PURPOSE
BEACON CRC evaluated encorafenib plus cetuximab with or without binimetinib versus investigators' choice of irinotecan or FOLFIRI plus cetuximab in patients with BRAFV600E–mutant metastatic colorectal cancer (mCRC), after progression on 1-2 prior regimens. In the previously reported primary analysis, encorafenib, binimetinib plus cetuximab (ENCO/BINI/CETUX; triplet) and encorafenib plus cetuximab (ENCO/CETUX; doublet) regimens improved overall survival (OS) and objective response rate (ORR; by blinded central review) versus standard of care. The purpose of this analysis was to report updated efficacy and safety data.
METHODS
In this open-label, phase III trial, 665 patients with BRAF V600E–mutant mCRC were randomly assigned 1:1:1 to receive triplet, doublet, or control. Primary end points were OS and independently reviewed ORR comparing triplet to control. OS for doublet versus control was a key secondary end point. Updated analyses include 6 months of additional follow-up and ORR for all randomized patients.
RESULTS
Patients received triplet (n = 224), doublet (n = 220), or control (n = 221). Median OS was 9.3 months (95% CI, 8.2 to 10.8) for triplet and 5.9 months (95% CI, 5.1 to 7.1) for control (hazard ratio [HR], 0.60 [95% CI, 0.47 to 0.75]). Median OS for doublet was 9.3 months (95% CI, 8.0 to 11.3) (HR v control, 0.61 [95% CI, 0.48 to 0.77]). Confirmed ORR was 26.8% (95% CI, 21.1% to 33.1%) for triplet, 19.5% (95% CI, 14.5% to 25.4%) for doublet, and 1.8% (95% CI, 0.5% to 4.6%) for control. Adverse events were consistent with the prior primary analysis, with grade ≥ 3 adverse events in 65.8%, 57.4%, and 64.2% for triplet, doublet, and control, respectively.
CONCLUSION
In the BEACON CRC study, encorafenib plus cetuximab improved OS, ORR, and progression-free survival in previously treated patients in the metastatic setting compared with standard chemotherapy. Based on the primary and updated analyses, encorafenib plus cetuximab is a new standard care regimen for previously treated patients with BRAF V600E mCRC.
INTRODUCTION
Mutations in BRAF are recurrently detected in human cancer, including melanoma, colorectal, thyroid, non–small-cell lung, and hairy cell leukemia.1 BRAF encodes a serine/threonine protein kinase that is part of the RAS/RAF/MEK/ERK pathway. The majority of mutations in BRAF result in V600E substitution, and these patients generally have a poor prognosis.2,3 Approximately 10% of patients with metastatic colorectal cancer (mCRC) have a BRAF mutation, with recent estimates ranging from as low as 5% to as high as 21%.1,4-10 BRAF V600E mutation results in downstream phosphorylation of MEK and ERK, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway, which drives tumor cell proliferation and survival.1
CONTEXT
Key Objective
The BEACON trial evaluated the safety and efficacy of encorafenib plus cetuximab with or without binimetinib in previously treated patients with BRAF V600E–mutant metastatic colorectal cancer (mCRC). This paper reports updated data from BEACON sponsored by Array BioPharma in collaboration with Merck, ONO Pharmaceutical, and Pierre Fabre. Array BioPharma was acquired by Pfizer in July 2019.
Knowledge Generated
Encorafenib plus cetuximab (with or without binimetinib) demonstrated significantly improved survival and tumor response, compared with standard chemotherapy plus cetuximab, with a similar initially reported safety profile. The binimetinib addition did not increase overall efficacy and was associated with additional MEK inhibitor-related adverse events.
Relevance
Encorafenib plus cetuximab may be a new regimen for patients with BRAF V600E–mutant mCRC whose disease progressed after one or two prior regimens. Further work may be warranted to explore if this regimen may serve as a backbone for the addition of other targeted agents and/or chemotherapy for BRAF–mutant mCRC.
New therapeutic strategies for the BRAF V600E–mutant mCRC population are warranted as standard cytotoxic combinations result in a modest benefit in the first-line setting and limited benefits in the second-line and beyond.11,12 BRAF inhibitor monotherapy in BRAF–mutant mCRC has low response rates.13-15 Suboptimal response to BRAF inhibitor monotherapy is linked to incomplete inhibition of MAPK signaling in CRC cell lines.16,17 BRAF inhibition results in a rapid release of feedback-suppressed epidermal growth factor receptor (EGFR)–mediated MAPK signaling in in vitro studies of BRAF V600E–mutant CRC cells, leading to a rebound in MAPK activation and continued cell proliferation. BRAF and EGFR inhibitor combinations result in synergistic inhibition of tumor growth in BRAF V600E–mutant CRC xenograft models, and subsequent clinical studies of EGFR-targeted monoclonal antibodies combined with BRAF inhibition suggested improved activity compared with single-agent BRAF inhibitors.16-19 The addition of a MEK inhibitor to BRAF inhibition has also been found to increase the inhibition of the MAPK pathway and produce potentially greater antitumor activity in preclinical and clinical studies.19,20
Encorafenib is a BRAF inhibitor with prolonged pharmacodynamic activity compared with other available BRAF inhibitors.21 The doublet combination of the BRAF inhibitor encorafenib and the anti-EGFR monoclonal antibody cetuximab showed promise in early clinical trials.22,23 The BEACON CRC study evaluated whether the combination of encorafenib plus cetuximab with or without the MEK inhibitor binimetinib could improve overall survival (OS) compared with standard therapy in patients with BRAF V600E–mutated mCRC whose disease has progressed after one or two prior lines of therapy. The study was a randomized, three-arm, phase III study that evaluated encorafenib plus cetuximab with or without binimetinib versus investigators' choice of irinotecan plus cetuximab or FOLFIRI (folinic acid, fluorouracil, and irinotecan) plus cetuximab in 665 patients with BRAF V600E–mutant mCRC whose disease had progressed after one or two prior regimens. In a prespecified analysis, encorafenib plus cetuximab with or without binimetinib significantly improved OS and objective response rate (ORR) in patients with BRAF V600E mCRC compared with current standard of care. This study marked the first evidence of survival benefit for a chemotherapy-free targeted treatment regimen in prospectively biomarker-defined patients with mCRC,20,24 defining a new standard of care for patients with previously treated BRAF V600E mCRC. Complete analyses on OS and ORR as well as analyses of some prognostic subgroups may require long-term follow-up. Herein, we report updated post hoc efficacy and safety results as well as exploratory subgroup analyses of the BEACON CRC trial.
METHODS
Study Design and Participants
The design and primary analyses have been previously published (ClinicalTrials.gov identifier: NCT02928224).24 The BEACON CRC study is a global, multicenter, randomized, open-label, phase III trial comparing encorafenib (300 mg once a day) plus binimetinib (45 mg twice a day) plus cetuximab (400 mg/m2 initial dose and then 250 mg/m2 once a week) (ENCO/BINI/CETUX or triplet combination), encorafenib plus cetuximab (ENCO/CETUX or doublet combination), with the control arm of investigators' choice of either cetuximab combined with irinotecan alone (180 mg/m2 on days 1 and 15) or cetuximab combined with FOLFIRI. Eligible patients were randomly assigned in a 1:1:1 ratio and stratified by ECOG performance status (0/1), prior use of irinotecan (yes/no), and cetuximab formulation (United States–licensed v European Union–approved).
Patients with histologically or cytologically confirmed mCRC with centrally confirmed BRAF V600E mutation and progression after one or two prior treatment regimens for metastatic disease were eligible for this trial. Prior BRAF, MEK, or EGFR inhibitors were not permitted. The full list of inclusion and exclusion criteria is in the study Protocol (online only).
Study End Points
The primary end points of the trial were OS for the triplet combination compared with the control and ORR (by blinded independent central review) for the triplet combination compared with the control. OS for the doublet arm compared with the control arm was defined as a key secondary end point. Other secondary end points included progression-free survival (PFS), duration of response (DOR; defined as time from first radiographic evidence of response [complete or partial response] to the earliest documented disease progression or death due to any cause), safety, and a comparison of ORR and OS between the doublet and triplet arms. Tumor assessments were performed according to RECIST v1.125 prior to random assignment and every 6 weeks from the date of random assignment for the first 24 weeks of treatment and then every 12 weeks thereafter until disease progression, withdrawal of consent, initiation of subsequent anticancer therapy, patient lost to follow-up, or death, regardless of whether study treatment had been discontinued. Assessments deemed as responses were confirmed with subsequent imaging obtained at least 4 weeks after the first response. The central review of imaging data was performed retrospectively by readers blinded to treatment assignment. The incidence and severity of adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
The BEACON CRC study was conducted in accordance with the requirements of each country's regulatory authorities as well as the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines, as defined by the International Council for Harmonisation. All patients who participated in the trial provided written informed consent. This trial was approved by the institutional review board or independent ethics committee at each center.
Statistical Analysis
An updated analysis was conducted after an additional 6 months of follow-up relative to the primary analysis (February 11, 2019) with a data cutoff of August 15, 2019. Time-to-event end points and ORR were analyzed based on all randomly assigned patients (ie, intention to treat population). Safety was evaluated by assessments of adverse events and laboratory abnormalities in patients who received at least one dose of trial drug and had at least one post-treatment safety assessment. Subgroup analyses were performed for OS and ORR for all randomly assigned patients who had available data at baseline for a given subgroup. For OS, Cox proportional hazards models were used to estimate the hazard ratio (HR) and corresponding 95% CI for each subgroup, comparing the treatment arms in a pairwise manner. The Kaplan–Meier method was used to calculate the median OS, and corresponding 95% CIs were determined for each subgroup. Forest plots were generated for visual representation of these results. For ORR, response rates (the number of patients achieving an overall best response of complete response or partial response divided by the total number of patients) by Blinded Independent Central Review per RECIST v1.1 were calculated for each subgroup by treatment arm.
Results of this post hoc analysis are descriptive with no formal hypothesis tests conducted. The study was not powered to formally compare the results of the triplet combination with the doublet combination. Additional details regarding the trial design, sample size calculations, and analysis methods have been previously published.24
RESULTS
Patients
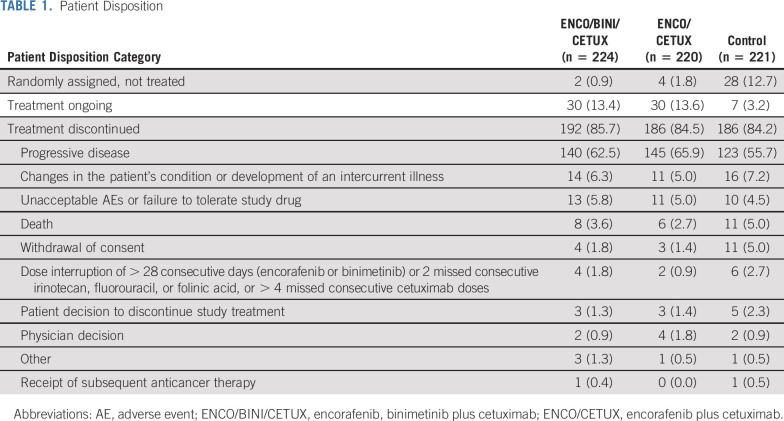
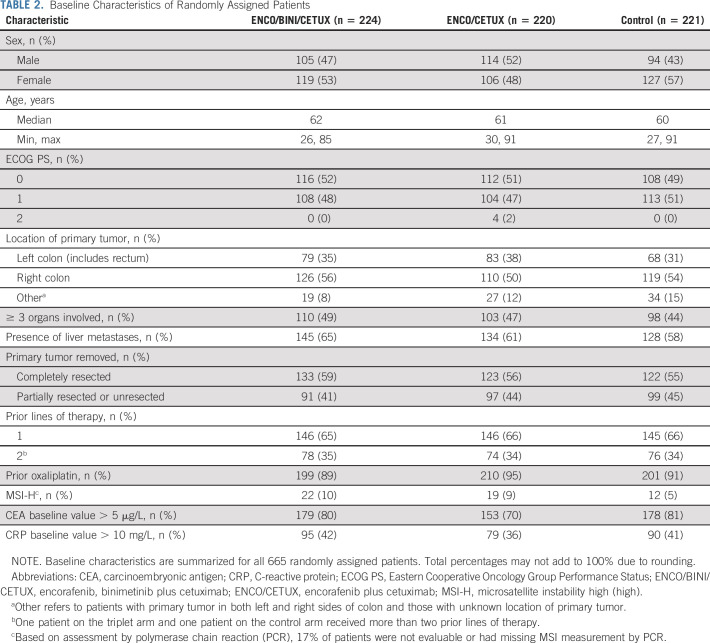
A total of 665 patients were enrolled from May 2017 to January 2019 and were randomly assigned to receive the triplet combination (n = 224), doublet combination (n = 220), or one of the control regimens (n = 221) (Table 1). The primary analysis was based on a data cutoff of February 11, 2019 (Data Supplement, online only) and has been previously published.24 At the time of the data cutoff date for the current report, 13.4% of patients in the triplet arm, 13.6% in the doublet arm, and 3.2% in the control arm were receiving study treatment. Overall, baseline demographic and clinical characteristics were generally similar in the three randomized phase III treatment arms, although there were trends to higher multiorgan involvement, elevated carcinoembryonic antigen (> 5 µg/L), and C-reactive protein (CRP) > 10 mg/L in the triplet arm (Table 2).
TABLE 1.
Patient Disposition
TABLE 2.
Baseline Characteristics of Randomly Assigned Patients
Efficacy
The median duration of follow-up for survival was 12.8 months across the arms as of the data cutoff date for this analysis (August 15, 2019). The Kaplan–Meier curves for OS are presented in Figure 1. The triplet combination resulted in a median OS of 9.3 months (95% CI, 8.2 to 10.8) compared with 5.9 months (95% CI, 5.1 to 7.1) for the control group, with a 40% reduction in the hazard of death on study compared with the control arm (HR of 0.60; 95% CI, 0.47 to 0.75). Similarly, the doublet combination resulted in a median OS of 9.3 months (95% CI, 8.0 to 11.3), with a 39% reduction in the risk of death compared with control with a HR of 0.61 (95% CI, 0.48 to 0.77). The OS results were similar between triplet and doublet with a HR of 0.95 (95% CI, 0.74 to 1.21).
FIG 1.
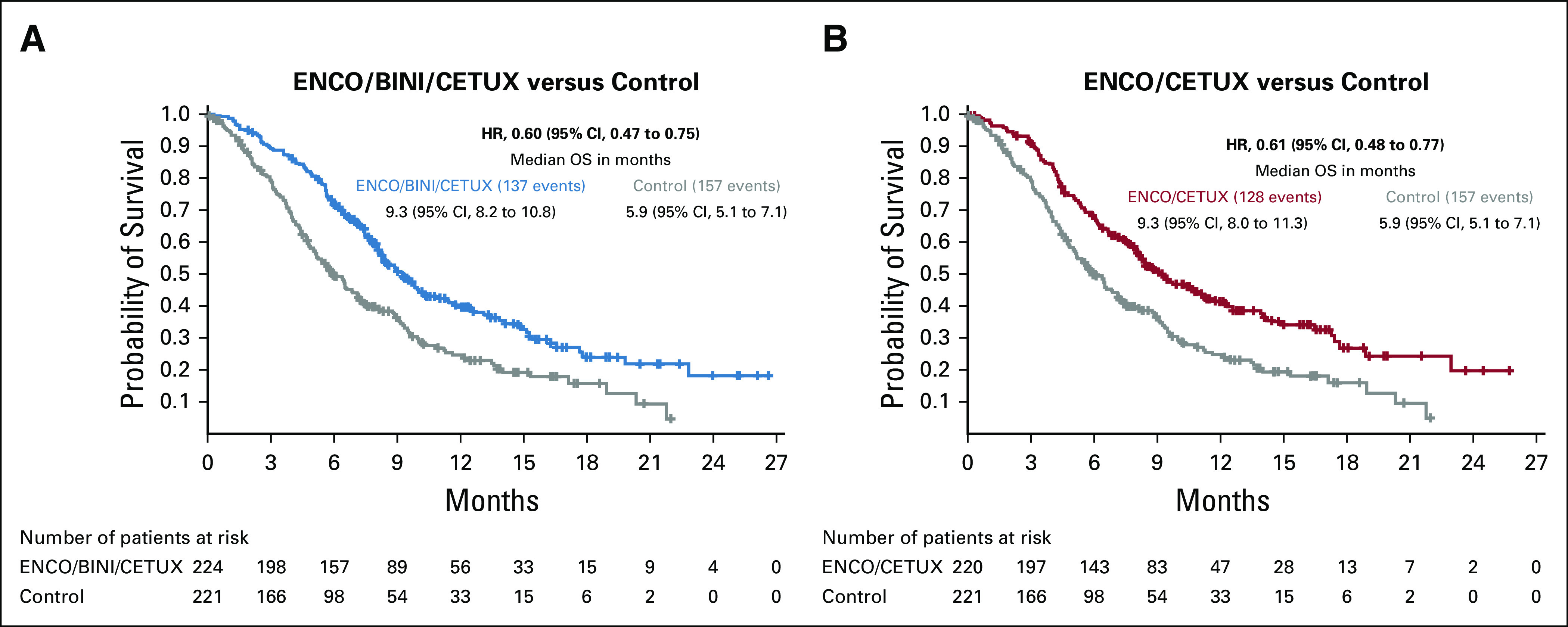
Overall survival results. (A) ENCO/BINI/CETUX versus control. (B) ENCO/CETUX versus control. ENCO/BINI/CETUX, encorafenib, binimetinib plus cetuximab; ENCO/CETUX, encorafenib plus cetuximab; HR, hazard ratio; OS, overall survival.
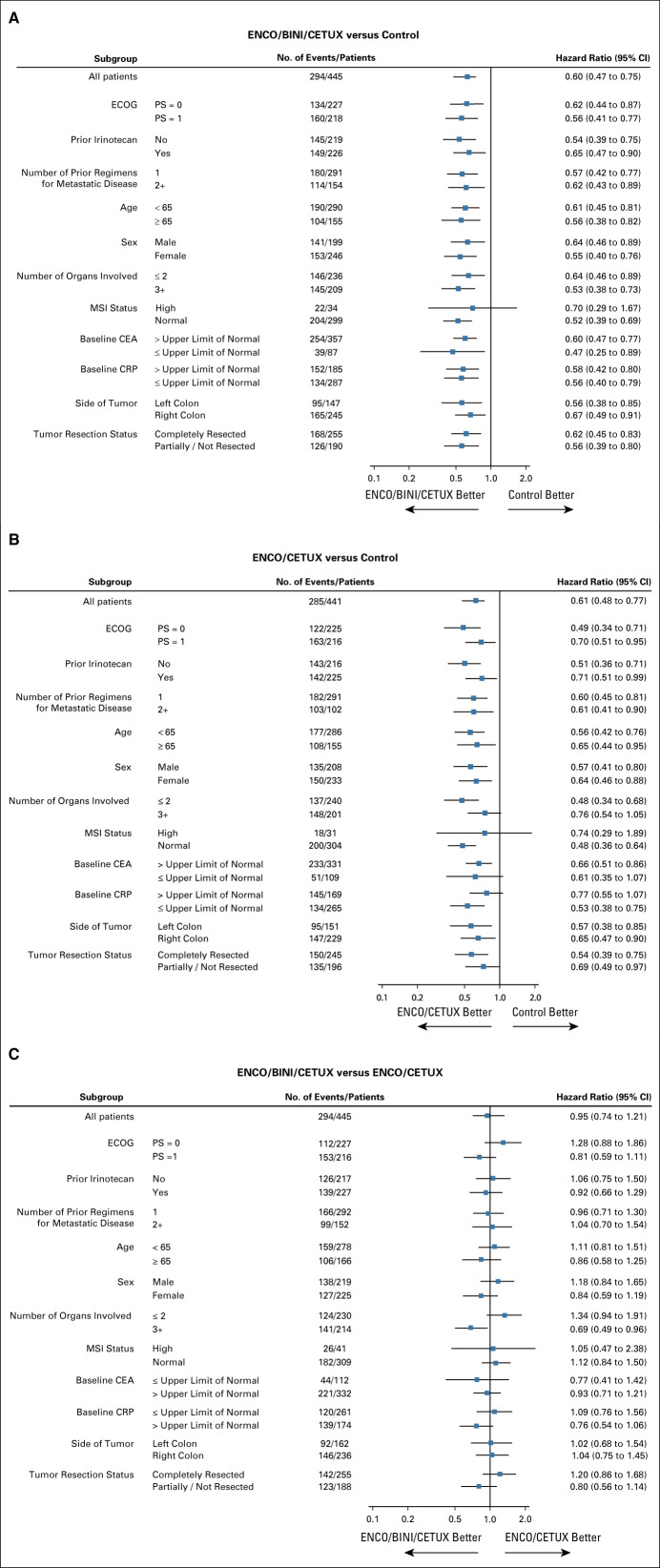
The results for subgroups were generally consistent with the overall analysis (Fig 2). Both triplet and doublet showed improved OS compared with control in all subgroups. The triplet group was compared with doublet in a separate subgroup analysis. The results for patients with high baseline levels of CRP (HR, 0.76 [95% CI, 0.54 to 1.06]), ECOG performance status of 1 (HR, 0.81 [95% CI, 0.59 to 1.11]), incompletely resected primary tumor (HR, 0.80 [95% CI, 0.56 to 1.14]), and ≥3 organ involvement (HR, 0.69 [95% CI, 0.49 to 0.96]) appeared to favor triplet therapy relative to doublet therapy.
FIG 2.
Subgroup analysis of overall survival. (A) ENCO/BINI/CETUX versus control. (B) ENCO/CETUX versus control. (C) ENCO/BINI/CETUX versus ENCO/CETUX. CEA, carcinoembryonic antigen; CRP, C-reactive protein; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ENCO/BINI/CETUX, encorafenib, binimetinib plus cetuximab; ENCO/CETUX, encorafenib plus cetuximab; MSI, microsatellite instability.
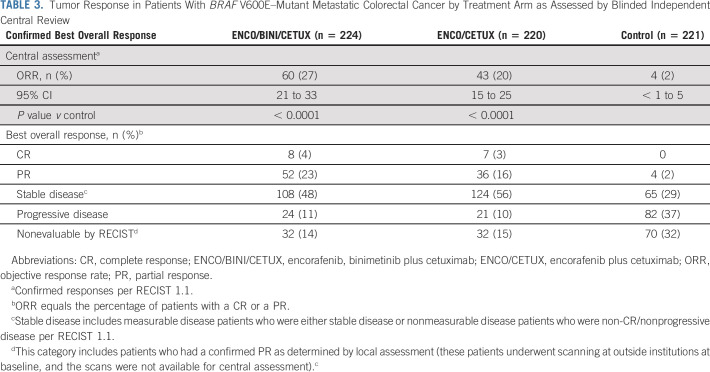
Confirmed ORR results by blinded independent review based on all randomly assigned patients were 26.8% (95% CI, 21.1 to 33.1) for triplet, 19.5% (95% CI, 14.5 to 25.4) for doublet, and 1.8% (95% CI, 0.5 to 4.6) for control (Table 3). Responses assessed by local investigators were similar to central review. The response rates for patients with only 1 prior line of therapy were 28% (95% CI, 21 to 36) for the triplet therapy, 20% (95% CI, 14 to 27) for the doublet combination, and 2% (95% CI, < 1 to 6) for the control. For patients with more than one prior line of therapy, response rates were 24% (95% CI, 15 to 35) for the triplet therapy, 19% (95% CI, 11 to 30) for the doublet combination, and 1% (95% CI, < 1 to 7) for the control. Patients in the triplet arm had a median DOR of 4.4 months (95% CI, 3.7 to 7.3), patients in the doublet arm had a median DOR of 5.5 months (95% CI, 4.1 to 8.3), and the four patients with a response in the control arm had a median DOR of 5.5 months (95% CI, 2.6 to NR). Nineteen of the 60 confirmed responders (31.7%) in the triplet arm had a response that was at least 6 months in duration, and another 4 (6.7%) had responses that were < 6 months in duration, but still ongoing at the data cutoff. In the doublet arm, 16 of the 43 confirmed responders (37%) had a response that lasted for at least 6 months in duration, with another 4 (9%) that were ongoing, but < 6 months in duration. In the control arm, one of the four confirmed responders had a response that was at least 6 months in duration and no ongoing responses. Waterfall plots showing the best percentage change from baseline in the sum of the diameters of the target lesions in each treatment arm are provided in the Data Supplement.
TABLE 3.
Tumor Response in Patients With BRAF V600E–Mutant Metastatic Colorectal Cancer by Treatment Arm as Assessed by Blinded Independent Central Review
Both the triplet and doublet combinations prolonged PFS by blinded independent central review relative to the control arm; median PFS was 4.5 months (95% CI, 4.2 to 5.4), 4.3 months (95% CI, 4.1 to 5.4), and 1.5 months (95% CI, 1.5 to 1.9) for triplet, doublet, and control, respectively (Fig 3), with HRs of 0.42 (95% CI, 0.33 to 0.53) for triplet compared with the control and 0.44 (95% CI, 0.35 to 0.55) for doublet compared with the control.
FIG 3.
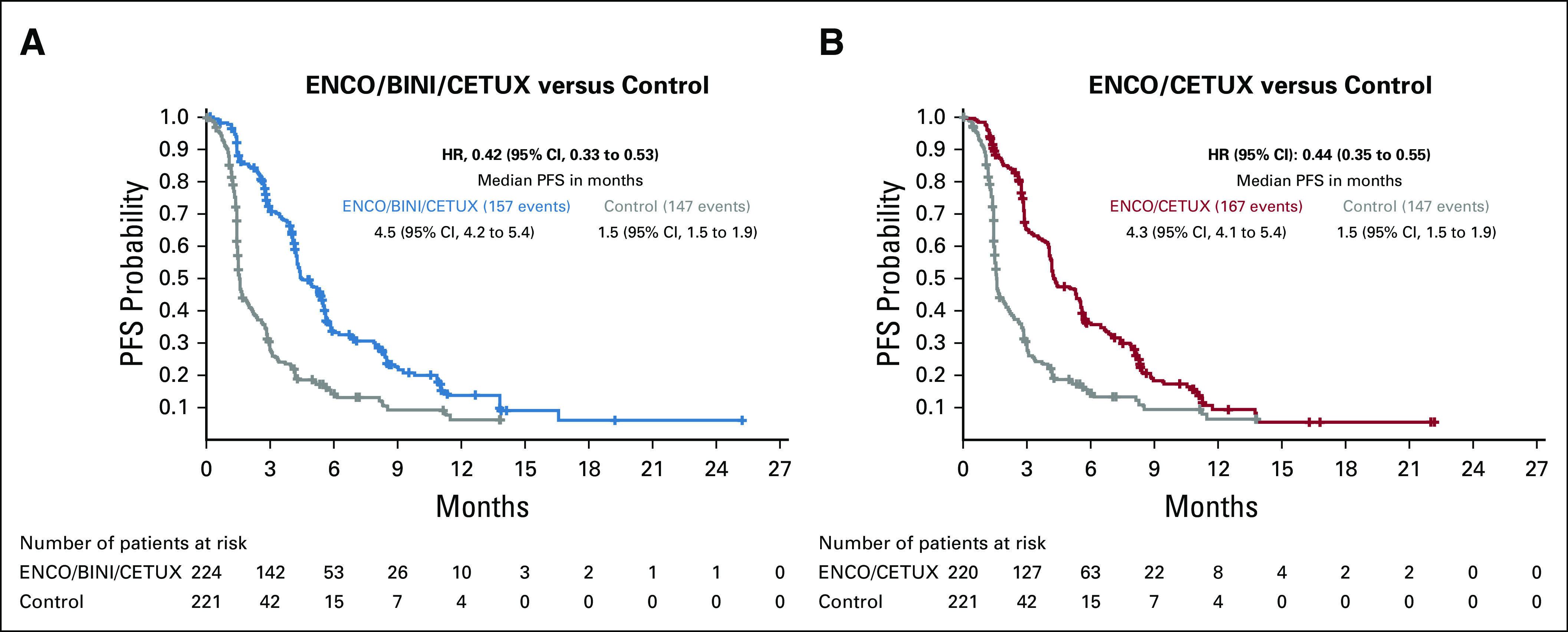
Progression-free survival by blinded independent central review. (A) ENCO/BINI/CETUX versus control. (B) ENCO/CETUX versus control. ENCO/BINI/CETUX, encorafenib, binimetinib plus cetuximab; ENCO/CETUX, encorafenib plus cetuximab; HR, hazard ratio; PFS, progression-free survival.
After study drug discontinuation, subsequent systemic treatments were received by 104 (46%) of 224 patients in the triplet arm, 99 (45%) of 220 in the doublet arm, and 104 (47%) of 221 in the control arm. In the triplet arm, subsequent systemic therapies of interest were fluorouracil (25.0%), irinotecan (24.6%), folinic acid (16.5%), bevacizumab (9.8%), oxaliplatin (6.7%), cetuximab (5.8%), regorafenib (5.4%), and TAS-102 (4.0%). In the doublet arm, subsequent systemic therapies of interest included irinotecan (26.8%), fluorouracil (25.9%), bevacizumab (12.7%), oxaliplatin (8.2%), aflibercept (5.5%), regorafenib (5.5%), capecitabine (4.1%), cetuximab (4.1%), and TAS-102 (2.3%). In the control arm, subsequent systemic therapies of interest were fluorouracil (19.9%), irinotecan (16.3%), cetuximab (14.5%), oxaliplatin (13.1%), bevacizumab (10.9%), vemurafenib (9.5%), TAS-102 (7.2%), and regorafenib (5.0%). BRAF/MEK/EGFR inhibitor combinations were administered in four patients (2%), one patient (0.5%), and 18 patients (8%), whereas BRAF/EGFR inhibitor combinations were administered in 3 patients (1%), 1 patient (0.5%), and 4 patients (2%) in the triplet, doublet, and control arms, respectively. Subsequent immunotherapy was received by < 10% of patients in each treatment arm.
Safety
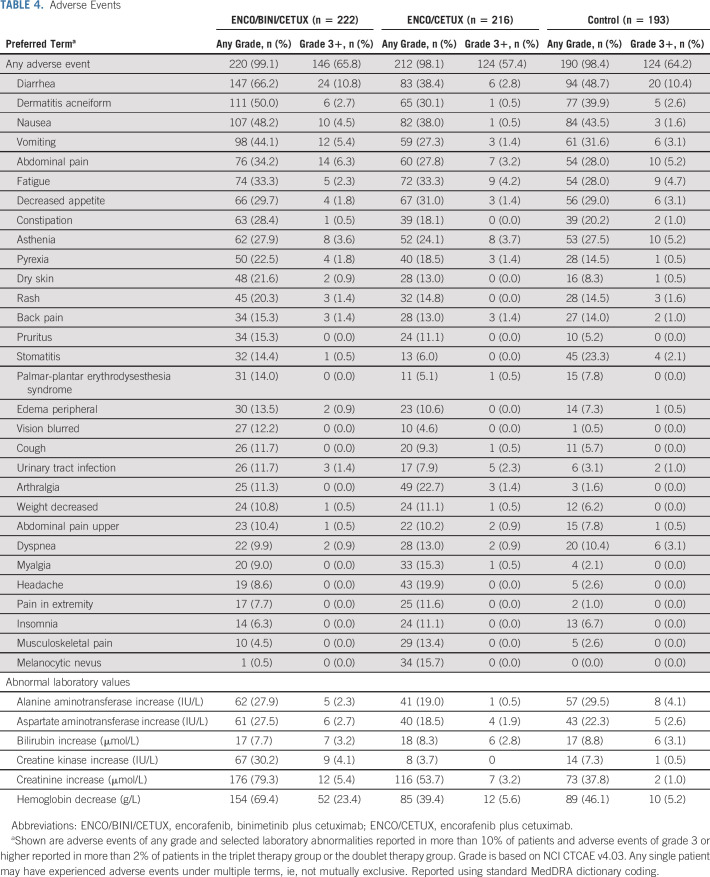
Safety results for the triplet and doublet arms relative to the control arm are consistent with the primary analysis and show no new safety signals. The median duration of exposure to study drugs was 21 weeks in the triplet arm, 19 weeks in the doublet arm, and 7 weeks in the control arm. The most frequently occurring adverse events regardless of assessed causality are summarized in Table 4. The most frequently reported adverse events (all grades) in the triplet arm were diarrhea (66.2%), dermatitis acneiform (50.0%), nausea (48.2%), anemia (45.9%), vomiting (44.1%), abdominal pain (34.2%), fatigue (33.3%), decreased appetite (29.7%), constipation (28.4%), and asthenia (27.9%). In the doublet arm, the most frequently reported adverse events were diarrhea (38.4%), nausea (38.0%), fatigue (33.3%), decreased appetite (31.0%), and dermatitis acneiform (30.1%). In the control arm, the most frequently reported adverse events were diarrhea (48.7%), nausea (43.5%), dermatitis acneiform (39.9%), vomiting (31.6%), decreased appetite (29.0%), fatigue (28.0%), abdominal pain (28.0%), and asthenia (27.5%). Dermatitis acneiform, diarrhea, nausea, constipation, and vomiting were reported at a higher incidence (> 10.0% difference in incidence) in the triplet arm than the doublet arm, whereas headache, arthralgia, and melanocytic nevus were reported at a higher incidence in the doublet arm than the triplet arm. Lab abnormalities of interest in > 10% of patients are reported in Table 4. Grade 3 or greater adverse events were observed in 66%, 57%, and 64% of patients treated with the triplet, doublet, and control regimens, respectively. Discontinuation of all therapy primarily due to an adverse event was seen in 9% of patients in the triplet arm, 9% in the doublet arm, and 11% in the control arm. Deaths resulting from AEs occurred in 5%, 4%, and 4% of patients treated with the triplet, doublet, and control regimens, respectively. Investigators deemed three of the deaths to be at least possibly related to treatment: one death was from colonic perforation (triplet), one was from anaphylaxis (control), and one was from respiratory failure (control).
TABLE 4.
Adverse Events
DISCUSSION
In these updated analyses of the phase III BEACON CRC study, the doublet encorafenib plus cetuximab regimen had similar overall efficacy to the triplet regimen, indicating that this regimen could be effectively used in patients with BRAF V600E–mutant mCRC whose disease had progressed after one or two prior regimens. Both the triplet and doublet regimens had an acceptable safety profile and significant clinically meaningful benefits relative to the control arm. Both regimens improved OS, ORR, and PFS compared with investigators' choice of cetuximab plus irinotecan-based chemotherapy in patients with BRAF V600E–mutant mCRC whose disease had progressed after one or two prior regimens. The control arm performed as expected based on prior experience in a similar population and published prospective and retrospective analyses.1,11,26-28 An assessment of subsequent treatment following BEACON suggests that additional lines of therapy did not differ between the triplet and doublet treatment arms and so were unlikely to have a major influence on the OS results.
Safety results for the triplet and doublet arms relative to the control arm are consistent with the primary analysis and the known safety profile of MEK, BRAF, and EGFR inhibitors, and no new safety signals were observed. Both treatment arms were similar in the rate of adverse events. Despite a longer median duration of exposure to study treatment in the triplet and doublet arms relative to the control arm (21, 19, and 7 weeks in the triplet, doublet, and control arms, respectively), the frequency of grade 3 or higher toxicity was slightly higher in the control and triplet arms than in the doublet arm. Binimetinib as part of the triple combination does add some additional toxicity associated with MEK inhibition but also had a mitigating effect in some specific toxicities (eg, headache, arthralgia, myalgia, and melanocytic nevi). Class-related toxicities of MEK inhibitors including serous retinopathy and left ventricular dysfunction occurred at rates similar to that previously described20,29 and were managed with treatment interruptions with or without subsequent dose reduction. The triplet and doublet regimens had similar rates of treatment discontinuation.
The study was not powered to compare the 2 experimental arms directly; however, the updated descriptive analyses comparing the triplet and doublet arms showed similar efficacy in the overall population across end points including OS and PFS. These results suggest that the doublet regimen is sufficient to maximize the OS benefit and is the optimal regimen for this patient population. The United States Food and Drug Administration approved the doublet for the treatment of BRAF V600E–mutant mCRC after prior therapy in April 2020. The doublet regimen will be a useful therapeutic backbone to explore the utility of novel targeted agents and/or chemotherapy combinations for patients with BRAF V600E–mutant mCRC.
Subgroup analyses suggested that patients with several poor prognostic indicators may benefit from the addition of binimetinib across primary and secondary end points, including the contribution to higher response rates (Fig 2C). For example, elevations in the tumor marker CRP are associated with poor outcomes in patients with mCRC,30,31 and the subgroup of patients with elevated CRP appeared to have better OS outcomes with the triplet regimen versus the doublet regimen (Fig 2C). Interestingly, specific toxicities were lower in the triplet regimen. Further prospective research is warranted to validate these observations and better define the relative benefits of the triplet and doublet regimens.
In conclusion, these updated analyses of the BEACON CRC study confirmed that encorafenib plus cetuximab with or without binimetinib improved OS, ORR, and PFS in previously treated patients with BRAF V600E–mutant mCRC compared with standard chemotherapy. Encorafenib plus cetuximab thus had similar OS efficacy with or without binimetinib, indicating that encorafenib plus cetuximab could be effectively used as the new standard of care for previously treated patients with BRAF V600E–mutant mCRC. Further work to explore whether this regimen may also serve as a suitable backbone for the addition of other targeted agents and/or chemotherapy may be warranted.
ACKNOWLEDGMENT
We thank the patients and their families, as well as the participating study teams, for making this study possible. J.D. Cox, PhD, of Mayville Medical Communications provided medical writing and editorial assistance. Caudex provided fact checking and editorial assistance with funding from Pfizer. A list of Steering Committee members, Data Monitoring Committee members, and Principal Investigators from participating enrolling centers, as well as a plain language summary, can be found in the Supplement.
SUPPORT
Supported by the Cancer Center Core Grant P30 CA 008748 to Memorial Sloan-Kettering Cancer Center. The BEACON trial was sponsored by Pfizer and was conducted with support from Merck KGaA Darmstadt, Germany (for sites outside of North America), ONO Pharmaceutical, and Pierre Fabre.
CLINICAL TRIAL INFORMATION
Registration: Clinical Trials.gov identifier: NCT02928224; EudraCT2015-005805-35.
See accompanying editorial on page 259
AUTHOR CONTRIBUTIONS
Conception and design: Josep Tabernero, Axel Grothey, Eric Van Cutsem, Takayuki Yoshino, Jayesh Desai, Fortunato Ciardiello, Ashwin Gollerkeri, Kati Maharry, Janna Christy-Bittel, Scott Kopetz
Administrative support: Harpreet Wasan
Collection and assembly of data: Josep Tabernero, Eric Van Cutsem, Rona Yaeger, Takayuki Yoshino, Jayesh Desai, Fortunato Ciardiello, Fotios Loupakis, Yong Sang Hong, Neeltje Steeghs, Tormod Guren, Pilar Garcia-Alfonso, Elena Elez, Ashwin Gollerkeri, Kati Maharry, Janna Christy-Bittel, Scott Kopetz
Data analysis and interpretation: Josep Tabernero, Axel Grothey, Eric Van Cutsem, Rona Yaeger, Harpreet Wasan, Takayuki Yoshino, Jayesh Desai, Fortunato Ciardiello, Fotios Loupakis, Neeltje Steeghs, Tormod Guren, Hendrik-Tobias Arkenau, Pilar Garcia-Alfonso, Elena Elez, Kati Maharry, Janna Christy-Bittel, Scott Kopetz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E–Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Josep Tabernero
Consulting or Advisory Role: Bayer, Boehringer Ingelheim, Eli Lilly, MSD, Merck Serono, Novartis, Sanofi, Taiho Pharmaceutical, Merrimack, Peptomyc, Rafael Pharmaceuticals, Symphogen, Chugai Pharma, Ipsen, Merus, Pfizer, Seattle Genetics, Array BioPharma, AstraZeneca, BeiGene, Genentech, Genmab, Halozyme, Imugene Limited, Inflection Biosciences Limited, Kura, Menarini, Molecular Partners, Pharmacyclics, ProteoDesign SL, Roche, Seattle Genetics, Servier, VCN Biosciences, Biocartis, Foundation Medicine, HalioDX SAS, Roche Diagnostics
Axel Grothey
Honoraria: Elsevier, Aptitude Health
Consulting or Advisory Role: Genentech (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), Eli Lilly (Inst), Boston Biomedical (Inst), Amgen (Inst), Array BioPharma (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst)
Research Funding: Genentech (Inst), Bayer (Inst), Pfizer (Inst), Eisai (Inst), Eli Lilly (Inst), Boston Biomedical (Inst), Daiichi Sankyo (Inst), Array BioPharma (Inst)
Travel, Accommodations, Expenses: Genentech, Bayer, Bristol-Myers Squibb, Boston Biomedical, Amgen, Boehringer Ingelheim, Merck Sharp & Dohme
Eric Van Cutsem
Consulting or Advisory Role: Array, Bayer, Biocartis, Eli Lilly, Ipsen, Roche, Servier, Bristol-Myers Squibb, Celgene, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Sirtex, Taiho, Pierre Fabre
Research Funding: Amgen (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Eli Lilly (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Ipsen (Inst), Merck (Inst), Merck KGaA (Inst), Servier (Inst), Bristol-Myers Squibb (Inst)
Rona Yaeger
Research Funding: Array BioPharma, GlaxoSmithKline, Novartis
Travel, Accommodations, Expenses: Array BioPharma
Harpreet Wasan
Honoraria: Merck KGaA, Celgene, Sirtex Medical, Servier, Array BioPharma, Shire, Genentech, ERYTECH Pharma, Amgen, Zymeworks, Pierre Fabre
Consulting or Advisory Role: Roche Pharma AG, Sirtex Medical, ERYTECH Pharma, Shire, Incyte
Speakers' Bureau: Sirtex Medical, Celgene, Merck KGaA, Servier
Research Funding: Sirtex Medical (Inst), Merck KGaA (Inst), Pfizer (Inst), Merck Sharp & Dohme (Inst)
Takayuki Yoshino
Research Funding: Chugai Pharma (Inst), Sanofi (Inst), Sumitomo Dainippon (Inst), GlaxoSmithKline (Inst)
Jayesh Desai
Consulting or Advisory Role: Bionomics, Eli Lilly, Eisai, BeiGene, Ignyta (Inst)
Research Funding: Roche (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Bionomics (Inst), MedImmune (Inst), BeiGene (Inst), Eli Lilly (Inst), Bristol-Myers Squibb (Inst)
Fortunato Ciardiello
Consulting or Advisory Role: Genentech, Merck KGaA, Bayer, Amgen, Pfizer
Research Funding: Merck KGaA (Inst), Genentech (Inst), Servier (Inst), Symphogen (Inst), Amgen (Inst), Bayer (Inst), Merck Sharp & Dohme (Inst), Bristol-Myers Squibb (Inst), Ipsen (Inst)
Neeltje Steeghs
Consulting or Advisory Role: AIMM Therapeutics (Inst), Boehringer Ingelheim (Inst), Ellipses Pharma (Inst)
Research Funding: AB Science (Inst), Abbvie (Inst), Actuate Therapeutics (Inst), Amgen (Inst), Array (Inst), AstraZeneca/MedImmune (Inst), Bayer (Inst), Blueprint Medicines (Inst), Boehringer Ingelheim (Inst), Bristol-Myers Squibb (Inst), Cantargia (Inst), Cytovation (Inst), Deciphera (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), Incyte (Inst), InteRNA (Inst), Lilly (Inst), Merck Sharp & Dohme (Inst), Merus (Inst), Novartis (Inst), Pfizer (Inst), Pierre Fabre (Inst), Roche (Inst), Sanofi (Inst), Taiho (Inst), Takeda (Inst)
Hendrik-Tobias Arkenau
Employment: Sarah Cannon Research Institute UK and HCA Healthcare UK
Advisory Boards: Roche, Servier, BioNTech, Beigene, Guardant, Bayer, iOnctura, Bicycle
Ashwin Gollerkeri
Employment: Pfizer
Kati Maharry
Employment: Pfizer
Janna Christy-Bittel
Employment: Pfizer (at time of research)
Scott Kopetz
Stock and Other Ownership Interests: MolecularMatch, Navire
Consulting or Advisory Role: Roche, Genentech, EMD Serono, Merck, Karyopharm Therapeutics, Amal Therapeutics, Navire Pharma, Symphogen, Holy Stone, Biocartis, Amal Therapeutics, Amgen, Novartis, Eli Lilly, Boehringer Ingelheim
Research Funding: Amgen (Inst), Sanofi (Inst), Biocartis (Inst), Guardant Health (Inst), Array BioPharma (Inst), Genentech (Inst), EMD Serono (Inst), MedImmune (Inst), Novartis (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Davies H Bignell GR Cox C, et al. : Mutations of the BRAF gene in human cancer. Nature 417:949-954, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Kayhanian H Goode E Sclafani F, et al. : Treatment and survival outcome of BRAF-mutated metastatic colorectal cancer: A retrospective matched case-control study. Clin Colorectal Cancer 17:e69-e76, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Tran B Kopetz S Tie J, et al. : Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 117:4623-4632, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorbye H Dragomir A Sundström M, et al. : High BRAF mutation frequency and marked survival differences in subgroups according to KRAS/BRAF mutation status and tumor tissue availability in a prospective population-based metastatic colorectal cancer cohort. PLoS One 10:e0131046, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Roock W Claes B Bernasconi D, et al. : Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol 11:753-762, 2010 [DOI] [PubMed] [Google Scholar]
- 6.The AACR Project GENIE Consortium : AACR project GENIE: Powering precision medicine through an international consortium, Cancer Discov 7:818-831, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Cancer Genome Atlas Research Network : Comprehensive genomic characterization of squamous cell lung. Nature 489:519-525, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaeger R Chatila WK Lipsyc MD, et al. : Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell 33:125-136.e3, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barras D Missiaglia E Wirapati P, et al. : BRAF V600E mutant colorectal cancer subtypes based on gene expression. Clin Cancer Res 23:104-115, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Clarke CN, Kopetz ES: BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: Clinical characteristics, clinical behavior, and response to targeted therapies. J Gastrointest Oncol 6:660-667, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris V Overman MJ Jiang Z-Q, et al. : Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin Col Cancer Res 13:164-171, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson AB Venook AP Al-Hawary MM, et al. : NCCN guidelines insights: Colon cancer, version 2.2018. J Natl Compr Canc Netw 16:359-369, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopetz S Desai J Chan E, et al. : Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol 33:4032-4038, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyman DM Puzanov I Subbiah V, et al. : Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 373:726-736, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seligmann JF Fisher D Smith CG, et al. : Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: Analysis from 2530 patients in randomised clinical trials. Ann Oncol 28:562-568, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Prahallad A Sun C Huang S, et al. : Unresponsiveness of colon cancer to BRAF (V600E) inhibition through feedback activation of EGFR. Nature 483:100-103, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Corcoran RB Ebi H Turke AB, et al. : EGFR-mediated reactivation of MAPK signaling contributes to insensitivity of BRAF-mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2:227-235, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong DS Morris VK El Osta B, et al. : Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAF V600E mutation. Cancer Discov 6:1352-1365, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corcoran RB Andre T Atreya CE, et al. : Combined BRAF, EGFR, and MEK inhibition in patients with BRAF V600E-mutant colorectal cancer. Cancer Discov 8:428-443, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Cutsem E Huijberts S Grothey A, et al. : Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E–mutant metastatic colorectal cancer: Safety lead-in results from the phase III BEACON colorectal cancer study. J Clin Oncol 37:1460-1469, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delord JP Robert C Nyakas M, et al. : Phase I dose-escalation and -expansion study of the BRAF inhibitor encorafenib (LGX818) in metastatic BRAF-mutant melanoma. Clin Cancer Res 23:5339-5348, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Tabernero J Van Geel R Guren TK, et al. : Phase 2 results: Encorafenib (ENCO) and cetuximab (CETUX) with or without alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC). J Clin Oncol 34:3544-3544, 2016. (15_suppl)27573652 [Google Scholar]
- 23.van Geel RMJM Tabernero J Elez E, et al. : A phase Ib dose-escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF-mutant colorectal cancer. Cancer Discov 7:610-619, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopetz S Grothey A Yaeger R, et al. : Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med 381:1632-1643, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer EA Therasse P Bogaerts J, et al. : New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Chibaudel B Bonnetain F Shi Q, et al. : Alternative end points to evaluate a therapeutic strategy in advanced colorectal cancer: Evaluation of progression-free survival, duration of disease control, and time to failure of strategy—an Aide et Recherche en Cancerologie Digestive Group Study. J Clin Oncol 29:4199-4204, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Kopetz S McDonough SL Lenz H-J, et al. : Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J Clin Oncol 35, 2017. (suppl; abstr 3505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowland A Dias MM Wiese MD, et al. : Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer 112:1888-1894, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dummer R Ascierto PA Gogas HJ, et al. : Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 19:1315-1327, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Pathak S Nunes QM Daniels IR, et al. : Is C-reactive protein useful in prognostication for colorectal cancer? A systematic review. Colorectal Dis 16:769-776, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Thomsen M Kersten C Sorbye H, et al. : Interleukin-6 and C-reactive protein as prognostic biomarkers in metastatic colorectal cancer. Oncotarget 7:75013-75022, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]