Abstract
Background relevance:
A plethora of literature is available regarding the clinical trials for natural products however; no information is available for critical assessments of the quality of these clinical trials.
Aim of study:
This is a first time report to critically evaluate the efficacy, safety and large scale applications of up-to-date clinical trials for diabetes, based on the three scales of Jadad, Delphi, and Cochrane.
Methodology:
An in-depth and extensive literature review was performed using various databases, journals, and books. The keywords searched included, “clinical trials,” “clinical trial in diabetes,” “diabetes,” “natural products in diabetes,” “ethnopharmacological relevance of natural products in diabetes,” etc.
Results:
Based on eligibility criteria, 16 plants with 74 clinical trials were found and evaluated. Major drawbacks observed were; “non-randomization and blindness of the studies,” “non-blindness of patients/healthcare/outcome assessors,” “lack of patient compliance and co-intervention reports,” “missing information regarding drop-out/withdrawal procedures,” and “inappropriate baseline characteristics.” Principal component analysis and Pearson correlation revealed four components with %variability; PC1: 23.12, PC2: 15.83, PC3: 13.11, and PC4: 11.38 (P ≤ .000). According to descriptive statistics, “non-blinding of outcome assessors” was the major drawback (82%) whereas, “not mentioning the timing of outcome assessment” was observed lowest (6.8%). An in-house quality grading (scale 0–24) classified these clinical trials as; poor (67.6%), acceptable (19.9%), and good quality trials (13.5%).
Conclusion:
Proper measures in terms of more strict regulations with pharmacovigilance of plants are utmost needed in order to achieve quality compliance of clinical trials.
Keywords: clinical trials, Delphi, diabetes, Jadad, natural products, systemic review
1. Introduction
“Diabetes Mellitus,” a term coined from Greek language where “Diabetes” stands for “a passer through” and “Mellitus” for “sweet.”[1] Diabetes mellitus (DM) is a metabolic disorder which leads to chronic hyperglycemia, the pathogenesis for which may include defects in insulin secretion, action or both.[2] Chronic autoimmune disease is considered a dominant cause behind insulin-dependent-diabetes (IDDM) which selectively destructs insulin secreting pancreatic β-cells and is treated by insulin. Non-insulin dependent diabetes mellitus (NIDDM) or type 2 diabetes is caused; due to insufficient insulin secretion via dysfunctional pancreatic β-cell, or insulin dysfunction due to decreased insulin sensitivity. First line treatment for NIDDM includes diet control and lifestyle modification; however, in case the diseases progresses, the use of oral hypoglycemic drugs is considered the next approach for treatment.[3,4] According to International Diabetes Federation (IDF) report in 2019; the estimated population with diabetes was 463 million adults which has been projected to raise up to 578 M adults by 2030 and 700 M by 2045.[5] This urges a proper treatment plan in order to lessen the prevalence of diabetes. The researchers are focusing more on natural products strategies in order to find an appropriate cure for diabetes. Several natural products have been successfully utilized to reduce the blood glucose level in the shape of pre-clinical and clinical studies.[6] For instance, Magnesium (Mg) has been applied to recover Mg deficiencies and help relieve insulin resistance,[7] cinnamon (known for insulin-like effect) has been reported to decrease blood sugar,[8] and zinc has been studied to regulate insulin receptors and extend insulin action.[9] Likewise, numerous plants have been observed with prominent folklore applications in various communities such as; bitter melon is considered a traditional plant to treat diabetes in Asia, South America, India, the Caribbean and East Africa,[10] and fenugreek is used since long to cure diabetes in Mediterranean, Asian, North African, and European communities.[11] Clinical research is a wide term that describe studies or trials conducted in human population[12] with a vital role in developing new treatments and advancing medical knowledge. Clinical trials are classified on the basis of goal of study; to treat, prevent, or reduce incidence of a disease.[13] With regard to natural products, the history of clinical trials dates back to 1990 and a huge amount of clinical trials has been published since then. Post-prandial blood sugar levels, fasting blood glucose, HbA1c, and insulin sensitivity are amongst the tangible, realistic, and varied examples of measurable metrics which reflects the substantial picture of “the effect of these natural products on diabetes patients.” Fenugreek, gymnema, aloe, neem, and various other natural products are the outcome of such clinical trial which were allocated in various part of the world, illustrating positive antidiabetic effects via different pathways.[14–18] However, most of the natural products were unable to make access to the market due to various factors. The quality of clinical trials and its evaluation based on the pre-set standards is one of the important factors to declare the fate of these natural product. Herein, the authors took a challenge to scrutinize all the natural products, with an established ethnopharmacological background and reported clinical trial in diabetes, for evaluation of the quality of these clinical trials according to standard scales of Delphi, Jadad, and Cochrane based review scales.
2. Materials and methods
2.1. Databases and search strategy
Electronic databases; Scopus, PubMed, Google Scholar, Science Direct, and E-portal of Imam Abdulrahman Bin Faisal University library. Journals; Journal of medicinal plants research, Journal of ethnobiology and ethnomedicine, Asian journal of Plant sciences, Journal of diabetes and complications, BMC complementary and alternative medicines, Natural product research, Journal of Ethnopharmacology, Diabetes, Journal of diabetes, etc Books; Herbalism, phytochemistry and ethnopharmacology, indigenous drugs of India, etc.
The data selection/extraction/analysis and evaluation was performed by a group of graduate pharmacists, academicians, researchers, and physicians who were expert in the field of clinical trials and patient's treatment related to diabetes. The literature was double checked for any multiple publications/duplications, incomplete/ineligible study and scored according to the pre-defined scales (Jadad, Delphi, and Cochrane scale).
2.2. Keywords searched
Randomized clinical trial, clinical trials, diabetes mellitus, Aloe vera, Panax quinquefolius, American ginseng, Vaccinium myrtillus, Bilberry, Cinnamomum cassia, Cinnamon, Trigonella foenum-graecum, Fenugreek, Allium sativum, Garlic, Gymnema sylvestre, Gymnema, Karela, Grifola frondosa, Maitake, Azadirachta indica, Neem, Opuntia fuliginosa, Nopal, onion, Allium cepa, Plantago ovata, Psyllium, Curcuma longa, Turmeric, Eleutherococcus senticosus, Acanthopanax senticosus, ginseng, Syzygium cumini, Jambolana, Jambolan, Bitter melon.
2.3. Inclusion criteria
The inclusion criteria consisted of; “studies reported in English language only and reporting the natural products clinical trials for diabetes humans,” “natural products with established folklore uses and applied practically in diabetes trials,” “any clinical trial for diabetes using natural products irrespective of randomization, blinding, phase (I–V) applied, statistical model used, outcome and assessor blinding, and negative or positive outcomes,” “clinical trials reporting the use of natural products alongwith conventional medications.”
For ethnopharmacological relevance, a list of natural products was collected and final selection was based on the ethnopharmacological uses of these natural products. The relevant reports, based on community surveys, interviews, and collection of data from local inhabitants and healers in that particular community, were extracted.
2.4. Exclusion criteria
The criteria for literature exclusion was; “any study reporting diabetes clinical trials without application of natural products,” “clinical studies reported in diabetes using natural products with lack of any prior ethnopharmacological or folklore use,” “natural products with established ethnopharmacological uses in diabetes; however, yet to be evaluated in a proper clinical trial for diabetes,” “any preclinical, duplicated, and incomplete clinical trial,” “Phase-0 clinical trials,” and “clinical trials in diabetes using minerals, vitamins, or conventional drugs only.”
2.5. Review period
An extensive literature search strategy was applied which started in September 2018 and continued till February 2020. The literature was updated on regular basis, for any new information added to database, till final preparation of manuscript.
2.6. Ethical review
The ethical approval was not necessary as the study did not include any animal or human subjects.
2.7. Patient consent
The study did not involve any patients and no consent form was required.
2.8. Search result
The literature search resulted a total of 1073 articles which were confined to 74 studies, following a proper scrutiny of the eligible articles as per pre-defined criteria (Fig. 1).
Figure 1.
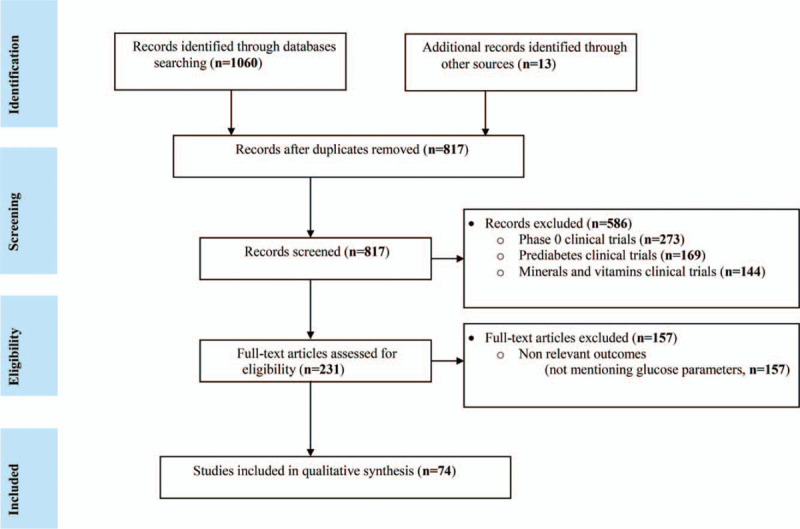
PRISMA flow-diagram for literature search and selection.
3. Literature search
The selected literature was downloaded, properly arranged, and studied in-depth for extraction of the relevant data. The bulk of this literature with relevant data is arranged in proper sections as mentioned below;
3.1. Ethnopharmacological relevance and evidence of the plants used in diabetes
Plants with ethnopharmacological relevance, part/s used for folklore purposes, and the community where the plants were applied for the treatment of various ailments have been reported in detail in Table 1.
Table 1.
Ethnopharmacological relevance of the selected antidiabetic plants.
| Herbs/plants | Botanical name | Synonyms | Part used | Ethnopharmacological relevance |
| Aloe | Aloe vera | Aloe barbadensis/chinensis/ elongate/indica Royle, Aloe | Leaves, dried sap (fluid), gel | Central and south America and Mexican communities, central Uganda[46] |
| American ginseng | Panax quinquefolius | Panax quinquefolius, Panacis quinquefolius, ginseng | Roots | different parts of the world[47] and Quebec[48] |
| Bilberry | Vaccinium myrtillus | Myrtillus niger and sylvaticus, Vaccinium oreophilum | Fresh fruit | America and Europe,[49] Canada[48] |
| Cinnamon | Cinnamomum aromaticum; Cinnamomum cassia | Cinnamomum aromaticum/ longifolium/medium, cinnamon | Bark, leaf | [50,51] |
| Fenugreek | Trigonella foenum-graecum | Foenum-graecum officinale, Trigonella tibetana (Alef.) | Seeds | Iran[52–54] |
| Garlic | Allium sativum | Allium controversum Schrad, Allium longicuspis Regel | Bulb, leaves | [55–58] |
| Gymnema | Gymnema sylvestre | gurmarbooti, gurmar, periploca | Dried roots and leaves | India and Africa,[59] India, America[47] and Canada[48] |
| Jambolan seeds | Syzygium cumini; Syzygium cumini jambolana | Calyptranthes caryophyllifolia/ cumin/cuminodora/ jambolana | Fruit, leaves, dried seed and bark | India,[60,61] Khulna, Bangladesh[62] |
| Bitter melon | Momordica charantia | karela, bitter gourd | Fruit, leaf and whole plant | Dhaka, Bangladesh,[63] Indian Jodhpur and Rajasthan communities[64] |
| Maitake | Grifola frondosa | Maitake mushroom | Fruits | Asia,[65] China[66] |
| Neem | Azadirachta indica | Neem, neem tree or Indian lilac | Seeds/leaves/bark | India[67–69] |
| Nopal | Opuntia fuliginosa/ streptacantha | Prickly pear cactus, nopal | Flowers, fruits | Morocco,[70] Latinos and Hispanics[71] |
| Onion | Allium cepa | Allium angolense/aobanum/cepaeum | Leaves, bulb, oil and seeds | Tamilnadu, India,[69] Palestine[72] |
| Psyllium | Plantago ovata | plantago seeds, psyllium husk | Seeds, husk | Mexico[73,74] |
| Siberian Ginseng | Eleutherococcus senticosus; Acanthopanax senticosus | Acanthopanax asperatus, Eleutherococcus asperatus | Bark, roots | [75,76] |
| Turmeric | Curcuma longa/ domestica; Curcuma aromatica | Amomum curcuma Jacq, Curcuma domestica Valeton, curcumin | Whole plant, fresh rhizome | [67], India[77] |
3.2. Clinical trials of plants used for diabetes
This section describes in detail, the clinical trials reported for natural products in diabetes. A comprehensive information regarding the part of the plant used in the study, mechanisms of action reported, and final results observed are given in Table 2.
Table 2.
Details regarding plant, its part used, mechanism reported and results observed in clinical trials during diabetes.
| Plant | Clinical trail | Part used | Sample size (n) | Intervention method in intervened groups | Period of treatment | Mechanism reported | Results |
| Aloe vera | A1 | Juice from gel | 72 | 1 tablespoonful aloe juice BID | 42 days | N/A | ↓Blood sugar and triglyceride levels[78] |
| A2 | Juice from gel | 72 | 1 tablespoonful aloe juice BID + 2 glibenclamide (5 mg) tablets | 42 days | N/A | ↓ Glucose and ↓ triglyceride[79] | |
| A3 | Leaves extracted gel powder | 67 | Aloe capsules (300 mg BID) | 2 months | ↓Insulin resistance | ↓HbA1c, LDL, and total cholesterol[80] | |
| A4 | Extract as tablet | 44 | Aloe extract tablets (1000 mg OD) | 2 months | N/A | No reduction in fasting blood sugar, HbA1c, total cholesterol, triglycerides, HDL/LDL[81] | |
| A5 | Powder of the leaves extracted gel | 90 | First intervention: Group 1: no treatment. Group 2: aloe powder (100 mg) Group 3: aloe powder (200 mg) | 3 months | ↑Effectiveness of insulin | ↓Fasting and post prandial blood glucose and lipid profile[82] | |
| Second intervention: nutrition to group 2 and 3. | 3 months | ||||||
| American ginseng | B1 | Opaque gelatin capsule | 39 | Konjac-glucomannan blend fiber (6g/day) + ginseng (3g/day) | 12 weeks with 4 weeks washout period (Crossover) | ↑insulin secretion | ↓ HbA1c and lipid panel[83] |
| B2 | Ginseng root extract | 74 | Capsules (total 3g/day) | 12 weeks | N/A | Safe in T2DM patient with CVS risk (Mucalo et al., 2014) | |
| B3 | Gelatin capsules | 19 | Ginseng capsules (3g) + oral glucose challenge (25g) in each visit | 4 visits (1 week interval between each visit) | ↓Digestion and ↑insulin secretion | Change in glycaemia[84] | |
| B4 | Root of American ginseng | 10 | Either placebo or ginseng 3, 6, or 9 g randomly/each visit | 16 visits with a 3 days interval | ↓Digestion and ↑insulin secretion | No effect on post prandial glycaemia[85] | |
| B5 | Dried whole root extract | 24 | Ginseng capsules (3g/day) | 8 weeks with 4 weeks washout period | ↑Insulin secretion | ↓HbA1c, fasting blood glucose[86] | |
| Bilberry | C1 | Fruit extract | 8 | 0·47 g of Mirtoselect (equal to 50 g of fresh bilberries) | Single dose with 2 weeks washout period | ↓Carbohydrate digestion or absorption | ↓Postprandial glycaemia and insulin[87] |
| Cinnamon | D1 | Capsule | 60 | Cinnamon capsules (500 mg BID) | 3 months | NA | No change in glucose and lipid profile[88] |
| D2 | Aqueous extract as capsule | 60 | 1, 3, 6 g cinnamon daily | 40 days | ↑Stimulation of insulin | ↓Serum glucose and lipid profile[89] | |
| D3 | Whole bark extract as capsule | 25 | Cinnamon 1500 mg/day | 6–7 weeks | ↑Insulin sensitivity | No improvement in glucose[90] | |
| D4 | Capsule | 109 | Cinnamon capsule (1g/day) | 90 days | N/A | ↓HbA1c[91] | |
| D5 | Aqueous cinnamon extract | 79 | Capsule (112 mg of aqueous cinnamon extract TID) | 4 months | N/A | ↓Fasting glucose[92] | |
| D6 | Capsule | 14 | Giving cinnamon capsule 1.5g/day | 30 days | NA | ↓Glucose, triglycerides and cholesterol[93] | |
| D7 | Bark extract as tablet | 66 | Placebo/cinnamon extract at 120 or 360 g/day. | 3 months | NA | ↓Fasting blood glucose and HbA1c[94] | |
| D8 | Capsule | 72 | Cinnamon (1 g/day) | 90 days | ↑Insulin stimulated tyrosine phosphorylation | No significant differences in glucose profile or number of hypoglycemic episodes[95] | |
| D9 | Bark extract as capsule | 44 | Cinnamon supplement (3g/day). | 8 weeks | ↑Insulin stimulated tyrosine phosphorylation | No significant difference in glycemic indicators between arms of the study[96] | |
| D10 | Bark powder as capsule | 58 | Cinnamon (2 g/day) | 12 weeks | ↑Glucose transporter (GLUT4) and receptor proteins | ↓HbA1c and blood pressure[97] | |
| Fenugreek | E1 | Seed extract (Fenfuro-TM) | 174 | Fenfuro capsule (500 mg BID) | 90 days | NA | ↓Post-prandial blood glucose and FBG[98] |
| E2 | Seeds soaked in hot water | 60 | Fenugreek seeds soaked in hot water (10g/day) | 6 months | ↑ Insulin release | ↓FBG levels and HbA1c[99] | |
| E3 | Dried ripe seed capsule | 69 | Fenugreek saponins 6 capsules (TFGs) TID (0.35g/cap) | 12 weeks | NA | ↓Glycemia and CSQS in the treated group[100] | |
| E4 | Seed powder | 24 | Powdered fenugreek seeds (10 g/day) yogurt or with hot water. | 8 weeks | ↑Insulin | ↓FBG[101] | |
| E5 | Hydro-alcoholic seeds extract | 25 | Hydro-alcoholic extract (1g/ day) | 2 months | ↑Insulin release | ↓HbA1c and insulin resistance[102] | |
| E6 | Seed powder | 80 | Fenugreek powder (25g/day) | 2 months | ↑ Insulin release | ↓FBS and HbA1c[103] | |
| E7 | Seed powder | 30 | Two sachet of Polyherbal formulation (PHF) containing fenugreek 2.5 g | 40 days | ↑Insulin | ↓FBG and HbA1c[104] | |
| Garlic | F1 | Aged garlic extract (Kyolic) | 26 | 1200 mg of Aged garlic extract daily | 4 weeks with 4 weeks washout | N/A | No extra benefit for adding aged garlic[105] |
| F2 | Aqueous extract capsule | 32 | Capsules (combination of 200 mg turmeric and 200 mg garlic). Three groups; group 1 (1.2g), group 2 (1.6g) and group 3 (2.4g) daily | 12 weeks | ↑ Insulin secretion | ↓Glucose profile[106] | |
| F3 | Garlic powder tablets (Allicor) | 60 | 300 mg Allicor/day | 4 weeks | ↑Insulin secretion | ↓Blood glucose[107] | |
| F4 | Bulb extract (Lasuna) capsule | 60 | Garlic capsules (250 mg BID) added to standard therapy | 12 weeks | ↑Insulin secretion and sensitivity | ↓Glycemic level[108] | |
| F5 | N/A | 96 | Capsules (50 mg/day) added to standard medication | 12 weeks | N/A | ↓Fasting blood glucose[109] | |
| F6 | Garlic (KWAI) tablet | 60 | Tablets (300 mg TID) | 24 weeks | ↑ Insulin secretion | ↓Fasting blood sugar[110] | |
| F7 | Garlic tablet | 210 | 5 groups received garlic (300, 600, 900, 1200, and 1500 mg/day), one took metformin and one was placebo. | 24 weeks | N/A | ↓FBS and HbA1c[111] | |
| F8 | Kyolic aged garlic extract | 48 | Extract (3g/day) | 3 months | ↑ Insulin secretion | No change in blood glucose[17] | |
| F9 | Bulb extract (Lasuna) capsules | 60 | Capsules (250 mg BID) + metformin. | 12 weeks | Sulfur containing metabolites i.e. allicin and its derivatives | Reduction in fasting blood glucose when used with metformin (250 mg)[112] | |
| Gymnema | G1 | Water-soluble leaves extract | 60 | 2 capsules daily (200 mg/cap) | 2–30 months | ↑Endogenous insulin | ↓Insulin requirements[14] |
| G2 | Water-soluble leaves extract | 47 | Capsule (400 mg/day) | 18–20 months | Beta cells regeneration | Reduced glucose and glycosylated Hgb[15] | |
| G3 | Beta Fast GXR (leaves extract) | 100 | Tablets (400 mg BID) | 90 days | ↑Insulin levels due to regeneration of the pancreatic beta cells | ↓Postprandial plasma glucose, HbA1c and pre-prandial plasma glucose concentrations[113] | |
| G4 | Om Santal Adivasi (OSA) | 11 | Capsules (1 g/day) | 6 days | ↑Insulin secretion | ↓Fasting glucose and ↑serum insulin[114] | |
| G5 | Leaves powder | 20 | Powder (6 g/day) | One month | N/A | ↓Blood glucose and postprandial blood glucose levels[115] | |
| Jambolan | H1 | Dried leaves tea | 27 | Group 1: Syzygium cumini leaves tea (2g/ day) + placebo tablets, Group 2: placebo tea + glyburide tablets (5 mg BID), Group3: placebo tea & tablet | 28 days | N/A | No hypoglycemic effect observed[116] |
| H2 | Dried leaves tea | 27 | Syzygium cumini leaves tea (2g/day) + placebo tablets, placebo tea + glyburide tablets (5 mg BID), placebo tea + placebo tablet | 28 days | N/A | ↓Hyperglycemic effect[117] | |
| Bitter Melon | I1 | Powdered whole karela fruit | 8 | Powder 50 mg/kg BID | 7 days | N/A | Enhanced glucose tolerance[118] |
| I2 | Dried powder of fruit pulp in capsule | 129 | Karela 0.5 g/day, 1 g/day, 2 g/day or metformin 1 g/day. | 4 weeks | N/A | ↓Fructosamine however, the hypoglycemic effect was less than metformin[119] | |
| I3 | Fresh whole fruit in tablet | 50 | Tablets (6 g/day) + standard medication | 4 weeks | N/A | No significant changes observed[120] | |
| I4 | Methanolic fruit soft extract | 15 | Extract (200 mg BID) + half dose metformin/glibenclamide | 7 days | N/A | ↑Hypoglycemic action[121] | |
| I5 | Dried fruit pulp capsule | 95 | Karela (2/4 g/day) or glibenclamide (5 mg/day) | 10 weeks | N/A | ↓HbA1c and plasma glucose[122] | |
| I6 | Extract of fruits/tissue cultures | 9 | Vegetable insulin doses (10/20/30 IU) | One day (single dose) | N/A | ↓Blood glucose[123] | |
| I7 | Dried fruit pulp capsule | 24 | Capsule (2000 mg/day) | 3 months | ↑Insulin secretion | ↓HA1c and insulin AUC[124] | |
| I8 | Charantia Ampalaya capsules | 40 | 2 capsule TID | 3 months | N/A | No significant effect observed[125] | |
| I9 | Fresh unripe fruit juice | 50 | Rosiglitazone (4 mg/day) or bitter melon juice (55 mL/24 h) | 6 months | N/A | No change in serum glucose[126] | |
| Maitake | J1 | Fruit bodies in caplets | Two | First participant; MMP caplet (500 mg TID) reduced to 2caplets/ day | 5 months | ↑Effect on insulin receptors | ↑Glycemic control[127] |
| Second participant; MMP caplet (500 mg TID) | 3 months | ||||||
| Neem | K1 | Powder leave aqueous extract | 400 | Neem extracts (5 mL/day) | 2 months | ↓Carbohydrate absorption from gut | ↓Fasting blood sugar level[18] |
| K2 | Powder leave aqueous extract | 90 | Capsules (2 g/day) of tulsi leaf powder, neem leaf powder or mixture of both | 3 months | N/A | ↓Diabetic symptoms[128] | |
| K3 | Seeds powder | 55 | Moringa oleifera (8g) or neem (6g) per day | 40 days | N/A | ↓Fasting and postprandial blood glucose[129] | |
| Nopal | L1 | N/A | Study 1: 7 | Meal containing 50 g carbohydrates from glucose or dehydrated nopal | 1 visit/each meal (1 week washout period | N/A | ↓Plasma glucose-dependent insulinotropic peptide peaks and serum insulin[130] |
| Study 2: 14 | High-carbohydrate breakfast or high-soy-protein breakfast, with/without 300 g of nopal | 4 visits (1 week washout period between meals) | |||||
| L2 | Fresh and tender stems | 32 | Group 1: broiled nopal stems (500 g), group 2: only water (400 mL), Group 3: nopal, water and broiled squash | Single dose, 1 week washout between each intervention. | ↑Insulin sensitivity is suggested | ↓Glucose and ↑hypoglycemia[131] | |
| L3 | Stems | 28 | 500 g of nopal | Single dose | N/A | ↓Serum glucose and insulin[132] | |
| L4 | Dehydrated extract, capsulated | 6 | 30 capsules of dehydrated nopal extract (10.1 g) | Single dose | N/A | No hypoglycemic effects observed[132] | |
| L5 | Heated blended crude stem | 8 | 5 interventions; 4 for nopal stems (entire broiled, blended broiled, blended crude, and heated blended) and 1 water as placebo. | 5 separate interventions (72 hs between them) | N/A | ↓Serum glucose[132] | |
| L6 | Dried, capsulated | 10 | 30 nopal capsules | Single dose | N/A | No hypoglycemic effect observed[132] | |
| 14 | 10 nopal capsules TID. | 1 week | |||||
| Onion | M1 | Fresh onion cut into slices | 84 | Crude fresh slices (100 g), standard diabetic treatment, or 15 ml of water. | Single dose | Improved and regenerated cells | ↓Blood glucose and FBG[133] |
| Psyllium | N1 | Psyllium pre-mixed in cookies | 77 | Cookies containing either flaxseed/psyllium/placebo (10 g/day) | 12 weeks | N/A | ↓FBG and HbA1c[134] |
| N2 | Psyllium pre-mixed in cookies | 51 | cookies containing either flaxseed/ psyllium/placebo (10g per day) | 12 weeks | N/A | ↓FBG and HbA1c[135] | |
| N3 | Fiber psyllium (Metamucil) | 37 | Psyllium (3.4 g BID), psyllium (6.8 g BID) or placebo. | 12 weeks | ↓Carbohydrate absorption | ↓FBG and HbA1c[136] | |
| N4 | Soluble fiber | 40 | Soluble fiber (10.5 g) daily | 8 weeks | ↓ CHO absorption/digestion | ↓Glucose level[137] | |
| N5 | Psyllium fiber | 18 | 6.8 g psyllium twice in the first visit and placebo in the crossover visits. | One day of treatment for each group (crossover) | ↓Access of glucose to the gut | ↓PBG and insulin concentrations[138] | |
| Siberian ginseng | O1 | Purified solution of extract | 75 | Extract of Siberian ginseng (480 mg/day), American ginseng (480 mg/day), or placebo | 3 months | ↑Glucose induced insulin secretion | ↓Fasting and post prandial blood sugar[139] |
| Turmeric | P1 | (Sina Curcumin) | 70 | Curcumin (80 mg/day) | 3 months | N/A | ↓HbA1c, FBG, TG, and BMI[140] |
| P2 | Capsule | 100 | Curcuminoids capsule (150 mg BID) | 3 months | ↓Serum A-FABP levels | ↓ Blood glucose with anti-diabetic effects[141] | |
| P3 | Rhizomes | 60 | 2 g turmeric + standard metformin therapy. | 4 weeks | ↑Beta cell stimulation | ↓Fasting plasma glucose[16] | |
| P4 | Extracts isolated from rhizome | 100 | Curcuminoids (300 mg/day) | 3 months | ↓BG and ↑insulin resistance | ↓Fasting blood glucose[142] |
3.3. Evaluation of clinical trials based on various scales
Jadad scale, is a tool used to assess the quality of clinical trials based on the three key features of randomization, masking, and accountability of all patients including the withdrawals. The Jadad system evaluates and scores a study on a scale of 0 to 5, based on the pre-defined factors as mentioned in Jadad scale (Table 3). Delphi uses numerous items to measure the quality of a clinical trial. Though a deficiency of a numerical scale for calculation of final score do exist in this system, yet it is widely implemented scale in most of the studies. Delphi uses nine designated questions in order to count the number of positive responses (Table 3). Cochrane back review group developed a scale which is considered the most comprehensive and uncritical among all the available scales. It is considered a quality rating standard scale, followed in most of the review studies for evaluation of the clinical trials. This system also has a lack of numerical scale needed to finalize a value for a clinical trial. As evident from Table 3, few of the points in the three mentioned scales are overlapping. In addition, each scale do possess positive and negative aspects hence, it becomes difficult to decide a best-fit scale for evaluation. To overcome the loopholes present in these scale the authors utilized a novel approach i.e. to apply the three scales together for evaluation of the quality of each clinical trial on individual basis. To maintain uniformity and ease of application in calculating the individual and final scores for each clinical trial, herein we calculated the responses from these two scales in terms of numerical values 0 to 9 (Delphi system) and 0 to 10 (Cochrane based review scale) which sum up to a final value of 0 to 24 points.[19] The scales are applied to evaluate each individual trial and the drawbacks observed per each scale are reported given in Table 4.
Table 3.
The scales used for evaluation of clinical trials.
| Cochrane back review group list | Final Delphi list | Jadad score calculation |
| Was the method of randomization adequate? | 1. Treatment allocation(a) Was a method of randomization performed?(b) Was the treatment allocation concealed? | Was the study described as randomized (this includes words such as randomly, random, and randomization)? |
| Was the treatment allocation concealed? | Was the method used to generate the sequence of randomization described and appropriate (table of random numbers, computer generated, etc.)? | |
| Were the groups similar at baseline regarding the most important prognostic indicators? | 2. Were the groups similar at baseline regarding the most important prognostic indicators? | Was the study described as double blind? |
| Was the patient blinded to the intervention? | 3. Were the eligibility criteria specified? | Was the method of double blinding described and appropriate (identical placebo, active placebo, dummy, etc)? |
| Was the care provider blinded to the intervention? | 4. Was the outcome assessor blinded? | Was there a description of withdrawals and dropouts? |
| Was the outcome assessor blinded to the intervention? | 5. Was the care provider blinded? | Deduct one point if the method used to generate the sequence of randomization was described and it was inappropriate (patients were allocated alternately, or according to date of birth, hospital number, etc). |
| Were co-interventions avoided or similar? | 6. Was the patient blinded? | Deduct one point if the study was described as double blind but the method of blinding was inappropriate (e.g., comparison of tablet vs injection with no double dummy). |
| Was the compliance acceptable in all groups? | 7. Were point estimates and measures of variability presented for the primary outcome measures? | |
| Was the drop-out rate described and acceptable? | 8. Did the analysis include an intention-to-treat analysis? | |
| Was the timing of the outcome assessment in all groups similar? |
Table 4.
limitations, individual and total score calculated for each clinical trial based on Jadad, Delphi and Cochrane scales.
| Jadad deficiencies | Delphi deficiencies | Cochrane deficiencies | |||||||||||||||||||||||||||
| Plant | Study # | a | b | c | d | e | Jadad Score[5] | a | b | c | d | e | f | g | h | i | Delphi score[9] | a | b | c | d | e | f | g | h | i | j | Cochrane score[10] | Total score[24] |
| Aloe vera | A1 | X | X | X | X | 1 | X | X | X | X | 3 | X | X | X | X | X | X | 1 | 5 | ||||||||||
| A2 | X | X | X | X | 1 | X | X | X | X | 3 | X | X | X | X | X | X | 1 | 5 | |||||||||||
| A3 | 5 | X | X | X | 6 | X | X | 8 | 19 | ||||||||||||||||||||
| A4 | 5 | X | X | X | 5 | X | X | X | 6 | 16 | |||||||||||||||||||
| A5 | X | X | X | X | X | 0 | X | X | X | X | X | X | X | 1 | X | X | X | X | X | X | X | X | X | 0 | 1 | ||||
| American ginseng | B1 | X | X | 3 | X | X | X | 6 | X | X | X | 7 | 16 | ||||||||||||||||
| B2 | 5 | X | X | X | 6 | X | X | 8 | 19 | ||||||||||||||||||||
| B3 | X | X | X | 1 | X | X | X | X | 4 | X | X | X | X | X | 4 | 9 | |||||||||||||
| B4 | X | X | 3 | X | X | X | X | X | 3 | X | X | X | X | X | 3 | 9 | |||||||||||||
| B5 | 5 | X | 8 | X | X | 7 | 20 | ||||||||||||||||||||||
| Bilberry | C1 | X | X | 2 | X | 7 | X | X | X | 5 | 14 | ||||||||||||||||||
| Cinnamon | D1 | X | X | 2 | 9 | X | X | 7 | 18 | ||||||||||||||||||||
| D2 | X | X | X | X | 0 | X | X | X | X | 4 | X | X | X | X | X | X | 1 | 5 | |||||||||||
| D3 | X | X | X | 2 | X | X | X | 5 | X | X | X | 6 | 13 | ||||||||||||||||
| D4 | X | X | 3 | X | X | X | 6 | X | X | X | 7 | 16 | |||||||||||||||||
| D5 | X | 3 | X | X | 6 | X | X | 7 | 16 | ||||||||||||||||||||
| D6 | X | X | X | X | 0 | X | X | X | X | 5 | X | X | X | X | X | X | X | 1 | 6 | ||||||||||
| D7 | X | X | 1 | X | X | 6 | X | X | X | 4 | 11 | ||||||||||||||||||
| D8 | X | X | 2 | X | 7 | X | X | X | 5 | 14 | |||||||||||||||||||
| D9 | X | 3 | X | 8 | X | 9 | 20 | ||||||||||||||||||||||
| D10 | 5 | 9 | 10 | 24 | |||||||||||||||||||||||||
| Fenugreek | E1 | X | 4 | X | X | 5 | X | X | 7 | 16 | |||||||||||||||||||
| E2 | X | X | X | 2 | X | X | 6 | X | X | X | X | X | 3 | 11 | |||||||||||||||
| E3 | 5 | X | 7 | X | 8 | 20 | |||||||||||||||||||||||
| E4 | X | X | X | X | 1 | X | X | X | X | X | X | X | -1 | X | X | X | X | X | X | X | X | -3 | -3 | ||||||
| E5 | X | X | 2 | X | X | X | 4 | X | X | X | X | X | 2 | 8 | |||||||||||||||
| E6 | X | X | X | 1 | X | X | X | X | X | 3 | X | X | X | X | X | X | 3 | 7 | |||||||||||
| E7 | X | X | X | X | 1 | X | X | X | X | X | X | 3 | X | X | X | X | X | X | 3 | 7 | |||||||||
| Garlic | F1 | X | X | 1 | X | X | 6 | X | X | 6 | 13 | ||||||||||||||||||
| F2 | X | X | 1 | X | 7 | X | X | 6 | 14 | ||||||||||||||||||||
| F3 | X | X | 1 | 9 | X | X | 6 | 16 | |||||||||||||||||||||
| F4 | X | X | X | X | X | 0 | X | X | X | X | X | X | 3 | X | X | X | X | X | X | X | 3 | 6 | |||||||
| F5 | X | X | X | X | 0 | X | X | X | X | X | X | 3 | X | X | X | X | X | X | X | X | -1 | 2 | |||||||
| F6 | X | X | X | X | 1 | X | X | X | X | X | 3 | X | X | X | X | 5 | 9 | ||||||||||||
| F7 | X | X | X | 1 | X | X | X | X | 4 | X | X | X | X | 4 | 9 | ||||||||||||||
| F8 | X | X | X | X | X | 0 | X | X | X | X | X | X | 3 | X | X | X | X | X | X | X | X | 0 | 3 | ||||||
| F9 | X | X | X | X | 0 | X | X | X | X | X | 4 | X | X | X | X | X | X | X | 1 | 5 | |||||||||
| Gymnema | G1 | X | X | X | X | 1 | X | X | X | X | X | 4 | X | X | X | X | X | 5 | 10 | ||||||||||
| G2 | X | X | X | X | X | 0 | X | X | X | X | X | X | 3 | X | X | X | X | X | X | 4 | 7 | ||||||||
| G3 | X | X | X | X | 1 | X | X | X | X | X | X | 3 | X | X | X | X | X | 5 | 9 | ||||||||||
| G4 | X | X | X | X | X | 0 | X | X | X | X | X | X | X | 2 | X | X | X | X | X | X | X | 3 | 5 | ||||||
| G5 | X | X | X | X | X | 0 | X | X | X | X | X | X | 3 | X | X | X | X | X | X | 4 | 7 | ||||||||
| Jambolan | H1 | X | X | 2 | X | 8 | X | X | X | X | X | 5 | 15 | ||||||||||||||||
| H2 | X | X | 2 | X | X | X | X | X | 3 | X | X | X | X | X | 2 | 7 | |||||||||||||
| Bitter Melon | I1 | X | X | X | X | 0 | X | X | X | X | X | 0 | X | X | X | X | X | X | -1 | -1 | |||||||||
| I2 | 5 | X | X | 6 | X | X | 7 | 18 | |||||||||||||||||||||
| I3 | X | X | X | 1 | X | X | X | 5 | X | X | X | X | 5 | 11 | |||||||||||||||
| I4 | X | X | X | X | X | 0 | X | X | X | X | X | X | X | -4 | X | X | X | X | X | X | X | X | X | -8 | -12 | ||||
| I5 | X | X | 1 | X | X | 6 | X | X | 6 | 13 | |||||||||||||||||||
| I6 | X | X | X | X | X | 0 | X | X | X | X | X | X | X | -3 | X | X | X | X | X | X | X | -2 | -5 | ||||||
| I7 | 5 | X | 7 | X | 8 | 20 | |||||||||||||||||||||||
| I8 | 5 | 9 | 10 | 24 | |||||||||||||||||||||||||
| I9 | X | X | X | X | 0 | X | X | X | X | X | -1 | X | X | X | X | X | X | X | -3 | -4 | |||||||||
| Maitake | J1 | X | X | X | X | X | 0 | X | X | X | X | X | X | X | X | 1 | X | X | X | X | X | X | X | 3 | 4 | ||||
| Neem | K1 | X | X | X | 1 | X | X | X | X | X | X | 2 | X | X | X | X | X | X | 2 | 5 | |||||||||
| K2 | X | X | X | X | X | 0 | X | X | X | X | X | X | 3 | X | X | X | X | X | X | 4 | 7 | ||||||||
| K3 | X | X | X | X | X | 0 | X | X | X | X | X | X | 3 | X | X | X | X | X | X | 4 | 7 | ||||||||
| Nopal | L1 | X | X | X | X | 1 | X | X | X | X | X | X | 0 | X | X | X | X | X | X | 1 | 2 | ||||||||
| L2 | X | X | X | X | 0 | X | X | X | X | X | X | -2 | X | X | X | X | X | X | X | -4 | -6 | ||||||||
| L3 | X | X | X | 2 | X | X | X | X | 3 | X | X | X | X | 3 | 8 | ||||||||||||||
| L4 | X | X | X | 2 | X | X | X | X | X | 0 | X | X | X | X | X | 1 | 3 | ||||||||||||
| L5 | X | X | X | 2 | X | X | X | 3 | X | X | X | X | 3 | 8 | |||||||||||||||
| L6 | X | X | X | 2 | X | X | X | X | X | 0 | X | X | X | X | X | X | -1 | 1 | |||||||||||
| Onion | M1 | X | X | X | X | X | 0 | X | X | X | X | X | X | X | 0 | X | X | X | X | X | X | X | X | 1 | 1 | ||||
| Psyllium | N1 | X | X | 3 | X | X | X | X | 5 | X | X | X | X | 6 | 14 | ||||||||||||||
| N2 | X | X | 3 | X | X | X | X | X | 3 | X | X | X | X | 5 | 11 | ||||||||||||||
| N3 | X | X | 1 | X | X | X | 5 | X | X | X | X | 4 | 10 | ||||||||||||||||
| N4 | X | X | X | 1 | X | X | X | X | X | X | 2 | X | X | X | X | X | X | X | X | 1 | 4 | ||||||||
| N5 | X | X | X | X | 0 | X | X | X | X | X | X | 2 | X | X | X | X | X | X | X | 3 | 5 | ||||||||
| Siberian ginseng | O1 | X | X | X | 0 | X | X | 7 | X | X | X | X | X | 4 | 11 | ||||||||||||||
| Turmeric | P1 | X | 3 | X | X | 7 | X | X | 8 | 18 | |||||||||||||||||||
| P2 | X | X | X | X | 0 | X | X | X | X | X | 2 | X | X | X | X | X | X | X | 1 | 3 | |||||||||
| P3 | X | X | X | X | 0 | X | X | X | X | X | 2 | X | X | X | X | X | X | X | 1 | 3 | |||||||||
| P4 | 5 | X | X | 7 | X | X | 8 | 20 | |||||||||||||||||||||
3.4. Statistical analysis
For principal component analysis (PCA), four components (scree plot Fig. 2) were observed with %variability (individual and total); PC1: 23.12 (23.12), PC2: 15.83 (38.96), PC3: 13.11 (52.07), and PC4: 11.38 (63.45). A total of 63.45% variability with Kaiser–Meyer–Olkin Measure of Sampling Adequacy (0.659) and Bartlett's test chi square value of 289.58 (P ≤ .000) was observed (Table 5). Around 23.12% variability was observed for the drawbacks; “non-randomized and non-blinded studies,” “lack of patient and care provider blinding,” “concealed treatment allocation,” and “lack of proper drop-out procedures mentioned in the studies.” Whereas 15% variation was due to; “lack of/no compliance report for patients” and “no information about co-interventions applied in the study.” Furthermore, no information about the “intention-to-treat-analysis” and “timing of outcome assessment,” was loaded in PC3 with individual variation of 13.11%. Finally, “non-blindness of the outcome assessor” contributed 11.38% to the total variance (63.455%). A three-dimensional representation of the factors loaded in components is shown in Figure 3.
Figure 2.
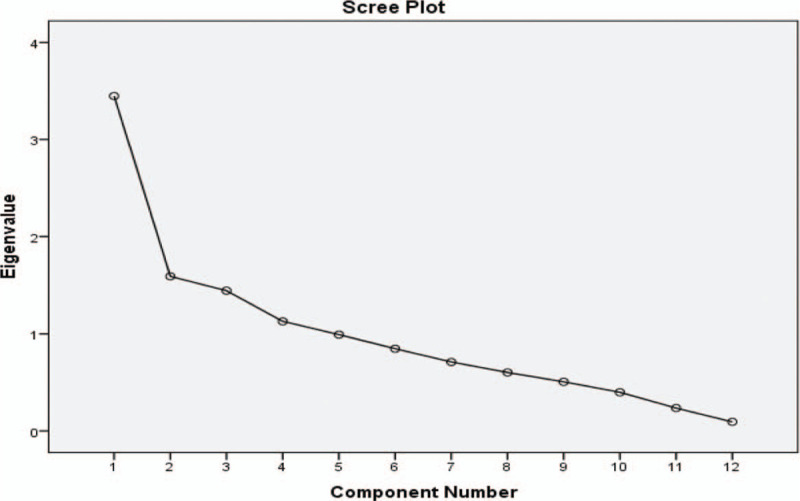
Scree plant, representing the number of possible components for factors loading.
Table 5.
Principal components loading (PCA) for factors analyzed in clinical studies and KMO and Bartlett's test.
| Factors | PC1 | PC2 | PC3 | PC4 |
| (A) Clinical trial was randomized or not? | 0.610 | 0.141 | 0.140 | −0.463 |
| (B) Clinical trial was blinded or non-blinded? | 0.665 | −0.556 | 0.010 | −0.032 |
| (C) Treatment allocation was concealed or not? | 0.806 | −0.141 | −0.213 | 0.123 |
| (D) The outcome assessor was blinded or not? | 0.270 | 0.399 | −0.157 | −0.610 |
| (E) Patient was blinded in the study or not? | 0.866 | −0.286 | −0.217 | 0.125 |
| (F) The care provider was blinded or not? | 0.634 | 0.186 | −0.543 | 0.281 |
| (G) The intention to treat analysis, was mentioned in clinical trial? | 0.416 | −0.066 | 0.602 | −0.197 |
| (H) Proper drop out procedure was mentioned? | 0.640 | 0.249 | 0.320 | −0.098 |
| (I) Was the patient compliance for the clinical trial reported? | 0.285 | 0.624 | 0.328 | 0.206 |
| (J) Was the timing of outcome assessment mentioned? | 0.111 | −0.267 | 0.628 | 0.352 |
| (K) Was the baseline characteristics for the group mentioned? | 0.233 | 0.121 | 0.203 | 0.299 |
| (L) Was any co-interventions mentioned? | 0.168 | 0.654 | −0.068 | 0.356 |
| Variability % | 23.12 | 15.83 | 13.11 | 11.38 |
| Cumulative % | 23.12 | 38.96 | 52.07 | 63.455 |
| KMO and Bartlett's test | ||
| Kaiser–Meyer–Olkin measure of sampling adequacy | 0.659 | |
| Bartlett's test of sphericity | Approx. Chi-square | 289.58 |
| degree of freedom | 66 | |
| Significance | 0.000 |
Figure 3.
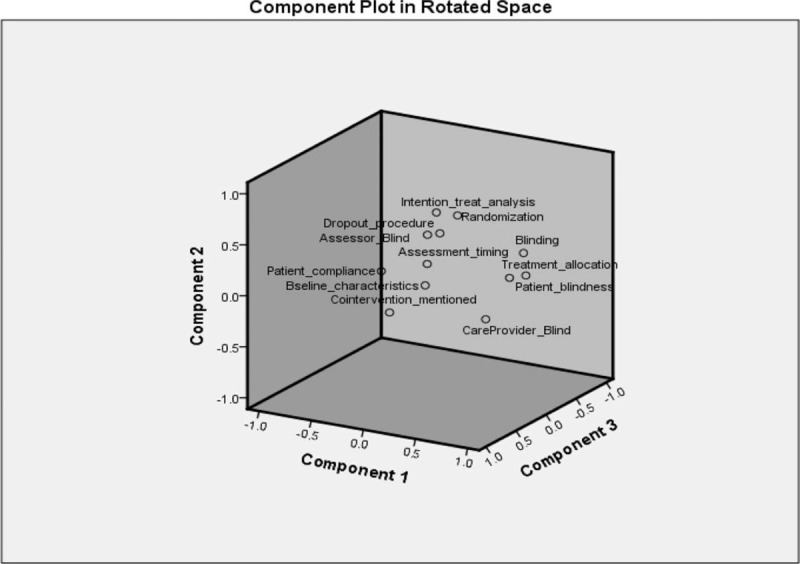
Scree plot and component loadings for factors.
For Pearson's correlation (Table 6), none of the pair was observed with a negative correlation however, the drawback of “mentioning the baseline characteristics for the groups” was found to have no significant correlation with any other drawback extracted from the study. With the same concept of evidence, PCA showed no loading with significant Eigen value for this drawback. The reason is due to sparse distribution of current drawback in the reported clinical trials. The Pearson's correlation between “intention-to-treat-analysis” and “outcome assessment method” again confirms the loading for both the drawbacks in PC3, and so on. The descending order of the drawbacks in these clinical trials, based on PCA and Pearson's correlation may be constructed as;
Table 6.
Pearson correlation matrix for factors observed in clinical trials (letter A-L represents the points as mentioned in Table 3).
| A | B | C | D | E | F | G | H | I | J | K | L | |
| A | 1 | |||||||||||
| B | 0.394∗ | 1 | ||||||||||
| C | 0.305∗ | 0.459∗ | 1 | |||||||||
| D | 0.285† | −0.052 | 0.181 | 1 | ||||||||
| E | 0.389∗ | 0.771∗ | 0.699∗ | 0.067 | 1 | |||||||
| F | 0.157 | 0.176 | 0.656∗ | 0.207 | 0.600∗ | 1 | ||||||
| G | 0.252† | 0.223 | 0.242† | 0.108 | 0.248† | −0.034 | 1 | |||||
| H | 0.423∗ | 0.252† | 0.383∗ | 0.184 | 0.372∗ | 0.251† | 0.330∗ | 1 | ||||
| I | 0.222 | −0.076 | 0.105 | 0.063 | 0.043 | 0.155 | 0.149 | 0.343∗ | 1 | |||
| J | −0.062 | 0.115 | 0.115 | −0.144 | 0.029 | −0.106 | 0.270† | 0.138 | 0.039 | 1 | ||
| K | 0.108 | 0.140 | 0.095 | 0.037 | 0.154 | 0.052 | 0.052 | 0.099 | 0.180 | 0.070 | 1 | |
| L | 0.087 | −0.151 | 0.012 | 0.081 | 0.077 | 0.261† | 0.013 | 0.154 | 0.278∗ | −0.051 | 0.080 | 1 |
[”Non-randomized and non-blinded,” “concealed treatment allocation,” “non-blindness of patient and care provider,” “lack of drop out procedures”] > [“lack of patients compliance and co-interventions reports”] > [“non-inclusion of intention-to-treat-analysis and timing of outcome assessment”] > [“outcome assessor non-blind”] > [missing to mention the baseline characteristics of the groups].
Figure 4 represents the descending order of occurrence for these drawbacks.
Figure 4.
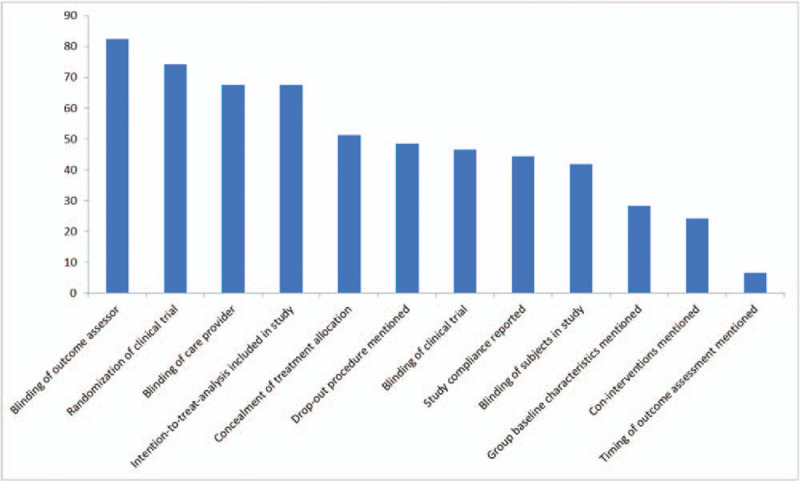
Descending order for drawbacks based upon “no” or “not reported” responses extracted from clinical.
4. Scoring of clinical trials (jadad, Delphi, and, cochrane scales)
For simplicity and leniency, an internal grading scale (points) was applied; 6 or below (any negative value) “very poor quality clinical trial,” 7 to 12 “poor quality clinical trial,” 13 to 18 “acceptable quality clinical trial,” and 19 to 24 “good quality clinical trial.” Beside the two excellent studies (D10, I8) the percentage for good quality clinical trial observed was 13.5%. The very poor and poor quality clinical trials makes the major proportion (67.6%) of these studies (Table 7).
Table 7.
Scoring for clinical trials based on in-house grading scale (0–24 points).
| Quality of trial based on assigned scale | Frequency (N) | Percent (%) | Cumulative Percent |
| Very poor quality clinical trials (6 and below, i.e., negative value) | 25 | 33.8 | 33.8 |
| Poor quality clinical trials[7–12] | 25 | 33.8 | 67.6 |
| Acceptable quality clinical trials[13–18] | 14 | 18.9 | 86.5 |
| Good quality clinical trials[19–24] | 10 | 13.5 | 100.0 |
| Total | 74 | 100.0 |
5. Discussion
This study presents a generalized view of the major drawbacks observed in the clinical trials selected and evaluated. The forthcoming discussion is a section-wise classification with extensive explanation of the drawbacks extracted;
5.1. Selection and identification of the plant source
To commence a clinical trial, the identification and quality of the source (natural product) is utmost important. Sufficient background information regarding the identity of the selected source is very essential. Plants may vary in terms of quality and quantity of the active principles present which is due to variation in geographical origins. Hence, the differences in environment, temperature, irrigation, salinity, stress, altitude, and seasonal variation may affect the composition of plant phytochemicals. A lack of very basic and essential information about the herbs used (family, genus, species and geographical origin, phytochemistry and quantification, and mechanism of actions, etc) was witnessed in these studies, which are strictly needed for a herb to be worked on as mentioned by Heinrich et al.[20] Such information regarding the species and its phytochemistry may help researchers and stakeholders to dig deeper at mechanistic and molecular level to isolate the compound of interest for an effective control of diabetes and related comorbidities. The proper evidence regarding ethnopharmacological and ethnomedicinal value of the plants reported,[21,22] is of prime importance.
5.2. Part of natural product used and its phytochemistry
Every individual part of a plant may vary with respect to the other part of the same plant in terms of nature of active ingredients or even the amount of similar active ingredient.[23] For instance, the antidiabetic effect was studied for seeds and leaves of Jambolan in these clinical trials. The seeds were observed with prominent antidiabetic effect whereas, the leaves for the same plant exhibited a lack of activity in diabetes. This is a self-explanatory evidence to choose wisely during selection of plant part as it may vary in phytochemistry.
5.3. Effects of extraction methods, temperatures and solvents upon the final dosage form preparation
The process-selection for dosage form preparation (extract, tea, infusion, essential oil, etc), exerts a subtle difference in the final outcomes of a study. The phenomenon has been mentioned in very detail in previous reports.[24–26] For example, Nopal was applied in the form of steamed as well as broiled steamed dosage form in these reported clinical trials. A proper explanation is missing regarding part, dosage form of Nopal used, and the effect of heat/difference in composition of steamed and broiled steamed Nopal dosage form in terms of active drug amount and therapeutic effects.
5.4. Use of toxic and hazardous solvent for dosage form preparation
The use of toxic and hazardous solvents for extraction may be problematic. One of the clinical trial for fenugreek applied hydro-alcoholic solvent for extract preparation. Another clinical study for bitter melon applied a technique for proper phytochemical screening which is interesting to see however, the same study utilized toxic solvents of carbon tetra chloride and benzene for extraction which are well known to release highly toxic phosgene fumes when heated. Hence, toxicity profile needs to be reported for these extracts in preclinical studies. An effective widely used alternative approach now-a-days is, the use of green solvent and green extraction.[27]
5.5. Clinical phase selection for study (Phase 0-V)
Phase-0 is a preliminary step in any clinical study where sub-therapeutic dosing is tested in about ten individuals in order to determine the pharmacokinetics and pharmacodynamics profile.[28] This supportive data helps to safely administer any herb or herbal product in human participants, without compromising the subject's rights and values. The data regarding toxicity and sub-therapeutic dosing, that is, Phase-0, is not included in any clinical trial mentioned here. In addition, the clinical trials in this systemic review are mostly reported at a preliminary level of Phase-I/II which necessitates a major data regarding Phase III–V.
5.6. Blinding and concealment of study
Natural products such as cinnamon, onion, and garlic possesses a particular smell/aroma and taste, the masking of which is utmost important to avoid any ambiguities/biasness in a study. Furthermore, to avoid any data manipulation, clinical studies needs to be of double-blind nature where both; the care provider/product administrator and outcome assessor are blinded towards the product used, protocols applied, subjects group assigned, and parameters/data to be studied.[29,30] Unfortunately, majority of the clinical trials herein were observed to be non-blinded or single blinded on behalf of patients, health care providers or outcome assessors.
5.7. Duration and compliance of clinical trials
The duration for these clinical trials ranged from week to months including 6 (cinnamon) and 20 months (gymnema) which are too lengthy and may be pose risks in certain conditions. No doubt, diabetic patients are asked to continue antidiabetic medications on a regular basis however, the co-administration of a natural product with conventional medication may produce unavoidable circumstance due to potential herb-drug interactions in such chronic used patient. Thus more lengthy studies with frequent dosing may lead to non-compliance which was observed in all of the clinical trials reported here.[31–33]
5.8. Potential herb–drug interactions
Severe or life threatening adverse effects have been reported when herbs are co-administered with conventional medicines. For example, bitter melon alongwith oral hypoglycemic may decrease blood glucose level[34,35] whereas, Aloe vera alongwith insulin/oral hypoglycemic potentiates the hypoglycemic effect hence, needs a proper caution.[34,36] These clinical trials were unable to explain; how to co-administer the plant with conventional drugs? What is the half-life, excretion ratio/rate, plasma protein binding, and clearance mechanism for extract/herbs studied? Most of the clinical trials used Metformin (half-life: 6.2 h); however, none of them mentioned the dosage frequency for the natural product, information about co-administration, dosage-gap, half-life of natural products used, metabolic pathways and enzymes involved, as well as any herb-drug interactions for the products studied. For example, clinical trial for turmeric and metformin observed a pronounced effect, yet no any prior information were available for such effect.
5.9. Dose of the natural product used
A dose of 1 to 6 g/day was observed in most of these clinical trials which apparently seems too high for any health related condition treatment. The question rises of how the powder/extract was converted to tablet/capsule with such a high amount? Based on size availability, “000” capsule is able to encompass a dose up to 1 g (subjected to natural product density as it may can reduce to 700/800 mg in case of dense powders). Above all, administering the allowable powder amount/capsule with a frequency of three time/day is still unable to deliver a dose of 3 g or above whereas, a number of clinical trials reported a dose up to 6 g/day. In particular, patient using conventional drugs (on conventional therapy) were given such high doses 3 to 4 times/day. This is a high risk trial with more chances and risks of life threatening herb-drug interactions. These studies completely skipped/failed to report proper information for managing the high doses/day in capsules, especially cinnamon (6 g), fenugreek (10 g), and nopal (500 g).
5.10. Placebo or psychological effect
Improper masking in some cases may produce psychological effects. One of the reported study using coconut oil for a placebo group observed alike effects in the all groups[37] which was explained due to a psychological phenomenon related to placebo. Most of the clinical trials reported herein were observed with a lack of proper coding/process, for natural products carrying intense smell.
6. Future perspectives and recommendations
6.1. Ethnopharmacological relevance of the plant with the study
To conduct a herbal clinical trial, it is essential to know its source (family, genus, species), ethnobotanical/ethnopharmacological relevance with the study, part of the plant used and phytochemical profile, preclinical, and toxicity data available as well as the information regarding the PKs and PDs of the herbal active ingredients.[20–22]
6.2. Identification and geographical information
Geographical location may affect the quality and quantity of active ingredient in a plant thus proper identification of geographical locations alongwith preliminary quality variation and standardization studies play a vital role for unification of the source intended for any clinical trial.[38,39] The pharmacological activities and toxicity profile must be ensured in animal and in vitro models as this will ease the uniformity of dose applied for an optimum activity of the plant.
6.3. A need for extraction or isolation does exist?
Albeit, it's a tiresome job with much investment to isolate an active chemical whereas, herbal extract/powders are easy to apply/use which adds an additional advantage of synergism. This made a dogma shift more toward the use of herbs and herbal products. However, when it comes to composition, quantity, mechanism of action, and PKs and PDs of the active drug, its tricky at times to study herbal samples as they are mixture of multicomponent.[40] Thus isolation of active principle is highly recommended as it is far better in order to evaluate the effect of a herbal drug at molecular or genetic level and to market the lead molecule as a potential drug candidate. For instance, the hypoglycemic polypeptide-p or p-insulin in Momordica charantia[41] is yet to be marketed. Likewise, eleutherosides presence makes Siberian ginseng more effective compared to Panax ginseng as a proper mechanism has been proposed for eleutherosides.[42]
6.4. Selection and preparation of final dosage
Due to variation in extraction tools (shorter and greener extraction) and parameters (temperature, solvent, particle size, length of extraction, pressure used during extraction and combination of solvents), the final dosage form may vary in concentration and activity of the active drug.[24–26] Powder samples (more surface area) are known to enhance the solubility, absorption, PKs, and PDs of the herbal drugs or extracts. For example, the clinical trial for psyllium observed a marked difference in activity for soluble fibers Vs whole seeds. Similarly, bitter melon juice showed pronounced effect compared to the fruit powder. For temperature effect, dehydrated Nopal study showed no results whereas, for drying effect, shade dried bitter melon revealed no effect compared to fresh and unripe fruit. Currently, the researchers are focusing to prepare the nano-dosage forms (nano-gels, micelles, nanoparticles and nanoemulsions) which are more target-oriented and effective at low doses. Curcumin-nano-micelle in one clinical trial exhibited excellent results.
6.5. Dataset available for clinical phases studied (Phase 0-V)
Proper literature data for Phase 0-V is necessary. Phase-0, though necessary to determine the sub therapeutic/starting dose/toxicity and PKs in human, is often not mandatory for plants with well-known ethnopharmacological background. Thus background information is very important to decide the clinical trials phase.[43]
6.6. Implementation of plan for short duration, multicenter and large scale study
Clinical trials with shorter duration are more effective, particularly in a population where the patient is either asked to pause/drop the conventional therapy during a clinical study or the subjects are maintained on conventional and natural products together. This will avoid unnecessary life threatening consequences due to herb–drug interactions.[29,30]
6.7. Strategy for treating co-markers or stress-related-markers in diabetes
A high level of stress prevalence have been reported in diabetic population. Various byproduct such as Amadori, advanced glycation end products (glyoxal, methyl glyoxal, fructosamine, etc), or inflammatory (interleukin-1β, insulin-like growth factor 1 [IGF-1] and monocyte chemoattractant protein 1, etc) and stress-related markers (thiobarbituric acid-reactive substances, glutathione, Catalase, Superoxide dismutase, etc) may be interesting and fruitful to study. These clinical trials may help eliminate/reduce the burden of diabetes and its comorbidities.[44,45]
6.8. Translation of the clinical trials into special populations
In addition to adult male/female subjects, it would be more helpful to conduct these clinical trials with caution in special population (pregnant, breast feeding women, and geriatric).
6.9. The need for a mechanistic approach
The herbs/herbal mixture may be challenging to determine the phytochemical profile hence, isolation is more preferable to overcome this loophole. However, if not required, a proper phytochemistry, suggested mechanism of action, metabolizing enzymes, and clearance pathways are least to be determined for any herbal product to be studied in a clinical trial.
7. Conclusion
Apart from the loopholes and drawbacks of the clinical studies reported, the authors worked to add few additional important points (Table 8), which includes; the availability of data regarding ethnopharmacological relevance, pharmacovigilance of medicinal plant (identification of plant and its part to be used with proper phytochemical profile) and intention to focus on co-markers in diabetes. These points may facilitate researchers to plan a clinical trial with high quality and uniformity.
Table 8.
Additional points suggested by authors for quality of clinical trials and its evaluation.
| Ethnopharmacological relevance of the plant | Proper preliminary literature or data (in vitro animal models, cell culture studies, etc) needed to establish the role in diabetes |
| Identification and taxonomy of planta. information regarding taxonomy | Identification of the correct plant and its part via authentic sources including herbarium, taxonomist, botanist and botanical gardens |
| b. information about plant part to use | Phytochemical screening for different plant parts with in vivo and in vitro models of pharmacological activities |
| c. quality variation and standardization | Quality variation for the same plant in terms of different geographical origins need to be standardized through green, short and reproducible analytical and pharmacological tools |
| Pharmacovigilance data of plant | Safety and efficacy data for Phase-0 with an extensive preclinical model for toxicity studies needed in order to rule out the side and adverse effects at a proper dose |
| Inclusion of co-markers treatment | Various non-conventional markers of stress, inflammation, glycation end products, etc, needs to be evaluated alongside the conventional markers |
8. Final note
The aim of the study was purely to evaluate the quality of reported clinical trials for natural products in diabetes and it does not aim to establish superiority of one clinical trial over the other nor does it intend to provide a comparison for the quality of researchers.
Author contributions
RA conceived the idea and designed the study with LHA and HNA. RA and all authors conducted literature review and wrote the introduction. RA, LHA, HNA along with MAA wrote the methodology. All the authors contributed in evaluation of each and individual clinical trial and scoring them on an excel sheet. LHA and HNA alongwith AFA, LSA, KNA, and HJA wrote the body parts including; ethnopharmacological relevance, clinical trials evaluation and schedules. RA wrote the discussion and future prospective whereas RA along with LHA and MAA performed statistical analysis. RA edited the final draft and all the authors reviewed and approved the final manuscript.
Conceptualization: Rizwan - Ahmad, Lina Hussain AlLehaibi.
Data curation: Rizwan - Ahmad, Lina Hussain AlLehaibi, Hind Nasser AlSuwaidan.
Formal analysis: Rizwan - Ahmad.
Funding acquisition: Majed A Alkhathami.
Investigation: Rizwan - Ahmad.
Methodology: Rizwan - Ahmad, Ali Fuad Alghiryafi, Lyla Shafiq AlMubarak, Khawlah Nezar AlKhalifah, Hawra Jassim AlMubarak.
Resources: Hind Nasser AlSuwaidan, Ali Fuad Alghiryafi, Khawlah Nezar AlKhalifah, Majed A Alkhathami.
Software: Hawra Jassim AlMubarak.
Supervision: Rizwan - Ahmad.
Validation: Rizwan - Ahmad.
Writing – original draft: Rizwan - Ahmad, Lina Hussain AlLehaibi.
Writing – review & editing: Rizwan - Ahmad, Hind Nasser AlSuwaidan, Ali Fuad Alghiryafi, Lyla Shafiq AlMubarak, Khawlah Nezar AlKhalifah, Hawra Jassim AlMubarak, Majed A Alkhathami.
Footnotes
Abbreviations: A-FABP = adipocyte-fatty acid binding protein, ATP = Adenosine triphosphate, AUC = Area Under The Curve, BG = Blood glucose , BMI = Body mass index, CSQS = clinical symptomatic quantitative scores, DM = Diabetes Mellitus, DSR = division of scientific research, FBG = fasting blood glucose, FDA = Food and Drug administration, FFA = Free fatty acids, GLUT4 = Glucose transporter type 4, GSH = Glutathione, HbA1c = Glycosylated Hemoglobin, Glycated Hemoglobin, Glycohemoglobin, HDL cholesterol = High-density lipoprotein cholesterol, Good cholesterol, HOMA-IR = Homeostatic Model Assessment of Insulin Resistance, IDDM = Insulin dependent diabetes mellitus, IDF = International Diabetes Federation, IGF-1 = insulin-like growth factor 1, IRB = Instuitional Review Board, KMO = Kaiser-Meyer-Olkin, LDL cholesterol = Low-density lipoproteins cholesterol. Bad cholesterol, MC = Momordica charantia, Mg = magnesium, MMP = maitake mushroom polysaccharides, NIDDM = non-insulin dependent diabetes mellitus, NIH = National Institute of Health, PBG = post-prandial blood glucose, PCA = principal component analysis, SOD = Superoxide dismutase, T1DM = type-1 diabetes mellitus, T2DM = type-2 diabetes mellitus, TBARS = thiobarbituric acid-reactive substances, TG = triglyceride.
How to cite this article: Ahmad R, AlLehaibi LH, AlSuwaidan HN, Alghiryafi AF, Almubarak LS, AlKhalifah KN, AlMubarak HJ, Alkhathami MA. Evaluation of clinical trials for natural products used in diabetes: An evidence-based systemic literature review. Medicine. 2021;100:16(e25641).
RA, LHA, and HNA contributed equally in to this work.
Funding source: No funding source (Governmental or Private organization) is available for the study.
The authors have no conflict of interest to disclose.
Consent to publish: A written informed consent for publication was obtained.
Availability of data and materials: The data used in the study will be provided upon request.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Jaded deficiencies: a; randomization mentioned, b; randomization method, c; double blind words, d; double blind method, e; description of withdrawals and dropouts. Delphi scale deficiencies: a; randomization performed, b; treatment allocation concealed, c; similarity at baseline, d; eligibility criteria specified, e; outcome assessor blinded, f; care provider blinded, g; patient blinded, h; point estimates and of variability presented for the primary outcome measured, I; intention-to-treat analysis. Cochrane scale deficiencies: a; randomization adequate, b; treatment allocation concealed, c; similarity at baseline, d; patient blinded, e; care provider blinded, f; outcome assessor blinded, g; co-interventions avoided or similar, h; compliance acceptable, I; description of withdrawals and dropouts, j; similarity in timing of the outcome assessment.
Correlation is significant at the .01 level (2-tailed).
Correlation is significant at the .05 level (2-tailed).
References
- [1].Piero MN. Diabetes mellitus—a devastating metabolic disorder. Asian J Biomed Pharm Sci 2015;4:01–7. [Google Scholar]
- [2].Ozougwu O. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J Physiol Pathophysiol 2013;4:46–57. [Google Scholar]
- [3].Warjeet Singh L. Traditional medicinal plants of Manipur as anti-diabetics. J Med Plants Res 2011;5:677–87. [Google Scholar]
- [4].A S, M N. A review of types 1 and 2 diabetes mellitus and their treatment with insulin. Am J Ther [Internet] 2006;13:349–61. [DOI] [PubMed] [Google Scholar]
- [5].Federation ID. IDF Diabetes Atlas—2019. International Diabetes Federation; 2019. 144 p. [Google Scholar]
- [6].Necyk C, Zubach-Cassano L. Natural health products and diabetes: a practical review. Can J Diabetes [Internet] 2017;41:642–7. [DOI] [PubMed] [Google Scholar]
- [7].Barbagallo M, Dominguez LJ. Diabetes and clinical research magnesium and type 2 diabetes: an update ClinMed. Int J Diabetes Clin Res 2015;2:01–5. [Google Scholar]
- [8].Medagama AB, Bandara R. The use of Complementary and Alternative Medicines (CAMs) in the treatment of diabetes mellitus: Is continued use safe and effective? Nutr J 2014;13:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bjorklund G, Dadar M, Pivina L, et al. The role of zinc and copper in insulin resistance and diabetes mellitus. Curr Med Chem 2020;27:6643–57. [DOI] [PubMed] [Google Scholar]
- [10].Joseph B, Jini D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pacific J Trop Dis 2013;3:93–102. [Google Scholar]
- [11].Marles RJ, Farnsworth NR. Antidiabetic plants and their active constituents. Phytomedicine [Internet] 1995;2:137–89. [DOI] [PubMed] [Google Scholar]
- [12].Akhondzadeh S. The importance of clinical trials in drug development. Avicenna J Med Biotechnol 2016;8(4): [PMC free article] [PubMed] [Google Scholar]
- [13].Barnes RW. Understanding investigative clinical trials. J Vasc Surg 1989;9:609–18. [DOI] [PubMed] [Google Scholar]
- [14].Shanmugasundaram ERB, Rajeswari G, Baskaran K, et al. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J Ethnopharmacol 1990;30:281–94. [DOI] [PubMed] [Google Scholar]
- [15].Baskaran K, Ahamath BK, Shanmugasundaram KR, et al. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J Ethnopharmacol 1990;30:295–305. [DOI] [PubMed] [Google Scholar]
- [16].Maithili Karpaga Selvi N, Sridhar MG, Swaminathan RP, et al. Efficacy of turmeric as adjuvant therapy in type 2 diabetic patients. Indian J Clin Biochem 2015;30:180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Balamash K, Albar O, Wang Q, et al. Effect of Kyolic® aged garlic extract on glycaemia, lipidaemia and oxidative stress in patients with type 2 diabetes mellitus. J Diabetes Res Clin Metab 2012;1:18. [Google Scholar]
- [18].Dineshkumar B, Analava M, Manjunatha M. Antidiabetic and hypolipidaemic effects of few common plants extract in type 2 diabetic patients at Bengal. Int J Diabetes Metab 2010;18:59–65. [Google Scholar]
- [19].Berger VW, Alperson SY. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials 2009;4:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Heinrich M, Appendino G, Efferth T, et al. Best practice in research—overcoming common challenges in phytopharmacological research. J Ethnopharmacol [Internet] 2020;246:112230. [DOI] [PubMed] [Google Scholar]
- [21].Almubayedh H, Ahmad R. Ethnopharmacology, phytochemistry, biological activities, and therapeutic applications of Cedrela serrata Royle: a mini review. J Ethnopharmacol [Internet] 2020;246:112206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Almubayedh H, Ahmad R. Ethnopharmacological uses, phytochemistry, biological activities of Debregeasia salicifolia: a review. J Ethnopharmacol [Internet] 2019;231:179–86. [DOI] [PubMed] [Google Scholar]
- [23].Shaw D, Graeme L, Pierre D, et al. Pharmacovigilance of herbal medicine. J Ethnopharmacol [Internet] 2012;140:513–8. [DOI] [PubMed] [Google Scholar]
- [24].Ahmad R, Ahmad N, Al-Anaki WS, et al. Solvent and temperature effect of accelerated solvent extraction (ASE) coupled with ultra-high-pressure liquid chromatography (UHPLC-PDA) for the determination of methyl xanthines in commercial tea and coffee. Food Chem [Internet] 2020;311:126021. [DOI] [PubMed] [Google Scholar]
- [25].Ahmad R, Ahmad N, Shehzad A. Solvent and temperature effects of Accelerated solvent extraction (ASE) coupled with Ultra-high pressure liquid chromatography (UHPLC-DAD) technique for determination of Thymoquinone in commercial food samples of black seeds (Nigella sativa). Food Chem [Internet] 2019;309:125740. [DOI] [PubMed] [Google Scholar]
- [26].Ahmad R, Ahmad N, Shehzad A. Solvent and temperature effects of accelerated solvent extraction (ASE) with Ultra-high pressure liquid chromatography (UHPLC-PDA) technique for determination of Piperine and its ICP-MS analysis. Ind Crops Prod [Internet] 2019;136:37–49. [Google Scholar]
- [27].Vian M, Breil C, Vernes L, et al. Green solvents for sample preparation in analytical chemistry. Curr Opin Green Sustain Chem [Internet] 2017;5:44–8. [Google Scholar]
- [28].Mahan VL. Clinical trial phases. Int J Clin Med 2014;5:1374–83. [Google Scholar]
- [29].Ahmad R. Current clinical status of osteopathy: study based on retrospective evidences of six years, a systemic review. Annu Res Rev Biol 2017;20:01–20. [Google Scholar]
- [30].Ahmad R. Current clinical status of homeopathy: an evidence based retrospective six years review. Annu Res Rev Biol 2018;22:01–15. [Google Scholar]
- [31].Furman BL, Candasamy M, Bhattamisra SK, et al. Reduction of blood glucose by plant extracts and their use in the treatment of diabetes mellitus; discrepancies in effectiveness between animal and human studies. J Ethnopharmacol 2020;247:2020. [DOI] [PubMed] [Google Scholar]
- [32].European Medicines Agency (EMA). Guideline on clinical investigation of medicinal products in the treatment or prevention of Diabetes Mellitus. Eur Med Agency [Internet] 2012;44:01–28. [Google Scholar]
- [33].Zheng W, Chang B, Chen J. Improving participant adherence in clinical research of traditional Chinese medicine. Evid Based Complement Altern Med 2014;2014:376058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Posadzki P, Watson L, Ernst E. Herb-drug interactions: an overview of systematic reviews. Br J Clin Pharmacol 2013;75:603–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hui H, Tang G, Go VLW. Hypoglycemic herbs and their action mechanisms. Chin Med 2009;4:01–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Surjushe A, Vasani R, Saple D. Aloe vera: a short review. Indian J Dermatol 2008;53:163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ahmad R, Naqvi AA, Al-Bukhaytan HM, et al. Evaluation of aromatherapy with lavender oil on academic stress: a randomized placebo controlled clinical trial. Contemp Clin Trials Commun [Internet] 2019;14:100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ahmad R, Ahmad N, Amir M, et al. Quality Variation and standardization of black pepper (Piper nigrum): a comparative geographical evaluation based on instrumental and metabolomics analysis. Biomed Chromatogr [Internet] 2020;34:e4772. [DOI] [PubMed] [Google Scholar]
- [39].Ahmad R, Ahmad N, Mohd AM, et al. Variation in Nigella sativa quality and its standardization via instrumental analysis: A study based on geographical origin. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2020;48:1141–54. [Google Scholar]
- [40].Adebajo AC, Iwalewa EO, Obuotor EM, et al. Pharmacological properties of the extract and some isolated compounds of Clausena lansium stem bark: anti-trichomonal, antidiabetic, anti-inflammatory, hepatoprotective and antioxidant effects. J Ethnopharmacol 2009;122:10–9. [DOI] [PubMed] [Google Scholar]
- [41].Raman A, Lau C. Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine [Internet] 1996;2:349–62. [DOI] [PubMed] [Google Scholar]
- [42].Yara-Varón E, Li Y, Balcells M, et al. Vegetable oils as alternative solvents for green oleo-extraction, purification and formulation of food and natural products. Molecules 2017;22:01–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].World Health Organization (WHO). Operational guidance: information needed to support clinical trials. Geneva: World Heal Organ; 2005, p. 15. [Google Scholar]
- [44].Mooy JM, De Vries H, Grootenhuis PA, et al. Major stressful life events in relation to prevalence of undetected type 2 diabetes: The Hoorn Study. Diabetes Care 2000;23:197–201. [DOI] [PubMed] [Google Scholar]
- [45].Tian M, Qing C, Cao XZ, et al. Differentailly expressed genes (DEGs) between cDNA libraries AfD0 and AfR5. J Chem Inf Model [Internet] 2016;27:449–55. [Google Scholar]
- [46].Ssenyange CW, Namulindwa A, Oyik B, et al. Plants used to manage type ii diabetes mellitus in selected districts of central Uganda. Afr Health Sci 2015;15:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dey L, Attele AS, Yuan CS. Alternative therapies for type 2 diabetes. Altern Med Rev 2002;7:45–58. [PubMed] [Google Scholar]
- [48].Haddad PS, Depot M, Settaf A, et al. Comparative study on the medicinal plants most recommended by traditional practitioners in Morocco and Canada. J Herbs Spices Med Plants 2003;10:25–45. [Google Scholar]
- [49].Tracy TS, Kingston RL. Herbal Products: Toxicology and Clinical Pharmacology. 2nd ed.2007;Germany: Springer Science and Business Media. Humana Press, XIII, 288 p. [Google Scholar]
- [50].Tarak D, Namsa ND, Tangjang S, et al. An inventory of the ethnobotanicals used as anti-diabetic by a rural community of Dhemaji district of Assam, Northeast India. J Ethnopharmacol [Internet] 2011;138:345–50. [DOI] [PubMed] [Google Scholar]
- [51].Almubayedh H, Ahmad R, Naqvi AA, et al. Ethnopharmacological uses and public knowledge regarding Cinnamomum zeylanicum in Khobar, Saudi Arabia. J Pharm Bioallied Sci 2018;10:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mardaninejad S, Janghorban M, Vazirpour M. Collection and identification of medicinal plants used by the indigenous people of Mobarakeh (Isfahan), southwestern Iran. J Herb Drugs 2013;4:23–32. [Google Scholar]
- [53].Amiri MS, Joharchi MR. Ethnobotanical investigation of traditional medicinal plants commercialized in the markets of Mashhad, Iran. Avicenna J phytomed [Internet] 2013;3:254–71. [PMC free article] [PubMed] [Google Scholar]
- [54].Asadi-Samani M, Moradi MT, Mahmoodnia L, et al. Traditional uses of medicinal plants to prevent and treat diabetes; an updated review of ethnobotanical studies in Iran. J Nephropathol 2017;6:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ahmad M, Khan MA, Arshad M, et al. Ethnophytotherapical approaches for the treatment of diabetes by the local inhabitants of District Attock (Pakistan). Ethnobot Leafl 2005;2005:24. [Google Scholar]
- [56].Rachid A, Rabah D, Farid L, et al. Ethnopharmacological survey of medicinal plants used in the traditional treatment of diabetes mellitus in the North Western and South Western Algeria. J Med Plants Res 2012;6:2041–50. [Google Scholar]
- [57].Keter LK, Mutiso PC. Ethnobotanical studies of medicinal plants used by Traditional Health Practitioners in the management of diabetes in Lower Eastern Province, Kenya. J Ethnopharmacol [Internet] 2012;139:74–80. [DOI] [PubMed] [Google Scholar]
- [58].Eddouks M, Maghrani M, Lemhadri A, et al. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J Ethnopharmacol 2002;82:97–103. [DOI] [PubMed] [Google Scholar]
- [59].Leach MJ. Gymnema sylvestre for diabetes mellitus: a systematic review. J Altern Complement Med 2007;13:977–83. [DOI] [PubMed] [Google Scholar]
- [60].Elavarasi S, Saravanan K. Ethnobotanical study of plants used to treat Diabetes by tribal people of Kolli hills, namakkal district,Tamilnadu, Southern India. Int J PharmTech Res 2012;4:404–11. [Google Scholar]
- [61].Musharaf K. Ethnobotanical studies on plant resources of Sheikh Maltoon, District Mardan, Pakistan. Med Plant Res 2014. [Google Scholar]
- [62].Mahabub Nawaz MD, Hossain AH, Karim M, et al. An ethnobotanical survey of Jessore district in Khulna division, Bangladesh. Am J Sustain Agric 2009;3:238–43. [Google Scholar]
- [63].Ocvirk S, Kistler M, Khan S, et al. Traditional medicinal plants used for the treatment of diabetes in rural and urban areas of Dhaka, Bangladesh—an ethnobotanical survey. J Ethnobiol Ethnomed 2013;9:01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Goyal M. Traditional plants used for the treatment of diabetes mellitus in Sursagar constituency, Jodhpur, Rajasthan - An ethnomedicinal survey. J Ethnopharmacol [Internet] 2015;174:364–8. [DOI] [PubMed] [Google Scholar]
- [65].Singh DR. A review on different benefits of mushroom. IOSR J Pharm Biol Sci 2017;12:107–11. [Google Scholar]
- [66].Khatun S. Research on mushroom as a potential source of nutraceuticals: a review on indian perspective. Am J Exp Agric 2012;2:47–73. [Google Scholar]
- [67].Devi S, Kumar D, MK. Ethnobotanical values of antidiabetic plants of M.P. Region, India. J Med Plants Stud 2016;4:26–8. [Google Scholar]
- [68].Atawodi SE, Atawodi JC. Azadirachta indica (neem): a plant of multiple biological and pharmacological activities. Phytochem Rev 2009;8:601–20. [Google Scholar]
- [69].Thirumalai T, Beverly CD, Sathiyaraj K, et al. Ethnobotanical Study of Anti-diabetic medicinal plants used by the local people in Javadhu hills Tamilnadu, India. Asian Pac J Trop Biomed 2012;2:S910–3. [Google Scholar]
- [70].Tahraoui A, El-Hilaly J, Israili ZH, et al. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J Ethnopharmacol 2007;110:105–17. [DOI] [PubMed] [Google Scholar]
- [71].Amirehsani KA, Wallace DC. Tés, Licuados, and Cápsulas: herbal self-care remedies of Latino/Hispanic immigrants for type 2 diabetes. Diabetes Educ 2013;39:828–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jaradat N. Ethnopharmacological survey of natural products in Palestine. An-Najah Univ J Res – Nat Sci 2005;19:13–67. [Google Scholar]
- [73].Hernandez-Galicia E, Aguilar-Contreras A, Aguilar-Santamaria L, et al. Studies on hypoglycemic activity of Mexican medicinal plants. Proc West Pharmacol Soc 2002;45:118–24. [PubMed] [Google Scholar]
- [74].Arenas PM, Molares S, Aguilar Contreras A, et al. Ethnobotanical, micrographic and pharmacological features of plant-based weight-loss products sold in naturist stores in Mexico City: the need for better quality control. Acta Bot Brasil 2013;27:560–79. [Google Scholar]
- [75].Yadav MK, Pradesh U. Ethno medicinal plants with antidiabetic activity: a review Mukesh Kumar Yadav ∗ 1. Gyanendra Tripathi 2 Parul Tripathi 3 2015;6:4570–8. [Google Scholar]
- [76].Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care 1989;12:553–64. [DOI] [PubMed] [Google Scholar]
- [77].Velayudhan KC, Dikshit N, Abdul Nizar M. Ethnobotany of turmeric (Curcuma longa L.). Indian J Tradit Knowl 2012;11:607–14. [Google Scholar]
- [78].Yongchaiyudha S, Rungpitarangsi V, Bunyapraphatsara N, et al. Antidiabetic activity of Aloe vera L. juice. I. Clinical trial in new cases of diabetes mellitus. Phytomedicine 1996;3:241–3. [DOI] [PubMed] [Google Scholar]
- [79].Bunyapraphatsara N, Yongchaiyudha S, Rungpitarangsi V, et al. Antidiabetic activity of Aloe vera L. juice II. Clinical trial in diabetes mellitus patients in combination with glibenclamide. Phytomedicine 1996;3:245–8. [DOI] [PubMed] [Google Scholar]
- [80].Huseini HF, Kianbakht S, Hajiaghaee R, et al. Anti-hyperglycemic and anti-hypercholesterolemic effects of aloe vera leaf gel in hyperlipidemic type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Planta Med 2012;78:311–6. [DOI] [PubMed] [Google Scholar]
- [81].Zarrintan A, Mobasseri M, Zarrintan A, et al. Effects of aloe vera supplements on blood glucose level and lipid profile markers in type 2 diabetic patients—a randomized clinical trial. Pharm Sci [Internet] 2015;21:65–71. [Google Scholar]
- [82].Choudhary M, Kochhar A, Sangha J. Hypoglycemic and hypolipidemic effect of Aloe vera L. in non-insulin dependent diabetics. J Food Sci Technol 2014;51:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jenkins AL, Morgan LM, Bishop J, et al. Co-administration of a konjac-based fibre blend and American ginseng (Panax quinquefolius L.) on glycaemic control and serum lipids in type 2 diabetes: a randomized controlled, cross-over clinical trial. Eur J Nutr 2018;57:2217–25. [DOI] [PubMed] [Google Scholar]
- [84].Vuksan V, Sievenpiper JL, Koo VYY, et al. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med 2000;160:1009–13. [DOI] [PubMed] [Google Scholar]
- [85].Vuksan V, Stavro MP, Sievenpiper JL, et al. Similar postprandial glycemic reductions with escalation of dose and administration time of American ginseng in type 2 diabetes. Diabetes Care 2000;23:1221–6. [DOI] [PubMed] [Google Scholar]
- [86].Vuksan V, Xu ZZ, Jovanovski E, et al. Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a double-blind, randomized, cross-over clinical trial. Eur J Nutr [Internet] 2019;58:1237–45. [DOI] [PubMed] [Google Scholar]
- [87].Hoggard N, Cruickshank M, Moar KM, et al. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36% wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J Nutr Sci 2013;2:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Blevins SM, Leyva MJ, Brown J, et al. Effect of cinnamon on glucose and lipid. Diabetes Care 2007;30:2236–7. [DOI] [PubMed] [Google Scholar]
- [89].Khan A, Safdar M, Ali Khan MM, et al. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003;26:3215–8. [DOI] [PubMed] [Google Scholar]
- [90].Vanschoonbeek K, Thomassen BJW, Senden JM, et al. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr 2006;136:977–80. [DOI] [PubMed] [Google Scholar]
- [91].Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. J Am Board Fam Med 2009;22:507–12. [DOI] [PubMed] [Google Scholar]
- [92].Mang B, Wolters M, Schmitt B, et al. Effects of a cinnamon extract on plasma glucose, HbA1c, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest 2006;36:340–4. [DOI] [PubMed] [Google Scholar]
- [93].Khan R, Khan Z, Shah S. Cinnamon may reduce glucose, lipid and cholesterol level in type 2 diabetic individuals. Pakistan J Nutr 2010;9:430–3. [Google Scholar]
- [94].Lu T, Sheng H, Wu J, et al. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr Res [Internet] 2012;32:408–12. [DOI] [PubMed] [Google Scholar]
- [95].Altschuler JA, Casella SJ, MacKenzie TA, et al. The effect of cinnamon on A1C among adolescents with type 1 diabetes. Diabetes Care 2007;30:813–6. [DOI] [PubMed] [Google Scholar]
- [96].Vafa M, Mohammadi F, Shidfar F, et al. Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients. Int J Prev Med [Internet] 2012;3:531–6. [PMC free article] [PubMed] [Google Scholar]
- [97].Akilen R, Tsiami A, Devendra D, et al. Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: a randomized, placebo-controlled, double-blind clinical trial. Diabet Med 2010;27:1159–67. [DOI] [PubMed] [Google Scholar]
- [98].Verma N, Usman K, Patel N, et al. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (FenfuroTM) in patients with type 2 diabetes. Food Nutr Res 2016;60:01–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ranade M, Mudgalkar N. A simple dietary addition of fenugreek seed leads to the reduction in blood glucose levels: a parallel group, randomized single-blind trial. Ayu 2017;38:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lu FR, Shen L, Qin Y, et al. Clinical observation on trigonella foenum-graecum L. total saponins in combination with sulfonylureas in the treatment of type 2 diabetes mellitus. Chin J Integr Med 2008;14:56–60. [DOI] [PubMed] [Google Scholar]
- [101].Kassaian N, Azadbakht L, Forghani B, et al. Effect of Fenugreek seeds on blood glucose and lipid profiles in type 2 diabetic patients. Int J Vit Nutr Res 2009;79:04–9. [DOI] [PubMed] [Google Scholar]
- [102].Gupta A, Gupta R, Lal B. Effect of Trigonella foenum-graecum (Fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double blind placebo controlled study. J Assoc Physicians India 2001;49:1057–61. [PubMed] [Google Scholar]
- [103].Sundaram G, Ramakrishnan T, Parthasarathy H, et al. Fenugreek, diabetes, and periodontal disease: A cross-link of sorts!. J Indian Soc Periodontol 2018;22:122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zarvandi M, Rakhshandeh H, Abazari M, et al. Safety and efficacy of a polyherbal formulation for the management of dyslipidemia and hyperglycemia in patients with advanced-stage of type-2 diabetes. Biomed Pharmacother 2017;89:69–75. [DOI] [PubMed] [Google Scholar]
- [105].Atkin M, Laight D, Cummings MH. The effects of garlic extract upon endothelial function, vascular inflammation, oxidative stress and insulin resistance in adults with type 2 diabetes at high cardiovascular risk. A pilot double blind randomized placebo controlled trial. J Diabetes Complications [Internet] 2016;30:723–7. [DOI] [PubMed] [Google Scholar]
- [106].Sukandar EY, Permana H, Adnyana IK, et al. Clinical study of turmeric (Curcuma longa L.) and Garlic (Allium sativum L) extracts as antihyperglycemic and antihyperlipidemic agent in type-2 diabetes-dyslipidemia patients. Int J Pharmacol 2010;6:438–45. [Google Scholar]
- [107].Sobenin IA, Nedosugova LV, Filatova LV, et al. Metabolic effects of time-released garlic powder tablets in type 2 diabetes mellitus: the results of double-blinded placebo-controlled study. Acta Diabetol 2008;45:01–6. [DOI] [PubMed] [Google Scholar]
- [108].Kumar R, Chhatwal S, Arora S, et al. Antihyperglycemic, antihyperlipidemic, anti-inflammatory and adenosine deaminase-lowering effects of garlic in patients with type 2 diabetes mellitus with obesity. Diabetes Metab Syndr Obes Targets Ther 2013;6:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Manafikhi R, Kalie L, Lahdo R. Effects of garlic supplementation on fasting blood sugar. HbA1c and lipid profile in type 2 diabetics receiving metformin and glyburide 2015;3:11–8. [Google Scholar]
- [110].Ashraf R, Khan RA, Ashraf I. Garlic (Allium sativum) supplementation with standard antidiabetic agent provides better diabetic control in type 2 diabetes patients. Pak J Pharm Sci 2011;24:565–70. [PubMed] [Google Scholar]
- [111].Khan R. Effects of garlic on blood glucose levels and HbA1c in patients with type 2 diabetes mellitus. J Med plant Res 2011;5:2922–8. [Google Scholar]
- [112].Chhatwal S, Sharma R, Sharma G, et al. To study the antihyperglycaemic and lipid lowering effect of garlic as an adjunct to metformin in patients of type 2 diabetes mellitus with obesity. Int J Basic Clin Pharmacol 2012;1:22. [Google Scholar]
- [113].Joffe DJ, Freed SH. Effect of extended release gymnema sylvestre leaf extract (Beta Fast GXR) alone or in combination with oral hypoglycemics or insulin regimens for type 1 and type 2 diabetes. Diabetes Control Newsl 2001;30. [Google Scholar]
- [114].Al-Romaiyan A, Liu B, Asare-Anane H, et al. A novel Gymnema sylvestre extract stimulates insulin secretion from human islets in vivo and in vitro. Phyther Res 2010;24:1370–6. [DOI] [PubMed] [Google Scholar]
- [115].Paliwal R, Kathori S, Upadhyay B. Effect of gurmar (Gymnema sylvestre) powder intervention on the blood glucose levels among diabetics. Stud Ethno-Med 2009;3:133–5. [Google Scholar]
- [116].Teixeira CC, Fuchs FD, Weinert LS, et al. The efficacy of folk medicines in the management of type 2 diabetes mellitus: results of a randomized controlled trial of Syzygium cumini (L.) Skeels. J Clin Pharm Ther 2006;31:01–5. [DOI] [PubMed] [Google Scholar]
- [117].Teixeira CC, Weinert LS, Barbosa DC, et al. Syzygium cumini (L.) skeels in the treatment of type 2 diabetes [2]. Diabetes Care 2004;27:3019–20. [DOI] [PubMed] [Google Scholar]
- [118].Akhtar MS. Trial of Momordica charantia linn (Karela) powder in patients with maturity-onset diabetes. J Pak Med Assoc 1982;32:106–7. [PubMed] [Google Scholar]
- [119].Fuangchan A, Sonthisombat P, Seubnukarn T, et al. Hypoglycemic effect of bitter melon compared with metformin in newly diagnosed type 2 diabetes patients. J Ethnopharmacol 2011;134:422–8. [DOI] [PubMed] [Google Scholar]
- [120].John AJ, Cherian R, Subhash HS, et al. Evaluation of the efficacy of bitter gourd (Momordica charantia) as an oral hypoglycemic agent: a randomized controlled clinical trial [3]. Indian J Physiol Pharmacol 2003;47:363–5. [PubMed] [Google Scholar]
- [121].Tongia A, Tongia SK, Dave M. Phytochemical determination and extraction of momordica charantia fruit and its hypoglycemic potentiation of oral hypoglycemic drugs in diabetes mellitus (NIDDM). Indian J Physiol Pharmacol 2004;48:241–4. [PubMed] [Google Scholar]
- [122].Rahman IU, Khan RU, Rahman KU, et al. Lower hypoglycemic but higher antiatherogenic effects of bitter melon than glibenclamide in type 2 diabetic patients. Nutr J 2015;14:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Baldwa VS, Bhandari CM, Pangaria A, et al. Clinical trial in patients with diabetes mellitus of an insulin-like compound obtained from plant source. Ups J Med Sci 1977;82:39–41. [DOI] [PubMed] [Google Scholar]
- [124].Cortez-Navarrete M, Martínez-Abundis E, Pérez-Rubio KG, et al. Momordica charantia administration improves insulin secretion in type 2 diabetes mellitus. J Med Food 2018;21:672–7. [DOI] [PubMed] [Google Scholar]
- [125].Dans AML, Villarruz MVC, Jimeno CA, et al. The effect of Momordica charantia capsule preparation on glycemic control in type 2 diabetes mellitus needs further studies. J Clin Epidemiol 2007;60:554–9. [DOI] [PubMed] [Google Scholar]
- [126].Inayat-ur-Rahman, Malik SA, Bashir M, et al. Serum sialic acid changes in non-insulin-dependant diabetes mellitus (NIDDM) patients following bitter melon (Momordica charantia) and rosiglitazone (Avandia) treatment. Phytomedicine 2009;16:401–5. [DOI] [PubMed] [Google Scholar]
- [127].Konno S, Tortorelis DG, Fullerton SA, et al. A possible hypoglycaemic effect of maitake mushroom on type 2 diabetic patients [5]. Diabet Med 2001;18:1010. [DOI] [PubMed] [Google Scholar]
- [128].Kochhar A, Sharma N, Sachdeva R. Effect of supplementation of Tulsi (Ocimum sanctum) and Neem (Azadirachta indica) leaf powder on diabetic symptoms, anthropometric parameters and blood pressure of non insulin dependent male diabetics. Stud Ethno-Med 2009;3:05–9. [Google Scholar]
- [129].Kumari DJ. Hypoglycaemic effect of Moringa oleifera and Azadirachta indica in type 2 diabetes mellitus. Bioscan 2010;5:211–4. [Google Scholar]
- [130].López-Romero P, Pichardo-Ontiveros E, Avila-Nava A, et al. The effect of Nopal (Opuntia Ficus Indica) on postprandial blood glucose, incretins, and antioxidant activity in mexican patients with type 2 diabetes after consumption of two different composition breakfasts. J Acad Nutr Diet [Internet] 2014;114:1811–8. [DOI] [PubMed] [Google Scholar]
- [131].Castañeda-Andrade I, Gonzalez-Sanchez J, Frati-Munari AC. Hypoglycemic effect of an Opuntia streptacanta Lemaire dialysate. J Prof Assoc Cactus Dev 1997;2:73–5. [Google Scholar]
- [132].Knishinsky R. Prickly pear cactus medicine: Treatments for diabetes, cholesterol, and the immune system. 144 pages, Published by Inner Traditions/Bear & Co; 2004 Jun 7. [Google Scholar]
- [133].Eldin IMT, Ahmed EM, Abd EHM. Preliminary study of the clinical hypoglycemic effects of Allium cepa (Red Onion) in type 1 and type 2 diabetic patients. Environ Health Insights 2010;4:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Soltanian N, Janghorbani M. Effect of flaxseed or psyllium vs. placebo on management of constipation, weight, glycemia, and lipids: a randomized trial in constipated patients with type 2 diabetes. Clin Nutr ESPEN [Internet] 2019;29:41–8. [DOI] [PubMed] [Google Scholar]
- [135].Noureddin S, Mohsen J, Payman A. Effects of psyllium vs. placebo on constipation, weight, glycemia, and lipids: a randomized trial in patients with type 2 diabetes and chronic constipation. Complement Ther Med 2018;40:01–7. [DOI] [PubMed] [Google Scholar]
- [136].Feinglos MN, Gibb RD, Ramsey DL, et al. Psyllium improves glycemic control in patients with type-2 diabetes mellitus. Bioact Carbohydrates Diet Fibre 2013;1:156–61. [Google Scholar]
- [137].Abutair AS, Naser IA, Hamed AT. Soluble fibers from psyllium improve glycemic response and body weight among diabetes type 2 patients (randomized control trial). Nutr J [Internet] 2016;15:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Pastors JG, Blaisdell PW, Balm TK, et al. Psyllium fiber reduces rise in postprandial glucose and insulin concentrations in patients with non-insulin-dependent diabetes. Am J Clin Nutr 1991;53:1431–5. [DOI] [PubMed] [Google Scholar]
- [139].Enno F, Gleske J. Siberian Ginseng results in beneficial effects on glucose metabolism in diabetes type 2 patients: a double blind placebo-controlled study in comparison to Panax Ginseng. Int J Clin Nutr [Internet] 2013;1:11–7. [Google Scholar]
- [140].Rahimi HR, Mohammadpour AH, Dastani M, et al. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J phytomed 2016;6:567. [PMC free article] [PubMed] [Google Scholar]
- [141].Na LX, Yan BL, Jiang S, et al. Curcuminoids target decreasing serum adipocyte-fatty acid binding protein levels in their glucose-lowering effect in patients with type 2 diabetes. Biomed Environ Sci 2014;27:902–6. [DOI] [PubMed] [Google Scholar]
- [142].Na LX, Li Y, Pan HZ, et al. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol Nutr Food Res 2013;57:1569–77. [DOI] [PubMed] [Google Scholar]


