.
METHODS AND MATERIALS
A library of reference commercialized HA fillers was selected to cover the full spectrum of product indications. Gels were assessed in terms of size of released HA fragments as a readout of gel integrity, degree of modification, cohesivity, and rheological properties.
RESULTS
The elastic modulus G′ often used to characterize fillers was shown not to follow macroscopic mechanical properties. To improve the mechanical characterization of fillers, Strength and Stretch scores were developed and tested. The Strength score defined the ability of a filler to sustain constant viscoelasticity over a wide range of constraints and represented the filler mechanical resilience. The Stretch score measured the propensity of a filler to deform in view to improve implant adaptation to facial animation for natural-looking results.
CONCLUSION
Strength and Stretch scores sorted rheological parameters to macroscopic cohesivity assays more accurately than G′ and may thus help predict the gel behavior once implanted and submitted to facial dynamics.
Soft tissue fillers are injectable soft gels aimed to counteract skin depression and changes because of tissue ageing and loss.1,2 They help by reducing the intensity of skin folds, wrinkles, lines, and creating facial volume in specific areas. Among all dermal fillers, hyaluronic acid (HA)-based gels have garnered increased attention over the past decades because of immediate and natural-looking visual effects on skin as well as being proven to be safe, long lasting, and easy-to-use alone or in combined treatments.3–5 Many HA dermal fillers are generally composed of high-molecular weight (Mw) HA, a naturally occurring polysaccharide in the skin, containing the repetition of d-glucuronic acid and d-N-acetyl glucosamine disaccharide units, crosslinked with difunctional molecules, or crosslinkers, on the carboxyl or the hydroxyl moieties.6,7 According to their chemical compositions, such as HA concentration, Mw of the HA, and the crosslinker content, each commercially available HA-based gel exhibits unique viscoelastic and biophysical properties intended to match its product indications.8–10 Hyaluronic acid chains are sensitive to manufacturing process parameters such as high temperatures and strong acidic and alkali pH.11 Indeed, the usual manufacturing conditions (heat, alkali pH, and sterilization) are prone to degrade HA gels12 and release low-Mw soluble HA (sHA) fragments. Accordingly, the manufactured HA gels may significantly differ in its final in vivo characteristics with potential safety issues.13,14 Therefore, there is an increasing need to master the mechanical properties of the gels, anticipate their safety profiles, and develop mild manufacturing conditions to ensure HA integrity throughout the process.12,15 TEOXANE Laboratories (Geneva, Switzerland) introduced a range of 4 Resilient HA fillers (TEOSYAL RHA®) manufactured with a unique technology (Preserved Network) that is specifically designed to improve HA chain integrity throughout the gel manufacturing, thus better preserving long (high Mw) HA chains that in turn require low amounts of crosslinker to achieve clinically desirable mechanical properties and durability. These less rigidly crosslinked HA chains are presumed to allow implants to better accompany and adapt to mechanical deformations such as muscle movements driving dynamic facial motion.16,17 As the primary function of all implants is to fill skin wrinkles and folds and restore facial volumes with good biointegration, their mechanical behavior is a key feature of their clinical use and performance. It is therefore essential to characterize their rheological profiles accurately. Traditionally, analysis of HA gels is limited to the elastic modulus G′, the viscous modulus G″, the phase angle δ (or tan δ), being connected to the former quantities (tan δ = G″/G′), and the complex viscosity η*.9,10,18,19 Such data are obtained with oscillatory dynamic rheology and is typically measured in nearly static conditions20: a few Pascals (1–10 Pa) or few percentages of deformation (0.1%–1%). This fall within the linear viscoelastic region (LVER), where G′, G″, and δ are constant. Hence, these parameters are not necessarily representative of the mechanical conditions to which a filler gel is subjected throughout its medical use, that is, being injected through a thin needle (delivering high shear rates), integration into the skin, and adapting to facial movements and their dynamics over several months (requiring elasticity, deformability, and cohesivity depending on the location in which they are placed).21
Consequently, 2 new rheological parameters were introduced to gain more insights into the performance of gel fillers.22 The first rheological parameter, termed as “Strength,” reflects how far, in terms of stresses and deformations, a gel preserves its G′. In other words, the Strength score reflects 2 rheological dimensions: the gel G′ and the range of stresses (or deformations) the G′ is maintained, namely the LVER. The second parameter, termed as “Stretch,” represents the ability of a gel to deform and adapt to stresses due, for instance, to tissue stretching.
In this article, comparative studies were performed on a series of commercially available 1,4-butanediol diglycidylether (BDDE)-crosslinked HA dermal fillers to assess their biophysical properties. To anticipate possible residual low Mw HA fragments resulting from the crosslinking reaction and possibly affecting the biophysical behavior of gels in situ, an analysis of extractable sHA was performed for Mw limits of 30, 100, and 250 kDa, as well as an analysis of the degree of modification (MoD) of the studied gels. Macrostructural cohesivity tests were then performed on the gels to assess the capacity of the gels to sustain constraints that could be encountered during gel life according to their product indications. The HA gel library was eventually assessed using common rheological parameters as well as the new Strength and Stretch scores.
Materials and Methods
Materials
A library of marketed dermal fillers was compared in this study. All fillers were HA gels crosslinked with BDDE and produced with different manufacturing parameters. The studied library included a wide class of fillers indicated for superficial or mid-to-deep wrinkles (with dermal injection depths) or deeper indications for volumizers (e.g., subdermal and supraperiosteal injection depth). The studied products are classified in Table 1. Fillers were chosen from among the market leaders and belong to following ranges: Restylane® (RES) gels manufactured with the NASHA™ technology and with the OBT™ technology, also known as the XpresHAn™ technology in the United States, or Juvéderm® gels manufactured with the Vycross® technology (VYC) and containing a majority of low Mw HA chains.21,23 These fillers were compared with TEOSYAL RHA® products (RHA) manufactured using the Preserved Network Technology.
TABLE 1.
Library of the Studied Dermal Fillers Classified According to Their Clinical Indications, Their Manufacturer, Their Process Technology, and Their Hyaluronic Acid Concentration
| Indication* | Filler | Abbreviation | Manufacturer | Technology | HA Concentration (mg/mL)* | Batch References | Needle Size† |
| Fillers for superficial wrinkles | Restylane® SkinboostersTM Vital | RESSV | GALDERMA | NASHATM | 20 | 17633-1 | 29G × 1/2″ |
| Juvederm® VoliteTM | VYC-12L | ALLERGAN | Vycross® | 12 | V12LA90739 | 32G × 1/2″ | |
| Juvederm® VolbellaTM | VYC-15L | ALLERGAN | Vycross® | 15 | V15LA90261 | 30G × 1/2″ | |
| TEOSYAL RHA® 1 | RHA1 | TEOXANE | Preserved Network | 15 | TPRL-192512A | 30G × 1/2″ | |
| Fillers for mid-to-deep wrinkles | Restylane® Lido | RES | GALDERMA | NASHATM | 20 | 17604-1 | 29G × 1/2″ |
| Restylane® RefyneTM | RESREF | GALDERMA | OBTTM/XpresHAnTM Technology | 20 | 17523 | 30G × 1/2″ | |
| Restylane® DefyneTM | RESDEF | GALDERMA | OBTTM/XpresHAnTM Technology | 20 | 17360 | 27G × 1/2″ | |
| Juvederm® VoliftTM | VYC-17.5L | ALLERGAN | Vycross® | 17.5 | V17LA90320 | 30G × 1/2″ | |
| TEOSYAL RHA® 2 | RHA2 | TEOXANE | Preserved Network | 23 | TP30L-192321A | 30G × 1/2″ | |
| TEOSYAL RHA® 3 | RHA3 | TEOXANE | Preserved Network | 23 | TP27L-192312A | 27G × 1/2″ | |
| Volumizers | Restylane® LyftTM Lido | RESLYFT | GALDERMA | NASHATM | 20 | 17460-1 | 29G × 1/2″ |
| Juvederm® VolumaTM | VYC-20L | ALLERGAN | Vycross® | 20 | VB20A80852 | 27G × 1/2″ | |
| TEOSYAL RHA® 4 | RHA 4 | TEOXANE | Preserved Network | 23 | TPUL-192311A | 27G × 1/2″ |
As indicated in the dermal filler package insert.
Needles provided with the filler.
sHA, soluble hyaluronic acid.
Chemical Gel Characterization: Soluble Hyaluronic Acid Distribution and Degree of Modification
Extractable Soluble Hyaluronic Acid Analysis
The Mw, the dispersity (Mw/Mn), and the quantity of sHA released from HA gels were determined by size exclusion chromatography equipped with a multiangle laser light scattering detection (Dawn Neon MALS, Wyatt Technology Corp) and a refractive index detection (Optilab dRI, Wyatt Technology Corp). The instrumentation used an Agilent Infinity LC system equipped with a dual set of size exclusion columns OHpak LB-806M held in series flow (Shodex). A pH 7.2 mobile phase of sodium nitrate was used as eluent at a flow rate of 0.3 mL/min. Refractive index increment (dn/dc) was set at 0.165 mL/g. Chromatograms were obtained and analyzed using ASTRA software (Wyatt Technology Corp).
For sample preparation, 0.5 g of gel was diluted in 5 mL of mobile phase for sHA extraction at 37°C. The diluted gel was filtered at 0.45 μm to remove insoluble aggregates, and 100 μL of the filtrate were injected.
Degree of Modification of the Fillers
The MoD of the studied fillers was assessed using1 H NMR. The gels were precipitated in isopropanol and dried for 6 hours under vacuum. The dried HA residues were dissolved at 10 mg/mL in D2O. Hyaluronidase of 50 μL (Type VI-S from bovine testes, 3 kU/mL in D2O) was added to degrade the gels for 18 hours at 37°C. The analysis was conducted on a 400 MHz Bruker Avance spectrometer. The MoD was determined as previously described.24
Mechanical Tests
Shear Cohesivity Assays in PBS With Methylene Blue
The assay of shear cohesivity in PBS (as inspired by a previously published protocol25) was performed at 22 ± 1°C. First, all the products used for this test were transferred into a 1 mL plastic syringes (Schott TopPac, Schott Schweiz AG, Switzerland) containing 1 g of gel sample. A 15 g/L stock solution of methylene blue of 2 μL was placed in another syringe. Both syringes were connected together and a series of back-and-forth extrusion cycles were performed to homogeneously stain gels without incorporating air bubbles. Stained gels were stored vertically at 6°C overnight. For cohesivity tests, gels were extruded from the needle-less syringe in a 500 mL glass beaker containing 300 mL PBS, pH = 7.3 (Braun Medical AG, Crissier, Switzerland). Immediately after the gel extrusion, gel coils were gently and constantly stirred for 30 seconds, by pouring an extra 200 mL PBS in the beaker at an average flow rate of 6.7 mL/s. Video recordings of the gel under shear started at the same time as the gel stirring. Gel cohesion/dispersion was then visually assessed by 5 scientists, blinded to the product being assessed, according to the proposed Gavard–Sundaram cohesivity scale.25 The results are reported as the mean score ± SDs.
Compression Test
A DHR2 rheometer (TA Instruments, France) equipped with a parallel flat plate geometry (40-mm diameter, anodized aluminum, TA Instruments, France) was used for mechanical compression assessments. The software associated with the rheometer was TRIOS (TA Instruments, France). For each of the filler, 2 g of gel was deposited on center of the Peltier plate at 25°C. The initial gap was set to 2.60 mm, and the gel was left to recover for 60 seconds. The gel was then compressed at a constant speed of 100 μm/s to 70% of the initial gap to limit gel expulsion from the geometries. The gel's mechanical resistance to compression was measured at the end of the compression course.
Rheology Assays
Dynamic Oscillatory Rheology—Strength Score
Dynamic oscillatory rheological measurements were performed using the same rheometer equipped with a rough Peltier plate and rough parallel plate geometry (50 and 25-mm diameter, 250-μm rough, stainless steel, PMP Mécanique de Precision, France). For each of the filler, 0.50 g of gel was slowly extruded through the syringe with the needle provided by the manufacturer on a rheometer plate. An oscillatory stress sweep test was performed at a temperature of 25°C over a stress range of 1 to 1,500 Pa at the oscillation frequency of 1 Hz and at 25°C, covering stresses within and beyond the LVER. A preconditioning step was performed to equilibrate the gel at a working temperature of 25°C for 70 seconds and at a working gap of 500 μm between the geometry and the rheometer plate. The values of the elastic modulus G′, the viscous modulus G″, the viscoelastic parameter δ, and the complex viscosity η* were measured at a stress σ = 5 Pa. The Strength score, characterizing the ability of a gel to maintain its G′ over a range of stresses, was then calculated by integrating the area under the curve of G′ from 1 Pa up to the stress value (in Pa) for which a decrease of 10% of initial G′ was observed. This range of stresses was considered as the LVER of the gel. Ten percent decrease was taken as a marker of significant G′ drop. It was verified that this decrease was not an artefact, that is, the G′ continued to drop when stress further increased.
Creep Measurement and Stretch Score
The Stretch test was performed using a creep measurement that consisted in applying a constant shear stress on the gels at 25°C and measuring the resulting deformation over time. A preconditioning step was performed to equilibrate the gel at a working temperature of 25°C during 70 seconds and at a working gap of 0.5 mm between the parallel flat plate geometry. After equilibrium, the Stretch test was performed at a stress of 5 Pa at 25°C with the same gap for 900 seconds. The deformation curve was obtained, and the Stretch score was calculated from the slope of the steady-state viscous creep deformation part of the strain curve.
Data Analysis
All measurements were performed in triplicate. Mean values expressed with SDs were obtained after 3 measurements on 3 different gels for each of the given filler.
Results
Chemical Gel Characterization: Soluble Hyaluronic Acid Distribution and Degree of Modification
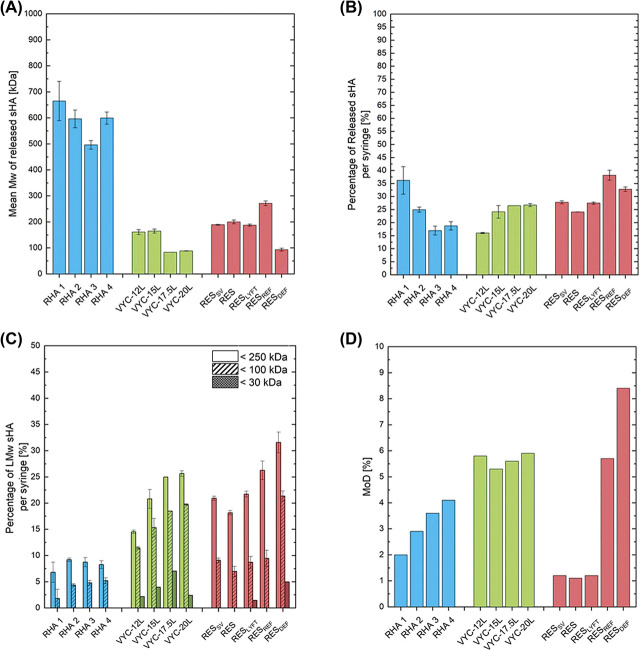
The sHA, the fraction of HA that was released from the gels and freely soluble in aqueous solution, was quantified in terms of the mean Mw of the released sHA, the overall sHA quantity per syringe of gel, and the quantity of low Mw fragments of sHA per syringe of gel (Figure 1). RHA fillers released sHA chains on average about twice longer than RES fillers and 3 time longer than VYC fillers (Figure 1A). The percentage of released HA per syringe of gel ranged between 16% and 38% (Figure 1B). The quantity of low Mw sHA fragments released from the studied gels is reported in Figure 1C. The quantity of sHA released per syringe of gel with a Mw < 250 kDa ranged between 6.8% for RHA 1% to 31.5% for RESDEF, with a Mw < 100 kDa ranging between 1.8% for RHA 1% to 11.6% for RESDEF, and with a Mw < 30 kDa ranged between 0% for RHA product line, RESSV, RES, and RESREF to 7.0% for VYC-17.5L. RHA products exhibited the lowest low Mw sHA content with less than 9.2% of the total HA content in one syringe being sHA <250 kDa, less than 5.2% of the total HA content in one syringe being sHA <100 kDa, and no sHA <30 kDa. VYC and RES products presented similar low Mw sHA content with 14.5% to 31.5% of the total HA content in one syringe being <250 kDa, 7.0% to 21.3% of the total HA content in one syringe being <100 kDa, and 0% to 7% of the total HA content in one syringe being <30 kDa. Figure 1D showed the MoD of the studied gels. NASHA products presented the lowest MoD values between 1.1% and 1.2%. RHA products exhibited MoD ranging between 2% and 4.1%. The MoD of Vycross products ranged between 5.3% and 5.9%, similarly to RESREF. RESDEF presented the highest MoD of the investigated products at 8.4%.
Figure 1.
Characterizations of the investigated gels. Size exclusion chromatography analysis of sHA released from the gels after extraction to assess (A) the mean Mw of the released sHA fragments, (B) the percentage of released sHA per syringe, and (C) the percentage of released low Mw sHA per syringe in the distribution ranges of [0–250 kDa], [0–100 kDa], and [0–30 kDa]. (D) 1H NMR analysis of the gels to assess the MoD of the enzymatically digested gels. sHA, soluble hyaluronic acid; MoD, degree of modification.
Mechanical Tests
Two different mechanical tests were performed on the library of fillers. A cohesivity test under shear in aqueous buffer was first performed according to a previously published procedure (See Supplemental Digital Content 1, Figure SI 1A–C, http://links.lww.com/DSS/A642, Gavard–Sundaram cohesivity test).25 After implementation of a mild shear, the cohesivity of resulting gel mixtures was assessed and visually graded from “fully dispersed” to “fully cohesive” according to a validated 5-grade scale.25,26 The results are visually and quantitatively represented in Supplemental Digital Content 1 (see Figure SI 1B and 1C, http://links.lww.com/DSS/A642). Gels exhibited strikingly different behaviors under the shear test. Interestingly, in this cohesivity model, the highest score was observed with a noncrosslinked 1.5 MDa HA solution (23 mg/mL); RHA products displayed the closest results to this unreacted HA product showing the highest cohesivity scores among all the studied gels. VYC-12L, RES, and RESLYFT presented the lowest cohesivity scores with no visible residual gel particles in the buffer. Regarding the RHA family of products, those intended for superficial placement (corresponding to the less crosslinked products) showed the highest gel shear cohesivity; surprisingly, the opposite trend was observed for VYC products that can be due to a major role of the HA concentration (and no differentiation in the crosslinking rate).
In complement to this cohesivity test under shear, a compression test was also performed to assess the resistance of gels submitted to increasing compression forces (see Supplemental Digital Content 1, Figure SI 2A, http://links.lww.com/DSS/A642, Figure, compression test). As the gel is progressively compressed, its resistance to compression is assessed that can translate into a lifting capacity of the gels (see Supplemental Digital Content 1, Figure SI 2B, http://links.lww.com/DSS/A642). The maximal force at the end of the compression test is reported in Supplemental Digital Content 1 (see Figure SI 2C, http://links.lww.com/DSS/A642). Unlike the NASHA range of products, the RHA, OBT, and VYC fillers presented increasing resistance values from the more superficial to the deeper indication product arguably because of an increasing crosslinking rate and/or HA concentration. RHA 4 presented a higher resistance to compression than all other studied fillers (see Supplemental Digital Content 1, Figure SI 2C, http://links.lww.com/DSS/A642).
Rheology Assessments
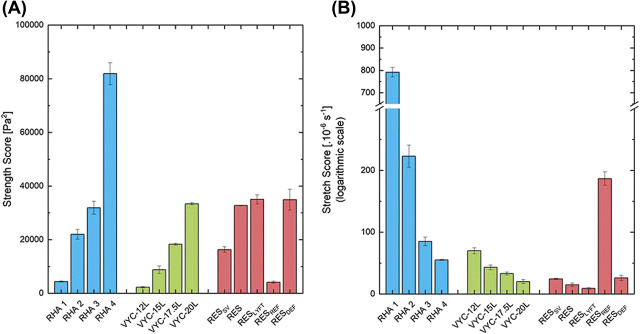
The conventional rheological quantities, namely elastic modulus G′, phase angle δ, and complex viscosity η*, plus the “Strength” and “Stretch” scores were measured on the library of fillers. The results are summarized in Supplemental Digital Content 1 (see Figures SI 3 and 4, http://links.lww.com/DSS/A642, rheological properties) and Figure 2. As expected in the studied filler library, a wide range of G′ was measured, in nearly static conditions, ranging from ∼60 to ∼800 Pa (see Supplemental Digital Content 1, Figure SI 3A, http://links.lww.com/DSS/A642). Within each filler range, volumizers displayed the highest G’. The LVER of a filler represents the range of stress (or deformation) values over which its viscoelastic parameters (such as G′) are maintained constant and can freely recover. Beyond the LVER, the gels start to lose their mechanical and rheological properties (see Methods and Supplemental Digital Content 1, Figure SI 3C, http://links.lww.com/DSS/A642). The range of studied gels followed a consistent and expected trend with increasing LVER measured for products intended for the placement in the deep dermis or for volumization (see Supplemental Digital Content 1, Figure SI 3C, http://links.lww.com/DSS/A642). RHA 3 and 4 displayed the highest LVER from ∼200 to ∼300 Pa, respectively, compared with the other studied fillers, meaning that RHA 3 and 4 are able to withstand larger deformations and stresses, such as what is expected in deep dermis, and fully recover. Consequently, as the facial tissues are dynamic, the Strength score was introduced in view to take into account both the G′ value, highlighting the gel elastic resistance to deformation or stresses that is measured in nearly static conditions, and how far the G′ is maintained in terms of stresses and deformations, in other words, the LVER. Practically, the Strength score was obtained by integrating the G′ plot over its entire LVER (see Supplemental Digital Content 1, Figure, SI 3D, http://links.lww.com/DSS/A642). For instance, RHA 4, VYC-20L, and RESLYFT presented G′ values of ∼263, ∼305, and ∼807 Pa, respectively, and maintained a constant G′, the LVER, up to stresses of ∼308, ∼104, and ∼46 Pa, respectively (see Supplemental Digital Content 1, Figure SI 3C, http://links.lww.com/DSS/A642). RHA 4 exhibited the highest Strength scores of 81.9 104 ± 4.1, with VYC-20L and RESLYFT scores being 33.4 104 ± 0.4 and 35.0 104 Pa2 ± 1.7, respectively.
Second, the Stretch characteristics of each gel were obtained by creep measurement, and scores were measured by the slope of the steady-state viscous response of the creep curve. Supplemental Digital Content 1 (see Figure SI 4, http://links.lww.com/DSS/A642) shows results from gels intended for superficial wrinkle filling that require a high rate of deformation to likely produce natural outcomes. RHA 1 displayed the highest Stretch scores among selected fillers, more than 10 times higher than the other superficial fillers reflecting its ability to deform after the application of a stress more rapidly and more largely.
When comparing fillers altogether, the RHA products exhibited the largest Strength and Stretch ranges (Figure 2A–B). Within each filler range (RHA, VYC, and RES), the gels intended for more superficial indications (e.g., RHA 1, RHA 2, VYC-12L, RESREF, and RESSV) displayed the highest Stretch scores, whereas volumizers exhibited the highest Strength scores. Interestingly, despite their high Strength scores, RHA 3 and RHA 4 also displayed Stretch scores at least similar to or higher than the studied Vycross products, NASHA products, and RESDEF.
Figure 2.
Scores of (A) Strength and (B) Stretch of the gel library.
Discussion
Hyaluronic Acid Fragments From the Gels and Degree of Modification
The production of sHA freely soluble in aqueous media and released from the gel after HA chain clipping was used as a readout of the impact of the different formulations and manufacturing technologies on HA fillers because a direct measurement of HA Mw is not possible for gels. Soluble HA may originate from the noncrosslinked HA added to the crosslinked HA in the formulation and/or small fragments of HA generated by the crosslinking reaction or the sterilization step.11 In terms of manufacturing technologies, RHA fillers released the longest sHA chains, consistent with the Preserved Network technology aimed to preserve high Mw HA chains. In terms of low Mw sHA fragments, VYC products presented more of the shortest sHA chains, consistently with the predominant use of low Mw HA during the Vycross manufacturing process,23 along with RESDEF. A previous study conducted on sHA showed that the released fragments were mainly constituted of small, soluble, and crosslinked HA fragments of ∼100 to 200 kDa likely generated during the crosslinking step, except for RHA 4 that was also composed of ∼50 % wt/wt of linear high Mw (Mw ∼1.1 MDa) sHA,26 resulting in a high mean Mw of the RHA 4 and RHA products in general. Finally, a low MoD was sufficient for RHA products to obtain gels with a high cohesivity. Altogether, these results illustrate that manufacturing technologies used to produce HA fillers strongly influence their contents in long (high Mw) versus short (low Mw) HA chains. Because excess of low Mw HA has been described as potentially proinflammatory and triggers the immune system,27 further studies may illustrate whether the high Mw of sHA released from RHA gels also has a positive impact on their safety profiles.26
Mechanical Tests
Biomaterial cohesivity defined as the energy required to break a material apart may be challenging to measure, standardize, and interpret physiologically.25,28–31 In this study, the library of gels was submitted to 2 quantitative evaluations of their macroscopic mechanical resistance using a recent shear cohesivity test in an aqueous buffer and a compression test.25,29,30 The shear cohesivity test describes the behavior of a gel implant under moderate shear in a hydrophilic medium. Physiologically, this test may illustrate the ability of a gel to resist fragmentation after multiple facial shear (e.g., on smiling or blinking) as one gel implant. In addition to this shear cohesivity test in an aqueous buffer, a compression test was performed to highlight the ability of a gel to sustain the tissues' pressure or external compression and therefore to create volume. In contrast to the common belief that G′ is a readout of the gel firmness, the compression test was developed to probe the actual firmness, cohesivity, and, as a consequence, the projection capacity of the investigated fillers.10 For instance, RES and RESLYFT, which presented the highest G′ values, also presented a mechanical resistance to compression close to RHA 1 and the lowest resistance to shear cohesivity. Filler cohesivity relies on the cumulative effect of weak, noncovalent, and reversible interactions between crosslinked HA chains10 that dissipate the energy generated by tissue shear or compression. These results strongly suggest that the high Mw long HA chains and specific crosslinked structure of RHA products contribute to maximizing these interactions thus increasing RHA gel cohesivity in both these mechanical tests.
Rheology Assessments
Recently published studies highlighted the tissue integration ability of a filler as the concomitant effects of a high cohesivity and a low viscosity.21,26,31 As a result, fillers with both high cohesivity and low viscosity are expected to spread easily without disaggregating when deposited in situ.21 Among fillers assessed in this study, RHA displayed the highest shear cohesivity (see Supplemental Digital Content 1, Figure SI 1C, http://links.lww.com/DSS/A642) and among the lowest viscosity (see Supplemental Digital Content 1, Figure SI 3B, http://links.lww.com/DSS/A642) for each class of product indications, thus supporting their enhanced tissue integration in situ previously reported in a published case series.32 Other, classical viscoelastic properties were also assessed. Within each product family, G′ scores increased from the more superficial to the deeper-indication product and were the highest for volumizers. Regarding the discrepancy between G′ and cohesivity and mechanical test results, increasing G′ values may simply reflect the increased crosslinking rate within each product range and/or higher HA concentration. This discrepancy likely arises from how G′ is measured: at low stresses or deformations that are nearly static conditions.20 Such conditions are not representative of the harsher conditions to which implants are submitted once implanted in situ, which may explain why G′ is poorly predictive of filler mechanical resistance and their macroscopic behaviors. Overall, the results confirm that G′ values are only partially informative20 and should be accompanied by the LVER that characterizes how far, in terms of stress or deformation, the G′ value is maintained. The Strength scores was specifically designed to reflect the ability of a gel to maintain its mechanical properties and viscoelasticity, or its G′, over a wide range of stresses (or deformations), namely the LVER. The parameter arose from a simple observation that the resistance to compression of the investigated fillers followed the width of their LVER (see Supplemental Digital Content 1, Figure SI 2C and Figure SI 3C, http://links.lww.com/DSS/A642). As a general trend, Strength scores increased with intended injection depth, from superficial fine lines to volumizing (Figure 2A). This is clearly in line with the need to maintain strong elastic behavior over a wide range of stresses for volumizing effects. To the best of our knowledge, Strength scores better reflect the mechanical resistance of a gel, and thus may be more predictive of implant behavior in situ. For example, RHA 4 that presented a G′ value similar to VYC-20L and lower than RESLYFT, but had the largest LVER, and thus the highest Strength score, also presented the highest resistance to compression and cohesivity. Moreover, it can be readily assessed by the means of a rheometer. It is noteworthy that the Strength score is more adapted for the comparison of fillers intended for similar product indications.
The second parameter, the Stretch score, assesses the gel deformation ability when submitted to a constant stress. To illustrate the Stretch score meaning, a physiological parallel can be made with dynamic facial expressions such as smiling: a temporary mechanical stress caused by facial muscles is applied to the gel as long as the subject smiles. The gel will more or less rapidly and easily deform in response to this facial stress. The creep profiles of the studied gels typically followed Burgers type behavior.33,34 A small instantaneous elastic response was first observed, later followed by a steady-state viscous response.35 The Stretch score was obtained by measuring the slope of the steady-state viscous response of the creep curve, which is linear, for the ease of measurement. It is noteworthy that RHA 1 presented a Stretch score significantly higher than all other fillers. The Stretch score was only second to a noncrosslinked high Mw HA solution (Mw = 1.5 MDa) (data not shown), a result in agreement with shear cohesivity results. Thus, a better similarity between RHA manufactured according to the PNT and native HA chains can be raised. To illustrate this statement, RHA 4 presented a high Stretch score similar to dermal fillers indicated for more superficial or moderate to deep wrinkle filling such as VYC-12L and VYC-15L, suggesting that this high Strength score volumizer is at the same time amenable to accompanying deformations and stresses. Future studies are required to confirm the prediction that RHA 4 could be potentially adapted to volumizing dynamic superficial fat compartments of the face.36
Conclusion
According to their compositions (crosslinking content, HA Mw, concentration, etc.) and the crosslinking technology (temperature, pH, etc.), every HA-based soft tissue filler bears unique properties aimed to match its product designation. An important focus was made on the characterization of low Mw HA fragments that are released from the gels and are potentially proinflammatory. Moreover, cohesivity tests highlighted the gap between gel macroscopic behaviors and classical rheological parameters. This study demonstrates that filler selection based solely on common viscoelastic quantities such as G′ hinders the ability to predict implant behavior in situ because these quantities are measured in nearly static conditions. The concepts of Strength and Stretch were thus specifically developed to overcome this shortcoming and provide injectors with more informative rheological parameters that characterize a filler global performance for specific indications. Indeed, once injected in situ, dermal fillers are exposed to a broad range of stresses and deformations exceeding the conditions of measurement of traditional rheological parameters such as G’. Consequently, the gels must retain their performance when subjected to challenges such as the facial dynamism to provide long-term and repeatable natural-looking aesthetic results.
RHA products were produced using the Preserved Network technology that improved the preservation of long (high Mw) HA chains, thus requiring low amounts of crosslinker to ensure filler stabilization and implant durability.17 This technology gave rise to optimized mechanical gel properties well adapted to the respective product uses, as highlighted by their high mechanical resistance and well-balanced Strength and Stretch scores directly in line with each of the filler's indication.
Acknowledgments
The authors wish to express thanks to Dr. Kevin Legent and Jimmy Mergy (Teoxane) for the fruitful discussions.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the full text and PDF versions of this article on the journal's Web site (www.dermatologicsurgery.org).
All authors are employed by Teoxane SA.
Contributor Information
Mélanie Gallet, Email: m.gallet@teoxane.com.
Elodie Tremblais, Email: e.tremblais@teoxane.com.
Patrick Trévidic, Email: patrick.trevidic@orange.fr.
References
- 1.Sánchez-Carpintero I, Candelas D, Ruiz-Rodríguez R. Dermal fillers: types, indications, and complications. Actas Dermo-Sifiliogr (English Edition) 2010;101:381–93. [DOI] [PubMed] [Google Scholar]
- 2.Sadick N, Sorhaindo L. The utility of soft tissue fillers in clinical dermatology: treatment of fine wrinkles and skin defects. Expert Rev Med Devices 2007;4:559–65. [DOI] [PubMed] [Google Scholar]
- 3.Dai X, Li L, Peterson W, Baumgartner RR, et al. Safety and effectiveness of hyaluronic acid dermal filler in correction of moderate-to-severe nasolabial folds in Chinese subjects. Clin Cosmet Invest Dermatol 2019;12:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John HE, Price RD. Perspectives in the selection of hyaluronic acid fillers for facial wrinkles and aging skin. Patient Preference Adherence 2009;3:225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg 2009;25:86–94. [DOI] [PubMed] [Google Scholar]
- 6.Khunmanee S, Jeong Y, Park H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J Tissue Eng 2017;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol 2004;83:317–25. [DOI] [PubMed] [Google Scholar]
- 8.Santoro S, Russo L, Argenzio V, Borzacchiello A. Rheological properties of cross-linked hyaluronic acid dermal fillers. J Appl Biomater Biomech 2011;9:127–36. [DOI] [PubMed] [Google Scholar]
- 9.Edsman K, Nord LI, Ohrlund A, Larkner H, et al. Gel properties of hyaluronic acid dermal fillers. Dermatol Surg 2012;38:1170–9. [DOI] [PubMed] [Google Scholar]
- 10.Pierre S, Liew S, Bernardin A. Basics of dermal filler rheology. Dermatol Surg 2015;41(Suppl 1):S120–6. [DOI] [PubMed] [Google Scholar]
- 11.Stern R, Kogan G, Jedrzejas MJ, Soltes L. The many ways to cleave hyaluronan. Biotechnol Adv 2007;25:537–57. [DOI] [PubMed] [Google Scholar]
- 12.Baek J, Fan Y, Jeong S-H, Lee H-Y, et al. Facile strategy involving low-temperature chemical cross-linking to enhance the physical and biological properties of hyaluronic acid hydrogel. Carbohydr Polym 2018;202:545–53. [DOI] [PubMed] [Google Scholar]
- 13.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol 2006;85:699–715. [DOI] [PubMed] [Google Scholar]
- 14.Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol 2015;2015:563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol 2014;5:101-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman-Janette J, Taylor SC, Cox SE, Weinkle SH, et al. Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: a 64-week, prospective, multicenter, controlled, randomized, double-blind and within-subject study. J Cosmet Dermatol 2019;18:1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rzany B, Converset-Viethel S, Hartmann M, Larrouy JC, et al. Efficacy and safety of 3 new resilient hyaluronic acid fillers, crosslinked with decreased BDDE, for the treatment of dynamic wrinkles: results of an 18-month, randomized controlled trial versus already available comparators. Dermatol Surg 2019;45:1304–14. [DOI] [PubMed] [Google Scholar]
- 18.Fagien S, Bertucci V, von Grote E, Mashburn JH. Rheologic and physicochemical properties used to differentiate injectable hyaluronic acid filler products. Plast Reconstr Surg 2019;143:707e–20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavard Molliard S, Albert S, Mondon K. Key importance of compression properties in the biophysical characteristics of hyaluronic acid soft-tissue fillers. J Mech Behav Biomed Mater 2016;61:290–8. [DOI] [PubMed] [Google Scholar]
- 20.Öhrlund Å. Evaluation of rheometry amplitude sweep cross-over point as an index of flexibility for HA fillers. J Cosmet Dermatol Sci Appl 2018;8:47–54. [Google Scholar]
- 21.Gavard Molliard S, Bon Bétemps J, Hadjab B, Topchian D, et al. Key rheological properties of hyaluronic acid fillers: from tissue integration to product degradation. Plast Aesthet Res 2018;5. [Google Scholar]
- 22.Bourdon F, Meunier S. Process for evaluating the mechanical performance of a filler gel. In: WO2016150974A1; 2015. [Google Scholar]
- 23.Goodman GJ, Swift A, Remington BK. Current concepts in the use of voluma, volift, and volbella. Plast Reconstr Surg 2015;136:139s–48s. [DOI] [PubMed] [Google Scholar]
- 24.Wende FJ, Gohil S, Nord LI, Helander Kenne A, et al. 1D NMR methods for determination of degree of cross-linking and BDDE substitution positions in HA hydrogels. Carbohydr Polym 2017;157:1525–30. [DOI] [PubMed] [Google Scholar]
- 25.Sundaram H, Rohrich RJ, Liew S, Sattler G, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six U.S. Food and drug administration-approved fillers. Plast Reconstr Surg 2015;136:678–86. [DOI] [PubMed] [Google Scholar]
- 26.La Gatta A, Salzillo R, Catalano C, D'Agostino A, et al. Hyaluronan-based hydrogels as dermal fillers: the biophysical properties that translate into a “volumetric” effect. PLoS One 2019;14:e0218287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baeva LF, Lyle DB, Rios M, Langone JJ, et al. Different molecular weight hyaluronic acid effects on human macrophage interleukin 1β production. J Biomed Mater Research A 2014;102:305–14. [DOI] [PubMed] [Google Scholar]
- 28.Rosenholm JB, Peiponen KE, Gornov E. Materials cohesion and interaction forces. Adv Colloid Interf Sci 2008;141:48–65. [DOI] [PubMed] [Google Scholar]
- 29.Borrell M, Leslie DB, Tezel A. Lift capabilities of hyaluronic acid fillers. J Cosmet Laser Ther 2011;13:21–7. [DOI] [PubMed] [Google Scholar]
- 30.Edsman K, Kenne AH. Re: lift capabilities of hyaluronic acid fillers by marcos borrell, dustin B. Leslie & ahmet tezel (J cosmet laser ther. 2011;13:21-27). J Cosmet Laser Ther 2011;13:123–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edsman KLM, Ohrlund A. Cohesion of hyaluronic acid fillers: correlation between cohesion and other physicochemical properties. Dermatol Surg 2018;44:557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Micheels P, Sarazin D, Besse S, Eliasd B. Comparison of two Swiss-designed hyaluronic acid gels: six-month clinical follow-up. J Drugs Dermatol 2017;16:154–61. [PubMed] [Google Scholar]
- 33.Dhar P, Katiyar A, Maganti LS. Smart viscoelastic and self-healing characteristics of graphene nano-gels. J Appl Phys 2016;120:214304. [Google Scholar]
- 34.Li J, Liu H, Wang C, Huang G. A facile method to fabricate hybrid hydrogels with mechanical toughness using a novel multifunctional cross-linker. RSC Adv 2017;7:35311–9. [Google Scholar]
- 35.Ferry JD. Viscoelastic Properties of Polymers: John Wiley & Sons; 1980. [Google Scholar]
- 36.Schenck TL, Koban KC, Schlattau A, Frank K, et al. The functional anatomy of the superficial fat compartments of the face: a detailed imaging study. Plast Reconstr Surg 2018;141:1351–9. [DOI] [PubMed] [Google Scholar]