Abstract
Background
There is a need to standardize monitoring in obstetric research of twin pregnancies. Identification of birth weight discordance (BWD), defined as a difference in the birth weights of twins, is a well‐documented phenomenon in twin pregnancies. Ultrasound for the diagnosis of BWD informs complex decision making including whether to intervene medically (via laser photo coagulation) or deliver the twins to avoid fetal morbidities or even death. The question is, how accurate is this measurement?
Objectives
To determine the diagnostic accuracy (sensitivity and specificity) of ultrasound estimated fetal weight discordance (EFWD) of 20% and 25% using different estimated biometric ultrasound measurements compared with the actual BWD as the reference standard in twin pregnancies.
Search methods
The search for this review was performed on 15 March 2019. We searched CENTRAL, MEDLINE (Ovid), Embase (Ovid), seven other databases, conference proceedings, reference lists and contacted experts. There were no language or date restrictions applied to the electronic searches, and no methodological filters to maximize sensitivity.
Selection criteria
We selected cohort‐type studies with delayed verification that evaluated the accuracy of biometric measurements at ultrasound scanning of twin pregnancies that had been proposed for the diagnosis of estimated BWD, compared to BWD measurements after birth as a reference standard. In addition, we only selected studies that considered twin pregnancies and applied a reference standard for EFWD for the target condition of BWD.
Data collection and analysis
We screened all titles generated by electronic database searches. Two review authors independently assessed the abstracts of all potentially relevant studies. We assessed the identified full papers for eligibility, and extracted data to create 2 × 2 tables. Two review authors independently performed quality assessment using the QUADAS‐2 tool. We excluded studies that did not report data in sufficient detail to construct 2 × 2 tables, and where this information was not available from the primary investigators. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 39 eligible studies with a median study sample size of 140. In terms of risk of bias, there were many unclear statements regarding patient selection, index test and use of proper reference standard. Twenty‐one studies (53%) were of methodological concern due to flow and timing. In terms of applicability, most studies were of low concern.
Ultrasound for diagnosis of BWD in twin pregnancies at 20% cut‐off
Twenty‐two studies provided data for a BWD of 20% and the summary estimate of sensitivity was 0.51 (95% CI 0.42 to 0.60), and the summary estimate of specificity was 0.91 (95% CI 0.89 to 0.93) (8005 twin pregnancies; very low‐certainty evidence).
Ultrasound for diagnosis of BWD in twin pregnancies at 25% cut‐off
Eighteen studies provided data using a BWD discordance of 25%. The summary estimate of sensitivity was 0.46 (95% CI 0.26 to 0.66), and the summary estimate of specificity was 0.93 (95% CI 0.89 to 0.96) (6471 twin pregnancies; very low‐certainty evidence).
Subgroup analyses were possible for both BWD of 20% and 25%. The diagnostic accuracy did not differ substantially between estimation by abdominal circumference and femur length but femur length had a trend towards higher sensitivity and specificity. Subgroup analyses were not possible by sex of twins, chorionicity or gestational age due to insufficient data.
Authors' conclusions
Very low‐certainty evidence suggests that EFWD identified by ultrasound has low sensitivity but good specificity in detecting BWD in twin pregnancies. There is uncertain diagnostic value of EFWD; this review suggests there is insufficient evidence to support this index as the sole measure for clinical decision making to evaluate the prognosis of twins with growth discordance. The diagnostic accuracy of other measures including amniotic fluid index and umbilical artery Doppler resistive indices in combination with ultrasound for clinical intervention requires evaluation. Future well‐designed studies could also evaluate the impact of chorionicity, sex and gestational age in the diagnostic accuracy of ultrasound for EFWD.
Plain language summary
Ultrasound during pregnancy for predicting differences in birth weight between twins
Background
Birth weight differences of more than 20% in twins is associated with poor outcomes for the mother and baby. Clinicians measure the estimated fetal weight differences by ultrasound before birth and compare it to differences in birth weight after the babies are born. In this review, we summarized data on whether the ultrasound measurements are accurate enough to predict birth weight differences in twins.
Study characteristics
We searched medical databases to March 2019 for studies comparing ultrasound measurements to birth weight differences and we identified 39 studies. Twenty‐two studies provided data on birth weight differences of 20% and 18 studies provided data on birth weight differences of 25%.
Quality of the evidence
We assessed the quality of individual studies using a tool called "Quality Assessment of Diagnostic Accuracy Studies" (QUADAS‐2) and the overall quality by a recommended method called GRADE to find out the reliability of the evidence.
Key results
We found that ultrasound estimation of fetal weight differences compared to birth weight differences was not reliable. On average, ultrasound detected birth weight differences of 20% and 25% only half the time. The quality of evidence was very low.
There is insufficient evidence to support the use of ultrasound as the sole measure for detecting birth weight differences in twins, or poor outcomes. The diagnostic accuracy of other measures including amniotic fluid volume (the fluid surrounding the babies in the womb) or Doppler studies (which use sound waves to detect the movement of blood in the babies' blood vessels and the umbilical cord) in combination with ultrasound to inform clinical decisions needs to be evaluated. Future well‐designed studies could also research the impact of whether the babies share a placenta (or not), the sex of the babies, and gestational age (time from woman's last menstrual period), in the diagnostic accuracy of ultrasound for estimated birth weight differences.
Summary of findings
Background
Target condition being diagnosed
Birth weight discordance (BWD), defined as a difference in the birth weights of twins, is a well‐documented phenomenon in twin pregnancies (Bagchi 2006; Mahony 2006). Estimated fetal weight discordance (EFWD) estimated by ultrasound is measured using the formula: ((larger twin estimated weight – smaller twin estimated weight)/larger twin estimated weight) × 100. EFWD of 20% or greater has been shown to have significant impact on fetal and perinatal outcomes. Growth discordance may occur as a physiological BWD, when both twins are appropriate‐for‐gestational‐age, or as pathological BWD when at least one twin is small‐for‐gestational age (Appleton 2007).
BWD is one of the major risk factors for adverse fetal, neonatal and maternal outcomes (Demissie 2002; Kilic 2006; Wen 2006). Demissie and colleagues found that the odds ratio of fetal death varied from 1.26 to 12.75 and the odds ratio of neonatal death varied from 1.02 to 3.43, depending on the degree of BWD. Kilic and colleagues reported higher frequency of mortality, sepsis, polycythaemia, hypoglycaemia, anaemia and respiratory distress syndrome among discordant twins. Wen and coworkers reported higher odds of maternal hypertension, eclampsia and other medical complications associated with BWD. Depending on the chorionicity, degree of discordance and the threshold used, BWD occurs in about 15% to 30% of twin gestations (Lewi 2008; Lopriore 2012; Miller 2012). Fetal growth abnormalities are associated with multiple conditions that affect fetal or neonatal well‐being, such as twin‐to‐twin transfusion syndrome (TTTS), chromosomal aberrations or structural defects. Excluding these conditions, discordant growth by itself is recognized as an independent risk factor for adverse perinatal outcomes (Mazhar 2010; Morin 2011; Suzuki 2009). BWD of 20% and 25% are the most common thresholds used in the literature to identify adverse perinatal outcomes (Jahanfar 2016a). One study suggested that BWD of 30% and greater is significantly associated with adverse perinatal outcomes irrespective of chorionicity (Jahanfar 2016b).
Several mechanisms have been proposed for growth discordance of fetuses exposed to the same intrauterine environment (Victoria 2001). For monochorionic (MC) twin pregnancies, the mechanism is explained through conditions such as TTTS and intrauterine growth retardation (IUGR). These two conditions result in greater risk of fetal and neonatal mortality compared to dichorionic (DC) twins. Technology is available to manage TTTS including amnio‐reduction and septotomy (Behrendt 2016), hence monitoring is usually started as early as 20 weeks.
In the case of DC twin pregnancies, intrauterine growth restriction and placenta pathology can cause growth discordance. Abnormal cord insertion and insufficient placental implantation in the uterine wall are other proposed mechanisms for BWD. These conditions are non‐treatable; hence, the main management is to determine fetal well‐being and decide the optimal time for delivery to save the growth discordant twins.
If the accuracy of ultrasound in assessing the 20% to 25% cut‐off is known, it helps the complex decision making that the clinician goes through in deciding whether to intervene medically (via laser photo coagulation) or deliver the twins to avoid fetal morbidities or even death.
Index test(s)
Ultrasound has been an important tool in the diagnosis of BWD since 1972 (Hoopmann 2011; Woo 1939). Diagnostic ultrasound is a sophisticated electronic technology, which utilizes pulses of high‐frequency sound to produce an image. In the past, before ultrasound became available, abdominal palpation was used, which is extremely poor for detecting growth discordance. Radiological examination is not recommended since it is not safe for the fetus.
Diagnosis of EFWD can be made via two modalities of ultrasound.
A series of diagnostic two‐dimensional (2D) ultrasound examinations is used to assess fetal growth of both twins; identify chorionicity; and diagnose problems with cord, amnion layers, congenital abnormalities and TTTS. Doppler ultrasound is used to detect abnormal blood flow patterns in fetal/placenta circulation which may indicate poor fetal prognosis (Alfirevic 2003).
Three‐dimensional (3D) ultrasound to facilitate the assessment of the placenta, such as surface‐rendered imaging and volume measurement, quantitative and qualitative assessments of the vascularization, and blood flow of the placenta (Hata 2011).
The standard diagnostic test for fetal growth discordance is ultrasound. Growth discordance can be estimated by measuring crown rump length (CRL: the distance measured from the top of the head to the bottom of the buttocks) at or before 12 weeks of gestation (Tai 2007). Later, during the second and third trimesters, other ultrasound measures such as biparietal diameter (BPD), abdominal circumference (AC) and femur length (FL) are used to estimate fetal weight and calculate the degree of BWD (Simoes 2011).
It is still unclear whether first or second trimester findings can accurately predict BWD, what sonographic parameters assessed at each trimester are reliable to assess for discordance or whether any particular ultrasound modality (e.g. 2D or 3D) is superior to the other.
Apart from EFWD, information on chorionicity is essential for the management of a twin pregnancy for the following reasons.
MC twins are at greater risk of fetal morbidity and mortality due to shared vascularization.
If monochorionicity is suspected at early ultrasound screening, subsequent screening should be serially scheduled during the second and third trimester.
Early ultrasound detection narrows down the differential diagnosis for the underlying causes such as placenta sharing and subsequent vascular anastomosis as an underlying cause of EFWD. Better prognosis is expected for DC twin pregnancies as they do not share placentas (Miller 2012). In simpler terms, MC twins have higher risk of complications and should be assessed separately.
Second trimester ultrasound screening, from 16 to 24 weeks' gestation is useful in measuring AC, amniotic fluid pockets, identification of dividing membrane between amniotic sacs and umbilical artery Doppler studies (Giles 1998; Rizzo 1993).
Third trimester ultrasound, after 24 weeks' gestation, aims at identifying discordance or insufficient fetal growth. Identification of a discordant twin is crucial if one of the twin pairs is small‐for‐gestational age rather than both being appropriate‐for‐gestational‐age.
It should be noted that there is overlap in the use of these diagnostic tests from one trimester to another.
The standard of practice is to identify the growth discordance in twin pregnancies using ultrasound (2D or 3D, or both) using measurements (CRL, BPD, AC or FL) in either early (for CRL) or late pregnancy (for BPD, AC and FL) and compare with the actual birth weight. Since EFWD of 20% and 25% are associated with adverse perinatal outcomes, we studied the diagnostic accuracy of ultrasound at those threshold values.
Clinical pathway
In the management of a twin pregnancy, evaluation of fetal growth is particularly important because growth restriction and prematurity are major causes of morbidity and mortality reported in twins compared with a singleton pregnancy (Adegbite 2004). Most clinicians start monitoring MC twins for growth delay and discordant growth from 18 weeks' gestation every two to three weeks. Each twin's growth, amniotic volume and fetal bladder volume are monitored for signs of oligohydramnios or polyhydramnios. in contrast, in DC twins, fetal growth monitoring by ultrasound usually begins after 20 weeks of gestation every four to six weeks as fetal growth deceleration leading to discordance is optimally detected between 20 and 28 weeks of gestation (Gonzalez‐Quintero 2003).
When discordant growth is identified in the absence of TTTS or congenital abnormalities, intensive fetal monitoring of fetal well‐being is employed until delivery becomes indicated. In mildly discordant twins, expectant management by frequent fetal assessment is preferable to preterm delivery, given the limitation of ultrasound diagnosis of growth discordance. In one large non‐randomized study of 2399 twin pregnancies, the sonographic diagnosis of discordance greater than 25% was a poor predictor of BWD, fetal loss after 22 weeks' and 28 weeks' gestation, perinatal death and preterm birth before 34 weeks of gestation (D'Antonio 2014). In complicated twin pregnancies, in particular those with fetal growth restriction and discordant growth, fetal well‐being assessment by a non‐stress test (NST), biophysical profile (BPP), amniotic fluid index (AFI) measurement and Doppler velocimetry may be useful to identify which fetuses would benefit from early delivery (Devoe 1995).
Ideally, a diagnostic test is expected to correctly identify all patients with the assessed condition and to exclude all patients without it; that is, to have a sensitivity and specificity of 100%. In practice, however, it is extremely rare to find a test with equally high sensitivity and specificity, when compared to the current gold standard (BWD). For most tests, there is usually a trade‐off between the measures. An approach to the test might vary depending on the clinical context, which relates to the consequences of missed diagnosis and magnitude of subsequent interventions if the test is positive. If clinical priority would be to avoid missed diagnosis, an adequate test in that case would be expected to have a high sensitivity (low false‐negative results), with lower specificity (higher number of false‐positive results). Thus, no woman with a growth discordant twin pregnancy is missed by a test, but some still get unnecessary interventions (intensive surveillance, early delivery, consequential issues pertaining to prematurity such as admission to the neonatal intensive care unit, costs associated with care for the premature baby and increased distress for parents). An alternative approach gives priority to a test that avoids unnecessary invasive interventions. In this scenario, the emphasis should be on a high specificity (close to 100%) with lower sensitivity (preferably above 50%), which will rule out growth discordance of the twins, but will not detect some women with this condition. Ruling out growth discordance eliminates the need for further clinical intervention. Ideally, a test with high sensitivity and specificity is most useful in a clinical setting, which will help in efficient patient counselling and ongoing pregnancy management. Improving the selection of patients who might benefit from interventions such as laser coagulation or amnioreduction or early delivery would be of considerable benefit to neonates, mothers, families and the community.
Rationale
Guidelines provide conflicting advice about the time, frequency and type of ultrasound measurements that reliably diagnose growth discordance. According to the American College of Obstetricians and Gynecologists (ACOG), growth discordance is defined as a difference of AC of 20 mm or estimated fetal weight (EFW) difference of 20% (ACOG Committee 2004). The Society of Obstetricians and Gynecologists of Canada recommends that the EFW be derived from bi‐parietal diameter with AC or a combination of AC and FL (Okun 2000). ACOG does not provide recommendations on the frequency of ultrasound examinations for twins while the green guideline from the Royal College of Obstetricians and Gynecologists advocates screening ultrasound for MC twins every two to three weeks from 16 weeks’ gestation onwards (NICE 2011).
For DC twins, the issues related to feto‐placental perfusion leading to growth discordance can be clarified by screening ultrasound. However, there are no guidelines on the optimal gestation at which to start screening, or the frequency and gestations for subsequent screenings. Moreover, gender is an important factor that has interaction with BWD and perinatal outcomes (Melamed 2009; Miller 2012). Identifying the gender mix by ultrasound improves predictability of antenatal outcomes (Di 2007). Thus, the accuracy of ultrasound to identify gender is also crucial. As a part of this review, we investigated the impact of gender on BWD as a confounding factor.
The variation in recommendations made in the guidelines arises from the fact that the ability to estimate abnormal growth is challenging. It is not clear if the antenatal prediction of a critical BWD by fetal weight estimation formulae ((birth weight of heavier twin – birth weight of lighter twin)/birth weight of heavier twin) two to three weeks prior to delivery (Caravello 1997), provides an accurate estimation of BWD (Kalish 2003; Klam 2003; van Mieghem 2009). The pooled sensitivity for ultrasound prediction of BWD of 25% or greater, in three studies, was 63% for a false‐positive rate of 2% (Caravello 1997; Diaz‐Garcia 2010; Gernt 2001).
The existing uncertainties are further complicated by an inconclusive predictive value of early (first‐second trimester) versus late detection (two to three weeks prior to delivery) of growth discordance, use of different sonographic estimates (AC versus FL), and reliance on the retrospective nature of study designs and small sample sizes of existing literature. Literature suggests that biometric measurements of EFWD at early gestation or during the second trimester have significantly different precisions (Banks 2008; Tai 2007). Moreover, the efficacy of a single biometric measurement, such as CRL or AC with or without other measurement(s), in predicting BWD, is controversial (Banks 2008; Bhide 2009; Chamberlain 1991; Chitkara 1985). The most popular current methods for predicting discordant growth in twin gestations have limited accuracy when held to a standard for discordance that requires a birth weight difference of at least 20% (Caravello 1997), as sonography tends to underestimate the degree of discordance (Chang 2006).
We reviewed and summarized the evidence for the diagnostic accuracy of antenatal ultrasound for EFWD compared to the BWD to inform clinical practice.
Objectives
To determine the diagnostic accuracy (sensitivity and specificity) of ultrasound estimated fetal weight discordance (EFWD) of 20% and 25% using different estimated biometric ultrasound measurements compared with the actual BWD as the reference standard in twin pregnancies.
Secondary objectives
To explore heterogeneity related to gestational age, sex and chorionicity. We planned to perform subgroup analyses for:
assessing the sensitivity and specificity of available diagnostic ultrasound tests in subgroups of twin pregnancies at various gestational ages by week (less than 28 weeks, 28 to 32 weeks, 32 to 36 weeks and more than 36 weeks);
assessing the sensitivity and specificity of each diagnostic ultrasound test in twin pregnancies with same‐sex versus opposite‐sex twins;
assessing the sensitivity and specificity of each diagnostic ultrasound test in dichorionic diamniotic versus monochorionic diamniotic twins.
We anticipate the following potential sources of heterogeneity.
Clinical factors: characteristics of study population (gestational age, chorionicity, inclusion of high‐risk pregnancy complicated by underlying maternal–fetal conditions, breastfeeding).
Methodological factors: study design (patient selection, prospective versus retrospective studies, time of test performance (time between index test and reference standard), clinical settings (tertiary centre versus community health care), multiple testing versus single testing for diagnosis of high‐risk pregnancy complicated by maternal–fetal conditions).
Other factors: geographic area (high‐, middle‐ and low‐income countries), year of publication.
Methods
Criteria for considering studies for this review
Types of studies
We included cohort‐type studies with delayed verification design that assessed EFWD of 20% compared to BWD of 20% and EFWD of 25% compared to BWD of 25%. We included only studies that reported both the index test and reference standard.
Participants
Twin pregnancies with ultrasound measurements to determine EFWD at any stage of pregnancy. The twin pregnancies included any type of chorionicity, any type of conception, and any maternal age and body mass index. We excluded pregnancies with inappropriate comparisons that were likely to distort an assessment of the diagnostic value of antenatal ultrasound (e.g. pregnancies that includes twins with one stillbirth, or other multiple pregnancies such as triplets or quadruplets).
Index tests
Any type of biometric measurements assessing EFWD, when either used as a single measurement, combinations or defined formulas, inclusive of CRL, BPD, AC and FL. The measurements were eligible if performed using transabdominal or transvaginal ultrasound, machines of any brand based on 2D or 3D ultrasound methods.
Target conditions
The target condition was BWD in twin pregnancies, which occurs when there is a disparity in birth weight between the larger and smaller infants of a twin set. For this review, we included studies that had investigated growth discordance of 20% or greater and growth discordance of 25% or greater.
Reference standards
Ultrasound EFWD of 20% was used to diagnose a prespecified BWD of 20% and an ultrasound EFWD of 25% was used to diagnose a prespecified BWD of 25%. BWD was calculated using the formula: ((larger estimated weight – smaller estimated weight)/larger estimated weight) × 100 and was categorized as those below or above a 20% threshold. Birth weight was accepted as estimated by using either electronic or mechanical scales of any type (bench‐top, portable, hanging, compact or not identified) from any manufacturer. Measurements were considered if performed in the hospital (labour ward, nursery or newborn intensive care unit) by trained medical personnel (doctor, nurse, midwife, paramedic). We included only the measurements performed within seven days of birth. Where available, the data on scale calibration, and whether baby was wet or dried before weighing were recorded and incorporated in the QUADAS‐2 quality assessment tool.
Search methods for identification of studies
We adopted a comprehensive search of multiple sources for eligible studies. We searched for unpublished and published studies using databases and conference proceedings as outlined below.
A librarian developed the search strategy following the recommendations in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (de Vet 2013). The searches were not limited to particular types of study design or have language or publication date restrictions. The search strategy incorporated words in the title, abstract, text words across the record and the subject headings. The search strategies for major databases are presented in Appendix 1.
Electronic searches
We searched the following electronic databases from inception to March 2020.
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 3) via the Cochrane Library.
MEDLINE via Ovid (from 1946).
Embase via Ovid (from 1980).
CINAHL (from 1982).
ISI Web of Science Core Collection (from 1900).
Trip Database (from 1997).
PubMed Systematic Reviews subset (from 1946).
DARE and NHS EED via the University of York (1994 to 2015).
HTA (2003 to 2018).
Prospero via the University of York (2011).
Searching other resources
Additional searches included:
a hand search of Australasian Journal of Ultrasound in Medicine (2009); Canadian Journal of Medical Sonography (2013), the reference lists of all the included studies and the seminal reviews from the field; and
communication with at least five experts in the field asking them to review our reference list and identify any studies that may be missing.
Data collection and analysis
We performed data extraction and handling, assessment of methodological quality and statistical analyses based on the recommendations of the Diagnostic Test Accuracy (DTA) group and their Internet‐based tutorials (methods.cochrane.org/sdt/dta-author-training-online-learning).
Selection of studies
One review author scanned the titles of studies identified by our search to remove any clearly irrelevant articles and scanned the titles and abstracts of the remaining studies to select potentially relevant articles. Two review authors independently reviewed full‐text versions of the articles selected by title and abstract and assessed their eligibility for inclusion. We resolved any disagreements by discussion and, if necessary, with a third review author, who was an expert in the field and in methodological aspects of Cochrane systematic reviews.
When we identified the reports that updated previous publications of the same study population at different recruitment points, the earlier records were classified as excluded. The most complete data set that superseded previous publications were used to avoid double counting participants or studies.
We retrieved missing data by contacting the authors of identified studies directly to clarify the study eligibility. Potentially relevant studies in languages other than English were translated where possible.
For excluded studies, we documented the reasons for exclusion with details of which criteria were not met. We produced Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification tables.
A single failed eligibility criterion was sufficient for a study to be excluded from the review.
Data extraction and management
We used a structured, piloted form to extract data from included studies. Two review authors independently extracted study characteristics and resolved disagreements by discussion. If disagreements persisted, a third review author resolved the issues. We extracted information on: author, year of publication, journal; study design; timing of data collection (prospective, retrospective); setting (inpatients, outpatients); study population (age, parity, obesity); type of index test and reference standard and data on index and reference test operators. We used the reported number of true positives (TP), false negatives (FN), true negatives (TN) and false positives (FP) to construct a 2 × 2 table for each index test. If these values were not reported, we attempted to reconstruct the 2 × 2 tables from the summary estimates presented in the article. We entered data into Review Manager 5, and used this to graphically display the quality assessment, study level data as forest plots and summary estimates as summary receiver operating characteristics (SROC) plots with studies and summary points (Review Manager 2014).
Assessment of methodological quality
We used the Quality Assessment of Diagnostic Test Accuracy Studies‐2 (QUADAS‐2) to assess the methodological quality of included studies. The QUADAS‐2 tool was applied in four phases: summarize the review question, tailor the tool and produce review‐specific guidance, construct a flow diagram for the primary study, and judge bias and applicability (Whiting 2011). Each paper was judged at 'low', 'high' or 'unclear' risk of bias for each of four domains, and concerns about applicability were assessed in three domains. The review‐specific QUADAS‐2 tool and explanatory document are presented in Appendix 2. Two review authors independently piloted the review‐specific tool to rate three of the included studies. The tool was utilized if there was a high level of agreement at the pilot stage. Similarly, two review authors independently applied the QUADAS‐2 tool to the full text of each study. We resolved disagreements by discussion, or if needed, by a third review author. We used Review Manager 5 to construct methodological quality summary graphs (Review Manager 2014).
We assessed the certainty of evidence and reported it according to GRADE for diagnostic test studies (Schünemann 2019; Schünemann 2020a; Schünemann 2020b). We used GRADEpro software to generate 'Summary of findings' tables (GRADEpro GDT).
The prevalences for assessing pretest probability were the median, lower and higher quartiles of the prevalences of the target condition (BWD) in the included studies (Oerbekke 2020).
Statistical analysis and data synthesis
We performed statistical analyses following recommendations presented in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Macaskill 2013).
The unit of analysis in the included studies was twin pregnancy, rather than twins themselves, as BWD is a single calculated measure estimated using biometric measurements of two fetuses. We extracted the absolute counts of TP, FP, FN and TN from each study. TP was defined as ultrasound positive for EFWD greater than or equal to 20% or 25% by biometric ultrasound measurements (CRL, AC, FL) confirmed by BWD of greater than or equal to 20% or 25% after birth. FP was defined as ultrasound positive for EFWD, as defined above, without BWD after birth. FN was a negative ultrasound for EFWD of greater than or equal to 20% or 25% with the diagnosis of BWD of greater than or equal to 20 or 25%. Finally, TN was a negative ultrasound for EFWD of 20% or 25% and without BWD of 20% or 25% after birth.
Two review authors extracted the counts separately and discussed disagreements before engaging a third review author to resolve any disagreements. We then transferred the data into Review Manager 5 to produce plots and estimates (Review Manager 2014).
We used the counts (TP, FP, FN and TN) to construct 2 × 2 tables to estimate sensitivity and specificity with 95% confidence intervals (CIs) for each study. We plotted estimates of the sensitivities and specificities, with their 95% CIs, in forest plots (Chu 2006). The results of the index tests were dichotomous (positive or negative). The bivariate random‐effects approach enabled us to calculate the summary estimates of sensitivity and specificity with 95% CI) (Deeks 2019; Macaskill 2013). The bivariate model also deals with variation beyond chance in sensitivity and specificity between studies and any correlation that may exist between sensitivity and specificity. We calculated summary estimates of sensitivity and specificity using 'xtmelogit' using ‘metandi’ and MIDAS packages (Dwamena 2009; Harbord 2007; Harbord 2009) for Stata (Stata Corp, College Station, TX). We generated forest plots with 95% CIs for sensitivity and specificity and SROC plots in Review Manager 5 (Review Manager 2014).
When a meta‐analysis was possible, we planned to use test‐level covariates in the bivariate logit‐normal model to identify statistically significant differences but there were insufficient data for covariates of sex and chorionicity. We planned to stratify our analysis using categories of gestational age (below or equal to 24 weeks of gestation, below or equal to 32 weeks of gestation, below or equal to 37 weeks of gestation). However, the data were too variable, and the recorded gestational age was presented as ranges rather than one cut‐off point for gestational age. Hence, we decided not to pool the data in respect of gestational age. We calculated positive (LR+) and negative (LR–) likelihood ratios by using summary sensitivity and specificity.
Investigations of heterogeneity
We investigated heterogeneity for the diagnostic tests where there were sufficient data (more than 10 included studies). Steps taken were as follows.
We planned to start the investigation of heterogeneity through visual examination of the forest plots by grouping according to covariates all the items listed as potential sources of heterogeneity (e.g. type of ultrasound, year of publication, geographic areas (high‐income versus low‐ and middle‐income countries), consecutive enrolment, blinding of the operators to clinical data, modifications applied to the widely accepted method of imaging techniques (such as 2D and 3D acquisition), number of index test operators and missing data. There were insufficient data to allow grouping according to the above covariates.
We planned to investigate heterogeneity by meta‐regression if data were available for the covariates; type of ultrasound (2D, 3D), gender determination (yes/no), gestational age (continuous variable), type of study (prospective versus retrospective), chorionicity determination (yes/no), estimated BWD threshold (10% or greater versus 20% or greater) and time frame between index test and delivery. There were insufficient data to allow grouping according to the above covariates.
We planned to investigate methodological sources of heterogeneity using meta‐regression analysis. These were to be listed as verification bias, incorporation bias, diagnostic review bias, and clinical review bias. Prespecified variables were inclusive of clinical settings (tertiary centre versus community health care) and high‐risk pregnancy complicated by underlying maternal–fetal conditions (yes/no). Maternal complications included hypertensive disorders, gestational diabetes and antepartum haemorrhage. Fetal complications includes TTTS, major structural congenital anomalies, stillbirth of one twin and IUGR. We were unable to perform meta‐regression analyses for the above‐mentioned covariates due to lack of data.
Sensitivity analyses
We planned to conduct sensitivity analyses based on 'type of study design' (prospective versus retrospective study designs) and the individual quality items of QUADAS‐2 tool, but sufficient data were not available.
Assessment of reporting bias
We did not plan to use funnel plots to evaluate the impact of publication bias or other biases associated with small studies, because, according to Leeflang and colleagues, the tests commonly used in interventional systematic reviews for publication bias are not useful for diagnostic testing reviews (Leeflang 2008). Deeks and colleagues verified that the use of an asymmetric effective sample size plot to detect publication bias lacks power in situations where sample variability is present (Deeks 2005). We attempted to use unpublished data to minimise reporting bias.
Results
Results of the search
Results of database searches to March 2019 yielded 3566 records. Of these, we removed 17 duplicates and 3480 records at the title and abstract level (Figure 1). Of the remaining 69 articles, we excluded 30 at full paper screening due to a variety of reasons (abstract only, irrelevant outcomes, inappropriate comparator, inappropriate patient population, literature review, duplicate records and inappropriate indication; see Characteristics of excluded studies table). We finally included 39 studies, all of which evaluated the index test of EFWD by ultrasound measurements compared to the reference standard of actual birth weight measured at birth (Bimson 2012; Bimson 2014a; Bimson 2014b; Blickstein 1989; Blickstein 1996; Caravello 1997; Chamberlain 1991; Chang 2006; Chauhan 1995; Crane 1980; D'Antonio 2013a; D'Antonio 2014; Danon 2008; Diaz‐Garcia 2010; Fox 2011; Hehir 2017; Hill 1994; Hoopmann 2011; Jensen 1995; Johansen 2014; Kalish 2003; Kim 2015; Klam 2005; Machado 2007; MacLean 1992; Murray 2014; Nakayama 2014; O'Connor 2013; Reberdao 2010; Sayegh 1993; Secher 1985; Shahshahan 2011; Simoes 2011; Storlazzi 1987; van de Waarsenburg 2015; van Mieghem 2009; Watson 1991; Zipori 2016; Zuckerwise 2015).
1.
PRISMA flow diagram outlining the study selection process.
The list and details of the included studies are presented in the Characteristics of included studies table. The 39 eligible studies had a median sample size of 140 and range of 38 to 2161 women per study. Of these studies, 16 were conducted in Europe, seven in Asia, 14 in North America and two in other geographical areas. Nine studies were conducted at university hospitals, eight studies were at tertiary centres, 11 studies were conducted in hospitals, six studies were conducted at other settings and five studies did not indicate their settings. Three studies were published prior to 1990, eight were published between 1991 and 2000, eight between 2001 and 2010, and the remaining 14 after 2010. All studies used ultrasound by 2D, and none evaluated EFWD using 3D ultrasound.
Methodological quality of included studies
We assessed all 39 studies using the QUADAS‐2 framework (Figure 2; Figure 3). All studies were observational cohort‐type studies with delayed verification. From the studies that reported BWD, four received financial support. There were no studies whose authors declared a conflict of interest. Thirty‐one studies gave no information on either of these two potential biases.
2.
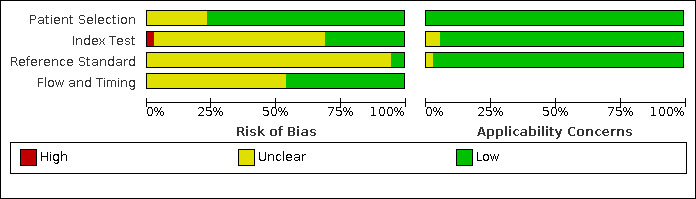
QUADAS‐2 risk of bias and applicability concerns graph including review authors' judgements about each domain presented as percentages across included studies.
3.
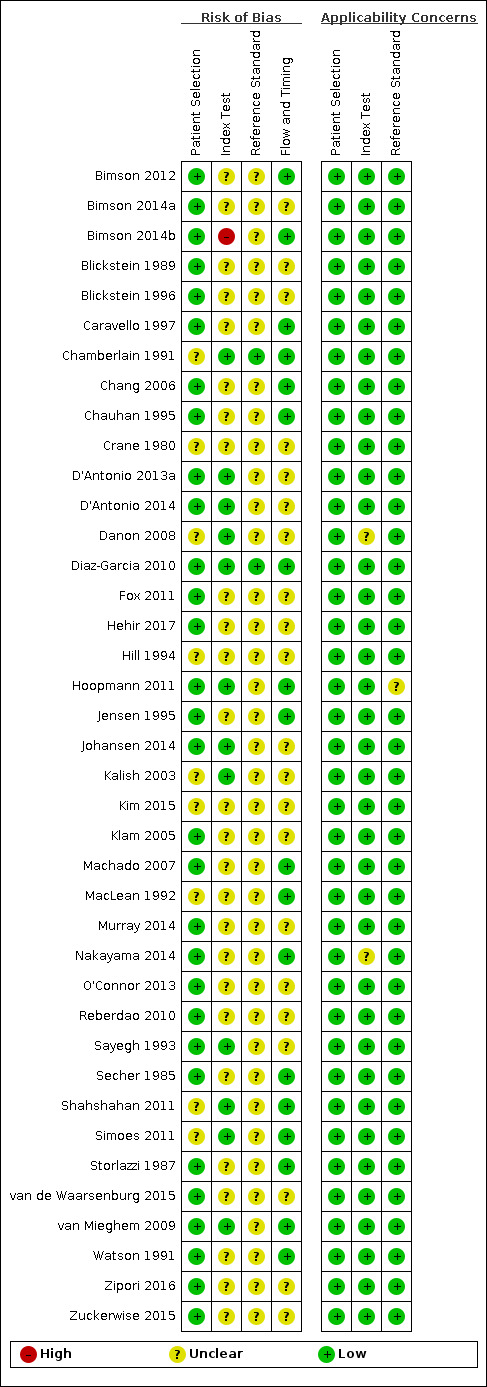
QUADAS‐2 risk of bias and applicability concerns summary including review authors’ judgements about each domain for each included study.
In terms of risk of bias, there were many unclear statements regarding patient selection, index test, use of proper reference standard, and flow and timing elements of the studies. In terms of applicability, most studies were high quality, and there was low concern. There was one study at high risk of bias based on the judgments made about the index test. There were no studies at high risk of bias based on the judgements made about the reference standard, and, in 37 (90%), the risk was unclear. Twenty‐one studies (53%) were of methodological concern due to flow and timing.
We judged 36 studies (92%) at low concern of applicability concerns in all three domains (Figure 3). Two studies had an unclear risk of bias for the index test (Danon 2008; Nakayama 2014), and one study with an unclear risk of bias for a reference standard (Hoopmann 2011).
We used the GRADE approach to evaluate the certainty of the evidence. For both diagnostic performance of ultrasound at 20% and 25% BWD, the certainty of evidence was downgraded one level for risk of bias and two levels for inconsistency to give a final estimation of very low certainty of evidence.
Findings
1. Diagnostic performance of ultrasound (20% difference in birth weight as reference)
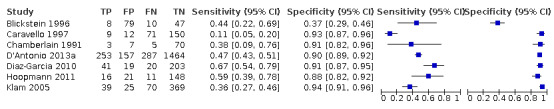
Twenty‐two studies that estimated EFWD by ultrasound measurements provided data for a discordance of equal to or greater than 20% (Bimson 2014a; Bimson 2014b; Blickstein 1996; Chamberlain 1991; Chang 2006; Chauhan 1995; D'Antonio 2013a; Diaz‐Garcia 2010; Fox 2011; Hill 1994; Jensen 1995; Johansen 2014; Kalish 2003; Kim 2015; Machado 2007; MacLean 1992; Secher 1985; Shahshahan 2011; Storlazzi 1987; van de Waarsenburg 2015; van Mieghem 2009; Watson 1991). Median BWD of 20% prevalence across the studies was 18% (range 9% to 50%). The findings and certainty of evidence are detailed in Table 1.
Summary of findings 1. Ultrasound for diagnosis of birth weight discordance in twin pregnancies at 20% cut‐off.
| Sensitivity | 0.51 (95% CI 0.42 to 0.60) | Prevalencesa | 18% | 15% | 28% | ||||||
| Specificity | 0.91 (95% CI 0.89 to 0.93) | ||||||||||
| Outcome |
No. of studies and participants |
Study design | Factors that may decrease certainty | Effect per 1000 women tested | |||||||
| Risk of bias | Indirectness | Inconsistency | Imprecision | Publication bias | Pretest probability of 18% | Pretest probability of 15% | Pretest probability of 28% | Test accuracy (certainty of the evidence) | |||
|
True positives (women with diagnosis of birth weight discordance) |
22 studies, 1462 participants | Cohort‐type studies with delayed verification (cohort type accuracy study) | Very seriousb | Not serious | Very seriousc | Not serious | None | 92 (76 to 108) | 77 (63 to 90) | 143 (118 to 168) | ⊕⊝⊝⊝ Very lowd |
|
False negatives (women incorrectly classified as not having diagnosis of birth weight discordance) |
88 (72 to 104) | 73 (60 to 87) | 137 (112 to 162) | ||||||||
| True negatives (women without diagnosis of birth weight discordance) | 22 studies, 6453 participants |
Cohort‐type studies with delayed verification (cohort type accuracy study) | Very seriousb | Not serious | Very seriousc | Not serious | None | 746 (730 to 763) | 774 (757 to 791) | 655 (641 to 670) | ⊕⊝⊝⊝ Very lowd |
| False positives (women incorrectly classified as having diagnosis of birth weight discordance) | 74 (57 to 90) | 76 (59 to 93) | 65 (50 to 79) | ||||||||
CI: confidence interval. aThe prevalence used to represent the pretest probability are the median, first quartile and third quartile of the prevalences of included studies. bIn more than 50% of the studies there were unclear statements regarding index test, use of proper reference standard and flow and timing elements; in 1/3 of the studies, it was unclear how the participants were selected. cVery high unexplained heterogeneity in terms of sensitivity ranging from 0.16 to 1.00. dGRADE certainty of evidence downgraded one level for risk of bias and two levels for inconsistency.
Study level estimates of sensitivities and specificities are shown in Figure 4.
4.
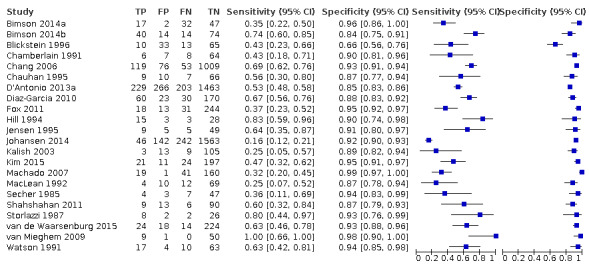
A forest plot representing study level sensitivities and specificities that used an estimated fetal weight discordance (EFWD) of 20%.
The sensitivities ranged between 16% and 100%, whereas the specificities ranged between 66% and 99%. The summary estimate of sensitivity was 0.51 (95% CI 0.42 to 0.60), and the summary estimate of specificity was 0.91 (95% CI 0.89 to 0.93) (Table 3). The summary receiver operating plot with the summary point is illustrated in Figure 5. Most of the studies on the SROC plot were close to the Y axis indicating high specificity but variable sensitivity. The confidence regions were narrow for specificity but not sensitivity. The large prediction region indicated the variability of the diagnostic accuracy of future studies. The LR+ for EFWD by ultrasound was 5.9 (95% CI 4.3 to 8.1), and for LR– was 0.53 (95% CI 0.44 to 0.64).
1. Meta‐analysis summary.
| Groups | No. of studies | Sensitivity (95% CI) | Specificity (95% CI) | LR+ | LR– |
| BWD of 20% | 22 | 0.51 (0.42 to 0.60) | 0.91 (0.89 to 0.93) | 5.9 (4.3 to 8.1) | 0.53 (0.44 to 0.64) |
| EFWD by AC | 7 | 0.57 (0.48 to 0.66) | 0.84 (0.72 to 0.92) | 3.6 (1.8 to 7.4) | 0.51 (0.38 to 0.68) |
| EFWD by FL | 7 | 0.60 (0.53 to 0.67) | 0.87 (0.84 to 0.90) | 4.6 (3.4 to 6.1) | 0.46 (0.38 to 0.56) |
| BWD of 25% | 18 | 0.46 (0.26 to 0.66) | 0.93 (0.89 to 0.96) | 6.7 (3.0 to 14.9) | 0.58 (0.39 to 0.88) |
| EFWD by AC | 7 | 0.42 (0.27 to 0.58) | 0.88 (0.76 to 0.94) | 3.3 (1.6 to 6.9) | 0.67 (0.51 to 0.87) |
| EFWD by FL | 4 | 0.55 (0.44 to 0.65) | 0.91 (0.89 to 0.92) | 5.8 (4.3 to 7.9) | 0.50 (0.40 to 0.64) |
AC: abdominal circumference; BWD: birth weight discordance; CI: confidence interval; EFWD: estimated fetal weight discordance; FL: femur length; LR+: likelihood ratio of a positive test; LR–: likelihood ratio of a negative test.
5.
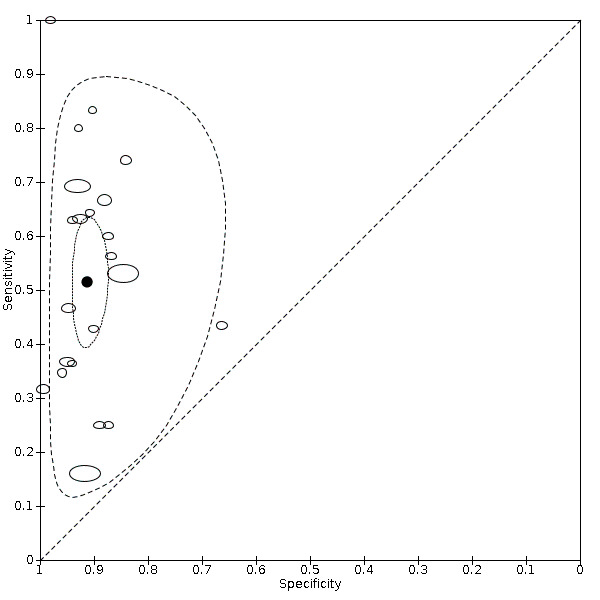
Summary receiver operating characteristic plot of studies assessing the accuracy of ultrasound based on an estimated fetal weight discordance (EFWD) of 20%. Each study is represented as an ellipse with size of the ellipse adjusted to the sample size of the study. The filled circle represents the summary point indicating summary sensitivity and specificity of the meta‐analytic estimate. Dotted closed line represents 95% confidence region and the dashed line represents the 95% prediction region around the summary point.
Subgroup analyses of the 20% discordance based on measurements by abdominal circumference versus femur length
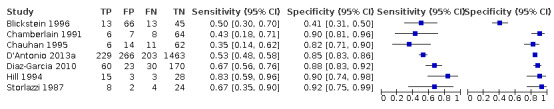
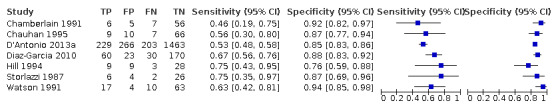
Of the 22 studies that reported estimates with 20% cut‐off, seven used AC (Blickstein 1996; Chamberlain 1991; Chauhan 1995; D'Antonio 2013a; Diaz‐Garcia 2010; Hill 1994; Storlazzi 1987) (Figure 6). Median disease prevalence across the studies was 26.3 (range 15.3 to 36.7). For AC as a measurement for EFWD, the summary estimates of sensitivity was 0.57 (95% CI 0.48 to 0.66), and the summary estimate of specificity was 0.84 (95% CI 0.72 to 0.92) (Table 3). The LR+ for EFWD by ultrasound was 3.6 (95% CI 1.8 to 7.4), and for LR– was 0.51 (95% CI 0.38 to 0.68).
6.
A forest plot representing sensitivities and specificities of the studies that used ultrasound abdominal circumference to detect estimated fetal weight discordance (EFWD) of 20%. FN: false negative; FP: false positive; TN: true negative; TP: true positive.
Among the 22 studies that reported estimates with 20% difference in birth weight as reference, seven studies used FL for EFWD (Chamberlain 1991; Chauhan 1995; D'Antonio 2013a; Diaz‐Garcia 2010; Hill 1994; Storlazzi 1987; Watson 1991). The study level sensitivity and specificity for ultrasound using FL are depicted in Figure 7. For FL as a measurement for EFWD, the summary estimate of sensitivity was 0.60 (95% CI 0.53 to 0.67), and the summary estimate of specificity was 0.87 (95% CI 0.84 to 0.90) (Table 3). LR+ was 4.6 (3.4 to 6.1), and LR– was 0.46 (0.38 to 0.56).
7.
A forest plot representing sensitivities and specificities of the studies that used femoral length by ultrasound to detect estimated fetal weight discordance (EFWD) of 20%. FN: false negative; FP: false positive; TN: true negative; TP: true positive.
No subgroup analyses were possible based on gestational age, sex discordance or chorionicity due to lack of sufficient data for analysis.
2. Diagnostic performance of ultrasound (25% difference in birth weight as reference)
Eighteen studies that EFWD by ultrasound measurements provided data using a 25% difference in birth weight as reference (Bimson 2014a; Bimson 2014b; Blickstein 1996; Caravello 1997; Chamberlain 1991; Chang 2006; Crane 1980; D'Antonio 2013a; Danon 2008; Diaz‐Garcia 2010; Hoopmann 2011; Klam 2005; Nakayama 2014; Reberdao 2010; Sayegh 1993; Simoes 2011; van Mieghem 2009; Zipori 2016). Study level estimates of sensitivities and specificities are shown in Figure 8. The sensitivities ranged between 1% and 100% whereas the specificities ranged between 79% and 100%. The summary estimate of sensitivity was 0.46 (95% CI 0.26 to 0.66), and the summary estimate of specificity was 0.93 (95% CI 0.89 to 0.96) (Table 3). The summary receiver operating plot along with the summary point is illustrated in Figure 9. Most of the studies on the SROC plot were close to the Y axis indicating high specificity but variable sensitivity. The confidence regions were narrow for specificity but not sensitivity. The large prediction region indicated the variability of the diagnostic accuracy of future studies. The LR+ for EFWD by ultrasound was 6.7 (95% CI 3.0 to 14.9) and for LR– was 0.58 (95% CI 0.39 to 0.88). Findings and the certainty of evidence (very low) are detailed in the Table 2.
8.
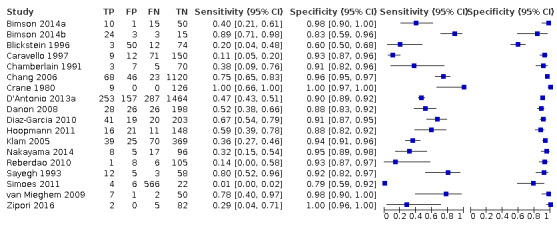
A forest plot representing sensitivities and specificities of all the studies that used an estimated fetal weight discordance (EFWD) of 25%. FN: false negative; FP: false positive; TN: true negative; TP: true positive.
9.
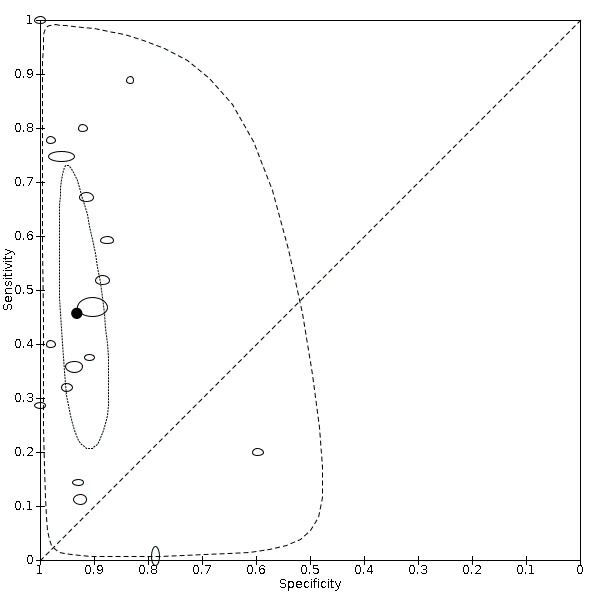
Summary receiver operating characteristic plot of studies assessing the accuracy of ultrasound based on an estimated fetal weight discordance (EFWD) of 25%. Each study is represented as an ellipse with size of the ellipse adjusted to the sample size of the study. The filled circle represents the summary point indicating summary sensitivity and specificity of the meta‐analytic estimate. Dotted closed line represents 95% confidence region and the dashed line represents the 95% prediction region around the summary point.
Summary of findings 2. Ultrasound for diagnosis of birth weight discordance in twin pregnancies at 25% cut‐off.
| Sensitivity | 0.46 (95% CI 0.26 to 0.66) | Prevalencesa | 19% | 9% | 27% | ||||||
| Specificity | 0.93 (95% CI 0.89 to 0.96) | ||||||||||
| Outcome |
No. of studies and participants |
Study design | Factors that may decrease certainty | Effect per 1000 women tested | |||||||
| Risk of bias | Indirectness | Inconsistency | Imprecision | Publication bias | Pretest probability of 19% | Pretest probability of 9% | Pretest probability of 27% | Test accuracy (certainty of the evidence) | |||
|
True positives (women with diagnosis of birth weight discordance) |
18 studies, 1679 participants | Cohort‐type studies with delayed verification (cohort type accuracy study) | Very seriousb | Not serious | Very seriousc | Not serious | None | 87 (49 to 125) | 41 (23 to 59) | 124 (70 to 178) | ⊕⊝⊝⊝ Very lowd |
|
False negatives (women incorrectly classified as not having diagnosis of birth weight discordance) |
103 (65 to 141) | 49 (31 to 67) | 146 (92 to 200) | ||||||||
| True negatives (women without diagnosis of birth weight discordance) | 18 studies 4792 participants | Cohort‐type studies with delayed verification (cohort type accuracy study) | Very seriousb | Not serious | Very seriousc | Not serious | None | 753 (721 to 778) | 846 (810 to 874) | 679 (650 to 701) | ⊕⊝⊝⊝ Very lowd |
| False positives (women incorrectly classified as having diagnosis of birth weight discordance) | 57 (32 to 89) | 64 (36 to 100) | 51 (29 to 80) | ||||||||
CI: confidence interval. aThe prevalence used to represent the pretest probability are the median, first quartile and third quartile of the prevalences of included studies. bAt least 50% of the studies had unclear statements regarding index test, use of proper reference standard and flow and timing elements. cVery high unexplained heterogeneity in terms of sensitivity ranging from 0.1 to 1.00. dGRADE certainty of evidence downgraded one level for risk of bias and two levels for inconsistency.
Subgroup analyses of the 25% discordance based on measurements of abdominal circumference measurement versus femur length
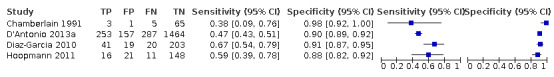
Among the studies that reported estimates with 25% difference in birth weight as reference, seven studies used AC (Blickstein 1996; Caravello 1997; Chamberlain 1991; D'Antonio 2013a; Diaz‐Garcia 2010; Hoopmann 2011; Klam 2005). Study level sensitivity and specificity for ultrasound using AC are depicted in Figure 10. The pooled estimate of sensitivity was 0.42 (95% CI 0.27 to 0.58) whereas that of the specificity was 0.88 (95% CI 0.76 to 0.94) (Table 3). LR+ was 3.3 (95% CI 1.6 to 6.9) and LR– was 0.67 (95% CI 0.51 to 0.87).
10.
A forest plot representing sensitivities and specificities of the studies that used ultrasound abdominal circumference to detect estimated fetal weight discordance (EFWD) of 25%. FN: false negative; FP: false positive; TN: true negative; TP: true positive.
Among the studies that reported estimates with 25% difference in birth weight as reference, we only found four studies used FL (Chamberlain 1991; D'Antonio 2013a; Diaz‐Garcia 2010; Hoopmann 2011). Study level sensitivity and specificity for ultrasound using FL are depicted in Figure 11. The pooled estimate of sensitivity was 0.55 (95% CI 0.44 to 0.65) whereas that of the specificity was 0.91 (95% CI 0.89 to 0.92) (Table 3). LR+ was 5.8 (95% CI 4.3 to 7.9) and LR– was 0.50 (95% CI 0.40 to 0.64).
11.
A forest plot representing sensitivities and specificities of the studies that used ultrasound femoral length to detect estimated fetal weight discordance (EFWD) of 25%. FN: false negative; FP: false positive; TN: true negative; TP: true positive.
No subgroup analyses were possible based on gestational age, sex discordance or chorionicity due to lack of sufficient data for analysis.
Discussion
Summary of main results
We evaluated the diagnostic accuracy of EFWD of 20% and 25% using biometric ultrasound measurements compared with BWD as the reference standard. We identified 39 studies that matched our inclusion criteria. The summary estimate of sensitivity was 0.51 (95% CI 0.42 to 0.60), and the summary estimate of specificity was 0.91 (95% CI 0.89 to 0.93) at 20% discordance in BWD (Table 3). The summary estimate of sensitivity was 0.46 (95% CI 0.26 to 0.66), and the summary estimate of specificity was 0.93 (95% CI 0.89 to 0.96) at 25% discordance in BWD. Sensitivity and specificity did not differ substantively between the two cut‐off points (20% and 25%). We found that there was high variability in sensitivity ranging from 0.1 to 1.0 but specificity was high with tight ranges for both 20% and 25% discordances in BWD. The certainty of the evidence was very low due to high risk of bias in the included studies and inconsistency.
Subgroup analyses were possible for ultrasound measurements using AC and FL for both 20% and 25% discordance in BWD. The sensitivities and specificities were not substantially different between these subgroups for both discordance cut‐offs, but sensitivity and specificity were marginally better in studies that used FL for EFWD estimation. No subgroup analyses were possible based on gestational age, sex discordance or chorionicity due to lack of sufficient data for analysis.
Overall, this systematic review found very low‐certainty evidence that ultrasound EFWD discordance between twins has poor sensitivity but good specificity in predicting actual BWD and suggested that FL performs slightly better than AC.
Leombroni 2017 reviewed limited data sources (PubMed, Embase and CINAHL) and fewer studies (20). The review authors could not perform comprehensive data synthesis for each AC discordance cut‐off and were unable to report data on FL measurement. They concluded that the optimal diagnostic performance of AC discordance was for prediction of BW discordance of 25% or greater, with a sensitivity of 70.8% and specificity of 86.4%. Our findings suggest that there was no difference in ultrasound diagnosis of EFWD between 20% and 25% discordance.
Strengths and weaknesses of the review
The strength of this review is implementing a comprehensive search in a variety of databases and a large number of publications reviewed. We followed the standard recommendations of the Cochrane DTA (methods.cochrane.org/sdt/). We provide the most up‐to‐date overall assessment of ultrasound in predicting BWD in twins.
There are several limitations to this review.
We found considerable heterogeneity in sensitivity of the EFWD measurement by ultrasound. The low sensitivity of EFWD would miss a significant number of twins with BWD, which would be clinically consequential. However, twins often have regular screening so a missed diagnosis in one scan could potentially be detected in a subsequent scan.
We were able to analyze only two cut‐off points (20% and 25%).
Lack of stratification by gestational age; gestational age at ultrasound examination is of great importance when assessing the predictive accuracy of ultrasound for growth discordance. We were unable to pool the data based on different gestational age due to the large variation in the extracted data. According to the literature, growth discrepancy in twins usually occurs in the third trimester (Puccio 2014). Hence, an ultrasound assessment performed early in pregnancy cannot reliably predict discordance.
Unable to conduct a subgroup analysis based on chorionicity due to the failure of included studies in reporting chorionicity. Since the pathophysiology of growth discordance differs in MC and DC twins, lack of chorionicity information in our analysis may be reflected in the predictive accuracy of ultrasound in identifying twins affected by abnormal growth.
Studies included in the analysis used different EFWD formulae (Hadlock, Shepard, Ferrero) to predict fetal weight, and some studies did not specify which formula they used to measure AC or FL. The absence of stratification according to each specific ultrasound weight formula might have hypothetically changed the approximation of EFW for each twin, consequently affecting the estimated EFWD.
It is also worth noting that some of the included studies are old; hence the technology used at the time may not have created as sharp an image as the new ultrasound machine.
Applicability of findings to the review question
Performing ultrasound on 1000 twin pregnancies to detect BWD of 20% or more with a median prevalence of BWD of 18% and using a summary sensitivity of 0.51 and specificity of 0.91, 88 women will miss a correct diagnosis (FN) and miss a chance to have beneficial interventions while 74 women will be diagnosed FP and may be subject to unnecessary medical interventions. The FN rate increases as the prevalence increases from 18% and more women will have a missed diagnosis but the FP rate decreases (Table 1).
Similarly, performing ultrasound on 1000 twin pregnancies to detect BWD of 25% or more with a median prevalence of BWD of 19% and using a summary sensitivity of 0.46 and specificity of 0.93, 103 women will miss a correct diagnosis (FN) and miss a chance to have beneficial interventions while 57 women will be diagnosed FP and may be subject to unnecessary interventions (Table 2).
BWD is associated with adverse perinatal outcomes in twin pregnancy (Jahanfar 2017a). Therefore, it is crucial to measure EFWD during routine ultrasound examinations. Our findings suggest that ultrasound EFWD has low sensitivity but good specificity in detecting discordance in actual BWD. There are a few points to consider when interpreting the result of this review. First, the reference standard that we used in this review (BWD) is a reliable reference standard, and so the findings are applicable to the review question. However, it should be noted that the tests were performed in a health clinic or tertiary hospitals, and our findings are, therefore, applicable only in these settings. Publications selected for this review included twin pregnancies with viable fetuses. Hence, the results of our review are not applicable to pregnancies with stillbirth, triplets or quadruplets. We were careful not to include the studies that did not define BWD or did not report the EFWD formula as identified by our review ((larger estimated weight – smaller estimated weight)/larger estimate weight) × 100). In addition, we included only the measurements performed within seven days of birth and measured by a healthcare professional using either electronic or mechanical scales. Examinations included in the analysis were 2D ultrasounds performed during any stage of pregnancy. Since the test works best in the third trimester, it is unlikely that it would be useful for identifying and treating TTTS.
Authors' conclusions
Implications for practice.
Very low‐certainty evidence suggests that estimated fetal weight discordance (EFWD) identified by ultrasound has a low sensitivity but good specificity in detecting birth weight discordance (BWD) in twin pregnancies. There is uncertain diagnostic value of EFWD; this review suggests there is insufficient evidence to support this index as the sole measure for clinical decision making to evaluate the prognosis of twins with growth discordance. The diagnostic accuracy of other measures including amniotic fluid Index and umbilical artery Doppler resistive indices in combination with ultrasound for clinical intervention needs evaluation. Other clinical factors that should be considered are gestational age, chorionicity and real‐time information about twin's heart rates and are important in the management of twin pregnancies with growth discordance. Currently, ultrasound is the only technology available to identify BWD. Until a better technology or test with better diagnostic accuracy is available, serial ultrasound to investigate the growth of fetuses is necessary to evaluate the degree of intertwin growth discordance.
Implications for research.
Well‐designed prospective cohort studies with delayed verification that assess the diagnostic performance of EFWD is needed. Stratification by gestational age, with different fetal weight discordance values (20% and 25%), chorionicity information and sex discordance information are needed and may be useful to reliably determine the diagnostic accuracy of ultrasound in predicting growth discordance in twin pregnancies.
History
Protocol first published: Issue 3, 2017 Review first published: Issue 3, 2021
Acknowledgements
As part of the prepublication editorial process, the review has been commented on by three peers (an editor and two referees who are external to the editorial team) and a member of the Pregnancy and Childbirth Group's international panel of consumers. The authors are grateful to the following peer reviewers for their time and comments: Gabriele Saccone, Department of Neuroscience, Reproductive Sciences and Dentistry, School of Medicine, University of Naples Federico II, Naples, Italy; Alexis Shub, Mercy Hospital for Women, Melbourne, Australia.
We thank Ursula Ellis for preparing the search strategies for this review. We also thank Vicki Nisenblat for her contribution to the protocol for this review.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. Search strategy
CENTRAL
(ultraso* or sonogra* or doppler) and (twin* or (multiple and (birth* or pregnanc*)) and (weigh* or size or grow* or discordan*))
Ovid MEDLINE
1. ultrasonography/ or exp ultrasonography, doppler/ or exp ultrasonography, prenatal/
2. Laser‐Doppler Flowmetry/
3. ultraso*.mp.
4. sonogra*.mp.
5. doppler.mp.
6. acoustic microscop*.mp
7. us.fs.
8. or/1‐7
9. exp Twins/ or Twin discordance/ or exp Pregnancy, Multiple/
10. twin*.mp.
11. (multiple adj3 (pregnanc* or birth*)).mp.
12. or/9‐11
13. exp Birth Weight/ or Fetal Weight/ or exp Fetal Growth Retardation/ or exp Infant, Low Birth Weight/
14. (weigh* adj3 (discordan* or differ*)).mp.
15. bwd.mp.
16. (growth adj3 (discordan* or differ* or retard*)).mp.
17. ((birth or newborn or neonat* or fetal or foetal or fetus or foetus) adj3 (weigh* or grow* or size)).mp.
18. or/13‐17
19. 8 and 12 and 18
Ovid Embase
1. echography/ or doppler echography/ or doppler flowmetry/ or exp fetus echography/
2. ultrasound/
3. exp fetal ultrasound monitor/
4. exp ultrasound transducer/
5. echograph/ or exp doppler device/ or exp ultrasound scanner/
6. (ultraso* or sonogra* or doppler or acoustic microscop*).mp.
7. or/1‐6
8. exp twins/ or exp multiple pregnancy/
9. twin*.mp.
10. (multiple adj3 (pregnanc* or birth*)).mp.
11. or/8‐10
12. exp birth weight/
13. exp intrauterine growth retardation/
14. (weigh* adj3 (discordan* or differ*)).mp.
15. bwd.mp.
16. (growth adj3 (discordan* or differ* or retardat*)).mp.
17. exp prenatal growth/
18. ((birth or newborn or neonat* or fetal or foetal or fetus or foetus) adj3 (weigh* or grow* or size)).mp.
19. or/12‐18
20. 7 and 11 and 19
CINAHL
1. (MH "Ultrasonography") OR (MH "Ultrasonography, Doppler+") OR (MH "Ultrasonography, Prenatal+")
2. ultraso* or sonogra* or doppler or acoustic microscop*
3. twins
4. (MH "Twins") OR (MH "Pregnancy, Multiple+")
5. twin* or (multiple N3 (pregnanc* OR birth*)
6. (MH "Birth Weight") OR (MH "Fetal Weight")
7. (MH "Fetal Growth Retardat*")
8. weigh* N3 (discordan* or differ*)
9. bwd
10. growth N3 (discordan* OR differ* or retard*)
11. (birth or newborn or neonat* or fetal or foetal or fetus or foetus) N3 (weigh* or grow* or size)
12. s1 or s2
13. s3 or s4 or s5
14. s6 or s7 or s8 or s9 or s10 or s11
15. s12 and s13 and s14
Web of Science Core Collection
TOPIC: (ultraso* or sonogra* or doppler) AND TOPIC: (twin* or (multiple NEAR/2 birth*) or (multiple NEAR/2 pregnanc*)) AND TOPIC: (weigh* or size or grow* or discordan*)
DARE, NHS EED, HTA, Prospero, Trip database, PubMed systematic review subset
(ultraso* or sonogra* or doppler) and (twin* or (multiple and (birth* or pregnanc*)) and (weigh* or size or grow* or discordan*))
Appendix 2. QUADAS‐2 risk of bias assessment tool
| Domain 1 –participant selection | |
| Description | Describe methods of participant selection; describe included participants (previous testing, presentation, intended use of index test and setting) |
| Type of bias assessed | Selection bias, spectrum bias |
| Review question | Twin pregnancies with ultrasound measurements at any stage of pregnancy. The twin pregnancies included any type of chorionicity, any type of conception, any body mass index, any maternal age and any maternal condition |
| Information collected | Study objectives, study population, selection (inclusion/exclusion criteria), study design, clinical presentation, age, number enrolled and number available for analysis, setting, place and period of the study |
| Signalling question 1 | Did the study avoid inappropriate exclusions? |
| Yes | If all the study participants with alive twin pregnancies were included |
| No | If the study selected participants based on particular clinical features. This section should include something indicated that the population was preselected and hence may not reflect the accurate findings applied to general population. Example: only participants with or without specific medical conditions; participants selected based on body‐mass index; only participants attending certain number of previous ultrasound examinations; only participants selected on the basis of other antenatal tests |
| Unclear | If the study did not provide a clear definition of the selection (inclusion/exclusion) criteria and 'no' judgement was not applicable |
| Signalling question 2 | Was a consecutive or random sample of participants enrolled? |
| Yes | If a consecutive sample or a random sample of the eligible participants was included in the study |
| No | If a non‐consecutive sample or non‐random sample of the eligible participants was included in the study |
| Unclear | If this information was unclear |
| Signalling question 3 | Was a case‐control design avoided? |
| Yes | If the study had a single set of inclusion criteria, defined by the clinical presentation (i.e. only participants with alive twin pregnancy) |
| No | If the study had > 1 set of inclusion criteria in respect to clinical presentation (i.e. participants with alive twin pregnancy and participants with singleton pregnancies or additional group of participants used as historical cohort) |
| Unclear | If it was unclear whether a case‐control deign was avoided or not |
| Risk of bias | Could the selection of participants have introduced bias? |
| High | If 'no' classification for any of the above 3 questions |
| Low | If 'yes' classification for all the above 3 questions |
| Unclear | If 'unclear' classification for any of the above questions and 'high' risk judgement was not applicable |
| Concerns about applicability | Are there concerns that the included participants do not match the review question? |
| High | If the study population differed from the population defined in the review question in terms of demographic features and comorbidity |
| Low | If the study included only clinically relevant population that would have undergone the index test in real practice |
| Unclear | If this information was unclear; only studies with sufficient information on study population were included, therefore, none of the included studies were anticipated to be classified as 'unclear' |
| Domain 2 – index test | |
| Description | Describe the index test, how it was conducted and interpreted |
| Type of bias assessed | Test review bias, clinical review bias, interobserver variation bias |
| Review question | Any type of ultrasound method and any type of biometric measurements for assessment of estimated fetal weight |
| Information collected | Index test name, description of positive case definition by index test as reported, examiners (number, level of expertise, blinding), interobserver variability, conflict of interests |
| Signalling question 1 | Were the index test results interpreted without knowledge of the results of the reference standard? |
| Yes | If the operators interpreting the index test were unaware of the results of reference standard. All the index tests are expected to be performed and interpreted before execution of the reference standard; therefore, none of the included studies are anticipated be classified as 'Yes' in response to this question |
| No | If the operators interpreting the index test were not blinded to the results of reference standard |
| Unclear | If this information was unclear |
| Signalling question 2 | Did the study provide a clear prespecified definition of what was considered to be a 'positive' result of index test? |
| Yes | If study provided clear definition of positive findings, which was defined before execution/interpretation of index test |
| No | If definition of the positive result was not provided or if study described the findings derived from the index test and not defined prior to its execution |
| Unclear | If it was unclear whether the criteria were prespecified or not |
| Signalling question 3 | Was the level of expertise of the index test operator provided? |
| Yes | If test was performed/interpreted either by single operator or interpreted after collegial discussion of the case |
| No | If test was performed/interpreted independently by several operators |
| Unclear | If this information was unclear |
| Signalling question 4 | Were the same clinical data available when the index test results were interpreted as would be available when the test is used in practice? |
| Yes | If operators performing/interpreting the test were aware of suspected birth weight discordance or of the clinical history (or both), but were not aware of the results of other imaging tests or of previous diagnosis of birth weight discordance, including the results of previous ultrasounds |
| No | If operators performing/interpreting the test were not blinded to the results of other imaging tests or tests raising suspicion for birth weight discordance |
| Unclear | If this information was unclear |
| Risk of bias | Could the conduct or interpretation of the index test have introduced bias? |
| High | If 'no' classification for any of the above 4 questions |
| Low | If 'yes' classification for all the above 4 questions |
| Unclear | If 'unclear' classification at least for 1 question and 'high' risk judgement was not applicable |
| Concerns about applicability | Are there concerns that the index test, its conduct or interpretation differ from the review question? |
| High | We plan not to consider the studies where index tests other than ultrasound estimated fetal weight measurements were included or where index test looked at other target conditions not specified in the review (e.g. studies aimed at identifying maternal diseases); therefore, none of the included studies is expected to be classified as 'high concern' |
| Low | We considered all types of ultrasound methods and biometric parameters as eligible; therefore, all the included studies are expected to be classified as 'low concern' |
| Unclear | Only studies with sufficient information on index was included; therefore, none of the included studies were classified as 'unclear' |
| Domain 3 –reference standard | |
| Description | Describe the reference standard, how it was conducted and interpreted |
| Type of bias assessed | Verification bias, bias in estimation of diagnostic accuracy due to inadequate reference standard |
| Review question | Target condition – birth weight discordance |
| Information collected | Target condition, prevalence of target condition in the sample, reference standard, description of positive case definition by reference test as reported, examiners (number, level of expertise, blinding) |
| Signalling question 1 | Is the reference standard likely to correctly classify the target condition? |
| Yes | If the study reported ≥ 1 of the following: calibration of scales, electronic scales, timing of weighing in relation to delivery or drying of neonate before weighing |
| No | If the reference standard did not classify the target condition correctly; considering the inclusion criteria, none of the studies are expected to be classified as 'no' for this item |
| Unclear | If information on execution of the reference standard, its interpretation or operators was unclear |
| Signalling question 2 | Were the reference standard results interpreted without knowledge of the results of the index tests? |
| Yes | If operators performing the reference test were unaware of the results of index test |
| No | If operators performing the reference test were aware of the results of index test |
| Unclear | If this information was unclear |
| Risk of bias | Could the reference standard, its conduct or its interpretation have introduced bias? |
| High | If 'no' classification for any of the above 2 questions |
| Low | If 'yes' classification for all the above 2 questions |
| Unclear | If 'unclear' classification for any of the above 2 questions and 'high risk' judgement was not applicable |
| Concerns about applicability | Are there concerns that the target condition as defined by the reference standard does not match the question? |
| High | We excluded studies where participants did not have ultrasound measurement; therefore, none of the included studies are expected to be classified as 'high concern' |
| Low | Considering the inclusion criteria, all the studies are expected to be classified as 'low concern' |
| Unclear | Only studies where scales to assess birth weight and universal formula to calculate birth weight discordance served as a reference test was included; therefore, none of the included studies is expected to be classified as 'unclear concern' |
| Domain 4 –flow and timing | |
| Description | Describe any participants who did not receive the index tests or reference standard or who were excluded from the 2 × 2 table, describe the interval and any interventions between index tests and the reference standard |
| Type of bias assessed | Disease progression bias, bias of diagnostic performance due to missing data |
| Review question | Birth weight discordance assessed within 7 days of delivery |
| Information collected | Time interval between index test and reference standard, withdrawals (overall number of reported and if were explained) |
| Signalling question 1 | Was there an appropriate interval between index test and reference standard? |
| Yes | If time interval was reported and was < 7 days |
| No | We excluded all studies where the time interval was > 7 days; therefore, none of the included studies is anticipated to be classified as 'no' for this item |
| Unclear | If time interval was not stated clearly, but authors description allowed the reader to assume that the interval was reasonably short |
| Signalling question 2 | Did all participants receive the same reference standard? |
| Yes | If all participants underwent birth weight assessment and there are sufficient data to calculate birth weight discordance; considering the inclusion criteria, all the studies are expected to be classified as 'yes' for this item |
| No | If all participants did not undergo birth weight assessment or if only a subset of participants had this assessment, but the information on this population was not available in isolation |
| Unclear | If this information was unclear |
| Signalling question 3 | Were all participants included in the analysis? |
| Yes | If all the participants were included in the analysis or if the participants were excluded prior to execution of index test or if the withdrawals were < 2% of the enrolled population (arbitrary selected cut‐off) |
| No | If any participants were excluded from the analysis because of uninterpretable results, inability to undergo either index test or reference standard or unclear reasons |
| Unclear | None of the studies expected to be classified as 'unclear' for this item |
| Risk of bias | Could the patient flow have introduced bias? |
| High | If 'no' classification for any of the above 3 questions |
| Low | If 'yes' classification for all the above 3 questions |
| Unclear | If 'unclear' classification for any of the above 3 questions and 'high risk' judgement was not applicable |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 Cut‐off 20% | 22 | 8005 |
| 2 20% abdominal circumference | 7 | 2846 |
| 3 20% femur length | 7 | 2791 |
| 4 Cut‐off 25% | 18 | 6471 |
| 5 25% abdominal circumference | 7 | 3614 |
| 6 25% femur length | 4 | 2714 |
1. Test.
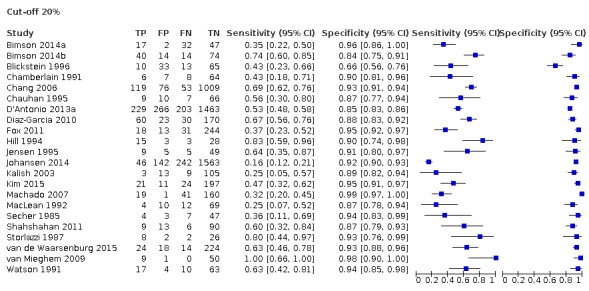
Cut‐off 20%
2. Test.
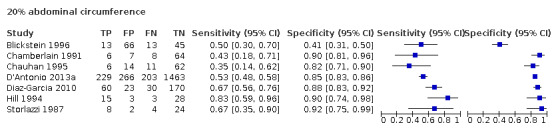
20% abdominal circumference
3. Test.
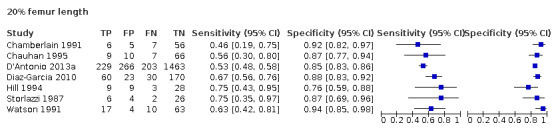
20% femur length
4. Test.
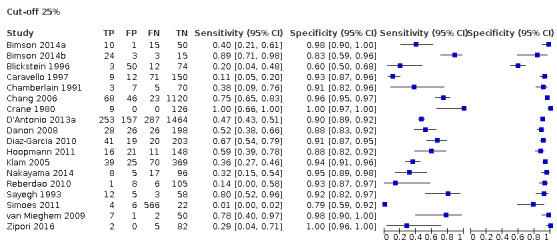
Cut‐off 25%
5. Test.
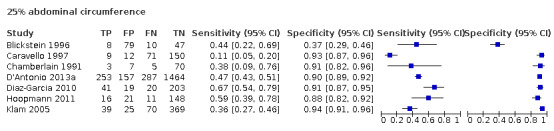
25% abdominal circumference
6. Test.
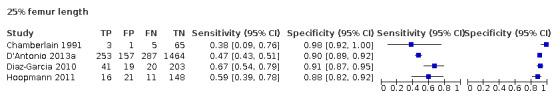
25% femur length
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bimson 2012.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with documented births; GA ≥ 24 weeks at delivery; US within 7 days of delivery | ||
| Patient characteristics and setting | 241 twins, GA: mean 35.8 (SD 2.4) weeks | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | No excluded women indicated. US within 7 days of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bimson 2014a.
| Study characteristics | |||
| Patient Sampling | DADC twin pregnancies with EFW within 2 weeks of delivery in the 3rd trimester | ||
| Patient characteristics and setting | 231 twin sets (January 2006 to March 2013); GA: mean 36 weeks 2 days (SD 2 weeks), DADC twins | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | EFW within 2 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Bimson 2014b.
| Study characteristics | |||
| Patient Sampling | DADC twins | ||
| Patient characteristics and setting | 336 twin sets (January 2006 March 2013); GA: N/A; DADC twins | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | 1st trimester CRL, 2nd and 3rd trimester EFW | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Blickstein 1989.
| Study characteristics | |||
| Patient Sampling | Twin pairs delivered after 32 weeks' gestation and 1 US within 2 weeks of delivery | ||
| Patient characteristics and setting | 178 twin pregnancies (1 September 1983 to 31 August 1986); GA: discordants: mean 36.4 (SD 2.3) weeks, concordants: mean 37.1 (SD 2.3) weeks; hospital | ||
| Index tests | AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women without AC, FL in both twins, and congenital malformations in either twin. US was within 2 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Blickstein 1996.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with complete sets of US measurements within 2 weeks of delivery delivered at Kaplan Hospital, Rehovot, Israel | ||
| Patient characteristics and setting | 90 twin pregnancies with US within 2 weeks of delivery; GA: mean 36.1 (SD 2.9) weeks; hospital | ||
| Index tests | EFW and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women who had measurements performed > 2 weeks before delivery and had incomplete measurements | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Caravello 1997.
| Study characteristics | |||
| Patient Sampling | Live‐born twin pairs born at tertiary centre with GA > 23 weeks, no anomalies and US within 3 weeks of delivery | ||
| Patient characteristics and setting | 242 twin pregnancies; GA: mean 32.8 (SD 4.1) weeks; tertiary centre | ||
| Index tests | EFW or AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | US within 3 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chamberlain 1991.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with last US performed within 7–14 days of delivery | ||
| Patient characteristics and setting | 85 twin pregnancies (enrolled from January 1985 to December 1988); GA at delivery: 36.9 weeks; hospital | ||
| Index tests | EFW using either AC alone or AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women had an interval between US and delivery > 14 days, intrauterine death, BW was not within 6 hours of delivery or EFW was unable to be determined | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chang 2006.
| Study characteristics | |||
| Patient Sampling | Twin births with GA ≥ 24 weeks, and US within 28 days of delivery | ||
| Patient characteristics and setting | 1257 twin pregnancies (January 1991 to August 2002); GA: mean 35.2 (SD 2.6) weeks; hospital | ||
| Index tests | EFWD using a combination of BPD, AC, HC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women had incomplete data. US was within 28 days of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chauhan 1995.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with FL, BPD and AC within 72 hours of delivery at medical centre | ||
| Patient characteristics and setting | 92 twin gestations; GA: mean 32.3 (SD 4.5) weeks; hospital | ||
| Index tests | EFW using FL, BPD or AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | EFW within 72 hours of delivery. Excluded women were those with fetal death or known structural or chromosomal abnormality | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Crane 1980.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies | ||
| Patient characteristics and setting | 18 twin pairs; GA: N/A; university | ||
| Index tests | BPD | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women not indicated | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
D'Antonio 2013a.
| Study characteristics | |||
| Patient Sampling | All twin pregnancies registering for antenatal care by 11 weeks | ||
| Patient characteristics and setting | 2161 twin pregnancies (enrolled 2000–2010); large regional cohort of 9 hospitals over ten years | ||
| Index tests | EFW using HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women not described | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
D'Antonio 2014.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies registering for routine antenatal care by 11 weeks at antenatal care | ||
| Patient characteristics and setting | 2399 twin pregnancies; GA: N/A; antenatal care in hospital | ||
| Index tests | EFW based on HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with termination of pregnancy, structural or chromosomal abnormalities, pregnancies of unknown chorionicity, monochorionic, monoamniotic, and higher‐order multiple gestations | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Danon 2008.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies > 500 g; GA > 24 weeks: absence of fetal malformations or hydrops | ||
| Patient characteristics and setting | 278 twin pregnancies and 834 pregnancies in control; GA: mean 34.2 (SD 2.6); tertiary centre | ||
| Index tests | EFW using Hadlock equation | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | EFW within 3 days of delivery. Excluded women had pregnancies complicated by gestational or pregestational diabetes | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Diaz‐Garcia 2010.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with delivery ≥ 22 weeks and 1 US within 15 weeks of delivery | ||
| Patient characteristics and setting | 283 participants (3‐year period); GA mean 33.29 (SD 4.12) weeks; tertiary referral centre | ||
| Index tests | EFWD using a combination of formulas including BPD, HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women did not have a 1st trimester US, had chromosomal abnormalities or congenital malformations | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Fox 2011.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies > 24 weeks cared for and delivered in maternal‐fetal medicine practice centre | ||
| Patient characteristics and setting | 343 twin deliveries (August 2005 to June 2010). Delivered by Maternal‐Fetal Medicine practice | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with monoamniotic twins, GA < 24 weeks, pregnancies with didelphic or unicornuate uterus, and those with major fetal anomalies | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Hehir 2017.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with 2 weekly US surveillance from 24 weeks' gestation with surveillance of monochorionic twins 2‐weekly from 16 weeks at an academic perinatal centre | ||
| Patient characteristics and setting | 1001 twin pairs (May 2007 to October 2009); GA: discordant: mean 35.3 (SD 3.0) weeks, concordant: mean 36.3 (SD 2.4) weeks; perinatal centres | ||
| Index tests | EFW based on BPD, HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with monochorionic pregnancies with TTTS, a major structural abnormality, fetal aneuploidy or monoamnionicity | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Hill 1994.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US ≥ 15 weeks | ||
| Patient characteristics and setting | 203 twin gestations; GA: 24.4–39.7 weeks, hospital | ||
| Index tests | EFW from HC and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women not reported | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Hoopmann 2011.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US at 18–25 weeks' gestation and 1 US within 14 days prior to delivery | ||
| Patient characteristics and setting | 196 twin pregnancies; GA: median 35.6 (interquartile range 32.4–37.0); university obstetrics/gynaecology department | ||
| Index tests | EFW using BPD, HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Fetuses with structural or chromosomal defects were excluded. EFW within 14 days of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Jensen 1995.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies delivered at university hospital with final US within 7 days of delivery | ||
| Patient characteristics and setting | 73 twin pregnancies (1 January 1990 to 31 March 1993); GA: mean 37 (range 19–40) weeks; university hospital | ||
| Index tests | EFW using BPD and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | US was within 7 days of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Johansen 2014.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with 2 live fetuses and chorionicity determined at the time of NT scan | ||
| Patient characteristics and setting | 2038 diamniotic twin pregnancies (1 January 2004 to 31 December 2006); GA: N/A; Department of Obstetrics/Gynaecology | ||
| Index tests | CRL discordance | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with pregnancies of unknown chorionicity, monochorionic monoamniotic pregnancies and pregnancies with known reduction from a higher number of multiples | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Kalish 2003.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US at 11–14 weeks' gestation | ||
| Patient characteristics and setting | 157 dichorionic twin pregnancies (April 2000 to April 2002); GA: mean 35.5 (SD 2.7) weeks; medical centre | ||
| Index tests | CRL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with monochorionic pregnancies, chromosomal anomalies and the loss of 1 or both fetuses | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Kim 2015.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with delivery at ≥ 35 weeks' gestations in medical centre | ||
| Patient characteristics and setting | 253 twin pregnancies (January 2007 to December 2013); GA: N/A; medical centre | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women had complicated pregnancies such as preterm labour, premature rupture of membranes, placenta previa, pre‐eclampsia, diabetes, TTTS, monoamniotic twin and congenital fetal anomaly | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Klam 2005.
| Study characteristics | |||
| Patient Sampling | Diamniotic twin pregnancies with 2 live births from tertiary care centre | ||
| Patient characteristics and setting | 503 diamniotic twin pregnancies (1 April 1994 to 11 January 2002); GA: growth discordant twins: mean 34.4 (SD 2.9) weeks, growth concordant twins: mean 35.6 (SD 2.9) weeks; tertiary care centre | ||
| Index tests | EFW and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with chromosomal and structural anomalies, pregnancies complicated by TTTS and intrauterine death of 1 or both fetuses | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Machado 2007.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US at university medical school | ||
| Patient characteristics and setting | 221 twin pregnancies; GA: mean 35.6 (SD 2.7) weeks; medical school | ||
| Index tests | EFW with HC, AC, BPD and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with fetal malformation, TTTS, fetal death or unknown outcome | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
MacLean 1992.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies beyond 28 weeks' gestation, with on 2 US and 1 within 3 weeks of delivery recruited from antenatal clinic | ||
| Patient characteristics and setting | 107 twin pregnancies (1984–1987); GA: mean 36.1 (SD 2.4) weeks; antenatal clinic | ||
| Index tests | EFW using AC formula and AC and BPD formula | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | US within 3 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Murray 2014.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies from academic centres with 2‐weekly US from 24 weeks' gestation with surveillance of monochorionic twins 2‐weekly from 16 weeks | ||
| Patient characteristics and setting | 960 twin pairs without TTTS; GA: N/A; academic centres | ||
| Index tests | EFW, BPD, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with twin pairs with TTTS | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Nakayama 2014.
| Study characteristics | |||
| Patient Sampling | Monochorionic twin pregnancies delivered at single referral centre, CRL at 8–10 weeks' gestation | ||
| Patient characteristics and setting | 126 MCDA twin pregnancies; GA: mean 37.4 weeks; single referral centre | ||
| Index tests | CRL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with terminated pregnancies, 1 or both fetal death before 10 weeks, twin‐reversed arterial perfusion sequence, triplets or reduction of high‐order multiples, and anomalies diagnosed before 10 weeks' gestation | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
O'Connor 2013.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with CRL in 1st trimester, with BPD, HC, AC and FL measured. Included women had 2‐weekly growth scans from 24 weeks' gestation | ||
| Patient characteristics and setting | 1001 twin pregnancies (May 2007 to October 2009); GA: N/A; perinatal centres | ||
| Index tests | EFW, BPD, AC, FL and CRL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women had monoamnionicity, a major structural abnormality or fetal aneuploidy | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Reberdao 2010.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US within 2 weeks of delivery, and both twins were alive at antenatal diagnosis centre | ||
| Patient characteristics and setting | 124 twin pairs; GA: N/A; antenatal diagnosis centre | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | US within 2 weeks of delivery. Excluded women not reported | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Sayegh 1993.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US at GA > 23 weeks | ||
| Patient characteristics and setting | 78 twin pairs; GA: N/A; general hospital | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with congenital anomalies | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Secher 1985.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies delivered at university hospital | ||
| Patient characteristics and setting | 80 twin pregnancies; GA: mean 251 (SD 22) days; hospital | ||
| Index tests | EFW using BPD and abdominal diameter | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women not reported | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Shahshahan 2011.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with US at 7–14 week of pregnancy at hospital | ||
| Patient characteristics and setting | 118 twin pregnancies; GA: mean 33.86 (SD 2.36) weeks; hospital | ||
| Index tests | CRL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with monochorionic twins, and with 1st or 2nd trimester pregnancy terminations | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Simoes 2011.
| Study characteristics | |||
| Patient Sampling | Twin gestations with US within 2 weeks before birth and both twins were born alive at university hospital | ||
| Patient characteristics and setting | 661 twin pregnancies (1 January 1994 to 30 June 2008); GA: concordant: mean 35.6 (SD 2.2) weeks, appropriate‐for‐gestational‐age: mean 35.7 (SD 1.0) weeks, small‐for‐gestational‐age: mean 33.5 (SD 2.7) weeks; hospital | ||
| Index tests | EFW and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | US measured within 2 weeks before birth | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Storlazzi 1987.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies at university health centre, with US every 2 weeks, and US within 2 weeks of delivery | ||
| Patient characteristics and setting | 43 twin pregnancies; GA: 33.5 weeks; university health centre | ||
| Index tests | EFW based on BPD and AC or FL and AC | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with congenital anomalies. US within 2 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
van de Waarsenburg 2015.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with antenatal surveillance at university medical centre | ||
| Patient characteristics and setting | 281 twin pregnancies (2008–2011); GA: median 35 + 0 weeks; medical centre | ||
| Index tests | EFW based on HC, AC and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with monoamniotic twin pregnancies, selective feticide, congenital disorders, intrauterine fetal death, GA at birth < 22 weeks, or BW < 500 g | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
van Mieghem 2009.
| Study characteristics | |||
| Patient Sampling | MCDA twin pregnancies with EFW at 16, 20 and 26 weeks' gestation, and within 2 weeks of birth at university hospital | ||
| Patient characteristics and setting | 68 MCDA twin pregnancies; GA 35.7 weeks; hospital | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with intrauterine fetal death or twin‐reverse arterial perfusion sequence. EFW within 2 weeks of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Watson 1991.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with fetal weight within 14 days of delivery | ||
| Patient characteristics and setting | 94 twin pregnancies; GA: mean 33 (SD 2.5) weeks; hospital | ||
| Index tests | BPD, mean abdominal diameters and FL | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with congenital anomalies. EFW within 14 days of delivery | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Zipori 2016.
| Study characteristics | |||
| Patient Sampling | MCDA twin gestations with NT and CRL measurements on US at 11–13 weeks' gestation | ||
| Patient characteristics and setting | 89 twin pregnancies (August 2003 to August 2012); GA: mean 34.1 (SD) 3.3 weeks; hospital | ||
| Index tests | EFW, CRL and NT | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with lethal anomalies at the time of scan, loss of 1 or both twins prior to scan or altered chorionicity findings | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Zuckerwise 2015.
| Study characteristics | |||
| Patient Sampling | Twin pregnancies with MCDA placentation with antenatal care at single academic centre | ||
| Patient characteristics and setting | 73 twin pairs; GA: non‐discordant: mean 34.6 (SD 4.0) weeks, discordant: mean 32.4 (SD 4.6) weeks; academic centre | ||
| Index tests | EFW | ||
| Target condition and reference standard(s) | Birth weight discordance | ||
| Flow and timing | Excluded women with pregnancies complicated by TTTS | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
AC: abdominal circumference; BPD: biparietal diameter; BW: birth weight; CRL: crown rump length; DADC: diamniotic dichorionic; EFW: estimated fetal weight; FL: femur length; GA: gestational age; HC: head circumference; MCDA: monochorionic diamniotic; N/A: not available; NT: nuchal translucency; SD: standard deviation; TTTS: twin‐to‐twin syndrome; US: ultrasound.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allaf 2014 | Research question did not include the reference standard. |
| Banks 2008 | Index test difference in birth weight as reference did not match research question. |
| Ben‐Ami 2015 | Birthweight discordance cut‐off did not match research question. |
| Bora 2009 | Research question did not include the reference standard. |
| Brown 1987 | Birthweight discordance cut‐off did not match research question. |
| Chauhan 1996 | Research question did not include the reference standard. |
| Chauhan 2004 | Unable to construct 2 × 2 table from data. |
| D'Antonio 2013b | Research question did not include the reference standard. |
| Esinler 2017 | Research question did not include the reference standard. |
| Fajardo‐Expósito 2011 | Index test cut‐off did not match research question. |
| Gaziano 1998 | Study did not address the index test. |
| Harper 2012a | Index test cut‐off did not match research question. |
| Harper 2012b | Study did not address the index test. |
| Harper 2012c | Research question did not include the reference standard. |
| Harper 2013 | Study did not address the index test. |
| Jensen 1992 | Study did not address the index test. |
| Kremkau 1978 | Article was not a published study. |
| Leombroni 2017 | Article was a systematic review and meta‐analysis. |
| Nakano 2015 | Research question did not include the reference standard. |
| O'Brien 1986 | Study did not address the index test. |
| Ocer 2011 | Unable to construct 2 × 2 table from data. |
| Papaioannou 2011 | Research question did not include the reference standard. |
| Ropacka‐Lesiak 2012 | Study did not address the index test. |
| Senoo 2000 | Research question did not include the reference standard. |
| Shivkumar 2015 | Research question did not include the reference standard. |
| Snijder 1998 | Research question did not include the reference standard. |
| Stirrup 2016 | Research question did not include the reference standard. |
| Suzuki 2009 | Birthweight discordance cut‐off did not match research question. |
| Weissmann‐Brenner 2012 | Birthweight discordance cut‐off did not match research question. |
| Xu 1995 | Research question did not include the reference standard. |
Differences between protocol and review
We were unable to identify an adequate number of papers looking into 18% or 30% BWD. Therefore, we analyzed the cut‐off points of 20% and 25%.
We reanalyzed data using MIDAS package in STATA.
Contributions of authors
SJ: conceptualized the idea, wrote the review, incorporated reviewers' comments, and edited the text.
JH: made clinical recommendations.
SH: made clinical recommendations.
IA: made methodological and analytical recommendations.
MN: helped with the screening process.
CR: helped with quality assessment and data extraction.
MP: reviewed and revised the final draft, reanalyzed data including subgroup analyses, assisted in making revisions according to the DTA editorial team.
Sources of support
Internal sources
-
DTA group, Other
DTA group provided technical support
External sources
No sources of support supplied
Declarations of interest
SJ: none.
JH: none.
SH: none.
IA: none.
MN: none.
CR: none.
MP: none.
New
References
References to studies included in this review
Bimson 2012 {unpublished data only}
- Bimson B, Schwartz RA, Morrissey M, Lust B, Brustman L. How accurate is ultrasound in predicting birth weight discordance in twin gestations? Reproductive Sciences. 59th Annual Scientific Meeting of the Society for Gynecologic Investigation, San Diego, CA, United States 2012;19(3 Suppl 1):146A. [Google Scholar]
Bimson 2014a {published data only}
- Bimson BE, Brustman L, Murphy E, Foroutan J, Herrera K, Rosenn B. Does ultrasound accurately predict growth discordance in dichorionic diamniotic twins? Reproductive Sciences. 61st Annual Scientific Meeting of the Society for Gynecologic Investigation, Florence, Italy 2014;21(3 Suppl):169A-70A. [Google Scholar]
Bimson 2014b {published data only}
- Bimson BE, Brustman L, Murphy E, Herrera K, Foroutan J, Rosenn B. Using ultrasound, what best predicts pathologic discordance in diamniotic dichorionic twins? Reproductive Sciences. 61st Annual Scientific Meeting of the Society for Gynecologic Investigation, Florence, Italy 2014;21(3):169A. [Google Scholar]
Blickstein 1989 {published data only}
- Blickstein I, Friedman A, Caspi B, Lancet M. Ultrasonic prediction of growth discordancy by intertwin difference in abdominal circumference. International Journal of Gynaecology and Obstetrics 1989;29(2):121-4. [DOI] [PubMed] [Google Scholar]
Blickstein 1996 {published data only}
- Blickstein I, Manor M, Levi R, Goldchmit R. Is intertwin birth weight discordance predictable? Gynecologic and Obstetric Investigation 1996;42(2):105-8. [DOI] [PubMed] [Google Scholar]
Caravello 1997 {published data only}
- Caravello JW, Chauhan SP, Morrison JC, Magann EF, Martin JN Jr, Devoe LD. Sonographic examination does not predict twin growth discordance accurately. Obstetrics and Gynecology 1997;89(4):529-33. [DOI] [PubMed] [Google Scholar]
Chamberlain 1991 {published data only}
- Chamberlain P, Murphy M, Comerford FR. How accurate is antenatal sonographic identification of discordant birthweight in twins? European Journal of Obstetrics, Gynecology, and Reproductive Biology 1991;40(2):91-6. [DOI] [PubMed] [Google Scholar]
Chang 2006 {published data only}
- Chang YL, Chang TC, Chang SD, Cheng PJ, Chao AS, Hsieh PC, et al. Sonographic prediction of significant intertwin birth weight discordance. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2006;127(1):35-40. [DOI] [PubMed] [Google Scholar]
Chauhan 1995 {published data only}
- Chauhan SP, Washburne JF, Roberts WE, Magann EF, Morrison JC, Martin JN. Estimates of birth-weight among twins by the use of femur length alone. Journal of Maternal-fetal Investigation 1995;5(2):113-6. [Google Scholar]
Crane 1980 {published data only}
- Crane JP, Tomich PG, Kopta M. Ultrasonic growth patterns in normal and discordant twins. Obstetrics and Gynecology 1980;55(6):678-83. [PubMed] [Google Scholar]
D'Antonio 2013a {published data only}
- D'Antonio F, Khalil A, Dias T, Thilaganathan B. Weight discordance and perinatal mortality in twins: analysis of the Southwest Thames Obstetric Research Collaborative (STORK) multiple pregnancy cohort. Ultrasound in Obstetrics & Gynecology 2013;41(6):643-8. [DOI] [PubMed] [Google Scholar]
D'Antonio 2014 {published data only}
- D'Antonio F, Khalil A, Thilaganathan B. Second-trimester discordance and adverse perinatal outcome in twins: the STORK multiple pregnancy cohort. BJOG 2014;121(4):422-9. [DOI] [PubMed] [Google Scholar]
Danon 2008 {published data only}
- Danon D, Melamed N, Bardin R, Meizner I. Accuracy of ultrasonographic fetal weight estimation in twin pregnancies. Obstetrics and Gynecology 2008;112(4):759-64. [DOI] [PubMed] [Google Scholar]
Diaz‐Garcia 2010 {published data only}
- Diaz-Garcia C, Bernard JP, Ville Y, Salomon LJ. Validity of sonographic prediction of fetal weight and weight discordance in twin pregnancies. Prenatal Diagnosis 2010;30(4):361-7. [DOI] [PubMed] [Google Scholar]
Fox 2011 {published data only}
- Fox NS, Saltzman DH, Schwartz R, Roman AS, Klauser CK, Rebarber A. Second-trimester estimated fetal weight and discordance in twin pregnancies. Journal of Ultrasound in Medicine 2011;30(8):1095-101. [DOI] [PubMed] [Google Scholar]
Hehir 2017 {published data only}
- Hehir MP, Breathnach FM, Hogan JL, McAuliffe FM, Geary MP, Daly S, et al. Prenatal prediction of significant intertwin birthweight discordance using standard second and third trimester sonographic parameters. Acta Obstetricia et Gynecologica Scandinavica 2017;96(4):472-8. [DOI] [PubMed] [Google Scholar]
Hill 1994 {published data only}
- Hill LM, Guzick D, Chenevey P, Boyles D, Nedzesky P. The sonographic assessment of twin growth discordancy. ACOG Current Journal Review 1994;2(8):20. [PubMed] [Google Scholar]
Hoopmann 2011 {published data only}
- Hoopmann M, Kagan KO, Yazdi B, Grischke EM, Abele H. Prediction of birth weight discordance in twin pregnancies by second-and third-trimester ultrasound. Fetal Diagnosis and Therapy 2011;30(1):29-34. [DOI] [PubMed] [Google Scholar]
Jensen 1995 {published data only}
- Jensen OH, Jenssen H. Prediction of fetal weights in twins. Acta Obstetricia et Gynecologica Scandinavica 1995;74(3):177-80. [DOI] [PubMed] [Google Scholar]
Johansen 2014 {published data only}
- Johansen ML, Oldenburg A, Rosthøj S, Cohn Maxild J, Rode L, Tabor A. Crown-rump length discordance in the first trimester: a predictor of adverse outcome in twin pregnancies? Ultrasound in Obstetrics & Gynecology 2014;43(3):277-83. [DOI] [PubMed] [Google Scholar]
Kalish 2003 {published data only}
- Kalish RB, Chasen ST, Gupta M, Sharma G, Perni SC, Chervenak FA. First trimester prediction of growth discordance in twin gestations. American Journal of Obstetrics & Gynecology 2003;189(3):706-9. [DOI] [PubMed] [Google Scholar]
Kim 2015 {published data only}
- Kim JH, Sung JH, Kim JY, Chang KH, Choi SJ, Oh SY, et al. Pregnancy outcome of isolated cases of inter-twin fetal weight discordance estimated by antenatal ultrasound. International Journal of Gynecology & Obstetrics 2015;131(S5):E461. [Google Scholar]
Klam 2005 {published data only}
- Klam SL, Rinfret D, Leduc L. Prediction of growth discordance in twins with the use of abdominal circumference ratios. American Journal of Obstetrics & Gynecology 2005;192(1):247-51. [DOI] [PubMed] [Google Scholar]
Machado 2007 {published data only}
- Machado RC, Brizot ML, Liao AW, Cabar FR, Zugaib M. Prenatal sonographic prediction of twin growth discordance. Twin Research and Human Genetics 2007;10(1):198-201. [DOI] [PubMed] [Google Scholar]
MacLean 1992 {published data only}
- MacLean M, Mathers A, Walker JJ, Cameron AD, Howat R. The ultrasonic assessment of discordant growth in twin pregnancies. Ultrasound in Obstetrics & Gynecology 1992;2(1):30-4. [DOI] [PubMed] [Google Scholar]
Murray 2014 {published data only}
- Murray A, Breathnach F, McAuliffe F, Geary M, Daly S, Higgins J, et al. 187: Prediction of significant birth weight discordance in twin pregnancies with second and third trimester ultrasound. American Journal of Obstetrics & Gynecology 2014;210(1):S104. [Google Scholar]
Nakayama 2014 {published data only}
- Nakayama S, Ishii K, Kawaguchi H, Yamamoto R, Murata M, Hayashi S, et al. Perinatal complications of monochorionic diamniotic twin gestations with discordant crown-rump length determined at mid-first trimester. Journal of Obstetrics and Gynaecology Research 2014;40(2):418-23. [DOI] [PubMed] [Google Scholar]
O'Connor 2013 {published data only}
- O'Connor C, McAuliffe FM, Breathnach FM, Geary M, Daly S, Higgins JR, et al. Prediction of outcome in twin pregnancy with first and early second trimester ultrasound. Journal of Maternal-fetal & Neonatal Medicine 2013;26(10):1030-5. [DOI] [PubMed] [Google Scholar]
Reberdao 2010 {published data only}
- Reberdao MA, Martins L, Torgal M, Viana R, Seminova T, Casal E, et al. The source of error in the estimation of intertwin birth weight discordance. Journal of Perinatal Medicine 2010;38(6):671-4. [DOI] [PubMed] [Google Scholar]
Sayegh 1993 {published data only}
- Sayegh SK, Warsof SL. Ultrasonic prediction of discordant growth in twin pregnancies. Fetal Diagnosis and Therapy 1993;8(4):241-6. [DOI] [PubMed] [Google Scholar]
Secher 1985 {published data only}
- Secher NJ, Kaern J, Hansen PK. Intrauterine growth in twin pregnancies: prediction of fetal growth retardation. Obstetrics and Gynecology 1985;66(1):63-8. [PubMed] [Google Scholar]
Shahshahan 2011 {published data only}
- Shahshahan Z, Hashemi M. Crown-rump length discordance in twins in the first trimester and its correlation with perinatal complications. Journal of Research in Medical Sciences 2011;16(9):1224. [PMC free article] [PubMed] [Google Scholar]
Simoes 2011 {published data only}
- Simoes T, Julio C, Cordeiro A, Cohen A, Silva A, Blickstein I. Abdominal circumference ratio for the diagnosis of intertwin birth weight discordance. Journal of Perinatal Medicine 2011;39(1):43-6. [DOI] [PubMed] [Google Scholar]
Storlazzi 1987 {published data only}
- Storlazzi E, Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Ultrasonic diagnosis of discordant fetal growth in twin gestations. Obstetrics and Gynecology 1987;69(3 Pt 1):363-7. [PubMed] [Google Scholar]
van de Waarsenburg 2015 {published data only}
- de Waarsenburg MK, Hack KE, Rijpma RJ, Mulder EJ, Pistorius L, Derks JB. Ultrasonographic prediction of birth weight discordance in twin pregnancies. Prenatal Diagnosis 2015;35(9):906-12. [DOI] [PubMed] [Google Scholar]
van Mieghem 2009 {published data only}
- Mieghem T, Deprest J, Klaritsch P, Gucciardo L, Done E, Verhaeghe J, et al. Ultrasound prediction of intertwin birth weight discordance in monochorionic diamniotic twin pregnancies. Prenatal Diagnosis 2009;29(3):240-4. [DOI] [PubMed] [Google Scholar]
Watson 1991 {published data only}
- Watson WJ, Valea FA, Seeds JW. Sonographic evaluation of growth discordance and chorionicity in twin gestation. American Journal of Perinatology 1991;8(05):342-4. [DOI] [PubMed] [Google Scholar]
Zipori 2016 {published data only}
- Zipori Y, Reidy K, Gilchrist T, Doyle LW, Umstad MP. The outcome of monochorionic diamniotic twins discordant at 11 to 13+ 6 weeks' gestation. Twin Research and Human Genetics 2016;19(6):692-6. [DOI] [PubMed] [Google Scholar]
Zuckerwise 2015 {published data only}
- Zuckerwise L, Nayeri U, Abdel-Razeq S, Copel J, Bahtiyar MO. Doppler abnormalities in monochorionic diamniotic twin pregnancies with discordant growth. Journal of Perinatology 2015;35(6):387. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allaf 2014 {published data only}
- Allaf MB, Campbell WA, Vintzileos AM, Haeri S, Javadian P, Shamshirsaz AA, et al. Does early second-trimester sonography predict adverse perinatal outcomes in monochorionic diamniotic twin pregnancies? Journal of Ultrasound in Medicine 2014;33(9):1573-8. [DOI] [PubMed] [Google Scholar]
Banks 2008 {published data only}
- Banks CL, Nelson SM, Owen P. First and third trimester ultrasound in the prediction of birthweight discordance in dichorionic twins. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008;138(1):34-8. [DOI] [PubMed] [Google Scholar]
Ben‐Ami 2015 {published data only}
- Ben-Ami I, Daniel-Spiegel E, Battino S, Melcer Y, Floeck A, Geipel A, et al. The association of crown rump length discrepancy with birthweight discordance in spontaneous versus IVF monochorionic twins: a multicenter study. Prenatal Diagnosis 2015;35(9):864-9. [DOI] [PubMed] [Google Scholar]
Bora 2009 {published data only}
- Bora SA, Bourne T, Bottomley C, Kirk E, Papageorghiou AT. Twin growth discrepancy in early pregnancy. Ultrasound in Obstetrics & Gynecology 2009;34(1):38-42. [DOI] [PubMed] [Google Scholar]
Brown 1987 {published data only}
- Brown CE, Guzick DS, Leveno KJ, Santos-Ramos R, Whalley PJ. Prediction of discordant twins using ultrasound measurement of biparietal diameter and abdominal perimeter. Obstetrics and Gynecology 1987;70(5):677-81. [PubMed] [Google Scholar]
Chauhan 1996 {published data only}
- Chauhan SP, Cowan BD, Brost BD, Washburne JF, Magann EF, Morrison JC. Estimating birth weight in twins. Comparison of eight sonographic models. Journal of Reproductive Medicine 1996;41(6):403-8. [PubMed] [Google Scholar]
Chauhan 2004 {published data only}
- Chauhan SP, Shields D, Parker D, Sanderson M, Scardo JA, Magann EF. Detecting fetal growth restriction or discordant growth in twin gestations stratified by placental chorionicity. Journal of Reproductive Medicine 2004;49(4):279-84. [PubMed] [Google Scholar]
D'Antonio 2013b {published data only}
- D'Antonio F, Khalil A, Mantovani E, Thilaganathan B. Embryonic growth discordance and early fetal loss: the STORK multiple pregnancy cohort and systematic review. Human Reproduction 2013;28(10):2621-7. [DOI] [PubMed] [Google Scholar]
Esinler 2017 {published data only}
- Esinler D, Aldemir OB, Alici Davutoglu E, Karahanoglu E, Salihoglu KN, Kuzu E, et al. A new mathematical formula to predict the foetal weight in twin pregnancies: a comparison of it with 19 different formulas. Journal of Obstetrics and Gynaecology 2017;37(1):53-7. [DOI] [PubMed] [Google Scholar]
Fajardo‐Expósito 2011 {published data only}
- Fajardo-Expósito MA, Hervías B, González FB, Melero-Jiménez V, Quintero-Prado R, Facio-Fernández MC, et al. First trimester fetal head and trunk volume predict growth disturbance in twin pregnancy. Prenatal Diagnosis 2011;31(6):543-7. [DOI] [PubMed] [Google Scholar]
Gaziano 1998 {published data only}
- Gaziano E, Gaziano C, Brandt D. Doppler velocimetry determined redistribution of fetal blood flow: correlation with growth restriction in diamniotic monochorionic and dizygotic twins. American Journal of Obstetrics & Gynecology 1998;178(6):1359-67. [DOI] [PubMed] [Google Scholar]
Harper 2012a {published data only}
- Harper L, Roehl K, Odibo A, Cahill A. 359: Crown-rump length discordance and adverse pregnancy outcomes in dichorionic twins. American Journal of Obstetrics and Gynecology 2012;206(1):S168-9. [Google Scholar]
Harper 2012b {published data only}
- Harper L, Weis M, Odibo A, Roehl K, Macones G, Cahill A. 137: Are normally grown twin pregnancies with birth weight discordance at risk for adverse outcomes? American Journal of Obstetrics and Gynecology 2012;206(1):S73. [Google Scholar]
Harper 2012c {published data only}
- Harper LM, Roehl KA, Odibo AO, Cahill AG. First-trimester growth discordance and adverse pregnancy outcome in dichorionic twins. Ultrasound in Obstetrics & Gynecology 2012;41(6):627-31. [DOI] [PubMed] [Google Scholar]
Harper 2013 {published data only}
- Harper LM, Weis MA, Odibo AO, Roehl KA, Macones GA, Cahill AG. Significance of growth discordance in appropriately grown twins. American Journal of Obstetrics & Gynecology 2013;208(5):393. e1-5. [DOI] [PubMed] [Google Scholar]
Jensen 1992 {published data only}
- Jensen OH. Doppler velocimetry in twin pregnancy. European Journal of Obstetrics & Gynecology 1992;45(1):9-12. [DOI] [PubMed] [Google Scholar]
Kremkau 1978 {published data only}
- Kremkau FW, Nelson LH. Diagnostic ultrasound and its obstetric applications. American Family Physician 1978;17(5):148-57. [PubMed] [Google Scholar]
Leombroni 2017 {published data only}
- Leombroni M, Liberati M, Fanfani F, Pagani G, Familiari A, Buca D, et al. Diagnostic accuracy of ultrasound in predicting birth weight discordance in twin pregnancy: systematic review and meta-analysis. Ultrasound in Obstetrics & Gynecology 2017;50(4):442-50. [DOI] [PubMed] [Google Scholar]
Nakano 2015 {published data only}
- Nakano JC, Liao AW, Brizot MdeL, Miyadahira M, Francisco RP, Zugaib M. Fetal growth according to different reference ranges in twin pregnancies with placental insufficiency. Clinics 2015;70(12):816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 1986 {published data only}
- O'Brien WF, Knuppel RA, Scerbo JC, Rattan PK. Birth weight in twins: an analysis of discordancy and growth retardation. Obstetrics and Gynecology 1986;67(4):483-6. [PubMed] [Google Scholar]
Ocer 2011 {published data only}
- Ocer F, Aydin Y, Atis A, Kaleli S. Factors affecting the accuracy of ultrasonographical fetal weight estimation in twin pregnancies. Journal of Maternal-fetal & Neonatal Medicine 2011;24(9):1168-72. [DOI] [PubMed] [Google Scholar]
Papaioannou 2011 {published data only}
- Papaioannou GI, Syngelaki A, Maiz N, Ross JA, Nicolaides KH. Prediction of outcome in dichorionic twin pregnancies at 6-10 weeks' gestation. American Journal of Obstetrics & Gynecology 2011;205(4):348. e1-5. [DOI] [PubMed] [Google Scholar]
Ropacka‐Lesiak 2012 {published data only}
- Ropacka-Lesiak M, Breborowicz G, Dera A. Blood flow changes in dichorionic twins with growth discordance. Twin Research and Human Genetics 2012;15(6):781-7. [DOI] [PubMed] [Google Scholar]
Senoo 2000 {published data only}
- Senoo M, Okamura K, Murotsuki J, Yaegashi N, Uehara S, Yajima A. Growth pattern of twins of different chorionicity evaluated by sonographic biometry. Obstetrics and Gynecology 2000;95(5):656-61. [DOI] [PubMed] [Google Scholar]
Shivkumar 2015 {published data only}
- Shivkumar S, Himes KP, Hutcheon JA, Platt RW. An ultrasound-based fetal weight reference for twins. American Journal of Obstetrics & Gynecology 2015;213(2):224. e1-9. [DOI] [PubMed] [Google Scholar]
Snijder 1998 {published data only}
- Snijder MJ, Wladimiroff JW. Fetal biometry and outcome in monochorionic vs. dichorionic twin pregnancies; a retrospective cross-sectional matched-control study. Ultrasound in Medicine and Biology 1998;24(2):197-201. [DOI] [PubMed] [Google Scholar]
Stirrup 2016 {published data only}
- Stirrup OT, Khalil A, D'Antonio F, Thilaganathan B. Patterns of second-and third-trimester growth and discordance in twin pregnancy: analysis of the Southwest Thames Obstetric Research Collaborative (STORK) Multiple Pregnancy Cohort. Fetal Diagnosis and Therapy 2016;41(2):100-7. [DOI] [PubMed] [Google Scholar]
Suzuki 2009 {published data only}
- Suzuki S, Shimizu E, Kinoshita M, Araki S. Accuracy of ultrasonographic fetal weight estimation in Japanese twin pregnancies. Journal of Medical Ultrasonics 2009;36(3):157-8. [DOI] [PubMed] [Google Scholar]
Weissmann‐Brenner 2012 {published data only}
- Weissmann-Brenner A, Weisz B, Achiron R, Shrim A. Can discordance in CRL at the first trimester predict birth weight discordance in twin pregnancies? Journal of Perinatal Medicine 2012;40(5):489-93. [DOI] [PubMed] [Google Scholar]
Xu 1995 {published data only}
- Xu B, Deter RL, Milner LL, Hill RM. Evaluation of twin growth status at birth using individualized growth assessment: comparison with conventional methods. Journal of Clinical Ultrasound 1995;23(5):277-86. [DOI] [PubMed] [Google Scholar]
Additional references
ACOG Committee 2004
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics, Society for Maternal-Fetal Medicine, ACOG Joint Editorial Committee. ACOG Practice Bulletin #56: Multiple gestation: complicated twin, triplet, and high-order multifetal pregnancy. Obstetrics and Gynecology 2004;104(4):869-83. [DOI] [PubMed] [Google Scholar]
Adegbite 2004
- Adegbite AL, Castille S, Ward S, Bajoria R. Neuromorbidity in preterm twins in relation to chorionicity and discordant birth weight. American Journal of Obstetrics and Gynecology 2004;190(1):156-8. [DOI] [PubMed] [Google Scholar]
Alfirevic 2003
- Alfirevic Z, Sundberg K, Brigham S. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD003252. [DOI: 10.1002/14651858.CD003252] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Appleton 2007
- Appleton C, Pinto L, Centeno M, Clode N, Cardoso C, Graca LM, et al. Near term twin pregnancy: clinical relevance of weight discordance at birth. Journal of Perinatal Medicine 2007;35(1):62-6. [DOI] [PubMed] [Google Scholar]
Bagchi 2006
- Bagchi S, Salihu HM. Birth weight discordance in multiple gestations: occurrence and outcomes. Journal of Obstetrics and Gynaecology 2006;26(4):291-6. [DOI] [PubMed] [Google Scholar]
Behrendt 2016
- Behrendt N, Galan HL. Twin-twin transfusion and laser therapy. Current Opinion in Obstetrics & Gynecology 2016;28(2):79-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhide 2009
- Bhide A, Sankaran S, Sairam S, Papageorghiou AT, Thilaganathan B. Relationship of intertwin crown-rump length discrepancy to chorionicity, fetal demise and birth-weight discordance. Ultrasound in Obstetrics and Gynecology 2009;34(2):131-5. [DOI] [PubMed] [Google Scholar]
Chitkara 1985
- Chitkara U, Berkowitz GS, Levine R, Riden DJ, Fagerstrom RM Jr, Chervenak FA, et al. Twin pregnancy: routine use of ultrasound examinations in the prenatal diagnosis of intrauterine growth retardation and discordant growth. American Journal of Perinatology 1985;2(1):49-54. [DOI] [PubMed] [Google Scholar]
Chu 2006
de Vet 2013
- Vet HC, Eisinga A, Riphagen II, Aertgeerts B, Pewsner D. Chapter 7: Searching for studies. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2013. Available from methods.cochrane.org/sdt/handbook-dta-reviews.
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882-93. [DOI] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated August 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Demissie 2002
- Demissie K, Ananth CV, Martin J, Hanley ML, MacDorman MF, Rhoads GG, et al. Fetal and neonatal mortality among twin gestations in the United States: the role of intrapair birth weight discordance. Obstetrics and Gynecology 2002;100(3):474-80. [DOI] [PubMed] [Google Scholar]
Devoe 1995
- Devoe LD, Wre DJ. Antenatal assessment of twin gestation. Seminars in Perinatology 1995;19(5):413. [DOI] [PubMed] [Google Scholar]
Di 2007
- Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gender Medicine 2007;4(1):19-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dwamena 2009
- Dwamena B. MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies, Statistical Software Components S456880, Boston College Department of Economics. ideas.repec.org/c/boc/bocode/s456880.html (accessed prior to 26 January 2021).
Gernt 2001
- Gernt PR, Mauldin JG, Newman RB, Durkalski VL. Sonographic prediction of twin birth weight discordance. Obstetrics and Gynecology 2001;97(1):53-6. [DOI] [PubMed] [Google Scholar]
Giles 1998
- Giles WB. Doppler ultrasound in multiple pregnancies. Bailliere's Clinical Obstetrics and Gynaecology 1998;12(1):77-89. [DOI] [PubMed] [Google Scholar]
Gonzalez‐Quintero 2003
- Gonzalez-Quintero VH, Luke B, O'Sullivan MJ, Misiunas R, Anderson E. Antenatal factors associated with significant birthweight discordancy in twin gestations. American Journal of Obstetrics and Gynecology 2003;189(3):83. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 1 July 2019. Available at gradepro.org.
Harbord 2007
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. [DOI] [PubMed] [Google Scholar]
Harbord 2009
- Harbord RM, Whiting P. Metandi: meta-analysis of diagnostic accuracy using hierarchical logistic regression. Stata Journal 2009;9:211-29. [Google Scholar]
Hata 2011
- Hata T, Tanaka H, Noguchi J, Hata K. Three-dimensional ultrasound evaluation of the placenta. Placenta 2011;32(2):105-15. [DOI] [PubMed] [Google Scholar]
Jahanfar 2016a
- Jahanfar S, Lim K. A systematic review of birth weight discordance and perinatal outcomes. Fetal and Maternal Medicine Review 2016;1(1):1-31. [Google Scholar]
Jahanfar 2016b
- Jahanfar S, Lim K. Optimal threshold for birth weight discordance. Journal of Perinatology 2016;2:1-9. [DOI] [PubMed] [Google Scholar]
Jahanfar 2017a
- Jahanfar S, Lim K, Ovideo-Joekes E. Birth weight discordance and adverse perinatal outcomes. Journal of Perinatal Medicine 2017;45(5):603-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kilic 2006
- Kilic M, Aygun C, Kaynar-Tuncel E, Küçüködük S. Does birth weight discordance in preterm twins affect neonatal outcome? Journal of Perinatology 2006;26(5):268-72. [DOI] [PubMed] [Google Scholar]
Klam 2003
- Klam S, Rinfret DE, Leduc L. Abdominal circumference ratio: a useful predictor of twin birth weight discordance. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl):231. [Google Scholar]
Leeflang 2008
- Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM, Cochrane Diagnostic Test Accuracy Working Group. Systematic reviews of diagnostic test accuracy. Annals of Internal Medicine 2008;149(12):889-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewi 2008
- Lewi L, Gucciardo L, Huber A, Jani J, Van Mieghem T, Done E, et al. Clinical outcome and placental characteristics of monochorionic diamniotic twin pairs with early- and late-onset discordant growth. American Journal of Obstetrics and Gynecology 2008;199(5):511.e1-7. [DOI] [PubMed] [Google Scholar]
Lopriore 2012
- Lopriore E, Pasman SA, Klumper FJ, Middeldorp JM, Walther FJ, Oepkes D, et al. Placental characteristics in growth-discordant monochorionic twins: a matched case-control study. Placenta 2012;33(3):171-4. [DOI] [PubMed] [Google Scholar]
Macaskill 2013
- Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
Mahony 2006
- Mahony R, Foley M, Mcauliffe F, Mcparland P, Carroll S. Birth weight discordance, obstetric and perinatal outcome in twin pregnancies at term (37 weeks gestation). American Journal of Obstetrics and Gynecology 2006;195(6):S114. [Google Scholar]
Mazhar 2010
- Mazhar SB, Kanwal S. Twin birth weight discordance: associated factors and outcome. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP 2010;20(6):391-4. [PubMed] [Google Scholar]
Melamed 2009
- Melamed N, Yogev Y, Glezerman M. Effect of fetal sex on pregnancy outcome in twin pregnancies. Obstetrics and Gynecology 2009;114(5):1085-92. [DOI] [PubMed] [Google Scholar]
Miller 2012
- Miller J, Chauhan SP, Abuhamad AZ. Discordant twins: diagnosis, evaluation and management. American Journal of Obstetrics and Gynecology 2012;206(1):10-20. [DOI] [PubMed] [Google Scholar]
Morin 2011
- Morin L, Lim K. Ultrasound in twin pregnancies. Journal of Obstetrics and Gynaecology Canada : JOGC 2011;33(6):643-56. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Care Excellence. Multiple pregnancy: the management of twin and triplet pregnancies in the antenatal period multiple pregnancy: the management of twin and triplet pregnancies in the antenatal period (CG129). www.nice.org.uk/guidance/cg129 (accessed October 2014).
Oerbekke 2020
- Oerbekke MS, Jenniskens K, Scholten RJ, Hooft L. Data sources and methods used to determine pretest probabilities in a cohort of Cochrane diagnostic test accuracy reviews. BMC Medical Research Methodology 2020;20(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okun 2000
- Okun N, Pastuzak A, Seaward G, Wilkinson J, Wilson D, Amankwah K, et al. The SOCG consensus statement: management of twin pregnancies (part 1). sogc.org/wp-content/uploads/2013/01/91E-CONS1-July2000.pdf (accessed October 2014).
Puccio 2014
- Puccio G, Giuffre M, Piccione M, Piro E, Malerba V, Corsello G. Intrauterine growth pattern and birthweight discordance in twin pregnancies: a retrospective study. Italian Journal of Pediatrics 2014;40:43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rizzo 1993
- Rizzo G, Arduini D, Romanini C. Uterine artery Doppler velocity waveforms in twin pregnancies. Obstetrics and Gynecology 1993;82(6):978-83. [PubMed] [Google Scholar]
Schünemann 2019
Schünemann 2020a
- Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2020;122:142-52. [DOI] [PubMed]
Schünemann 2020b
- Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. Journal of Clinical Epidemiology 2020;122:129-41. [DOI] [PubMed] [Google Scholar]
Tai 2007
- Tai J, Grobman WA. The association of crown-rump length discordance in twin gestations with adverse perinatal outcomes. American Journal of Obstetrics and Gynecology 2007;197(4):369.e1-4. [DOI] [PubMed] [Google Scholar]
Victoria 2001
- Victoria A, Mora G, Arias F. Perinatal outcome, placental pathology, and severity of discordance in monochorionic and dichorionic twins. Obstetrics and Gynecology 2001;97(2):310-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wen 2006
- Wen SW, Tan H, Walker M. The association between intratwin birthweight discordance and preterm birth in twin pregnancy. Australian & New Zealand Journal of Obstetrics & Gynaecology 2006;46(5):402-6. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI] [PubMed] [Google Scholar]
Woo 1939
- Woo J. A short history of the development of ultrasound in obstetrics and gynecology. www.ob-ultrasound.net/history3.html (accessed 28 January 2017).
References to other published versions of this review
Jahanfar 2017b
- Jahanfar S, Ho JJ, Jaafar SH, Abraha I, Nisenblat V, Ellis UM, et al+. Ultrasound for diagnosis of birth weight discordance in twin pregnancies. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD012553. [DOI: 10.1002/14651858.CD012553] [DOI] [PMC free article] [PubMed] [Google Scholar]


