PURPOSE
ADJUVANT-CTONG1104 (ClinicalTrials.gov identifier: NCT01405079), a randomized phase III trial, showed that adjuvant gefitinib treatment significantly improved disease-free survival (DFS) versus vinorelbine plus cisplatin (VP) in patients with epidermal growth factor receptor (EGFR) mutation-positive resected stage II-IIIA (N1-N2) non–small-cell lung cancer (NSCLC). Here, we report the final overall survival (OS) results.
METHODS
From September 2011 to April 2014, 222 patients from 27 sites were randomly assigned 1:1 to adjuvant gefitinib (n = 111) or VP (n = 111). Patients with resected stage II-IIIA (N1-N2) NSCLC and EGFR-activating mutation were enrolled, receiving gefitinib for 24 months or VP every 3 weeks for four cycles. The primary end point was DFS (intention-to-treat [ITT] population). Secondary end points included OS, 3-, 5-year (y) DFS rates, and 5-year OS rate. Post hoc analysis was conducted for subsequent therapy data.
RESULTS
Median follow-up was 80.0 months. Median OS (ITT) was 75.5 and 62.8 months with gefitinib and VP, respectively (hazard ratio [HR], 0.92; 95% CI, 0.62 to 1.36; P = .674); respective 5-year OS rates were 53.2% and 51.2% (P = .784). Subsequent therapy was administered upon progression in 68.4% and 73.6% of patients receiving gefitinib and VP, respectively. Subsequent targeted therapy contributed most to OS (HR, 0.23; 95% CI, 0.14 to 0.38) compared with no subsequent therapy. Updated 3y DFS rates were 39.6% and 32. 5% with gefitinib and VP (P = .316) and 5y DFS rates were 22. 6% and 23.2% (P = .928), respectively.
CONCLUSION
Adjuvant therapy with gefitinib in patients with early-stage NSCLC and EGFR mutation demonstrated improved DFS over standard of care chemotherapy. Although this DFS advantage did not translate to a significant OS difference, OS with adjuvant gefitinib was one of the longest observed in this patient group compared with historic data.
INTRODUCTION
Adjuvant chemotherapy following surgery improves survival in patients with early-stage non–small-cell lung cancer (NSCLC).1-3 The current standard of care for patients with stage II-IIIA (N1-N2) NSCLC is surgery followed by adjuvant cisplatin-based chemotherapy (vinorelbine plus cisplatin [VP]), irrespective of epidermal growth factor receptor (EGFR) mutation status.4-8 The 5-year overall survival (OS) rate in these patients is poor, estimated to be between 36% and 49%, with a median (m) survival time of 35.0-58.9 months as per the International Association for the Study of Lung Cancer (IASLC) staging system (7th edition).9 In addition, recurrence rates are high (> 30%),10 often because of distant metastases, and adjuvant therapy may be of benefit in NSCLC patients with potential micrometastases.11
CONTEXT
Key Objective
There are no overall survival (OS) data on adjuvant target therapy for resected early-stage non–small-cell lung cancer (NSCLC). To the best of our knowledge, the ADJUVANT CTONG1104 study provides the first report of OS data with 80 months' follow up.
Knowledge Generated
The median OS was 75.5 months in patients treated with adjuvant gefitinib. It is one of the longest durations of OS reported to date, although it was not significantly different to adjuvant chemotherapy. The updated median disease-free survival was 30.8 months, with a risk reduction in disease recurrence or death of 44%. Subsequent therapy greatly influenced OS.
Relevance
Adjuvant therapy with gefitinib is an important treatment option in patients with resected early (stage II-IIIA [N1-N2]) epidermal growth factor receptor (EGFR)-mutant NSCLC. Findings from this study suggest that adjuvant EGFR-tyrosine kinase inhibitor therapy may be a suitable alternative to standard-of-care chemotherapy in these patients.
EGFR is often mutated in NSCLC; in advanced NSCLC, EGFR tyrosine kinase inhibitors (TKIs) are the standard treatment for patients with EGFR mutations.7 Treatment with TKIs has resulted in longer progression-free survival, compared with conventional chemotherapy, when used in patients with advanced disease.12 Patients with EGFR-mutant early-stage NSCLC (IB-IIIA) may also benefit from adjuvant EGFR-TKI therapy. The recent ADJUVANT-CTONG1104 study (ClinicalTrials.gov identifier: NCT01405079) was a randomized phase III trial that demonstrated significantly higher disease-free survival (DFS) with adjuvant gefitinib treatment than standard VP chemotherapy (mDFS, 28.7 months v 18.0 months; hazard ratio [HR] 0.60, 95% CI, 0.42 to 0.87) in patients with EGFR-mutation-positive resected stage II-IIIA (N1-N2) NSCLC.13 In the recent phase II EVAN study, DFS in stage III, N2 patients treated with adjuvant erlotinib was superior than for those treated with adjuvant chemotherapy (mDFS, 42.4 months v 21.0 months; HR, 0.268; 95% CI, 0.136 to 0.531).14 The SELECT study also demonstrated improved 2-year DFS with adjuvant erlotinib in a similar patient population; 2-year DFS rates were 88% with erlotinib compared with 76% in historic genotype-matched controls.15
There are currently limited data on OS in early-stage NSCLC patients receiving EGFR-TKI adjuvant therapy; the ADJUVANT-CTONG1104 study is the first phase III study to compare OS after treatment with adjuvant EGFR-TKI versus standard chemotherapy in this patient population. Here, we present the final OS results from the ADJUVANT-CTONG1104 study.
METHODS
Patients
Full details of the ADJUVANT study have been described previously.13 Briefly, patients of age 18-75 years diagnosed with stage II-IIIA (N1-N2) NSCLC with an EGFR-activating mutation who had undergone complete resection were enrolled in the study. Overall, 222 patients were enrolled from 27 sites and randomly assigned according to the Pocock and Simon minimization method,16 using the Windows 2008 Server IIS, and ASP and SQL Server 2008 database. All patients were randomly assigned 1:1 to receive adjuvant gefitinib (n = 111) or doublet chemotherapy (n = 111) from September 2011 to April 2014, and stratified by lymph node status (N stage: N1 and N2) and EGFR mutation status (exon 19 deletion or exon 21 L858R) by the random assignment system.
Treatment
Patients received 250 mg adjuvant gefitinib once daily for 24 months, or vinorelbine (25 mg/m2 on days 1 and 8) plus cisplatin (75 mg/m2 on day 1) every 3 weeks for four cycles.
Subsequent therapy because of disease progression was also recorded. Subsequent therapy was categorized into targeted therapy (EGFR-TKIs); other treatment, including chemotherapy, surgery, radiotherapy, or other interventional therapies; or no subsequent treatment.
Assessment
The intention-to-treat (ITT) population included all subjects who were randomly assigned. The per-protocol (PP) population included all subjects who were randomly assigned and received at least one dose of investigational drug treatment. Follow-up interval for all patients was 3 months in the first 3 years, and then, every 6 months. The primary end point was DFS in the ITT population. Secondary end points included OS, 3- and 5-year DFS, and 5-year OS rate; subsequent therapy data were collected for post hoc analysis. OS was defined as the time from the date of random assignment to death, for any reason. Patients who were still alive or lost to follow-up were censored on the date of the last confirmation of their survival.
Statistical Analysis
The sample size calculation has been described previously,12 and was estimated for DFS with no hierarchy analysis assigned to OS based on the statistical analysis plan for the final OS analysis. The final pre-planned OS, the updated DFS, 3- and 5-year DFS, and 5-year OS rate analyses were conducted on the ITT population from the date of last patient enrollment, with follow-up for at least 5 years. Post hoc analyses of subsequent therapies were conducted on patients who experienced an event of DFS, based on investigator evaluation of the tumor response from patients' medical records. Missing values were excluded from the analysis unless otherwise specified. All tests were two-sided with a nominal type I error (α) of 5%. Significance levels (P values) were not adjusted for multiplicity. All analyses were performed using SPSS25.0 and R statistical packages (version 3.4.3).
The Kaplan-Meier method was used to describe OS and DFS, and the cumulative proportion surviving at 3 and 5 years. A two-sided logrank test was used to compare OS and DFS between treatment groups. When two survival curves crossed, the two-stage procedure was an alternative method used to estimate the survival curves. The Cox proportional hazard model (performed at the α = .05 level, forward stepwise [likelihood ratio method]) was used to calculate the unstratified HR for treatment, while considering the effects of age, sex, lymph node status, and EGFR mutation. The χ2 test was used to compare response between the subsequent treatment groups, and the PP population used for the sensitivity analysis. EGFR mutation and lymph node status were not considered as strata in the Kaplan-Meier analysis, and HRs for the treatment groups were unstratified for the ITT and PP populations. Data cutoff was on April 19, 2020.
RESULTS
Patients and Study Treatment
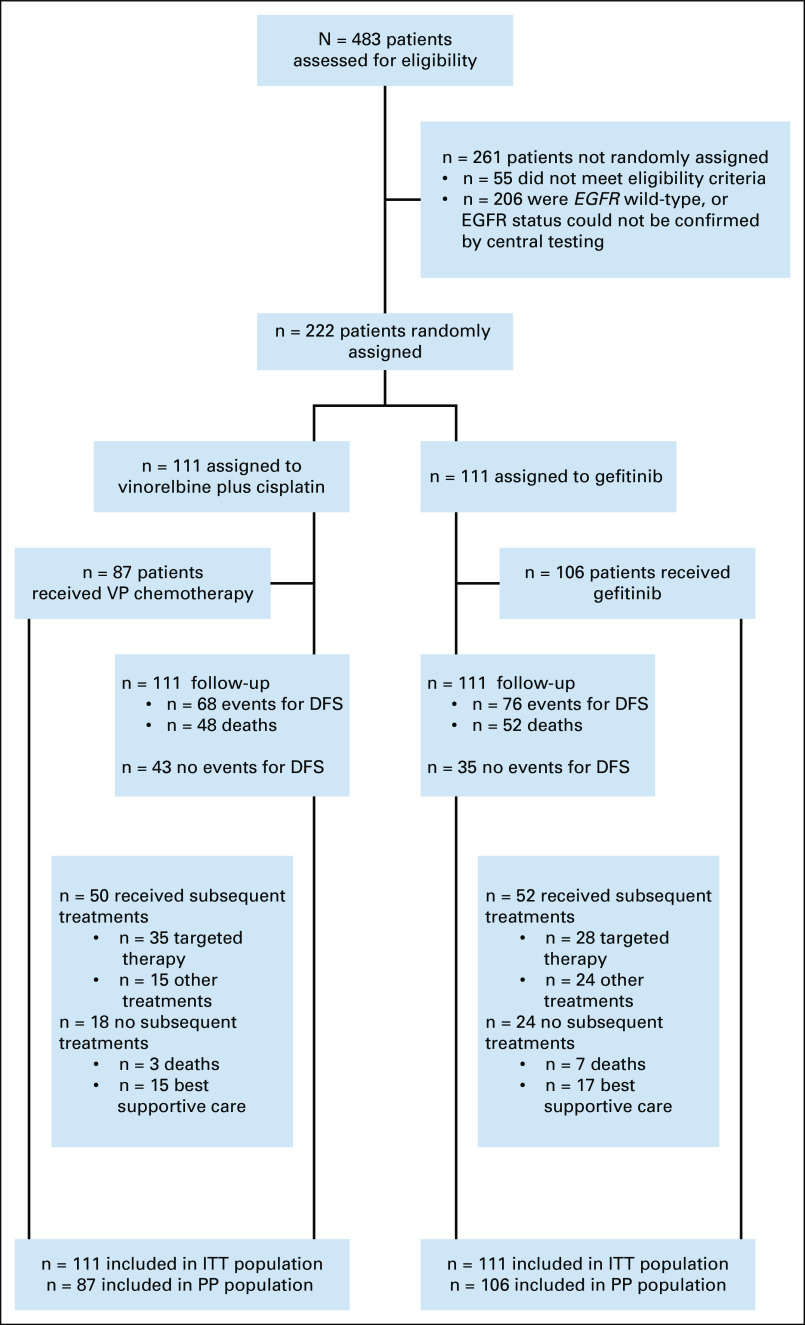
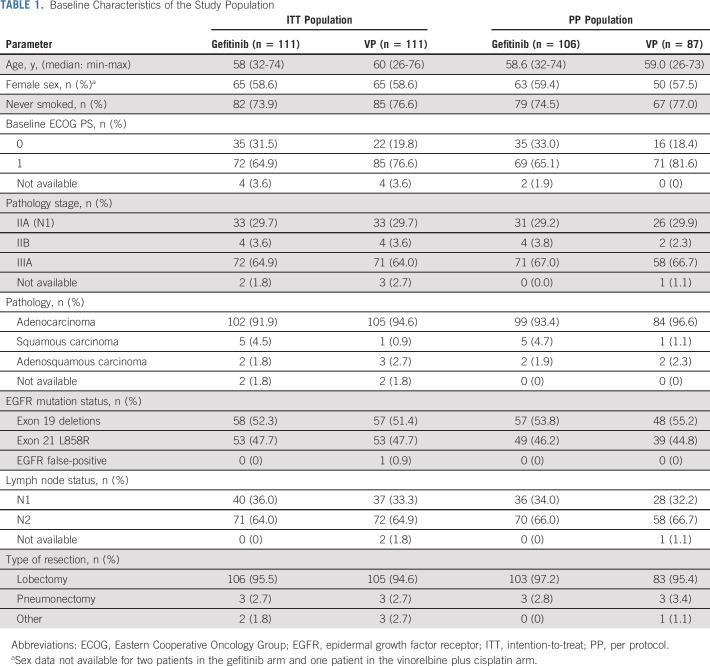
A total of 483 subjects were enrolled between September 19, 2011, and April 24, 2014, from 27 centers across China. Of these, 222 were randomly assigned 1:1 to receive gefitinib or VP and included in the ITT analysis (Fig 1). Full patient demographics have been reported previously.13 Briefly, age, sex, baseline Eastern Cooperative Oncology Group performance status, pathology stage, and EGFR mutation status were similar between the two arms (Table 1).13
FIG 1.
Consort diagram showing patient disposition for the ADJUVANT-CTONG1104 study (data cutoff: April 19, 2020). DFS, disease-free survival; EGFR, epidermal growth factor receptor; ITT, intention-to-treat; PP, per protocol; VP, vinorelbine plus cisplatin.
TABLE 1.
Baseline Characteristics of the Study Population
Final OS Analysis
At data cutoff, median follow-up was 80.0 months, and 100 (45.0%) deaths were reported in the ITT population (n = 222), occurring in 46.8% (52/111) of patients receiving gefitinib and 43.2% (48/111) receiving VP (Fig 1). Median overall survival (mOS) was 75.5 months (95% CI, 46.6 to not calculable [NC]) in the gefitinib arm and 62.8 months (95% CI, 45.8 to NC) in the VP arm; OS was not statistically significantly different between the two arms (HR, 0.92; 95% CI, 0.62 to 1.36; P = .674; Fig 2A). In addition, no statistically significant differences were observed in 5-year OS rates between the gefitinib and VP arms (53.2% v 51.2%, respectively, P = .784; Fig 2A). Among patients who received adjuvant gefitinib, the 5-year OS rate in those with N1 and N2 disease was 61.4% and 49.3%, respectively.
FIG 2.
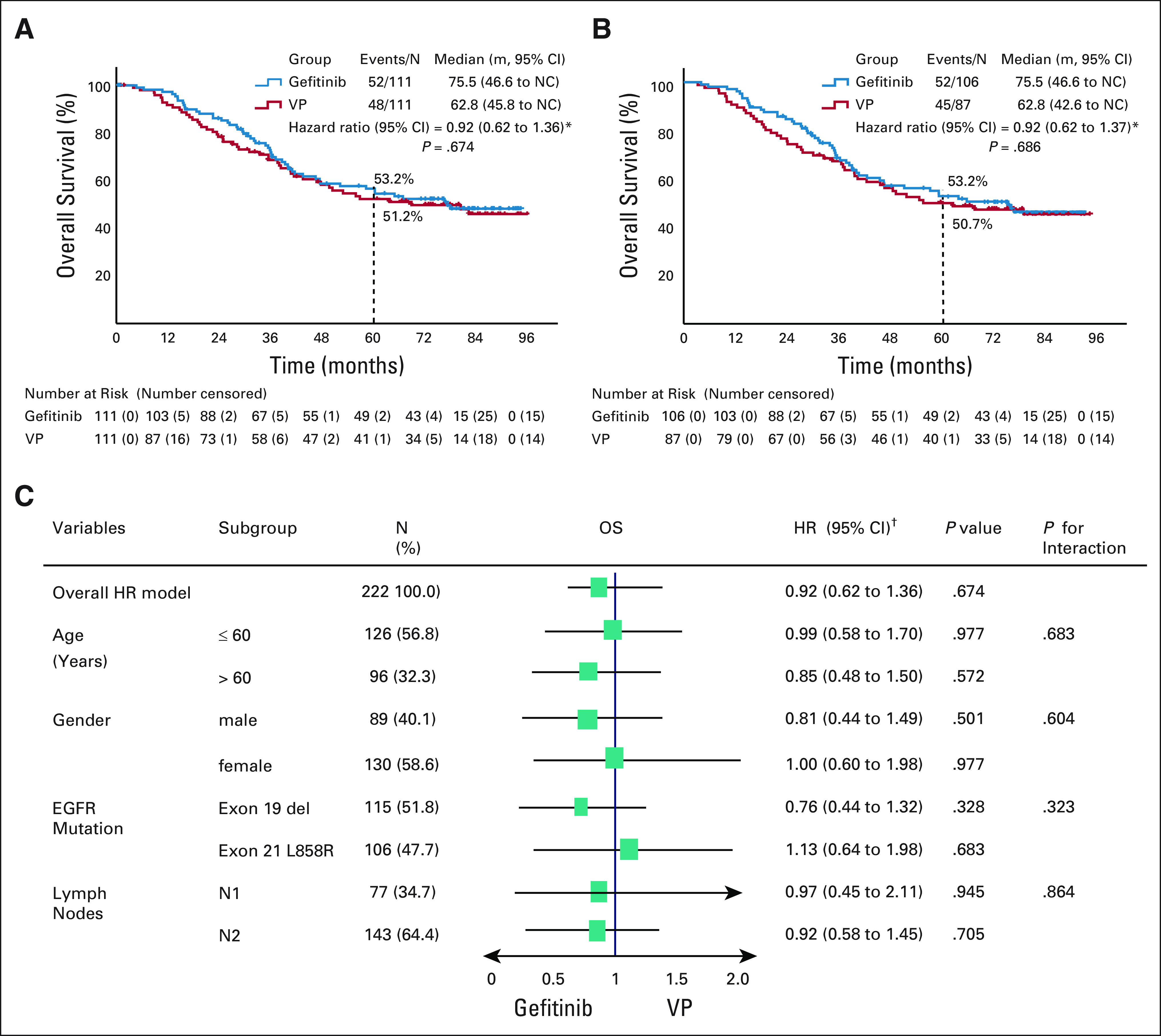
Overall survival in the (A) ITT population and (B) PP population, and (C) subgroup analysis between gefitinib and VP groups. EGFR, epidermal growth factor receptor; HR, hazard ratio; ITT, intention-to-treat; PP, per protocol; VP, vinorelbine plus cisplatin. *Univariate test; †multivariate test.
In the PP population, mOS was 75.5 months (95% CI, 46.6 to NC) in the gefitinib arm and 62.8 months (95% CI, 42.6 to NC) in the VP arm (HR, 0.92; 95% CI, 0.62 to 1.37; P = .686; Fig 2B). Again, no statistically significant differences were observed in 5-year OS rates between the two arms (53.2% v 50.7%, respectively, P = .731; Fig 2B).
Subgroup analysis of OS found no statistically significant differences in predefined subgroups, including age, sex, lymph node involvement, and EGFR mutation type, between gefitinib and VP (Fig 2C).
Impact of Subsequent Treatment
Among the ITT population, 144 patients experienced an event of disease relapse or death. In patients experiencing disease progression, 68.4% (52/76) and 73.6% (50/68) in the gefitinib and VP arms, respectively, received subsequent therapy (Fig 3A). Of these patients, 36.8% (28/76) and 51.5% (35/68) in the gefitinib and VP arms, respectively, received targeted (any TKI) therapy alone, or in combination with chemotherapy or local treatment.
FIG 3.
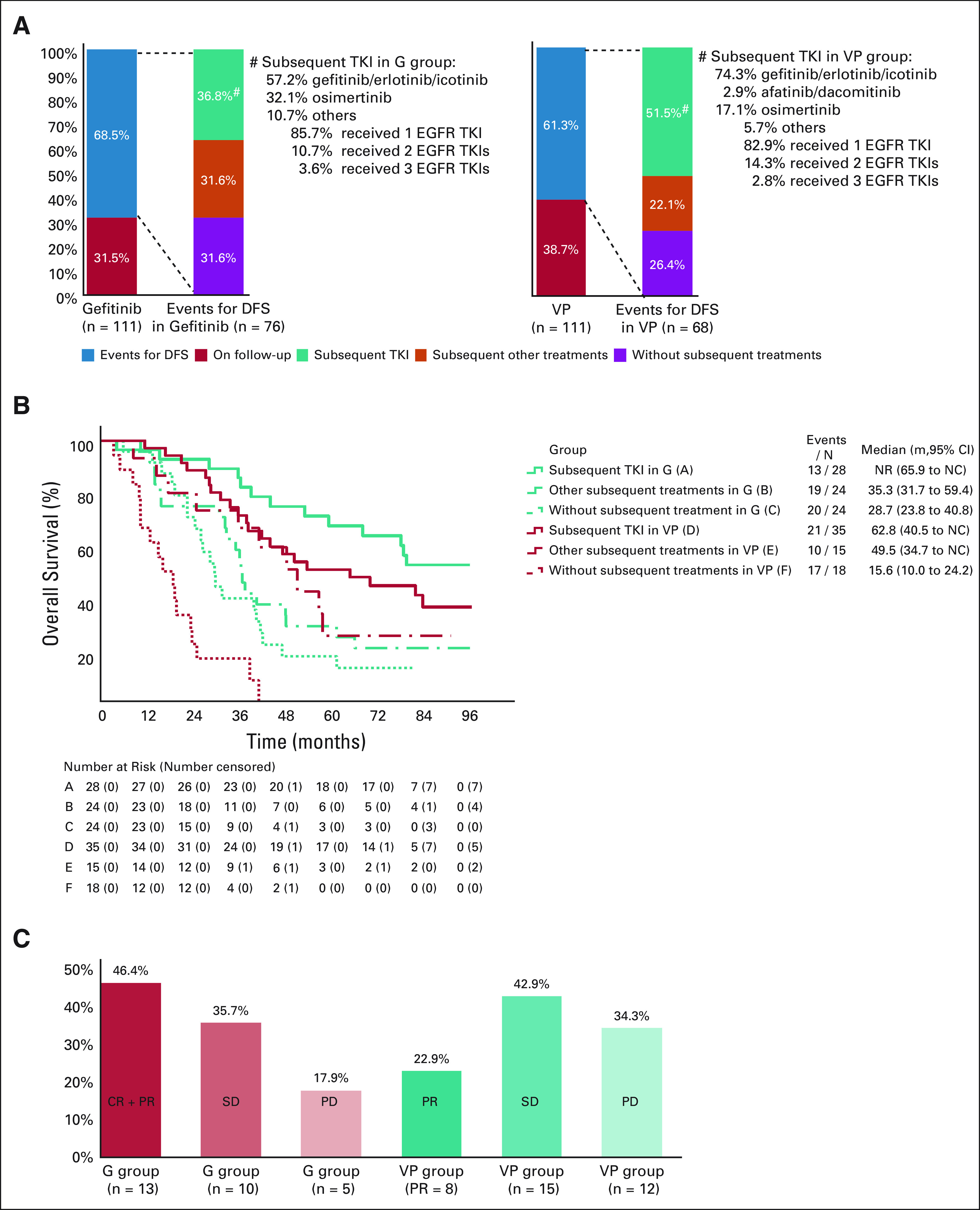
Subsequent treatment in the (A) ITT population, (B) overall survival, and (C) objective response rate for subsequent treatments. CR, complete response; DFS, disease-free survival; EGFR, epidermal growth factor receptor; NC, not calculable; NR, not reached; ITT, intention-to-treat; PD, progressive disease; PR, partial response; SD, stable disease; TKI, tyrosine kinase inhibitor; VP, vinorelbine plus cisplatin. *Univariate test.
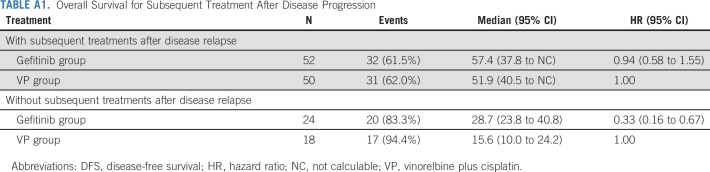
In the gefitinib group, the mOS of patients with disease progression who received subsequent treatment was 57.4 months (95% CI, 37.8 to NC) and 28.7 months (95% CI, 23.8 to 40.8) in those who did not receive subsequent treatment. In the VP arm, mOS was 51.9 months with subsequent treatment (95% CI, 40.5 to NC) and 15.6 months without subsequent treatment (95% CI, 10.0 to 24.2) (Appendix Table A1, online only).
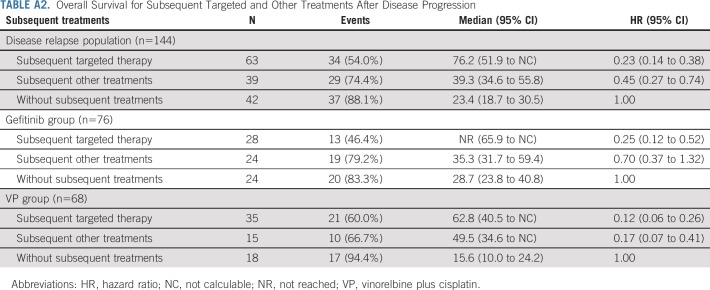
The mOS in patients who experienced disease progression and received subsequent targeted therapy (63/144) was 76.2 months (95% CI, 51.9 to NC) and 39.3 months (95% CI, 34.6 to 55.8) in those who received subsequent other therapy (39/144). In patients who did not receive subsequent therapy (42/144), the mOS was 23.4 months (95% CI, 18.7 to 30.5) (Appendix Table A2, online only).
In patients who received subsequent treatment with TKIs in the gefitinib arm (group A), mOS was not reached (NR; 95% CI, 65.9 to NC; Fig 3B). For patients treated with other subsequent treatments (group B) and no subsequent treatments (group C), mOS was 35.3 months (95% CI, 31.7 to 59.4) and 28.7 months (95% CI, 23.8 to 40.8), respectively. In the VP arm, mOS was 62.8 (95% CI, 40.5 to NC), 49.5 (95% CI, 34.7 to NC), and 15.6 (95% CI, 10.0 to 24.2) months in patients receiving subsequent TKIs (group D), subsequent other therapy (group E), and no subsequent treatment (group F), respectively.
The post hoc analysis of overall response rate (ORR) of complete or partial response was 46.4% (13/28) in patients who received subsequent TKI re-treatment in the gefitinib arm; 35.7% (10/28) and 17.9% (5/28) of these patients reported stable disease and progressive disease, respectively (Fig 3C). For patients in the VP arm who received subsequent TKI therapy, partial response was reported in 22.9% (8/35), stable disease in 42.9% (15/35), and progressive disease in 34.3% (12/35) (Fig 3C).
Updated DFS Analysis
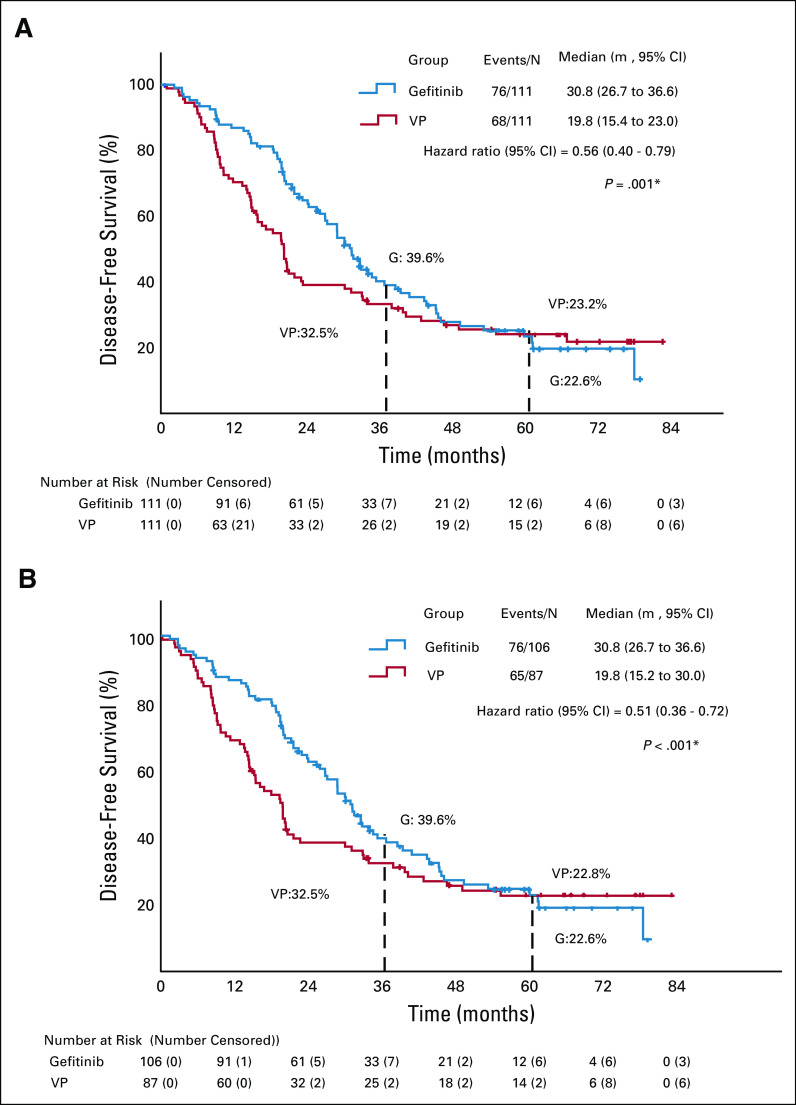
The 3- and 5-year DFS rates in the ITT population were 39.6% and 22.6% with gefitinib, and 32.5% and 23.2% with VP, respectively. mDFS was significantly longer in the gefitinib than in the VP arm (30.8, 95% CI, 26.7 to 36.6 months v 19.8, 95% CI, 15.4 to 23.0 months, respectively; HR, 0.56; 95% CI, 0.40 to 0.79; P = .001) (Fig 4A).
FIG 4.
Updated disease-free survival for the (A) ITT and (B) PP populations. HR, hazard ratio; ITT, intention-to-treat; PP, per protocol; VP, vinorelbine plus cisplatin. *Univariate test.
In the PP population, 3- and 5-year DFS rates were 39.6% and 22.6% with gefitinib, and 32.5% and 22.8% with VP, respectively. The updated mDFS was 30.8 (95% CI, 26.7 to 36.6) and 19.8 (95% CI, 15.2 to 30.0) months in the gefitinib and VP arms, respectively (HR, 0.51; 95% CI, 0.36 to 0.72; P < .001) (Fig 4B).
DISCUSSION
The ADJUVANT-CTONG1104 study is the first randomized phase III study that compares OS after treatment with adjuvant EGFR-TKI versus standard chemotherapy in patients with EGFR-mutant stage II-III NSCLC. We report mOS of 75.5 months in patients in the adjuvant gefitinib arm, which to date is one of the longest durations of OS observed with adjuvant therapy, compared with existing data from the IASLC database.9 Although demonstrating a numerical advantage, mOS with adjuvant gefitinib was not significantly different to that for adjuvant VP (mOS, 75.5 months v 62.8 months; HR, 0.92; 95% CI, 0.62 to 1.36; P = .674; Fig 2A). A sensitivity analysis of predefined subgroups confirmed these results, finding no statistically significant differences between the two treatment arms when adjusted for age, sex, lymph node involvement, or EGFR mutation type.
The results of this study highlight the importance of targeted therapy as an adjuvant treatment in early-stage NSCLC. The 5-year OS rate with adjuvant gefitinib in the ADJUVANT study was 53.2%, with N2 disease accounting for 64.0% of patients in this treatment arm. In patients with N2 disease receiving adjuvant gefitinib, the 5-year OS rate was 49.3%, and 61.4% for those with N1 disease. This compares with 38% (N2) and 50% (N1) in the IASLC database.9 It must be noted, however, that the EGFR mutation status of patients on the IASLC database is unknown, which is also reflective of the wider paucity of real-world data for postoperative adjuvant therapy in patients with EGFR-mutant stage II-III NSCLC.
We also presented updated mDFS for the ADJUVANT study, which showed a statistically significant improvement in the gefitinib versus VP arm (30.8 v 19.8 months, respectively; HR, 0.56; 95% CI, 0.40 to 0.79). However, the DFS benefits in the ITT population did not translate to a significant difference in OS. One important possible reason is that after disease recurrence, patients may experience many later lines of treatment, the efficacy of which will contribute to OS, but this makes it increasingly challenging to achieve statistically significant differences in OS in an adjuvant setting. Taken together, this may support the proposition of DFS being a surrogate for OS in an adjuvant treatment setting, as currently used in breast cancer treatment.17,18 Another possible explanation for the lack of separation in OS between treatment groups is that, of the patients experiencing disease progression, more received subsequent treatment in the VP arm than those in the gefitinib arm (73.6% v 68.4%, P = .501). This may have had an effect in dampening the difference in OS between the two treatment arms. This observation is supported by the prolonged OS experienced by patients receiving subsequent treatment compared with those not receiving any subsequent treatment in the gefitinib and VP arms (Appendix Table A1). In addition, more patients in the VP arm crossover received subsequent EGFR-TKI therapy than in the gefitinib arm after disease recurrence (51.5% v 36.8%). This observation is supported by the prolonged OS experienced by patients receiving subsequent EGFR-TKI compared with those receiving other subsequent treatment in the VP arm (mOS, 62.8 months; 95% CI, 40.5 to NC and mOS, 49.5 months; 95% CI, 34.7 to NC, respectively).
We also sought to determine whether patients who received adjuvant EGFR-TKI would still respond to subsequent EGFR-TKI treatment and obtain a survival benefit. Our finding was that in the adjuvant gefitinib arm, 36.8% of patients received subsequent EGFR-TKI, with a response rate of 46.4% and disease control rate of 82.1%. However, in the VP arm, 51.5% of patients received subsequent EGFR-TKI, with a response rate of 22.9% and disease control rate of 65.8%. Although the ORRs with VP appear low, we would advise caution in any interpretation of these data, because the sample size is small. Nevertheless, these findings still indicate that EGFR-mutant lung cancer maintained sensitivity to EGFR-TKIs at re-treatment, which may have contributed to the prolonged OS in both arms of the study.19 In general, patients who received more subsequent lines of EGFR-TKIs had a longer OS (mOS, NR; 95% CI, 65.9 to NC and mOS, 62.8 months; 95% CI, 40.5 to NC for the gefitinib and VP arms, respectively; Fig 3B). These results suggest that the modality of adjuvant targeted therapy followed by subsequent targeted therapy may provide longer OS and may be a better sequential treatment model for patients with resected stage II-IIIA (N1-N2) NSCLC with an EGFR mutation. In our trial, approximately 48% of patients who received adjuvant chemotherapy did not receive EGFR-TKIs despite harboring EGFR mutations. The most important reason for this observation is because of drug accessibility because most patients in China incur out-of-pocket expenses for EGFR-TKI treatment. A similar observation was made in a recent real-world survey conducted in China, where approximately 31% of EGFR-mutant patients with advanced NSCLC did not receive EGFR-TKI treatment.20
Currently, most adjuvant trials are designed with 2 years of drug exposure.13-15,21 The CTONG-1103 erlotinib study used neoadjuvant erlotinib for 42 days and postoperative treatment for 1 year,13,22 whereas ADAURA investigated the use of osimertinib (a third-generation TKI) for 3 years post-resection.23 Our previous analysis showed that adjuvant gefitinib had a unique spatial-temporal treatment failure pattern, with recurrence with gefitinib increasing at a steady rate 12 months post-surgery, and a first peak of extracranial metastases occurring 24-36 months post-surgery.24 The optimal duration of adjuvant targeted therapy treatment remains under discussion and needs further exploration.
The ADAURA study recently demonstrated that adjuvant osimertinib provides a statistically significant and clinically meaningful improvement in DFS in patients with stage IB-IIIA EGFR-mutant NSCLC (HR, 0.20; 99.12% CI, 0.14 to 0.30; P < .001), and that a consistent improvement in DFS was observed regardless of whether patients did or did not receive prior adjuvant chemotherapy (HR, 0.16; 95% CI, 0.10 to 0.26 and HR, 0.23; 95% CI, 0.13 to 0.40, respectively).25 Together with the results of our study designed to directly compare adjuvant gefitinib with adjuvant chemotherapy, it is unclear whether chemotherapy remains necessary in an adjuvant setting. Future OS outcomes of the ADAURA study will be of interest in light of those for the ADJUVANT-CTONG1104 study reported here, given the impact of increasingly effective therapies providing longer survival after disease progression. Taken together, we suggest that the standard of care for resected stage II-IIIA (N1-N2) EGFR-mutant NSCLC may be changed from adjuvant therapy to adjuvant EGFR-TKI treatment without prior adjuvant chemotherapy.
One of the limitations of this study is that patients were only recruited from China; thus, these results cannot be used to generalize across broader populations. Another limitation is that we did not test for biomarkers of relapse mechanisms in patients treated with adjuvant gefitinib or adjuvant VP. Because of the inequities in subsequent treatments between groups, and the small sample sizes in the post hoc analysis, it was not possible to draw firm conclusions about the differential efficacy of subsequent EGFR-TKI therapy in relation to patient characteristics and treatment decisions. Furthermore, the median follow-up for OS was 80 months, resulting in a lost-to-follow up rate of 5.4% (12/222); these patients were included as censored data in the OS analysis.
In conclusion, the DFS advantage with adjuvant gefitinib did not translate to a significant difference in OS. However, adjuvant therapy with gefitinib is an important treatment option for patients with resected stage II-IIIA (N1-N2) NSCLC, demonstrating improved DFS over standard-of-care chemotherapy. Final OS results from the ADAURA trial with the third-generation EGFR-TKI osimertinib are anticipated, and further studies to elucidate personalized approaches to adjuvant EGFR-TKI therapy in patients with resected stage II-IIIA (N1-N2) NSCLC are warranted.
ACKNOWLEDGMENT
The authors would like to thank all study participants and centers. Editorial assistance in manuscript preparation was provided by Tim Stentiford BSc (Hons), PgDip (SciComm), CMPP, and Joyce Lee PhD of Nucleus Global Shanghai, China, funded by AstraZeneca.
APPENDIX
TABLE A1.
Overall Survival for Subsequent Treatment After Disease Progression
TABLE A2.
Overall Survival for Subsequent Targeted and Other Treatments After Disease Progression
SUPPORT
Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine; National Health and Family Planning Commission of People's Republic of China; and AstraZeneca China.
CLINICAL TRIAL INFORMATION
See accompanying editorial on page 697
AUTHOR CONTRIBUTIONS
Conception and design: Wen-Zhao Zhong, Si-Yang Liu, Yi-Long Wu
Provision of study materials or patients: Wen-Zhao Zhong, Qun Wang, Wei-Min Mao, Song-Tao Xu, Lin Wu, Yu-Cheng Wei, Yong-Yu Liu, Chun Chen, Ying Cheng, Fan Yang, Sheng-Xiang Ren, Xiao-Fei Li, Jian Li, Cheng Huang, Zhi-Dong Liu, Shun Xu, Ke-Neng Chen, Shi-Dong Xu, Lun-Xu Liu, Ping Yu, Hai-Tao Ma, Yi-Long Wu
Collection and assembly of data: All authors
Data analysis and interpretation: Wen-Zhao Zhong, Qun Wang, Wei-Min Mao, Song-Tao Xu, Yu-Cheng Wei, Chun Chen, Fan Yang, Xiao-Fei Li, Jian Li, Cheng Huang, Zhi-Dong Liu, Shi-Dong Xu, Lun-Xu Liu, Ping Yu, Bu-Hai Wang, Hai-Tao Ma, Hong-Hong Yan, Xue-Ning Yang, Si-Yang Liu, Qing Zhou, Yi-Long Wu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Gefitinib Versus Vinorelbine Plus Cisplatin as Adjuvant Treatment for Stage II-IIIA (N1-N2) EGFR-Mutant NSCLC: Final Overall Survival Analysis of CTONG1104 Phase III Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Wen-Zhao Zhong
Honoraria: AstraZeneca, Roche
Fan Yang
Consulting or Advisory Role: AstraZeneca, Roche
Qing Zhou
Honoraria: AstraZeneca, Roche
Yi-Long Wu
Honoraria: AstraZeneca, Lilly, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim
Research Funding: Boehringer Ingelheim, Roche, Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Burdett S Pignon JP Tierney J, et al. : Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev CD011430, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng H Li XJ Wang XJ, et al. : A meta-analysis of adjuvant EGFR-TKIs for patients with resected non-small cell lung cancer. Lung Cancer 137:7-13, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Yuan Y Huang Q Gu C, et al. : Disease-free survival improved by use of adjuvant EGFR tyrosine kinase inhibitors in resectable non-small cell lung cancer: an updated meta-analysis. J Thorac Dis 9:5314-5321, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisters KM Evans WK Azzoli CG, et al. : Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol 25:5506-5518, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Xiu-Yi Zhi Jin-Ming Yu, and Yuan-Kai Shi. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version). Cancer 121:3165-3181, 2015. Cancer 122:162, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Eberhardt WE De Ruysscher D Weder W, et al. : 2nd ESMO Consensus Conference in Lung Cancer: Locally advanced stage III non-small-cell lung cancer. Ann Oncol 26:1573-1588, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Ettinger DS Wood DE Aggarwal C, et al. : NCCN guidelines insights: Non-small cell lung cancer, version 1.2020. J Natl Compr Canc Netw 17:1464-1472, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Kris MG Gaspar LE Chaft JE, et al. : Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol 35:2960-2974, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Goldstraw P Chansky K Crowley J, et al. : The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 11:39-51, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Colt HG Murgu SD Korst RJ, et al. : Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e437S-e454S, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Deng XF Jiang L Liu QX, et al. : Lymph node micrometastases are associated with disease recurrence and poor survival for early-stage non-small cell lung cancer patients: a meta-analysis. J Cardiothorac Surg 11:28, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mok TS Wu YL Thongprasert S, et al. : Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947-957, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Zhong WZ Wang Q Mao WM, et al. : Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): A randomised, open-label, phase 3 study. Lancet Oncol 19:139-148, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Yue D Xu S Wang Q, et al. : Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): A randomised, open-label, phase 2 trial. Lancet Res Med 6:863-873, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Pennell NA Neal JW Chaft JE, et al. : SELECT: A phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol 37:97-104, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pocock SJ, Simon R: Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 31:103-115, 1975 [PubMed] [Google Scholar]
- 17.Gyawali B, Hey SP, Kesselheim AS: Evaluating the evidence behind the surrogate measures included in the FDA's table of surrogate endpoints as supporting approval of cancer drugs. EClinicalMedicine 21:100332, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauguen A Pignon JP Burdett S, et al. : Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: A re-analysis of meta-analyses of individual patients' data. Lancet Oncol 14:619-626, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oxnard GR Janjigian YY Arcila ME, et al. : Maintained sensitivity to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer recurring after adjuvant erlotinib or gefitinib. Clin Cancer Res 17:6322-6328, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q Song Y Zhang X, et al. : A multicenter survey of first-line treatment patterns and gene aberration test status of patients with unresectable stage IIIB/IV nonsquamous non-small cell lung cancer in China (CTONG 1506). BMC Cancer 17:462, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly K Altorki NK Eberhardt WE, et al. : Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): A randomized, double-blind, phase III trial. J Clin Oncol 33:4007-4014, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Zhong WZ Chen KN Chen C, et al. : Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): A randomized phase II study. J Clin Oncol 37:2235-2245, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Wu YL Herbst RS Mann H, et al. : ADAURA: Phase III, double-blind, randomized study of osimertinib versus placebo in egfr mutation-positive early-stage NSCLC after complete surgical resection. Clin Lung Cancer 19:e533-e536, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Xu ST Xi J-J Zhong WZ, et al. : The unique spatial-temporal treatment failure patterns of adjuvant gefitinib therapy: A post hoc analysis of the ADJUVANT trial (CTONG 1104). J Thorac Oncol 14:503-512, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Wu YL Tsuboi M He J, et al. : Osimertinib in resected EGFR-mutated non–small-cell lung cancer. N Engl J Med 383:1711-1723, 2020 [DOI] [PubMed] [Google Scholar]