Abstract
Background
Stillbirth is generally defined as a death prior to birth at or after 22 weeks' gestation. It remains a major public health concern globally. Antenatal interventions may reduce stillbirths and improve maternal and neonatal outcomes in settings with high rates of stillbirth. There are several key antenatal strategies that aim to prevent stillbirth including nutrition, and prevention and management of infections.
Objectives
To summarise the evidence from Cochrane systematic reviews on the effects of antenatal interventions for preventing stillbirth for low risk or unselected populations of women.
Methods
We collaborated with Cochrane Pregnancy and Childbirth's Information Specialist to identify all their published reviews that specified or reported stillbirth; and we searched the Cochrane Database of Systematic Reviews (search date: 29 Feburary 2020) to identify reviews published within other Cochrane groups. The primary outcome measure was stillbirth but in the absence of stillbirth data, we used perinatal mortality (both stillbirth and death in the first week of life), fetal loss or fetal death as outcomes. Two review authors independently evaluated reviews for inclusion, extracted data and assessed quality of evidence using AMSTAR (A Measurement Tool to Assess Reviews) and GRADE tools. We assigned interventions to categories with graphic icons to classify the effectiveness of interventions as: clear evidence of benefit or harm; clear evidence of no effect or equivalence; possible benefit or harm; or unknown benefit or harm or no effect or equivalence.
Main results
We identified 43 Cochrane Reviews that included interventions in pregnant women with the potential for preventing stillbirth; all of the included reviews reported our primary outcome 'stillbirth' or in the absence of stillbirth, 'perinatal death' or 'fetal loss/fetal death'. AMSTAR quality was high in 40 reviews with scores ranging from 8 to 11 and moderate in three reviews with a score of 7.
Nutrition interventions
Clear evidence of benefit: balanced energy/protein supplementation versus no supplementation suggests a probable reduction in stillbirth (risk ratio (RR) 0.60, 95% confidence interval (CI) 0.39 to 0.94, 5 randomised controlled trials (RCTs), 3408 women; moderate‐certainty evidence).
Clear evidence of no effect or equivalence for stillbirth or perinatal death: vitamin A alone versus placebo or no treatment; and multiple micronutrients with iron and folic acid versus iron with or without folic acid.
Unknown benefit or harm or no effect or equivalence: for all other nutrition interventions examined the effects were uncertain.
Prevention and management of infections
Possible benefit for fetal loss or death: insecticide‐treated anti‐malarial nets versus no nets (RR 0.67, 95% CI 0.47 to 0.97, 4 RCTs; low‐certainty).
Unknown evidence of no effect or equivalence: drugs for preventing malaria (stillbirth RR 1.02, 95% CI 0.76 to 1.36, 5 RCTs, 7130 women, moderate certainty in women of all parity; perinatal death RR 1.24, 95% CI 0.94 to 1.63, 4 RCTs, 5216 women, moderate‐certainty in women of all parity).
Prevention, detection and management of other morbidities
Clear evidence of benefit: the following interventions suggest a reduction: midwife‐led models of care in settings where the midwife is the primary healthcare provider particularly for low‐risk pregnant women (overall fetal loss/neonatal death reduction RR 0.84, 95% CI 0.71 to 0.99, 13 RCTs, 17,561 women; high‐certainty), training versus not training traditional birth attendants in rural populations of low‐ and middle‐income countries (stillbirth reduction odds ratio (OR) 0.69, 95% CI 0.57 to 0.83, 1 RCT, 18,699 women, moderate‐certainty; perinatal death reduction OR 0.70, 95% CI 0.59 to 0.83, 1 RCT, 18,699 women, moderate‐certainty).
Clear evidence of harm: a reduced number of antenatal care visits probably results in an increase in perinatal death (RR 1.14 95% CI 1.00 to 1.31, 5 RCTs, 56,431 women; moderate‐certainty evidence).
Clear evidence of no effect or equivalence: there was evidence of no effect in the risk of stillbirth/fetal loss or perinatal death for the following interventions and comparisons: psychosocial interventions; and providing case notes to women.
Possible benefit: community‐based intervention packages (including community support groups/women's groups, community mobilisation and home visitation, or training traditional birth attendants who made home visits) may result in a reduction of stillbirth (RR 0.81, 95% CI 0.73 to 0.91, 15 RCTs, 201,181 women; low‐certainty) and perinatal death (RR 0.78, 95% CI 0.70 to 0.86, 17 RCTs, 282,327 women; low‐certainty).
Unknown benefit or harm or no effect or equivalence: the effects were uncertain for other interventions examined.
Screening and management of fetal growth and well‐being
Clear evidence of benefit: computerised antenatal cardiotocography for assessing infant's well‐being in utero compared with traditional antenatal cardiotocography (perinatal mortality reduction RR 0.20, 95% CI 0.04 to 0.88, 2 RCTs, 469 women; moderate‐certainty).
Unknown benefit or harm or no effect or equivalence: the effects were uncertain for other interventions examined.
Authors' conclusions
While most interventions were unable to demonstrate a clear effect in reducing stillbirth or perinatal death, several interventions suggested a clear benefit, such as balanced energy/protein supplements, midwife‐led models of care, training versus not training traditional birth attendants, and antenatal cardiotocography. Possible benefits were also observed for insecticide‐treated anti‐malarial nets and community‐based intervention packages, whereas a reduced number of antenatal care visits were shown to be harmful. However, there was variation in the effectiveness of interventions across different settings, indicating the need to carefully understand the context in which these interventions were tested.
Further high‐quality RCTs are needed to evaluate the effects of antenatal preventive interventions and which approaches are most effective to reduce the risk of stillbirth. Stillbirth (or fetal death), perinatal and neonatal death need to be reported separately in future RCTs of antenatal interventions to allow assessment of different interventions on these rare but important outcomes and they need to clearly define the target populations of women where the intervention is most likely to be of benefit. As the high burden of stillbirths occurs in low‐ and middle‐income countries, further high‐quality trials need to be conducted in these settings as a priority.
Plain language summary
What are the most effective interventions during pregnancy for preventing stillbirth?
What is stillbirth?
A stillbirth is generally defined as the death of a baby before birth, at or after 22 weeks of development.
Why is this important?
Stillbirth can be very upsetting for families. It is most common in low‐ and middle‐income countries but also affects people in high‐income countries. Numbers of stillbirths have not fallen much in the last 20 years and, despite the high numbers, it is not widely recognised as a global health problem. It is important to raise awareness of effective methods of preventing stillbirths, particularly in low‐ and middle‐income countries.
What did we do?
Cochrane systematic reviews of interventions aim to answer specific medical questions based on up‐to‐date research studies. We searched for all Cochrane systematic reviews that assessed ways of preventing stillbirth during pregnancy to produce an overview of Cochrane evidence on preventing stillbirth.
What evidence did we find?
We found 43 Cochrane reviews that assessed 61 different ways of preventing stillbirth during pregnancy, or infant deaths around the time of birth. However, few of these provided any clear evidence of an effect during pregnancy to reduce the risk of stillbirth or infant death.
We grouped them into four different areas: nutrition, preventing infection, managing mothers' other healthcare problems, and looking after the baby before it is born.
Nutrition
‐ Giving mothers balanced energy and protein supplements to increase the growth of the baby, particularly in undernourished pregnant women, probably reduces stillbirth by 40%.
‐ For Vitamin A alone versus placebo (sham) or no treatment, and multiple micronutrients with iron and folic acid compared with iron with or without folic acid, there was clear evidence of no effect.
Prevention and management of infections
‐ Insecticide‐treated anti‐malarial nets versus no nets may reduce loss of the baby in the womb (fetus) by 33%.
Prevention, detection and management of other healthcare problems
‐ Where midwives were the primary healthcare provider, particularly for low‐risk pregnant women, loss of the fetus or infant deaths fell by 16%.
‐ Having a trained traditional birth attendant versus having an untrained traditional birth attendant probably reduces stillbirth in rural populations of low‐ and middle‐income countries by 31% and infant death by 30%.
‐ A reduced number of antenatal care visits probably results in an increase in infant death around the time of birth.
‐ Community‐based intervention packages (including community‐support groups/women's groups, community mobilisation and home visits, or training traditional birth attendants who made home visits) may reduce stillbirth by 19%.
Checking the baby before birth
‐ Cardiotocography measures the baby's heart rate and contractions in the womb. It can be recorded automatically by computer or manually, with pen and paper. Computerised cardiotocography to monitor baby’s well‐being in the womb, by measuring contractions, probably reduces the rate of infant deaths around the time of birth by 80% when compared with traditional cardiotocography.
We were uncertain about the effects of other methods.
What does this mean?
We found a large number of reviews but few produced clear evidence. The effectiveness of the methods used to prevent stillbirth varied depending on where they took place, highlighting that it is important to understand how they were tested. The findings cannot be applied to women in general and across all global settings.
Background
Description of the condition
An antepartum fetal death, also known as stillbirth (a term preferred by the community (Froen 2011)), is defined by the International Classification of Diseases 11th revision (ICD‐11; WHO 2020), as a fetus that has suffered an intrauterine death after the 24th week of gestation and before the onset of labour, although definitions very widely (Lawn 2016). Global estimates indicate that at least 2.6 million (uncertainty range 2.08 million to 3.79 million) stillbirths occurred in the last trimester of pregnancy in 2008 (when the fetus was at least 1000 g in birthweight or at least the 28th week of gestation), with more than 55% of stillbirths occurring in the antepartum period (Cousens 2011). Advances in care during pregnancy are required to reduce the risk of antepartum stillbirths (1.46 million) and to address pregnancy hypertension, maternal infectious diseases and fetal growth restriction (Lawn 2011). Early stillbirths (20 weeks up to 28 completed weeks of gestation) are rarely counted in low‐income countries (Flenady 2016; Lawn 2016). The vast majority (98%) of these stillbirths are from low‐ and middle‐income countries. Over half of all stillbirths (55%) occur in rural Sub‐Saharan Africa and South Asia, particularly in settings where the number of skilled birth attendants and caesarean sections are significantly lower than in urban settings (Lawn 2011). Third‐trimester stillbirths approximate three million early neonatal deaths every year (Lawn 2011). Despite this large burden, stillbirths have been ignored in global statistics and global health policy, were not included in the Millennium Development Goals (MDGs; UN 2010), are not included in the Sustainable Developmental Goals (SDGs; UN 2015), nor in estimates of the global burden of disease. Furthermore, most countries generally under‐report or do not include stillbirths in their vital statistics reporting systems (Blencowe 2016). MDG5 (to improve maternal health) has shown the least progress among all MDGs (UN 2010). Maternal mortality is correlated with stillbirth; in low‐ and middle‐ income countries, prolonged labour, infections and haemorrhage, asphyxia and trauma are the leading causes of maternal death or stillbirth (McClure 2007; Weiner 2003). Major risk factors for stillbirths in high‐income countries are maternal overweight and obesity (body mass index of 25 kg/m² or higher), maternal age over 35 years, primiparity and smoking (Flenady 2011).
The ICD‐10 defines early fetal death as the reporting of the death of a fetus with a "birthweight of 500 g or more; if birthweight is unknown, by gestational age of 22 completed weeks or more; or, if both criteria are unknown, by crown‐heel length of 25 cm or more" (WHO 2020). The World Health Organization (WHO), for international comparability, defines stillbirth as the reporting of late fetal deaths at a birthweight of 1000 g or more, or 28 or more completed weeks of gestation and a body length of at least 35 cm. In this overview, we define the term 'stillbirth' to include all fetal deaths at a birthweight of at least 500 g or at 22 weeks of gestation or later. We define miscarriage as occurring before 22 weeks of gestation. Our main focus for this overview is to assess antenatal interventions to prevent stillbirth during pregnancy; we excluded interventions for stillbirth during the intrapartum period (death that occurs after the onset of labour but before birth), as this will be covered in a separate overview review.
Description of the interventions
In low‐ and middle‐income countries, the most common causes of stillbirths are infections such as syphilis, gram‐negative infections and malaria in first pregnancy within malaria‐endemic areas; gestational hypertensive disorders, especially poor management of pre‐eclampsia and eclampsia; obstructed or prolonged labour with associated asphyxia, infection and birth injury; and low availability of caesarean section (Lawn 2016). In high‐income countries, the majority of stillbirths occur prior to the onset of labour with the main causes being related to placental pathology (Flenady 2011). However, a specific cause is not identified in up to 70% of stillbirths depending on the system used to classify these deaths and the level of investigation undertaken, even in high‐income countries where placental pathological examinations and autopsies are available (Flenady 2011).
Bhutta and colleagues reviewed 35 potential interventions to prevent stillbirths, of which they strongly recommended 10 for implementation: periconceptional folic acid fortification, insecticide‐treated bed nets or intermittent preventive treatment for malaria prevention, syphilis detection and treatment, detection and management of hypertensive disease of pregnancy, detection and management of diabetes in pregnancy, detection and management of fetal growth restriction, routine induction to prevent post‐term pregnancies, skilled care at birth, basic emergency obstetric care and comprehensive emergency obstetric care (Bhutta 2011).
In this overview review, we focused on interventions during antenatal care to prevent stillbirth during pregnancy. These include the following interventions.
Nutritional interventions: periconceptional folate supplementation, vitamin A supplementation, vitamin C supplementation, vitamin D supplementation, vitamin E supplementation, vitamin supplementation for preventing miscarriage, calcium supplementation, iodine supplementation, magnesium supplementation, zinc supplementation, multiple micronutrient supplementation, energy and protein intake in pregnancy, marine oil and other prostaglandin precursors
Prevention and management of infection: insecticide‐treated nets for preventing malaria, drugs for preventing malaria
Prevention, detection and management of other morbidities: smoking cessation, support for women at increased risk of low birthweight, women carrying their own case notes, midwife‐led care, traditional birth attendant training, alternative versus standard packages of antenatal care, group antenatal care, community‐based intervention packages, diuretics for preventing pre‐eclampsia, nitric oxide for preventing pre‐eclampsia and its complications, progesterone for preventing pre‐eclampsia and its complications, antioxidants for preventing pre‐eclampsia, altered dietary salt, screening for gestational diabetes mellitus, diet and exercise for preventing gestational diabetes mellitus, screening for thyroid dysfunction, treating periodontal disease and testing for placental dysfunction.
Screening and management of fetal growth and well‐being: ultrasound for fetal assessment in early pregnancy, routine ultrasound in late pregnancy, fetal movement counting, fetal and umbilical Doppler ultrasound, utero‐placental Doppler ultrasound, fetal and umbilical Doppler ultrasound, antenatal cardiotocography for fetal assessment and symphysial fundal height measurement (SFH) in pregnancy for detecting abnormal fetal growth.
How the intervention might work
1. Nutritional interventions
The nutritional status of pregnant women is important for a healthy pregnancy outcome (WHO 2016). Inadequate dietary intake can lead to adverse perinatal outcome due to increasing requirement of macro‐ and micronutrients during pregnancy (Abu‐Saad 2010; De Onis 1998). Di Mario and colleagues reviewed risk factors for stillbirth in low‐ and middle‐income countries and concluded that maternal nutritional status is one of the factors significantly associated with stillbirth (Di Mario 2007). Balanced energy protein intake improves fetal growth and reduces the risk of fetal and neonatal death under maternal undernutritional conditions (Imdad 2011). Folic acid supplementation before pregnancy and during the first two months of pregnancy reduces the risk of neural tube defects (NTDs), which account for a small proportion of NTD‐related stillbirths (Blencowe 2010). Replacing iron‐folic acid supplements with multiple micronutrient supplements in the package of health and nutrition interventions delivered to mothers during pregnancy will improve the impact of supplementation on fetal growth and development and on birthweight (Shrimpton 2009). While the immediate association between stillbirth and nutritional interventions is limited in accurate and robust evidence, nutritional interventions during pregnancy are closely related to perinatal and neonatal outcomes. For example, low maternal serum zinc levels during pregnancy are associated with an increased risk of low birthweight and small‐for‐gestational age (Wang 2015). An increased dietary intake of fruits and vegetables or vitamin C during pregnancy has been associated with increases in fetal growth and birth weight (Jang 2018). Vitamin D supplementation is associated with a possible reduction in the risk of pre‐eclampsia and preterm birth and may increase birthweight (Perez‐Lopez 2015). Vitamin E has a preventive effect on many maternal and perinatal complications such as pre‐eclampsia, growth restriction, preterm premature rupture of membranes and serious neonatal morbidities (Rumbold 2006). Calcium supplementation is associated with a significant benefit in the prevention of pre‐eclampsia (Hofmeyr 2018). Magnesium deficiency especially has been linked with pre‐eclampsia and preterm birth, which have higher rates of perinatal and neonatal mortality relevant to stillbirth (Chein 1996). Iodine supplementation during pregnancy has been shown to increase birthweight, reduces maternal and fetal hypothyroidism and improves intellectual development (Zimmermann 2012).
2. Prevention and management of infections
Infections such as TORCH infections including Toxoplasmosis, Other (syphilis, varicella‐zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), Herpes, malaria and various others are a leading cause of stillbirth worldwide and account for about half of the stillbirths in low‐ and middle‐income countries (Di Mario 2007; McClure 2009; Schmid 2007; Van Geertruyden 2004). Syphilis may cause congenital syphilis by being transmitted to the fetus transplacentally or by placental infection which results in the decrease of blood flow to the fetus and also causes fetal death (Goldenberg 2003). A review of nine hospital studies found that placental malaria was associated with twice the risk of stillbirth, indicating that placental damage is the likely cause for many of the fetal deaths with maternal malaria (Van Geertruyden 2004). A Cochrane Review concluded that the prevention of malaria in pregnancy through chemoprophylaxis or intermittent preventive treatment (IPT) is associated with reductions in low birthweight and severe maternal anaemia and increased mean birthweight in the first two pregnancies (Radeva‐Petrova 2014). Chloroquine has not been found to have any harmful effects on the fetus when used in the recommended doses for malaria prophylaxis or chemoprophylaxis; pregnancy is not a contraindication to malaria prophylaxis with chloroquine or hydroxychloroquine.
3. Prevention, detection and management of other morbidities
Globally, pre‐eclampsia/eclampsia, which occurs in about 6% of pregnancies and decreases blood flow, causing poor fetal growth and hypoxia, often results in stillbirths (McClure 2009). A population‐based study has shown that pregnancy‐induced hypertension is associated with increased risk of stillbirth and neonatal mortality (Ananth 2010). Existing interventions for reducing the risk of pre‐eclampsia include calcium and aspirin used for prevention; and use of anti‐hypertensive drugs and magnesium sulphate for management of pre‐eclampsia/eclampsia (Jabeen 2011). Even though there are no treatments available to reduce the incidence of pre‐eclampsia; the stillbirth rates could be substantially reduced with screening and medical management, including early labour (Menzies 2007).
The prevalence of gestational diabetes mellitus continues to rise. Gestational diabetes mellitus is associated with increased risk of macrosomia, large‐for‐gestational age, perinatal mortality, pre‐eclampsia and caesarean section (Wendland 2012). A differential diagnosis of gestational diabetes mellitus is obtained if women fall within one or more of the following thresholds at any time during pregnancy: fasting plasma glucose 5.1 to 6.9 mmol/L (92‐125 mg/dL), one‐hour plasma glucose of 10.0 mmol/L (180 mg/dL) or higher following a 75 g oral glucose load and two‐hour plasma glucose 8.5 to 11.0 mmol/L (153‐199 mg/dL) following a 75 g oral glucose load (WHO 2013). While earlier studies showed an association between gestational diabetes mellitus and stillbirth, recent studies could not verify this association and current evidence is inconsistent (Rosenstein 2012).
Tobacco smoking during pregnancy is a potentially preventable cause of adverse pregnancy outcomes, including placental abruption, stillbirth, preterm birth (less than 37 weeks' gestation) and low birthweight (less than 2500 g; Hammoud 2005; Salihu 2007; US 2004). Nicotine and other harmful compounds in cigarettesrestrict the supply of oxygen and other essential nutrients, restricting fetal growth (Crawford 2008).
Post‐term pregnancy is associated with an increased rate of stillbirth (Galal 2012; Norwitz 2007). The major cause of perinatal morbidity and mortality in post‐term pregnancy is presumed to be the progressive uteroplacental insufficiency (Hussain 2011; Sanchez‐Ramos 2003).
Periodontal diseases are relatively common during pregnancy and have been linked to adverse pregnancy outcomes including preterm birth, pre‐eclampsia and low birthweight, but there is no clear evidence that this link exists, as several intervention studies could not demonstrate improvements in pregnancy outcomes after treatment (Srinivas 2012).
4. Screening and management of fetal growth and well‐being
Screening and management for detecting fetal compromise, especially impaired growth and distress, have been developed to identify problems during pregnancy (Haws 2009). These interventions include detection of intrauterine growth restriction through clinical examination such as ultrasound screening or fundal height measurement. Symphisical fundal height measurements aim for the detection of fetuses with poor growth as delay in the diagnosis of this fetal condition may lead to stillbirth (Challis 2002). Fetal hypoxia or compromise can lead to reduction in fetal movements, which can be identified in pregnant women with formal assessment of fetal movement counting or fetal phonocardiography (Bhutta 2011). Also, some advanced technologies for assessing adverse perinatal risks have been developed to detect umbilical vascular flow patterns such as Doppler velocimetry, which measures blood flow dynamics in uterine, umbilical and fetal arteries (Alfirevic 2015; Haws 2009; Hoffman 2009).
Why it is important to do this overview
For women and their families who experience stillbirths, the impact can be devastating (Heazell 2016). In countries with a high burden of stillbirths, there are interventions that can substantially reduce stillbirths and could also improve maternal and neonatal outcomes (Bhutta 2011). By implementing improvements in pregnancy‐related care, large reductions in stillbirths can be achieved in low‐ and middle‐income countries (Goldenberg 2011; Pattinson 2011). This overview of Cochrane systematic reviews brings together evidence on the interventions and strategies aimed at preventing stillbirths during pregnancy.
Objectives
To summarise the evidence from Cochrane systematic reviews on the effects of antenatal interventions for preventing stillbirth for low risk or unselected populations of women.
Methods
Criteria for considering reviews for inclusion
Types of studies
In this overview of reviews, we have included all published Cochrane systematic reviews of randomised controlled trials (RCTs) of antepartum interventions aiming to prevent stillbirth/perinatal mortality/fetal loss/fetal death as long as stillbirth is listed as a primary or secondary outcome. Cochrane Reviews are regularly updated and employ methods to minimise bias (Moher 2007; Shea 2007).
Types of participants
We included either low‐risk populations, or all pregnant women (i.e. unselected populations). We have excluded reviews that included only women in high‐risk groups, for example, women at risk of imminent very preterm birth or HIV‐positive pregnant women.
Types of interventions
We included all types of interventions used for preventing stillbirths in the antenatal period for pregnant women. The interventions include: nutrition interventions; interventions for prevention and management of infections; interventions for prevention, detection and management of other morbidities; and interventions for screening and management of fetal growth and well‐being.
Types of outcomes
Primary outcomes
Stillbirth, perinatal mortality or fetal loss/fetal death, as defined by the study authors, or any combination of two or all of these.
In the absence of stillbirth data or if there were limited numbers of stillbirth data for an outcome, we used perinatal mortality, fetal loss and fetal death as outcomes.
Secondary outcomes
Low birthweight (LBW), less than 2500 g
Small‐for‐gestational age (SGA) or intrauterine growth restriction (IUGR), as defined by the study authors
Neonatal intensive care unit (NICU) stay
Search methods for identification of reviews
We collaborated with the Cochrane Pregnancy and Childbirth Information Specialist to identify all their published reviews that specified or reported stillbirth/fetal loss or perinatal mortality as an outcome. We initially screened a list of 873 reviews, protocols and registered titles listed with the Group. We also searched the Cochrane Database of Systematic Reviews (date of last search: 29 Feburary 2020) to identify reviews published within other Cochrane groups (see Appendix 1).
Data collection and analysis
The methodology for data collection and analysis was based on Chapter V of the Cochrane Handbook of Systematic Reviews of Interventions (Pollock 2019).
Selection of reviews
Two review authors independently assessed for inclusion all the potential Cochrane systematic reviews in order to identify the relevant reviews that assess the effects of antenatal interventions that aim to prevent stillbirth during pregnancy, reviewing the objectives and methods, including outcomes and participants. We only included Cochrane systematic reviews if they reported our primary outcome stillbirth, fetal death or perinatal mortality. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
Two review authors independently extracted data from the reviews using a predefined data extraction form and another review author verified the extracted data. We resolved discrepancies through discussion or, if needed, through arbitration by a third review author. If any information from the reviews was unclear or missing, we accessed the published papers of the individual trials. If we could not obtain the information from the published papers, we contacted the individual review authors or authors of the original papers for further details.
Assessment of methodological quality of included reviews
Two review authors independently assessed the quality of evidence in the included reviews and the methodological quality of the systematic reviews. We resolved discrepancies through discussion or, if needed, through arbitration by a third review author.
Quality of included reviews
We assessed the methodological quality of each systematic review using the AMSTAR (A Measurement Tool to Assess Reviews) instrument (Shea 2007). AMSTAR evaluates the methods used in a review against 11 distinct criteria and assesses the degree to which review methods are unbiased.
Each item on AMSTAR is rated as yes (clearly done), no (clearly not done), cannot answer, or not applicable. These criteria, and the way they assess review quality, are as follows.
Was an 'a priori' design provided? (Yes: the research question and inclusion criteria were established before conducting the review.)
Was there duplicate study selection and data extraction? (Yes: at least two people working independently extracted the data and the method was reported for reaching consensus if disagreements arose.)
Was a comprehensive literature search performed? (Yes: at least two electronic sources were searched; details of the databases, years searched and search strategy were provided; the search was supplemented by searching of reference lists of included studies, and specialised registers, and by contacting experts.)
Was the status of publication (i.e. grey literature) used as an inclusion criterion? (Yes: authors searched for reports irrespective of publication type. They did not exclude reports based on publication from the systematic review. No: the authors stated that they excluded studies from the review based on publication status.)
Was a list of studies (included and excluded provided)? (Yes: a list was provided.)
Were the characteristics of the included studies provided? (Yes: data on participants, interventions and outcomes were provided, and the range of relevant characteristics reported.)
Was the scientific quality of the included studies assessed and reported? (Yes: predetermined methods of assessing quality were reported.)
Was the scientific quality of the included studies used appropriately in formulating conclusions? (Yes: the quality, and limitations, of included studies were used in the analysis, conclusions and recommendations of the review.)
Were the methods used to combine the findings of studies appropriate? (Yes: if results were pooled statistically, heterogeneity was assessed and used to inform the decision of the statistical model to be used. If heterogeneity was present, the appropriateness of combining studies was considered by review authors.)
Was the likelihood of publication bias assessed? (Yes: publication bias was explicitly considered and assessed.)
Was the conflict of interest stated? (Yes: source of funding or support for the systematic review AND for each of the included studies was clearly acknowledged)
For all items a rating of 'yes' is considered adequate. A review that adequately meets all of the 11 criteria is considered to be a review of the highest quality. For this overview, we considered reviews that achieved scores of between 8 to 11 as high quality; scores of 4 to 7 as moderate quality; and scores of 0 to 3 as low quality. Two review authors independently assessed the quality of the included reviews using AMSTAR and another review author verified this assessment. We resolved differences by discussion and consensus and, if needed, through arbitration by a third review author. We identified and discussed differences in quality between reviews, and used the review quality assessment to interpret the results of reviews when synthesised in this overview.
Quality of evidence in the included reviews
We did not re‐evaluate the risks of bias among the individual trials included in the eligible systematic reviews as it is a component of all Cochrane Reviews (Higgins 2011a). We used the GRADE assessment from the pooled outcome data as assessed by authors in a particular systematic review. GRADE integrates the review author’s judgment on risk of bias and the pooled estimates of individual trials. According to the criteria described in the GRADE Handbook, we performed GRADE assessment ourselves when the review authors had not assessed it (Schünemann 2013).
We did not reassess the GRADE assessment for our primary outcomes in the included systematic reviews where it was reported by review authors. If review authors did not assess GRADE, we made a new assessment ourselves. As we included a large number of systematic reviews, we created figures by assigning graphic icons to present the direction of review effect estimates with our confidence on estimates (see Figure 1). To assign a graphic icon, we considered GRADE judgements and the pooled summary statistics with 95% confidence intervals. The graphic icons indicate mutually exclusive assessment categories such as clear evidence of benefit, clear evidence of harm, clear evidence of no effect or equivalence, possible benefit, possible harm, and unknown benefit or harm or no effect or equivalence. The clear evidence of benefit, harm and no effect or equivalence refers to GRADE moderate‐ or high‐certainty evidence with narrow confidence intervals. The possible benefit or possible harm refers to GRADE low‐certainty evidence with clear benefit or clear harm (the confidence interval does not cross the line of no effect) or GRADE moderate‐ to high‐certainty evidence with wide confidence intervals not crossing the line of no effect respectively. We considered GRADE low, moderate‐ or high‐certainty evidence with wide confidence intervals crossing the line of no effect, low‐certainty evidence with no effect or equivalence, or very low‐certainty evidence, as unknown benefit or harm or no effect or equivalence. To define 'clear evidence of no effect or equivalence', we considered a confidence interval of risk ratio (RR) within the range of 0.75 to 1.25 as sufficiently narrow to indicate a minimal effect relative to the comparator.
1.
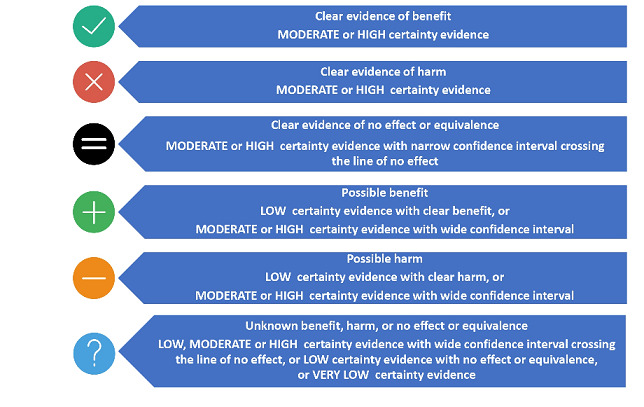
Explanation of certainty of evidence for graphic icons (all icons by Freepik at www.flaticon.com)
Data synthesis
We summarised the characteristics of included reviews in tables (see Table 1; Table 2; Table 3; Table 4) as well as the AMSTAR ratings for each separate review (see Table 5; Table 6; Table 7; Table 8). We also provided individual review narrative summaries of the relevant results for the individual reviews (Table 9; Table 10; Table 11; Table 12).
1. Characteristics of included Cochrane systematic reviews: nutritional interventions.
| Review title | Date last searched in the review | Number of studies included (number of participants in included studies) | Review question/objective | Study design | Types of participants | Interventions | Relevant outcomes (stillbirth definition used in the review) | Overall AMSTAR score and relevant GRADE assessment in reported in review |
| Effects and safety of periconceptional folate supplementation for preventing birth defects (De‐Regil 2015) | August 2015 | 5 studies 7391 women |
To examine whether periconceptional folate supplementation reduces the risk of neural tube and other congenital anomalies (including cleft palate) without causing adverse outcomes in mothers or babies | RCTs | Women who become pregnant were ≤ 12 weeks pregnant, independent of age and parity or history of neural tube defect‐affected pregnancy | Oral supplements of folate alone and with other vitamins and minerals given on a daily or intermittent (1, 2 or 3 times a week on non‐consecutive days) basis and compared with receiving a placebo, no supplementation or other vitamins and minerals but no folate. |
|
AMSTAR: 9 GRADE: not assessed for relevant outcomes |
| Vitamin A supplementation during pregnancy for maternal and newborn outcomes (McCauley 2015) | March 2015 | 19 studies > 310,000 women |
To review the effects of supplementation of vitamin A, or one of its derivatives, during pregnancy, alone or in combination with other vitamins and micronutrients, on maternal and newborn clinical outcomes. | RCTs Cluster‐RCTs Quasi‐RCTs |
Pregnant women receiving vitamin A supplementation either in areas with endemic vitamin A deficiency or in areas with adequate intake as defined by the WHO global database on vitamin A deficiency | Vitamin A supplementation, alone or in combination with other supplements compared with a control group, (placebo, no treatment or another intervention) |
|
AMSTAR: 10 GRADE:
|
| Vitamin C supplementation in pregnancy (Rumbold 2015a) | March 2015 | 29 studies 24,300 women |
To evaluate the effects of vitamin C supplementation, alone or in combination with other separate supplements on pregnancy outcomes, adverse events, side effects and use of health resources | RCTs Quasi‐RCTs |
All pregnant women receiving either vitamin C supplementation or control either in areas where there is inadequate dietary intake or where there is presumed adequate intake | Vitamin C supplementation, alone or in combination with other separate supplements compared with placebo, no placebo or other supplements |
|
AMSTAR: 9 GRADE:
|
| Vitamin D supplementation for women during pregnancy (Palacios 2019) | July 2018 | 30 studies 7033 women |
To examine whether oral supplements with vitamin D alone or in combination with calcium or other vitamins and minerals given to women during pregnancy can safely improve maternal and neonatal outcomes | RCTs Quasi‐RCTs |
Pregnant women of any gestational or chronological age, parity (number of births) and number of fetuses. Pregnant women with pre‐existing conditions (i.e. gestational diabetes) were excluded | Vitamin D supplementation during pregnancy irrespective of dose, duration or time of commencement of supplementation. |
|
AMSTAR: 10 GRADE:
|
| Vitamin E supplementation in pregnancy (Rumbold 2015b) | March 2015 | 21 studies 22,129 women |
To assess the effects of vitamin E supplementation, alone or in combination with other separate supplements, on pregnancy outcomes, adverse events, side effects and use of health services | RCTs Quasi‐RCTs |
Pregnant women receiving vitamin E supplementation or control, living in areas where there is either inadequate dietary intake of vitamin E or where is presumed adequate intake | Vitamin E supplementation, alone or in combination with other separate supplements compared with placebo, no placebo or other supplements |
|
AMSTAR: 9 GRADE: stillbirth, moderate‐certainty evidence |
| Vitamin supplementation for preventing miscarriage (Balogun 2016) | November 2015 | 40 studies 276,820 women |
To determine the effectiveness and safety of any vitamin supplementation, on the risk of spontaneous miscarriage | RCTs Quasi‐RCTs Cluster‐RCTs |
Pregnant women (< 20 weeks' gestation) or women in the reproductive age group planning on becoming pregnant in the near future, regardless of whether they are at low or high risk of having a miscarriage | Comparisons of specific vitamin(s), alone or in combination with other agents with either placebo, other vitamin(s), no vitamin(s) or other interventions for the prevention of miscarriage |
|
AMSTAR: 9 GRADE: not assessed for the comparisons of interest |
| Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy (Hofmeyr 2019) | July 2018 | 1 study 1355 women |
To determine the effect of calcium supplementation, given before or early in pregnancy and for at least the first half of pregnancy, on pre‐eclampsia and other hypertensive disorders, maternal morbidity and mortality, and fetal and neonatal outcomes | RCTs | Women of child bearing age but not yet pregnant, and women in the early stages of pregnancy (up to approximately 12 weeks' gestation). Low or high risk population for pre‐eclampsia | Calcium supplementation with or without additional supplements or treatments, compared with placebo, no intervention, or the same additional supplements or treatments |
|
AMSTAR: 9 GRADE:
|
| Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems (Hofmeyr 2018) | September, 2017 | 27 studies 18,064 women |
To determine, from the best available evidence, the effect of calcium supplementation during pregnancy on the risk of hypertensive disorders and related maternal and fetal or neonatal adverse outcomes | RCTs Quasi‐RCTs |
Pregnant women, regardless of the risk of hypertensive disorders of pregnancy. Women with diagnosed hypertensive disorders of pregnancy were excluded as wells as women with multiple pregnancy | Supplementation with high‐dose calcium (≥ 1 g/d elemental calcium ) or low‐dose calcium (< 1 g/d elemental calcium) from at the latest 34 weeks of pregnancy, compared with placebo, no treatment. Comparison of different dosages of calcium |
|
AMSTAR: 10 GRADE: not assessed for relevant outcomes |
| Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy outcomes (Buppasiri 2015) | September 2014 | 25 studies 17,842 women |
To determine the effect of calcium supplementation on maternal, fetal and neonatal outcomes (other than for preventing or treating hypertension) | RCTs | Pregnant women who received any calcium supplementation | Calcium supplementation during pregnancy compared with placebo or no treatment |
|
AMSTAR: 9 GRADE:
|
| Iodine supplementation for women during the preconception, pregnancy and postpartum period (Harding 2017) | November 2017 | 14 studies > 2700 women |
To assess the benefits and harms of supplementation with iodine, alone or in combination with other vitamins and minerals, for women in the preconceptional, pregnancy or postpartum period on their and their children’s outcomes | RCTs Cluster‐RCTs Quasi‐RCTs |
Women who become pregnant, or pregnant or postpartum women of any chronological age and parity (number of births), regardless as to the iodine status of the study population or setting | Injected or oral iodine supplementation (such as tablets, capsules, drops) during preconception, pregnancy or the postpartum period irrespective of compound, dose, frequency or duration |
|
AMSTAR: 11 GRADE:
|
| Magnesium supplementation in pregnancy (Makrides 2014) | March 2013 | 10 studies 9090 women |
To assess the effects of magnesium supplementation during pregnancy on maternal, neonatal and paediatric outcomes | RCTs Quasi‐RCTs |
Women with normal or high‐risk pregnancies | Magnesium orally administered at any time during the antenatal period, regardless of dose |
|
AMSTAR: 8 GRADE: not assessed |
| Zinc supplementation for improving pregnancy and infant outcome (Ota 2015b) | October 2014 | 21 studies > 17,000 women and their babies |
|
RCTs | Normal pregnant women with no systemic illness. Women who may have had normal zinc levels or they may have been, or likely to have been, zinc deficient | Routine zinc supplementation vs no zinc supplementation or placebo |
|
AMSTAR: 9 GRADE:
|
| Multiple‐micronutrient supplementation for women during pregnancy (Keats 2019) | February 2018 | 21 studies 142,496 women |
To evaluate the benefits of oral MMN supplementation during pregnancy on maternal, fetal, and infant outcomes | RCTs Cluster‐RCTs |
Pregnant women of any gestation. HIV‐positive women were excluded. | MMN supplementation with iron and folic acid compared with supplementation with iron, with or without folic acid |
|
AMSTAR: 10 GRADE:
|
| Antenatal dietary education and supplementation to increase energy and protein intake (Ota 2015a) | January 2015 | 17 studies 9030 women |
To assess the effects of dietary advice, supplementation, or restriction on gestational weight gain, pre‐eclampsia, and/or pregnancy outcomes | RCTs | Pregnant women, for the assessment of dietary restriction, pregnant women with either high pregnancy weight or high gestational weight gain | Specific advice to increase dietary energy and protein intakes, energy and/or protein supplementation, or prescription of low energy diet |
|
AMSTAR: 10 GRADE:
|
| Omega‐3 fatty acid addition during pregnancy (Middleton 2018) | August 2018 | 70 studies 19,927 women |
To assess the effects of omega‐3 LCPUFA, as supplements or as dietary additions, during pregnancy on maternal, perinatal, and neonatal outcomes and longer‐term outcomes for mother and child | RCTs Quasi‐RCTs |
Pregnant women, regardless of their risk for pre‐eclampsia, preterm birth or IUGR | Omega‐3 fatty acids (usually fish or algal oils) compared with placebo or no omega‐3 fatty acids. Trials that assessed omega‐3 fatty acid co‐interventions (e.g. omega‐3 with another agent). Studies or study arms that compared omega‐3 doses or types of omega‐3 (e.g. DHA vs EPA) directly |
|
AMSTAR: 10 GRADE:
|
| Lipid‐based nutrient supplements for maternal, birth, and infant developmental outcomes (Das 2018) | May 2018 | 4 studies 8018 women |
To assess the effects of LNS for maternal, birth and infant outcomes in pregnant women. Secondary objectives were to explore the most appropriate composition, frequency and duration of LNS administration | RCTs, Quasi‐RCTs |
Women with singleton pregnancy of any age and parity, living in stable or emergency settings | Interventions involving the provision of LNS for point‐of‐use food fortification or direct consumption, irrespective of dose, frequency and duration vs no intervention, placebo, or another intervention |
|
AMSTAR: 11 GRADE:
|
| AMSTAR: A Measurement Tool to Assess Reviews; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; IUGR: interuterine growth restriction; LBW: low birthweight; LCPUFA: long‐chain polyunsaturated fatty acids; LNS: lipid‐based nutritional supplements; MMN: multiple‐micronutrient; NICU: neonatal intensive care unit; RCT: randomised controlled trial; SGA: small‐for‐gestational age; WHO: World Health Organization | ||||||||
2. Characterstics of included Cochrane systematic reviews: prevention and management of infection.
| Review title | Date last searched in the review | Number of studies included (number of participants in included studies) | Review question/objective | Study design | Types of participants | Interventions |
Relevant outcomes (stillbirth definition used in the review) |
Overall AMSTAR score and relevant GRADE assessment |
| Insecticide‐treated nets for preventing malaria in pregnancy (Gamble 2006) | February 2009 |
5 studies 6759 women |
To compare ITNs with no nets or untreated nets on preventing malaria in pregnancy | RCTs | Pregnant women in malaria‐endemic areas | ITNs compared to no nets or untreated nets |
|
AMSTAR: 7 GRADE: not assessed |
| Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment (Radeva‐Petrova 2014) | June 2014 | 17 studies 14,481 women |
In malaria‐endemic areas, to assess the effects in pregnant women of:
|
RCTs Quasi‐RCTs |
Pregnant women living in endemic malaria areas | Any antimalarial drug chemoprevention regimen given to pregnant women |
|
AMSTAR: 9 GRADE:
|
| AMSTAR: A Measurement Tool to Assess Reviews; ITN: insecticide‐treated net; LBW: low birthweight; RCT: randomised controlled trial | ||||||||
3. Characteristics of included Cochrane systematic reviews: prevention, detection, and management of other morbidities.
| Review title | Date last searched in the review | Number of studies included (number of participants in included studies) | Review question/objective | Study design | Types of participants | Interventions |
Relevant outcomes (stillbirth definition used in the review) |
Overall AMSTAR score and relevant GRADE assessment |
| Psychosocial interventions for supporting women to stop smoking in pregnancy (Chamberlain 2017) | November 2015 | 88 studies > 28,000 women |
To assess the effects of smoking cessation interventions during pregnancy on smoking behaviour and perinatal health outcomes | RCTs, Cluster‐RCTs, Quasi‐RCT, Randomised cross‐over trials | Women who are currently smoking or have recently quit smoking and are pregnant
Implementation strategies to support pregnant women to stop smoking |
|
|
AMSTAR: 9 GRADE: not assessed for relevant outcomes |
| Pharmacological interventions for promoting smoking cessation during pregnancy (Coleman 2015) | July 2015 | 9 studies 2210 women |
To determine the efficacy and safety of smoking cessation pharmacotherapies (including NRT), varenicline and bupropion), other medications, or ENDS when used for smoking cessation in pregnancy. | RCTs | Women who are pregnant and who also smoke | Pharmacological treatments aimed at promoting smoking cessation including, but not exclusive to, treatments that have been proven effective in non‐pregnant adults (e.g. NRT, bupropion, varenicline; and ENDS used to promote smoking cessation. |
|
AMSTAR: 8 GRADE: not assessed |
| Giving women their own case notes to carry during pregnancy (Brown 2015) | August 2015 | 4 studies 1176 women |
To evaluate the effects of giving women their own case notes to carry during pregnancy on administrative outcomes, maternal satisfaction and control, health‐related behaviours and clinical outcomes | RCTs Cluster‐RCTs |
Pregnant women from the time of their first antenatal visit to the end of the postpartum period | Any intervention that involved giving women their own case notes to carry during their pregnancy from the time of their first antenatal visit through the time of hospital admission for the birth of the baby and into the postpartum period |
|
AMSTAR: 8 GRADE:
|
| Midwife‐led continuity models versus other models of care for childbearing women (Sandall 2016) | January 2016 | 15 studies 17,674 women |
To compare midwife‐led models of care with other models of care for childbearing women and their infants and to determine whether the effects of midwife‐led care are influenced by:
|
RCTs Quasi‐RCTs Cluster‐RCTs |
Pregnant women | Midwife‐led models of care compared to other or shared care on the basis of the lead professional in the antepartum and intrapartum periods |
|
AMSTAR: 9 GRADE:
|
| Traditional birth attendant training for improving health behaviours and pregnancy outcomes (Sibley 2012) | June 2012 | 9 studies > 32,000 women |
To assess the effects of TBA training on TBA and maternal behaviours thought to mediate positive pregnancy outcomes, as well as on maternal, perinatal, and newborn mortality and morbidity | RCTs Quasi‐RCTs, Cluster‐RCTs |
|
TBA training |
|
AMSTAR: 9 GRADE: not assessed for relevant outcomes |
| Alternative versus standard packages of antenatal care for low‐risk pregnancy (Dowswell 2015) | March 2015 | 7 studies 60,724 women |
To compare the effects of antenatal care programmes providing a reduced number of antenatal care visits for low‐risk women with programmes providing the standard schedule of visits, and to assess the views of the care providers and the women receiving antenatal care | RCTs Quasi‐RCTs |
Pregnant women attending antenatal care clinics and considered to be at low risk of developing complications during pregnancy and labour | Provision of a schedule of reduced number of visits, with or without goal‐oriented antenatal care, compared with a standard schedule of visits |
|
AMSTAR: 9 GRADE:
|
| Group versus conventional antenatal care for women (Catling 2015) | October 2014 | 4 studies 2350 women |
|
RCTs Quasi‐RCTs Cluster‐RCTs |
Pregnant women accessing antenatal care | Group antenatal care compared with conventional antenatal care (1‐1 basis) |
|
AMSTAR: 10 GRADE:
|
| Diuretics for preventing pre‐eclampsia (Churchill 2007) | May 2010 | 5 studies 1836 women |
To ascertain if the use of diuretics in pregnancy prevents the onset of pre‐eclampsia | RCTs | Pregnant women, both at high and low risk of pre‐eclampsia but without pre‐eclampsia at trial entry | Prophylactic administration of diuretics of any group during pregnancy when used in order to prevent pre‐eclampsia |
|
AMSTAR: 8 GRADE: not assessed |
| Nitric oxide for preventing pre‐eclampsia and its complications (Meher 2007) | February 2012 | 7 studies 389 women |
To determine the effectiveness and safety of nitric oxide for preventing pre‐eclampsia and its complications | RCTs | Pregnant women were included, regardless of gestation at trial entry. | Studies were included if they were comparisons of any nitric oxide agent with any of the following:
|
|
AMSTAR: 8 GRADE: not assessed |
| Progesterone for preventing pre‐eclampsia and its complications (Meher 2006) | January 2011 | 10 studies 4659 women |
To assess the effects of progesterone, or any other progestogen, for prevention of pre‐eclampsia and its complications | RCTs | Pregnant women with normal blood pressure or high blood pressure without proteinuria were included, regardless of gestation at trial entry. | The following comparisons were included:
|
|
AMSTAR: 8 GRADE: not assessed |
| Antioxidants for preventing pre‐eclampsia (Rumbold 2008) | April 2013 | 13 studies 16,606 women |
To determine the effectiveness and safety of any antioxidant supplementation during pregnancy on the risk of:
|
RCTs | Pregnant women considered to be at low, moderate or high risk of developing pre‐eclampsia |
|
|
AMSTAR: 9 GRADE: not assessed |
| Altered dietary salt for preventing pre‐eclampsia, and its complications (Duley 2005) |
October 2009 | 2 studies 603 women |
To assess the effects of altered dietary salt on the risk of developing pre‐eclampsia and its complications and to compare the effects of one form of alteration with another, such as restricted salt intake with increased salt intake, and to compare the effects of altered salt intake with other measures for prevention of pre‐eclampsia | RCTs | Women who had normal or high blood pressure without proteinuria during pregnancy were included, regardless of gestation at trial entry | Any comparison of altered dietary salt intake with normal salt intake during pregnancy was included, as were comparisons of one form of alteration with another, such as restricted salt intake with increased salt intake, and comparisons of dietary salt intake with other measures for prevention of pre‐eclampsia |
|
AMSTAR: 7 GRADE: not assessed |
| Community‐based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes (Lassi 2015) | May 2014 | 26 studies | To assess the effectiveness of community‐based intervention packages in reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. | Community‐based trials RCTs Quasi‐RCTs |
Women of reproductive age group, particularly pregnant women at any period of gestation | Intervention packages that included additional training of outreach workers namely, lady health workers/visitors, community midwives, community/village health workers, facilitators or TBAs in maternal care during pregnancy, delivery and in the postpartum period; and routine newborn care |
|
AMSTAR: 9 GRADE: not assessed |
| Screening for gestational diabetes mellitus based on different risk profiles and settings for improving maternal and infant health (Tieu 2017) | June 2017 | 2 studies 4523 women |
To assess the effects of screening for GDM based on different risk profiles and settings on maternal and infant outcomes | RCTs Quasi‐RCTs |
Pregnant women, women already diagnosed with (GDM) in their current pregnancy and with pre‐existing (type 1 or 2) diabetes mellitus were excluded. | Different protocols, guidelines or programmes for screening for GDM based on different risk profiles and settings, compared with the absence of screening, or compared with other protocols, guidelines or programmes for screening |
|
AMSTAR: 10 GRADE:
|
| Combined diet and exercise interventions for preventing gestational diabetes mellitus (Shepherd 2017) | November 2016 | 23 studies 8918 women and 8709 infants |
To assess the effects of diet interventions in combination with exercise interventions for pregnant women for preventing GDM, and associated adverse health consequences for the mother and her infant/child | RCTs Cluster‐RCTs |
Pregnant women regardless of age, gestation, parity or plurality. Studies involving women with pre‐existing GDM, type 1 or type 2 diabetes were excluded. | Any type of dietary advice with any type of exercise intervention (i.e. exercise advice, providing exercise sessions) compared with no intervention (i.e. standard care). |
|
AMSTAR: 10 GRADE:
|
| Screening and subsequent management for thyroid dysfunction pre‐pregnancy and during pregnancy for improving maternal and infant health (Spencer 2015) | July 2015 | 2 studies 26,408 women | To assess the effects of different screening methods (and subsequent management) for thyroid dysfunction pre‐pregnancy and during pregnancy on maternal and infant outcomes. | RCTs | Women, either pre‐pregnancy or during pregnancy (including both singleton and multiple pregnancies). Women with a pre‐existing diagnosis of thyroid dysfunction were excluded. |
|
|
AMSTAR: 10 GRADE:
|
| Treating periodontal disease for preventing adverse birth outcomes in pregnant women (Iheozor‐Ejiofor 2017) | October 2016 | 15 studies 7161 women |
To assess the effects of treating periodontal disease in pregnant women in order to prevent or reduce perinatal and maternal morbidity and mortality | RCTs | Pregnant women considered to have periodontal disease (diagnoses of gingivitis and periodontitis) after dental examination | Treatment for periodontal disease, performed by a dentist, dental hygienist or therapist, either singly or in combination with counselling on oral hygiene, antiseptic oral agents, topical or systemic antimicrobial therapies compared with either placebo (for adjunctive treatment), no treatment or alternative treatments |
|
AMSTAR: 11 GRADE:
|
| Use of biochemical tests of placental function for improving pregnancy outcome (Heazell 2015) | July 2015 | 3 studies 740 women |
To assess whether clinicians' knowledge of the results of biochemical tests of placental function is associated with improvement in fetal or maternal outcome of pregnancy | RCTs Quasi‐RCTs |
All pregnant women, regardless of whether deemed to be high risk or low risk for pregnancy complications, or unselected participants by the study investigators. Women who had pregnancies complicated by chromosomal or structural anomaly were excluded. | Comparison of women who had placental function tests (biochemical test of placental function carried out using the woman's maternal biofluid, either alone or in combination with other placental function test/s) and the results were available to their clinicians with women who either did not have the tests, or the tests were done but the results were not available to the clinicians |
|
AMSTAR: 10 GRADE:
|
| AMSTAR: A Measurement Tool to Assess Reviews; CBT: cognitive behavioural therapy; ENDS: electronic nicotine delivery systems; GDM: gestational diabetes mellitus; LBW: low birthweight; MI: motivational interviewing; NICU: neonatal intensive care unit; NRT: nicotine replacement therapy; RCT: randomised controlled trial; SGA: small‐for‐gestational age; TBA: traditional birth attendant | ||||||||
4. Characteristics of included Cochrane systematic reviews: screening and management of fetal growth and well‐being.
| Review title | Date last searched in the review | Number of studies included (number of participants in included studies) | Review question/objective | Study design | Types of participants | Interventions |
Relevant outcomes (stillbirth definition used in the review) |
Overall AMSTAR score and relevant GRADE assessment |
| Ultrasound for fetal assessment in early pregnancy (Whitworth 2015) | March 2015 | 11 studies 37,505 women |
To assess whether routine early pregnancy ultrasound for fetal assessment influences the diagnosis of fetal malformations, multiple pregnancies, the rate of clinical interventions, and the incidence of adverse fetal outcome when compared with the selective use of early pregnancy ultrasound | RCTs Quasi‐RCTs |
Women with early pregnancies, i.e. < 24 weeks' gestation | Routine ultrasound examination compared with selective ultrasound examination |
|
AMSTAR: 9 GRADE: perinatal mortality, low‐certainty evidence |
| Routine ultrasound in late pregnancy (after 24 weeks' gestation) (Bricker 2015) | May 2015 | 13 studies 34,980 Women |
To assess the effects on obstetric practice and pregnancy outcome of routine late pregnancy ultrasound, defined as > 24 weeks' gestation, in women with either unselected or low‐risk pregnancies | RCTs, Quasi‐RCTs | Women in late pregnancy (after 24 weeks’ gestation) in both unselected populations and designated low‐risk populations | Routine ultrasound examination in late pregnancy (after 24 weeks' gestation) to assess one, some or all of the following: fetal size; amniotic fluid volume; placental site; placental grading; fetal structural anatomy; fetal presentation |
|
AMSTAR: 8 GRADE:
|
| Fetal movement counting for assessment of fetal wellbeing (Mangesi 2015) | May 2015 | 5 studies 71,458 women |
To compare the outcome of pregnancy when fetal movement counting is done routinely, selectively, or not at all, and using various methods | RCTs Cluster‐RCTs |
Pregnant women who had reached the gestational age of fetal viability, as defined in the trial setting |
|
|
AMSTAR: 8 GRADE: not assessed for relevant outcomes |
| Fetal and umbilical Doppler ultrasound in normal pregnancy (Alfirevic 2015) | February 2015 | 5 studies 14,624 women |
To assess the effects of routine fetal and umbilical Doppler ultrasound, or a combination of uterine Doppler ultrasound and umbilical Doppler ultrasound, in unselected and low‐risk pregnancies on obstetric practice and pregnancy | RCTs Quasi‐RCTs |
Pregnant women in both unselected and low‐risk populations | Routine Doppler ultrasound of the fetal and umbilical artery circulation in pregnancy in unselected or low‐risk populations |
|
AMSTAR: 9 GRADE:
|
| Utero‐placental Doppler ultrasound for improving pregnancy outcome (Stampalija 2010) | June 2010 | 2 studies 4993 women |
To assess whether the use of utero‐placental Doppler ultrasound (uterine arteries and placental vessels) improves the outcome of low‐ and high‐risk pregnancies | RCTs Quasi‐RCTs |
Pregnant women, considered to be either low or high risk, who had utero‐placental Doppler ultrasound performed at 1st or 2nd trimester of pregnancy | Doppler ultrasound of the utero‐placental circulation (uterine, arcuate, radial and spiral arteries) in pregnancies at high and low risk |
|
AMSTAR: 8 GRADE: not assessed |
| Antenatal cardiotocography for fetal assessment (Grivell 2015) | June 2015 | 6 studies 2105 women | To assess the effectiveness of antenatal CTG in improving outcomes for babies and also how effective computerised CTG might be | RCTs, Quasi‐RCTs |
All pregnant women and their babies. | CTG performed in the antenatal period to assess fetal well‐being
|
|
AMSTAR: 8 GRADE:
|
| Symphysial fundal height (SFH) measurement in pregnancy for detecting abnormal fetal growth (Robert Peter 2015) | July, 2015 | 1 study 1639 women |
To compare SFH measurement with serial ultrasound measurement of fetal parameters or clinical palpation to detect abnormal fetal growth (IUGR and large‐for‐gestational age), and improving perinatal outcome | RCTs | Pregnant women with singleton fetuses who are of ≥ 20 weeks' gestation | Tape measurement of SFH |
|
AMSTAR: 7 GRADE:
|
| AMSTAR: A Measurement Tool to Assess Reviews; CTG: cardiotocography; IUGR: interuterine growth restriction; LBW: low birthweight; NICU: neonatal intensive care unit;RCT: randomised controlled trial; SFH: symphysial fundal height; SGA: small‐for‐gestational age | ||||||||
5. AMSTAR ratings for each Cochrane systematic review: nutritional intervention.
| Review title | 1.* | 2.* | 3.* | 4.* | 5.* | 6.* | 7.* | 8.* | 9.* | 10.* | 11.* | Total score (out of a maximum of 11) |
| Effects and safety of periconceptional folate supplementation for preventing birth defects (De‐Regil 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | NA | Yes | 9 |
| Vitamin A supplementation during pregnancy for maternal and newborn outcomes (McCauley 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
| Vitamin C supplementation in pregnancy (Rumbold 2015a) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Vitamin D supplementation for women during pregnancy (Palacios 2019) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 |
| Vitamin E supplementation in pregnancy (Rumbold 2015b) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Vitamin supplementation for preventing miscarriage (Balogun 2016) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy (Hofmeyr 2019) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA | Yes | 9 |
| Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems (Hofmeyr 2018) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 |
| Calcium supplementation (other than for preventing or treating hypertension ) for improving pregnancy and infant outcomes (Buppasiri 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Iodine supplementation for women during the preconception, pregnancy and postpartum period (Harding 2017) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| Magnesium supplementation in pregnancy (Makrides 2014) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | 8 |
| Zinc supplementation for improving pregnancy and infant outcome (Ota 2015b) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Multiple‐micronutrient supplementation for women during pregnancy (Keats 2019) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 |
| Antenatal dietary education and supplementation to increase energy and protein intake (Ota 2015a) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
| Omega‐3 fatty acid addition during pregnancy (Middleton 2018) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 |
| Lipid‐based nutrient supplements for maternal, birth, and infant developmental outcomes (Das 2018) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| AMSTAR: A Measurement Tool to Assess Reviews | ||||||||||||
* criteria for AMSTAR:
1. A prior design
2. Duplicate selection and extraction
3. Comprehensive literature search
4. Searched for reports regardless of publication type or language
5. Excluded/included list provided
6. Characteristics of included studies provided
7. Quality assessment of included studies assessed and presented
8. Quality used appropriately in formulating conclusions
9. Methods used to combine studies appropriate
10. Publication bias assessed
11. Conflict of interests stated
NA = not applicable
6. AMSTAR ratings for each Cochrane systematic reviews: prevention and management of infection.
| Review titles | 1.* | 2.* | 3.* | 4.* | 5.* | 6.* | 7.* | 8.* | 9.* | 10.* | 11.* |
Total score (out of maximum of 11) |
| Insecticide‐treated nets for preventing malaria in pregnancy (Gamble 2006) |
Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | NA | No | 7 |
| Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment (Radeva‐Petrova 2014) |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | 9 |
| AMSTAR: A Measurement Tool to Assess Reviews | ||||||||||||
* criteria for AMSTAR:
1. A prior design
2. Duplicate selection and extraction
3. Comprehensive literature search
4. Searched for reports regardless of publication type or language
5. Excluded/included list provided
6. Characteristics of included studies provided
7. Quality assessment of included studies assessed and presented
8. Quality used appropriately in formulating conclusions
9. Methods used to combine studies appropriate
10. Publication bias assessed
11. Conflict of interests stated
NA = not applicable
7. AMSTAR ratings for each Cochrane systematic review: prevention, detection, and management of other morbidities.
| Review titles | 1.* | 2.* | 3.* | 4.* | 5.* | 6.* | 7.* | 8.* | 9.* | 10.* | 11.* | Total score (out of a maximum of 11) |
| Psychosocial interventions for supporting women to stop smoking in pregnancy (Chamberlain 2017) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Pharmacological interventions for promoting smoking cessation during pregnancy (Coleman 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | NA | No | 8 |
| Giving women their own case notes to carry during pregnancy (Brown 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | NA | No | 8 |
| Midwife‐led versus other models of care for childbearing women (Sandall 2016) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Traditional birth attendant training for improving health behaviours and pregnancy outcomes (Sibley 2012) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | NA | Yes | 9 |
| Alternative versus standard packages of antenatal care for low‐risk pregnancy (Dowswell 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Group versus conventional antenatal care for women (Catling 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 |
| Diuretics for preventing pre‐eclampsia (Churchill 2007) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | NA | No | 8 |
| Nitric oxide for preventing pre‐eclampsia and its complications (Meher 2007) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | NA | No | 8 |
| Progesterone for preventing pre‐eclampsia and its complications (Meher 2006) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | NA | No | 8 |
| Antioxidants for preventing pre‐eclampsia (Rumbold 2008) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Altered dietary salt for preventing pre‐eclampsia, and its complications (Duley 2005) | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | NA | No | 7 |
| Community‐based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes (Lassi 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Screening for gestational diabetes mellitus based on different risk profiles and settings for improving maternal and infant health (Tieu 2017) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | Yes | 10 |
| Combined diet and exercise interventions for preventing gestational diabetes mellitus (Shepherd 2017) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 |
| Screening and subsequent management for thyroid dysfunction pre‐pregnancy and during pregnancy for improving maternal and infant health (Spencer 2015) | Yes | yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 10 |
| Treating periodontal disease for preventing adverse birth outcomes in pregnant women (Iheozor‐Ejiofor 2017) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| Use of biochemical tests of placental function for improving pregnancy outcome (Heazell 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 |
| AMSTAR: A Measurement Tool to Assess Reviews | ||||||||||||
* criteria for AMSTAR:
1. A prior design
2. Duplicate selection and extraction
3. Comprehensive literature search
4. Searched for reports regardless of publication type or language
5. Excluded/included list provided
6. Characteristics of included studies provided
7. Quality assessment of included studies assessed and presented
8. Quality used appropriately in formulating conclusions
9. Methods used to combine studies appropriate
10. Publication bias assessed
11. Conflict of interests stated
NA = not applicable
8. AMSTAR ratings for each Cochrane systematic review: screening and management of fetal growth and well‐being.
| Review titles | 1. * | 2.* | 3.* | 4.* | 5.* | 6.* | 7.* | 8.* | 9.* | 10.* | 11.* | Total score (out of a maximum of 11) |
| Ultrasound for fetal assessment in early pregnancy (Whitworth 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Routine ultrasound in late pregnancy (after 24 weeks' gestation) (Bricker 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | 8 |
| Fetal movement counting for assessment of fetal wellbeing (Mangesi 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | NA | No | 8 |
| Fetal and umbilical Doppler ultrasound in normal pregnancy (Alfirevic 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 9 |
| Utero‐placental Doppler ultrasound for improving pregnancy outcome (Stampalija 2010) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | NA | No | 8 |
| Antenatal cardiotocography for fetal assessment (Grivell 2015) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | NA | No | 8 |
| Symphysial fundal height (SFH) measurement in pregnancy for detecting abnormal fetal growth (Robert Peter 2015) | Yes | Yes | Yes | Yes | NA | Yes | Yes | No | Yes | NA | No | 7 |
| AMSTAR: A Measurement Tool to Assess Reviews | ||||||||||||
* criteria for AMSTAR:
1. A prior design
2. Duplicate selection and extraction
3. Comprehensive literature search
4. Searched for reports regardless of publication type or language
5. Excluded/included list provided
6. Characteristics of included studies provided
7. Quality assessment of included studies assessed and presented
8. Quality used appropriately in formulating conclusions
9. Methods used to combine studies appropriate
10. Publication bias assessed
11. Conflict of interests stated
NA = not applicable
9. Results by individual review: nutritional interventions.
| Folic acid supplementation (De‐Regil 2015) | |||
| Comparison | Outcome | No. of studies, no. of women | Results |
| Supplementation with any folate vs no intervention, placebo or other micronutrients without folate | Stillbirth | 4 studies, 6597 women | RR 1.05, 95% CI 0.54 to 2.05, no evidence of a difference GRADEa: very low |
| Supplementation with any folate vs no intervention, placebo or other micronutrients without folate | LBW | 2 studies, 5048 women | RR 1.13, 95% CI 0.84 to 1.52, no evidence of a difference |
| Supplementation with any folate vs no intervention, placebo or other micronutrients without folate | SGA | Outcome not reported | |
| Supplementation with any folic acid vs no intervention, placebo or other micronutrients without folate | NICU admission | Outcome not reported | |
| Vitamin A supplementation (McCauley 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Vitamin A alone vs placebo or no treatment | Stillbirth | 2 studies, 122,850 women | RR 1.04, 95% CI 0.98 to 1.10, evidence of no difference GRADEa: moderate |
| Vitamin A alone vs placebo or no treatment | Perinatal death | 1 study, 76,176 women | RR 1.01, 95% CI 0.95 to 1.07, evidence of no difference GRADEb: high |
| Vitamin A alone vs placebo or no treatment | LBW | 4 studies, 14,599 women | RR 1.02, 95% CI 0.89 to 1.16, no evidence of a difference |
| Vitamin A alone vs placebo or no treatment | SGA | Outcome not reported | |
| Vitamin A alone vs placebo or no treatment | NICU admission | Outcome not reported | |
| Vitamin A with other micronutrients vs micronutrient supplements without vitamin A | Stillbirth | 2 studies, 866 women | RR 1.41, 95% CI 0.57 to 3.47, no evidence of a difference GRADEa: very low |
| Vitamin A with other micronutrients vs micronutrient supplements without vitamin A | Perinatal death | 1 study, 179 women | RR 0.51, 95% CI 0.10 to 2.69, no evidence of a difference GRADEa: moderate |
| Vitamin A with other micronutrients vs micronutrient supplements without vitamin A | LBW | 1 study, 594 women | RR 0.67, 95% CI 0.47 to 0.96 (P = 0.03), reduction in LBW for women receiving vitamin A with other micronutrients |
| Vitamin A with other micronutrients vs micronutrient supplements without vitamin A | SGA | Outcome not reported | |
| Vitamin A with other micronutrients vs micronutrient supplements without vitamin A | NICU admission | Outcome not reported | |
| Vitamin C supplementation (Rumbold 2015a) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Vitamin C supplementation alone or in combination with other supplements (all trials) | Stillbirth | 11 studies, 20,038 women | RR 1.15, 95% CI 0.89 to 1.49, no evidence of a difference GRADEb: moderate |
| Vitamin C supplementation alone or in combination with other supplements (all trials) | Perinatal death | 7 studies, 17,271 women | RR 1.07, 95% CI 0.77 to 1.49, no evidence of a difference GRADEa: very low |
| Vitamin C supplementation alone or in combination with other supplements (all trials) | IUGR | 12 studies, 20,361 women | RR 0.98, 95% CI 0.91 to 1.06, evidence of no difference |
| Vitamin C supplementation alone or in combination with other supplements (all trials) | NICU admission | 9 studies, 18,371 women | RR 1.02, 95% CI 0.96 to 1.09, evidence of no difference |
| Vitamin C supplementation alone or in combination with other supplements (all trials) | LBW | Outcome not reported | |
| Vitamin C supplementation alone or in combination with other supplements (all trials) | SGA | Outcome not reported | |
| Vitamin D supplementation (Palacios 2019) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Vitamin D alone vs no treatment/placebo (no vitamins or minerals) | Stillbirth | 3 studies, 584 women | RR 0.35, 95% CI 0.06 to 1.98, no evidence of a difference GRADEa: very low |
| Vitamin D alone vs no treatment/placebo (no vitamins or minerals) | LBW | 5 studies, 697 women | RR 0.55, 95% CI 0.35 to 0.87 (P = 0.01), reduction in LBW for women receiving vitamin D alone |
| Vitamin D alone vs no treatment/placebo (no vitamins or minerals) | SGA | Outcome not reported | |
| Vitamin D alone vs no treatment/placebo (no vitamins or minerals) | NICU admission | Outcome not reported | |
| Vitamin E supplementation (Rumbold 2015b) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Any vitamin E supplementation | Stillbirth | 9 studies, 19,023 women | RR 1.17, 95% CI 0.88 to 1.56, no evidence of a difference GRADEb: moderate |
| Any vitamin E supplementation | Perinatal death | 6 studies, 16,923 women | RR 1.09, 95% CI 0.77 to 1.54, no evidence of a difference GRADEa: very low |
| Any vitamin E supplementation | IUGR | 11 studies, 20,202 women | RR 0.98, 95% CI 0.91 to 1.06, evidence of no difference |
| Any vitamin E supplementation | NICU admission | 8 studies, 17,594 women | RR 1.01, 95% CI 0.95 to 1.08, evidence of no difference |
| Any vitamin E supplementation | LBW | Outcome not reported | |
| Any vitamin E supplementation | SGA | Outcome not reported | |
| Vitamin supplementation for preventing miscarriage (Balogun 2016) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Multivitamin vs control | Stillbirth | 1 study, 5021 women | RR 0.83, 95% CI 0.58 to 1.17, no evidence of a difference GRADEa: low |
| Multivitamin vs control | Total fetal loss | 1 study, 5021 women | RR 0.83, 95% CI 0.58 to 1.17, no evidence of a difference GRADEa: low |
| Multivitamin plus vitamin E vs multivitamin without vitamin E or control | Stillbirth | 1 study, 823 women | RR 0.88, 95% CI 0.39 to 1.98, no evidence of a difference GRADEa: low |
| Multivitamin plus vitamin E vs multivitamin without vitamin E or control | Total fetal loss | 1 study, 823 women | RR 0.92, 95% CI 0.46 to 1.83, no evidence of a difference GRADEa: low |
| Folic acid plus iron vs iron | Stillbirth | 1 study, 75 women | RR 0.38, 95% CI 0.02 to 9.03, no evidence of a difference GRADEa: low |
| Folic acid plus iron vs iron | Total fetal loss | 1 study, 75 women | RR 0.23, 95% CI 0.01 to 4.59, no evidence of a difference GRADEa: low |
| Folic acid plus iron and antimalarials vs iron and antimalarials | Total fetal loss | 1 study, 160 women | RR 13.0, 95% CI 0.74 to 226.98, no evidence of a difference GRADEa: low |
| Any comparison | LBW | Outcome not reported | |
| Any comparison | SGA | Outcome not reported | |
| Any comparison | NICU admission | Outcome not reported | |
| Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy (Hofmeyr 2019) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Calcium supplementation vs placebo (before and/or early pregnancy only) | Stillbirth | 1 study, 579 women | RR 0.78, 95% CI 0.48 to 1.27, no evidence of a difference GRADEa: low |
| Calcium supplementation vs placebo (before and/or early pregnancy only) | Pregnancy loss, stillbirth or neonatal death before discharge | 1 study, 632 women | RR 0.82, 95% CI 0.61 to 1.10, no evidence of a difference GRADEb: low |
| Calcium supplementation vs placebo (before and/or early pregnancy only) | Perinatal death and/or NICU admission for > 24 h | 1 study, 508 women | RR 1.11, 95% CI 0.77 to 1.60, no evidence of a difference GRADEb: low |
| Calcium supplementation vs placebo (before and/or early pregnancy only) | LBW | Outcome not reported | |
| Calcium supplementation vs placebo (before and/or early pregnancy only) | SGA | Outcome not reported | |
| Calcium supplementation (preventing hypertensive disorders) (Hofmeyr 2018) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium | Stillbirth or death before discharge from hospital | 11 studies, 15,665 women | RR 0.90, 95% CI 0.74 to 1.09, no evidence of a difference GRADEa: very low |
| Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium | LBW | 9 studies, 14,883 women | RR 0.85, 95% CI 0.72 to 1.01 (P = 0.06), evidence of no difference |
| Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium | SGA | 4 studies, 13,615 women | RR 1.05, 95% CI 0.86 to 1.29, no evidence of a difference |
| Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium | NICU admission | 4 studies, 13,406 women | RR 1.05, 95% CI 0.94 to 1.18, no evidence of a difference |
| Low‐dose calcium supplementation (< 1 g/d) with or without co‐supplements vs placebo or no treatment | Stillbirth or death before discharge from hospital | 5 studies, 1025 women | RR 0.48, 95% CI 0.14 to 1.67, no evidence of a difference GRADEa: very low |
| Low‐dose calcium supplementation (< 1 g/d) with or without co‐supplements vs placebo or no treatment | LBW | 2 studies, 134 women | RR 0.20, 95% CI 0.05 to 0.88 (P = 0.033), reduction in LBW for women receiving low‐dose calcium supplementation during pregnancy |
| Low‐dose calcium supplementation (< 1 g/d) with or without co‐supplements vs placebo or no treatment | SGA | 4 studies, 854 women | RR 0.81, 95% CI 0.54 to 1.21, no evidence of a difference |
| Low‐dose calcium supplementation (< 1 g/d) with or without co‐supplements vs placebo or no treatment | NICU admission | 1 study, 422 women | RR 0.44, 95% CI 0.20 to 0.99 (P = 0.047), reduction in NICU admission for women receiving low‐dose calcium supplementation during pregnancy |
| Calcium supplementation (other than for preventing or treating hypertension) (Buppasiri 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Calcium supplementation vs placebo or no treatment | Stillbirth or fetal death | 6 studies, 15,269 women | RR 0.91, 95% CI 0.72 to 1.14, no evidence of a difference GRADEa: low |
| Calcium supplementation vs placebo or no treatment | LBW | 6 studies, 14,162 women | RR 0.93, 95% CI 0.81 to 1.07, evidence of no difference |
| Calcium supplementation vs placebo or no treatment | IUGR | 6 studies, 1701 women | RR 0.83, 95% CI 0.61 to 1.13, no evidence of a difference |
| Calcium supplementation vs placebo or no treatment | NICU admission | 4 studies, 14,062 women | RR 1.05, 95% CI 0.94 to 1.18, evidence of no difference |
| Iodine supplementation (Harding 2017) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Any supplement containing iodine vs same supplement without iodine or no intervention/placebo | Perinatal death | 2 studies, 457 women | RR 0.66, 95% CI 0.42 to 1.03, no evidence of a difference GRADEb: low |
| Any supplement containing iodine vs same supplement without iodine or no intervention/placebo | LBW | 2 studies, 377 women | RR 0.56, 95% CI 0.26 to 1.23, no evidence of a difference |
| Any supplement containing iodine vs same supplement without iodine or no intervention/placebo | SGA | 2 studies, 377 women | RR 1.26, 95% CI 0.77 to 2.05, no evidence of a difference |
| Any supplement containing iodine vs same supplement without iodine or no intervention/placebo | Stillbirth | Outcome not reported | |
| Any supplement containing iodine vs same supplement without iodine or no intervention/placebo | NICU admission | Outcome not reported | |
| Magnesium supplementation (Makrides 2014) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Magnesium supplementation vs no magnesium | Stillbirth | 4 studies, 5526 women | RR 0.73, 95% CI 0.43 to 1.25, no evidence of a difference GRADEa: low |
| Magnesium supplementation vs no magnesium | LBW | 5 studies, 5577 women | RR 0.95, 95% CI 0.83 to 1.09, evidence of no difference |
| Magnesium supplementation vs no magnesium | SGA | 3 studies, 1291 women | RR 0.76, 95% CI 0.54 to 1.07, no evidence of a difference |
| Magnesium supplementation vs no magnesium | NICU admission | 3 studies, 1435 women | RR 0.74, 95% CI 0.50 to 1.11, no evidence of a difference |
| Zinc supplementation (Ota 2015b) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Zinc supplementation vs no zinc (with or without placebo) | Stillbirth or neonatal death | 8 studies, 5100 women | RR 1.12, 95% CI 0.86 to 1.46, no evidence of a difference GRADEb: low |
| Zinc supplementation vs no zinc (with or without placebo) | LBW | 14 studies, 5643 women | RR 0.93, 95% CI 0.78 to 1.12, evidence of no difference |
| Zinc supplementation vs no zinc (with or without placebo) | SGA | 8 studies, 4252 women | RR 1.02, 95% CI 0.94 to 1.11, evidence of no difference |
| Zinc supplementation vs no zinc (with or without placebo) | NICU admission | Outcome not reported | |
| Multiple micronutrient supplementation (Keats 2019) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Multiple micronutrients with iron and folic acid vs iron with or without folic acid | Stillbirth | 17 studies, 97,927 women | RR 0.95, 95% CI 0.86 to 1.04, evidence of no difference GRADEb: high |
| Multiple micronutrients with iron and folic acid vs iron with or without folic acid | Perinatal mortality | 15 studies, 63,922 women | RR 1.00, 95% CI 0.90 to 1.11, evidence of no difference GRADEb: high |
| Multiple micronutrients with iron and folic acid vs iron with or without folic acid | LBW | 18 studies, 68,801 women | RR 0.88, 95% CI 0.85 to 0.91 (P < 0.00001), reduction in LBW for women receiving multiple micronutrient supplementation vs iron with or without folic acid |
| Multiple micronutrients with iron and folic acid vs iron with or without folic acid | SGA | 17 studies, 57,348 women | RR 0.92, 95% CI 0.88 to 0.97 (P = 0), reduction in SGA for women receiving multiple micronutrient supplementation vs iron with or without folic acid |
| Multiple micronutrients with iron and folic acid vs iron with or without folic acid | NICU admission | Outcome not reported | |
| Energy and protein intake (Ota 2015a) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Nutritional advice during pregnancy | Stillbirth | 1 study, 431 women | RR 0.37, 95% CI 0.07 to 1.90, no evidence of a difference GRADEb: low |
| Nutritional advice during pregnancy | LBW | 1 study, 300 women | RR 0.04, 95% CI 0.01 to 0.14 (P < 0.00001), reduction in LBW for women receiving nutritional advice during pregnancy |
| Nutritional advice during pregnancy | SGA | 1 study, 404 women | RR 0.97, 95% CI 0.45 to 2.11, no evidence of a difference |
| Nutritional advice during pregnancy | NICU admission | Outcome not reported | |
| Balanced protein/energy supplementation in pregnancy | Stillbirth | 5 studies, 3408 women | RR 0.60, 95% CI 0.39 to 0.94 (P = 0.024), reduction in stillbirth for women receiving balanced protein/energy supplementation in pregnancy GRADEb: moderate |
| Balanced protein/energy supplementation in pregnancy | SGA | 7 studies, 4408 women | RR 0.79, 95% CI 0.69 to 0.90 (P = 0.0004), reduction in SGA for women receiving balanced protein/energy supplementation in pregnancy |
| Balanced protein/energy supplementation in pregnancy | LBW | Outcome not reported | |
| Balanced protein/energy supplementation in pregnancy | NICU admission | Outcome not reported | |
| High protein supplementation in pregnancy | Stillbirth | 1 study, 529 women | RR 0.81, 95% CI 0.31 to 2.15, no evidence of a difference GRADEb: low |
| High protein supplementation in pregnancy | SGA | 1 study, 505 women | RR 1.58, 95% CI 1.03 to 2.41, (P = 0.04), increase in SGA for women receiving high protein supplementation during pregnancy |
| High protein supplementation in pregnancy | LBW | Outcome not reported | |
| High protein supplementation in pregnancy | NICU admission | Outcome not reported | |
| Isocaloric balanced protein supplementation in pregnancy | Stillbirth | Outcome not reported | |
| Isocaloric balanced protein supplementation in pregnancy | LBW | Outcome not reported | |
| Isocaloric balanced protein supplementation in pregnancy | SGA | Outcome not reported | |
| Isocaloric balanced protein supplementation in pregnancy | NICU admission | Outcome not reported | |
| Omega‐3 fatty acid addition during pregnancy (Middleton 2018) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Omega‐3 vs no omega‐3 | Stillbirth | 16 studies, 7880 women | RR 0.94, 95% CI 0.62 to 1.42, no evidence of a difference GRADEa: very low |
| Omega‐3 vs no omega‐3 | Perinatal death | 10 studies, 7416 women | RR 0.75, 95% CI 0.54 to 1.03, no evidence of a difference GRADEb: low |
| Omega‐3 vs no omega‐3 | LBW | 15 studies, 8449 women | RR 0.90, 95 % CI 0.82 to 0.99 (P = 0.034), decrease in LBW for women receiving omega‐3 fatty acids during pregnancy |
| Omega‐3 vs no omega‐3 | SGA/IUGR | 8 studies, 6907 women | RR 1.01, 95% CI 0.90 to 1.13, evidence of no difference |
| Omega‐3 vs no omega‐3 | NICU admission | 9 studies, 6920 women | RR 0.92, 95% CI 0.83 to 1.03, evidence of no difference |
| Lipid‐based nutrient supplements (LNS) (Das 2018) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Lipid‐based nutrient supplements vs iron folic acid | Stillbirth | 3 studies, 5575 women | RR 1.14, 95% CI 0.52 to 2.48, no evidence of a difference GRADEa: low |
| Lipid‐based nutrient supplements vs iron folic acid | LBW | 3 studies, 4826 women | RR 0.87, 95% CI 0.72 to 1.05, possible reduction, but also slight increase |
| Lipid‐based nutrient supplements vs iron folic acid | SGA | 3 studies, 4823 women | RR 0.94, 95% CI 0.89 to 0.99 (P = 0.015), decrease in SGA for women receiving LNS during pregnancy |
| Lipid‐based nutrient supplements vs iron folic acid | NICU admission | Outcome not reported | |
| CI: confidence interval; CTG: cardiotocography; IUGR: interuterine growth restriction; LBW: low birthweight; LNS: lipid‐based nutrient supplements; NICU: neonatal intensive care unit; RR: risk ratio; SGA: small‐for‐gestational age | |||
aGRADE assessed by review overview authors because it was not reported in the original review; bGRADE rating reported in the original review.
10. Results by individual review: prevention and management of infection.
| Insecticide‐treated nets for preventing malaria (Gamble 2006) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Insecticide‐treated nets vs no nets (all) | Fetal loss | 5 studies | RR 0.68, 95% CI 0.48 to 0.98 (P = 0.04), reduction in fetal loss for women receiving intervention of insecticide‐treated nets GRADEa: low |
| Insecticide‐treated nets vs no nets (First or second pregnancy) | Fetal loss | 4 studies | RR 0.67, 95% CI 0.47 to 0.97 (P = 0.03), reduction in fetal loss for first or second pregnancy for women receiving intervention of insecticide‐treated nets GRADEa: low |
| Insecticide‐treated nets vs no nets (Fifth or greater pregnancy) | Fetal loss | 1 study | RR 1.02, 95% CI 0.17 to 6.23, no evidence of a difference GRADEa: very low |
| Insecticide‐treated nets vs no nets (all) | LBW | 4 studies | RR 0.80, 95% CI 0.64 to 1.00 (P = 0.05), a possible reduction |
| Insecticide‐treated nets vs no nets (First or second pregnancy) | LBW | 3 studies | RR 0.77, 95% CI 0.61 to 0.98 (P = 0.03), reduction in LBW for first or second pregnancy for women receiving intervention of insecticide‐treated nets |
| Insecticide‐treated nets vs no nets (Fifth or greater pregnancy) | LBW | 1 study | RR 1.12, 95% CI 0.56 to 2.24, no evidence of a difference |
| Insecticide‐treated nets vs no nets | SGA | Outcome not reported | |
| Insecticide‐treated nets vs no nets | NICU admission | Outcome not reported | |
| Drugs for preventing malaria (Radeva‐Petrova 2014) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Preventive antimalarials vs placebo/no intervention (women of all parity groups) | Stillbirth | 5 studies, 7130 women | RR 1.02, 95% CI 0.76 to 1.36, evidence of no difference GRADEb: moderate |
| Preventive antimalarials vs placebo/no intervention (women in first or second pregnancy) | Stillbirth | 4 studies, 2703 women | RR 0.97, 95% CI 0.63 to 1.49, no evidence of a difference GRADEb: low |
| Preventive antimalarials vs placebo/no intervention (women of all parity groups) | Perinatal death | 4 studies, 5216 women | RR 1.24, 95% CI 0.94 to 1.63, evidence of no difference GRADEb: moderate |
| Preventive antimalarials vs placebo/no intervention (women in first or second pregnancy) | Perinatal death | 2 studies, 1620 women | RR 0.73, 95% CI 0.54 to 1.00 (P = 0.05), no evidence of a difference GRADEb: low |
| Preventive antimalarials vs placebo/no intervention (women of all parity groups) | LBW | 4 studies, 3644 women | RR 1.06, 95% CI 0.89 to 1.27, no evidence of a difference |
| Preventive antimalarials vs placebo/no intervention (women in first or second pregnancy) | LBW | 10 studies, 3619 women | RR 0.73, 95% CI 0.61 to 0.87 (P = 0.00065), reduction in LBW for women receiving preventive antimalarials |
| Preventive antimalarials vs placebo/no intervention | SGA | Outcome not reported | |
| Preventive antimalarials vs placebo/no intervention | NICU admission | Outcome not reported | |
| CI: confidence interval; IUGR: interuterine growth restriction; LBW: low birthweight; NICU: neonatal intensive care unit; RR: risk ratio; SGA: small‐for‐gestational age | |||
aGRADE assessed by review overview authors because it was not reported in the original review; bGRADE rating reported in the original review.
11. Results by individual review: prevention, detection, and management of other morbidities.
| Smoking cessation (Chamberlain 2017; Coleman 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Interventions for smoking cessation in pregnancy vs control | Stillbirth | 8 studies, 6170 women | RR 1.20, 95% CI 0.76 to 1.90, evidence of no difference GRADEa: high |
| Interventions for smoking cessation in pregnancy vs control | Perinatal death | 4 studies, 4465 women | RR 1.13, 95% CI 0.72 to 1.77, evidence of no difference GRADEa: moderate |
| Interventions for smoking cessation in pregnancy vs control | LBW | 18 studies, 9402 women | RR 0.83, 95% CI 0.72 to 0.94 (P = 0.0037), reduction in LBW for women receiving interventions for smoking cessation |
| Interventions for smoking cessation in pregnancy vs control | NICU admission | 8 studies, 2100 women | RR 0.78, 95% CI 0.61 to 0.98 (P = 0.035), reduction in NICU admission for women receiving interventions for smoking cessation |
| Interventions for smoking cessation in pregnancy vs control | SGA | Outcome not reported | |
| Nicotine replacement therapy vs control | Stillbirth | 4 studies, 1777 women | RR 1.24, 95% CI 0.54 to 2.84, no evidence of a difference GRADEa: low |
| Nicotine replacement therapy vs control | LBW | 6 studies, 2037 women | RR 0.74, 95% CI 0.41 to 1.34, no evidence of a difference |
| Nicotine replacement therapy vs control | NICU admission | 4 studies, 1756 women | RR 0.90, 95% CI 0.64 to 1.27, no evidence of a difference |
| Nicotine replacement therapy vs control | SGA | Outcome not reported | |
| Women carrying their own case notes (Brown 2015) | |||
| Comparison | Outcome | No. of studies (no. women) | Results |
| Case notes vs control | Stillbirth or neonatal death | 2 studies, 713 women | RR 1.00, 95% CI 0.99 to 1.01, evidence of no difference GRADEb: moderate |
| Case notes vs control | NICU admission | 1 study, 501 women | RR 1.18, 95% CI 0.36 to 3.83, no evidence of a difference |
| Case notes vs control | LBW | Outcome not reported | |
| Case notes vs control | SGA | Outcome not reported | |
| Midwife‐led care (Sandall 2016) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Midwife‐led vs other models of care for childbearing women and their infants | Fetal loss/neonatal death before 24 weeks | 11 studies, 15,645 women | RR 0.81, 95% CI 0.67 to 0.98 (P = 0.03), reduction in fetal loss/neonatal death before 24 weeks for women receiving midwife‐led care GRADEa: high |
| Midwife‐led vs other models of care for childbearing women and their infants | Fetal loss/neonatal death equal to/after 24 weeks | 12 studies, 17,359 women | RR 1.00, 95% CI 0.67 to 1.49, no evidence of a difference GRADEa: moderate |
| Midwife‐led vs other models of care for childbearing women and their infants | Overall fetal loss and neonatal death | 13 studies, 17,561 women | RR 0.84, 95% CI 0.71 to 0.99 (P = 0.04), reduction in overall fetal loss/neonatal death for women receiving midwife‐led care GRADEb: high |
| Midwife‐led vs other models of care for childbearing women and their infants | LBW | 7 studies, 11,458 women | RR 0.96, 95% CI 0.82 to 1.13, evidence of no difference |
| Midwife‐led vs other models of care for childbearing women and their infants | NICU admission | 13 studies, 17,561 women | RR 0.90, 95% CI 0.78 to 1.04, evidence of no difference |
| Midwife‐led vs other models of care for childbearing women and their infants | SGA | Outcome not reported | |
| Traditional birth attendant training (Sibley 2012) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Trained vs untrained traditional birth attendants | Stillbirth | 1 study, 18,699 women | OR 0.69, 95% CI 0.57 to 0.83 (P = 0.00011), reduction in stillbirth for women receiving care from trained traditional birth attendants GRADEa: moderate |
| Trained vs untrained traditional birth attendants | Perinatal death | 1 study, 18,699 women | OR 0.70, 95% CI 0.59 to 0.83 (P < 0.0001), reduction in perinatal death for women receiving care from trained traditional birth attendants GRADEa: moderate |
| Trained vs untrained traditional birth attendants | LBW | Outcome not reported | |
| Trained vs untrained traditional birth attendants | SGA | Outcome not reported | |
| Trained vs untrained traditional birth attendants | NICU admission | Outcome not reported | |
| Additionally trained vs trained traditional birth attendants | Stillbirth | 2 studies, 27,594 women | RR 0.99, 95% CI 0.76 to 1.28, evidence of no difference GRADEa: moderate |
| Additionally trained vs trained traditional birth attendants | Perinatal death | 1 study, 24,007 women | OR 0.79, 95% CI 0.61 to 1.02, evidence of no difference GRADEa: moderate |
| Additionally trained vs trained traditional birth attendants | LBW | Outcome not reported | |
| Additionally trained vs trained traditional birth attendants | SGA | Outcome not reported | |
| Additionally trained vs trained traditional birth attendants | NICU admission | Outcome not reported | |
| Alternative vs standard packages of antenatal care (Dowswell 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Reduced number of antenatal care visits/goal‐oriented vs standard antenatal care visits | Perinatal death | 5 studies, 56431 women | RR 1.14, 95% CI 1.00 to 1.31 (P = 0.05), increase in perinatal death for women with reduced number of antenatal care visits GRADEb: moderate |
| Reduced number of antenatal care visits/goal‐oriented vs standard antenatal care visits | LBW | 6 studies | RR 1.04, 95% CI 0.97 to 1.11, evidence of no difference |
| Reduced number of antenatal care visits/goal‐oriented vs standard antenatal care visits | SGA | 4 studies, 43,045 | RR 0.99, 95% CI 0.91 to 1.09, evidence of no difference |
| Reduced number of antenatal care visits/goal‐oriented vs standard antenatal care visits | NICU admission | 5 studies, 43048 women | RR 0.89, 95% CI 0.79 to 1.02, evidence of no difference |
| Group vs conventional antenatal care (Catling 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Group antenatal care vs individual antenatal care | Perinatal death | 3 studies, 1943 women | RR 0.63, 95% CI 0.32 to 1.25, no evidence of a difference GRADEb: low |
| Group antenatal care vs individual antenatal care | LBW | 3 studies, 1935 women | RR 0.92, 95% CI 0.68 to 1.23, no evidence of a difference |
| Group antenatal care vs individual antenatal care | SGA | 2 studies, 1473 women | RR 0.92, 95% CI 0.68 to 1.24, no evidence of a difference |
| Group antenatal care vs individual antenatal care | NICU admission | 2 studies, 1315 women | RR 1.48, 95% CI 0.63 to 3.45, no evidence of a difference |
| Diuretics for preventing pre‐eclampsia (Churchill 2007) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Diuretic vs placebo or no treatment | Stillbirth | 5 studies, 1836 women | RR 0.60, 95% CI 0.27 to 1.34, no evidence of a difference GRADEa: low |
| Diuretic vs placebo or no treatment | Perinatal death | 5 studies, 1836 women | RR 0.72, 95% CI 0.40 to 1.27, no evidence of a difference GRADEa: low |
| Diuretic vs placebo or no treatment | SGA | 1 study, 20 women | Not estimable |
| Diuretic vs placebo or no treatment | LBW | Outcome not reported | |
| Diuretic vs placebo or no treatment | NICU admission | Outcome not reported | |
| Nitric oxide for preventing pre‐eclampsia and its complications (Meher 2007) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Nitric oxide vs placebo/no intervention | Perinatal or neonatal death | 2 studies, 114 women | RR 0.25, 95% CI 0.03 to 2.34, no evidence of a difference GRADEa: low |
| Nitric oxide vs placebo/no intervention | SGA | 2 studies, 108 women | RR 0.78, 95% CI 0.36 to 1.70, no evidence of a difference |
| Nitric oxide vs placebo/no intervention | NICU admission | 1 study, 68 women | RR 1.05, 95% CI 0.25 to 4.35, no evidence of a difference |
| Nitric oxide vs placebo/no intervention | LBW | Outcome not reported | |
| Progesterone for preventing pre‐eclampsia and its complications (Meher 2006) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Progesterone vs placebo/no treatment | Fetal or neonatal death | 4 studies | RR 1.34, 95% CI 0.78 to 2.31, no evidence of a difference GRADEa: very low |
| Progesterone vs placebo/no treatment | SGA | 1 study, 168 women | RR 0.83, 95% CI 0.19 to 3.57, no evidence of a difference |
| Progesterone vs placebo/no treatment | NICU admission | 1 study | RR 1.06, 95% CI 0.83 to 1.35, no evidence of a difference |
| Progesterone vs placebo/no treatment | LBW | Outcome not reported | |
| Antioxidants for preventing pre‐eclampsia (Rumbold 2008) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Any antioxidants vs control or placebo | Miscarriage or stillbirth | 4 studies, 5144 women | RR 1.32, 95% CI 0.92 to 1.90, no evidence of a difference GRADEa: low |
| Any antioxidants vs control or placebo | SGA | 5 studies, 5271 women | RR 0.83, 95% CI 0.62 to 1.11, no evidence of a difference |
| Any antioxidants vs control or placebo | NICU admission | 1 study, 2714 women | RR 1.11, 95% CI 0.95 to 1.29, no evidence of a difference |
| Any antioxidants vs control or placebo | LBW | Outcome not reported | |
| Altered dietary salt (Duley 2005) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Low vs normal salt intake in pregnancy | Perinatal death | 2 studies, 409 women | RR 1.92, 95% CI 0.18 to 21.03, no evidence of a difference GRADEa: moderate |
| Low vs normal salt intake in pregnancy | SGA | 1 study, 242 women | RR 1.50, 95% CI 0.73 to 3.07, no evidence of a difference |
| Low vs normal salt intake in pregnancy | NICU admission | 1 study, 361 women | RR 0.98, 95% CI 0.69 to 1.40, no evidence of a difference |
| Low vs normal salt intake in pregnancy | LBW | Outcome not reported | |
| Community‐based intervention packages (Lassi 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Community‐based intervention vs control | Stillbirth | 15 studies, 201,181 women | RR 0.81, 95% CI 0.73 to 0.91 (P = 0.00021), reduction in stillbirth for women receiving community‐based intervention GRADEa: low |
| Community‐based intervention vs control | Perinatal mortality | 17 studies, 282,327 women | RR 0.78, 95% CI 0.70 to 0.86 (P < 0.00001), reduction in perinatal mortality for women receiving community‐based intervention GRADEa: low |
| Community‐based intervention vs control | LBW | Outcome not reported | |
| Community‐based intervention vs control | SGA | Outcome not reported | |
| Community‐based intervention vs control | NICU admission | Outcome not reported | |
| Screening for gestational diabetes (Tieu 2017) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Primary care screening vs secondary care screening | Stillbirth | 1 study, 690 women | RR 1.10, 95% CI 0.10 to 12.12, no evidence of a difference GRADEa: low |
| Primary care screening vs secondary care screening | Perinatal death | 1 study, 690 women | RR 1.10, 95% CI 0.10 to 12.12, no evidence of a difference GRADEb: very low |
| Primary care screening vs secondary care screening | NICU admission | 1 study, 690 women | RR 0.99, 95% CI 0.58 to 1.69, no evidence of a difference |
| Primary care screening vs secondary care screening | LBW | Outcome not reported | |
| Primary care screening vs secondary care screening | SGA | Outcome not reported | |
| Diet and exercise for preventing gestational diabetes (Shepherd 2017) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Combined diet and exercise interventions vs standard care | Stillbirth | 5 studies, 4783 women | RR 0.69, 95% CI 0.35 to 1.36, no evidence of a difference GRADEa: very low |
| Combined diet and exercise interventions vs standard care | Perinatal death | 2 studies, 3757 women | RR 0.82, 95% CI 0.42 to 1.63, no evidence of a difference GRADEb: low |
| Combined diet and exercise interventions vs standard care | SGA | 6 studies, 2434 women | RR 1.20, 95% CI 0.95 to 1.52, no evidence of a difference |
| Combined diet and exercise interventions vs standard care | NICU admission | 4 studies, 4549 women | RR 1.03, 95% CI 0.93 to 1.14, evidence of no difference |
| Combined diet and exercise interventions vs standard care | LBW | Outcome not reported | |
| Screening and management for thyroid dysfunction (Spencer 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Universal screening vs case finding in pregnancy for any thyroid dysfunction | Fetal and neonatal death | 1 study, 4516 women | RR 0.92, 95% CI 0.42 to 2.02, no evidence of a difference GRADEb: moderate |
| Universal screening vs case finding in pregnancy for any thyroid dysfunction | LBW | 1 study, 4516 women | RR 0.97, 95% CI 0.74 to 1.27, no evidence of a difference |
| Universal screening vs case finding in pregnancy for any thyroid dysfunction | NICU admission | 1 study, 4516 women | RR 1.04, 95% CI 0.81 to 1.34, no evidence of a difference |
| Universal screening vs case finding in pregnancy for any thyroid dysfunction | SGA | Outcome not reported | |
| Periodontal treatment (Iheozor‐Ejiofor 2017) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Periodontal treatment vs no treatment | Perinatal death | 7 studies, 5320 women | RR 0.85, 95% CI 0.51 to 1.43, no evidence of a difference GRADEb: very low |
| Periodontal treatment vs no treatment | LBW | 7 studies, 3470 women | RR 0.67, 95% CI 0.48 to 0.95 (P = 0.024), reduction in LBW for women receiving periodontal treatment |
| Periodontal treatment vs no treatment | SGA | 3 studies, 3610 women | RR 0.97, 95% CI 0.81 to 1.16, evidence of no difference |
| Periodontal treatment vs no treatment | NICU admission | Outcome not reported | |
| Periodontal treatment vs alternative periodontal treatment | Perinatal death | 2 studies, 855 women | RR 1.06, 95% CI 0.60 to 1.85, no evidence of a difference GRADEb: low |
| Periodontal treatment vs alternative periodontal treatment | LBW | 1 study, 756 women | RR 1.39, 95% CI 0.92 to 2.09, no evidence of a difference |
| Periodontal treatment vs alternative periodontal treatment | SGA | Outcome not reported | |
| Periodontal treatment vs alternative periodontal treatment | NICU admission | Outcome not reported | |
| Biochemical tests of placental function (Heazell 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Test of placental function vs standard care | Miscarriage or stillbirth | 2 studies, 740 women | RR 0.56, 95% CI 0.16 to 1.88, no evidence of a difference GRADEb: very low |
| Test of placental function vs standard care | SGA | 1 study, 118 women | RR 0.44, 95% CI 0.16 to 1.19, no evidence of a difference |
| Test of placental function vs standard care | NICU admission | 1 study, 118 women | RR 0.32, 95% CI 0.03 to 3.01, no evidence of a difference |
| Test of placental function vs standard care | LBW | Outcome not reported | |
| CI: confidence interval; IUGR: interuterine growth restriction; LBW: low birthweight; NICU: neonatal intensive care unit; OR: odds ratio; RR: risk ratio; SGA: small‐for‐gestational age | |||
aGRADE assessed by review overview authors because it was not reported in the original review; bGRADE rating reported in the original review.
12. Results by individual review: screening and management of fetal growth and well‐being.
| Ultrasound for fetal assessment in early pregnancy (Whitworth 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Routine/revealed vs selective/concealed ultrasound in early pregnancy | Perinatal death (all babies) | 10 studies, 35,735 women | RR 0.89, 95% CI 0.70 to 1.12, no evidence of a difference GRADEb: low |
| Routine/revealed vs selective/concealed ultrasound in early pregnancy | LBW | 8 study, 19,337 women | RR 1.04, 95% CI 0.82 to 1.33, no evidence of a difference |
| Routine/revealed vs selective/concealed ultrasound in early pregnancy | SGA | 3 studies, 17,105 women | RR 1.05, 95% CI 0.81 to 1.35, no evidence of a difference |
| Routine/revealed vs selective/concealed ultrasound in early pregnancy | NICU admission | 8 studies, 19,088 women | RR 0.95, 95% CI 0.88 to 1.02, evidence of no difference |
| Routine ultrasound in late pregnancy (Bricker 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Routine ultrasound > 24 weeks vs no/concealed/selective ultrasound > 24 weeks | Stillbirth | 6 studies, 28,107 women | RR 1.18, 95% CI 0.51 to 2.70, no evidence of a difference GRADEa: very low |
| Routine ultrasound > 24 weeks vs no/concealed/selective ultrasound > 24 weeks | Perinatal mortality | 8 studies, 30,675 women | RR 1.01, 95% CI 0.67 to 1.54, no evidence of a difference GRADEb: moderate |
| Routine ultrasound > 24 weeks vs no/concealed/selective ultrasound > 24 weeks | LBW | 3 studies, 4510 women | RR 0.92, 95% CI 0.71 to 1.18, no evidence of a difference |
| Routine ultrasound > 24 weeks vs no/concealed/selective ultrasound > 24 weeks | SGA | 4 studies, 20,293 women | RR 0.98, 95% CI 0.74 to 1.28, no evidence of a difference |
| Routine ultrasound > 24 weeks vs no/concealed/selective ultrasound > 24 weeks | NICU admission | 5 studies, 12,915 women | RR 1.01, 95% CI 0.91 to 1.14, evidence of no difference |
| Serial ultrasound and Doppler ultrasound vs selective ultrasound | Stillbirth | 1 study, 2834 women | RR 0.84, 95% CI 0.36 to 1.93, no evidence of a difference GRADEa: low |
| Serial ultrasound and Doppler ultrasound vs selective ultrasound | Perinatal mortality | 1 study, 2834 women | RR 0.59, 95% CI 0.30 to 1.17, no evidence of a difference. GRADEa: low |
| Serial ultrasound and Doppler ultrasound vs selective ultrasound | LBW | 1 study, 2834 women | RR 1.14, 95% CI 0.85 to 1.52, no evidence of a difference |
| Serial ultrasound and Doppler ultrasound vs selective ultrasound | SGA | 1 study, 2834 women | RR 1.36, 95% CI 1.10 to 1.68 (P = 0.0046), increase in SGA for women receiving serial ultrasound and Doppler ultrasound |
| Serial ultrasound and Doppler ultrasound vs selective ultrasound | NICU admission | 1 study, 2834 women | RR 0.95, 95% CI 0.69 to 1.30, no evidence of a difference |
| Fetal movement counting (Mangesi 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Fetal movement counting vs hormonal analysis | Stillbirth | 1 study, 1191 women | RR 3.19, 95% CI 0.13 to 78.20, no evidence of a difference GRADEa: very low |
| Fetal movement counting vs hormonal analysis | LBW | Outcome not reported | |
| Fetal movement counting vs hormonal analysis | SGA | Outcome not reported | |
| Fetal movement counting vs hormonal analysis | NICU admission | Outcome not reported | |
| Fetal and umbilical Doppler ultrasound (Alfirevic 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| All routine Doppler ultrasound vs no Doppler ultrasound (fetal/umbilical vessels only) | Stillbirth | 2 studies, 6877 women | RR 0.34, 95% CI 0.12 to 0.95 (P = 0.04), reduction in stillbirth for women who received fetal/umbilical vessels Doppler ultrasound GRADEb: moderate |
| All routine Doppler ultrasound vs no Doppler ultrasound (fetal/umbilical vessels+uterine artery) | Stillbirth | 2 studies, 5276 women | RR 1.41, 95% CI 0.44 to 4.46, no evidence of a difference GRADEb: low |
| All routine Doppler ultrasound vs no Doppler ultrasound (fetal/umbilical vessels only) | Perinatal mortality | 2 studies, 5907 women | RR 0.48, 95% CI 0.21 to 1.07 (P = 0.074), evidence of no difference GRADEa: moderate |
| All routine Doppler ultrasound vs no Doppler ultrasound (fetal/umbilical vessels+uterine artery) | Perinatal mortality | 2 studies, 5276 women | RR 1.16, 95% CI 0.29 to 4.56, no evidence of a difference GRADEa: very low |
| All routine doppler ultrasound vs no Doppler ultrasound (fetal/umbilical vessels only) | NICU admission | 2 studies, 5002 women | RR 0.99, 95% CI 0.82 to 1.18, no evidence of a difference |
| All routine doppler ultrasound vs no Doppler ultrasound (fetal/umbilical vessels+uterine artery) | NICU admission | 1 study, 2475 women | RR 1.01, 95% CI 0.67 to 1.53, no evidence of a difference |
| Single Doppler ultrasound assessment vs no Doppler ultrasound (fetal/umbilical vessels only) | Stillbirth | 1 study, 3891 women | RR 0.40, 95% CI 0.08 to 2.06, no evidence of a difference GRADEa: low |
| Single Doppler ultrasound assessment vs no Doppler ultrasound (fetal/umbilical vessels only) | Perinatal mortality | 1 study, 3891 women | RR 0.36, 95% CI 0.13 to 0.99 (P = 0.047), reduction in perinatal mortality for women receiving single Doppler ultrasound GRADEa: low |
| Multiple Doppler ultrasound assessments vs no Doppler ultrasound (Fetal/umbilical vessels+uterine artery) | Stillbirth | 2 studies, 5276 women | RR 1.41, 95% CI 0.44 to 4.46, no evidence of a difference GRADEa: low |
| Multiple Doppler ultrasound assessments vs no Doppler ultrasound (Fetal/umbilical vessels only) | Perinatal mortality | 1 study, 2016 women | RR 0.79, 95% CI 0.21 to 2.93, no evidence of a difference GRADEa: low |
| Multiple Doppler ultrasound assessments vs no Doppler ultrasound (Fetal/umbilical vessels+uterine artery) | Perinatal mortality | 2 studies, 5276 women | RR 1.16, 95% CI 0.29 to 4.56, no evidence of a difference GRADEa: low |
| Multiple Doppler ultrasound assessments vs no Doppler ultrasound (Fetal/umbilical vessels only) | NICU admission | 1 study, 2016 women | RR 0.92, 95% CI 0.56 to 1.52, no evidence of a difference |
| Multiple Doppler ultrasound assessments vs no Doppler ultrasound (Fetal/umbilical vessels+uterine artery) | NICU admission | 1 study, 2475 women | RR 1.01, 95% CI 0.67 to 1.53, no evidence of a difference |
| Any Doppler ultrasound vs no Doppler ultrasound | LBW | Outcome not reported | |
| Any Doppler ultrasound vs no Doppler ultrasound | SGA | Outcome not reported | |
| Utero‐placental Doppler ultrasound (Stampalija 2010) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Uterine artery Doppler ultrasound vs no Doppler ultrasound, 2nd trimester | Stillbirth | 2 studies, 5003 women | RR 1.44, 95% CI 0.38 to 5.49, no evidence of a difference GRADEa: low |
| Uterine artery Doppler ultrasound vs no Doppler ultrasound, 2nd trimester | Perinatal mortality | 2 studies, 5009 women | RR 1.61, 95% CI 0.48 to 5.39, no evidence of a difference GRADEa: low |
| Uterine artery Doppler ultrasound vs no Doppler ultrasound, 2nd trimester | IUGR | 2 studies, 5006 women | RR 0.98, 95% CI 0.64 to 1.50, no evidence of a difference |
| Uterine artery Doppler ultrasound vs no Doppler ultrasound, 2nd trimester | NICU admission | 2 studies, 5001 women | RR 1.12, 95% CI 0.92 to 1.37, no evidence of a difference |
| Uterine artery Doppler ultrasound vs no Doppler ultrasound, 2nd trimester | LBW | Outcome not reported | |
| Antenatal cardiotocography for fetal assessment (Grivell 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Traditional antenatal CTG vs no antenatal CTG | Perinatal mortality | 4 studies, 1627 women | RR 2.05, 95% CI 0.95 to 4.42, no evidence of a difference GRADEb: low |
| Traditional antenatal CTG vs no antenatal CTG | NICU admission | 2 studies, 883 women | RR 1.08, 95% CI 0.84 to 1.39, no evidence of a difference |
| Computerised antenatal CTG vs traditional antenatal CTG | Perinatal mortality | 2 studies, 469 women | RR 0.20, 95% CI 0.04 to 0.88 (P = 0.034), reduction in perinatal mortality for women receiving computerised antenatal CTG GRADEb: moderate |
| Traditional antenatal CTG vs no antenatal CTG or computerised antenatal CTG | LBW | Outcome not reported | |
| Traditional antenatal CTG vs no antenatal CTG or computerised antenatal CTG | SGA | Outcome not reported | |
| Symphysial fundal height measurement (SFH) in pregnancy (Robert Peter 2015) | |||
| Comparison | Outcome | No. of studies, no. women | Results |
| Tape measurement vs clinical palpation | Perinatal death | 1 study, 1639 women | RR 1.25, 95% CI 0.38 to 4.07, no evidence of a difference GRADEb: low |
| Tape measurement vs clinical palpation | Neonatal detection of small‐for‐dates | 1 study, 1639 women | RR 1.32, 95% CI 0.92 to 1.90, no evidence of a difference |
| Tape measurement vs clinical palpation | NICU admission | 1 study, 1639 women | RR 1.06, 95% CI 0.70 to 1.61, no evidence of a difference |
| Tape measurement vs clinical palpation | LBW | Outcome not reported | |
| CI: confidence interval; CTG: cardiotocography; IUGR: interuterine growth restriction; LBW: low birthweight; NICU: neonatal intensive care unit; RR: risk ratio; SGA: small‐for‐gestational age | |||
aGRADE assessed by review overview authors because it was not reported in the original review; bGRADE rating reported in the original review.
We assigned graphic icons to communicate the direction of review effect estimates and our confidence in the available data. This is the framework adopted by Medley and colleagues in their overview on 'Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews' (Medley 2018), and was based on graphics produced by the WHO to describe different types of workers and their roles in maternal and newborn care (optimizemnh.org/optimizing-health-worker-roles-maternal-newborn-health). We used six graphic icons to indicate mutually exclusive assessment categories (see Figure 1), the results of these assessments are presented below in the results section. We adapted the model slightly in this overview: we changed the 'unknown harm or benefit' graphic icon in the framework to include both high‐ and moderate‐certainty evidence and to also include unknown evidence of no effect or equivalence.
Clear evidence of benefit (moderate‐ or high‐certainty evidence with confidence intervals (CIs) not crossing the line of no effect).
Clear evidence of harm (moderate‐ or high‐certainty evidence with CIs not crossing the line of no effect).
Clear evidence of no effect or equivalence (moderate‐ or high‐certainty evidence with narrow CIs crossing the line of no effect).
Possible benefit (low‐certainty evidence with clear benefit, or moderate or high‐certainty evidence with wide CIs not crossing the line of no effect).
Possible harm (low‐certainty evidence with clear harm, or moderate or high‐certainty evidence with wide CIs not crossing the line of no effect).
Unknown benefit or harm or no effect or equivalence (low, moderate or high‐certainty evidence with wide CIs crossing the line of no effect, or low‐certainty evidence with no effect or equivalence, or very low‐certainty evidence).
Results
Description of included reviews
In this overview review we searched for Cochrane systematic reviews and identified Cochrane systematic reviews of interventions for pregnancy and childhood health. We found a total of 873 Cochrane systematic reviews (including titles, protocols and full reviews). After screening titles and abstracts, we excluded 807 titles and retrieved 66 titles in full text for further assessment (see Table 13 for list of reasons for exclusion). Figure 2 gives a flow diagram outlining the selection process and numbers of reviews. After further selection, quality assessment, categorisation of targeted primary outcome and exclusion of duplications, we included 43 reviews.
13. Reason for excluded study.
| Name of review | Reason for exclusion |
| Alexander 2010 | No relevant outcomes for stillbirth |
| Balogun 2016 | Intervention is after birth, no outcome for stillbirth |
| Bergel 2002 | No relevant outcomes for stillbirth |
| Crowther 2010 | No relevant outcomes for stillbirth |
| Demicheli 2015 | No relevant outcomes for stillbirth |
| East 2019 | High‐risk population |
| Gagnon 2007 | No relevant outcomes for stillbirth |
| Jahanfar 2015 | No relevant outcomes for stillbirth |
| Kramer 2006 | No relevant outcomes for stillbirth |
| Lagarde 2009 | No relevant outcomes for stillbirth |
| McBain 2015 | No relevant outcomes for stillbirth |
| Meher 2006a | No relevant outcomes for stillbirth |
| Muktabhant 2015 | No relevant outcomes for stillbirth |
| Nabhan 2008 | No relevant outcomes for stillbirth |
| Nabhan 2015 | No relevant outcomes for stillbirth |
| Pattinson 2005 | Not related to antenatal intervention, and no relevant outcomes for stillbirth |
| Peña‐Rosas 2015a | No relevant outcomes for stillbirth |
| Peña‐Rosas 2015b | No relevant outcomes for stillbirth |
| Salam 2015 | No relevant outcomes for stillbirth |
| Sangkomkamhang 2015 | No relevant outcomes for stillbirth |
| Stade 2009 | No relevant outcomes for stillbirth |
| Van Lonkhuijzen 2012 | No relevant outcomes for stillbirth |
| Walker 2001 | No relevant outcomes for stillbirth |
2.
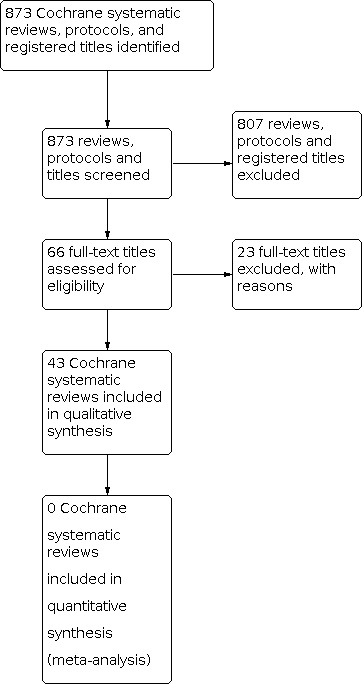
Flow diagram outlining the selection process and numbers of reviews
The titles of the 43 Cochrane Reviews are listed below in alphabetical order.
Altered dietary salt for preventing pre‐eclampsia, and its complications (Duley 2005)
Alternative versus standard packages of antenatal care for low‐risk pregnancy (Dowswell 2015)
Antenatal cardiotocography for fetal assessment (Grivell 2015)
Antenatal dietary education and supplementation to increase energy and protein intake (Ota 2015a)
Antioxidants for preventing pre‐eclampsia (Rumbold 2008)
Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy (Hofmeyr 2019)
Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems (Hofmeyr 2018)
Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes (Buppasiri 2015)
Combined diet and exercise interventions for preventing gestational diabetes mellitus (Shepherd 2017)
Community‐based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes (Lassi 2015)
Diuretics for preventing pre‐eclampsia (Churchill 2007)
Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment (Radeva‐Petrova 2014)
Effects and safety of periconceptional folate supplementation for preventing birth defects (De‐Regil 2015)
Fetal and umbilical Doppler ultrasound in normal pregnancy (Alfirevic 2015)
Fetal movement counting for assessment of fetal wellbeing (Mangesi 2015)
Giving women their own case notes to carry during pregnancy (Brown 2015)
Group versus conventional antenatal care for women (Catling 2015)
Insecticide‐treated nets for preventing malaria in pregnancy (Gamble 2006)
Iodine supplementation for women during the preconception, pregnancy and postpartum period (Harding 2017)
Lipid‐based nutrient supplements for maternal, birth, and infant developmental outcomes (Das 2018)
Magnesium supplementation in pregnancy (Makrides 2014)
Midwife‐led continuity models versus other models of care for childbearing women (Sandall 2016)
Multiple‐micronutrient supplementation for women during pregnancy (Keats 2019)
Nitric oxide for preventing pre‐eclampsia and its complications (Meher 2007)
Omega‐3 fatty acid addition during pregnancy (Middleton 2018)
Pharmacological interventions for promoting smoking cessation during pregnancy (Coleman 2015)
Progesterone for preventing pre‐eclampsia and its complications (Meher 2006)
Psychosocial interventions for supporting women to stop smoking in pregnancy (Chamberlain 2017)
Routine ultrasound in late pregnancy (after 24 weeks' gestation) (Bricker 2015)
Screening and subsequent management for thyroid dysfunction pre‐pregnancy and during pregnancy for improving maternal and infant health (Spencer 2015)
Screening for gestational diabetes mellitus based on different risk profiles and settings for improving maternal and infant health (Tieu 2017)
Symphysial fundal height (SFH) measurement in pregnancy for detecting abnormal fetal growth (Robert Peter 2015)
Traditional birth attendant training for improving health behaviours and pregnancy outcomes (Sibley 2012)
Treating periodontal disease for preventing adverse birth outcomes in pregnant women (Iheozor‐Ejiofor 2017)
Ultrasound for fetal assessment in early pregnancy (Whitworth 2015)
Use of biochemical tests of placental function for improving pregnancy outcome (Heazell 2015)
Utero‐placental Doppler ultrasound for improving pregnancy outcome (Stampalija 2010)
Vitamin A supplementation during pregnancy for maternal and newborn outcomes (McCauley 2015)
Vitamin C supplementation in pregnancy (Rumbold 2015a)
Vitamin D supplementation for women during pregnancy (Palacios 2019)
Vitamin E supplementation in pregnancy (Rumbold 2015b)
Vitamin supplementation for preventing miscarriage (Balogun 2016)
Zinc supplementation for improving pregnancy and infant outcome (Ota 2015b)
We summarised the characteristics of included studies in Table 1, Table 2, Table 3 and Table 4.
Objectives and scope of the reviews
All included reviews aimed to evaluate the impact of some specific antenatal interventions on adverse maternal, fetal, neonatal and infant outcomes. Although the outcomes varied in these reviews, we only included reviews where stillbirth or perinatal mortality or fetal loss were reported. Other outcomes reported included low birthweight, small‐for‐gestational age or intrauterine growth restriction and admission to NICU in this review.
Among 43 included reviews:
43 reviews reported stillbirth or perinatal mortality or fetal loss/fetal death, or a combination of one or all of these (Alfirevic 2015; Balogun 2016; Bricker 2015; Brown 2015; Buppasiri 2015; Catling 2015; Chamberlain 2017; Churchill 2007; Coleman 2015; Das 2018; De‐Regil 2015; Dowswell 2015; Duley 2005; Gamble 2006; Grivell 2015; Harding 2017; Heazell 2015; Hofmeyr 2018; Hofmeyr 2019; Iheozor‐Ejiofor 2017; Keats 2019; Lassi 2015; Makrides 2014; Mangesi 2015; McCauley 2015; Meher 2006; Meher 2007; Middleton 2018; Ota 2015a; Ota 2015b; Palacios 2019; Radeva‐Petrova 2014; Robert Peter 2015; Rumbold 2008; Rumbold 2015a; Rumbold 2015b; Sandall 2016; Shepherd 2017; Sibley 2012; Spencer 2015; Stampalija 2010; Tieu 2017; Whitworth 2015)
25 reviews reported low birthweight (Bricker 2015; Buppasiri 2015; Catling 2015; Chamberlain 2017; Coleman 2015; Das 2018; De‐Regil 2015; Dowswell 2015; Gamble 2006; Harding 2017; Hofmeyr 2018; Iheozor‐Ejiofor 2017; Keats 2019; Makrides 2014; McCauley 2015; Meher 2006; Middleton 2018; Ota 2015a; Ota 2015b; Palacios 2019; Radeva‐Petrova 2014; Sandall 2016; Sibley 2012; Spencer 2015; Whitworth 2015)
25 reviews reported small‐for‐gestational age or intrauterine growth restriction (Bricker 2015; Buppasiri 2015; Catling 2015; Churchill 2007; Das 2018; Dowswell 2015; Duley 2005; Keats 2019; Harding 2017; Heazell 2015; Hofmeyr 2018; Iheozor‐Ejiofor 2017; Makrides 2014; Meher 2006; Meher 2007; Middleton 2018; Ota 2015a; Ota 2015b; Robert Peter 2015; Rumbold 2008; Rumbold 2015a; Rumbold 2015b; Shepherd 2017; Stampalija 2010; Whitworth 2015)
28 reviews reported admission to NICU (Alfirevic 2015; Bricker 2015; Brown 2015; Buppasiri 2015; Catling 2015; Chamberlain 2017; Churchill 2007; Coleman 2015; Dowswell 2015; Duley 2005; Grivell 2015; Heazell 2015; Hofmeyr 2018; Hofmeyr 2019; Makrides 2014; Meher 2006; Meher 2007; Middleton 2018; Robert Peter 2015; Rumbold 2008; Rumbold 2015a; Rumbold 2015b; Sandall 2016; Shepherd 2017; Spencer 2015; Stampalija 2010; Tieu 2017; Whitworth 2015).
Sixteen reviews evaluated the effects of interventions on both stillbirth and perinatal mortality (Alfirevic 2015; Bricker 2015; Chamberlain 2017; Churchill 2007; Hofmeyr 2019; Keats 2019; Lassi 2015; McCauley 2015; Middleton 2018; Radeva‐Petrova 2014; Rumbold 2015a; Rumbold 2015b; Sibley 2012; Shepherd 2017; Stampalija 2010; Tieu 2017), and these reviews prioritised results of stillbirth. Reviews that did not report the outcome of stillbirth assessed perinatal mortality instead (Catling 2015; Dowswell 2015; Duley 2005; Grivell 2015; Harding 2017; Iheozor‐Ejiofor 2017; Meher 2007; Robert Peter 2015; Whitworth 2015).
Study characteristics and populations
The study designs included: randomised controlled trials (RCTs) (Alfirevic 2015; Balogun 2016; Bricker 2015; Brown 2015; Buppasiri 2015; Catling 2015; Chamberlain 2017; Churchill 2007; Coleman 2015; Das 2018; De‐Regil 2015; Dowswell 2015; Duley 2005; Gamble 2006; Grivell 2015; Harding 2017; Heazell 2015; Hofmeyr 2018; Hofmeyr 2019; Iheozor‐Ejiofor 2017; Keats 2019; Lassi 2015; Makrides 2014; Mangesi 2015; McCauley 2015; Meher 2006; Meher 2007; Middleton 2018; Ota 2015a; Ota 2015b; Palacios 2019; Radeva‐Petrova 2014; Robert Peter 2015; Rumbold 2008; Rumbold 2015a; Rumbold 2015b; Sandall 2016; Shepherd 2017; Sibley 2012; Spencer 2015; Stampalija 2010; Tieu 2017; Whitworth 2015), quasi‐RCTs (Alfirevic 2015; Balogun 2016; Bricker 2015; Catling 2015; Chamberlain 2017; Das 2018; Dowswell 2015; Grivell 2015; Harding 2017; Heazell 2015; Hofmeyr 2018; Lassi 2015; Makrides 2014; McCauley 2015; Middleton 2018; Palacios 2019; Radeva‐Petrova 2014; Rumbold 2015a; Rumbold 2015b; Sandall 2016; Sibley 2012; Tieu 2017; Whitworth 2015), cluster‐RCTs (Balogun 2016; Brown 2015; Catling 2015; Chamberlain 2017; Harding 2017; Keats 2019; Mangesi 2015; McCauley 2015; Sandall 2016; Shepherd 2017; Sibley 2012) and randomised cross‐over trials (Chamberlain 2017). RCTs are regarded as the gold standard study design for evaluating the effect of an intervention. The range of the number of included trials ranged from one (Hofmeyr 2019; Robert Peter 2015), to 86 (Chamberlain 2017), and the number of participants included ranged from 389 (Meher 2007), to over 310,000 (McCauley 2015).
Interventions
1. Nutritional interventions
We included 16 reviews that assessed nutritional interventions (Balogun 2016; Buppasiri 2015; De‐Regil 2015; Das 2018; Harding 2017; Hofmeyr 2018; Hofmeyr 2019; Keats 2019; Makrides 2014; McCauley 2015; Middleton 2018; Ota 2015a; Ota 2015b; Palacios 2019; Rumbold 2015a; Rumbold 2015b).
2. Prevention and management of infection
Interventions for prevention and management of infection included two reviews on malaria prevention (Gamble 2006; Radeva‐Petrova 2014).
3. Prevention, detection and management of other morbidities
There were 18 reviews on prevention, detection and management of major morbidities during the antenatal period (Brown 2015; Catling 2015; Chamberlain 2017; Churchill 2007; Coleman 2015; Dowswell 2015; Duley 2005; Heazell 2015; Iheozor‐Ejiofor 2017; Lassi 2015; Meher 2006; Meher 2007; Rumbold 2008; Sandall 2016; Shepherd 2017; Sibley 2012; Spencer 2015; Tieu 2017).
4. Screening and management of fetal growth and well‐being
We included seven reviews for screening and management of fetal growth and well‐being (Alfirevic 2015; Bricker 2015; Grivell 2015; Mangesi 2015; Robert Peter 2015; Stampalija 2010; Whitworth 2015).
Methodological quality of included reviews
Methodological quality of included systematic reviews
We used the AMSTAR rating scale to assess the methodological quality in each included review (Shea 2007). The Cochrane Handbook for Systematic Reviews of Interventions specifies a standard protocol specifying the methods, such as the search strategy should be comprehensive, data extraction and management should be carried out independently by at least two authors, methods for data synthesis should be specified, reasons for excluding studies and characteristics of included studies should be described, the quality of methodological of included studies should be determined, and data should be analysed and findings should be reported.
Of all included reviews, we rated 40 as high quality with an AMSTAR score ranging from 8 to 11 (Alfirevic 2015; Balogun 2016; Bricker 2015; Brown 2015; Buppasiri 2015; Catling 2015; Chamberlain 2017; Churchill 2007; Coleman 2015; Das 2018; De‐Regil 2015; Dowswell 2015; Grivell 2015; Keats 2019; Harding 2017; Heazell 2015; Hofmeyr 2018; Hofmeyr 2019; Iheozor‐Ejiofor 2017; Lassi 2015; Makrides 2014; Mangesi 2015; McCauley 2015; Meher 2006; Meher 2007; Middleton 2018; Ota 2015a; Ota 2015b; Palacios 2019; Radeva‐Petrova 2014; Rumbold 2008; Rumbold 2015a; Rumbold 2015b; Sandall 2016; Shepherd 2017; Sibley 2012; Spencer 2015; Stampalija 2010; Tieu 2017; Whitworth 2015) and three as moderate quality with a score of 7 (Duley 2005; Gamble 2006; Robert Peter 2015).
As all 43 included reviews were from the Cochrane Library, they included only RCTs (individual or cluster‐RCTs) or quasi‐RCTs. The methodological quality was generally high, as assessed by AMSTAR.
For AMSTAR ratings for each Cochrane systematic review, see Table 5 for nutritional interventions; Table 6 for prevention and management of infection; Table 7 for prevention, detection and management of other morbidities; and Table 8 for screening and management of fetal growth and well‐being.
Certainty of evidence
Forty‐two out of 43 (98%) Cochrane systematic reviews used the domain‐based evaluation for assessment of risk of bias as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
We rated most of the included reviews at low risk of bias in terms of sequence generation and allocation concealment (risk of selection bias) (Balogun 2016; Bricker 2015; Buppasiri 2015; Das 2018; De‐Regil 2015; Duley 2005; Gamble 2006; Hofmeyr 2019; Iheozor‐Ejiofor 2017; Middleton 2018; Meher 2007; Ota 2015b; Rumbold 2008; Rumbold 2015a; Sandall 2016; Shepherd 2017; Sibley 2012; Spencer 2015; Whitworth 2015). But some reviews failed to provide evidence of the treatment allocation procedure (Churchill 2007; Lassi 2015; Meher 2006a; Ota 2015a; Radeva‐Petrova 2014). Most of the participants in the included studies of the following reviews were blinded to treatment allocation (risks of performance and detection bias) (Balogun 2016; Buppasiri 2015; De‐Regil 2015; Keats 2019; Hofmeyr 2018; Hofmeyr 2019; Middleton 2018; Ota 2015b; Radeva‐Petrova 2014; Rumbold 2008; Rumbold 2015a; Shepherd 2017). Some reviews reported loss to follow‐up data or attrition and risk of incomplete data outcome (Alfirevic 2015; Churchill 2007; Dowswell 2015; Duley 2005; Gamble 2006; Keats 2019; Meher 2006; Ota 2015b; Rumbold 2008; Rumbold 2015a). Heterogeneity amongst included studies was very high in one review (Chamberlain 2017), but was reported low in one review (De‐Regil 2015).
We evaluated pooled outcome data from each systematic review using GRADE assessments. We did not reassess the GRADE assessment for our primary outcomes in the included systematic reviews where it was reported by review authors. If review authors did not assess GRADE, we made a new assessment ourselves. As we included a large number of systematic reviews, we created figures by assigning graphic icons to present the direction of review effect estimates with our confidence on estimates (see Figure 3; Figure 4; Figure 5; Figure 6), as outlined in the Methods in Assessment of methodological quality of included reviews.
3.
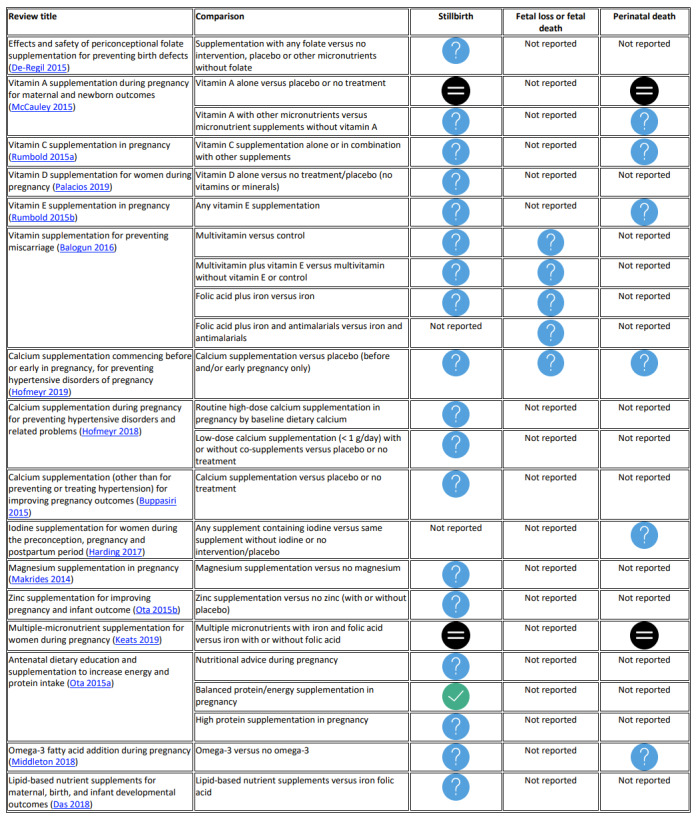
Certainty of evidence for nutritional interventions.
Note: a green tick for clear benefit, a black equals sign for clear evidence of no effect or equivalence, and a blue question mark graphic icon for unknown benefit or harm or no effect or equivalence (see Figure 1)
4.
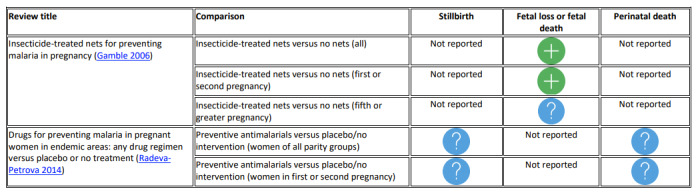
Certainty of evidence for intervention of prevention and management of infection.
Note: a green plus sign for possible benefit, and a blue question mark graphic icon for unknown benefit or harm or no effect or equivalence (see Figure 1)
5.
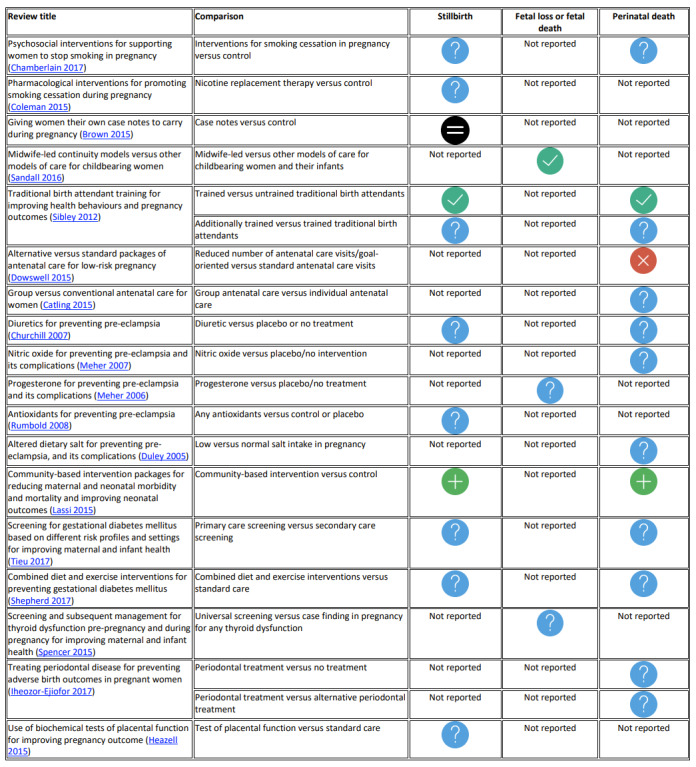
Certainty of evidence for intervention of prevention, detection and management of other morbidities.
Note: a green tick for clear benefit, a red‐cross for clear harm, a black equals sign for clear evidence of no effect or equivalence, a green plus sign for possible benefit, and a blue question mark graphic icon for unknown benefit or harm or no effect or equivalence (see Figure 1)
6.
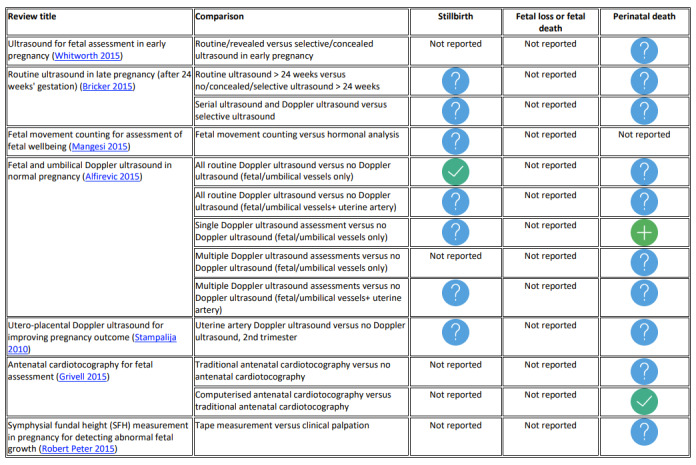
Certainty of evidence for intervention of screening and management of fetal growth and well‐being.
Note: a green tick for clear benefit, a green plus sign for possible benefit, and a blue question mark graphic icon for unknown benefit or harm or no effect or equivalence (see Figure 1)
Effect of interventions
Nutritional interventions (16 reviews)
We included 16 Cochrane systematic reviews on nutritional interventions in this overview (Balogun 2016; Buppasiri 2015; Das 2018; De‐Regil 2015; Palacios 2019; Keats 2019; Harding 2017; Hofmeyr 2018; Hofmeyr 2019; Middleton 2018; Makrides 2014; McCauley 2015; Ota 2015a; Ota 2015b; Rumbold 2015a; Rumbold 2015b). See Table 9 for all results relating to dietary interventions.
1. Folic acids
De‐Regil 2015 included five RCTs with 7391 women who became pregnant or were 12 weeks pregnant or less. This review assessed the effects and safety of folate supplementation alone or in combination with other vitamins or minerals for pregnancy outcomes: stillbirth and low birthweight. No RCTs reported on small‐for‐gestational age and admission to NICU.
Primary outcomes
Unknown benefit or harm: there may be little to no effect on the risk of stillbirth between women receiving supplementation with folic acid and those not, but the evidence is very uncertain (risk ratio (RR) 1.05, 95% confidence interval (CI) 0.54 to 2.05, 4 RCTs; 6597 women; very low‐certainty evidence).
Secondary outcomes
Two RCTs assessed the effects of this intervention on low birthweight and reported little to no difference between intervention and control groups (RR 1.13, 95% CI 0.84 to 1.52; 5048 women).
2. Vitamin A supplementation (two comparisons)
McCauley 2015 included 19 RCTs, cluster‐RCTs and quasi‐RCTs that randomised over 310,000 pregnant women, who received vitamin A supplementation or one of its derivatives, and who lived in either an area of endemic vitamin A deficiency or in an area with adequate intakes. This review evaluated stillbirth, perinatal death and low birthweight. There were no studies assessing the effect of vitamin A supplementation during pregnancy on small‐for‐gestation age and admission to NICU.
2.1. Vitamin A alone versus placebo or no treatment
Primary outcomes
Clear evidence of no effect or equivalence: only two RCTs of vitamin A alone during pregnancy compared with placebo or no treatment reported the effect of this intervention on stillbirth, and showed that there is probably no reduction for stillbirth (RR 1.04, 95% CI 0.98 to 1.10; 122,850 women; moderate‐certainty evidence) or perinatal death (RR 1.01, 95% CI 0.95 to 1.07; 1 RCT, 76,176 women; high‐certainty evidence).
Secondary outcomes
There was also evidence of little to no difference in low birthweight (RR 1.02, 95% CI 0.89 to 1.16; 4 RCTs, 14,599 women).
2.2. Vitamin A with other micronutrient versus micronutrient supplements without vitamin A
Primary outcomes
Unknown benefit or harm or no effect or equivalence: the evidence is very uncertain about the effect of Vitamin A on stillbirth (RR 1.41, 95% CI 0.57 to 3.47; 2 RCTs, 866 women; very low‐certainty evidence). The evidence suggests that vitamin A supplementation in combination with other micronutrients compared to micronutrients without vitamin A, probably does not reduce perinatal death, although the CI is wide and so we cannot be certain there is no effect (RR 0.51, 95% CI 0.10 to 2.69; 1 RCT, 179 women; moderate‐certainty evidence).
Secondary outcomes
There may be a reduction in low birthweight for women receiving vitamin A with other micronutrients (RR 0.67, 95% CI 0.47 to 0.96; 1 RCT, 594 women).
3. Vitamin C supplementation
Rumbold 2015a included 29 RCTs and quasi‐RCTs, that randomised 24,300 pregnant women. This review evaluated the effects of vitamin C supplementation, alone or in combination with other supplements on pregnancy outcomes: stillbirth, neonatal death, perinatal death, infant death, intrauterine growth restriction and admission to NICU. There were no studies assessing the effects of this intervention on low birthweight and small‐for‐gestational age.
Primary outcomes
Unknown benefit or harm or no effect or equivalence: vitamin C supplementation administered alone or in combination with other separate supplements compared with placebo, no placebo or other supplements, probably does not lead to a reduction in stillbirth, although the CI is wide and so we cannot be certain it has no effect (RR 1.15, 95% CI 0.89 to 1.49; 11 RCTs, 20,038 women; moderate‐certainty evidence). The evidence is very uncertain about the effect of vitamin C supplementation administered alone or in combination with other separate supplements compared with placebo, no placebo or other supplements on perinatal death (RR 1.07, 95% CI 0.77 to 1.49; 7 RCTs, 17,271 women, very low‐certainty evidence).
Secondary outcomes
There may be no effect on intrauterine growth restriction (RR 0.98, 95% CI 0.91 to 1.06; 12 RCTs, 20,361 women) or admission to NICU (RR 1.02, 95% CI 0.96 to 1.06; 9 RCTs, 18,371 women) with vitamin C supplementation administered alone or in combination with other separate supplements and placebo, no placebo or other supplements.
4. Vitamin D supplementation
Palacios 2019 included 30 RCTs and quasi‐RCTs, with 7033 pregnant women. This review examined the effect of oral vitamin D supplementation versus no treatment/placebo on stillbirth and low birthweight. There were no studies assessing the effect of vitamin D supplementation on small‐for‐gestational age or admission to NICU.
Primary outcomes
Unknown benefit or harm: we are very uncertain about the effects of Vitamin D supplementation alone during pregnancy compared with no treatment/placebo (no vitamin or mineral) on stillbirth (RR 0.35, 95% CI 0.06 to 1.98; 3 RCTs, 584 women; very low‐certainty evidence).
Secondary outcomes
Vitamin D supplementation probably results in a reduction in low birthweight (RR 0.55, 95% CI 0.35 to 0.87; 5 RCTs, 697 women; moderate‐certainty evidence).
5. Vitamin E supplementation
Rumbold 2015b included 21 RCTs and quasi‐RCTs that randomised 22,129 pregnant women, who received vitamin E supplementation alone or in combination with other separate supplements during pregnancy. This review assessed the effects of vitamin E supplementation on pregnancy outcomes: stillbirth, perinatal mortality, intrauterine growth restriction and admission to NICU. No studies assessed the effect of vitamin E supplementation on low birthweight or small‐for‐gestational age.
Primary outcomes
Unknown benefit or harm or no effect or equivalence: administration of any vitamin E supplementation alone or in combination with other supplements during pregnancy probably does not reduce stillbirth, although the CI is wide and so we cannot be certain there is no effect (RR 1.17, 95% CI 0.88 to 1.56; 9 RCTs, 19,023 women; moderate‐certainty evidence). The evidence is very uncertain about the effect of this intervention on perinatal death (RR 1.09, 95% CI 0.77 to 1.54; 6 RCTs, 16,923 women, very low‐certainty evidence).
Secondary outcomes
There was no reduction in intrauterine growth restriction (RR 0.98, 95% CI 0.91 to 1.06; 11 RCTs, 20,202 women), or in admission to NICU (RR 1.01, 95% CI 0.95 to 1.08; 8 RCTs, 17,594 women) for women receiving any vitamin E supplementation alone or in combination with other supplements during pregnancy.
6. Vitamin supplementation for preventing miscarriage (four comparisons)
Balogun 2016 included 40 RCTs, cluster‐RCTs and quasi‐RCTs that randomised 276,820 pregnant women. This review assessed the effectiveness and safety of any vitamin supplementation on the risk of spontaneous miscarriage. It reported stillbirth and total fetal loss. In this overview we have reported only comparisons not covered by individual vitamin reviews or only reporting low‐risk populations.
Primary outcomes
6.1. Multivitamin versus control
Unknown benefit or harm: there may be little to no difference in stillbirth (RR 0.83, 95% CI 0.58 to 1.17; 1 RCT, 5021 women; low‐certainty evidence) or total fetal loss (RR 0.83, 95% CI 0.58 to 1.17; 1 RCT, 5021 women; low‐certainty evidence) for women receiving multivitamins compared with control.
6.2 Multivitamin plus vitamin E versus multivitamin without vitamin E or control
Unknown benefit or harm: for women receiving multivitamin plus vitamin E compared with women receiving multivitamin without vitamin E or control there may be little or no difference in risk of stillbirth (RR 0.88, 95% CI 0.39 to 1.98; 1 RCT, 823 women; low‐certainty evidence) or total fetal loss (RR 0.92, 95% CI 0.46 to 1.83; 1 RCT, 823 women; low‐certainty evidence).
6.3. Folic acid plus iron versus iron
Unknown benefit or harm: there was little or no difference in risk of stillbirth (RR 0.38, 95% CI 0.02 to 9.03; 1 RCT, 75 women; low‐certainty evidence) or total fetal loss (RR 0.23, 95% CI 0.01 to 4.59; 1 RCT, 75 women; low‐certainty evidence) for women receiving folic acid plus iron compared with women receiving only iron.
6.4. Folic acid plus iron and antimalarials versus iron and antimalarials
Unknown benefit or harm: one RCT compared women who received folic acid plus iron and antimalarials with women who received iron and antimalarials and there was little or no difference in risk of total fetal loss (RR 13.0, 95% CI 0.74 to 226.98; 160 women; low‐certainty evidence).
7. Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy
Hofmeyr 2019 included one RCT that randomised 1355 non‐pregnant women with previous pre‐eclampsia, of whom 651 became pregnant. This review assessed calcium supplementation commencing before or early in pregnancy for preventing hypertensive disorders during pregnancy. Participants received 500 mg calcium or placebo from enrolment until 20 weeks' gestation followed by 1.5 mg/day calcium for all women after 20 weeks. This review determined the effects of the intervention on pregnancy loss/stillbirth or neonatal death before discharge and perinatal death or NICU admission, or both, for more than 24 hours. Low birthweight and small‐for‐gestation age were not reported.
Primary outcomes
Unknown benefit or harm: there was little or no difference in risk of stillbirth (RR 0.78, 95% CI 0.48 to 1.27; 1 RCT, 579 women; low‐certainty evidence); pregnancy loss, stillbirth or neonatal death before discharge (RR 0.82, 95% CI 0.61 to 1.10; 1 RCT, 632 women; low‐certainty evidence) or perinatal death or NICU admission, or both, for more than 24 hours (RR 1.11, 95% CI 0.77 to 1.60; 1 RCT, 508 women; low‐certainty evidence) for women receiving calcium before and early in pregnancy compared with women receiving placebo.
Secondary outcomes
Secondary outcomes were not reported.
8. Calcium supplementation for preventing hypertensive disorders (two comparisons)
Hofmeyr 2018 included 27 RCTs that randomised 18,064 pregnant women, who received high‐dose calcium supplementation (≥ 1 g/day of elemental calcium), and 12 RCTs and quasi‐RCTs that randomised 2334 pregnant women, who received low‐dose calcium supplementation (≤ 1 g/day of elemental calcium) from at the latest 34 weeks of pregnancy. This review determined the effects of high‐ and low‐dose calcium supplementation during pregnancy for preventing hypertensive disorders and related problems of pregnancy and neonatal adverse outcomes: stillbirth or death before discharge from hospital, low birthweight, small‐for‐gestation age and admission to NICU.
8.1. High‐dose calcium supplementation (≥ 1 g/day) in pregnancy for preventing hypertensive disorders
Primary outcomes
Unknown benefit or harm: the evidence is very uncertain about the effect of high‐dose calcium supplementation compared with placebo treatment on stillbirth (RR 0.90, 95% CI 0.74 to 1.09; 11 RCTs, 15,665 women; very low‐certainty evidence).
Secondary outcomes
The intervention may reduce low birthweight (RR 0.85, 95% CI 0.72 to 1.01; 9 RCTs, 14,883 women) but may have no effect on small‐for‐gestational age (RR 1.05, 95% CI 0.86 to 1.29; 4 RCTs, 13,615 women) and admission to NICU (RR 1.05, 95% CI 0.94 to 1.18; 4 RCTs, 13,406 women).
8.2. Low‐dose calcium supplementation (< 1 g/day) in pregnancy for preventing hypertensive disorders
Primary outcomes
Unknown benefit or harm: the evidence is very uncertain about the effect of low‐dose calcium supplementation during pregnancy on stillbirth (RR 0.48, 95% CI 0.14 to 1.67; 5 RCTs, 1025 women; very low‐certainty evidence).
Secondary outcomes
There may be a reduction in the risk of low birthweight (RR 0.20, 95% CI 0.05 to 0.88; 2 RCTs, 134 women) and NICU admission (RR 0.44, 95% CI 0.20 to 0.99; 1 RCT, 422 women) for women receiving low‐dose calcium supplementation. However, there was little to no effect on small‐for‐gestational age (RR 0.81, 95% CI 0.54 to 1.21; 4 RCTs, 854 women).
9. Calcium supplementation other than for preventing or treating hypertension
Buppasiri 2015 included 25 RCTs with 17,842 women, who received calcium supplementation during pregnancy. This review determined the effect of calcium supplementation on maternal, fetal and neonatal outcomes (other than for preventing or treating hypertension) including stillbirth or fetal death, low birthweight, intrauterine growth restriction and admission to NICU.
Primary outcomes
Unknown benefit or harm: there may be little to no effect of calcium supplementation in reducing stillbirth or fetal death (RR 0.91, 95% CI 0.72 to 1.14; 6 RCTs, 15,269 women; low‐certainty evidence).
Secondary outcomes
Calcium supplementation during pregnancy compared with control probably does not reduce low birthweight (RR 0.93, 95% CI 0.81 to 1.07, 6 RCTs, 14,162 women; moderate‐certainty evidence), intrauterine growth restriction (RR 0.83, 95% CI 0.61 to 1.13, 6 RCTs, 1701 women) or NICU admission (RR 1.05, 95% CI 0.94 to 1.18, 4 RCTs, 14,062 women).
10. Iodine supplementation
Harding 2017 included 14 RCTs, cluster‐RCTs and quasi‐RCTs that randomised over 2700 women. This review assessed the effects of iodine supplementation for women in the periconceptional, pregnancy, or postpartum period on pregnancy and infant outcomes: perinatal mortality, low birthweight and small‐for‐gestational age. Admission to NICU was not assessed.
Primary outcomes
Unknown benefit or harm: there may be little to no difference in perinatal mortality (RR 0.66, 95% CI 0.42 to 1.03; 2 RCTs, 457 women; low‐certainty evidence) for women receiving any supplement with iodine compared with women receiving the same supplement without iodine, no intervention, or placebo.
Secondary outcomes
Iodine supplementation to women in the periconceptional, pregnancy, or postpartum period may result in little or no difference in low birthweight (RR 0.56, 95% CI 0.26 to 1.23; 2 RCTs, 377 women; low‐certainty evidence), or small‐for‐gestational age (RR 1.26, 95% CI 0.77 to 2.05; 2 RCTs, 377 women).
11. Magnesium supplementation
Makrides 2014 included 10 RCTs and quasi‐RCTs that randomised 9090 women with normal or high‐risk pregnancies. This review assessed the effects of magnesium supplementation during pregnancy on maternal, neonatal and paediatric outcomes: stillbirth, low birthweight, small‐for‐gestation age and admission to NICU.
Primary outcomes
Unknown benefit or harm: there may be little to no difference in risk of stillbirth (RR 0.73, 95% CI 0.43 to 1.25; 4 RCTs, 5526 women; low‐certainty evidence) for women receiving magnesium supplementation in pregnancy compared to no magnesium supplementation.
Secondary outcomes
There may be no effect on low birthweight (RR 0.95, 95% CI 0.83 to 1.09; 5 RCTs, 5577 women) and little to no differences in small‐for‐gestational age (RR 0.76, 95% CI 0.54 to 1.07; 3 RCTs, 1291 women), or admission to NICU (RR 0.74, 95% CI 0.50 to 1.11; 3 RCTs, 1435 women) in women receiving magnesium supplementation in pregnancy compared to no magnesium supplementation
12. Zinc supplementation
Ota 2015b included 21 RCTs with over 17,000 normal pregnant women with no systemic diseases and their babies. This review assessed the effects of zinc supplementation during pregnancy (before 27 weeks' gestation) on maternal, fetal, neonatal and infant outcomes: stillbirth or neonatal death, small‐for‐gestational age and low birthweight. There were no studies assessing the effects of zinc supplementation on admission to NICU.
Primary outcomes
Unknown benefit or harm: there may be little to no difference in stillbirth or neonatal death (RR 1.12, 95% CI 0.86 to 1.46; 8 RCTs, 5100 women; low‐certainty evidence) between pregnant women administered routine zinc supplementation and women who did not receive zinc.
Secondary outcomes
There is probably no reduction in low birthweight (RR 0.93, 95% CI 0.78 to 1.12; 14 RCTs, 5643 women; moderate‐certainty evidence) and small‐for‐gestational age (RR 1.02, 95% CI 0.94 to 1.11; 8 RCTs, 4252 women; moderate‐certainty evidence) in pregnant women administered routine zinc supplementation compared with those who did not receive zinc.
13. Multiple micronutrient supplementation
Keats 2019 included 21 RCTs and cluster‐RCTs that randomised 142,496 pregnant women (women who were HIV‐positive were excluded). This review evaluated the benefits of multiple‐micronutrient supplementation with iron and folic acid for pregnant women on stillbirth, perinatal mortality, low birthweight and small‐for‐gestational age. No studies assessed the effect of the intervention on admission to NICU.
Primary outcomes
Clear evidence of no effect or equivalence: there was no reduction in stillbirth for women who received multiple micronutrients supplementation with iron and folic acid in pregnancy compared to women who received iron with or without folic acid (RR 0.95, 95% CI 0.86 to 1.04; 17 RCTs, 97,927 women; high‐certainty evidence) and perinatal death (RR 1.00, 95% CI 0.90 to 1.11; 15 RCTs, 63,922 women, high‐certainty evidence).
Secondary outcomes
The intervention reduced the risk of low birthweight (RR 0.88, 95% CI 0.85 to 0.91; 18 RCTs, 68,801 women; high‐certainty evidence) and probably reduced small‐for‐gestational age (RR 0.92, 95% CI 0.88 to 0.97; 17 RCTs, 57,348 women; moderate‐certainty evidence).
14. Energy and protein (four comparisons)
Ota 2015a included 17 RCTs involving 9030 pregnant women with either high pregnancy weight or high gestational weight gain. This review assessed the effects of dietary advice, supplementation, or restriction on gestational weight gain, pre‐eclampsia and/or pregnancy outcomes: stillbirth, low birthweight and small‐for‐gestational age but effects on admission to the NICU were not reported.
14.1. Nutritional advice during pregnancy
Primary outcomes
Unknown benefit or harm: only one RCT with 431 women, who received specific advice to increase dietary energy and protein intake, reported on stillbirth and found little to no difference compared with no nutritional education or normal care (RR 0.37, 95% CI 0.07 to 1.90; low‐certainty evidence).
Secondary outcomes
Nutritional advice during pregnancy was associated with a reduction in low birthweight in one RCT (RR 0.04, 95% CI 0.01 to 0.14; 300 women). One RCT reported small‐for‐gestational age and found little to no difference in small‐for‐gestational age (RR 0.97, 95% CI 0.45 to 2.11; 404 women; low‐certainty evidence).
14.2. Balanced protein/energy supplementation in pregnancy
Primary outcomes
Clear evidence of benefit: 12 RCTs assessed the effectiveness of balanced protein/energy supplementation given to pregnant women. Balanced energy/protein supplementation probably reduces stillbirth (RR 0.60, 95% CI 0.39 to 0.94; 5 RCTs, 3408 women; moderate‐certainty evidence).
Secondary outcomes
The intervention of balanced energy/protein supplementation probably reduces the risk of small‐for‐gestational‐age at birth (RR 0.79, 95% CI 0.69 to 0.90; 7 RCTs, 4408 women; moderate‐certainty evidence). These effects of balanced protein/energy supplementation did not appear greater in undernourished women and had little to no effect in reducing preterm birth.
14.3. High protein supplementation in pregnancy
Primary outcomes
Unknown benefit or harm: when high protein supplementation was administered to pregnant women, there was little or no difference in stillbirth (RR 0.81, 95% CI 0.31 to 2.15; 1 RCT, 529 women; low‐certainty evidence).
Secondary outcomes
In one RCT with 505 women there is probably an increase in small‐for‐gestational age for women receiving high protein supplementation during pregnancy (RR 1.58, 95% CI 1.03 to 2.41; moderate‐certainty evidence).
14.4. Isocaloric balanced protein supplementation in pregnancy
Two RCTs involving 184 women assessed the effect of isocaloric balanced protein supplementation versus protein replaced by an equal quantity of non‐protein energy in pregnancy, but did not report on stillbirth, fetal growth, or admission to NICU.
15. Omega‐3 fatty acid addition during pregnancy
Middleton 2018 included 70 RCTs that randomised 19,927 pregnant women, regardless of their risk for pre‐eclampsia, preterm birth or intrauterine growth restriction. This review estimated the effects of omega‐3 long‐chain polyunsaturated fatty acids (LCPUFA) supplementation or dietary addition during pregnancy on stillbirth, perinatal death, low birthweight, small‐for‐gestational age and admission to NICU.
Primary outcomes
Unknown benefit or harm: the evidence is very uncertain about the effect of omega‐3 on stillbirth (RR 0.94, 95% CI 0.62 to 1.42; 16 RCTs, 7880 women; very low‐certainty evidence) or perinatal death (RR 0.75, 95% CI 0.54 to 1.03; 10 RCTs, 7416 women; low‐certainty evidence).
Secondary outcomes
The intervention probably does not reduce small‐for‐gestational age/intrauterine growth restriction (RR 1.01, 95% CI 0.90 to 1.13; 8 RCTs, 6907 women; moderate‐certainty evidence) or admission to NICU (RR 0.92, 95% CI 0.83 to 1.03; 9 RCTs, 6920 women; moderate‐certainty evidence). However, omega‐3 LCPUFA supplementation during pregnancy showed a reduced risk of low birthweight (RR 0.90, 95% CI 0.82 to 0.99; 15 RCTs, 8449 women; high‐certainty evidence).
16. Lipid‐based nutrient supplements
Das 2018 included four RCTs that randomised 8018 women with a singleton pregnancy. This review assessed the effect of ready‐to‐use lipid‐based nutrient supplements (LNS) for maternal, birth and infant outcomes in pregnant women. The outcomes stillbirth, low birthweight and small‐for‐gestational age were reported, but not perinatal death and admission to NICU.
Primary outcomes
Unknown benefit or harm: there was little to no difference in stillbirth (RR 1.14, 95% CI 0.52 to 2.48; 3 RCTs, 5575 women; low‐certainty evidence) for LNS versus iron and folic acid.
Secondary outcomes
There may be a reduction in low birthweight, although the CI also indicates a slight increase (RR 0.87, 95% CI 0.72 to 1.05; 3 RCTs, 4826 women; moderate‐certainty evidence) for LNS versus iron and folic acid. The intervention probably slightly reduces the risk of small‐for‐gestational age compared with iron folic acid supplementation (RR 0.94, 95% CI 0.89 to 0.99; 3 RCTs, 4823 women; moderate‐certainty evidence).
Prevention and management of infection (two reviews)
We included two Cochrane systematic reviews on prevention and management of infection in this overview (Gamble 2006; Radeva‐Petrova 2014). See Table 10 for all results relating to prevention and management of infection.
1. Insecticide‐treated nets for preventing malaria (two comparisons)
Gamble 2006 included five RCTs with 6759 pregnant women who lived in malaria‐endemic areas. This review analysed the effects of insecticide‐treated nets to prevent malaria in pregnancy on fetal loss and low birthweight. There were no studies assessing the effect of this intervention on small‐for‐gestational age and NICU admission.
1.1. Insecticide‐treated nets versus no nets (all)
Primary outcomes
Possible benefits: using insecticide‐treated nets was found to possibly reduce the risk of fetal loss (RR 0.68, 95% CI 0.48 to 0.98; 5 RCTs; low‐certainty evidence).
Secondary outcomes
Using insecticide‐treated nets may reduce low birthweight (RR 0.80, 95% CI 0.64 to 1.00; 4 RCTs).
1.2. Insecticide‐treated nets versus no nets (first or second pregnancy, fifth or greater pregnancy)
Primary outcomes
Possible benefits:Gamble 2006 observed a possible reduction in fetal loss with insecticide‐treated nets used in first or second pregnancies compared with no nets (RR 0.67, 95% CI 0.47 to 0.97; 4 RCTs; low‐certainty evidence).
Unknown benefit or harm: there may be little to no difference on the risk of fetal loss in pregnant women with four or more previous pregnancies (RR 1.02, 95% CI 0.17 to 6.23; 1 RCT).
Secondary outcomes
Insecticide‐treated nets used in first or second pregnancies showed a reduction in the risk of low birthweight (RR 0.77, 95% CI 0.61 to 0.98; 3 RCTs). There may be little to no difference on the risk of low birthweight in pregnant women with four or more previous pregnancies (RR 1.12, 95% CI 0.56 to 2.24; 1 RCT).
2. Drugs for preventing malaria (two comparisons)
Radeva‐Petrova 2014 included 17 RCTs or quasi‐RCTs with 14,481 pregnant women living in a malaria‐endemic area. This review assessed the efficacy of drugs given to prevent malaria in pregnant women on stillbirth, perinatal mortality and low birthweight. There were no studies reporting the efficacy of drugs on small‐for‐gestational age and admission to NICU.
2.1. Any antimalarial drug prevention versus placebo/no intervention (women of all parity groups)
Primary outcomes
Unknown no effect or equivalence: there is probably no reduction in stillbirth with any antimalarial drug prevention administered to pregnant women of all parity groups (RR 1.02, 95% CI 0.76 to 1.36; 5 RCTs, 7130 women; moderate‐certainty evidence) or perinatal death (RR 1.24, 95% CI 0.94 to 1.63; 4 RCTs, 5216 women; moderate‐certainty evidence), indicating that antimalarial drugs had little impact on preventing stillbirth or other pregnancy‐related outcomes, although the CIs are wide and so we cannot be certain there is no effect.
Secondary outcomes
There may be little to no difference in low birthweight (RR 1.06, 95% CI 0.89 to 1.27; 4 RCTs, 3644 women; low‐certainty evidence) for pregnant women of all parity groups receiving antimalarial drug prevention compared with placebo or no intervention.
2.2. Any antimalarial drug prevention versus placebo/no intervention (women in first or second pregnancy)
Primary outcomes
Unknown benefit or harm: there may be little to no differences in stillbirth with any antimalarial drug administered to women in their first or second pregnancies compared with placebo or no intervention, (RR 0.97, 95% CI 0.63 to 1.49; 4 RCTs, 2703 women; low‐certainty evidence) or perinatal death (RR 0.73, 95% CI 0.54 to 1.00; 2 RCTs, 1620 women; low‐certainty evidence).
Secondary outcomes
This intervention showed a possible reduction in low birthweight (RR 0.73, 95% CI 0.61 to 0.87; 10 RCTs, 3619 women; moderate‐certainty evidence) for women in their first or second pregnancy receiving any antimalarial drug prevention compared with those who received placebo or no intervention.
Prevention, detection and management of other morbidities (18 reviews)
We included 18 Cochrane systematic reviews on prevention, detection and management of other morbidities in this overview (Brown 2015; Catling 2015; Chamberlain 2017; Churchill 2007; Coleman 2015; Dowswell 2015; Duley 2005; Heazell 2015; Iheozor‐Ejiofor 2017; Lassi 2015; Meher 2006; Meher 2007; Rumbold 2008; Sandall 2016; Shepherd 2017; Sibley 2012; Spencer 2015; Tieu 2017). See Table 11 for all results relating to prevention, detection and management of other morbidities.
1. Smoking cessation (two comparisons)
Chamberlain 2017 included 86 RCTs involving over 28,000 pregnant women in any care setting, women seeking a pregnancy consultation, and health professionals, with respect to smoking cessation. This review examined the impact of promoting smoking cessation during pregnancy, including cognitive behaviour therapy, educational and motivational interviewing approaches, stages of change‐based interventions, feedback of fetal health measurement, provision and rewards and incentives for smoking cessation and provision of nicotine replacement therapy or other pharmacological agents on mothers' and infants' outcomes: stillbirth, perinatal death, low birthweight and admission to NICU.
Coleman 2015 assessed pharmacological interventions for promoting smoking cessation during pregnancy, in a review that included nine RCTs with 2210 women. This review assessed the effect of pharmacological treatments (i.e. bupropion and varenicline as well as other drugs) for smoking cessation on pregnancy outcomes including stillbirth, low birthweight and admission to NICU.
There were no studies reporting the effect of the interventions on small‐for‐gestational age.
1.1. Interventions for smoking cessation in pregnancy versus control
Primary outcomes
Unknown benefit or harm or no effect or equivalence: for pregnant women receiving interventions for smoking cessation during pregnancy there is probably no reduction in stillbirth (RR 1.20, 95% CI 0.76 to 1.90; 8 RCTs, 6170 women; high‐certainty evidence) or perinatal death (RR 1.13, 95% CI 0.72 to 1.77; 4 RCTs, 4465 women; moderate‐certainty evidence) compared with those who did not, although the CIs are wide and so we cannot be certain there is no effect.
Secondary outcomes
Interventions for smoking cessation in pregnancy may reduce the risk of low birthweight (RR 0.83, 95% CI 0.71 to 0.94; 18 RCTs, 9402 women) and admission to NICU (RR 0.78, 95% CI 0.61 to 0.98; 8 RCTs, 2100 women).
1.2. Nicotine replacement therapy versus control
Primary outcomes
Unknown benefit or harm: there may be little to no difference in risk of stillbirth for women receiving nicotine replacement therapy for promoting smoking cessation during pregnancy (RR 1.24, 95% CI 0.54 to 2.84; 4 RCTs, 1777 women; low‐certainty evidence).
Secondary outcomes
There may be little to no effect on the risk of low birthweight (RR 0.74, 95% CI 0.41 to 1.34; 6 RCTs, 2037 women) and admission to NICU (RR 0.90, 95% CI 0.64 to 1.27; 4 RCTs, 1756 women) for women receiving nicotine replacement therapy compared with control.
2. Women carrying their own case notes
Brown 2015 included four RCTs and cluster‐RCTs involving 1176 pregnant women. This review evaluated the effects of giving women their own case notes to carry during pregnancy on administrative outcomes, maternal satisfaction and control, health‐related behaviours and clinical outcomes: stillbirth or neonatal birth and admission to NICU. There were no studies reporting the effects of the intervention on low birthweight or small‐for‐gestational age.
Primary outcomes
Clear evidence of no effect or equivalent: there is probably no reduction in stillbirth or neonatal death (RR 1.00, 95% CI 0.99 to 1.01; 2 RCTs, 713 women; moderate‐certainty evidence) for women carrying their own case notes compared with control.
Secondary outcomes
There may be little to no difference in admission to NICU (RR 1.18, 95% CI 0.36 to 3.83; 1 RCT, 501 women) for women carrying their own case notes compared to control.
3. Midwife‐led care
Sandall 2016 included 15 RCTs, quasi‐RCTs and cluster‐RCTs with 17,674 pregnant women. This review assessed the effectiveness of midwife‐led models of care for childbearing women and their infants on fetal loss, low birthweight and admission to NICU. There were no studies reporting the effect of the intervention on small‐for‐gestational age.
Primary outcomes
Clear evidence of benefit: midwife‐led models of care for childbearing women and their infants in comparison to other models of care were more likely to result in reduced fetal loss or neonatal death before 24 weeks (RR 0.81, 95% CI 0.67 to 0.98; 11 RCTs, 15,645 women; high‐certainty evidence) and overall fetal loss and neonatal death (RR 0.84, 95% CI 0.71 to 0.99; 13 RCTs, 17,561 women; high‐certainty evidence).
Unknown benefit or harm: there may be little to no difference in reducing fetal loss or neonatal death equal to or after 24 weeks (RR 1.00, 95% CI 0.67 to 1.49, 12 RCTs, 17,359 women, moderate‐certainty evidence).
Secondary outcomes
The intervention did not reduce risk of low birthweight infant (RR 0.96, 95% CI 0.82 to 1.13, 7 RCTs, 11,458 women) or admission to NICU (RR 0.90, 95% CI 0.78 to 1.04, 13 RCTs, 17,561 women).
4. Traditional birth attendant training (two comparisons)
Sibley 2012 included nine RCTs, quasi‐RCTS and cluster‐RCTs with more than 32,000 women. This review assessed the effects of traditional birth attendant training in combination with improved heath services on positive pregnancy outcomes, stillbirth and perinatal death. There were no studies reporting on low birthweight, small‐for‐gestational age or admission to NICU.
Primary outcomes
Clear evidence of benefit: one cluster‐RCT that randomised 18,699 pregnant women and that compared trained versus untrained traditional birth attendants to mediate positive pregnancy outcomes showed a probable reduction in stillbirth (odds ratio (OR) 0.69, 95% CI 0.57 to 0.83; moderate‐certainty evidence) and perinatal death (OR 0.70, 95% CI 0.59 to 0.83; moderate‐certainty evidence).
Primary outcomes
Unknown no effect or equivalence: there was probably no reduction in the risk of stillbirth (RR 0.99, 95% CI 0.76 to 1.28; 2 RCTs, 27,594 women; moderate‐certainty evidence) or perinatal mortality (OR 0.79, 95% CI 0.61 to 1.02; 1 RCT, 24,007 women; moderate‐certainty evidence) for additionally trained traditional birth attendant versus trained traditional birth attendant, although the CIs are wide and so we cannot be certain there is no effect.
5. Alternative versus standard packages of antenatal care
Dowswell 2015 included seven RCTs and quasi‐RCTs with 60,724 pregnant women who attended antenatal care clinics and were considered to be at low risk of complications during pregnancy and labour. This review assessed the effects of alternative packages of antenatal care programmes on perinatal death, low birthweight, small‐for‐gestational age and admission to NICU.
Primary outcomes
Clear evidence of harm: there is probably an increase in perinatal death for women with reduced number of antenatal care visits (RR 1.14, 95% CI 1.00 to 1.31; 5 RCTs; 56,431 women; moderate‐certainty evidence).
Secondary outcomes
There was evidence of no reduction in low birthweight (RR 1.04, 95% CI 0.97 to 1.11; 6 RCTs), small‐for‐gestational age (RR 0.99, 95% CI 0.91 to 1.09; 4 RCTs; moderate‐certainty evidence) and admission to NICU (RR 0.89, 95% CI 0.79 to 1.02; 5 RCTs; 43,048 babies).
6. Group versus conventional antenatal care
Catling 2015 included four RCTs, cluster‐RCTs and quasi‐RCTs that randomised 2350 women. This review compared the effects of group antenatal care versus conventional antenatal care on psychosocial, physiological, labour and birth outcomes for women and their babies and on care provider satisfaction. Perinatal mortality, low birthweight, small‐for‐gestational age and admission to NICU were assessed.
Primary outcomes
Unknown benefit or harm: there may be little to no difference in perinatal mortality with group antenatal care compared with individual antenatal care (RR 0.63, 95% CI 0.32 to 1.25; 3 RCTs, 1943 women; low‐certainty evidence).
Secondary outcomes
There may be little to no difference between group antenatal care and individual antenatal care for the outcomes low birthweight (RR 0.92, 95% CI 0.68 to 1.23; 3 RCTs, 1935 women; moderate‐certainty evidence), small‐for‐gestational age (RR 0.92, 95% CI 0.68 to 1.24; 2 RCTs, 1473 women), or admission to NICU (RR 1.48, 95% CI 0.63 to 3.45; 2 RCTs, 1315 women; moderate‐certainty evidence).
7. Diuretics for preventing pre‐eclampsia
Churchill 2007 included five RCTs that randomised 1836 pregnant women, both at high and low risk of pre‐eclampsia but without pre‐eclampsia at trial entry. This review examined whether the use of diuretics during pregnancy prevents the onset of pre‐eclampsia on stillbirth, perinatal death and small‐for‐gestational age. No studies reported effects of receiving diuretics during pregnancy on low birthweight or admission to NICU.
Primary outcomes
Unknown benefit or harm: there may be little to no difference between receiving diuretics versus placebo or no treatment in reducing stillbirth (RR 0.60, 95% CI 0.27 to 1.34; 5 RCTs, 1836 women; low‐certainty evidence) and perinatal death (RR 0.72, 95% CI 0.40 to 1.27; 5 RCTs, 1836 women; low‐certainty evidence).
Secondary outcomes
Small‐for‐gestational age was not estimable (1 RCT, 20 women).
8. Nitric oxide for preventing pre‐eclampsia and its complications
Meher 2007 included seven RCTs that randomised 389 pregnant women. This review determined the effectiveness and safety of nitric oxide agents on perinatal or neonatal mortality, small‐for‐gestational age and admission to NICU. The outcome low birthweight was not reported.
Primary outcomes
Unknown benefit or harm: nitric oxide agents administered to women during pregnancy may result in little to no difference in reducing perinatal or neonatal death (RR 0.25, 95% CI 0.03 to 2.34; 2 RCTs, 114 women; low‐certainty evidence).
Secondary outcomes
There was little to no clear difference between intervention and control group for the outcomes small‐for‐gestational age (RR 0.78, 95% CI 0.36 to 1.70, 2 RCTs, 108 women) or admission to NICU (RR 1.05, 95% CI 0.25 to 4.35; 1 RCT, 68 women).
9. Progesterone for preventing pre‐eclampsia and its complications
Meher 2006 included 10 RCTs with 4659 pregnant women with normal blood pressure or high blood pressure without proteinuria. This review assessed the effects of progesterone or any other progesterone to prevent pre‐eclampsia and its complications on fetal or neonatal death, small‐for‐gestational age and admission to NICU. Low birthweight was not reported.
Primary outcomes
Unknown benefit or harm: the evidence is very uncertain about the effect of receiving progesterone during pregnancy versus placebo or no treatment on fetal or neonatal death (RR 1.34, 95% CI 0.78 to 2.31; 4 RCTs; very low‐certainty evidence).
Secondary outcomes
Progesterone during pregnancy compared with placebo or no treatment may result in little to no difference in the risk of small‐for‐gestational age (RR 0.83, 95% CI 0.19 to 3.57; 1 RCT, 168 women) or admission to NICU (RR 1.06, 95% CI 0.83 to 1.35; 1 RCT).
10. Antioxidants for preventing pre‐eclampsia
Rumbold 2008 included 13 RCTs that randomised 16,606 pregnant women considered to be at low, moderate or high risk of developing pre‐eclampsia. This review assessed the effectiveness and safety of any antioxidant supplementation during pregnancy on stillbirth, small‐for‐gestational age and admission to NICU. Low birthweight was not reported.
Primary outcomes
Unknown benefit or harm: antioxidants for preventing pre‐eclampsia administered to pregnant women may have little to no effect in reducing miscarriage or stillbirth (RR 1.32, 95% CI 0.92 to 1.90; 4 RCTs, 5144 women; low‐certainty evidence) compared with control or placebo.
Secondary outcomes
Antioxidants for preventing pre‐eclampsia may result in little to no difference in small‐for‐gestational age (RR 0.83, 95% CI 0.62 to 1.11; 5 RCTs, 5271 women) or admission to NICU (RR 1.11, 95% CI 0.95 to 1.29; 1 RCT, 2714 women) compared with control or placebo.
11. Altered dietary salt
Duley 2005 included two RCTs with 603 pregnant women who had normal or high blood pressure without proteinuria during pregnancy. This review assessed the effects of altered dietary salt on the risk of developing pre‐eclampsia and its complications: perinatal death, small‐for‐gestational age and admission to NICU.
Primary outcomes
Unknown benefit or harm: low versus normal intake of dietary salt for preventing pre‐eclampsia in pregnant women with normal or high blood pressure probably makes little to no difference in perinatal death (RR 1.92, 95% CI 0.18 to 21.03; 2 RCTs, 409 women; moderate‐certainty evidence).
Secondary outcomes
There was little to no effect altered dietary salt on the risk of small‐for‐gestational age (RR 1.50, 95% CI 0.73 to 3.07; 1 RCT, 242 women) or admission to NICU (RR 0.98, 95% CI 0.69 to 1.40; 1 RCT, 361 women) compared with control.
12. Community‐based intervention packages
Lassi 2015 included 26 community‐based RCTs and quasi‐RCTs involving pregnant women at any period of gestation. This review assessed the effectiveness of community‐based intervention packages (community support groups/women's groups, community mobilisation and home visitation, or training traditional birth attendants who made home visits) in reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes: stillbirth and perinatal mortality. The effects of community‐based intervention packages in reducing low birthweight, small‐for‐gestational age and NICU admission were not reported.
Primary outcomes
Possible benefit: community‐based intervention may reduce stillbirth (RR 0.81, 95% CI 0.73 to 0.91; 15 RCTs, 201,181 women; low‐certainty evidence) and perinatal mortality (RR 0.78, 95% CI 0.70 to 0.86; 17 RCTs, 282,327 women; low‐certainty evidence).
13. Screening for gestational diabetes
Tieu 2017 included one RCT and one quasi‐RCT with a total of 4523 women. This review assessed the effects of screening for gestational diabetes mellitus on different risk profiles and settings on maternal and infant outcomes: stillbirth, perinatal mortality and admission to NICU. Low birthweight and small‐for‐gestational age were not reported.
Primary outcomes
Unknown benefit or harm: there may be little or no differences in stillbirth (RR 1.10, 95% CI 0.10 to 12.12; 1 RCT, 690 women; low‐certainty evidence) and the evidence is very uncertain for perinatal mortality (RR 1.10, 95% CI 0.10 to 12.12; 1 RCT, 690 women; very low‐certainty evidence) for women receiving primary care screening (screening appointment for gestational diabetes mellitus at their local general practitioner’s clinic) compared with secondary care screening (screening appointment for gestational diabetes mellitus at the hospital women attended for antenatal care).
Secondary outcomes
The intervention did not show a reduction in admission to NICU (RR 0.99, 95% CI 0.58 to 1.69; 1 RCT, 690 women) compared with control.
14. Diet and exercise for preventing gestational diabetes
Shepherd 2017 included 23 RCTs and cluster‐RCTs with 8918 women and 8709 infants that assessed the effects of combined diet and exercise intervention for preventing gestational diabetes mellitus and associated adverse health consequences. The effects of this intervention on stillbirth, perinatal mortality, small‐for‐gestational age and admission to NICU were reported. Low birthweight was not reported.
Primary outcomes
Unknown benefit or harm: the evidence is very uncertain on whether combined diet and exercise for preventing gestational diabetes mellitus makes any difference to stillbirth (RR 0.69, 95% CI 0.35 to 1.36; 5 RCTs, 4783 women; very low‐certainty evidence) and may have little to no effect on perinatal mortality (RR 0.82, 95% CI 0.42 to 1.63; 2 RCTs, 3757 women; low‐certainty evidence).
Secondary outcomes
Diet and exercise for preventing gestational diabetes made little to no difference in the risk of small‐for‐gestational age (RR 1.20, 95 CI 0.95 to 1.52; 6 RCTs 2434 women) and did not appear to reduce admission to NICU (RR 1.03, 95% CI 0.93 to 1.14; 4 RCTs, 4549 women).
15. Screening and management for thyroid dysfunction
Spencer 2015 included two RCTs with a total of 26,408 women. The review assessed the effects of different screening methods for thyroid dysfunction pre‐pregnancy and during pregnancy on maternal and infant outcomes: fetal and neonatal death, low birthweight and admission to NICU. Small‐for ‐gestational age was not reported.
Primary outcomes
Unknown benefit or harm or no effect or equivalence: there is probably little to no difference in fetal and neonatal death (RR 0.92, 95% CI 0.42 to 2.02; 1 RCT, 4516 women; moderate‐certainty evidence) for women receiving universal screening compared with case finding in pregnancy for any thyroid dysfunction.
Secondary outcomes
For low birthweight (RR 0.97, 95% CI 0.74 to 1.27; 1 RCT, 4516 women) or admission to NICU (RR 1.04, 95% CI 0.81 to 1.34; 1 RCT, 4516 women) there may be little or no difference for women receiving universal screening compared with case finding in pregnancy for any thyroid dysfunction.
16. Periodontal treatment (two comparisons)
Iheozor‐Ejiofor 2017 included 15 RCTs with 7161 women. This review assessed the effects of treating periodontal diseases in pregnant women in order to prevent or reduce perinatal and maternal morbidities and mortality. The outcomes perinatal mortality, low birthweight and small‐for‐gestational age were reported, but not admission to NICU.
16.1. Periodontal treatment versus no treatment
Primary outcomes
Unknown benefit or harm: the evidence was very uncertain about the effect of periodontal treatment for perinatal mortality (RR 0.85, 95% CI 0.51 to 1.43; 7 RCTs, 5320 women; very low‐certainty evidence) for women receiving periodontal treatment compared with no treatment.
Secondary outcomes
Periodontal treatment did not reduce the risk of small‐for‐gestational age compared with no treatment (RR 0.97, 95% CI 0.81 to 1.16; 3 RCTs, 3610 women; low‐certainty evidence). However, this intervention reduced the risk of low birthweight (RR 0.67, 95% CI 0.48 to 0.95; 7 RCTs, 3470 women; low‐certainty evidence).
16.2. Periodontal treatment versus alternative periodontal treatment
Primary outcomes
Unknown benefit or harm: there may be little to no difference for periodontal treatment versus alternative treatment on perinatal mortality (RR 1.06, 95% CI 0.60 to 1.85; 2 RCTs, 855 women; low‐certainty evidence).
Secondary outcomes
We are uncertain about the effect of periodontal treatment on low birthweight (RR 1.39, 95% CI 0.92 to 2.09; 1 RCT, 756 women; very low‐certainty evidence) compared with alternative periodontal treatment. Small‐for‐gestational age was not reported for this intervention.
17. Biochemical tests of placental function
Heazell 2015 included three RCTs and quasi‐RCTs that randomised 740 women. This review assessed whether clinicians' knowledge of the results of biochemical tests of placental function were associated with improvement in fetal and maternal outcomes of pregnancy such as stillbirth, small‐for‐gestational age or admission to NICU. Low birthweight was not reported. Placental function was tested using biochemical tests, e.g. measuring maternal oestrogen or human placental lactogen (hPL) levels, using maternal biofluids, alone or in combination with other tests for placental function.
Primary outcomes
Unknown benefit or harm: the evidence is very uncertain about the effect on miscarriage or stillbirth (RR 0.56, 95% CI 0.16 to 1.88; 2 RCTs, 740 women; very low‐certainty evidence) for women who had placental functional tests compared with women receiving standard care.
Secondary outcomes
There may be little to no difference in the risk of small‐for‐gestational age for women who had placental functional tests compared with women who received standard care (RR 0.44, 95% CI 0.16 to 1.19; 1 RCT, 118 women; low‐certainty evidence) or admission to NICU (RR 0.32, 95% CI 0.03 to 3.01; 1 RCT, 118 women).
Screening and management of fetal growth and well‐being (seven reviews)
We included seven Cochrane systematic reviews on screening and management of fetal growth and well‐being in this overview (Alfirevic 2015; Bricker 2015; Grivell 2015; Mangesi 2015; Robert Peter 2015; Stampalija 2010; Whitworth 2015). See Table 12 for all results relating to screening and management of fetal growth and well‐being.
1. Ultrasound for fetal assessment in early pregnancy
Whitworth 2015 included 11 RCTs and quasi‐RCTs that randomised 37,505 women with early pregnancies, (less than 24 weeks' gestation). This review assessed the effects of routine early pregnancy ultrasound for fetal assessment on perinatal mortality, low birthweight, small‐for‐gestational age and admission to NICU.
Primary outcomes
Unknown benefit or harm: there may be little to no difference between receiving routine or revealed versus selective or concealed ultrasound in early pregnancy for reducing perinatal death (RR 0.89, 95% CI 0.70 to 1.12; 10 RCTs, 35,735 women; low‐certainty evidence).
Secondary outcomes
There may be little or no difference in the risk of low birthweight (RR 1.04, 95% CI 0.82 to 1.33; 8 RCTs, 19,337 women) or small‐for‐gestational age (RR 1.05, 95% CI 0.81 to 1.35; 3 RCTs, 17,105 women) and no reduction in admission to NICU (RR 0.95, 95% CI 0.88 to 1.02; 8 RCTs, 19,088 women).
2. Routine ultrasound in late pregnancy (two comparisons)
Bricker 2015 included 13 RCTs and quasi‐RCTs with 34,980 women who received late pregnancy ultrasound (after 24 weeks' gestation). This review assessed the effects on obstetric practice and pregnancy outcome of routine late pregnancy ultrasound on stillbirth, perinatal mortality, low birthweight, small‐for‐gestational age and admission to NICU.
2.1. Routine ultrasound after 24 weeks' gestation versus no/concealed/selective ultrasound after 24 weeks' gestation
Primary outcomes
Unknown benefit or harm or no effect or equivalence: the evidence is very uncertain about the effect of routine ultrasound in late pregnancy (after 24 weeks' gestation) administered to women in late pregnancy on stillbirth (RR 1.18, 95% CI 0.51 to 2.70, 6 RCTs, 28,107 women; very low‐certainty evidence). Routine ultrasound in late pregnancy (after 24 weeks' gestation) probably makes little to no difference to perinatal mortality (RR 1.01, 95% CI 0.67 to 1.54, 8 RCTs, 30,675 women; moderate‐certainty evidence).
Secondary outcomes
There may be little to no difference in risk of low birthweight for women receiving routine ultrasound in late pregnancy (after 24 weeks' gestation; RR 0.92, 95% CI 0.71 to 1.18; 3 RCTs, 4510 women) or small‐for‐gestational age (RR 0.98, 95% CI 0.74 to 1.28; 4 RCTs, 20,293 women), but no reduction in admission to NICU (RR 1.01, 95% CI 0.91 to 1.14; 5 RCTs, 12,915 women).
Primary outcomes
Unknown benefit or harm: serial ultrasound and Doppler ultrasound administered to women in late pregnancy compared with selective ultrasound examination may have little to no effect on stillbirth (RR 0.84, 95% CI 0.36 to 1.93; 1 RCT, 2834 women; low‐certainty evidence) or perinatal mortality (RR 0.59, 95% CI 0.30 to 1.17; 1 RCT, 2834 women; low‐certainty evidence).
Secondary outcomes
There may be little to no difference between serial ultrasound and Doppler ultrasound compared with selective ultrasound for the outcomes low birthweight (RR 1.14, 95% CI 0.85 to 1.52; 1 RCT, 2834 women) and admission to NICU (RR 0.95, 95% CI 0.69 to 1.30; 1 RCT, 2834 women). However, there was an increase in the risk of small‐for‐gestational age for women receiving serial ultrasound and Doppler ultrasound (RR 1.36, 95% CI 1.10 to 1.68; 1 RCT, 2834 women).
3. Fetal movement counting
Mangesi 2015 included five RCTs and cluster‐RCTs that randomised 71,458 pregnant women who had reached the gestational age of fetal viability. This review evaluated the effects of fetal movement counting on stillbirth. The effect of fetal movement counting versus hormonal analysis was not assessed on low birthweight, small‐for‐gestational age and admission to NICU.
Primary outcomes
Unknown benefit or harm: in one RCT, the evidence was very uncertain about the risk of stillbirth for women receiving fetal movement counting compared to those receiving hormonal analysis (RR 3.19, 95% CI 0.13 to 78.20; 1 RCT, 1191 women; very low‐certainty evidence).
4. Fetal and umbilical Doppler ultrasound (five comparisons)
Alfirevic 2015 included five RCTs and quasi‐RCTs that randomised 14,624 pregnant women in both unselected and low‐risk populations. This review assessed the effects of routine fetal and umbilical Doppler ultrasound on stillbirth, perinatal mortality and admission to NICU. There were no comparisons that reported the effects of this intervention on low birthweight or small‐for gestational age.
4.1. All routine Doppler ultrasound versus no Doppler ultrasound (fetal/umbilical vessels only ‐ subgroup analysis)
Primary outcomes
Clear evidence of benefit: all routine Doppler ultrasound used only in fetal or umbilical vessels compared with no Doppler ultrasound probably reduces stillbirth (RR 0.34, 95% CI 0.12 to 0.95; 2 RCTs, 6877 women; moderate‐certainty evidence). However, it should be noted that in the main review (Alfirevic 2015), data for stillbirth were not pooled due to clinical heterogeneity. Data were presented separately for subgroups of fetal/umbilicla vessels only and fetal/umbilical + uterine artery. There was evidence of a difference between subgroups. Although the subgroup analysis of fetal/umbilical vessels only showed that Doppler may have improved rates of stillbirth, the subgroup analysis of fetal/umbilical vessels + uterine artery found no such differences. This result should therefore be treated with caution.
Unknown benefit or harm or no effect or equivalence: all routine Doppler ultrasound probably does not reduce perinatal mortality (RR 0.48, 95% CI 0.21 to 1.07; 2 RCTs, 5907 women; moderate‐certainty evidence), although the CI is wide and so we cannot be certain it has no effect .
Secondary outcomes
All routine Doppler ultrasound used only in fetal or umbilical vessels compared with no Doppler ultrasound did not reduce the risk of admission to NICU (RR 0.99, 95% CI 0.82 to 1.18; 2 RCTs, 5002 women).
4.2. All routine Doppler ultrasound versus no Doppler ultrasound (fetal/umbilical vessels + uterine artery ‐ subgroup analysis)
Primary outcomes
Unknown benefit or harm: there was little to no difference for women receiving all routine Doppler ultrasound in fetal or umbilical vessels in combination with uterine artery Doppler ultrasound compared with those receiving no Doppler ultrasound for stillbirth (RR 1.41, 95% CI 0.44 to 4.46; 2 RCTs, 5276 women; low‐certainty evidence). We are uncertain about the effect on perinatal mortality due to very low certainty evidence (RR 1.16, 95% CI 0.29 to 4.56; 2 RCTs, 5276 women; very low‐certainty evidence).
Secondary outcomes
There may be little to no difference in admission to NICU (RR 1.01, 95% CI 0.67 to 1.53; 1 RCT, 2475 women).
4.3. Single Doppler ultrasound assessment versus no Doppler ultrasound (fetal/umbilical vessels only)
Primary outcomes
Possible benefits: perinatal mortality may be reduced in women receiving single Doppler ultrasound used only in fetal or umbilical vessels (RR 0.36, 95% CI 0.13 to 0.99; 1 RCT, 3891 women; low‐certainty evidence).
Unknown benefit or harm: there may be little to no difference for women receiving single Doppler ultrasound assessment compared with those receiving no Doppler ultrasound in stillbirth (RR 0.40, 95% CI 0.08 to 2.06; 1 RCT, 3891 women; low‐certainty evidence).
4.4. Multiple Doppler ultrasound assessments versus no Doppler ultrasound (fetal/umbilical vessels only)
Primary outcomes
Unknown benefit or harm: there may be little to no difference in the risk of perinatal mortality for pregnant women receiving multiple Doppler ultrasound used only in fetal or umbilical vessels (RR 0.79, 95% CI 0.21 to 2.93; 1 RCT, 2016 women; low‐certainty evidence) compared with those who received no Doppler ultrasound.
Secondary outcomes
Multiple Doppler ultrasound used only in fetal or umbilical vessels did not reduce the risk of admission to NICU (RR 0.92, 95% CI 0.56 to 1.52; 1 RCT, 2016 women) compared with control.
4.5. Multiple Doppler ultrasound assessments versus no Doppler ultrasound (fetal/umbilical vessels + uterine artery)
Primary outcomes
Unknown benefit or harm: there may be little to no difference in the risk of stillbirth for women receiving multiple Doppler ultrasound in fetal or umbilical vessels in combination with uterine artery Doppler ultrasound assessments compared with those receiving no Doppler ultrasound (RR 1.41, 95% CI 0.44 to 4.46; 2 RCTs, 5276 women; low‐certainty evidence) or perinatal mortality (RR 1.16, 95% CI 0.29 to 4.56; 2 RCTs, 5276 women; low‐certainty evidence).
Secondary outcomes
There may be little to no difference in admission to NICU (RR 1.01, 95% CI 0.67 to 1.53; 1 RCT, 2475 women) between intervention and control group.
5. Utero‐placental Doppler ultrasound
Stampalija 2010 included two RCTs and quasi‐RCTs involving 4993 pregnant women who were considered to be either low or high risk, who had utero‐placental Doppler ultrasound performed at first or second trimester of pregnancy. This review assessed the effects of utero‐placental Doppler ultrasound on stillbirth, perinatal mortality, intrauterine growth restriction and admission to NICU.
Primary outcomes
Unknown benefit or harm: there may be little to no difference for women who received utero‐placental Doppler ultrasound assessment compared with those who had no Doppler ultrasound in the second trimester in stillbirth (RR 1.44, 95% CI 0.38 to 5.49; 2 RCTs, 5003 women; low‐certainty evidence) or perinatal mortality (RR 1.61, 95% CI 0.48 to 5.39; 2 RCTs, 5009 women; low‐certainty evidence).
Secondary outcomes
There may be little to no difference for women who received utero‐placental Doppler ultrasound assessment on intrauterine growth restriction (RR 0.98. 95% CI 0.64 to 1.50; 2 RCTs, 5006 women) or admission to NICU (RR 1.12, 95% CI 0.92 to 1.37; 2 RCTs, 5001 women) compared with those who had no Doppler ultrasound.
6. Antenatal cardiotocography (CTG) for fetal assessment (two comparisons)
Grivell 2015 included six RCTs and quasi‐RCTs involving 2105 pregnant women and their babies. This review assessed the effects of antenatal CTG for fetal assessment on perinatal mortality and admission to NICU. No comparisons reported the effects of the intervention on stillbirth, low birthweight or small‐for gestational age.
6.1. Traditional antenatal CTG versus no antenatal CTG
Primary outcomes
Unknown benefit or harm: there may be little to no difference for pregnant women at increased risk of pregnancy‐related complications who received traditional antenatal CTG in perinatal mortality (RR 2.05, 95% CI 0.95 to 4.42; 4 RCTs, 1627 women; low‐certainty evidence).
Secondary outcomes
There may be little to no difference on admission to NICU with traditional antenatal CTG (RR 1.08, 95% CI 0.84 to 1.39; 2 RCTs, 883 women; low‐certainty evidence) compared with no antenatal CTG.
6.2. Computerised antenatal CTG versus traditional antenatal CTG
Primary outcomes
Clear evidence of benefit: computerised antenatal CTG for assessing infants' well‐being in utero during pregnancy probably reduces perinatal mortality (RR 0.20, 95% CI 0.04 to 0.88; 2 RCTs, 469 women; moderate‐certainty evidence) compared with traditional antenatal CTG.
7. Symphysial fundal height measurement in pregnancy
Robert Peter 2015 included one RCT that randomised 1639 pregnant women with singleton fetuses of 20 weeks' gestation and above. This review assessed the effects of symphysial fundal height (SFH) with serial ultrasound measurement of fetal parameters or clinical palpation to detect abnormal fetal growth and outcomes: perinatal mortality, neonatal detection of small‐for‐dates and admission to NICU. The outcomes stillbirth and low birthweight were not reported.
Primary outcomes
Unknown benefit or harm: there may be little to no differences between tape measurement and clinical palpation in reducing perinatal mortality (RR 1.25, 95% CI 0.38 to 4.07; 1 RCT, 1639 women; low‐certainty evidence).
Secondary outcomes
The intervention made little to no difference on neonatal detection of small‐for‐dates (RR 1.32, 95% CI 0.92 to 1.90; 1 RCT, 1639 women; low‐certainty evidence) or the risk of admission to NICU (RR 1.06, 95% CI 0.70 to 1.61; 1 RCT, 1639 women; low‐certainty evidence).
Discussion
We identified 43 reviews investigating the effectiveness of various interventions during pregnancy for preventing stillbirth. The methodological quality of the included systematic reviews was found to be high according to AMSTAR quality ratings.
Summary of main results
In the 43 included Cochrane systematic reviews, we summarised the certainty of the evidence for our primary outcomes: stillbirth, fetal loss or fetal death, and perinatal death. Six graphic icons indicate our confidence in and interpretation of the available evidence (Figure 1). For moderate‐ or high‐certainty evidence, we used three graphic icons: a green tick for clear benefit, a red‐cross for clear harm, and a black equals sign for clear evidence of no effect or equivalence. For possible benefit and possible harm, we used a green plus sign and a yellow minus sign respectively. A blue question mark graphic icon indicates unknown benefit or harm or no effect or equivalence.
Nutritional interventions
The certainty of evidence and its direction of effect for all nutritional interventions are presented in Figure 3.
Stillbirth
Of all systematic reviews that reported stillbirth, only one systematic review reported clear benefit of the nutritional intervention of balanced protein/energy supplementation in pregnancy (Ota 2015a). We found the following interventions from two systematic reviews that showed a clear evidence of no effect or equivalence with a comparator: vitamin A alone versus placebo or no treatment (McCauley 2015); and multiple micronutrients with iron and folic acid versus iron with or without folic acid (Keats 2019).
Fourteen systematic reviews consisting of eighteen nutritional interventions reported evidence for the outcome of stillbirth that we categorised to be of unknown benefit, harm, or evidence of no effect or equivalence because of moderate‐ or low‐certainty evidence with wide confidence intervals crossing the line of no effect or very low‐certainty evidence.
Supplementation with any folate versus no intervention, placebo or other micronutrients without folate (De‐Regil 2015)
Vitamin A with other micronutrients versus micronutrient supplements without vitamin A (McCauley 2015)
Vitamin C supplementation alone or in combination with other supplements (Rumbold 2015a)
Vitamin D alone versus no treatment/placebo (no vitamins or minerals) (Palacios 2019)
Vitamin E supplementation (Rumbold 2015b)
Multivitamin versus control, multivitamin plus vitamin E versus multivitamin without vitamin E or control, folic acid plus iron versus iron (Balogun 2016)
Calcium supplementation versus placebo (before and/or early pregnancy only) (Hofmeyr 2019)
Routine high‐dose calcium supplementation in pregnancy by baseline dietary calcium, low‐dose calcium supplementation (< 1 g/day) with or without co‐supplements versus placebo or no treatment (Hofmeyr 2018)
Calcium supplementation versus placebo or no treatment (Buppasiri 2015)
Magnesium supplementation versus no magnesium (Makrides 2014)
Zinc supplementation versus no zinc (with or without placebo) (Ota 2015b)
Nutritional advice during pregnancy, high protein supplementation in pregnancy (Ota 2015a);
Omega‐3 versus no omega‐3 (Middleton 2018)
Lipid‐based nutrient supplements versus iron folic acid (Das 2018)
None of the systematic reviews reported clear evidence of harm, possible benefit or possible harm for any nutritional interventions.
The systematic reviews that assessed two interventions: folic acid plus iron and antimalarials versus iron and antimalarials (Balogun 2016), and any supplement containing iodine versus same supplement without iodine or no intervention/placebo (Harding 2017), did not assess the outcome of stillbirth.
Fetal loss or fetal death
Two systematic reviews assessed the effects of five nutritional interventions on prevention of fetal loss or fetal death that we categorised to be of unknown benefit, harm, or evidence of no effect or equivalence because of effects of estimates with wide confidence intervals and moderate/low‐ or very low‐certainty evidence: multivitamin versus control, multivitamin plus vitamin E versus multivitamin without vitamin E or control, folic acid plus iron versus iron, folic acid plus iron and antimalarials versus iron and antimalarials (Balogun 2016); and calcium supplementation versus placebo (before and/or early pregnancy only) (Hofmeyr 2019). None of the other systematic reviews reported the outcome of fetal loss or fetal death.
Perinatal death
For perinatal death prevention, seven systematic reviews assessed the effect of eight nutritional interventions. We categorised two interventions as moderate‐ or high‐certainty evidence that showed a clear evidence of no effect or equivalence with a comparator: vitamin A alone versus placebo or no treatment (McCauley 2015); multiple micronutrients with iron and folic acid versus iron with or without folic acid (Keats 2019). We found six nutritional interventions categorised to be of unknown benefit, harm, or evidence of no effect or equivalence because of moderate/low‐ or very low‐certainty evidence with wide confidence intervals: vitamin C supplementation alone or in combination with other supplements (Rumbold 2015a); any vitamin E supplementation (Rumbold 2015b); calcium supplementation versus placebo (before and/or early pregnancy only) (Hofmeyr 2019); any supplement containing iodine versus same supplement without iodine or no intervention/placebo (Harding 2017); omega‐3 versus no omega‐3 (Middleton 2018); and vitamin A with other micronutrients versus micronutrient supplements without vitamin A (McCauley 2015).
Prevention and management of infection
There were only two systematic reviews that assessed the impact of prevention and management of infection interventions on the reduction of stillbirth, fetal loss or fetal death and perinatal death (Figure 4).
We categorised one systematic review to be of unknown benefit, harm, or evidence of no effect or equivalence because of moderate or low GRADE certainty of evidence with a wide confidence interval crossing the line of no effect: preventive antimalarials versus placebo/no intervention (women of all parity groups; women in first or second pregnancy) (Radeva‐Petrova 2014).
We found evidence of possible benefit for reducing fetal loss or fetal death in the following interventions: insecticide‐treated nets versus no nets (all), and insecticide‐treated nets versus no nets (first or second pregnancy) (Gamble 2006). Another intervention included in this systematic review was assessed as evidence of unknown benefit, harm, or evidence of no effect or equivalence due to GRADE low‐ or very low‐certainty with a wide confidence interval crossing the line of no effect: insecticide‐treated nets versus no nets (fifth or greater pregnancy) (Gamble 2006).
Prevention, detection and management of other morbidities
The certainty of evidence and its direction for all interventions of prevention, detection and management of other morbidities are summarised in Figure 5.
Stillbirth
Only one systematic review that reported stillbirth showed a clear benefit of the intervention due to moderate certainty evidence (the confidence interval did not cross the line of no effect): trained versus untrained traditional birth attendants (Sibley 2012). We found the following intervention from a systematic review that showed a clear evidence of no effect or equivalence with a comparator: case notes versus control (Brown 2015). Only one intervention reported possible benefit for the effect of the community‐based interventions (Lassi 2015).
The following interventions were considered to be of unknown benefit, harm, or evidence of no effect or equivalence due to high‐, moderate‐ or low‐certainty evidence with wide confidence intervals crossing the line of no effect or very low‐certainty evidence: interventions for smoking cessation in pregnancy versus control (Chamberlain 2017); additionally trained versus trained traditional birth attendants (Sibley 2012); nicotine replacement therapy versus control (Coleman 2015); diuretic versus placebo or no treatment (Churchill 2007); any antioxidants versus control or placebo (Rumbold 2008); primary care screening versus secondary care screening (Tieu 2017); combined diet and exercise interventions versus standard care (Shepherd 2017); and test of placental function versus standard care (Heazell 2015).
Fetal loss or fetal death
Only three systematic reviews assessed the effect of interventions of prevention, detection and management of other morbidities on reduction of fetal loss or fetal death. A clear evidence of benefit was found for midwife‐led interventions for childbearing women and their infants (Sandall 2016). We categorised two systematic reviews as having evidence of unknown benefit, harm, or evidence of no effect or equivalence: progesterone versus placebo/no treatment (Meher 2006); universal screening versus case finding in pregnancy for any thyroid dysfunction (Spencer 2015).
Perinatal death
For perinatal death, we categorised one intervention as showing clear evidence of benefit: trained versus untrained traditional birth attendants (Sibley 2012); one intervention as a clear evidence of harm: reduced number of antenatal care visits/goal‐oriented versus standard antenatal care visits (Dowswell 2015) and one intervention as a evidence of possible benefit: community‐based intervention versus control (Lassi 2015).
The following interventions reported evidence for the outcome of perinatal death that we categorised to be of unknown benefit, harm, or evidence of no effect or equivalence due to moderate/low‐certainty evidence with wide confidence intervals crossing the line of no effect or very low‐certainty evidence.
Interventions for smoking cessation in pregnancy versus control (Chamberlain 2017)
Additionally trained versus trained traditional birth attendants (Sibley 2012)
Group antenatal care versus individual antenatal care (Catling 2015)
Diuretic versus placebo or no treatment for preventing pre‐eclampsia (Churchill 2007)
Nitric oxide versus placebo/no intervention for preventing pre‐eclampsia (Meher 2007)
Low versus normal salt intake in pregnancy (Duley 2005).
Primary care screening versus secondary care screening for gestational diabetes mellitus (Tieu 2017)
Combined diet and exercise interventions versus standard care for gestational diabetes mellitus (Shepherd 2017)
Periodontal treatment versus no treatment, periodontal treatment versus alternative periodontal treatment (Iheozor‐Ejiofor 2017)
Screening and management of fetal growth and well‐being
The certainty of evidence and its direction for screening and management of fetal growth and well‐being related interventions are described in Figure 6.
Seven systematic reviews reported the outcome of stillbirth or perinatal death, but no reviews reported fetal loss or fetal death.
Stillbirth
We found one systematic review that reported stillbirth with an intervention that we classified as showing clear evidence of benefit due to moderate‐ or high‐certainty evidence (the confidence interval did not cross the line of no effect): all routine Doppler ultrasound versus no Doppler ultrasound (fetal/umbilical vessels only) (Alfirevic 2015). However, this finding should be viewed with caution because it is based on a subgroup analysis as data were not pooled for the main analysis due to clinical heterogeneity.
We categorised the following interventions targeting stillbirth to be of unknown benefit, harm, or unknown or no effect or equivalence because of moderate/low‐certainty evidence with wide confidence intervals crossing the line of no effect or very low‐certainty evidence.
Routine ultrasound after 24 weeks versus no/concealed/selective ultrasound after 24 weeks, serial ultrasound and Doppler ultrasound versus selective ultrasound (Bricker 2015)
Fetal movement counting versus hormonal analysis (Mangesi 2015)
All routine Doppler ultrasound versus no Doppler ultrasound (fetal/umbilical vessels + uterine artery), single Doppler ultrasound assessment versus no Doppler ultrasound (fetal/umbilical vessels only), multiple Doppler ultrasound assessments versus no Doppler ultrasound (fetal/umbilical vessels + uterine artery) (Alfirevic 2015)
Utero‐placental Doppler ultrasound versus no Doppler ultrasound, second trimester (Stampalija 2010).
Perinatal death
For perinatal death reduction, we found one intervention with a clear evidence of benefit: computerised antenatal CTG versus traditional antenatal CTG (Grivell 2015), and one intervention as a possible benefit: single Doppler ultrasound assessment versus no Doppler ultrasound (Fetal/umbilical vessels only) (Alfirevic 2015).
We categorised the following interventions as having evidence of unknown benefit, harm, or unknown equivalence due to moderate/low‐certainty evidence with wide confidence intervals crossing the line of no effect or very low‐certainty evidence.
Routine/revealed versus selective/concealed ultrasound in early pregnancy (Whitworth 2015)
Routine ultrasound after 24 weeks versus no/concealed/selective ultrasound after 24 weeks (Bricker 2015)
Serial ultrasound and Doppler ultrasound versus selective ultrasound (Bricker 2015)
All routine Doppler ultrasound versus no Doppler ultrasound (fetal/umbilical vessels only) (Alfirevic 2015)
All routine Doppler ultrasound versus no Doppler ultrasound (fetal/umbilical vessels + uterine artery), multiple Doppler ultrasound assessments versus no Doppler ultrasound (fetal/umbilical vessels only), multiple Doppler ultrasound assessments versus no Doppler ultrasound (fetal/umbilical vessels + uterine artery) (Alfirevic 2015)
Utero‐placental Doppler ultrasound versus no Doppler ultrasound, second trimester (Stampalija 2010)
Traditional antenatal CTG versus no antenatal CTG (Grivell 2015)
Tape measurement versus clinical palpation (Robert Peter 2015)
Overall completeness and applicability of evidence
We identified a total of 43 Cochrane Reviews that focused on 61 different comparisons for preventing stillbirth for this overview. The overview addresses a broad question about the effectiveness of various interventions during pregnancy on stillbirth. All reviews involved the appropriate types of participants, interventions, comparators and outcome measures. However, only seven interventions showed an effect during pregnancy to reduce the risk of stillbirth, perinatal death or fetal loss. Although the number of Cochrane Reviews included in this overview was large, most of the results were derived from a small number of trials, therefore, limiting the evidence to support the effectiveness of intervention during pregnancy on stillbirth. The findings should be interpreted with caution.
While the available evidence to support the interventions to reduce the risk of stillbirth provided by this review is limited, we believe that it would be useful to understand the association between the interventions and their effects. The findings of this overview are applicable to near future international policy agenda and practice. However, the evidence suggests that seven prevention interventions during pregnancy were only beneficial in specific target populations or settings.
Balance protein/energy supplementation in pregnancy appeared effective particularly in undernourished pregnant women. The evidence suggests this intervention is unlikely to be effective in overweight pregnant women or in those who exhibit high weight gain.
Insecticide‐treated nets were effective when targeted at women with a number of previous pregnancies and conducted in settings where malaria is endemic. Therefore, they may only be applicable and may have a much larger impact when applied in malaria‐endemic areas.
Midwife‐led care models were effective only for fetal death before 24 weeks of gestation, administered in settings where a midwife is the primary healthcare provider to provide care for childbearing women, particularly for low‐risk pregnant women. The applicability of this intervention to other settings where, for example, medical doctors are the major healthcare providers should be considered.
Traditional birth attendant intervention was conducted in rural populations of low‐ and middle‐income countries, where traditional birth attendants were accessible and preferred to assist women during pregnancy and labour, and post‐partum. The effects of this intervention are unknown in settings with lower numbers of traditional birth attendants, lack of access to health facilities or in urban populations. The results should be interpreted with caution since it was derived from one study conducted in 2005.
Community‐based intervention packages including community support groups/women's groups, community mobilisation and home visitation, or training traditional birth attendants who made home visits were mostly applied in low‐ and middle‐income countries.
All routine Doppler ultrasound, particularly using fetal/umbilical vessels only targeted unselected and low‐risk pregnant women. Studies assessing the effects of this intervention were published between 1994 and 1997. The relevance of this intervention for its implication to current practice thus should be considered.
Computerised antenatal CTG was performed in high‐income countries, where CTG may be feasible and affordable. Moreover, the participants in the trials administered with this intervention were women at risk of complications only, therefore it is not clear if this intervention can be evaluated or would be beneficial for low‐risk women living in low‐ and middle‐income countries. Furthermore, the quality of trials was low, and so results should be interpreted with caution.
The findings may slightly differ across target populations and settings. Most of the included studies were conducted in low‐ and middle‐income countries and it is therefore not clear if the findings can be applied to the general population of pregnant women and in all contexts globally for reducing the risk of stillbirth. Most of the interventions were more system‐ or community‐based rather than individual interventions. Our results show that broader interventions such as nutrition, models of care, and community‐based interventions like insect nets may be more effective than more screening, monitoring or individual interventions.
Quality of the evidence
In this overview, we used the AMSTAR rating scale to assess the overall quality of evidence and methodology in each Cochrane Review. The results of AMSTAR are described in Table 5, Table 6, Table 7 and Table 8. The AMSTAR scale uses three levels of quality: high, moderate and low. Of the included reviews, we assessed 40 as having high scores between 8 to 11 and four as moderate with a score of 7.
Forty‐two out of 43 (98%) Cochrane systematic reviews used the domain‐based evaluation for assessment of risk of bias as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We rated most of the included reviews at low risk of bias in terms of sequence generation and allocation concealment (risk of selection bias) (Balogun 2016; Bricker 2015; Buppasiri 2015; Das 2018; De‐Regil 2015; Duley 2005; Gamble 2006; Hofmeyr 2019; Iheozor‐Ejiofor 2017; Middleton 2018; Meher 2007; Ota 2015b; Rumbold 2008; Rumbold 2015a; Sandall 2016; Shepherd 2017; Sibley 2012; Spencer 2015; Whitworth 2015). But some reviews failed to provide evidence of the treatment allocation procedure (Churchill 2007; Lassi 2015; Meher 2006a; Ota 2015a; Radeva‐Petrova 2014). Most of the participants in the included studies of the following reviews were blinded to treatment allocation (risks of performance and detection bias) (Balogun 2016; Buppasiri 2015; De‐Regil 2015; Keats 2019; Hofmeyr 2018; Hofmeyr 2019; Middleton 2018; Ota 2015b; Radeva‐Petrova 2014; Rumbold 2008; Rumbold 2015a; Shepherd 2017). Some reviews reported loss to follow‐up data or attrition and risk of incomplete data outcome (Alfirevic 2015; Churchill 2007; Dowswell 2015; Duley 2005; Gamble 2006; Keats 2019; Meher 2006; Ota 2015b; Rumbold 2008; Rumbold 2015a). Heterogeneity amongst included studies was very high in one review (Chamberlain 2017), but was reported low in one review (De‐Regil 2015).
We evaluated pooled outcome data from each systematic review using GRADE assessments. We did not reassess the GRADE assessment for our primary outcomes in the included systematic reviews where it was reported by review authors. If review authors did not assess GRADE, we made a new assessment ourselves. As we included a large number of systematic reviews, we created figures by assigning graphic icons to present the direction of review effect estimates with our confidence on estimates (Figure 3; Figure 4; Figure 5; Figure 6), as outlined in the Methods in Assessment of methodological quality of included reviews.
Potential biases in the overview process
At all stages of conducting this overview, we considered a number of potential biases. We attempted to reduce the risk of bias in several ways: two review authors independently applied the eligibility criteria and assessed the reviews for inclusion, extracted data, and assessed the scientific quality of reviews according to AMSTAR. Review authors who are also authors of included reviews were not involved in the selection or AMSTAR assessment of the particular review. We reached consensus through virtual consultation with a third review author. We included only reviews that included individual RCTs, cluster‐RCTs, quasi‐RCTs or cross‐over trials to limit the risk of bias that may be reported by observational data and narrative reviews. Although all included reviews used a standard methodological quality assessment to assess the risk of bias of included trials, where information was incomplete or data reporting errors were suspected, we referred to the original study reports from the Cochrane Reviews.
At the time when this overview was completed, a few of the potential reviews had not yet finished. Two of the 43 included Cochrane Reviews (Duley 2005; Gamble 2006), had not conducted new searches since 2009. One review (Gamble 2006), from the Cochrane Infectious Diseases, could not be searched from Cochrane Pregnancy and Childbirth's Trials Register (via the Information Specialist). Therefore, the findings we have reported in this overview do not include the new study results from these reviews.
Agreements and disagreements with other studies or reviews
In this overview, the included systematic reviews differed in terms of their setting and this, together with differences in assessments of quality, may account for disagreements in findings relating to stillbirth/fetal loss/perinatal death. For example, a pooled analysis of a Cochrane Review for promoting calcium supplementation commencing before or early in pregnancy for preventing hypertensive disorder during pregnancy showed no clear evidence to support this intervention in reducing stillbirth (Hofmeyr 2019). The findings of this review are in agreement with evidence from another two reviews, which assessed the effectiveness of this intervention on preventing or treating hypertension and related problems during pregnancy (Hofmeyr 2018; Buppasiri 2015). Ota 2015a assessed the effectiveness of protein and energy supplementation in pregnancy and reported that balanced protein/energy supplementation during pregnancy was significantly associated with a 40% reduction of stillbirths, but there was no clear evidence in reducing stillbirths when pregnant women received high‐protein supplementation.
Bhutta 2011 reviewed 35 potential interventions to prevent stillbirths and recommended 10 interventions. For nutritional interventions, they recommended periconceptional folic acid fortification. This is in disagreement with the findings of two included reviews in our overview, which saw no clear evidence in the reduction of stillbirths for women receiving folic acid supplementation (Balogun 2016; De‐Regil 2015). However, we identified that balanced energy and protein supplementation were effective in reducing stillbirth (Ota 2015a).
Authors' conclusions
Implications for practice.
This overview summarises the evidence from Cochrane systematic reviews of randomised controlled trials (RCTs) of antepartum interventions aiming to prevent stillbirth, perinatal mortality, fetal loss and fetal death, and can be used by researchers, clinicians, decision makers or policy makers to assist them in decision‐making and knowledge translation. While most interventions were unable to demonstrate a clear effect in reducing stillbirth or perinatal death, several interventions suggested a clear benefit, such as, balanced energy/protein supplements, midwife‐led models of care, training versus not training traditional birth attendants, and antenatal cardiotocography. Possible benefits were also observed for insecticide‐treated anti‐malarial nets and community‐based intervention packages, whereas a reduced number of antenatal care visits were shown to be harmful. However, there was variation in effectiveness of interventions across different settings, indicating the need to carefully understand the context in which these interventions were tested.
Further high‐quality RCTs are needed to evaluate the effects of antenatal preventive interventions and which approaches are most effective to reduce the risk of stillbirth. Stillbirth (or fetal death), perinatal and neonatal death needs to be reported separately in future RCTs of antenatal interventions to allow assessment of different interventions on these rare but important outcomes and they need to clearly define the target populations of women where the intervention is most likely to be of benefit. As the high burden of stillbirths occurs in low‐ and middle‐income countries, further high‐quality trials are needed to be conducted in these settings as a priority.
Implications for research.
Research efforts should be focused on high‐quality RCTs to evaluate the effects of prevention interventions, including technology‐based interventions, on measuring stillbirth and to ensure the accuracy of the evidence. Future research should be conducted to clarify which approaches are more effective to reduce the risk of stillbirth. Moreover, stillbirth, perinatal mortality, fetal loss or fetal death should be investigated as a primary or secondary outcome, measured by World Health Organization definitions, in new RCTs, to ensure that the best evidence is readily available. It would be helpful to report all losses before birth (presumably after some reasonably early gestational age) as a trial outcome. Future trials are needed, especially focusing in specific areas and target populations of women who are eligible to receive the interventions to further generalise the findings. Because the burden of stillbirth is more in low‐ and middle‐income countries, more high‐quality trials should be conducted in these countries and future trials are required before these interventions can be expanded to other settings.
There is also a need for the assessment of risk factors associated with the outcome of stillbirth, and assessment of adverse effects related to the interventions should be taken into account.
What's new
| Date | Event | Description |
|---|---|---|
| 18 December 2020 | Amended | Edited to resolve a format error in Figure 2. |
History
Protocol first published: Issue 1, 2012 Review first published: Issue 12, 2020
Acknowledgements
As part of the pre‐publication editorial process, three peers (an editor and two referees who are external to the editorial team) commented on this review, as well as a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewer for her time and comments: Professor Caroline Homer and also to the other peer reviewer who wishes to remain anonymous. We also appreciate the support of Leanne Jones from Cochrane Pregnancy and Childbirth.
This work was supported by JSPS KAKENHI Grant‐in‐Aid for Scientific Research (B) Grant Number JP17H04452. We appreciated the support from Dr.João Paulo Souza in the protocol stage.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. Search strategy
#1 MeSH descriptor: [Stillbirth] explode all trees #2 MeSH descriptor: [Perinatal Mortality] explode all trees #3 MeSH descriptor: [Fetal Death] explode all trees #4 (stillbirth):ti,ab,kw #5 ("perinatal mortality"):ti,ab,kw #6 (fetal loss):ti,ab,kw #7 (fetal death):ti,ab,kw #8 "stillbirth" #9 "perinatal mortality" #10 "fetal death" #11 fetal loss #12 (pregnan*):ti,ab,kw #13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR # #14 #13 AND #12
Differences between protocol and review
Three additional review authors have joined the review team: Windy MV Wariki, Katharina da Silva Lopes and Md. Obaidur Rahman.
We planned to add to the relevant Cochrane Reviews the recent primary clinical trials, which had not yet been included in the reviews and which included our primary and secondary outcomes. However, since the majority of the reviews were recently updated, we included only published data included in the reviews.
We added a framework to the methods for data synthesis for summarising the evidence from the systematic reviews. This framework assigns graphic icons to communicate the direction of review effect estimates and our confidence in the available data. This is the framework adopted by Medley and colleagues in their overview on 'Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews' (Medley 2018), and was based on graphics produced by the World Health Organization to describe different types of workers and their roles in maternal and newborn care (optimizemnh.org/optimizing-health-worker-roles-maternal-newborn-health). We adapted this framework slightly, but still used graphic icons to indicate mutually exclusive assessment categories (Figure 1).
Clear evidence of benefit (moderate‐ or high‐certainty evidence with CIs not crossing line of no effect)
Clear evidence of harm (moderate‐ or high‐certainty evidence with CIs not crossing line of no effect)
Clear evidence of no effect or equivalence (moderate‐ or high‐certainty evidence with narrow CIs crossing the line of no effect)
Possible benefit (low‐certainty evidence with clear benefit, or moderate‐ or high‐certainty evidence with wide CIs not crossing the line of no effect)
Possible harm (low‐certainty evidence with clear harm, or moderate‐ or high‐certainty evidence with wide CIs not crossing the line of no effect)
Unknown benefit or harm or no effect or equivalence (low, moderate or high‐certainty evidence with wide CIs crossing the line of no effect, or low‐certainty evidence with no effect or equivalence, or very low‐certainty evidence)
Contributions of authors
Erika Ota (EO) and Rintaro Mori (RM) participated in the study design. EO, Md. Obaidur Rahman and Katharina da Silva Lopes drafted the review. Windy Wariki, Ruoyan Tobe‐Gai, RM, Philippa Middleton and Vicki Flenady provided critical comments and valuable suggestions.
Sources of support
Internal sources
Department of Reproductive Health and Research and Department of Technical Cooperation among Countries, World Health Organization, Geneva, Switzerland
The Grant of National Center for Child Health and Development 27B‐10, Japan
JSPS KAKENHI Grant‐in‐Aid for Scientific Research(B)Grant Number JP17H04452, Japan
External sources
Scheme for Academic Mobility and Exchange (SAME) program of Ministry of Research, Technology, and Higher Education of Indonesia. 2018, Indonesia
Declarations of interest
Erika Ota: author of 'Vitamin supplementation for preventing miscarriage', 'Antenatal dietary education and supplementation to increase energy and protein intake', 'Zinc supplementation for improving pregnancy and infant outcome', 'Vitamin E supplementation in pregnancy', 'Vitamin C supplementation in pregnancy', and 'Iodine supplementation for women during the preconception pregnancy and postpartum period'.
Katharina da Silva Lopes: author of 'Vitamin supplementation for preventing miscarriage'.
Md. Obaidur Rahman: none known.
Philippa Middleton: author of 'Omega‐3 fatty acid addition during pregnancy' and 'Zinc supplementation for improving pregnancy and infant outcome'.
Vicki Flenady: I have a Career Development Fellowship grant for my salary from the National Health and Medical Research Council Australia.
Windy MV Wariki: none known.
Ruoyan Tobe‐Gai: author of 'Antenatal dietary education and supplementation to increase energy and protein intake', and 'Zinc supplementation for improving pregnancy and infant outcome'.
Rintaro Mori: author of 'Giving women their own case notes to carry during pregnancy.', 'Vitamin supplementation for preventing miscarriage', 'Antenatal dietary education and supplementation to increase energy and protein intake', and 'Zinc supplementation for improving pregnancy and infant outcome'
Edited (no change to conclusions)
References
References to included reviews
Alfirevic 2015
- Alfirevic Z, Stampalija T, Medley N. Fetal and umbilical Doppler ultrasound in normal pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD001450. [DOI: 10.1002/14651858.CD001450.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balogun 2016
- Balogun OO, da Silva Lopes K, Ota E, Takemoto Y, Rumbold A, Takegata M, et al. Vitamin supplementation for preventing miscarriage. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD004073. [DOI: 10.1002/14651858.CD004073.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bricker 2015
- Bricker L, Medley N, Pratt JJ. Routine ultrasound in late pregnancy (after 24 weeks' gestation). Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD001451. [DOI: 10.1002/14651858.CD001451.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2015
- Brown HC, Smith HJ, Mori R, Noma H. Giving women their own case notes to carry during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD002856. [DOI: 10.1002/14651858.CD002856.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buppasiri 2015
- Buppasiri P, Lumbiganon P, Thinkhamrop J, Ngamjarus C, Laopaiboon M, Medley N. Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD007079. [DOI: 10.1002/14651858.CD007079.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Catling 2015
- Catling CJ, Medley N, Foureur M, Ryan C, Leap N, Teate A, et al. Group versus conventional antenatal care for women. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD007622. [DOI: 10.1002/14651858.CD007622.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamberlain 2017
- Chamberlain C, O'Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD001055. [DOI: 10.1002/14651858.CD001055.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Churchill 2007
- Churchill D, Beevers GD, Meher S, Rhodes C. Diuretics for preventing pre-eclampsia. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD004451. [DOI: 10.1002/14651858.CD004451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleman 2015
- Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD010078. [DOI: 10.1002/14651858.CD010078.pub2] [DOI] [PubMed] [Google Scholar]
Das 2018
- Das JK, Hoodbhoy Z, Salam RA, Bhutta AZ, Valenzuela-Rubio NG, Weise Prinzo Z, et al. Lipid-based nutrient supplements for maternal, birth, and infant developmental outcomes. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD012610. [DOI: 10.1002/14651858.CD012610.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2015
- De-Regil LM, Peña‐Rosas JP, Fernandez-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD007950. [DOI: 10.1002/14651858.CD007950.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowswell 2015
- Dowswell T, Carroli G, Duley L, Gates S, Gülmezoglu AM, Khan-Neelofur D, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD000934. [DOI: 10.1002/14651858.CD000934.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2005
- Duley L, Henderson-Smart D, Meher S. Altered dietary salt for preventing pre-eclampsia, and its complications. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No: CD005548. [DOI: 10.1002/14651858.CD005548] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gamble 2006
- Gamble CL, Ekwaru JP, ter Kuile FO. Insecticide-treated nets for preventing malaria in pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD003755. [DOI: 10.1002/14651858.CD003755.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grivell 2015
- Grivell RM, Alfirevic Z, Gyte GM, Devane D. Antenatal cardiotocography for fetal assessment. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD007863. [DOI: 10.1002/14651858.CD007863.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harding 2017
- Harding KB, Peña‐Rosas JP, Webster AC, Yap CM, Payne BA, Ota E, et al. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD011761. [DOI: 10.1002/14651858.CD011761.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heazell 2015
- Heazell AE, Whitworth M, Duley L, Thornton JG. Use of biochemical tests of placental function for improving pregnancy outcome. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD011202. [DOI: 10.1002/14651858.CD011202.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2018
- Hofmeyr GJ, Lawrie TA, Atallah AN, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD001059. [DOI: 10.1002/14651858.CD001059.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2019
- Hofmeyr GJ, Manyame S, Medley N, Williams MJ. Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD011192. [DOI: 10.1002/14651858.CD011192.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iheozor‐Ejiofor 2017
- Iheozor‐Ejiofor Z, Middleton P, Esposito M, Glenny AM. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD005297. [DOI: 10.1002/14651858.CD005297.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keats 2019
- Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD004905. [DOI: 10.1002/14651858.CD004905.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassi 2015
- Lassi ZS, Bhutta ZA. Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD007754. [DOI: 10.1002/14651858.CD007754.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Makrides 2014
- Makrides M, Crosby DD, Bain E, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD000937. [DOI: 10.1002/14651858.CD000937.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mangesi 2015
- Mangesi L, Hofmeyr GJ, Smith V, Smyth RM. Fetal movement counting for assessment of fetal wellbeing. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD004909. [DOI: 10.1002/14651858.CD004909.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCauley 2015
- McCauley ME, Van den Broek N, Dou L, Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD008666. [DOI: 10.1002/14651858.CD008666.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2006
- Meher S, Duley L. Progesterone for preventing pre-eclampsia and its complications. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD006175. [DOI: 10.1002/14651858.CD006175] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2007
- Meher S, Duley L. Nitric oxide for preventing pre-eclampsia and its complications. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD006490. [DOI: 10.1002/14651858.CD006490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Middleton 2018
- Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD003402. [DOI: 10.1002/14651858.CD003402.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ota 2015a
- Ota E, Hori H, Mori R, Tobe-Gai R, Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD000032. [DOI: 10.1002/14651858.CD000032.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ota 2015b
- Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD000230. [DOI: 10.1002/14651858.CD000230.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palacios 2019
- Palacios C, Lostiuk LK, Peña‐Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD008873. [DOI: 10.1002/14651858.CD008873.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radeva‐Petrova 2014
- Radeva-Petrova D, Kayentao K, ter Kuile FO, Sinclair D, Garner P. Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD000169. [DOI: 10.1002/14651858.CD000169.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robert Peter 2015
- Robert Peter J, Ho JJ, Valliapan J, Sivasangari S. Symphysial fundal height (SFH) measurement in pregnancy for detecting abnormal fetal growth. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD008136. [DOI: 10.1002/14651858.CD008136.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rumbold 2008
- Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre-eclampsia. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD004227. [DOI: 10.1002/14651858.CD004227.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rumbold 2015a
- Rumbold A, Ota E, Nagata C, Shahrook S, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD004072. [DOI: 10.1002/14651858.CD004072.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rumbold 2015b
- Rumbold A, Ota E, Hori H, Miyazaki C, Crowther CA. Vitamin E supplementation in pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD004069. [DOI: 10.1002/14651858.CD004069.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandall 2016
- Sandall J, Soltani H, Gates S, Shennan A, Devane D. Midwife-led continuity models versus other models of care for childbearing women. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD004667. [DOI: 10.1002/14651858.CD004667.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepherd 2017
- Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD010443. [DOI: 10.1002/14651858.CD010443.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sibley 2012
- Sibley LM, Sipe TA, Barry D. Traditional birth attendant training for improving health behaviours and pregnancy outcomes. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD005460. [DOI: 10.1002/14651858.CD005460.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2015
- Spencer L, Bubner T, Bain E, Middleton P. Screening and subsequent management for thyroid dysfunction pre-pregnancy and during pregnancy for improving maternal and infant health. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011263. [DOI: 10.1002/14651858.CD011263.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stampalija 2010
- Stampalija T, Gyte GM, Alfirevic Z. Utero-placental Doppler ultrasound for improving pregnancy outcome. Cochrane Database of Systematic Reviews 2010, Issue 9. Art. No: CD008363. [DOI: 10.1002/14651858.CD008363.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tieu 2017
- Tieu J, McPhee AJ, Crowther CA, Middleton P, Shepherd E. Screening for gestational diabetes mellitus based on different risk profiles and settings for improving maternal and infant health. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD007222. [DOI: 10.1002/14651858.CD007222.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitworth 2015
- Whitworth M, Bricker L, Mullan C. Ultrasound for fetal assessment in early pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD007058. [DOI: 10.1002/14651858.CD007058.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to excluded reviews
Alexander 2010
- Alexander S, Boulvain M, Ceysens G, Haelterman E, Zhang WH. Repeat digital cervical assessment in pregnancy for identifying women at risk of preterm labour. Cochrane Database of Systematic Reviews 2010, Issue 6. Art. No: CD005940. [DOI: 10.1002/14651858.CD005940.pub2] [DOI] [PubMed] [Google Scholar]
Balogun 2016
- Balogun OO, O'Sullivan EJ, McFadden A, Ota E, Gavine A, Garner CD, et al. Interventions for promoting the initiation of breastfeeding. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD001688. [DOI: 10.1002/14651858.CD001688.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergel 2002
- Bergel E, Carroli G, Althabe F. Ambulatory versus conventional methods for monitoring blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD001231. [DOI: 10.1002/14651858.CD001231] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 2010
- Crowther CA, Crosby DD, Henderson-Smart DJ. Vitamin K prior to preterm birth for preventing neonatal periventricular haemorrhage. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD000229. [DOI: 10.1002/14651858.CD000229.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Demicheli 2015
- Demicheli V, Barale A, Rivetti A. Vaccines for women for preventing neonatal tetanus. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD002959. [DOI: 10.1002/14651858.CD002959.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
East 2019
- East CE, Biro MA, Fredericks S, Lau R. Support during pregnancy for women at increased risk of low birthweight babies. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD000198. [DOI: 10.1002/14651858.CD000198.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gagnon 2007
- Gagnon AJ, Sandall J. Individual or group antenatal education for childbirth or parenthood, or both. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD002869. [DOI: 10.1002/14651858.CD002869.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jahanfar 2015
- Jahanfar S, Jaafar SH. Effects of restricted caffeine intake by mother on fetal, neonatal and pregnancy outcomes. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD006965. [DOI: 10.1002/14651858.CD006965.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 2006
- Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD000180. [DOI: 10.1002/14651858.CD000180.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lagarde 2009
- Lagarde M, Haines A, Palmer N. The impact of conditional cash transfers on health outcomes and use of health services in low and middle income countries. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD008137. [DOI: 10.1002/14651858.CD008137] [DOI] [PMC free article] [PubMed] [Google Scholar]
McBain 2015
- McBain RD, Crowther CA, Middleton P. Anti-D administration in pregnancy for preventing Rhesus alloimmunisation. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD000020. [DOI: 10.1002/14651858.CD000020.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meher 2006a
- Meher S, Duley L. Rest during pregnancy for preventing pre-eclampsia and its complications in women with normal blood pressure. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD005939. [DOI: 10.1002/14651858.CD005939] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muktabhant 2015
- Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD007145. [DOI: 10.1002/14651858.CD007145.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabhan 2008
- Nabhan AF, Abdelmoula YA. Amniotic fluid index versus single deepest vertical pocket as a screening test for preventing adverse pregnancy outcome. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD006593. [DOI: 10.1002/14651858.CD006593.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabhan 2015
- Nabhan AF, Aflaifel N. High feedback versus low feedback of prenatal ultrasound for reducing maternal anxiety and improving maternal health behaviour in pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD007208. [DOI: 10.1002/14651858.CD007208.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pattinson 2005
- Pattinson RC, Say L, Makin JD, Bastos MH. Critical incident audit and feedback to improve perinatal and maternal mortality and morbidity. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No: CD002961. [DOI: 10.1002/14651858.CD002961.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peña‐Rosas 2015a
- Peña-Rosas JP, De-Regil LM, Gomez Malave H, Flores‐Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD009997. [DOI: 10.1002/14651858.CD009997.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peña‐Rosas 2015b
- Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD004736. [DOI: 10.1002/14651858.CD004736.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salam 2015
- Salam RA, Zuberi NF, Bhutta ZA. Pyridoxine (vitamin B6) supplementation during pregnancy or labour for maternal and neonatal outcomes. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD000179. [DOI: 10.1002/14651858.CD000179.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sangkomkamhang 2015
- Sangkomkamhang US, Lumbiganon P, Prasertcharoensuk W, Laopaiboon M. Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD006178. [DOI: 10.1002/14651858.CD006178.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stade 2009
- Stade BC, Bailey C, Dzendoletas D, Sgro M, Dowswell T, Bennett D. Psychological and/or educational interventions for reducing alcohol consumption in pregnant women and women planning pregnancy. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD004228. [DOI: 10.1002/14651858.CD004228.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Lonkhuijzen 2012
- Van Lonkhuijzen L, Stekelenburg J, Van Roosmalen J. Maternity waiting facilities for improving maternal and neonatal outcome in low-resource countries. Cochrane Database of Systematic Reviews 2012, Issue 10. Art. No: CD006759. [DOI: 10.1002/14651858.CD006759.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2001
- Walker GJ. Antibiotics for syphilis diagnosed during pregnancy. Cochrane Database of Systematic Reviews 2001, Issue 3. Art. No: CD001143. [DOI: 10.1002/14651858.CD001143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abu‐Saad 2010
- Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiologic Reviews 2010;32(1):5-25. [DOI] [PubMed] [Google Scholar]
Ananth 2010
- Ananth CV, Basso O. Impact of pregnancy-induced hypertension on stillbirth and neonatal mortality. Epidemiology 2010;21(1):118-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 2011
- Bhutta ZA, Yakoob MY, Lawn JE, Rizvi A, Friberg IK, Weissman E, et al. Stillbirths: what difference can we make and at what cost? Lancet 2011;377(9776):1523-38. [DOI] [PubMed] [Google Scholar]
Blencowe 2010
- Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. International Journal of Epidemiology 2010;39 Suppl 1:i110-i121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blencowe 2016
- Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Global Health 2016;4(2):e98-e108. [DOI] [PubMed] [Google Scholar]
Challis 2002
- Challis K, Osman NB, Nystrom L, Nordahl G, Bergstrom S. Symphysis-fundal height growth chart of an obstetric cohort of 817 Mozambican women with ultrasound-dated singleton pregnancies. Tropical Medicine and International Health 2002;7(8):678-84. [DOI] [PubMed] [Google Scholar]
Chein 1996
- Chein PF, Khan KS, Arnott N. Magnesium sulphate is the treatment of eclampsia and pre-eclampsia: an overview of the evidence from randomized trials. British Journal of Obstetrics and Gynaecology 1996;103:1085-91. [DOI] [PubMed] [Google Scholar]
Cousens 2011
- Cousens S, Blencowe H, Stanton C, Chou D, Ahmed S, Steinhardt L, et al. National, regional, and worldwide estimates of stillbirth rates in 2009 with trends since 1995: a systematic analysis. Lancet 2011;377(9774):1319-30. [DOI] [PubMed] [Google Scholar]
Crawford 2008
- Crawford JT, Tolosa JE, Goldenberg RL. Smoking cessation in pregnancy: why, how, and what next. Clinical Obstetrics and Gynecology 2008;51(2):419-35. [DOI] [PubMed] [Google Scholar]
De Onis 1998
- De Onis M, Villar J, Gulmezoglu M. Nutritional interventions to prevent intrauterine growth retardation: evidence from randomized controlled trials. European Journal of Clinical Nutrition 1998;52 Suppl 1:S83-S93. [PubMed] [Google Scholar]
Di Mario 2007
- Di Mario S, Say L, Lincetto O. Risk factors for stillbirth in developing countries: a systematic review of the literature. Sexually Transmitted Diseases 2007;34(7 Suppl):S11-S21. [DOI] [PubMed] [Google Scholar]
Flenady 2011
- Flenady V, Koopmans L, Middleton P, Frøen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet 2011;377(9774):1331-40. [DOI] [PubMed] [Google Scholar]
Flenady 2016
- Flenady V, Wojcieszek AM, Middleton P, Ellwood D, Erwich JJ, Coory M, et al. Stillbirths: recall to action in high-income countries. Lancet 2016;387(10019):691-702. [DOI] [PubMed] [Google Scholar]
Froen 2011
- Froen JF, Cacciatore J, McClure EM, Kuti O, Jokhio AH, Islam M, et al. Stillbirths: why they matter. Lancet 2011;377(9774):1353-66. [DOI] [PubMed] [Google Scholar]
Galal 2012
- Galal M, Symonds I, Murray H, Petraglia F, Smith R. Postterm pregnancy. Facts, Views & Vision in ObGyn 2012;4(3):175-87. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Goldenberg 2003
- Goldenberg RL, Thompson C. The infectious origins of stillbirth. American Journal of Obstetrics and Gynecology 2003;189(3):861-73. [DOI] [PubMed] [Google Scholar]
Goldenberg 2011
- Goldenberg RL, McClure EM, Bhutta ZA, Belizán JM, Reddy UM, Rubens CE, et al, Lancet's Stillbirths Series steering committee. Stillbirths: the vision for 2020. Lancet 2011;377(9779):1798-805. [DOI] [PubMed] [Google Scholar]
Hammoud 2005
- Hammoud AO, Bujold E, Sorokin Y, Schild C, Krapp M, Baumann P. Smoking in pregnancy revisited: findings from a large population-based study. American Journal of Obstetrics and Gynecology 2005;192:1856-63. [DOI] [PubMed] [Google Scholar]
Haws 2009
- Haws RA, Yakoob MY, Soomro T, Menezes EV, Darmstadt GL, Bhutta ZA. Reducing stillbirths: screening and monitoring during pregnancy and labour. BMC Pregnancy Childbirth 2009;9 Suppl 1:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heazell 2016
- Heazell AE, Siassakos D, Blencowe H, Burden C, Bhutta ZA, Cacciatore J, et al. Stillbirths: economic and psychosocial consequences. Lancet 2016;387(10018):604-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hoffman 2009
- Hoffman C, Galan HL. Assessing the 'at-risk' fetus: Doppler ultrasound. Current Opinion in Obstetrics and Gynecology 2009;21(2):161-6. [DOI] [PubMed] [Google Scholar]
Hussain 2011
- Hussain AA, Yakoob MY, Imdad A, Bhutta ZA. Elective induction for pregnancies at or beyond 41 weeks of gestation and its impact on stillbirths: a systematic review with meta-analysis. BMC Public Health 2011;11 Suppl 3:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imdad 2011
- Imdad A, Yakoob MY, Bhutta ZA. The effect of folic acid, protein energy and multiple micronutrient supplements in pregnancy on stillbirths. BMC Public Health 2011;11 Suppl 3:S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jabeen 2011
- Jabeen M, Yakoob MY, Imdad A, Bhutta ZA. Impact of interventions to prevent and manage preeclampsia and eclampsia on stillbirths. BMC Public Health 2011;11 Suppl 3:S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jang 2018
- Jang W, Kim H, Lee BE, Chang N. Maternal fruit and vegetable or vitamin C consumption during pregnancy is associated with fetal growth and infant growth up to 6 months: results from the Korean Mothers and Children’s Environmental Health (MOCEH) cohort study. Nutrition Journal 2018;17(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawn 2011
- Lawn JE, Blencowe H, Pattinson R, Cousens S, Kumar R, Ibiebele I, et al. Stillbirths: where? When? Why? How to make the data count? Lancet 2011;377(9775):1448-63. [DOI] [PubMed] [Google Scholar]
Lawn 2016
- Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 2016;387(10018):587-603. [DOI] [PubMed] [Google Scholar]
McClure 2007
- McClure EM, Goldenberg RL, Bann CM. Maternal mortality, stillbirth and measures of obstetric care in developing and developed countries. International Journal of Gynecology & Obstetrics 2007;96(2):139-46. [DOI] [PubMed] [Google Scholar]
McClure 2009
- McClure EM, Saleem S, Pasha O, Goldenberg RL. Stillbirth in developing countries: a review of causes, risk factors and prevention strategies. Journal of Maternal-Fetal and Neonatal Medicine 2009;22(3):183-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Medley 2018
- Medley N, Vogel JP, Care A, Alfirevic Z. Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD012505. [DOI: 10.1002/14651858.CD012505.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Menzies 2007
- Menzies J, Magee LA, Li J, MacNab YC, Yin R, Stuart H, et al. Instituting surveillance guidelines and adverse outcomes in preeclampsia. Obstetrics & Gynecology 2007;110(1):121-7. [DOI] [PubMed] [Google Scholar]
Moher 2007
- Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. PLoS Medicine 2007;4(3):e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norwitz 2007
- Norwitz ER, Snegovskikh VV, Caughey AB. Prolonged pregnancy: when should we intervene? Clinical Obstetrics and Gynecology 2007;50(2):547-57. [DOI] [PubMed] [Google Scholar]
Pattinson 2011
- Pattinson R, Kerber K, Buchmann E, Friberg IK, Belizan M, Lansky S, et al, for The Lancet's Stillbirths Series steering committee. Stillbirths: how can health systems deliver for mothers and babies? Lancet 2011;377(9777):1610-23. [DOI] [PubMed] [Google Scholar]
Perez‐Lopez 2015
- Perez-Lopez FR, Pasupuleti V, Mezones-Holguin E, Benites-Zapata VA, Thota P, Deshpande A, et al. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertility and Sterility 2015;103(5):1278-88.e4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pollock 2019
- Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration,. Available from https://www.training.cochrane.org/handbook.
Rosenstein 2012
- Rosenstein MG, Cheng YW, Snowden JM, Nicholson JM, Doss AE, Caughey AB. The risk of stillbirth and infant death stratified by gestational age in women with gestational diabetes. American Journal of Obstetrics and Gynecology 2012;206(4):309.e1-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rumbold 2006
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS, for the ACTSSG. Vitamins C and E and the risks of preeclampsia and perinatal complications. New England Journal of Medicine 2006;354:1796-806. [DOI] [PubMed] [Google Scholar]
Salihu 2007
- Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Human Development 2007;83(11):713-20. [DOI] [PubMed] [Google Scholar]
Sanchez‐Ramos 2003
- Sanchez-Ramos L, Olivier F, Delke I, Kaunitz AM. Labor induction versus expectant management for postterm pregnancies: a systematic review with meta-analysis. Obstetrics & Gynecology 2003;101(6):1312-8. [DOI] [PubMed] [Google Scholar]
Schmid 2007
- Schmid GP, Stoner BP, Hawkes S, Broutet N. The need and plan for global elimination of congenital syphilis. Sexually Transmitted Diseases 2007;34(7 Suppl):S5-S10. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html 2013.
Shea 2007
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrimpton 2009
- Shrimpton R, Huffman SL, Zehner ER, Darnton-Hill I, Dalmiya N. Multiple micronutrient supplementation during pregnancy in developing-country settings: policy and program implications of the results of a meta-analysis. Food and Nutrition Bulletin 2009;30:S556-S573. [DOI] [PubMed] [Google Scholar]
Srinivas 2012
- Srinivas SK, Parry S. Periodontal disease and pregnancy outcomes: time to move on? Journal of Women's Health 2012;21(2):121-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2010
- United Nations. Millennium Development Goals. UN Summit on the Millennium Development Goals (http://www.un.org/millenniumgoals) (accessed 2011).
UN 2015
- United Nations. Sustainable development goals. sdgs.un.org/goals.
US 2004
- US Department of Health and Human Services. The Health Consequences of Smoking. 2004 Surgeon General's Report. U.S. Department of Health and Human Services, 2004. [Google Scholar]
Van Geertruyden 2004
- Van Geertruyden JP, Thomas F, Erhart A, D'Alessandro U. The contribution of malaria in pregnancy to perinatal mortality. American Journal of Tropical Medicine and Hygiene 2004;71(2 Suppl):35-40. [PubMed] [Google Scholar]
Wang 2015
- Wang H, Hu YF, Hao JH, Chen YH, Su PY, Wang Y, et al. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: a population-based birth cohort study. Scientific reports 2015;5:11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiner 2003
- Weiner R, Ronsmans C, Dorman E, Jilo H, Muhoro A, Shulman C. Labour complications remain the most important risk factor for perinatal mortality in rural Kenya. Bulletin of the World Health Organization 2003;81(8):561-6. [PMC free article] [PubMed] [Google Scholar]
Wendland 2012
- Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes--a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy and Childbirth 2012;12:23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2013
- WHO. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. apps.who.int/iris/bitstream/handle/10665/85975/WHO_NMH_MND_13.2_eng.pdf?sequence=1 2013.
WHO 2016
- WHO. WHO recommendations on antenatal care for a positive pregnancy experience. www.who.int/publications/i/item/9789241549912 2016. [PubMed]
WHO 2020
- WHO. KD3B.0 Antepartum fetal death. ICD-11 for Mortality and Morbidity Statistics. Eleventh revision (Version:09/2020) 2020;Available from:https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1947342847 Accessed on December 14, 2020..
Zimmermann 2012
- Zimmermann MB. The effects of iodine deficiency in pregnancy and infancy. Paediatric and Perinatal Epidemiology 2012;26 Suppl 1:108-17. [PMID: ] [DOI] [PubMed] [Google Scholar]


