PURPOSE
To examine the incidence and risk factors for de novo atrial fibrillation (AF) after allogeneic hematopoietic cell transplantation (HCT) and to describe the impact of AF on HCT-related outcomes.
METHODS
A retrospective cohort study design was used to examine AF and associated outcomes in 487 patients who underwent allogeneic HCT from 2014 to 2016 and to characterize patient- and HCT-related risk factors. A nested case-control study design was used to describe the association between pre-HCT echocardiographic measures and future AF events.
RESULTS
The median age at HCT was 52.4 years (18.1-78.6); the median time to AF was 117.5 days (4.0-1,405.0). The 5-year cumulative incidence of AF was 10.6%. Older (≥ 50 years) age (hazard ratio [HR], 2.76; 95% CI, 1.37 to 5.58), HLA-unrelated donor (HR, 2.20; 95% CI, 1.18 to 4.12), dyslipidemia (HR, 2.40; 95% CI, 1.23 to 4.68), and pre-HCT prolonged QTc interval (HR, 2.55; 95% CI, 1.38 to 4.72) were independent risk factors for AF. Despite having comparable left ventricular systolic function, patients who developed AF were significantly more likely to have lower left atrial ejection fraction, left atrial reservoir function, and elevated tricuspid regurgitant jet velocity prior to HCT, compared with patients who did not. The incidence rate of stroke after AF was 143 per 1,000 person-years. In adjusted analyses, AF was associated with a 12.8-fold (HR, 12.76; 95% CI, 8.76 to 18.57) risk of all-cause mortality and 15.8-fold (HR, 15.78; 95% CI, 8.70 to 28.62) risk of nonrelapse mortality.
CONCLUSION
The burden of AF after allogeneic HCT population is substantial, and the development of AF is associated with poor survival. We identified important associations between patient demographics, pre-HCT cardiac parameters, HCT-related exposures, and risk of AF, setting the stage for targeted prevention strategies during and after HCT.
INTRODUCTION
Hematopoietic cell transplantation (HCT) is an established and effective treatment for hematological disorders and malignancies.1 Improvement in HCT strategies during the past five decades has led to an increasing number of long-term survivors.2,3 There are currently an estimated 250,000 HCT survivors in the United States, a number that will double within the next 10 years.4 Despite improvements in long-term outcomes, HCT survivors continue to have substantially higher mortality rates compared with the general population.5-7 In particular, the risk of cardiovascular-related mortality is more than twice that of the general population,6-8 and the magnitude of risk increases with time from HCT.8 In HCT survivors, cardiovascular complications such as myocardial infarction, stroke, and heart failure are a leading cause of long-term morbidity,9 and there are well-described pre-HCT (eg, anthracycline chemotherapy and chest radiation), conditioning-related (eg, high-dose cyclophosphamide), and post-HCT (eg, de novo comorbidities [hypertension, diabetes, and dyslipidemia]) risk factors for these health conditions.10-11
CONTEXT
Key Objective
To examine the incidence and risk factors for atrial fibrillation (AF) after allogeneic hematopoietic cell transplantation (HCT) and describe the impact of AF on HCT-related outcomes.
Knowledge Generated
The 5-year cumulative incidence of AF was 10.6%. Older (≥ 50 years) age at HCT, having an HLA-unrelated donor, dyslipidemia, and pre-HCT prolonged QTc interval were independent risk factors for AF. Despite having comparable left ventricular systolic function, patients who developed AF were more likely to have lower left atrial ejection fraction, left atrial reservoir function, and elevated tricuspid regurgitant jet velocity prior to HCT, compared with patients who did not. AF was associated with a > 15-fold risk of nonrelapse mortality.
Relevance
This information may help set the stage for informed decision making between healthcare providers and high-risk patients prior to HCT and consideration of innovative monitoring approaches for these patients during HCT or in the community after HCT.
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the general population.12 AF is associated with a five-fold increased risk of stroke, three-fold increased risk of heart failure, and two-fold increased risk of dementia and death in the general population,13,14 and there are established clinical (eg, older age and hypertension) and ECG (eg, prolonged QTc interval and abnormal P axis) risk factors for AF.15-18 Studies in nononcology patients have further shown that echocardiographic measures of left atrial (LA) dysfunction (eg, low LA ejection fraction [EF] and abnormal reservoir strain) may provide incremental value in determining future AF risk.19,20 There is a paucity of information on the incidence and risk factors of de novo AF in HCT patients, especially in those undergoing allogeneic HCT, and there have been no studies to examine the association between pre-HCT echocardiographic parameters and future AF risk. In these patients, it is especially important to understand outcomes following the onset of AF, given the increasing indications for HCT and greater numbers of older (> 65 years) individuals referred for higher-risk allogeneic HCT.
For the current study, we used both a retrospective cohort study design and a nested case-control approach to describe the incidence of de novo AF and its associated health outcomes after HCT and to evaluate the role of patient demographics, chronic comorbidities, pre-HCT treatment–related exposures, transplant conditioning, and cardiac (electrocardiographic and echocardiographic) risk factors associated with the development of AF after allogeneic HCT.
METHODS
Cohort Analysis
A total of 599 consecutive patients underwent allogeneic HCT at City of Hope (COH) between January 1, 2014, and December 31, 2016. Patients included in the current study were identified from the COH long-term follow-up research protocol, which ensures the active and comprehensive follow-up of all patients who undergo HCT at COH. The human subjects committee at COH approved the protocol, and informed consent was obtained according to the Declaration of Helsinki. Children (< 18 years at HCT; n = 65), individuals with a prior history of AF (n = 34), or those undergoing a second HCT (n = 13) were excluded from the current study; 487 patients were included in the retrospective cohort analysis. The median follow-up for the cohort was 3.1 years (range, 0-6.0), representing 1,400 person-years of follow-up, and 81% of the cohort was followed through December 31, 2019 (if alive), or death.
Medical records maintained at COH were the primary source of data and were used to abstract demographics (age, sex, and race or ethnicity), medical history (hypertension, diabetes, dyslipidemia, thyroid disorder, and cardiovascular disease [eg, heart failure and coronary artery disease]), pre-HCT treatment (chemotherapy and radiation), indication for and relapse risk at HCT,21 HCT-related exposures (conditioning agents and intensity,22 HLA match [related and unrelated], stem-cell source, and graft v host disease [GVHD] prophylaxis), and post-HCT outcomes (acute GVHD severity23 and cause-specific mortality). Pre-HCT ECGs obtained per standard of care were also evaluated for heart rate, PR interval, and QTc interval; 468 patients (96.1% of the cohort) had available ECGs. Information on vital status and cause of death was obtained from the National Death Index and COH medical records.
Case definition of AF, including categorization of lone versus paroxysmal or persistent AF, was according to established guidelines for the management of patients with AF,14 which required ECG documentation of an irregular R-R interval, the absence of distinct repeating P waves, and irregular atrial activity, or was based on physician documentation of AF followed by initiation of medical management. Individuals were considered to have pre-HCT diabetes, hypertension, dyslipidemia, or hypothyroidism if they were on medications for management of these conditions in the 4 weeks prior to HCT.
The cumulative incidence of AF after HCT was calculated taking into consideration the competing risk of death for right-censored data.24 The time to AF was computed from the start of conditioning therapy to onset of AF; for those who did not develop AF, the follow-up ended on the date of death or the date of last contact (censored: December 31, 2019), whichever came first. We calculated the incidence rate of stroke after AF onset, reported as the number of events per person-years of follow-up.
Fine-Gray proportional subdistributional hazard models (hazard ratios [HRs], 95% CI) were used to estimate the relationship between clinically relevant variables and risk of AF, accounting for death as competing risk.24 Univariate analyses were performed initially with patient demographics, comorbidities, pre-HCT ECG parameters, and HCT-related exposures. Multivariable regression analysis was conducted by including all variables in univariate analyses with P < .1 and then using backward stepwise elimination to obtain the final model with only the significant (P < .05) independent variables; the multivariable analysis was limited to the 468 patients with available ECGs.
In addition, we examined the effect of AF onset on nonrelapse mortality (NRM) and relapse-related mortality treating AF as a time-varying covariate, with each outcome serving as competing risk in the Fine-Gray model.24 Cox proportional hazards regression was used to examine the relationship between AF and overall survival, treating AF as a time-varying covariate. Multivariable Fine-Gray regression analysis was used to examine the association between AF onset and cause-specific mortality, adjusting for pre-HCT prognostic CHARGE-AF score (derived from age at HCT, race, height, weight, blood pressure, current smoking, use of antihypertensive medication, diabetes, and history of cardiovascular disease),16 severity of acute GVHD after HCT, conditioning intensity, and stem-cell donor type. Data were analyzed using SAS Version 9.4 software (SAS Institute, Cary, NC). All statistical analyses were two-sided, and a P value < .05 was considered statistically significant.
Case-Control Analysis
To examine the association between pre-HCT echocardiographic measures and risk of AF, we first determined the number of patients with AF who had available pre-HCT echocardiograms. Of the 50 patients who developed AF, 39 (78%) had an echocardiogram that was available and interpretable; the median time from echocardiogram to the start of conditioning was 17 days (range, 0-82). Of the 437 individuals who did not develop AF, 389 (89%) had available echocardiograms. Of note, there were no statistically significant differences in clinical and treatment characteristics between individuals with and without pre-HCT echocardiograms. Cases (all patients who developed AF with echocardiograms, n = 39) were matched to up to three controls (did not develop AF and had an echocardiogram, n = 97). Cases and controls were matched on age at HCT (± 5 years), sex, and length of follow-up (control follow-up was equivalent to or exceeded that of the case). We elected to use a nested case control design because of the time commitment (approximately 2 hours) required for central review of each echocardiogram, the need to match for length of follow-up, and variables (eg, age and sex) that may affect echocardiographic measures of interest. All echocardiograms were reviewed by a single observer (L.C.) who was blinded to the case or control status. Measurements included left ventricular (LV) dimensions, wall thickness, volumes, mitral velocity, and tricuspid regurgitant (TR) jet velocity; and LA dimensions, volumes, and strain; LV and LA EF% was calculated as (Volmax − Volmin)/Volmax × 100. Twenty echocardiograms were randomly selected for quality control evaluation, confirming previously reported high intraobserver reliability.25-27 We used multivariable conditional logistic regression to compare echocardiographic indices between cases and controls and to estimate the odds ratio (OR) of AF occurrence. Variables in the models included echocardiographic variables (LA EF [< 45%], TR [> 2.8 m/s], and reservoir function [<39%]) and relevant treatment-related differences between the two groups. Data were analyzed using SPSS Version 26.0 (IBM Corp, Armonk, NY).
RESULTS
Patient and Clinical Characteristics
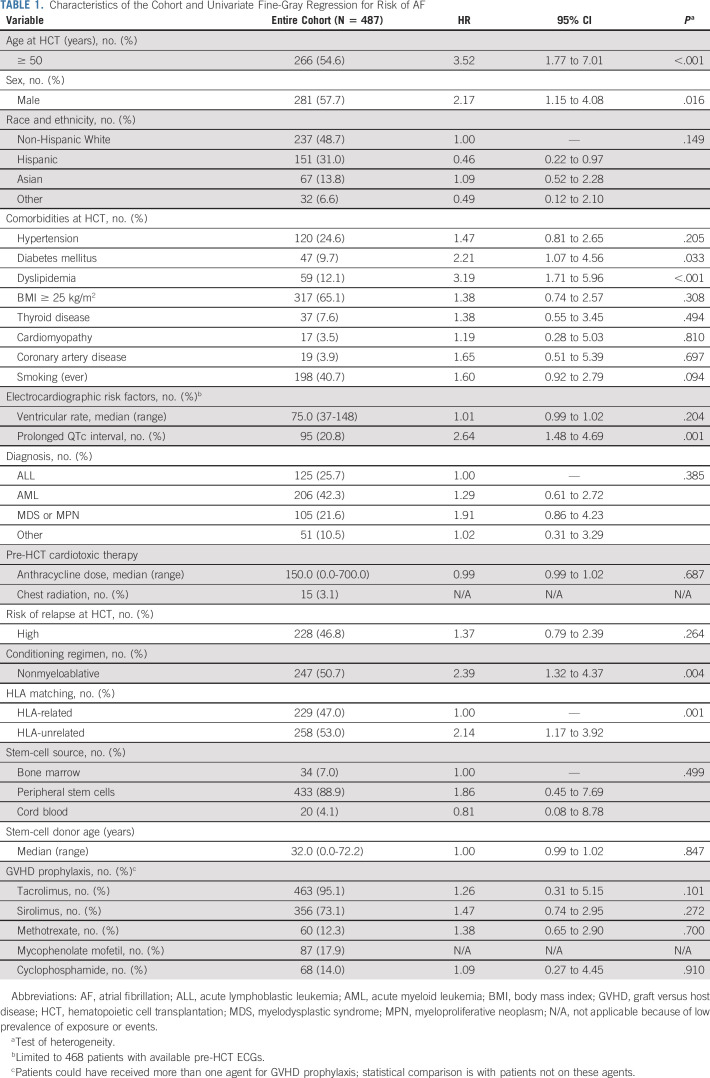
The characteristics of the cohort are summarized in Table 1. The median age at HCT was 52.4 years (range, 18.1-78.6); the majority of patients were male (57.7%) and non-Hispanic White (48.7%). Primary diagnoses included acute myeloid leukemia (42.3%), acute lymphoblastic leukemia (25.7%), myelodysplastic syndrome or myeloproliferative neoplasm (21.6%), and others (10.5%); 53.2% were at low risk of relapse at HCT, 50.7% received nonmyeloablative conditioning, and 53.0% had an HLA-unrelated donor; the most common GVHD prophylaxis medications were tacrolimus (95.1%) and sirolimus (73.1%).
TABLE 1.
Characteristics of the Cohort and Univariate Fine-Gray Regression for Risk of AF
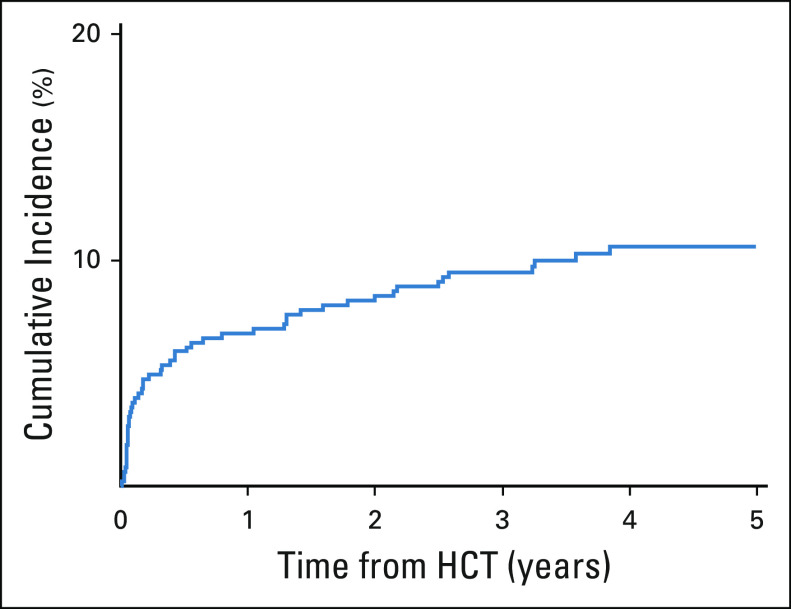
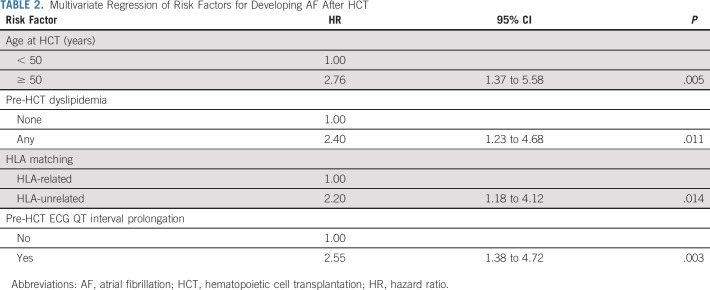
The cumulative incidence of AF at 1 and 5 years was 6.8% and 10.6%, respectively (Fig. 1); the median time to AF was 117.5 days (range, 4.0-1,405.0). Of the 50 patients who developed AF, three (6%) had lone AF and 47 (94%) had paroxysmal or persistent AF. Univariate analysis using Fine-Gray regression showed that older (≥ 50 years) age at HCT (HR, 3.52; 95% CI, 1.77 to 7.01), male (HR, 2.17; 95% CI, 1.15 to 4.08), diabetes (HR, 2.21; 95% CI, 1.07 to 4.56), dyslipidemia (HR, 3.19; 95% CI, 1.71 to 5.96), pre-HCT prolonged QTc interval (HR, 2.64; 95% CI, 1.48 to 4.69), HLA-unrelated donor (HR, 2.14; 95% CI, 1.17 to 3.92), and nonmyeloablative conditioning (HR, 2.39; 95% CI, 1.32 to 4.37) are significantly associated with AF risk (Table 1). In the multivariable regression model, older (≥ 50 years) age (HR, 2.76; 95% CI, 1.37 to 5.58), having an HLA-unrelated donor (HR, 2.20; 95% CI, 1.18 to 4.12), dyslipidemia (HR, 2.40; 95% CI, 1.23 to 4.68), and prolonged QTc interval (HR, 2.55; 95% CI, 1.38 to 4.72) were significant independent predictors of AF risk (Table 2).
FIG 1.
Cumulative incidence of AF after allogeneic HCT. AF, atrial fibrillation; HCT, hematopoietic cell transplantation.
TABLE 2.
Multivariate Regression of Risk Factors for Developing AF After HCT
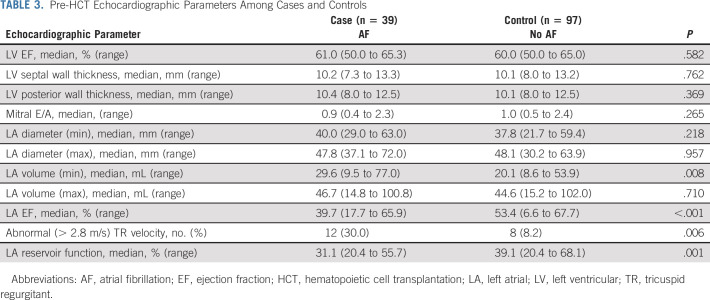
An examination of echocardiograms obtained before HCT revealed that despite having comparable LV EF (Table 3), patients who developed AF had significantly lower median LA EF (39.7% v 53.4%, P < .001) and LA reservoir function (31.1% v 39.1%, P = .001) and had a higher prevalence of abnormal TR jet velocity (30.8% v 8.2%, P = .006), compared with those who did not develop AF. In the multivariable regression model, having an abnormal (< 45%) LA EF, low (< 39%) LA reservoir function, or elevated (> 2.8 m/s) TR jet velocity was significantly associated with the odds of developing AF (LA EF: OR, 12.7, P < .001; LA reservoir function: OR, 3.8, P = .010; TR jet velocity: OR, 4.2, P = .023); having two or more abnormal indices was associated with an 18.0-fold (OR, 18.0, P < .001) odds of developing AF after HCT.
TABLE 3.
Pre-HCT Echocardiographic Parameters Among Cases and Controls
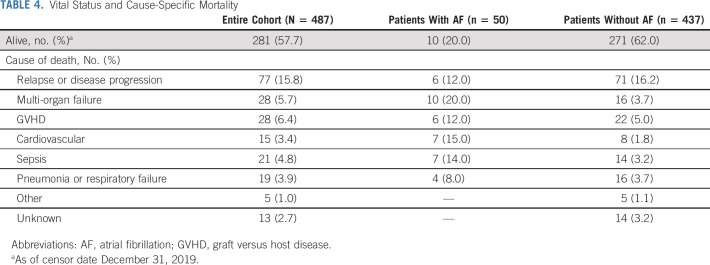
Five patients developed stroke during 34.8 person-years of follow-up after AF onset. The incidence rate of stroke after AF was 143 per 1,000 person-years. The overall 1- and 5-year survival probabilities for the entire cohort were 75.4% and 55.8%, respectively. The most common cause of death was relapse or progression; the prevalence of nonrelapse cause-specific mortality differed between patients with and without AF (Table 4). In adjusted analyses, AF was associated with a 12.8-fold (HR, 12.76; 95% CI, 8.76 to 18.57) risk of all-cause mortality and 15.8-fold (HR, 15.78; 95% CI, 8.70 to 28.62) risk of NRM. There were no differences in the risk of relapse-related mortality between the two groups (HR, 1.08; 95% CI: 0.43 to 2.68).
TABLE 4.
Vital Status and Cause-Specific Mortality
DISCUSSION
In this contemporary cohort of patients undergoing allogeneic HCT, there was a high burden of AF soon after the start of conditioning that persisted into long-term survivorship. One in ten patients developed AF at a median of 117 days after HCT, and the steepest increase in the incidence of AF was in the first year after HCT. Outcomes in patients who developed AF were poor, largely because of the high risk of nonrelapse-related mortality. Similar to what has been reported in nononcology populations,28-31 we found older age, pre-HCT dyslipidemia, and prolonged QTc interval to be significant independent risk factors for AF. Specific to the HCT population, patients who had an HLA-unrelated donor had an especially high risk of AF. We also identified pre-HCT echocardiographic predictors of future AF risk. Overall, our findings speak to the importance of comprehensive risk assessment for AF prior to HCT, focusing on patient-related, clinical, and HCT-related risk factors, as well as key cardiac parameters, allowing for implementation of prevention and treatment strategies to mitigate adverse cardiovascular outcomes after HCT.
To date, studies examining AF after HCT have been limited to patients undergoing autologous HCT,32-38 representing an older population with greater comorbidities and a higher proportion of pre-HCT cardiotoxic (eg, chest radiation and anthracyclines) exposures, compared with patients undergoing allogeneic HCT. To our knowledge, our study is the first to describe the incidence and risk factors for AF in a contemporary cohort of allogeneic patients, allowing us to capture evolving HCT practices (eg, expanding donor stem-cell source, use of nonmyeloablative conditioning, increasingly older age at HCT, and expanded indications for HCT). Finally, we excluded patients with a history of AF prior to conditioning, allowing us to characterize the incidence and predictors of de novo AF after HCT.
We identified significant and independent HCT-related risk factors that warrant an additional study. Specifically, we found that patients who had an unrelated donor had a > 2-fold risk of AF, compared with individuals who had related donors. This risk may be driven by the higher incidence of GVHD in these patients,39-41 and the resultant complex multi-organ (eg, renal and vascular) dysfunction that may persist after HCT. Pre-HCT cardiotoxic exposures such as chest radiation or anthracyclines were not associated with AF risk, which may be due to the low dose and low prevalence of these exposures in our cohort. Although there has been increased recognition of the arrhythmogenic potential of newer anticancer therapies (eg, protein kinase and checkpoint inhibitors) in patients with hematologic malignancies,42 < 5% of our cohort were treated with these agents prior to or shortly after HCT. It remains to be seen whether increased utilization of these therapies in the future will add to the already high burden of AF after allogeneic HCT.
Studies in nononcology populations have highlighted the association between echocardiographic LA dimensions or volumes and a range of cardiovascular outcomes, including AF and cardiovascular mortality.43-46 Speckle tracking echocardiography, initially developed for the left ventricle, has been applied to assess LA deformation (LA strain) and can provide important clinical information on LA performance and tissue health.47-49 To our knowledge, the current study is the first to examine the association between these novel echocardiographic parameters and the risk of AF in the oncology setting. We used a standardized protocol to perform a blinded review of echocardiograms and were careful to consider parameters that would be obtained as part of standard of care. We found that pre-HCT low (< 45%) LA EF, low (< 39%) LA reservoir function, and elevated (> 2.8 m/s) TR jet velocity were significantly associated with AF risk. Although there are currently no established recommendations for the use of echocardiography to screen for AF risk in the general population, there may be a role for its use in certain high-risk populations, such as those undergoing allogeneic HCT.
Patients who developed AF had especially poor outcomes after HCT. The risk of all-cause mortality was nearly thirteen-fold higher, whereas the risk of NRM was more than fifteen-fold higher in patients who developed AF compared with those who did not. More than one third of NRM in patients with AF was attributable to cardiovascular causes or multi-organ failure. It is important to note that our multivariable models included the calculated CHARGE-AF score16,50 for each patient, accounting for well-established risk factors for all-cause and cardiovascular mortality in the general population. Our study highlights the need for HCT-specific comprehensive (demographic, cardiac, and HCT-related risk factors) prognostic scores that may guide screening and prevention of AF prior to HCT. These strategies would have to balance the relative benefits and harms of pre-emptive management, including anticoagulation in the setting of prolonged thrombocytopenia or hepatotoxicity from medications (eg, amiodarone) used to manage AF in patients at risk for sinusoidal obstruction syndrome or liver GVHD during HCT.
The findings from this study have to be considered in the context of its limitations. Our study relied on retrospectively collected patient and treatment information, which limited the number of variables that could be reliably included in our analyses, including the chronic use of beta-blockers or other preventive pharmacotherapies in the months to years after HCT. We were careful to include objective variables that could be accurately extracted from medical records, using a strategy that has been successfully implemented to describe cardiovascular outcomes in HCT.9,51,52 We used a standardized definition for AF, which would allow future studies to compare rates and outcomes reported in our population with theirs. That said, we acknowledge that the incidence of AF reported in our study may be an underestimate of the true incidence of disease in this population, given that some patients may be asymptomatic. All patients who developed AF in our cohort required medical intervention (eg, pharmacologic rate and/or rhythm control or cardiac resynchronization) and thus represent clinically relevant events. Of note, anticoagulation was rarely in used because of contraindications such as profound thrombocytopenia or organ (eg, hepatorenal) dysfunction or because of a low CHA2DS2-VASc score14 in a minority (< 5%) of individuals.
In summary, to our knowledge, our study describes for the first time the incidence of AF in allogeneic HCT patients and identifies novel associations between pretreatment echocardiographic abnormalities and HCT-related risk factors for AF in these patients. We also highlight the poor survival outcomes in patients who develop AF, emphasizing the need for increasing awareness regarding AF after allogeneic HCT. The information from the current study may help set the stage for informed decision making between healthcare providers and high-risk patients prior to HCT and consideration of innovative monitoring (eg, wireless wearable ECG) for these patients during HCT or in the community after HCT. The growing number of patients undergoing allogeneic HCT (more than 25,000 per year in the United States and Europe alone)53,54 makes the development of personalized transplant strategies imperative, to ensure that these patients live long and healthy lives well beyond the immediate HCT period.
PRIOR PRESENTATION
Presented in part at the American Heart Association meeting, Philadelphia, PA, November 16-18, 2019.
SUPPORT
Supported in part by grants from the Lymphoma/Leukemia Society Scholar Award for Clinical Research (2315-17; S.H.A.) and NIH/NHLBI: R01 HL150069 (S.H.A.).
AUTHOR CONTRIBUTIONS
Conception and design: Ellen K. Chang, Saro H. Armenian
Financial support: Saro H. Armenian
Administrative support: Jennifer Berano Teh, Saro H. Armenian, Stephen J. Forman
Provision of study materials or patients: Jennifer Berano Teh, Ryotaro Nakamura, Saro H. Armenian, Stephen J. Forman
Collection and assembly of data: Ellen K. Chang, Jennifer Berano Teh, Aleksi Iukuridze, Kelly Peng, Ryotaro Nakamura, LiYing Cai, Saro H. Armenian
Data analysis and interpretation: Ellen K. Chang, Dayana Chanson, Jennifer Berano Teh, Stephen J. Forman, Ryotaro Nakamura, F. Lennie Wong, LiYing Cai, Saro H. Armenian
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Atrial Fibrillation in Patients Undergoing Allogeneic Hematopoietic Cell Transplantation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Stephen J. Forman
Stock and Other Ownership Interests: MustangBio, Lixte Biotechnology
Consulting or Advisory Role: Alimera Sciences, Lixte Biotechnology, MustangBio
Research Funding: MustangBio
Patents, Royalties, Other Intellectual Property: MustangBio
Ryotaro Nakamura
Consulting or Advisory Role: Viracor, Magenta Therapeutics, Kadmon, Napajen Pharma
Research Funding: Helocyte, Miyarisan pharmaceutical
Travel, Accommodations, Expenses: Kyowa Hakko Kirin, Alexion Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Copelan EA: Hematopoietic stem-cell transplantation. N Engl J Med 354:1813-1826, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Shapiro CL: Cancer Survivorship. N Engl J Med 379:2438-2450, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Miller KD Nogueira L Mariotto AB, et al. : Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 69:363-385, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Majhail NS Tao L Bredeson C, et al. : Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transpl 19:1498-1501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankar SM Carter A Sun CL, et al. : Health care utilization by adult long-term survivors of hematopoietic cell transplant: report from the Bone Marrow Transplant Survivor Study. Cancer Epidemiol Biomarkers Prev 16:834-839, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Bhatia S Robison LL Francisco L, et al. : Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood 105:4215-4222, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wingard JR Majhail NS Brazauskas R, et al. : Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 29:2230-2239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow EJ Mueller BA Baker KS, et al. : Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med 155:21-32, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Armenian SH Sun CL Vase T, et al. : Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood 120:4505-4512, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armenian SH, Bhatia S: Cardiovascular disease after hematopoietic cell transplantation--lessons learned. Haematologica 93:1132-1136, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Armenian SH, Chow EJ: Cardiovascular disease in survivors of hematopoietic cell transplantation. Cancer 120:469-479, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonorezos ES Stillwell EE Calloway JJ, et al. : Arrhythmias in the setting of hematopoietic cell transplants. Bone Marrow Transpl 50:1212-1216, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin EJ Virani SS Callaway CW, et al. : Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 137:e67-e492, 2018 [DOI] [PubMed] [Google Scholar]
- 14.January CT Wann LS Alpert JS, et al. : 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 64:e1-e76, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Brunner KJ Bunch TJ Mullin CM, et al. : Clinical predictors of risk for atrial fibrillation: implications for diagnosis and monitoring. Mayo Clin Proc 89:1498-1505, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Alonso A Krijthe BP Aspelund T, et al. : Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2:e000102, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnabel RB Sullivan LM Levy D, et al. : Development of a risk score for atrial fibrillation (Framingham Heart Study): A community-based cohort study. Lancet 373:739-745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chamberlain AM Agarwal SK Folsom AR, et al. : A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 107:85-91, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramkumar S Ochi A Kawakami H, et al. : Echocardiographic risk assessment to guide screening for atrial fibrillation. J Am Soc Echocardiogr 32:1259-1267, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Siontis KC, Geske JB, Gersh BJ: Atrial fibrillation pathophysiology and prognosis: insights from cardiovascular imaging. Circ Cardiovasc Imaging 8:e003020, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Sorror ML Storb RF Sandmaier BM, et al. : Comorbidity-age index: A clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol 32:3249-3256, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacigalupo A Ballen K Rizzo D, et al. : Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl 15:1628-1633, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Przepiorka D Weisdorf D Martin P, et al. : 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 15:825-828, 1995 [PubMed] [Google Scholar]
- 24.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 25.Armenian SH Mertens L Slorach C, et al. : Prevalence of anthracycline-related cardiac dysfunction in long-term survivors of adult-onset lymphoma. Cancer 124:850-857, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armenian SH Rinderknecht D Au K, et al. : Accuracy of a novel handheld wireless platform for detection of cardiac dysfunction in anthracycline-exposed survivors of childhood cancer. Clin Cancer Res 24:3119-3125, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Armenian SH Gelehrter SK Vase T, et al. : Screening for cardiac dysfunction in anthracycline-exposed childhood cancer survivors. Clin Cancer Res 20:6314-6323, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staerk L Sherer JA Ko D, et al. : Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 120:1501-1517, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Middeldorp ME Ariyaratnam J Lau D, et al. : Lifestyle modifications for treatment of atrial fibrillation. Heart 106:325-332, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Mandyam MC Soliman EZ Alonso A, et al. : The QT interval and risk of incident atrial fibrillation. Heart Rhythm 10:1562-1568, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang N Gong M Tse G, et al. : Prolonged corrected QT interval in predicting atrial fibrillation: A systematic review and meta-analysis. Pacing Clin Electrophysiol 41:321-327, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo JD Krone R Rich MW, et al. : Supraventricular tachyarrhythmias after hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Bone Marrow Transpl 34:615-619, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Steuter JA Villanueva ML Loberiza FR, et al. : Factors affecting the development of atrial fibrillation and atrial flutter (AF/AFL) following autologous hematopoietic SCT (auto-HSCT). Bone Marrow Transpl 48:963-965, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Singla A Hogan WJ Ansell SM, et al. : Incidence of supraventricular arrhythmias during autologous peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 19:1233-1237, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathur P Paydak H Thanendrarajan S, et al. : Atrial fibrillation in hematologic malignancies, especially after autologous hematopoietic stem cell transplantation: Review of risk factors, current management, and future directions. Clin Lymphoma Myeloma Leuk 16:70-75, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Farmakis D, Parissis J, Filippatos G: Insights into onco-cardiology: Atrial fibrillation in cancer. J Am Coll Cardiol 63:945-953, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Chiengthong K Lertjitbanjong P Thongprayoon C, et al. : Arrhythmias in hematopoietic stem cell transplantation: A systematic review and meta-analysis. Eur J Haematol 103:564-572, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Fatema K Gertz MA Barnes ME, et al. : Acute weight gain and diastolic dysfunction as a potent risk complex for post stem cell transplant atrial fibrillation. Am J Hematol 84:499-503, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Beatty P Anasetti C Hansen J, et al. : Marrow transplantation from unrelated donors for treatment of hematologic malignancies: effect of mismatching for one HLA locus. Blood 81:249-253, 1993 [PubMed] [Google Scholar]
- 40.Sasazuki T Juji T Morishima Y, et al. : Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med 339:1177-1185, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Petersdorf EW Gooley TA Anasetti C, et al. : Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood 92:3515-3520, 1998 [PubMed] [Google Scholar]
- 42.Herrmann J: Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol 17:474-502, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benjamin EJ D'Agostino RB Belanger AJ, et al. : Left atrial size and the risk of stroke and death: The Framingham heart study. Circulation 92:835-841, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Gottdiener JS Kitzman DW Aurigemma GP, et al. : Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons ≥65 years of age (The ardiovascular Health Study). Am J Cardiol 97:83-89, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Takemoto Y Barnes ME Seward JB, et al. : Usefulness of left atrial volume in predicting first congestive heart failure in patients ≥65 years of age with well-preserved left ventricular systolic function. Am J Cardiol 96:832-836, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Hoit BD: Left atrial size and function: role in prognosis. J Am Coll Cardiol 63:493-505, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Saha SK Anderson PL Caracciolo G, et al. : Global left atrial strain correlates with CHADS2 risk score in patients with atrial fibrillation. J Am Soc Echocardiogr 24:506-512, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Cameli M Mandoli GE Loiacono F, et al. : Left atrial strain: A useful index in atrial fibrillation. Int J Cardiol 220:208-213, 2016 [DOI] [PubMed] [Google Scholar]
- 49.Donal E Lip GYH Galderisi M, et al. : EACVI/EHRA expert consensus document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J 17:355-383, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Christophersen IE Yin X Larson MG, et al. : A comparison of the CHARGE-AF and the CHA2DS2-VASc risk scores for prediction of atrial fibrillation in the Framingham Heart Study. Am Heart J 178:45-54, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armenian SH Sun CL Francisco L, et al. : Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol 26:5537-5543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armenian SH Sun CL Shannon T, et al. : Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood 118:6023-6029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Passweg JR Baldomero H Basak GW, et al. : The EBMT activity survey report 2017: a focus on allogeneic HCT for nonmalignant indications and on the use of non-HCT cell therapies. Bone Marrow Transpl 54:1575-1585, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Souza A Fretham C Lee SJ, et al. : Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transpl 26:e177-e182, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]