Abstract
Background
Venous thromboembolism (VTE) is a common condition in hospital patients. Considerable controversy is ongoing regarding optimal initial warfarin dosing for patients with acute deep venous thrombosis (DVT) and pulmonary embolism (PE). Achieving a therapeutic international normalized ratio (INR) with warfarin as soon as possible is important because this minimizes the duration of parenteral medication necessary to attain immediate anticoagulation, and it potentially decreases the cost and inconvenience of treatment. Although a 5‐mg loading‐dose nomogram tends to prevent excessive anticoagulation, a 10‐mg loading‐dose nomogram may achieve a therapeutic INR more quickly. This is an update of a review first published in 2013.
Objectives
To evaluate the efficacy of a 10‐mg warfarin nomogram compared with a 5‐mg warfarin nomogram among patients with VTE.
Search methods
For this update the Cochrane Vascular Trials Search Co‐ordinator searched the Specialised Register (last searched September 2015) and the Cochrane Register of Studies (CENTRAL (2015, Issue 8). Clinical trials databases were also searched. The review authors searched PubMed (last searched 11 June 2015) and LILACS (last searched 11 June 2015). In addition, the review authors contacted pharmaceutical companies.
Selection criteria
Randomized controlled studies comparing warfarin initiation nomograms of 10 and 5 mg in patients with VTE.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. The review authors contacted study authors for additional information.
Main results
Four trials involving 494 participants were included. Three studies involving 383 participants provided data on the proportion of participants who had achieved a therapeutic INR by day five. Significant benefit of a 10‐mg warfarin nomogram was observed (risk ratio (RR) 1.27, 95% confidence interval (CI) 1.05 to 1.54; moderate quality evidence), although with substantial heterogeneity (I2 = 90%). The review authors analyzed each study separately because it was not possible to perform a subgroup analysis by inpatient or outpatient status. One study showed significant benefit of a 10‐mg warfarin nomogram for the proportion of outpatients with VTE who had achieved a therapeutic INR by day five (RR 1.78, 95% CI 1.41 to 2.25), with the number needed to treat for an additional beneficial outcome (NNTB = 3, 95% CI 2 to 4); another study showed significant benefit of a 5‐mg warfarin nomogram in outpatients with VTE (RR 0.58, 95% CI 0.36 to 0.93) with NNTB = 5 (95% CI 3 to 28); a third study, consisting of both inpatients and outpatients, showed no difference (RR 1.08, 95% CI 0.65 to 1.80).
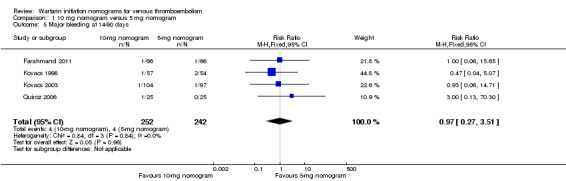
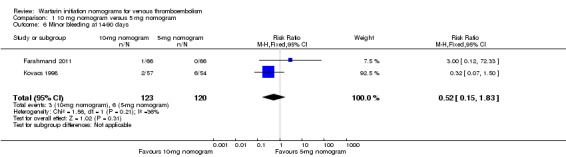
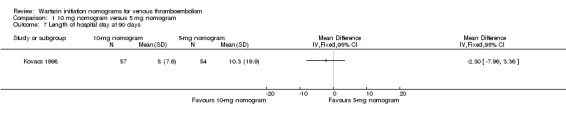
No difference was observed in recurrent venous thromboembolism at 90 days when the warfarin nomogram of 10 mg was compared with the warfarin nomogram of 5 mg (RR 1.48, 95% CI 0.39 to 5.56; 3 studies, 362 participants, low quality evidence); no difference was observed in major bleeding at 14 to 90 days (RR 0.97, 95% CI 0.27 to 3.51; 4 studies, 494 participants, moderate quality evidence). No difference was observed in minor bleeding at 14 to 90 days (RR 0.52, 95% CI 0.15 to 1.83; 2 studies, 243 participants, very low quality evidence) or in length of hospital stay (mean difference (MD) ‐2.3 days, 95% CI ‐7.96 to 3.36; 1 study, 111 participants, low quality evidence).
Authors' conclusions
In patients with acute thromboembolism (DVT or PE) aged 18 years or older, considerable uncertainty surrounds the use of a 10‐mg or a 5‐mg loading dose for initiation of warfarin to achieve an INR of 2.0 to 3.0 on the fifth day of therapy. Heterogeneity among analyzed studies, mainly caused by differences in types of study participants and length of follow‐up, limits certainty surrounding optimal warfarin initiation nomograms.
Plain language summary
Warfarin initiation nomograms of 5 mg and 10 mg for venous thromboembolism
Backgound
Venous thromboembolism is the presence of a blood clot that blocks a blood vessel within the venous system; it includes deep vein thrombosis (DVT) and pulmonary embolism (PE), which can be fatal. Venous thromboembolism occurs in 40% to 60% of patients after major orthopedic surgery.
Venous thromboembolism is usually treated for a minimum of five days with an anticoagulant administered by injection-either unfractionated heparin or low molecular weight heparin-together with warfarin dose titration. Achieving a therapeutic international normalized ratio (INR) as soon as possible while taking warfarin is important because this minimizes the duration of medication given via injections, infusions, etc, necessary to attain immediate anticoagulation and potentially decreases costs and inconvenience. Although a 5‐mg loading‐dose nomogram tends to prevent excessive anticoagulation, a 10‐mg loading‐dose nomogram may achieve a therapeutic INR more quickly.
Study characteristics and key results
This review of four studies with a total of 494 participants (current until June 2015) found that in patients with acute thromboembolism aged 18 years or older, considerable uncertainty surrounds the use of a 10‐mg or a 5‐mg loading dose for initiation of warfarin to achieve an INR of 2.0 to 3.0 on the fifth day of therapy. A benefit of 10‐mg warfarin compared with 5‐mg warfarin was observed for the proportion of patients with VTE who had achieved a therapeutic INR by day five but the quality of the evidence was moderate due to the differences among analyzed studies. In addition, no differences were observed between 5‐mg and 10‐mg nomograms for recurrent venous thromboembolism, major and minor bleeding, and length of hospital stay. Studies with high‐quality evidence regarding the efficacy of 5‐mg and 10‐mg warfarin nomograms are needed.
Quality of the evidence
The quality of the evidence was moderate for therapeutic INR and major bleeding, low for recurrent VTE and length of hospital stay, and very low for minor bleeding. The main reason for downgrading the quality of the evidence was differences between the studies in types of study participants and length of follow‐up.
Summary of findings
Summary of findings for the main comparison. 10 mg warfarin nomogram compared to 5 mg warfarin nomogram for venous thromboembolism.
| 10‐mg warfarin nomogram compared with 5‐mg warfarin nomogram for venous thromboembolism | ||||||
| Patient or population: patients with venous thromboembolism. Settings: outpatients and inpatients with venous thromboembolism. Intervention: 10‐mg warfarin nomogram. Comparison: 5‐mg warfarin nomogram. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 5‐mg warfarin nomogram | 10‐mg warfarin nomogram | |||||
| Therapeutic INR INR TEST Follow‐up: 14 to 90 days | Study population | RR 1.27 (1.05 to 1.54) | 383 (3 studies) | ⊕⊕⊕⊝ moderate1,2,3 | ||
| 473 per 1000 | 601 per 1000 (497 to 729) | |||||
| Moderate | ||||||
| 470 per 1000 | 597 per 1000 (493 to 724) | |||||
| Recurrent venous thromboembolism sonography or lung scan or chest computed tomography Follow‐up: 14 to 90 days | Study population | RR 1.48 (0.39 to 5.56) | 362 (3 studies) | ⊕⊕⊝⊝ low4,5,6,7 | ||
| 17 per 1000 | 25 per 1000 (7 to 95) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Major bleeding fall in hemoglobin of > 20 g/L or transfusion of 2 or more units of red cells Follow‐up: 14 to 90 days | Study population | RR 0.97 (0.27 to 3.51) | 494 (4 studies) | ⊕⊕⊕⊝ moderate5,8,9 | ||
| 17 per 1000 | 16 per 1000 (4 to 58) | |||||
| Moderate | ||||||
| 13 per 1000 | 13 per 1000 (4 to 46) | |||||
| Minor bleeding it is not major bleeding Follow‐up: 14‐90 days | Study population | RR 0.52 (0.15 to 1.83) | 243 (2 studies) | ⊕⊝⊝⊝ very low5,10,11 | ||
| 50 per 1000 | 26 per 1000 (8 to 92) | |||||
| Length of hospital stay record of hospitalization Follow‐up: median 3 months | The mean length of hospital stay in the intervention groups was 2.3 lower (7.96 lower to 3.36 higher) | 111 (1 study) | ⊕⊕⊝⊝ low5,12,13 | result of a single study | ||
| *The assumed risk (e.g., the median control group risk across studies) is the assumed risk as calculated by the GRADEpro software. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 In Kovacs 2003 and Farahmand 2011, the participants were outpatients. In Quiroz 2006, the participants were inpatients and outpatients. It was not possible to conduct a subgroup analysis because we were unable to obtain the data of outpatients and inpatients separately. Therefore, heterogeneity affects the interpretation of results 2 The objective is to obtain therapeutic INR on the fifth day. Kovacs 2003 reported one death, and in Quiroz 2006 and Farahmand 2011, no deaths were reported. We believe that the primary outcome (therapeutic INR on the fifth day) is an intermediate variable (surrogate) 3 We used the warfarin nomogram of 5 mg and 10 mg, which is twice the dose. In Kovacs 2003, the therapeutic INR with 5 mg was 45% and with the 10‐mg nomogram was almost double 4 Recurrent VTE is an important outcome variable (hard variable) for the patient. Heterogeneity of 47% (moderate) is explained by variations in the ages of participants in the three studies 5 In Kovacs 1998, the control group received warfarin dosed according to usual practice 6 The 95% CI is wide: 0.39 to 5.56 7 Confounding variables (e.g., age) in the results have not been adjusted 8 The 95% CI is wide: 0.27 to 3.51 9 There is variety in the ages of participants and in follow‐up time (14 to 90 days) 10 Heterogeneity is 36%; the studies had results in opposite directions 11 The results had wide confidence intervals 12 The 10‐mg warfarin nomogram was compared with usual practice 13 Result of a single study
Background
Description of the condition
Venous thromboembolism (VTE) is a common condition among hospitalized patients. The main manifestations are deep venous thrombosis (DVT) and pulmonary embolism (PE). Without prophylaxis, the incidence of objectively confirmed, hospital‐acquired DVT is approximately 10% to 40% among medical or general surgical patients, rising to 40% to 60% after major orthopedic surgery. One quarter to one third of these DVTs are much more likely to produce symptoms and to result in PE (Geerts 2004; White 2003).
Deep vein thrombosis and PE are usually treated with heparin-either unfractionated heparin (UFH) or low molecular weight heparin (LMWH)- over a minimum of five days, concurrently with warfarin dose titration (Bhutia 2013; Büller 2004; Erkens 2010; Hirsh 2001; Hirsh 2003; Merli 2001; Quinlan 2004).
Description of the intervention
Management of VTE has improved substantially in the past 10 years. Conventional therapy consists of UFH or LMWH for five to seven days, together with oral anticoagulation with warfarin given for a minimum of three months (Levine 1996; Weinmann 1994). LMWH facilitates outpatient treatment, and warfarin is usually initiated within 24 hours. Clinical trials have demonstrated that LMWH may be safely discontinued after five days once the international normalized ratio (INR) has remained greater than 1.9 for 24 hours (Levine 1996; The Columbus Investigators 1997). Warfarin is the oral anticoagulant most commonly used all over the world; it exerts its antithrombotic effects by inhibiting activation of the vitamin K-dependent clotting factors II, VII, IX and X. More recent evidence, however, suggests that reduction of prothrombin (factor II), which has a half‐life of 60 to 72 hours, and possibly of factor X is more important than reduction of factors VII and IX for the antithrombotic effect of warfarin. This suggestion that the antithrombotic effect of warfarin is reflected in lower levels of factor II forms the basis for overlapping UFH or LMWH with administration of warfarin until the INR is prolonged into the therapeutic range during treatment of patients with VTE (Baglin 2005; Baker 2004; Hirsh 2003).
Prothrombin time (PT) monitoring of warfarin treatment is very imprecise when expressed as a PT ratio (calculated as a simple ratio of the patient’s plasma value to that of normal control plasma) because thromboplastins can vary markedly in their responsiveness to warfarin. It has contributed to clinically important differences in oral anticoagulant dosing among countries. Recognition of these shortcomings in PT monitoring stimulated the development of the INR standard for monitoring of oral anticoagulant therapy, and adoption of this standard improved the safety of oral anticoagulant therapy and its ease of monitoring (Hirsh 2003).
The role of warfarin in anticoagulation continues to evolve even after 50 years of experience with its use. During this time, much effort has been spent improving the safety and efficacy profile of warfarin through intensified anticoagulation monitoring and patient education. Although much has been accomplished, many controversies remain regarding the best dosing strategies. One such controversy that has resurfaced involves establishing an optimal dosing strategy for the initiation of warfarin therapy. One of the reasons that warfarin initiation remains complex is its high interpatient and intrapatient variability. Factors such as age, gender, ethnicity, indication for anticoagulation, vitamin K intake, body weight and interacting medication(s) can contribute to this variability. Age < 55 years, male gender, African American race, vitamin K intake > 400 µg/day, and body weight ≥ 91 kg have been shown to be independently associated with warfarin maintenance doses > 5 mg. In addition, patients who are malnourished or elderly, who have low albumin levels or body weight, or who are taking interacting drugs may have lower warfarin requirements (Holbrook 2005).
The American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (Ansell 2008) recommend initiation of oral anticoagulation with doses between 5 mg and 10 mg for the first one or two days for most individuals, with subsequent dosing based on the INR response. Additionally, the guidelines suggest that a starting dose of < 5 mg might be appropriate in elderly patients, in patients with impaired nutrition, in those with liver disease and congestive heart failure, and in patients who are at high risk of bleeding. Over the past decade, the debate over the most appropriate starting dose of warfarin has generated two primary dosing strategies: warfarin nomograms using 10‐mg and 5‐mg loading doses. Several studies have compared initiation of warfarin with these loading‐dose nomograms, reflecting controversial results (Eckhoff 2004).
Ongoing research is investigating pharmacogenetic dosing for warfarin initiation (e.g., COAG trial; GIFT trial), and although administrative bodies such as the US Food and Drug Association suggest that a patient's pharmacogenetics should be considered upon initiation of warfarin if this information is available, more research is needed before pharmacogenetic dosing can become the standard of care (FDA 2007).
Why it is important to do this review
Considerable controversy continues regarding optimal initial warfarin dosing for patients with acute DVT and PE. Achieving a stable INR on warfarin as soon as possible is important because it minimizes the duration of parenteral medication necessary to attain immediate anticoagulation and potentially decreases cost and inconvenience. However, excessive anticoagulation with warfarin requires a readjustment of the dose downward and may require a prolonged course of an injected anticoagulant. Although a 5‐mg loading‐dose nomogram tends to prevent excessive anticoagulation, a 10‐mg loading‐dose nomogram may achieve a therapeutic INR more quickly (Quiroz 2006).
Objectives
To evaluate the efficacy of a 10‐mg warfarin nomogram compared with a 5‐mg warfarin nomogram among patients with venous thromboembolism.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Patients with a diagnosis of objectively confirmed acute thromboembolism (DVT or PE), aged 18 years or older. Studies that contain a mix of patients with acute thromboembolism and other conditions requiring warfarin treatment will be included only if the data for patients with acute thromboembolism are available.
Types of interventions
Warfarin initiation nomograms of 10 mg and 5 mg.
Types of outcome measures
Primary outcomes
The proportion of patients with an INR of 2.0 to 3.0 on the fifth day of initial therapy with warfarin.
Secondary outcomes
Recurrent venous thromboembolism (RVTE) as measured by sonography, lung scan, or chest computed tomography.
Major bleeding as defined by fall in hemoglobin of > 20 g/L or transfusion of two or more units of red cells.
Minor bleeding (other bleeding not categorized as major bleeding).
Length of hospital stay.
Search methods for identification of studies
We identified relevant trials regardless of language or publication status (published and unpublished, in press, and in progress), from 1989 onward. We excluded trials published before 1989 because before that year, most clinical centers were still using PT instead of INR (Linkins 2003).
Electronic searches
For this update the Cochrane Vascular Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched 16 September 2015) and the Cochrane Register of Studies (CRS) (http://www.metaxis.com/CRSWeb/Index.asp) (CENTRAL (2015, Issue 8)). See Appendix 1 for details of the search strategy used to search the CRS. The Specialised Register is maintained by the Trials Search Co‐ordinator and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The following trial databases were searched by the TSC ( September 2015) for details of ongoing and unpublished studies using the term nomogram:
World Health Organization International Clinical Trials Registry http://apps.who.int/trialsearch/;
ClinicalTrials.gov http://clinicaltrials.gov/; and
ISRCTN register (http://www.isrctn.com/)
The authors searched PubMed (last searched 11 June 2015) using the search strategy shown in Appendix 2 and LILACS (last searched 11 June 2015; Appendix 3).
Searching other resources
To help identify unpublished and ongoing trials, we contacted researchers and organizations including the American Society of Hematology, the American College of Chest Physicians (ACCP), and the pharmaceutical companies Bristol‐Myers Squibb Perù S.A. and Sanofi Aventis.
We searched the reference lists of relevant trials and reviews identified through electronic searches.
Data collection and analysis
Selection of studies
Two review authors (PG and WR) independently screened the search results using article titles and abstracts (if available). The full text of selected articles were retrieved and scrutinized to ensure that multiple publications of the same study were identified. The same review authors (PG and WR) then independently selected articles for inclusion according to a standardized form used to assess the eligibility of trials. Disagreements were resolved through discussion with the third review author (CLM).
A kappa statistic was calculated for measuring agreement between two authors in making simple inclusion and exclusion decisions (Higgins 2011).
Data extraction and management
Two review authors (PG and WR) independently extracted the data using standard forms. Differences were resolved through discussion with the third review author (CLM). Attempts were made to obtain missing data from the trial authors (Crowther 1997; Harrison 1997; Quiroz 2006), but unfortunately no response was received.
Assessment of risk of bias in included studies
Two review authors (PG and WR) independently assessed the risk of bias of each trial, recorded the information in a table and provided a narrative description in the text.
Risks of bias were assessed according to Higgins 2011 and using the domains: random sequence generation, allocation concealment, blinding (participants, care providers, or outcome assessors), incomplete outcome data, selective reporting and other bias. For each of these domains, we assessed whether the study was at high risk of bias, low risk of bias, or unclear risk of bias using the guidance provided by Higgins 2011. Disagreements were resolved by consensus.Trial authors (Crowther 1997, Farahmand 2011, Harrison 1997, Kovacs 1998, Kovacs 2003, and Quiroz 2006) were contacted for clarification when information was unclear. Unfortunately, a response was not received, except from the authors of the Farahmand 2011, Kovacs 1998 and Kovacs 2003 studies.
Measures of treatment effect
For dichotomous outcomes, we expressed results as risk ratio (RR) with 95% confidence interval (CI).
For continuous scales of measurement we used the mean difference (MD) with 95% CI.
Dealing with missing data
Requests for relevant missing data from the authors of the following trials were met with no response: Crowther 1997; Harrison 1997; and Quiroz 2006. We did receive a response from the authors of the Farahmand 2011; Kovacs 1998; and Kovacs 2003 studies. In addition, we asked Kovacs 1998 about the proportion of patients with an INR of 2 to 3 on the fifth day of initial therapy with warfarin; Kovacs 1998 could not locate the original data sheets because their colleague Dr. Cruickshank, who performed the data analysis, died several years ago (García 2011 [pers comm]).
In the case of duplicate publications and companion papers of a primary study, we tried to maximize yield of information by simultaneously evaluating all available data. In cases of doubt, the publication with the highest quality and the most complete information was given priority.
Assessment of heterogeneity
Heterogeneity amongst trials was investigated with the I2 test statistic (> 50% was considered to indicate substantial heterogeneity). We attempted to determine potential reasons for any heterogeneity found.
Assessment of reporting biases
Publication bias was not analyzed using the planned funnel plot method because only four trials were evaluated.
Data synthesis
We analyzed the data using Review Manager 5.1 (Review Manager 5.1).
We pooled dichotomous data using the risk ratio. Continuous data were combined using mean differences.
We used fixed‐effect and random‐effects meta‐analytic models.
Subgroup analysis and investigation of heterogeneity
We used subgroup analysis to explore possible sources of heterogeneity (e.g., participants, interventions, study quality). Heterogeneity among participants could be related to age and status as outpatients and inpatients with VTE.
Sensitivity analysis
We performed a sensitivity analysis based on the four parameters of trial methodological quality. We also performed a sensitivity analysis by applying the random‐effects model to ensure the robustness of the model chosen and susceptibility to outliers.
Summary of findings
We used GRADEpro software (http://gdt.guidelinedevelopment.org) to help us create the Table 1 and reported the outcomes therapeutic INR, recurrent VTE, major bleeding, minor bleeding and length of hospital stay. We downgraded the evidence from 'high quality' by one or two levels for serious or very serious study limitations (risk of bias), indirectness and inconsistency of evidence, imprecision of effect estimates or potential publication bias according to the Cochrane Handbook for Systematic Reviews (Higgins 2011) and GRADE (Atkins 2004).
Results
Description of studies
Results of the search
See Figure 1.
1.
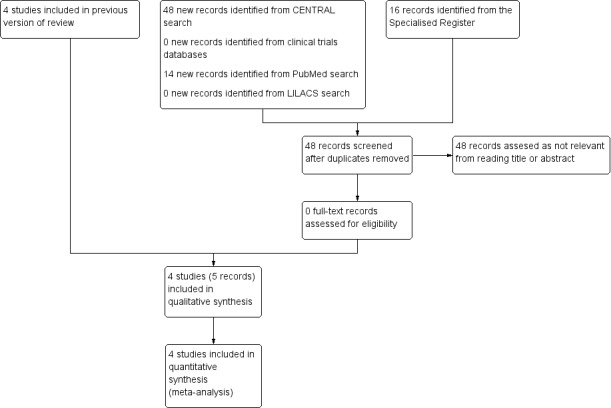
Study flow diagram.
For this update no studies were retrieved from the database searches that were not in the Specialised Register. No relevant ongoing or unpublished studies were retrieved from the search of the clinical trials databases.
Included studies
No additional studies were included in this update.
Four studies met the review inclusion criteria: Farahmand 2011; Kovacs 1998; Kovacs 2003; and Quiroz 2006. All four studies were published as full reports. For details of these studies see the Characteristics of included studies table.
The four studies were randomized and were conducted in nine study centers in four countries (Canada, Iran, Switzerland, and USA).
Demographic characteristics of the four studies were not similar. The participants in Kovacs 1998 were inpatients with VTE, the participants in Kovacs 2003 and Farahmand 2011 were outpatients, and in Quiroz 2006, the participants were outpatients and inpatients. In Kovacs 1998, those randomly assigned to standard practice were significantly older than those randomly assigned to nomogram.
Three studies (Farahmand 2011; Kovacs 2003; Quiroz 2006) compared a 10‐mg warfarin nomogram with a 5‐mg nomogram. One trial (Kovacs 1998) compared a 10‐mg nomogram with the usual practice of the attending physicians; according to the information requested from the original investigators, the usual practice consisted of warfarin initiation of 5 mg in "most of cases" (García 2012b [pers comm]).
Only three studies (Farahmand 2011; Kovacs 2003; Quiroz 2006) evaluated the proportion of patients with an INR of 2.0 to 3.0 on the fifth day of initial therapy with warfarin.
Three studies (Kovacs 1998; Kovacs 2003; Quiroz 2006) evaluated the incidence of RVTE but at different times. Kovacs 1998 and Kovacs 2003 evaluated the incidence of RVTE at 90 days, and Quiroz 2006 evaluated the incidence at 14 days of treatment.
All four included studies (Farahmand 2011; Kovacs 1998; Kovacs 2003; Quiroz 2006) evaluated the incidence of major bleeding. Follow‐up ranged between 14 and 90 days. Two studies (Farahmand 2011; Kovacs 1998) evaluated the incidence of minor bleeding but at different times. Kovacs 1998 evaluated minor bleeding at three months, and Farahmand 2011 evaluated the incidence of minor bleeding at 14 days of treatment.
Only one study (Kovacs 1998) evaluated the length of hospital stay.
Excluded studies
No additional studies were excluded in this update.
We excluded six studies (Crowther 1997; Crowther 1999; Crowther 2002; Harrison 1997; Pineo 1997; Shulman 1984). See also Characteristics of excluded studies table.
In two studies, the group of participants were a mixed group with regard to the reason for warfarin initiation. In the study by Crowther 1999, 29% of participants in the 10‐mg nomogram group and 41% of participants in the 5‐mg nomogram group did not have VTE; in Harrison 1997, 28% of participants in the 10‐mg nomogram group and 12.5% of participants in the 5‐mg nomogram group did not have VTE. We contacted the authors of these studies (Crowther 1999; Harrison 1997) for clarification but unfortunately did not receive a response. For both studies, we were unable to identify data for the VTE patients. Other reasons for exclusion included that the participants did not have VTE and the reason for warfarin initiation was unknown (Crowther 1997; Pineo 1997); investigators did not use INR as an outcome measure (Shulman 1984); and researchers did not use 5‐mg and 10‐mg initiation nomograms (Crowther 2002).
Risk of bias in included studies
See also the 'Risk of bias in included studies' summary (Figure 2).
2.
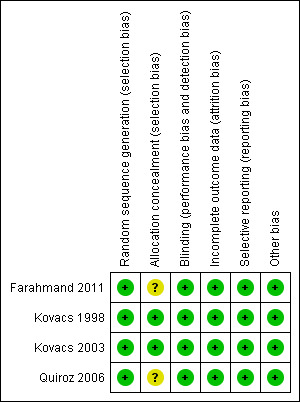
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The four included studies (Farahmand 2011; Kovacs 1998; Kovacs 2003; Quiroz 2006) had acceptable quality standards. Inclusion and exclusion criteria were clear, as was the analysis undertaken.
Allocation
Participants were allocated to the corresponding study group according to random sequences generated by computer‐based randomization programs (Farahmand 2011; Kovacs 1998; Kovacs 2003; Quiroz 2006). Moreover, the methods used to conceal the allocation sequence were adequate in Kovacs 1998 and Kovacs 2003 because they were performed by using sets of sequentially numbered, opaque, sealed envelopes in Kovacs 2003 and by applying central randomization with computer‐generated blocks of four or six in Kovacs 1998. Therefore, the risk of selection bias was low for Kovacs 1998 and Kovacs 2003 (defined as presenting low risk of bias for these two domains: "random sequence generation" and "allocation concealment"). In Farahmand 2011 and Quiroz 2006, methods used to conceal allocation sequence were unclear.
Blinding
Blinding was double (physician‐patient) in Kovacs 2003 and Farahmand 2011. Single blinding (adjudication of outcome events) was used in Quiroz 2006. Kovacs 1998 also used single blinding because the attending physicians were unaware that their method of ordering warfarin was being monitored.
Incomplete outcome data
Follow‐up was complete and no losses to follow‐up were reported in all of the included studies.
Selective reporting
No selective reporting was identified as the prespecified outcomes (primary and secondary) of the four included studies were reported in the prespecified way.
Other potential sources of bias
Upon review of the studies (Farahmand 2011; Kovacs 1998; Kovacs 2003; Quiroz 2006), no other potential sources of bias were identified.
Effects of interventions
See: Table 1
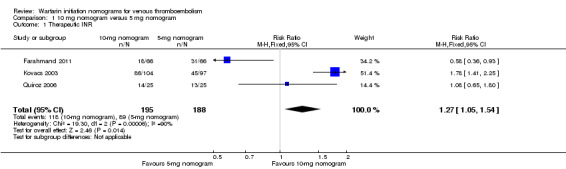
Three studies including 383 participants overall (Farahmand 2011; Kovacs 2003; and Quiroz 2006) provided data on the proportions of participants who had achieved a therapeutic INR by day five. Significant benefit of a 10‐mg warfarin nomogram was observed (RR 1.27, 95% CI 1.05 to 1.54) (Figure 3), although with substantial heterogeneity (I2 = 90%). A sensitivity analysis was performed using the random‐effects model, and no difference was observed (RR 1.06, 95% CI 0.52 to 2.16). We explored possible sources of heterogeneity, including participants' age, inclusion of outpatients and inpatients, and study size. Participants in Kovacs 2003 (mean age 55.3 years) were older than participants in Quiroz 2006 (mean age 50.5 years) and Farahmand 2011 (mean age 47.5 years). In Kovacs 2003 and Farahmand 2011, the participants were outpatients with VTE, but in Quiroz 2006, the participants were outpatients and inpatients. Kovacs 2003 (201 participants) is a larger study than Farahmand 2011 (132 participants) and Quiroz 2006 (50 participants). We analyzed each study separately because it was not possible to perform a subgroup analysis. Kovacs 2003 showed significant benefit of the 10‐mg nomogram in achieving therapeutic INR by day five (RR 1.78, 95% CI 1.41 to 2.25) and with the number needed to treat for an additional beneficial outcome (NNTB = 3, 95% CI 2 to 4). Quiroz 2006 showed no difference (RR 1.08, 95% CI 0.65 to 1.80), but Farahmand 2011 showed significant benefit of the 5‐mg nomogram (RR 0.58, 95% CI 0.36 to 0.93) compared with NNTB = 5 (95% CI 3 to 28).
3.

Forest plot of comparison: 1 10‐mg nomogram versus 5‐mg nomogram, outcome: 1.1 therapeutic INR on fifth day.
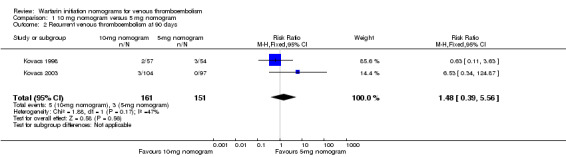
Two studies with 161 participants overall (Kovacs 1998; Kovacs 2003) provided data on the RVTE at 90 days (Figure 4). No difference was observed in RVTE when nomograms of 10 mg were compared with nomograms of 5 mg of warfarin (RR 1.48, 95% CI 0.39 to 5.56) and with moderate heterogeneity (I2 = 47%). We explored the possible sources of heterogeneity, including participants' age, whether participants were outpatients or inpatients, and study size. Mean age was different among participants in Kovacs 1998 (10‐mg nomogram group: 57 years vs standard practice group: 64.5 years; P = 0.036) and was older than that of participants in Kovacs 2003 (mean age 55.3 years). In Kovacs 1998, the participants were inpatients, but in Kovacs 2003, they were outpatients. Kovacs 2003 (201 participants) was a larger study than Kovacs 1998 (111 participants). The review authors did not include Quiroz 2006 in this meta‐analysis because length of follow‐up in this study was different (14 days) from the 90‐day follow‐up described in the Kovacs studies. Quiroz 2006 reported no episodes of RVTE.
4.
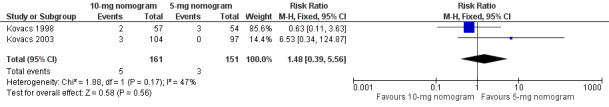
Forest plot of comparison: 1 10‐mg nomogram versus 5‐mg nomogram, outcome: 1.2 recurrent venous thromboembolism at 90 days.
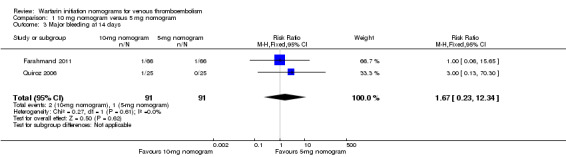
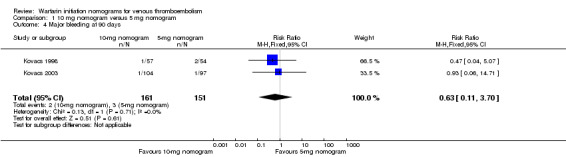
Two studies with an overall 91 participants (Farahmand 2011; Quiroz 2006) provided data on major bleeding at 14 days (Figure 5). No difference was observed (RR 1.69, 95% CI 0.22 to 13.04) and no heterogeneity was noted (I2 = 0%). Also two studies with an overall 161 participants (Kovacs 1998; Kovacs 2003) provided data on major bleeding at 90 days (Figure 6). No difference was observed (RR 0.62, 95% CI 0.10 to 3.78) and no heterogeneity was noted (I2 = 0%). We performed an analysis for major bleeding independent of follow‐up, and this meta‐analysis (Farahmand 2011; Kovacs 1998; Kovacs 2003; Quiroz 2006) showed no difference (RR 0.97, 95% CI 0.22 to 13.04) and no heterogeneity (I2 = 0%).
5.
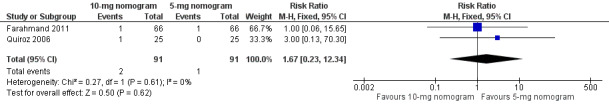
Forest plot of comparison: 1 10‐mg nomogram versus 5‐mg nomogram, outcome: 1.3 major bleeding at 14 days.
6.
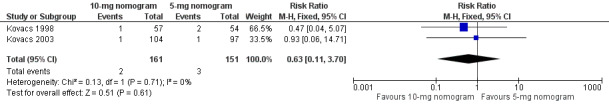
Forest plot of comparison: 1 10‐mg nomogram versus 5‐mg nomogram, outcome: 1.4 major bleeding at 90 days.
Two studies with 243 participants (Farahmand 2011; Kovacs 1998) provided data on minor bleeding at 14 to 90 days. No difference was observed in minor bleeding (RR 0.32, 95% CI 0.15 to 1.83).
Kovacs 1998 was the only study to provide data on the length of hospital stay. No difference was observed in length of hospital stay (MD ‐2.30 days, 95% CI ‐7.96 to 3.36).
In addition to planned outcomes, we report on two outcomes that were not prespecified in our protocol, namely, proportions of participants with supratherapeutic INR and mortality. The heterogeneity of the data for supratherapeutic INR and mortality prevented pooling.
All four included studies reported information on supratherapeutic INR. Farahmand 2011 reported the proportion of patients with INR > 3 and < 5 on the seventh day (10‐mg nomogram group [57.58%] vs 5‐mg nomogram group [12.12%]) and on the 14th day (10‐mg nomogram group [22.73%] vs 5‐mg nomogram group [10.61%]). Farahmand 2011 reported that no patients had an INR > 5 on the seventh and 14th days. Kovacs 1998 reported the proportion of patients with INR > 4.5 in the first week (10‐mg nomogram group [15.7%] vs standard practice group [11.1%]). Kovacs 2003 reported the proportion of patients with INR > 3 in the first four weeks (10‐mg nomogram group [77.88%] vs 5‐mg nomogram group [86.60%] (P > 0.2)) and the proportion of patients with INR > 5 in the first four weeks (10‐mg nomogram group [8.65%] vs 5‐mg nomogram group [11.34%], P > 0.2). Quiroz 2006 reported overall frequencies of INR > 3 within two weeks: 10‐mg nomogram group (3.7%) versus 5‐mg nomogram group (14.5%) (P < 0.001); Quiroz 2006 reported no patients with INR > 5 and no deaths.
Only two studies (Kovacs 1998; Kovacs 2003) reported mortality. Kovacs 1998 reported mortality at 90 days: 10‐mg nomogram group (8.77%) versus standard practice group (20.37%). Kovacs 2003 reported mortality at 90 days: 10‐mg nomogram group (0%) versus 5‐mg nomogram group (10.31%). The P values for these differences not were reported by study authors.
Discussion
Summary of main results
The objective of this review was to evaluate the efficacy of a 10 mg warfarin nomogram compared with a 5‐mg warfarin nomogram among patients with VTE. The RR on the proportion of patients who had achieved a therapeutic INR by day five comparing a 10‐mg warfarin nomogram with a 5‐mg nomogram was 1.27 (95% CI 1.05 to 1.54). However, this result is not consistent with an I2 of 90%. A sensitivity analysis was performed using the random‐effects model, and no difference was observed (RR 1.06, 95% CI 0.52 to 2.16). We analyzed each study separately because it was not possible to perform a subgroup analysis. Kovacs 2003 showed significant benefit of a 10‐mg warfarin nomogram for the proportion of patients who had achieved a therapeutic INR by day five (RR 1.78, 95% CI 1.41 to 2.25) with the number needed to treat for an additional beneficial outcome (NNTB = 3, 95% CI 2 to 4); Farahmand 2011 showed the significant benefit of a 5‐mg warfarin nomogram (RR 0.58, 95% CI 0.36 to 0.93) with NNTB = 5 (95% CI 3 to 28), and Quiroz 2006 showed no difference (RR 1.08, 95% CI 0.65 to 1.80). No difference was observed in RVTE at 90 days when the warfarin nomogram of 10 mg was compared with the warfarin nomogram of 5 mg (RR 1.48, 95% CI 0.39 to 5.56). Also no difference was observed in major bleeding at 14 days (RR 1.69, 95% CI 0.22 to 13.04) and at 90 days (RR 0.62, 95% CI 0.10 to 3.78). No difference was observed in minor bleeding at 14 to 90 days (RR 0.32, 95% CI 0.15 to 1.83) or in length of hospital stay (MD ‐2.30 days, 95% CI ‐7.96 to 3.36).
Overall completeness and applicability of evidence
The four included studies were insufficient to assess the objectives of the review. Results were inconsistent for primary and secondary outcomes. We analyzed each study separately because it was not possible to perform a subgroup analysis. Kovacs 2003 showed significant benefit of a 10‐mg nomogram in achieving therapeutic INR by day five, with NNTB = 3; Farahmand 2011 showed significant benefit of a 5‐mg warfarin nomogram with NNTB = 5 (95% CI 3 to 28), but it is necessary to emphasize that the patients were ambulatory with an average age of 55.3 and 47.5 years respectively, and studies were limited in power to perform safety assessment. This probably does not apply to elderly or hospitalized patients, who are likely to be more sick.
Quality of the evidence
According to the GRADE system, the quality of the evidence was moderate for therapeutic INR and major bleeding, low for recurrent VTE and length of hospital stay, and very low for minor bleeding. For therapeutic INR the quality of the evidence was moderate because the heterogeneity affect the interpretation of the results and the primary outcome was an intermediate variable. For major bleeding the quality of the evidence was moderate because there was variety in the ages of participants and in follow‐up time (14 to 90 days) and the confidence interval (CI) of the risk ratio was wide. For recurrent VTE the quality of the evidence was low because to the variations in the ages of participants in the three studies (heterogeneity), the CI of RR was wide and the confounding variables (e.g. ages) in the results have not been adjusted. For length of hospital stay the quality of the evidence was low because the 10‐mg nomogram was compared with usual practice and the result was from single study; and for minor bleeding the quality of the evidence was very low because the studies had results in opposite directions (inconsistency), in Kovacs 1998, the control group received warfarin dosed according to usual practice, variety in follow‐up time (14 to 90 days) and the results had wide CI. See also Table 1.
Potential biases in the review process
Bias in this review is not discarded despite the developed methodology. We were unable to obtain data on the proportions of outpatients who had achieved a therapeutic INR from the studies by Kovacs 1998 and Quiroz 2006.
Agreements and disagreements with other studies or reviews
Mahtani 2012 was the first updated systematic review that evaluated twelve randomized controlled trials (Ageno 2001; Anderson 2007; Caraco 2008; Crowther 1999; Gedge 2000; Harrison 1997; Hillman 2005; Kovacs 2003; Quiroz 2006; Roberts 1999; Shine 2003; Burmester 2011) consisting of 1570 patients for whom anticoagulation was commenced with warfarin, and different loading doses and different regimens were compared. Although considerable heterogeneity was noted across the twelve studies in terms of design quality, loading dose protocols, patient populations, outcome measures, and length of follow‐up, the authors grouped them into four clinically relevant categories: 5 mg versus 10 mg; 5 mg versus other doses; and age‐adjusted and genotype loading doses.
In the category 5‐mg versus 10‐mg loading dose, similar to the comparison in this Cochrane review, the authors assessed four studies (Crowther 1999; Harrison 1997; Kovacs 2003; Quiroz 2006) with an overall 355 patients with VTE and other pathologies. Kovacs 1998 was not included in the review by Mahtani 2012 but was included in our review. Crowther 1999 and Harrison 1997 were not included in our Cochrane review because our review included VTE patients only. According to Mahtani 2012, all four studies reported a therapeutic INR by day five, and meta‐analysis revealed no overall difference between 5‐mg versus 10‐mg loading doses (RR 1.17, 95% CI 0.77 to 1.77, P = 0.46, I2 = 83%). Mahtani 2012 suggested that one possible reason for this result was that studies reported the proportion as single or two consecutive INR measures. In the two studies that used single INR measures (Harrison 1997; Kovacs 2003), the 10‐mg warfarin nomogram led more patients to a therapeutic INR by day five (RR 1.49, 95% CI 1.01 to 2.21, P = 0.05, I2 = 72%), although heterogeneity remained high. These results were similar to our review where a significant benefit of 10 mg warfarin nomogram was observed too (RR 1.27, 95% CI 1.05 to 1.54; I2 = 90%). We did not evaluate the mean values of two consecutive INRs at day five however Mahtani 2012 did evaluate those reported in two other studies (Crowther 1999; Quiroz 2006) and showed that the 10‐mg loading dose did not lead to more patients in range on day five (RR 0.86, 95% CI 0.62 to 1.19, P = 0.37, I2 = 22%). Another possible reason for not showing an overall difference between 5‐mg versus 10‐mg loading doses is that the nomograms employed for Crowther 1999 and Harrison 1997 were different from those used by Kovacs 2003 and Quiroz 2006. Mahtani 2012 reported that the number of serious adverse events was reported by three studies (Harrison 1997; Kovacs 2003; Quiroz 2006), but power was insufficient to allow evaluation of the effects of the nomograms on these, and variation in the follow‐up period used for evaluation of these adverse events was considerable. Again, this was very similar to our review.
In the category 5 mg versus other doses, Mahtani 2012 assessed two studies (Ageno 2001; Shine 2003) with a total of 322 patients with VTE and other pathologies. Shine 2003 assessed 90 inpatients with DVT, PE, and other pathologies and compared 5 mg (standard dose) with a calculated dose, which took account of age, weight, serum albumin, and malignancy. No difference in proportion achieving INR in range on or before day six was noted: 77% calculated dose compared with 63% with the 5‐mg dose (RR 1.22, 95% CI 0,88 to 1.70, P = 0.24). Also no differences in serious adverse events or in above‐range INR were noted. In our review, the studies did not report the therapeutic INR on day six.
In the age‐adjusted category, Mahtani 2012 assessed two studies (Gedge 2000; Roberts 1999) with a total of192 patients with VTE and other pathologies. Roberts 1999 reported that by day five more patients in the age‐adjusted group achieved a therapeutic INR (defined as in range on two consecutive days, or within 0.5) compared with the Fennerty protocol group (48% vs 22%, P = 0.02). In both studies, significantly fewer patients on the age‐adjusted group had high out‐of‐range INRs.
In the genotype loading dose category, Mahtani 2012 assessed four studies (Anderson 2007; Burmester 2011; Caraco 2008; Hillman 2005) with an overall 701 patients with VTE and other pathologies and comparing loading doses that took account of the patient genotype (genotype model). Only Anderson 2007 and Caraco 2008 used a comparison group similar to that of the interventions used in our Cochrane review. Anderson 2007 performed a prospective, randomized study with 206 participants that compared pharmacogenetically guided (18.8% of participants with DVT and/or PE) and standard empirical dosing (28.3% of participants with DVT and/or PE) in patients being initiated on oral anticoagulation. Standard dosing followed the 10‐mg warfarin nomogram of Kovacs 2003 except that the dose given on days three and four was 5 mg regardless of the INR on day three, whereas pharmacogenetically guided dosing followed a regression equation that included the three genetic variants of age, sex, and weight. This largely initiated study failed to achieve its primary endpoint of demonstrating a reduction by pharmacogenetically guided dosing in the per‐patient average percentage of INRs outside the therapeutic range (INR < 1.8 or > 3.2), which averaged 30.7% and 33.1% (P = 0.47) in the pharmacogenetic and standard groups respectively, and with three months of follow‐up. Of interest, more than one half (54%) of these out‐of‐range INRs were subtherapeutic. Also no difference was seen in the proportions of patients whose INRs were within the therapeutic range on the fifth day: 69.7% in the pharmacogenetic group and 68.3% in the standard dosing group (odds ratio [OR] 1.07, 95% CI 0.56 to 2.04, P = 0.85). Total adverse events (clinical events plus INR > 4) were not different between pharmacogenetic and standard groups. This study was the only study that compared a pharmacogenetic nomogram with the 10‐mg warfarin nomogram employed for the studies included in our review. In Caraco 2008, 191 patients with VTE and other pathologies were randomly assigned to receive warfarin by a validated algorithm, which is similar to the 5‐mg warfarin nomogram of our review, except that the doses given on days three and four were different ('control group', 96 patients), or to six different CYP2C9 genotype‐adjusted algorithms ('study group', 95 patients). The proportion of patients whose INRs were > 2 on the fifth day was 49% in study group and 11% in the control group (P < 0.001), and the study group experienced less minor bleeding (3.2% vs 12.5%, P < 0.002). Over‐anticoagulation was most prominent among control group patients who bled during the initiation phase with average INR of 4.89 ± 1.89 (95% CI 1.89 to 7.90). New thromboembolic events were not diagnosed in any groups. However, the results of this study should be interpreted carefully. The dose required to anticoagulate may vary in patients treated for different indications; this study included only patients with atrial fibrillation, DVT, and PE; therefore the applicability of the CYP2C9 genotype-based algorithms in patients with other indications should be confirmed; the algorithms used in this report required daily INR monitoring for the first eight days, which is almost never possible or required under non‐study conditions, and the proportion of patients with therapeutic INR in the control group was significantly lower than expected in comparison with similar arms from the other 5‐mg trials.
Overall, Mahtani 2012 suggest that considerable uncertainty still exists between a 10‐mg and a 5‐mg loading dose for initiation of warfarin in patients requiring anticoagulation; these investigators conclude that current evidence is insufficient to warrant genotype‐guided initiation, and adequately powered trials to detect effects on adverse events are warranted.
Authors' conclusions
Implications for practice.
In patients with acute thromboembolism aged 18 years or older, considerable uncertainty exists between the use of 10‐mg and 5‐mg loading doses for initiation of warfarin to achieve an INR of 2.0 to 3.0 on the fifth day of therapy. Heterogeneity among studies analyzed limits of certainty surrounding optimal warfarin initiation nomograms.
Implications for research.
Future clinical trials are warranted to compare the 5‐mg and 10‐mg warfarin nomograms in both inpatients and outpatients with venous thromboembolism. These should have sufficient numbers of participants to allow investigation of efficacy, safety, and cost‐effectiveness of the nomograms.
What's new
| Date | Event | Description |
|---|---|---|
| 11 November 2015 | New search has been performed | Searches were updated. No additional studies were included or excluded. |
| 11 November 2015 | New citation required but conclusions have not changed | Searches were updated. No additional studies were included or excluded. Minor changes made reflecting current Cochrane review standards. Conclusions not changed |
Acknowledgements
Editorial base staff of Cochrane Vascular.
Appendices
Appendix 1. CRS search strategy
| Search run on Wed Sep 16 2015 | ||
| #1 | MESH DESCRIPTOR Thrombosis | 1140 |
| #2 | MESH DESCRIPTOR Thromboembolism | 843 |
| #3 | MESH DESCRIPTOR Venous Thromboembolism | 168 |
| #4 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES | 1867 |
| #5 | (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*):TI,AB,KY | 15021 |
| #6 | MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES | 679 |
| #7 | (PE or DVT or VTE):TI,AB,KY | 3839 |
| #8 | ((vein* or ven*) near thromb*):TI,AB,KY | 5512 |
| #9 | (blood near3 clot*):TI,AB,KY | 2102 |
| #10 | (pulmonary near3 clot*):TI,AB,KY | 5 |
| #11 | (lung near3 clot*):TI,AB,KY | 4 |
| #12 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 | 19327 |
| #13 | MESH DESCRIPTOR Warfarin EXPLODE ALL TREES | 1014 |
| #14 | (warfarin* or Jantoven or Marevan or Lawarin or Waran or Warfant or coumadin* or coumarin* or coumadin or coumarin):TI,AB,KY | 2655 |
| #15 | ((vit* k) near3 antagon*):TI,AB,KY | 257 |
| #16 | #13 OR #14 OR #15 | 2806 |
| #17 | ((initial or initiation or induction or loading) near3 (dose* or dosage*)):TI,AB,KY | 6335 |
| #18 | ((dosing or dose* or dosage*) near5 (empirical or regime* or method* or protocol* or algorithm* or Kovacs or Fennerty or Tait)):TI,AB,KY | 40792 |
| #19 | MESH DESCRIPTOR Nomograms EXPLODE ALL TREES | 31 |
| #20 | Nomogram*:TI,AB,KY | 270 |
| #21 | #17 OR #18 OR #19 OR #20 | 46116 |
| #22 | #12 AND #16 AND #21 | 161 |
Appendix 2. Authors' PubMed search strategy
| #1 | Search Thrombosis[Mesh] | 145099 |
| #2 | Search Thromboembolism[Mesh] | 45054 |
| #3 | Search Venous Thromboembolism[Mesh] | 5319 |
| #4 | Search Venous Thrombosis[Mesh] | 45571 |
| #5 | Search thrombus*[tiab] OR thrombotic*[tiab] OR thrombolic*[tiab] OR thromboemboli*[tiab] OR thrombos*[tiab] OR embol*[tiab] | 254789 |
| #6 | Search Pulmonary Embolism[Mesh] | 32186 |
| #7 | Search PE[tiab] OR DVT[tiab] OR VTE[tiab] | 35494 |
| #8 | Search vein thromb*[tiab] OR venous thromb*[tiab] | 48272 |
| #9 | Search (vein*[ti] OR venous[ti]) AND thromb*[ti] | 29269 |
| #10 | Search ((((((((#1) OR #2) OR #3) OR #4) OR #5) OR #6) OR #7) OR #8) OR #9 | 333665 |
| #11 | Search warfarin[MeSH] | 15143 |
| #12 | Search warfarin[tiab] OR Jantoven[tiab] OR Marevan[tiab] OR Lawarin[tiab] OR Waran[tiab] OR Warfant[tiab] OR coumadin[tiab] OR coumarin[tiab] OR coumadin[tiab] OR coumarin[tiab] | 24984 |
| #13 | Search vitamin k antagon*[tiab] | 2771 |
| #14 | Search (vitamin k[ti] OR vit k[ti]) AND antagon*[ti] | 620 |
| #15 | Search (((#11) OR #12) OR #13) OR #14 | 31700 |
| #16 | Search Nomograms[MeSH] | 1467 |
| #17 | Search nomogram*[tiab] | 5136 |
| #18 | Search (initial[ti] OR initiation[ti] OR induction[ti] OR loading[ti]) AND (dose*[ti] OR dosage[ti]) | 3337 |
| #19 | Search (dosing[ti] OR dose*[ti] OR dosage*[ti]) AND (empirical[ti] OR regime*[ti] OR method[ti] OR protocol[ti] OR algorithm*[ti] OR Kovacs[ti] OR Fennerty[ti] OR Tait[ti]) | 6191 |
| #20 | Search (((#16) OR #17) OR #18) OR #19 | 14749 |
| #21 | Search ((#10) AND #15) AND #20 | 97 |
Appendix 3. Authors' LILACS search strategy
| #1 | venous AND thrombosis AND warfarin | 27 |
| #2 | embolism AND pulmonary AND warfarin | 16 |
Data and analyses
Comparison 1. 10 mg nomogram versus 5 mg nomogram.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Therapeutic INR | 3 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.05, 1.54] |
| 2 Recurrent venous thromboembolism at 90 days | 2 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.39, 5.56] |
| 3 Major bleeding at 14 days | 2 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.23, 12.34] |
| 4 Major bleeding at 90 days | 2 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.11, 3.70] |
| 5 Major bleeding at 14‐90 days | 4 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.27, 3.51] |
| 6 Minor bleeding at 14‐90 days | 2 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.15, 1.83] |
| 7 Length of hospital stay at 90 days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
Comparison 1 10 mg nomogram versus 5 mg nomogram, Outcome 1 Therapeutic INR.
1.2. Analysis.
Comparison 1 10 mg nomogram versus 5 mg nomogram, Outcome 2 Recurrent venous thromboembolism at 90 days.
1.3. Analysis.
Comparison 1 10 mg nomogram versus 5 mg nomogram, Outcome 3 Major bleeding at 14 days.
1.4. Analysis.
Comparison 1 10 mg nomogram versus 5 mg nomogram, Outcome 4 Major bleeding at 90 days.
1.5. Analysis.
Comparison 1 10 mg nomogram versus 5 mg nomogram, Outcome 5 Major bleeding at 14‐90 days.
1.6. Analysis.
Comparison 1 10 mg nomogram versus 5 mg nomogram, Outcome 6 Minor bleeding at 14‐90 days.
1.7. Analysis.
Comparison 1 10 mg nomogram versus 5 mg nomogram, Outcome 7 Length of hospital stay at 90 days.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Farahmand 2011.
| Methods |
Study design: randomized, controlled clinical trial. Method of randomization: randomization was performed on outpatients admitted for acute deep vein thrombosis between April 2005 and 2007 on the basis of a computer‐based randomization program; an Excel program was used for randomization of participants between two groups (García 2012a [pers comm]). Concealment of allocation: authors mentioned that investigators and coordinators were unaware of the group to which participants were allocated. They did not detail the concrete method that was used to conceal sequence. In light of the study conducted by Kovacs 2003, a specific nomogram was used in each group to determine the required warfarin dose on the basis of INR values reported during the study period. Blinded: double (physician–patient). Power calculation: the sample size was calculated to be about 63 participants in each treatment group, so that the study would have power of 95% to yield an SD of 1 for INR levels and an α level of 0.05. Because of the 95% sensitivity of Doppler ultrasound in detecting acute proximal DVT and possible dropouts, some three additional participants were recruited in each group. Number of participants randomly assigned: 132. Number of participants analyzed: 132. Number of withdrawals and reasons: none. Intention‐to‐treat analysis: yes. Source of funding: not stated. Potential financial conflicts of interest:none disclosed. |
|
| Participants |
Country: Iran. Number of centers: 1. Location: emergency department (ED) of Imam Khomeini Hospital in Tehran, Iran. Source of participants: 10‐mg nomogram group: 66 participants. 5‐mg nomogram group: 66 participants. Age: mean ± SD, years. 10‐mg nomogram group: 45.8 ± 14.5 years (P = 0.07). 5‐mg nomogram group: 47.2 ± 17.5 years. Sex: men/women, n/n. 10‐mg nomogram group: 32/34. 5‐mg nomogram group: 40/26. Inclusion criteria: consecutive outpatients with suspected acute VTE (deep venous thrombosis) in whom the diagnosis was confirmed by color Doppler ultrasound. Exclusion criteria: participants with INR levels > 1.4 before initiation of treatment, platelet count < 50,000, severe hypertension (systolic blood pressure ≥ 200 mm Hg or diastolic blood pressure ≥ 120 mm Hg), and active bleeding, along with participants younger than 18 years of age; those with a positive history of ocular or neurosurgical surgery, intracranial hemorrhage in the past 10 days, documented liver disease, and bleeding disorders; and those who had used anticoagulants in the past 2 weeks. |
|
| Interventions |
Treatment(s): Intervention: 66 participants were assigned to the 10‐mg nomogram group. Control: 66 participants were assigned to the 5‐mg nomogram group. Treatment was started with the subcutaneous injection of a single dose of enoxaparin (1.5 mg/kg) per day along with 5 or 10 mg warfarin, depending on the participant's group. The regimen was continued for 3 days; thereafter, the amount of prescribed warfarin was modified on the basis of INR levels. Throughout the study period, warfarin was prescribed early in the morning, and INR levels were evaluated at 10:00 AM. Duration: 14 days. |
|
| Outcomes |
Outcomes: INR was checked daily on the first 7 days of the study and on the 14th day. During this time, participants were asked daily for possible signs of bleeding. Third day: mean of INR. 10‐mg nomogram group: 2.0636 ± 0.5979. 5‐mg nomogram group: 1.5667 ± 0.4145, P = 0.000. Fourth day: mean of INR, 10‐mg nomogram group: 2.7758 ± 0.7764. 5‐mg nomogram group: 1.8652 ± 0.6560, P = 0.004. Fifth day: mean of INR. 10‐mg nomogram group: 3.2742 ± 0.5936. 5‐mg nomogram group: 2.1561 ± 0.6805, P = 0.912. Seventh day: mean of INR. 10‐mg nomogram group: 3.2394 ± 0.3687. 5‐mg nomogram group: 2.6970 ± 0.6411, P = 0.024. Fourteenth day: mean of INR. 10‐mg nomogram group: 3.2467 ± 0.3170. 5‐mg nomogram group: 2.7773 ± 0.3062, P = 0.769. Proportions of participants whose INRs were within the therapeutic range (2.0 to 3.0) on the fifth day: n (%). Data were taken from the published table. 10‐mg nomogram group: 18 (27.27%). 5‐mg nomogram group: 31 (46.97%). Incidence of major bleeding within 14 days of follow‐up: n (%) 10‐mg nomogram group: 1 (1.52%). 5‐mg nomogram group: 1 (1.52%). Incidence of minor bleeding within 14 days of follow‐up: n 10‐mg nomogram group: 1 (1.52%). 5‐mg nomogram group: 0. |
|
| Notes | To add any information: Outcomes not included at protocol: Proportion of participants with 3 < INR < 5 on the seventh day: n (%) 10‐mg nomogram group: 38 (57.58%). 5‐mg nomogram group: 8 (12.12%). Proportion of participants with 3 < INR < 5 on the fourteenth day: n (%) 10‐mg nomogram group: 15 (22.73%). 5‐mg nomogram group: 7 (10.61%). Proportion of participants with INR > 5 on the seventh and fourteenth days: n (%) 10‐mg nomogram group: 0%. 5‐mg nomogram group: 0%. Mortality: It was not reported. The nomograms of 5 mg and 10 mg are similar to those in the study of Kovacs 2003. It should be stressed that this is the first of such a study conducted on outpatients referred to an ED. A significant difference was noted between INR levels of the 2 groups on the third, fourth, and seventh days; those reported on days 1, 5, and 14, however, were not statistically different. Invitation to authors to add and audit review data: yes, extra data provided. García 2012a [pers comm]: "We use Excel program for randomisation of participants between two groups. In 5‐mg group, we have one case of GI bleeding (24‐year‐old illegal drug user man) and in 10‐mg group one case of GI bleeding (68‐year‐old man suffering from GI malignancy) and one case of nose bleeding." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study authors mentioned that participants were allocated to receive 5 or 10 mg warfarin on the basis of a computer‐based randomization program and used an Excel program for randomization of participants between two groups (García 2012a [pers comm]). |
| Allocation concealment (selection bias) | Unclear risk | The study authors mentioned that investigators and coordinators were unaware of the group to which participants were allocated. They did not detail the concrete method that was used to conceal sequence. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It is a double‐blind (physician‐patient) controlled trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed up for 14 days. Losses to follow‐up: none. |
| Selective reporting (reporting bias) | Low risk | The dates are objectively measured. |
| Other bias | Low risk | Probably free of other bias sources. |
Kovacs 1998.
| Methods |
Study design: randomized clinical trial. Method of randomization: randomization was computer‐generated (blocks of 4 or 6 participants) and stratified. It was performed for the type of heparin used (standard heparin or low molecular weight heparin) and according to whether VTE was a secondary diagnosis (i.e., developed while patient was in the hospital for another diagnosis) or the primary diagnosis (i.e., the reason for hospital admission). Concealment of allocation: yes. Randomization was central with computer‐generated blocks of 4 or 6. Blinded: single (for participants randomly assigned to the nomogram, study investigators ordered warfarin. The attending physicians were unaware that their method of ordering warfarin was being monitored. Study investigators did not order warfarin for participants on the standard arm.) Power calculation: unknown. Number of participants randomly assigned: 111. Number of participants analyzed: 111. Number of withdrawals and reasons: 0. Intention‐to‐treat analysis: yes. Source of funding: not stated. |
|
| Participants |
Country: Canada. Number of centers: 3. Location: teaching hospitals. Source of participants: Canada. 10‐mg nomogram group: 57 participants. Standard practice group: 54 participants. Age: mean age ± SD, years. 10‐mg nomogram group: 57 ± 17.6. Standard practice group: 64.5 ± 15.7 (P = 0.036). Sex: men/women: unknown. Inclusion criteria: consecutive patients,older than 18 years, with an objectively confirmed diagnosis of VTE: deep venous thrombosis (DVT) or pulmonary embolism (PE). Exclusion criteria: participants with baseline INR > 1.4, younger than 18 years of age, received oral anticoagulant therapy within the previous two weeks, pregnant or of childbearing potential, and not using adequate contraception. |
|
| Interventions |
Treatment(s): 10‐mg nomogram group: 57 participants received their warfarin dose as determined by nomogram. Control (standard practice group): 54 participants received their warfarin dose as determined by attending physicians according to their usual practice (see below for further information). Participants were treated with standard heparin according to a nomogram‐ or weight‐adjusted low molecular weight heparin. Warfarin or low molecular weight heparin therapy was initiated on the second day of heparin to ensure time to achieve a therapeutic activated partial thromboplastin time (aPTT). Duration: three months. |
|
| Outcomes |
Incidence of RVTE (3 months): n (%) 10‐mg nomogram group: 2 (3.51%). Standard practice group: 3 (5.56%). Incidence of major bleeding (3 months): n (%) 10‐mg nomogram group: 1 (1.75%). Standard practice group: 2 (3.51%). Incidence of minor bleeding (3 months): n (%) 10‐mg nomogram group: 2 (3.51%). Standard practice group: 6 (11.11%). Length of hospital stay: mean ± SD, days 10‐mg nomogram group: 8.0 ± 7.6. Standard practice group: 10.3 ± 19.9. |
|
| Notes | To add any information: Outcomes not included at protocol: Proportion of participants with INR > 4.5 on the first week: n (%) 10‐mg nomogram group: 9 (15.7%). Standard practice (5‐mg nomogram group): 6 (11.1%). Mortality at 90 days: n (%) 10‐mg nomogram group: 5 (8.77%). Standard practice (50 mg nomogram group): 11 (20.37%). Invitation to authors to add and audit review data: yes. According to the information requested of the original investigators, the usual practice consisted of a warfarin initiation dosing of 5 mg in most cases (García 2012b [pers comm]). We asked for the following additional information: proportions of participants with an INR of 2.0 to 3.0 on the fifth day of initial therapy with warfarin: "I have been looking for the info that you have requested and I cannot find the original data sheets. Unfortunately, my colleague Dr. Cruickshank who did the data analysis died several years ago" (García 2011 [pers comm]. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was central with computer‐generated blocks of 4 or 6. |
| Allocation concealment (selection bias) | Low risk | Randomization was central with computer‐generated blocks of 4 or 6. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single (in participants randomly assigned to the nomogram: study investigators ordered the warfarin). Attending physicians were unaware that their method of ordering warfarin was being monitored. Study investigators did not order warfarin for participants on the standard arm. Outcome measurements are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After discharge, all participants were followed up for three months. |
| Selective reporting (reporting bias) | Low risk | The dates are objectively measured. |
| Other bias | Low risk | Probably free of other bias sources. |
Kovacs 2003.
| Methods |
Study design: randomized controlled clinical trial. Method of randomization: the randomization sequence was computer‐generated by the trial statistician. Also the randomization was stratified by study center and the presence of active malignant disease. Concealment of allocation: yes. The details of the randomization sequence, which were not known to the investigators or to the study coordinator, were contained in sets of sequentially numbered, opaque, sealed envelopes. The outside of each envelope was marked only with the name of the hospital, whether the participant had a malignant condition, and a participant number. Blinded: double (physician‐patient). Power calculation: we calculated that 92 participants per group would be required to show a 0.5‐day difference in time to a therapeutic INR (90% power; two‐sided α = 0.05). Number of participants randomly assigned: 201. Number of participants analyzed: 201. Number of withdrawals and reasons: none. Intention‐to‐treat analysis: yes. Source of funding: not stated. Potential financial conflicts of interest:none disclosed. |
|
| Participants |
Country: Canada. Number of centers: 4. Location: thrombosis clinics of four Canadian academic centers. Source of participants: Canada. 10‐mg nomogram group: 104 participants. 5‐mg nomogram group: 97 participants. Age: mean age ± SD, years. 10‐mg nomogram group: 55 ± 17.4. 5‐mg nomogram group: 55.6 ± 17.2. Sex: men/women, n/n 10‐mg nomogram group: 65/39. 5‐mg nomogram group: 47/50. Inclusion criteria: outpatients, older than 18 years and with a diagnosis of objectively confirmed acute VTE:DVT or PE. Exclusion criteria: participants with baseline INR > 1.4, had thrombocytopenia (platelet count < 50 × 109 cells/mL), were younger than 18 years of age, required hospitalization, had received oral anticoagulant therapy within the previous two weeks, or were at high risk for major bleeding (as judged by the attending physician). |
|
| Interventions |
Treatment(s): Intervention: 104 participants were assigned to the 10‐mg nomogram group. Control: 97 participants were assigned to the 5‐mg nomogram group. Treatment was initiated on the first day (day 1) with subcutaneous low molecular weight heparin (dalteparin 200 U/kg of body weight) or tinzaparin (175 U/kg). Low molecular weight heparin was continued for a minimum of five daily injections until the INR was therapeutic (> 1.9). Duration: 90 days. If a patient did not have a therapeutic INR by day 5, the INR was measured daily until it was therapeutic. Local attending physicians directed management of warfarin monitoring from day 8 to day 90. |
|
| Outcomes |
Proportion of participants whose INRs were within the therapeutic range (2.0 to 3.0) on the fifth day: n (%) 10‐mg nomogram group: 86 (83%). 5‐mg nomogram group: 45 (46%), P < 0.001. Incidence of RVTE within 90 days of diagnosis: n (%) 10‐mg nomogram group: 3 (2.9%). 5‐mg nomogram group: 0 (0%), P = 0.09. Incidence of major bleeding within 90 days of diagnosis: n (%) 10‐mg nomogram group: 1 (0.96%). 5‐mg nomogram group: 1 (1.03%). |
|
| Notes | To add any information: Outcomes not included at protocol: Proportion of participants with INR > 3 in the first 4 weeks: n (%) 10‐mg nomogram group: 81 (77.88%). 5‐mg nomogram group: 84 (86.60%), P > 0.2. Proportion of participants with INR > 5 in the first 4 weeks: n (%) 10‐mg nomogram group: 9 (8.65%). 5‐mg nomogram group: 11 (11.34%), P > 0.2. Mortality at 90 days: n (%) 10‐mg nomogram group: 0. 5‐mg nomogram group: 1 (10.31%). The study included participants with cancer: n (%) 10‐mg nomogram group: 26 (25%). 5‐mg nomogram group: 22 (23%). This study was limited in its power for safety assessment. Invitation to authors to add and audit review data: not necessary. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization sequence was computer‐generated by the trial statistician. |
| Allocation concealment (selection bias) | Low risk | The details of the randomization sequence were contained in sets of sequentially numbered, opaque, sealed envelopes. The outside of each envelope was marked only with the name of the hospital, whether the participant had a malignant condition, and a participant number. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double (physician‐patient): An adjudication committee consisting of three study investigators evaluated all clinical events in a blinded fashion, and end points were determined by consensus. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed for three months. Losses to follow‐up: none. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
| Other bias | Low risk | Probably free of other bias sources. |
Quiroz 2006.
| Methods |
Study design: open‐label, randomized trial. Method of randomization: Participants were randomly assigned in a 1:1 computer‐generated allocation to one of the treatment groups. Blinded: single for outcome assessment. Power calculation: 90% two sided α = 0.05. Number of participants randomly assigned: 50. Number of participants analyzed: 50. Number of withdrawals and reasons: none. Intention‐to‐treat analysis: yes. Source of funding: pharmaceutical: Sanofi‐Syntelabo, New York. |
|
| Participants |
Country: USA, Switzerland. Number of centers: 2. Location: Massachussetts, Zurich. Source of participants: N: 50. 10‐mg nomogram group: 25 participants. Inpatients/outpatients: 14/11. 5‐mg nomogram group: 25 participants. Inpatients/outpatients: 9/16. Age: mean ± SD 10‐mg nomogram group: 50 ± 17 years. 5‐mg nomogram group : 51 ± 17 years. Sex: men/women 10‐mg nomogram group: 13/12. 5‐mg nomogram group: 14/11. Inclusion criteria: participants older than 18 years and with deep venous thrombosis (DVT) confirmed by venous ultrasound or pulmonary embolism (PE) by chest computed tomography. Exclusion criteria: Participants were unable to provide written consent, had received treatment with unfractionated heparin, warfarin, or low molecular weight heparin for > 36 hours, had life expectancies of < 3 months, were unable to participate in the 2‐week follow‐up office visits, were < 18 years of age, had estimated creatinine clearances of < 30 mL/min, had contraindications to anticoagulation, or were at high risk for major bleeding. |
|
| Interventions |
Treatment(s):
Intervention: 25 participants were assigned to the 10‐mg nomogram group. Control: 25 participants were assigned to the 5‐mg nomogram group. All participants received fondaparinux for ≥ 5 days as a bridge to warfarin using the 5‐mg or 10‐mg warfarin initiation nomogram. The fondaparinux dose was administered subcutaneously once daily of 5.0 mg for weight < 50 kg, 7.5 mg for weight from 50 to 100 kg, and 10.0 mg for weight > 100 kg. Duration: 14 days. |
|
| Outcomes |
Proportion of participants whose INRs were within the therapeutic range (2.0 to 3.0) on the fifth day: n (%) 10‐mg nomogram group: 14 (56%), P > 0.5. 5‐mg nomogram group: 13 (52%). Incidence of RVTE within 14 days of diagnosis: 10‐mg nomogram group: 0. 5‐mg nomogram group: 0. Incidence of major bleeding (14 days): n (%) 10‐mg nomogram group: 1 (4%). Participant with an abdominal hematoma on day 4, with an INR of 2.2 (P > 0.5). 5‐mg nomogram group: 0 (P > 0.5). |
|
| Notes |
Additional information: Outcomes not included at protocol: Overall frequencies of INR > 3 within 2 weeks: 10‐mg nomogram group: 3.7%. 5‐mg nomogram group: 14.5%. Proportion of participants with INR > 5 within 2 weeks: 0 Mortality within 2 weeks: 0 The study included participants with cancer: n (%) 10‐mg nomogram group: 4 (16%). 5‐mg nomogram group: 7 (28%). Invitation to authors to add and audit review data: yes. It is important to know numbers of outpatients and inpatients who achieved therapeutic INR. Unfortunately we did not receive a response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in a 1:1 computer‐generated allocation to one of the treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | This study was an open‐label, randomized trial. The details of the allocation concealment are not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single for adjudication of outcome events. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed for 14 days. |
| Selective reporting (reporting bias) | Low risk | The dates were objectively measured. |
| Other bias | Low risk | Probably free of other bias sources. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Crowther 1997 | 1. Types of participants: the diseases of participants are unknown. Probably participants were being given warfarin for reasons other than acute VTE. 2. Types of interventions: the nomograms are not explicitly indicated. NOTE: We contacted the author for clarification, but unfortunately we did not receive a response. |
| Crowther 1999 | 1. Types of participants: 29% of participants of 10‐mg nomogram group and 41% of participants of 5‐mg nomogram group did not have VTE. 2. Types of interventions: for a given day and INR, the dose is not explicitly indicated other than a dosage range; therefore it is uncertain as to the exact dose that each participant received. These nomograms could not be reproduced. NOTE: We contacted the author, but unfortunately we did not receive a response. |
| Crowther 2002 | 1. Types of interventions: Authors did not use warfarin initiation nomograms of 5 mg and 10 mg. 2. Objective: Authors did not evaluate the efficacy of warfarin initiation nomograms for VTE. |
| Harrison 1997 | 1. Types of participants: 12.5% of participants of 5‐mg nomogram group and 28% of participants of 10‐mg nomogram group did not have VTE (Crowther 1997a). 2. Types of interventions: For a given day and INR, the dose is not explicitly indicated other than a dosage range, and so it is uncertain as to the exact dose each participant received. These nomograms were published later in a letter and then could not be reproduced (Crowther 1997a; Kovacs 2003). NOTE: We contacted the author, but unfortunately we did not receive a response. |
| Pineo 1997 | Types of participants: they are not participants with a diagnosis of VTE. |
| Shulman 1984 | This study was carried out before the year 1989. It does not use the international normalized ratio (INR) as the outcome measure. |
Differences between protocol and review
According to updated guidelines, the quality of the studies has been assessed using the 'Risk of bias' tool from The Cochrane Collaboration (Higgins 2011).
The outcomes RVTE, major bleeding, and minor bleeding have been clarified through the addition of definitions.
Contributions of authors
P García conceived the idea for this systematic review and has coordinated its development. He screened search results, selected articles for inclusion, assessed trial eligibility, extracted data and assessed the risk of bias of included studies.
W Ruiz screened search results, selected articles for inclusion, assessed trial eligibility, extracted data, and assessed the risk of bias of included studies.
C Loza Munarriz resolved disagreements in trial selection, assessment of trial quality and data extraction.
P García, C Loza Munarriz and W Ruiz developed the search strategy.
Sources of support
Internal sources
No sources of support supplied
External sources
Iberoamerican Cochrane Center, Spain.
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
P García: has declared that he has received payments for educational presentations on Octaplex from Farmadual, on Ampholip from Pharmaris, on Cerezyme from Genzyme and on Ferinject from OmPharma S.A..These are not related to this review. W Ruiz: none known C Loza Munarriz: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Farahmand 2011 {published data only}
- Farahmand S, Saeedi M, Javadi H, Khashayar P. High doses of warfarin are more beneficial than its low doses in patients with deep vein thrombosis. American Journal of Emergency Medicine 2011;29(9):1222‐6. [DOI] [PubMed] [Google Scholar]
Kovacs 1998 {published data only}
- Kovacs MJ, Cruickshank M, Wells PS, Kim H, Chin‐Yee I, Morrow B, et al. A randomized assessment of a warfarin nomogram for initial oral anticoagulation post venous thromboembolic disease. Thrombosis and Haemostasis 1997;78(Suppl June):583. Abstract No. OC‐2383. [DOI] [PubMed] [Google Scholar]
- Kovacs MJ, Cruickshank M, Wells PS, Kim H, Chin‐Yee I, Morrow B, et al. Randomized assessment of a warfarin nomogram for initial oral anticoagulation after venous thromboembolic disease. Haemostasis 1998;28(2):62‐9. [DOI] [PubMed] [Google Scholar]
Kovacs 2003 {published data only}
- Kovacs MJ, Rodger M, Anderson DR, Morrow B, Kells G, Kovacs J, et al. Comparison of 10‐mg and 5‐mg warfarin initiation nomograms together with low‐molecular‐weight heparin for outpatient treatment of acute venous thromboembolism. Annals of Internal Medicine 2003;138(9):714‐9. [DOI] [PubMed] [Google Scholar]
Quiroz 2006 {published data only}
- Quiroz R, Gerhard‐Herman M, Kosowsky JM, DeSantis SM, Kucher N, Mckean SC, et al. Comparison of a single end point to determine optimal initial warfarin dosing (5 mg versus 10 mg) for venous thromboembolism. American Journal of Cardiology 2006;98(4):535‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Crowther 1997 {published data only}
- Crowther MA, Harrison L, Ginsberg JS, Massicotte MP, Johnson J, Willan A, et al. A randomized comparison of a 5 mg and 10 mg loading dose for the initiation of warfarin therapy. Thrombosis and Haemostasis 1997;78(Suppl June):583‐Abstract No OC‐2382. [Google Scholar]
Crowther 1999 {published data only}
- Crowther MA, Ginsberg JB, Kearon C, Harrison L, Johnson J, Massicotte P, et al. A randomized trial comparing 5‐mg and 10‐mg warfarin loading doses. Archives of Internal Medicine 1999;159(1):46‐8. [DOI] [PubMed] [Google Scholar]
Crowther 2002 {published data only}
- Crowther MA, Ginsberg JS, Gent M, Julian J, Constantini L, Kovacs M, et al. A randomized trial of two intensities of warfarin (international normalized ratio of 2.0 to 3.0 versus 3.1 to 4.0) for the prevention of recurrent thrombosis in patients with antiphospholipid antibodies. Blood 2002;100(2):148a. Abstract 555. [Google Scholar]
Harrison 1997 {published data only}
- Harrison L, Johnston M, Massicotte P, Crowther M, Moffat K, Hirsh J. Comparison of 5‐mg and 10‐mg loading doses in initiation of warfarin therapy. Annals of Internal Medicine 1997;126(2):133‐6. [DOI] [PubMed] [Google Scholar]
- Hirsh J. Comparison of 5 mg with 10 mg initial dose of warfarin for the induction of an oral anticoagulation [Vergleich einer 5‐mg‐ mit einer 10‐mg initialdosis zur einleitung einer oralen antikoagulation mit warfarin]. Hamostaseologie 1997;17:153‐5. [Google Scholar]
Pineo 1997 {published data only}
- Pineo GF, Hull RD, Brant R, Raskob G, Rosenbloom D. The use of a nomogram to control warfarin therapy: INR data. Thrombosis and Haemostasis 1997;78(Suppl June):700. [Google Scholar]
Shulman 1984 {published data only}
- Shulman S, Lockner D, Bergstrom K, Blomback M. Intensive initial oral anticoagulation and shorter heparin treatment in deep vein thrombosis. Thrombosis and Haemostasis 1984;52(3):276‐80. [PubMed] [Google Scholar]
Additional references
Ageno 2001
- Ageno W, Turpie AG, Steidl L, Ambrosini F, Cattaneo R, Codari RL, et al. Comparison of a daily fixed 2.5‐mg warfarin dose with a 5‐mg, international normalized ratio adjusted, warfarin dose initially following heart valve replacement. American Journal of Cardiology 2004;88(1):40‐4. [DOI] [PubMed] [Google Scholar]
Anderson 2007
- Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP, et al. Couma‐Gen Investigators. Randomized trial of genotype‐guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 2007;116(22):2563‐70. [DOI] [PubMed] [Google Scholar]
Ansell 2008
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G, American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines(8th Edition). Chest 2008;133(6 Suppl):160S‐98S. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1491‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baglin 2005
- Baglin TP, Keeling DM, Watson HG, the British Committee for Standards in Haematology. Guidelines on oral anticoagulation (warfarin): third edition, 2005 update. British Society for Haematology 2005;132(3):277‐85. [DOI] [PubMed] [Google Scholar]
Baker 2004
- Baker RI, Coughlin PB, Salem HH, Gallus AS, Harper PL, Wood EM, the Warfarin Reversal Consensus Group. Warfarin reversal: consensus guidelines, on behalf of the Australasian Society of Thrombosis and Haemostasis. Medical Journal of Australia 2004;181(9):492‐7. [DOI] [PubMed] [Google Scholar]
Bhutia 2013
- Bhutia S, Wong PF. Once versus twice daily low molecular weight heparin for the initial treatment of venous thromboembolism. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003074.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burmester 2011
- Burmester JK, Berg RL, Yale SH, Rottscheit CM, Glurich IE, Schmelzer JR, et al. A randomised controlled trial of genotype‐based coumadin initiation. Genetics in Medicine 2011;13(6):509‐18. [DOI] [PubMed] [Google Scholar]
Büller 2004
- Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(3 Suppl):401S‐28S. [DOI] [PubMed] [Google Scholar]
Caraco 2008
- Caraco Y, Blotnick S, Muszkat M. CYP2C9 genotype‐guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study. Clinical Pharmacology and Therapeutics 2008;83(3):460‐70. [DOI] [PubMed] [Google Scholar]
COAG trial
- Clarification of Optimal Anticoagulation Through Genetics (COAG). ClinicalTrials.gov Identifier: NCT00839657. [ClinicalTrials.gov Identifier: NCT00839657]
Crowther 1997a
- Crowther M, Harrison L, Hirsh J. Warfarin: less may be better. Annals of Internal Medicine 1997;127:332‐3. [Google Scholar]
Eckhoff 2004
- Eckhoff CD, Didomenico RJ, Shapiro NL. Initiating warfarin therapy: 5 mg versus 10 mg. Annals of Pharmacotherapy 2004;38(12):2115‐21. [DOI] [PubMed] [Google Scholar]
Erkens 2010
- Erkens PMG, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD001100.pub3] [DOI] [PubMed] [Google Scholar]
FDA 2007
- US Food, Drug Administration. FDA approves updated warfarin (coumadin) prescribing information. New genetic information may help providers improve initial dosing estimates of the anticoagulant for individual patients. http://www.fda.gov/bbs/topics/NEWS/2007/NEW01684.html. 16 Aug, 2007 (accessed 8 January 2013).
García 2011 [pers comm]
- García P. Proportion of patients with an INR of 2 to 3 on the fifth day of initial therapy with warfarin [personal communication]. Email to: M Kovacs 20 May 2011.
García 2012a [pers comm]
García 2012b [pers comm]
- García P. Giving warfarin according to usual practice [personal communication]. Email to: M Kovacs 22 August 2012.
Gedge 2000
- Gedge J, Orme S, Hampton KK, Channer KS, Hendra TJ. A comparison of a low‐dose warfarin induction regimen with the modified Fennerty regimen in elderly inpatients. Age and Ageing 2000;29:31‐4. [DOI] [PubMed] [Google Scholar]
Geerts 2004
- Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(3 Suppl):338S‐400S. [DOI] [PubMed] [Google Scholar]
GIFT trial
- Genetics Informatics Trial (GIFT) of Warfarin to Prevent DVT. ClinicalTrials.gov Identifier: NCT01006733. [ClinicalTrials.gov Identifier: NCT01006733]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hillman 2005
- Hillman MA, Wilke RA, Yale SH, Vidaillet HJ, Caldwell MD, Glurich I, et al. A prospective, randomized pilot trial of model‐based warfarin dose initiation using CYP2C9 genotype and clinical data. Clinical Medicine and Research 2005;3(3):137‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirsh 2001
- Hirsh J, Anand SS, Halperin JL, Fuster V. Guide to anticoagulant therapy. Heparin: a statement for healthcare professionals from the American Heart Association. Circulation 2001;103(24):2994‐3018. [DOI] [PubMed] [Google Scholar]
Hirsh 2003
- Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation 2003;107(12):1692‐711. [DOI] [PubMed] [Google Scholar]
Holbrook 2005
- Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, et al. Systematic overview of warfarin and Its drug and food interactions. Archives of Internal Medicine 2005;165(10):1095‐106. [DOI] [PubMed] [Google Scholar]
Levine 1996
- Levine M, Gent M, Hirsh J, Leclerc J, Anderson D, Weitz J, et al. A comparison of low‐molecular‐weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep‐vein thrombosis. New England Journal of Medicine 1996;334(11):677‐81. [DOI] [PubMed] [Google Scholar]
Linkins 2003
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta‐analysis. Annals of Internal Medicine 2003;139(11):893‐900. [DOI] [PubMed] [Google Scholar]
Mahtani 2012
- Mahtani KR, Heneghan CJ, Nunan D, Bankhead C, Keeling D, Ward AM, et al. Optimal loading dose of warfarin for the initiation of oral anticoagulation. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008685.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Merli 2001
- Merli G, Spiro TE, Olsson CG, Abildgaard U, Davidson BL, Eldor A, et al. Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Annals of Internal Medicine 2001;134(3):191‐202. [DOI] [PubMed] [Google Scholar]
Quinlan 2004
- Quinlan DJ, McQuillan A, Eikelboom JW. Low‐molecular‐weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta‐analysis of randomized controlled trials. Annals of Internal Medicine 2004;140(3):175‐83. [DOI] [PubMed] [Google Scholar]
Review Manager 5.1 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.1.. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Roberts 1999
- Roberts GW, Druskeit T, Jorgensen LE, Wing LM, Gallus AS, Miller C, et al. Comparison of an age adjusted warfarin loading protocol with empirical dosing and Fennerty's protocol. Australian and New Zealand Journal of Medicine 1999;29(5):731‐6. [DOI] [PubMed] [Google Scholar]
Shine 2003
- Shine D, Patel J, Kumar J, Malik A, Jaeger J, Maida M, et al. A randomized trial of initial warfarin dosing based on simple clinical criteria. Thrombosis and Haemostasis 2003;89(2):297‐304. [PubMed] [Google Scholar]
The Columbus Investigators 1997
- The Columbus Investigators. Low‐molecular‐weight heparin in the treatment of patients with venous thromboembolism. New England Journal of Medicine 1997;337(10):657‐62. [DOI] [PubMed] [Google Scholar]
Weinmann 1994
- Weinmann EE, Salzman EW. Deep‐vein thrombosis. New England Journal of Medicine 1994;331(24):1630‐41. [DOI] [PubMed] [Google Scholar]
White 2003
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(23 Suppl 1):I‐4‐I‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Garcia 2009
- Garcia P, Ruiz W, Loza Munarriz C. Warfarin initiation nomograms for venous thromboembolism. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007699] [DOI] [Google Scholar]
Garcia 2013
- Garcia P, Ruiz W, Loza Munarriz C. Warfarin initiation nomograms for venous thromboembolism. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD007699.pub2] [DOI] [PubMed] [Google Scholar]


