PURPOSE
Patients with the activated B-cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) historically showed inferior survival with standard rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Phase II studies demonstrated that adding the immunomodulatory agent lenalidomide to R-CHOP improved outcomes in ABC-type DLBCL. The goal of the global, phase III ROBUST study was to compare lenalidomide plus R-CHOP (R2-CHOP) with placebo/R-CHOP in previously untreated, ABC-type DLBCL.
METHODS
Histology and cell-of-origin type were prospectively analyzed by central pathology prior to random assignment and study treatment. Patients with ABC-DLBCL received lenalidomide oral 15 mg/d, days 1-14/21 plus standard R-CHOP21 versus placebo/R-CHOP21 for six cycles. The primary end point was progression-free survival (PFS) per independent central radiology review.
RESULTS
A total of 570 patients with ABC-DLBCL (n = 285 per arm) were stratified by International Prognostic Index score, age, and bulky disease, and randomly assigned to R2-CHOP or placebo/R-CHOP. Baseline demographics were similar between arms. Most patients completed six cycles of treatment: 74% R2-CHOP and 84% placebo/R-CHOP. The most common grade 3/4 adverse events for R2-CHOP versus placebo/R-CHOP were neutropenia (60% v 48%), anemia (22% v 14%), thrombocytopenia (17% v 11%), and leukopenia (14% v 15%). The primary end point of PFS was not met, with a hazard ratio of 0.85 (95% CI, 0.63 to 1.14) and P = .29; median PFS has not been reached for either arm. PFS trends favoring R2-CHOP over placebo/R-CHOP were seen in patients with higher-risk disease.
CONCLUSION
ROBUST is the first DLBCL phase III study to integrate biomarker-driven identification of eligible ABC patients. Although the ROBUST trial did not meet the primary end point of PFS in all patients, the safety profile of R2-CHOP was consistent with individual treatments with no new safety signals.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) comprises one-third of patients with mature B-cell non-Hodgkin lymphoma as the most common type of aggressive lymphoma.1,2 Standard first-line therapy for advanced-stage DLBCL currently relies on the anti-CD20 antibody rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).3-5 Although > 50% of patients experience long-term disease control with R-CHOP, approximately 30% in remission ultimately relapse at increasing rates, upon which outcomes are poor.3-6 Numerous trials attempted to improve outcomes by investigating alternate regimens, adding combination agents,7-9 and exchanging rituximab for type II anti-CD20 antibody obinutuzumab10; to date, none have demonstrated a clinically significant improvement.
CONTEXT
Key Objective
What is the potential improvement in outcomes with the combination of lenalidomide plus rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) over standard R-CHOP in previously untreated patients with diffuse large B-cell lymphoma who had activated B-cell-like disease (typically associated with worse prognosis)?
Knowledge Generated
The global, phase III ROBUST study did not meet the primary end point for significantly improved progression-free survival with the lenalidomide/R-CHOP combination over control R-CHOP, although response rates were very high (91% overall response rate) in both study arms and median overall survival was not reached. The safety profile of lenalidomide plus R-CHOP was generally well tolerated, with no new safety signals with the addition of lenalidomide.
Relevance
Despite the lack of statistically significant efficacy benefit of lenalidomide with R-CHOP, these study results provide support for ongoing and future analyses to further evaluate the potential effect of pharmacokinetics or dosing, molecular classification, and mutational status in patients with diffuse large B-cell lymphoma.
DLBCL is a heterogeneous disease with two major biologically distinct pathophysiologic entities based on cell-of-origin (COO) and classified as germinal center B-cell-like (GCB) and activated B-cell-like (ABC) subtypes by gene expression profiling (GEP).11,12 These were discovered by GEP,11,13 then later translated into an immunohistochemistry (IHC) algorithm categorizing them as GCB and non-GCB.14 GEP-based models recognize a third “unclassified” category that cannot be assigned to either main subtype with sufficient confidence.15 Patients with ABC-DLBCL treated with R-CHOP historically demonstrated inferior survival (5-year overall survival [OS]: approximately 50% ABC v approximately 80% GCB; P < .001).16
Preclinical studies established lenalidomide's antiproliferative activity in ABC-DLBCL cells through increasing interferon-stimulated gene transcription and activation of immunomodulatory mechanisms.17-19 Phase II studies showed activity of lenalidomide monotherapy with tolerable safety in relapsed or refractory DLBCL.20,21 Coupled with analyses demonstrating a significant clinical response in non-GCB versus GCB-type DLBCL,22 these studies provided the basis for further evaluation of first-line lenalidomide with R-CHOP (R2-CHOP). Results from two independent, single-arm, phase II studies (REAL07 and MC078E) of R2-CHOP suggested that improved survival may be achieved in non-GCB DLBCL, and with manageable safety.23,24 MC078E compared R2-CHOP-treated patients with contemporaneous R-CHOP-only controls, demonstrating that adding lenalidomide may improve survival in non-GCB DLBCL.24 Longer follow-up of REAL07/MC078E combined non-GCB data showed durable efficacy with 5-year progression-free survival (PFS) of 65% and 5-year OS of 74%.25 These initial results23,24 provided proof-of-concept for ROBUST (ClinicalTrials.gov identifier: NCT02285062; EudraCT number 2013-004054-21), which prospectively compared efficacy and safety of first-line R2-CHOP with placebo/R-CHOP in ABC-type DLBCL.
METHODS
Patients
Eligible patients with CD20+, ABC-type DLBCL were of age 18-80 years, Eastern Cooperative Oncology Group performance status ≤ 2, Ann Arbor stage II-IV disease, and International Prognostic Index (IPI) score of ≥ 2. ABC subtype was determined using the NanoString Lymphoma Subtyping Test performed on NanoString's nCounter Dx analysis system (NanoString Technologies, Inc, Seattle, WA).13 Additional eligibility criteria are provided in the Appendix (online only).
Trial Design or Treatments
ROBUST was a multicenter, international, randomized, double-blind, phase III trial (Appendix Fig A1, online only). During screening, the central pathology laboratory confirmed disease diagnosis and CD20 status, and identified COO subtype as ABC or non-ABC (GCB and unclassified). Following eligibility confirmation, patients were stratified by IPI score (2 v ≥ 3), age (< 65 v ≥ 65 years), and bulky disease (< 7 cm [nonbulky] v ≥ 7 cm [bulky]), and randomly assigned 1:1 to R2-CHOP or placebo/R-CHOP.
Lenalidomide dose was selected based on risk-benefit considerations from proof-of-concept studies (REAL07 and MC078E).23,24 Treatment included lenalidomide (or placebo) 15 mg oral on days 1-14 of every 21-day cycle plus R-CHOP21 (rituximab 375 mg/m2 intravenous [IV] day −1 or 1, cyclophosphamide 750 mg/m2 IV day 1, doxorubicin 50 mg/m2 IV day 1, vincristine 1.4 mg/m2 [maximum 2.0 mg total] IV day 1, and prednisone [or prednisolone] 100 mg oral days 1-5 [IV day 1 of prednisone or prednisolone, or equivalent methylprednisolone or dexamethasone dose]). Treatment was continued for six cycles, or until intolerability, inadequate response, disease progression, consent withdrawal, or death, whichever occurred first. Two additional rituximab doses (1 dose/21-day cycle) were permitted at cycles 7 and 8 if prespecified and considered standard of care per local practice. Investigators could prospectively give prespecified local radiotherapy consolidation after chemotherapy to treat a particular bulky disease site (≥ 7 cm) or large mass.
Neutropenia prophylaxis with either granulocyte-colony stimulating factor or granulocyte macrophage-colony stimulating factor was required every cycle per local practices. Additional prophylaxis recommendations are in the Appendix. Growth factor prophylaxis was recommended, and blood product transfusions were allowed per protocol in accordance with ASCO/European Society for Medical Oncology guidelines.26,27
All patients received the same lenalidomide starting dose regardless of baseline creatinine clearance levels. Lenalidomide dose adjustments were planned to manage toxicity (Appendix). Rituximab and chemotherapy dose modifications were allowed per clinical practice of the investigator's institution per approved prescribing information.
The trial adhered to Good Clinical Practice per the International Conference on Harmonisation Guideline E6 under ethical principles of the Declaration of Helsinki. Study conduct followed guidance from each site's institutional review board, independent ethics committee, and regulatory authorities. All patients provided written informed consent before trial enrollment.
Efficacy and Safety Assessments
For the primary efficacy analysis, the intent-to-treat population included all randomly assigned patients regardless of receiving study treatment. The primary end point was PFS per 2014 International Working Group criteria28 (amended from the original protocol following 2007 International Working Group criteria29), as assessed by Independent Radiology Adjudication Committee with Food and Drug Administration censoring rules applied.30 Investigator-assessed results provided additional sensitivity analyses.
PFS was defined as the time from random assignment to objective disease progression or death from any cause, whichever occurred first. Secondary end points were event-free survival (EFS; key secondary), OS, response rates, duration of response, time to next lymphoma treatment, and safety. EFS was defined as the time from random assignment to initiation of disease progression, relapse from complete response, initiation of subsequent antilymphoma therapy, or death because of any cause. Response assessments included computed tomography and positron emission tomography scans and evaluation of laboratory or clinical data.
The safety population included all patients receiving ≥ 1 dose of any study treatment. Treatment-emergent adverse events (TEAEs) were coded per the Medical Dictionary for Drug Regulatory Activities v21.0 and classified by National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 (tumor flare reaction and skin rash per v3.0).
Statistical Analyses
Superiority in PFS was defined as achieving hazard ratio (HR) = 0.625 for R2-CHOP over placebo/R-CHOP (ie, 37.5% risk reduction in disease progression) for an estimated median PFS improvement of 24 months with placebo/R-CHOP to 38 months with R2-CHOP (two-sided P < .05). The study was powered to measure 192/560 PFS events for 90% power and included interim futility analysis at 50% (96 events). If the event rate fell < 2 events/mo before reaching 192 events, final analysis was performed when ≥ 170 events occurred (86% power).
Demographics or characteristics and safety were summarized using descriptive statistics, and categorical variables using frequency tabulations. Time-to-event end points using an HR with two-sided 95% CI were estimated by Kaplan-Meier procedure, stratified log-rank test for treatment efficacy, and Cox proportional hazards model. Binary end points (eg, response rate) were summarized in frequency and percent by arm; stratified Cochran-Mantel-Haenszel test evaluated treatment efficacy. All statistical analyses used SAS software version ≥ 9.2 (SAS Institute, Cary, NC).
RESULTS
Patients
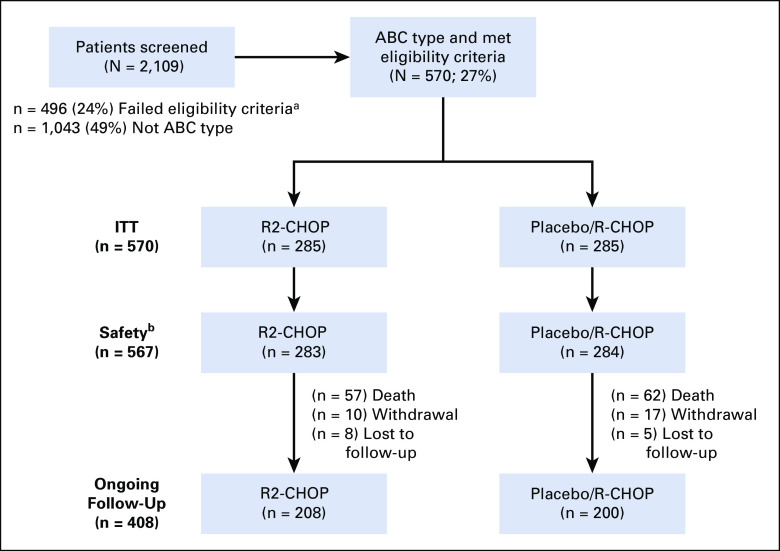
From February 17, 2015 to August 3, 2017, the central pathology laboratory screened samples from 2,109 patients; 570 patients with ABC-DLBCL met eligibility criteria for enrollment at 257 active study centers in 21 countries (Fig 1). Of 2,109 screened patients, exclusions were predominantly because 49% were non-ABC subtype and 24% failed other eligibility criteria (main reasons: 8% inadequate lymph node or biopsy specimen available, 5% non-Ann Arbor stage II-IV, 4% non-IPI ≥ 2, and 3% unable to adhere to protocol requirements, 1% because of a small or insufficient core or tissue biopsy).
FIG 1.
R2-CHOP and placebo/R-CHOP CONSORT diagram (flow of patients from screening to analysis). aMain reasons for failing eligibility criteria: 8% inadequate lymph node or biopsy specimen available, 5% not Ann Arbor stage II-IV, 4% not International Prognostic Index ≥ 2, and 3% unable to adhere to protocol requirements. bTwo R2-CHOP and one placebo/R-CHOP patients were randomly assigned, but never received lenalidomide/placebo or R-CHOP. ABC, activated B-cell-like; ITT, intention to treat; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R2-CHOP, lenalidomide plus R-CHOP.
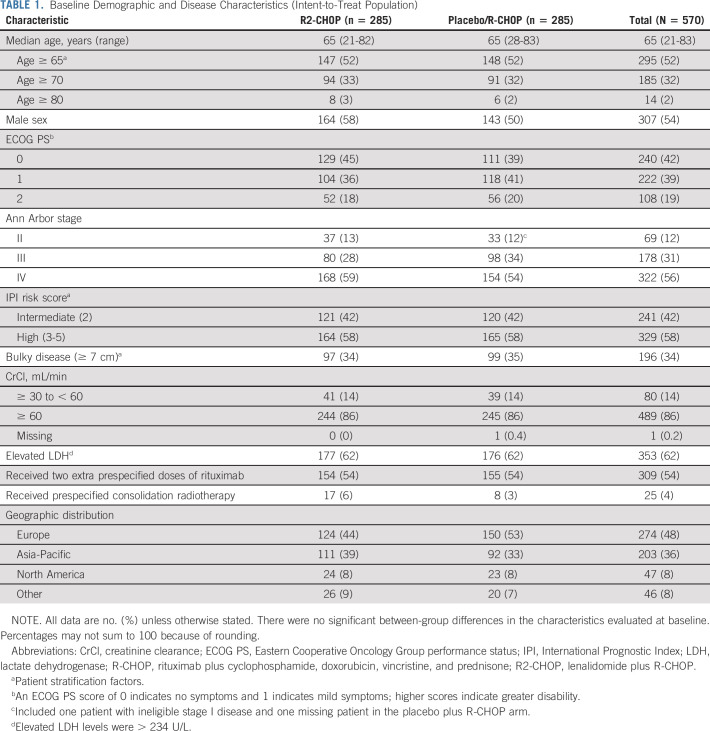
In the intent-to-treat analysis, 285 patients in each arm were randomly assigned 1:1 to experimental R2-CHOP and control placebo/R-CHOP groups. Baseline demographics were similar between arms. Overall, patients had a median age of 65 years (52% ≥ 65 and 2% ≥ 80 years of age); 42% IPI 2/58% IPI ≥ 3 score; 88% stage III/IV disease; and 34% bulky disease (Table 1). Median time from initial diagnosis or biopsy date to treatment was 31 days for both arms (R2-CHOP: range, 6-114 days; placebo/R-CHOP: range, 8-98 days). Median follow-up time for all surviving patients was 27.1 months (range, 0-47 months).
TABLE 1.
Baseline Demographic and Disease Characteristics (Intent-to-Treat Population)
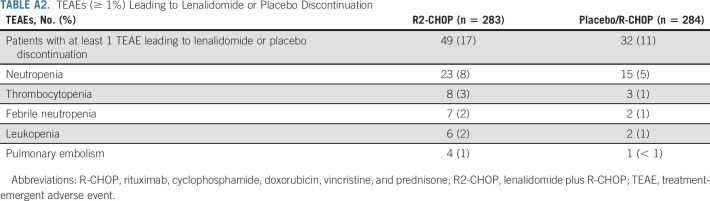
Two patients on R2-CHOP and one on placebo/R-CHOP were randomly assigned but never received treatment, and therefore are excluded from the safety population. Adverse events (AEs) were the most frequent reason for discontinuation of lenalidomide or placebo (17% R2-CHOP v 11% placebo/R-CHOP; Appendix Table A2, online only).
Efficacy
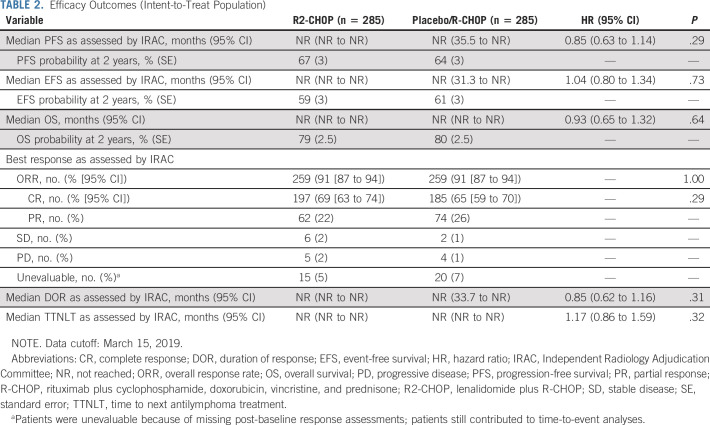
Primary end point.
The primary end point of PFS was not met (HR, 0.85; 95% CI, 0.63 to 1.14; P = .29; Table 2; Fig 2A). Median PFS was not reached in either arm; 2-year PFS was 67% for R2-CHOP and 64% for placebo/R-CHOP.
TABLE 2.
Efficacy Outcomes (Intent-to-Treat Population)
FIG 2.
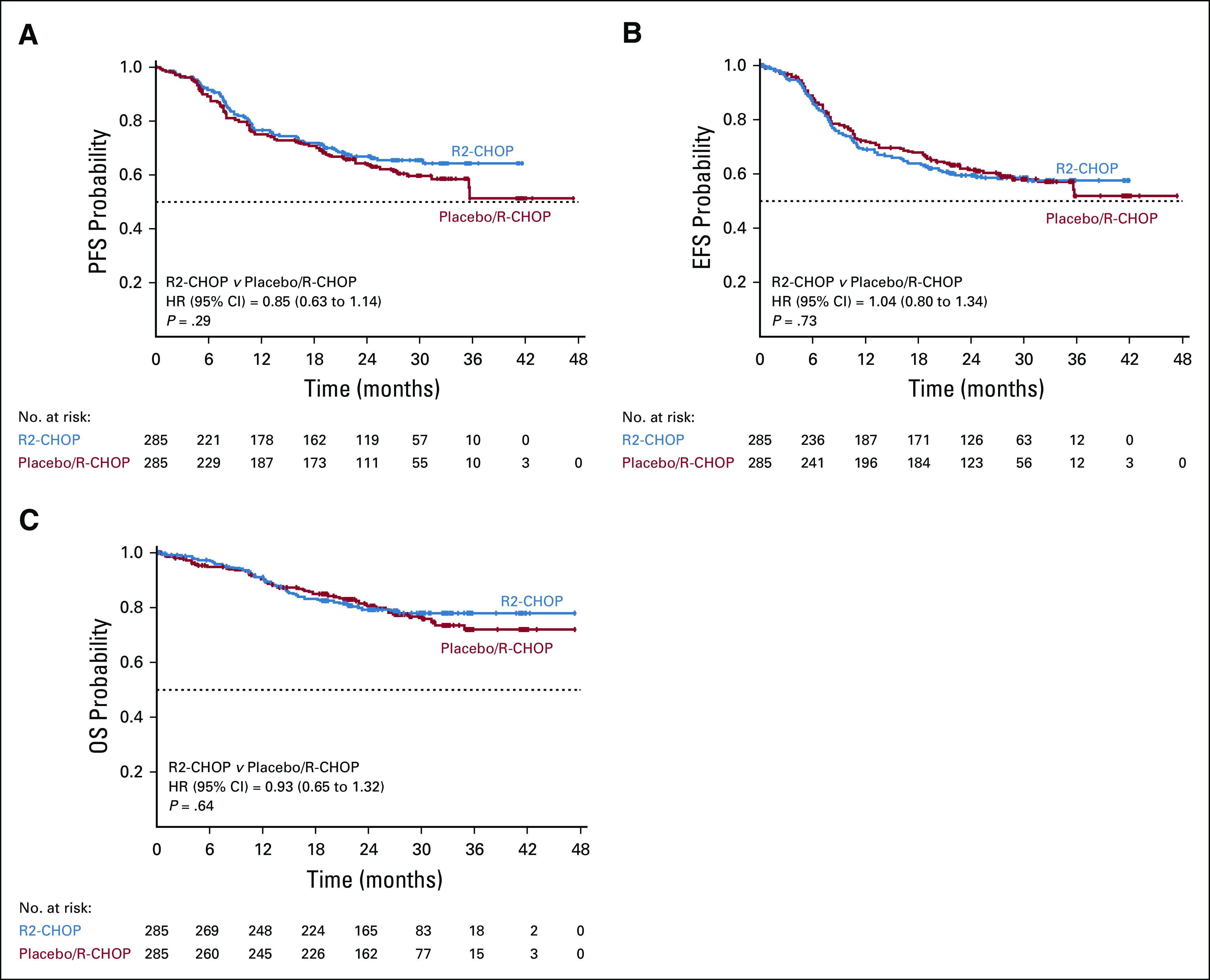
PFS, EFS, and OS in the intent-to-treat population: (A) progression-free survival by Independent Radiology Adjudication Committee (IRAC) assessment; (B) event-free survival by IRAC assessment; (C) OS. EFS, event-free survival; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R2-CHOP, lenalidomide plus R-CHOP.
Secondary and exploratory efficacy end points.
The key secondary efficacy end point EFS was also not met (HR, 1.04 [95% CI, 0.80 to 1.34]; P = .73); medians were not reached (Table 2; Fig 2B). EFS included n = 10 R2-CHOP and n = 8 placebo/R-CHOP with stable disease or positron emission tomography-positive partial responses who initiated new therapy. OS data were immature; estimated 2-year OS rates were 79% R2-CHOP and 80% placebo/R-CHOP; medians were not reached (Table 2; Fig 2C). Of patients who died, 93/119 deaths (78%) were because of progressive disease (< 2% each from AEs or other causes). Overall response rates were 91% for both arms, with 69% versus 65% complete responses for R2-CHOP versus placebo/R-CHOP, respectively (Table 2). Median time to next antilymphoma treatment was not reached in either arm.
Exploratory subgroup analyses of PFS suggested a positive trend in 2-year PFS favoring R2-CHOP (v placebo/R-CHOP) in patients with IPI ≥ 3 (59% v 50%, P = .09; Fig 3), nonbulky disease (73% v 66%, P = .05), and lower baseline creatinine clearance 30 to < 60 mL/min (69% v 45%, P = .03; Fig 4).
FIG 3.
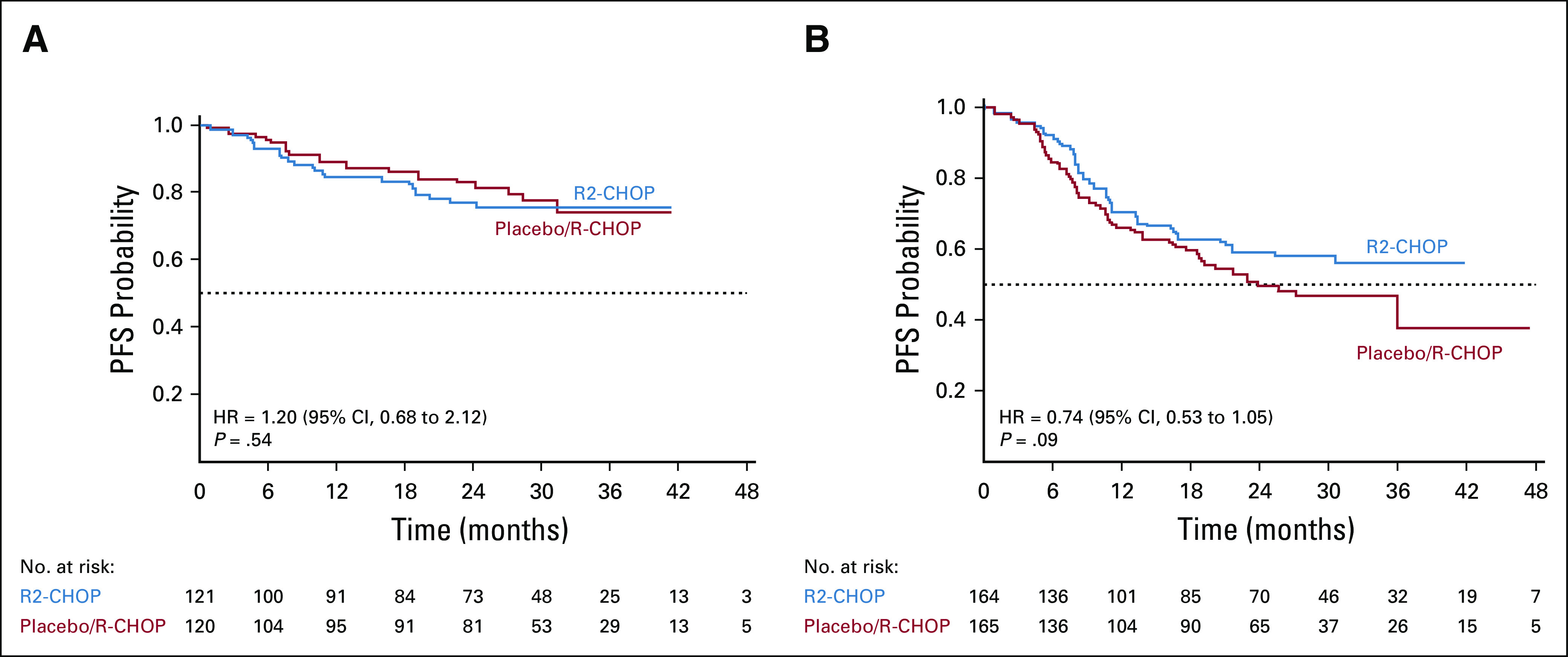
PFS based on IPI status (A) IPI = 2 and (B) IPI ≥ 3 (intent-to-treat population). HR, hazard ratio; IPI, International Prognostic Index; PFS, progression-free survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R2-CHOP, lenalidomide plus R-CHOP.
FIG 4.
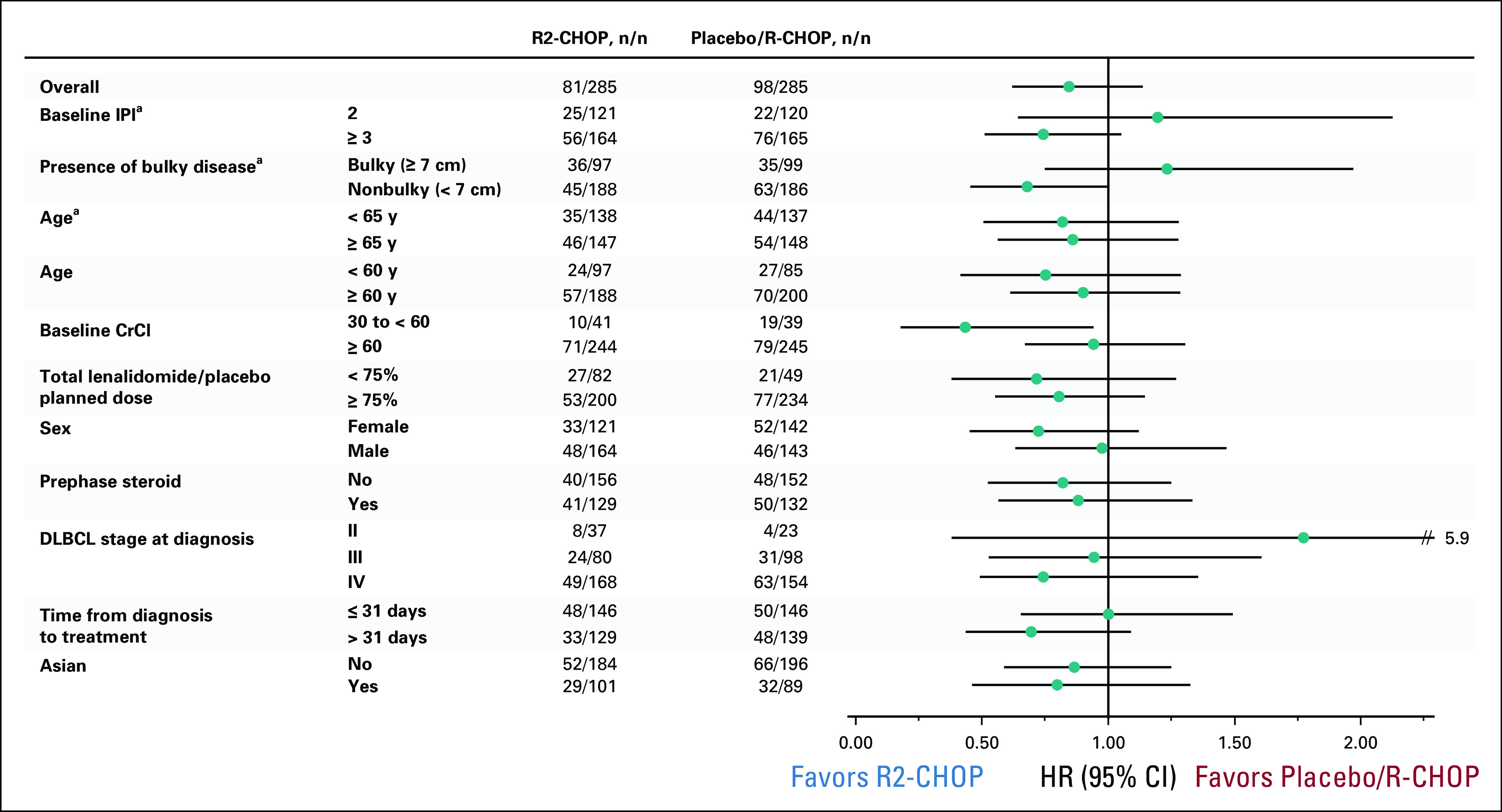
Subgroup analyses of progression-free survival by Independent Radiology Adjudication Committee in the intent-to-treat population treated with R2-CHOP versus placebo/R-CHOP. aPrespecified stratification factor. CrCl, creatinine clearance; DLBCL, diffuse large B-cell lymphoma; HR, hazard ratio; IPI, International Prognostic Index; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R2-CHOP, lenalidomide plus R-CHOP.
Safety
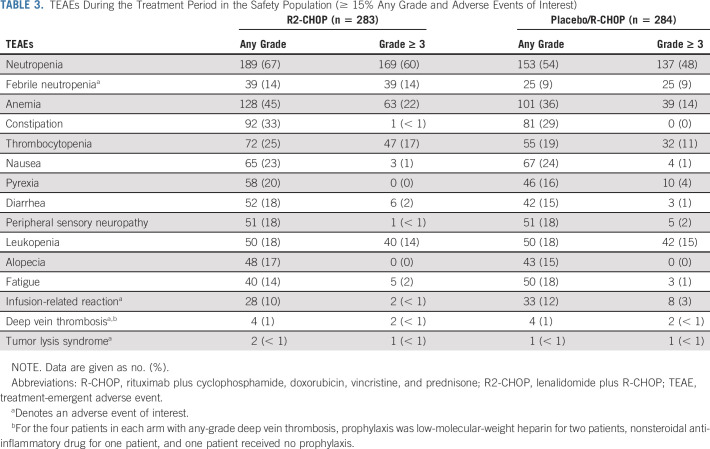
The safety population included 283 R2-CHOP and 284 placebo/R-CHOP patients. Treatment in both arms was given for a median of 18.1 weeks (range, 0.3-29.0 weeks). Overall, 89% of R2-CHOP and 91% of placebo/R-CHOP patients completed six cycles of R-CHOP backbone, and 75% R2-CHOP and 84% placebo/R-CHOP completed six cycles of both lenalidomide or placebo and R-CHOP. The median relative dose intensity of lenalidomide or placebo was 15.0 mg/d for both arms; individual R-CHOP components showed similar dose intensities between arms. More than 80% of patients in both arms received a relative dose intensity of > 90% lenalidomide or placebo.
Nearly all patients experienced ≥ 1 any-grade TEAE (99% R2-CHOP and 98% placebo/R-CHOP patients), and 78% R2-CHOP and 71% placebo/R-CHOP patients had ≥ 1 grade ≥ 3 TEAE. Serious TEAEs were observed in 37% R2-CHOP versus 31% placebo/R-CHOP patients. In the respective R2-CHOP versus placebo/R-CHOP arms, dose reductions of lenalidomide or placebo were reported in 26% versus 16% of patients (mainly because of AEs) at a median time to first dose reduction of 72 and 53 days. Dose interruptions of lenalidomide or placebo were reported in 79% versus 73% of patients (mainly because of AEs), and median time to first dose interruption was 22 days for both arms. Discontinuation rates for R2-CHOP versus placebo/R-CHOP, respectively, because of AEs were 17% versus 11%, predominantly because of neutropenia (8% v 5%; Appendix Table A2). Dose reductions or delays because of individual R-CHOP components were similar in both arms.
Most common grade 3/4 TEAEs (≥ 10%) for R2-CHOP versus placebo/R-CHOP, respectively, were neutropenia (60% v 48%), anemia (22% v 14%), thrombocytopenia (17% v 11%), leukopenia (14% v 15%), febrile neutropenia (14% v 9%), and lymphopenia (11% v 8%; Table 3). More than 89% of patients in each arm received concomitant growth factors throughout the six cycles of study treatment (95% during cycle 1).
TABLE 3.
TEAEs During the Treatment Period in the Safety Population (≥ 15% Any Grade and Adverse Events of Interest)
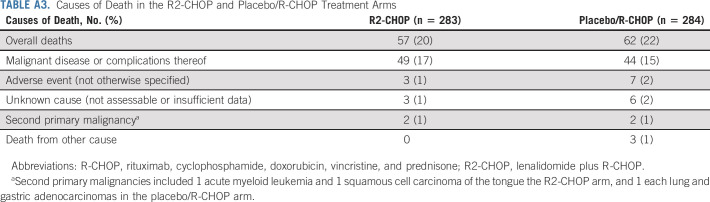
During the entire study` duration, 57 patients (20%) receiving R2-CHOP and 62 (22%)` receiving placebo/R-CHOP died; the primary cause was because of malignant disease or complications thereof (49 [17%] and 44 [16%], respectively; Appendix Table A3, online only). All other causes occurred in < 3% of patients per arm (because of AEs, unknown reasons, second primary malignancies [SPMs], or other). Death was because of SPMs for 2 patients/arm (R2-CHOP: acute myeloid leukemia and squamous cell carcinoma of the tongue; placebo/R-CHOP: lung and gastric adenocarcinomas).
DISCUSSION
In patients with ABC-DLBCL from ROBUST, adding lenalidomide to R-CHOP did not improve efficacy over placebo/R-CHOP. Response rates were very high (91% overall response rate) overall, median OS was not reached, and survival or SPMs continue to be followed. The R2-CHOP safety profile was generally well tolerated (no new safety signals), consistent with known profiles for individual agents.
At a median 27.1-month follow-up for survivors, placebo/R-CHOP results were interesting in that control patients had better outcomes than originally projected. Median PFS/OS were not reached; 2-year PFS and OS were 64% and 80%, respectively. Longer PFS for control patients were recently confirmed by the GOYA study, evaluating cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab or obinutuzumab; median PFS was not reached for patients with ABC-DLBCL (median 29-month follow-up), and 3-year PFS was 58% R-CHOP and 61% obinutuzumab plus cyclophosphamide, doxorubicin, vincristine, and prednisone.10 Multiple studies showed that R-CHOP-treated patients with ABC or non–GCB-type DLBCL expect to have 2-year PFS of ≤ 40% and 2-year OS of approximately 50%,16,24 whereas more recent studies experienced a similar phenomenon to ROBUST. Bortezomib plus R-CHOP failed to show improved PFS over R-CHOP in non-GCB patients (per IHC) from the PYRAMID phase II study (2-year PFS: 78% R-CHOP v 82% bortezomib/R-CHOP)8 or in the REMoDL-B phase III study of patients with ABC-DLBCL (per GEP), with 30-month PFS of 65% R-CHOP versus 73% bortezomib/R-CHOP.7 The randomized phase III PHOENIX study of ibrutinib/R-CHOP versus placebo/R-CHOP was similarly disappointing with no significant difference between arms for the EFS primary end point (or other survival end points) in either non-GCB patients prospectively selected by IHC or ABC patients retrospectively evaluated by GEP.9 Exploratory analyses from PHOENIX identified a treatment interaction for EFS, PFS, and OS favoring the ibrutinib-containing arm in patients < 60 years of age. Additional exploratory evaluations of PFS and OS in ROBUST based on age cutoffs of < 60 and ≥ 60 years of age showed no significant treatment interaction (Fig 4, Appendix Fig A2, online only). Although these independent phase II-III studies used various techniques for identifying non-GCB or ABC types, interestingly, R-CHOP control arms performed similarly to the active treatment arm, and with OS rates ≥ 80% after 2-3 years of follow-up.7-9
In ROBUST, there was a positive trend for PFS favoring R2-CHOP in 58% of patients with IPI score ≥ 3. This trend was not observed in the E1412 study, although IPI cutoffs evaluated in E1412 varied by including IPI groups of 2-3 versus 4-5.31 Despite the difference, we continue to believe that IPI prognostic factors remain valid in the post-rituximab era for patients receiving R-chemotherapy-based treatment (except ≥ 1 extranodal disease site),32 historically showing 3-year PFS of 59% for IPI 3 and 56% for IPI 4-5.33
Prior studies of lenalidomide in DLBCL supported prioritizing treatment in ABC-type patients based on disease biology and clinical benefit.22-24 However, there appears to be additional complexity within COO subtypes,34 leading to possible variable outcomes independent of IPI. Regional variability in ABC-type patient proportions worldwide may also contribute some differences,35 although this remains to be determined with further study. Based on molecular classifier analyses within ABC type, further subgroups may show differential outcomes. Ongoing analyses of biomarker and mutational profiles for ROBUST's ABC patients will help identify whether variable genetic profiles affected outcomes. Moreover, further classification of types may help provide a biologic basis for novel/novel drug combinations in DLBCL.
A potential limitation here was median time from initial diagnosis (or first biopsy) to treatment initiation. Although COO sample identification was streamlined to 2.4 calendar days for time from central pathology sample receipt to COO results being provided to the study site,35 median time from diagnosis to treatment initiation was longer at 31 days. In this global study, many patients were referred from smaller community practices to treatment centers. It is a common practice for referral centers to re-review pathology considering diagnostic difficulties in non-Hodgkin lymphoma. This can cause an additional delay, apart from the need to access and submit tissue for central review and COO assay. Similarly, many referral centers require fluorescent in situ hybridization for double-hit or triple-hit lymphoma, typically not done at community practices for additional delays. It is possible that this longer time may have led to selection bias for patients with less high-risk disease. Recently, the E1412 study of first-line R2-CHOP versus R-CHOP alone reported a median time from diagnosis to treatment of 21 days; patients were enrolled without prospective COO selection, and efficacy outcomes showed a significant PFS difference between arms, irrespective of COO subtype.31 Recently reported evidence from large patient cohorts from the University of Iowa and Mayo Clinic (n = 986) and LYSA LNH-2003 (n = 1,444) showed that diagnosis-to-treatment interval (DTI) was an important clinical factor in newly diagnosed DLBCL.36 Patients who received anthracycline-based immunochemotherapy demonstrated a significant association between shorter DTI, worse clinical factors (elevated lactate dehydrogenase, poor Eastern Cooperative Oncology Group performance status, B symptoms, and higher IPI) and lower EFS rate at 24 months. The converse was also true; patients with longer DTI demonstrated improved 24-month EFS, meaning that longer times to initiate treatment represented a higher willingness to wait for treatment (ie, lower-risk disease, better overall health, and fitness), increasing the likelihood of better outcomes. A similar association was reported in GOYA study patients; shorter PFS was observed for patients with < 15 days from diagnosis to random assignment and < 8 days from diagnosis to screening, potentially because of higher-risk disease.37
Although REAL07 and MC078E phase II studies of lenalidomide + R2-CHOP suggested similar outcomes despite different dosing schema,23,24 a potential limitation in ROBUST may be from the lower total lenalidomide dose. Indeed, studies in relapsed or refractory DLBCL demonstrating activity of single-agent lenalidomide mainly used the dose of 25 mg daily. However, other studies have used the 20 mg daily dose with rituximab38; the proper lenalidomide dose in combination with other agents remains an open issue.
Overall, the safety profile for both ROBUST arms was as expected, with no new safety findings. Most patients completed six cycles in both arms (74% R2-CHOP and 84% placebo/R-CHOP), with 80% of patients receiving > 90% relative dose intensity of lenalidomide or placebo. The most common grade 3/4 TEAEs were neutropenia, anemia, and thrombocytopenia for both arms, although > 89% of patients received concomitant growth factors throughout treatment.
Despite the lack of benefit of lenalidomide with R-CHOP observed here, ongoing and future analyses will further evaluate the potential effect of pharmacokinetics or dosing, molecular classification, and mutational status. The meaning of IPI findings and why worse prognosis patients had better PFS when receiving lenalidomide remain to be further elucidated. It is also important to address the role of timing from diagnosis to initial treatment and further evaluate genetic classifiers that may inherently affect outcomes. These data will broaden our understanding to support future assessments of next-generation immunomodulatory agents (CELMoDs), which have displayed promising preclinical activity in B-cell lymphoma.18
ACKNOWLEDGMENT
We would like to dedicate this work to the memory of Bertrand Coiffier after his untimely passing. Prof Coiffier was a foremost expert in the field and has our utmost respect for his significant contributions to the ROBUST study and to the field overall. Thank you to patients, families, caregivers, and investigators who participated in the ROBUST clinical study. Thank you to the Fondazione Italiana Linfomi (FIL) and Mayo Clinic for providing significant contributions and support for the study. Thank you to the international board of expert pathologists for providing histopathology review, and independent expert hematologist for clinical assessment and imaging review. Thank you to the data monitoring committee (DMC) that served as an independent expert advisory group to evaluate safety and efficacy throughout the study, including Bertrand Coiffier, MD (chair); Martin Dreyling MD; David Maloney MD, PhD; John Leonard MD; and Weichung Shih, PhD. The authors received editorial support in the preparation of this manuscript from Julie Kern, PhD, CMPP of Bio Connections LLC, funded by Celgene Corporation. The authors directed development of the manuscript and are fully responsible for all content and editorial decisions for this manuscript.
Appendix Supplemental Methods
Patients
Patients with unclassified or germinal center B-cell-like (GCB)-type diffuse large B-cell lymphoma (DLBCL) were excluded. Patient tissue biopsies were prospectively evaluated by central pathology to confirm CD20+, ABC-type DLBCL status. Cell-of-origin (COO) type and histology were analyzed from formalin-fixed, paraffin-embedded excisional or surgical or core needle biopsy samples. Histology was classified by the following World Health Organization 2008 subclassifications2: not otherwise specified (NOS), associated with chronic inflammation, or with positive Epstein-Barr virus in the elderly. Patient age was 18-80 years; patients older than 80 years could be included if their Eastern Cooperative Oncology Group (ECOG) performance status (PS) was ≤ 1, each individual organ system score was ≤ 2 per the modified Cumulative Illness Rating Scale for comorbidities, and patients were otherwise eligible for full-dose rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) per local practices. Additional patient inclusion criteria included: ECOG PS ≤ 2; Ann Arbor stage II-IV disease; International Prognostic Index (IPI) score of ≥ 2; bi-dimensionally measurable disease by computed tomography (CT) or magnetic resonance imaging (MRI) with at least one lesion ≥ 1.5 cm in the longest diameter and measurable in two perpendicular directions; and candidacy for six cycles of R-CHOP21. Laboratory requirements were the following: absolute neutrophil count (ANC) ≥ 1.5 × 109/L; platelets ≥ 75 × 109/L; hemoglobin ≥ 7.5 g/dL (4.7 mmol/L); serum aspartate or alanine transaminase ≤ 3.0 upper limit of normal (ULN); serum total bilirubin ≤ 2.0 mg/dL (34 μmol/L); and calculated creatinine clearance (Cockcroft-Gault formula) of ≥ 30 mL/min.
Two negative pregnancy tests were required for females of childbearing potential, and all patients were required to prevent pregnancy via abstinence or use of contraception.
Exclusion criteria were unclassified or GCB-type DLBCL; active central nervous system or meningeal lymphoma; post-transplant lymphoproliferative disorder cases; transformed NHL or composite DLBCL or follicular lymphoma; active infection with hepatitis B or C or human immunodeficiency virus; left ventricular ejection fraction < 45%; grade ≥ 2 peripheral neuropathy; history of other malignancies (unless disease-free for ≥ 5 years); life expectancy < 6 months; unwillingness to take venous thromboembolic prophylaxis; and prior use of lenalidomide.
Trial Design and Treatments
The lenalidomide dose was selected based on risk-benefit considerations from two proof-of-concept studies, which administered lenalidomide 15 mg/day, days 1-14 in the REAL07 study and 25 mg/day, days 1-10 in the MC078E study.23,24 Because the efficacy outcomes were similar, the lower dose from the REAL07 study was selected for ROBUST based on the best data on feasibility and lower incidence of severe adverse events (AEs), suggesting a better benefit:risk profile.
Lenalidomide dose reductions to the following levels were allowed: 10, 5, and 2.5 mg. If lenalidomide dosing was interrupted for toxicity or the cycle delayed, lenalidomide could be restarted only if the laboratory parameters recovered as follows: ANC ≥ 1.0 × 109/L; platelet count ≥ 75 × 109/L; and any other nonhematologic AE resolved to grade ≤ 2 (except where noted below). A mandatory lenalidomide hold was required in the instance of grade 4 neutropenia lasting ≥ 7 days or grade 3/4 febrile neutropenia, grade 4 thrombocytopenia, grade 2 or 3 nondesquamating rash, grade 2 allergic reaction or hypersensitivity, grade ≥ 3 constipation, grade ≥ 3 venous thrombosis or embolism, grade ≥ 2 tumor lysis syndrome, elevated aspartate or alanine transferase (> 3 × ULN) or bilirubin (≥ 3 × ULN), or another grade ≥ 3 nonhematologic AE. Lenalidomide was permanently discontinued in the instance of grade ≥ 3 desquamating or grade 4 nondesquamating rash, and grade ≥ 3 allergic reaction or hypersensitivity. For other, lenalidomide-related nonhematologic AEs grade ≥ 3, lenalidomide was withheld until the AE resolved to grade ≤ 2, and then restarted at the same or next lower dose level per the investigator’s discretion.
For patients at risk for CNS involvement, CNS lymphoma prophylaxis with four to eight doses of concomitant intrathecal methotrexate and/or cytarabine during study treatment was recommended. Patients were recommended to receive prophylaxis for tumor lysis syndrome (allopurinol, rasburicase, or equivalent per institutional guidelines) and be well hydrated during the first week of treatment. For patients at high risk for a venous thromboembolic event, prophylactic anticoagulation therapy with low-molecular-weight heparin, heparin, or warfarin was recommended. Premedication with an antiemetic for nausea prophylaxis, acetaminophen or diphenhydramine for rituximab infusion reaction prophylaxis, and prophylaxis for Pneumocystis were permitted per local practice. Growth factor and blood product transfusions were allowed during treatment and follow-up periods, and were used in accordance with the American Society of Clinical Oncology/European Society for Medical Oncology guidelines.26,27
Efficacy and Safety Assessments
The key secondary end point was event-free survival, which was defined as the time from random assignment to initiation of disease progression, relapse from complete response (CR), initiation of subsequent antilymphoma therapy, or death because of any cause. CR was defined as the percentage of patients who ever achieved a CR after initiating study treatment and prior to initiation of subsequent antilymphoma treatment.
Response assessments included CT and positron emission tomography scans and evaluation of laboratory or clinical data after cycle 3; once 3-4 weeks after completing cycle 6; every 12 weeks from week 34 to 154 (± 1 week); and weeks 180, 206, 258, and 310 (± 2 weeks) until first progression.
Supplemental Results
Futility analysis.
An interim futility analysis of PFS at the 50% information level (96 events) was performed with a data cutoff of June 30, 2017. The data monitoring committee (composed of independent, external oncologists and a biostatistician) met on November 6, 2017, to review the interim analysis data and agreed to allow the study to continue as planned.
Safety.
The incidence of treatment-emergent adverse events (TEAEs) was analyzed for patients by age < 65 years (n = 274) and ≥ 65 years (n = 293) and summarized by treatment arm for the safety population. In general, the incidences of the TEAEs were similar between the two age subgroups. For the more commonly occurring AEs in the two treatment arms, notable (≥ 10 percentage points) differences observed between the two age subgroups (< 65 and ≥ 65 years, respectively), with the higher incidence in the older ≥ 65 years subgroup, were as follows: anemia (37.2% and 52.7% in the R2-CHOP arm; 29.9% and 40.8% in placebo/R-CHOP arm), febrile neutropenia (8.8% and 18.5% in R2-CHOP), constipation (27.0% and 37.7% in R2-CHOP), diarrhea (13.1% and 23.3% in R2-CHOP; 9.5% and 19.7% in placebo/R-CHOP), and peripheral edema (5.1% and 18.5% in R2-CHOP).
FIG A1.
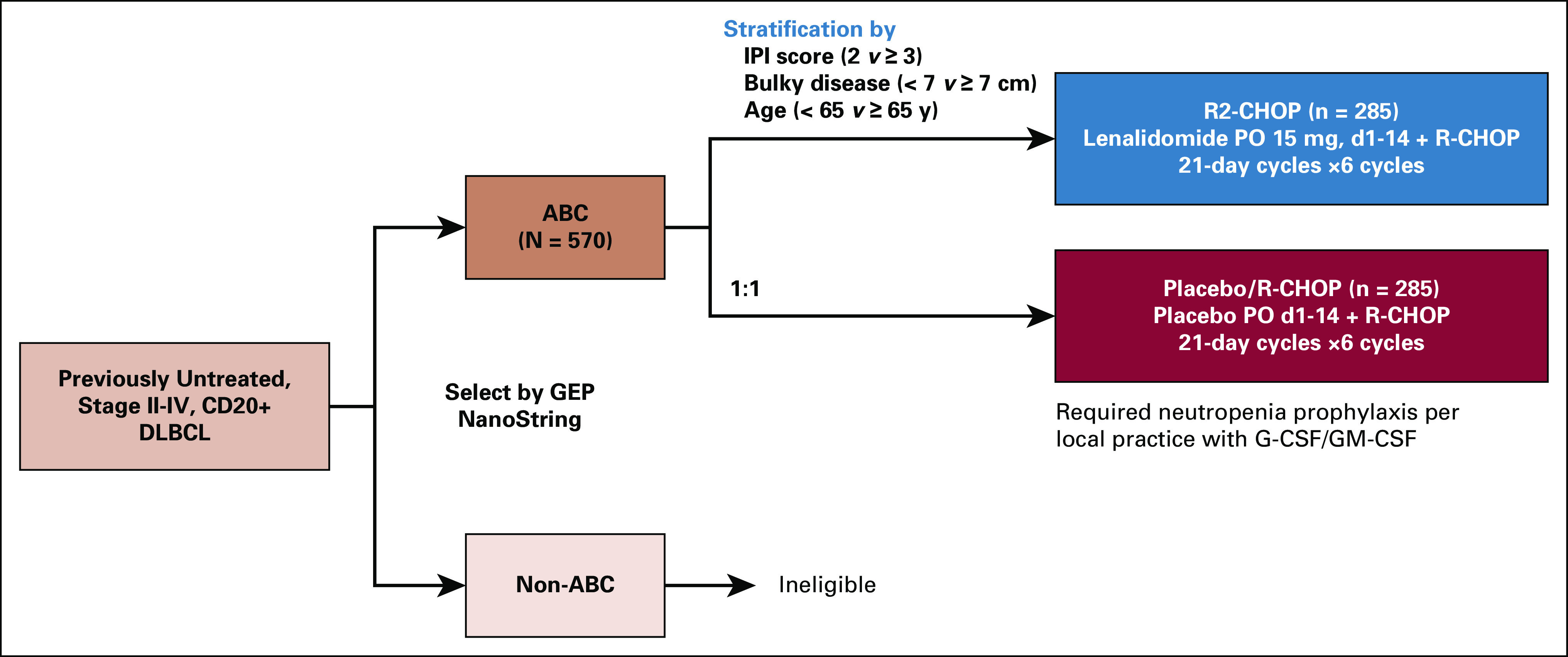
ROBUST study design (ClinicalTrials.gov identifier: NCT02285062; EudraCT 2013-004054-21). Following confirmation of CD20 positivity and identification of ABC-type by gene expression profiling, patients were stratified by IPI score, bulky disease, and age, and randomly assigned 1:1 to R2-CHOP or placebo/R-CHOP. Patients with non–ABC-type DLBCL were ineligible for the study. Treatment included the following: lenalidomide (or placebo) 15 mg oral (PO) on days 1-14 of every 21-day dosing cycle plus R-CHOP21 (rituximab 375 mg/m2 intravenous [IV] day −1 or 1, cyclophosphamide 750 mg/m2 IV day 1, doxorubicin 50 mg/m2 IV day 1, vincristine 1.4 mg/m2 [maximum 2.0 mg total] IV day 1, and prednisone [or prednisolone] 100 mg PO days 1-5 [IV day 1 was acceptable]). Treatment was continued until six cycles were complete, or until intolerability, inadequate response, disease progression, or withdrawal of consent, whichever occurred first. Two additional doses of single-agent rituximab (one dose per 21-day cycle) were permitted if prespecified and considered standard of care per local practice. ABC, activated B-cell-like; DLBCL, diffuse large B-cell lymphoma; IPI, International Prognostic Index; IV, intravenous; PO, oral; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R2-CHOP, lenalidomide plus R-CHOP.
FIG A2.
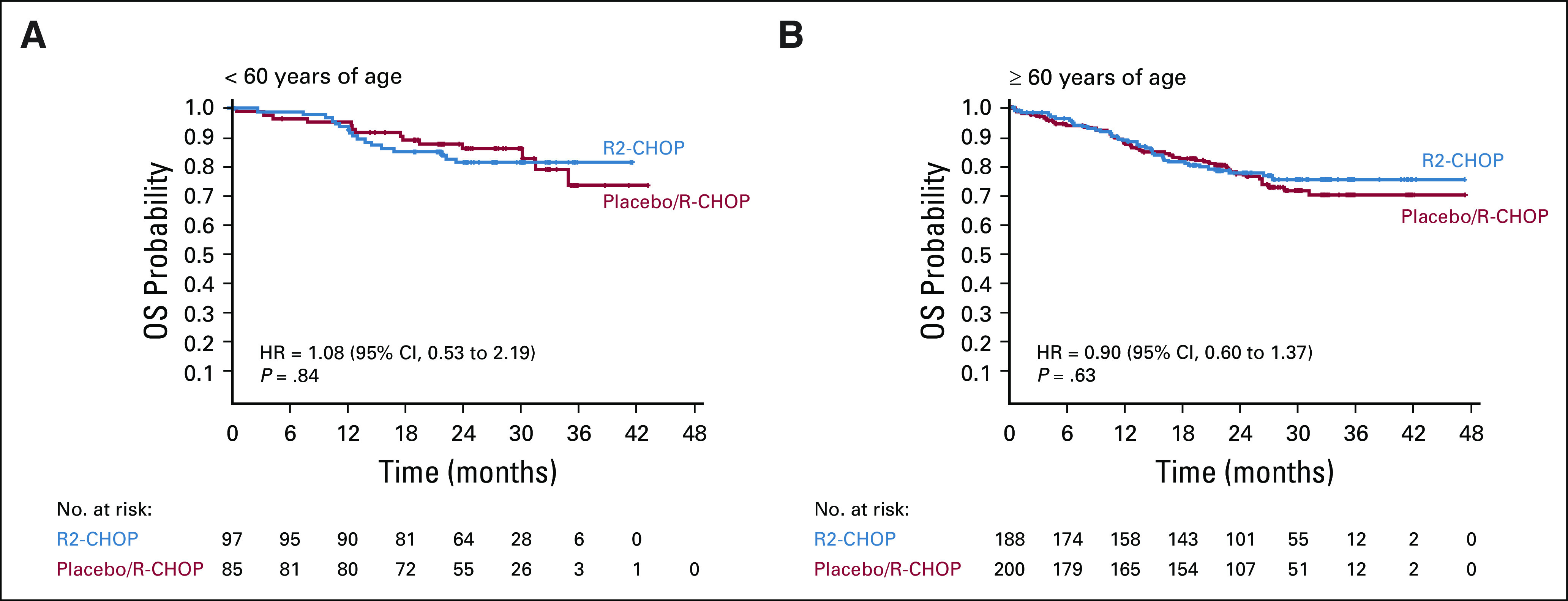
Overall survival based on treatment group and age < 60 years (A) and ≥ 60 years (B) (ITT population). R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R2-CHOP, lenalidomide plus R-CHOP.
TABLE A1.
List of ROBUST Study Investigators
TABLE A2.
TEAEs (≥ 1%) Leading to Lenalidomide or Placebo Discontinuation
TABLE A3.
Causes of Death in the R2-CHOP and Placebo/R-CHOP Treatment Arms
Grzegorz S. Nowakowski
Consulting or Advisory Role: Celgene, MorphoSys, Genentech, Selvita, Debiopharm Group, Kite/Gilead
Research Funding: Celgene, NanoString Technologies, MorphoSys
Annalisa Chiappella
Honoraria: Celgene, Gilead-Kite, Janssen Oncology, Roche, Servier
Consulting or Advisory Role: Celgene, Gilead-Kite, Janssen-Cilag, Takeda, iQone
David W. Scott
Consulting or Advisory Role: Celgene, Janssen, Abbvie, AstraZeneca
Research Funding: Janssen, Roche/Genentech, NanoString Technologies
Patents, Royalties, Other Intellectual Property: Named inventor on a pending patent describing gene expression profiling in prognostication in classical Hodgkin lymphoma. As a member of the LLMPP I am potentially a named inventor on a pending patent on the use of gene expression profiling to assign cell-of-origin in diffuse large B-cell lymphoma. I am a named inventor on a pending patent on the use of gene expression profiling to determine the proliferation signature in mantle cell lymphoma. Named inventor on a pending patent describing using gene expression profiling to identify molecular subtypes of GCB-DLBCL.
Wojciech Jurczak
Research Funding: Janssen-Cilag, Acerta Pharma/AstraZeneca, Merck, Loxo, TG Therapeutics, BeiGene
Muhit Özcan
Honoraria: Takeda
Research Funding: Janssen, Celgene, Takeda, Bayer, Merck, Archigen Biotech, Roche, Pharmacyclics, Abbvie
Travel, Accommodations, Expenses: Takeda, Sanofi, Roche, Bristol-Myers Squibb, Abdi Ibrahim, Amgen, Janssen
David Belada
Consulting or Advisory Role: Roche, Gilead Sciences, Janssen-Cilag, Takeda, MorphoSys, Debiopharm Group
Research Funding: Roche, Gilead Sciences, Janssen-Cilag, Takeda, MorphoSys, Pharmacyclics, Archigen Biotech
Travel, Accommodations, Expenses: Gilead Sciences, Takeda, Roche
Juan Miguel Bergua
Consulting or Advisory Role: Daiichi Sankyo
Travel, Accommodations, Expenses: Roche/Genentech
Dok Hyun Yoon
Honoraria: Celltrion, Roche, Janssen, Amgen, Celgene, Samyang, Kirin Pharmaceuticals
Consulting or Advisory Role: Roche, Janssen, Amgen¸Celgene, Green Cross
Research Funding: Samyang, Abclone, Roche/Genentech, Janssen Oncology, Amgen, Genmab, Boryung
Federica Cavallo
Honoraria: Takeda, Janssen-Cilag, Gilead Sciences
Consulting or Advisory Role: Janssen-Cilag, Gilead Sciences
Travel, Accommodations, Expenses: Celgene
Kazuhito Yamamoto
Honoraria: Kyowa Hakko Kirin, Takeda, Janssen, Bristol-Myers Squibb, Celgene, Sumitomo Dainippon, Ono Pharmacuetical, Chugai Pharma, Novartis, Otsuka, Mundipharma, Eisai, MSD, Meiji Seika Kaisha, Sanofi, Nippon Shinyaku, Abbvie, GlaxoSmithKline
Consulting or Advisory Role: Ono Pharmaceutical, Meiji Seika Kaisha, Chugai Pharma, Bristol-Myers Squibb, Kyowa Hakko Kirin, Takeda, Celgene, HUYA Bioscience International, Stemline Therapeutics, Eisai, Janssen, AstraZeneca, Daiichi Sankyo, Abbvie
Research Funding: Chugai Pharma, Novartis, ARIAD, Takeda, Gilead Sciences, Abbvie, Ono Pharmaceutical, Celgene, Solasia Pharma, MSD, Eisai, Zenyaku Kogyo, Bayer, SymBio Pharmaceuticals, AstraZeneca, Incyte, Mundipharma, Yakult Pharmaceutical
Koji Izutsu
Honoraria: Takeda, Chugai Pharma, Eisai, Janssen, Abbvie, Novartis, MSD, Dainippon Sumitomo Pharma, Ono Pharmaceutical, Mundipharma, HUYA Bioscience International, AstraZeneca, Bayer, Bristol-Myers Squibb, Kyowa Hakko Kirin, Fujifilm, Celgene
Consulting or Advisory Role: Bayer, Celgene, AstraZeneca
Research Funding: Eisai, Chugai Pharma
Koji Kato
Honoraria: Takeda, MSD, Kyowa-Kirin, Janssen, Celgene, Ono, Mundi, Dainippon-Sumitomo, Bristol-Myers Squibb
Consulting or Advisory Role: AbbVie, AstraZeneca, Celgene, Chugai, Eisai, Janssen, Novartis, Daiichi Sankyo
Research Funding: Chugai, Takeda, Kyowa Kirin, AbbVie, Novartis, Eisai, Janssen, Celgene, Ono, Novartis, Daiichi Sankyo
Myron Czuczman
Employment: Celgene
Stock and Other Ownership Interests: Celgene
Sarah Hersey
Stock and Other Ownership Interests: Novartis, Johnson & Johnson, Bristol-Myers Squibb
Other Relationship: Bristol-Myers Squibb
Adrian Kilcoyne
Employment: Celgene
Stock and Other Ownership Interests: Celgene
Jacqueline Russo
Employment: Celgene/Bristol-Myers Squibb, Kite Pharma
Stock and Other Ownership Interests: Celgene/Bristol-Myers Squibb, Kite Pharma
Krista Hudak
Employment: Bristol-Myers Squibb, Novartis
Stock and Other Ownership Interests: Bristol-Myers Squibb, Novartis
Jingshan Zhang
Employment: Celgene, Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Celgene
Steve Wade
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Thomas E. Witzig
Consulting or Advisory Role: Karyopharm Therapeutics, Abbvie/Genentech, Seattle Genetics, Celgene, Incyte, Epizyme, Cellectar, Tessa Therapeutics, Portola Pharmaceuticals, MorphoSys, ADC Therapeutics
Research Funding: Celgene, Acerta Pharma, Kura Oncology, Acrotech Biopharma, Karyopharm Therapeutics
Patents, Royalties, Other Intellectual Property: I am co-inventor on a patent application filed by Mayo Clinic and pending on the combination of CRM1 inhibitors with salicylates. Please note—simply filed—not even close to being granted.
Umberto Vitolo
Consulting or Advisory Role: Gilead Sciences, Janssen, Celgene, Regeneron
Speakers' Bureau: Gilead Sciences, Celgene, Abbvie, Roche, Janssen Oncology
Travel, Accommodations, Expenses: Celgene, Gilead Sciences, Roche
No other potential conflicts of interest were reported.
See accompanying editorial on page 1314
PRIOR PRESENTATION
Presented in part as an oral presentation (abstract #005) at the 2019 International Conference for Malignant Lymphoma, Lugano, Switzerland, June 18-22, 2019.
SUPPORT
Supported by Celgene Corporation, a Bristol-Myers Squibb Company, Princeton, NJ. This study was conducted with the scientific support of the Fondazione Italiana Linfomi and the Mayo Clinc.
CLINICAL TRIAL INFORMATION
ClinicalTrials.gov identifier: NCT02285062; EudraCT 2013-004054-21.
STUDY GROUPS
A comprehensive list of ROBUST study investigators and institutions is shown in Appendix Table A1 (online only).
DATA SHARING STATEMENT
Data requests may be submitted to Celgene, A Bristol Myers Squibb Company at https://vivli.org/ourmember/celgene/ and must include a description of the research proposal.
AUTHOR CONTRIBUTIONS
Conception and design: Grzegorz S. Nowakowski, Annalisa Chiappella, David W. Scott, Wojciech Jurczak, Jun Zhu, Myron Czuczman, Thomas E. Witzig, Umberto Vitolo
Administrative support: Juan Miguel Bergua, Adrian Kilcoyne
Provision of study materials or patients: Wojciech Jurczak, Jie Jin, Juan Miguel Bergua, Francesco Piazza, Anna Lia Molinari, Kazuhito Yamamoto
Collection and assembly of data: Grzegorz S. Nowakowski, Annalisa Chiappella, Randy D. Gascoyne, David W. Scott, Qingyuan Zhang, Muhit Özcan, Xiaonan Hong, Jun Zhu, Jie Jin, David Belada, Juan Miguel Bergua, Francesco Piazza, Heidi Mócikova, Anna Lia Molinari, Federica Cavallo, Monica Tani, Kazuhito Yamamoto, Koji Izutsu, Koji Kato, Myron Czuczman, Sarah Hersey, Adrian Kilcoyne, Jacqueline Russo, Krista Hudak, Thomas E. Witzig, Umberto Vitolo
Data analysis and interpretation: Grzegorz S. Nowakowski, Annalisa Chiappella, David W. Scott, Jie Jin, Dok Hyun Yoon, Federica Cavallo, Monica Tani, Kazuhito Yamamoto, Koji Izutsu, Myron Czuczman, Sarah Hersey, Adrian Kilcoyne, Jacqueline Russo, Krista Hudak, Jingshan Zhang, Steve Wade, Thomas E. Witzig, Umberto Vitolo
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
ROBUST: A Phase III Study of Lenalidomide Plus R-CHOP Versus Placebo Plus R-CHOP in Previously Untreated Patients With ABC-Type Diffuse Large B-Cell Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Grzegorz S. Nowakowski
Consulting or Advisory Role: Celgene, MorphoSys, Genentech, Selvita, Debiopharm Group, Kite/Gilead
Research Funding: Celgene, NanoString Technologies, MorphoSys
Annalisa Chiappella
Honoraria: Celgene, Gilead-Kite, Janssen Oncology, Roche, Servier
Consulting or Advisory Role: Celgene, Gilead-Kite, Janssen-Cilag, Takeda, iQone
David W. Scott
Consulting or Advisory Role: Celgene, Janssen, Abbvie, AstraZeneca
Research Funding: Janssen, Roche/Genentech, NanoString Technologies
Patents, Royalties, Other Intellectual Property: Named inventor on a pending patent describing gene expression profiling in prognostication in classical Hodgkin lymphoma. As a member of the LLMPP I am potentially a named inventor on a pending patent on the use of gene expression profiling to assign cell-of-origin in diffuse large B-cell lymphoma. I am a named inventor on a pending patent on the use of gene expression profiling to determine the proliferation signature in mantle cell lymphoma. Named inventor on a pending patent describing using gene expression profiling to identify molecular subtypes of GCB-DLBCL.
Wojciech Jurczak
Research Funding: Janssen-Cilag, Acerta Pharma/AstraZeneca, Merck, Loxo, TG Therapeutics, BeiGene
Muhit Özcan
Honoraria: Takeda
Research Funding: Janssen, Celgene, Takeda, Bayer, Merck, Archigen Biotech, Roche, Pharmacyclics, Abbvie
Travel, Accommodations, Expenses: Takeda, Sanofi, Roche, Bristol-Myers Squibb, Abdi Ibrahim, Amgen, Janssen
David Belada
Consulting or Advisory Role: Roche, Gilead Sciences, Janssen-Cilag, Takeda, MorphoSys, Debiopharm Group
Research Funding: Roche, Gilead Sciences, Janssen-Cilag, Takeda, MorphoSys, Pharmacyclics, Archigen Biotech
Travel, Accommodations, Expenses: Gilead Sciences, Takeda, Roche
Juan Miguel Bergua
Consulting or Advisory Role: Daiichi Sankyo
Travel, Accommodations, Expenses: Roche/Genentech
Dok Hyun Yoon
Honoraria: Celltrion, Roche, Janssen, Amgen, Celgene, Samyang, Kirin Pharmaceuticals
Consulting or Advisory Role: Roche, Janssen, Amgen¸Celgene, Green Cross
Research Funding: Samyang, Abclone, Roche/Genentech, Janssen Oncology, Amgen, Genmab, Boryung
Federica Cavallo
Honoraria: Takeda, Janssen-Cilag, Gilead Sciences
Consulting or Advisory Role: Janssen-Cilag, Gilead Sciences
Travel, Accommodations, Expenses: Celgene
Kazuhito Yamamoto
Honoraria: Kyowa Hakko Kirin, Takeda, Janssen, Bristol-Myers Squibb, Celgene, Sumitomo Dainippon, Ono Pharmacuetical, Chugai Pharma, Novartis, Otsuka, Mundipharma, Eisai, MSD, Meiji Seika Kaisha, Sanofi, Nippon Shinyaku, Abbvie, GlaxoSmithKline
Consulting or Advisory Role: Ono Pharmaceutical, Meiji Seika Kaisha, Chugai Pharma, Bristol-Myers Squibb, Kyowa Hakko Kirin, Takeda, Celgene, HUYA Bioscience International, Stemline Therapeutics, Eisai, Janssen, AstraZeneca, Daiichi Sankyo, Abbvie
Research Funding: Chugai Pharma, Novartis, ARIAD, Takeda, Gilead Sciences, Abbvie, Ono Pharmaceutical, Celgene, Solasia Pharma, MSD, Eisai, Zenyaku Kogyo, Bayer, SymBio Pharmaceuticals, AstraZeneca, Incyte, Mundipharma, Yakult Pharmaceutical
Koji Izutsu
Honoraria: Takeda, Chugai Pharma, Eisai, Janssen, Abbvie, Novartis, MSD, Dainippon Sumitomo Pharma, Ono Pharmaceutical, Mundipharma, HUYA Bioscience International, AstraZeneca, Bayer, Bristol-Myers Squibb, Kyowa Hakko Kirin, Fujifilm, Celgene
Consulting or Advisory Role: Bayer, Celgene, AstraZeneca
Research Funding: Eisai, Chugai Pharma
Koji Kato
Honoraria: Takeda, MSD, Kyowa-Kirin, Janssen, Celgene, Ono, Mundi, Dainippon-Sumitomo, Bristol-Myers Squibb
Consulting or Advisory Role: AbbVie, AstraZeneca, Celgene, Chugai, Eisai, Janssen, Novartis, Daiichi Sankyo
Research Funding: Chugai, Takeda, Kyowa Kirin, AbbVie, Novartis, Eisai, Janssen, Celgene, Ono, Novartis, Daiichi Sankyo
Myron Czuczman
Employment: Celgene
Stock and Other Ownership Interests: Celgene
Sarah Hersey
Stock and Other Ownership Interests: Novartis, Johnson & Johnson, Bristol-Myers Squibb
Other Relationship: Bristol-Myers Squibb
Adrian Kilcoyne
Employment: Celgene
Stock and Other Ownership Interests: Celgene
Jacqueline Russo
Employment: Celgene/Bristol-Myers Squibb, Kite Pharma
Stock and Other Ownership Interests: Celgene/Bristol-Myers Squibb, Kite Pharma
Krista Hudak
Employment: Bristol-Myers Squibb, Novartis
Stock and Other Ownership Interests: Bristol-Myers Squibb, Novartis
Jingshan Zhang
Employment: Celgene, Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Celgene
Steve Wade
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Thomas E. Witzig
Consulting or Advisory Role: Karyopharm Therapeutics, Abbvie/Genentech, Seattle Genetics, Celgene, Incyte, Epizyme, Cellectar, Tessa Therapeutics, Portola Pharmaceuticals, MorphoSys, ADC Therapeutics
Research Funding: Celgene, Acerta Pharma, Kura Oncology, Acrotech Biopharma, Karyopharm Therapeutics
Patents, Royalties, Other Intellectual Property: I am co-inventor on a patent application filed by Mayo Clinic and pending on the combination of CRM1 inhibitors with salicylates. Please note—simply filed—not even close to being granted.
Umberto Vitolo
Consulting or Advisory Role: Gilead Sciences, Janssen, Celgene, Regeneron
Speakers' Bureau: Gilead Sciences, Celgene, Abbvie, Roche, Janssen Oncology
Travel, Accommodations, Expenses: Celgene, Gilead Sciences, Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Armitage JO, Weisenburger DD: New approach to classifying non-Hodgkin's lymphomas: Clinical features of the major histologic subtypes. Non-Hodgkin's lymphoma classification project. J Clin Oncol 16:2780-2795, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH Campo E Harris NL, et al. : WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France, International Agency for Research on Cancer, 2008 [Google Scholar]
- 3.Coiffier B Lepage E Briere J, et al. : CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235-242, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Habermann TM Weller EA Morrison VA, et al. : Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 24:3121-3127, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Tilly H Gomes da Silva M Vitolo U, et al. : Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v116-v125, 2015. (suppl 5) [DOI] [PubMed] [Google Scholar]
- 6.Wang Y Farooq U Link BK, et al. : Late relapses in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol 37:1819-1827, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies A Cummin TE Barrans S, et al. : Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): An open-label, randomised, phase 3 trial. Lancet Oncol 20:649-662, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonard JP Kolibaba KS Reeves JA, et al. : Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol 35:3538-3546, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Younes A Sehn LH Johnson P, et al. : Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol 37:1285-1295, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitolo U Trneny M Belada D, et al. : Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol 35:3529-3537, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Alizadeh AA Eisen MB Davis RE, et al. : Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503-511, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Rosenwald A Wright G Chan WC, et al. : The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346:1937-1947, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Scott DW Wright GW Williams PM, et al. : Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood 123:1214-1217, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hans CP Weisenburger DD Greiner TC, et al. : Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275-282, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Wright G Tan B Rosenwald A, et al. : A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A 100:9991-9996, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenz G Wright G Dave SS, et al. : Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 359:2313-2323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamberlain PP Lopez-Girona A Miller K, et al. : Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat Struct Mol Biol 21:803-809, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Hagner PR Man HW Fontanillo C, et al. : CC-122, a pleiotropic pathway modifier, mimics an interferon response and has antitumor activity in DLBCL. Blood 126:779-789, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang LH Kosek J Wang M, et al. : Lenalidomide efficacy in activated B-cell-like subtype diffuse large B-cell lymphoma is dependent upon IRF4 and cereblon expression. Br J Haematol 160:487-502, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Wiernik PH Lossos IS Tuscano JM, et al. : Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol 26:4952-4957, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Witzig TE Vose JM Zinzani PL, et al. : An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann Oncol 22:1622-1627, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Ilizaliturri FJ Deeb G Zinzani PL, et al. : Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer 117:5058-5066, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Vitolo U Chiappella A Franceschetti S, et al. : Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: Results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol 15:730-737, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Nowakowski GS LaPlant B Macon WR, et al. : Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-cell lymphoma: A phase II study. J Clin Oncol 33:251-257, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Castellino A Chiappella A LaPlant BR, et al. : Lenalidomide plus R-CHOP21 in newly diagnosed diffuse large B-cell lymphoma (DLBCL): Long-term follow-up results from a combined analysis from two phase 2 trials. Blood Cancer J 8:108, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford J Caserta C Roila F, et al. : Hematopoietic growth factors: ESMO clinical practice guidelines for the applications. Ann Oncol 21:v248-v251, 2010. (suppl 5) [DOI] [PubMed] [Google Scholar]
- 27.Smith TJ Bohlke K Lyman GH, et al. : Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33:3199-3212, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD Fisher RI Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32:3059-3068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheson BD Pfistner B Juweid ME, et al. : Revised response criteria for malignant lymphoma. J Clin Oncol 25:579-586, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Food and Drug Administration (FDA), Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research (US) : Guidance for Industry: Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. Rockville, MD, US Food and Drug Administration, 2007 [Google Scholar]
- 31.Nowakowski GS Hong F Scott DW, et al. : Addition of lenalidomide to R-CHOP (R2CHOP) improves outcomes in newly diagnosed diffuse large B-cell lymphoma (DLBCL): First report of ECOG-ACRIN1412 a randomized phase 2 US intergroup study of R2CHOP vs R-CHOP. Hematol Oncol (ICML Abstract) 37:37-38, 2019. (suppl; abstr 006) [Google Scholar]
- 32.Shipp MA Harrington DP Anderson JR, et al. : A predictive model for aggressive non-Hodgkin's lymphoma: International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 329:987-994, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Ziepert M Hasenclever D Kuhnt E, et al. : Standard International Prognostic Index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol 28:2373-2380, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Chapuy B Stewart C Dunford AJ, et al. : Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 24:679-690, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nowakowski GS Chiappella A Witzig TE, et al. : Variable global distribution of cell-of-origin from the ROBUST phase 3 study in diffuse large B-cell lymphoma. Haematologica 105:e72-e75, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurer MJ Ghesquieres H Link BK, et al. : Diagnosis-to-treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for bias in clinical trials. J Clin Oncol 36:1603-1610, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szafer-Glusman E Liu J Peale FV Jr, et al. : A simulation analysis to evaluate the effect of prospective biomarker testing on progression-free survival (PFS) in DLBCL. Blood 130:abstract 419, 2017 [Google Scholar]
- 38.Zinzani PL Pellegrini C Gandolfi L, et al. : Combination of lenalidomide and rituximab in elderly patients with relapsed or refractory diffuse large B-cell lymphoma: A phase 2 trial. Clin Lymphoma Myeloma Leuk 11:462-466, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data requests may be submitted to Celgene, A Bristol Myers Squibb Company at https://vivli.org/ourmember/celgene/ and must include a description of the research proposal.