PURPOSE
The role of maintenance therapy for gastric (GC) or gastroesophageal junction cancer (GEJC) is unclear. We investigated avelumab (anti–programmed death ligand-1 [PD-L1]) maintenance after first-line induction chemotherapy for GC/GEJC.
PATIENTS AND METHODS
JAVELIN Gastric 100 was a global, open-label, phase III trial. Eligible patients had untreated, unresectable, human epidermal growth factor receptor 2–negative, locally advanced or metastatic GC or GEJC. Patients without progressive disease after 12 weeks of first-line chemotherapy with oxaliplatin plus a fluoropyrimidine were randomly assigned 1:1 to avelumab 10 mg/kg every 2 weeks or continued chemotherapy, stratified by region (Asia v non-Asia). The primary end point was overall survival (OS) after induction chemotherapy in all randomly assigned patients or the PD-L1–positive randomly assigned population (≥ 1% of tumor cells; 73-10 assay).
RESULTS
A total of 805 patients received induction; 499 were randomly assigned to avelumab (n = 249) or continued chemotherapy (n = 250). Median OS was 10.4 months (95% CI, 9.1 to 12.0 months) versus 10.9 months (95% CI, 9.6 to 12.4 months) and 24-month OS rate was 22.1% versus 15.5% with avelumab versus chemotherapy, respectively (hazard ratio [HR], 0.91; 95% CI, 0.74 to 1.11; P = .1779). In the PD-L1–positive population (n = 54), the HR for OS was 1.13 (95% CI, 0.57 to 2.23; P = .6352). In an exploratory analysis of the PD-L1–positive population, defined as combined positive score ≥ 1 (22C3 assay; n = 137), median OS was 14.9 months (95% CI, 8.7 to 17.3 months) with avelumab versus 11.6 months (95% CI, 8.4 to 12.6 months) with chemotherapy (unstratified HR, 0.72; 95% CI, 0.49 to 1.05). With avelumab and chemotherapy, treatment-related adverse events (TRAEs) occurred in 149 (61.3%) and 184 (77.3%) patients, including grade ≥ 3 TRAEs in 31 (12.8%) and 78 (32.8%) patients, respectively.
CONCLUSION
JAVELIN Gastric 100 did not demonstrate superior OS with avelumab maintenance versus continued chemotherapy in patients with advanced GC or GEJC overall or in a prespecified PD-L1–positive population.
INTRODUCTION
The prognosis for patients with advanced gastric cancer (GC) remains poor.1 International guidelines recommend platinum plus a fluoropyrimidine doublet or triplet chemotherapy regimens for first-line treatment of unresectable advanced or metastatic human epidermal growth factor receptor 2 (HER2)–negative GC or gastroesophageal junction cancer (GEJC)2-4; however, durations of progression-free survival (PFS; median, approximately 6 months) and overall survival (OS; median, 9-18 months) are short.5-9 Although maintenance therapy improves PFS and OS in several tumors,10-13 its role in GC/GEJC has not been established.14-16 Recently, anti–programmed death-1 (PD-1) immune checkpoint inhibitors nivolumab and pembrolizumab were approved for patients with previously treated advanced GC or GEJC in different regions.17-21
CONTEXT
Key Objective
We performed a phase III trial to determine if administering avelumab maintenance therapy after induction chemotherapy would improve outcomes versus continued chemotherapy in patients with advanced gastric cancers.
Knowledge Generated
JAVELIN Gastric 100 did not demonstrate superior overall survival (primary end point) with avelumab maintenance versus continued chemotherapy in all randomly assigned patients or in a predefined programmed death ligand-1–positive population. However, avelumab maintenance had a milder adverse event profile than continued chemotherapy and showed evidence of clinical activity, including prolonged duration of response and potentially increased benefit in some subgroups.
Relevance
To our knowledge, this is the first phase III trial of switch maintenance treatment with an immune checkpoint inhibitor in patients with advanced gastric cancers, and its results are informative for design of future trials. Additional studies are needed to identify patients with gastric cancers who can derive greater benefit from checkpoint inhibitor therapy than standard chemotherapy.
Avelumab is an anti–programmed death ligand-1 (PD-L1) antibody that has shown antitumor activity and a tolerable safety profile in patients with various solid tumors.22-27 In a phase Ib cohort, avelumab switch maintenance therapy exhibited encouraging activity in patients with advanced GC or GEJC without disease progression after first-line chemotherapy,28 supporting further investigation. We report the primary analysis of the phase III JAVELIN Gastric 100 trial of avelumab switch maintenance therapy after first-line induction chemotherapy compared with continuation of first-line chemotherapy for advanced HER2-negative GC/GEJC.
PATIENTS AND METHODS
Patients
Eligible patients for induction chemotherapy had histologically confirmed, unresectable, locally advanced or metastatic adenocarcinoma of the stomach or GEJ, had not received chemotherapy for locally advanced or metastatic disease, and had measurable disease per RECIST (version 1.1). Other key inclusion criteria were age ≥ 18 years, Eastern Cooperative Oncology Group performance status of 0 or 1, and recently obtained (≤ 6 months) tumor specimen. Key exclusion criteria included HER2-positive tumor, prior immune checkpoint inhibitor therapy, and untreated or symptomatic brain metastasis. Full eligibility criteria are provided in the Protocol (online only).
The trial was conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, the International Conference on Harmonisation Guidelines for Good Clinical Practice, and the Declaration of Helsinki. All patients provided written informed consent before enrollment. The trial Protocol and all amendments were approved by the institutional review board or ethics committee at each participating center.
Study Design and Treatment
JAVELIN Gastric 100 (ClinicalTrials.gov identifier: NCT02625610) was an open-label, multicenter, randomized phase III trial. All patients received first-line induction therapy for up to 12 weeks with one of three regimens: oxaliplatin 85 mg/m2 intravenously (IV) and leucovorin 200 mg/m2 (or equivalent levoleucovorin dose) in accordance with label instructions and local guidelines, followed by fluorouracil (FU) 2,600 mg/m2 by continuous infusion over 24 hours on day 1, every 2 weeks; oxaliplatin 85 mg/m2 IV and leucovorin 400 mg/m2 (or equivalent levoleucovorin dose), followed by FU 400 mg/m2 IV on day 1 and FU 2,400 mg/m2 by continuous infusion over 46 to 48 hours on days 1 to 2, every 2 weeks; or oxaliplatin 130 mg/m2 IV on day 1 and capecitabine 1,000 mg/m2 orally twice daily for 2 weeks, followed by a 1-week rest period, every 3 weeks. Patients without progressive disease (PD) per RECIST (version 1.1) after induction chemotherapy, confirmed by an independent radiologist, were randomly assigned 1:1 to either switch maintenance therapy with avelumab 10 mg/kg IV every 2 weeks or continuation of the same chemotherapy. Random assignment was stratified by region (Asia v non-Asia). All patients received best supportive care (BSC). In the chemotherapy arm, patients unable to tolerate further combination chemotherapy could receive capecitabine, FU plus leucovorin, or oxaliplatin alone. Patients considered ineligible for further chemotherapy received BSC only. Patients received antihistamine/acetaminophen pretreatment before the first four avelumab infusions. Avelumab dose reductions were not permitted; changes in infusion rate and dose delays were permitted. Dose modifications of chemotherapy were permitted in accordance with labeling instructions and local guidelines. All randomly assigned patients continued assigned treatment until PD, unacceptable toxicity, withdrawal of consent, or any other criterion for withdrawal occurred.
End Points and Assessments
The primary end point was OS (time from random assignment to death resulting from any cause). OS was assessed in all randomly assigned patients and in randomly assigned patients with PD-L1–positive tumors. For the primary analysis, as prespecified in the statistical analysis plan, PD-L1 status was assessed centrally at baseline using the PD-L1 immunohistochemical (IHC) 73-10 performance evaluation–only assay (Agilent Technologies/Dako, Carpinteria, CA), and PD-L1–positive status was defined as PD-L1 protein expression in ≥ 1% of tumor cells. Secondary end points included PFS (time from random assignment to first documentation of PD per RECIST [version 1.1] according to investigator assessment or death resulting from any cause, whichever occurred first), best overall response (best response among all tumor assessments from baseline [at random assignment, after induction chemotherapy] per RECIST [version 1.1]), duration of response (time from first documentation of objective response in the maintenance phase until PD per RECIST [version 1.1] or death), and safety. Tumors were assessed radiologically at baseline, every 6 weeks for the first 12 months, and every 12 weeks thereafter. In a post hoc exploratory subset analysis, PD-L1 expression in both tumor and immune cells (lymphocytes and macrophages) was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies/Dako) according to the manufacturer’s instructions, with PD-L1–positive status defined as combined positive score (CPS) ≥ 1.29 Microsatellite instability (MSI) status (exploratory analysis) was assessed using the Idylla MSI assay (Biocartis, Mechelen, Belgium). Adverse events (AEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). Immune-related AEs were identified using a prespecified list of preferred terms followed by comprehensive medical review.
Statistical Analysis
The original primary objective was to show superior OS or PFS of avelumab maintenance over continuation of first-line chemotherapy in all randomly assigned patients. In June 2018 (before interim analysis and availability of patient data), based on results from the phase Ib study of avelumab in GC and GEJC, which showed longer OS in the PD-L1–positive population,28 the primary objective was amended to show the superiority of avelumab maintenance over continuation of first-line chemotherapy in prolonging OS in all randomly assigned patients or in the randomly assigned PD-L1–positive population, enabling formal statistical analysis of OS in both populations. PFS became a secondary objective. The number of patients enrolled in the induction phase was driven by the observed induction failure rate to allow approximately 466 patients to be randomly assigned. For OS in all randomly assigned patients, assuming median OS of 10.5 and 15.0 months in the chemotherapy and avelumab arms, respectively, corresponding to a hazard ratio (HR) of 0.70, and with a dropout rate of 5%, 356 events (deaths) were required to achieve 90% power in a log-rank test with one-sided α of 2%. This calculation included an interim efficacy analysis, performed after 75% of OS events had occurred. Interim and primary analyses used a sequential α-spending function approach per Lan and DeMets with an O’Brien and Fleming boundary function. For analysis of OS in the PD-L1–positive population, a median OS of 10.5 and 19.3 months was assumed in the chemotherapy and avelumab arms, respectively, corresponding to an HR of 0.54. The primary end point was considered positive if null hypothesis testing for OS in either the overall or PD-L1–positive population was rejected. An imbalanced type I error allocation was used for the two primary hypotheses to control the error rate at 2.5% (one sided), with 2% and 0.5% (one sided) allocated to the overall and PD-L1–positive populations, respectively. Calculations were performed using EAST (version 6.4; Cytel, Cambridge, MA) and R software (R Foundation, Vienna, Austria). Dual primary hypothesis testing of OS was analyzed with a closed testing procedure using weighted Bonferroni tests. If the OS comparison in one population was significant, the α value would be recycled for the OS comparison in the other population. OS and PFS were estimated using the Kaplan-Meier method. Objective response rates (ORRs; proportion with a confirmed best overall response of complete response [CR] or partial response [PR]) by treatment group were compared using the Cochran-Mantel-Haenszel test, accounting for stratification, with a one-sided α level of 0.025; two-sided 95% CIs were calculated using the Clopper-Pearson method. Safety was assessed in all patients who received ≥ one dose of randomly assigned treatment in the maintenance phase.
RESULTS
Patients and Treatment
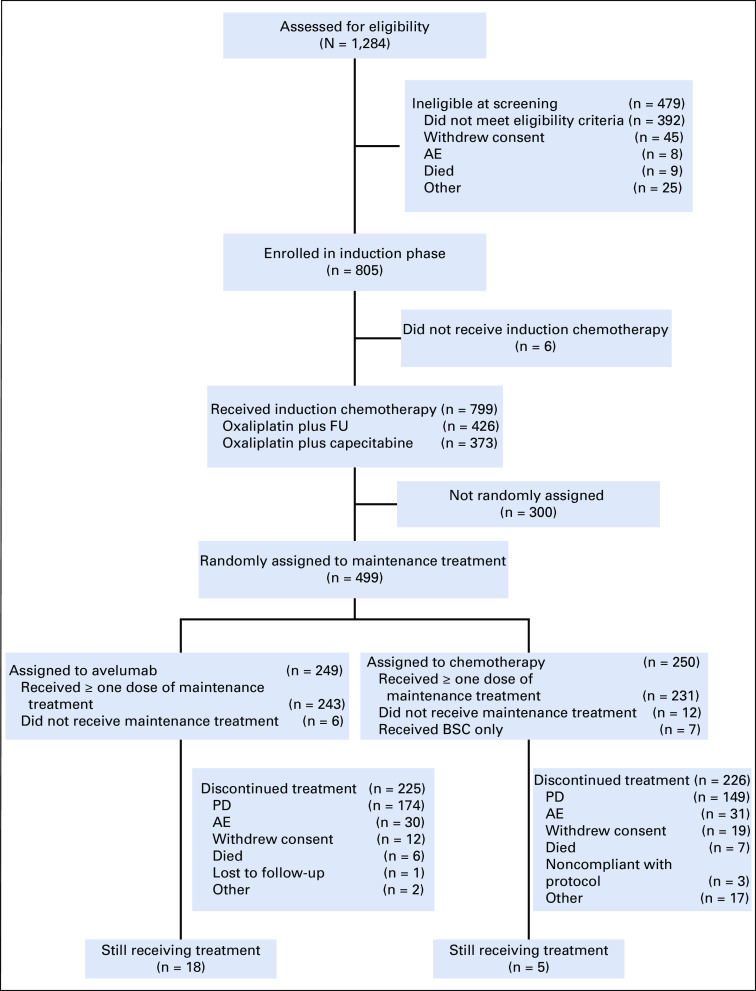
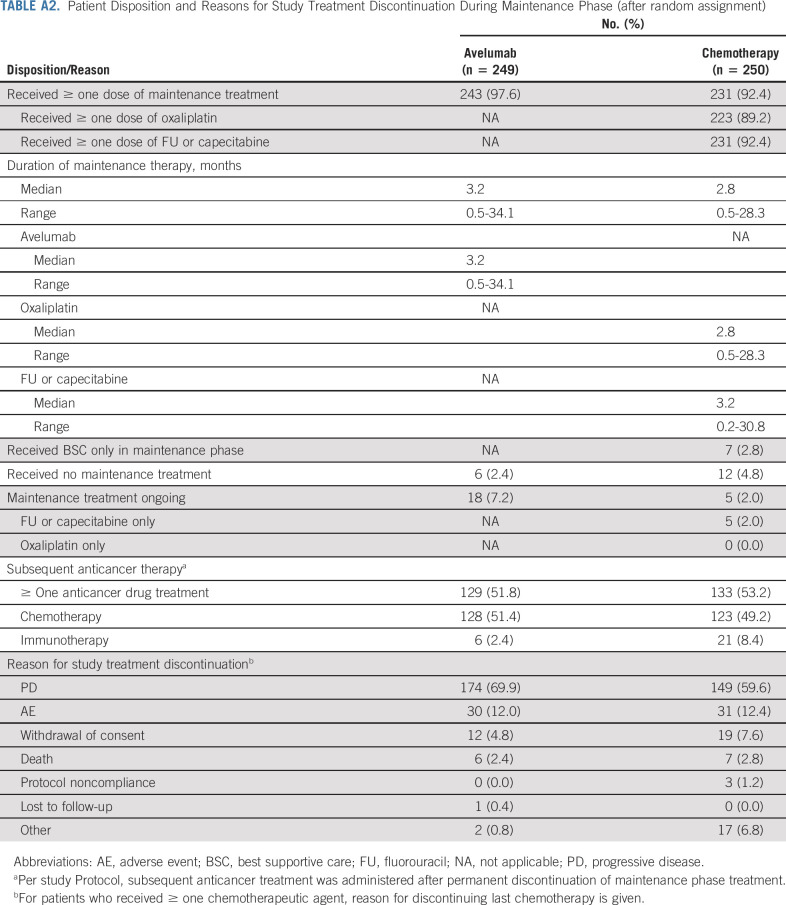
Between December 31, 2015, and November 29, 2017, 805 patients enrolled at 178 sites in 17 countries (Appendix Table A1, online only) entered the 12-week induction phase (Fig 1). Subsequently, 499 patients with disease control were randomly assigned to avelumab maintenance (n = 249) or continued chemotherapy (n = 250), including 30 and 24 patients, respectively, with PD-L1–positive tumors based on a prespecified definition (expression in ≥ 1% of tumor cells; 73-10 assay). In the chemotherapy arm, seven patients (2.8%) were considered unsuitable for further chemotherapy and received BSC only. Baseline characteristics were similar between arms (Table 1). At data cutoff on September 13, 2019, 18 (7.2%) and five (2.0%) patients were still receiving study treatment in the avelumab and chemotherapy arms, respectively (Appendix Table A2, online only). Median duration of treatment in the maintenance phase was 3.2 months (range, 0.5-34.1 months) in the avelumab arm and 2.8 months (range, 0.5-28.3 months) in the chemotherapy arm, and median follow-up for OS was 24.1 and 24.0 months, respectively (minimum, 18 months in both arms). In the avelumab and chemotherapy arms, subsequent immunotherapy was received by 2.4% and 8.4% of patients, respectively, and subsequent chemotherapy was received by 51.4% and 49.2% of patients, respectively.
FIG 1.
CONSORT diagram. AE, adverse event; BSC, best supportive care; FU, fluorouracil; PD, progressive disease.
TABLE 1.
Baseline Demographic and Clinical Characteristics
Efficacy
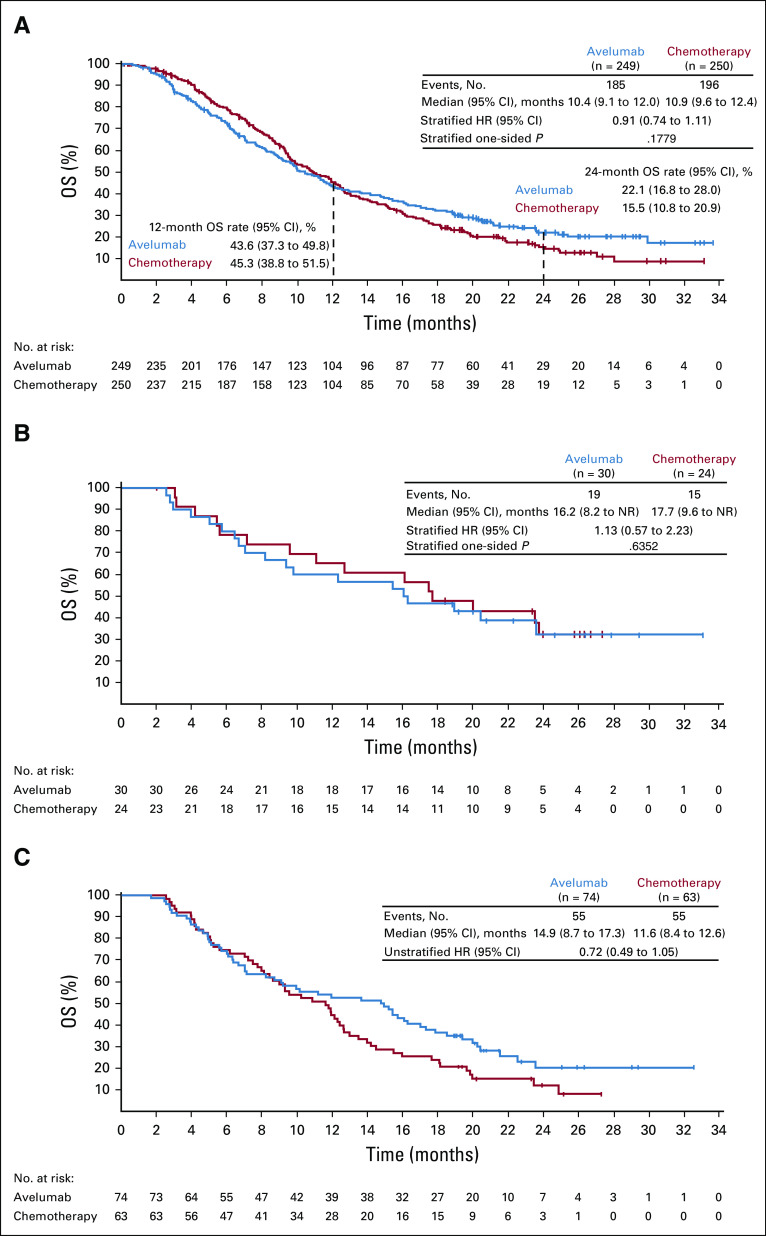
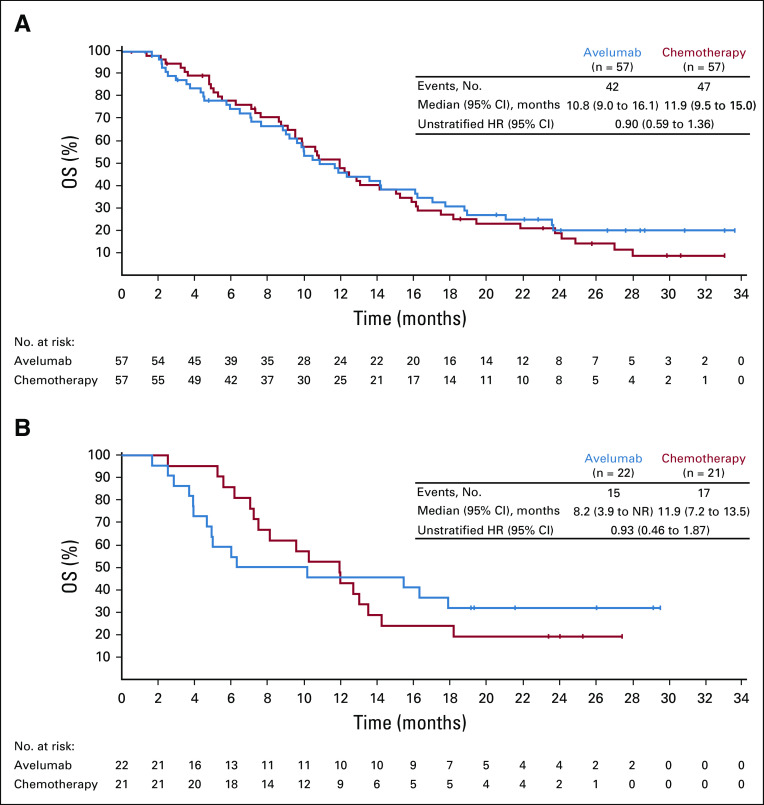
OS was not significantly different in the avelumab and chemotherapy arms (Fig 2). In all randomly assigned patients, median OS (measured from random assignment [ie, after 12 weeks of induction chemotherapy]) was 10.4 months (95% CI, 9.1 to 12.0 months) in the avelumab arm and 10.9 months (95% CI, 9.6 to 12.4 months) in the chemotherapy arm (HR, 0.91; 95% CI, 0.74 to 1.11; one-sided P = .1779); 24-month OS rates were 22.1% (95% CI, 16.8 to 28.0) versus 15.5% (95% CI, 10.8 to 20.9), respectively. In the prespecified PD-L1–positive population (≥ 1% of tumor cells; 73-10 assay; 54 [12.3%] of 438 evaluable patients), median OS was 16.2 months (95% CI, 8.2 months to not reached [NR]) in the avelumab arm and 17.7 months (95% CI, 9.6 months to NR) in the chemotherapy arm (HR, 1.13; 95% CI, 0.57 to 2.23; one-sided P = .6352). OS trends were similar in most Protocol-specified subgroups (Fig 3), including Asian patients (n = 114; HR, 0.90; 95% CI, 0.59 to 1.36; Appendix Fig A1A, online only); however, an OS difference was seen in two prespecified populations: patients with no metastatic sites after induction chemotherapy (n = 114; HR, 0.52; 95% CI, 0.28 to 0.98) and the small subset with MSI-high tumors (n = 13; HR, 0.27; 95% CI, 0.06 to 1.25). In an exploratory analysis performed in the subset of evaluable patients with PD-L1–positive tumors, defined as CPS ≥ 1 using the 22C3 assay (137 [64.3%] of 213 evaluable patients), median OS was 14.9 months (95% CI, 8.7 to 17.3 months) with avelumab and 11.6 months (95% CI, 8.4 to 12.6 months) with chemotherapy (unstratified HR, 0.72; 95% CI, 0.49 to 1.05; Fig 2C). In the subset with PD-L1–high tumors based on the 22C3 assay (CPS ≥ 10; n = 43), no evidence of avelumab benefit was seen (Appendix Fig A1B).
FIG 2.
Overall survival (OS; measured from random assignment after 12 weeks of induction chemotherapy) in (A) all randomly assigned patients, (B) prespecified programmed death ligand-1 (PD-L1)–positive population (tumor cell PD-L1 expression, ≥ 1% cutoff; 73-10 assay), and (C) exploratory subset of patients with PD-L1–positive tumors based on combined positive score (≥ 1 cutoff; 22C3 assay). HR, hazard ratio; NR, not reached.
FIG 3.
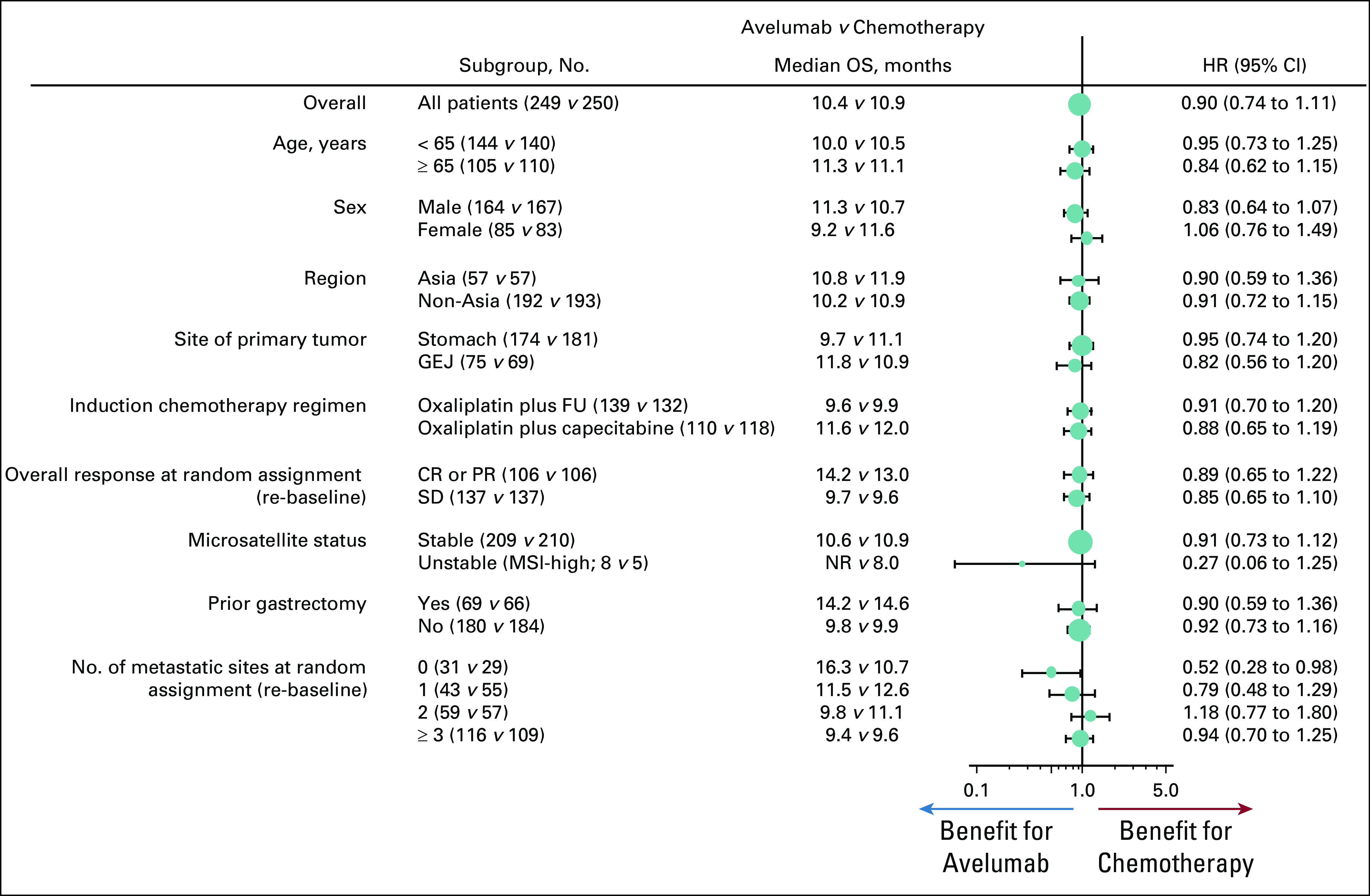
Overall survival (OS; measured from random assignment after 12 weeks of induction chemotherapy [ie, re-baseline]) in subgroups. Hazard ratios (HRs) were calculated for a univariable unstratified model. In the microsatellite instability (MSI)–high subgroup, 12-month OS rate was 75.0% (95% CI, 31.5 to 93.1) in the avelumab arm and 40.0% (95% CI, 5.2 to 75.3) in the chemotherapy arm. CR, complete response; FU, fluorouracil; GEJ, gastroesophageal junction; NR, not reached; PR, partial response; SD, stable disease.
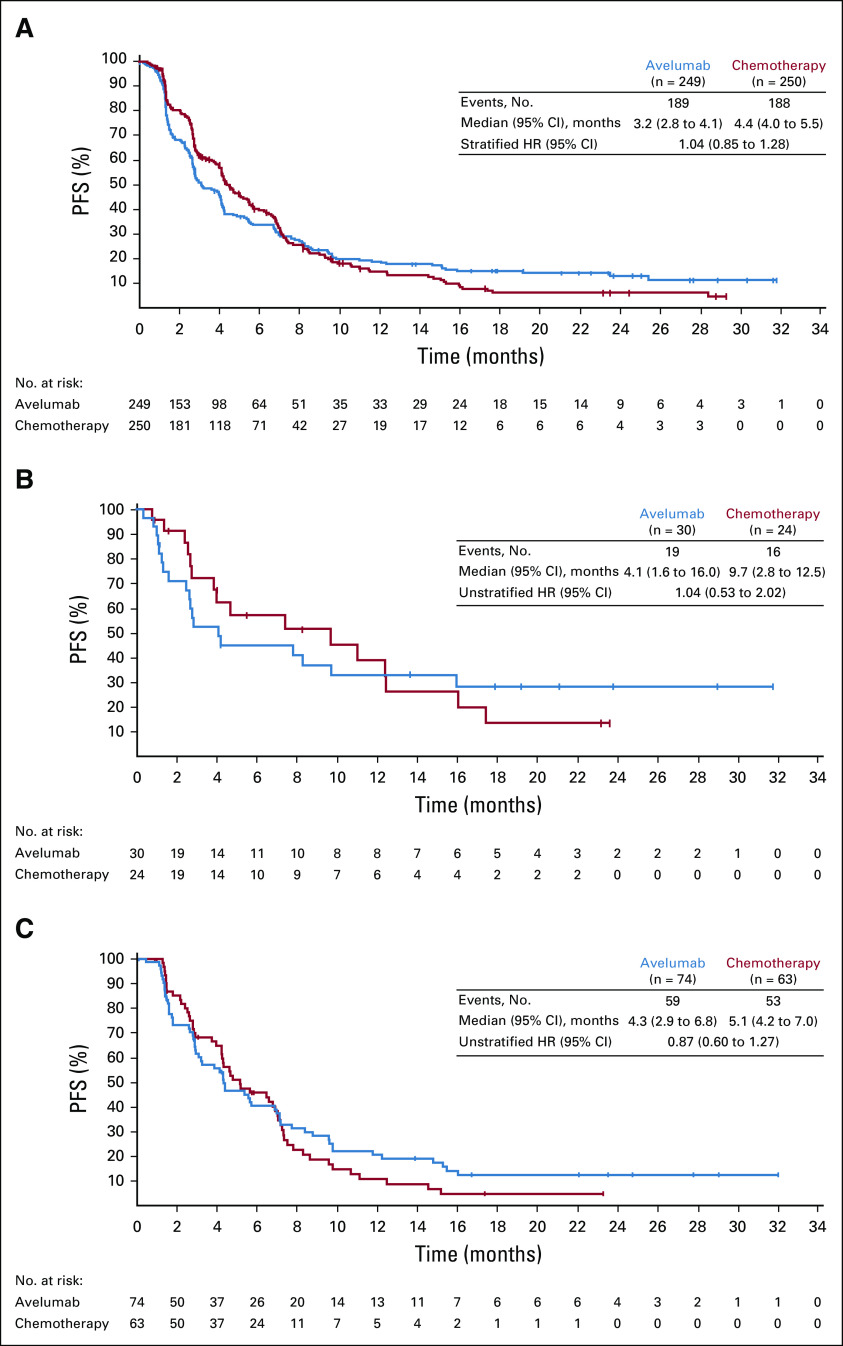
In all randomly assigned patients, median PFS (after random assignment) was 3.2 months (95% CI, 2.8 to 4.1 months) in the avelumab arm and 4.4 months (95% CI, 4.0 to 5.5 months) in the chemotherapy arm (HR, 1.04; 95% CI, 0.85 to 1.28; Appendix Fig A2A, online only). In the prespecified PD-L1–positive population (≥ 1% of tumor cells; 73-10 assay), median PFS was 4.1 months (95% CI, 1.6 to 16.0 months) in the avelumab arm and 9.7 months (95% CI, 2.8 to 12.5 months) in the chemotherapy arm (HR, 1.04; 95% CI, 0.53 to 2.02; Appendix Fig A2B). In the exploratory subset with PD-L1–positive tumors, defined as CPS ≥ 1 (22C3 assay), median PFS was 4.3 months (95% CI, 2.9 to 6.8 months) in the avelumab arm and 5.1 months (95% CI, 4.2 to 7.0 months) in the chemotherapy arm (unstratified HR, 0.87; 95% CI, 0.60 to 1.27; Appendix Fig A2C).
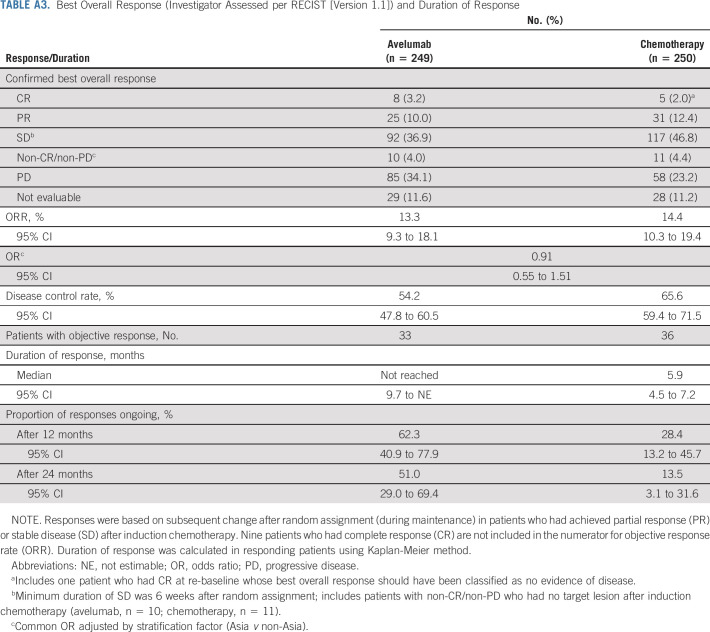
The ORR, representing additional or deepening tumor responses after random assignment in patients who had achieved PR or stable disease with induction chemotherapy, was 13.3% (95% CI, 9.3 to 18.1) in the avelumab arm and 14.4% (95% CI, 10.3 to 19.4) in the chemotherapy arm (Appendix Table A3, online only). At random assignment, 10 patients had CR after induction chemotherapy and were not included in the numerator for ORR because these patients no longer had tumors to monitor, except for one patient in the chemotherapy arm misclassified as having CR during the maintenance phase. Within this subgroup, three patients in the avelumab arm and two patients in the chemotherapy arm maintained no evidence of disease at time of data cutoff. Median time to response was 16.1 weeks (range, 5.6-96.4 weeks) with avelumab versus 6.4 weeks (range, 3.3-116.0 weeks) with chemotherapy. Median duration of response achieved after random assignment was not reached (95% CI, 9.7 months to not estimable) with avelumab versus 5.9 months (95% CI, 4.5 to 7.2 months) with chemotherapy (Fig 4). The probability of ongoing response at 12 months with avelumab versus chemotherapy was 62.3% (95% CI, 40.9 to 77.9) versus 28.4% (95% CI, 13.2 to 45.7), and at 24 months, it was 51.0% (95% CI, 29.0 to 69.4) versus 13.5% (95% CI, 3.1 to 31.6), respectively.
FIG 4.
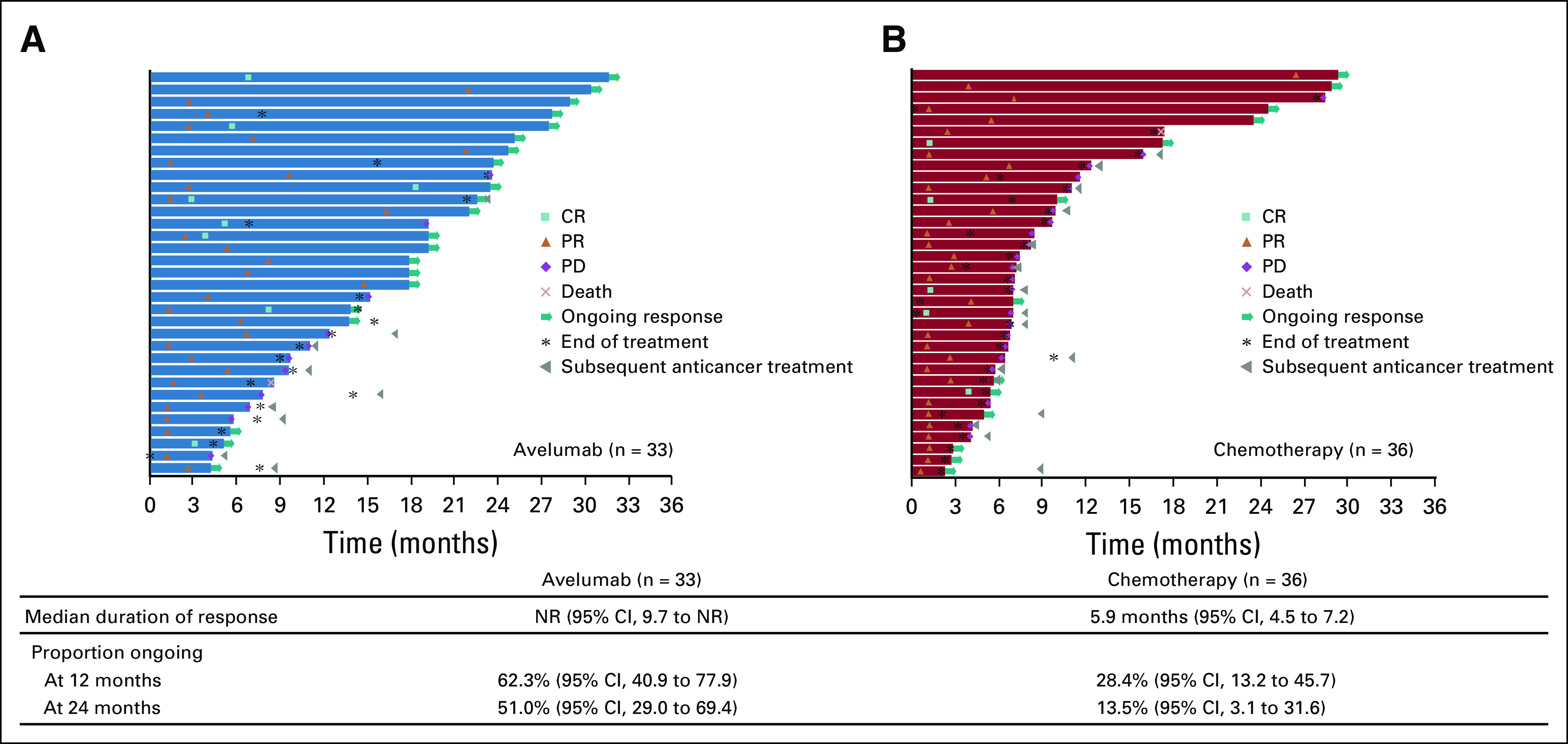
Time to and duration of response (investigator assessed per RECIST [version 1.1]) during the maintenance phase (after random assignment) in (A) avelumab and (B) chemotherapy arms. Responses were based on subsequent change after random assignment (during maintenance) in patients who had achieved partial response (PR) or stable disease (SD) after induction chemotherapy. Excludes 10 patients with complete response (CR) during induction chemotherapy. NR, not reached; PD, progressive disease.
Safety
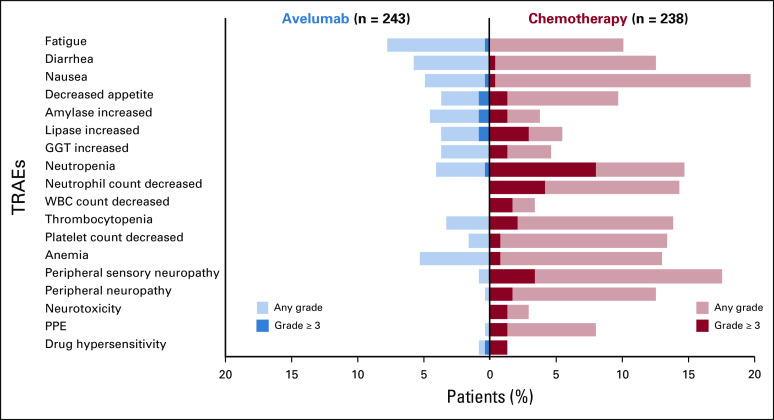
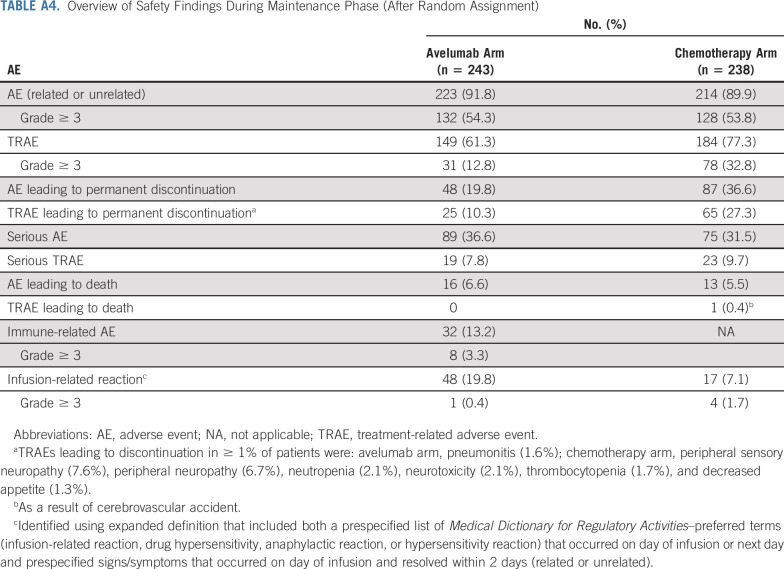
During the maintenance phase, AEs of any causality occurred in 223 (91.8%) of 243 avelumab-treated patients and in 214 (89.9%) of 238 patients treated in the chemotherapy arm, including grade ≥ 3 AEs in 132 (54.3%) and 128 patients (53.8%), respectively (Appendix Table A4, online only). In the avelumab and chemotherapy arms, treatment-related AEs (TRAEs) of any grade occurred in 149 (61.3%) versus 184 patients (77.3%), including grade ≥ 3 TRAEs in 31 (12.8%) and 78 patients (32.8%), respectively. The most common grade ≥ 3 TRAEs in the avelumab arm were increased amylase, increased lipase, asthenia, colitis, decreased appetite, hypertension, and pneumonitis (n = 2 each [0.8%]), and in the chemotherapy arm, they were neutropenia (n = 19 [8.0%]), decreased neutrophil count (n = 10 [4.2%]), and peripheral sensory neuropathy (n = 8 [3.4%]; Fig 5). In the avelumab and chemotherapy arms, serious TRAEs occurred in 19 (7.8%) versus 23 (9.7%) patients, and TRAEs led to permanent discontinuation in 25 (10.3%) versus 65 (27.3%) patients, respectively. A TRAE led to death in one patient in the chemotherapy arm (cerebrovascular event). In the avelumab arm, 32 patients (13.2%) had an immune-related AE, including grade ≥ 3 events in eight patients (3.3%). The most frequent immune-related AEs of any grade (≥ 2.0%) were hypothyroidism (n = 7 [2.9%]), pneumonitis (n = 6 [2.5%]), and rash (n = 5 [2.1%]).
FIG 5.
Treatment-related adverse events (TRAEs) that occurred at any grade in ≥ 10% or grade ≥ 3 in ≥ 1% of patients in either arm during the maintenance phase (after random assignment). GGT, γ-glutamyltransferase; PPE, palmar-plantar erythrodysesthesia syndrome.
DISCUSSION
The JAVELIN Gastric 100 trial did not meet its primary objective of demonstrating superior OS with switch maintenance avelumab versus continued chemotherapy in patients with advanced GC of GEJC who had disease control after first-line induction chemotherapy, either in the overall or prespecified PD-L1–positive population (≥ 1% of tumor cells; 73-10 assay). Nonsignificant trends toward a higher 24-month OS rate (22.1% v 15.5%) and longer durations of response (probability of ongoing response at 24 months, 51.0% v 13.5%) compared with chemotherapy were observed. On the basis of exploratory subset analyses, OS differences with avelumab versus chemotherapy were seen in subgroups with no metastatic sites at random assignment; in a small subset of patients with MSI-high tumors, although the 95% CI for the HR (0.06 to 1.25) overlaps with 1; and in patients with PD-L1–positive tumors, defined as CPS ≥ 1 (22C3 assay), accounting for PD-L1 protein expression in tumor cells, lymphocytes, and macrophages, and thus representing possible areas for further evaluation. Avelumab showed favorable safety versus continued chemotherapy, including lower rates of grade ≥ 3 TRAEs (12.8% v 32.8%), permanent discontinuations because of TRAEs (10.3% v 27.3%), and reductions in treatment-related gastrointestinal AEs, hematologic AEs, and neuropathy. The safety profile of avelumab was consistent with previous avelumab monotherapy studies.22-26,28,30
Anti–PD-1 antibodies are approved for later-line treatment of GC and GEJC, but to our knowledge, no phase III has shown statistical superiority compared with chemotherapy in any line.4,14,31 In the analysis of OS in all randomly assigned patients, the Kaplan-Meier curve was lower in the avelumab versus chemotherapy arm at initial time points, but the curves crossed at approximately 12 months, and the OS curve was higher for avelumab at later time points. In the phase III KEYNOTE-062 trial, which compared first-line pembrolizumab monotherapy versus chemotherapy in patients with PD-L1–positive GC or GEJC, defined as CPS ≥ 1 (22C3 assay), OS curves had a generally similar shape to those in JAVELIN Gastric 100, although the initial detrimental effect on OS with pembrolizumab versus chemotherapy was more marked, likely reflecting differences in study design and population enrichment between trials.31 Specifically, in JAVELIN Gastric 100, avelumab maintenance was administered only to patients who had disease control after first-line induction chemotherapy (ie, chemotherapy-sensitive patients), whereas in KEYNOTE-062, first-line pembrolizumab was administered to patients with PD-L1–positive tumors (CPS ≥ 1). In addition, in JAVELIN Gastric 100, OS was measured from random assignment after 12 weeks of induction chemotherapy, whereas in KEYNOTE-062, OS was measured from enrollment. In an exploratory hypothesis-generating analysis of patients with PD-L1–positive tumors, defined as CPS ≥ 1 (22C3 assay), in JAVELIN Gastric 100, OS was similar in the avelumab and chemotherapy arms until 12 months, when the curves diverged, suggesting that this subgroup may have excluded those who had worse outcomes with avelumab during initial treatment. In KEYNOTE-062, OS differences for pembrolizumab versus chemotherapy were increased in patients with PD-L1–high tumors (CPS ≥ 10); this was not seen in our study, suggesting that high PD-L1 may not predict increased benefit in patients with disease control after chemotherapy. However, few patients had tumors with CPS ≥ 10 in JAVELIN Gastric 100 (n = 43), limiting interpretation.
To our knowledge, JAVELIN Gastric 100 is the first phase III trial of switch maintenance treatment with an immune checkpoint inhibitor in GC/GEJC, and its results are informative for future trials. The low proportion of evaluable patients with PD-L1–positive tumors based on a prespecified definition (12.4% with ≥ 1% PD-L1–positive tumor cells; 73-10 assay) meant that the analysis of OS in this population was underpowered. This proportion was smaller than that in the phase Ib study of avelumab performed in a similar setting (33.3%)28 and in a phase III trial of third-line avelumab versus chemotherapy (JAVELIN Gastric 300; 26.8%)32 but is comparable to proportions with ≥ 1% PD-L1–positive tumor cells in the ATTRACTION-2 (ClinicalTrials.gov identifier: NCT02267343; 13.5%; 28-8 assay) and KEYNOTE-059 studies (ClinicalTrials.gov identifier: NCT02335411; 12.5%; 22C3 assay).17,29 However, assessment of PD-L1 expression in both tumor and immune cells via CPS may be more useful. A majority of patients had disease control with induction chemotherapy, and nearly all patients subsequently randomly assigned to the chemotherapy arm received continued chemotherapy in the maintenance phase (92.4%) rather than BSC alone, which may reflect the relative fitness of patients who achieve disease control. Of note, median duration of chemotherapy, including induction treatment, was approximately 6 months.
In conclusion, the JAVELIN Gastric 100 trial did not achieve its primary objective of OS improvement with maintenance avelumab in patients with disease control after induction chemotherapy for advanced GC/GEJC. However, results suggest potential activity in selected patient subsets and a favorable safety profile, providing guidance for future studies in this challenging disease.
ACKNOWLEDGMENT
We thank Ilaria Conti, Sara Georges, Mary Ruisi, and Silke Scheller of Merck KGaA, Darmstadt, Germany for their contributions to the study as well as the patients and their families and the investigators, coinvestigators, and study teams at each of the participating centers. Medical writing assistance was provided by Mark Holland of ClinicalThinking and funded by Merck KGaA, Darmstadt, Germany and Pfizer.
Appendix
TABLE A1.
List of JAVELIN Gastric 100 Investigators
TABLE A2.
Patient Disposition and Reasons for Study Treatment Discontinuation During Maintenance Phase (after random assignment)
TABLE A3.
Best Overall Response (Investigator Assessed per RECIST [Version 1.1]) and Duration of Response
TABLE A4.
Overview of Safety Findings During Maintenance Phase (After Random Assignment)
FIG A1.
Overall survival (OS; measured from random assignment after 12 weeks of induction chemotherapy) in (A) Asian patients and (B) subset with programmed death ligand-1–high tumors based on the 22C3 assay (combined positive score ≥ 10; 22C3 assay). HR, hazard ratio; NR, not reached.
FIG A2.
Progression-free survival (PFS; measured from random assignment to first documentation of progressive disease per RECIST [version 1.1] according to investigator assessment or death resulting from any cause, whichever occurred first) in (A) all randomly assigned patients, (B) prespecified programmed death ligand-1 (PD-L1)–positive population (≥ 1% of tumor cells; 73-10 assay), and (C) exploratory subset with PD-L1–positive tumors (combined positive score ≥ 1; 22C3 assay). HR, hazard ratio.
PRIOR PRESENTATION
Presented in part at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium, San Francisco, CA, January 23-25, 2020
SUPPORT
Supported by Merck KGaA, Darmstadt, Germany, as part of an alliance between Merck KGaA and Pfizer.
CLINICAL TRIAL INFORMATION
NCT02625610 (JAVELIN Gastric 100)
J.T. and Y.-J.B. contributed equally to this work.
DATA SHARING STATEMENT
For all new products or new indications approved in both the European Union and the United States after January 1, 2014, Merck KGaA, Darmstadt, Germany will share patient-level and study-level data after deidentification, as well as redacted study Protocols and clinical study reports from clinical trials in patients. These data will be shared with qualified scientific and medical researchers, upon researcher's request, as necessary for conducting legitimate research. Such requests must be submitted in writing to the company’s data sharing portal. More information can be found at https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. Where Merck KGaA has a co-research, co-development or co-marketing/co-promotion agreement or where the product has been out-licensed, it is recognized that the responsibility for disclosure may be dependent on the agreement between parties. Under these circumstances, Merck KGaA will endeavor to gain agreement to share data in response to requests.
AUTHOR CONTRIBUTIONS
Conception and design: Markus Moehler, Narikazu Boku, Huiling Xiong, Julien Taieb, Yung-Jue Bang
Administrative support: Mikhail Dvorkin, Mustafa Özgüroğlu, Janet Hong
Provision of study material or patients: Markus Moehler, Mikhail Dvorkin, Mustafa Özgüroğlu, Min-Hee Ryu, Alina S. Muntean, Sara Lonardi, Marina Nechaeva, Arinilda C. Bragagnoli, Hasan S. Coşkun, Antonio Cubillo Gracian, Toshimi Takano, Rachel Wong, Gina M. Vaccaro, Zev A. Wainberg, Janet Hong, Julien Taieb, Yung-Jue Bang
Collection and assembly of data: Mikhail Dvorkin, Narikazu Boku, Mustafa Özgüroğlu, Min-Hee Ryu, Alina S. Muntean, Sara Lonardi, Marina Nechaeva, Arinilda C. Bragagnoli, Hasan S. Coşkun, Antonio Cubillo Gracian, Toshimi Takano, Rachel Wong, Howard Safran, Gina M. Vaccaro, Zev A. Wainberg, Huiling Xiong, Janet Hong, Julien Taieb, Yung-Jue Bang
Data analysis and interpretation: Markus Moehler, Mikhail Dvorkin, Narikazu Boku, Mustafa Özgüroğlu, Hasan S. Coşkun, Rachel Wong, Howard Safran, Gina M. Vaccaro, Matthew R. Silver, Huiling Xiong, Janet Hong, Julien Taieb, Yung-Jue Bang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Markus Moehler
Honoraria: Taiho Pharmaceutical, Eli Lilly/ImClone, Amgen, Roche/Genentech, Merck KGaA, Darmstadt, Germany, MSD Oncology, Bristol Myers Squibb, AstraZeneca/MedImmune, Servier
Consulting or Advisory Role: Bayer, Merck Sharp & Dohme, Merck KGaA, Darmstadt, Germany, Amgen, Taiho Pharmaceutical, Nordic Group, Pfizer, Yakult, Roche, Eli Lilly, Servier
Research Funding: Amgen (Inst), Leap Therapeutics (Inst), Merck KGaA, Darmstadt, Germany (Inst), Jennerex (Inst), AstraZeneca (Inst), Merck Sharp & Dohme (Inst)
Travel, Accommodations, Expenses: Amgen, Merck KGaA, Darmstadt, Germany, Roche, Bayer, American Society of Clinical Oncology, German Cancer Society, Merck Sharp & Dohme, European Society for Medical Oncology
Narikazu Boku
Honoraria: Taiho Pharmaceutical, Ono Pharmaceutical, Bristol Myers Squibb Japan
Research Funding: Bristol Myers Squibb (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst)
Mustafa Özgüroğlu
Honoraria: Astellas Pharma, Novartis, Janssen Oncology (Inst)
Consulting or Advisory Role: MSD Oncology, AstraZeneca
Speakers’ Bureau: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Min-Hee Ryu
Honoraria: Dae Hwa Pharmaceutical, Bristol Myers Squibb, Eli Lilly, Ono Pharmaceutical, Merck Sharp & Dohme, Taiho Pharmaceutical, Novartis
Consulting or Advisory Role: Dae Hwa Pharmaceutical, Bristol Myers Squibb, Eli Lilly, Ono Pharmaceutical, Merck Sharp & Dohme, Taiho Pharmaceutical, Novartis
Sara Lonardi
Consulting or Advisory Role: Amgen, Merck KGaA, Darmstadt, Germany, Eli Lilly, Servier
Speakers’ Bureau: Roche, Eli Lilly, Bristol Myers Squibb, Servier, Merck Serono
Research Funding: Amgen, Merck KGaA, Darmstadt, Germany
Hasan S. Coşkun
Consulting or Advisory Role: Nestle Health Science
Speakers’ Bureau: Eli Lilly, Astellas Pharma
Travel, Accommodations, Expenses: Pfizer, Amgen, Bristol Myers Squibb
Toshimi Takano
Honoraria: Daiichi Sankyo, Eisai, Pfizer, Eli Lilly, Chugai Pharma, Kyowa Hakko Kirin, Celltrion Healthcare
Research Funding: Taiho Pharmaceutical (Inst), Novartis (Inst), Ono Pharmaceutical (Inst), Merck Sharp & Dohme (Inst), Merck KGaA, Darmstadt, Germany (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Bristol Myers Squibb (Inst), Chugai Pharma (Inst), Kyowa Hakko Kirin (Inst)
Gina M. Vaccaro
Consulting or Advisory Role: Bayer, Exelixis, Taiho Pharmaceutical, AstraZeneca, Incyte, Amgen, Novartis, Celgene, Astellas Pharma, QED Therapeutics
Research Funding: Celgene, Merck Sharp & Dohme, Eli Lilly, Astellas Pharma, EMD Serono, Inc (an affiliate of Merck KGaA, Darmstadt, German), Incyte, Bristol Myers Squibb, Array BioPharma, Newlink Genetics, Polaris, Boston Scientific
Zev A. Wainberg
Consulting or Advisory Role: Array BioPharma, Five Prime Therapeutics, Novartis, Eli Lilly, Merck & Co, Merck KGaA, Darmstadt, Germany, Bristol Myers Squibb, Bayer, AstraZeneca/MedImmune, Ipsen, Macrogenics
Research Funding: Novartis (Inst), Plexxikon (Inst), Pfizer (Inst), Merck & Co (Inst), Five Prime Therapeutics (Inst)
Travel, Accommodations, Expenses: Eli Lilly, Merck & Co, Bayer
Matthew R. Silver
Employment: EMD Serono Research & Development Institute, Inc (an affiliate of Merck KGaA, Darmstadt, Germany)
Huiling Xiong
Employment: EMD Serono Research & Development Institute, Inc (an affiliate of Merck KGaA, Darmstadt, Germany)
Janet Hong
Employment: EMD Serono Research & Development Institute, Inc (an affiliate of Merck KGaA, Darmstadt, Germany), Alnylam (I)
Stock and Other Ownership Interests: EMD Serono, Alnylam (I), Bristol Myers Squibb, Sanofi
Julien Taieb
Consulting or Advisory Role: Roche, Merck KGaA, Darmstadt, Germany, Amgen, Celgene, Eli Lilly, Servier, Sirtex Medical, Merck Sharp & Dohme, Pierre Fabre
Speakers’ Bureau: Servier, Amgen, Roche/Genentech, Sanofi, Merck KGaA, Darmstadt, Germany, Eli Lilly, Merck Sharp & Dohme, Pierre Fabre
Yung-Jue Bang
Consulting or Advisory Role: Samyang, BeiGene, Green Cross, Taiho Pharmaceutical, Merck KGaA, Darmstadt, Germany, AstraZeneca/MedImmune, Novartis, MSD Oncology, Bayer, Hanmi, Genentech/Roche, Eli Lilly, Daiichi Sankyo, Astellas Pharma, Bristol Myers Squibb, Genexine, GlaxoSmithKline
Research Funding: AstraZeneca/MedImmune (Inst), Novartis (Inst), Genentech/Roche (Inst), Merck Sharp & Dohme (Inst), Merck KGaA, Darmstadt, Germany (Inst), Bayer (Inst), GlaxoSmithKline (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Eli Lilly (Inst), Boehringer Ingelheim (Inst), Macrogenics (Inst), Boston Biomedical (Inst), Five Prime Therapeutics (Inst), CKD (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), BeiGene (Inst), Curis (Inst), Green Cross (Inst), Daiichi Sankyo (Inst), Astellas Pharma (Inst), Genexine (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Leiting JL, Grotz TE: Advancements and challenges in treating advanced gastric cancer in the West. World J Gastrointest Oncol 11:652-664, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth EC Verheij M Allum W, et al. : Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v38-v49, 2016. (suppl 5) [DOI] [PubMed] [Google Scholar]
- 3.Muro K Lordick F Tsushima T, et al. : Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic oesophageal cancer: A JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 30:34-43, 2019 [DOI] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: Gastric cancer (version 4.2019). https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
- 5.Koizumi W Narahara H Hara T, et al. : S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol 9:215-221, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Lordick F Kang YK Chung HC, et al. : Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol 14:490-499, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Digklia A, Wagner AD: Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol 22:2403-2414, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner AD Syn NL Moehler M, et al. : Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 8:CD004064, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsina M Moehler M Hierro C, et al. : Immunotherapy for gastric cancer: A focus on immune checkpoints. Target Oncol 11:469-477, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Ciuleanu T Brodowicz T Zielinski C, et al. : Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet 374:1432-1440, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Coleman RL Oza AM Lorusso D, et al. : Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:1949-1961, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirza MR Monk BJ Herrstedt J, et al. : Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 375:2154-2164, 2016 [DOI] [PubMed] [Google Scholar]
- 13. Poveda A, Floquet A, Ledermann JA, et al: Final overall survival (OS) results from SOLO2/ENGOT-ov21: A phase III trial assessing maintenance olaparib in patients (pts) with platinum-sensitive, relapsed ovarian cancer and a BRCA mutation. J Clin Oncol 38, 2020 (suppl; abstr 6002)
- 14.Bang YJ Cho JY Kim YH, et al. : Efficacy of sequential ipilimumab monotherapy versus best supportive care for unresectable locally advanced/metastatic gastric or gastroesophageal junction cancer. Clin Cancer Res 23:5671-5678, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Meulendijks D de Groot JW Los M, et al. : Bevacizumab combined with docetaxel, oxaliplatin, and capecitabine, followed by maintenance with capecitabine and bevacizumab, as first-line treatment of patients with advanced HER2-negative gastric cancer: A multicenter phase 2 study. Cancer 122:1434-1443, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Eren OO Ozturk MA Sonmez OU, et al. : Safety, feasibility, and efficacy of capecitabine maintenance in patients with advanced gastric cancer: A retrospective study. Am J Ther 23:e1493-e1497, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Kang YK Boku N Satoh T, et al. : Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:2461-2471, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Bang YJ Kang YK Catenacci DV, et al. : Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: Results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 22:828-837, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. doi: 10.1001/jamaoncol.2018.0013. Fuchs CS, Doi T, Jang RW, et al: Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 4:e180013, 2018 [Erratum: JAMA Oncol 5:579, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muro K Chung HC Shankaran V, et al. : Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol 17:717-726, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Shitara K Özgüroğlu M Bang YJ, et al. : Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 392:123-133, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Gulley JL Rajan A Spigel DR, et al. : Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): Dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 18:599-610, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Disis ML Taylor MH Kelly K, et al. : Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase 1b results from the JAVELIN Solid Tumor trial. JAMA Oncol 5:393-401, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel MR Ellerton J Infante JR, et al. : Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 19:51-64, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Tourneau C Hoimes C Zarwan C, et al. : Avelumab in patients with previously treated metastatic adrenocortical carcinoma: Phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer 6:111, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keilholz U Mehnert JM Bauer S, et al. : Avelumab in patients with previously treated metastatic melanoma: Phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer 7:12, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan R Thomas A Nemunaitis JJ, et al. : Efficacy and safety of avelumab treatment in patients with advanced unresectable mesothelioma: Phase 1b results from the JAVELIN Solid Tumor trial. JAMA Oncol 5:351-357, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung HC Arkenau HT Lee J, et al. : Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: Phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer 7:30, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulangara K Zhang N Corigliano E, et al. : Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med 143:330-337, 2019 [DOI] [PubMed] [Google Scholar]
- 30. Hassan R, Thomas A, Nemunaitis JJ, et al: Avelumab in patients with previously treated mesothelioma: Updated phase 1b results from the JAVELIN Solid Tumor trial. J Clin Oncol 36, 2018 (suppl; abstr 166) [Google Scholar]
- 31. doi: 10.1001/jamaoncol.2020.3370. Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 Phase 3 randomized clinical trial. JAMA Oncol 6:1-10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bang YJ Ruiz EY Van Cutsem E, et al. : Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN Gastric 300. Ann Oncol 29:2052-2060, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For all new products or new indications approved in both the European Union and the United States after January 1, 2014, Merck KGaA, Darmstadt, Germany will share patient-level and study-level data after deidentification, as well as redacted study Protocols and clinical study reports from clinical trials in patients. These data will be shared with qualified scientific and medical researchers, upon researcher's request, as necessary for conducting legitimate research. Such requests must be submitted in writing to the company’s data sharing portal. More information can be found at https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. Where Merck KGaA has a co-research, co-development or co-marketing/co-promotion agreement or where the product has been out-licensed, it is recognized that the responsibility for disclosure may be dependent on the agreement between parties. Under these circumstances, Merck KGaA will endeavor to gain agreement to share data in response to requests.