PURPOSE
It remains controversial whether primary tumor resection (PTR) before chemotherapy improves survival in patients with colorectal cancer (CRC) with asymptomatic primary tumor and synchronous unresectable metastases.
PATIENTS AND METHODS
This randomized phase III study investigated the superiority of PTR followed by chemotherapy versus chemotherapy alone in relation to overall survival (OS) in patients with unresectable stage IV asymptomatic CRC and three or fewer unresectable metastatic diseases confined to the liver, lungs, distant lymph nodes, or peritoneum. Chemotherapy regimens of either mFOLFOX6 plus bevacizumab or CapeOX plus bevacizumab were decided before study entry. The primary end point was OS, which was analyzed by intention-to-treat.
RESULTS
Between June 2012 and September 2019, a total of 165 patients were randomly assigned to either chemotherapy alone (84 patients) or PTR plus chemotherapy (81 patients). When the first interim analysis was performed in September 2019 with 50% (114/227) of the expected events observed among 160 patients at the data cutoff date of June 5, 2019, the Data and Safety Monitoring Committee recommended early termination of the trial because of futility. With a median follow-up of 22.0 months, median OS was 25.9 months (95% CI, 19.9 to 31.5) in the PTR plus chemotherapy arm and 26.7 (95% CI, 21.9 to 32.5) in the chemotherapy-alone arm (hazard ratio, 1.10; 95% CI, 0.76 to 1.59; one-sided P = .69). Three postoperative deaths occurred in the PTR plus chemotherapy arm.
CONCLUSION
Given that PTR followed by chemotherapy showed no survival benefit over chemotherapy alone, PTR should no longer be considered a standard of care for patients with CRC with asymptomatic primary tumors and synchronous unresectable metastases.
INTRODUCTION
Colorectal cancer (CRC) is one of the leading causes of death from cancer worldwide.1 At diagnosis, most patients with stage IV disease have unresectable tumors and can undergo palliative treatment only. For those with both an asymptomatic primary tumor and unresectable metastatic disease, the initial treatment strategy is controversial. Some researchers have reported that the benefits of primary tumor resection (PTR) on overall survival (OS) are unclear and that the morbidity and mortality associated with tumor resection should be avoided because of the delay in initiating chemotherapy, which can negatively impact survival.2-4
CONTEXT
Key Objective
The initial treatment strategy remains controversial in patients with incurable advanced colorectal cancer (CRC). This phase III randomized controlled study evaluated the survival benefit of adding upfront primary tumor resection (PTR) to standard chemotherapy for unresectable stage IV CRC patients with both an asymptomatic primary tumor and up to three unresectable metastases.
Knowledge Generated
Additional upfront PTR showed no superiority over chemotherapy alone, with median overall survival (OS) at slightly more than 2 years in both arms. The study was terminated early, at the interim analysis, because patients assigned to upfront PTR plus chemotherapy were unlikely to have improved OS over standard chemotherapy alone.
Relevance
Despite recent retrospective studies reporting significantly better OS in patients with CRC who underwent PTR compared with those who did not, additional upfront PTR should not be considered as standard initial treatment for patients with asymptomatic primary CRC and synchronous unresectable metastatic disease.
If opting for resection in patients with both an asymptomatic primary tumor and unresectable metastatic disease, then upfront PTR is preferable to avoid tumor-related complications that can otherwise develop during chemotherapy.5,6 Moreover, recent studies have reported significantly better OS in patients undergoing PTR than in those who do not undergo this treatment.5,7-10 However, these studies were all retrospective in design. To the best of our knowledge, no randomized controlled trials (RCTs) have demonstrated a survival benefit of upfront PTR over chemotherapy alone in patients with incurable advanced CRC.11-15
We conducted this randomized phase III study (JCOG1007, iPACS study; UMIN identifier: UMIN000008147)16 to evaluate the survival benefit of adding upfront PTR to standard chemotherapy for patients with CRC with an asymptomatic primary tumor and synchronous unresectable metastases.
PATIENTS AND METHODS
Systemic Design and Patients
iPACS was an open-label, randomized, phase III trial conducted by the Japan Clinical Oncology Group (JCOG; JCOG1007).16 The study Protocol (online only) was approved by the institutional review boards of all participating hospitals before the study commenced. Patients provided written informed consent before enrollment.
Eligible patients were of age 20-74 years with histologically proven primary colon cancer, rectosigmoid cancer, or upper rectal adenocarcinoma and with between one and three unresectable metastatic diseases confined to the liver, lungs, distant lymph nodes, or peritoneum evident on computed tomography (CT) or chest X-ray photograph. Tumors were staged according to the Japanese Classification of Colon and Rectal Carcinoma (7th edition)17 and the TNM classification (7th edition).18
Procedures
In the chemotherapy-alone arm, chemotherapy was started within 14 days of enrollment. In the PTR plus chemotherapy arm, resection of the primary tumor was performed within 21 days of enrollment. Surgery was performed under laparoscopy or laparotomy. The level of lymph node dissection was kept in the range of D1-D3, which was needed for resection of the primary tumor. Between 8 and 56 days after surgery, chemotherapy with either mFOLFOX6 plus bevacizumab or CapeOX plus bevacizumab was initiated. The mFOLFOX6 or CapeOX regimen was selected by each investigator before random assignment. Detailed chemotherapy methods can be found in the Protocol.
Treatment was discontinued if disease progression was diagnosed clinically or by imaging, if a serious adverse event (AE) occurred, if a treatment course was delayed for more than 28 days owing to an AE, if an AE meant a subsequent dose reduction was needed after the second reduction, if the patient declined treatment, or if judged necessary by the attending physician for other reasons.
For patients assigned to chemotherapy alone, palliative surgery was performed if deemed necessary because of intestinal obstruction, perforation, fistulation, or hemorrhage associated with the primary tumor. Additionally, for patients in either arm, surgery to achieve R0 was performed if the tumors were deemed resectable because all noncurable factors identified upon registration responded well to chemotherapy.
Outcomes
The primary end point was OS. Secondary end points were progression-free survival (PFS), AEs, proportion of patients who underwent R0 resection, and proportion of patients who underwent palliative surgery.
AEs were assessed according to the CTCAE version 4.0. Patients were assessed twice a month from baseline for AEs via verbal interview, physical examination, and blood tests, including a complete blood cell count and assessments of liver and renal function, until disease progression. Chest and abdominal CT and measurements of carcinoembryonic antigen and carbohydrate antigen 19-9 were performed every 8 weeks.
Random Assignment
Patients were randomly assigned (1:1; centrally by the JCOG Data Center) to PTR plus chemotherapy or chemotherapy alone by a minimization method with a random component to balance the arms based on institution, tumor location (colon, rectosigmoid colon v upper rectum), sex (male v female), and performance status (0 v 1).
Statistical Analysis
This study was designed to confirm the superiority of PTR plus chemotherapy compared with chemotherapy alone in relation to OS. At the start of the study, we hypothesized that the median survival time (MST) in the PTR plus chemotherapy arm would be greater by 4 months than an MST of 20 months in the chemotherapy-alone arm (hazard ratio [HR], 0.83). The total targeted sample size was 770 patients, with a one-sided alpha of 5% and a power of 75%, with 647 events expected to occur during the 5 years of accrual and 3 years of follow-up.
However, as of October 2017, 5 years and 4 months after initiating enrollment, only 18.6% (143 of 770) of the required patients had been enrolled. Therefore, we concluded that the following amendments could be made: (1) change the add-on effect of the test treatment on the standard treatment from 4 months to 8 months taking the high mortality of PTR into account and (2) change the MST of the standard treatment arm from 20 months to 24 months based on longer OS reported by recent large-scale studies on unresectable advanced or recurrent CRC (SOFT and WJOG4407)19,20 and expected minimal treatment effect in guidelines.21,22 Furthermore, the power was reduced from 75% to 70% considering the feasibility of the study. Based on these amendments, the planned sample size was recalculated to a total of 280 patients (227 deaths) in both arms, with MST of 24 months versus 32 months (HR, 0.75), an accrual period of 8.5 years, a follow-up period of 3 years, one-sided α = 5% (which is higher than that of 2.5% recommended by the ICH E9 guideline), and a power of 70%. A one-sided test was used because it was not important to determine whether adding PTR is significantly inferior to the standard treatment.
Two interim analyses were planned, with adjustments for repeated comparisons taken into account with the Lan and DeMets method and the O'Brien-Fleming type α spending function.23 The first interim analysis was planned for the date at which half of the planned sample size had been enrolled, and the second interim analysis was planned 1 year after the completion of enrollment. The prespecified stopping criteria for futility in the study Protocol were as follows: if the survival curve for PTR plus chemotherapy was below that for chemotherapy alone (ie, HR, > 1.0), study termination owing to futility would be considered, taking into account various factors such as the toxicity profile in both arms and precision of the estimated HR. Predictive probability was calculated as reference. Data from all randomly assigned patients were analyzed for OS and PFS on an intention-to-treat basis. Preplanned subgroup analyses were performed. Post hoc supplementary analysis was done in per-protocol set. All statistical analyses were performed using SAS 9.4.
RESULTS
Between June 2012 and September 2019, a total of 165 patients were enrolled and randomly assigned to chemotherapy alone (84 patients) or PTR plus chemotherapy (81 patients; Fig 1) at 38 cancer centers in Japan. Two patients in the chemotherapy-alone arm were ineligible (one with a second primary tumor and one because of lack of inclusion laboratory findings) as were three patients in the PTR plus chemotherapy arm (one with a second primary tumor and two because of deviation from the specified surgical methods). Four patients assigned to PTR plus chemotherapy did not undergo PTR (Fig 1). Defined chemotherapy was not delivered in 15 patients (in the chemotherapy-alone arm, three patients declined unrelated to AEs and two had rapid progression of the disease; in the PTR plus chemotherapy arm, five had rapid progression of the disease, three died, one had an AE, and one declined unrelated to AEs; Fig 1). Of the 165 randomly assigned patients, the 19 who did not receive the study treatment after random assignment were excluded from the safety population (five in the chemotherapy-alone arm and 14 in the PTR plus chemotherapy arm). The median time from enrollment to starting the study treatment was 9 days (IQR, 8-12) in the chemotherapy arm and 13 days (IQR, 10-16) in the PTR plus chemotherapy arm.
FIG 1.
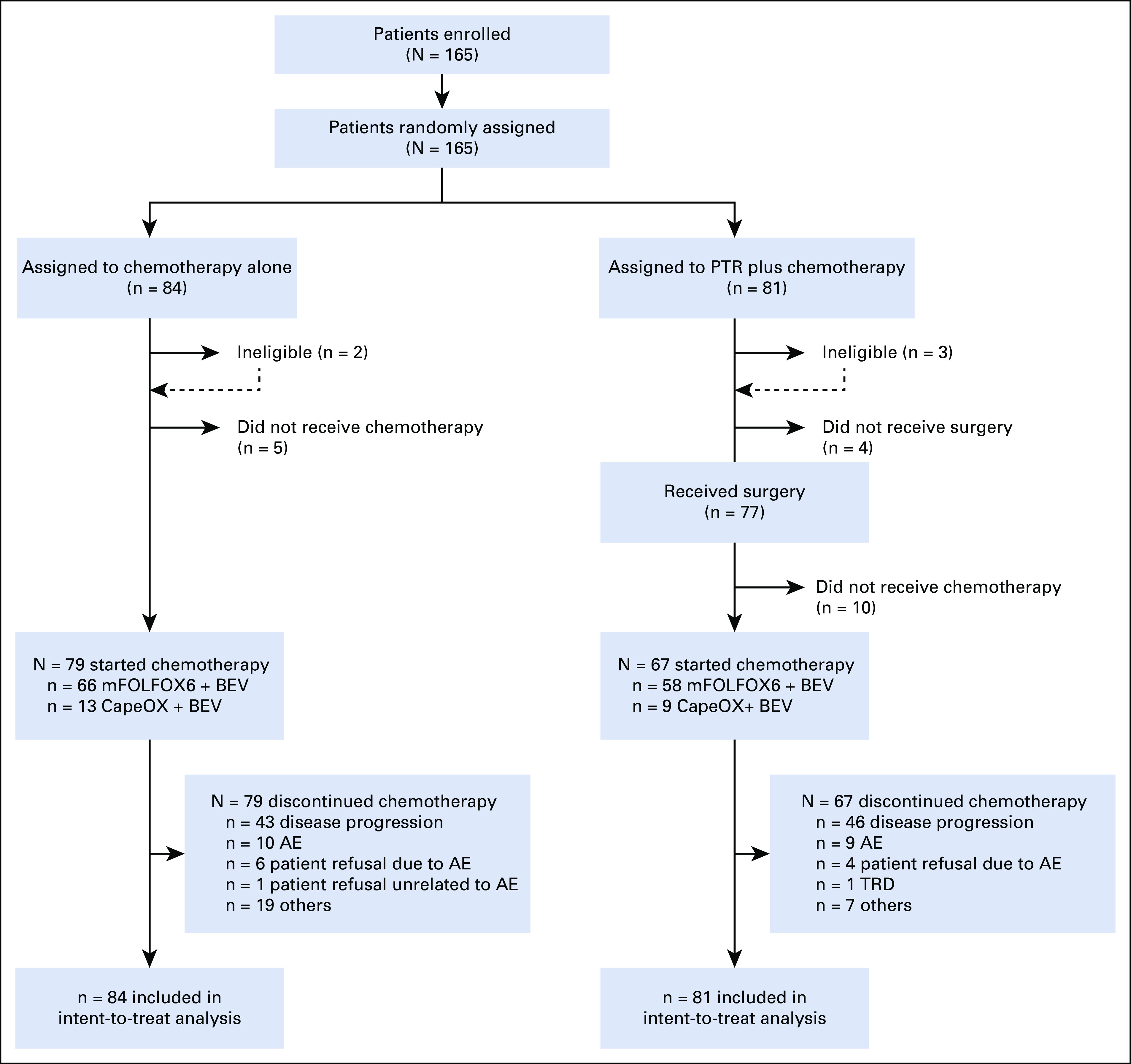
CONSORT diagram. AE, adverse event; TRD, treatment-resistant depression.
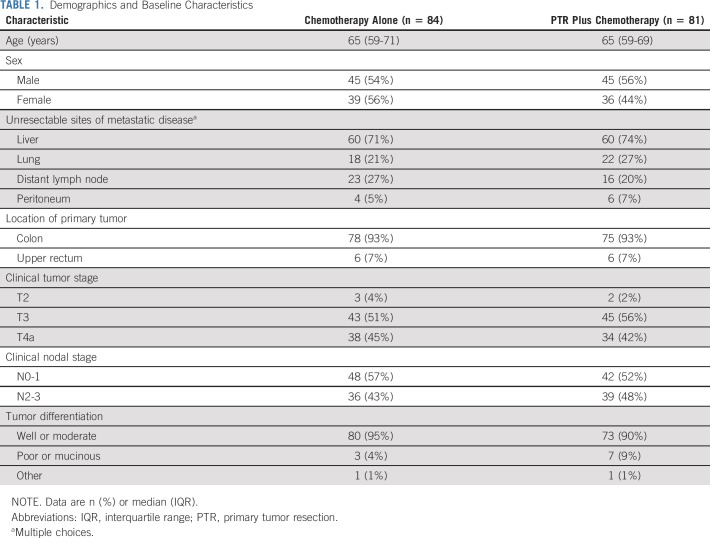
Patients were well balanced between the two arms, including tumor location (colon and rectosigmoid: 78 [93%] in the chemotherapy-alone arm and 75 [93%] in the PTR plus chemotherapy arm). The most frequent unresectable metastatic disease was liver metastasis in 120 of 165 (73%) patients. The distribution of unresectable metastatic diseases was similar in both arms (Table 1).
TABLE 1.
Demographics and Baseline Characteristics
The first interim analysis was conducted in September 2019 for the 160 enrolled patients based on data as of April 2019 (median follow-up, 22.0 months). The Data and Safety Monitoring Committee recommended early termination of the study according to the prespecified stopping criteria on the basis of futility, with 114 (50%) of the expected 227 events reported, because the predictive probability of OS being significantly higher in the PTR plus chemotherapy arm than in the chemotherapy-alone arm would be 12.3% at the final analysis, even if accrual continued to the planned number. Median OS was 26.7 months (95% CI, 21.9 to 32.5) for patients assigned to chemotherapy alone and 25.9 months (19.9 to 31.5) for those assigned to PTR plus chemotherapy (HR, 1.10; 95% CI, 0.76 to 1.59; one-sided P = .69 by the stratified log-rank test; Fig 2A). Supplementary analysis in a per-protocol analysis that excluded five patients deemed ineligible and 17 patients who did not receive planned chemotherapy out of 160 registrations by April 4, 2019, yielded similar findings (HR, 0.99, 95% CI, 0.66 to 1.47).
FIG 2.
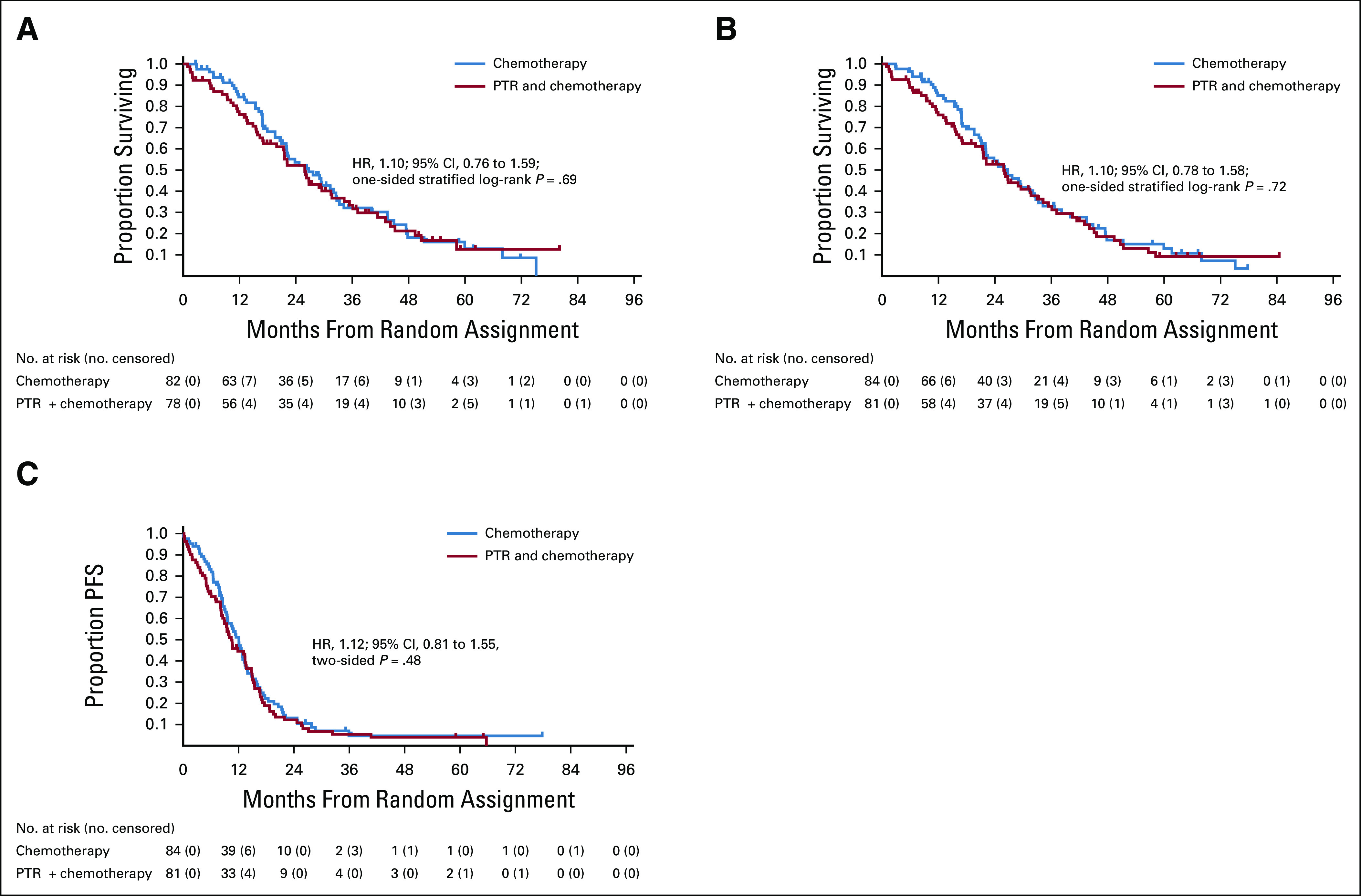
Kaplan-Meier curves in the intention-to-treat population. (A) OS at the first interim analysis (data cutoff date June 5, 2019). (B) OS at the updated analysis (data cutoff date November 26, 2019). (C) PFS at the updated analysis (data cutoff date November 26, 2019). HR, hazard ratio; OS, overall survival; PFS, progression-free survival; PTR, primary tumor resection.
The updated analysis was conducted for the 165 patients based on data as of November 2019 (median follow-up, 22.1 months). OS at 3 years for all randomly assigned patients was 33.0% (22.5 to 43.9) for patients assigned to chemotherapy alone compared with 32.9% (22.2 to 44.0) for those assigned to PTR plus chemotherapy. Median OS was 26.4 months (21.9 to 32.1) for patients assigned to chemotherapy alone and 25.9 months (19.9 to 31.7) for those assigned to PTR plus chemotherapy (HR, 1.11; 95% CI, 0.78 to 1.58; one-sided P = .72 by the stratified log-rank test; Fig 2B). Seventy-three of 84 patients (87%) assigned to chemotherapy alone and 75 of 81 (93%) assigned to PTR plus chemotherapy experienced disease progression. Median PFS was 12.1 months (9.5 to 13.2) with chemotherapy alone and 10.4 months (8.3 to 13.4) with PTR plus chemotherapy (HR, 1.12; 95% CI, 0.81 to 1.55; two-sided P = .48; Fig 2C). In the prespecified subgroup analysis of OS, there was a prominent difference depending on performance status, PS0 and PS1, but the number of patients with PS1 was very small. Other than the abovementioned factors, there were no other notable differences between the arms (Fig 3).
FIG 3.
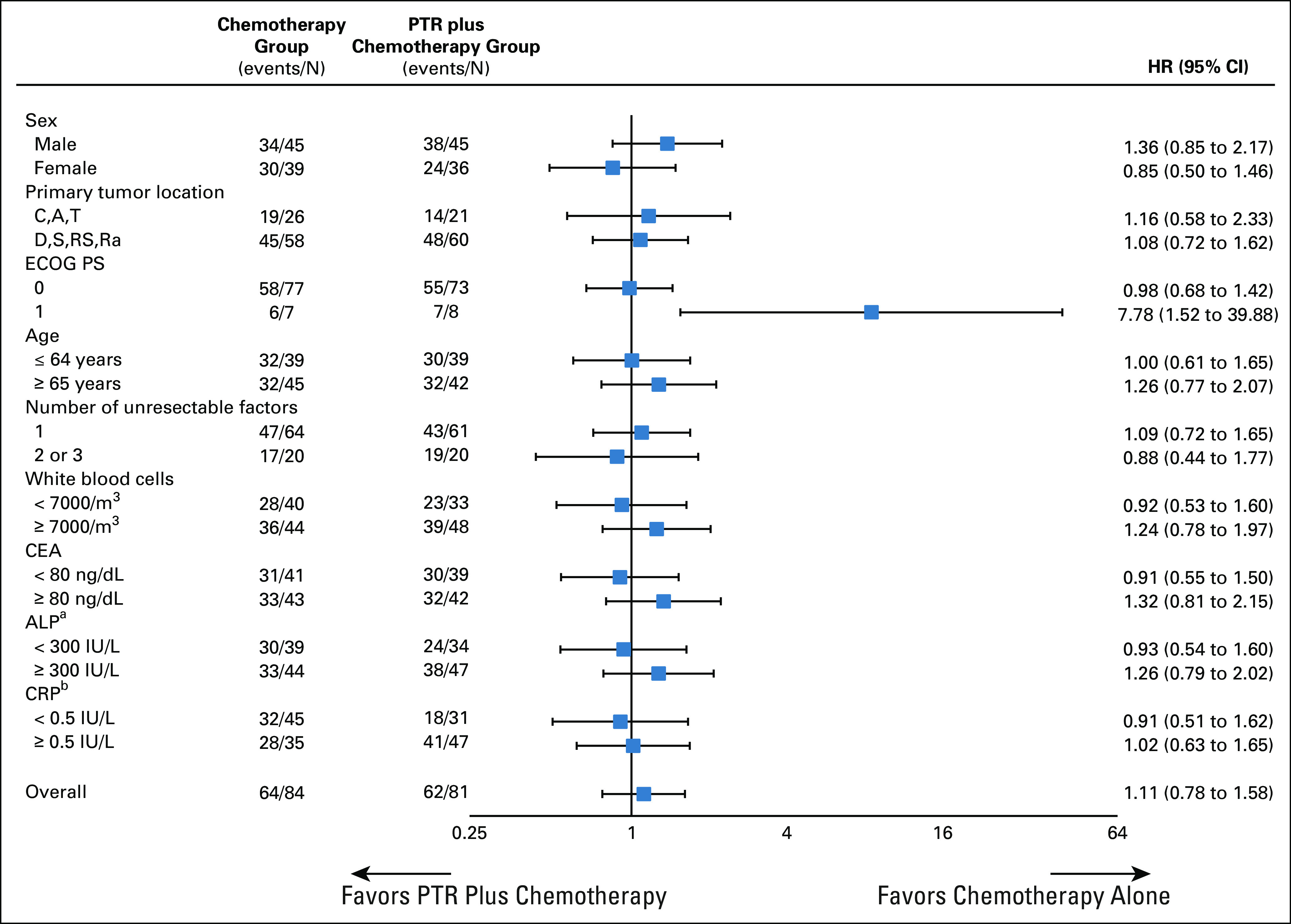
Forest plot of OS. Subgroup analyses of OS were performed using patient baseline characteristics. Data cutoff date was November 26, 2019. aOne patient missing in the chemotherapy group. bFour patients missing in the chemotherapy group and three patients missing in the PTR plus chemotherapy group. ALP, alkaline phosphatase; CEA, carcinoembryonic antigen; CRP, C-reactive protein; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; OS, overall survival; PTR, primary tumor resection.
Grade 2 or worse AEs occurred in 29 of 77 (38%) patients who underwent PTR. The incidence of major surgery-related complications of grade 3 or worse was as follows: fever (one patient; 1%), intra-abdominal abscess (one patient; 1%), postoperative bleeding (one patient; 1%), bilirubinemia (one patient; 1%), anastomotic leakage (three patients; 4%), abnormal alanine aminotransferase concentration (seven patients; 9%), and increased AST (13 patients; 17%). One patient underwent reoperation because of anastomotic leakage. Hospital death, defined as death during the hospital stay for PTR or death from any cause within 30 days after surgery, occurred in three patients (4%) because of aggressive progression of the unresectable hepatic tumor (hepatorrhagia) or postoperative complications (multiple organ failure, thromboembolism) (two treatment-related deaths) (Table 2). Five of 82 (6%) eligible patients assigned to chemotherapy alone and two of 78 (3%) eligible patients assigned to PTR plus chemotherapy underwent R0 resection because of the partial or near-complete response of metastatic lesions to chemotherapy. Additionally, PTR and metastasectomy with curative intent were safely performed without any postoperative complications (data not shown). In the chemotherapy-alone arm, 11 of 84 (13%) patients underwent palliative surgery for symptoms linked to the primary tumor.
TABLE 2.
Mortality and Morbidity Post-PTR
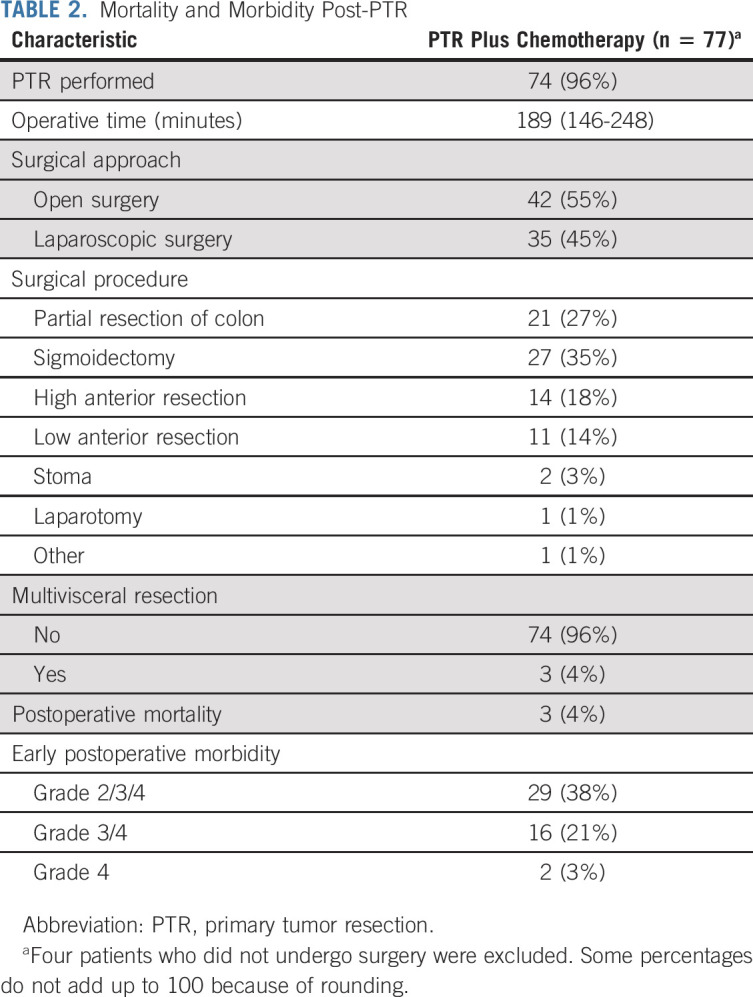
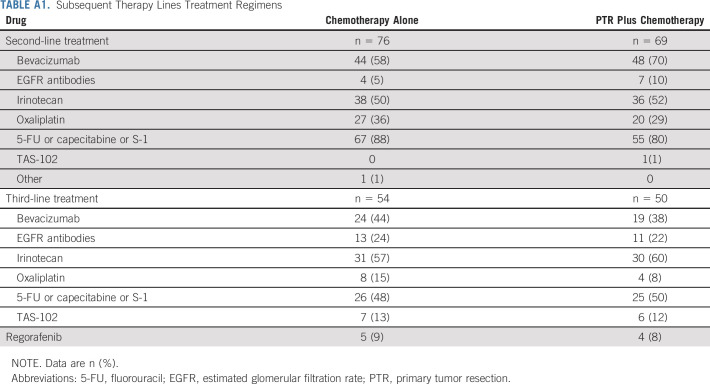
Table 3 shows AEs associated with chemotherapy. Median treatment cycles were 13 in both arms. Grade 2 or higher nonhematological AEs were more frequent in the PTR plus chemotherapy arm than in the chemotherapy-alone arm (58/67 [87%] patients v 61/79 [77%] patients). Grade 3 or higher nonhematological AEs were more frequent in the PTR plus chemotherapy arm than in the chemotherapy-alone arm (32/67 [48%] patients v 27/79 [34%] patients). One treatment-related death 31 days after initiation of protocol treatment was reported in a patient assigned to PTR plus chemotherapy (sudden duodenal perforation of unknown cause during the fifth cycle of chemotherapy). The median time to commencing chemotherapy after PTR was 34 days (IQR, 30-39). Chemotherapy was discontinued in all patients who received defined chemotherapy in both arms. There was no significant difference in chemotherapy regimen or the number of subsequent therapy lines between the two groups (Appendix Table A1, online only).
TABLE 3.
Hematological and Nonhematological Adverse Events Associated With Chemotherapy
DISCUSSION
The iPACS is the first RCT to address the role of PTR in patients with metastatic CRC cancer. The results did not show survival benefit of additional upfront PTR over chemotherapy alone in asymptomatic patients with incurable advanced CRC. Median OS was slightly more than 2 years regardless of whether patients with three or fewer unresectable metastatic diseases immediately received chemotherapy or underwent PTR before receiving chemotherapy. The study was terminated early at the interim analysis because patients assigned to PTR plus chemotherapy were unlikely to have improved OS compared with those assigned to chemotherapy alone. Chemotherapy-related morbidity was higher and more severe, 4% of the patients died of complications after surgery in the PTR plus chemotherapy arm, and 87% of patients in the chemotherapy-alone arm were able to avoid surgery entirely.
Although numerous recent papers have suggested a survival benefit of PTR compared with not undergoing it,5,7-9 retrospective comparative survival analysis without random assignment or clear explanation of the indications for resection is at great risk for bias and misleading results.4,24 In an observational cohort study using an appropriate epidemiologic and statistical methodology, Alawadi et al4 found no survival benefit of PTR among patients with unresectable metastatic CRC. RCTs should remain the gold standard for determining the efficacy of new cancer therapies.25 Around the time we began this trial or slightly after, there was much anticipation regarding the results of several ongoing RCTs.11-15 Elucidation of the true clinical significance of PTR for this patient population is evidently an important unmet need worldwide.
In this trial, 11 of the 84 (13%) patients initially assigned to chemotherapy alone underwent palliative surgery for symptoms linked to primary tumors. We found that 87% of patients in the chemotherapy-alone arm did not develop symptoms from the primary tumor that required surgery or problems related to the primary tumor resulting in death. This result is very similar to that of NSABP C-10, a phase II study26 that demonstrated acceptable toxicity profiles and efficacy of upfront chemotherapy with mFOLFOX6 and bevacizumab for asymptomatic unresectable metastatic CRC. On the other hand, in the present study, grade 2 or worse AEs after PTR occurred in 38% of patients, whereas grade 3 or worse AEs occurred in 21% of patients who underwent PTR. Hospital death after PTR occurred in 4% because of postoperative complications. PTR was associated with more frequent and more severe chemotherapy-related nonhematological AEs. Subgroup analysis also found no populations that benefited from PTR. The median time to commencing chemotherapy after PTR was 34 days. Thus, PTR not only delayed the start of effective systemic therapy but also increased the risk of severe complications and mortality.
This study has some limitations. First, the planned power of 70% was not sufficient and the planned sample size was not achieved because the study was terminated early according to the recommendation by the Data and Safety Monitoring Committee because of overall futility and ethical reasons, which then limited the statistical power supporting our conclusions. Our study had many intrinsic difficulties in patient accrual because of its strict eligibility criteria, patient preferences, and biases of individual clinicians, which led to poor acceptance of random assignment. With this reduced power, we opted to terminate the study, even though there is a lack of consensus over whether PTR is beneficial for asymptomatic patients with unresectable stage IV. The CAIRO411 and SYNCHRONOUS12 trials are ongoing in the West, with patient recruitment now completed in the SYNCHRONOUS trial12 (personal communication), and it is hoped that the comprehensive results of these trials will clearly demonstrate the role of PTR for these patients. Second, the quality of the study was partially impaired because five (3%) patients were deemed ineligible and 19 (12%) did not receive planned PTR and chemotherapy. This could have affected the outcomes, but the HR for death was essentially unchanged in patients assigned to PTR plus chemotherapy when calculated in a per-protocol analysis. Third, assessment of quality of life was not done, which is a crucial consideration for patients with a limited lifespan and their caregivers when choosing the optimum treatment strategy. Fourth, patients with lower rectal cancer were not included in this study. Surgery becomes more challenging in lower rectal cancer, and although laparoscopic surgery is rapidly becoming more widespread, only some facilities are conducting clinical trials of it for lower rectal cancer. Therefore, because the heterogeneity of risks could not be ruled out if we were to include lower rectal cancer in the study, we decided not to include it. Finally, asymptomatic patients with either tumors that could not be endoscopically traversed or circumferential lesions were enrolled in this study as long as there was no evidence of obstruction on X-ray. However, 13% of patients underwent palliative surgery for symptoms linked to the primary tumor in the chemotherapy-alone arm. Therefore, our results might be applied cautiously to patients with colonoscopic findings of nontraversable lesions at diagnosis that can cause luminal obstruction attributable to progression or fibrosis from response to treatment.27
In conclusion, PTR followed by chemotherapy had no survival benefit over chemotherapy alone in the present study. Therefore, PTR should no longer be considered standard of care among patients with CRC with an asymptomatic primary tumor and synchronous unresectable metastases.
ACKNOWLEDGMENT
The authors sincerely thank the participating patients and their families, and the investigators, research nurses, study coordinators, operation staff, and the members of the Japan Clinical Oncology Group (JCOG) Data Center and JCOG Operations Office for their support in managing the data (Ayaka Nakano).
APPENDIX
TABLE A1.
Subsequent Therapy Lines Treatment Regimens
See accompanying editorial on page 1095
PRIOR PRESENTATION
Presented at the 2020 ASCO GI Cancers Symposium, San Francisco, CA, January 23-25, 2020.
SUPPORT
Supported in part by National Cancer Center Research and Development Funds (23-A-19, 26-A-4, 29-A-3, 2020-J-3) and the Applied Research for Innovative Treatment of Cancer (H25-007) from the Ministry of Health of Japan. These funding sources had no role in the design, execution, analyses, interpretation of the data, or decision to submit results.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Yukihide Kanemitsu, Kohei Shitara, Junki Mizusawa, Tetsuya Hamaguchi, Koji Komori, Akio Shiomi, Yasuhiro Shimada, Hiroshi Katayama, Haruhiko Fukuda
Financial support: Yukihide Kanemitsu, Koji Komori
Administrative support: Kenji Katsumata
Provision of study materials or patients: Satoshi Ikeda, Hitoshi Ojima, Hideyuki Ike, Ryoji Hyakudomi, Yasuhiro Shimada
Collection and assembly of data: Yukihide Kanemitsu, Kohei Shitara, Tetsuya Hamaguchi, Dai Shida, Koji Komori, Satoshi Ikeda, Hitoshi Ojima, Hideyuki Ike, Akio Shiomi, Jun Watanabe, Yasumasa Takii, Takashi Yamaguchi, Kenji Katsumata, Masaaki Ito, Junji Okuda, Ryoji Hyakudomi, Haruhiko Fukuda
Data analysis and interpretation: Yukihide Kanemitsu, Kohei Shitara, Junki Mizusawa, Tetsuya Hamaguchi, Akio Shiomi, Yasuhiro Shimada, Hiroshi Katayama, Haruhiko Fukuda
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients With Asymptomatic, Synchronous Unresectable Metastases (JCOG1007; iPACS): A Randomized Clinical Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Yukihide Kanemitsu
Honoraria: Chugai Pharma, Ethicon, Covidien, Intuitive Surgical
Consulting or Advisory Role: Covidien
Kohei Shitara
Honoraria: Novartis, Abbvie, Yakult Pharmaceutical
Consulting or Advisory Role: Astellas Pharma, Lilly, Bristol-Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Novartis, Abbvie, GlaxoSmithKline
Research Funding: Dainippon Sumitomo Pharma, Lilly, MSD, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma, Ono Pharmaceutical, Astellas Pharma, Medi Science
Junki Mizusawa
Honoraria: Chugai Pharma, Taiho Pharmaceutical
Tetsuya Hamaguchi
Honoraria: Chugai Pharma, Merck Serono, Takeda, Taiho Pharmaceutical, Ono Pharmaceutical, Yakult Pharmaceutical, Lilly, Sanofi, Bristol-Myers Squibb Japan, Bayer, Fujifilm, Novartis
Research Funding: Taiho Pharmaceutical, Ono Pharmaceutical, Chugai Pharma, Eisai, BeiGene, Astellas Pharma
Jun Watanabe
Speakers' Bureau: Covidien Japan, Johnson & Johnson/Janssen
Masaaki Ito
Stock and Other Ownership Interests: A-Traction
Honoraria: KOTOBUKI Medical Inc, Applied Medical Japan, Olympus, Covidien Japan, Sanofi, Johnson & Johnson K.K. Medical Company, Tsumura & Co, Miyarisan Pharmaceutical Co, Ltd, Medical Leaders Co, Ltd, Taiho Pharmaceutical, Daiichi Sankyo Company, Limited, Chugai Pharma
Research Funding: Fujita Medical Instruments Co, Ltd, Intelligent Surfaces, Inc, Olympus, Muranaka Medical Instruments Co, Ltd, Kawasumi Laboratories, Incorporated, Indivumed
Patents, Royalties, Other Intellectual Property: Muranaka Medical Instruments Co, EBM Co
Yasuhiro Shimada
Honoraria: Chugai Pharma, Ono Pharmaceutical, Taiho Pharmaceutical, Lilly Japan, Sanofi
Research Funding: Taiho Pharmaceutical, MSD KK
Haruhiko Fukuda
Honoraria: Chugai Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 383: 1490-1502, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Sarela AI Guthrie JA Seymour MT, et al. : Non-operative management of the primary tumor in patients with incurable stage IV colorectal cancer. Br J Surg 88:1352-1356, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Poultsides GA Servais EL Saltz LB, et al. : Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol 27:3379-3384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alawadi Z Phatak UR Hu CY, et al. : Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer 123:1124-1133, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galizia G Lieto E Orditura M, et al. : First-line chemo- therapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch Surg 143:352-358, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Michel P Roque I Di Fiore F, et al. : Colorectal cancer with non-resectable synchronous metastases: Should the primary tumor be resected? Gastroenterol Clin Biol 28:434-437, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Ahmed S Leis A Fields A, et al. : Survival impact of surgical resection of primary tumor in patients with stage IV colorectal cancer: Results from a large population-based cohort study. Cancer 120:683-691, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Gresham G Renouf DJ Chan M, et al. : Association between palliative resection of the primary tumor and overall survival in a population-based cohort of metastatic colorectal cancer patients. Ann Surg Oncol 21:3917-3923, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Ishihara S Hayama T Yamada H, et al. : Prognostic impact of primary tumor resection and lymph node dissection in stage IV colorectal cancer with unresectable metastasis: A propensity score analysis in a multicenter retrospective study. Ann Surg Oncol 21:2949-2955, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Stillwell AP, Buettner PG, Ho YH: Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg 34: 797-780, 2010 [DOI] [PubMed] [Google Scholar]
- 11.tLam-Boer J Mol L Verhoef C, et al. : The CAIRO4 study: The role of surgery of the primary tumor with few or absent symptoms in patients with synchronous unresectable metastases of colorectal cancer–a randomized phase III study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer 14: 741, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahbari NN Lordick F Fink C, et al. : Resection of the primary tumor versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS–a randomized con- trolled multicentre trial (ISRCTN30964555). BMC Cancer 12: 142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biondo S Frago R Kreisler E, et al. : Impact of resection versus no resection of the primary tumor on survival in patients with colorectal cancer and synchronous unresectable metastases: Protocol for a randomized multicenter study (CR4). Int J Colorectal Dis 32: 1085-1090, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Mehdi K: Colectomy in Patients With Asymptomatic and Unresectable Stage IV Colon Cancer (CLIMAT). https://clinicaltrials.gov/ct2/show/NCT02363049. [Google Scholar]
- 15.Chen G: Palliative Resection of Asymptomatic Primary Tumor Following Effective Induction Chemotherapy in Colorectal Cancer Patients With Unresectable Distant Metastasis: A Multi-Center, Prospective, Randomized Controlled Study. https://clinicaltrials.gov/ct2/show/study/NCT02149784?term=NCT02149 784&rank=1, 2014 [Google Scholar]
- 16.Moritani K Kanemitsu Y Shida D, et al. : A randomized controlled trial comparing primary tumor resection plus chemotherapy with chemotherapy alone in incurable stage IV colorectal cancer: JCOG1007 (iPACS study). Jpn J Clin Oncol 50:89-93, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Japanese Society of Cancer of the Colon and Rectum General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum, and Anus (ed 7). Tokyo, Japan, Kanehara, 2006. (in Japanese) [Google Scholar]
- 18.Sobin L, Wittekind C: TNM Classification of Malignant Tumors (ed 7) New York, NY, Wiley-Liss, 2009 [Google Scholar]
- 19.Yamada Y Takahari D Matsumoto H, et al. : Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): An open-label, non-inferiority, randomized phase 3 trial. Lancet Oncol 14:278-286, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Yamazaki K Nagase M Tamagawa H, et al. : Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 27:1539-1546, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Ellis LM Bernstein DS Voest EE, et al. : American Society of Clinical Oncology Perspective: Raising the bar for clinical trials by defining clinically meaningful outcomes. J Clin Oncol 20:1277-1280, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Sobrero AF Pastorino A Sargent DJ, et al. : Raising the bar for antineoplastic agents: How to choose threshold values for superiority trials in advanced solid tumors. Clin Cancer Res 21:1036-1043, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Lan KKG, DeMets DL: Discrete sequential boundaries for clinical trials. Biometrika 70:659-663, 1983 [Google Scholar]
- 24.Shida D Boku N Tanabe T, et al. : Primary tumor resection for stage IV colorectal cancer in the era of targeted chemotherapy. J Gastrointest Surg 23:2144-2150, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Booth CM Karim S Peng Y, et al. : Radical treatment of the primary tumor in metastatic bladder cancer: Potentially dangerous findings from observational data. J Clin Oncol 36:533-535, 2018 [DOI] [PubMed] [Google Scholar]
- 26.McCahill LE Yothers G Sharif S, et al. : Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: Definitive analysis of NSABP trial C-10. J Clin Oncol 30:3223-3228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto T Hasegawa S Matsumoto S, et al. : Overcoming the challenges of primary tumor management in patients with metastatic colorectal cancer unresectable for cure and an asymptomatic primary tumor. Dis Colon Rectum 57:679-686, 2014 [DOI] [PubMed] [Google Scholar]