PURPOSE
SEQUOIA compared efficacy and safety of adding pegilodecakin (PEG), a pegylated recombinant human interleukin (IL)-10, with folinic acid, fluorouracil, and oxaliplatin (FOLFOX) in patients following progression on first-line gemcitabine-containing therapy with metastatic pancreatic ductal adenocarcinoma (PDAC).
PATIENTS AND METHODS
SEQUOIA, a randomized, global phase III study, compared FOLFOX with PEG + FOLFOX as second line in gemcitabine-refractory PDAC. Patients were randomly assigned 1:1 (PEG + FOLFOX:FOLFOX) and stratified by prior gemcitabine and region. Eligible patients had only one prior gemcitabine-containing treatment. Primary end point was overall survival (OS). Secondary end points included progression-free survival (PFS), response evaluation per Response Evaluation Criteria in Solid Tumor (RECIST) 1.1, and safety. Exploratory analyses included biomarkers related to immune activation.
RESULTS
Between March 1, 2017, and September 9, 2019, 567 patients were randomly assigned PEG + FOLFOX (n = 283) or FOLFOX (n = 284). Most (94.7%) patients received prior gemcitabine plus nab paclitaxel. OS was similar comparing PEG + FOLFOX versus FOLFOX (median: 5.8 v 6.3 months; hazard ratio = 1.045; 95% CI, 0.863 to 1.265). Also, PFS (median 2.1 v 2.1 months; hazard ratio = 0.981; 95% CI, 0.808 to 1.190) and objective response rate (4.6% v 5.6%) were similar between the treatment arms. Most common (≥ 35%) treatment-emergent adverse events in PEG + FOLFOX versus FOLFOX were thrombocytopenia (55% v 20%), anemia (40% v 16%), fatigue (61% v 45%), neutropenia (39% v 28%), abdominal pain (37% v 29%), nausea (45% v 41%), neuropathy (37% v 38%), and decreased appetite (35% v 31%). Exploratory analyses revealed increases in total IL-18, interferon (IFN)-γ, and granzyme B and decreases in transforming growth factor (TGF)-β with the addition of PEG.
CONCLUSION
PEG added to FOLFOX did not improve efficacy in advanced gemcitabine-refractory PDAC. Safety findings were consistent as previously observed from PEG with chemotherapy; toxicity was manageable and tolerable. Exploratory pharmacodynamic results were consistent with immunostimulatory signals of the IL-10R pathway.
INTRODUCTION
The treatment landscape for metastatic pancreatic cancer as outlined in ESMO and NCCN guidelines (2014-2016) has focused on chemotherapeutic agents such as gemcitabine-based combination with nab-paclitaxel or folinic acid, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) for first-line patients.1 The introduction of FOLFIRINOX in 20112 and nab-paclitaxel in 20133 as first-line treatment greatly improved survival of metastatic pancreatic cancer. For metastatic pancreatic cancer refractory to first-line gemcitabine chemotherapy, oxaliplatin or irinotecan (nanoliposomal irinotecan)–based therapy can be considered.1
CONTEXT
Key Objective
In patients with advanced gemcitabine-refractory pancreatic cancer, we assessed efficacy and safety of pegilodecakin (PEG), a pegylated recombinant human interleukin (IL)-10, used in combination with folinic acid, fluorouracil, and oxaliplatin (FOLFOX).
Knowledge Generated
This global phase III randomized study was powered to detect overall survival (OS) and assess safety. The results showed no OS improvement by adding PEG to FOLFOX. No new safety signals were observed. Exploratory analyses were consistent with immunostimulatory signals of IL-10R pathway with a significant increase in interferon (IFN)-γ, granzyme B, and IL-18 compared with baseline in the experimental arm (PEG + FOLFOX) not observed in the control arm (FOLFOX).
Relevance
SEQUOIA is the largest randomized study that confirmed acceptable safety profile and provided immune biomarker information to improve understanding of this first-in-class agent, PEG in pancreatic cancer. The lack of OS benefit underscores the need for further translational efforts to better select patients who are likely to respond in this immune-refractory tumor.
As second-line therapy in gemcitabine-refractory pancreatic ductal adenocarcinoma (PDAC), folinic acid, fluorouracil, and oxaliplatin (FOLFOX) has demonstrated acceptable tolerability and clinical benefit.4-7 A meta-analysis of six studies (n = 258) with FOLFOX in patients with pancreatic cancer demonstrated similar efficacy with a median overall survival of 6.3 months.8 Despite the progress observed with FOLFOX and irinotecan, there still remains a need for additional therapeutic options for patients with metastatic pancreatic cancer, such as immunotherapies.
Advances have been made with checkpoint inhibitors such as CD40,9 PD-1,10 and CTLA-411 in a variety of solid tumors; however, this approach has not been successful in PDAC, except for the rare MSI-high cases, almost exclusively in patients with Lynch syndrome.12,13 Functional characterization of interleukin (IL)-10 has revealed its direct regulation of MHC class II antigens and the growth of T-cells, B-cells, and mast cells.14 Previous studies have demonstrated that increased IL-10 can result in T-cell-mediated tumor rejection.15 Preclinical experience with pegylated recombinant murine IL-10 (PEG-rMuIL-10) led to expansion of tumor-specific intratumoral CD8+ T-cells in mice and directly enhanced the cytotoxic activity of CD8+ T-cells in vitro.16
Early trials with a PEGylated recombinant IL-10 termed pegilodecakin (PEG-hIL-10) in patients with advanced solid tumors demonstrated signs of antitumor activity, with PEG monotherapy providing lasting partial responses in 27% of these heavily pretreated patients with renal cell carcinoma (RCC).17 In phase I study IVY, PEG revealed a tolerable safety profile with single-agent activity in RCC and uveal melanoma.18 A different cohort of IVY demonstrated that PEG + FOLFOX exhibited clinical activity in patients with gemcitabine-refractory metastatic PDAC, with objective response of three (15.8%) out of 19 patients, including two (10.5%) complete responses (CRs), and 1-year and 2-year survival rates of 43% and 28.8%, respectively.19 Grade ≥ 3 thrombocytopenia (56%) and anemia (44%) were managed by dose modification.19
The immune activation previously observed across tumor types and promising results in PDAC led to further investigation of PEG with FOLFOX in patients with metastatic PDAC in this randomized, global, phase III trial. Here, we report the results of SEQUOIA, which compared efficacy and safety of PEG + FOLFOX versus FOLFOX in inoperable gemcitabine-refractory PDAC.
PATIENTS AND METHODS
Patients
The study was conducted across 133 study sites by 143 oncology physicians. Approximately 566 patients were randomly assigned 1:1 ratio (PEG + FOLFOX:FOLFOX). Eligible patients were male or nonpregnant, nonlactating female of age ≥ 18 years with metastatic pancreatic adenocarcinoma in addition to histological diagnosis of pancreatic adenocarcinoma or histological or cytological diagnosis of adenocarcinoma consistent with pancreas origin in conjunction with either the presence of a mass in the pancreas or a history of pancreatic adenocarcinoma, measurable disease by Response Evaluation Criteria in Solid Tumor (RECIST) v.1.1, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and documented tumor progression during or following gemcitabine-containing treatment of metastatic disease. Chemotherapy or investigational therapy must be completed at least 2 weeks before random assignment, including recovery from toxicity to grade 1 or baseline. With the exception of gemcitabine-containing regimen, no prior therapy was permitted. Patients with pancreatic islet neoplasm, acinar cell carcinoma, nonadenocarcinoma, adenocarcinoma of the biliary tree, or cystadenocarcinoma and intolerance of gemcitabine-containing therapies (ie, < 8 weeks of treatment) were excluded. Patients had adequate organ function by hematological laboratory assessments at baseline (platelets ≥ 100 × 109/L, hemoglobin ≥ 9.0 g/dL, and absolute neutrophil count ≥ 1.5 × 109/L). All patients completed an informed consent form. Human investigations were performed in accordance with the principles outlined in the Good Clinical Practice (GCP) guidelines of the International Conference on Harmonisation (ICH), the Declaration of Helsinki, and local ethical and regulatory requirements.
Treatment
Patients were randomly assigned 1:1 to PEG + FOLFOX or FOLFOX. Random assignment was stratified according to region (NA, EU, and others) and prior therapy (gemcitabine-containing therapy). Patients randomly assigned to PEG + FOLFOX received PEG subcutaneous (SQ) dose at days (D)1-5 (rest on D6 and 7), D8-12 (rest on 13 and 14) plus FOLFOX regimen (2-hour infusion of dl-leucovorin [400 mg/m2] plus oxaliplatin [85 mg/m2] followed by bolus 5-FU [400 mg/m2] and 46- to 48-hour infusion 5-FU [2,400 mg/m2]) initiated on D1 of 14-day cycle for up to 12 cycles or until progressive disease (PD) by RECIST v.1.1. PEG was administered at two fixed doses (0.4 mg [patients weighing ≤ 80 kg] and 0.8 mg [patients weighing > 80 kg]) on the basis of phase I findings.18 Patients on PEG + FOLFOX were permitted to continue maintenance PEG therapy at higher dose after FOLFOX discontinuation in the absence of tumor progression. Patients randomly assigned to FOLFOX started on D1 of a 14-day cycle for up to 12 cycles or until PD by RECISTv1.1. Crossover to PEG + FOLFOX was not permitted.
Assessments
Tumor response images in CT scans were assessed using RECISTv.1.1 guidelines and were performed at baseline, week 8 (± 3 days), and every 8 weeks (± 3 days) until PD by RECISTv.1.1. All CT and MRI scans were collected prospectively and submitted to a central imaging reader for archiving and storage, allowing possible further review at the end of the study at the request of Eli Lilly and Company. Since pseudoprogression may be experienced by patients in immune-oncology treatments, patients on PEG + FOLFOX had a radiographic scan 4 weeks after PD to determine if tumor size had decreased or PD had continued. A confirmatory scan was required when clinical deterioration was suspected to be PD. CA19-9 serum levels were evaluated over time to assess for changes in response to study treatment. Increased CA19-9 was not used as evidence for PD or removing patients from study treatment. Hematologic and blood chemistry laboratory tests were performed centrally on D1 of cycles 1-12, D13 of cycles 1, 2, and 4, and every 28D of remaining cycles. PEG pharmacokinetic (PK) samples were collected from patients randomly assigned to PEG + FOLFOX on D1 of cycles 1, 2, 3, and 5 and D13 of cycles 1, 2, and 4. AEs were recorded and graded according to the National Cancer Institute Common Terminology Criteria (NCI-CTCAE) version 4.03, evaluated at every patient visit from baseline until short-term follow-up, and characterized by severity.
End Points
Primary end point was overall survival (OS). Secondary end points included progression-free survival (PFS), disease control rate, duration of response by RECISTv.1.1, and safety. Exploratory end points included analyses of biomarkers related to tumor response, immune activation, and clinical efficacy outcome. Circulating immune-related proteins were assessed by Nexelis using immunoassay performed on serum samples. The T-cell Receptor (TCR) repertoire was assessed by short-read RNA sequencing performed by Omniseq using blood samples collected in Paxgene RNA tubes.
Statistical Analysis
Primary end point, OS, was evaluated using a log-rank test stratified by region and prior therapy in the intent-to-treat (ITT) population. Final analysis was planned at 393 OS events, which provided approximately 85% power, assuming an OS hazard ratio (HR) of 0.74 and two-sided α = .05 to detect superiority of PEG + FOLFOX. One efficacy interim analysis was planned at approximately 70% of final events. PFS was assessed by investigator and summarized in the same manner as OS. Objective response rate was summarized and compared using a Cochran-Mantel-Haenszel two-sided test stratified by region and prior therapy. Unless otherwise noted, all hypothesis tests were performed at two-sided 0.05 level, and all confidence intervals used a 95% CI. The safety and exposure were assessed in all patients who received any amount of study drug (safety population). PK analyses were conducted for patients who received at least one dose of investigational product and had samples collected. An independent data review committee reviewed the efficacy interim data and safety data periodically. SAS (v9.4 or later; SAS Institute, Cary, NC) was used for statistical analyses. Translational research (TR) analyses were based on the subset of patients from the ITT population of whom a valid baseline and on-treatment time point cytokines' result had been obtained (translational research [TR] population). Unstratified Cox regression analysis was performed to assess the treatment effect for each subgroup in the treatment arm compared with the control arm.
RESULTS
Baseline Demographic and Clinical Characteristics
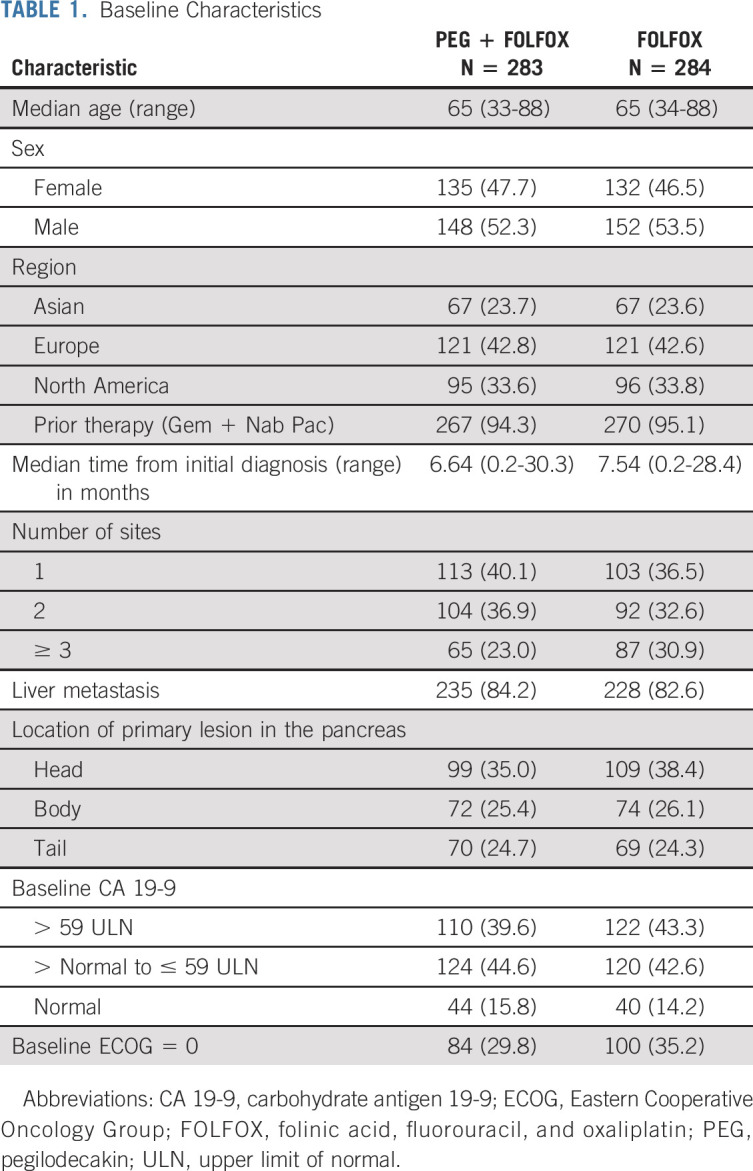
Five hundred sixty-seven patients were randomly assigned between March 1, 2017, and September 9, 2019. Two hundred eighty-three patients were randomly assigned to PEG + FOLFOX and 284 patients to FOLFOX, and 529 patients received any study treatment (Fig 1). There were 229 patients who were screened but not randomly assigned to treatment. All patients had ECOG 0 or 1. The median patient age was 65 years, and a majority of patients (94.7%) received prior gemcitabine plus nab-paclitaxel. All baseline disease characteristics were well-balanced between the arms (Table 1).
FIG 1.
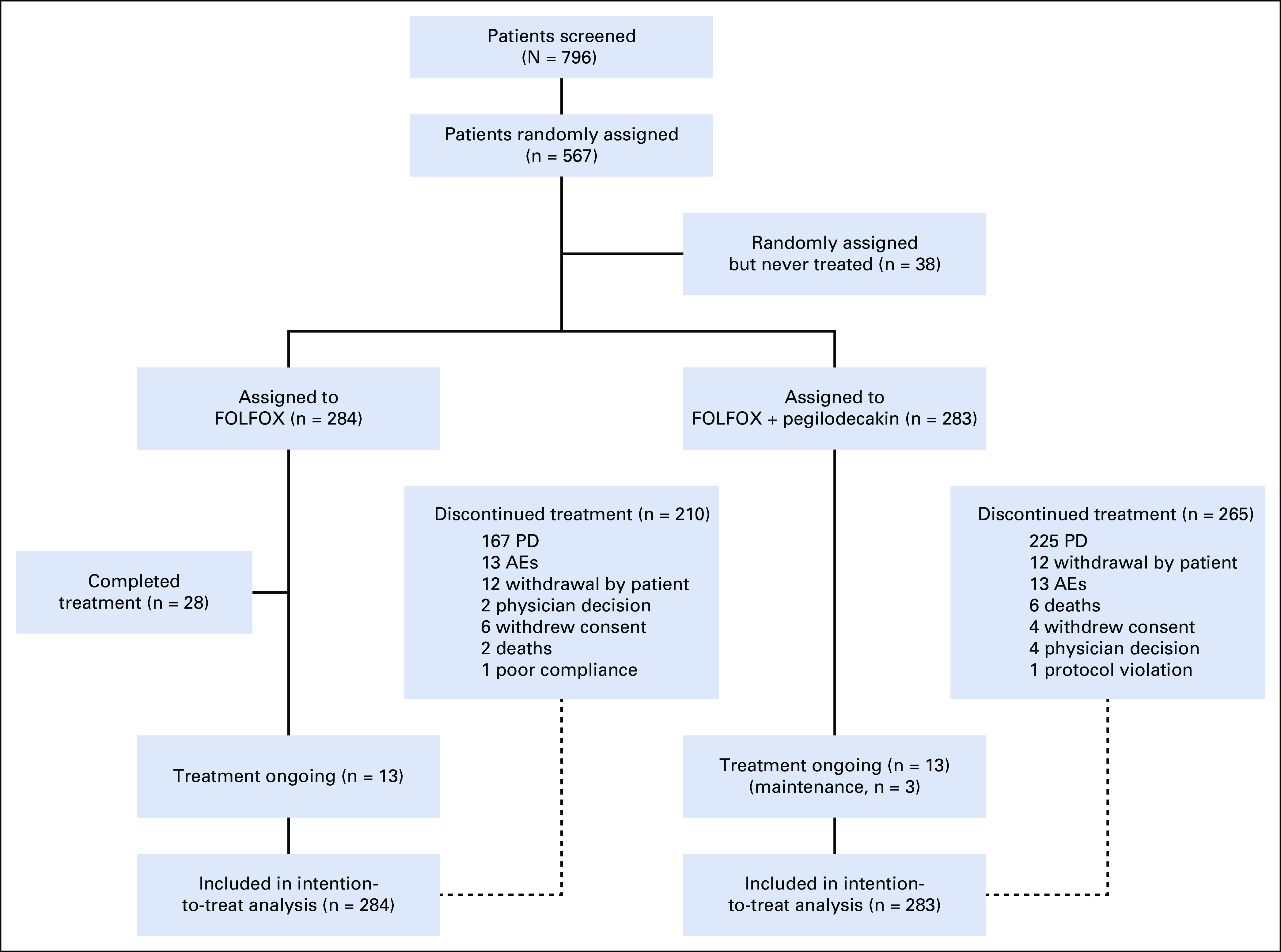
CONSORT diagram. This profile of the SEQUOIA trial depicts the schema of the trial including random assignment and patient disposition. Thirty-six of 40 patients who received maintenance therapy had received 12 cycles of FOLFOX treatment (all components or partial components). The remaining four patients received 5, 9, 10, and 11 cycles of FOLFOX treatment, respectively. AE, adverse event; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; PD, progressive disease.
TABLE 1.
Baseline Characteristics
Efficacy
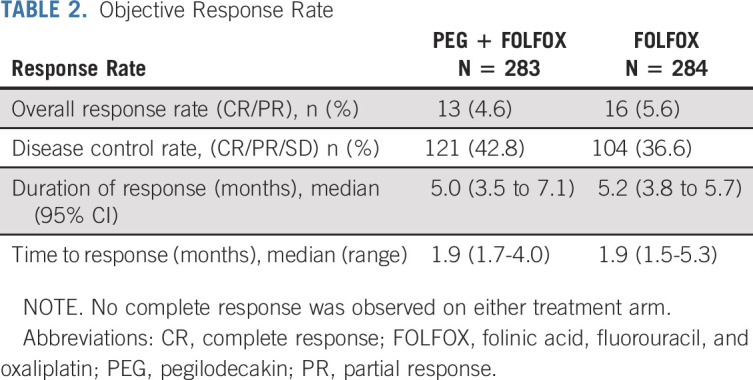
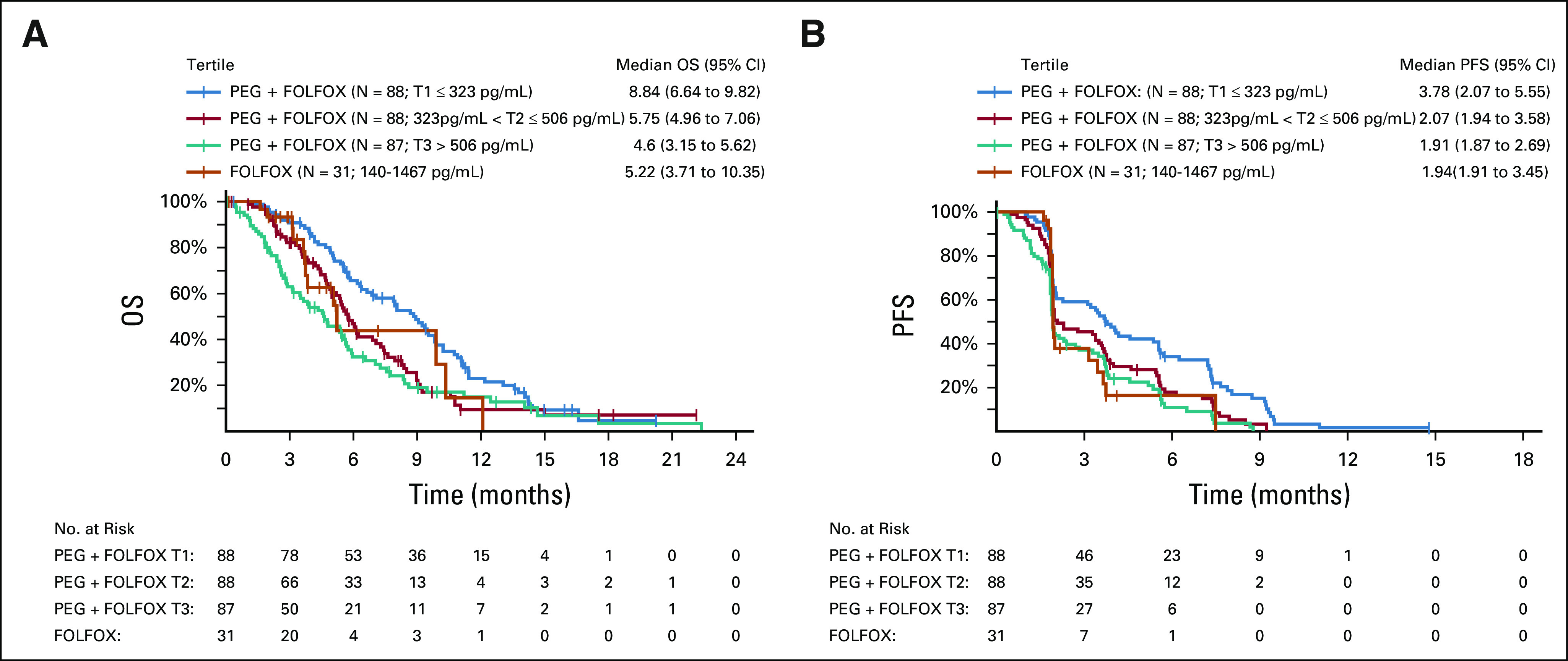
By the time of interim analysis (approximately 70% of final events), the Data Monitoring Committee recommended the study to continue without modification. Final OS was analyzed using cutoff date of September 9, 2019. In ITT population, 431 OS events occurred (220 on PEG + FOLFOX and 211 on FOLFOX). The median follow-up was 15.0 months in the PEG + FOLFOX group and 14.5 months in the FOLFOX group. Median OS was similar between PEG + FOLFOX (5.8 months) and FOLFOX (6.3 months) with HR = 1.05 (95% CI, 0.86 to 1.27), and the estimated OS rate at 1-year per Kaplan-Meier analysis was 14.7% in the PEG + FOLFOX group and 19.1% in the FOLFOX group (Fig 2A). Subgroup analysis of OS using prespecified stratification factors was consistent with the overall population, with an expected range of variability observed across the subgroups (Fig 3). PFS by investigator revealed no meaningful difference between treatment arms. The median PFS was 2.1 months in both arms (HR = 0.98; 95% CI, 0.81 to 1.19; Fig 2B). The overall response rate (ORR) was 4.6% compared with 5.6% of PEG + FOLFOX and FOLFOX arms, respectively. No CRs were observed in either treatment arm. Duration of response and time to response were similar between the two arms (Table 2).
FIG 2.
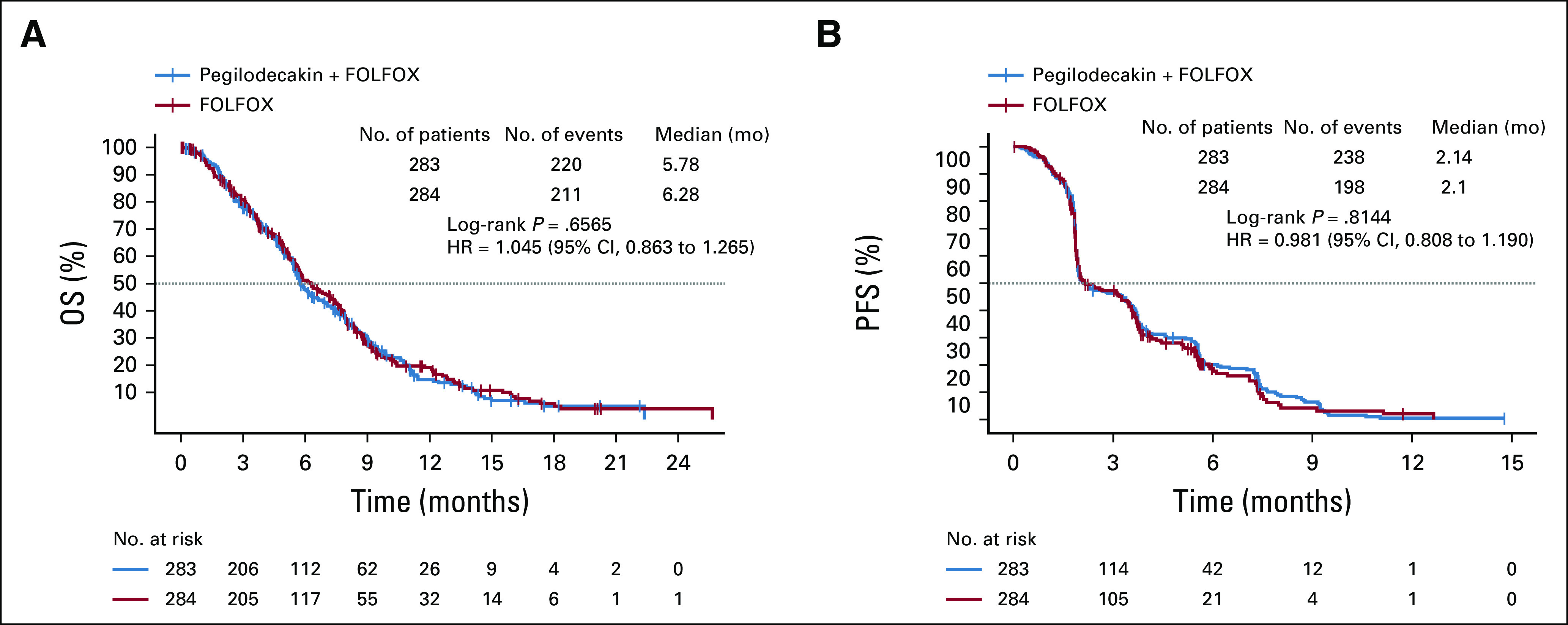
OS and PFS. (A) Kaplan-Meier plot compares OS in the ITT population between patients who received FOLFOX and patients who were provided FOLFOX with PEG. (B) Kaplan-Meier plot compares progression-free survival between patients who received FOLFOX and patients who were provided FOLFOX with PEG. FOLFOX, folinic acid, fluorouracil, and oxaliplatin; ITT, intent-to-treat; OS, overall survival; PEG, pegilodecakin; PFS, progression-free survival.
FIG 3.
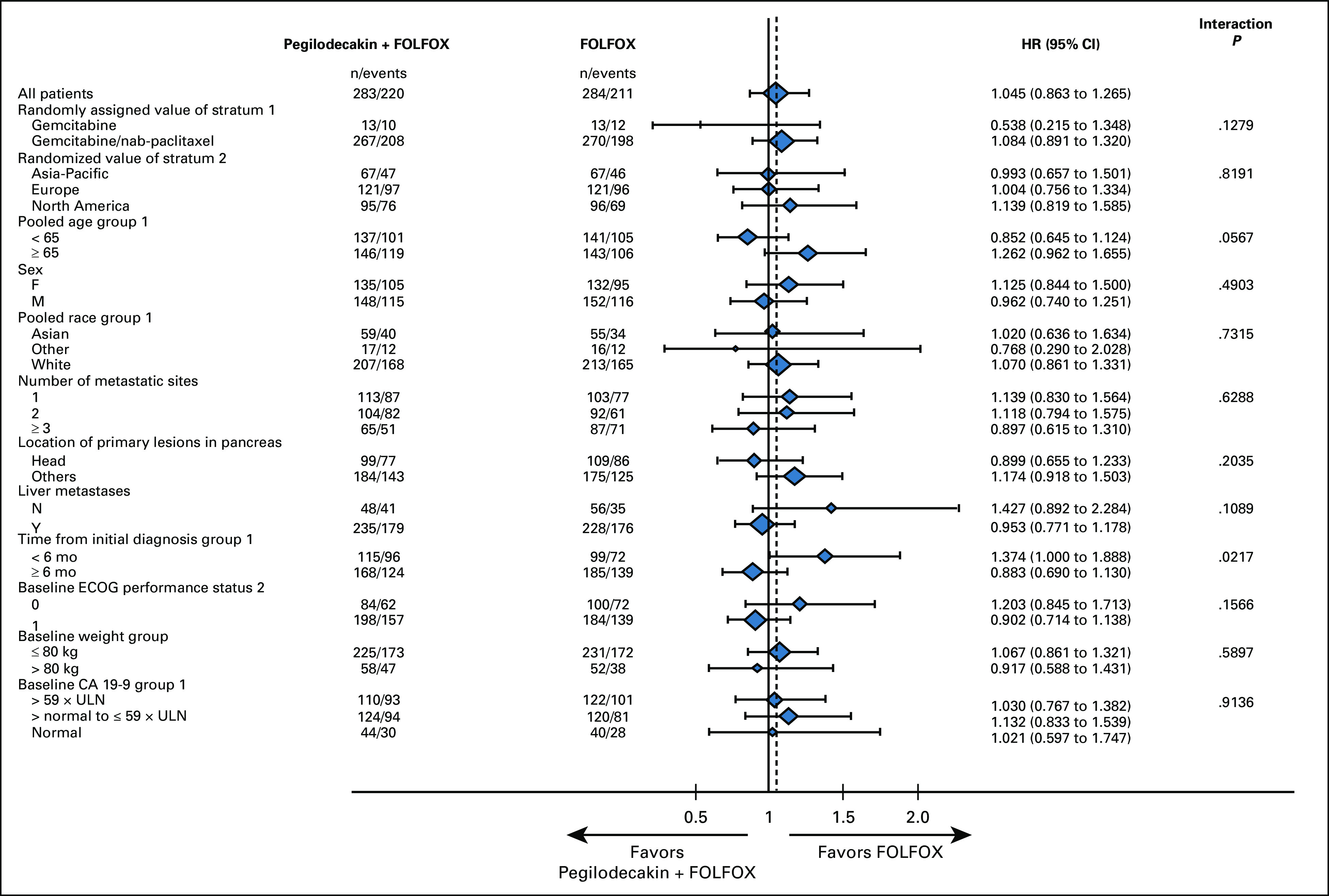
Prespecified subgroup analysis for OS. The forest plot depicts the different stratification factors used for OS subgroup analyses. CA 19-9, carbohydrate antigen 19-9; ECOG, Eastern Cooperative Oncology Group; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; OS, overall survival; ULN, upper limit of normal.
TABLE 2.
Objective Response Rate
Exposure to Treatment and Dose Intensity
For patients who received at least one dose of PEG, the median PEG treatment duration was 10 weeks (range: 6.7-21.3). The median relative dose intensity of PEG (prior to maintenance period) in patients ≤ 80 kg was 96.7% (IQR: 83.3 to 100.0), and in patients > 80 kg, it was 90.5% (IQR: 75.0 to 100.0) (Appendix Table A1, online only). Median relative dose intensity of each component of FOLFOX was similar between the arms (92.5%-100.0% [PEG + FOLFOX] and 98.6%-100.0% [FOLFOX]). This indicates that the addition of PEG did not cause a notable reduction in FOLFOX intensity (Appendix Table A2, online only).
Patient Disposition
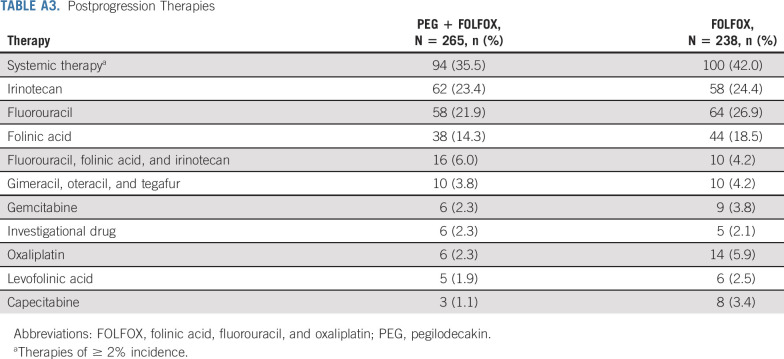
At the time of cutoff, 265 patients on PEG + FOLFOX versus 210 on FOLFOX discontinued study treatment. The most common reason for treatment discontinuation was disease progression (clinical or radiological; 67.1% v 58.8%). AEs (3.9% v 4.6%) and deaths (2.1% v 0.7%) resulting in discontinuation of all treatment were low and similar between PEG + FOLFOX and FOLFOX treatment arms (Fig 1). Frequency of subsequent anticancer therapy was balanced between treatment arms. Overall, 36% of patients on PEG + FOLFOX versus 42% of patients on FOLFOX continued on other systemic therapies following treatment discontinuation. The most prominent postprogression therapies included fluorouracil (22% v 27%) and irinotecan (23% v 24%) on PEG + FOLFOX and FOLFOX, respectively (Appendix Table A3, online only).
Safety
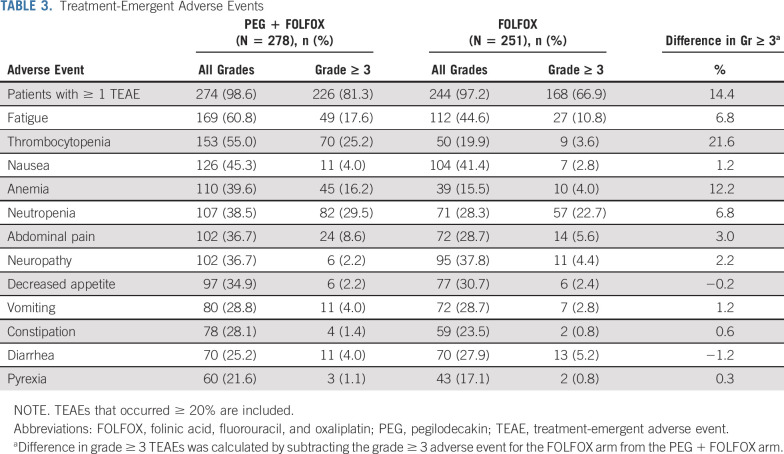
In the safety population, most common (≥ 35%) treatment-emergent adverse events (TEAEs) in PEG + FOLFOX versus FOLFOX were thrombocytopenia (55% v 20%), anemia (40% v 16%), fatigue (61% v 45%), neutropenia (39% v 28%), abdominal pain (37% v 29%), nausea (45% v 41%), neuropathy (37% v 38%), and decreased appetite (35% v 31%). TEAEs grade ≥ 3 occurred in 74.5% (394 of 529) of patients. Grade ≥ 3 AEs 5% or higher on PEG + FOLFOX compared with FOLFOX were thrombocytopenia (25.2% v 3.6%), anemia (16.2% v 4.0%), neutropenia (29.5% v 22.7%), and fatigue (17.6% v 10.8%; Table 3).
TABLE 3.
Treatment-Emergent Adverse Events
Grade ≥ 3 severe bleeding related to thrombocytopenia occurred in 0.7% of patients on PEG + FOLFOX. PEG + FOLFOX arm had a higher incidence of RBC transfusions compared with FOLFOX in relation to anemia (18.0% v 6.0%). Furthermore, anemia warranted the use of erythropoiesis-stimulating agents (ESAs) in both treatment arms (5.8% [PEG + FOLFOX] and 2.0% [FOLFOX]). One patient on PEG + FOLFOX required ESA and RBC transfusion (0.4% [PEG + FOLFOX]). Percentage of any grade and grade ≥ 3 potential immune-related AEs (irAEs) that led to high-dose corticosteroid use in the PEG + FOLFOX arm was similar to that of the FOLFOX arm (any grade: 3.6% v 3.2%; grade ≥ 3: 1.8% v 1.6%). Although a higher percentage of patients had neutropenia on PEG + FOLFOX, febrile neutropenia was observed with PEG + FOLFOX (1.4%) and FOLFOX (0.8%). Granulocyte colony stimulating factor (G-CSF) or granulocyte macrophage colony stimulating factor (GM-CSF) use was similar between PEG + FOLFOX and FOLFOX (10.4% v 11.6%).
Incidence of serious adverse events (SAEs) was numerically higher on PEG + FOLFOX versus FOLFOX (43.2% v 36.7%), with most frequent SAEs in PEG + FOLFOX compared with FOLFOX of pyrexia (4.7% v 2.8%), abdominal pain (4.3% v 2.0%), sepsis (3.6% v 1.6%), and vomiting (2.5% v 2.0%). Overall incidence of deaths because of an AE was low but increased in PEG + FOLFOX (6.8%) compared with FOLFOX (2.4%). There were 2 deaths because of AE (sepsis) on PEG + FOLFOX, which were deemed related to study treatment.
Dose Modifications
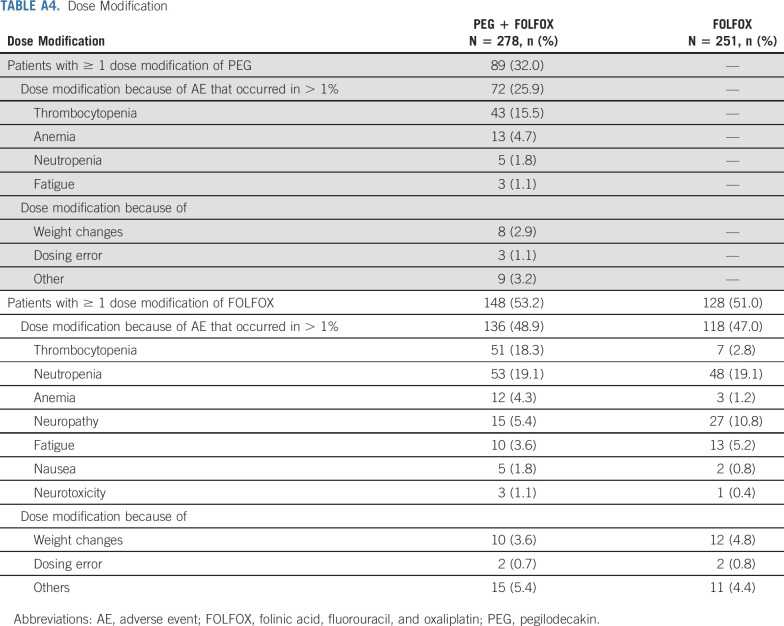
Dose adjustment of PEG is mostly PEG schedule reduction (schedule change, eg, from 5 days on, 2 days off to 4 days on, 3 days off) and dose omission. Of all PEG-treated patients, 32% experienced dose modification (Appendix Table A4, online only). Seventy-two (25.9%) patients had PEG dose modification because of AE. The most common AE (> 5%) that led to PEG dose adjustment was thrombocytopenia (15.5%). Of PEG-treated patients, 48.6% had PEG dose delay. The most common AEs that led to PEG dose delay included thrombocytopenia (18.7%), neutropenia (6.8%), fatigue (7.2%), and anemia (5.8%).
In the safety population, the percentage of patients with any dose level reduction of FOLFOX was comparable between the PEG + FOLFOX arm and the FOLFOX arm (53.2% v 51.0%). The percentage of patients with TEAEs that resulted in FOLFOX dose reductions was also comparable between PEG + FOLFOX and FOLFOX (48.9% v 47%). The most common TEAEs (> 5%) that led to FOLFOX reduction on PEG + FOLFOX versus FOLFOX were thrombocytopenia (18.3% v 2.8%) and neutropenia (19.1% v 19.1%; Appendix Table A4).
Exploratory Analyses and Immune Activation
PEG serum concentration (897 samples) was available for 238 patients. Majority (97%) were observations from 5 days on 2 days off schedule with only 27 (3% of total) and three (< 1% of total) observed samples from patients on 4 days on 3 days off and 3 days on 4 days off schedules, respectively. There were 743 (83%) and 148 (16%) observations following 0.4 and 0.8 mg doses, respectively. Higher trough mean concentrations were observed with 0.8 mg dose compared with 0.4 mg dose. A majority of observed PEG concentrations (73%) were above 1 ng/mL, which was previously identified as trough PEG concentration associated with activity.17 Observed PEG trough serum concentrations were higher in patients > 80 kg assigned to the higher dose (0.8 mg PEG). Increased PEG concentration (minimum concentration in steady state [Cmin, ss]: > 4.379 ng/mL) or increased exposure (steady-state area under the curve [AUCss]) in steady state did not improve survival probability (Appendix Fig A1, online only).
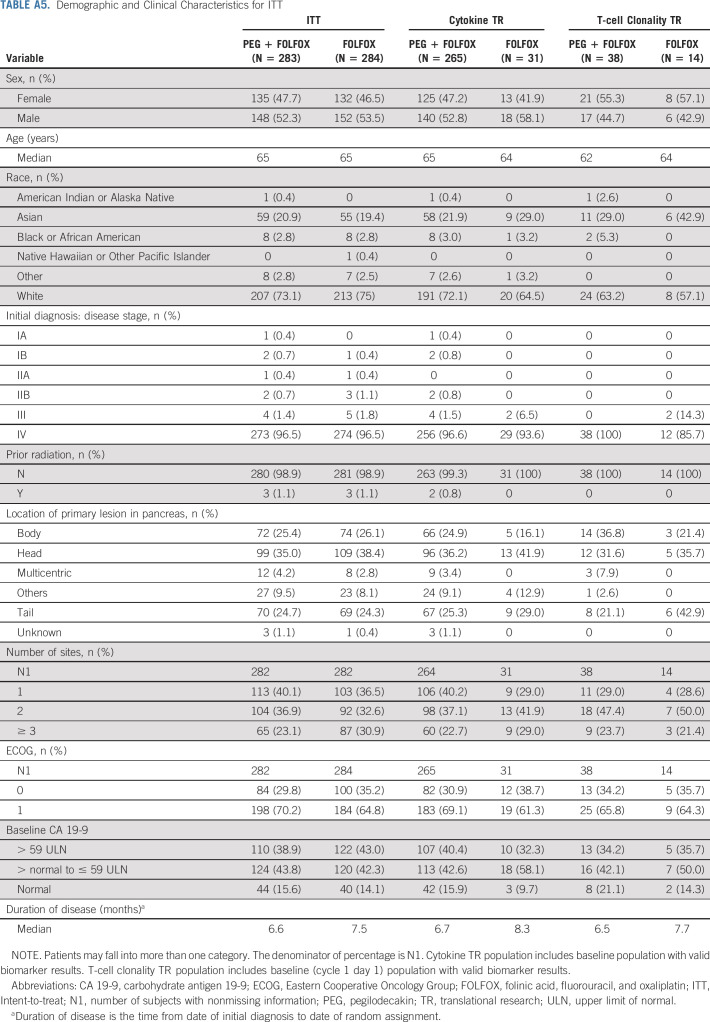
Biomarkers were analyzed for their potential association with immune activation, cytokine population described in Appendix Table A5 (online only) and clinical outcomes shown in Appendix Figure A2 (online only). Exploratory analysis was performed on levels of interferon gamma (IFN)-γ, granzyme B, total-IL-18, and transforming growth factor (TGF)-β (Fig 4). Cytokine levels were comparable between FOLFOX and PEG + FOLFOX at baseline assessment for all four tested cytokines. There was an increase from baseline in granzyme B, IFNγ, and IL-18, with PEG + FOLFOX at C1D13, C2D13, and C4D13, whereas no such change was observed with FOLFOX (Fig 4). There was a decrease in TGFβ with PEG + FOLFOX at C1D13, C2D13, and C4D13 (Fig 4D). Smaller decreases in TGFβ were observed with FOLFOX. Additionally, comparison of the biomarker fold-changes from baseline between treatment arms suggested an impact because of PEG's pharmacodynamics at both C1D13 and C2D13 in all cytokines. C2D13 fold-change of IL-18 was analyzed in 214 patients to assess its relationship to clinical outcome of PFS and OS (Appendix Fig A3, online only). Patients with the largest IL-18 fold-increases from baseline (third tertile, T3) had the longest OS and PFS times on the PEG arm. However, since there were samples from only 31 control arm patients, relationships by IL-18 fold-changes within the control arm could not be similarly assessed.
FIG 4.
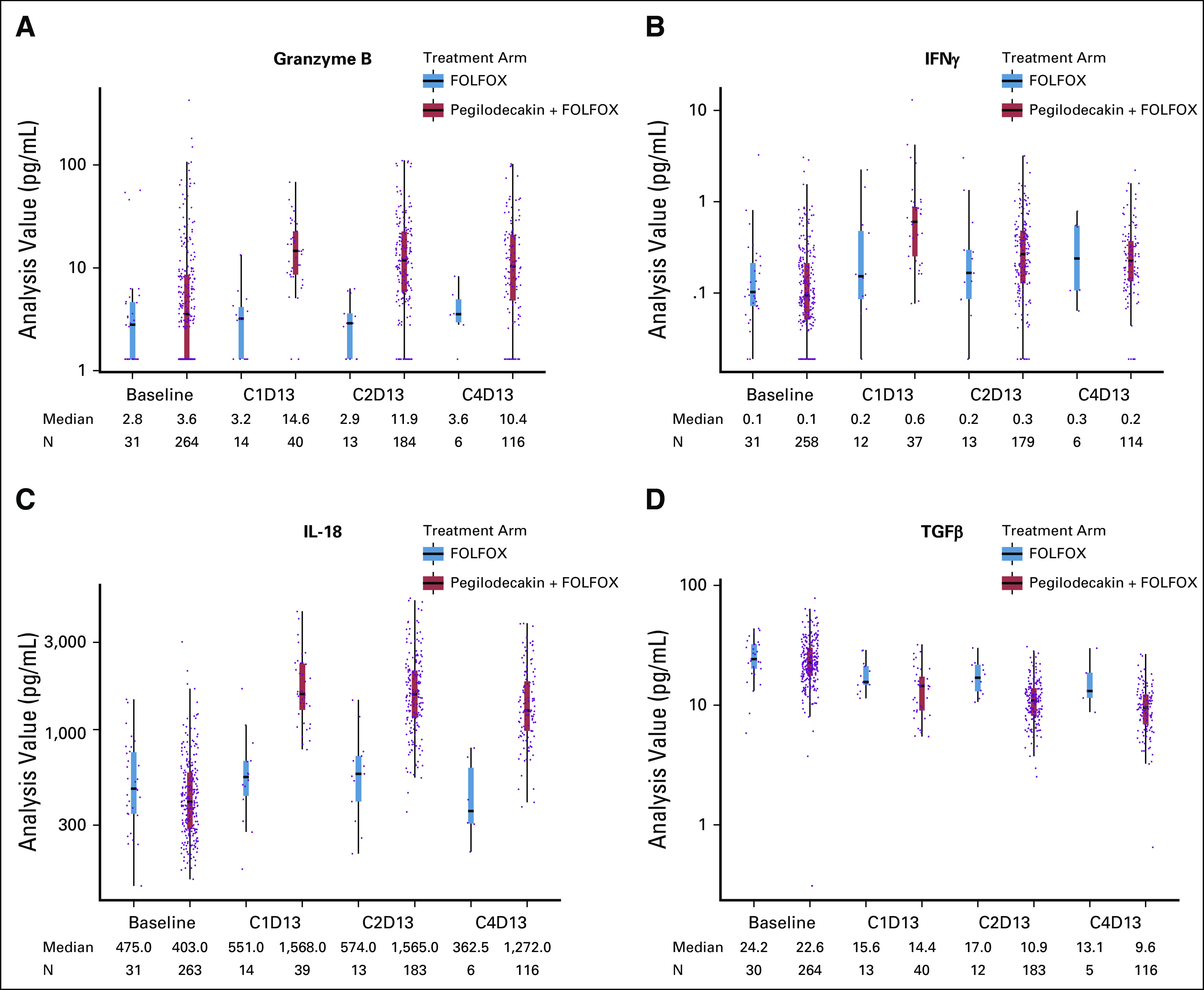
Cytokine expression. The boxplots depict the level of expression of the given cytokine at baseline, cycle 1 day 13 (C1D13), cycle 2 day 13 (C2D13), and cycle 4 day 13 (C4D13) for the control arm, FOLFOX, in gray and the experimental arm, PEG + FOLFOX, in red. Dots represent an individual patient sample, and the median is plotted for each category as the horizontal line on the bar and is listed below the figure. Circulating levels of granzyme B (A), IFNγ (B), IL-18 (C), and TGFβ (D) are shown. FOLFOX, folinic acid, fluorouracil, and oxaliplatin; IFN, interferon; IL, interleukin; PEG, pegilodecakin; TGF, transforming growth factor.
Baseline total-IL-18 was analyzed in 294 patients to assess its relationship to clinical outcome PFS and OS. The first tertile (T1; 1%-33% quantile) subgroup with the lowest total-IL-18 protein levels was associated with the longest OS (Appendix Fig A4A, online only) and PFS (Appendix Fig A4B). Since there were samples from only 31 control arm patients, relationships by baseline IL-18 levels within the control arm could not be similarly assessed.
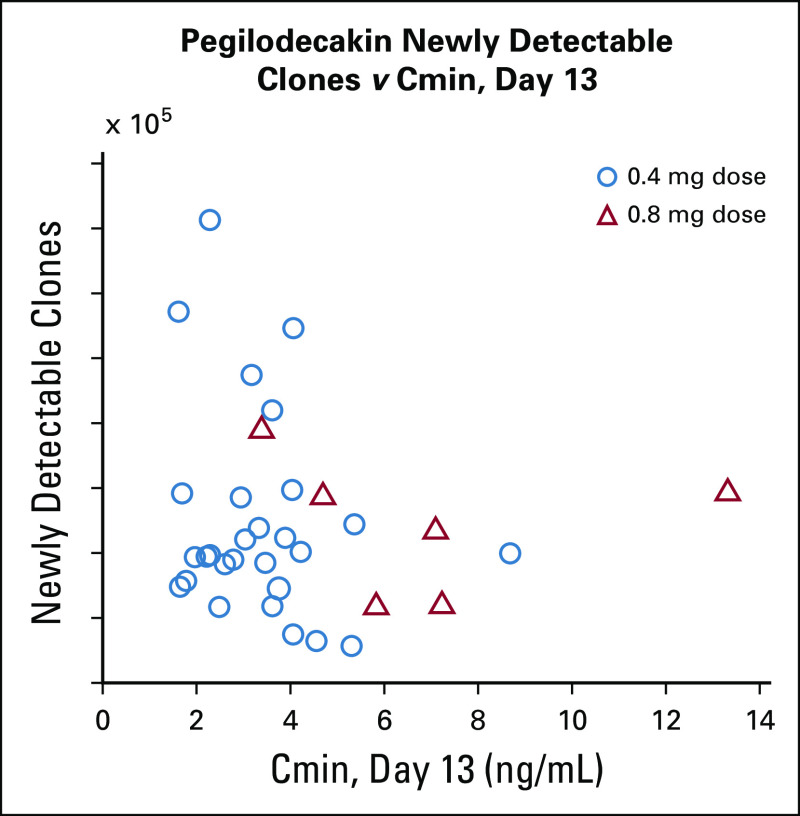
TCR clonality analysis was performed to evaluate newly detectable TCR clones that were below the level of detection by the assay at baseline, but were detectable after treatment initiation, TCR population described in Appendix Table A5. We were unable to demonstrate a relationship between the numbers of newly detectable clones at C2D13 and measured PEG serum concentrations (Appendix Fig A5, online only). There was a slight trend for greater numbers of newly detectable TCR clones observed on treatment in the experimental arm (PEG + FOLFOX) compared with the control arm (FOLFOX) at C2D13 and C4D13 (Appendix Fig A6A, online only). Higher numbers of newly detectable on-treatment clones appeared to be associated with the best overall response (Appendix Fig A6B) and longer PFS (Appendix Fig A6C) and OS (Appendix Fig A6D) in the experimental arm. Conclusions cannot be made in the FOLFOX arm because of the small patient population available for analysis.
DISCUSSION
Progress in treatment options for second-line gemcitabine-refractory metastatic PDAC remains poor. Despite promising safety and efficacy data from the phase I study IVY with PEG + FOLFOX,19 SEQUOIA results did not demonstrate improvement in OS, PFS, or ORR in this large, randomized, phase III trial. Meta-analysis of six different studies with 258 patients confirmed the benefit of FOLFOX regimen, the control arm of SEQUOIA, with an observed median overall survival of 6.3 months.8 To our knowledge, SEQUOIA remains the largest randomized phase III second-line metastatic PDAC study with the combination of FOLFOX as the control arm.20
Combination of PEG + FOLFOX demonstrated a safety profile consistent with that previously observed with PEG and FOLFOX combinations in patients with PDAC in the phase 1 study (IVY).19 However, overall toxicity was higher with the combination treatment than with FOLFOX alone. Grade ≥ 3 AEs that had an increased incidence of ≥ 5% with addition of PEG to FOLFOX included thrombocytopenia (25.2% v 3.6%), anemia (16.2% v 4.0%), neutropenia (29.5% v 22.7%), and fatigue (17.6% v 10.8%). Limited grade 3 and/or 4 irAEs were observed. The discontinuation rate for PEG because of AEs was low (4.6%), allowing for high relative dose intensity for PEG + FOLFOX. Exposure analysis revealed that attained doses were in excess of target nadir and higher PEG dose did not improve survival.
Previous analysis of 83 cytokines using multiplex panels demonstrated PEG's induction of markers of CD8+ T-cell immunity, with Th1 and Th2 upregulation, reduction of immune suppressive cytokine TGFβ, and direct activation of PD-1 + CD8+ T cells (assessed by granzyme B).21 Therefore, exploratory analysis in this study included Th1 cytokines IFNγ and IL-18, as well as TGFβ and granzyme B. Exploratory results of SEQUOIA were consistent with previously published findings for PEG,21 in line with immunostimulatory signals of IL-10R pathway, with increased IFNγ, IL-18, and granzyme B and decreased TGFβ in the experimental arm (PEG + FOLFOX) compared with the control arm (FOLFOX). Larger on-treatment fold increases from baseline in IL-18 levels correlated with better clinical outcomes in the PEG-treated patients. Because of the use of different assays and other factors, it cannot be determined with confidence whether the fold-changes in marker levels in the PEG-treated patients were similar in SEQUOIA compared with the earlier trial. Consistent with prognostic relationships previously reported in the literature,22-24 lower baseline IL-18 protein levels exhibited a possible correlation with OS.
TCR clonality analysis in this trial is unique, because it was performed in a relatively large number of patients from the experimental arm (PEG + FOLFOX; n = 36) and also included samples from the control arm (FOLFOX; n = 10). Assessment of newly detectable clones (not detected at baseline) after treatment initiation did identify relationships of interest, with increased on-treatment newly detectable clones observed in patients with better overall response or longer PFS and/or OS on the experimental arm (PEG + FOLFOX). It cannot be determined based on these data whether the newly detectable TCR sequences were actually present at baseline at undetectable levels in the peripheral blood and then clonally expanded to detectable levels on treatment or whether these receptor sequences only developed in the body after treatment initiation. Modeling was also performed using a small sample size (n = 46) to assess the relationship between PEG blood concentration and increased newly detectable T-cell clones. No clear associations were observed between study drug concentration (AUCss or Cmin) and newly detectable T-cell clones, which may be in part due to the nature of the clonal T-cell analysis. Recent findings in humans with cancer revealed that < 36% of the tumor-infiltrating lymphocyte (TIL) clonal sequences are detectable in the peripheral blood and nearly 12% of TIL sequences detected in the blood pretreatment were not detected in the tumor pretreatment,25 demonstrating some of the limitations of detectability and interpretation of the TCR sequencing. Therefore, examining T-cell clones in peripheral blood, without tissue biopsy, has limitations in that it only detects the effects of PEG on circulating T-cells and may not represent those which are truly TILs.
Safety and biological effects reported in SEQUOIA of the combination arm were consistent with prior PEG studies.17,18,21 Given the on-target toxicity of anemia and thrombocytopenia, elevation of immune biomarkers, and the attained PEG dose in excess of target nadir, stimulating the IL-10 pathway in combination with FOLFOX did not benefit second-line pancreatic cancer in this patient population. This global phase III SEQUOIA study did not demonstrate efficacy benefit for PEG in combination with FOLFOX in second-line metastatic PDAC.
ACKNOWLEDGMENT
Kristi Gruver, employee of Eli Lilly and Company, provided medical writing assistance. All writing assistance was funded by Eli Lilly and Company. We thank all the patients who contributed to this study and to all the staff who worked on this project.
APPENDIX
FIG A1.
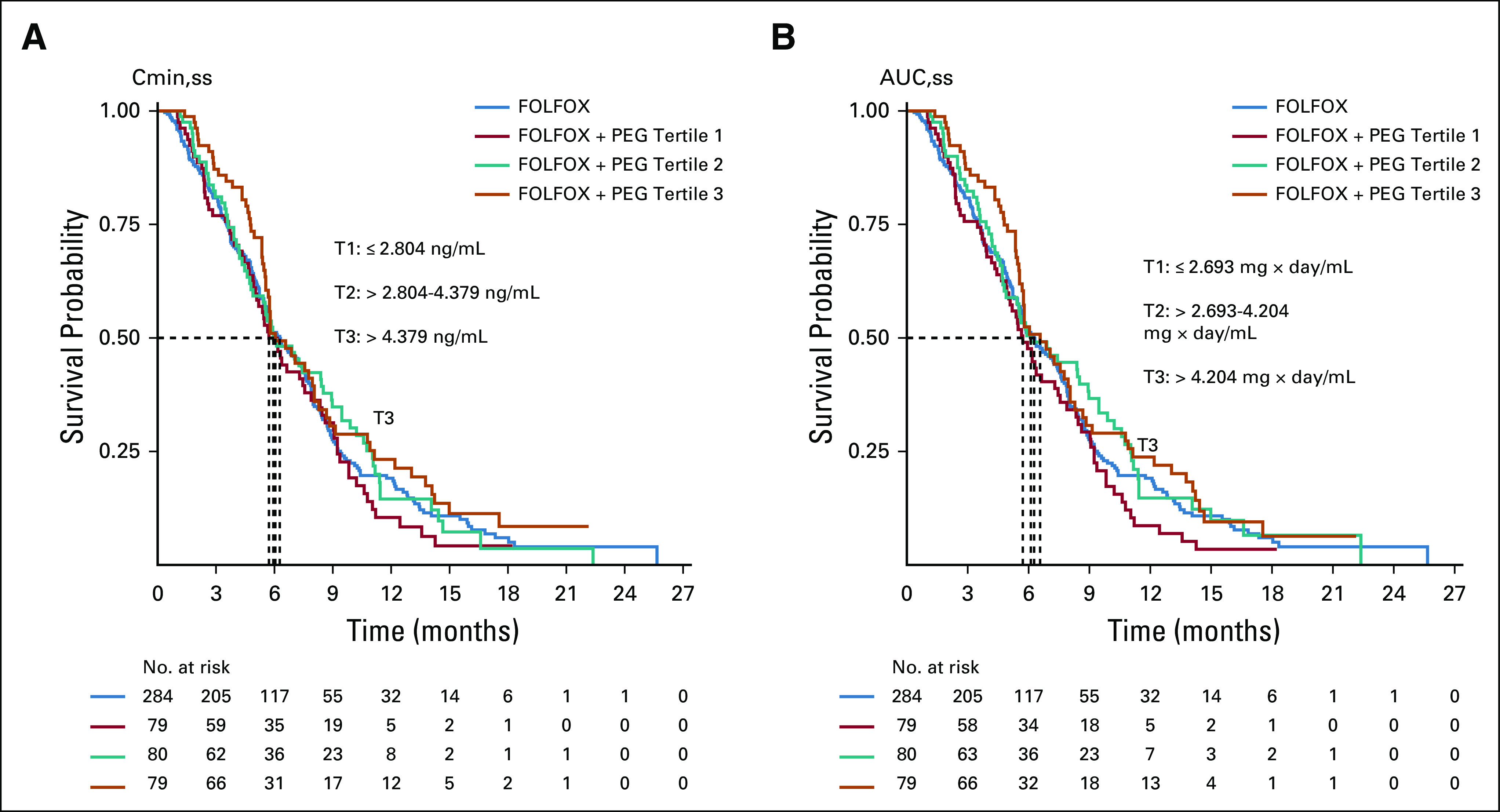
Kaplan Meier plot demonstrates no significant association between survival probability and pegilodecakin concentration (A) or exposure at steady state (B). FOLFOX, folinic acid, fluorouracil, and oxaliplatin; PEG, pegilodecakin.
FIG A2.
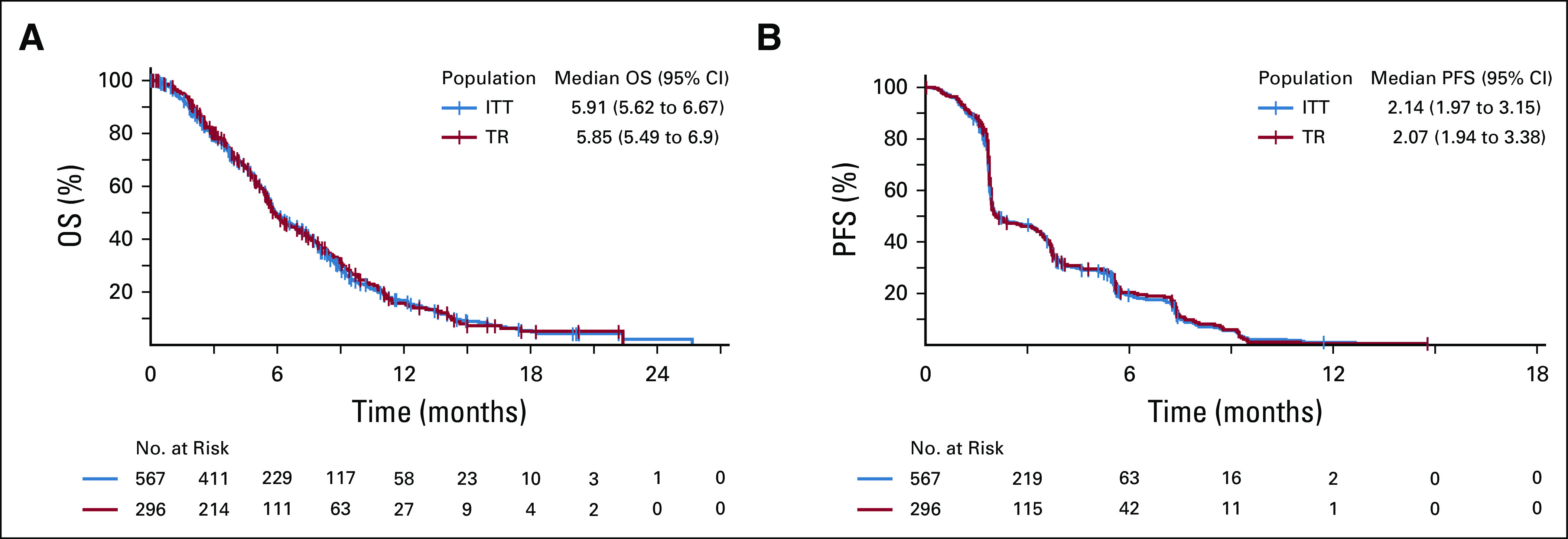
Kaplan-Meier plots of overall survival (A) and progression-free survival (B) for ITT population and Cytokine TR populations. ITT, intention to treat; OS, overall survival; PEG, pegilodecakin; PFS, progression-free survival; TR, translational research.
FIG A3.
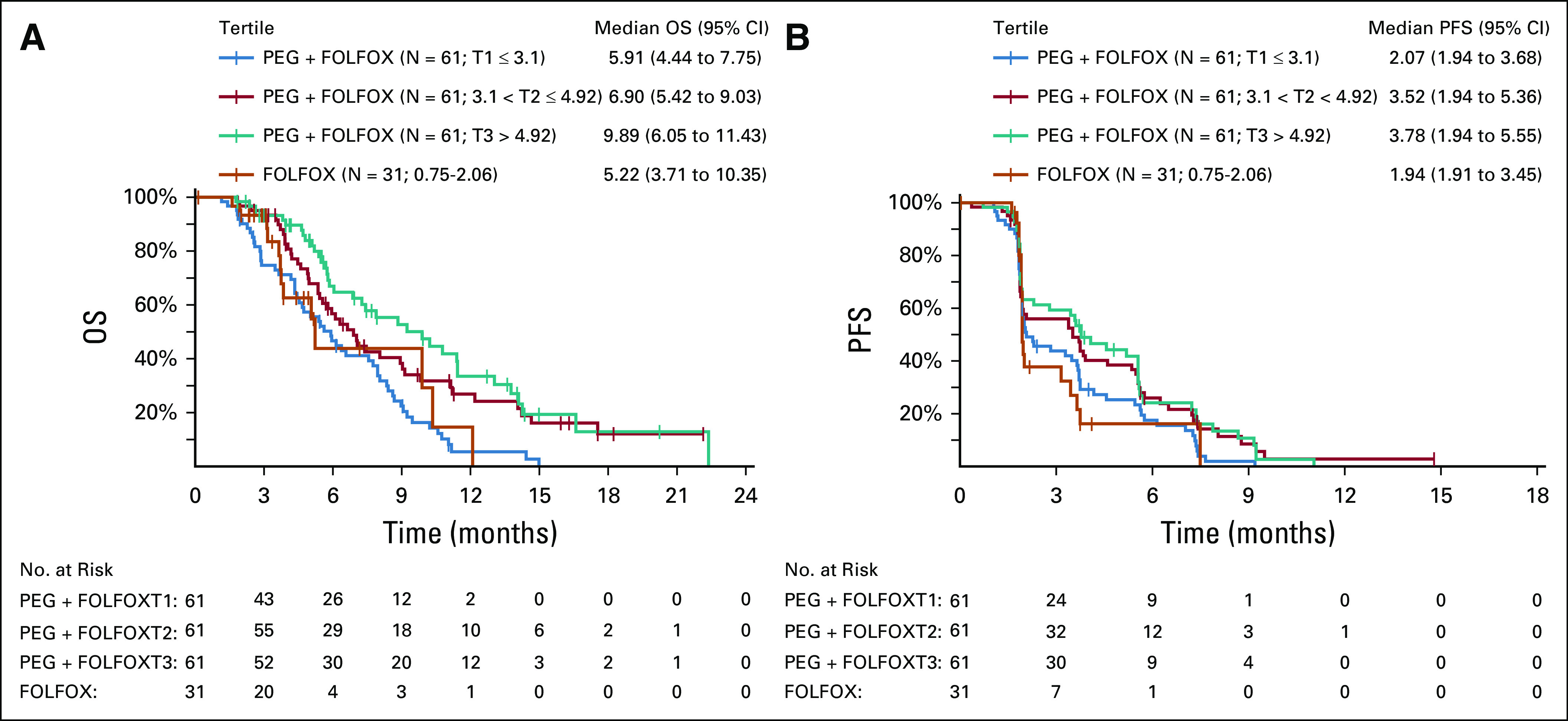
Fold change in IL-18 expression is depicted for 214 patients in relationship to OS (A) and PFS (B). FOLFOX, folinic acid, fluorouracil, and oxaliplatin; IL, interleukin; OS, overall survival; PEG, pegilodecakin; PFS, progression-free survival.
FIG A4.
Baseline IL-18 expression is plotted for 294 patients in relationship to OS (A) and PFS (B). FOLFOX, folinic acid, fluorouracil, and oxaliplatin; IL, interleukin; OS, overall survival; PEG, pegilodecakin; PFS, progression-free survival.
FIG A5.
The level of Cmin was analyzed in relationship to newly-detectable T-cell clonal receptor clones in patients treated with pegilodecakin + FOLFOX (N = 36) or FOLFOX (N = 10) at cycle 2 day 13. Dose of 0.4 mg is indicated by circles and 0.8 mg is indicated by triangles. Cmin, pegilodecakin concentration; FOLFOX, folinic acid, fluorouracil, and oxaliplatin.
FIG A6.
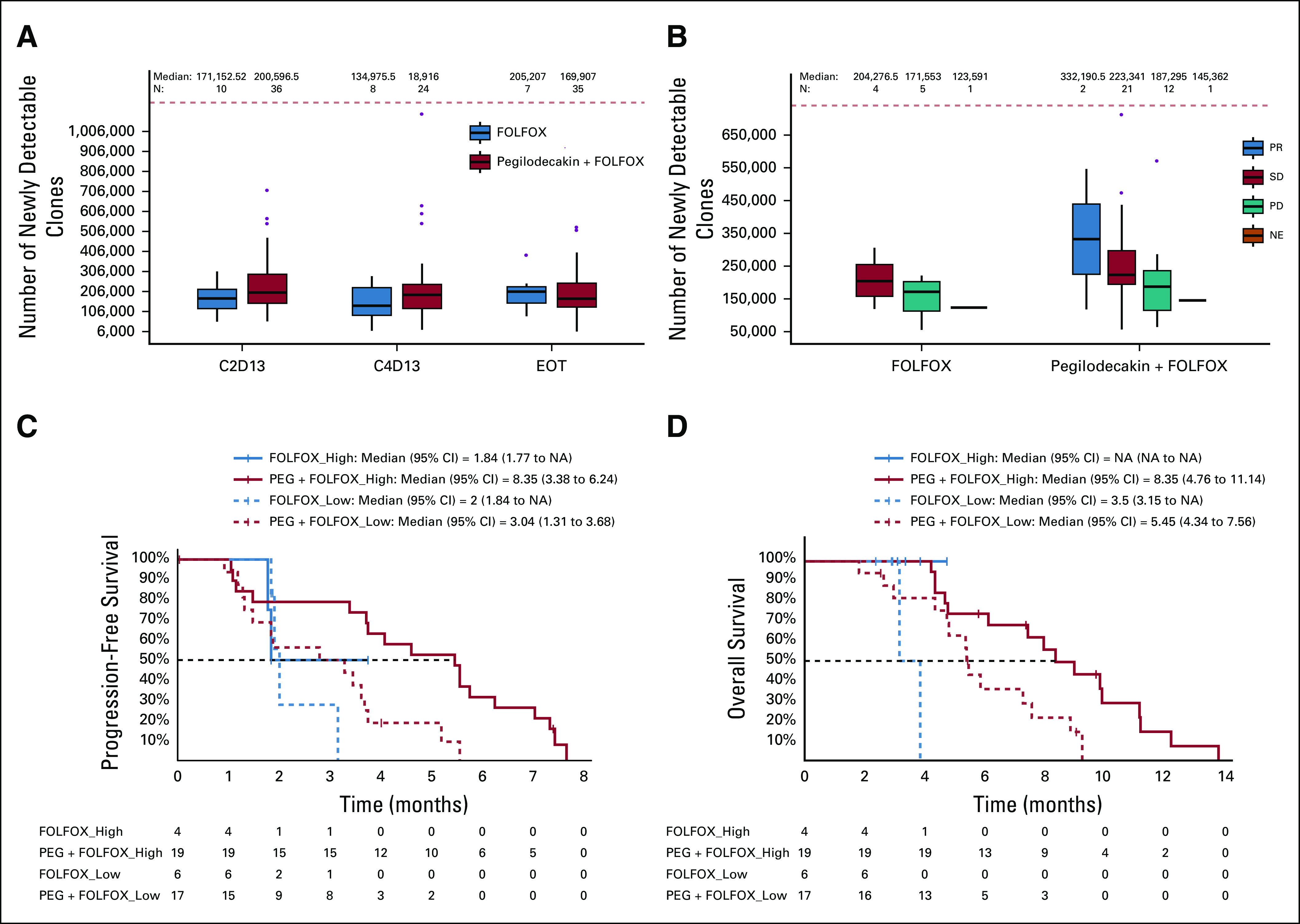
The number of copies of the T-cell receptor amino acid sequence was evaluated in order to assess the number of copies of that T-cell clone in a given patient. The frequency of each amino acid sequence was compared between samples on treatment, end of treatment, and baseline. Clones were considered newly-detectable if not detected at baseline, but detected after treatment initiation. (A) The newly-detectable T-cell clones in patient samples were analyzed at baseline, C2D13, C4D13, and EOT comparing between the control arm (FOLFOX) and the experimental arm (PEG + FOLFOX). Wilcoxon tests were used for each group of data. (B) Newly-detectable T-cell clones were assessed in relationship to best clinical response of PR (blue), SD (red), or PD (green). One patient on both treatment arms was NE (orange). Wilcoxon test of SD versus PD patients in the experimental arm; no comparisons were statistically significant. The experimental arm (PEG + FOLFOX; red) and the control arm (FOLFOX; blue) were split into two groups by level of newly-detectable clone counts (“high” [solid line] and “low” [dashed line] in respect to the median) and plotted in relationship to progression-free survival (C) and overall survival (D) at C2D13. C2D13, cycle 2 day 13; C4D13, cycle 4 day 13; EOT, end of treatment; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; NE, not evaluable; PD, progressive disease; PEG, pegilodecakin; PR, partial response; SD, stable disease.
TABLE A1.
PEG Exposure and Dose Intensity
TABLE A2.
FOLFOX Exposure and Dose Intensity
TABLE A3.
Postprogression Therapies
TABLE A4.
Dose Modification
TABLE A5.
Demographic and Clinical Characteristics for ITT
DISCLAIMER
J.R.H. received grants, personal fees, and nonfinancial support from ARMO BioSciences, a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study.
PRIOR PRESENTATION
Presented as an oral presentation at the 2020 American Society of Clinical Oncology Gastrointestinal Cancers Symposium, San Francisco, CA January 25-27, 2020.
SUPPORT
Supported by Eli Lilly and Company.
CLINICAL TRIAL INFORMATION
NCT02923921 (SEQUOIA).
DATA SHARING STATEMENT
Lilly provides access to all individual data collected during the trial, after anonymization, with the exception of PK or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided in www.vivli.org.
AUTHOR CONTRIBUTIONS
Conception and design: J. Randolph Hecht, Rebecca R. Hozak, Sujata Rao, Baek-Yeol Ryoo
Administrative support: Yong Lin
Provision of study materials or patients: Johanna Bendell, Hao-Wen Sim, Teresa Macarulla, Charles D. Lopez, Richard Greil
Collection and assembly of data: J. Randolph Hecht, Sara Lonardi, Johanna Bendell, Hao-Wen Sim, Teresa Macarulla, Charles D. Lopez, Eric Van Cutsem, Andres J. Muñoz Martin, Joon Oh Park, Richard Greil, Hong Wang, Rebecca R. Hozak, Sujata Rao, Baek-Yeol Ryoo
Data analysis and interpretation: J. Randolph Hecht, Hao-Wen Sim, Teresa Macarulla, Charles D. Lopez, Eric Van Cutsem, Andres J. Muñoz Martin, Joon Oh Park, Richard Greil, Hong Wang, Rebecca R. Hozak, Ivelina Gueorguieva, Yong Lin, Sujata Rao, Baek-Yeol Ryoo
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase III Study of FOLFOX Alone or With Pegilodecakin as Second-Line Therapy in Patients With Metastatic Pancreatic Cancer that Progressed After Gemcitabine (SEQUOIA)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
J. Randolph Hecht
Honoraria: Ipsen, Merck, Acrotech Biopharma
Research Funding: ARMO BioSciences, Halozyme, Amgen, Merck, Abbvie, Advaxis, Astellas Pharma, Forty Seven, Immunomedics, Lilly, Gritstone Oncology, Tesaro/GSK, Arcus Biosciences
Travel, Accommodations, Expenses: Advaxis, Lilly, Amgen
Sara Lonardi
Consulting or Advisory Role: Amgen, Merck Serono, Lilly, SERVIER, AstraZeneca, Incyte, Daiichi Sankyo
Speakers' Bureau: Roche, Lilly, Bristol-Myers Squibb, Servier, Merck Serono, Pierre Fabre, GlaxoSmithKline, Amgen
Research Funding: Amgen, Merck Serono
Johanna Bendell
Consulting or Advisory Role: Gilead Sciences, Genentech/Roche, Bristol-Myers Squibb, Five Prime Therapeutics, Lilly, Merck, MedImmune, Celgene, EMD Serono, Taiho Pharmaceutical, Macrogenics, GlaxoSmithKline, Novartis, OncoMed, Leap Therapeutics, TG Therapeutics, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Bayer, Incyte, Apexigen, Array BioPharma, Sanofi, ARMO BioSciences, Ipsen, Merrimack, Oncogenex, FORMA Therapeutics, Arch Oncology, Prelude Therapeutics, Phoenix Biotech, Cyteir, Molecular Partners, Innate Pharma, Torque, Tizona Therapeutics, Inc, Janssen, Tolero Pharmaceuticals, TD2, Amgen, Seattle Genetics, Moderna Therapeutics, Tanabe Research, Beigene, Continuum Clinical, Cerulean Pharma, Kyn therapeutics, Bicycle Therapeutics, Relay Therapeutics, Evelo Therapeutics, Fusion Pharmaceuticals
Research Funding: Lilly, Genentech/Roche, Incyte, Gilead Sciences, Bristol-Myers Squibb, Leap Therapeutics, AstraZeneca/MedImmune, Boston Biomedical, GlaxoSmithKline, Novartis, Array BioPharma, Taiho Pharmaceutical, Celgene, OncoMed, Daiichi Sankyo, Bayer, Apexigen, Kolltan Pharmaceuticals, SynDevRx, Merck, Macrogenics, Five Prime Therapeutics, EMD Serono, TG Therapeutics, Boehringer Ingelheim, Forty Seven, Stem CentRx, Onyx, Sanofi, Takeda, Abbott/AbbVie, Eisai, Celldex, Agios, ARMO BioSciences, CytomX Therapeutics, Nektar, Ipsen, Merrimack, Tarveda Therapeutics, Tyrogenex, Oncogenex, Marshall Edwards, Pieris Pharmaceuticals, Mersana, Calithera Biosciences, Blueprint Medicines, Gritstone Oncology, Evelo Therapeutics, FORMA Therapeutics, Forty Seven, EMD Serono, Merus, Jacobio, eFFECTOR Therapeutics, Novocure, Sorrento Therapeutics, Arrys Therapeutics, TRACON Pharma, Sierra Oncology, Innate Pharma, Prelude Therapeutics, Arch Oncology, Harpoon therapeutics, Phoenix Biotech, Unum Therapeutics, Vyriad, Harpoon therapeutics, Cyteir, Molecular Partners, Innate Pharma, ADC Therapeutics, Torque, Tizona Therapeutics, Inc, Janssen, Amgen, BeiGene, Pfizer, Millenium Pharamceuticals, ImClone Systems, Acerta Pharma, Rgenix, Bellicum Pharmaceuticals, Arcus Biosciences, Gossamer Bio, Seattle Genetics, Tempest Therapeutics, Shattuck Labs, Synthorx, Revolution Medicines, Bicycle Therapeutics, Zymeworks, Relay Therapeutics, Evelo Therapeutics, Scholar Rock, NGM Biopharmaceuticals, Numab, AtlasMedx, Treadwell Therapeutics, IGM, MabSpace Biosciences, Hutchison MediPharma, Repare Therapeutics, NeoImmuneTech, Regeneron, PureTech
Travel, Accommodations, Expenses: Merck, Roche/Genentech, Celgene, Daiichi Sankyo, Gilead Sciences, Bristol-Myers Squibb, Lilly, MedImmune, Taiho Pharmaceutical, Novartis, OncoMed, Boehringer Ingelheim, ARMO BioSciences, Ipsen, FORMA Therapeutics
Hao-Wen Sim
Research Funding: Abbvie, Bristol-Myers Squibb
Teresa Macarulla
Consulting or Advisory Role: Sanofi/Aventis, Shire, Celgene, Roche, Baxalta, QED Therapeutics, Baxter, Incyte, Servier, Lilly, Ipsen
Research Funding: Celgene, Agios, ASLAN Pharmaceuticals, Bayer, Roche, Genentech, Astra Zeneca, Halozyme, Immunomedics, Lilly, Merrimack, Millennium, Novartis, Novocure, Pfizer, Pharmacyclics
Travel, Accommodations, Expenses: Merck, H3 Biomedicine, Sanofi, Celgene, Servier
Charles D. Lopez
Consulting or Advisory Role: Boston Scientific, Celgene, Boston Biomedical, Pfizer, Exelixis, Astellas Pharma
Research Funding: Taiho Pharmaceutical
Travel, Accommodations, Expenses: RenovoRx
Eric Van Cutsem
Consulting or Advisory Role: Bayer, Lilly, Roche, Servier, Bristol-Myers Squibb, Celgene, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Halozyme, Array BioPharma, Biocartis, GlaxoSmithKline, Daiichi Sankyo, Pierre Fabre, Sirtex Medical, Taiho Pharmaceutical, Incyte
Research Funding: Amgen, Bayer, Boehringer Ingelheim, Lilly, Novartis, Roche, Celgene, Ipsen, Merck, Merck KGaA, SERVIER, Bristol-Myers Squibb
Andres J. Muñoz Martin
Consulting or Advisory Role: Celgene, Sanofi, Bristol-Myers Squibb/Pfizer, LEO Pharma, Daiichi Sankyo, Bayer, Halozyme
Speakers' Bureau: Rovi
Research Funding: Sanofi, LEO Pharma
Patents, Royalties, Other Intellectual Property: Risk assessment model in venous thromboembolism in cancer patients
Travel, Accommodations, Expenses: Celgene, Roche, Merck Serono
Joon Oh Park
Consulting or Advisory Role: Celgene, Merck Serono, SERVIER, AstraZeneca, GI Innovation
Speakers' Bureau: Celgene
Research Funding: Celgene, MedPacto
Richard Greil
Honoraria: Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol-Myers Squibb, MSD, Sandoz, Abbvie, Gilead Sciences, Daiichi Sankyo
Consulting or Advisory Role: Celgene, Novartis, Roche, Bristol-Myers Squibb, Takeda, Abbvie, AstraZeneca, Janssen, MSD, Merck, Gilead Sciences, Daiichi Sankyo
Research Funding: Celgene, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol-Myers Squibb, MSD, Sandoz, Gilead Sciences, Roche
Travel, Accommodations, Expenses: Roche, Amgen, Janssen-Cilag, AstraZeneca, Novartis, MSD, Celgene, Gilead Sciences, Bristol-Myers Squibb, Abbvie, Daiichi Sankyo
Hong Wang
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Rebecca R. Hozak
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Patents, Royalties, Other Intellectual Property: Patent published and pending related to ramucirumab
Ivelina Gueorguieva
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Yong Lin
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Sujata Rao
Employment: Lilly
Stock and Other Ownership Interests: Lilly
Travel, Accommodations, Expenses: Lilly
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ducreux M Cuhna AS Caramella C, et al. : Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v56-68, 2015(suppl 5) [DOI] [PubMed] [Google Scholar]
- 2.Conroy T Desseigne F Ychou M, et al. : FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817-1825, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD Ervin T Arena FP, et al. : Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691-1703, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berk V Ozdemir N Ozkan M, et al. : XELOX vs. FOLFOX4 as second line chemotherapy in advanced pancreatic cancer. Hepatogastroenterology 59:2635-2639, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Chung JW Jang HW Chung MJ, et al. : Folfox4 as a rescue chemotherapy for gemcitabine-refractory pancreatic cancer. Hepatogastroenterology 60:363-367, 2013 [PubMed] [Google Scholar]
- 6.Yoo C Hwang JY Kim JE, et al. : A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer 101:1658-1663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosn M Saroufim A Kattan J, et al. : Sequential FOLFOX-6 and gemcitabine for locally advanced and/or metastatic pancreatic cancer. Med Oncol 29:2831-2837, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Wainberg Z Feeney K Lee MA, et al. : Meta-analysis of OS for pancreatic cancer patients receiving 5-FU and oxaliplatin-based therapy after failing first-line gemcitabine-containing therapy. J Clin Oncol 37, 2019(suppl; abstr 202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piechutta M, Berghoff AS: New emerging targets in cancer immunotherapy: The role of Cluster of Differentiation 40 (CD40/TNFR5). ESMO Open 4:e000510, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patnaik A Kang SP Rasco D, et al. : Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 21:4286-4293, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Royal RE Levy C Turner K, et al. : Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 33:828-833, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laghi L Beghelli S Spinelli A, et al. : Irrelevance of microsatellite instability in the epidemiology of sporadic pancreatic ductal adenocarcinoma. PLoS One 7:e46002, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu ZI Shia J Stadler ZK, et al. : Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: Challenges and recommendations. Clin Cancer Res 24:1326-1336, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spits H, de Waal Malefyt R: Functional characterization of human IL-10. Int Arch Allergy Immunol 99:8-15, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Moore KW de Waal Malefyt R Coffman RL, et al. : Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683-765, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Mumm JB Emmerich J Zhang X, et al. : IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell 20:781-796, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Naing A Papadopoulos KP Autio KA, et al. : Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J Clin Oncol 34:3562-3569, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naing A Wong DJ Infante JR, et al. : Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): A multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol 20:1544-1555, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht JR Naing A Falchook GS, et al. : Overall survival of PEGylated pegilodecakin with 5-FU/LV and oxaliplatin (FOLFOX) in metastatic pancreatic adenocarcinoma (PDAC). J Clin Oncol 36:4119, 2018 [Google Scholar]
- 20.Sonbol MB Firwana B Wang Z, et al. : Second-line treatment in patients with pancreatic ductal adenocarcinoma: A meta-analysis. Cancer 123:4680-4686, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Naing A Infante JR Papadopoulos KP, et al. : PEGylated IL-10 (Pegilodecakin) Induces Systemic Immune Activation, CD8(+) T Cell Invigoration and Polyclonal T Cell Expansion in Cancer Patients. Cancer Cell 34:775-791 e3, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tangkijvanich P Thong-Ngam D Mahachai V, et al. : Role of serum interleukin-18 as a prognostic factor in patients with hepatocellular carcinoma. World J Gastroenterol 13:4345-4349, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawabata T Ichikura T Majima T, et al. : Preoperative serum interleukin-18 level as a postoperative prognostic marker in patients with gastric carcinoma. Cancer 92:2050-2055, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Carbone A Vizio B Novarino A, et al. : IL-18 paradox in pancreatic carcinoma: Elevated serum levels of free IL-18 are correlated with poor survival. J Immunother 32:920-931, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Yost KE Satpathy AT Wells DK, et al. : Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med 25:1251-1259, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Lilly provides access to all individual data collected during the trial, after anonymization, with the exception of PK or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided in www.vivli.org.