Supplemental Digital Content is available in the text.
Keywords: brain, endothelium, fibrin, incidence, myocardial infarction
Abstract
The Coronavirus disease 2019 (COVID)-19 pandemic has already affected millions worldwide, with a current mortality rate of 2.2%. While it is well-established that severe acute respiratory syndrome-coronavirus-2 causes upper and lower respiratory tract infections, a number of neurological sequelae have now been reported in a large proportion of cases. Additionally, the disease causes arterial and venous thromboses including pulmonary embolism, myocardial infarction, and a significant number of cerebrovascular complications. The increasing incidence of large vessel ischemic strokes as well as intracranial hemorrhages, frequently in younger individuals, and associated with increased morbidity and mortality, has raised questions as to why the brain is a major target of the disease. COVID-19 is characterized by hypercoagulability with alterations in hemostatic markers including high D-dimer levels, which are a prognosticator of poor outcome. Together with findings of fibrin-rich microthrombi, widespread extracellular fibrin deposition in affected various organs and hypercytokinemia, this suggests that COVID-19 is more than a pulmonary viral infection. Evidently, COVID-19 is a thrombo-inflammatory disease. Endothelial cells that constitute the lining of blood vessels are the primary targets of a thrombo-inflammatory response, and severe acute respiratory syndrome coronavirus 2 also directly infects endothelial cells through the ACE2 (angiotensin-converting enzyme 2) receptor. Being highly heterogeneous in their structure and function, differences in the endothelial cells may govern the susceptibility of organs to COVID-19. Here, we have explored how the unique characteristics of the cerebral endothelium may be the underlying reason for the increased rates of cerebrovascular pathology associated with COVID-19.
Coronavirus disease 2019 (COVID-19) is a zoonotic outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) that has affected 126 million+ people and caused ≈2.7 million deaths worldwide, approximately 20% of these deaths being from the United States alone.1 As an acute respiratory disease, it was initially characterized by fever, dry cough, headache, shortness of breath (dyspnea) leading to progressive respiratory failure and pneumonia.2 However, there is an established link between COVID-19 and neurological symptoms in up to 50% of patients, including loss of smell and taste, necrotizing encephalitis, seizures, and rarely, Guillain-Barre syndrome.3 With a median time to onset of neurological symptoms of only 2 days, there is also an increased risk of mortality and lower rates of discharge home among these patients.4 Acute cerebrovascular disease with a relatively low mean age (ranging from 45 to 67 years) is a significant complication of COVID-19,3 which is associated with an 8-fold greater risk of stroke compared with influenza.5 The incidence of acute cerebrovascular complications was 5.7% in patients with severe COVID-196; in fact, cerebrovascular disease is thought to be an independent predictor of mortality after SARS-CoV2 infection.7
That the overall incidence of large vessel occlusions is 2-fold higher than in normal acute ischemic stroke (AIS) cases and they occur among patients from all age groups, even those without risk factors or comorbidities, strongly implicates COVID-19-related hypercoagulability as the underlying cause.5 Severe COVID-19 is associated with a cytokine storm, endothelial activation, and coagulopathy leading to multiorgan dysfunction.8 Despite these advances in the understanding of how SARS-CoV2 infection causes morbidity and mortality, the underlying mechanisms and phenotype of COVID-19 associated ischemic and hemorrhagic stroke are as yet unclear.
The endothelium has a pivotal role in cerebrovascular disease. Endothelial dysfunction occurs after stroke and leads to oxidative stress, inflammation, increased vascular tone, blood-brain barrier (BBB) damage, and further thrombovascular complications in the brain. Endothelial damage as a result of denudation, fluctuations in shear stresses, or inflammation can also trigger onset of stroke.9 Here, we will evaluate whether COVID-19-related endothelial dysfunction is more severe in the brain, and whether this may explain the propensity for stroke after SARS-CoV2 infection.
Cause of Stroke in the COVID-19 Pandemic
COVID-19 is strongly associated with ischemic stroke, intracerebral hemorrhage, and cerebral venous sinus thrombosis, all of which are linked to poor outcome.10 The number of people who have experienced neurological complication worldwide is estimated to be between 10 000 and 50 000.3 Metadata reveals that the worldwide incidence of stroke after SARS-CoV2 infection is 1.4%. Ischemic stroke with multiple cerebral infarctions and of cryptogenic origin is the most frequent type of stroke11 and mortality among patients with COVID-19 with AIS is as high as 50%.12
Table I in the Data Supplement summarizes the worldwide incidence of stroke in patients with COVID-19, as presented in the current literature. In the United Kingdom, data from 125 patients with COVID-19 showed that 46% sustained ischemic strokes and 7% sustained intracerebral hemorrhages (ICHs).13 In the Netherlands, 5/184 patients studied had an AIS.14 Yaghi et al15 and Oxley et al16 have both reported stroke patients with COVID-19 in New York were younger than historical non-COVID-19 stroke patients and also presented with more severe strokes likely because of a majority of them being proximal large vessel occlusions in the brain. This has been confirmed by another recent report of fatal ischemic strokes in patients aged under 55.17 The incidence of large vessel occlusions in the brain is increased by 46.9% among COVID-19 cases.5 Alarmingly, a Turkish study revealed that the mean time from onset of COVID-19 symptoms to stroke diagnosis was only 2 days.18 A majority of these strokes are reported to be cryptogenic, while some are cardioembolic in origin.19 There are also reports of small infarctions with dense microthrombi in postmortem samples.20 Patients with COVID-19 with receive routine therapies including thrombolysis, endovascular thrombectomy, and antiplatelet therapy; however, the incidence of hemorrhagic transformation is as high as 10.3% in a subset of patients.21
In another study from New York, 33/755 (4.4%) of patients were diagnosed with ICH; 26 of these patients had occlusive infarctions that were converted into ICH. It affirms that patients with COVID-19 at increased risk of thrombosis are also at risk of hemorrhage. Among these cases, all 5 patients who received therapeutic anticoagulation sustained fatal large parenchymal ICH involving mass effect and herniation. The neurovascular effects of COVID-19 are complex and additional preclinical studies and randomized clinical trials are needed to find the risk-benefit ratio of therapeutic anticoagulants such as unfractionated heparin. Further investigations are also needed about the use of thrombolytic therapy for patients with COVID-19 with large acute cerebral infarcts, to understand the risk of hemorrhagic transformation.22
ACE2: the Receptor for SARS-CoV2
SARS-CoV and SARS-CoV2 share 79.6% sequence homology and both have the same entry cell receptor, namely ACE2 (angiotensin-converting enzyme 2). It is a metallopeptidase that is binds the S1 spike glycoprotein of SARS-CoV2,2 and the binding affinity of SARS-CoV2 for human ACE2 is 10-20-fold greater than that of SARS-CoV.23 There are also sequence differences in the S1 glycoprotein, as known potent antibodies against the ACE2 binding domain for SARS-CoV do not bind to the ACE2 binding domain of SARS-CoV-2.24 Further, unlike other human coronaviruses, SARS-CoV2 has a furin-like cleavage site on the S-protein that aids in endosomal membrane fusion and virus egress. This feature is predicted to provide a gain-of-function to SARS-CoV2, increasing its efficiency of human to human transmission.25 Collectively, these findings may explain why the clinicopathological manifestations of SARS-CoV2 infection are worse than that of SARS-CoV.
Organotropism of SARS-CoV2 Is Based on ACE2 Expression
Recent literature shows that SARS-CoV-2 RNA is detectable not only in the respiratory tract, but also the kidneys, liver, heart, and brain, and organotropism may determine the disease course after infection.26 SARS-CoV2 viral RNA has been detected solely in the cerebrospinal fluid of a small number of patients diagnosed with COVID-19-associated encephalitis.25 A recent German study documented the presence of viral RNA or proteins in 53% of postmortem brain samples from patients with COVID-19.27 The virus has also been detected using immunohistochemistry in brain specimens from patients with COVID-19 in another study.28
In humans, ACE2 expression is present but not limited to oral and nasal mucosa, nasopharynx, liver, kidney, and brain. At the cellular level, ACE2 showed high surface expression on lung alveolar epithelial cells and macrophages, upper and stratified epithelial cells of the oesophagus, as well as small intestine enterocytes. ACE2 is highly expressed in the non-neuronal goblet/secretory cells and ciliated receptor cells of the nasal epithelium.29 There is now clear evidence that SARS-CoV-2 gains access to the central nervous system via the neuronal-mucosal interface.30 The virus likely travels through the cribriform plate to the olfactory bulb, after which it can move via the synapses to the cortex, and then to the brain stem. ACE2 receptors are also expressed by glial cells and neurons, which makes them another potential target of SARS-CoV2. These findings may explain the peripheral neuronal symptoms of COVID-19 such as loss of smell and taste,31 but they do not explain the cerebrovascular complications caused by the disease.
Immunohistochemical studies of postmortem brain samples of patients with COVID-19 showed that ACE2 was highly expressed in lung and brain parenchymal capillaries, both organs which were highly thrombosed, but not in the kidney which was largely unaffected in this cohort.20 Although it was believed that ACE2 is universally expressed in arterial and venous endothelial cells and arterial smooth muscle cells,29 recent reports suggest that it may be the pericytes encapsulating capillaries and other small vessels that express ACE2.32 Crosstalk between pericytes and endothelial cells is vital for maintaining microvascular integrity and for local homeostasis.33 Therefore, although it is not definitive as to whether the virus enters the brain via cerebral endothelial cells expressing ACE2, inflammation and release of damage signals triggering endothelial damage followed by BBB damage and pericyte mediated entry of the virus can still be an explanation for the associated cerebrovascular pathology.
ACE2 As a Target of SARS-CoV2 Infection
ACE2 is a transmembrane type I glycoprotein and a key effector in the renin angiotensin system. ACE2 is a carboxypeptidase that cleaves the leucyl residue from decapeptide Ang (angiotensin) I and octapeptide Ang II to produce Ang1-9 and Ang1-7, respectively. Ang1-7 subsequently acts on the Mas receptor to regulate important physiological functions in several tissues such as the heart, kidney, and brain. The ACE2-Ang1-7-Mas axis is neuroprotective while the ACE/AngII/AngII type 1 receptor activation causes inflammation and endothelial dysfunction in cerebral arteries and the microcirculation. In fact, the brain has its own unique self-contained renin angiotensin system (review by Regenhardt et al34). The AngII-AngII type 1 receptor axis is associated with several prothrombotic functions as well as proinflammatory cytokine secretion. These are discussed in detail in later sections and illustrated in the Figure.
Figure.
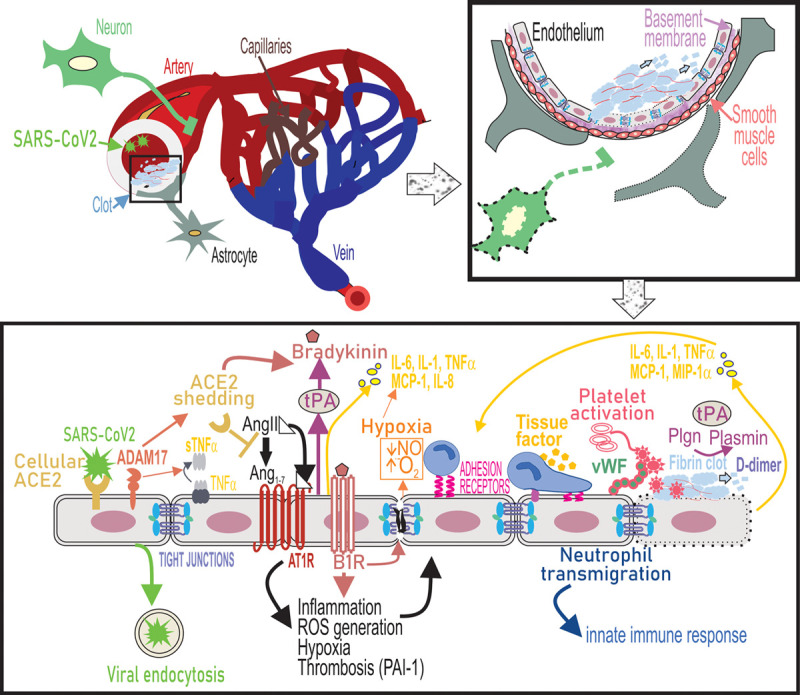
Endothelial dysfunction underlies cerebrovascular complications of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection. SARS-CoV2 binds to ACE2 receptors on the endothelial cells of large arteries and is endocytosed into the brain. Simultaneously, upregulation of ADAM17 (ADAM metallopeptidase domain 17) leads to shedding of ACE2 (angiotensin-converting enzyme 2) from the cell surface and as a result cell-bound ACE2-mediated conversion of Ang II (angiotensin II) to Ang1-7 is lost. ADAM-17 also functions as a TNF-α (tumor necrosis factor-α), convertase to release soluble TNF-α. Ang II binds to Ang II type 1 receptor (AT1R) and activation of AT1R triggers a range of intracellular cytokine signaling processes that lead to increased ROS generation and suppressed nitric oxide (NO) production from the endothelium, which causes vasoconstriction and potentiates oxidative stress causing endothelial activation. ACE2 depletion increases levels of bioactive bradykinin metabolite and binding of bradykinin to B2 receptors on endothelial cells promotes endothelial tight junction disruption. Ang II-AT1R signaling promotes gene expression of inflammatory cytokines including IL (interleukin)-6, TNF-α, MCP (monocyte chemoattractant protein)-1, and IL-8 via NF-κB signaling. Loss of ACE2 and therefore accumulation of AngII also induces tissue factor (TF) and PAI-1 (plasminogen activator inhibitor-1) expression by endothelial cells causes a shift in the PAI-1/tPA (tissue-type plasminogen activator) balance to a prothrombotic state. Endothelial activation also leads to upregulation of leukocyte adhesion molecules including VCAM-1 (vascular cell adhesion molecule), which promotes neutrophil transmigration. Hypoxia, cytokine activation, and oxidative stress promote platelet activation and release of VWF (von Willebrand Factor), which culminates in the formation of a fibrin clot on the endothelial cell surface. Fibrin in turn provides a cofactor for tPA-driven plasminogen activation that results in plasmin formation. Plasmin also cleaves the bradykinin precursor to yield bradykinin. These events trigger a thromboinflammatory loop that further causes endothelial dysfunction, leading to cerebrovascular pathologies. MIP-1α indicates macrophage inflammatory protein.
ACE2 can be cleaved by 2 proteases, ADAM17 (ADAM metallopeptidase domain 17) and TMPRSS2 (transmembrane serine protease 2). When ACE2 is cleaved by TMPRSS2, it leads to downregulation of ACE2 expression and facilitates viral endocytosis.35 Therefore, in the context of COVID-19, ACE2 has a dichotomous role-first as a receptor for SARS-CoV-2 entry; and then following infection, ACE2 downregulation leads to increased AngII.36 Indeed, AngII levels are elevated in plasma and positively correlated with viral load in patients with COVID-19.37 In another study, plasma levels of AngII were elevated in 90.2% of all patients positive for COVID-19 all of whom were critically ill.38 In rodents, deficiency of ACE2 and therefore reduced Ang1-7 production leads to disruption of endothelial function in cerebral arteries and augments oxidative stress39 (Figure). ACE2 downregulation also adversely affects cerebral autoregulation. MR perfusion-weighted brain imaging in ICU-COVID-19 patients with unexplained encephalopathy has shown bilateral frontotemporal hypoperfusion and 2 of these patients sustained single acute ischemic strokes.40
The Vascular Endothelium—a Gatekeeper for SARS-CoV2 Infection
The vascular endothelium is a layer of cells lining blood vessels. Endothelial cells are metabolically active and have diverse yet integral roles in regulating hemostatic balance, control of vasomotor tone, inflammation, vascular permeability, coagulation, and the immune response. Specifically, the endothelium maintains vascular tone by regulating production of vasodilators such as nitric oxide and prostacyclin that also have antithrombotic properties, and vasoconstrictors, for example, ET-1 (endothelin-1), Ang II, and reactive oxidative species. Endothelial cells regulate thrombosis by controlling the release of prothrombotic molecules including VWF (von Willebrand Factor) that promotes platelet aggregation, and plasminogen activator inhibitor-1 that inhibits fibrinolysis.41 Emerging literature continues to suggest a key role for dysregulated endothelial function in SARS-CoV-2 infection.42
Endothelial cells are highly heterogeneous, and their function varies between organs as well as between different vascular beds in the same organ. The cerebral endothelium is particularly unique since it is a critical component of the BBB wherein these cells tightly regulate the movement of ions, molecules, and cells. The human brain has a total length of ≈400 miles of vasculature which includes arteries, arterioles, capillaries, venules, and veins,43 this extensive vascular network further explains how SARS-CoV2-mediated endothelial damage might be concentrated in the brain. Cerebral endothelial cells are unique in that they are held together by tight junctions that limit the paracellular movement of solutes between the blood and the brain44 with pericytes located opposite these junctions. A range of enzymes, including alkaline phosphatase, γ-glutamyl transpeptidase, and Na,K-ATPase are present in higher concentration in cerebral endothelial cells when compared with endothelial cells from other tissues. Further, thrombomodulin is highly expressed in endothelial cells lining most blood vessels in all organs, but in the brain, it is preferentially expressed in the endothelium of arteries and veins, and virtually absent in brain capillaries. Endothelial protein C receptor, that is located adjacent to thrombomodulin and amplifies protein C activation 10-fold also has a similar expression profile.45
Although a sophisticated system of adequate collateral circulation protects the brain from isolated arterial occlusion, endothelial damage changes the vascular wall and hence causes local flow disturbances, combined with platelet activation and augmented procoagulant activity. These in turn further aggravate the vascular lesion. Obstruction of large cerebral arteries such as that seen during SARS-CoV2 infection thus triggers downstream reductions in microvascular flow, further platelet-fibrin deposition causing microvascular obstruction and increased BBB permeability. Exposure of plasma to astrocyte secreted tissue factor initiates (extrinsic) coagulation system activation and thrombosis. Platelet-fibrin thrombi further occlude capillaries within the microvascular bed while the microvascular endothelium upregulates adhesion molecules such as VCAM-1 (vascular cell adhesion molecule) to enhance leukocyte transmigration across the BBB. Therefore, during ischemia, altered blood flow in large brain vessels in turn affects the microvasculature, thereby propagating thrombosis of the ischemic cerebral vascular beds.46 In support of this, a recent study has found that systemic endothelium-dependent microvascular vasodilator responses and perfusion are reduced early after onset of severe COVID-19.47
Evidence for Endothelial Infection of SARS-CoV2
SARS-CoV-2 can directly invade endothelial cells and induce vascular endothelialitis leading to vascular thrombosis.48 A detailed histopathologic analysis in a small set of lung specimens from deceased patients with COVID-19 and those of deceased influenza patients showed 9× more alveolar capillary microthrombi per square centimeter of vascular lumen area in the former group. These specimens had severe endothelial injury evidenced by disrupted intercellular junctions, cell swelling, and detachment from the basal membrane. In addition to perivascular inflammation, that SARS-CoV2 was also visible inside these cells indicates that the binding of the virus itself may exacerbate endothelial injury in COVID-19.49 These findings were corroborated by Varga and co-workers who showed SARS-CoV2 inclusion structures in endothelial cells, inflammatory cells associated with the endothelium as well as apoptotic endothelial cells in the heart, small bowel, and lung. Pulmonary endothelial cells were swollen, had disrupted cell membranes and intercellular junctions, and were detached from the basal membrane. Endothelialitis, vascular thrombosis with microangiopathy, and occlusion of alveolar capillaries in these specimens also correlated with increased frequency of sprouting and intussusceptive angiogenesis.48 In a new study, endothelial expression of IL-6, TNF-α (tumor necrosis factor-α), intercellular cell adhesion molecule (ICAM)-1, and caspase-1 was significantly elevated in lung tissue from patients with COVID-19, relative to those from H1N1 patients and control groups.50
Paniz-Mondolfi and colleagues used transmission electron microscopy to show the presence of isolated 80 to 110 nm viral particles in small vesicles of endothelial cells. Blebbing of viral particles on the endothelial wall indicated transcellular penetration of the virus across the brain microvascular endothelial cells. Viral particles concentrated in cytoplasmic vacuoles were seen near the basement membrane within neurons of the frontal lobe indicating penetration of the viral particles across the BBB and into neural bodies.51 Therefore, while retrograde axonal transport may still be an alternative means of central nervous system entry causing peripheral neurological manifestations, SARS-CoV2 entry to the brain via the hematogenous route may explain central neurological involvement.52 Microscopic data showing detachment of viable endothelial cells from the basement membrane and toward the lumen of the vessel is unique to ischemic strokes caused by SARS-CoV2 infection and further confirm that endothelial disruption is the primary mechanism of neurological damage caused by this virus.19 Meinhardt and colleagues recently reported an 18% incidence of microthrombi and localized brain infarcts within which there were SARS-CoV S protein immunoreactive endothelial cells.30 Although a Belgian study found no direct evidence of endothelialitis in COVID-19 brain tissue, cerebral hemorrhage, focal ischemic necrosis, as well as edema and vascular congestion were observed.53
Hernández-Fernández et al reported interesting postmortem findings in 2 patients with COVID-19 who succumbed to ICH. These brain specimens showed loss of endothelial cells and consequent vessel wall damage in small arterioles, capillaries, and venules with signs of increased neurovascular permeability and localized inflammation. Endothelial activation was not associated with sporadic cerebral small vessel disease,19 suggesting direct effects of viral infection on the cerebral endothelium as a possible mechanism of ICH in these patients. This has been further confirmed by findings of perivascular and intraparenchymal microbleeds together with intravascular thrombosis and transendothelial lymphocyte and monocyte infiltration in brain specimens of patients with COVID-19.54
Thrombo-Inflammatory manifestations of COVID-19
COVID-19 promotes a hypercoagulable state. Elevated levels of D-dimer, a breakdown product of cross-linked fibrin, are reflective of thrombin formation and intravascular fibrin precipitating in coagulopathy. D-dimer concentrations were elevated and median prothrombin time was prolonged in patients who died of COVID-19, when compared with those that recovered55 and platelet counts are reduced in all patients with COVID-19.56 In patients who have 5× higher D-dimer levels than normal (ie, >2500 ng/mL), there is a 7× increase in thrombosis risk and the risk of progression to critical illness and need for mechanical ventilation is doubled. Remarkably, elevated D-dimer levels are associated with a 15-fold higher chance of succumbing to the infection.57 Seventy-six percent of patients with COVID-19 hospitalized in New York City had elevated D-dimer levels, and 45% of these patients developed severe illness.58 Abnormally high D-dimer levels correlate with inflammatory biomarkers, including CRP (C-reactive protein) and erythrocyte sedimentation rate,56 confirming that COVID-19 is in part, a thrombo-inflammatory disease.
Release of VWF from Weibel-Palade bodies indicates substantial endothelial activation, as once secreted, it can tether platelets and leucocytes to the vessel wall. Published data show 5-fold elevation of plasma VWF antigen and activity42,59 and a positive correlation between mortality and VWF levels.59 Soluble P-selectin and elevated plasminogen activator inhibitor-1 levels in these patients further indicate endothelial activation and impaired fibrinolysis.59 Changes in these hemostatic parameters are evidence that endothelial dysfunction and platelet activation are highly prevalent in COVID-19-associated coagulopathy and their correlation with disease severity leads us to think that these processes have important roles in the progression of disease.
The occurrence of circulating antiphospholipid antibodies (aPL-ab) in patients with COVID-19 linked with both venous thromboembolism and higher disease severity has raised the question about the role of aPL-ab in thrombogenesis during SARS-CoV2 infection. A British group reported the presence of aPL-ab in 5 of 6 patients with AIS studied and suggested that these antibodies may serve as biomarkers to predict the risk of AIS.60 aPL-ab such as anti-β2-glycoprotein (GP)-I interact with β2GP1 expressed on the endothelial cell membrane and create a proinflammatory and procoagulant endothelium. Complement activation by aPL-ab also promotes thrombosis by increasing tissue factor expression through the binding of C5a and membrane attack complex to specific receptors on endothelial cells.61
Unlike the pathological manifestations of viral-induced acute respiratory distress syndrome, in COVID-19 lung specimens, there is evidence of complement deposition within the lung microvasculature. Activation of the membrane attack complex causing microvascular endothelial cell injury may be the underlying cause for thrombosis and fibrin deposition in these specimens.62 Cugno et al63 have recently shown that plasma levels of complement proteins C5a and soluble C5b-9 levels were increased in a subset of patients with COVID-19; further, VWF levels significantly correlated with soluble C5b-9 levels, suggesting endothelial activation.
Hyperfibrinolysis
During COVID-19, changes in the balance between levels of activators and inhibitors of fibrinolysis can result in fibrinolysis shutdown but can also cause the opposite; that is, hyperfibrinolysis due to excessive plasmin-mediated fibrin cleavage.64 There is evidence from 44 patients with COVID-19 that the plasminogen pathway is disrupted, with impaired clot lysis seen in 57% of patients.65 Plasminogen levels are also independently increased in comorbid conditions of COVID-19 including hypertension and diabetes.66 Concentrations of both tPA (tissue-type plasminogen activator) and plasminogen activator inhibitor-1 have been shown to be increased in COVID-19 plasma samples in a new study.63
tPA-driven plasminogen activation results in plasmin formation. Plasmin has the capacity to cleave furin sites in the S protein of SARS-CoV-2, thereby enhancing its virulence. Plasmin also cleaves the bradykinin precursor (HMWK [high-molecular weight kininogen]) to yield bradykinin67 (Figure). Similarly, in vitro, binding of SARS-CoV-2 spike protein to human ACE2 enhances its proteolytic activity for bradykinin.68
Central nervous system expression of B1 and B2 receptors is widespread, including neurons, glia, and endothelial cells; both receptors are upregulated in the ischemic brain. When bradykinin binds to either receptor, it enhances intracellular calcium release and downregulated claudin-5, a tight junction protein, loss of which causes BBB damage.69 Bradykinin also promotes poststroke inflammation triggering the release of arachidonic acid and activating COX (cyclooxygenase) enzymes.69 Hyperfibrinolysis induces BBB leakage in a plasmin- and bradykinin-dependent manner, by activation of B2 receptors that are highly expressed in the cerebral endothelium (Figure). tPA infusion for thrombolysis in patients with stroke stimulates significant bradykinin generation67 and the higher risk for hemorrhagic transformation after tPA administration correlates with a concomitant increase in expression of bradykinin receptors in the ischemic brain.67
Hypoxia
COVID-19 and acute respiratory distress syndrome–related pneumonia causes silent hypoxemia, which is associated with an increased dependence on mechanical ventilation and a higher mortality rate.55 However, some patients with COVID-19 have signs of hypoxia before any signs of respiratory distress. Endothelial dysfunction, impaired oxygen transfer by hemoglobin, and reduced gas exchange in alveoli due to presence of microthrombi causing hypoxic vasoconstriction may be the underlying causes.70
In the context of the brain, hypoxic ischemic encephalopathy and encephalitis linked with neuropsychiatric symptoms has also been reported in 31% of patients presenting with COVID-19-related neurological complications.13 Postmortem brain tissue from patients with COVID-19 has also shown distinctive hypoxic ischemic damage and neuronal loss occurring in the cerebral cortex, hippocampus, and cerebellar Purkinje cell layer in the absence of any thrombi or encephalitis.28 Prolonged hypoxia sustained during SARS-CoV2 infection can cause death of oligodendrocytes and subsequent demyelination, as well as white matter microhemorrhages.71
Besides white matter damage, the effect of prolonged hypoxia on the cerebral endothelium is well characterized; it results in BBB disruption and capillary damage, which can produce microhemorrhages.72 The adult mammalian brain has a low glycolytic capacity, and because it has a disproportionately large bodily oxygen requirement, is more sensitive to hypoxia/ischemia than most organs.73 Hypoxia also suppresses endothelial thrombomodulin production.74 Therefore, we are of the opinion that the effect of hypoxia on the cerebral endothelium could also explain why large vessel ischemic strokes, particularly affecting the MCA75 are prevalent after SARS-CoV2 infection.
Hypercytokinemia
After SARS-CoV2 infection, uncontrolled proinflammatory cytokine production or a cytokine storm, is associated with increased vascular permeability, multiorgan failure, and eventually death. Excessive proinflammatory cytokine release can also promote vascular thrombosis by activating the coagulation cascade commencing with thrombin generation (Figure). This leads us to think that the thromboinflammatory loop might be the reason for the concomitant upregulation of excessive proinflammatory cytokine release and serum markers of hypercoagulable state in patients with severe COVID-19.76
Poor outcome associated with a cytokine storm is further evidenced by the report that IL (interleukin)-2 receptor, IL-6, IL-8, IL-10, and TNF-α were all significantly higher in patients who subsequently died of COVID-19 compared with those who survived.55 A separate study identified that critically ill patients with COVID-19 had higher plasma levels of IL-2, IL-7, IL-10, GCSF (granulocyte-colony stimulating factor), IP (interferon-γ inducible protein)-10, MCP (monocyte chemoattractant protein)-1, MIP (macrophage inflammatory protein)-1α, and TNFα.77
IL-6, IL-1, and TNF-α are associated with acute endothelial dysfunction. In patients with COVID-19, increased IL-6 levels correlate with disease severity and a procoagulant profile, suggesting that IL-6 may have an important role in endothelial dysfunction in this disease.78 A chemokine secreted by T-cells and macrophages, IL-6 also activates coagulation pathways, thereby disrupting procoagulant-anticoagulant homeostasis. IL-6 is frequently produced in response to viral infection, which can precipitate onset of stroke; IL-6 concentration independently predicts early neurological deterioration after stroke in humans. Cerebrospinal fluid and serum levels of IL-6 are rapidly increased after the ischemic event in patients with stroke and correlate with infarct volume.79 IL-6, TNFα, IL-1, and MCP-1 are also known to regulate postischemic inflammation.80 ACE2 receptor downregulation leads to increased Ang II levels; and AngII binds to its receptor AngII type 1 receptor and promotes gene expression of several inflammatory cytokines including IL-6, TNF-α, MCP-1 and IL-8 via NF-κB signaling (Figure). Hence, we think it is possible that ACE2 downregulation and consequent increase in AngII is responsible for the cytokine storm in SARS-CoV2 infections.
Elevated levels of C-reactive protein or CRP have been observed in >80% of severe COVID-19 cases and compared with recovered patients, CRP levels were 10-fold higher in deceased patients.81. Similarly, a sharp rise in CRP levels within 1 day of stroke onset also predicts a poor outcome and correlates with cerebrovascular and cardiovascular events.82 In experimental settings, CRP downregulates endothelial nitric oxide synthase activity causing endothelial cell dysfunction; it also facilitates Ang II/AT1 receptor–induced vascular smooth muscle cell migration and proliferation and increases reactive oxidative species production. In vitro, high concentrations of CRP elicit significant proinflammatory effects in umbilical vein and coronary artery endothelial cells, inducing high levels of expression of adhesion molecules ICAM-1, VCAM-1, and E-selectin.83 Consistently, a recent systematic review of the literature found that patients with COVID-19 with good neurological outcomes had lower mean CRP levels.84
Concluding Remarks
COVID-19 is now denoted as a multiorgan disease with cardiovascular, gastrointestinal, neurological, hematopoietic, and immunologic dysregulation. Although COVID-19 results in a lower mortality rate than other zoonotic coronavirus outbreaks such as SARS and Middle East Respiratory Syndrome, it is more contagious. Also, unlike SARS and Middle East Respiratory Syndrome in which neurological complications are rarely reported, COVID-19 has neurological manifestations in up to 36.7% of cases.6 Global epidemiological data suggest that the elderly and those with comorbidities are more susceptible to death from COVID-19, but alarmingly, in the United States, 20% of hospitalized patients and 12% of ICU patients were aged 20 to 44 years.85
In patients with COVID-19, uncontrolled cytokine release, endothelial disruption, and upregulation of procoagulant activity are likely further compounded by hypoxia resulting in a positive thrombo-inflammatory feedback loop culminating in both thrombosis and hemorrhage. Here, we have drawn on the current medical and scientific literature to postulate why the cerebral endothelium may be the underlying reason for the brain being a major target for SARS-CoV2 infection. First, the brain has an extensive vascular network because of substantially higher nutrient and oxygen demands. Second, there is higher ACE2 expression in the cerebral vasculature of patients with COVID-19 with endothelialitis.54 And lastly, the presence of viral-like particles in brain capillary endothelium and its visible budding across endothelial cells makes it highly plausible that the microvascular endothelium is the site of virus entry into the brain.51 Targeting endothelial dysfunction underlying neurovascular-thrombotic complications associated with COVID-19 may be a strategy to reduce mortality associated with this disease.
Affiliation
Sources of Funding
This work was supported by the National Health and Medical Research Council (NHMRC) Project grant APP1141046 awarded to Dr Nandurkar.
Disclosures
Dr Nandurkar received fees from Boehringer-Ingelheim and Pfizer-BMS for consultancy, unrelated to the work in this article. The other author reports no conflicts.
Supplemental Materials
Online Table I
Supplementary Material
Footnotes
This manuscript was sent to Ajay K. Wakhloo, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.032711.
For Sources of Funding and Disclosures, see page 1902.
References
- 1.World Health Organization. Who Coronavirus Disease (COVID-19) Dashboard. 2020. Accessed March 29, 2021. https://covid19.who.int/.
- 2.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frontera JA, Sabadia S, Lalchan R, Fang T, Flusty B, Millar-Vernetti P, Snyder T, Berger S, Yang D, Granger A, et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York city. Neurology. 2021;96:e575–e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridman S, Bullrich MB, Jimenez-Ruiz A, Costantini P, Shah P, Just C, Vela-Duarte D, Linfante I, Sharifi-Razavi A, Karimi N, et al. Stroke risk, phenotypes, and death in COVID-19: systematic review and newly reported cases. Neurology. 2020;95:e3373–e3385. [DOI] [PubMed] [Google Scholar]
- 6.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchi A, Mazzoni LN, Busoni S, Pinna N, Albanesi M, Cavigli E, Cozzi D, Poggesi A, Miele V, Fainardi E, et al. Assessment of cerebrovascular disease with computed tomography in COVID-19 patients: correlation of a novel specific visual score with increased mortality risk [published online November 28, 2020]. Radiol Med. 2020. doi: 10.1007/s11547-020-01313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colling ME, Kanthi Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc Med. 2020;25:471–478. doi: 10.1177/1358863X20932640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu X, De Silva TM, Chen J, Faraci FM. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res. 2017;120:449–471. doi: 10.1161/CIRCRESAHA.116.308427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verstrepen K, Baisier L, De Cauwer H. Neurological manifestations of COVID-19, SARS and MERS. Acta Neurol Belg. 2020;120:1051–1060. doi: 10.1007/s13760-020-01412-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2020;16:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bach I, Surathi P, Montealegre N, Abu-Hadid O, Rubenstein S, Redko S, Gupta S, Hillen M, Patel P, Khandelwal P, et al. Stroke in COVID-19: a single-centre initial experience in a hotspot of the pandemic. Stroke Vasc Neurol. 2020;5:331–336. doi: 10.1136/svn-2020-000525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, Sultan M, Easton A, Breen G, Zandi M, et al. ; CoroNerve Study Group. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kananeh MF, Thomas T, Sharma K, Herpich F, Urtecho J, Athar MK, Jabbour P, Shah SO. Arterial and venous strokes in the setting of COVID-19. J Clin Neurosci. 2020;79:60–66. doi: 10.1016/j.jocn.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tun CA, UnlUba SY, Alemdar M, AkyUz E. Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci. 2020;77:227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA, Collado-Jiménez R, Ayo-Martín Ó, Barrena C, Molina-Nuevo JD, García-García J, Lozano-Setién E, Alcahut-Rodriguez C, et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143:3089–3103. doi: 10.1093/brain/awaa239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, Hernandez T, Stock A, Zhao Z, Al Rasheed M, et al. Pathophysiology of SARS-COV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The mount sinai COVID-19 autopsy experience. medRxiv. Preprint posted online May 22, 2020. doi: 10.1101/2020.05.18.20099960 [Google Scholar]
- 21.Tan YK, Goh C, Leow AST, Tambyah PA, Ang A, Yap ES, Tu TM, Sharma VK, Yeo LLL, Chan BPL, et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. 2020;50:587–595. doi: 10.1007/s11239-020-02228-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dogra S, Jain R, Cao M, Bilaloglu S, Zagzag D, Hochman S, Lewis A, Melmed K, Hochman K, Horwitz L, et al. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29:104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, Lu L, Jiang S, Yang Z, Wu Y, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, Mushumba H, Fitzek A, Allweiss L, Dandri M, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF, Jr, Sabeti P. Neuropathological features of COVID-19. N Engl J Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5 [DOI] [PubMed] [Google Scholar]
- 31.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L, Mäe MA, Muhl L, Sun Y, Pietilä R, Nahar K, Liébanas EV, Fagerlund MJ, Oldner A, Liu J, et al. Pericyte-specific vascular expression of SARS-COV-2 receptor ACE2 – implications for microvascular inflammation and hypercoagulopathy in COVID-19 patients. bioRxiv. Preprint posted online July 26 2020. doi: 10.1101/2020.05.11.088500 [Google Scholar]
- 33.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16.e1–27.e1. doi: 10.1016/j.cell.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regenhardt RW, Bennion DM, Sumners C. Cerebroprotective action of angiotensin peptides in stroke. Clin Sci (Lond). 2014;126:195–205. doi: 10.1042/CS20130324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao L, Sakagami H, Miwa N. ACE2: the key molecule for understanding the pathophysiology of severe and critical conditions of COVID-19: demon or angel? Viruses. 2020;12:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miesbach W. Pathological role of angiotensin II in severe COVID-19. TH Open. 2020;4:e138–e144. doi: 10.1055/s-0040-1713678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z, Hu R, Zhang C, Ren W, Yu A, Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit Care. 2020;24:290. doi: 10.1186/s13054-020-03015-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peña Silva RA, Chu Y, Miller JD, Mitchell IJ, Penninger JM, Faraci FM, Heistad DD. Impact of ACE2 deficiency and oxidative stress on cerebrovascular function with aging. Stroke. 2012;43:3358–3363. doi: 10.1161/STROKEAHA.112.667063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17:121. doi: 10.1186/s12933-018-0763-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel SC, Wagner S, Cooper G, Rodnitzky RL. Conn PM, ed. The vasculature of the human brain. In: Neuroscience in Medicine. 2003. Humana Press; 129–163. [Google Scholar]
- 44.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a [DOI] [PubMed] [Google Scholar]
- 45.Laszik Z, Mitro A, Taylor FB, Jr, Ferrell G, Esmon CT. Human protein C receptor is present primarily on endothelium of large blood vessels: implications for the control of the protein C pathway. Circulation. 1997;96:3633–3640. doi: 10.1161/01.cir.96.10.3633 [DOI] [PubMed] [Google Scholar]
- 46.del Zoppo GJ. Virchow’s triad: the vascular basis of cerebral injury. Rev Neurol Dis. 2008;5(suppl 1):S12–S21. [PMC free article] [PubMed] [Google Scholar]
- 47.Sabioni L, De Lorenzo A, Lamas C, Muccillo F, Castro-Faria-Neto HC, Estato V, Tibirica E. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc Res. 2021;134:104119. doi: 10.1016/j.mvr.2020.104119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagashima S, Mendes MC, Camargo Martins AP, Borges NH, Godoy TM, Miggiolaro AFRDS, da Silva Dezidério F, Machado-Souza C, de Noronha L. Endothelial dysfunction and thrombosis in patients with COVID-19-brief report. Arterioscler Thromb Vasc Biol. 2020;40:2404–2407. doi: 10.1161/ATVBAHA.120.314860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen S, Lu H, Liu Z, Yuan W. Comment on “Central Nervous System involvement by severe acute respiratory syndrome coronavirus -2 (SARS-CoV-2)”. J Med Virol. 2020;92:1399–1400. doi: 10.1002/jmv.25991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Remmelink M, De Mendonça R, D’Haene N, De Clercq S, Verocq C, Lebrun L, Lavis P, Racu ML, Trépant AL, Maris C, et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24:495. doi: 10.1186/s13054-020-03218-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirschenbaum D, Imbach LL, Jane Rushing E, Frauenknecht KBM, Gascho RTD, Victor Ineichen B, Keller E, Kohler S, Lichtblau M, Reimann RR, et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol Appl Neurobiol. 2021;47:454–459. doi: 10.1111/nan.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan NC, Weitz JI. COVID-19 coagulopathy, thrombosis, and bleeding. Blood. 2020;136:381–383. doi: 10.1182/blood.2020007335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berger JS, Kunichoff D, Adhikari S, Ahuja T, Amoroso N, Aphinyanaphongs Y, Cao M, Goldenberg R, Hindenburg A, Horowitz J, et al. Prevalence and outcomes of D-Dimer elevation in hospitalized patients with COVID-19. Arterioscler Thromb Vasc Biol. 2020;40:2539–2547. doi: 10.1161/ATVBAHA.120.314872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, Humphries F, Jäger HR, Losseff NA, Perry RJ, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samudra AN, Dwyer KM, Selan C, Freddi S, Murray-Segal L, Nikpour M, Hickey MJ, Peter K, Robson SC, Sashindranath M, et al. CD39 and CD73 activity are protective in a mouse model of antiphospholipid antibody-induced miscarriages. J Autoimmun. 2018;88:131–138. doi: 10.1016/j.jaut.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 62.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cugno M, Meroni PL, Gualtierotti R, Griffini S, Grovetti E, Torri A, Lonati P, Grossi C, Borghi MO, Novembrino C, et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J Autoimmun. 2021;116:102560. doi: 10.1016/j.jaut.2020.102560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seheult JN, Seshadri A, Neal MD. Fibrinolysis shutdown and thrombosis in severe COVID-19. J Am Coll Surg. 2020;231:203–204. doi: 10.1016/j.jamcollsurg.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, Nydam TL, Moore PK, McIntyre RC., Jr. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231:193.e1–203.e1. doi: 10.1016/j.jamcollsurg.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ji HL, Zhao R, Matalon S, Matthay MA. Elevated Plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev. 2020;100:1065–1075. doi: 10.1152/physrev.00013.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marcos-Contreras OA, Martinez de Lizarrondo S, Bardou I, Orset C, Pruvost M, Anfray A, Frigout Y, Hommet Y, Lebouvier L, Montaner J, et al. Hyperfibrinolysis increases blood-brain barrier permeability by a plasmin- and bradykinin-dependent mechanism. Blood. 2016;128:2423–2434. doi: 10.1182/blood-2016-03-705384 [DOI] [PubMed] [Google Scholar]
- 68.Lu J, Sun PD. High affinity binding of SARS-CoV-2 spike protein enhances ACE2 carboxypeptidase activity. J Biol Chem. 2020;295:18579–18588. doi: 10.1074/jbc.RA120.015303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gauberti M, Potzeha F, Vivien D, Martinez de Lizarrondo S. Impact of bradykinin generation during thrombolysis in ischemic stroke. Front Med (Lausanne). 2018;5:195. doi: 10.3389/fmed.2018.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmed S, Zimba O, Gasparyan AY. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow’s triad. Clin Rheumatol. 2020;39:2529–2543. doi: 10.1007/s10067-020-05275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parry AH, Wani AH, Yaseen M. Neurological dysfunction in Coronavirus Disease-19 (COVID-19). Acad Radiol. 2020;27:1329–1330. doi: 10.1016/j.acra.2020.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Erecińska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128:263–276. doi: 10.1016/s0034-5687(01)00306-1 [DOI] [PubMed] [Google Scholar]
- 74.Ogawa S, Shreeniwas R, Brett J, Clauss M, Furie M, Stern DM. The effect of hypoxia on capillary endothelial cell function: modulation of barrier and coagulant function. Br J Haematol. 1990;75:517–524. doi: 10.1111/j.1365-2141.1990.tb07792.x [DOI] [PubMed] [Google Scholar]
- 75.Ntaios G, Michel P, Georgiopoulos G, Guo Y, Li W, Xiong J, Calleja P, Ostos F, González-Ortega G, Fuentes B, et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the Global COVID-19 Stroke Registry. Stroke. 2020;51:e254–e258. doi: 10.1161/STROKEAHA.120.031208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Du F, Liu B, Zhang S. COVID-19: the role of excessive cytokine release and potential ace2 down-regulation in promoting hypercoagulable state associated with severe illness. J Thromb Thrombolysis. 2021;51:313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Z, Li J, Chen D, Gao R, Zeng W, Chen S, Huang Y, Huang J, Long W, Li M, et al. Dynamic interleukin-6 level changes as a prognostic indicator in patients with COVID-19. Front Pharmacol. 2020;11:1093. doi: 10.3389/fphar.2020.01093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, del Zoppo GJ, Hallenbeck JM, Rothwell NJ, Hopkins SJ, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92:2409–2411. doi: 10.1002/jmv.26097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winbeck K, Poppert H, Etgen T, Conrad B, Sander D. Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke. 2002;33:2459–2464. doi: 10.1161/01.str.0000029828.51413.82 [DOI] [PubMed] [Google Scholar]
- 83.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165 [DOI] [PubMed] [Google Scholar]
- 84.Wijeratne T, Sales C, Karimi L, Crewther SG. Acute ischemic stroke in COVID-19: a case-based systematic review. Front Neurol. 2020;11:1031. doi: 10.3389/fneur.2020.01031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sahu KK, Mishra AK, Lal A. Trajectory of the COVID-19 pandemic: chasing a moving target. Ann Transl Med. 2020;8:694. doi: 10.21037/atm-20-2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Jones S, Bilaloglu S, Hochman K, Raz E, Galetta S, et al. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. doi: 10.1016/j.jns.2020.116923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khandelwal P, Mufti F, Tiwari A, Singla A, Dmytriw A, Piano M, Quilici L, Pero G, Renieri L, Limbucci N. Incidence, characteristics and outcomes of large vessel stroke in COVID-19 cohort: a multicentric international study. SSRN. 2020;12Https://ssrn.Com/abstract=3617195 or http://dx.Doi.Org/10.2139/ssrn.3617195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, Wang D, Mao L, Jin H, Hu B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siegler JE, Cardona P, Arenillas JF, Talavera B, Guillen AN, Chavarria-Miranda A, de Lera M, Khandelwal P, Bach I, Patel P, et al. Cerebrovascular events and outcomes in hospitalized patients with COVID-19: the svin COVID-19 multinational registry [published online september 30, 2020]. Int J Stroke. doi: 10.1177/1747493020959216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richter D, Krogias C, Eyding J, Bartig D, Grau A, Weber R. Comparison of stroke care parameters in acute ischemic stroke patients with and without concurrent COVID-19. A Nationwide analysis. Neurol Res Pract. 2020;2:48. doi: 10.1186/s42466-020-00095-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, et al. ; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kvernland A, Kumar A, Yaghi S, Raz E, Frontera J, Lewis A, Czeisler B, Kahn DE, Zhou T, Ishida K, et al. Anticoagulation use and hemorrhagic stroke in SARS-COV-2 patients treated at a New York healthcare system [published online August 24, 2020]. Neurocrit Care. doi: 10.1007/s12028-020-01077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77:1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Modin D, Claggett B, Sindet-Pedersen C, Lassen MCH, Skaarup KG, Jensen JUS, Fralick M, Schou M, Lamberts M, Gerds T, et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142:2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


