Abstract
Background.
Periodontal disease and diabetes are widespread comorbid conditions that are detrimental to oral and overall health. Dentists’ performing chairside screenings for undiagnosed diabetes mellitus (UDM) can be beneficial to both patients and providers. The authors determined UDM rates in a population-based study and whether UDM and periodontal disease were independently associated.
Methods.
Data from 7,343 participants in the Atherosclerosis Risk in Communities study visit 4 were used to determine rates of UDM by periodontal status, edentulism, and body mass index. The authors used a χ2 test or analysis of variance, along with a 2-stage logistic regression model, to determine relationships with UDM. UDM was defined as no self-reported diabetes and blood glucose levels (fasting glucose ≥ 126 milligrams/deciliter or nonfasting glucose > 200 mg/dL). Periodontal disease was defined using the Periodontal Profile Classes system adapted to stages and the Centers for Disease Control and Prevention and American Academy of Periodontology index.
Results.
UDM rates overall were 5.6%. The highest rates occurred in patients who were obese and edentulous (12.6%) and obese and had severe periodontal disease (12.2%). Significant associations were found for UDM and severe periodontal disease (Periodontal Profile Classes system stage IV) (odds ratio, 1.78; 95% confidence interval, 1.10 to 2.88). Edentulism was significantly associated with UDM in the Periodontal Profile Classes system model (odds ratio, 1.87; 95% confidence interval, 1.27 to 2.75) and Centers for Disease Control and Prevention and American Academy of Periodontology index (odds ratio, 1.70; 95% confidence interval, 1.08 to 2.67). Hyperglycemia was found in participants of all body mass index categories.
Conclusions.
UDM is significantly associated with obesity, edentulism, and periodontitis. These characteristics could help dentists identify patients at higher risk of developing DM. Patients without these characteristics still have UDM, so dentists performing chairside diabetes screening for all patients would yield additional benefit.
Practical Implications.
Dental offices are a major point of contact within the US health care system. Diabetes screening in this setting can provide important health information with direct relevance to patient care.
Keywords: Periodontal disease, undiagnosed diabetes, body mass index
Diabetes mellitus (DM) is one of the largest global health problems of the 21st century.1 Characterized by hyperglycemia that can damage multiple organ systems, including the eyes, kidneys, heart, blood vessels, nerves, and oral cavity, DM affects 8.8% of adults (n = 424.9 million) aged 20 through 79 years worldwide,1 and in 50% (n = 212.4 million) the diabetes is undiagnosed.1
DM is a leading cause of cardiovascular disease, blindness, kidney failure, and lower limb amputation in almost all high-income countries.2 In the United States, 9% of adults aged 20 through 79 years (n = 30 million) have DM, and 24% the diabetes is undiagnosed.1,3 Rates of DM have doubled in the past 20 years and are rising rapidly among older adults, racial minorities, and people who are obese.4 In addition, the estimated cost of undiagnosed diabetes mellitus (UDM) in the United States is $31.7 billion,5 despite being a largely preventable disease. There is an urgent need for early identification and treatment of people with UDM to avoid detrimental health outcomes and high health care expenditures.
Periodontal disease is a common chronic infection of the oral cavity, referenced as the sixth complication of DM.6 Pathogenic infection and host immune response are 2 critical factors for periodontal disease severity and progression.7 Periodontal therapy has resulted in a modest reduction in hemoglobin A1c levels8 and, conversely, hemoglobin A1c levels are a predictable indicator of severity of periodontal disease.9 Certain subtypes of periodontal disease are independently associated with prevalent diabetes,10 and these conditions share common risk factors, such as increased age11 and increased weight or levels of obesity.12,13 People who are obese are 35% more likely to have periodontal disease12 and 10 times more likely to develop type 2 diabetes than people of normal weight.14 Obesity is an important predictor of periodontal disease, and this relationship is likely mediated by insulin resistance.15 Causal relationships among periodontitis, diabetes, and obesity are unclear, but studies indicate that these diseases might have shared inflammation-related patho-physiological pathways.16
Dental offices serve as a major contact point within the US health care system.17 Considering that approximately 65% of US adults visit a dentist yearly18 compared with approximately 55% who visit a primary care physician,19 dentists’ screening for UDM could be 1 approach to improving patients’ health. Strauss and colleagues20 reported that 93% of patients with moderate to severe periodontal disease from national data (National Health and Nutrition Examination Survey 2003–2004) meet the American Diabetes Association’s guidelines for diabetes testing, and that one-half of these patients had contact with a dentist in the past year.21 Screening is likely to result in earlier detection of diabetes, reducing associated morbidity and mortality.22 Knowledge of patients’ diabetes status is also relevant to oral health care, as this information could change the dental treatment plan.23 For example, diabetes can result in increased progression of periodontal disease, as well as complications from invasive dental treatments.17 Diabetes screening by dentists has ample support in the literature, is relatively simple to perform, is well-received by patients and providers,24 and can provide important health information with direct relevance to oral health care,25 but this practice has not been largely adopted.
Our study objectives were to observe the rates of UDM in this population-based study, determine whether aspects of periodontal disease are independently associated with prevalent UDM, and, if so, interpret how our findings might relate to clinical practice.
METHODS
We used data from the Atherosclerosis Risk in Communities (ARIC) study,26 a large population-based longitudinal cohort that began recruitment in 1987, enrolling 15,792 people aged 45 through 64 years in 4 US cities. ARIC has followed these people to study the progression and sequelae of atherosclerosis. Extensive information was collected, including demographic characteristics, vital signs, medical history, medications, laboratory results and biomarkers, and a dental examination. All study participants provided written informed consent and the institutional review board on research involving human participants at the University of North Carolina or at each study performance site reviewed and approved the protocol. The University of North Carolina at Chapel Hill Office of Human Research Ethics approved the administrative annual review of protocol 94–0790 for continued analysis of the data on January 7, 2020.
There were 11,656 ARIC participants seen at the fourth clinical visits in 1996 through 1998. We excluded participants with missing information and those who self-reported a physician’s diagnosis of diabetes or had a high fasting blood glucose levels during visits 2 and 3 (reducing the number of participants to 10,123). Validity and reliability of diabetes in the ARIC study are good.27 For the periodontal analysis, we focused on data from the 6,793 participants who underwent a dental examination at visit 4 or were edentulous (n = 2,082), for a total of 8,875 participants. Of these 8,875 participants, 1,325 were excluded because they reported that a physician told them they had diabetes, and an additional 182 participants were excluded because they did not respond to the question about diabetes or had missing fasting glucose level information at visit 4. Finally, we excluded 25 people who were neither African American nor white, resulting in 7,343 participants in our study.
The dental examination included a full-mouth periodontal examination with 6 sites per tooth for the following measures: gingival recession, probing depth, gingival inflammation, and bleeding on probing. The dental examiners were calibrated against a standard examiner as well as one another. Details of the ARIC study objectives and methodology have been described previously.28
Exposures and outcomes
UDM was defined as no self-reported diabetes diagnosis, which is a “no” answer to the question “Has a doctor ever said you had any of the following? Diabetes or sugar in the blood?”; negative tests for fasting blood glucose level greater than 126 milligrams/deciliter or nonfasting blood glucose level greater than 200 mg/dL (2-hour oral glucose tolerance test) in ARIC examinations before visit 4; and a positive test at visit 4.
We defined periodontal disease per the Periodontal Profile Classes system adapted to stages (PPC-Stages).29 PPC-Stages is a newer and validated periodontal disease definition index with 7 stages or subtypes of periodontal disease. The first 4 stages are PPC-Stage I, health/incidental; PPC-Stage II, mild periodontal disease; PPC-Stage III, moderate periodontal disease; and PPC-Stage IV, severe periodontal disease. The final 3 stages are PPC-Stage V, mild tooth loss and high gingival inflammation; PPC-Stage VI, moderate tooth loss and reduced periodontium; and PPC-Stage VII, severe tooth loss. Although the stages are numbered, PPC-Stages represent mutually exclusive and exhaustive clinically homogenous categorical groups. PPC-Stages are more predictive of associations between periodontal disease and systemic conditions than traditional periodontal definitions10 and have increased heritability estimates.30 The design and validation of the PPC-Stages have been described in more detail previously.31 People who are edentulous also visit dental offices, but being edentulous is not part of PPC-Stages. To include this group in our study, we added a separate edentulous category to PPC-Stages and the Centers for Disease Control and American Academy of Periodontology (CDC-AAP) index.
We compared the PPC-Stages’ definition of periodontal disease with that of the CDC-AAP index,32 which is a traditional 4-level index (that is, healthy, mild, moderate, and severe disease). Severe periodontitis is defined as having 2 or more interproximal sites with clinical attachment level 6 millimeters or greater (not on the same tooth) and 1 or more interproximal sites with probing depth (PD) 5 mm or greater; moderate periodontitis is defined as 2 or more interproximal sites with clinical attachment level 4 mm or greater (not on the same tooth) or 2 or more interproximal sites with PD 5 mm or greater, also not on the same tooth; and mild periodontitis is defined as 2 or more interproximal sites with clinical attachment level 3 mm or greater and 2 or more interproximal sites with PD 4 mm or greater (not on the same tooth) or 1 site with PD 5 mm or greater.32
Other variables of interest
Age, sex, race, body mass index (BMI), and lipid profile were measured according to published methods.33 A variable representing race and ethnicity (African American or white) and ARIC examination field center was also included to adjust for ethnic, regional, and dental examiner differences. Education was self-reported during ARIC visit 1 and categorized as basic (≤ 11 years), intermediate (12–16 years), and advanced (17–21 years). A 3-level smoking status was used in this report, including never smoker, former smoker, and current smoker at visit 4. Smoking status was obtained via interview of ARIC participants. BMI was interpreted using the CDC standard weight status categories for adults as follows: BMI less than 18.5 is underweight, BMI from 18.5 to less than 25 is normal weight, BMI from 25 to less than 30 is overweight, and BMI 30 or higher is obese.34 Hypertension was defined as the mean of 2 blood pressure readings at the visit (with systolic blood pressure cutoff of ≥ 140 millimeters of mercury and diastolic blood pressure cutoff of ≥ 90 mm Hg) or use of hypertension medication. Level of high-density lipoprotein (HDL) and triglycerides was dichotomized at less than 40 mg/dL and greater than 200 mg/dL, respectively. Whether participants took statins was also recorded.28
Statistical analysis
We first described the characteristics of the sample using counts and percentages or means and standard deviations, as appropriate. To screen the demographic and periodontal disease characteristics for potential relationships with UDM, χ2 test or analysis of variance was used. To determine the relationships with UDM, a 2-stage logistic regression model was used; model 1 included demographic characteristics, and model 2 added each of the 2 periodontal disease characterizations. Odds ratios (OR) with 95% confidence intervals (CIs) were reported after adjusting for race and center, sex, smoking status (3 levels), BMI, education, HDL, triglycerides, hypertension, and statins. All analyses were performed using SAS software, Version 9.4 (SAS Institute).
RESULTS
Rates of UDM at visit 4 were 5.8% overall, but the rate was 5.6% in study participants who had undergone a dental examination or were edentulous and 6.3% in those without a dental examination (P < .17). Table 1 provides demographic characteristics, including race, sex, age, BMI, smoking status, educational level, and diabetes risk factors (that is, HDL, triglycerides, hypertension, and taking statins) for participants with UDM versus those without diabetes for all visit 4 participants and those with a periodontal examination or edentulous. Overall, the results for participants with a periodontal examination were similar to those for all participants at visit 4. For the 7,343 study participants, those with a higher prevalence of UDM appear to be African American in Mississippi and North Carolina, male, have a higher BMI, and have a basic level of education. In addition, they are likely to have low HDL, triglycerides, and hypertension. The race and center variable not only designates race, but also regional, cultural, and dental examiner differences.
Table 1.
Demographic characteristics for visit 4 Atherosclerosis Risk in Communities study participants who were not diabetic (all participants versus participants who were edentulous and underwent a periodontal examination or were edentulous) according to their diabetes status at visit 4 (undiagnosed versus not diabetic).
| CHARACTERISTIC | VISIT 4 PARTICIPANTS (N = 10,123) | PARTICIPANTS WITH PERIODONTAL EXAMINATION OR EDENTULISM (N = 7,343) | ||||
|---|---|---|---|---|---|---|
| Undiagnosed | Not Diabetic | P Value | Undiagnosed | Not Diabetic | P Value | |
| Center (Race), No. (%) | NA* | NA | < .0001 | NA | NA | < .0001 |
| Jackson, Mississippi (African American) | 155 (9) | 1,639 (91) | NA | 103 (8) | 1,169 (92) | NA |
| North Carolina (white) | 109 (5) | 2,228 (95) | NA | 84 (5) | 1,645 (95) | NA |
| North Carolina (African American) | 16 (8) | 185 (92) | NA | 11 (8) | 125 (92) | NA |
| Maryland (white) | 175 (6) | 2,596 (94) | NA | 121 (6) | 1,893 (94) | NA |
| Suburban Minneapolis, Minnesota (white) | 131 (4) | 2,857 (96) | NA | 92 (4) | 2,077 (96) | NA |
| Sex, No. (%) | NA | NA | .0004 | NA | NA | .008 |
| Female | 289 (5) | 5396 (95) | NA | 201 (5) | 3,842 (95) | NA |
| Male | 299 (7) | 4139 (93) | NA | 211 (6) | 3,089 (94) | NA |
| Age, Years, Mean (Standard Deviation) | 63.0 (5.5) | 62.7 (5.7) | .20 | 62.9 (5.6) | 62.7 (5.7) | .32 |
| Body Mass Index, Mean (Standard Deviation) | 31.6 (5.9) | 28.2 (5.3) | < .0001 | 31.7 (6.0) | 28.2 (5.3) | < .0001 |
| Smoking Status, No. (%) | NA | NA | .01 | NA | NA | .06 |
| Never | 249 (6) | 4,230 (94) | NA | 170 (5) | 3,102 (95) | NA |
| Former | 247 (6) | 3,591 (94) | NA | 174 (6) | 2,586 (94) | NA |
| Current | 61 (4) | 1,330 (96) | NA | 46 (5) | 963 (95) | NA |
| Education, No. (%) | NA | NA | .0001 | NA | NA | .001 |
| Basic | 141 (8) | 1,655 (92) | NA | 102 (8) | 1,226 (92) | NA |
| Intermediate | 242 (6) | 4,049 (94) | NA | 163 (5) | 2,983 (95) | NA |
| Advanced | 203 (5) | 3,820 (95) | NA | 145 (5) | 2,714 (95) | NA |
| High-Density Lipoprotein, No. (%) | NA | NA | < .0001 | NA | NA | < .0001 |
| ≥ 40 milligrams/deciliter | 294 (11) | 2,411 (89) | NA | 212 (11) | 1,781 (89) | NA |
| < 40 mg/dL | 294 (4) | 7,124 (96) | NA | 200 (4) | 5,150 (96) | NA |
| Triglycerides, No. (%) | NA | NA | < .0001 | NA | NA | < .0001 |
| > 200 mg/dL | 194 (12) | 1,433 (88) | NA | 134 (11) | 1,056 (89) | NA |
| ≤ 200 mg/dL | 394 (5) | 8,102 (95) | NA | 278 (5) | 5,875 (95) | NA |
| Statins, No. (%) | NA | NA | .22 | NA | NA | .60 |
| Yes | 86 (7) | 1,227 (93) | NA | 55 (6) | 862 (94) | NA |
| No | 502 (6) | 8,291 (94) | NA | 357 (6) | 6,056 (94) | NA |
| Hypertension, No. (%) | NA | NA | < .0001 | NA | NA | < .0001 |
| Yes | 304 (9) | 3,250 (91) | NA | 206 (8) | 2,256 (92) | NA |
| No | 283 (4) | 6,237 (96) | NA | 205 (4) | 4,642 (96) | NA |
NA: Not applicable.
Table 2 details the demographic characteristics and risk factors for participants in each PPC-Stage plus edentulous category. Stage I (health) and edentulous are the largest groups, and stage IV (severe disease) is the smallest group; the demographic characteristics and risk factors differ among the stages. For example, African American participants in Mississippi are predominately in stage V or are edentulous and least likely to be in stages I through III; African American participants in North Carolina are predominately in stage VI or edentulous and least likely to be in stages III, II, and V. White participants in Minnesota are predominately in stages I and III and least likely to be in stage IV. Participants with a basic level of education are predominately edentulous or in stage VII and are least likely to be in stages III and II. Table 3 provides the same information according to the CDC-AAP index designations. The size of the groups differs from PPC-Stages, as the healthy group is the smallest and the moderate disease group is the largest. The healthy group is more likely to be white, female, and a never smoker, but the education levels are more balanced, although the demographic characteristics for the severe disease group are similar to those for the PPC-Stages.
Table 2.
Demographic characteristics and clinical tests for Atherosclerosis Risk in Communities visit 4 participants according to PPC-Stages.*
| VARIABLE | PPC-STAGE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| I (N = 1,741) | II (N = 964) | III (N = 918) | IV (N = 419) | V (N = 568) | VI (N = 707) | VII (N = 768) | Edentulous (N = 1,258) | P VALUE | |
| Center (Race), No. (%) | NA† | NA | NA | NA | NA | NA | NA | NA | < .0001 |
| Jackson, Mississippi (African American) | 36 (3) | 14 (1) | 6 (0) | 158 (12) | 401 (32) | 87 (7) | 213 (17) | 357 (28) | NA |
| North Carolina (white) | 617 (36) | 300 (17) | 118 (7) | 72 (4) | 44 (3) | 214 (12) | 128 (7) | 236 (14) | NA |
| North Carolina (African American) | 15 (11) | 10 (7) | 6 (4) | 16 (12) | 9 (7) | 31 (23) | 22 (16) | 27 (20) | NA |
| Maryland (white) | 266 (13) | 369 (18) | 238 (12) | 135 (7) | 64 (3) | 217 (11) | 249 (12) | 476 (24) | NA |
| Suburban Minneapolis, Minnesota (white) | 805 (37) | 268 (12) | 547 (25) | 35 (2) | 50 (2) | 155 (7) | 151 (7) | 158 (7) | NA |
| Sex, No. (%) | NA | NA | NA | NA | NA | NA | NA | NA | < .0001 |
| Female | 1,176 (29) | 450 (11) | 412 (10) | 153 (4) | 310 (8) | 389 (10) | 438 (11) | 715 (18) | NA |
| Male | 565 (17) | 514 (16) | 506 (15) | 266 (8) | 258 (8) | 318 (10) | 330 (10) | 543 (16) | NA |
| Age, Years, Mean (Standard Deviation) | 61.7 (5.4) | 62.3 (5.9) | 62.9 (5.6) | 61.8 (5.8) | 61.5 (5.5) | 63.7 (5.6) | 63.0 (5.5) | 64.2 (5.7) | < .0001 |
| Body Mass Index, Mean (Standard Deviation) | 27.2 (4.7) | 28.2 (4.8) | 28.0 (4.9) | 29.3 (6.0) | 29.7 (5.9) | 28.5 (5.0) | 29.3 (6.0) | 29.0 (6.0) | < .0001 |
| Smoking Status, No. (%) | NA | NA | NA | NA | NA | NA | NA | NA | < .0001 |
| Never | 961 (29) | 528 (16) | 332 (10) | 192 (6) | 291 (9) | 288 (9) | 277 (8) | 403 (12) | NA |
| Former | 633 (23) | 357 (13) | 413 (15) | 142 (5) | 174 (6) | 284 (10) | 296 (11) | 461 (17) | NA |
| Current | 117 (12) | 54 (5) | 145 (14) | 65 (6) | 62 (6) | 107 (11) | 158 (16) | 301 (30) | NA |
| Education, No. (%) | NA | NA | NA | NA | NA | NA | NA | NA | < .0001 |
| Basic | 88 (7) | 75 (6) | 54 (4) | 92 (7) | 133 (10) | 111 (8) | 207 (16) | 568 (43) | NA |
| Intermediate | 733 (23) | 429 (14) | 410 (13) | 167 (5) | 183 (6) | 346 (11) | 376 (12) | 502 (16) | NA |
| Advanced | 917 (32) | 459 (16) | 453 (16) | 160 (6) | 250 (9) | 250 (9) | 185 (6) | 185 (6) | NA |
| High-Density Lipoprotein, No. (%) | NA | NA | NA | NA | NA | NA | NA | NA | < .0001 |
| ≥ 40 milligrams/deciliter | 334 (17) | 301 (12) | 273 (14) | 127 (6) | 130 (7) | 215 (11) | 223 (11) | 390 (20) | NA |
| < 40 mg/dL | 1,407 (26) | 663 (15) | 645 (12) | 292 (5) | 438 (9) | 492 (9) | 545 (10) | 868 (16) | NA |
| Triglycerides, No. (%) | NA | NA | NA | NA | NA | NA | NA | NA | < .0001 |
| > 200 mg/dL | 299 (25) | 186 (16) | 156 (13) | 33 (3) | 56 (5) | 129 (11) | 128 (11) | 203 (17) | NA |
| ≤ 200 mg/dL | 1,442 (23) | 778 (13) | 762 (12) | 386 (6) | 512 (8) | 578 (9) | 640 (10) | 1,055 (17) | NA |
| Statins, No. (%) | NA | NA | NA | NA | NA | NA | NA | NA | .009 |
| Yes | 219 (24) | 131 (14) | 120 (13) | 39 (4) | 45 (5) | 94 (10) | 94 (10) | 175 (19) | NA |
| No | 1,521 (24) | 833 (13) | 798 (12) | 380 (6) | 520 (8) | 613 (10) | 670 (10) | 1,078 (17) | NA |
| Hypertension, No. (%) | NA | NA | NA | NA | NA | NA | NA | NA | < .0001 |
| Yes | 444 (18) | 292 (12) | 246 (10) | 171 (7) | 243 (10) | 255 (10) | 275 (11) | 536 (22) | NA |
| No | 1,294 (27) | 668 (14) | 669 (14) | 247 (5) | 321 (7) | 451 (9) | 486 (10) | 711 (15) | NA |
PPC-Stages: Periodontal Profile Classes system adapted to stages.
NA: Not applicable.
Table 3.
Demographic characteristics and clinical tests for Atherosclerosis Risk in Communities visit 4 participants according to CDC-AAP* index.
| CHARACTERISTIC | CDC-AAP INDEX | |||||
|---|---|---|---|---|---|---|
| Healthy (N = 701) | Mild (N = 1,874) | Moderate (N = 2,483) | Severe (N = 1,027) | Edentulous (N = 1,258) | P Value | |
| Center (Race), No. (%) | NA† | NA | NA | NA | NA | < .0001 |
| Jackson, Mississippi (African American) | 83 (7) | 352 (28) | 311 (24) | 169 (13) | 357 (28) | NA |
| North Carolina (white) | 251 (15) | 560 (32) | 537 (31) | 145 (8) | 236 (14) | NA |
| North Carolina (African American) | 8 (6) | 23 (17) | 36 (26) | 42 (31) | 27 (20) | NA |
| Maryland (white) | 156 (8) | 347 (17) | 660 (33) | 375 (19) | 476 (24) | NA |
| Suburban Minneapolis, Minnesota (white) | 202 (9) | 589 (27) | 932 (43) | 288 (13) | 158 (7) | NA |
| Sex, No. (%) | NA | NA | NA | NA | NA | < .0001 |
| Female | 517 (13) | 1,205 (30) | 1,288 (30) | 378 (9) | 715 (18) | NA |
| Male | 184 (6) | 669 (20) | 1,255 (38) | 649 (20) | 543 (16) | NA |
| Age, Years, Mean (Standard Deviation) | 61.8 (5.5) | 61.6 (5.6) | 62.8 (5.6) | 63.0 (5.6) | 64.1 (5.7) | < .0001 |
| Body Mass Index, Mean (Standard Deviation) | 28.3 (5.6) | 28.0 (5.2) | 28.4 (5.0) | 28.5 (5.4) | 29.0 (6.0) | < .0001 |
| Smoking Status, No. (%) | NA | NA | NA | NA | NA | < .0001 |
| Never | 426 (13) | 1,065 (33) | 1,058 (32) | 320 (10) | 403 (12) | NA |
| Former | 208 (8) | 614 (22) | 1,039 (38) | 438 (16) | 461 (17) | NA |
| Current | 47 (5) | 144 (14) | 292 (29) | 225 (22) | 301 (30) | NA |
| Education, No. (%) | NA | NA | NA | NA | NA | < .0001 |
| Basic | 77 (6) | 176 (13) | 325 (24) | 182 (14) | 568 (43) | NA |
| Intermediate | 342 (11) | 771 (25) | 1,082 (34) | 449 (14) | 502 (16) | NA |
| Advanced | 281 (10) | 925 (32) | 1,072 (38) | 396 (14) | 185 (6) | NA |
| High-Density Lipoprotein, No. (%) | NA | NA | NA | NA | NA | < .0001 |
| ≥ 40 milligrams/deciliter | 130 (7) | 413 (21) | 738 (37) | 322 (16) | 390 (20) | NA |
| < 40 mg/dL | 571 (11) | 1,461 (27) | 1,745 (33) | 705 (13) | 868 (16) | NA |
| Triglycerides, No. (%) | NA | NA | NA | NA | NA | .18 |
| > 200 mg/dL | 114 (10) | 307 (26) | 425 (33) | 141 (12) | 203 (17) | NA |
| ≤ 200 mg/dL | 587 (10) | 1,567 (25) | 2,058 (36) | 886 (14) | 1,055 (17) | NA |
| Statins, No. (%) | NA | NA | NA | NA | NA | .50 |
| Yes | 84 (9) | 222 (24) | 307 (33) | 129 (14) | 175 (19) | NA |
| No | 614 (10) | 1,650 (26) | 2,174 (34) | 897 (14) | 1,078 (17) | NA |
| Hypertension, No. (%) | NA | NA | NA | NA | NA | < .0001 |
| Yes | 236 (10) | 614 (25) | 748 (30) | 328 (13) | 536 (22) | NA |
| No | 459 (9) | 1,254 (26) | 1,724 (36) | 699 (14) | 711 (15) | NA |
CDC-AAP: Centers for Disease Control and Prevention and American Academy of Periodontology.
NA: Not applicable.
Table 4 explores the relationships among demographics, risk factors, and UDM (model 1), as well as the additional contribution (model 2) of the 2 periodontal disease measures (PPC-Stages plus edentulous and the CDC-AAP index plus edentulous). Model 1 indicated that BMI, African American participants in Mississippi, HDL, triglycerides, and hypertension were significantly associated with UDM. Model 2 found that when PPC-Stages and being edentulous were added to model 1, the type III P value was significant (P = .009) and both being edentulous (OR, 1.87; 95% CI, 1.27 to 2.75) and PPC-Stage IV (OR, 1.78; 95% CI, 1.10 to 2.88) were significant, indicating that they were independently associated with UDM. When the CDC-AAP index plus edentulous was added to model 1, the type III P value was .007 and only being edentulous was independently associated with UDM (OR, 1.70; 95% CI, 1.08 to 2.67).
Table 4.
Odds ratios (95% confidence intervals) for associations between demographic variables, periodontal disease, and undiagnosed diabetes.
| VARIABLE | DATA |
|---|---|
| Demographics | NA§ |
| Model 1, OR* (95% CI†)‡ | NA |
| Body mass index (n = 7,343) | 1.08 (1.07 to 1.10) |
| Age (n = 7,343) | 1.02 (1.00 to 1.04) |
| Male (n = 3,300) | 1.12 (0.88 to 1.42) |
| Center (race) | NA |
| Suburban Minneapolis, Minnesota (white) (n = 2,169) | [Reference] |
| North Carolina (African American) (n = 136) | 1.97 (0.99 to 3.93) |
| North Carolina (white) (n = 1,729) | 1.19 (0.87 to 1.63) |
| Maryland (white) (n = 2,014) | 1.26 (0.94 to 1.69) |
| Jackson, MS (African American) (n = 1,272) | 2.02 (1.44 to 2.81) |
| Education | NA |
| Advanced (n = 2,859) | [Reference] |
| Intermediate (n = 3,146) | 0.98 (0.70 to 1.24) |
| Basic (n = 1,328) | 1.11 (0.83 to 1.48) |
| Smoking status | NA |
| Never (n = 3,272) | [Reference] |
| Former (n = 2,760) | 1.13 (0.90 to 1.42) |
| Current (n = 1,009) | 0.99 (0.71 to 1.37) |
| High-density lipoprotein < 40 milligrams/deciliter (n = 1,993) | 2.35 (1.86 to 2.98) |
| Triglycerides > 200 mg/dL (n = 1,190) | 2.16 (1.69 to 2.74) |
| Statins (n = 917) | 0.95 (0.70 to 1.29) |
| Hypertension (n = 2,462) | 1.45 (1.17 to 1.80) |
| Periodontal Profile Classes System Adapted to Stages¶ | NA |
| Model 2 (model 1 + Periodontal Profile Classes system adapted to stages), OR (95% CI) | NA |
| Stage I, health (n = 1,741) | [Reference] |
| Stage II, mild (n = 964) | 1.10 (0.73 to 1.66) |
| Stage III, moderate (n = 918) | 0.88 (0.55 to 1.38) |
| Stage IV, severe (n = 419) | 1.78 (1.10 to 2.88) |
| Stage V mild TL# (n = 568) | 1.19 (0.73 to 1.94) |
| Stage VI, moderate TL and reduced periodontium (n = 707) | 1.24 (0.81 to 1.92) |
| Stage VII, severe TL (n = 768) | 1.38 (0.90 to 2.11) |
| Edentulous (n = 1,258) | 1.87 (1.27 to 2.75) |
| Type III P value | .009 |
| Centers for Disease Control and Prevention and American Academy of Periodontology Index | NA |
| Model 2 (model 1 + Centers for Disease Control and Prevention and American Academy of Periodontolgy), OR (95% CI) | NA |
| Health (n = 701) | [Reference] |
| Mild disease (n = 1,874) | 0.91 (0.58 to 1.42) |
| Moderate disease (n = 2,483) | 1.22 (0.80 to 1.87) |
| Severe disease (n = 1,027) | 1.21 (0.75 to 1.95) |
| Edentulous (n = 1,258) | 1.70 (1.08 to 2.67) |
| Type III P value | .007 |
OR: Odds ratio.
CI: Confidence interval.
Also adjusted for race and center.
NA: Not applicable.
Statistically significant
TL: Tooth loss.
DISCUSSION
Severe periodontal disease (PPC-Stage IV) was moderately but significantly associated with UDM after adjusting for demographic characteristics and known diabetes risk factors, but the CDC-AAP index was not related to UDM. We also found that study participants who were edentulous were also more likely to have UDM (Table 4). Both of these associations can be useful when targeting patients for diabetes screening in dental offices. As we indicated earlier in this article, researchers have reported previously that obesity is also a risk factor for diabetes. Table 5 provides the rates of UDM according to PPC-Stage, edentulousness, and obesity. Participants who were obese were more strongly associated with UDM than those who were not obese (9.9% versus 3.7%), and among patients who were obese, those who were edentulous were more likely to have UDM (12.6% versus 9.9%). For every PPC-Stage, patients who were obese were approximately twice as likely to have UDM than patients who were not obese at that stage. In their 2011 study, Lalla and colleagues35 proposed that dentists screen for diabetes in high-risk groups (that is, those who are overweight or obese, have a family history of diabetes, hypertension, or high cholesterol). Our findings from model 1 in Table 4 would support this proposition, as all of these conditions were significantly associated with UDM except family history, which was not available to be in the model. Although more participants who were obese in our study had UDM, there were also participants without obesity and with UDM. Table 5 provides the percentage of study participants without obesity and with UDM by PPC-Stage. Overall, 3.7% of participants without obesity had UDM, but those classified in PPC-Stages IV through VII were more likely to have UDM.
Table 5.
Rates of undiagnosed diabetes according to PPC-Stages* plus edentulous for study participants who were and were not obese.
| PPC-STAGES | ALL (%) | NOT OBESE (%) | OBESE (%) |
|---|---|---|---|
| I | 3.4 | 2.0 | 8.1 |
| II | 4.9 | 2.1 | 11.6 |
| III | 3.6 | 2.3 | 6.6 |
| IV | 8.4 | 5.9 | 12.2 |
| V | 6.5 | 5.0 | 8.9 |
| VI | 5.7 | 4.9 | 7.2 |
| VII | 6.5 | 4.0 | 10.8 |
| Edentulous | 8.8 | 6.6 | 12.6 |
| Total | 5.6 | 3.7 | 9.9 |
PPC-Stages: Periodontal Profile Classes system adapted to stages.
The Figure shows a decreasing prevalence of fasting glucose levels less than 126 mg/dL (pre-diabetes), an increasing prevalence of fasting glucose levels from 126 through 239 mg/dL (diabetes), and a positive trend between increasing BMI and high fasting glucose levels (≥ 240 mg/dL). However, even patients who were underweight and normal weight had hyperglycemia. Our data indicate that dentists who perform chairside screening for diabetes detect UDM in all patients regardless of BMI, confirming the conclusions of a 2014 study by Genco and colleagues,17 that screening for diabetes and prediabetes in the dental setting might provide an important benefit to patients. If diabetes screening for all dental patients is not possible, clinicians should screen patients who are obese as well as patients who are not but who are edentulous or have more severe subtypes of periodontal disease. Although patients who are edentulous might not use dental services on a regular basis, our findings indicate they are approximately twice as likely to have UDM as dentate patients. Considering many more patients see dentists than physicians annually, dentists performing diabetes screening could detect cases of UDM and refer them for medical care. We recommend screening for diabetes in all patients and not just those who are obese.
Figure.
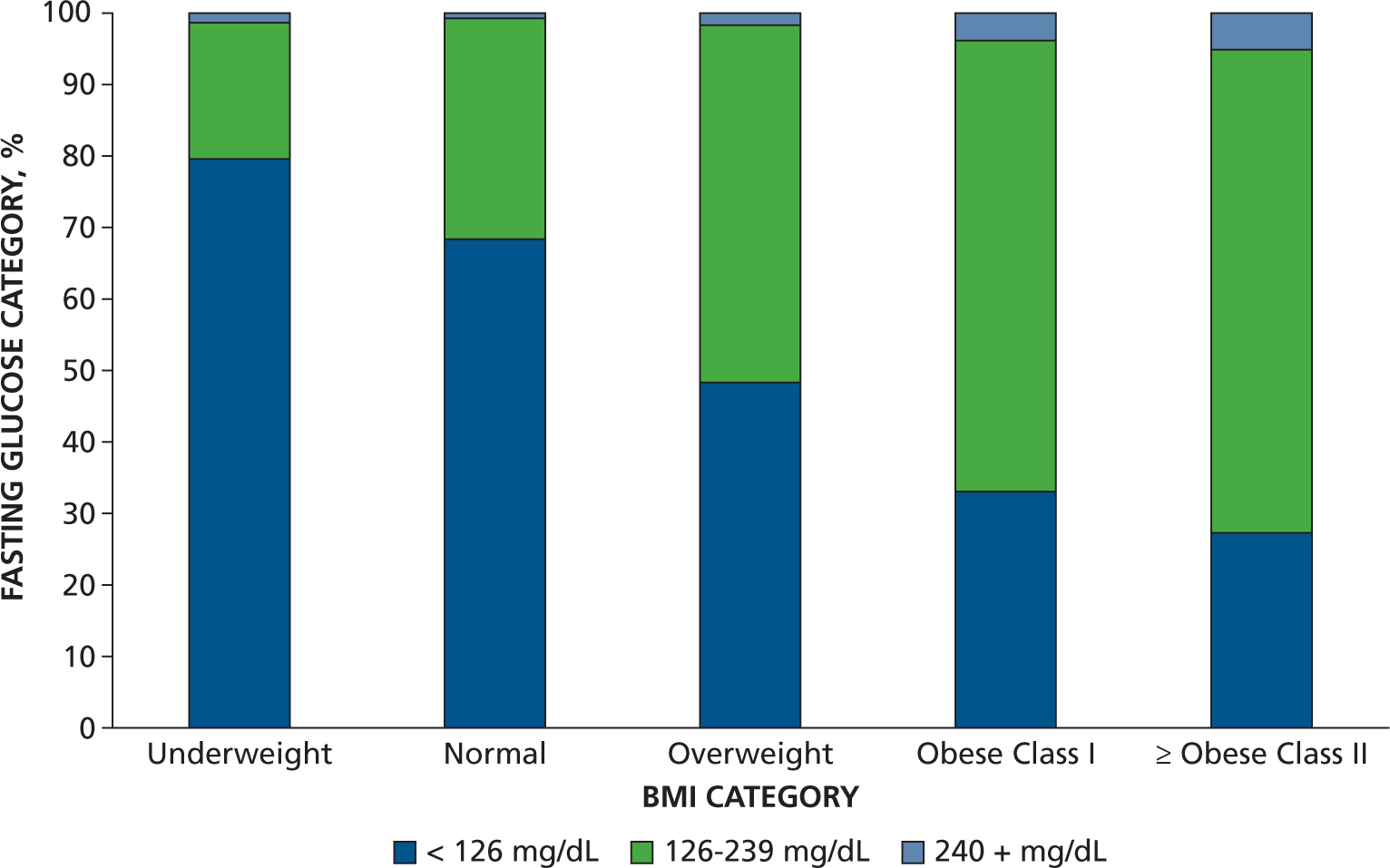
Percentage fasting glucose category according to body mass index (BMI) category (underweight, normal, overweight, obese class I, obese class II and higher). Fasting glucose levels were divided into the following categories: < 126 milligrams/deciliter = normal; 126–239 mg/dL = diabetes; 240+ mg/dL = potential diabetic emergency (ketoacidosis).
Strengths and limitations
Study strengths include a large sample size from a community cohort with multiple biomarkers, medical histories, rigorous methodology, and thorough periodontal examinations that measured all teeth at 6 sites per tooth. Limitations include that ARIC participants had a mean age of 63 years and represent an older group; the national mean age is 36 years. However, national data (National Health and Nutrition Examination Survey 2003–2004) indicate that the mean age of people with periodontal disease is 54 years21; ARIC participants are approximately 9 years older. Study participants were selected randomly to be representative of their communities. Over time, however, they have become less representative owing to mortality, loss to follow-up, and changes in the communities. They might remain representative of others who had the same life experiences but are no longer considered to be representative of their communities. We did not include prediabetes in our study because there was no question in the study protocol to detect whether participants had been told that they had prediabetes.
To evaluate whether our estimates of UDM were inflated, we investigated whether undergoing the periodontal examination made a difference in the odds of having UDM. Major reasons for not undergoing the examination included edentulism, refusal of examination, or ineligibility. Participants who did not undergo the periodontal examination were 34% more likely to have UDM (OR, 1.34; 95% CI, 1.12 to 1.60). This indicates that we did not overestimate the likelihood of having UDM in participants who underwent the periodontal examination. Both of these models were adjusted for race and center, sex, age, smoking (3 levels), BMI, and education (data not shown).
The PPC-Stages definition of periodontal disease predicted significant associations with UDM, and the traditional CDC-AAP index in its standard form found no significant relationship except for the participants who were edentulous. We did not expect to find a relationship between the CDC-AAP index and UDM because this classification is infrequently associated with systemic diseases and conditions, but it was the best definition that we could find for comparison. We searched for clinically relevant periodontal definitions, such as the 2014 update to the 1999 Classification of Periodontal Diseases and Conditions that the AAP proposed,36 as well as the 2017 index from the World Workshop on Periodontal and Peri-Implant Disease Classification.37 However, we were unable to use these definitions because they lack a “healthy” category for reference in this age group. Heterogeneous definitions of periodontal disease have complicated the interpretation of epidemiologic studies in the past, but we think our results showed that PPC-Stages improve measurement of the phenotype and indicate that there are subtypes of periodontal disease that help specify associations between periodontal subtypes and systemic diseases.
CONCLUSIONS
Severe periodontal disease and edentulousness are moderately associated with UDM when using the PPC-Stages definition of periodontal disease. Although screening for medical conditions in dental offices is a complex issue, our study results indicate that screening can benefit the health of dental patients referred for medical care. The greatest benefit would come from dentists screening all patients, but if this is not possible, screening all patients who are obese, patients who are not but have severe periodontal disease, and patients who are edentulous would result in the greatest likelihood of detecting UDM.
Acknowledgments
The Atherosclerosis Risk in Communities study was funded in whole or in part by grants HHSN268201700001I, HHSN268201700002I, HHSN26820173I, HHSN268201700004I, and HHSN268201700005I from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH) and the US Department of Health and Human Services. The Atherosclerosis Risk in Communities dental study was funded by grants R01-DE021418 from the National Institute of Dental and Craniofacial Research, NIH, R01-DE021986 from the National Institute of Dental and Craniofacial Research, and USA UL1-TR001111 from the National Center for Research Resources, NIH. Dr. Zhang received funding from grant R00DE027086 from the National Institute of Dental and Craniofacial Research, NIH.
The authors thank the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions.
ABBREVIATION KEY
- ARIC
Atherosclerosis Risk in Communities.
- BMI
Body mass index.
- CDC
Centers for Disease
- AAP
Control and Prevention and American Academy of Periodontology.
- DM
Diabetes mellitus.
- HDL
High-density lipoprotein.
- PPC
Periodontal Profile
- Stages
Classes system adapted to stages.
- PD
Probing depth.
- TL
Tooth loss.
- UDM
Undiagnosed diabetes mellitus.
Footnotes
This article has an accompanying online continuing education activity available at: http://jada.ada.org/ce/home.
Disclosure. None of the authors reported any disclosures.
Contributor Information
Kamaira H. Philips, Division of Oral and Craniofacial Health Sciences, Adams School of Dentistry, University of North Carolina at Chapel Hill, Koury Oral Health Sciences Building, 385 S. Columbia St, CB 7450, Chapel Hill, NC 27599.
Shaoping Zhang, Periodontics Department, College of Dentistry, University of Iowa, Iowa City, IA..
Kevin Moss, Division of Oral and Craniofacial Health Sciences, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC..
Katharine Ciarrocca, Division of Diagnostic Sciences-Oral Medicine, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC..
James D. Beck, Division of Comprehensive Oral Health-Periodontology, Adams School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC..
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 8th ed.) Brussels, Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33-(suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, Division of Diabetes Translation; 2017. [Google Scholar]
- 4.Fang M. Trends in the prevalence of diabetes among US adults: 1999–2016. Am J Prev Med. 2018;55(4):497–505. [DOI] [PubMed] [Google Scholar]
- 5.Dall TM, Yang W, Gillespie K, et al. The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes, and pre-diabetes. Diabetes Care. 2019:dc181226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Löe H. Periodontal disease: the sixth complication of diabetes mellitus. Diabetes Care. 1993;16(1):329–334. [PubMed] [Google Scholar]
- 7.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2014;15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Clin Periodontol. 2013;40(suppl 14):S153–S163. [DOI] [PubMed] [Google Scholar]
- 9.Lim L, Tay F, Sum C, Thai A. Relationship between markers of metabolic control and inflammation on severity of periodontal disease in patients with diabetes mellitus. J Clin Periodontol. 2007;34(2):118–123. [DOI] [PubMed] [Google Scholar]
- 10.Beck JD, Moss KL, Morelli T, Offenbacher S. Periodontal profile class is associated with prevalent diabetes, coronary heart disease, stroke, and systemic markers of C-reactive protein and interleukin-6. J Periodontol. 2018; 89(2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayo Clinic. Diabetes. Available at: https://www.mayoclinic.org/diseases-conditions/diabetes/symptoms-causes/syc-20371444. Accessed July 20, 2019.
- 12.Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol. 2010;81(12):1708–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aung K, Lorenzo C, Hinojosa MA, Haffner SM. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab. 2014; 99(2):462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001; 161(13):1581–1586. [DOI] [PubMed] [Google Scholar]
- 15.Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76(11 suppl):2075–2084. [DOI] [PubMed] [Google Scholar]
- 16.Pischon N, Heng N, Bernimoulin J-P, Kleber BM, Willich SN, Pischon T. Obesity, inflammation, and periodontal disease. J Dent Res. 2007;86(5):400–409. [DOI] [PubMed] [Google Scholar]
- 17.Genco RJ, Schifferle RE, Dunford RG, Falkner KL, Hsu WC, Balukjian J. Screening for diabetes mellitus in dental practices: a field trial. JADA. 2014;145(1):57–64. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Table 78: dental visits in the past year, by selected characteristics:—United States, selected years 1997–2016. Available at: https://www.cdc.gov/nchs/data/hus/2017/078.pdf. Accessed August 3, 2019.
- 19.Centers for Disease Control and Prevention. National Ambulatory Medical Care Survey: 2016 National Summary Tables. Available at: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf. Accessed August 3, 2019.
- 20.Strauss SM, Russell S, Wheeler A, Norman R, Borrell LN, Rindskopf D. The dental office visit as a potential opportunity for diabetes screening: an analysis using NHANES 2003–2004 data. J Public Health Dent. 2010; 70(2):156–162. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 2003–2004. Hyattsville, MD: National Center for Health Statistics; 2004. [Google Scholar]
- 22.Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000;16(4):230–236. [DOI] [PubMed] [Google Scholar]
- 23.Vernillo AT. Dental considerations for the treatment of patients with diabetes mellitus. JADA. 2003;134(suppl 1):24S–33S. [DOI] [PubMed] [Google Scholar]
- 24.Barasch A, Safford MM, Qvist V, Palmore R, Gesko D, Gilbert G; for The Dental Practice-Based Research Network Collaborative Group Random blood glucose testing in dental practice: a community-based feasibility study from The Dental Practice-Based Research Network. JADA. 2012;143(3):262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barasch A, Gilbert GH, Spurlock N, Funkhouser E, Persson LL, Safford MM; for the DPBRN Collaborative Group. Random plasma glucose values measured in community dental practices: findings from the Dental Practice-Based Research Network. Clin Oral Investig. 2013;17(5): 1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 27.Schneider AL, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the atherosclerosis risk in communities study. Am J Epidemiol. 2012;176(8):738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. Relationship of periodontal disease to carotid artery intima-media wall thickness: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2001; 21(11):1816–1822. [DOI] [PubMed] [Google Scholar]
- 29.Beck JD, Philips K, Moss K, Divaris K, Morelli T, Offenbacher S. Advances in precision oral health. Periodontology 2000. 2020;82(1):268–285. [DOI] [PubMed] [Google Scholar]
- 30.Agler CS, Moss K, Philips KH, et al. Biologically defined or biologically informed traits are more heritable than clinically defined ones: the case of oral and dental phenotypes. Adv Exp Med Biol. 2019;1197: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morelli T, Moss KL, Beck J, et al. Derivation and validation of the periodontal and tooth profile classification system for patient stratification. J Periodontol. 2017; 88(2):153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eke PI, Dye BA, Wei L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86(5):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck JD, Moss KL, Morelli T, Offenbacher S. Periodontal profile class is associated with prevalent diabetes, coronary heart disease, stroke, and systemic markers of C-reactive protein and interleukin-6. J Periodontol. 2018; 89(2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Defining adult overweight and obesity. Available at: https://www.cdc.gov/obesity/adult/defining.html; 2017. Accessed October 1, 2019.
- 35.Lalla E, Kunzel C, Burkett S, Cheng B, Lamster I. Identification of unrecognized diabetes and pre-diabetes in a dental setting. J Dent Res. 2011;90(7):855–860. [DOI] [PubMed] [Google Scholar]
- 36.American Academy of Periodontology Task Force Report on the Update to the 1999 Classification of Periodontal Diseases and Conditions. J Periodontol. 2015; 86(7):835–838. [DOI] [PubMed] [Google Scholar]
- 37.Caton JG, Armitage G, Berglundh T. A new classification scheme for periodontal and peri-implant diseases and conditions: Introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45(supplement 20): S1–S8. [DOI] [PubMed] [Google Scholar]


