PURPOSE
High-grade nonmuscle invasive bladder cancer (HRNMIBC) is a heterogeneous disease. Treatments include intravesical maintenance Bacillus Calmette-Guerin (mBCG) and radical cystectomy (RC). We wanted to understand whether a randomized trial comparing these options was possible.
MATERIALS AND METHODS
We conducted a two-arm, prospective multicenter randomized study to determine the feasibility in Bacillus Calmette-Guerin-naive patients. Participants had new high-risk HRNMIBC suitable for both treatments. Random assignment was stratified by age, sex, center, stage, presence of carcinoma in situ, and prior low-risk bladder cancer. Qualitative work investigated how to maintain equipoise. The primary outcome was the number of patients screened, eligible, recruited, and randomly assigned.
RESULTS
We screened 407 patients, approached 185, and obtained consent from 51 (27.6%) patients. Of these, one did not proceed and therefore 50 were randomly assigned (1:1). In the mBCG arm, 23/25 (92.0%) patients received mBCG, four had nonmuscle invasive bladder cancer (NMIBC) after induction, three had NMIBC at 4 months, and four received RC. At closure, two patients had metastatic BC. In the RC arm, 20 (80.0%) participants received cystectomy, including five (25.0%) with no tumor, 13 (65.0%) with HRNMIBC, and two (10.0%) with muscle invasion in their specimen. At follow-up, all patients in the RC arm were free of disease. Adverse events were mostly mild and equally distributed (15/23 [65.2%] patients with mBCG and 13/20 [65.0%] patients with RC). The quality of life (QOL) of both arms was broadly similar at 12 months.
CONCLUSION
A randomized controlled trial comparing mBCG and RC will be challenging to recruit into. Around 10% of patients with high-risk HRNMIBC have a lethal disease and may be better treated by primary radical treatment. Conversely, many are suitable for bladder preservation and may maintain their prediagnosis QOL.
INTRODUCTION
Bladder cancer (BC) is a common malignancy and one of the most expensive to manage.1,2 Around 25% of patients present with high-grade nonmuscle invasive (NMI) tumors. Rates of progression to muscle invasion vary between 15% and 50%.3 Around 20% of patients may die from BC.4 Progression risks increase with carcinoma in situ (CIS), invasion into the lamina propria, or prostatic urethral involvement. Consequently, these features can be used to identify high-risk high-grade NMIBC (HRNMIBC).3
CONTEXT
Key Objective
We wanted to understand whether a randomized trial comparing intravesical maintenance Bacillus Calmette-Guerin (mBCG) and radical cystectomy (RC) for high-grade nonmuscle invasive bladder cancer (HRNMIBC) was possible.
Knowledge Generated
An RCT comparing mBCG and RC will be difficult to conduct. In the mBCG arm, 23/25 (92.0%) patients received mBCG, four had nonmuscle invasive bladder cancer (NMIBC) after induction, three had NMIBC at 4 months, four received RC, and two developed metastases. In the RC arm, 20/25 (80.0%) participants received cystectomy, including five (25.0%) with no tumor, 13 (65.0%) with HRNMIBC, and two (10.0%) with muscle invasion in their specimen. All patients in the RC arm were free of disease at closure.
Relevance
Around 10% of patients with new high-risk HRNMIBC have a lethal disease and may be better treated by primary radical treatment. Conversely, many are suitable for bladder preservation and may maintain their prediagnosis quality of life.
The treatment options for HRNMIBC are intravesical immunotherapy (using maintenance intravesical Bacillus Calmette-Guerin [mBCG]) and radical cystectomy (RC).5,6 Although mBCG avoids bladder removal, it leaves patients at risk of progression and may affect health-related quality of life (HRQOL) through local symptoms and anxiety.7 RC removes the risk of local disease progression, but maybe overtreatment for nonprogressing tumors. Many patients develop postoperative complications after RC, around 3% die after 90 days,8 and others have a reduction in HRQOL.9,10
RC and mBCG have not been directly compared, hampering decision making and exposing patients to overtreatment or undertreatment.11 As RCTs comparing radical surgical with nonsurgical options are challenging to complete,12,13 we undertook a feasibility study to assess whether recruitment was possible. We embedded qualitative components exploring patient experience and clinician equipoise. We hypothesized that recruitment and random assignment would not be possible.
MATERIALS AND METHODS
Patients and Random Assignment
BRAVO was a multicenter, parallel-group, mixed-methods, individually randomized, controlled feasibility study14 run through seven NHS cancer networks. Eligible patients were ≥ 18 years old with a new diagnosis of high-grade15 or grade 316 NMI urothelial cell carcinoma (UCC) (either pTa, pTis, or pT1). One or more criteria from presence of pTis, lympho(vascular) invasion, residual grade 3/high UCC on re-resection, multifocal disease (> 3 tumors), young age (< 65 years old), initial tumor size > 3 cm (or > 5 g in histology specimen), or pT1 stage were also needed. Participants were consented by clinicians and randomly assigned (1:1) by the Clinical Trials Research Unit. Random assignment was stratified by age (< 75, ≥ 75), sex, center, highest transurethral resection of bladder tumor stage (pTa/pTis, pT1), the presence of CIS, and previous low-risk BC. Re-resection was performed if the transurethral resection of bladder tumor specimen had T1 disease or Ta/Tis disease without detrusor muscle.17 It was not possible to blind treatment allocation. The study has ethical approval (16/YH/0268).
Treatment and Follow-Up
Successful mBCG was defined as ≥ 4 induction doses and at least 12 months of maintenance treatment using the SWOG Protocol.18 mBCG could continue in the presence of HRNMIBC at first cystoscopy after induction of Bacillus Calmette-Guerin (BCG) or in the presence of low-risk nonmuscle invasive bladder cancer (NMIBC) at any time. The presence of HRNMIBC or invasive BC after induction required the cessation of mBCG. Rigid cystoscopy with biopsy and bladder washings or urine cytology was mandated at the first check. Thereafter, cystoscopic approach was per local protocol. Dose adjustment for BCG was not permissible. Please see our methods report14 and Protocol (Data Supplement, online only) for more details.
RC included removal of the prostate or seminal vesicles in men, and uterus or cervix or fallopian tubes and anterior vaginal wall in women. Pelvic lymphadenectomy included, at least, regional lymph nodes up to the ureteric crossing of the common iliac vessels. To minimize surgical variation,19 only surgeons with ≥ 10 years' RC experience or reported (British Association of Urological Surgeons data set20) outcome data on ≥ 10 RCs per year for the last 2 years (or 20 in the last year), with a median length of stay under 16 days and a 90-day post-RC mortality rate of < 10%, were used.
Follow-up included three monthly clinic review and chest or abdominal or pelvis computed tomography scans 52 weeks after random assignment. HRQOL questionnaires were administered prior to random assignment and at 3 and 6 months after random assignment. For those randomly assigned early to the study, HRQOL results were collected at 12 months after random assignment. Measures included EuroQuol-5D (EQ-5D),21 EORTC QLQ-C30,22 and either EORTC QLQ-BLM30 (RC cohort) or EORTC QLQ-NMIBC24 (for BCG). Decision regret questionnaires asking about trial participation and acceptance of allocated treatments were completed at 12 months. Deaths, complications, and toxicities (adverse events [AEs]), and related unexpected serious AEs up to 12 months after random assignment, or 3 months after the last participant was randomly assigned were collected. Please see ref. 14 and the Protocol (Data Supplement).
Outcomes
Primary outcomes were the number of patients screened, eligible, recruited, and randomly assigned in the study. Secondary outcomes included the acceptance rates of allocated treatments, 12-month mBCG compliance, feasibility of collecting (including optimal schedule and likely distribution of) HRQOL data, and to explore the reasons expressed by patients for declining recruitment.
Sample Size
Sample size was set to give confidence that recruitment for a definitive trial could be met. A formal power calculation was not appropriate. The primary end point of a phase III trial would be cancer-specific survival. We estimated that 506 participants would be required to have 80% power to show a superiority hazard ratio of 0.626 (based on improvement in 5-year cancer-specific survival from 0.7 with mBCG to 0.8 for RC), assuming a 3-year accrual period, 5 years of follow-up, and accounting for 5% loss to follow-up. For this feasibility study, pilot data suggested a pool of 200 eligible patients per year, of which we estimated we would need to randomly assign approximately 25% to meet the rate required in a definitive trial. Thus, we aimed to recruit a minimum of 60 participants from seven centers (and their associated district general hospitals) over an 18-month period. This assumed a 6-month setup (recruitment rate approximately four patients per center per year) and a rate of approximately 11 patients per center per year thereafter.
Analyses
Quantitative analyses focused on descriptive statistics and CI estimation and were conducted on the intention-to-treat population; formal hypothesis testing was not undertaken.
Summary statistics were calculated for screening, eligibility, recruitment, and random assignment to provide estimates for the definitive trial. To understand acceptability of the study, uptake of allocated treatment and compliance with 12-month mBCG were summarized descriptively. Follow-up rates for self-reported outcomes were reported together with 95% CIs to understand the feasibility of collecting quality of life (QOL) data and to inform the sample size calculation for a definitive trial.
Qualitative Interviews
Qualitative analyses were conducted from interviews with eight doctors, six nurse specialists, and 29 patients (14 had received RC and 15 BCG). A manuscript summarizing these results is in preparation (M. Twiddy, personal communication, January 2020).
RESULTS
Screened, Eligible, and Randomly Assigned Populations
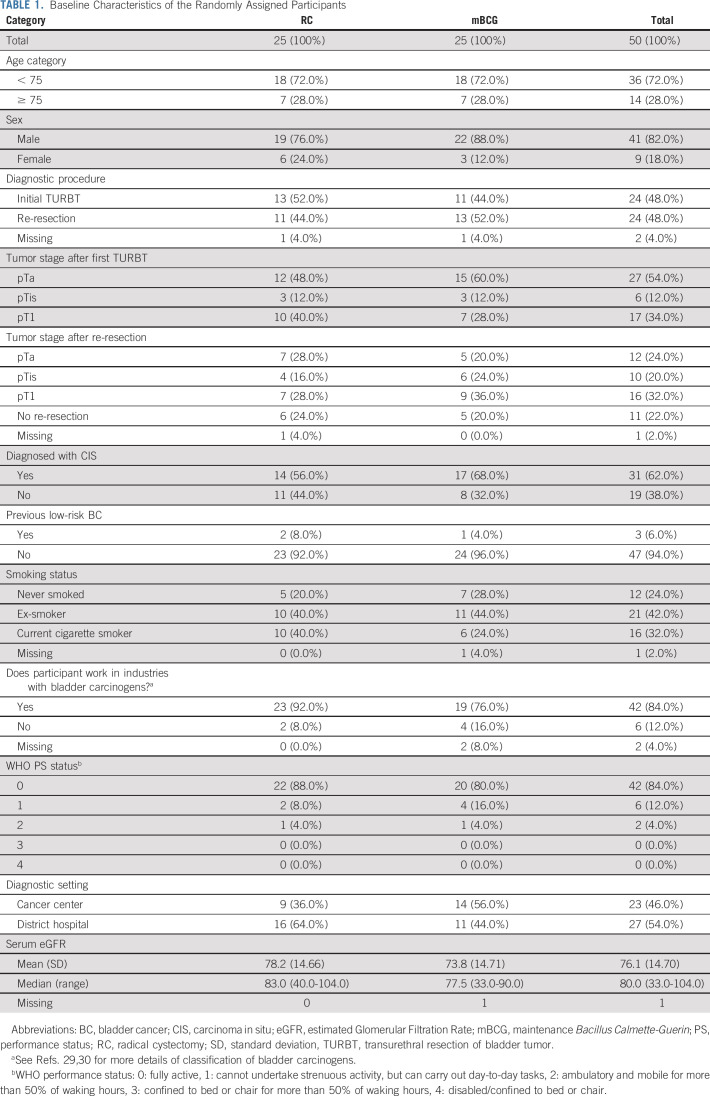
In total, 407 patients were screened and 215 (52.8%) patients were found eligible (Fig 1, Data Supplement) between October 2016 and March 2018. The commonest reasons for ineligibility were prior HRNMIBC and/or BCG, NMIBC lacking additional risk factors, another malignancy, or the patient was unsuitable for both treatments. Investigators approached 185/215 eligible patients and 51 agreed to be randomly assigned (27.6%). Patients declined random assignment because of one or more of the following: mBCG preference (77 [50.0%]), RC preference (39 [25.3%]), dislike of random assignment (27 [17.5%]), concerns about study participation (8 [5.2%]), or did not specify (3 [1.9%]). One participant did not proceed to random assignment (and chose mBCG outside of the study). Consequently, 25 patients were randomly assigned to mBCG and 25 to RC. Recruitment was halted after 18 months (as per statistical plan). The randomly assigned cohort were typical for BC patients: most were men (4:1 ratio), between the age of 60-80 years (82.0%), and with a smoking history (74.0%, Table 1 and Data Supplement).
FIG 1.
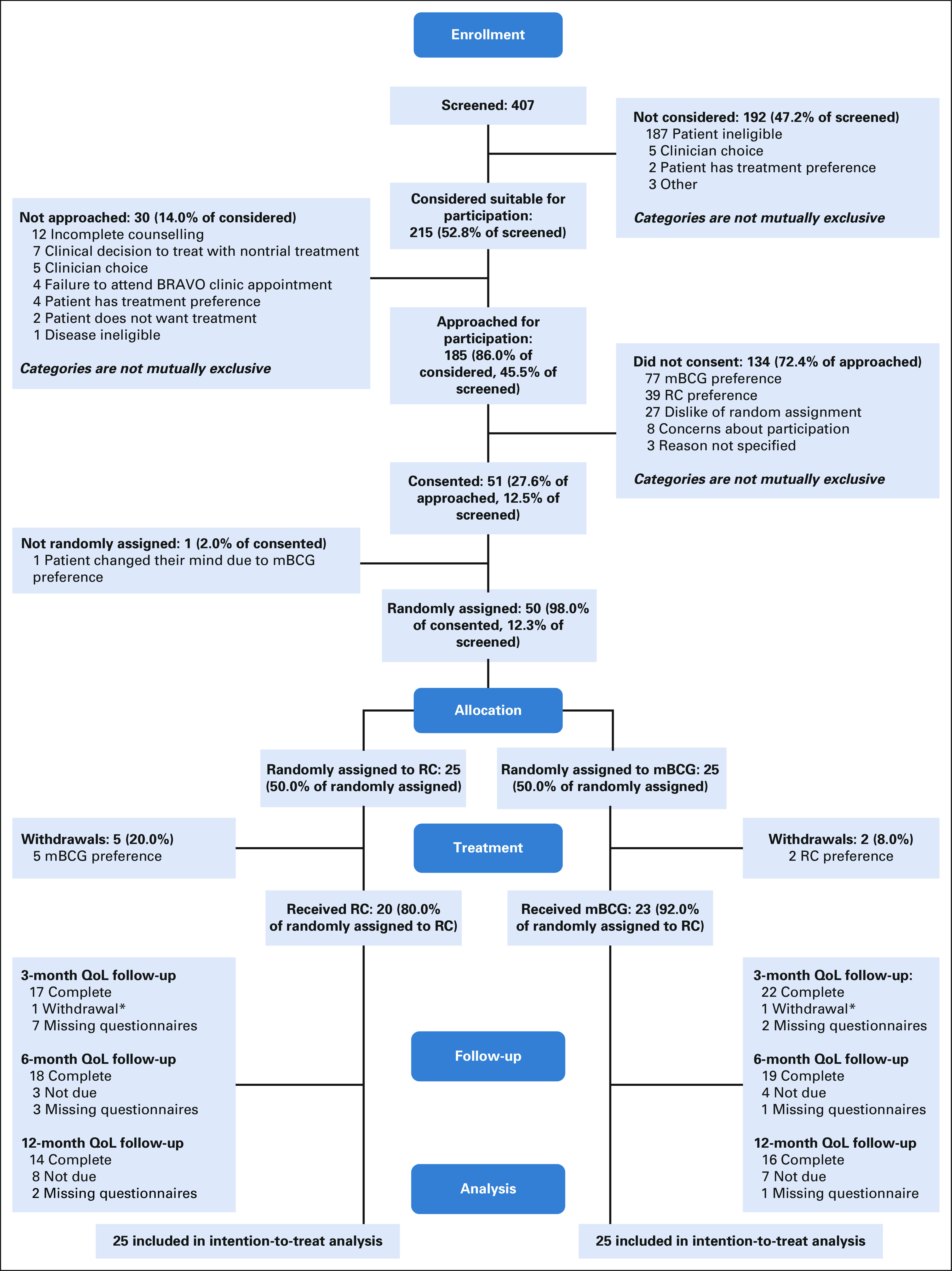
Consort diagram for the BRAVO feasibility study. mBCG, maintenance Bacillus Calmette-Guerin; QOL, quality of life; RC, radical cystectomy.
TABLE 1.
Baseline Characteristics of the Randomly Assigned Participants
Maintenance BCG
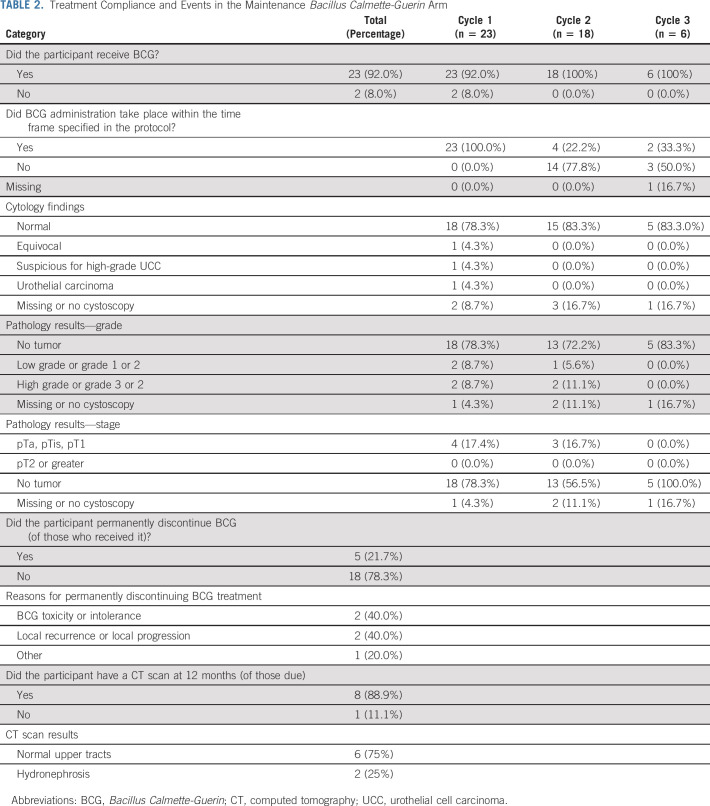
Follow-up concluded in July 2018. In the mBCG arm, 2/25 (8% of randomly assigned) participants did not commence treatment and 23/25 (92.0% of randomly assigned) started BCG, all within 4 weeks of random assignment (Table 2). Of these, 22 (95.7%) participants received all induction doses and 1 had 2 omissions because of a urinary infection or BCG-itis before refusing further BCG (Data Supplement). All 18 who received cycle 2 and 5/6 (83.3%) participants reaching cycle 3 received three instillations. Cycle commencement was delayed in 14/18 (77.8%) and 3/6 (50.0%) participants for cycle 2 and 3, respectively. In cycle 3, one patient had three omissions because of BCG-related toxicities and feeling too unwell for treatment. Cystoscopy during induction (ie, after 6 BCG instillations) revealed low-grade (2/22 [9.1%]) and high-grade (2/22 [9.1%)] NMIBC in four patients. At 4 months, low-grade pTa and high-grade pT1 NMIBC were present in one and two patients, respectively. At follow-up, 1/25 (4.0%) patient had progressed to distant metastatic UCC (at 12 months after random assignment), 4/25 (16.0%) patients had received RC (two because of initial preference for RC, one because of ineffectiveness of BCG post-induction (6 months after random assignment: RC histology was pT3 N1 UCC), and one for CIS (at 12 months after random assignment), and one patient was receiving hyperthermic mitomycin C for BCG-unresponsive CIS (9 months after random assignment). Fifty-one AEs were seen in 15/23 (65.2%) patients. The average number of AEs per person was 2.6 (standard deviation [SD] = 0.96) and 1.9 (SD = 0.64) for induction and cycle 2, respectively; one patient experienced two AEs at cycle 3. Most AEs were mild (grade 1 or 2), such as urinary frequency (15/51; 29.4%) or dysuria (13/51; 25.5%). However, 2 AEs were grade 3 during induction and AEs led to unplanned hospital admission in 3/13 (23.1%) patients during induction and in 1/8 (12.5%) patient during cycle 2 (0 in cycle 3). In total, of those who commenced BCG treatment, 5 (21.7%) permanently discontinued BCG.
TABLE 2.
Treatment Compliance and Events in the Maintenance Bacillus Calmette-Guerin Arm
Radical Cystectomy
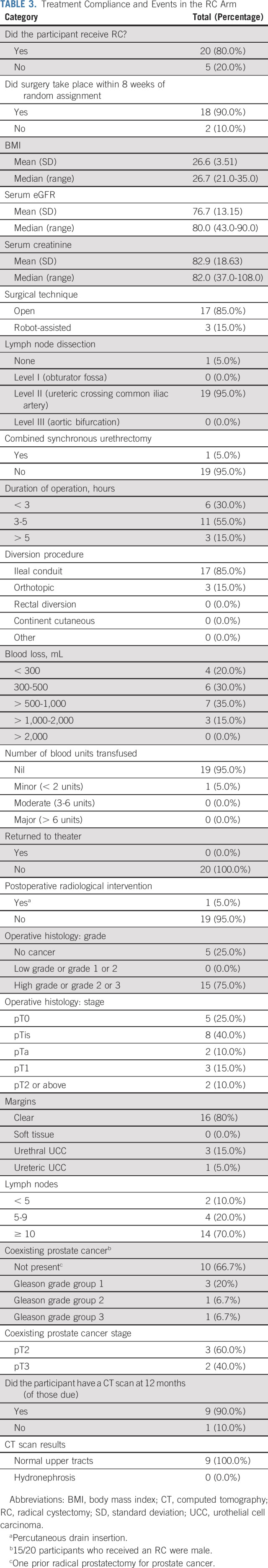
In the RC arm, 20/25 (80%) patients received primary surgery (Table 3; including 18 within 8 weeks of random assignment and one who deferred RC until after coronary angioplasty) and five (20.0%) chose mBCG. Most surgery was via an open approach (17/20 [85.0%]) using an ileal conduit for urinary drainage (17/20 [85.0%]). One case included synchronous urethrectomy. One patient required blood transfusion (5.0%) and one required radiological guided drain insertion for a pelvic collection (Clavien-Dindo grade 3a: 5.0%). Five (25.0%) participants experienced five AEs relating to cystectomy (Data Supplement hemorrhage or bleeding [two], small bowel injury [one], nerve injury [one], and difficult dissection [one]). Six (30.0%) participants experienced 9 postoperative complications (chest infection [three], wound infection [one], ileus [three] urine leak [one], and constipation [one]), and consequently, 4/20 (20.0%) participants had an increased length of hospital stay. There were no deaths within the follow-up period. By 6 weeks after surgery, nine patients had developed a complication (anastomotic leak [one], constipation [three], fever [two] and sepsis [three], ileus [one], and wound [one] or urinary infection [one]), three patients had a prolonged length of stay, and four patients required readmission. By 20 weeks, a further five complications (in three patients) were observed (abdominal pain [one], fever [one], sepsis [two], and low B12 and folate [one]). No further complications were observed by week 52. Overall, complications were seen in 13/20 (65.0%) participants, of which two were Clavien-Dindo grade 3a, one grade 3b, and one grade 4a. Histology revealed high-grade urothelial carcinoma in 15/20 patients (Table 3, 75.0%), including muscle invasion in 2/20 (pT2N0 in 10.0%) and NMIBC in 13 (pT1 in three, pTis in eight, and pTa in two). No tumor was found in five cases. Lymphadenectomy was performed in 19/20 patients, with a median of 10 nodes (range, 4-27) identified by histopathology. No nodal metastases were detected. Four patients had UCC at the urethral (three) or ureteric (one) resection margins. Prostate cancer was present in five men (pT2 in three and pT3 in two, Table 3), excluding one who had undergone prior radical prostatectomy. Of the 10 patients with 52-week follow-up, nine were disease-free without hydronephrosis and data for one patient were missing. The median eGFR at 52 weeks was 72.0 mL/min.
TABLE 3.
Treatment Compliance and Events in the RC Arm
HRQOL Data
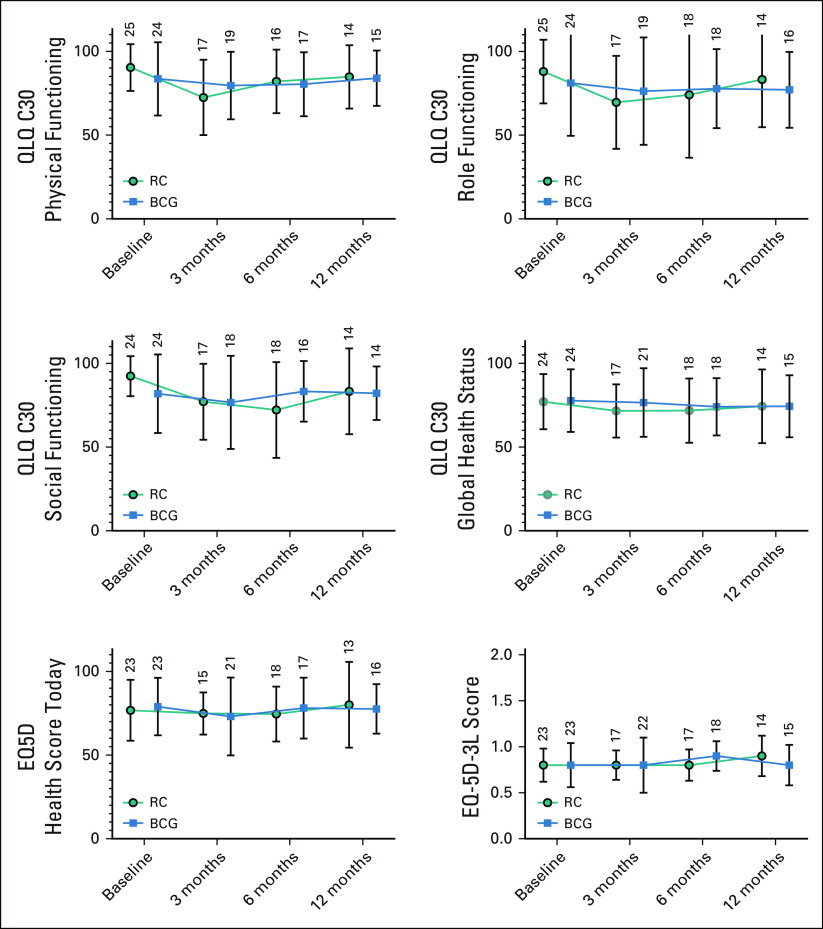
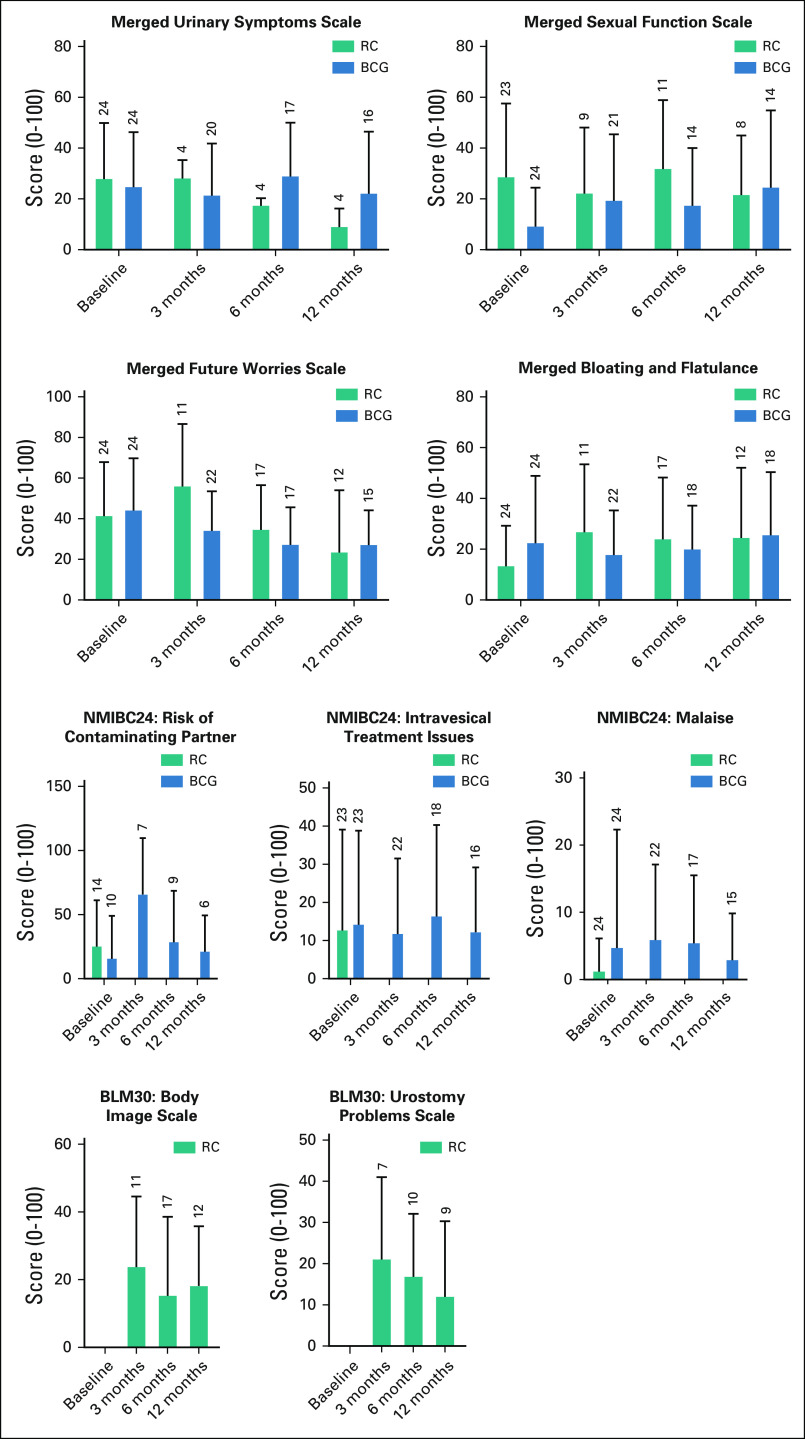
HRQOL questionnaires were completed in 98.0%, 78.0%, 74.0%, and 60.0% at baseline, 3, 6, and 12 months, respectively. Although powered comparative analyses of HRQOL are not possible and the EORTC Bladder tools are not directly comparable, global trends were apparent (Figs 2 and 3). In general, few changes in EQ-5D-3L were seen (Data Supplement). Within QLQ-C30 functional domains (Data Supplement), the RC cohort had a reduction in QOL at 3 months, which recovered to baseline between 6 and 12 months. Little change was seen with mBCG cohort over 12 months. Similarly, few changes were seen in the QLQ-C30 symptom scores in both arms. Changes were seen in treatment-specific questionnaires (Data Supplement, Fig 3). For mBCG, there were small increases in the urinary symptom scores at 6 months and concerns about contaminating a partner at 3 months (although this reduced by 12 months), and small reductions in future worry scores on the NMIBC24 questionnaire with time. For the RC cohort, most symptoms improved (lower scores) from 3 to 12 months, except sexual function, which did not change. Decision regret questionnaires were completed by 39/50 (78.0%) participants at 12 months (Data Supplement). There was little regret at entering the study; “It was the right decision” (mean = 13.5, SD = 18.3, lower scores indicating less regret) and “I would go for the same choice if I had to do it over again” (mean 11.5, SD = 17.3, lower scores indicating less regret). With respect to individual treatments, patients in the BCG arm had a higher regret score; “I regret the choice that was made” (mean = 22.1, SD = 15.6 v mean = 13.2, SD = 27.8), compared with the RC cohort.
FIG 2.
Generic health-related quality of life for patients in the BRAVO feasibility study as measured using the EuroQuol-5D (EQ5D) and EORTC QLQ-C30 questionnaires. The EORTC-QLQ-C30 scores and the EQ-5D-3L Health score today range from 0 to 100, with high scores indicating better self-reported health. The EQ-5D-3L Score is calculated using dimensions from the questionnaire and high scores indicating better self-reported health. The number of completed questionnaires is shown above each column (see the Data Supplement for more details). BCG, Bacillus Calmette-Guerin; RC, radical cystectomy.
FIG 3.
Bladder cancer-specific EORTC PROMs questionnaire outcomes. Scores range from 0 to 100. A high score for urinary symptoms, future worries, and abdominal bloating and flatulence represents a high level of symptomatology. A high score for sexual functioning represents a high level of functioning. For individual items (malaise, intravesical treatment issues and risk of contaminating partner, body image scale, and urostomy problems), a high score is interpreted as worse. Merged scores are generated from the matching scales from either questionnaire and presented together. The number of completed questionnaires is shown above each column (see the Data Supplement for more details). BCG, Bacillus Calmette-Guerin; RC, radical cystectomy.
Feasibility of Conducting a Phase III RCT
We randomly assigned 50 of the planned 60 patients within the 18-month window. However, 47/50 (94.0%) patients were from a single network (22 from the cancer center and 25 from the neighboring community hospitals). Four of the seven cancer networks did not randomly assign a patient during recruitment. Of those recruited, seven did not accept their allocated treatments (compliance 86.0%); two of these also withdrew from further data collection and completion of questionnaires. A further participant withdrew from trial treatment 121 days after random assignment.
DISCUSSION
We report the first prospective randomized comparison of mBCG and RC. We suspected challenging recruitment and so undertook this feasibility study.7,23 We ran training sessions using mock patient consultations, lectures challenging beliefs, and focusing upon equipoise. Many HRNMIBCs are diagnosed in district hospitals, before onward referral to the nearest cancer center. Patients meet multiple clinical staff before deciding treatment, each of whom might influence choice. Recruitment was successful in one network, which accounted for 47/50 randomly assigned patients. Half of these were diagnosed at district hospitals, suggesting clinicians in this network were in equipoise. Other networks struggled to recruit, suggesting either a lack of equipoise, enthusiasm, or logistical difficulties. Evidence to understand this may be derived from the screening logs. These reported few patients expressed treatment preference at diagnosis (1.0% in the Data Supplement), similar rates of screening between networks, and that many patients (62.7%) had preference by the time they were approached regarding BRAVO. Rates of preference were similar between networks, suggesting that failure to recruit was multifactorial. Treatment acceptance was high (43/50, 86%) and comparable to other surgical versus nonsurgical trials (eg, 78% in ProtecT24). Fewer patients accepted RC than BCG, likely reflecting its irreversibility and greater physical impact.
Our data reveal important insights into the disease and challenge preconceptions. First, BCG is often the default first-line treatment because clinicians feel patients are unfit for RC.25,26 We found that although 2/3 of screened patients were > 70 years old and 3/4 had a smoking history, most (around 80% of the total considered population) were judged fit for either treatment by urological and anesthetic staff. Second, clinicians often manage HRNMIBC as a nonlethal disease. We found that most new high-grade NMI cancers were of high progression risk (as defined by the presence of one or more adverse prognostic risk factors). In the participants randomly assigned to RC, 2/20 (10.0%) had muscle invasive BCs and one had Gleason 4 + 3 = 7 T3a prostate cancer in their final histology. These patients had received re-resection and cross-sectional imaging,17 were reviewed by a multidisciplinary meeting, and had seen specialist uro-oncologists. In the BCG arm, two participants developed BC metastases during study follow-up (total 2/23 with metastatic BC). Thus, 10% of patients with new HRNMIBC have a potentially lethal disease. Our findings reflect nonrandomly assigned population-based observations suggesting a 14% difference in BC-specific survival between BCG and immediate RC at 10 years, that metastatic BC is present in cystectomy specimens in around 5% of cases, and long-term cancer-specific survival rates of around 90% with immediate RC.11,27,28 Finally, patients fear RC as they perceive a low HRQOL. Few data support this fear. Our HRQOL analysis was limited as not all patients were followed up for 12 months; however, some trends in changes were noted over time. Specifically, within the first 3 months, HRQOL may be superior with mBCG to RC, but differences may disappear by 12 months. Conversely, at 12 months, there was slightly more decision regret and slightly lower emotional function scores in the BCG cohort, suggesting ongoing concerns about uncertainty in BC outcomes. These data support population-based surveys regarding HRQOL in patients with the BC spectrum9,10 and clinical trial data measuring recovery13 after RC.
Although these findings may suggest that RC has superior oncological outcomes with limited impact of HRQOL, our data do not support RC as the standard of care for all patients. The study was designed to test the feasibility of a larger trial, and so findings with respect to UCC outcomes or impact of HRQOL are underpowered and need to be interpreted cautiously. In the RC cohort, 25% of patients had no tumor in their cystectomy specimen (suggesting possible overtreatment) and only 10% had invasive cancer. Although longer-term outcomes of the 65% with NMI are unknown, it is likely that many would not develop progressive disease and would not need RC. In keeping with this, most patients in the BCG arm kept their bladders in situ and there was little change in their HRQOL during treatment. Although HRQOL and decision regret in the mBCG cohort may change with longer follow-up, at closure, there were no major differences between each arm. As such, we suggest our findings be used to inform patients about the relative risks of each approach, and recommend use of an individualized risk-adaptive approach.
ACKNOWLEDGMENT
We gratefully acknowledge the ongoing support of participants, principal investigators, research nurses, MDT coordinators, data managers, and other site staff who have been responsible for setting up, recruiting participants, and collecting the data for the study. This study was funded by Yorkshire Cancer Research (Study S388). The funder had no role in the design, analysis, or collection of the data; in writing the manuscript; or in the decision to submit the manuscript for publication. We are grateful for the study oversight provided by the sponsor Sheffield Teaching Hospitals NHS Trust and the members of the TSC. We thank the Independent steering group members: Alison Birtle (Chair), Simon Fulford, and Chris Metcalfe.
DISCLAIMER
J.W.F.C. has received reimbursement for consultancy from Astra Zeneca, Roche, and Janssen, speaker fees from BMS, MSD, Nucleix, and Roche, and honoraria for membership of an advisory board for Ferring. J.W.F.C. is funded by an NIHR Research Professorship. The remaining authors declare no potential conflicts of interest.
CLINICAL TRIAL INFORMATION
ISRCTN12509361 Registered September 6, 2016.
STUDY GROUPS
BRAVO study group members: Zahir Abbasi, Linzi Bone, Angela Brocklehurst, Nicolas Bryan, Lindsay Caygill, Jeremy Crew, Russsell Dowde, Jan Farrell, Jonathan Gill, Lisa Gledhill, Fredddie Hamdy, Susan Hill, Helen Hothersall, Anne Kay, Phil Kelly, Bernadette Kilbane, Sanjeev Kotwal, Kate Linton, Stephen Littler, Tiago Mendonca, Suzanne Miles, Stephen Mitchell, Peter Murphy, Vicky Murray, Naledi Mzwimbi, Alan Paul, Ramanan Rajasundaram, Julie Rawlings, Hannah Roberts, Helen Robertshaw, Wendy Robson, Derek Rosario, Kully Sandhu, Paul Sagar, Alison Shaw, Rajindrah Singh, Nick Smith, Tina Soar, Zoe Storton, Beverley Taylor, Francis Thomas, Nicky Thomas, Suresh Venugopal, Rachel Walker, Louise Weatherley, and Susan Wormley.
AUTHOR CONTRIBUTIONS
Conception and design: James W. F. Catto, Michelle Collinson, Heather Poad, Maureen Twiddy, Mark Johnson, Rohit Chahal, Matt Simms, Phillip Koenig, Julia M. Brown
Financial support: James W. F. Catto
Administrative support: James W. F. Catto, Kathryn Gordon
Provision of study materials or patients: James W. F. Catto, Kathryn Gordon, Rohit Chahal, Matt Simms, Richard Bell, Phillip Koenig, Louise Goodwin
Collection and assembly of data: James W. F. Catto, Kathryn Gordon, Michelle Collinson, Heather Poad, Mark Johnson, Sunjay Jain, Rohit Chahal, Mohantha Dooldeniya, Richard Bell, Phillip Koenig, Samantha Conroy, Louise Goodwin, Aidan P. Noon, Julie Croft, Julia M. Brown
Data analysis and interpretation: James W. F. Catto, Michelle Collinson, Maureen Twiddy, Rohit Chahal, Phillip Koenig, Julie Croft, Julia M. Brown
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Radical Cystectomy Against Intravesical BCG for High-Risk High-Grade Nonmuscle Invasive Bladder Cancer: Results From the Randomized Controlled BRAVO-Feasibility Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
James W. F. Catto
Honoraria: Abbott Laboratories
Consulting or Advisory Role: AstraZeneca/MedImmune, Janssen, Ferring
Speakers' Bureau: ASCO, Roche, AstraZeneca/MedImmune, MSD Oncology, Nucleix
Travel, Accommodations, Expenses: eau
Mark Johnson
Research Funding: Astellas Pharma
Julia M. Brown
Research Funding: Roche
Other Relationship: NIHR
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cumberbatch MGK Jubber I Black PC, et al. : Epidemiology of bladder cancer: A systematic review and contemporary update of risk factors in 2018. Eur Urol 74:784-795, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Leal J Luengo-Fernandez R Sullivan R, et al. : Economic burden of bladder cancer across the European Union. Eur Urol 69:438-447, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Sylvester RJ van der Meijden AP Oosterlinck W, et al. : Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49:466-475, 2006; discussion 475-477 [DOI] [PubMed] [Google Scholar]
- 4.Thomas F Noon AP Rubin N, et al. : Comparative outcomes of primary, recurrent, and progressive high-risk non-muscle-invasive bladder cancer. Eur Urol 63:145-154, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Babjuk M Burger M Comperat EM, et al. : European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in Situ)—2019 update. Eur Urol 76:639-657, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Malmstrom PU Sylvester RJ Crawford DE, et al. : An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol 56:247-256, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Edmondson AJ Birtwistle JC Catto JWF, et al. : The patients' experience of a bladder cancer diagnosis: A systematic review of the qualitative evidence. J Cancer Surviv 11:453-461, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pang KH Groves R Venugopal S, et al. : Prospective implementation of enhanced recovery after surgery protocols to radical cystectomy. Eur Urol 73:363-371, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Mason SJ Downing A Wright P, et al. : Health-related quality of life after treatment for bladder cancer in England. Br J Cancer 118:1518-1528, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardt J Filipas D Hohenfellner R, et al. : Quality of life in patients with bladder carcinoma after cystectomy: First results of a prospective study. Qual Life Res 9:1-12, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Klaassen Z Kamat AM Kassouf W, et al. : Treatment strategy for newly diagnosed T1 high-grade bladder urothelial carcinoma: New insights and updated recommendations. Eur Urol 74:597-608, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Paramasivan S Huddart R Hall E, et al. : Key issues in recruitment to randomised controlled trials with very different interventions: A qualitative investigation of recruitment to the SPARE trial (CRUK/07/011). Trials 12:78, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catto JWF Khetrapal P Ambler G, et al. : Multidomain quantitative recovery following radical cystectomy for patients within the robot-assisted radical cystectomy with intracorporeal urinary diversion versus open radical cystectomy randomised controlled trial: The first 30 patients. Eur Urol 74:531-534, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Oughton JB Poad H Twiddy M, et al. : Radical cystectomy (bladder removal) against intravesical BCG immunotherapy for high-risk non-muscle invasive bladder cancer (BRAVO): A protocol for a randomised controlled feasibility study. BMJ Open 7:e017913, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eble JN Sauter G Epstein JI, et al. : World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon, France, IARC Press, 2004 [Google Scholar]
- 16.Histological Typing of Urinary Bladder Tumours. Geneva, Switzerland, World Health Organization, 1973 [Google Scholar]
- 17.Cumberbatch MGK Foerster B Catto JWF, et al. : Repeat transurethral resection in non-muscle-invasive bladder cancer: A systematic review. Eur Urol 73:925-933, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Lamm DL Blumenstein BA Crissman JD, et al. : Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized Southwest Oncology Group Study. J Urol 163:1124-1129, 2000 [PubMed] [Google Scholar]
- 19.Konety BR Dhawan V Allareddy V, et al. : Impact of hospital and surgeon volume on in-hospital mortality from radical cystectomy: Data from the health care utilization project. J Urol 173:1695-1700, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Boustead GB Fowler S Swamy R, et al. : Stage, grade and pathological characteristics of bladder cancer in the UK: British Association of Urological Surgeons (BAUS) urological tumour registry. BJU Int 113:924-930, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Rabin R, de Charro F: EQ-5D: A measure of health status from the EuroQol Group. Ann Med 33:337-343, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Aaronson NK Ahmedzai S Bergman B, et al. : The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365-376, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Elliott D Husbands S Hamdy FC, et al. : Understanding and improving recruitment to randomised controlled trials: Qualitative research approaches. Eur Urol 72:789-798, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Hamdy FC Donovan JL Lane JA, et al. : 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 375:1415-1424, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Goossens-Laan CA Leliveld AM Verhoeven RH, et al. : Effects of age and comorbidity on treatment and survival of patients with muscle-invasive bladder cancer. Int J Cancer 135:905-912, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Noon AP Albertsen PC Thomas F, et al. : Competing mortality in patients diagnosed with bladder cancer: Evidence of undertreatment in the elderly and female patients. Br J Cancer 108:1534-1540, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gontero P Sylvester R Pisano F, et al. : Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guerin: Results of a retrospective multicenter study of 2451 patients. Eur Urol 67:74-82, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Hautmann RE, Volkmer BG, Gust K: Quantification of the survival benefit of early versus deferred cystectomy in high-risk non-muscle invasive bladder cancer (T1 G3). World J Urol 27:347-351, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Cumberbatch MG Cox A Teare D, et al. : Contemporary occupational carcinogen exposure and bladder cancer: A systematic review and meta-analysis. JAMA Oncol 1:1282-1290, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Reed O Jubber I Griffin J, et al. : Occupational bladder cancer: A cross section survey of previous employments, tasks and exposures matched to cancer phenotypes. PLoS One 15:e0239338, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]