PURPOSE
Lenalidomide combined with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) (R2CHOP) in untreated diffuse large B-cell lymphoma (DLBCL) has shown promising activity, particularly in the activated B-cell–like (ABC) subtype. Eastern Cooperative Oncology Group (ECOG)-ACRIN trial E1412 was a randomized phase II study comparing R2CHOP versus R-CHOP in untreated DLBCL.
PATIENTS AND METHODS
Patients with newly diagnosed DLBCL, stage II bulky-IV disease, International Prognostic Index (IPI) ≥ 2, and ECOG performance status ≤ 2 were eligible and randomly assigned 1:1 to R2CHOP versus R-CHOP for six cycles. Tumors were analyzed using the NanoString Lymph2Cx for cell of origin. The primary end point was progression-free survival (PFS) in all patients with the co-primary end point of PFS in ABC-DLBCL. Secondary end points included overall response rate (ORR), complete response (CR) rate, and overall survival (OS).
RESULTS
Three hundred forty-nine patients were enrolled; 280 patients (145 R2CHOP and 135 R-CHOP) were evaluable: 94 were ABC-DLBCL, 122 germinal center B-cell–like-DLBCL, 18 unclassifiable, and 46 unknowns. Baseline characteristics were well-balanced between arms, and the median age was 66 (range, 24-92); 70% of patients had stage IV disease; 34%, 43%, and 24% had IPI 2, 3, and 4 or 5, respectively. Myelosuppression was more common in the R2CHOP arm. The ORR and CR rate were 92% and 68% in R-CHOP and 97% (P = .06) and 73% (P = .43) in the R2CHOP arm, respectively. The median follow-up was 3.0 years; R2CHOP was associated with a 34% reduction in risk of progression or death versus R-CHOP (hazard ratio [HR], 0.66 95% CI, 0.43 to 1.01) and 3-year PFS of 73% versus 61%, one-sided P = .03, and an improvement in OS (83% and 75% at 3 years; HR, 0.67; one-sided P = .05). The PFS HR for R2CHOP was 0.67 for ABC-DLBCL, one-sided P = .1.
CONCLUSION
In this signal-seeking study, the addition of lenalidomide to R-CHOP (R2CHOP) improved outcomes in newly diagnosed DLBCL including patients with ABC-DLBCL.
INTRODUCTION
Approximately 40% of patients with diffuse large B-cell lymphoma (DLBCL) will relapse after initial chemoimmunotherapy, the majority of which will succumb to the disease.1-3 Consequently, improving frontline therapy in DLBCL has been a focus of intense effort in the last decade.4 Much of the effort has focused on recognizing the molecular heterogeneity of DLBCL. Gene expression profiling (GEP) identifies three cell-of-origin (COO) subtypes: activated B-cell–like (ABC), germinal center B-cell–like (GCB), and unclassifiable subtypes, of which ABC subtype was shown to be associated with worse outcomes in retrospective studies.5
CONTEXT
Key Objective
The addition of novel agents to rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and selection of patients based on cell of origin (COO) have been postulated as the best ways to improve outcomes in diffuse large B-cell lymphoma (DLBCL). Lenalidomide has been a prime candidate for addition to R-CHOP (R2CHOP).
Knowledge Generated
R2CHOP has been tested in two independent randomized trials: a phase III study (ROBUST) in activated B-cell–like (ABC)-DLBCL and a phase II study (E1412) in all DLBCL regardless of COO, allowing recruitment without the delay of patients with rapidly progressive disease. E1412 also used a higher lenalidomide dose among other differences.
Although E1412 showed the potential benefit of adding lenalidomide to R-CHOP, considering it was a signal-seeking study and a larger ROBUST study did not show any benefit, the results seen in E1412 are not practice changing.
Relevance
The E1412 results provide impetus to study lenalidomide and novel lenalidomide analogues and highlight the importance of trial design when incorporating biomarkers in frontline studies.
Lenalidomide has single-agent activity in relapsed and refractory DLBCL,6,7 and it can be safely combined with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) (R2CHOP) without affecting the dose intensity of chemoimmunotherapy. R2CHOP showed promising results in single-arm phase II studies, particularly in non-GCB-DLBCL.8,9
Based on these results, the Eastern Cooperative Oncology Group (ECOG) developed a randomized phase II study (E1412) comparing R2CHOP with R-CHOP in patients with newly diagnosed DLBCL. The design of E1412 had three principal goals. The first was to evaluate the activity of lenalidomide with R-CHOP in all patients with DLBCL, regardless of molecular subtype. Preclinical studies of lenalidomide suggested preferential direct cytotoxicity in ABC subtype. However, lenalidomide could have a potential impact on other mechanisms such as immunomodulation and enhancing antibody-dependent cytotoxicity in GCB subtype.10 Indeed, lenalidomide shows activity in GCB subtype in a relapsed or refractory (R/R)-DLBCL, albeit less so than in ABC subtype.7 Interestingly, the benefit of lenalidomide as maintenance after R-CHOP was seen primarily in the GCB subtype, suggesting a different mechanism of action in GCB subtype.11 The second goal was to maximize synergy with chemoimmunotherapy early in the cycle while allowing hematological recovery. A higher dose of lenalidomide (25 mg) was selected for days 1-10 of a 21-day cycle based on data from phase I and single-arm phase II studies.8,12 Most of the data regarding single activity of lenalidomide in the R/R-DLBCL were based on a 25 mg daily dose.6,7 The third goal was to facilitate easy enrollment of patients in a large intergroup study without delay. We therefore elected not to use real-time biomarker selection for COO to assess patient eligibility. Centers participating in the study were allowed to use local laboratory and pathology to determine eligibility, and the study assumed that some of the patients would be rendered ineligible after central pathology review. Here, we present the results of the E1412 study.
PATIENTS AND METHODS
This prospective randomized multicenter phase II study was conducted as a US Intergroup Study led by ECOG-ACRIN with participation of SWOG and Alliance and supported by NCI. This study was approved by the IRB at respective participating institutions, and all patients provided written study consent.
Patients
Eligible patients were adults (≥ 18 years) with newly diagnosed, untreated, histologically proven DLBCL, measurable, stage II bulky (> 10 cm) to IV, ECOG performance status 0-2, and International Prognostic Index (IPI) 2-5. Patients with severe systemic symptoms or compressive disease were allowed treatment with up to 1 mg/kg/day prednisone for ≤ 7 days before beginning the study treatment. Adequate organ function was required: absolute neutrophil count (ANC) > 1,500/μL, platelet count > 100,000/μL, total bilirubin < 1.5 times upper limit of normal (ULN) (if bilirubin was > 1.5 × ULN, the direct bilirubin must have been normal), alkaline phosphatase ≤ 3 × ULN, aspartate transferase ≤ 3 × ULN (unless evidence of liver involvement, than < 5 times normal), creatinine ≤ 2 × ULN (or creatinine clearance > 30 mL/min), and left ventricular ejection fraction of > 45%.
Exclusion criteria included: known CNS lymphoma, history of deep venous thrombosis or embolism, or known thrombophilia (unless willing to be on full anticoagulation—Warfarin or low-molecular-weight heparin) if randomly assigned to the R2CHOP arm, and AIDS-related conditions (other than the presenting DLBCL).
Central Pathology Review
Patients were required to have histologically and immunophenotypically confirmed DLBCL expressing CD20 by central pathology review based on WHO criteria at the time of study initiation.13 Patients with transformed lymphoma and composite lymphoma in the diagnostic tissue (concomitant DLBCL and follicular lymphoma or another low-grade lymphoma component) were excluded, as were patients with primary mediastinal large B-cell lymphoma. Patients with tumors of DLBCL morphology and MYC translocation alone or in combination with BCL2 and/or BCL6 translocations by fluorescence in situ hybridization (FISH) (so-called double-hit and triple-hit lymphomas) were eligible; MYC FISH testing before study enrollment was not required. In the original study design, the central pathology review was conducted after study entry. However, after enrollment of 208 patients, high ineligibility rate by central pathology review (74 of 208 or 35% of patients) primarily because of composite histology and alternative diagnosis (n = 47) and/or inadequate tissue or its quality for central confirmation of diagnosis (n = 27) became apparent. The study was amended to require rapid real-time pathology review for eligibility, thereby reducing ineligibility as described elsewhere.14 In brief, a real-time central pathology review was performed within 48 hours and centers were allowed to enroll patients only if a combined morphologic and immunophenotypic diagnosis of DLBCL was confirmed by study pathologists (step 0). Following this amendment, 214 patients were screened and 147 patients were enrolled. Importantly, this requirement did not affect the median time from diagnosis to treatment (an important prognostic variable)15; it was 21 days before and after the institution of the real-time pathology eligibility requirement.
Cell-of-Origin Assessment
Paraffin-embedded tumor tissues from the initial biopsies were analyzed for COO by the NanoString Lymph2Cx GEP assay16 performed centrally (D.W.S.).
Treatment and Supportive Care
Patients received R-CHOP21 (one dose each of rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, and vincristine 1.4 mg/m2 all on day 1, and prednisone 100 mg/m2 once a day on days 1-5 of each cycle every 21 days × 6 cycles). Patients in the R2CHOP arm, in addition to RCHOP21, received 25 mg of lenalidomide daily days 1-10 of each cycle. All patients received prophylactic G-CSF (pegfilgrastim or filgrastim per local practice), and patients taking lenalidomide received aspirin 325 mg once a day as thromboembolism prophylaxis. Patients unable to take aspirin or with history of thrombosis or pulmonary embolism were required to be on full anticoagulation. Antiemetics, tumor lysis prophylaxis, and other supportive care were applied per local practice. ANC and platelet count ≥ 1,500/μL and ≥ 75,000/μL at the time of retreatment were required, respectively. Lenalidomide was not held or reduced midcycle regardless of ANC nadir unless ANC was < 500/μL > 7 days and/or platelet nadir was below 10,000/μL or < 25,000/μL for 7 days to maintain dose intensity of lenalidomide.
Statistical Analysis
Patients were randomly assigned 1:1 and stratified by IPI (2 of 3 v 4 of 5) and age (< 60 v ≥ 60 years old) to R2CHOP21 versus R-CHOP for six cycles (Fig 2A). The primary end point was progression-free survival (PFS) defined as the occurrence of progression or death from the time of random assignment. With anticipated 289 evaluable patients (102 ABC-DLBCL), the study was designed to have 89% power at one-sided 0.1 significance level with 89 PFS events for detecting a hazard ratio (HR) of 0.59 in all patients and 81% power at one-sided 0.125 significance level with 40 PFS events for an HR of 0.52 in ABC-DLBCL. Secondary end points included overall response rate (ORR), complete response (CR) rate,17 and overall survival (OS). Cochran-Mantel-Haenszel (CMH) test was used to compare ORR and CR rate stratified on IPI and age. Kaplan-Meier method was used to estimate PFS and OS, and stratified log-rank test and Cox model were used to compare between arms and estimate HR. All randomly assigned patients who were eligible and received the study treatment were deemed evaluable and included in the primary analysis. Intent-to-treatment analysis, which excludes only pathology ineligible patients, was conducted as the secondary analysis. One-sided P-values were reported for PFS and OS end points per study design. Two-sided P-values were presented for all other efficacy end points and all safety outcomes (Appendix Table A1, online only).
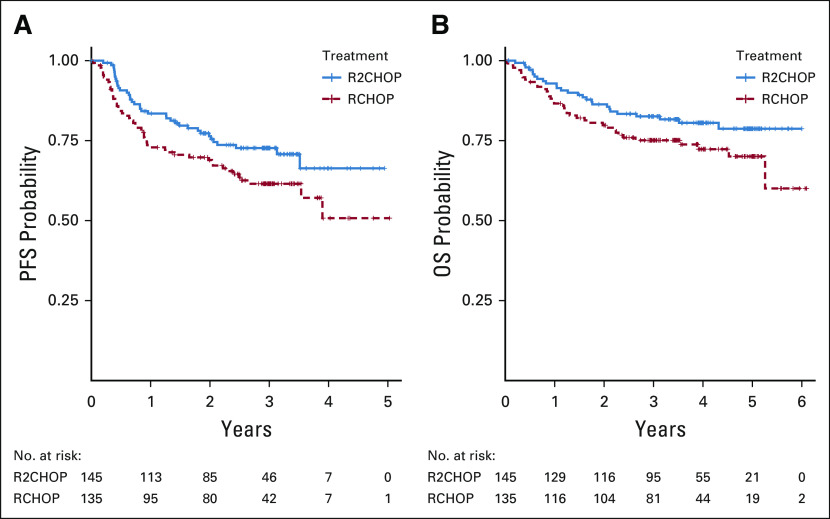
FIG 2.
Kaplan-Meier estimate of (A) PFS for all patients, n = 280 and (B) OS for all patients, n = 280. OS, overall survival; PFS, progression-free survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R2CHOP, lenalidomide plus R-CHOP.
RESULTS
Patient Characteristics and Disposition
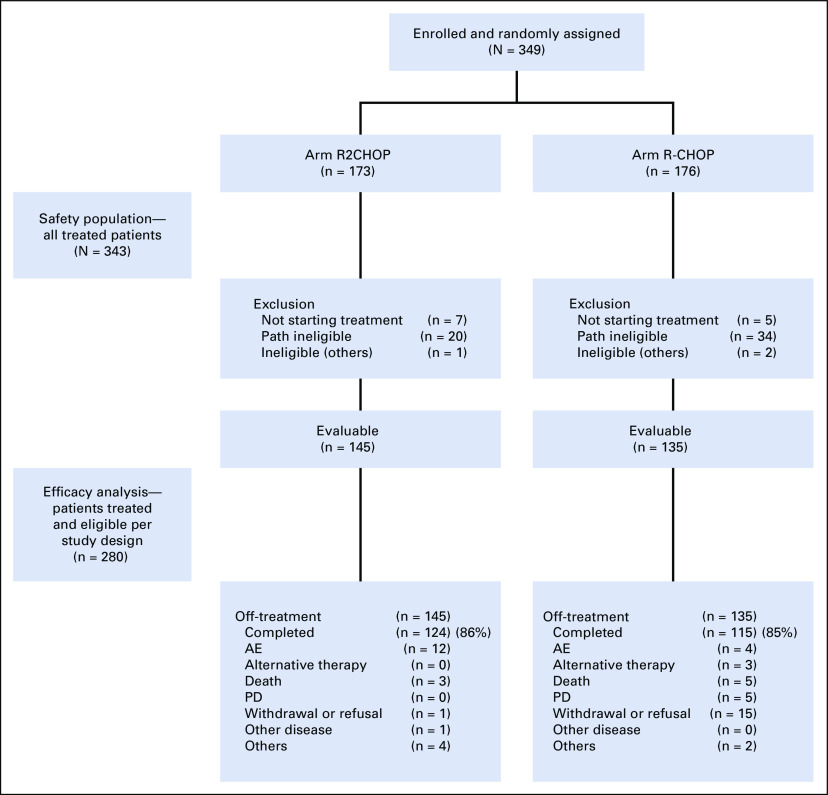
Between August 2013 and January 2017, 349 patients were enrolled and randomly assigned in the study. Twelve patients did not start planned treatment (seven in the R2CHOP arm and five in the R-CHOP arm) because of consent withdrawal (n = 5), ineligibility (n = 5), death (n = 1), and the need to start alternative therapy urgently (n = 1). The safety analysis population included all 337 treated patients, 166 in the R2CHOP arm and 171 in the R-CHOP arm. Fifty-four patients were deemed ineligible for efficacy evaluation secondary to central pathology review exclusion because of lack of material either for confirmation of local diagnosis or for ancillary studies required by the study or to ineligible diagnoses. Three patients were deemed ineligible because of not meeting other eligibility criteria (Fig 1).
FIG 1.
E1412 CONSORT diagram. AE, adverse event; PD, progression of disease; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R2CHOP, lenalidomide plus R-CHOP.
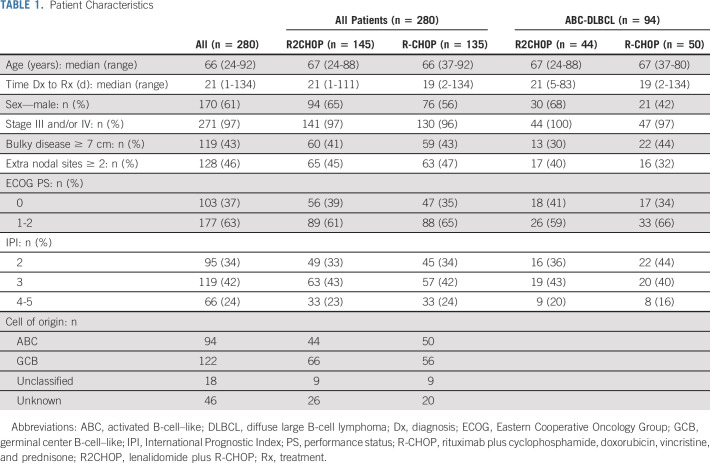
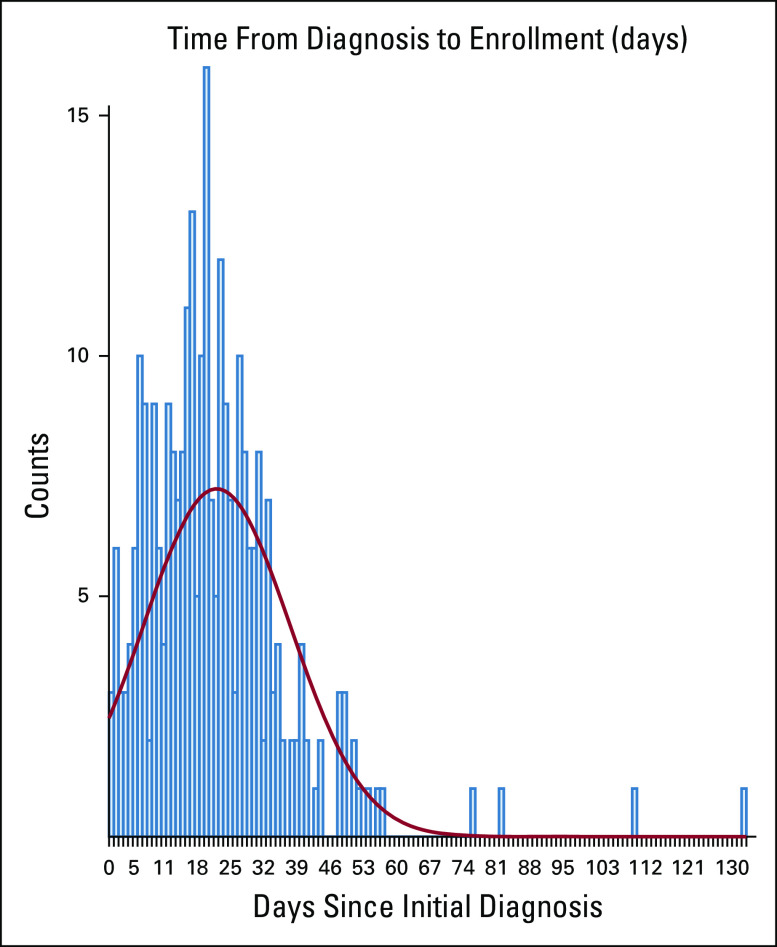
Two hundred eighty patients (145 in R2CHOP and 135 in R-CHOP) were evaluable (eligible and treated) for efficacy analysis. Baseline patient characteristics were balanced between arms with a median age of 66 (range, 24-92). The majority of patients had relatively high-risk disease: 96% had stage III or IV, 46% had ≥ 2 extra-nodal site involvement, and 24% had a high IPI of 4 or 5 (Table 1). The clinical characteristics were also balanced in the patients with ABC analyzed for co-primary end point (Table 1). The median time from diagnosis to treatment was 21 days (21 days in R2CHOP and 19 days in the R-CHOP arm); only 22% of patients were treated more than 31 days from diagnosis (Appendix Fig A2, online only).
TABLE 1.
Patient Characteristics
COO Analysis
COO analysis was attempted in all patients with available material by Lymph2Cx, and the results were obtained for 234 patients of 280 evaluable patients: 122 patients (52%) had GCB-DLBCL, 94 (40%) had ABC-DLBCL, and 18 (8%) were unclassifiable (Table 1). COO by GEP was unable to be assessed in 46 of 280 (16%) evaluable patients because of insufficient material (n = 34), low RNA quantity (n = 10), or low RNA quality (n = 2).
Efficacy
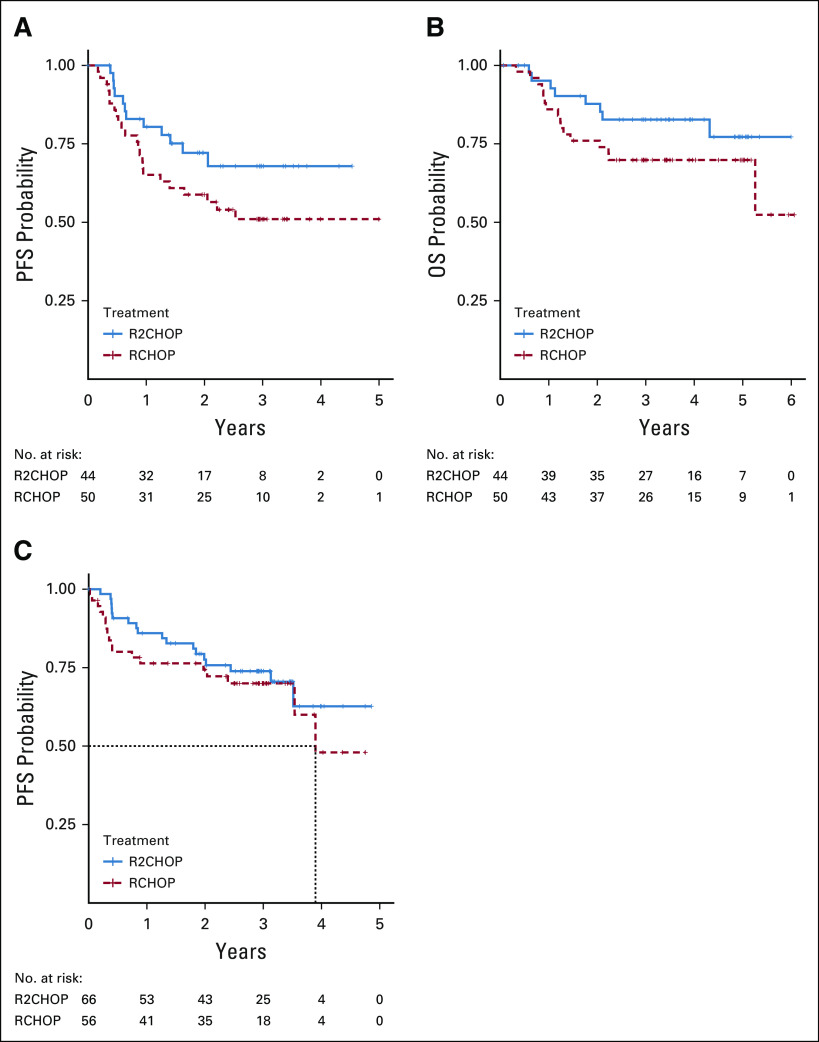
The ORR and CR rate were 92% and 68% in R-CHOP and 97% (P = .06) and 73% (P = .43) in R2CHOP arms, respectively. The median follow-up was 3.0 years. The study met its prespecified primary and co-primary end points. R2CHOP was associated with 34% reduction in risk of progression or death compared with R-CHOP, an HR of 0.66 with one-sided 90% CI < 0.88 (95% CI, 0.43 to 1.01), and P (one-sided) = .03 (Fig 2A). The 1-, 2-, and 3-year PFS was 84% versus 73%, 76% versus 69%, and 73% versus 62% for R2CHOP versus R-CHOP, respectively. OS was also superior with R2CHOP versus R-CHOP with a 3-year OS of 83% versus 75% (one-sided P = .05), respectively (Fig 2B). Analysis of PFS by COO using GEP showed improved outcome with R2CHOP in the 94 patients with ABC (HR = 0.64; one-sided 90% CI upper limit, 1.01; two-sided 95% CI, 0.31 to 1.29; one-sided P = .1) (Fig 3A). In the 122 patients with GCB, the HR was 0.82 (one-sided 90% CI upper limit, 1.27; two-sided 95% CI, 0.43 to 1.59) (Fig 3C). The subset analysis of PFS showed that R2CHOP was associated with a benefit in most groups (Fig 4). However, the power of analysis was limited by a relatively small number of patients in most subgroups. Outcomes in the intend to treat population and in patients age < 60 and ≥ 60 years are shown in Appendix Table A1 and Appendix Figure A2.
FIG 3.
Kaplan-Meier estimate of (A) PFS in patients with ABC, n = 94; (B) OS for patients with ABC; and (C) PFS in patients with GCB. ABC, activated B-cell–like; GCB, germinal center B-cell–like; OS, overall survival; PFS, progression-free survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R2CHOP, lenalidomide plus R-CHOP.
FIG 4.
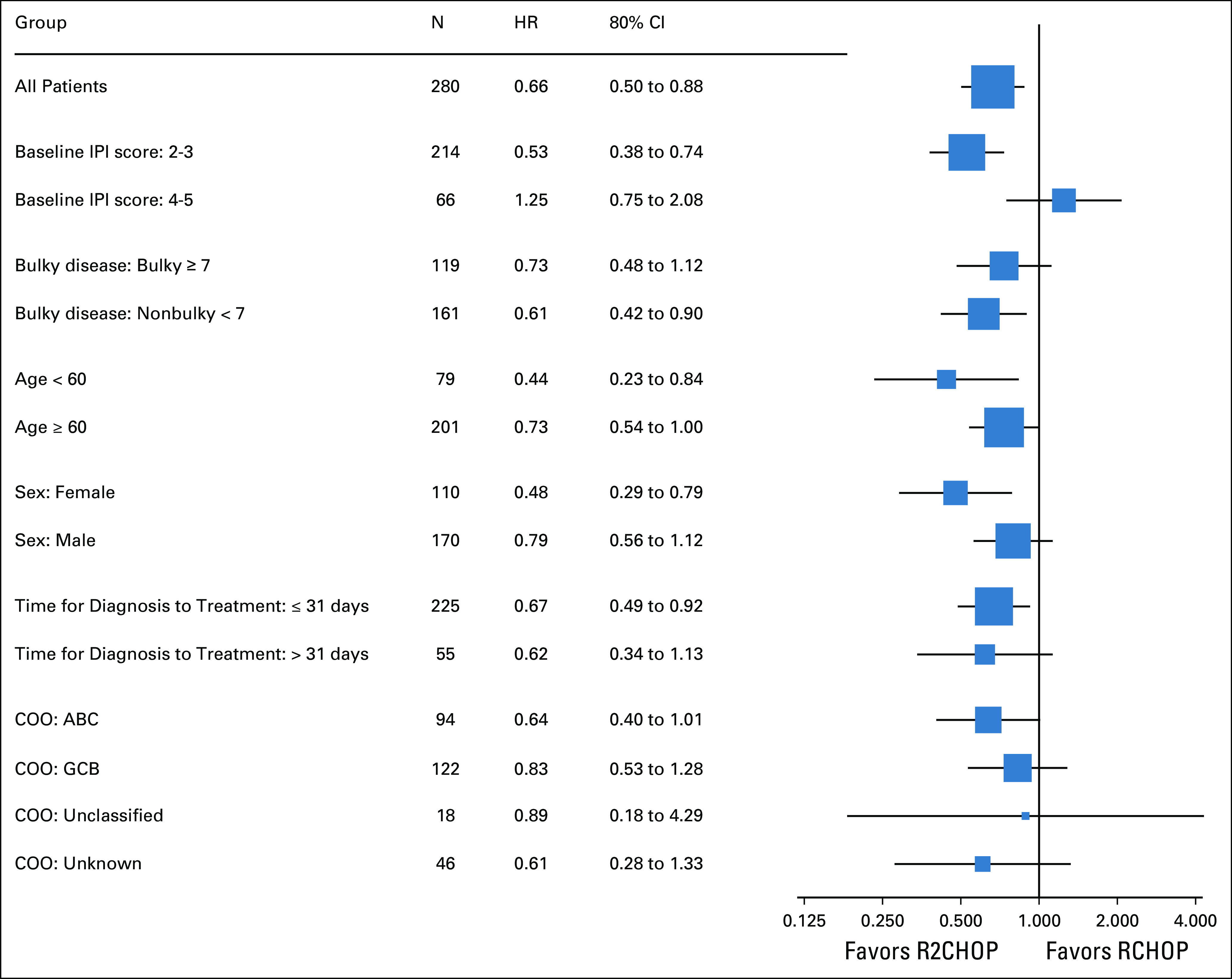
Forest plot of treatment effect (HR, 80% CI) within subsets of interest. ABC, activated B-cell–like; COO, cell of origin; GCB, germinal center B-cell–like; HR, hazard ratio; IPI, International Prognostic Index.
Safety and Dose Modifications
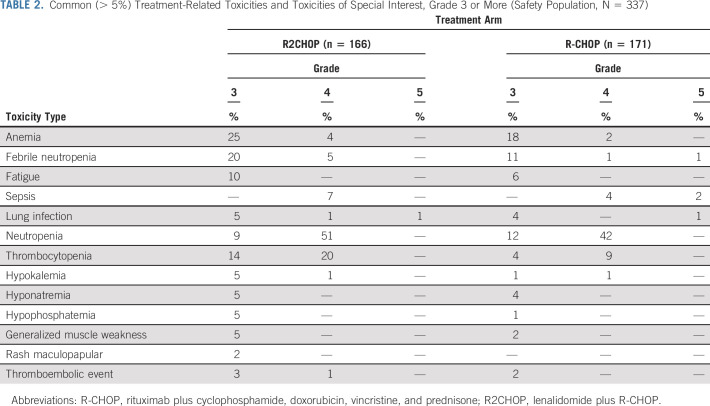
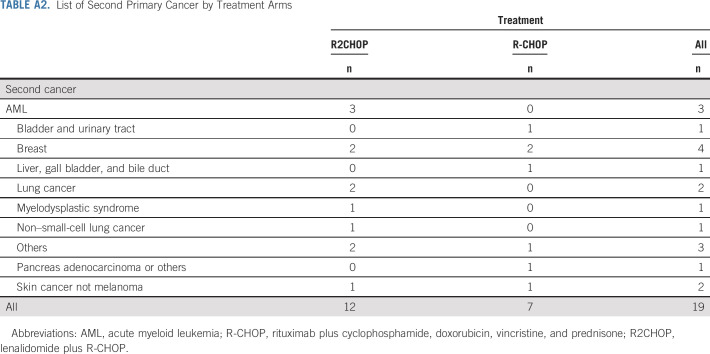
Adverse events (AEs) were largely as expected with R-CHOP. Significantly different rates of grade ≥ 3 AEs between R2CHOP and R-CHOP arms were found for diarrhea (6% v 1%, P = .005), anemia (29% v 20%, P = .03), febrile neutropenia (25% v 14%, P = .003), thrombocytopenia (34% v 13%, P < .001), and electrolyte abnormalities (5% v 2%, P = .06), respectively (Table 2). 86% and 85% of patients completed the intended six cycles of therapy in R2CHOP and R-CHOP arms, respectively. AEs were the reason for discontinuation of intended treatment in 12 (8%) patients in the R2CHOP arm (discontinuation of lenalidomide alone in seven and R-CHOP and lenalidomide in five) and four (3%) patients in the R-CHOP arm (discontinuation of R-CHOP). There were nine treatment-related deaths, two in the R2CHOP arm (lung infection, n = 2) and seven in the R-CHOP arm (lung infection n = 1, sepsis or febrile neutropenia n = 5, and adult respiratory distress syndrome n = 1). To date, there have been 19 secondary neoplasms: 12 in the R2CHOP arm and seven in the R-CHOP arm (Appendix Table A2, online only).
TABLE 2.
Common (> 5%) Treatment-Related Toxicities and Toxicities of Special Interest, Grade 3 or More (Safety Population, N = 337)
DISCUSSION
Lenalidomide has long been a prime candidate to be combined with chemoimmunotherapy in frontline therapy of DLBCL based on its novel mechanisms of activity, single-agent activity, synergy with rituximab and chemotherapy, and modest toxicity profile allowing safe combination with R-CHOP. Single arm phase II studies combining lenalidomide with R-CHOP show promising results in patients with newly diagnosed and untreated DLBCL, particularly in the ABC subtype.4,8,9
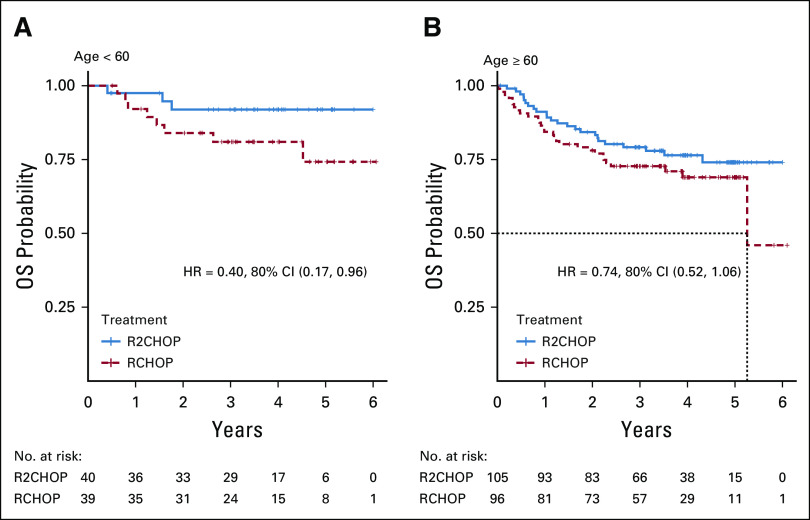
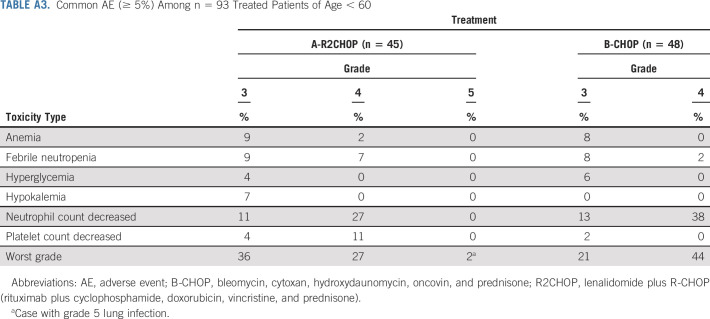
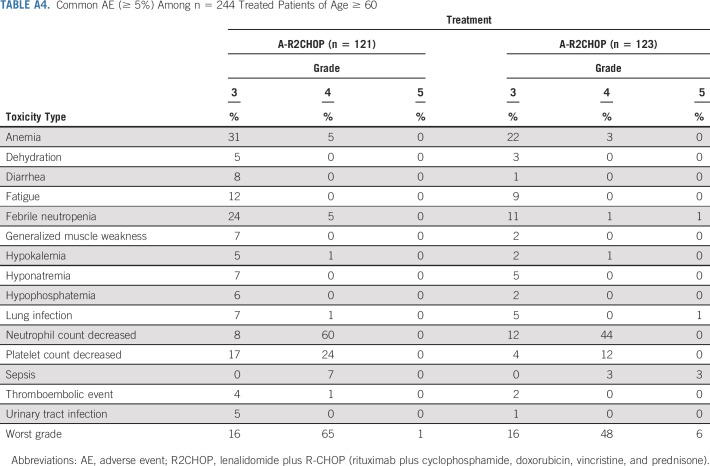
In the current randomized study, the addition of lenalidomide to R-CHOP resulted in a 34% reduction in risk of progression or death in all comers when compared with R-CHOP alone and in the co-primary end point of improved PFS in ABC subtype of DLBCL. Subset analysis showed mostly consistent benefit from adding lenalidomide across the clinical subsets, albeit small numbers of patients in each subgroup warrant caution in interpretation. A subset analysis from a randomized phase III study of ibrutinib plus R-CHOP versus R-CHOP (PHOENIX) in patients with non-GCB DLBCL suggested better outcomes in patients < 60 years of age.18 A similar subset analysis of E1412 demonstrated trend toward more pronounced benefit from addition of lenalidomide in younger patients (Fig 4, Fig A2) with less toxicity than in older patients (Appendix Tables A3 and A4, online only); however, a small number of patients limit interpretation of this finding.
The E1412 study was designed as a signal-seeking study to be validated in a further phase III study and to test the best platform for future studies. The recently presented phase III ROBUST study,19 evaluating R2CHOP versus R-CHOP plus placebo, was not designed as the validation study for E1412 because it was conducted at the same time and with several major differences. Important differences between E1412 and ROBUST were the targeted patient population, lenalidomide dose and schedule, and the ability to promptly enroll high-risk patients, with E1412 being a smaller study with wider confidence intervals.19,20 Although these differences could have played a role in the discordant results of both trials, the experience with E1412 allows one to draw important implications for future frontline studies in DLBCL. The study was designed to allow for accrual of high-risk, rapidly progressing patients based on local laboratories and local pathology review, but later changed to a rapid central pathology review for patient enrollment. The initial design was based on emerging evidence that time from diagnosis to treatment is a critical variable and that complicated trials with central review of biomarkers or other baseline data are biased toward patients who can wait, and such patients tend to have better outcomes.15 The strategy applied in E1412, allowing local laboratory parameters to assess eligibility criteria, local pathology, and utilization of an integral yet not real-time biomarker, as well as allowing pretreatment with steroids in symptomatic patients, was successful in overcoming some of the obstacles for enrollment of rapidly progressing and sick patients with the median time from diagnosis to treatment of 21 days. Because of this strategy, the study was designed to compensate for some initially enrolled patients found to be ineligible by central pathology review, either because of not meeting diagnostic criteria or because of sites unable to submit adequate tissue to verify diagnosis and assess COO. In E1412, ineligibility because of these factors was 35% before amendment (20% because of composite DLBCL or alternative diagnosis, and 15% because of site unable to submit diagnostic tissue for retrospective review, despite study eligibility criteria requiring confirmation that tissue is available for central pathology review and biomarker assessment). This high ineligibility rate by central pathology review resulted in the development of a rapid registration step 0 real-time central pathology review that was able to be performed without a negative impact on the time from diagnosis to treatment. The experience with step 0 rapid central pathology review in E1412 as US intergroup study was published elsewhere, demonstrating the feasibility of this approach.14
There are two broad strategies of incorporating new agents into R-CHOP model: to extend duration of targeted agent exposure or to maximize exposure early in the cycle.4 E1412 used the latter approach to maximize synergy with chemoimmunotherapy early in the cycle while allowing hematological recovery. The dose of lenalidomide was 25 mg daily, which was supported by effectiveness in R/R-DLBCL.6,7 This strategy of a higher dose but administered for only 10 of the 21 days was indeed successful in E1412, and while associated with a higher incidence of neutropenia, febrile neutropenia, and thrombocytopenia, these toxicities did not result in an increase in rates of treatment-related deaths or bleeding complications. Overall treatment discontinuations did not differ between arms, and while 12 (9%) patients discontinued study treatment because of AEs in R2CHOP, this number included seven patients who discontinued lenalidomide only—representing only 5% of treated patients. Although optimal dose versus duration of exposure may depend on the experimental agent added to R-CHOP, R2CHOP in E1412 provides an example of the incorporation of a potentially myelosuppressive agent early in the cycle at doses shown to be effective in relapse and refractory setting. Although the number of secondary malignancies was small, careful further follow-up is required in this regard.
E1412 was designed with co-primary end point of improvement of PFS in ABC-DLBCL, as preclinical studies and single-arm trials indicated that this population is particularly likely to benefit from the addition of lenalidomide.8 Indeed, the improvement of outcomes in patients with the ABC subtype had the largest impact on the overall positive study results. It is now established that DLBCL subtypes defined by COO are themselves molecularly heterogenous with different underlying genetic changes.21,22 Recently published data from a single-arm R2CHOP study identified a specific molecular signature linked to the benefit from the addition of lenalidomide to R-CHOP that was seen in both ABC and GCB subtypes of DLBCL, albeit enriched in the ABC subtype.23 This observation is consistent with the clinical results in E1412 and supports the notion that COO appears to be inadequate to support a precision medicine approach in DLBCL. Although further molecular studies evaluating mutation clusters and other molecular markers associated with benefit of lenalidomide in E1412 are ongoing, E1412 demonstrates both feasibility and the need for evaluation of the effects of new therapy in all molecular subsets of DLBCL while allowing for a co-primary end point in a molecular subset of highest interest.
In summary, the ECOG-ACRIN E1412 study demonstrated a potential clinical benefit of adding lenalidomide to R-CHOP in newly diagnosed patients regardless of COO and in patients with ABC-DLBCL. This benefit was consistent across subgroups and translated to OS benefit of R2CHOP. No new safety signals were observed. Although the E1412 results are not practice changing, considering its signal-seeking nature and conflicting results from phase III ROBUST study, these results support further studies of lenalidomide and/or novel lenalidomide analogues in frontline therapy of DLBCL.
APPENDIX
FIG A1.
Time from diagnosis to treatment in E1412.
FIG A2.
OS in patients age ≥ 60. HR, hazard ratio; OS, overall survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R2CHOP, lenalidomide plus R-CHOP.
TABLE A1.
PFS and OS Outcomes in the ITT Population Analysis
TABLE A2.
List of Second Primary Cancer by Treatment Arms
TABLE A3.
Common AE (≥ 5%) Among n = 93 Treated Patients of Age < 60
TABLE A4.
Common AE (≥ 5%) Among n = 244 Treated Patients of Age ≥ 60
Grzegorz S. Nowakowski
Consulting or Advisory Role: Celgene/BMS, MorphoSys, Genentech, Roche Selvita, Debiopharm Group, Kite/Gilead, Karyopharm Therapeutics
Research Funding: Celgene/BMS, NanoString Technologies, MorphoSys Roche, Seattle Genetics
Fangxin Hong
Consulting or Advisory Role: Merck Sharp & Dohme
David W. Scott
Consulting or Advisory Role: Celgene/BMS, Janssen, AbbVie, AstraZeneca
Research Funding: Janssen, Roche/Genentech, NanoString Technologies
Patents, Royalties, Other Intellectual Property: Named inventor on a pending patent describing gene expression profiling in prognostication in classical Hodgkin lymphoma, As a member of the LLMPP I am potentially a named inventor on a pending patent on the use of gene expression profiling to assign cell-of-origin in diffuse large B-cell lymphoma, I am a named inventor on a pending patent on the use of gene expression profiling to determine the proliferation signature in mantle cell lymphoma, Named inventor on a pending patent describing using gene expression profiling to identify molecular subtypes of GCB-DLBCL
Rebecca L. King
Research Funding: Celgene/BMS
Thomas M. Habermann
Consulting or Advisory Role: Celgene/BMS, Kite/Gilead
Nina Wagner-Johnston
Consulting or Advisory Role: ADC Therapeutics, Bayer, Regeneron, Calibr, Verastem, Gilead Sciences
Research Funding: Merck, Novartis/Pfizer, Genentech, Astex Pharmaceuticals, Juno Therapeutics, Regeneron, Acerta Pharma, ADC Therapeutics
Carla Casulo
Honoraria: Gilead Sciences
Research Funding: Celgene/BMS, Verastem, Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences, Roche
James L. Wade
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Celgene/BMS, Abbott, GlaxoSmithKline, Johnson & Johnson, Novartis
Consulting or Advisory Role: New Century Health
Jonathon B. Cohen
Consulting or Advisory Role: Celgene/BMS, Seattle Genetics, Abbvie, Janssen, Loxo, Kite/Gilead, AstraZeneca, Cellectar, Aptitude Medical
Research Funding: Bristol-Myers Squibb, Janssen, Novartis, Takeda, AI Therapeutics, Genentech, ASH, Lymphoma Research Foundation, Loxo, Bioinvent, AstraZeneca
Nadia Khan
Honoraria: Janssen
Consulting or Advisory Role: Incyte
Research Funding: Bristol-Myers Squibb
Jennifer E. Amengual
Honoraria: Janssen, Daiichi Sankyo, Karyopharm Therapeutics
Research Funding: Appia Pharmaceuticals
Travel, Accommodations, Expenses: Epizyme
John P. Leonard
Consulting or Advisory Role: Celgene/BMS, Bristol-Myers Squibb, Gilead Sciences, Epizyme, Bayer, Genentech/Roche, ADC Therapeutics, MEI Pharma, AstraZeneca, Merck, Morphosys, Nordic Nanovector, Karyopharm Therapeutics, Sutter Medical Group, Miltenyi Biotec, Regeneron, Bayer, Akcea Therapeutics, Sandoz, Nordic Nanovector, Genmab, Abbvie, Incyte
Research Funding: Research Funding: Celgene/BMS, Alliance for Clinical Trials in Oncology, Takeda, Pfizer, National Cancer Institute, Janssen Oncology, Epizyme
Travel, Accommodations, Expenses: BeiGene
Jonathan W. Friedberg
Consulting or Advisory Role: Astellas Pharma, Novartis
Research Funding: Seattle Genetics
Patents, Royalties, Other Intellectual Property: Patient on bone marrow microenvironment signals
Lale Kostakoglu
Consulting or Advisory Role: Roche/Genentech
Brad S. Kahl
Consulting or Advisory Role: Celgene/BMS, Juno Therapeutics, Abbvie, Pharmacyclics, Acerta Pharma, ADC Therapeutics, Pharmacyclics, Genentech, Roche, AstraZeneca, BeiGene, Bayer, MEI Pharma, TG Therapeutics, Kite/Gilead, MorphoSys, Janssen
Research Funding: Genentech, Acerta Pharma, ADC Therapeutics, Celgene/BMS
Travel, Accommodations, Expenses: Celgene/BMS, Juno Therapeutics, Genentech/Roche, Abbvie, Millennium, Seattle Genetics
Thomas E. Witzig
Consulting or Advisory Role: Karyopharm Therapeutics, Abbvie/Genentech, Seattle Genetics, Celgene/BMS, Incyte, Epizyme, Cellectar, Tessa Therapeutics, Portola Pharmaceuticals, MorphoSys, ADC Therapeutics
Research Funding: Celgene/BMS, Acerta Pharma, Kura Oncology, Acrotech Biopharma, Karyopharm Therapeutics
Patents, Royalties, Other Intellectual Property: T.E.W. is co-inventor on a patent application filed by Mayo Clinic and pending on the combination of CRM1 inhibitors with salicylates.
No other potential conflicts of interest were reported.
See accompanying editorial on page 1314
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
SUPPORT
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O'Dwyer, MD, and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: U10CA180820, U10CA180794, UG1CA232760, UG1CA233230, UG1CA233339, UG1CA233196, U10CA180821,UG1CA233277, UG1CA233247, U10CA180888, UG1CA189830, UG1CA189863, and UG1CA189971.
B.S.K. and T.E.W. are co-senior authors of this work.
AUTHOR CONTRIBUTIONS
Conception and design: Grzegorz S. Nowakowski, Fangxin Hong, William R. Macon, Thomas M. Habermann, R. F. Little, Brad S. Kahl, Thomas E. Witzig
Administrative support: Thomas M. Habermann, Brad S. Kahl
Provision of study materials or patients: Grzegorz S. Nowakowski, Nina Wagner-Johnston, James L. Wade, Jonathon B. Cohen, Nadia Khan, Jonathan W. Friedberg, Thomas E. Witzig
Collection and assembly of data: Grzegorz S. Nowakowski, David W. Scott, William R. Macon, Carla Casulo, James L. Wade, Gauri G. Nagargoje, C. M. Reynolds, Jonathon B. Cohen, Jennifer E. Amengual, Kristy L. Richards, John P. Leonard, Brad S. Kahl, Thomas E. Witzig
Data analysis and interpretation: Grzegorz S. Nowakowski, Fangxin Hong, David W. Scott, William R. Macon, Rebecca L. King, Thomas M. Habermann, Nina Wagner-Johnston, C. M. Reynolds, Jonathon B. Cohen, Nadia Khan, John P. Leonard, Jonathan W. Friedberg, Lale Kostakoglu, Thomas E. Witzig
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Addition of Lenalidomide to R-CHOP Improves Outcomes in Newly Diagnosed Diffuse Large B-Cell Lymphoma in a Randomized Phase II US Intergroup Study ECOG-ACRIN E1412
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Grzegorz S. Nowakowski
Consulting or Advisory Role: Celgene/BMS, MorphoSys, Genentech, Roche Selvita, Debiopharm Group, Kite/Gilead, Karyopharm Therapeutics
Research Funding: Celgene/BMS, NanoString Technologies, MorphoSys Roche, Seattle Genetics
Fangxin Hong
Consulting or Advisory Role: Merck Sharp & Dohme
David W. Scott
Consulting or Advisory Role: Celgene/BMS, Janssen, AbbVie, AstraZeneca
Research Funding: Janssen, Roche/Genentech, NanoString Technologies
Patents, Royalties, Other Intellectual Property: Named inventor on a pending patent describing gene expression profiling in prognostication in classical Hodgkin lymphoma, As a member of the LLMPP I am potentially a named inventor on a pending patent on the use of gene expression profiling to assign cell-of-origin in diffuse large B-cell lymphoma, I am a named inventor on a pending patent on the use of gene expression profiling to determine the proliferation signature in mantle cell lymphoma, Named inventor on a pending patent describing using gene expression profiling to identify molecular subtypes of GCB-DLBCL
Rebecca L. King
Research Funding: Celgene/BMS
Thomas M. Habermann
Consulting or Advisory Role: Celgene/BMS, Kite/Gilead
Nina Wagner-Johnston
Consulting or Advisory Role: ADC Therapeutics, Bayer, Regeneron, Calibr, Verastem, Gilead Sciences
Research Funding: Merck, Novartis/Pfizer, Genentech, Astex Pharmaceuticals, Juno Therapeutics, Regeneron, Acerta Pharma, ADC Therapeutics
Carla Casulo
Honoraria: Gilead Sciences
Research Funding: Celgene/BMS, Verastem, Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences, Roche
James L. Wade
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Celgene/BMS, Abbott, GlaxoSmithKline, Johnson & Johnson, Novartis
Consulting or Advisory Role: New Century Health
Jonathon B. Cohen
Consulting or Advisory Role: Celgene/BMS, Seattle Genetics, Abbvie, Janssen, Loxo, Kite/Gilead, AstraZeneca, Cellectar, Aptitude Medical
Research Funding: Bristol-Myers Squibb, Janssen, Novartis, Takeda, AI Therapeutics, Genentech, ASH, Lymphoma Research Foundation, Loxo, Bioinvent, AstraZeneca
Nadia Khan
Honoraria: Janssen
Consulting or Advisory Role: Incyte
Research Funding: Bristol-Myers Squibb
Jennifer E. Amengual
Honoraria: Janssen, Daiichi Sankyo, Karyopharm Therapeutics
Research Funding: Appia Pharmaceuticals
Travel, Accommodations, Expenses: Epizyme
John P. Leonard
Consulting or Advisory Role: Celgene/BMS, Bristol-Myers Squibb, Gilead Sciences, Epizyme, Bayer, Genentech/Roche, ADC Therapeutics, MEI Pharma, AstraZeneca, Merck, Morphosys, Nordic Nanovector, Karyopharm Therapeutics, Sutter Medical Group, Miltenyi Biotec, Regeneron, Bayer, Akcea Therapeutics, Sandoz, Nordic Nanovector, Genmab, Abbvie, Incyte
Research Funding: Research Funding: Celgene/BMS, Alliance for Clinical Trials in Oncology, Takeda, Pfizer, National Cancer Institute, Janssen Oncology, Epizyme
Travel, Accommodations, Expenses: BeiGene
Jonathan W. Friedberg
Consulting or Advisory Role: Astellas Pharma, Novartis
Research Funding: Seattle Genetics
Patents, Royalties, Other Intellectual Property: Patient on bone marrow microenvironment signals
Lale Kostakoglu
Consulting or Advisory Role: Roche/Genentech
Brad S. Kahl
Consulting or Advisory Role: Celgene/BMS, Juno Therapeutics, Abbvie, Pharmacyclics, Acerta Pharma, ADC Therapeutics, Pharmacyclics, Genentech, Roche, AstraZeneca, BeiGene, Bayer, MEI Pharma, TG Therapeutics, Kite/Gilead, MorphoSys, Janssen
Research Funding: Genentech, Acerta Pharma, ADC Therapeutics, Celgene/BMS
Travel, Accommodations, Expenses: Celgene/BMS, Juno Therapeutics, Genentech/Roche, Abbvie, Millennium, Seattle Genetics
Thomas E. Witzig
Consulting or Advisory Role: Karyopharm Therapeutics, Abbvie/Genentech, Seattle Genetics, Celgene/BMS, Incyte, Epizyme, Cellectar, Tessa Therapeutics, Portola Pharmaceuticals, MorphoSys, ADC Therapeutics
Research Funding: Celgene/BMS, Acerta Pharma, Kura Oncology, Acrotech Biopharma, Karyopharm Therapeutics
Patents, Royalties, Other Intellectual Property: T.E.W. is co-inventor on a patent application filed by Mayo Clinic and pending on the combination of CRM1 inhibitors with salicylates.
No other potential conflicts of interest were reported.
REFERENCES
- 1.Coiffier B Lepage E Briere J, et al. : CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235-242, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Habermann TM Weller EA Morrison VA, et al. : Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 24:3121-3127, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Farooq U Maurer MJ Thompson CA, et al. : Clinical heterogeneity of diffuse large B cell lymphoma following failure of front-line immunochemotherapy. Br J Haematol 179:50-60, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nowakowski GS Blum KA Kahl BS, et al. : Beyond RCHOP: A blueprint for diffuse large B cell lymphoma research. J Natl Cancer Inst 108:djw257, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alizadeh AA Eisen MB Davis RE, et al. : Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503-511, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Witzig TE Vose JM Zinzani PL, et al. : An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann Oncol 22:1622-1627, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Czuczman MS Trneny M Davies A, et al. : A phase 2/3 multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator's choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res 23:4127-4137, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowakowski GS LaPlant B Macon WR, et al. : Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-cell lymphoma: A phase II study. J Clin Oncol 33:251-257, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Vitolo U Chiappella A Franceschetti S, et al. : Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: Results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol 15:730-737, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Riches JC, Gribben JG: Mechanistic and clinical aspects of lenalidomide treatment for chronic lymphocytic leukemia. Curr Cancer Drug Targets 16:689-700, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Thieblemont C Howlett S Casasnovas RO, et al. : Lenalidomide maintenance for diffuse large B-cell lymphoma patients responding to R-CHOP: Quality of life, dosing, and safety results from the randomised controlled REMARC study. Br J Haematol 189:84-96, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowakowski GS LaPlant B Habermann TM, et al. : Lenalidomide can be safely combined with R-CHOP (R2CHOP) in the initial chemotherapy for aggressive B-cell lymphomas: phase I study. Leukemia 25:1877-1881, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow SH Campo E Harris NL, et al. : WHO classification of tumours of haematopoietic and lymphoid tissues, in: Bosman FT Jaffe ES Lakhani SR, et al. (eds): World Health Organization Classification of Tumours. Lyon, France, IARC, 2008 [Google Scholar]
- 14.King RL Nowakowski GS Witzig TE, et al. : Rapid, real time pathology review for ECOG/ACRIN 1412: A novel and successful paradigm for future lymphoma clinical trials in the precision medicine era. Blood Cancer J 8:27, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurer MJ Ghesquieres H Link BK, et al. : Diagnosis-to-Treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for bias in clinical trials. J Clin Oncol 36:1603-1610, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott DW Wright GW Williams PM, et al. : Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood 123:1214-1217, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson BD Pfistner B Juweid ME, et al. : Revised response criteria for malignant lymphoma. J Clin Oncol 25:579-586, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Younes A Sehn LH Johnson P, et al. : Randomized phase III trial of ibrutinib and rituximab plus Cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol 37:1285-1295, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitolo U Witzig TE Gascoyne RD, et al. : . ROBUST: First report of phase III randomized study of lenalidomide/R‐CHOP (R2‐CHOP) vs placebo/R‐CHOP in previously untreated ABC‐type diffuse large B‐cell lymphoma. Hematol Oncol 37:36-37, 2019 [Google Scholar]
- 20.Nowakowski GS Chiappella A Witzig TE, et al. : Variable global distribution of cell-of-origin from the ROBUST phase III study in diffuse large B-cell lymphoma. Haematol 105:e72-e75, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapuy B Stewart C Dunford AJ, et al. : Author correction: Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 24:1290-1291, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Schmitz R Wright GW Huang DW, et al. : Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med 378:1396-1407, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartert KT Wenzl K Krull JE, et al. : Targeting of Inflammatory Pathways with R2CHOP in High-Risk DLBCL. Leukemia 10.1038/s41375-020-0766-4 [epub ahead of print on March 5, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]