Abstract
We aimed to assess the respiratory virus characteristics and forecasts among young children with acute respiratory tract infection (ARTI) in west China.
This retrospective study investigated the epidemic characteristics of respiratory viruses among 11,813 paediatric ARTI patient samples (mean age, 2.25 years) between March 2018 and March 2020.
The ratio of boys to girls was 1.36. The 2 predominant viruses were influenza (Flu) A and respiratory syncytial virus (RSV) in both years, with Flu A accounting for 47.3% and 47.5% in the first and second years and RSV accounting for 32.7% and 24.7% of the positive samples in the first and second years, respectively. The Flu B positive rates were 10.9% and 13.1%, and those of the other 4 viruses were <7%. The most common virus was RSV in children below 5 years and Flu A in those between 5 and 10 years. Flu A and RSV demonstrated pronounced seasonality, and their infection rates increased from October. During the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic, isolation measures led to a decline in the number of ARTI cases.
This study provides surveillance data of the respiratory viruses in west China. It could guide medical staff in implementing necessary prevention and management strategies before future viral outbreaks.
Keywords: epidemiology, respiratory infection, virus
1. Introduction
Acute respiratory tract infection (ARTI) is the leading cause of morbidity and mortality in young children.[1] In 2015, 138 million ARTI cases, 22 million severe ARTI cases, and 0.9 million ARTI-related deaths occurred globally. The majority of ARTI cases can be attributed to viral infection, and among the diverse types of respiratory viruses, respiratory syncytial virus (RSV), adenovirus (ADV), influenza virus (Flu), and parainfluenza virus (PIV) are the most common ones.[2–5] According to World Health Organization statistics, RSV infection causes the hospitalization of 3.2 million people, and 1/15th of them eventually die.[6] Flu exhibits an obvious seasonality in positive infection rates and accounts for 7% of ARTI cases, 5% of ARTI-related hospital admissions, and 4% of ARTI-related deaths in children <5 years.[7] ADV infection can be observed all year round and accounts for 5% to 10% of lower respiratory tract infections in children.[8]
After the outbreak of severe acute respiratory syndrome (SARS) in 2003, China Information System for Disease Control and Prevention, the notifiable disease reporting system, was overhauled. However, the sudden emergence of SARS coronavirus-2 (SARS-CoV-2) highlighted the presence of new threats and need for a more effective surveillance system.[9] Daily broadcasting of the coronavirus disease (COVID-19) epidemic situation has aroused the public's interest in the spread of respiratory viruses. People have realized that common clinical viruses such as RSV, ADV, and Flu should also be routinely monitored because these viruses are a serious threat to health. Moreover, broadcasts regarding the spread of several infectious diseases have often covered the overall situation in the whole country; however, there is a lack of broadcasted information regarding the epidemic situation in specific provinces or cities. In addition, the data obtained were often related to confirmed positive severe cases that were reported by local medical institutions, but the data on many mild outpatient cases were not included, resulting in the loss of a proportion of positive case-related data. Therefore, we considered that it is quite important to review and update the data related to the epidemiological characteristics of the respiratory viruses in our region.
A comprehensive and up-to-date epidemiological database on viral ARTI would be vital for clinical management especially in terms of issuing a warning concerning the possible timing of the next respiratory viral outbreak.[10] Furthermore, it could alert clinicians regarding the need to prioritize virus detection in children because early identification of a virus can ensure appropriate treatment within an appropriate duration (2.5–4 days)[11] and the avoidance of antibiotic misuse. Therefore, we conducted a retrospective study on the detection results of respiratory viruses in children with ARTI with the aim of determining the etiologies of viral ARTI in west China. Further, through the analysis of the prevalence of 7 respiratory viruses in the past 2 years, we aimed to identify the populations with higher positive rates of respiratory viruses, common types of viruses, epidemic seasons, and changes in the viral positivity rate before and during the outbreak of SARS-CoV-2.
2. Methods
2.1. Study population
The present study was conducted in compliance with the principles of the Declaration of Helsinki and was approved by the Medical ethics committee of West China Second University Hospital of Sichuan University. This study was conducted between March 3, 2018 and March 3, 2020, as no samples that were positive for any virus were detected from March 4 to June 3, 2020. The study population included children aged 0 to 10 years with suspected ARTIs who visited pediatricians at the West China Second University Hospital of Sichuan University. The inclusion criteria required that the patient have at least 2 of the following signs or symptoms: fever (>38.0 °C), pharyngeal erythema, sore throat, cough, hoarseness, nasal discharge, a purulent pharyngeal exudate, an increased respiratory rate, or wet rales. Patients (N = 11,813) had nasopharyngeal swabs collected in the outpatient department and sent to the hospital laboratory for testing. Upon receiving the nasopharyngeal swabs, the laboratory technicians processed and tested the samples immediately.
2.2. Specimen detection
Specimens collected from each child were tested for all 7 most common respiratory viruses, among which PIV l, PIV 2, PIV 3, RSV, and ADV were detected by a direct immunofluorescence assay using the D2 Ultra DFA Respiratory Virus Screening and ID Kit (Diagnostic Hybrids, Inc., Athens, GA), and Flu A and Flu B were detected by the colloidal gold method using the Influenza A&B Antigen Detection Kit (Wondfo Biotech Co., Ltd, Guangzhou, China).
2.3. Viral identification
For the identification of PIV l, PIV 2, PIV 3, RSV, and ADV, samples were observed by direct immunofluorescence staining. Samples were considered qualified only when the number of epithelial cells was >20, which was judged by confirming the presence of at least 2 epithelial cells in each random field at 200 times magnification. When >2 green, fluorescent cells were observed in the visual field, the samples were deemed to be positive. When samples were tested for Flu A and F1u B by the colloidal gold method, it was ensured that the quality control line appeared regardless of whether the test line appeared, and the result was deemed to be positive if the test line appeared.
2.4. Statistical analysis
The constituent ratios were calculated as follows:
Data were analyzed with SPSS Statistics 19 (SPSS, Inc., Chicago, IL). Distribution normality was checked for all the variables, including age and sex. The comparison of the age was analyzed by the Mann–Whitney U test. Due to unequal group sizes and data variance, a chi-square test or Fisher exact test was used to analyze the associations between age groups and sex groups with positivity for respiratory viruses. A P value <.05 was considered statistically significant.
3. Results
A total of 11,813 samples were collected during the 2-year study period. The socio-demographic variables associated with all the samples are outlined in Table 1. In the first year (March 3, 2018–March 3, 2019), 82.8% (4294/5184) of the patients were <5 years old. The mean age of the children was 2.2 ± 2.5 years old, with more than half (58.1%) of the patients being <1 year old. In the second year (March 4, 2019–March 3, 2020), 81.7% (5414/6629) of the patients were <5 years. The mean age of the children was 2.3 ± 2.5 years old, with 45.4% of the patients being <1 year old. There were no significant differences in either the mean age (Z = –1.723, P = .09) or constituent ratio of age (χ2 = 2.68, P = .10) between the 2 years. The total number of samples from boys was higher than that from girls (χ2 = 188, P < .001). The ratio of boys to girls was 1.36 and 1.37 in the 2 years, respectively, without any statistical differences (χ2 = 0.04, P = .85).
Table 1.
Distribution of respiratory viruses according to age and gender in different years.
| March 3, 2018–March 3, 2019 | March 4, 2019–March 3, 2020 | |||||||||
| Age n (%) | Gendern (%) | Agen (%) | Gendern (%) | |||||||
| Variables | <5 years | 5–10 years | Boy | Girl | Totaln (%) | <5 years | 5–10 years | Boy | Girl | Totaln (%) |
| Samples (% of total sample received) | ||||||||||
| Total sample received | 4294 (82.8) | 890 (17.2) | 2987 (57.6) | 2197 (42.4) | 5184 (100.0) | 5414 (81.7) | 1215 (18.3) | 3831 (57.8) | 2798 (42.2) | 6629 (100.0) |
| Positive samples | 1822 (35.1) | 501 (9.7) | 1340 (25.8) | 983 (19.0) | 2323 (44.8) | 2116 (31.9) | 690 (10.4) | 1656 (25.0) | 1150 (17.3) | 2806 (42.3) |
| Respiratory virus identified (% of total positive samples based on age/sex) | ||||||||||
| Influenza A | 712 (39.1) | 387 (77.2) | 600 (44.8) | 499 (50.8) | 1099 (47.3) | 861 (40.7) | 472 (68.4) | 791 (47.8) | 542 (47.1) | 1333 (47.5) |
| Influenza B | 170 (9.3) | 83 (16.6) | 148 (11.0) | 105 (10.7) | 253 (10.9) | 224 (10.6) | 143 (20.7) | 216 (13.0) | 151 (13.1) | 367 (13.1) |
| Parainfluenza virus 1 | 15 (0.8) | 3 (0.6) | 9 (0.7) | 9 (0.9) | 18 (0.8) | 48 (2.3) | 5 (0.7) | 34 (2.1) | 19 (1.7) | 53 (1.9) |
| Parainfluenza virus 2 | 0 | 0 | 0 | 0 | 0 | 15 (0.7) | 4 (0.6) | 9 (0.5) | 10 (0.9) | 19 (0.7) |
| Parainfluenza virus 3 | 107 (5.9) | 5 (1.0) | 65 (4.9) | 47 (4.8) | 112 (4.8) | 184 (8.7) | 6 (0.9) | 107 (6.5) | 83 (7.2) | 190 (6.8) |
| Respiratory syncytial virus | 752 (41.3) | 8 (1.6) | 463 (34.6) | 297 (30.2) | 760 (32.7) | 671 (31.7) | 21 (3.0) | 414 (25.0) | 278 (24.2) | 692 (24.7) |
| Adenovirus | 66 (3.6) | 15 (3.0) | 55 (4.1) | 26 (2.6) | 81 (3.5) | 113 (5.3) | 39 (5.7) | 85 (5.1) | 67 (5.8) | 152 (5.4) |
The overall positive detection rate of all the samples was 44.8% in the first year and 42.3% in the second year, without significant differences (χ2 = 2.87, P = .09). The 2 predominant viruses were Flu A and RSV in both years, with Flu A accounting for 47.3% (n = 1099) and 47.5% (n = 1333) in the first and second year and RSV accounting for 32.7% (n = 760) and 24.7% (n = 692) of the positive samples in the first and second year, respectively. The third predominant virus was Flu B, and the positive rates of the other 4 viruses were low. Although there was no significant difference in the overall positive rates between the 2 years, especially in the case of the positive rates of Flu A (χ2 = 0.01, P = .93), the positive rate of RSV in the first year was significantly higher than that in the second year (χ2 = 22.59, P < .001).
Further, 35.1% of the children who were positive for viruses were below 5 years old in the first year. In this age group, Flu A and RSV were significantly more common (χ2 = 1174, P < .001; χ2 = 1260, P < .001). The proportion of RSV cases decreased to 1.6% (χ2 = 543.4, P < .001) while that of Flu A increased significantly to 77.2% (χ2 = 64.7, P < .001) in the 5 to 10-year-old group. In the second year, 31.9% of the children who were positive for viruses were below 5 years old. Flu A and RSV, which accounted for 40.7% and 31.7% of the total positive viral samples, respectively, were significantly more common (χ2 = 1574, P < .001; χ2 = 1120, P < .001). However, Flu B was more common than RSV (20.7% vs 10.6%, χ2 = 81.63, P < .001) in the 5 to 10-year-old age group. In both the years, the positive virus detection rates in children from the <5 years group (>30%) were significantly higher than those in the higher age group (around 10%).
Among the children who visited our hospital because of ARTI, the number of boys was significantly higher than that of girls. Among all the positive samples, 25.8% were from boys and 19.0% were from girls in the first year; 25.0% were from boys and 17.3% were from girls in the second year. The constituent ratio of boys to girls exhibited no statistical significance between the 2 years (χ2 = 0.93, P = .34). The positive detection rates in boys were not significantly different from those in girls (44.9% vs 44.7%, χ2 = 0.003, P = .96 and 43.2% vs 41.1%, χ2 = 1.22, P = .27 in the 2 years, respectively), and the differences in positive serology rates between the sexes were not statistically significant among the 7 viruses (χ2 = 12.00, P = .36 and χ2 = 14.00, P = .37).
The overall distribution of samples in both the years is presented in Fig. 1. The seasonal distributions of each respiratory virus in the first and second years are presented in Figs. 2 and 3, respectively. Flu A and RSV demonstrated pronounced seasonality, with peak infection occurring in autumn and winter (from October to December) and the lowest activity level occurring in spring and summer (from April to August). Flu B exhibited a small peak in January and March, but the number of positive cases in the other months was small. Because of the higher total number of children affected in the second year, the monthly positive numbers in the second year were always higher than those in the first year apart from in January. There were 586 positive samples in January 2019, while in comparison, the positive number in January 2020 decreased significantly to 354. In order to eliminate the influence of the difference in the total number of children tested, we converted the number of infections to the positive rate per month. The numbers of cases affected by other viruses were too small to detect any seasonality; therefore, we only analyzed the positive rates of Flu A and RSV. As shown in Fig. 4, the monthly positive rates of Flu A and RSV were consistent during the 2 years with obvious seasonality.
Figure 1.
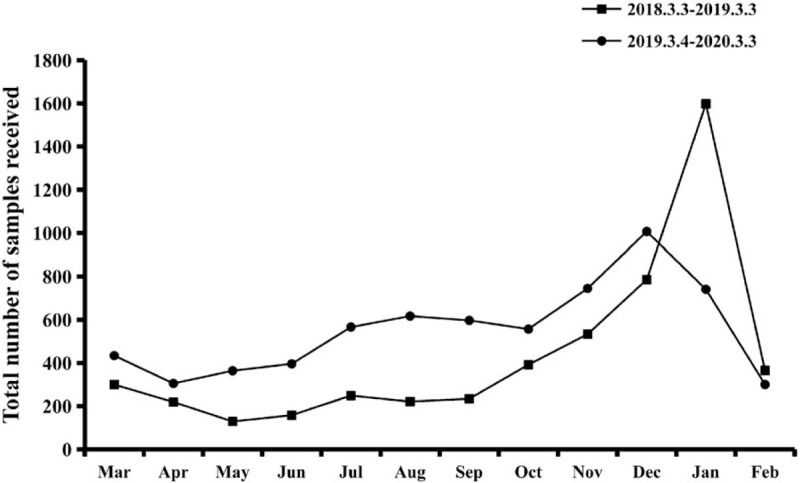
Monthly activity patterns of the total number of respiratory viruses from March 3, 2018 to March 3, 2020.
Figure 2.
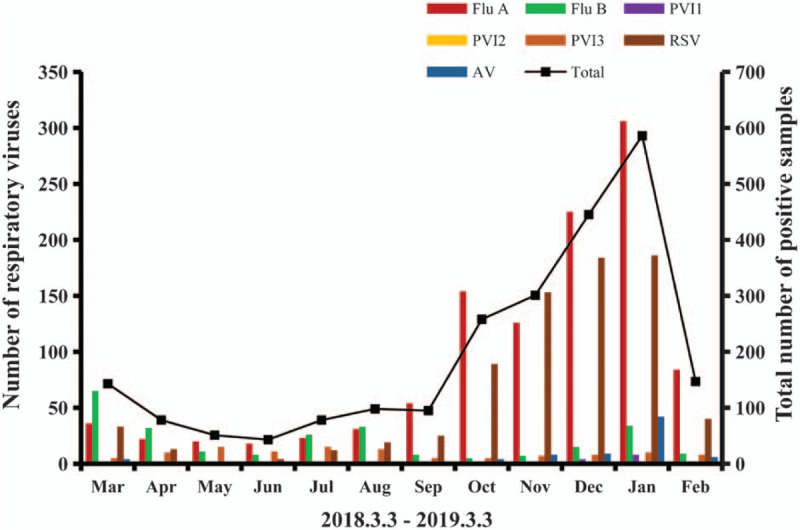
Monthly activity patterns of the 7 common respiratory viruses in the first year. ADV = adenovirus, Flu A = influenza A; Flu B = influenza B; PIV l = parainfluenza virus 1; PIV 2 = parainfluenza virus 2; PIV 3 = parainfluenza virus 3; RSV = respiratory syncytial virus.
Figure 3.
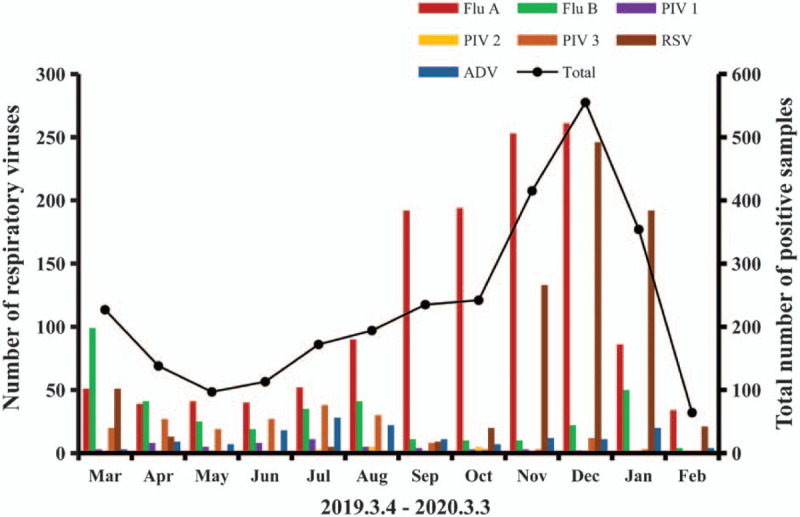
Monthly activity patterns of the 7 common respiratory viruses in the second year. ADV = adenovirus; Flu A = influenza A; Flu B = influenza B; PIV l = parainfluenza virus 1; PIV 2 = parainfluenza virus 2; PIV 3 = parainfluenza virus 3; RSV = respiratory syncytial virus.
Figure 4.
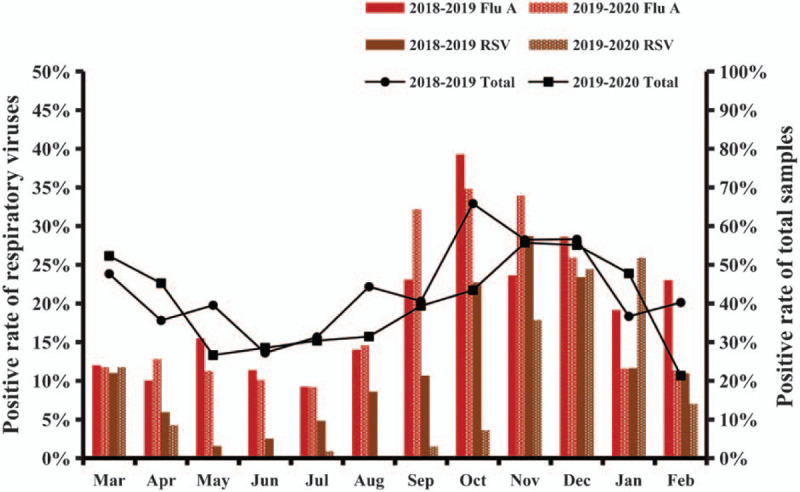
The positive rates of the respiratory viruses and total number of samples in each month. ADV = adenovirus; Flu A = influenza A; Flu B = influenza B; PIV l = parainfluenza virus 1; PIV 2 = parainfluenza virus 2; PIV 3 = parainfluenza virus 3; RSV = respiratory syncytial virus.
4. Discussion
Several types of viruses can cause ARTI with various clinical symptoms. The early detection of a virus is not only beneficial in terms of recovery from the resulting infection[12] but is also conducive to the control of virus outbreaks so as to avoid a wider spread of infection. Besides, it also greatly aids in epidemiological investigation.[13]
We found that the total number of children with ARTI in the second year was greater than that in the first year. In both the years, there were more affected boys than girls with similar ratios of boys to girls. This indicates that boys may be more vulnerable to ARTI than girls[14] owing to greater activity levels that makes them more likely to be exposed to the viruses. The average age of the population was only 2.2 to 2.3 years old, and nearly 50% of the children were <1 year old. There may be 2 reasons for this. First, the heavier burden of viral respiratory infections in children <1 year might be a result of an immature immune system and the waning of maternal antibodies after 6 months old. Children usually develop antibodies against viral infections by around 2.5 to 3.5 years of age.[15] Second, there is a potential bias from addressability to the hospital by parents of young children compared with those of parents of older children, who might be more likely in some settings to manage the child at home or at the general practitioner office.
Previous epidemiological data have revealed that RSV was the most predominant respiratory virus in children.[16] However, our data revealed that Flu A was the most common virus in our region. This could be a result of the usage of more sensitive detection methods or the seasonally variable nature of virus distributions. Our results are aligned with those of a study in which Flu A was found to be the most predominant virus among Chinese young children, followed by RSV.[17] Considering that some children have been vaccinated against influenza, the positive rate of influenza A may be higher in the absence of the vaccine. In our study, RSV was predominantly detected in children <5 years old, while Flu A and B were mainly found in those from 5 to 10 years old, suggesting that RSV infection is likely to be spread within the family or community while Flu A and B infections might mainly be spread in school.
The characteristics of geographical areas could significantly affect the observed burden of each respiratory virus.[9] The monthly positive rates in the 2 years were mostly similar, and the monthly trends were consistent apart from in January. The highest burden of viral infection in the first year was recorded in January, while in comparison, the positive number of samples decreased significantly in January 2020, mainly due to measures implemented against the spread SARS-CoV-2, such as strict home isolation and the long-term wearing of masks, which could have effectively blocked the spread of the viruses. The sharp decrease in the number of infections was consistent with the data provided by the China National Influenza Center on June 5, 2020,[18] proving that the measures taken by our country against SARS-CoV-2 were completely effective.[19] Compared with the positive rates of other viruses, those of Flu A and RSV were higher throughout the study period. We were aware that Flu A and RSV exhibit obvious seasonality. The positive rates increased when the weather became colder (autumn and winter) and decreased when the weather was warmer (spring and summer). The total number of positive samples increased from October in the past 2 years. Therefore, it is reasonable to infer that the infection rates of respiratory viruses in 2020 may gradually increase from October if less strict isolation measures were to be implemented because of the blockage of SARS-CoV-2 infection in China.
Due to the timely adoption of various isolation measures, the SARS-CoV-2 infection that broke out in late 2019 did not widely break out in our region. Besides, few positive cases have been observed in children and adolescents because they exhibit a more favourable clinical course than adults.[20] From the start of the outbreak to June 3, 2020, our hospital has tested all the presumed cases for the SARS-CoV-2 nucleic acid, as well as performed SARS-CoV-2 antibody tests for 21,241 hospitalized patients and their families, but none of the test results were positive. Therefore, the possible influence of SARS-CoV-2 on the positive rates of the 7 respiratory viruses in children could be excluded.
In summary, this study provides important virus surveillance information related to the ARTI burden and seasonality among young children in west China. The variety of respiratory viruses detected in this study highlights the need for a monitoring database to track ARTI and its etiological pathogens among children of different ages and sexes. Judging from the epidemic seasons of the respiratory viruses in our region, we infer that the infection rate of respiratory viruses in 2020 may gradually increase from October onwards, and therefore, individuals could implement corresponding preventive measures in advance to reduce the risk of viral infection. There remain some limitations of this study. First, the study's population only included children who visited our hospital for a check-up; children with mild symptoms are more likely to have been managed at home or at the general practitioner's office. This could affect the positive serology rates among elder children. Second, we only counted limited factors: the final positive serology rates of the viruses; some contributing factors affecting the positive rates, such as more exposure of boys than girls; and coverage of the influenza vaccine. Third, the isolation measures taken during the epidemic period of SARS-CoV-2 certainly had an impact on the positive rate of the virus in the second year.
Acknowledgments
The authors are grateful for the paediatricians in West China Second University Hospital of Sichuan University for their instructive suggestions and helpful comments. Thanks for the support by Sichuan University (grant number SCU2019C4198).
Author contributions
Conceptualization: Yongmei Jiang.
Data curation: Yifei Duan, Jinlan He.
Formal analysis: Yali Cui.
Funding acquisition: Yali Cui.
Methodology: Yifei Duan, Jinlan He, Yali Cui.
Supervision: Wensheng Li.
Validation: Wensheng Li.
Writing – original draft: Yifei Duan, Jinlan He, Yongmei Jiang.
Writing – review & editing: Yifei Duan, Jinlan He, Wensheng Li, Yongmei Jiang.
Footnotes
Abbreviations: ADV = adenovirus, ARTI = acute respiratory tract infection, Flu = influenza, PIV = parainfluenza virus, RSV = respiratory syncytial virus, SARS = severe acute respiratory syndrome, SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2.
How to cite this article: Duan Y, He J, Cui Y, Li W, Jiang Y. Characteristics and forecasting of respiratory viral epidemics among children in west China. Medicine. 2021;100:16(e25498).
This work was supported by the Fundamental Research Funds for the Central Universities (grant number SCU2019C4198).
The authors have no conflicts of interest to disclose.
Ethical Guidelines: The content of this study does not contain personal information that can be identified.
Data Availability Statement: The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Posted History: This manuscript was previously posted to Authorea: DOI: 10.22541/au.159654462.28679476.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Wang X, Li Y, O’Brien KL, et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: a systematic review and modelling study. Lancet Global health 2020;8:e497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Luo HJ, Huang XB, Zhong HL, et al. Epidemiological characteristics and phylogenic analysis of human respiratory syncytial virus in patients with respiratory infections during 2011-2016 in southern China. Int J Infect Dis 2020;90:05–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kong DC, Zheng YX, Jiang CY, et al. [Analysis of adenovirus infection in acute respiratory tract infection cases in Shanghai from 2015 to 2019]. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:733–7. [DOI] [PubMed] [Google Scholar]
- [4].Ma Y, Liu K, Yin Y, et al. The phylodynamics of seasonal influenza A/H1N1pdm virus in China between 2009 and 2019. Front Microbiol 2020;11:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wen S, Yu M, Zheng G, et al. Changes in the etiology of viral lower respiratory tract infections in hospitalized children in Wenzhou, China: 2008-2017. J Med Virol 2020;92:982–7. [DOI] [PubMed] [Google Scholar]
- [6].Pangesti KNA, El Ghany MA, Kesson AM, et al. Respiratory syncytial virus in the Western Pacific Region: a systematic review and meta-analysis. J Glob Health 2019;9:020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lafond KE, Nair H, Rasooly MH, et al. Global role and burden of influenza in pediatric respiratory hospitalizations, 1982-2012: a systematic analysis. PLoS Med 2016;13:e1001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jin Y, Zhang RF, Xie ZP, et al. Prevalence of adenovirus in children with acute respiratory tract infection in Lanzhou, China. Virol J 2013;10:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dong Y, Wang L, Burgner DP, et al. Infectious diseases in children and adolescents in China: analysis of national surveillance data from 2008 to 2017. BMJ 2020;369:m1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hui DS, Ng SS. Recommended hospital preparations for future cases and outbreaks of novel influenza viruses. Expert Rev Respir Med 2020;14:41–50. [DOI] [PubMed] [Google Scholar]
- [11].Moesker FM, van Kampen JJA, Aron G, et al. Diagnostic performance of influenza viruses and RSV rapid antigen detection tests in children in tertiary care. J Clin Virol 2016;79:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kolawole O, Oguntoye M, Dam T, et al. Etiology of respiratory tract infections in the community and clinic in Ilorin. Nigeria 2017;10:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Giamberardin HI, Homsani S, Bricks LF, et al. Clinical and epidemiological features of respiratory virus infections in preschool children over two consecutive influenza seasons in southern Brazil. J Med Virol 2016;88:1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yassine HM, Sohail MU, Younes N, et al. Systematic Review of the Respiratory Syncytial Virus (RSV) prevalence, genotype distribution, and seasonality in children from the Middle East and North Africa (MENA) region. Microorganisms 2020;8:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yew SM, Tan KL, Yeo SK, et al. Molecular epidemiology of respiratory viruses among Malaysian Young children with a confirmed respiratory infection during 2014-2015. J Thorac Dis 2019;11:4626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lu L, Robertson G, Ashworth J, et al. Epidemiology and phylogenetic analysis of viral respiratory infections in Vietnam. Front Microbiol 2020;11:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li Y, Wang J, Wang C, et al. Characteristics of respiratory virus infection during the outbreak of 2019 novel coronavirus in Beijing. Int J Infect Dis 2020;96:266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weekly Influenza Report; 2020. Available at: http://ivdc.chinacdc.cn/cnic/zyzx/lgzb/202006/t20200605_217160.htm. Accessed June 5, 2020. [Google Scholar]
- [19].Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020;145:e20200702. [DOI] [PubMed] [Google Scholar]
- [20].De Rose DU, Piersigilli F, Ronchetti MP, et al. Novel Coronavirus disease (COVID-19) in newborns and infants: what we know so far. Ital J Pediatr 2020;46:56. [DOI] [PMC free article] [PubMed] [Google Scholar]


