Abstract
To evaluate the clinical and imaging findings of papillary breast neoplasm and review the pathologic correlation at a tertiary center.
Retrospective study of patients diagnosed with benign and malignant papillary lesions between 2008 to 2018. 147 patients were identified with histology diagnosis of papillary lesions. The clinical, imaging, and pathological characteristics were reviewed.
Patient cohort included 147 women diagnosed with papillary lesions (mean age at diagnosis 53.8 years) and were divided into 3 histology groups (benign, atypical, and malignant). Common clinical presentations were breast lump (n = 60) and nipple discharge (n = 29), 48 patients were asymptomatic.
Only 37 were detected as a mass lesion on mammogram. The presence of mass lesion on mammogram was the most common feature in all 3 papillary lesion groups, and with the presence of asymmetric density, were the 2 mammographic features significantly associated (P < .05) with malignancy.
All lesions were detected on ultrasound. The most common sonographic features for all 3 groups were the presence of a mass and irregular shape. Among all the sonographic features assessed, larger size, presence of vascularity and absence of dilated ducts were significantly associated (P < .05) with malignancy.
Feature pattern recognition of the variety of benign, atypical and malignant papillary neoplasm on ultrasound and mammogram, with emphasis on size, presence of vascularity and dilated ducts on ultrasound and presence of mass, and architectural distortion on mammogram, is important in the assessment of patients with suspected ductal lesions to facilitate optimal treatment and surgical care.
Keywords: breast neoplasms, mammography, papilloma, ultrasonography
1. Introduction
Papillary lesions of the breast are a heterogeneous group of breast lesions that are difficult to diagnose. The main diagnostic concern is differentiating benign and malignant lesions, which can be challenging both on imaging as well as on histopathological examination. Benign papillary lesions incur an increased risk of breast carcinoma to women, of about 1.5 to 2.0 increased risk for those with solitary papillomas, and the risk is even higher in those with multiple papillomatosis.[1,2]
They have variable pathological characteristics. Pathologically they appear as arborescent or tree-like lesions made up of fibrovascular stalks lined with epithelial cells with or without interspersed myoepithelial layer. Papillary lesions can be classified into a spectrum of intraductal papilloma, papillomatosis, papilloma with atypia, papilloma with DCIS (ductal carcinoma in situ), encapsulated papillary carcinoma and invasive micropapillary carcinoma.[3] A benign diagnosis on fine needle aspiration or core needle biopsy may not completely exclude malignancy especially if it manifests as focal carcinoma in-situ or abruption of the myoepithelial layer.[4,5] Bianchi et al recommended further surgery or vacuum assisted excision if core needle biopsy (14-gauge) were to diagnose benign intraductal papilloma without atypia in order to reduce the risk of delaying a malignant diagnosis.[6] However, Han et al, in a study with 511 cases of intraductal papilloma, revealed that the rate of upgrading to malignancy from a benign papilloma in the absence of atypia is very low.[7]
Mammographic and sonographic features are not sensitive or specific enough to allow accurate differentiation between benign and malignant lesions.[5] Benign intraductal papilloma on mammography may demonstrate a dense well-circumscribed mass with microcalcifications, but this is non-specific. On sonography, a dilated duct with a solid mass within may be detected, but often the only finding is of a well-defined hypoechoic mass. Malignant lesions may also present with the latter sonographic appearance. Han et al found that sonographic findings of papillary lesions correlated well with pathologic findings in most cases with respect to relationship between the mass and the duct.[8] Non-parallel orientation, echogenic halo, posterior acoustic enhancement, and associated microcalcification on mammography have been suggested as more specific features for malignancy.[1,2]
In conclusion, little is known to characterize papillary lesions on ultrasound and mammogram. Hence, the aim of this study was to review the spectrum of papillary breast lesions via imaging, clinical and histopathological examination; and assess if any specific imaging feature could assist in the differentiation of malignant and benign papillary lesions.
2. Methods
This was an institutional board approved cross sectional retrospective study of which patient informed consent was waived. A total of 147 patients were identified from the pathology records (n = 11,318) of University Malaya Medical Centre (UMMC) from 2008 to 2018 of which the patients either had core biopsy, surgical excision or mastectomy (see Table 1). Out of this group, 32 did not have imaging stored in the PACS (picture archiving and communication system). Analysis was performed with the available imaging data, whilst maintaining the overarching aim of studying the population of papillary patients in our patient group.
Table 1.
Diagnostic techniques utilized in the 147 cases obtained via pathology records.
| Diagnostic technique | N (%) |
| Core biopsy | 55 (38.8) |
| Excision biopsy | 7 (4.8) |
| Hookwire localization | 34 (23.1) |
| VAB | 1 (0.7) |
| Microdochectomy | 5 (3.4) |
| Wide local excision/ Breast conserving surgery | 16 (10.9) |
| Mastectomy | 23 (15.6) |
| Missing information | 4 (2.7) |
The available imaging (ultrasound, mammogram) for the other patients was assessed by 2 breast radiologists with 8 (MTRH) and 6 (FF) years of experience. The 2 readers were blinded to each other's assessment of the images. Cohen Kappa coefficient was measured to assess inter-rater agreement. The imaging findings of the papillary lesions were assessed with the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) criteria.[1,2]
Mammography was performed with standard images of craniocaudal and mediolateral oblique (MLO) views for each breast using Mammomat Novation digital mammography machine (Siemens, Erlangen, Germany). After July 2014, mammograms were also done with Selenia Dimensions (Hologic, Bedford, Mass) Digital Breast Tomosynthesis machine, in which 2D Full Field Digital Mammography as well as tomosynthesis images were produced for each patient. The mammogram features assessed were presence of mass, shape, margins, mass density, calcification, asymmetric density, architectural distortion and breast density according to the 5th ACR BI-RADS atlas.[4,5]
Ultrasonography was performed with Phillips iU22 units (Philips Medical Systems, Bothell, WA) utilizing Philips L12–5 high-frequency linear ultrasound probe. Operators of the machine were radiologists and sonographers with at least 5 years’ experience in scanning and interpreting breast ultrasound. Static and color Doppler images of lesions were generated and stored into the hospital's PACS. The sonographic features assessed were presence of mass, papillary lesion appearance (based on paper by Han et al [6]), shape, orientation, margins, echogenicity, posterior acoustic features, calcification, presence of dilated duct, vascularity and debris.
The clinical, imaging, and pathological characteristics were summarized using means and ranges for continuous variables and percentages for categorical variables.
The mammography and sonographic findings were correlated with histology, which were grouped into benign, atypical and malignant. Statistical analysis was performed via Chi-Squared test with Cohen Kappa being used for inter-rater agreement using SPSS version 20 (SPSS, Inc., Chicago, Illinois). Results with P value of <.05 were considered statistically significant.
3. Results
3.1. Demographics (see Table 1)
The age range of the patients was 21 to 87 years of age. The most prevalent age group was between 41 to 50 years. For the benign cases, the age range is from 21 to 78 years, whereas for the malignant, it was between 40 to 87 years. There was significant difference in age distribution (P = .01), with younger age group in the benign cases and older age group in the malignant cases. In terms of ethnicity, 50% of the patients were Chinese, 27% Malays, and 20% Indian. 3% of the patients were foreigners and from the Iban race. No statistically significant relationship between the diagnosis subtype (benign, atypical, or malignant) with race (P = .29).
3.2. Clinical
Commonest clinical presentations in this group of patients were breast lump (n = 60, 40.8%) and nipple discharge (n = 29 19.7%). However, it is notable that a large number of patients (n = 48, 32.7%) were asymptomatic (see Table 2).
Table 2.
Clinical presentation of the patients.
| Symptom | N (%) |
| Asymptomatic | 48 (32.7) |
| Nipple discharge | 17 (11.6) |
| Bloody nipple discharge | 12 (8.2) |
| Breast lump | 60 (40.8) |
| Discharge and lump | 8 (5.4) |
| Mastalgia | 2 (1.4) |
3.3. Pathology
The cases were divided into 3 groups, which were benign, atypical, and malignant. The benign group was made up of intraductal papilloma and papillomatosis. Malignant group included papilloma with DCIS, encapsulated papillary carcinoma, and invasive micropapillary carcinoma. (see Table 3) There were 100 benign, 5 atypical, and 42 malignant cases.
Table 3.
Pathological spectrum of papillary lesions in our study population.
| Lesion | N (%) | |
| Benign | Intraductal papilloma | 84 (57.1) |
| Papillomatosis | 16 (10.9) | |
| Atypical | Atypical papilloma | 5 (3.4) |
| Malignant | Papilloma with DCIS | 9 (6.1) |
| Intraductal papillary carcinoma | 28 (19.1) | |
| Invasive Micropapillary carcinoma | 5 (3.4) | |
3.4. Ultrasound
Majority of the patients had ultrasound performed (see Table 4), with a total of 106 scans available for retrospective review. The size on ultrasound, for the benign lesions range between 0.3 to 0.6 cm and 4.2 to 7.8 cm for malignan lesions. Malignant lesions had significantly larger diameters compared to benign lesions (P < .001). (see Table 5).
Table 4.
Sonographic features of the papillary lesions.
| a Descriptive sonographic features | ||||||
| Feature | BENIGN | MALIGNANT | ATYPICAL | P value | ||
| Mass lesion (%)presentabsent | 69 (89.6)8 (10.4) | 26 (100)0 | 3 (100)0 | .20 | ||
| Papillary lesion appearance a (%)IntraluminalExtraductalSolidMixed | 21 (36.2)13 (22.4)24 (41.4)0 | 7 (31.9)1 (4.5)14 (63.6)0 | 1 (33.3)2 (66.7)00 | <.05∗ | ||
| Shape (%)OvalRoundIrregular | 27 (46.5)3 (5.2)28 (48.3) | 7 (31.8)2 (9.1)13 (59.1) | 003 (100) | .36 | ||
| orientation (%)ParallelNon-parallel | 33 (56.9)25 (43.1) | 8 (36.4)14 (63.6) | 2 (66.7)1 (33.3) | .23 | ||
| margins (%)CircumscribedIndistinctMicrolobulatedAngular | 24 (41.4)23 (39.7)6 (10.3)5 (8.6) | 6 (27.3)9 (40.9)5 (22.7)2 (9.1) | 02 (66.7)1 (33.3)0 | .51 | ||
| echogenicity (%)hypoechoicisoechoiccomplex | 30 (51.7)1 (1.7)27 (46.6) | 8 (36.4)014 (63.6) | 1 (33.3)02 (66.7) | .67 | ||
| posterior acoustic features(%)EnhancementShadowingAbsent | 20 (34.5)3 (5.2)35 (60.3) | 7 (37.9)4 (1.8)11 (50) | 01 (33.3)2 (66.7) | .20 | ||
| mass calcification (%)PresentAbsent | 4 (6.9)54 (93.1) | 3 (14.3)18 (85.7) | 03 (100) | .50 | ||
| Dilated duct (%)presentabsentprominent | 21 (33.3)34 (54)8 (12.7) | 2 (8.7)19 (82.6)2 (8.7) | 1 (33.3)2 (66.7)0 | .15.04# | ||
| Vascularity (%)presentabsent | 7 (17.9)32 (82.1) | 9 (52.9)8 (47.1) | 0 2 (100) | .02∗ | ||
| Debris (%)presentabsent | 4 (7)53 (93) | 1 (5)19 (95) | 03 (100) | .86 | ||
Table 5.
Ultrasonographic size of papillary lesions assessed according to HPE.
| Median (IQR) | |
| Benign | 1.00 (0.65–1.35) |
| Atypical | 1.10 (N/A) |
| Malignant | 1.9 (0.70–3.10) |
Among all the sonographic features assessed, the presence of vascularity was significantly associated (P = .02) with malignant papillary lesions. There were more malignant cases with vascularity (52.9%) compared with benign (17.9%) and atypical groups (0%). When the statistical analysis was repeated with only the benign and malignant groups, absence of dilated ducts (82.6% malignant/54% benign) was associated with malignancy, (P = .04).
3.5. Mammography
The presence of mass lesion was the most common feature in all the 3 papillary lesion groups on mammography, 32.7% of benign, 78.3% of malignant, and 100% of atypical cases (see Table 6). This feature, as well as asymmetric density were the only mammographic features significantly associated (P < .01 and P = .03 respectively) with malignant lesions. About 9.7% (3 cases) of the benign group showed asymmetric density on mammography compared to approximately 40% (8 cases) in the malignant group. BI-RADS density B was the most common breast parenchymal density on mammogram overall with density D being the least common. Repeat statistical analysis using only the benign and malignant groups did not yield any further statistical significance.
Table 6.
Mammographic features of the papillary lesions.
| Feature | BENIGN | MALIGNANT | ATYPICAL | P value |
| Mass lesion (%) | <.01∗ | |||
| present | 17 (32.7) | 18 (78.3) | 2 (100) | |
| absent | 35 (67.3) | 5 (21.7) | 0 | |
| Shape (%) | .20 | |||
| Oval | 4 (20) | 3 (18.8) | 0 | |
| Round | 4 (20) | 0 | 1 (50) | |
| Irregular | 12 (60) | 13 (81.2) | 1 (50) | |
| Margins (%) | .34 | |||
| Circumscribed | 6 (30) | 2 (12.5) | 1 (50) | |
| Indistinct | 10 (50) | 5 (31.2) | 1 (50) | |
| Microlobulated | 1 (5) | 3 (18.8) | 0 | |
| Spiculated | 3 (15) | 6 (37.5) | 0 | |
| Mass density (%) | .13 | |||
| Low | 0 | 0 | 0 | |
| Equal | 8 (44.4) | 2 (13.3) | 1 (50) | |
| High | 10 (55.6) | 13 (86.7) | 1 (50) | |
| Mass calcification (%) | .15 | |||
| Benign | 6 (30) | 3 (18.8) | 2 (100) | |
| Suspicious | 4 (20) | 6 (37.5) | 0 | |
| Absent | 10 (50) | 7 (43.7) | 0 | |
| Asymmetric density (%) | .03∗ | |||
| present | 3 (9.7) | 8 (40) | 0 | |
| absent | 28 (90.3) | 12 (60) | 2 (100) | |
| Architectural distortion (%) | .05 | |||
| present | 1 (3.3) | 2 (10.5) | 1 (50) | |
| absent | 29 (96.7) | 17 (89.5) | 1 (50) | |
| Density | .38 | |||
| A | 10 (20) | 3 (14.3) | 1 (50) | |
| B | 24 (48) | 12 (57.1) | 0 | |
| C | 10 (20) | 6 (28.6) | 1 (50) | |
| D | 6 (12) | 0 | 0 |
Figures 1–6 are case examples with images of a selection of papillary lesions in this patient group.
Figure 1.
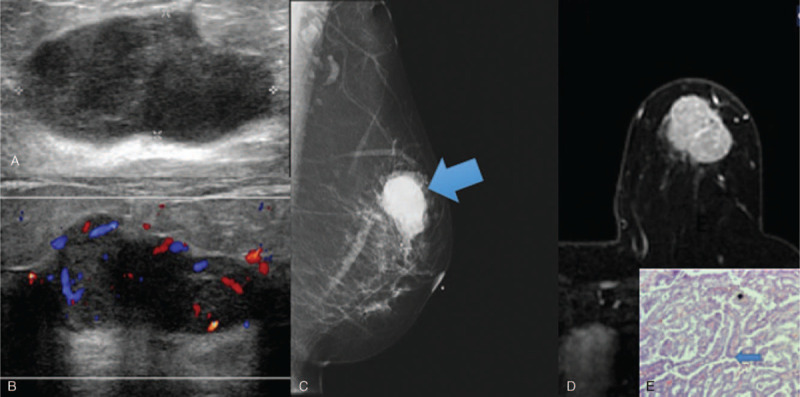
Invasive papillary carcinoma solid mass sonographic appearance. 50 year old lady presented with a mass in the left breast. Figure 1A showed a lobulated hypoechoic mass, with increased vascularity on Doppler images (Fig. 1B). Mammogram (Fig. 1C) demonstrates a high density mass in the left upper quadrant. Figure 1D is of subtracted MRI image post gadolinium, with a large heterogeneously enhancing mass which shows washout on the delayed phase (Type 3 curve). Histopathological examination (Figure 1E) demonstrates numerous papillary structures (blue arrow). (Magnification 40x).
Figure 6.
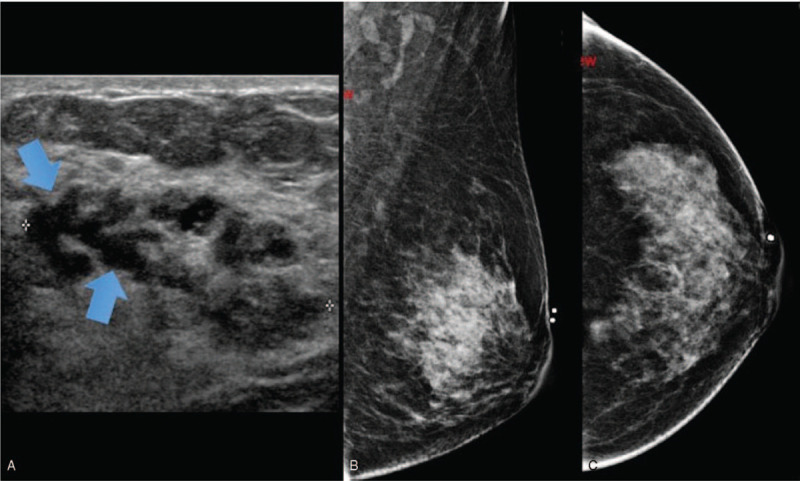
Papilloma with DCIS solid mass appearance. 48 year old lady presented with left breast lump and nipple discharge. Figure 6A showed a heterogeneous mass in the left breast with tubular hypoechoic areas within (arrows) suggestive of ducts. No increased vascularity seen. Left mammogram (Fig. 6B (MLO)) did not show any mass or asymmetric density. Histopathological examination (Fig. 6C) demonstrates papillary lesion (arrows) with papillary architecture. (Magnification 40x).
Figure 2.
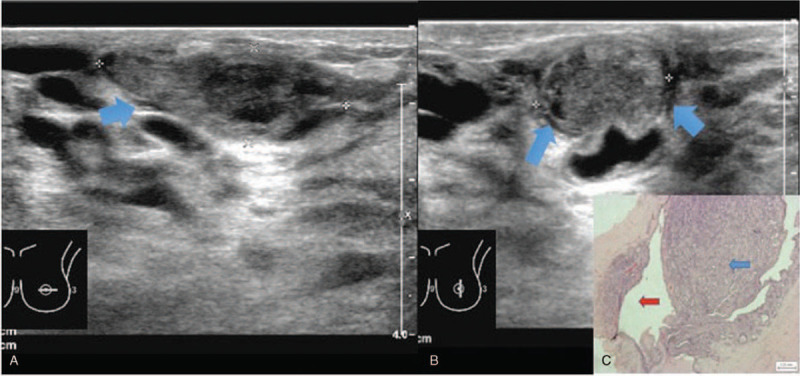
Intraductal papilloma solid mass with anechoic rim sonographic appearance. 30 year old lady presented with left nipple discharge. Figure 2A and 2B showed a solid mass with anechoic rim (arrows) on ultrasound images. Doppler image (Fig. 2C) of the mass revealed peripheral but no internal vascularity. Histopathological examination (Fig. 2D) demonstrates intraductal papilloma (blue arrow) within a duct (red arrow). (Magnification 20x).
Figure 4.
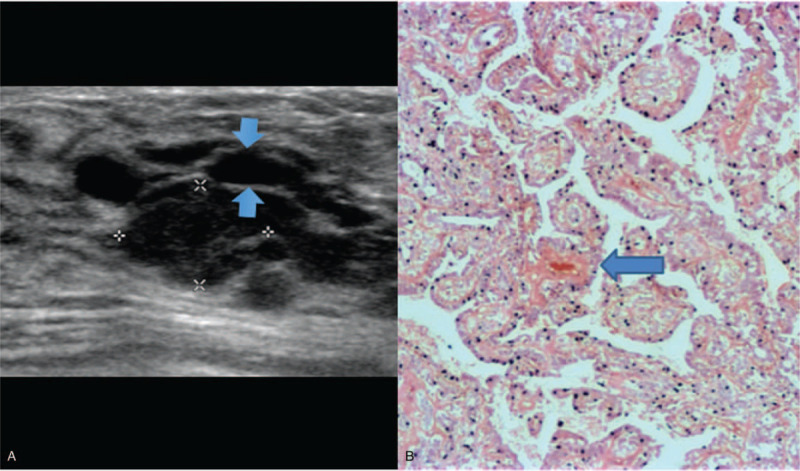
Papillomatosis solid type with anechoic rim appearance. 26 year old lady presented with nipple discharge and lump in the left breast. Figure 4A showed a solid hypoechoic mass measuring 1.3 × 0.8 cm with anechoic rim noted at the superior aspect of the mass (arrow), and no increased vascularity. Histopathological examination (Fig. 4B) demonstrates a high magnification image of an Intraductal papilloma with benign papillary structures and fibrovascular cores [blue arrow]. (Magnification 40x) The papillary processes are seen in several ducts [not seen in images].
Inter-rater agreement between the 2 radiologists were performed for some of the features on ultrasound (echogenicity, presence of dilated ducts, vascularity, and presence of debris) and mammogram (asymmetric density, architectural distortion, calcification, and breast density). Both radiologists reviewed the images separately, and the findings noted by each were compared and analyzed with Cohen Kappa on SPSS. For ultrasound, Kappa scores of between 0.60 to 0.88 were found, indicating substantial and almost perfect agreement. Similar scores ranging from 0.64 to 0.96 were noted for mammogram features.
4. Discussion
Papillary lesions constitute less than 10% of all benign breast lesions with the malignant spectrum being even rarer, at approximately 2% of breast cancers.[7] The uncommon nature of these lesions has brought about a less understanding of characterizations through mammogram and ultrasound. Hence, we ventured to investigate the spectrum of papillary disease in our center.
Using Han et al[9] method of classifying the papillary lesions into 4 subtypes (type I, intraluminal mass; type II, extraductal mass; type III, purely solid mass; and type IV, mixed) we found an almost equal number of intraluminal and solid masses in the benign group (21 cases, 36.2%), whereas the malignant group predominantly consisted of solid masses (14 cases (63.6%). The difference in the proportions was statistically significant, therefore, we can conclude that malignant papillary lesions are more likely to appear solid morphologically. However, the results we obtained demonstrates that both benign and malignant lesions have overlap in their appearances, and thus it is not possible to differentiate them solely on ultrasound morphology.
Size was found to be a significant parameter when benign and malignant lesions were compared, with malignant lesions tending to be larger in size. Our findings are similar to that of Kuzmiak et al, who noted that lesions larger than 1 cm tended to have a higher risk of malignancy.[8] This is contrary to findings by Cheng et al who did not find size to be significantly associated with the risk of malignancy.[9]
In our study, apart from size, intralesional vascularity was significantly more commonly seen in malignant papillomas. Figures 1 and 5 demonstrate 2 of the malignant cases with internal vascularity. The presence of Doppler signal within the papilloma has been reported to be commonly seen due to vascularity from a feeding pedicle, and therefore this increased vascularity can be seen even in benign lesions, as seen in Figure 3.[4,5] Characteristic Doppler flow pattern of a vascular pedicle with branching vessels has been described.[6] However, Kim et al study which was similar to ours did not include vascularity in the assessment, and they hypothesized that this feature may actually have a significant impact in the assessment of papillary lesions.[7] More recent studies by Kuzmiak et al and Choi et al also found intralesional vascularity to be a feature more frequently seen in malignant lesions.[9]
Figure 5.
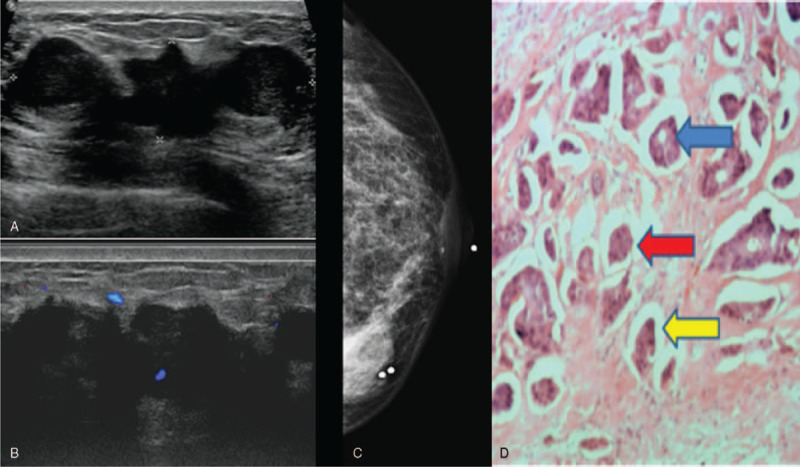
Invasive micropapillary carcinoma solid mass appearance. 49 year old lady presented with left sided nipple discharge. Figure 5A showed a large solid hypoechoic mass which was lobulated with irregular margins. Doppler images demonstrated increased vascularity (Fig. 5B). Mammogram (Fig. 5C) showed a high density mass with irregular margins in the left lower inner quadrant. Histopathological examination (Fig. 5D) demonstrates invasive tumor cells in vague glands [blue arrow] and nests [red arrows], most of which appear within clear spaces [yellow arrow]. (Magnification 40x).
Figure 3.
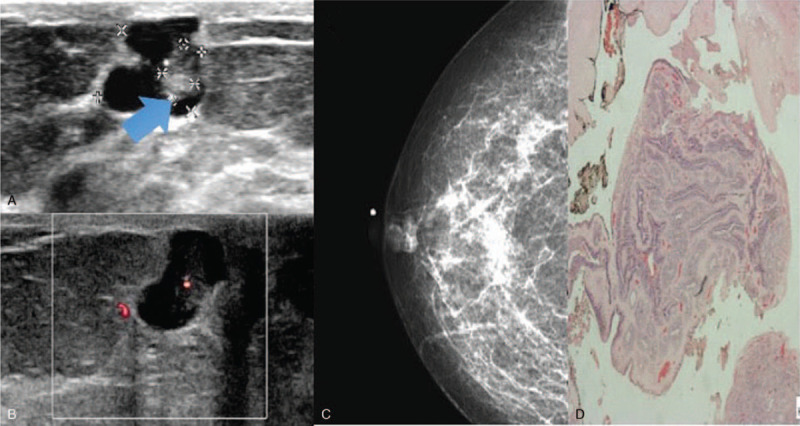
Intraductal papilloma intracystic sonographic appearance. 45 year old lady presented with a mass in the right breast. Figure 3A showed an intraluminal mass with an intracystic appearance, increased vascularity noted on Doppler images (Fig. 3B). Mammogram (Fig. 3C) demonstrated a lobulated high density mass in the right retroareolar region. Histopathological examination (Fig. 3D) demonstrates intraductal papilloma within a duct. (Magnification 20x).
When the data was scrutinized further by removing the atypical group, dilated ducts were found to have statistical significance, with malignant lesions more likely to be associated with the absence of dilated ducts. This was not noted in previous studies and may be explained by infiltration or compression of the ducts by the solid mass causing its collapse. It has been shown that benign lesions including intraductal papillomas are the more common cause of nipple discharge, which may explain the increased likelihood of dilated ducts in benign lesions.[8]
The rest of the BI-RADS sonographic features did not show any association, suggesting that these imaging findings may not assist in differentiating benign from malignant lesions. It is important to note that this result may be due to the small numbers of cases obtained during the time frame of this study. Kuzmiak et al stated that apart from size and vascularity, the features of non-circumscribed margin, hypoechoic pattern, and the presence of posterior features were helpful to differentiate benign and malignant lesions.[10] Choi et al also found boundary and echo patterns to be significant discriminating features on ultrasound.[11] Whereas Kim et al demonstrated that nonparallel orientation, echogenic halo, posterior enhancement and associated calcification were findings attributable to papillary carcinoma, with high sensitivity and positive predictive value when at least one of the features were detected on sonography.[12] Raza et al, in their paper looking at ultrasound of breast masses in general, also stated that nonparallel lesions and echogenic halo were features favoring malignancy.[9]
Our study showed similar results to Kuzmiak et al in terms of size and to both Kuzmiak et al and Choi et al in terms of intralesional vascularity.[8] In their study Lam et al did not show significant features to help predict malignancy during sonographic assessment.[13]
Mammographic assessment demonstrated that the presence of a mass and asymmetric density were features significantly associated with malignancy. In the benign group, most of the papillary lesions did not have any corresponding lesion on mammography, whereas more of the malignant lesions had a mass detectable on mammogram. This could be related to the inherent density of the breast, as more of the patients in the benign group were in the C and D categories, and this was fewer in the malignant group. Denser breasts may obscure the presence of a mass, especially those of lower density.[14] The significance of asymmetric density is likely related to the presence of the mass on mammogram. Although according to BI-RADS criteria, asymmetric density is the presence of a density on mammogram without the definite borders or conspicuity of a mass.[15] Lam et al study also looked at mammographic features of papillary lesions according to BI-RADS criteria but they did not find any feature which could significantly differentiate benignity and malignancy.[1,2,12] Choi et al revealed in their study that lesion visibility and lesion density are factors significantly associated with malignancy on mammography.[16] This study also noted the presence of a mass to be significantly associated with malignancy.
The feature of architectural distortion was almost significant (P = .05) on mammography, suggesting that benign papillary lesions in this population generally do not distort the breast parenchyma. This is a commonly expected phenomenon in benign lesions, as it is usually papillary DCIS or carcinoma which show features of architectural distortion.[9] The remaining mammographic features did not show statistical significance. However, it is of importance to note that most of the papillary lesions, whether benign or malignant demonstrated irregular shape on mammography. Also, a large percentage of benign papillary lesions had indistinct margins. These 2 features add to the common knowledge that the assessment of papillary lesions often cause diagnostic dilemmas due to overlapping features between benign and malignant lesions.[14,17] A meta-analysis investigating factors that could underestimate the risk of malignancy after a nonmalignant biopsy results showed that positive mammographic findings was a significant factor.[18]
In view of the complexity of defining benign and malignant papillary lesions, the authors have also worked on a computer aided diagnosis method to assist with this.[19] A computational method was used to extract features of papillary lesions from ultrasound images which were then used for quantitative image assessment. The model achieved a 98% accuracy and the authors also developed a breast papillary index to further characterize lesions into benign or malignant class. This novel technique is in line with current interest in deep learning and artificial intelligence.
Interobserver agreement for some of the ultrasound and mammography features was performed, and this ranged from moderate agreement to near perfect agreement, thus reflecting that the detection of the features of intraductal papilloma was generally reproducible.
The most important limitation of this study includes the absence of PACS records of some of the patients due to en bloc loss of a large number of patient images during migration of PACS systems. The small number of cases is also a limiting factor, but this may be reflected by the low incidence of papillary lesions in the population, therefore increasing the period of study would be useful.
5. Conclusion
Ultrasonographic and mammographic features play an important role in differentiating benign and malignant papillary lesions and both modalities should be recommended in the assessment of patients with suspected papillary lesions. Size, vascularity and presence of dilated ducts on ultrasound, and the presence of mass as well as asymmetric density on mammogram are features significantly associated to malignant papillary lesions in this study and should be taken into serious consideration when present. Papillary lesions in general require thorough assessment as the presence of papillary lesions is known to be associated with a higher breast carcinoma risk.[17]
Author contributions
Conceptualization: Kartini Rahmat, Farhana Fadzli.
Data curation: Marlina Tanty Ramli, Teoh Kean Hooi, See Mee Hoong, Nur Aishah Mohd Taib.
Formal analysis: Farhana Fadzli, Marlina Tanty Ramli, Teoh Kean Hooi, Ahmad Nazran Fadzli, See Mee Hoong.
Funding acquisition: Kartini Rahmat.
Investigation: Farhana Fadzli, Kartini Rahmat, Marlina Tanty Ramli, Teoh Kean Hooi, Norlisah Mohd Ramli.
Methodology: Farhana Fadzli, Teoh Kean Hooi, Ahmad Nazran Fadzli, See Mee Hoong.
Project administration: Farhana Fadzli, Marlina Tanty Ramli, Faizatul Izza Rozalli.
Supervision: Kartini Rahmat, Faizatul Izza Rozalli, Norlisah Mohd Ramli, Nur Aishah Mohd Taib.
Writing – original draft: Farhana Fadzli.
Writing – review & editing: Farhana Fadzli, Kartini Rahmat, Marlina Tanty Ramli, Faizatul Izza Rozalli, Teoh Kean Hooi, Ahmad Nazran Fadzli, See Mee Hoong, Norlisah Mohd Ramli, Nur Aishah Mohd Taib.
Footnotes
Abbreviations: ACR = American College of Radiology, BI-RADS = breast imaging reporting and data system, DCIS = ductal carcinoma in situ, PACS = picture archiving and communication system.
How to cite this article: Fadzli F, Rahmat K, Ramli MT, Rozalli FI, Hooi TK, Fadzli AN, Hoong SM, Ramli NM, Taib NA. Spectrum of imaging findings of papillary breast disease: a radiopathological review in a tertiary center. Medicine. 2021;100:16(e25297).
This study was funded by University of Malaya Grants: RP052B-17HTM, FP017–2019A, BK052-2017 and FRGS/1/2019/SKK03/UM/01/1.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
P value <.05.
P value <.05 obtained by analyzing benign vs malignant.
Kruskal–Wallis test demonstrated P < .001.
References
- [1].Muttarak M, Lerttumnongtum P, Chaiwun B, et al. Spectrum of papillary lesions of the breast: clinical, imaging, and pathologic correlation. Am J Roentgenol 2008;191:700–7. [DOI] [PubMed] [Google Scholar]
- [2].Eiada R, Chong J, Kulkarni S, et al. Papillary lesions of the breast: MRI, ultrasound, and mammographic appearances. Am J Roentgenol 2012;198:264–71. [DOI] [PubMed] [Google Scholar]
- [3].Ueng S-H, Mezzetti T, Tavassoli FA. Papillary neoplasms of the breast: a review. Archives Pathol Lab Med 2009;133:893–907. [DOI] [PubMed] [Google Scholar]
- [4].Collins L, Schnitt S. Papillary lesions of the breast: selected diagnostic and management issues. Histopathology 2008;52:20–9. [DOI] [PubMed] [Google Scholar]
- [5].Lam WWM, Chu WCW, Tang APY, et al. Role of radiologic features in the management of papillary lesions of the breast. Am J Roentgenol 2006;186:1322–7. [DOI] [PubMed] [Google Scholar]
- [6].Bianchi S, Bendinelli B, Saladino V, et al. Non-malignant breast papillary lesions-B3 diagnosed on ultrasound-guided 14-gauge needle core biopsy: analysis of 114 cases from a single institution and review of the literature. Pathol Oncol Res 2015;21:535–46. [DOI] [PubMed] [Google Scholar]
- [7].Han S-H, Kim M, Chung YR, et al. Benign intraductal papilloma without atypia on core needle biopsy has a low rate of upgrading to malignancy after excision. J Breast Cancer 2018;21:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Han B-K, Choe YH, Ko Y-H, et al. Benign papillary lesions of the breast: sonographic-pathologic correlation. J Ultrasound Med 1999;18:217–23. [DOI] [PubMed] [Google Scholar]
- [9].Kim TH, Kang DK, Kim SY, et al. Sonographic differentiation of benign and malignant papillary lesions of the breast. J Ultrasound Med 2008;27:75–82. [DOI] [PubMed] [Google Scholar]
- [10].Liberman L, Menell JH. Breast imaging reporting and data system (BI-RADS). Radiologic Clin 2002;40:409–30. [DOI] [PubMed] [Google Scholar]
- [11].Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS (Atlas, Breast imaging reporting and data system). 2013;Reston, VA: Am Coll Radiol, 39-48. [Google Scholar]
- [12].Brookes MJ, Bourke AG. Radiological appearances of papillary breast lesions. Clin Radiol 2008;63:1265–73. [DOI] [PubMed] [Google Scholar]
- [13].Lee S-J, Trikha S, Moy L, et al. ACR appropriateness criteria evaluation of nipple discharge. J Am Coll Radiol 2017;14:S138–53. [DOI] [PubMed] [Google Scholar]
- [14].Kuzmiak CM, Lewis MQ, Zeng D, et al. Role of sonography in the differentiation of benign, high-risk, and malignant papillary lesions of the breast. J Ultrasound Med 2014;33:1545–52. [DOI] [PubMed] [Google Scholar]
- [15].Cheng T-Y, Chen C-M, Lee M-Y, et al. Risk factors associated with conversion from nonmalignant to malignant diagnosis after surgical excision of breast papillary lesions. Annals Surg Oncol 2009;16:3375–9. [DOI] [PubMed] [Google Scholar]
- [16].Ganesan S, Karthik G, Joshi M, et al. Ultrasound spectrum in intraductal papillary neoplasms of breast. Br J Radiol 2006;79:843–9. [DOI] [PubMed] [Google Scholar]
- [17].Choi SH, Jo S, Kim D-H, et al. Clinical and imaging characteristics of papillary neoplasms of the breast associated with malignancy: a retrospective cohort study. Ultrasound Med Biol 2014;40:2599–608. [DOI] [PubMed] [Google Scholar]
- [18].Wen X, Cheng W. Nonmalignant breast papillary lesions at core-needle biopsy: a meta-analysis of underestimation and influencing factors. Annals Surg Oncol 2013;20:94–101. [DOI] [PubMed] [Google Scholar]
- [19].Raghavendra U, Koh JEW, Gudigar A, et al. Development of breast papillary index for differentiation of benign and malignant lesions using ultrasound images. J Amb Intel Human Comput 2020;01–9. [Google Scholar]


