Abstract
Background:
Thyroid autoimmune disease (TAI) has been verified to be related to multiple adverse pregnancy outcomes. A growing number of evidences highlight the protective roles of glucocorticoid on the treatments of TAI. This meta-analysis aimed to study whether it is beneficial to add glucocorticoid treatment in infertile women with TAI when they are undergoing assisted reproductive technology (ART).
Methods:
We conducted a systematic search in PubMed, EMBASE, Cochrane Library, China National Knowledge Infrastructure (CNKI), WanFang database, Weipu China Science and Technology Journal Databases (VIP database) up to September 10, 2020. The Revman 5.3 software was utilized for data statistics. We used a random-effects model to analyze data and the odds ratio (OR) combining with 95% confidence interval (95% CI) were employed to reveal the results.
Results:
Three publications with 237 antithyroid antibody (ATA)-positive and 384 ATA-negative women were included in the final analysis. Overall, glucocorticoid therapy showed satisfying effects on improving clinical pregnancy rate (OR = 4.63, 95% CI [2.23, 9.58], I2 = 0.0%, P < .0001) and live birth rate (OR = 3.19, 95% CI [1.13, 9.04], I2 = 0.0%, P = .03) of ATA-positive women compared with control group. However, it seems that glucocorticoid showed no significant difference in the abortion rate (OR = 0.62, 95% CI [0.09, 4.32], I2 = 35%, P = .64) and oocyte recovery (OR = 2.26, 95% CI [−1.46, 5.99], I2 = 79%, P < .0001) between the 2 groups.
Conclusions:
Glucocorticoid may improve the pregnancy outcomes of ART women with ATA positive, but there is no significant reduction in the risk of miscarriage. Due to the limited enrolled references, glucocorticoid adjuvant therapy should be applied after more randomized controlled trials.
Keywords: assisted reproductive technology, glucocorticoid supplementation, thyroid autoimmune disease, woman infertility
1. Introduction
Thyroid autoimmune disease (TAI) is defined as the disorder of normal thyroid autoimmune function caused by the presence of thyroid autoantibodies (ATA), including thyroglobulin antibodies (Tg-Ab) and thyroid peroxidase antibodies (TPO-Ab).[1,2] In women of childbearing age, the prevalence of TPO-Ab positive, Tg-Ab positive, and TPO-Ab/Tg-Abs double positive were 4.4%, 3.4%, and 6.9%, respectively.[3] Noteworthy, Unuane et al[4] also found the prevalence of TAI was higher in infertile women as compared to fertile women (19% vs 13%, P = .047). Some studies have also indicated ATA is associated with infertility and other poor pregnancy outcomes, such as pregnancy loss, recurrent abortion, premature delivery, placental abruption, postpartum depression, or even neonatal respiratory distress syndrome.[5,6] It is considered as an independent marker of the failure of assisted reproductive technology (ART).[7,8] However, the conflicting data remain on the influence of ATA on the pregnancy outcomes of infertile women, especially on the adverse outcomes of ART.[9–11]
A retrospective study investigated 873 euthyroid women with ART and found no significant difference in delivery rate, pregnancy loss, and biochemical pregnancy between ATA-positive group and ATA-negative group.[9] The result was also supported by the recent studies of Chen et al[10] and Kris et al.[11] Conversely, theories have been put forward that ATA positive in euthyroid women with infertility was associated with adverse outcomes of pregnancy, and even caused the failure of ART.[12–14] The link between ATA and reproductive outcomes remains debated in various studies, and underlying mechanisms are still unclear and required to be more in-depth investigated.
Glucocorticoids, playing the crucial role on normal embryo implantation and fetal placental development, are used to treat autoimmune diseases, such as systemic lupus erythematosus and antiphospholipid syndrome.[15] Some studies have revealed medium and small dose glucocorticoid could reduce the level of ATA and improve pregnancy successful rate of ART in infertile women with TAI.[16–19] But Robertson and his colleagues[20] questioned the safety and efficacy of glucocorticoids, arguing that it is unnecessary and probably harmful unless in the state of over-immune pathology. In view of these inconsistent findings, we conducted a meta-analysis to investigate the effect of glucocorticoids on reproductive outcomes in ATA-positive women undergoing ART.
2. Methods
2.1. Study search methodology
Up to December 31, 2020, the literature search was performed by PubMed, EMBASE, Cochrane Library, China National Knowledge Infrastructure (CNKI), WanFang database, Weipu China Science and Technology Journal Databases (VIP database). There is no language and time limitation imposed in the literature search. Literature was filtered utilizing combinations of the following key terms: “prednisone” or “prednisolone” or “corticosteroid” or “glucocorticoid” or “antithyroid autoimmunity” or “anti-thyroid antibodies” or “thyroperoxidase antibody” or “thyroglobulin antibodies” or “anti-thyroid antibodies” or“ thyroid autoimmune disease” or “IVF” or “ICSI” or “ART” or “assisted reproductive technology” or “infertility” or “infertility” or “pregnancy outcome” or “clinical outcome.” Relevant references were also manually retrieved to identify qualified references.
2.2. Study selection
The inclusion criterias were as follows:
-
(1)
prospective or retrospective studies with a control group;
-
(2)
the subjects were infertile women with TPO-Ab or TG-Ab positive without thyroid dysfunction following ART;
-
(3)
obtain the compared results of pregnancy outcomes between women treated with glucocorticoid and no glucocorticoid (or placebo);
-
(4)
outcome indicators include pregnancy rate, live birth rate, or abortion rate.
When studies from the same institution were searched, the one with more sample size and the most credible and complete information was enrolled. Besides, case reports, meeting abstracts, conference proceedings, repeated publications were also excluded.
2.3. Data extraction
The following data were extracted from the literature: first author, country, years of publication, study population, study method, study design, study period, infertility causes, ART type, thyroid autoantibody detection method, reference value, and intervention measures. The main outcome index is the clinical pregnancy rate. The secondary index is the live birth rate and abortion rate. Clinical pregnancy was defined as a pregnancy sac with a heartbeat observed by ultrasound. Miscarriage was defined as the loss of clinical pregnancy. The 3 included articles in this paper all adopted the Newcastle-Ottawa quality assessment scale (NOS) for quality assessment.
2.4. Statistical analysis
All statistical analyses were performed using RevMan Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Odds ratio (OR) and corresponding 95% confidence interval (CI) were used to describe the results. Heterogeneity was determined by I2 statistics, and the value less than 50% was considered as no significant heterogeneity. The random-effect model was employed to calculate the summary-effect estimates, and the significance level was set at P < .05. The study was approved by the ethics review committee of the First Affiliated Hospital of Chongqing Medical University.
3. Results
3.1. Search results
Figure 1 summarizes the process of literature retrieval and research selection. A total of 212 articles were retrieved in this study, of which 22 publications were obtained by manual retrieval. After removing duplicates and reviewing titles and abstracts, 67 relevant articles were selected. Among them, we excluded 42 studies without ART, 12 studies without glucocorticoid-treated, and 10 studies that were meta-analyses or reviews. Finally, only 3 articles published between 2000 and 2020 met inclusion and exclusion criteria, including 237 ATA-positive and 384 ATA-negative women. The basic information of the included studies is shown in Table 1, and the quality evaluation is illustrated in Table 2.
Figure 1.
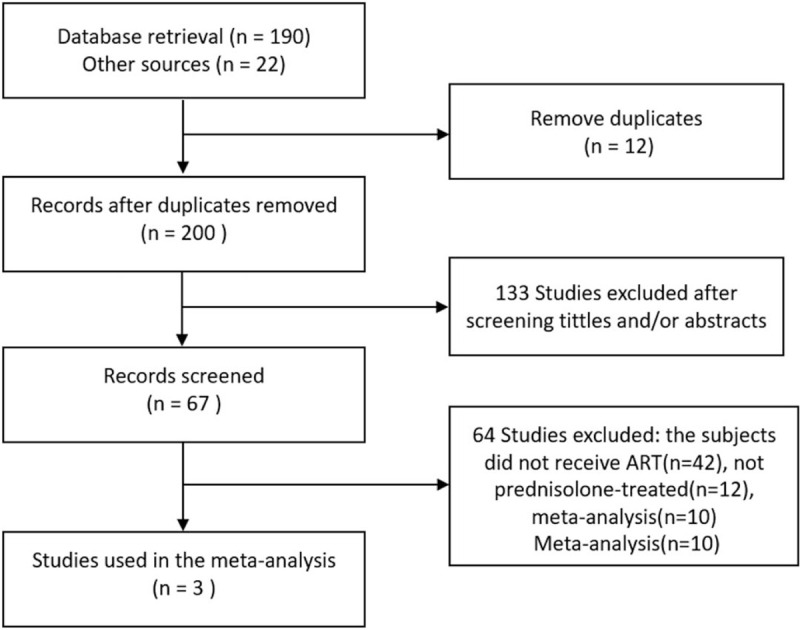
Flow chart of study selection.
Table 1.
Characteristics of the included studies.
| First author (year),Country (reference) | Revelli et al (2009) Italy[17] | Turi et al (2010) Italy[18] | Litwicka et al (2015) Italy[19] |
| Period | Between February 2004 and May 2008 | Between January 2006 and August 2008 | Between January 2011 and April 2012 |
| Patients | 129 ATA + infertile women undergoing IVF, 36 received prednisone treatment and 38 did not | 48 ATA + infertile women undergoing IUI, 24 received prednisone treatment and 24 received placebo control | 60 ATA + infertile women undergoing ICSI, 30 received prednisone treatment and 30 did not |
| Study design | Retrospective study | Prospective study | Prospective study |
| Causes of infertility | Pelvic endometriosis, reduced ovarian reserve, tubal disease, idiopathic infertility, male related infertility, PCO, hyperprolactinemia | Unclear | Tubal factor, male factor, idiopathic infertility |
| Conception method | IVF (“long” protocol) | IUI (ovulation induction) | ICSI (“long” protocol) |
| Assays used values for thyroid autoantibodies | Pharmacia Diagnostic commercial kits (Pharmacia, Sweden) using an immunofluorescence assay | TPO-Ab was determined using a radioimmunoassay kit (Brahms GmbH, Hennigsdorf, Germany). | TPO and TG antibodies were assayed using enzyme linked immunoassay kits (Pharmacia; Upjohn Diagnostics, Freiburg, Germany) |
| Cut-off values for thyroid autoantibodies | TgAb: 0–40 UI/mL, TPOAb: 0–35 UI/mL | TPOAb 0–100 U/mL | TPOAb 0–18 UI/mLTGAb 0–40 UI/mL |
| Intervention | The treated group received 10 mg P (P was increased to 30 mg/d for 5 d starting the day of ET, and subsequently returned to 10 mg/d until the day of hCG test) until 10 wk of gestational age. After this time, the option to continue the treatment was left to doctors taking care of the pregnancy. | Prednisone was administered orally at 10 mg/d in the first week, 5 mg/d in the second week, 2.5 mg/d in the third week, and 2.5 mg/d 3 times per week in the fourth week before IUI. The control group received a placebo. | prednisolone was started from the day of oocyte retrieval and continued until the day of the pregnancy test. In the case of a positive test, this regimen was continued during the first pregnancy trimester |
| Outcomes | PR AR OPR NOR | PR AR LBR | NOR PR AR LBR |
Table 2.
Quality assessment of included studies.

3.2. Age, TSH, and recovery number of oocytes
As illustrated in Figure 2, there were no significant differences in age (MD = −0.32, 95% confidence interval [95% CI] [−1.49, 0.85], I2 = 32%, P = .59) (Fig. 2A) and serum TSH (MD = −0.23, 95% CI [−0.69, 0.23], I2 = 82%, P = .32) (Fig. 2B) between the treatment group and the control group. Besides, the number of retrieved oocytes was slightly higher in the treatment group than the control group (MD = 2.26, 95% CI [−1.46, 5.99], I2 = 79%, P = .23) (Fig. 2C).
Figure 2.
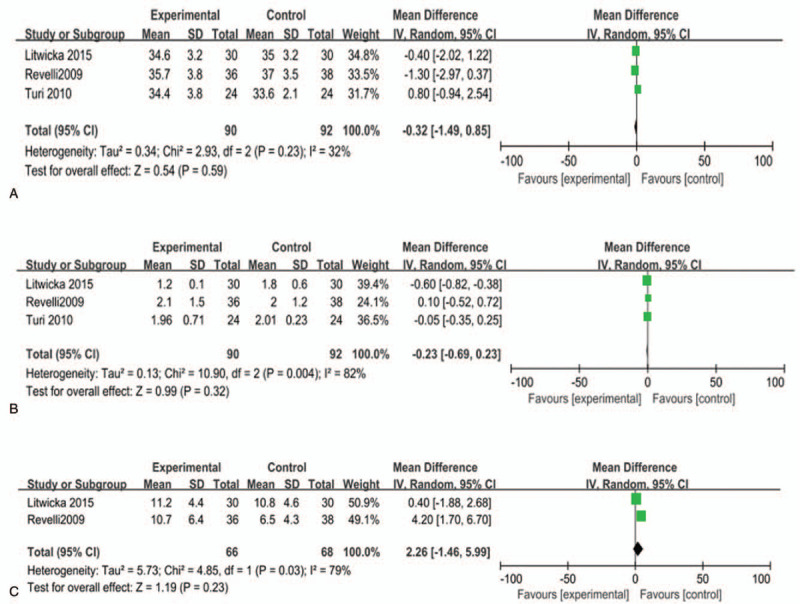
Relationship between glucocorticoid therapy and (A) age, (B) TSH, and (C) number of oocytes retrieved. P < .05 was considered statistically significant.
3.3. Ovarian response and embryo quality
In the study of Revelli et al,[17] it was found that the ovarian response to stimulation (Gn dose/oocyte) in the glucocorticoid-treated group was remarkably higher than that in the untreated group (448 ± 520 vs 1028 ± 1141). Another study of Litwicka[19] showed that the embryo quality in the glucocorticoid group was slightly higher than that in the untreated group (Table 3). Unfortunately, neither of these 2 studies was further explored; therefore, the effect of glucocorticoid on oocyte number and embryo therapy remains to be discussed.
Table 3.
Participant characteristics in the included studies.
| Author, year of publication | Age (yr, intervention vs controls) | TSH (mU/L, intervention vs controls) | Number of oocytes retrieved (intervention vs controls) | Gn dose/oocyte (IU, intervention vs controls) | Good quality embryos (intervention vs controls) |
| Litwicka 2015[19] | 34.6 ± 3.2 vs 35.0 ± 3.2 | 1.2 ± 0.1 vs 1.8 ± 0.6 | 11.2 ± 4.4 vs 10.8 ± 4.6 | Unstated | 1.0 ± 0.8 vs 0.8 ± 0.6 |
| Turi 2010[18] | 34.4 ± 3.8 vs 33.6 ± 2.1 | 1.96 ± 0.71 vs 2.01 ± 0.23 | Unstated | Unstated | Unstated |
| Revelli 2009[17] | 35.7 ± 3.8 vs 37.0 ± 3.5 | 2.1 ± 1.5 vs 2.0 ± 1.2 | 10.7 ± 6.4 vs 6.5 ± 4.3 | 448 ± 520 vs 1028 ± 1141 | Unstated |
3.4. Pregnancy rate
Three studies reported the effect of glucocorticoid supplementation on the pregnancy rate in infertile women with TAI undergoing ART.[17–19] The pregnancy rate was obviously increased in the glucocorticoid group compared with the control group (OR = 4.63, 95% CI [2.23, 9.58], I2 = 0.0%, P < .0001) (Fig. 3A).
Figure 3.
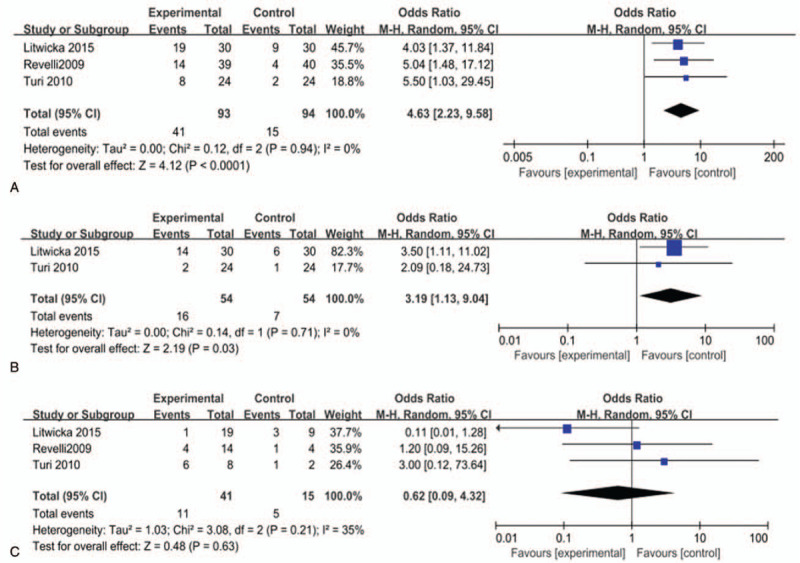
Effect of glucocorticoid treatment on (A) pregnancy rate, (B) live birth rate, and (C) miscarriage rate. P < .05 was considered statistically significant.
3.5. Live birth rate
Two studies investigated the effect of glucocorticoid supplementation on the live birth rate in infertile women with TAI undergoing ART.[18,19] The pooled effect revealed a significantly increased live births rate among women treated with glucocorticoid (OR = 3.19, 95% CI [1.13, 9.04], I2 = 0.0%, P = .03) (Fig. 3B).
3.6. Miscarriage rate
Three studies reported the roles of glucocorticoid supplementation on the abortion rate in infertile women with TAI undergoing ART.[17–19] Our meta-analysis revealed there was no significant decrease in the abortion pregnancy rate in the glucocorticoid group, compared with the control group (OR = 0.62, 95% CI [0.09, 4.32], I2 = 35%, P = .63) (Fig. 3C).
4. Discussion
In this review, we compared the effect of glucocorticoid on reproductive outcomes in infertile women with ATA positive and normal thyroid function undergoing ART. The administration of glucocorticoid improved pregnancy rate and live birth rate of women with ATA positive. Only one of the articles concluded that glucocorticoid reduced the abortion rate after in vitro fertilization (IVF) in women with TAI.[19] However, another 2 articles believed that the abortion rate of ATA-positive patients was not influenced by glucocorticoid therapy. Additionally, we found the number of retrieved oocytes varied indistinctively following the glucocorticoid treatment, but the ovarian stimulation response and embryo quality were slightly higher than those in control group. It is probably promising to improve the development and quality of embryos with glucocorticoid supplements. However, few studies have further discussed the treatment strategy, and more relevant studies are needed to address the issue in the future.
Several studies emphasized the importance of ATA in many aspects of embryogenesis and embryo implantation. The presence of ATA in ovarian follicles may cause the antibody-mediated cytotoxicity and the damage to the maturing oocyte, then reduce the quality of oocytes and developmental potential.[2,21,22] Besides, Zhong et al[12] speculated that TPO-Ab may interfere with fertilization and affect embryonic development by binding to the egg and/or embryonic surfaces. Meanwhile, the ATA in the endometrium may obstruct the embryo implantation, resulting in embryonic loss. Vissenberg et al[2] also found that TPO-Ab can diffuse through the placental barrier in late pregnancy.
It is well known that controlled ovarian stimulation in IVF can increase the demand for thyroid hormone in patients with TAI, which may change thyroid function and greatly increase the risk of subclinical hypothyroidism.[5,23] Besides, it has been demonstrated that increasing proportion of peripheral blood NK cells (PNK) and cytotoxicity are associated with infertility and IVF failure.[24] Brouillet et al[25] thought the failure of IVF was closely related to the high level of C-reactive protein before embryo implantation. Thus, a growing number of studies suggested that glucocorticoid may regulate the symptoms of thyroid autoimmune disorder, reduce the level of ATA and the number of natural killer cells (NK) which may be helpful for IVF outcomes. Additionally, glucocorticoid is also capable to improve the response of ovary, improve the quality of the embryo, and intrauterine environment through anti-inflammatory and immuno-suppressive treatment.[15,26,27,28]
There are still several shortcomings in this study. First, some factors of infertility such as endometriosis, premature ovarian failure are closely related to the presence of ATA, which need stratified research to explore the effect of glucocorticoid treatment.[29] Secondly, the potential interference factors of this study are not clear because the scheme of ART was not unified. One study reported a higher prevalence of ATA in infertile women with 3 or more IVF failures.[30] Another also reported that intracytoplasmic sperm injection (ICSI) could overcome the negative effect of TAI on embryo quality,[21] resulting in a higher implantation rate. Thirdly, the treatment effect of glucocorticoid is not limited to antithyroid antibodies. Other autoantibodies positive, such as antinuclear antibodies and anticardiolipin antibodies, can increase the incidence of repeated IVF-ET failure.[31] Then, due to the strict inclusion and exclusion criteria, the sample size is limited, and the research design is not rigorous enough. Therefore, further researches remain expected to determine the different effects of glucocorticoids on the types and titers of antibodies, the time and dosage of drug usage, as well as the potential complications and risks associated with glucocorticoids.
5. Conclusion
In conclusion, there was a particular association between thyroid autoantibodies and adverse pregnancy outcomes in ART. We found that glucocorticoid therapy increases pregnancy rate, but does not seem to reduce the risk of miscarriage. Further studies are expected to demonstrate whether glucocorticoid adjuvant therapy is potential to improve reproductive outcomes associated with ATA positive. It is hoped that this meta-analysis can play a positive role in clinical work, so as to remind us to carry out early screening and diagnosis for infertile women with ATA positive, and to better realize the management of patients in ART with thyroid autoimmune diseases for improving pregnancy outcomes.
Author contributions
Data curation: Guangqin Zhou.
Formal analysis: Guangqin Zhou.
Funding acquisition: Weihong Li.
Methodology: Meiying Zhou.
Software: Meiying Zhou.
Validation: Xuan Duan.
Writing – original draft: Guangqin Zhou.
Writing – review & editing: Xuan Duan.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ART = assisted reproductive technology, ATA = antithyroid antibody, CNKI = China National Knowledge Infrastructure, ICSI = intracytoplasmic sperm injection, IVF = in vitro fertilization, NOS = The Newcastle-Ottawa quality assessment scale, OR = odds ratio, TAI = thyroid autoimmune disease, Tg-Ab = thyroglobulin antibodies, TPO-Ab = thyroid peroxidase antibodies, VIP database = Weipu China Science and Technology Journal Databases.
How to cite this article: Zhou G, Zhou M, Duan X, Li W. Glucocorticoid supplementation improves reproductive outcomes in infertile women with antithyroid autoimmunity undergoing ART: A meta-analysis. Medicine. 2021;100:16(e25554).
The National Natural Science Foundation of China (Grant No. 81501335) and Medical Scientific Research Projects of Chongqing Municipal Health and Family Planning Commission (No. 2017MSXM015).
The authors have no conflicts of interest to disclose.
Authors can provide all of datasets analyzed during the study on reasonable request.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Alan P, Kristin B, Samantha B, et al. The role of immunotherapy in in vitro fertilization: a guideline. Fertil Steril 2018;110:387–400. [DOI] [PubMed] [Google Scholar]
- [2].Vissenberg R, Manders VD, Mastenbroek S, et al. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum Reprod Update 2015;21:378–87. [DOI] [PubMed] [Google Scholar]
- [3].Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489–99. [DOI] [PubMed] [Google Scholar]
- [4].Unuane D, Velkeniers B, Anckaert E, et al. Thyroglobulin autoantibodies: is there any added value in the detection of thyroid autoimmunity in women consulting for fertility treatment? Thyroid 2013;23:1022–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alexander EK, Pearce EN, Brent GA, et al. 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017;27:315–89. [DOI] [PubMed] [Google Scholar]
- [6].Carvalheiras G, Faria R, Braga J, et al. Fetal outcome in autoimmune diseases. Autoimmun Rev 2012;11:A520–30. [DOI] [PubMed] [Google Scholar]
- [7].Bussen S, Steck T, Dietl J. Increased prevalence of thyroid antibodies in euthyroid women with a history of recurrent in-vitro fertilization failure. Hum Reprod 2000;15:545–8. [DOI] [PubMed] [Google Scholar]
- [8].Bellver J, Soares SR, Alvarez C, et al. The role of thrombophilia and thyroid autoimmunity in unexplained infertility, implantation failure, and recurrent spontaneous abortion. Hum Reprod 2008;23:278–84. [DOI] [PubMed] [Google Scholar]
- [9].Kutteh WH, Schoolcraft WB, Scott RT. Antithyroid antibodies do not affect pregnancy outcome in women undergoing assisted reproduction. Hum Reprod 1999;14:2886–90. [DOI] [PubMed] [Google Scholar]
- [10].Chen X, Mo ML, Huang CY, et al. Association of serum autoantibodies with pregnancy outcome of patients undergoing first IVF/ICSI treatment: a prospective cohort study. J Reprod Immunol 2017;122:14–20. [DOI] [PubMed] [Google Scholar]
- [11].Poppe K, Autin C, Veltri F, et al. Thyroid autoimmunity and intracytoplasmic sperm injection outcome: a systematic review and meta-analysis. J Clin Endocrinol Metab 2018;103:1755–66. [DOI] [PubMed] [Google Scholar]
- [12].Zhong YP, Ying Y, Wu HT, et al. Relationship between antithyroid antibody and pregnancy outcome following in vitro fertilization and embryo transfer. Int J Med Sci 2012;9:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf) 2011;74:513–9. [DOI] [PubMed] [Google Scholar]
- [14].Kumru P, Erdogdu E, Arisoy R, et al. Effect of thyroid dysfunction and autoimmunity on pregnancy outcomes in low risk population. Arch Gynecol Obstet 2015;291:1047–54. [DOI] [PubMed] [Google Scholar]
- [15].Michael AE, Papageorghiou AT. Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Hum Reprod Update 2008;14:497–517. [DOI] [PubMed] [Google Scholar]
- [16].Suxian L, Ping C, Jianchang J, et al. Evaluation the effect of prednison in the treatment of antithyroid antibodies induced recurrent spontaneous abortion. China Mod Doctor 2016;54:92–4. [Google Scholar]
- [17].Revelli A, Casano S, Piane DL, et al. A retrospective study on IVF outcome in euthyroid patients with anti-thyroid antibodies: effects of levothyroxine, acetylsalicylic acid and prednisolone adjuvant treatments. Reprod Biol Endocrinol 2009;7:137.1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Turi A, Giannubilo SR, Zanconi S, et al. Preconception steroid treatment in infertile women with antithyroid autoimmunity undergoing ovarian stimulation and intrauterine insemination: a double-blind, randomized, prospective cohort study. Clin Therap 2010;32:2415–21. [DOI] [PubMed] [Google Scholar]
- [19].Litwicka K, Arrivi C, Varricchio MT, et al. In women with thyroid autoimmunity, does low-dose prednisolone administration, compared with no adjuvant therapy, improve in vitro fertilization clinical results? J Obstet Gynaecol Res 2015;41:722–8. [DOI] [PubMed] [Google Scholar]
- [20].Robertson SA, Jin M, Yu D, et al. Corticosteroid therapy in assisted reproduction – immune suppression is a faulty premise. Hum Reprod 2016;31:2164–73. [DOI] [PubMed] [Google Scholar]
- [21].Monteleone P, Parrini D, Faviana P, et al. Female infertility related to thyroid autoimmunity: the ovarian follicle hypothesis. Am J Reprod Immunol 2011;66:108–14. [DOI] [PubMed] [Google Scholar]
- [22].Andrisani A, Sabbadin C, Marin L, et al. The influence of thyroid autoimmunity on embryo quality in women undergoing assisted reproductive technology. Gynecol Endocrinol 2018;34:01–4. [DOI] [PubMed] [Google Scholar]
- [23].Poppe K, Unuane D, Miguel D’Haeseleer, et al. Thyroid function after controlled ovarian hyperstimulation in women with and without the hyperstimulation syndrome. Fertil Steril 2011;96:241–5. [DOI] [PubMed] [Google Scholar]
- [24].Karami N, Boroujerdnia MG, Nikbakht R, et al. Enhancement of peripheral blood CD56 dim cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J Reprod Immunol 2012;95:87–92. [DOI] [PubMed] [Google Scholar]
- [25].Brouillet S, Boursier G, Anav A, et al. C-reactive protein and ART outcomes: a systematic review. Hum Reprod Update 2020;26:753–73. [DOI] [PubMed] [Google Scholar]
- [26].Boomsma Carolien M, Keay Stephen D, Macklon Nick S. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst Rev 2012;CD005996.1-59. [DOI] [PubMed] [Google Scholar]
- [27].Hviid MM, Macklon N, et al. Immune modulation treatments-where is the evidence? Fertil Steril: official Journal of the American Fertility Society, Pacific Coast Fertility Society, and the Canadian Fertility and Andrology Society 2017;107:1284–93. [DOI] [PubMed] [Google Scholar]
- [28].Alhalabi M, Samawi S, Taha A, et al. Prednisolone improves implantation in ICSI patients with high peripheral CD69 + NK Cells. Hum Reprod 2011;26:i219. [Google Scholar]
- [29].Poppe K, Glinoer D, Van Steirteghem A, et al. Thyroid dysfunction and autoimmunity in infertile women. Thyroid 2002;12:997–1001. [DOI] [PubMed] [Google Scholar]
- [30].Bussen S, Steck T, Dietl J. Increased prevalence of thyroid antibodies in euthyroid women with a history of recurrent in vitro fertilization failure. Hum Reprod 2000;15:545–8. [DOI] [PubMed] [Google Scholar]
- [31].Geva E, Amit A, Liat-Lerner-Eva L, et al. Prednisone and aspirin improve pregnancy rate in patients with reproductive failure and autoimmune antibodies: a prospective study. Am J Reprod Immunol 2015;43:36–40. [DOI] [PubMed] [Google Scholar]


