Abstract
Importance:
One-third of ischemic strokes have no identifiable cause after standard evaluation. In 2014, Hart et al termed these “embolic strokes of undetermined source” (ESUS) and argued that this entity would respond to anticoagulation. Two recent trials did not uphold this hypothesis, leading to questions about the ESUS concept.
Observations:
We propose that ESUS remains a useful concept, the clinical impact of which can be enhanced by considering two subsets defined by their likelihood of responding to anticoagulation. Recent studies indicate that some ESUS cases result from subclinical atrial fibrillation, atrial cardiopathy, unrecognized myocardial infarction, patent foramen ovale, or cancer, while other cases result from nonstenosing large-artery atherosclerosis, aortic atherosclerosis, or non-atherosclerotic vasculopathies. Evidence suggests that anticoagulation will prove superior to antiplatelet therapy for the former group of etiologies but not the latter, suggesting the need for personalized therapy.
Conclusions and Relevance:
Although the ESUS concept as currently constructed cannot guide treatment, efforts to better understand ESUS and develop therapies tailored to specific mechanisms are likely to help reduce the burden of stroke.
Stroke accounts for 10% of all deaths worldwide and leads to substantial long-term disability.1 Most strokes are ischemic and up to one-third of ischemic strokes do not have a known cause after standard evaluation.2,3 For decades, such strokes were referred to as cryptogenic strokes; in 2014, Hart et al proposed the term “embolic stroke of undetermined source” (ESUS).4 These investigators elucidated that most cryptogenic strokes share the clinical and radiographic appearance of a distant embolic source. The ESUS concept has been a conceptual spur to scientific advancement in vascular neurology; since 2014, over 200 published studies have shed light on various possible underlying mechanisms of ESUS, its natural history, and optimal therapies to prevent recurrence. As part of this effort, two randomized trials recently indicated that there is no overall benefit of anticoagulant therapy in preventing recurrent stroke after ESUS.5,6 These results have diminished the hope that ESUS might be a single entity which can be addressed with a unified treatment approach. Nevertheless, strokes that fit the ESUS definition remain a substantial challenge,7 suggesting that additional research into their pathophysiology and management remains important. In this article, we will review recent evidence indicating a previously underappreciated heterogeneity in the mechanisms of ESUS (Figure 1) and outline the potential benefits of tailoring treatments to patients’ individual characteristics.
Figure 1.
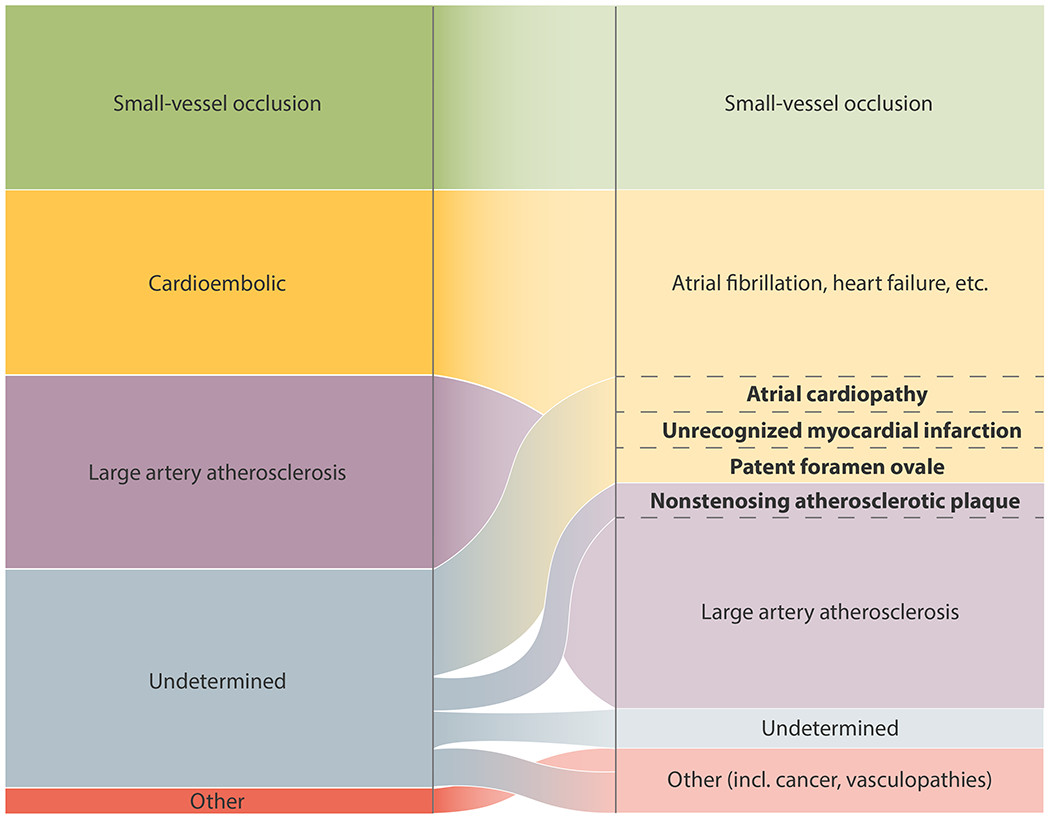
Currently Accepted Etiologies of Ischemic Stroke and Their Likely Reclassification Based on Emerging Evidence.
Original Formulation of the Concept of Embolic Stroke of Undetermined Source
The central thesis of the ESUS concept was that most cryptogenic strokes are thromboembolic and that such strokes are a therapeutically relevant entity likely to benefit from anticoagulation. Hart et al hypothesized that currently occult sources of thromboembolism are mostly comprised of various cardiac abnormalities, venous thromboembolism via a patent foramen ovale (PFO), and nonstenosing atherosclerotic plaque. They argued that embolization from all of the above sources mostly consists of thrombus and thus anticoagulation should prevent recurrence better than antiplatelet therapy. On the basis of this formulation, two major trials, NAVIGATE ESUS and RE-SPECT ESUS, were launched to compare non-vitamin K antagonist oral anticoagulant (NOAC) drugs versus aspirin in patients with recent ESUS. If validated, such an approach would have obviated the need for extensive testing to identify the proximal cause of the ischemic event, allowing the broad category of ESUS to be treated with the same NOAC drugs that clinicians feel comfortable prescribing for atrial fibrillation (AF). Unfortunately, neither NAVIGATE ESUS nor RE-SPECT ESUS demonstrated a reduction in stroke recurrence with anticoagulation.5,6 The results of these trials have understandably raised questions about the utility of the ESUS concept and the role of anticoagulant therapy in patients with strokes of undetermined source.8,9 Here, we propose that ESUS remains a useful concept, the clinical impact of which can be enhanced by considering two subgroups defined by their likelihood of responding to anticoagulation (Figure 2).
Figure 2.
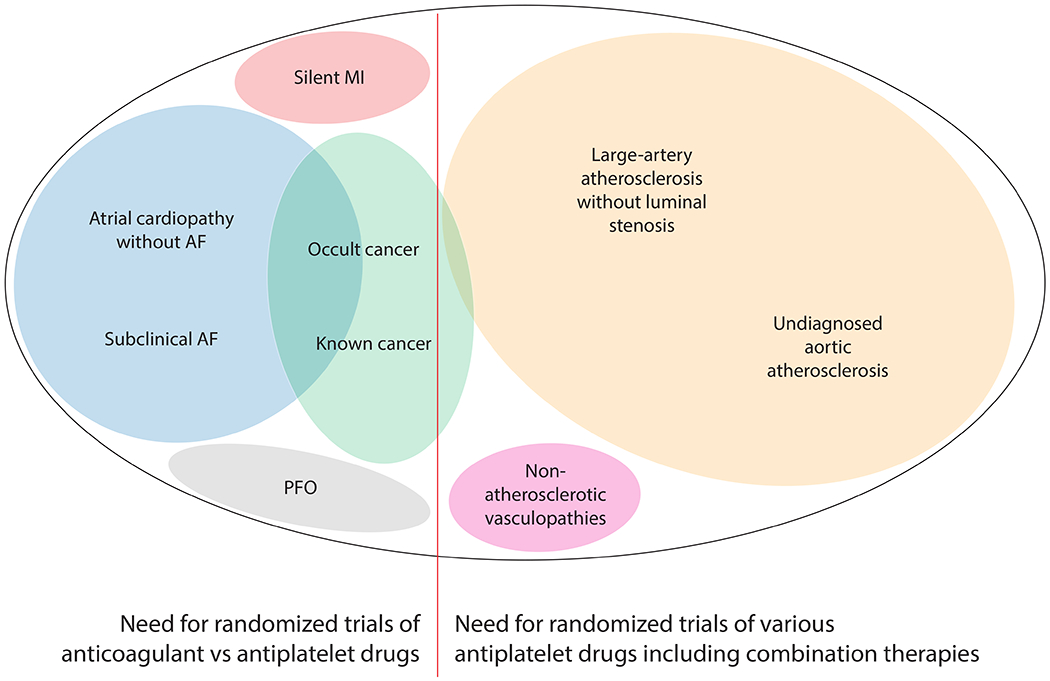
Potential Occult Sources of Currently Unexplained Ischemic Stroke, Their Overlap, and Their Expected Response to Antithrombotic Drugs.
Occult Embolic Mechanisms Likely to Respond to Anticoagulant Therapy
Subclinical Atrial Fibrillation
A leading candidate for an occult mechanism of ESUS is subclinical AF. This common arrhythmia often occurs in a paroxysmal and asymptomatic form10 and may cause a stroke but then revert back to sinus rhythm when the patient presents for evaluation, leading clinicians to label the stroke as ESUS. Such a scenario spurred numerous studies of continuous heart-rhythm monitoring over the past few decades. These studies show that prolonged heart-rhythm monitoring establishes a new diagnosis of AF in 10-20% of patients with recent stroke,11 a much higher rate than seen with only clinical follow-up.12 The yield increases with the duration of monitoring and approaches 30% after 3 years of continuous monitoring.13 Based on these studies, recent guidelines make a moderate recommendation for post-discharge heart-rhythm monitoring.14–16
It is not fully established that AF detected after ESUS was responsible for the preceding stroke. For example, post-stroke AF may be triggered by damage to central autonomic pathways.17 However, many post-ESUS AF cases are probably related to the preceding stroke. The incidence of new AF diagnosis after hospitalization for ischemic stroke, particularly cryptogenic stroke, appears to be higher than after hospitalization for hemorrhagic stroke or other non-stroke conditions, arguing for some degree of pathogenic connection.18 It remains unclear whether anticoagulation is superior to antiplatelet therapy for secondary stroke prevention in patients with subclinical AF detected after ESUS. This hypothesis will probably not be tested in patients with prior stroke, given that approximately 90% of physicians treated these patients with anticoagulation in the CRYSTAL-AF and EMBRACE trials.12,13 It is hoped that the NoAH and ARTESiA trials,19,20 which are comparing anticoagulant and antiplatelet therapy in stroke-free patients with subclinical AF, will shed more light on this question soon.
Several other questions remain about the relationship between subclinical AF and stroke. First, what about the 70% of ESUS patients with no AF even after prolonged heart-rhythm monitoring?13 Clearly, subclinical AF does not account for most cases of ESUS. Second, how could only a few minutes of subclinical AF lead to an increased risk of stroke months later?21 In many of these cases, AF occurs for the first time after the stroke.22,23 These findings undermine the notion of a direct, causal relationship between AF and stroke22 and have led to the hypothesis that underlying atrial disease may cause stroke in the absence of arrhythmia.
Atrial Cardiopathy
AF rarely develops in a healthy atrium and usually occurs in the setting of an abnormal atrial substrate (Figure 3B, 3E).24 It is possible that such an abnormal atrial substrate—referred to as atrial cardiopathy, atrial cardiomyopathy, or atrial myopathy—forms a nidus for thromboembolism even before AF occurs.25–28 In support of this hypothesis, multiple studies have found associations between markers of left atrial dysfunction and ischemic stroke in the absence of AF.29–32 Markers of atrial cardiopathy are most strongly associated with embolic-appearing strokes.33,34 Even though anticoagulant therapy has not proven superior to antiplatelet therapy for preventing stroke recurrence in patients without known AF,5,35 post hoc analyses have found a benefit in the subset of patients with elevated NT-proBNP31 or an enlarged left atrium.36 These findings suggest that atrial cardiopathy that has not manifested with AF may be an underlying mechanism of ESUS and that anticoagulant therapy may prove superior to standard antiplatelet therapy in this subset of ESUS patients. The ongoing ARCADIA trial is testing this hypothesis.37
Figure 3. Imaging Findings Associated with Potential Occult Sources of Currently Unexplained Ischemic Stroke.
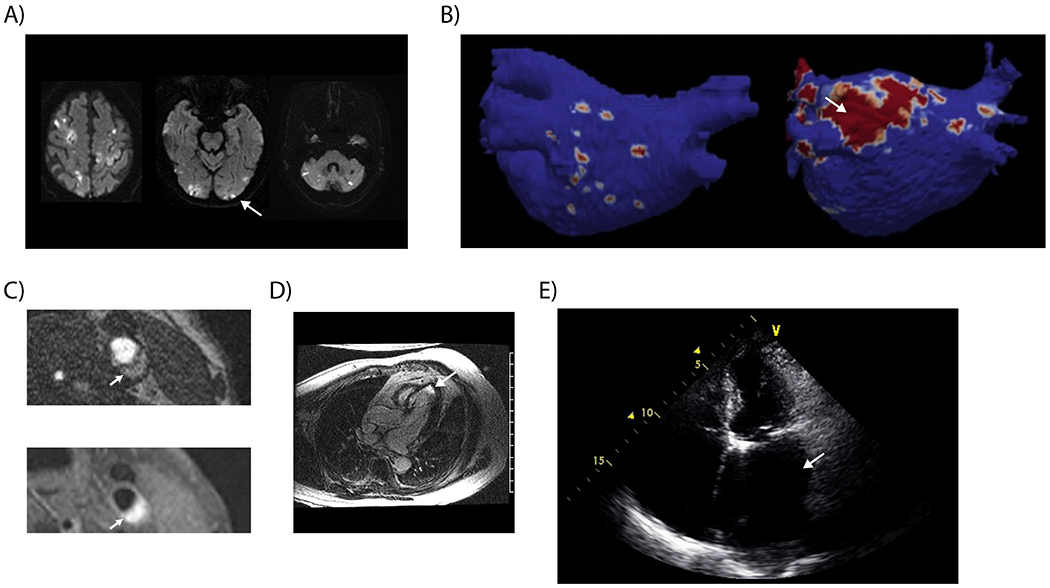
(A) Diffusion-weighted magnetic resonance imaging demonstrating acute infarction in all three major arterial territories of the brain, a finding suspicious for cancer-related hypercoagulability. (B) Cardiac magnetic resonance imaging demonstrating significant areas of atrial fibrosis (image on right) compared to an atrium with minimal fibrosis (image on left).73 (C) Hemorrhage within nonstenosing atherosclerotic plaque demonstrated on 3-dimensional time-of-flight magnetic resonance angiography (upper image) and T1 CUBE FS sequences (lower image). (D) Cardiac magnetic resonance imaging demonstrating late gadolinium enhancement consistent with MI in an ESUS patient with no clinical history of MI. (E) Echocardiography demonstrating a severely dilated left atrium in an ESUS patient without known atrial fibrillation.
Unrecognized Myocardial Infarction
In addition to unrecognized atrial abnormalities, derangements in ventricular tissue may also form a nidus for thromboembolism. Traditionally, myocardial infarction (MI) has been thought to increase the risk of stroke for about 1 month.4,38 However, recent evidence indicates that MI is a much broader risk factor for stroke. First, the risk of stroke appears to be elevated for longer than 1 month after MI, continuing for at least 3 months.39 Second, unrecognized MI, which comprises at least one-third of all MIs,40 may also be a stroke risk factor. Similar to clinically recognized MI, unrecognized MI leads to myocardial scar formation41 which may be capable of inducing thrombosis and subsequent cardiac embolism and resultant ischemic stroke (Figure 3D). In support of this hypothesis, markers of unrecognized MI are more common in patients with ESUS than in those with non-cardioembolic stroke.42,43 This category of cardiac disease may be amenable to anticoagulation given the recent results of the COMPASS trial, which found that anticoagulant therapy is more beneficial than antiplatelet therapy alone for reducing ischemic stroke in patients with clinically apparent coronary artery disease.44 Further studies may be warranted to determine whether anticoagulant therapy, alone or in combination with antiplatelet therapy, is more effective than antiplatelet therapy alone in patients with ESUS and evidence of unrecognized or distant MI.
Patent Foramen Ovale
Besides acquired cardiac risk factors such as atrial cardiopathy and unrecognized MI, there is strengthening evidence that PFO, a congenital risk factor, is causally related to ESUS. Numerous studies over the past 30 years have reported conflicting results regarding the association between PFO and stroke. There is a strong association in case-control studies but no significant association in cohort studies.45 PFO is not listed as a definite stroke etiology in any of the major stroke etiological classification systems,38,46,47 including the original ESUS formulation.4 On the other hand, a meta-analysis of five randomized trials indicated that percutaneous PFO closure reduced the risk of recurrent stroke in young patients with cryptogenic stroke and evidence of a PFO.48 The benefit seen with specific PFO-targeted treatment supports the longstanding hypothesis that PFO can be a causal mechanism of stroke. The presumed mechanism of PFO-related stroke is passage of an embolus from the venous circulation through the PFO to the arterial circulation, which suggests that anticoagulant therapy may also prevent PFO-related stroke. A pooled analysis of several randomized trials supports a protective effect of anticoagulation in patients with cryptogenic stroke and PFO,49 although the more recently announced RE-SPECT ESUS trial did not find such a benefit in its subjects with PFO.6 Thus, further analysis will be necessary to determine the benefit of anticoagulation in specific subgroups such as young patients with a large PFO.
Cancer
In addition to cardiac pathology, systemic disorders may also be underappreciated mechanisms of ESUS. A common example is cancer, which affects approximately 40% of people over their lifetime. Cancer appears to increase the risk of stroke, including even in the period before the cancer is detected. In a large population-based cohort, the risk of ischemic stroke increased starting about 5 months before a cancer diagnosis, suggesting that some cryptogenic strokes may be caused by occult cancer.50 Cancer likely increases stroke risk through several mechanisms, including hypercoagulability, iatrogenic effects of cancer treatments, and diagnosis-related factors such as reduced antithrombotic use.51 Of these risk factors, hypercoagulability may be the most important because stroke risk in cancer patients is highest soon after diagnosis, when cancer-mediated hypercoagulability generally peaks, and stroke is most strongly associated with cancer types classically associated with hypercoagulability and subsequent venous thromboembolism.52,53 About 50% of cancer-associated strokes are considered ESUS and most demonstrate infarctions in multiple vascular territories (Figure 3A).54 Transcranial Doppler ultrasound and autopsy studies implicate cardiac emboli from nonbacterial thrombotic endocarditis in many of these strokes, but in real-world practice nonbacterial thrombotic endocarditis is rarely confirmed and thus these patients are often classified as ESUS. Other possible occult stroke mechanisms in the cancer population include paradoxical embolism, tumor embolism, and cerebral intravascular coagulation leading to in situ cerebral artery thromboses. Aside from tumor embolism, all of these cancer-specific mechanisms may preferentially respond to anticoagulant therapy. However, cancer patients are predisposed to systemic and intracranial bleeding due to the destructive effects of tumors, frequent invasive procedures, and chemotherapy-induced thrombocytopenia, so the risks of anticoagulation in these patients is likely higher than in the general ESUS population. Therefore, the presumed benefit of anticoagulation in patients with ESUS and cancer will need to be tested in a randomized trial, which would likely be feasible based on the results of the TEACH pilot trial.55
Occult Embolic Mechanisms Unlikely to Respond to Anticoagulant Therapy
Nonstenosing Large-Artery Atherosclerosis
The mechanisms discussed above would be expected to respond to anticoagulant therapy. The neutral results of NAVIGATE ESUS and RE-SPECT ESUS suggest either that anticoagulation is not effective in subclinical AF, atrial cardiopathy, unrecognized MI, PFO, and cancer, or that any benefit of anticoagulation in these subsets is offset by its lack of efficacy in other mechanistic subsets of the ESUS population. Recent data suggest that a substantial proportion of ESUS cases may be the result of large-artery atherosclerotic disease that goes unrecognized because it does not cause significant stenosis of the arterial lumen. Multiple studies have found a higher prevalence of nonstenosing atherosclerotic plaque ipsilateral to a cryptogenic brain infarction compared to the contralateral hemisphere.56–59 Atherosclerosis of the intracranial and cervical large arteries has traditionally been defined using angiography of the arterial lumen, and the major stroke etiological classification systems define a large-artery origin of stroke based on whether there is ≥50% luminal stenosis.4,38,46,47 However, recent imaging advances allow more detailed characterization of the vessel wall and the plaque itself, revealing other features of atherosclerosis (Figure 3C). High-risk features such as soft plaque or intraplaque hemorrhage are detectable on computed tomography and magnetic resonance imaging and it is likely that many cases of ESUS result from rupture of such high-risk, nonstenosing plaques. In addition to nonstenosing plaque of the cervical and intracranial arteries, there is an association between aortic atherosclerosis and stroke.60 Patients with stroke due to aortic atherosclerosis may often be labeled as ESUS because transesophageal echocardiography is infrequently performed after stroke.61 The authors of the original ESUS concept presciently recognized these mechanisms, but argued that thrombin-rich clots on the surface of atherosclerotic plaques would respond to anticoagulant therapy. However, they acknowledged that “some embolic sources under the ESUS umbrella might have a variable response to anticoagulation.” Subgroup analyses from the NAVIGATE ESUS trial indicated that such heterogeneity was in fact present; for example, a benefit was seen with rivaroxaban in the ~10% of subjects with left atrial diameter >4.6 cm. That the trial found no overall benefit suggests that the effects of anticoagulation in subgroups such as this were diluted by other groups, most likely those with large-artery atherosclerosis. This subset of the ESUS population would not be expected to benefit from anticoagulant therapy more than antiplatelet therapy given that several randomized trials have found no reduction in recurrent stroke with anticoagulant versus antiplatelet therapy in patients with large-artery atherosclerotic stenosis or aortic atherosclerosis.62,63
Non-Atherosclerotic Vasculopathies
Although atherosclerosis is the most common cause of vasculopathy leading to stroke, other pathophysiological processes can also lead to vasculopathy of the cerebral circulation. Dissection of the cervicocephalic arteries causing downstream embolization or hypoperfusion is a well-known mechanism of stroke, especially in younger patients. Dissections are usually recognized on non-invasive vessel imaging; however, focal dissections, particularly nonstenosing ones or those involving the intracranial medium-sized arteries, can be missed.64 Recent neck trauma or respiratory infections can trigger dissections and may serve as a useful clue to the diagnosis. The CADISS trial demonstrated similar efficacy of antiplatelet and anticoagulant drugs for preventing stroke in patients with symptomatic carotid- and vertebralartery dissections.65 Infectious and inflammatory vasculopathies are also important considerations in ESUS, particularly if systemic signs are present or multiple vascular territories are involved. Varicella zoster vasculopathy is an increasingly recognized mechanism of stroke and has also been implicated in the development of temporal arteritis.66 Systemic or primary central nervous system vasculitis is a rare form of vasculopathy, although it may be underappreciated in ESUS because it is often limited to the small- and medium-sized arteries, thereby necessitating brain biopsy for definitive diagnosis. It remains to be established whether directed immunosuppressive and/or antiviral therapy improve the natural history of these non-atherosclerotic vasculopathies. Neither infectious nor inflammatory vasculopathies would be expected to benefit from anticoagulant therapy and harm might occur in the forms of vasculopathy predisposed to hemorrhage, such as amyloid beta-related angiitis.67 Based on these considerations, it is likely that ESUS patients whose mechanism involves lesions of the cervical or intracranial arteries do not benefit more from anticoagulant therapy than antiplatelet therapy.
Interactions between Underlying Mechanisms of Stroke
Mechanisms of stroke often do not occur in isolation. Many share risk factors, so their co-occurrence would be expected more often than by chance alone. In some cases, such coexisting mechanisms may augment each other and work synergistically to cause thromboembolism.
Atrial Fibrillation, Atrial Cardiopathy, and Cancer
The atrial abnormalities that predispose to AF and thromboembolism include mechanical dysfunction and chamber dilatation that lead to stasis of blood as well as tissue fibrosis that affects the normal homeostasis of pro- versus anticoagulant activity in the left atrium. At the same time, active malignancy leads to a hypercoagulable state. It may be that these two conditions work synergistically, with atrial cardiopathy providing a reservoir of slow-flowing arterial blood and cancer contributing a systemic hypercoagulability that in combination lead to a particularly heightened risk of arterial thromboembolism.68,69
Atherosclerosis and Cancer
Cancer promotes atherosclerotic plaque formation and rupture through heightened systemic inflammation.70 Radiation, a standard treatment for many cancers, also predisposes to atherosclerosis and subsequent stroke risk, especially if the radiation fields include the heart or large arteries of the head, neck, and mediastinum. Radiation-induced vascular injury generally develops several years after treatment and is a major concern in pediatric and young adult cancer survivors. In one study of survivors of Hodgkin lymphoma, radiation to the neck and mediastinum more than doubled the long-term risk of cerebrovascular events.71
Conclusions and Future Directions
The 2014 article by Hart et al formulated a concise definition of ESUS that facilitates a standardized approach to diagnosing stroke etiology.4 Although it does not appear that this definition alone can serve as a therapeutic guide, it may provide a useful foundation for additional diagnostic advances that could in turn allow more effective, tailored treatment to prevent stroke. A great deal of work remains before this is possible. Although subgroup analyses must always be viewed with caution, such subgroup analyses from NAVIGATE ESUS and RE-SPECT ESUS will be important to preliminarily evaluate current hypotheses and generate further hypotheses about mechanisms that may respond to anticoagulant therapy. Additional information about the benefits of anticoagulation in specific subpopulations with PFO will be particularly important. The ARCADIA trial will hopefully shed light on whether anticoagulation can reduce stroke recurrence in patients with atrial cardiopathy. The ATTICUS trial is evaluating the benefit of anticoagulation in ESUS patients with a variety of cardiac abnormalities, such as left atrial enlargement, PFO, or a high risk of AF (defined by the CHA2DS2-VASc score).72 The ARTESiA and NoAH trials will provide important knowledge about the benefit of anticoagulation in patients with isolated episodes of subclinical AF. Based on the results of the TEACH pilot trial,55 a phase 2/3 trial of anticoagulation versus antiplatelet therapy may be warranted in patients with ESUS and active malignancy. In the meantime, earlier-phase exploratory research is required to better understand the relationship between unrecognized MI and stroke, and to identify optimal markers of high-risk nonstenosing plaque. Such work may set the stage for future trials of anticoagulant therapy in those with ESUS and unrecognized MI and trials of aggressive atherosclerosis treatment in those with high-risk plaque. Although the results of the recent ESUS trials may be disappointing, the evolutionary success of mechanical thrombectomy for acute ischemic stroke highlights the importance of avoiding nihilism and working to improve patient selection and personalized therapy. It remains highly likely that continued efforts to better understand ESUS and develop therapies tailored to specific mechanisms will lead to further reductions in the burden of stroke.
Acknowledgments
The authors acknowledge Monica L. Chen for her assistance in creating the figures in this article.
Funding
Dr. Kamel is supported by NIH/NINDS grants R01NS097443, R01NS104143, and U01NS095869 and by the Michael Goldberg Research Fund. Dr. Merkler is supported by AHA grant 18CDA34110419 and by the Leon Levy Fellowship in Neuroscience. Dr. Iadecola is supported by NIH/NINDS grants R01NS034179, R01NS037853, R37NS089323, R01NS095441, and R01NS100447 and by the Feil Family Foundation. Dr. Gupta is supported by NIH/NINDS grants R01NS092802 and R01NS105144. Dr. Navi is supported by NIH/NINDS grant K23NS091395 and by the Florence Gould Endowment for Discovery in Stroke.
Role of Funder
No funding source was involved in the writing of this article. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication
Footnotes
Conflict of Interest Disclosures
Dr. Kamel serves on the Steering Committee for Medtronic’s Stroke AF trial, has served on an advisory board for Roivant Sciences, and receives in-kind study support for the ARCADIA trial from the BMS-Pfizer Alliance and Roche Diagnostics. Dr. Iadecola serves on the scientific advisory board of Broadview Ventures.
References
- 1.Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1(5):e259–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. 2017;48(4):867–872. [DOI] [PubMed] [Google Scholar]
- 3.Marnane M, Duggan CA, Sheehan OC, et al. Stroke subtype classification to mechanism-specific and undetermined categories by TOAST, A-S-C-O, and Causative Classification system. Stroke. 2010;41(8):1579–1586. [DOI] [PubMed] [Google Scholar]
- 4.Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13(4):429–438. [DOI] [PubMed] [Google Scholar]
- 5.Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. N Engl J Med. 2018;378(23):2191–2201. [DOI] [PubMed] [Google Scholar]
- 6.Diener HC, Sacco RL, Easton JD, Granger CB, Cronin L, Grauer C, Cotton D, Brueckmann M. RE-SPECT ESUS: Dabigatran versus acetylsalicyclic acid for stroke prevention in patients with embolic stroke of undetermined source. Abstract presented at World Stroke Congress, Montreal, 2018. https://cmoffice.kenes.com/cmsearchableprogrammeV15/conferencemanager/programme/personid/anonymous/WSC18/normal/b833d15f547f3cf698a5e922754684fa334885ed#!abstractdetails/0000087910. Int J Stroke. Epub 2018 Oct 20. [Google Scholar]
- 7.Li L, Yiin GS, Geraghty OC, et al. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol. 2015;14(9):903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harloff A, Schlachetzki F. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. N Engl J Med. 2018;379(10):986–987. [DOI] [PubMed] [Google Scholar]
- 9.Girgis M, Jelaidan I. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. N Engl J Med. 2018;379(10):986. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler PD, Glotzer TV, Daoud EG, et al. Incidence of newly detected atrial arrhythmias via implantable devices in patients with a history of thromboembolic events. Stroke. 2010;41(2):256–260. [DOI] [PubMed] [Google Scholar]
- 11.Sposato LA, Cipriano LE, Saposnik G, Ruiz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14(4):377–387. [DOI] [PubMed] [Google Scholar]
- 12.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370(26):2467–2477. [DOI] [PubMed] [Google Scholar]
- 13.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486. [DOI] [PubMed] [Google Scholar]
- 14.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. [DOI] [PubMed] [Google Scholar]
- 15.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. [DOI] [PubMed] [Google Scholar]
- 16.Culebras A, Messe SR, Chaturvedi S, Kase CS, Gronseth G. Summary of evidence-based guideline update: prevention of stroke in nonvalvular atrial fibrillation: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(8):716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114(9):1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witsch J, Merkler AE, Chen ML, et al. Incidence of Atrial Fibrillation in Patients With Recent Ischemic Stroke Versus Matched Controls. Stroke. 2018;49(10):2529–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopes RD, Alings M, Connolly SJ, et al. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137–145. [DOI] [PubMed] [Google Scholar]
- 20.Kirchhof P, Blank BF, Calvert M, et al. Probing oral anticoagulation in patients with atrial high rate episodes: Rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am Heart J. 2017;190:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120–129. [DOI] [PubMed] [Google Scholar]
- 22.Brambatti M, Connolly SJ, Gold MR, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129(21):2094–2099. [DOI] [PubMed] [Google Scholar]
- 23.Martin DT, Bersohn MM, Waldo AL, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36(26):1660–1668. [DOI] [PubMed] [Google Scholar]
- 24.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114(9):1483–1499. [DOI] [PubMed] [Google Scholar]
- 25.Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47(3):895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberger JJ, Arora R, Green D, et al. Evaluating the atrial myopathy underlying atrial fibrillation: identifying the arrhythmogenic and thrombogenic substrate. Circulation. 2015;132(4):278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcus GM, Dewland TA. Premature atrial contractions: a wolf in sheep’s clothing? J Am Coll Cardiol. 2015;66(3):242–244. [DOI] [PubMed] [Google Scholar]
- 28.Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol. 2015;65(20):2239–2251. [DOI] [PubMed] [Google Scholar]
- 29.Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30(10):2019–2024. [DOI] [PubMed] [Google Scholar]
- 30.Folsom AR, Nambi V, Bell EJ, et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: the Atherosclerosis Risk In Communities study. Stroke. 2013;44(4):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longstreth WT Jr., Kronmal RA, Thompson JL, et al. Amino terminal pro-B-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke. 2013;44(3):714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamel H, Soliman EZ, Heckbert SR, et al. P-wave morphology and the risk of incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45(9):2786–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamel H, O’Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the Atherosclerosis Risk In Communities study. Ann Neurol. 2015;78(5):670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamel H, Hunter M, Moon YP, et al. Electrocardiographic left atrial abnormality and risk of stroke: Northern Manhattan Study. Stroke. 2015;46(11):3208–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444–1451. [DOI] [PubMed] [Google Scholar]
- 36.Gladstone DJ, Healey J, Swaminathan B, Connolly S, Hart R. Rivaroxaban compared with aspirin in ESUS patients with left atrial enlargement or other risk factors for atrial fibrillation: subgroup analysis of the NAVIGATE ESUS trial. Abstract presented at World Stroke Congress, Montreal, 2018. Int J Stroke. Epub 2018. Oct 20. [Google Scholar]
- 37.Kamel H, Longstreth WT Jr., Tirschwell DL, et al. The AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke randomized trial: Rationale and methods. Int J Stroke. 2018:1747493018799981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. [DOI] [PubMed] [Google Scholar]
- 39.Merkler AE, Diaz I, Wu X, et al. Duration of Heightened Ischemic Stroke Risk After Acute Myocardial Infarction. J Am Heart Assoc. 2018;7(22):e010782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med. 1995;122(2):96–102. [DOI] [PubMed] [Google Scholar]
- 41.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113(23):2733–2743. [DOI] [PubMed] [Google Scholar]
- 42.Merkler AE, Gialdini G, Murthy SB, et al. Association Between Troponin Levels and Embolic Stroke of Undetermined Source. J Am Heart Assoc. 2017;6(9):e005905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaghi S, Chang AD, Ricci BA, et al. Early Elevated Troponin Levels After Ischemic Stroke Suggests a Cardioembolic Source. Stroke. 2018;49(1):121–126. [DOI] [PubMed] [Google Scholar]
- 44.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017;377(14):1319–1330. [DOI] [PubMed] [Google Scholar]
- 45.Ma B, Liu G, Chen X, Zhang J, Liu Y, Shi J. Risk of stroke in patients with patent foramen ovale: an updated meta-analysis of observational studies. J Stroke Cerebrovasc Dis. 2014;23(5):1207–1215. [DOI] [PubMed] [Google Scholar]
- 46.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME, Hennerici MG. The ASCOD phenotyping of ischemic stroke (Updated ASCO Phenotyping). Cerebrovasc Dis. 2013;36(1):1–5. [DOI] [PubMed] [Google Scholar]
- 47.Ay H, Benner T, Arsava EM, et al. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke. 2007;38(11):2979–2984. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad Y, Howard JP, Arnold A, et al. Patent foramen ovale closure vs. medical therapy for cryptogenic stroke: a meta-analysis of randomized controlled trials. Eur Heart J. 2018;39(18):1638–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kasner SE, Swaminathan B, Lavados P, et al. Rivaroxaban or aspirin for patent foramen ovale and embolic stroke of undetermined source: a prespecified subgroup analysis from the NAVIGATE ESUS trial. Lancet Neurol. Epub 2018. Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navi BB, Reiner AS, Kamel H, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. Epub 2018. Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navi BB, Iadecola C. Ischemic stroke in cancer patients: A review of an underappreciated pathology. Ann Neurol. 2018;83(5):873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navi BB, Reiner AS, Kamel H, et al. Association between incident cancer and subsequent stroke. Ann Neurol. 2015;77(2):291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Navi BB, Reiner AS, Kamel H, et al. Risk of Arterial Thromboembolism in Patients With Cancer. J Am Coll Cardiol. 2017;70(8):926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navi BB, Singer S, Merkler AE, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014;83(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navi BB, Marshall RS, Bobrow D, et al. Enoxaparin vs Aspirin in Patients With Cancer and Ischemic Stroke: The TEACH Pilot Randomized Clinical Trial. JAMA Neurol. 2018;75(3):379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freilinger TM, Schindler A, Schmidt C, et al. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging. 2012;5(4):397–405. [DOI] [PubMed] [Google Scholar]
- 57.Gupta A, Gialdini G, Lerario MP, et al. Magnetic resonance angiography detection of abnormal carotid artery plaque in patients with cryptogenic stroke. J Am Heart Assoc. 2015;4(6):e002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta A, Gialdini G, Giambrone AE, et al. Association between nonstenosing carotid artery plaque on MR angiography and acute ischemic stroke. JACC Cardiovasc Imaging. 2016;9(10):1228–1229. [DOI] [PubMed] [Google Scholar]
- 59.Coutinho JM, Derkatch S, Potvin AR, et al. Nonstenotic carotid plaque on CT angiography in patients with cryptogenic stroke. Neurology. 2016;87(7):665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331(22):1474–1479. [DOI] [PubMed] [Google Scholar]
- 61.Giruparajah M, Bosch J, Vanassche T, et al. Global survey of the diagnostic evaluation and management of cryptogenic ischemic stroke. Int J Stroke. 2015;10(7):1031–1036. [DOI] [PubMed] [Google Scholar]
- 62.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352(13):1305–1316. [DOI] [PubMed] [Google Scholar]
- 63.Amarenco P, Davis S, Jones EF, et al. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45(5):1248–1257. [DOI] [PubMed] [Google Scholar]
- 64.Fukuhara K, Ogata T, Ouma S, et al. Impact of initial symptom for accurate diagnosis of vertebral artery dissection. Int J Stroke. 2015;10 Suppl A100:30–33. [DOI] [PubMed] [Google Scholar]
- 65.Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361–367. [DOI] [PubMed] [Google Scholar]
- 66.Gilden D, White T, Khmeleva N, et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology. 2015;84(19):1948–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishii M, Lavi E, Kamel H, Gupta A, Iadecola C, Navi BB. Amyloid beta-Related Central Nervous System Angiitis Presenting With an Isolated Seizure. Neurohospitalist. 2014;4(2):86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rahman F, Ko D, Benjamin EJ. Association of Atrial Fibrillation and Cancer. JAMA Cardiol. 2016;1(4):384–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63(10):945–953. [DOI] [PubMed] [Google Scholar]
- 70.Tapia-Vieyra JV, Delgado-Coello B, Mas-Oliva J. Atherosclerosis and Cancer; A Resemblance with Far-reaching Implications. Arch Med Res. 2017;48(1):12–26. [DOI] [PubMed] [Google Scholar]
- 71.De Bruin ML, Dorresteijn LD, van’t Veer MB, et al. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101(13):928–937. [DOI] [PubMed] [Google Scholar]
- 72.Geisler T, Poli S, Meisner C, et al. Apixaban for treatment of embolic stroke of undetermined source (ATTICUS randomized trial): Rationale and study design. Int J Stroke. 2017;12(9):985–990. [DOI] [PubMed] [Google Scholar]
- 73.Reprinted from: Zghaib T, Keramati A, Chrispin J. Multimodal Examination of Atrial Fibrillation Substrate. JACC Clin Electrophysiol. 2018;4(1):59–68. With permission from Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]


