Supplemental Digital Content is available in the text.
Keywords: cerebrovascular disorders, intracranial hemorrhages, neuroimaging, stroke, venous thrombosis
Abstract
Background and Purpose:
Stroke is reported as a consequence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in several reports. However, data are sparse regarding the details of these patients in a multinational and large scale.
Methods:
We conducted a multinational observational study on features of consecutive acute ischemic stroke, intracranial hemorrhage, and cerebral venous or sinus thrombosis among SARS-CoV-2–infected patients. We further investigated the risk of large vessel occlusion, stroke severity as measured by the National Institutes of Health Stroke Scale, and stroke subtype as measured by the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria among patients with acute ischemic stroke. In addition, we explored the neuroimaging findings, features of patients who were asymptomatic for SARS-CoV-2 infection at stroke onset, and the impact of geographic regions and countries’ health expenditure on outcomes.
Results:
Among the 136 tertiary centers of 32 countries who participated in this study, 71 centers from 17 countries had at least 1 eligible stroke patient. Of 432 patients included, 323 (74.8%) had acute ischemic stroke, 91 (21.1%) intracranial hemorrhage, and 18 (4.2%) cerebral venous or sinus thrombosis. A total of 183 (42.4%) patients were women, 104 (24.1%) patients were <55 years of age, and 105 (24.4%) patients had no identifiable vascular risk factors. Among acute ischemic stroke patients, 44.5% (126 of 283 patients) had large vessel occlusion; 10% had small artery occlusion according to the TOAST criteria. We observed a lower median National Institutes of Health Stroke Scale (8 [3–17] versus 11 [5–17]; P=0.02) and higher rate of mechanical thrombectomy (12.4% versus 2%; P<0.001) in countries with middle-to-high health expenditure when compared with countries with lower health expenditure. Among 380 patients who had known interval onset of the SARS-CoV-2 and stroke, 144 (37.8%) were asymptomatic at the time of admission for SARS-CoV-2 infection.
Conclusions:
We observed a considerably higher rate of large vessel occlusions, a much lower rate of small vessel occlusion and lacunar infarction, and a considerable number of young stroke when compared with the population studies before the pandemic. The rate of mechanical thrombectomy was significantly lower in countries with lower health expenditures.
Since the emergence of the coronavirus disease 2019 (COVID-19) pandemic, several cases of cerebrovascular events were reported among patients with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).1 Studies presented the incidence and prevalence of acute ischemic stroke (AIS), intracranial hemorrhage (ICH), and cerebral venous or sinus thrombosis (CVST) in SARS-CoV-2–infected patients.2–6
Many studies proposed coagulopathy as the underlying pathophysiological mechanism for the cerebrovascular events.7,8 Small case series demonstrated a higher proportion of large vessel occlusions (LVOs),2,9 or cryptogenic strokes,4,10 with elevated d-dimer level, liver enzymes, and inflammatory or renal failure biomarkers among the patients who experienced SARS-CoV-2 infection.4,5 Additionally, most of the studies noted a higher severity and mortality rate among stroke patients diagnosed with SARS-CoV-2 compared with others.4,11,12
To present a more comprehensive overview of stroke among patients infected with SARS-CoV-2, we devised a multinational multiple-phase study. In the first phase, we estimated the risk of stroke among the SARS-CoV-2–infected hospitalized patients. 13 In the current study, we aimed to present more details on the features and characteristics of our expanded multinational stroke cohort with prior SARS-CoV-2 infection. We further investigated the risk of LVO, stroke severity as measured by the National Institutes of Health Stroke Scale (NIHSS), and stroke subtype as measured by the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria in the entire cohort, as well as different geographic regions.
Methods
Study Design
The details of the study design are available in Document I in the Data Supplement. The authors declare that all supporting data are available within the article. Additional data that support the findings of this study are available from the corresponding author upon reasonable request. This multicenter, multinational prospective and observational study was conducted and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology,14 and Enhancing the Quality and Transparency of Health Research guidelines.15 The study protocol was designed by the investigators at the Neuroscience Institute of Geisinger Health System, Pennsylvania, and received approval by the Institutional Review Board of Geisinger Health System and other participating institutions. Investigators from 6 continents including North America (Canada, 6 states of the United States, and Mexico), South America (Brazil), Europe (Belgium, Croatia, Czech Republic, Finland, France, Germany, Greece, Ireland, Italy, Norway, Portugal, Spain, Sweden, and Switzerland), Asia (India, Iran, Iraq, Israel, Lebanon, Singapore, South Korea, Turkey, and the United Arab Emirates), Oceania (Australia and New Zealand), and Africa (Egypt, Nigeria, and Uganda) responded to our invitation. The centers were included by nonprobability sampling, and data were included until June 10, 2020.
Participants
We included consecutive SARS-CoV-2–infected adult patients who had imaging-confirmed subsequent stroke16—AIS, intracerebral hemorrhage, subarachnoid hemorrhage (SAH), and CVST. The preferred diagnostic criteria for SARS-CoV-2 were defined according to the World Health Organization interim guidance.17 Ischemic or hemorrhagic strokes were defined in the presence of a rapid onset of a neurological deficit with evidence of acute ischemic or hemorrhagic lesions on computed tomography or magnetic resonance imaging. Patients who had transient stroke-like symptoms (transient ischemic attack) without acute lesions on computed tomography or magnetic resonance imaging were not included in this study due to the high diagnostic error. 18–20
The inclusion of the stroke patients was based on the confirmed prior infection by SARS-CoV-2 (and not symptom presentation). Patients who initially presented to the hospital with stroke-related chief complaints and asymptomatic SARS-CoV-2 infection were detected by admission tests, those who had a stroke while being hospitalized for SARS-CoV-2 infection, or patients with stroke-related admission who had confirmed prior diagnosis of SARS-CoV-2 were included in this study.
Data Element and Processing
Collaborators were asked to provide data according to a core protocol. The age, sex, vascular risk factors and comorbidities (ie, hypertension, diabetes, ischemic heart disease, atrial fibrillation, carotid stenosis, chronic kidney disease, cardiac ejection fraction <40%, active neoplasms, rheumatological diseases, smoking status, and history of transient ischemic attack or stroke), and laboratory findings (ie, the count for white blood cells, neutrophils, lymphocytes, and platelets, C-reactive protein, blood urea nitrogen, creatinine, alanine transaminase, aspartate transaminase, lactic acid dehydrogenase, fibrinogen, and D-dimer) were requested for the patients with stroke. We also obtained additional data including the onset of the stroke and SARS-CoV-2 infection diagnosis, the initiation of mechanical ventilation (if applicable), length of hospital stays, and patient disposition—defined as still in the hospital, in-hospital death, being discharged to home, acute rehabilitation service, or nursing home. The details of neurological symptoms and investigations, imaging-based localization of the lesion(s), use of antiplatelets or anticoagulants before the stroke, the NIHSS, the ICH score, administration of intravenous thrombolysis (IVT), and mechanical thrombectomy were also requested. The severity of the stroke according to NIHSS was defined as no stroke symptoms (NIHSS score, 0), minor (NIHSS score, 1–4), moderate (NIHSS score, 5–15), moderate-to-severe (NIHSS score, 16–20), and severe stroke (NIHSS score, 21–42).21 The TOAST criteria were defined as large artery atherosclerosis, cardioembolism, small artery occlusion, other determined etiology, and undetermined etiology.22In addition, the lesions on diffusion-weighted imaging or computed tomography images were categorized as lacunar,23 embolic/large vessel atherothromboembolism,24,25 vasculitis pattern,26 or other phenotypes (borderzone or equivocal lesions). In this study, the AISs due to LVOs are referred as occlusion of the internal carotid artery, middle cerebral artery at M1 and M2, anterior cerebral artery at A1, posterior cerebral artery at P1, intracranial vertebral artery, or basilar artery.27 Brain imaging findings were evaluated by local radiologists with expertise in neuroimaging. To determine the interval between the infection and stroke, the onset of SARS-CoV-2 was considered as either the symptom onset or the day of taking the sample with a positive result, whichever was first. The interval was considered as zero if the infection was diagnosed at the same visit as the onset of stroke. Countries were considered as either low or middle and high health expenditure based on the World Health Organization reports.28 The countries’ annual health expenditure of above US $1000 per capita (2015–2017; Table I in the Data Supplement) and total health expenditure of above US $10 000 per capita (2010–2017; Figure I in the Data Supplement) were considered as the cutoff.
Outcome Measures
The primary outcome measures in this study were the presence versus absence of LVO, stroke severity as measured by NIHSS, and stroke subtype as measured by the TOAST criteria among the AIS patients.
We further compared the groups of the patients with AIS or intraparenchymal hemorrhage (IPH) according to their age (younger versus older than 55 years and younger versus older than 65 years),29 sex, geographic regions (America, Europe, Asia, and the Middle East), countries’ health expenditure (low versus middle and high income), imaging findings, and the interval of stroke onset and infection diagnosis (same day versus others). We did not analyze the disposition and length of stay as outcome measures since many patients were still in the acute phase or admitted in long-term acute care hospitals at the closure of our study.
Statistical Analyses and Modeling
We used descriptive statistics to summarize the data. Demographic data, comorbidities, laboratory findings, and neurological investigations were reported as medians and interquartile range, mean and SDs, and under stratified categories when possible. The equality of the variances was assessed by Leven test. Categorical variables were reported as absolute frequencies and valid percentages. The comparisons between categorical variables were conducted with the Pearson χ2 test, while the differences among continuous variables were assessed by independent t test and ANOVA. A post hoc z test on the adjusted residuals and Cramér phi, Tukey, or Dunnett tests were used to demonstrate the degree and direction of the associations in comparison of means, while post hoc comparison of medians was conducted by the Dunn-Bonferroni approach to compare subgroups. All tests were performed using IBM SPSS Statistics, version 26,30 and P<0.05 was considered statistically significant. Bonferroni correction was used for adjusting all P values in multiple comparisons.
Results
Collaborators from 136 tertiary centers of 32 countries participated in this prospective study. Among them, 71 centers from 17 countries had at least 1 stroke patient eligible included in this study. One center in the Middle East could not provide data by the deadline. The rest of the centers did not have stroke patients who met our inclusion criteria (Document I in the Data Supplement). We received data on 432 patients—America: 114 (26.4%), Europe: 82 (19.0%), Middle East: 228 (52.8%), and Asia: 8 (1.9%). Among them, 203 (47.0%) patients were from countries with middle-to-high health expenditure. The mean age for the entire cohort was 65.7±15.7 years. Of 432 patients, the majority were men—249 (57.6%), P<0.001. A total of 144 (37.8%) of 380 patients with a known interval of stroke and infection presented to the hospital with chief complaints of stroke-related symptoms, with asymptomatic or SARS-CoV-2 infection. Among the 430 patients with complete comorbidity profiles, 105 (24.4%) patients had no identifiable vascular risk factors at the time of stroke incidence. Demographic characteristics under each stroke subtype are presented in Table 1.
Table 1.
The Baseline Characteristics, Comorbidities, and Laboratory Findings Among SARS-CoV-2 Infected Patients With Stroke
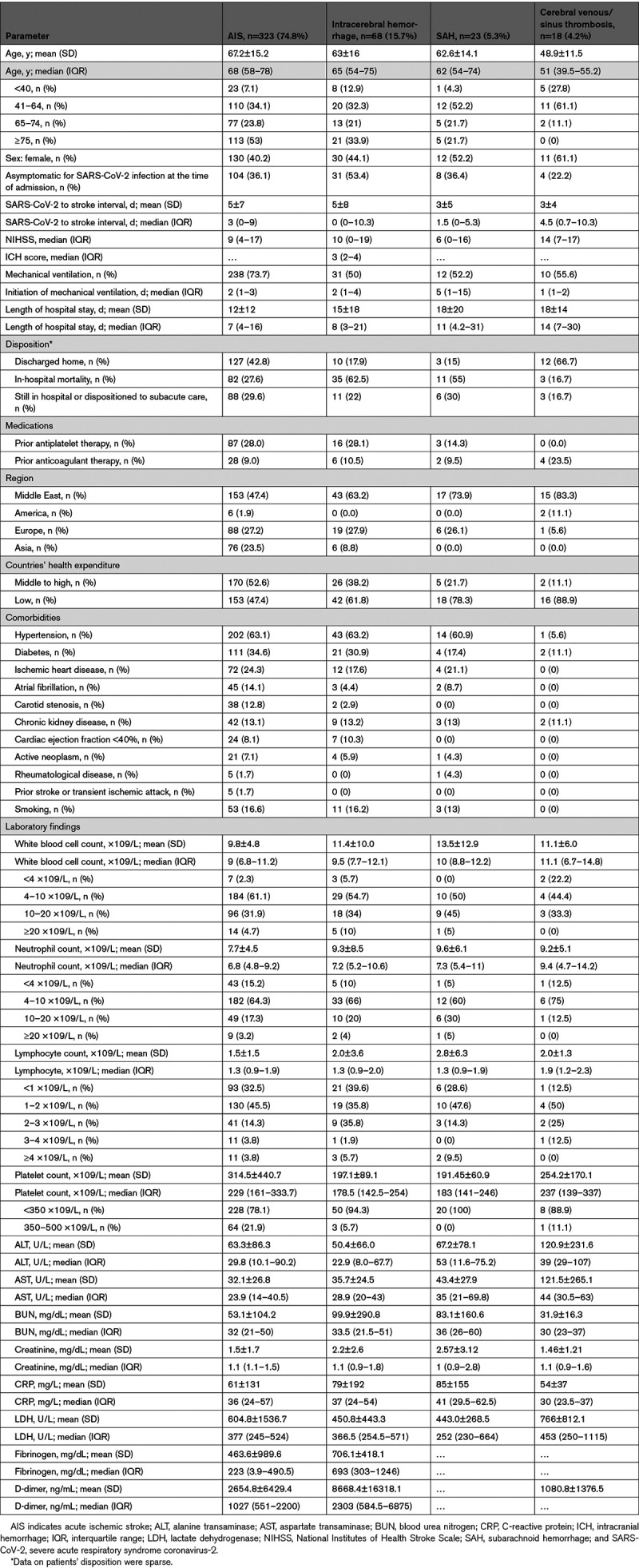
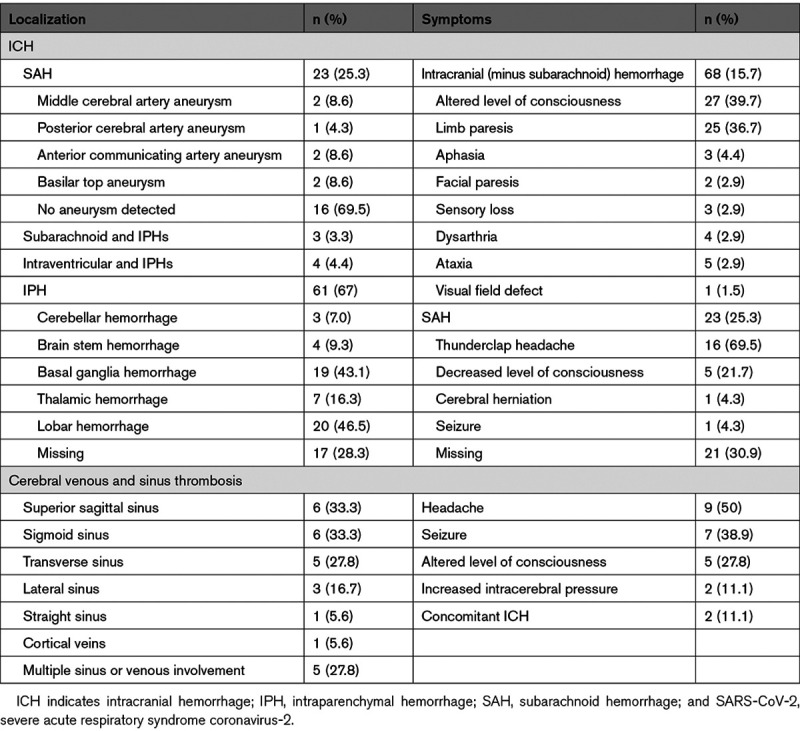
Overall, 323 (74.8%) patients had AIS, 91 (21.1%) ICH, and 18 (4.2%) CVST. Among the patients with ICH (Table 2), 3 (3.3%) had simultaneous SAH and IPH without any evidence of aneurysm, and 4 (4.4%) were presented with simultaneous intraventricular hemorrhage and IPH. Isolated SAH occurred in 23 (25.3%) and isolated IPH in 61 (67%) of the patients with hemorrhagic stroke. Among 23 patients with isolated SAH, 16 (69.5%) had no evidence of aneurysm. Among the 18 patients with CVST, 5 (27.8%) had multiple cerebral sinuses and veins involvements.
Table 2.
Localization and Presenting Symptoms Regarding the SARS-CoV-2 Infected Patients With ICH and Cerebral Sinus and Venous Thrombosis
The distribution of AIS subtypes according to the TOAST classification was the following: large artery atherosclerosis (33%), cardioembolism (27%), small vessel occlusion (SVO; 10%), other determined etiology (8%), and undetermined etiology (22%; Table 3). The subgroups of the patients according to the TOAST classification were different in terms of age, sex, the prevalence of LVO, imaging patterns, and need for mechanical ventilation. We observed a lower median of D-dimer among patients with large artery atherosclerosis compared with those with cardioembolic strokes (486.5 [371.5–1422.5] versus 1100.0 [955.0–2355.0] ng/mL; P=0.04). There were no significant differences in terms of lactate dehydrogenase or fibrinogen among the TOAST subgroups.
Table 3.
Neurological Findings Among Patients With Acute Ischemic Stroke
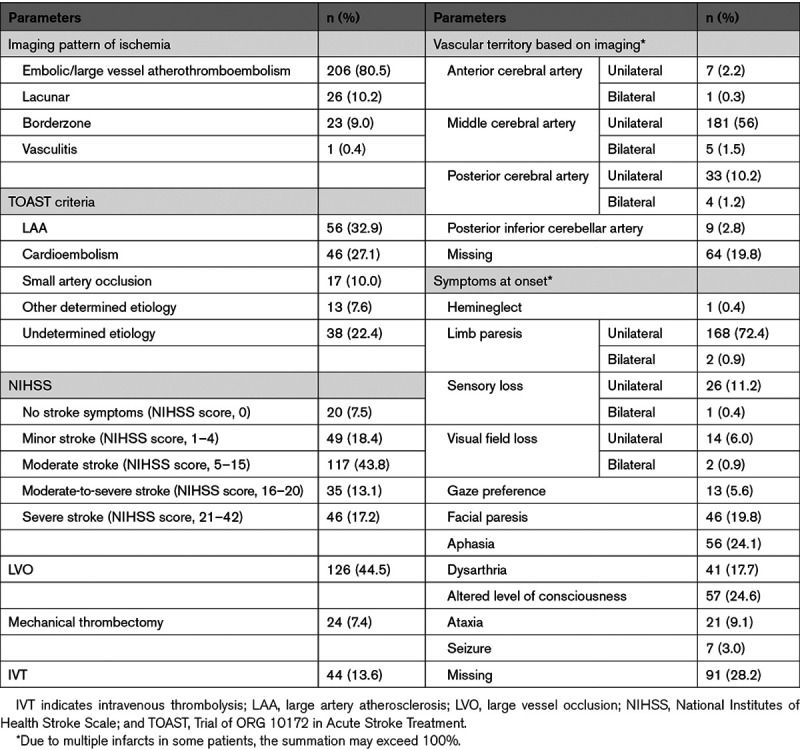
Of 283 AIS patients with confirmed data on the site of vascular occlusion, 126 (44.5%) had LVO (Table 4). In comparison with those without LVO, patients with LVO had higher prevalence of large artery atherosclerosis based on TOAST criteria (58.2% versus 9.6%), higher embolic/large vessel atherothromboembolism (88.4% versus 73.3%), and lower lacunar pattern (1.8% versus 17%) on imaging. Patients with LVO also had higher rates of IVT (22.2% versus 8.9%) and mechanical thrombectomy (19% versus 0%).
Table 4.
Baseline Characteristics and Neuroimaging Findings Under Each Outcome Measures
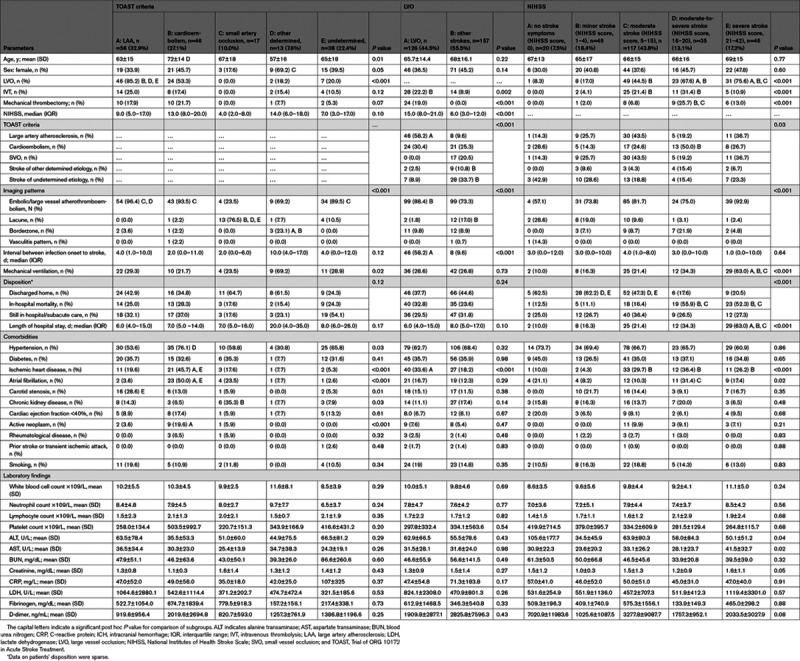
The median of NIHSS among AIS patients was 9 (4–17; Table 1). The risk of LVO increased from 8.3% among patients with no stroke symptoms (NIHSS score, 0) to 75.6% among patients with severe stroke (NIHSS score, >21; Table 4). IVT (31.4%) and mechanical thrombectomy (25.4%) were more prevalent among patients with moderate-to-severe stroke (NIHSS score, 16–20). The need for mechanical ventilation increased from 10% among patients with no stroke symptoms to 63% among patients with severe stroke.
We observed similar rates of LVOs and IVT in various geographic regions (Tables II and III in the Data Supplement). However, the rate of mechanical thrombectomy was significantly lower in the Middle Eastern countries and countries with lower health expenditure—2.6% in the Middle East versus 21.1% in Europe (P<0.001) and 2% in countries with lower health expenditure versus 12.4% in countries with higher health expenditure (P<0.001). We also detected a higher NIHSS in countries with lower health expenditure (11.0 [5.0–17.0] versus 8.0 [3.0–17.0]; P=0.02). Similarly, when comparing different regions, patients in America and Europe had a lower NIHSS than those in the Middle East (7.0 [0.0–16.0] in America and 8.0 [4.0–18.0] in Europe versus 12.0 [6.0–17.0] in the Middle East; P=0.06).
AIS patients were grouped based on sex and age (Tables IV through VI in the Data Supplement). Of 323 patients with AIS, 59.8% were men, and 36.2% were <55 years of age. Women had a lower rate of smoking and chronic kidney disease and higher NIHSS. Patients above 55 years had a higher proportion of hypertension, atrial fibrillation, ischemic heart disease, and carotid stenosis. Patients >65 years of age also had a higher rate of cardiac ejection fraction of <40%. Patients who were asymptomatic for SARS-CoV-2 infection at the stroke onset had a higher in-hospital mortality and a higher median of D-dimer (Table VII in the Data Supplement). The subgroups of AIS patients based on neuroimaging findings were different in terms of the proportion of LVO, TOAST criteria, and NIHSS categories (Table VIII in the Data Supplement).
Discussion
To our knowledge, this is to date the largest study that comprehensively presents the characteristics and stroke subtypes of stroke in SARS-CoV-2–infected patients at a multinational level. The results of our work indicated a relatively high number of young AIS patients, male predominance, asymptomatic SARS-CoV-2 infection in more than one-third of the AIS patients, a higher proportion of LVO strokes, and a low rate of small artery occlusion and lacunar infarcts. We also noted significant differences regarding the TOAST criteria in both regional and health expenditure subgroups, as well as higher NIHSS among countries with lower health expenditure.
Regarding the characteristics of patients with AIS, 44.5% (126 of 283 patients) had LVOs, without any age or sex predominance. This rate is comparable to a similar report from New York on stroke patients with SARS-CoV-2 infection.31 In general, LVOs accounted for 24% to 46% of AIS worldwide.32 If the definition of the LVOs is limited to internal carotid artery, middle cerebral artery (M1 and M2), anterior cerebral artery (A1), posterior cerebral artery (P1), intracranial vertebral artery, and basilar artery, similar to our study, the risk of LVO drops to 24% to 38%.33,34 Considering this definition, we observed a considerably higher rate of LVOs among our patients. In our study, 13.6% of AIS patients received IVT and 7.4% underwent mechanical thrombectomy. These rates are similar to the multinational study on 174 AIS SARS-CoV-2–infected patients (12.7% IVT, 6.9% IVT and thrombectomy, and 5.2% mechanical thrombectomy).35
Based on the TOAST classification, large artery atherosclerosis accounts for 33% of strokes in our study, which is higher than reports from worldwide population-based studies (19%–23%).36,37 SVO accounted for stroke etiology in 10% of our patients, and analysis of neuroimaging patterns showed 10.2% lacunar infarcts. These rates are lower than worldwide population-based studies—21% to 44% SVO37,38 and 21% to 30% lacunar infarcts.39,40 In Europe, SVO was present among 4.1% of patients in this study versus 12% to 31% of stroke patients in previous population studies.41–47 Lacunar infarcts were detected in 9.3% of our AIS patients versus 14% to 31% of other population studies.43,48–50 In the North and South America, these rates were 5.3% versus 15% to 18%37,51 for SVO, and 9.1% versus 13% to 18% for lacunar infarcts.37,40 Similarly, in the Middle Eastern countries, we observed 18.3% SVO versus 20% to 25% in previous reports52–55 and 11.3% lacunar infarcts versus 19% to 26% in the previous population studies.55–57
Higher rates of LVO and large artery atherosclerosis strokes and lower rates of SVO and lacunar infarcts among patients in our study may present a predilection of the virus for inducing a certain type of stroke. Similar to our findings, other reports on SARS-CoV-2–infected stroke patients suggested a lower rate of SVO and lacunar infarct and a higher risk of LVO strokes among infected patients with SARS-CoV-2.58–60 However, lacunar infarctions and SVO are more likely to produce milder deficits.39,61–63 During the COVID-19 pandemic, patients with mild-to-moderate stroke symptoms were less likely to present at medical centers.4,59 In addition, less severe stroke symptoms, mostly in critically ill patients or overwhelmed health centers, were more likely to be underdiagnosed. Our observation of a higher median NIHSS in countries with lower health expenditure and those in Middle Eastern countries may reflect a lower capacity of these centers for the diagnosis of mild stroke patients in the pandemic. It may also indicate that patients with mild stroke symptoms refused to present to the hospitals. In addition, we realized similar rates of LVOs and IVT in various geographic regions but a considerably lower rate of thrombectomy in countries with lower health expenditures. This observation may highlight a care disparity among countries. Future studies such as CASCADE64 (Call to Action: SARS-CoV-2 and Cerebrovascular Disorders) are required to shed light on changes in stroke care protocols and hospitalization rate during the pandemic and compare it to the available infrastructure in each region.
Of notice, our study results showed a considerable number of young strokes. Although the definition of young stroke is debatable, the majority of the studies considered 50 or 55 years as the cutoff.65 We realized that 36% of the AIS patients in our study were <55 years of age and 46% were <65 years of age (Tables V and VI in the Data Supplement). These proportions are considerably higher than the population-based reports before the pandemic (12.9%–20.7%).66,67 The median age of AIS patients in our study was 68 (58–78) years. A case series from New York on 32 AIS patients with SARS-CoV-2 showed a median of 63 years for these patients. This finding was significantly lower than AIS patients without SARS-CoV-2 in the same study and same interval (median, 70 years) or the historical cohort of AIS patients presented to the same center in 2019 (median, 68.5 years).31 A multinational study on 174 AIS patients with SARS-CoV-2 infection reported a median age of 71 years.35
Regarding cerebral venous sinus thrombosis and ICH, we had 18 stroke patients with CVST; the average age of patients was 49 years, 78% were <55 years of age, and >60% were women. Classically CVST is considered to occur in young adults, with the predilection of women.68 However, the sex ratio varies widely—44.7% to 83.4% in women.69 A systematic review on the sex ratio of 23 638 patients with CVST demonstrated an increasing trend among women (54.8% before 1981 to 69.8% after 2001), likely due to increased use of oral contraceptives.70 Even though CVST patients in our study were younger than patients with other stroke subtypes, they were older than previously reported CVST patients without SARS-CoV-2 infection.69–74 In addition, only 27.8% of the patients in our study had multiple sinus or venous involvement, which is considerably lower than previous reports in non–SARS-CoV-2–infected patients.69,73,75 One reason might be the severe condition of the patients with multiple CVSTs that prevent the proper diagnosis of these patients.
Our study reported 91 patients with ICH. Among the patients with SAH (23), no aneurysm was detected in 69.5% of patients, which is higher than the reported 15% (5%–34%) spontaneous SAH among patients without SARS-CoV-2 infection.76 We observed that 27.9% of IPH patients had no vascular risk factors or comorbidities. These patients had higher ICH score and younger age in comparison with other patients with IPH.
This work has several limitations. Despite that we included centers from multiple countries and presented a comprehensive panel of patients’ characteristics, some of the specific laboratory parameters related to rare stroke causes (eg, antiphospholipid antibodies) were not included in this study. The collaborators tried to identify SARS-CoV-2 patients who presented with stroke as the first and only symptom, but the difficulty in measuring all symptoms related to COVID-19 (such as fatigue, anosmia, and ageusia) should be taken into consideration. In addition, not all the stroke patients in this study had a final disposition outcome, which limited our conclusion about in-hospital mortality. Although attempts were made to minimize the selection bias by including patients from different ethnicities, ecological conditions, and health care systems, this study may suffer from selection bias and low power in some subgroups. The authors attempt to assess the quality of data by Risk of Bias in Exposure Studies tool13; however, heterogeneity may exist among data obtained from multiple settings and multiple countries. Further studies that include a control population are warranted.
Conclusions
In conclusion, we observed a considerably higher rate of LVOs and a much lower rate of SVO and lacunar infarction when compared with the prior population studies. We also observed a relatively high number of young stroke and a high number of asymptomatic SARS-CoV-2 patients at stroke onset. The rate of mechanical thrombectomy was significantly lower in countries with lower health expenditures.
Acknowledgments
We appreciate the efforts of all other health care providers and administrators who contributed data for this study.
Sources of Funding
None.
Disclosures
The coauthors report the following supports outside the current study: Dr Punter reports personal fees from Alexion Pharmaceuticals. Dr Arenillas reports personal fees from Boehringer Ingelheim, Pfizer, Bayer, Amgen, Daiichi-Sankyo, and Medtronic. Dr Aguiar de Sousa reports nonfinancial support from Boehringer Ingelheim. Dr Bonati reports grants from Swiss National Science Foundation, Swiss Heart Foundation, Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung sowie der medizinischen Bildauswertung, and AstraZeneca and personal fees from Claret Medical and InnovHeart. Dr Cereda reports other supports from iSchemaView and Bayer. Dr De Marchis reports other supports from Bayer and Pfizer. Dr Gonçalves reports grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil. Dr Leker reports other supports from BI, Abbott, and iSchemaView. The other authors report no conflicts.
Supplemental Materials
Online Document I
Online Figure I
Online Tables I–VIII
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AIS
- acute ischemic stroke
- CASCADE
- Call to Action: SARS-CoV-2 and Cerebrovascular Disorders
- COVID-19
- coronavirus disease 2019
- CVST
- cerebral venous or sinus thrombosis
- ICH
- intracranial hemorrhage
- IPH
- intraparenchymal hemorrhage
- IVT
- intravenous thrombolysis
- LVO
- large vessel occlusion
- NIHSS
- National Institutes of Health Stroke Scale
- SAH
- subarachnoid hemorrhage
- SVO
- small-vessel occlusion
- TOAST
- Trial of ORG 10172 in Acute Stroke Treatment
This manuscript was sent to Marc Fisher, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.032927.
Preprint posted on medRxiv. doi: https://doi.org/10.1101/2020.08.05.20169169.
For Sources of Funding and Disclosures, see page e128.
The podcast and transcript are available at https://www.ahajournals.org/str/podcast.
Contributor Information
Shima Shahjouei, Email: sshimashah@gmail.com.
Georgios Tsivgoulis, Email: tsivgoulisgiorg@yahoo.gr.
Ghasem Farahmand, Email: ghasem.farahmand89@gmail.com.
Eric Koza, Email: EKoza@som.geisinger.edu.
Ashkan Mowla, Email: mowla@usc.edu.
Alireza Vafaei Sadr, Email: vafaei.sadr@gmail.com.
Arash Kia, Email: arash.kia@mssm.edu.
Alaleh Vaghefi Far, Email: alalehvaghefifar@gmail.com.
Stefania Mondello, Email: stm_mondello@hotmail.com.
Achille Cernigliaro, Email: achille.cernigliaro@regione.sicilia.it.
Annemarei Ranta, Email: anna.ranta@otago.ac.nz.
Martin Punter, Email: Martin.Punter@ccdhb.org.nz.
Faezeh Khodadadi, Email: selmakhodadadi@gmail.com.
Soheil Naderi, Email: soheilnaaderi@gmail.com.
Mirna Sabra, Email: myrnasabra@hotmail.com.
Mahtab Ramezani, Email: drramezani23@gmail.com.
Ali Amini Harandi, Email: noorian@gmail.com.
Oluwaseyi Olulana, Email: OOlulana@som.geisinger.edu.
Durgesh Chaudhary, Email: dpchaudhary@geisinger.edu.
Aicha Lyoubi, Email: Aicha.Lyoubi@ch-stdenis.fr.
Bruce C.V. Campbell, Email: bruce.campbell@mh.org.au.
Juan F. Arenillas, Email: juanfarenillas@gmail.com.
Daniel Bock, Email: daniel.bock@klinikumfrankfurt.de.
Joan Montaner, Email: joan.montaner@vhir.org.
Saeideh Aghayari Sheikh Neshin, Email: S.Salehizadeh@yahoo.com.
Diana Aguiar de Sousa, Email: dianasousa@campus.ul.pt.
Matthew S. Tenser, Email: Matthew.tenser@med.usc.edu.
Ana Aires, Email: ana.aires.mail@gmail.com.
Orkhan Alizada, Email: alizadaorhan@gmail.com.
Elsa Azevedo, Email: eazevedo@med.up.pt.
Nitin Goyal, Email: ngoyal@uthsc.edu.
Zabihollah Babaeepour, Email: dr.zabih.bp@gmail.com.
Gelareh Banihashemi, Email: gelarehbaniha@gmail.com.
Leo H. Bonati, Email: leo.bonati@usb.ch.
Carlo W. Cereda, Email: carlo.cereda@eoc.ch.
Jason J. Chang, Email: jjwchang@hotmail.com.
Miljenko Crnjakovic, Email: miljacdr@gmail.com.
Gian Marco De Marchis, Email: gian.demarchis@usb.ch.
Massimo Del Sette, Email: massimo.del.sette@galliera.it.
Seyed Amir Ebrahimzadeh, Email: dr.sajedy@gmail.com.
Mehdi Farhoudi, Email: farhoudi_m@yahoo.com.
Ilaria Gandoglia, Email: ilaria.gandoglia@galliera.it.
Bruno Gonçalves, Email: brunogs@gmail.com.
Christoph J. Griessenauer, Email: christoph.griessenauer@gmail.com.
Mehmet Murat Hanci, Email: murath@istanbul.edu.tr.
Aristeidis H. Katsanos, Email: ar.katsanos@gmail.com.
Christos Krogias, Email: christos.krogias@ruhr-uni-bochum.de.
Ronen R. Leker, Email: leker@hadassah.org.il.
Lev Lotman, Email: lotmanl@amc.edu.
Jeffrey Mai, Email: Jeffreymai@gmail.com.
Shailesh Male, Email: smale@umn.edu.
Konark Malhotra, Email: konark.malhotra@yahoo.com.
Branko Malojcic, Email: bmalojcic@gmail.com.
Teresa Mesquita, Email: tmesquita@chlo.min-saude.pt.
Asadollah Mir Ghasemi, Email: asadmirghassemi@gmail.com.
Hany Mohamed Aref, Email: haref30@hotmail.com.
Zeinab Mohseni Afshar, Email: zeinabafshar710@gmail.com.
Jusun Moon, Email: moonzoos@naver.com.
Mika Niemelä, Email: mika.niemela@hus.fi.
Behnam Rezai Jahromi, Email: bsabayan@mgh.harvard.edu.
Lawrence Nolan, Email: nolanl@amc.edu.
Abhi Pandhi, Email: drpandhiab@gmail.com.
Jong-Ho Park, Email: neurocraft.jhp@gmail.com.
João Pedro Marto, Email: joao.pedro.seabra.marto@gmail.com.
Francisco Purroy, Email: fpurroygarcia@gmail.com.
Sakineh Ranji-Burachaloo, Email: sranji@sina.tums.ac.ir.
Nuno Reis Carreira, Email: nuno.reiscarreira@gmail.com.
Manuel Requena, Email: m.requenaruiz@gmail.com.
Marta Rubiera, Email: mrubifu@hotmail.com.
Seyed Aidin Sajedi, Email: dr.sajedy@gmail.com.
João Sargento-Freitas, Email: jsargentof@hotmail.com.
Vijay K. Sharma, Email: drvijay@singnet.com.sg.
Thorsten Steiner, Email: thorsten_steiner@med.uni-heidelberg.de.
Kristi Tempro, Email: temprok@amc.edu.
Guillaume Turc, Email: g.turc@ch-sainte-anne.fr.
Yasaman Ahmadzadeh, Email: yassi.ahmdzdh@gmail.com.
Mostafa Almasi-Dooghaee, Email: A_mostafa108@yahoo.com.
Farhad Assarzadegan, Email: assarfarhad@gmail.com.
Arefeh Babazadeh, Email: babazade95.a@gmail.com.
Humain Baharvahdat, Email: humainbv@gmail.com.
Fabricio Buchadid Cardoso, Email: fabriciobuchdid@yahoo.com.br.
Apoorva Dev, Email: apurva.dev22@gmail.com.
Mohammad Ghorbani, Email: harirchm@tums.ac.ir.
Ava Hamidi, Email: hamidiava@gmail.com.
Zeynab Sadat Hasheminejad, Email: z.hasheminejad7097@gmail.com.
Sahar Hojjat-Anasri Komachali, Email: sahar.ansari2099@yahoo.com.
Fariborz Khorvash, Email: fkhorvash@gmail.com.
Firas Kobeissy, Email: firasko@gmail.com.
Hamidreza Mirkarimi, Email: hamid.mirkarimi@gmail.com.
Elahe Mohammadi-Vosough, Email: mv.elahe@gmail.com.
Debdipto Misra, Email: dmisra@geisinger.edu.
Ali Reza Noorian, Email: noorian@gmail.com.
Peyman Nowrouzi-Sohrabi, Email: peyman.nowroozi2@gmail.com.
Sepideh Paybast, Email: sepideh.paybast@yahoo.com.
Leila Poorsaadat, Email: leilamd@yahoo.com.
Mehrdad Roozbeh, Email: mehrdadroozbeh@gmail.com.
Behnam Sabayan, Email: bsabayan@mgh.harvard.edu.
Saeideh Salehizadeh, Email: S.Salehizadeh@yahoo.com.
Alia Saberi, Email: aliasaberigums@gmail.com.
Mercedeh Sepehrnia, Email: mercede978@yahoo.com.
Fahimeh Vahabizad, Email: fahime.vahabizad@gmail.com.
Thomas Alexandre Yasuda, Email: yasuda85@gmail.com.
Mojdeh Ghabaee, Email: mojdeh.ghabaee@gmail.com.
Nasrin Rahimian, Email: dr.nasrin.rahimian@gmail.com.
Mohammad Hossein Harirchian, Email: harirchm@tums.ac.ir.
Afshin Borhani-Haghighi, Email: neuro.ab@gmail.com.
Mahmoud Reza Azarpazhooh, Email: Reza.azarpazhooh@lhsc.on.ca.
Rohan Arora, Email: Rarora@northwell.edu.
Saeed Ansari, Email: saeedansari81@yahoo.com.
Venkatesh Avula, Email: venkateshavula87@gmail.com.
Jiang Li, Email: jli@geisinger.edu.
Vida Abedi, Email: vabedi@geisinger.edu.
References
- 1.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweid A, Hammoud B, Weinberg JH, Oneissi M, Raz E, Shapiro M, DePrince M, Tjoumakaris S, Gooch MR, Herial NA, et al. Letter: thrombotic neurovascular disease in COVID-19 patients. Neurosurgery. 2020;87:E400–E406. doi: 10.1093/neuros/nyaa254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. SARS2-CoV-2 and stroke in a new york healthcare system. Stroke. 2020;120:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morassi M, Bagatto D, Cobelli M, D’Agostini S, Gigli GL, Bnà C, Vogrig A. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267:2185–2192. doi: 10.1007/s00415-020-09885-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Anna L, Kwan J, Brown Z, Halse O, Jamil S, Kalladka D, Venter M, Banerjee S. Characteristics and clinical course of Covid-19 patients admitted with acute stroke. J Neurol. 2020;267:3161–3165. doi: 10.1007/s00415-020-10012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrios-López JM, Rego-García I, Muñoz Martínez C, Romero-Fábrega JC, Rivero Rodríguez M, Ruiz Giménez JA, Escamilla-Sevilla F, Mínguez-Castellanos A, Fernández Pérez MD. Ischaemic stroke and SARS-CoV-2 infection: a causal or incidental association? Neurologia. 2020;35:295–302. doi: 10.1016/j.nrl.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escalard S, Maïer B, Redjem H, Delvoye F, Hébert S, Smajda S, Ciccio G, Desilles J-P, Mazighi M, Blanc R, et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19. Stroke. 2020;120:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valderrama EV, Humbert K, Lord A, Frontera J, Yaghi S. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke. 2020;51:e124–e127. doi: 10.1161/STROKEAHA.120.030153 [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, Wang D, Mao L, Jin H, Hu B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, Glaser A, Elsayegh D. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahjouei S, Naderi S, Li J, Khan A, Chaudhary D, Farahmand G, Male S, Griessenauer C, Sabra M, Mondello S, et al. Risk of stroke in hospitalized SARS-CoV-2 infected patients: a multinational study. EBioMedicine. 2020;59:102939. doi: 10.1016/j.ebiom.2020.102939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 15.Equator Network. Enhancing the QUAlity and Transparency Of health Research [Internet]. 2019. Univ. Oxford; https://www.equator-network.org [Google Scholar]
- 16.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, et al. ; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. Interim Guid. 2020:1–7. [Google Scholar]
- 18.Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis. 2008;26:630–635. doi: 10.1159/000166839 [DOI] [PubMed] [Google Scholar]
- 19.Sadighi A, Stanciu A, Banciu M, Abedi V, Andary NE, Holland N, Zand R. Rate and associated factors of transient ischemic attack misdiagnosis. eNeurologicalSci. 2019;15:100193. doi: 10.1016/j.ensci.2019.100193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadarajan V, Perry RJ, Johnson J, Werring DJ. Transient ischaemic attacks: mimics and chameleons. Pract Neurol. 2014;14:23–31. doi: 10.1136/practneurol-2013-000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ver Hage A. The NIH stroke scale: a window into neurological status. Com Nurs. Spectr. 2011;24:44–49. [Google Scholar]
- 22.Adams H, Bendixen B, Kappelle L, Biller J, Love B, Gordon D, Marsh E. Classification of subtype of acute ischemic stroke. Stroke. 1993;23:35–41. [DOI] [PubMed] [Google Scholar]
- 23.Potter GM, Marlborough FJ, Wardlaw JM. Wide variation in definition, detection, and description of lacunar lesions on imaging. Stroke. 2011;42:359–366. doi: 10.1161/STROKEAHA.110.594754 [DOI] [PubMed] [Google Scholar]
- 24.Wessels T, Röttger C, Jauss M, Kaps M, Traupe H, Stolz E. Identification of embolic stroke patterns by diffusion-weighted MRI in clinically defined lacunar stroke syndromes. Stroke. 2005;36:757–761. doi: 10.1161/01.STR.0000158908.48022.d7 [DOI] [PubMed] [Google Scholar]
- 25.Bang OY, Ovbiagele B, Liebeskind DS, Restrepo L, Yoon SR, Saver JL. Clinical determinants of infarct pattern subtypes in large vessel atherosclerotic stroke. J Neurol. 2009;256:591–599. doi: 10.1007/s00415-009-0125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel Razek AA, Alvarez H, Bagg S, Refaat S, Castillo M. Imaging spectrum of CNS vasculitis. Radiographics. 2014;34:873–894. doi: 10.1148/rg.344135028 [DOI] [PubMed] [Google Scholar]
- 27.Waqas M, Mokin M, Primiani CT, Gong AD, Rai HH, Chin F, Rai AT, Levy EI, Siddiqui AH. Large vessel occlusion in acute ischemic stroke patients: a dual-center estimate based on a broad definition of occlusion site. J Stroke Cerebrovasc Dis. 2020;29:104504. doi: 10.1016/j.jstrokecerebrovasdis.2019.104504 [DOI] [PubMed] [Google Scholar]
- 28.Global Health Expenditure Database [Internet]. [cited 2020 Jun 30]. https://apps.who.int/nha/database/Select/Indicators/en
- 29.Putaala J. Young stroke. Helsinki Univ. 2016;1:1–15. [Google Scholar]
- 30.IBM. Downloading IBM SPSS Statistics 26. Ibm. 2020. [Google Scholar]
- 31.Yaghi S, Ishida K, Torres J, Grory B Mac, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;120:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rennert RC, Wali AR, Steinberg JA, Santiago-Dieppa DR, Olson SE, Pannell JS, Khalessi AA. Epidemiology, natural history, and clinical presentation of large vessel ischemic stroke. Neurosurgery. 2019;85(suppl_1):S4–S8. doi: 10.1093/neuros/nyz042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malhotra K, Gornbein J, Saver JL. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: a review. Front Neurol. 2017;8:651. doi: 10.3389/fneur.2017.00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dozois A, Hampton L, Kingston CW, Lambert G, Porcelli TJ, Sorenson D, Templin M, VonCannon S, Asimos AW. PLUMBER Study (prevalence of large vessel occlusion strokes in mecklenburg county emergency response). Stroke. 2017;48:3397–3399. doi: 10.1161/STROKEAHA.117.018925 [DOI] [PubMed] [Google Scholar]
- 35.Ntaios G, Michel P, Georgiopoulos G, Guo Y, Li W, Xiong J, Calleja P, Ostos F, González-Ortega G, Fuentes B, et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the global COVID-19 stroke registry. Stroke. 2020;51:e254–e258. doi: 10.1161/STROKEAHA.120.031208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ornello R, Degan D, Tiseo C, Di Carmine C, Perciballi L, Pistoia F, Carolei A, Sacco S. Distribution and temporal trends from 1993 to 2015 of ischemic stroke subtypes: a systematic review and meta-analysis. Stroke. 2018;49:814–819. doi: 10.1161/STROKEAHA.117.020031 [DOI] [PubMed] [Google Scholar]
- 37.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, et al. ; INTERSTROKE Investigators. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3 [DOI] [PubMed] [Google Scholar]
- 38.Al-Rukn S, Mazya M, Akhtar N, Hashim H, Mansouri B, Faouzi B, Aref H, et al. Stroke in the Middle-East and North Africa: a 2-year prospective observational study of stroke characteristics in the region—results from the Safe Implementation of Treatments in Stroke (SITS)–Middle-East and North African (MENA). Int J Stroke. 2019;14:715–722. [DOI] [PubMed] [Google Scholar]
- 39.M P, L N, P D, B L. Outcomes from ischemic stroke subtypes classified by the Oxfordshire Community Stroke Project: a systematic review. Eur J Phys Rehabil Med. 2011;47:19–23. [PubMed] [Google Scholar]
- 40.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, et al. ; INTERSTROKE Investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed] [Google Scholar]
- 41.Li L, Yiin GS, Geraghty OC, Schulz UG, Kuker W, Mehta Z, Rothwell PM; Oxford Vascular Study. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol. 2015;14:903–913. doi: 10.1016/S1474-4422(15)00132-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yesilot Barlas N, Putaala J, Waje-Andreassen U, Vassilopoulou S, Nardi K, Odier C, Hofgart G, Engelter S, Burow A, Mihalka L, et al. Etiology of first-ever ischaemic stroke in European young adults: the 15 cities young stroke study. Eur J Neurol. 2013;20:1431–1439. doi: 10.1111/ene.12228 [DOI] [PubMed] [Google Scholar]
- 43.Ihle-Hansen H, Thommessen B, Wyller TB, Engedal K, Fure B. Risk factors for and incidence of subtypes of ischemic stroke. Funct Neurol. 2012;27:35–40. [PMC free article] [PubMed] [Google Scholar]
- 44.Hauer AJ, Ruigrok YM, Algra A, van Dijk EJ, Koudstaal PJ, Luijckx G-J, Nederkoorn PJ, van Oostenbrugge RJ, Visser MC, Wermer MJ, et al. Age-specific vascular risk factor profiles according to stroke subtype. J Am Heart Assoc. 2017;6:e005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carrera E, Maeder-Ingvar M, Rossetti AO, Devuyst G, Bogousslavsky J; Lausanne Stroke Registry. Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: the Lausanne Stroke Registry. Cerebrovasc Dis. 2007;24:97–103. doi: 10.1159/000103123 [DOI] [PubMed] [Google Scholar]
- 46.Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, Glahn J, Brandt T, Hacke W, Diener HC. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–2566. doi: 10.1161/hs1101.098524 [DOI] [PubMed] [Google Scholar]
- 47.Wafa HA, Wolfe CDA, Rudd A, Wang Y. Long-term trends in incidence and risk factors for ischaemic stroke subtypes: prospective population study of the South London Stroke Register. PLoS Med. 2018;15:e1002669. doi: 10.1371/journal.pmed.1002669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czlonkowska A, Ryglewicz D, Weissbein T, Baranska-Gieruszczak M, Hier DB. A prospective community-based study of stroke in Warsaw, Poland. Stroke. 1994;25:547–551. doi: 10.1161/01.str.25.3.547 [DOI] [PubMed] [Google Scholar]
- 49.Alzamora MT, Sorribes M, Heras A, Vila N, Vicheto M, Forés R, Sánchez-Ojanguren J, Sancho A, Pera G; “ISISCOG Study Group”. Ischemic stroke incidence in Santa Coloma de Gramenet (ISISCOG), Spain. A community-based study. BMC Neurol. 2008;8:5. doi: 10.1186/1471-2377-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Consoli D, Wolfe CD, Giroud M, Rudd A, Burger I, Ghetti A, et al. ; European BIOMED Study of Stroke Care Group. Risk factors and outcome of subtypes of ischemic stroke. Data from a multicenter multinational hospital-based registry. The European Community Stroke Project. J Neurol Sci. 2006;244:143–150. doi: 10.1016/j.jns.2006.01.016 [DOI] [PubMed] [Google Scholar]
- 51.Porcello Marrone LC, Diogo LP, de Oliveira FM, Trentin S, Scalco RS, de Almeida AG, Gutierres Ldel C, Marrone AC, da Costa JC. Risk factors among stroke subtypes in Brazil. J Stroke Cerebrovasc Dis. 2013;22:32–35. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.022 [DOI] [PubMed] [Google Scholar]
- 52.Saber H, Thrift AG, Kapral MK, Shoamanesh A, Amiri A, Farzadfard MT, Behrouz R, Azarpazhooh MR. Incidence, recurrence, and long-term survival of ischemic stroke subtypes: a population-based study in the Middle East. Int J Stroke. 2017;12:835–843. doi: 10.1177/1747493016684843 [DOI] [PubMed] [Google Scholar]
- 53.Khorvash F, Khalili M, Rezvani Habibabadi R, Sarafzadegan N, Givi M, Roohafza H, Yadgarfar G, Dehghani L, Taheri M, Saadatnia M. Comparison of acute ischemic stroke evaluation and the etiologic subtypes between university and nonuniversity hospitals in Isfahan, Iran. Int J Stroke. 2019;14:613–619. doi: 10.1177/1747493019828648 [DOI] [PubMed] [Google Scholar]
- 54.Senel GB, Elmali AD, Mehrvar K, Farhoudi M, Aboutalebi M, Rezaei M, Ince B. A survey from Turkey and Iran on comparison of risk factors and etiology in ischemic stroke. Iran J Neurol. 2019;18:176–178. [PMC free article] [PubMed] [Google Scholar]
- 55.Lutski M, Zucker I, Shohat T, Tanne D. Characteristics and outcomes of young patients with first-ever ischemic stroke compared to older patients: the National Acute Stroke ISraeli Registry. Front Neurol. 2017;8:421. doi: 10.3389/fneur.2017.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumral E, Özkaya B, Sagduyu A, Şirin H, Vardarli E, Pehliva M. The ege stroke registry: a hospital-based study in the aegean region, Izmir, Turkey. Cerebrovasc Dis. 1998;8:278–288. doi: 10.1159/000015866 [DOI] [PubMed] [Google Scholar]
- 57.Ghandehari K, Izadi-Mood Z. Khorasan stroke registry: analysis of 1392 stroke patients. Arch Iran Med. 2007;10:327–334. doi: 07103/AIM.009 [PubMed] [Google Scholar]
- 58.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jain R. Evolving neuroimaging findings during COVID-19. Am. J. Neuroradiol. 2020;41:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegler JE, Heslin ME, Thau L, Smith A, Jovin TG. Falling stroke rates during COVID-19 pandemic at a comprehensive stroke center: cover title: falling stroke rates during COVID-19. J Stroke Cerebrovasc Dis. 2020;29:104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Favate AS, Younger DS. Epidemiology of ischemic stroke. Neurol Clin. 2016;34:967–980. doi: 10.1016/j.ncl.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 62.Wilterdink JL, Bendixen B, Adams HP, Jr, Woolson RF, Clarke WR, Hansen MD. Effect of prior aspirin use on stroke severity in the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Stroke. 2001;32:2836–2840. doi: 10.1161/hs1201.099384 [DOI] [PubMed] [Google Scholar]
- 63.Pittock SJ, Meldrum D, Hardiman O, Thornton J, Brennan P, Moroney JT. The Oxfordshire Community Stroke Project classification: correlation with imaging, associated complications, and prediction of outcome in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2003;12:1–7. doi: 10.1053/jscd.2003.7 [DOI] [PubMed] [Google Scholar]
- 64.Abootalebi S, Aertker BM, Andalibi MS, Asdaghi N, Aykac O, Azarpazhooh MR, Bahit MC, Barlinn K, Basri H, Shahripour RB, et al. Call to Action: SARS-CoV-2 and Cerebrovascular Disorders (CASCADE). J Stroke Cerebrovasc Dis. 2020;29:104938. doi: 10.1016/j.jstrokecerebrovasdis.2020.104938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.F. C. Defining young stroke. YoungStroke Inc. [Internet]. 2017:1–15. http://youngstroke.org/wp-content/definingyoungstroke
- 66.Cabral NL, Freire AT, Conforto AB, Dos Santos N, Reis FI, Nagel V, Guesser VV, Safanelli J, Longo AL. Increase of stroke incidence in young adults in a middle-income country: a 10-year population-based study. Stroke. 2017;48:2925–2930. doi: 10.1161/STROKEAHA.117.018531 [DOI] [PubMed] [Google Scholar]
- 67.Kissela BM, J.C. K, Alwell K, Moomaw JC, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, Rosa FDLRL, et al. Age at stroke. Neurol 1781-1787. 2012;79:1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo Y, Tian X, Wang X. Diagnosis and Treatment of cerebral venous thrombosis: a review. Front Aging Neurosci. 2018;10:2. doi: 10.3389/fnagi.2018.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunes HN, Cokal BG, Guler SK, Yoldas TK, Malkan UY, Demircan CS, Yon MI, Yoldas Z, Gunes G, Haznedaroglu IC. Clinical associations, biological risk factors and outcomes of cerebral venous sinus thrombosis. J Int Med Res. 2016;44:1454–1461. doi: 10.1177/0300060516664807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zuurbier SM, Middeldorp S, Stam J, Coutinho JM. Sex differences in cerebral venous thrombosis: a systematic analysis of a shift over time. Int J Stroke. 2016;11:164–170. doi: 10.1177/1747493015620708 [DOI] [PubMed] [Google Scholar]
- 71.Dentali F, Poli D, Scoditti U, Di Minno MN, De Stefano V, Stefano VD, Siragusa S, Kostal M, Palareti G, Sartori MT, et al. ; Cerebral Vein Thrombosis International Study Investigators. Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study. J Thromb Haemost. 2012;10:1297–1302. doi: 10.1111/j.1538-7836.2012.04774.x [DOI] [PubMed] [Google Scholar]
- 72.Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012;43:3375–3377. doi: 10.1161/STROKEAHA.112.671453 [DOI] [PubMed] [Google Scholar]
- 73.Sidhom Y, Mansour M, Messelmani M, Derbali H, Fekih-Mrissa N, Zaouali J, Mrissa R. Cerebral venous thrombosis: clinical features, risk factors, and long-term outcome in a Tunisian cohort. J Stroke Cerebrovasc Dis. 2014;23:1291–1295. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.025 [DOI] [PubMed] [Google Scholar]
- 74.Ferro JM, Aguiar de Sousa D. Cerebral venous thrombosis: an update. Curr Neurol Neurosci Rep. 2019;19:74. doi: 10.1007/s11910-019-0988-x [DOI] [PubMed] [Google Scholar]
- 75.Sassi SB, Touati N, Baccouche H, Drissi C, Romdhane NB, Hentati F. Cerebral venous thrombosis: a Tunisian Monocenter Study on 160 patients. Clin Appl Thromb Hemost. 2017;23:1005–1009. doi: 10.1177/1076029616665168 [DOI] [PubMed] [Google Scholar]
- 76.Kim YW, Lawson MF, Hoh BL. Nonaneurysmal subarachnoid hemorrhage: an update. Curr Atheroscler Rep. 2012;14:328–334. doi: 10.1007/s11883-012-0256-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


