PURPOSE:
IMpower133 (ClinicalTrials.gov identifier: NCT02763579), a randomized, double-blind, phase I/III study, demonstrated that adding atezolizumab (anti-programmed death-ligand 1 [PD-L1]) to carboplatin plus etoposide (CP/ET) for first-line (1L) treatment of extensive-stage small-cell lung cancer (ES-SCLC) resulted in significant improvement in overall survival (OS) and progression-free survival (PFS) versus placebo plus CP/ET. Updated OS, disease progression patterns, safety, and exploratory biomarkers (PD-L1, blood-based tumor mutational burden [bTMB]) are reported.
PATIENTS AND METHODS:
Patients with untreated ES-SCLC were randomly assigned 1:1 to receive four 21-day cycles of CP (area under the curve 5 mg per mL/min intravenously [IV], day 1) plus ET (100 mg/m2 IV, days 1-3) with atezolizumab (1,200 mg IV, day 1) or placebo, and then maintenance atezolizumab or placebo until unacceptable toxicity, disease progression, or loss of clinical benefit. Tumor specimens were collected; PD-L1 testing was not required for enrollment. The two primary end points, investigator-assessed PFS and OS, were statistically significant at the interim analysis. Updated OS and PFS and exploratory biomarker analyses were conducted.
RESULTS:
Patients received atezolizumab plus CP/ET (n = 201) or placebo plus CP/ET (n = 202). At the updated analysis, median follow-up for OS was 22.9 months; 302 deaths had occurred. Median OS was 12.3 and 10.3 months with atezolizumab plus CP/ET and placebo plus CP/ET, respectively (hazard ratio, 0.76; 95% CI, 0.60 to 0.95; descriptive P = .0154). At 18 months, 34.0% and 21.0% of patients were alive in atezolizumab plus CP/ET and placebo plus CP/ET arms, respectively. Patients derived benefit from the addition of atezolizumab, regardless of PD-L1 immunohistochemistry or bTMB status.
CONCLUSION:
Adding atezolizumab to CP/ET as 1L treatment for ES-SCLC continued to demonstrate improved OS and a tolerable safety profile at the updated analysis, confirming the regimen as a new standard of care. Exploratory analyses demonstrated treatment benefit independent of biomarker status.
INTRODUCTION
Nearly two-thirds of patients with small-cell lung cancer (SCLC) have extensive-stage (ES) disease at diagnosis, which is associated with poor prognosis and a 5-year survival rate of < 7%.1 Platinum chemotherapy (carboplatin [CP] or cisplatin) with etoposide (ET) has remained the standard treatment for > 2 decades.2,3 Despite initial high response rates, median survival is limited to approximately 10 months.4 Recently, immune checkpoint inhibitors have demonstrated improved outcomes in patients with extensive-stage small-cell lung cancer (ES-SCLC).5-7 Based on the double-blinded, randomized phase III IMpower133 study,5 the programmed death-ligand 1 (PD-L1) inhibitor atezolizumab, in combination with CP and ET (CP/ET), was approved for the first-line (1L) treatment of ES-SCLC by the US Food and Drug Administration (FDA)8 and the European Medicines Agency.9 Results from the randomized, phase III CASPIAN study also recently led to the FDA approval of the combination of the PD-L1 inhibitor, durvalumab, plus platinum and ET for the 1L treatment of ES-SCLC.7 In addition, based on durable responses observed in nonrandomized studies, the programmed death-1 (PD-1) inhibitors nivolumab6 and pembrolizumab10,11 both received US FDA accelerated approval for the third-line treatment of ES-SCLC.
CONTEXT
Key Objective
Based on results of the pivotal IMpower133 study, atezolizumab (anti–programmed death-ligand 1 [anti–PD-L1]) in combination with carboplatin and etoposide (CP/ET) was approved for the first-line treatment of extensive-stage small-cell lung cancer (ES-SCLC). Updated findings at a preplanned overall survival (OS) analysis of IMpower133, representing an additional 9 months of follow-up from the primary analysis, are reported.
Knowledge Generated
With a median follow up of 22.9 months, updated results from IMpower133 continued to show OS improvement with atezolizumab plus CP/ET and a safety profile comparable to that of placebo plus CP/ET. Exploratory analyses also suggest that clinical benefit was seen in atezolizumab plus CP/ET-treated patients with ES-SCLC independent of biomarker (blood-based tumor mutational burden or PD-L1) status.
Relevance
Updated analyses from IMpower133 further support atezolizumab plus CP/ET as a new standard of care for untreated ES-SCLC.
Atezolizumab is a humanized monoclonal anti–PD-L1 antibody that inhibits PD-L1 engagement with PD-1 and B7.1.12 In the landmark IMpower133 study, 1L treatment with either atezolizumab or placebo plus CP/ET was compared in patients with ES-SCLC.5,13 At the primary analysis, with a median follow-up for overall survival (OS) of 13.9 months, atezolizumab was associated with a significant improvement in OS with a median of 12.3 months in the atezolizumab plus CP/ET arm and of 10.3 months in the placebo plus CP/ET arm (hazard ratio [HR], 0.70; 95% CI, 0.54 to 0.91; P = .007).5 The median progression-free survival (PFS) was 5.2 months in the atezolizumab plus CP/ET and 4.3 months in the placebo plus CP/ET arm (HR, 0.77; 95% CI, 0.62 to 0.96; P = .02).5
A preplanned updated OS analysis with an additional 9 months of follow-up was conducted; median follow-up for OS was 22.9 months. Updated analyses of safety and OS by blood-based tumor mutational burden (bTMB) levels, as well as exploratory analyses of OS according to PD-L1 expression levels and patterns of disease progression, are reported.
PATIENTS AND METHODS
Study Design and Patients
IMpower133 was a global, phase I/III double-blind, randomized, placebo-controlled trial, whose design and primary results have been previously reported (ClinicalTrials.gov identifier: NCT02763579).5 The trial was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. Protocol approval was obtained from independent review boards or ethics committees for each site. All patients provided written informed consent.
Briefly, eligible patients had histologically or cytologically confirmed chemotherapy-naive ES-SCLC. A computed tomography or magnetic resonance imaging scan of the head was required at screening. Patients with treated asymptomatic brain metastases were eligible, and those with active or untreated CNS metastases were excluded from the study.
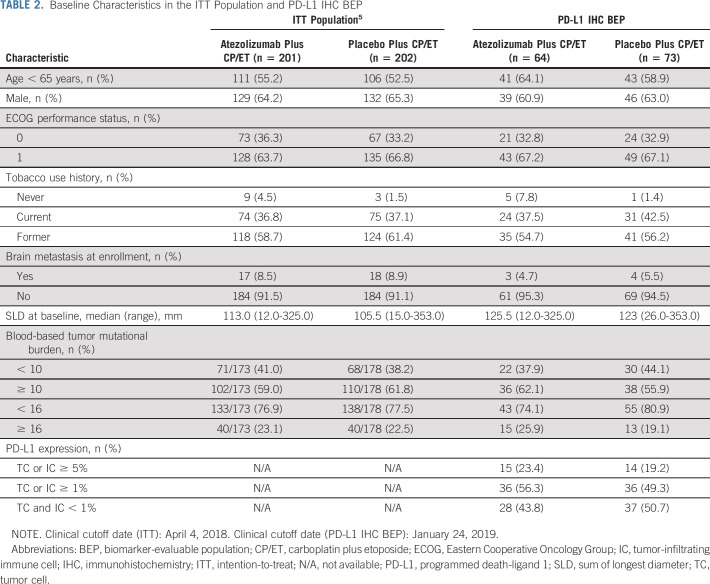
Patients were stratified by sex, Eastern Cooperative Oncology Group performance status (0 v 1), and presence of brain metastases (yes v no) at enrollment. Available tumor specimens retrieved using any method (eg, fine needle aspirations, tissue biopsies, bronchoscopy, or cytological samples) were collected. Exploratory biomarker analyses according to PD-L1 expression levels on both tumor cells (TCs) and tumor-infiltrating immune cells (ICs) were conducted in the biomarker-evaluable population (BEP). Prespecified secondary analyses according to bTMB status were conducted in the BEP.
Procedures
Eligible patients were randomly assigned in a 1:1 ratio to receive four 21-day cycles of CP/ET (CP: area under the curve of 5 mg/mL/min, intravenous [IV] on day 1 of each cycle; ET: 100 mg/m2 of body surface area, IV on days 1-3 of each cycle) plus either IV atezolizumab 1,200 mg (atezolizumab plus CP/ET) or IV placebo (placebo plus CP/ET) on day 1 of each cycle (induction phase), followed by the same dose of IV atezolizumab or placebo during a maintenance phase until unacceptable toxicity or disease progression. Treatment beyond disease progression was allowed if patients experienced clinical benefit.
End Points, Assessments, and Statistical Analysis
The two primary end points were OS and investigator-assessed PFS per RECIST version 1.1 (RECIST 1.1) in the intention-to-treat (ITT) population. Key secondary end points were investigator-assessed overall response rate (ORR) per RECIST 1.1, duration of response (DOR), and safety.
Exploratory end points included assessment of efficacy based on PD-L1 expression levels and bTMB as previously described.5 PD-L1 testing was performed using the PD-L1 immunohistochemical (SP263) assay on a Ventana BenchMark ULTRA automated staining platform according to the manufacturer's instructions.14 Tumor tissue slides (slide age ≤ 1 year) were used for the SP263 analyses. For these exploratory analyses, including updated analyses of OS and PFS, results are considered descriptive in nature.
Samples were considered to be positive for PD-L1 expression if the percentage of TC expressing PD-L1 at any intensity was ≥ 1% or if the proportion of tumor area occupied by IC expressing PD-L1 at any intensity was ≥ 1%. As there are no PD-L1 immunohistochemistry (IHC)-validated cutoffs in SCLC, PD-L1 prevalence was evaluated in a stepwise fashion in line with non-SCLC (NSCLC) PD-L1 IHC cutoffs: ≥ 1%, ≥ 5%, ≥ 25%, and ≥ 50% TC or IC. Because of the limited PD-L1 prevalence observed in the study, exploratory OS by PD-L1 status was evaluated at the ≥ 1% and ≥ 5% TC or IC cutoffs, whereas PFS and ORR were assessed at the ≥ 1% TC or IC cutoff.
Tumor assessments and statistical analyses were previously described5; a brief description is included in the Data Supplement (online only).
Patients who received at least one dose of study treatment were included in the safety analysis. Adverse events (AEs) were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. The investigator determined whether AEs were related to the trial regimen.
Patterns of disease progression were evaluated in the ITT population. Patients with disease progression were evaluated for sites of progression, including at existing lesions (target and nontarget lesions) and new lesions.
RESULTS
Baseline Characteristics
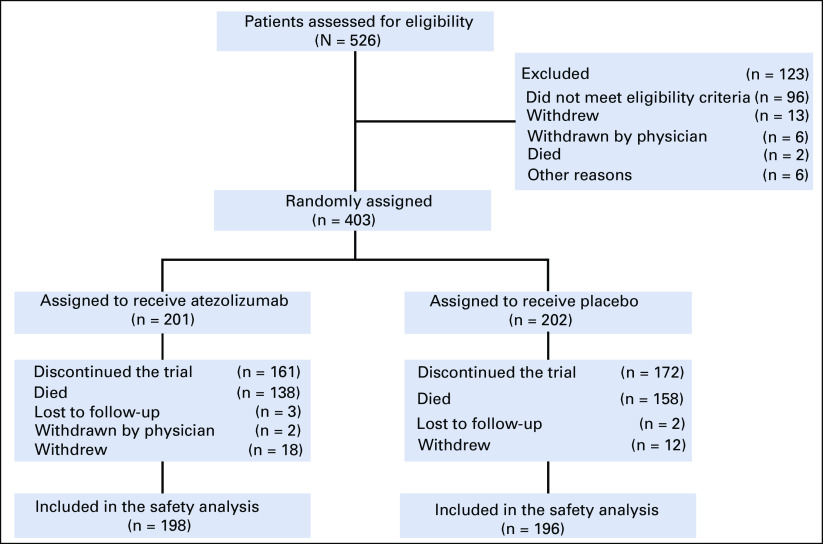
At the data cutoff for this preplanned analysis (clinical cutoff date: January 24, 2019), follow-up had continued for an additional 9 months after the primary analysis. The median duration of follow-up for OS was 23.1 months (range, 0-29.5 months) in the atezolizumab plus CP/ET arm and 22.6 months (range, 0-30.7 months) in the placebo plus CP/ET arm. Patient disposition and reasons for study discontinuation are shown in Figure 1. Patient baseline characteristics were previously described and were generally well-balanced between the treatment arms.5
FIG 1.
Patient flow diagram in IMpower133. All patients who underwent random assignment were included in the intention-to-treat analysis. Patients were assigned to receive either atezolizumab plus carboplatin plus etoposide or placebo plus carboplatin plus etoposide. One patient assigned to the placebo group received a dose of atezolizumab and was included in the atezolizumab group in the safety analyses. Clinical cutoff date: January 24, 2019.
Efficacy
Updated Kaplan-Meier estimates for OS in the ITT population are shown in Figure 2A. The median OS was 12.3 months (95% CI, 10.8 to 15.8) in the atezolizumab plus CP/ET arm and 10.3 months (95% CI, 9.3 to 11.3) in the placebo plus CP/ET arm (HR, 0.76; 95% CI, 0.60 to 0.95). OS at 12 months demonstrated a survival increase of 12.9% in the atezolizumab plus CP/ET arm (51.9%) compared with the placebo plus CP/ET arm (39.0%). Similarly, at 18 months, 13.0% more patients were alive in the atezolizumab plus CP/ET arm (34.0%) than with placebo plus CP/ET (21.0%). Consistent with results observed at the primary analysis,5 the addition of atezolizumab was associated with consistent OS benefit across the majority of subgroups (Fig 2B). At the updated analysis, confirmed ORRs in the ITT population were 60.2% in the atezolizumab plus CP/ET arm (95% CI, 53.1 to 67.0) versus 64.4% (95% CI, 57.3 to 71.0; descriptive P = .3839) in the placebo plus CP/ET arm. The median DOR was 4.2 months (95% CI, 4.1 to 4.5) in the atezolizumab plus CP/ET arm versus 3.9 months (95% CI, 3.1 to 4.2) in the placebo plus CP/ET arm (HR, 0.67; 95% CI, 0.51 to 0.88; descriptive P = .0037).
FIG 2.
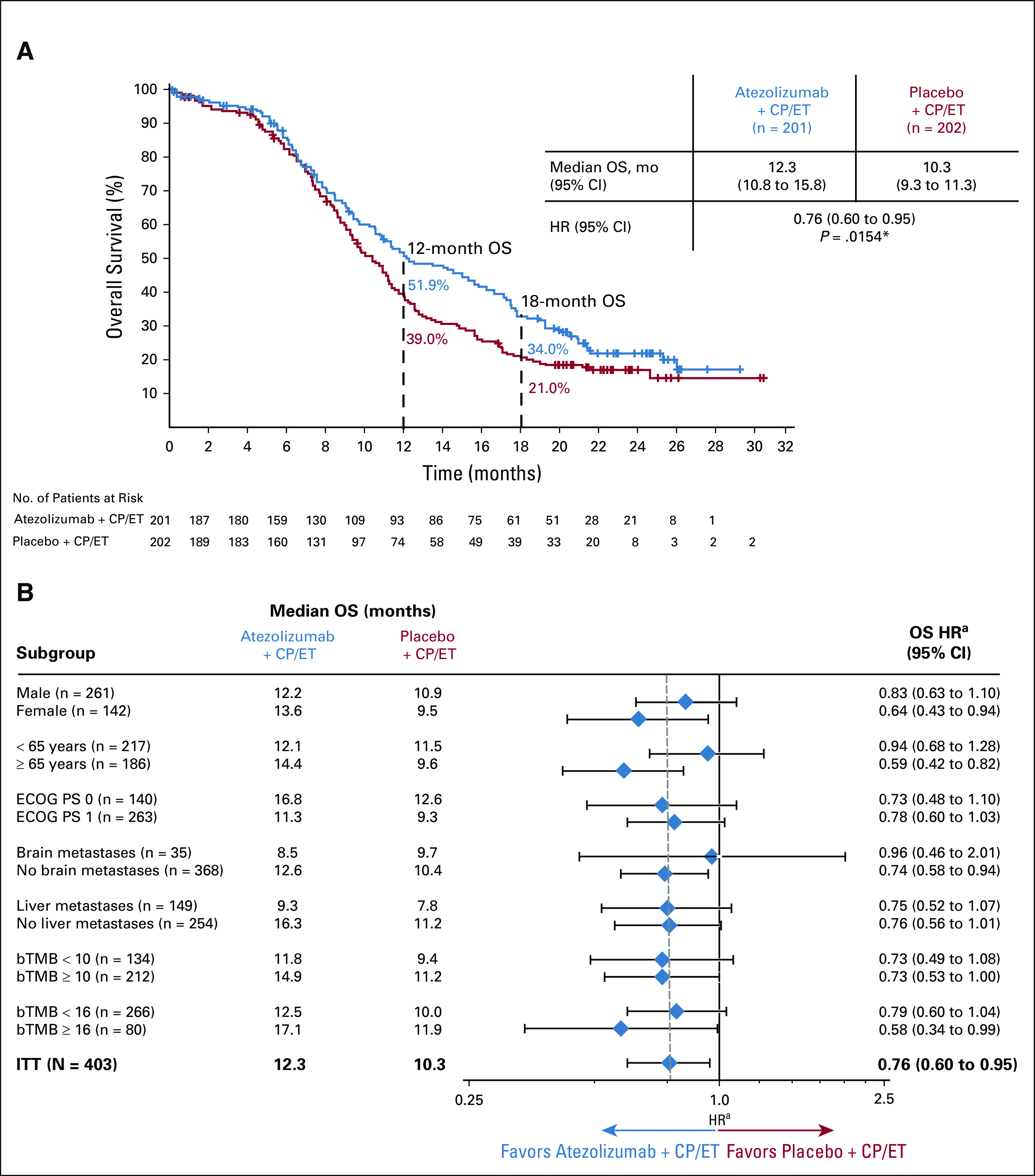
(A) Kaplan-Meier analysis of overall survival (OS) at the updated analysis of the intention-to-treat (ITT) population and (B) OS by baseline characteristics. A total of 57 patients had unknown bTMB score. These patients comprised the nonbiomarker evaluable population, which included 28 patients who had a bTMB result with a maximum somatic allele frequency < 1%, 15 patients who had a sample that either failed quality control at the testing vendor or had median exon coverage < 800, and 14 patients who did not submit a plasma sample at baseline. *P value used for descriptive purposes only. aHazard ratios (HRs) are unstratified for patient subgroups and are stratified for the ITT population. Clinical cutoff date: January 24, 2019. bTMB, blood-based tumor mutational burden; CP/ET, carboplatin plus etoposide; ECOG PS, Eastern Cooperative Oncology Group performance status.
At the updated analysis, 181 patients (90.0%) in the atezolizumab plus CP/ET arm and 194 patients (96.0%) in the placebo plus CP/ET arm had RECIST-defined disease progression. Median PFS in the ITT population at the updated analysis was 5.2 months (95% CI, 4.4 to 5.6) in the atezolizumab plus CP/ET arm and 4.3 months (95% CI, 4.2 to 4.5) in the placebo plus CP/ET arm (HR, 0.77; 95% CI, 0.63 to 0.95). Disease progression occurred with the following patterns in the atezolizumab plus CP/ET and placebo plus CP/ET arms, respectively: 57.7% and 64.9% at existing lesions, 42.8% and 49.0% at new lesions, and 20.9% and 28.2% at both new and existing lesions. The patterns of progression in specific organs were generally similar between arms (Data Supplement). The most common sites of new lesions (occurring in ≥ 10% of patients) were the CNS, lung, lymph nodes, and liver; the incidence of new lesions at these sites was similar between arms (Data Supplement). Among patients treated with PCI in the atezolizumab plus CP/ET (n = 22) and placebo plus CP/ET (n = 22) arms, respectively, disease progression occurred in 68.2% and 77.3% at existing lesions, 45.5% and 54.5% at new lesions, and 27.3% and 31.8% at both new and existing lesions. The patterns of progression in specific organs in patients treated with or without PCI are shown in the Data Supplement. Among the patients who received PCI, one patient in each arm developed new brain lesions. Among patients who did not receive PCI, 21.1% and 22.2% developed new brain lesions in the atezolizumab plus CP/ET and placebo plus CP/ET arms, respectively.
Safety
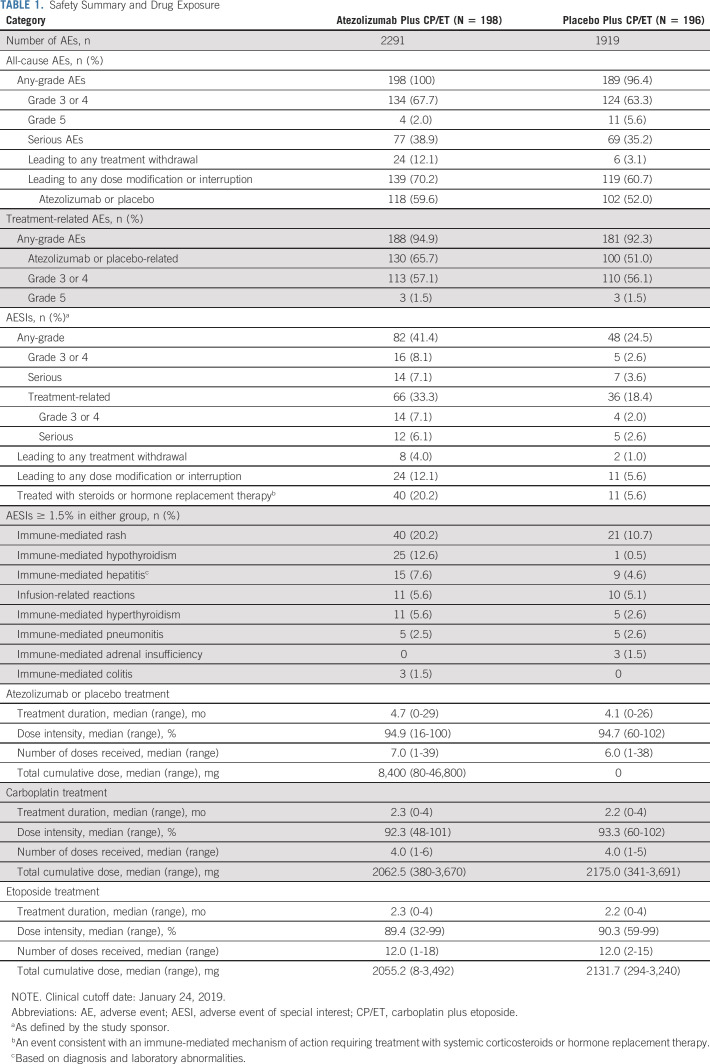
Similar to the findings in the primary analysis, the median duration of treatment was 4.7 months (range, 0-29 months) with atezolizumab and 4.1 months (range, 0-26 months) with placebo (Table 1). Patients received a median of 7.0 doses (range, 1-39 doses) of atezolizumab and 6.0 doses (range, 1-38 doses) of placebo. AEs, including treatment-related AEs, are listed in Table 1 and were comparable with safety reported at the primary analysis.5 Immune-related AEs that were consistent with an immune-mediated mechanism of action and required treatment with systemic corticosteroids occurred in 40 patients (20.2%) in the atezolizumab plus CP/ET arm versus 11 patients (5.6%) in the placebo plus CP/ET arm (Table 1). The most common immune-related AEs, irrespective of treatment given for the event, in the atezolizumab plus CP/ET and placebo plus CP/ET arms were rash (20.2% v 10.7%), hypothyroidism (12.6% v 0.5%), hepatitis (7.6% v 4.6%), and infusion-related reactions (5.6% v 5.1%). Immune-related pneumonitis occurred in five patients (2.5% v 2.6%) in each arm.
TABLE 1.
Safety Summary and Drug Exposure
Exploratory Biomarker Analyses
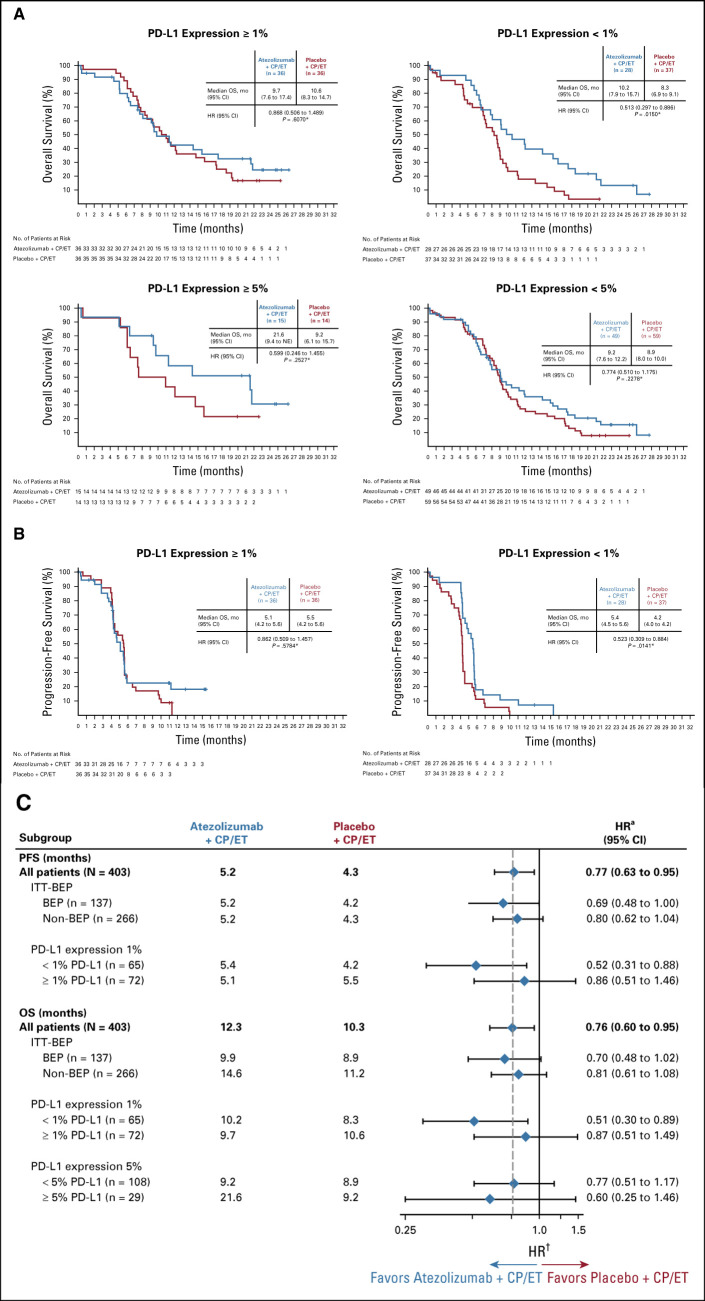
The PD-L1 IHC BEP comprised 34% of the ITT population: 64 patients (32%) from the atezolizumab plus CP/ET arm and 73 patients (36%) from the placebo plus CP/ET arm (Data Supplement). Baseline characteristics in the BEP are summarized in Table 2 and were generally well balanced. The prevalence for PD-L1 expression levels was 47.4% (n = 65) for PD-L1 expression on < 1% of TC and IC, 52.6% (n = 72) for PD-L1 expression on ≥ 1% on TC or IC, and 21.2% (n = 29) for PD-L1 expression on ≥ 5% TC or IC (Data Supplement). PD-L1 expression was observed mostly on IC, with limited expression on TC (Data Supplement). Because of the limited number of patients whose tumors had PD-L1 expression ≥ 5%, exploratory analysis of OS by PD-L1 status was evaluated only at the 1% and 5% TC or IC cutoffs. In the subgroup of patients with PD-L1 expression < 1% TC and IC, an OS benefit was observed with atezolizumab plus CP/ET (median OS, 10.2 months; 95% CI, 7.9 to 15.7) compared with placebo plus CP/ET (8.3 months; 95% CI, 6.9 to 9.1; HR, 0.51; 95% CI, 0.30 to 0.89; Figs 3 A-3C). The median OS in patients with PD-L1 expression ≥ 1% TC or IC was 9.7 months (95% CI, 7.6 to 17.4) in the atezolizumab plus CP/ET arm compared with 10.6 months (95% CI, 8.3 to 14.7) in the placebo plus CP/ET arm (HR, 0.87; 95% CI, 0.51 to 1.49) (Figs 3A-3C). An OS benefit was observed in patients with tumors containing PD-L1 expression ≥ 5% TC or IC (Figs 3A-3C). In this subgroup, the median OS was 21.6 months (95% CI, 9.4 to not evaluable) with atezolizumab plus CP/ET compared with 9.2 months (95% CI, 6.1 to 15.7) with placebo plus CP/ET (HR, 0.60; 95% CI, 0.25 to 1.46).
TABLE 2.
Baseline Characteristics in the ITT Population and PD-L1 IHC BEP
FIG 3.
Kaplan-Meier analysis of (A) overall survival (OS) and (B) progression-free survival (PFS) and (C) subgroup analysis of PFS (clinical cutoff date: April 4, 2018) and OS (clinical cutoff date: January 24, 2019) by programmed death-ligand 1 (PD-L1) expression in the biomarker-evaluable population (BEP). *P values used for descriptive purposes only. aHazard ratios (HRs) are unstratified for patient subgroups and stratified for the intention-to-treat (ITT) population. CP/ET, carboplatin plus etoposide; NE, not evaluable.
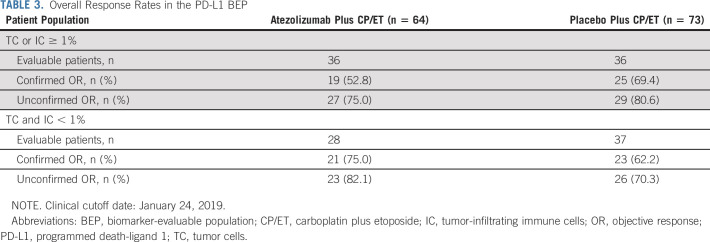
Similarly, a PFS benefit with atezolizumab plus CP/ET (median PFS, 5.4 months; 95% CI, 4.5 to 5.6) compared with placebo plus CP/ET (4.2 months; 95% CI, 4.0 to 4.2) was observed in patients with tumors containing PD-L1 expression < 1% TC or IC (HR, 0.52; 95% CI, 0.31 to 0.88; Figs 3B-3C). The median PFS in patients with tumors containing PD-L1 expression ≥ 1% TC or IC was 5.1 months (95% CI, 4.2 to 5.6) in the atezolizumab plus CP/ET arm compared with 5.5 months (95% CI, 4.2 to 5.6) in the placebo plus CP/ET arm (HR, 0.86; 95% CI, 0.51 to 1.46) (Figs 3B-3C). Confirmed ORRs in the PD-L1 negative subgroup (defined by PD-L1 expression < 1% TC and IC) were 75.0% and 62.2% with atezolizumab plus CP/ET and placebo plus CP/ET, respectively. Responses in the PD-L1 positive subgroup (defined by PD-L1 expression ≥ 1% TC or IC) were 52.8% and 69.4% with atezolizumab plus CP/ET and placebo plus CP/ET, respectively (Table 3).
TABLE 3.
Overall Response Rates in the PD-L1 BEP
Consistent with the previously reported bTMB results, updated analyses showed an OS benefit in both the bTMB high and low subgroups using the prespecified bTMB cutoffs of 10 and 16, respectively (Fig 2B).5 The overlap of patients with bTMB high and PD-L1 high was limited, as 55.9% (n = 38/68) of PD-L1 positive patients (defined by a PD-L1 IHC result of ≥ 1% TC or IC) were also bTMB high (defined by a bTMB score≥ 10) (Data Supplement).
DISCUSSION
IMpower133 was the first randomized study in > 3 decades to show improved survival in patients with untreated ES-SCLC, leading to regulatory approvals of atezolizumab plus CP/ET in the 1L setting in the United States8 and European Union.9 The updated OS results, after an additional 9 months of follow-up, continued to demonstrate improved clinical benefit of atezolizumab plus CP/ET compared with placebo plus CP/ET. At both 12 and 18 months, 13% more patients were alive in the atezolizumab plus CP/ET arm than in the placebo plus CP/ET arm. The OS benefit was observed in most patient subgroups. Consistent with previously reported results,5 there appeared to be an imbalance in OS benefit among patients with baseline brain metastases compared with patients without baseline brain metastases. The number of patients enrolled in the study with brain metastases was small, which may be because of the requirement that brain metastases be treated prior to enrollment; further trials are needed to investigate the role of immunotherapy in patients with SCLC who have brain metastases.
There continued to be fewer patients in the atezolizumab plus CP/ET arm than in the placebo plus CP/ET arm with radiographic progression. Patterns of progression were similar between arms, with no difference in the incidence of new brain metastases. However, it should be noted that evaluation of the patterns of progression considered metastatic sites individually rather than the combination(s) of patterns at sites (eg, isolated v diffused progression or unique visceral involvement). With longer follow-up, the safety profile of atezolizumab plus CP/ET remained consistent with that previously reported. Improvements in health-related quality of life in patients in the atezolizumab plus CP/ET arm compared with patients in the placebo plus CP/ET arm were previously reported13 and suggest that the addition of atezolizumab did not increase toxicity related to CP/ET or adversely contribute to symptom burden.
PD-L1 expression in SCLC is highly variable, and published results indicate that expression ranges from 2% to 83%, depending on the assay and scoring algorithm used.15 In this study, using the SP263 assay, the prevalence of PD-L1 expression ≥ 1% on TC or IC within the BEP was 53%, whereas the prevalence of PD-L1 expression ≥ 5% on TC or IC was 21%. Furthermore, PD-L1 expression was more frequently observed on IC than on TC; this finding differs from that for NSCLC, for which PD-L1 expression is observed on both TC and IC.16 The pattern of PD-L1 expression primarily on IC in SCLC was also seen in the phase III CASPIAN (ClinicalTrials.gov identifier: NCT03043872) study, which also demonstrated an improvement in OS similar to that observed in IMpower133 when the anti–PD-L1 antibody durvalumab was added to platinum plus etoposide (EP) versus placebo plus EP in patients with untreated ES-SCLC.7,17 The data from both IMpower133 and CASPIAN suggest that the immune biology of SCLC may be more similar to that of diseases such as triple-negative breast cancer18 and urothelial carcinoma,19 for which PD-L1 expression is primarily on IC (limited PD-L1 expression on TC).
In the exploratory analysis in IMpower133, an OS benefit was observed across PD-L1 subgroups. These findings were also noted in other similar phase III studies. In CASPIAN, durvalumab plus EP demonstrated improved OS compared with placebo plus EP across PD-L1 subgroups.17 In KEYNOTE-604, analyses of OS by PD-L1 subgroup appeared to show a similar benefit in OS with pembrolizumab plus EP compared with placebo plus EP across PD-L1 subgroups.20 Results from these studies suggest that PD-L1 expression does not appear to be a predictive biomarker for PD-L1/PD-1 checkpoint inhibitor plus chemotherapy in 1L ES-SCLC. An updated exploratory analysis by bTMB in IMpower133 continued to demonstrate that atezolizumab plus CP/ET resulted in improved benefit over placebo plus CP/ET, independent of bTMB levels. However, similar to NSCLC and other indications,21 in ES-SCLC, bTMB and PD-L1 IHC appear to identify distinct patient populations. Together, these data suggest that bTMB and PD-L1 status should not be used for patient treatment decisions for the atezolizumab plus CP/ET regimen, as currently neither biomarker appears to be predictive of outcomes.
A caveat with the exploratory biomarker PD-L1 IHC analysis was the limited size of the BEP, with only 34% of the enrolled study population included in the PD-L1 analyses. This limitation can be partially attributed to poor tissue quality (including crush artifact) and sample collection methods (eg, fine needle aspirates) that destroy tissue architecture and limit IHC analyses.22 Various sample collection methods were allowed with the intention of making the study population more reflective of the general population of patients with ES-SCLC, given how diagnoses are made in clinical practice (bronchoscopy, cytological samples, and fine needle aspiration).
Given the limitations of the PD-L1 IHC analyses and the current lack of identification of a biomarker for checkpoint inhibitors in SCLC, additional studies are necessary to further evaluate potential biomarkers and associations with outcomes.17,23,24
In conclusion, with a follow-up of 22.9 months, updated results continued to demonstrate an improvement in OS with atezolizumab plus CP/ET and a similar safety profile compared with placebo plus CP/ET in patients with ES-SCLC. Although the PD-L1 analyses were limited to a subset of the ITT population, no numerical difference in efficacy outcomes was observed across the PD-L1 IHC subgroups. Overall, the data suggest that clinical benefit was observed in patients with ES-SCLC treated with 1L atezolizumab plus CP/ET, independent of bTMB or PD-L1 biomarker status.
ACKNOWLEDGMENT
We thank the patients participating in this trial and their families and the nurses, research coordinators, data managers, and clinical study site investigators. Medical writing assistance for this manuscript was provided by Preshita Gadkari, PhD, of Health Interactions, Inc, and funded by F. Hoffmann-La Roche, Ltd.
PRIOR PRESENTATION
Presented at the European Society for Medical Oncology 2019 Congress, Barcelona, Spain, September 27 - October 1, 2019 (abstract 2374).
SUPPORT
Sponsored by F. Hoffmann-La Roche Ltd/Genentech, Inc, a member of the Roche Group.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Martin Reck, Tony Mok, Arnaud Scherpereel, Niels Reinmuth, Marina Chiara Garassino, Sivuonthanh Lam, Mark McCleland, Leora Horn
Provision of study materials or patients: Martin Reck, Aaron S. Mansfield, Arnaud Scherpereel, Niels Reinmuth, Marina Chiara Garassino, Makoto Nishio, Francisco Orlandi, Jorge Alatorre-Alexander, Ticiana Leal, Ying Cheng
Collection and assembly of data: Martin Reck, Aaron S. Mansfield, Tony Mok, Arnaud Scherpereel, Niels Reinmuth, Marina Chiara Garassino, Javier De Castro Carpeno, Makoto Nishio, Francisco Orlandi, Jorge Alatorre-Alexander, Ticiana Leal, Jong-Seok Lee, Sivuonthanh Lam, Mark McCleland
Data analysis and interpretation: Stephen V. Liu, Martin Reck, Aaron S. Mansfield, Tony Mok, Arnaud Scherpereel, Niels Reinmuth, Marina Chiara Garassino, Javier De Castro Carpeno, Raffaele Califano, Francisco Orlandi, Jorge Alatorre-Alexander, Ticiana Leal, Ying Cheng, Sivuonthanh Lam, Mark McCleland, Yu Deng, See Phan, Leora Horn
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Stephen V. Liu
Consulting or Advisory Role: Pfizer, Celgene, Lilly, Taiho Pharmaceutical, Bristol Myers Squibb, AstraZeneca, Takeda, HERON, Regeneron, Apollomics, G1 Therapeutics, Guardant Health, Inivata, Janssen Oncology, Tempus, Ignyta, ARIAD, Loxo, PharmaMar, Roche, MSD Oncology, Boehringer Ingelheim, Catalyst Pharmaceuticals, Blueprint Medicines
Research Funding: Genentech/Roche, Pfizer, Threshold Pharmaceuticals, Clovis Oncology, Corvus Pharmaceuticals, Esanex, Bayer, OncoMed, Merck, Lycera, AstraZeneca, Molecular Partners, Blueprint Medicines, Lilly, Rain Therapeutics, Alkermes, Bristol Myers Squibb, Ignyta, Turning Point Therapeutics, RAPT, Spectrum Pharmaceuticals
Travel, Accommodations, Expenses: AstraZeneca, Roche/Genentech, MSD Oncology
Martin Reck
Consulting or Advisory Role: Lilly, MSD Oncology, Merck Serono, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Roche/Genentech, AbbVie, Amgen, Mirati Therapeutics, Samsung Bioepis
Speakers' Bureau: Roche/Genentech, Lilly, MSD Oncology, Merck Serono, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Pfizer, Novartis, Amgen
Aaron S. Mansfield
Consulting or Advisory Role: Genentech, Bristol Myers Squibb, AbbVie, AstraZeneca
Research Funding: Novartis, Bristol Myers Squibb
Travel, Accommodations, Expenses: AbbVie, Roche
Tony Mok
Employment: The Chinese University of Hong Kong
Leadership: Sanomics Limited, Hutchison MediPharma, AstraZeneca
Stock and Other Ownership Interests: Sanomics Limited, Hutchison MediPharma
Honoraria: AstraZeneca, Roche/Genentech, Lilly, Bristol Myers Squibb, Boehringer Ingelheim, Novartis, Merck Sharp & Dohme, Pfizer, Merck Serono, SFJ Pharmaceuticals Group, ACEA Biosciences, Vertex, Celgene, Ignyta, Fishawack Facilitate Ltd, Takeda, Janssen, Hutchison MediPharma
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Lilly, Merck Serono, Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Novartis, Clovis Oncology, Vertex, SFJ Pharmaceuticals Group, ACEA Biosciences, Merck Sharp & Dohme, geneDecode, Oncogenex, Celgene, Ignyta, Cirina, Hutchison MediPharma
Research Funding: AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, SFJ Pharmaceuticals Group, Roche, Merck Sharp & Dohme, Clovis Oncology, Bristol Myers Squibb, Xcovery
Arnaud Scherpereel
Consulting or Advisory Role: MSD Oncology, Roche, Bristol Myers Squibb, AstraZeneca/MedImmune
Speakers' Bureau: Roche Pharma AG, AstraZeneca, Bristol Myers Squibb, MSD Oncology
Travel, Accommodations, Expenses: MSD Oncology, Bristol Myers Squibb, Roche SA
Marina Chiara Garassino
Honoraria: MSD Oncology, AstraZeneca/MedImmune, GlaxoSmithKline, Takeda, Roche, Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Novartis, Takeda, Roche, Tiziana Life Sciences, Sanofi, Celgene, Daiiki Sankyo, Inivata, Incyte, Pfizer, Seattle Genetics, Lilly, GlaxoSmithKline, Bayer, Blueprint Medicines, Janssen
Speakers' Bureau: AstraZeneca, Takeda, MSD Oncology, Celgene, Incyte, Roche, Bristol Myers Squibb, Otsuka, Lilly
Research Funding: Bristol Myers Squibb, MSD, Roche/Genentech, AstraZeneca/MedImmune, AstraZeneca, Pfizer, GlaxoSmithKline, Novartis, Merck, Incyte, Takeda, Spectrum Pharmaceuticals, Blueprint Medicines, Lilly, AstraZeneca, Ipsen, Turning Point Therapeutics, Janssen, Exelixis, MedImmune, Array BioPharma, Sanofi
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca
Javier De Castro Carpeno
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Roche, Pfizer, Pierre Fabre, Takeda, Tesaro, Teva
Research Funding: Roche
Travel, Accommodations, Expenses: AstraZeneca Spain: Merck Sharp & Dohme, Roche
Raffaele Califano
Stock and Other Ownership Interests: The Christie Private Care
Honoraria: AstraZeneca, Boehringer Ingelheim, Lilly Oncology, Roche, Pfizer, MSD, BMS, Takeda, Novartis
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Lilly Oncology, Roche, Pfizer, MSD, BMS, Takeda, Novartis
Speakers' Bureau: AstraZeneca, Roche, Pfizer, MSD, BMS, Takeda, Novartis
Research Funding: Roche, AstraZeneca, Pfizer, Clovis, Lilly Oncology, MSD, BMS, AbbVie, Takeda, Novartis
Travel, Accommodations, Expenses: Roche, Lilly Oncology, MSD, Takeda
Makoto Nishio
Honoraria: Pfizer, Bristol Myers Squibb Japan, Ono Pharmaceutical, Chugai Pharma, Taiho Pharmaceutical, AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Lilly, Nippon Kayaku
Consulting or Advisory Role: Novartis, Ono Pharmaceutical, Chugai Pharma, Taiho Pharmaceutical, Bristol-Myers Squibb Japan, Daiichi Sankyo, Lilly, AstraZeneca, MSD, AbbVie, Takeda Pharmaceutical Company Limited, Teijin Pharma
Research Funding: Novartis, Ono Pharmaceutical, Chugai Pharma, Bristol Myers Squibb, Taiho Pharmaceutical, Pfizer, AstraZeneca, Lilly, MSD, Merck Biopharma
Francisco Orlandi
Honoraria: Roche/Genentech
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Bristol Myers Squibb, MSD Oncology, Lilly, Pfizer, Bristol Myers Squibb, Novartis, Sanofi
Speakers' Bureau: AstraZeneca/MedImmune, Roche
Research Funding: AstraZeneca/MedImmune, Amgen, Genentech/Roche, Boehringer Ingelheim, Astellas Medivation, MSD Oncology, Bristol Myers Squibb, Celltrion, Pfizer, mAbxience, Nektar, Sanofi
Travel, Accommodations, Expenses: Pfizer, MSD Oncology, AstraZeneca/MedImmune, Roche, Bristol Myers Squibb, Genentech/Roche, AstraZeneca/MedImmune, Genentech/Roche
Jorge Alatorre-Alexander
Honoraria: Bristol Myers Squibb (Mexico), AstraZeneca, Roche/Genentech
Consulting or Advisory Role: MSD Oncology, AstraZeneca, Roche/Genentech, Bristol Myers Squibb, Novartis
Speakers' Bureau: Roche/Genentech, AstraZeneca, MSD
Travel, Accommodations, Expenses: AstraZeneca, Roche
Ticiana Leal
Consulting or Advisory Role: Takeda, AbbVie, AstraZeneca, Genentech, Bayer, BeyondSpring Pharmaceuticals, Bristol Myers Squibb, Merck, Takeda, Genentech, InVisionFirst-Lung, Merck, Bristol-Myers Squibb, Novocure
Travel, Accommodations, Expenses: Takeda, AbbVie, Bristol Myers Squibb, Genentech, Mirati Therapeutics, Mirati Therapeutics, AstraZeneca, Mirati Therapeutics, Takeda, Merck,
See Phan
Employment: Genentech
Stock and Other Ownership Interests: Roche
Research Funding: Genentech
Patents, Royalties, Other Intellectual Property: Use of Avastin for the treatment of metastatic breast cancer
Expert Testimony: Genentech
Travel, Accommodations, Expenses: Genentech
Leora Horn
Employment: AstraZeneca
Consulting or Advisory Role: Merck, Xcovery, Genentech, AstraZeneca, Incyte, EMD Serono, Tesaro, Pfizer, Amgen, Bayer
Research Funding: Boehringer Ingelheim, Xcovery
Travel, Accommodations, Expenses: Xcovery
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bernhardt EB, Jalal SI: Small cell lung cancer. Cancer Treat Res 170:301-322, 2016 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer (Version 1.2019), 2019 [Google Scholar]
- 3.Armstrong SA, Liu SV: Dashing decades of defeat: Long anticipated advances in the first-line treatment of extensive-stage small cell lung cancer. Curr Oncol Reports 22:20, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Foster NR Renfro LA Schild SE, et al. : Multitrial evaluation of progression-free survival as a surrogate end point for overall survival in first-line extensive-stage small-cell lung cancer. J Thorac Oncol 10:1099-1106, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horn L Mansfield AS Szczęsna A, et al. : First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220-2229, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Antonia SJ Lopez-Martin JA Bendell J, et al. : Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol 17:883-895, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Paz-Ares L Dvorkin M Chen Y, et al. : Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 394:P1929-P1939, 2019 [DOI] [PubMed] [Google Scholar]
- 8.TECENTRIQ (Atezolizumab) [Package Insert]. South San Francisco, CA, Genentech, 2019 [Google Scholar]
- 9.TECENTRIQ (Atezolizumab) [Summary of Product Characteristics]. Grenzach-Wyhlen, Germany, Roche Registration GmbH, 2019 [Google Scholar]
- 10.Ott PA Elez E Hiret S, et al. : Pembrolizumab in patients with extensive-stage small-cell lung cancer: Results from the phase Ib KEYNOTE-028 study. J Clin Oncol 35:3823-3829, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Chung HC Piha-Paul SA Lopez-Martin J, et al. : Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic small-cell lung cancer: Results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol 15:618-627, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS Soria JC Kowanetz M, et al. : Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansfield A Każarnowicz A Karaseva N, et al. : Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): A randomized phase I/III trial. Ann Oncol 31:310-317, 2020 [DOI] [PubMed] [Google Scholar]
- 14.VENTANA PD-L1 (SP263) Assay (CE-IVD) [Package Insert]. Tucson, AZ, Ventana Medical Systems, 2018 [Google Scholar]
- 15.Tian Y Zhai X Han A, et al. : Potential immune escape mechanisms underlying the distinct clinical outcome of immune checkpoint blockades in small cell lung cancer. J Hematol Oncol 12:67, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowanetz M Zou W Gettinger SN, et al. : Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti–PD-L1). Proc Natl Acad Sci USA 115:E10119-E10126, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paz-Ares L Goldman JW Garassino MC, et al. : PD-L1 expression, patterns of progression and patient-reported outcomes (PROs) with durvalumab plus platinum-etoposide in ES-SCLC: Results from CASPIAN. Annals Oncol 30(suppl 5):v851-v934, 2019 [Google Scholar]
- 18.Emens L Loi S Rugo H, et al. : IMpassion130: Efficacy in immune biomarker subgroups from the global, randomized, double-blind, placebo-controlled, phase III study of atezolizumab+ nab-paclitaxel in patients with treatment-naïve, locally advanced or metastatic triple-negative breast cancer, Cancer Res 79, 2019. (suppl; abstr GS1-04) [Google Scholar]
- 19.Petrylak DP Powles T Bellmunt J, et al. : A phase Ia study of MPDL3280A (anti-PDL1): Updated response and survival data in urothelial bladder cancer (UBC), J Clin Oncol 33, 2015. (suppl; abstr 4501) [Google Scholar]
- 20.Rudin CM Awad MM Navarro A, et al. : Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 38:2369-2379, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandara DR Paul SM Kowanetz M, et al. : Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 24:1441-1448, 2018 [DOI] [PubMed] [Google Scholar]
- 22.VanderLaan PA: Fine‐needle aspiration and core needle biopsy: An update on 2 common minimally invasive tissue sampling modalities. Cancer Cytopathol 124:862-870, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Gadgeel SM Pennell NA Fidler MJ, et al. : Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J Thorac Oncol 13:1393-1399, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellmann MD Callahan MK Awad MM, et al. : Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 33:853-861, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]