PURPOSE
Programmed death 1 (PD-1) pathway inhibitors have not been prospectively evaluated in patients with non–clear cell renal cell carcinoma (nccRCC). The phase II KEYNOTE-427 study (cohort B) was conducted to assess the efficacy and safety of single-agent pembrolizumab, a PD-1 inhibitor, in advanced nccRCC.
METHODS
Patients with histologically confirmed, measurable (Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1) nccRCC and no prior systemic therapy received pembrolizumab 200 mg intravenously once every 3 weeks for ≤ 24 months. The primary end point was objective response rate (ORR) per RECIST v1.1.
RESULTS
Among enrolled patients (N = 165), 71.5% had confirmed papillary, 12.7% had chromophobe, and 15.8% had unclassified RCC histology. Most patients (67.9%) had intermediate or poor International Metastatic RCC Database Consortium risk status and tumors with programmed death ligand 1 (PD-L1) combined positive score (CPS) ≥ 1 (61.8%). The median time from enrollment to database cutoff was 31.5 months (range, 22.7-38.8). In all patients, the ORR was 26.7%. The median duration of response was 29.0 months; 59.7% of responses lasted ≥ 12 months. The ORR by CPS ≥ 1 and CPS < 1 status was 35.3% and 12.1%, respectively. The ORR by histology was 28.8% for papillary, 9.5% for chromophobe, and 30.8% for unclassified. Overall, the median progression-free survival was 4.2 months (95% CI, 2.9 to 5.6); the 24-month rate was 18.6%. The median overall survival was 28.9 months (95% CI, 24.3 months to not reached); the 24-month rate was 58.4%. Overall, 69.7% of patients reported treatment-related adverse events, most commonly pruritus (20.0%) and hypothyroidism (14.5%). Two deaths were treatment related (pneumonitis and cardiac arrest).
CONCLUSION
First-line pembrolizumab monotherapy showed promising antitumor activity in nccRCC. The safety profile was similar to that observed in other tumor types.
INTRODUCTION
Worldwide, it is estimated that more than 400,000 people will be diagnosed with kidney cancer in 2020.1 Because the most common type of kidney cancer is renal cell carcinoma (RCC) and approximately 70% of patients with RCC have clear cell histology (ccRCC), most approved therapies were developed in the ccRCC population.2 The remaining cases of RCC, broadly defined as non–clear cell renal cell carcinoma (nccRCC), compose a heterogeneous group of tumors that originate from the kidney and lack effective therapies.3 Most clinical trials in patients with nccRCC have been conducted to explore antivascular endothelial growth factor (VEGF) therapies in predominantly papillary RCC populations, and objective response rates (ORR) were low (< 15%).2,3 The data for mammalian target of rapamycin (mTOR) inhibitors suggest even lower overall efficacy in patients with nccRCC.3 Because of the limited positive clinical trial data for antiangiogenic and mTOR-targeted agents in patients with nccRCC, the National Comprehensive Cancer Network (NCCN) treatment guidelines recommend participation in a clinical trial as a preferred strategy for patients with nccRCC.2
CONTEXT
Key Objective
To determine if it is possible to treat advanced or metastatic non–clear cell renal cell carcinoma (nccRCC) with pembrolizumab monotherapy in the first-line setting.
Knowledge Generated
Pembrolizumab monotherapy demonstrated promising antitumor activity and survival in patients treated in the first-line setting of advanced or metastatic nccRCC. Pembrolizumab monotherapy demonstrated an objective response rate of 26.7% in the overall nccRCC population, which was consistent across key subgroups including International Metastatic RCC Database risk groups, patients with varying histologic subtypes, and patients with tumors with high-programmed death ligand 1 status. The median progression-free survival was 4.2 months, and the median overall survival was 28.9 months.
Relevance
Pembrolizumab monotherapy may be a potential treatment option for nccRCC.
Cytokine-based immunotherapies such as interleukin 2 and interferon α were beneficial in only a small group of patients with RCC and showed virtually no activity in patients with nccRCC.4-7 As understanding of the role of immune evasion in RCC has improved, more recent treatment approaches for patients with advanced RCC have used immune checkpoint inhibitors that target the programmed death 1 (PD-1) and the cytotoxic T-lymphocyte–associated antigen (CTLA-4) pathways. Therefore, it was likely that there was therapeutic potential in inhibiting the PD-1 pathway in patients with nccRCC. The phase II KEYNOTE-427 study (ClinicalTrials.gov identifier: NCT02853344) was conducted to evaluate the efficacy and safety of the PD-1 inhibitor pembrolizumab as monotherapy for first-line treatment of patients with advanced ccRCC (cohort A) and patients with advanced nccRCC (cohort B). Analysis of cohort A showed that pembrolizumab monotherapy has considerable antitumor activity in previously untreated patients with ccRCC.8 Herein, we present the results of pembrolizumab monotherapy in previously untreated patients with nccRCC (cohort B).
METHODS
Study Design and Objectives
KEYNOTE-427 (ClinicalTrials.gov identifier: NCT02853344) was an international, single-arm, open-label, multicohort, multicenter phase II trial performed at 61 sites in 10 countries. Patients enrolled in the study were administered intravenous pembrolizumab 200 mg once every 3 weeks until disease progression, unacceptable toxicity, or a total treatment duration of 24 months (maximum of 35 doses). The primary objective was to estimate ORR per RECIST, version 1.1 (RECIST v1.1) as assessed by blinded independent central review (BICR) in patients with nccRCC.
The Protocol and its amendments were approved by the appropriate institutional review board or independent ethics committee at each site. The trial was conducted per Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent.
Patient Characteristics
Eligible patients were ≥ 18 years with newly diagnosed or recurrent stage IV nccRCC as determined by the investigator and measurable disease per RECIST v1.1. Diagnosis of nccRCC was retrospectively confirmed by central pathology review. Subtype histology of nccRCC was determined by central pathology review. Patients must not have received prior systemic therapy for metastatic disease and must have maintained a Karnofsky performance status score ≥ 70 within 10 days before initiating treatment. Prior neoadjuvant and/or adjuvant therapy for RCC was allowed if it was completed more than 12 months before allocation and if it did not include a PD-1 pathway blocker. Exclusion criteria are provided in the Data Supplement (online only).
Study Assessments
The primary end point was ORR, per RECIST v1.1 as assessed by BICR. Secondary end points were duration of response (DOR), disease control rate (DCR; defined as the sum of complete responders, partial responders, and patients with stable disease lasting ≥ 6 months), progression-free survival (PFS; defined as the time from first day of study treatment to first documented disease progression per RECIST v1.1 or death, whichever occurred first), overall survival (OS; defined as the time from first day of study treatment to time of death) per RECIST v1.1 by BICR, and safety and tolerability. Exploratory end points were ORR, DOR, and DCR in relation to (1) histology, (2) International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk status, (3) programmed death ligand 1 (PD-L1) combined positive score (CPS), and (4) sarcomatoid differentiation.
Tumor imaging was performed using computed tomography or magnetic resonance imaging of the chest, abdomen, or pelvis. Imaging assessments were performed at week 12, then every 6 weeks until week 54, and every 12 weeks thereafter. Baseline bone imaging was necessary for confirmation of complete response (CR) and was performed at screening and at weeks 18, 30, 42, and 54, and then every 24 weeks thereafter. Response was assessed according to RECIST v1.1 based on BICR.
Assessment of PD-L1 expression and sarcomatoid differentiation is described in the Data Supplement.
Safety was monitored throughout the study and for 30 days after the last dose of pembrolizumab and graded per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4. Any adverse events (AEs) associated with pembrolizumab exposure with immunologic etiology were recorded as immune-mediated AEs. Patients were monitored for any serious AEs and immune-mediated AEs for up to 90 days after study completion.
Statistical Analyses
ORR was calculated as the proportion of patients in the analysis population who experienced CR or partial response (PR). The 95% CIs were calculated using the Clopper-Pearson method based on binomial distribution. The Kaplan-Meier method for censored data was used to estimate OS, PFS, and DOR from the date of the first exposure to pembrolizumab to the database cutoff date. The DOR analysis population included all responders. No statistical adjustments were performed for multiple comparisons.
RESULTS
Patients
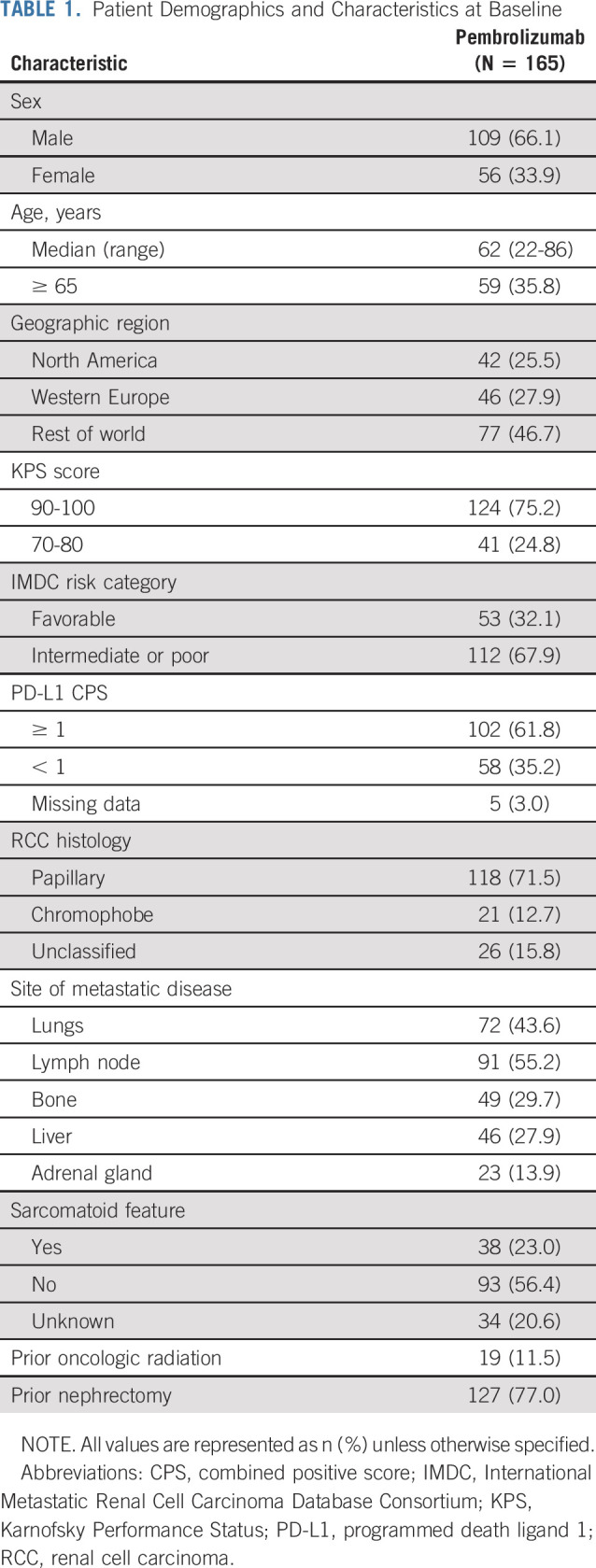
In total, 165 patients were enrolled across nine countries in cohort B. The median time from enrollment to database cutoff was 31.5 months (range, 22.7-38.8). The median age was 62 years (range, 22-86), and 66.1% of patients were male (Table 1). The median duration of therapy was 6.9 months (range, 0.03-29.2). The PD-L1 expression status was CPS ≥ 1 in 61.8% (n = 102) of patients. Fifty-three patients (32.1%) and 112 patients (67.9%) were classified into favorable and intermediate or poor IMDC risk categories, respectively.
TABLE 1.
Patient Demographics and Characteristics at Baseline
At data cutoff on February 24, 2020, treatment was ongoing in two patients (1.2%), had been discontinued in 139 patients (84.2%), and was completed in 24 patients (14.5%). Most patients (66.1%) discontinued treatment because of progressive disease (57.0%) or clinical progression (9.1%). Treatment was discontinued because of an AE in 25 patients (15.2%); 16 patients (9.7%) discontinued treatment because of a treatment-related AE (Data Supplement Figure S1, online only). Other reasons for discontinuation were patient withdrawal (n = 3) and treatment with other anticancer therapy (n = 1).
Efficacy Outcomes in the Total Population
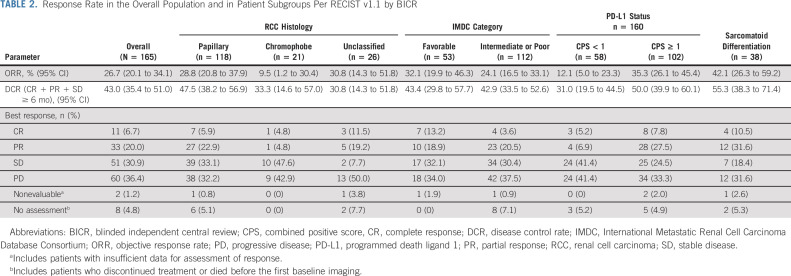
The ORR was 26.7% (95% CI, 20.1 to 34.1) in the total population: 11 patients (6.7%) achieved CR and 33 patients (20.0%) achieved PR; the DCR was 43.0% (Table 2). Ninety-one patients (55.2%) had a reduction in target lesions; 20 patients (12.1%) had reductions ≥ 80%, and seven patients (4.2%) had 100% target lesion reduction (Fig 1A).
TABLE 2.
Response Rate in the Overall Population and in Patient Subgroups Per RECIST v1.1 by BICR
FIG 1.
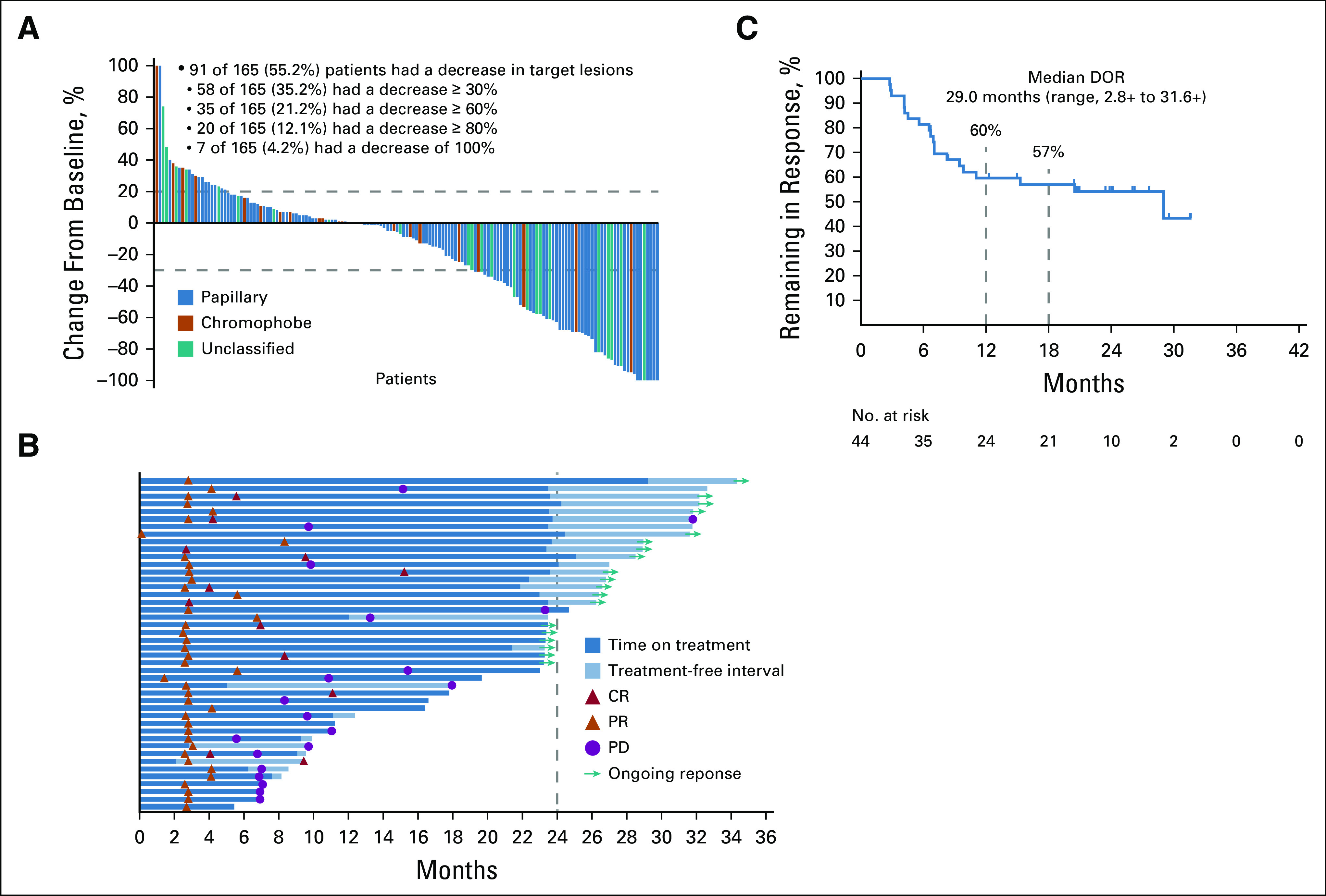
Maximum change from baseline in target lesions (A),a time to response and response duration (B), and Kaplan-Meier estimate of DOR (C) based on blinded independent central review. aMaximum change from baseline in target lesions by central review was assessed for patients who received ≥ 1 dose of pembrolizumab, had baseline imaging with measurable disease per RECIST v1.1, and had a postbaseline assessment (n = 155). +, ongoing response; CR, complete response; DOR, duration of response; PD, progressive disease; PR, partial response.
The median time to response was 2.8 months (range, 0.1-8.3), and the median DOR was 29.0 months (range, 2.8-31.6 +). By Kaplan-Meier estimate, the percentage of responders with response durations ≥ 12 months and ≥ 18 months was 59.7% and 57.0%, respectively (Fig 1B). Of the 44 patients who experienced a CR or PR, 19 had an ongoing response at data cutoff (Fig 1B) and 20 had experienced subsequent progressive disease per BICR (Fig 1C). For the other five responders, two discontinued treatment because of an AE (acute coronary syndrome and hemorrhagic stroke), two because of radiographic progression, and one because of clinical progression.
The median PFS for the entire nccRCC group was 4.2 months (95% CI, 2.9 to 5.6) (Fig 2A); 12- and 24-month PFS rates were 24.7% and 18.6%, respectively. The 12- and 24-month OS rates were 73.2% and 58.4%, respectively; the median OS was 28.9 months (95% CI, 24.3 months to not reached; Fig 2B).
FIG 2.
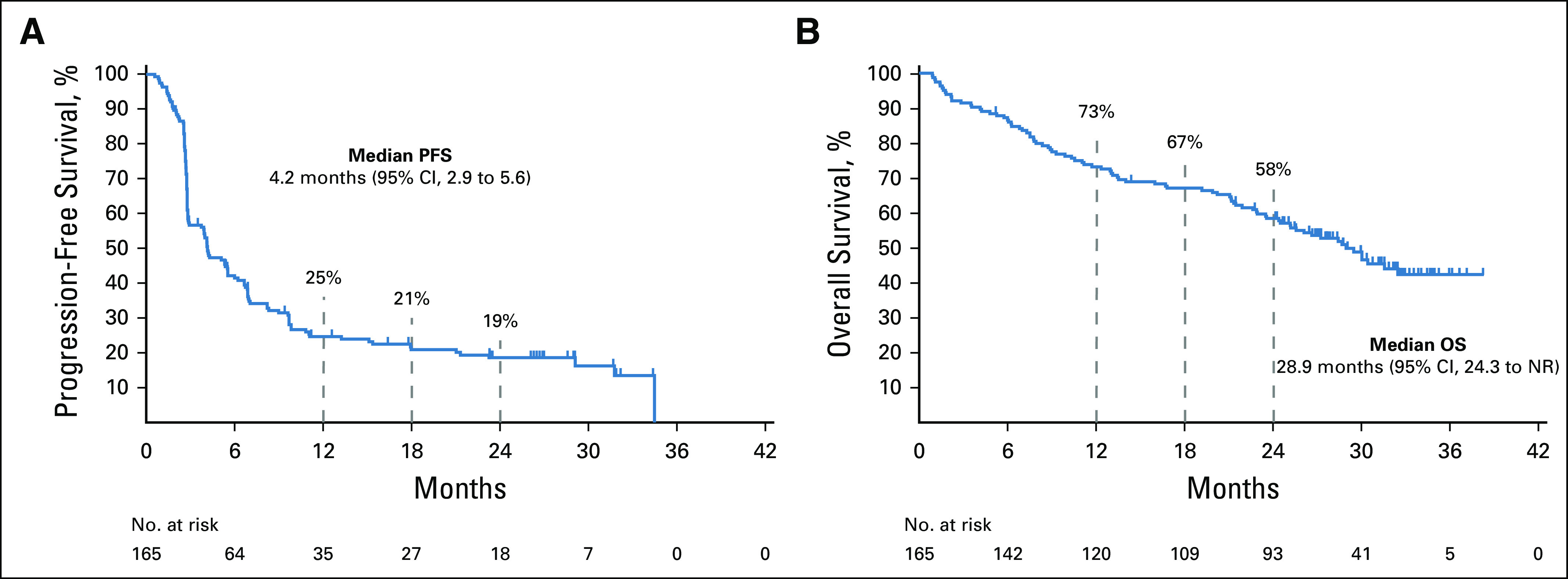
Kaplan-Meier curves for (A) progression-free survival and (B) overall survival. NR, not reached; OS, overall survival; PFS, progression-free survival.
Efficacy Outcomes by PD-L1 Expression
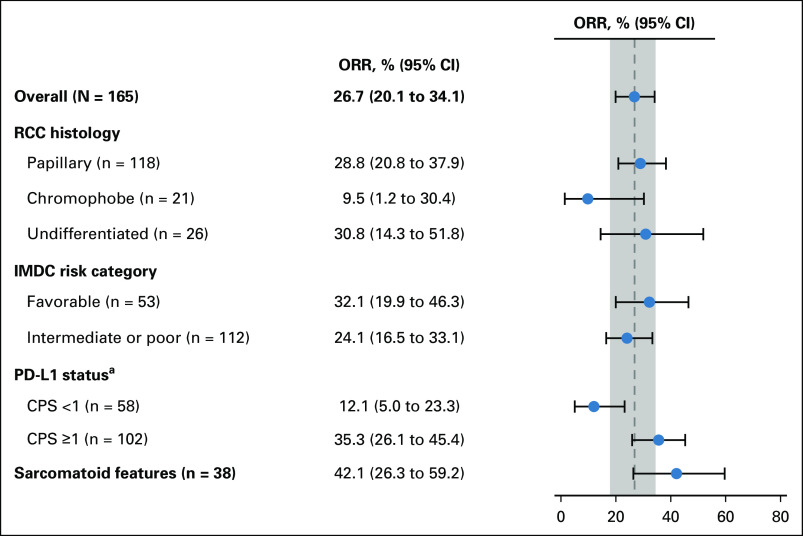
For patients with CPS ≥ 1 (n = 102), the confirmed ORR was 35.3% (95% CI, 26.1 to 45.4) (Fig 3; Table 2). The DCR was 50.0% (95% CI, 39.9 to 60.1). The median DOR was 29.0 months (range, 2.8 + to 31.6 +), the median PFS was 5.6 months (95% CI, 2.9 to 8.3), and the median OS was 30.0 months (95% CI, 22.9 to not reached) (Data Supplement Table S1, online only). For patients with CPS < 1 (n = 58), the confirmed ORR was 12.1% (95% CI, 5.0 to 23.3) (Fig 3; Table 2). The DCR was 31.0% (95% CI, 19.5 to 44.5). The median DOR was 9.5 months (range, 2.8 to 26.0 +), the median PFS was 3.7 months (95% CI, 2.8 to 4.2), and the median OS was 26.6 months (95% CI, 19.2 months to not reached) (Data Supplement Table S1).
FIG 3.
ORR by patient subgroup. aFive patients had missing PD-L1 status. CPS, combined positive score; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; ORR, objective response rate; PD-L1, programmed death ligand 1; RCC, renal cell carcinoma.
Efficacy Outcomes by Histology
The confirmed ORRs for patients with papillary, chromophobe, and unclassified histology were 28.8% (95% CI, 20.8% to 37.9%), 9.5% (95% CI, 1.2% to 30.4%), and 30.8% (95% CI, 14.3% to 51.8%), respectively (Fig 3;Table 2). The median DOR ranged from 29.0 months to not reached (Data Supplement Table S1). For patients with papillary histology, the DCR was 47.5% (95% CI, 38.2% to 56.9%), the median PFS was 5.5 months (95% CI, 3.9 to 6.9), and the median OS was 31.5 (95% CI, 25.5 to not reached) (Table 2; Data Supplement Table S1). For patients with chromophobe histology, the DCR was 33.3% (95% CI, 14.6% to 57.0%), the median PFS was 3.9 months (95% CI, 2.6 to 6.9), and the median OS was 23.5 months (95% CI, 9.3 to not reached) (Table 2, Data Supplement Table S1). For patients with unclassified histology, the DCR was 30.8% (95% CI, 14.3% to 51.8), the median PFS was 2.8 months (95% CI, 2.8 to 5.1), and the median OS was 17.6 months (95% CI, 7.5 to not reached) (Data Supplement Table S1).
Efficacy by Sarcomatoid Differentiation
Among patients with sarcomatoid differentiation (n = 38), the confirmed ORR was 42.1% (95% CI, 26.3% to 59.2%) (Fig 3; Table 2). The DCR was 55.3% (95% CI, 38.3% to 71.4%) (Table 2). The median DOR was 15.3 months (range, 2.8 + to 29.5 +), the median PFS was 6.9 months (95% CI, 2.8 to 15.4), and the median OS was 25.5 months (95% CI, 13.1 to 30.0) (Data Supplement Table S1).
Efficacy Outcomes by IMDC Risk Category
For patients with favorable IMDC risk (n = 53), the confirmed ORR was 32.1% (95% CI, 19.9% to 46.3%) (Fig 3; Table 2). The DCR was 43.4% (95% CI, 29.8% to 57.7%). The median DOR was 11.0 months (range, 2.8 to 27.7 +), the median PFS was 5.3 months (95% CI, 2.9 to 8.2), and the median OS was not reached (95% CI, 30.4 to not reached) (Data Supplement Table S1).
In the intermediate or poor IMDC risk subgroup (n = 112), the confirmed ORR was 24.1% (95% CI, 16.5% to 33.1%) (Fig 3; Table 2). The DCR was 42.9% (95% CI, 33.5% to 52.6%). The median DOR was 29.0 months (range, 2.8 to 31.6 +), the median PFS was 4.0 months (95% CI, 2.8 to 6.2), and the median OS was 24.5 months (95% CI, 16.7 to 30.0) (Data Supplement Table S1).
Safety
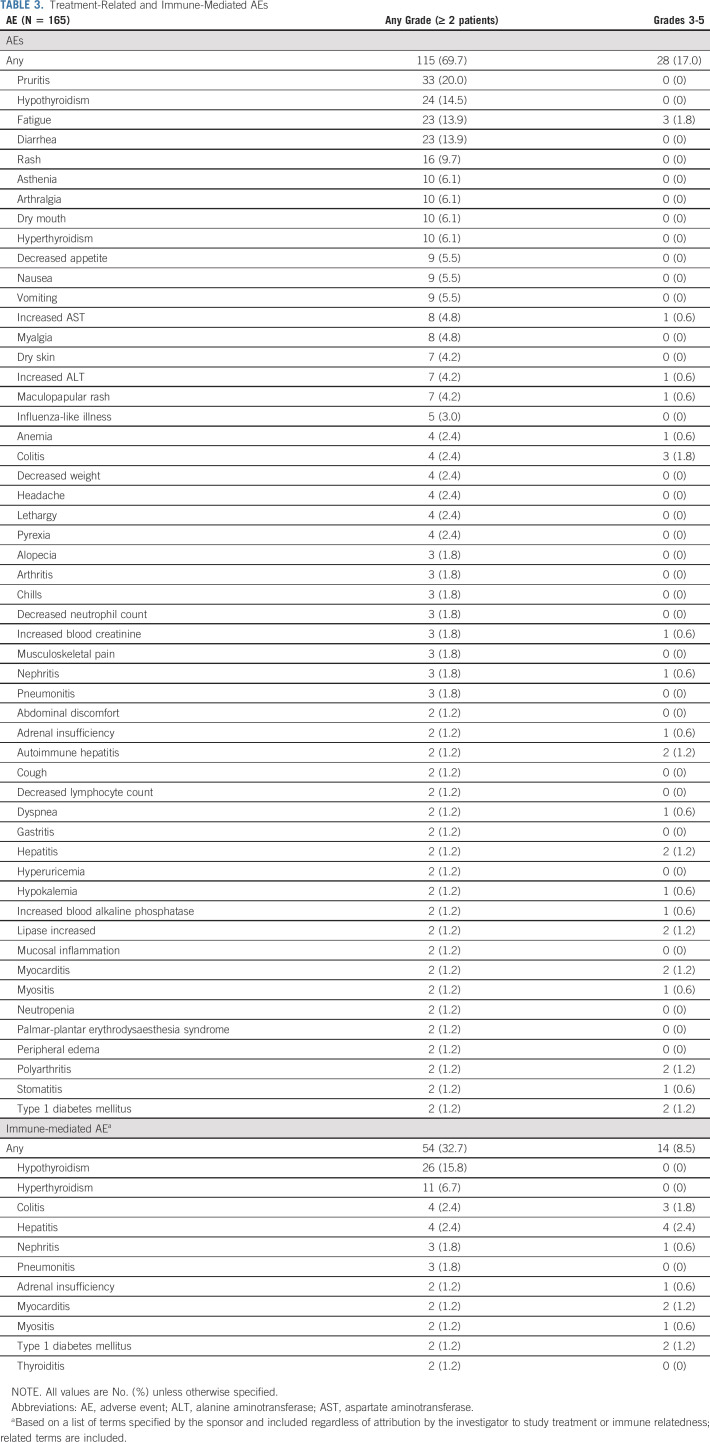
A total of 69.7% of patients experienced treatment-related AEs of any grade; 17% experienced treatment-related AEs of grade 3-5 (Table 3). The most commonly reported treatment-related AEs of any grade were pruritus (20.0%), hypothyroidism (14.5%), fatigue (13.9%), and diarrhea (13.9%). Colitis (1.8%) and fatigue (1.8%) was the most commonly reported grade 3-5 treatment-related AE. Discontinuation because of a treatment-related AE was reported for 16 patients (9.7%) (Data Supplement Table S2, online only). Eight patients died of AEs, two of which were considered related to treatment (pneumonia and cardiac arrest); six deaths (pneumonia, ischemic stroke, respiratory failure, bleeding from esophageal varices left ventricular failure, and multiple organ dysfunction syndrome) were not considered related to treatment. Immune-mediated AEs were reported in 32.7% of patients (grade 1 or 2, 40 of 54 patients) (Table 3). The most commonly reported immune-mediated AEs were hypothyroidism (15.8%), hyperthyroidism (6.7%), colitis (2.4%), and hepatitis (2.4%). Hepatitis (2.4%) was the most commonly reported grade 3-5 immune-mediated AE.
TABLE 3.
Treatment-Related and Immune-Mediated AEs
Systemic corticosteroids were used for the management of 71 immune-related AE episodes. Eighteen episodes (25.4%) were managed with a high starting dose of corticosteroids (≥ 40 mg/d prednisone or equivalent), and four (5.6%) were managed with a low starting dose of corticosteroids (< 40 mg/d prednisone or equivalent). The remaining 49 episodes (69.0%) did not necessitate treatment with corticosteroids.
DISCUSSION
The single-arm, phase II KEYNOTE-427 study is the first and largest interventional clinical study conducted in a cohort of patients with previously untreated advanced nccRCC. First-line pembrolizumab monotherapy showed promising antitumor activity (ORR = 26.7%) in the overall nccRCC population, consistent results across IMDC risk groups, and promising activity in selected patient subgroups with tumors with high PD-L1 expression, papillary or unclassified histology, and sarcomatoid differentiation. The median DOR was 29.0 months, with most patients achieving a response for ≥ 12 months. The median PFS was 4.2 months, the median OS was 28.9 months, and the 24-month OS rate was 58.4%. These findings are consistent with those of other studies of PD-1/PD-L1 inhibitors, both as monotherapy and in combination treatment.9-12
The results of the current study show that pembrolizumab monotherapy provides durable antitumor activity in untreated patients with nccRCC. Furthermore, the confirmation of an nccRCC diagnosis by central pathology in KEYNOTE-427 provides confidence in our results, given the histologic diversity of these cancers.
Because the focus of RCC clinical trials of targeted systemic therapies has predominantly been on patients with ccRCC, the NCCN kidney cancer guidelines indicate that enrollment in clinical trials is the preferred strategy for patients with nccRCC.2 Current standard-of-care systemic treatment options recommended by NCCN guidelines for patients with nccRCC include sunitinib, everolimus, and cabozantinib.2 The phase II ASPEN and ESPN trials were conducted to assess efficacy and safety of sunitinib versus everolimus in patients with nccRCC, and recommendations were primarily based on the results of the primary end point of PFS.13,14 Notably, nccRCC histology in ASPEN and ESPN was not confirmed by central pathology review. Tumor response was evaluated as a secondary end point in both trials. In the ASPEN trial, the ORR was 18% (PR, n = 9) in the sunitinib arm and 9% (CR, n = 2; PR, n = 4) in the everolimus arm.13 In the ESPN trial, the ORR was 9% (PR, n = 3) with first-line sunitinib and 3% (PR, n = 1) with first-line everolimus.14 Despite guideline recommendations for these regimens, the results of these approaches demonstrate that more effective treatment options for nccRCC are needed.
The safety profile of pembrolizumab monotherapy in this study is generally consistent with what has been observed in other tumor types.15 Overall, 69.7% of patients reported treatment-related AEs of any grade and 17% reported treatment-related AEs of grade 3-5. The most reported immune-mediated AEs were hypothyroidism, hyperthyroidism, colitis, and hepatitis, consistent with a recent systematic review and meta-analysis of PD-1/PD-L1 inhibitors.16 Two of the eight deaths that occurred in the study were considered related to treatment (pneumonitis and cardiac arrest). Although rare, serious and potentially fatal cases of pneumonitis and cardiotoxicity can occur with the use of immune checkpoint inhibitors such as pembrolizumab.17,18
The antitumor activity of pembrolizumab monotherapy was also demonstrated across key patient subgroups. ORR in the overall nccRCC population was similar to ORRs in the favorable and intermediate or poor IMDC risk groups, suggesting that antitumor activity was generally consistent across IMDC risk categories. When evaluated by RCC histology, ORRs were higher for patients with papillary and unclassified RCC than for patients with chromophobe RCC. This result is similar to that reported in a retrospective analysis of nivolumab in nccRCC, in which the highest response rate was observed in patients with unclassified histology, followed by papillary and chromophobe RCC.10 Although there were few patients in this study with chromophobe RCC (n = 21) to draw meaningful conclusions, it is unclear why these patients seem to have a poorer response with anti-PD-1 therapies because this histologic subtype is traditionally associated with better survival than other RCC subtypes.19 Given the rarity of chromophobe histology, the majority of studies are retrospective with substantial heterogeneity; therefore, there is no consensus on the optimal therapy for patients with chromophobe histology.20 The effectiveness of pembrolizumab might also be influenced by PD-L1 status. Despite differing methodologies (eg, choice of antibody) and positivity thresholds (eg, ≥ 5% positivity), PD-L1 expression has been reported in 11%-20% of samples from patients with nccRCC.21-25 Following the same methodology as used in this study, PD-L1 expression, defined as CPS ≥ 1, in the KEYNOTE-426 and KEYNOTE-427 cohort A studies was reported in 47% and 60% of patients with ccRCC, respectively.8,26 Although responses in this study were observed in patients with CPS ≥ 1 and those with CPS < 1, the ORR was three times higher in patients with CPS ≥ 1 (35.3%) than in those with CPS < 1 (12.1%). The results of this study also showed relatively high response rates in patients with sarcomatoid differentiation (ORR, 42.1%). Recently reported results of ongoing studies suggest that sarcomatoid differentiation and DNA and RNA analyses of the tumor microenvironment may play a role in predicting response to PD-1 or PD-L1 inhibitors.11,27,28 Data from the current study suggest that sarcomatoid differentiation may be a histologic biomarker for response to checkpoint inhibitor therapy in patients with nccRCC, as was observed in patients with ccRCC.
The current study has several limitations. First, the single-arm study design limits comparisons of response rate and survival outcomes with currently recommended regimens. Second, the heterogeneity of the nccRCC patient population makes subgroup analyses difficult to identify which patients achieve the greatest benefit. Furthermore, despite central pathology review, we were unable to subgroup patients into papillary type I and type II.
In conclusion, pembrolizumab monotherapy showed promising antitumor activity and survival for patients with nccRCC. The safety and tolerability of pembrolizumab monotherapy are consistent with those reported in previous studies. Given the lack of established therapy for nccRCC and favorable ORR relative to VEGF and mTOR therapies, pembrolizumab monotherapy may be a potential treatment option for nccRCC. Additional studies to evaluate antitumor activity of immune checkpoint blockade coupled with studies to validate tissue-based biomarkers of response will better elucidate the role of pembrolizumab treatment in nccRCC.
ACKNOWLEDGMENT
The authors thank the patients and their families and all investigators and site personnel. The authors also thank Scot Ebbinghaus, Karla Rodriguez-Lopez, Trevor Newhook, Jialin Xu, Sabrina Wan, Susmita Gupta, Kate Fogarty, Yuying Hwang and Xiaoqi Du (employees of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ), and Sabina Signoretti (Brigham and Women's Hospital, Boston, MA) for their contributions to the study. Medical writing and/or editorial assistance was provided by Robert Steger, PhD, and Matthew Grzywacz, PhD, of ApotheCom (Yardley, PA). This assistance was funded by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ.
PRIOR PRESENTATION
Presented in part at the ASCO20 Virtual Meeting, May 29-31, 2020; presented at the 2019 ASCO Genitourinary Cancers Symposium, San Francisco, CA, February 14-16, 2019; presented at the 2019 ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2019; presented at the 2019 ESMO Annual Meeting, Barcelona, Spain, September 27-October 1, 2019.
SUPPORT
Supported by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ. This work was also supported by a grant from the National Cancer Institute, USA Kidney SPORE (5P50CA101942).
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The data sharing policy for Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the Engagezone site or via email to dataaccess@merck.com.
AUTHOR CONTRIBUTIONS
Conception and design: David F. McDermott, Rodolfo F. Perini, Charles Schloss, Michael B. Atkins
Provision of study materials or patients: Georg A. Bjarnason, Daniel Castellano, Michael B. Atkins
Collection and assembly of data: Jae-Lyun Lee, Marek Ziobro, Cristina Suarez, Przemyslaw Langiewicz, Vsevolod Borisovich Matveev, Pawel Wiechno, Rustem Airatovich Gafanov, Piotr Tomczak, Frederic Pouliot, Frede Donskov, Boris Yakovlevich Alekseev, Sang Joon Shin, Georg A. Bjarnason, Charles Schloss
Data analysis and interpretation: Jae-Lyun Lee, Marek Ziobro, Przemyslaw Langiewicz, Pawel Wiechno, Rustem Airatovich Gafanov, Frederic Pouliot, Frede Donskov, Boris Yakovlevich Alekseev, Sang Joon Shin, Georg A. Bjarnason, Daniel Castellano, Rachel Kloss Silverman, Rodolfo F. Perini, Charles Schloss, Michael B. Atkins
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non–Clear Cell Renal Cell Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David F. McDermott
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Genentech/Roche, Pfizer, Exelixis, Novartis, Array BioPharma, Peloton Therapeutics, EMD Serono, Jounce Therapeutics, Alkermes, Lilly, Eisai, Calithera Biosciences, Iovance Biotherapeutics
Research Funding: Prometheus, Bristol-Myers Squibb, Merck, Genentech, Novartis, Alkermes, Peloton Therapeutics
Other Relationship: Beth Israel Deaconess Medical Center
(OPTIONAL) Uncompensated Relationships: X4 Pharmaceuticals, AVEO
Jae-Lyun Lee
Stock and Other Ownership Interests: Myovant Sciences
Honoraria: Bristol-Myers Squibb, Astellas Pharma, Pfizer, AstraZeneca, MSD
Consulting or Advisory Role: Pfizer, Sanofi/Aventis, Bristol-Myers Squibb, Alteogen, GI Innovation, MSD, Merck, AstraZeneca
Research Funding: Pfizer, Janssen, Novartis, Bristol-Myers Squibb, Roche/Genentech, AstraZeneca/MedImmune, MSD, Bayer Schering Pharma, Seattle Genetics
Marek Ziobro
Consulting or Advisory Role: Bristol-Myers Squibb, MSD, Pierre Fabre, Pierre Fabre, Ipsen
Speakers' Bureau: Bristol-Myers Squibb, MSD, Novartis, Roche
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Roche, Novartis, Pierre Fabre
Cristina Suarez
Consulting or Advisory Role: Bristol-Myers Squibb, Ipsen, Sanofi, Pfizer, EUSA Pharma, Astellas Pharma, Novartis, Merck Sharp & Dohme
Speakers' Bureau: Bristol-Myers Squibb, Ipsen, PfizerRoche/Genentech, AstraZeneca, Merck Sharp & Dohme
Research Funding (Institution): Astellas Pharma, Roche/Genentech, Exelixis, AstraZeneca, Bristol-Myers Squibb, Pfizer, Novartis, Janssen Oncology, Calithera Biosciences, AB Science, Arog, AVEO, Bayer, SFJ Pharmaceuticals Group, Blueprint Medicines, Clovis Oncology, Boehringer Ingelheim, Cougar Biotechnology, Deciphera, GlaxoSmithKline, Incyte, Karyopharm Therapeutics, MedImmune, Nanobiotix, Millennium, Puma Biotechnology, Teva
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Roche, Ipsen
Vsevolod Borisovich Matveev
Honoraria: Ipsen, Bayer, Bristol-Myers Squibb, AstraZeneca, Janssen, Astellas Pharma, MSD
Expert Testimony: Bristol-Myers Squibb, Bayer, MSD
Pawel Wiechno
Honoraria: Pfizer, Astellas Pharma, Bristol-Myers Squibb, Bayer, Janssen-Cilag, Merck
Consulting or Advisory Role: Merck, Pfizer, Bayer, Bristol-Myers Squibb, Astellas Pharma, Janssen-Cilag
Travel, Accommodations, Expenses: Pfizer, Astllas
Rustem Airatovich Gafanov
Honoraria: Janssen, Astellas Pharma, Bayer, Sanofi, MSD, Bristol-Myers Squibb, Pfizer
Consulting or Advisory Role: Janssen, Astellas Pharma, Bayer, Sanofi, MSD, Bristol-Myers Squibb, Pfizer
Speakers' Bureau: Janssen, Astellas Pharma, Bayer, Sanofi, MSD, Bristol-Myers Squibb, Pfizer
Frederic Pouliot
Stock and Other Ownership Interests: Allogene Therapeutics
Honoraria: Amgen, Astellas Pharma, Janssen, Bayer, Genzyme, Sanofi, Abbvie, Ferring
Consulting or Advisory Role: Amgen, Pfizer, Sanofi, Astellas Pharma, Progenics, Bayer, Janssen, Genzyme
Speakers' Bureau: Astellas Pharma, Bayer, Amgen, Sanofi, Janssen
Research Funding: Sanofi, Astellas Pharma, Tersera, Janssen, Bayer
Patents, Royalties, Other Intellectual Property: Transcriptional system and uses therof for single cell detection
Other Relationship: Merck, Progenics
Frede Donskov
Research Funding: Pfizer, Ipsen, MSD Oncology
Boris Yakovlevich Alekseev
Honoraria: Asrtrazeneca, Astellas Pharma, Ferring, Eisai, Janssen, Bayer, MSD, Merck, Pfizer, Roche, Sanofi, Bristol-Myers Squibb
Consulting or Advisory Role: AstraZeneca, Astellas Pharma, Bayer, Bristol-Myers Squibb, Ferring, Janssen, Merck, Sanofi, Pfizer, MSD, Roche, Eisai
Speakers' Bureau: Janssen, Sanofi, Ferring, Astellas Pharma, Pfizer, AstraZeneca, Bayer, Merck, Bristol-Myers Squibb, MSD, Eisai, Roche
Research Funding: AstraZeneca, Merck, Sanofi, Bayer, Astellas Pharma, Janssen, Bristol-Myers Squibb, Bavarian Nordic, Pfizer, ICON Clinical Research, Eisai, MSD, Roche
Travel, Accommodations, Expenses: AstraZeneca, Astellas Pharma, Bayer, Bristol-Myers Squibb, Janssen, MSD, Pfizer, Sanofi
Georg A. Bjarnason
Stock and Other Ownership Interests: Merck
Honoraria: Pfizer, Novartis, Bristol-Myers Squibb, Eisai, Ipsen
Consulting or Advisory Role: Pfizer, Novartis, Bristol-Myers Squibb, Eisai, Ipsen
Research Funding: Pfizer, Merck
Travel, Accommodations, Expenses: Pfizer, Novartis
Daniel Castellano
Consulting or Advisory Role: Janssen Oncology, Roche/Genentech, Astellas Pharma, AstraZeneca, Pfizer, Novartis, Ipsen, Bristol-Myers Squibb, MSD Oncology, Bayer, Lilly, Sanofi, Pierre Fabre, Boehringer Ingelheim
Research Funding: Janssen Oncology
Travel, Accommodations, Expenses: Pfizer, Roche, Bristol-Myers Squibb, AstraZeneca
Rachel Kloss Silverman
Employment: Merck
Stock and Other Ownership Interests: Merck
Travel, Accommodations, Expenses: Merck
Rodolfo F. Perini
Employment: Merck
Stock and Other Ownership Interests: Merck
Charles Schloss
Employment: Merck
Stock and Other Ownership Interests: Merck
Michael B. Atkins
Stock and Other Ownership Interests: Werewolf Pharma, Pyxis
Consulting or Advisory Role: Genentech, Novartis, Bristol-Myers Squibb, Merck, Exelixis, Eisai, Agenus, Arrowhead Pharmaceuticals, Werewolf Pharma, Surface Oncology, Iovance Biotherapeutics, Immunocore, Pyxis, Pneuma Respiratory, Leads Biolabs, Fathom Biotechnology, Aveo, Cota Healthcare, Neoleukin Therapeutics, Adagene, Idera, Ellipses Pharma, AstraZeneca, PACT Pharma, Third Rock Ventures
Research Funding: Bristol-Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.Du Z Chen W Xia Q, et al. : Trends and projections of kidney cancer incidence at the global and national levels, 1990-2030: A Bayesian age-period-cohort modeling study. Biomark Res 8:16, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology-Kidney Cancer. V.1.2021, National Comprehensive Cancer Network, 2020. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf [DOI] [PubMed] [Google Scholar]
- 3.Zhang T Gong J Maia MC, et al. : Systemic therapy for non-clear cell renal cell carcinoma. Am Soc Clin Oncol Educ Book 37:337-342, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ Bacik J Mariani T, et al. : Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol 20:2376-2381, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ Bacik J Murphy BA, et al. : Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 20:289-296, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Fyfe G Fisher RI Rosenberg SA, et al. : Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 13:688-696, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Upton MP Parker RA Youmans A, et al. : Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother 28:488-495, 2005 [DOI] [PubMed] [Google Scholar]
- 8.McDermott DF Lee J-L Bjarnason GA, et al. : First-line pembrolizumab (pembro) monotherapy in advanced clear cell renal cell carcinoma (ccRCC): Updated follow-up for KEYNOTE-427 cohort A. J Clin Oncol 2020 (suppl; abstr 5069) [Google Scholar]
- 9.De Giorgi U Carteni G Giannarelli D, et al. : Safety and efficacy of nivolumab for metastatic renal cell carcinoma: Real-world results from an expanded access programme. BJU Int 123:98-105, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Koshkin VS Barata PC Zhang T, et al. : Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer 6:9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGregor BA McKay RR Braun DA, et al. : Results of a multicenter phase II study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features. J Clin Oncol 38:63-70, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chahoud J Msaouel P Campbell MT, et al. : Nivolumab for the treatment of patients with metastatic non-clear cell renal cell carcinoma (nccRCC): A single-institutional experience and literature meta-analysis. Oncologist, 25:252-258, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong AJ Halabi S Eisen T, et al. : Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial. Lancet Oncol 17:378-388, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tannir NM Jonasch E Albiges L, et al. : Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): A randomized multicenter phase 2 trial. Eur Urol 69:866-874, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.KEYTRUDA® (pembrolizumab) for Injection, for Intravenous Use. Whitehouse Station, NJ, Merck Sharp & Dohme Corp, 2020 [Google Scholar]
- 16.Baxi S Yang A Gennarelli RL, et al. : Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ 360:k793, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui PF Ma JX Wang FX, et al. : Pneumonitis and pneumonitis-related death in cancer patients treated with programmed cell death-1 inhibitors: A systematic review and meta-analysis. Ther Clin Risk Manag 13:1259-1271, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varricchi G Galdiero MR Marone G, et al. : Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2:e000247, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricketts CJ De Cubas AA Fan H, et al. : The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep 23:313-326.e5, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papanikolaou D Ioannidou P Koukourikis P, et al. : Systemic therapy for chromophobe renal cell carcinoma: A systematic review. Urol Oncol 38:137-149, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Choueiri TK Fay AP Gray KP, et al. : PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol 25:2178-2184, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erlmeier F Hartmann A Autenrieth M, et al. : PD-1/PD-L1 expression in chromophobe renal cell carcinoma: An immunological exception? Med Oncol 33:120, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Motoshima T Komohara Y Ma C, et al. : PD-L1 expression in papillary renal cell carcinoma. BMC Urol 17:8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chipollini J Azizi M Peyton CC, et al. : Implications of programmed death ligand-1 positivity in non-clear cell renal cell carcinoma. J Kidney Cancer VHL 5:6-13, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z Peng S Xie H, et al. : Prognostic and clinicopathological significance of PD-L1 in patients with renal cell carcinoma: A meta-analysis based on 1863 individuals. Clin Exp Med 18:165-175, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Rini BI Plimack ER Stus V, et al. : Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116-1127, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Rini BI Motzer RJ Powles T, et al. : Atezolizumab (atezo) + bevacizumab (bev) versus sunitinib (sun) in pts with untreated metastatic renal cell carcinoma (mRCC) and sarcomatoid (sarc) histology: IMmotion151 subgroup analysis. J Clin Oncol 37, 2019. (suppl; abstr 4512) [Google Scholar]
- 28.Choueiri TK Albiges L Haanen JBAG, et al. : Biomarker analyses from JAVELIN Renal 101: Avelumab + axitinib (A+Ax) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). J Clin Oncol 37, 2019. (suppl; abstr 101) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy for Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the Engagezone site or via email to dataaccess@merck.com.