Abstract
Background
Obesity is a highly and increasingly prevalent chronic condition for which drugs are commonly prescribed to improve health.
Objectives
To assess the long‐term effects of approved anti‐obesity medications in clinical trials of at least one‐year duration.
Search methods
MEDLINE, EMBASE, The Cochrane Library, the Current Science Meta‐register of Controlled Trials and reference lists were searched. Drug manufacturers and two obesity experts were contacted.
Selection criteria
Double‐blind, randomised placebo‐controlled trials of approved anti‐obesity agents that 1) included patients over 18 years, 2) used an intention‐to‐treat analysis, and 3) had follow‐up of one year or more. Both weight loss and weight maintenance trials were included. Abstracts, pseudo‐randomised trials, head‐to‐head trials and open‐label studies were excluded.
Data collection and analysis
Two reviewers independently assessed all potentially relevant reports for inclusion and methodological quality. Data were extracted using double data entry. The primary outcome measure was weight loss.
Main results
Sixteen orlistat (n = 10,631), 10 sibutramine (n = 2623) and four rimonabant trials (n = 6365) met inclusion criteria. Attrition rates averaged 30% to 40%. Compared to placebo, orlistat reduced weight by 2.9 kg (95% confidence interval (CI) 2.5 to 3.2 kg), sibutramine by 4.2 kg (95% CI 3.6 to 4.7 kg), and rimonabant by 4.7 kg (95% CI 4.1 to 5.3 kg). Patients on active drug therapy were significantly more likely to achieve 5% and 10% weight loss thresholds. Placebo‐controlled weight losses were consistently lower in patients with diabetes. Orlistat reduced diabetes incidence, improved total cholesterol, LDL‐cholesterol, blood pressure, and glycaemic control in patients with diabetes but increased rates of gastrointestinal side effects and slightly lowered HDL levels. Sibutramine improved HDL and triglyceride levels but raised blood pressure and pulse rate. Rimonabant improved HDL‐cholesterol, triglyceride and blood pressure levels and glycaemic control in patients with diabetes but increased the risk of mood disorders.
Authors' conclusions
Orlistat, sibutramine and rimonabant have been studied in trials of one year or longer. Internal validity of studies was limited by high attrition rates. All three antiobesity agents are modestly effective in reducing weight and have differing effects on cardiovascular risk and adverse effects profiles. Longer and more methodologically rigorous studies of anti‐obesity drugs that are powered to examine endpoints such as mortality and cardiovascular morbidity are required.
Plain language summary
Long‐term drug pharmacotherapy for obesity and overweight
This review assessed the long‐term benefits and risks of approved anti‐obesity drugs in clinical trials of 1 to 4 years duration. Sixteen orlistat (10,631 patients), 10 sibutramine (2623 patients) and four rimonabant (6635 patients) studies were examined. High drop‐out rates (30% to 40%) were a limitation of nearly all studies. Compared to placebo, all three drugs reduced weight by around five kg or less and orlistat reduced the number of high‐risk patients who developed diabetes. No data to show that any of the three drugs lowers the risk of death or cardiovascular disease were found. The most prominent side effects were gastrointestinal for orlistat, cardiovascular for sibutramine (raised blood pressure and/or pulse rate) and psychiatric for rimonabant (mood disorders). In Europe, rimonabant is contraindicated for patients with severe depression and/or patients who are treated with antidepressive medications. Rimonabant is furthermore not recommended for patients with other untreated psychiatric conditions. We conclude that: 1. average weight losses with current anti‐obesity agents appear modest but may be of clinical benefit, and 2. better studies designed to examine mortality and cardiovascular morbidity are required to fully evaluate any potential benefit of such agents.
Background
Description of the condition
Obesity is a highly and increasingly prevalent chronic condition that is associated with significant morbidity and mortality. Globally, over 300 million individuals are obese and an additional 800 million are overweight (Haslam 2005). In many countries, the adult prevalence of obesity has risen above 20% (IOTF 2007). In the United States, Eastern Mediterranean, and Pacific Islands, the prevalence ranges from approximately 30% to over 70% (Flegal 2002; IOTF 2007). Prevalence rises with age and is higher in females and certain ethnic populations, such as American Indians, Hispanic Americans and Pacific Islanders (Flegal 2002; Kopelman 2000; WHO 1998). Obesity and overweight are also becoming a major concern in children and adolescents (Haslam 2005; Ogden 2002).
In addition to increased total mortality, obesity is associated with a number of chronic conditions including coronary artery disease, stroke, type 2 diabetes, heart failure, dyslipidaemia, hypertension, reproductive and gastrointestinal cancers, gallstones, fatty liver disease, osteoarthritis, and sleep apnea (Birmingham 1999; Calle 1999; Kenchaiah 2002; Manson 1995; UTD 2001; Williamson 1993). There is also a significant psychosocial stigma associated with being obese (Bray 1998). In many countries, the economic burden of obesity‐related illness is substantial, with estimates ranging from 2% to 7% of total health care expenditures and billions of dollars in direct and indirect costs to society (Birmingham 1999; Seidell 1996).
Body mass index (BMI) is the most widely used measurement to quantify degree of overweight and obesity because it is easily calculated (weight in kilograms divided by the square of the height in meters) and available in many epidemiological studies. According to the World Health Organization (WHO) criteria, overweight is defined as a BMI of 25 to 29.9 kg/m2 and obesity as 30 kg/m2 or greater. Obesity is further subdivided into mild (30 to 34.9 kg/m2), moderate (35 to 39.9 kg/m2) and severe (40 kg/m2 or greater) categories. Mortality rates and risk of cardiovascular disease rise with increasing degrees of overweight and obesity; marked increases in risk of death occur when BMI levels reach 29 to 30 kg/m2 or greater (Calle 1999; Manson 1995; Stevens 1998). In non‐smokers with BMI levels greater than 40 kg/m2, the 14‐year relative risk of death is 2.6 times higher for men and 2.0 times higher for women compared to non‐smokers with BMI levels between 23.5 and 24.9 kg/m2 (Calle 1999). Obesity is a particularly strong risk factor for the development of type 2 diabetes. Compared to a baseline BMI of less than 22 kg/m2, a BMI greater than 35 kg/m2 increases the 10‐year odds ratio of developing type 2 diabetes by 41 in men and 30 in women (Field 2001). The pattern of fat deposition is also an important prognostic factor, particularly in the elderly, with increased cardiovascular risk observed in those with central or visceral fat accumulation (typically measured by the waist circumference or waist‐hip ratio) (Kissebah 1994; Rexrode 1998; Rimm 1995; Visscher 2002). In fact, central measures of adiposity may be more strongly associated with cardiovascular events than BMI (INTERHEART 2005).
Before‐after case series, cohort studies and randomised controlled trials have demonstrated that weight loss in overweight and obese participants ‐ even as little as 5% to 10% of initial body weight ‐ is associated with an improvement in cardiovascular risk factors (Blackburn 1995; Colditz 1995; Goldstein 1992; Wadden 1993) and a reduction in the incidence of type 2 diabetes in high‐risk individuals (DPP 2002). Cohort studies examining the relationship between weight loss and long‐term mortality have shown mixed results (Andres 1993; Williamson 1993). Many studies have failed to distinguish between voluntary and involuntary weight loss or fat loss and overall weight loss. Those studies making such distinctions have generally found that voluntary weight loss or fat loss in overweight or obese participants leads to decreased mortality rates (Allison 1999; French 1999; Williamson 1995). To date, no randomised controlled trial has been performed that confirms these findings, although studies examining hard cardiovascular endpoints and mortality are underway ( Look AHEAD; Padwal 2007).
Non‐pharmacological methods of obesity therapy, which include dietary modification, exercise and behavioural modification, have demonstrated short‐term effectiveness. Unfortunately, long‐term recidivism rates are high (NIH 1993) and effectiveness is also limited by a compensatory slowing of the metabolic rate (Leibel 1995). Surgical procedures such as gastric bypass and banding have the greatest long‐term success rates (approximately 20% weight loss after ten years) but are currently indicated only for the very obese (BMI greater than 40 kg/m2 or BMI 35 to 40 kg/m2 with an obesity‐related disorder) (SOS 2004). Operative mortality rates are generally less than one percent in appropriate patients and high‐volume centres, but long‐term gastrointestinal adverse effects and other complications may occur (Greenway 2000).
Description of the intervention
Obesity guidelines currently recommend that drug therapy be considered for patients with a BMI greater than or equal to 30 kg/m2 or a BMI of 27 to 30 kg/m2 with one or more obesity‐related disorders (Lau D 2007; Lau DC 2007; Padwal 2007; US Guidelines 1998). Drugs should be used in conjunction with non‐pharmacological therapy. Approved anti‐obesity medications can be divided into three broad categories: 1. Inhibitors of intestinal fat absorption. Orlistat, a drug that inhibits pancreatic and other lipases, is the only agent currently available in this class. Side effects are related to malabsorption of fat within the gastrointestinal tract and include steatorhea, bloating, and oily discharge. Fecal incontinence and malabsorption of fat‐soluble vitamins, such as vitamin A, D, E, and K, have also been reported (McNeely 1998). 2. Medications that act to suppress appetite, increase satiety, or increase thermogenesis, primarily by modifying central nervous system neurotransmission of norepinephrine, dopamine and serotonin. This category includes sibutramine, phentermine, mazindol, diethylpropion, benzphetamine, phendimetrazine, fenfluramine, and dexfenfluramine. The latter two agents have been associated with a higher risk of cardiac valvulopathy and pulmonary hypertension and are no longer available (Abenhaim 1996; Connolly 1997; Jick 1998;Khan 1998; Weissman 1998). Sibutramine, which inhibits re‐uptake of serotonin and norepinephrine, is the most widely used agent in this category and primarily acts to suppress appetite. The most common adverse effects of sibutramine are related to increased adrenergic activity and include dry mouth, headache, insomnia, and constipation (Luque 1999). Sibutramine may also cause increases in blood pressure and heart rate. Potential concerns regarding cardiac arrhythmias and cardiac mortality have been raised and the drug has been reviewed by several regulatory agencies and deemed safe to remain on the market (Health Canada 2002; Wooltorton 2002). 3. Inhibitors of the endocannabinoid system. Rimonabant, the first of this class of drugs, is approved in the European Union and other countries. Rimonabant acts by both central and peripheral mechanisms to reduce food intake and body weight (Padwal 2007). An increased incidence of mood disorders is the major adverse effect. In Europe, rimonabant is contraindicated for patients with severe depression and/or patients who are treated with antidepressive medications; it is not recommended for patients with other untreated psychiatric conditions.
Orlistat, sibutramine and rimonabant are the three medications approved for long‐term use.
Why it is important to do this review
It has been suggested that obesity be considered a chronic illness requiring long‐term therapy similar to hypertension or dyslipidaemia (Bray 2000; NTF 1994). The majority of randomised‐controlled trials (RCTs) evaluating anti‐obesity medications have been of short duration. However, short‐term efficacy is clearly a sub‐optimal endpoint, especially if most patients regain weight over the long term when therapy is stopped. A meta‐analysis of 108 primarily short‐term studies published up to December 1999 found that average antiobesity drug‐induced weight losses compared to placebo were modest, never exceeding four kg for any one agent (Haddock 2002).
This review of obesity pharmacotherapy focuses on 'long‐term' studies, which we define as one‐year or greater. For a chronic illness like obesity, it is more relevant to evaluate drug efficacy over the long‐term. In addition, since weight losses achieved with lifestyle intervention are modest and recidivism rates are high (Lau 2007; Lau DC 2007), there is potential for even greater use of drug therapy, particularly given rising obesity prevalence rates. It is also important to determine if the modest weight reductions associated with drug therapy translate into a reduced cardiovascular morbidity, cardiovascular mortality and overall mortality. In addition, it is important to quantify the degree of improvement in cardiovascular risk factors (that is blood pressure, lipid profiles and glycaemic control) reported with antiobesity drug therapy.
This review represents an update of a previous Cochrane review (Padwal 2003). Major changes can be inspected in Appendix 6.
Other systematic reviews and Health Technology Assessments in this area may also be of interest to the reader (Arterburn 2004; Li 2005; Norris 2005; O'Meara 2001; O'Meara 2002). A Cochrane review specifically on rimonabant has also recently been published (Curioni 2006).
Objectives
To assess the effects of Long‐term pharmacotherapy for obesity and overweight.
Primary Objective
In placebo‐controlled clinical trials of at least one year duration, to determine the efficacy of single or combination anti‐obesity drug therapy in reducing weight, cardiovascular morbidity (stroke, myocardial infarction), cardiovascular mortality and overall mortality.
Secondary Objectives
In placebo‐controlled clinical trials of at least one year duration, to determine:
The efficacy of antiobesity drugs in reducing waist circumference and body mass index (BMI) levels.
The impact of antiobesity drug therapy on cardiovascular risk factors. These include blood pressure, lipid parameters and glycaemic control.
The efficacy of anti‐obesity drug therapy in reducing weight and improving glycaemic control in patients with type 2 diabetes.
The major adverse events associated with each antiobesity agent.
Methods
Criteria for considering studies for this review
Types of studies
Only double‐blind (blinding of participants and care providers) RCTs of anti‐obesity agents were considered for inclusion. Quasi‐randomised, open‐label, and cross‐over trials were not included. Studies had to 1) enroll overweight or obese patients (defined below), 2) include a placebo control group, 3) report an intention‐to‐treat analysis and 4) have a minimum follow‐up period of one year (from the point of randomizations). Studies published in abstract form only were not included. No language or publication restrictions were applied. Previously, our search included head‐to‐head clinical trials but, due to the length of this review and large number of placebo‐controlled studies, the decision was made to focus on placebo‐controlled trials only.
Types of participants
Adults (age 18 or over) with either:
body mass index (BMI) 30 kg/m2 or greater;
BMI 27 kg/m2 or greater plus one or more obesity‐related co‐morbidity.
Types of interventions
Weight loss and weight maintenance studies evaluating the pharmacologic therapy of obesity including the following medications: sibutramine, phentermine, mazindol, diethylpropion, benzphetamine, phendimetrazine, benzocaine rimonabant and orlistat. Drugs excluded from this review include off‐label therapy (e.g. fluoxetine, sertraline, bupropion, topiramate, metformin), those with high addiction potentials that preclude long‐term use (amphetamine/dexamphetamine and methamphetamine), investigational/herbal/alternative compounds, and drugs withdrawn from the market due to unacceptable side effect profiles (fenfluramine, dexfenfluramine, phenylpropanolamine).
Types of outcome measures
Primary outcomes
weight loss, expressed as number of kilograms lost, percentage of baseline weight lost, or both.
Secondary outcomes
weight loss expressed as the proportion of patients achieving 5% and 10% weight loss (5% and 10% responders), change in BMI and change in waist circumference;
total and cardiovascular mortality;
myocardial infarction (fatal and nonfatal);
stroke (fatal and nonfatal);
medication intolerance (percentage withdrawn from therapy due to adverse events);
change in blood pressure;
change in lipid profile (total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, triglycerides);
change in glycosylated haemoglobin concentration (Hb A1C);
side effects of therapy.
Search methods for identification of studies
Electronic searches
The Cochrane Library (issue 4, 2006);
MEDLINE (until December Week 3, 2006);
EMBASE (until week 51, 2006);
metaRegister of Controlled Trials (www.controlled‐trials.com; until December 2006).
Electronic searches were performed with the aid of a medical librarian. Search strategy available upon request.
The described search strategy (see for a detailed search strategy under Appendix 1) were used for MEDLINE. For use with EMBASE, The Cochrane Library and the other databases this strategy will be slightly adapted.
Searching other resources
reference lists of original studies, narrative reviews and systematic reviews;
drug manufacturers and two experts in the field of obesity were contacted in an effort to identify unpublished studies.
Data collection and analysis
Selection of studies
Two reviewers (RP or DR) performed electronic searches and screened the initial results. Articles that clearly did not meet the inclusion criteria were rejected on initial review. If uncertainty existed, the full text of the article was reviewed. Two reviewers (RP and SL or DR) independently assessed all potentially relevant studies for inclusion using pre‐designed data abstraction forms. Disagreements were resolved by consensus. Reviewers were not blinded to the journal, author, or institution of publication. Inter‐rater agreement was assessed using Cohen's kappa coefficient (Cohen 1960).
Data extraction and management
Two reviewers (RP or SL/DR) independently extracted and recorded data. Discrepancies were rechecked twice more. Data not presented in written form were extrapolated from graphs if possible. If the published article provided inadequate information for a given endpoint, the primary author was contacted by e‐mail at least twice. An additional request was made to the pharmaceutical company if data elements were still missing.
Assessment of risk of bias in included studies
The Verhagen Delphi list for quality assessment of RCTs was used as a guide to assess study quality (Verhagen 1998) and was independently judged by two authors (RP and SL or DR). The nine criteria are as follows: 1. Was a method of randomizations performed? 2. Was the treatment allocation concealed? 3. Were the groups similar at baseline regarding the most important prognostic indicators? 4. Were the eligibility criteria specified? 5. Was the outcome assessor blinded? 6. Was the care provider blinded? 7. Was the patient blinded? 8. Were point estimates and measures of variability presented for the primary outcome variables? 9. Did the analysis include an intention‐to‐treat analysis (ITT)?
The assessment of ITT analysis included an assessment of the study attrition rate (Hollis 1999) for the primary endpoint of weight loss . Although an acceptable attrition rate is difficult to define and depends both on the study and outcome in question, to conveniently define a specific threshold, we arbitrarily deemed an attrition rate of less than 15% per study arm as acceptable. We have reported methodological quality in a descriptive fashion rather than using a numeric quality score, as such scores can be inaccurate and poorly reproducible when used to differentiate between high and low quality studies (Juni 2001).
Measures of treatment effect
We calculated a risk difference for dichotomous outcomes and a weighted mean difference for continuous outcomes. This calculation was performed using data at the end of follow‐up for each individual study.
Two different types of study designs are generally used in obesity pharmacotherapy ‐ so called 'weight loss' and 'weight maintenance' studies. The latter type of study examines the impact of the drug on weight after a weight loss induction phase that uses a low or very‐low calorie diet. This induction phase typically lasts between 1 to 6 months and is performed in all patients. Weight maintenance studies consistently tend to include the weight losses achieved during the induction phase in the overall weight changes reported for each study arm. Because randomization typically occurs after the induction phase and the weight loss achieved during this phase, when reported, appears equivalent between study arms, the effect of this practice on the overall placebo‐subtracted mean difference in weight tends to be negligible. In addition, many weight loss trials included a short 'run‐in' phase during which patients are treated with placebo and diet. This is performed to either stratify randomizations by the degree of weight loss achieved during the run‐in phase or exclude patients not able to achieve a predefined amount of weight. Accordingly, this practice blurs the distinction between weight loss and weight maintenance trials. Therefore, in contrast to our previous version of this review (Padwal 2003), we analysed separately published weight loss and weight maintenance trials together.
Dealing with missing data
Quantitative analyses of outcomes were based on intention‐to‐treat (ITT) results. In studies with high attrition rates, we preferentially abstracted results reported in a last‐observation‐carried‐forward (LOCF) fashion, in which the last observation on record was used as a surrogate for the final value. This is a commonly reported, more conservative analysis than an analysis involving completers only. When quantitative pooling was not possible or deemed inappropriate, results were presented in narrative fashion.
If studies reported mean baseline and mean final values in the control or intervention groups (with associated standard errors or deviations), but did not report the standard deviation associated with this difference, we computed this as follows (for the control and intervention arms separately): 1. We took the difference between mean final and mean initial measurements as the mean change in the variable (delta V). 2. The standard deviation (SD) associated with change in delta V was calculated using the following formula: square root of [(SDpre)2 + (SDpost)2 ‐ 2r(SDpre*SDpost)], where SDpre was the standard deviation of the mean baseline measurement, SDpost was the standard deviation of the mean follow‐up measurement, and was the correlation between the baseline and follow‐up values. As studies did not report r, and its true value is unknown (ranging between 0 and 1), we used 0.5 as an estimation of its value. We tested this assumption by performing sensitivity analyses on the outcomes of systolic blood pressure, total cholesterol, and fasting glucose using 0.25 and 0.75 as values for r.
If the study reported only the mean change in a variable for the treatment and control groups and its associated P‐value, we computed the standard deviations for delta V for each study arm by assuming that both study arms had equal variances. If the P‐value was reported as less than a certain value, we used 1/100 less than that value as a conservative estimate of the true P‐value. For example, if the reported P‐value was less than 0.01, we estimated the true P‐value to be 0.0099. Z‐scores were estimated by assuming a normal distribution. If the sample size of the study arm was less than 100, we assumed a t‐distribution instead of a normal distribution.
Assessment of heterogeneity
A chi‐squared test for heterogeneity was performed and the I2 statistic calculated for each outcome. A random‐effects model was preferentially used, partly to incorporate any observed heterogeneity among trials. If the I2 statistic demonstrated significant heterogeneity (over 50%), we did not quantitatively pool the results unless the observed statistical heterogeneity was judged to be of little clinical relevance (that is clinically insignificant between‐study differences and consistently concordant results across studies).
Assessment of reporting biases
If the number of studies was greater than 10 for a given drug, a funnel plot was used to assess for small study bias.
Data synthesis
A random‐effects model was employed using the RevMan 4.2.9 program.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analyses (for cardiovascular morbidity/mortality endpoints and weight loss) included:
patients with type 2 diabetes;
body mass index (BMI) strata (less than 30 kg/m2, 30 to 34.9 kg/m2, 35 to 39.9 kg/m2, 40 kg/m2 or greater);
patients at high cardiovascular risk (that is known cardiovascular disease or risk factors).
If significant statistical heterogeneity was present and deemed clinically significant, we planned to assess the impact of study size, study length, baseline BMI and baseline cardiovascular risk on this heterogeneity. In the absence of individual patient data, we knew a priori that meta‐regression analysis would not be possible.
Sensitivity analysis
We performed separate analyses using a fixed‐effect model and by varying the correlation coefficient r (as noted above).
Results
Description of studies
Results of the search
Search results are summarized in the Quality of Reporting of Meta‐analyses (QUORUM) flow diagram (Figure 1; Moher 1999). From the date of the last search (December 2002) to December 2006, 27 potentially relevant trials were identified and five orlistat, five sibutramine and four rimonabant studies met final inclusion criteria. These were added to the 11 orlistat and five sibutramine trials previously identified. Cohen's kappa coefficient for inter‐rater agreement measured 0.95 for trial selection and 0.85 for study quality.
1.
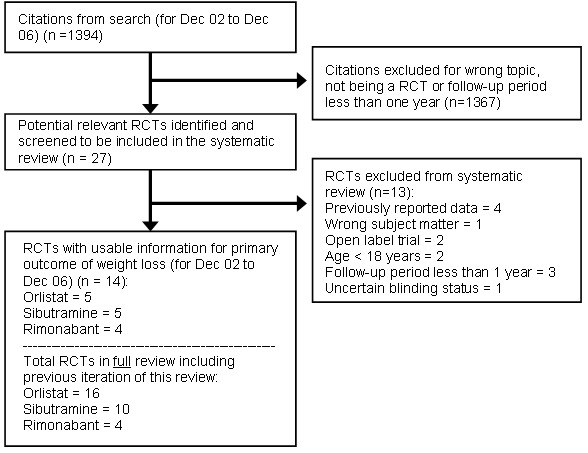
QUOROM statement.
This QUOROM (quality of reporting of meta‐analyses) diagram reflects the updated search only (December 2002 to December 2006)
Orlistat
Population and setting
The 16 trials included 10 631 participants with an average body mass index (BMI) of 36.3 kg/m2, weight of 104 kg, and age of 47 years ( Bakris 2002; Berne 2004; Broom 2002; Davidson 1999; Derosa 2003; Finer 2000; Hauptman 2000; Hollander 1998; Kelley 2002; Krempf 2003; Lindgarde 2000; Miles 2002; Rossner 2000; Sjostrom 1998; Swinburn 2005; XENDOS). Study size ranged from 50 to 3305 participants, 66% of whom were female and 89% Caucasian. The country of origin for each study is listed in Characteristics of included studies.
Nine studies limited enrolment to higher risk populations: four recruited patients with type 2 diabetes on stable doses of oral hypoglycaemic agents or insulin (Berne 2004; Hollander 1998; Kelley 2002; Miles 2002) and five enrolled obese patients with at least one cardiovascular risk factor (hypertension, dyslipidaemia, type 2 diabetes, or impaired glucose tolerance) (Bakris 2002; Broom 2002; Derosa 2003; Lindgarde 2000; Swinburn 2005). In the XENDOS trial, the largest study, 21% of patients had impaired glucose tolerance (XENDOS).
Exclusion criteria common to most studies were obesity of endocrine origin, uncontrolled hypertension, treatment with drugs affecting body weight, pregnant or lactating women, women of childbearing potential not on contraceptives, significant psychiatric or medical illness, previous bariatric surgery, and weight loss of greater than 3 to 4 kg in the three months prior to screening.
Most trials included a single‐blind, placebo run‐in phase, which varied in duration from 2 to 5 weeks and many required a compliance rate of 75% or greater during the run‐in phase before randomizations into the actual trial.
The four orlistat weight maintenance studies represented continuations of weight loss trials in which patients were placed on a weight maintenance diet during their second year (Davidson 1999; Hauptman 2000; Rossner 2000; Sjostrom 1998).
Interventions
The dose of orlistat used in all trials was 120 mg tid, which is the standard dose recommended for use in clinical practice. Two studies also included 60 mg tid study arms, showing efficacy and tolerability that was intermediate between that of placebo and 120 mg tid study arms (Hauptman 2000; Rossner 2000). The remainder of this review will focus on results obtained using 120 mg tid dosage regimen.
A standardized, low fat (less than 30% of caloric intake), hypocaloric diet and encouragement to exercise were the main co‐interventions (see Characteristics of included studies). A typical diet derived 30% of calories from fat, 50% from carbohydrates, and 20% from protein, with maximum cholesterol content of 300 mg/day.
During the second year of weight maintenance, diets differed between trials but, in general, were increased by approximately 200 to 300 kcal/day in those patients still losing weight and remained unaltered in those patients in whom weight remained stable. At the beginning of the weight maintenance phase, patients in the orlistat group were re‐randomised to receive placebo, 60 mg tid and 120 mg tid in one study (Davidson 1999). In a second study, all patients completing year one were re‐randomised to orlistat 120 mg tid or placebo (Sjostrom 1998). In the final two studies, patients remained in the same groups to which they were assigned during year one (orlistat 60 mg, 120 mg and placebo) (Hauptman 2000; Rossner 2000).
Outcomes
Fourteen trials reported weight change as the primary outcome. This was commonly reported as the percentage of baseline weight lost, absolute number of kilograms lost and the percentage of patients losing 5% and 10% of initial body weight. In the remaining two trials, diabetes incidence (XENDOS) and change in the Framingham cardiovascular risk score (Swinburn 2005) were the primary endpoints. Other commonly reported outcomes included change in cholesterol, fasting glucose and blood pressure levels and gastrointestinal side effects.
Total mortality, cardiovascular morbidity, and cardiovascular mortality were not reported as outcomes in any of the trials. Other endpoints are summarized in the table Characteristics of included studies.
Sibutramine
Population and setting
The 10 sibutramine studies included 2623 participants (range 86 to 485) with an average BMI of 35.1 kg/m2, weight of 97 kg, and age of 45 years (see table Characteristics of included studies). Seven weight loss trials (Hauner 2004; McMahon 2000; McMahon 2002; McNulty 2003; Sanchez‐Reyes 2004; Smith 2001) and three weight maintenance trials (Apfelbaum 1999; James 2000; Mathus‐Vliegen 2005) were identified, with follow‐up periods for the randomizations phase ranging between 1 to 1.5 years. Seventy‐three percent of the participants were female and 95% were Caucasian. Most non‐Caucasian participants came from two trials, in which 26% of participants were African American (McMahon 2000; McMahon 2002). Two studies limited enrolment to hypertensive patients with controlled blood pressure (McMahon 2000; McMahon 2002) and three enrolled patients with type 2 diabetes (Kaukua 2003; McNulty 2003; Sanchez‐Reyes 2004). Exclusion criteria were similar to ones described above for the orlistat studies.
Further details regarding the study populations and settings are reported in the table Characteristics of included studies.
Interventions and outcomes
The dose of sibutramine ranged between 10 to 20 mg, with the most common dose being 15 mg. If results for both 15 and 20 mg arms were reported, we used the 15 mg results because the 20 mg dose is no longer used. Dietary modification with or without advice to exercise were common co‐interventions.
Four weight loss trials included a single‐blind, placebo run‐in phase, which varied from 2 to 10 weeks in duration (McMahon 2000; McMahon 2002; Smith 2001; Kaukua 2003). Randomizations were restricted to those participants that could follow dietary advice (Smith 2001) or those that achieved 75% compliance during the run‐in phase (McMahon 2000).
The weight maintenance studies included initial calorie‐restricted induction phases that varied from 1 to 6 months (see table Characteristics of included studies). Patients able to lose a pre‐defined amount of weight entered the randomizations phase of the study.
Specific outcomes for each study are summarized in the table Characteristics of included studies and were similar to those presented in orlistat studies. All studies reported either percent or absolute weight loss. Blood pressure and pulse rate were also commonly reported. No data on cardiovascular morbidity or mortality were found. In addition to reporting overall weight loss, the weight maintenance studies reported the proportion of patients achieving successful weight maintenance, defined as maintenance of 80% to 100% of the weight lost during the induction phase.
Rimonabant
Population and setting
The four rimonabant studies included 6635 participants (range 1036 to 3045) with an average BMI of 36.5 kg/m2, weight of 102 kg, and age of 48 years (see table Characteristics of included studies). All studies reported results for a one‐year weight loss phase; one study also reported outcomes for a second year of weight maintenance in which rimonabant‐treated patients were re‐randomised to continue taking rimonabant or switch to placebo (RIO‐North America). Seventy‐three percent of the participants were female and 87% were Caucasian.
Further details regarding the study populations and settings are reported in the table Characteristics of included studies. One study enrolled patients with dyslipidaemia (RIO‐Lipids), one enrolled patients with diabetes (RIO‐Diabetes), and the other two commonly included patients with dyslipidaemia or hypertension (RIO‐Europe; RIO‐North America). Exclusion criteria were similar to ones described above for the orlistat studies.
Interventions and outcomes
All studies included a placebo arm, a rimonabant 5 mg arm and a rimonabant 20 mg arm. The 20 mg dose is the one used in clinical practice; therefore all abstracted outcomes reflect this dose. Dietary modification with or without advice to exercise were common co‐interventions.
All trials included a 4‐week single‐blind, placebo run‐in phase, and restricted randomizations to those participants completed the run‐in phase and were adherent.
Specific outcomes for each study are summarized in the table Characteristics of included studies. All studies reported either percent or absolute weight loss. No data on cardiovascular morbidity or mortality were found.
Ongoing studies
The major ongoing studies in this area are summarized in the table Characteristics of ongoing studies. CRESCENDO and SCOUT, involving rimonabant and sibutramine, respectively, are the largest antiobesity studies to‐date and are designed to evaluate the effect of these agents on cardiovascular morbidity and mortality.
Included studies
A total of 30 double‐blind, placebo‐controlled RCTs, including 16 orlistat (n = 10,631), 10 sibutramine (n = 2623) and four rimonabant studies (n = 6635) were included in the final review and are detailed below. Twenty‐seven studies were financially supported by the drug manufacturer.
Twenty‐seven studies (16 orlistat, seven sibutramine and four rimonabant) were weight loss trials, in which drug therapy was used in conjunction with a weight loss diet for 1 to 4 years. One rimonabant and four orlistat weight loss trials also contained a second weight maintenance year. The three remaining sibutramine trials were weight maintenance studies with follow‐up periods of one and 1.5 years from the point of randomizations.
Excluded studies
The most pertinent excluded trials are summarized in the table Characteristics of excluded studies.The degree of weight loss reported in all excluded studies was similar to that of studies included in this review. No excluded studies examined the effect of a given drug on cardiovascular morbidity or mortality.
Risk of bias in included studies
All studies
Methodological quality is summarized in the corresponding tables of methodological quality for each drug (see Appendix 2; Appendix 3; Appendix 4). A "?" in the table indicates that the study did not mention this quality indicator in sufficient detail to confirm that it was done. Studies were all of similar quality.
Allocation, blinding and baseline similarity
Eligibility criteria were reported in all studies. In all studies, co‐interventions appeared to be equally applied to intervention and control arms. "Double‐blinding" was assumed to refer to blinding of patients and blinding of care providers, although this was not explicitly stated in any of the trials. Blinding of outcome assessors was not specifically mentioned in any study.
Selective reporting
Secondary endpoints were inconsistently reported, and sometimes reported in only a subgroup of patients or were not reported in an extractable manner. Given these limitations, our analysis includes only those data which were extractable from a given study. Readers should keep this in mind, as, in particular, studies may have only reported full results for endpoints that significantly differed from placebo.
Follow‐up and exclusions
The major methodological limitation was high attrition rates, as detailed below.
Orlistat
Weight loss studies
Allocation, blinding and baseline similarity
Five trials adequately described methods of randomizations and allocation concealment (Berne 2004; Derosa 2003; Finer 2000; Sjostrom 1998; XENDOS). The remaining studies merely stated that randomizations was performed without giving further details. Five studies reported baseline similarity from the point of entry into the run‐in phase and not from the point of randomizations (Broom 2002; Davidson 1999; Hollander 1998; Krempf 2003; Lindgarde 2000). In the remaining studies, the groups were similar at baseline. All studies reported point estimates and measures of variability for the primary outcome of weight loss.
In many studies, baseline weight was defined as the weight measured at the beginning of the run‐in period, rather than at the point of randomizations. Thus, the absolute change in weight (mean final weight minus mean baseline weight) in both study arms was inflated because weight lost during the run‐in period was included in this calculation. However, both study arms lost similar amounts of weight during the run‐in phase of each trial. Therefore, the overall mean difference in weight between treatment and control arms was not affected.
Selective reporting
All trials reported some measure of variability for the primary endpoint of weight loss.
Follow‐up and exclusions
Only two trials met the definition of true intention‐to‐treat (ITT) analysis (Berne 2004; Derosa 2003); the remaining 14 studies reported attrition rates of over 20% (range for all studies 0% to 66%; approximate average for all 16 trials was 30%). In the largest and longest trial, nearly 60% of patients dropped out over the four year follow‐up period (XENDOS). The most common reasons for premature withdrawal were treatment refusal, loss to follow‐up, and adverse effects.
Weight maintenance studies
Patients entering year two of the study already represented a highly select population because of the high attrition rates observed during the first year of each study. For example, in the two trials that did not re‐randomise patients, only 52% and 60% of patients were followed for the full two years (Hauptman 2000; Rossner 2000). A last‐observation‐carried‐forward (LOCF) ITT analysis was again used in the weight loss maintenance phase of each trial. No study reported the baseline characteristics of patients entering the second year of the trial.
Sibutramine
Allocation, blinding and baseline similarity
Three of ten trials reported methods of randomizations and allocation concealment in adequate detail (James 2000; ; Mathus‐Vliegen 2005; Smith 2001). Study groups were similar at baseline in all studies. Two studies included weight loss achieved during the 6‐month run‐in phase in the analysis (James 2000; Mathus‐Vliegen 2005).
Selective reporting
One trial did not provide any measures of variability for weight loss (McMahon 2000). We were able to obtain this from the drug manufacturer.
Follow‐up and exclusions
Similar to the orlistat studies described above, all trials reported a LOCF ITT analysis for weight loss. Attrition rates were high in nine of ten studies (see Characteristics of included studies), ranging from 11% to 51% per study arm and averaging almost 40%. Therefore, only one study was deemed to have fully met criteria for a true ITT analysis (that is an acceptable drop‐out rate) (Kaukua 2003).
Rimonabant
Allocation, blinding and baseline similarity
Two of four trials reported methods of randomizations and allocation concealment in adequate detail (RIO‐Diabetes; RIO‐Europe). Study groups were similar at baseline in all studies.
Selective reporting
All trials reported some measure of variability for the primary endpoint of weight loss.
Follow‐up and exclusions
Similar to orlistat and sibutramine studies, all trials reported a LOCF ITT analysis for weight loss. Attrition rates were high in all studies (see Characteristics of included studies), ranging from 32% to 49% per study arm and averaging 40%. Therefore, no study was deemed to fully meet criteria for a true ITT analysis (that is an acceptable drop‐out rate).
Effects of interventions
General comments
The number of patients included in a given endpoint analysis may be lower than the overall number of patients studied and varies according to study attrition rates, lack of endpoint reporting, and our ability to abstract data for that endpoint. The Characteristics of included studies contains a list of the endpoints reported for each study. If an endpoint is reported in this table but is not included in the meta‐analysis section, it is because the endpoint was not reported in an extractable manner or was not reported in a manner that facilitated inclusion (e.g., reported as an on‐treatment rather than an intention‐to‐treat result or reported only in a subset of patients).
Studies did not report results by body mass index (BMI) strata. Therefore, this analysis was not performed.
The results of our two sensitivity analyses (using a fixed‐effect model and by varying the correlation coefficient r) were essentially identical to the main results and, for the sake of brevity, are not detailed below.
Heterogeneity
Statistical heterogeneity (I2 of 50% or greater) was present in several anthropometric outcomes but was not judged to be clinically relevant (see Methods section). Substantial statistical heterogeneity was also present when analysing the effects of orlistat and rimonabant on glycaemic control in patients with and without diabetes. For orlistat, this heterogeneity was attenuated and did not appear clinically relevant when limiting pooling to patients with diabetes alone. For rimonabant, glycaemic control results are reported only for the single trial involving patients with type 2 diabetes.
Orlistat
Body weight
All sixteen studies reported greater reductions in weight in the orlistat group compared to the placebo group. Orlistat‐treated patients lost 2.9 kg (95% confidence interval (CI) 2.5 to 3.2 kg; 15 studies) or 2.9% (95% CI 2.5 to 3.4%; 13 studies) more weight than placebo‐treated patients. Placebo‐subtracted absolute weight losses were slightly greater in patients with lower baseline cardiovascular (CV) risk (3.0 kg, 95% CI 2.4 to 3.6 kg; 7 studies) compared to patients with higher baseline risk (2.8 kg, 95% CI 2.4 to 3.1 kg; 8 studies). Similar results were obtained for percentage weight loss as the outcome: 3.4% (95% CI 2.8 to 4.0%; 6 studies) for lower risk patients versus 2.7% (95% CI 2.1 to 3.3%; 7 studies) for higher risk patients.
In patients with diabetes, orlistat reduced weight by 2.6% (95% CI 2.2 to 3.1%; 5 studies) or 2.3 kg (95% CI 1.6 to 3.0 kg; 4 studies) compared to placebo therapy (Berne 2004; Hollander 1998; Kelley 2002; Lindgarde 2000; Miles 2002).
All trials reported that a greater percentage of participants in the orlistat group achieved 5% and 10% weight loss compared to placebo. Pooling results from 14 trials showed that 21% (95% CI 18% to 24%) more participants in the orlistat group achieved 5% weight loss. Pooled data from 13 studies demonstrated that 12% (95% CI 9% to 14%) more orlistat‐treated patients achieved 10% weight loss.
Waist circumference and body mass index
Orlistat significantly reduced waist circumference (2.1 cm, 95% CI 1.3 to 2.9 cm; 9 studies) and BMI (1.1 kg/m2, 95% CI 0.7 to 1.4 kg/m2 ; 3 studies) compared to placebo.
Blood pressure
Orlistat resulted in placebo‐subtracted systolic blood pressure reductions of 1.5 mm Hg (95% CI 0.9 to 2.2 mm Hg; 13 studies) and diastolic blood pressure reductions of 1.4 mm Hg (95% CI 0.7 to 2.0 mm Hg; 12 studies).
Glycaemic parameters
Orlistat reduced the incidence of type 2 diabetes from 9.0% to 6.2% (hazard ratio 0.63, 95% CI 0.46 to 0.86) in the XENDOS trial (XENDOS). This benefit was primarily observed in the patients with impaired glucose tolerance at baseline.
In trials that included patients with and without diabetes, orlistat reduced fasting glucose levels by 0.1 to 0.5 mmol/L (statistically significant in 4 of 6 studies). In patients with diabetes, orlistat reduced fasting glucose and Hb A1C levels by 1.0 mmol/L (95% CI 0.6 mmol/L to 1.5 mmol/L; 5 studies) and 0.4% (95% CI 0.2 to 0.6%; 5 studies), respectively.
A more detailed analysis of obesity pharmacotherapy in patients with type 2 diabetes is provided in a separate Cochrane review (Norris 2005).
Lipid parameters
Compared to placebo, orlistat reduced total cholesterol levels by 0.32 mmol/L (95% CI 0.28 to 0.37 mmol/L; 13 studies), LDL‐cholesterol levels by 0.26 mmol/L (95% CI 0.22 to 0.30 mmol/L; 13 studies) and HDL cholesterol levels by 0.03 mmol/L (95% CI 0.02 to 0.04 mmol/L; 11 studies). The change in triglyceride levels were not significantly different from placebo (‐0.03 mmol/L, 95% CI +0.07 mmol/L to ‐0.12 mmol/L; 11 studies).
Change in Framingham risk score
One study evaluated the effect of orlistat on the change in Framingham cardiovascular risk score and found nearly identical changes to that of placebo with no significant difference between study arms (Swinburn 2005).
Orlistat weight maintenance studies
During the weight maintenance phase of each study, both orlistat and placebo study arms showed similar amounts of weight regain, but the weight differential observed after the weight loss phase was preserved. Changes in serum lipids and glucose values during the weight maintenance phase were similar to those described for the weight loss phase of each trial (Hauptman 2000; Rossner 2000; Sjostrom 1998).
Adverse effects
Gastrointestinal (GI) events were the predominant side effect associated with orlistat therapy. The categorization of outcomes and detail of reporting of GI adverse events varied between trials. Over 80% of orlistat‐treated patients experienced at least one GI side effect, with an absolute frequency that was 24% (95% CI 20% to 29%; 14 studies) higher than patients on placebo. The most commonly reported GI events were fatty/oily stool, fecal urgency and oily spotting, each occurring at frequency rates of 15% to 30% in most studies. Approximately 5% of orlistat‐treated patients discontinued therapy due to GI side effects, which was 2% (95% CI 1% to 3%; 12 studies) higher than patients taking placebo.
Fecal incontinence was a reported side effect of orlistat therapy but only three trials reported this complication as a separate endpoint (Hauptman 2000; Rossner 2000; Sjostrom 1998), with an incidence rate of 7%. This was 6% (95% CI 5% to 8%) higher than the frequency of fecal incontinence in patients on placebo. Levels of fat‐soluble vitamins (A, D, E) and beta‐carotene were reportedly lowered by orlistat therapy, with vitamin D the most frequently affected (Finer 2000; Hauptman 2000; Hollander 1998; Sjostrom 1998). However, no study reported the occurrence of clinically significant vitamin deficiency, although patients were routinely advised to take a multivitamin pill daily.
Sibutramine
Weight loss
Patients on sibutramine therapy lost 4.2 kg (95% CI 3.6 to 4.7 kg; 8 studies) or 4.3% (95% CI 3.7% to 5.0%; 10 studies) more weight than those taking placebo. Placebo‐subtracted weight loss for patients at higher CV risk was 4.3 kg (95% CI 3.6 to 5.0 kg; 5 studies) or 4.5% (95% CI 3.8 to 5.2%; 5 studies). Corresponding values for lower risk patients were 4.0 kg (95% CI 3.0 to 5.0 kg; 5 studies) or 3.9% (95% CI 2.1% to 5.7%; 3 studies). In addition, sibutramine treatment increase the frequency of successful 5% responders by 32% (95% CI 27% to 37%; 7 studies) and 10% responders by 18% (95% CI 11% to 25%; 7 studies) compared to placebo.
In patients with diabetes, sibutramine reduced weight by 5.0% (95% CI 3.8 to 6.2%; 3 studies) or 4.9 kg (95% CI 3.6 kg to 6.2 kg; 3 studies) compared to placebo therapy (Kaukua 2003; McNulty 2003; Sanchez‐Reyes 2004).
The three weight maintenance studies reported that 10% to 30% more sibutramine‐treated patients achieved successful weight maintenance compared to placebo (successful weight maintenance defined as maintaining 80% to 100% of the initial weight loss). This achieved statistical significance (P<0.05) in all three studies (Apfelbaum 1999; James 2000; Mathus‐Vliegen 2005).
Waist circumference and body mass index
Sibutramine‐treated patients demonstrated placebo‐subtracted reductions in BMI of 1.5 kg/m2 (95% CI 1.3 kg/m2 to 1.8 kg/m2; 5 studies) and waist circumference by 4.0 cm (95% CI 3.3 cm to 4.7 cm; 8 studies).
Glycaemic parameters
Overall, changes in glycaemic parameters were inconsistently reported and, when reported, were not significantly different from placebo in any study even in patients with diabetes. Data were commonly not reported in an extractable format, which limited quantitative pooling.
Lipid parameters
Compared to placebo, sibutramine increased HDL cholesterol levels by 0.04 mmol/L (95% CI 0.01 to 0.08 mmol/L; 5 studies) and reduced triglyceride levels by 0.18 mmol/L (95% CI 0.07 to 0.30 mmol/L; 4 studies).
Data for total cholesterol and LDL‐cholesterol were not consistently reported or extractable in the majority of studies. Placebo‐subtracted changes in these two endpoints were not statistically significant in any study.
Adverse effects
Blood pressure and pulse rate
Sibutramine increased systolic blood pressure by 1.7 mm Hg (95% CI 0.1 to 3.3 mm Hg; 7 studies), diastolic blood pressure by 2.4 mm Hg (95% CI 1.5 to 3.3 mm Hg; 7 studies) and pulse rate by 4.5 beats/min (95% CI 3.5 to 5.6 beats/min; 7 studies) compared to placebo.
Other adverse effects
Insomnia, nausea, dry mouth, and constipation were more common in patients on sibutramine therapy, occurring at frequency rates of 7% to 20%.
Rimonabant
Weight loss
Patients on rimonabant therapy lost 4.7 kg (95% CI 4.1 to 5.3 kg; 4 studies) more weight than those taking placebo. All studies enrolled patients with cardiovascular risk factors; therefore, sensitivity analysis according to baseline CV risk was not performed. Rimonabant treatment increased the number of 5% weight‐loss responders by 33% (95% CI 29% to 37%; 4 studies) and 10% responders by 19% (95% CI 15% to 23%; 7 studies) compared to placebo.
Waist circumference
Rimonabant reduced waist circumference by 3.9 cm (95% CI 3.3 to 4.5 cm; 4 studies) compared to placebo.
Blood pressure
Rimonabant reduced placebo‐subtracted systolic blood pressure by 1.8 mm Hg (95% CI 0.8 to 2.8 mm Hg; 3 studies) and diastolic blood pressure by 1.2 mm Hg (95% CI 0.5 to 1.9 mm Hg; 3 studies) more than placebo.
Glycaemic parameters
Fasting glucose levels were reduced in RIO‐Diabetes by 1 mmol/L (95% CI 0.6 to 1.3 mmol/L) and haemoglobin A1C levels reduced by 0.7% (95% CI 0.6 to 0.8). When reported, no clinically or statistically significant reductions were demonstrated in the other studies.
Lipid parameters
Compared to placebo, rimonabant increased HDL cholesterol levels by 0.1 mmol/L (95% CI 0.08 to 0.11 mmol/L; 4 studies) and reduced triglyceride levels by 0.24 mmol/L (95% CI 0.17 to 0.30 mmol/L; 4 studies). Non‐significant changes in LDL (‐0.05 mmol/L, 95% CI ‐0.12 to +0.01 mmol/L; 4 studies) and total cholesterol (‐0.04 mmol/L, 95% CI ‐0.11 to +0.03; 4 studies) levels were demonstrated after quantitative pooling of data.
Adverse effects
The frequency of serious adverse effects was 6% in rimonabant‐treated patients, which was 2% (95% CI 0% to 3%; 4 studies) higher than those taking placebo. Fourteen percent of patients on rimonabant discontinued therapy due to adverse events, which was 6% (95% CI 5% to 8%; 4 studies) greater than placebo. The most concerning adverse effect was an increased incidence of psychiatric disorders (depression, anxiety, irritability, aggression), which occurred in 6% of patients receiving rimonabant and was 3% (95% CI 2% to 5%; 4 studies) more likely in patients receiving rimonabant compared to placebo.
Discussion
In summary, meta‐analysis of long‐term RCTs involving orlistat, sibutramine and rimonabant demonstrates that each drug results in average placebo‐subtracted weight reductions of approximately 5 kg or less. No data on the effect of these agents on mortality or cardiovascular morbidity were found. Weight maintenance studies for each agent report similar amounts of weight regain in both active treatment and placebo study arms, such that the original weight differential between groups is maintained. Orlistat reduces total cholesterol, LDL‐cholesterol, blood pressure, diabetes incidence and improves glycaemic control but increases the risk of gastrointestinal side effects and slightly lowers HDL levels. Sibutramine improves HDL‐cholesterol and triglyceride levels but increases blood pressure and pulse rate. Rimonabant improves HDL, triglyceride and blood pressure levels and glycemic control in patients with diabetes but increases the risk of mood disorders.
Previous studies have demonstrated that weight loss is more difficult to achieve in patients with diabetes, possibly because of the underlying disease state or because medications used to treat diabetes tend to increase weight (Wing 1987). We found that studies enrolling patients with diabetes reported slightly smaller amounts of weight loss with orlistat and rimonabant therapy, a finding that was not seen with sibutramine therapy. Despite this finding, both orlistat and rimonabant improved glycaemic parameters in patients with diabetes whereas sibutramine did not. The underlying reasons for and the clinical significance of these findings are unclear. One potential contributor to improved glycaemic control with rimonabant therapy is an increase in adiponectin levels (RIO‐North America). Further data are needed, ideally from head‐to‐head clinical trials of all three agents, before more definitive conclusions can be made.
High attrition rates in both treatment and control groups compromise the internal validity of many studies. Authors attempt to address this limitation by using a last‐observation‐carried‐forward (LOCF) analysis. Such an analysis can bias results in either direction, depending on the differential dropout rates in treatment and control arms and the reasons for withdrawal. It is difficult to compensate for such high attrition rates by using any form of analysis. Bias may be introduced into the results of these studies, and should be kept in mind when interpreting the results of this review. A recent study using administrative data from British Columbia, Canada found poor persistence rates with orlistat and sibutramine in a 'real world' setting (Padwal R 2007). In nearly 3500 users of sibutramine and 17,000 users of orlistat, persistence rates at one year were less than 10% and at two years, less than 2%. Overall, data from within and outside of the clinical trial setting suggest that a lack of adherence to therapy is a major limiting factor to the efficacy and effectiveness of antiobesity drug therapy.
Gastrointestinal side effects were the predominant side effect of orlistat therapy, with most studies reporting that side effects were mild and transient and decreased as patients adjusted to a low fat diet. However, high study attrition rates may reflect a differential drop‐out of patients unable to tolerate the medication and may partly explain the improved tolerance of patients remaining in the study.
The increase in blood pressure and heart rate observed with sibutramine therapy are of potential concern, particularly on a population‐wide basis where even mild increases in blood pressure can be expected to result in an increase in cardiovascular events in a population already at risk. A small rise blood pressure may have a detrimental effect on patients with pre‐existing cardiovascular disease, a patient population excluded from these trials. This further underscores the need for studies examining mortality and cardiovascular morbidity and the ongoing SCOUT trial should provide further information (SCOUT 2005). If sibutramine is prescribed, careful blood pressure monitoring is recommended.
The increased incidence of mood disorders with rimonabant mandates careful post‐marketing surveillance, particularly because psychiatric illness commonly coexists with obesity (Lau DC 2007; NIH 1993). As the patient population enrolled in the RIO studies was carefully screened to exclude patients with major psychiatric disease, the risk of mood disorders with rimonabant therapy may have been underestimated. In Europe, rimonabant is contraindicated for patients with severe depression and/or patients who are treated with antidepressive medications; moreover it is not recommended for patients with other untreated psychiatric conditions.
If one assumes that the observed results of this review are valid and reproducible outside of the clinical trial setting, is the mild degree of weight loss achieved of clinical benefit? Current evidence suggests that it may be, particularly in high‐risk subgroups, but more definitive data are needed. Weight reduction of approximately 5% to 10% of initial body weight is associated with improvements in blood pressure, lipid and glucose parameters (Blackburn 1995; Goldstein 1992) but RCT data examining the impact of weight reduction on cardiovascular events and mortality are lacking and studies are ongoing (CRESCENDO 2005; ; Look AHEAD; SCOUT 2005). Recently, RCTs involving treatments such as intensive lifestyle modification (diet plus exercise), acarbose, metformin, orlistat, troglitazone and rosiglitazone have reduced diabetes incidence in high risk patients, the majority of whom were overweight or obese (Buchanan 2002; Chiasson 2002; DPP 2002; DREAM 2006; Tuomilehto 2001). Intensive lifestyle modification led to the largest reduction in risk of 58% in two studies (DPP 2002; Tuomilehto 2001). Although these studies are not directly comparable due to differences in patient populations and treatment regimens, weight loss for all trials was modest, ranging from 0.8 to 5.6 kg greater in the intervention arms compared to control arms. These data suggest that small amounts of weight loss in this high‐risk population are associated with a significant reduction in the incidence of diabetes. Whether this benefit is sustained over longer follow‐up periods remains to be seen. It should also be noted that the observed results can only be attributed to the entire randomised intervention (diet/exercise +/‐ drug therapy), rather than just the observed reduction in weight.
Studying morbidity/mortality endpoints is vital to confirming a favourable benefit/risk ratio for antiobesity drugs as drugs that improve surrogate endpoints (such as weight loss) may not ultimately improve more clinically relevant outcomes (Padwal 2007; Padwal RS 2007). Similarly, the clinical significance of the reduction in diabetes incidence observed with pharmacotherapy, including orlistat in the XENDOS trial, is uncertain at this time. The reduction in diabetes incidence wanes when the drug is stopped, suggesting a masking/delaying effect rather than a true preventive effect (Padwal 2005). RCTs examining the effect of pharmacotherapy on diabetes‐related microvascular and macrovascular endpoints as well as mortality are required. Trials such as SCOUT and CRESCENDO will provide much needed information on the effect of antiobesity agents on hard morbidity and mortality endpoints (CRESCENDO 2005; SCOUT 2005).
Limitations
The major methodological issues have been discussed above. All studies in this review showed a positive treatment effect. We did not find any negative or neutral studies. This raises the possibility of publication bias. The vast majority of trials were funded by pharmaceutical companies and this may increase the potential for positive results (Lexchin 2003). We were unable to locate unpublished data by contacting study authors and drug manufacturers. We generated a funnel plot of orlistat studies (Figure 2) to assess for publication or small study bias (Egger 1997; Sterne 2001). This shows a scattering of points near the midpoint and apex of the pyramid and a paucity of points at the bottom. This indicates that the impact of all types of small studies (positive, negative or neutral) may be underestimated in this meta‐analysis. However, the limited number of studies included in this review may limit overall interpretation and accuracy of the funnel plot. The number of sibutramine and rimonabant studies was too small to warrant generation of funnel plots.
2.
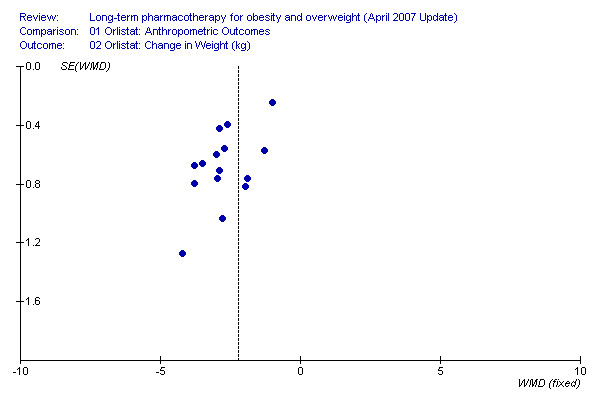
Orlistat funnel plot.
Statistical heterogeneity was present when quantitative pooling was performed for several outcomes. This was addressed by using a random‐effects meta‐analysis and by not combining outcomes when the heterogeneity was felt to be clinically significant. As we did not have access to individual patient data, we could not perform meta‐regression analysis to further investigate the cause of the observed heterogeneity. It is likely that differences in patient populations, cointerventions, trial duration and drug dose all were contributing factors.
The majority of the patients in this review were middle‐aged and Caucasian. Extrapolation to patients of different ethnic background and to the elderly should be made with caution.
Due to the lack of data, we were not able to draw any conclusions regarding the relative efficacy of antiobesity agents in different ranges of BMI levels and in patients with pre‐existing cardiovascular disease. As mentioned above, the effect of antiobesity drugs on cardiovascular morbidity and mortality is unknown. The role of combination anti‐obesity therapy was not reviewed, nor was the role of anti‐obesity pharmacotherapy in children and adolescents. Effects on health‐related quality of life were not reviewed.
Authors' conclusions
Implications for practice.
The following issues should be considered before prescribing an antiobesity agent: 1. The available evidence is limited in two major ways. Internal validity is limited by high attrition rates. External validity is limited by the enrolment of highly selected patient populations into clinical trials and also by data showing poor long‐term persistence in 'real world' settings. 2. The decision to prescribe involves a careful assessment of the risks and benefits. The average amount of weight lost is modest and most patients will remain significantly obese or overweight even with drug therapy. Current antiobesity agents are costly, each drug has associated adverse effects, and the ultimate effect on cardiovascular morbidity and mortality remains unknown. Balanced against these factors are the potential for modest improvements in the cardiovascular risk profile that varies according to each agent and the possibility that the patient will have a good response (that is 10% weight loss or more). In addition, realistic minimum weight loss goals of 5% to 10% should be set and it should be noted that there is accumulating evidence that even such modest amounts of weight loss are beneficial. 3. A minority of patients (10% to 20%) do achieve weight loss of 10% or more, although it is difficult to predict which patients will respond to this extent. Since near‐maximal weight loss was achieved by three to six months in most trials, one should discontinue therapy at this point if significant weight loss and/or improvement in comorbidity has not occurred. 4. Drug therapy should be used in conjunction with lifestyle modification. 5. Although we did not formally systematically review head‐to‐head trials, there appear to be no definitive data demonstrating that one particular agent is clearly more efficacious than another (Padwal 2007).
Therefore, initial therapy can be guided by the following factors:
patient preference;
local costs, availability and drug plan coverage;
patient comorbidity and the adverse effect profiles.
A summary is provided in Appendix 5. Orlistat reduces LDL levels, diabetes incidence, glucose levels and blood pressure and is not associated with major systemic toxicities. It is likely to be most useful in patients with prediabetes/diabetes, elevated LDL levels or pre‐existing cardiovascular disease but should be avoided in patients with chronic gastrointestinal problems. Sibutramine acts primarily upon satiety and is useful when lack of satiety is a problem. It also may be preferentially used in patients with dyslipidaemia (high triglycerides/low HDL levels) but should be avoided in patients with a history of cardiovascular disease and poorly controlled hypertension until further data are available. Rimonabant might be particularly useful in patients with the metabolic syndrome, dyslipidaemia (high triglycerides/low HDL levels), diabetes (based on RIO‐Diabetes) and hypertension. Originally developed as a dual antiobesity drug and smoking cessation agent, rimonabant may also be of use in an obese patient who is concurrently trying to quit smoking. It should be noted that this latter developmental program has since been discontinued and the drug is not indicated solely for smoking cessation. Rimonabant should be avoided in patients with previous psychiatric disease, patients on antidepressants and individuals with significant liver dysfunction.
Implications for research.
Further study is needed to evaluate:
the effectiveness, efficacy, and safety of existing antiobesity medications over longer follow‐up periods, including their impact on mortality and cardiovascular morbidity. This needs to be done in a methodologically rigorous manner, with minimization of attrition rates.
the efficacy and effectiveness of combination drug therapy over the long‐term.
which patients respond best to which agent.
newer, more effective, and better tolerated anti‐obesity drugs.
the effect of anti‐obesity therapy on other obesity‐related comorbidites such as sleep apnea, arthritis, and cancer.
What's new
| Date | Event | Description |
|---|---|---|
| 1 November 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 14 February 2008 | New search has been performed | This is an update of the Cochrane review first published in issue 4, 2003. Major changes include: The previous search included head‐to‐head clinical trials but, due to the length of this review and large number of placebo‐controlled studies, the decision was made to focus on placebo‐controlled trials only. In contrast to the previous version of this review we analysed separately published weight loss and weight maintenance trials together. From the date of the last search (December 2002) to December 2006, 29 potentially relevant trials were identified and five orlistat, five sibutramine and four rimonabant studies met final inclusion criteria. These were added to the 11 orlistat and five sibutramine trials previously identified. |
Acknowledgements
The authors would like to acknowledge the help of Ms. Jeannette Buckingham in helping to update our search and to study authors who provided supplemental data.
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms were free text terms; exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = substitute for one or no characters; tw = textword; ab = abstract; ti = titel; kf = Keyword Heading Word; ot = original titel; pt = publication type; sh = MeSH: Medical subject heading (MEDLINE medical index term); adj = adjacency. Finally searches were limited to January 2003 to December 2006. Later combined with the findings of the first Cochrane review of this study. Part I: Drugs 1 (orlistat or xenical).tw,ot. 2 ("Ro 18 0647" or Ro 18‐0647 or Ro 180647 or Ro18647).tw,ot. 3 96829‐58‐2.rn. 4 (sibutramin$ or arcalion).tw,ot. 5 (Bts 54 524 or Bts 54524 or Bts54524).tw,ot. 6 (reductil or medaria or meridia).tw,ot. 7 106650‐56‐0.rn. 8 (rimonabant or acomplia or zimulti).tw,ot. 9 (Sr 141716 or Sr141716 or Sr 141716a or Sr141716a).tw,ot. 10 158681‐13‐1.rn. 11 or/1‐10 Part II: RCT/CCT (sensitive search) 12 exp Randomized Controlled Trials as topic/ 13 Randomized Controlled Trial.pt. 14 exp Controlled Clinical Trials as topic/ 15 Controlled Clinical Trial.pt. 16 exp Random Allocation/ 17 exp Double‐Blind Method/ 18 exp Single‐Blind Method/ 19 or/12‐18 20 exp Clinical Trials as topic/ 21 Clinical Trial.pt. 22 (clinic$ adj25 trial$).tw,ot. 23 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind)).tw,ot. 24 exp Placebos/ 25 (placebo$ or random).tw,ot. 26 exp Research Design/ 27 (latin adj3 square).tw,ot. 28 or/20‐27 29 comparative study.pt. 30 exp Evaluation Studies as topic/ 31 Evaluation Studies.pt. 32 exp Follow‐Up Studies/ 33 exp Prospective Studies/ 34 (control$ or prospectiv$ or volunteer$).tw,ot. 35 exp Cross‐Over Studies/ 36 or/29‐35 37 19 or 28 or 36 Part III: I and II 38 11 and 37 39 limit 38 to animal 40 limit 38 to humans 41 39 not 40 42 38 not 41 |
Appendix 2. Methodological quality (Orlistat)
| Study | Randomization OK | Allocation Conceal | Baseline Similarity | Eligib Crit Spec | Patient Blinded | Care Provider Blind | Outcome Assess Blind | Primary Outcome Rep | ITT Analysis |
| Bakris | ? | ? | Y | Y | Y | Y | ? | Y | N |
| Berne | Y | Y | Y | Y | Y | Y | ? | Y | Y |
| Broom | ? | ? | ? | Y | Y | Y | ? | Y | N |
| Davidson | ? | ? | ? | Y | Y | Y | ? | Y | N |
| Derosa | Y | Y | Y | Y | Y | Y | ? | Y | Y |
| Lindgarde | ? | ? | ? | Y | Y | Y | ? | Y | N |
| Krempf | ? | ? | ? | Y | Y | Y | ? | Y | N |
| Finer | Y | Y | Y | Y | Y | Y | ? | Y | N |
| Rossner | ? | ? | Y | Y | Y | Y | ? | Y | N |
| Sjostrom | Y | Y | Y | Y | Y | Y | ? | Y | N |
| Hauptman | ? | ? | Y | Y | Y | Y | ? | Y | N |
| Hollander | ? | ? | ? | Y | Y | Y | ? | Y | N |
| Kelley | ? | ? | Y | Y | Y | Y | ? | Y | N |
| Miles | ? | ? | Y | Y | Y | Y | ? | Y | N |
| Torgerson | Y | Y | Y | Y | Y | Y | ? | N | N |
| Swinburn | Y | ? | Y | Y | Y | Y | ? | Y | N |
Appendix 3. Methodological quality (Sibutramine)
| Study | Randomization OK | Alloc Concealment | Baseline Similarity | Eligiblity Crit Spec | Patient Blinded | Care Provider Blind | Outcome Assess Blind | Primary Outcome Rep | ITT Analysis |
| Apfelbaum | ? | ? | Y | Y | Y | Y | ? | Y | N |
| Hauner | Y | ? | Y | Y | Y | Y | ? | Y | N |
| James | Y | Y | Y | Y | Y | Y | ? | Y | N |
| Krakua | ? | ? | Y | Y | Y | Y | ? | Y | Y |
| Mathus‐Vliegen | Y | Y | Y | Y | Y | Y | ? | Y | N |
| McNulty | ? | ? | Y | Y | Y | Y | ? | Y | N |
| McMahon 2000 | ? | ? | Y | Y | Y | Y | ? | N | N |
| McMahon 2002 | ? | ? | Y | Y | Y | Y | ? | Y | N |
| Sanchez‐Reyes | ? | ? | Y | Y | Y | Y | ? | Y | N |
| Smith | Y | Y | Y | Y | Y | Y | ? | Y | N |
Appendix 4. Methodological quality (Rimonabant)
| Study | Randomization OK | Allocation Conceal | Baseline Similarity | Eligib Crit Spec | Patient Blinded | Care Provider Blind | Outcome Assess Blind | Primary Outcome Rep | ITT Analysis |
| RIO‐Diabetes | Y | Y | Y | Y | Y | Y | ? | Y | N |
| RIO‐Europe | Y | Y | Y | Y | Y | Y | ? | Y | N |
| RIO‐Lipids | N | N | Y | Y | Y | Y | ? | Y | N |
| RIO‐NA | ? | ? | Y | Y | Y | Y | ? | Y | N |
Appendix 5. Practical approach to prescribing antiobesity drugs
| Drug | Dose | Potential uses | Avoid in | Comments |
| Orlistat | 120 mg three times daily | Prediabetes, diabetes, elevated LDL cholesterol, hypertension, pre‐existing CVD | Chronic malabsorption or GI disease | Prescribe concurrent multivitamin. Half‐strength available OTC in US. |
| Sibutramine | 10‐15 mg once daily | Lack of satiety major barrier to weight reduction, dyslipidemia (high triglyceride/low HDL) | Uncontrolled hypertension, tachycardia, pre‐existing CVD | Monitor blood pressure. |
| Rimonabant | 20 mg once daily | Dyslipidemia (high triglyceride/low HDL), diabetes, metabolic syndrome, hypertension | History of psychiatric illness, liver impairment | Monitor for mood disorders. |
Appendix 6. Major changes to the first published version of this review
| Overview |
| This is an update of the Cochrane review first published in issue 4, 2003. Major changes include: The previous search included head‐to‐head clinical trials but, due to the length of this review and large number of placebo‐controlled studies, the decision was made to focus on placebo‐controlled trials only. In contrast to the previous version of this review we analysed separately published weight loss and weight maintenance trials together. From the date of the last search (December 2002) to December 2006, 29 potentially relevant trials were identified and five orlistat, five sibutramine and four rimonabant studies met final inclusion criteria. These were added to the 11 orlistat and five sibutramine trials previously identified. |
Data and analyses
Comparison 1. Orlistat: Anthropometric Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Orlistat: Change in Weight (%) | 13 | 6196 | Mean Difference (IV, Random, 95% CI) | ‐2.93 [‐3.35, ‐2.50] |
| 2 Orlistat: Change in Weight (kg) | 14 | 9457 | Mean Difference (IV, Random, 95% CI) | ‐2.87 [‐3.21, ‐2.53] |
| 3 Orlistat: 5% Responders (absolute % difference) | 14 | 9389 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [0.18, 0.24] |
| 4 Orlistat: 10% Responders (absolute % difference) | 13 | 8857 | Risk Difference (M‐H, Random, 95% CI) | 0.12 [0.09, 0.14] |
| 5 Orlistat: Change in Waist Circumference (cm) | 9 | 4631 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐2.86, ‐1.26] |
| 6 Orlistat: Change in Body Mass Index (kg/m2) | 3 | 1276 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.40, ‐0.71] |
| 7 Orlistat: Sensitivity Analysis According to Baseline CV Risk (Absolute Weight Loss) | 15 | 9833 | Mean Difference (IV, Random, 95% CI) | ‐2.75 [‐3.13, ‐2.36] |
| 7.1 Change in Weight in Lower Risk Population (kg) | 7 | 6655 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐3.59, ‐2.41] |
| 7.2 Change in Weight in Higher Risk Population (kg) | 8 | 3178 | Mean Difference (IV, Random, 95% CI) | ‐2.59 [‐3.16, ‐2.02] |
| 8 Orlistat: Sensitivity Analysis According to Baseline CV Risk (% Weight Loss) | 13 | 6196 | Mean Difference (IV, Random, 95% CI) | ‐2.93 [‐3.35, ‐2.50] |
| 8.1 Change in Weight in Lower Risk Population (%) | 6 | 3378 | Mean Difference (IV, Random, 95% CI) | ‐3.42 [‐4.01, ‐2.83] |
| 8.2 Change in Weight in Higher Risk Population (%) | 7 | 2818 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐3.25, ‐2.11] |
1.1. Analysis.
Comparison 1 Orlistat: Anthropometric Outcomes, Outcome 1 Orlistat: Change in Weight (%).
1.2. Analysis.
Comparison 1 Orlistat: Anthropometric Outcomes, Outcome 2 Orlistat: Change in Weight (kg).
1.3. Analysis.
Comparison 1 Orlistat: Anthropometric Outcomes, Outcome 3 Orlistat: 5% Responders (absolute % difference).
1.4. Analysis.
Comparison 1 Orlistat: Anthropometric Outcomes, Outcome 4 Orlistat: 10% Responders (absolute % difference).
1.5. Analysis.
Comparison 1 Orlistat: Anthropometric Outcomes, Outcome 5 Orlistat: Change in Waist Circumference (cm).
1.6. Analysis.
Comparison 1 Orlistat: Anthropometric Outcomes, Outcome 6 Orlistat: Change in Body Mass Index (kg/m2).
1.7. Analysis.
Comparison 1 Orlistat: Anthropometric Outcomes, Outcome 7 Orlistat: Sensitivity Analysis According to Baseline CV Risk (Absolute Weight Loss).
1.8. Analysis.
Comparison 1 Orlistat: Anthropometric Outcomes, Outcome 8 Orlistat: Sensitivity Analysis According to Baseline CV Risk (% Weight Loss).
Comparison 2. Orlistat: Change in Blood Pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Orlistat: Change in Systolic Blood Pressure (mm Hg) | 13 | 6965 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐2.19, ‐0.86] |
| 2 Orlistat: Change in Diastolic Blood Pressure (mm Hg) | 12 | 8322 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.03, ‐0.74] |
2.1. Analysis.
Comparison 2 Orlistat: Change in Blood Pressure, Outcome 1 Orlistat: Change in Systolic Blood Pressure (mm Hg).
2.2. Analysis.
Comparison 2 Orlistat: Change in Blood Pressure, Outcome 2 Orlistat: Change in Diastolic Blood Pressure (mm Hg).
Comparison 3. Orlistat: Change in Lipid Parameters.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Orlistat: Change in Total Cholesterol Levels | 13 | 5206 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.37, ‐0.28] |
| 2 Orlistat: Change in LDL cholesterol levels | 13 | 5206 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.30, ‐0.22] |
| 3 Orlistat: Change in HDL cholesterol Levels | 11 | 4152 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.04, ‐0.02] |
| 4 Orlistat: Change in Triglyceride Levels | 11 | 4456 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.12, 0.07] |
3.1. Analysis.
Comparison 3 Orlistat: Change in Lipid Parameters, Outcome 1 Orlistat: Change in Total Cholesterol Levels.
3.2. Analysis.
Comparison 3 Orlistat: Change in Lipid Parameters, Outcome 2 Orlistat: Change in LDL cholesterol levels.
3.3. Analysis.
Comparison 3 Orlistat: Change in Lipid Parameters, Outcome 3 Orlistat: Change in HDL cholesterol Levels.
3.4. Analysis.
Comparison 3 Orlistat: Change in Lipid Parameters, Outcome 4 Orlistat: Change in Triglyceride Levels.
Comparison 4. Orlistat: Subgroup Analysis in Diabetes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in Weight (%) | 5 | 1678 | Mean Difference (IV, Random, 95% CI) | ‐2.61 [‐3.06, ‐2.16] |
| 2 Change in Weight (kg) | 4 | 1737 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐1.00, ‐1.60] |
| 3 Change in Fasting Glucose Levels (mmol/L) | 5 | 1678 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.49, ‐0.57] |
| 4 Orlistat: Change in HgbA1c (%) | 5 | 1678 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.59, ‐0.18] |
4.1. Analysis.
Comparison 4 Orlistat: Subgroup Analysis in Diabetes, Outcome 1 Change in Weight (%).
4.2. Analysis.
Comparison 4 Orlistat: Subgroup Analysis in Diabetes, Outcome 2 Change in Weight (kg).
4.3. Analysis.
Comparison 4 Orlistat: Subgroup Analysis in Diabetes, Outcome 3 Change in Fasting Glucose Levels (mmol/L).
4.4. Analysis.
Comparison 4 Orlistat: Subgroup Analysis in Diabetes, Outcome 4 Orlistat: Change in HgbA1c (%).
Comparison 5. Orlistat: GI Adverse Events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Orlistat: Overall GI Adverse Events (Absolute % Difference) | 14 | 8938 | Risk Difference (M‐H, Random, 95% CI) | 0.24 [0.20, 0.29] |
| 2 Orlistat: Fecal Incontinence (Absolute % Difference) | 4 | 1636 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.05, 0.08] |
| 3 Orlistat: Discontinuation Due to GI Side Effects (Absolute % Difference) | 12 | 5994 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.01, 0.03] |
5.1. Analysis.
Comparison 5 Orlistat: GI Adverse Events, Outcome 1 Orlistat: Overall GI Adverse Events (Absolute % Difference).
5.2. Analysis.
Comparison 5 Orlistat: GI Adverse Events, Outcome 2 Orlistat: Fecal Incontinence (Absolute % Difference).
5.3. Analysis.
Comparison 5 Orlistat: GI Adverse Events, Outcome 3 Orlistat: Discontinuation Due to GI Side Effects (Absolute % Difference).
Comparison 6. Sibutramine: Anthropometric Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sibutramine: Change in Weight (%) | 8 | 1725 | Mean Difference (IV, Random, 95% CI) | ‐4.34 [‐5.01, ‐3.67] |
| 1.1 Weight Loss Studies | 7 | 1536 | Mean Difference (IV, Random, 95% CI) | ‐4.54 [‐5.11, ‐3.96] |
| 1.2 Weight Maintenance Studies | 1 | 189 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐4.44, ‐0.36] |
| 2 Sibutramine: Change in Weight (kg) | 10 | 2348 | Mean Difference (IV, Random, 95% CI) | ‐4.16 [‐4.73, ‐3.59] |
| 2.1 Weight Loss Studies | 7 | 1536 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐4.77, ‐3.64] |
| 2.2 Weight Maintenance Studies | 3 | 812 | Mean Difference (IV, Random, 95% CI) | ‐4.01 [‐5.73, ‐2.28] |
| 3 Sibutramine: 5% Responders (absolute % difference) | 7 | 1464 | Risk Difference (M‐H, Random, 95% CI) | 0.32 [0.27, 0.37] |
| 3.1 Weight Loss Studies | 6 | 1304 | Risk Difference (M‐H, Random, 95% CI) | 0.32 [0.27, 0.38] |
| 3.2 Weight Maintenance Studies | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.31 [0.18, 0.45] |
| 4 Sibutramine: 10% Responders (absolute % difference) | 7 | 1464 | Risk Difference (M‐H, Random, 95% CI) | 0.18 [0.11, 0.25] |
| 4.1 Weight Loss Studies | 6 | 1304 | Risk Difference (M‐H, Random, 95% CI) | 0.17 [0.10, 0.23] |
| 4.2 Weight Maintenance Studies | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.31 [0.16, 0.45] |
| 5 Sibutramine: Change in Waist Circumference (cm) | 8 | 1837 | Mean Difference (IV, Random, 95% CI) | ‐3.99 [‐4.70, ‐3.28] |
| 5.1 Weight Loss Studies | 6 | 1237 | Mean Difference (IV, Random, 95% CI) | ‐3.97 [‐4.92, ‐3.03] |
| 5.2 Weight Maintenance Studies | 2 | 600 | Mean Difference (IV, Random, 95% CI) | ‐4.11 [‐5.52, ‐2.70] |
| 6 Sibutramine: Change in Body Mass Index (kg/m2) | 5 | 956 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐1.79, ‐1.30] |
| 7 Sibutramine: Sensitivity Analysis According to Baseline CV Risk (Absolute Weight Loss) | 10 | 2348 | Mean Difference (IV, Random, 95% CI) | ‐4.16 [‐4.73, ‐3.59] |
| 7.1 Change in Weight in Lower Risk Population (kg) | 5 | 1470 | Mean Difference (IV, Random, 95% CI) | ‐3.99 [‐5.04, ‐2.95] |
| 7.2 Change in Weight in Higher Risk Population (kg) | 5 | 878 | Mean Difference (IV, Random, 95% CI) | ‐4.28 [‐4.97, ‐3.58] |
| 8 Sibutramine: Sensitivity Analysis According to Baseline CV Risk (% Weight Loss) | 8 | 1725 | Mean Difference (IV, Random, 95% CI) | ‐4.34 [‐5.01, ‐3.67] |
| 8.1 Change in Weight in Lower Risk Population (%) | 3 | 847 | Mean Difference (IV, Random, 95% CI) | ‐3.87 [‐5.68, ‐2.06] |
| 8.2 Change in Weight in Higher Risk Population (%) | 5 | 878 | Mean Difference (IV, Random, 95% CI) | ‐4.53 [‐5.23, ‐3.83] |
6.1. Analysis.
Comparison 6 Sibutramine: Anthropometric Outcomes, Outcome 1 Sibutramine: Change in Weight (%).
6.2. Analysis.
Comparison 6 Sibutramine: Anthropometric Outcomes, Outcome 2 Sibutramine: Change in Weight (kg).
6.3. Analysis.
Comparison 6 Sibutramine: Anthropometric Outcomes, Outcome 3 Sibutramine: 5% Responders (absolute % difference).
6.4. Analysis.
Comparison 6 Sibutramine: Anthropometric Outcomes, Outcome 4 Sibutramine: 10% Responders (absolute % difference).
6.5. Analysis.
Comparison 6 Sibutramine: Anthropometric Outcomes, Outcome 5 Sibutramine: Change in Waist Circumference (cm).
6.6. Analysis.
Comparison 6 Sibutramine: Anthropometric Outcomes, Outcome 6 Sibutramine: Change in Body Mass Index (kg/m2).
6.7. Analysis.
Comparison 6 Sibutramine: Anthropometric Outcomes, Outcome 7 Sibutramine: Sensitivity Analysis According to Baseline CV Risk (Absolute Weight Loss).
6.8. Analysis.
Comparison 6 Sibutramine: Anthropometric Outcomes, Outcome 8 Sibutramine: Sensitivity Analysis According to Baseline CV Risk (% Weight Loss).
Comparison 7. Sibutramine: Change in Blood Pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sibutramine: Change in Systolic Blood Pressure (mm Hg) | 7 | 1906 | Mean Difference (IV, Random, 95% CI) | 1.69 [0.11, 3.28] |
| 1.1 Weight Loss Studies | 6 | 1442 | Mean Difference (IV, Random, 95% CI) | 1.02 [‐0.39, 2.43] |
| 1.2 Weight Maintenance Studies | 1 | 464 | Mean Difference (IV, Random, 95% CI) | 4.3 [1.57, 7.03] |
| 2 Sibutramine: Change in Diastolic Blood Pressure (mm Hg) | 7 | 1906 | Mean Difference (IV, Random, 95% CI) | 2.42 [1.51, 3.32] |
| 2.1 Weight Loss Studies | 6 | 1442 | Mean Difference (IV, Random, 95% CI) | 2.15 [1.26, 3.03] |
| 2.2 Weight Maintenance Studies | 1 | 464 | Mean Difference (IV, Random, 95% CI) | 3.9 [1.98, 5.82] |
7.1. Analysis.
Comparison 7 Sibutramine: Change in Blood Pressure, Outcome 1 Sibutramine: Change in Systolic Blood Pressure (mm Hg).
7.2. Analysis.
Comparison 7 Sibutramine: Change in Blood Pressure, Outcome 2 Sibutramine: Change in Diastolic Blood Pressure (mm Hg).
Comparison 8. Sibutramine: Change in Lipid Parameters.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sibutramine: Change in HDL cholesterol (mmol/L) | 5 | 977 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.01, 0.08] |
| 1.1 Weight Loss Studies | 4 | 818 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.03, 0.10] |
| 1.2 Weight Maintenance Studies | 1 | 159 | Mean Difference (IV, Random, 95% CI) | 0.01 [0.00, 0.02] |
| 2 Sibutramine: Change in Triglycerides (mmol/L) | 4 | 785 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.30, ‐0.07] |
| 2.1 Weight Loss Studies | 3 | 626 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.40, ‐0.03] |
| 2.2 Weight Maintenance Studies | 1 | 159 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.31, ‐0.01] |
8.1. Analysis.
Comparison 8 Sibutramine: Change in Lipid Parameters, Outcome 1 Sibutramine: Change in HDL cholesterol (mmol/L).
8.2. Analysis.
Comparison 8 Sibutramine: Change in Lipid Parameters, Outcome 2 Sibutramine: Change in Triglycerides (mmol/L).
Comparison 9. Sibutramine: Subgroup Analysis in Diabetes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in Weight (%) | 3 | 450 | Mean Difference (IV, Random, 95% CI) | ‐4.99 [‐6.20, ‐3.78] |
| 2 Change in Weight (kg) | 3 | 450 | Mean Difference (IV, Random, 95% CI) | ‐4.91 [‐6.18, ‐3.64] |
9.1. Analysis.
Comparison 9 Sibutramine: Subgroup Analysis in Diabetes, Outcome 1 Change in Weight (%).
9.2. Analysis.
Comparison 9 Sibutramine: Subgroup Analysis in Diabetes, Outcome 2 Change in Weight (kg).
Comparison 10. Sibutramine: Change in Heart Rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in Heart Rate (beats/min) | 7 | 1658 | Mean Difference (IV, Random, 95% CI) | 4.53 [3.49, 5.57] |
| 1.1 Weight Loss Studies | 5 | 1035 | Mean Difference (IV, Random, 95% CI) | 4.16 [2.86, 5.45] |
| 1.2 Weight Maintenance Studies | 2 | 623 | Mean Difference (IV, Random, 95% CI) | 5.44 [2.94, 7.94] |
10.1. Analysis.
Comparison 10 Sibutramine: Change in Heart Rate, Outcome 1 Change in Heart Rate (beats/min).
Comparison 11. Rimonabant: Anthropometric Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rimonabant: Change in Weight (kg) | 4 | 4099 | Mean Difference (IV, Random, 95% CI) | ‐4.67 [‐5.26, ‐4.07] |
| 2 Rimonabant: 5% Responders (absolute % difference) | 4 | 4099 | Risk Difference (M‐H, Random, 95% CI) | 0.33 [0.29, 0.37] |
| 3 Rimonabant: 10% Responders (absolute % difference) | 4 | 4099 | Risk Difference (M‐H, Random, 95% CI) | 0.19 [0.15, 0.23] |
| 4 Rimonabant: Change in Waist Circumference (cm) | 4 | 4098 | Mean Difference (IV, Random, 95% CI) | ‐3.89 [‐4.47, ‐3.30] |
11.1. Analysis.
Comparison 11 Rimonabant: Anthropometric Outcomes, Outcome 1 Rimonabant: Change in Weight (kg).
11.2. Analysis.
Comparison 11 Rimonabant: Anthropometric Outcomes, Outcome 2 Rimonabant: 5% Responders (absolute % difference).
11.3. Analysis.
Comparison 11 Rimonabant: Anthropometric Outcomes, Outcome 3 Rimonabant: 10% Responders (absolute % difference).
11.4. Analysis.
Comparison 11 Rimonabant: Anthropometric Outcomes, Outcome 4 Rimonabant: Change in Waist Circumference (cm).
Comparison 12. Rimonabant: Change in Blood Pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rimonabant: Change in Systolic Blood Pressure (mm Hg) | 3 | 2273 | Mean Difference (IV, Random, 95% CI) | ‐1.78 [‐2.81, ‐0.76] |
| 2 Rimonabant: Change in Diastolic Blood Pressure (mm Hg) | 3 | 2273 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐1.93, ‐0.54] |
12.1. Analysis.
Comparison 12 Rimonabant: Change in Blood Pressure, Outcome 1 Rimonabant: Change in Systolic Blood Pressure (mm Hg).
12.2. Analysis.
Comparison 12 Rimonabant: Change in Blood Pressure, Outcome 2 Rimonabant: Change in Diastolic Blood Pressure (mm Hg).
Comparison 13. Rimonabant: Change in Lipid Parameters.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rimonabant: Change in Total Cholesterol (mmol/L) | 3 | 2223 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 2 Rimonabant: Change in LDL Cholesterol (mmol/L) | 3 | 2223 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.12, 0.01] |
| 3 Rimonabant: Change in HDL Cholesterol (mmol/L) | 4 | 4050 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.08, 0.11] |
| 4 Rimonabant: Change in Triglycerides (mmol/L) | 4 | 4049 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.30, ‐0.17] |
13.1. Analysis.
Comparison 13 Rimonabant: Change in Lipid Parameters, Outcome 1 Rimonabant: Change in Total Cholesterol (mmol/L).
13.2. Analysis.
Comparison 13 Rimonabant: Change in Lipid Parameters, Outcome 2 Rimonabant: Change in LDL Cholesterol (mmol/L).
13.3. Analysis.
Comparison 13 Rimonabant: Change in Lipid Parameters, Outcome 3 Rimonabant: Change in HDL Cholesterol (mmol/L).
13.4. Analysis.
Comparison 13 Rimonabant: Change in Lipid Parameters, Outcome 4 Rimonabant: Change in Triglycerides (mmol/L).
Comparison 14. Rimonabant: Adverse Events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rimonabant: Discontinuation due to Adverse Event (Absolute % Difference) | 4 | 4105 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.05, 0.08] |
| 2 Rimonabant: Serious Adverse Event (Absolute % Difference) | 4 | 4105 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.00, 0.03] |
| 3 Rimonabant: Psychiatric Disorders (Absolute % Difference) | 4 | 4105 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.02, 0.05] |
14.1. Analysis.
Comparison 14 Rimonabant: Adverse Events, Outcome 1 Rimonabant: Discontinuation due to Adverse Event (Absolute % Difference).
14.2. Analysis.
Comparison 14 Rimonabant: Adverse Events, Outcome 2 Rimonabant: Serious Adverse Event (Absolute % Difference).
14.3. Analysis.
Comparison 14 Rimonabant: Adverse Events, Outcome 3 Rimonabant: Psychiatric Disorders (Absolute % Difference).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Apfelbaum 1999.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight maintenance trial. | |
| Participants | 205 participants underwent 4 week VLCD. Those who lost 6 or more kg (n=160) were randomized for 1 y treatment. Mean age 38y. Mean BMI 35.5 kg/m2. Mean weight 104 kg. France. | |
| Interventions | Sibutramine 10 mg daily (n=352). Attrition rate 34%. Placebo (n=78). Attrition rate 42%. | |
| Outcomes | weight loss, % wieght maintenance, waist circumference, 5 and 10% responders, triglycerides, HDL chol, Total/HDL chol ratio, LDL chol, blood pressure, pulse rate | |
| Notes | Co‐intervention: diet counseling. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bakris 2002.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 554 patients with HTN. 1 year follow‐up. Mean age 53 y. Mean BMI 35.6 kg/m2. Mean weight 101 kg. US. | |
| Interventions | Orlistat 120 mg tid (n=278). Attrition rate 42%. Placebo (n=278). Attrition rate 61%. | |
| Outcomes | weight loss, 5% responders, BMI, waist circ, lipid profile, blood pressure, insulin | |
| Notes | Co‐interventions: 600 kcal/d deficit, exercise, educational package | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Berne 2004.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 220 patients with DM2 on oral agents. 1 year follow‐up. Mean age 59 y. Mean BMI 32.7 kg/m2. Mean weight 96 kg. Sweden. | |
| Interventions | Orlistat 120 mg tid (n=111). Attrition rate 14%. Placebo (n=109). Attrition rate 14%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, A1c, lipid profile, blood pressure, heart rate, insulin, apolipoprotein B | |
| Notes | Co‐interventions: 600 kcal/d deficit, exercise, educational package | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Broom 2002.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 531 patients with HTN, IGT or dyslipidemia. 1 year follow‐up. Mean age 46 y. Mean BMI 37.1 kg/m2. Mean weight 101 kg. UK. | |
| Interventions | Orlistat 120 mg tid (n=265). Attrition rate 30%. Placebo (n=266). Attrition rate 40%. | |
| Outcomes | weight loss, 5% and 10% responders, BMI, waist circ, lipid profile, blood pressure, glucose, OGTT score | |
| Notes | Co‐interventions: 600‐900 kcal/d deficit diet, food diary | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Davidson 1999.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. Second year weight maintenance. | |
| Participants | 892 patients with 1 year follow‐up and a second weight maintenance year. Mean age 46 y. Mean BMI 37.1 kg/m2. Mean weight 101 kg. UK. | |
| Interventions | Orlistat 120 mg tid (n=668). Attrition rate 31%. Placebo (n=224). Attrition rate 41%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, lipid profile, blood pressure, glucose, insulin | |
| Notes | Co‐interventions: 500‐800 kcal/d deficit diet, food diary, exercise counseling. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Derosa 2003.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 50 patients with dyslipidemia. 1 year follow‐up. Mean age 52 y. Mean BMI 31.9 kg/m2. Mean weight 95 kg. Italy. | |
| Interventions | Orlistat 120 mg tid (n=27). Attrition rate 7%. Placebo (n=23). Attrition rate 0%. | |
| Outcomes | weight loss, waist circ, BMI, lipid profile, blood pressure | |
| Notes | Co‐interventions: 1500 kcal/d diet, exercise. Data for fluvastatin and orlistat/fluvastatin arm not presented in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Finer 2000.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 228 patients followed for 1 year. Mean age 41 y. Mean BMI 36.8 kg/m2. Mean weight 98 kg. UK. | |
| Interventions | Orlistat 120 mg tid (n=114). Attrition rate 36%. Placebo (n=114). Attrition rate 42%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, lipid profile, blood pressure, glucose, insulin | |
| Notes | Co‐interventions: 600‐900 kcal/d deficit diet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hauner 2004.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 362 patients from primary care. 54 week follow‐up. Mean age 43 y. Mean BMI 35.3 kg/m2. Mean weight 100 kg. Germany. | |
| Interventions | Sibutramine 15 mg daily (n=180). Attrition rate 40%. Placebo (n=182). Attrition rate 48%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, waist‐hip ratio, lipid profile, blood pressure, heart rate | |
| Notes | Co‐interventions: 500‐1000 kcal/d deficit diet. Food diary. Exercise. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hauptman 2000.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. Second year weight maintenance. | |
| Participants | 635 patients with 1‐year follow‐up and second weight maintenance year. Mean age 42 y. Mean BMI 36.0 kg/m2. Mean weight 101 kg. US. | |
| Interventions | Orlistat 60 mg tid (n=213). Attrition rate 28%. Orlistat 120 mg tid (n=210). Attrition rate 28%. Placebo (n=212). Attrition rate 42%. | |
| Outcomes | weight loss, 5% and 10% responders, lipid profile, blood pressure, glucose, insulin | |
| Notes | Co‐interventions: 1200‐1500 kcal/d diet. Food diary. Exercise. Educational video. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hollander 1998.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 322 patients with DM2 followed for 1 year. Mean age 55 y. Mean BMI 34.3 kg/m2. Mean weight 100 kg. US. | |
| Interventions | Orlistat 120 mg tid (n=63). Attrition rate 15%. Placebo (n=159). Attrition rate 28%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, lipid profile, apolipoproteins, A1c, glucose, insulin | |
| Notes | Co‐interventions: 500 kcal/d deficit diet. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
James 2000.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight maintenance trial. | |
| Participants | 605 patients received sibutramine and diet for 6 mo. 5% responders (n=467) then randomized to continue sibutramine or take placebo for 18 mo. Mean age 41 y. Mean BMI 36.7 kg/m2. Mean weight 102 kg. Eight European centers. | |
| Interventions | Sibutramine 10‐20 mg daily (n=352). Attrition rate 42% Placebo (n=115). Attrition rate 50%. | |
| Outcomes | weight loss, weight maintenance, 5 and 10% responders, waist circumference, waist/hip ratio, lipid profile, uric acid, glucose, insulin, C‐peptide, A1c, blood pressure | |
| Notes | Co‐interventions: 600 kcal/d deficit diet and exercise counseling. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kaukua 2003.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 236 patients with DM2. 52 week follow‐up. Mean age 53 y. Mean BMI 35.7 kg/m2. Mean weight 99 kg. Finland. | |
| Interventions | Sibutramine 15 mg daily (n=114). Attrition rate 11%. Placebo (n=122). Attrition rate 11%. | |
| Outcomes | HRQL (primary), weight loss, A1c, blood pressure, heart rate | |
| Notes | Co‐interventions: 700 kcal/d deficit diet. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kelley 2002.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 550 patients with DM2. 1 year follow‐up. Mean age 58 y. Mean BMI 35.7 kg/m2. Mean weight 102 kg. US. | |
| Interventions | Orlistat 120 mg tid (n=274). Attrition rate 50%. Placebo (n=276). Attrition rate 54%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, lipid panel, A1c, glucose, blood pressure | |
| Notes | Co‐interventions: 600‐800 kcal/d deficit diet, exercise counselling, food records. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Krempf 2003.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 696 patients followed for 18 months. Mean age 41 y. Mean BMI 36.1 kg/m2. Mean weight 97 kg. France. | |
| Interventions | Orlistat 120 mg tid (n=346). Attrition rate 35%. Placebo (n=350). Attrition rate 43%. | |
| Outcomes | weight loss, waist circ, lipid panel, A1c, glucose, blood pressure | |
| Notes | Co‐intervention: 20% energy reduced diet increased by 10% if weight stable; food diary | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lindgarde 2000.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 376 patients with DM2, HTN or dyslipidemia. 1 year follow‐up. Mean age 53 y. Mean BMI 33.2 kg/m2. Mean weight 96 kg. Sweden. | |
| Interventions | Orlistat 120 mg tid (n=190). Attrition rate 16%. Placebo (n=186). Attrition rate 12%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, waist‐hip ratio, lipid panel, A1c, glucose, blood pressure | |
| Notes | Co‐interventions: 600‐900 kcal/d deficit diet, exercise, educational package. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mathus‐Vliegen 2005.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight maintenance trial. | |
| Participants | 221 patients on VLCD for 3 mo. Those who lost 10% or more weight (n=189) randomized to sibutramine or placebo for 18 mo. Mean age 43 y. Mean BMI 36.6 kg/m2. Mean weight 105 kg. Dutch. | |
| Interventions | Sibutramine 10‐15 mg daily (n=94). Attrition rate 35%. Placebo (n=95). Attrition rate 39%. | |
| Outcomes | weight loss, weight maintenance, waist circ, hip circ, BMI, waist/hip ratio, lipid profile, glucose, uric acid, blood pressure, pulse rate | |
| Notes | Co‐intervention: 600 kcal/d deficit diet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
McMahon 2000.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 224 patients with controlled HTN. 36% African American. 52 week follow‐up. Mean age 53 y. Mean BMI 34.3 kg/m2. Mean weight 97 kg. US. | |
| Interventions | Sibutramine 20 mg daily (n=170). Attrition rate 22%. Placebo (n=169). Attrition rate 19%. | |
| Outcomes | weight loss, 5% and 10% responders, BMI, waist circ, lipid profile, glucose, blood pressure, heart rate | |
| Notes | Co‐intervention: diet counseling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McMahon 2002.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 220 patients with controlled HTN. 15% African American. 52 week follow‐up. Mean age 51 y. Mean BMI 33.9 kg/m2. Mean weight 98 kg. US. | |
| Interventions | Sibutramine 20 mg daily (n=146). Attrition rate 42%. Placebo (n=74). Attrition rate 51%. | |
| Outcomes | weight loss, 5% and 10% responders, BMI, waist circ, waist‐hip ratio, lipid profile, blood pressure, heart rate | |
| Notes | Co‐intervention: diet counseling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McNulty 2003.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 194 patients with DM2. 52 week follow‐up. Mean age 49 y. Mean BMI 36.6 kg/m2. Mean weight 103 kg. UK, Canada, France, Belgium. | |
| Interventions | Sibutramine 15 mg daily (n=68). Attrition rate 28%. Sibutramine 20 mg daily (n=62). Attrition rate 21%. Placebo (n=64). Attrition rate 28%. | |
| Outcomes | weight loss, 5% and 10% responders, BMI, waist circ, waist‐hip ratio, lipid profile, A1c, glucose, insulin, blood pressure, heart rate | |
| Notes | Co‐intervention: diet counseling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Miles 2002.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 516 patients with DM2 on oral hypoglycemics. 52 week follow‐up. Mean age 53 y. Mean BMI 35.4 kg/m2. Mean weight 102 kg. US, Canada. | |
| Interventions | Orlistat 120 mg tid (n=255). Attrition rate 35%. Placebo (n=261). Attrition rate 44%. | |
| Outcomes | weight loss, 5% and 10% responders, BMI, lipid profile, A1c, glucose, blood pressure | |
| Notes | Co‐interventions: 600‐800 kcal/d deficit diet and exercise. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
RIO‐Diabetes.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 1047 patients with DM2 on oral agents. 1 year follow‐up. Mean age 56 y. Mean BMI 34.2 kg/m2. Mean weight 98 kg. 11 countries. | |
| Interventions | Rimonabant 20 mg daily (n=339). Attrition rate 32%. Rimonabant 5 mg daily (n=358). Attrition rate 35%. Placebo (n=348). Attrition rate 34%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, lipid profile, glucose, A1c, insulin, blood pressure, insulin resistance | |
| Notes | Co‐interventions: 600 kcal/d deficit diet and exercise counseling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
RIO‐Europe.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 1507 patients. 41% hypertensive and 61% dyslipidemic. 1 year follow‐up. Mean age 45 y. Mean BMI 36.0 kg/m2. Mean weight 101 kg. Europe and US. | |
| Interventions | Rimonabant 20 mg daily (n=599). Attrition rate 39%. Rimonabant 5 mg daily (n=603). Attrition rate 37%. Placebo (n=305). Attrition rate 42%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, lipid profile, glucose, insulin, blood pressure, insulin resistance | |
| Notes | Co‐interventions:600 kcal/d deficit diet and exercise counseling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
RIO‐Lipids.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 1036 patients with untreated dyslipidemia. 1 year follow‐up. Mean age 48 y. Mean BMI 34.0 kg/m2. Mean weight 96 kg. Europe and NA. | |
| Interventions | Rimonabant 20 mg daily (n=346). Attrition rate 36%. Rimonabant 5 mg daily (n=345). Attrition rate 40%. Placebo (n=342). Attrition rate 37%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, lipid profile, apolipoprotein B:A‐1 ratio, metabolic syndrome, adiponectin, leptin, c‐reactive protein, glucose, insulin, blood pressure | |
| Notes | Co‐interventions:600 kcal/d deficit diet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
RIO‐North America.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. Second year weight maintenance | |
| Participants | 3045 patients. 30% hypertensive and 63% dyslipidemic. 2 year follow‐up. Mean age 45 y. Mean BMI 37.6 kg/m2. Mean weight 104 kg. Europe and US. | |
| Interventions | Rimonabant 20 mg daily (n=1222). Attrition rate 45%. Rimonabant 5 mg daily (n=1216). Attrition rate 49%. Placebo (n=607). Attrition rate 49%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, lipid profile, glucose, insulin, blood pressure, insulin resistance | |
| Notes | Co‐interventions:600 kcal/d deficit diet and exercise counseling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rossner 2000.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. Second year weight maintenance. | |
| Participants | 729 patients followed for 1 year with a second weight maintenance year. Mean age 44 y. Mean BMI 35.1 kg/m2. Mean weight 98 kg. Europe. | |
| Interventions | Orlistat 60 mg tid (n=242). Attrition rate 25%. Orlistat 120 mg tid (n=244). Attrition rate 26%. Placebo (n=243). Attrition rate 35%. | |
| Outcomes | weight loss, 5% and 10% responders, BMI, lipid profile, lipoprotein a, glucose, insulin, blood pressure, satisfaction | |
| Notes | Co‐interventions:600 kcal/d deficit diet and food intake diary. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sanchez‐Reyes 2004.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 86 patients with DM2 on sulfonylurea treatment. 52 week follow‐up. Mean age 47 y. Mean BMI 30 kg/m2. Mean weight 74 kg. Mexico | |
| Interventions | Sibutramine 10 mg daily (n=44). Attrition rate 45%. Placebo (n=42). Attrition rate 45%. | |
| Outcomes | weight loss, 5% and 10% responders, waist circ, lipid profile, A1c, uric acid, glucose, blood pressure, heart rate | |
| Notes | Co‐interventions: diet and exercise counseling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sjostrom 1998.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. Second year weight maintenance. | |
| Participants | 688 patients followed for 1 year with a second weight maintenance year. Mean age 45 y. Mean BMI 36.1 kg/m2. Mean weight 100 kg. Europe. | |
| Interventions | Orlistat 120 mg tid (n=345). Attrition rate 17%. Placebo (n=343). Attrition rate 20%. | |
| Outcomes | weight loss, 5% and 10% responders, lipids, glucose, insulin, blood pressure | |
| Notes | Co‐interventions: 600‐900 kcal/d deficit diet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Smith 2001.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 485 patients from primary care. 52 week follow‐up. Mean age 42 y. Mean BMI 32.7 kg/m2. Mean weight 87 kg. UK. | |
| Interventions | Sibutramine 10 mg daily (n=161). Attrition rate 42%. Sibutramine 15 mg daily (n=161). Attrition rate 49%. Placebo (n=163). Attrition rate 51%. | |
| Outcomes | weight loss, 5% and 10% responders, BMI, waist circ, waist‐hip ratio, total cholesterol, triglycerides, glucose, uric acid, blood pressure, heart rate | |
| Notes | Co‐intervention: diet counseling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Swinburn 2005.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 339 patients with one or more cardiovascular risk factor(s). 1 year follow‐up. Mean age 52 y. Mean BMI 37.8 kg/m2. Mean weight 108 kg. Australia and New Zealand. | |
| Interventions | Orlistat 120 mg tid (n=170). Attrition rate 22%. Placebo (n=169). Attrition rate 19%. | |
| Outcomes | weight loss, 10‐year risk of cardiovascular disease based on Framingham model (primary), waist circ, lipid panel, A1c, glucose, blood pressure, HRQL, | |
| Notes | Co‐intervention: diet and exercise counseling. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
XENDOS.
| Methods | Randomized, double‐blind, placebo‐controlled. Weight loss trial. | |
| Participants | 3305 patients (21% with impaired glucose tolerance) 4 years follow‐up. Mean age 43 y. Mean BMI 37.3 kg/m2. Mean weight 111 kg. Sweden. | |
| Interventions | Orlistat 120 mg tid (n=1650). Attrition rate 48%. Placebo (n=1655). Attrition rate 66%. | |
| Outcomes | Diabetes incidence (primary), weight loss, waist circ, 5% and 10% responders, A1c, glucose, lipid profile, blood pressure, insulin, fibrinogen, PAI‐1 | |
| Notes | Co‐intervention: 800 kcal/d deficit diet and exercise counseling. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Derosa 2002 | Open‐label randomized trial (n=87) of orlistat alone, simvastatin alone, and orlistat plus simvastatin for the treatment of hypercholesterolemia. No placebo arm. |
| Gaciong 2005 | Short‐term (12 weeks), open‐label cohort study of 2225 obese Polish patients. |
| Gilbert 1983 | Pseudorandomized one‐year trial using diethylpropion |
| Hanefeld 2002 | A trial of orlistat vs. placebo in patients with Type 2 diabetes. Follow‐up period after randomization too short (44 weeks). Results similar to trials included in the review. |
| Heymsfield 2000 | Pooled results from three orlistat trials already included in the review. |
| Hill 1999 | Orlistat weight maintenance trial lasting one year. Did not use intention‐to‐treat analysis. Results are not presented for 36% of patients randomized. |
| Hsieh 2005 | One‐year placebo‐controlled RCT of orlistat. Unable to determine if the study was blinded. Attempts to contact the author to clarify were unsuccessful. |
| James 1997 | Preliminary publication of Finer 2000 |
| Lucas 2003 | Subgroup analysis of 5 previous sibutramine trials. Unable to determine which trials. Trials may already have been included in review. |
| Poston 2003 | Open‐label 1‐year trial of orlistat |
| Redmon 1999 | Patients were randomized to phentermine plus fenfluramine vs. dual placebo for two years. Did not contain a phentermine only arm |
| Redmon 2003 | Open‐label 1‐year trial involving sibutramine |
| Redmon 2005 | Open‐label 1‐year trial involving sibutramine |
| Rossner 2001 | Duplicate publication of James et al. Lancet 2000;356:2119‐25. (Swedish) |
| Scholze 2002 | Non‐randomized cohort study of 12 weeks duration in 6360 obese Germans. |
| Toubro 2001 | Duplicate publication of James et al. Lancet 2000;356:2119‐25. (Danish) |
| Wadden 2001 | A 16‐week comparison of different dietary and lifestyle modification regimens in sibutramine treated patients. Not a comparison of sibutramine vs. placebo or another drug. |
| Wadden 2005 | A three‐arm one‐year open‐label study of behavioural modification +/‐ pharmacotherapy with sibutramine in 224 adults. Excluded because open label. Results of sibutramine arm similar to this review. |
| Weintraub 1992 | A mulitmodal intervention study lasting four years that included a comparison of combination therapy with phentermine/ fenfluramine and placebo. Only 24 weeks of this period was double‐blind. |
| Wirth 2001 | Follow‐up period after randomization too short (44 weeks). Results showed slightly lower efficacy compared to sibutramine studies included in this review. Also included an intermittent sibutramine arm. |
| Wirth 2005 | XXL trial. Study of 15 549 patients on orlistat. No control arm |
| Zavoral 1998 | Pooled data from five randomized controlled trials of orlistat. It is not known how many of these studies were subsequently published (and included in the present review). Attempts to contact the author to clarify and obtain unpublished data were unsuccessful. |
Characteristics of ongoing studies [ordered by study ID]
AUDITOR 2005.
| Trial name or title | AUDITOR |
| Methods | |
| Participants | 600 overweight patients 55 years or older with the metabolic syndrome |
| Interventions | Rimonabant 20 mg daily versus placebo |
| Outcomes | Change in carotid intima media thickness; major cardiovascular events are secondary outcomes. |
| Starting date | August 2005 |
| Contact information | Sanofi‐Aventis |
| Notes | 24‐26 months follow‐up |
CRESCENDO 2005.
| Trial name or title | CRESCENDO |
| Methods | |
| Participants | 17 000 patients 55 years or older with central adiposity and cardiovascular risk factors |
| Interventions | Rimonabant 20 mg daily versus placebo |
| Outcomes | MI, stroke, cardiovascular death |
| Starting date | December 2005 |
| Contact information | Sanofi‐Aventis |
| Notes |
SCOUT 2005.
| Trial name or title | SCOUT |
| Methods | |
| Participants | 9000 patients with central obesity and cardiovascular disease or risk factors |
| Interventions | Sibutramine 10‐15 mg daily versus placebo |
| Outcomes | MI, stroke, cardiovascular mortality, cardiac arrest |
| Starting date | December 2002 |
| Contact information | Abbott |
| Notes | 5 year follow‐up |
STRADIVARIUS 2005.
| Trial name or title | STRADIVARIUS |
| Methods | |
| Participants | 800 patients 18 years or older with central adiposity and cardiovascular risk factors |
| Interventions | Rimonabant 20 mg daily versus placebo |
| Outcomes | Intravascular ultrasound |
| Starting date | January 2005 |
| Contact information | Sanofi‐Aventis |
| Notes | Over 2 year follow‐up |
Contributions of authors
RAJ PADWAL: registered the topic, performed the literature search and collected articles, reviewed articles for inclusion and methodological quality, performed data entry and statistical calculations, and was the primary author for the initial and final drafts.
DIANA RUCKER: performed the literature search for the update, collected articles, reviewed articles for inclusion and quality, performed data entry and statistical calculations and co‐authored the update.
STEPHANIE LI: collected articles, reviewed articles for inclusion and methodological quality, performed data entry and co‐wrote the final draft.
CINTIA CURIONI and DAVID LAU: co‐designed the review, provided expert advice and co‐wrote the initial and final drafts.
Sources of support
Internal sources
Department of Medicine, University of Alberta, Canada.
External sources
No sources of support supplied
Declarations of interest
R. Padwal: none known. D. Rucker: none known. S. Li: none known. C.Curioni: none known. D. Lau: David Lau owns common shares in GlaxoSmithKline and Eli Lilly. He is a consultant to Abbott Laboratories, Ltd., AstraZeneca Canada Inc., Merck Frosst Canada Inc., Bristol‐Myers Squibb Canada, Eli Lilly Canada Inc., Oryx Pharmaceuticals Inc., Pfizer Canada Inc., sanofi‐aventis Canada Inc., Servier Canada Inc. and Solvay Pharma Inc.; and has received speaker fees from Abbott Laboratories, Ltd., AstraZeneca Canada Inc., GlaxoSmithKline, Merck Frosst Canada Inc., Merck/Schering, Eli Lilly Canada Inc., sanofi‐aventis Canada Inc. and Novo Nordisk Canada Inc.; research grants from AstraZeneca Canada Inc., Bristol Myers Squibb, Dainippon Pharmaceuticals, GlaxoSmithKline, Pfizer Canada Inc., and Sanofi‐Aventis Canada Inc.; and travel assistance to attend international meetings from Abbott Laboratories, Ltd., AstraZeneca Canada Inc. and Sanofi‐Aventis Canada Inc.
Edited (no change to conclusions)
References
References to studies included in this review
Apfelbaum 1999 {published data only}
- Apfelbaum M, Vague P, Ziegler O, Hanotin C, Thomas F, Leutenegger E. Long‐term maintenance of weight loss after a very‐low‐calorie diet: a randomized blinded trial of the efficacy and tolerability of sibutramine. American Journal of Medicine 1999;106:179‐84. [DOI] [PubMed] [Google Scholar]
Bakris 2002 {published data only}
- Bakris G, Calhoun D, Egan B, Hellmann C, Dolker M, Kingma I. Orlistat improves blood pressure control in obese subjects with treated but inadequately controlled hypertension. Journal of Hypertension 2002;20:2257‐67. [DOI] [PubMed] [Google Scholar]
Berne 2004 {published data only}
- Berne C. A randomized study of orlistat in combination with a weight management programme in obese patients with type 2 diabetes treated with metformin. Diabet Med 2005;22:612‐8. [DOI] [PubMed] [Google Scholar]
Broom 2002 {published data only}
- Broom I, Wilding J, Stott P, Myers N. Randomised trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK Multimorbidity Study. International Journal of Clinical Practice 2002;56:494‐9. [PubMed] [Google Scholar]
Davidson 1999 {published data only}
- Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat. Journal of the American Medical Association 1999;281:235‐42. [DOI] [PubMed] [Google Scholar]
Derosa 2003 {published data only}
- Derosa G, Mugellini A, Ciccarelli L, Fogari R. Randomized, double‐blind, placebo‐controlled comparison of the action of orlistat, fluvastatin, or both on anthropometric measurements, blood pressure and lipid profile in obese patients with hypercholesterolemia prescribed a standardized diet. Clin Ther 2003;25:1107‐1122. [DOI] [PubMed] [Google Scholar]
Finer 2000 {published data only}
- Finer N, James WPT, Kopelman PG, Lean MEJ, Williams G. One‐year treatment of obesity: a randomized, double‐blind, placebo‐controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. International Journal of Obesity and Related Metabolic Disorders 2000;24:306‐13. [DOI] [PubMed] [Google Scholar]
Hauner 2004 {published data only}
- Hauner H, Meier M, Wendland G, et al. Weight reduction by sibutramine in obese subjects in primary care medicine: the SAT study. Exp Clin Endocrinol Diabetes 2004;112:201‐7. [DOI] [PubMed] [Google Scholar]
Hauptman 2000 {published data only}
- Hauptman J, Lucas C, Boldrin MN, Collins H, Segal K. Orlistat in the long‐term treatment of obesity in primary care settings. Archives of Family Medicine 2000;9:160‐7. [DOI] [PubMed] [Google Scholar]
Hollander 1998 {published data only}
- Hollander PA, Elbein SC, Hirsch IB, Kelley D, McGill J, Taylor T, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. Diabetes Care 1998;21:1288‐94. [DOI] [PubMed] [Google Scholar]
James 2000 {published data only}
- James WPT, Astrup A, Finer N, Hilsted J, Kopelman P, Rossner S, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Lancet 2000;356:2119‐25. [DOI] [PubMed] [Google Scholar]
Kaukua 2003 {published data only}
- Kaukua JK, Pekkarinen TA, Rissanen AM. Health‐related quality of life in a randomised placebo‐controlled trial of sibutramine in obese patients with type II diabetes. Int J Obes Relat Metab Disord 2004;28:600‐5. [DOI] [PubMed] [Google Scholar]
Kelley 2002 {published data only}
- Kelley DE, Bray GA, Pi‐Sunyer FX, Klein S, Hill J, Miles J, Hollander P. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin‐treated type 2 diabetes. Diabetes Care 2002;25:1033‐41. [DOI] [PubMed] [Google Scholar]
Krempf 2003 {published data only}
- Krempf M, Louvet JP, Allanic H, et al. Weight reduction and long‐term maintenance after 18 months treatment with orlistat for obesity. Int J Obese Relat Met Disord 2003;27:591‐7. [DOI] [PubMed] [Google Scholar]
Lindgarde 2000 {published data only}
- Lindgarde F. The effect of orlistat on body weight and coronary heart disease risk profile in obese patients: The Swedish Multimorbidity Study. Journal of Internal Medicine 2000;248:245‐54. [DOI] [PubMed] [Google Scholar]
Mathus‐Vliegen 2005 {published data only}
- Mathus‐Vliegen EM for the Balance Study Group. Long‐term maintenance of weight loss with sibutramine in a GP setting following a specialist guided very‐low‐calorie diet: a double‐blind, placebo‐controlled, parallel group study. European Journal of Clinical Nutrition 2005;59(Suppl 1):S31‐39. [DOI] [PubMed] [Google Scholar]
McMahon 2000 {published data only}
- McMahon FG, Fujioka K, Singh BN, Mendel CM, Rowe E, Rolston K, et al. Efficacy and safety of sibutramine in obese white and African American patients with hypertension. Archives of Internal Medicine 2000;160:2185‐91. [DOI] [PubMed] [Google Scholar]
McMahon 2002 {published data only}
- McMahon FG, Weinstein SP, Rowe E, Ernst KR, Johnson F, Fujioka K. Sibutramine is safe and effective for weight loss in obese patients whose hypertension is well controlled by angiotensin converting enzyme inhibitors. Journal of Human Hypertension 2002;16:5‐11. [DOI] [PubMed] [Google Scholar]
McNulty 2003 {published data only}
- McNulty SJ, Ur E, Williams G, et al. A randomized trial of sibutramine in the management of obese type 2 diabetic patients treated with metformin. Diabetes Care 2003;26:125‐131. [DOI] [PubMed] [Google Scholar]
Miles 2002 {published data only}
- Miles JM, Leiter L, Hollander P, et al. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes Care 2002;25:1123‐28. [DOI] [PubMed] [Google Scholar]
RIO‐Diabetes {published data only}
- Scheen AJ, Finer N, Hollander P, Jensen MD, Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 2006;368:1660‐72. [DOI] [PubMed] [Google Scholar]
RIO‐Europe {published data only}
- Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid‐1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1‐year experience from the RIO‐Europe study. Lancet 2005;365:1389‐97. [DOI] [PubMed] [Google Scholar]
RIO‐Lipids {published data only}
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005;353:2121‐34. [DOI] [PubMed] [Google Scholar]
RIO‐North America {published data only}
- Pi‐Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid‐1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients. JAMA 2006;295:761‐5. [DOI] [PubMed] [Google Scholar]
Rossner 2000 {published data only}
- Rossner S, Sjostrom L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. Obesity Research 2000;8:49‐61. [DOI] [PubMed] [Google Scholar]
Sanchez‐Reyes 2004 {published data only}
- Sanchez‐Reyes L, Fanghanel G, Yamamoto J, et al. Use of sibutramine in overweight adult Hispanic patients with type 2 diabetes mellitus: a 12‐month, randomized, double‐blind, placebo‐controlled clinical trial. Clin Ther 2004;26:1427‐35. [DOI] [PubMed] [Google Scholar]
Sjostrom 1998 {published data only}
- Sjostrom L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HPF, et al. Randomised placebo‐controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet 1998;352:167‐73. [DOI] [PubMed] [Google Scholar]
Smith 2001 {published data only}
- Smith IG, Goulder MA. Randomized placebo‐controlled trial of long‐term treatment with sibutramine in mild to moderate obesity. Journal of Family Practice 2001;50:505‐12. [PubMed] [Google Scholar]
Swinburn 2005 {published data only}
- Swinburn BA, Carey D, Hills AP, et al. Effect of orlistat on cardiovascular disease risk in obese adults. Diab Obes Metab 2005;7:254‐62. [DOI] [PubMed] [Google Scholar]
XENDOS {published data only}
- Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. Xenical in the prevention of diabetes in obese subjects (XENDOS) study. Diabetes Care 2004;1:155‐61. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Derosa 2002 {published data only}
- Derosa G, Mugellini A, Ciccarelli L, Rinaldi A, Fogari R. Effects of orlistat, simvastatin, and orlistat plus simvastatin in obese patients with hypercholesterolemia: a randomized, open‐label trial. Current Therapeutic Research, Clinical and Experimental 2002;63:621‐33. [Google Scholar]
Gaciong 2005 {published data only}
- Gaciong Z, Placha G. Efficacy and safety of sibutramine in 2225 subjects with cardiovascular risk factors: short‐term, open‐labl, observational study. Journal of Hum Hypertens 2005;19:737‐43. [DOI] [PubMed] [Google Scholar]
Gilbert 1983 {published data only}
- Gilbert S, Garrow JS. A prospective controlled trial of outpatients treatment for obesity. Human Nutrition. Clinical Nutrition 1983;37:21‐9. [PubMed] [Google Scholar]
Hanefeld 2002 {published data only}
- Hanefeld M, Sachse G. The effects of orlistat on body weight and glycaemic control in overweight patients with type 2 diabetes: a randomized, placebo‐controlled trial.. Diabetes, Obesity and Metabolism 2002;4:415‐23. [DOI] [PubMed] [Google Scholar]
Heymsfield 2000 {published data only}
- Heymsfield SB, Segal KR, Hauptman J, Lucas CP, Boldrin MN, Rissanen A, et al. Effects of weight loss with orlistat on glucose tolerance and progression to type 2 diabetes in obese adults. Archives of Internal Medicine 2000;160:1321‐26. [DOI] [PubMed] [Google Scholar]
Hill 1999 {published data only}
- Hill JO, Hauptman J, Anderson JW, Fujioka K, O'Neil PM, Smith DK, et al. Orlistat, a lipase inhibitor, for weight maintenance after conventional dieting: a 1‐y study. American Journal of Clinical Nutrition 1999;69:1108‐16. [DOI] [PubMed] [Google Scholar]
Hsieh 2005 {published data only}
- Hsieh CJ, Wang PW, Liu RT, et al. Orlistat for obesity: benefits beyond weight loss. Diabetes Res Clin Pract 2005;67:78‐83. [DOI] [PubMed] [Google Scholar]
James 1997 {published data only}
- James WPT, Avenell A, Broom J, Whitehead J. A one‐year trial to assess the value of orlistat in the management of obesity. International Journal of Obesity and Related Metabolic Disorders 1997;21(Suppl 3):S24‐30. [PubMed] [Google Scholar]
Lucas 2003 {published data only}
- Lucas CP, Boldrin MN, Reaven GM. Effect of orlistat added to diet (30% of calories from fat) on plasma lipids, glucose, and insulin in obese patients with hypercholesterolemia. Am J Cardiol 2003;91:961‐4. [DOI] [PubMed] [Google Scholar]
Poston 2003 {published data only}
- Poston WS, Reeves RS, Haddock CK, et al. Weight loss in obese Mexican Americans treated for 1‐year with orlistat and lifestyle modification. Int J Obes Relat Metab Disord 2003;27:1486‐93. [DOI] [PubMed] [Google Scholar]
Redmon 1999 {published data only}
- Redmon JB, Raatz SK, Kwong CA, Swanson JE, Thomas W, Bantle JE. Pharmacologic induction of weight loss to treat type 2 diabetes. Diabetes Care 1999;22:896‐903. [DOI] [PubMed] [Google Scholar]
Redmon 2003 {published data only}
- Redmon JB, Raatz SK, Reck JP, et al. One‐year outcome of a combination of weight loss therapies for subjects with type 2 diabetes: a randomized trial.. Diabetes Care 2003;26:2505‐11. [DOI] [PubMed] [Google Scholar]
Redmon 2005 {published data only}
- Redmon JB, Reck KP, Raatz SK, et al. Two‐year outcome of a combination of weight loss therapies for type 2 diabetes. Diabetes Care 2005;28:1311‐5. [DOI] [PubMed] [Google Scholar]
Rossner 2001 {published data only}
- Rossner S. Sibutramine ‐ antidepressive agent tested against obesity. Ladartidningen 2001;98:1802‐3. [PubMed] [Google Scholar]
Scholze 2002 {published data only}
- Scholze J. Treating obesity with sibutramine under practical conditions. Dtsch Med Wochenschr 2002;127:606‐10. [DOI] [PubMed] [Google Scholar]
Toubro 2001 {published data only}
- Toubro S, Hansen DL, Hilsted JC, Porsborg PA, Astrup AV. The effect of sibutramine for the maintenance of weight loss: a randomised, clinical, controlled study. Ugeskrift for Laeger 2001;163:2395‐40. [PubMed] [Google Scholar]
Wadden 2001 {published data only}
- Wadden TA, Berkowitz RI, Sarwer DB, Prus‐Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity. Archives of Internal Medicine 2001;161:218‐27. [DOI] [PubMed] [Google Scholar]
Wadden 2005 {published data only}
- Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med 2005;353:2111‐20. [DOI] [PubMed] [Google Scholar]
Weintraub 1992 {published data only}
- Weintraub M. Long‐term weight control: The national heart, lung, and blood institute funded multimodal intervention study. Clinical Pharmacology and Therapeutics 1992;51:581‐585. [DOI] [PubMed] [Google Scholar]
Wirth 2001 {published data only}
- Wirth A, Krause J. Long‐term weight loss with sibutramine. Journal of the American Medical Association 2001;286:1331‐9. [DOI] [PubMed] [Google Scholar]
Wirth 2005 {published data only}
- Wirth A. Reduction of body weight and co‐morbidities by orlistat: the XXL ‐ Primary Health Care Trial. Diab Obes Metab 2005;7:21‐7. [DOI] [PubMed] [Google Scholar]
Zavoral 1998 {published data only}
- Zavoral JH. Treatment with orlistat reduces cardiovascular risk in obese patients. J Hypertens 1998;16:2013‐7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
AUDITOR 2005 {published data only}
- ClinicalTrials.gov. Atherosclerosis underlying development assessed by intima‐media thickness in patients on rimonabant (AUDITOR). http://www.clinicaltrials.gov/ct/show/NCT00228176?order=1. Accessed March 2007. December 2005.
CRESCENDO 2005 {published data only}
- ClinicalTrials.gov. CRESCENDO: comprehensive rimonabant evaluation study of cardiovascular endpoints and outcomes. http://www.clinicaltrials.gov/ct/show/NCT00263042?order=3 (accessed March 2007) December 2005.
SCOUT 2005 {published data only}
- James WP. The SCOUT study: risk‐benefit profile of sibutramine in overweight high‐risk cardiovascular patients. Eur Heart J 2005;7(suppl L):L44‐8. [Google Scholar]
STRADIVARIUS 2005 {published data only}
- ClinicalTrials.gov. STRADIVARIUS (Strategy to reduce atherosclerosis development involving administration of rimonabant ‐ the intravascular ultrasound study). http://www.clinicaltrials.gov/ct/show/NCT00124332?order=1. Accessed March 2007 December 2005.
Additional references
Abenhaim 1996
- Abenhaim L, Moride Y, Brenot F, et al. Appetite‐suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med 1996;335:609‐16. [DOI] [PubMed] [Google Scholar]
Allison 1999
- Allison DB, Zannolli R, Faith MS, Heo M, Pietrobelli A, VanItallie TB, et al. Weight loss increases and fat loss decreases all‐cause mortality rate: results from two independent cohort studies. International Journal of Obesity and Related Metabolic Disorders 1999;23:603‐11. [DOI] [PubMed] [Google Scholar]
Andres 1993
- Andres R, Muller DC, Sorkin JD. Long‐term effects of change in body weight on all‐cause mortality. Annals of Internal Medicine 1993;119:737‐43. [DOI] [PubMed] [Google Scholar]
Arterburn 2004
- Arterburn DE, Crane PK, Veenstra DL. The efficacy and safety of sibutramine for weight loss: a systematic review. Arch Intern Med 2004;164:994‐1003. [DOI] [PubMed] [Google Scholar]
Birmingham 1999
- Birmingham CL, Muller JL, Palepu A, Spinelli JJ, Anis AH. The cost of obesity in Canada. Canadian Medical Association Journal 1999;160:483‐8. [PMC free article] [PubMed] [Google Scholar]
Blackburn 1995
- Blackburn G. Effect of degree of weight loss on health benefits. Obesity Research 1995;3(Supp 2):2112S‐5S. [DOI] [PubMed] [Google Scholar]
Bray 1998
- Bray GA. Obesity: a time bomb to be defused. Lancet 1998;352:160‐1. [DOI] [PubMed] [Google Scholar]
Bray 2000
- Bray GA, Tartaglia LA. Medicinal strategies in the treatment of obesity. Nature 2000;404:672‐7. [DOI] [PubMed] [Google Scholar]
Buchanan 2002
- Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, et al. Preservation of pancreatic beta‐cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high‐risk hispanic women. Diabetes 2002;51:2796‐803. [DOI] [PubMed] [Google Scholar]
Calle 1999
- Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr. Body‐mass index and mortality in a prospective cohort of U.S. adults. New England Journal of Medicine 1999;341:1097‐105. [DOI] [PubMed] [Google Scholar]
Chiasson 2002
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP‐NIDDM randomised trial. Lancet 2002;359:2072‐7. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Colditz 1995
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Annals of Internal Medicine 1995;122:481‐6. [DOI] [PubMed] [Google Scholar]
Connolly 1997
- Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine‐phentermine. N Engl J Med 1997;337:581‐8. [DOI] [PubMed] [Google Scholar]
Curioni 2006
- Curioni C, Andre C. Rimonabant for overweight or obesity. Cochrane Database of Systematic Reviews 2006, Issue 4. [Art. No.:CD006162] [DOI] [PMC free article] [PubMed] [Google Scholar]
DPP 2002
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346:393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
DREAM 2006
- The DREAM Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096‐105. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Field 2001
- Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, et al. Impact of overweight on the risk of developing common chronic diseases during a 10‐year period. Archives of Internal Medicine 2001;161:1581‐6. [DOI] [PubMed] [Google Scholar]
Flegal 2002
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999‐2000. Journal of the American Medical Association 2002;288:1723‐27. [DOI] [PubMed] [Google Scholar]
French 1999
- French SA, Folsom AR, Jeffery RW, Williamson DF. Prospective study of intentionality of weight loss and mortality in older women: the Iowa Women's Health Study. American Journal of Epidemiology 1999;149:504‐14. [DOI] [PubMed] [Google Scholar]
Goldstein 1992
- Goldstein DJ. Beneficial effects of modest weight loss. International Journal of Obesity and Related Metabolic Disorders 1992;16:397‐415. [PubMed] [Google Scholar]
Greenway 2000
- Greenway FL. Obesity surgery. Endocrinology and Metabolism Clinics of North America 1996;25:1005‐27. [DOI] [PubMed] [Google Scholar]
Haddock 2002
- Haddock CK, Poston WSC, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. International Journal of Obesity and Related Metabolic Disorders 2002;26:262‐73. [DOI] [PubMed] [Google Scholar]
Haslam 2005
- Haslam DW, James WPT. Obesity. Lancet 2005;366:1197‐209. [DOI] [PubMed] [Google Scholar]
Health Canada 2002
- Health Canada. Health Canada investigates safety of MERIDIA® (Sibutramine). http://www.hc‐sc.gc.ca/english/protection/warnings/2002/2002_21e.htm.
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. British Medical Journal 1999;319:670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
INTERHEART 2005
- Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case control study. Lancet 2005;366:1640‐49. [DOI] [PubMed] [Google Scholar]
IOTF 2007
- International Obesity Task Force. Global obesity map. Available at http://www.iotf.org/database/documents/GlobalAdultmapswithtop5ineachregion_000.ppt. Accessed January 2007.
Jick 1998
- Jick H, Vasilakis C, Weinrauch LA, Meier CR, Jick SS, Derby LE. A population‐based study of appetite‐suppressant drugs and the risk of cardiac‐valve regurgitation. New England Journal of Medicine 1998;339:719‐24. [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Smith GD, Altman DG. Assessing the quality of randomised controlled trials. In: Egger M, Smith GD, Altman DG editor(s). Systematic Reviews in Health Care. Second Edition. London: BMJ Publishing Group, 2001:87‐108. [Google Scholar]
Kenchaiah 2002
- Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin, EJ, Larson MG, et al. Obesity and risk of heart failure. New England Journal of Medicine 2002;347:305‐13. [DOI] [PubMed] [Google Scholar]
Khan 1998
- Khan MA, Herzog CA, Peter JV, Hartley GG, Madlon‐Kay R, Dick CD, et al. The prevalence of cardiac valvular insufficiency assessed by transthoracic echocardiography in obese patients treated with appetite‐suppressant drugs. New England Journal of Medicine 1998;339:713‐18. [DOI] [PubMed] [Google Scholar]
Kissebah 1994
- Kissbah AH, Krakower GR. Regional adiposity and morbidity. Physiological Reviews 1994;74:761‐811. [DOI] [PubMed] [Google Scholar]
Kopelman 2000
- Kopelman PG. Obesity as a medical problem. Nature 2000;404:635‐43. [DOI] [PubMed] [Google Scholar]
Lau 2007
- Lau DCW. Synopsis of the 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ 2007;176:1103‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau D 2007
- Lau DCW, Douketis JD, Morrison KM, et al. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ 2007;176:S1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau DC 2007
- Lau DCW, Douketis JD, Morrison KM, et al. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children. CMAJ 2007;176:S1‐130. Available at www.cmaj.ca/cgi/content/full/176/8/S1/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leibel 1995
- Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995;332:621‐8. [DOI] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326:1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2005
- Li Z, Maglione M, Tu W, et al. Meta‐analysis: pharmacologic treatment of obesity. Ann Intern Med 2005;142:532‐46. [DOI] [PubMed] [Google Scholar]
Look AHEAD
- The Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610‐28. [DOI] [PubMed] [Google Scholar]
Luque 1999
- Luque CA, Rey JA. Sibutramine: a serotonin‐norepinephrine reuptake‐inhibitor for the treatment of obesity. Annals of Pharmacotherapy 1999;33:968‐78. [DOI] [PubMed] [Google Scholar]
Manson 1995
- Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, et al. Body weight and mortality among women. New England Journal of Medicine 1995;333:677‐85. [DOI] [PubMed] [Google Scholar]
McNeely 1998
- McNeely W, Benfield P. Orlistat. Drugs 1998;52:241‐49.. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup D. Improving the quality of reporting of meta‐analysis of randomized controlled trials: the QUOROM statement. Lancet 1999;354:1896‐900. [DOI] [PubMed] [Google Scholar]
NIH 1993
- NIH Technology Assessment Conference Panel. Methods for voluntary weight loss and control. Annals of Internal Medicine 1993;119:764‐70. [PubMed] [Google Scholar]
Norris 2005
- Norris SL, Zhang X, Avenell A, Gregg E, Schmid CH, Lau J. Pharmacotherapy for weight loss in adults with type 2 diabetes. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
NTF 1994
- The National Task Force on Prevention and Treatment of Obesity. Towards prevention of obesity: research directions. Obes Res 1994;2:571‐584. [DOI] [PubMed] [Google Scholar]
O'Meara 2001
O'Meara 2002
Ogden 2002
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999‐2000. Journal of the American Medical Association 2002;288:1728‐32. [DOI] [PubMed] [Google Scholar]
Padwal 2003
- Padwal R, Li SK, Lau DCW. Long‐term pharmacotherapy for obesity and overweight. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI] [PubMed] [Google Scholar]
Padwal 2005
- Padwal R, Majumdar SR, Johnson JA, Varney J, McAlister FA. A systematic review of drug therapy to delay or prevent type 2 diabetes. Diabetes Care 2005;28:736‐44. [DOI] [PubMed] [Google Scholar]
Padwal 2007
- Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine and rimonabant. Lancet 2007;369:71‐7. [DOI] [PubMed] [Google Scholar]
Padwal R 2007
- Padwal R, Kezouh A, Levine M, Etminan M. Long‐term persistence with orlistat and sibutramine in a population‐based cohort. Int J Obes (Lond) 2007:In press. [DOI] [PubMed] [Google Scholar]
Padwal RS 2007
- Padwal RS. Antiobesity drug therapy: A call for more rigorous endpoint evaluation. Future Drugs 2007;4:221‐6. [Google Scholar]
Rexrode 1998
- Rexrode KM, Hennekens CH, Willett WC, Colditz GA, Stampfer MJ, Rich‐Edwards JW, et al. A prospective study of body mass index, weight change, and risk of stroke in women. Journal of the American Medical Association 1997;277:1539‐45. [DOI] [PubMed] [Google Scholar]
Rimm 1995
- Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, Willett WC. Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, et al. Body size and fat distribution as predictors of coronary heart disease among middle‐aged and older US men. American Journal of Epidemiology 1995;141:1117‐27. [DOI] [PubMed] [Google Scholar]
Seidell 1996
- Seidell JC. The impact of obesity on health status: some implications for health care costs. International Journal of Obesity and Related Metabolic Disorders 1996;19(Suppl 6):S13‐S16. [PubMed] [Google Scholar]
SOS 2004
- Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683‐93. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases. In: Egger M, Smith GD, Altman DG editor(s). Systematic Reviews in Health Care. Second Edition. London: BMJ Publishing Group, 2001. [Google Scholar]
Stevens 1998
- Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body‐mass index and mortality. New England Journal of Medicine 1998;338:1‐7. [DOI] [PubMed] [Google Scholar]
Tuomilehto 2001
- Toumilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne‐Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344:1343‐50. [DOI] [PubMed] [Google Scholar]
US Guidelines 1998
- Expert Panel on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Archives of Internal Medicine 1998;158:1855‐67. [DOI] [PubMed] [Google Scholar]
UTD 2001
- Bray GA. Health hazards associated with obesity. UpToDate Clinical Reference Library [CD‐ROM] 2001; Vol. Version 9.1.
Verhagen 1998
- Verhagen AP, Vet HC, Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. Journal of Clinical Epidemiology 1998;51:1235‐31. [DOI] [PubMed] [Google Scholar]
Visscher 2002
- Visscher TL, Seidell JC, Molarius A, Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist‐hip ratio and waist circumference as predictors of all‐cause mortality among the elderly: the Rotterdam study. International Journal of Obesity and Related Metabolic Disorders 2001;25:1730‐5. [DOI] [PubMed] [Google Scholar]
Wadden 1993
- Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Annals of Internal Medicine 1993;119:688‐93. [DOI] [PubMed] [Google Scholar]
Weissman 1998
- Weissman NJ, Tighe JF Jr, Gottdiener JS, Gwynne JT. An assessment of heart‐valve abnormalities in obese patients taking dexfenfluramine, sustained‐release dexfenfluramine, or placebo. New England Journal of Medicine 1998;339:725‐32. [DOI] [PubMed] [Google Scholar]
WHO 1998
- World Health Organisation (WHO). Obesity: Preventing and managing the Global Epidemic ‐ Report of a WHO Consultation on Obesity, 3‐5 June 1997. WHO/NUT/NCD/98.1 1998. [PubMed]
Williamson 1993
- Williamson DF, Pamuk ER. The association between weight loss and increased longevity. A review of the evidence. Annals of Internal Medicine 1993;119:731‐36. [DOI] [PubMed] [Google Scholar]
Williamson 1995
- Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in never‐smoking overweight US white women aged 40‐64 years. American Journal of Epidemiology 1995;141:1128‐41. [DOI] [PubMed] [Google Scholar]
Wing 1987
- Wing RR, Marcus MD, Epstein LH, Salata R. Type 2 diabetic subjects lose less weight than their overweight spouses.. Diabetes Care 2000;23:1499‐1504. [DOI] [PubMed] [Google Scholar]
Wooltorton 2002
- Wooltorton 2002. Obesity drug sibutramine (Meridia): hypertension and cardiac arrhythmias. Canadian Medical Association Journal 2002;166:1307‐8. [PMC free article] [PubMed] [Google Scholar]


