Abstract
Background
Cervical artery dissection (CeAD) is a pathological bleed or tear, or both, in the wall of the carotid or vertebral arteries as they course through the neck, and is a leading cause of stroke in young people.
Objectives
To assess the effectiveness of surgical and radiological interventions versus best medical treatment alone for treating symptomatic cervical artery dissection.
Search methods
We performed comprehensive searches of the Cochrane Stroke Group Trials Register (last searched March 2020), the Cochrane Central Register of Controlled Trials (CENTRAL), 2020, Issue 4, in the Cochrane Library (searched March 2020), MEDLINE (1946 to March 2020) and Embase (1974 to March 2020). We searched relevant ongoing trials and research registers (searched March 2020), checked references in all relevant papers for additional eligible studies, and contacted authors and researchers in the field.
Selection criteria
Randomised controlled trials (RCTs) and controlled clinical trials (CCTs) of either surgical or endovascular intervention for the management of symptomatic CeAD were eligible for inclusion. Only studies with anticoagulants or antiplatelet treatment as the control group were included. Two review authors planned to independently extract data.
Data collection and analysis
Primary outcomes were ipsilateral stroke and disability. Secondary outcomes were death, any stroke, or transient ischaemic attack, residual stenosis (> 50%), recurrence of cervical dissection, expanding pseudoaneurysm, or major bleeding. We analysed the studies according to the first choice of treatment. We planned to assess for risk of bias and apply GRADE criteria for any included studies.
Main results
We did not find any completed RCTs or CCTs undertaken in this area of research.
Authors' conclusions
No RCTs or CCTs compared either surgery or endovascular therapy with control. Thus, there is no available evidence to support their use for the treatment of extracranial cervical artery dissection in addition to antithrombotic therapy in people who continue to have neurological symptoms when treated with antithrombotic therapy alone.
Plain language summary
Surgical and radiological interventions for treating symptomatic extracranial cervical artery dissection
Review question
This review was conducted to establish whether a surgical operation or a keyhole endovascular treatment, such placement of stent in addition to blood‐thinning medication would improve outcomes in people with cervical artery dissection for whom treatment with blood‐thinning medication alone does not improve persistent or worsening symptoms of stroke. The outcomes which we wished to assess are permanent brain damage and long‐term disability from stroke.
Background
Cervical artery dissection is a tear in the wall of the blood vessels in the neck that supply blood to the brain. There are two of each artery, a carotid artery on the right and one on the left of the neck, and a vertebral artery on the right and one on the left of the neck. When tears occur in the walls of these arteries, clots can form inside the artery. These clots can then break away from the artery wall and travel to the brain to cause a stroke. The standard way of treating tears in these arteries is to give patients medications which thin the blood and reduce clot formation. However, sometimes patients continue to get symptoms of stroke, and their condition worsens despite being on maximum dose medication.
Search date
We performed a thorough and comprehensive review of the literature to look for clinical trials which could help us to answer the review question. The evidence is current to March 2020.
Study characteristics
We looked for trials which were conducted in an objective way and designed specifically to compare either surgery or radiological intervention to medical therapy alone.
Key results
We did not find any trials that matched the criteria of the review. Therefore, there is no available objective evidence that an operation or a radiological procedure can benefit patients who continue to have symptoms of stroke despite medical therapy. We did find a number of reports from experienced groups of doctors that operations and radiological interventions were safe in their hands. However, we found no evidence that this could be applicable to other hospitals and clinical teams. Therefore, large scale, well‐conducted studies are required to answer the review question.
Certainty of the evidence
There is no good quality evidence to guide clinicians on the best treatment for people with cervical artery dissection who remain symptomatic despite optimal medical therapy.
Background
Cervical artery dissection (CeAD) is a pathological bleed or tear, or both, in the wall of the carotid or vertebral arteries at a point in their anatomical course through the neck. Since these arteries form the blood supply to the brain, when an intramural haematoma occurs in their wall, the risk of stroke is extremely high. Stroke as a result of CeAD accounts for 2% to 2.5% of all ischaemic strokes (Debette 2009). CeAD is a significant cause of stroke in young and middle‐aged people and accounts for 8% to 25% of stroke in people under the age of 45 years. Stroke in this age group has a profound socioeconomic consequence and long‐term quality of life implications (Schievink 2001; Schwartz 2009). The underlying pathogenesis is still not clearly defined, and so efforts to reduce incidence, recurrence, and severity are challenging to implement (Biller 2014).
Although two‐thirds of patients present with a stroke or transient ischaemic attack (TIA), patients can present with local symptoms such as neck pain, headache, Horner syndrome, cranial nerve palsy, tinnitus, or cervical root impairment (Engelter 2013). The pathophysiological process of intramural haematoma can lead to narrowing of the arterial lumen, resulting in stenosis or vessel occlusion, thereby yielding cerebral ischaemic events. However, these cerebral ischaemic events are more often embolic rather than due to haemodynamic compromise. Emboli arise from a thrombus that forms on the injured intima (Engelter 2015).
There is no consensus on how best to manage people with CeAD. Considering that most patients present with ischaemic events and the pathological process relates to clot formation or embolisation, then it makes sense to treat patients with antithrombotic or antiplatelet medication. Anticoagulant and antiplatelet drugs are both safe treatment options, and a randomised controlled trial (RCT) has demonstrated them to be equivalent to each other (CADISS Trial Investigators 2015). Many physicians opt to treat people with CeAD by using medical therapy alone and reserve surgical or endovascular intervention for those in whom neurological symptoms do not resolve or reoccur (Arnold 2011; Caplan 2008). However, advances in technology have inspired some physicians to reduce their threshold to use endovascular techniques to restore vessel wall integrity and reduce the recurrence of stroke (Cohen 2012; Fava 2008; Jeon 2010). Surgery, in turn, is mostly limited to patients who develop neurological symptoms or have a progressive clinical course despite medical therapy, as long as they have anatomically accessible lesions and endovascular treatment is contraindicated (Engelter 2015). These clinical management algorithms, however, are not based on level one evidence and are influenced by anecdotal experience or local expertise. A systematic review of the relevant literature is therefore required to inform evidence‐based practice, particularly in those patients for whom medical therapy alone fails to stop development of neurological symptoms.
Description of the condition
The cervical arteries are located in the neck and give blood supply to the head and brain. The cervical arteries comprise of bilateral carotid arteries anteriorly, and bilateral vertebral arteries posteriorly. The cervical artery consists of three layers, the adventitia (outer layer), the media (middle layer), and the intima (inner layer). Arterial dissection, which is essentially a tear in layers of the artery, occurs when the structural integrity of the arterial wall is compromised, allowing blood to collect between the intimal and medial layers as an intramural haematoma (Béjot 2014; Schievink 2001). The resulting tear leads to separation of the intima and media layers. Blood then flows from the central vessel channel (the true lumen), into a newly formed channel (the false lumen). If the true lumen becomes compromised, blood cannot flow into the brain, and this can lead to symptoms of a stroke. The more common mechanism by which cervical artery dissection can lead to stroke is where a clot, which builds up in the false lumen, can embolise (break off) and travel to the brain, blocking blood flow to small blood vessels within the brain (Blum 2015).
The annual incidence of symptomatic spontaneous cervical artery dissection is reportedly 2.5 to 3 per 100,000 (Schievink 2001). However, the actual incidence is under‐reported as many cases are undiagnosed because they are asymptomatic or associated with mild transient neurological symptoms, such as temporary slurring of speech, numbness, or pins and needles in the face or limbs. In young and middle‐aged people, cervical dissection, which includes dissection of the carotid or vertebral arteries, or both, is particularly belligerent (Debette 2009). It accounts for as many as one in four ischaemic strokes, and it is the leading cause of stroke in people under the age of 45 years (Debette 2009). This age group has experienced the fastest growth rate of stroke compared to any other age group. In the 10 years between 1994 and 2005, the incidence of stroke increased by 43.8% (Kissela 2012). This is the age group in which people are most productive and at a time in their lives when a stroke is particularly devastating due to the psychological, social, and economic consequences for themselves, their families, and society as a whole (Smajlović 2015).
Description of the intervention
All patients receive oral medical therapy regardless of whether they need additional or subsequent surgical or radiological intervention. Medical treatments are focused on prevention of clot propagation to prevent extension of the dissection and to facilitate arterial wall healing. The choice of drugs include:
antiplatelet drugs (acetylsalicylic acid, ticlopidine, clopidogrel, sulfinpyrazone, dipyridamole);
full‐dose anticoagulants (intravenous or subcutaneous fractionated or unfractionated heparin, oral warfarin (coumarin), or oral factor Xa inhibitors or oral direct thrombin inhibitors);
thrombolytic agents (intravenous, intra‐arterial, local or systemic).
It is important to note that we anticipate that all patients (including those who receive surgical or radiological interventions) receive medical therapy in line with international treatment guidelines (Brott 2011).
Most study authors state that candidates for procedural therapy include those with recurrent ischaemia despite medical treatment, patients with contraindications to anticoagulants or antiplatelet medications, and patients with significantly compromised cerebral blood flow (Debette 2009; Georgiadis 2006; Medel 2014; Xianjun 2012).
The specific interventions under review were surgery or radiological treatment, or both, with medical therapy, compared with medical therapy treatment alone.
Surgical intervention
Surgical interventions include a variety of procedures that require open surgical wounds to access the diseased cervical artery. The American Heart Association/American Stroke Association (AHA/ASA) recommends surgical repair only for internal carotid artery dissection and not vertebral artery dissection (Brott 2011). The AHA/ASA also fails to clarify when exactly medical therapy alone is inadequate and surgical intervention should be considered.
The diseased portion of the carotid artery that contains dissection is either repaired directly, removed (resected), or bypassed.
Primary artery repair is performed by removal of the clot (i.e. thromboendarterectomy) and then the artery is repaired with patchplasty using autologous vein (a segment of the patient's own vein) or a synthetic patch.
If the damaged arterial segment is resected, it is replaced with an autologous interposition vein graft.
If bypass is the procedure of choice, a tube (conduit) is sewn (anastomosed) to a healthy portion of artery proximal to the dissection and to a healthy portion of artery distal to the dissection.
Radiological intervention
Radiological interventions (endovascular) include patients treated with angioplasty, stent, or stent‐supported angioplasty, whereby a metal cage is deployed within the artery, which acts as a scaffold that is used to tack down the intimal flap and to reinforce the cervical arterial wall. The procedure is minimally invasive, with the stent delivered through a small puncture wound in the skin. The skin puncture is made in the groin or the arm to access the underlying femoral or brachial artery, respectively. Guidewires and catheters are then used to cannulate the cervical arteries under X‐ray guidance, and the stent is delivered over the guidewire and into the diseased artery.
International guidelines, in particular those from the AHA/ASA, recommend that angioplasty and stenting be considered when ischaemic neurologic symptoms have not responded to antithrombotic therapy after acute dissection (Brott 2011). Numerous studies have investigated endovascular repair of internal carotid artery dissection and vertebral artery dissection, with successful results regarding safety and recurrent stroke (Ahlhelm 2012; Asif 2015; Hassan 2012; Hernández‐Durán 2014; Kashiwazaki 2013; Ohta 2011; Pham 2011; Sadato 2010; Stella 2010; Xianjun 2012). Therefore, although the indications for endovascular repair are not clear, these treatments remain options for patients who do not respond to medical therapy and in patients with severe ischaemic symptoms who are not candidates for thrombolytics, anticoagulation, or antiplatelet agents.
How the intervention might work
At baseline, all symptomatic patients with unilateral or bilateral dissection are placed on antithrombotic medication, either antiplatelet or anticoagulation, or a combination of both. The primary objective is to prevent stroke and long‐term neurological complications. Specifically, the intention when using these medications is to prevent thrombotic occlusion of the artery and reduce the risk of distal embolisation. Extension of intramural haematoma is rare, but still possible, during antithrombotic therapy. However, there is a paucity of evidence to demonstrate which medication is superior. The Cervical Artery Dissection in Stroke Study (CADISS) found that anticoagulation was not superior to antiplatelet therapy. However, the population of 250 participants was underpowered, with statistical estimates calculating that at least 10,000 participants should be required to demonstrate robust evidence (CADISS Trial Investigators 2015). If patients continue to have evolving neurological symptoms despite maximum medical therapy, then surgical or radiological intervention is required to arrest neurological deficit and prevent stroke. Other postulated benefits of surgical or radiological intervention are enhanced arterial wall strength, prevention of recurrence, more rapid resolution of symptoms, and prevention of pseudoaneurysm formation.
Why it is important to do this review
At present, there is no consensus on how to treat people with symptomatic cervical artery dissection. There are no randomised trials individually comparing either anticoagulants or antiplatelet drugs with control. Therefore, there is no primary evidence to support the routine use of antithrombotic therapies for the treatment of extracranial cervical artery dissection. A previous Cochrane Review found evidence that anticoagulants were associated with a trend towards reduced risk of death and disability compared to antiplatelet therapy, but this was offset by a non‐significant risk of major intracerebral and extracerebral bleeding (Lyrer 2010). The CADISS group randomly compared antiplatelet and anticoagulant medications to each other. They found no statistically significant difference in stroke or death between those treated with antiplatelet medicines versus those treated with anticoagulants (CADISS Trial Investigators 2015). However, the CADISS trial was underpowered to elicit such a difference. Although they reported that patients with resolved neurological symptoms could be treated safely with both antiplatelet and anticoagulant medication, they did find that 2% of the patient population had a stroke or died despite medical therapy. Furthermore, several meta‐analyses of observational data on antithrombotic agents in cervical artery dissection have reported much higher stroke rates than in CADISS. Thus the stroke rate in CADISS might be falsely low, rendering alternative intervention strategies all the more relevant (CADISS Trial Investigators 2015).
Due to a lack of robust evidence, treatment algorithms describing when and how to intervene for patients who remain symptomatic with evolving neurological defects despite medical management, have not been adequately developed. Factors which confound therapeutic decisions and which are particularly pertinent in the setting of cervical artery dissection include the patients' young age, the possibility of an underlying connective tissue disorder, and the increased risk of stroke when surgical or radiological intervention is undertaken during the acute phase.
Some authors have demonstrated favourable long‐term results from surgical intervention for symptomatic cervical artery dissection (Martinelli 2017; Vishteh 1998). However, others have not been so positive and have blamed poor outcomes on difficulties in the preparation of the dissected carotid artery and associated cranial nerve injury (Müller 2000). Poor surgical outcomes have inspired the application of radiological interventions, and some authors have reported promising results, but there is a lack of robust evidence (Donas 2008). The evidence pertaining to radiological intervention arises from case reports and cases series. Although collation of this data demonstrates good technical success, the majority of reported cases are on traumatic dissection, which represents a different disease aetiology and patient population than those with spontaneous dissection (Pham 2011). Therefore, currently, there is an absence of consensus on how best to prevent long‐term neurological complications, stroke, and death in people with symptomatic cervical artery dissection.
Objectives
To assess the effectiveness of surgical and radiological interventions versus best medical treatment alone for treating symptomatic cervical artery dissection.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and CCTs assessing the effects of open surgical repair or radiological repair of symptomatic extracranial cervical artery dissection, or both, compared with medical therapy alone, were eligible. We planned to include trials only if the individuals (or other units) followed in the trial were definitely or possibly assigned prospectively to one of two (or more) alternative forms of treatment using either random allocation or some quasi‐random method of distribution. A trial is classified as an RCT only if the author(s) explicitly states that the assignment was randomised. If they do not expressly state that the trial was randomised, but randomisation cannot be ruled out, the report is classified as a CCT (controlled clinical trial). The classification CCT is also applied to quasi‐randomised studies, where the method of allocation is known but is not considered strictly random. Examples of quasi‐random methods of assignment include alternation, date of birth, and medical record number (Lefebvre 2019).
Types of participants
We planned to include participants with a clinical diagnosis of symptomatic extracranial carotid artery or vertebral artery dissections, or both, if associated with active neurological symptoms. Neurological symptoms included stroke, transient ischaemic attack or local neurological deficit, haemodynamic brain ischaemia, and/or expansion of concomitant pseudoaneurysm with neurological symptoms.
Diagnosis may be made by duplex scanning, magnetic resonance imaging (MRI), computerised tomographic (CT) arteriography or digital subtraction angiography, and studies using these methods were eligible if the following diagnostic signs were present in the extracranial carotid or vertebral artery, or both.
Mural haematoma.
Pseudoaneurysms.
Long tapering stenosis.
Intimal flap.
Double lumen.
Occlusion revealing a pseudoaneurysm or long tapering stenosis after recanalisation.
We did not exclude studies based on age, gender, stage or severity of the condition, aetiology, or comorbidities.
Types of interventions
We planned to include trials comparing surgical or radiological interventions with best medical therapy.
We planned to include the following comparisons.
Radiological intervention plus medical therapy versus medical therapy alone.
Surgical intervention plus medical therapy versus medical therapy alone.
We did not examine thrombolysis specifically, but we did not exclude patients who have had thrombolysis prior to surgical or radiological intervention.
We planned to include trials comparing radiological versus surgical interventions as long as both arms had best medical therapy alone as a comparator. If they compared directly to each other without the control of best medical therapy alone, we planned to include these trials in a narrative description but not to include them in a meta‐analysis.
Types of outcome measures
Primary outcomes
Ipsilateral stroke (for vertebral dissection, an ipsilateral event is defined as a recurrent event in the vertebrobasilar territory). The World Health Organization (WHO) defines stroke as "rapidly developing clinical signs of focal (or global) disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than that of vascular origin" (Hatano 1976).
-
Disability (defined according to the modified Rankin Scale (mRS) UK‐TIA Study Group 1988):
mRS 0,1 and 2 are judged as no or minor disability*;
mRS 3, 4 and 5 as major disability;
mRS 6 are dead.
*People who are able to return to their original or similar job (part‐time or full‐time) are classified as having no disability.
We planned to undertake subgroup analyses for both primary outcomes at early (0 to 1 month), midterm (1 to 3 months), late (3 months to 1 year) and extended (> 1 year) time points.
Secondary outcomes
Length of survival
Length of survival free from any stroke, or transient ischaemic attack (including amaurosis fugax)
Residual stenosis (> 50%)
Time to recurrence of cervical dissection, in either the same artery or any of the other three extracranial cervical arteries
Expanding pseudoaneurysm
Major bleeding
The International Society on Thrombosis and Haemostatis defines major bleeding as fatal bleeding or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular, pericardial or intramuscular with compartment syndrome, or bleeding causing a fall in haemoglobin concentration of 1.24 units or more, or leading to transfusion of two or more units of whole blood or red cells (Schulman 2005).
Search methods for identification of studies
See the 'Specialised register' section at the Cochrane Stroke Group website. We searched for trials in all languages and arranged for the translation of relevant articles where necessary.
Electronic searches
We searched the Cochrane Stroke Group trials register and the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library; 2020, Issue 4) (Appendix 1)
MEDLINE Ovid (from 1946; last searched 24 March 2020) (Appendix 2)
Embase Ovid (from 1974; last searched 24 March 2020) (Appendix 3)
We modelled the subject strategies for databases on the search strategy designed for MEDLINE Ovid in (Appendix 2). We combined all search strategies deployed with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and CCTs (as described in the Cochrane Handbook for Systematic Reviews of Interventions (hereafter referred to as the Cochrane Handbook), Chapter 4, Lefebvre 2019).
We searched the following ongoing trials registers in March 2020.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/) (Appendix 4)
World Health Organization (WHO) International Clinical Trials Registry Platform (who.int/ictrp/en/) (Appendix 5)
Stroke Trials Registry (www.strokecenter.org/trials/).
Searching other resources
In an effort to identify further published, unpublished and ongoing trials, we:
checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials and used the Web of Science Cited Reference Search to forward track relevant references;
contacted original authors for clarification and further data if trial reports were unclear;
where necessary, contacted experts, trialists, or organisations in the field to obtain additional information of any unpublished or ongoing clinical trials.
Data collection and analysis
Selection of studies
Two review authors (NH, SS) independently screened titles and abstracts of the references obtained as a result of our searching activities. We retrieved the full‐text articles for the remaining references. Two review authors (NH, SS) independently screened the full‐text articles to identify studies for inclusion, and also identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, we consulted a third review author (FJ). We collated multiple reports of the same study so that each study, not each reference, is the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram (Liberati 2009).
Data extraction and management
Two review authors (NH, SS) planned to independently extract data from included studies, using an adapted data extraction form provided by the Cochrane Stroke Group.
Assessment of risk of bias in included studies
Two review authors (NH, FJ) planned to independently assess the risk of bias for each study using the criteria outlined in the Cochrane Handbook (Higgins 2011). We planned to resolve any disagreements by discussion or by involving another review author (SS). We planned to assess the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We planned to grade the risk of bias for each domain as 'high', 'low', or 'unclear', and provide information from the study report together with a justification for our judgment in the 'Risk of bias' tables.
Measures of treatment effect
Dichotomous data
We planned to express the results for dichotomous outcome measures using risk ratio (RR) and associated 95% confidence intervals (CIs) to reflect the uncertainty of the point estimate of effects.
Continuous data
For continuous outcome measures, we planned to calculate the mean and standard deviation with the corresponding 95% CIs. We planned to use the standardised mean difference (SMD) with 95% CI to combine outcomes from trials that measured outcomes using different scales (Higgins 2019).
Time‐to‐event data
We planned to use survival analysis to report time‐to‐event data and the intervention effect expressed as a hazard ratio (HR) and associated 95% CIs. The methods we planned to use to analyse time‐to‐event data would have been guided by those described by Parmar 1998 and Tierney 2007.
Unit of analysis issues
We planned to consider the unit of analysis within each trial to be each participant.
We planned to consider the number of individual participants as the unit of analysis. If information had been available from cluster‐randomised trials, we planned to include this data, but we would have adjusted the results when the unit of analysis in the trial was presented as the total number of individual participants, instead of the number of clusters. We planned to adjust the results using the mean cluster size and intracluster correlation coefficient (ICC) (McKenzie 2019). We planned to assess data from cross‐over trials at the cross‐over point, if possible. In the case of meta‐analysis, we planned to combine data from individually randomised trials using the generic inverse variance method, as described in Chapter 16 (section 16.3) of the Cochrane Handbook (McKenzie 2019).
Dealing with missing data
We planned to record missing and unclear data for each included study. If possible, we planned to perform all analyses using an intention‐to‐treat approach; that is, we planned to analyse all participants and their outcomes within the groups to which they were allocated, regardless of whether they received the intervention. If necessary, we planned to contact study authors to request missing data.
Assessment of heterogeneity
We planned to quantify statistical heterogeneity using the I2 statistic. We would have considered statistical heterogeneity to be present if I2 was greater than 60%. We planned to treat heterogeneity by using random‐effects or sensitivity analysis, or both: if the heterogeneity was being caused by one or two studies with peripheral results that were in conflict with the rest of the studies, we would have carried out analyses with and without these studies as part of the sensitivity analysis.
Assessment of reporting biases
We planned to investigate publication bias using funnel plots if there were 10 or more studies included in the review, as recommended by the Cochrane Handbook (Page 2019).
Data synthesis
If we had considered studies to be sufficiently similar, we planned to conduct a meta‐analysis by pooling the appropriate data using Review Manager 5 (Review Manager 2014).
We planned to use a fixed‐effect meta‐analysis for synthesising data where it was reasonable to assume that trials were estimating the same underlying treatment effect. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, we planned to use a random‐effects meta‐analysis to produce an overall summary where the average treatment effect was clinically meaningful. If we identified substantial clinical, methodological, or statistical heterogeneity across included trials, we would not have reported pooled results from the meta‐analysis, but instead, we planned to use a narrative approach to data synthesis (Deeks 2019).
Subgroup analysis and investigation of heterogeneity
We planned to limit subgroup analyses to primary outcomes. Planned subgroup analyses included:
vertebral artery dissection versus carotid artery dissection;
single versus multiple concomitant cervical arterial dissection;
presence versus absence of connective tissue disorder;
traumatic (including iatrogenic), that is, as a result of injury versus spontaneous (i.e. without a precipitating cause), where data are available;
surgical intervention with optimal medical management versus radiological intervention with optimal medical management.
Sensitivity analysis
We planned to repeat the analyses including high‐quality trials only. For the purpose of this review, trials we judged to be at 'low risk of bias' for sequence generation and allocation concealment would have been classified as high‐quality trials. We also planned to repeat the analyses including RCTs only.
Summary of findings and assessment of the certainty of the evidence
We planned to create a 'Summary of findings' table using the outcomes as described in the Types of outcome measures section. We have included an example table (Table 1). We planned to use methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook (Schünemann 2019), using GRADEproGDT software (GRADEproGDT). We planned to use the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004).
1. Template 'Summary of findings' table.
| Surgical and radiological interventions for treating symptomatic extracranial cervical artery dissection | ||||||
|
Participants or population: people with symptomatic extracranial cervical artery dissection Setting: hospital, emergency Intervention: surgical or radiological cervical artery repair Comparison: medical therapy (antiplatelet/anticoagulant/both) | ||||||
| Outcomes |
Anticipated absolute effects (95% CI) |
Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Ipsilateral stroke (primary outcome 1) | No eligible trials were available | |||||
| Disability (defined according to the modified Rankin Scale: mRS ‐ primary outcome 2) | No eligible trials were available | |||||
| Length of survival | No eligible trials were available | |||||
| Length of survival from any stroke, or transient ischaemic attack (including amaurosis fugax) | No eligible trials were available | |||||
| Residual stenosis (> 50%) | No eligible trials were available | |||||
| Time to recurrence of cervical dissection | No eligible trials were available | |||||
| Expanding pseudoaneurysm | No eligible trials were available | |||||
| Major bleeding | No eligible trials were available | |||||
| CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
We planned to justify all decisions to downgrade the quality of studies using footnotes, and we planned to make comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
The search generated 13,000 references. We identified and removed 591 duplicates. Two authors (NH, SS) then reviewed the titles and abstracts of the remaining 12,409 studies. NH and SS each undertook the search independently and included or excluded studies based on study type and PICO (an acronym that stands for Patient (or Population or Problem), Intervention, Comparison, Outcome), as outlined in the protocol (Hynes 2018). Once each author had reviewed all the titles and abstracts, we met to review the conflicts which were generated. We resolved these conflicts without the need for a third review author. Of the 12,409 studies reviewed, we only carried forward 11 studies to full‐text review.
NH and SS independently reviewed the 11 full texts and included or excluded studies based on study type and PICO, as outlined in the PRISMA flow diagram. (Figure 1)
1.
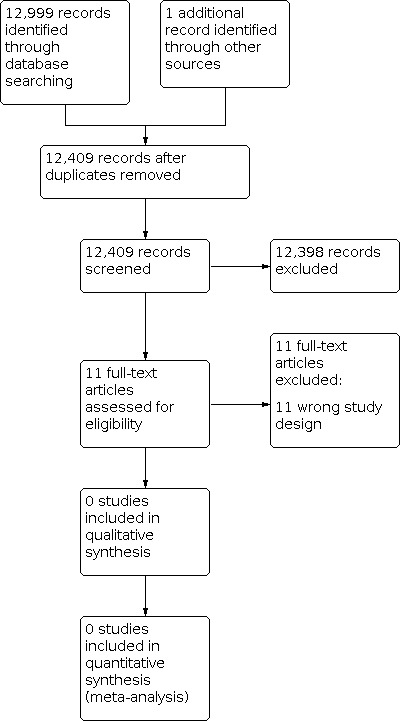
Study flow diagram.
Included studies
None of the studies met the inclusion criteria of the peer‐reviewed Cochrane systematic review protocol submitted to the Cochrane Stroke Group. If we had included studies, the included studies would have been assessed and analysed using methods detailed in the review protocol to include assessment of the risk of bias, measurement of the treatment effect, unit of analysis issues, dealing with missing data, assessment of heterogeneity, data synthesis, and subgroup and sensitivity analyses (Hynes 2018).
Excluded studies
Following the full‐text review, we excluded all 11 studies from further analysis (Asif 2015; Chiche 2005; Edgell 2005; Fava 2008; Jensen 2017; Joo 2005; Juszkat 2015; Lavallee 2007; Marnat 2016; Martinelli 2017; Schievink 1994). We provide the reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
We did not identify any studies which met the inclusion criteria. Therefore, it was not possible to assess the risk of bias. Had we included studies we had planned to assess the following domains of bias.
Allocation
We did not identify any studies which met the inclusion criteria.
Blinding
We did not identify any studies which met the inclusion criteria.
Incomplete outcome data
We did not identify any studies which met the inclusion criteria.
Selective reporting
We did not identify any studies which met the inclusion criteria.
Other potential sources of bias
We did not identify any studies which met the inclusion criteria.
Effects of interventions
We did not identify any study which met the inclusion criteria. Therefore we were unable to perform any of the planned analyses and we were not able to compare the effectiveness of surgical intervention to medical management or the effectiveness of radiological intervention to medical management in patients with symptomatic cervical artery dissection.
Discussion
There is a lack of evidence in the management of CeAD in people who develop neurological symptoms or experience progressive neurological deterioration despite antithrombotic therapy. The only randomised controlled trials we found in this systematic review enrolled participants with CeAD and randomised them to either antiplatelet medication or anticoagulation. Since the participants in these trials were presenting with their first episode of CeAD, they did not match the PICO for this review. Therefore, we did not analyse them.
The CADISS study was the most noteworthy RCT in this field (CADISS Trial Investigators 2015). This trial compared antiplatelets to anticoagulants in people with CeAD, which became clinically apparent within seven days before randomisation. The primary outcome in this trial was ipsilateral stroke or death from all causes within three months. The target enrolment of 250 patients was reached (118 carotid, 132 vertebral) and 90% (n = 224) of those enrolled presented with stroke or transient ischaemic attack. The trialists found no difference between antiplatelet and anticoagulant drugs in their efficacy at preventing the primary endpoint of stroke. They conclude that stroke was rare in both groups at only 2%, and that this rate was lower than seen in observational trials. At first glance, one could argue that this undermines the need for the systematic review which we sought to undertake, as there is seldom a need for either endovascular or surgical intervention. However, on closer inspection, it can be noted that 52 patients, which constitutes more than 20% of the study population, were found to have been incorrectly diagnosed. On central review of the imaging, these 52 patients were subsequently found not to have radiographic evidence of CeAD and should therefore not have been enrolled in the study. Not only does this weaken the power of the study conclusions, it potentially underestimates the requirement for secondary intervention, because once these patients were excluded, the stroke rate rose by 50%.
One of the issues with an RCT in CeAD is that to sufficiently power a study, large numbers of participants are required for the clinical endpoint of stroke or neurological symptoms. This is all the more pertinent when attempting to enrol patients in whom medical therapy alone has failed to prevent or ameliorate neurological symptoms because the population at risk is even smaller. Estimates based on the meta‐analyses ‐ in particular, four meta‐analyses which are based on observational studies comparing antiplatelet to antithrombotic medication ‐ suggest that an RCT based purely on clinical outcomes may require a sample size as large as 2000 participants (Kennedy 2012; Lyrer 2010; Menon 2008; Sarikaya 2013). Therefore, alternative surrogate outcome markers may be needed to compare treatments in this patient population. An example of a proxy marker for ischaemic events secondary to CeAD is Diffusion‐weighted MRI (DWI). An RCT comparing surgery to endovascular stenting in carotid stenosis demonstrated that usage of DWI as an alternative outcome marker revealed equivalent results as the clinical study (Bonati 2010). Similarly, when investigating carotid revascularisation, adding new silent microbleeds in MRI is useful as a proxy for intracranial haemorrhage (Bonati 2008). The use of imaging endpoints has also been tested in CeAD, whereby magnetic resonance surrogates are being used to compare aspirin to anticoagulant treatment (vitamin K antagonists), in an open‐labelled, multicentre, non‐inferiority RCT (Droste 2001).
Imaging endpoints are not the only ones which have been used to supplement clinical or neurological endpoints. The Biomarkers and Antithrombotic Treatment in Cervical Artery Dissection (TREAT‐CAD) study, which has been recruiting participants since September 2013, aims at demonstrating non‐inferiority of aspirin compared with vitamin K antagonists in CeAD patients. The endpoints in this study will include the biomarkers MMP9 and TIMP2, in addition to a composite outcome of clinical or imaging endpoints of brain ischaemia, bleeds, or death (TREAT‐CAD 2014). MMP9, or Matrix metallopeptidase 9, is collagenase, i.e. an enzyme which breaks down collagen in the arterial wall, thereby compromising the integrity of the vessel structure and its resistance to dissection. TIMP2 is Tissue inhibitor of metalloproteinases 2, which is a protein that is a natural antagonist of MMPs. The expansion of endpoints and the prospect for reducing the scale of the numbers needed to recruit may make it more feasible to run RCTs and CCTs, especially when evaluating invasive therapies.
Endovascular intervention is usually reserved for CeAD patients with recurrent ischaemic events despite antithrombotic therapy, when ruptured infarction is impending, in ruptured dissecting aneurysm, or iatrogenic CeAD (Engelter 2015). Although we did not identify any RCTs or CCTs comparing endovascular therapy and antithrombotic treatment to antithrombotic therapy alone, we did find reports on endovascular treatment using angioplasty and stenting in CeAD patients which did not meet the inclusion criteria (Ahlhelm 2012; Asif 2015; Assadian 2004; Bernardo 2019; Edgell 2005; Fava 2008; Jensen 2017; Joo 2005; Juszkat 2015; Lavallee 2007; Marnat 2016; Martinelli 2017; Rahme 2013; Traenka 2018). One systematic review of endovascular stenting in extracranial CeAD identified 140 patients (153 arteries) with extracranial internal carotid dissection (ICAD) and 10 patients (12 arteries) with extracranial vertebral artery dissection (VAD) (Pham 2011). The aetiology was mixed with 48% (n = 64) traumatic, 37% (n = 49) spontaneous, and 16% (n = 21) iatrogenic. The combined technical success rate was high at 99% for ICAD and 100% for VAD with low peri‐procedural complications (1.3% for ICAD and 0% for VAD). However, thrombosis of the stents occurred in three ICAD cases (3/150, 2%) and one VAD case (1/7, 14%). Within a mean follow‐up period of 17.7 months (range 1 month to 72 months), there were no deaths, and neurological complications occurred in 1.4% for ICAD and zero cases for VAD. The participant population included in these cases was not the same as that chosen in our review; that is, active neurological symptoms despite antithrombotic therapy. In 70% of all the reported cases, endovascular treatment was chosen because of contraindications for the use of anticoagulants or because of the severity of the haemodynamic compromise with or without failure of the antithrombotic treatment alone.
A report from the SWISS Registry included 62 patients with symptomatic CeAD who were treated with intravenous thrombolysis (IVT) alone (24 patients) versus those who received endovascular therapy (38 patients) (Traenka 2018). In the group of 38 patients who received endovascular therapy, some received endovascular therapy in addition to IVT (25 patients), while the remaining 13 patients had endovascular therapy without bridging with IVT. n the same paper, the authors report the outcome of a meta‐analysis of retrospective studies comparing endovascular therapy (102 participants) to IVT alone (110 participants). Overall they found a non‐significant trend towards higher recanalisation rates in endovascular‐treated patients. Interpretation of their outcomes was restricted by low numbers and the retrospective nature of the observation data, leading the authors to postulate that in future prospective studies with higher numbers and more advanced endovascular devices, endovascular therapy may prove to be superior. However, they cautioned about the use of endovascular therapy in combination with IVT, which appeared to increase the risk of symptomatic intracranial haemorrhage. This latter observation was echoed by a more recent publication of 109 participants (Bernardo 2019). In this study of consecutive patients with cervical artery dissection‐related acute ischaemic stroke and intracranial occlusion from the Acute STroke Registry and Analysis of Lausanne (ASTRAL), 24 had endovascular treatment, 38 received IVT alone, and 47 had no revascularisation treatment. The authors found that endovascular treatment with prior intravenous thrombolysis may increase the risk of major radiological but not symptomatic intracranial haemorrhage. They did not find a clear superiority with endovascular treatment but they did conclude that endovascular therapy should not be withheld in these patients. An additional rare indication for endovascular stenting was the rupture of an extracranial dissecting aneurysm (Goyal 2009). From this collated data it would appear that endovascular treatment seems relatively safe; however, an RCT would be needed to identify superiority over antithrombotic treatment alone. In the absence of level one evidence, endovascular treatment continues to be reserved for CeAD patients in whom antithrombotic therapy alone has failed, especially those patients who demonstrate rapid deterioration with impending haemodynamic infarction, those with ruptured dissecting aneurysm, or those with iatrogenic CeAD. However, one must bear in mind that these choices are not evidenced‐based and are subject to selective reporting bias and confounded by the experience of the operator, their training and individual preferences and skills.
Five studies, two of which were brought to full‐text review but were ultimately excluded, reported on surgical intervention (Chiche 2005; Ehrenfeld 1976; Mokri 1990; Schievink 1994; Zelenock 1982). However, none of these studies compared the surgical outcome to medical therapy alone. The numbers are very small, with the number of surgical cases ranging from three (Zelenock 1982), to 22 (Schievink 1994), and the studies are very old. A Cochrane systematic review reported collated data across cases series and found 135 CeAD patients who had arterial surgery for ICAD (Lyrer 2003). Ten (7.4%) patients died and seven (5.2%) had a residual neurological deficit which rendered them disabled. In particular, seven (5.2%) patients suffered from a stroke and two (1.5%) experienced an intracranial haemorrhage. These morbidity and mortality rates exceed those reported from antithrombotic agents. The adverse outcomes from surgery could be due to either treatment effects or more likely, they represent selection bias because patients who had surgery were likely to be more severely affected at presentation, may have had more extensive disease or may have demonstrated disease progression despite medical therapy, for example, severe, recurrently symptomatic stenosis or persistent emboli (Rao 2011). Surgical treatment, when undertaken, reportedly carries risks of early occlusion, stroke, and cranial nerve injuries (Müller 2000). Therefore, with the limited observational data that it is available, it has been suggested that surgery is restricted to those cases of progression of symptoms where the lesions are anatomically accessible or endovascular therapy is contraindicated, or both (Rao 2011).
Summary of main results
We did not identify any trials for inclusion.
Overall completeness and applicability of evidence
We did not identify any trials for inclusion.
Quality of the evidence
We did not identify any trials for inclusion.
Potential biases in the review process
We did not identify any trials for inclusion. The authors of the review do not have any conflicts of interest that may compromise their identification of suitable studies or their ability to objectively review resultant data, had it been available to analyse.
Agreements and disagreements with other studies or reviews
We believe that in adhering to the recommended Cochrane search and review methods, we have identified all data of interest to the review question. We did not identify any trials for inclusion and we were therefore unable to synthesise evidence that could be used in a comparison with other studies or reviews.
Authors' conclusions
Implications for practice.
No conclusions can be drawn about surgical or radiological interventions for CeAD in symptomatic patients from this review, and consequently, there are no implications for practice. Although observational data suggest that endovascular therapy can be used in CeAD when antithrombotic therapy fails or haemodynamic infarction is impending, and that surgical intervention can be considered if endovascular therapy is a contraindication and the lesion is anatomically accessible, the level of evidence is insufficient to support this. In the absence of robust evidence, CeAD in patients with evolving neurological symptoms despite medical therapy remains a condition where a bedside clinician should use, on a case‐by‐case basis, the best clinical judgment, and adopt a stepped care approach (Arnold 2011).
Implications for research.
The absence of any evidence points to the need for an RCT. However, the logistical difficulties of such an undertaking should not be underestimated. The Cervical Artery Dissection and Ischemic Stroke Patients (CADISP) group estimated that for a comparison of antiplatelet and anticoagulation drugs, a large sample size of approximately 2800 participants would be required to provide sufficient clinical endpoints to detect a difference of 5% in the number of patients dead or disabled from 20% to 15% (25% relative odds reduction) (Engelter 2007). To recruit such numbers and obtain sufficient funding, international collaboration will be necessary. This is all the more challenging when trying to recruit a subgroup who are neurologically symptomatic despite antithrombotic therapy. Given the anticipated difficulties in recruitment, there are high risks of a type II error or premature cessation of the trial. Even with ample participant numbers and fiscal support, several other methodological issues still exist. These include the choice of an endpoint, which has implications for study power and clinical expectations. The combination of death and disability was used by the CADISP study group to calculate the number of 2800 participants required in an RCT. This is in contrast to most contemporary RCTs in the area of acute stroke treatment, which normally employ total or partial recovery as the primary endpoint (NINDS 1995). It is likely that, in an RCT, the event rate would be lower than the death and disability rate obtained from meta‐analysis of historical series; therefore, proxy endpoints such as imaging or biomarker endpoints are necessary.
History
Protocol first published: Issue 9, 2018 Review first published: Issue 2, 2021
Acknowledgements
We acknowledge the Cochrane Stroke Group for the guidance and support they provided during the preparation of this review.
Appendices
Appendix 1. CENTRAL search strategy
#1MeSH descriptor: [Carotid Artery Injuries] this term only #2MeSH descriptor: [Carotid Artery, Internal, Dissection] this term only #3MeSH descriptor: [Vertebral Artery Dissection] this term only #4MeSH descriptor: [Vertebral Artery] this term only #5MeSH descriptor: [Neck Injuries] explode all trees #6(((carotid or vertebr* or cervical) near/2 arter* near/3 (dissect* or damag* or injur* or lesion* or laceration* or trauma* or ruptur* or wound))):ti,ab,kw (Word variations have been searched) #7{or #1‐#6} #8MeSH descriptor: [Carotid Arteries] explode all trees #9MeSH descriptor: [Carotid Artery Diseases] this term only #10MeSH descriptor: [Carotid Artery Thrombosis] this term only #11{or #8‐#10} #12MeSH descriptor: [Aneurysm, Dissecting] this term only #13MeSH descriptor: [Aneurysm, False] this term only #14MeSH descriptor: [Aneurysm, Ruptured] this term only #15MeSH descriptor: [Wounds, Nonpenetrating] explode all trees #16((traumatic near/5 (dissection or aneurysm or pseudoaneurysm))):ti,ab,kw (Word variations have been searched) #17((blunt near/5 (injur* or trauma))):ti,ab,kw (Word variations have been searched) #18(dissecting aneurysm):ti,ab,kw (Word variations have been searched) #19MeSH descriptor: [Rupture, Spontaneous] this term only #20{or #12‐#19} #21#11 and #20 #22#7 or #21
Appendix 2. MEDLINE search strategy
1. carotid artery injuries/ or carotid artery, internal, dissection/ 2. vertebral artery dissection/ 3. vertebral artery/in or exp neck injuries/ 4. ((carotid or vertebr$ or cervical) adj2 arter$ adj3 (dissect$ or damag$ or injur$ or lesion$ or laceration$ or trauma$ or ruptur$ or wound)).tw. 5. or/1‐4 6. exp carotid arteries/ or carotid artery diseases/ or carotid artery thrombosis/ or vertebral artery/ 7. (carotid$ or vertebr$ or cervical$).tw. 8. 6 or 7 9. aneurysm, dissecting/ or aneurysm, false/ or aneurysm, ruptured/ 10. exp wounds, nonpenetrating/ 11. (traumatic adj5 (dissection or aneurysm or pseudoaneurysm)).tw. 12. (blunt adj5 (injur$ or trauma)).tw. 13. dissecting aneurysm.tw. 14. rupture, spontaneous/ or rupture/ 15. spontaneous dissection.tw. 16. or/9‐15 17. 16 and 8 18. 5 or 17 19. randomized controlled trial.pt. 20. controlled clinical trial.pt. 21. randomized.ab. 22. placebo.ab. 23. randomly.ab. 24. trial.ab. 25. groups.ab. 26. or/19‐25 27. 18 and 26
Appendix 3. Embase search strategy
1. carotid artery injury/ 2. vertebral artery/ or neck injury/ 3. artery dissection/ 4. ((carotid or vertebr$ or cervical) adj2 arter$ adj3 (dissect$ or damag$ or injur$ or lesion$ or laceration$ or trauma$ or ruptur$ or wound)).tw. 5. or/1‐4 6. exp carotid artery/ or exp carotid artery disease/ or carotid artery obstruction/ or carotid artery thrombosis/ 7. (carotid$ or vertebr$ or cervical$).tw. 8. 6 or 7 9. aneurysm rupture/ or exp carotid artery aneurysm/ or dissecting aneurysm/ or false aneurysm/ 10. (traumatic adj5 (dissection or aneurysm or pseudoaneurysm)).tw. 11. dissecting aneurysm.tw. 12. rupture/ 13. spontaneous dissection.tw. 14. 9 or 10 or 11 or 12 or 13 15. 14 and 8 16. 5 or 15 17. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/ 18. Randomization/ 19. Controlled clinical trial/ or "controlled clinical trial (topic)"/ 20. control group/ or controlled study/ 21. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 22. Crossover Procedure/ 23. Double Blind Procedure/ 24. Single Blind Procedure/ or triple blind procedure/ 25. placebo/ or placebo effect/ 26. (random$ or RCT or RCTs).tw. 27. (controlled adj5 (trial$ or stud$)).tw. 28. (clinical$ adj5 trial$).tw. 29. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 30. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 31. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 32. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 33. (cross‐over or cross over or crossover).tw. 34. (placebo$ or sham).tw. 35. trial.ti. 36. (assign$ or allocat$).tw. 37. controls.tw. 38. or/17‐37 39. 16 and 38
Appendix 4. CT.gov search strategy
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; ) n=28 INFLECT EXACT "Interventional" [STUDY‐TYPES] AND ( Cervical Artery OR Vertebral Artery ) [DISEASE] AND INFLECT EXACT ( "Adult" OR "Older Adult" ) [AGE‐GROUP]
Appendix 5. WHO search strategy
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; ) n=42 Basic search: artery dissection Synonyms: artery dissection, ANEURYSM DISSECTING, Aneurysm Dissecting, Aneurysm Dissecting [Disease/Finding], Aneurysm;dissecting, Aneurysms Dissecting, Arterial dissection, Arterial dissection (disorder), Artery NOS dissection, artery; dissection, Dissecting Aneurysm, Dissecting aneurysm (morphologic abnormality), Dissecting aneurysm of artery, Dissecting aneurysm of artery (disorder), Dissecting aneurysm of artery NOS, Dissecting Aneurysms, Dissection of artery, dissection of artery (diagnosis), Dissection of artery (disorder), dissection; artery
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asif 2015 | Wrong study design Cases series. Correct patient population but wrong study design |
| Chiche 2005 | Wrong study design Cases series of surgical treatment for vertebral artery dissection. Correct patient population. No comparator |
| Edgell 2005 | Wrong study design Retrospective case series |
| Fava 2008 | Wrong study design Cases series. No control group. Mixed population: 11 spontaneous and 1 traumatic dissection |
| Jensen 2017 | Wrong study design and wrong patient population Retrospective case series of Intra‐cranial carotid and vertebral dissections. No control |
| Joo 2005 | Wrong study design Mixed patient population (trauma and spontaneous, extra and intra‐cerebral). Retrospective series. No comparator |
| Juszkat 2015 | Wrong study design Cases series. Mixed spontaneous and traumatic. No comparator |
| Lavallee 2007 | Wrong study design This is a retrospective series of consecutive patients from a hospital database |
| Marnat 2016 | Wrong study design and wrong patient population Retrospective review of a prospective multicentre database, looking at a subgroup of patients. Patient population had intracranial rather than cervical dissections |
| Martinelli 2017 | Wrong study design Case series. Mixed spontaneous and traumatic medical treatment. Endovascular and surgical treatment. No comparator |
| Schievink 1994 | Wrong study design Case series. Retrospective review of 22 patients. No comparator. Mixed population of spontaneous and traumatic |
Differences between protocol and review
A number of changes have been made to the review since it was at protocol stage.
We clarified that it is not failure of medical therapy per se that necessitates surgical or radiological intervention. Rather, it is continued development of neurological symptoms despite maximum medical therapy that prompts surgical or radiological intervention.
We defined our primary outcomes as discrete events rather than events at multiple early, midterm, late and extended time points. However, we had planned to do subgroup analyses to assess events at discrete time points.
We updated three of the secondary outcomes to clarify that they are time‐to‐event variables rather than continuous or dichotomous variables. These outcomes are:
length of survival;
length of survival free from any stroke, or transient ischaemic attack (including amaurosis fugax);
time to recurrence of cervical dissection, in either the same artery or any of the other three extracranial cervical arteries.
We added major bleeding to the 'Summary of findings' table as an adverse event outcome (Table 1).
Contributions of authors
NH: designed and drafted the protocol; acquired trial reports; performed trial selection; drafted the review and will undertake future review updates.
EPK: designed and drafted the protocol.
SS: designed and drafted the protocol; drafted the review and will undertake future review updates.
FJ: designed and drafted the protocol; drafted the review and will undertake future review updates.
Declarations of interest
NH: has no conflict of interest which will effect this review.
EPK: none known.
SS: has no conflict of interest which will effect this review.
FJ: none known.
New
References
References to studies excluded from this review
Asif 2015 {published data only}
- Asif KS, Lazzaro MA, Teleb MS, Fitzsimmons BF, Lynch J, Zaidat O. Endovascular reconstruction for progressively worsening carotid artery dissection. Journal of Neurointerventional Surgery 2015;7(1):32-9. [DOI] [PubMed] [Google Scholar]
Chiche 2005 {published data only}
- Chiche L, Praquin B, Koskas F, Kieffer E. Spontaneous dissection of the extracranial vertebral artery: Indications and long-term outcome of surgical treatment. Annals of Vascular Surgery 2005;19(1):5-10. [DOI] [PubMed] [Google Scholar]
Edgell 2005 {published data only}
- Edgell RC, Abou-Chebl A, Yadav JS. Endovascular management of spontaneous carotid artery dissection. Journal of Vascular Surgery 2005;42(5):854-60. [DOI] [PubMed] [Google Scholar]
Fava 2008 {published data only}
- Fava M, Meneses L, Loyola S, Tevah J, Bertoni H, Huete I, et al. Carotid artery dissection: endovascular treatment. Report of 12 patients. Catheterization and Cardiovascular Interventions 2008;71(5):694-700. [DOI] [PubMed] [Google Scholar]
Jensen 2017 {published data only}
- Jensen J, Salottolo K, Frei D, Loy D, McCarthy K, Wagner J, et al. Comprehensive analysis of intra-arterial treatment for acute ischemic stroke due to cervical artery dissection. Journal of Neurointerventional Surgery 2017;9(7):654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joo 2005 {published data only}
- Joo JY, Ahn JY, Chung YS, Han IB, Chung SS, Yoon PH, et al. Treatment of intra- and extracranial arterial dissections using stents and embolization. CardioVascular and Interventional Radiology 2005;28(5):595-602. [DOI] [PubMed] [Google Scholar]
Juszkat 2015 {published data only}
- Juszkat R, Liebert W, Stanislawska K, Tomczyk T, Wronka J, Wasik N, et al. Internal carotid artery dissection treated with self-expandable stents: a single-centre experience. CardioVascular and Interventional Radiology 2015;38:1451-7. [DOI] [PubMed] [Google Scholar]
Lavallee 2007 {published data only}
- Lavallee PC, Mazighi M, Saint-Maurice JP, Meseguer E, Abboud H, Klein IF, et al. Stent-assisted endovascular thrombolysis versus intravenous thrombolysis in internal carotid artery dissection with tandem internal carotid and middle cerebral artery occlusion. Stroke 2007;38(8):2270-4. [DOI] [PubMed] [Google Scholar]
Marnat 2016 {published data only}
- Marnat G, Mourand I, Eker O, Machi P, Arquizan C, Riquelme C, et al. Endovascular management of tandem occlusion stroke related to internal carotid artery dissection using a distal to proximal approach: insight from the RECOST study. American Journal of Neuroradiology 2016;37(7):1281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinelli 2017 {published data only}
- Martinelli O, Venosi S, BenHamida J, Malaj A, Belli C, Irace FG, et al. Therapeutical options in the management of carotid dissection. Annals of Vascular Surgery 2017;41:69-76. [DOI] [PubMed] [Google Scholar]
Schievink 1994 {published data only}
- Schievink WI, Piepgras DG, McCaffrey TV, Mokri B. Surgical treatment of extracranial carotid artery dissecting aneurysms. Neurosurgery 1994;35:809-15. [DOI] [PubMed] [Google Scholar]
Additional references
Ahlhelm 2012
- Ahlhelm F, Benz RM, Ulmer S, Lyrer P, Stippich C, Engelter S. Endovascular treatment of cervical artery dissection: ten case reports and review of the literature. Interventional Neurology 2012;1(3-4):143-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnold 2011
- Arnold M, Fischer U, Bousser MG. Treatment issues in spontaneous cervico-cephalic artery dissection. International Journal of Stroke 2011;6(3):213-8. [DOI] [PubMed] [Google Scholar]
Assadian 2004
- Assadian A, Senekowitsch C, Rotter R, Zölss C, Strassegger J, Hagmüller GW. Long-term results of covered stent repair of internal carotid artery dissections. Journal of Vascular Surgery 2004;40(3):484-7. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Béjot 2014
- Béjot Y, Aboa-Eboulé C, Debette S, Pezzini A, Tatlisumak T, Engelter S, CADISP Group. Characteristics and outcomes of patients with multiple cervical artery dissection. Stroke 2014;45(1):37-41. [DOI] [PubMed] [Google Scholar]
Bernardo 2019
- Bernardo F, Nannoni S, Strambo D, Puccinelli F, Saliou G, Michel P, et al. Efficacy and safety of endovascular treatment in acute ischemic stroke due to cervical artery dissection: a 15-year consecutive case series. International Journal of Stroke 2019;14(4):381-9. [DOI] [PubMed] [Google Scholar]
Biller 2014
- Biller J, Sacco RL, Albuquerque FC, Demaerschalk BM, Fayad P, Long PH, American Heart Association Stroke Council. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(10):3155-74. [DOI] [PubMed] [Google Scholar]
Blum 2015
- Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Archives of Neuroscience 2015;2(4):e26670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonati 2008
- Bonati LH, Lyrer PA, Fluri F. Cerebral microbleeds after carotid revascularization - a prospective MRI study. Cerebrovascular Diseases 2008;26 Suppl 2:55.
Bonati 2010
- Bonati LH, Jongen LM, Haller S, Flach HZ, Dobson J, Nederkoorn PJ, ICSS-MRI study group. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurology 2010;9(4):353-62. [DOI] [PubMed] [Google Scholar]
Brott 2011
- Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Journal of the American College of Cardiology 2011;57(8):e16-94. [DOI] [PubMed] [Google Scholar]
CADISS Trial Investigators 2015
- CADISS Trial Investigators. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurology 2015;14(4):361-7. [DOI] [PubMed] [Google Scholar]
Caplan 2008
- Caplan LR. Dissections of brain-supplying arteries. Nature Clinical Practice Neurology 2008;4(1):34-42. [DOI] [PubMed] [Google Scholar]
Cohen 2012
- Cohen JE, Gomori JM, Itshayek E, Spektor S, Shoshan Y, Rosenthal G, et al. Single-center experience on endovascular reconstruction of traumatic internal carotid artery dissections. Journal of Trauma and Acute Care Surgery 2012;72(1):216-21. [DOI] [PubMed] [Google Scholar]
Debette 2009
- Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurology 2009;8(7):668-78. [DOI] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Donas 2008
- Donas KP, Mayer D, Guber I, Baumgartner R, Genoni M, Lachat M. Endovascular repair of extracranial carotid artery dissection: current status and level of evidence. Journal of Vascular and Interventional Radiology 2008;19(12):1693-8. [DOI] [PubMed] [Google Scholar]
Droste 2001
- Droste DW, Junker K, Stogbauer F, Lowens S, Besselmann M, Braun B, et al. Clinically silent circulating microemboli in 20 patients with carotid or vertebral artery dissection. Cerebrovascular Diseases 2001;12(3):181-5. [DOI] [PubMed] [Google Scholar]
Ehrenfeld 1976
- Ehrenfeld WK, Wylie EJ. Spontaneous dissection of the internal carotid artery. Archives of Surgery 1976;111:1294-301. [DOI] [PubMed] [Google Scholar]
Engelter 2007
- Engelter ST, Brandt T, Debette S, Caso V, Lichy C, Pezzini A, Cervical Artery Dissection in Ischemic Stroke Patients (CADISP) Study Group. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke 2007;38(9):2605-11. [DOI] [PubMed] [Google Scholar]
Engelter 2013
- Engelter ST, Grond-Ginsbach C, Metso TM, Metso AJ, Kloss M, Debette S, Cervical Artery Dissection and Ischemic Stroke Patients Study Group. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology 2013;80(21):1950-7. [DOI] [PubMed] [Google Scholar]
Engelter 2015
- Engelter ST, Traenka C, Von Hessling A, Lyrer PA. Diagnosis and treatment of cervical artery dissection. Neurology Clinics 2015;33:421–41. [DOI] [PubMed] [Google Scholar]
Georgiadis 2006
- Georgiadis D, Caso V, Baumgartner RW. Acute therapy and prevention of stroke in spontaneous carotid dissection. Clinical and Experimental Hypertension 2006;28(3-4):365-70. [DOI] [PubMed] [Google Scholar]
Goyal 2009
- Goyal MS, Derdeyn CP. The diagnosis and management of supraaortic arterial dissections. Current Opinion in Neurology 2009;22(1):80-9. [DOI] [PubMed] [Google Scholar]
GRADEproGDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version Accessed 8 February 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hassan 2012
- Hassan AE, Zacharatos H, Souslian F, Suri MF, Qureshi AI. Long-term clinical and angiographic outcomes in patients with cervico-cranial dissections treated with stent placement: a meta-analysis of case series. Journal of Neurotrauma 2012;29(7):1342-53. [DOI] [PubMed] [Google Scholar]
Hatano 1976
- Hatano S. Experience from a multicentre stroke register: a preliminary report. Bulletin of the World Health Organization 1976;54(5):541. [PMC free article] [PubMed] [Google Scholar]
Hernández‐Durán 2014
- Hernández-Durán S, Ogilvy CS. Clinical outcomes of patients with vertebral artery dissection treated endovascularly: a meta-analysis. Neurosurgical Review 2014;37(4):569-77. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2019
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Jeon 2010
- Jeon P, Kim BM, Kim DI, Shin YS, Kim KH, Park SI, et al. Emergent self-expanding stent placement for acute intracranial or extracranial internal carotid artery dissection with significant hemodynamic insufficiency. American Journal of Neuroradiology 2010;31(8):1529-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kashiwazaki 2013
- Kashiwazaki D, Ushikoshi S, Asano T, Kuroda S, Houkin K. Long-term clinical and radiological results of endovascular internal trapping in vertebral artery dissection. Neuroradiology 2013;55(2):201-6. [DOI] [PubMed] [Google Scholar]
Kennedy 2012
- Kennedy F, Lanfranconi S, Hicks C, Reid J, Gompertz P, Price C, et al. Antiplatelets vs anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology 2012;79(7):686-9. [DOI] [PubMed] [Google Scholar]
Kissela 2012
- Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology 2012;79(17):1781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Public Library of Science Medicine 2009;6(7):1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lyrer 2003
- Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD000255. [DOI: 10.1002/14651858.CD000255] [DOI] [PubMed] [Google Scholar]
Lyrer 2010
- Lyrer P, Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD000255. [DOI: 10.1002/14651858.CD000255.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 2019
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Chapter 9: Summarizing study characteristics and preparing for synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Medel 2014
- Medel R, Starke RM, Valle-Giler EP, Martin-Schild S, El Khoury R, Dumont AS. Diagnosis and treatment of arterial dissections. Current Neurology and Neuroscience Reports 2014;14(1):419. [DOI] [PubMed] [Google Scholar]
Menon 2008
- Menon R, Kerry S, Norris JW, Markus HS. Treatment of cervical artery dissection: a systematic review and meta-analysis. Journal of Neurology, Neurosurgery and Psychiatry 2008;79(10):1122-7. [DOI] [PubMed] [Google Scholar]
Mokri 1990
- Mokri B. Traumatic and spontaneous extracranial internal carotid artery dissections. Journal of Neurology 1990;237:356-61. [DOI] [PubMed] [Google Scholar]
Müller 2000
- Müller BT, Luther B, Hort W, Neumann-Haefelin T, Aulich A, Sandmann W. Surgical treatment of 50 carotid dissections: indications and results. Journal of Vascular Surgery 2000;31(5):980-8. [DOI] [PubMed] [Google Scholar]
NINDS 1995
- The National Institute of Neurological Disorders and Stroke rt-PA Study Group. Tissue plasminogen activator for acute ischemic stroke. New England Journal of Medicine 1995;333:1581–7. [DOI] [PubMed] [Google Scholar]
Ohta 2011
- Ohta H, Natarajan SK, Hauck EF, Khalessi AA, Siddiqui AH, Hopkins LN, et al. Endovascular stent therapy for extracranial and intracranial carotid artery dissection: single-center experience. Journal of Neurosurgery 2011;115(1):91-100. [DOI] [PubMed] [Google Scholar]
Page 2019
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Pham 2011
- Pham MH, Rahme RJ, Arnaout O, Hurley MC, Bernstein RA, Batjer HH, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery 2011;68(4):856-66. [DOI] [PubMed] [Google Scholar]
Rahme 2013
- Rahme RJ, Aoun SG, McClendon J Jr, El Ahmadieh TY, Bendok BR. Spontaneous cervical and cerebral arterial dissections: diagnosis and management. Neuroimaging Clinics of North America 2013;23(4):661-71. [DOI] [PubMed] [Google Scholar]
Rao 2011
- Rao AS, Makaroun MS, Marone LK, Cho JS, Rhee R, Chaer RA. Long-term outcomes of internal carotid artery dissection. Journal of Vascular Surgery 2011;54(2):370-4. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sadato 2010
- Sadato A, Maeda S, Hayakawa M, Kato Y, Sano H, Hirose Y, et al. Endovascular treatment of vertebral artery dissection using stents and coils: its pitfall and technical considerations. Minimally Invasive Neurosurgery 2010;53(5-6):243-9. [DOI] [PubMed] [Google Scholar]
Sarikaya 2013
- Sarikaya H, da Costa BR, Baumgartner RW, Duclos K, Touzé E, Bray JM, et al. Antiplatelets versus anticoagulants for the treatment of cervical artery dissection: Bayesian meta-analysis. PLoS One 2013;8(9):e72697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schievink 2001
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. New England Journal of Medicine 2001;344(12):898-906. [DOI] [PubMed] [Google Scholar]
Schulman 2005
- Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. Journal of Thrombosis and Haemostasis 2005;3(4):692-4. [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Schwartz 2009
- Schwartz NE, Vertinsky AT, Hirsch KG, Albers GW. Clinical and radiographic natural history of cervical artery dissections. Journal of Stroke and Cerebrovascular Diseases 2009;18:416–23. [DOI] [PubMed] [Google Scholar]
Smajlović 2015
- Smajlović D. Strokes in young adults: epidemiology and prevention. Vascular Health and Risk Management 2015;11:157-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stella 2010
- Stella N, Palombo G, Filippi F, Fantozzi C, Taurino M. Endovascular treatment of common carotid artery dissection via the superficial temporal artery. Journal of Endovascular Therapy 2010;17(4):569-73. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Traenka 2018
- Traenka C, Jung S, Gralla J, Kurmann R, Stippich C, Simonetti BG, et al. Endovascular therapy versus intravenous thrombolysis in cervical artery dissection ischemic stroke - results from the SWISS registry. European Stroke Journal 2018;3(1):47-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
TREAT‐CAD 2014
- TREAT-CAD. Biomarkers and Antithrombotic Treatment in Cervical Artery Dissection - TREAT-CAD. https://clinicaltrials.gov/show/nct02046460 2014. [DOI] [PMC free article] [PubMed]
UK‐TIA Study Group 1988
- UK-TIA Study Group. United Kingdom Transient Ischaemic Attack (UK-TIA) aspirin trial: interim results. BMJ 1988;296(6618):316. [PMC free article] [PubMed] [Google Scholar]
Vishteh 1998
- Vishteh AG, Marciano FF, David CA, Schievink WI, Zabramski JM, Spetzler RF. Long-term graft patency rates and clinical outcomes after revascularization for symptomatic traumatic internal carotid artery dissection. Neurosurgery 1998;43(4):761-7. [DOI] [PubMed] [Google Scholar]
Xianjun 2012
- Xianjun H, Zhiming Z. A systematic review of endovascular management of internal carotid artery dissections. Interventional Neurology 2012;1(3-4):164-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zelenock 1982
- Zelenock GB, Kazmers A, Whitehouse WM, Graham LM, Erlandson EE, Cronenwett JL. Extracranial internal carotid artery dissections. Archives of Surgery 1982;117:425-32. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hynes 2018
- Hynes N, Kavanagh EP, Tawfick W, Sultan S, Jordan F. Surgical and radiological interventions for treating symptomatic extracranial cervical artery dissection. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD013118. [DOI: 10.1002/14651858.CD013118] [DOI] [PMC free article] [PubMed] [Google Scholar]


