Abstract
Background
Non‐compliance is a significant problem among people with serious mental disorders, presenting a challenge for mental health professionals. Prompts such as telephone calls, visits, and a posted referral letter to patients are currently used to encourage patient attendance at clinics and/or compliance with medication. More recently, the use of information and communication technology (ICT)‐based prompting methods have increased. Methods include mobile text message (SMS ‐ short message service), e‐mail or use of any other electronic device with the stated purpose of encouraging compliance.
Objectives
To investigate the effects of ICT‐based prompting to support treatment compliance in people with serious mental illness compared with standard care.
Search methods
We searched the Cochrane Schizophrenia Group’s Trials Register (31st May 2011 and 9th July 2012) which is based on regular searches of CINAHL, BIOSIS, AMED, EMBASE, PubMed, MEDLINE, PsycINFO, and registries of clinical trials. Also, we inspected references of all identified studies for further trials and contacted authors of trials for additional information.
Selection criteria
Relevant randomised controlled trials involving adults with serious mental illness, comparing any ICT‐based prompt or combination of prompts by automatic or semi‐automatic system compared with standard care.
Data collection and analysis
Review authors reliably assessed trial quality and extracted data. We calculated risk ratio (RR) with 95% confidence intervals (CI) using a fixed‐effect model. For continuous outcomes, we estimated the mean difference (MD) between groups, again with 95% confidence intervals. A 'Summary of findings' table using GRADE was created, and we assessed included studies for risk of bias.
Main results
The search identified 35 references, with 25 studies, but we could only include two studies with a total of 358 participants. The studies had a moderate risk of bias, and therefore risk overestimating any positive effects of ICT‐based prompting. Both included studies compared semi‐automatised ICT‐based prompting intervention with standard care groups in mental health outpatient care. The interventions were SMS‐message and an electronic assistant device. One included study reported our primary outcome, compliance.
There was not any clear evidence that ICT‐based prompts increase improvement in compliance (stop taking medication within six months n = 320, RR 1.11 CI 0.96 to 1.29, moderate quality evidence). There was some low quality evidence that ICT‐based prompts have small effects for: mental state (average change in specific symptom scores within three months n = 251, MD ‐0.30 CI ‐0.53 to ‐0.07; severity of illness within three months n = 251, MD ‐0.10 CI ‐0.13 to ‐0.07 and six months n = 251, MD ‐0.10 CI ‐0.13 to ‐0.07; average change in depressive scores within six months n = 251, RR 0.00 CI ‐0.28 to 0.28; global symptoms within three months n = 251, MD ‐0.10 CI ‐0.38 to ‐0.07; negative symptoms within three months n = 251, MD ‐0.10 CI ‐0.38 to 0.18 and six months n = 251, MD ‐0.30 CI ‐0.58 to 0.02, low quality evidence). Level of insight improved more among people receiving ICT‐based prompt compared with those in the control group at six months (n = 251, MD ‐0.10 CI ‐0.13 to ‐0.07). ICT‐based prompts also increased quality of life (average change in quality of life within six months n = 251, RR 0.50 CI 0.19 to 0.81, moderate quality evidence).
Based on the existing data, there is no evidence that either intervention is less acceptable than the other (n = 347, 2 RCTs, RR 1.46 CI 0.70 to 3.05, low quality evidence). Included studies did not report outcomes of service utilisation, behaviour, costs or adverse events.
Authors' conclusions
The evidence base on the effects of ICT‐based prompts is still inconclusive. Data to clarify ICT‐based prompting effects are awaited from an ongoing trial, but further well‐conducted trials considering the different ICT‐based prompts are warranted.
Keywords: Adult, Humans, Patient Compliance, Reminder Systems, Medication Adherence, Mental Disorders, Mental Disorders/psychology, Mental Disorders/therapy, Randomized Controlled Trials as Topic, Text Messaging
Plain language summary
Using information and communication‐based prompting for patients with serious mental illness
Recently there has been an increase in the use of ICT (Information Communication Technology) for the delivery of information to people with severe mental illness. ICT is considered to be any technical means of delivering information and communication and can include use of telephones, television, radio, computers and hand‐held devices.
People with severe mental health problems often have difficulties with 'treatment compliance' i.e. following their treatment programme. They can have difficulty remembering to take medication or appointment times. Unpleasant side effects of medication can also lead to people stopping medication, and a lack of insight into their illness can mean they do not see the need to follow treatments. Non‐compliance with treatment can lead to poor health outcomes and even relapses and hospitalisation. There are several methods healthcare professionals use to help people with serious mental illness improve compliance; once such method is prompting. The purpose of prompting is to help patients to follow the treatment instructions and keep the treatment appointment times by using reminders via telephone calls, personal visits or posted referral letter. More recently, Information and technology‐based prompts are being used. This review investigates the effectiveness of ICT‐based prompts in order to support treatment compliance among patients with serious mental illness. A search for randomised controlled trials was run in 2012. Two trials that compared the use of ICT prompting compared with standard care could be included. Review authors rated the quality of data in these as 'moderate' or 'low'. Because of the small amount of data, it is impossible to say whether ICT‐based prompts are effective. Only one trial measured medication compliance. The study suggested that ICT‐based prompts may help people take their medication, but clear evidence of a benefit is missing. There were some positive effects for patient insight. However, insight was only better in the medium term and appeared to show no difference in the short term. Further, some positive effect was found in patient satisfaction with treatment, although the results for the analyses were imprecise. Also, mental state and quality of life showed minor improvement. There were no clear evidence that either intervention is less acceptable than the other. Outcomes such as service use, behaviour, costs or adverse effects were not presented in the studies. There is an ongoing trial, but additional well‐conducted trials are needed.
Summary of findings
Summary of findings for the main comparison. INFORMATION AND COMMUNICATION TECHNOLOGY (ICT) FOR PROMPTING/SUPPORT + STANDARD CARE compared with STANDARD CARE for treatment compliance for people with serious mental illness.
| INFORMATION AND COMMUNICATION TECHNOLOGY (ICT) FOR PROMPTING/SUPPORT + STANDARD CARE compared with STANDARD CARE for treatment compliance for people with serious mental illness | ||||||
| Patient or population: patients with treatment compliance for people with serious mental illness Settings: Mental health outpatient care Intervention: ICT FOR PROMPTING / SUPPORT + STANDARD CARE Comparison: STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | ICT FOR PROMPTING / SUPPORT + STANDARD CARE | |||||
| Compliance with medication: Would stop taking medication Morisky Green Adherence Questionnaire (MAQ) Follow‐up: 6 months | Low1 | RR 1.11 (0.96 to 1.29) | 320 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| 300 per 1000 | 336 per 1000 (285 to 393) | |||||
| Moderate1 | ||||||
| 600 per 1000 | 672 per 1000 (570 to 786) | |||||
| High1 | ||||||
| 900 per 1000 | 1000 per 1000 (855 to 1000) | |||||
| Service utilisation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Nature of measure is unclear. |
| Adverse effects/events | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome. |
| Mental state: Average change in specific symptom scores: depressive scores degree of change CGI‐SCH‐DC Follow‐up: 6 months | The mean mental state: average change in specific symptom scores: depressive scores degree of change in the intervention groups was 0 higher (0.28 lower to 0.28 higher) | 251 (1 study) | ⊕⊕⊝⊝ low3 | |||
| Acceptability (of intervention): Leaving the studies early – any reason Loss to follow‐up or leaving the study early | Study population | RR 1.46 (0.7 to 3.05) | 347 (2 studies) | ⊕⊕⊝⊝ low4 | Length of follow‐up varies between 6 to18 months. | |
| 50 per 1000 | 73 per 1000 (35 to 152) | |||||
| Moderate | ||||||
| 94 per 1000 | 137 per 1000 (66 to 287) | |||||
| Quality of life: Average change Euroquol 5D, visual analogue scale Follow‐up: 6 months | The mean quality of life: average change in the intervention groups was 0.5 higher (0.19 to 0.81 higher) | 251 (1 study) | ⊕⊕⊕⊝ moderate5 | |||
| Costs | See comment | See comment | Not estimable | 0 (0) | See comment | No study reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Unclear data: these analyses were performed with excluded sub‐sample. 2 Risk of bias: 22% of participants were excluded from the analysis (analyses performed with excluded sub‐sample); results of MAQ written emphasising significantly greater results. 3 Risk of bias: 22% of participants were excluded from the analysis. 4 Risk of bias: unclear blinding; incomplete outcome data; selective reporting. 5 Risk of bias: 22% of participants were excluded from the analysis. The results based on the MANSA were not used in SoF; the results might change the estimate if usable.
Background
Description of the condition
Schizophrenia is a prevalent serious mental illness. It is a severe chronic psychiatric disorder affecting approximately 1% of the population (Buchanan 2005). Schizophrenia is characterised by psychotic symptoms that alter the person’s perception, thoughts, affect and behaviour (NICE 2009). The major features of schizophrenia include, for example, disorganised thinking and behaviour, abnormal interpretation of reality, impaired ability to relate to self and others, and impaired ability to function (Eby 2005). It is estimated that around 24 million people worldwide are affected by schizophrenia and more than half of those people are not receiving appropriate treatment (WHO 2011). For those offered treatment, non‐compliance is a significant problem. It has been found that at least half of people prescribed medication do not comply with it (McIntosh 2006), and that stopping medication is a common precursor of relapse (Robinson 1999).
On an individual level, the illness can cause suffering and social exclusion, while on a societal level, schizophrenia causes costs on a global scale (Nicholl 2010). Non‐compliance with treatment has consequences on different levels, including economic. Other factors affecting total cost are suicide attempts, cognitive symptoms and hostility (Hong 2009). Non‐attendance at healthcare appointments is a major problem across health services. For example, in the UK, a total of 6.8 million outpatient appointments were missed (Health and Social Care Information Centre 2012), and the estimated direct cost of missed appointments in 2004 was £185 million for general practitioner appointments, £34 million for practice nurse appointments, and around £575 million for missed hospital appointments (Department of Health UK 2004).
Description of the intervention
Prompts to encourage attendance at clinics or compliance with medication are often used in day‐to‐day practice by diligent carers (Reda 2010). These may take the form of telephone prompting (Crespo‐Iglesias 2006), with or without specific visits to the home (Thi Phan 1995), or issuing a copy of the referral letter to the appointee (Kitcheman 2008). Prompts may also be embedded within complex care packages (Reda 2010). At its most simple, information and communication technology (ICT)‐based prompting may, for example, be a mobile text message, telephone call, e‐mail or use of any other electronic device with the stated purpose of encouraging compliance. Looking beyond mental health, text messaging has been used for smoking cessation (Haug 2009) or reduction (Berkman 2011), and changing behaviour related to safer sex and sun safety (Fjeldsoe 2009). However, prompting has less often been used for patients with severe mental disorder (Pijnenborg 2010).
How the intervention might work
The use of ICT‐based prompts is being examined across health services and new evidence is rapidly emerging. A Cochrane review of people with mental health problems found that using a prompt, especially a letter, can encourage outpatient attendance (Reda 2010). It has been found that telephone prompting can increase the attendance rate in mental health community services (MacDonald 2000). Sawyer 2002 found that in adolescent services, telephone reminders reduced non‐attendance from 20% to 8%. Prompts should be simple, encouraging patients to adherence (Reda 2010).
Telephone‐based prompts may encourage mental health patients to keep treatment appointments. Calling patients before the appointment has improved attendance at appointments (Crespo‐Iglesias 2006). From staff’s point of view, implementation of any system would be easier if it does not require extra effort, it is easy to use, acceptable, less expensive and shows clear benefit. A simple ICT‐based application might have potential to support patient compliance and meet all requirements for an easily implemented system in psychiatric services. Any system may be ‘stand‐alone’, automated, semi‐automated or fully manual and managed by staff or carers.
Why it is important to do this review
Compliance with treatment is challenging for people with schizophrenia (Anderson 2010) and even a modest improvement could be very cost‐effective. In relation to using ICT‐based attendance prompts for people with schizophrenia, some encouraging results have been found but we know of no systematic review of evidence. Mental health services are becoming more community‐based (Department of Health AU 2010; Department of Health UK 2010; EU 2008; WHO 2005), which highlights the importance of maintaining contact as people with more severe mental illness move outside institutions. In light of these changes and the implications illnesses such as schizophrenia have for individuals and societies, effective interventions to increase compliance with services need to be sought. ICT interventions in health care are evolving rapidly. Societies are changing and greater numbers of doctors and patients are found to be comfortable with using technology‐driven care solutions (Atherton 2009).
Objectives
To investigate the effects of information and communication technology‐based prompting to support treatment compliance in people with serious mental illness compared with standard care.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials. If a trial had been described as 'double blind' but implied randomisation, we would have included such trials in sensitivity analysis. If their inclusion did not result in a substantive difference, they would have remained in the analyses. If their inclusion did result in statistically significant differences, we would not add the data from these lower quality studies to the results of the better trials, but would have presented such data within a subcategory. We excluded quasi‐randomised studies allocating by alternate days of the week. Where people were given additional treatments within ICT‐based prompting, we only included data if the adjunct treatment was evenly distributed between groups and it was only the ICT‐based prompting that was randomised.
Types of participants
The focus of this review is people with the diagnosis of serious mental illness. Adults, however defined, with schizophrenia or related disorders, including schizophreniform disorder, schizoaffective disorder and delusional disorder, again, by any means of diagnosis. We also included studies involving those with 'serious/chronic mental illness' or 'psychotic illness'.
We were interested in making sure that information is as relevant to the current care of people with schizophrenia as possible. We therefore proposed, if possible, to highlight the current clinical state (acute, early post‐acute, partial remission, remission) as well as the stage (prodromal, first episode, early illness, persistent), and as to whether the studies primarily focused on people with particular problems (for example, negative symptoms, treatment‐resistant illnesses). Participants were not to be excluded due to age, nationality, gender, duration of illness or treatment setting.
Types of interventions
1. ICT for prompting support (as a sole method) without standard professional care
ICT for prompt or combination of prompts by automatised or semi‐automatised system, such as mobile text message, e‐mail or other electronic device that has the stated purpose of encouraging compliance. Where such a system was used as a part of package of care, these studies were excluded and their results will be reported a future review.
2. ICT for prompting support and standard professional care
ICT for prompt or combination of prompts by automatised or semi‐automatised system, such as mobile text message, e‐mail or other electronic device that has the stated purpose of encouraging compliance and standard professional care.
3. Prompting support with no technology
Any prompt or combination of prompts not using technology. For example, this could be by letter, personal visit, financial or other rewards that has the stated purpose of encouraging compliance.
4. Standard professional care
Standard professional care of where the study was undertaken.
Types of outcome measures
We divided all outcomes into short term (less than six months), medium term (seven to 12 months) and long term (over one year).
Primary outcomes
1. Compliance
1.1 Loss to follow‐up ‐ loss of contact with the psychiatric care team (including loss to follow‐up in outpatients and failure of psychiatric team to re‐establish contact) 1.2 Compliance with medication 1.3 Relapse (both incidence of and time to relapse) 1.4 Attendance of appointments
Secondary outcomes
1. Service utilisation
1.1 Admitted to psychiatric hospital 1.2 Mean days spent in psychiatric hospital per month 1.3 Number of contacts with own doctor for mental health problems 1.4 Number of contacts with psychiatric outpatient services 1.5 Crisis attendance due to mental health problems
2. Mental state
2.1 Clinically important change in general mental state 2.2 Average change in general mental state scores 2.3 Clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia, depression, mania) 2.4 Average change in specific symptom scores
3. Insight
3.1 Clinically important change in average level of insight of his/her illness and need for treatment 3.2 Average change in level of insight scores 3.3 Clinically important change in specific aspects of insight 3.4 Average change in specific aspects of insight
4. Behaviour
4.1 Clinically important change in general behaviour 4.2 Average change in general behaviour scores 4.3 Clinically important change in specific aspects of behaviour 4.4 Average change in specific aspects of behaviour
5. Quality of life
5.1 Clinically important change in quality of life 5.2 Average change in quality of life scores 5.3 Clinically important change in specific aspects of quality of life 5.4 Average change in specific aspects of quality of life
6. Functioning
6.1 Clinically important change in general functioning 6.2 Average change in general functioning scores 6.3 Clinically important change in specific aspects of functioning 6.4 Average change in specific aspects of functioning
7. Satisfaction with treatment
7.1 Clinically important change in satisfaction with treatment 7.2 Average change in social satisfaction with treatment scores 7.3 Clinically important change in specific aspects of satisfaction with treatment 7.4 Average change in specific aspects of satisfaction with treatment
8. Acceptability of intervention
8.1 Leaving the studies early – any reason 8.2 Leaving the studies early – specific technological reason
9. Costs
9.1 Direct costs 9.2 Indirect costs
10. Adverse effects
10.1 Suicide attempts 10.2 Death (all causes) 10.3 Adverse effects ‐ any
11. 'Summary of findings' table
We used the GRADEpro approach to interpret findings (Schünemann 2008) and used GRADE profiler to import data from RevMan 5 (Review Manager) to create Table 1. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision making. We selected the following main outcomes for inclusion in the 'Summary of findings' table, and, in preference, chose one binary outcome from each category. Because this was not always possible, we also used continuous outcomes.
Compliance with medication: would stop taking medication (medium term)
Service utilisation (not reported, since nature of measure was unclear)
Adverse effects/events (no study reported this outcome)
Mental state: average change in specific symptom scores: depressive scores degree of change (medium term)
Acceptability (of intervention): leaving the studies early – any reason, loss to follow‐up or leaving the study early (length of follow‐up varied between six to 18 months)
Quality of life: average change (medium term)
Costs (no study reported this outcome)
Search methods for identification of studies
Electronic searches
1. Cochrane Schizophrenia Group’s Trials Register
The Trials Search Coordinator (TSC) searched the Cochrane Schizophrenia Group’s Registry of Trials (31st May 2011 and 9th July 2012) using the following phrase:
(*appointment* or *attend* or *remind* or *prompt*) and (*letter* or *phone* or *text* or *email* or *e‐mail* or *sms* or *visit* or call* or *system* or *messeng* or *msn*) and (*computer* or *internet* or *ict* or *electronic* or *online* or *virtual* or *world wide web* or *second life* or *facebook* or *twitter* or *blog* or *messeng* or *msn* or *sms*) in title, abstract, index terms of REFERENCE
The Cochrane Schizophrenia Group’s Registry of Trials is compiled by systematic searches of major resources (including AMED, BIOSIS, CINAHL, EMBASE, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, hand‐searches, grey literature, and conference proceedings (see Group Module). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
Searching other resources
1. Reference searching
We inspected references of all identified studies for further relevant studies.
2. Personal contact
We contacted the first authors of the two included studies for more detailed data, conference presentation, results tables (Hulsbosch 2008; Montes 2011). We also contacted the authors (Berns 2001) for more details about their methods and participants.
Data collection and analysis
Selection of studies
Review authors KK, MV, HH and LK independently inspected citations from the searches and identified relevant abstracts. Where there were disputes, the full report was acquired for more detailed scrutiny. Full reports of abstracts meeting the review criteria were obtained and inspected by KK, MV, HH, and LK. Disagreements were clarified with assistance from CEA. Where it was not possible to find out answers to all questions, we also contacted authors of the study for clarification.
Data extraction and management
1. Extraction
Review authors KK, MV, HH and LK extracted data from included studies. We identified potentially relevant abstracts from the search. Any disagreement was discussed, decisions documented and authors of studies were contacted for clarification. With remaining problems CEA helped to clarify issues and these final decisions were documented. Once the full articles were obtained, we (KK, MV, HH, LK, CEA) decided whether the studies met the review criteria. If disagreement was not resolved by discussion, we sought further information and these trials were added to the list of those awaiting assessment.
If data were presented only in graphs and figures, we did extract these data but included them only if review authors independently found the same result. Where necessary, we tried to contact trial authors through an open‐ended request in order to obtain missing information or clarification.
2. Management
2.1 Forms
Review authors extracted data onto standard, simple forms.
2.2 Scale‐derived data
We aimed to include continuous data from rating scales only if: a. the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and b. the measuring instrument had not been written or modified by one of the trialists for that particular trial.
Ideally, the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). This was not always realised or reported clearly. In Description of studies we noted if we were unsure of the validity of the instruments.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. Endpoint and change data would have been combined in the analysis as we used mean differences (MD) rather than standardised mean differences throughout (Higgins 2011). However, there were no endpoint data in the analysis.
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion: a) standard deviations and means are reported in the paper or obtainable from the authors; b) when a scale starts from the finite number zero, the standard deviation, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996); c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS) (Kay 1986), which can have values from 30 to 210), the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have a finite start and endpoint and these rules can be applied. Skewed endpoint data from studies of less than 200 participants would have been entered in other data tables within the data analyses section rather than into a statistical analysis. Skewed endpoint data pose less of a problem when looking at means if the sample size is large and we entered data from trials with over 200 participants into statistical analysis.
When continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We did not entered skewed change data into analysis, but presented the skewed data in Table 2.
1. Skewed data.
| Reference | Outcome | Outcome measure | Experimental | Control | Total | ||||
| Mean | SD | Total | Mean | SD | Total | ||||
| Montes 2011 | Compliance with medication ‐ short term | DAI‐10 | 2 | 0.3 | 99 | 0.4 | 0.3 | 152 | 251 |
| Montes 2011 | Mental state ‐ short term | CGI‐SCH‐SI | ‐0.2 | 0.1 | 99 | ‐0.1 | 0.1 | 152 | 251 |
| Montes 2011 | Mental state ‐ medium term | CGI‐SCH‐SI | ‐0.2 | 0.1 | 99 | ‐0.1 | 0.1 | 152 | 251 |
| Hulsbosch 2008 | Functioning ‐ medium term | HoNOS | 9.68 | 5.14 | 34 | 9.13 | 4.25 | 38 | 72 |
| Hulsbosch 2008 | Satisfaction (with treatment) ‐ medium term | CQ‐Index | 5.23 | 4.68 | 35 | 4.68 | 1.45 | 38 | 73 |
CGI‐SCH‐SI ‐ The Clinical Global Impression – Schizophrenia Scale ‐ Severity of illness CQ‐Index ‐ Consumer Quality Index DAI‐10 ‐ Drug Attitude Inventory 10‐item version HoNOS ‐ Health of the Nation Outcome Scale
2.5 Common measure
To facilitate comparison between trials, we converted variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This was done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for ICT‐based prompting. Where keeping to this makes it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'Not un‐improved'), we reported data where the left of the line indicates an unfavourable outcome. This was noted in the relevant graphs.
Assessment of risk of bias in included studies
Review authors KK, MV, HH, LK and KW‐S worked independently to assess risk of bias by using criteria described in the Cochrane Collaboration Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
In case of disagreement, the final rating was made by consensus, with the involvement of CEA. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain further information. Non‐concurrence in quality assessment was reported, but if disputes arose as to which category a trial was to be allocated, again, resolution was made by discussion.
We noted the level of risk of bias in both the text of the review, in the Risk of bias in included studies for each study and in the Table 1 for the key outcomes.
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). The Number Needed to Treat/Harm (NNT/H) statistic with its confidence intervals is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta‐analyses and interpretation (Hutton 2009). For binary data presented in the Table 1, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we estimated mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference (SMD)). However, if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and we calculated effect size and transform the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
If we had found cluster trials where clustering was not accounted for in primary studies, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review, if we do include cluster trials we will seek to contact first authors of studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). If we had found cluster trials where clustering had been incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
The binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC is not reported it would be assumed to be 0.1 (Ukoumunne 1999). We would have sought statistical advice to do this.
If cluster studies had been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, if we had included cross‐over trials, we would have only used data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we would have presented the additional treatment arms in comparisons. If data were binary these would have simply been added and combined within the two‐by‐two table. If data were continuous, we would have combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where the additional treatment arms were not relevant, these data would not have been reproduced.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses, (except for the outcome 'leaving the study early'). If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we would marked such data with (*) to indicate that such a result may well be prone to bias.
2. Binary
In the case where attrition for a binary outcome is between 0% and 50% and where these data were not clearly described, data were presented on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). Those leaving the study early were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ was used for those who did not. A sensitivity analysis was undertaken to test how prone the primary outcomes are to change when 'completer' data only are compared to the ITT analysis using the above assumptions. Review authors calculated binary data for Analysis 1.1 and Analysis 1.16.
1.1. Analysis.
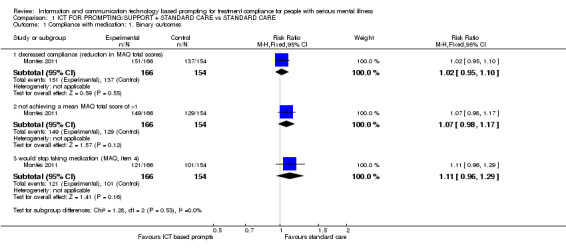
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 1 Compliance with medication: 1. Binary outcomes.
1.16. Analysis.
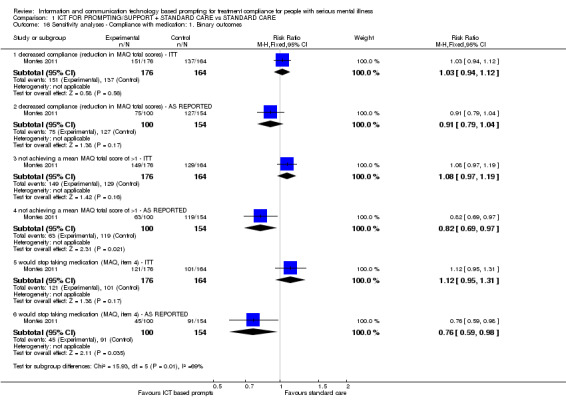
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 16 Sensitivity analyses ‐ Compliance with medication: 1. Binary outcomes.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50% and completer‐only data were reported, we have reproduced these.
3.2 Standard deviations
If standard deviations (SD) were not reported, we first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals available for group means, and either a P value or 't' value available for differences in mean, we can calculate them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When only the SE is reported, SDs were calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges or other statistics. If these formulae did not apply, we would have calculated the SDs according to a validated imputation method which was based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We would have examined the validity of the imputations in a sensitivity analysis excluding imputed values. However, a sensitivity analysis was not done because of the limited data.
3.3 Last observation carried forward
We anticipated in one study that the method of last observation carried forward (LOCF) was employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data were used in the trial and less than 50% of the data have been assumed, we reproduced these data and indicated in the Characteristics of included studies that they were the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
If we had included more than two studies we would have considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We would simply inspect all studies for clearly outlying people or situations which we had not predicted would arise. When such situations or participant groups arose, we would have fully discussed these.
2. Methodological heterogeneity
Again, if we had included more than two studies we would have considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We would simply inspect all studies for clearly outlying methods which we had not predicted would should arise. If such methodological outliers had been present we would have fully discussed these.
3. Statistical heterogeneity
3.1 Visual inspection
If we had included more than two studies we would have visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
Heterogeneity between studies would have been investigated by considering the I2 method alongside the Chi2 P value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. P value from Chi2 test, or a confidence interval for I2). We interpreted an I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistics, as evidence of substantial levels of heterogeneity (Section 9.5.2 ‐ Higgins 2011). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We would not have used funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we would have sought statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. For this reason we chose to use fixed‐effect model for all analyses. The reader is, however, able to choose to inspect the data using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses ‐ only primary outcomes
1.1 Clinical state, stage or problem
We proposed to undertake this review and provide an overview of the effects of information and communication technology based prompting for people with schizophrenia in general. We also tried to report data on subgroups of people in the same clinical state, stage, with similar problems and settings.
2. Investigation of heterogeneity
If inconsistency was high, this was reported. First, we investigated whether data had been entered correctly. Second, if data were correct, the graph was visually inspected and outlying studies were successively removed to see if homogeneity is restored. For this review we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, data were presented. If not, data were not pooled and issues were discussed. We know of no supporting research for this 10% cut‐off but are investigating use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity were obvious, we simply stated hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For the primary outcomes we included these studies and if there was no substantive difference when the implied randomised studies to those with better description of randomisation, then all data were employed from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding the people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption compared with completer data only. If there was a substantial difference, we reported results and discussed them but continue to employ our assumption.
Where assumptions had to be made regarding missing SD data (see Dealing with missing data), we compared the findings on primary outcomes when we used our assumption compared with complete data only. A sensitivity analysis was undertaken to test how prone results were to change when 'completer' data only were compared to the imputed data using the above assumption. If there was a substantial difference, we have reported results and discussed them but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that were judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available), allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then data from these trials were included in the analysis.
4. Imputed values
If we had included cluster‐randomised trials, we planned to undertake a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect.
If substantial differences were noted in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we would not have pooled data from the excluded trials with the other trials contributing to the outcome, but presented them separately.
5. Fixed and random effects
All data were synthesised using a fixed‐effect model. We also synthesised data for the primary outcome using a random‐effects model to evaluate whether this alters the significance of the result.
Results
Description of studies
For more detailed description of each study, please refer to the Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies.
Results of the search
We identified a total of 35 references. After detailed inspection we found that three references were duplicates, leaving 32 references to 25 trials. Only two trials, however, met our inclusion criteria (Figure 1).
1.
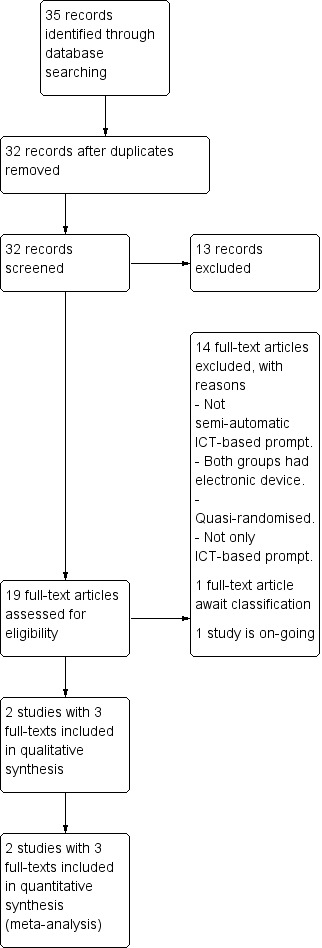
Study flow diagram.
Included studies
The two trials ‐ Hulsbosch 2008 and Montes 2011 ‐ included a total of 358 participants. See Characteristics of included studies for detailed descriptions of studies.
1. Participants
People in the two studies were diagnosed with schizophrenia (Montes 2011), schizoaffective disorder or mood disorder, such as uni‐ and bipolar depression (Hulsbosch 2008), and in both cases operational criteria (DSM IV) was used. The average age was around 40 to 46 years, and both men and woman were included. Participants were clinically stabile outpatients who were considered to have poor adherence to antipsychotic therapy (Montes 2011). In Hulsbosch 2008 the average year in care was just over a decade. In Montes 2011 participants were outpatients in Mental Health Centres receiving antipsychotic medication.
2. Interventions
Hulsbosch 2008 compared a telecare group, i.e. a digital communication device plus care as usual with care as usual. The device included regular or crisis consultation via web cam, computerised information about treatment plans, laboratory results, reminders and scheduled appointments, and support by significant others. Participants in the control group received standard counselling (care as usual). The duration of the intervention was 18 months with measurement points at zero, nine and 18 months.
The Montes 2011 study compared daily text message (short message service ‐ SMS) on mobile phone to remind the person to take medication with a control (no SMS) ‐ both supplementary to standard care. The reminder SMS message was sent daily at 10 am or 2 pm. The duration of the intervention was three months with measurement points at zero, three and six months.
3. Outcomes
A variety of scales were used to assess clinical response. In Hulsbosch 2008 the primary outcome was patient satisfaction, while Montes 2011 was primarily interested in adherence to antipsychotic medication (this concurs with our primary outcome for this review). However, neither study reported economic outcomes, behaviour, acceptability of intervention or adverse effects.
Details of scales that provided usable data are shown below.
3.1 Compliance
3.1.1 Morisky Green Adherence Questionnaire (MAQ)
MAQ was developed to measure medication adherence. The instrument is a self‐reported scale consisting of four items about medication adherence and history. Items are scored as zero (no) or one (yes). Lower scores indicate higher compliance, so that maximum score for compliance is one and if a person receives three points or more, he/she could be considered as non‐compliant (Morisky 1986). One study reported data from this scale.
3.1.2 Drug Attitude Inventory 10‐item version (DAI‐10)
DAI‐10 assesses subjective responses to medication. The instrument evaluates if the patient is satisfied with treatment and whether the person understands how treatment affects them. DAI‐10 has 10 items and responses are true and false, or yes (one) or no (two). Higher scores indicate a positive subjective attitude to medication and treatment (Hogan 1983.) One study reported data from this scale.
3.2 Mental state
3.2.1 Clinical Global Impressions Scale (CGI‐SCH‐SI and CGI‐SCH‐DC)
CGI‐S is a part of a CGI‐instrument (Guy 1976). It measures severity of illness at the current time of assessment (the past seven days) and treatment response over time ‐ based on clinical judgement. It is a seven‐point scale. Higher scores indicate higher severity. Information is collected by interviewing patients and other sources and by direct observation. One study reported data from this scale.
3.3 Insight
3.3.1 Unawareness of Mental Disorder (SUMD)
SUMD (Amador 1991; Amador 1994) assesses the dimension of insight or awareness of patients of their illness. Higher scores indicate poor awareness. Information is collected by using semi‐structured interview. One study reported data from this scale.
3.2 Quality of life
3.2.1 Euroquol 5D, visual analogue scale (Euroquol VAS)
The Euroqol 5D is a standardised instrument measuring health outcomes, as health‐related quality of life. One part of this instrument is Euroquol VAS, which is a visual analogue scale. In Euroqol VAS, people are asked to rate their current health state. The scale is from zero to 100. High scores indicate best health state (Brooks 1996). One study reported data from this scale.
3.2.2 Quality of life (MANSA)
The Mansa is a shortened version of the Lancashire Quality of Life Profile (Nieuwenhuizen 1998; Nieuwenhuizen 2001; Oliver 1991). The Mansa consists of 16 questions with four dichotomous questions and 12 items with one to seven points (Priebe 1999). The total score for the Mansa is the average of all subjective items (a minimum value, maximum value seven). Higher scores indicate better quality of life. One study reported data from this scale.
3.2.3 Loneliness Scale
The Loneliness Scale (de Jong Gierveld 1999) rates quality of life to gain an insight into feelings of loneliness. It consists of 11 items answered on a five‐point scale (one to five) . The total score is the sum of all individual items with a minimum of 11 and maximum of 55. A high score indicates high loneliness. One study reported data from this scale.
3.3 Functioning
3.3.1 Computer Use and Experience Scale (CUE)
Computer experience is based on the Computer Use and Experience Scale (CUE) (Potosky 1998). The version employed consists of 11 items. The total score is determined by summation of all item scores (minimum 11, maximum 55). Higher scores indicate a higher level of computer experience. One study reported data from this scale.
3.3.2 Working Alliance Inventory‐Short Revised (WAI‐SR)
The Working Alliance Inventory‐Short Revised (WAI‐SR) is a recently‐refined measure that assesses three key aspects of the therapeutic alliance (Hatcher 2006; Munder 2010). The WAI‐SR examines the working relationship between client and counsellor. The WAI‐SR consists of 12 items. The items are answered using a five‐point scale (one to five). The total scores are obtained by summing item scores. The total score of the WAI‐SR is between 12 and 60. A higher score is indicative of a good working relationship. One study reported data from this scale.
3.3.3 Psychological and social functioning (HoNOS)
The HoNOS used in this review is a Dutch adaptation of the original version (Mulder 2004a; Mulder 2004b). The questionnaire consists of 12 items but the Dutch version has three additional questions (maniforme disinhibition, motivation and treatment compliance/adherence to medication). The HoNOS can be completed by an assessor (physician, nurse or other mental health worker). The scale is designed to be used by clinicians before and after interventions, so that changes attributable to the interventions (outcomes) can be measured. In this study, the Netherlands version with 15 questions was used. All items use a five‐point Likert scale from zero to four. The total score for HoNOS is the sum score on the 15 items, with a minimum of zero and a maximum of 60. A high score indicates many problems in functioning. One study reported data from this scale.
3.4 Satisfaction with treatment
3.4.1 Camberwell Assessment of Needs ‐ Short Appraisal Schedule (CANSAS)
Unmet needs were assessed using the Camberwell Assessment of Need Short Appraisal Schedule (Slade 1999) which assesses needs in 22 health and social domains. Each domain is rated as either an unmet need (current serious problem, regardless of any help given), met need (no/moderate problem because of help given), no need, or not known. The unmet need score is the total number of unmet needs (range zero to 22, with a high score being worse). One study reported data from this scale.
3.4.2 CQ‐Index
CQ‐Index measures clients’ appreciation of accessibility of care (van Wijngaarden 2007). Questions can be used on the basis of a three‐point scale (zero to two). Summation of the three item scores gives the total score varying from zero to six points. Higher scores indicate higher degrees of satisfaction. The questionnaire consists of 72 questions. All answers were assigned a one to four‐point score. Compilation of scores can range from one to four. One study reported data from this scale.
3.4.3 GGZ Thermometer
This Dutch instrument (Kerzman 2003) assesses how satisfied clients are with mental health services provided with a three‐point scale (zero to two). Summation of the three item scores gives the total score. The score varies from zero to a maximum of six. Higher scores indicate higher degrees of satisfaction. One study reported data from this scale.
3.5 Acceptability (of intervention)
In this review, we assessed acceptability of intervention by measuring how many patients left the study early for any reason.
Excluded studies
We excluded 14 studies. Two had to be excluded because they were not randomised (Lassen 2011; MacDonald 2000a). Pijnenborg 2010 was A‐B design and was not randomised but did, however, investigate a semi‐automated SMS‐based system and would have been of great interest if allocation of intervention had been randomised. This group of researchers, however, gave a first group one intervention and followed this with the second set. We also excluded Cramer 1999 because both groups had ICT‐based prompting and less than 50% of participants had schizophrenia. In addition, eight studies had to be excluded because the intervention was not automatised or semi‐automatised ICT‐based prompting (Burgoyne 1983; Crespo‐Iglesias 2006; Frangou 2005; Hansson 2008; Kluger 1983; Montes 2009; Nietert 2009; Rossi 1994; Tidey 2011). In the Velligan 2008 study the intervention was not only semi‐automatised ICT‐based prompting, but also Cognitive adaptation training (CAT), so we could not be sure if effects were because of the ICT‐based prompt alone.
Studies awaiting classification
It was not possible to include Berns 2001 as further information was needed on intervention, methods and participants. We have not received the necessary information and were unable to make a decision regarding inclusion.
Ongoing studies
Välimäki 2012 is an ongoing study in which several of the review authors are involved (KK, MV, HH, CEA). Randomisation was completed in 2012 and data collection for follow‐up closes in late 2014.
Risk of bias in included studies
Our overall impression of risk of bias of included studies is shown in Figure 2. Our suggestion is that there is, at the very least, a moderate risk of bias and therefore a risk of overestimating any positive effects of ICT‐based prompting.
2.
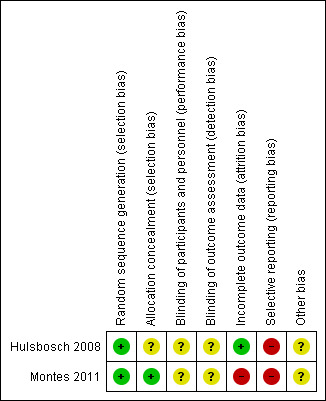
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both included studies were stated to be randomised. They were classified as 'low risk', because descriptions of allocation methods were included in the reports. Hulsbosch 2008 used block‐randomisation and Montes 2011 stated that "Randomisation codes were computer generated by our statistician and sealed in envelopes labelled with consecutive numbers. Envelopes were opened by the investigator in a ascending order and patients were allocated to the intervention or control group".
Blinding
Montes 2011 stated that the blinding was open‐label, so this was rated as 'unclear'. Hulsbosch 2008 did not report whether blinding had been used and we rated this as 'unclear'. Blinding of outcome assessment was rated 'unclear' in the Montes 2011 study because there were no information regarding this aspect of methodology.
Incomplete outcome data
Hulsbosch 2008 used the Intention‐to‐treat (ITT) method of analysis. The attrition rate was 23%. In Montes 2011 the attrition rate was 1%. However, Montes 2011 was rated as 'high risk', because the analysis sample did not consist of all the enrolled participants. "A total of 340 patients were included in the study. Twenty patients were excluded from the analysis due to major protocol deviations. An additional group of 66 patients were classified as not properly exposed to the intervention and also excluded. The analysis sample population comprised a total of 254 patients, 100 in the SMSG and 154 in the CG".
Selective reporting
We did not have protocols for either of the included studies. Both were classified as 'high risk'. Hulsbosch 2008 did not report all instruments (CUE). The trial author, however, sent data of the instrument after we contacted him. In Montes 2011, the study analysis sample did not consist of all participants. Results were written slightly emphasising significantly greater results. For example, "Nevertheless, the improvement was significantly greater only in item 4: percentage of affirmative responses of 45% in the SMSG versus 59.1% in CG". "There was a significantly greater improvement at month 3 in negative (3.3 vs. 3.5), cognitive (3.3 vs. 3.6) and global symptoms (3.2 vs. 3.5) as assessed by the CGI‐SCH‐DG in the SMSG than in the CG, respectively (P<0.05)". "After 3 months of intervention, improvement in quality of life was significantly greater in the SMSG (mean changes from baseline EQ‐5D visual analogue scale (VAS) of 6.6, 95%CI 6.38, 6.82) compared to the CG (3.1, 95%CI 2.91, 3.29), P = 0.03".
Other potential sources of bias
There is no clear source of other biases. In both cases we classified trials as 'unclear'. Montes 2011 was funded by the pharmaceutical company (AstraZeneca, Spain) but we were not at all sure if this would effect results.
Effects of interventions
See: Table 1
1. COMPARISON 1: INFORMATION AND COMMUNICATION TECHNOLOGY (ICT) FOR PROMPTING SUPPORT + STANDARD PROFESSIONAL CARE versus STANDARD CARE
We calculated risk ratios (RR) for dichotomous data and mean differences (MD) for continuous data, with their respective 95% confidence intervals (CIs).
1.1 Compliance with medication
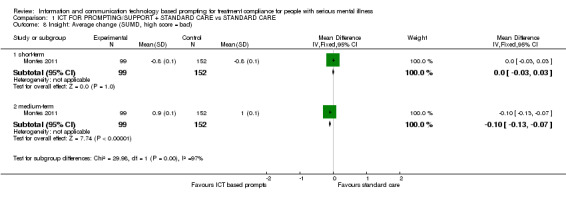
Montes 2011 measured compliance with medication by using MAQ and DAI‐10 questionnaires. In agreement with the trial authors, we found no differences between the ICT‐based prompting group and standard care group in whatever way compliance was measured. Reduction in MAQ total scores showed no difference (n = 320, RR 1.02 CI 0.95 to 1.10). The same number of people in both groups achieved a specific mean MAQ total scores in three months (n = 320, RR 1.07 CI 0.98 to 1.17). No differences were found between intervention group and standard care group when people were asked if they would stop taking their medication (n = 320, RR 1.11 CI 0.96 to 1.29, Analysis 1.1). Neither were differences found in MAQ short‐term results (n = 251, MD 0.30 CI 0.27 to 0.33) or the medium‐term data (n = 254, MD 0.30 CI CI 0.27 to 0.33). Moreover, we did not find that compliance with medication would be better in either group when measured with DAI‐10 (n = 251, MD medium term 1.40 CI 1.32 to 1.48, Analysis 1.2).
1.2. Analysis.
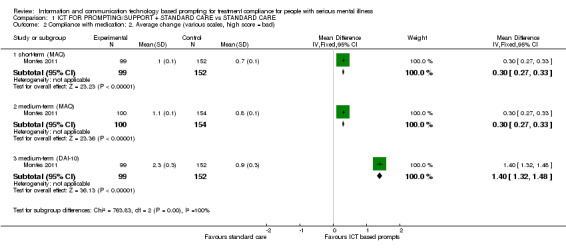
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 2 Compliance with medication: 2. Average change (various scales, high score = bad).
1.2 Mental state: Average change in specific symptom scores
Only Montes 2011 reported average change in specific symptom scores. Differences were found between ICT‐based prompting and standard care groups in short and medium terms. For example, average change in specific symptom scores measured by CGI‐SCH‐DC showed that there were differences in cognitive symptoms in the short term (n = 251, MD ‐0.30 CI ‐0.53 to ‐0.07). There were no differences in the medium‐term scores (n = 251, MD 0.00 CI ‐0.25 to 0.25). Severity of illness measured by CGI‐SCH‐SI showed differences in the short term (n = 251, MD ‐0.10 CI ‐0.13 to ‐0.07) and medium term (n = 251, MD ‐0.10 CI ‐0.13 to ‐0.07, Analysis 1.3).
1.3. Analysis.
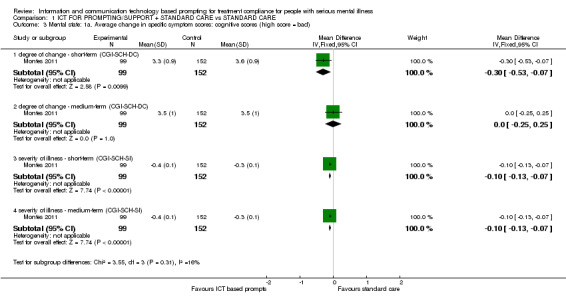
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 3 Mental state: 1a. Average change in specific symptom scores: cognitive scores (high score = bad).
There were no differences in average change of depression scores as measured by CGI‐SCH‐DC (n = 251, MD short term ‐0.20 CI ‐0.45 to 0.05, n = 251, MD medium term 0.00 CI ‐0.28 to 0.28), Analysis 1.4).
1.4. Analysis.
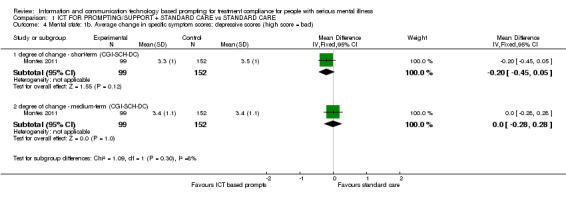
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 4 Mental state: 1b. Average change in specific symptom scores: depressive scores (high score = bad).
Average change in global symptoms were better in the intervention group in the short term (n = 251, MD ‐0.30 CI ‐0.53 to ‐0.07). There were no differences in medium term (n = 251, MD ‐0.20 CI ‐0.46 to 0.06). Severity of illness, measured by CGI‐SCH‐SI, also favoured the intervention group (n = 251, MD medium term ‐0.10 CI ‐0.13 to ‐0.07**, Table 2).
Average change of negative symptoms measured by CGI‐SCH‐DC showed no differences between groups in the short term (n = 251, MD ‐0.20 CI ‐0.44 to 0.04). Findings were similar for the medium term (n = 251, MD 0.00 CI ‐0.25 to 0.25). Severity of illness measured by CGI‐SCH‐SI, however, showed differences in negative symptoms for both periods (n = 251, MD short term ‐0.10 CI ‐0.38 to 0.18; n=251, MD medium term ‐0.30 CI ‐0.58 to 0.02, Analysis 1.6)
1.6. Analysis.
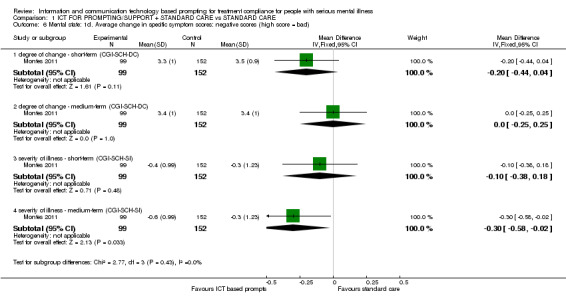
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 6 Mental state: 1d. Average change in specific symptom scores: negative scores (high score = bad).
There were no differences between two groups in positive symptoms measured by CGI‐SCH‐DC (n = 251, MD short term ‐0.20 CI ‐0.45 to 0.05; n = 251, MD medium term 0.10 CI ‐0.15 to 0.35). CGI‐SCH‐SI, however, again showed differences in short‐term severity of illness (n = 251, MD ‐0.10 CI ‐0.13 to ‐0.07). No differences were found on severity of illness by the medium term (n = 251, MD 0.00 CI ‐0.03 to 0.03, Analysis 1.7)
1.7. Analysis.
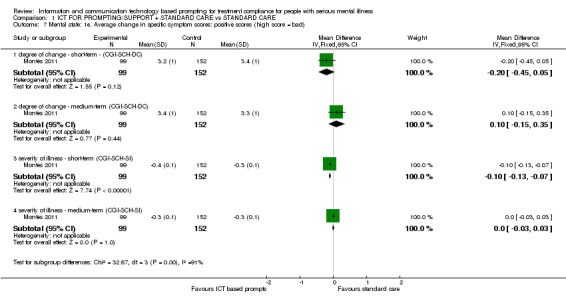
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 7 Mental state: 1e. Average change in specific symptom scores: positive scores (high score = bad).
1.3 Insight: Average change in level of insight scores
Only Montes 2011 reported average change in level of insight score (measured by SUMD). They found a difference between groups in the medium term. Level of insight improved more among people receiving ICT‐based prompt compared with those in control group (n = 251, MD ‐0.10 CI ‐0.13 to ‐0.07, Analysis 1.8).
1.8. Analysis.
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 8 Insight: Average change (SUMD, high score = bad).
1.4 Quality of life: Average change in quality of life scores
Both included studies reported average change in quality of life scores. Hulsbosch 2008 used Loneliness Scale and MANSA. Montes 2011, however, measured quality of life by EQ‐5D‐VAS. Measured by this scale, differences were apparent (n = 251, MD short term 3.50 CI 3.21 to 3.79; n = 251, MD medium term 0.50 CI 0.19 to 0.81, Analysis 1.9). There were no differences, however, between ICT‐based prompting and standard care in either the medium term (n = 72, MD 0.22 CI ‐0.09 to 0.53), or long term when quality of life was measured with MANSA (n = 72, MD 0.05 CI ‐0.35 to 0.45, Analysis 1.9), or the Loneliness Scale (n = 73, MD medium term ‐2.17 CI ‐6.81 to 2.47; n = 72, MD long term ‐2.32 CI ‐7.89 to 3.25, Analysis 1.10).
1.9. Analysis.
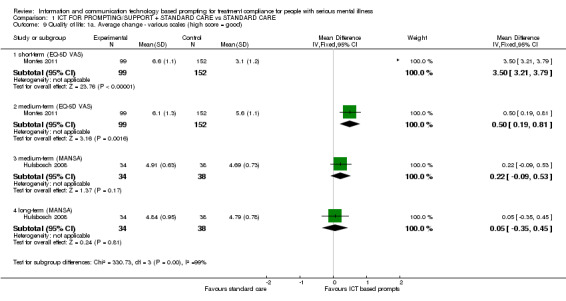
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 9 Quality of life: 1a. Average change ‐ various scales (high score = good).
1.10. Analysis.
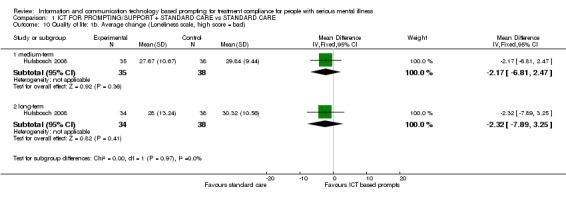
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 10 Quality of life: 1b. Average change (Loneliness scale, high score = bad).
1.5 Functioning: Average change in specific aspects of functioning
Effects on functioning were reported in Hulsbosch 2008 measured by Computer Use and Experience Scale (CUE), WAI‐SR and HoNOS. Computer experience measured by CUE, showed a difference in the medium term (n = 71, MD 6.60 CI 1.25 to 11.95) but not long term (n = 71, MD 4.63 CI ‐0.60 to 9.86). It was the other way round when functioning was measured by the WAI‐S (n = 73, MD medium term 1.91 CI ‐1.47 to 5.29; n = 71, MD long term 6.16, CI 2.47 to 9.85, Analysis 1.11). Problems in functioning measured by HoNOS, showed no differences (n = 72, MD long term ‐0.93 CI ‐2.82 to 0.96, Analysis 1.12*).
1.11. Analysis.
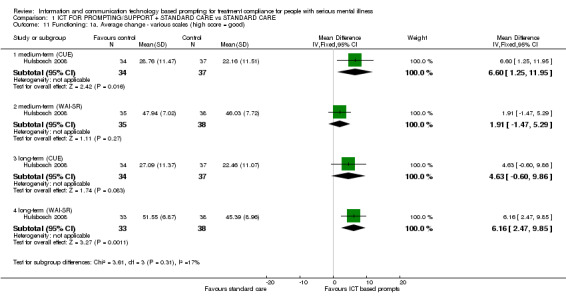
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 11 Functioning: 1a. Average change ‐ various scales (high score = good).
1.12. Analysis.
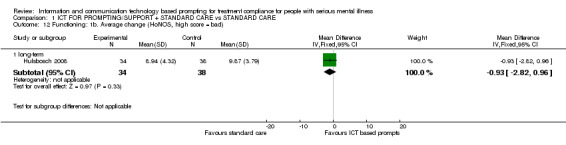
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 12 Functioning: 1b. Average change (HoNOS, high score = bad).
*Long term data measured by HoNOS were not totally normally distributed, but not skewed.
1.6 Satisfaction: Average change in general satisfaction with treatment scores
Only Hulsbosch 2008 reported average change in general satisfaction measured (by CANSAS, CQ‐Index and GGZ‐T). There were no differences between intervention and standard care groups in unmet needs measured by CANSAS (n = 73, MD medium term ‐0.20 CI ‐1.57 to 1.17; n = 72, MD long term ‐0.64 CI ‐2.00 to 0.72, Analysis 1.13**). Regarding the general satisfaction with treatment, we found no differences in GGZ‐T scores (n = 73, MD medium term 0.35 CI ‐1.35 to 2.05; n = 71, MD long term 1.59 CI 0.16 to 3.02). This also applies to CQ‐Index scores (n = 71, MD long term 0.72 CI 0.07 to 1.37, Analysis 1.14).
1.13. Analysis.
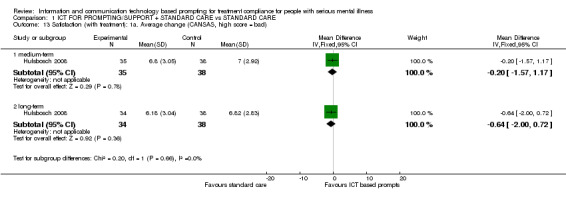
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 13 Satisfaction (with treatment): 1a. Average change (CANSAS, high score = bad).
1.14. Analysis.
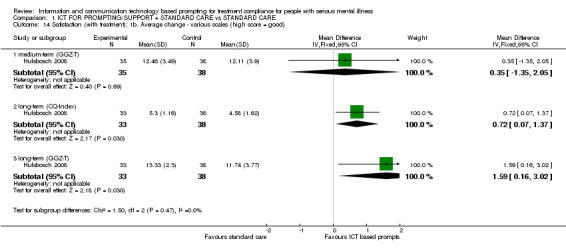
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 14 Satisfaction (with treatment): 1b. Average change ‐ various scales (high score = good).
**Data were not totally normally distributed, however, it was not skewed.
1.7 Acceptability of the intervention ‐ leaving the studies early
Acceptability of intervention was measured by loss to follow‐up or leaving the study early. There were no differences between groups (n = 347, RR 1.46 CI 0.70 to 3.05, Analysis 1.15).
1.15. Analysis.
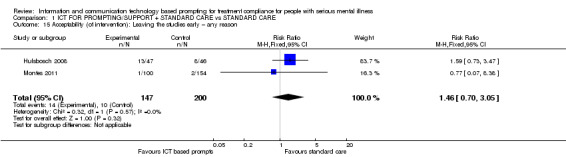
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 15 Acceptability (of intervention): Leaving the studies early – any reason.
1.8 Missing outcomes
Included studies did not report all outcomes we wished to find. Changes in service utilisation, behaviour, direct and indirect costs, adverse effects/events, including suicide attempts and death for all causes were missing.
Many outcomes were reported solely as continuous data and not as binary. This may indicate that the trials were more researcher‐driven than designed to provide data for clinicians, consumers and policymakers. In case that calculation of binary data were possible, analysis was calculated by the review authors. This was done in Analysis 1.1 (ISMS group 25% 0f 100 = 25. Total 100‐25 + 66 participants who were excluded from the analysis + 10 not meeting inclusion criteria. Control group 17.5% of 154 = 30. Total 154 ‐ 30 + 10) and Analysis 1.16 (ISMS group 25% 0f 100 = 25. Total 100 ‐ 25 + 66 participants who were excluded from the analysis + 10 not meeting inclusion criteria. Control group 17.5% of 154 = 30. Total 154 ‐ 30 + 10).
1.9 Skewed data
Skewed data were excluded from the analysis and is presented in Table 2.
2.0 Fixed and random effect
Results did not alter when we synthesised data for the primary outcome using a random‐effects model.
Discussion
Summary of main results
1. COMPARISON 1: INFORMATION AND COMMUNICATION TECHNOLOGY (ICT) FOR PROMPTING SUPPORT + STANDARD PROFESSIONAL CARE versus STANDARD CARE
Please see Table 1.
1.1 Compliance with medication
This was a primary outcome of this review. All data were from Montes 2011 and were both binary and continuous. The latter were only for a subset of the total number randomised (78%). By both measures, we found no clear evidence that ICT‐based prompting would promote compliance with medication compared with standard care. The binary outcome, used within the Table 1 was graded as being of 'moderate' quality.
1.2 Mental state
Overall, there is a suggestion that mental state might be helped by the ICT‐based prompting, but more and better data are needed. The CGI‐SCH‐SI measure used by Montes 2011 on the subset of people who were not excluded from the analysis tended to suggest positive outcomes for the ICT‐based promoting group. We have included one mental state outcome in the Table 1 and graded this outcome as being of 'low' quality, largely due to issues with selective reporting. The other measure (CGI‐SCH‐DC) tended to be more equivocal.
If such a simple intervention was really to improve mental state in terms of cognition, depressive, positive and negative symptoms, or overall, this would be most important. Montes 2011 has generated an important hypothesis but not, as yet, proven it.
1.3 Insight
According to Montes 2011, ICT‐based prompts may improve medium‐term insight. Again, this could be important as insight ‐ or lack of it ‐ has direct clinical impact. However, the result is based on one trial of unclear quality.
1.4 Quality of life
Both included studies (Hulsbosch 2008; Montes 2011) reported quality of life but used different measures. For the various measures of quality of life, there was a clear difference between intervention versus standard care groups for one measure (see Table 1). Employing an inconsistent variety of instruments makes evaluation more difficult.
In the Table 1, we have graded this as 'moderate' on quality of evidence, since the analysis sample does not consist of all the participants enrolled in study of Montes 2011 (22% participants excluded).
1.5 Functioning
One small trial Hulsbosch 2008 reported functioning. This was evaluated in many areas, including the use of computer, therapeutic alliance and evaluation of psychological and social functioning. However, only medium‐term CUE and long‐term WAI‐SR improved.
1.6 Satisfaction with treatment
Hulsbosch 2008 measured satisfaction with treatment. ICT‐based prompts did not clearly promote satisfaction.
1.7 Acceptability of the intervention ‐ leaving the studies early
Where it comes to loss to follow‐up or leaving the study early, there is no evidence that either treatment is less or more acceptable than the other. This was evaluated in both studies, but neither reported it directly as an outcome. In the Table 1, we rated this outcome as being of 'low' quality because, for Montes 2011, analysis did not consist of all randomised participants. Just over 39% of participants in the intervention group were excluded because of failure to receive the intervention correctly. This large proportion may indicate something about acceptability of the simple SMS intervention.
1.8 Missing outcomes
Neither included study reported behaviour, costs or adverse effects ‐ all secondary outcomes of this review. Costs and adverse effects were particularly important to us and were pre‐stated to be included in the Table 1. On the other hand, both studies reported several other important outcomes. However, instruments and outcomes varied between included studies (Table 3). The only outcome that both trials evaluated was quality of life. but they did not measure it with the same instrument. This confusion of outcomes is not helpful. Consistency and co‐operation between researchers could greatly increase the potency of any findings. Without this, opportunities to highlight any real effects are diminished.
2. Trial outcomes.
| Trial ► | Hulsbosch 2008 | Montes 2011 | |
| Domain ▼ | Scale ▼ | ||
| Compliance with medication | DAI‐10 | ✓ | |
| MAQ | ✓ | ||
| Quality of life | EQ‐5D VAS | ✓ | |
| Loneliness Scale | ✓ | ||
| MANSA | ✓ | ||
| Functioning | CUE | ✓ | |
| HoNos | ✓ | ||
| WAI‐SR | ✓ | ||
| Insight | SUMD | ✓ | |
| Satisfaction with treatment | CANSAS | ✓ | |
| CQ Index | ✓ | ||
| GZZ Thermometer | ✓ | ||
| Severity of illness | CGI‐SCH‐DC | ✓ | |
| CGI‐SCH‐SI | ✓ | ||
| Total estimate of questionnaires/person | 8 | 6 | |
| Total estimate of questions/person | 167 | 28 | |
CANSAS ‐ Camberwell Assessment of Needs ‐ Short Appraisal Schedule CGI‐SCH‐DC ‐ The Clinical Global Impression – Schizophrenia Scale ‐ degree of change CGI‐SCH‐SI ‐ The Clinical Global Impression – Schizophrenia Scale ‐ severity of illness CQ Index ‐ Consumer Quality Index CUE ‐ Computer Use and Experience Scale DAI‐10 ‐ Drug Attitude Inventory 10‐item version EQ‐5D VAS ‐ EQ‐5D visual analogue scale GZZ Thermometer ‐ Voor waardering door cliënten HoNos ‐ Health of the Nation Outcome Scale MANSA ‐ Manchester Short Assessment of Quality of Life MAQ ‐ Morisky Green Adherence Questionnaire SUMD ‐ Unawareness of Mental Disorder WAI‐SR ‐ Working Alliance Inventory‐Short Revised
Overall completeness and applicability of evidence
1. Completeness
There were only two relatively small studies looking at the effects of semi‐automatic information and communication technology‐based prompting to support treatment compliance. Both specifically focused on people with severe mental illness and one (Montes 2011), had compliance as its primary outcome. All data, however, are incomplete and several important estimates of effect are completely missing. Behaviour, costs and adverse effects are important and it is perfectly feasible that they could be effected by the prompt. Välimäki 2012 states that their trial will include evaluation of adverse effects.
2. Applicability
With more confidence in the findings, and more consistent and clinically meaningful positive results, in many cultures of care, the ICT‐based prompting interventions described in the two included studies could be implemented.
Quality of the evidence
Both included studies (Hulsbosch 2008 and Montes 2011) were randomised, but blinding of outcome assessment, participants or personnel was not used. One study (Hulsbosch 2008) reported random sequence generation, while allocation concealment was unclear in both studies. In Hulsbosch 2008, the attrition rate was 23% and ITT analysis was used, whereas in Montes 2011 the study attrition rate was very low at only 1%. In Hulsbosch 2008 all CUE measurements were not reported, but the data were sent to us when requested. On the other hand, in Montes 2011, all the data were available, although its interpretation was unclear. We did not find any other clear biases. However, the study by Montes 2011 was funded by pharmaceutical company. In addition, we do not have the protocols of the included studies available, so we are not able to evaluate whether there are any differences between the protocols and actual studies. We felt that the overall quality was not strong and could easily have been considerably improved by compliance with CONSORT.
Potential biases in the review process
The process of searching for studies was thorough. We followed the review protocol in study selection, data extraction and analysis. However, we only worked with published reports in this review and may be perpetuating a publishing bias. In addition, out of two included studies, one was from the Netherlands, only available in Dutch language, and not published in peer‐reviewed international journal (Hulsbosch 2008). We contacted the author and he helpfully translated crucial parts of the study and we were able to extract data needed for the review. We did change our list of outcomes to be included in the Table 1. We added costs and adverse effects and that was before appreciating all the data, but we do not have this recorded and could have been influenced by findings. We think not and no data are available, but this issue should be considered by the reader. In addition, the search date was in July 2012, which should be taken into consideration.
Agreements and disagreements with other studies or reviews
This Cochrane systematic review investigating the effects of any prompt to encourage appointment attendance for people with serious mental illness (Reda 2010) showed that a simple prompt to attend clinic, very close to the time of the appointment, probably encourages attendance. The results of this review do not contradict that finding. Our review also indicated the need for more research in this area where fair testing of simple measures could be so revealing.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
There is no clear evidence for or against using modern technology prompting systems for treatment compliance for people with schizophrenia. People with schizophrenia may wish to volunteer to be involved in adaption and fair testing of any innovations before they are implemented without evaluation.
2. For clinicians
This review was unable to provide sufficient evidence to inform clinicians about the value of information and communication technology‐based prompting to support treatment compliance for people with serious mental illness. Clinicians need to follow the development of this area in great detail. User‐friendly and cost‐effective technology already exists in this area (Sims 2012), and clinicians need to be aware of the possible positive and negative effects of these innovations for people with serious mental illness.
3. For commissioners or policymakers
There is no evidence for or against using information and communication technology‐based prompting to support treatment compliance for people with serious mental illness. Moreover, no data exist about the economic consequences of using this technology in clinical practice. We do have a potential conflict of interest in saying that more high‐quality studies should be undertaken in this area to explore the costs of this novel intervention and variations of approach. Several of the review authors are involved on one such study (Välimäki 2012), but many more are needed. Policymakers need to follow the development of technology‐based prompting systems as the area is developing fast, and inexpensive and effective innovations may be available.
Implications for research.
1. General
Following CONSORT for good reporting of randomisation, identification of participants, and description of interventions would have helped to increase the data available for this review. A minimum requirement should be that all data should at least be presented in numbers to enable statistical analysis. Continuous data should be reported with mean, standard deviations, and the number of participants should be clearly stated. Both studies placed too much reliance on scales for which there was no concordance whatsoever (Table 3), and also reported continuous measures at the expense of binary data. We remain unsure of the clinical meaning of the measures used. We are not clear that either report of the trials convincingly describe the clinical utility of the measures. It is important that the needs of researchers are met to measure fine‐grain changes in outcomes. This should not be at the expense of outcomes stipulated to be of importance to clinicians and people with the target problems.
2. Specific
2.1 Reviews
We identified several interesting studies that suggested other topics which could be the focus of reviews in this broad area (Table 4).
3. Review titles suggested by excluded studies.
| Title | Reference |
| Cognitive adaption training for people with schizophrenia | Velligan 2008 |
| Computer‐mediated structuring of patient‐key worker communication for people with schizophrenia | Hansson 2008 |
| Contingency management for people with schizophrenia | Tidey 2011 |
| Medication monitoring for people with schizophrenia | Frangou 2005 |
| Orientation statements for people with schizophrenia | Kluger 1983 |
| Pharmacy‐mediated monitoring for people with schizophrenia | Nietert 2009 |
| Telephone prompting for people with schizophrenia* | Burgoyne 1983, Crespo‐Iglesias 2006, Kluger 1983, Montes 2009, Rossi 1994 |
| Visual feedback techniques for people with schizophrenia | Cramer 1999 |
* Already within Reda 2010
2.2 Trials
The evidence base on the effects of ICT‐based prompts is still inconclusive. Data to clarify ICT‐based prompting effects are awaited from an ongoing trial, but further well‐conducted trials considering the different ICT‐based prompts are warranted. Välimäki 2012 is one such study but there are an enormous number of comparisons and variations of ICT‐based prompting interventions that are possible to test in this area. The Välimäki 2012 prompt is consumer‐tailored. This is different to what has gone before and it will be interesting to see its acceptability. However, there are now many other types of prompts ‐ commercial or non‐commercial ‐ available.
Future trials should employ well‐standardised ICT‐based interventions with clear definitions of the content of interventions. Methodology should also be well‐described and the use of CONSORT should be obligatory.
Acknowledgements
The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods section of their reviews. We have used this text as the basis of what appears here and adapted it as required. We also wish to thank Claire B Irving and Samantha Roberts for their valuable help and assistance during the review process.
We would particularly like to thank the kindness of Dr. Jorge Maurino and Dr. Lex Hulsbosch for sharing information about their trials. We look forward to any future collaboration and remain truly grateful for their help.
Data and analyses
Comparison 1. ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Compliance with medication: 1. Binary outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 decreased compliance (reduction in MAQ total scores) | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.10] |
| 1.2 not achieving a mean MAQ total score of >1 | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.98, 1.17] |
| 1.3 would stop taking medication (MAQ, item 4) | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.96, 1.29] |
| 2 Compliance with medication: 2. Average change (various scales, high score = bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 short‐term (MAQ) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.27, 0.33] |
| 2.2 medium‐term (MAQ) | 1 | 254 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.27, 0.33] |
| 2.3 medium‐term (DAI‐10) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [1.32, 1.48] |
| 3 Mental state: 1a. Average change in specific symptom scores: cognitive scores (high score = bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 degree of change ‐ short‐term (CGI‐SCH‐DC) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.53, ‐0.07] |
| 3.2 degree of change ‐ medium‐term (CGI‐SCH‐DC) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.25, 0.25] |
| 3.3 severity of illness ‐ short‐term (CGI‐SCH‐SI) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.13, ‐0.07] |
| 3.4 severity of illness ‐ medium‐term (CGI‐SCH‐SI) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.13, ‐0.07] |
| 4 Mental state: 1b. Average change in specific symptom scores: depressive scores (high score = bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 degree of change ‐ short‐term (CGI‐SCH‐DC) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.45, 0.05] |
| 4.2 degree of change ‐ medium‐term (CGI‐SCH‐DC) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.28, 0.28] |
| 5 Mental state: 1c. Average change in specific symptom scores: global scores (high score = bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 degree of change ‐ short‐term (CGI‐SCH‐DC) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.53, ‐0.07] |
| 5.2 degree of change ‐ medium‐term ‐ (CGI‐SCH‐DC) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.46, 0.06] |
| 5.3 severity of illness ‐ short‐term (CGI‐SCH‐SI) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.23, ‐0.17] |
| 5.4 severity of illness ‐ medium‐term (CGI‐SCH‐SI) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.13, ‐0.07] |
| 6 Mental state: 1d. Average change in specific symptom scores: negative scores (high score = bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 degree of change ‐ short‐term (CGI‐SCH‐DC) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.44, 0.04] |
| 6.2 degree of change ‐ medium‐term (CGI‐SCH‐DC) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.25, 0.25] |
| 6.3 severity of illness ‐ short‐term (CGI‐SCH‐SI) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.38, 0.18] |
| 6.4 severity of illness ‐ medium‐term (CGI‐SCH‐SI) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.3 [‐0.58, ‐0.02] |
| 7 Mental state: 1e. Average change in specific symptom scores: positive scores (high score = bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 degree of change ‐ short‐term ‐ (CGI‐SCH‐DC) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.45, 0.05] |
| 7.2 degree of change ‐ medium‐term (CGI‐SCH‐DC) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.15, 0.35] |
| 7.3 severity of illness ‐ short‐term (CGI‐SCH‐SI) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.13, ‐0.07] |
| 7.4 severity of illness ‐ medium‐term (CGI‐SCH‐SI) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8 Insight: Average change (SUMD, high score = bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 short‐term | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8.2 medium‐term | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.13, ‐0.07] |
| 9 Quality of life: 1a. Average change ‐ various scales (high score = good) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 short‐term (EQ‐5D VAS) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | 3.50 [3.21, 3.79] |
| 9.2 medium‐term (EQ‐5D VAS) | 1 | 251 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.19, 0.81] |
| 9.3 medium‐term (MANSA) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.09, 0.53] |
| 9.4 long‐term (MANSA) | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.35, 0.45] |
| 10 Quality of life: 1b. Average change (Loneliness scale, high score = bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 medium‐term | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐2.17 [‐6.81, 2.47] |
| 10.2 long‐term | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐2.32 [‐7.89, 3.25] |
| 11 Functioning: 1a. Average change ‐ various scales (high score = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 medium‐term (CUE) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 6.60 [1.25, 11.95] |
| 11.2 medium‐term (WAI‐SR) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 1.91 [‐1.47, 5.29] |
| 11.3 long‐term (CUE) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 4.63 [‐0.60, 9.86] |
| 11.4 long‐term (WAI‐SR) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 6.16 [2.47, 9.85] |
| 12 Functioning: 1b. Average change (HoNOS, high score = bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 long‐term | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐2.82, 0.96] |
| 13 Satisfaction (with treatment): 1a. Average change (CANSAS, high score = bad) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 medium‐term | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.57, 1.17] |
| 13.2 long‐term | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐2.00, 0.72] |
| 14 Satisfaction (with treatment): 1b. Average change ‐ various scales (high score = good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 medium‐term (GGZ‐T) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐1.35, 2.05] |
| 14.2 long‐term (CQ‐Index) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [0.07, 1.37] |
| 14.3 long‐term (GGZ‐T) | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [0.16, 3.02] |
| 15 Acceptability (of intervention): Leaving the studies early – any reason | 2 | 347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.70, 3.05] |
| 16 Sensitivity analyses ‐ Compliance with medication: 1. Binary outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 decreased compliance (reduction in MAQ total scores) ‐ ITT | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.94, 1.12] |
| 16.2 decreased compliance (reduction in MAQ total scores) ‐ AS REPORTED | 1 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.04] |
| 16.3 not achieving a mean MAQ total score of >1 ‐ ITT | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.97, 1.19] |
| 16.4 not achieving a mean MAQ total score of >1 ‐ AS REPORTED | 1 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.97] |
| 16.5 would stop taking medication (MAQ, item 4) ‐ ITT | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.95, 1.31] |
| 16.6 would stop taking medication (MAQ, item 4) ‐ AS REPORTED | 1 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.59, 0.98] |
1.5. Analysis.
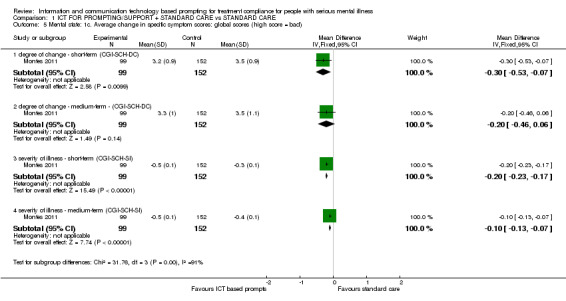
Comparison 1 ICT FOR PROMPTING/SUPPORT + STANDARD CARE vs STANDARD CARE, Outcome 5 Mental state: 1c. Average change in specific symptom scores: global scores (high score = bad).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hulsbosch 2008.
| Methods | Allocation: randomised ‐ block randomisation. Blindness: no. Duration: 18 months. Setting: outpatient. |
|
| Participants | Diagnosis: people with schizophrenia, schizoaffective, depressive or bipolar disorders. N = 93. Age: average age ˜ 45 years. Sex: both. History: clients of two outpatient teams. Inclusion: severe mentally ill (DSM‐IV), duration of illness two years or longer associated with limitations in daily life. Exclusion: homeless, living in sheltered homes, long‐term hospitalised, insufficient comprehension of Dutch language. |
|
| Interventions | 1. Electronic assistant device (including web cam, computerised information about treatment, such as; treatment plans, lab results, reminders and scheduled appointments, support by significant others via web cam) + usual care. N = 47. 2. Usual care without electronic assistant device. N = 46. |
|
| Outcomes | Quality of life: MANSA, Social isolation (Loneliness Scale). Functioning: CUE (Computer use), WAI‐SR (Therapeutic alliance), HoNos (Global functioning). Satisfaction with treatment: CANSAS (Fulfilment of needs), CQ Index (Experiences of care), GZZ Thermometer (Client satisfaction). Data not able to use: Service utilisation (No clear description of the outcome in English language |
|
| Notes | Total report in English is not available. We contacted the authors for further information relating to the use of scales and clarification of study outcomes, the authors provided us with this information and we were able to include this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation, by using block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate was 23%. ITT analysis was used. |
| Selective reporting (reporting bias) | High risk | We do not have protocol of the study available. All instruments were not reported (CUE). However, author sent the data of the instrument. |
| Other bias | Unclear risk | No clear other bias. Research protocol was not available. |
Montes 2011.
| Methods | Allocation: randomised by using computer‐generated codes and sealed envelopes which were opened in an ascending order by investigator. Blindness: open‐label. Duration: 24 weeks. Setting: 56 mental health outpatient centres throughout Spain. |
|
| Participants | Diagnosis: schizophrenia (DSM‐IV). N = 320, analysis sample population N = 254. Age: average age ˜ 40 years (SD ˜ 11). Sex: male and female. History: outpatients with schizophrenia. Inclusion: 18 ‐ 65 years old, clinically stabilized, using a single oral antipsychotic medication and considered to be poor adherent (at least one affirmative answer of Morisky‐Green questionnaire ‐MAQ) to antipsychotic monotherapy, follow‐up as an outpatient, availability of a cell phone capable to receive SMS‐messages. Exclusion: patients receiving long‐acting injectable antipsychotic treatment. |
|
| Interventions | 1. SMS group: daily SMS on cell phone to remind to take medication, during 12 weeks. N = 166, analysis sample N = 100. 2. Control group: standard care and no message or any other intensive approach for increasing medication compliance. N = 154. Duration: intervention 12 weeks, follow‐up 6 months. |
|
| Outcomes | Compliance: Loss to follow‐up. Compliance with medication: MAQ (adherence to medication), DAI‐10 (attitude toward medication), Insight: SUMD (insight of the illness), CGI‐SCH‐SI (clinical global impression ‐ schizophrenia ‐ severity of illness), CGI‐SCH‐DC (clinical global impression ‐ schizophrenia ‐ degree of change). Quality of life: EQ‐5D VAS (health‐related quality of life). |
|
| Notes | 340 patients were enrolled and 320 were randomised, however, 86 patients were excluded from the analysis (n = 20 reason of major protocol deviations, n = 66 not properly exposed to the intervention). *Analyse 1.16 was calculated by reviewers (SMS group 25% 0f 100 = 25. Total 100 were added 66 participants who were excluded from the analysis. Control group 17.5% of 154 = 30). *Analyse 1.17 was calculated by reviewers (SMS group were added 66 participants who were excluded from the analysis). *Analyse 1.18 was calculated by reviewers (SMS group 45% 0f 100 = 45. Total 100 were added 66 participants who were excluded from the analysis 1. We had to contact the authors to ask more information on intervention, published reports, number of participants and data (MAQ and DAI‐10). The information was obtained and we were able to include this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised. "Randomisation codes were computer generated by our statistician and sealed in envelopes labelled with consecutive numbers. Envelopes were opened by the investigator in a ascending order and patients were allocated to the intervention or control group". |
| Allocation concealment (selection bias) | Low risk | "Envelopes were opened by the investigator in a ascending order and patients were allocated to the intervention or control group". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate was 1%. Analysis sample do not consist all the enrolled participants. "A total of 340 patients were included in the study. Twenty patients were excluded from the analysis due to major protocol deviations. An additional group of 66 patients were classified as not properly exposed to the intervention and also excluded. The analysis sample population comprised a total of 254 patients, 100 in the SMSG and 154 in the CG". |
| Selective reporting (reporting bias) | High risk | We do not have protocol of the study available. Analysis sample population do not consists all participants. Results are written emphasising significantly greater results; "Nevertheless, the improvement was significantly greater only in item 4: percentage of affirmative responses of 45% in the SMSG versus 59.1% in CG". "There was a significantly greater improvement at month 3 in negative (3.3 vs. 3.5), cognitive (3.3 vs. 3.6) and global symptoms (3.2 vs. 3.5) as assessed by the CGI‐SCH‐DG in the SMSG than in the CG, respectively (P<0.05)". "After 3 months of intervention, improvement in quality of life was significantly greater in the SMSG (mean changes from baseline EQ‐5D visual analogue scale (VAS) of 6.6, 95%CI 6.38, 6.82) compared to the CG (3.1, 95%CI 2.91, 3.29), P = 0.03". |
| Other bias | Unclear risk | No clear other bias. Funded by pharmaceutical industry ‐ AstraZeneca, Spain. |
Rating scale abbreviations CANSAS ‐ Camberwell Assessment of Needs ‐ Short Appraisal Schedule CQ Index ‐ Consumer Quality Index CUE ‐ Computer Use and Experience Scale DAI‐10 ‐ Drug Attitude Inventory 10‐item version DSM‐IV ‐ Diagnostic and Statistical Manual of Mental Disorders EQ‐5D VAS ‐ EQ‐5D visual analogue scale GZZ Thermometer ‐ Voor waardering door cliënten HoNos ‐ Health of the Nation Outcome Scale MANSA ‐ Manchester Short Assessment of Quality of Life MAQ ‐ Morisky Green Adherence Questionnaire SUMD ‐ Unawareness of Mental Disorder WAI‐SR ‐ Working Alliance Inventory‐Short Revised General abbreviations ITT ‐ intention to treat N ‐ number of participants SD ‐ standard deviation SMS ‐ short message service vs ‐ versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Burgoyne 1983 | Allocation: randomised. Participants: severe mental illness. Intervention: telephone prompt vs. no prompt, not semi‐automatic ICT‐based prompt. |
| Cramer 1999 | Allocation: randomised. Participants: less than 50% with schizophrenia. Intervention: compliance support via visual feedback, both groups had electronic device. |
| Crespo‐Iglesias 2006 | Allocation: randomised. Participants: all people with psychiatry appointment. Intervention: telephone call vs. assume no telephone call, not semi‐automatic ICT‐based prompt. |
| Frangou 2005 | Allocation: randomised. Participants: schizophrenia. Intervention: e‐monitoring (medication dispenser transmitting to research team) vs. pill counting vs. usual care, not semi‐automatic ICT‐based prompt. |
| Hansson 2008 | Allocation: randomised. Participants: schizophrenia or related psychotic disorder. Intervention: DIALOG (computer‐mediated structuring patient‐key worker communicating to patient satisfaction with 8 life domains) vs usual care, not semi‐automatic ICT‐based prompt. |
| Kluger 1983 | Allocation: randomised. Participants: psychiatric outpatients. Intervention: telephone prompt vs. orientation statement + prompt vs. orientation statement vs. usual care, not semi‐automatic ICT‐based prompt. |
| Lassen 2011 | Allocation: quasi‐randomised. Participants: schizophrenia or related psychotic disorder. Intervention: acceptance and commitment therapy or treatment as usual + treatment as usual, not semi‐automatic ICT‐based prompt. |
| MacDonald 2000a | Allocation: not randomised. Participants: psychiatric outpatients. Intervention: telephone prompting vs. usual care, not semi‐automatic ICT‐based prompt. |
| Montes 2009 | Allocation: randomised. Participants: schizophrenia. Intervention: telephone call vs. usual care, not semi‐automatic ICT prompt. |
| Nietert 2009 | Allocation: randomised. Participants: people with mixed serious medical diagnoses, 1 % psychosis. Intervention: system to prompt pharmacist to prompt clinicians via telephone vs. facsimile vs. usual care, not semi‐automatic ICT prompt. |
| Pijnenborg 2010 | Allocation: quasi‐randomised, not randomised. Participants: schizophrenia or related disorders. Intervention: SMS‐message semi‐automated goal directed prompts vs. delay instigation waiting list control full stop. |
| Rossi 1994 | Allocation: randomised. Participants: severe mental illness. Intervention: telephone prompt vs. assignment only vs. assignment with written prompt vs. control, not semi‐automatic ICT prompt. |
| Tidey 2011 | Allocation: randomised. Participants: schizophrenia. Intervention: contingency management vs. non‐contingent reinforcement, not semi‐automatic ICT prompt. |
| Velligan 2008 | Allocation: randomised. Participants: schizophrenia outpatients. Intervention: full‐CAT vs. pharm‐CAT vs. treatment as usual, not (ONLY) semi‐automatic ICT prompt. |
CAT ‐ cognitive adaption training DIALOG ‐ computer‐mediated structuring patient‐key worker communicating tool full‐CAT ‐ cognitive adaption training to improve multiple areas of adaptive functioning ICT ‐ information and communication technology pharm‐CAT ‐ cognitive adaption training for medication adherence SMS ‐ short message service vs ‐ versus
Characteristics of studies awaiting assessment [ordered by study ID]
Berns 2001.
| Methods | Allocation: randomised. |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder or bipolar disorder. |
| Interventions | 1. Assistive device. 2. No assistive device. |
| Outcomes | 1. Neuropsychological function. 2. Quality of life. 3. Disability. |
| Notes | We contacted the authors for further information that is needed on intervention, methods and participants, we did not receive a reply. |
Characteristics of ongoing studies [ordered by study ID]
Välimäki 2012.
| Trial name or title | "Mobile telephone text messages to encourage compliance with medication and to follow up with people with psychosis: A multi‐centre randomised controlled two armed trial". "Mobile.Net" (132581) |
| Methods | Allocation: randomised. |
| Participants | Diagnosis: psychosis. N = 3100, 1550 for both arms. Age: 18‐65 years. Sex: male and female. Inclusion: antipsychotic medication, discharged from psychiatric hospital, have mobile phone, able to use Finnish language, able to give written informed consent to participate. Exclusion: unable to use the Finnish language, unable to give written informed consent to participate, planned non‐acute treatment period or visit in psychiatric hospital, planned non‐acute treatment period or visit in‐psychiatric hospital, forensic patients. |
| Interventions | 1. Semi‐automatic SMS‐messages. 2. Treatment as usual. |
| Outcomes | Compliance: Drop‐outs. Service utilisation: use of social and health care services, involuntary treatment in psychiatric hospital, use of coercive measures, use of specialised mental health in‐ and outpatient care, use of primary health care, healthcare centres, use of reimbursements of National Health Insurance. Quality of life: Q‐LES‐Q. Satisfaction with treatment: CSQ. Acceptability of intervention: patient requests to stop the text‐messages. Adverse effects: adverse events. |
| Starting date | 01/06/2011 |
| Contact information | Professor Maritta Välimäki University of Turku, Finland. http://www.controlled‐trials.com/ISRCTN27704027 |
| Notes | Estimated completion date: 31/12/2013. Total N revised to 1000. Recruitment now closed (12‐2012), completion of follow‐up end 2014. |
CSQ ‐ Client Satisfaction Questionnaire SMS ‐ short message service Q‐LES‐Q ‐ Quality of Life Enjoyment and Satisfaction Questionnaire
Differences between protocol and review
We modified the definition of the intervention and based on this modification, manual telephone calls were excluded. We had not accounted for this in the original protocol but do not feel that this compromises our investigation.
We added two outcomes to the 'Summary of findings' table, which were not listed in the protocol version. The reason for this was that our knowledge for ICT‐based prompts increased during the process of conducting review. In the protocol there were five outcomes. In the final version costs and adverse effects were added. This was done before seeing all data, but we do not have a record of this. Readers may choose, therefore to disregard this emphasis.
Contributions of authors
Kaisa Kauppi ‐ protocol production, analysis, data interpretation, data extraction, analysis and writing the final report of the review. Maritta Välimäki ‐ initiation of the review, protocol production, searching, data extraction, analysis, data interpretation and writing the final report of the review. Heli Hätönen ‐ protocol production, analysis, data interpretation, data extraction, analysis and writing the final report of the review. Lauri Kuosmanen ‐ protocol production, analysis, data interpretation, data extraction and writing the final report of the review. Katja Warwick‐Smith ‐ protocol production and writing the final report of the review. Clive Adams ‐ comments on drafts of the protocol, data interpretation, data extraction and writing the final report of the review.
Sources of support
Internal sources
-
The University of Turku, Finland.
University of Turku provided research facilities to conduct the review activities.
University of Nottingham, UK.
External sources
-
Academy of Finland, Finland.
(132581)
-
Turku University Hospital, The Hospital District of Southwest Finland, Finland.
(EVO 13893)
-
Satakunta Hospital District, State Subsidy System, Finland.
(EVO 12/2010, 81096)
Finnish Cultural Foundation, Foundations’ Professor Pool, Finland.
University of Turku, Finland.
University of Turku Foundation, Finland.
Declarations of interest
Kaisa Kauppi, Maritta Välimäki, Heli Hätönen and Clive Adams are involved in a study about ICT‐based interventions, especially prompting in psychiatric care, which is the ongoing study in this review (Välimäki 2012). The roles are specified below. Our opinions are actually quite open. Prior opinion at the time of completing the protocol, is that any kinds of prompt may increase treatment compliance for people with schizophrenia.
Kaisa Kauppi ‐ research assistant with an ongoing PhD‐dissertation linked to Välimäki 2012. Maritta Välimäki ‐ leader of the study Välimäki 2012. Heli Hätönen ‐ worked as a project researcher ‐ Välimäki 2012. Lauri Kuosmanen ‐ no declarations of interest. Katja Warwick‐Smith ‐ no declarations of interest. Clive Adams ‐ has helped design Välimäki 2012.
New
References
References to studies included in this review
Hulsbosch 2008 {published and unpublished data}
- Hulsbosch AM, Kroon H. Randomised controlled trial of home automation in mental health. Available from http: //www.trialregister.nl/trialreg/index.asp 2008.
Montes 2011 {published data only}
- Maurino J, Tesoro A, Diez T, Gomez‐Beneyto M. Short message service (SMS)‐based strategy to improve antipsychotic adherence among patients with schizophrenia. Value in Health 2010:242.
- Montes JM, Gmez Beneyto M, Tesoro A, Dez T, Maurino J. Short message service (sms)‐based strategy to improve antipsychotic adherence in schizophrenia. 19th European Congress of Psychiatry, Vienna 2011.
- Montes JM, Medina E, Gomez‐Beneyto M, Maurino J. A short message service (SMS)‐based strategy for enhancing adherence to antipsychotic medication in schizophrenia. Psychiatry Research 2012;200(2‐3):84‐95. [PUBMED: 22901437] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Burgoyne 1983 {published data only}
- Burgoyne RW, Acosta FX, Yamamoto J. Telephone prompting to increase attendance at a psychiatric outpatient clinic. American Journal of Psychiatry 1983;140:345‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cramer 1999 {published data only}
- Cramer JA, Rosenheck R. Enhancing medication compliance for people with serious mental illness. Journal of Nervous and Mental Disease 1999;187(1):53‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crespo‐Iglesias 2006 {published data only}
- Crespo‐Iglesias JM, Gonzalez Garcia A, Santos Miguelez B, Naya Fernandez S. Improvement of the attendance to the first psychiatric appointments after telephone reminder [Mejoria de la asistencia a las primeras citas en psiquiatria tras aviso telefonico]. Anales de Psiquiatria 2006;22(3):95‐101. [MEDLINE: ] [Google Scholar]
Frangou 2005 {published data only}
- Frangou S, Sachpazidis I, Stassinakis A, Sakas G. Telemonitoring of medication adherence in patients with schizophrenia. Telemedicine Journal and E‐Health 2005;11(6):675‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hansson 2008 {published data only}
- Hansson L, Svensson B, Bjorkman T, Bullenkamp J, Lauber C, Martinez‐Leal R, et al. What works for whom in a computer‐mediated communication intervention in community psychiatry? Moderators of outcome in a cluster randomized trial. Acta Psychiatrica Scandinavica 2008;118(5):404‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kluger 1983 {published data only}
- Kluger MP, Karras A. Strategies for reducing missed initial appointments in a community mental health center. Community Mental Health Journal 1983;19:137‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lassen 2011 {published data only}
- Lassen WE. The effects of acceptance and commitment therapy (act) on anxiety in people with psychosis. Sciences and Engineering 2011;71(11‐B):7093. [MEDLINE] [Google Scholar]
MacDonald 2000a {published data only}
- MacDonald J, Brown N, Ellis P. Using telephone prompts to improve initial attendance at a community mental health centre. Psychiatric Services 2000;51(6):812‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Montes 2009 {published data only}
- Montes J, Saiz Ruiz J, Claret J, Maurino J, Diez T. Effect of a nurse telephone follow‐up on therapeutic adherence of patients with schizophrenia. World Psychiatry 2009; Vol. 8, issue Suppl 1.
Nietert 2009 {published data only}
Pijnenborg 2010 {published data only}
- Pijnenborg GHM, Withaar FK, Brouwer WH, Timmerman ME, Bosch RJ, Evans JJ. The efficacy of SMS text messages to compensate for the effects of cognitive impairments in schizophrenia. British Journal of Clinical Psychology 2010;49:259‐74. [DOI] [PubMed] [Google Scholar]
Rossi 1994 {published data only}
- Rossi E. Effects of a simple strategy on attendance and satisfaction with medication clinic services in the chronically mentally ill. PhD dissertation submitted to the United States International University, USA 1994:103.
Tidey 2011 {published data only}
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Reid N. Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacology 2011;217(2):279‐87. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Velligan 2008 {published data only}
- Velligan DI, Diamond PM, Mintz J, Maples N, Li X, Zeber J, et al. The use of individually tailored environmental supports to improve medication adherence and outcomes in schizophrenia. Schizophrenia Bulletin 2008; Vol. 34, issue 3:483‐93. [DOI] [PMC free article] [PubMed]
References to studies awaiting assessment
Berns 2001 {published data only}
- Berns S. Electronic assistive device to help patients with schizophrenia. National Alliance for Research on Schizophrenia and Depression. Available from http: //www.mhsource.com/narsad/bd/studyops.html 2001.
References to ongoing studies
Välimäki 2012 {published and unpublished data}
- Välimäki M. Mobile telephone text messages to encourage compliance with medication and to follow up with people with psychosis: A multi‐centre randomised controlled two armed trial. http://www.controlled‐trials.com/ISRCTN27704027 2011. [DOI: 10.1186/ISRCTN27704027] [DOI]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amador 1991
- Amador XF, Strauss DH, Yale SA. Awareness of illness in schizophrenia. Schizophrenia Bulletin. 1991;17(1):113‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amador 1994
- Amador XE, Flaum M, Andreasen NC. Awareness of illness in schizophrenia and schizoaffective and mood disorders. Archives of General Psychiatry 1994;51(10):826‐36. [PUBMED: 7944872] [DOI] [PubMed] [Google Scholar]
Anderson 2010
- Anderson KH, Ford S, Robson D, Cassis J, Rodrigues C, Gray R. An exploratory, randomized controlled trial of adherence therapy for people with schizophrenia. International Journal of Mental Health Nursing 2010;19(5):340‐9. [PUBMED: 20887608] [DOI] [PubMed] [Google Scholar]
Atherton 2009
- Atherton H, Car J, Meyer B. Email for the management of healthcare appointments and attendance reminders. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007981] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berkman 2011
- Berkman ET, Dickenson J, Falk EB, Lieberman MD. Using SMS text messaging to assess moderators of smoking reduction: Validating a new tool for ecological measurement of health behaviors. Health Psychology 2011;30(2):186‐94. [PUBMED: 21401252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [PUBMED: 9302962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'etude des Indices d'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] [PubMed] [Google Scholar]
Brooks 1996
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37(1):53‐72. [PUBMED: 10158943] [DOI] [PubMed] [Google Scholar]
Buchanan 2005
- Buchanan RW, Carpenter WT. Concept of schizophrenia. In: Sadock BJ, Sadock VA editor(s). Kaplan & Sadock´s Comprehensive Textbook of Psychiatry. 8th Edition. Vol. II, Philadelphia, USA: Lippincott Williams & Wilins, 2005:1329–45. [Google Scholar]
de Jong Gierveld 1999
- Jong Gierveld J, Tilburg T. Manual of the Loneliness Scale. http://home.fsw.vu.nl/tg.van.tilburg/manual_loneliness_scale_1999.html 1999.
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Department of Health AU 2010
- Department of Health, Victorian Government, Melbourne. Mental Health Bill 2010, Information sheet 1: Supported decision making, improving patient participation and carer involvement. http: //www.health.vic.gov.au/mentalhealth/mhactreview/information_sheet1.pdf (accessed 15 August 2011).
Department of Health UK 2004
- Department of Health. Missed appointments guidance. GP Bulletin 2004, issue 33.
Department of Health UK 2010
- Department of Health. The NHS Choices Annual Report 2010. http: //www.nhs.uk/aboutNHSChoices/professionals/developments/Documents/annual‐report/annual‐report‐2010.pdf (accessed 15 August 2011).
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [PUBMED: 1453246] [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21(19):2971‐80. [PUBMED: 12325113] [DOI] [PubMed] [Google Scholar]
Eby 2005
- Eby L, Brown N. Mental Health Nursing Care. New Jersey: Pearson Education, 2005. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involvingcross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
EU 2008
- European Union. European pact for mental health and well‐being. EU high‐level conference “Together for mental health and wellbeing” 2008.
Fjeldsoe 2009
- Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short‐message service. American Journal of Preventive Medicine 2009;36(2):165‐73. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- Brozek J, Oxman J, Schunemann H. GRADEpro. Version 3.2 for Windows. Cochrane IMS, 2008.
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impression (CGI). ECDEU Assessment Manual for Psychopharmacology. Rockville, MD, U.S. Department of Health, Education, and Welfare 1976.
Hatcher 2006
- Hatcher RL, Gillaspy JA. Development and validation of a revised short version of the working alliance inventory. Psychotherapy Research 2006;16:12‐25. [Google Scholar]
Haug 2009
- Haug S, Meyer C, Schorr G, Bauer S, John U. Continuous individual support of smoking cessation using text messaging: a pilot experimental study. Nicotine & Tobacco Research 2009;11(8):915‐23. [DOI] [PubMed] [Google Scholar]
Health and Social Care Information Centre 2012
- Hospital Episode Statistics:Hospital Outpatient Activity 2011‐12 Summary report. https://catalogue.ic.nhs.uk/publications/hospital/outpatients/hosp‐outp‐acti‐11‐12/hosp‐outp‐acti‐11‐12‐summ‐repo‐rep.pdf.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hogan 1983
- Hogan TP, Awad AG, Eastwood R. A self‐report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychological Medicine 1983;13:177‐83. [DOI] [PubMed] [Google Scholar]
Hong 2009
- Hong J, Windmeijer F, Novick D, Haro JP, Brown J. The cost of relapse in patients with schizophrenia in the European SOHO (Schizophrenia Outpatient Helath Outcomes) study. Progress in Neuro‐Psychopharmacalogy & Biological Psychiatry 2009;33:835–41. [DOI] [PubMed] [Google Scholar]
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Kerzman 2003
- Kerzman T, Kok I, Wijngaarden B. De GGZ Thermometer Nader Onderzocht: De Evaluatie van een Vragenlijstvoor Cliëntwaardering in de Volwassenenzorg. Trimbos‐instituut, Utrecht, 2003. [Google Scholar]
Kitcheman 2008
- Kitcheman J, Adams CE, Pervaiz A, Kader I, Mohandas D, Brookes G. Does an encouraging letter encourage attendance at psychiatric out‐patient clinics? The Leeds PROMPTS randomized study. Psychological Medicine 2008;5:717‐23. [DOI] [PubMed] [Google Scholar]
Leucht 2005
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacDonald 2000
- MacDonald J, Brown N, Ellis P. Using telephone promts to improve initial attendance at a community mental health centre. Psychiatric Services 2000;51(6):812‐4. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
McIntosh 2006
- McIntosh AM, Conlon L, Lawrie SM, Stanfied AC. Compliance therapy for schizophrenia. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD003442.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morisky 1986
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Medical Care 1986;24(1):67‐74. [DOI] [PubMed] [Google Scholar]
Mulder 2004a
- Mulder CL, Staring ABP, Loos J, Buwalda V, Kuijpers D, Sytema S, et al. De Health of the NationsOutcome Scale (HoNOS) als instrument voor 'routine outcome assessment. Tijdschrift voor Psychiatrie. 2004;46(5):273‐85. [Google Scholar]
Mulder 2004b
- Mulder CL, Staring ABP, Loos J, Buwalda VJA, Kuijpers D, Sytema S, et al. No English title found [De Health of the NationsOutcome Scale (HoNOS) in Nederlandse bewerking: Handleiding Rotterdam: O3 Onderzoekscentrum GGZ Rijnmond. 2004b]. http://www.euronet.nl/sogg/honos.htm 2004.
Munder 2010
- Munder T, Wilmers F, Leonhart R, Linster HW, Barth J. Working Alliance Inventory‐Short Revised (WAISR): psychometric properties in outpatients and inpatients. Clinical Psychology and Psychotherapy 2010;17(3):231‐9. [DOI: 10.1002/cpp.658] [DOI] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Clinical Excellence. Schizophrenia. Core interventions in the treatment and management of schizophrenia in adults in primary and secondary care. Available from http: //www.nice.org.uk 2009.
Nicholl 2010
- Nicholl D, Akhras K, Diels J, Schadrack J. Burden of schizophrenia in recently diagnosed patients: healthcare utilisation and cost perspective. Current Medical Research & Opinion 2010;26(4):943–55. [DOI] [PubMed] [Google Scholar]
Nieuwenhuizen 1998
- Nieuwenhuizen CV, Schene A, Boevink W, Wolf J. The Lancashire Quality of Life Profile: First experiences in The Netherlands. Community Mental Health Journal 1998;34(5):513‐24. [DOI] [PubMed] [Google Scholar]
Nieuwenhuizen 2001
- Nieuwenhuizen C, Schene AH, Koeter MWJ, Huxley PJ. The Lancashire Quality of Life Profile: modification and psychometric evaluation. Social Psychiatry and Psychiatric Epidemiology 2001;36(1):36‐44. [DOI] [PubMed] [Google Scholar]
Oliver 1991
- Oliver JP. The Social Care Directive: development of a quality of life profile for use in community services for the mentally ill. Social Work and Social Sciences Review 1991;3:5‐45. [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Potosky 1998
- Potosky D, Bobko P. The Computer Experience and Understanding Scale: a self‐report measure of computer experience. Computers in Human Behavior 1998;14:337‐48. [Google Scholar]
Priebe 1999
- Priebe S, Huxley P, Knight S, Evans S. Application and results of the Manchester Short Assessment of Quality of Life (MANSA). International Journal of Social Psychiatry 1999;45(1):7‐12. [PUBMED: 10443245] [DOI] [PubMed] [Google Scholar]
Reda 2010
- Reda S, Rowett M, Makhoul S. Prompts to encourage appointment attendance for people with serious mental illness. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 1999
- Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Archives of Psychiatry 1999;56(3):241‐7. [DOI] [PubMed] [Google Scholar]
Sawyer 2002
- Sawyer SM, Zalan A, Bond LM. Telephone reminders improve adolescent clinic attendance: A randomised controlled trial. Journal of Paediatrics and Child Health 2002;38(1):79‐83. [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83. [Google Scholar]
Sims 2012
- Sims H, Sanghara H, Hayes D, Wandiembe S, Finch M, Jakobsen H, Tsakanikos E, Okocha CI, Kravariti E. Text message reminders of appointments: a pilot intervention at four community mental health clinics in London. Psychiatric Services 2012;36(2):161‐8. [PUBMED: 22302334] [DOI] [PubMed] [Google Scholar]
Slade 1999
- Slade M, Beck A, Bindman J, Thornicroft G, Wright S. Routine clinical outcome measures for patients with severe mental illness: CANSAS and HoNOS. British Journal of Psychiatry 1999;174:404‐8. [PUBMED: 10616605] [DOI] [PubMed] [Google Scholar]
Thi Phan 1995
- Thi Phan T. Enhancing client adherence to psychotropic medication regiments: a psychosocial nursing approach. International Journal of Psychiatric Nursing Research 1995;2(1):147‐72. [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organistation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
van Wijngaarden 2007
- Wijngaarden B, Kok I, Meije D, Fotiadis L. Een Consumer Quality Index voor Kortdurende Ambulante GGZ: De ontwikkeling en Psychometrische Kwaliteiten van een Vragenlijst voor het Meten van Cliëntervaringen ‐ Verslag van een Pilotstudie. http://www.centrumklantervaringzorg.nl/fileadmin/site/ckz/publicaties/CQI_ambulante_GGZ.pdf. Trimbos‐instituut, Utrecht.
WHO 2005
- World Health Organization. WHO Resource Book on Mental Health, Human Rights and Legislation. Stop Exclusion, Dare to Care. World Health Organization, 2005. [Google Scholar]
WHO 2011
- World Health Organization. Schizophrenia. http: //www.who.int/mental_health/management/schizophrenia/en/ (accessed 15 August 2011).
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]


