Abstract
Background:
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread throughout the world. In this study, we aimed to identify the risk factors for severe COVID-19 to improve treatment guidelines.
Methods:
A multicenter, cross-sectional study was conducted on 313 patients hospitalized with COVID-19. Patients were classified into two groups based on disease severity (nonsevere and severe) according to initial clinical presentation. Laboratory test results and epidemiological and clinical characteristics were analyzed using descriptive statistics. Univariate and multivariate logistic regression models were used to detect potential risk factors associated with severe COVID-19.
Results:
A total of 289 patients (197 nonsevere and 92 severe cases) with a median age of 45.0 (33.0, 61.0) years were included in this study, and 53.3% (154/289) were male. Fever (192/286, 67.1%) and cough (170/289, 58.8%) were commonly observed, followed by sore throat (49/289, 17.0%). Multivariate logistic regression analysis suggested that patients who were aged ≥ 65 years (OR: 2.725, 95% confidence interval [CI]: 1.317–5.636; P = 0.007), were male (OR: 1.878, 95% CI: 1.002–3.520, P = 0.049), had comorbid diabetes (OR: 3.314, 95% CI: 1.126–9.758, P = 0.030), cough (OR: 3.427, 95% CI: 1.752–6.706, P < 0.001), and/or diarrhea (OR: 2.629, 95% CI: 1.109–6.231, P = 0.028) on admission had a higher risk of severe disease. Moreover, stratification analysis indicated that male patients with diabetes were more likely to have severe COVID-19 (71.4% vs. 28.6%, χ2 = 8.183, P = 0.004).
Conclusions:
The clinical characteristics of those with severe and nonsevere COVID-19 were significantly different. The elderly, male patients with COVID-19, diabetes, and presenting with cough and/or diarrhea on admission may require close monitoring to prevent deterioration.
Keywords: Clinical feature, Coronavirus disease 2019, Diabetes, Risk factor, Severe acute respiratory syndrome coronavirus 2
Introduction
Coronavirus disease 2019 (COVID-19) refers to an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has resulted in huge economic losses and already become a global threat. Most patients diagnosed with COVID-19 present with fever and cough, which are the most common symptoms.[1–3] The clinical spectrum of COVID-19 appears to be wide, encompassing severe pneumonia, acute respiratory distress syndrome, or multiple organ failure and death.[1] Although the disease is mild in most cases, the proportion with severe COVID-19 is 24.3%.[4] As of July 22, 2020, the global mortality rate was estimated to be 4.1% according to the WHO,[5] far below that reported with severe acute respiratory syndrome (SARS; more than 10%) and the Middle East respiratory syndrome (>35%).[6] Previous studies of COVID-19 have included single site studies, such as in Wuhan,[1,2,7,8] Beijing,[9] and Shenzhen,[10] and multi-center studies,[3,11–13] primarily focusing on epidemiological and clinical features. Few publications have focused on severe cases specifically. The rapidly increasing number of patients, especially those with severe or critical COVID-19, has created a major public health challenge. It is therefore urgent that critical patients be studied and identified early, medical resources be allocated rationally, and treatment plans be adjusted in a timely manner to enhance the efficacy and reduce the risk of death resulting from COVID-19.
Therefore, this multi-center cross-sectional study was conducted. We analyzed the laboratory test results and epidemiological and clinical characteristics of 313 patients hospitalized with COVID-19 across 12 provinces/municipalities in China to identify risk factors and clinical features associated with severe COVID-19, with the intention of providing guidance for early diagnosis and timely treatment.
Methods
Ethical approval
The protocol for this study was approved by the Institutional Ethics Review Board of Peking University People's Hospital (No. 2020PHB051–01). Written informed consent was waived by the Ethics Review Board due to the urgent need for clinical data collection and investigation.
Study design and participants
In this retrospective study, the sample size was calculated based on the assumed proportion of severe cases among all COVID-19 subjects (assumed to be 0.25 based on a previous study),[4] with a type I error level of 0.05.[14,15] Hence, the sample size was estimated to be 306 for this study.
From February 22 to March 29, 2020, we collected information of 313 patients hospitalized for COVID-19 from 25 designated hospitals across 12 provinces/municipalities in China. During the study period, the management of COVID-19 patients was more standardized than during the onset of the disease, including diagnosis, laboratory tests, and daily documents (eg, case report form). All participants recruited from the hospitals were laboratory-confirmed cases with positive high-throughput sequencing or real-time reverse-transcription polymerase chain reaction (RT-PCR) assay results from respiratory secretions.[16] Patients were classified into four groups (mild, moderate, severe, and critical) according to the “Diagnosis and Treatment Protocol for Novel Coronavirus Infection-Induced Pneumonia Version 6 (Trial)” established by the National Health Commission of the People's Republic of China.[16]
Data collection
Patient data were extracted from electronic medical records using a standardized data collection form, the modified translated version of the WHO/International Severe Acute Respiratory and Emerging Infection Consortium case record form for severe acute respiratory infections.[8] These data, including demographics, clinical features (including smoking history, exposure history, comorbidities, and signs and symptoms), laboratory findings, and radiological characteristics, were collected on the day of hospital admission. Information concerning the treatments applied during hospitalization was collected, and the outcomes were defined as of March 29, 2020.
To secure data validity and quality, the study team and tools were established. A coordinator was designated to manage the local medical records in each hospital. All information was extracted from the medical records by the coordinator and entered into SO JUMP (https://www.wjx.cn/), a professional online platform for designing, distributing, and collecting data. All data were cross-checked by a team of experienced respiratory clinicians. If any core data were missing, a query was immediately sent to the coordinator, who would then contact the patient's attending clinicians and update the data in SO JUMP.
Laboratory procedures and treatments
SARS-CoV-2 RNA was detected by the local centers for disease control and prevention, health institutions, and hospitals. Laboratory confirmation was conducted using real-time RT-PCR.[2] Sputum and throat swab specimens collected from patients were analyzed using real-time RT-PCR for SARS-CoV-2 RNA within three hours. Virus detection was performed twice at least 24 h apart.
Laboratory examinations included a complete blood count, a blood chemistry (including liver and renal function, creatine kinase, and glucose), myocardial enzymes, procalcitonin, and C-reactive protein. Radiological assessments were conducted using chest X-rays or computed tomography (CT). Each patient was examined on the day of admission to the designated hospital.
Noninvasive and invasive ventilation were applied based on the severity of hypoxemia. Other treatments, including the administration of inotropes/vasopressors, neuromuscular blocking agents, renal replacement therapy and the use of extracorporeal membrane oxygenation (ECMO) were also applied for some patients.
Definitions
To better understand the clinical features, and to provide guidance for the early diagnosis and timely treatment of severe COVID-19, we combined mild and moderate cases into the nonsevere group and the severe and critical cases into the severe group. According to the “Diagnosis and Treatment Protocol for Novel Coronavirus Infection-Induced Pneumonia Version 6 (Trial)”,[16] the criteria for discharge were as follows: absence of fever for at least three days, substantial improvement in both lungs on chest CT, clinical remission of respiratory symptoms, and two lower respiratory tract specimens negative for SARS-CoV-2 RNA obtained at least 24 h apart. Exposure history was defined as exposure to live/dead animals, exposure to people with COVID-19 infection, or exposure to people who had recently visited Wuhan.
Statistical analysis
For continuous variables, the Shapiro-Wilk normality test was performed for each. Normally distributed continuous variables are presented as means and standard deviations. Continuous variables that are not normally distributed are presented as medians (Q1, Q3). The t tests or Wilcoxon rank-sum tests were then applied. Categorical variables are summarized as counts and percentages, and χ2 or Fisher exact tests were used. Univariate and multivariate logistic regression models were performed to detect potential risk factors associated with severe COVID-19. The statistically significant risk factors from the univariate logistic regression analysis, along with sex and age, were included in the final multivariate models. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were included in the univariate and multivariate regression analyses. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). The statistical significance level was set at 0.05.
Results
Demographic and clinical characteristics
By March 29, 2020, a total of 313 laboratory-confirmed COVID-19 patients were recruited from 25 hospitals across 12 provinces/municipalities in China. For this study, three with duplicated information, four with missing data (eg, sex, age), and 17 who did not meet the clinical classification criteria were excluded from the final analysis. Therefore, 289 patients were included in this retrospective study [Figure 1].
Figure 1.
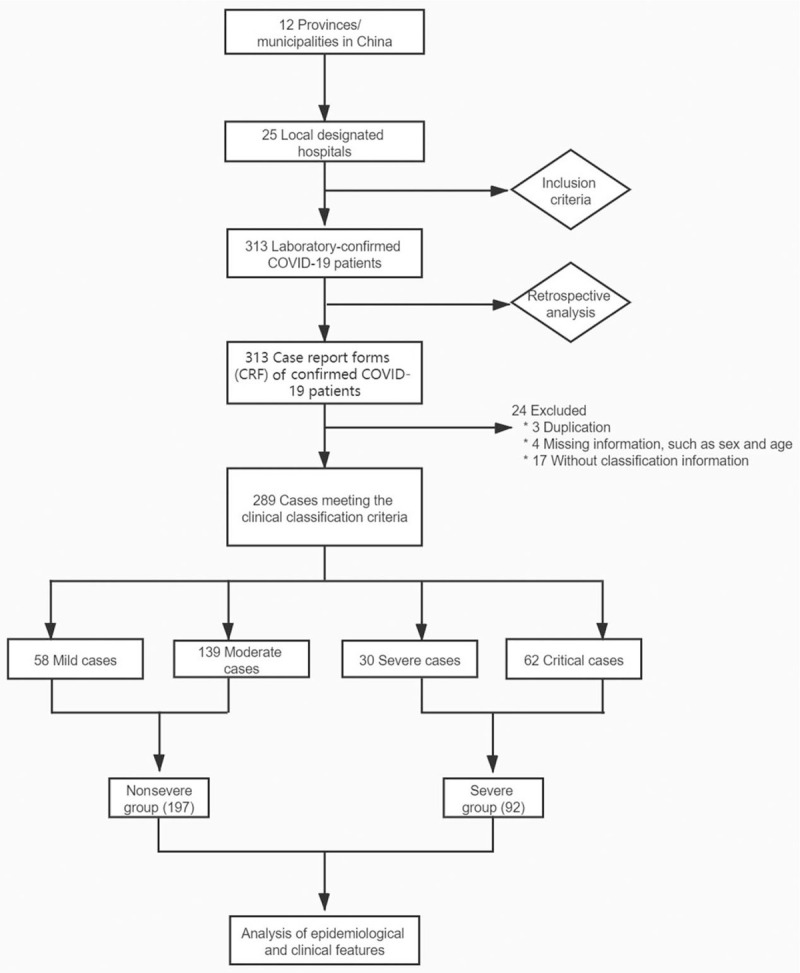
Recruitment flowchart of COVID-19 patient. COVID-19: Coronavirus disease 2019.
The demographic and clinical features of the patients are shown in Table 1. The patients were aged from one month to 91 years, the median age was 45.0 (33.0, 61.0) years, 20.4% (59/289) were aged ≥ 65 years, and there were slightly more male patients (154/289, 53.3%). Of the 289 patients, 98 (33.9%) were residents of Wuhan, Hubei province, 8 (2.8%) were from other areas in Hubei province, and 183 (63.3%) were from outside of Hubei province. A history of recent travel to an epidemic area, contact with confirmed cases of COVID-19, and contact with wildlife was documented in 56.5% (157/278), 50.0% (97/194), and 1.0% (2/208) of the patients, respectively. The most common symptoms in the laboratory-confirmed patients were fever (192/286, 67.1%) and cough (170/289, 58.8%), followed by sore throat (49/289, 17.0%), shortness of breath (42/289, 14.5%), fatigue/malaise (42/289, 14.5%), diarrhea (38/289, 13.1%), myalgia (35/289, 12.1%), headache (30/289, 10.4%), runny nose (23/289, 8.0%), vomiting/nausea (21/289, 7.3%), chest pain (19/289, 6.6%), arthralgia (15/289, 5.2%), wheezing (11/289, 3.8%), bleeding (6/289, 2.1%), and abdominal pain (5/289, 1.7%). Only one patient (1/289, 0.3%) developed altered consciousness. Moreover, the most common comorbidity was diabetes (25/289, 8.7%), followed by chronic cardiovascular disease (18/289, 6.2%) and chronic pulmonary disease (12/289, 4.2%).
Table 1.
Characteristics of patients with COVID-19.
| Characteristics | All patients (n = 289) | Nonsevere (n = 197) | Severe (n = 92) | Statistics | P |
| Age (years) | 45.0 (33.0, 61.0) | 40.0 (30.0, 50.0) | 60.0 (45.5, 69.5) | 43.912∗ | < 0.001 |
| Age (years) | – | <0.001 | |||
| 0–14 | 8 (2.8) | 7 (3.6) | 1 (1.1) | ||
| 15–29 | 48 (16.6) | 41 (20.8) | 7 (7.6) | ||
| 30–49 | 115 (39.8) | 93 (47.2) | 22 (23.9) | ||
| 50–64 | 59 (20.4) | 35 (17.8) | 24 (26.1) | ||
| ≥ 65 | 59 (20.4) | 21 (10.7) | 38 (41.3) | ||
| Sex | 3.117† | 0.077 | |||
| Male | 154 (53.3) | 98 (49.7) | 56 (60.9) | ||
| Female | 135 (46.7) | 99 (50.3) | 36 (39.1) | ||
| Smoking history | 7 (2.4) | 2 (1.0) | 5 (5.4) | – | 0.035 |
| Healthcare worker | 23/233 (9.9) | 22/177 (12.4) | 1/56 (1.8) | 5.417† | 0.020 |
| Region of residence | – | 0.001 | |||
| Wuhan | 98 (33.9) | 58 (29.4) | 40 (43.5) | ||
| Other cities in Hubei Province | 8 (2.8) | 2 (1.0) | 6 (6.5) | ||
| Cities outside Hubei Province | 183 (63.3) | 137 (69.5) | 46 (50.0) | ||
| Exposure history | |||||
| Recently visited epidemic area | 157/278 (56.5) | 102/192 (53.1) | 55/86 (64.0) | 2.833† | 0.092 |
| Contact with confirmed cases | 97/194 (50.0) | 84/153 (54.9) | 13/41 (31.7) | 6.958† | 0.008 |
| Contact with wildlife | 2/208 (1.0) | 2/155 (1.3) | 0 (0) | – | 1.000 |
| Comorbidity | |||||
| Diabetes | 25 (8.7) | 9 (4.6) | 16 (17.4) | 13.049† | <0.001 |
| Chronic cardiac disease | 18 (6.2) | 5 (2.5) | 13 (14.1) | 14.430† | <0.001 |
| Chronic pulmonary disease | 12 (4.2) | 5 (2.5) | 7 (7.6) | – | 0.058 |
| Chronic kidney disease | 5 (1.7) | 1 (0.5) | 4 (4.3) | – | 0.037 |
| Malignant neoplasm | 5 (1.7) | 3 (1.5) | 2 (2.2) | – | 0.655 |
| Chronic hematologic disease | 3 (1.0) | 0 (0) | 3 (3.3) | – | 0.032 |
| Chronic liver disease | 3 (1.0) | 3 (1.5) | 0 (0) | – | 0.554 |
| Obesity | 3 (1.0) | 2 (1.0) | 1 (1.1) | – | 1.000 |
| Dementia | 2 (0.7) | 2 (1.0) | 0 (0) | – | 1.000 |
| Malnutrition | 1 (0.3) | 0 (0) | 1 (1.1) | – | 0.318 |
| Signs and symptoms | |||||
| Fever | 192/286 (67.1) | 141/194 (72.7) | 53/92 (57.6) | 6.497† | 0.011 |
| Cough | 170 (58.8) | 99 (50.3) | 71 (77.2) | 18.763† | <0.001 |
| Sore throat | 49 (17.0) | 37 (18.8) | 12 (13.0) | 1.467† | 0.226 |
| Fatigue/malaise | 42 (14.5) | 29 (14.7) | 13 (14.1) | 0.018† | 0.894 |
| Shortness of breath | 42 (14.5) | 20 (10.2) | 22 (23.9) | 9.561† | 0.002 |
| Diarrhea | 38 (13.1) | 20 (10.2) | 18 (19.6) | 4.866† | 0.027 |
| Myalgia | 35 (12.1) | 21 (10.7) | 14 (15.2) | 1.224† | 0.269 |
| Headache | 30 (10.4) | 18 (9.1) | 12 (13.0) | 1.029† | 0.310 |
| Rhinitis | 23 (8.0) | 18 (9.1) | 5 (5.4) | 1.173† | 0.279 |
| Vomiting/nausea | 21 (7.3) | 15 (7.6) | 6 (6.5) | 0.111† | 0.739 |
| Chest pain | 19 (6.6) | 16 (8.1) | 3 (3.3) | 2.413† | 0.120 |
| Arthralgia | 15 (5.2) | 13 (6.6) | 2 (2.2) | – | 0.157 |
| Wheezing | 11 (3.8) | 6 (3.0) | 5 (5.4) | – | 0.335 |
| Bleeding | 6 (2.1) | 5 (2.5) | 1 (1.1) | – | 0.668 |
| Abdominal pain | 5 (1.7) | 3 (1.5) | 2 (2.2) | – | 0.655 |
| Altered consciousness | 1 (0.3) | 1 (0.5) | 0 (0) | – | 1.000 |
| Heart rate (>100 beats per min) | 45/256 (17.6) | 28/170 (16.5) | 17/86 (19.8) | 0.428† | 0.513 |
| Respiratory rate (>24 breaths per min) | 19/258 (7.4) | 2/172 (1.2) | 17/86 (19.8) | 29.090† | <0.001 |
| SaO2 (>93%) | 206/238 (86.6) | 148/148 (100.0) | 58/90 (64.4) | 60.797† | <0.001 |
| PaO2/FiO2 (>300 mmHg) | 43/69 (62.3) | 38/38 (100.0) | 5/31 (16.1) | 51.142† | <0.001 |
Data are presented as median (Q1, Q3), n (%), or n/N (%). COVID-19: Coronavirus disease 2019; PaO2/FiO2: Arterial partial pressure of oxygen/fraction of inspired oxygen; SaO2: Arterial oxygen saturation; –: Not applicable. ∗Z values. †χ2 values.
As indicated in Table 1, 197 (68.2%) and 92 (31.8%) of the patients were classified into the nonsevere and severe group, respectively. Between the two groups, the following parameters were significantly different: age (40.0 [30.0, 50.0] years vs. 60.0 [45.5, 69.5] years, Z = 43.912, P < 0.001); region of residence (P = 0.001); prevalence of contact with confirmed cases (54.9% vs. 31.7%, χ2 = 6.958, P = 0.008); presence of cough (50.3% vs. 77.2%, χ2 = 18.763, P < 0.001), shortness of breath (10.2% vs. 23.9%, χ2 = 9.561, P = 0.002), and diarrhea (10.2% vs. 19.6%, χ2 = 4.866, P = 0.027); arterial oxygen saturation (SaO2) > 93% (100.0% vs. 64.4%, χ2 = 60.797, P < 0.001); and arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) > 300 mm Hg (100.0% vs. 16.1%, χ2 = 51.142, P < 0.001). Compared to the nonsevere group, the patients in the severe group more commonly had comorbidities, including diabetes (17.4% vs. 4.6%, χ2 = 13.049, P < 0.001), chronic cardiac disease (14.1% vs. 2.5%, χ2 = 14.430, P < 0.001), chronic kidney disease (4.3% vs. 0.5%, P = 0.037), and chronic hematologic disease (3.3% vs. 0, P = 0.032). Tachypnea (respiratory rate > 24 breaths per min) (19.8% vs. 1.2%, χ2 = 29.090, P < 0.001) and a history of smoking (5.4% vs. 1.0%, P = 0.035) were also more common in the severe group than in the nonsevere group.
Laboratory and radiological findings
The laboratory and radiological findings of patients diagnosed with COVID-19 are listed in Table 2. Most patients (164/239, 68.6%) had normal peripheral white blood cell counts [(4–9.9)× 109/L] on admission. Lymphopenia was observed in 40.8% (95/233) of the patients. Procalcitonin < 0.5 ng/mL and C-reactive protein ≥ 10 mg/mL occurred in 76.4% (84/110) and 57.0% (90/158) of the patients, respectively. Severe cases had more prominent laboratory abnormalities, such as reduced hemoglobin levels (64.9% vs. 45.8%, χ2 = 6.397, P = 0.011), lymphopenia (54.2% vs. 33.3%, χ2 = 9.650, P = 0.002) and elevated creatinine (11.6% vs. 2.1%, P = 0.019), blood urea nitrogen (18.4% vs. 5.2%, P = 0.038), glucose (42.9% vs. 25.3%, χ2 = 4.166, P = 0.041), procalcitonin (40.8% vs. 9.8%, χ2 = 14.449, P < 0.001), and C-reactive protein (71.2% vs. 48.5%, χ2 = 7.772, P = 0.005) levels, as compared with nonsevere cases.
Table 2.
Laboratory and radiological findings of patients with COVID-19.
| Items | All patients (n = 289) | Nonsevere (n = 197) | Severe (n = 92) | χ2 | P |
| Laboratory findings | |||||
| Hemoglobin (g/L) | 6.397 | 0.011 | |||
| <130 | 97/181 (53.6) | 49/107 (45.8) | 48/74 (64.9) | ||
| ≥130 | 84/181 (46.4) | 58/107 (54.2) | 26/74 (35.1) | ||
| White blood cell count (×109/L) | 4.552 | 0.103 | |||
| <4 | 57/239 (23.8) | 44/158 (27.8) | 13/81 (16.0) | ||
| 4–9.9 | 164/239 (68.6) | 104/158 (65.8) | 60/81 (74.1) | ||
| ≥10 | 18/239 (7.5) | 10/158 (6.3) | 8/81 (9.9) | ||
| Lymphocyte count (×109/L) | 9.650 | 0.002 | |||
| <1 | 95/233 (40.8) | 50/150 (33.3) | 45/83 (54.2) | ||
| ≥1 | 138/233 (59.2) | 100/150 (66.7) | 38/83 (45.8) | ||
| Neutrophil count (×109/L) | 2.336 | 0.126 | |||
| <1.8 | 29/209 (13.9) | 22/132 (16.7) | 7/77 (9.1) | ||
| ≥1.8 | 180/209 (86.1) | 110/132 (83.3) | 70/77 (90.9) | ||
| Platelet count (×109/L) | – | 0.070 | |||
| <100 | 8/184 (4.3) | 2/107 (1.9) | 6/77 (7.8) | ||
| ≥100 | 176/184 (95.7) | 105/107 (98.1) | 71/77 (92.2) | ||
| Alanine aminotransferase (U/L) | 2.043 | 0.153 | |||
| <40 | 147/196 (75.0) | 92/117 (78.6) | 55/79 (69.6) | ||
| ≥40 | 49/196 (25.0) | 25/117 (21.4) | 24/79 (30.4) | ||
| Aspartate aminotransferase (U/L) | 3.258 | 0.071 | |||
| <40 | 144/178 (80.9) | 88/103 (85.4) | 56/75 (74.7) | ||
| ≥40 | 34/178 (19.1) | 15/103 (14.6) | 19/75 (25.3) | ||
| Total bilirubin (μmol/L) | 0.724 | 0.395 | |||
| <17.1 | 136/170 (80.0) | 79/96 (82.3) | 57/74 (77.0) | ||
| ≥17.1 | 34/170 (20.0) | 17/96 (17.7) | 17/74 (23.0) | ||
| Creatinine (μmol/L) | – | 0.019 | |||
| <133 | 153/163 (93.9) | 92/94 (97.9) | 61/69 (88.4) | ||
| ≥133 | 10/163 (6.1) | 2/94 (2.1) | 8/69 (11.6) | ||
| Blood urea nitrogen (mmol/L) | – | 0.038 | |||
| <7.5 | 122/134 (91.0) | 91/96 (94.8) | 31/38 (81.6) | ||
| ≥7.5 | 12/134 (9.0) | 5/96 (5.2) | 7/38 (18.4) | ||
| Glucose (mmol/L) | 4.166 | 0.041 | |||
| <7.0 | 92/133 (69.2) | 68/91 (74.7) | 24/42 (57.1) | ||
| ≥7.0 | 41/133 (30.8) | 23/91 (25.3) | 18/42 (42.9) | ||
| Creatine kinase (U/L) | 3.160 | 0.075 | |||
| <185 | 133/151 (88.1) | 81/88 (92.0) | 52/63 (82.5) | ||
| ≥185 | 18/151 (11.9) | 7/88 (8.0) | 11/63 (17.5) | ||
| Myoglobin (ng/mL) | 2.854 | 0.091 | |||
| <75 | 60/79 (75.9) | 29/34 (85.3) | 31/45 (68.9) | ||
| ≥75 | 19/79 (24.1) | 5/34 (14.7) | 14/45 (31.1) | ||
| Troponin T (ng/mL) | 0.315 | 0.574 | |||
| <0.1 | 36/50 (72.0) | 20/29 (69.0) | 16/21 (76.2) | ||
| ≥0.1 | 14/50 (28.0) | 9/29 (31.0) | 5/21 (23.8) | ||
| Procalcitonin (ng/mL) | 14.449 | <0.001 | |||
| <0.5 | 84/110 (76.4) | 55/61 (90.2) | 29/49 (59.2) | ||
| ≥0.5 | 26/110 (23.6) | 6/61 (9.8) | 20/49 (40.8) | ||
| C-reactive protein (mg/L) | 7.772 | 0.005 | |||
| <10 | 68/158 (43.0) | 51/99 (51.5) | 17/59 (28.8) | ||
| ≥10 | 90/158 (57.0) | 48/99 (48.5) | 42/59 (71.2) | ||
| Radiological findings | |||||
| Ground-glass opacity | 184/211 (87.2) | 133/151 (88.1) | 51/60 (85.0) | 0.365 | 0.546 |
| Infiltration | 136/184 (73.9) | 97/135 (71.9) | 39/49 (79.6) | 1.117 | 0.291 |
| Air bronchogram | 59/184 (32.1) | 43/136 (31.6) | 16/48 (33.3) | 0.048 | 0.827 |
| Interlobular septal thickening | 57/185 (30.8) | 41/137 (29.9) | 16/48 (33.3) | 0.193 | 0.660 |
| Reversed halo sign | 27/182 (14.8) | 24/136 (17.6) | 3/46 (6.5) | 3.367 | 0.066 |
| Mosaic sign | 16/183 (8.7) | 14/137 (10.2) | 2/46 (4.3) | – | 0.365 |
| Tractive bronchiectasis | 8/184 (4.3) | 7/136 (5.1) | 1/48 (2.1) | – | 0.683 |
Data are presented as n/N (%). COVID-19: Coronavirus disease 2019; –: Not applicable.
Although there were no statistically significant differences in chest CT or radiography results between the severe and nonsevere cases, for patients infected with COVID-19, the most common patterns on chest CT were ground-glass opacities (184/211, 87.2%) and infiltration (136/184, 73.9%), followed by air bronchogram (59/184, 32.1%) and interlobular septal thickening (57/185, 30.8%). The chest CTs of two patients are shown in Supplementary Figure 1.
Treatments and outcomes
In our study, three kinds of anti-infection medications were used to treat patients with COVID-19 [Table 3]. Antivirals, antibiotics, and antifungals were used in 95.8% (253/264), 62.2% (155/249) and 8.1% (20/247) of patients, respectively. Antibiotics (67.4% vs. 49.3%, χ2 = 7.091, P = 0.008) were used more often in nonsevere group, while antifungal agents (21.6% vs. 2.3%, χ2 = 25.969, P < 0.001) were used more often in the severe group. In addition, noninvasive and invasive mechanical ventilation, renal replacement therapy, inotropes/vasopressors, neuromuscular blocking agents, and ECMO were only used in the severe group.
Table 3.
Treatments and outcomes of patients with COVID-19.
| Items | All patients (n = 289) | Nonsevere (n = 197) | Severe (n = 92) | χ2 | P |
| Treatments | |||||
| Antiviral treatment | 253/264 (95.8) | 168/177 (94.9) | 85/87 (97.7) | – | 0.348 |
| Antibiotic treatment | 155/249 (62.2) | 120/178 (67.4) | 35/71 (49.3) | 7.091 | 0.008 |
| Antifungal treatment | 20/247 (8.1) | 4/173 (2.3) | 16/74 (21.6) | 25.969 | <0.001 |
| Noninvasive ventilation | 62/270 (23.0) | 0 (0) | 62/89 (69.7) | ||
| Invasive ventilation | 6/270 (2.2) | 0 (0) | 6/89 (6.7) | ||
| Renal replacement therapy | 4/270 (1.5) | 0 (0) | 4/89 (4.5) | ||
| Inotropes/vasopressors | 4/270 (1.5) | 0 (0) | 4/89 (4.5) | ||
| Neuromuscular blocking agents | 4/270 (1.5) | 0 (0) | 4/89 (4.5) | ||
| ECMO | 2/270 (0.7) | 0 (0) | 2/89 (2.2) | ||
| Outcomes | – | 0.002 | |||
| Discharged alive | 154/218 (70.6) | 112/148 (75.7) | 42/70 (60.0) | ||
| Hospitalization | 51/218 (23.4) | 30/148 (20.3) | 21/70 (30.0) | ||
| Transfer to other facility | 8/218 (3.7) | 6/148 (4.1) | 2/70 (2.9) | ||
| Death | 5/218 (2.3) | 0/148 (0) | 5/70 (7.1) | ||
Data are presented as n/N (%). COVID-19: Coronavirus disease 2019; ECMO: Extracorporeal membrane oxygenation; –: Not applicable.
As of March 29, 2020, five patients had died during hospitalization, 154 patients were discharged, 51 patients were still hospitalized, and eight patients were transferred to other designated hospitals [Table 3]. The percentages of patients who were discharged in nonsevere and severe groups were 75.7% (112/148) and 60.0% (42/70), respectively, while the percentages of patients who were still hospitalized were 20.3% (30/148) vs. 30.0% (21/70), who had transferred to other facilities were 4.1% (6/148) vs. 2.9% (2/70), and who had died were 0 vs. 7.1% (5/70), respectively, in nonsevere group and severe group.
Risk factors for patients with severe COVID-19
The univariate analysis results [Table 4] showed that older age (≥ 65 years; OR, 5.898; 95% CI: 3.192–10.900; P < 0.001), and smoking history (OR, 5.603; 95% CI: 1.066–29.450; P = 0.042) were associated with severe COVID-19, and being a health care worker (OR, 0.128; 95% CI: 0.017–0.973; P = 0.047), being a resident in cities outside Hubei province (OR, 0.487; 95% CI: 0.289–0.822; P = 0.007), having close contact with confirmed patients (OR, 0.381; 95% CI: 0.184–0.792; P = 0.010) were less likely to be associated with severe COVID-19. Patients with chronic cardiac disease (OR, 6.319; 95% CI: 2.180–18.310; P = 0.001) and diabetes (OR, 4.398; 95% CI: 1.863–10.380; P = 0.001) were also more likely to be in the severe group. In addition, the presence of fever (OR, 0.511; 95% CI: 0.304–0.859; P = 0.011), cough (OR, 3.347; 95% CI: 1.909–5.867; P < 0.001), respiratory rate > 24 breaths per min (OR, 20.933; 95% CI: 4.711–93.067; P < 0.001), and some laboratory results, including higher hemoglobin (OR, 0.458; 95% CI: 0.249–0.842; P = 0.012) and lymphocyte count (OR, 0.422; 95% CI: 0.244–0.731; P = 0.002) were associated with the severity of COVID-19; and elevated glucose (OR, 2.218; 95% CI: 1.024–4.802; P = 0.043), blood urea nitrogen (OR, 4.110; 95% CI: 1.216–13.890; P = 0.023), creatinine (OR, 6.033; 95% CI: 1.239–29.370; P = 0.026), procalcitonin (OR, 6.322; 95% CI: 2.286–17.480; P < 0.001), and C-reactive protein levels (OR, 2.625; 95% CI: 1.320–5.221; P = 0.006) were associated with severe COVID-19.
Table 4.
Risk factors associated with severe COVID-19.
| Univariate | Multivariate | |||||
| Parameters | OR | 95% CI | P | OR | 95% CI | P |
| Baseline characteristics | ||||||
| Age (≥ 65 years vs. < 65 years) | 5.898 | 3.192–10.900 | <0.001 | 2.725 | 1.317–5.636 | 0.007 |
| Sex (male vs. female) | 1.571 | 0.950–2.599 | 0.078 | 1.878 | 1.002–3.520 | 0.049 |
| Smoking history (yes vs. no) | 5.603 | 1.066–29.450 | 0.042 | 2.252 | 0.360–14.090 | 0.385 |
| Healthcare worker (yes vs. no) | 0.128 | 0.017–0.973 | 0.047 | |||
| Region of residence | ||||||
| Wuhan | 1.000 | – | – | |||
| Other cities in Hubei Province | 4.350 | 0.835–22.660 | 0.081 | |||
| Cities outside Hubei Province | 0.487 | 0.289–0.822 | 0.007 | |||
| Exposure history | ||||||
| Recently visited epidemic area (yes vs. no) | 1.565 | 0.927–2.642 | 0.093 | 1.638 | 0.870–3.084 | 0.126 |
| Contact with confirmed patients (yes vs. no) | 0.381 | 0.184–0.792 | 0.010 | |||
| Comorbidities | ||||||
| Diabetes (yes vs. no) | 4.398 | 1.863–10.380 | 0.001 | 3.314 | 1.126–9.758 | 0.030 |
| Chronic cardiac disease (yes vs. no) | 6.319 | 2.180–18.310 | 0.001 | 3.533 | 0.989–12.642 | 0.052 |
| Chronic pulmonary disease (yes vs. no) | 3.162 | 0.976–10.250 | 0.055 | |||
| Chronic kidney disease (yes vs. no) | 8.909 | 0.982–80.860 | 0.052 | |||
| Malignant neoplasm (yes vs. no) | 1.437 | 0.236–8.751 | 0.694 | |||
| Obesity (yes vs. no) | 1.072 | 0.096–11.970 | 0.955 | |||
| Signs and symptoms | ||||||
| Fever (yes vs. no) | 0.511 | 0.304–0.859 | 0.011 | 0.541 | 0.278–1.051 | 0.070 |
| Cough (yes vs. no) | 3.347 | 1.909–5.867 | <0.001 | 3.427 | 1.752–6.706 | <0.001 |
| Sore throat (yes vs. no) | 0.649 | 0.321–1.312 | 0.228 | |||
| Fatigue/malaise (yes vs. no) | 0.954 | 0.470–1.933 | 0.895 | |||
| Shortness of breath (yes vs. no) | 2.781 | 1.429–5.411 | 0.003 | 1.326 | 0.557–3.156 | 0.523 |
| Diarrhea (yes vs. no) | 2.153 | 1.077–4.301 | 0.030 | 2.629 | 1.109–6.231 | 0.028 |
| Myalgia (yes vs. no) | 1.504 | 0.727–3.112 | 0.271 | |||
| Headache (yes vs. no) | 1.492 | 0.686–3.243 | 0.313 | |||
| Rhinitis (yes vs. no) | 0.572 | 0.205–1.590 | 0.284 | |||
| Vomiting/nausea (yes vs. no) | 0.847 | 0.317–2.257 | 0.739 | |||
| Chest pain (yes vs. no) | 0.381 | 0.108–1.343 | 0.133 | |||
| Arthralgia (yes vs. no) | 0.315 | 0.070–1.424 | 0.133 | |||
| Wheezing (yes vs. no) | 1.830 | 0.544–6.158 | 0.329 | |||
| Bleeding (yes vs. no) | 0.422 | 0.049–3.664 | 0.434 | |||
| Abdominal pain (yes vs. no) | 1.437 | 0.236–8.751 | 0.694 | |||
| Respiratory rate (>24 breaths per min vs. ≤24 breaths per min) | 20.933 | 4.711–93.067 | <0.001 | |||
| Heart rate (>100 beats per min vs. ≤100 beats per min) | 1.249 | 0.641–2.436 | 0.513 | |||
| Laboratory parameters | ||||||
| Hemoglobin (≥130 g/dL vs. <130 g/dL) | 0.458 | 0.249–0.842 | 0.012 | |||
| WBC count (4.0 × 109–9.9 × 109/L vs. <4.0 × 109/L) | 1.953 | 0.974–3.915 | 0.059 | |||
| WBC count (≥10.0 × 109/L vs. <4.0 × 109/L) | 1.387 | 0.519–3.704 | 0.514 | |||
| Lymphocyte count (≥1 × 109/L vs. <1 × 109/L) | 0.422 | 0.244–0.731 | 0.002 | |||
| Neutrophil count (≥1.8 × 109/L vs. <1.8 × 109/L) | 2.000 | 0.812–4.928 | 0.132 | |||
| Platelet count (≥100 × 109/L vs. <100 × 109/L) | 0.225 | 0.044–1.149 | 0.073 | |||
| Alanine aminotransferase (≥40 U/L vs. <40 U/L) | 1.606 | 0.836–3.083 | 0.155 | |||
| Aspartate aminotransferase (≥40 U/L vs. <40 U/L) | 1.990 | 0.935–4.236 | 0.074 | |||
| Total bilirubin (≥17.1 mmol/L vs. <17.1 mmol/L) | 1.386 | 0.652–2.945 | 0.396 | |||
| Creatinine (≥133 μmol/L vs. <133 μmol/L) | 6.033 | 1.239–29.370 | 0.026 | |||
| Blood urea nitrogen (≥7.5 mmol/L vs. <7.5 mmol/L) | 4.110 | 1.216–13.890 | 0.023 | |||
| Glucose (≥7.0 mmol/L vs. <7.0 mmol/L) | 2.218 | 1.024–4.802 | 0.043 | |||
| Creatine kinase (≥185 U/L vs.<185 U/L) | 2.448 | 0.892–6.718 | 0.082 | |||
| Myoglobin (≥75 ng/mL vs. <75 ng/mL) | 2.619 | 0.838–8.188 | 0.098 | |||
| Troponin T (≥0.1 ng/mL vs. <0.1 ng/mL) | 0.694 | 0.194–2.487 | 0.575 | |||
| Procalcitonin (≥0.5 ng/mL vs. <0.5 ng/mL) | 6.322 | 2.286–17.480 | <0.001 | |||
| C-reactive protein (≥10 mg/L vs. <10 mg/L) | 2.625 | 1.320–5.221 | 0.006 | |||
CI: Confidence interval; COVID-19: Coronavirus disease 2019; OR: Odds ratio; WBC: White blood cell; –: Not applicable.
To further analyze the risk factors for severe COVID-19, multivariate regression models were used. The results indicated that being ≥ 65 years old (OR, 2.725; 95% CI: 1.317–5.636; P = 0.007), male (OR, 1.878; 95% CI: 1.002–3.520; P = 0.049), having comorbid diabetes (OR, 3.314; 95% CI: 1.126–9.758; P = 0.030) or chronic cardiac disease (OR, 3.533; 95% CI: 0.989–12.642; P = 0.052, marginal significance), and presenting with cough (OR, 3.427; 95% CI: 1.752–6.706; P < 0.001) and/or diarrhea (OR, 2.629; 95% CI: 1.109–6.231; P = 0.028) on admission were significantly positively correlated with severe COVID-19. Moreover, COVID-19 patients with and without comorbid diabetes were stratified by sex for both groups [Supplementary Table 1], and male patients with diabetes were found to be more likely to develop severe COVID-19 (71.4% vs. 28.6%; χ2 = 8.183; P = 0.004).
Discussion
This multi-center retrospective study covered 313 laboratory-confirmed COVID-19 patients from 25 hospitals across 12 provinces/municipalities in China. Our study included patients from a vast area of China, not only Wuhan, and the definition of COVID-19 from Diagnosis and Treatment Protocol for Novel Coronavirus Infection-Induced Pneumonia Version 6 (Trial) was used. However, the cases were divided into two groups instead of four (severe vs. nonsevere) according to our study objective, which was to better understand the clinical features of COVID-19 and provide guidance to improve early diagnosis and timely treatment of severe cases.
A total of 40.8% of patients in this study were aged ≥ 50 years. This finding is consistent with previous studies, which have shown that older age is as an important predictor of severe illness or even death in COVID-19[1,8,17] and is similar to the findings with SARS[18] and Middle East Respiratory Syndrome.[19] Since the majority of the elderly population have weak immune function, proinflammatory responses may be prolonged and the risk of serious outcomes may be increased.[20] Among the 289 cases included in this study, we also observed that COVID-19 was more frequently diagnosed in male than in female, consistent with recent COVID-19 cases.[3,7] Additionally, Jaillon et al[21] have reported that the X chromosome and sex hormones play a role in reducing susceptibility to viral, bacterial, and fungal infections in female. In addition, 50% of patients diagnosed with COVID-19 in this study had a history of exposure to confirmed cases, which is consistent with previous studies showing that COVID-19 infection is spread by human-to-human transmission.[1,3,17]
Consistent with other studies,[7,8,17] severe COVID-19 was found to occur more often in those with comorbid chronic cardiac disease or diabetes in our study. It has also been associated with more serious outcomes in influenza and other respiratory viral infections.[22] Patients with diabetes usually have weaker immune function,[23] suggesting that daily blood glucose control and supportive immune system therapies should be maintained to effectively reduce the severity of this disease in these patients.
The most two common symptoms in this study were fever and cough, which is consistent with previous findings.[1,3] In our study, presenting with fever, cough, shortness of breath, diarrhea, and a higher respiratory rate (>24 breaths per min) were more frequently found in severe COVID-19 cases than in nonsevere cases. These results suggest that patients with these symptoms on admission should be closely monitored to achieve better outcomes.
In terms of laboratory tests, lymphopenia was more commonly observed in patients with severe COVID-19 in this study. This was consistent with a previous study,[17] which suggested that SARS-CoV-2 might selectively attack lymphocytes, initiating a series of immune responses. Moreover, higher levels of glucose, blood urea nitrogen, and creatinine were observed in severe COVID-19 cases, which was consistent with the finding that patients with diabetes are more likely to have severe COVID-19. Analogous to the report by Guan et al,[3] we found that patients with severe COVID-19 were more likely to have procalcitonin ≥0.5 ng/mL and C-reactive protein ≥10 mg/L, suggesting the potential for serious outcomes from severe COVID-19. Hence, early identification and timely treatment of severe cases is essential.
Antiviral drugs and antibiotics were more commonly used for patients with COVID-19 in this study, which was similar to some previous findings.[1,2,17] Antibiotics were used more often in nonsevere COVID-19 cases, whereas antifungal drugs were used more in severe COVID-19 cases. Other medications administered more in severe COVID-19 cases included inotropes/vasopressors and neuromuscular blocking agents. This suggests that these drugs should be available for prompt treatment in COVID-19 patients. Furthermore, Richardson et al[24] reported that baricitinib may be a potential treatment option for COVID-19, and Wang et al[25] found that remdesivir and chloroquine could effectively inhibit SARS-CoV-2 in vitro. However, Wang et al[26] carried out a randomized, double-blind, placebo-controlled, multicenter trial with severe COVID-19 patients and the results showed that remdesivir was not significantly associated with clinical benefits. In the current study, 62 patients with severe COVID-19 received noninvasive ventilation and 6 patients with severe COVID-19 received invasive ventilation, whereas no patients with nonsevere COVID-19 received either treatment. Renal replacement therapy and ECMO were also only used in severe COVID-19 patients. Based on the above results, we suggest that comprehensive therapies are necessary in patients with severe COVID-19.
This study, however, has some limitations. First, due to the heavy workload of front-line physicians, not all patients hospitalized for COVID-19 during the study period from these 25 hospitals were included. Additionally, a much more comprehensive understanding of COVID-19 could have been obtained if patients from other provinces/municipalities in China were included. Second, since our study was conducted soon after the onset of COVID-19, a full picture of the disease could not be obtained. Therefore, the 289 cases included in this study might not be generalizable, especially since there were few mild or asymptomatic cases included in this study. Hence, the study might be biased towards more severe COVID-19. Third, in this study, the valid response rates were 80.6% (233/289) and 67.1% (194/289) for whether being a healthcare worker and having closely contact with confirmed cases, respectively. There might be a potential bias for the analysis of those two risk factors. Therefore, interpretation of these two risk factors needs to be cautious. Further observations of the natural history of COVID-19 are needed, particularly from outbreaks outside of China, to obtain more information on the epidemiological and clinical features of COVID-19.
In conclusion, we identified that fever and cough were the most two common symptoms in patients with COVID-19, while the latter was more likely to be associated with severe COVID-19. Elevated procalcitonin level (≥0.5 ng/mL) and C-reactive protein (≥10 mg/L) were also potential risk factors for severe COVID-19. Patients with diabetes, especially men, more often developed severe COVID-19, potentially resulting in poor clinical prognosis. Therefore, further investigations that include rigorous observations and comprehensive therapies for patients diagnosed with severe COVID-19 are necessary.
Funding
This project was supported by the grants from Emergency Research Project on COVID-19 Prevention and Control, Xiamen University (Nos. 20720200017 and 20720200032).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Jiang N, Liu YN, Bao J, Li R, Ni WT, Tan XY, Xu Y, Peng LP, Wang XR, Zeng YM, Liu DS, Xue Q, Li JS, Hu K, Zheng YL, Gao ZC. Clinical features and risk factors associated with severe COVID-19 patients in China. Chin Med J 2021;134:944–953. doi: 10.1097/CM9.0000000000001466
Supplemental digital content is available for this article.
References
- 1.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun PF, Qie SY, Liu ZJ, Ren JZ, Xi JN. Clinical characteristics of 5732 patients with 2019-nCoV infection. SSRN Electronic Journal 2020. Available from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3539664. [Accessed February 6, 2021]. [Google Scholar]
- 5.Coronavirus disease 2019 (COVID-19) situation report-184. Geneva: World Health Organization, 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200722-covid-19-sitrep-184.pdf?sfvrsn=7680210a_2. [Accessed July 23, 2020]. [Google Scholar]
- 6.Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, et al. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses 2019; 11:59.doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect 2020; 80:401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Liu Y, Liu L, Wang X, Luo N, Li L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at Hospital Admission in Shenzhen, China. J Infect Dis 2020; 221:1770–1774. doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect 2020; 80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med 2020; 201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Tang Y, Mo Y, Li S, Lin D, Yang Z, et al. A diagnostic model for coronavirus disease 2019 (COVID-19) based on radiological semantic and clinical features: a multi-center study. Eur Radiol 2020; 30:4893–4902. doi: 10.1007/s00330-020-06829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. New York: A John Wiley & Sons, Inc.; 2004. [Google Scholar]
- 15.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998; 17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Diagnosis and Treatment Protocol for Novel Coronavirus Infection-Induced Pneumonia Version 6 (Trial). Beijing: National Health Commission of the People's Republic of China. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202002/208334a208326dd202094d202329df202351d202007da202008aefc202002.shtml. [Accessed April 28, 2020]. [Google Scholar]
- 17.Cao JL, Tu WJ, Cheng WL, Yu L, Liu YK, Hu XY, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis 2020; 71:748–755. doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi KW, Chau TN, Tsang O, Tso E, Chiu MC, Tong WL, et al. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med 2003; 139:715–723. doi: 10.7326/0003-4819-139-9-200311040-00005. [DOI] [PubMed] [Google Scholar]
- 19.Hong KH, Choi JP, Hong SH, Lee J, Kwon JS, Kim SM, et al. Predictors of mortality in Middle East respiratory syndrome (MERS). Thorax 2018; 73:286–289. doi: 10.1136/thoraxjnl-2016-209313. [DOI] [PubMed] [Google Scholar]
- 20.Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis 2005; 41 Suppl 7:S504–S512. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 21.Jaillon S, Berthenet K, Garlanda C. Sexual Dimorphism in Innate Immunity. Clin Rev Allergy Immunol 2019; 56:308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 22.Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013; 310:1711–1720. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 23.Noble JA. Immunogenetics of type 1 diabetes: a comprehensive review. J Autoimmun 2015; 64:101–112. doi: 10.1016/j.jaut.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019- nCoV acute respiratory disease. Lancet 2020; 395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus 2019-nCoV) in vitro. Cell Res 2020; 30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang YM, Zhang DY, Du GH, Du RH, Zhao JP, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


