Supplemental Digital Content is available in the text.
Keywords: Diabetes, Air pollution, Ozone, Fine particulate matter, African Americans
Background:
Diabetes is especially prevalent among African Americans. Prior studies suggest that long-term exposure to ambient air pollution may be associated with greater incidence of diabetes, but results remain heterogeneous. Few studies have included large numbers of African Americans.
Methods:
We assessed diabetes status and concentrations of 1- and 3-year fine particulate matter (PM2.5) and ozone (O3) among African American participants of the Jackson Heart Study at visits 1 (2000–2004, N = 5128) and 2 (2005–2008, N = 2839). We used mixed-effect modified Poisson regression to estimate risk ratios (RRs) and 95% confidence intervals (CIs) of incidence of diabetes by visit 2 and prevalence ratios (PRs) of the association between air pollution exposure and prevalent diabetes at visits 1 and 2. We adjusted for potential confounding by patient characteristics, as well as inverse probability weights of diabetes at visit 2, accounting for clustering by census tract.
Results:
We observed associations between incident diabetes and interquartile range increase in 1-year O3 (RR 1.34, 95% CI = 1.11, 1.61) and 3-year O3 (RR 0.88, 95% CI = 0.76, 1.02). We observed associations between prevalent diabetes and 1-year PM2.5 (PR 1.08, 95% CI = 1.00, 1.17), 1-year O3 (PR 1.18, 95% CI = 1.10, 1.27), and 3-year O3 (PR 0.95, 95% CI = 0.90, 1.01) at visit 2.
Conclusions:
Our results provide some evidence of positive associations between indicators of long-term PM2.5 and O3 exposure and diabetes. This study is particularly relevant to African Americans, who have higher prevalence of diabetes but relatively few studies of environmental pollution risk factors.
What this study adds.
Diabetes is a major health issue for African Americans; air pollution may affect whether a person has diabetes. We examined associations between long-term (1- and 3-year) exposures to fine particulate matter (PM2.5) and ozone (O3) with new and existing diabetes among 5,128 African Americans enrolled in the Jackson Heart Study. We observed associations between 1-year PM2.5 and O3 exposure and existing diabetes at visit 2, but only O3 was associated with new diabetes. Three-year O3 was inversely associated with diabetes. This study concurs with the few previous similar studies and is among the first to be conducted among African Americans.
Background
Diabetes is a major source of morbidity and mortality in the United States and is a risk factor for cardiovascular disease (CVD). Diabetes shares many risk factors with CVD, such as obesity, lack of physical activity, and poor diet.1 Diabetes is also more common among African Americans compared to European Americans; as of 2010, 13.5% of African-American men and 15.4% of African-American women have been diagnosed with diabetes, compared to 7.7% of European-American men and 6.2% of European-American women.1
Short- and long-term exposure to ambient air pollution, originating from vehicular and industrial sources, has been shown to increase risk of CVD, possibly via inflammatory and oxidative stress pathways.2 Long-term exposure to ambient air pollution may also influence diabetes risk through similar mechanisms.3,4 Recent reviews have identified long-term exposure to air pollutants, especially fine particulate matter (PM2.5) as being associated with prevalent and incident diabetes.5–9 However, several studies have failed to find an association between long-term exposure to fine particulate matter (PM2.5) and incident diabetes,4,10,11 or found associations between PM2.5 and diabetes prevalence, but not incidence.12 Others have found stronger associations in women compared to men.3,5 Most studies have been conducted using PM2.5 as a pollutant of interest; Jerrett et al and Renzi et al observed associations between incident diabetes and ozone (O3).11,13 Although African Americans are at high risk for diabetes, especially in the southern US,14 only two of the prior studies specifically examined the association between traffic-related pollution and diabetes in African-American women,4,13 and neither were specifically in the southern US. An increase of 10 µg/m3 PM2.5 concentration was associated with an IRR of 1.48 (95% confidence interval [CI] = 0.95, 2.31) for incident diabetes4 and an increase of 6.7 ppb O3 concentration was associated with an HR of 1.18 (95% CI = 1.04, 1.34) of incident diabetes.13
We examined the associations between indicators of long-term exposure to ambient air pollution and prevalence and incidence of diabetes among African Americans living in the southern state of Mississippi. Specifically, we estimated residential concentrations of fine particulate matter (PM2.5) and ozone (O3) and residential proximity to major roadways among African American participants in the Jackson Heart Study (JHS). We examined both prevalent and incident diabetes and estimated exposure for the 1 and 3 years before each study visit. JHS participants lived in the tri-county area of Jackson, Mississippi, an area that includes both urban and rural neighborhoods. Given the disproportionate burden of diabetes among African Americans, this study is important in understanding potential adverse health effects of environmental pollutants among this high-risk population.
Methods
Data source
The JHS is a prospective cohort study of the etiology of CVD, which included 5,301 African Americans 21–94 years of age at the time of recruitment (2000–2004) living in the tri-county Jackson, Mississippi Metropolitan Statistical Area, as previously described.15,16 This area includes Jackson, the capital and largest city in the state of Mississippi (population was 184,256 as of 2000, and approximately 70% of the population was African American [census.gov]). The JHS study area also includes surrounding urban and rural areas in Hinds, Madison, and Rankin counties. Traffic was the major source of ambient air pollution in this area.17 Upon enrollment (visit 1), participants completed an in-home interview and a clinic visit.15,16 Participants underwent an additional interview and clinic visit approximately 4 years (visit 2) after enrollment. All participants provided written informed consent. Institutional Review Boards at the University of Mississippi Medical Center, Jackson State University, and Tougaloo College approved the JHS protocol. The Institutional Review Board at Indiana University approved the current analysis of de-identified data.
Exposure
Our primary exposures of interest were mean levels of PM2.5 and O3 1 and 3 years before visits 1 and 2 at participants’ ZIP codes of residence. These were based on 24-hour mean PM2.5 and 8-hour O3 concentrations, estimated at the census tract and aggregated to ZIP code. Our secondary exposure of interest was residential proximity to major roadways with Census Feature Class Code A1 (major roads with limited access, such as interstate highways) or A2 (major roads without limited access, such as US highways) roads.
We obtained geocoded address information from JHS participants for visits 1 and 2. We generated estimates of PM2.5 and O3 concentrations using the Bayesian space-time downscaling fusion modeling approach that was developed by the U.S. Environmental Protection Agency and its partners. Model development, background, and initial evaluation of the downscaling model have been published previously.18–20 Briefly, this technique uses numerical output from the CMAQ model, which uses meteorology, emissions, and chemical and physical interactions of pollutants to estimate pollutant concentration at specified grids. These estimates are then fused with data from national, state, and local monitoring networks. The downscaling framework generates predictions of O3 daily maximum 8-hour average ozone concentrations in parts per billion (ppb), and daily 24-hour average concentrations of PM2.5 at centroids of US Census tracts21; Census tracts are relatively fixed statistical subdivisions of a county, containing about 4,000 residents. We then applied geo-imputation approaches to convert daily, census tract level concentrations of PM2.5 and O3 to obtain 1- and 3-year mean PM2.5 and O3 concentrations at participants’ ZIP (postal) code of residence. ZIP codes are typically larger than Census tracts; JHS aggregates pollutant levels to ZIP codes due to confidentiality concerns.
For sensitivity analyses, we calculated the Euclidian distance to roadways, defined as US Census feature class A1 and A2. We categorized distance to A1 or A2 roads as <150 m, 150–299 m, 300–999 m, and ≥1,000 m. We used the cutpoints of 150 and 300 m because particulate matter concentration from highway traffic pollution has been shown to decrease by 50% at 150 m and to fade to background level after 300 m.22 We included an additional cutpoint of 1,000 m so our results may be comparable to reference categories in several previous articles.23–26 We also examined distance to A1 or A2 roadways as a log-transformed continuous outcome.
Outcome
For all analyses, we defined diabetes as fasting blood glucose ≥126 mg/dL, HbA1c ≥6.5%, or use of diabetes medications (insulin or oral diabetes medications) at a particular visit.27 Participants were asked to bring in all medications used within the previous 2 weeks, which were then catalogued by trained research staff, and participants answered questions regarding medication use. Incident diabetes was defined as new cases of diabetes at visit 2 among participants without diabetes at visit 1. Prevalent diabetes was defined as diabetes at a given visit regardless of previous diabetes status.
Covariates
We included the following potential confounders in the associations between air pollution exposure indicators and diabetes: age, sex, body mass index (BMI; continuous), smoking status (never, former, current), education (less than high school, high school/GED, college degree/certificate, graduate/professional school), sleep score, likely occupational exposure to air pollution (farming, production, construction, or military considered likely occupational exposures), neighborhood socioeconomic status (SES, continuous z-score), physical activity (poor, moderate, ideal), nutritional status (poor, moderate, ideal), family history of diabetes, and year of examination. We created a modified version of the Pittsburgh Sleep Quality Index,28 using sleep quantity (number of hours of sleep per day) and quality (snoring, stopping breathing during sleep, and overall sleep quality on a scale of 1–5) to create a sleep score for each visit. Neighborhood SES (NSES) was defined based on census-tract level data from the US Census in 2000 as described in Dubowitz et al29 and was converted to a z-score, as described by Diez Roux et al30 and evaluated as a continuous variable. Physical activity and nutritional status were defined as poor, moderate, or good, based on Life’s Simple Seven criteria.31,32 Although geographic information was not available, we used an indicator variable indicating census tract to account for clustering by census tract. Age, BMI, sleep score, and NSES were available at both visits; all other covariates were only available at visit 1. We adjusted for covariates at the most recent relevant visit that they were available.
Statistical analyses
We excluded participants for whom we were not able to determine diabetes status at visit 1 (n = 66) (Figure 1). Exposure data were only available beginning in 2001; we excluded those for whom we could not calculate exposure data due to date of enrollment or inability to geocode address (n = 113). For analyses at visit 2, we excluded those who were lost to follow-up (n = 1101), who were missing information on diabetes status (n = 861), or for whom we could not assign exposure data (n = 568). For analyses on incident diabetes at visit 2, we excluded 739 participants with diabetes at visit 1. Participants could be excluded for more than one reason.
Figure 1.
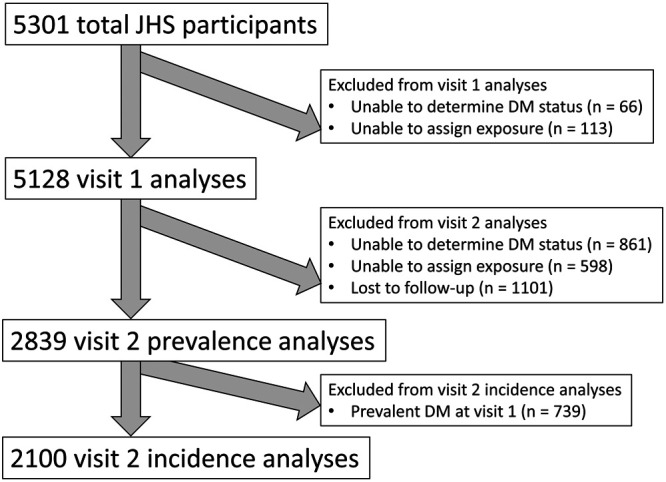
Flow diagram showing exclusion criteria and numbers of JHS participants in each analysis. Participants could be excluded for more than one reason.
First, we calculated the Pearson correlation between PM2.5 and O3 concentrations to contextualize results from different pollutants. For all other analyses, we used mixed-effects modified Poisson regression with robust standard errors33 to directly estimate the cumulative prevalence ratio (PR) (at each visit) and cumulative risk ratio (RR) of incident diabetes (visit 2 only) and indicators of exposure to ambient air pollution, accounting for clustering by census tract. In primary analyses, we used interquartile range (IQR) change in 1-year and 3-year PM2.5 and O3 concentrations as our exposures of interest. To correct for potential selection bias at visit 2 due to loss to follow-up between visits 1 and 2, we adjusted full visit 2 models for inverse probability weights of diabetes given following visit 1 variables that were different between those missing and not missing diabetes status at visit 2: diabetes status at visit 1, age, BMI, smoking status, nutrition status, education, chronic kidney disease, hypertension, family history of diabetes, and NSES. We also used mixed-effect models to account for clustering by census tract. We present results from four models: (1) unadjusted, accounting for clustering by census tract; (2) adjusted for anthropometric and health covariates (age, sex, BMI, smoking status, sleep score, occupational exposure to air pollution, physical activity, nutritional status, family history of diabetes, and year of examination) and accounting for clustering by census tract; (3) adjusted for model 2 covariates plus socioeconomic covariates (education and NSES) and inverse probability weight of diabetes for visit 2 analyses; and (4) adjusted for model 2 covariates plus additional pollutants (adjustment for O3 where PM2.5 was exposure of interest and vice versa).
Sensitivity analyses
In sensitivity analyses, we used residential proximity to A1 or A2 roads (in categories of <150 m, 150–299 m, 300–999 m, and ≥1,000 m, as well as a natural log-transformed continuous variable) as our exposure of interest. Second, as many participants were missing diabetes status at visit 2, we compared descriptive characteristics of those missing and not missing diabetes status at visit 2. Third, we plotted 1-year and 3-year PM2.5 and O3 concentrations by year to examine potential temporal trends. Fourth, as previous studies have reported differential associations between air pollution exposure and diabetes among men and women,3,5 we report associations stratified by sex. Fifth, as one-time fasting blood glucose or HbA1c may overestimate diabetes, we have conducted a sensitivity analysis limiting our definition of diabetes to those taking antidiabetic medication.
Results
For analyses using PM2.5 and O3 as exposures of interest, we analyzed 5,128 participants with complete PM2.5, O3, and diabetes information at visit 1 and 2,839 at visit 2. Table 1 shows descriptive characteristics of participants by diabetes status at visit 1. Those with diabetes tended to be older, more likely to be female, had higher BMI, were more likely to be physically inactive, and were more likely to have a family history of diabetes. Other characteristics were comparable across visit 1 diabetes status. Prevalence of diabetes was 21.8% at visit 1 and 33.2% at visit 2. Among participants free of diabetes at visit 1, 12.5% developed diabetes by visit 2 (Table 2).
Table 1.
Descriptive characteristics of JHS participants at visit 1 by diabetes status (n = 5128).
| Diabetes at visit 1 (n = 1,117) | No diabetes at visit 1 (n = 4,011) | Total (N = 5,128) | |
|---|---|---|---|
| Mean (SD) or n (%) | Mean (SD) or n (%) | ||
| Age (years) | 60.6 (10.6) | 53.8 (13.0) | 55.2 (12.8) |
| Female | 736 (65.9%) | 2,513 (62.7%) | 3,249 (63.4%) |
| Mean BMI (kg/m2) | 34.2 (7.2) | 31.1 (7.1) | 31.8 (7.3) |
| Smoking status | |||
| Never | 726 (65.3) | 2,738 (68.9) | 3,464 (68.1) |
| Former | 264 (23.7) | 687 (17.3) | 951 (18.7) |
| Current | 122 (11.0) | 547 (13.8) | 669 (13.2) |
| Education | |||
| Less than high school | 247 (22.8%) | 733 (19.2%) | 980 (20.0%) |
| High school/GED | 407 (37.6%) | 1,411 (37.0%) | 1,818 (37.1%) |
| College | 252 (23.3%) | 1,056 (27.7%) | 1,308 (26.7%) |
| Graduate/professional school | 178 (16.4%) | 619 (16.2%) | 797 (16.3%) |
| Sleep score | |||
| Excellent | 69 (6.2%) | 298 (7.5%) | 367 (7.2%) |
| Good | 572 (51.7%) | 2,066 (52.1%) | 2,638 (52.0%) |
| Fair | 414 (37.4%) | 1,450 (36.5%) | 1,864 (36.7%) |
| Poor | 52 (4.7%) | 154 (3.9%) | 206 (4.1%) |
| Occupational exposurea | 266 (23.8) | 827 (20.6) | 1,093 (21.3) |
| NSES | −0.56 (5.0) | −0.16 (7.1) | −0.2 (5.0) |
| Nutritional status | |||
| Poor | 441 (43.3%) | 2,181 (60.2%) | 2,622 (56.5%) |
| Intermediate | 559 (54.9%) | 1,413 (39.0%) | 1,972 (42.5%) |
| Ideal | 19 (1.9%) | 30 (0.8%) | 49 (1.1%) |
| Physical activity | |||
| Poor | 655 (58.7%) | 1,869 (46.6%) | 2,524 (49.3%) |
| Intermediate | 311 (27.9%) | 1,306 (32.6%) | 1,617 (31.6%) |
| Ideal | 150 (13.4%) | 833 (20.8%) | 983 (19.2%) |
| Family history of diabetes | 766 (68.6%) | 1,870 (46.6%) | 2,636 (51.4%) |
aOccupational exposure to air pollution includes those with occupations in farming, production, construction, or military.
Table 2.
Distribution of exposures and outcomes of participants in the JHS at visit 1 (2000–2004) and visit 2 (2005–2008).
| Visit 1 (N = 5,128) | Visit 2 (N = 2,839) | |
|---|---|---|
| Median (IQR) or n (%) | Median (IQR) or n (%) | |
| Prevalence of diabetes | 1,117 (21.8%) | 943 (33.2%) |
| Incidence of diabetesa | NA | 262 (12.5%) |
| 1-year PM2.5 concentration (µg/m3) | 12.2 (0.8) | 12.1 (0.8) |
| 3-year PM2.5 concentration (µg/m3) | 12.4 (0.4) | 12.3 (0.5) |
| 1-year O3 concentration (ppb) | 40.6 (2.3) | 40.8 (2.5) |
| 3-year O3 concentration (ppb) | 40.9 (1.07) | 42.2 (0.7) |
aIncidence defined as new cases of diabetes since prior visit among those at risk (without prevalent diabetes): visit 2 incidence calculated as 262 new cases of diabetes among 2,100 at risk, visit 3 calculated as 123 new cases among 1,605 at risk.
PM2.5 concentrations were slightly higher than the current Environmental Protection Agency’s National Ambient Air Quality Standard primary standard of 12 µg/m3 but below the standard at the time of the study of 15 µg/m3 (www.epa.gov/criteria-air-pollutants/naaqs-table). PM2.5 1-year and 3-year mean concentrations were similar between visits 1 (median 12.2 µg/m3, IQR = 0.8 and median 12.4 µg/m3, IQR = 0.4, respectively) and 2 (median 12.1 µg/m3, IQR = 0.8 and median 12.3 µg/m3, IQR = 0.5, respectively). One-year and 3-year O3 concentrations had medians of 40.6 (IQR = 2.3) and 40.9 (IQR = 1.1) ppb, respectively at visit 1, and had medians of 40.8 (IQR = 2.5) and 42.2 (IQR = 0.7) ppb at visit 2. All PM2.5 measures were moderately or strongly correlated with each other, as were visit 1 O3 1-year and 3-year concentrations. However, O3 concentrations at visit 2 were weakly correlated with those at visit 1. Three-year O3 concentrations at visit 2 were inversely correlated with PM2.5 concentrations (Table 3).
Table 3.
Correlation matrix for PM2.5 and O3 concentrations.
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | |||||
|---|---|---|---|---|---|---|---|---|
| 1-year mean PM2.5 | 3-year mean PM2.5 | 1-year mean PM2.5 | 3-year mean PM2.5 | 1-year mean O3 | 3-year mean O3 | 1-year mean O3 | 3-year mean O3 | |
| Visit 1 | ||||||||
| 1-year mean PM2.5 | 1.00 | |||||||
| 3-year mean PM2.5 | 0.86 | 1.00 | ||||||
| Visit 2 | ||||||||
| 1-year mean PM2.5 | 0.39 | 0.50 | 1.00 | |||||
| 3-year mean PM2.5 | 0.41 | 0.52 | 0.76 | 1.00 | ||||
| Visit 1 | ||||||||
| 1-year mean O3 | 0.12 | 0.050 | −0.085 | −0.094 | 1.00 | |||
| 3-year mean O3 | −0.064 | −0.028 | −0.14 | −0.12 | 0.94 | 1.00 | ||
| Visit 2 | ||||||||
| 1-year mean O3 | 0.075 | 0.19 | 0.64 | 0.18 | 0.0077 | −0.036 | 1.00 | |
| 3-year mean O3 | −0.32 | −0.36 | −0.20 | −0.12 | 0.15 | 0.21 | 0.056 | 1.00 |
We did not observe evidence of associations between PM2.5 or O3 concentrations and prevalent diabetes at visit 1 after adjustment for covariates (Table 4). At visit 2, in fully adjusted models (model 3) an IQR increase in 1-year PM2.5 concentration was associated with prevalence of diabetes (PR 1.08, 95% CI = 1.00, 1.17) and an IQR increase in 1-year O3 concentration was associated with increased prevalence (PR 1.18, 95% CI = 1.10, 1.27) of diabetes. Three-year O3 concentrations were inversely associated with diabetes (PR 0.95, 95% CI = 0.90, 1.01). An IQR increase in 1-year O3 concentration was associated with an increase in diabetes incidence (RRadj 1.32, 95% CI = 1.08, 1.59); while 3-year O3 was inversely associated (RRadj 0.88, 95% CI = 0.76, 1.02). Results for O3 were consistent in model 4 after adjustment for PM2.5 concentrations, but results for PM2.5 were attenuated after adjustment for O3 concentrations.
Table 4.
Results from modified Poisson regression models of associations between IQRa increase in 1-year and 3-year mean PM2.5 and O3 concentrations and prevalence and incidence of diabetes among participants in the JHS at visits 1 and 2.
| PM2.5 | O3 | |||
|---|---|---|---|---|
| 1-year mean | 3-year mean | 1-year mean | 3-year mean | |
| Visit 1 | ||||
| Prevalence of diabetes (N = 5,128), PR (95% CI) | ||||
| Model 1b | 1.15 (1.07, 1.24) | 1.09 (1.04, 1.14) | 1.03 (0.99, 1.07) | 1.00 (0.98, 1.02) |
| Model 2c | 1.00 (0.92, 1.09) | 1.00 (0.95, 1.06) | 1.03 (0.99, 1.06) | 1.01 (1.00, 1.03) |
| Model 3d | 1.03 (0.94, 1.12) | 1.01 (0.96, 1.07) | 1.03 (1.00, 1.06) | 1.01 (1.00, 1.03) |
| Model 4e | 1.02 (0.93, 1.11) | 1.01 (0.95, 1.06) | 1.03 (1.00, 1.06) | 1.01 (1.00, 1.03) |
| Visit 2 | ||||
| Prevalence of diabetes (N = 2,839), PR (95% CI) | ||||
| Model 1b | 1.26 (1.16, 1.36) | 1.06 (0.99, 1.13) | 1.31 (1.19, 1.44) | 0.87 (0.81, 0.93) |
| Model 2c | 1.14 (1.04, 1.23) | 0.99 (0.93, 1.05) | 1.21 (1.11, 1.32) | 0.92 (0.86, 0.98) |
| Model 3d | 1.08 (1.00, 1.17) | 0.98 (0.91, 1.05) | 1.18 (1.10, 1.27) | 0.95 (0.90, 1.01) |
| Model 4e | 0.98 (0.91, 1.07) | 0.98 (0.91, 1.05) | 1.20 (1.09, 1.31) | 0.95 (0.90, 1.01) |
| Incidence of diabetes (N = 2,100), RR (95% CI) | ||||
| Model 1b | 1.12 (0.93, 1.35) | 1.01 (0.88, 1.17) | 1.28 (1.06, 1.54) | 0.89 (0.79, 0.99) |
| Model 2c | 1.07 (0.89, 1.28) | 0.96 (0.85, 1.09) | 1.30 (1.08, 1.57) | 0.88 (0.76, 1.01) |
| Model 3d | 1.09 (0.90, 1.32) | 0.97 (0.86, 1.10) | 1.32 (1.08, 1.59) | 0.88 (0.76, 1.02) |
| Model 4e | 0.96 (0.86, 1.08) | 0.96 (0.81, 1.13) | 1.34 (1.11, 1.61) | 0.88 (0.76, 1.01) |
aIQRs are as follows: Visit 1—0.83 µg/m3 for 1-year PM2.5, 0.39 µg/m3 for 3-year PM2.5, 2.28 ppb for 1-year O3, 1.07 ppb for 3-year O3; Visit 2—0.81 µg/m3 for 1-year PM2.5, 0.47 µg/m3 for 3-year PM2.5, 2.54 ppb for 1-year O3, 0.66 ppb for 3-year O3.
bModel 1 unadjusted, accounting for clustering by census tract.
cModel 2 adjusted for age, sex, BMI, smoking status, sleep score, occupational exposure to air pollution, physical activity, nutritional status, family history of diabetes, and year, accounting for clustering by census tract.
dModel 3 adjusted for Model 2 covariates plus education, NSES, and inverse probability weight of diabetes given diabetes status at visit 1 (visit 2 only), accounting for clustering by census tract.
eModel 4 adjusted for model 3 covariates plus other pollutant measure (models with PM2.5 concentrations as exposure of interest adjusted for O3 concentrations and vice versa), accounting for clustering by census tract.
In sensitivity analyses, we did not find evidence of associations between residential distance to A1 or A2 roads and prevalent or incident diabetes (Table S1; http://links.lww.com/EE/A126). Additional adjustment for potential confounders did not substantially change our estimates. We then compared characteristics of 861 participants missing diabetes status at visit 2 with those of 3,344 not missing diabetes status at visit 2. We observed that participants missing diabetes status at visit 2 had poorer health indicators, including older age at baseline, and were more likely to have chronic kidney disease and hypertension but were less likely to have diabetes at visit 1 (Table S2; http://links.lww.com/EE/A126). Compared to those with complete visit 2 information, those who were lost to follow-up also had poorer health indicators; they were more likely to have chronic kidney disease (10.9% compared to 4.6%) and a history of CVD (16.2% compared to 9.3%). They also were more likely to have had diabetes at visit 1 (26.3% compared to 20.6%) but had no significant differences in PM2.5 or O3 concentrations. We did not observe major temporal trends in 1-year or 3-year PM2.5 or O3 (Figure S1; http://links.lww.com/EE/A126).
In sex-stratified analyses, we did not observe any associations in adjusted models at visit 1 (Table S3; http://links.lww.com/EE/A126). At visit 2, women had an increased prevalence of diabetes per IQR increase in 1-year PM2.5 (PR 1.11, 95% CI = 1.02, 1.21), and per IQR increase in 1-year O3 (PR 1.20, 95% CI = 1.10, 1.31), and a decreased incidence of diabetes per IQR increase in 3-year O3 (RRadj 0.85, 95% CI = 0.76, 0.95). Men had an increased prevalence (PR 1.16, 95% CI = 1.02, 1.31) and incidence (RRadj 1.42, 95% CI = 1.07, 1.87) of diabetes per IQR increase in 1-year O3. When considering only those who used diabetic medications, prevalence was 15.2% at visit 1, 23.8% at visit 2, and incidence at visit 2 was 8.2%. Visit 1 results were similar to those from the main analysis, except slightly stronger associations between visit 1 diabetes prevalence and IQR increase in 1-year (PR 1.06, 95% CI = 1.01, 1.11) and 3-year O3 (PR 1.03, 95% CI = 1.01, 1.05) (Table S4; http://links.lww.com/EE/A126). Visit 2 prevalence results were somewhat attenuated, and incidence results were similar.
Discussion
In this prospective cohort study of exposure to air pollutants and diabetes among African Americans, we observed evidence of positive associations between 1-year O3 concentrations and incident and prevalent diabetes at visit 2. We also observed associations between 1-year PM2.5 concentrations and prevalent diabetes at visit 2. However, we observed inverse associations between 3-year O3 concentrations and prevalent diabetes at visit 2.
We observed a high prevalence of diabetes (21.8% at visit 1 and 33.2% at visit 2). The state of Mississippi is among the states with the highest prevalence of diabetes, and African-American Mississippians had an estimated prevalence of diabetes of 20.4% at the time of JHS.14 This is similar to the prevalence observed in this study at visit 1. diabetes prevalence increases with age, although prevalence at visit 2 in our study was higher than expected.
Although few studies examine O3 exposure in relation to diabetes, our results for 1-year O3 concentration and incident diabetes largely agree with previously published studies, although we observed a somewhat stronger magnitude. Similar to our results, Renzi et al11 observed associations between incident diabetes and O3 (HR for 10-ppb increase 1.01, 95% CI = 1.00, 1.02) in an Italian cohort. Likewise, Jerrett et al13 observed associations between O3 and incident diabetes (HR for IQR of 6.7 ppb increase 1.18, 95% CI = 1.04, 1.34) in a cohort of African American women. Prior studies examining associations between NO2, another traffic-related gaseous pollutant, and diabetes have had mixed results.3,34 However, we observed a possible inverse association between 3-year O3 concentration and diabetes prevalence at visit 2, and between 3-year O3 and incident diabetes among women. Three-year O3 concentrations were very weakly correlated with 1-year O3 concentrations and were inversely correlated with PM2.5 concentrations. It is possible that 3-year O3 concentration is not an accurate proxy for air pollution exposure, as it is temporally variable and may reflect other factors, such as meteorology or seasonality.
We did not observe associations between PM2.5 and incident diabetes, but we observed an association between PM2.5 concentrations and prevalent diabetes at visit 2. This was somewhat stronger for women in sensitivity analyses. One previous study on associations between PM2.5 and diabetes among African-American women observed associations between PM2.5 and diabetes incidence at concentrations that were considerably higher than those observed in the present study.4 It is possible effects are more pronounced at higher concentrations and in urban areas. The present study was conducted in a mixed urban/rural area with moderate PM2.5 concentrations and limited exposure variability. This may have hindered our ability to observe meaningful associations with PM2.5 levels. Prevalence results largely agree with previous studies, most of which have reported associations between PM2.5 and prevalence of diabetes.3,5–9,12 We did not observe associations at visit 1 after confounder adjustment and had substantial loss to follow-up at visit 2, especially among those with poorer health. Air pollution exposure is one of many potential contributors to diabetes; it is possible that many diabetes cases at visit 1 were due to other issues, including comorbidities, which may have attenuated observed associations.
In sensitivity analyses, we did not observe associations between residential distance to road and diabetes, as did Puett et al.10 However, their study only observed associations among those who lived <50 m from a road; we did not have a large enough number of participants living <50 m from a major roadway to consider that category in our analysis. Previous studies have observed positive associations between PM and diabetes prevalence12,35 and incidence.36 However, other studies have found null associations between PM and diabetes.4,10
The major important limitation of this study is that many participants were missing relevant data to establish diabetes status at visit 2 and many participants were lost to follow-up between the first and second visits. It is possible that those missing diabetes status at visit 2 and those lost to follow-up were more likely to have or develop diabetes compared to those with complete data at both visits. To compensate for this possible bias, we adjusted visit 2 models for inverse probability weights of diabetes given factors that vary between those missing and not missing diabetes status at visit 2. We were unable to distinguish between type 1 and type 2 diabetes, which may have different associations with air pollutants. However, type 2 diabetes makes up approximately 90%–95% of diagnosed diabetes in adults, so we assume our results reflect associations with type 2 diabetes.37 Additionally, there may be exposure misclassification. Participants may not spend all their time at their residences, exposures had low spatial resolution (ZIP code-level), approximately 18% of participants moved between visit 1 and visit 2, and we do not have residential history before enrollment in the JHS. These participants were assigned air pollution exposure at their visit 2 address. These are common issues in using residential measures of air pollution exposure, which are still the standard metric used in air pollution epidemiology, despite their potential for exposure misclassification. We also do not have information on the date of diagnosis of diabetes; air pollution exposure information may not be in the appropriate temporal window. PM2.5 and O3 may not be the only pollutants of interest. NO2 may also be associated with diabetes, as previously demonstrated.34 Components of PM2.5 may also be of interest. However, JHS restricts access to residential data to outside researchers, to protect participant confidentiality. Therefore, we were unable to examine additional pollutants at the time of publication. Additionally, there may be residual confounding due to factors such as traffic noise. We adjusted for sleep, which may be affected by noise, and also used distance to road as a proxy exposure metric of both noise and traffic-related pollution exposure. We did not observe associations between distance to road and diabetes prevalence or incidence; this indicates traffic noise is not a major contributor to diabetes in this population. Prevalence of diabetes was high at baseline, leading to relatively small population at risk. However, this was not an usually high prevalence for African Americans living in Mississippi. We also conducted a sensitivity analysis on those who had been prescribed diabetes medications, and are thus not likely to be false-positives and observed mainly attenuated results.
Conclusions
In this prospective cohort study of African-American adults in Jackson, Mississippi, we observed evidence of associations between 1-year O3 concentrations and incident diabetes at visit. We also observed associations between PM2.5 concentrations and prevalent diabetes at visit 2 and between 1-year O3 concentrations and prevalent diabetes at visit 2. Further research should investigate the associations between diabetes various pollutants, specifically with long-term O3 concentrations, in African-American populations.
ACKNOWLEDGMENTS
We wish to thank the staff and participants of the Jackson Heart Study.
Supplementary Material
Footnotes
Published online 22 April 2021
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I/HHSN26800001), and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I, and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD).
G.A.W. has received consulting income from the Health Effects Institute (Boston, MA) and serves as a visiting scientist at Google, LLC (Mountain View, CA). The other authors have no conflicts to report.
Datasets analyzed for this study are not publicly available as they contain protected medical information and personally identifiable information. Information on requesting data can be found here: https://www.jacksonheartstudy.org/Research/Study-Data/Data-Access (accessed October 19, 2020). Analytic code is available upon request from the corresponding author.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; the U.S. Department of Health and Human Services; or the U.S. Environmental Protection Agency.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013; 127:e6–e245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barclay J, Hillis G, Ayres J. Air pollution and the heart: cardiovascular effects and mechanisms. Toxicol Rev. 2005; 24:115–123 [DOI] [PubMed] [Google Scholar]
- 3.Brook RD, Jerrett M, Brook JR, Bard RL, Finkelstein MM. The relationship between diabetes mellitus and traffic-related air pollution. J Occup Environ Med. 2008; 50:32–38 [DOI] [PubMed] [Google Scholar]
- 4.Coogan PF, White LF, Jerrett M, et al. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012; 125:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eze IC, Hemkens LG, Bucher HC, et al. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect. 2015; 123:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Xu L, Shan Z, Teng W, Han C. Association between air pollution and type 2 diabetes: an updated review of the literature. Ther Adv Endocrinol Metab. 2019; 10:2042018819897046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F, Chen G, Huo W, et al. Associations between long-term exposure to ambient air pollution and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Environ Pollut. 2019; 252:1235–1245 [DOI] [PubMed] [Google Scholar]
- 8.Rao X, Montresor-Lopez J, Puett R, Rajagopalan S, Brook RD. Ambient air pollution: an emerging risk factor for diabetes mellitus. Curr Diab Rep. 2015; 15:603. [DOI] [PubMed] [Google Scholar]
- 9.Yang BY, Fan S, Thiering E, et al. Ambient air pollution and diabetes: a systematic review and meta-analysis. Environ Res. 2020; 180:108817. [DOI] [PubMed] [Google Scholar]
- 10.Puett RC, Hart JE, Schwartz J, Hu FB, Liese AD, Laden F. Are particulate matter exposures associated with risk of type 2 diabetes? Environ Health Perspect. 2011; 119:384–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renzi M, Cerza F, Gariazzo C, et al. Air pollution and occurrence of type 2 diabetes in a large cohort study. Environ Int. 2018; 112:68–76 [DOI] [PubMed] [Google Scholar]
- 12.Park SK, Adar SD, O’Neill MS, et al. Long-term exposure to air pollution and type 2 diabetes mellitus in a multiethnic cohort. Am J Epidemiol. 2015; 181:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerrett M, Brook R, White LF, et al. Ambient ozone and incident diabetes: a prospective analysis in a large cohort of African American women. Environ Int. 2017; 102:42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danaei G, Friedman AB, Oza S, Murray CJ, Ezzati M. Diabetes prevalence and diagnosis in US states: analysis of health surveys. Popul Health Metr. 2009; 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuqua SR, Wyatt SB, Andrew ME, et al. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005; 154 Suppl 6S6–S18 [PubMed] [Google Scholar]
- 16.Taylor HA, Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005; 154 Suppl 6S6–S4 [PubMed] [Google Scholar]
- 17.Yerramilli A, Dodla VB, Desamsetti S, et al. Air quality modeling for the urban Jackson, mississippi region using a high resolution WRF/Chem model. Int J Environ Res Public Health. 2011; 8:2470–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berrocal VJ, Gelfand AE, Holland DM. A spatio-temporal downscaler for output from numerical models. J Agric Biol Environ Stat. 2010; 15:176–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berrocal VJ, Gelfand AE, Holland DM. Space-time data fusion under error in computer model output: an application to modeling air quality. Biometrics. 2012; 68:837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrocal VJ, Gelfand AE, Holland DM. A bivariate space-time downscaler under space and time misalignment. Ann Appl Stat. 2010; 4:1942–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirabelli MC, Vaidyanathan A, Flanders WD, Qin X, Garbe P. Outdoor PM2.5, ambient air temperature, and asthma symptoms in the past 14 days among adults with active Asthma. Environ Health Perspect. 2016; 124:1882–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002; 52:1032–1042 [DOI] [PubMed] [Google Scholar]
- 23.Kirwa K, Eliot MN, Wang Y, et al. Residential proximity to major roadways and prevalent hypertension among postmenopausal women: results from the Women’s Health Initiative San Diego Cohort. J Am Heart Assoc. 2014; 3:e000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Eliot MN, Kuchel GA, et al. Long-term exposure to ambient air pollution and serum leptin in older adults: results from the MOBILIZE Boston study. J Occup Environ Med. 2014; 56:e73–e77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Eliot MN, Koutrakis P, et al. Ambient air pollution and depressive symptoms in older adults: results from the MOBILIZE Boston study. Environ Health Perspect. 2014; 122:553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wellenius GA, Boyle LD, Coull BA, et al. Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: results from the MOBILIZE Boston Study. J Am Geriatr Soc. 2012; 60:2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010; 33Suppl 1S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28:193–213 [DOI] [PubMed] [Google Scholar]
- 29.Dubowitz T, Ghosh-Dastidar M, Eibner C, et al. The Women’s Health Initiative: The food environment, neighborhood socioeconomic status, BMI, and blood pressure. Obesity (Silver Spring). 2012; 20:862–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001; 345:99–106 [DOI] [PubMed] [Google Scholar]
- 31.Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical activity and cardiovascular disease in African Americans in atherosclerosis risk in communities. Med Sci Sports Exerc. 2013; 45:901–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010; 121:586–613 [DOI] [PubMed] [Google Scholar]
- 33.Petersen MR, Deddens JA. A comparison of two methods for estimating prevalence ratios. BMC Med Res Methodol. 2008; 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coogan PF, White LF, Yu J, et al. Long term exposure to NO2 and diabetes incidence in the Black Women’s Health Study. Environ Res. 2016; 148:360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care. 2010; 33:2196–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krämer U, Herder C, Sugiri D, et al. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect. 2010; 118:1273–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamin EJ, Virani SS, Callaway CW, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018; 137:e67–e492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


