PURPOSE
Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate that targets aminopeptidases and rapidly and selectively releases alkylating agents into tumor cells. The phase II HORIZON trial evaluated the efficacy of melflufen plus dexamethasone in relapsed and refractory multiple myeloma (RRMM), a population with an important unmet medical need.
PATIENTS AND METHODS
Patients with RRMM refractory to pomalidomide and/or an anti-CD38 monoclonal antibody received melflufen 40 mg intravenously on day 1 of each 28-day cycle plus once weekly oral dexamethasone at a dose of 40 mg (20 mg in patients older than 75 years). The primary end point was overall response rate (partial response or better) assessed by the investigator and confirmed by independent review. Secondary end points included duration of response, progression-free survival, overall survival, and safety. The primary analysis is complete with long-term follow-up ongoing.
RESULTS
Of 157 patients (median age 65 years; median five prior lines of therapy) enrolled and treated, 119 patients (76%) had triple-class–refractory disease, 55 (35%) had extramedullary disease, and 92 (59%) were refractory to previous alkylator therapy. The overall response rate was 29% in the all-treated population, with 26% in the triple-class–refractory population. In the all-treated population, median duration of response was 5.5 months, median progression-free survival was 4.2 months, and median overall survival was 11.6 months at a median follow-up of 14 months. Grade ≥ 3 treatment-emergent adverse events occurred in 96% of patients, most commonly neutropenia (79%), thrombocytopenia (76%), and anemia (43%). Pneumonia (10%) was the most common grade 3/4 nonhematologic event. Thrombocytopenia and bleeding (both grade 3/4 but fully reversible) occurred concomitantly in four patients. GI events, reported in 97 patients (62%), were predominantly grade 1/2 (93%); none were grade 4.
CONCLUSION
Melflufen plus dexamethasone showed clinically meaningful efficacy and a manageable safety profile in patients with heavily pretreated RRMM, including those with triple-class–refractory and extramedullary disease.
INTRODUCTION
Despite the introduction of novel therapies and regimens that have improved outcomes in multiple myeloma (MM),1,2 almost all patients will relapse.1,3 After relapse, treatment choice is usually determined by the class of and response to previous treatment and patient characteristics.2,3 Although class switching is generally prioritized, this is becoming increasingly difficult, not least because novel agents are commonly administered in combination in earlier treatment lines, resulting in disease resistant to multiple drug classes as early as second-line therapy.2,3
CONTEXT
Key Objective
To evaluate whether melphalan flufenamide (melflufen) plus dexamethasone is effective and safe in patients with heavily pretreated relapsed and refractory multiple myeloma (RRMM), a population with a high unmet medical need.
Knowledge Generated
In this pivotal, phase II study, melflufen plus dexamethasone showed meaningful efficacy in heavily pretreated patients with RRMM, including patients with triple-class–refractory disease and those with extramedullary disease. The safety profile of melflufen plus dexamethasone was consistent with previously reported data and was characterized primarily by clinically manageable hematologic toxicities.
Relevance
As new combinations of antimyeloma drugs are introduced in earlier lines of therapy, patients with RRMM often have disease that is refractory to multiple drugs. Therefore, drugs with novel mechanisms of action are urgently needed. Melflufen, when combined with dexamethasone, has the potential to fill this unmet medical need by providing a novel mechanism of action, clinically meaningful efficacy, and manageable safety in patients with RRMM.
Outcomes are particularly poor for patients with high-risk cytogenetics, extramedullary disease, and MM resistant to multiple drug classes, including those with triple-class–refractory disease who represent groups with a high unmet need.1,3,4 Furthermore, patients with relapsed and refractory multiple myeloma (RRMM) may have comorbidities because of age, disease symptoms, and cumulative toxicities stemming from previous therapies.5,6 There is an urgent requirement for agents with novel mechanisms of action that are effective, safe, and tolerable and that maintain quality of life in patients with aggressive and resistant disease.
Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate that targets aminopeptidases and rapidly and selectively releases alkylating agents into tumor cells.7-12 Melflufen is rapidly and passively taken up by cells because of its high lipophilicity, thereby circumventing the development of transporter-associated resistance.8,11,13 Intracellular aminopeptidases hydrolyze melflufen to release hydrophilic alkylating moieties.11 Melflufen and its metabolites melphalan and desethyl-melflufen have equipotent alkylating potential.11 Unlike previous aminopeptidase-targeting therapies that directly inhibit aminopeptidase activity, melflufen takes a novel approach by leveraging increased aminopeptidase activity to selectively direct potent cytotoxic agents into tumor cells.11,14,15 Melflufen and its metabolites trigger robust and irreversible DNA damage, have antiangiogenic effects, induce apoptosis—resulting in potent antitumor activity in myeloma cells, including those with resistance to melphalan, bortezomib, and dexamethasone—and, importantly, retain activity in myeloma cells with absent or impaired p53 function.8-10,16 Melflufen may also have activity in other hematologic malignancies (including immunoglobulin light chain amyloidosis and leukemia) and solid tumors (including breast cancer and ovarian cancer).11
The phase I/II, multicenter O-12-M1 trial established the dosage of melflufen plus dexamethasone in patients who had RRMM, received a median of four previous lines of therapy (including lenalidomide and bortezomib), and had disease refractory to their last line of therapy.17 In 45 patients treated with infusional melflufen 40 mg administered on day 1 of each 28-day cycle and once weekly dexamethasone dosed at 40 mg, the overall response rate (ORR) was 31%, the median duration of response (DOR) was 8.4 months, the median progression-free survival (PFS) was 5.7 months, and the median overall survival (OS) was encouraging at 20.7 months. The safety profile of melflufen was characterized primarily by hematologic toxicities that were clinically manageable with appropriate dose delays, dose reductions, and supportive care. Based on these results, the efficacy and safety of melflufen plus dexamethasone were therefore evaluated in the current study in a larger population with heavily pretreated, resistant, and poor-risk RRMM, including those with triple-class–refractory disease, for whom few effective treatment options exist.3
PATIENTS AND METHODS
Study Design and Participants
HORIZON (OP-106; ClinicalTrials.gov identifier: NCT02963493) was a pivotal, single-arm, multicenter, phase II study of melflufen plus dexamethasone in patients with RRMM refractory to pomalidomide and/or an anti-CD38 monoclonal antibody. Patients were enrolled from December 28, 2016, to October 14, 2019, at 17 sites (see the Data Supplement, online only). Eligible adult patients had an Eastern Cooperative Oncology Group performance status score of 0-2, a previous diagnosis of MM with disease progression, and measurable disease (serum monoclonal protein ≥ 5 g/L, urine monoclonal protein ≥ 200 mg per 24 hours, or serum immunoglobulin-free light chain ≥ 100 mg/L, and abnormal serum immunoglobulin kappa to lambda–free light chain ratio) at study entry. Patients had received at least two prior lines of therapy, including an immunomodulatory agent and proteasome inhibitor, and were refractory to pomalidomide and/or an anti-CD38 monoclonal antibody. RRMM was defined as disease that was nonresponsive (ie, did not achieve a minimal response or better, or developed progressive disease with treatment) while on primary or salvage therapy or progressed within 60 days of last therapy.18 Please see the Data Supplement for full eligibility criteria. Patients received once-monthly melflufen 40 mg as a 30-minute central intravenous infusion on day 1 of each 28-day cycle in combination with oral dexamethasone 40 mg (20 mg for patients age ≥ 75 years) once-weekly administered on days 1, 8, 15, and 22 of each 28-day cycle until disease progression, unacceptable toxicity, or the patient or treating physician determined it was not in the patient's best interest to continue. Melflufen dose reduction for drug-related toxicities was allowed in 10 mg increments each cycle from 40 mg down to 30 mg and from 30 mg down to 20 mg (see the Data Supplement).
This study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation guidelines for Good Clinical Practice. The Protocol was reviewed and approved by national regulatory authorities and an independent ethics committee or institutional review board at each study center. Each patient provided written informed consent.
Outcomes
The primary end point was ORR, defined as the proportion of patients achieving a confirmed response of stringent complete response (sCR), complete response (CR), very good partial response (VGPR), or partial response (PR) as their best response per International Myeloma Working Group (IMWG) uniform response criteria, as assessed by the investigator.18 Response, confirmed response, and confirmed progression were subsequently verified by an independent review committee.18 Secondary end points included DOR, PFS, OS, clinical benefit rate (CBR), best response, time to response, time to progression, time to next treatment, and safety (defined in the Data Supplement). All response categories required confirmation with two consecutive assessments (see the Data Supplement). Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events, version 4.03. AE frequency and relationship to study treatment were summarized.
Statistical Analysis
Planned enrollment was 150 patients. ORR and associated two-sided exact 95% CI19 were estimated for all patients treated (all-treated population). With a sample size of 150 patients and an assumed ORR of 30%, the exact 95% CI was estimated to range between 23% and 38%. CBR and disease stabilization were also summarized. Time-to-event end points were summarized using the Kaplan-Meier method in the all-treated population. Median and estimated 95% CIs were constructed using the methods of Brookmeyer and Crowley20; duration of follow-up was estimated by the reverse Kaplan-Meier methods of Schemper and Smith.21 See the Data Supplement for patient censoring and handling of missing data.
A preplanned subgroup analysis was performed in patients with triple-class–refractory MM (refractory to or intolerant of at least one immunomodulatory drug, at least one proteasome inhibitor, and at least one anti-CD38 monoclonal antibody). With a sample size of 150 patients, 104-120 patients with triple-class–refractory disease were expected; the primary end point was considered met if the lower bound of the 95% CI for the ORR was higher than 15%. Additional subgroup analyses, including extramedullary disease, are described in the Data Supplement. Extramedullary disease was assessed at baseline for patients with known or suspected extramedullary disease and to confirm a response achieved by M-protein or for suspected progression per IMWG uniform response criteria.18
RESULTS
Patients
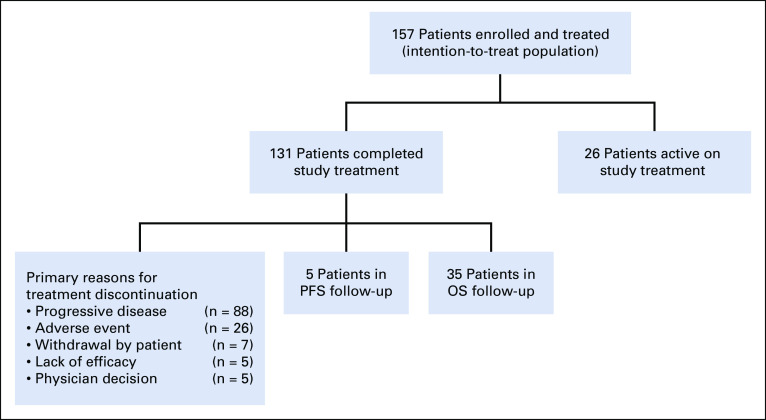
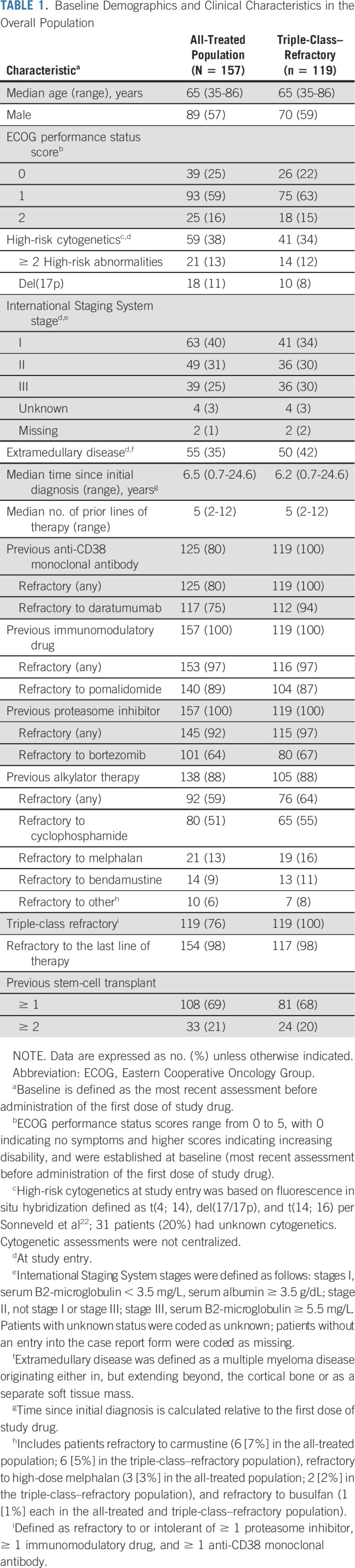
In total, 157 patients were enrolled in the study, received at least one dose of study medication, and were included in the all-treated population. At the data cutoff date (January 14, 2020), 131 patients (83%) had discontinued treatment—the most common primary reasons for discontinuation were disease progression (n = 88; 56%) and AEs (n = 26; 17%)—and 26 patients (17%) remained on treatment (Fig 1). The median duration of treatment with melflufen plus dexamethasone was 3.8 months (range, 0.9-22.7 months). At baseline, the median age was 65 years, patients had received a median of five prior lines of therapy, 154 patients (98%) had disease that was refractory to the last line of therapy received, 119 (76%) had triple-class–refractory disease, and 92 (59%) had MM that was refractory to prior alkylator therapy (Table 1). Overall, 59 patients (38%) had high-risk cytogenetics, 39 (25%) had International Staging System stage III disease, and 55 (35%) had extramedullary disease.
FIG 1.
Trial profile. OS, overall survival; PFS, progression-free survival.
TABLE 1.
Baseline Demographics and Clinical Characteristics in the Overall Population
Efficacy
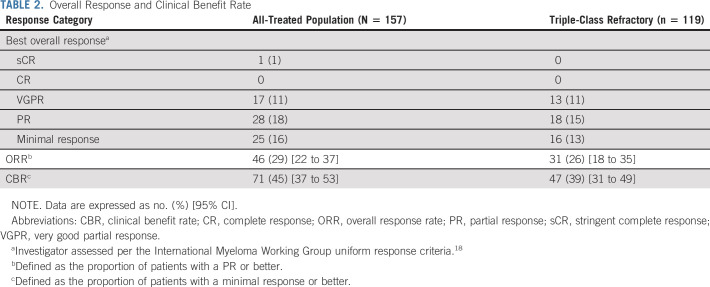
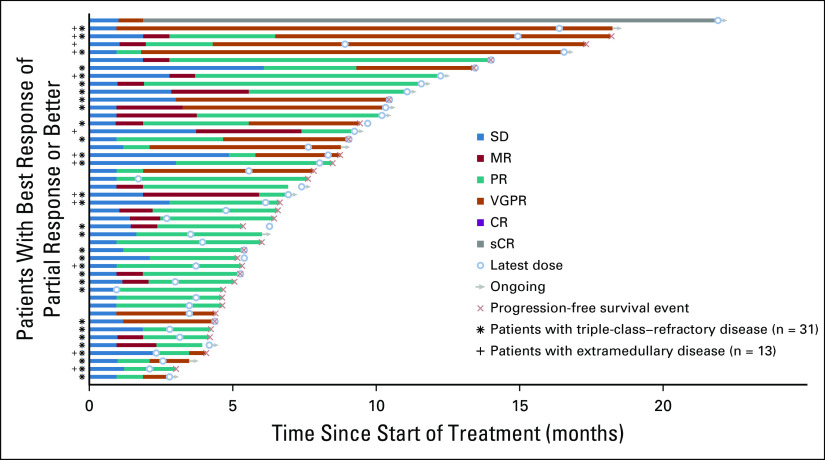
The ORR per investigator assessment was 29% (95% CI, 22% to 37%), with one patient achieving an sCR, 17 a VGPR, and 28 a PR (Table 2). An additional 25 patients achieved a minimal response for a CBR of 45% (95% CI, 37% to 53%). In the triple-class–refractory population, the ORR was 26% (95% CI, 18% to 35%), with 13 patients achieving a VGPR and 18 a PR. The ORR per independent review committee was 30% (95% CI, 23% to 38%) overall and 26% (95% CI, 18% to 35%) in the triple-class–refractory population (Data Supplement). Reduction in M-protein was observed in 118 of the 145 patients (81.4%) (Data Supplement). In the all-treated and triple-class–refractory populations, the median time to PR or better was 1.9 months (range, 1.0-7.4 months) and 1.9 months (range, 1.0-6.1 months), respectively, and the median duration of PR or better was 5.5 months (95% CI, 3.9 to 7.6 months) and 4.4 months (95% CI, 3.4 to 7.6 months), respectively (Fig 2 and Data Supplement).
TABLE 2.
Overall Response and Clinical Benefit Rate
FIG 2.
Duration of response to melflufen plus dexamethasone. Data on patients in the all-treated population (n = 46), triple-class–refractory population (asterisk; n = 31), and extramedullary subgroup (dagger; n = 13) who achieved a PR or better as the best response. Open circles indicate the latest dose of melflufen received; arrows indicate patients still receiving treatment at the data cutoff date; orange Xs indicate progression-free survival events. CR, complete response; MR, minimal response; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
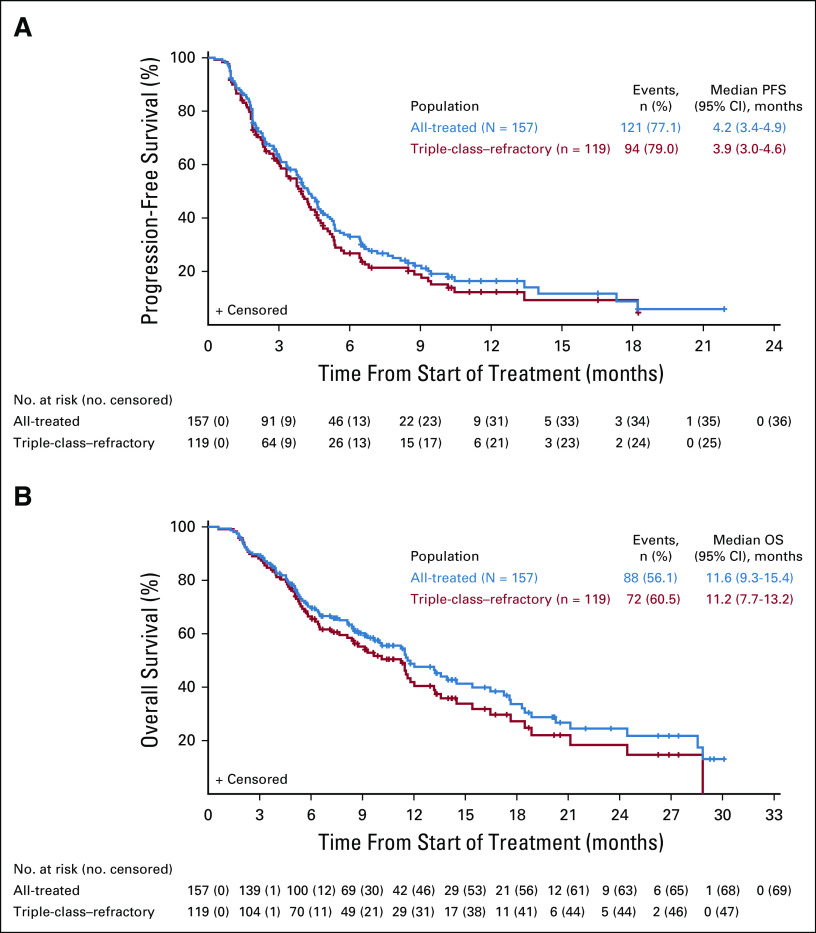
In the all-treated and triple-class–refractory populations, the median PFS was 4.2 months (95% CI, 3.4 to 4.9 months) and 3.9 months (95% CI, 3.0 to 4.6 months), respectively (Fig 3A). The median OS was 11.6 months (95% CI, 9.3 to 15.4 months) and 11.2 months (95% CI, 7.7 to 13.2 months), with an estimated 1-year event-free rate of 48.8% (95% CI, 39.6% to 57.4%) and 41.9% (95% CI, 31.6% to 51.8%), respectively (Fig 3B), at a median follow-up of 14 months (range, 10.8-18.7 months). Among responders, the median PFS was 8.5 months (95% CI, 5.4 to 13.4 months) and 8.5 months (95% CI, 5.3 to 13.4 months), and the median OS was 17.6 months (95% CI, 13.2 to 28.9 months) and 16.5 months (95% CI, 11.5 to 18.5 months) in the all-treated and triple-class–refractory populations, respectively (Data Supplement). Among patients in the all-treated population and the triple-class–refractory group (n = 70 and n = 52, respectively) who discontinued the study and initiated a new myeloma therapy, the median time to next therapy was 8.2 months (95% CI, 7.2 to 10.8 months) and 7.9 months (95% CI, 6.9 to 10.9 months), respectively. The median time to next therapy or death was 5.8 months (95% CI, 4.8 to 7.1 months) in the all-treated population and 5.3 months (95% CI, 4.5 to 6.3 months) in the triple-class–refractory group.
FIG 3.
PFS and OS. Kaplan-Meier analysis of (A) PFS and (B) OS in the all-treated (N = 157) and triple-class–refractory (n = 119) populations. OS, overall survival; PFS, progression-free survival.
In a subgroup analysis, 19 of the 54 patients (35%) age 65-74 years and 8 of the 25 patients (32%) older than 75 years achieved a PR or better. In addition, a PR or better was achieved in 13 of the 55 patients (24%) with extramedullary disease and 12 of the 59 patients (20%) with high-risk cytogenetics (Data Supplement). Among patients with MM refractory to previous alkylator therapy, the ORR was 21% (19 of the 92 patients achieved a PR or better, including one sCR, six VGPRs, and 12 PRs) and the CBR was 34% (Data Supplement). Among patients refractory to an alkylator in one previous line of therapy (n = 60), the ORR was 28% (CBR, 40%). In patients refractory to alkylators in multiple previous lines of therapy (n = 32), the ORR was 6% (CBR, 22%). Median PFS and OS in the subgroups analyzed were consistent with those of the all-treated population (Data Supplement).
Safety
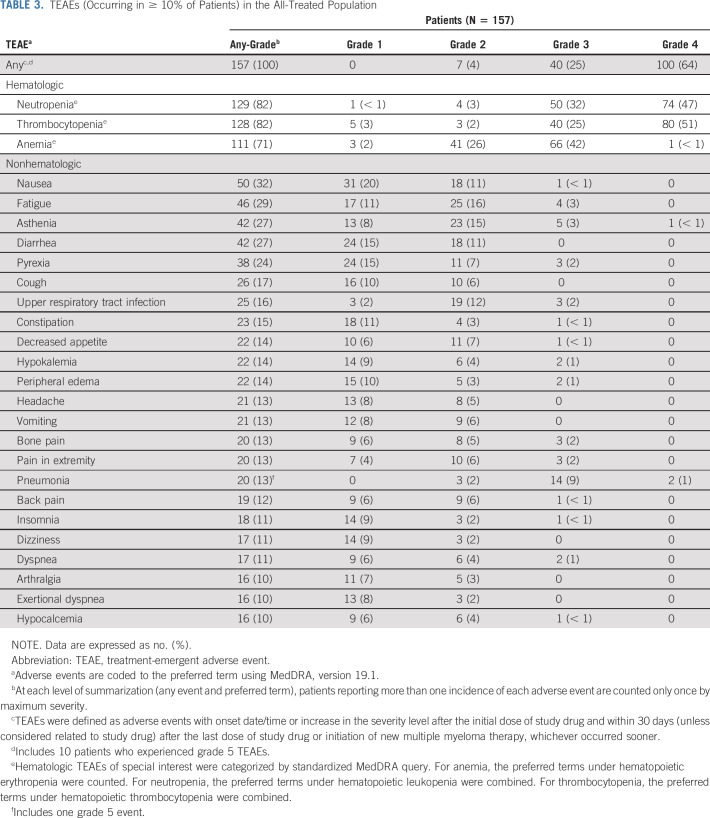
Treatment-emergent AEs (TEAEs) were reported in all 157 patients (100%) in the all-treated population, with 149 (95%) reporting at least one melflufen-related TEAE (Table 3 and Data Supplement). Grade ≥ 3 TEAEs occurred in 150 patients (96%), most commonly neutropenia (124 [79%]), thrombocytopenia (120 [76%]), and anemia (67 [43%]). Any-grade and grade 3/4 bleeding events with concurrent grade 3/4 thrombocytopenia occurred in 25 patients (16%) and four patients (3%), respectively. The most common nonhematologic treatment-emergent grade 3/4 events included pneumonia (16 [10%]; grade 3, 14 [9%]; grade 4, two [1%]) and hypophosphatemia (eight [5%]; grade 3, eight [5%]; grade 4, 0). Grade 3/4 neutropenia with concurrent grade 3/4 infections occurred in 18 patients (11%); of these, 11 (7%) had pneumonia (Data Supplement). GI events occurred in 97 patients overall and were grade 1/2 in 90 of the 97 patients (93%) and grade 3 in seven of the 97 patients (7%). No grade 4 events were reported. The most common any-grade GI events included nausea (50 [32%]), diarrhea (42 [27%]), constipation (23 [15%]), and vomiting (21 [13%]). Mucositis occurred in one patient (1%; grade 1 event), and there were no reports of alopecia or neuropathy.
TABLE 3.
TEAEs (Occurring in ≥ 10% of Patients) in the All-Treated Population
Serious TEAEs occurred in 77 patients (49%), most commonly pneumonia (14 [9%]) and febrile neutropenia (eight [5%]; Data Supplement). Second primary malignancies occurred in five patients; of these, four had malignancies with cutaneous manifestations (two patients with basal cell carcinoma, one patient with squamous cell carcinoma, and one patient with basal cell carcinoma, squamous cell carcinoma, and malignant melanoma; see the Data Supplement). One patient developed myelodysplasia after having received 17 cycles of study medication and in the context of multiple prior cycles of alkylator-based therapy, including stem-cell transplant prior to study entry. Moreover, the review of fluorescence in situ hybridization studies from the screening bone marrow confirmed pre-existing abnormalities supporting a subclinical myelodysplastic syndrome that was likely treatment-related and not otherwise apparent. No other cases of myelodysplastic syndromes were seen. Overall, 10 patients (6%) died from TEAEs. Most commonly, general physical health deterioration was associated with progressive disease (n = 3; 2%) and respiratory failure (n = 2; 1%; Data Supplement). None of the deaths were considered related to melflufen.
The average (standard deviation) monthly dose of melflufen received was 37.8 mg (± 4.0). TEAEs leading to melflufen dose reductions occurred in 42 patients (27%), most commonly thrombocytopenia (n = 22; 14%) and neutropenia (n = 5; 3%). While on study, 102 patients (65%) received concomitant RBC or platelet transfusion support, with 68 (43%) receiving platelet transfusion support only and 106 (68%) receiving concomitant growth factor support (Data Supplement). Overall, 34 patients (22%) had at least one TEAE leading to melflufen treatment discontinuation, most commonly thrombocytopenia (n = 16) and neutropenia (n = 5; Data Supplement). Overall, 95 patients (61%) experienced at least one dose delay, and the median number of treatment cycles with a dose delay was one (range, 0-9).
DISCUSSION
In this study, melflufen plus dexamethasone demonstrated meaningful efficacy and a manageable safety profile in patients with heavily pretreated RRMM. These findings build substantially on previously reported results17 but in a population that is more aligned with current treatment practice in the relapsed and refractory as well as highly resistant disease setting (ie, patients refractory to an anti-CD38 monoclonal antibody and/or pomalidomide, as well as exposed and refractory to prior lenalidomide, dexamethasone, and proteasome inhibitors). Durable responses were seen in this heavily pretreated population with a high proportion of extramedullary disease and high-risk cytogenetic features. Although the median DOR was 5.5 months, the median PFS among responders was encouragingly longer at 8.5 months. Furthermore, the median time to first response was 1.9 months, but many patients achieved their best response beyond 2 months of treatment. Altogether, these data support the notion that the clinical benefit of melflufen plus dexamethasone improves with longer treatment duration.
The ORR of 29% was consistent among high-risk patient subgroups, including those with triple-class–refractory disease (26%), those with extramedullary disease (24%), and patients age 75 years or older (32%), which is encouraging given the reported ORRs (10%-31%) in patients refractory to anti-CD38 monoclonal antibody therapy and/or with extramedullary disease at relapse.3,4,23-25 In fact, this is the largest population with extramedullary disease reported to date in a prospective study.4,26,27 Subgroup analyses showed sufficient efficacy in 60 patients refractory to an alkylator in one previous line of therapy with an ORR of 28%, while the ORR was only 6% in the 32 patients refractory to alkylators in two or more previous lines. Melflufen may have a mechanism of action that is different from that of other alkylators.8,11 For example, melflufen induced cell death more effectively than melphalan in TP53-mutated cell lines and in cells from patients with TP53-mutated RRMM, suggesting that the mechanism of cytotoxicity of melflufen—but not that of other alkylators—is independent of p53 function.8,11,16 Unlike other newer agents that work via immune-based mechanisms (including chimeric antigen receptor T cell therapy, belantamab mafodotin, iberdomide, and isatuximab), melflufen adds a unique mechanism of action to the treatment landscape in relapsed disease as a potent and novel cytotoxic agent targeting myeloma more broadly while providing meaningful clinical efficacy and a manageable safety profile for heavily pretreated RRMM.8,10,28-30
The safety profile of melflufen primarily consisted of hematologic AEs, consistent with previous results.17 Despite cytopenias being common, the incidence of significant bleeding events or infections was low. Hematologic AEs were generally reversible and clinically manageable with dose adjustments, dose delays, growth factor use, platelet transfusions, and appropriate supportive care. Nonhematologic grade 3/4 AEs were infrequent, with infections being the most common. Moreover, the frequency of infections was generally consistent with the expected rates of infections in heavily pretreated patients.23,27,31 Specifically, the 10% rate of grade 3/4 pneumonia reported in HORIZON was similar to 9%-11% reported with pomalidomide plus dexamethasone, bortezomib plus dexamethasone, and selinexor plus dexamethasone in RRMM.23,27,31 GI toxicities, a common reason for treatment discontinuation with other agents,23 were infrequent, primarily grade 1/2, and did not lead to melflufen treatment cessation in HORIZON in any patient. Encouragingly, alopecia and treatment-emergent peripheral neuropathy were not reported. Patients were therefore able to tolerate treatment, with rates of discontinuation from AEs lower than or comparable with other studies (which range from 6% to 33%) in this patient population and with a prolonged median duration of treatment, together with the added convenience of monthly infusions, which is an especially important consideration in the current era of COVID-19.23,27,28
In conclusion, the results from HORIZON suggest that melflufen has the potential to be an important therapeutic option in RRMM by providing a novel mechanism of action, clinically meaningful efficacy, and manageable safety when combined with dexamethasone in heavily pretreated patients.32 Based on these results, the efficacy and safety of melflufen plus dexamethasone versus pomalidomide plus dexamethasone are being further evaluated in OCEAN (OP-103), a randomized, global, phase III multicenter study (ClinicalTrials.gov identifier: NCT03151811) for patients in earlier relapse.33 Studies of melflufen plus dexamethasone in combination with bortezomib or daratumumab are also ongoing, with promising results to date.34
ACKNOWLEDGMENT
The authors especially thank the patients and their families for participating in this trial and all the study investigators and coordinators for their contributions to this work. The authors also thank Jakob Obermüller and Hanan Zubair (Oncopeptides AB, Stockholm, Sweden) for data management as well as Katherine Mills-Lujan, PhD, CMPP, and Jennifer Leslie, PhD, CMPP, of Team 9 Science for providing medical editorial assistance under the guidance of the authors, which was funded by Oncopeptides AB in accordance with Good Publications Practice (GPP3) guidelines.
APPENDIX
TABLE A1.
HORIZON (OP-106) Investigators and Recruitment Sites
PRIOR PRESENTATION
Presented in part at the 25th European Hematology Association annual congress, virtual format, June 11-21, 2020; abstract EP945.
SUPPORT
This study was sponsored by Oncopeptides AB, which also provided support for manuscript editorial assistance.
CLINICAL TRIAL INFORMATION
See accompanying article on page 836
DATA SHARING STATEMENT
Oncopeptides commits to share clinical study data with qualified researchers to enable enhancement of public health. As such, Oncopeptides will share anonymized patient-level data on request or if required by law or regulation. Qualified scientific and medical researchers can request patient-level data for studies of Oncopeptides pharmaceutical substances listed on ClinicalTrials.gov and approved by health authorities in the United States and the EU. Patient-level data for studies of newly approved pharmaceutical substances or indications can be requested 9 months after US Food and Drug Administration and European Medicines Agency approval. Such requests are assessed at Oncopeptides' discretion, and the decisions depend on the scientific merit of the proposed request, data availability, and the purpose of the proposal. The applicants should be willing to submit both positive and negative findings to a scientific journal. If Oncopeptides agrees to share clinical data for research purposes, the applicant is required to sign an agreement for data sharing before data release, to ensure that the patient data are de-identified. In case of any risk of re-identification on anonymized data despite measures to protect patient confidentiality, the data will not be shared. The patients' informed consent will always be respected. If the anonymization process will provide futile data, Oncopeptides will have the right to refuse the request. Oncopeptides will provide access to patient-level clinical trial analysis datasets in a secured environment upon execution of the data sharing agreement.
Oncopeptides will also provide the Protocol, statistical analysis plan, and the clinical study report synopsis if needed. For additional information or requests for access to Oncopeptides clinical trial data for research purposes, please contact us at medinfo@oncopeptides.com.
AUTHOR CONTRIBUTIONS
Conception and design: Paul G. Richardson, Catriona Byrne, Johan Harmenberg, María-Victoria Mateos
Provision of study materials or patients: Paul G. Richardson, Albert Oriol, Alessandra Larocca, Joan Bladé, Michele Cavo, Paula Rodriguez-Otero, Xavier Leleu, Omar Nadeem, John W. Hiemenz, Hani Hassoun, Cyrille Touzeau, Adrián Alegre, Agner Paner, Christopher Maisel, Amitabha Mazumder, Anastasios Raptis, Jan S. Moreb, Kenneth C. Anderson, Jacob P. Laubach, María-Victoria Mateos
Collection and assembly of data: Paul G. Richardson, Albert Oriol, Alessandra Larocca, Joan Bladé, Michele Cavo, Paula Rodriguez-Otero, Xavier Leleu, Omar Nadeem, John W. Hiemenz, Hani Hassoun, Cyrille Touzeau, Adrián Alegre, Agner Paner, Christopher Maisel, Amitabha Mazumder, Anastasios Raptis, Jan S. Moreb, Kenneth C. Anderson, Jacob P. Laubach, Marcus Thuresson, María-Victoria Mateos
Data analysis and interpretation: Paul G. Richardson, Albert Oriol, Alessandra Larocca, Joan Bladé, Michele Cavo, Paula Rodriguez-Otero, Xavier Leleu, John W. Hiemenz, Cyrille Touzeau, Adrián Alegre, Agne Paner, Anastasios Raptis, Jan S. Moreb, Kenneth C. Anderson, Jacob P. Laubach, Sara Thuresson, Marcus Thuresson, Catriona Byrne, Johan Harmenberg, Nicolaas A. Bakker, María-Victoria Mateos
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects for the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Melflufen and Dexamethasone in Heavily Pretreated Relapsed and Refractory Multiple Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I =Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Paul G. Richardson
Consulting or Advisory Role: Celgene, Janssen, Takeda, Karyopharm Therapeutics, Oncopeptides, Sanofi, Jazz Pharmaceuticals, SecuraBio
Research Funding: Celgene, Takeda, Bristol-Myers Squibb, Oncopeptides
Albert Oriol
Consulting or Advisory Role: Celgene, Janssen, Amgen, Sanofi, GlaxoSmithKline
Speakers' Bureau: Amgen, Celgene
Alessandra Larocca
Honoraria: Amgen, Bristol-Myers Squibb, Celgene, Janssen, GlaxoSmithKline
Consulting or Advisory Role: Bristol-Myers Squibb, Celgene, Janssen, Takeda
Joan Bladé
Honoraria: Janssen, Celgene, Amgen, Takeda, Oncopeptides
Michele Cavo
Honoraria: Janssen, Bristol-Myers Squibb, Celgene, Sanofi, GlaxoSmithKline, Takeda, Amgen, Oncopeptides, Abbvie, Karyopharm Therapeutics, Adaptive Biotechnologies
Consulting or Advisory Role: Janssen, Bristol-Myers Squibb, Celgene, Sanofi, GlaxoSmithKline, Takeda, Amgen, Oncopeptides, Abbvie, Karyopharm Therapeutics, Adaptive Biotechnologies
Speakers' Bureau: Janssen, Celgene
Paula Rodriguez-Otero
Honoraria: Janssen, Celgene, Amgen, Oncopeptides, Sanofi, Abbvie, GlaxoSmithKline, Kite Pharma
Consulting or Advisory Role: Janssen, Celgene, Amgen, Takeda, Oncopeptides, Sanofi, AbbVie, GlaxoSmithKline, Kite Pharma
Xavier Leleu
Honoraria: Janssen-Cilag, Celgene, Amgen, Novartis, Bristol-Myers Squibb, Takeda, Sanofi, Abbvie, Merck, Roche, Karyopharm Therapeutics, Carsgen Therapeutics Ltd, Oncopeptides, GlaxoSmithKline
Consulting or Advisory Role: Janssen-Cilag, Celgene, Amgen, Takeda, Bristol-Myers Squibb, Novartis, Merck, Gilead Sciences, Abbvie, Roche, Karyopharm Therapeutics, Oncopeptides, Carsgen Therapeutics Ltd, GlaxoSmithKline
Travel, Accommodations, Expenses: Takeda
Omar Nadeem
Consulting or Advisory Role: Janssen, Celgene, Sanofi, Takeda, Adaptive Biotechnologies
Hani Hassoun
Consulting or Advisory Role: Novartis
Research Funding: Takeda, Janssen
Cyrille Touzeau
Honoraria: Abbvie, Celgene, Amgen, Takeda, Janssen, Sanofi, Novartis, GlaxoSmithKline
Consulting or Advisory Role: Novartis, Amgen, Celgene, Abbvie, Takeda, Janssen, GlaxoSmithKline
Research Funding: Abbvie
Adrián Alegre
Leadership: Amgen, Janssen-Cilag, Celgene-BMS, Takeda, Sanofi, GlaxoSmithKline, ONCOPETIDES
Agne Paner
Honoraria: Amgen, Celgene, Janssen
Consulting or Advisory Role: Takeda, Celgene, Amgen, Karyopharm Therapeutics, Oncopetides
Christopher Maisel
Stock and Other Ownership Interests: Actinium Pharmaceuticals, Karyopharm Therapeutics, Amgen
Honoraria: Bristol-Myers Squibb, Karyopharm Therapeutics, Takeda, Janssen Oncology, Kite/Gilead, Oncopetides, GlaxoSmithKline
Speakers' Bureau: Amgen, Bristol-Myers Squibb, Karyopharm Therapeutics, Takeda, Janssen Oncology, Kite/Gilead
Amitabha Mazumder
Honoraria: Karyopharm Therapeutics
Speakers' Bureau: Karyopharm Therapeutics
Anastasios Raptis
Consulting or Advisory Role: intellisphere, integra
Jan S. Moreb
Consulting or Advisory Role: Oncopeptide
Kenneth C. Anderson
Stock and Other Ownership Interests: C4 Therapeutics, OncoPep
Consulting or Advisory Role: Celgene, Millennium, Gilead Sciences, Bristol-Myers Squibb, Janssen Oncology, Sanofi, Tolero Pharmaceuticals, Precision Biosciences
Patents, Royalties, Other Intellectual Property: C4 Therapeutics, OncoPep
Jacob P. Laubach
Research Funding: Abbvie, Bristol-Myers Squibb, Genentech, Janssen Research & Development, Carsgen, Millennium
Sara Thuresson
Employment: Oncopeptides
Stock and Other Ownership Interests: Oncopeptides
Consulting or Advisory Role: Oncopeptides
Marcus Thuresson
Employment Oncopeptides
Stock and Other Ownership Interests: Oncopeptides
Consulting or Advisory Role: Oncopeptides
Catriona Byrne
Consulting or Advisory Role: Oncopeptides
Travel, Accommodations, Expenses: Oncopeptides
Johan Harmenberg
Leadership: Oncopeptides
Stock and Other Ownership Interests: Oncopeptides
Consulting or Advisory Role: Oncopeptides, Ectin AB
Travel, Accommodations, Expenses: Oncopeptides
Nicolaas A. Bakker
Employment: Oncopeptides
Stock and Other Ownership Interests: Oncopeptides
Honoraria: Oncopeptides
María-Victoria Mateos
Honoraria: Janssen-Cilag, Celgene, Amgen, Takeda, GlaxoSmithKline, Abbvie/Genentech, Adaptive Biotechnologies
Consulting or Advisory Role: Takeda, Janssen-Cilag, Celgene, Amgen, Abbvie, GlaxoSmithKline, Pharmamar-zeltia
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kumar SK Dimopoulos MA Kastritis E, et al. : Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia 31:2443-2448, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Moreau P San Miguel J Sonneveld P, et al. : Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv52-iv61, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Gandhi UH Cornell RF Lakshman A, et al. : Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 33:2266-2275, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimenez-Segura R Granell M Gironella M, et al. : Pomalidomide-dexamethasone for treatment of soft-tissue plasmacytomas in patients with relapsed/refractory multiple myeloma. Eur J Haematol 102:389-394, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Sonneveld P: Management of multiple myeloma in the relapsed/refractory patient. Hematology Am Soc Hematol Educ Program 2017:508-517, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chim CS Kumar SK Orlowski RZ, et al. : Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia 32:252-262, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wickström M Haglund C Lindman H, et al. : The novel alkylating prodrug J1: Diagnosis directed activity profile ex vivo and combination analyses in vitro. Invest New Drugs 26:195-204, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Chauhan D Ray A Viktorsson K, et al. : In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells. Clin Cancer Res 19:3019-3031, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strese S Wickstrom M Fuchs PF, et al. : The novel alkylating prodrug melflufen (J1) inhibits angiogenesis in vitro and in vivo. Biochem Pharmacol 86:888-895, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Ray A Ravillah D Das DS, et al. : A novel alkylating agent Melflufen induces irreversible DNA damage and cytotoxicity in multiple myeloma cells. Br J Haematol 174:397-409, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wickström M Nygren P Larsson R, et al. : Melflufen—A peptidase-potentiated alkylating agent in clinical trials. Oncotarget 8:66641-66655, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miettinen JJ Kumari R Traustadottir GA, et al. : Aminopeptidase expression in multiple myeloma associates with disease progression and sensitivity to melflufen. Presented at the 25th European Hematology Association Annual Conference, June 11-21, 2020 (virtual poster EP897) [DOI] [PMC free article] [PubMed]
- 13.Pinto V Bergantim R Caires HR, et al. : Multiple myeloma: Available therapies and causes of drug resistance. Cancers (Basel) 12:407, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wickström M Larsson R Nygren P, et al. : Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci 102:501-508, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore HE Davenport EL Smith EM, et al. : Aminopeptidase inhibition as a targeted treatment strategy in myeloma. Mol Cancer Ther 8:762-770, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Slipicevic A Munawar U Aschan J, et al. : Melflufen efficacy in multiple myeloma with TP53 aberrations. Presented at the American Association for Cancer Research (AACR) Annual Meeting, June 22-24, 2020 (Virtual Annual Meeting II, abstr 1843)
- 17.Richardson P Bringhen S Voorhees P, et al. : Melflufen plus dexamethasone in relapsed and refractory multiple myeloma (O-12-M1): A multicentre, international, open-label, phase 1-2 study. Lancet Haematol 7:e395-e407, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Rajkumar SV Harousseau JL Durie B, et al. : Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 117:4691-4695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleiss JL: Statistical methods for rates and proportions (ed 2). New York, NY, Wiley, 1981 [Google Scholar]
- 20.Brookmeyer R, Crowley J: A confidence interval for the median survival time. Biometrics 38:29-42, 1982 [Google Scholar]
- 21.Schemper M, Smith TL: A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343-346, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Sonneveld P Avet-Loiseau H Lonial S, et al. : Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 127:2955-2962, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chari A Vogl DT Gavriatopoulou M, et al. : Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med 381:727-738, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Usmani SZ Weiss BM Plesner T, et al. : Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 128:37-44, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichinohe T Kuroda Y Okamoto S, et al. : A multicenter phase 2 study of pomalidomide plus dexamethasone in patients with relapsed and refractory multiple myeloma: The Japanese MM-011 trial. Exp Hematol Oncol 5:11, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raje N Berdeja J Lin Y, et al. : Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med 380:1726-1737, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimopoulos MA Palumbo A Corradini P, et al. : Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): A phase 3b study in refractory multiple myeloma. Blood 128:497-503, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lonial S Lee HC Badros A, et al. : Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol 21:207-221, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Lonial S Van de Donk NW Popat R, et al. : A phase 1b/2a study of the CELMoD iberdomide (CC-220) in combination with dexamethasone in patients with relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk 19:E52-E53, 2019 [Google Scholar]
- 30.Attal M Richardson PG Rajkumar SV, et al. : Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 394:2096-2107, 2019 [DOI] [PubMed] [Google Scholar]
- 31.San-Miguel JF Hungria VT Yoon SS, et al. : Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: A multicentre, randomised, double-blind phase 3 trial. Lancet Oncol 15:1195-1206, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Richardson PG San Miguel JF Moreau P, et al. : Interpreting clinical trial data in multiple myeloma: Translating findings to the real-world setting. Blood Cancer J 8:109, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schjesvold F Robak P Pour L, et al. : OCEAN: A randomized phase III study of melphalan flufenamide + dexamethasone to treat relapsed refractory multiple myeloma. Future Oncol 16:631-641, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Ocio EM Efebera YA Granell M, et al. : ANCHOR (OP-104): Updated efficacy and safety from a phase 1/2 study of melflufen and dexamethasone plus bortezomib or daratumumab in patients with relapsed/refractory multiple myeloma (RRMM) refractory to an IMiD or a proteasome inhibitor (PI). Blood 133, 2019(abstr 3124) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Oncopeptides commits to share clinical study data with qualified researchers to enable enhancement of public health. As such, Oncopeptides will share anonymized patient-level data on request or if required by law or regulation. Qualified scientific and medical researchers can request patient-level data for studies of Oncopeptides pharmaceutical substances listed on ClinicalTrials.gov and approved by health authorities in the United States and the EU. Patient-level data for studies of newly approved pharmaceutical substances or indications can be requested 9 months after US Food and Drug Administration and European Medicines Agency approval. Such requests are assessed at Oncopeptides' discretion, and the decisions depend on the scientific merit of the proposed request, data availability, and the purpose of the proposal. The applicants should be willing to submit both positive and negative findings to a scientific journal. If Oncopeptides agrees to share clinical data for research purposes, the applicant is required to sign an agreement for data sharing before data release, to ensure that the patient data are de-identified. In case of any risk of re-identification on anonymized data despite measures to protect patient confidentiality, the data will not be shared. The patients' informed consent will always be respected. If the anonymization process will provide futile data, Oncopeptides will have the right to refuse the request. Oncopeptides will provide access to patient-level clinical trial analysis datasets in a secured environment upon execution of the data sharing agreement.
Oncopeptides will also provide the Protocol, statistical analysis plan, and the clinical study report synopsis if needed. For additional information or requests for access to Oncopeptides clinical trial data for research purposes, please contact us at medinfo@oncopeptides.com.