Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and the resulting COVID‐19 pandemic present important diagnostic challenges. Several diagnostic strategies are available to identify or rule out current infection, identify people in need of care escalation, or to test for past infection and immune response. Point‐of‐care antigen and molecular tests to detect current SARS‐CoV‐2 infection have the potential to allow earlier detection and isolation of confirmed cases compared to laboratory‐based diagnostic methods, with the aim of reducing household and community transmission.
Objectives
To assess the diagnostic accuracy of point‐of‐care antigen and molecular‐based tests to determine if a person presenting in the community or in primary or secondary care has current SARS‐CoV‐2 infection.
Search methods
On 25 May 2020 we undertook electronic searches in the Cochrane COVID‐19 Study Register and the COVID‐19 Living Evidence Database from the University of Bern, which is updated daily with published articles from PubMed and Embase and with preprints from medRxiv and bioRxiv. In addition, we checked repositories of COVID‐19 publications. We did not apply any language restrictions.
Selection criteria
We included studies of people with suspected current SARS‐CoV‐2 infection, known to have, or not to have SARS‐CoV‐2 infection, or where tests were used to screen for infection. We included test accuracy studies of any design that evaluated antigen or molecular tests suitable for a point‐of‐care setting (minimal equipment, sample preparation, and biosafety requirements, with results available within two hours of sample collection). We included all reference standards to define the presence or absence of SARS‐CoV‐2 (including reverse transcription polymerase chain reaction (RT‐PCR) tests and established clinical diagnostic criteria).
Data collection and analysis
Two review authors independently screened studies and resolved any disagreements by discussion with a third review author. One review author independently extracted study characteristics, which were checked by a second review author. Two review authors independently extracted 2x2 contingency table data and assessed risk of bias and applicability of the studies using the QUADAS‐2 tool. We present sensitivity and specificity, with 95% confidence intervals (CIs), for each test using paired forest plots. We pooled data using the bivariate hierarchical model separately for antigen and molecular‐based tests, with simplifications when few studies were available. We tabulated available data by test manufacturer.
Main results
We included 22 publications reporting on a total of 18 study cohorts with 3198 unique samples, of which 1775 had confirmed SARS‐CoV‐2 infection. Ten studies took place in North America, two in South America, four in Europe, one in China and one was conducted internationally. We identified data for eight commercial tests (four antigen and four molecular) and one in‐house antigen test. Five of the studies included were only available as preprints.
We did not find any studies at low risk of bias for all quality domains and had concerns about applicability of results across all studies. We judged patient selection to be at high risk of bias in 50% of the studies because of deliberate over‐sampling of samples with confirmed COVID‐19 infection and unclear in seven out of 18 studies because of poor reporting. Sixteen (89%) studies used only a single, negative RT‐PCR to confirm the absence of COVID‐19 infection, risking missing infection. There was a lack of information on blinding of index test (n = 11), and around participant exclusions from analyses (n = 10). We did not observe differences in methodological quality between antigen and molecular test evaluations.
Antigen tests
Sensitivity varied considerably across studies (from 0% to 94%): the average sensitivity was 56.2% (95% CI 29.5 to 79.8%) and average specificity was 99.5% (95% CI 98.1% to 99.9%; based on 8 evaluations in 5 studies on 943 samples). Data for individual antigen tests were limited with no more than two studies for any test.
Rapid molecular assays
Sensitivity showed less variation compared to antigen tests (from 68% to 100%), average sensitivity was 95.2% (95% CI 86.7% to 98.3%) and specificity 98.9% (95% CI 97.3% to 99.5%) based on 13 evaluations in 11 studies of on 2255 samples. Predicted values based on a hypothetical cohort of 1000 people with suspected COVID‐19 infection (with a prevalence of 10%) result in 105 positive test results including 10 false positives (positive predictive value 90%), and 895 negative results including 5 false negatives (negative predictive value 99%).
Individual tests
We calculated pooled results of individual tests for ID NOW (Abbott Laboratories) (5 evaluations) and Xpert Xpress (Cepheid Inc) (6 evaluations). Summary sensitivity for the Xpert Xpress assay (99.4%, 95% CI 98.0% to 99.8%) was 22.6 (95% CI 18.8 to 26.3) percentage points higher than that of ID NOW (76.8%, (95% CI 72.9% to 80.3%), whilst the specificity of Xpert Xpress (96.8%, 95% CI 90.6% to 99.0%) was marginally lower than ID NOW (99.6%, 95% CI 98.4% to 99.9%; a difference of −2.8% (95% CI −6.4 to 0.8))
Authors' conclusions
This review identifies early‐stage evaluations of point‐of‐care tests for detecting SARS‐CoV‐2 infection, largely based on remnant laboratory samples. The findings currently have limited applicability, as we are uncertain whether tests will perform in the same way in clinical practice, and according to symptoms of COVID‐19, duration of symptoms, or in asymptomatic people. Rapid tests have the potential to be used to inform triage of RT‐PCR use, allowing earlier detection of those testing positive, but the evidence currently is not strong enough to determine how useful they are in clinical practice.
Prospective and comparative evaluations of rapid tests for COVID‐19 infection in clinically relevant settings are urgently needed. Studies should recruit consecutive series of eligible participants, including both those presenting for testing due to symptoms and asymptomatic people who may have come into contact with confirmed cases. Studies should clearly describe symptomatic status and document time from symptom onset or time since exposure. Point‐of‐care tests must be conducted on samples according to manufacturer instructions for use and be conducted at the point of care. Any future research study report should conform to the Standards for Reporting of Diagnostic Accuracy (STARD) guideline.
Plain language summary
How accurate are rapid tests, performed during a health‐care visit (point‐of‐care), for diagnosing COVID‐19?
Why is this question important?
People with suspected COVID‐19 need to know quickly whether they are infected, so that they can self‐isolate, receive treatment, and inform close contacts. Currently, COVID‐19 infection is confirmed by sending away samples, taken from the nose and throat, for laboratory testing. The laboratory test, called RT‐PCR, requires specialist equipment, may require repeat healthcare visits, and typically takes at least 24 hours to produce a result.
Rapid point‐of‐care tests can provide a result ‘while you wait’, ideally within two hours of providing a sample. This could help people isolate early and reduce the spread of infection.
What did we want to find out?
We were interested in two types of rapid point‐of‐care tests, antigen and molecular tests. Antigen tests identify proteins on the virus, often using disposable devices. Molecular tests detect the virus’s genetic material, using small portable or table‐top devices. Both test the same nose or throat samples as RT‐PCR tests.
We wanted to know whether rapid point‐of‐care antigen and molecular tests are accurate enough to replace RT‐PCR for diagnosing infection, or to select people for further testing if they have a negative result.
What did we do?
We looked for studies that measured the accuracy of rapid point‐of‐care tests compared with RT‐PCR tests to detect current COVID‐19 infection. Studies could assess any rapid antigen or molecular point‐of‐care test, compared with a reference standard test. The reference standard is the best available method for diagnosing the infection; we considered RT‐PCR test results and clinically defined COVID‐19 as reference tests. People could be tested in hospital or the community. Studies could test people with or without symptoms.
Tests had to use minimal equipment, be performed safely without risking infection from the sample, and have results available within two hours of the sample being collected. Tests could be used in small laboratories or wherever the patient is (in primary care, urgent care facilities, or in hospital).
How did studies assess diagnostic test accuracy?
Studies tested participants with the rapid point‐of‐care tests. Participants were classified as known to have – and not to have ‐ COVID‐19, by RT‐PCR in all studies. Studies then identified false positive and false negative errors in the point‐of‐care test results, compared to RT‐PCR. False positive tests incorrectly identified COVID‐19 when it was not present, potentially leading to unnecessary self‐isolation and further testing. False negatives missed COVID‐19 when it was present, risking delayed self‐isolation and treatment, and spread of infection.
What we found
We found 18 relevant studies. Ten studies took place in North America, four in Europe, two in South America, one in China and one in multiple countries.
Nine studies deliberately included a high percentage of people with confirmed COVID‐19 or included only people with COVID‐19. Fourteen studies did not provide any information about the people providing the samples for testing and 12 did not provide any information about where people were tested.
None of the studies reported includedsamples from people without symptoms.
Main results
Five studies reported eight evaluations of five different antigen tests. Overall, there was considerable variation between the results of the antigen tests in how well they detected COVID‐19 infection. Tests gave false positive results in less than 1% of samples.
Thirteen evaluations of four different molecular tests correctly detected an average of 95% of samples with COVID‐19 infection. Around 1% of samples gave false positive results.
If 1000 people had molecular tests, and 100 (10%) of them really had COVID‐19:
‐ 105 people would test positive for COVID‐19. Of these, 10 people (10%) would not have COVID‐19 (false positive result).
‐ 895 people would test negative for COVID‐19. Of these, 5 people (1%) would actually have COVID‐19 (false negative result).
We noted a large difference in COVID‐19 detection between the two most commonly evaluated molecular tests.
How reliable were the results of the studies?
Our confidence in the evidence is limited.
‐ Three‐quarters of studies did not follow the test manufacturers’ instructions, so may have found different results if they had.
‐ Often, studies did not use the most reliable methods or did not report enough information for us to judge their methods. This may have affected estimates of test accuracy, but it is impossible to identify by how much.
‐ A quarter of studies were published early online as ‘preprints’ and are included in the review. Preprints do not undergo the normal rigorous checks of published studies, so we are uncertain how reliable they are.
What are the implications of this review?
Studies provided little information about their participants, so it is not possible to tell if the results can be applied to people with no symptoms, mild symptoms, or who were hospitalised with COVID‐19. Accurate rapid tests would have the potential to select people for RT‐PCR testing or to be used where RT‐PCR is not available. However, the evidence currently is not strong enough and more studies are urgently needed to be able to say if these tests are good enough to be used in practice.
How up‐to‐date is this review?
This review includes evidence published up to 25 May 2020. Because new research is being published in this field, we will update this review soon.
Summary of findings
Summary of findings 1. Diagnostic accuracy of point‐of‐care antigen and molecular‐based tests for the diagnosis of SARS‐CoV‐2 infection.
| Question | What is the diagnostic accuracy of rapid point‐of‐care antigen and molecular‐based tests for the diagnosis of SARS‐CoV‐2 infection? | |||||
| Population | Adults or children suspected of:
or populations undergoing screening for SARS‐CoV‐2 infection, including
|
|||||
| Index test | Any rapid antigen or molecular‐based test for diagnosis of SARS‐CoV‐2 meeting the following criteria:
|
|||||
| Target condition | Detection of current SARS‐CoV‐2 infection | |||||
| Reference standard | For COVID‐19 cases: positive RT‐PCR alone or clinical diagnosis of COVID‐19 based on established guidelines or combinations of clinical features For non‐COVID‐19 cases: repeated negative RT‐PCR or pre‐pandemic sources of samples |
|||||
| Action |
False negative results mean missed cases of COVID‐19 infection, with either delayed or no confirmed diagnosis and increased risk of community transmission due to false sense of security False positive results lead to unnecessary self‐isolation or quarantine, with the potential for new infection to be acquired |
|||||
| Quantity of evidence | Number of studies | Total samples | Total samples with confirmed SARS‐CoV‐2 | |||
| 18 | 3198 | 1775 | ||||
| Limitations in the evidence | ||||||
| Risk of bias |
Participants: high or unclear risk in 16 studies (89%) Index test: high or unclear risk in 14 studies (78%) Reference standard: unclear risk in 10 studies (56%) Flow and timing: high or unclear risk in 15 studies (83%) |
|||||
| Concerns about applicability |
Participants: high concerns in 13 studies (72%) Index test: high concerns in 13 studies (72%) Reference standard: high concerns in 17 studies (94%) |
|||||
| Findings | ||||||
| Antigen tests | ||||||
| Evaluations (studies) | Samples | Confirmed SARS‐CoV‐2 samples |
Average sensitivity (95% CI) [Range] |
Average specificity (95% CI) [Range] |
||
| 8 (5) | 943 | 596 | 56.2 (29.5 to 79.8) [0% to 94%]a |
99.5 (98.1 to 99.9) [90% to 100%] |
||
| Average sensitivity and specificity applied to a hypothetical cohort of 1000 patientsa | ||||||
| Prevalence of COVID‐19 | TP | FP | FN | TN | PPVb | NPVc |
| 5% | 28a | 5 | 22a | 945 | 85% (68% to 95%)a | 98% (97% to 99%) |
| 10% | 56a | 5 | 44a | 896 | 92% (82% to 97%)a | 95% (94% to 97%)a |
| 20% | 112a | 4 | 88a | 796 | 97% (91% to 99%)a | 90% (88% to 92%)a |
| Rapid molecular tests | ||||||
| Evaluations (studies) | Samples | Confirmed SARS‐CoV‐2 samples |
Average sensitivity (95% CI) [Range] |
Average specificity (95% CI) [Range] |
||
| 13 (11) | 2255 | 1179 | 95.2 (86.7 to 98.3) [68% to 100%] |
98.9 (97.3 to 99.5) [92% to 100%] |
||
| Average sensitivity and specificity applied to a hypothetical cohort of 1000 patients | ||||||
| Prevalence of COVID‐19 | TP | FP | FN | TN | PPVb(95% CI) | NPVc(95% CI) |
| 5% | 48 | 10 | 2 | 940 | 83% (71% to 91%) | 100% (99% to 100%) |
| 10% | 95 | 10 | 5 | 890 | 90% (83% to 95%) | 99% (99% to 100%) |
| 20% | 190 | 9 | 10 | 791 | 95% (92% to 98%) | 99% (98% to 99%) |
| Pooled results for individual tests | ||||||
| Tests | Evaluations | Samples |
SARS‐CoV‐2 cases |
Sensitivity (95% CI) | Specificity (95% CI) | |
| Shenzhen Bioeasy Ag assay | 2 | 238 | 162 | 89.5 (83.7 to 93.8) | 100 (95.3 to 100) | |
| ID NOW | 5 | 1003 | 496 | 76.8 (72.9 to 80.3) | 99.6 (98.4 to 99.9) | |
| Xpert Xpress | 6 | 919 | 479 | 99.4 (98.0 to 99.8) | 96.8 (90.6 to 99.0) | |
| Average sensitivity and specificity applied to a hypothetical cohort of 1000 patients where 100 have COVID‐19 infection (10% prevalence) | ||||||
| Tests | TP | FP | FN | TN | PPVb(95% CI) | NPVc(95% CI) |
| Shenzhen Bioeasy Ag assay | 90 | 0 | 11 | 900 | 100% (96% to 100%) | 99% (98% to 99%) |
| ID NOW | 77 | 4 | 23 | 896 | 96% (89% to 99%) | 97% (96% to 98%) |
| Xpert Xpress | 99 | 29 | 1 | 871 | 77% (69% to 84%) | 100% (99% to 100%) |
| Ag: antigen;CI: confidence interval; FN: false negative; FP: false positive;NPV: negative predictive value; PPV: positive predictive value; RT‐PCR: reverse transcription polymerase chain reaction; TN: true negative; TP: true positive | ||||||
aAs there is high heterogeneity in the estimates of sensitivity, the values observed in practice could vary considerably from these figures. bPPV (positive predictive value) defined as the percentage of positive rapid test results that are truly positive according to the reference standard diagnosis. cNPV (negative predictive value) defined as the percentage of negative rapid test results that are truly negative according to the reference standard diagnosis.
Background
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and the resulting COVID‐19 pandemic present important diagnostic evaluation challenges. These range from: understanding the value of signs and symptoms in predicting possible infection; assessing whether existing biochemical and imaging tests can identify infection or people needing critical care; and evaluating whether new biomarker tests can accurately identify current infection, rule out infection, identify people in need of care escalation, or test for past infection and immunity.
We are creating and maintaining a suite of living systematic reviews to cover the roles of tests and patient characteristics in the diagnosis of COVID‐19. This review summarises evidence for the accuracy of rapid antigen and molecular tests, suitable for use at the point of care, as alternatives to standard laboratory‐based reverse transcription polymerase chain reaction (RT‐PCR), that are relied on for identifying current infection. If sufficiently accurate, point‐of‐care tests may have a greater impact on public health than RT‐PCR as they do not require the same technical expertise and laboratory capacity. These tests can be undertaken locally, avoiding the need for centralised testing facilities that rarely meet the needs of patients, caregivers, health workers and society as a whole, especially in low‐ and middle‐income countries. As these are rapid tests, their results can be returned within the same clinical encounter, facilitating timely decisions concerning the need for isolation.
Target condition being diagnosed
COVID‐19 is the disease caused by infection with the SARS‐CoV‐2 virus. The key target conditions for this suite of reviews are current SARS‐CoV‐2 infection, current COVID‐19 disease, and past SARS‐CoV‐2 infection. The tests included in this review concern the identification of current infection.
For current infection, the severity of the disease is of importance. SARS‐CoV‐2 infection can be asymptomatic (no symptoms); mild or moderate (symptoms such as fever, cough, aches, lethargy but without difficulty breathing at rest); severe (symptoms with breathlessness and increased respiratory rate indicative of pneumonia); or critical (requiring respiratory support due to severe acute respiratory syndrome (SARS) or acute respiratory distress syndrome (ARDS). People with COVID‐19 pneumonia (severe or critical disease) require different patient management, and it is important to be able to identify them. Viral load may also be an indicator of disease severity (Zheng 2020), and whilst the accuracy of antigen and molecular tests have the potential to be affected by participant viral load, the main aim of rapid testing is not to establish viral load. In this review, we therefore consider the role of point‐of‐care tests for detecting SARS‐CoV‐2 infection of any severity.
Index test(s)
The primary consideration for the eligibility of tests for inclusion in this review is that they should detect current infection and should have the capacity to be performed at the ‘point of care’ or in a ‘near‐patient’ testing role. There is an ongoing debate around the specific use and definitions of these terms, therefore for the purposes of this review, we consider ‘point‐of‐care’ and ‘near patient’ to be synonymous, but for consistency and avoidance of confusion, we use the term ‘point‐of‐care’ throughout.
We have adapted a definition of point‐of‐care testing, namely that it “refers to decentralized testing that is performed by a minimally trained healthcare professional near a patient and outside of central laboratory testing” (WHO 2018), with the additional caveat that test results must be available within a single clinical encounter (Pai 2012). The key criteria for test inclusion are therefore:
the equipment for running and or reading the assay must be portable or easily transported, although mains power may be required;
minimal sample preparation requirements, for example, single‐step mixing, with no requirement for additional equipment or precise sample volume transfer unless a disposable automatic fill or graduated transfer device is used;
minimal biosafety requirements, for example, personal protective equipment (PPE) for sample collector and test operator, good ventilation and a biohazard bag for waste disposal;
no requirement for a temperature‐controlled environment; and
test results available within two hours of sample collection.
Tests for detection of current infection that are currently suitable for use at the point of care include antigen tests and molecular‐based tests. Both types of test use the same respiratory‐tract samples acquired by swabbing, washing or aspiration as for laboratory‐based RT‐PCR. Rapid antigen tests use lateral flow immunoassays, which are disposable devices, usually in the form of plastic cassettes akin to a pregnancy test. Viral antigen is captured by dedicated antibodies that are either colloidal gold‐ or fluorescent‐labelled. Antigen detection is indicated by visible lines appearing on the test strip (colloidal gold‐based immunoassays, or CGIA), or through fluorescence, which can be detected using an immunofluorescence analyser (fluorescence immunoassays or FIA). Molecular‐based tests to detect viral ribonucleic acid (RNA) have historically been laboratory‐based assays using RT‐PCR technology (see Alternative test(s)). In recent years, automated, single‐step RT‐PCR methods have been developed, as well as other nucleic acid amplification methods, such as isothermal amplification, that do not require the sophisticated thermo cycling involved in RT‐PCR (Carter 2020). These technological advances have allowed molecular technologies to be developed that are suitable for use in a point‐of‐care context (Kozel 2017).
Following the emergence of COVID‐19 there has been prolific industry activity to develop accurate tests. The Foundation for Innovative Diagnostics (FIND) and Johns Hopkins Centre for Health Security have maintained online lists of these and other molecular‐based tests for SARS‐CoV‐2 (FIND 2020). At the time of writing (19 July 2020), FIND listed 48 rapid antigen tests, 32 of which are described as "commercialized" and 21 have been identified as having regulatory approval. A total of 113 molecular tests were described as automated, including both laboratory‐based assays and assays suitable for use outside of a laboratory setting (i.e. near or at the point of care). Further information from FIND indicates that 47 of the 113 assays were categorised as point‐of‐care or near point‐of‐care tests, including 26 with regulatory approval. This classification was based on the information provided to FIND by the test manufacturers and does not necessarily mean that these tests meet the criteria for point‐of‐care tests that we have specified for this review. The numbers of tests of these types will increase over time.
Clinical pathway
Patients may be tested for infection when they present with symptoms, or have had known exposure to COVID‐19, or during screening for COVID‐19. The standard approach to diagnosis of COVID‐19 infection is through laboratory‐based testing of swab samples taken from the upper respiratory (e.g. nasopharynx, oropharynx) or lower respiratory tract (e.g. bronchoalveolar lavage or sputum) with RT‐PCR. RT‐PCR is the primary method for detecting infection during the acute phase of the illness while the virus is still present (whether people are symptomatic or asymptomatic), but can give false negative results (Arevalo‐Rodriguez 2020). Both the World Health Organiation (WHO) and the China CDC (National Health Commission of the People's Republic of China), have produced case definitions for COVID‐19 that include the presence of convincing clinical evidence when RT‐PCR is negative (Appendix 1). The most recent case definition from the China CDC also includes positive serology tests.
Prior test(s)
Signs and symptoms are used in the initial diagnosis of suspected COVID‐19 infection and to help identify those who require a test for RT‐PCR. A number of key symptoms have been associated with mild to moderate COVID‐19, including: troublesome dry cough (for example, coughing more than usual over a one‐hour period, or three or more coughing episodes in 24 hours), fever greater than 37.8 °C, diarrhoea, headache, breathlessness on light exertion, muscle pain, fatigue, and loss of sense of smell and taste. However, the recently published review of signs and symptoms found good evidence for the accuracy for these symptoms alone or in combination to be lacking (Struyf 2020).
Where people are asymptomatic but are being tested on the basis of epidemiological risk factors, such as exposure to someone with confirmed SARS‐CoV‐2, no prior tests will have been conducted.
Role of index test(s)
For most settings in which testing for acute SARS‐CoV‐2 infection takes place, results of laboratory‐based RT‐PCR tests are unlikely to be available within a single clinical encounter. Point‐of‐care tests potentially have a role either as a replacement for RT‐PCR (if sufficiently accurate), or as a means of triaging and rapid management (quarantine or treatment, or both), with confirmatory RT‐PCR testing for negative results. Obtaining quick results within a healthcare visit will allow more appropriate decisions about isolation and healthcare interventions. If accurate, tests may also be considered for screening at‐risk populations, for example in airport settings or in local outbreaks.
Alternative test(s)
This review is one of seven planned reviews that cover the range of tests and characteristics being considered in the management of COVID‐19 (Deeks 2020; McInnes 2020). Full details of the alternative tests and evidence of their accuracy will be summarised in these reviews. Tests that might be considered as alternatives to point‐of‐care tests are considered here.
Laboratory‐based molecular tests
RT‐PCR tests for SARS‐CoV‐2 identify viral ribonucleic acid (RNA). Reagents for RT‐PCR were rapidly produced once the viral RNA sequence was published (Corman 2020). Testing is undertaken in central laboratories and can be very labour‐intensive, with several points along the path of performing a single test where errors may occur, although some automation of parts of the process is possible. The amplification process requires thermal cycling equipment to allow multiple temperature changes within a cycle, with cycles repeated up to 40 times until viral DNA is detected (Carter 2020). Although the amplification process for RT‐PCR can be completed in a relatively short timeframe, the stages of extraction, sample processing and data management (including reporting) mean that test results are typically only available in 24 to 48 hours. Where testing is undertaken in a centralised laboratory, transport times increase this further. The time to result for fully automated RT‐PCR assays is shorter than for manual RT‐PCR, however most assays still require sample preparation steps that make them unsuitable for use at the point of care. Other nucleic acid amplification methods, including loop‐mediated isothermal amplification (LAMP), or CRISPR‐based nucleic acid detection methods, that allow amplification at a constant temperature are also being developed (Carter 2020). These methods have the potential to reduce the time to produce test results after extraction and sample processing to minutes, but the time for the whole process may still be significant. Laboratory‐based molecular tests are most often applied to upper and lower respiratory samples although they are also being used on faecal and urine samples.
Antibody tests
Serology tests to measure antibodies to SARS‐CoV‐2 have been evaluated in people with active infection and in convalescent cases (Deeks 2020a). Antibodies are formed by the body's immune system in response to infections, and can be detected in whole blood, plasma or serum. Antibody tests are available for laboratory use including enzyme‐linked immunosorbent assay (ELISA) methods, or more advanced chemiluminescence immunoassays (CLIA). There are also rapid lateral flow assays (LFA)s for antibody testing that use a minimal amount of whole blood, plasma or serum on a testing strip as opposed to the respiratory specimens that are used for rapid antigen tests; all assays for antibody detection are considered in Deeks 2020a.
Rationale
It is essential to understand the clinical accuracy of tests and diagnostic features to identify the best way they can be used in different settings to develop effective diagnostic and management pathways. The suite of Cochrane 'living systematic reviews' summarises evidence on the clinical accuracy of different tests and diagnostic features, grouped according to the research questions and settings that we are aware of. Estimates of accuracy from these reviews will help inform diagnosis, screening, isolation, and patient‐management decisions.
As the COVID‐19 pandemic progresses, earlier, fast and reliable detection of active SARS‐CoV‐2 infection is key to reducing community transmission. New biomarker tests are being developed and evidence is accumulating at an unprecedented rate. Point‐of‐care testing provides a potentially attractive route to increasing testing rates; however their potential to have an impact on patient care and help reduce transmission depends not only on the time it takes to report the test result, but on test performance and frequency of testing. We are aware of two other reviews on this topic (Green 2020; Subsoontorn 2020). One rapid review of point‐of‐care tests relied on performance data from manufacturers’ instructions for use documents (Green 2020). A systematic review of nucleic acid amplification ‘point‐of‐care tests’ selected studies for inclusion based on the use of isothermal techniques (i.e. not requiring thermal cycling), with apparently no consideration for the feasibility of deploying the tests in a point‐of‐care environment (Subsoontorn 2020). A comprehensive systematic review of the clinical performance of tests suitable for use at the point of care is therefore urgently needed. We will update this review as often as is feasible to ensure that it provides current evidence about the accuracy of point‐of‐care tests.
Please note, this review follows a generic protocol that covers six of the seven Cochrane COVID‐19 DTA reviews (Deeks 2020). The Background and Methods sections of this review therefore use some text that was originally published in the protocol (Deeks 2020), and text that overlaps some of our other reviews (Deeks 2020a; Struyf 2020).
Objectives
To assess the diagnostic accuracy of rapid point‐of‐care antigen and molecular‐based tests to determine if a person presenting in the community or in primary or secondary care has current SARS‐CoV‐2 infection.
Secondary objectives
Where data are available, we will investigate potential sources of heterogeneity that may influence diagnostic accuracy (either by stratified analysis or meta‐regression) according to index test, participant characteristics (length and severity of symptoms, and viral load), study setting, study design and reference standard used.
Methods
Criteria for considering studies for this review
Types of studies
We applied broad eligibility criteria in order to include all patient groups (that is, if patient population was unclear, we included the study) and all variations of a test.
We included studies of all designs that produce estimates of test accuracy or provide data from which we can compute estimates, including the following.
Studies restricted to participants confirmed to either have (or to have had) the target condition (to estimate sensitivity) or confirmed not to have (or have had) the target condition (to estimate specificity). These types of studies may be excluded in later review updates.
Single‐group studies, which recruit participants before disease status has been ascertained.
Multi‐group studies, where people with and without the target condition are recruited separately (often referred to as two‐gate or diagnostic case‐control studies).
Studies based on either patients or samples.
We excluded studies from which we could not extract data to compute either sensitivity or specificity.
We carefully considered the limitations of different study designs in the quality assessment and analyses.
We included studies reported in published articles and as preprints.
Participants
We included studies recruiting people presenting with suspicion of current SARS‐CoV‐2 infection or those recruiting populations where tests were used to screen for disease (for example, contact tracing or community screening).
We also included studies that recruited people known to have SARS‐CoV‐2 infection and known not to have SARS‐CoV‐2 infection (i.e. cases only or multi‐group studies).
We excluded small studies with fewer than 10 samples or participants. Although the size threshold of 10 is arbitrary, such small studies are likely to give unreliable estimates of sensitivity or specificity and may be biased.
Index tests
We included studies evaluating any rapid antigen or molecular‐based test for diagnosis of SARS‐CoV‐2, if it met the criteria outlined in the Background, that is, requiring minimal equipment, sample preparation, and biosafety considerations, with results available within two hours of sample collection.
Target conditions
The target condition was current SARS‐CoV‐2 infection (either symptomatic or asymptomatic). We also refer to SARS‐CoV‐2 infection as ‘COVID‐19 infection’.
Reference standards
We anticipated that studies would use a range of reference standards to define both the presence and absence of SARS‐CoV‐2 infection but were unclear at the start of the review exactly what methods we would encounter. For the QUADAS‐2 (Quality Assessment tool for Diagnostic Accuracy Studies; Whiting 2011), assessment we categorised each method of defining the presence of SARS‐CoV‐2 according to the risk of bias (the chances that it would misclassify the presence or absence of infection) and whether it defined COVID‐19 in an appropriate way that reflected cases encountered in practice. Likewise, we considered the risk of bias in definitions of the absence of SARS‐CoV‐2, and whether the definition included all those who would be tested in practice.
Evaluations of molecular tests generally consider agreement between molecular assays, for example, agreement of a new rapid test against a more standard RT‐PCR test. For the purposes of this review, we considered RT‐PCR to be the ‘reference standard’ against which the rapid tests were compared, and present results as ‘sensitivity’ and ’specificity’ as opposed to percentage agreement. The result of further RT‐PCR analysis of discrepant cells (samples with results disagreeing on the rapid test and the RT‐PCR) were also considered in sensitivity analyses. As discrepant analysis involves retesting only a subsample of patients selected according to index and reference standard results, it can introduce bias (Hadgu 1999). Retesting of all samples with a second test in a composite reference standard would be preferable when there are concerns over the accuracy of the first reference test.
Search methods for identification of studies
Electronic searches
We conducted a single literature search to cover our suite of Cochrane COVID‐19 diagnostic test accuracy (DTA) reviews (Deeks 2020; McInnes 2020).
We conducted electronic searches using two primary sources. Both of these searches aimed to identify all published articles and preprints related to COVID‐19, and were not restricted to those evaluating biomarkers or tests. Thus, there are no test terms, diagnosis terms, or methodological terms in the searches. Searches were limited to 2019 and 2020, and for this version of the review have been conducted to 25 May 2020.
Cochrane COVID‐19 Study Register searches
We used the Cochrane COVID‐19 Study Register (covid-19.cochrane.org/), for searches conducted from inception of the Register to 28 March 2020. At that time, the register was populated by searches of PubMed, as well as trials registers at ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
Search strategies were designed for maximum sensitivity, to retrieve all human studies on COVID‐19 and with no language limits. See Appendix 2.
COVID‐19 Living Evidence Database from the University of Bern
From 28 March 2020, we used the COVID‐19 Living Evidence database from the Institute of Social and Preventive Medicine (ISPM) at the University of Bern (www.ispm.unibe.ch), as the primary source of records for the Cochrane COVID‐19 DTA reviews. This search includes PubMed, Embase, and preprints indexed in bioRxiv and medRxiv databases. The strategies as described on the ISPM website are described here (ispmbern.github.io/covid-19/). See Appendix 3. To ensure comprehensive coverage we also downloaded records from the ‘Bern feed’ from 1 January to 28 March 2020 and de‐duplicated them against those obtained via the Cochrane COVID‐19 Study Register.
The decision to focus primarily on the Bern feed was because of the exceptionally large numbers of COVID‐19 studies available only as preprints. The Cochrane COVID‐19 Study Register has undergone a number of iterations since the end of March and we anticipate moving back to the Register as the primary source of records for subsequent review updates.
Searching other resources
We identified Embase records through the Centers for Disease Control and Prevention (CDC), Stephen B Thacker CDC Library, COVID‐19 Research Articles Downloadable Database (www.cdc.gov/library/researchguides/2019novelcoronavirus/researcharticles.html), and de‐duplicated them against the Cochrane COVID‐19 Study Register up to 28 March 2020. See Appendix 4.
We also checked our search results against two additional repositories of COVID‐19 publications including:
the Evidence for Policy and Practice Information and Co‐ordinating Centre (EPPI‐Centre) 'COVID‐19: Living map of the evidence' (eppi.ioe.ac.uk/COVID19_MAP/covid_map_v4.html);
the Norwegian Institute of Public Health 'NIPH systematic and living map on COVID‐19 evidence' (www.nornesk.no/forskningskart/NIPH_diagnosisMap.html)
Both of these repositories allow their contents to be filtered according to studies potentially relating to diagnosis, and both have agreed to provide us with updates of new diagnosis studies added. For this iteration of the review, we examined all diagnosis studies from either source up to 25 May 2020.
We appeal to researchers to supply details of additional published or unpublished studies at the following email address, which we will consider for inclusion in future updates (coviddta@contacts.bham.ac.uk).
Data collection and analysis
Selection of studies
A team of experienced systematic review authors from the University of Birmingham screened the titles and abstracts of all records retrieved from the literature searches. Two review authors independently screened studies in Covidence. A third, senior review author resolved any disagreements. We tagged all records selected as potentially eligible according to the Cochrane COVID‐19 DTA review(s) that they might be eligible for and we then exported them to separate Covidence reviews for each review title.
We obtained the full texts for all studies flagged as potentially eligible. Two review authors independently screened the full texts for one of the COVID‐19 biomarker reviews (molecular, antigen or antibody tests). We resolved any disagreements on study inclusion through discussion with a third review author.
Data extraction and management
One review author extracted the characteristics of each study, which a second review author checked. Items that we extracted are listed in Appendix 5. In addition, we coded tests according to complexity, regardless of the nature of the test (antigen or molecular test), as follows:
low: one sample preparation step and up to two test steps;
moderate: two sample preparation steps and up to three test steps;
high: more than two sample preparation steps and more than three test steps.
Two review authors independently carried out this classification, with referral to a third review author if necessary.
Both review authors independently performed data extraction of 2x2 contingency tables of the number of true positives, false positives, false negatives and true negatives. They resolved disagreements by discussion. Where possible, we separately extracted data according to viral load, and for molecular assays, before and after re‐analysis of samples in discrepant cells.
We encourage study authors to contact us regarding missing details on the included studies (coviddta@contacts.bham.ac.uk).
Assessment of methodological quality
Two review authors independently assessed risk of bias and applicability concerns using the QUADAS‐2 checklist tailored to this review (Appendix 6; Whiting 2011). The two review authors resolved any disagreements by discussion.
Ideally, studies should prospectively recruit a representative sample of participants presenting with signs and symptoms of COVID‐19, either in community or primary care settings or to a hospital setting, and they should clearly record the time of testing after the onset of symptoms. Studies in asymptomatic people at risk of infection should document time from exposure. Studies should perform tests in their intended use setting, using appropriate samples with or without viral transport medium and within the time period following specimen collection as indicated in the 'instructions for use' document. Tests should be performed by relevant personnel (e.g. healthcare workers), and should be interpreted blinded to the final diagnosis (presence or absence of SARS‐CoV‐2). The reference standard diagnosis should be blinded to the result of the rapid test, and should not incorporate the result of the index test. We did not consider a comparison of a rapid molecular‐based test against an RT‐PCR assay to be at risk of incorporation bias. If the reference standard includes clinical diagnosis of COVID‐19 for RT‐PCR‐negative patients, then established criteria should be used. Studies including samples from participants known not to have COVID‐19 should use pre‐pandemic sources or contemporaneous samples with at least one RT‐PCR‐negative test result. Data should be reported for all study participants, including those where the result of the rapid test was inconclusive, or participants in whom the final diagnosis of COVID‐19 was uncertain. Studies should report whether results relate to participants (one sample per participant), or samples (multiple samples per participant).
Statistical analysis and data synthesis
We analysed rapid antigen and molecular tests separately. If studies evaluated multiple tests in the same samples, we included them multiple times. We present estimates of sensitivity and specificity for each test brand using paired forest plots, and summarise results using average sensitivity and specificity in tables as appropriate. There were only sufficient studies to make formal comparisons (based on between‐study comparisons) for studies using two brands of molecular tests (ID NOW (Abbott Laboratories) and Xpert Xpress (Cepheid Inc)).
We estimated summary sensitivities and specificities with 95% confidence intervals (CI) using the bivariate model (Reitsma 2005), via the meqrlogit command of Stata/SE 16.0. When few studies were available, we simplified models by first assuming no correlation between sensitivity and specificity estimates and secondly by setting near‐zero variance estimates of the random effects to zero (Takwoingi 2017). In cases where there was only one study per test, we reported individual sensitivities and specificities with 95% CI constructed using the binomial exact method.
Where studies presented only estimates of sensitivity, we fitted univariate random effects logistic regression models. In a small number of instances where a model failed to converge (usually when there were very small numbers of studies or the sensitivity/specificity estimates were all very high), we computed estimates and CI by summing the counts of TP, FP, FN and TN across 2x2 tables. These analyses are clearly marked in the tables. We present all estimates with 95% confidence intervals.
Investigations of heterogeneity
We examined heterogeneity between studies by visually inspecting the forest plots of sensitivity and specificity. Where adequate data were available, we investigated heterogeneity related to viral load, test brand, and sample type by including indicator variables in the random‐effects logistic regression models. Absolute differences between the sensitivity or specificity and the P values were reported from the model. In instances where only one study was available per test or when tests were being directly compared following summing of counts of the 2x2 tables, we performed test comparison using the two‐sample test of proportions.
Sensitivity analyses
We performed three sensitivity analyses. First, estimation of sensitivity for molecular tests was made with and without studies that only evaluated samples with RT‐PCR‐confirmed SARS‐CoV‐2 (and thus did not estimate specificity). Secondly, comparisons were made between analyses using the primary reference standard and analyses using results adjusted after sample retesting with a second RT‐PCR test, either for discrepant cells (discrepant analysis) or for all samples. Thirdly, we restricted our analysis comparing ID NOW (Abbott Laboratories) and Xpert Xpress (Cepheid Inc) to studies that compared the tests in the same samples.
Assessment of reporting bias
We made no formal assessment of reporting bias.
Summary of findings
We summarised key findings in a 'Summary of findings' table indicating the strength of evidence for each test and findings, and highlighted important gaps in the evidence.
Updating
We are aware of additional studies published since the search date of 25 May 2020 and plan to update this review imminently. We have already completed searches for the update up until 22 June 2020, and screening of those is ongoing.
Results
Results of the search
We screened 19,092 unique records (published or preprints) for inclusion in the complete suite of reviews to assist in the diagnosis of COVID‐19 (Deeks 2020; McInnes 2020). Of 808 records selected for further assessment for inclusion in any of the four molecular, antigen or antibody test reviews, we assessed 90 full‐text reports for inclusion in this review. See Figure 1 for the PRISMA flow diagram of search and eligibility results (McInnes 2018; Moher 2009). We included 18 studies from 22 reports in this review, and we excluded 68 publications that did not meet our inclusion criteria. Exclusions were mainly because of index tests not meeting our criteria for use at the point of care (n = 36) or ineligible study designs (n = 21). The reasons for exclusion of all 68 publications are provided in Characteristics of excluded studies.
1.
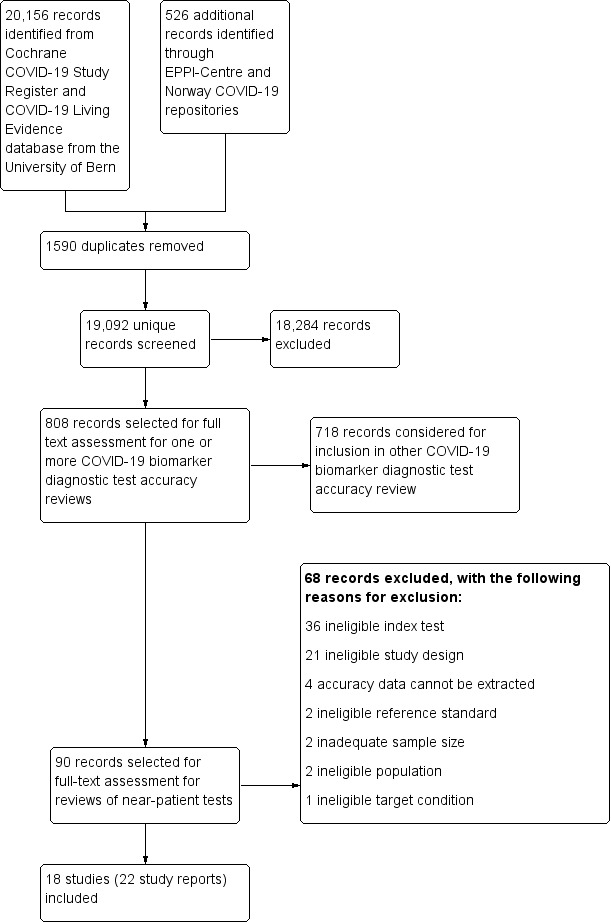
Study flow diagram
We contacted the authors of three included studies for further information (Diao 2020; Porte 2020; Weitzel 2020 [A]), and received replies and the requested information in regard to all three.
The 22 included study reports relate to 18 separate studies, four studies having both preprints and subsequent journal publications (Broder 2020; Mertens 2020; Porte 2020; Smithgall 2020 [A]). Of the 18 studies, five are available only as preprints. (Please note when naming studies, we use the letters [A], [B], [C] etc. in square brackets to indicate data on different tests evaluated in the same study).
Description of included studies
The 18 studies include a total of 3198 unique samples, with 1775 samples with RT‐PCR‐confirmed SARS‐CoV‐2 (some samples were analysed by more than one index test). Five studies evaluated antigen tests (Diao 2020; Lambert‐Niclot 2020; Mertens 2020; Porte 2020; Weitzel 2020 [A]) and 13 studies evaluated molecular tests (Assennato 2020; Broder 2020; Harrington 2020; Hogan 2020; Lieberman 2020; Loeffelholz 2020; Mitchell 2020; Moore 2020; Moran 2020; Rhoads 2020; Smithgall 2020 [A]; Wolters 2020; Zhen 2020 [A]). Summary study characteristics are presented in Table 2 with further details of study design and index test details in Appendix 7 and Appendix 8. Full details are provided in the Characteristics of included studies table.
1. Description of studies.
| Antigen tests | Molecular tests | ||
| Participants | 5 | 13 | |
| Overall sample size | Median (IQR) | 112 (96 to 198) | |
| Range | 26 to 524 | ||
| Overall number of SARS‐CoV‐2 positive samples | Median (IQR) | 85 (50 to 119) | |
| Range | 13 to 220 | ||
| Sample size | Median (IQR) | 138 (127 to 239) | 103 (88 to 172) |
| Range | 111 to 328 | 26 to 524 | |
| Number of SARS‐CoV‐2 positive samples | Median (IQR) | 94 (82 to 132) | 58 (46 to 96) |
| Range | 80 to 208 | 13 to 220 | |
| Setting | Hospital A & E | 2 (40%) | 1 (8%) |
| Mixed | 0 (0%) | 3 (31%) | |
| Unclear | 3 (60%) | 9 (69%) | |
| Patient group | Acute (A&E presentation) | 2 (40%) | 1 (8%) |
| Unclear | 3 (60%) | 12 (92%) | |
| Study design | |||
| Recruitment structure | Single group ‐ sensitivity and specificity | 3 (60%) | 6 (46%) |
| Single group ‐ sensitivity only | 0 (0) | 2 (15%) | |
| Two or more groups ‐ sensitivity and specificity | 2 (40%) | 5 (38%) | |
| Reference standard for presence of SARS‐CoV‐2 | All RT‐PCR positive | 5 (100%) | 13 (100%) |
| Reference standard for absence of SARS‐CoV‐2 | COVID suspects (double RT‐PCR negative) | 1 (20%) | 0 (0%) |
| COVID suspects (single RT‐PCR negative) | 4 (80%) | 11 (85%) | |
| Not applicable | 0 (0) | 2 (15%) | |
| Tests | |||
| Number of tests per study | 1 | 4 (80%) | 11 (84.6%) |
| 2 | 0 (0) | 2 (15.4%) | |
| 4 | 1 (20%) | 0 (0) | |
| Test technology, antigen tests onlya | Colloidal‐gold immunoassay | 4 (50%) | N/A |
| Fluorescent immunoassay | 4 (50%) | N/A | |
| Sample type | Nasal only | 0 (0%) | 1 (8%) |
| Nasopharyngeal only | 3 (60%) | 6 (46%) | |
| Nasopharyngeal + oropharyngeal combined | 2 (40%) | 1 (8%) | |
| Nasopharyngeal or nasal | 0 (0) | 3 (23%) | |
| Nasopharyngeal or oropharyngeal | 0 (0) | 1 (8%) | |
| Mixed (3 or more types) | 0 (0) | 1 (8%) | |
| IQR: interquartile range; RT‐PCR: reverse transcriptase polymerase chain reaction | |||
aAs a % of antigen test evaluations (n = 8).
The median sample size of the included studies is 112 (interquartile range (IQR) 96 to 198) and median number of SARS‐CoV‐2 confirmed samples included is 85 (IQR 50 to 119). The majority of studies (10/18) were conducted in the USA, four in Europe, two in South America, one in China and one study included samples from more than one country.
Participant characteristics
Studies predominantly selected samples from those submitted to laboratories for routine RT‐PCR testing with limited detail of the participants providing the samples. Three studies included samples from participants in emergency department or urgent care settings, three included samples from participants presenting in mixed settings (inpatient, outpatient or emergency department), and 12 did not report any details of setting in which study participants presented.
Four studies included samples from symptomatic patients, only one of which provided any information on the type of symptoms experienced and time from symptom onset (median 2 days; IQR 1 to 4; range 0 to 12; Porte 2020). Three additional studies provided basic demographic data such as age or gender, and the remaining 14 provided no information on participant characteristics.
All five studies evaluating antigen tests reported results for SARS‐CoV‐2‐confirmed samples with high and low viral load as defined by the cycle threshold (Ct) value from the reference standard. In one study (Diao 2020), the proportion with high viral load was 27% (cut‐off ≤ 30 Ct), and in the other four (using a cut‐off of ≤ 25 Ct) it ranged from 48% to 74% (Appendix 7). Four studies reporting five molecular assay evaluations, reported proportions with high viral load ranging from 33% (Mitchell 2020), to 60% (Smithgall 2020 [A]). All four studies defined high viral load as Ct of 30 or less. Ct values were missing for some samples in Porte 2020.
Study designs
We found it difficult to fully ascertain whether samples were included in studies with or without knowledge of whether patients did or did not have COVID‐19 infection. All studies defined the presence or absence of COVID‐19 infection based on RT‐PCR, with a single (n = 17) or two (n = 1) negative RT‐PCR results used to confirm the absence of infection. One study used paired nasopharyngeal swabs for RT‐PCR and nasal swabs for the index test (Harrington 2020); all other studies used the same respiratory sample for the RT‐PCR and for the index test.
Nine studies appeared to include series of samples submitted for laboratory testing regardless of the RT‐PCR result, but only Harrington 2020 reported including consecutive samples, and only Mertens 2020 randomly selected samples. The number of samples in these single‐group studies ranged from 26 to 524 with between 13 and 208 samples with confirmed SARS‐CoV‐2 (median prevalence 50%; IQR 41% to 68%).
Seven studies described deliberate separate sampling of RT‐PCR‐positive and RT‐PCR‐negative samples, for example, to ‘enrich’ for positive samples, to reach a stated ratio of positive to negative samples, or to represent a range of Ct values on RT‐PCR. We designated these studies as two‐group studies. Sample sizes of these studies ranged from 88 to 481 with between 57 and 220 samples with confirmed SARS‐CoV‐2 (median prevalence 60%; IQR 46% to 66%).
Two studies included only samples with confirmed SARS‐CoV‐2, thus only allowing estimation of sensitivity; 35 samples in Broder 2020, and 96 in Rhoads 2020.
Index tests
Fifteen studies evaluated only one test, three compared two or more tests using the same samples (two with two tests each, and one with four tests). In total the 18 studies reported on a total of 23 test evaluations. Appendix 9 provides details extracted from the manufacturer’s instructions for use documents for all included tests.
Antigen tests
Five studies reported eight evaluations of antigen tests (4 CGIA and 4 FIA), seven of which evaluated one of five commercially produced tests (produced by Beijing Savant, Shenzhen Bioeasy, Coris BioConcept, Liming Bio‐Products and RapiGEN Inc.) and one classified as using an in‐house CGIA method (full identification details for all tests is provided in Appendix 8). Contact with the study author indicates that this study reports the development of the Shenzhen Bioeasy assay (Diao 2020), but it is not clear whether the commercially available assay is identical to the one reported in the study or whether it has undergone further refinement. Only two studies provided product codes for the tests evaluated (Porte 2020; Weitzel 2020 [A];Appendix 8). The Beijing Savant, Coris BioConcept, Shenzhen Bioeasy and in‐house assays all target the nucleocapsid protein; this information was not reported for the Liming Bio‐Products and RapiGEN Inc.assays (Appendix 8). We have not been able to identify any information for either the Beijing Savant or Liming Bio‐Products assays online.
Two of the five studies used only nasopharyngeal swab samples, two used both nasopharyngeal and oropharyngeal swab samples from all patients (Porte 2020; Weitzel 2020 [A]), and one study (Mertens 2020), used mixed swab samples including nasopharyngeal swabs, nasopharyngeal aspirate and bronchoalveolar lavage. All studies used samples either in viral transport medium (n = 4) or in saline solution (n = 1; Diao 2020). The Coris BioConcept assay, evaluated in two studies (Lambert‐Niclot 2020; Mertens 2020), is the only one to document instructions for use for swabs in viral transport medium (VTM); the use of VTM is not mentioned in the instructions for use documents for any of the other assays (Appendix 9). Samples were tested "soon" after collection in Lambert‐Niclot 2020, after a defined period of refrigerated storage in Porte 2020 or frozen storage in Weitzel 2020 [A]; two studies did not report sample storage and timing of testing.
Molecular tests
Thirteen studies reported 15 evaluations of four different commercially available rapid molecular tests: six evaluating ID NOW (Abbott Laboratories), seven evaluating Xpert Xpress (Cepheid Inc), and one evaluation each of Accula (Mesa Biotech Inc.) and SAMBA II (Diagnostics for the Real World). None of the studies reported product codes for the tests evaluated (Appendix 8). One study of Xpert Xpress used the 'research use only' (RUO) version of the test, but reported that the RUO version contains the same reagents as the 'emergency use authorisation' (EUA) version. The RUO test allows the user to view the amplification curves for the RdRp gene as well as for the E‐gene and N2 targets whereas the EUA version restricts the amplification curves to E and N2 only. ID NOW and SAMBA‐II use isothermal techniques, Xpert Xpress is based on RT‐PCR, and Accula is described as a PCR plus LFA.
In the 13 studies, seven used only nasopharyngeal (n = 6) or nasal (n = 1) swab samples, one used both nasopharyngeal and oropharyngeal swab samples from all patients, and the remaining five evaluations used mixed swab samples including nasopharyngeal or nasal swabs (n = 3), nasopharyngeal or oropharyngeal swabs (n = 1), or multiple sample types including tracheal aspirate (n = 1). One study reported direct swab testing (Harrington 2020), 10 used either swabs in viral transport medium (n = 5), viral transport medium or saline (n = 4), or viral transport medium or gelatin‐lactalbumin‐yeast (GLY) medium (n = 1), and two did not report whether any transport medium was used. Five of 13 studies reported testing immediately (n = 1), or within 48 (n = 1) or 72 hours (n = 3) of sample collection. Four studies reported testing after a period of frozen storage, and four did not describe sample storage or timing of testing at all. Two of the four manufacturers document instructions for use for samples in transport medium (for the Xpert Xpress and SAMBA II assays) and two explicitly recommend against the use of viral transport medium (ID NOW and Accula), although at the time of the test evaluations, some viral transport media were documented as acceptable for ID NOW. Although immediate sample testing is preferred, all manufacturers document acceptable period of refrigerated storage of between 24 hours (ID NOW) and seven days (Xpert Xpress). See Appendix 9.
Across the 23 test evaluations of antigen or molecular tests, only one reported testing outside of a centralised laboratory setting, where direct swab testing (using ID NOW (Abbott Laboratories)) was carried out by on‐site medical personnel or laboratory personnel at local laboratories (Harrington 2020).
Our own assessment of test complexity across test types classified SAMBA II as high complexity (more than two sample preparation steps and more than three test steps), Shenzhen Bioeasy FIA, ID NOW and Accula as moderate complexity and the other antigen tests and Xpert Xpress as low complexity (one sample preparation step and up to two test steps).
Methodological quality of included studies
We report the overall methodological quality assessed using the QUADAS‐2 tool for all included studies (n = 18) in Figure 2 (Whiting 2011). See Appendix 10 for a plot of study‐level ratings by quality.
2.
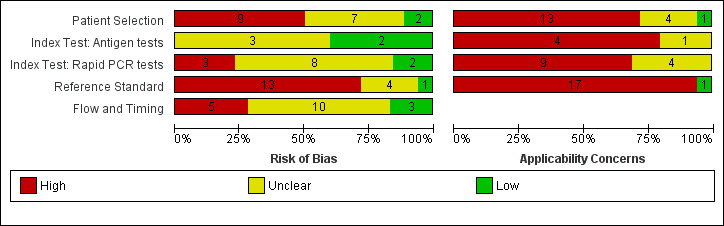
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies. Numbers in the bars indicate the number of studies
We considered the risk of bias in the individual studies and whether the results were likely to be applicable to standard use of the tests. We did not judge any study at low risk of bias and we had concerns about the applicability of results in all studies. We considered risk of bias to be high in nine (50%) studies because of how they selected samples and in 13 (72%) because they considered that one negative RT‐PCR was sufficient to confirm the absence of COVID‐19 infection. Lack of details in reporting meant we could not clearly assess whether there was a risk of bias through performance of the index test in 11 (61%) studies, or from the way in which the study was undertaken and analysed in 10 (56%). We judged that there were high concerns about the applicability of the evidence related to participants in 13 (72%) studies, to the index test in 13 (72%) studies and to the reference standard in 17 (94%) studies. We did not observe differences in methodological quality between antigen and molecular test evaluations. Explanations of how we reached these judgements are given below and in the Characteristics of included studies table.
Participant selection
We judged only two studies to be at low risk of bias, and in seven (39%) the risk was unclear because of poor reporting. The remaining 50% (9/18) we judged to be at high risk of bias because of deliberate sampling of participants based on the reference standard result; two of which also only included samples with confirmed COVID‐19 infection. We were not able to judge the appropriateness of study exclusions (16/18) or inclusions (11/18) where selection was based on the availability of laboratory samples with no participant eligibility criteria specified. Numbers per group are not mutually exclusive.
We had high concerns about the applicability of the selected participants in 13/18 studies (72%), meaning that the participants who were recruited were unlikely to be similar to those in whom the test would be used in clinical practice. This was largely because of the use of deliberate sampling; and sample inclusion based on the availability of residual and sometimes frozen samples, created unrepresentative participant samples. We judged only one study recruiting participants presenting to urgent care or emergency departments as likely to have selected an appropriate patient group.
Index tests
Figure 2 demonstrates similar patterns in risk of bias and applicability of the index test for studies of both antigen and rapid molecular tests. We observed low risk of bias in four studies that clearly described interpretation of the index test blinded to results of the reference standard, and used prespecified test thresholds. There was high risk of bias in three studies because the manufacturer’s prespecified threshold for the Xpert Xpress test (re‐testing of samples with presumptive positive results) was not followed. The risk of bias was unclear in 11 studies because we could not judge whether interpretation of the index test was undertaken with knowledge of whether individuals did or did not have COVID‐19 infection.
Thirteen studies did not carry out testing as it would occur in practice: four studies used trained, centralised laboratory staff and not local laboratory or healthcare personnel; one test could not be purchased (Diao 2020); and 11 because the test was not conducted within the manufacturer instructions for use (these categories are not mutually exclusive). Four studies tested samples in a viral transport medium that was not covered by the manufacturer instructions for use, five used frozen samples, one reported heat inactivation of samples prior to direct testing and two reported a testing timeframe beyond that recommended.
The remaining five studies provided inadequate information to make a judgement; three of them did conduct the test within the manufacturer instructions for use but none of them clearly described the setting for testing or personnel conducting the test.
Reference standards
Only one study used an appropriate reference standard to define the presence or absence of COVID‐19 infection (two negative PCR results required to confirm the absence of COVID‐19) and implemented it in ways that prevented bias (Diao 2020). One additional study reported two RT‐PCR results for all study participants (Moore 2020), and two did not include non‐COVID‐19 cases. We considered that the remaining 14 did not use an adequate reference standard, putting them at high risk of bias (Figure 2). Eight studies reported blinded RT‐PCR interpretation and 10 (56%) provided insufficient information about blinding of the reference standard to the index test to judge risk of bias.
RT‐PCR is unlikely to falsely classify participants as having COVID‐19 (low risk of false positive), but may miss true cases leading to false positives on the index test when a single RT‐PCR alone is used as a reference standard. Four studies (22%) used a second RT‐PCR test for samples with discrepant results (FP and FN) to address this. However, selective re‐testing could miss additional cases of COVID‐19 infection, and is likely to lead to distorted results. One study (Moore 2020), used a second RT‐PCR test in all samples and furthermore carried out a record review for all cases with discrepant results in order to verify whether participants were truly considered to have had COVID‐19 infection.
We judged 17 of the 18 studies to raise concerns for applicability (94%) because of defining the presence of COVID‐19 infection based on a single RT‐PCR‐positive result. These studies will have excluded individuals who are RT‐PCR‐negative but have exposure and clinical features that meet the case definitions for COVID‐19.
Flow and timing
Only three studies were at low risk of bias for participant flow and timing, one (Porte 2020), used a Standards of Reporting Diagnostic Accuracy Studies (STARD)‐style participant flow diagram and checklist (Bossuyt 2015), to fully report outcomes for all samples. Five studies were at high risk of bias because of exclusion of samples following invalid index test results (they did not carry out any retesting).
Unclear risk of bias was present in 10 (56%) studies because of lack of clarity around participant inclusion and exclusion from analyses. Six studies were unclear regarding whether the analysis was participant‐based or sample‐based (where there is a possibility of multiple samples per participant overstating the precision of estimates).
Conflicts of interest
In six studies all authors declared no conflicts of interest, although one study that evaluated an ‘in‐house’ test included a co‐author affiliated to a test manufacturing company. Eight studies did not provide a conflict of interest statement (one of these included co‐authors affiliated to the test manufacturer) and in the four remaining studies at least one author declared conflicts of interest in relation to the test.
Eleven studies provided no funding statement, five reported no funding sources to declare, and two reported one or more public funding sources. Two studies reported receipt of test kits or reagents ‘in kind’ from test manufacturers.
Findings
Of the 18 included studies, three reported evaluations of more than one test using the same samples (Table 2). In order to include all results from all tests in these analyses we have treated results from different tests of the same samples within a study as separate data points, such that data are available on 23 test evaluations (8 evaluations of antigen tests in 5 studies and 15 evaluations of rapid molecular tests in 13 studies). The results table (Table 3), identifies where estimates are based on multiple assessments of the same samples by including both the number of test evaluations and the number of studies. The numbers of true positives, false positives, and total samples with and without confirmed SARS‐CoV‐2 infection are based on test result counts.
2. Summary of analyses of test accuracy.
| Test |
Evaluations (studies) |
Samples | Cases | Average sensitivity, % (95% CI) | Average specificity, % (95% CI) |
| Antigen tests | |||||
| All | 8 (5) | 1180 | 762 | 56.2 (29.5 to 79.8) | 99.5 (98.1 to 99.9) |
| Subgroup analysis by viral load | |||||
| High viral load | 7 (5) | 400 | 400 | 93.2 (63.6 to 99.1) | N/A |
| Low viral load | 7 (5) | 341 | 341 | 32.6 (17.5 to 52.6) | N/A |
| Difference(95% CI) | −60.6 (−83.0 to −38.2), P < 0.001 | N/A | |||
| Subgroup analysis by testa | |||||
| Beijing Savant FIA | 1 | 109 | 78 | 16.7 (9.2 to 26.8) | 100 (88.8 to 100) |
| Coris Bioconcept CGIAb | 2 | 466 | 226 | 54.4 (47.9 to 60.8) | 99.6 (97.7 to 99.9) |
| Liming CGIA | 1 | 19 | 9 | 0 (0 to 33.6) | 90.0 (55.5 to 99.7) |
| RapiGEN CGIA | 1 | 109 | 79 | 62.0 (50.4 to 72.7) | 100 (88.4 to 100) |
| Shenzhen Bioeasy FIAb | 2 | 238 | 162 | 89.5 (83.8 to 93.3) | 100 (95.2 to 100) |
| In‐house FIA | 1 | 239 | 208 | 67.8 (61.0 to 74.1) | 100 (88.8 to 100) |
| Subgroup analysis by sample type | |||||
| Nasopharyngeal only | 3 | 705 | 434 | 59.4 (50.7 to 67.5) | 99.6 (97.4 to 99.9) |
| Molecular tests | |||||
| All studies with 2x2 data | 13 (11) | 2194 | 1113 | 95.2 (86.7 to 98.3) | 98.9 (97.3 to 99.5) |
| All studiesc,d | 15 (13) | 1244 | 1244 | 95.5 (88.5 to 98.4) | N/A |
| Sensitivity analysis before and after discrepant analysis | |||||
| Before | 4 | 1280 | 536 | 98.2 (87.0 to 99.8) | 97.8 (94.8 to 99.1) |
| After | 4 | 1280 | 547 | 99.5 (79.9 to 100) | 99.6 (98.7 to 99.9) |
| Subgroup analyses by viral load | |||||
| High viral loadb | 5 (4) | 151 | 151 | 100 (97.5 to 100) | N/A |
| Low viral load | 5 (4) | 142 | 142 | 93.3 (46.7 to 99.6) | N/A |
| Subgroup analyses by testa | |||||
| Abbott – ID NOW | 5 | 1003 | 496 | 76.8 (72.9 to 80.3) | 99.6 (98.4 to 99.9) |
| Cepheid – Xpert Xpress | 6 | 919 | 479 | 99.4 (98.0 to 99.8) | 96.8 (90.6 to 99.0) |
| Difference(95% CI) | 22.6 (18.8 to 26.3), P < 0.001 | −2.8 (−6.4 to 0.8), P = 0.13 | |||
| Mesa Biotech – Accula | 1 | 100 | 50 | 68.0 (53.3 to 80.5) | 100 (92.9 to 100) |
| DRW – SAMBA II | 1 | 172 | 88 | 98.9 (93.8 to 100) | 96.4 (89.9 to 99.3) |
| Direct comparisons by test | |||||
| Abbott – ID NOWa,b | 2 | 220 | 145 | 79.3 (71.8 to 85.6) | 100 (95.2 to 100) |
| Cepheid – Xpert Xpressa,b | 2 | 221 | 146 | 98.6 (95.1 to 99.8) | 97.3 (90.7 to 99.7) |
| Difference(95% CI)e | 19.3 (12.5 to 26.2), P < 0.001 | −2.7 (−6.3 to 1.0), P = 0.15 | |||
| Sample type | |||||
| Nasopharyngeal onlyc | 6 (5) | 600 | 343 | 87.1 (71.6 to 94.7) | 100 (98.6 to 100) |
| CGIA: colloidal gold immunoassay; CI: confidence intervals; FIA: fluorescent immunoassay; DRW: Diagnostics for the Real World | |||||
aSee Appendix 9 for details of product codes, where available (these were not necessarily reported in studies but we obtained them from manufacturer instructions for use documents). b2x2 tables combined prior to calculating estimates. cSeparate pooling of sensitivity and/or specificity. dThis includes two studies that only include COVID‐19 positive cases. eTwo‐sample test of proportions.
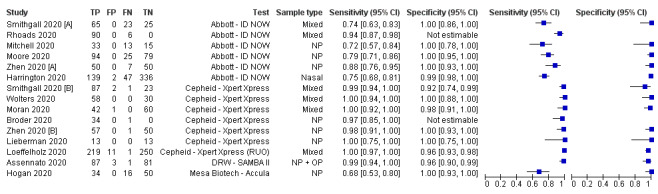
We undertook analyses separately for antigen tests and for molecular‐based tests. We present results for all analyses in Table 3. Forest plots of study data for the primary analyses are in Figure 3 and Figure 4. Full identification details for all assays are provided in Appendix 8 and Appendix 9); for brevity, the antigen assays are referred to by the manufacturer name. Subgroup analyses according to viral load are in Figure 5 and Figure 6, and rapid molecular test results before and after discrepant analysis are in Figure 7.
3.
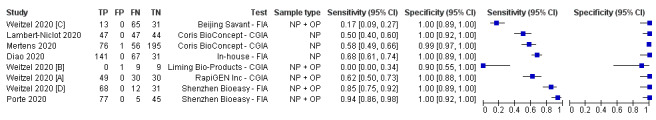
Forest plot of studies evaluating antigen tests. Studies grouped by test (FIA: fluorescence immunoassays; CGIA: colloidal gold‐based immunoassays; NP: nasopharyngeal; OP: oropharyngeal)
4.
Forest plot of studies evaluating rapid molecular tests. Studies grouped by test and sample type (NP: nasopharyngeal; OP: oropharyngeal; RUO: research use only)
5.
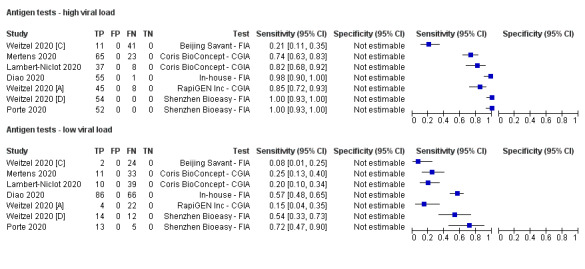
Forest plot of studies evaluating antigen tests according to viral load: high (≤ 25 Ct) versus low viral load (≤ 30 Ct in Diao 2020). Studies grouped by test
6.
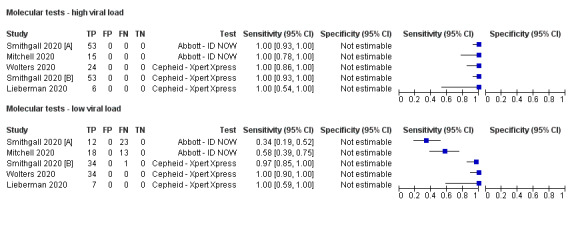
Forest plot of studies evaluating rapid molecular tests according to viral load: high (≤ 30 Ct) versus low viral load. Studies grouped by test
7.
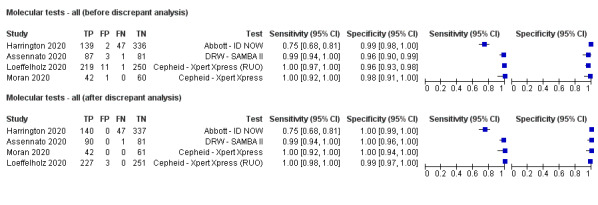
Forest plot of studies of molecular tests before and after discrepant analysis. Studies grouped by test (DRW: Diagnostics for the Real World; RUO: research use only)
Accuracy of antigen tests overall and by test
Average sensitivity across the eight evaluations of antigen tests was 56.2% (95% CI 29.5 to 79.8%), and average specificity 99.5% (95% CI 98.1% to 99.9%; 943 samples, including 596 samples with confirmed SARS‐CoV‐2; Table 3). However, Figure 3 shows considerable heterogeneity in sensitivity, with results across studies ranging from 0% to 94%. The average value should therefore be interpreted with caution as there may be real differences in sensitivity between the test brands. The two assays with lowest sensitivity (Liming Bio‐Products and Beijing Savant assays) do not now appear to be commercially available. Pooled results for the two tests with two studies each suggested higher sensitivity for the Shenzhen Bioeasy FIA (89.5%, 95% CI 83.7% to 93.8%) than the Coris BioConcept CGIA (54.4%, 95% CI 47.7% to 61.0%), but these tests were not evaluated in the same studies and other factors may explain the observed differences. Similar, unknown factors may explain differences between the Shenzhen Bioeasy and Coris BioConcept assays and the other tests for which only single studies were available. Specificities were consistent and high, with point estimates of 99% or 100% in seven evaluations, and one study estimating specificity as 90% but with a 95% confidence interval that included 100%.
Accuracy of rapid molecular tests overall and by test
Average sensitivity and specificity for the 13 rapid molecular test evaluations that included samples with and without SARS‐CoV‐2, were 95.2% (95% CI 86.7% to 98.3%) and 98.9% (95% CI 97.3% to 99.5%; 2255 samples, 1179 with confirmed SARS‐CoV‐2). Adding the two 'cases only' studies made little difference to the average sensitivity (95.5%, 95% CI 88.5% to 98.4%; 1244 cases). We excluded these two studies from further analyses (Broder 2020; Rhoads 2020).
Figure 4 demonstrates heterogeneity in sensitivity estimates (ranging from 68% to 100%), with consistently high specificities (92% to 100%, but with upper limits of 95% CIs of 99% or 100% in every study). Of the four different molecular tests evaluated, two were evaluated in one study each. The sensitivity and specificity of the Accula test were 68.0% (95% CI 53.3% to 80.5%) and 100% (95% CI 92.9% to 100%; 100 samples, 50 with confirmed SARS‐CoV‐2). For SAMBA II, sensitivity and specificity were 98.9% (95% CI 93.8% to 100%) and 96.4% (95% CI 89.9% to 99.3%; 172 samples, 88 with confirmed SARS‐CoV‐2).
The ID NOW and Xpert Xpress tests were evaluated in five studies (1003 samples, 496 with confirmed SARS‐CoV‐2) and six studies (919 samples, 479 with confirmed SARS‐CoV‐2), respectively. Pooled analysis showed the Xpert Xpress test to have higher sensitivity (99.4%, 95% CI 98.0% to 99.8%) in comparison to ID NOW (76.8%, 95% CI 72.9% to 80.3%), a difference of 22.6 (95% CI 18.8 to 26.3) percentage points (Table 3). Whilst the specificity of Xpert Xpress (96.8%, 95% CI 90.6 % to 99.0%) was marginally lower than ID‐NOW (99.6%, 95% CI 98.4% to 99.9%) the difference was of a magnitude that can be explained by chance (difference of −2.8, 95% CI −6.4 to 0.8) percentage points (P = 0.13)). Restricting the analysis to the two studies that compared the two tests in the same patients gave very similar results (difference in sensitivity of 19.3% (95% CI 12.5% to 26.2%) and difference in specificity of −2.7 percentage points (95% CI −6.3 to 1.0), based on 221 samples, 146 with SARS‐CoV‐2; Smithgall 2020 [A]; Zhen 2020 [A])). (This analysis used the two‐sample test of proportions).
Subgroup analyses by sample type
Adequate data for different sample types were available for studies using nasopharyngeal samples only. We observed similar average sensitivity (59.4%, 95% CI 50.7% to 67.5%) and specificity (99.6%, 95% CI 97.4% to 99.9%) for three evaluations of antigen tests (705 samples, 434 with confirmed SARS‐CoV‐2). For six evaluations of molecular tests, average sensitivity appeared lower compared to the overall pooled estimate (87.1%, 95% CI 71.6% to 94.7%) with little change in specificity (Table 3).
Subgroup analyses by viral load
We extracted sensitivity data according to viral load from seven evaluations of antigen tests (three with the assistance of the study authors) and five evaluations of molecular tests. Ct threshold for high viral load was 25 or less for four of the five antigen studies and 30 or less for the remaining antigen evaluation and for all of the molecular assay evaluations. We observed a large difference in sensitivity in the high viral load group (400 with confirmed SARS‐CoV‐2) for antigen tests (difference of 60.6 percentage points (95% CI 38.2, 83.0) compared to low viral load (341 samples with confirmed SARS‐CoV‐2) that was beyond that expected by chance (P < 0.001) (Table 3; Figure 5).
For molecular tests, all sensitivity estimates for the high viral load subgroups were 100% (based on 151 samples with confirmed SARS‐CoV‐2) compared to between 34% and 100% for low viral load subgroups (summary sensitivity 93.3%, 95% CI 46.7% to 99.6%; 142 samples with confirmed SARS‐CoV‐2; Table 3; Figure 6). The evaluations with the lowest sensitivities both evaluated ID NOW, with reported sensitivity estimates of 34% (35 samples with confirmed SARS‐CoV‐2 in Smithgall 2020 [A]), and 58% (based on 31 samples with confirmed SARS‐CoV‐2 in Mitchell 2020). Sensitivity in the three evaluations of Xpert Xpress ranged from 97% (35 samples with confirmed SARS‐CoV‐2 in Smithgall 2020 [B]) to 100% (in Lieberman 2020 and Wolters 2020, with 7 and 34 samples with confirmed SARS‐CoV‐2 respectively).
Sensitivity analysis of the impact of discrepant analysis
Four evaluations of molecular tests (in 1566 samples) reported results before and after discrepant analysis where selected samples were re‐tested with either the same (Harrington 2020; Moran 2020), or an alternative RT‐PCR assay (Assennato 2020; Loeffelholz 2020), three of which also reported re‐testing of samples with the index test (Assennato 2020; Harrington 2020; Moran 2020; Table 4; Figure 7).
3. Effect of sample re‐testing.
| Study | Index test (target genes) | First RT‐PCR | Target gene | Second RT‐PCR | Target gene | False positives | False negatives | Index test re‐test | Reference standard re‐test |
| Discrepant analysis | |||||||||
| Assennato 2020 | SAMBA II (ORF1ab, N2) | PHE Cambridge (Wuhan) assay | RdRp, E gene | PHE Colindale RT‐PCR assay | RdRp 'different region' | 3 → 0 | 1 → 1 | Yes; same results obtained | Yes; 3 FPs (reclassified as TP), all borderline positive for ≥ 1 target gene on either RT‐PCR test 1 FN (remained FN), positive on both RT‐PCR assays |
| Harrington 2020 | ID NOW (RdRp) | Abbott RealTime | Not stated | Same RT‐PCR | Same | 2 → 0 | 47 no‐retest | 1 FP reclassified as TN with repeat sampling 1 FP not re‐tested | 1 FP reclassified as TP 1 FP reclassified as TN (both with repeat sampling) |
| Loeffelholz 2020 | Xpert Xpress (RUO) (E, N2) | RT‐PCR varied by site:
|
By assay
|
One of:
|
By assay:
|
11 → 3 | 1 → 0 | None reported | 1 FN re‐classified as TN (inconclusive positive on Quest assay; negative on CDC assay) 3 FP remained as FP (2 negative on NY assay, 1 negative on Charité Virologie assay; all confirmed negative with Hologic Panther Fusion) 8 FP re‐classified as TP (all negative on Charité Virologie assay; positive on re‐test with Roche Tib Molbiol assay) |
| Moran 2020 | Xpert Xpress (E, N2) | Roche cobas 6800 | ORF1, E | Same RT‐PCR | Same | 1 → 0 | 0 | 1 FP reclassified as TN (was initially E gene negative and low positive for N2; negative for both targets on re‐test) | 1 FP 'repeatedly negative' on RT‐PCR re‐test (re‐classified as TN based on index re‐test) |
| Additional studies reporting sample re‐testing (not discrepant analysis) | |||||||||
| Broder 2020 | Xpert Xpress (E, N2) | Roche cobas 6800 | ORF1a, E | modified CDC protocol | NR | 0 | 1 | None reported No presumptive positive results reported | Yes; 1 FN (became TN) |
| Hogan 2020 | Accula (N) | In‐house SHC assay | E gene | N/A | N/A | 0 | 16 | Yes; 1 TP remained as TP; faint positive Accula test line was repeated on re‐test | None reported |
| Lieberman 2020 | Xpert Xpress (E, N2) | CDC EUA‐based in‐house test (positive if 1 of 2 targets detected) | NI, N2 | N/A | N/A | 0 | 0 | Yes; 1 presumptive positive (E‐gene only positive) became positive (N‐gene only positive) on re‐test | None reported |
| Moore 2020 | ID NOW (RdRp) | Modified CDC RT‐PCR | N1, N2 | Abbott RealTime | N, RdRp | 0 → 0 | 25 → 31 | None reported | All samples tested with both RT‐PCR assays 25 FN remained as FN (2 were inconclusive but considered positive on CDC assay, confirmed positive with Abbott RealTime assay) 6 TN reclassified as FN (negative on CDC assay, confirmed positive with Abbott RealTime assay) All 8 discordant results between the two RT‐PCR's were confirmed SARS‐CoV‐2 positive based on record review |
| Wolters 2020 | Xpert Xpress (E, N2) | In‐house assays at three laboratories | By laboratory:
|
Same RT‐PCR per laboratory | Same | 0 → 2 | 0 | None reported 1 presumptive positive considered TP by review team | 2 TP samples (both positive on only one target; 1 presumptive positive (E positive) and 1 positive (N2 positive)) re‐classified as FP; both considered SARS‐CoV‐2 negative on RT‐PCR re‐test *authors note that viral loads were at the limit of detection for Xpert Xpress and that multiple freeze‐thaw steps of samples could have had a significant impact on detection. |
| CDC: center for disease control; EUA: emergency use authorisation; FN: false negative; FP: false positive; PHE: Public Health England; RT‐PCR: reverse transcriptase polymerase chain reaction; RUO: research use only; TN: true negative; TP: true positive | |||||||||
Discrepant analysis always works to reduce the number of samples deemed to be false negative or false positive errors. Discrepant analysis reduced the false negative proportion (1‐sensitivity) from 1.8% to 0.5% and the false positive rate (1‐specificity) from 2.2% to 0.4%. Three of the four studies reporting initially ‘false positive’ results reported zero false positives after sample re‐testing and one reported a drop in false positives from 11 to 3 (Loeffelholz 2020; Table 4). One of the two studies reporting re‐testing of initially ‘false negative’ results reported reclassification as true negative on re‐testing, and in the other the false negative remained as a false negative. Given the bias inherent in choosing the reference test dependent on the observed results, we caution against these findings.
An additional study tested all samples with two different RT‐PCR assays, and hence used a more accurate reference standard in all samples, not just samples with discrepant results (Moore 2020), in which six initial true negatives were reclassified as false negatives after the second RT‐PCR. Had discrepant analysis been undertaken these misclassifications would have been missed, further underlining the methodological flaws inherent to discrepant analysis.
Other sources of heterogeneity
We planned to evaluate the effect of other sources of heterogeneity, including study design, reference standard, length and severity of symptoms, and setting. However, additional formal investigations using meta‐regression were not possible because of limited data, lack of reporting or lack of variability across the studies in these features (Appendix 11). Only one study reported the median time to testing after symptom onset, none reported symptom severity, and three reported the setting in which tests were conducted. All studies used RT‐PCR alone as the reference standard for diagnosing COVID‐19 infection.
We anticipate revisiting the effect of study design and including a more detailed investigation by sample type in future iterations of this review.
Discussion
This is the first version of a Cochrane living review summarising the accuracy of point‐of‐care antigen and molecular tests for detecting current SARS‐CoV‐2 infection. This version of the review is based on published studies, or studies available as preprints, up until 25 May 2020. We are continually identifying new published studies, and plan regular updates of this review.
Summary of main results
We included data from 18 studies including 3198 samples (including 1775 samples with confirmed SARS‐CoV‐2). Five studies, reporting eight test evaluations, considered antigen tests and 13 studies, reporting 15 test evaluations, considered rapid molecular tests. Key findings are presented in the Table 1.
We summarise five key findings from this review.
A significant proportion of antigen and molecular assays that are suitable for use at the point of care do not have any published or preprint reports of accuracy. This review has evaluated data from five commercial antigen tests, two of which we could not identify as available for purchase, and four molecular assays. These represent a small proportion of assays currently available. We have identified 24 additional studies of rapid antigen or molecular tests published or available as preprints up until 22 June 2020, which we will appraise for inclusion in the review update, but there still remain no published data for the majority of tests on the current FIND list.
The design and execution of studies limits the strength of conclusions that we are currently able to draw, either for antigen or for molecular tests. It is unclear whether the limitations in the primary studies will lead to over‐ or under‐estimates of test accuracy, thus all results we report should be interpreted with a high degree of caution. Half of studies used deliberate sampling based on the presence or absence of confirmed COVID‐19 infection, and the majority selected samples from those submitted to laboratories for routine RT‐PCR testing with little to no detail of the participants who provided the samples in relation to either symptom status or time from symptom onset. It is impossible to determine the effect of inclusion decisions based on the availability of residual or remnant samples. It was not always clear how many samples were included from each participant, and the analysis had to be undertaken on a per‐sample basis, which will have overestimated the precision of the estimates. RT‐PCR was the only reference standard for diagnosing the presence of SARS‐COV‐2 infection so that we are unable to comment on the accuracy of rapid tests for diagnosing infection in those who are RT‐PCR negative but meet case definition criteria for the presence of infection. The use of a second RT‐PCR assay to determine the disease status of samples with discrepant results following rapid molecular testing is likely to introduce further bias.
Three‐quarters of studies conducted tests outside of manufacturers’ instructions for use, particularly in regard to sample storage and use of transport media, and with tests conducted in centralised laboratories rather than at the point of care, so that test accuracy in a clinical setting remains unknown. We considered five tests, including one molecular assay, to have low complexity in terms of minimal sample preparation and test steps, and the other four to have moderate (n = 3) or high (n = 1) complexity, which could also affect how well the observed accuracy translates into practice. We did not include interpretation steps in our assessment of test complexity; however the use of reader devices, for example for FIAs, could be considered to further add to complexity.
On average, the sensitivity of antigen tests was relatively poor (56.2%, 95% CI 29.5 to 79.8%), but with consistently high specificities (average 99.5%, 95% CI 98.1 to 99.9%). However, there is considerable heterogeneity in sensitivities between studies, and with limited data for individual tests. We observed large differences in sensitivity according to viral load and suspect that differences in the distribution of samples with high and low viral load between studies may have affected overall accuracy estimates. Combined with methodological limitations and other unknown factors, it is not possible to state with any certainty whether any test is superior to the others. There is a suggestion of higher sensitivity in two studies of the Shenzhen Bioeasy fluorescent immunoassay (sensitivity 89.5%, 95% CI 83.8%, 93.3%), that was maintained in subgroup analysis by viral load (one of the two obtained over 90% of samples during the first week of symptoms). An additional study reporting the development of this assay reported lower sensitivity overall (68%, 95% CI 61, 74%), however it included a much lower proportion of samples with high viral load (27% compared to 68 to 74% in the other two studies). Subgroup analysis suggested the test performed similarly to the other two studies when restricted to high and low viral load subgroups. All three studies included high percentages of samples with confirmed SARS‐CoV‐2, and more data is needed to determine whether test performance for this assay can be repeated in clinical practice.
On average, the sensitivity for the rapid molecular tests was 95.2% (95%CI 86.7%, 98.3%) with specificity 98.9% (95% CI 97.3, 99.5%). Although the average estimates are based on twice as much data as for the antigen tests, the evaluations are subject to the same methodological limitations, and we do not know how the assays would perform in any specific clinical setting when used in people suspected of having COVID‐19 infection or of having been exposed to a confirmed case.
Most of the evaluations of molecular tests were of ID NOW or Xpert Xpress. Summary sensitivity for Xpert Xpress (99.4%, 95% CI 98.0 to 99.8%) was 22.6 percentage points higher than that of ID NOW, a magnitude of difference that was more or less maintained in the two direct comparisons of the two assays. Concerns over risk of bias would suggest that this high rate of sensitivity might be an over‐estimate. However as both sets of studies have similar methodological limitations, it is probably reasonable to presume that some difference in sensitivity between tests would be maintained if these sources of bias were removed. The difference in specificity between the tests is small (ID NOW being 2.8% more specific compared to Xpert Xpress), but potentially important especially if used in a low‐prevalence setting. However, this would not be an issue should test positives be confirmed by a laboratory‐based RT‐PCR assay. Concerns about the applicability of study participants and index tests brings into question whether similar differences in test performance would be observed in practice.
As stated above, we did not undertake a formal comparison between antigen and molecular assays because of the lack of direct head‐to‐head comparisons of the two test types. However, the possible effect of the observed differences in accuracy can be illustrated by applying the summary estimates of test accuracy to a hypothetical cohort of 1000 people suspected of COVID‐19 infection (Table 1). If 100 people had confirmed SARS‐COV‐2 infection (prevalence of 10%), the average sensitivity and specificities of antigen tests mean that 5 of 61 people with a positive test result would be false positives (positive predictive value (PPV) 92%) while 44 of 940 people with negative test results would be falsely negative (negative predictive value (NPV) 95%). As there is high heterogeneity in the estimates of sensitivity, the values observed in practice could vary considerably from these figures. For molecular assays at the same prevalence, 10 of 105 positive test results would be false positive (PPV 90%), and 5 of 895 with negative results would be falsely negative (NPV 99%).
Small decreases (to 5%) or increases (to 20%) in prevalence make little difference to the absolute number of false positive results, but have a large relative effect when considered in relation to the number of positive test results (PPV ranging 85% to 97% for antigen tests and 83% to 95% for molecular assays). The NPV (percentage of negative test results that are truly negative) for the molecular assays is not affected by these prevalence changes in the same way because of the relatively high sensitivity and relatively low‐prevalence scenarios considered. Wider variation is observed for antigen tests (98% to 90%). This shows how even in a low‐prevalence setting, tests with poor sensitivity can have a considerable impact on the level of confidence that can be had in a negative test result. However, we emphasise that these numbers are not based on any evidence comparing antigen and molecular tests in the same samples.
We saw a similar pattern of results when applying summary results for individual tests with wide variations in sensitivity and only small differences in specificities (Table 1).
Strengths and weaknesses of the review
Our review used a broad search screening all articles concerning COVID‐19. We undertook all screening and eligibility assessments, QUADAS‐2 assessments (Whiting 2011), and data extraction of study findings independently and in duplicate. Whilst we have reasonable confidence in the completeness and accuracy of the findings up until the search date, should errors be noted please inform us at coviddta@contacts.bham.ac.uk so that we can check and correct in our next update.
We identified one other systematic review of point‐of‐care tests for detection of SARS‐CoV‐2 that is currently available only as a preprint (Subsoontorn 2020). The review did not consider antigen tests or RT‐PCR‐based tests (such as Xpert Xpress), instead focusing on molecular tests that do not require the use of a thermal cycler. We undertook a careful assessment of test complexity to ensure that included tests were suitable for use at the point of care. This assessment included explicit consideration of sample preparation and biosafety requirements as well as time to test result. The application of these index test criteria led to the exclusion of the majority of the 31 RT‐LAMP or CRISPr assay evaluations that were included in Subsoontorn 2020. Evaluations of alternative laboratory‐based molecular technologies are under consideration for inclusion in another review in our series of Cochrane COVID‐19 DTA reviews. An additional seven studies included in Subsoontorn 2020 became available after our search cut‐off and are already under consideration for inclusion in the review update.
Weaknesses of the review primarily reflect the weaknesses in the primary studies and their reporting. Many studies omitted descriptions of participants, and key aspects of study design and execution. In order to include data for all tests in pooled analyses we have had to include some samples multiple times. We have been explicit about these issues where they arose. It is possible that eligible studies have been missed by our search strategy however we believe the risk to be very low considering our broad approach to identification of literature.
Around a quarter (5/18) of the studies we have included are currently only available as preprints, and as yet, have not undergone peer review. As published versions of these studies are identified in the future, we will double‐check study descriptions, methods and findings, and update the review as required.
Applicability of findings to the review question
We have concerns about the applicability of the evidence that we have identified for point‐of‐care tests.
Due to lack of reporting, we do not know whether tests perform in the same way or differently according to whether those being tested have symptoms of COVID‐19, and if so how long they have experienced those symptoms for, or are asymptomatic. Studies appeared to include remnant or residual samples for testing and many selectively included high percentages of samples with RT‐PCR‐confirmed SARS‐CoV‐2. In reality, point‐of‐care tests will be considered for use in much lower prevalence settings. Methodological work on diagnostic test evaluations has shown that independently of prevalence, tests do not necessarily exhibit the same sensitivity and specificity in different prevalence settings (Usher‐Smith 2016). This can be because of differences in the case‐mix or ‘spectrum’ of disease (e.g. viral load). However, the mechanisms in action can be complex and difficult to clearly identify (Leeflang 2013).
We also had concerns about the way in which many of the tests evaluated were performed outside of manufacturer instructions for use, and not in fact at the point of care.
Great caution should be taken in applying these results outside of the individual study contexts.
Authors' conclusions
Implications for practice.
In the Role of index test(s) section, we suggested two main roles for point‐of‐care testing.
As a replacement for laboratory‐based RT‐PCR, if sufficiently accurate. Evidence included to date suggests that some rapid molecular tests might have accuracy levels approximating those of laboratory‐based RT‐PCR. However, many of the data come from two‐group studies with deliberate over‐sampling of cases and concerns over the applicability of the evidence. We cannot be certain as to whether any test performs sufficiently well for this role.
As a triage to RT‐PCR, allowing earlier detection and rapid management (self‐isolation, quarantine or therapeutic intervention) of those testing positive; those with negative results waiting for the laboratory‐based RT‐PCR result. On current evidence of test performance (using average observed sensitivities and specificities), rapid tests could only perform this type of triage role in higher‐prevalence settings (i.e. 20% or higher) because of the (relative) risk of false positive results in lower‐prevalence settings. Although average test specificities are high for both antigen and for molecular tests, unnecessary quarantine measures for 1 in 6 (83% PPV), or even 1 in 10 (90% PPV), people with a positive test result would seem a relatively high price to pay for a rapid result. The 99% to 100% specificities observed for individual tests would need to be replicated in well designed field studies, and following manufacturers’ instructions for use, before any test could be recommended for use as a triage test.
Alternatively, serial testing (over a number of days), or combinations of different rapid tests (e.g. an antigen test followed by a rapid molecular test) on the same sample may provide a useful testing strategy; however, additional evidence of the performance of any such diagnostic strategy would be needed. In the absence of further evidence, and in low prevalence settings, both positive and negative results from any of the rapid point‐of‐care tests included in this review would need to be followed up with a laboratory‐based RT‐PCR.
Ultimately, decisions around rapid testing will be driven not only by diagnostic accuracy but by acceptable levels of test complexity, time to result, and acceptability to those being tested, all of which might vary according to the setting in which the tests are to be used. In settings where RT‐PCR is not available, rapid tests may have a role if accpetable performance targets for diagnostic test accuracy, such as those laid out in WHO's priority target product profiles for COVID‐19 diagnostics, can be met (WHO 2020c).
Implications for research.
A considerable volume of research has already emerged for point‐of‐care tests for COVID‐19 infection. However further prospective and comparative evaluations of individual tests, either alone or in combination, and in clinically relevant settings are urgently needed. These settings include those where people with signs and symptoms present for testing as well as those involving testing asymptomatic people who may have come into contact with confirmed cases. Reliable and ideally rapid diagnostic tests are the keystone to good track and trace programmes, as a means of implementing necessary self‐isolation or quarantine and reducing community transmission. Studies should recruit consecutive series of eligible participants and should clearly describe symptomatic status, and should document time from symptom onset or time since exposure. Point‐of‐care tests must be conducted in accordance with manufacturer instructions for use, and across the spectrum of point‐of‐care settings and test operators.
We observed a number of studies of molecular assays employing discrepant analysis to confirm the disease status of samples with false positive results in particular. There is a considerable risk of this type of selective re‐testing leading to distorted results. If there is sufficient concern about the reliability of a single RT‐PCR test then all samples should be tested with two RT‐PCR assays. Finally, any future research study needs to be clear about eligibility and exclusion decisions throughout the whole diagnostic pathway, and should conform to the updated Standards for Reporting of Diagnostic Accuracy (STARD) guideline (Bossuyt 2015).
History
Review first published: Issue 8, 2020
Acknowledgements
Members of the Cochrane COVID‐19 Diagnostic Test Accuracy Review Group include:
the project team (Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MMG, Spijker R, Hooft L, Van den Bruel A, McInnes MDF, Emperador D, Dittrich S, Cunningham J);
-
the systematic review teams for each review:
Molecular, antigen, and antibody tests (Adriano A, Beese S, Dretzke J, Ferrante di Ruffano L, Harris I, Price M, Taylor‐Phillips S)
Signs and symptoms (Stuyf T, Domen J, Horn S)
Routine laboratory markers (Yang B, Langendam M, Ochodo E, Guleid F, Holtman G, Verbakel J, Wang J, Stegeman I)
Imaging tests (Salameh JP, McGrath TA, van der Pol CB, Frank RA, Prager R, Hare SS, Dennie C, Jenniskens K, Korevaar DA, Cohen JF, van de Wijgert J, Damen JAAG, Wang J);
the wider team of systematic reviewers from University of Birmingham, UK who assisted with title and abstract screening across the entire suite of reviews for the diagnosis of COVID‐19 (Agarwal R, Baldwin S, Berhane S, Herd C, Kristunas C, Quinn L, Scholefield B).
The editorial process for this review was managed by Cochrane's EMD Editorial Service in collaboration with Cochrane Infectious Diseases. We thank Helen Wakeford, Anne‐Marie Stephani and Deirdre Walshe for their comments and editorial management. We thank Sarah Hodgkinson for comments on the Abstract. We thank Robin Featherstone and Douglas M Salzwedel for comments on the search and Mike Brown and Paul Garner for sign‐off comments. We thank Denise Mitchell for her efforts in copy‐editing this review.
Thank you also to peer referees Kristien Verdonck, Tivani Mashamba‐Thompson, Fergus Macbeth, consumer referee Brian Duncan and methodological referees Mia Schmidt‐Hansen and Jo Leonardi‐Bee, for their insights.
The editorial base of Cochrane Infectious Diseases is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK Government’s official policies.
The authors thank Dr Mia Schmidt‐Hansen who was the Cochrane Diagnostic Test Accuracy (DTA) Contact Editor for this review; the clinical and methodological referees; the Cochrane DTA Editorial Team; and Anne Lawson who copy‐edited the protocol.
Jonathan Deeks is a UK National Institute for Health Research (NIHR) Senior Investigator Emeritus. Yemisi Takwoingi is supported by a NIHR Postdoctoral Fellowship. Jonathan Deeks, Jacqueline Dinnes, Yemisi Takwoingi, Clare Davenport and Malcolm Price are supported by the NIHR Birmingham Biomedical Research Centre. This paper presents independent research supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Appendices
Appendix 1. Summary of World Health Organization and Chinese National Health Commission Guidelines for the diagnosis of SARS‐CoV‐2
Table A: World Health Organization guidelines for the diagnosis of SARS‐CoV‐2a
Includes laboratory testing guidelines and global surveillance guidelines
| Date range (2020) | Definition of confirmed case | Definition of confirmed non‐case | Definition of suspect case | Definition of probable case | Role of serology in testing |
| 10‐30 January | 10‐30 January: no documentation to define at this time (before first date of global guidelines)
31 January onwards: a confirmed case is a person with laboratory confirmation of COVID‐19 infection, irrespective of clinical signs and symptoms. No prescribed test in laboratory guidelines, suggested tests from 10 January include broad coronavirus RT‐PCR (with sequencing of precise virus in test positives), whole genome sequencing, broad coronavirus serology on paired samples, microscopy, culture (Lab 10 January). Four suggested tests from 17 January: broad coronavirus RT‐PCR (with sequencing of precise virus in test positives), NAAT for SARS‐CoV‐2 when it becomes available, whole genome sequencing, and broad coronavirus serology on paired samples. States that once specific NAAT assays are developed and validated, confirmation will be based on specific detection of unique sequences of viral nucleic acid by RT‐PCR. |
None stated | No definition of 'suspect case' at this time, but case definitions for surveillance are defined as a combination of symptoms and exposure, with more severe symptoms requiring less evidence for exposure | No definition at this time | Serological testing may be useful to confirm immunologic response to a pathogen from a specific viral group, e.g. coronavirus. Best results from serologic testing requires the collection of paired serum samples (in the acute and convalescent phase) from cases under investigation. |
| 31 January‐26 February | None stated | Suspect case defined as combination of symptoms and exposure, with more severe symptoms requiring less evidence for exposure | A suspect case with inconclusive laboratory results or is test‐positive using a pan‐coronavirus assay without laboratory evidence of other respiratory pathogens (global 31 January) | ||
| 27 February‐1 March | None stated | Suspect case defined as combination of symptoms and exposure, with more severe symptoms requiring less evidence for exposure, OR defined by symptoms requiring hospitalisation and an absence of alternative explanation | A suspected case with inconclusive laboratory results (global 27 February) | ||
| 2 March‐19 March | A person with laboratory confirmation of COVID‐19 infection, irrespective of clinical signs and symptoms (global 31 January, 27 February, 20 March)
Laboratory confirmation of cases by NAAT specific to SAR‐CoV‐2 such as real‐time reverse‐transcription polymerase chain reaction (rRT‐PCR) with confirmation by nucleic acid sequencing when necessary. The viral genes targeted so far include the N, E, S and RdRP genes. In areas with no known COVID‐19 virus circulation confirmation requires:
Discordant results should be resampled. In areas where COVID‐19 virus is widely spread a simpler algorithm might be adopted (e.g. RT‐PCR of a single discriminatory target) |
One or more negative result does not rule out the possibility of COVID‐19 virus infection | In cases where NAAT assays are negative and there is a strong epidemiological link to COVID‐19 infection, paired serum samples (in the acute and convalescent phase) could support diagnosis once validated serology tests are available. Serological assays will play an important role in research and surveillance but are not currently recommended for case detection. |
||
| 19 March‐present | Probable case A suspect case for whom testing for the COVID‐19 virus is inconclusive OR A suspect case for whom testing could not be performed for any reason. | ||||
| NAAT: nucleic acids amplification test; RT‐PCR: reverse transcription polymerase chain reaction. | |||||
aSource data from Laboratory testing of 2019 novel coronavirus (2019‐nCoV) in suspected human cases: interim guidance, World Health Organization. 10 January, 17 January, 2 March, 19 March, 21 March 2020 (WHO 2020a), and Global surveillance for COVID‐19 caused by human infection with COVID‐19 virus, interim guidance, 31 January, 27 February, and 20 March 2020 (WHO 2020b).
Table B: Summary of Chinese National Health Commission guidelines for diagnosis and treatment for novel coronavirus pneumonia (trial versions 1‐7)
| Dates in effect | Definition of confirmed case | Definition of confirmed non‐case | Definition of suspect case | Role of serology in testing |
| 16‐17 January 2020 (version 1) | Cases (not confirmed cases) defined as virus genome highly homologous to coronaviruses | Not defined | Observation cases: defined as combination of exposure in Wuhan and symptoms focused on pneumonia, leukopenia and lack of improvement. | No role |
| 18 January‐2 March (versions 2, 3, 4, 5, 5 revised, and 6) | Suspect cases with either
|
Suspect cases can be ruled out after 2 consecutive negative respiratory tract nucleic acid tests taken at least 24 hours apart. | Suspect cases: combination of exposure (such as residence in/travel to Wuhan or exposure to a confirmed case within 14 days of onset) AND clinical features (such as symptoms: fever, respiratory symptoms, and tests: chest imaging, white blood cell and lymphocyte count). Exact definition varies slightly with version | No role |
| 3 March‐present (version 7) | Suspect cases with either
|
Suspect cases can be ruled out after 2 negative NAATs, taken at least 24 hours apart, and the NCP virus‐specific IgM and IgG are negative after 7 days from onset. | Suspect cases: combination of exposure (such as residence in/travel to Wuhan or exposure to a confirmed case within 14 days of onset) AND clinical features (such as symptoms: fever, respiratory symptoms, and tests: chest imaging, white blood cell and lymphocyte count). | Part of definition of cases and confirmed non‐cases |
| NAAT: nucleic acids amplification test; NCP: novel coronavirus pneumonia; RT‐PCR: reverse transcription polymerase chain reaction; Source: Table from Cheng 2020 | ||||
Appendix 2. Cochrane COVID‐19 Study Register searches
| Source | Strategy |
| CT.gov | COVID‐19a |
| WHO ICTRP | Health topic: 2019‐nCov / COVID‐19 |
| PubMed | (("2019 nCoV"[tiab] OR 2019nCoV[tiab] OR "2019 novel coronavirus"[tiab] OR "COVID 19"[tiab] OR COVID19[tiab] OR "new coronavirus"[tiab] OR "novel coronavirus"[tiab] OR "novel corona virus"[tiab] OR "SARS CoV‐2"[tiab] OR (Wuhan[tiab] AND (coronavirus[tiab] OR "corona virus"[tiab])) OR "COVID‐19"[Supplementary Concept] OR "severe acute respiratory syndrome coronavirus 2"[Supplementary Concept]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms])) NOT (editorial[pt] OR comment[pt] OR letter[pt] OR newspaper article[pt]) |
aAutomatic term mapping links results for 2019‐nCoV, 2019 novel coronavirus, SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Appendix 3. Living search from the University of Bern
The following information is taken from the university of Bern website (see: ispmbern.github.io/covid-19/living-review/collectingdata.html).
The register is updated daily and CSV file downloads are made available.
1 April 2020
From 1 April 2020, we will retrieve the curated BioRxiv/MedRxiv dataset (connect.medrxiv.org/relate/content/181).
26 to 31 March 2020
MEDLINE: (\"Wuhan coronavirus\" [Supplementary Concept] OR \"COVID‐19\" OR \"2019 ncov\"[tiab] OR ((\"novel coronavirus\"[tiab] OR \"new coronavirus\"[tiab]) AND (wuhan[tiab] OR 2019[tiab])) OR 2019‐nCoV[All Fields] OR (wuhan[tiab] AND coronavirus[tiab])))))
Embase: (nCoV or 2019‐nCoV or ((new or novel or wuhan) adj3 coronavirus) or covid19 or covid‐19 or SARS‐CoV‐2).mp.
BioRxiv/MedRxiv: ncov or corona or wuhan or COVID or SARS‐CoV‐2
With the kind support of the Public Health & Primary Care Library PHC (www.unibe.ch/university/services/university_library/faculty_libraries/medicine/public_health_amp_primary_care_library_phc/index_eng.html), and following guidance of the Medical Library Association (www.mlanet.org/p/cm/ld/fid=1713).
1 January 2020 to 25 March 2020
MEDLINE: ("Wuhan coronavirus" [Supplementary Concept] OR "COVID‐19" OR "2019 ncov"[tiab] OR (("novel coronavirus"[tiab] OR "new coronavirus"[tiab]) AND (wuhan[tiab] OR 2019[tiab])) OR 2019‐nCoV[All Fields] OR (wuhan[tiab] AND coronavirus[tiab])))))
Embase: ncov OR (wuhan AND corona) OR COVID
BioRxiv/MedRxiv: ncov or corona or wuhan or COVID
Appendix 4. CDC Library, COVID‐19 Research Articles Downloadable Database
Embase records from the Stephen B. Thacker CDC Library, COVID‐19 Research articles Downloadable database
Records were obtained by the CDC library by searching Embase through Ovid using the following search strategy.
| Source | Strategy |
| Embase | coronavir* OR corona virus* OR betacoronavir* OR covid19 OR covid 19 OR nCoV OR novel CoV OR CoV 2 OR CoV2 OR sarscov2 OR 2019nCoV OR wuhan virus*).mp. OR ((wuhan OR hubei OR huanan) AND (severe acute respiratory OR pneumonia*) AND outbreak*).mp. OR Coronavirus infection/ OR coronavirinae/ OR exp betacoronavirus/ Limits: 2020‐ OR (novel coronavir* OR novel corona virus* OR covid19 OR covid 19 OR nCoV OR novel CoV OR CoV 2 OR CoV2 OR sarscov2 OR 2019nCoV OR wuhan virus*).mp. OR ((wuhan OR hubei OR huanan) AND (severe acute respiratory OR pneumonia*) AND outbreak*).mp. OR ((wuhan OR hubei OR huanan) AND (coronavir* OR betacoronavir*)).mp. Limits: 2019‐ |
Appendix 5. Data extraction items
| Patient sampling items | Patient characteristics and setting items | Index test items | Reference standard items | Flow and timing items | Notes items |
| A1 Purpose | B1 Setting | D1.1 Test name (please include product code if reported) | E1 Reference standard for cases including threshold | F1 What was the time interval between index and reference tests? | G1 Funding |
| A2 Design (and description of groups labelled [1] [2] …) | B2 Location (include name of institution if available) | D1.2 Manufacturer | E1.1 RT‐PCR genetic targets | F2 Did all patients receive the same reference standard? | G2 Publication status |
| A3 Recruitment | B3 Country | D1.3 Antigen or genetic target | E2 Samples used | F3 Missing data | G3 Source (preprint or Journal name) |
| A4 Were cases recruited prospectively or retrospectively? | B4 Dates | D1.4 Antibodies used | E3 Timing of reference standard | F4 Uninterpretable results | G4 Study author CoI (including any manufacturer affiliations) |
| A5 Sample size (virus/COVID cases) | B5 Symptoms and severity | D1.5 POC or laboratory | E4 Was it blind to index test? | F5 Indeterminate results (index) | G5 Comment |
| A6 Inclusion and exclusion criteria | B6 Demographics | D1.6 Test method | E5 Did it incorporate index test? | F5.1 Indeterminate results (reference) | |
| A7 Comment | B7 Exposure history | D1.7 When were samples taken? | E6 Reference standard for non‐cases | F6 Samples or patients | |
| B8 Comment | D1.8 Samples used (include who collected by) | E7 Samples used | F7 Comment | ||
| Nom‐COVID patients (if additional groups) | D1.8.1 Transport media (volume and manufacturer detail) | E8 Timing of reference standard | |||
| C1.1 Group name | D1.8.2 Sample storage and timing of test | E9 Was it blind to index test? | |||
| C1.2 Source and time | D1.9 Who applied the test (include reported training/e)? | E10 Did it incorporate index test? | |||
| C1.3 Characteristics | D1.10 How was positive defined? | E11 Comment | |||
| C2.1 Group name | D1.11 Blinded to reference standard | ||||
| C2.2 Source and time | D1.12 Threshold predefined | ||||
| C2.3 Characteristics | D1.13 Comment | ||||
| CoI: conflict of interest; POC: point of care; RT‐PCR: reverse transcription polymerase chain reaction | |||||
Appendix 6. Criteria for assessment of study quality (QUADAS‐2)
| DOMAIN: Participant selection | |
| Was a consecutive or random sample of patients enrolled? | This will be similar for all index tests, target conditions, and populations. Yes: if a study explicitly stated that all participants within a certain time frame were included; that this was done consecutively; or that a random selection was done. No: if it was clear that a different selection procedure was employed; for example, selection based on clinician's preference, or based on institutions, or based on result of RT‐PCR Unclear: if the selection procedure was not clear or not reported |
| Was a case‐control design avoided? | This will be similar for all index tests, target conditions, and populations. Yes: if a study explicitly stated that all participants came from the same group of (suspected) patients. No: if it was clear that a different selection procedure was employed for the participants depending on their COVID‐19 status or SARS‐CoV‐2 infection status; or if only participants with SARS‐CoV‐2 infection were included Unclear: if the selection procedure was not clear or not reported. |
| Did the study avoid inappropriate exclusions? | Studies may have excluded patients, or selected patients in such a way that they avoided including those who were difficult to diagnose or likely to be borderline. Although the inclusion and exclusion criteria will be different for the different index tests, inappropriate exclusions and inclusions will be similar for all index tests: for example, only elderly patients excluded, or children (as sampling may be more difficult). This needs to be addressed on a case‐by‐case basis. Yes: if a high proportion of eligible patients was included without clear selection. No: if a high proportion of eligible patients was excluded without providing a reason; if, in a retrospective study, participants without index test or reference standard results were excluded. Unclear: if the exclusion criteria were not reported. |
| Did the study avoid inappropriate inclusions? | Some laboratory studies may have intentionally included groups of patients in whom the accuracy was likely to differ, such as those with particularly low or high viral loads, or who had other diseases, such that the sample over‐represented these groups. This needs to be addressed on a case‐by‐case basis. Yes: if samples included were likely to be representative of the spectrum of disease. No: if the study oversampled patients with particular characteristics likely to affect estimates of accuracy. Unclear: if the exclusion criteria were not reported. |
| Could the selection of patients have introduced bias? | High: if one or more signalling questions were answered with no, as any deviation from the selection process may lead to bias. Low: if all signalling questions were answered with yes. Unclear: all other instances |
| Is there concern that the included participants do not match the review question? | High: for two‐group studies that included healthy or other disease controls, whether pre‐pandemic or contemporaneous; studies that only included people with COVID‐19 (whether RT‐PCR‐confirmed only, participants meeting official guideline criteria); Low: for single‐group studies recruiting participants with signs and symptoms of COVID‐19; or for two‐group studies where control groups suspected of COVID‐19 were separately recruited. Unclear: if a description about the participants was lacking. |
| DOMAIN: Index tests | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes: if blinding was explicitly stated or index test was recorded before the results from the reference standard were available. No: if it was explicitly stated that the index test results were interpreted with knowledge of the results of the reference standard. Unclear: if blinding was unclearly reported. |
| If a threshold was used, was it prespecified? | Yes: if the test was dichotomous by nature, or if the threshold was stated in the methods section, or if study authors stated that the threshold as recommended by the manufacturer was used. No: if a receiver operating characteristic curve was drawn or multiple threshold reported in the results section; and the final result was based on one of these thresholds. Unclear: if threshold selection was not clearly reported. |
| Could the conduct or interpretation of the index test have introduced bias? | High: if one or more signalling questions were answered with no, as even in a laboratory situation knowledge of the reference standard may lead to bias. Low: if all signalling questions were answered with yes. Unclear: all other instances |
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | For all test types, if index test is 'in‐house' or not commercially available, then state 'High'. If any test procedures used in the study diverged from IFU ((use of VTM, or testing outwith stated time limit), also state High If testing carried out in centralised laboratory and not near patient then state High. Evaluations that withheld the name of the test, or that used mixed sample types or did not report the evaluation setting, state Unclear If samples used and any sample processing steps are in accordance with test IFU, or if study describes conducting the test according to the manufacturer's protocol, state Low |
| DOMAIN: Reference standard | |
| Is the reference standard likely to correctly classify the target condition? | We will define acceptable reference standards using a consensus process once the list of reference standards that have been used has been obtained from the eligible studies. For COVID‐19 cases Yes: RT‐PCR; confirmed or suspected case using official criteria (WHO, CDC) or a clearly set out combination of signs/symptoms/exposure No: RT‐PCR not used, or if inadequate combination of clinical characteristics used in PCR‐negatives, e.g. computed tomography alone Unclear: if definition of COVID‐19 was not reported For absence of COVID‐19 Yes: if at least 2 negative RT‐PCR results reported if suspected COVID‐19 based on signs/symptoms; single negative RT‐PCR test for asymptomatic contacts or contemporaneous controls with no clinical suspicion of COVID‐19; only pre‐pandemic sources of control samples used. No: single RT‐PCR or number of negative RT‐PCRs not reported for COVID‐19 suspects; no RT‐PCR reported (untested) for asymptomatic contacts or contemporaneous controls Unclear: if timing of control samples (pre‐pandemic or contemporaneous) was not reported |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes: if it was explicitly stated that the reference standard results were interpreted without knowledge of the results of the index test, or if the result of the index test was obtained after the reference standard. No: if it was explicitly stated that the reference standard results were interpreted with knowledge of the results of the index test or if the index test was used to make the final diagnosis. Unclear: if blinding was unclearly reported. |
| Did the definition of the reference standard incorporate results from the index test(s)? | Yes: if results from the index test were a component of the reference standard definition. No: if the reference standard did not incorporate the index standard test. Unclear: if it was unclear whether the results of the index test formed part of the reference standard. |
| Could the conduct or interpretation of the reference standard have introduced bias? | High: if one or more signalling questions were answered with no. Low: if all signalling questions were answered with yes. Unclear: all other instances |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Applicability was judged primarily on the definition of disease‐positive. High: if RT‐PCR alone used to define cases Low: if clinical criteria, including RT‐PCR, were used to define cases, regardless of whether official criteria were used, as long as the criteria were explicitly described. Unclear: if definition of COVID‐19 cases was not provided, including if some clinically diagnosed cases were included but the clinical criteria used were not described. |
| DOMAIN: Flow and timing | |
| Was there an appropriate interval between index test and reference standard? | Yes: if same swab used, or swabs obtained at same time regardless of freezing (which is covered under index applicability) No: if different samples used with more than 24 hours between collection times Unclear: if can't tell |
| Did all participants receive the same reference standard? | Yes: if all participants received the same reference standard (clearly no differential verification). No: if (part of) the index test‐positives or index test‐negatives received a different reference standard. Unclear: if it was not reported |
| Were all participants included in the analysis? | Yes: if it is clear that all eligible participants were included in the analyses. No: if after the inclusion/exclusion process, participants were removed from the analyses for different reasons: no reference standard done, no index test done, intermediate results of both index test or reference standard, indeterminate results of both index test or reference standard, samples unusable. Unclear: if it is not possible to determine whether all participants were included (e.g. from a STARD‐style participant flow diagram) |
| Did all participants receive a reference standard? | Yes: if all participants received a reference standard (clearly no partial verification). No: if only (part of) the index test positives or index test negatives received the complete reference standard. Unclear: if it was not reported |
| Were results presented per participant? | Yes: if either only one sample per participant (regardless of disaggregation of results over time), or if multiple samples per participant but results are disaggregated by time period (at least week by week) No: if multiple samples per participant and results are not disaggregated by time period Unclear: if it is not possible to tell whether results presented are per participant or per sample |
| Could the participantflow have introduced bias? | High: if one or more signalling questions were answered with no. Low: if all signalling questions were answered with yes. Unclear: all other instances |
| CDC: Centers for Disease Control; ICU: intensive care unit; IFU: instructions for use; RT‐PCR: real‐time polymerase chain reaction; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; VTM: viral transport medium; WHO: World Health Organization | |
Footnotes
Appendix 7. Summary study details
| Study |
Study design Inclusion criteria |
Setting Country (Recruitment dates) |
Participant characteristics | Reference standard | Reference samples and timing |
Missing data Uninterpretable results Indeterminate results |
| Antigen tests | ||||||
|
Diao 2020 (Preprint) 239 samples (208 cases) for NP swab 20 samples (19 cases ) for urine |
Single group Samples from cases of suspected SARS‐CoV‐2 infection |
Not stated China (Not stated) |
Not stated High viral load (≤ 30 Ct): 56, 27% (reported for 208 samples) |
RT‐PCR (Daan Gene kit) Threshold < 40 Ct; threshold < 30 Ct also investigated Target: ORF1ab and N gene |
As for index test; NP swab Timing of reference: not stated Interval from/to index test: done in parallel |
Not reported Not reported Reference: "indeterminate" category introduced into the reference standard for values between 30 and 40 Index: not reported |
|
Lambert‐Niclot 2020 (Accepted manuscript) 138 samples (94 cases) |
Single group Samples submitted for RT‐PCR testing |
Not stated France (1‐15 April 2020) |
Not stated High viral load (≤ 25 Ct): 45, 48% (reported for 94 samples) |
RT‐PCR (different kits used) Target: E gene |
As for index test; NP swab Timing of reference: within a few hours after collection Interval from/to index test: same sample, both tests conducted within a few hours |
4 samples in cobas VTM gave invalid results so all samples in cobas medium were excluded Same as above Reference: none reported Index: control lines "barely visible" for 9 positive and 8 negative tests |
|
Mertens 2020 (Preprint) 328 samples (132 cases) |
Single group Samples from cases of suspected SARS‐CoV‐2 infection |
Not stated (university laboratory) Belgium (19‐30 March 2020) |
Not reported High viral load (≤ 25 Ct): 88, 67% (reported for 132 samples) |
qRT‐PCR (4 different kits used) Target:
|
As for index test; respiratory specimens (322 NP swabs, 4 NP aspirate and 2 BAL) Timing of reference: analysed at time of collection Interval from/to index test: same samples used; "some delay" between PCR and antigen testing |
No None reported Reference: none reported Index: weak T lines considered positive |
|
Porte 2020 (Preprint) 127 samples (82 cases) |
Two groups; deliberate sampling of PCR‐positive and negative cases 2:1 Samples from cohort of suspected COVID‐19 cases (n = 1453); patients with respiratory symptoms and/or fever and an epidemiological risk factor for SARS‐CoV‐2 infection (travel or contact with case) |
Outpatients at private hospital emergency room Chile (16‐21 March 2020) |
Cough 94 (74.6%);
Fever 77 (61.1%);
Median duration of symptoms 2 days (IQR 1–4; range 0‐12);
68 male (53.5%);
median age 38 years (IQR 29.5–44; range 1–91) High viral load (≤ 25 Ct): 52, 74% (reported for 70 samples) |
RT‐PCR (COVID‐19 Genesig Real‐Time PCR assay (Primer Design Ltd., Chander's Ford, UK)) Threshold ≤ 40 Ct Target: not stated |
As for index test; OP and NP swabs Timing of reference: median 2 days post‐symptom onset (IQR 1‐4; range 0‐12) Interval from/to index test: same sample used; within 48 hours |
No Not reported Reference: patients Index: not reported |
|
Weitzel 2020 [A] (Preprint) 111 samples (80 cases) |
Two groups; deliberate sampling of PCR‐positive and negative cases 2:1 Samples from patients with respiratory symptoms and/or fever attending a private hospital ED |
ED (private hospital) Chile (16‐26 March 2020) |
Respiratory symptoms and/or fever 50 male (45%); median age 40 years High viral load (≤ 25 Ct): 54, 68% (reported for 80 samples) |
RT‐PCR (COVID‐19 Genesig Real‐Time PCR assay (Primerdesign Ltd., Chander's Ford, UK)) Threshold ≤ 40Ct Target: RdRp gene |
As for index test; NOP swabs Timing of reference: as for index test; median 2 days (IQR 1‐5) Interval from/to index test: same samples; index tests conducted after frozen storage |
2 invalid excluded 2 invalid results in 2 tests due to insufficient liquid migration Reference: none reported Index: visual interpretation of the Savant assay (using manufacturer‐supplied UV torch) was reportedly difficult under daylight conditions; manufacturer's fluorescence reader not available in Chile |
| Rapid molecular tests | ||||||
|
Assennato 2020 (Preprint) 172 samples (88 cases; 91 after retesting) |
Single group Samples from symptomatic individuals with suspected COVID‐19 sent for routine laboratory diagnosis |
Not stated; supplied by PHE UK (Not stated) |
Symptomatic | RT‐PCR (2 different kits used);
Target:
|
As for index; combined nose and throat swab in VTM Timing of reference: not stated Interval from/to index test: not stated; likely reference carried out for routine diagnostic testing |
None reported None reported Reference: all samples tested with second RT‐PCR, including 3 FP and 1 FN (see Table 4) assays Index: 3 FP and 1 FN result retested using SAMBA‐II (see Table 4) |
|
Broder 2020 (Accepted manuscript) 35 samples (35 cases) |
Single group (cases) Samples positive on RT‐PCR with lower range of viral load (E target Ct ≥ 30) |
Not stated (laboratory) USA (Not stated) |
All lower viral load | RT‐PCR (Roche cobas 6800 SARS‐CoV‐2 assay) Target: E gene (unclear if other genetic targets as well) |
As for index test; NP swab Timing of reference: not stated (presumably on presentation) Interval from/to index test: same samples; index test within 3 days of reference |
None reported None reported Reference: samples positive on reference were tested by in‐house assay using modified CDC protocol Index: none reported |
|
Harrington 2020 (Accepted manuscript) 524 samples (186 cases) |
Single group Symptomatic patients, diagnostic criteria for COVID‐19 |
EDs (n = 3) or urgent care centres (n = 2) USA (Not stated) |
Not stated | RT‐PCR (Abbott RealTime SARS‐CoV‐2 (ACOV) assay performed on the Abbott m2000 system (Abbott Molecular Inc. Des Plaines, IL) Threshold: not stated Target: not stated |
NP swabs (paired) Timing of reference: not reported Interval from/to index test: simultaneous swab collection |
None reported None reported Reference: 2 initial FPs had repeat sampling (see Table 4) Index: none reported |
|
Hogan 2020 (Preprint) 100 samples (50 cases) |
Single group Samples from adult patients from 1 hospital and paediatric and adult samples from surrounding hospitals |
Not stated (clinical virology laboratory) USA (7‐13 April 2020) |
Not stated | RT‐PCR (in‐house SHC assay ) Target: E gene |
As for index test; NP swab Timing of reference: not stated Interval from/to index test: not stated but implies that both tests undertaken in laboratory soon after sample collection |
None reported 3 invalid results were re‐tested; 1 positive and 2 negative Reference: none reported Index: 1 known RT‐PCR‐positive sample that showed a faint positive test line was re‐tested and again showed the same faint test line (considered positive) |
|
Lieberman 2020 (Accepted manuscript) 169 samples (87 cases); data for Xpert Xpress available for only 26 samples (13 cases) |
Single group Samples submitted for clinical diagnostic testing (not all samples analysed for all tests) |
Not stated, sampled from laboratory USA (Not stated) |
Not stated High viral load (≤ 30 Ct): 6, 46% (reported for 13 samples) |
RT‐PCR (UW CDC EUA‐based in‐house test) Threshold: positive if 1 of 2 targets detected ‐ presume at <40 Ct Target: NI, N2 genes |
As for index test; NP swab Timing of reference: not stated Interval from/to index test: all testing conducted within 72 h |
None reported; additional data reported comparing Panther Fusion with DiaSorin Simplexa Not stated Reference: inconclusive' results (i.e. 1 genetic target detected) considered positive due to the high specificity of all assays and limited cross‐reactivity seen for SARS‐CoV‐2 primer sets Index: same as above |
|
Loeffelholz 2020 (Accepted manuscript) 486 samples (220 cases) |
Two groups; deliberate sampling to enrich for positive specimens Suspected patients referred for COVID‐19 testing at 7 sites according to the local criteria; one site (LAC+USC) tested specimens from a 4‐day point prevalence survey of patients presenting with COVID‐19 symptoms |
Not stated USA, UK, France, Italy (1 March‐2 April 2020) |
Adults at all sites (all age groups at New York City Dept. Health and Mental Hygiene and Niguarda Hospital) | RT‐PCR (8 different kits used at different sites)
Target:
Tie‐breaker methods (for discrepant results), included: Hologic Panther Fusion (San Diego, USA), Tib‐Molbiol LightMix Modular Wuhan Coronavirus E‐gene RT‐PCR (Roche, Basel, Switzerland); and the CDC assay (IDT primers and probes) |
As for index test Timing of reference: as for index test Interval from/to index test: same samples but index test performed after frozen storage for undefined period of time (except at University Hospital, Newark where specimens were tested in real time, within 2 h by the index test) |
4 Xpert Xpress test results were lost permanently due to a single instrument computer malfunction and 1 invalid result excluded 1 Xpert Xpress test was invalid due to a cartridge error (inadequate sample volume) Reference: specimens with inconclusive results by a test, and those with discrepant results between index and the RT‐PCR tests were analysed by a third RT‐PCR method (see Table 4). 12 specimens (8 NPS, 4 NPS/OPS) were inconclusive and considered positive for data analysis purposes in the study. 1 NPS specimen was inconclusive by the Quest SARS‐CoV‐2 rRT‐PCR test and negative by the Xpert test. This specimen was negative by a tie‐breaker NAAT. Index: presumptive positive results on index test were not reanalysed by the index test, but all discrepant results were reanalysed by a third RT‐PCR method |
|
Mitchell 2020 (Accepted manuscript) 61 samples (46 cases) |
Single group Samples positive and negative on 1/2 SARS‐CoV‐2 RT‐PCR assays |
Not stated; 2 independent laboratories USA (Not stated) |
Not stated High viral load (≤ 30 Ct): 15, 33% (reported for 46 samples) |
RT‐PCR (2 different in‐house kits used) CDC EUA and NY RT‐PCR Target: not stated |
As for index test Timing of reference: as for index test Interval from/to index test: same samples but used at different times (samples used for index test stored at −80 ℃) |
None reported None reported Reference: none reported Index: none reported |
|
Moore 2020 (Preprint) 200 samples (125 cases) |
Two groups; consecutive (n = 94), then deliberate sampling of all PCR‐positive samples plus the next PCR‐negative sample after each positive Samples from symptomatic (fever or cough or shortness of breath) adult and paediatric outpatients, ED patients, and inpatients |
Mixed (outpatients, ED patients and inpatients) USA (27 March‐9 April 2020) |
79 (39.5%) hospitalised including 29 in ICU, 76 (38%) ambulatory care including 55 seen in a designated COVID‐19 screening clinic, and 45 (23%) seen at ED; 92 male (46%); mean age 50 years (SD 17) |
RT‐PCR (2 different methods used in all samples)
Threshold: [1] positive result required Ct <40 for both targets; negative if neither target detected and positive amplification curve for control (RP) gene; inconclusive if only one target detected at Ct<40, and test repeated [2] amplification curves reported as detected or not detected; Target: [1]N1, N2 genes [2]N, RdRp genes |
As for index test; NP swab Timing of reference: not stated Interval from/to index test: all 3 tests conducted within 72 h of sample collection |
2 invalid excluded 2 results were invalid on ID Now and were not retested (excluded) Reference: discordant results on RT‐PCR had record review to determine presence/absence COVID‐19 infection Index: none reported |
|
Moran 2020 (Accepted manuscript) 103 samples (42 cases) |
Single group Specimens collected from inpatients and ambulatory patients at the University of Chicago |
Mixed (inpatient and ambulatory); samples selected from central laboratory USA (Not stated) |
Not stated | RT‐PCR (Roche cobas SARS‐CoV‐2 assay on the cobas 6800 system (Roche Molecular Systems, Branchburg, NJ)) Target: ORF1, E genes |
As for index; nasal or NP swabs Timing of reference: not stated Interval from/to index test: same sample and appear to have both been conducted soon after sample collection |
None reported None reported Reference: single FP was retested on RT‐PCR and found to be repeatedly negative Index: single FP was retested with index test and considered negative on both targets |
|
Rhoads 2020 (Accepted manuscript) 96 samples (96 cases) |
Single group (cases); Samples positive using standard of care testing |
Not stated; includes self‐collected and provided‐collected samples USA (Not stated) |
Not stated | Standard of care testing for original samples; remnant samples re‐tested with modified CDC RT‐PCR (using 7500 Fast instrument and using alternate RNA extraction Threshold: samples with one positive target detected considered positive instead of "inconclusive" Target: N1 and N2 genes |
As for index test Timing of reference: as for index test Interval from/to index test: same samples used |
None reported None reported Reference: RT‐PCR detected only 1/2 targets for 2 samples (both considered positive and diagnosed as positive on original sample testing); both were negative on index test) Index: none reported |
|
Smithgall 2020 [A] (Published) 113 samples (88 cases) |
Two groups; deliberate sampling of samples with high, medium and low Ct values on the reference standard RT‐PCR Patients undergoing routine clinical testing by RT‐PCR |
Mixed; in‐patient and ED USA (8‐13 April 2020) |
111 adults (range 23‐101 years; mean 65 years for RT‐PCR‐positive and 43 years for RT‐PCR‐negative; 2 paediatric (age 1 day and 5 days); 61 male (54%) High viral load (≤ 30 Ct): 53, 60% (reported for 88 samples) |
RT‐PCR (cobas SARS‐CoV‐2 assay on the 6800 platform (Roche Diagnostics, Indianapolis, IN)); Threshold: not stated, all Ct values < 37 on both target genes Target: ORF1 a/b, E‐gene |
As for index test Timing of reference: as for index test Interval from/to index test: simultaneous; same samples used |
None reported None reported Reference: none reported Index: 1 sample was a presumptive positive based on detection of E‐gene target but not the N2 target |
|
Wolters 2020 (Accepted manuscript) 88 samples (58 cases) |
Two groups; deliberate sampling according to target gene Samples selected from laboratories on the basis of E gene or RdRp on RT‐PCR (n = 88) |
Not stated; 3 laboratories The Netherlands (January‐March 2020) |
Not stated High viral load (≤ 30 Ct): 24, 41% (reported for 58 samples) |
In‐house RT‐PCR (3 different kits at different laboratories) Target:
|
As for index test Timing of reference: as for index test Interval from/to index test: same samples used; index text seems to have been conducted after frozen storage |
None reported None reported Reference: re‐testing of the FN and presumptive positive samples with RT‐PCR (see Table 4); Index: 1 sample was positive only on N2 gene (considered negative according to IFU) and one was positive only on E gene (considered presumptive positive, requiring re‐testing according to IFU). Both samples were re‐tested on RT‐PCR only |
|
Zhen 2020 [A] (Accepted manuscript) 108 samples (58 cases) |
Two groups; deliberate sampling to represent the true positivity rate at study authors' institution (50%‐60%), and to span low and high viral loads Samples from symptomatic patients of all ages and gender |
Not stated; selected from laboratory USA (March‐April 2020) |
Symptomatic; all ages and gender |
RT‐PCR (Hologic Panther Fusion SARS‐CoV‐2 assay) Target: 2 regions of ORF1ab; either positive |
As for index; NP swabs Timing of reference: not stated Interval from/to index test: not stated in exact terms; delay between index and reference only for GenMark assay, as 88 samples tested at time of collection with ePlex then frozen before testing with all other assays |
1 invalid excluded 1 specimen with invalid result on ID Now excluded from that dataset Reference: none reported; no re‐testing conducted Index: none reported; no re‐testing conducted |
| BAL: bronchoalveolar lavage; CDC: Centers for Disease Control; Ct: cycle threshold; ED: emergency department; EUA: emergency use authorisation; FN: false negative; FP: false positive; ICU: intensive care unit; IFU: instructions for use; IQR: interquartile range; NAAT: nucleic acids amplification test; NOP: naso‐oropharyngeal; NP: nasopharyngeal; OP: oropharyngeal; PHE: Public Health England; qRT‐PCR: quantitative reverse transcription polymerase chain reaction; RT‐PCR: reverse transcription polymerase chain reaction; UV: ultraviolet; UW: University of Washington; VTM: viral transport medium | ||||||
Appendix 8. Summary index test details
| Study | Index test (manufacturer) | Test method Target | Sample details | Test operator Test threshold |
| Antigen tests | ||||
| Diao 2020 | Not stated (in‐house; co‐author affiliated to Bioeasy) | FIA Nucleocapsid protein (N‐antigen) |
Samples tested: NP in saline Timing of sampling: not stated Timing of test: not reported Storage: not reported |
Not stated; presumably lab staff Threshold: mean value of the fluorescence signal plus 5 SD |
| Lambert‐Niclot 2020 | COVID‐19 Ag Respi‐Strip CORIS (BioConcept, Gembloux, Belgium) Product code NR |
CGIA SARS‐CoV‐2 NP |
Samples tested: NP in VTM Timing of sampling: not stated Timing of test: not stated (soon after collection) Storage: none; no cooling or freezing step used |
Not stated; presumably lab staff Threshold: as per manufacturer |
| Mertens 2020 | COVID‐19 Ag Respi‐Strip (Coris BioConcept (Belgium)) Product code NR |
CGIA SARS‐CoV and SARS‐CoV‐2 highly conserved nucleoprotein |
Samples tested: NP in VTM Timing of sampling: not stated Timing of test: not described Storage: not reported |
Laboratory technician Threshold: visible reddish‐purple band appearing at the Test line position (T) |
| Porte 2020 | Diagnostic Kit for 2019‐Novel Coronavirus (2019‐nCoV) Ag Test (Bioeasy Biotechnology Co., Shenzhen, China) Catalogue no.YRLF04401025, lot no. 2002N408 |
CGIA SARS‐CoV‐2 nucleocapsid protein |
Samples tested: NP + OP in VTM Timing of sampling: not stated Timing of test: not described Storage: not reported |
Laboratory technician Threshold: as per manufacturer |
| Weitzel 2020 [A] | [A] Biocredit COVID‐19 Ag One Step SARS‐CoV‐2 Antigen Test (RapiGEN Inc., Anyang‐si, Gyeonggi‐do, Republic of Korea) Catalogue no. G61RHA20; Lot No. H073001SD |
CGIA Not reported in study |
Samples tested: NP + OP in VTM Timing of sampling: not stated Timing of test: not described Storage: not reported |
Single trained laboratory technician under BSL2 cabinet; visual outputs read by 2 independent observers with referral to third if needed Threshold: as per manufacturer |
| Weitzel 2020 [B] | [B] COVID‐19 Antigen Rapid Test Device StrongStep COVID‐19 Antigen Test (Liming Bio‐Products Co., Jiangsu, China) Catalogue no. 500200; Lot No. 2003014 |
CGIA Not reported in study |
As above | |
| Weitzel 2020 [C] | [C] Huaketai New Coronavirus (SARS‐CoV‐2) N Protein Detection Kit (fluorescence immunochromatography) (Savant Biotechnology Co., Beijing, China) Catalogue no. BCT‐HKT‐050; Lot No. 20031501 |
FIA N protein |
As above plus test required use of manufacturer supplied UV torch due to unavailability of reader device in Chile | |
| Weitzel 2020 [D] | [D] Diagnostic Kit for 2019‐Novel Coronavirus (2019‐nCoV) Ag Test (Bioeasy Biotechnology Co., Shenzhen, China). Catalogue no. YRLF04401025; Lot No. 2002N408 |
FIA N protein |
As above | |
| Rapid molecular tests | ||||
| Assennato 2020 | SAMBA II SARS‐CoV‐2 Test (Diagnostics for the Real World) Product code not reported |
Isothermal PCR ORF1ab, N2 genes |
Samples tested: NP + OP in VTM Timing of sampling: not stated Timing of test: not stated Storage: not stated |
Not stated; presumably laboratory staff Threshold: as per manufacturer; either target present |
| Broder 2020 | GeneXpert Xpress SARS‐CoV‐2 assay (Cepheid Inc) Product code not reported |
RT‐ PCR Not stated (E gene) |
Samples tested: NP Timing of sampling: not stated Timing of test: within 3 days of RT‐PCR Storage: not stated |
Not stated; presumably lab staff Threshold: as per manufacturer |
| Harrington 2020 | ID Now COVID‐19 assay (Abbott Laboratories) Product code not reported |
Isothermal PCR Not stated |
Samples tested: NP (no VTM) Timing of sampling: not stated Timing of test: not stated (soon after collection) Storage: none |
On‐site medical personnel (urgent care centres); laboratory personnel at each separate location (EDs)
‐ 2 sites reportedly experienced users of ID Now (one ED and 1 urgent care centre) and 3 sites received training) Threshold: as per manufacturer |
| Hogan 2020 | Accula SARS‐CoV‐2 POCT (Mesa Biotech, Inc., San Diego, CA) Product code not reported |
RT‐PCR N gene |
Samples tested: NP in VTM or saline Timing of sampling: not stated Timing of test: not stated (possibly soon after collection) Storage: not stated |
Not stated; performed at the SHC Clinical Virology Laboratory Threshold: as per manufacturer |
| Lieberman 2020 [A] | [A] Xpert Xpress (Cepheid) Study also evaluate 4 additional tests not eligible for this review Product code not reported |
RT‐PCR [A] E, N2 genes |
Samples tested: NP in VTM Timing of sampling: not stated Timing of test: < 72 h Storage: 4 °C with no freeze‐thaws |
Not stated; presumably lab staff Threshold: any 1 of 2 targets detected was considered positive for all assays; Xpert Xpress data extracted as per IFU definition (positive = both targets or N gene positive) |
| Loeffelholz 2020 | Cepheid Xpert Xpress SARS‐CoV‐2 (RUO version) (Cepheid Europe) Product code not reported |
RT‐PCR Nucleocapsid gene (N2) and the envelope gene (E) (also detects RdRp but this does not contribute to positivity) |
Samples tested: mixed Timing of sampling: not stated Timing of test: not stated; except 1 site < 2 h (n = 21) Storage: stored at −80 °C; except 1 site tested in real time (n = 21) |
Not stated; presumably lab staff Threshold: as per manufacturer (if both targets are detected, or, if only N2 is detected, the test reports a positive result. If only the E target is detected the test reports a presumptive positive result because this target is shared among some members of the sarbecovirus subgenus of coronaviruses) |
| Mitchell 2020 | ID NOW COVID‐19 (Abbott, Chicago, USA) Product code not reported |
Isothermal PCR Not stated |
Samples tested: NP in VTM Timing of sampling: not stated Timing of test: not stated Storage: stored at −80 ℃ |
Certified laboratory personnel Threshold: as per manufacturer |
| Moore 2020 | ID NOW (Abbott Laboratories) Product code not reported |
Isothermal PCR RdRp gene |
Samples tested: NP in VTM Timing of sampling: not stated Timing of test: < 72 h from collection Storage: none, or stored at 4 °C (if testing could not be completed on the same day) |
Not stated; presumably lab staff Threshold: as per manufacturer |
| Moran 2020 | Xpert Xpress SARS‐CoV‐2 assay (Cepheid, Sunnyvale, CA) Product code not reported |
RT‐PCR E, N (N2 region) genes |
Samples tested: NP or nasal Timing of sampling: not stated Timing of test: not stated Storage: not stated |
Not stated; presumably lab staff Threshold: as per manufacturer |
| Rhoads 2020 | [A] ID Now (Abbott; Chicago, USA) Product code not reported Also evaluates [B] Simplexa (Diasorin; Saluggia, Italy); not eligible for this review |
Isothermal PCR Not stated |
Samples tested: NP in VTM or nasal in saline Timing of sampling: not stated Timing of test: not stated Storage: not stated |
Not stated; presumably lab staff Threshold: as per manufacturer |
| Smithgall 2020 [A] | [A] ID Now (Abbott) Product code not reported |
Isothermal PCR [A] RdRp gene |
Samples tested: NP in VTM or nasal in saline Timing of sampling: not stated Timing of test: within 48 h collection Storage: stored at 4 °C |
Not stated; presumably lab staff Threshold: as per manufacturer |
| Smithgall 2020 [B] | [B] Xpert Xpress (Cepheid) Product code not reported |
RT‐PCR [B] N2, E genes |
||
| Wolters 2020 | Xpert Xpress SARS‐CoV‐2 (Cepheid Europe) Product code not reported |
RT‐PCR E‐gene (sarbeco‐specific) and N2‐gene (SARS‐CoV‐2‐specific) |
Samples tested: NP or OP in VTM or GLY Timing of sampling: not stated Timing of test: not stated Storage: stored at −80 ℃ |
Not stated; presumably lab staff Threshold: as per manufacturer: E‐gene only positive specimens considered ‘SARS‐CoV‐2 presumptive positive’ and require retesting, N2 only positives deemed positive |
| Zhen 2020 [A] | [A] Xpert Xpress SARS‐CoV‐2 (Cepheid) Product code not reported |
RT‐ PCR [A] N2, E genes |
Samples tested: NP in VTM Timing of sampling: not stated Timing of test: for routine testing up to 72 h; 20 samples tested prospectively after collection on all systems Storage: for routine testing (ePlex) stored at 2‐8 ⁰C; then stored at −80 ⁰C (ID Now, Xpert Xpress and Hologic RT‐PCR); 20 samples tested prospectively after collection on all systems |
Not stated; presumably lab staff Threshold: as per manufacturer |
| Zhen 2020 [B] | [B] ID NOW COVID‐19 (Abbott) Product code not reported Also evaluates [C] ePlex SARS‐CoV‐2 Test (GenMark); not eligible for this review |
Isothermal PCR [B] RdRp gene |
||
| CGIA: colloidal gold immunoassay; ED: emergency department; EUA: Emergency Use Authorisation; FIA: fluorescent immunoassay; GLY: gelatin‐lactalbumin‐yeast; IFU: instructions for use; NP: nasopharyngeal; OP: oropharyngeal; PCR: polymerase chain reaction; RT‐PCR: reverse transcription polymerase chain reaction; SD: standard deviation; UV: ultraviolet; VTM: viral transport medium | ||||
Appendix 9. Index test details from manufacturer instructions for use documents
| Index testa | Type of assay Throughput Time to result | Equipment Kit storage | Sample types | Transport medium | Sample storage | Test interpretation |
| Antigen tests | ||||||
| SARS‐Cov‐2 Antigen Fluorescence Rapid Detection Kit (Beijing Savant) IFU: not obtained; no mention of any COVID tests on website |
IFU not obtained | IFU not obtained | IFU not obtained | IFU not obtained | IFU not obtained | IFU not obtained |
| COVID‐19 Ag Respi‐Strip (Coris BioConcept) IFU: 5723/TB/V03 |
CGIA (paper strip method) Single test 15 min |
Paper strips in a bottle with desiccant; LY‐S dilution buffer (3.5 mL or 15 mL; tubes and stoppers); 4 to 30 °C |
NPs or culture extracted solution; samples must be liquid | A gel or a sponge matrix can be used | ASAP, any delay may result in a low signal intensity. If not, store frozen at −20 °C | Visual; read through collection tube. Control line only (negative), T line (with or without control (positive), no control line (invalid) |
| COVID‐19 Antigen Rapid Test Device (StrongStep®) (Liming Bio‐Products Co., Ltd) IFU: obtained via Weitzel 2020 [A]; REF 500200 v1 |
CGIA Single test 15 min |
Test device, extraction buffer vial, extraction tubes, workstation for holding tubes 2‐30°C |
NP or OP | Not mentioned in IFU | ASAP; can be held in clean, dry plastic tube or sleeve up to 72 h at 15‐30 °C, or 2‐8 °C before processing | Visual; 2 coloured bands for positive; control band only for negative; test line only is invalid |
| BIOCREDIT COVID‐19 Ag (RapiGEN Inc) IFU: I‐H0734‐E00(2020.04.03) |
CGIA Single test 5‐8 min |
Test device, assay diluent tube and filter cap, swab for NP collection; 1‐40 °C |
NP swab | Not mentioned in IFU | Test ASAP after collection; if storage required then 2‐8 °C for up to 12 h, or −20 °C for up to 24 h | Visual; control line only (negative), control and test lines (positive), no control line (invalid) |
| BIOEASY 2019‐nCoV Ag Fluorescence Rapid Test Kit (Time‐Resolved Fluorescence) (Shenzhen Bioeasy Biotechnology Co, Ltd) IFU: TS‐IU‐F027‐A2 (YRLF04401025/ YRLF04401050/ YRLF04401100) |
FIA Single test 10 min |
Test card, extraction solution, extraction tube, dripper, swab and ID chip. Test runs on immunofluorescence analyser (supplied separately), transfer pipette also required | Nasal swabs, throat swabs and deep sputum samples | Not mentioned in IFU | ASAP after collection, or store at 2‐8 °C for ≤ 24 h; or store at −70 °C for longer periods. Avoid repeated freezing and thawing (no more than 3 times). | Automatic; positive if both detection line and control line detect a fluorescent signal, and the detection line detection value is ≥ 0.005 ng/mL; negative if fluorescent signal on control line only; invalid if no fluorescent signal, or signal only on test line |
| Rapid molecular testsa | ||||||
| ID NOW COVID‐19 (Abbott Diagnostics Scarborough Inc) IFU: IN190000 v1 |
Isothermal nucleic acid amplification 1 cartridge per run 5‐13 min |
Sample receiver (with elution/lysis buffer), test base (with 2 sealed reaction tubes, each containing a lyophilised pellet), transfer cartridge for transfer of the eluted sample to the test base, positive and negative control swabs; requires ID NOW Instrument | Throat, nasal, NP and OP swabs (direct testing or in listed VTM) | Early versions of IFU documents multiple options but now not recommended (ID NOW COVID‐19 Product Insert, IN190000 Rev.3 2020/04:6‐8) | ASAP after collection, otherwise hold in original package at room temperature (15‐30 °C) for up to 2 h. If longer then store at 2‐8 °C for up to 24 h from collection. No mention of frozen storage | Automatic; results displayed on the instrument screen as positive, negative or presence or absence of COVID‐19 Viral RNAs cannot be determined |
| Xpert Xpress SARS‐CoV‐2 test (Cepheid) IFU: XPRSARS‐COV2‐10 |
Automated RT‐PCR 1‐80 cartridges according to GeneExpert system used 45 min |
Single‐use disposable cartridges that hold the RT‐PCR reagents and host the RT‐PCR process, transfer pipette; run on GeneExpert System | NP swab in VTM | Swab stored in viral transport tube containing 3 mL transport medium | Store at room temperature (15–30 °C) for up to 8 h or refrigerate (2–8 °C) up to 7 days until testing performed | Automatic; displayed positive (N2+ and E+, or N2+ only), presumptive positive (E+ only), negative (both negative), no result (repeat test), instrument error |
| Accula SARS‐Cov‐2 Test (Mesa Biotech Inc.) IFU: LBL‐60058 Rev A (COV4100) |
RT‐PCR + LFA 1 cartridge per run 30 min |
Each test kit contains: test cassette, SARS‐CoV‐2 buffer (5.0 mL), single‐use fixed volume pipette, positive + negative control swabs; Accula or Silaris dock required to run test | Throat swab and nasal swab per test; direct testing only * check this ‐ Hogan 2020 reports use of NP swabs only | Not recommended and will invalidate the test | Prepared sample (in buffer vial) may be stored at room temperature for up to 24 h or refrigerated (2‐8b °C) and tested within 72 h of sample collection. Sample may be stored for up to 1 week at −20 °C | Visually interpretation (shown as blue test and control lines on exterior of test cassette): positive (any test line at T position, with or without control line C, but with no negative control line), negative (control line only with no negative control line), invalid (appearance of negative control line or all lines absent) |
| SAMBA II COVID‐19 Test (Diagnostics for the Real World Ltd) IFU: REF 8500‐12 |
Isothermal PCR Single test per run 1.5 h |
Each test set contains 4 cartridges for extraction, amplification and detection of the amplification products, 2 ml SCoV buffer, fixed volume pipette, 300 μL + pipette tips or transfer pipettes 300 μL, sample collection tube and sample card; SAMBA II Assay Module and Tablet module both required to run the test; 2‐37°C |
Combined nose and throat swabs, NP/OP swabs | Direct testing or UTM/VTM can be used; no limitations on type of VTM recorded in IFU | Store at 2‐30 °C for up to 18 h prior to testing. Freezing of samples should be avoided |
Automatic; presented and stored on the connected tablet ‐ Tablet module result: negative, positive, invalid, halted, read failure or no results; Visual reading of test strip: internal control line only (Negative), ≥ 1 of 2 test lines (ORF and or N lines) with or without internal control line (positive), no lines (invalid); other combinations possible in rare cases |
| ASAP: as soon as possible; CGIA: colloidal gold immunoassay; IFU: instructions for use; NP: nasopharyngeal; NPS: nasopharyngeal swab; OP: oropharyngeal; RNA: ribonucleic acid; RT‐PCR: reverse transcription polymerase chain reaction; UVM: universal transfer medium; VTM: viral transport medium | ||||||
| aThe reported product codes are as reported in the instructions for use documents and may diverge from those evaluated in the included studies (product codes were reported in only two of 18 studies). | ||||||
Appendix 10. Study‐level assessments of study quality
8.
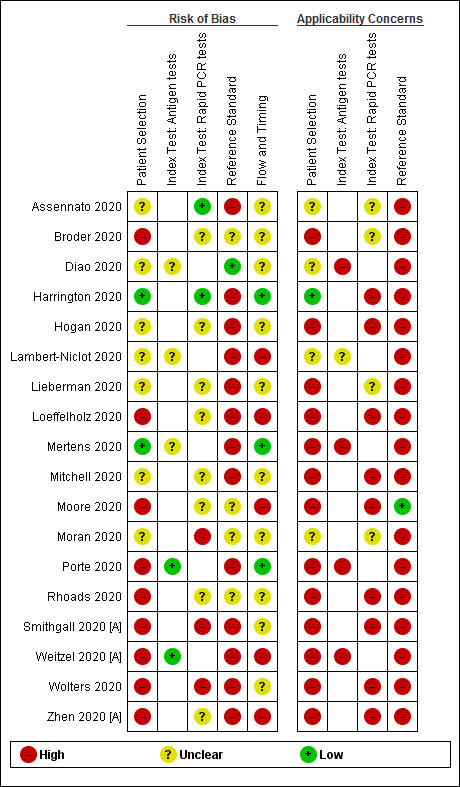
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Appendix 11. Planned heterogeneity investigations
| Test subgroups | Number of studies (test evaluations) | Number of COVID‐19 cases | Number of non COVID‐19 cases |
| Study design | |||
| Antigen tests | |||
| Single group – sensitivity and specificity | 3 (3) | 434 | 271 |
| Two or more groups – sensitivity and specificity | 5 (2) | 328 | 147 |
| Rapid molecular tests | |||
| Single group – sensitivity and specificity | 6 (6) | 425 | 561 |
| Two or more groups – sensitivity and specificity | 5 (7) | 688 | 520 |
| Sample type | |||
| Antigen tests | |||
| NP only | 3 (3) | 434 | 271 |
| NP+OP | 5 (2) | 328 | 147 |
| Mixed (3 or more types) | 0 (0) | n/a | n/a |
| Rapid molecular tests | |||
| NP only | 6 (7) | 529 | 595 |
| NP+OP | 1 (1) | 88 | 84 |
| Mixed (3 or more types) | 4 (5) | 496 | 402 |
| NP: nasopharyngeal; OP: oropharyngeal | |||
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 Antigen tests ‐ All | 8 | 1180 |
| 2 Antigen tests ‐ high viral load | 7 | 400 |
| 3 Antigen tests ‐ low viral load | 7 | 341 |
| 4 Molecular tests ‐ all | 15 | 2325 |
| 5 Molecular tests ‐ all (before discrepant analysis) | 4 | 1280 |
| 6 Molecular tests ‐ all (after discrepant analysis) | 4 | 1280 |
| 7 Molecular tests ‐ high viral load | 5 | 151 |
| 8 Molecular tests ‐ low viral load | 5 | 142 |
1. Test.
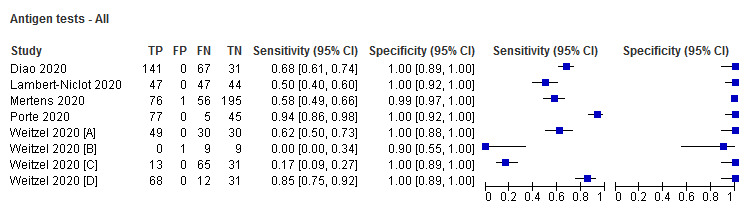
Antigen tests ‐ All
2. Test.
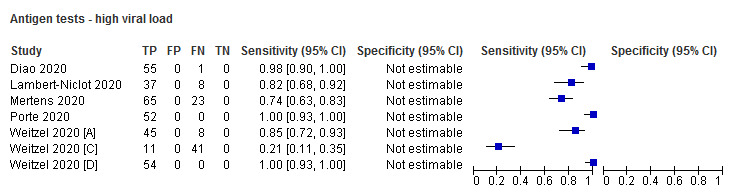
Antigen tests ‐ high viral load
3. Test.
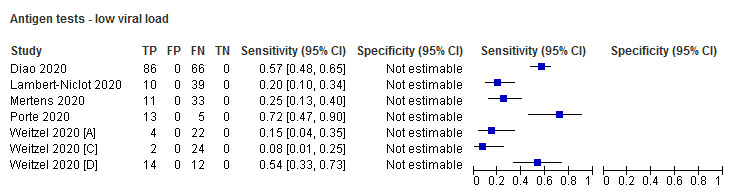
Antigen tests ‐ low viral load
4. Test.
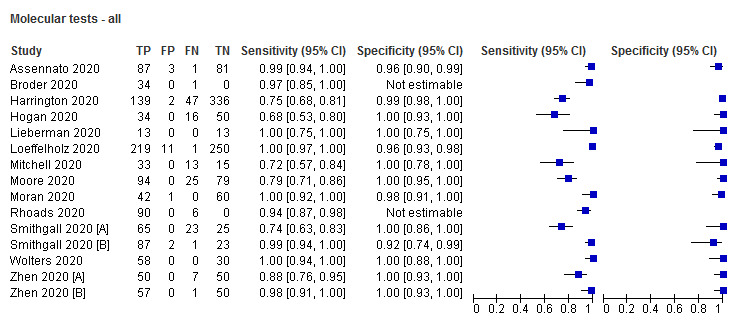
Molecular tests ‐ all
5. Test.
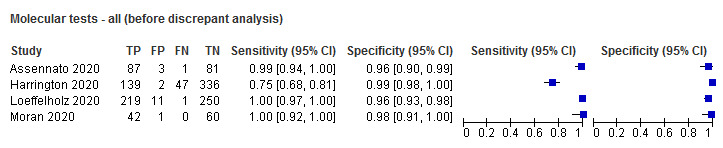
Molecular tests ‐ all (before discrepant analysis)
6. Test.
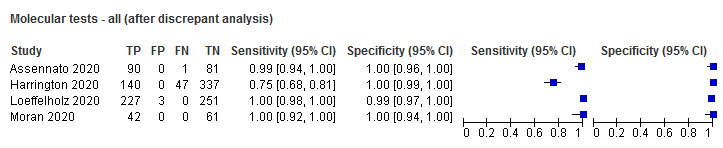
Molecular tests ‐ all (after discrepant analysis)
7. Test.
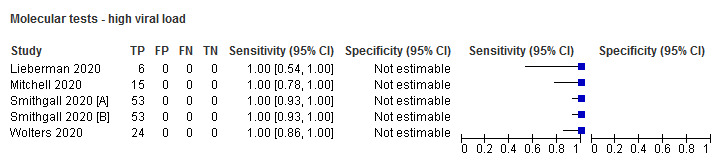
Molecular tests ‐ high viral load
8. Test.
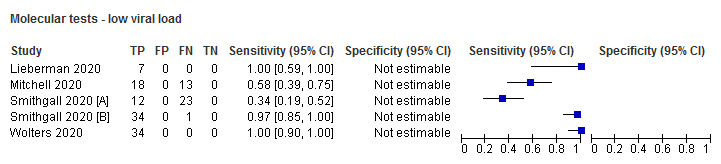
Molecular tests ‐ low viral load
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Assennato 2020.
| Study characteristics | |||
| Patient Sampling | Single‐group study to estimate sensitivity and specificity:
‐ samples from symptomatic individuals with suspected COVID‐19 sent for routine laboratory diagnosis; supplied via PHE (n = 172) Recruitment: not stated Prospective or retrospective: retrospective Number of samples (samples with confirmed SARS‐CoV‐2): 172 (88) |
||
| Patient characteristics and setting | Setting: not stated; supplied by PHE Location: PHE, Cambridge Laboratory (samples from East of England) Country: UK Dates: not stated Symptoms and severity: symptomatic; no further details Demographics: not stated Exposure history: not stated |
||
| Index tests | Test name: SAMBA II SARS‐CoV‐2 Test Manufacturer: Diagnostics for the Real World Antigen target: ORF1ab, N2 Antibody: N/A Test method: rapid PCR Samples used: combined nose and throat swab samples, provided as VTM Transport media: samples diluted 1:2 with SAMBA SCoV buffer Sample storage: not stated Test operator: not stated; presume laboratory staff Definition of test positivity: as per manufacturer; either target present Blinding reported: yes; states that samples were rendered anonymous and provided blinded for the purpose of test validation Timing of samples: not stated |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR; (1) Cambridge RdRp gene (Wuhan) assay on the Rotor gene Q real‐time PCR assay routinely used by PHE; Ct ≤ 36 considered positive. (2) Samples also tested with the PHE Colindale (Reference Laboratory) assay Definition of non‐COVID cases: Single RT‐PCR negative Genetic target(s): (1) RdRp, E gene, (2) RdRp 'different region' Samples used: combined nose and throat swab in VTM; same as for index test Timing of reference standard: not stated; Cambridge assay seems to have been part of routine testing near to time of sample collection; not clear if Colindale assay was at a later date after a period of storage Blinded to index test: not stated but seems yes for Cambridge assay Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: not stated; seems likely reference was carried out for routine diagnostic testing All participants received same reference standard: yes (all samples underwent both RT‐PCR tests) Missing data: none reported Uninterpretable results: none reported Indeterminate results (index test): 3 FP and 1 FN result retested using SAMBA‐II; same results obtained on repeat Indeterminate results (reference standard): 3 FP and 1 FN result were re‐tested ‐ all 3 FPS found to be borderline positive for ≥ 1 target gene on either Colindale or Cambridge (Wuhan) test (reclassified as TP) ‐ the FN result remained positive on both RT‐PCR assays Unit of analysis: refers to participants rather than samples |
||
| Comparative | |||
| Notes | Funding: RKG is funded by Wellcome Senior Fellowship In Clinical Science award no WT108082AIA Publication status: preprint Source: medRxiv Author COI: no COI statement reported; 3 co‐authors are affiliated to test manufacturer |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Broder 2020.
| Study characteristics | |||
| Patient Sampling | Single‐group study to estimate sensitivity:
‐ samples positive on Roche cobas 6800 assay in lower range of viral load (E target Ct ≥ 30) (n = 35) Recruitment: not stated; deliberate sampling according to viral load Prospective or retrospective: unclear Number of samples (samples with confirmed SARS‐CoV‐2): 35 (35) |
||
| Patient characteristics and setting | Setting: not stated Location: not stated; author institution Emory University School of Medicine, Atlanta Country: USA Dates: not stated Symptoms and severity: not stated; lower viral load Demographics: not stated Exposure history: not stated |
||
| Index tests | Test name: GeneXpert Xpress SARS‐CoV‐2 assay (no product code reported) Manufacturer: Cepheid Antigen target: not stated E gene Antibody: N/A Test method: rapid PCR Samples used: NP swabs in VTM Transport media: not stated Sample storage: within 3 days of initial testing (with RT‐PCR) Test operator: not stated; presume laboratory staff Definition of test positivity: not stated; “all specimens were tested using the manufacturer’s protocol”, no mention of presumptive positives Blinding reported: not stated Timing of samples: not stated |
||
| Target condition and reference standard(s) | Reference standard: Roche cobas 6800 SARS‐CoV‐2 assay Definition of non‐COVID cases: N/A Genetic target(s): E gene (unclear if other genetic targets as well) Samples used: NP swabs (as for index test) Timing of reference standard: not stated; presume on presentation Blinded to index test: not stated; presume yes Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: same samples; index within 3 days of reference All participants received same reference standard: yes Missing data: none reported Uninterpretable results: none reported Indeterminate results (index test): none reported Indeterminate results (reference standard): discrepancies resolved using modified CDC RT‐PCR; 1 FN confirmed as disease negative (i.e. a TN) Unit of analysis: not stated; refers only to samples |
||
| Comparative | |||
| Notes | Funding: no funding described Publication status: accepted manuscript Source: Journal of Clinical Microbiology Author COI: Dr. Kraft participated on a Roche advisory board regarding COVID serology. All other study authors have no conflicts |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Diao 2020.
| Study characteristics | |||
| Patient Sampling | Single group estimating sensitivity and specificity for detecting active disease
‐ samples from cases of suspected SARS‐CoV‐2 infection (n = 239) Recruitment: not stated if participants were consecutive Prospective or retrospective: retrospective Number of samples (samples with confirmed SARS‐CoV‐2): 239 (208) |
||
| Patient characteristics and setting | Setting: hospital (inpatients) Location: 7 centres, including General Hospital of Central Theatre Command, Wuhan No.7 People’s Hospital, Wuhan Pulmonary Hospital, Hubei Maternal and Child Hospital, Taikang Hospital, Hanyang Hospital and Wuguo Hospital. Urine study done in Southwest Hospital in Chongqing Country: China Dates: not stated Symptoms and severity: not stated Demographics: not stated Exposure history: not stated |
||
| Index tests | Test name: not stated Manufacturer: in house (but study authors affiliated to Bioeasy Technology) Antibody: monoclonal antibody Antigen target: nucleocapsid protein (N‐antigen) Test method: FIA (fluorescence immunochromatographic); requires immunofluorescence analyser Samples used: NP (all), urine (subgroup) Transport media: samples diluted and mixed in 500 μL saline solution; 100 μL transferred to the sample well of the test card Sample storage: not reported Test operator: not stated; presume laboratory staff Definition of test positivity: cut‐off value was determined by testing 100 nasal swab samples of healthy people and calculated as the mean value of the fluorescence signal plus 5 SD. Blinding reported: done in parallel; blinded Timing of samples: not stated |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR (Daan Gene kit); performed on ABI Prism 7500 and Light Cycler 480 real‐time PCR system. Threshold < 40 Ct; threshold < 30 Ct also investigated
Definition of non‐COVID cases: all participants underwent 3 nucleic acid tests, and the results of each nucleic acid test were verified by 2 COVID‐19 nucleic acid test kits. Genetic target(s): ORF1ab and N gene Samples used: NP swab, same as for index test Timing of reference standard: not stated Blinded to index test: done in parallel; blinded Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: done in parallel All participants received same reference standard: yes Missing data: not reported Uninterpretable results: not reported Indeterminate results (index test): none reported Indeterminate results (reference standard): none described Unit of analysis: participants |
||
| Comparative | |||
| Notes | Funding: this research was supported by grants from National Key R&D Program of China (2016YFA0502204); Chongqing Health Commission COVID‐19 Project (2020ZX01). Publication status: preprint (not peer‐reviewed) Source: medRxiv preprint Author COI: study authors declare no COI present; 1 affiliated to Shenzhen Bioeasy Biotechnology Co. Ltd. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Harrington 2020.
| Study characteristics | |||
| Patient Sampling | Single‐group study to estimate sensitivity and specificity:
‐ symptomatic patients meeting diagnostic criteria for COVID‐19 (n = 524) Recruitment: consecutive Prospective or retrospective: unclear; presume prospective Number of samples (samples with confirmed SARS‐CoV‐2): 524 (186) |
||
| Patient characteristics and setting | Setting: ED (n = 3) or urgent (immediate) care centres (n = 2) Location: not stated; author institutions Loyola University Medical Centre, Cedars‐Sinai Medical Centre Country: USA Dates: not reported Symptoms and severity: not stated Demographics: not stated Exposure history: not stated |
||
| Index tests | Test name: ID NOW COVID‐19 assay (no product code provided) Manufacturer: Abbott Antigen target: not stated Antibody: N/A Test method: not stated; isothermal PCR Samples used: nasal swabs (provider collected) Transport media: none; direct testing after heat inactivation Sample storage: ED swabs transported in sterile transport containers (using cups or conical tubes) Test operator: on‐site medical personnel (urgent care centres); laboratory personnel at each separate location (EDs) ‐ 2 sites reportedly experienced users of ID NOW (one ED and one urgent care centre) and 3 sites received training) Definition of test positivity: as per manufacturer Blinding reported: yes (RT‐PCR performed at separate central lab) Timing of samples: not stated; on presentation |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR (Abbott RealTime SARS‐CoV‐2 (ACOV) assay performed on the Abbott m2000 system (Abbott Molecular Inc. Des Plaines, IL); threshold not stated Definition of non‐COVID cases: not specifically stated; presume yes as central lab used Genetic target(s): not stated Samples used: NP swabs Timing of reference standard: VTM (no detail) Blinded to index test: not stated, transferred to central clinical laboratory; samples heat inactivated for 30 min at 60 °C prior to testing Incorporated index test: no (paired collection with swabs for index test) |
||
| Flow and timing | Time interval between index and reference tests: simultaneous swab collection (different swabs for index and reference) All participants received same reference standard: yes Missing data: none reported Uninterpretable results: none reported Indeterminate results (index test): none reported Indeterminate results (reference standard): 2 initial FPs had repeat sampling: ‐ 1 retested on RT‐PCR only and was positive (designated as TP) ‐ 1 retested on RT‐PCR and ID NOW and was negative on both (designated as FP based on original sampling) Unit of analysis: participants |
||
| Comparative | |||
| Notes | Funding: study authors received "received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors" Publication status: accepted manuscript Source: Journal of Clinical Microbiology Author COI: COI not mentioned |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hogan 2020.
| Study characteristics | |||
| Patient Sampling | Single‐group design to estimate sensitivity and specificity
‐ samples from adult patients from 1 hospital and paediatric and adult samples from surrounding hospitals Recruitment: unclear; equal numbers of positive and negative RT‐PCR samples (suspect deliberate sampling by PCR result) Prospective or retrospective: not stated Number of samples (samples with confirmed SARS‐CoV‐2): 100 (50) |
||
| Patient characteristics and setting | Setting: hospital; not stated if inpatient or outpatient (samples selected from clinical virology laboratory) Location: Stanford Health Care (hospital), and surrounding hospitals (not named) Country: USA Dates: 7‐13 April 2020 Symptoms and severity: not stated Demographics: not stated Exposure history: not stated |
||
| Index tests | Test name: Accula SARS‐CoV‐2 POCT (no product code reported) Manufacturer: Mesa Biotech, Inc., San Diego, CA Antigen target: N gene Antibody: N/A Test method: rapid PCR Samples used: NP swabs in VTM (n = 37) or saline (n = 63, including 37 positive on RT‐PCR) Transport media: not stated; 10 μL of VTM or saline was transferred to 60 μL of SARS‐CoV‐2 buffer within a biosafety cabinet (not covered by manufacturer IFU) Sample storage: not stated; testing appears to have been conducted soon after sample collection Test operator: not stated; presume laboratory staff Definition of test positivity: as per manufacturer Blinding reported: not stated Timing of samples: not stated |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR; in‐house SHC assay (cites Hogan 2020 10.1016/j.jcv.2020.104383:104383) Definition of non‐COVID cases: single RT‐PCR negative Genetic target(s): E gene Samples used: NP swabs, same as for index test Timing of reference standard: not stated Blinded to index test: not stated Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: not stated but implies that both tests undertaken in laboratory soon after sample collection All participants received same reference standard: yes Missing data: none reported Uninterpretable results: 3 invalid results were re‐tested; 1 positive and 2 negative Indeterminate results (index test): 1 known RT‐PCR‐positive sample that showed a faint positive test line was re‐tested and again showed the same faint test line (considered positive) Indeterminate results (reference standard): none reported Unit of analysis: refers to participants |
||
| Comparative | |||
| Notes | Funding: study authors report no specific funding Publication status: preprint Source: medRxiv Author COI: authors declare no COI present |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Lambert‐Niclot 2020.
| Study characteristics | |||
| Patient Sampling | Single‐group study to estimate sensitivity and specificity:
‐ samples submitted for RT‐PCR testing (n = 138) Recruitment: not stated Prospective or retrospective: unclear; testing conducted prospectively Number of samples (samples with confirmed SARS‐CoV‐2): 138 (94) |
||
| Patient characteristics and setting | Setting: not stated Location: samples collected from virology laboratories of 3 university hospital groups from Assistance‐Publique‐Hôpitaux de Paris (APHP), (Saint‐Antoine‐Tenon‐Trousseau, Saint‐Louis‐Lariboisière and Kremlin Bicêtre‐Paul Brousse) Country: France Dates: 1‐15 April 2020 Symptoms and severity: not stated Demographics: not stated Exposure history: not stated |
||
| Index tests | Test name: COVID‐19 Ag Respi‐Strip CORIS (no product code) Manufacturer: BioConcept, Gembloux, Belgium Antigen target: SARS‐CoV‐2 NP Antibody: monoclonal antibodies Test method: CGIA Samples used: NP swabs in VTM (collection process not described) Transport media: either of: COPAN UTM 3 mL, Virocult 1 mL, Eswab Amies 1 mL, 4MRT 3 mL, 0.9% NaCl buffer and cobas ROCHE Sample storage: no cooling or freezing step used Test operator: not stated; presume laboratory staff Definition of test positivity: not stated; as per manufacturer Blinding reported: not stated Timing of samples: not stated; presume on presentation |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR (different kits used including RealStar Altona®, Anatolia®, cobas 6800 Roche®, Allplex™ 2019‐nCoV Assay Seegene®) Definition of non‐COVID cases: single negative PCR Genetic target(s): E gene Samples used: NP swabs (same as for index) Timing of reference standard: within a few hours after collection; time post onset of symptoms not reported Blinded to index test: unclear Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: same sample, both tests conducted within a few hours All participants received same reference standard: yes (different kits) Missing data: none reported Uninterpretable results: 4 samples collected in cobas VTM gave invalid results and all samples in cobas medium were excluded Indeterminate results (index test): control lines reported as "barely visible" for 9 positive and 8 negative tests Indeterminate results (reference standard): none reported Unit of analysis: not reported, but samples tested on day of collection so considered to be 1 per participant |
||
| Comparative | |||
| Notes | Funding: no funding sources reported Publication status: accepted manuscript Source: Journal of Clinical Microbioloby Author COI: no conflict of interest statement reported |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Lieberman 2020.
| Study characteristics | |||
| Patient Sampling | Single‐group study to estimate sensitivity and specificity:
‐ samples submitted for clinical diagnostic testing (n = 169; not all samples analysed for all tests) Recruitment: not stated Prospective or retrospective: retrospective (residual samples) Number of samples (samples with confirmed SARS‐CoV‐2): 169 (87) |
||
| Patient characteristics and setting | Setting: not stated; sampled from laboratory Location: Washington State Public Health Laboratory Country: USA Dates: not stated Symptoms and severity: not stated Demographics: not stated Exposure history: not stated |
||
| Index tests | Test name: Xpert Xpress Manufacturer: Cepheid Antigen target: E, N2 Antibody: N/A Test method: rapid PCR Samples used: NP swabs (collection not described) Transport media: 300 μL of VTM sample Sample storage: all same‐sample comparisons were performed on specimens stored at 4 °C for < 72 h with no freeze‐thaws Test operator: not stated; presume laboratory staff Common panel of 26 specimens tested at UW by the UW CDC EUA‐based LDT or at LabCorp Seattle Definition of test positivity: 1 of 2 targets detected was considered positive for all assays; Xpert Xpress data extracted as per IFU definition (positive = both targets or N gene positive; E‐gene‐positive requires retest) Blinding reported: not stated Timing of samples: not stated Also evaluates: [B] Hologic Panther Fusion RUO, [C] Hologic Panther Fusion EUA, [D] Diasorin Simplexa, [E] Roche cobas 6800 in same 26 samples and in additional residual specimens (n = 115) at UW (different N per test) |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR; UW CDC EUA‐based in‐house test (positive if 1 of 2 targets detected ‐ presume at < 40 Ct) Definition of non‐COVID cases: single negative PCR Genetic target(s): NI, N2 Samples used: NP swabs, as for index test Timing of reference standard: not stated Blinded to index test: not stated Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: all testing conducted within 72 h All participants received same reference standard: yes Missing data: none reported; review team excluded data for 28 specimens comparing Panther Fusion with DiaSorin Simplexa Uninterpretable results: not stated Indeterminate results (index test): ‘Inconclusive' results (i.e. 1 genetic target detected) were considered positive due to the high specificity of all assays and limited cross‐reactivity seen for SARS‐CoV‐2 primer sets. For Xpert Xpress only 12/13 were positive according to IFU specifications on first test (both targets present, or N gene positive); on retesting the presumptive positive became positive (detection of E‐gene but not N‐gene) Indeterminate results (reference standard): as for index test Unit of analysis: not stated, only refers to samples |
||
| Comparative | |||
| Notes | Funding: no funding statement reported Publication status: accepted manuscript Source: Journal of Clinical Microbioloby Author COI: no COI statement reported |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Loeffelholz 2020.
| Study characteristics | |||
| Patient Sampling | 2‐group study to estimate sensitivity and specificity for diagnosis of active disease
‐ suspected patients referred for COVID‐19 testing at 7 sites according to the local criteria (n = 486); sampled to enrich for RT‐PCR‐positive specimens (not further described) Recruitment: convenience (in addition, 1 site (LAC+USC) tested specimens from a 4‐day point prevalence survey of patients presenting with COVID‐19 symptoms) Prospective or retrospective: retrospective Number of samples (samples with confirmed SARS‐CoV‐2): 486 (220) |
||
| Patient characteristics and setting | Setting: not stated Location: 7 sites: Johns Hopkins University, Baltimore; LAC+USC Medical Centre, University of Southern California, Los Angeles; Manchester University NHS Foundation Trust Manchester; Mondor Hospital, Paris; New York City Dept. Health and Mental Hygiene, NYC; Niguarda Hospital, Milan; University Hospital, Newark. Country: USA, UK, France, Italy Dates: 1 March‐2 April 2020 Symptoms and severity: not stated Demographics: adults at all sites except New York City Dept. Health and Mental Hygiene and Niguarda Hospital where all age groups were tested (ages not stated) Exposure history: not stated |
||
| Index tests | Test name: Cepheid Xpert Xpress SARS‐CoV‐2 (RUO version, no product code reported) Manufacturer: Cepheid Europe Antigen target: nucleocapsid gene (N2) and the envelope gene (E) (RUO version also detects RdRp gene but this does not contribute to definition of positive) Antibody: N/A Test method: automated point‐of‐care PCR Samples used: swabs (NP (n = 339), OP (n = 15), combined NP/OP in the same transport vial (n = 97)), and TA (n = 30):
Transport media: VTM (swabs), diluted in saline (TA). 1 site (Manchester) pretreated specimens with an equal volume (≥ 30‐< 50% (w/w)) of a guanidine hydrochloride buffer and heated at 80 °C Sample storage: stored at −80 °C prior to index test, except at 1 site (University Hospital, Newark) where specimens were tested in real time, within 2 h by the Xpert test (n = 21). Test operator: not stated; presume laboratory staff Definition of test positivity: as per manufacturer: if both targets are detected, or if only N2 is detected, the test reports a positive result. If only the E target is detected the test reports a presumptive positive result "because this target is shared among some members of the sarbecovirus subgenus of coronaviruses". The RUO version of the test shows the amplification curves and PCR cycle threshold for all 3 genetic targets. The study reports that "The EUA test version cartridge contains the same reagents as the RUO cartridge. The only difference between the tests is the software which in the EUA version allows the user to see amplification curves and results for the N2 and E targets only". Blinding reported: not stated Timing of samples: not stated, presume on presentation |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR (sites using each kit not reported, added by review team based on number of samples per site and per RT‐PCR kit)
Definition of non‐COVID cases: yes (performed prior to index test) Genetic target(s): different targets depending on RT‐PCR test used:
Tie‐breaker methods (for discrepant results), included: Hologic Panther Fusion (San Diego, USA), Tib‐Molbiol LightMix Modular Wuhan Coronavirus E‐gene RT‐PCR (Roche, Basel, Switzerland); and the CDC assay (IDT primers and probes) Samples used: as for index test Timing of reference standard: as for index test Blinded to index test: no storage; tested in real time Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: same samples but index performed after frozen storage for undefined period of time except at University Hospital, Newark where specimens were tested in real time, within 2 h by the Xpert test All participants received same reference standard: no Missing data: 4 Xpert Xpress test results were lost permanently due to a single instrument computer malfunction Uninterpretable results: 1 Xpert Xpress test was invalid due to a cartridge error (inadequate sample volume) Indeterminate results (index test) presumptive positive results on Xpert Xpress were not reanalysed by Xpert Xpress, but all discrepant results were reanalysed by a third RT‐PCR method Indeterminate results (reference standard): specimens with inconclusive results by a test, and those with discrepant results between Xpert and the RT‐PCR tests were analysed by a third RT‐PCR method 1 FN result was inconclusive on Quest SARS‐CoV‐2, and negative on CDC RT‐PCR; re‐considered as TN Of 11 FPs (including 1 presumptive positive on Xpert Xpress), 2 were negative on both New York SARS‐CoV‐2 and Panther Fusion (remained as FPs), and 9 were negative on in‐house RT‐PCR but positive on Roche RT‐PCR (reclassified as TP) In addition, 12 specimens (8 NP, 4 NP/OP) were inconclusive by the NY (RT)‐ PCR Diagnostic Panel and considered positive for data analysis purposes in the study. Of these, 11 were positive by the Xpert test and 1 was presumptive positive (EUA version of Xpert test). In 4 of these only the N1 target was detected and in 8 only the N2 target was detected by the New York EUA method, all with Ct values > 36 One NP specimen was inconclusive by the Quest SARS‐CoV‐2 rRT‐PCR test and negative by the Xpert test. The Quest test reports inconclusive if only a single target (N1 or N3) is detected. They were unable to determine which target was detected by the Quest test. This specimen was negative by a tie‐breaker NAAT. Unit of analysis: not stated; only samples reported |
||
| Comparative | |||
| Notes | Funding: not stated; presume funded by test manufacturer (see COI statement) Publication status: accepted manuscript Source: Journal of Clinical Microbiolobyogy Author COI: the study was designed and supervised by the sponsor, Cepheid. Data were collected by investigators at each study site, and statistical analyses were performed by a Cepheid author. Cepheid authors wrote the first draft of the manuscript. All study authors vouch for the accuracy and completeness of the data reported. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Mertens 2020.
| Study characteristics | |||
| Patient Sampling | Single‐group study to estimate sensitivity and specificity for diagnosis of active disease:
‐ samples from patients suspected of SARS‐COV‐2 infections (n = 328) Recruitment: random sampling of samples submitted to 3 laboratories 322/328 NP samples (NP swabs) were randomly selected Prospective or retrospective: retrospectively Number of samples (samples with confirmed SARS‐CoV‐2): 328 (132) |
||
| Patient characteristics and setting | Setting: unclear; samples from university laboratories (discussion states that no outpatient population has been sampled, therefore assume inpatients and HCW samples) Location: laboratories at Université Libre de Bruxelles (LHUB‐ULB), UZ Leuven and Centre Hospitalier Universitaire Sart‐Tilman (CHU) Liège Country: Belgium Dates: 19‐30 March 2020 Symptoms and severity: not reported Demographics: not reported Exposure history: unclear; 53/328 samples were from HCW |
||
| Index tests | Test name: COVID‐19 Ag Respi‐Strip Manufacturer: Coris BioConcept (Belgium) Antigen target: SARS‐CoV and SARS‐CoV‐2 highly conserved nucleoprotein Antibody: monoclonal antibodies directed against SARS‐CoV and SARS‐CoV‐2 highly conserved nucleoprotein antigen Test method: immunochromatographic assay using colloidal gold (CGIA) Samples used: remnant respiratory specimens (322 NP swabs, 4 NP aspirate and 2 BAL) Transport media: NP: flocked swab + UTM 3 mL (or 1 mL of Amies) (Copan, Brescia, Italy); NPA: 3 mL VTM (veal infusion broth (Difco, Becton Dickinson, Sparks, MD, USA) supplemented with bovine albumin (Sigma Aldrich, St Louis, MO, USA)) BAL: N/A Sample storage: not described Test operator: laboratory technician Definition of test positivity: visible reddish‐purple band appearing at the Test line position (T) Blinding reported: not stated Timing of samples: not clear |
||
| Target condition and reference standard(s) | Reference standard: qRT‐PCR: RealStar SARS‐CoV‐2 RT‐PCR Kit from Altona‐diagnostics with a cut‐off set at 40 Ct (LHUB‐ULB); Roche LC480 thermocycler using Taqman Fast Virus 1‐Step Master Mix (Thermo Fisher) (Liege); QuantStudio Dx (Thermo Fisher Scientific) or Panther Fusion (PF, Hologic, San Diego, USA) (UZ Leuven) Definition of non‐COVID cases:
Samples used: as for index test (respiratory specimens (322 NP swabs, 4 NP aspirate and 2 BAL) Timing of reference standard: not stated; same samples as for index test but analysed at time of collection Blinded to index test: yes (undertaken for diagnostic purposes at time of collection) Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: same samples used; discussion report 'some delay' between PCR and antigen testing All participants received same reference standard: yes but different RT‐PCR kits Missing data: no Uninterpretable results: none reported; discussion reports some difficulties in visualising the strip through the closed tube requiring the lab technician to open the test tube in the laminar air flow cabinet and pull out the strip with forceps Indeterminate results (index test): weak T lines considered positive Indeterminate results (reference standard): none reported; sensitivity can be extracted for cases with Ct values < or > 25 (high vs lower viral load) Unit of analysis: refers to participants |
||
| Comparative | |||
| Notes | Funding: not stated Publication status: preprint (not peer‐reviewed) Sourcepreprint server (medRxiv) Author COI: the IVD medical device has been developed by the investigator Pascal Mertens, Henri Magein, and Justine Bouzet working for Coris BioConcept (potential conflict of interest declared even though they don’t have any share in this company); Thierry Leclipteux was involved in the development of this test and is the CEO of Coris Bioconcept (potential conflict of interest declared). All scientific investigators that are external to Coris BioConcept declare having no conflict of interest. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mitchell 2020.
| Study characteristics | |||
| Patient Sampling | Single‐group study to estimate sensitivity and specificity for diagnosis of active disease:
‐ samples positive and negative on 1 of 2 SARS‐CoV‐2 RT‐PCR assays Recruitment: not stated; suggests possible deliberate sampling of positive cases Prospective or retrospective: retrospective (residual samples) Number of samples (samples with confirmed SARS‐CoV‐2): 61 (46) |
||
| Patient characteristics and setting | Setting: not stated; 2 independent laboratories (Class II biosafety cabinet (BSC)) Location: not stated; author institutions University of Pittsburgh School of Medicine, Pittsburgh and Laboratory of Viral Diseases, Wadsworth Centre, New York State Department of Health, Albany, NY Country: USA Dates: not stated Symptoms and severity: not stated Demographics: not stated Exposure history: not stated |
||
| Index tests | Test name: ID NOW COVID‐19 (product code not reported) Manufacturer: Abbott, Chicago, USA Antigen target: not stated Antibody: N/A Test method: not stated (should be isothermal PCR) Samples used: NP samples (residual samples) Transport media: VTM; no further detail (no longer covered on IFU) Sample storage: stored at −80 ℃ prior to testing Test operator: certified laboratory personnel Definition of test positivity: not stated; as per manufacturer Blinding reported: not stated Timing of samples: not stated |
||
| Target condition and reference standard(s) | Reference standard: CDC EUA or the New York EUA RT‐PCR assays Definition of non‐COVID cases: single RT‐PCR negative Genetic target(s): not stated Samples used: as for index test Timing of reference standard: as for index test Blinded to index test: not stated; samples analysed at or near time of collection Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: same samples but used at different times (samples used for index test stored at −80 ℃) All participants received same reference standard: no, either the CDC EUA or the New York EUA assays Missing data: none reported Uninterpretable results: none reported Indeterminate results (index test): none reported Indeterminate results (reference standard): none reported Unit of analysis: not stated; only samples reported |
||
| Comparative | |||
| Notes | Funding: not stated Publication status: accepted manuscript Source: Journal of Clinical Virology Author COI: COI not mentioned by study authors |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Moore 2020.
| Study characteristics | |||
| Patient Sampling | 2‐group study to estimate sensitivity and specificity:
‐ samples from symptomatic (fever or cough or shortness of breath) adult and paediatric outpatients, ED patients, and inpatients Recruitment: consecutive (first 94 participants), then all PCR‐positive samples plus the next PCR‐negative sample after each positive sample, to a total of 200 samples Prospective or retrospective: retrospective (participant and sample details extracted from the electronic medical record) Number of samples (samples with confirmed SARS‐CoV‐2): 200 (125) |
||
| Patient characteristics and setting | Setting: mixed (outpatients, ED patients and inpatients) Location: Rush University Medical Centre (RUMC) or Rush Oak Park Hospital (ROPH), Chicago Country: USA Dates: 27 March‐9 April 2020 Symptoms and severity: 79 (39.5%) hospitalised including 29 in ICU, 76 (38%) ambulatory care including 55 seen in a designated COVID‐19 screening clinic), and 45 (23%) seen at ED Demographics: mean age 50 years (SD 17 years), 92 (46%) men Exposure history: not stated |
||
| Index tests | Test name: ID NOW (no product code) Manufacturer: Abbott Antigen target: RdRp Antibody: N/A Test method: rapid PCR (isothermal) Samples used: NP swabs in 3 mL VTM (collection not reported) Transport media: M4‐RT VTM (Remel, Lenexa, KS) Sample storage: stored at 4 °C if all testing could not be completed on the same day; all tests completed within 72 h of collection Test operator: not stated; presume laboratory staff Definition of test positivity: as per manufacturer Blinding reported: not stated Timing of samples: not stated; presumably on presentation but no information on symptom status |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR; 2 methods used in the study
Record review used to verify status of 8 samples positive on RealTime assay and negative (6) or inconclusive (2) on CDC assay (all considered disease‐positive) Definition of non‐COVID cases: single RT‐PCR negative Genetic target(s):
Samples used: NP swabs in VTM, as for index test Timing of reference standard: not stated Blinded to index test: not stated Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: all 3 tests conducted within 72 h of sample collection All participants received same reference standard: no? (all received both RT‐PCR tests, only discordant results on RT‐PCR had record review) Missing data: none reported Uninterpretable results: 2 results were invalid on ID NOW and were not retested (excluded) Indeterminate results (index test): none reported Indeterminate results (reference standard): discordant results between 2 RT‐PCR assays had record review to determine presence/absence COVID‐19 infection Unit of analysis: participants (specimens from 200 unique participants) |
||
| Comparative | |||
| Notes | Funding: none reported (some reagents supplied from NIH) Publication status: preprint Source: medRxiv Author COI: no COI statement was reported |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Moran 2020.
| Study characteristics | |||
| Patient Sampling | Single‐group study to estimate sensitivity and specificity:
‐ specimens collected from inpatients and ambulatory patients at the University of Chicago Recruitment: not stated Prospective or retrospective: not stated Number of samples (samples with confirmed SARS‐CoV‐2): 103 (42) |
||
| Patient characteristics and setting | Setting: inpatient and ambulatory; samples selected from central laboratory Location: Clinical Microbiology Laboratory, University of Chicago Country: USA Dates: not stated Symptoms and severity: not stated Demographics: not stated Exposure history: not stated |
||
| Index tests | Test name: Xpert Xpress SARS‐CoV‐2 assay (no product code) Manufacturer: Cepheid, Sunnyvale, CA Antigen target: E, N (N2 region) Antibody: N/A Test method: rapid PCR Samples used: 8 nasal and 95 NP swabs Transport media: none described Sample storage: not stated Test operator: not stated; presume laboratory staff Definition of test positivity: not stated; re‐testing using Xpert Xpress was undertaken for an N‐gene positive result due discrepancy with RT‐PCR (not in line with IFU recommendation) Blinding reported: not stated Timing of samples: not stated |
||
| Target condition and reference standard(s) | Reference standard: Roche cobas SARS‐CoV‐2 assay on the cobas 6800 system (Roche Molecular Systems, Branchburg, NJ) Definition of non‐COVID cases: single RT‐PCR negative Genetic target(s): ORF1, E Samples used: nasal and NP swabs; same as for index test Timing of reference standard: not stated Blinded to index test: not stated Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: not stated; same sample and appear to have both been conducted soon after sample collection All participants received same reference standard: yes Missing data: none reported Uninterpretable results: none reported Indeterminate results (index test): single FP (negative on E gene and low positive on N gene) was retested with Xpert Xpress and considered negative on both targets Indeterminate results (reference standard): single FP was retested on RT‐PCR and found to be repeatedly negative Unit of analysis: refers to participants |
||
| Comparative | |||
| Notes | Funding: none described Publication status: accepted manuscript Source: Journal of Clinical Microbioloby Author COI: no COI statement was reported |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Porte 2020.
| Study characteristics | |||
| Patient Sampling | 2‐group study to estimate sensitivity and specificity for diagnosis of active disease:
‐ samples from suspected COVID‐19 cases (n = 1453) with deliberate sampling of PCR‐positive and negative cases on a 2:1 basis (n = 127) Recruitment: convenience sampling Prospective or retrospective: retrospectively Number of samples (samples with confirmed SARS‐CoV‐2): 127 (82) |
||
| Patient characteristics and setting | Setting: outpatients attending ED at private medical centre (hospital) Location: Clínica Alemana, Santiago Country: Chile Dates: 16‐21 March 2020 Symptoms and severity: cough 94 (74.6%) Fever 77 (61.1%) Median duration of symptoms of 2 days (IQR 1–4; range 0‐12) Duration of symptoms: day 0‐3 91 (72.2%); day 4‐7 27 (22.4%); day ≥ 8 8 (6.3%) Demographics: 68 male (53.5%), median age 38 years (IQR 29.5–44; range 1–91) Exposure history: not stated |
||
| Index tests | Test name: diagnostic Kit for 2019‐Novel Coronavirus (2019‐nCoV) Ag Test (Cat. N° YRLF04401025, lot N° 2002N408) Manufacturer: Bioeasy Biotechnology Co., Shenzhen, China Antigen target: SARS‐CoV‐2 nucleocapsid protein Antibody: not stated Test method: FIA Samples used: remnant (?) OP and NP swabs in 3 mL UTM Transport media: UTM‐RT System, Copan Diagnostics, Murrieta, CA, USA Sample storage: stored at 4 °C and tested within 48 h Test operator: laboratory technician Definition of test positivity: not stated; test "automatically delivers a positive or negative qualitative result" Positive or negative defined qualitatively Blinding reported: yes Timing of samples: on presentation Within 48 h of the PCR test but it doesn't say when PCR test was performed (median duration of symptoms reported in D9) |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR (COVID‐19 Genesig Real‐Time PCR assay (Primer Design Ltd., Chandler's Ford, UK)); Ct ≤ 40 considered positive Definition of non‐COVID cases: single RT‐PCR negative Genetic target(s): not stated Samples used: as for index test; same OP and NP swabs used Timing of reference standard: median 2 d post symptom onset (IQR 1‐4; range 0‐12) Blinded to index test: yes (index test done within 48 h of PCR test) Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: same sample used; within 48 h All participants received same reference standard: yes Missing data: no Uninterpretable results: not reported Indeterminate results (index test): not reported Indeterminate results (reference standard): not reported Unit of analysis: participants |
||
| Comparative | |||
| Notes | Funding: this work did not receive funding Publication status: preprint (not peer‐reviewed) Source: SSRN Author COI: all study authors declare no competing interests |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Rhoads 2020.
| Study characteristics | |||
| Patient Sampling | Single‐group study to estimate sensitivity:
‐ samples positive using standard of care testing (n = 96)
(14 negative controls (UTM) included to control for carry‐over contamination only) Recruitment: convenience Prospective or retrospective: retrospective (remnant samples) Number of samples (samples with confirmed SARS‐CoV‐2): 96 (96) |
||
| Patient characteristics and setting | Setting: not stated; includes self‐collected and provided‐collected samples Location: not stated; author institutions University Hospitals Cleveland Medical Centre and Case Western Reserve University Country: USA Dates: not stated Symptoms and severity: not stated Demographics: not stated Exposure history: not stated |
||
| Index tests | Test name: ID NOW (product codes not reported) Manufacturer: Abbott; Chicago, USA Also reports evaluation of Diasorin Simplexa (not eligible for this review) Antigen target: not stated Antibody: N/A Test method: isothermal PCR Samples used: nasal swabs (self‐collected) and NP swabs (provider collected); all remnant samples Transport media: nasal swabs (2 mL normal saline) and NP swabs (3 mL UTM) Sample storage: not stated Test operator: not stated; presume laboratory staff Definition of test positivity: not stated; as per manufacturer Blinding reported: not stated Timing of samples: not stated |
||
| Target condition and reference standard(s) | Reference standard: standard of care testing for original samples; remnant samples re‐tested with modified CDC RT‐PCR (using 7500 Fast instrument and using alternate RNA extraction method (Maxwell RSC 6 instrument with Viral TNA Kit (Cat# AS1330; Promega, Madison, USA)); samples with 1 positive target detected considered positive instead of "inconclusive" Definition of non‐COVID cases: as for index test Genetic target(s): N1 and N2 Samples used: as for index test Timing of reference standard: as for index test Blinded to index test: as for index test Incorporated index test: as for index test |
||
| Flow and timing | Time interval between index and reference tests: same samples used All participants received same reference standard: yes Missing data: none reported Uninterpretable results: none reported Indeterminate results (index test): none reported Indeterminate results (reference standard): RT‐PCR detected only 1 of 2 targets for 2 samples (both considered positive (diagnosed as positive on original sample testing); both were negative on index test) Unit of analysis: not stated; only samples reported |
||
| Comparative | |||
| Notes | Funding: no outside funding used to support the investigation Publication status: accepted manuscript Source: Journal of Clinical Microbioloby Author COI: COI not mentioned by study authors |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Smithgall 2020 [A].
| Study characteristics | |||
| Patient Sampling | 2‐group study to estimate sensitivity and specificity:
‐ patients undergoing routine clinical testing by RT‐PCR (n = 113) Recruitment: unclear; describes deliberate sampling of samples with high, medium and low Ct values on the reference standard RT‐PCR Prospective or retrospective: unclear; residual swabs used but testing undertaken within 48 h of sample collection Number of samples (samples with confirmed SARS‐CoV‐2): 113 (88) |
||
| Patient characteristics and setting | Setting: inpatient and ED (n from each not reported) Location: not stated; author institution is Columbia University Irving Medical Centre Country: USA Dates: 8‐13 April 2020 Symptoms and severity: not stated Demographics: 111 adult (range 23‐101 years; average 65 years for RT‐PCR‐positive and 43 years for RT‐PCR‐negative); 2 paediatric (age 1 day and 5 days) 61, 54% male Exposure history: not stated |
||
| Index tests | Test name: [A] ID NOW (see Smithgall 2020 [B] for details of comparator test)
(product codes not reported) Manufacturer: [A] Abbott Antigen target: [A] RdRp gene Antibody: N/A Test method: [A] isothermal PCR Samples used: residual NP swabs (collection not described) Transport media: 3 mL VTM (M4RT VTM; ThermoFisher Scientific, Waltham, MA) or UTM (UTM; Becton Dickinson and Co., Franklin Lakes, NJ) Sample storage: stored at 4 °C; testing completed within 48 h of sample collection Test operator: not stated; presume laboratory staff Definition of test positivity: automated as per manufacturer Blinding reported: not stated Timing of samples: not stated; presume on admission or presentation at ED |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR with cobas SARS‐CoV‐2 assay on the 6800 platform (Roche Diagnostics, Indianapolis, IN); threshold not stated, all Ct values < 37 on both target genes Definition of non‐COVID cases: not stated; presume single RT‐PCR negative Genetic target(s): ORF1 a/b, E‐gene Samples used: as for index test Timing of reference standard: as for index test Blinded to index test: as for index test Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: simultaneous; same samples used All participants received same reference standard: yes Missing data: none reported Uninterpretable results: Indeterminate results (index test): Xpert: 1 sample was a presumptive positive based on detection of E‐gene target but not the N2 target Indeterminate results (reference standard): none reported Unit of analysis: participants |
||
| Comparative | |||
| Notes | Funding: none reported Publication status: published Source: Journal of Clinical Virology Author COI: study authors report no conflicts of interest present |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Smithgall 2020 [B].
| Study characteristics | |||
| Patient Sampling | See Smithgall 2020 [A] for full study details and QUADAS‐2 entries | ||
| Patient characteristics and setting | See Smithgall 2020 [A] for full study details and QUADAS‐2 entries | ||
| Index tests | Test name: [B] Xpert Xpress (product codes not reported) (see Smithgall 2020 [A] for details of comparator test) Manufacturer: [B] Cepheid Antigen target: [B] N2, E genes Antibody: N/A Test method: [B] automated RT‐PCR Samples used: residual NP swabs (collection not described) Transport media: 3 mL VTM (M4RT VTM; ThermoFisher Scientific, Waltham, MA) or UTM (UTM; Becton Dickinson and Co., Franklin Lakes, NJ) Sample storage: stored at 4 °C; testing completed within 48 h of sample collection. Test operator: not stated; presume laboratory staff Definition of test positivity: automated as per manufacturer Blinding reported: not stated Timing of samples: not stated; presume on admission or presentation at ED |
||
| Target condition and reference standard(s) | See Smithgall 2020 [A] for full study details and QUADAS‐2 entries | ||
| Flow and timing | See Smithgall 2020 [A] for full study details and QUADAS‐2 entries | ||
| Comparative | |||
| Notes | See Smithgall 2020 [A] for full study details and QUADAS‐2 entries | ||
Weitzel 2020 [A].
| Study characteristics | |||
| Patient Sampling | Single‐group study to estimate sensitivity and specificity:
‐ samples from patients with respiratory symptoms and/or fever attending a private hospital ED Recruitment: convenience with deliberate sampling of positive cases to ensure a 2:1 distribution reported (5276 samples processed during study period) Prospective or retrospective: retrospective Number of samples (samples with confirmed SARS‐CoV‐2): 111 (80) *17 samples included in Porte 2020 |
||
| Patient characteristics and setting | Setting: ED (private hospital) Location: Clínica Alemana de Santiago Country: Chile Dates: 16 March‐26 April 2020 Symptoms and severity: respiratory symptoms and/or fever; no further detail Demographics: median age 40 years; 50, 45% male (median age 38 years, 43% male for all samples tested during period) Exposure history: none reported |
||
| Index tests |
Weitzel 2020 [A] entry is for test [A] in the list below Test name: [A] Biocredit COVID‐19 Ag One Step SARS‐CoV‐2 Antigen Test (RapiGEN Inc., Anyang‐si, Gyeonggi‐do, Republic of Korea) [B] COVID‐19 Antigen Rapid Test Device StrongStep COVID‐19 Antigen Test (Liming Bio‐Products Co., Jiangsu, China) [C] Huaketai New Coronavirus (SARS‐CoV‐2) N Protein Detection Kit (Fluorescence immunochromatography) (Savant Biotechnology Co., Beijing, China), [D] Diagnostic Kit for 2019‐Novel Coronavirus (2019‐nCoV) Ag Test (Fluorescence Immunochromatographic Assay) (Bioeasy Biotechnology Co., Shenzhen, China). Manufacturer: [A] RapiGEN Inc., Anyang‐si, Gyeonggi‐do, Republic of Korea [B] Liming Bio‐Products Co., Jiangsu, China [C] Savant Biotechnology Co., Beijing, China [D] Bioeasy Biotechnology Co., Shenzhen, China Antigen target: not reported in study Antibody: not reported in study Test method: [A] and [B] CGIA [C] and [D] FIA Samples used: NOP swabs in 3 mL UTM Transport media: UTM‐RT System (Copan Diagnostics, Murrieta, CA, USA) Sample storage: stored at −80 °C; index tests applied on 28 and 29 April 2020 Test operator: single, trained laboratory technician under BSL2 cabinet; visual outputs read by 2 independent observers with referral to third if needed Definition of test positivity: as per manufacturer; Beijing Savant test required use of manufacturer supplied UV torch due to unavailability of reader device in Chile Blinding reported: yes; blinding stated Timing of samples: median 2 days (IQR 1‐5 days); 88% (96/109) during the first week of symptoms |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR; COVID‐19 Genesig Real‐Time PCR assay (Primerdesign Ltd., Chandler's Ford, UK). Ct ≤ 40 considered positive Definition of non‐COVID cases: single PCR negative Genetic target(s): RdRp Samples used: NOP swabs; as for index Timing of reference standard: as for index test; median 2 days (IQR 1‐5 days) Blinded to index test: yes; prior to index Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: same samples; index tests conducted after frozen storage All participants received same reference standard: yes Missing data: none reported; evaluation of Liming test was discontinued after initial poor performance (zero TP) Uninterpretable results: 2 tests had invalid results due to insufficient liquid migration (2 results excluded for each test) Indeterminate results (index test): visual interpretation of the Beijing Savant assay (using manufacturer supplied UV torch) was reportedly difficult under daylight conditions; manufacturer's fluorescence reader not available in Chile. Indeterminate results (reference standard): none reported Unit of analysis: participants |
||
| Comparative | |||
| Notes | Funding: study authors report that the work received no funding; Savant Biotechnology Co. provided test kits free of charge Publication status: preprint Source: medRxiv Author COI: all authors declare no competing interests |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Weitzel 2020 [B].
| Study characteristics | |||
| Patient Sampling | See Weitzel 2020 [A] for full study details and QUADAS entries | ||
| Patient characteristics and setting | |||
| Index tests |
Weitzel 2020 [B] entry is for test [B] in the list below; see Weitzel 2020 [A] for full study details and QUADAS entries Test name: [A] Biocredit COVID‐19 Ag One Step SARS‐CoV‐2 Antigen Test (RapiGEN Inc., Anyang‐si, Gyeonggi‐do, Republic of Korea) [B] COVID‐19 Antigen Rapid Test Device StrongStep COVID‐19 Antigen Test (Liming Bio‐Products Co., Jiangsu, China) [C] Huaketai New Coronavirus (SARS‐CoV‐2) N Protein Detection Kit (Fluorescence immunochromatography) (Savant Biotechnology Co., Beijing, China), [D] Diagnostic Kit for 2019‐Novel Coronavirus (2019‐nCoV) Ag Test (Fluorescence Immunochromatographic Assay) (Bioeasy Biotechnology Co., Shenzhen, China). Manufacturer: [A] RapiGEN Inc., Anyang‐si, Gyeonggi‐do, Republic of Korea [B] Liming Bio‐Products Co., Jiangsu, China [C] Savant Biotechnology Co., Beijing, China [D] Bioeasy Biotechnology Co., Shenzhen, China Antigen target: not reported in study Antibody: not reported in study Test method: [A] and [B] CGIA [C] and [D] FIA Samples used: NOP swabs in 3 mL UTM Transport media: UTM‐RT System (Copan Diagnostics, Murrieta, CA, USA) Sample storage: stored at −80 °C; index tests applied on 28 and 29 April 2020 Test operator: single, trained laboratory technician under BSL2 cabinet; visual outputs read by 2 independent observers with referral to third if needed Definition of test positivity: as per manufacturer; Savant test required use of manufacturer supplied UV torch due to unavailability of reader device in Chile Blinding reported: yes; blinding stated Timing of samples: median 2 days (IQR 1‐5 days); 88% (96/109) during the first week of symptoms |
||
| Target condition and reference standard(s) | See Weitzel 2020 [A] for full study details and QUADAS entries | ||
| Flow and timing | See Weitzel 2020 [A] for full study details and QUADAS entries | ||
| Comparative | |||
| Notes | |||
Weitzel 2020 [C].
| Study characteristics | |||
| Patient Sampling | See Weitzel 2020 [A] for full study details and QUADAS entries | ||
| Patient characteristics and setting | See Weitzel 2020 [A] for full study details and QUADAS entries | ||
| Index tests |
Weitzel 2020 [C] entry is for test [C] in the list below; see Weitzel 2020 [A] for full study details and QUADAS entries Test name: [A] Biocredit COVID‐19 Ag One Step SARS‐CoV‐2 Antigen Test (RapiGEN Inc., Anyang‐si, Gyeonggi‐do, Republic of Korea) [B] COVID‐19 Antigen Rapid Test Device StrongStep COVID‐19 Antigen Test (Liming Bio‐Products Co., Jiangsu, China) [C] Huaketai New Coronavirus (SARS‐CoV‐2) N Protein Detection Kit (Fluorescence immunochromatography) (Savant Biotechnology Co., Beijing, China), [D] Diagnostic Kit for 2019‐Novel Coronavirus (2019‐nCoV) Ag Test (Fluorescence Immunochromatographic Assay) (Bioeasy Biotechnology Co., Shenzhen, China). Manufacturer: [A] RapiGEN Inc., Anyang‐si, Gyeonggi‐do, Republic of Korea [B] Liming Bio‐Products Co., Jiangsu, China [C] Savant Biotechnology Co., Beijing, China [D] Bioeasy Biotechnology Co., Shenzhen, China Antigen target: not reported in study Antibody: not reported in study Test method: [A] and [B] CGIA [C] and [D] FIA Samples used: NOP swabs in 3 mL UTM Transport media: UTM‐RT System (Copan Diagnostics, Murrieta, CA, USA) Sample storage: stored at −80 °C; index tests applied on 28 and 29 April 2020 Test operator: single, trained laboratory technician under BSL2 cabinet; visual outputs read by 2 independent observers with referral to third if needed Definition of test positivity: as per manufacturer; Savant test required use of manufacturer supplied UV torch due to unavailability of reader device in Chile Blinding reported: yes; blinding stated Timing of samples: median 2 days (IQR 1‐5 days); 88% (96/109) during the first week of symptoms |
||
| Target condition and reference standard(s) | See Weitzel 2020 [A] for full study details and QUADAS entries | ||
| Flow and timing | See Weitzel 2020 [A] for full study details and QUADAS entries | ||
| Comparative | |||
| Notes | |||
Weitzel 2020 [D].
| Study characteristics | |||
| Patient Sampling | See Weitzel 2020 [A] for full study details and QUADAS entries | ||
| Patient characteristics and setting | See Weitzel 2020 [A] for full study details and QUADAS entries | ||
| Index tests |
Weitzel 2020 [D] entry is for test [D] in the list below; see Weitzel 2020 [A] for full study details and QUADAS entries Test name: [A] Biocredit COVID‐19 Ag One Step SARS‐CoV‐2 Antigen Test (RapiGEN Inc., Anyang‐si, Gyeonggi‐do, Republic of Korea) [B] COVID‐19 Antigen Rapid Test Device StrongStep® COVID‐19 Antigen Test (Liming Bio‐Products Co., Jiangsu, China) [C] Huaketai New Coronavirus (SARS‐CoV‐2) N Protein Detection Kit (Fluorescence immunochromatography) (Savant Biotechnology Co., Beijing, China), [D] Diagnostic Kit for 2019‐Novel Coronavirus (2019‐nCoV) Ag Test (Fluorescence Immunochromatographic Assay) (Bioeasy Biotechnology Co., Shenzhen, China). Manufacturer: [A] RapiGEN Inc., Anyang‐si, Gyeonggi‐do, Republic of Korea [B] Liming Bio‐Products Co., Jiangsu, China [C] Savant Biotechnology Co., Beijing, China [D] Bioeasy Biotechnology Co., Shenzhen, China Antigen target: not reported in study Antibody: not reported in study Test method: [A] and [B] CGIA [C] and [D] FIA Samples used: NOP swabs in 3 mL UTM Transport media: UTM‐RT System (Copan Diagnostics, Murrieta, CA, USA) Sample storage: stored at −80°C; index tests applied on 28 and 29 April 2020 Test operator: single, trained laboratory technician under BSL2 cabinet; visual outputs read by 2 independent observers with referral to third if needed Definition of test positivity: as per manufacturer; Savant test required use of manufacturer supplied UV torch due to unavailability of reader device in Chile Blinding reported: yes; blinding stated Timing of samples: median 2 days (IQR 1‐5 days); 88% (96/109) during the first week of symptoms |
||
| Target condition and reference standard(s) | See Weitzel 2020 [A] for full study details and QUADAS entries | ||
| Flow and timing | See Weitzel 2020 [A] for full study details and QUADAS entries | ||
| Comparative | |||
| Notes | |||
Wolters 2020.
| Study characteristics | |||
| Patient Sampling | 2‐group study to estimate sensitivity and specificity for diagnosis of active disease:
‐ samples selected from laboratories on the basis of presence/absence of 2 genetic targets on RT‐PCR: SARS‐CoV‐2 E‐gene +/RdRp gene + (n = 30); SARS‐CoV‐2 E‐gene +/RdRp gene – (n = 28); SARS‐CoV‐2 E‐gene ‐/RdRp gene (n = 30)
(A separate set of samples were tested in triplicate at all 3 laboratories to determine limits of detection and analytical specificity) Recruitment: not stated; deliberate sampling used Prospective or retrospective: retrospective Sample size (cases): 88 (58) |
||
| Patient characteristics and setting | Setting: not stated; 3 laboratories Location: Radboud UMC in Nijmegen, PAMM in Veldhoven and the RIVM in Bilthoven Country: The Netherlands Dates: January‐March 2020 Symptoms and severity: not stated Demographics: not stated Exposure history: not stated |
||
| Index tests | Test name: Cepheid Xpert Xpress SARS‐CoV‐2 (product code not reported) Manufacturer: Cepheid Europe Antigen target: E‐gene (sarbeco‐specific) and N2‐gene (SARS‐CoV‐2‐specific) Antibody: N/A Test method: not stated (it should be automated PCR) Samples used: NP or mid‐turbinate, and OP swabs Transport media: UTM or GLY medium; no further details Sample storage: stored at −80 ℃ Test operator: not stated; presume laboratory staff Definition of test positivity: as per manufacturer; reported E‐gene‐only positive specimens as presumptive positive but no re‐testing with Xpert Xpress was reported. N2‐only positives were considered positive (but re‐tested with RT‐PCR) Blinding reported: not stated (see comment section) Timing of samples: not stated |
||
| Target condition and reference standard(s) | Reference standard: in‐house RT‐PCR:
Radboud UMC Lab: MagNApure 96 (Roche) (isolation platform); MagNApure 96 DNA and Viral NA Small Volume (extraction kit); Roche LC480 II (PCR platform); Life Technologies Taqman FastVirus 1‐step mastermix (RT‐PCR mastermix)
PAMM Lab: Roche cobas 4800 (isolation platform); CT/NG extraction protocol (extraction kit); Roche LC480 II (PCR platform); Roche LightCycler Multiplex RNA Virus Master (RT‐PCR mastermix);
RIVM Lab: BioMérieux NucliSens (isolation platform); easyMAG EasyMAG extraction reagents (extraction kit); Thermo Fisher QuantStudio 6 (PCR platform); Life Technologies Taqman FastVirus 1‐step mastermix (RT‐PCR mastermix) Definition of non‐COVID cases: yes (performed prior to index test) Genetic target(s): Radboud UMC lab: E‐gene and RdRp‐gene PAMM Lab: started with E‐gene and RdRp‐gene and mid‐March moved on to E‐gene testing only RIVM Lab: started with E‐gene and RdRp‐gene and at the beginning of April moved on to E‐gene and CDC N1‐gene primer and probes Samples used: as for index test Timing of reference standard: as for index test Blinded to index test: storage prior to freezing was not reported; samples were analysed at or near time of collection ("processed … in the routine diagnostic procedure using the locally implemented RT‐PCR") Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: same samples used; index text seems to have been conducted after frozen storage All participants received same reference standard: no, 3 different in‐house PCR based on the laboratory Missing data: none reported Uninterpretable results: none reported Indeterminate results (index test): 1 sample was positive only on N2 gene (positive according to IFU) and 1 was positive only on E gene (presumptive positive, requires re‐testing according to IFU). Both samples were re‐tested on RT‐PCR only Indeterminate results (reference standard): re‐testing of the two ‘FN’ samples (one TP and 1 presumptive positive according to IFU definition) with RT‐PCR found both samples to be disease‐negative (reclassed as 1 TN and 1 FP); study authors note that the viral loads of these samples are at the limit of detection for Xpert Xpress and that multiple freeze‐thaw steps of samples could have had a significant impact on detection. Unit of analysis: not stated; only samples reported |
||
| Comparative | |||
| Notes | Funding: not stated Publication status: accepted manuscript Source: Journal of Clinical Virology Author COI: the study authors declare no COI present |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Unclear | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zhen 2020 [A].
| Study characteristics | |||
| Patient Sampling | 2‐group study to estimate sensitivity and specificity:
‐ samples from symptomatic patients of all ages and gender Recruitment: not stated; specimens selected to represent the true positivity rate at authors' institution (50% to 60%), and to span low and high viral loads Prospective or retrospective: mixed; included frozen samples (n = 88) and prospectively tested (n = 20) Number of samples (samples with confirmed SARS‐CoV‐2):108 (58) |
||
| Patient characteristics and setting | Setting: not stated; selected from laboratory Location: not stated; authors' institutions were Northwell Health Laboratories, and Dept Pathology and Laboratory Medicine, The Donald and Barbara Zucker School of Medicine Country: USA Dates: March‐April 2020 Symptoms and severity: "symptomatic"; no further details Demographics: not stated (all ages and genders) Exposure history: not stated |
||
| Index tests |
Zhen 2020 [A] is the entry for test [A] from the list below Test name: [A] Xpert® Xpress SARS‐CoV‐2 [B] ID NOW COVID‐19 (no product codes reported) Manufacturer: [A] Cepheid, [B] Abbott Antigen target: [A] N2, E; [B] RdRp Antibody: N/A Test method: rapid PCR Samples used: NP swabs Transport media: UTM (various manufacturers) Sample storage: on collection, stored at 2‐8 ⁰C for up to 72 h; after routine testing, stored at −80 ⁰C 88 samples tested using ePlex on collection, then frozen prior to testing with ID NOW, Xpert Xpress and Hologic RT‐PCR; 20 samples tested prospectively after collection on all systems Test operator: not stated; presume laboratory staff Definition of test positivity: not stated; states “testing was performed according to the manufacturer’s instructions” but no presumptive positives reported Blinding reported: not stated Timing of samples: not stated Study also evaluates [C] GenMar kePlex® SARS‐CoV‐2 Test (not eligible for this review) |
||
| Target condition and reference standard(s) | Reference standard: RT‐PCR; Hologic Panther Fusion SARS‐CoV‐2 assay, performed according to manufacturer's IFU Definition of non‐COVID cases: single RT‐PCR Genetic target(s): 2 regions of ORF1ab; either positive Samples used: NP swabs; same as for index test Timing of reference standard: not stated Blinded to index test: not stated Incorporated index test: no |
||
| Flow and timing | Time interval between index and reference tests: not stated in exact terms; delay between index and reference only for GenMark assay, as 88 samples tested at time of collection with ePlex then frozen before testing with all other assays. All participants received same reference standard: yes Missing data: none reported Uninterpretable results: 1 specimen with invalid result on ID NOW was excluded from that dataset Indeterminate results (index test): none reported; no re‐testing conducted Indeterminate results (reference standard): none reported; no re‐testing conducted Unit of analysis: not stated only refers to samples |
||
| Comparative | |||
| Notes | Funding: none stated; study authors thank Cepheid for providing the reagents used Publication status: accepted manuscript Source: Journal of Clinical Microbioloby Author COI: Gregory Berry has previously given education seminars for Abbott, Cepheid, and Hologic, Inc. and has received Honorariums |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Did the study avoid inappropriate inclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Antigen tests) | |||
| DOMAIN 2: Index Test (Rapid PCR tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Reference standard does not incorporate result of index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Did all participants receive a reference standard? | Yes | ||
| Were results presented per patient? | Unclear | ||
| Could the patient flow have introduced bias? | High risk | ||
Zhen 2020 [B].
| Study characteristics | |||
| Patient Sampling | See Zhen 2020 [A] for full study details and QUADAS entries | ||
| Patient characteristics and setting | See Zhen 2020 [A] for full study details and QUADAS entries | ||
| Index tests |
Zhen 2020 [B] is the entry for test [B] from the list below, see Zhen 2020 [A] for full study details and QUADAS entries Test name: [A] Xpert® Xpress SARS‐CoV‐2 [B] ID NOWCOVID‐19 (no product codes reported) Manufacturer: [A] Cepheid, [B] Abbott Antigen target: [A] N2, E; [B] RdRp Antibody: N/A Test method: rapid PCR Samples used: NP swabs Transport media: UTM (various manufacturers) Sample storage: on collection, stored at 2‐8 ⁰C for up to 72 h; after routine testing, stored at −80 ⁰C 88 samples tested using ePlex on collection, then frozen prior to testing with ID NOW, Xpert Xpress and Hologic RT‐PCR; 20 samples tested prospectively after collection on all systems Test operator: not stated; presume laboratory staff Definition of test positivity: not stated; states “testing was performed according to the manufacturer’s instructions” but no presumptive positives reported Blinding reported: not stated Timing of samples: not stated Study also evaluates [C] GenMar kePlex® SARS‐CoV‐2 Test (not eligible for this review) |
||
| Target condition and reference standard(s) | See Zhen 2020 [A] for full study details and QUADAS entries | ||
| Flow and timing | See Zhen 2020 [A] for full study details and QUADAS entries | ||
| Comparative | |||
| Notes | Funding: none stated; study authors thank Cepheid for providing the reagents used Publication status: accepted manuscript Source: Journal of Clinical Microbioloby Author COI: Gregory Berry has previously given education seminars for Abbott, Cepheid, and Hologic, Inc. and has received Honorariums |
||
BAL: bronchoalveolar lavage; CDC: Center for Disease Control; CGIA: colloidal gold immunoassay; COI: conflict of interest; Ct: cycle threshold; ED: Emergency Department; EUA: emergency use authorisation; FIA: fluorescence immunochromatographic; FN: false negative; FP: false positive; GLY: Glucose‐Lactalbumin‐Yeast; HCW: healthcare worker; ICU: intensive care unit; IFU: instructions for use; IQR: interquartile range; LDT: laboratory‐developed test; N/A: not applicable; NAAT: nucleic acids amplification test; NIH: National Institutes of Health; NOP: naso‐oropharyngeal; NP: nasopharyngeal; OP: oropharyngeal; PCR: polymerase chain reaction; PHE: Public Health England; qRT‐PCR: quantitative reverse transcription polymerase chain reaction; RNA: ribonucleic acid; RT‐PCR: reverse transcription polymerase chain reaction; SD: standard deviation; TA: tracheal aspirate; TN: true negative; TP: true positive; UTM: universal transport medium; UV: ultraviolet; UW: University of Washington; VTM: viral transport medium;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ai 2020 | Ineligible index test |
| Anahtar 2020 | Ineligible index test |
| Arumugam 2020 | Ineligible index test |
| Baek 2020 | Ineligible index test |
| Barra 2020 | Ineligible study design |
| Basu 2020 | Ineligible reference standard |
| Behrmann 2020 | Accuracy data cannot be extracted |
| Bordi 2020 | Ineligible study design |
| Broughton 2020 | Ineligible index test |
| Callahan 2020 | Accuracy data cannot be extracted |
| Chandler‐Brown 2020 | Ineligible study design |
| Colson 2020 | Inadequate sample size |
| Comar 2020 | Ineligible reference standard |
| Crone 2020 | Ineligible index test |
| Curti 2020 | Ineligible study design |
| Ding 2020 | Ineligible study design |
| Dohla 2020 | Ineligible index test |
| Farfan 2020 | Ineligible study design |
| Francis 2020 | Ineligible study design |
| Freire‐Paspuel 2020 | Ineligible study design |
| Ganguli 2020 | Ineligible population |
| Giamarellos‐Bourboulis 2020 | Ineligible study design |
| Gonzalez‐Gonzalez 2020 | Ineligible study design |
| Grant 2020 | Ineligible index test |
| Hass 2020 | Ineligible target condition |
| Hogan 2020a | Ineligible index test |
| Hu 2020 | Ineligible index test |
| Huang 2020 | Ineligible index test |
| Jiang 2020 | Ineligible index test |
| Joung 2020 | Ineligible index test |
| Kalikiri 2020 | Ineligible index test |
| Kim 2019 | Ineligible study design |
| Konrad 2020 | Ineligible study design |
| Kurstjens 2020 | Ineligible index test |
| Lalli 2020 | Inadequate sample size |
| Lamb 2020 | Ineligible study design |
| Lee 2020 | Ineligible index test |
| Lin 2020 | Ineligible population |
| Lowe 2020 | Ineligible index test |
| Lu 2020 | Ineligible study design |
| Lu 2020a | Ineligible index test |
| Mahari 2020 | Ineligible study design |
| Marzinotto 2020 | Accuracy data cannot be extracted |
| McCormick‐Baw 2020 | Ineligible index test |
| McRae 2020 | Ineligible index test |
| Mei 2020 | Ineligible index test |
| Noerz 2020 | Ineligible index test |
| Osterdahl 2020 | Ineligible index test |
| Paden 2020 | Ineligible study design |
| Pellanda 2020 | Ineligible index test |
| Pfefferle 2020 | Ineligible study design |
| Seo 2020 | Accuracy data cannot be extracted |
| Smyrlaki 2020 | Ineligible index test |
| St Hilaire 2020 | Ineligible index test |
| Tan 2020 | Ineligible study design |
| Visseaux 2020 | Ineligible index test |
| Wang 2020 | Ineligible index test |
| Wang 2020a | Accuracy data cannot be extracted |
| Wee 2020 | Ineligible study design |
| Xue 2020 | Ineligible index test |
| Yan 2020 | Ineligible index test |
| Yang 2020 | Ineligible index test |
| Yu 2020 | Ineligible index test |
| Yu 2020a | Ineligible index test |
| Zamecnik 2020 | Ineligible index test |
| Zeng 2020 | Ineligible study design |
| Zhang 2020 | Ineligible index test |
| Zhao 2020 | Ineligible study design |
Differences between protocol and review
We planned to check the following websites for eligible index tests, however these did not prove to be very accessible or easy to use and, after initial review, were not further considered:
National Institute for Health Research (NIHR) Innovation Observatory (www.io.nihr.ac.uk/)
We planned to check the following evidence repository for additional eligible studies however, the EPPI‐Centre and Norwegian Institute of Public Health resources proved to be more accessible therefore we decided to prioritise our other sources of evidence.
Meta‐evidence (meta-evidence.co.uk/the-role-of-evidence-synthesis-in-covid19/)
We intended for two authors to independently perform data extraction, however one review author extracted study characteristics, and a second author checked them. Contingency table data were extracted independently by two review authors as planned.
We planned to evaluate the effect of additional sources of heterogeneity, including study design, reference standard, length and severity of symptoms, and setting. However, additional formal investigations using meta‐regression were not possible because of limited data, lack of reporting or lack of variability across the studies in these features.
We planned to conduct a sensitivity analysis excluding studies that are solely published as preprints. We have inadequate study numbers to allow this at present but will reconsider for the next update.
Contributions of authors
JJD was the contact person with the editorial base. JDI co‐ordinated contributions from the co‐authors and wrote the final draft of the review. JJD, JDi, YT, CD, STP, IH, AA, LFR, MP, JDr, SB screened papers against eligibility criteria. RS conducted the literature searches. JDi and AA appraised the quality of papers. JDi and AA extracted data for the review and sought additional information about papers. JDi entered data into Review Manager 2014. JDi, JJD and SB, analysed and interpreted data. JJD, JDi, YT, CD, STP, RS, ML, LH, AVB, DE, SD, JC worked on the methods sections and commented on the draft review. JJD and JDi responded to the comments of the referees. JJD is the guarantor of the update.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
University of Birmingham, UK
External sources
-
Department for International Development, UK
Project number: 300342‐104
National Institute for Health Research (NIHR), UK
NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham, UK
Declarations of interest
Jonathan J Deeks: none known
Jacqueline Dinnes: none known
Yemisi Takwoingi: none known
Clare Davenport: none known
Mariska MG Leeflang: none known
René Spijker: the Dutch Cochrane Centre (DCC) has received grants for performing commissioned systematic reviews. In no situation, the commissioner had any influence on the results of the work.
Lotty Hooft: none known
Ann Van den Bruel: none known
Devy Emperador: is employed by FIND with funding from DFID and KFW. FIND is a global non‐for profit product development partnership and WHO Diagnostic Collaboration Centre. It is FIND’s role to accelerate access to high quality diagnostic tools for low resource settings and this is achieved by supporting both R&D and access activities for a wide range of diseases, including COVID‐19. .FIND has several clinical research projects to evaluate multiple new diagnostic tests against published Target Product Profiles that have been defined through consensus processes. These studies are for diagnostic products developed by private sector companies who provide access to know‐how, equipment/reagents, and contribute through unrestricted donations as per FIND policy and external SAC review.
Sabine Dittrich: is employed by FIND with funding from DFID and Australian Aid. FIND is a global non‐for profit product development partnership and WHO Diagnostic Collaboration Centre. It is FIND’s role to accelerate access to high quality diagnostic tools for low resource settings and this is achieved by supporting both R&D and access activities for a wide range of diseases, including COVID‐19. .FIND has several clinical research projects to evaluate multiple new diagnostic tests against published Target Product Profiles that have been defined through consensus processes. These studies are for diagnostic products developed by private sector companies who provide access to know‐how, equipment/reagents, and contribute through unrestricted donations as per FIND policy and external SAC review.
Ada Adriano: none known
Sophie Beese: none known
Janine Dretzke: none known
Lavinia Ferrante di Ruffano: none known
Isobel Harris: none known
Malcolm Price: none known
Sian Taylor‐Phillips: none known
Sarah Berhane: is funded by NIHR Birmingham Biomedical Research Centre.
Jane Cunningham: none known
New
References
References to studies included in this review
Assennato 2020 {published data only}
- Assennato SM, Ritchie AV, Nadala C, Goel N, Zhang H, Datir R, et al. Performance evaluation of the point-of-care SAMBA II SARS-CoV-2 test for detection of SARS-CoV-2. medRxiv [Preprint] 24 May 2020. [DOI: 10.1101/2020.05.24.20100990] [DOI] [PMC free article] [PubMed] [Google Scholar]
Broder 2020 {published data only}
- Broder K, Babiker A, Myers C, White T, Jones H, Cardella J, et al. Test agreement between Roche cobas 6800 and Cepheid GeneXpert Xpress SARS-CoV-2 assays at high cycle threshold ranges. Journal of Clinical Microbiology 2020;58:e01187-20. [DOI: 10.1128/JCM.01187-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder KJ, Babiker A, Myers C, White T, Jones H, Cardella J, et al. Test agreement between Roche cobas 6800 and Cepheid GeneXpert Xpress SARS-CoV-2 assays at high cycle threshold ranges. bioRxiv [Preprint] 5 May 2020:1-13. [DOI: 10.1101/2020.05.05.078501] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diao 2020 {published data only}
- Diao B, Wen K, Chen J, Liu Y, Yuan Z, Han C, et al. Diagnosis of acute respiratory syndrome coronavirus 2 infection by detection of nucleocapsid protein. medRxiv [Preprint] 10 March 2020:1-13. [DOI: 10.1101/2020.03.07.20032524] [DOI] [Google Scholar]
Harrington 2020 {published data only}
- Harrington A, Cox B, Snowdon J, Bakst J, Ley E, Grajales P, et al. Comparison of Abbott ID NOW and Abbott m2000 methods for the detection of SARS-CoV-2 from nasopharyngeal and nasal swabs from symptomatic patients. Journal of Clinical Microbiology 2020;58(8):e00798-20. [DOI: 10.1128/JCM.00798-20.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogan 2020 {published data only}
- Hogan CA, Garamani N, Lee AS, Tung JK, Sahoo MK, Huang C, et al. Comparison of the Accula SARS-CoV-2 test with a laboratory-developed assay for detection of SARS-CoV-2 RNA in clinical nasopharyngeal specimens. bioRxiv [Preprint] 2020. [DOI: 10.1101/2020.05.12.092379v1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambert‐Niclot 2020 {published data only}
- Lambert-Niclot S, Cuffel A, Le Pape S, Vauloup-Fellous C, Morand-Joubert L, Roque-Afonso AM, et al. Evaluation of a rapid diagnostic assay for detection of SARS CoV-2 antigen in nasopharyngeal swab. Journal of Clinical Microbiology 2020;58(8):e00977-20. [DOI: 10.1128/JCM.00977-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lieberman 2020 {published data only}
- Lieberman JA, Pepper G, Naccache SN, Huang ML, Jerome KR, Greninger AL. Comparison of commercially available and laboratory developed assays for in vitro detection of SARS-CoV-2 in clinical laboratories. Journal of Clinical Microbiology 2020;58(8):e00821-20. [DOI: 10.1128/JCM.00821-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loeffelholz 2020 {published data only}
- Loeffelholz MJ, Alland D, Butler-Wu SM, Pandey U, Perno CF, Nava A, et al. Multicenter evaluation of the Cepheid Xpert Xpress SARS-CoV-2 test. Journal of Clinical Microbiology 2020;58(8):e00926-20. [DOI: 10.1128/JCM.00926-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mertens 2020 {published data only}
- Mertens P, De Vos N, Martiny D, Jassoy C, Mirazimi A, Cuypers L, et al. Development and potential usefulness of the COVID-19 Ag Respi-Strip diagnostic assay in a pandemic context. medRxiv [Preprint] 24 April 2020:1-29. [DOI: 10.1101/2020.04.24.20077776] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2020 {published data only}
- Mitchell SL, George KS. Evaluation of the COVID19 ID NOW EUA assay. Journal of Clinical Virology 2020;128:104429. [DOI: 10.1016/j.jcv.2020.104429] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2020 {published data only}
- Moore NM, Li H, Schejbal D, Lindsley J, Hayden M. Comparison of two commercial molecular tests and a laboratory-developed modification of the CDC 2019-nCOV RT-PCR assay for the qualitative detection of SARS-CoV-2 from upper respiratory tract specimens. medRxiv [Preprint] 2020:1-22. [DOI: 10.1101/2020.05.02.20088740] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moran 2020 {published data only}
- Moran A, Beavis KG, Matushek SM, Ciaglia C, Francois N, Tesic V, et al. The detection of SARS-CoV-2 using the Cepheid Xpert Xpress SARS-CoV-2 and Roche cobas SARS-CoV-2 assays. Journal of Clinical Microbiology 2020;58(8):e00772-20. [DOI: 10.1128/JCM.00772-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Porte 2020 {published data only}
- Porte L, Legarraga P, Vollrath V, Aguilera X, Munita JM, Araos R, et al. Evaluation of novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. papers.ssrn.com/abstract=3569871 14 April 2020:1-23. [DOI] [PMC free article] [PubMed]
Rhoads 2020 {published data only}
- Rhoads DD, Cherian SS, Roman K, Stempak LM, Schmotzer CL, Sadri N. Comparison of Abbott ID NOW, Diasorin Simplexa, and CDC FDA EUA methods for the detection of SARS-CoV-2 from nasopharyngeal and nasal swabs from individuals diagnosed with COVID-19. Journal of Clinical Microbiology 2020;58(8):e00760-20. [DOI: 10.1128/JCM.00760-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smithgall 2020 [A] {published data only}
- Smithgall MC, Scherberkova I, Whittier S, Green D. Comparison of Cepheid Xpert Xpress and Abbott ID Now to Roche cobas for the rapid detection of SARS-CoV-2. bioRxiv [Preprint] 25 April 2020:1-16. [DOI: 10.1101/2020.04.22.055327] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithgall MC, Scherberkova I, Whittier S, Green DA. Comparison of Cepheid Xpert Xpress and Abbott ID Now to Roche Cobas for the rapid detection of SARS-CoV-2. Journal of Clinical Virology 2020;128:104428. [DOI: 10.1016/j.jcv.2020.104428] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smithgall 2020 [B] {published data only}
- Smithgall MC, Scherberkova I, Whittier S, Green DA. Comparison of Cepheid Xpert Xpress and Abbott ID Now to Roche Cobas for the rapid detection of SARS-CoV-2. Journal of Clinical Virology 2020;128:104428. [DOI: 10.1016/j.jcv.2020.104428] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weitzel 2020 [A] {published data only}
- Weitzel T, Legarraga P, Iruretagoyena M, Pizarro G, Vollrath V, Araos R, et al. Head-to-head comparison of four antigen-based rapid detection tests for the diagnosis of SARS-CoV-2 in respiratory samples. bioRxiv [Preprint] 30 May 2020:1-21. [DOI: 10.1101/2020.05.27.119255] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weitzel 2020 [B] {published data only}
- Weitzel T, Legarraga P, Iruretagoyena M, Pizarro G, Vollrath V, Araos R, et al. Head-to-head comparison of four antigen-based rapid detection tests for the diagnosis of SARS-CoV-2 in respiratory samples. bioRxiv [Preprint] 30 May 2020:1-21. [DOI: 10.1101/2020.05.27.119255] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weitzel 2020 [C] {published data only}
- Weitzel T, Legarraga P, Iruretagoyena M, Pizarro G, Vollrath V, Araos R, et al. Head-to-head comparison of four antigen-based rapid detection tests for the diagnosis of SARS-CoV-2 in respiratory samples. bioRxiv [Preprint] 30 May 2020:1-21. [DOI: 10.1101/2020.05.27.119255] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weitzel 2020 [D] {published data only}
- Weitzel T, Legarraga P, Iruretagoyena M, Pizarro G, Vollrath V, Araos R, et al. Head-to-head comparison of four antigen-based rapid detection tests for the diagnosis of SARS-CoV-2 in respiratory samples. bioRxiv [Preprint] 30 May 2020:1-21. [DOI: 10.1101/2020.05.27.119255] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolters 2020 {published data only}
- Wolters F, Van de Bovenkamp J, Van den Bosch B, Van den Brink S, Broeders M, Chung NH, et al. Multi-center evaluation of Cepheid Xpert(R) Xpress SARS-CoV-2 point-of-care test during the SARS-CoV-2 pandemic. Journal of Clinical Virology 2020;128:104426. [DOI: 10.1016/j.jcv.2020.104426] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhen 2020 [A] {published data only}
- Zhen W, Smith E, Manji R, Schron D, Berry GJ. Clinical evaluation of three sample-to-answer platforms for the detection of SARS-CoV-2. Journal of Clinical Microbiology 2020;58(8):e00783-20. [DOI: 10.1128/JCM.00783-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhen 2020 [B] {published data only}
- Zhen W, Smith E, Manji R, Schron D, Berry GJ. Clinical evaluation of three sample-to-answer platforms for the detection of SARS-CoV-2. Journal of Clinical Microbiology 2020;58(8):e00783-20. [DOI: 10.1128/JCM.00783-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ai 2020 {published data only}
- Ai JW, Zhang HC, Xu T, Wu J, Zhu M, Yu YQ, et al. Optimizing diagnostic strategy for novel coronavirus pneumonia, a multi-center study in Eastern China. medRxiv [Preprint] 17 February 2020:1-18. [DOI: 10.1101/2020.02.13.20022673] [DOI] [Google Scholar]
Anahtar 2020 {published data only}
- Anahtar MN, McGrath GE, Rabe BA, Tanner NA, White BA, Lennerz JK, et al. Clinical assessment and validation of a rapid and sensitive SARS-CoV-2 test using reverse-transcription loop-mediated isothermal amplification. medRxiv [Preprint] 18 May 2020:1-22. [DOI: 10.1101/2020.05.12.20095638] [DOI] [PMC free article] [PubMed]
Arumugam 2020 {published data only}
- Arumugam A, Faron ML, Yu P, Markham C, Wong S. A rapid COVID-19 RT-PCR detection assay for low resource settings. bioRxiv [Preprint] 30 April 2020:1-13. [DOI: 10.1101/2020.04.29.069591] [DOI] [Google Scholar]
Baek 2020 {published data only}
- Baek YH, Um J, Antigua KJ, Park JH, Kim Y, Oh S, et al. Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerging Microbes & Infections 2020;9(1):998-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barra 2020 {published data only}
- Barra GB, Ticiane Henriques SR, Goes MP, Henriques JR, Nery LF. Analytical sensibility and specificity of two RT-qPCR protocols for SARS-CoV-2 detection performed in an automated workflow. medRxiv [Preprint] 10 March 2020:1-5. [DOI: 10.1101/2020.03.07.20032326] [DOI] [PMC free article] [PubMed] [Google Scholar]
Basu 2020 {published data only}
- Basu A, Zinger T, Inglima K, Woo KM, Atie O, Yurasits L, et al. Performance of Abbott ID NOW COVID-19 rapid nucleic acid amplification test in nasopharyngeal swabs transported in viral media and dry nasal swabs, in a New York City academic institution. Journal of Clinical Microbiology 2020;58(8):e01136-20. [DOI: 10.1128/JCM.01136-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Behrmann 2020 {published data only}
- Behrmann O, Bachmann I, Spiegel M, Schramm M, El Wahed AA, Dobler G, et al. Rapid detection of SARS-CoV-2 by low volume real-time single tube reverse transcription recombinase polymerase amplification using an exo probe with an internally linked quencher (exo-IQ). Clinical Chemistry 8 May 2020 [Epub ahead of print]:hvaa116. [DOI: 10.1093/clinchem/hvaa116] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bordi 2020 {published data only}
- Bordi L, Piralla A, Lalle E, Giardina F, Colavita F, Tallarita M, et al. Rapid and sensitive detection of SARS-CoV-2 RNA using the Simplexa COVID-19 direct assay. Journal of Clinical Virology 2020;128:104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Broughton 2020 {published data only}
- Broughton JP, Deng X, Yu G, Fasching CL, Singh J, Streithorst J, et al. Rapid detection of 2019 novel coronavirus SARS-CoV-2 using a CRISPR-based DETECTR lateral flow assay. medRxiv [Preprint] 27 March 2020:1-28. [DOI: 10.1101/2020.03.06.20032334] [DOI] [Google Scholar]
Callahan 2020 {published data only}
- Callahan CJ, Lee R, Zulauf K, Tamburello L, Smith KP, Previtera J, et al. Open development and clinical validation of multiple 3D-printed sample-collection swabs: rapid resolution of a critical COVID-19 testing bottleneck. medRxiv [Preprint] 7 May 2020:1-16. [EMBASE: 10.1101/2020.04.14.20065094] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CJ, Lee R, Zulauf KE, Tamburello L, Smith KP, Previtera J, et al. Open development and clinical validation of multiple 3D-printed nasopharyngeal collection swabs: rapid resolution of a critical COVID-19 testing bottleneck. Journal of Clinical Microbiology 2020;58(8):e00876-20. [DOI: 10.1128/JCM.00876-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chandler‐Brown 2020 {published data only}
- Chandler-Brown D, Bueno AM, Atay O, Tsao DS. A highly scalable and rapidly deployable RNA extraction-free COVID-19 assay by quantitative Sanger sequencing. medRxiv [Preprint] 10 April 2020:1-15. [DOI: 10.1101/2020.04.07.029199] [DOI] [Google Scholar]
Colson 2020 {published data only}
- Colson P, Lagier JC, Baudoin JP, Bou Khalil J, La Scola B, Raoult D. Ultrarapid diagnosis, microscope imaging, genome sequencing, and culture isolation of SARS-CoV-2. European Journal of Clinical Microbiology & Infectious Diseases 2020;39(8):1601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Comar 2020 {published data only}
- Comar M, Brumat M, Concas MP, Argentini G, Bianco A, Bicego L, et al. COVID-19 experience: first Italian survey on healthcare staff members from a Mother-Child Research hospital using combined molecular and rapid immunoassays test. medRxiv [Preprint] 22 April 2020:1-12. [DOI: 10.1101/2020.04.19.20071563] [DOI] [Google Scholar]
Crone 2020 {published data only}
- Crone MA, Priestman M, Ciechonska M, Jensen K, Sharp DJ, Randell P, et al. A new role for Biofoundries in rapid prototyping, development, and validation of automated clinical diagnostic tests for SARS-CoV-2. medRxiv [Preprint] 12 May 2020:1-31. [DOI: 10.1101/2020.05.02.20088344] [DOI] [Google Scholar]
Curti 2020 {published data only}
- Curti L, Pereyra-Bonnet F, Gimenez CA. An ultrasensitive, rapid, and portable coronavirus SARS-CoV-2 sequence detection method based on CRISPR-Cas12. bioRxiv [Preprint] 2 March 2020:1-10. [DOI: 10.1101/2020.02.29.971127] [DOI] [Google Scholar]
Ding 2020 {published data only}
- Ding X, Yin K, Li Z, Liu C. All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) assay: a case for rapid, ultrasensitive and visual detection of novel coronavirus SARS-CoV-2 and HIV virus. bioRxiv [Preprint] 21 March 2020:1-19. [DOI: 10.1101/2020.03.19.998724] [DOI] [Google Scholar]
Dohla 2020 {published data only}
- Dohla M, Boesecke C, Schulte B, Diegmann C, Sib E, Richter E, et al. Rapid point-of-care testing for SARS-CoV-2 in a community screening setting shows low sensitivity. Public Health 2020;182:170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farfan 2020 {published data only}
- Farfan MJ, Torres JP, Oryan M, Olivares M, Gallardo P, Salas C. Optimizing RT-PCR detection of SARS-CoV-2 for developing countries using pool testing. medRxiv [Preprint] 17 April 2020:1-10. [DOI: 10.1101/2020.04.15.20067199] [DOI] [PubMed] [Google Scholar]
Francis 2020 {published data only}
- Francis R, Le Bideau M, Jardot P, Grimaldier C, Raoult D, Khalil JY, et al. High speed large scale automated isolation of SARS-CoV-2 from clinical samples using miniaturized co-culture coupled with high content screening. bioRxiv [Preprint] 19 May 2020:1-23. [DOI: 10.1101/2020.05.14.097295] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freire‐Paspuel 2020 {published data only}
- Freire-Paspuel B, Vega-Marino P, Velez A, Cruz M, Bereguiain MA. High sensitivity CDC EUA SARS-CoV-2 kit-based End Point-PCR assay. medRxiv [Preprint] 18 May 2020:1-7. [DOI: 10.1101/2020.05.11.20098590] [DOI] [Google Scholar]
Ganguli 2020 {published data only}
- Ganguli A, Mostafa A, Berger J, Aydin M, Sun F, Valera E, et al. Rapid isothermal amplification and portable detection system for SARS-CoV-2. bioRxiv [Preprint] 21 May 2020:1-31. [DOI: 10.1101/2020.05.21.108381] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giamarellos‐Bourboulis 2020 {published data only}
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host & Microbe 2020;27(6):992-1000 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez‐Gonzalez 2020 {published data only}
- Gonzalez-Gonzalez E, Lara-Mayorga IM, Rodriguez-Sanchez IP, Yee-de Leon F, Garcia-Rubio A, Garciamendez-Mijares CE, et al. Scaling diagnostics in times of COVID-19: rapid prototyping of 3D-printed water circulators for Loop-mediated Isothermal Amplification (LAMP) and detection of SARS-CoV-2 virus. medRxiv [Preprint] 19 June 2020:1-39. [DOI: 10.1101/2020.04.09.20058651] [DOI] [Google Scholar]
Grant 2020 {published data only}
- Grant PR, Turner MA, Shin GY, Nastouli E, Levett LJ. Extraction-free COVID-19 (SARS-CoV-2) diagnosis by RT-PCR to increase capacity for national testing programmes during a pandemic. bioRxiv [Preprint] 9 April 2020:1-6. [Google Scholar]
Hass 2020 {published data only}
- Hass KN, Bao M, He Q, Park M, Qin P, Du K. Integrated Micropillar Polydimethylsiloxane Accurate CRISPR Detection (IMPACT) system for rapid viral DNA sensing. bioRxiv [Preprint] 20 March 2020:1-10. [DOI: 10.1101/2020.03.17.994137] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogan 2020a {published data only}
- Hogan CA, Sahoo MK, Huang C, Garamani N, Stevens B, Zehnder J, et al. Comparison of the Panther Fusion and a laboratory-developed test targeting the envelope gene for detection of SARS-CoV-2. Journal of Clinical Virology 2020;127:104383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2020 {published data only}
- Hu X, Deng Q, Li J, Chen J, Wang Z, Zhang X, et al. Development and clinical application of a rapid and sensitive loop-mediated isothermal amplification test for SARS-CoV-2 infection. medRxiv [Preprint] 29 May 2020:1-28. [DOI: 10.1101/2020.05.20.20108530] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2020 {published data only}
- Huang WE, Lim B, Hsu CC, Xiong D, Wu W, Yu Y, et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microbial Biotechnology 2020;13(4):950-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2020 {published data only}
- Jiang M, Pan W, Arastehfar A, Fang W, ling L, Fang H, et al. Development and validation of a rapid single-step reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) system potentially to be used for reliable and high-throughput screening of COVID-19. medRxiv [Preprint] 27 March 2020:1-12. [DOI: 10.1101/2020.03.15.20036376] [DOI] [PMC free article] [PubMed] [Google Scholar]
Joung 2020 {published data only}
- Joung J, Ladha A, Saito M, Segel M, Bruneau R, Huang MW, et al. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. medRxiv [Preprint] 8 May 2020:1-21. [DOI: 10.1101/2020.05.04.20091231] [DOI] [Google Scholar]
Kalikiri 2020 {published data only}
- Kalikiri MK, Hasan M, Mirza F, Xaba T, Tang P, Lorenz S. High-throughput extraction of SARS-CoV-2 RNA from nasopharyngeal swabs using solid-phase reverse immobilization beads. medRxiv [Preprint] 11 April 2020:1-5. [DOI: 10.1101/2020.04.08.20055731] [DOI] [Google Scholar]
Kim 2019 {published data only}
- Kim JH, Kang M, Park E, Chung DR, Kim J, Hwang ES. A simple and multiplex Loop-Mediated isothermal Amplification (LAMP) assay for rapid detection of SARS-CoV. Biochip Journal 2019;13(4):341-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konrad 2020 {published data only}
- Konrad R, Eberle U, Dangel A, Treis B, Berger A, Bengs K, et al. Rapid establishment of laboratory diagnostics for the novel coronavirus SARS-CoV-2 in Bavaria, Germany, February 2020. Euro Surveillance 2020;25(9):2000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurstjens 2020 {published data only}
- Kurstjens S, Van der Horst A, Herpers R, Geerits MW, Kluiters-de Hingh YC, Göttgens E-L, et al. Rapid identification of SARS-CoV-2-infected patients at the emergency department using routine testing. bioRxiv [Preprint] 4 April 2020:1-21. [DOI: 10.1101/2020.04.20.20067512] [DOI] [PubMed] [Google Scholar]
Lalli 2020 {published data only}
- Lalli MA, Chen X, Langmade SJ, Fronick CC, Sawyer CS, Burcea LC, et al. Rapid and extraction-free detection of SARS-CoV-2 from saliva with colorimetric LAMP. medRxiv [Preprint] 11 May 2020:1-25. [DOI: 10.1101/2020.05.07.20093542] [DOI] [Google Scholar]
Lamb 2020 {published data only}
- Lamb LE, Bartolone SN, Ward E, Chancellor MB. Rapid detection of novel coronavirus (COVID-19) by reverse transcription-loop-mediated isothermal amplification. medRxiv [Preprint] 24 February 2020:1-17. [DOI: 10.1101/2020.02.19.20025155] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2020 {published data only}
- Lee JY, Best N, McAuley J, Porter JL, Seemann T, Schultz MB, et al. Validation of a single-step, single-tube reverse transcription-loop-mediated isothermal amplification assay for rapid detection of SARS-CoV-2 RNA. bioRxiv [Preprint] 30 April 2020:1-32. [DOI: 10.1101/2020.04.28.067363] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2020 {published data only}
- Lin CY, Hwang D, Chiu NC, Weng LC, Liu HF, Mu JJ, et al. Increased detection of viruses in children with respiratory tract infection using PCR. International Journal of Environmental Research and Public Health 2020;17(2):564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowe 2020 {published data only}
- Lowe CF, Matic N, Ritchie G, Lawson T, Stefanovic A, Champagne S, et al. Detection of low levels of SARS-CoV-2 RNA from nasopharyngeal swabs using three commercial molecular assays. Journal of Clinical Virology 2020;128:104387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2020 {published data only}
- Lu R, Wu X, Wan Z, Li Y, Zuo L, Qin J, et al. Development of a novel reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. Virologica Sinica 2020;35(3):344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2020a {published data only}
- Lu R, Wu X, Wan Z, Li Y, Jin X, Zhang C. A novel reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. International Journal of Molecular Sciences 2020;21(8):2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahari 2020 {published data only}
- Mahari S, Roberts A, Shahdeo D, Gandhi S. eCovSens-Ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCOVID-19 antigen, a spike protein domain 1 of SARS-CoV-2. bioRxiv [Preprint] 11 May 2020:1-20. [DOI: 10.1101/2020.04.24.059204] [DOI] [Google Scholar]
Marzinotto 2020 {published data only}
- Marzinotto S, Mio C, Cifu A, Verardo R, Pipan C, Schneider C, et al. A streamlined approach to rapidly detect SARS-CoV-2 infection, avoiding RNA extraction. medRxiv [Preprint] 11 April 2020:1-10. [DOI: 10.1101/2020.04.06.20054114] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCormick‐Baw 2020 {published data only}
- McCormick-Baw C, Morgan K, Gaffney D, Cazares Y, Jaworski K, Byrd A, et al. Saliva as an alternate specimen source for detection of SARS-CoV-2 in symptomatic patients using Cepheid Xpert Xpress SARS-CoV-2. Journal of Clinical Microbiology 2020;58(8):e01109-20. [DOI: 10.1128/JCM.01109-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
McRae 2020 {published data only}
- McRae MP, Simmons GW, Christodoulides NJ, Lu Z, Kang SK, Fenyo D, et al. Clinical decision support tool and rapid point-of-care platform for determining disease severity in patients with COVID-19. medRxiv [Preprint] 22 April 2020:1-32. [DOI: 10.1101/2020.04.16.20068411] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mei 2020 {published data only}
- Mei X, Lee HC, Diao K, Huang M, Lin B, Liu C, et al. Artificial intelligence-enabled rapid diagnosis of COVID-19 patients. medRxiv [Preprint] 7 May 2020:1-30. [DOI: 10.1101/2020.04.12.20062661] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noerz 2020 {published data only}
- Noerz D, Fischer N, Schultze A, Kluge S, Mayer-Runge U, Aepfelbacher M, et al. Clinical evaluation of a SARS-CoV-2 RT-PCR assay on a fully automated system for rapid on-demand testing in the hospital setting. Journal of Clinical Virology 2020;128:104390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osterdahl 2020 {published data only}
- Osterdahl MF, Lee KA, Ni LM, Wilson S, Douthwaite S, Horsfall R, et al. Detecting SARS-CoV-2 at point of care: preliminary data comparing Loop-mediated Isothermal Amplification (LAMP) to PCR. medRxiv [Preprint] 4 April 2020:1-9. [DOI: 10.1101/2020.04.01.20047357] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paden 2020 {published data only}
- Paden CR, Tao Y, Queen K, Zhang J, Li Y, Uehara A, et al. Rapid, sensitive, full genome sequencing of severe acute respiratory syndrome virus coronavirus 2 (SARS-CoV-2). bioRxiv [Preprint] 24 April 2020:1-13. [DOI: 10.1101/2020.04.22.055897] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pellanda 2020 {published data only}
- Pellanda LC, Wendland EM, McBride AJ, Tovo-Rodrigues L, Ferreira MR, Dellagostin OA, et al. Sensitivity and specificity of a rapid test for assessment of exposure to SARS-CoV-2 in a community-based setting in Brazil. medRxiv [Preprint] 10 May 2020:1-10. [DOI: 10.1101/2020.05.06.20093476] [DOI] [Google Scholar]
Pfefferle 2020 {published data only}
- Pfefferle S, Reucher S, Norz D, Lutgehetmann M. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. EuroSurveillance 2020;25(9):2000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seo 2020 {published data only}
- Seo G, Lee G, Kim MJ, Baek SH, Choi M, Ku KB, et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 2020;14(4):5135-42. [DOI] [PubMed] [Google Scholar]
Smyrlaki 2020 {published data only}
- Smyrlaki I, Ekman M, Lentini A, Vondracek M, Papanicoloau N, Aarum J, et al. Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-qPCR. medRxiv [Preprint] 12 May 2020:1-18. [DOI: 10.1101/2020.04.17.20067348] [DOI] [PMC free article] [PubMed] [Google Scholar]
St Hilaire 2020 {published data only}
- St Hilaire BG, Durand NC, Mitra N, Pulido SG, Mahajan R Blackburn A, et al. A rapid, low cost, and highly sensitive SARS-CoV-2 diagnostic based on whole genome sequencing. bioRxiv [Preprint] 11 May 2020:1-29. [DOI: 10.1101/2020.04.25.061499] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2020 {published data only}
- Tan X, Lin C, Zhang J, Khaing OM, Fan X. Rapid and quantitative detection of COVID-19 markers in micro-liter sized samples. bioRxiv [Preprint] 22 April 2020:1-17. [DOI: 10.1101/2020.04.20.052233] [DOI] [Google Scholar]
Visseaux 2020 {published data only}
- Visseaux B, Le Hingrat Q, Collin G, Bouzid D, Lebourgeois S, Le Pluart D, et al. Evaluation of the QIAstat-Dx Respiratory SARS-CoV-2 Panel, the first rapid multiplex PCR commercial assay for SARS-CoV-2 detection. Journal of Clinical Microbiology 2020;58(8):e00630-20. [DOI: 10.1128/JCM.00630-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020 {published data only}
- Wang X, Zhong M, Liu Y, Ma P, Dang L, Meng Q, et al. Rapid and sensitive detection of COVID-19 using CRISPR/Cas12a-based detection with Naked Eye Readout, CRISPR/Cas12a-NER. Science Bulletin (Beijing) 5 May 2020 [Epub ahead of print]. [DOI: 10.1016/j.scib.2020.04.041] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020a {published data only}
- Wang X, Yao H, Xu X, Zhang P, Zhang M, Shao J, et al. Limits of detection of six approved RT-PCR kits for the novel SARS-coronavirus-2 (SARS-CoV-2). Clinical Chemistry 2020;66(7):977-9. [DOI: 10.1093/clinchem/hvaa099] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wee 2020 {published data only}
- Wee SK, Sivalingam SP, Yap EPH. Rapid direct nucleic acid amplification test without RNA extraction for SARS-CoV-2 using a portable PCR thermocycler. bioRxiv [Preprint] 20 April 2020:1-12. [DOI: 10.1101/2020.04.17.042366] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xue 2020 {published data only}
- Xue G, Li S, Zhang W, Du B, Cui J, Yan C, et al. Reverse-transcription recombinase-aided amplification assay for rapid detection of the 2019 novel coronavirus (SARS-CoV-2). Analytical Chemistry 2020;92(14):9699-705. [DOI: 10.1021/acs.analchem.0c01032] [DOI] [PubMed] [Google Scholar]
Yan 2020 {published data only}
- Yan C, Cui J, Huang L, Du B, Chen L, Xue G, et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clinical Microbiology and Infection 2020;26(6):773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2020 {published data only}
- Yang W, Dang X, Wang Q, Xu M, Zhao Q, Zhou Y, et al. Rapid detection of SARS-CoV-2 using reverse transcription RT-LAMP method. medRxiv [Preprint] 3 March 2020:1-25. [DOI: 10.1101/2020.03.02.20030130] [DOI] [Google Scholar]
Yu 2020 {published data only}
- Yu L, Wu S, Hao X, Dong X, Mao L, Pelechano V, et al. Rapid detection of COVID-19 coronavirus using a Reverse Transcriptional Loop-Mediated Isothermal Amplification (RT-LAMP) diagnostic platform. Clinical Chemistry 2020;66(7):975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2020a {published data only}
- Yu L, Wu S, Hao X, Li X, Liu X, Ye S, et al. Rapid colorimetric detection of COVID-19 coronavirus using a Reverse Transcriptional Loop-Mediated Isothermal Amplification (RT-LAMP) diagnostic platform: iLACO. medRxiv [Preprint] 24 February 2020:1-19. [DOI: 10.1101/2020.02.20.20025874] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zamecnik 2020 {published data only}
- Zamecnik CR, Rajan JV, Yamauchi KA, Mann SA, Sowa GM, Zorn KC, et al. ReScan, a multiplex diagnostic pipeline, pans human sera for SARS-CoV-2 antigens. medRxiv [Preprint] 13 May 2020:1-21. [DOI: 10.1101/2020.05.11.20092528] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2020 {published data only}
- Zeng W, Liu G, Ma H, Zhao D, Yang Y, Liu M, et al. Biochemical characterization of SARS-CoV-2 nucleocapsid protein. Biochemical and Biophysical Research Communications 2020;527(3):618-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2020 {published data only}
- Zhang Y, Odiwuor N, Xiong J, Sun L, Nyaruaba RO, Wei H, et al. Rapid molecular detection of SARS-CoV-2 (COVID-19) virus RNA using colorimetric LAMP. medRxiv [Preprint] 29 February 2020:1-14. [DOI: 10.1101/2020.02.26.20028373] [DOI] [Google Scholar]
Zhao 2020 {published data only}
- Zhao Z, Cui H, Song W, Ru X, Zhou W, Yu X. A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. bioRxiv [Preprint] 27 February 2020:1-18. [DOI: 10.1101/2020.02.22.961268] [DOI] [Google Scholar]
Additional references
Arevalo‐Rodriguez 2020
- Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, Zambrano-Achig P, Campo R, Ciapponi A, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. medRxiv [Preprint] 2020:1-26. [DOI: 10.1101/2020.04.16.20066787] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bossuyt 2015
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI: 10.1136/bmj.h5527] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2020
- Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, Saveson CJ, et al. Assay techniques and test development for COVID-19 diagnosis. ACS Central Science 2020;6(5):591-605. [DOI: 10.1021/acscentsci.0c00501] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2020
- Cheng MP, Yansouni CP, Basta NE, Desjardins M, Kanjilal S, Paquette K, et al. Serodiagnostics for severe acute respiratory syndrome-related coronavirus-2: a narrative review. Annals of Internal Medicine (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Corman 2020
- Corman V, Bleicker T, Brünink S, Drosten C, Landt O, Koopmans M, et al. Diagnostic detection of Wuhan coronavirus 2019 by real-time RT-PCR - protocol and preliminary evaluation as of Jan 13, 2020. Available from www.who.int/docs/default-source/coronaviruse/wuhan-virus-assay-v1991527e5122341d99287a1b17c111902.pdf?sfvrsn=d381fc88_2 2020.
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 27 April 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2020
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, Spijker R, et al. Diagnosis of SARS-CoV-2 infection and COVID-19: accuracy of signs and symptoms; molecular, antigen, and antibody tests; and routine laboratory markers. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013596. [DOI: 10.1002/14651858.CD013596] [DOI] [Google Scholar]
Deeks 2020a
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD013652. [DOI: 10.1002/14651858.CD013652] [DOI] [PMC free article] [PubMed] [Google Scholar]
FIND 2020
- FIND. SARS-COV-2 Diagnostic pipeline. www.finddx.org/covid-19/pipeline/ Accessed 19 July 2020.
Green 2020
- Green K, Graziadio S, Turner P, Fanshawe T, Allen J, on behalf of the Oxford COVID-19 Evidence Service Team Centre. Molecular and antibody point-of-care tests to support the screening, diagnosis and monitoring of COVID-19. Available at www.cebm.net/oxford-covid-19/ 7 April 2020.
Hadgu 1999
- Hadgu A. Discrepant analysis: a biased and an unscientific method for estimating test sensitivity and specificity. Journal of Clinical Epidemiology 1999;52(12):1231-7. [DOI: 10.1016/s0895-4356(99)00101-8] [DOI] [PubMed] [Google Scholar]
Kozel 2017
- Kozel TR, Burnham-Marusich AR. Point-of-care testing for infectious diseases: past, present, and future. Journal of Clinical Microbiology 2017;55(8):2313-20. [DOI: 10.1128/JCM.00476-17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leeflang 2013
- Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ : Canadian Medical Association Journal 2013;185(11):E537-44. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McInnes 2018
- McInnes MD, Moher D, Thombs BD, McGrath TA, Bossuyt PM, PRISMA-DTA Group. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA Statement. JAMA 2018;319(4):388-96. [DOI: 10.1001/jama.2017.19163] [PMID: ] [DOI] [PubMed] [Google Scholar]
McInnes 2020
- McInnes M, Leeflang MM, Salameh J-P, McGrath T, Van der Pol CB, Frank RA, et al. Imaging tests for the diagnosis of COVID-19. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD013639. [DOI: 10.1002/14651858.CD013639] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Medicine 2009;6(7):1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pai 2012
- Pai NP, Vadnais C, Denkinger C, Engel N, Pai M. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Medicine 2012;9(9):e1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982-90. [DOI: 10.1016/j.jclinepi.2005.02.022] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Stata [Computer program]
- Stata. Version 15. College Station, TX, USA: StataCorp, 2017. Available at www.stata.com.
Struyf 2020
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Subsoontorn 2020
- Subsoontorn P, Lohitnavy M, Kongkaew C. The diagnostic accuracy of nucleic acid point-of-care tests for human coronavirus: a systematic review and meta-analysis. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.07.09.20150235] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takwoingi 2017
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta-analysis of diagnostic test accuracy with few studies or sparse data. Statistical Methods in Medical Research 2017;26(4):1896-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Usher‐Smith 2016
- Usher-Smith JA, Sharp SJ, Griffin SJ. The spectrum effect in tests for risk prediction, screening, and diagnosis. BMJ 2016;353:i3139. [DOI: 10.1136/bmj.i3139] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI] [PubMed] [Google Scholar]
WHO 2018
- World Health Organization (WHO). Diagnostic assessment: in vitro diagnostic medical devices (IVDs) used for the detection of high-risk human papillomavirus (HPV) genotypes in cervical cancer screening. Licence: CC BY-NC-SA 3.0 IGO. Available at apps.who.int/iris/handle/10665/272282 2018.
WHO 2020a
- World Health Organization. Laboratory testing of 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance. Available from www.who.int/publications/i/item/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 2020.
WHO 2020b
- Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance. Available from www.who.int/publications/i/item/global-surveillance-for-covid-19-caused-by-human-infection-with-covid-19-virus-interim-guidance 2020.
WHO 2020c
- COVID-19 Target product profiles for priority diagnostics to support response to the COVID-19 pandemic v.0.1. Available from www.who.int/publications/m/item/covid-19-target-product-profiles-for-priority-diagnostics-to-support-response-to-the-covid-19-pandemic-v.0.1 2020.
Zheng 2020
- Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ 2020;369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]


