Abstract
Background
Calcineurin inhibitors used in kidney transplantation for immunosuppression have adverse effects that may contribute to nephrotoxicity and increased cardiovascular risk profile. Fish oils are rich in very long chain omega‐3 fatty acids, which may reduce nephrotoxicity by improving endothelial function and reduce rejection rates through their immuno‐modulatory effects. They may also modify the cardiovascular risk profile. Hence, fish oils may potentially prolong graft survival and reduce cardiovascular mortality.
Objectives
This review aimed to look at the benefits and harms of fish oil treatment in ameliorating the kidney and cardiovascular adverse effects of CNI‐based immunosuppressive therapy in kidney transplant recipients.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register (up to 17 March 2016) through contact with the Information Specialist using search terms relevant to this review.
Selection criteria
All randomised controlled trials (RCTs) and quasi‐RCTs of fish oils in kidney transplant recipients on a calcineurin inhibitor‐based immunosuppressive regimen. RCTs of fish oil versus statins were included.
Data collection and analysis
Data was extracted and the quality of studies assessed by two authors, with differences resolved by discussion with a third independent author. Dichotomous outcomes were reported as risk ratio (RR) and continuous outcome measures were reported as the mean difference (MD) with 95% confidence intervals using the random effects model. Heterogeneity was assessed using a Chi2 test on n‐1 degrees of freedom and the I2 statistic. Data not suitable for pooling were tabulated and described.
Main results
Fifteen studies (733 patients) were suitable for analysis. All studies were small and had variable methodology. Fish oil did not significantly affect patient or graft survival, acute rejection rates, or calcineurin inhibitor toxicity when compared to placebo. Overall SCr was significantly lower in the fish oil group compared to placebo (5 studies, 237 participants: MD ‐30.63 µmol/L, 95% CI ‐59.74 to ‐1.53; I2 = 88%). In the subgroup analysis, this was only significant in the long‐course (six months or more) group (4 studies, 157 participants: MD ‐37.41 µmol/L, 95% CI ‐69.89 to ‐4.94; I2 = 82%). Fish oil treatment was associated with a lower diastolic blood pressure (4 studies, 200 participants: MD ‐4.53 mm Hg, 95% CI ‐7.60 to ‐1.45) compared to placebo. Patients receiving fish oil for more than six months had a modest increase in HDL (5 studies, 178 participants: MD 0.12 mmol/L, 95% CI 0.03 to 0.21; I2 = 47%) compared to placebo. Fish oil effects on lipids were not significantly different from low‐dose statins. There was insufficient data to analyse cardiovascular outcomes. Fishy aftertaste and gastrointestinal upset were common but did not result in significant patient drop‐out.
Authors' conclusions
There is insufficient evidence from currently available RCTs to recommend fish oil therapy to improve kidney function, rejection rates, patient survival or graft survival. The improvements in HDL cholesterol and diastolic blood pressure were too modest to recommend routine use. To determine a benefit in clinical outcomes, future RCTs will need to be adequately powered with these outcomes in mind.
Keywords: Humans, Calcineurin Inhibitors, Blood Pressure, Blood Pressure/drug effects, Blood Pressure/physiology, Fish Oils, Fish Oils/adverse effects, Fish Oils/therapeutic use, Graft Rejection, Graft Rejection/prevention & control, Graft Survival, Graft Survival/drug effects, Kidney, Kidney/drug effects, Kidney/physiology, Kidney Transplantation, Kidney Transplantation/mortality, Lipids, Lipids/blood, Randomized Controlled Trials as Topic
Plain language summary
Fish oil for kidney transplant recipients
This review set out to assess any benefit or harm in using fish oil to reduce the risk of kidney damage and heart disease in people who have had a kidney transplant and are receiving standard drugs to prevent rejection. Information from 15 studies was used and showed that fish oils provide a slight improvement in HDL cholesterol and diastolic blood pressure. These studies did not provide enough information on the differences in the risk of death, heart disease, kidney transplant rejection or kidney function between patients receiving fish oils and those receiving placebo. There appeared to be no harmful effects of taking fish oil. The benefits of taking fish oil after a kidney transplant are a mild improvement in some heart disease risk factors. There was not enough information to show any benefit in preventing heart disease or reduction in kidney function. Larger, better studies are needed before regular use of fish oil can be recommended.
Background
Description of the intervention
Fish oils contain high levels of polyunsaturated fatty acids (PUFAs) that may reduce cardiovascular risk and improve kidney transplant graft survival but they are not currently routinely used by kidney transplant recipients. There are two types of PUFAs, namely omega‐6 (n‐6 PUFA) and omega‐3 (n‐3 PUFA). n‐6 PUFA (linoleic acid) is mainly derived from vegetable oils such as corn oil and sunflower oil. The n‐3 PUFAs (linolenic acid) include α‐linolenic acid (ALA, C18:3 n‐3), eicosapentaenoic acid (EPA, C20:5 n‐3) and docosahexaenoic acid (DHA, C22:6 n‐3). While ALA is available from certain plants such as linseed, soy, flaxseed and walnut, EPA and DHA are derived from fish and fish oils. The typical Western diet is abundant in plant n‐6 PUFA relative to n‐3 PUFA, with a ratio of approximately 10:1 (Kris‐Etherton 2000). However, humans lack the enzymes to convert n‐6 to n‐3 PUFA, and convert only about 5% of ALA to EPA or DHA (Brenna 2002).
Evidence is accumulating in the general population that the very long chain n‐3 PUFAs (EPA/DHA) found in fatty fish or fish oil supplements are beneficial in the prevention of cardiovascular disease (Psota 2006). This is probably the most common indication for fish oil supplementation. Potential benefits include antiarrhythmic, antithrombotic (decreased platelet aggregation), antiatherosclerotic and anti‐inflammatory actions, lowering of blood pressure, and improvements in endothelial function and lipid profile (Din 2004). The common side effects of fish oil include gastrointestinal upset and fishy aftertaste. The inhibition of platelet aggregation theoretically increases the risk of bleeding but its clinical significance is debatable.
There are special circumstances in the kidney transplant population that increase the risk of graft dysfunction and cardiovascular disease that may be reduced by fish oil. The majority of kidney transplant immunosuppressive regimens include corticosteroids and a calcineurin‐inhibitor (CNI), such as cyclosporin A (CSA) and tacrolimus. CNIs can cause endothelial dysfunction by reducing production of vasodilators (nitric oxide and prostaglandins, particularly prostacyclin) and increased release of vasoconstrictors (endothelin and thromboxane A2). In the kidney, this imbalance in the arachidonic acid‐derived eicosanoid system results in a concentration‐dependent vasoconstriction of the glomerular arterioles, decreasing renal plasma flow and glomerular filtration rate (GFR). CNI vasoconstriction causes dose‐dependent azotaemia, increasing the risk of acute tubular necrosis and poor graft function. Chronically, CNIs can cause progressive kidney disease with tubular atrophy, striped tubulointerstitial fibrosis and an arteriolopathy characterised by hyalinosis of the afferent arteriole (Ader 1998; Andoh 1997; Andoh 1998; de Mattos 2000). This increased risk of kidney failure due to CNI nephrotoxicity is apparent in non‐kidney organ recipients (Ojo 2003).
Corticosteroid and CNI use is associated with a higher prevalence of hypertension (Braun 2003), where CSA has been shown to stimulate the renin‐angiotensin system and enhance sympathetic nervous system activity (Ader 1998). Furthermore, an abnormal lipid profile occurs in 50% to 80% of kidney transplant recipients treated with prednisolone and CSA, characterised by an increase in total cholesterol, low‐density lipoprotein (LDL), very low‐density lipoprotein, and apolipoprotein B levels (Braun 2003). Hypertension is an independent risk factor for graft failure (Opelz 1998) and cardiovascular disease is an important contributor to mortality in kidney transplant recipients. For example, 38% of deaths in kidney transplant recipients in Australia and New Zealand are due to cardiovascular causes, where the risk of death is 5 to 10 times that of the age‐matched population under 60 (McDonald 2003).
How the intervention might work
The potential specific benefits of fish oil for the kidney transplant population include:
Reversing the endothelial dysfunction caused by CNI‐induced disturbance in the eicosanoid pathway: Fish oil treatment is associated with a decreased synthesis of thromboxane and increased synthesis of prostacyclin, and reduced CSA‐induced kidney dysfunction in rats (Norris 1990).
Immunomodulatory effects: Fish oil has been shown to reduce the pro‐inflammatory cytokines involved in acute transplant rejection such as TNF‐α, IL‐1 and IL‐2 (Endres 1989; Ford 1991; Endres 1993; Noronha 1992; Noronha 1993; Vandenbroecke 1991; Yard 1994), enhance the immunosuppressive effects of cyclosporine (Kelley 1989), inhibit delayed type hypersensitivity in rat models of cardiac transplant (Otto 1990), and slow the deterioration of graft function in chronic vascular rejection (Sweny 1989).
Why it is important to do this review
A recent large observational study looking at plasma n‐3 PUFA levels in kidney transplant recipients in a Norwegian population noted reduced cardiovascular mortality with higher n‐3 PUFA levels (Eide 2015). Furthermore, high levels of n‐PUFA were also associated with better kidney allograft survival (Eide 2016). These observations were based on a population with a generally high dietary intake of marine n‐3 PUFA.
Hence, fish oil may potentially prolong graft survival in addition to lowering cardiovascular risk. The addition of fish oil to kidney transplant protocols may therefore improve both short‐ and long‐term outcomes for kidney transplant recipients.
Objectives
This review aimed to look at the benefits and harms of fish oil treatment in ameliorating the kidney and cardiovascular adverse effects of CNI‐based immunosuppressive therapy in kidney transplant recipients.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) examining fish oils in kidney transplant recipients. The first period of randomised cross‐over studies were included.
Types of participants
Inclusion criteria
All recipients of cadaveric or living kidney transplants on a CNI‐based immunosuppressive protocol.
Exclusion criteria
CNI‐free transplant immunosuppression protocol
Multi‐organ combined transplants, e.g. liver‐kidney, pancreas‐kidney
Types of interventions
Fish oil versus control oil
Fish oil versus statin
Early versus late introduction (> three months)
Short‐course versus long‐course (> three months)
Types of outcome measures
Patient survival/death: yes/no
Graft failure, defined as creatinine clearance (CrCl)/GFR < 15 mL/min OR dialysis: yes/no
Acute rejection (biopsy proven) present: yes/no
CNI toxicity (biopsy proven) present: yes/no
Cardiovascular events (stroke, myocardial infarction and cardiovascular death): yes/no
Adverse effects (gastrointestinal upset, taste, breath): yes/no
Compliance (percentage drop‐out rate during study period) and satisfaction (quality of life assessment by standard validated method e.g. the SF‐36)
Kidney function (GFR, CrCl, serum creatinine (SCr))
Blood pressure (systolic, diastolic, mean arterial pressure (MAP))
Serum lipid levels (total cholesterol, LDL, HDL, triglycerides)
For dichotomous outcomes, the events were combined to 12 months (or earlier for shorter studies). For continuous outcomes, the data was assessed at the 12‐month time point (or earlier time point for shorter studies).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register (up to 17 March 2016) through contact with the Information Specialist using search terms relevant to this review. The Cochrane Kidney and Transplant Specialised Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The review was undertaken by four authors. The search strategy described was used to obtain titles and abstracts of studies that were potentially relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable. However, studies and reviews that included relevant data or information on studies were retained initially. Two authors independently assessed the retrieved abstracts and, if necessary, the full text of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out by the same authors independently using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of the same study existed, reports were grouped together and the publication with the most complete data was used. Disagreements were resolved in consultation with a third author.
Assessment of risk of bias in included studies
For this update the following items were used to assess the risk of bias (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Results for dichotomous outcomes (death, graft failure, acute rejection, CNI toxicity, cardiovascular events, non‐compliance with patient drop‐out) were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (kidney function, blood pressure, lipid levels), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales had been used. Adverse effects were tabulated and assessed descriptively, as there was insufficient data to calculate a RR or MD.
Dealing with missing data
Further information required from the original authors were requested by written correspondence and information obtained in this manner was included in the review.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance, and also using the I2 statistic (Higgins 2003).
Assessment of reporting biases
Funnel plots were to be used to assess for the potential existence of small study bias (Higgins 2011) however there were insufficient studies to do this.
Data synthesis
Data was pooled using the random effects model but the fixed effects model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to assess possible sources of heterogeneity. This was only possible for duration of treatment, dividing treatment courses into short (three months or less) and long (more than three months). The other subgroups which were considered but not analysed due to lack of data include:
Heterogeneity among participants related to patient age, underlying risk of graft failure (high if panel reactive antibodies > 50%, previous transplant, cold ischaemia > 24 hours) or diabetes.
Heterogeneity in treatments related to the dose of fish oil (g/d) or CNI (as assessed by serum CSA or tacrolimus level), duration and timing of treatment initiation.
Blood pressure control and specifically the use of calcium channel antagonists, renin‐angiotensin antagonists or both.
Different immunosuppressive combinations.
Results
Description of studies
Results of the search
2007 review
A total of 65 reports were identified after searching the Specialised Register, CENTRAL, MEDLINE and EMBASE. Of these, 45 reports (44 studies) were excluded and 20 reports (15 studies) were included.
2016 review update
A search of the Specialised Register identified 16 new reports: seven new reports of five existing included studies (Busnach 1998; Homan van der Heide 1990a; Homan van der Heide 1990b; Homan van der Heide 1992; Kooijmans‐Coutinho 1996); four new reports of two existing excluded studies (Alexander 2005; Levi 1992); four reports of four new excluded studies (Alexander 2006; Alexander 2008; Ramezani 2011; Romo 2012); and one recently completed study which is yet to publish any results (NCT01744067).
See Figure 1.
1.

Study flow diagram.
Included studies
Of the 15 included studies (733 patients), 13 compared fish oil to control/placebo (658 patients) (Bennett (high) 1995; Bennett (low) 1995; Berthoux 1992; Busnach 1998; Hernandez 2002; Homan van der Heide 1990a; Homan van der Heide 1990b; Homan van der Heide 1992; Homan van der Heide 1993; Kooijmans‐Coutinho 1996; Maachi 1995; Santos 2000; Schut 1992a; Schut 1992b; Yoa 1994), and two compared fish oil to statin treatment (75 patients) (Castro 1997; Rodriguez 1997).
Bennett (high) 1995 is the same study as Bennett (low) 1995. The high dose arm is analysed compared to half of the control group for continuous outcomes. Dichotomous outcomes analysed together. Results for low and high dose corn oil were combined (n = 50) in the published report.
Schut 1992a is the same study as Schut 1992b. Schut 1992a presents the data for patients receiving cyclosporin A and Schut 1992b presents the data for patients receiving cyclosporin A plus prednisone.
Four studies (Homan van der Heide 1990a; Homan van der Heide 1990b; Homan van der Heide 1992; Homan van der Heide 1993) reported median rather than mean values for several continuous data variables and were not suitable for meta‐analysis. Their continuous outcomes were assessed in a separate table but their dichotomous data was included in the analysis. All available studies contained small patient numbers and reporting on outcome measures was highly variable. The duration of treatment and follow‐up was also variable, ranging from one to 12 months. No study exceeded 12 months in duration. The dose of fish oil ranged from 2 g/d to 18 g/d, comprising 0.6 g/d to 5.4 g/d of EPA and DHA. Three studies examined delayed introduction of fish oil (Bennett (high) 1995; Homan van der Heide 1990b; Schut 1992a). One study (Hernandez 2002) used soy oil as the control, which is potentially a weak source ALA. However, we considered ALA to be sufficiently different from EPA/DHA to include this study. Two studies were identified which compared fish oil with statins. Both were small, single centre studies with late introduction of fish oil. Castro 1997 compared low‐dose simvastatin 10 mg/d to fish oil (duration three months), while Rodriguez 1997 compared lovastatin 20 mg/d with fish oil (six months).
The reporting of outcome measures was variable (Table 1 ‐ Summary of reported outcome measures). The primary outcomes of interest for most of these studies were kidney function and graft survival (except for the statin studies). The definition of the end points were also variable. Graft failure/loss was generally not defined. Reporting on acute rejection was either in terms of rejection episodes or number of patients with rejection. Some studies did not specify biopsy‐proven rejection to define acute rejection episodes. Only Hernandez 2002 provided unpublished data on biopsy‐proven CNI toxicity, whereas in Bennett (low) 1995 it was physician‐diagnosed. Cardiovascular events were not a primary outcome measure for any of the included studies but data on cardiovascular death could be obtained from the reported cause of death. Adverse effects were not quantified but merely stated if present. Some studies reported drop‐out rates from non‐compliance but no standard quality of life assessment was used. The method of reporting kidney function was inconsistent, with some reports using SCr and CrCl, while others used nuclear GFR (methodology also varied e.g. EDTA, iothalamate, DTPA, inulin). Where data for different time points were available, only data at the latest follow‐up was analysed.
1. Summary of reported outcome measures.
| Study ID | Patient survival | Graft survival | Acute rejection | CNI toxicity | Adverse effects | Compliance | Kidney function | Blood pressure | Lipid profile |
| Bennett (high) 1995; Bennett (low) 1995 | X | X | X | ‐ | ‐ | ‐ | X | ‐ | X |
| Berthoux 1992 | X | X | ‐ | ‐ | ‐ | ‐ | X | X | X |
| Busnach 1998 | X | X | X | ‐ | ‐ | X | X | X | X |
| Castro 1997 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X |
| Hernandez 2002 | X | X | X | X | X | X | X | X | X |
| Homan van der Heide 1990a | X | X | X | ‐ | X | ‐ | X | X | ‐ |
| Homan van der Heide 1990b | X | X | X | ‐ | ‐ | X | X | X | ‐ |
| Homan van der Heide 1992 | X | X | X | ‐ | ‐ | X | X | X | ‐ |
| Homan van der Heide 1993 | X | X | X | X | X | X | X | ‐ | |
| Kooijmans‐Coutinho 1996 | X | X | X | ‐ | X | X | X | X | ‐ |
| Maachi 1995 | X | X | X | ‐ | X | X | X | ‐ | X |
| Rodriguez 1997 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X |
| Santos 2000 | X | X | X | ‐ | X | X | X | X | X |
| Schut 1992a; Schut 1992b | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X | ‐ |
| Yoa 1994 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | ‐ | X |
CNI ‐ calcineurin inhibitor
Excluded studies
A total of 48 studies did not meet our inclusion criteria and were excluded (see Characteristics of excluded studies). The main reasons for exclusion were:
Not randomised: 40 studies
Wrong population: 1 study
Wrong intervention: 5 studies
Other: 2 studies (full‐text publication not available; crossover study with no outcomes of interest reported or available)
Risk of bias in included studies
All studies were small and had variable methodology (Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was judged to be at low risk of bias in one study (Busnach 1998) and unclear in the remaining 14 studies.
Allocation concealment was judged to be at ow risk of bias in two studies (Busnach 1998; Kooijmans‐Coutinho 1996) and unclear in the remaining 13 studies.
Intention‐to‐treat analysis was not always explicitly stated but was apparent in 10 studies (Busnach 1998; Castro 1997; Homan van der Heide 1990a; Homan van der Heide 1992; Homan van der Heide 1993; Kooijmans‐Coutinho 1996; Rodriguez 1997; Santos 2000; Schut 1992a; Schut 1992b; Yoa 1994).
Blinding
Eight studies were judged to be at low risk of performance bias (Bennett (high) 1995; Bennett (low) 1995; Busnach 1998; Hernandez 2002; Homan van der Heide 1990a; Homan van der Heide 1990b; Homan van der Heide 1992; Homan van der Heide 1993; Kooijmans‐Coutinho 1996) (using fish flavour or similar capsules). Three studies were judge to be at high risk of bias (Berthoux 1992; Castro 1997; Maachi 1995), and four studies were judged to be unclear (Rodriguez 1997; Santos 2000; Schut 1992a; Schut 1992b; Yoa 1994)
Three studies were judged to be at low risk of detection bias (Busnach 1998; Hernandez 2002; Kooijmans‐Coutinho 1996); two were judged to be at high risk of bias (Berthoux 1992; Castro 1997), and the remaining 10 studies were judged to be unclear (Bennett (high) 1995; Bennett (low) 1995; Homan van der Heide 1990a; Homan van der Heide 1990b; Homan van der Heide 1992; Homan van der Heide 1993; Maachi 1995; Rodriguez 1997; Santos 2000; Schut 1992a; Schut 1992b; Yoa 1994).
Incomplete outcome data
Studies of three months or less showed complete follow‐up of patients, with the exception of Homan van der Heide 1990b (14% lost to follow‐up). In studies lasting longer than six months, patients lost to follow‐up ranged from 0% to 32%. Eight studies were judged to be at low risk of attrition bias (Busnach 1998; Castro 1997; Homan van der Heide 1990a; Homan van der Heide 1992; Kooijmans‐Coutinho 1996; Santos 2000; Schut 1992a; Schut 1992b; Yoa 1994); one study was judge to be at high risk of bias (Bennett (high) 1995; Bennett (low) 1995); and the remaining six studies were judged to be at unclear risk (Berthoux 1992; Hernandez 2002; Homan van der Heide 1990b; Homan van der Heide 1993; Maachi 1995; Rodriguez 1997).
Selective reporting
Five studies were judged to be at low risk of reporting bias (Bennett (high) 1995; Bennett (low) 1995; Busnach 1998; Hernandez 2002; Kooijmans‐Coutinho 1996; Santos 2000), nine studies were judged to be at high risk of bias (Berthoux 1992; Castro 1997; Homan van der Heide 1990a; Homan van der Heide 1990b; Homan van der Heide 1992; Homan van der Heide 1993; Rodriguez 1997; Schut 1992a; Schut 1992b; Yoa 1994), and one study was unclear (Maachi 1995).
Effects of interventions
Fish oil versus control
All‐cause mortality
There were six deaths in 12 studies where survival information was available (Analysis 1.1 (12 studies, 645 participants): RR 1.65, 95% CI 0.34 to 8.10; I2 = 0%). Deaths were limited to three studies (Busnach 1998; Homan van der Heide 1993; Kooijmans‐Coutinho 1996). Of the six deaths, there were three deaths in each of the fish oil and control groups. All patients died with a functioning graft. The cause of deaths were:
1.1. Analysis.
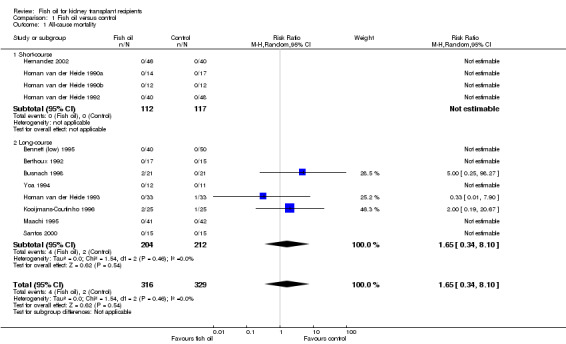
Comparison 1 Fish oil versus control, Outcome 1 All‐cause mortality.
Fish oil group ‐ intestinal infarction (1), haemorrhagic shock post‐removal of polycystic native kidney (1), not specified (1)
Control group ‐ myocardial infarction (1), not specified (2).
Graft loss
Twelve studies reported data on graft survival. Two studies in the short‐course and five studies in the long‐course reported one or more graft loss events. Graft survival was not affected by fish oil (Analysis 1.2 (12 studies, 640 participants): RR 0.91, 95% CI 0.51 to 1.63; participants = 640; studies = 12; I2 = 0%).
1.2. Analysis.

Comparison 1 Fish oil versus control, Outcome 2 Graft loss.
Acute rejection
Nine studies contained data on acute rejection but data was only pooled from the eight studies reporting the proportion of patients with rejection as a dichotomous outcome within the study period. Pooled data from three short‐course and five long‐course studies showed no significant difference in acute rejection (Analysis 1.3 (8 studies, 482 participants): RR 1.00, 95% CI 0.80 to 1.25; I2 = 0%). There was also no significant difference between short‐course (Analysis 1.3.1(3 studies, 197 participants): RR 1.21, 95% CI 0.82 to 1.79; I2 = 1%) and long‐course (Analysis 1.3.2 (5 studies, 285 participants): RR 0.92, 95% CI 0.70 to 1.20; I2 = 0%) subgroups. However Homan van der Heide 1993 found less rejection episodes in the fish oil group at 12 months (8 versus 20, P = 0.029). The largest difference occurred in the second and third months after transplantation (1 versus 9, P = 0.016).
1.3. Analysis.

Comparison 1 Fish oil versus control, Outcome 3 Acute rejection.
Calcineurin inhibitor toxicity
Hernandez 2002 reported biopsy‐proven CNI toxicity. In this three‐month study, there was no significant difference between fish oil and control (Analysis 1.4 (1 study, 90 participants): RR 1.19, 95% CI 0.56 to 2.51).
1.4. Analysis.

Comparison 1 Fish oil versus control, Outcome 4 CNI toxicity.
Calcineurin inhibitor levels
A post‐hoc analysis of CNI levels was performed to look for any potential differences in CNI levels between the fish oil and control groups. No significant difference was detected in the six studies reporting trough CSA (Co) levels at the end of the studies (Analysis 1.5 (6 studies, 275 participants): MD 4.25 ng/mL, 95% CI ‐11.57 to 20.07; I2 = 17%).
1.5. Analysis.
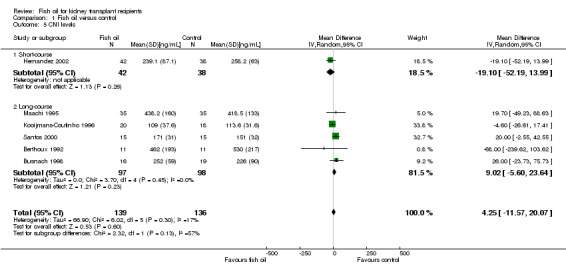
Comparison 1 Fish oil versus control, Outcome 5 CNI levels.
Cardiovascular events
None of the studies specifically reported myocardial infarction, stroke or cardiovascular death. Three studies had data on myocardial infarction (Bennett (low) 1995; Homan van der Heide 1993; Kooijmans‐Coutinho 1996), with only one event in the fish oil and control groups each. Myocardial infarction was not a pre‐specified end‐point in these studies but was reported as a reason why patients were not evaluated at the end of the study. One study noted no stroke events (Bennett (low) 1995). Cardiovascular causes of death could be gleaned from the description of the causes of death in some studies. However, events were too infrequent for a reliable estimate to be made.
Adverse effects
None of the included studies quantitatively reported rates of adverse effects. Reported adverse effects are presented in Table 2: Fish oil versus control: adverse effects. Two studies reported no adverse effects (Bennett (low) 1995; Santos 2000). Seven studies reported a fishy aftertaste as the most common problem, with one (Hernandez 2002) reporting a fishy aftertaste in 70% of patients. Gastrointestinal upset such as bloating, nausea, vomiting and diarrhoea was reported in four studies (Berthoux 1992; Homan van der Heide 1993; Kooijmans‐Coutinho 1996; Rodriguez 1997). Significant bleeding problems were not encountered.
2. Fish oil versus control: adverse effects.
| Study ID | Fishy taste | GI upset | Breath | Bleeding | Other |
| Bennett (high) 1995; Bennett (low) 1995 | No | No | No | No | ‐ |
| Berthoux 1992 | Yes | Yes | No | No | ‐ |
| Hernandez 2002 | Yes (70%) | No | No | No | ‐ |
| Homan van der Heide 1990a | Yes | No | No | No | ‐ |
| Homan van der Heide 1992 | Yes | No | No | No | ‐ |
| Homan van der Heide 1993 | Yes | Yes | No | No | "Pyrosis" Swallowing problems |
| Kooijmans‐Coutinho 1996 | Yes (1 patient in control group) | Yes | No | No | ‐ |
| Maachi 1995 | Yes | No | No | No | ‐ |
| Rodriguez 1997 | No | Yes | No | No | ‐ |
| Santos 2000 | No | No | No | No | ‐ |
GI ‐ gastrointestinal
Compliance and satisfaction
Reporting of patient compliance was variable, with attempts at monitoring non‐compliance having included pill counting, self‐monitoring and biochemical measurements. The biochemical assays have included measuring plasma EPA as a percentage of total fatty acids (Bennett (low) 1995) and analyses of plasma cholesterol esters (Homan van der Heide 1993). However, these data could not be pooled due to the lack of a uniform and consistent measure of compliance among the studies. Hence, only data regarding significant non‐compliance resulting in patient drop‐out was sufficient for analysis. Information on such non‐compliant patients was available in eight RCTs (two short‐course and six long‐course) (Analysis 1.8 (8 studies, 412 participants): RR 1.88, 95% CI 0.56 to 6.26; I2 = 0%). Patient satisfaction was not assessed quantitatively or qualitatively in any of the included studies.
1.8. Analysis.
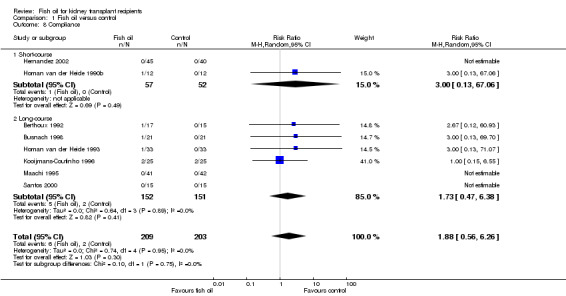
Comparison 1 Fish oil versus control, Outcome 8 Compliance.
Kidney function
Serum creatinine
Overall SCr was significantly lower in the fish oil group compared to control (Analysis 1.9 (5 studies, 237 participants): MD ‐30.63 µmol/L, 95% CI ‐59.74 to ‐1.53; I2 = 88%). In the subgroup analysis, this was only significant in the long‐course group (Analysis 1.9.2 (4 studies, 157 participants): MD ‐37.41 µmol/L, 95% CI ‐69.89 to ‐4.94; I2 = 82%). There was also significant heterogeneity among the studies.
1.9. Analysis.

Comparison 1 Fish oil versus control, Outcome 9 Serum creatinine.
Three other studies used median values, which were not pooled (Table 1). Two (Homan van der Heide 1990a; Homan van der Heide 1992) showed no significant difference between fish oil and control, while another (Homan van der Heide 1990b) found a small difference favouring fish oil (120 μmol/L versus 147 μmol/L; P < 0.05).
Creatinine clearance
Overall there was no significant difference in CrCl between fish oil and control (Analysis 1.10 (8 studies, 353 participants): MD ‐0.61 mL/min, 95% CI ‐5.67 to 4.45; I2 = 0%). There was also no difference in subgroup analyses.
1.10. Analysis.

Comparison 1 Fish oil versus control, Outcome 10 Creatinine clearance.
Of the three studies reporting median values, two (Homan van der Heide 1990a; Homan van der Heide 1992) found no significant difference however (Homan van der Heide 1990b) found a better CrCl in the fish oil group (88.4 mL/min versus 79.5 mL/min; P < 0.05).
Glomerular filtration rate
Overall there was no significant difference in GFR between fish oil and control (Analysis 1.11 (9 studies, 343 participants): MD 2.18 mL/min, 95% CI ‐2.90 to 7.26; I2 = 25%).
1.11. Analysis.
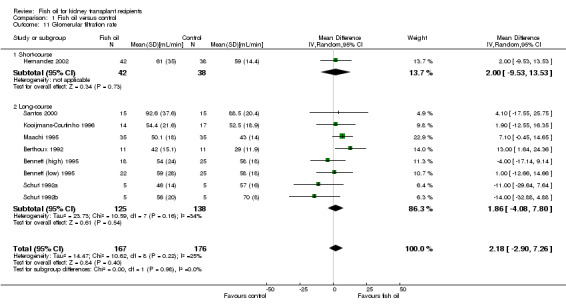
Comparison 1 Fish oil versus control, Outcome 11 Glomerular filtration rate.
Of the three studies reporting median GFR, Homan van der Heide 1990a and Homan van der Heide 1990b found no difference between the two groups while Homan van der Heide 1993 reported an improved GFR with fish oil (53 mL/min versus 40 mL/min; P = 0.038).
Blood pressure
Systolic pressure, diastolic pressure and MAP were reported variably among the included studies. Generally, MAP was defined as systolic + (2 x diastolic)/3.
Concomitant antihypertensive medication use was poorly reported. Therefore, data on antihypertensive medication use could not be analysed.
Systolic/diastolic blood pressure
There was no significant difference in systolic blood pressure between fish oil and control (Analysis 1.12 (4 studies, 200 participants): MD 2.45 mm Hg, 95% CI ‐5.93 to 10.83; I2 = 66%); significant heterogeneity was evident. There was a modest but significant reduction in diastolic blood pressure in the fish oil group (Analysis 1.13 (4 studies, 200 participants): MD ‐4.53 mm Hg, 95% CI ‐7.60 to ‐1.45; I2 = 0%).
1.12. Analysis.

Comparison 1 Fish oil versus control, Outcome 12 Systolic blood pressure.
1.13. Analysis.
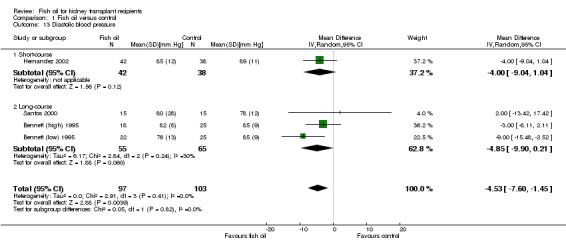
Comparison 1 Fish oil versus control, Outcome 13 Diastolic blood pressure.
Mean arterial blood pressure
There was a non‐significant reduction in MAP in fish oil‐treated patients (Analysis 1.14 (4 studies, 138 participant): MD ‐3.45 mm Hg, 95% CI ‐7.43 to 0.53; I2 = 0%).
1.14. Analysis.

Comparison 1 Fish oil versus control, Outcome 14 Mean arterial pressure.
Of the four studies reporting median values (Table 1), three (Homan van der Heide 1990a; Homan van der Heide 1990b; Homan van der Heide 1992) found no difference in MAP between fish oil and control groups, while one (Homan van der Heide 1993) found a lower MAP with fish oil (103 mm Hg versus 118 mm Hg; P = 0.0011).
Serum lipids
The full lipid profile was not reported in all the included studies, hence variable patient numbers were available for each parameter for analysis.
Total cholesterol
There was no significant difference in TC between fish oil and control (Analysis 1.15 (6 studies, 260 participants): MD ‐0.11 mmol/L, 95% CI ‐0.36 to 0.14; I2 = 26%).
1.15. Analysis.
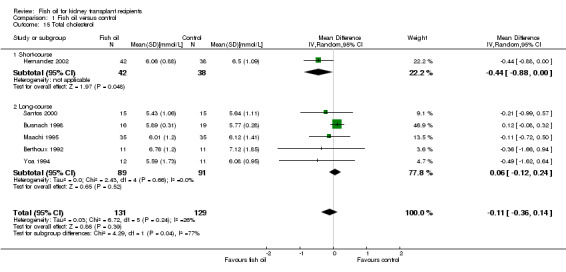
Comparison 1 Fish oil versus control, Outcome 15 Total cholesterol.
LDL cholesterol
There was no significant difference in LDL cholesterol between the two groups (Analysis 1.16.2 (3 studies, 120 participants): MD 0.30 mmol/L, 95% CI ‐0.62 to 1.22; I2 = 93%). The high heterogeneity was attributed to Bennett (low) 1995, however removal of this study from the analysis did not change the significance (MD ‐0.10 mmol/L, 95% CI ‐0.40 to 0.20; I2 = 0%).
1.16. Analysis.
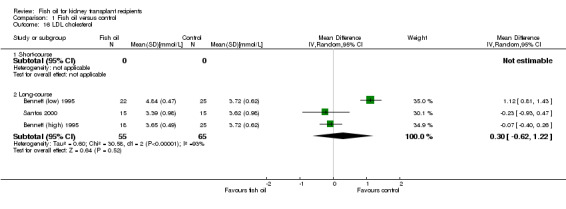
Comparison 1 Fish oil versus control, Outcome 16 LDL cholesterol.
HDL cholesterol
Overall there was no significant difference in HDL cholesterol between fish oil and control (Analysis 1.17 (6 studies, 258 participants): MD 0.09 mmol/L, 95% CI ‐0.01 to 0.19; I2 = 59%). Subgroup analysis showed long‐course fish oil had a small but significant increase in HDL compared to control (Analysis 1.17.2 (5 studies, 178 participants): MD 0.12 mmol/L, 95% CI 0.03 to 0.21; I2 = 47%).
1.17. Analysis.
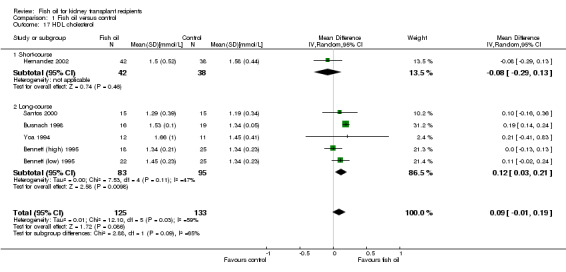
Comparison 1 Fish oil versus control, Outcome 17 HDL cholesterol.
Triglycerides
Overall there was no significant difference in triglycerides between fish oil and control (Analysis 1.18 (MD ‐0.26 mmol/L, 95% CI ‐0.58 to 0.05; participants = 260; studies = 6; I2 = 73%). There was substantial heterogeneity which could not be attributed to any one study.
1.18. Analysis.

Comparison 1 Fish oil versus control, Outcome 18 Triglycerides.
Fish oil versus statins
Two studies compared fish oil with statins. Castro 1997 compared the effects of simvastatin (10 mg/d) with fish oil (6 g/d) (50% EPA/DHA) in 43 patients over three months. Rodriguez 1997 compared fish oil (2 g/d) (30% EPA/DHA) with lovastatin (20 mg/d) in 34 patients over three months.
Total cholesterol was higher in the fish oil group but this was not significant (Analysis 2.1 (2 studies, 75 participants): MD 0.36 mmol/L, 95% CI ‐0.10 to 0.82; I2 = 0%).
2.1. Analysis.
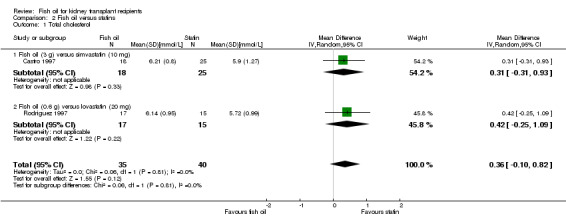
Comparison 2 Fish oil versus statins, Outcome 1 Total cholesterol.
LDL cholesterol was higher in the fish oil group but this was not significant (Analysis 2.2 (2 studies, 75 participants): MD 0.41 mmol/L, 95% CI ‐0.06 to 0.88; I2 = 0%).
2.2. Analysis.
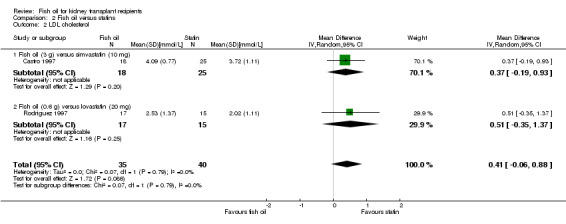
Comparison 2 Fish oil versus statins, Outcome 2 LDL cholesterol.
HDL cholesterol was lower in the fish oil group but this was not significant (Analysis 2.3 (2 studies, 75 participants): MD ‐0.18 mmol/L, 95% CI ‐0.39 to 0.02; I2 = 44%).
2.3. Analysis.
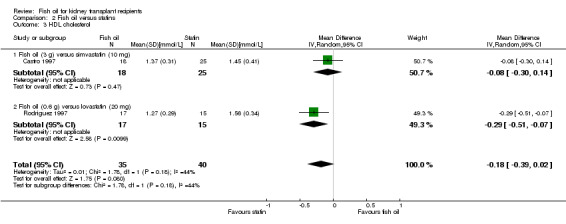
Comparison 2 Fish oil versus statins, Outcome 3 HDL cholesterol.
Triglycerides were higher in the fish oil group but this was not significant (Analysis 2.4 (2 studies, 75 participants): MD 0.18 mmol/L, 95% CI ‐0.18 to 0.55; I2 = 0%).
2.4. Analysis.

Comparison 2 Fish oil versus statins, Outcome 4 Triglycerides.
Patient subgroups
Subgroup analysis could only be performed dividing studies into short (three months or less) and long (greater than three months) courses of treatment. The only difference found was the better HDL result in the long‐course as described. When we re‐analysed the data using the different patient numbers for the continuous variables reported in Hernandez 2002 (see Table of included studies), i.e. 45/40 versus 42/38, no significant change in the any of the results were found.
The results for those studies that only provided median and range for short‐ and long‐course treatment are presented in Table 3: Short course fish oil versus control/miscellaneous and Table 4: Long course fish oil versus control/miscellaneous.
3. Short‐course fish oil versus control/miscellaneous.
| Study ID | Serum creatinine: median (range) | Creatinine clearance: median (range) | GFR: median (range) | Blood pressure (MAP): median (range) | Lipids | Other |
| Homan van der Heide 1990a | Fish oil: 170 (93 to 224) Control: 172 (101 to 540) P = NS | Fish oil: 51 (35 to 75) Control: 48 (10 to 88) P = NS |
Fish oil: 44 (26 to 60) Control: 40 (10 to 80) P = NS |
Fish oil: 106 (82 to 137) Control: 107 (80 to 132) P = NS |
Not reported | ‐ |
| Homan van der Heide 1990b | Fish oil: 120 (88 to 159) Control: 147 (106 to 189) P < 0.05 |
Fish oil: 88.4 (57.7 to 158.6) Control: 79.5 (52.3 to 113.3) P < 0.05 |
Fish oil: 68 (29 to 93) Control: 60 (32 to 84) Per cent change Fish oil: 20.3 (8.9 to 33.3) Control: ‐2.6 (‐38 to ‐7.2) P < 0.01 |
Fish oil: 98 (76 to 106) Control: 109 (103 to 116) Per cent change Fish oil: ‐8.6 (‐23.8 to 0) Control: 0.9 (‐6.9 to 9.4) P < 0.01 |
Not reported | ‐ |
| Homan van der Heide 1992 | Fish oil: 163 (93 to 406) Control: 200 (100 to 850) P = NS |
Fish oil: 53 (22 to 80) Control: 49 (12 to 88) P = NS |
Not reported | Fish oil: 105 (76 to 137) Control: 108 (76 to 142) P = NS |
Not reported | Rejecting patients SCr median (range) Fish oil: 183 (127 to 406) Control: 283 (132 to 860) P < 0.05 CrCl median (range) Fish oil: 43 (22 to 69) Control: 27 (12 to 50) P < 0.05 |
CrCl ‐ creatinine clearance; NS ‐ not significant; SCr ‐ serum creatinine
4. Long‐course fish oil versus control/miscellaneous.
| Study ID | Serum creatinine | Creatinine clearance | GFR (median) | Mean arterial pressure (range) | Lipids | Other |
| Homan van der Heide 1993 | Not reported | Not reported | Fish oil: 53 mL/min Control: 40 mL/min P = 0.038 |
Fish oil: 103 (80 to 141) Control: 118 (98 to 131) P = 0.0011 | Not reported | ‐ |
Fixed effects model
This model gave different results for the following outcomes.
Fish oil versus controls
LDL cholesterol was higher with fish oil treatment (MD 0.49 mmol/L, 95% CI 0.27 to 0.70; I2 = 93%).
HDL cholesterol was higher with fish oil treatment (MD 0.14 mmol/L, 95% CI 0.10 to 0.19; I2 = 59%).
Triglyceride was lower with fish oil treatment (MD ‐0.43 mmol/L, 95% CI ‐0.54 to ‐0.32; I2 = 73%).
Fish oil versus statins
HDL cholesterol was higher with statin treatment (MD ‐0.18 mmol/L, 95% CI ‐0.34 to ‐0.03; P = 0.02).
Discussion
Summary of main results
In kidney transplant recipients on CSA‐based immunosuppression, fish oil treatment has no effect on patient survival, graft survival, acute rejection and CNI toxicity. There was a modest lowering of diastolic blood pressure, and increased HDL in patients treated for six months or more. Clinical events were small and whilst an effect was not seen, a benefit cannot be ruled out by this analysis. The data on cardiovascular events was particularly limited as these were often not pre‐specified end points. Where event data is reported, it has been used, but should be interpreted with caution because it is unclear whether cardiovascular event data was carefully collected for all patients.
The lowering of diastolic blood pressure was a consistent finding, with no heterogeneity. No data on antihypertensive medication use was available to allow stratification by antihypertensives in this analysis to explore the specific influences of calcium‐channel or renin‐angiotensin antagonists. Calcium channel blockers are known to reduce CSA mediated vasoconstriction, while renin‐angiotensin inhibitors are capable of affecting GFR. There was evidence for heterogeneity in the finding of a higher HDL in the long course group (I2 = 47%). The discrepancy between the I2 and the Chi2 test for heterogeneity (Chi2 = 7.53, P = 0.11) reflects the lower power of the latter test to detect true heterogeneity when the number of studies is low (Higgins 2003). Such heterogeneity may arise from the use of different fish oil doses and duration, and different methods in measuring serum lipids.
There was no effect on kidney function and a discrepancy existed between serum creatinine and the other measures of kidney function. While SCr appeared lower with fish oil, there was substantial heterogeneity (I2 = 88%; Chi2 = 33.96, P < 0.00001). In addition to some of the potential sources listed above, heterogeneity between studies may have been impacted by the differing methods of measuring serum creatinine. Given that nuclear GFR is a better measure of kidney function in the transplant population, this is the more important measure on which to base conclusions. A fishy aftertaste was the most common adverse effect followed by gastrointestinal upset. Bleeding did not seem to be a problem despite the potential antithrombotic effect of fish oil. There was a suggestion of poorer compliance with fish oil compared to control but it was not statistically significant and did not significantly effect drop‐out rates. The overall deficiencies in patient blinding made the comparisons of adverse effects and compliance difficult to assess. Patient satisfaction or quality of life assessments were not performed by any of the studies. With the exception of HDL cholesterol, there was no difference in short versus longer course treatment. There was also no difference in early versus late introduction of fish oils.
The fixed effects model showed different results for the lipid outcomes of LDL, HDL and triglycerides. This model is based on the assumption that the true effect of treatment (in both magnitude and direction) is the same value in every study, that there is no statistical heterogeneity, and that the observed differences are due to chance. However, there was significant heterogeneity among the studies included for these variables, with an I2 ranging from 44% to 94%. As the cause of heterogeneity is not readily apparent and the typical treatment effect is not known, the random effects model appears more applicable. However, it does introduce uncertainty as to whether a true effect may be missed.
Quality of the evidence
There are several limitations of this review. Many of the studies were of poor or average quality due to small patient numbers, inadequate randomisation or allocation concealment, and lack of blinding (Figure 2). Some data from one group publishing several studies were expressed in a way that could not be incorporated in our analysis (Homan van der Heide 1990a; Homan van der Heide 1990b; Homan van der Heide 1992; Homan van der Heide 1993). There were several potential sources of heterogeneity, including differing doses of fish oils, duration of treatment, and timing of initiation of treatment. The studies were too small for these issues to be adequately explored through subgroup analyses. The majority of studies were conducted in the early to mid‐1990s, prior to the common use of tacrolimus. Therefore, differences between CSA and tacrolimus could not be examined.
Agreements and disagreements with other studies or reviews
A recent study in rats has demonstrated that DHA can increase the bioavailability of CSA possibly through inhibition of intestinal CYP 3A enzyme responsible for the first pass metabolism (Hirunpanich 2006). In our post‐hoc analysis, we found that fish oil did not effect CSA levels. However, there are limitations to this finding. Firstly, the doses of CSA were not kept constant, as it is usual practice to adjust doses according to levels. Secondly, the studies included in the analysis where a benefit was significant were different to those which reported CSA levels. Thirdly, the use of a single trough level to indicate CNI exposure has limitations. The question whether fish oil influences CSA levels is difficult to answer as all but one of the included studies were not set up to assess CSA pharmacokinetics. In the only study to do so, fish oil treated patients had a higher maximum serum concentration (Cmax) than control patients but the area under curve (AUC) was not significantly higher despite similar trough levels (Busnach 1998). As AUC is probably a better indicator of overall drug exposure, the higher Cmax may not necessarily translate to increased CNI toxicity in the long term. However, effects on other outcomes such as blood pressure are theoretically possible but the lower diastolic blood pressure found in our analysis suggests that this positive effect of fish oil may have outweighed any possible adverse pharmacokinetic interactions, or that CNI dose adjustments have compensated for this possible effect.
Overall, this analysis agrees with a published meta‐analysis of fish oil supplementation in kidney transplantation (Tatsioni 2005), which demonstrated no difference in clinical outcomes except a modest reduction in triglycerides. The same studies were included in that analysis, however we have included CrCl data from the study by Yoa 1994 which was only reported in an earlier abstract. They included one study that we excluded (Urakaze 1989) that reported rejection episodes, GFR, lipid and blood pressure data. We excluded the latter study as it was unclear which patients received CSA and which did not. This study was included in their analysis of triglycerides, which found a significant lowering by fish oils whereas we did not. These authors also used different statistical methodology for continuous variables. Rather than calculating MD, these authors calculated the net change in triglyceride for each study (Bonis 2005; Tatsioni 2005) and evaluated the aggregate. Although they appear to include the two studies comparing fish oil to statins in their publication (Tatsioni 2005), they do separate these from the placebo comparison in a separate publication (Bonis 2005).
Systematic reviews of n‐3 PUFAs in the general population have demonstrated conflicting results. A reduction in triglycerides, increased HDL and increased LDL has been found with n‐3 PUFA supplementation (Balk 2006) but a systematic review by Hooper 2006 showed no reduction in mortality or cardiovascular events. In this review, studies of ALA, EPA and DHA were pooled together, and composite end‐points were analysed. In the review by Wang 2006 fish oil reduced rates of all‐cause mortality, cardiac and sudden death, and possibly stroke. These authors analysed ALA separately from fish‐derived n‐3 PUFAs (EPA/DHA) and found that ALA was not associated with these positive findings, suggesting that ALA is sufficiently different from EPA/DHA to be analysed separately. We did not include studies using ALA in our analysis.
An important issue in these studies is the dose of fish oil used, which varied from 0.6 to 5.4 g/d (EPA + DHA). Active n‐3 PUFAs (EPA, DHA) constitute only about 30% to 50% of common fish oil supplements, although higher doses may be available. This, and the duration and timing of treatment, may have contributed to the heterogeneity seen in a number of comparisons. There is some suggestion of a dose‐dependent effect of fish oil. In the review by Balk 2006 there was an association between the dose of fish oil and reduction in TG. In our analysis, only one study directly compared two different doses (2.7 g/d and 5.4 g/d of EPA/DHA) and found no difference with the higher dose (Bennett (high) 1995).
Authors' conclusions
Implications for practice.
There is insufficient evidence from currently available RCTs to recommend fish oil supplementation to improve kidney function, rejection rates, graft survival or patient survival in kidney transplantation. A dose of at least 6 g/d for longer than three months may be useful for improving HDL and lowering diastolic blood pressure. However, the safety and availability of conventional lipid modifying and antihypertensive agents makes fish oil an unlikely first‐line choice. Fish oils were well tolerated with only minor adverse effects.
Implications for research.
Clearly, the studies included in this meta‐analysis were too small, and the number of events too few to draw conclusions regarding benefit. These questions can only be answered by larger RCTs powered for specific clinical outcomes and of adequate duration. The introduction of non‐CNI immunosuppressive protocols (such as those based on sirolimus) means that further studies with these medications are needed to assess the effects of fish oils in this subset of kidney transplant recipients. It is recommended that future RCTs use a higher dose of fish oil to compare to controls, ideally 6 g/d or more. No studies have assessed the benefits fish oil as "add‐on" therapy to statins and a subgroup of kidney transplant recipients with recurrent IgA nephropathy may also derive benefit from fish oil treatment and could be evaluated. Further studies on the effect of fish oil on CSA pharmacokinetics may be useful and future studies should report CNI levels (including Cmax) and doses, and AUC where possible.
What's new
| Date | Event | Description |
|---|---|---|
| 17 March 2016 | New citation required but conclusions have not changed | New search, 1 ongoing study identified |
| 17 March 2016 | New search has been performed | New search; subheadings added; risk of bias assessment included |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 12 May 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank the library staff at Austin Health for their assistance.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Fish oil versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 12 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.34, 8.10] |
| 1.1 Short‐course | 4 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Long‐course | 8 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.34, 8.10] |
| 2 Graft loss | 12 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.51, 1.63] |
| 2.1 Short‐course | 4 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.33, 4.17] |
| 2.2 Long‐course | 8 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.45, 1.65] |
| 3 Acute rejection | 8 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.80, 1.25] |
| 3.1 Short‐course | 3 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.82, 1.79] |
| 3.2 Long‐course | 5 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.70, 1.20] |
| 4 CNI toxicity | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Short‐course | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Long‐course | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 CNI levels | 6 | 275 | Mean Difference (IV, Random, 95% CI) | 4.25 [‐11.57, 20.07] |
| 5.1 Short‐course | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐19.10 [‐52.19, 13.99] |
| 5.2 Long‐course | 5 | 195 | Mean Difference (IV, Random, 95% CI) | 9.02 [‐5.60, 23.64] |
| 6 Myocardial infarction | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Long‐course | 3 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.11, 9.37] |
| 7 Cardiovascular death | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Long‐course | 3 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.11, 9.37] |
| 8 Compliance | 8 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.56, 6.26] |
| 8.1 Short‐course | 2 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 67.06] |
| 8.2 Long‐course | 6 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.47, 6.38] |
| 9 Serum creatinine | 5 | 237 | Mean Difference (IV, Random, 95% CI) | ‐30.63 [‐59.74, ‐1.53] |
| 9.1 Short‐course | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐8.84 [‐26.31, 8.63] |
| 9.2 Long‐course | 4 | 157 | Mean Difference (IV, Random, 95% CI) | ‐37.41 [‐69.89, ‐4.94] |
| 10 Creatinine clearance | 8 | 353 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐5.67, 4.45] |
| 10.1 Short‐course | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐15.73, 8.33] |
| 10.2 Long‐course | 7 | 273 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐5.52, 5.63] |
| 11 Glomerular filtration rate | 9 | 343 | Mean Difference (IV, Random, 95% CI) | 2.18 [‐2.90, 7.26] |
| 11.1 Short‐course | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐9.53, 13.53] |
| 11.2 Long‐course | 8 | 263 | Mean Difference (IV, Random, 95% CI) | 1.86 [‐4.08, 7.80] |
| 12 Systolic blood pressure | 4 | 200 | Mean Difference (IV, Random, 95% CI) | 2.45 [‐5.93, 10.83] |
| 12.1 Short‐course | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐13.46, 1.46] |
| 12.2 Long‐course | 3 | 120 | Mean Difference (IV, Random, 95% CI) | 5.97 [‐1.59, 13.53] |
| 13 Diastolic blood pressure | 4 | 200 | Mean Difference (IV, Random, 95% CI) | ‐4.53 [‐7.60, ‐1.45] |
| 13.1 Short‐course | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐9.04, 1.04] |
| 13.2 Long‐course | 3 | 120 | Mean Difference (IV, Random, 95% CI) | ‐4.85 [‐9.90, 0.21] |
| 14 Mean arterial pressure | 4 | 138 | Mean Difference (IV, Random, 95% CI) | ‐3.45 [‐7.43, 0.53] |
| 14.1 Short‐course | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐4.70 [‐9.85, 0.45] |
| 14.2 Long‐course | 3 | 58 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐7.87, 4.66] |
| 15 Total cholesterol | 6 | 260 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.36, 0.14] |
| 15.1 Short‐course | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.88, ‐0.00] |
| 15.2 Long‐course | 5 | 180 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.12, 0.24] |
| 16 LDL cholesterol | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Short‐course | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Long‐course | 3 | 120 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.62, 1.22] |
| 17 HDL cholesterol | 6 | 258 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.01, 0.19] |
| 17.1 Short‐course | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.29, 0.13] |
| 17.2 Long‐course | 5 | 178 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.03, 0.21] |
| 18 Triglycerides | 6 | 260 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.58, 0.05] |
| 18.1 Short‐course | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.49, 0.31] |
| 18.2 Long‐course | 5 | 180 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.67, 0.07] |
1.6. Analysis.

Comparison 1 Fish oil versus control, Outcome 6 Myocardial infarction.
1.7. Analysis.

Comparison 1 Fish oil versus control, Outcome 7 Cardiovascular death.
Comparison 2. Fish oil versus statins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total cholesterol | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.10, 0.82] |
| 1.1 Fish oil (3 g) versus simvastatin (10 mg) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.31, 0.93] |
| 1.2 Fish oil (0.6 g) versus lovastatin (20 mg) | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.25, 1.09] |
| 2 LDL cholesterol | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.06, 0.88] |
| 2.1 Fish oil (3 g) versus simvastatin (10 mg) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.19, 0.93] |
| 2.2 Fish oil (0.6 g) versus lovastatin (20 mg) | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.35, 1.37] |
| 3 HDL cholesterol | 2 | 75 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.39, 0.02] |
| 3.1 Fish oil (3 g) versus simvastatin (10 mg) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.30, 0.14] |
| 3.2 Fish oil (0.6 g) versus lovastatin (20 mg) | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.51, ‐0.07] |
| 4 Triglycerides | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.18, 0.55] |
| 4.1 Fish oil (3 g) versus simvastatin (10 mg) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.17, 0.67] |
| 4.2 Fish oil (0.6 g) versus lovastatin (20 mg) | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.75, 0.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bennett (high) 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not reported |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 43 (32%) |
| Selective reporting (reporting bias) | Low risk | Outcomes relevant to our review were reported |
Bennett (low) 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: not reported |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 43 (32%) |
| Selective reporting (reporting bias) | Low risk | Outcomes relevant to our review were reported |
Berthoux 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 3 (9%) |
| Selective reporting (reporting bias) | High risk | BP reported but no data available |
Busnach 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation (sponsor) |
| Allocation concealment (selection bias) | Low risk | Closed envelopes |
| Intention‐to‐treat analysis | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors and data analysis blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported; BP not reported but data no longer available |
Castro 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | High risk | Only lipids were reported |
Hernandez 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central (method not specified) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12/86 (14%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes relevant to this review reported |
Homan van der Heide 1990a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | High risk | Unable to use acute rejection data; median data reported for some continuous outcomes |
Homan van der Heide 1990b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 3 (14%) |
| Selective reporting (reporting bias) | High risk | Median data reported for some continuous outcomes |
Homan van der Heide 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded; placebo flavour matched |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | High risk | Median data reported for some continuous outcomes |
Homan van der Heide 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded; placebo flavour matched |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 6 (9%) |
| Selective reporting (reporting bias) | High risk | Median data reported for some continuous outcomes |
Kooijmans‐Coutinho 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Intention‐to‐treat analysis | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only first 3 months then code broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes relevant to this review were reported |
Maachi 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 2 (2%) |
| Selective reporting (reporting bias) | Unclear risk | Outcomes relevant to this review were reported |
Rodriguez 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Intention‐to‐treat analysis | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: 2 (6%) |
| Selective reporting (reporting bias) | High risk | Only lipid results reported |
Santos 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: low sodium diet advised |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes relevant to this review were reported |
Schut 1992a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | High risk | Only BP and GFR reported |
Schut 1992b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | High risk | Only BP and GFR reported |
Yoa 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions: none |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Intention‐to‐treat analysis | Low risk | Yes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | High risk | Only lipids and CrCl reported |
AHA ‐ American Heart Association; BP ‐ blood pressure; CAN ‐ chronic allograft nephropathy; CNI ‐ calcineurin inhibitor; COPD ‐ chronic obstructive pulmonary disease; CrCl ‐ creatinine clearance; CSA ‐ cyclosporin A; DHA ‐ docosahexaenoic acid; EPA ‐ eicosapentaenoic acid; GFR ‐ glomerular filtration rate; HDL ‐ high density lipoprotein; LDL ‐ low density lipoprotein; MAP ‐ mean arterial pressure; M/F ‐ male/female; MI ‐ myocardial infarction; RCT ‐ randomised controlled trial; SCr ‐ serum creatinine; SD ‐ standard deviation; TC ‐ total cholesterol; TG ‐ triglycerides
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| A‐Echevarria 2004 | Non‐interventional, non‐RCT, cross‐sectional dietary assessment study, examining dietary influences on the plasma profile of fatty acids. |
| Alexander 2005 | This RCT has dual therapy (arginine + canola oil) in the intervention arm. Fish oil is not used, although canola oil does contain small amounts of omega‐3. Arginine alone may have impact on outcome measures. |
| Alexander 2006 | Dual intervention with arginine and fish oil. Arginine alone may have impact on outcome measure |
| Alexander 2008 | Dual intervention with arginine and fish oil |
| Arnadottir 1997 | Non‐RCT, review article on treatment of hyperlipidaemia in kidney transplant recipients |
| Balasubramaniam 1998 | A conference abstract looking at treatment of donors, not recipients |
| Bellomo 2002 | Non‐RCT, conference paper on nutritional support in ICU patients with kidney failure |
| Bennett 1992 | Non‐RCT, review article on arachidonic acid metabolism in transplant‐associated acute kidney failure |
| Bloomgarden 2004 | Non‐RCT, review article |
| Butani 2000 | Non‐RCT, case report on the effect of fish oil in post‐transplantation IgA nephropathy |
| Clark 1994 | Non‐RCT, review article on the treatment of lupus nephritis |
| Davis 2002 | Non‐RCT, review article on kidney disease and liver transplantation |
| de Mattos 1996 | Non‐RCT, review article on immunosuppressive medications |
| Donadio 1997 | Non‐RCT, review article on IgA nephropathy |
| Donadio 2002 | Non‐RCT, review article on IgA nephropathy |
| Endres 1995 | Non‐RCT, review article on n‐3 fatty acids |
| Endres 1996 | Non‐RCT, review article on n‐3 PUFA and human cytokine synthesis |
| Farbakhsh 2005 | Non‐RCT, review article on dyslipidaemia in CKD |
| Gerster 1995 | Non‐RCT, review article on fish oil in enteral nutrition |
| Grekas 2001 | Non‐RCT. There is no control group in this clinical trial of dyslipidaemia, where treatment consists of statin + fish oil |
| Grimble 2001 | Non‐RCT, review article on stress proteins |
| Hansen 1995a | Non‐RCT, cohort study in stable transplants looking at the effects of fish oil on kidney haemodynamics |
| Hansen 1995b | No control group ‐ All patients and "controls" received fish oil. No randomisation |
| Hejaili 2003 | Non‐RCT, review article on lupus nephritis |
| Homan van der Heide 1994 | Non‐RCT. Journal note/commentary on another trial |
| Julian 1997 | Non‐RCT, conference paper on IgA nephropathy |
| Kasiske 2003 | Non‐RCT, K/DOQI practice guidelines on treatment of dyslipidaemia in CKD |
| Kasiske 2004 | Non‐RCT, K/DOQI practice guidelines on treatment of dyslipidaemia in kidney transplantation |
| Kho 1989 | Non‐RCT, study examining effects of dopamine infusion and fish oil administration on urinary prostaglandins |
| Kobashigawa 1997 | Non‐RCT. Review article on hyperlipidaemia in solid organ transplantation |
| Levi 1992 | Fish oil versus corn oil crossover trial, which did not include any of the outcome measures examined in the review |
| Maes 2000 | Non‐RCT, short survey on IgA nephropathy |
| Mathis 2004 | Non‐RCT, review article on drug‐related dyslipidaemia after kidney transplantation |
| Naber 2003 | Non‐RCT, conference paper on lipids in artificial nutrition |
| Nakamura 1998 | Non‐RCT, uncontrolled cohort study on the effects of fish oil on hyperlipidaemia post transplantation |
| Ramezani 2011 | Immunosuppression protocol could not be determined (in relation of CNI exposure) and the lipid data presented represents change in values rather than absolute numbers. No further information was available from study authors. Authors contacted 24 July 2015 ‐ no reply as of 10 Feb 2016 |
| Rodicio 1993 | No fish oil intervention |
| Romo 2012 | Abstract only available. All patients received omega‐3, with the control group receiving placebo and intervention group receiving atorvastatin. Unclear if all patients on CNI |
| Schiele 1999 | Non‐RCT, review article on IgA nephropathy |
| Shah 1994 | Non‐RCT, no fish oil intervention |
| Shihab 1996 | Non‐RCT, review article on cyclosporine nephropathy |
| Singer 2004 | Non‐RCT study of parenteral fish oil given to donors and recipients |
| Soylu 2002 | Non‐RCT, conference paper on paediatric kidney transplant experience |
| Urakaze 1989 | Not all patients were on CNI (separate data for patients on CNI not provided) |
| Wierzbicki 1999 | Non‐RCT, review article on lipid lowering in transplantation |
| Young 1995 | Non‐kidney transplant patients. Conference paper |
| Zak 1996 | Non‐RCT, non‐transplant patients, trial on fish oil in diabetic patients |
| Zolotarski 2003 | Non‐RCT, conference paper on effects of parenteral fish oil given to donors and kidney transplant recipients |
CKD ‐ chronic kidney disease; CNI ‐ calcineurin inhibitor; ICU ‐ intensive care unit; RCT ‐ randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01744067.
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double blind (subject, caregiver, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Active comparator Omega‐3 fatty acids 2,7 g omega‐3 fatty acids / day: Omacor 1 g 1 capsule by mouth 3 times a day for 44 weeks. Placebo comparator Placebo: 1 capsule containing 1 g of olive oil by mouth 3 times a day for 44 weeks. |
| Outcomes | Primary outcome measures Glomerular filtration rate. Time frame: 44 weeks. Iohexol clearance Secondary outcome measures Proteinuria. Time frame: 44 weeks. Both ACR and FEPR Inflammation in the renal transplant. Time frame: 44 weeks. Degree of total inflammation in renal transplant biopsies, scores by PI and rescored by two pathologists. Fibrosis in the renal transplant. Time frame: 44 weeks. As for inflammation Blood pressure. Time frame: 44 weeks. Heart rate variability. Time frame: 44 weeks. Flow mediated dilation. Time frame: 44 weeks. Pulse wave velocity and augmentation index. Time frame: 44 weeks. Blood glucose. Time frame: 44 weeks. HbA1c and oral glucose tolerance test Lipids. Time frame: 44 weeks. Total, LDL and HDL cholesterol, triglyceride and ratios Body composition. Time frame: 44 weeks. Visceral fat volume and weight, visceral to subcutaneous fat ratio. Bone mineral density. Time frame: 44 weeks. Regular BMD in the lumbar spine, hips, femur and arms and also selected to trabecular bone. Body mass index. Time frame: 44 weeks. Vitamin D levels. Time frame: 44 weeks. Fatty acid composition in plasma and renal tissue. Time frame: 44 weeks. Tacrolimus pharmacokinetics. Time frame: 12 weeks. Substudy of 15 patients, where we study tacrolimus trough levels, Tmax and AUC at the end of the ORENTRA trial, after a minimum of 4 weeks wash‐out and again after 4 weeks of 2.7 g omega‐3 fatty acid supplementation Other outcomes Incidence of post‐transplant complications. Time frame: 44 weeks + 8 weeks. Adverse events. Time frame: 44 weeks + 8 weeks. Adverse reactions. Time frame: 44 weeks. Frequency of clinically significant safety laboratory variables [Time Frame: 44 weeks]. Especially INR and tacrolimus trough concentrations. Follow‐up by local nephrologist plus five Telephone Controls during follow‐up. Quality of life. Time frame: 44 weeks. The participants fill out SF‐36 at baseline and the 1 year post transplant control for both safety reasons and measurement of differences between the groups with regards to quality of life. Food questionnaire. Time frame: 44 weeks. Two specially designed food questionnaires to obtain data on dietary habits in general and type and amount of fish consumed Comorbidity, concomitant medication and life‐style factor interview. Time frame: 44 weeks. |
| Notes |
Differences between protocol and review
Risk of bias assessment tool has replaced the Quality checklist.
Contributions of authors
AL: researched background, established protocol, performed search and reviewed identified studies, co‐author of review KM: researched background, established protocol, performed search and reviewed identified studies, co‐author of review MR: conflict arbitration as independent third author, co‐author of review MF: project supervisor, proof‐reading, editorial comments
Declarations of interest
Andy KH Lim: none known
Karen J Manley: none known
Matthew A Roberts: none known
Margaret B Fraenkel: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bennett (high) 1995 {published and unpublished data}
- Bennett WM, Carpenter CB, Shapiro ME, Strom TB, Hefty D, Tillman M, et al. Delayed omega‐3 fatty acid supplements in renal transplantation. A double‐blind, placebo‐controlled study. Transplantation 1995;59(3):352‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Bennett (low) 1995 {published and unpublished data}
- Bennett WM, Carpenter CB, Shapiro ME, Strom TB, Hefty D, Tillman M, et al. Delayed omega‐3 fatty acid supplements in renal transplantation. A double‐blind, placebo‐controlled study. Transplantation 1995;59(3):352‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Berthoux 1992 {published data only}
- Berthoux F, Guerrin C, Bertoux P, Alamarine E. A randomised trial with omega‐3 fatty acid‐fish oil (FO) in renal transplants [abstract]. Nephrology Dialysis Transplantation 1990;5(8):745. [CENTRAL: CN‐00260575] [Google Scholar]
- Berthoux FC, Guerin C, Burgard G, Berthoux P, Alamartine E. One‐year randomized controlled trial with omega‐3 fatty acid‐fish oil in clinical renal transplantation. Transplantation Proceedings 1992;24(6):2578‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Busnach 1998 {published and unpublished data}
- Busnach G, Stragliotto E, Minetti E, Perego A, Brando B, Broggi ML, et al. Effect of n‐3 polyunsaturated fatty acids on cyclosporine pharmacokinetics in kidney graft recipients: a randomised placebo‐controlled study. Journal of Nephrology 1998;11(2):87‐93. [MEDLINE: ] [PubMed] [Google Scholar]
- Busnach G, Stragliotto E, Perego A, Brando B, Civati G. Cyclosporine pharmacokinetics modifications with n‐3 PUFA treatment: a randomized placebo‐controlled study in kidney graft recipients [abstract]. Nephrology 1997;3(Suppl 1):S443. [PubMed] [Google Scholar]
Castro 1997 {published data only}
- Castro R, Queiros J, Fonseca I, Pimentel JP, Henriques AC, Sarmento AM, et al. Therapy of post‐renal transplantation hyperlipidaemia: comparative study with simvastatin and fish oil. Nephrology Dialysis Transplantation 1997;12(10):2140‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hernandez 2002 {published and unpublished data}
- Hernandez D, Guerra R, Milena A, Garcia S, Garcia C, Abreu P, et al. Dietary fish oil does not influence acute rejection rate and graft survival after renal transplantation a randomized placebo‐controlled study. Nephrology Dialysis Transplantation 2002;17(5):897‐904. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Homan van der Heide 1990a {published data only}
- Homan van der Heide JJ, Bilo HJ, Donker AJ, Wilmink JM, Sluiter WJ, Tegzess AM. Dietary supplementation with fish oil modifies renal reserve filtration capacity in postoperative, cyclosporin A‐treated renal transplant recipients. Transplant International 1990;3(3):171‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Homan van der Heide JJ, Bilo HJG, Tegzess AM, Donker AJM. Omega‐3 polynsaturated fatty acids improve renal function in renal transplant recipients treated with cyclosporin‐A [abstract]. Kidney International 1989;35(1):516. [CENTRAL: CN‐00626077] [Google Scholar]
Homan van der Heide 1990b {published data only}
- Homan van der Heide JJ, Bilo HJ, Sluiter WJ, Donker AJ, Tegzess AM. Dietary fish‐oil Improves renal reserve capacity after amino‐acid infusion in acute postoperative cyclosporin‐treated renal transplant recipients [abstract]. Nephrology Dialysis Transplantation 1990;5(4):308. [CENTRAL: CN‐00260597] [Google Scholar]
- Homan van der Heide JJ, Bilo HJ, Tegzess AM, Donker AJ. The effects of dietary supplementation with fish oil on renal function in cyclosporine‐treated renal transplant recipients. Transplantation 1990;49(3):523‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Homan van der Heide JJ, Bilo HJG, Sluiter WJ, Donker AJM, Tegzess AM. Dietary fish oil improves renal reserve filtration capacity (RRFC) after amino acid infusion in ciclosporin‐treated renal transplant patients. [abstract]. Kidney International 1990;37(1):614. [CENTRAL: CN‐00615877] [Google Scholar]
- Homan van der Heide JJ, Bilo HJG, Tegzess AM, Donker AJM. Omega‐3 polyunsaturated fatty acids improve renal function in renal transplant recipients treated with cyclosporin A [abstract]. Kidney International 1989;35(6):1432. [CENTRAL: CN‐00775629] [Google Scholar]
Homan van der Heide 1992 {published data only}
- Homan van der Heide J, Bilo HJG, Donker AJM, Wilmink JM, Tegzess AM. Better renal function, lower mean arterial pressure (MAP) and less rejection episodes in cyslosporin A (CsA)‐treated renal transplant recipients fed dietary fish oil (FO) [abstract]. Kidney International 1991;40(5):979. [Google Scholar]
- Homan van der Heide JJ, Bilo HJ, Donker AJ, Wilmink JM, Sluiter WJ, Tegzess AM. The effects of dietary supplementation with fish oil on renal function and the course of early postoperative rejection episodes in cyclosporine‐treated renal transplant recipients. Transplantation 1992;54(2):257‐63. [MEDLINE: ] [PubMed] [Google Scholar]
- Homan van der Heide JJ, Bilo HJG, Donker AJM, Wilmink JM, Sluiter WJ, Tegzess AM. Dietary fish oil modifies renal function during early postoperative rejection episodes in cyclosporin (CsA) treated renal allograft recipients [abstract]. Kidney International 1990;38(6):1233. [Google Scholar]
Homan van der Heide 1993 {published data only}
- Homan van der Heide JJ, Bilo HJ, Donker JM, Wilmink JM, Tegzess AM. Effect of dietary fish oil on renal function and rejection in cyclosporine‐treated recipients of renal transplants. New England Journal of Medicine 1993;329(11):769‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kooijmans‐Coutinho 1996 {published data only}
- Kooijmans‐Coutinho MF, Homan van der Heide JJ, Rischen‐Vos J, Hermans J, Es LA, Woude FJ. The effects of dietary fish oil in cyclosporine‐A treated renal transplant recipients [abstract]. Journal of the American Society of Nephrology 1994;5(3):1018. [DOI] [PubMed] [Google Scholar]
- Kooijmans‐Coutinho MF, Rischen‐Vos J, Hermans J, Arndt JW, Woude FJ. Dietary fish oil in renal transplant recipients treated with cyclosporin‐A: no beneficial effects shown. Journal of the American Society of Nephrology 1996;7(3):513‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maachi 1995 {published data only}
- Maachi K, Berthoux P, Burgard G, Alamartine E, Berthoux F. Results of a 1‐year randomized controlled trial with omega‐3 fatty acid fish oil in renal transplantation under triple immunosuppressive therapy. Transplantation Proceedings 1995;27(1):846‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Rodriguez 1997 {published data only}
- Rodriguez AP, Bonis E, Gonzalez‐Posada JM, Torres A, Perez L, Dominguez ML, et al. Treatment of hyperlipidemia after renal transplantation: Comparative effect of lovastatin and omega‐3 fatty acids [Tratamiento de la dislipemia postrasplante renal: efecto comparativo de la lovastatina y acidos grasos pliinsaturados omega‐3]. Nefrologia 1997;17(1):49‐54. [EMBASE: 1997130521] [Google Scholar]
Santos 2000 {published data only}
- Santos J, Queiros J, Silva F, Cabrita A, Rodrigues A, Henriques AC, et al. Effects of fish oil in cyclosporine‐treated renal transplant recipients. Transplantation Proceedings 2000;32(8):2605‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schut 1992a {published data only}
- Schut NH, Bilo HJ, Popp‐Snijders C, Goedhart PT, Wilmink JM. Erythrocyte deformability, endothelin levels, and renal function in cyclosporin‐treated renal transplant recipients: effects of intervention with fish oil and corn oil. Scandinavian Journal of Clinical & Laboratory Investigation 1993;53(5):499‐506. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schut NH, Hardeman MR, Goedhart PT, Bilo HJ, Wilmink JM. Blood viscosity measurements are not sensitive enough to detect changes in erythrocyte deformability in cyclosporin‐treated patients and its subsequent reversal with fish and corn oil. Clinical Hemorheology 1993;13(4):465‐72. [EMBASE: 1993223288] [Google Scholar]
- Schut NH, Hardeman MR, Wilmink JM. Decrease of erythrocyte deformability in cyclosporine‐treated renal transplant patients: correction with fish oil as well as corn oil. Transplant International 1992;5 Suppl 1:S536‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schut 1992b {published data only}
- Schut NH, Bilo HJ, Popp‐Snijders C, Goedhart PT, Wilmink JM. Erythrocyte deformability, endothelin levels, and renal function in cyclosporin‐treated renal transplant recipients: effects of intervention with fish oil and corn oil. Scandinavian Journal of Clinical & Laboratory Investigation 1993;53(5):499‐506. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schut NH, Hardeman MR, Goedhart PT, Bilo HJ, Wilmink JM. Blood viscosity measurements are not sensitive enough to detect changes in erythrocyte deformability in cyclosporin‐treated patients and its subsequent reversal with fish and corn oil. Clinical Hemorheology 1993;13(4):465‐72. [EMBASE: 1993223288] [Google Scholar]
- Schut NH, Hardeman MR, Wilmink JM. Decrease of erythrocyte deformability in cyclosporine‐treated renal transplant patients: correction with fish oil as well as corn oil. Transplant International 1992;5 Suppl 1:S536‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yoa 1994 {published data only}
- Corda C, Mounie J, Mousson C, Charfeddine K, Chulopin JM, Escousse A. Randomised double blind study of the possible protective effects by fish oil polyunsaturated omega 3 fatty acids, against cyclosporine A (CyA) induced renal damage in kidney transplants [abstract]. Fundamental & Clinical Pharmacology 1992;6(4‐5):223. [CENTRAL: CN‐00305208] [Google Scholar]
- Yoa RG, Corda C, Rapin JR, Santona L, Goudonnet H, Rifle G, et al. Hemorheological benefits of omega‐3 polyunsatured fatty acids on erythrocyte deformability in renal transplanted patients. Clinical Hemorheology 1994;14(5):663‐75. [EMBASE: 1994288697] [Google Scholar]
References to studies excluded from this review
A‐Echevarria 2004 {published data only}
- Aldamiz‐Echevarria L, Vallo A, Sanjurjo P, Elorz J, Prieto JA, Ruiz JI, et al. Influence of diet on atherogenic risk in children with renal transplants. Pediatric Nephrology 2004;19(9):1039‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alexander 2005 {published data only}
- Alexander JW, Metze TJ, Goodman HR, Greenberg NA, First MR, McIntosh MJ, et al. Transplant success and immunomodulating diets [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00415168]
- Alexander JW, Metze TJ, Goodman HR, McIntosh MJ, Zeng L, Cardi MA, et al. Transplant success and immunomodulating diets [abstract]. American Journal of Transplantation 2003;3(Suppl 5):562. [CENTRAL: CN‐00444140] [Google Scholar]
- Alexander JW, Metze TJ, McIntosh MJ, Goodman HR, First MR, Munda R, et al. The influence of immunomodulatory diets on transplant success and complications. Transplantation 2005;79(4):460‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Alexander JW, Ofstedal T, Stanley L, Erickson A, Greenberg N, First MR, et al. L‐arginine and canola oil improve outcome after renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00444141]
Alexander 2006 {published data only}
- Alexander JW, Goodman HR, Cardi M, Austin J, Goel S, Safdar S, et al. Simultaneous corticosteroid avoidance and calcineurin inhibitor minimization in renal transplantation. Transplant International 2006;19(4):295‐302. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alexander 2008 {published data only}
- Alexander JW, Goodman HR, Succop P, Light JA, Kuo PC, Moser AB, et al. Influence of long chain polyunsaturated fatty acids and ornithine concentrations on complications after renal transplant. Experimental & Clinical Transplantation 2008;6(2):118‐26. [MEDLINE: ] [PubMed] [Google Scholar]
Arnadottir 1997 {published data only}
- Arnadottir M, Berg AL. Treatment of hyperlipidemia in renal transplant recipients. Transplantation 1997;63(3):339‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Balasubramaniam 1998 {published data only}
- Balasubramaniam J, Balasubramaniam S, Palaniappan N. Effect of eicosapentanoic acid (EPA) therapy to donors in prevention of ischemic renal injury in live donor renal transplantation ‐ a study [abstract]. 35th Congress. European Renal Association. European Dialysis and Transplantation Association; 1998 Jun 6‐9; Rimini, Italy. 1998:371. [CENTRAL: CN‐00483112]
Bellomo 2002 {published data only}
- Bellomo R. How to feed patients with renal dysfunction. Blood Purification 2002;20(3):296‐303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bennett 1992 {published data only}
- Bennett WM. Therapeutic implications of arachidonic acid metabolism in transplant‐associated acute renal failure. Renal Failure 1992;14(3):261‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bloomgarden 2004 {published data only}
- Bloomgarden ZT. Aspects of blood pressure, lipid, and glycemic treatment. Diabetes Care 2004;27(1):264‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Butani 2000 {published data only}
- Butani L, Palmer J. Effect of fish oil in a patient with post‐transplantation IgA nephropathy. Nephrology Dialysis Transplantation 2000;15(8):1264‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clark 1994 {published data only}
- Clark WF. Treatment of lupus nephritis: immunosuppression, general therapy, dialysis and transplantation. Clinical & Investigative Medicine ‐ Medecine Clinique et Experimentale 1994;17(6):588‐601. [MEDLINE: ] [PubMed] [Google Scholar]
Davis 2002 {published data only}
- Davis CL, Gonwa TA, Wilkinson AH. Pathophysiology of renal disease associated with liver disorders: implications for liver transplantation. Part 1. Liver Transplantation 2002;8(2):91‐109. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Mattos 1996 {published data only}
- Mattos AM, Olyaei AJ, Bennett WM. Pharmacology of immunosuppressive medications used in renal diseases and transplantation. American Journal of Kidney Diseases 1996;28(5):631‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Donadio 1997 {published data only}
- Donadio JV Jr, Grande JP. Immunoglobulin A nephropathy: a clinical perspective. Journal of the American Society of Nephrology 1997;8(8):1324‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Donadio 2002 {published data only}
- Donadio JV, Grande JP. IgA nephropathy. New England Journal of Medicine 2002;347(10):738‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Endres 1995 {published data only}
- Endres S, Caterina R, Schmidt EB, Kristensen SD. n‐3 polyunsaturated fatty acids: update 1995. European Journal of Clinical Investigation 1995;25(9):629‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Endres 1996 {published data only}
- Endres S, Schacky C. n‐3 polyunsaturated fatty acids and human cytokine synthesis. Current Opinion in Lipidology 1996;7(1):48‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Farbakhsh 2005 {published data only}
- Farbakhsh K, Kasiske BL. Dyslipidemias in patients who have chronic kidney disease. Medical Clinics of North America 2005;89(3):689‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gerster 1995 {published data only}
- Gerster H. The use of n‐3 PUFAs (fish oil) in enteral nutrition. International Journal for Vitamin & Nutrition Research 1995;65(1):3‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Grekas 2001 {published data only}
- Grekas D, Kassimatis E, Makedou A, Bacharaki D, Bamichas G, Tourkantonis A. Combined treatment with low‐dose pravastatin and fish oil in post‐renal transplantation dislipidemia. Nephron 2000;88(4):329‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grimble 2001 {published data only}
- Grimble RF. Stress proteins in disease: metabolism on a knife edge. Clinical Nutrition 2001;20(6):469‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hansen 1995a {published data only}
- Hansen JM, Hoy CE, Strandgaard S. Fish oil and cyclosporin A‐induced renal hypoperfusion in kidney‐transplanted patients. Nephrology Dialysis Transplantation 1995;10(9):1745‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Hansen 1995b {published data only}
- Hansen JM, Lokkegaard H, Hoy CE, Fogh‐Andersen N, Olsen NV, Strandgaard S. No effect of dietary fish oil on renal hemodynamics, tubular function, and renal functional reserve in long‐term renal transplant recipients. Journal of the American Society of Nephrology 1995;5(7):1434‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hejaili 2003 {published data only}
- Hejaili FF, Moist LM, Clark WF. Treatment of lupus nephritis. Drugs 2003;63(3):257‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Homan van der Heide 1994 {published data only}
- Homan van der Heide JJ, Lynch CT. Dietary fish oil after renal transplantation. Annals of Internal Medicine 1994;120(6 Suppl 2):41. [EMBASE: 1994084101] [Google Scholar]
Julian 1997 {published data only}
- Julian BA, Bake AW. Treatment options in IgA nephropathy. Nephrology 1997;3(1):103‐8. [EMBASE: 1997113212] [Google Scholar]
Kasiske 2003 {published data only}
- Kasiske B, Cosio FG, Beto J, Chavers B, Grimm Jr R, Levin A, et al. K/DOQI clinical practice guidelines for managing dyslipidemias in chronic kidney disease. American Journal of Kidney Diseases 2003;41(4 Suppl 3):i‐s91. [EMBASE: 2003140838] [Google Scholar]
Kasiske 2004 {published data only}
- Kasiske B, Cosio FG, Beto J, Bolton K, Chavers BM, Grimm Jr R, et al. Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: A report from the Managing Dyslipidemias in Chronic Kidney Disease Work gGroup of the National Kidney Foundation Disease Outcomes Quality Initiative. American Journal of Transplantation 2004;4 Suppl 7:13‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kho 1989 {published data only}
- Kho TL, Leunissen KM, Wirtz JJ, Dries PJ, Noordzij TZ, Rijnders A, et al. Cyclosporine and urinary prostaglandins. Transplantation Proceedings 1989;21(1 Pt 2):1504‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Kobashigawa 1997 {published data only}
- Kobashigawa JA, Kasiske BL. Hyperlipidemia in solid organ transplantation. Transplantation 1997;63(3):331‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levi 1992 {published data only}
- Levi M, Wilmink J, Buller HR, Surachno J, Cate JW. Impaired fibrinolysis in cyclosporine‐treated renal transplant patients. Analysis of the defect and beneficial effect of fish‐oil. Transplantation 1992;54(6):978‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Levi M, Wilmink JM, Cate JW. Defective fibrinolysis in patients using cyclosporine after renal transplantation; Beneficial effects of fish oil [abstract]. Kidney International 1991;40(5):974. [Google Scholar]
Maes 2000 {published data only}
- Maes B. IgA nephropathy. Tijdschrift voor Geneeskunde 2000;56(20):1496‐508. [EMBASE: 2000383155] [Google Scholar]
Mathis 2004 {published data only}
- Mathis AS, Dave N, Knipp GT, Friedman GS. Drug‐related dyslipidemia after renal transplantation. American Journal of Health‐System Pharmacy 2004;61(6):565‐87. [MEDLINE: ] [PubMed] [Google Scholar]
Naber 2003 {published data only}
- Naber AH. Sense and nonsense of lipids in artificial nutrition. Scandinavian Journal of Gastroenterology ‐ Supplement 2003;38(239):11‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamura 1998 {published data only}
- Nakamura H, Asano T, Suzuki S, Tokonabe S, Hayakawa M. Evaluation of ethyl icosapentate in the treatment of hypercholesterolemia in kidney transplant recipients. Transplantation Proceedings 1998;30(7):3047‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramezani 2011 {published data only}
- Ramezani M, Nazemian F, Shamsara J, Koohrokhi R, Mohammadpour AH. Effect of omega‐3 fatty acids on plasma level of 8‐isoprostane in kidney transplant patients. Journal of Renal Nutrition 2011;21(2):196‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rodicio 1993 {published data only}
- Rodicio JL, Morales JM, Ruilope LM. Lipophilic dihydropyridines provide renal protection from cyclosporin toxicity. Journal of Hypertension ‐ Supplement 1993;11(6):S21‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Romo 2012 {published data only}
- Romo JC, Hernandez AM, Toussaint G, Lopez S, Navarro B, Velasquez L, et al. Treatment of dyslipidemia in renal transplant children, a comparative, prospective, randomized study [abstract]. Blood Purification 2012;33(1‐3):215. [EMBASE: 70723740] [Google Scholar]
Schiele 1999 {published data only}
- Schiele J, Nowack R, Julian BA, Woude FJ. Treatment of immunoglobulin A nephropathy. Annales de Medicine Interne 1999;150(2):127‐36. [MEDLINE: ] [PubMed] [Google Scholar]
Shah 1994 {published data only}
- Shah B, Nair S, Sirsat RA, Ashavaid TF, Nair KG. Dyslipidemia in patients with chronic renal failure and in renal transplant patients. Journal of Postgraduate Medicine 1994;40(2):57‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Shihab 1996 {published data only}
- Shihab FS. Cyclosporine nephropathy: pathophysiology and clinical impact. Seminars in Nephrology 1996;16(6):536‐47. [MEDLINE: ] [PubMed] [Google Scholar]
Singer 2004 {published data only}
- Singer P, Zolotarski V, Yussim A, Lustig S, Attal‐Singer J, Cohen J. Renal effects of parenteral fish oil administered to heart‐beating organ donors and renal‐transplant recipients: a tolerance study. Clinical Nutrition 2004;23(4):597‐603. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Soylu 2002 {published data only}
- Soylu A, Kavucku S, Turkmen M, Bora S, Gulay H. Eight‐year experience in pediatric renal transplantation. Transplantation Proceedings 2002;34(6):2062‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Urakaze 1989 {published data only}
- Urakaze M, Hamazaki T, Kashiwabara H, Omori K, Fischer S, Yano S. Favorable effects of fish oil concentrate on risk factors for thrombosis in renal allograft recipients. Nephron 1989;53(2):102‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Urakaze M, Hamazaki T, Yano S, Kashiwabara H, Oomori K, Yokoyama T. Effect of fish oil concentrate on risk factors of cardiovascular complications in renal transplantation. Transplantation Proceedings 1989;21(1 Pt 2):2134‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Wierzbicki 1999 {published data only}
- Wierzbicki AS. The role of lipid lowering in transplantation. International Journal of Clinical Practice 1999;53(1):54‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Young 1995 {published data only}
- Young JB. Fish oil and antioxidants after heart transplantation: future strategies or eye of newt and wing of bat revisited?. Journal of Heart & Lung Transplantation 1995;14(6 Pt 2):S250‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Zak 1996 {published data only}
- Zak A, Zeman M, Tvrzicka E, Stolba P. Effects of fish oils in patients with type 2 diabetes with associated dyslipidaemia [Ucinky rybich oleju na nektere metabolicke parametry nemocnych s diabetes mellitus 2. typu a pruvodnou dyslipidemii]. Casopis Lekaru Ceskych 1996;135(11):354‐9. [EMBASE: 1996174498] [PubMed] [Google Scholar]
Zolotarski 2003 {published data only}
- Zolotarski V, Cohen J, Sharabani E, Bar Nathan N, Yussim A, Lustig S, et al. Parenteral fish oil administered to heart‐beating organ donors and to renal transplant recipients: effects on renal function. Transplantation Proceedings 2003;35(2):624. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01744067 {published data only}
- Eide IA. The effects of n‐3 polyunsaturated fatty acids on renal and cardiovascular risk markers in renal transplant recipients: a randomized double blinded placebo controlled intervention study. www.clinicaltrials.gov/ct2/show/NCT01744067 (accessed 1 March 2016).
Additional references
Ader 1998
- Ader JL, Rostaing L. Cyclosporin nephrotoxicity: pathophysiology and comparison with FK‐506. Current Opinion in Nephrology & Hypertension 1998;7(5):539‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Andoh 1997
- Andoh TF, Burdmann EA, Bennett WM. Nephrotoxicity of immunosuppressive drugs: experimental and clinical observations. Seminars in Nephrology 1997;17(1):34‐45. [MEDLINE: ] [PubMed] [Google Scholar]
Andoh 1998
- Andoh TF, Bennett WM. Chronic cyclosporine nephrotoxicity. Current Opinion in Nephrology & Hypertension 1998;7(3):265‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Balk 2006
- Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega‐3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis 2006;189(1):19‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonis 2005
- Bonis PA, Chung M, Tatsioni A, Sun Y, Kupelnick B, Lichtenstein A, et al. Effects of omega‐3 fatty acids on organ transplantation. Evidence Report: Technology Assessment (Summary) 2005, (115):1‐11. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Braun 2003
- Braun WE. The medical management of renal transplant recipients. In: Johnson RJ, Feehally J editor(s). Comprehensive Clinical Nephrology. 2nd Edition. London: Mosby, 2003:1105‐10. [Google Scholar]
Brenna 2002
- Brenna JT. Efficiency of conversion of alpha‐linolenic acid to long chain n‐3 fatty acids in man. Current Opinion in Clinical Nutrition & Metabolic Care 2002;5(2):127‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Mattos 2000
- Mattos AM, Olyaei AJ, Bennett WM. Nephrotoxicity of immunosuppressive drugs: Long‐term consequences and challenges for the future. American Journal of Kidney Diseases 2000;35(2):333‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Din 2004
- Din JN, Newby DE, Flapan AD. Omega 3 fatty acids and cardiovascular disease‐fishing for a natural treatment. BMJ 2004;328(7430):30‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eide 2015
- Eide IA, Jenssen T, Hartmann A, Diep LM, Dahle DO, Reisaeter AV, et al. The association between marine n‐3 polyunsaturated fatty acid levels and survival after renal transplantation. Clinical Journal of The American Society of Nephrology: CJASN 2015;10(7):1246‐56. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eide 2016
- Eide IA, Jenssen T, Hartmann A, Diep LM, Dahle DO, Reisaeter AV, et al. Plasma levels of marine n‐3 polyunsaturated fatty acids and renal allograft survival. Nephrology Dialysis Transplantation 2016;31(1):160‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Endres 1989
- Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, Meer JW, et al. The effect of dietary supplementation with n‐3 polyunsaturated fatty acids on the synthesis of interleukin‐1 and tumor necrosis factor by mononuclear cells. New England Journal of Medicine 1989;320(5):265‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Endres 1993
- Endres S, Meydani SN, Ghorbani R, Schindler R, Dinarello CA. Dietary supplementation with n‐3 fatty acids suppresses interleukin‐2 production and mononuclear cell proliferation. Journal of Leukocyte Biology 1993;54(6):599‐603. [MEDLINE: ] [PubMed] [Google Scholar]
Ford 1991
- Ford HR, Hoffman RA, Tweardy DJ, Kispert P, Wang S, Simmons RL. Evidence that production of interleukin 6 within the rejecting allograft coincides with cytotoxic T lymphocyte development. Transplantation 1991;51(3):656‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7417):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirunpanich 2006
- Hirunpanich V, Katagi J, Sethabouppha B, Sato H. Demonstration of docosahexaenoic acid as a bioavailability enhancer for CYP3A substrates: in vitro and in vivo evidence using cyclosporin in rats. Drug Metabolism & Disposition 2006;34(2):305‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hooper 2006
- Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ 2006;332(7544):752‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelley 1989
- Kelley VE, Kirkman RL, Bastos M, Barrett LV, Strom TB. Enhancement of immunosuppression by substitution of fish oil for olive oil as a vehicle for cyclosporine. Transplantation 1989;48(1):98‐102. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kris‐Etherton 2000
- Kris‐Etherton PM, Taylor DS, Yu‐Poth S, Huth P, Moriarty K, Fishell V, et al. Polyunsaturated fatty acids in the food chain in the United States. American Journal of Clinical Nutrition 2000;71(1 Suppl):179S‐88S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McDonald 2003
- McDonald S, Russ G. Deaths. 26th Report of the ANZDATA Registry. Adelaide: Australia and New Zealand Dialysis and Transplant Registry, 2003:15‐23. [Google Scholar]
Noronha 1992
- Noronha IL, Eberlein‐Gonska M, Hartley B, Stephens S, Cameron JS, Waldherr R. In situ expression of tumor necrosis factor‐alpha, interferon‐gamma, and interleukin‐2 receptors in renal allograft biopsies. Transplantation 1992;54(6):1017‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Noronha 1993
- Noronha IL, Hartley B, Cameron JS, Waldherr R. Detection of IL‐1 beta and TNF‐alpha message and protein in renal allograft biopsies. Transplantation 1993;56(4):1026‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Norris 1990
- Norris SH. Renal eicosanoids. Seminars in Nephrology 1990;10(1):64‐88. [MEDLINE: ] [PubMed] [Google Scholar]
Ojo 2003
- Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. New England Journal of Medicine 2003;349(10):931‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Opelz 1998
- Opelz G, Wujciak T, Ritz E. Association of chronic kidney graft failure with recipient blood pressure (Collaborative Transplant Study). Kidney International 1998;53(1):217‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Otto 1990
- Otto DA, Kahn DR, Hamm MW, Forrest DE, Wooten JT. Improved survival of heterotopic cardiac allografts in rats with dietary n‐3 polyunsaturated fatty acids. Transplantation 1990;50(2):193‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Psota 2006
- Psota TL, Gebauer SK, Kris‐Etherton P. Dietary omega‐3 fatty acid intake and cardiovascular risk. American Journal of Cardiology 2006;98(4A):3i‐18i. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sweny 1989
- Sweny P, Wheeler DC, Lui SF, Amin NS, Barradas MA, Jeremy JY, et al. Dietary fish oil supplements preserve renal function in renal transplant recipients with chronic vascular rejection. Nephrology Dialysis Transplantation 1989;4(12):1070‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Tatsioni 2005
- Tatsioni A, Chung M, Sun Y, Kupelnick B, Lichtenstein AH, Perrone R, et al. Effects of fish oil supplementation on kidney transplantation: A systematic review and meta‐analysis of randomized, controlled trials. Journal of the American Society of Nephrology 2005;16(8):2462‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vandenbroecke 1991
- Vandenbroecke C, Caillat‐Zucman S, Legendre C, Noel LH, Kreis H, Woodrow D, et al. Differential in situ expression of cytokines in renal allograft rejection. Transplantation 1991;51(3):602‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2006
- Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, et al. n‐3 fatty acids from fish or fish oil supplements, but not alpha‐linolenic acid, benefit cardiovascular disease outcomes in primary‐ and secondary‐prevention studies: a systematic review. American Journal of Clinical Nutrition 2006;84(1):5‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yard 1994
- Yard BA, Kooymans‐Couthino M, Paape ME, Bruijn JA, Daha MR, Es LA, et al. Analysis of cytokine production by graft‐infiltrating cells isolated from rejecting renal allografts. Transplantation 1994;57(1):153‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lim 2007a
- Lim AK, Manley KJ, Roberts MA, Fraenkel MB. Fish oil for kidney transplant recipients. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005282.pub2] [DOI] [PubMed] [Google Scholar]
Lim 2007b
Manley 2005
- Manley K, Fraenkel M, Lim A, Roberts MA. Fish oil for kidney transplant recipients. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005282] [DOI] [PubMed] [Google Scholar]


