Abstract
Background
Overall survival rates are disappointing for women with early poor prognosis breast cancer. Autologous transplantation of bone marrow or peripheral stem cells (in which the woman is both donor and recipient) has been considered a promising technique because it permits use of much higher doses of chemotherapy.
Objectives
To compare the effectiveness and safety of high‐dose chemotherapy and autograft (either autologous bone marrow or stem cell transplantation) with conventional chemotherapy for women with early poor prognosis breast cancer.
Search methods
We searched the Cochrane Breast Cancer Group Specialised Register, MEDLINE (1966 to October 2015), EMBASE (1980 to October 2015), the World Health Organization's International Clinical Trials Registry Search Platform, and ClinicalTrials.gov on the 21 October 2015.
Selection criteria
Randomised controlled trials (RCTs) comparing high‐dose chemotherapy and autograft (bone marrow transplant or stem cell rescue) versus chemotherapy without autograft for women with early poor prognosis breast cancer.
Data collection and analysis
Two review authors selected RCTs, independently extracted data and assessed risks of bias. We combined data using a Mantel‐Haenszel fixed‐effect model to calculate pooled risk ratios (RRs) and 95% confidence intervals (CIs). We assessed the quality of the evidence using GRADE methods. Outcomes were survival rates, toxicity and quality of life.
Main results
We included 14 RCTs of 5600 women randomised to receive high‐dose chemotherapy and autograft (bone marrow transplant or stem cell rescue) versus chemotherapy without autograft for women with early poor prognosis breast cancer. The studies were at low risk of bias in most areas.
There is high‐quality evidence that high‐dose chemotherapy does not increase the likelihood of overall survival at any stage of follow‐up (at three years: RR 1.02, 95% CI 0.95 to 1.10, 3 RCTs, 795 women, I² = 56%; at five years: RR 1.00, 95% CI 0.96 to 1.04, 9 RCTs, 3948 women, I² = 0%; at six years: RR 0.94, 95% CI 0.81 to 1.08, 1 RCT, 511 women; at eight years: RR1.17, 95% CI 0.95 to 1.43, 1 RCT, 344 women; at 12 years: RR 1.18, 95% CI 0.99 to 1.42, 1 RCT, 382 women).
There is high‐quality evidence that high‐dose chemotherapy improves the likelihood of event‐free survival at three years (RR 1.19, 95% CI 1.06 to 1.34, 3 RCTs, 795 women, I² = 56%) but this effect was no longer apparent at longer duration of follow‐up (at five years: RR 1.04, 95% CI 0.99 to 1.09, 9 RCTs, 3948 women, I² = 14%; at six years RR 1.04, 95% CI 0.87 to 1.24, 1 RCT, 511 women; at eight years: RR 1.27, 95% CI 0.99 to 1.64, 1 RCT, 344 women; at 12 years: RR 1.18, 95% CI 0.95 to 1.45, 1 RCT, 382 women).
Treatment‐related deaths were much more frequent in the high‐dose arm (RR 7.97, 95% CI 3.99 to 15.92, 14 RCTs, 5600 women, I² = 12%, high‐quality evidence) and non‐fatal morbidity was also more common and more severe in the high‐dose group. There was little or no difference between the groups in the incidence of second cancers at four to nine years' median follow‐up (RR 1.25, 95% CI 0.90 to 1.73, 7 RCTs, 3423 women, I² = 0%, high‐quality evidence). Women in the high‐dose group reported significantly worse quality‐of‐life scores immediately after treatment, but there were few statistically significant differences between the groups by one year.
The primary studies were at low risk of bias in most areas, and the evidence was assessed using GRADE methods and rated as high quality for all comparisons.
Authors' conclusions
There is high‐quality evidence of increased treatment‐related mortality and little or no increase in survival by using high‐dose chemotherapy with autograft for women with early poor prognosis breast cancer.
Plain language summary
High‐dose chemotherapy and bone marrow or stem cell transplantation for early poor prognosis breast cancer using a woman's own cells (autologous)
Background
Women with breast cancer who have multiple positive lymph nodes when first diagnosed are at high risk of recurrence. Conventional chemotherapy has limited success and is unsafe in high doses as it damages the bone marrow. One treatment considered promising was to give women very high doses of chemotherapy followed by transplantation of stem cells to regenerate their bone marrow. Cochrane review authors examined the evidence, which is current to October 2015.
Study characteristics
We included 14 randomised controlled trials (5600 women) which compared high‐dose chemotherapy versus conventional chemotherapy in women with early breast cancer and with a high risk of recurrence. We defined these as women with breast cancer that has spread to multiple local lymph nodes without any evidence of spread beyond local lymph nodes.
All studies reported their source of funding. Eight studies were funded by non‐profit organisations, one by a public health insurance company, one by industry sources and four by a combination of non‐profit organisations and industry sources. Four of the studies reported that authors had no potential conflict of interest, six reported that one or more of their authors had received some kind of support from pharmaceutical companies, and four did not mention whether any of their authors had any potential conflict of interest.
Key results
Using high‐dose chemotherapy has little or no effect on increasing survival. Although rates of event‐free survival were higher in the high‐dose arm over three‐year follow‐up, this effect was not apparent at longer follow‐up. Treatment‐related deaths were much more common in the high‐dose group. Side‐effects were also more common and more severe in the high‐dose group. We did not find an effect on the number of women developing second cancers.
Quality of the evidence
The evidence was of high quality.
Summary of findings
Summary of findings for the main comparison. High‐dose chemotherapy versus chemotherapy without bone marrow transplant or stem cell rescue.
| High‐dose chemotherapy versus chemotherapy without bone marrow transplant or stem cell rescue | ||||||
| Population: women with early poor prognosis breast cancer Setting: Tertiary Intervention: High‐dose chemotherapy Comparison: Chemotherapy without bone marrow transplant or stem cell rescue (standard chemotherapy) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with standard chemotherapy | Risk with high dose chemotherapy | |||||
| Overall survival at 5‐year follow‐up | 672 per 1000 | 672 per 1000 (645 to 698) | RR 1.00 (0.96 to 1.04) | 3566 (8 RCTs) |
⨁⨁⨁⨁ HIGH | |
| Event‐free survival at 5‐year follow‐up | 578 per 1000 | 601 per 1000 (572 to 630) | RR 1.04 (0.99 to 1.10) | 3566 (8 RCTs) | ⨁⨁⨁⨁ HIGH | |
| Treatment‐related mortality | 2 per 1000 | 14 per 1000 (7 to 28) | RR 7.97 (3.99 to 15.92) | 5600 (14 RCTs) | ⨁⨁⨁⨁ HIGH | Most deaths occurred within the first year of treatment |
| Second cancers at 4 ‐ 9‐year median follow‐up | 25 per 1000 | 31 per 1000 (23 to 43) | RR 1.25 (0.90 to 1.73) | 3423 (7 RCTs) | ⨁⨁⨁⨁ HIGH | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the median risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Background
Description of the condition
Breast cancer is the most common cancer occurring in women and is the primary cause of cancer deaths among women worldwide (Ferlay 2015; WHO 2000). Its incidence is increasing in most countries (Bray 2004). The lifetime risk of a woman developing breast cancer is about one in eight in the United States and one in nine in England and Wales (ACS 2002; DOH 2002).
In women with early breast cancer, all detectable cancer is restricted to the breast or local lymph nodes (Clarke 2008). Women who have multiple positive lymph nodes when they are first diagnosed are at high risk of recurrent disease. Without adjuvant chemotherapy, the median recurrence rate at five years is over 60% for women with four to nine positive nodes and over 70% for women with more than 10 positive nodes (Nemoto 1980). Established chemotherapy regimens have a high failure rate, improving the chance of 10‐year survival by about 11% for women under 50 and about 3% for older women (Clarke 2008).
Description of the intervention
Researchers from the 1970s onwards described a dose‐response relationship in the action of chemotherapy drugs against cancer (Frei 1980); thus it was observed that in the treatment of breast cancer the percentage of women responding to therapy is positively associated with the dose intensity of the drugs received, dose intensity being a function of both the dose and the timing of the chemotherapy regimen (Hryniuk 1986). The technique of autologous bone marrow or peripheral blood stem cell transplant (autograft) was considered an exciting development because it addressed the problem of bone‐marrow toxicity which had previously limited the dose of chemotherapy drugs that could be safely given. The use of autograft with chemotherapy permitted the administration of doses many times higher than could otherwise be used. Results of animal studies were encouraging, as were non‐randomised patient trials which commenced during the 1980s and which achieved prolonged survival times using high‐dose chemotherapy and autograft for women with advanced breast cancer (Antman 1992; Peters 1988; Williams 1992).
Why it is important to do this review
Evidence from non‐randomised studies has been criticised for participant selection bias and other design weaknesses (Eddy 1992). The first randomised trials began in 1991. Several randomised controlled trials have now been carried out among women with early poor prognosis breast cancer, which is defined as breast cancer that has spread to multiple local lymph nodes without any evidence of distant metastases (spread). The aim of this review was to consider these studies with respect to the relative effectiveness and safety of the treatments they compare.
Objectives
To compare the effectiveness and safety of high dose chemotherapy and autograft (either autologous bone marrow or stem cell transplantation) with conventional chemotherapy for women with early poor prognosis breast cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials were eligible for the review.
Types of participants
Women of any age with early poor prognosis breast cancer either at first diagnosis or as a recurrence, whether or not previously treated with chemotherapy. We include women with any size of breast tumour. We define 'early poor prognosis breast cancer' in this review as breast cancer that has spread to multiple local lymph nodes without any evidence of distant metastases
Types of interventions
High‐dose chemotherapy with autologous bone marrow or stem cell transplantation versus conventional chemotherapy, regardless of the duration of therapy. We define 'high‐dose chemotherapy' as chemotherapy sufficient to require bone marrow transplantation or stem cell rescue. We define 'conventional therapy' as chemotherapy at a lower dose than the high‐dose therapy and without bone marrow transplant or stem cell rescue.
Types of outcome measures
Primary outcomes
Overall survival (measured at 3, 4, 5, 6 or more years)
Event‐free survival (no evidence of recurrence of breast cancer: measured at 3, 4, 5, 6 or more years)
Secondary outcomes
Treatment‐related mortality
Morbidity such as non‐haematological toxicities, e.g. nausea and vomiting, white cell measures, new malignancies
Quality‐of‐life measures
Search methods for identification of studies
Electronic searches
We searched the following databases on 21 October 2015:
The Cochrane Breast Cancer Group's (CBCG's) Specialised Register. Details of search strategies used by the CBCG for the identification of studies and the procedures used to code references are outlined in the CBCG's module at www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html. We retrieved trials with the text‐words bone marrow transplantation, stem cell transplantation, stem cell support, autologous stem cell support, high dose chemotherapy, and chemotherapy.
MEDLINE (via OvidSP) until October 2015 (See Appendix 1).
EMBASE (via Embase.com) until October 2015 (See Appendix 2).
The World Health Organization's (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/Default.aspx) for prospectively‐registered and ongoing trials until October 2015 (See Appendix 3).
ClinicalTrials.gov (clinicaltrials.gov/ct2/search/advanced) until October 2015 (See Appendix 4).
Searching other resources
We searched the reference lists of articles found by the above search strategy.
Data collection and analysis
Selection of studies
For the 2015 review update, two review authors (JM and MA or AL) undertook study selection, and independently screened all articles retrieved by the search. Three review authors (JM, MA or AL and CF) independently assessed whether the studies met the inclusion criteria, with disagreements resolved by discussion.
For previous versions of the review, three review authors (CF, RB and JMB), one of whom (RB) is a content expert, had undertaken study selection. CF screened the titles and abstracts of articles found in the search, and discarded studies that were clearly ineligible; however, the aim was to be overly inclusive rather than risk losing relevant studies. CF obtained copies of the full‐text articles and made copies for RB in which details of the authors and institutions were struck out and the Results section removed: however, despite this RB was able to recognise the studies since this is such a small field in terms of publications. We sought further information from the authors of the primary studies where papers contained insufficient information to make a decision about eligibility.
Data extraction and management
For the 2015 review update, two review authors (JM and MA or CF) independently extracted information using data extraction sheets designed by CF, and resolved discrepancies by discussion. We collected the following information from each study: country where the study was conducted, source of funding, design and methods of the study, study population, inclusion criteria, description of the high‐dose chemotherapy and conventional therapy, outcomes measured and study results. Where possible, we sought missing data from the authors.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risks of bias using the Cochrane 'Risk of bias' assessment tool (www.cochrane‐handbook.org) to assess: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. We resolved disagreements by discussion or by recourse to a third review author. We described all judgements fully and presented the conclusions in the 'Risk of bias' table, which was incorporated into the interpretation of review findings by means of sensitivity analyses.
Measures of treatment effect
Some of the available data were immature (e.g. five‐year outcomes were estimated when not all participants had been randomised for five years). Where trialists reported survival rates based on immature data, we used these rates in our tables of comparison. We have noted this in the Characteristics of included studies table of where applicable.
Where results were available only as percentages or only presented in graphs, we calculated the numerators and denominators from the data available and completed the tables of comparison accordingly. We have noted this in the Characteristics of included studies table of where applicable.
Overall survival, event‐free survival, treatment‐related mortality and morbidity are all presented as risk ratios (RRs) with 95% confidence intervals (CIs). We have described quality‐of‐life measures in narrative form.
Several studies have multiple publications and have reported outcomes at different follow‐up times. The data included in this review are the most mature data available for each study.
Unit of analysis issues
All analyses were per woman randomised.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible, and tried to obtain missing data from the original trialists. Where these were unobtainable, we did not impute individual values and analysed only the available data.
Assessment of heterogeneity
We assessed statistical heterogeneity by inspecting the scatter in the data points on the graphs and the overlap in their confidence intervals and, more formally, by checking the I² value (Higgins 2003). This measure describes the percentage of total variation across studies that is due to heterogeneity rather than to chance. Interpretation of a given degree of heterogeneity will differ according to whether the estimates show the same direction of effect but we planned to interpret the I² measure as follows: 0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. Where there were 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We assembled the most complete data set feasible. We assessed clinical heterogeneity between trials through evaluation of potential differences between participants, interventions and outcomes within each study. Where trials appeared to be clinically comparable, we pooled the data to obtain a risk ratio using the Mantel‐Haenszel method in a fixed‐effect model (Mantel 1959), with 95% CIs.
We did not combine quality‐of‐life outcomes because only descriptive data were available.
Subgroup analysis and investigation of heterogeneity
We did not conduct any subgroup analyses. A priori, we had planned to look at the possible contribution of differences in trial design to any meta‐analyses with an I² value of 50% or more.
Sensitivity analysis
We conducted sensitivity analyses by repeating the analyses excluding studies which differed in respect of study quality or chemotherapy regimen, in order to examine the stability of the results.
As there was considerable variation in prognostic factors between participants in the 14 studies, we also conducted a post hoc sensitivity analysis by lymph node status, restricting analysis to studies that included women with 10 or more positive lymph nodes.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using Guideline Development Tool software (GRADEpro GDT). This table highlighted the overall quality of the body of evidence for the main review outcomes, using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We justified our judgements about evidence quality (high, moderate or low), and documented and incorporated them into the reporting of results for each outcome.
Results
Description of studies
The included studies differed in several ways, and are described in detail below. Refer to the Characteristics of included studies table.
Results of the search
In the updated search conducted in October 2015, we screened 1385 records, and retrieved 11 potentially relevant articles for further assessment:
-
Nine articles were new publications relating to studies already included in the review (CALGB 2005; Dutch Intergp 2003 x2; GABG 2004; IBCSG 2006; JCOG 2001; MCG 2001; PEGASE 01 2003; WSG 2005).
Five of these nine articles reported new data on survival outcomes (IBCSG 2006; GABG 2004; JCOG 2001; MCG 2001) or quality of life (PEGASE 01 2003).
The other four articles reported on outcomes not of interest to the current review, and we have added them as additional references to the relevant studies (CALGB 2005; Dutch Intergp 2003 x 2).
One article was the first publication of a study previously categorised as ongoing (NCT00002772).
One study we excluded because it was not an RCT (Sportès 2009).
See PRISMA flowchart (Figure 1).
1.
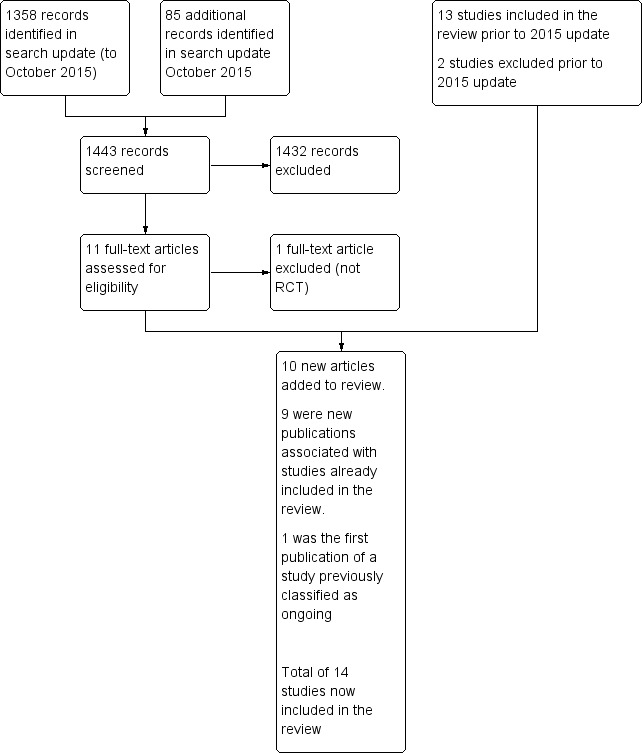
Study flow diagram.
Altogether we include 14 studies in the updated review and exclude three. All the included studies were randomised trials comparing an experimental group (experimental arm) receiving high‐dose chemotherapy and autologous bone marrow or stem cell transplantation with a control group (control arm) on a lower dose of chemotherapy for the treatment of early poor prognosis breast cancer. All of the included trials have been published in full text.
Included studies
Trial design and setting
Six of the trials were European, three were international, four were North American, and one was Japanese. The 14 trials randomised 5600 women, of whom only 47 (< 1%) were not included in the analyses (see comments on methodological quality below). The five largest trials accounted for 3322 of the women randomised (ACCOG 2004; CALGB 2005; Dutch Intergp 2003; ECOG 2003; NCT00002772). Five of the trials involved between 300 and 400 women (or just over) (IBCSG 2006; GABG 2004; MCG 2001; PEGASE 01 2003; WSG 2005) and one analysed 279 women (ICCG 2005). The other three analysed fewer than 100 women each; in one case this was because it was a pilot study (Dutch pilot 1998) and in another it was due to accrual difficulties (MDACC 2000).
In one study women who relapsed in the control arm became eligible for high‐dose treatment and transplant (CALGB 2005).
Eleven studies reported using stratification, as follows: seven stratified according to menopausal status (CALGB 2005; Dutch Intergp 2003; Dutch pilot 1998; ECOG 2003; IBCSG 2006; ICCG 2005; JCOG 2001), four according to the number of positive axillary nodes (ACCOG 2004; ICCG 2005; JCOG 2001; MCG 2001), six according to participating institution (CALGB 2005; ECOG 2003; IBCSG 2006; ICCG 2005; JCOG 2001, WSG 2005), three according to hormone receptor status (CALGB 2005; ECOG 2003; IBCSG 2006), two according to age (Dutch Intergp 2003; ECOG 2003), two according to clinical stage (CALGB 2005; MDACC 2000), two according to tumour size (Dutch Intergp 2003; WSG 2005), one according to type of primary therapy (NCT00002772) and one according to clinical response to initial chemotherapy (Dutch pilot 1998).
All studies reported their source of funding. Eight studies were funded by non‐profit organisations (CALGB 2005; Dutch pilot 1998; ECOG 2003; GABG 2004; JCOG 2001, MDACC 2000; NCT00002772; PEGASE 01 2003), one by a public health insurance company (Dutch Intergp 2003), one by industry sources (WSG 2005) and four by a combination of non‐profit organisations and industry sources (ACCOG 2004; IBCSG 2006; ICCG 2005; MCG 2001). Four of the studies (CALGB 2005; GABG 2004; IBCSG 2006; MCG 2001) reported that authors had no potential conflict of interest, six (ACCOG 2004; ECOG 2003; MDACC 2000; PEGASE 01 2003; NCT00002772; WSG 2005) reported that one or more of their authors had received some kind of support from pharmaceutical companies, and four studies did not mention whether any of their authors had any potential conflict of interest (Dutch Intergp 2003; Dutch pilot 1998; ICCG 2005; JCOG 2001;).
Participants
All participants were women with early poor prognosis breast cancer, having evidence of multiple axillary lymph node involvement and no evidence of distant metastasis. There was considerable variation between participants in the 14 studies with respect to specific prognostic factors, notably their differing number of positive lymph nodes. Five of the studies included women with four or more positive lymph nodes (ACCOG 2004; Dutch Intergp 2003; ICCG 2005; MCG 2001; PEGASE 01 2003), seven included only women with 10 or more positive nodes (CALGB 2005; ECOG 2003; GABG 2004; JCOG 2001; MDACC 2000; NCT00002772; WSG 2005), one included women with either 10 or more positive nodes or else at least five positive nodes plus an additional risk factor (IBCSG 2006), and the other study (Dutch pilot 1998) specified that women have "extensive axillary node metastasis as evidenced by a positive infraclavicular node biopsy". The number of positive nodes required for inclusion in the trials ranged from "at least four" to "at least ten". In the Dutch Pilot study (1998), a positive axillary apex node on infraclavicular lymph node biopsy was taken as evidence of multiple axillary lymph node involvement.
Median age, where stated, was 43 to 47 years, but ages ranged from 22 to 66 years. The trials used a variety of means to identify and exclude women with distant metastases. Five required participants to have bone marrow aspiration and biopsy (CALGB 2005; ECOG 2003; JCOG 2001; MDACC 2000; NCT00002772); they also required, as did the Dutch Intergp 2003 and the WSG 2005 studies, a normal chest X‐Ray, bone scan and liver ultrasound. The ICCG 2005 study as well as the NCT00002772 study required women to have a normal bone scan. The Dutch pilot 1998 study stated that CT scans and bone marrow biopsies werenot done.
Details of the prognostic characteristics of trial participants are given in Table 2. Table 3 briefly outlines breast cancer staging.
1. Prognostic factors of women in the included studies.
| Study ID | Median Age | Tumour | Median nodes positive | Minimum nodes positive | > 9 nodes | Oestro positive | Progest. positive | Other | Premenop'sal |
| ACCOG 2004 | 45 | 3 cm max. | 9 | 4 | 45% | 31% (ER or PR +ve) | 31% (ER or PR +ve) | 43% receptor unknown | ‐ |
| CALGB 2005 | 45 | 3 cm median | 14 (range 10 ‐ 52) | 10 | 100% | 69% | ‐ | ‐ | ‐ |
| Dutch pilot 1998 | 45 | T1 5%; T2 30%; T3 45%; T4 10%; Tx 10% | ‐ | N/A: Had pre‐op chemo | N/A | 20% | 25% | 54% receptor unknown | 83% |
| Dutch Intergp 2003 | 45.7 | T1 22%; T2 60%; T3 16% | ‐ | 4 | 35.8% | 65% | 53% | 28% oestrogen receptor negative | ‐ |
| ECOG 2003 | 44 | ‐ | ‐ | 10 | ‐ | 60% | 59% | 46% > 14 +ve nodes | 72% |
| GABG 2004 | ‐ | ‐ | ‐ | 10 | 100% | 60% | 40% | ‐ | 58% |
| IBCSG 2006 | 46 | T1 26%; T2 51%; T3 20% | 13 | 5 ‐ 10 depending on other prognostic factors | 73% | ‐ | ‐ | 40% oestrogen & progesterone receptor ‐ve | 67% |
| ICCG 2005 | 47 (range 24 ‐ 60) | T1 28%; T2 54%; T3 14%; unknown 4% | 9 (range 4 ‐ 36) | 4 | 45% | 43% | 25% | 38% receptor status not known | 70% |
| JCOG 2001 | 46 | ‐ | 16 (range 10 ‐ 49) | 10 | 100% | ‐ | ‐ | ‐ | 74% |
| MDACC 2000 | 45 | ‐ | ‐ | 10 at diagnosis or 4 after initial chemo | > 60% | 50% | 45% | 5% receptor unknown | 68% |
| MCG 2001 | ‐ | ‐ | ‐ | 4 | 62% | ‐ | ‐ | ‐ | ‐ |
| PEGASE 01 2003 | 46 (mean) | ‐ | 13 | 8 | ? | 31% | ‐ | ‐ | 68% |
| NCT00002772 | Not stated. 45% were aged 40 ‐ 49 yrs | 20% had T3 tumour | 8% were N2 | ‐ | ‐ | ‐ | ‐ | 66% ER/PgR +ve; 8% receptor unknown | ‐ |
| WSG 2005 | 47 | Mean size 3.3 ‐ 3.5 cm | 17 ‐ 18 | 10 | 100% | 63% | ‐ | ‐ | 53% |
+ve = positive ‐ve = negative ER = estrogen receptor NA = not applicable PR = progestogen receptor
2. Breast cancer staging.
| Stage | What stage means |
| I | Breast tumour 2 cm or less in diameter and does not appear to have spread beyond the breast |
| IIA | Breast tumour over 2 cm in diameter OR has spread to the axillary (underarm) lymph nodes on the same side as the breast cancer. The nodes are not stuck to one another or to the surrounding tissues |
| IIB | Breast tumour over 2 cm in diameter AND has spread to the axillary nodes on the same side as the breast cancer. The nodes are not stuck together or to the surrounding tissues. OR the tumour is larger than 5 cm in diameter (and nodes are clear) |
| IIIA | Breast tumour over 5 cms in diameter AND has spread to the axillary lymph nodes on the same side OR tumour has spread to the lymph nodes on the same side as the breast cancer and the nodes are stuck to each other or to the surrounding tissues |
| IIIB | Breast tumour has spread to chest wall or skin OR tumour has spread to internal mammary lymph nodes on the same side as breast tumour |
| IV | Tumour has spread from breast to distant sites or to supraclavicular (above collarbone) lymph nodes |
Most of the studies randomised women soon after full or partial mastectomy and axillary node dissection. The exception was Dutch pilot 1998, which enrolled women for a course of preoperative chemotherapy but excluded before randomisation any women whose disease progressed during the chemotherapy. In one of the American studies (MDACC 2000), women who presented with advanced local disease received a course of preoperative chemotherapy and were eligible for randomisation if they had more than four positive nodes at subsequent surgery. Both Dutch trials, as well as the Japanese trial (JCOG 2001), excluded women who had had prior chemotherapy or radiotherapy. The NCT00002772 trial excluded women who had had radiotherapy, chemotherapy or hormonal therapy for breast cancer or had had chemotherapy for any previous malignancy.
We sought additional information regarding the study design and results from all the principal investigators. We received replies from the Anglo Celtic Oncology Group (ACCOG 2004), the Netherlands Working Party on Autologous Transplantation in Solid Tumours (Dutch Intergp 2003; Dutch pilot 1998), the Eastern Co‐operative Oncology Group (ECOG 2003), the German Breast Cancer Study group (GABG 2004), the MD Anderson group (MDACC 2000), the International Breast Cancer Study Group (IBCSG 2006), the International Collaborative Cancer Group (ICCG 2005), the Michelangelo Cooperative Group (MCG 2001) and the Japanese Clinical Oncology Group (JCOG 2001). However, not all of the requested information was provided: we categorise missing information on trial design as 'Not stated' in the Characteristics of included studies.
Interventions
There was considerable variation among the chemotherapy regimens used. Most of the trials delivered an initial course of chemotherapy at conventional doses to all women. This served as an 'induction' course to women in the high‐dose groups, who went on to receive a high‐dose myeloablative regimen followed by the infusion of stem cells to 'rescue' the bone marrow. In some cases women in the control arms went on to have additional cycles of conventional‐dose chemotherapy and in other cases the conventional treatment was modified in some way to increase the strength or intensity of the dose (CALGB 2005; MCG 2001; WSG 2005). Two trials (IBCSG 2006; MCG 2001) gave the high‐dose arm multiple cycles of high‐dose therapy without a lower‐dose induction course.
Initial chemotherapy
In most of the trials both arms received the same initial chemotherapy. Five trials gave all women an initial course of pre‐ and/or postoperative chemotherapy comprising three to four cycles of cyclophosphamide with anthracycline (doxorubicin or epirubicin) and fluorouracil (CALGB 2005; Dutch pilot 1998; Dutch Intergp 2003; ICCG 2005; PEGASE 01 2003). Two trials gave this regimen for six cycles (ECOG 2003; JCOG 2001) and one for eight cycles (MDACC 2000). Two gave multiple cycles of cyclophosphamide and epirubicin only (GABG 2004; WSG 2005), and one (ACCOG 2004) used four cycles of doxorubicin alone for the initial chemotherapy. Doses in the control arm varied: see Table 4. During this initial phase of chemotherapy, women randomised to receive the high‐dose treatment had a course of granulocyte colony‐stimulating factor (GCSF) to stimulate the production of white cells which were then harvested for later transplantation.
3. Control arm ‐ chemotherapy doses (per m²).
| Study | Phase 1 | Phase 2 |
| ACCOG 2004 | doxorubicin 75 mg 4 cycles | cyclophosphamide methotrexate fluorouracil 8 cycles (doses not stated) |
| CALGB 2005 | cyclophosphamide 600 mg doxorubicin 60 mg fluorouracil 1200 mg 4 cycles | cyclophosphamide 900 mg cisplatin 90 mg carmustine 90 mg 1 cycle with GCSF |
| Dutch Intergp 2003 | cyclophosphamide 500 mg epirubicin 90 mg fluorouracil 500 mg 5 cycles | ‐ |
| Dutch pilot 1998 | cyclophosphamide 500 mg epirubicin 90 mg fluorouracil 500 mg 4 cycles | ‐ |
| ECOG 2003 | cyclophosphamide 1400 mg (po) doxorubicin 60 mg fluorouracil 1000 mg X 6 cycles | ‐ |
| GABG 2004 | cyclophosphamide 600 mg epirubicin 90 mg 4 cycles | cyclophosphamide 1 gm methotrexate 80 gm fluorouracil 1200 mg 3 cycles |
| IBCSG 2006 | doxorubicin 60mg or epirubicin 90 mg cyclophosphamide 600 mg 3 cycles | cyclophosphamide 1400 mg (po) fluorouracil 1200 mg methotrexate 80 mg 3 cycles |
| ICCG 2005 | cyclophosphamide 600 mg epirubicin 50 mg fluorouracil 500 mg 1 cycle | cyclophosphamide 1200 mg epirubicin 100 mg fluorouracil 1000 mg 5 cycles |
| JCOG 2001 | cyclophosphamide 500 mg doxorubicin 40 mg fluorouracil 500 mg 6 cycles | ‐ |
| MCG 2001 | epirubicin 120 mg 3 cycles | cyclophosphamide 600 mg methotrexate 40 mg fluorouracil 600 mg 6 cycles |
| MDACC 2000 | cyclophosphamide 500 mg doxorubicin 50 mg fluorouracil 1 gm 8 cycles | ‐ |
| PEGASE 01 2003 | cyclophosphamide 500 mg epirubicin 100 mg fluorouracil 500 mg 4 cycles | ‐ |
| NCT00002772 | sequential administration of 3 cycles each of doxorubicin 80 mg/m², paclitaxel 200 mg/m², and cyclophosphamide 3 g/m² (total 9 cycles over 18 weeks), with a cumulative doxorubicin dose of 240 mg/m² | ‐ |
| WSG 2005 | cyclophosphamide 600 mg epirubicin 90 mg 4 cycles X 2 weekly | cyclophosphamide 600 mg methotrexate 40 mg fluorouracil 600 mg 3 cycles X 2 weekly with GCSF |
The control arm
In five of the trials the women in the control arm had no further chemotherapy after the initial phase mentioned above. In two (Dutch Intergp 2003; ICCG 2005) the control arm had a continuation of the initial chemotherapy. In two trials (ACCOG 2004; GABG 2004) they received a standard course of a different chemotherapy, cyclophosphamide, methotrexate and fluorouracil (CMF). The control arm in one trial (CALGB 2005) were given an "intermediate level" version of the high‐dose therapy along with a course of GCSF to stimulate white cell production. In another (WSG 2005) they had a dose‐dense regimen supported by GCSF, comprising two further cycles of the initial chemotherapy followed by three cycles of CMF, all at two‐week intervals.
In three trials where the two arms did not have any of their treatment in common, the control arm received two combination therapies in sequence: in the Italian trial (MCG 2001) the control arm received three cycles of epirubicin followed by six of CMF; in the IBCSG 2006 trial they received four cycles of epirubicin and cyclophosphamide (EC) followed by three cycles of CMF. In NCT00002772 the control group received sequential dose‐dense and dose‐escalated chemotherapy consisting of three cycles of doxorubicin, paclitaxel, and cyclophosphamide, with filgrastim support. The study authors noted that this regimen is non‐standard.
The experimental arm
In most studies, after the initial chemotherapy described above, the experimental arm went on to receive one or two cycles of high‐dose chemotherapy. The high‐dose therapy for most trials comprised cyclophosphamide with thiotepa or etoposide or carmustine, with or without a platinum‐based drug (cisplatin or carboplatin) or mitoxantrone. Doses varied: see Table 5 and Characteristics of included studies tables.
4. High‐dose chemo regimes (all doses per m² unless otherwise stated).
| Study | Initial phase | High‐dose cycle 1 | High‐dose cycle 2 | High‐dose cycle 3 | High‐dose cycle 4 | Regimen |
| ACCOG 2004 | 4 cycles of doxorubicin (as control arm) followed by: | cyclophosphamide 4 gm | cyclophosphamide 6 gm thiotepa 800 mg | ‐ | ‐ | Divided doses over 4 days |
| CALGB 2005 | 4 cycles of cyclophosphamide, doxorubicin and fluorouracil (as control arm) followed by: | cyclophosphamide 5.625 gm cisplatin 165 mg carmustine 600 mg | ‐ | ‐ | ‐ | Divided doses over 3 days |
| Dutch Intergp 2003 | 4 cycles of cyclophosphamide, epirubicin and fluorouracil (doses as control arm) followed by: | cyclophosphamide 6 gm thiotepa 480 mg carboplatin 1600 mg | ‐ | ‐ | ‐ | Divided doses over 4 days |
| Dutch pilot 1998 | 4 cycles of cyclophosphamide, epirubicin and fluorouracil (as control arm) followed by: | cyclophosphamide 6 gm thiotepa 480 mg carboplatin 1600 mg | ‐ | ‐ | ‐ | Divided doses over 4 days |
| ECOG 2003 | 6 cycles of cyclophosphamide, doxorubicin and 5FU (as control arm) followed by: | cyclophosphamide 6 gm thiotepa 800 mg | ‐ | ‐ | ‐ | Continuous infusion over 4 days |
| GABG 2004 | 4 cycles of cyclophosphamide and epirubicin (as control arm) followed by: | cyclophosphamide 6 gm thiotepa 600 mg mitoxantrone 40 mg | ‐ | ‐ | ‐ | Divided doses over 4 days |
| IBCSG 2006 | No common path with control group protocol | epirubicin 200 mg cyclophosphamide 4 gm | As cycle 1 | As cycle 1 | 3 X 21‐day cycles | |
| ICCG 2005 | 2 cycles of cyclophosphamide, epirubicin and fluorouracil (as control arm cycles 1 and 2) | cyclophosphamide 6 gm thiotepa 600 mg carboplatin 800 mg | ‐ | ‐ | ‐ | Continuous infusion over 4 days |
| JCOG 2001 | 6 cycles of cyclophosphamide, doxorubicin and fluorouracil (as control arm), followed by: | cyclophosphamide 6 gm thiotepa 600 mg | ‐ | ‐ | ‐ | ‐ |
| MCG 2001 | No common path with control group protocol | cyclophosphamide 7 gm | methotrexate 8gm | epirubicin 120 mg X 2 | thiotepa 600 mg melphalan 160 ‐ 180 mg | 4 high‐dose treatments in sequence |
| MDACC 2000 | 8 cycles of cyclophosphamide, doxorubicin and fluorouracil (as control arm), followed by: | cyclophosphamide 5.25 gm cisplatin 165 mg etoposide 1.2 gm | As cycle 1 | ‐ | ‐ | Divided doses over 3 days. 2nd cycle given when haematologically safe |
| PEGASE 01 2003 | 4 cycles of cyclophosphamide, epirubicin and fluorouracil (as control arm), followed by: | cyclophosphamide 120 mg mitoxantrone 45 mg alkeran 140 mg | ‐ | ‐ | ‐ | ‐ |
| NCT00002772 | 4 cycles of doxorubicin 80 mg/m² and cyclophosphamide 600 mg/m² (AC) every 3 weeks | STAMP I or STAMP V HDC regimen. STAMP I consisted of cyclophosphamide 1.85 g/m²/d and cisplatin 55 mg/m²/d, followed by carmustine 600 mg/m²; STAMP V consisted of cyclophosphamide 1.5 g/m²/d, carboplatin 200 mg/m²/d, and thiotepa 125 mg/m²/d |
‐ | ‐ | ‐ | ‐ |
| WSG 2005 | 2 cycles of cyclophosphamide and epirubicin (as control arm) | cyclophosphamide 3 gm epirubicin 90 mg thiotepa 400 mg | As cycle 1 | ‐ | ‐ | High‐dose cycles over 28 days |
As noted above, three studies differed in design by giving different initial chemotherapy to the experimental arm. In one (MCG 2001) the experimental arm had a sequence of cyclophosphamide, methotrexate, epirubicin and thiotepa with melphalan, all at high doses, and in the second (IBCSG 2006) they had three cycles of high‐dose epirubicin and cyclophosphamide; in the third (NCT00002772) they received four cycles of doxorubicin and cyclophosphamide.
In all cases high‐dose therapy was supported by autologous peripheral blood progenitor cell transplantation or bone marrow transplantation or both, using the cells or marrow harvested during the initial phase of chemotherapy.
Radiotherapy
All women in 10 of the trials received a course of loco‐regional radiotherapy after chemotherapy (ACCOG 2004; CALGB 2005; Dutch Intergp 2003; Dutch pilot 1998; ECOG 2003; ICCG 2005; MDACC 2000; PEGASE 01 2003; NCT00002772; WSG 2005), and this was introduced as a protocol change in another study (GABG 2004). In the Italian trial (MCG 2001) only women who had had conservative surgery received radiotherapy, and in another (IBCSG 2006) it was mandatory after breast‐conserving surgery but recommended for all women. The Japanese trial (JCOG 2001) did not include radiotherapy as part of the protocol.
Tamoxifen
Trial protocols for tamoxifen varied. It was prescribed for all women in seven trials (ACCOG 2004; Dutch Intergp 2003; Dutch pilot 1998; IBCSG 2006; ICCG 2005; JCOG 2001; MCG 2001). Other trials did not prescribe it for women who had hormone receptor‐negative disease (CALGB 2005; ECOG 2003; GABG 2004; WSG 2005) or were premenopausal (MDACC 2000; PEGASE 01 2003; NCT00002772). The duration of treatment, where specified, was five years for all trials except Dutch Intergp 2003 (in which the duration of treatment was initially two years but was changed to five years during the course of the trial), Dutch pilot 1998 (in which the duration of treatment was two years) and JCOG 2001 (in which the duration of treatment was "at least two years").
Outcomes
All studies measured overall survival (i.e. survival with or without recurrence), and all specified the number of deaths caused by treatment toxicity.
All studies measured event‐free survival (i.e. survival without breast cancer recurrence). Two studies differed by including the incidence of other cancers in events for this outcome, without separately reporting the data relating to breast cancer alone (CALGB 2005; GABG 2004).
With regard to non‐fatal toxicity, eight studies (Dutch Intergp 2003; Dutch pilot 1998; ACCOG 2004; ECOG 2003; ICCG 2005; MDACC 2000; NCT00002772; WSG 2005) described the side effects experienced by women on both standard and high‐dose regimens. Six trials reported long‐term toxicity which included the incidence of second cancers (CALGB 2005; Dutch Intergp 2003; Dutch pilot 1998; IBCSG 2006; ICCG 2005; MDACC 2000).
Limited quality‐of‐life data have been published (ACCOG 2004; CALGB 2005; Dutch Intergp 2003; Dutch pilot 1998; PEGASE 01 2003; WSG 2005).
Mature data on overall survival and event‐free survival have been published for five studies. Four of these have followed up all women for three years (Dutch Intergp 2003; MDACC 2000; PEGASE 01 2003; WSG 2005) and one has followed up all women for five years (Dutch pilot 1998). The other studies had median follow‐up times ranging from three to over 11 years and reported estimated survival rates at differing follow‐up periods, based on their results to date. See Table 6.
5. Data maturity.
| Study ID | Data maturity | Median follow‐up |
| ACCOG 2004 | No | 4 years |
| CALGB 2005 | No | 7.3 years |
| Dutch pilot 1998 | 5 years | 6.9 years |
| Dutch Intergp 2003 | 3 years | 7 years |
| ECOG 2003 | No | 6.1 years |
| GABG 2004 | No | 6.1 years |
| IBCSG 2006 | No | 8.3 years |
| ICCG 2005 | No | 4.2 years |
| JCOG 2001 | No | 63 months |
| MDACC 2000 | 3 years | 11.9 years |
| MCG 2001 | No | 11.33 years |
| PEGASE 01 2003 | 3 years | 3.25 years |
| NCT00002772 | No | 5.8 years |
| WSG 2005 | 3 years | 4 years |
Only six studies (Dutch Intergp 2003; Dutch pilot 1998; ECOG 2003; ICCG 2005; MDACC 2000; NCT00002772) reported data comparing the adverse effects of the different doses after the end of chemotherapy or during long‐term follow‐up, or both.
Excluded studies
We excluded three studies, one because it compared two experimental chemotherapy regimens and did not include a control group receiving conventional chemotherapy (Bergh 2000), one because the trial results have been retracted after an investigation for breach of scientific integrity (Bezwoda 1999), and one because it was not an RCT (Sportès 2009).
Risk of bias in included studies
Allocation
Sequence generation
We rated 10 trials at low risk of bias for sequence generation; all used computerised methods. The other four trials did not describe the methods used, and we rated them at unclear risk.
Allocation concealment
We rated 10 trials at low risk of bias for allocation concealment; most used remote allocation. The other four trials did not describe the methods used, and we rated them at unclear risk.
Blinding
Dutch Intergp 2003 conducted a centralised review of pathological specimens in a blinded fashion, but otherwise blinding was not mentioned in any group. As it appears unlikely (but not impossible) that blinding would influence our primary review outcomes, we rated all studies at unclear risk of bias in this domain.
Incomplete outcome data
We rated all studies at low risk of bias in this domain, as in all cases 95% to 100% of women randomised were included in the analysis.
Selective reporting
All studies reported all expected outcomes and were rated at low risk of bias in this domain.
Other potential sources of bias
We identified no other potential source of bias for 10 studies, and we rated them at low risk for this domain. Four studies reported issues that could potentially cause bias, and we rated these at unclear risk.
For details on risks of bias please see Figure 2; Figure 3 and Characteristics of included studies
2.
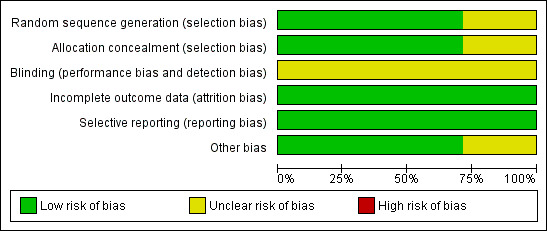
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
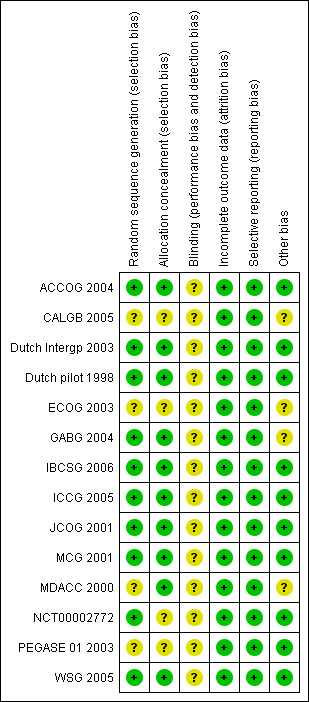
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
A total of 5600 women were analysed in 14 studies, of whom 2800 were randomised to receive high‐dose chemotherapy with stem cell transplantation (experimental group) and 2800 were randomised to conventional treatment (control group).
High‐dose chemotherapy with autograft versus chemotherapy without bone marrow transplant or stem cell rescue
Primary outcomes
1. Overall survival
Refer to Figure 4.
4.
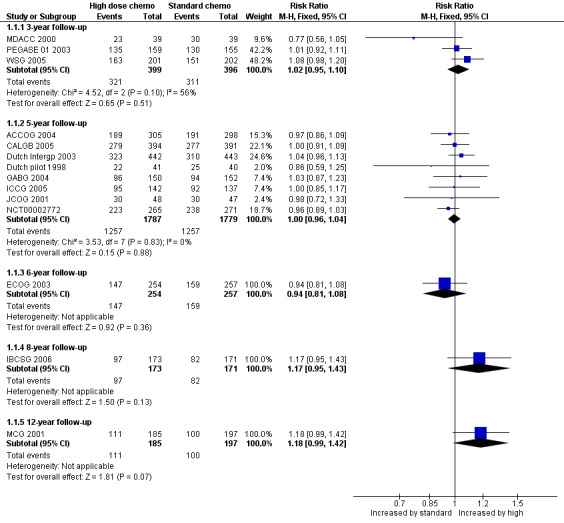
Forest plot of comparison: 1 High‐dose chemotherapy versus standard chemotherapy, outcome: 1.1 Overall survival.
Three‐year follow‐up
We pooled three studies for this outcome. There was no evidence of a difference between the groups (risk ratio (RR) 1.02, 95% confidence interval (CI) 0.95 to 1.10, 3 RCTs, 795 women, I² = 56%; Analysis 1.1). There was moderate statistical heterogeneity for this finding. Heterogeneity appeared to be attributable to MDACC 2000, which was the smallest study in the review but did not differ from other studies in any obvious way. Exclusion of this study from analysis did not substantially affect the findings (RR 1.05, 95% CI 0.98 to 1.13, 2 RCTs, 717 women, I² = 0%).
1.1. Analysis.

Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 1 Overall survival.
Five‐year follow‐up
We pooled eight studies for this outcome. There was no evidence of a difference between the groups (RR 1.00, 95% CI 0.96 to 1.04, 8 RCTs, 3566 women; I² = 0%; Analysis 1.1).
Six‐year follow‐up
One study (ECOG 2003) reported this outcome. There was no evidence of a difference between the groups (RR 0.94, 95% CI 0.81 to 1.08, 1 RCT, 511 women; Analysis 1.1).
Eight‐year follow‐up
One study (IBCSG 2006) reported this outcome. There was no evidence of a difference between the groups (RR 1.17, 95% CI 0.95 to 1.43, 1 RCT, 344 women; Analysis 1.1).
12‐year follow‐up
One study (MCG 2001) reported this outcome.There was no evidence of a difference between the groups (RR 1.18, 95% CI 0.99 to 1.42, 1 RCT, 382 women; Analysis 1.1).
2. Event‐free survival
Refer to Figure 5.
5.

Forest plot of comparison: 1 High‐dose chemotherapy versus standard chemotherapy, outcome: 1.2 Event‐free survival.
Three‐year follow‐up
We pooled three studies for this outcome. There was a statistically significant difference between the groups, favouring the high‐dose group (RR 1.19, 95% CI 1.06 to 1.34, 3 RCTs, 795 participants, I² = 56%; Analysis 1.2). There was moderate statistical heterogeneity for this finding. Heterogeneity appeared to be attributable to MDACC 2000, which was the smallest study in the review but did not differ from other studies in any obvious way. Exclusion of this study from analysis did not substantially affect the findings (RR 1.24, 95% CI 1.10 to 1.40, 2 RCTs, 717 women, I² = 0%).
1.2. Analysis.

Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 2 Event‐free survival.
Five‐year follow‐up
We pooled eight studies for this outcome. There was no evidence of a difference between the groups (RR 1.04, 95% CI 0.99 to 1.10, 9 RCTs, 3566 women, I² = 25%; Analysis 1.2).
Six‐year follow‐up
One study (ECOG 2003) reported this outcome. There was no evidence of a difference between the groups (RR 1.04 (95% CI 0.87 to 1.24, 1 RCT, 511 women; Analysis 1.2).
Eight‐year follow‐up
One study (IBCSG 2006) reported this outcome. There was no evidence of a difference between the groups (RR 1.27, 95% CI 0.99 to 1.64, 1 RCT, 344 women; Analysis 1.2).
12‐year follow‐up
One study (MCG 2001) reported this outcome.There was no evidence of a difference between the groups (RR 1.18, 95% CI 0.95 to 1.45, 1 RCT, 382 women; Analysis 1.2).
Secondary outcomes
1. Treatment‐related deaths
There were 68 deaths attributed to treatment toxicity among the 2800 women who were randomised to receive high‐dose chemotherapy with stem cell transplantation, and five among the 2800 women in the control arms. There were significantly fewer treatment‐related deaths in the control group (RR 7.97, 95% CI 3.99 to 15.92, 14 RCTs, 5600 women, I² = 12%; Analysis 1.3). There were no treatment‐related deaths in three of the trials (Dutch pilot 1998; JCOG 2001; WSG 2005).
1.3. Analysis.
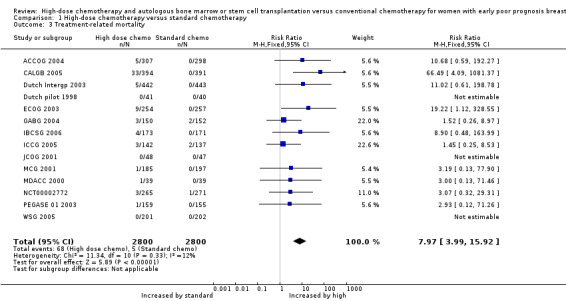
Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 3 Treatment‐related mortality.
Treatment‐related deaths were accounted for as follows:
Deaths in the high‐dose arm: most of the deaths occurred in one study (CALGB 2005), where there were 33 treatment‐related deaths in the high‐dose arm (33/394). Most of the deaths in this study were caused by acute infection, pulmonary toxicity or renal failure (haemolytic‐uraemic syndrome). However there were also three late treatment‐related deaths, one due to acute myeloid leukaemia secondary to treatment and two due to pulmonary fibrosis. There were five treatment‐related deaths in the high‐dose arm in the ACCOG 2004 study, four related to infection and one to pulmonary fibrosis (5/307). In the Dutch Intergp 2003 trial one woman died of cardiac arrhythmia during the preliminary standard‐dose chemotherapy (before receiving high‐dose treatment) and a further four women died within 100 days of autograft, two of septicaemia and two from cardiac problems (5/442). Nine women died in the ECOG 2003 study within eight weeks of stem cell transplantation; in six cases these women had been given stem cells from the bone marrow rather than from the peripheral circulation (9/254). There was one death during the transplant procedure in the PEGASE 01 2003 trial, (1/159) and one death from interstitial pneumonia in the Italian trial (MCG 2001) (1/185). In the small American trial (MDACC 2000) one woman died from treatment‐related sepsis (1/39). There were two acute and two late treatment‐related deaths in the IBCSG 2006 trial (4/173). Two women in the ICCG 2005 trial died of liver failure caused by hepatic veno‐occlusive disease and one died from cardiomyopathy (3/142). In the German trial (GABG 2004) there were three treatment‐related deaths, caused by lung toxicity, cardiac toxicity and acute myeloid leukaemia (suspected to be chemotherapy‐induced) (3/150). Three deaths occurred as a result of treatment‐related toxicity in NCT00002772, one due to acute respiratory distress syndrome during induction of chemotherapy and two during transplantation (one from pneumonia and the other from complications of veno‐occlusive disease).
Deaths in the standard‐dose arm: Five women in the standard‐dose groups died as a result of treatment toxicity, two in the ICCG 2005 trial (2/137), two in the GABG 2004 trial (2/152), and one in the NCT00002772 trial, in which the cause of death was a cardiac event (1/271). In the ICCG 2005 trial one woman died three months after randomisation as a result of neutropenia associated with colitis, and another died suddenly 10 days after chemotherapy, possibly due to anthracycline cardiotoxicity.
2. Morbidity
Five studies described the adverse effects of conventional‐dose chemotherapy (which was given to all women in these studies), and also described the additional toxicity experienced by women who went on to receive high‐dose therapy (Dutch Intergp 2003; Dutch pilot 1998; ECOG 2003; ICCG 2005; MDACC 2000). Three studies compared toxicity between the control and high‐dose arms (ACCOG 2004; NCT00002772; WSG 2005). Another study, in which the women in the two arms had no treatment in common (IBCSG 2006), compared the worst toxic effects experienced by the two arms. The German study (GABG 2004) systematically registered toxicity only in the high‐dose arm.
Toxicity was much higher in the high‐dose group, notably (as expected) neutropenia, often accompanied by fever and infection. The Dutch pilot 1998 study was the only one to report on the length of the hospital inpatient stay for high‐dose therapy: 90% of women required up to 18 days in hospital after transplantation to allow their bone marrow to regenerate.
Classic treatment side‐effects such as fatigue, vomiting, mucositis and diarrhoea were common with both regimens, although all were more common and more severe with high‐dose treatment. Two studies mentioned hair loss, which was universal in the high‐dose groups. High‐dose therapy was also more likely to induce menopause. Three studies mentioned that about 75% to 80% of women in the high‐dose arm and 60% of women in the control arm were postmenopausal after therapy (Dutch Intergp 2003; IBCSG 2006; PEGASE 01 2003), while another noted that all women in the high‐dose arm became amenorrhoeic (WSG 2005). All premenopausal participants in ICCG 2005 became amenorrhoeic after chemotherapy.
Organ toxicities affected more women in the high‐dose arms; these included cardiac, pulmonary, renal, hepatic, bladder, skin and neurological complications. Most of these toxicities were not severe and were reversible; one study reported no major organ toxicities in either arm (WSG 2005). However, some toxicities were fatal, as described above, and in addition there were a number of women who suffered ongoing treatment‐related morbidities such as peripheral neuropathy, congestive heart failure, pulmonary fibrosis and radiation‐induced pneumonitis; such long‐term complications affected women in both groups but were more common in the high‐dose group. See Table 7 for details.
6. Non‐fatal morbidity ‐ descriptive data.
| Study ID | Haemopoietic | Gastrointestinal | Pulmonary | Cardiac events | Neurological | Other toxicity | Late/ long term | Second cancers | Trialist's summary |
| ACCOG 2004 | Standard chemo: Grade 4 neutropenia 15% | Haemorrhage ≥ grade 2: High‐dose arm 8% Control arm 1% Platelet‐related toxicity ≥ grade 3: High‐dose arm 19% Control arm 1% Neutrophil‐related toxicity ≥ grade 4: High‐dose arm 21% Control arm 22% | Nausea ≥ grade 3: High‐dose arm 30% Control arm 27% Vomiting ≥ grade 4: High‐dose arm: 14% Control arm: 2% Diarrhoea ≥ grade 3: High‐dose arm 23% Control arm 1% | Rhythm toxicity ≥ grade 2: High‐dose arm 2% Functional toxicity ≥ grade 2: High‐dose arm 2% Pericardial toxicity ≥ grade 1: High‐dose arm 1% | Cortical neurotoxicity ≥ 1 High‐dose arm 2% Control arm 1% Constipation ≥ 3: High‐dose arm 2% Control arm 1% | Both trial arms: Menopausal symptoms common. High‐dose arm: several cases of shingles, which responded to acyclovir. Nitrogen or creatinine disorder ≥ grade 2 2% Proteinuria ≥ grade 2 2% Haematuria ≥ grade 2 5% Allergy ≥ grade 2 8% Skin problem ≥ grade 3 6% Infection ≥ grade 3 28% Local pain ≥ grade 2 6% Control arm: Haematuria ≥ grade 2 2% Allergy ≥ grade 2 1% Skin problem ≥ grade 3 2% Infection ≥ grade 3 4%% | ‐ | ‐ | ‐ |
| CALGB 2005 | Leukopenia and thrombocytopenia common in both groups but more severe and persistent in HDC arm | ‐ | Toxicity ≥ grade 3: High‐dose arm: > 10% Control arm: "infrequent" | ‐ | Toxicity ≥ grade 3: High‐dose arm: > 10% Control arm: "infrequent" | Hepatic toxicity ≥ grade 3: High‐dose arm: > 10% Control arm: "infrequent" | ‐ | By median 7.5 yrs High‐dose arm: 16 second cancers (4%) (including acute myeloid leukaemia or myelodysplatic syndrome 7; breast cancer 5) Control arm: 20 second cancers (5%) (including acute myeloid leukaemia or myelodysplastic syndrome 4; breast cancer 8) 9/13 breast cancers considered new primaries |
‐ |
| Dutch pilot 1998 | High‐dose chemo: all hospitalised for 13 ‐ 30 days for haemopoietic recovery. Median neutropenic fever 5 days Standard chemo: neutropenic fever after 4% of cycles | High‐dose: mucositis 85% (severe in 22%), diarrhoea common. Standard chemo: Mild nausea and vomiting, mucositis (28% of cycles), diarrhoea (4% of cycles) | ‐ | See long‐term events | ‐ | Both arms: alopecia 100%, fatigue common, lymphoedema of arm in 20% High‐dose: ovarian failure 100%, radiation pneumonitis 10%, Standard dose: radiation pneumonitis 2% | High‐dose arm: 1 case hypothyroidism, 1 case auto‐antibody production Control arm: 1 case hypothyroidy, 1 myocardial infarction | At median follow‐up of 7 years: High‐dose arm: 4/41 (basal cell skin cancer 1, colon 1, myelodysplastic syndrome 2) Control arm: 1/40 (colon) | High‐dose: "Moderately well tolerated but substantial though reversible toxic effects". Standard dose: "Mild toxicity" |
| Dutch Intergp 2003 | High‐dose: transfusion‐dependent 100% Standard chemo: fever and neutropenia requiring antibiotics 1% of episodes | High‐dose: nausea and vomiting 100% Standard chemo: moderate or severe mucositis < 1% of courses, moderate or severe nausea 10% of courses | ‐ | High‐dose: cardiac arrhythmia 1/442, possible heart failure 1/442 | ‐ | High‐dose: high fever (necessitating early termination of treatment): 4 women (1%) | ‐ | By median follow‐up 7 years: High‐dose arm: 28/442 women (29 cancers: breast 17, melanoma 3, uterine 3, non melanoma skin cancer 3, head and neck 1, oesophagus 1, pancreas 1). Control arm: 26/443 women (27 cancers: breast cancer 15, melanoma 2, nonmelanoma skin cancer 1, myelodysplasia or leukaemia 1, ovarian 1, uterine 1, head and neck 2, lung 1, stomach 1, papil vater 1, unclear 1) | High‐dose: "Well tolerated" |
| ECOG 2003 | High‐dose: leukopenia 98%, granulocytopenia 94%, thrombocytopenia 97%, anaemia 62%, Standard chemo: granulocytopenia and thrombocytopenia 90% (grade 3 or 4) | High‐dose: nausea 32%, vomiting 16%, diarrhoea 22%, stomatitis 37% Standard chemo: nausea 11%, vomiting 8%, stomatitis 4% (all grade 3 or 4) | Standard chemo: 1% (grade 3 or 4) | ‐ | Standard chemo: 6% (grade 3 or 4) | High‐dose: infection 21%, liver effects 13%, skin effects 11%, diabetes 14% Standard dose: hyperglycaemia 2%, phlebitis 1%, hepatotoxicity 1% (all grade 3 or 4) | ‐ | By median 6.1 years: High‐dose: 15/254 (ovary 2, myelodysplastic syndrome or acute myelogenous leukaemia 9, nonmelanoma skin cancer 2, cervix 1, sarcoma 1) Control arm: 9/257 (thyroid 1, kidney 2, melanoma 2, nonmelanoma skin cancer 1, myeloma 1, endometrium 1, non‐Hodgkin's lymphoma 1) | ‐ |
| GABG 2004 | ‐ | High‐dose: Grade 3 or 4 gastrointestinal toxicity < 1%; Grade 3 or 4 oral mucosal toxicity 5% | Grade 3 or 4 toxicity < 1% | ‐ | High‐dose: Grade 3 or 4 toxicity nil | High‐dose: Grade 3 or 4 toxicity: Bladder < 1%; kidney nil; liver nil | ‐ | ‐ | ‐ |
| IBCSG 2006 | High‐dose: myelosuppression Standard dose: neutropenia | High‐dose: nausea and vomiting; mucositis | ‐ | ‐ | ‐ | ‐ | Permanent amenorrhoea: High‐dose arm 77/95 (81% overall, age < 40 years 61%; age > 40 years 96%); Standard‐dose arm 61/98 (63% overall age < 40 years 24%; age > 40 years 84%) | By median 8.3 years: High‐ dose: 6/173 (1 AML (with breast cancer recurrence), 2 melanoma, 1 endometrium, 1 ovary, 1 head and neck) Control arm: 2/171 (1 melanoma, 1 unstated) | High‐dose: Overall toxicities Grade 3 1%; Grade 4 98%; Standard dose: Overall toxicities Grade 3: 41%, Grade 4: 35% |
| ICCG 2005 | High‐dose: leucopenia and thrombocytopenia presumed 100% but nadir count not always available (grade 3 or 4) Control group: (second half of course): leucopenia 14%, thrombocytopenia 0% (grade 3 or 4) | High‐dose: nausea and vomiting 46%, mucositis 22% (grade 3 or 4) Control group (second half of course): nausea and vomiting 5%, mucositis 2% (grade 3 or 4) | High‐dose: Pulmonary embolus 1/143; respiratory failure requiring ventilator 1/143 | High‐dose: severe cardiac arrhythmia 2% (3/143) | ‐ | High‐dose: hair loss 100%, fever (no infection) 17%, infection 24%, "other" 28% (grade 3 or 4), deep vein thrombosis 1/143 Control group (second half of course): hair loss 9%, fever (no infection) 0%, infection 3%, "other" 5% (grade 3 or 4), deep vein thrombosis 1/138 | After chemotherapy: 227 toxic events occurred (127 in high‐dose arm, 110 in control arm), of which 30% related to tamoxifen. Of the others, 7 events deemed life‐threatening (5 in high‐dose group, 2 in control arm) All premenopausal women developed amenorrhoea following completion of chemotherapy | High‐dose: 2/143 (breast 1, ovarian 1) Control arm: 1/138 (ovarian) | |
| JCOG 2001 | High‐dose: All 34 women receiving HDC actually developed grade 4 leukopenia and grade 4 neutropenia; 27 (79%) developed grade 4 and the other 7 grade 3 thrombocytopenia. Standard dose: 7 women (8%) developed grade 4 neutropenia |
High‐dose: vomiting 62%, diarrhoea 29%, mucositis 15%, (grade 3 or 4) | ‐ | High‐dose: grade 3 arrhythmia 3%, | ‐ | High‐dose: Grade 3 or 4 infection: 6% | ‐ | ‐ | ‐ |
| MDACC 2000 | High‐dose: Length of hospital stay not stated. Standard dose: 22% admitted with infection or fever | High‐dose: mild/moderate vomiting 80%, mild/moderate diarrhoea 58%, mild/moderate mucositis 83%. Standard dose: Nausea and vomiting moderate 75%, severe 16%. Diarrhoea moderate 19%, severe 8%. Mucousitis moderate 36%, severe 10% | High‐dose: 1 case (severe) | High‐dose: moderate/severe 8%. Standard dose: 1 woman (1%) had myocardial infarction | High‐dose: hearing loss 2 cases (6%) ‐ 1 permanent, mild/moderate peripheral neuropathy 11% | High‐dose: Renal: 25% (22% mild, < 3% severe), hepatic (mild/moderate) 31%, bladder (moderate) 25%, skin (mild) 8% | High‐dose: 1 case of avascular necrosis Standard dose: 1 woman (1%) had cerebrovascular accident, 1 (1%) had hepatic fibrosis | High‐dose: 1 case of acute myeloid leukaemia | "Overall there was greater and more frequent morbidity associated with high dose chemotherapy" |
| MCG 2001 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| PEGASE 01 2003 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| NCT00002772 | High‐dose: 62% had haematologic toxicity during induction and 92% had it during transplantation. 3 women had myelodysplastic syndrome Controls: 59% had haematologic toxicity 2 women had myelodysplastic syndrome |
‐ | ‐ | ‐ | ‐ | ‐ | High‐dose: 44% of women experienced grade 3 or 4 nonhaematologic toxicity during induction while 80% experienced grade 3 or 4 nonhematologic toxicity during transplantation. Control arm: Approximately 63% experienced grade 3 or 4 nonhaematologic toxicity, most commonly fatigue, nausea and vomiting, infection, febrile neutropenia, mucositis, and sensory neuropathy |
‐ | High‐dose: 44% had grade 3 or 4 nonhaematologic toxicity during inductio; 80% experienced grade 3 or 4 nonhaematologic toxicity during transplantation. Controls: 63% had grade 3 or 4 nonhaematologic toxicity, most commonly fatigue, nausea and vomiting, infection, febrile neutropenia, mucositis, and sensory neuropathy |
| WSG 2005 | ‐ | High‐dose arm: nausea 25%, mucositis 18%, diarrhoea 5% Control arm: nausea 10%, mucositis 10%, diarrhoea 2% (all grade 3 or 4, percentages are approximate) | High‐dose arm: 1% Control arm 2% (grade 3 or 4, percentages are approximate) | High‐dose arm: 3% Control arm: 1% (grade 3 or 4, percentages are approximate) | ‐ | High‐dose arm: grade 3 or 4 skin toxicity 3%, amenorrhoea 100% Control arm: grade 3 or 4 skin toxicity 2% | ‐ | ‐ | Both high‐dose chemotherapy and dose‐dense conventional chemotherapy are feasible with tolerable toxicity in a multicentre setting |
Several trials compared the incidence of second primary malignancies in women from the two trial arms at variable durations of follow‐up (CALGB 2005; Dutch Intergp 2003; Dutch pilot 1998; IBCSG 2006, ICCG 2005; MDACC 2000; NCT00002772). These included second breast cancers and haematological malignancies as well as other types of cancer. A meta‐analysis of these results showed no significant difference between the two arms in the incidence of second cancers at a median of four to nine years of follow‐up (RR 1.25, 95% CI 0.90 to 1.73, 7 RCTs, 3423 women, I² = 0%; Analysis 1.4).
1.4. Analysis.

Comparison 1 High‐dose chemotherapy versus standard chemotherapy, Outcome 4 Second cancers.
3. Quality of Life
Women from five of the trials took part in related quality‐of‐life studies (ACCOG 2004; CALGB 2005; Dutch Intergp 2003; PEGASE 01 2003, WSG 2005) and in addition a subset of women drawn from both Dutch trials participated in a study of chemotherapy‐related cognitive impairment. The ACCOG 2004 study analysed quality‐of‐life data relating to 84 women on the high‐dose treatment arm and 82 on the standard‐dose arm. There was no significant difference between the groups: at six months women in both arms of the trial reported a significant deterioration in quality of life, but at one year both groups reported a quality of life very similar to the pre‐treatment baseline. Both groups were significantly less tense and worried at follow‐up assessments than they were at baseline. At five years the investigators reported that quality of life associated with high‐dose therapy was only transiently lower than that associated with conventional dose therapy. More detailed analysis is planned.
A subset of 210 women from the CALGB 2005 trial was enrolled in a companion quality‐of‐life study, using telephone‐based questionnaires. The high‐dose group reported significantly worse overall quality of life at three months, but the difference between the groups narrowed until there was minimal difference among survivors at one year. Quality of life improved in both groups over the follow‐up time.
Quality of life data were also available from 96 women in the WSG 2005 trial. Again, quality of life scores were worse in the high‐dose arm but recovered by three weeks after the second cycle of high‐dose therapy. This outcome was not followed up beyond that point.
Women participating in the Dutch Intergp 2003 trial were sent regular quality‐of‐life questionnaires which were completed for at least four years by 58 women in the conventional arm and 46 in the high‐dose arm. The high‐dose arm scored significantly lower on several measures just after chemotherapy, but there was no significant difference between the arms six months later. Scores improved consistently in both arms over time with no significant difference between them. However, at four years more than 20% of women in both groups reported fatigue, sore muscles, decreased sexual interest and sweating as adverse effects of therapy.
A further study in the Dutch Intergp 2003 trial surveyed 413 women three years after chemotherapy. There was no difference between the two arms in their mean vitality score at one, two or three years, and their scores never fell below those of the reference population. Women in the high‐dose arm had a slightly lower haemoglobin level during the three years following chemotherapy, but overall this did not correlate well with reports of fatigue: 81% of women reporting fatigue did not have a low haemoglobin.
The PEGASE 01 2003 trialists found that women in the high‐dose arm recorded a strong deterioration in quality of life during treatment and that physical functioning was significantly better for women in the control arm. At three months after completion of radiotherapy the differences between the two arms had disappeared. However, at one year follow‐up women in the high‐dose arm had physical and functional scores below their baseline values, which were significantly lower than the scores of women in the control arm.
The effect of chemotherapy on cognitive functioning was evaluated in a report in which 102 women took part, 34 from the high‐dose arm of either one of the Dutch trials, 34 from the standard‐dose arm of the same trials, and 34 from a control group of women with stage I breast cancer not treated with chemotherapy. Psychoneurological tests administered at a median of two years after treatment showed cognitive impairment in 32% of the high‐dose group, 17% of the standard‐dose group and 9% of the control group. The difference between the high‐dose group and the control group was statistically significant (P = 0.006) but the difference between the high‐dose arm and the standard‐dose group was not (P = 0.056). It was observed that the women who reported cognitive problems were not necessarily the same ones who were objectively identified as being cognitively impaired, and that self‐reported cognitive problems were more related to anxiety, depression and psychological distress. At four years the tests were repeated with a subset of the original participants and the results suggested that cognitive dysfunction after chemotherapy may be transient; however, there was a high attrition rate of initially cognitively impaired women in the high‐dose arm.
Statistical heterogeneity
We noted moderate or high statistical heterogeneity in two instances: namely, event‐free survival and overall survival at three years. In both cases the results of MDACC 2000 differed in direction from those of other trials, although the 95% confidence interval overlapped the confidence interval of the summary effect measure. When MDACC 2000 was omitted from the meta‐analyses, there was no statistically significant heterogeneity. It is unclear why the results of MDACC 2000 were relatively less favourable for the high‐dose arm; however, it was the smallest trial in the review, which limits the strength of its findings.
Sensitivity analyses
Study quality:
We conducted sensitivity analyses excluding studies which did not clearly report satisfactory methods of randomisation and allocation concealment (CALGB 2005; ECOG 2003; MDACC 2000; PEGASE 01 2003), studies which did not state that they analysed all randomised participants by intention‐to‐treat (MCG 2001) and studies which did not indicate that prognostic factors were balanced between the two arms (MCG 2001; JCOG 2001). These analyses did not change the statistical significance of the results.
Chemotherapy regimen:
Over half of the treatment‐related deaths were in CALGB 2005, which noted that deaths occurred more frequently in centres that did fewer than 50 transplants. It was one of only two studies to use carmustine as part of the high‐dose regimen. It differed from the other studies in that women in the conventional arm of the study were given their final cycle of chemotherapy at an "intermediate" dose with GCSF (growth factor) support, and has been described as "a comparison between high and intermediate dose" chemotherapy regimens (Antman 1992). Excluding CALGB 2005 from the analysis did not change the statistical significance of any of the results. As noted above, there was considerable variation between the chemotherapy regimens used. We conducted sensitivity analyses omitting firstly studies that used upfront high‐dose chemotherapy with no induction cycles for the high dose group (IBCSG 2006; MCG 2001), and secondly studies where the 'conventional' chemotherapy was modified in some way to increase the strength or intensity of the dose (CALGB 2005;MCG 2001;NCT00002772WSG 2005). In neither case did these omissions affect the statistical significance of the results.
Lymph node status
Sensitivity analysis restricted to studies that randomised women with 10 or more nodes (with and without the Dutch pilot 1998 study and IBCSG 2006 in both groups) did not affect the statistical significance of the results.
Assessment of publication bias
A funnel plot for the outcome of treatment‐related mortality was not suggestive of publication bias (Figure 6).
6.
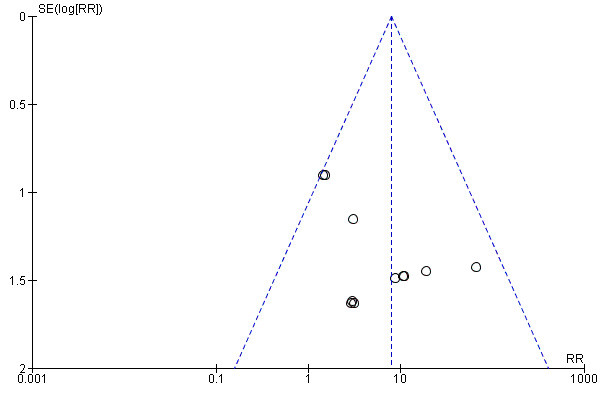
Funnel plot of comparison: 1 High‐dose chemotherapy versus standard chemotherapy, outcome: 1.3 Treatment‐related mortality.
Discussion
Summary of main results
These studies tested the hypothesis that women with early poor prognosis breast cancer would benefit from treatment with high‐dose chemotherapy with autologous bone marrow or stem cell support. There was statistically significant evidence of increased event‐free survival for women in the high‐dose group at three year follow‐up, but there was no good evidence of a difference between the groups at other time points. There was no evidence of a difference between the groups in overall survival rates at any stage of follow‐up and in fact there was evidence of harm, with greater numbers of treatment‐related deaths and adverse events occurring in the high‐dose arms.
CALGB 2005 dominates the results with respect to treatment‐related deaths: the increased mortality is thought to have occurred because of the pulmonary and hepatic toxicity of carmustine used in this study and the relative inexperience of some transplant centres. However, sensitivity analysis excluding this trial did not negate the statistical significance of the results for this outcome, which continued to favour the group that received conventional chemotherapy.
Overall completeness and applicability of evidence
Many researchers have suggested that while most studies have not demonstrated an overall benefit, there may be subgroups that benefit from high‐dose therapy. The Dutch Intergp 2003 study reported improved event‐free survival at five years in women with more than 10 positive nodes in the high‐dose arm that was of borderline statistical significance by the log‐rank test (P = 0.05, hazard ratio for relapse 0.71 (95% CI 0.5 to 1.0)). However, two of the other studies in this review reported survival rates for women with at least 10 nodes at five and six years respectively, and neither showed any statistically significant advantage for the high‐dose group (CALGB 2005; ECOG 2003). Nor did subgroup analyses of women with 10 or more positive lymph nodes in two other trials (ACCOG 2004; IBCSG 2006) find any statistically significant survival benefit for the high‐dose group.
Unplanned subgroup analyses in the Dutch Intergp 2003 study unexpectedly showed a statistically significant benefit in event‐free survival for women in the high‐dose group with lower expression of the HER2/neu gene, which is associated with cell division (P = 0.002 at five‐year follow‐up). Younger age and lower histological grade were also associated with more responsiveness to high‐dose chemotherapy in this study. Retrospective analysis of data from the German study (WSG 2005) found that younger women with large higher‐grade tumours benefited most from high‐dose treatment in their study, although subgroups were very small. More recently, this group has proposed a specific biomarker (Y‐box binding protein YB‐1) as a method of identifying women who might benefit from high‐dose chemotherapy (Gluz 2009). As the authors noted, their subgroup analyses were unplanned (and therefore must be viewed with caution), but may indicate promising areas for investigation. No evidence of a difference between the groups was reported in other subgroup analyses of baseline prognostic factors such as age, tumour size and grade, menopausal status, surgery type and hormone receptor status (ACCOG 2004; ECOG 2003; IBCSG 2006).
There is also the question of statistical power. Are these 14 trials of over 5000 women sufficient to answer the question of effectiveness? In order to detect a 10% improvement in event‐free survival (estimating that 50% of women with early poor prognosis breast cancer will progress without additional treatment by five years, which would decrease to 40% with treatment), it would be necessary to recruit 407 women to each arm of the study with 95% confidence of detecting a difference with 80% power. Therefore, the total number of women in this systematic review is sufficient to detect a difference by five years, and at least one of the individual studies (Dutch Intergp 2003) has the statistical power to detect a 10% difference However, as noted below, five years of follow‐up may not be long enough to reach a conclusion about the relative efficacy of treatments.
Is it appropriate to pool the results of these 14 studies? They randomised women with differing prognoses, in particular with respect to their number of positive lymph nodes. There was also variability in the chemotherapy regimens used. However, inspection of the forest plots shows very little heterogeneity except for three‐year survival outcomes, which were influenced by the differing findings of the smallest trial in the review (MDACC 2000). Sensitivity analysis excluding studies with lower‐risk participants(fewer than 10 nodes) negated the statistically significant benefit shown for high‐dose therapy in event‐free survival rates at three years. This was probably due to reduced power in the meta‐analysis rather than clinical differences in the participants, since there was no statistical heterogeneity and sensitivity analyses allowing for these differences did not change the statistical significance of any other results. Thus it would appear that pooling the results of the studies was appropriate.
Although high‐dose chemotherapy with autograft is associated with considerable morbidity and its role in the treatment of breast cancer has not yet been fully defined, it has often been viewed as a worthwhile treatment for women with poor prognosis or advanced disease (Nieto 2000). As a result, many women in the USA were treated outside of a clinical trial: data from the Autologous Blood and Marrow Transplant Registry suggest that in the USA during the 1990s over 40,000 women with breast cancer received this treatment, yet fewer than 1000 were recruited to clinical trials (ABMTR 2002). This review underscores the need for randomised controlled trials as the only reliable method of establishing effective treatments for women with breast cancer.
Quality of the evidence
Most of the primary studies in this review were at low risk of bias in all but one of the domains assessed. The exception was blinding, which appears unlikely to influence our outcomes of interest. Many of the findings were based on estimated data as study follow‐up was not complete in all women, but findings were largely consistent across trials and it appears unlikely that further data will change the overall finding.
The overall quality of the evidence was high for all comparisons. There was no serious indirectness, inconsistency, imprecision or evidence of publication bias.
However, the ECOG 2003 trialists noted that subgroup analysis excluding women with minor protocol violations showed a longer time to recurrence in the high‐dose group, and they also commented, as did the WSG 2005 trialists, on an apparent late divergence in survival rates. Extended follow‐up will be important to determine whether such differences persist or increase. The Dutch Intergp 2003 trialists suggested that additional follow‐up of five to 10 years might be required before a definitive conclusion about overall survival could be made. We do not plan to update this review unless further compelling evidence emerges.
Potential biases in the review process
The statistical methods used in our review are not ideal, as we have pooled data at specific time points rather than pooling all data to calculate hazard ratios. However as the data mature our findings are consistent with those of the individual patient data meta‐analysis mentioned below (Berry 2011).
Agreements and disagreements with other studies or reviews
The data from 15 RCTs of high‐dose chemotherapy have been combined in an overview and meta‐analysis of individual patient data (Berry 2011). This review included all of the RCTs in our updated review and reached very similar conclusions: "Adjuvant high dose chemotherapy with autologous hematopoietic stem‐cell transplantation prolongs relapse‐free survival in high‐risk primary breast cancer compared with control, but this does not translate into a significant overall survival benefit. Whether high dose chemotherapy benefits patients in the context of targeted therapies is unknown".
Two subsequent systematic reviews (Wang 2012;Pedrazzoli 2015) also reached similar conclusions.
Authors' conclusions
Implications for practice.
There is high‐quality evidence that high‐dose chemotherapy with autologous bone marrow or peripheral stem cell transplant does not improve survival in women with early poor prognosis breast cancer. There is high‐quality evidence of an increased risk of treatment‐related deaths with high‐dose chemotherapy. Using this intervention in the context of clinical trials could still be warranted.
Implications for research.
It is unclear whether further studies are warranted unless more compelling evidence emerges of a beneficial effect of high‐dose chemotherapy, or until there are further encouraging technical or scientific developments. As trial authors suggest, it may be valuable to study subgroups of women across studies in order to establish whether high‐dose chemotherapy has a role in specific clinical circumstances.
What's new
| Date | Event | Description |
|---|---|---|
| 22 May 2016 | Review declared as stable | This review will no longer be updated in the future. This is because the findings of this review have remained consistent, it is highly unlikely that new studies will be conducted on this topic, and breast cancer management has changed and now involves personalising therapy based on the sub‐type of breast cancer |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 21 October 2015 | New citation required but conclusions have not changed | One study previously classified as ongoing (NCT00002772) has now been published and the data have been incorporated into the review. Five studies have been updated with new data (IBCSG 2006; GABG 2004; JCOG 2001; MCG 2001; PEGASE 01 2003). This review update added 536 participants to the analysis |
| 21 October 2015 | New search has been performed | Performed search for new studies on 21 October 2015 |
| 5 August 2008 | Amended | Converted to new review format. |
| 23 May 2005 | New citation required and conclusions have changed | First review publication |
Acknowledgements
We would like to acknowledge Dr Russell Basser for his contribution as an author in pre‐2016 versions of this review.
For the 2016 update of this review, we would like to thank the staff of the Editorial Office of the Cochrane Breast Cancer Group, especially Melina Willson and Slavica Berber.
The following individuals have provided help and advice with previous versions of this review: Dr Mark Jefferies, Oncology Department, Christchurch Hospital, Christchurch, New Zealand, and members of the Cochrane Menstrual Disorders and Subfertility Group (now the Gynaecology and Fertility Group).
We also thank the study investigators who provided additional information and were generally helpful.
Appendices
Appendix 1. MEDLINE search strategy
| # ▲ | Searches |
| 1 | randomised controlled trial.pt. |
| 2 | randomized controlled trial.pt. |
| 3 | controlled clinical trial.pt. |
| 4 | randomized.ab. |
| 5 | randomised.ab. |
| 6 | placebo.ab. |
| 7 | randomly.ab. |
| 8 | trial.ab. |
| 9 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 |
| 10 | exp Breast Neoplasms/ |
| 11 | (locally adj6 advance* adj6 breast adj6 cancer$).mp. |
| 12 | (locally adj6 advance* adj6 breast adj6 carcinoma$).mp. |
| 13 | (locally adj6 advance* adj6 breast adj6 neoplas$).mp. |
| 14 | (locally adj6 advance* adj6 breast adj6 tumour$).mp. |
| 15 | (locally adj6 advance* adj6 breast adj6 tumor$).mp. |
| 16 | (early adj6 breast adj6 cancer$).mp. |
| 17 | (early adj6 breast adj6 carcinoma$).mp. |
| 18 | (early adj6 breast adj6 neoplas$).mp. |
| 19 | (early adj6 breast adj6 tumour$).mp. |
| 20 | (early adj6 breast adj6 tumor$).mp. |
| 21 | 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 |
| 22 | high dose chemotherap*.mp. |
| 23 | high‐dose chemotherap*.mp. |
| 24 | chemotherap*.mp. |
| 25 | exp Cyclophosphamide/ |
| 26 | exp Doxorubicin/ |
| 27 | exp Methotrexate/ |
| 28 | exp Fluorouracil/ |
| 29 | 22 or 23 or 24 or 25 or 26 or 27 or 28 |
| 30 | stem cell transplantation*.mp. |
| 31 | exp Hematopoietic Stem Cell Transplantation/ |
| 32 | stem cell support.mp. |
| 33 | autologous stem cell support.mp. |
| 34 | exp Stem Cell Transplantation/ |
| 35 | exp Bone Marrow Transplantation/ |
| 36 | exp Transplantation, Autologous/ |
| 37 | bone marrow transplant*.mp. |
| 38 | (bone marrow adj6 transplant*).mp. |
| 39 | (stem cell adj6 (transplant* or support or rescue)).mp. |
| 40 | 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 |
| 41 | 29 and 40 |
| 42 | 9 and 21 and 41 |
| 43 | Animals/ |
| 44 | Humans/ |
| 45 | 43 not 44 |
| 46 | 42 not 45 |
Appendix 2. EMBASE search strategy
random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR (doubl* AND blind*) OR (singl* AND blind*) OR assign* OR allocat* ORvolunteer*or AND 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'randomized controlled trial'/exp OR 'single blind procedure'/exp
'locally advanced breast cancer'
'locally advanced breast neoplasm'
'locally advanced breast carcinoma'
'locally advanced breast tumour'
'locally advanced breast tumor'
'early breast cancer'
'early breast neoplasm'
'early breast carcinoma'
'early breast tumour'
'early breast tumor'
'breast cancer'/exp OR 'breast cancer'
#2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12
'high dose chemotherapy'
'high dose chemotherapies'
chemotherap*
'high dose' NEAR/6 chemotherap*
#14 OR #15 OR #16 OR #17
'stem cell transplantation'/exp OR 'stem cell transplantation'
'stem cell transplant'
'stem cell support'
'autologous stem cell support'
'stem cell rescue'
'bone marrow transplantation'/exp OR 'bone marrow transplantation'
'bone marrow transplant'/exp OR 'bone marrow transplant'
'bone marrow' NEAR/6 transplant*
'stem cell' NEAR/6 (transplant* OR support OR rescue)
#19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27
#18 AND #28
#1 AND #13 AND #29
#30 AND [humans]/lim AND [embase]/lim
Appendix 3. WHO ICTRP
Basic Searches:
1. High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer
2. Breast cancer AND chemotherap* AND bone marrow transplant*
3. Breast cancer AND chemotherap* AND stem cell transplant*
Advanced Searches:
1. Title: High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer
Recruitment Status: ALL
2. Condition: early breast cancer*
Intervention: chemotherap* AND (stem cell transplantation* OR bone marrow transplantation* OR stem cell support OR autologous stem cell support)
Recruitment Status: ALL
3. Condition: locally advanced breast cancer*
Intervention: chemotherap* AND (stem cell transplantation* OR bone marrow transplantation* OR stem cell support OR autologous stem cell support)
Recruitment Status: ALL
Appendix 4. ClinicalTrials.gov
Basic Searches:
1. High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer
2. Breast cancer AND chemotherapy AND bone marrow transplant
3. Breast cancer AND chemotherapy AND stem cell transplant
Advanced Searches:
1. Title: High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All studies
2. Condition: early breast cancer
Intervention: chemotherapy AND (stem cell transplantation OR bone marrow transplantation OR stem cell support OR autologous stem cell support)
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All studies
3. Condition: locally advanced breast cancer
Intervention: chemotherapy AND (stem cell transplantation OR bone marrow transplantation OR stem cell support OR autologous stem cell support)
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All studies
Data and analyses
Comparison 1. High‐dose chemotherapy versus standard chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 3‐year follow‐up | 3 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.10] |
| 1.2 5‐year follow‐up | 8 | 3566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.96, 1.04] |
| 1.3 6‐year follow‐up | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.08] |
| 1.4 8‐year follow‐up | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.95, 1.43] |
| 1.5 12‐year follow‐up | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.42] |
| 2 Event‐free survival | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 3‐year follow‐up | 3 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.06, 1.34] |
| 2.2 5‐year follow‐up | 8 | 3566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.99, 1.10] |
| 2.3 6‐year follow‐up | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.24] |
| 2.4 8‐year follow‐up | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.99, 1.64] |
| 2.5 12‐year follow‐up | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.95, 1.45] |
| 3 Treatment‐related mortality | 14 | 5600 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.97 [3.99, 15.92] |
| 4 Second cancers | 7 | 3423 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.90, 1.73] |
| 4.1 By median 4‐ to 5‐year follow‐up | 2 | 817 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.61, 8.99] |
| 4.2 By median 6‐year follow‐up | 1 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.75, 3.78] |
| 4.3 By median 7‐year follow‐up | 3 | 1751 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.69, 1.51] |
| 4.4 By median 8‐ to 9‐year follow‐up | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.61, 14.49] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACCOG 2004.
| Methods | Randomised controlled trial Number of dropouts pre‐randomisation: Not stated Stratified by: Number of positive nodes (4 ‐ 9 or 10+) Number of women randomised: 605 (307 high dose, 298 standard dose) Number of women analysed: 603 Number of women not analysed: 2 in high‐dose arm lost to follow‐up Number of breaches of protocol/ failure to receive prescribed treatment: 39 (27 in high‐dose arm did not receive HDC; 12 in conventional‐dose arm did not complete treatment ‐ of these, 5 received high‐dose treatment elsewhere) Intention‐to‐treat analysis: Yes Number of centres: 34 Source of funding: NHS executive (West Midlands); Biotechnology company (AMGEN) Years: 2/95 ‐ 6/99 Countries: UK, Ireland, Belgium, New Zealand | |
| Participants | INCLUDED: Women aged > 18 with operable Stage II or IIIa breast cancer with 4+ involved lymph nodes, ECOG performance status 0/1, normal haematological and biochemical parameters and no other malignant disease. Adequate surgery mandatory | |
| Interventions | After randomisation all women received 4 standard cycles of doxorubicin (75 mg/m²) then HDC or CDC. HDC group received PBPC mobilisation (cyclophosphamide 4.0 gm/m² + filgrastim) followed by a single cycle of PBPC‐supported HDC (cyclophosphamide 6.0 gm/m², thiotepa 800 mg/m² + filgrastim). CDC group received conventional course of CMF (cyclophosphamide, methotrexate and fluorouracil) All women had radiotherapy on completion of radiotherapy and tamoxifen for 5 years if oestrogen receptor status positive or unknown. Otherwise at discretion of treating physician | |
| Outcomes | Overall survival Event‐free survival Quality of Life (EORTC Quality of Life Questionnaire) Cost effectiveness | |
| Notes | Power calculation: 300 participants in total would give power to detect a 12% survival difference at 5 years, assuming a survival with conventional chemotherapy (A‐CMF) of 50% at 10 years. Rapid accrual enabled inclusion of 600 women. Data are immature as in the most recent trial publication not all participants have completed 5‐year follow‐up. 5‐year survival data presented in the tables of comparison have been calculated from 5‐year percentages reported by trialists at median of 6 years' follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation programme used |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned to their treatment by telephone from the trial management office." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 603/605 (over 99%) of randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
CALGB 2005.
| Methods | Randomised controlled trial Stratified by: Participating institution, disease stage, hormone receptor status, menopausal status Number entered: 874 Number of dropouts pre‐randomisation: 100 (reasons: 26 had recurrent breast cancer, 2 died from chemo toxicity, 10 never received treatment, 25 were denied insurance cover for transplant, 15 withdrew, 5 were ineligible, 3 other reasons, 14 were removed for medical reasons) Number of women randomised: 785 (394 high dose, 391 controls) Number of women analysed: 785 Number of women not analysed: None Number of breaches of protocol/failure to receive prescribed treatment: 112 minor protocol violations (54 in HDC, 58 in CDC) and 2 major protocol violations (2 women in HDC arm received CDC) included in analysis. 94% of HDC arm and 95% of CDC arm received prescribed treatment Intention‐to‐treat analysis: Yes Number of centres: 40 Source of funding: Public Health Service Grants Years: 1/91 ‐ 5/98 Countries: Canada and USA | |
| Participants | INCLUDED: Women with operable Stage II or IIIa breast cancer with 10+ involved axillary lymph nodes, within 8 weeks of definitive surgery No evidence of metastasis: Negative CTs, bone scans, bone marrow biopsies, chemistry panel. > 18 years and "physiologically" < 55 years of age. performance status of CALGB 0 or 1 or Karnofsky 80% ‐ 100%. Normal bone marrow, cardiac, pulmonary, renal function No serious medical/psychiatric condition, evidence of adequate financial resources to cover treatments (e.g. insurance coverage) Radical or modified mastectomy or lumpectomy with level 1/11 axillary dissection required not > 8 weeks prior to CAF initiation EXCLUDED: Bilateral, inflammatory or metastatic breast cancer Prior chemotherapy or radiotherapy | |
| Interventions | 2 ‐ 8 weeks after primary surgery all women received 3 cycles of standard dose CAF chemotherapy (cyclophosphamide 600 mg/m²; doxorubicin 60 mg/m²; fluorouracil 1200 mg/m²). Women were then re‐evaluated and if disease‐free were randomised to HDC or CDC. HDC group had bone marrow harvest before or after a 4th cycle of standard‐dose CAF and GCSF‐primed PBPC harvest after the 4th cycle. They then received a course of HDC (cyclophosphamide 5625 mg/m², cisplatin 165 mg/m², carmustine 600 mg/m²) with both bone marrow and PBPC support, plus GCSF. The CDC group completed the 4th cycle of CAF then received an intermediate level dose of cyclophosphamide (900 mg/m²), cisplatin (90 m/m²) and carmustine (90 mg/m²), plus GCSF All women received local‐regional radiation therapy and those with positive or unknown hormone receptor status received tamoxifen for 5 years following completion of chemotherapy | |
| Outcomes | Disease‐free survival Treatment toxicity (including death, infections, thrombocytopaenia, pulmonary drug toxicity, renal dysfunction, hepatic dysfunction) within 60 days of therapy Quality of life (companion study CALGB 9066). Used the Functional Living Index‐Cancer (FLIC) scale and the McCorkle Symptom Distress Scale Overall survival | |
| Notes | Power calculation: 380 participants per arm give 90% power to detect 15% absolute difference in disease‐free survival at 5 years (P = 0.05) Participants relapsing on CDC eligible for HDC, but post‐relapse transplant not part of protocol Data are immature. 3‐year survival data in our tables of comparison have been calculated from percentages reported by trialists at median of 37 months' follow‐up, and 5‐year data have been calculated from 5‐year percentage survivals quoted by trialists at median of 7.3 years' follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Methods not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | 112 women initially declared ineligible (with minor protocol violations) but subsequently randomised |
Dutch Intergp 2003.
| Methods | Randomised controlled trial Stratification: According to nodal status (4 ‐ 9 or > 9), age, menopausal status, and tumour size Number of dropouts pre‐randomisation: Not stated Number of women randomised: 885 (442 high dose, 443 standard dose) Number of women analysed: 885 Number of women not analysed: None Number of breaches of protocol/failure to complete prescribed treatment: 37 women found to be ineligible (4 had prior radiation, 2 had evidence of distant metastases, 1 had prior cervical cancer, 30 had abnormalities in lab tests); all stayed in the study. 2 women declined chemotherapy after randomisation (1 in each group). 45 women in the high‐dose group did not receive high‐dose chemotherapy (15 refused, 5 had psychological problems, 9 had medical complications, 6 had disease progression, 1 had venous access problems, 1 had insufficient progenitor cells harvested, 1 had early death, 7 for unknown reasons). Of 397 women who received high dose treatment, 6 did not receive the full dose due to complications: high fever (4), cardiac arrhythmia (1), possible heart failure (1). Control arm: 1 refused any chemotherapy Intention‐to‐treat analysis: Yes Number of centres: 10 Source of funding: Health Care Insurers' Council Years: 8/93 ‐ 7/99 Country: The Netherlands | |
| Participants | INCLUDED: Mastectomy or breast‐conserving surgery, < 6 weeks post‐op Women < 56 years, WHO functional status 0 or 1 No prior chemotherapy or radiotherapy Post‐mastectomy or breast‐conserving surgery for Stage II or III breast cancer No distant metastases (assessed by chest X‐Ray, isotope bone scan, liver ultrasound) Adequate organ function At least 4 positive axillary lymph nodes | |
| Interventions | Women randomised to the HDC group received 4 cycles of FEC (fluorouracil 500 mg/m², epirubicin 90 mg/m², cyclophosphamide 500 mg/m²) and 1 cycle of CTC (cyclophosphamide 6 g/m², thiotepa 480 mg/m², carboplatin 1600 mg/m²) with PBPC support Women randomised to the CDC group received 5 courses of FEC All women received radiation therapy and all received tamoxifen for 2 years post‐surgery, subsequently increased to 5 years for hormone receptor‐positive women | |
| Outcomes | Relapse‐free survival Overall survival Toxicity Quality of life (unpublished data) Cost effectiveness | |
| Notes | Power calculation: 880 participants give 90% power to detect a reduction in hazard of 24% after 571 events (progression‐free survival 30% to 40%) 3‐year data are mature: 3‐year survival results in our tables estimated from graphs published at median 57 month follow‐up 5‐year data immature: 5‐year event‐free survival results in our tables based on 5‐year actuarial rates reported as percentages by trialists at median follow‐up of 57 months. 5‐year overall survival results in our tables estimated from graphs published at median 57 month follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Treatment allocation by phone call to centralised trial office |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | A centralised review of pathological specimens was carried out in a blinded fashion. Otherwise blinding was not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | Quote: "In two patients data were lacking on infectious complications and in three patients data were lacking on bacterial cultures. In 99% of patients (437 patients) sufficient data could be retrieved from the medical records and case record forms. Ultimately, 392 patients actually received high‐dose chemotherapy. Reasons not to proceed with the high‐dose regimen were an infected central venous catheter prior to high‐dose chemotherapy in three patients, venous access problems in one patient and catheter‐unrelated in the remaining patients." |
| Other bias | Low risk | No other potential bias identified |
Dutch pilot 1998.
| Methods | Randomised controlled trial
Stratification: Yes, according to a) whether clinically complete response to initial chemotherapy and b) postmenopausal status
Number of women who had upfront 3 cycles chemotherapy: 97
Number of dropouts pre‐randomisation: 16 (11 reluctant to undergo high‐dose therapy; 5 unresponsive to FEC)
Number of women randomised: 81
Number of women analysed: 81 (41 high dose, 40 standard dose)
Number of women not analysed: None
Number of breaches of protocol/failure to receive prescribed treatment: 6 (5 HDC arm refused HDC; 1 HDC arm failed to mobilise PBPCs so unsuitable for HDC)
Intention‐to‐treat analysis: Yes
Number of centres: 2 institutions, Phase II study
Source of funding: Schumaker‐Kramer Foundation
Years: 4/91 ‐ 12/95 Country: The Netherlands |
|
| Participants | INCLUDED: Women < 60 years with operable breast cancer Extensive involvement of level III axillary lymph notes as evidenced by positive axillary apex node on infraclavicular lymph node biopsy No distant metastases: normal CXR, liver U/S, bone scan Normal bone marrow, renal and hepatic functions WHO performance status of 0 or 1. Initial criterion: Evidence of at least a minimal clinical or subjective response to upfront 3 cycles FEC chemo Later criteria: No evident progression of disease during upfront 3 cycles of FEC chemo EXCLUDED: Evidence of disease progression during initial chemotherapy (but before randomisation), defined as increase in tumour size of 25% or more, or appearance of new lesion | |
| Interventions | Women were assessed for appropriate breast surgery. All women received 3 courses of FEC (5 fluorouracil 500 mg/m², epirubicin 120 mg/m², cyclophosphamide 500 mg/m²) At this stage women were clinically assessed to exclude those with no response to initial chemo. As this evaluation proved poorly reproducible, it was decided to exclude only those women with signs of disease progression After surgery, women were randomised to receive HDC or CDC. The HDC arm received a 4th cycle of GCSF‐primed FEC followed by PBPC harvest. They then received HDC (cyclophosphamide 6 g/m², thiotepa 480 mg/m², carboplatin 1600 mg/m²) with PBPC support. The control group received a 4th cycle of FEC if they were judged to have chemosensitive disease All women received radiation therapy and tamoxifen for 2 years. | |
| Outcomes | Disease‐free survival Overall survival Toxicity | |
| Notes | Power calculation: designed to provide 80% power to predict 30% increase in progression‐free survival at 4 years (30% ‐ 60%) 5‐year survival rates based on percentages reported in text. Follow‐up complete to 5 years. 7‐year survival rates based on median follow‐up of 6.9 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Treatment allocation by phone call to centralised trial office |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | Seems to be free of selective reporting |
| Other bias | Low risk | No other potential bias identified |
ECOG 2003.
| Methods | Randomised controlled trial
Stratification: By institution, oestrogen receptor status, age, menopausal status
Number of women randomised: 540 (270 high dose, 270 standard dose)
Number of women analysed: 511
Number of women not analysed: 29 (28 due to major protocol violations: No bone scan (1), positive resected margins (5), no bone marrow biopsy (6), inflammatory carcinoma or peau d'orange (5), suspected metastasis (1), prior invasive Ca breast (1), diabetes (1), prior therapeutic oophorectomy (2), no documented LVE (1) or no documented pulmonary function test (3) at baseline, residual axillary disease (1), < 10 positive nodes (1); 1 due to having no data submitted)
Number of breaches of protocol: 28 major protocol violations as above, 94 minor protocol violations (e.g. documentation failures) but still included in analysis: 45 in high‐dose arm, 49 in control arm
Number who failed to receive prescribed treatment: 14% of high‐dose group did not receive a transplant and 7% underwent transplantation outside the study. 7% of control group received some form of transplantation therapy
Intention‐to‐treat analysis: 29 women not included in primary analysis due to major protocol violations or lack of data, as above.
Number of centres: Not stated
Source of funding: supported in part by grants from the Public Health Service, the National Cancer Institute, National Institutes of Health, and the Department
of Health and Human Services.
Years: 1991 ‐ 1998 Country: USA |
|
| Participants | INCLUDED: Women aged 15 ‐ 60 years with stage II or III epithelial breast cancer, within 12 weeks of breast surgery, with histologically free surgical margins and at least 10 positive ipsilateral lymph nodes
Negative bone marrow biopsy and bone scan or received 1 ‐ 2 cycles of doxorubicin based chemotherapy prior to trial entry
Normal LVE and FEV
ECOG performance status 0 or 1 EXCLUDED: Any evidence of metastatic disease, serious organ dysfunction, pregnancy or breast feeding, prior malignancy Any prior therapy for breast cancer except tamoxifen for up to 21 days and/or 1 or 2 cycles of doxorubicin‐based chemotherapy Currently taking HRT |
|
| Interventions | All women had 6 cycles of cyclophosphamide 100 mg/m² orally for 14 days, and doxorubicin 30 mg/m² I/V and fluorouracil 500 mg/m² I/V on days 1 and 8 in 28‐day cycles. Women randomised to the HDC group then received 1 cycle of high‐dose CTM (cyclophosphamide 6 gm/m² and thiotepa 800 mg/m², continuously for 4 days with autologous stem cell support. All women had a course of radiotherapy to the chest wall and regional lymphatics, plus tamoxifen for 5 years if hormone receptor positive | |
| Outcomes | Event‐free survival, overall survival, time to recurrence, toxicity | |
| Notes | Data immature: 6‐year results in our tables based on percentage survival figures at median follow‐up 6.1 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 511/540 (95%) randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | "The primary analysis was originally planned to include the subgroup of eligible patients. However, owing to the high rates of ineligibility, this policy was reviewed in July 1999, whereupon we decided to divide protocol violations into major and minor categories and to include patients with minor violations in the primary analysis." |
GABG 2004.
| Methods | Randomised controlled trial
Stratified by clinical centre
Number of dropouts pre‐randomisation: None mentioned
Number of women randomised: 307 (150 high dose, 152 standard dose, 5 (who were subsequently excluded) not stated)
Number of women analysed: 302, including 18 who breached entry criteria (5 in high‐dose arm, 13 in control arm)
Number of women not analysed: 5 (excluded from analysis because of lack of co‐operation after randomisation)
Number of breaches of protocol/ failure to receive prescribed treatment: 20/150 (13%) of HDC arm did not complete treatment (11 refused, 8 had recurrence or died, 1 reason unknown); 16/152 CDC arm did not complete treatment (8 refused, 3 had recurrence or died, 2 due to side effects, 3 unknown)
Intention‐to‐treat analysis: No
Number of centres: 33 (plus 3 centres which were excluded because the 5 women they enrolled did not co‐operate)
Source of funding: the Dr Mildred Scheel Stiftung der Deutschen Krebshilfe, the Hamburger Krebsgesellschaft and the Erich and Gertrud Roggenbuck Foundation.
Years: 1993 ‐ 2000 Country: Germany |
|
| Participants | INCLUDED: Women with > 10 positive axillary lymph nodes found at mastectomy or breast‐conserving surgery, aged not more than 60, Karnofsky index of at least 70 EXCLUDED: Women with distant metastases, heart disease or reduced lung function | |
| Interventions | All women had 4 cycles of EC (epirubicin 90 mg/m², cyclophosphamide 600 mg/m²). Women randomised to the HDC group then received 1 cycle of high‐dose CTM (cyclophosphamide 6 gm/m² , thiotepa 600 mg/m², mitoxanthrone 40 mg/m²) with PBPC support
Women randomised to the CDC group then received 3 cycles of CMF (cyclophosphamide 1000 mg/m², methotrexate 80 mg/m², 5‐fluorouracil 1200 mg/m²)
Both groups had tamoxifen for 5 years if their hormone receptor status was oestrogen‐ or progesterone‐positive Application of radiotherapy not specified in protocol until 1998: from then on, radiotherapy recommended after both mastectomy and breast ‐conserving surgery, to start within 3 ‐ 6 weeks postoperatively |
|
| Outcomes | Event‐free survival
Absolute survival
Second cancer Toxicity |
|
| Notes | Power calculation: 320 participants would give 80% power to detect an improvement in the 5‐year event‐free survival rate from 25% to 40% (P = 0.05)
Randomisation stopped at 307 in 2000 due to low recruitment Data are immature: 4‐year data in our tables based on results at median 3.8 year follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization code was produced by the statistical center using a computerized random‐number generator. The clinical center was used as a stratification criterion, and within each center block, randomization with varying block size was performed." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomised treatment was communicated centrally by phone after registration of the patient, guaranteeing concealment of the randomised treatment." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Obviously, blinding was not possible, and the statistician was also aware of the treatment." This appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 302/307 (98%) randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | More women in the high‐dose arm than in the control arm had in excess of 16 positive lymph nodes (52% compared to 38% in the control arm) |
IBCSG 2006.
| Methods | Randomised controlled trial
Stratified by menopausal status, hormone receptor status, institution
Number of dropouts pre‐randomisation: None mentioned
Number of women randomised: 344 (173 high dose, 171 standard dose)
Number of women analysed: 344
Number of women not analysed: None
Number of breaches of protocol/failure to receive prescribed treatment: None stated
Intention‐to‐treat analysis: Yes
Number of centres: 17
Source of funding: Grants plus industry support
Years: 1995 ‐ 2000 Countries: Australia, New Zealand, Italy, Switzerland, Hong Kong, Slovenia |
|
| Participants | INCLUDED: Women with stage 2 or stage 3 breast cancer, having at least 10 positive nodes OR at least 5 positive nodes and oestrogen receptor‐negative OR at least 5 positive nodes and an operable T3 tumour | |
| Interventions | HDC arm had 3 cycles of epirubicin 200 mg/m² and cyclophosphamide 4 gm/m² with PBPC support CDC arm had 3 cycles of doxorubicin 60 mg/m² or epirubicin 90 mg/m² and cyclophosphamide 600 mg/m² then 3 cycles of cyclophosphamide 100 mg/m², fluorouracil 600 mg/m² and methotrexate 40 mg/m² After completing chemotherapy all women had tamoxifen 20 mg daily for 5 years | |
| Outcomes | Event‐free survival Overall survival Treatment‐related death Toxicity | |
| Notes | Data immature: 8‐year data in our tables based on 8‐year estimates reported by trialists at median follow‐up 8.3 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted centrally (at the coordinating centres in Bern, Switzerland, and Sydney, Australia). A permuted blocks randomization schedule was produced by use of pseudorandom numbers generated by a congruence method." |
| Allocation concealment (selection bias) | Low risk | Carried out by central data centre |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
ICCG 2005.
| Methods | Randomised controlled trial
Number of women randomised: 281 (143 high dose, 138 standard dose)
Number of women analysed: 279
Number of women not analysed: 2 (lost to follow‐up)
Number of breaches of protocol/failure to receive prescribed treatment: 68 (30 in high‐dose arm, due to toxicity (5), centre error (1), refusal of treatment (10), recurrence (2), death (2), other reasons (10); 38 in control arm, due to toxicity (13), intercurrent illness (2), centre error (2), refusal of treatment (2), recurrence (3), death (2), other reasons (14))
Intention‐to‐treat analysis: 279 women analysed by intention‐to‐treat
Number of centres: 8
Source of funding: Cancer Research UK, Pharmacia, Amgen
Years: 1993 ‐ 2001 Countries: UK, Italy, Spain, Australia |
|
| Participants | INCLUDED: Women with primary breast cancer, T1 ‐ T4, aged 60 or less, with at least 4 positive axillary nodes after complete surgical resection and no metastatic disease on bone scan EXCLUDED: Women with overt metastatic disease or abnormal bone marrow, hepatic or renal function or WHO performance status > 1 | |
| Interventions | All women had 1 3‐week cycle of FEC (cyclophosphamide 600 mg/m², epirubicin 50 mg/m², fluorouracil 600 mg/m²) followed by 2 4‐week cycles of FEC (as above but 2 doses per cycle) Women randomised to the HDC arm then received cyclophosphamide 6 gm/m², epirubicin 50 mg/m² and carboplatin 800 mg/m² with PBSC support Women randomised to the control arm had a further 3 4‐week cycles of FEC as above Women who had had conservative surgery and some who had had a mastectomy received radiotherapy. All received tamoxifen for 5 years | |
| Outcomes | Disease‐free survival Overall survival Toxicity | |
| Notes | Power calculation: 300 participants would show an improvement from 30% ‐ 45% in 5‐year survival with 80% power (α = 0.05) Accrual failed following early reports from other trials Data immature: 5‐year data in our tables based on 5‐year estimates by trialists at median 50 months follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Centres randomised their patients by telephoning the ICCG Data Centre. The randomisation method used was adapted minimisation, where the weighted probabilities ensure a random component to the allocation. Stratification factors were centre, menopausal status and number of axillary nodes involved (4–9, 10+)". |
| Allocation concealment (selection bias) | Low risk | By telephone to central data centre |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 279/281 (99%) randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
JCOG 2001.
| Methods | Randomised controlled trial
Stratified by: Number of axillary nodes positive, menopausal status, institution
Number of women randomised: 97 (49 high dose, 47 standard dose)
Number of women analysed: 95
Number of women not analysed: 2 (ineligible: 1 was stage IV, 1 enrolled too late)
Number of breaches of protocol/failure to receive prescribed treatment: 17 (2 on standard arm: 1 wanted high‐dose chemotherapy, 1 refused any chemotherapy; 15 on high‐dose arm: 7 had recurrent breast cancer, 7 no reason stated, 1 was ineligible)
Intention‐to‐treat analysis: 95/97 participants analysed by intention‐to‐treat
Number of centres: 8
Source of funding: Grants‐in‐Aid from the Ministry of Health, Labor and Welfare of Japan, and the Science and Technology Agency
Years: 1993 ‐ 99 Country: Japan |
|
| Participants | INCLUDED: Women with stage I to IIIB breast cancer, postoperatively (all had radical mastectomy) ≥ 10 positive axillary nodes (median 16; range 10 ‐ 49) Age 15 ‐ 55 Grade 0 or 1 performance status Negative bone marrow aspiration or biopsy, adequate bone marrow, hepatic, renal, cardiac and respiratory function EXCLUDED: Previous chemotherapy, radiotherapy or endocrine therapy Median age was 46 72 (of 95) were premenopausal | |
| Interventions | After randomisation, all women had 6 cycles of CAF (cyclophosphamide 500 mg/m², adriamycin 40 mg/m², fluorouracil 500 mg/m²) HDC arm then had cyclophosphamide 6 gm/m² and thiotepa 600 mg/m² Both arms: tamoxifen for at least 2 years | |
| Outcomes | Relapse‐free survival Overall survival Treatment toxicity | |
| Notes | 1. Power calculation: 90% power to detect 30% increase in relapse‐free survival at 5 years of a 60% power to detect a 20% increase (P = 0.05) (1‐sided logrank)
2. Large number of withdrawals: Over 17% withdrew before treatment
3. 2 ineligible women were randomised and then excluded from event‐free survival analysis Data immature ‐ median follow‐up 5.25 years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "eligible patients were randomly assigned to the STD or HDC arm at the time of enrolment by minimization method to balance the numbers of positive axillary nodes (10–19 or 20–), menopausal status (pre or post) and institution between the arms." |
| Allocation concealment (selection bias) | Low risk | Allocation by phone call to centralised trial office |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 95/97 (98%) randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
MCG 2001.
| Methods | Randomised controlled trial
Number of dropouts pre‐randomisation: Not stated
Number randomised: 398 (196 high dose, 202 standard dose)
Stratified by number of nodes: 4 ‐ 9 or > 9
Number analysed: 382
Number of women not analysed: 16 (ineligible)
Number of breaches of protocol/failure to receive prescribed treatment: 16 refused treatment (5 in standard‐dose arm and 11 in high‐dose arm)
Intention‐to‐treat analysis: The 382 eligible participants were analysed by intention‐to‐treat
Number of centres: Multicentre
Source of funding: Italian Association for Cancer Research and pharmaceutical companies
Years: 1993 ‐ 98 Country: Italy |
|
| Participants | INCLUDED: Post‐operative women with breast cancer with at least 4 positive axillary lymph nodes Aged < 60 | |
| Interventions | Women randomised to the high‐dose arm received 1 cycle of cyclophosphamide 7 gm/m², then 1 cycle of methotrexate 8 gm/m², then 2 cycles of epirubicin 120 mg/m², then 1 cycle of thiotepa 600 mg/m² plus melphalan 160 ‐ 180 mg/m² plus PBPC transplant Women in the control arm received 3 cycles of epirubicin 120 mg/m² then 6 cycles of CMF (cyclophosphamide 600 mg/m², methotrexate 40 mg/m² and fluorouracil 600 mg/m²) Both arms: tamoxifen for 5 years Women who had had conservative surgery received locoregional radiotherapy | |
| Outcomes | Progression‐free survival
Overall survival
Treatment‐related mortality Toxicity |
|
| Notes | Power calculation: 80% power to detect a 15% increase in progression‐free survival at 5 years Progression‐free survival not defined; this outcome is reported as event‐free survival in this review 12‐year data in our tables based on percentages for survival reported by trialists at median follow‐up 136 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Low risk | Allocation by fax to centralised trial office |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 382/398 (96%) randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
MDACC 2000.
| Methods | Randomised controlled trial
Stratified by: Stage
Number randomised: 78 (39 high dose, 39 standard dose)
Number analysed: 78
Number of women not analysed: None
Number of breaches of protocol/failure to receive prescribed treatment: High‐dose arm: 10 (of 39) did not proceed to high‐dose chemotherapy: (1 was denied insurance cover, 3 refused the treatment, 1 developed hepatitis B, 1 was ineligible, 4 relapsed before high‐dose treatment). Of the women who proceeded to high‐dose therapy, 4 received only 1 of the 2 scheduled cycles (3 for toxicity and 1 refused) and 2 received different high‐dose regimens from those dictated by the protocol
Control arm: 3 (of 39) withdrew, electing to receive high‐dose treatment at other institutions
Intention‐to‐treat analysis: Yes
Number of centres: Not stated
Financed by: Supported in part by Public Health Service grant from the National Cancer Institute, National Institutes of Health, Department of Health
and Human Services, the Nylene Eckles Professorship in Breast Cancer Research, and the Nellie B. Connally Chair in Breast Cancer.
Years: 1/90 ‐ 11/97 Country: USA |
|
| Participants | INCLUDED: 1. Women with operable Stage II or III breast and 10+ positive axillary lymph nodes, having had no preoperative chemotherapy OR 2. Women with stage III or locally‐advanced breast cancer who had had 4 cycles of preoperative chemotherapy and at least 4 positive axillary lymph nodes found at surgery Aged < 65 Adequate liver function, renal function, cardiac function, pulmonary function EXCLUDED: Comorbid condition that excludes possibility of high‐dose chemotherapy Prior chemotherapy or radiation HIV positive Evidence of distant metastasis | |
| Interventions | Women entered the trial in 2 ways: a) Postoperative women were randomised before receiving any chemotherapy b) Women who had chemotherapy preceding surgical resection were randomised before commencing postoperative chemotherapy All women received a total of 8 cycles of FAC (5‐fluorouracil 1000 mg/m², doxorubicin 50 mg/m², cyclophosphamide 500 mg/m²) Women randomised to high‐dose chemotherapy went on to receive 2 cycles of CEP (cyclophosphamide 5250 mg/m², cisplatin 165 mg/m², etoposide 1200 mg/m²) with autologous stem cell/bone marrow support. The control group did not receive any further chemotherapy. All women received radiotherapy. Those who were aged > 50 with oestrogen receptor‐positive tumours received tamoxifen for 5 years | |
| Outcomes | Time to relapse Overall survival Treatment toxicity | |
| Notes | Power calculation: Needed 40 participants in each arm to 80% power to detect 30% improvement in 3‐year relapse‐free survival (P = 0.05) OR Needed observation of 24 participants who failed to respond to treatment (the latter was achieved) Text of trial publication differs from flow chart with respect to number of women who withdrew from treatment in high‐dose group Figures given here are derived from the text Results in our tables based on percentage estimates reported for 3‐year survival. Data are mature: median follow‐up 6.5 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Randomization was performed by remote computer access; blocks of four patients (1122, 1221, 1212, 2121, etc.) were used in random order to ensure balance between the two treatment arms." |
| Allocation concealment (selection bias) | Low risk | Remote allocation. "Access to the computerized randomization program was restricted to research nurses and was not available to treating physicians." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Unclear risk | 10/39 women randomised to high‐dose arm did not receive high‐dose chemo |
NCT00002772.
| Methods | Randomised control trial Number of dropouts pre‐randomisation: Not stated Number randomised: 536 (265 high dose, 271 standard dose) Stratified by: Primary therapy i.e. mastectomy without radiotherapy, mastectomy with radiotherapy after chemotherapy, or breast‐conserving therapy with radiotherapy after chemotherapy Number analysed: 536 Number of women not analysed: None Number of breaches of protocol/failure to receive prescribed treatment: None stated Intention‐to‐treat analysis: Yes Number of centres: Multicentre Source of funding: National Cancer Institute, Department of Health and Human Services Years: 1996 ‐ 2001 Country: USA |
|
| Participants | Women with breast cancer who had completed modified radical mastectomy or breast‐conserving surgery with axillary dissection within 12 weeks of registration. Initially, the study included patients with four to nine involved lymph nodes. In March 2000, the study was amended to include patients with 10 or more involved lymph nodes. Patients with ipsilateral internal mammary or supraclavicular lymph node involvement and patients with T4 tumours were excluded. | |
| Interventions | High‐dose arm: doxorubicin and cyclophosphamide X 4 followed by high‐dose STAMP I or STAMP V (depending on centre) with autograft STAMP I consisted of cyclophosphamide 1.85 g/m²/d and cisplatin 55 mg/m²/d, each for 3 days (days 6, 5, and 4), followed by carmustine 600 mg/m² (day 3). STAMP V consisted of cyclophosphamide 1.5 g/m²/d, carboplatin 200 mg/m²/d, and thiotepa 125 mg/m²/d for 4 days (days 7 through 4). Control arm: doxorubicin, paclitaxel and cyclophosphamide X 3 of each in intensive sequential doses supported by GCSF |
|
| Outcomes | Disease‐free survival Overall survival Toxicity |
|
| Notes | Initial plan was to enrol 1000 women as approximately 350 events were required to achieve 90% power to be able to detect 45% improvement in the transplantation arm. The process of enrolment began in July 1996 and was stopped in February 2001 as the data from transplantation trials in breast cancer were not very encouraging 5‐year data immature; median follow‐up 70 months (max 102 months) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
PEGASE 01 2003.
| Methods | Randomised controlled trial
Number of dropouts pre‐randomisation: Not stated
Number of women randomised: 314 (159 high dose, 155 standard dose)
Number of women analysed: 314
Number of women not analysed: None
Number of breaches of protocol/failure to receive prescribed treatment: 15 did not complete high‐dose treatment
Intention‐to‐treat analysis: Yes
Number of centres: Not stated
Source of funding: Ligue Nationale Contre le Cancer, Association pour la Recherche sur le Cancer, Fondation de France
Years: 12/94 ‐ 12/98 Country: France |
|
| Participants | INCLUDED: Postoperative women with breast cancer < 60 years > 7 nodes involved No metastatic disease on clinical examination | |
| Interventions | All women received 4 cycles of FEC (fluorouracil 500 mg/m²; cyclophosphamide 500 mg/m²; epirubicin 100 mg/m²) Women in the high‐dose arm then received 1 cycle of CMA (cyclophosphamide 120 mg/kg; mitoxantrone 40 mg/m²; alkeran 100 mg/m²) plus PBPC transplant (harvested during 2nd or 3rd FEC cycles with GCSF priming) Women in the CDC arm had no further chemotherapy All women had radiotherapy, and postmenopausal hormone receptor‐positive women also had tamoxifen | |
| Outcomes | Event‐free survival Overall survival Toxicity Quality of life Cost effectiveness | |
| Notes | Power calculation: 90% power to detect a 20% increase in disease‐free survival at 3 years (P = 0.05) Data are immature. 3‐year survival results in our tables are based on 3‐year percentages reported by the trialists at a median follow‐up of 39 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
WSG 2005.
| Methods | Randomised controlled trial
Method of randomisation: Remotely generated, "with a random permuted block design and stratification by tumour size (<4 cm or >/=4 cm) and by centre." Allocation concealment: Done centrally by telephone or fax Number of dropouts pre‐randomisation: Not stated Number of women randomised: 403 (201 high dose, 202 standard dose) Number of women analysed: 403 Number of women not analysed: None Number of breaches of protocol/failure to receive prescribed treatment: High‐dose arm: 8 received no protocol therapy, 3 crossed to opposite arm, 1 was male (*see Participants), 4 had metastases, 14 had no documented radiotherapy. Control arm: 7 received no protocol treatment, 1 crossed to opposite arm, 2 had metastases, 19 had no documented radiotherapy Intention‐to‐treat analysis: Yes Number of centres: 6 Source of funding: Amgen, Pharmacia and Lederle Years: 6/95 ‐ 6/02 Country: Germany |
|
| Participants | INCLUDED:
Women* with breast cancer within 6 weeks of complete resection, No metastases on CXR, liver US, bone scan. Adequate organ function performance status < 2
18 ‐ 60 years
At least 10 axillary nodes involved * One man was randomised: it is unclear whether this was a breach of protocol |
|
| Interventions | All women received 2 cycles of EC (cyclophosphamide 600 mg/m²; epirubicin 90 mg/m²) 2 weeks apart with GCSF priming Women in the high‐dose arm then received tandem EC‐thiotepa (cyclophosphamide 3000; epirubicin 90, thiotepa 400 mg/m² X 2 cycles 28 days apart) plus PBPC transplants (harvested after EC with GCSF priming) Women in the CDC arm had 4 further cycles of EC 2 weeks apart with GCSF priming ("dose‐intense" regimen) All women had radiotherapy, and postmenopausal hormone receptor‐positive women also had tamoxifen. | |
| Outcomes | Event‐free survival
Overall survival
Toxicity Quality of life: European Organisation for Research and Treatment of Cancer quality‐of‐life C30 questionnaire (only administered to "about" the first 200 participants) |
|
| Notes | Power calculation: 80% power to detect a 10% absolute reduction in event‐free survival after 3 years ASCO abstract at 34.6‐month follow‐up gives estimated survival for 2 and 4 years only. 3‐year data reported in our tables are estimated from graphs in 2003 ASCO slide presentation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Remotely generated, with a random permuted‐block design and stratification by tumour size (<4 cm or >/=4 cm) and by centre." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was done centrally by telephone or fax in the WSG study office" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; however this appears unlikely to influence primary review outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No other potential bias identified |
CAF = cyclophosphamide, doxorubicin & fluorouracil CDC = Conventional adjuvant chemotherapy CMF = Cyclophosphamide, Methotrexate and 5‐Fluorouracil CXR = chest X‐ray EC = epirubicin & cyclophosphamide EORTC = European Organisation for Research and Treatment of Cancer FEC = fluorouracil, epirubicin & cyclophosphamide GCSF = Granulocyte colony‐stimulating factor HDC = High‐dose chemotherapy LVE = Left ventricular ejection fraction (cardiac function test) PBPC = Peripheral blood progenitor cells PPC = Peripheral blood progenitor cells STAMP I = cyclophosphamide, cisplatin and carmustine STAMP V = cyclophosphamide, carboplatin and thiotepa US = ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bergh 2000 | This study did not have a control group receiving conventional‐dose chemotherapy: both arms of the study were treated with experimental therapies. Participants with bony micro‐metastases were not excluded from the study. |
| Bezwoda 1999 | This study was formally withdrawn from the scientific domain at the request of the University of Witwatersrand in South Africa in February 2000 after an investigation into possible serious breaches of scientific honesty and integrity. The data presented at ASCO 1999 are incorrect. |
| Sportès 2009 | Not an RCT |
Characteristics of ongoing studies [ordered by study ID]
Adkins/Isaacs 1998.
| Trial name or title | Isaacs |
| Methods | |
| Participants | 4 ‐ 9 positive lymph nodes Stage II/IIIA |
| Interventions | High‐dose arm: adriamycin X 4 then high‐dose cyclophosphamide, then 1 cycle of STAMP with autograft Control arm: adriamycin X 4 then CMF X 6 |
| Outcomes | Disease‐free survival Overall survival |
| Starting date | Accrued 1996 ‐ 98 |
| Contact information | |
| Notes | Some preliminary data presented ASCO 1999 but not available for review. |
BCIRG 2002.
| Trial name or title | BCIRG 002 (RP56976‐321) |
| Methods | |
| Participants | 4+ positive lymph nodes Stage II/III Age ≤ 65 |
| Interventions | High‐dose arm: TAC X 4, then high‐dose mitoxantrone, cyclophosphamide and vinorelbine Control arm: TAC X 6 |
| Outcomes | Disease‐free survival Overall survival Toxicity Quality of life |
| Starting date | |
| Contact information | Chuck Vogel; Miguel Martin |
| Notes | Enrolled: 476 |
PEGASE 06.
| Trial name or title | Pegase 06 |
| Methods | |
| Participants | 8+ positive lymph nodes |
| Interventions | High‐dose arm: High‐dose EC X 4 Control arm: FEC X 6 |
| Outcomes | |
| Starting date | December 2000 |
| Contact information | Prof. P. Pouillart, Institute Curie Paris |
| Notes | Target population: 400 |
Seeber 2000.
| Trial name or title | |
| Methods | |
| Participants | ? 10+ positive lymph nodes |
| Interventions | High‐dose arm: EX X 3 then high‐dose cyclophosphamide, carboplatin, thiotepa and mitoxantrone Control arm: EC X 3; CMF X 3 |
| Outcomes | |
| Starting date | |
| Contact information | Dr Seeber, West German Cancer Center of Essen |
| Notes | For possible submission for publication 2003 |
HDC = High‐dose chemotherapy AC = doxorubicin CAF = cyclophosphamide, doxorubicin and fluorouracil CMF = cyclophosphamide, methotrexate and fluorouracil GCSF = Granulocyte colony‐stimulating factor EC = epirubicin and cyclophosphamide FEC = fluorouracil, epirubicin and cyclophosphamide CET = cyclophosphamide, epirubicin and thiotepa STAMP I = cyclophosphamide, cisplatin and carmustine STAMP V = cyclophosphamide, carboplatin and thiotepa TAC = docetaxel, doxorubicin & cyclophosphamide
Differences between protocol and review
For the 2016 update of the review we made the following changes:
Differentiated the outcomes as primary and secondary
Undertook 'Risk of bias' assessment with the Cochrane 'Risk of bias' tool
Added formal assessment of publication bias (by means of a funnel plot)
Utilised GRADE methods to assess and summarise the quality of the evidence
Edited the text to clarify that we conducted a post hoc sensitivity analysis by number of lymph nodes.
The rationale for the changes was to conform to current Cochrane methodological standards and in accordance with the advice of the statistician who peer‐reviewed the 2016 update.
For the 2007 update of the review, we made the following change:
Tables of comparisons edited to include each study at only one point of follow‐up for each outcome. For each study we chose the follow‐up time with the most mature data, with preference given to published data.
Contributions of authors
For the 2016 update of this review, Jane Marjoribanks, Maimoona Azhar and Anne Lethaby conducted the search, selected the studies, extracted the data and/or updated the text. CIndy Farquhar checked the study selection and commented on drafts.
For previous versions of the review: Cindy Farquhar drafted the protocol, searched for and selected the studies, extracted the data and wrote the text of the review. Jane Marjoribanks selected the studies, extracted the data, entered the data, completed the included and excluded studies table, assisted with the writing of the document. Russell Basser edited the protocol, selected the studies, extracted the data and commented on the draft on several occasions, particularly providing content advice. Anne Lethaby commented on the draft of the protocol, provided statistical advice and commented on the draft on several occasions. Jane Marjoribanks updated the review.
Sources of support
Internal sources
University of Auckland, New Zealand.
External sources
RAND Corporation, Santa Monica, California (Supported by the Robert Wood Johnson Foundation Grant #044128), USA.
Declarations of interest
Cindy Farquhar: No conflict of interest
Jane Marjoribanks: No conflict of interest
Anne Lethaby: No conflict of interest
Maimoona Azhar: No conflict of interest
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
ACCOG 2004 {published data only}
- Forbes AJ, Foster E, Lind MJ, Twelves C, Wilson CB, Crown JP, et al. Quality of life in the Anglo‐Celtic randomised trial of high dose adjuvant chemotherapy. Breast Cancer Research and Treatment 2001;64(1):129. [Abstract 553] [Google Scholar]
- Leonard RCF, Lind M, Twelves C, Coleman R, Belle S, Wilson C, et al. Anglo‐Celtic Cooperative Oncology Group. Conventional adjuvant chemotherapy versus single‐cycle autograft‐supported, high‐dose, late‐intensification chemotherapy in high‐risk breast cancer patients: a randomised trial. Journal of the National Cancer Institute 2004;96(14):1076‐83. [DOI] [PubMed] [Google Scholar]
CALGB 2005 {published data only}
- Hurd DD, Peters WP. Randomized, comparative study of high‐dose (with autologous bone marrow support) versus low‐dose cyclophosphamide, cisplatin, and carmustine as consolidation to adjuvant cyclophosphamide, doxorubicin, and fluorouracil for patients with operable Stage II or III breast cancer involving 10 or more axillary lymph nodes (CALGB Protocol 9082). Journal of the National Cancer Institute Monographs 1995;19:41‐4. [PubMed] [Google Scholar]
- Marks LB, Cirrincione C, Fitzgerald TJ, Laurie F, Glicksman AS, Vredenburgh J, et al. Impact of high‐dose chemotherapy on the ability to deliver subsequent local‐regional radiotherapy for breast cancer: analysis of cancer and leukemia group B protocol 9082. International Journal of Radiation Oncology Biology Physics 2010;76(5):1305‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppercorn J, Herndon J, Kornblith AB, Peters W, Ahles T, Vredenburgh J, et al. The Cancer and Leukaemia Group B (CALGB) and The Southwestern Oncology Group (SWOG). Quality of life among patients with stage II and III breast carcinoma randomized to receive high‐dose chemotherapy with autologous bone marrow support or intermediate‐dose chemotherapy. Cancer 2005;104(8):1580‐9. [DOI] [PubMed] [Google Scholar]
Dutch Intergp 2003 {published data only}
- Buijs C, Rodenhuis S, Seynaeve CM, Hoesel QG, WE, Smit WJ, et al. Prospective study of long‐term impact of adjuvant high‐dose and conventional‐dose chemotherapy on health‐related quality of life. Journal of Clinical Oncology 2007;25(34):5403‐9. [DOI] [PubMed] [Google Scholar]
- Cottu OH, Cuvier C, Laurence V, Espié M. High‐dose adjuvant chemotherapy for breast cancer: state of the art. Cancer Futures 2001;12(1):27‐30. [PubMed] [Google Scholar]
- Vries EGE, Mastenbroek CC, Rodenhuis S. High‐dose chemotherapy for breast cancer. Annals of Internal Medicine 1997;126(11):917‐8. [DOI] [PubMed] [Google Scholar]
- Nieboer P, Vries EGE, Mulder NH, Rodenhuis S, Bontenbal M, Wall E, et al. Factors influencing catheter‐related infections in the Dutch multicenter study on high‐dose chemotherapy followed by peripheral SCT in high‐risk breast cancer patients. Bone Marrow Transplantation 2008;42(7):475‐81. [DOI] [PubMed] [Google Scholar]
- Rodenhuis S, Bontenbal M, Beex LVAM, Wagstaff J, Richel DJ, Nooij MA, et al. High‐dose chemotherapy with hematopoietic stem‐cell rescue for high‐risk breast cancer. New England Journal of Medicine 2003;349(1):7‐15. [DOI] [PubMed] [Google Scholar]
- Rodenhuis S, Bontenbal M, Hoesel QGCM, Smit WM, Nooij MA, Voest EE, et al. Netherland Working Party on Autologous Transplantation in Solid Tumours. Efficacy of high‐dose alkylating chemotherapy in HER2/neu‐negative breast cancer. Annals of Oncology 2006;17(4):588‐96. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Rosenbrand RM, Rhijn D, Rodenhuis S, et al. Late effects of adjuvant chemotherapy on cognitive function: a follow‐up study in breast cancer patients. Annals of Oncology 2002;13(9):1387‐97. [DOI] [PubMed] [Google Scholar]
- Dam FSAM, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, et al. Impairment of cognitive function in women receiving adjuvant treatment for high‐risk breast cancer: high‐dose versus standard‐dose chemotherapy. Journal of the National Cancer Institute 1998;90(3):210‐7. [DOI] [PubMed] [Google Scholar]
Dutch pilot 1998 {published data only}
- Rodenhuis S, Richel DJ, Wall E, Schornagel JH, Baars JW, Koning CCE, et al. Randomised trial of high‐dose chemotherapy and haemopoietic progenitor‐cell support in operable breast cancer with extensive axillary lymph‐node involvement. Lancet 1998;352:515‐21. [DOI] [PubMed] [Google Scholar]
- Schrama JG, Faneyte IF, Schornagel JH, Baars JW, Peterse JL, Vijver MJ, et al. Randomized trial of high dose chemotherapy and hematopoietic progenitor‐cell support in operable breast cancer with extensive lymph node involvement: final analysis with 7 years of follow‐up. Annals of Oncology 2002;13(5):689‐98. [DOI] [PubMed] [Google Scholar]
ECOG 2003 {published data only}
- Tallman MS, Gray R, Robert NJ, LeMaistre CF, Osborne CK, Vaughan WP, et al. Conventional adjuvant chemotherapy with or without high‐dose chemotherapy and autologous stem‐cell transplantation in high‐risk breast cancer. New England Journal of Medicine 2003;349(1):17‐26. [DOI] [PubMed] [Google Scholar]
GABG 2004 {published data only}
- Scherwath A, Mehnert A, Schleimer B, Schirmer L, Fehlauer F, Kreienberg R, et al. Neuropsychological function in high‐risk breast cancer survivors after stem‐cell supported high‐dose therapy versus standard‐dose chemotherapy: evaluation of long‐term treatment effects. Annals of Oncology 2006;17(3):415‐23. [DOI] [PubMed] [Google Scholar]
- Zander AR, Krüger W, Schmoor C, Kröger N, Möbus V, Frickhofen N, et al. High‐dose chemotherapy with autologous hematopoietic stem‐cell support compared with standard‐dose chemotherapy in breast cancer patients with 10 or more positive lymph nodes: first results of a randomized trial. Journal of Clinical Oncology 2004;22(12):1‐11. [DOI] [PubMed] [Google Scholar]
- Zander AR, Schmoor C, Kröger N, Krüger W, Möbus V, Frickhofen N, et al. Randomized trial of high‐dose adjuvant chemotherapy with autologous hematopoietic stem‐cell support versus standard‐dose chemotherapy in breast cancer patients with 10 or more positive lymph nodes: overall survival after 6 years of follow‐up. Annals of Oncology 2008;19(6):1082‐9. [DOI] [PubMed] [Google Scholar]
IBCSG 2006 {published data only}
- Colleoni M, Sun Z, Martinelli G, Basser RL, Coates AS, Gelber RD, et al. International Breast Cancer Study Group. The effect of endocrine responsiveness on high‐risk breast cancer treated with dose‐intensive chemotherapy: results of International Breast Cancer Study Group Trial 15‐95 after prolonged follow‐up. Annals of Oncology 2009;20(8):1344‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Breast Cancer Study Group. Multicycle dose‐intensive chemotherapy for women with high‐risk primary breast cancer: results of International Breast Cancer Study Group Trial 15‐95. Journal of Clinical Oncology 2006;24(3):370‐8. [DOI] [PubMed] [Google Scholar]
ICCG 2005 {published data only}
- Coombes RC, Howell A, Emson M, Peckitt C, Gallagher C, Bengala C, et al. High dose chemotherapy and autologous stem cell transplantation as adjuvant therapy for primary breast cancer patients with four or more lymph nodes involved: long‐term results of an international randomised trial. Annals of Oncology 2005;16(5):726‐34. [DOI] [PubMed] [Google Scholar]
JCOG 2001 {unpublished data only}
- Tokuda Y, Tajima T, Narabayashi M, Takeyama K, Watanabe T, Fukutomi T, et al. Randomized phase III study of high‐dose chemotherapy (HDC) with autologous stem cell support as consolidation in high‐risk postoperative breast cancer: Japan Clinical Oncology Group (JCOG9208). Poster Presentation: Asco Online: www.asco.org/. American Society of Clinical Oncology, 2001 (Accessed 24th July 2002).
- Tokuda Y, Tajima T, Narabayashi M, Takeyama K, Watanabe T, Fukutomi T, et al. Autologous Bone Marrow Transplantation Study Group, Breast Cancer Study Group of the Japan Clinical Oncology Group (JCOG). Phase III study to evaluate the use of high‐dose chemotherapy as consolidation of treatment for high‐risk postoperative breast cancer: Japan Clinical Oncology Group study, JCOG 9208. Cancer Science 2008;99(1):145‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
MCG 2001 {unpublished data only}
- Cottu PH, Cuvier C, Laurence V, Espié M. High‐dose adjuvant chemotherapy for breast cancer: state of the art. Cancer Futures 2001;12(1):27‐30. [PubMed] [Google Scholar]
- Gianni A, Bonadonna G. Five‐year results of the randomized clinical trial comparing standard versus high‐dose myeloablative chemotherapy in the adjuvant treatment of breast cancer with>3 positive nodes (LN+). Asco Online: www.asco.org/. American Society of Clinical Oncology, 2001 (Accessed 27the June 2002).
- Gianni AM, Bonadonna G, Michelangelo Cooperative Group. Updated 12‐year results of a randomized clinical trial comparing standard‐dose to high‐dose myeloablative chemotherapy in the adjuvant treatment of breast cancer with more than three positive nodes (LN+). Journal of Clinical Oncology 2007 ASCO Annual Meeting Proceedings (Post‐Meeting Edition);25(18S (June 20 Supplement)):549. [Google Scholar]
MDACC 2000 {published data only}
- Hanrahan EO, Broglio K, Frye D, Buzdar AU, Theriault RL, Valero V, et al. Randomized trial of high‐dose chemotherapy and autologous hematopoietic stem cell support for high‐risk primary breast carcinoma. Cancer 2006;106(11):2327‐36. [DOI] [PubMed] [Google Scholar]
- Hortobagyi GN, Buzdar AU, Theriault RL, Valero V, Frye D, Booser DJ, et al. Randomized trial of high‐dose chemotherapy and blood cell autografts for high‐risk primary breast carcinoma. Journal of the National Cancer Institute 2000;92(3):225‐33. [DOI] [PubMed] [Google Scholar]
NCT00002772 {published data only}
- Moore HCF, Green SJ, Gralow JR, Bearman SI, Lew D, Barlow WE, et al. Intensive dose‐dense compared with high‐dose adjuvant chemotherapy for high‐risk operable breast cancer: Southwest Oncology Group/Intergroup study 9623. Journal of Clinical Oncology 2007;25(13):1677‐82. [DOI] [PubMed] [Google Scholar]
- NCT00002772. NCI high priority trial: Phase III randomized study of intensive sequential doxorubicin, paclitaxel and cyclophosphamide versus doxorubicin and cyclophosphamide followed by STAMP I and STAMP V combination chemotherapy with autologous stem cell rescue in women with primary breast cancer and at least 4 involved axillary lymph nodes. https://clinicaltrials.gov/ct2/show/NCT00002772. National Cancer Institute, 2001 (Accessed 21st June 2002).
PEGASE 01 2003 {published data only}
- Marino P, Roche H, Biron P, Janvier M, Spaeth D, Fabbro M, et al. Deterioration of quality of life of high‐risk breast cancer patients treated with high‐dose chemotherapy: The PEGASE 01 quality of life study. Value in Health 2008;11(4):709‐18. [DOI] [PubMed] [Google Scholar]
- Roche H, Viens P, Biron P, Lotz JP, Asselain B, PEGASE Group. High‐dose chemotherapy for breast cancer: the French PEGASE experience. Cancer Control 2003;10(1):42‐7. [DOI] [PubMed] [Google Scholar]
WSG 2005 {published data only}
- Gluz O, Mengele K, Schmitt M, Kates R, Diallo‐Danebrock R, Neff F, et al. Y‐box–binding protein YB‐1 identifies high‐risk patients with primary breast cancer benefiting from rapidly cycled tandem high‐dose adjuvant chemotherapy. Journal of Clinical Oncology 2009;27(36):6144‐51. [DOI] [PubMed] [Google Scholar]
- Nitz UA, Mohrmann S, Fischer J, Lindemann W, Berdel WE, Jackisch C, et al. West German Study Group. Comparison of rapidly cycled tandem high‐dose chemotherapy plus peripheral‐blood stem‐cell support versus dose‐dense conventional chemotherapy for adjuvant treatment of high‐risk breast cancer: results of a multicentre phase III trial. Lancet 2005;366(9501):1935‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bergh 2000 {published data only}
- Bergh J, Wiklund T, Erikstein B, Lidbrink E, Lindman H, Malmstrom P, et al. Tailored fluorouracil, epirubicin and cyclophosphamide compared with marrow‐supported high‐dose chemotherapy as adjuvant treatment for high‐risk breast cancer: a randomised trial. The Lancet 2000;356(9239):1384‐91. [DOI] [PubMed] [Google Scholar]
- Brandberg Y. Scandinavian Breast Cancer study group: Quality of life in women with breast cancer randomised to adjuvant treatment with marrow supported high dose chemotherapy with Ctcb (Bmt) or tailored FEC therapy: The SBG 9401 study, Radiumhemmet, Karolinska Hospital. Asco Online : www.asco.org. American Society of Clinical Oncology, 2000 (Accessed 24th July 2002).
- Wilking N, Lidbrink E, Wiklund T, Erikstein B, Lindman H, Malmström P, et al. Scandinavian Breast Group, study SBG 9401. Long‐term follow‐up of the SBG 9401 study comparing tailored FEC‐based therapy versus marrow‐supported high‐dose therapy. Annals of Oncology 2007;18(4):694‐700. [DOI] [PubMed] [Google Scholar]
Bezwoda 1999 {published data only}
- Bezwoda W. Randomised, controlled trial of high dose chemotherapy (HD‐CNVp) versus standard dose (CAF) chemotherapy for high‐risk, surgically treated, primary breast cancer. Asco Online: www.asco.org. American Society of Clinical Oncologists, 1999 (Accessed 29th August 2002).
Sportès 2009 {published data only}
- Sportès C, Steinberg SM, Liewehr DJ, Gea‐Banacloche J, Danforth DN, Avila DN, et al. Strategies to improve long‐term outcome in stage IIIB inflammatory breast cancer: multimodality treatment including dose‐intensive induction and high‐dose chemotherapy. Biology of Blood and Marrow Transplantation 2009;15(8):963‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Adkins/Isaacs 1998 {published data only}
- Isaacs RE, Adkins DR, Spitzer G, Freeman S, Pecora AL, Weaver C. A phase III multi‐institution randomized study comparing standard adjuvant chemotherapy to intensification with high‐dose chemotherapy (HDC) and autologous peripheral blood progenitor cell (PBPC) rescue in patients with stage II/IIIA breast cancer with 4‐9 involved axillary lymph nodes. Asco Online: www.asco.org/. American Society of Clinical Oncology, 1999 (Accessed 24th July 2002).
BCIRG 2002 {published data only}
- Nabholtz JM (PI). A multicenter phase III randomized trial comparing docetaxel in combination with doxorubicin and cyclophosphamide (TAC) with TAC followed by high dose chemotherapy with mitoxantrone, cyclophosphamide and vinorelbine (HDCT) with autologous peripheral stem cell transplantation and G‐CSF in adjuvant treatment of operable breast cancer with 4 or more positive axillary nodes. http://www.trioncology.org/studies/bcirg‐002/. Breast Cancer International Research Group, (Accessed 25th June 2002).
PEGASE 06 {published data only}
- Pouillart P (PI). Randomized multicentric exploratory study phase III evaluating the contribution of the therapeutic intensification with autotransplantation of hematopoietic cells in non metastatic breast cancer with ganglionic invasion [Etude prospective multicentrique randomisée de phase III évaluant l'apport de l'intensification thérapeutic avec autotransplantation de cellules hématopoïétiques dans les cancers du sein non métastasés avec envahissement ganglionnaire (N=8)]. FNCLCC website: www.fnclcc.fr. Fédération Nationale des Centres contre le Cancer, 2002 (Accessed 20th August 2002).
- Roche H, Viens P, Biron P, Lotz JP, Asselain B, PEGASE Group. High‐dose chemotherapy for breast cancer: the French PEGASE experience. Cancer Control 2003;10(1):42‐7. [DOI] [PubMed] [Google Scholar]
Seeber 2000 {unpublished data only}
- Nieto Y, Champlin RE, Wingard JR, Vredenburgh JJ, Elias AD, Richardson P, et al. Status of high‐dose chemotherapy for breast cancer: a review. Biology of Blood and Marrow Transplantation 2000;6(5):476‐95. [DOI] [PubMed] [Google Scholar]
- West German Cancer Center of Essen [pers comm]. [Personal communication]. Personal communication with Regina Schleucher 26th July 2003.
Additional references
ABMTR 2002
- IBMTR [pers comm]. Autologous Bone Marrow Transplant Registry: www.ibmtr.org. Melodee Nugent, personal communication with Cindy Farquar 16th May 2002.
ACS 2002
- American Cancer Society. Breast cancer facts and figures. American Cancer Society website: www.cancer.org 2002 (Accessed 6th September 2002).
Antman 1992
- Antman K, Ayash L, Elias A, Wheeler C, Hunt M, Eder JP, et al. A phase II study of high dose cyclophosphamide, thiopeta and carboplatinum with autologous marrow support in women with measurable advanced breast cancer responding to standard dose therapy. Journal of Clinical Oncology 1992;10(1):102‐10. [DOI] [PubMed] [Google Scholar]
Berry 2011
- Berry DA, Ueno NT, Johnson MM, Lei X, Caputo J, Rodenhuis S, et al. High‐dose chemotherapy with autologous stem‐cell support as adjuvant therapy in breast cancer: overview of 15 randomized trials. Journal of Clinical Oncology 2011;29(24):3214‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bray 2004
- Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. European Journal of Cancer 2002;38(1):99‐166. [DOI] [PubMed] [Google Scholar]
Clarke 2008
- Early Breast Cancer Trialists' Collaborative Group. Multi‐agent chemotherapy for early breast cancer. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD000487.pub2] [DOI] [PubMed] [Google Scholar]
DOH 2002
- National Health Service. Cancer Screening Programmes. Department of Health website: www.cancerscreening.nhs.uk. Department of Health, 2002 (Accessed 6th September 2002).
Eddy 1992
- Eddy DM. High‐dose chemotherapy with autologous bone marrow transplantation for the treatment of metastatic breast cancer. Journal of Clinical Oncology 1992;10(4):657‐70 [Erratum in: Journal of Clinical Oncology 1992 Oct;10(10):1655‐8]. [DOI] [PubMed] [Google Scholar]
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. [DOI] [PubMed] [Google Scholar]
Frei 1980
- Frei E III, Canellos GP. Dose: A critical factor in cancer chemotherapy. American Journal of Medicine 1980;69(4):585‐94. [DOI] [PubMed] [Google Scholar]
Gluz 2009
- Gluz O, Mengele K, Schmitt M, Kates R, Diallo‐Danebrock R, Neff F, et al. Y‐box–binding protein YB‐1 identifies high‐risk patients with primary breast cancer benefiting from rapidly cycled tandem high‐dose adjuvant chemotherapy. Journal of Clinical Oncology 2009;27(36):6144‐51. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from www.gradepro.org.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hryniuk 1986
- Hryniuk W, Levine MN. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer. Journal of Clinical Oncology 1986;4(8):1162‐70. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel WH. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. [PubMed] [Google Scholar]
Nemoto 1980
- Nemoto T, Vana J, Bedwani R, Baker HW, McGregor FH, Murphy GP. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer 1980;45(12):2917‐24. [DOI] [PubMed] [Google Scholar]
Nieto 2000
- Nieto Y, Champlin RE, Wingard JR, Vredenburgh JJ, Elias AD, Richardson P, et al. Status of High‐Dose Chemotherapy for Breast Cancer: A Review. Biology of Blood and Marrow Transplantation 2000;6(5):476‐95. [DOI] [PubMed] [Google Scholar]
Pedrazzoli 2015
- Pedrazzoli P, Martino M, Delfanti S, Generali D, Giovanni Rosti G, Bregni M, et al. European Group for Blood and Marrow Transplantation (EBMT), Solid Tumor Working Party. High‐dose chemotherapy with autologous hematopoietic stem cell transplantation for high‐risk primary breast cancer. Journal of the National Cancer Institute. Monographs 2015;2015(51):70‐5. [DOI] [PubMed] [Google Scholar]
Peters 1988
- Peters WP, Shpall EJ, Jones RB, Olsen GA, Bast RC, Gockerman JP, et al. High dose combination alkylating agents with bone marrow support as initial treatment for metastatic breast cancer. Journal of Clinical Oncology 1988;6(9):1368‐76. [DOI] [PubMed] [Google Scholar]
Wang 2012
- Wang J, Zhang Q, Zhou R, Chen B, Ouyang J. High‐dose chemotherapy followed by autologous stem cell transplantation as a first‐line therapy for high‐risk primary breast cancer: a meta‐analysis. PLoS ONE 2012;7(3):e33388. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Globocan. www‐depdb.iarc.fr/who/menu.htm 2000 (Accessed 19th May 2003).
Williams 1992
- Williams SF, Gilewski T, Mick R, Bitran JD. High dose consolidation therapy with autologous stem cell rescue in Stage IV breast cancer: A follow‐up report. Journal of Clinical Oncology 1992;10(11):1743‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Farquhar 2003
- Farquhar C, Basser R, Marjoribanks J, Lethaby A. High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003139] [DOI] [PubMed] [Google Scholar]
Farquhar 2005
- Farquhar C, Marjoribanks J, Basser R, Lethaby A. High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003139.pub2] [DOI] [PubMed] [Google Scholar]


