PURPOSE
Osimertinib is a third-generation, CNS-active, irreversible, oral epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) that potently and selectively inhibits both EGFR-TKI-sensitizing and T790M resistance mutations. We assess the cardiac failure risk in patients receiving osimertinib by evaluating the available data.
METHODS
Post hoc analyses of cardiac data from (1) studies in patients with advanced non–small-cell lung cancer, FLAURA (osimertinib, n = 279; comparator EGFR-TKI, n = 277) and AURA3 (osimertinib, n = 279; chemotherapy, n = 140), and (2) a pooled data set of patients treated with osimertinib 80 mg from across the clinical trial program (n = 1,142), including cardiac failure-related adverse events and left ventricular ejection fraction (LVEF) reductions. An LVEF pharmacokinetic or pharmacodynamic analysis of the pooled data set was performed. The sponsor's global safety database was analyzed for cardiac failure-related adverse events, and a literature search was conducted.
RESULTS
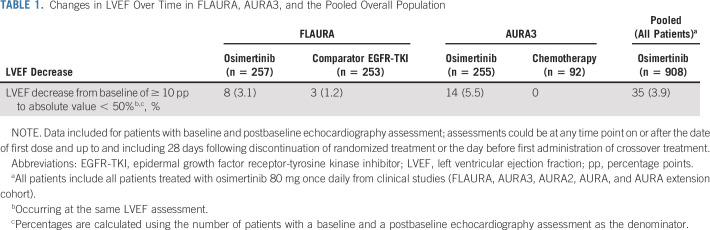
Decreases in LVEF from a baseline of ≥ 10 percentage points to an absolute value of < 50% following osimertinib treatment were observed in 8 (3.1%) and 14 (5.5%) patients in FLAURA and AURA3, respectively, and in 35 (3.9%) patients in the pooled population. Most events were asymptomatic and resolved without treatment of the event or osimertinib discontinuation. The pharmacokinetic or pharmacodynamic analysis did not indicate a relationship between exposure to osimertinib and decreases in LVEF from baseline. The database and literature search showed no specific trend or pattern that was suggestive of a safety issue in patients receiving osimertinib.
CONCLUSION
These data do not suggest a causal relationship between osimertinib and cardiac failure. However, because of LVEF decreases that were observed in patients with cardiac risk factors before osimertinib treatment, cardiac monitoring, including an assessment of LVEF at baseline and during osimertinib treatment, is advised.
INTRODUCTION
Osimertinib is a third-generation, irreversible, oral epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) that potently and selectively inhibits both EGFR mutations (EGFRm; Ex19del/L858R) and T790M resistance mutations and has demonstrated efficacy in non–small-cell lung cancer (NSCLC) CNS metastases.1-5 In the phase III FLAURA study (ClinicalTrials.gov identifier: NCT02296125), osimertinib provided statistically significant superior median progression-free survival (PFS) and overall survival (OS) versus comparator EGFR-TKIs (erlotinib or gefitinib) in patients with previously untreated EGFRm-advanced NSCLC.3,6 PFS outcomes for osimertinib versus comparator, respectively, were 18.9 months versus 10.2 months (hazard ratio [HR], 0.46; 95% CI, 0.37 to 0.57; P < .001), and OS outcomes were 38.6 months (95% CI, 34.5 to 41.8) versus 31.8 months (95% CI, 26.6 to 36.0), respectively (HR, 0.80; 95% CI, 0.64 to 1.00; P = .046).3,6 In the phase III AURA3 study (ClinicalTrials.gov identifier: NCT02151981), osimertinib provided statistically significant superior median PFS versus platinum therapy plus pemetrexed (10.1 months v 4.4 months; HR 0.30; 95% CI, 0.23 to 0.41; P < .001) in patients with T790M-positive advanced NSCLC who had progressed on first-line EGFR-TKI therapy.2 Osimertinib is approved globally as the first-line treatment of patients with EGFRm-advanced NSCLC7-9 and in patients with T790M-positive NSCLC following disease progression on or after EGFR-TKI therapy.7,8 Across the FLAURA and AURA studies, osimertinib demonstrated a manageable and consistent tolerability profile.2,3,10,11
CONTEXT
Key Objective
Cardiovascular adverse events (AEs) have been associated with cancer therapies. By analyzing the cardiac safety data from across the osimertinib clinical program, we attempt to conclude whether a causal relationship exists between cardiac-related AEs and treatment with osimertinib.
Knowledge Generated
From the analysis, it was found that a low proportion of patients receiving osimertinib across the clinical study program had reported a cardiac-related AE, the majority of which were asymptomatic. In addition, data from the global safety database demonstrated no specific trend or pattern for cardiac failure-related AEs in patients receiving osimertinib.
Relevance
Our findings indicate that the key factor in the development of cardiac failure is a pre-existing cardiac risk factor, rather than the receipt of osimertinib. Therefore, in line with standard practice, cardiac monitoring, including assessment of left ventricular ejection fraction, is advised in patients with cardiac risk factors.
Cardiovascular adverse events (AEs) have been associated with cancer therapies and can be classified into two distinct types.12 Type 1 cardiotoxicity, mainly caused by anthracyclines, results in cumulative dose-related and irreversible myocardial oxidative stress or damage with identifiable ultrastructural abnormalities. Type 2, observed with human epidermal growth factor receptor 2 (HER2) targeting agents such as trastuzumab, does not occur in all patients, is not dose-related or associated with ultrastructural myocardial changes, and is reversible.12 HER2 operates by maintaining heart function and mediating cell survival pathways that regulate the physiological adaptive response to cardiac stress.13,14 Given the potential for cardiovascular AEs with HER2-targeting therapies, it is important to identify patients at risk of developing cardiac failure, who are receiving such therapies. Furthermore, congestive heart failure (CHF) is one of the most common comorbidities in patients with lung cancer.15 Echocardiography, multigated acquisition (MUGA) scans, and cardiac magnetic resonance imaging (MRI) can assess ejection fraction (EF) and other measures of cardiac function.16 However, EF assessment is inherently complicated by the inability to differentiate between the effects of treatment-related cardiotoxicity versus other physiological variables, and techniques such as cardiac ultrasound can be limited by inter- and intraobserver variability.17
In in vitro studies, osimertinib and its metabolites were shown to inhibit HER2. However, osimertinib inhibited HER2 with a potency of six- to ten-fold less than other HER2 inhibitors afatinib and lapatinib, suggesting that clinical exposure may not be sufficient to target HER2 (Appendix, online only). Osimertinib is also a weak inhibitor of the cardiac potassium ion channel, Kv11.1, inhibiting hERG-encoded potassium channel function with an in vitro IC50 of 0.69 μM. However, only marginal, transient QTcR increases were seen in dogs at clinically relevant plasma concentrations.18 Extensive cardiac evaluation in the clinical studies led to the detection and characterization of QTc prolongation (not associated with any adverse clinical outcomes or cardiac failure) as an adverse drug reaction for osimertinib. As such, this is featured in the US Food and Drug Administration label, and clinical trial program has been adjusted accordingly.8,19
Data linking HER2-targeting therapies to cardiac failure events are unclear or inconclusive. For example, in trastuzumab studies, cardiac safety outcomes were dependent on factors such as concomitant treatments and long- or short-term dosing.16,20 Similarly, mixed results were seen in studies with other drugs such as lapatinib, sunitinib, and afatinib.21-24 The reasons for these inconsistencies are unknown, although HER2-targeting therapies may not cause direct cardiotoxicity but instead inhibit antioxidant systems that maintain normal heart function, particularly during oxidative stress conditions as induced by anthracyclines.25 Conversely, a possible cardioprotective effect of small-molecule HER2 inhibitors may be implicated.26,27
We present a comprehensive overview of cardiac failure data from key osimertinib studies in first- and second-line advanced NSCLC2,3,10 and from postapproval AE case reports.
METHODS
Study Design, Patients, and Treatment
Post hoc analyses of cardiac contractility and cardiac failure AEs were performed using FLAURA and AURA3 data and data from a pooled population of patients who received osimertinib 80 mg once daily in FLAURA and the AURA trials (FLAURA [ClinicalTrials.gov identifier: NCT02296125], n = 2,793; AURA3 [ClinicalTrials.gov identifier: NCT02151981], n = 2,792; AURA2 [ClinicalTrials.gov identifier: NCT02094261], n = 21010; AURA extension, n = 20128; AURA [ClinicalTrials.gov identifier: NCT01802632], n = 17311). Objective measurements of left ventricular ejection fraction (LVEF) were assessed for changes in values, with a corresponding assessment of symptomatic cardiac failure AEs; as decreased EF AEs are based on LVEF decreases alone, these were considered part of the objective LVEF analysis above.
The osimertinib clinical program study designs (FLAURA [NCT02296125], AURA3 [NCT02151981], AURA2 [NCT02094261], and AURA [NCT01802632]) have been previously reported (Appendix Table A1, online only).2,3,10,11,28 In all studies, AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0, and LVEF measurements were taken at baseline, every 12 weeks postbaseline, and as clinically indicated. All studies were conducted in accordance with the Declaration of Helsinki and guidelines on Good Clinical Practice, protocols were approved by local ethics committees at each participating center, and all patients provided written consent.
Search of Company Global Safety Database and Published Literature
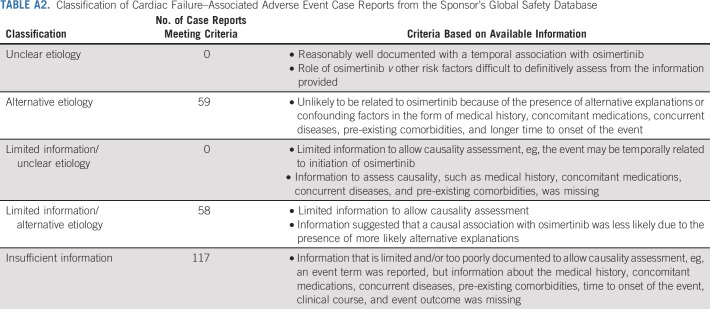
The sponsor's (AstraZeneca, Cambridge, UK) global safety database contains all AE reports from spontaneous sources (eg, healthcare professionals, regulatory authorities, literature, and consumers), regardless of meeting regulatory authorities' definition of serious, in addition to reports from clinical studies, defined as serious. The database was searched for cardiac failure-related terms, cumulative to August 31, 2018, using the Standardized Medical Dictionary for Regulatory Activities Query (SMQ) of cardiac failure, which includes the Medical Dictionary for Regulatory Activities (MedDRA) Preferred Terms (PTs) of EF decreased, cardiac failure, and chronic heart failure (HF) and the cardiomyopathy SMQ, which also includes the PTs of EF decreased as well metabolic cardiomyopathy. The reports retrieved were assessed and placed into five categories based on their contents (Appendix Table A2, online only).
A search of literature databases (including Embase and Pharmaspectra), cumulative up to August 31, 2018, was also conducted to obtain literature reports of cardiac failure in patients receiving osimertinib.
Clinical Trial Data Analysis
Only data from patients with both a baseline and ≥ 1 follow-up LVEF assessment were included in the analyses and comprised LVEF data obtained at any time point on or after the first dose and ≤ 28 days following treatment discontinuation. The end point for LVEF changes was defined as LVEF decrease from baseline of ≥ 10 percentage points (pps) to an LVEF absolute value < 50%, where the baseline was defined as the last nonmissing measurement before dosing.
Cardiac failure effects reported as AEs were defined using the SMQs of cardiac failure and cardiomyopathy. Any differences in the absolute incidence of these cardiac failure-related AEs when comparing treatment arms in FLAURA and AURA3 were based on exposure-adjusted event rate (per 100 patient-years) to account for longer osimertinib exposure.
Pharmacokinetic or Pharmacodynamic Analysis
An LVEF pharmacokinetic (PK) or pharmacodynamic (PD) analysis evaluated potential relationships between systemic exposure of osimertinib or the osimertinib metabolite, AZ5104, and changes in LVEF data obtained from all patients from FLAURA and the AURA trials who had received osimertinib 80 mg and had available baseline and postdose LVEF data.
RESULTS
Clinical Trial Data from FLAURA
LVEF measurements.
In FLAURA, 257/279 (92.1%) and 253/277 (91.3%) patients receiving osimertinib and comparator EGFR-TKI, respectively, had baseline and ≥ 1 follow-up LVEF assessment available and were included in this analysis. The median (range) duration of treatment was 16.2 months (0.1-27.4) and 11.5 months (0-26.2) in the osimertinib and comparator EGFR-TKI arms, respectively.
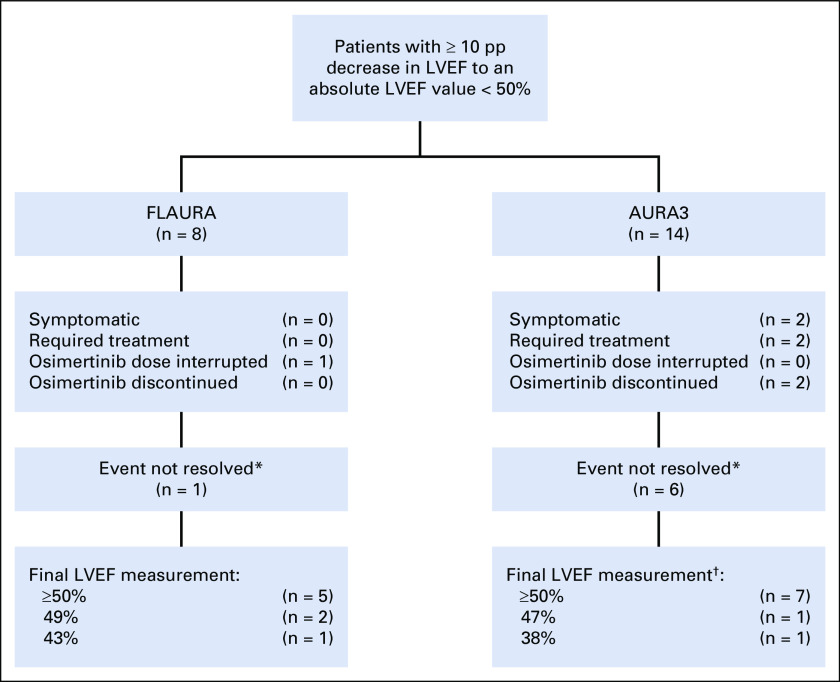
The median maximum change from baseline in LVEF was −3.0 pp in the osimertinib arm both at week 48 (n = 199) and 72 (n = 124) (range, −3 to −1 pp) and in the comparator EGFR-TKI arm at week 96 (n = 18) (range, −3 to 0 pp) (Fig 1A). Eight (3.1%) and three (1.2%) patients in the osimertinib and comparator EGFR-TKI arms, respectively, had an LVEF decrease from baseline ≥ 10 pp to an LVEF absolute value < 50% (Table 1). All eight patients with decreases in LVEF while taking osimertinib recovered while on full dose of osimertinib without occurrence of cardiac symptoms. Clinical management and outcomes for patients in the osimertinib arm are summarized in Figure 2; 3/8 patients (38%) had cardiomyopathy risk factors from their medical history (such as atrial fibrillation, hypertension, hypothyroidism, pulmonary embolism, and type II diabetes mellitus).
FIG 1.
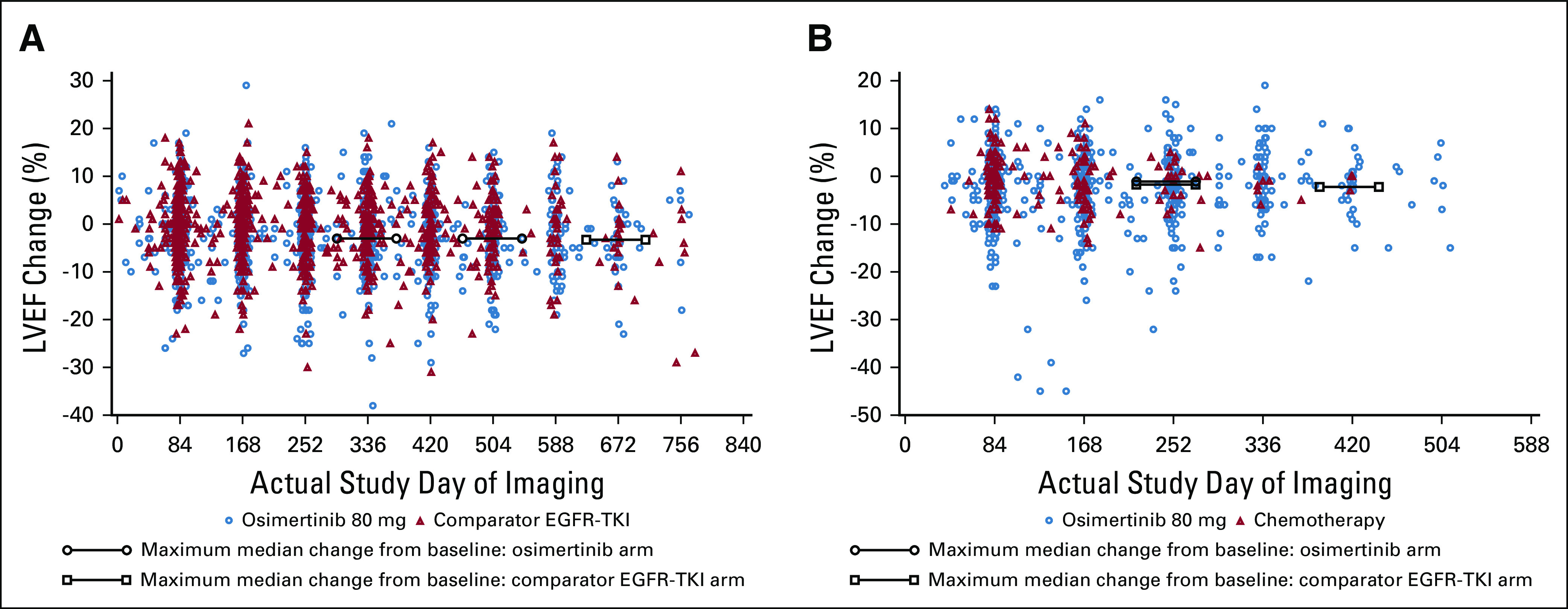
Box plots of relative change from baseline in LVEF over time in (A) FLAURA; (B) AURA3. EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; LVEF, left ventricular ejection fraction.
TABLE 1.
Changes in LVEF Over Time in FLAURA, AURA3, and the Pooled Overall Population
FIG 2.
Medical management and clinical outcomes for patients with ≥ 10 pp decrease in LVEF to an absolute value < 50% in FLAURA and AURA3. *Events being marked not resolved is because of the event not having had resolution marked in the database by the time of the data cutoff. †Final LVEF measurements were not available for five patients. LVEF, left ventricular ejection fraction; pp, point percentage.
Cardiac failure-related AEs.
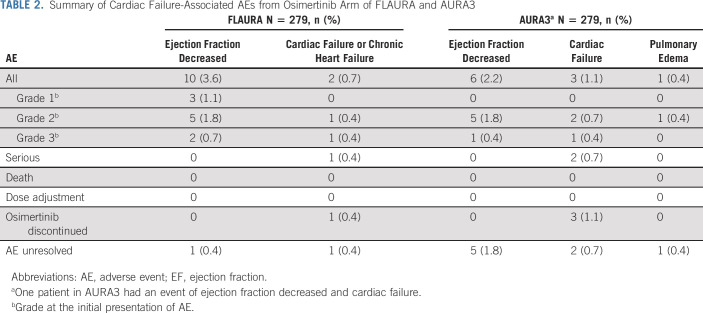
Cardiac failure SMQ AEs were reported in a low but numerically higher proportion of patients in the osimertinib arm (n = 12; 4.3%) versus the comparator EGFR-TKI arm (n = 5; 1.8%). In the osimertinib arm, cardiac failure-related AEs included EF decreased in 10 (3.6%) patients and cardiac failure or chronic HF in 2 (0.7%) patients (Table 2). EF decreased AEs are based on the measured LVEF decreases alone and are therefore part of the objective LVEF analysis above. Cardiac failure or chronic HF was reported as AEs in two patients, one of whom had a serious CTCAE grade 3 event (chronic HF) that resolved after discontinuation of osimertinib and was judged to be possibly related to osimertinib by the investigator. This patient (72 years, female, Asian) had a medical history of atrial fibrillation, hypertension, valvular heart disease, hyperlipidemia, and chronic HF. The other patient had a nonserious grade 2 event, not judged to be drug-related.
TABLE 2.
Summary of Cardiac Failure-Associated AEs from Osimertinib Arm of FLAURA and AURA3
Cardiomyopathy SMQ AEs were reported in 10 (3.6%) patients in the osimertinib arm and 6 (2.2%) in the comparator EGFR-TKI arm. With osimertinib, cardiomyopathy AEs comprised the same EF decreased events (in the same patients) described earlier. Further information regarding clinical management and outcomes for the cardiac failure-related AEs are shown in Table 2.
AURA3
LVEF.
In AURA3, 255/279 (91.4%) and 92/136 (67.6%) patients receiving osimertinib and chemotherapy, respectively, had a baseline and a postbaseline LVEF assessment. The median (range) duration of treatment was 8.1 months (0.2-18.5) and 4.2 months (0.4-14.5) in the osimertinib and chemotherapy arms, respectively.
The maximum median change from baseline in LVEF was −2.0 pp in the osimertinib arm at cycle 9 (n = 207; range, −45 to +16 pp) and cycle 13 (n = 120; range, −24 to +16 pp) versus −1.5 pp in the chemotherapy arm at cycle 9 (n = 44; range, −14 to +11 pp) (Fig 1B). Fourteen (5.5%) patients in the osimertinib arm had an LVEF decrease from baseline ≥ 10 pp to an LVEF absolute value < 50% versus no patients in the chemotherapy arm (Table 1). For these 14 patients, the median time to the onset of the first LVEF decrease from baseline ≥ 10 pp to an absolute value < 50% was 5.5 months. Follow-up LVEF measurements were available for 13/14 patients: six recovered while remaining on osimertinib and LVEF values remained unchanged in two patients. Of the remaining five patients, two discontinued treatment as the LVEF reductions were associated with AE reports of cardiac failure and three continued study drug with no clear evidence of causality of the LVEF decreases. Most of the 14 patients had a history of cardiomyopathy risk factors. Twelve patients were asymptomatic. Further details of the LVEF decreases for all 14 patients are presented in Figure 2.
Cardiac failure-related AEs.
Cardiac failure SMQ AEs were reported in nine (3.2%) patients receiving osimertinib and no patients receiving chemotherapy. AEs with osimertinib included EF decreased in six (2.2%) patients, cardiac failure in three (1.1%) patients, and pulmonary edema in one (0.4%) patient; one patient had both an AE of EF decreased and cardiac failure (Table 2). In three patients with cardiac failure AEs (grade 3 [n = 1] and grade 2 [n = 2]), two serious events were judged to be possibly related to osimertinib by the investigator, and all patients discontinued osimertinib. Two of these patients had cardiac-related medical histories, including atrial fibrillation and hypokinesia in the anterolateral and inferior wall of the left ventricle. The pulmonary edema AE observed in one patient was grade 2, nonserious, and not drug-related.
Cardiomyopathy SMQ AEs were reported in six (2.2%) patients in the osimertinib arm; all were EF decreased, as described earlier, and no patients in the chemotherapy arm.
Pooled Analysis for all Osimertinib-Treated Patients from Clinical Studies
LVEF.
Across all studies, 908 patients had baseline and ≥ 1 follow-up LVEF assessment. LVEF decreased ≥ 10 pp from baseline to an absolute LVEF absolute value < 50% occurred in 35 (3.9%) patients (Table 1).
Cardiac failure-related AEs.
Of the 1,142 patients treated with osimertinib 80 mg once daily across all studies, the cardiac failure-related AE incidence was 30 (2.6%).
PK or PD analysis.
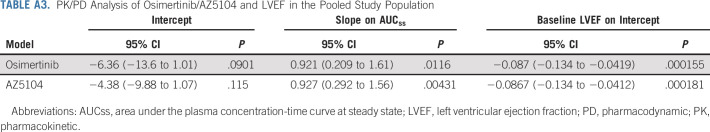
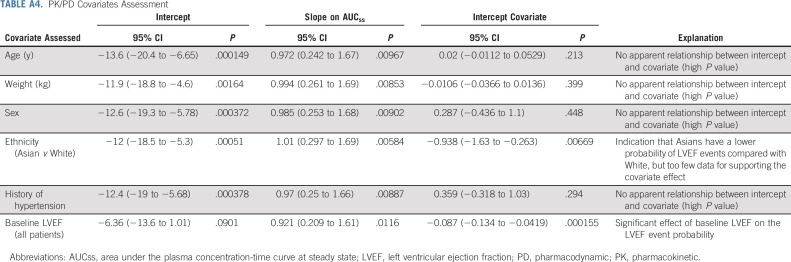
The PK or PD analysis evaluating potential relationships between systemic exposure of osimertinib or AZ5104 and changes in LVEF in the pooled study population showed no statistically significant evidence of any quantifiable exposure-related effects on change from baseline LVEF and LVEF decrease incidence (based on the predefined threshold of P < .001; Appendix Table A3, online only). Model-based covariate assessments identified that patients of white ethnicity, older age, and with a medical history of hypertension were more likely to experience an LVEF event (Appendix Table A4, online only). No patient with any of the other medical histories assessed (myocardial infarction, ischemic heart disease, aortic stenosis, and cardiomyopathy) had an LVEF event.
Search of Published Literature and Company Global Safety Database
From the sponsor's global safety database, 252 AEs of cardiac failure or relevant MedDRA PTs reported in 234 case reports were identified. These comprised 188 reports from postapproval sources (noninterventional postmarketing study, n = 119; spontaneous, n = 60; literature case reports, n = 9) and 46 reports from clinical trials (sponsor, n = 40; nonsponsor, n = 6).
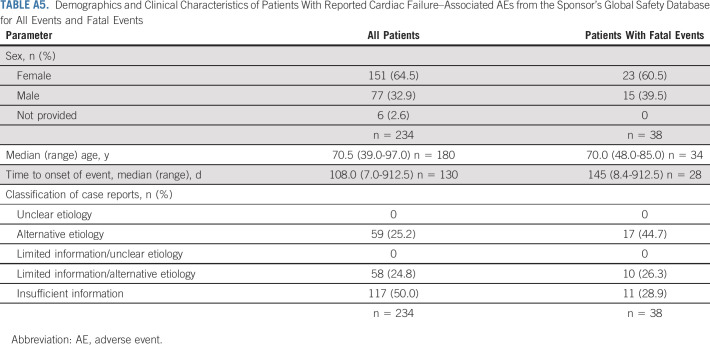
Of the 234 case reports with cardiac failure-associated AEs, the median (range) time to event onset from the first osimertinib dose was 108.0 (7.0-912.5) days (Appendix Table A5, online only). The number of events decreased over time (Fig 3A); however, most patients had T790M-positive disease; given the median PFS is approximately 10 months, the proportion of patients on treatment diminished over time, and hence, interpretation should be made with caution.
FIG 3.
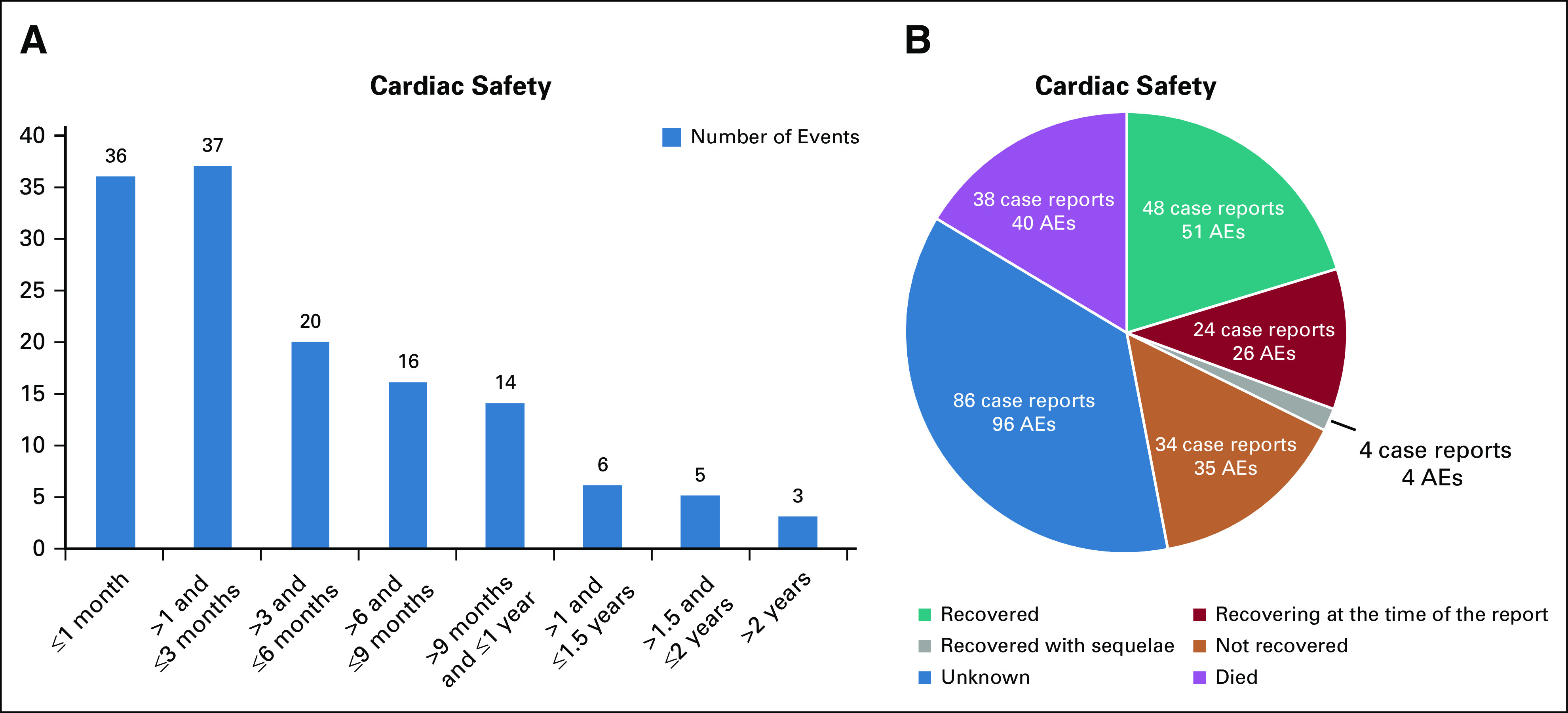
(A) Times to onset of events (n = 130 case reports containing 137 AEs)* and (B) outcome for cardiac failure-related AEs from the sponsor's global safety database. *Information on time to onset of events was not reported or unknown in 104 case reports containing 115 AEs. AEs, adverse events.
Of the 234 case reports, 117 (50.0%) contained insufficient information to ascertain any relationship between the AE and osimertinib. The remaining reports contained more than one contributory factor or condition that increased the patient's risk of developing cardiac events (59 [25.2%] reports of alternative etiologies; 58 [24.8] reports of limited information or alternative etiologies) (Appendix Table A5).
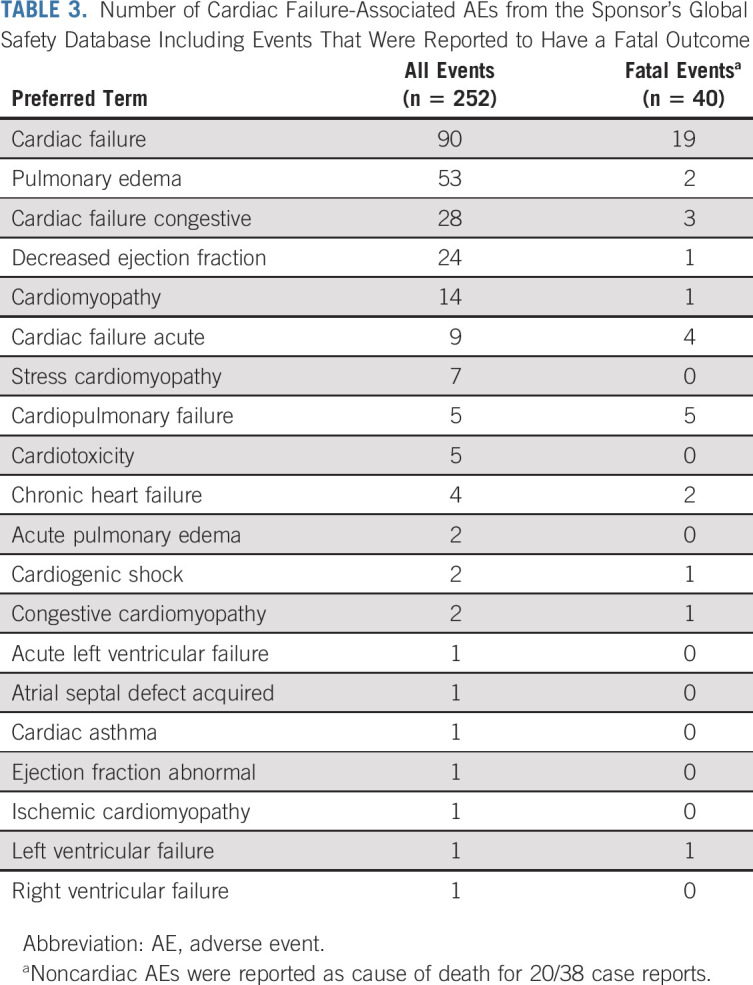
Of the 252 AEs, 209 (82.9%) were reported as serious, the most common being cardiac failure, pulmonary edema, cardiac failure congestive, EF decreased, and cardiomyopathy (Table 3).
TABLE 3.
Number of Cardiac Failure-Associated AEs from the Sponsor's Global Safety Database Including Events That Were Reported to Have a Fatal Outcome
Event outcome data were available in 148 (63.2%) case reports with 156 AEs (Fig 3B). Of all AEs, the outcome was fatal (16%), not recovered (14%), recovered (20%), recovering (10%), and recovered with sequelae (2%). In most reports where the outcome was recovered, recovering, or recovered with sequelae, osimertinib was either discontinued (57 reports) or temporarily stopped (14 reports).
Fatal cardiac failure-associated AEs are presented in Table 3. Of the 38 case reports with fatal events, 11 reports contained limited information and/or were poorly documented; information on patients' demographics, relevant medical history, concurrent diseases, concomitant medications, time to onset, or event clinical course were either unavailable or not reported to enable an assessment of the relationship between the event and osimertinib. The remaining 27 reports contained more than one contributory factor such as previous medical history (such as cardiac failure, hypertension, ex-tobacco user, mitral valve prolapse or incompetence, and pulmonary embolism), concurrent conditions (such as atrioventricular block, hyperlipidemia, diabetes mellitus, and CHF), and concomitant medications that could increase the patient's risk of developing cardiac events despite osimertinib treatment (17 case reports of alternative etiologies and 10 case reports of limited information or alternative etiologies). Further details are presented in Appendix Table A5. In summary, no specific trend or pattern in the occurrence or nature of the fatal cardiac failure events was noted that was suggestive of a safety issue in patients receiving osimertinib.
DISCUSSION
This analysis represents a comprehensive evaluation of cardiac failure data in patients with advanced NSCLC receiving osimertinib. A low proportion (3.9%) of patients receiving osimertinib across the clinical study program had an LVEF decrease ≥ 10 pp from baseline to an LVEF absolute value < 50 pp. In FLAURA, all LVEF events were asymptomatic in the osimertinib arm and did not result in dose modifications. In AURA3, only 0.7% (2/279) of patients had a symptomatic LVEF event that resulted in osimertinib discontinuation. Evaluation of these events has not identified a causal relationship between effects on changes in cardiac contractility and osimertinib.
Across the program, the majority of cardiac failure-related AEs were asymptomatic EF decreases that resolved without dose modification of osimertinib. Symptomatic events included two patients in AURA3 with LVEF decrease AEs, which led to treatment for EF decrease and osimertinib dose modification in both patients, one of whom had a medical history of hypokinesia in the anterolateral and inferior wall of the left ventricle. There were also three patients reporting cardiac failure leading to osimertinib discontinuation, two of whom had cardiac medical histories including atrial fibrillation and hypokinesia in the anterolateral and inferior wall of the left ventricle. In FLAURA, other AEs included one cardiac failure case (possibly related to osimertinib, as assessed by the investigator), where the patient had medical history including hypertension and chronic HF, and one pulmonary edema case (unrelated to osimertinib).
Data from the global safety database also demonstrated no specific trend or pattern for cardiac failure-related AEs in patients receiving osimertinib. Of note, half of the case reports from postapproval sources contained limited information that did not allow for a causal relationship with osimertinib to be evaluated, and the remaining case reports had multiple factors or concomitant medications that would have increased the patient's cardiac event risk. A CHF case report during osimertinib treatment has been published.29 Potentially confounding factors (disease progression, previous bevacizumab and platinum-based chemotherapy treatment) were identified, and further information is needed to perform a meaningful causal assessment.29
As previously noted, cardiac failure is considered a potential safety concern for some cancer therapies targeting the HER2 pathway, particularly for trastuzumab in combination with anthracyclines.16,20 However, there is inconclusive evidence or no clear association for an effect on LVEF with other HER2-targeting therapies, such as lapatinib, sunitinib, and afatinib.16,21-24 Similar to the osimertinib data presented here, an analysis of cardiac failure with afatinib, a treatment for patients with EGFRm metastatic NSCLC, reported low rates of protocol-defined cardiac failure and clinically significant LVEF reductions, comparable to those seen with the control treatment arms. The authors concluded that afatinib was not associated with cardiac failure or clinically significant LVEF reductions in the clinical trial program; therefore, no evidence of direct or primary cardiac or cardiovascular toxicity was found.24
Preclinical studies have also suggested a lack of or uncertain association between osimertinib and cardiotoxicity. As stated previously, osimertinib and its metabolites were shown in vitro to inhibit HER2, though osimertinib was approximately six- to ten-fold less potent versus other HER2 inhibitors. For the metabolites, AZ5104 and AZ7550, the geometric mean exposure was approximately 10% of that of osimertinib at steady state with a greater potency against HER2 observed for AZ5104, comparable with afatinib and lapatinib; however, the clinical relevance of this activity is unknown (Appendix). Furthermore, no clinically significant changes in cardiac contractility with osimertinib were observed in in vivo dog and guinea pig models.18
From this review, there is no evidence suggesting that osimertinib has direct (often referred to as type I) treatment-related cardiotoxicity, as seen with anthracycline cardiotoxicity or ionizing radiation.12,30 As this type of cardiotoxicity is related to the total exposure or cumulative dose, it will, given sufficient exposure, become apparent and no cumulative dose-related effects were observed with osimertinib. Subtler, and more difficult to define and characterize, are transient and reversible toxicities,12 which may occur as an isolated event, making cause and effect difficult or impossible to prove in a clinical setting that involves end-stage patients. In some settings, as with trastuzumab, patients may remain unaffected for unusually long exposure periods, while others have drug-related toxicity much earlier or even during the active exposure period.17,31 In such instances, causal relationship to the drug cannot be definitively identified and must be satisfied with a description that is quantitatively meaningful and qualitatively useful, while also being clinically relevant. However, with osimertinib, the incidence of observed asymptomatic LVEF declines fall within the expected range, and symptomatic declines are rare and can often be attributed to other causes.
A further limitation of defining cardiac events is that the parameters used to define them often rely on suboptimal measuring tools. In a statistical modeling study, the cumulative false-positive rate over four assessments (1-year period) for assessing cardiotoxicity with cardiac ultrasound was 3.6%, very similar to the percentage observed in the osimertinib studies (4%).17 For longer exposure or more assessments as in the osimertinib studies, it could be expected that this false-positive rate may be higher; however, those studies used a MUGA scan or echocardiogram test and requested that, where possible, the same machine and operator were used. As with any uncommon event evaluated with imperfect tools, the rarer the true incidence of the event, the more likely false-positives will occur. If the event incidence is high, some are likely to be missed by measurement-related false negativity. Failure to appreciate false-positive results may trigger confusion and result in surveillance recommendations that are costly and unnecessary and may cause patients responding well to their treatment to receive alternate regimens.17,32
Also of note is the documented higher prevalence of cardiac failure in patients with advanced NSCLC versus patients without cancer.33 In this analysis, risk factors for cardiomyopathy were present in 38% and 36% of patients in FLAURA and AURA3, respectively, who had an LVEF decrease ≥ 10 pp from baseline to an LVEF absolute value < 50%. Furthermore, 25% of patients reporting cardiac failure-related AEs with osimertinib from the global safety database had confounding factors such as pre-existing comorbidities and/or concomitant medications that could have contributed to the patient having a higher risk of experiencing a cardiac event.
Recently, a retrospective single-center cohort study in Japan reported osimertinib-associated cardiac AEs in 123 patients with advanced NSCLC and confirmed EGFR mutations.34 They reported six (4.9%) instances of severe cardiac AEs (CTCAE grade ≥ 3); half of these patients had EF declines (2.4%) and the remaining patients had valvular or ischemic disease. As previously discussed, these numbers are within the expected false-positive range; selection bias and medical conditions beyond osimertinib may have contributed to these findings, as most (5/6) events occurred in patients with pre-existing cardiovascular disease or risk factors.34,35
In reporting these data, certain limitations should be considered, including the post hoc nature of the analyses and limited follow-up assessments in some patients. In AURA3, there was an imbalance in the number of patients with LVEF assessments in each arm, with the relative difference between arms increasing with each cycle. This imbalance is most likely because of the 2:1 randomization in favor of osimertinib and longer duration of exposure in the osimertinib arm, reflecting the shorter time to progression events in the chemotherapy arm. Also, some data were missing and/or incorrectly documented information for case reports from postapproval sources.
In light of these data and conclusions, routine LVEF surveillance in the absence of clinical indications is not indicated. However, as stated in the osimertinib-prescribing information, cardiac monitoring, including LVEF assessment, should be performed at baseline and during treatment in patients with cardiac risk factors and in those who develop cardiac signs or symptoms during treatment; osimertinib should be discontinued in the case of symptomatic CHF.8 In addition, cardio-oncologic guidelines for other anticancer treatments recommend screening before treatment to identify patients at increased cardiotoxicity risk and throughout the treatment pathway.36,37 Such risk assessment should include clinical history and cardiac function assessment using, for example, biomarkers, echocardiography, or cardiac MRI.37 Repeated LVEF assessment shortly after and during treatment should be conducted if a patient has a significant LVEF decrease (> 10%) to a value that does not fall below the lower limit of normal. If a significant LVEF decrease occurs, then standard therapeutic interventions (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, in combination with beta-blockers) are recommended, unless contraindicated.
In conclusion, this comprehensive evaluation did not identify a causal relationship between osimertinib treatment and cardiac failure in patients with advanced NSCLC. Our findings indicate that the key element in the development of cardiac failure is a pre-existing cardiac risk factor. Therefore, in line with standard practice, cardiac monitoring, including assessment of LVEF, is advised in cancer patients with cardiac risk factors.
ACKNOWLEDGMENT
The authors would like to thank all patients and their families. They would like to acknowledge Bernadette Tynan, MSc, of iMed Comms, Macclesfield, UK, an Ashfield Company, part of UDG Healthcare plc for medical writing support, under the direction of the authors that was funded by AstraZeneca in accordance with Good Publications Practice (GPP3) guidelines.
APPENDIX
TABLE A1.
Study Designs of the Osimertinib Clinical Trial Program
TABLE A2.
Classification of Cardiac Failure–Associated Adverse Event Case Reports from the Sponsor’s Global Safety Database
TABLE A3.
PK/PD Analysis of Osimertinib/AZ5104 and LVEF in the Pooled Study Population
TABLE A4.
PK/PD Covariates Assessment
TABLE A5.
Demographics and Clinical Characteristics of Patients With Reported Cardiac Failure–Associated AEs from the Sponsor’s Global Safety Database for All Events and Fatal Events
SUPPORT
Supported by AstraZeneca (Cambridge, United Kingdom) the manufacturer of osimertinib: FLAURA (NCT02296125), AURA3 (NCT02151981), AURA2 (NCT02094261), and AURA (NCT01802632).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Michael S. Ewer, Sri Harsha Tekumalla, Andrew Walding
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cardiac Safety of Osimertinib: A Review of Data
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Michael S. Ewer
Consulting or Advisory Role: Bayer, AstraZeneca, Boehringer Ingelheim
Patents, Royalties, Other Intellectual Property: Author of book “Cancer and the Heart”
Sri Harsha Tekumalla
Employment: AstraZeneca
Stock and Other Ownership Interests: Gilead Sciences, AstraZeneca
Andrew Walding
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Kwame N. Atuah
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cross DA Ashton SE Ghiorghiu S, et al. : AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4:1046-1061, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mok TS Wu YL Ahn MJ, et al. : Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med 376:629-640, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soria JC Ohe Y Vansteenkiste J, et al. : Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113-125, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Wu YL Ahn MJ Garassino MC, et al. : CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: Data from a randomized phase III trial (AURA3). J Clin Oncol 36:2702-2709, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Reungwetwattana T Nakagawa K Cho BC, et al. : CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non–small-cell lung cancer. J Clin Oncol 36:3290-3297, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Ramalingam SS Vansteenkiste J Planchard D, et al. : Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382:41-50, 2020 [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency : TAGRISSO (Osimertinib) Summary of Product Characteristics, 2018. https://www.ema.europa.eu/en/documents/product-information/tagrisso-epar-product-information_en.pdf [Google Scholar]
- 8.US Food and Drug Administration : TAGRISSO (Osimertinib) Highlights of Prescribing Information, 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208065s008lbl.pdf [Google Scholar]
- 9.Japanese Ministry of Health, Labour and Welfare : TAGRISSOTM (Osimertinib) Prescribing Information, 2019. https://www.astrazeneca.com/media-centre/press-releases/2018/tagrisso-approved-in-japan-for-1st-line-treatment-of-egfr-mutated-non-small-cell-lung-cancer-21082018.html#! [Google Scholar]
- 10.Goss G Tsai CM Shepherd FA, et al. : Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 17:1643-1652, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Jänne PA Yang JC Kim DW, et al. : AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 372:1689-1699, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Ewer MS, Lippman SM: Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J Clin Oncol 23:2900-2902, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Perry MC Dufour CR Eichner LJ, et al. : ERBB2 deficiency alters an E2F-1-dependent adaptive stress response and leads to cardiac dysfunction. Mol Cell Biol 34:4232-4243, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negro A, Brar BK, Lee KF: Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog Horm Res 59:1-12, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Islam KM Jiang X Anggondowati T, et al. : Comorbidity and survival in lung cancer patients. Cancer Epidemiol Biomarkers Prev 24:1079-1085, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Ponde NF, Lambertini M, de Azambuja E: Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open 1:e000073, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewer MS, Herson J: False positive cardiotoxicity events in cancer-related clinical trials: Risks related to imperfect noninvasive parameters. Chemotherapy 63:324-329, 2018 [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency : Assessment Report, EMA, 2015. https://www.ema.europa.eu/en/documents/assessment-report/tagrisso-epar-public-assessment-report_en.pdf [Google Scholar]
- 19.Ritter JM: Cardiac safety, drug-induced QT prolongation and torsade de pointes (TdP). Br J Clin Pharmacol 73:331-334, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seidman A Hudis C Pierri MK, et al. : Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 20:1215-1221, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Perez EA Koehler M Byrne J, et al. : Cardiac safety of lapatinib: Pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc 83:679-686, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Narayan V Keefe S Haas N, et al. : Prospective evaluation of sunitinib-induced cardiotoxicity in patients with metastatic renal cell carcinoma. Clin Cancer Res 23:3601-3609, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewer MS Suter TM Lenihan DJ, et al. : Cardiovascular events among 1090 cancer patients treated with sunitinib, interferon, or placebo: A comprehensive adjudicated database analysis demonstrating clinically meaningful reversibility of cardiac events. Eur J Cancer 50:2162-21670, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Ewer MS Patel K O’Brien D, et al. : Cardiac safety of afatinib: A review of data from clinical trials. J Clin Oncol 32:e19037, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belmonte F Das S Sysa-Shah P, et al. : ErbB2 overexpression upregulates antioxidant enzymes, reduces basal levels of reactive oxygen species, and protects against doxorubicin cardiotoxicity. Am J Physiol Heart Circ Physiol 309:H1271-H1280, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spector NL Yarden Y Smith B, et al. : Activation of AMP-activated protein kinase by human EGF receptor 2/EGF receptor tyrosine kinase inhibitor protects cardiac cells. Proc Natl Acad Sci U S A 104:10607-10612, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shell SA Lyass L Trusk PB, et al. : Activation of AMPK is necessary for killing cancer cells and sparing cardiac cells. Cell Cycle 7:1769-1775, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Yang JC Ahn MJ Kim DW, et al. : Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol 35:1288-1296, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Watanabe H Ichihara E Kano H, et al. : Congestive heart failure during osimertinib treatment for epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC). Intern Med 56:2195-2197, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gramatyka M, Sokol M: Radiation metabolomics in the quest of cardiotoxicity biomarkers: The review. Int J Radiat Biol 96:349-359, 2020 [DOI] [PubMed] [Google Scholar]
- 31.Ewer MS, Ewer SM: Trastuzumab cardiotoxiciy: The age-old balance of risk and benefit. Br J Cancer 115:1441-1442, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ewer MS Gibbs HR Swafford J, et al. : Cardiotoxicity in patients receiving transtuzumab (Herceptin): Primary toxicity, synergistic or sequential stress, or surveillance artifact? Semin Oncol 26:96-101, 1999 [PubMed] [Google Scholar]
- 33.Duarte CW Lindner V Francis SA, et al. : Visualization of cancer and cardiovascular disease co-occurrence with network methods. JCO Clin Cancer Inform 1:1-12, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Kunimasa K Kamada R Oka T, et al. : Cardiac adverse events in EGFR-mutated non-small cell lung cancer treated with osimertinib. 2:1-10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piper-Vallillo AJ, Sequist LV: Cardiac risk-informed treatment of EGFR-mutant lung cancer with osimertinib. 1:179-181, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamorano JL Lancellotti P Rodriguez Munoz D, et al. : 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 37:2768-2801, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Armenian SH Lacchetti C Barac A, et al. : Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 35:893-911, 2017 [DOI] [PubMed] [Google Scholar]