INTRODUCTION
The outcome of B-cell precursor acute lymphoblastic leukemia (ALL) is different in children and adults, with overall survival (OS) rates at 5 years ranging from 90% to 45%.1,2 Significant needs also remain unmet in patients with B-cell non-Hodgkin lymphoma (NHL), with rates of refractory disease up to 20% according to histology in the rituximab era.3-5 In both ALL and NHL, many patients fail not only front-line but also salvage treatments, including allogeneic hematopoietic stem cell transplantation (alloHSCT). The therapeutic scenario for these patients with relapsed/refractory (R/R) disease is evolving, and immunotherapy is at the forefront. It took many years to move from the first to the current generation of bispecific antibodies that are changing the therapeutic landscape of acute leukemias and lymphomas.6,7 Today the ability to produce recombinant antibodies allows the generation of bispecific antibodies with defined pharmacological properties (Fig 1).8 Herein, we have reviewed the clinical development of antibodies designed to redirect the cytotoxic potential of nonantigen-specific T cells on specific antigens, such as CD19 and CD20 expressed on the cell surface of precursor and mature B lymphocytes.
FIG 1.
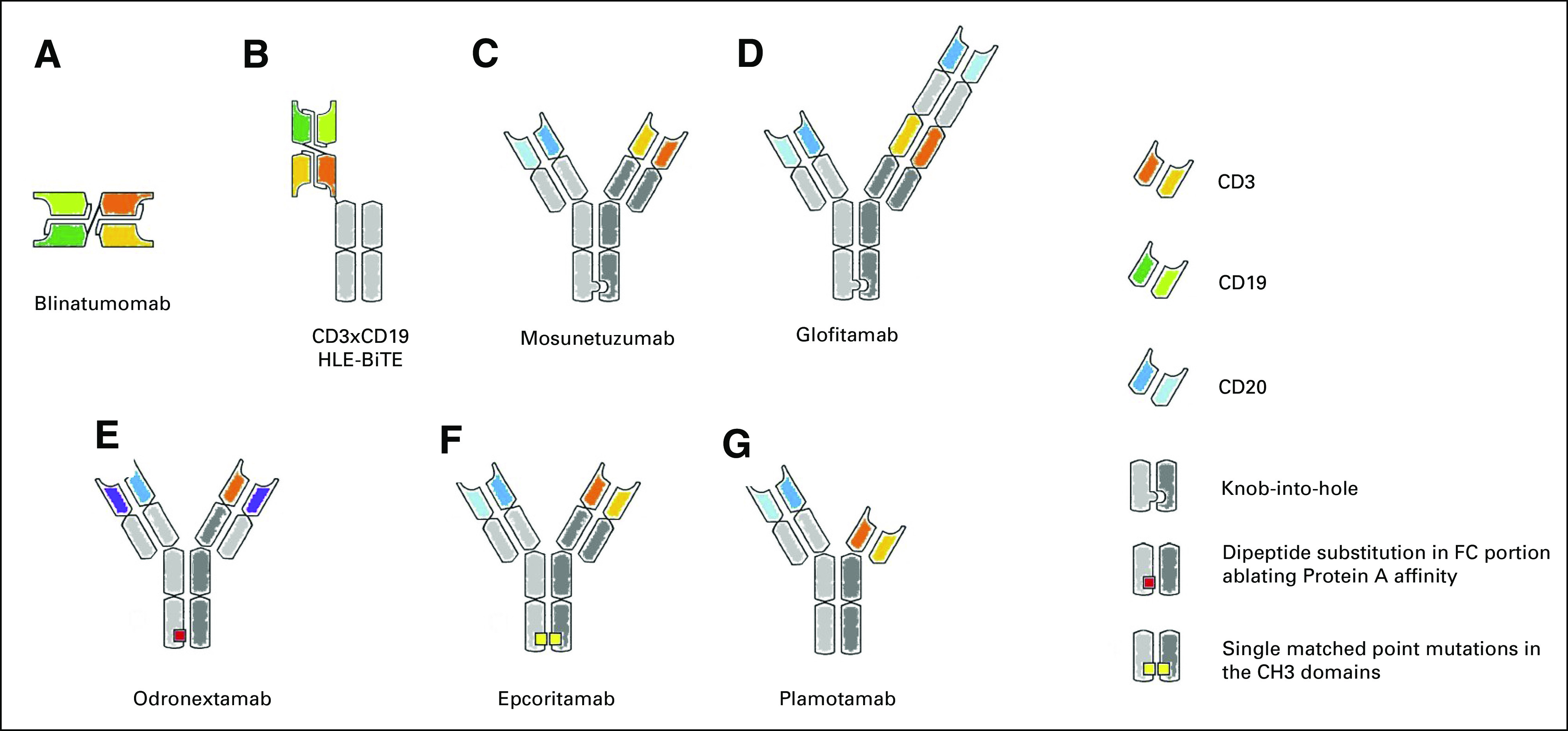
Main T cell–redirecting bispecific antibodies in clinical development. (A) Blinatumomab, the first bispecific T-cell engager (BiTE), is a tandem single-chain variable fragment (scFv). (B) To increase the half-life, the CD3xCD20 BiTE it is linked to a silent fragment crystallizable region (constant; FC) portion to form the half-life extended (HLE)-BiTE. (C, D) The knob-into-hole technology facilitates the correct pairing of FC portion of mosunetuzumab and glofitamab; this latter is characterized also by an asymmetric 2:1 format that incorporates bivalent binding to CD20 and monovalent binding to CD3 (CrossMAb). (E) Design of odronextamab exploits differences in the affinities of the immunoglobulin isotypes for Protein A coupled with the use of common light chain, allowing efficient large-scale purification. (F) In the Duo-Body, each parental antibody contains single matched point mutations in the constant region of the heavy chain 3 (CH3) domains, which allows the correct reassembly after in vitro separation (controlled fragment antigen-binding [Fab]-arm exchange). (G) Plamotamab uses FC domain variants that spontaneously form stable, heterodimeric bispecific antibodies allowing the use of standard antibody production methods. Different from the other molecules, the FC domain is functional.
CONTEXT
Key Objective
Will the use of T cell–redirected bispecific antibodies change the immunotherapy landscape of acute lymphoblastic leukemia and lymphoma?
Knowledge Generated
Blinatumomab, the first bispecific T-cell engager (BiTE), has been approved for relapsed/refractory acute lymphoblastic leukemia. It is more effective and better tolerated than conventional chemotherapy. It is the first antileukemic drug approved for the treatment of minimal residual disease, a new treatment paradigm in medical oncology. Blinatumomab and other BiTEs are currently evaluated also in relapse/refractory lymphomas. The preliminary results are promising, and BiTEs may become an alternative to chimeric antigen receptor T cells.
Relevance
The antineoplastic activity and the relative ease of use of BiTEs make them an attractive therapeutic option in front-line treatment. BiTEs could reduce the indication to allogeneic and autologous transplantation. At the same time, they could improve the transplantation outcome when given either as a prophylaxis or as a postrelapse treatment.
BLINATUMOMAB: THE FIRST BISPECIFIC T-CELL ENGAGER
Blinatumomab, the first bispecific T-cell engager (BiTE) among these revolutionary molecules,9 is an antibody composed of two single-chain variable antibody fragments (scFy) connected by a flexible linker. It binds specifically to CD19 expressed by precursor and mature B lymphocytes and CD3 expressed on the surface of T cells.9-12 This results in cytotoxic CD3+ T-cell engagement against CD19-expressing cells, bypassing the barrier physiologically represented by the unique, antigen-specific T-cell receptor and the major histocompatibility complex. CD19 antigen is widely expressed during normal B-cell ontogeny; therefore, it is the most reliable surface marker for B cells and a good target antigen in ALL, chronic lymphocytic leukemia, and NHL.13,14 Blinatumomab has a molecular weight of 54 kDa and a half-life of approximately 2 hours. It is metabolized in the bloodstream by protein cleavage into amino acids without any renal or hepatic clearance.15 Because of its short pharmacokinetics, a continuous intravenous infusion (CI) is required.16,17 Preclinical studies showed that there is no target saturation and that one T cell could engage more CD19+ cells.12
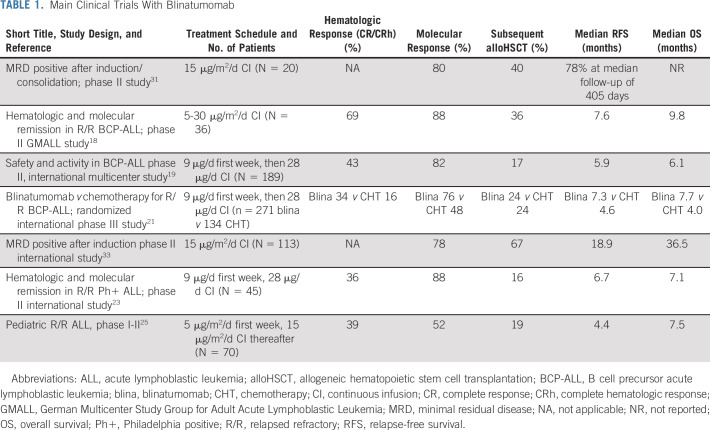
BLINATUMOMAB FOR R/R ALL: FROM CLINICAL TRIALS TO REAL-WORLD EXPERIENCE
Phase II studies led to the identification of the appropriate dose in R/R adult ALL (Table 1). The current treatment schedule is based on a ramp-up with 9 μg daily the first week followed by 28 μg daily for 28 days each subsequent cycle. Major achievements of blinatumomab in phase II studies have been the high proportion of complete hematologic response (CR/CRh), ranging from 43% to 69%, and the minimal residual disease (MRD) negativity obtained in approximately 80% of responders. About 40% of remitters could proceed to alloHSCT, and patients in first relapse did apparently better compared with second relapse or after a previous alloHSCT.18 The relapse-free survival (RFS) and OS ranged from 5-8 months and 6-10 months, respectively.18,19 A comparative analysis of these results also suggested a benefit for blinatumomab compared with a historical cohort,20 but the regular approval by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for R/R Philadelphia (Ph)-negative ALL came from a phase III trial, the TOWER trial (ClinicalTrials.gov identifier: NCT02013167). Compared with the salvage chemotherapy arm, blinatumomab-treated patients had a median OS of 7.7 months versus 4.4 months (P = .011), and OS resulted in favor of blinatumomab in each patient subgroup, such as age, prior salvage treatment, or prior alloHSCT. The CR rate was 34% versus 16% (P < .001), and event-free survival was 31% versus 12% at 6 months (P < .001). Among patients who achieved CR, 76% were MRD negative in the blinatumomab treatment group versus 48% in the chemotherapy group.21 The main blinatumomab toxicities were the cytokine release syndrome (CRS), neurotoxicity, and hypogammaglobulinemia.
TABLE 1.
Main Clinical Trials With Blinatumomab
After the European approval for the treatment of R/R Ph-negative ALL, blinatumomab has been made available to patients via an expanded access program. To minimize blinatumomab-related toxicities, patients (60% relapsed and 40% refractory) were pretreated with debulking chemotherapy or dexamethasone. Complete response was achieved by 51% (85% of these patients with MRD response), and 41% proceeded to alloHSCT. At 24 months after blinatumomab initiation, the median estimate of OS was 40% (36% after censoring for alloHSCT). Overall, the results obtained in this real-life setting confirm those achieved by clinical trials.22
The efficacy of blinatumomab has also been evaluated in R/R Ph-positive ALL in a multicenter single-arm trial (ALCANTARA; ClinicalTrials.gov identifier: NCT02000427). CR/CRh was achieved by 36% of patients during the first two cycles, including some with the T315I mutation. Among responders, 88% of patients achieved a complete MRD response. Median RFS and OS were 6.7 and 7.1 months, and 44% of responders proceeded to alloHSCT.23 A propensity score analysis was performed to compare outcomes of patients treated with blinatumomab in the ALCANTARA study with those of an external cohort of patients receiving standard chemotherapy. A higher rate of CR/CRh (36% v 25%) and better OS with a hazard ratio of 0.81 (95% CI, 0.57 to 1.14) was seen after blinatumomab.24 The results in R/R Ph-positive patients are in keeping with those obtained in Ph-negative ALL. Although confirming the efficacy of blinatumomab in terms of hematologic and molecular response, the impact on duration of response remains less impressive.
These results have been confirmed in the pediatric setting. In a phase I/II dose-escalation/dose-expansion trial, blinatumomab was studied in patients with Ph-negative ALL, refractory or relapsed after at least two lines of therapy or alloHSCT. The pediatric recommended dose is 5 μg/m2/d for the first 7 days, then 15 μg/m2/d.25 Complete remis-sion was achieved by 39% of the patients, with MRD negativity in 52% of responders. Median RFS and OS were 4.4 and 7.5 months, respectively. The long-term follow-up of this study at 24 months showed a survival of 20%, and there was no difference in OS between transplanted and nontransplanted responders.26 When this study was compared with three historical comparator groups, single-agent blinatumomab treatment was associated with longer OS in comparison with standard chemotherapy.27 The randomized, phase III AALL1331 trial (ClinicalTrials.gov identifier: NCT02101853), conducted by the Children’s Oncology Group in patients < 30 years old, showed that blinatumomab is superior to standard chemotherapy as post-reinduction consolidation before alloHSCT, resulting in fewer and less-severe toxicities, higher rates of MRD response, greater likelihood of proceeding to alloHSCT, and improved RFS and OS.28
Blinatumomab proved effective even in some rare chemorefractory ALL subtypes, such as those bearing the t(17;19) and the related TCF3-HLF fusion gene, which is usually characterized by a high rate of treatment failure despite treatment intensification and alloHSCT.29
BLINATUMOMAB IS THE FIRST DRUG APPROVED FOR THE TREATMENT OF MRD
In both childhood and adult ALL, the persistence of MRD represents the most informative prognostic factor of poor outcome.30 The early results obtained in the R/R setting showed the ability of blinatumomab to induce not only hematologic but also molecular remission.18 For this reason, blinatumomab has also been extensively tested in patients with molecular evidence of MRD in first or later CR. In the first phase II pilot study conducted in 21 MRD-positive patients, a major MRD response was achieved in 80% of patients after one cycle of treatment. Most notably, 10 out of 11 patients with a high MRD load (≥ 10−2) achieved a molecular remission. The clinical outcome after achieving molecular remission was not different for patients having or not a subsequent alloHSCT. It is worth noting that a reduced transplant-related mortality was observed in this small cohort, suggesting that the sequence of inducing MRD response by blinatumomab followed by alloHSCT is safe. An RFS estimate of 61% at a median observation of 33 months was reported.31,32 A subsequent larger, confirmatory multicenter phase II study of 116 patients was performed enrolling adults with ALL in hematologic CR with a high MRD level (≥ 10−3). After one cycle of blinatumomab, the primary end point was achieved by 78% of patients.33 In a long-term follow-up analysis, the median OS was 36.5 months, and it was not reached among patients who achieved a complete MRD response after the first cycle of blinatumomab.34 On the basis of these results, in March 2018, the FDA granted the first approval to treat a patient in the MRD setting, confirmed on January 2019 by the EMA.
Before country-specific reimbursement, blinatumomab was made available to MRD-positive patients under an expanded access program. MRD assessment was undertaken as per local clinical practice, including flow cytometry and polymerase chain reaction. After blinatumomab, 68% proceeded to alloHSCT, being in CR in 88% of cases before transplantation. The median RFS was 27.6 months, and at 24 months the OS was 65%. These results confirmed that in a real-life setting, two cycles of blinatumomab are able to induce molecular response in the majority of patients.35
Blinatumomab proved effective when given alone or in combination with donor lymphocyte infusion to pediatric36,37 and adult patients with ALL relapsed after alloHSCT.38,39 Blinatumomab is also currently being investigated to prevent leukemia relapse post alloHSCT in high-risk patients for biologic characteristics (ClinicalTrials.gov identifier: NCT03114865), or MRD positivity (ClinicalTrials.gov identifier: NCT03982992), or both (ClinicalTrials.gov identifier: NCT02807883). On the same rationale, targeting leukemic blasts with BiTE against CD123 (ClinicalTrials.gov identifier: NCT02715011) and CD33 (ClinicalTrials.gov identifier: NCT02520427) is currently under investigation in the setting of acute myeloid leukemia,40 where other post-transplant strategies are rapidly developing.41
WHAT TO DO IN SECOND REMISSION AFTER BLINATUMOMAB: alloHSCT OR MAINTENANCE?
In both phase II and TOWER studies, the option to proceed to alloHSCT was left free after two cycles of blinatumomab. However, the available results do not show a clear clinical benefit for patients proceeding to transplantation.19,21 In particular, the results of the TOWER study after censoring for alloHSCT did not show a significant increase in terms of OS. In addition, blinatumomab continuation therapy (≥ six cycles) and maintenance were associated with an increased OS and RFS compared with patients not receiv-ing maintenance independently from consolidation with alloHSCT.42 In patients treated for MRD positivity in first CR, the OS was not different in patients who did or did not undergo transplantation, whereas for those treated in second or later CR, the outcome of patients who did not undergo transplantation was inferior.33 Nonetheless, in a long-term analysis of a phase II study conducted in the MRD setting, younger patients (≤ 35 years) who underwent alloHSCT had a better 3-year survival compared with those who did not (62% v 22%).43 In the real-life setting, alloHSCT after blinatumomab salvage therapy has been confirmed as an effective postremission treatment, with RFS at 2 years of 40%.44 No unexpected toxicities or increase rate of graft-versus-host disease grades II to IV were reported. At our institution, for patients < 55 years old, the alloHSCT remains the first option.
BLINATUMOMAB WITH CHEMOTHERAPY OR OTHER DRUGS IN FRONT-LINE TREATMENT
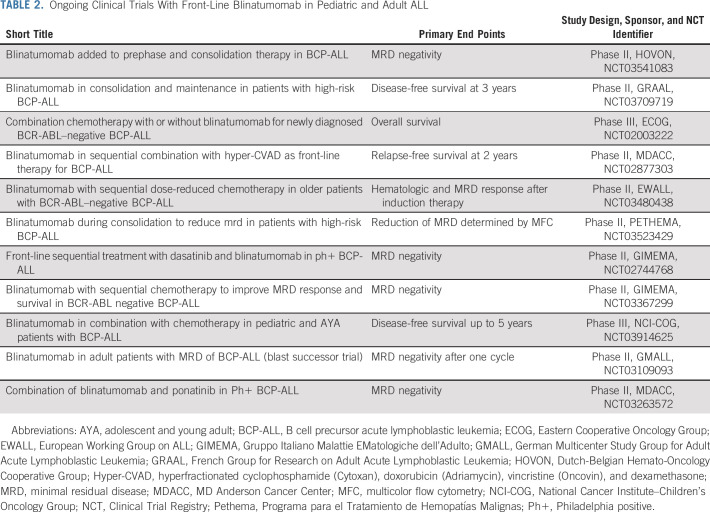
Several trials are currently evaluating blinatumomab as part of front-line treatment (Table 2; Fig 2). In this setting, blinatumomab is particularly attractive, because it is highly active in MRD-positive patients and well tolerated in patients undergoing alloHSCT, with no additional risk of venous occlusive disease or any other transplant-related toxicity.44 Thus, it is expected that incorporation of this immunotherapy will lead to a significant improvement of the cure rate in adult patients with ALL. Two phase III studies in adult (ClinicalTrials.gov identifier: NCT02003222) and pediatric (ClinicalTrials.gov identifier: NCT03914625) ALL are currently ongoing, with blinatumomab randomly added to conventional chemotherapy with the aim to improve RFS. Several phase II studies have been launched by cooperative study groups (Table 2). The primary end point is to increase the rate and depth of MRD negativity as a powerful surrogate of more robust clinical end points, such as RFS and OS. In these studies, blinatumomab has been added at different points during treatment, ranging from prephase to consolidation or maintenance. The results of these studies will help to establish the correct positioning of blinatumomab in the future up-front therapeutic regimens.
TABLE 2.
Ongoing Clinical Trials With Front-Line Blinatumomab in Pediatric and Adult ALL
FIG 2.
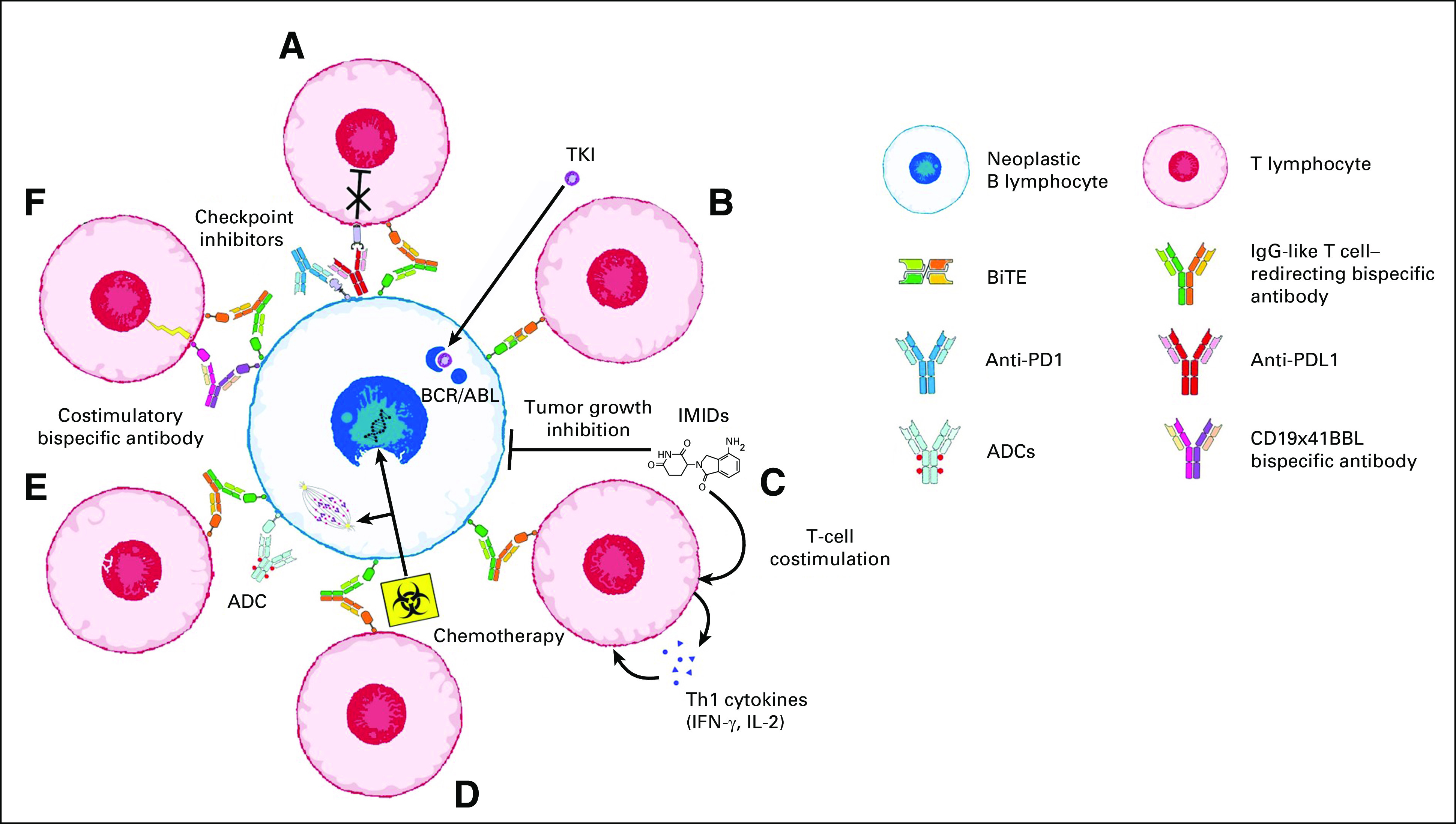
Combined use of T cell–redirecting bispecific antibodies in B-cell precursor acute lymphoblastic leukemia and B-cell non-Hodgkin lymphomas. Ongoing clinical trials (see Tables 2 and 3 for details) are evaluating the therapeutic activity of T cell–redirecting bispecific antibodies used in combination with (A) checkpoint inhibitors; (B) tyrosine kinase inhibitors (TKIs); (C) immunomodulatory drugs (IMIDs); (D) chemotherapy; (E) antibody-drug conjugates (ADCs); and (F) costimulatory bispecific antibodies.
The GIMEMA LAL2116 D-ALBA trial (ClinicalTrials.gov identifier: NCT02744768) evaluated a front-line chemotherapy-free regimen using the combination of dasatinib and blinatumomab for Ph-positive ALL. Dasatinib, 140 mg daily, was administered as induction for 85 days, followed by a postinduction consolidation with blinatumomab. At the end of the second cycle of blinatumomab, a molecular response was reported in 54% of patients, which further increased to 68% and 80% after the third and fourth cycles, respectively. The 12-month OS and RFS were 94% and 87%, respectively.45 A phase II trial using blinatumomab and ponatinib combination in patients > 60 years with previously untreated Ph-positive ALL is currently ongoing (ClinicalTrials.gov identifier: NCT03263572). Another intriguing combination is the use of blinatumomab after treatment with inotuzumab ozogamicin. A phase II study has been launched by the National Cancer Institute in newly diagnosed older adults or adults with R/R ALL (ClinicalTrials.gov identifier: NCT03739814).
The results from these studies may lead to the development of innovative chemotherapy-free treatment modalities for patients with ALL.
BLINATUMOMAB FOR R/R B-CELL NHL
Patients with R/R NHL were the first in whom blinatumomab was tested as a salvage treatment.9 In a phase I study, the maximum tolerated dose was identified as 60 μg/m2/d given by CI for 6 weeks, and strategies to mitigate the toxicity were implemented. Among the 35 patients treated at the target dose, the overall response rate (ORR) was 69% (CR rate, 37%), with an excellent durability of response (median response, 404 days).46 High response rates were observed in most lymphoma subtypes, including follicular (80%), mantle cell (71%), and diffuse large B-cell (DLBCL) lymphomas (55%). Despite the weekly dose escalation and preemptive dexamethasone treatment, neurotoxicity was the most relevant adverse event, occurring in 71% of the patients (22% grade 3, 0% grade 4-5). Although transient and without long-term effects, neurologic events represented the limiting toxicity.46,47
The encouraging ORR prompted further studies. In a phase II trial, 23 patients with DLBCL were treated with stepwise doses (9-28-112 μg/d, with weekly dose increases), with a target dose of 112 μg/d by CI for 8 weeks. The ORR among the 21 evaluable patients after one cycle was 36% (CR, 16%), and the exposure to at least 1 week of treatment at the target dose of 112 μg/d appeared to be required for efficacy. However, the need for 2 weeks of treatment at subtherapeutic doses (9-28 μg/d) limited the potential efficacy of the molecule in this setting of rapidly proliferating lymphomas, and a more aggressive dose escalation schedule was hampered by severe neurotoxicity.48 In another phase II study, blinatumomab was evaluated as a bridge to autologous HSCT in patients with aggressive NHL not responding to platinum-based salvage therapy. Stepwise blinatumomab (9-28-112 μg/d) was given in a 70-day cycle and optional second 28-day cycle. Of the 41 patients enrolled, 68% were refractory to the first salvage therapy, and a high rate of discontinuation due to disease progression (41%) was observed during the first treatment cycle. Despite that the exposure to blinatumomab was lower than anticipated, the ORR after 12 weeks was 37% (CR, 22%), and 20% of patients subsequently received autologous transplantation.49 Additional studies to treat residual disease with blinatumomab are ongoing in the front-line setting,50 and an MRD-driven therapy for DLBCL post autologous transplantation was terminated early because of low accrual (ClinicalTrials.gov identifier: NCT03298412). In some patients, the response achieved by blinatumomab was durable. The long-term results of the first phase I study showed that patients treated with ≥ 60 μg/m2 (n = 25) achieved an OS of 5.8 years, with six patients disease free after > 7 years.51 In another study with patients with DLBCL, 62% of complete responders were alive at 18 months.52 Thus, the meaningful long-term survival achieved with the first-in-class BiTE construct increases the hope that bispecific antibodies may lead to the cure of a proportion of chemotherapy-resistant NHL.
MECHANISMS OF RESISTANCE TO BLINATUMOMAB
Resistance to blinatumomab is attributable to characteristics of the disease or the patient’s immune system or both. Loss of target antigen expression has been proposed as a common mechanism of resistance to either chimeric antigen receptor T cells (CARTs) or blinatumomab. In retrospective analyses, performed in patients experiencing treatment failure on blinatumomab, < 20% had ALL recurrence with CD19-negative blasts.18,32,53 Alternative splicings or truncated CD19 variants can explain this loss of expression, but recently a disrupted CD19 membrane export in the post–endoplasmic reticulum compartment has been indicated as a possible molecular basis for CD19 loss on the cell surface.54 A myeloid lineage switch is also possible, particularly in the case of KMT2A(MLL)-rearranged ALL.55
Alternatively, an excessive number of T regulatory cells (T-regs) can play a key detrimental role on the therapeutic effect of blinatumomab. A threshold of T-regs < 10% in the peripheral blood has been proposed to identify patients with the highest likelihood to respond to blinatumomab.56 An increased expression of programmed death-ligand 1 (PD-L1) on T cells represents an additional potential immune escape mechanism.57 Two studies are exploring if the addition of pembrolizumab to blinatumomab, to enhance the activity of T cells and hence of blinatumomab, will improve the ORR in adults with R/R BCP-ALL (ClinicalTrials.gov identifiers: NCT03160079 and NCT03512405).
It should be remembered that in ALL a bone marrow blast infiltration > 50% is associated with a reduced probability to achieve CR.19 Thus, the optimal ratio between the CD3+ T cells and the target CD19+ cells is probably crucial.
These data have practical implications, because treatment strategies to tackle blinatumomab resistance may change according to the CD19 antigen loss. The therapeutic approaches may indeed vary from the combination of blinatumomab with checkpoint inhibitors58-60 in CD19-positive relapses to the use of CARTs with different affinity for CD19.61 The use of other immunotherapies, such as inotuzumab, in CD19-negative cases62 or bicistronic CARTs targeting CD19 and CD22,63,64 will be an alternative option.
NEW T CELL–REDIRECTED BISPECIFIC ANTIBODIES FOR B-CELL NHL
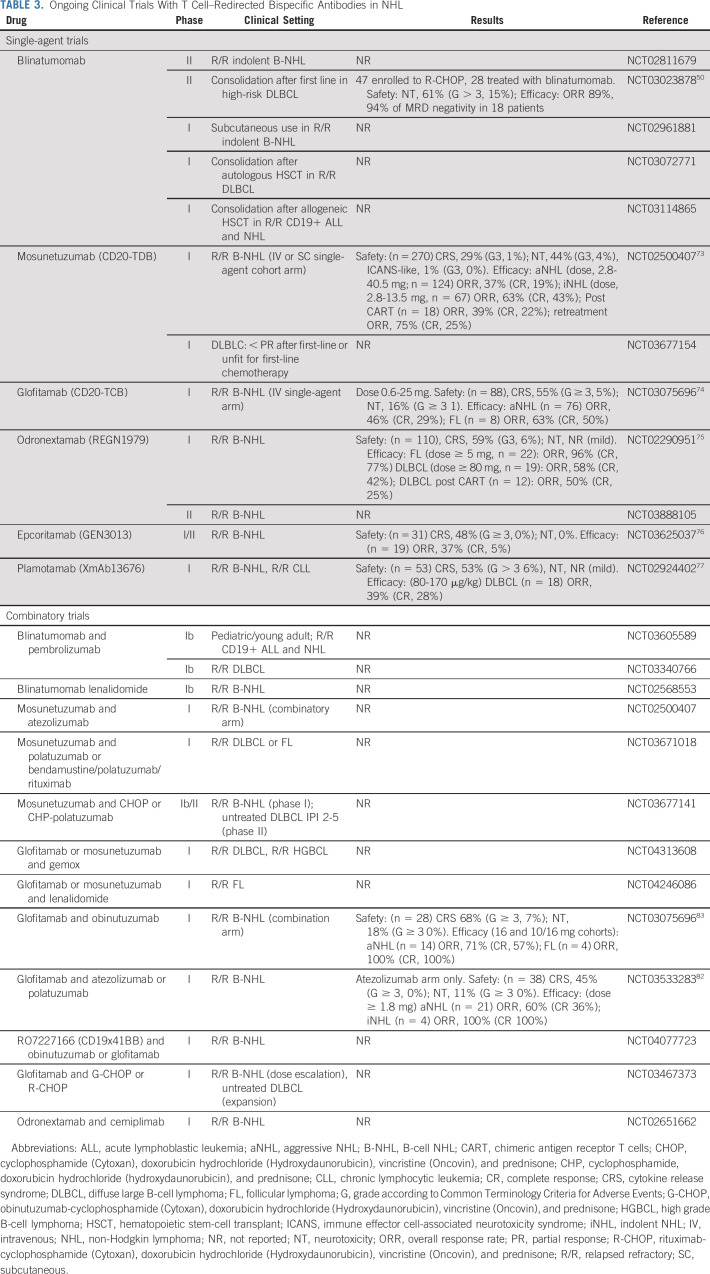
Although CARTs and checkpoint inhibitors rapidly achieved approval, no bispecific antibody has obtained an authorization market for NHL so far. Reasons for this delay included administration hurdles (ie, CI and need of repeated hospitalization) and difficulties in generating antibodies able to guarantee a relevant activity while avoiding immunogenicity.51,65 New scFV-based molecules have been generated to enable a once-weekly dosing as the next-generation BiTE antibody constructs modified by fusion to an Fc domain (extend half-life BiTE).66 However, near-physiologic half-life of > 10 days could be obtained by using a fully human or humanized immunoglobulin form, with or without a functional FC region (Fig 1). This was possible thanks to innovative technical solutions facing the issues of heterodimerization of heavy chains, as well as the correct coupling between heavy and light chains and the strategies of antibody purification.8 Among these, the knob-into-hole technology was developed to solve the heavy-chain mispairing and allowed the generation of the full-length IgG1-like molecule with near-native architecture CD3xCD20 mosunetuzumab (CD20-TDB, RG7828, RO7030816).67 The use of CrossMab technology, developed to allow a correct light chain mispairing, led to the generation of the 2:1 format bispecific antibody glofitamab (RG6026, CD20-TCB, RO7082859), in which two CD20 moieties are coupled with CD3.68 Other proprietary technologies have been developed allowing the generation of the fully human IgG4 CD3xCD20 bispecific antibody odronextamab (REGN1979),69 or the full-length CD3xCD20 bispecific IgG1 epcoritamab (GEN3013, DuoBody-CD3xCD20).70 Although all the IgG-like molecules discussed are provided by a nonfunctional FC portion, plamotamab (XmAb13676), a full-length CD3xCD20 bispecific antibody, was generated to permit the binding to FC receptor and thus enhance the T cell–mediated tumor killing.71 Many other molecules are in a preclinical development72; hereafter, we will briefly overview the early clinical results so far available with the previously described new molecules (Table 3). Compared with blinatumomab, most of the IgG-like molecules have been associated with lower incidence of neurotoxicity, observed in 21%-44% of the patients (grade ≥ 3: 0%-4%), but higher CRS, occurring in 29%-59% of the cases (grade ≥ 3: 1%-6%).73-77 With the aim to mitigate toxicity, additional strategies have been investigated. The increased antigen avidity of the 2:1 format of glofitamab allowed the use of anti-CD20 pretreatment to reduce antigen burden and, thus, toxicity.74 The use of subcutaneous administration of epcoritamab was reported to induce less cytokine secretion in a preclinical model, and this potentially safer route of administration is under clinical investigation.70,76 Beyond all the mitigation strategies adopted, CRS appears to be correlated with histology subtype and disease burden, with patients with bulky or leukemic disease being at higher risk. The future application of T cell–redirecting bispecific antibodies will largely depend on their improved safety profile and more practical and manageable delivery. It is worth noting the favorable toxicity profile of mosunetuzumab, which allowed its use also in the outpatient setting with severe CRS or neurological toxicity occurring in only 1% of cases.73
TABLE 3.
Ongoing Clinical Trials With T Cell–Redirected Bispecific Antibodies in NHL
Early efficacy data from IgG-like molecules confirmed the capability of bispecific antibodies to induce promising ORR (Table 3). With the limitation of the nature of most studies (ie, phase I dose-escalation trials), the reported ORR and CR rate for R/R aggressive lymphoma is in the range of 37%-58% and 19%-42%, respectively. As expected, indolent lymphoma showed a marked sensitivity to immunotherapy, with ORR ranging from 63% to 95% and CR rate of 43%-77% in the R/R setting.73-77 Most interestingly, bispecific antibodies were demonstrated to be active even in patients R/R to CD19-CART treatment and to be able to induce a second remission in those patients relapsing after achieving response to the first course of treatment.73,75
Emerging evidence is dissecting the pharmacodynamics of T cell–redirecting bispecific antibodies and could provide critical data to optimize the efficacy and dissociate activity from toxicity. Among the key findings, CD20 receptor occupancy proved to correlate with clinical response, thus informing the optimal biologic dose selection.78-80 In addition, the capability of T cells to be efficiently activated appears to be critical for tumor eradication, and strategies to further unleash T-cell activity or restore T-cell functionality are currently under investigation.81
Although many clinical trials with T cell–redirecting bispecific antibodies for NHL are currently ongoing as single-drug use, several others are investigating their potential synergism with different agents (Fig 2; Table 3).82,83 Suppression of T cell–mediated killing through PD-L1 was observed in NHL, and combination of checkpoint inhibitors with T cell–redirecting bispecific antibodies has been proved synergistic, preclinically.84 Similarly, a rational com-bination seems to be the concomitant use of positive costimulations, as in the case of RO7227166, in which the potent T-cell costimulation (41BBL) is strictly dependent on tumor antigen (CD19).85 Other strategies to costimulate T cells are represented by the combination with immunomodulatory agents such as lenalidomide. The investigation of the concomitant targeting of different antigens by adding antibody-drug conjugate is also ongoing. Last, the combination with standard chemoimmunotherapy may also provide evidence of synergism and may lead to the improvement of the most common first-line treatment schema (Fig 2; Table 3).
In conclusion, the innovative technology initially proposed by blinatumomab and then followed by other different forms of bispecific antibodies is changing the paradigm of immunotherapy and may represent a robust alternative to cellular therapy such as CARTs. Many issues remain open, such as the optimization of the pharmacokinetics of these compounds and a better control of toxicity that currently limits the treatment efficacy, particularly in NHL. The use of these drugs in combination or sequentially with chemotherapy, stem-cell transplantation, or other immune modulators most likely will improve the outcome of both ALL and NHL. In the years to come, with the intent to increase the efficacy and reduce the toxicity of intensive chemotherapy in both pediatric and adult high-risk patients, these novel immunotherapies will be specifically selected to maximize their benefit in different patient subsets. Most likely, BiTEs will be incorporated into the earlier phases of treatment (ClincalTrials.gov identifiers: NCT03367299 and NCT03792633), leaving a wider therapeutic role to CARTs in the R/R setting.86,87 Overall, the therapeutic landscape will likely change, particularly in some molecularly specific disease subsets, where target therapies combined with immunotherapy will reduce the role of chemotherapy and improve the outcome. In general, the clinical benefit will not be limited to the younger and more fit patients but will also apply to older and more frail patients.45,62
ACKNOWLEDGMENT
We thank Dr Vincenzo Mistrini for figure preparation.
EQUAL CONTRIBUTION
F.L. and G.G. contributed equally to this work.
SUPPORT
Supported by Associazione Italiana per la Ricerca sul Cancro-AIRC 5×1000 Program: “Metastatic disease: the key unmet need in oncology”-ISM-Code:21147 and the Associazione Italiana Lotta alla Leucemia, Linfoma e Mieloma (AIL) Sezione Bergamo.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Alessandro Rambaldi
Provision of study material or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Immunotherapy of Acute Lymphoblastic Leukemia and Lymphoma With T Cell–Redirected Bispecific Antibodies
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Federico Lussana
Honoraria: Amgen
Consulting or Advisory Role: Jazz Pharmaceuticals, Janssen Oncology, Shire
Travel, Accommodations, Expenses: Celgene/Jazz
Giuseppe Gritti
Consulting or Advisory Role: Autolus, Takeda, Gilead Sciences, IQvia
Speakers' Bureau: Amgen, Roche
Travel, Accommodations, Expenses: Roche, AbbVie, Janssen, Gilead Sciences
Alessandro Rambaldi
Honoraria: Amgen, Omeros
Consulting or Advisory Role: Amgen, Omeros, Novartis, Astellas Pharma, Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Celgene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pui C-H Yang JJ Hunger SP, et al. : Childhood acute lymphoblastic leukemia: Progress through collaboration. J Clin Oncol 33:2938-2948, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. doi: 10.1200/JCO.2017.77.3648. Bassan R, Bourquin J-P, DeAngelo DJ, et al: New approaches to the management of adult acute lymphoblastic leukemia. J Clin Oncol 36:3512-3527, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Tarella C Gueli A Delaini F, et al. : Rate of primary refractory disease in B and T-cell non-Hodgkin’s lymphoma: Correlation with long-term survival. PLoS One 9:e106745, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hübel K Ghielmini M Ladetto M, et al. : Controversies in the treatment of follicular lymphoma. HemaSphere 4:e317, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mondello P, Nowakowski GS: Treatment of aggressive B cell lymphomas: Updates in 2019. Curr Hematol Malig Rep 15:225-234, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Brennan M, Davison PF, Paulus H: Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science 229:81-83, 1985 [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann U, Kontermann RE: The making of bispecific antibodies. MAbs 9:182-212, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. doi: 10.3390/antib8030043. Wang Q, Chen Y, Park J, et al: Design and production of bispecific antibodies. Antibodies (Basel) 8:43, 2019. [DOI] [PMC free article] [PubMed]
- 9.Bargou R Leo E Zugmaier G, et al. : Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321:974-977, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Nagorsen D Kufer P Baeuerle PA, et al. : Blinatumomab: A historical perspective. Pharmacol Ther 136:334-342, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Löffler A Kufer P Lutterbüse R, et al. : A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 95:2098-2103, 2000 [PubMed] [Google Scholar]
- 12.Zhu M Wu B Brandl C, et al. : Blinatumomab, a bispecific t-cell engager (BiTE) for CD-19 targeted cancer immunotherapy: Clinical pharmacology and its implications. Clin Pharmacokinet 55:1271-1288, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Carter RH, Myers R: Germinal center structure and function: Lessons from CD19. Semin Immunol 20:43-48, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang K, Wei G, Liu D: CD19: A biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol 1:36, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klinger M Benjamin J Kischel R, et al. : Harnessing T cells to fight cancer with BiTE antibody constructs--past developments and future directions. Immunol Rev 270:193-208, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Nagel I Bartels M Duell J, et al. : Hematopoietic stem cell involvement in BCR-ABL1-positive ALL as a potential mechanism of resistance to blinatumomab therapy. Blood 130:2027-2031, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Portell CA, Wenzell CM, Advani AS: Clinical and pharmacologic aspects of blinatumomab in the treatment of B-cell acute lymphoblastic leukemia. Clin Pharmacol 5:5-11, 2013. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topp MS Gökbuget N Zugmaier G, et al. : Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol 32:4134-4140, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Topp MS Gökbuget N Stein AS, et al. : Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol 16:57-66, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Barlev A Lin VW Katz A, et al. : Estimating long-term survival of adults with Philadelphia chromosome-negative relapsed/refractory B-precursor acute lymphoblastic leukemia treated with blinatumomab using historical data. Adv Ther 34:148-155, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantarjian H Stein A Gökbuget N, et al. : Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 376:836-847, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boissel N Ribera J-M Chiaretti S, et al. : Treatment of adults with relapsed/refractory Philadelphia chromosome negative acute lymphoblastic leukemia with blinatumomab in a real-world setting: Results from the Neuf Study. Blood 134, 2019. (suppl 1; abstr 2627) [Google Scholar]
- 23.Martinelli G Boissel N Chevallier P, et al. : Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: Results from a phase II, single-arm, multicenter study. J Clin Oncol 35:1795-1802, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Rambaldi A Ribera J-M Kantarjian HM, et al. : Blinatumomab compared with standard of care for the treatment of adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia. Cancer 126:304-310, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Stackelberg A Locatelli F Zugmaier G, et al. : Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 34:4381-4389, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Gore L Locatelli F Zugmaier G, et al. : Survival after blinatumomab treatment in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Blood Cancer J 8:80, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locatelli F Whitlock JA Peters C, et al. : Blinatumomab versus historical standard therapy in pediatric patients with relapsed/refractory Ph-negative B-cell precursor acute lymphoblastic leukemia. Leukemia 34:2473-2478, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown PA, Ji L, Xu X, et al: A randomized phase 3 trial of blinatumomab vs. chemotherapy as post-reinduction therapy in high and intermediate risk (HR/IR) first relapse of B-acute lymphoblastic leukemia (B-ALL) in children and adolescents/young adults (AYAs) demonstrates superior efficacy and tolerability of blinatumomab: A report from Children’s Oncology Group Study AALL1331. Blood 134, 2019 (suppl 2; abstr LBA-1) [Google Scholar]
- 29.Mouttet B Vinti L Ancliff P, et al. : Durable remissions in TCF3-HLF positive acute lymphoblastic leukemia with blinatumomab and stem cell transplantation. Haematologica 104:e244-e247, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry DA Zhou S Higley H, et al. : Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: A meta-analysis. JAMA Oncol 3:e170580, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topp MS Kufer P Gökbuget N, et al. : Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 29:2493-2498, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Topp MS Gökbuget N Zugmaier G, et al. : Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood 120:5185-5187, 2012 [DOI] [PubMed] [Google Scholar]
- 33. doi: 10.1182/blood-2017-08-798322. Gökbuget N, Dombret H, Bonifacio M, et al: Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 131:1522-1531, 2018 [Erratum: Blood 133:2625, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gökbuget N Dombret H Giebel S, et al. : Minimal residual disease level predicts outcome in adults with Ph-negative B-precursor acute lymphoblastic leukemia. Hematology 24:337-348, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Boissel N Bassan R Ribera J-M, et al. : Treatment of adults with minimal residual disease (MRD) positive acute lymphoblastic leukemia with blinatumomab in a real-world setting: Results from the Neuf Study. Blood 134, 2019. (suppl 1; abstr 2624) [Google Scholar]
- 36.Schlegel P Lang P Zugmaier G, et al. : Pediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab. Haematologica 99:1212-1219, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handgretinger R Zugmaier G Henze G, et al. : Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia 25:181-184, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Khan MW, Gul Z: Blinatumomab may induce graft versus host leukemia in patients with pre-B ALL relapsing after hematopoietic stem cell transplant. Clin Case Rep 4:743-746, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durer S Durer C Shafqat M, et al. : Concomitant use of blinatumomab and donor lymphocyte infusion for mixed-phenotype acute leukemia: A case report with literature review. Immunotherapy 11:373-378, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herrmann M Krupka C Deiser K, et al. : Bifunctional PD-1 × αCD3 × αCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia. Blood 132:2484-2494, 2018 [DOI] [PubMed] [Google Scholar]
- 41. Craddock C, Jilani N, Siddique S, et al: Tolerability and clinical activity of post-transplantation azacitidine in patients allografted for acute myeloid leukemia treated on the RICAZA Trial. Biol Blood Marrow Transplant 22:385-390, 2016. [DOI] [PMC free article] [PubMed]
- 42.Rambaldi A Huguet F Zak P, et al. : Blinatumomab consolidation and maintenance therapy in adults with relapsed/refractory B-precursor acute lymphoblastic leukemia. Blood Adv 4:1518-1525, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Topp M Stein AS Zugmaier G, et al. : Long-term survival of adults with B-cell precursor (BCP) acute lymphoblastic leukemia (ALL) after treatment with blinatumomab and subsequent allogeneic hematopoietic stem cell transplantation (HSCT). J Clin Oncol 36, 2018. (15_suppl; abstr 7044) [Google Scholar]
- 44.Salhotra A Yang D Mokhtari S, et al. : Outcomes of allogeneic hematopoietic cell transplantation after salvage therapy with blinatumomab in patients with relapsed/refractory acute lymphoblastic leukemia. Biol Blood Marrow Transplant 26:1084-1090, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiaretti S Bassan R Vitale A, et al. : Dasatinib-blinatumomab combination for the front-line treatment of adult Ph+ ALL patients. Updated results of the Gimema LAL2116 D-Alba trial. Blood 134, 2019. (suppl 1; abstr 740) [Google Scholar]
- 46.Goebeler M-E Knop S Viardot A, et al. : Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: Final results from a phase I study. J Clin Oncol 34:1104-1111, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Stein AS Schiller G Benjamin R, et al. : Neurologic adverse events in patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab: management and mitigating factors. Ann Hematol 98:159-167, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viardot A Goebeler M-E Hess G, et al. : Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 127:1410-1416, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coyle L Morley NJ Rambaldi A, et al. : Open-label, phase 2 study of blinatumomab as second salvage therapy in adults with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Leuk Lymphoma 61: 2103-2112, 2020 [DOI] [PubMed] [Google Scholar]
- 50.Katz DA Chu MP David KA, et al. : Open-label, phase 2 study of blinatumomab after first-line rituximab-chemotherapy in adults with newly diagnosed, high-risk diffuse large B-cell lymphoma. Blood 134, 2019. (suppl 1; abstr 4077) [Google Scholar]
- 51.Dufner V Sayehli CM Chatterjee M, et al. : Long-term outcome of patients with relapsed/refractory B-cell non-Hodgkin lymphoma treated with blinatumomab. Blood Adv 3:2491-2498, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viardot A Hess G Bargou RC, et al. : Durability of complete response after blinatumomab therapy for refractory/relapsed aggressive B-cell non-Hodgkin lymphoma. Leuk Lymphoma, 2020 [DOI] [PubMed] [Google Scholar]
- 53.Aldoss I Song J Stiller T, et al. : Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol 92:858-865, 2017 [DOI] [PubMed] [Google Scholar]
- 54.Braig F Brandt A Goebeler M, et al. : Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood 129:100-104, 2017 [DOI] [PubMed] [Google Scholar]
- 55.Aldoss I, Song JY: Extramedullary relapse of KMT2A(MLL)-rearranged acute lymphoblastic leukemia with lineage switch following blinatumomab. Blood 131:2507, 2018 [DOI] [PubMed] [Google Scholar]
- 56.Duell J Dittrich M Bedke T, et al. : Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia 31:2181-2190, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Köhnke T Krupka C Tischer J, et al. : Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol 8:111, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster J Luskin MR Prince GT, et al. : Blinatumomab in combination with immune checkpoint inhibitors of PD-1 and CTLA-4 in adult patients with relapsed/refractory (R/R) CD19 positive B-cell acute lymphoblastic leukemia (ALL): Preliminary results of a phase I study. Blood 132, 2018. (suppl 1; abstr 557) [Google Scholar]
- 59.Schwartz M Damon LE Jeyakumar D, et al. : Blinatumomab in combination with pembrolizumab is safe for adults with relapsed or refractory b-lineage acute lymphoblastic leukemia: University of California Hematologic Malignancies Consortium Study 1504. Blood 134, 2019. (suppl 1; abstr 3880) [Google Scholar]
- 60.Feucht J Kayser S Gorodezki D, et al. : T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget 7:76902-76919, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghorashian S Kramer AM Onuoha S, et al. : Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med 25:1408-1414, 2019 [DOI] [PubMed] [Google Scholar]
- 62.Jabbour E Sasaki K Ravandi F, et al. : Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage. Cancer 124:4044-4055, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kantarjian H, Jabbour E: Incorporating immunotherapy into the treatment strategies of B-cell adult acute lymphoblastic leukemia: The role of blinatumomab and inotuzumab ozogamicin. Am Soc Clin Oncol Ed Book 38:574-578, 2018. [DOI] [PubMed]
- 64.Amrolia PJ Wynn R Hough RE, et al. : Phase I study of AUTO3, a bicistronic chimeric antigen receptor (CAR) T-cell therapy targeting CD19 and CD22, in pediatric patients with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL): Amelia Study. Blood 134, 2019. (suppl 1; abstr 2620) [Google Scholar]
- 65.Buhmann R Michael S Juergen H, et al. : Immunotherapy with FBTA05 (Bi20), a trifunctional bispecific anti-CD3 x anti-CD20 antibody and donor lymphocyte infusion (DLI) in relapsed or refractory B-cell lymphoma after allogeneic stem cell transplantation: Study protocol of an investigator-driven, open-label, non-randomized, uncontrolled, dose-escalating phase I/II-trial. J Transl Med 11:160, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lorenczewski G Friedrich M Kischel R, et al. : Generation of a half-life extended anti-CD19 BiTE antibody construct compatible with once-weekly dosing for treatment of CD19-positive malignancies. Blood 130, 2017. (suppl 1; abstr 2815) [Google Scholar]
- 67. Sun LL, Ellerman D, Mathieu M, et al: Anti-CD20/CD3 T cell–dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med 7:287ra70, 2015. [DOI] [PubMed]
- 68.Bacac M Colombetti S Herter S, et al. : CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res 24:4785-4797, 2018 [DOI] [PubMed] [Google Scholar]
- 69.Smith EJ Olson K Haber LJ, et al. : A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci Rep 5:17943, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engelberts PJ Hiemstra IH de Jong B, et al. : DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine 52:102625, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chu SY Lee S-H Rashid R, et al. : Immunotherapy with long-lived anti-CD20 × anti-CD3 bispecific antibodies stimulates potent T cell-mediated killing of human B cell lines and of circulating and lymphoid B cells in monkeys: A potential therapy for B cell lymphomas and leukemias. Blood 124:3111, 2014 [Google Scholar]
- 72.Lejeune M Köse MC Duray E, et al. : Bispecific, T-cell-recruiting antibodies in B-cell malignancies. Front Immunol 11:762, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schuster SJ Bartlett NL Assouline S, et al. : Mosunetuzumab induces complete remissions in poor prognosis non-Hodgkin lymphoma patients, including those who are resistant to or relapsing after chimeric antigen receptor T-cell (CAR-T) therapies, and is active in treatment through multiple lines. Blood 134, 2019. (suppl 1; abstr 6) [Google Scholar]
- 74.Dickinson MJ Morschhauser F Iacoboni G, et al. : CD20-TCB (RG6026), a novel “2:1” format T-cell-engaging bispecific antibody, induces complete remissions in relapsed/refractory B-cell non-Hodgkin’s lymphoma. Hematol Oncol 37:92-93, 2019. 31187519 [Google Scholar]
- 75.Bannerji R Allan JN Arnason JE, et al. : Clinical activity of REGN1979, a bispecific human, anti-CD20 x anti-CD3 antibody, in patients with relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma (B-NHL). Blood 134, 2019. (suppl 1; abstr 762) [Google Scholar]
- 76.Lugtenburg P Mous R Clausen MR, et al. : First-in-human, phase 1/2 trial to assess the safety and clinical activity of subcutaneous GEN3013 (DuoBody-CD3×CD20) in B-cell non-Hodgkin lymphomas. Blood 134, 2019. (suppl 1; abstr 758) [Google Scholar]
- 77.Patel K Michot J-M Chanan-Khan AA, et al. : Preliminary safety and anti-tumor activity of XmAb13676, an anti-CD20 x anti-CD3 bispecific antibody, in patients with relapsed/refractory non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Blood 134, 2019. (suppl 1; abstr 4079) [Google Scholar]
- 78.Li C-C Bender B Yin S, et al. : Exposure-response analyses indicate a promising benefit/risk profile of mosunetuzumab in relapsed and refractory non-Hodgkin lymphoma. Blood 134, 2019. (suppl 1; abstr 1285) [Google Scholar]
- 79.Bröske A-ME James I Belousov A, et al. : CD20-TCB, a novel T-cell-engaging bispecific antibody, induces T-cell-mediated killing in relapsed or refractory non-Hodgkin lymphoma: Biomarker results from a phase I dose-escalation trial. Blood 134, 2019. (suppl 1; abstr 5319) [Google Scholar]
- 80.Djebli N Jaminion F Laurent J, et al. : Population pharmacokinetics and novel exposure-response analyses to inform optimal biologic dose selection for CD20-TCB, a T-cell-engaging bispecific antibody, in relapsed or refractory B-cell non-Hodgkin lymphoma. Blood 134, 2019. (suppl 1; abstr 3799) [Google Scholar]
- 81.Golay J D’Amico A Borleri G, et al. : A novel method using blinatumomab for efficient, clinical-grade expansion of polyclonal T cells for adoptive immunotherapy. J Immunol 193:4739-4747, 2014 [DOI] [PubMed] [Google Scholar]
- 82.Hutchings M Gritti G Sureda A, et al. : CD20-TCB, a novel T-cell-engaging bispecific antibody, can be safely combined with the anti-PD-L1 antibody atezolizumab in relapsed or refractory B-cell non-Hodgkin lymphoma. Blood 134, 2019. (suppl 1; abstr 2871) [Google Scholar]
- 83.Morschhauser F Carlo-Stella C Offner F, et al. : Dual CD20-targeted therapy with concurrent CD20-TCB and obinutuzumab shows highly promising clinical activity and manageable safety in relapsed or refractory B-cell non-Hodgkin lymphoma: Preliminary results from a phase Ib trial Blood 134, 2019. (suppl 1; abstr 1584) [Google Scholar]
- 84.Kobold S Pantelyushin S Rataj F, et al. : Rationale for combining bispecific T cell activating antibodies with checkpoint blockade for cancer therapy. Front Oncol 8:285, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. doi: 10.1126/scitranslmed.aav5989. Claus C, Ferrara C, Xu W, et al: Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci Transl Med 11:eaav5989, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. doi: 10.1056/NEJMra1706169. June CH, Sadelain M: Chimeric antigen receptor therapy. N Engl J Med 379:64-73, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. doi: 10.1172/JCI138473. Magnani CF, Gaipa G, Federico Lussana F, et al: Sleeping beauty-engineered CAR T cells achieve anti-leukemic activity without severe toxicities. J Clin Invest 130:6021-6033, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]