PURPOSE
In relapsed and/or refractory multiple myeloma, daratumumab reduced the risk of progression or death by > 60% in POLLUX (daratumumab/lenalidomide/dexamethasone [D-Rd]) and CASTOR (daratumumab/bortezomib/dexamethasone [D-Vd]). Minimal residual disease (MRD) is a sensitive measure of disease control. Sustained MRD negativity and outcomes were evaluated in these studies.
METHODS
MRD was assessed via next-generation sequencing (10−5) at suspected complete response (CR), 3 and 6 months following confirmed CR (POLLUX), 6 and 12 months following the first dose (CASTOR), and every 12 months post-CR in both studies. Sustained MRD negativity (≥ 6 or ≥ 12 months) was evaluated in the intention-to-treat (ITT) and ≥ CR populations.
RESULTS
The median follow-up was 54.8 months in POLLUX and 50.2 months in CASTOR. In the ITT population, MRD-negativity rates were 32.5% versus 6.7% for D-Rd versus lenalidomide and dexamethasone (Rd) and 15.1% versus 1.6% for D-Vd versus bortezomib and dexamethasone (Vd; both P < .0001). Higher MRD negativity rates were achieved in ≥ CR patients in POLLUX (D-Rd, 57.4%; Rd, 29.2%; P = .0001) and CASTOR (D-Vd, 52.8%; Vd, 17.4%; P = .0035). More patients in the ITT population achieved sustained MRD negativity ≥ 6 months with D-Rd versus Rd (20.3% v 2.1%; P < .0001) and D-Vd versus Vd (10.4% v 1.2%; P < .0001), and ≥ 12 months with D-Rd versus Rd (16.1% v 1.4%; P < .0001) and D-Vd versus Vd (6.8% v 0%). Similar results for sustained MRD negativity were observed among ≥ CR patients. More patients in the daratumumab-containing arms achieved MRD negativity and sustained MRD negativity, which were associated with prolonged progression-free survival.
CONCLUSION
Daratumumab-based combinations induce higher rates of sustained MRD negativity versus standard of care, which are associated with durable remissions and prolonged clinical outcomes.
INTRODUCTION
Despite high response rates achieved in recent years in patients with multiple myeloma (MM) treated with combination therapies, most patients relapse and require subsequent therapy.1 Therefore, assays with greater precision and sensitivity that provide a more accurate assessment of disease state and the likelihood of relapse are needed.2
CONTEXT
Key Objective
Can minimal residual disease (MRD)–negative status and sustained MRD negativity in relapsed and/or refractory multiple myeloma (RRMM) serve as a predictive and prognostic end point for clinical outcomes? We conducted an exploratory analysis using the POLLUX and CASTOR studies, which represent the largest set of MRD data prospectively collected among patients with RRMM.
Knowledge Generated
Patients who achieved MRD-negative status had improved progression-free survival (PFS) compared with MRD-positive patients, and sustained MRD negativity was associated with improved PFS compared with patients who obtained MRD-negative status but did not maintain MRD durability.
The benefit of MRD negativity and durability occurred regardless of therapy, but daratumumab-based regimens enabled many patients with RRMM to attain deep and sustained MRD-negative responses, resulting in longer periods without disease progression.
Relevance
Achieving durable MRD negativity may predict long-term outcomes, as durable MRD negativity improves PFS and increases the time between treatment relapses for RRMM.
Detection of minimal residual disease (MRD) is emerging as an important tool to evaluate therapeutic efficacy in MM.3-5 Multiple analyses have demonstrated that MRD negativity is associated with prolonged progression-free survival (PFS) and overall survival (OS) in patients with newly diagnosed MM (NDMM).3,4,6 MRD is being investigated as a potential surrogate for established clinical end points, including PFS and OS, in MM.1,3,4,7 The International Myeloma Working Group (IMWG) has published guidelines for the uniform assessment and reporting of MRD negativity in MM.1 The guidelines recommend an MRD sensitivity threshold of at least 10−5 (one tumor cell in 100,000 normal cells) using next-generation sequencing (NGS) or next-generation flow cytometry.1 When measured sequentially, sustained MRD negativity provides an index of deep clinical responses that may enable a more robust assessment of disease control.1 Per IMWG criteria, sustained MRD negativity is a negative MRD result in bone marrow, confirmed ≥ 1 year apart.1 To date, sustained MRD negativity has not been prospectively reported in any large relapsed and/or refractory MM (RRMM) studies, and identifying therapies and regimens that drive sustained MRD negativity and how this affects long-term outcomes is of great clinical importance.
Daratumumab is a human immunoglobulin Gκ monoclonal antibody targeting CD38 that has a direct on-tumor and immunomodulatory mechanism of action.8-14 Daratumumab is approved as monotherapy and in combination with standard-of-care regimens (bortezomib and dexamethasone [Vd], lenalidomide and dexamethasone [Rd], carfilzomib and dexamethasone, and pomalidomide and dexamethasone) for treatment of RRMM in many countries.15 Daratumumab is also approved in combination with Rd; with bortezomib, thalidomide, and dexamethasone; and with bortezomib, melphalan, and prednisone for treatment of NDMM.15
In the primary analyses of two randomized, controlled, phase III trials in patients with RRMM who received ≥ 1 prior line of therapy, daratumumab showed a significant clinical benefit when added to standard-of-care regimens Rd (POLLUX; daratumumab/lenalidomide/dexamethasone [D-Rd] v Rd; median follow-up, 13.5 months) and Vd (CASTOR; daratumumab/bortezomib/dexamethasone [D-Vd] v Vd; median follow-up, 7.4 months).16,17 In POLLUX and CASTOR, after longer follow-up (median, 25.4 and 19.4 months, respectively), daratumumab-based therapies reduced the risk of disease progression or death by ≥ 59%.18,19 Responses deepened with longer follow-up in both studies, resulting in higher complete response or better (≥ CR) rates versus the control arms in POLLUX (51.2% v 21.0%; P < .0001) and CASTOR (28.8% v 9.8%; P < .0001).18,19
Assessment of MRD using NGS was a prespecified secondary end point in both studies (using clonoSEQ v.1.3; Adaptive Biotechnologies, Seattle, WA). Daratumumab combination therapy led to higher MRD negativity (10−5) rates (POLLUX: 26.2% v 6.4%, P < .0001; CASTOR: 11.6% v 2.4%, P < .0001) after the median follow-up of 25.4 and 19.4 months, respectively.18,19 Regardless of treatment group, patients achieving MRD negativity demonstrated prolonged PFS compared with MRD-positive patients.18,19
POLLUX and CASTOR are ongoing studies; these studies represent the largest set of MRD data prospectively collected among patients with RRMM. Until this analysis, the prognostic value of MRD in RRMM had not yet been evaluated. To our knowledge, this is the first report to assess whether MRD-negative status and sustained MRD negativity in RRMM may serve as a predictive and prognostic end point for clinical outcomes in the relapsed and/or refractory disease setting. Moreover, we evaluated the degree to which MRD negativity could be sustained with daratumumab plus standard-of-care regimens for RRMM, using clinical data after an extended follow-up period of approximately 4 years.
METHODS
Trial Design and Oversight
POLLUX (ClinicalTrials.gov identifier: NCT02076009)17 and CASTOR (ClinicalTrials.gov identifier: NCT02136134)16 are randomized, open-label, multicenter, phase III studies conducted in patients with RRMM (study designs have been published previously).16,17 Eligible patients in both studies had progressive disease per IMWG criteria20,21 during or after receipt of their last regimen, received ≥ 1 prior line of therapy, and had a partial response or better to ≥ 1 line of previous therapy.16,17 Patients refractory to lenalidomide were ineligible for POLLUX, and patients refractory to bortezomib or another proteasome inhibitor were ineligible for CASTOR.16,17 Trials were approved by independent ethics committees or institutional review boards at each site. Patients provided written informed consent, and trials were conducted in accordance with the principles of the Declaration of Helsinki and current International Conference on Harmonisation Good Clinical Practice guidelines.
Random Assignment and Study Treatment
Patients were randomly assigned (1:1) in POLLUX to D-Rd or Rd and in CASTOR to D-Vd or Vd. Treatment regimen descriptions are given in the Data Supplement (online only).
End Points and Assessments
In both studies, PFS was the primary efficacy end point. Tumor response and disease progression assessments were conducted using a central laboratory and a validated computer algorithm in accordance with IMWG response criteria. In the absence of central laboratory results, local laboratories could be used for confirmatory evaluations.
In accordance with the IMWG criteria, MRD was assessed at the time of suspected CR, 3 and 6 months following confirmed CR in POLLUX, and at the time of suspected CR and at 6 and 12 months following the first treatment dose in CASTOR (at the end and 6 months after the end of Vd background therapy, respectively). Additional MRD evaluation was required every 12 months post-CR in both studies (see the Data Supplement). MRD was assessed using bone marrow aspirate samples and evaluated via NGS using the clonoSEQ assay (v.2.0). Detailed methods are presented in the clonoSEQ assay technical information.22
Sustained MRD negativity was defined as the maintenance of MRD negativity in bone marrow confirmed ≥ 6 or ≥ 12 months apart and was evaluated in the intention-to-treat (ITT) population. Sustained MRD negativity was evaluated in patients who achieved ≥ CR to account for different sustained MRD negativity rates between treatment arms.
Statistical Analysis
Statistical methods supporting the sample sizes were described previously.16,17 PFS was compared between groups based on a stratified log-rank test. Hazard ratios (HRs) and 95% CIs were estimated using a Cox regression model with treatment as the sole explanatory variable, stratified by International Staging System (ISS; I, II, or III), number of prior lines of therapy (1 v 2 or 3 v > 3), and prior bortezomib (CASTOR) or lenalidomide (POLLUX) treatment (no v yes). MRD negativity rate was defined as the proportion of patients with negative MRD results at any time during treatment. A minimum cell input equivalent to the given sensitivity threshold was required to determine MRD negativity (eg, MRD at 10−5 required that ≥ 100,000 cells were evaluated). If MRD negativity was not achieved, a patient was considered MRD positive. This study focused on a sensitivity threshold of 10−5 for sustained MRD negativity based on the IMWG criteria,1 but thresholds of 10−4 and 10−6 were also evaluated. MRD negativity rates were compared using Fisher's exact test, and the chi-square estimate of the common odds ratio was provided.
In post hoc analyses of PFS and MRD status by response category among patients pooled from both studies and the daratumumab treatment arms (D-Rd and D-Vd) versus control arms (Rd and Vd), PFS was evaluated and a two-sided P value was presented. Additional methods are presented in the Data Supplement.
RESULTS
Patients
In total, 569 (D-Rd, n = 286; Rd, n = 283) patients in POLLUX and 498 (D-Vd, n = 251; Vd, n = 247) patients in CASTOR were randomly assigned to the daratumumab and control groups, respectively (Data Supplement). Patient baseline demographics and clinical characteristics were published previously and were generally well balanced.16,17 At clinical cutoff (August 26, 2019 for POLLUX; August 14, 2019 for CASTOR), the median duration of follow-up was 54.8 months (range, 0.0-61.9) in POLLUX and 50.2 months (range, 0.0-58.6) in CASTOR.
MRD Negativity and Durability
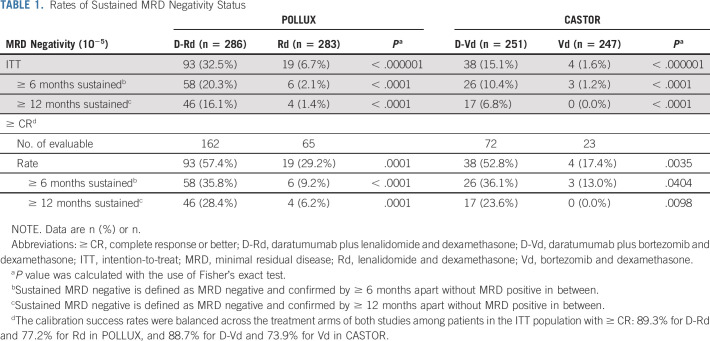
Daratumumab-based regimens induced a ≥ 4-fold higher MRD negativity rate (10−5) compared with control regimens in the ITT populations of both studies (POLLUX: D-Rd, 32.5% v Rd, 6.7%, P < .000001; CASTOR: D-Vd, 15.1% v Vd, 1.6%, P < .000001; Table 1). MRD negativity rates were also higher for the daratumumab-based regimens among patients achieving ≥ CR (D-Rd, 57.4% v Rd, 29.2%, P = .0001; D-Vd, 52.8% v Vd, 17.4%, P = .0035; Table 1). The overall numbers of patients achieving MRD negativity were higher in POLLUX compared with CASTOR.
TABLE 1.
Rates of Sustained MRD Negativity Status
For patients with MRD-negative (10−5) status, durability of this response was assessed. In each study, a higher proportion of patients in the ITT population achieved sustained MRD negativity for ≥ 6 months when treated with daratumumab (POLLUX: D-Rd, 20.3% v Rd, 2.1%, P < .0001; CASTOR: D-Vd, 10.4% v Vd, 1.2%, P < .0001; Table 1). Only six patients in the Rd arm and three in the Vd arm had prolonged MRD negativity for ≥ 6 months. Among patients who achieved ≥ CR, the proportion with sustained MRD negativity for ≥ 6 months remained higher for those treated with daratumumab (POLLUX: D-Rd, 35.8% v Rd, 9.2%, P < .0001; CASTOR: D-Vd, 36.1% v Vd, 13.0%, P = .0404; Table 1). Similarly, a higher proportion of patients achieved sustained MRD negativity for ≥ 12 months when treated with daratumumab (POLLUX: D-Rd, 16.1% v Rd, 1.4%, P < .0001; CASTOR: D-Vd, 6.8% v Vd, 0, P < .0001; Table 1) in the ITT population and among patients with CR or better (POLLUX, D-Rd, 28.4% v Rd, 6.2%, P = .0001; CASTOR: D-Vd, 23.6% v Vd, 0%, P = .0098).
Baseline demographics and clinical characteristics among daratumumab-treated patients with durable MRD negativity ≥ 12 months and not ≥ 12 months in POLLUX and CASTOR are summarized in the Data Supplement. Overall, because only four patients in the Rd arm of POLLUX and no patients in the Vd arm of CASTOR had durable MRD responses ≥ 12 months, identifying baseline features associated with these responses was not feasible. In both studies, the relative proportions of daratumumab-treated patients with sustained MRD negativity were generally similar within the demographic characteristics of age, sex, race, Eastern Cooperative Oncology Group performance status, type of disease, and ISS staging compared with baseline (Data Supplement). In POLLUX and CASTOR, fewer daratumumab-treated patients with durable MRD negativity lasting ≥ 12 months had high cytogenetic risk compared with standard cytogenetic risk (POLLUX, 8.8% v 91.2%, respectively; CASTOR, 33.3% v 66.7%; Data Supplement).
PFS and MRD Negativity
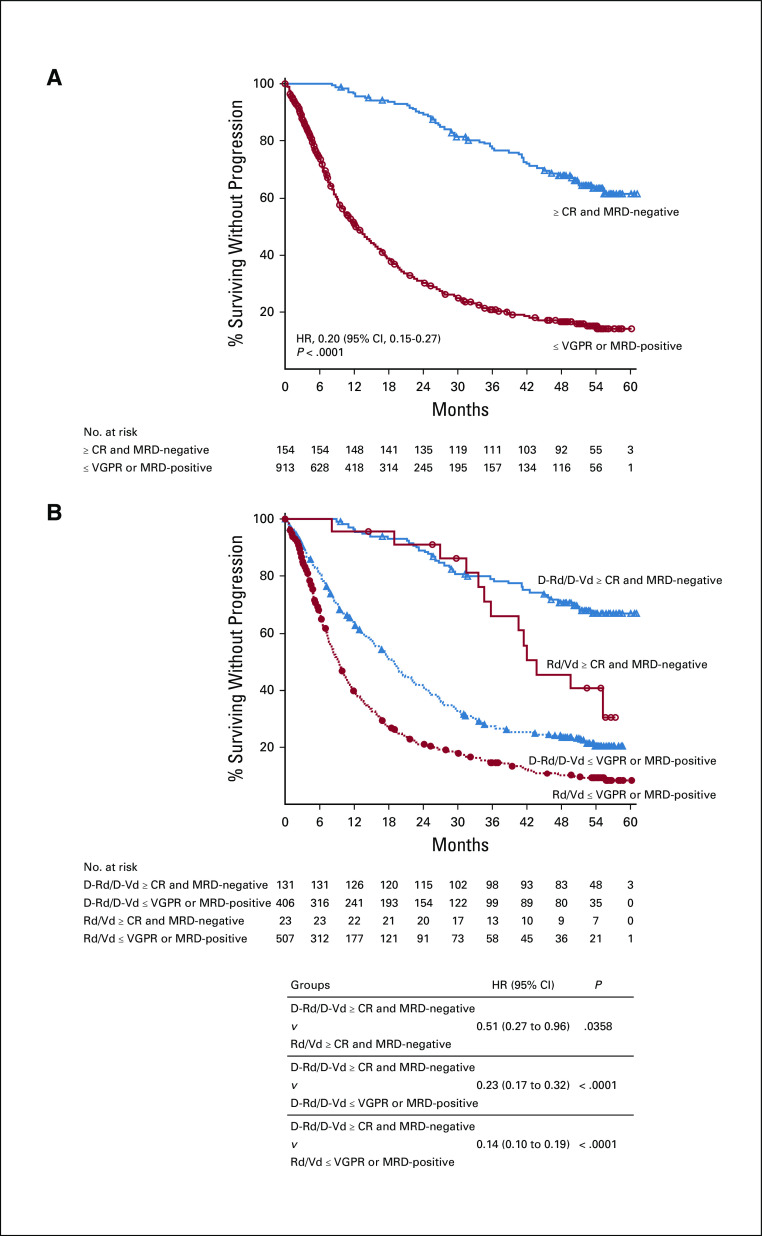
In the ITT populations of POLLUX and CASTOR, patients who achieved MRD negativity (10−5) had a lower risk of disease progression or death compared with MRD-positive patients (Fig. 1), consistent with previous findings based on shorter follow-up.18,19 In both studies, daratumumab-based regimens were associated with longer PFS for both MRD-negative and MRD-positive patients compared with the standard-of-care groups (Data Supplement).
FIG 1.

PFS based on MRD status (10−5) in POLLUX (A) and CASTOR (B). Shown are the results of the Kaplan-Meier estimates of PFS among patients in the ITT population based on the absence of MRD at a threshold of one tumor cell per 105 white cells. Blue lines show regimens containing daratumumab; red lines show standard-of-care regimens. D-Rd, daratumumab plus lenalidomide and dexamethasone; D-Vd, daratumumab plus bortezomib and dexamethasone; ITT, intention-to-treat; MRD, minimal residual disease; PFS, progression-free survival; Rd, lenalidomide and dexamethasone; Vd, bortezomib and dexamethasone.
In POLLUX and CASTOR, PFS was prolonged in patients who achieved sustained MRD negativity ≥ 6 months compared with MRD-positive patients regardless of treatment arm (Fig. 2). Consistent with these findings, achievement of sustained MRD negativity for ≥ 12 months demonstrated prolonged PFS for daratumumab-containing regimens versus MRD-positive patients in POLLUX and CASTOR (Fig. 3).
FIG 2.
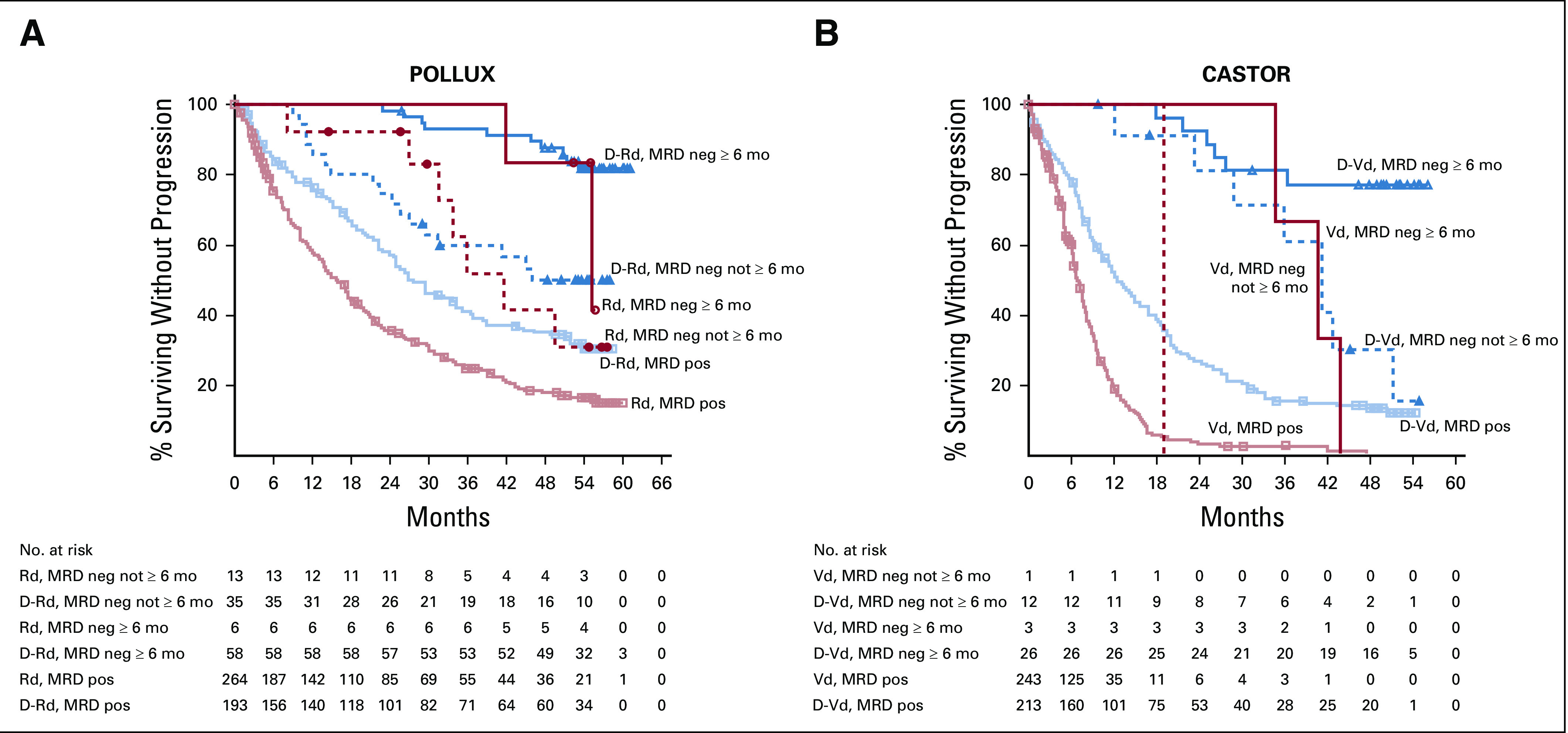
PFS based on sustained MRD negativity (10−5; ≥ 6 months) in the ITT populations of POLLUX (A) and CASTOR (B). Shown are the results of the Kaplan-Meier estimates of PFS among patients in the ITT population based on sustained MRD negativity ≥ 6 months at a threshold of one tumor cell per 105 white cells. Blue lines show regimens containing daratumumab; red lines show standard-of-care regimens. D-Rd, daratumumab plus lenalidomide and dexamethasone; D-Vd, daratumumab plus bortezomib and dexamethasone; ITT, intention-to-treat; MRD, minimal residual disease; PFS, progression-free survival; Rd, lenalidomide and dexamethasone; Vd, bortezomib and dexamethasone.
FIG 3.
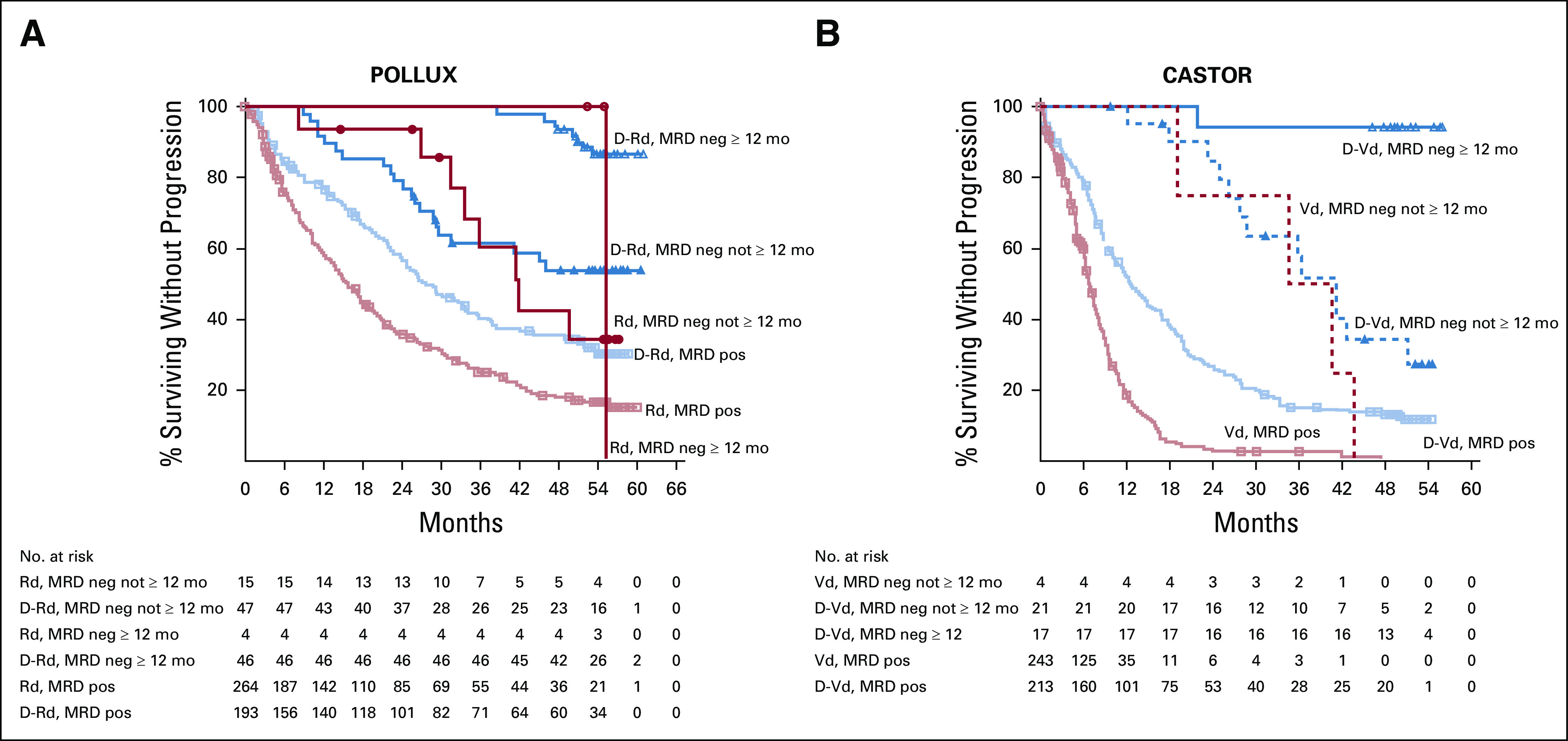
PFS based on sustained MRD negativity (10−5; ≥ 12 months) in the ITT populations of POLLUX (A) and CASTOR (B). Shown are the results of the Kaplan-Meier estimates of PFS among patients in the ITT population based on sustained MRD negativity ≥ 12 months at a threshold of one tumor cell per 105 white cells. Blue lines show regimens containing daratumumab; red lines show standard-of-care regimens. D-Rd, daratumumab plus lenalidomide and dexamethasone; D-Vd, daratumumab plus bortezomib and dexamethasone; ITT, intention-to-treat; MRD, minimal residual disease; PFS, progression-free survival; Rd, lenalidomide and dexamethasone; Vd, bortezomib and dexamethasone.
In a combined analysis of daratumumab-containing regimens (D-Rd and D-Vd; n = 537) versus standard of care (Rd and Vd; n = 530), patients who achieved MRD negativity (10−5) had prolonged PFS compared with MRD-positive patients (Data Supplement). PFS was also prolonged in patients with durable MRD negativity lasting ≥ 6 months or ≥ 12 months compared with patients who did not achieve sustained MRD negativity (Data Supplement).
In POLLUX and CASTOR, the median time to next therapy was longer for the daratumumab-containing groups versus the control groups among patients who were MRD-negative at any time prior to starting next therapy (Table 2). Patients who were MRD-positive had the shortest median times to subsequent anticancer therapy (TTSAT) and the lowest 48-month TTSAT rates (Table 2). Median TTSAT was improved for patients who achieved sustained MRD negativity ≥ 6 months and ≥ 12 months, with these groups showing the highest 48-month TTSAT rates (Table 2). For patients who were MRD-negative at any time before initiating subsequent anticancer therapy, the risk of disease progression or death on the next subsequent line of therapy (PFS2) was not different for the daratumumab groups versus the control groups in either study. Median PFS2 was longer for MRD-negative patients compared with MRD-positive patients; however, among patients who were MRD-positive before initiating subsequent therapy, the daratumumab groups had prolonged PFS2 versus the control groups (Table 2).
TABLE 2.
Time to Next Therapy and PFS on Next Subsequent Line of Therapy
PFS and MRD Negativity by Response Status
In post hoc analyses of combined POLLUX and CASTOR patients who achieved MRD negativity and ≥ CR (n = 154) versus patients who were MRD-positive or with a response worse than CR (n = 913; termed ≤ very good partial response), patients in the deepest level of response (≥ CR with MRD negativity) had improved PFS (HR, 0.20 [95% CI, 0.15 to 0.27], P < .0001; Figure 4A); this trend was maintained regardless of therapy regimen (Fig. 4B). These data are further supported by a time-varying survival model showing that ≥ CR with MRD negativity had an effect on PFS in both univariate and multivariate models (Data Supplement).
FIG 4.
PFS by response and MRD status (10−5) among (A) all patients in POLLUX and CASTOR and (B) in the pooled daratumumab-based combination groups versus control groups. Shown are the results of the Kaplan-Meier estimates of PFS among patients in the ITT population based on the absence of MRD at a threshold of one tumor cell per 105 white cells and on response category (≥ CR, ≤ VGPR). In panel A, blue line shows patients who achieve ≥ CR and MRD negativity at any time since random assignment; red line shows patients who achieve ≤ VGPR or are MRD-positive. In panel B, blue lines show regimens containing daratumumab; red lines show standard-of-care regimens. ≥ CR, complete response or better; D-Rd, daratumumab plus lenalidomide and dexamethasone; D-Vd, daratumumab plus bortezomib and dexamethasone; HR, hazard ratio; ITT, intention-to-treat; MRD, minimal residual disease; PFS, progression-free survival; Rd, lenalidomide and dexamethasone; Vd, bortezomib and dexamethasone; ≤ VGPR, very good partial response or worse.
DISCUSSION
This exploratory analysis represents the first randomized, controlled, prospective evaluation of the effect of sustained MRD negativity on PFS in RRMM, using the IMWG-recommended sensitivity threshold (10−5). We demonstrate that patients with RRMM who achieved MRD-negative status had improved PFS compared with MRD-positive patients. Furthermore, sustained MRD negativity was associated with improved PFS compared with patients who obtained MRD-negative status but did not maintain MRD durability. Although the benefit of MRD negativity and durability occurred regardless of therapy, daratumumab-containing regimens enabled a higher proportion of patients to achieve deep and durable responses; among patients who achieved MRD negativity, daratumumab drove more patients to maintain this state for ≥ 6 and ≥ 12 months, which was associated with prolonged PFS in both studies.
Although both POLLUX and CASTOR demonstrated durable MRD negativity with daratumumab-based regimens, inherent differences exist between the studies. With similar median follow-up times (POLLUX, 54.8 months; CASTOR, 50.2 months), longer PFS was observed with D-Rd (median of 45.0 months) compared with D-Vd (median of 16.7 months).23,24 Based on these data, patients treated with D-Rd would be expected to achieve higher rates of MRD negativity compared with D-Vd–treated patients. However, because of differences in study designs and patient populations, a direct comparison cannot be made between studies. Patients in POLLUX received treatment with D-Rd until disease progression, whereas patients in CASTOR received eight fixed cycles of D-Vd followed by daratumumab monotherapy until progression. Additionally, in POLLUX, patients received a median of one prior line of therapy versus two prior lines in CASTOR.
Although CR has generally been considered a favorable outcome for patients with MM, CR is not indicative of a cure, and most patients relapse.1 The disconnection between CR and long-term efficacy suggests that persistent disease remains undetected, and measuring deeper responses is necessary to predict and improve long-term outcomes. As shown here, achieving durable MRD negativity may predict long-term outcomes, as durable MRD negativity improves PFS and increases the time between treatment relapses for RRMM; this supports the concept that sustained MRD negativity may serve as a surrogate end point for PFS in ongoing and future clinical trials. Although ultrasensitive MRD assessment techniques are available, there is currently no consensus on how or when to use these methods for detecting and monitoring MRD status.1,25-27 Prospectively gathered clinical data will be useful in developing future paradigms for MRD analysis as a clinical practice decision tool.
The results of a pooled analysis of 609 patients enrolled in three studies of transplant-eligible or elderly patients with MM revealed the lack of a survival benefit for patients who achieved CR without MRD negativity.6 These findings highlight the limited value in using CR without MRD negativity as a prognostic marker for survival outcomes in MM.
In the ITT populations of POLLUX and CASTOR, PFS was prolonged in patients who achieved MRD negativity compared with MRD-positive patients, regardless of treatment regimen. Similar findings were observed for patients achieving sustained MRD negativity for ≥ 6 months and ≥ 12 months, compared with MRD-positive patients. Moreover, in a combined analysis including all patients from POLLUX and CASTOR, patients achieving CR or better and MRD negativity had prolonged PFS, regardless of therapy regimen.
Currently, PFS outcomes in both studies were similar between treatment arms for MRD-positive patients. However, daratumumab-containing regimens allowed more patients to achieve MRD-negative status (D-Rd, 32.5% v Rd, 6.7%; D-Vd, 15.1% v Vd, 1.6%), which was associated with prolonged long-term outcomes. Follow-up for OS is ongoing and will be assessed at the end of both studies to provide important insight regarding whether daratumumab-containing regimens provide a long-term OS benefit for patients with RRMM.
Studies in NDMM demonstrated that PFS and OS were prolonged (both P < .001) in patients who reached MRD-negative status, supporting the prognostic value of MRD negativity and its value as a surrogate marker for PFS and OS in patients with NDMM, including those who achieved CR.3,4,28 Achieving MRD negativity for a meaningful duration (eg, for > 6 or > 12 months) may represent a higher level of prognostic significance compared with standard testing. The findings presented here demonstrate a clear survival benefit for patients who reach durable MRD negativity for durations of ≥ 6 months or ≥ 12 months.
Our data represent the largest data set analyzed to date, but other studies have highlighted the role of sustained MRD negativity in MM and its association with survival outcomes.29-32 In a phase II study of carfilzomib, lenalidomide, and dexamethasone in patients with NDMM (N = 45), sustained MRD negativity was observed in 54% and 46% of patients after 1 and 2 years of maintenance therapy, respectively, which corresponded to an 82% PFS rate at 48 months.29 Finally, in a real-world retrospective analysis of 232 patients with MM who underwent autologous stem-cell transplantation, 51% of patients achieved durable MRD negativity 12 months post-transplant, which was associated with improved survival outcomes.32 Taken together, these findings support the utility of sustained MRD assessment in MM.
In summary, patients in the RRMM setting who achieved CR or better and MRD-negative status had prolonged PFS compared with patients who did not. In addition, daratumumab-based regimens enabled many patients with RRMM to attain deep and sustained MRD-negative responses, resulting in longer periods without disease progression.
ACKNOWLEDGMENT
We thank the patients who volunteered to participate in the POLLUX and CASTOR trials and their families, as well as the coinvestigators, staff members at the trial sites who cared for them, and clinical site coordinators. We also thank Jamie Bald for her support in sample operations, Linda Okonkwo and Chris Velas for contributions to the study, Huiling Pei for critical review of the manuscript, representatives of the sponsor who were involved in data collection and analyses, and Kristin Runkle, PhD, and Charlotte D. Majerczyk, PhD, of MedErgy for medical writing and editorial assistance.
PRIOR PRESENTATION
Presented in part at the 60th American Society of Hematology (ASH) Annual Meeting & Exposition, San Diego, CA, December 1-4, 2018; data in the current manuscript have been updated to a more recent sampling time point.
SUPPORT
This trial was supported by Janssen Research and Development, LLC.
CLINICAL TRIAL INFORMATION
NCT02136134 (CASTOR); NCT02076009 (POLLUX)
DATA SHARING STATEMENT
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
AUTHOR CONTRIBUTIONS
Conception and design: Hervé Avet-Loiseau, Jesus San-Miguel, Tineke Casneuf, Sagar Lonial, Philippe Moreau, Katja Weisel, Jon Ukropec, Christopher Chiu, Himal Amin, Steven Sun, Ming Qi, Rachel Kobos, Nizar J. Bahlis
Administrative support: Christopher Chiu, Sonali Trivedi
Provision of study materials or patients: Shinsuke Iida, Sagar Lonial, Saad Z. Usmani, Philippe Moreau, Torben Plesner, Katja Weisel, Nizar J. Bahlis
Collection and assembly of data: Hervé Avet-Loiseau, Jesus San-Miguel, Tineke Casneuf, Shinsuke Iida, Andrew Spencer, Philippe Moreau, Torben Plesner, Katja Weisel, Jon Ukropec, Christopher Chiu, Sonali Trivedi, Himal Amin, Rachel Kobos, Nizar J. Bahlis
Data analysis and interpretation: Hervé Avet-Loiseau, Jesus San-Miguel, Tineke Casneuf, Shinsuke Iida, Sagar Lonial, Saad Z. Usmani, Andrew Spencer, Philippe Moreau, Torben Plesner, Katja Weisel, Jon Ukropec, Christopher Chiu, Sonali Trivedi, Himal Amin, Maria Krevvata, Priya Ramaswami, Xiang Qin, Mia Qi, Steven Sun, Ming Qi, Rachel Kobos, Nizar J. Bahlis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Evaluation of Sustained Minimal Residual Disease Negativity With Daratumumab-Combination Regimens in Relapsed and/or Refractory Multiple Myeloma: Analysis of POLLUX and CASTOR
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jesus San-Miguel
Consulting or Advisory Role: Amgen, Celgene, Takeda, Bristol-Myers Squibb, MSD, Novartis, Sanofi, Janssen, Roche, AbbVie, GlaxoSmithKline, Karyopharm Therapeutics
Tineke Casneuf
Employment: Janssen
Stock and Other Ownership Interests: Johnson & Johnson
Patents, Royalties, Other Intellectual Property: 2 patent applications by J&J with a symbolic 1 dollar as compensation.
Travel, Accommodations, Expenses: Janssen
Shinsuke Iida
Honoraria: Takeda, Celgene, Janssen, Daiichi Sankyo, Ono Pharmaceutical, Sanofi
Consulting or Advisory Role: Sanofi, Ono Pharmaceutical, Takeda, Janssen, GlaxoSmithKline, AbbVie
Research Funding: Takeda, Chugai Pharma, Kyowa Hakko Kirin, Ono Pharmaceutical, Sanofi, Bristol-Myers Squibb Japan, Janssen, AbbVie
Sagar Lonial
Stock and Other Ownership Interests: TG Therapeutics
Consulting or Advisory Role: Celgene, Bristol-Myers Squibb, Janssen Oncology, Novartis, GlaxoSmithKline, Amgen, AbbVie, Takeda, Merck, Sanofi
Research Funding: Celgene, Bristol-Myers Squibb, Takeda
Other Relationship: TG Therapeutics
Saad Z. Usmani
Consulting or Advisory Role: Celgene, Amgen, Janssen Oncology, Seattle Genetics, Takeda, GlaxoSmithKline, Karyopharm Therapeutics, AbbVie, Skyline Diagnostics, Merck
Speakers' Bureau: Celgene, Takeda, Amgen, Janssen Oncology, Sanofi
Research Funding: Celgene, Array BioPharma, Janssen Oncology, Pharmacyclics, Sanofi, Bristol-Myers Squibb, Amgen, Seattle Genetics, Merck, Skyline Diagnostics, GlaxoSmithKline
Andrew Spencer
Honoraria: Janssen-Cilag
Consulting or Advisory Role: Janssen-Cilag
Speakers' Bureau: Janssen-Cilag
Research Funding: Janssen-Cilag
Philippe Moreau
Honoraria: Celgene, Takeda, Novartis, Janssen-Cilag, Amgen, GlaxoSmithKline
Consulting or Advisory Role: Celgene, Takeda, Janssen, Amgen, GlaxoSmithKline
Torben Plesner
Consulting or Advisory Role: Janssen, Celgene, Takeda, AbbVie, Genmab
Speakers' Bureau: Janssen
Research Funding: Janssen
Katja Weisel
Honoraria: Amgen, Bristol-Myers Squibb, Celgene, Janssen-Cilag, GlaxoSmithKline, Adaptive Biotechnologies, Karyopharm Therapeutics, Takeda, Sanofi, Roche/Genentech, Oncopeptides
Consulting or Advisory Role: Amgen, Adaptive Biotechnologies, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen-Cilag, Karyopharm Therapeutics, Sanofi, Takeda
Research Funding: Amgen, Celgene, Sanofi, Janssen-Cilag
Travel, Accommodations, Expenses: Amgen, Celgene, Bristol-Myers Squibb, Janssen-Cilag, GlaxoSmithKline, Takeda
Jon Ukropec
Employment: Janssen
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Johnson & Johnson/Janssen
Christopher Chiu
Employment: Janssen Research & Development, Genmab
Stock and Other Ownership Interests: Janssen Research & Development, Genmab
Patents, Royalties, Other Intellectual Property: Related to Daratumumab with Janssen, Related to Epcoritamab with Genmab
Himal Amin
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Maria Krevvata
Stock and Other Ownership Interests: Amicus Therapeutics
Priya Ramaswami
Employment: Johnson & Johnson
Xiang Qin
Employment: Janssen Research & Development
Mia Qi
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Steven Sun
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Ming Qi
Employment: Janssen R&D LLC
Stock and Other Ownership Interests: Janssen R&D LLC
Rachel Kobos
Employment: Janssen Oncology
Stock and Other Ownership Interests: Johnson & Johnson
Nizar J. Bahlis
Honoraria: Celgene, Janssen, AbbVie, Amgen, Sanofi, Takeda, Karyopharm Therapeutics
Consulting or Advisory Role: Janssen, Celgene, Amgen, Sanofi, Takeda, Pfizer, Karyopharm Therapeutics
Research Funding: Janssen, Celgene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kumar S Paiva B Anderson KC, et al. : International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17:e328-e346, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Mailankody S Korde N Lesokhin AM, et al. : Minimal residual disease in multiple myeloma: Bringing the bench to the bedside. Nat Rev Clin Oncol 12:286-295, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munshi NC Avet-Loiseau H Rawstron AC, et al. : Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: A meta-analysis. JAMA Oncol 3:28-35, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landgren O Devlin S Boulad M, et al. : Role of MRD status in relation to clinical outcomes in newly diagnosed multiple myeloma patients: A meta-analysis. Bone Marrow Transpl 51:1565-1568, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrot A Lauwers-Cances V Corre J, et al. : Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood 132:2456-2464, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahuerta JJ Paiva B Vidriales MB, et al. : Depth of response in multiple myeloma: A pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol 35:2900-2910, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gormley NJ, Farrell AT, Pazdur R: Minimal residual disease as a potential surrogate end point-lingering questions. JAMA Oncol. 3:18-20, 2017 [DOI] [PubMed] [Google Scholar]
- 8.de Weers M Tai YT van der Veer MS, et al. : Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840-1848, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Lammerts van Bueren J Jakobs D Kaldenhoven N, et al. : Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood 124:3474, 2014 [Google Scholar]
- 10.Overdijk MB Verploegen S Bogels M, et al. : Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 7:311-321, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overdijk MB Jansen JH Nederend M, et al. : The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. J Immunol 197:807-813, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Krejcik J Casneuf T Nijhof IS, et al. : Daratumumab depletes CD38+ immune-regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128:384-394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casneuf T Adams HC III van de Donk NWCJ, et al. : Deep immune profiling of patients treated with lenalidomide and dexamethasone with or without daratumumab. Leukemia 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams HC III Stevenaert F Krejcik J, et al. : High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytometry A 95:279-289, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DARZALEX® (Daratumumab) [Package Insert]. Horsham, PA, Janssen Biotech, 2020 [Google Scholar]
- 16.Palumbo A Chanan-Khan A Weisel K, et al. : Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375:754-766, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulos MA Oriol A Nahi H, et al. : Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375:1319-1331, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos M San Miguel J Belch A, et al. : Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of POLLUX. Haematologica 103:2088-2096, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer A Lentzsch S Weisel K, et al. : Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of CASTOR. Haematologica 103:2079-2087, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durie BGM Harousseau JL Miguel JS, et al. : International uniform response criteria for multiple myeloma. Leukemia 20:1467-1473, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Rajkumar SV Harousseau JL Durie B, et al. : Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 117:4691-4695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ClonoSEQ® Assay Technical Information. Seattle, WA, Adaptive Biotechnologies Corporation, 2018 [Google Scholar]
- 23.Weisel KC Sonneveld P Mateos MV, et al. : Efficacy and safety of daratumumab, bortezomib, and dexamethasone (D-Vd) versus bortezomib and dexamethasone (Vd) in first relapse patients (pts) with multiple myeloma (MM): Four-year update of CASTOR. Poster Presented at the 61st American Society of Hematology (ASH) Annual Meetings & Exposition, Orlando, FL, December 7-10, 2019
- 24.Kaufman JL Usmani SZ San-Miguel J, et al. : Four-year follow-up of the phase 3 pollux study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) alone in relapsed or refractory multiple myeloma (RRMM). Poster Presented at the 61st American Society of Hematology (ASH) Annual Meetings & Exposition, Orlando, FL, December 7-10, 2019
- 25.Nishihori T, Song J, Shain KH: Minimal residual disease assessment in the context of multiple myeloma treatment. Curr Hematol Malig Rep 11:118-126, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paiva B, Garcia-Sanz R, San Miguel JF: Multiple myeloma minimal residual disease. Cancer Treat Res 6:103-122, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Fulciniti M, Munshi NC, Martinez-Lopez J: Deep response in multiple myeloma: A critical review. Biomed Res Int 2015:832049, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attal M Lauwers-Cances V Hulin C, et al. : Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 376:1311-1320, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kazandjian D Korde NS Roschewski M, et al. : Sustained minimal residual disease negativity in newly diagnosed multiple myeloma (NDMM) patients treated with carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (DEX) followed by 2 years of lenalidomide maintenance (CRd-R): Updated results of a phase 2 study. Blood 128:4527, 2016 [Google Scholar]
- 30.Mailankody S Kazandjian D Korde N, et al. : Baseline mutational patterns and sustained MRD negativity in patients with high-risk smoldering myeloma. Blood Adv 1:1911-1918, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu J Liu J Chen M, et al. : Longitudinal flow cytometry identified “minimal residual disease” (MRD) evolution patterns for predicting the prognosis of patients with transplant-eligible multiple myeloma. Biol Blood Marrow Transpl 24:2568-2574, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Austin M Pawlyn C Woods H, et al. : Sustained MRD negativity at 12 months post-ASCT predicts outcomes for myeloma patients: A real world study. Poster Presented at the 23rd European Hematology Association (EHA) Annual Congress, Stockholm, Sweden, June 14-17, 2018 (abstr PS1326)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.