Supplemental Digital Content is available in the text.
Keywords: goal, intracranial hemorrhage, laboratories, reperfusion, stent
Abstract
Background and Purpose:
The Tigertriever is a novel, radially adjustable, fully visible, stentriever that permits the operator to align radial expansion with target vessel diameters. This multicenter trial compared the Tigertriever’s effectiveness and safety compared with established stent retrievers.
Methods:
Single arm, prospective, multicenter trial comparing the Tigertriever to efficacy and safety performance goals derived from outcomes in 6 recent pivotal studies evaluating the Solitaire and Trevo stent-retriever devices with a lead-in and a main-study phase. Patients were enrolled if they had acute ischemic stroke with National Institutes of Health Stroke Scale score ≥8 due to large vessel occlusion within 8 hours of onset. The primary efficacy end point was successful reperfusion, defined as core laboratory-adjudicated modified Thrombolysis in Cerebral Ischemia score 2b-3 within 3 passes of the Tigertriever. The primary safety end point was a composite of 90-day all-cause mortality and symptomatic intracranial hemorrhage. Secondary efficacy end points included 3-month good clinical outcome (modified Rankin Scale score 0–2) and first-pass successful reperfusion.
Results:
Between May 2018 and March 2020, 160 patients (43 lead-in, 117 main phase) at 17 centers were enrolled and treated with the Tigertriever. The primary efficacy end point was achieved in 84.6% in the main-study phase group compared with the 63.4% performance goal and the 73.4% historical rate (noninferiority P<0.0001; superiority P<0.01). The first pass successful reperfusion rate was 57.8%. After all interventions, successful reperfusion (modified Thrombolysis in Cerebral Ischemia score ≥2b) was achieved in 95.7% and excellent reperfusion (modified Thrombolysis in Cerebral Ischemia score 2c-3) in 71.8%. The primary safety composite end point rate of mortality and symptomatic intracranial hemorrhage was 18.1% compared with the 30.4% performance goal and the 20.4% historical rate (noninferiority P=0.004; superiority P=0.57). Good clinical outcome was achieved in 58% at 90 days.
Conclusions:
The Tigertriever device was shown to be highly effective and safe compared with Trevo and Solitaire devices to remove thrombus in patients with large-vessel occlusive stroke eligible for mechanical thrombectomy.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03474549.
Based on convergent evidence from major clinical trials of reperfusion efficacy and improved clinical outcomes, endovascular thrombectomy with stent retrievers has become the standard of care for patients with large vessel occlusion ischemic strokes.1–8 A meta-analysis of 5 randomized trials demonstrated the number needed to treat with thrombectomy is 1 in 2.6 to improve 3-month global disability outcome by 1 or more levels on the modified Rankin Scale (mRS).9 However, while quite substantial, the benefits conveyed by first-generation stent retrievers are constrained by less than maximal reperfusion rates achieved with these devices. In pooled, individual participant data meta-analyses of the pivotal trials, failure to achieve successful reperfusion (modified Thrombolysis in Cerebral Ischemia [mTICI] 2b-3) occurred in 29% of patients and failure to achieve complete reperfusion (mTICI 3) in 67%.9 Accordingly, developing additional endovascular thrombectomy devices with performance characteristics comparable or better than established stent retrievers is desirable.
The Tigertriever (Rapid Medical) is a novel operator-adjustable stent retriever that affords the interventionalist incremental control over the radial diameter and radial force of the thrombectomy basket. The design is intended to facilitate alignment of the mesh with the anatomy of the occluded vessel and to increase internalization of the thrombus within the device, thereby facilitating retrieval and reducing downstream embolization (Figure 1A; Figure I in the Data Supplement). The device has had CE mark, the European Union certification indicating conformity with health, safety, and environmental protection, since 2016 and has shown promising signals of efficacy and safety in preliminary case series reported by European centers.10–12
Figure 1.
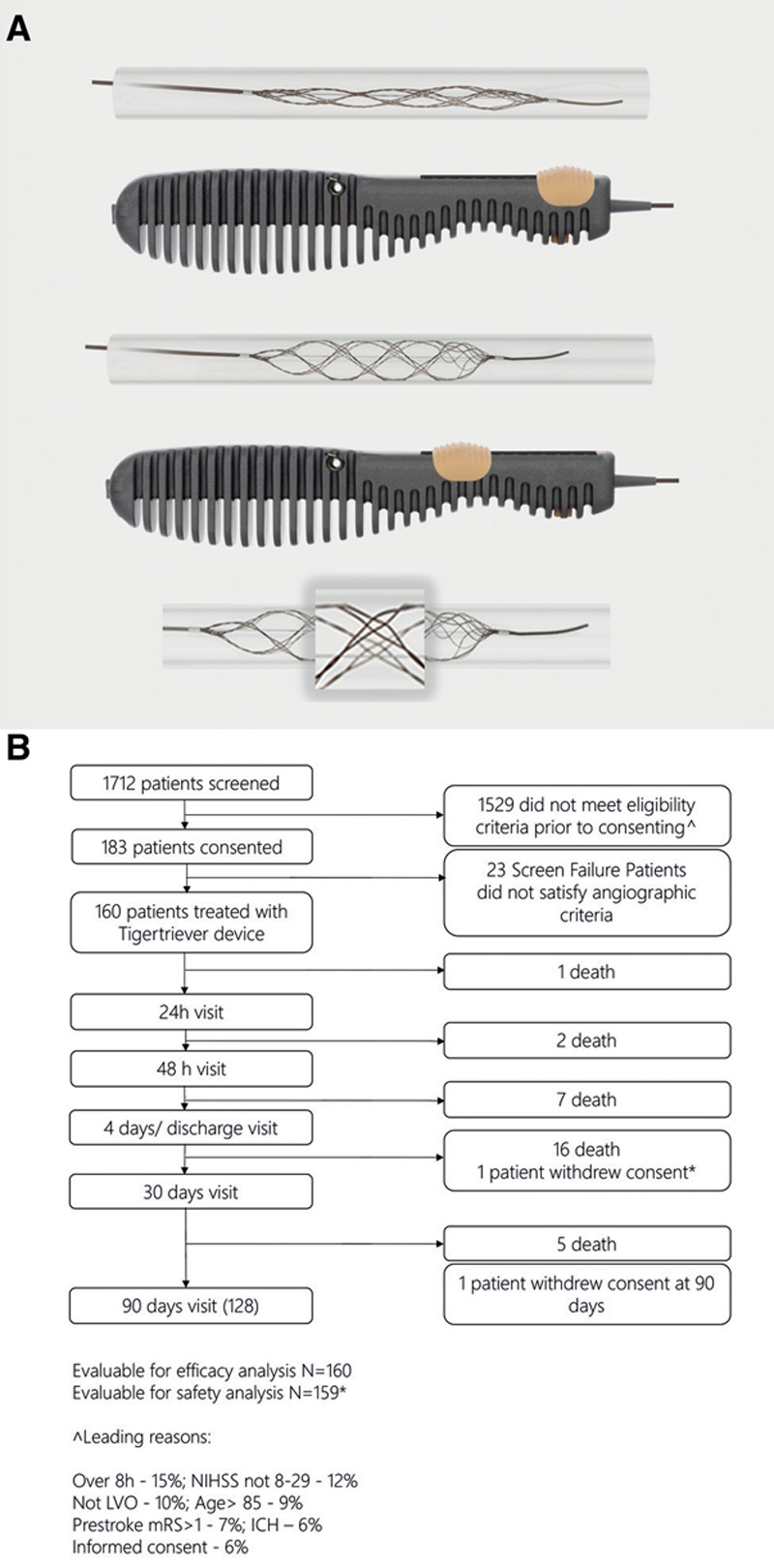
Tigertriever device and chart of patient flow through study. A, Tigertriever device including external handle for the operator to control the degree of expansion of the stent retriever. Fine wire mesh mounted on a flexible shaft. The design of the wire mesh (magnified view) is optimized to penetrate the clot and encapsulate it during retrieval. B, Patient screening, enrollment, treatment, and follow-up. ICH indicates intracranial hemorrhage; LVO, large vessel occlusion; mRS, modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
The TIGER trial (Treatment With Intent to Generate Endovascular Reperfusion) was a single-arm, multicenter, prospective study assessing the efficacy and safety of the Tiger-21 and Tiger-17 retrievers. The study employed an objective performance criterion, noninferiority design, comparing efficacy and safety of the Tigertriever with performance goals derived from adjudicated outcomes in 6 completed prospective trials of 2 FDA-approved predicate stent retrievers, Solitaire (Medtronic) and Trevo (Stryker).1–4,13,14
Methods
Study Design
The data that support the findings of this study are available from the corresponding author on reasonable request and with approval from the TIGER investigators. The primary TIGER Study hypothesis was that the Tigertriever device would achieve successful reperfusion performance within 3 attempts would be superior to the performance goal established using Bayesian meta-analysis of 6 recent pivotal trials of the Solitaire and Trevo stent-retriever devices—TREVO 2 (Thrombectomy Revascularization of Large Vessel Occlusions in Acute Ischemic Stroke),13 SWIFT (SOLITAIRE FR With the Intention for Thrombectomy),14 MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands),1 ESCAPE (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion With Emphasis on Minimizing CT to Recanalization Times),4 REVASCAT (Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset),3 and SWIFT PRIME (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment).2
The study was overseen by an executive committee, composed of academic investigators, and an independent data and safety monitoring board (DSMB) (Table I in the Data Supplement). The DSMB consisted of an independent stroke neurologist and 2 independent neurointerventionalists, who did not enroll patients, and a biostatistician. At the level of individual events, the DSMB adjudicated all safety end points, including hemorrhages and serious adverse events. At the level of aggregate study data, the DSMB acted in an expert supervisory capacity and monitored subject safety and the conduct of the study. Central readers at a core imaging laboratory assessed qualifying diagnostic catheter angiography to determine location of the target occlusion, and rated reperfusion grades on angiograms obtained after every pass of the Tigertriever device, every pass of rescue therapy, and end of the procedure. The core laboratory imaging readers also assessed baseline and 24-hour post-procedure computed tomography (CT) or magnetic resonance imaging scans to verify study entry criteria and determine the presence and type of any postprocedure intracranial hemorrhage. The sponsor, Rapid Medical, and its contracted Contract Research Organization (Genae Americas) were responsible for logistical operations, data management, and monitoring of the trial. The study protocol was approved by the institutional review board or ethics committee at each participating site.
Population and Participating Centers
All patients or their legally authorized representative provided written informed consent before enrollment. Patients aged 18 to 85 years were eligible for the study if they had acute ischemic stroke with new moderate-to-severe neurological deficits, angiographically confirmed occlusion in the intracranial internal carotid, middle cerebral (M1 or M2 segment), basilar, or vertebral artery, and could undergo endovascular therapy (at least 1 Tigertriever deployment in the target artery) within 8 hours of last known well. Full study entry criteria are shown in the Data Supplement (Table II in the Data Supplement). Key inclusion criteria included the following: prestroke mRS score ≤1; baseline National Institutes of Health Stroke Scale (NIHSS) score 8–29; Alberta Stroke Program Early CT Score 6–10 for CT-qualifying or diffusion restriction volume ≤50 mL for magnetic resonance imaging-qualifying anterior circulation cases; patient being either (1) eligible for, and received, IV tPA (intravenous tissue-type plasminogen activator) within 3 hours of symptom onset or (2) ineligible for IV tPA treatment; and anticipated device deployment within 8 hours of last known well. Key exclusion criteria included stenosis or occlusion in the deployment site or in a proximal vessel that would prevent device access to the thrombus or require angioplasty or stenting to enable device access to the thrombus. The treatment start inclusion criterion of within 8 hours, rather than also 8 to 24 hours with advanced imaging selection, was selected as initial trial design discussions and review with the Food and Drug Administration occurred before the reporting of results of late window trials. Physicians were trained in the use of the device on a bench vascular model before any procedures were done. In addition, participating sites that had no experience with the Tigertriever device were required to first participate in a lead-in phase before participating in the main-study phase for primary end point analysis. During the lead-in phase, up to 4 patients per clinical site were enrolled. The lead-in phase was completed by the site either (1) achieving 2 successive successful reperfusions (mTICI 2b or higher) or (2) performing 4 cases. Patients enrolled in the lead-in phase were consented, treated, and followed according to the clinical protocol, in the same manner as the main-study patients.
Procedure
Site of arterial access and anesthesia mode were based on operator preference at the enrolling center. The selection of guide catheter type (balloon guide catheter (BGC) versus non-BGC) and use of an intermediate catheter was also at the discretion of the operator. After placement of the guide catheter in the target vessel, a roadmap was constructed and the microcatheter was navigated across the clot. The Tigertriever was deployed and the amount of expansion of the device was based on vessel diameter and clot length.
Two versions of the Tigertriever device were available: The standard version (Tigertriever) has a net length of 32 mm (unexpanded form) and can expand up to 6 mm diameter, can be delivered through a microcatheter with an internal diameter of 0.021 inches. And a smaller version (Tigertriever 17) has a net length of 23 mm (unexpanded form) and can be delivered through a microcatheter with an internal diameter of 0.017 inches. It can expand up to 3 mm diameter. The device deployment, dwell time, and number of expansions/deflations were based on the instructions for use. The first pass was required to be with the Tigertriever for inclusion in the trial. Up to 3 passes of the Tigertriever were permitted to achieve successful reperfusion (mTICI, 2b-3). For each pass, a new Tigertriever device was used and the total number of passes and devices used were recorded. After 3 attempts, rescue therapy could be used by the operator. Rescue therapy could consist of use of another mechanical thrombectomy device, angioplasty, intraarterial tPA, or intracranial stenting at the discretion of the treating physician. The Tigertriever could not be used after a rescue attempt and for primary efficacy analysis when revascularization was unsuccessful after Tigertriever attempts it was considered a failure to achieve the end point. Imaging with CT or magnetic resonance imaging was obtained at 24 hours to assess for intracranial hemorrhage. NIHSS scores were obtained at 24 hours, 48 hours, 4 days (or discharge if sooner), and 90 days; mRS scores were obtained at 4 days (or discharge if sooner), 30 days, and 90 days.
Outcomes
Primary Efficacy End Point
The primary efficacy end point was successful reperfusion, defined as achieving mTICI 2b-3 within 3 passes with the Tigertriever without use of rescue therapy. All angiograms were interpreted by an experienced neurointerventionalist readers at the independent core imaging laboratory, who assigned scores using the mTICI scale.15 The core imaging laboratory mTICI ratings were used for assessment of the primary efficacy end point.
Primary Safety End Point
The primary safety end point was defined as the composite of all-cause mortality at 90±14 days and symptomatic intracranial hemorrhage (sICH) within 24 (18–36) hours from the study procedure. sICH was defined as any parenchymal hematoma type 2, remote intracerebral hemorrhage, subarachnoid hemorrhage, or intraventricular hemorrhage that is the predominant cause of an NIHSS deterioration of 4 or more points at 24 hours, as adjudicated by the DSMB (modified Heidelberg Bleeding Classification criteria).16 The independent core imaging laboratory assessed all 24-hour CT/MR images and radiologically classified hemorrhages as hemorrhagic infarction types 1 or 2, parenchymal hematoma types 1 or 2, remote intracerebral hemorrhage, subarachnoid hemorrhage, and intraventricular hemorrhage.
Secondary End Points
Prespecified secondary efficacy end points for the study were (1) good clinical outcome (mRS score 0–2 at 90 days; (2) first pass successful reperfusion (mTICI 2b-3); (3) health-related quality of life at 90 days, assessed with the EQ-5D2,17; and (4) granular level of disability at 90 days, assessed with the Academic Medical Center Linear Disability Score.18,19 Prespecified secondary safety end points were (1) asymptomatic intracranial hemorrhage within 24 hours (18–36 hours) of procedure; (2) neurological deterioration within 24 hours after procedure, defined as a NIHSS increase of 4 points or more; and (3) embolization to previously uninvolved vascular territories.
Statistical Analysis
The performance goal for the efficacy primary end point was defined as the incidence in the 6 trials cited below, minus a noninferiority statistical margin of 10%, the same clinically relevant threshold employed in the registration trials of stent-retrievers, SWIFT and TREVO 2,9,13 therefore, giving a performance goal (PG) of 73.4%−10%=63.4%. The pooled incidence was derived from the following 6 studies: TREVO 2, SWIFT,1 MR CLEAN, ESCAPE, REVASCAT, and SWIFT PRIME.1–4,9,13 For this efficacy end point, the primary noninferiority hypothesis test was performed at an overall 2-sided alpha level of 0.05 using exact binomial methods, in which the lower confidence bound on the observed incidence of reperfusion is compared with the PG. If noninferiority was demonstrated, superiority was then tested in a post hoc hierarchical manner. The study statistical design is shown graphically in Figure IIA in the Data Supplement.
The PG for safety primary end point was defined as the incidence in the 6 trials cited above, plus a statistical margin of 10%. Thus, the PG was 30.4% (18.2% mortality+2.2% sICH+10% noninferiority statistical margin). For primary safety, the hypothesis test was performed at an overall 2-sided alpha level of 0.05 using exact binomial methods, in which the upper confidence bound on the observed incidence of revascularization is compared with the PG.
The primary study population was the main-study phase group. Sensitivity analysis was performed at regulatory request on the combined lead-in/main-study phase population). To evaluate Tigertriever device performance in vessels with of varied size, the study population was divided into 2 vessel diameter categories, ≥2 and <2 mm, and performance compared across the groups.
Results
Between May 2018 and March 2020, 1712 patients were preliminarily screened, 183 consented before angiography, and 160 met angiographic and device access criteria for full study enrollment. Patients were enrolled at 17 study centers (16 US centers—159 patients, 1 Israeli center—1 patient; Table I in the Data Supplement) and included 43 lead-in phase and 117 main-study phase patients. Figure 1B shows the screening, enrollment, treatment, and follow-up flow of the study population.
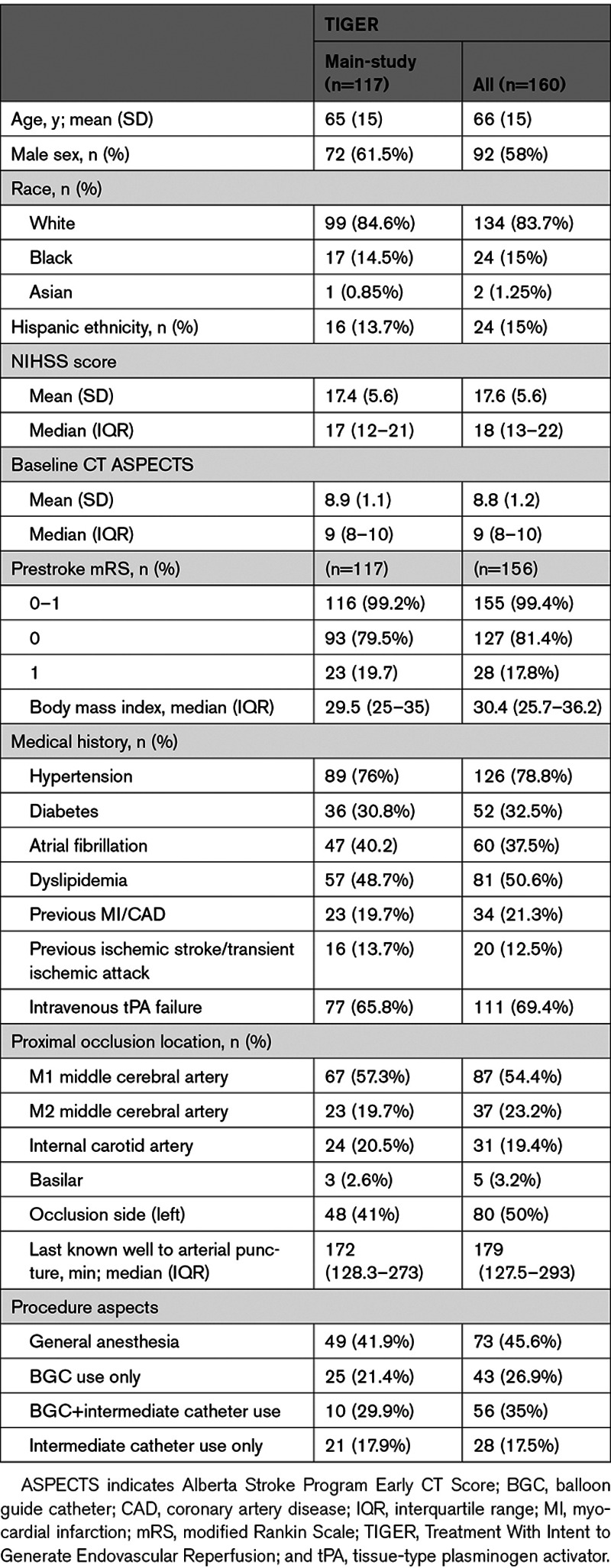
Patient baseline characteristics are shown for the main-study phase and combined lead-in/main-study phase cohorts in Table 1 (Table III in the Data Supplement shows patient characteristics for the lead-in phase patients alone; Tables IV and V in the Data Supplement compare patient characteristics and outcomes in the current trial with those of the 6 trials contributing to the performance goal and the more recent ARISE II trial [Analysis of Revascularization in Ischemic Stroke With EmboTrap]). In the main-study phase cohort, patient age was mean 65±15 years, 61.5% were men, and median baseline NIHSS 17 (interquartile range [IQR], 12–21). The median Alberta Stroke Program Early CT score was 9 (IQR, 8–10). Target occlusion location was in the anterior circulation in 97.4% of patients, with the most common sites the M1 middle cerebral artery (57.3%) and intracranial internal carotid artery (20.5%). Time from last known normal to puncture was 172 minutes (IQR, 128–273), and a majority (65.8%) of patients received intravenous t-PA before thrombectomy. A BGC was used in 29.9% of the procedures (21.4% BGC alone and 8.5% BGC+regional aspiration).
Table 1.
Patient and Procedure Characteristics for Main-Study and Combined Lead-In/Main-Study Patients
Primary Efficacy End Point and Reperfusion Results
In the primary analysis main-study phase population, the Tigertriever achieved the primary end point of successful reperfusion (mTICI 2b-3) within 3 passes and without rescue therapy in 99 of 117 patients, 84.6% (95% CI, 78.1%–91.2%), compared with the 63.4% performance goal and the 73.4% historical rate (noninferiority P<0.0001; superiority P<0.01; Table 2, Figure IIB in the Data Supplement).
Table 2.
Angiographic and Clinical Efficacy Outcomes in Main-Study and Combined Lead-In/Main-Study Patients

The full distribution of reperfusion outcomes is shown in Figure 2A. The rate of excellent reperfusion (mTICI 2c-3) within 3 passes of the Tigertriever was 63.2% and 71.8% after all interventions. The frequency of first pass excellent reperfusion (mTICI 2c-3) was 41.4% and of first pass substantial reperfusion (mTICI 2b-3) 57.8%. The mean number of passes with the Tigertriever was 1.8±0.9. The rates of reperfusion achieved with each device size (Tigertriever and Tigertriever 17) and in each target artery are reported in Text I in the in the Data Supplement.
Figure 2.
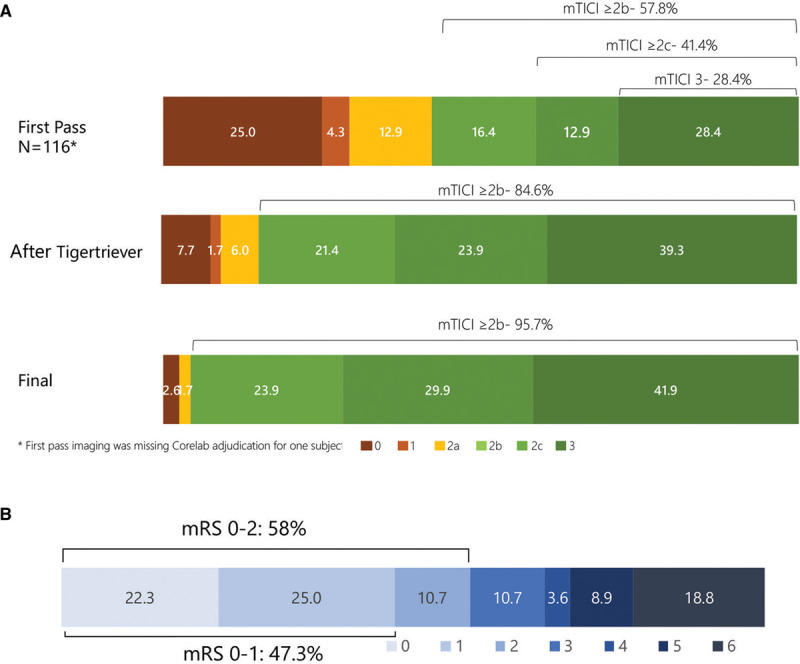
Reperfusion and functional outcomes in main-study phase of TIGER trial (Treatment With Intent to Generate Endovascular Reperfusion). A, Reperfusion degree after first pass, after up to 3 Tigertriever passes, and after rescue therapy. B, Distribution of 90-d global disability outcomes on the modified Rankin Scale (mRS). mRS was available from 114 patients out of the 117 of the main-study group. mTICI indicates modified Thrombolysis in Cerebral Ischemia.
Rescue therapy was employed in 33 of 117 (28.2%) main-study phase patients (frequency of other mechanical thrombectomy devices, angioplasty, intraarterial thrombolysis, and intracranial stenting shown in Table VI in the Data Supplement). In 5, at the interventionalist’s discretion, a different device was used before reaching 3 passes with Tigertriever. Per protocol, these were counted as failures despite not having not made 3 attempts with Tigertriever. In 13, the additional devices were used at interventionalist discretion to further improve reperfusion despite mTICI ≥2b having been attained within 3 passes of the Tigertriever. Per protocol, these were counted as Tigertriever successes in achieving mTICI≥2b Final reperfusion rates after all interventions were 95.7% mTICI 2b-3; 71.8% mTICI 2c-3; and 41.9% mTICI 3. Median time from arterial puncture to successful reperfusion (mTICI 2b-3) was 24 minutes (IQR, 16–38).
Primary efficacy end point and additional reperfusion results in lead-in patients did not statistically differ from main-study phase patient results (Table VII in the Data Supplement).
Primary Safety End Point
In the primary analysis main-study phase population, the rate for the primary safety composite end point of combined sICH and 90-day all-cause mortality was 18.1% (95% CI, 11.1%–25.1%, Table 3), compared with the PG of 30.4% and historical rate of 20.4% (noninferiority P=0.004; superiority P=0.57). The primary safety end point in lead-in patients did not statistically differ from main-study phase patient results (Table VIII in the Data Supplement).
Table 3.
Safety End Points in Main-Study and Combined Lead-In/Main-Study Patients

Secondary End Points
Secondary clinical efficacy outcomes are shown in Table 2. In main-study phase patients, good clinical outcome (functional independence, mRS score 0–2) was attained by 58.0% (95% CI, 48.3%–67.3%), a rate superior to the 43.5% in the pooled comparator trials (P=0.006). The full distribution of disability levels at 90 days is shown in Figure 2B. Health-related quality of life on the EQ-5D at 90 days was median 80 (IQR, 70–90) and granular disability level on the Academic Medical Center Linear Disability Score at 90 days was median 93.3 (IQR, 57.8–100).
Secondary safety outcomes are shown in Table 3. Neurological deterioration by 4 or more NIHSS points by 24 hours occurred in 7.7% (95% CI, 3.6%–14.2%). The rate of embolization to a previously uninvolved new territory was 2.6% (95% CI, 0.5%–7.4%), a rate superior to the 7.4% in the pooled comparator trials (P=0.0403). Asymptomatic intracranial hemorrhage within 24 hours occurred in 31.0% (95% CI, 22.7%–40.3%). (Additional data on radiological classification of hemorrhages are shown in Tables IX and X in the Data Supplement.)
Secondary clinical efficacy and safety results in lead-in patients did not statistically differ from main-study phase patient results (Tables III and IV in the Data Supplement). All adverse events are shown in Table XI in the Data Supplement.
Tigertriever Performance in Vessels of Different Size
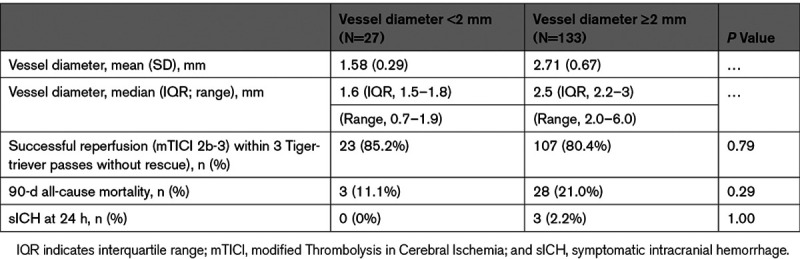
Tigertriever performance in smaller (<2 mm) and larger (≥2 mm) vessels is shown in Table 4. Median vessel diameters were 1.6 mm (IQR, 1.5–1.8) for smaller vessels and 2.5 mm (IQR, 2.2–3.0) for larger vessels. Rates of both the primary efficacy end point (successful reperfusion) and the primary safety end point components (sICH at 24 hours and all-cause mortality at 90 days) were comparable in both vessel size groups.
Table 4.
Tigertriever Performance in Vessels of Different Size Among Combined Lead-In/Main-Study Patients
Discussion
In this prospective, multicenter trial, the Tigertriever yielded high rates of reperfusion among patients with acute ischemic stroke swith large vessel occlusion, with substantial reperfusion achieved in >8 of every 10 patients with the Tigertriever alone and >9 of every 10 patients after additional interventions. The rate of successful reperfusion surpassed the predefined performance goal against the predicate Trevo and Solitaire devices derived from regulatory registration and additional pivotal trials. The high successful reperfusion rates with the Tigertriever device translated to favorable functional outcomes, with nearly 6 of every 10 patients achieving functional independence at day 90.
Patients enrolled in the TIGER study were similar to the comparator studies in several key features, including age, presenting deficit severity (NIHSS), extent of ischemic injury on initial imaging (Alberta Stroke Program Early CTs), vascular risk factors, rate of pretreatment with tPA, and frequency of target occlusion in internal carotid artery and M1 middle cerebral artery (Table IV in the Data Supplement). The frequency of M2 middle cerebral artery occlusions was mildly higher in the present study than several comparator studies but lower than in the most recent comparator investigation, ARISE II.20 The greater proportion of M2 occlusions reflects the increasing treatment of more distal vessel occlusions in clinical practice.21 M2 occlusions have features that make them both more difficult targets for EVT, including distal location, smaller size, reduced accessibility, and less difficult, including smaller thrombus burden. As the frequency of reperfusion of the M2 was similar to other occlusion sites in the present study, the distribution of target occlusions did not affect comparison with prior studies. Patients in this study were treated sooner after onset (last known well to puncture 2 hour 59 minutes) than in prior studies, in accord with increasing emphasis in national guidelines and national practice on accelerating speed of endovascular thrombectomy.6–8
Reperfusion rates were better in the current study than in the comparator studies in a variety of parameters, including first pass reperfusion, mean number of passes, reperfusion after up to 3 passes before rescue therapy, and final reperfusion after rescue therapy. First pass effect, defined as achieving mTICI 2c-3 reperfusion with one pass of the stent retriever, is likely to yield better patient outcomes and is a stringent metric to assess device performance.22 In the current study, a first pass effect was achieved in 41.4% of patients, which compares favorably to the rate of 25% in both the North American Solitaire Acute Stroke registry23 and Trevo acute ischemic stroke registry.24 In the ARISE II study, the first pass effect was seen in 40% of patients,20 which is comparable to the present study. In the current study, successful reperfusion at procedure end, including rescue therapies, was achieved in nearly every patient (96%), a value that elevates cerebral reperfusion success rates to those of cardiac reperfusion procedures.25,26
Faster time from puncture to achievement of reperfusion has been associated with improved clinical outcomes.27 The current study results demonstrate the median procedure time (from puncture to achievement of mTICI ≥2b) was 25 (IQR, 17–43) minutes, which compares favorably to the ARISE II study, where the median time was 35 (IQR, 24–58) minutes.20 This time was similar to the contact aspiration first pass thrombectomy time of median 25 minutes as reported in the COMPASS study (Aspiration Thrombectomy Versus Stent Retriever Thrombectomy as First-Line Approach for Large Vessel Occlusion) but shorter than the median 35 minutes reported for that trial’s stent retriever group.28 In experienced, high-volume centers performing over 48 thrombectomies annually, the median puncture-to-reperfusion time with the Trevo device was 67 (IQR, 42–105) minutes.29 The reduced procedure times with the Tigertriever may reflect in part the technological advancement of the operator being able to adjust the radial force expansion to interact with the clot in a more productive manner.
The use of a BGC in the proximal vessel of the target occlusion when prior stent retrievers are deployed has been shown to improve the first pass reperfusion effect and clinical outcomes.30 The proximal flow arrest may prevent distal embolization or non- target distal embolization. The unique design of the Tigertriever allows for more effective internalization of the thrombus into the interstices of the device. As the device is collapsed by the operator, it theoretically limits the chance of distal embolization as the clot is completely entwined in the retriever. The current study had a lower rate of BGC use at 35% in the intention to treat population, in comparison to the ARISE II trial in which this technique was used in 73.6% of patients.20 Despite the lower use of BGC in this study, there were only 4 patients (2.5%) who had an embolization to a new territory compared with 6.6% in ARISE II and to 7.4% in the pooled data from of MR CLEAN,1 ARISE II,20 REVASCAT,3 and TREVO 2.13
Clinical outcomes in the present study were favorable. Functional independence (mRS score 0–2) at 90 days was achieved in 58% of patients, a rate higher than in the comparator studies. Similarly, health-related quality of life showed a utility value of 0.80, higher than the 0.57 in the MR CLEAN trial.31 These high rates of good long-term functional outcome likely reflect reduced total brain ischemia time because of high achieved reperfusion rates, early last known well-to-punctures times, and rapid puncture-to-reperfusion times. The faster last known well-to-puncture times versus historical comparator trials likely reflects interval evolution in endovascular workflow and systems of care, while the increased perfusion rates and rapid puncture-to-reperfusion times likely at least in part reflect intrinsic properties of the Tigertriever itself.
Limitations
This study has limitations. The study design was a single-arm trial against objective performance criteria derived from pooled prior studies of predicate devices, rather than a randomized trial with a contemporaneous control group. This approach constrains precision in delineating how well the Tigertriever compares with any particular comparator device. Reflecting the single-arm design, the central imaging and site clinical outcome evaluators and were not blinded to treatment assignment. However, for imaging the use of a central core laboratory was employed to mitigate any resulting bias. Similarly, for clinical outcomes, the primary efficacy outcome was performed by certified raters using a structured assessment system (the Rankin Focused Assessment) and the congruence of rater assessments of global disability and patient-reported health-related quality of life indicates accurate scoring.
Conclusions
In this prospective, multicenter study with a unique operator-controlled stent retriever, the Tigertriever achieved successful reperfusion within 3 passes in >8 of every 10 patients, demonstrating noninferiority over a performance goal and superiority over actual historical rates derived from trials and prospective studies of established devices. In addition, first pass successful reperfusion was achieved in nearly 6 of every 10 patients and final successful reperfusion in >9 of every 10 patients. Rates of embolization to a new territory and sICH were low and rates of good global disability and health-related quality of life outcomes high. Efficacy and safety outcomes were similar in vessels with diameters <2 and ≥2 mm.
Acknowledgments
The members of the Data Safety Monitoring Board were Vineeta Singh, MD (Chair), Colin Derdeyn, MD, M. Shazam Hussain, MD, Scott Hamilton, PhD (Biostatistician).
Sources of Funding
The study was funded by Rapid Medical. The study was designed with the input from an academic steering committee. There was an independent imaging core laboratory, independent statistician, and data safety monitoring board. The sponsor provided operational support with on-site and remote monitoring.
Disclosures
Dr Gupta serves as Principal Investigator (PI) for the TIGER (Treatment With Intent to Generate Endovascular Reperfusion) Study (Rapid Medical), PI for the ASSIST Registry (Stryker Neurovascular), PI for the RECCLAIM II Study (Zoll), CLEAR Study (Vesalio; The Vesalio NeVa Stent Retriever Study for Treatment of Large Vessel Occlusion Strokes), Clinical Events Committee (CEC) for the MIND Trial (Penumbra), consultant for Cerenovous. Dr Saver discloses consultant or advisory boards from Medtronic, Stryker, Cerenovus, and Rapid Medical; institutional conflict of interest from University of California. The University of California has patent rights in retrieval devices for stroke. Dr Levy is consultant or advisory board or Ownership interest for Claret Medical, GLG consulting, Guidepoint Global, Imperative Care, Medtronic, StimMed, Misionix, Mosaic, Clarion, Stryker, NeXtGen Biologics, MEDX, Cognition Medical, Endostream Medical, Rapid Medical, Rebound Therapeutics, Three Rivers Medical, and received Honorarium for training from Medtronic and Penumbra. Dr Zaidat is a consultant for Stryker Neurovascular, Cerenovous, Penumbra, Rapid Medical and Medtronic. He has also received research grants from Penumbra and Stryker Neurovascular. Dr Yavagal is a consultant for Medtronic, Cerenovous, Rapid Medical, Vascular Dynamics, Poseydon, Neurosave, Neural Analytics and Galaxy Therapeutics. Dr Liebeskind is a consultant as the imaging core lab for Cerenovous, Genentech, Medtronic, Stryker and Rapid Medical. Dr Gross is a consultant for Medtronic and Microvention. Dr Jankowitz is a consultant for Medtronic and Stryker. Dr Snyder has reported he is a consultant for Toshiba, Medtronic, EV3, Abbott Vascular, Micrus, Boston Scientific, Codman, Zimmer, Stryker, Vital, Cannon. Significant financial interest in Endo Tex, Micrus, BSC EPI, Access Closure, Inc, Cordis, Primus. He is a major stockholder in Boston Scientific, Access Closure, Inc, Niagara Gorge Medical. Dr Siddiqui is a consultant for Amnis Therapeutics, Apellis Pharmaceuticals, Boston Scientific, Canon Medical Systems, United States, Inc, Cerebrotech Medical Systems, Inc, Cerenovous, Corindus, Inc, Endostream Medical, Ltd, Imperative Care, Inc, Integra Lifesciences Corp, IRRAS, Medtronic, Microvention, Minnetronix Neuro, Inc, Northwest University-DSMB for HEAT trial, Penumbra, Perflow Medical, Ltd, Q’Apel Medical, Inc, Rapid Medical, Rebound Therapeutics Corp, Serenity Medical, Inc, Silk Road Medical, StimMed, Stryker, Three Rivers Medical, Inc, VasSol, Vizai, Inc. W.L. Gore Associates. National PI/Steering Committee for Cerenovous NAPA Trial and ARISE II Trial, Medtronic SWIFT PRIME (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment) and SWIFT DIRECT Trials (Bridging Thrombolysis Versus Direct Mechanical Thrombectomy in Acute Ischemic Stroke), Microvention FRED Trial (The Flow Direction Endoluminal Device) and CONFIDENCE Study (Carotid Stent Trial to Evaluate the Safety and Efficacy of the Roadsaver Stent Used in Conjunction with the Nanoparasol Embolic Protection System for Patients at Increased Risk for Adverse Events From Carotid Endarterectomy), Medical University of South Carolina POSITIVE Trial (Perfusion Imaging Selection of Ischemic Stroke Patients for Endovascular Therapy), Penumbra 3D Separator Trial, COMPASS Trial (Aspiration Thrombectomy Versus Stent Retriever Thrombectomy as First-Line Approach for Large Vessel Occlusion), INVEST Trial. Financial interests in Adona Medical, Amnis Therapeutics, Bend IT Technologies, Ltd., BlinkTBI, Inc, Boston Scientific Corp (for purchase of Claret Medical), Buffalo Technology Partners, Inv., Cardinal Consultants, LLC, Cerebrotech Medical Systems, Inv. Cognition Medical, Endostreamt Medical, Ltd, Imperative Care, Inc, Instylla, Inc, International Medical Distribution Partners, IRRAS, LaunchNY Seed Fund Management, LLC, NeuroRadial Technologies, Inc, Neurovascular Diagnostics, Inc, Perflow Medical, Ltd, Q’Apel, Inc, Radical Catheter Technologies, Inc, Rebound Therapeutics Corp (Purchased 2019 by Integra Lifesciences Corp), Rist Neurovascular, Inc, Sense Diagnostics, Inc, Serenity Medical, Inc, Silk Road Medical, Spinnaker Medical, Inc, StimMed, Synchron, Three Rivers Medical, Inc, Truvic Medical, Inc, Vastrax, LLC, VICIS, Inc, Viseon, Inc, Vizai, Inc. Research grants as co-investigator NIH/NINDS 1RO1NS091075 Virtual intervention of aneurysms and Co-Principal Investigator NIH-NINDS R21 NS109575-01 Optimizing approaches to endovascular therapy of acute ischemic stroke. Dr Davies is a consultant for Medtronic, Microvention. Research support NIH RO1. Shares and ownership of Cerebrotech and Rist Neurovascular. Dr Hassan is a consultant for Medtronic, Stryker, Microvention, Penumbra, Cerenovous, Genentech, GE Healthcare, Scientia, Balt, Vizai, Insera therapeutics, Proximie, NovaSignal, Vesalio. PI COMPLETE Study (Penumbra; International Acute Ischemic Stroke Registry With the Penumbra System Aspiration Including the 3D Revascularization Device) and LVO SYNCHRONISE (Vizai; Observational Study of Automated Detection for Identification, Triage, and Timely Intervention in Large Vessel Occlusions). Steering Committee for SELECT (Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke), DAWN, SELECT 2 (Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke 2), EXPEDITE II (Evaluate a Next Generation Portable Diagnostic Platform for Determination and Immediate Triage of Emergency Large Vessel Stroke 2), EMBOLISE (Embolization of the Middle Meningeal Artery With ONYX Liquid Embolic System for Subacute and Chronic Subdural Hematoma), CLEAR. Grant support from GE Healthcare and Valley Baptist. Dr Hanel is a consultant for Rapid Medical, Medtronic, Stryker, Cerenovous, Balt, Phenox, Elum, MIVI, ThrombX, Endostream, RIST, REIST, Serenity, BendIT. Dr Malek is a consultant for Stryker, RAPID, InNeuroCo. Dr Mueller-Kronast reports no financial disclosures. Dr Starke has research support from NREG, Joe Niekro Foundation, Brain Aneurysm Foundation, Been Foundation and NIHS RO1 NS111119-01A1 and UL 1TR02736 and KL 2TR002737 through the Miami Clinical and Translational Science Institute, National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities. Unrestricted research grants from Medtronic. Consultant for Penumbra, Abbott, Medtronic, InNeuroCo and Cerenovous. Dr Bozorgchami is a consultant for Cerenovous. Dr Nesbit reports funding from Oregon Health Sciences. Dr Horikawa is a consultant for Terumo, Inc. Dr Priest is a consultant for Medtronic, Cerenovous and Stryker. Dr Vora is a consultant for Medtronic and Microvention. Dr Taqi is a consultant for Rapid Medical, Stryker and Medtronic. Dr Samaniego is a consultant for Rapid Medical, Medtronic and Micorvention. Dr Nossek is a consultant for Rapid Medical. Dr Dabus is a consultant for Microvention, Penumbra, Medtronic, Cerenovous, InNeuroCo. Dr Linfante is a consultant for Medtronic, Stryker, Cerenovous. He is a major shareholder for Three Rivers, Prolong Pharmaceuticals, Prometheus, InNeuroCo. Dr Puri is a consultant for Stryker, Cerenovous, Medtronic, Microvention, Q’Apel, Merit Medical, Arsenal Medical. He has received grant support from SBIR, NIH. Speaker Bureau for Merit Medical, Cerenovous, Q’Apel. Stock options from InNeuroCo, Galaxy Therapeutics, NTI, Agile Medical and Perfuze. Dr Starkman is a consultant for Medtronic, Microvention. He reports stock ownership with Rist Neurovascular and Cerebrotech. He has research support from the NIH. Dr Tateshima is a consultant for Medtronic, Stryker, Cerenovous, Balt, Phenox, Spartan Micro, and Irvine Neurovascular. He has research support from Biomedical Solutions, Inc. He reports educational support from MicroVention. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BGC
- balloon guide catheter
- CT
- computed tomography
- DSMB
- data and safety monitoring board
- IQR
- interquartile range
- IV tPA
- intravenous tissue-type plasminogen activator
- mRS
- modified Rankin Scale
- mTICI
- modified Thrombolysis in Cerebral Ischemia
- NIHSS
- National Institutes of Health Stroke Scale
- PG
- performance goal
- sICH
- symptomatic intracranial hemorrhage
- TIGER
- Treatment With Intent to Generate Endovascular Reperfusion
R. Gupta and J.L. Saver are co-first authors.
This manuscript was sent to Gert Kwakkel, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.034436.
For Sources of Funding and Disclosures, see page 1542.
Presented in part at the International Stroke Conference, virtual, March 17–19, 2021.
Contributor Information
Jeffrey L. Saver, Email: jsaver@mednet.ucla.edu.
Elad Levy, Email: eladlevy@buffalo.edu.
Osama O. Zaidat, Email: OOZaidat@Mercy.com.
Dileep Yavagal, Email: dyavagal@med.miami.edu.
David S. Liebeskind, Email: davidliebeskind@yahoo.com.
Ahmad Khaldi, Email: ahmad.khaldi@wellstar.org.
Bradley Gross, Email: grossb2@upmc.edu.
Michael Lang, Email: langmj3@upmc.edu.
Sandra Narayanan, Email: sandra.narayanan@gmail.com.
Brian Jankowitz, Email: bjankowitz@gmail.com.
Kenneth Snyder, Email: KSnyder@ubns.com.
Adnan Siddiqui, Email: as257@buffalo.edu.
Jason Davies, Email: jdavies@ubns.com.
Eugene Lin, Email: elin@mercy.com.
Ameer Hassan, Email: ameerehassan@gmail.com.
Ricardo Hanel, Email: ricardo.hanel@bmcjax.com.
Amin Aghaebrahim, Email: Amin.Aghaebrahim@bmcjax.com.
Ritesh Kaushal, Email: rit.kau@gmail.com.
Ali Malek, Email: armalekmd@gmail.com.
Nils Mueller-Kronast, Email: muellerkronast@gmail.com.
Robert Starke, Email: RStarke@med.miami.edu.
Hormozd Bozorgchami, Email: bozorgch@ohsu.edu.
Gary Nesbit, Email: nesbitg@ohsu.edu.
Masahiro Horikawa, Email: horikawa@ohsu.edu.
Ryan Priest, Email: priestr@ohsu.edu.
Jesse Liu, Email: liu@ohsu.edu.
Ronald F. Budzik, Email: rbudzik@riversiderad.com.
Peter Pema, Email: PPema@riversiderad.com.
Nirav Vora, Email: Nirav.Vora@ohiohealth.com.
M. Asif Taqi, Email: asiftaqi@icloud.com.
Edgar Samaniego, Email: edgar-samaniego@uiowa.edu.
Qingliang Tony Wang, Email: qwang@maimonidesmed.org.
Erez Nossek, Email: nossek@gmail.com.
Guilherme Dabus, Email: guilhermed@baptisthealth.net.
Italo Linfante, Email: linfante.italo@gmail.com.
Ajit Puri, Email: ajit.puri@umassmemorial.org.
Eitan Abergel, Email: e_abergel@rambam.health.gov.il.
Sidney Starkman, Email: starkman@ucla.edu.
Satoshi Tateshima, Email: STateshima@mednet.ucla.edu.
Ashutosh P. Jadhav, Email: ashu.jadhav@gmail.com.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, et al. ; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, et al. ; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, et al. ; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. ; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. ; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 7.Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, Schellinger PD, Toni D, de Vries J, White P, et al. European Stroke Organisation (ESO)- European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019;11:535–538. doi: 10.1136/neurintsurg-2018-014568 [DOI] [PubMed] [Google Scholar]
- 8.Mokin M, Ansari SA, McTaggart RA, Bulsara KR, Goyal M, Chen M, Fraser JF; Society of NeuroInterventional Surgery. Indications for thrombectomy in acute ischemic stroke from emergent large vessel occlusion (ELVO): report of the SNIS Standards and Guidelines Committee. J Neurointerv Surg. 2019;11:215–220. doi: 10.1136/neurintsurg-2018-014640 [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, et al. ; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 10.Kara B, Selcuk HH, Erbahceci Salik A, Zalov H, Yildiz O, Gul G, Balkan B. Single-center experience with the Tigertriever device for the recanalization of large vessel occlusions in acute ischemic stroke. J Neurointerv Surg. 2019;11:455–459. doi: 10.1136/neurintsurg-2018-014196 [DOI] [PubMed] [Google Scholar]
- 11.Gruber P, Diepers M, von Hessling A, Weber J, Kahles T, Anon J, Berberat J, Nedeltchev K, Liebeskind DS, Remonda L. Mechanical thrombectomy using the new Tigertriever in acute ischemic stroke patients - A Swiss prospective multicenter study. Interv Neuroradiol. 2020;26:598–601. doi: 10.1177/1591019920946499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Will L, Maus V, Maurer C, Weber A, Weber W, Fischer S. Mechanical thrombectomy in acute ischemic stroke using a maximally expandable stent retriever (Tigertriever): preliminary single center experience [published online June 11, 2020]. Clin Neuroradiol. doi: 10.1007/s00062-020-00919-w [DOI] [PubMed] [Google Scholar]
- 13.Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, Liebeskind DS, Smith WS; TREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO; SWIFT Trialists. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1 [DOI] [PubMed] [Google Scholar]
- 15.Goyal M, Fargen KM, Turk AS, Mocco J, Liebeskind DS, Frei D, Demchuk AM. 2C or not 2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J Neurointerv Surg. 2014;6:83–86. doi: 10.1136/neurintsurg-2013-010665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, Treurniet KM, Majoie CB, Marquering HA, Mazya MV, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 17.Feng YS, Kohlmann T, Janssen MF, Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30:647–673. doi: 10.1007/s11136-020-02688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holman R, Weisscher N, Glas CA, Dijkgraaf MG, Vermeulen M, de Haan RJ, Lindeboom R. The Academic Medical Center Linear Disability Score (ALDS) item bank: item response theory analysis in a mixed patient population. Health Qual Life Outcomes. 2005;3:83. doi: 10.1186/1477-7525-3-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisscher N, Vermeulen M, Roos YB, de Haan RJ. What should be defined as good outcome in stroke trials; a modified Rankin score of 0-1 or 0-2? J Neurol. 2008;255:867–874. doi: 10.1007/s00415-008-0796-8 [DOI] [PubMed] [Google Scholar]
- 20.Zaidat OO, Bozorgchami H, Ribó M, Saver JL, Mattle HP, Chapot R, Narata AP, Francois O, Jadhav AP, Grossberg JA, et al. Primary Results of the Multicenter ARISE II Study (Analysis of Revascularization in Ischemic Stroke With EmboTrap). Stroke. 2018;49:1107–1115. doi: 10.1161/STROKEAHA.117.020125 [DOI] [PubMed] [Google Scholar]
- 21.Noguiera RG, Mohammaden MH, Haussen DC, Budzik RF, Gupta R, Krajina A, English JD, Malek AR, Sarraj A, Narata AP, et al. Endovascular therapy in the distal neurovascular territory: results of a large prospective registry [published online December 15, 2020]. J Neurointerv Surg. doi: 10.1136/neurintsurg-2020-016851 [DOI] [PubMed] [Google Scholar]
- 22.Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, Mueller-Kronast N, English JD, Dabus G, Malisch TW, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke. 2018;49:660–666. doi: 10.1161/STROKEAHA.117.020315 [DOI] [PubMed] [Google Scholar]
- 23.Zaidat OO, Castonguay AC, Gupta R, Sun CJ, Martin C, Holloway WE, Mueller-Kronast N, English JD, Linfante I, Dabus G, et al. North American Solitaire Stent Retriever Acute Stroke registry: post-marketing revascularization and clinical outcome results. J Neurointerv Surg. 2018;10(suppl 1):i45–i49. doi: 10.1136/neurintsurg-2013-010895.rep [DOI] [PubMed] [Google Scholar]
- 24.Zaidat OO, Castonguay AC, Nogueira RG, Haussen DC, English JD, Satti SR, Chen J, Farid H, Borders C, Veznedaroglu E, et al. TREVO stent-retriever mechanical thrombectomy for acute ischemic stroke secondary to large vessel occlusion registry. J Neurointerv Surg. 2018;10:516–524. doi: 10.1136/neurintsurg-2017-013328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel RD, Saver JL. Evolution of reperfusion therapies for acute brain and acute myocardial ischemia: a systematic, comparative analysis. Stroke. 2013;44:94–98. doi: 10.1161/STROKEAHA.112.666925 [DOI] [PubMed] [Google Scholar]
- 26.Sun CH, Bhatt DL, Nogueira RG, Gupta R. Endovascular therapy for stroke: getting to the “heart” of the matter. Circulation. 2014;129:1152–1160. doi: 10.1161/CIRCULATIONAHA.113.003703 [DOI] [PubMed] [Google Scholar]
- 27.Jahan R, Saver JL, Schwamm LH, Fonarow GC, Liang L, Matsouaka RA, Xian Y, Holmes DN, Peterson ED, Yavagal D, et al. Association between time to treatment with endovascular reperfusion therapy and outcomes in patients with acute ischemic stroke treated in clinical practice. JAMA. 2019;322:252–263. doi: 10.1001/jama.2019.8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turk AS, 3rd, Siddiqui A, Fifi JT, De Leacy RA, Fiorella DJ, Gu E, Levy EI, Snyder KV, Hanel RA, Aghaebrahim A, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet. 2019;393:998–1008. doi: 10.1016/S0140-6736(19)30297-1 [DOI] [PubMed] [Google Scholar]
- 29.Nogueira RG, Haussen DC, Castonguay A, Rebello LC, Abraham M, Puri A, Alshekhlee A, Majjhoo A, Farid H, Finch I, et al. Site Experience and outcomes in the trevo acute ischemic stroke (TRACK) Multicenter Registry. Stroke. 2019;50:2455–2460. doi: 10.1161/STROKEAHA.118.024639 [DOI] [PubMed] [Google Scholar]
- 30.Zaidat OO, Mueller-Kronast NH, Hassan AE, Haussen DC, Jadhav AP, Froehler MT, Jahan R, Ali Aziz-Sultan M, Klucznik RP, Saver JL, et al. ; STRATIS Investigators. Impact of balloon guide catheter use on clinical and angiographic outcomes in the STRATIS Stroke Thrombectomy Registry. Stroke. 2019;50:697–704. doi: 10.1161/STROKEAHA.118.021126 [DOI] [PubMed] [Google Scholar]
- 31.Schreuders J, van den Berg LA, Fransen PS, Berkhemer OA, Beumer D, Lingsma HF, van Oostenbrugge RJ, van Zwam WH, Majoie CB, van der Lugt A, et al. ; MR CLEAN investigators. Quality of life after intra-arterial treatment for acute ischemic stroke in the MR CLEAN trial-Update. Int J Stroke. 2017;12:708–712. doi: 10.1177/1747493017706244 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


