Abstract
The research has correlated the risk factors of herpes zoster with some chronic diseases. This meta-analysis aimed to assess the incidence of herpes zoster in patients with diabetes mellitus.
We conducted a literature search using Web of Science and PubMed for articles published from January 1, 2000 to December 31, 2019. The incidence rate ratio and 95% confidence interval for herpes zoster associated with diabetes mellitus was calculated.
We included 5 cohort studies for a meta-analysis. The pooled incidences of herpes zoster in patients with diabetes mellitus and in patients without diabetes mellitus were 7.22 and 4.12 per 1000 person-years. The overall risk of developing herpes zoster was significantly higher in patients with diabetes mellitus when compared to those with no diabetes mellitus (incidence rate ratio = 1.60, 95% confidence interval = 1.33–1.93).
Patients with diabetes mellitus are substantially at increased risk for the development of herpes zoster. Patients with diabetes mellitus should take into consideration the vaccination to prevent herpes zoster.
Keywords: diabetes mellitus, herpes zoster, meta-analysis
1. Introduction
Herpes zoster is an infection caused by reactivation of the varicella-zoster virus, which is the same virus causing chickenpox as a primary infection.[1,2] When the varicella-zoster virus-related cell-mediated immunity declines, the varicella-zoster virus living in dorsal root ganglia is reactivated and then herpes zoster develops clinically.[1,2]
The most common and serious complications are the pain caused by acute phase and post-herpetic neuralgia of herpes zoster. The research has correlated the development of herpes zoster with numerous risk factors including older age, immune status, and some chronic diseases which include diabetes mellitus, chronic obstructive pulmonary disease, rheumatoid arthritis, and systemic lupus erythematosus.[3,4]
Patients with diabetes mellitus are at increased risk for developing microvascular and macrovascular complications. Also, immunological function in patients with diabetes mellitus may be impaired.[5–7] Impaired immunological function has placed these patients at risk for developing a variety of infections including herpes zoster.[8–11]
Epidemiological studies revealed that the likelihood of developing herpes zoster in patients with diabetes mellitus is higher as compared to those with no diabetes mellitus in the world. However, there is limited evidence regarding the incidence of herpes zoster in patients with diabetes mellitus. Without the incidence data, the attributable risk of herpes zoster associated with diabetes mellitus cannot be measured. Without the attributable risk data, health policy professionals cannot make an accurate decision on which populations are good candidates for vaccination against herpes zoster. Given the great debilitation and long-term complications caused by herpes zoster, how to make a decision to prevent herpes zoster depends on the updated evidence. Therefore, the purpose of the meta-analysis was to estimate the incidence of herpes zoster in patients with diabetes mellitus.
2. Methods
2.1. Search strategy
We conducted a literature search using Web of Science and PubMed for articles published from January 1, 2000 to December 31, 2019. The following keywords were used to find articles of interest: “diabetes mellitus,” “herpes zoster,” “incidence,” and “cohort study.” The inclusion criteria were used as follows:
-
(1)
participants with a baseline diagnosis of diabetes mellitus;
-
(2)
herpes zoster as a major outcome;
-
(3)
using event number of herpes zoster and person-years as parameters.
The end date for article search was December 31, 2019 (Fig. 1 as flow chart).
Figure 1.
Flow chart of article search.
2.2. Data extraction
The eligibility of each article was independently evaluated by 3 authors (SWL, CSL, and BFH) according to the required keywords and inclusion criteria. Data were extracted for the following variables: surname of the first author, year of publication, country where the research was performed, number of diabetes mellitus, number of non-diabetes mellitus, event number of herpes zoster, follow-up person-years, and incidence rate of herpes zoster. Disagreement was resolved by group discussion and consensus with the fifth author (KFL).
2.3. Statistical analysis
The association between diabetes mellitus and herpes zoster was expressed as incidence rate ratio (IRR) with 95% confidence interval (95% CI), which represented the relative risk of developing herpes zoster in patients with diabetes mellitus as compared to patients with no diabetes mellitus. The pooled IRR was estimated using the random effect model. Heterogeneity between the studies was evaluated using the I2 statistic. I2 > 50% indicates significant heterogeneity between studies. All statistical analyses were performed using Cochrane's Review Manager 5.3 software (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014). Two-tailed P < .05 was considered statistically significant.
2.3.1. Statement of ethics
The study was approved by the Research Ethics Committee at China Medical University and Hospital in Taiwan (CMUH-104-REC2-115).
3. Results
3.1. Characteristics of eligible studies
Table 1 demonstrated that 5 eligible cohort studies met the inclusion criteria.[11–15] The incidence of herpes zoster in patients with diabetes mellitus varied greatly between studies, ranging from 2.11 per 1000 person-years to 9.38 per 1000 person-years. The overall incidence of herpes zoster in patients with diabetes mellitus was 7.22 per 1000 person-years. The pooled incidence of herpes zoster in patients without diabetes mellitus was 4.12 per 1000 person-years.
Table 1.
Characteristics of eligible studies.
| Diabetes mellitus | Non-diabetes mellitus | |||||||
| Author | Country | Year | Event number of herpes zoster | Person-years | Incidence (1000 person-years) | Event number of herpes zoster | Person-years | Incidence (1000 person-years) |
| Suaya et al (2014) | USA | 2014 | 61,142 | 7,681,336 | 7.96 | 359,373 | 80,266,547 | 4.48 |
| Esteban-Vasallo et al (2014) | Spain | 2014 | 8012 | 854,158 | 9.38 | 58,772 | 13,541,935 | 4.34 |
| Muñoz-Quiles et al (2017) | Spain | 2017 | 14,104 | 1,516,559 | 9.3 | 55,334 | 8,137,353 | 6.8 |
| Chen et al (2017) | Taiwan | 2017 | 85 | 40,226 | 2.11 | 195 | 163,212 | 1.19 |
| Lai et al (2019) | Taiwan | 2019 | 1676 | 213,530 | 7.85 | 6057 | 897,807 | 6.75 |
| Overall | 7.22 | 4.12 | ||||||
3.2. Risk of herpes zoster associated with diabetes mellitus
Figure 2 revealed that the overall risk of developing herpes zoster was significantly higher in patients with diabetes mellitus compared to those with no diabetes mellitus (incidence rate ratio = 1.60, 95% confidence interval = 1.33–1.93).
Figure 2.
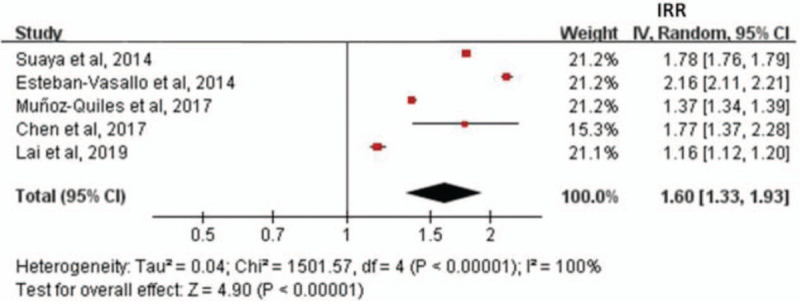
Forest plot revealing that the overall risk of developing herpes zoster was significantly higher in patients with diabetes mellitus when compared to those with no diabetes mellitus (IRR = 1.60, 95%CI = 1.33–1.93). 95%CI = 95% confidence interval, IRR = incidence rate ratio.
3.3. Pooled incidence of herpes zoster stratified by age group
The sub-analysis including 2 eligible studies demonstrated that the pooled incidence of herpes zoster was higher in patients aged ≥65 than that in patients aged 18 to 64 among both diabetes and non-diabetes groups. Patients aged ≥65 in diabetes group had the highest incidence rate of herpes zoster (11.91 per 1000 person-years) (Table 2).[11,13]
Table 2.
Pooled incidence of herpes zoster stratified by age group in diabetes group and non-diabetes group.
| Diabetes mellitus | Non-diabetes mellitus | ||||||
| Age group (yr) | Event number of herpes zoster | Person-years | Incidence (1000 person-years) | Event number of herpes zoster | Person-years | Incidence (1000 person-years) | Incidence rate ratio of herpes zoster∗ (95%CI) |
| 18–64 | 45,351 | 6,428,458 | 7.05 | 309,447 | 73,881,964 | 4.19 | 1.68 (1.67–1.70) |
| ≥65 | 17,467 | 1,466,409 | 11.91 | 55,983 | 7,282,388 | 7.69 | 1.55 (1.52–1.58) |
3.4. Assessment of study quality
We used the Newcastle-Ottawa Scale (NOS) system for quality assessment of the included studies.[16] All of 5 eligible studies were scored as 8 (Supplementary Table 1). The NOS score above 6 was regarded as a high-quality study.
4. Discussion
In the present meta-analysis, we found that patients aged ≥65 had a higher incidence rate of developing herpes zoster than those aged 18 to 64 among both diabetes and non-diabetes groups. It indicates that older age remains to be a risk factor of developing herpes zoster. We noted that patients with diabetes mellitus were associated with a 1.6-fold increased risk of the development of herpes zoster. We estimated that the attributable risk caused by diabetes mellitus was 0.0031 ([7.22 minus 4.12] divided by 1000, Table 1). That is, an absence of diabetes mellitus might reduce approximately 31 cases of herpes zoster per 10,000 persons per year. A cohort study revealed that the attributable risk caused by appendectomy was 0.00104.[17] That is, an absence of appendectomy might reduce approximately 10 cases of herpes zoster per 10,000 persons per year. These measures indicate that the attributable risk of developing herpes zoster is about 3-fold higher among patients with diabetes mellitus compared to patients with appendectomy. From a view of preventive medication, primary prevention is the best policy to prevent disease development. This task includes 2 components: modification of risk factors and vaccination. First, physicians should consider primary prevention of type 2 diabetes mellitus, such as unhealthy dietary pattern, decreased physical activity, sedentary lifestyle, smoking, and obesity, which account for the development of the majority of diabetes mellitus cases in industrialized countries.[18–20]. If these risk factors can be modified, the risk of developing diabetes mellitus might be decreased and subsequently the risk of developing herpes zoster might be further decreased. Second, little research is available about the efficacy and safety of vaccination against herpes zoster in patients with diabetes mellitus.[21] Data from the National Health Interview Survey revealed that among patients aged 60 and over with diabetes mellitus, only 27.2% had ever received a vaccine for herpes zoster.[22] A meta-analysis revealed that the recombinant zoster vaccine can prevent more cases of herpes zoster than the live attenuated zoster vaccine in adults ages 50 and older.[23] The Advisory Committee on Immunization Practices recommended that adults ages 50 and older should consider the recombinant zoster vaccine for the prevention of herpes zoster.[24] Therefore, we suggest that patients with diabetes mellitus should consider a recombinant zoster vaccine to prevent herpes zoster.
5. Limitation and strength
First, some studies revealed the incidence of herpes zoster in patients with diabetes mellitus, but they did not reveal the event number of herpes zoster and the follow-up person-years. These studies could not be included for our meta-analysis. We suggest that future research dealing with the incidence of herpes zoster should reveal the event number and the follow-up person-years. Second, a cohort study revealed that persons in the lowest hemoglobin A1c group (<5.0%) were at higher risk of developing herpes zoster than those in the reference group (hemoglobin A1c of 5.0–6.4%; adjusted odds ratio = 1.63; 95% confidence interval = 1.07–2.48).[25] We could not assess the incidence rate of herpes zoster stratified by the hemoglobin A1c levels due to lack of the data of hemoglobin A1c. Third, a cohort study revealed that patients who took short-term dipeptidyl peptidase-4 inhibitors were at a higher risk of developing herpes zoster (adjusted hazard ratio = 2.04, 95% confidence interval = 1.03–4.04).[26] We could not assess the incidence rate of herpes zoster stratified by anti-diabetic medications due to lack of the data of anti-diabetic medications. These limitations indicate that a future study should focus on the incidence rate of herpes zoster stratified by the status of glycemic control, and the type of anti-diabetic medications. Fourth, although only 5 cohort studies matched the inclusion criteria, our meta-analysis creates more concepts for other real-world databases to examine the association between diabetes mellitus and herpes zoster.
6. Conclusion
Patients with diabetes mellitus are at increased risk of the development of herpes zoster. Patients with diabetes mellitus should take into consideration the vaccination to prevent herpes zoster.
Author contributions
Shih-Wei Lai contributed to the conception of the article, initiated the draft of the article, and has approved the final draft submitted.
Yu-Hung Kuo, Cheng-Li Lin, and Kuan-Fu Liao conducted data analysis.
Chiu-Shong Liu, Bing-Fang Hwang, and Kuan-Fu Liao interpreted the data and contributed equally to the article.
Conceptualization: Shih-Wei Lai.
Data curation: Shih-Wei Lai.
Formal analysis: Shih-Wei Lai, Chiu-Shong Liu, Yu-Hung Kuo, Cheng-Li Lin, Bing-Fang Hwang, Kuan-Fu Liao.
Methodology: Shih-Wei Lai.
Supervision: Shih-Wei Lai.
Validation: Shih-Wei Lai.
Writing – original draft: Shih-Wei Lai.
Writing – review & editing: Shih-Wei Lai.
Supplementary Material
Footnotes
Abbreviations: 95%CI = 95% confidence interval, IRR = incidence rate ratio.
How to cite this article: Lai SW, Liu CS, Kuo YH, Lin CL, Hwang BF, Liao KF. The incidence of herpes zoster in patients with diabetes mellitus: a meta-analysis of cohort studies. Medicine. 2021;100:16(e25292).
This study was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004), China Medical University Hospital in Taiwan (DMR109-098), and MOST Clinical Trial Consortium for Stroke (MOST 108-2321-B-039-003). These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental digital content is available for this article.
95%CI = 95% confidence interval.
References
- [1].Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and postherpetic neuralgia. Am Fam Physician 2000;61:2437–44. [PubMed] [Google Scholar]
- [2].Saguil A, Kane S, Mercado M, et al. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician 2017;96:656–63. [PubMed] [Google Scholar]
- [3].Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc 2017;92:1806–21. [DOI] [PubMed] [Google Scholar]
- [4].Koshy E, Mengting L, Kumar H, et al. Epidemiology, treatment and prevention of herpes zoster: a comprehensive review. Indian J Dermatol Venereol Leprol 2018;84:251–62. [DOI] [PubMed] [Google Scholar]
- [5].Ayelign B, Negash M, Genetu M, et al. Immunological impacts of diabetes on the susceptibility of mycobacterium tuberculosis. J Immunol Res 2019;2019:6196532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hodgson K, Morris J, Bridson T, et al. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 2015;144:171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Okamoto S, Hata A, Sadaoka K, et al. Comparison of varicella-zoster virus-specific immunity of patients with diabetes mellitus and healthy individuals. J Infect Dis 2009;200:1606–10. [DOI] [PubMed] [Google Scholar]
- [8].Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 2005;41:281–8. [DOI] [PubMed] [Google Scholar]
- [9].Heymann AD, Chodick G, Karpati T, et al. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection 2008;36:226–30. [DOI] [PubMed] [Google Scholar]
- [10].Lin HF, Liao KF, Chang CM, et al. Anti-diabetic medication reduces risk of pulmonary tuberculosis in diabetic patients: a population-based cohort study in Taiwan. Kuwait Med J 2017;49:22–8. [Google Scholar]
- [11].Lai SW, Lin CL, Liao KF. Real-world database investigating the association between diabetes mellitus and herpes zoster in Taiwan. Medicine 2019;98:e15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen HH, Lin IC, Chen HJ, et al. Association of herpes zoster and type 1 diabetes mellitus. PLoS One 2016;11:e0155175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Suaya JA, Chen SY, Li Q, et al. Incidence of herpes zoster and persistent post-zoster pain in adults with or without diabetes in the United States. Open Forum Infect Dis 2014;1:ofu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Esteban-Vasallo MD, Dominguez-Berjon MF, Gil-Prieto R, et al. Sociodemographic characteristics and chronic medical conditions as risk factors for herpes zoster: a population-based study from primary care in Madrid (Spain). Hum Vaccin Immunother 2014;10:1650–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Munoz-Quiles C, Lopez-Lacort M, Ampudia-Blasco FJ, et al. Risk and impact of herpes zoster on patients with diabetes: a population-based study, 2009–2014. Hum Vaccin Immunother 2017;13:2606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wells G, Shea BJ, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [cited on June 1, 2020]. [Google Scholar]
- [17].Lai SW, Lin CL. Patients with appendectomy are at increased risk of herpes zoster: real-world data in Taiwan. Intern Emerg Med 2019;14:329–30. [DOI] [PubMed] [Google Scholar]
- [18].Bellou V, Belbasis L, Tzoulaki I, et al. Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PLoS One 2018;13:e0194127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Msopa E, Mwanakasale V. Identification of risk factors of diabetes mellitus in bank employees of selected banks in Ndola town. Diabetes Metab Syndr 2019;13:1497–504. [DOI] [PubMed] [Google Scholar]
- [20].Nguyen CT, Pham NM, Lee AH, et al. Prevalence of and risk factors for type 2 diabetes mellitus in Vietnam: a systematic review. Asia Pac J Public Health 2015;27:588–600. [DOI] [PubMed] [Google Scholar]
- [21].Papagianni M, Metallidis S, Tziomalos K. Herpes zoster and diabetes mellitus: a review. Diabetes Ther 2018;9:545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Villarroel MA, Vahratian A. Vaccination coverage among adults with diagnosed diabetes: United States, 2015. NCHS Data Brief 2016;265:01–8. [PubMed] [Google Scholar]
- [23].Tricco AC, Zarin W, Cardoso R, et al. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis. BMJ 2018;363:k4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018;67:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kobayashi D, Shimbo T, Noto H, et al. Low level of hemoglobin A1c and the increased incidence of herpes zoster: longitudinal study. Eur J Clin Microbiol Infect Dis 2019;38:1539–45. [DOI] [PubMed] [Google Scholar]
- [26].Chen HH, Lin CL, Yeh SY, et al. Short-term dipeptidyl peptidase-4 inhibitor use increases the risk of herpes zoster infection in Asian patients with diabetes. QJM 2016;109:91–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


