Abstract
This study aimed to investigate the effects of the basic treatment for heart failure and sequential treatment with rh-brain natriuretic peptide (rhBNP) alone or the combination of rhBNP and sacubitril/valsartan. Cardiac structure, pulmonary artery pressure, inflammation and oxidative stress in patients with acute heart failure were evaluated.
Three hundred patients with acute heart failure were included. According to the random number table method, the patients were divided into 3 groups of 100 patients per group: the standard treatment group (treated with an angiotensin-converting enzyme inhibitor, β receptor blocker, and corticosteroid antagonist), rhBNP group (basic treatment combined with rhBNP) and sequential treatment group (basic treatment for heart failure combined with rhBNP followed by sacubitril/valsartan). The changes in NT-probrain natriuretic peptide (BNP) levels, cardiac troponin T (cTnT) levels, cardiac structure, pulmonary artery pressure, and the levels inflammatory factors and oxidative stress factors were compared among the 3 groups at 1, 4, 12, and 36 weeks after treatment.
The sequential treatment group displayed superior outcomes than the standard treatment group and the rhBNP group in terms of left atrium diameter, left ventricular end diastolic volume, left ventricular ejection fraction, pulmonary artery pressure, NT-proBNP levels, and cTnT levels, which respond to damage to the heart structure and myocardium. This result may be related to the decreased levels of inflammatory factors and the correction of oxidative stress imbalance.
Sacubitril/valsartan significantly reduce the serum levels of inflammatory factors in patients with acute heart failure while decreasing the levels of oxidizing factors and increasing the levels of antioxidant factors. These changes may be one of the explanations for the better cardiac structure and better pulmonary artery pressure observed in the sequential treatment group.
Keywords: heart failure, heart structure, inflammatory factors, oxidative stress, recombinant human brain natriuretic peptide, sacubitril/valsartan
1. Introduction
Heart failure is a complex clinical syndrome characterized by low output syndrome and venous congestion syndrome that is caused by an absolute or relative decrease in cardiac output due to any abnormality in either the heart structure or function, resulting in the impairment of ventricular filling or ejection capacity and a decrease in cardiac pump function. Thus, the heart is unable to meet the needs of tissue metabolism in the body, resulting in hemorheology alterations. Heart failure is the most serious and terminal stage of various heart diseases, with a high incidence rate. Over 1 million people worldwide are diagnosed with heart failure every year. The 5-year survival rate is only 50%,[1–3] and it is a serious disease threatening human health.
Acute heart failure refers to the rapid occurrence or deterioration of heart failure symptoms and signs. Clinically, acute left heart failure is common but has a high mortality rate. Acute left heart failure refers to a significant decrease in myocardial contraction and an increase in cardiac load caused by acute attacks or aggravated left heart dysfunction, resulting in a sudden decrease in acute cardiac output, a sudden increase in pulmonary circulation pressure, and increased peripheral circulation resistance, resulting in a clinical syndrome of pulmonary congestion and acute pulmonary congestion, pulmonary edema, and cardiogenic shock accompanied by insufficient perfusion of tissues and organs.
The mechanism of heart failure is closely related to abnormal neurohumoral regulatory activities. Excessive activation of the sympathetic nervous system, the renin-angiotensin-aldosterone system and endothelin hormones promotes sodium and water retention and vasoconstriction, increases the heart load, and induces ventricular remodeling.[4–6] Currently, angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor antagonist (ARB), beta receptor blocker and aldosterone receptor antagonist constitute the “gold triangle” for the treatment of heart failure. ACEIs/ARBs and beta receptor blockers effectively reduce patient mortality.[7,8] However, the therapeutic effect of these drugs is still limited. In recent years, with the gradual increase in the understanding of the pathophysiological mechanism of heart failure, natriuretic peptide has been considered to play an important role in the occurrence and development of heart failure. Natriuretic peptides include atrial natriuretic peptide, brain natriuretic peptide (BNP), and C-type natriuretic peptide. Atrial natriuretic peptide is mostly synthesized and secreted in the atria, and its synthesis is mainly promoted by atrial wall stretching caused by changes in blood volume or cardiac transmural pressure. BNP is mainly synthesized and secreted in the ventricle, and its synthesis is mainly promoted by ventricular wall stretching caused by left ventricular pressure or volume overload.[5] C-type natriuretic peptide is mainly synthesized by vascular endothelial cells and may exert protective effects on ventricular remodeling after myocardial infarction.[9] These peptides are antagonistic regulatory hormones secreted by the body when cardiac function is impaired. They maintain heart function and fluid balance through physiological antagonism and inhibition of renin-angiotensin-aldosterone system, endocorticoid-1 and sympathetic nervous system (SNS). However, with the progressive deterioration of heart function, the excessive activation of the vascular neurohormonal system and the sodium-retaining water hormonal system has caused the circulatory system to exhibit a pathophysiological disorder. Eventually, it might contribute to the compensatory role of endogenous natriuretic peptide in cardio protection, leading to worsening heart failure. At this time, exogenous BNP is used to compensate for the lack of synthetic release of endogenous human BNP in the body, which quickly improves hemodynamic disorders, neurohormonal levels, clinical symptoms and signs in patients with acute decompensation and reduces patient mortality.[10]
Recombinant human BNP has been shown to be safe in patients with acute heart failure in VMAC, PROACTION, and ASCEND-HF studies, which can improve clinical and hemodynamic parameters but does not improve the prognosis.[11–13] Unfortunately, recombinant human BNP has a short half-life in circulating blood. However, the inhibition of enkephalinase increases the level of natriuretic peptide, promotes an increase in cyclic guanylic acid in myocardial cells, produces vasodilation, promotes urinary sodium excretion and diuretics, reduces sympathetic tone, inhibits cell proliferation, and reduces myocardial hypertrophy and cardiorenal protection.[5,14–19] A new drug target, enkephalinase, was discovered and produces significant benefits in large-scale clinical trials, becoming a new bright spot in the field of heart failure treatment. The first angiotensin receptor enkephalinase dual inhibitor (ARNI) drug was sacubitril/valsartan. Namely, LCZ696, a mixture of ARB and AHU377, the precursor substance of enkephalinase inhibitor (LBQ657), was composed of a 1:1 mixture. This protein mainly acts on both the natriuretic peptide and renin-angiotensin system (RAS), which synergistically relax blood vessels, promote urinary sodium excretion, reduce the heart load, and prevent cardiomyocyte hypertrophy and fibrosis, allowing LBQ657 and valsartan to reach steady-state concentrations within 3 days.
The PARAMOUNT study[20] shows that sacubitril/valsartan reduce the left atrial diameter, left atrial volume, and left atrial volume index in patients with heart failure. In the PARADIGM-HF study,[21] sacubitril/valsartan significantly reduced symptoms and improved activity tolerance in patients with chronic HFrEF heart failure while reducing patients’ sudden death rate, heart failure and hospitalization risks, all-cause mortality, intensive care for treatment, and the need for emergency treatment due to the exacerbation of the emergency. Meanwhile, it always maintains low levels of cardiac biomarkers (NT-proBNP and troponin) related to the cardiac load and myocardial injury. The results of the PROVE-HF study[22] confirmed that sacubitril/valsartan reverse cardiac remodeling while maintaining low levels of NT-proBNP. It significantly improved the key indicators of cardiac remodeling after 6 months, and treatment for 12 months will produce a greater benefit. The EVALUATE-HF study[23] confirmed that sacubitril/valsartan improve the structure and function of the left ventricle compared with enalapril. The PIONEER-HF study[24] confirmed that treatment with sacubitril/valsartan for 8 weeks in hemodynamically stable hospitalized patients with acute decompensated heart failure (ADHF) may further reduce NT-ProBNP levels and heart failure rehospitalization rates.
However, at present, very few studies have explored the clinical efficacy of the sequential recombinant human BNP and sacubitril/valsartan treatment for acute heart failure and its effect on oxidative stress and inflammatory factors.
2. Methods
2.1. Patients
This study was approved by the Medical Ethics Committee of Tianjin Union Medical Center, and all patients were informed and signed informed consent forms to participate in the experiment. All experiments were performed according to the provisions of the Declaration of Helsinki.
-
1.
Diagnostic criteria for heart failure and exclusion criteria. Referring to the 2018 Chinese Heart Failure Diagnosis and Treatment Guide, the following inclusion criteria were used: age ≥18 years, acute heart failure within 24 hours (patients with acute heart failure clinical grade bedside grade II to IV), an ejection fraction <40% (except for patients with massive mitral regurgitation), plasma B-type natriuretic peptide levels ≥400 pg/ml or N-terminal B-type natriuretic peptide levels ≥2000 pg/ml. The following exclusion criteria were used:
-
a.
symptomatic hypotension, systolic blood pressure ≤95 mm Hg;
-
b.
hyperkalemia (blood potassium >5.4 mmol/L);
-
c.
severe renal impairment (eGFR <30 ml/min/1.73 m2);
-
d.
severe liver impairment (Child-Pugh grade C);
-
e.
angioedema or unacceptable side effects when receiving previous ACEIs or ARBs;
-
f.
severe anemia;
-
g.
women who are pregnant, breastfeeding, or preparing for pregnancy; and
-
h.
cognitive impairment.
-
a.
-
2.
Grouping. All patients were diagnosed with heart failure from July 2018-May 2019 in the Department of Cardiology, Tianjin Union Medical Center and underwent regular outpatient follow-up. All the patients included in the present study were diagnosed with heart failure no more than 3 years prior to inclusion, and the ejection fraction was <40%. Patients were randomly divided into 3 groups: 100 patients aged 53 to 81 years were included in the standard group; 100 patients aged 55 to 77 years were included in the rhBNP group; and 100 patients aged 56 to 78 years were included in the sequential group.
2.2. Intervention methods
The standard group was administered the recommended basic treatment for heart failure (including ACEIs, β blockers, and mineralocorticoid antagonists). The rhBNP group was administered the basic treatment for heart failure combined with recombinant human BNP. The sequential group was administered the basic treatment for heart failure (excluding ACEI/ARB drugs) combined with sequential recombinant human BNP and sacubitril/valsartan treatment. According to the treatment requirements, patients were administered the basic treatment for heart failure (including ACEIs, β-blockers, and mineralocorticoid antagonists at specific dosages according to the patient's condition), recombinant human BNP through an intravenous injection of 1.5 μg/kg at a rate of 0.0075 μg/kg/min for 3 consecutive days, and sacubitril/valsartan (LCZ696) with a dose of gradually increased from 50 mg twice daily to 200 mg twice daily. The dose was doubled every 2 weeks until reaching the maximum tolerated dose or target dose. The target doses of drugs were as follows: fosinopril sodium 20 to 30 mg qd, benazepril hydrochloride 10 to 20 mg qd, valsartan 80 to 160 mg bid, irbesartan 300 mg qd, telmisartan 80 mg qd, and sacubitril/valsartan 200 mg bid.
2.3. Observation items and detection methods
The following indicators were tested before and 1, 4, 12, and 36 weeks after drug administration:
-
1.
Cardiac function assessment. Cardiac ultrasound that measured the left ventricular ejection fraction, left atrium volume, left ventricular end-diastolic volume (LVEDV), and pulmonary artery pressure. The numerical value of NT-ProBNP was calculated.
-
2.
Evaluation of relevant indexes of myocardial injury, including the detection of the cardiac troponin T (cTnT) level.
-
3.
Detection of inflammatory factors and oxidative stress indicators. The basic principle of ELISA is antigen-antibody binding, and experimental operations were performed in 96-well plates. The content of the substance to be tested in the sample was determined by conducting a quantitative analysis of the amount of colored product by measuring the absorbance. All ELISA kits were purchased from Wuhan Genmei Biotechnology Co. Ltd. Tumor necrosis factor alpha (TNF-α, #JYM0110Hu), interleukin-6 (IL-6, #JYM0140Hu), serum malondialdehyde (MDA, #JYM0375Hu), xanthine oxidase (XO, #JYM0488Hu), and glutathione-peroxidase (#JYM1124Hu) were determined according to the kit instructions. And the concentration of each factor was then calculated from a quantitative curve.
-
4.
End point event analysis.
Main efficacy endpoint events: first rehospitalization for heart failure and death from cardiovascular disease.
Secondary endpoints include life-threatening arrhythmia (ventricular tachycardia (VT) or ventricular fibrillation (VF)), and decrease of renal function (end-stage renal disease or a decrease in the eGFR by at least 50%).
Main safety risk endpoint events: severe hypotension, cough, hyperkalemia, and angioedema.
2.4. Statistical method
SPSS 25 statistical software was used to analyze the data, and the measurement data are presented as the means ± standard deviations and were analyzed using a t test. The count data were analyzed using the χ2 test. P < .05 was considered a statistically significant difference.
3. Results
3.1. General characteristics of the patients
Statistically significant differences in general characteristics, including sex, age, cause of heart failure, and cardiac function classification, were not observed between the 3 groups (P > .05) (Table 1).
Table 1.
General information of patients.
| Gender | Cause of heart failure | Cardiac function classification | |||||||||
| Group | Male | Female | Age (y) | CHD | MI | HBP | AF | SHD | II | III | IV |
| Standard | 52 | 48 | 67.10 ± 8.15 | 36 | 22 | 27 | 9 | 6 | 61 | 25 | 14 |
| rhBNP | 48 | 52 | 67.59 ± 8.01 | 38 | 21 | 28 | 9 | 4 | 53 | 32 | 15 |
| Sequential | 48 | 52 | 67.52 ± 7.93 | 42 | 20 | 25 | 10 | 3 | 60 | 24 | 16 |
3.2. Effect on heart structure
As shown in Figure 1, no statistically significant difference in left atrial diameters was observed among the 3 groups (P > .05, P = .47, P = .36, and P = .92). Statistically significant differences were not observed 1 week (P = .52, P = .05, P = .16), 4 weeks (P = .62, P = .82, and P = .50), or 12 weeks after treatment (P = .92, P = .96, and P = .88) among the 3 groups. The difference was statistically significant at 36 weeks after treatment, and patients the sequential treatment group showed a significant decrease (P < .05) in the left atrial diameter. However, a statistically significant difference in the left atrial diameter was not observed in the patients in the standard treatment group (P = .01) and the rhBNP group (P = .04).
Figure 1.
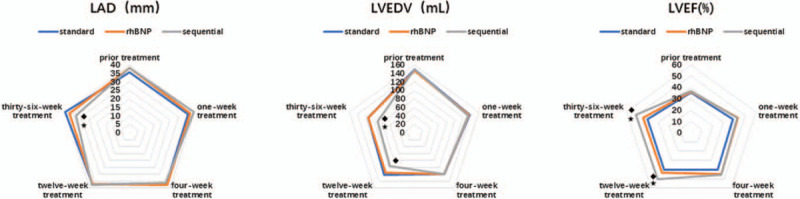
Changes in cardiac structure and the ejection fraction before and after treatment.  Compared with the standard treatment group, the rhBNP group exhibited a significant difference (P < .05).
Compared with the standard treatment group, the rhBNP group exhibited a significant difference (P < .05).  The sequential treatment group exhibited significant differences compared with the standard treatment group (P < .05).
The sequential treatment group exhibited significant differences compared with the standard treatment group (P < .05).  The sequential treatment group presented significant differences compared with the rhBNP group (P < .05). LAD = left atrial diameters, LVEDV = left ventricular end-diastolic volume, LVEF = left ventricular ejection fraction, rhBNP = recombinant human brain natriuretic peptide.
The sequential treatment group presented significant differences compared with the rhBNP group (P < .05). LAD = left atrial diameters, LVEDV = left ventricular end-diastolic volume, LVEF = left ventricular ejection fraction, rhBNP = recombinant human brain natriuretic peptide.
As shown in Figure 1, the LVEDV was not significantly different among the 3 groups (P > .05, P = .69, P = .94, and P = .74). A significant difference was no observed after 1 week of treatment (P = .90, P = .74, P = .85) or after 4 weeks of treatment (P = .99, P = .92, P = .91) among the 3 groups. Compared with the standard treatment group, the LVEDV of the sequential treatment group was significantly decreased after 12 weeks of treatment (P < .05, P = .03). At 36 weeks after treatment, the LVEDV of the sequential treatment group was significantly reduced (P < .05) compared with the standard treatment group (P = .03) and the rhBNP group (P = .04). As shown in Figure 1, the changes in left ventricular ejection fraction (LVEF) among the 3 groups before and after treatment showed a similar trend as the LVEDV. Before treatment and 1 week and 12 weeks after treatment, significant differences in LVEF were not observed in the standard treatment group compared with the rhBNP group (P = .72, P = .13, P = .14) the sequential treatment group (P = .66, P = .20, P = .10), and in the rhBNP group compared with the sequential treatment group (P = .92, P = .85, P = .85). After 12 weeks of treatment, the LVEF of the sequential treatment group was significantly higher than the standard treatment group (P = .007) and the rhBNP group (P = .049) (P < .05). Thirty-six weeks after treatment, the LVEF of the sequential treatment group continued to increase compared with the standard treatment group (P = .01) and the rhBNP group (P = .04), and both increases were statistically significant (P < .05).
3.3. Changes in pulmonary artery pressure
No significant difference in pulmonary artery pressure was observed among the 3 groups before treatment (41.29 ± 8.056, 42.6 ± 9.737, and 42.6 ± 4.278, P = .78, and P = .78, P > .99). After 1, 4, 12, and 36 weeks of treatment, the pulmonary artery pressure decreased compared with the measurement obtained prior to treatment (standard treatment group: 5.43 ± 0.976, 5.29 ± 1.799, and 5 ± 2.16, 5.29 ± 1.799; rhBHP group: 10.4 ± 2.074, 9.4 ± 3.286, 9.8 ± 2.775, and 8.8 ± 2.683; and sequential group: 14 ± 3.082, 15.4 ± 2.881, 14.4 ± 2.191, and 14.4 ± 2.191, respectively), and statistically significant differences were observed among the 3 groups, as shown in Figure 2. The decrease in pulmonary artery pressure of the sequential treatment group was greater than in the rhBNP group and the standard treatment group (P = .001, P = .02, P = .001, and P = .02). Compared with the standard treatment group (P = 0) and the rhBNP group (P = .02, P = .001, P = .01, and P = .001), the pulmonary artery pressure of the sequential treatment group was significantly decreased, particularly compared with the standard treatment group, as shown in Figure 2.
Figure 2.
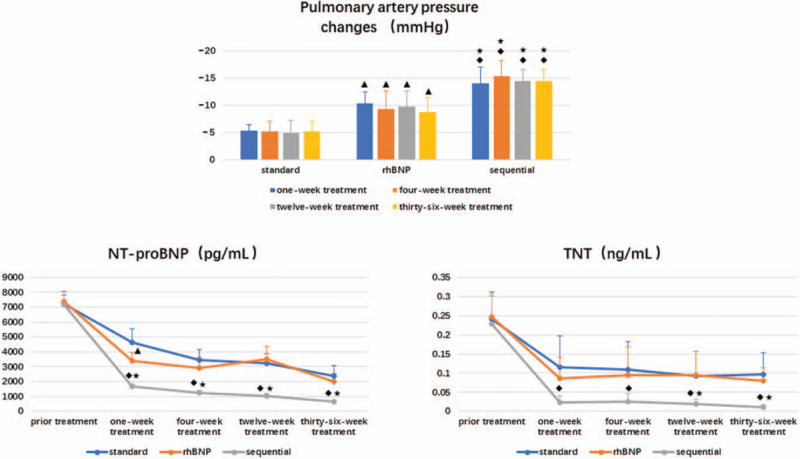
Decreased pulmonary arterial pressure and changes in the NT-proBNP and cTnT concentrations before and after treatment.  Compared with the standard treatment group, the rhBNP group presented a significant difference in these parameters (P < .05).
Compared with the standard treatment group, the rhBNP group presented a significant difference in these parameters (P < .05).  The sequential treatment group exhibited significant differences compared with the standard treatment group (P < .05).
The sequential treatment group exhibited significant differences compared with the standard treatment group (P < .05).  The sequential treatment group presented significant differences compared with the rhBNP group (P < .05). cTnT = cardiac troponin T, NT-proBNP = N-terminal B-type natriuretic peptide, rhBNP = recombinant human brain natriuretic peptide.
The sequential treatment group presented significant differences compared with the rhBNP group (P < .05). cTnT = cardiac troponin T, NT-proBNP = N-terminal B-type natriuretic peptide, rhBNP = recombinant human brain natriuretic peptide.
3.4. Evaluation of cardiac function and myocardial injury
NT-proBNP is one of the indicators of cardiac function, and a significant difference was not observed in this parameter among the 3 groups before treatment (P > .05, P = .67, P = .95, P = .64). Compared with the standard treatment group, the NT-proBNP level decreased significantly in the rhBNP group (P = .01), but this advantage disappeared over time (P = .14, P = .51, and P = .29). In the sequential treatment group, NT-proBNP levels decreased significantly beginning at 1 week after treatment and remained at a low level for 36 weeks compared with the standard treatment group (P = 0) and the rhBNP group (P = .001, P = 0, P = 0, and P = .002), with a statistically significant difference observed between groups (P < .05). The results mentioned above are presented in Figure 2.
cTnT reflects the degree of myocardial damage to a certain extent, and as shown in Figure 2, a significant difference in cTnT levels was not observed among the 3 groups before treatment (P > .05, P = .89, P = .75, and P = .68). The sequential treatment group showed a decreasing trend in the cTnT level, with lower levels than the standard treatment groups observed after treatment (P = .02, P = .04, P = .03, and P = .004; P < .05). Compared with the rhBNP group, the sequential treatment group showed significantly lower levels at 12 weeks after treatment (P = .04 and P = .01, P < .05).
3.5. Changes in inflammatory factors
Tumor necrosis factor (TNF) is a small protein secreted by macrophages. TNF-α is mainly secreted by mononuclear phagocytes and macrophages and participates in inflammation in humans. Using an ELISA to determine the effect of the treatments on cytokine secretion, we observed a decreasing trend in the TNF-α levels among the 3 groups after treatment, as shown in Figure 3. In particular, the decrease detected in the sequential group after 12 weeks of treatment was more obvious than in the standard treatment group (P = .001) and the rhBNP group (P = .03), and was statistically significant (P < .05); the aforementioned decrease (P = .003 and P = .04) was still observed in the sequential group compared with the other 2 groups persisted until 36 weeks after treatment.
Figure 3.
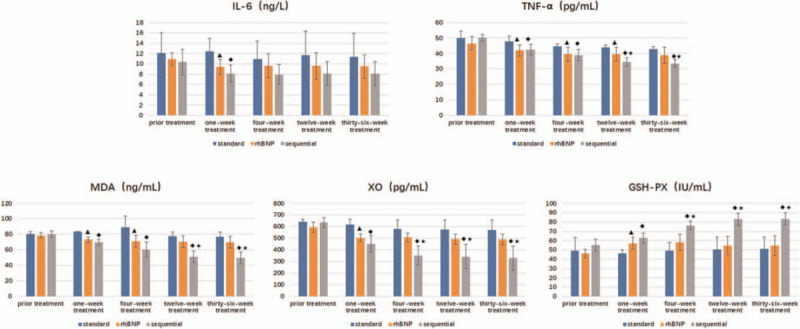
Changes in the concentrations of inflammatory factors and oxidative stress-related factors.  Compared with the standard treatment group, the rhBNP group exhibited significant differences in these parameters (P < .05).
Compared with the standard treatment group, the rhBNP group exhibited significant differences in these parameters (P < .05).  The sequential treatment group displayed significant differences compared with the standard treatment group (P < .05).
The sequential treatment group displayed significant differences compared with the standard treatment group (P < .05).  The sequential treatment group presented significant differences compared with the rhBNP group (P < .05). GSH-PX = glutathione-peroxidase, IL-6 = interleukin-6, MDA = serum malondialdehyde, rhBNP = recombinant human brain natriuretic peptide, TNF-α = tumor necrosis factor α, XO = xanthine oxidase.
The sequential treatment group presented significant differences compared with the rhBNP group (P < .05). GSH-PX = glutathione-peroxidase, IL-6 = interleukin-6, MDA = serum malondialdehyde, rhBNP = recombinant human brain natriuretic peptide, TNF-α = tumor necrosis factor α, XO = xanthine oxidase.
Interleukin is a lymphokine that facilitates the interaction between leukocytes and immune cells, and it a member of the same cytokine family as a blood cell growth factor. Interleukins play an important role in transmitting information, activating and regulating immune cells, mediating T and B cell activation, proliferation and differentiation, and regulating the inflammatory response. As shown in Figure 3, the IL-6 concentration of the 3 groups were lower after treatment than before treatment, but the differences among the 3 groups were not significant (P > .05).
3.6. Changes in oxidative stress factors
MDA is currently recognized as a parameter that indicates lipid peroxidation. XO catalyzes the degradation of xanthine to produce oxygen free radicals. GSH is a key substance in the cellular defense system, eliminating cell damage caused by oxygen free radicals. Glutathione-peroxidase deficiency increases oxidative stress and induces endothelial dysfunction and structural vascular abnormalities.[25] In the present study, the MDA concentration in the rhBNP group was significantly lower than in the standard treatment group at 1 and 4 weeks after treatment (P = .001, P = .04), but the long-term changes were not statistically significant (P = .01 and P = .18). The MDA concentration in the sequential treatment group showed a significant decreasing trend compared with the standard treatment group after 1 week of treatment (P < .001, P = .006, P < .001, P < .001). Meanwhile, its level decreased significantly after the 12th week of treatment compared with the rhBNP group (P = .004 and P = .003). In terms of changes in XO concentration, the rhBNP group exhibited a significant decrease compared with the standard treatment group at approximately 1 week of treatment (P = .01), but a further reduction was not observed in the remaining time period. However, the levels detected in the sequential treatment group at 1 week, 4 weeks, and 12 weeks and 36 weeks were significantly lower than in the standard treatment group (P = .001, P = .001, P = .001, and P = .002, respectively). At 4 weeks, 12 weeks, and 36 weeks after treatment, a significantly lower level of XO was observed than in the rhBNP group (P = .02, P = .04, and P = .03, respectively). The concentration of GSH-PX in patients with heart failure was higher after treatment than before treatment, and the rhBNP group displayed a significantly higher concentration than the standard treatment group at 1 week of treatment (P = .01). A significantly higher GSH-PX concentration was detected in the sequential treatment group than in the standard treatment group at 1 week, 4 weeks, 12 weeks, and 36 weeks of treatment (P = .002, P = .002, P = .004, and P = .004, respectively). The increase was more obvious after 4 weeks compared with the rhBNP group (P = .02, P = .01, and P = .01).
3.7. End-point events
One case of cardiovascular death each occurred in the standard treatment group and the rhBNP group in the course of the study, and 1 case of rehospitalization due to acute coronary syndrome occurred in the sequential treatment group. No worsening of renal function or new atrial fibrillation was reported.
3.8. Adverse reactions
All subjects tolerated recombinant human BNP and sacubitril/valsartan well, no adverse reactions related to the drug appeared, and the patients insisted on completing the whole course of medication.
4. Discussion
The mechanism underlying the development of heart failure is cardiac remodeling. During this process, the morphology and structure of cardiomyocytes and even the interstitial structure have changed. Cardiomyocyte hypertrophy is accompanied by an increase in the ratio of length to width. The wall contraction movement is not coordinated, the blood in the ventricle is not effectively emptied, and the pressure load is increased, which eventually causes the entire left ventricle to expand and the structural shape of the ventricle to change. Cardiac remodeling occurs throughout the development of heart failure and is also one of the main factors that determine cardiac function and patient prognosis. A significant positive correlation with the risk of death or hospitalization for heart failure has been observed.[26]
As shown in the present study, treated patients with heart failure exhibited an improved left atrial anterior-posterior diameter, LVEDV, and left ventricular ejection fraction. The sequential treatment group showed obvious advantages after 12 weeks of treatment. In terms of the improvement in pulmonary arterial pressure, the rhBNP group displayed a larger decrease than the standard treatment group, and the sequential treatment group showed a more obvious decreasing trend than the other 2 groups. According to the indicators of cardiac function and myocardial damage, such as NT-proBNP and cTnT, sequential treatment is suggested to be superior to rhBNP and standard treatment, particularly regarding the long-term effects. In summary, sequential treatment significantly improves patients’ left ventricular structure and even reverse ventricular remodeling, significantly reduce NT-proBNP and cTnT levels, and even reduce pulmonary artery pressure, showing obvious advantages.
When heart failure occurs, cardiomyocytes exhibit reduced energy production and utilization, and myocardial tissues of the failed heart are characterized by energy starvation. The levels of oxygen free radicals increase during heart failure, and oxygen free radical scavenging ability decreases. Free radicals should not be ignored in the process of myocardial remodeling and heart failure. Oxygen free radicals can induce mitochondrial swelling, particle formation, and even disintegration, disrupting cardiomyocyte energy production. Oxygen free radicals also cause myocardial excitation-contraction coupling disorders. Oxygen free radicals damage the structures responsible for myocardial mechanical work and the energy metabolism process required for myocardial cell activity, thereby reducing myocardial compliance, myocardial contraction and diastolic dysfunction and ultimately leading to the occurrence and development of heart failure.[27] A significant positive correlation was observed between NT-proBNP and MDA in heart failure patients.[28] XO inhibitor, has been shown to improve cardiovascular outcomes in patients with heart failure and coronary heart disease.[29] In the present study, higher concentrations of MDA and XO were detected in the serum of patients with heart failure before treatment, and the concentration measured after treatment was significantly lower than the value detected before treatment. A significant increase in the efficacy of the rhBNP treatment was observed after 1 week and the efficacy of the sequential treatment was also significantly increased at 1 week of treatment and persisted to 36 weeks of treatment. GSH has been shown to confer protection in a model of heart damage termed ischemia-reperfusion injury.[30] A low concentration of GSH was detected in patients with heart failure, and the concentration measured after treatment was significantly higher than the level detected before treatment. The increases in the rhBNP group and the sequential treatment group were more obvious, and the GSH concentration in the sequential treatment group was maintained at a high level. Oxidative stress may drive the development of heart disease through damaging cellular components such as proteins, DNA, and lipids. The stretching of myocytes and cardiac fibroblasts is increased by ROS production. Based on these results, rhBNP and sacubitril/valsartan quickly adjust the imbalance of oxidative stress/antioxidants in patients with heart failure, and the sequential treatment allows the effect on improving cardiac function to persist. Heart failure may lead to the abnormal expression of various inflammatory cytokines in patients, which plays an important role in the pathological development of heart failure. In patients with acute heart failure, inflammation often occurs in the early stage of onset. When myocardial cells are damaged, their necrosis, remodeling, and oxidative reactions will aggravate the condition of patients with acute heart failure. Oxidative stress may drive the development of heart disease through damaging the cellular components such as proteins, DNA, and lipids.[31] The stretching of myocytes and cardiac fibroblasts can be increased by ROS production.[32] Other study found that IL-6 could be a potential therapeutic target in specific heart failure subpopulations.[33] IL-6 is a lymphokine that targets T cells and fibroblasts, and its expression level is one of the more sensitive signs of the inflammatory response in the body. TNF-α is one of the most common proinflammatory factors secreted by monocytes, mast cells, macrophages, and other cells. It participates in disease progression by damaging the vascular endothelium, reducing myocardial contractility, and promoting myocardial cell apoptosis and ventricular remodeling. Blood TNF-α levels are increased in patients with many diseases, such as inflammatory myocarditis, acute myocardial infarction, unstable angina, and other types of heart injury. It is considered one of the protective responses to heart disease.[34] As shown in the present study, the levels of TNF-α and IL-6 were significantly reduced after treatment, and the sequential treatment group showed significantly reduced TNF-α levels. The reduction in TNF-α and IL-6 indicates that the inflammatory response of myocardial cells is reduced, and further myocardial damage is ameliorated. Thus, a correlation likely exists between the reductions in TNF-α and IL-6 levels and the preservation of myocardial function.
The present study has some limitations. First, the number of patients we included was not large and patients were not recruited from multiple centers. More patients must be included in subsequent studies. Second, novel signaling pathways have to be discovered and verified in future studies.
5. Conclusions
Sacubitril/valsartan significantly reduce the serum levels of inflammatory factors such as TNF-α in patients with acute heart failure while decreasing the levels of oxidizing factors such as MDA and XO and increasing the levels of antioxidants such as GSH-PX. These changes may explain why the sequential treatment group exhibited a better cardiac structure and better pulmonary artery pressure.
Acknowledgments
We are so appreciated for the support from Fundamental of Research Funds for Tianjin Union Medical Center in China (No. 2019YJ020).
Author contributions
Conceptualization: Zhihua Pang, Chang Pan, Zhuhua Yao.
Data curation: Zhihua Pang, Chang Pan, Zhuhua Yao, Ying Ren, Liuyang Tian, Jian Cui, Ximei Liu, Lijun Zhang, Ying Chen.
Formal analysis: Zhihua Pang, Chang Pan, Zhuhua Yao, Ying Ren, Liuyang Tian, Jian Cui, Ximei Liu, Lijun Zhang, Ying Chen.
Funding acquisition: Zhuhua Yao.
Methodology: Zhihua Pang.
Project administration: Zhuhua Yao.
Resources: Zhuhua Yao.
Writing – original draft: Zhihua Pang.
Footnotes
Abbreviations: ARB = angiotensin receptor antagonist, BNP = brain natriuretic peptide, IL-6 = interleukin-6, LVEDV = left ventricular end-diastolic volume, MDA = serum malondialdehyde, TNF-α = tumor necrosis factor α, XO = xanthine oxidase.
How to cite this article: Pang Z, Pan C, Yao Z, Ren Y, Tian L, Cui J, Liu X, Zhang L, Chen Y. A study of the sequential treatment of acute heart failure with sacubitril/valsartan by recombinant human brain natriuretic peptide: a randomized controlled trial. Medicine. 2021;100:16(e25621).
ZP and CP both the authors contributed equally.
This work was supported by Fundamental of Research Funds for Tianjin Union Medical Center in China (Nos. 2019YJ020).
This study was approved by Medical Ethics Committee of Tianjin Union Medical Center, approval number: (2018) No. (C03) and all patients provided written informed consent.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AF = atrial fibrillation, CHD = coronary heart disease, HBP = high blood pressure, MI = myocardial infarction, SHD = structural heart disease.
References
- [1].Writing Group M, Mozaffarian D, Benjamin EJ, et al. Executive summary: heart disease and stroke statistics--2016 update: a report from the American Heart Association. Circulation 2016;133:447–54. [DOI] [PubMed] [Google Scholar]
- [2].Moran AE, Forouzanfar MH, Roth GA, et al. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation 2014;129:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–33. [DOI] [PubMed] [Google Scholar]
- [4].Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J 2012;33:1058–66. [DOI] [PubMed] [Google Scholar]
- [5].Volpe M, Carnovali M, Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci (Lond) 2016;130:57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 2008;29:367–74. [DOI] [PubMed] [Google Scholar]
- [7].Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- [8].McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803–69. [DOI] [PubMed] [Google Scholar]
- [9].Del Ry S, Cabiati M, Vozzi F, et al. Expression of C-type natriuretic peptide and its receptor NPR-B in cardiomyocytes. Peptides 2011;32:1713–8. [DOI] [PubMed] [Google Scholar]
- [10].Chen HH. Heart failure: a state of brain natriuretic peptide deficiency or resistance or both!. J Am Coll Cardiol 2007;49:1089–91. [DOI] [PubMed] [Google Scholar]
- [11].Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- [12].Abraham WT, Cheng ML, Smoluk G. Vasodilation in the Management of Acute Congestive Heart Failure Study G. Clinical and hemodynamic effects of nesiritide (B-type natriuretic peptide) in patients with decompensated heart failure receiving beta blockers. Congest Heart Fail 2005;11:59–64. [DOI] [PubMed] [Google Scholar]
- [13].Peacock WF, Holland R, Gyarmathy R, et al. Observation unit treatment of heart failure with nesiritide: results from the proaction trial. J Emerg Med 2005;29:243–52. [DOI] [PubMed] [Google Scholar]
- [14].Volpe M. Natriuretic peptides and cardio-renal disease. Int J Cardiol 2014;176:630–9. [DOI] [PubMed] [Google Scholar]
- [15].Brutsaert EF, Biggs ML, Delaney JA, et al. Longitudinal assessment of N-terminal pro-B-type natriuretic peptide and risk of diabetes in older adults: the cardiovascular health study. Metabolism 2016;65:1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Standeven KF, Hess K, Carter AM, et al. Neprilysin, obesity and the metabolic syndrome. Int J Obes (Lond) 2011;35:1031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koizumi M, Watanabe H, Kaneko Y, et al. Impact of obesity on plasma B-type natriuretic peptide levels in Japanese community-based subjects. Heart Vessels 2012;27:287–94. [DOI] [PubMed] [Google Scholar]
- [18].Macheret F, Heublein D, Costello-Boerrigter LC, et al. Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J Am Coll Cardiol 2012;60:1558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gardner DG, Chen S, Glenn DJ, et al. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension 2007;49:419–26. [DOI] [PubMed] [Google Scholar]
- [20].Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–95. [DOI] [PubMed] [Google Scholar]
- [21].Sabe MA, Jacob MS, Taylor DO. A new class of drugs for systolic heart failure: the PARADIGM-HF study. Cleve Clin J Med 2015;82:693–701. [DOI] [PubMed] [Google Scholar]
- [22].Januzzi JL, Jr, Prescott MF, Butler J, et al. Association of change in N-Terminal Pro-B-Type natriuretic peptide following initiation of Sacubitril-Valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 2019;01–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vitale G, Romano G, Di Franco A, et al. Early effects of Sacubitril/Valsartan on exercise tolerance in patients with heart failure with reduced ejection fraction. J Clin Med 2019;8:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Velazquez EJ, Marrow DA, DeVore AD, et al. Angiotensin-Neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2018;380:539–48. [DOI] [PubMed] [Google Scholar]
- [25].Forgione MA, Cap A, Liao R, et al. Heterozygous cellular glutathione peroxidase deficiency in the mouse: abnormalities in vascular and cardiac function and structure. Circulation 2002;106:1154–8. [DOI] [PubMed] [Google Scholar]
- [26].Wang Y, Zhou R, Lu C, et al. Effects of the Angiotensin-Receptor Neprilysin inhibitor on cardiac reverse remodeling: meta-analysis. J Am Heart Assoc 2019;8:e012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].MacFarlane NG, Miller DJ. Depression of peak force without altering calcium sensitivity by the superoxide anion in chemically skinned cardiac muscle of rat. Circ Res 1992;70:1217–24. [DOI] [PubMed] [Google Scholar]
- [28].Verk B, Nemec Svete A, Salobir J, et al. Markers of oxidative stress in dogs with heart failure. J Vet Diagn Invest 2017;29:636–44. [DOI] [PubMed] [Google Scholar]
- [29].Yu W, Cheng JD. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front Pharmacol 2020;11:582680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Subramani J, Kundumani-Sridharan V, Hilgers RH, et al. Thioredoxin uses a GSH-independent route to deglutathionylate endothelial nitric-oxide synthase and protect against myocardial infarction. J Biol Chem 2016;291:23374–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].de Almeida A, de Almeida Rezende MS, Dantas SH, et al. Unveiling the role of inflammation and oxidative stress on age-related cardiovascular diseases. Oxid Med Cell Longev 2020;2020:1954398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Biglane JB, Becnel MF, Ventura HO, et al. Pharmacologic therapy for heart failure with reduced ejection fraction: closing the gap between clinical guidelines and practice. Prog Cardiovasc Dis 2017;60:187–97. [DOI] [PubMed] [Google Scholar]
- [33].Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail 2019;21:965–73. [DOI] [PubMed] [Google Scholar]
- [34].Ge Q, Zhao L, Liu C, et al. LCZ696, an Angiotensin Receptor-Neprilysin inhibitor, improves cardiac hypertrophy and fibrosis and cardiac lymphatic remodeling in transverse aortic constriction model mice. Biomed Res Int 2020;2020:7256862. [DOI] [PMC free article] [PubMed] [Google Scholar]


