PURPOSE
Genetic rearrangements of the tyrosine receptor kinase ROS proto-oncogene 1 (ROS1) are oncogenic drivers in non-small-cell lung cancer (NSCLC). We report the results of an updated integrated analysis of three phase I or II clinical trials (ALKA-372-001, STARTRK-1, and STARTRK-2) of the ROS1 tyrosine kinase inhibitor, entrectinib, in ROS1 fusion–positive NSCLC.
METHODS
The efficacy-evaluable population included adults with locally advanced or metastatic ROS1 fusion–positive NSCLC with or without CNS metastases who received entrectinib ≥ 600 mg orally once per day. Co-primary end points were objective response rate (ORR) assessed by blinded independent central review and duration of response (DoR). Secondary end points included progression-free survival (PFS), overall survival (OS), intracranial ORR, intracranial DoR, intracranial PFS, and safety.
RESULTS
In total, 161 patients with a follow-up of ≥ 6 months were evaluable. The median treatment duration was 10.7 months (IQR, 6.4-17.7). The ORR was 67.1% (n = 108, 95% CI, 59.3 to 74.3), and responses were durable (12-month DoR rate, 63%, median DoR 15.7 months). The 12-month PFS rate was 55% (median PFS 15.7 months), and the 12-month OS rate was 81% (median OS not estimable). In 24 patients with measurable baseline CNS metastases by blinded independent central review, the intracranial ORR was 79.2% (n = 19; 95% CI, 57.9 to 92.9), the median intracranial PFS was 12.0 months (95% CI, 6.2 to 19.3), and the median intracranial DoR was 12.9 months (12-month rate, 55%). The safety profile in this updated analysis was similar to that reported in the primary analysis, and no new safety signals were found.
CONCLUSION
Entrectinib continued to demonstrate a high level of clinical benefit for patients with ROS1 fusion–positive NSCLC, including patients with CNS metastases.
INTRODUCTION
Genetic rearrangements of the tyrosine receptor kinase ROS proto-oncogene 1 (ROS1) can result in constitutively active fusion proteins that act as oncogenic drivers.1,2 ROS1 fusions are found in 1%-2% of non-small-cell lung cancers (NSCLC) where it is considered a distinct disease subtype.1,3,4 Up to 40% of patients with ROS1 fusion–positive metastatic NSCLC have CNS metastases at diagnosis.5-8 Therefore, to maximize efficacy, novel targeted agents must demonstrate activity in the CNS.
CONTEXT
Key Objective
Were the efficacy and safety of entrectinib in patients with locally advanced or metastatic ROS proto-oncogene 1 (ROS1) fusion–positive non–small-cell lung cancer, including those with CNS metastases at baseline, confirmed in a larger population with a longer follow-up than previous reports?
Knowledge Generated
Entrectinib continued to have strong overall (objective response rate 67.1%; median duration of response 15.7 months) and intracranial (intracranial objective response rate 72.9% in patients with measurable baseline CNS metastases) efficacy in these patients. The safety profile was consistent with previous reports.
Relevance
Before the approval of entrectinib, there was an unmet need for a CNS-active treatment for patients with locally advanced or metastatic ROS1 fusion–positive non–small-cell lung cancer. As well as confirming the overall efficacy of entrectinib, our results provide strong evidence that entrectinib can treat existing CNS metastases and may have a potential CNS-protective effect in patients without CNS involvement at baseline. These data will help physicians to make more informed treatment decisions for their patients.
The tyrosine kinase inhibitor (TKI) crizotinib is approved as first-line therapy for metastatic ROS1 fusion–positive NSCLC. However, crizotinib has poor CNS penetration9,10 and is actively exported from the CNS by P-glycoprotein.11 Furthermore, almost half of patients with ROS1 fusion–positive NSCLC receiving crizotinib experience first progression solely in the CNS.7,8 The small-molecule TKI entrectinib is a potent inhibitor of ROS1, specifically designed to cross the blood-brain barrier and remain active within the CNS.12 Contrary to crizotinib, preclinical models confirmed that entrectinib is a weak P-glycoprotein substrate and therefore achieves high CNS concentrations, associated with strong efficacy in brain tumor models.11
The results from an integrated analysis of three prospective phase I or II clinical trials (ALKA-372-001, EudraCT 2012-000148-8; STARTRK-1, ClinicalTrials.gov identifier: NCT02097810; STARTRK-2, ClinicalTrials.gov identifier: NCT02568267) of entrectinib in patients with ROS1 fusion–positive NSCLC have been reported (data cutoff May 31, 2018).12 In the efficacy-evaluable population (n = 53), 77% (n = 41; 95% CI, 64 to 88) of patients responded to entrectinib, with a median duration of response (DoR) of 24.6 months (95% CI, 11.4 to 34.8). Among patients with baseline CNS disease (n = 20), the intracranial response rate was 55% (n = 11; 95% CI, 32 to 77) with a median intracranial DoR of 12.9 months (95% CI, 5.6 to not estimable [NE]). Entrectinib was well-tolerated with a manageable safety profile. Based on these positive findings, entrectinib was approved by the US Food and Drug Administration and the European Medicines Agency for patients with ROS1 fusion–positive NSCLC.
We report updated efficacy and safety data for entrectinib in patients with ROS1 fusion–positive NSCLC, based on an integrated analysis of ALKA-372-001, STARTRK-1, and STARTRK-2 with more patients and longer follow-up.
METHODS
Study Design and Patients
Full details of the study designs have been published previously (study protocols, available online).12 In brief, patients of age ≥ 18 years with locally advanced or metastatic NSCLC harboring ROS1 gene fusions were enrolled in one of two phase I studies (ALKA-372-001 or STARTRK-1) or a phase II global basket study (STARTRK-2). Patients enrolled before October 31, 2018, were included in the analysis to ensure that they had a follow-up of ≥ 6 months at the clinical cutoff date (May 1, 2019); patients who had discontinued the study or died before the 6-month follow-up were also included. Data for a cohort of 94 patients with ROS1 fusion–positive NSCLC enrolled in the same studies but with a follow-up of ≥ 12 months (enrollment cutoff November 30, 2017; data cutoff May 1, 2019) are presented in the Data Supplement, online only.
Enrolled patients had locally assessed measurable disease at baseline (RECIST version 1.1) and an Eastern Cooperative Oncology Group Performance Status 0-2. ROS1 gene fusions in ALKA-372-001 and STARTRK-1 were identified by local testing only, using fluorescence in situ hybridization, quantitative polymerase chain reaction, or DNA- or RNA-based next-generation sequencing. In STARTRK-2, patients enrolled via local testing were required to provide tumor tissue (unless biopsy was contraindicated) for independent next-generation sequencing. Patients with asymptomatic or pretreated and controlled CNS metastases were permitted.
All studies were conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. Written informed consent was obtained from all patients. Protocols for all studies were approved by relevant institutional review boards and/or ethics committees.
Treatment and Assessments
Patients received entrectinib orally until documented radiographic progression, unacceptable toxicity, or withdrawal of consent. Treatment could continue if the investigator decided that the patient continued to derive clinical benefit despite radiological disease progression. All 161 efficacy-evaluable patients received ≥ 1 dose of entrectinib ≥ 600 mg once a day; for six patients from the dose-escalation phase I studies, per-cycle dose intensity was below (n = 2) or above (n = 4) the intended 600 mg/day. Disease sites, including CNS metastases, were assessed by systematic imaging, via computed tomography or magnetic resonance imaging scanning, at screening, at end of cycle 1 (4 weeks), and every 8 weeks thereafter. Scans were evaluated by blinded independent central review (BICR) using RECIST version 1.1. Patients with investigator-assessed baseline CNS metastases had brain scans performed at every tumor assessment; CNS follow-up of patients without baseline CNS metastases was based on symptomatic progression or routine CNS scans where customary.
Safety was assessed by physical examination, laboratory tests, and adverse event (AE) monitoring. AEs were coded using Medical Dictionary for Regulatory Activities (version 14.0 or higher for individual studies; version 21.0 for integrated safety analysis) and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). If required, dose reductions could occur in decrements of 200 mg; a maximum of 2 dose reductions were allowed.
Outcomes
Co-primary end points were confirmed objective response rate (ORR) and BICR-assessed DoR. ORR was defined as the proportion of responders with a confirmed complete response (CR) or partial response (PR). DoR was measured from the date of first objective response to first documented radiographic disease progression or death because any cause.
Key secondary end points were progression-free survival (PFS) per BICR, overall survival (OS), and safety. PFS was defined as time from first dose of entrectinib to first documented radiographic disease progression or death from any cause. OS was defined as time from first dose of entrectinib to death from any cause. Additional prespecified secondary end points evaluated in patients with CNS metastases (measurable or nonmeasurable) at baseline per BICR were intracranial ORR, intracranial DoR, and intracranial PFS. Intracranial responders included patients with a CR or PR by BICR in CNS lesion(s) only. BICR-assessed intracranial DoR was measured from the date of first intracranial response to first documentation of radiographic CNS disease progression or date of death from any cause. Radiographic CNS disease progression was defined as occurrence of a new CNS lesion or progression of existing CNS lesions according to RECIST version 1.1; intracranial PFS was defined as the time from first dose of entrectinib to first documentation of radiographic CNS disease progression or death because of any cause.
Statistical Analysis
For primary and secondary outcomes, the integrated efficacy-evaluable population included patients with ROS1 fusion–positive NSCLC, who were ROS1 inhibitor–naïve, had measurable disease at baseline, received ≥ 1 dose of entrectinib, and had a follow-up of ≥ 6 months from onset of treatment. The ROS1-integrated safety-evaluable population included patients with ROS1 fusion–positive NSCLC who had received ≥ 1 dose of entrectinib, regardless of dose. The overall integrated safety-evaluable population included patients who had received ≥ 1 dose of entrectinib while enrolled in ALKA-372-001, STARTRK-1, STARTRK-2, or STARTRK-NG (ClinicalTrials.gov identifier: NCT02650401; ongoing pediatric phase I and II study).13
For the integrated analysis, assuming that the proportion of patients achieving an objective response by BICR was 70%, a sample size of ≥ 50 patients would yield a two-sided 95% CI with a precision of ≥ 17% (excluding a lower limit of 50% as observed with standard-of-care ROS1 fusion–positive NSCLC treatment, as determined in consultation with the US Food and Drug Administration). A response rate of ≥ 50% was considered clinically meaningful. Patient demographic and safety data were summarized descriptively. For response data, the number, percentage, and corresponding two-sided 95% Clopper–Pearson exact CIs were summarized. There was no formal hypothesis testing, and significance tests were not performed; there was no alpha spending for ORR or DoR end points. The Kaplan–Meier method was used to estimate time-to-event end points (DoR, PFS, and OS), with corresponding 95% CIs. Statistical evaluation was performed using the SAS software package (version 9.3 or higher).
RESULTS
Patients
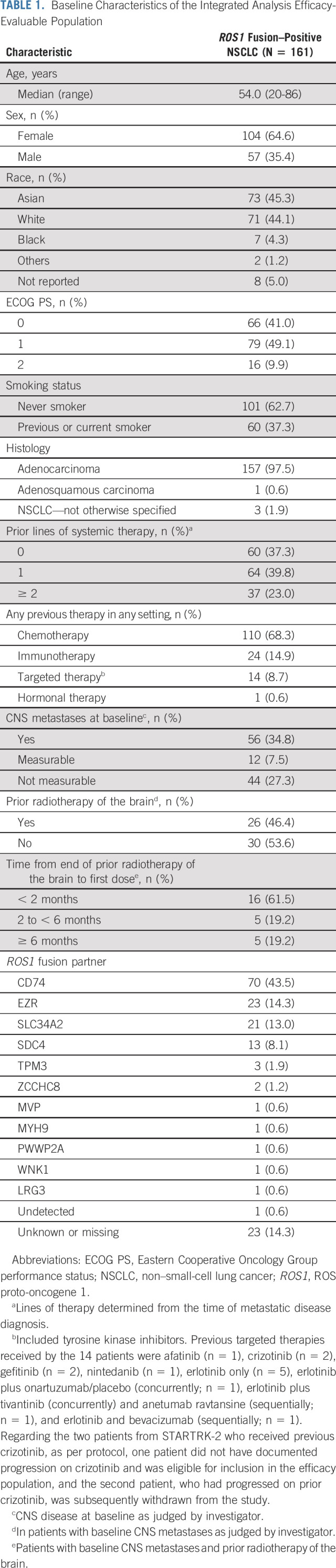
The integrated efficacy-evaluable population comprised 161 patients with a follow-up of ≥ 6 months: 145 were enrolled in STARTRK-2, 7 in STARTRK-1, and 9 in ALKA-372-001 (Data Supplement, online only). The median duration of follow-up was 15.8 months (IQR, 10.4-22.9). Patient baseline demographics or disease characteristics are summarized in Table 1. In total, 62.7% of patients had received ≥ 1 prior line of systemic therapy for metastatic disease. Baseline CNS lesions were present in 34.8% of patients (n = 56; measurable, n = 12) as assessed by investigator. Of these, 46.4% (n = 26) had completed prior brain radiotherapy. Type of radiotherapy received was available for 18 patients: 12 had whole brain radiotherapy; six had stereotactic radiotherapy only.
TABLE 1.
Baseline Characteristics of the Integrated Analysis Efficacy-Evaluable Population
Efficacy
Overall Efficacy.
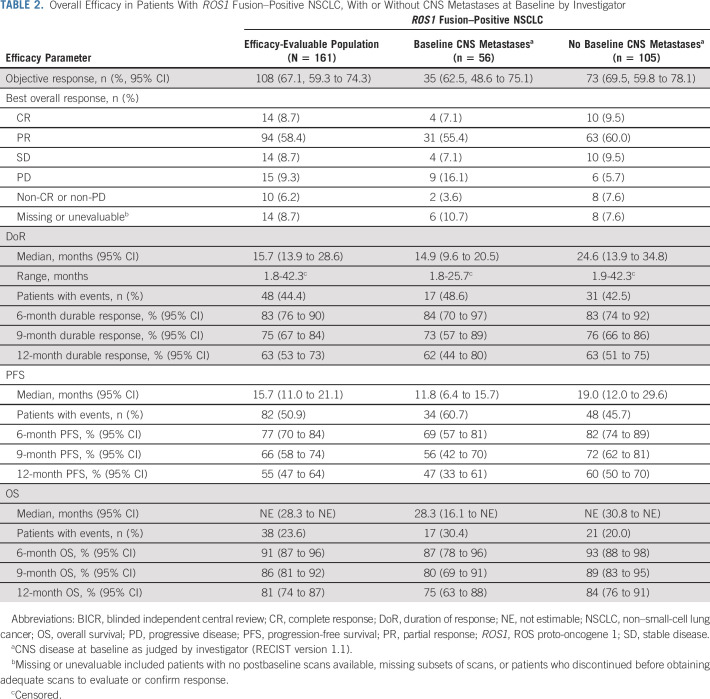
In the integrated efficacy-evaluable population (n = 161), the median duration of treatment was 10.7 months (IQR, 6.4-17.7). The majority of patients experienced a reduction in the size of the target lesions, as assessed by BICR (Fig 1A), and a confirmed objective response was achieved in 108 patients (67.1%, 95% CI, 59.3 to 74.3; Table 2): 14 CRs (8.7%) and 94 PRs (58.4%). A further 14 (8.7%) patients had stable disease. Responses occurred early (median time to response 0.95 months, range, 0.7-26.6 months), with most reported at the first follow-up imaging assessment (Data Supplement). The median time to first progressive disease or death (n = 23) was 2.8 months (range, 0.4-21.1 months; Data Supplement). Key baseline characteristics of responders and nonresponders are summarized in the Data Supplement.
FIG 1.
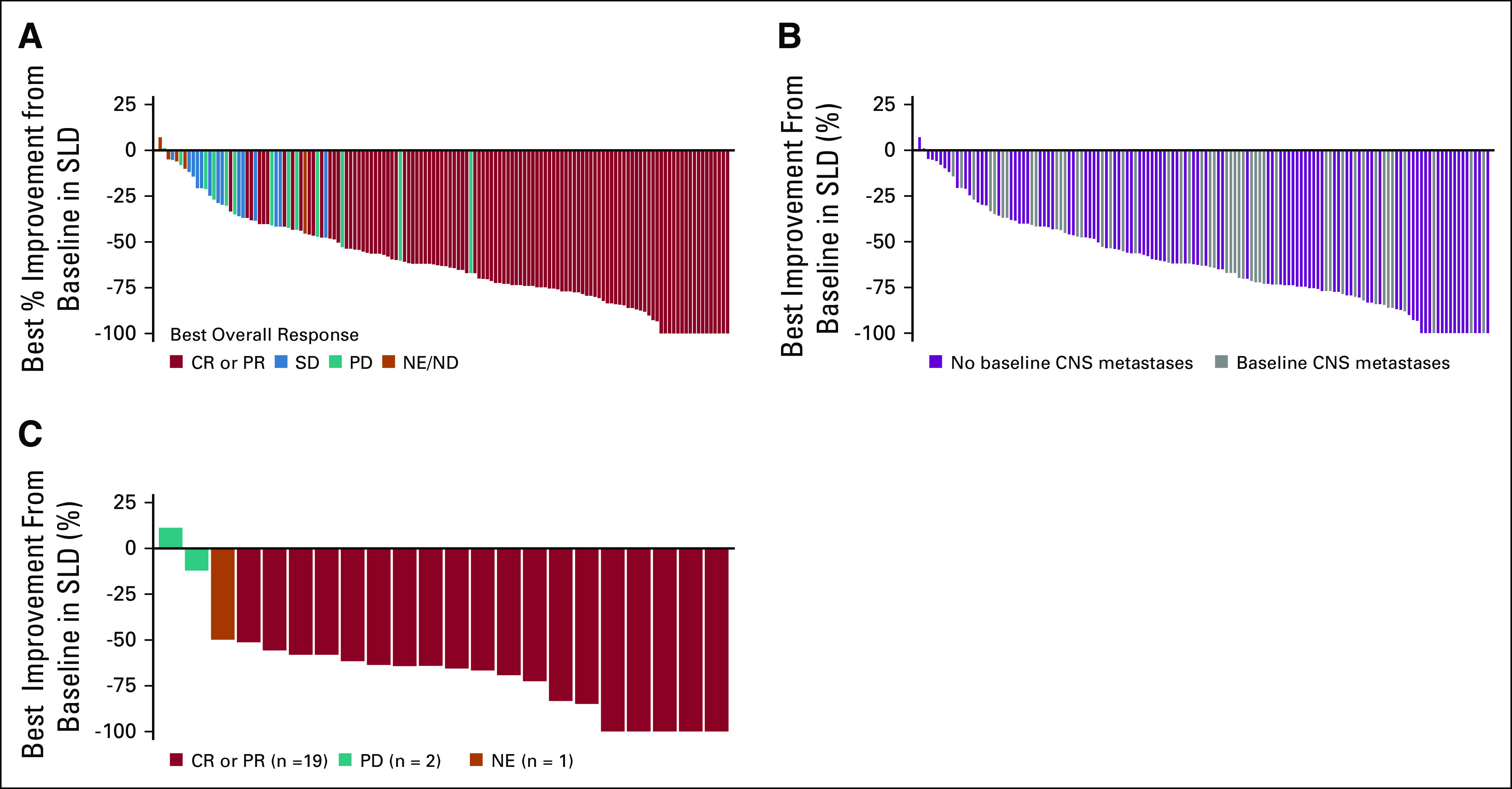
Waterfall plots of best response to entrectinib in patients with ROS proto-oncogene 1 (ROS1) fusion–positive lung cancers, shown as the maximum percent improvement in the sum of longest diameters of identified target lesions compared with baseline, as assessed by BICR: (A) the efficacy-evaluable population (patients without measurable disease were excluded); (B) patients with and without CNS metastases at baseline (investigator assessed); and (C) best intracranial responses in patients with measurable CNS metastases at baseline (BICR assessed). BICR, blinded independent central review; CR, complete response; ND, not determined; NE, not estimable; PD, progressive disease; PR, partial response; SD, stable disease; SLD, sum of longest diameter.
TABLE 2.
Overall Efficacy in Patients With ROS1 Fusion–Positive NSCLC, With or Without CNS Metastases at Baseline by Investigator
Responses occurred across fusion partners, with the highest observed for CD74 (72.9%; 95% CI, 60.9 to 82.8) and the lowest for SLC34A2 (57.1%; 95% CI, 34.0 to 78.2) (Data Supplement). ORR was consistent regardless of CNS disease status at baseline (as judged by investigator; Fig 1B), with an ORR of 62.5% in patients with CNS metastases and 69.5% in patients without CNS metastases (Table 2). Equivalent data for patients with and without baseline CNS metastases judged by BICR are shown in the Data Supplement.
Among all responders, the 12-month DoR rate was 63% (median DoR 15.7 months, 95% CI, 13.9 to 28.6) (Table 2; Fig 2A) and was similar between patients with and without CNS metastases at baseline. At the cutoff date, 48 (44.4%) responders had experienced an event (disease progression, n = 36; death, n = 12). In the overall population, PFS was maintained for at least 12 months in 55% of patients (Table 2; Fig 2B), with 82 (50.9%) patients experiencing an event by the cutoff date. The median PFS was 15.7 months (95% CI, 11.0 to 21.1). In the overall population, median time to CNS progression (exploratory end point; only radiologically confirmed CNS progression counted as an event) was NE (Fig 2C). Few patients without baseline CNS metastases had reported CNS progression confirmed by scans (n = 3/105; 2.9%), and 27 of 56 patients (48.2%) with baseline CNS metastases had CNS progression. OS remains immature with a 12-month OS rate of 81% (Table 2; Fig 2D); 38 (23.6%) patients died during follow-up (median OS was NE).
FIG 2.
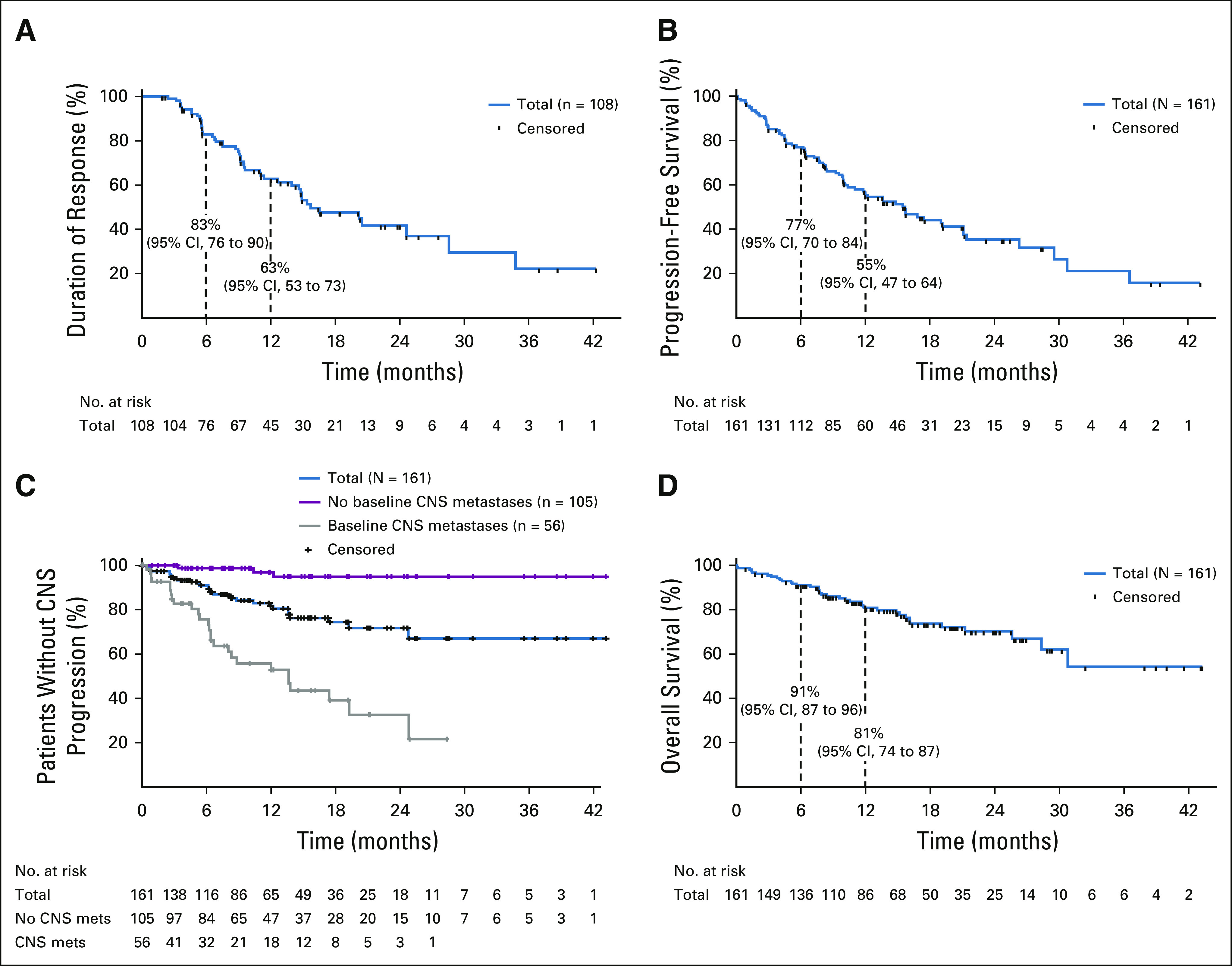
Time-to-event analyses for (A) duration of response; (B) progression-free survival; (C) time to CNS progression (deaths censored) in all patients (blue) and in patients with (gray) or without (red) baseline CNS metastases by investigator assessment; and (D) overall survival, in patients with ROS1 fusion–positive non–small-cell lung cancer (N = 161). For patients without CNS metastases at baseline, CNS progressive disease was detected either through scans triggered by symptoms suggestive of CNS disease or scans performed routinely at the investigator's discretion, since brain scans were not mandated by the protocol.
A cohort of 94 efficacy-evaluable patients with an extended follow-up of ≥ 12 months (enrollment cutoff: November 30, 2017; data cutoff: May 1, 2019) showed consistent findings: the ORR was 73.4% (95% CI, 63.3 to 82.0); the median DoR was 16.5 months (95% CI, 14.6 to 28.6); and the median PFS was 16.8 months (95% CI, 12.0 to 21.4; Data Supplement).
Intracranial Efficacy.
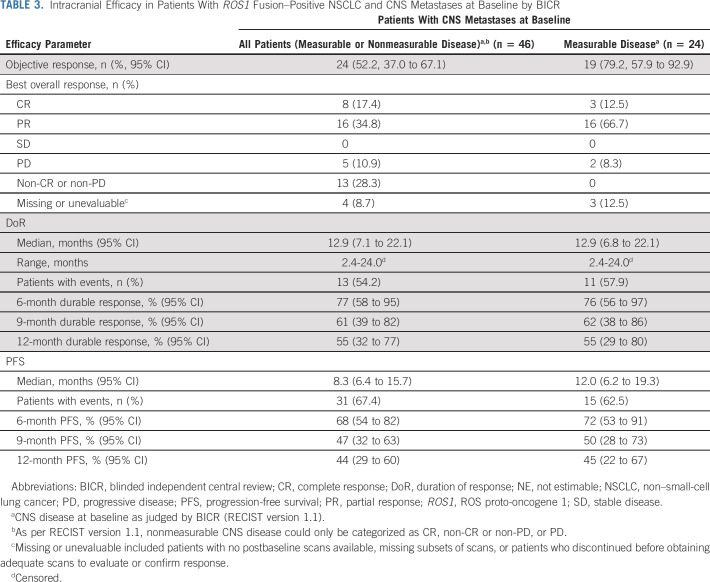
Following BICR of scans from patients with CNS disease at baseline by investigator assessment, CNS metastases were confirmed in 46 patients; of these, 24 had measurable disease. The intracranial ORR in all patients with baseline CNS metastases (measurable or nonmeasurable) was 52.2% (n = 24; 95% CI, 37.0 to 67.1), including eight patients (17.4%) with an intracranial CR. In the 24 patients with measurable CNS metastases at baseline, the intracranial ORR was 79.2% (n = 19, 95% CI, 57.9 to 92.9; Table 3; Fig 1C), including three patients (12.5%) with an intracranial CR. Among 24 responders with measurable or nonmeasurable CNS metastases, the median time to intracranial response was 0.95 months (range, 0.7-6.4); responses were durable, with a 12-month intracranial DoR rate of 55.0% (Table 3). The median (95% CI) intracranial PFS was 8.3 months (6.4 to 15.7) in patients with measurable or nonmeasurable CNS metastases; 31 patients (67.4%) experienced an event (disease progression, n = 24; death, n = 7). Intracranial PFS was maintained for at least 12 months in 44% of patients with measurable or nonmeasurable CNS metastases (Table 3). The intracranial ORR was 46.2% (95% CI, 26.6 to 66.6) among patients who had not received radiotherapy of the brain or had received brain radiotherapy at least 6 months before commencing entrectinib and 60.0% (95% CI, 36.1 to 80.9) among those who had received brain radiotherapy within the last 6 months (Data Supplement).
TABLE 3.
Intracranial Efficacy in Patients With ROS1 Fusion–Positive NSCLC and CNS Metastases at Baseline by BICR
Safety
The safety data cutoff for the ROS1 fusion–positive NSCLC population (October 31, 2018) was aligned to match that of the NTRK fusion–positive cohort (Data Supplement). Data for the overall safety-evaluable population regardless of gene fusion (N = 504) are presented in the Data Supplement. The ROS1 fusion–positive safety-evaluable population comprised 210 patients who had received ≥ 1 dose of entrectinib, regardless of dose. The median duration of treatment was 7.4 months (IQR, 3.6-14.1 months). Most patients (n = 208; 99.0%) experienced an AE. Treatment-related AEs (TRAEs) of any grade were reported by 196 patients (93.3%); the most frequent TRAEs are summarized by grade in Table 4. Nearly all of the TRAEs were grades 1-2, with the most frequent being dysgeusia (n = 90; 42.9%), dizziness (n = 72; 34.3%), and constipation (n = 66; 31.4%). The most common grade 3 TRAEs were weight increase (n = 17; 8.1%), ALT increase (n = 7; 3.3%), and diarrhea (n = 6; 2.9%). Seven patients (3.3%) experienced grade 4 TRAEs (hyperuricemia, n = 2; hypertriglyceridemia; limbic encephalitis; blood creatine phosphokinase myocardial band increased; anorectal disorder; myocarditis; all n = 1); there were no grade 5 TRAEs.
TABLE 4.
Treatment-Related Adverse Events by Grade in Patients With ROS1 Fusion–Positive NSCLC
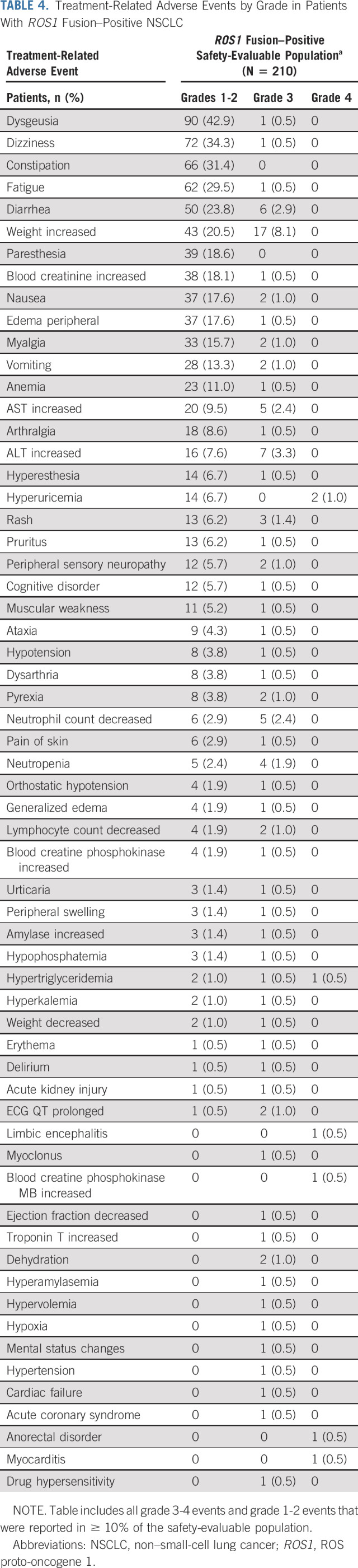
Serious AEs (SAEs) related to treatment were uncommon, affecting 23 (11.0%) patients (Data Supplement). The most common SAEs included pyrexia (n = 3; 1.4%), cognitive disorder (n = 2; 1.0%), and vomiting (n = 2; 1.0%). Sixteen (7.6%) patients discontinued study treatment because of AEs, with the most common events related to respiratory, thoracic, and mediastinal disorders (n = 4; 1.9%). TRAEs led to an entrectinib dose reduction in 61 (29.0%) patients, dose interruption in 64 (30.5%) patients, and discontinuation in 9 (4.3%) patients. The median dose intensity over the full treatment duration was 94.9% (IQR, 67.9-100).
DISCUSSION
This updated integrated analysis represents the largest prospective trial in patients with locally advanced or metastatic ROS1 fusion–positive NSCLC and supports the strong activity of entrectinib, both overall and within the CNS. A high proportion of enrolled patients were pretreated (62.7% had received ≥ 1 prior line of systemic therapy) and 34.8% had CNS metastases at baseline (per investigator), highlighting the relatively poor prognostic population included in the study. The overall confirmed ORR was 67.1%; most responses occurred by the first assessment at week 4 and were durable (12-month DoR rate, 63%). Moreover, a high proportion of patients with NSCLC present with CNS metastases,5-8 and outcomes for this subpopulation are consistently poorer than those for patients without CNS involvement.14 Our analysis shows that entrectinib achieves high response rates in patients with CNS metastases at baseline, with an overall ORR of 62.5% that was also durable (12-month DoR rate, 62%). Additionally, among all patients (N = 161), median time to CNS progression was NE, with only six patients developing their first lesions in the CNS. For patients without CNS metastases at baseline, brain scans were not mandated during treatment but were done as clinically indicated. CNS progressive disease in these patients could thus only be detected through routine scans performed at the investigator's discretion or through scans triggered by symptoms suggestive of CNS disease. Although data for this exploratory end point must be interpreted with caution, they are suggestive of a potential CNS-protective effect of entrectinib.
Entrectinib further induced an impressive intracranial ORR of 52.2% (n = 24, including eight CR) that was durable (12-month DoR rate, 55%) in these patients. The intracranial ORR was 79.2% in patients with measurable CNS metastases. Analysis of intracranial efficacy in the subcohort of patients who had not received radiotherapy of the brain or had received brain radiotherapy ≥ 6 months before commencing entrectinib showed an ORR of 46.2%, confirming that intracranial efficacy was not due to residual effects of radiotherapy on brain lesion size. We believe that the intracranial benefit shown in this integrated analysis is of particular clinical significance, given that robust evidence of intracranial activity with other available ROS1 inhibitors is limited (ceritinib: 25%, n = 2/815 and lorlatinib: 64%, n = 7/1116), and up to 40% of patients with ROS1 fusion–positive metastatic NSCLC present with CNS disease at baseline.7
The overall ORR for entrectinib in our analysis (67.1%) was consistent with that of other ROS1 inhibitors: crizotinib (72%, n = 38/53, PROFILE 1001 study17; 71.7%, n = 91/127, OxOnc study6), lorlatinib (62%, n = 13/2116), and ceritinib (62%, n = 20/3215) in TKI-naïve patients with ROS1 fusion–positive NSCLC. However, cross-trial comparisons should be considered with caution given the inherent differences between study populations.
The safety profile of entrectinib in this updated analysis was similar to that from the primary analysis.12 Most AEs were of low grade, manageable, and comparable with other ROS1 inhibitors,6,15-17 although ceritinib has been associated with high levels of GI toxicity.15,18 The rate of SAEs remained the same as in the primary analysis (11%),12 and no new safety signals were identified. Low rates of entrectinib discontinuation were observed and the high dose intensity indicates that any dose modifications had minimal impact on overall exposure, with most patients receiving the full planned dose.
Limitations of this study include a relatively small sample size and the single-arm design. Furthermore, there was no mandatory requirement for postprogression tissue collection and the profile of acquired resistance to entrectinib is yet to be characterized.
National Comprehensive Cancer Network guidelines recommend crizotinib or entrectinib as preferred initial TKIs and lorlatinib upon progression.19 In patients previously treated with crizotinib, around one third will respond to lorlatinib16; however, entrectinib is ineffective against the most frequently reported crizotinib-resistance mutation ROS1 G2032R.8,20 Considering the high risk of CNS metastases in NSCLC, entrectinib is well placed as a first-choice TKI rather than after progression on another TKI. Our data provide further confirmation that entrectinib is a CNS-penetrant compound, with intracranial efficacy in patients with ROS1 fusion–positive NSCLC with baseline CNS metastases, and limits the risk of CNS progression in those without baseline CNS metastases.
In conclusion, in this updated analysis, comprising more patients and a longer follow-up than the primary analysis, entrectinib continued to demonstrate a high level of clinical benefit for patients with ROS1 fusion–positive NSCLC, including those with CNS metastases at baseline.
ACKNOWLEDGMENT
The authors thank the patients, their families, and the participating study centers. We also acknowledge the valuable contribution of Harald Zeuner to this manuscript. Third-party medical writing assistance, under the direction of the authors, was provided by Lewis Cawkwell, PhD, of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd. Matthew Krebs acknowledges support from National Institute for Health Research (NIHR) Manchester Biomedical Research Centre and NIHR Manchester Clinical Research Facility at The Christie and Manchester Experimental Cancer Medicine Centre (Manchester, United Kingdom).
Rafal Dziadziuszko
Honoraria: Roche/Genentech, Novartis, Pfizer, Bristol-Myers Squibb, Takeda, AstraZeneca, MSD Oncology, Boehringer Ingelheim, Seattle Genetics
Travel, Accommodations, Expenses: Roche, AstraZeneca
Matthew G. Krebs
Honoraria: Roche
Consulting or Advisory Role: Roche, Achilles Therapeutics, Janssen, Seattle Genetics, OM Pharmaceutical Industries, Bayer
Speakers' Bureau: Roche
Research Funding: Roche
Travel, Accommodations, Expenses: AstraZeneca, BerGenBio, Immutep
Filippo De Braud
Honoraria: Roche, Pfizer, Bristol-Myers Squibb, Merck, MSD, Servier, Sanofi, Roche
Consulting or Advisory Role: Incyte, Teofarma, EMD Serono, Bristol-Myers Squibb, Nerviano Medical Sciences, Sanofi, Novartis Italy
Research Funding: Novartis, Roche, Merck Serono, Pfizer, Servier, Philogen, Loxo, Tesaro, Nerviano Medical Sciences, Kymab
Salvatore Siena
Stock and Other Ownership Interests: Guardant Health, Myriad Genetics
Consulting or Advisory Role: Amgen, Roche/Genentech, Bayer, Bristol-Myers Squibb, Clovis Oncology, Daiichi Sankyo, Incyte, Merck, Novartis, Seattle Genetics, CheckmAb
Patents, Royalties, Other Intellectual Property: Amgen
Travel, Accommodations, Expenses: Amgen, Bayer, Roche
Alexander Drilon
Honoraria: Medscape, OncLive, PeerVoice, Physicans' Education Resource, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, Peerview
Consulting or Advisory Role: Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Verastem, Takeda/Millennium, BerGenBio, MORE Health, Lilly, Abbvie, 14ner/Elevation Oncology, Remedica, Archer, Monopteros Therapeutics, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Melendi, Repare Therapeutics
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology
Robert C. Doebele
Employment: Rain Therapeutics
Leadership: Rain Therapeutics
Stock and Other Ownership Interests: Rain Therapeutics
Consulting or Advisory Role: GreenPeptide, AstraZeneca, Roche/Genentech, Bayer, Takeda, Rain Therapeutics, Anchiano, Blueprint Medicines, Foundation Medicine
Patents, Royalties, Other Intellectual Property: Licensing Fees from Abbott Molecular for Patent PCT/US2013/057495, Licensing fees for biologic materials from Genentech, Licensing fees for patent from Rain Therapeutics, Licensing fees for biologic materials from Foundation Medicine, Licensing fees for biologic materials from Black Diamond, Licensing fees for biologic materials from Pearl River, licensing fees for biologic materials from Voronoi
Travel, Accommodations, Expenses: Rain Therapeutics, Roche/Genentech
Manish R. Patel
Consulting or Advisory Role: Nektar
Research Funding: Merck KGaA, AstraZeneca, Fate Therapeutics, Vyriad
Byoung Chul Cho
Leadership: Gencurix, Interpark Bio
Stock and Other Ownership Interests: Theravance, Gencurix, Bridgebio, Kanaph Therapeutics, Cyrus Therapeutics, Interpark Bio
Consulting or Advisory Role: Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Yuhan, Pfizer, Janssen, Takeda, MSD, Ono Pharmaceutical, Lilly, Medpacto, Blueprint Medicines, Kanaph Therapeutics, Bridgebio, Cyrus Therapeutics, Guardant Health, Joseah BIO
Research Funding: Novartis, Bayer, AstraZeneca, Mogam Biotechnology Research Institue, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, MSD, Abbvie, Medpacto, GI Innovation, Lilly, Blueprint Medicines, Interpark Bio
Patents, Royalties, Other Intellectual Property: Champions Oncology
Other Relationship: DAAN Biotherapeutics
Stephen V. Liu
Consulting or Advisory Role: Genentech, Pfizer, Lilly, Bristol-Myers Squibb, AstraZeneca, Takeda, Regeneron, Apollomics, G1 Therapeutics, Guardant Health, Janssen Oncology, Roche, MSD Oncology, Jazz Pharmaceuticals, Blueprint Medicines, Inivata, PharmaMar, Daiichi Sankyo/UCB Japan, BeiGene
Research Funding: Genentech/Roche, Pfizer, Corvus Pharmaceuticals, Bayer, Merck, Lycera, AstraZeneca, Molecular Partners, Blueprint Medicines, Lilly, Rain Therapeutics, Alkermes, Bristol-Myers Squibb, Turning Point Therapeutics, RAPT, Spectrum Pharmaceuticals
Travel, Accommodations, Expenses: AstraZeneca, Roche/Genentech, MSD Oncology
Myung-Ju Ahn
Honoraria: AstraZeneca, Lilly, MSD, Takeda
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Lilly, Takeda, Alpha Pharmaceutical
Chao-Hua Chiu
Honoraria: AstraZeneca/MedImmune, Boehringer Ingelheim, Roche, Novartis, Chugai Pharma, Bristol-Myers Squibb, Ono Pharmaceutical, MSD, Lilly, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Ono Pharmaceutical
Consulting or Advisory Role: AstraZeneca/MedImmune, MSD, Novartis, Chugai Pharma, Lilly
Speakers' Bureau: AstraZeneca, Boehringer Ingelheimm, Roche, Novartis, Chugai Pharma, Bristol-Myers Squibb, MSD
Anna F. Farago
Employment: Novartis
Honoraria: Clinical Care Options, Medscape, Peerview, Research to Practice
Consulting or Advisory Role: Abbvie, Loxo, Genentech/Roche, Bayer, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Merck, Pfizer, H3 Biomedicine
Research Funding: PharmaMar, Abbvie, AstraZeneca, Bristol-Myers Squibb, Merck, Loxo, Ignyta, Amgen, Genentech/Roche, Bayer, Lilly
Travel, Accommodations, Expenses: Abbvie, Bayer, Loxo, Genentech/Roche, AstraZeneca, Boehringer Ingelheim, Merck, Bristol-Myers Squibb, Peerview, Medscape
Chia-Chi Lin
Honoraria: Novartis, Roche, Daiichi Sankyo, Lilly
Consulting or Advisory Role: Novartis, Boehringer Ingelheim, Blueprint Medicines, Daiichi Sankyo
Travel, Accommodations, Expenses: Lilly, Daiichi Sankyo, BeiGene, Novartis, Daiichi Sankyo
Christos S. Karapetis
Honoraria: Roche, Servier
Consulting or Advisory Role: AstraZeneca, Bristol-Myers Squibb, Roche, Eisai
Travel, Accommodations, Expenses: AstraZeneca
Yu-Chung Li
Honoraria: Bayer, AstraZeneca, Takeda, Novartis, Lilly, MSD, Roche
Research Funding: Roche
Travel, Accommodations, Expenses: MundiPharma, Taiho Pharmaceutical, MSD, Roche, Eisai, Boehringer Ingelheim, Sanomics Limited
Bann-mo Day
Employment: Genentech
Stock and Other Ownership Interests: Genentech
Research Funding: Genentech
Travel, Accommodations, Expenses: Genentech
David Chen
Employment: Genentech, Amgen
Stock and Other Ownership Interests: Genentech, Amgen
Travel, Accommodations, Expenses: Genentech, Amgen
Timothy R. Wilson
Employment: Genentech
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: Methods of treatment with Taselisib
Fabrice Barlesi
Honoraria: Genentech/Roche, Pfizer, Pierre Fabre, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Lilly, Novartis, Pierre Fabre, Merck Serono, MSD Oncology, Takeda, Bayer
Consulting or Advisory Role: Roche/Genentech, Pfizer, Novartis, Pierre Fabre, Bristol-Myers Squibb, AstraZeneca/MedImmune, Boehringer Ingelheim, Lilly, Merck Serono, MSD Oncology, Takeda, Bayer
Research Funding: Roche/Genentech, AstraZeneca/MedImmune, Bristol-Myers Squibb, Pierre Fabre, Abbvie, Amgen, Bayer, Boehringer Ingelheim, Eisai, Lilly, Ipsen, Innate Pharma, Novartis, Merck Serono, MSD Oncology, Pfizer, Sanofi/Aventis, Takeda
Patents, Royalties, Other Intellectual Property: Roche/Genentech, Bristol-Myers Squibb, AstraZeneca/MedImmune, MSD Oncology
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2020 ESMO Virtual Congress, September 19-21, 2020.
SUPPORT
Supported by F. Hoffmann-La Roche Ltd.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
AUTHOR CONTRIBUTIONS
Conception and design: Rafal Dziadziuszko, Salvatore Siena, Alexander Drilon, Robert C. Doebele, Byoung Chul Cho, Fabrice Barlesi
Provision of study materials or patients: Rafal Dziadziuszko, Matthew G. Krebs, Filippo De Braud, Salvatore Siena, Alexander Drilon, Robert C. Doebele, Byoung Chul Cho, Stephen V. Liu, Myung-Ju Ahn, Chao-Hua Chiu, Christos S. Karapetis
Collection and assembly of data: Rafal Dziadziuszko, Matthew G. Krebs, Salvatore Siena, Alexander Drilon, Robert C. Doebele, Stephen V. Liu, Chao-Hua Chiu, Anna F. Farago, Chia-Chi Lin, Christos S. Karapetis, Yu-Chung Li, Bann-mo Day, Timothy R. Wilson, Fabrice Barlesi
Data analysis and interpretation: Rafal Dziadziuszko, Matthew G. Krebs, Filippo De Braud, Salvatore Siena, Alexander Drilon, Robert C. Doebele, Manish R. Patel, Byoung Chul Cho, Stephen V. Liu, Myung-Ju Ahn, Chao-Hua Chiu, Christos S. Karapetis, Bann-mo Day, David Chen, Timothy R. Wilson, Fabrice Barlesi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Locally Advanced or Metastatic ROS1 Fusion–Positive Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rafal Dziadziuszko
Honoraria: Roche/Genentech, Novartis, Pfizer, Bristol-Myers Squibb, Takeda, AstraZeneca, MSD Oncology, Boehringer Ingelheim, Seattle Genetics
Travel, Accommodations, Expenses: Roche, AstraZeneca
Matthew G. Krebs
Honoraria: Roche
Consulting or Advisory Role: Roche, Achilles Therapeutics, Janssen, Seattle Genetics, OM Pharmaceutical Industries, Bayer
Speakers' Bureau: Roche
Research Funding: Roche
Travel, Accommodations, Expenses: AstraZeneca, BerGenBio, Immutep
Filippo De Braud
Honoraria: Roche, Pfizer, Bristol-Myers Squibb, Merck, MSD, Servier, Sanofi, Roche
Consulting or Advisory Role: Incyte, Teofarma, EMD Serono, Bristol-Myers Squibb, Nerviano Medical Sciences, Sanofi, Novartis Italy
Research Funding: Novartis, Roche, Merck Serono, Pfizer, Servier, Philogen, Loxo, Tesaro, Nerviano Medical Sciences, Kymab
Salvatore Siena
Stock and Other Ownership Interests: Guardant Health, Myriad Genetics
Consulting or Advisory Role: Amgen, Roche/Genentech, Bayer, Bristol-Myers Squibb, Clovis Oncology, Daiichi Sankyo, Incyte, Merck, Novartis, Seattle Genetics, CheckmAb
Patents, Royalties, Other Intellectual Property: Amgen
Travel, Accommodations, Expenses: Amgen, Bayer, Roche
Alexander Drilon
Honoraria: Medscape, OncLive, PeerVoice, Physicans' Education Resource, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, Peerview
Consulting or Advisory Role: Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Verastem, Takeda/Millennium, BerGenBio, MORE Health, Lilly, Abbvie, 14ner/Elevation Oncology, Remedica, Archer, Monopteros Therapeutics, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Melendi, Repare Therapeutics
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology
Robert C. Doebele
Employment: Rain Therapeutics
Leadership: Rain Therapeutics
Stock and Other Ownership Interests: Rain Therapeutics
Consulting or Advisory Role: GreenPeptide, AstraZeneca, Roche/Genentech, Bayer, Takeda, Rain Therapeutics, Anchiano, Blueprint Medicines, Foundation Medicine
Patents, Royalties, Other Intellectual Property: Licensing Fees from Abbott Molecular for Patent PCT/US2013/057495, Licensing fees for biologic materials from Genentech, Licensing fees for patent from Rain Therapeutics, Licensing fees for biologic materials from Foundation Medicine, Licensing fees for biologic materials from Black Diamond, Licensing fees for biologic materials from Pearl River, licensing fees for biologic materials from Voronoi
Travel, Accommodations, Expenses: Rain Therapeutics, Roche/Genentech
Manish R. Patel
Consulting or Advisory Role: Nektar
Research Funding: Merck KGaA, AstraZeneca, Fate Therapeutics, Vyriad
Byoung Chul Cho
Leadership: Gencurix, Interpark Bio
Stock and Other Ownership Interests: Theravance, Gencurix, Bridgebio, Kanaph Therapeutics, Cyrus Therapeutics, Interpark Bio
Consulting or Advisory Role: Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Yuhan, Pfizer, Janssen, Takeda, MSD, Ono Pharmaceutical, Lilly, Medpacto, Blueprint Medicines, Kanaph Therapeutics, Bridgebio, Cyrus Therapeutics, Guardant Health, Joseah BIO
Research Funding: Novartis, Bayer, AstraZeneca, Mogam Biotechnology Research Institue, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, MSD, Abbvie, Medpacto, GI Innovation, Lilly, Blueprint Medicines, Interpark Bio
Patents, Royalties, Other Intellectual Property: Champions Oncology
Other Relationship: DAAN Biotherapeutics
Stephen V. Liu
Consulting or Advisory Role: Genentech, Pfizer, Lilly, Bristol-Myers Squibb, AstraZeneca, Takeda, Regeneron, Apollomics, G1 Therapeutics, Guardant Health, Janssen Oncology, Roche, MSD Oncology, Jazz Pharmaceuticals, Blueprint Medicines, Inivata, PharmaMar, Daiichi Sankyo/UCB Japan, BeiGene
Research Funding: Genentech/Roche, Pfizer, Corvus Pharmaceuticals, Bayer, Merck, Lycera, AstraZeneca, Molecular Partners, Blueprint Medicines, Lilly, Rain Therapeutics, Alkermes, Bristol-Myers Squibb, Turning Point Therapeutics, RAPT, Spectrum Pharmaceuticals
Travel, Accommodations, Expenses: AstraZeneca, Roche/Genentech, MSD Oncology
Myung-Ju Ahn
Honoraria: AstraZeneca, Lilly, MSD, Takeda
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Lilly, Takeda, Alpha Pharmaceutical
Chao-Hua Chiu
Honoraria: AstraZeneca/MedImmune, Boehringer Ingelheim, Roche, Novartis, Chugai Pharma, Bristol-Myers Squibb, Ono Pharmaceutical, MSD, Lilly, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Ono Pharmaceutical
Consulting or Advisory Role: AstraZeneca/MedImmune, MSD, Novartis, Chugai Pharma, Lilly
Speakers' Bureau: AstraZeneca, Boehringer Ingelheimm, Roche, Novartis, Chugai Pharma, Bristol-Myers Squibb, MSD
Anna F. Farago
Employment: Novartis
Honoraria: Clinical Care Options, Medscape, Peerview, Research to Practice
Consulting or Advisory Role: Abbvie, Loxo, Genentech/Roche, Bayer, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Merck, Pfizer, H3 Biomedicine
Research Funding: PharmaMar, Abbvie, AstraZeneca, Bristol-Myers Squibb, Merck, Loxo, Ignyta, Amgen, Genentech/Roche, Bayer, Lilly
Travel, Accommodations, Expenses: Abbvie, Bayer, Loxo, Genentech/Roche, AstraZeneca, Boehringer Ingelheim, Merck, Bristol-Myers Squibb, Peerview, Medscape
Chia-Chi Lin
Honoraria: Novartis, Roche, Daiichi Sankyo, Lilly
Consulting or Advisory Role: Novartis, Boehringer Ingelheim, Blueprint Medicines, Daiichi Sankyo
Travel, Accommodations, Expenses: Lilly, Daiichi Sankyo, BeiGene, Novartis, Daiichi Sankyo
Christos S. Karapetis
Honoraria: Roche, Servier
Consulting or Advisory Role: AstraZeneca, Bristol-Myers Squibb, Roche, Eisai
Travel, Accommodations, Expenses: AstraZeneca
Yu-Chung Li
Honoraria: Bayer, AstraZeneca, Takeda, Novartis, Lilly, MSD, Roche
Research Funding: Roche
Travel, Accommodations, Expenses: MundiPharma, Taiho Pharmaceutical, MSD, Roche, Eisai, Boehringer Ingelheim, Sanomics Limited
Bann-mo Day
Employment: Genentech
Stock and Other Ownership Interests: Genentech
Research Funding: Genentech
Travel, Accommodations, Expenses: Genentech
David Chen
Employment: Genentech, Amgen
Stock and Other Ownership Interests: Genentech, Amgen
Travel, Accommodations, Expenses: Genentech, Amgen
Timothy R. Wilson
Employment: Genentech
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: Methods of treatment with Taselisib
Fabrice Barlesi
Honoraria: Genentech/Roche, Pfizer, Pierre Fabre, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Lilly, Novartis, Pierre Fabre, Merck Serono, MSD Oncology, Takeda, Bayer
Consulting or Advisory Role: Roche/Genentech, Pfizer, Novartis, Pierre Fabre, Bristol-Myers Squibb, AstraZeneca/MedImmune, Boehringer Ingelheim, Lilly, Merck Serono, MSD Oncology, Takeda, Bayer
Research Funding: Roche/Genentech, AstraZeneca/MedImmune, Bristol-Myers Squibb, Pierre Fabre, Abbvie, Amgen, Bayer, Boehringer Ingelheim, Eisai, Lilly, Ipsen, Innate Pharma, Novartis, Merck Serono, MSD Oncology, Pfizer, Sanofi/Aventis, Takeda
Patents, Royalties, Other Intellectual Property: Roche/Genentech, Bristol-Myers Squibb, AstraZeneca/MedImmune, MSD Oncology
No other potential conflicts of interest were reported.
REFERENCES
- 1.Davies KD, Doebele RC: Molecular pathways: ROS1 fusion proteins in cancer. Clin Cancer Res 19:4040-4045, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drilon A Jenkins C Iyer S, et al. : ROS1-dependent cancers—Biology, diagnostics and therapeutics. Nat Rev Clin Oncol, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergethon K Shaw AT Ou S-HI, et al. : ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 30:863-870, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dugay F Llamas-Gutierrez F Gournay M, et al. : Clinicopathological characteristics of ROS1- and RET-rearranged NSCLC in Caucasian patients: Data from a cohort of 713 non-squamous NSCLC lacking KRAS/EGFR/HER2/BRAF/PIK3CA/ALK alterations. Oncotarget 8:53336-53351, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazieres J Zalcman G Crino L, et al. : Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: Results from the EUROS1 cohort. J Clin Oncol 33:992-999, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Wu YL Yang JC Kim DW, et al. : Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol 36:1405-1411, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Patil T Smith DE Bunn PA, et al. : The incidence of brain metastases in stage IV ROS1-rearranged non-small cell lung cancer and rate of central nervous system progression on crizotinib. J Thorac Oncol 13:1717-1726, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gainor JF Tseng D Yoda S, et al. : Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis Oncol 10.1200/PO.17.00063 [DOI] [PMC free article] [PubMed]
- 9.Tang SC Nguyen LN Sparidans RW, et al. : Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer 134:1484-1494. 2014 [DOI] [PubMed] [Google Scholar]
- 10.Dagogo-Jack I, Shaw AT: Crizotinib resistance: Implications for therapeutic strategies. Ann Oncol 27:iii42-iii50, 2016. (suppl 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer H Ullah M de la Cruz CC, et al. : Entrectinib, a TRK/ROS1 inhibitor with anti-CNS tumor activity: Differentiation from other inhibitors in its class due to weak interaction with P-glycoprotein. Neuro Oncol 22:819-829, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drilon A Siena S Dziadziuszko R, et al. : Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: Integrated analysis of three phase 1-2 trials. Lancet Oncol 21:261-270, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai AV Robinson GW Basu EM, et al. : Updated entrectinib data from a phase 1/2 trial in children and adolescents with recurrent or refractory solid tumors, including primary central nervous system (CNS) tumors. Pediatr Blood Cancer 2020;67:e28742, 2020. (suppl 4)33049088 [Google Scholar]
- 14.Rodrigus P, de Brouwer P, Raaymakers E: Brain metastases and non-small cell lung cancer. Prognostic factors and correlation with survival after irradiation. Lung Cancer 32:129-136, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Lim SM Kim HR Lee J-S, et al. : Open-Label, multicenter, phase II study of ceritinib in patients with non-small-cell lung cancer harboring ROS1 rearrangement. J Clin Oncol 35:2613-2618, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Shaw AT Solomon BJ Chiari R, et al. : Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: A multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol 20:1691-1701, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Shaw AT Riely GJ Bang YJ, et al. : Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): Updated results, including overall survival, from PROFILE 1001. Ann Oncol 30:1121-1126, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa RB Costa RLB Talamantes SM, et al. : Systematic review and meta-analysis of selected toxicities of approved ALK inhibitors in metastatic non-small-cell lung cancer. Oncotarget 9:22137-22146, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. Non-small cell lung cancer (Version 1.2021) . https://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf [Google Scholar]
- 20.Katayama R Gong B Togashi N, et al. : The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat Commun 10:3604, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).