Abstract
Background:
While antibiotics are life-saving drugs, their use is not without risk, including adverse events and antibiotic resistance. The majority of US antibiotic prescriptions are prescribed in outpatient settings, making outpatient antibiotic prescribing an important antibiotic stewardship target. The primary objective of this study was to describe trends in US outpatient oral antibiotic prescriptions from 2011-2016.
Methods:
We estimated annual oral antibiotic prescription rates using national prescription dispensing count data from IQVIA Xponent divided by census estimates for 2011-2016. We calculated the ratio of broad- to narrow-spectrum prescriptions by dividing broad-spectrum prescription rates by narrow-spectrum prescription rates. We used Poisson models to estimate prevalence rate ratios comparing 2011 and 2016 antibiotic prescription rates and linear models to evaluate temporal trends throughout the study period.
Results:
Oral antibiotic prescription rates decreased 5% from 877 prescriptions per 1,000 persons in 2011 to 836 per 1,000 persons in 2016. During this period, rates of prescriptions dispensed to children decreased 13% while adult rates increased 2%. The ratio of broad- to narrow-spectrum antibiotics decreased from 1.62 in 2011 to 1.49 in 2016, driven by decreases in macrolides and fluoroquinolones. The proportion of prescriptions written by nurse practitioners and physician assistants increased during the study period; in 2016, these providers prescribed over one-quarter of all antibiotic prescriptions.
Conclusions:
Outpatient antibiotic prescription rates, especially of broad-spectrum agents, have decreased in recent years. Clinicians who prescribe to adults, including nurse practitioners and physician assistants, are important targets for antibiotic stewardship.
Keywords: Antibiotic, antibiotic stewardship, outpatient
Background
Antibiotics are life-saving drugs designed to treat bacterial infections and provide the safety-net needed for modern medical treatments, such as chemotherapy and organ transplantation. However, antibiotic use is not without risks. Annually, an estimated 200,000 emergency department visits are attributed to antibiotic-associated adverse events [1]. Additionally, antibiotic use is one of the primary modifiable drivers of antibiotic resistance. Antibiotic-resistant organisms are responsible for at least 2 million infections, 23,000 deaths, and $20 billion in excess direct healthcare costs in the United States each year [2]. An estimated 85-95% of antibiotic use in human healthcare occurs in the outpatient setting [3], making it an important antibiotic stewardship target.
An estimated 30% of outpatient antibiotics prescribed in the United States in 2010-2011 were unnecessary [4]. The National Action Plan for Combating Antibiotic-Resistant Bacteria set a goal of reducing inappropriate outpatient antibiotic use by 50% by 2020 [5]. This goal translates to reducing overall outpatient antibiotic use by 15% compared with 2010-2011 levels.
Efforts to improve antibiotic prescribing have targeted the outpatient setting, such as the Centers for Disease Control and Prevention’s (CDC) Core Elements of Outpatient Antibiotic Stewardship [6], released in 2016. Additionally, CDC has led ongoing education efforts since the mid-1990s to educate patients, parents, and clinicians on appropriate antibiotic use [7]. National estimates of 2011 outpatient antibiotic prescriptions were published to identify stewardship opportunities and set a baseline from which to track progress [8]. However, more recent estimates of large-scale, US antibiotic prescription trends are limited to convenience samples [9-11], making it difficult to evaluate progress in improving antibiotic prescribing nationally and to identify stewardship targets.
Additionally, antibiotic stewardship efforts have targeted the inappropriate use of broad-spectrum agents, i.e. those with activity against wider ranges of bacteria than necessary to treat an infection. Inappropriate broad-spectrum antibiotic use is concerning as these agents are needed for infections resistant to narrow-spectrum antibiotics and may be associated with higher risks of adverse events [12]. The US Food and Drug Administration (FDA) issued warnings about adverse events associated with macrolides and fluoroquinolones, broad-spectrum antibiotic classes [13, 14]. In addition, fluoroquinolones and cephalosporins are associated with increased risk of Clostridioides (formerly Clostridium) difficile infection compared with other antibiotic classes [15, 16]. In Europe, public health agencies have used ratios of broad- to narrow-spectrum antibiotics to evaluate progress in improving antibiotic agent selection [17]. National trends in US broad- versus narrow-spectrum antibiotic prescriptions have not been described. This approach may present an opportunity to examine potential improvements in antibiotic agent selection.
The first objective of this study was to describe trends in outpatient oral antibiotic prescriptions from 2011-2016 by year, region, patient age group and sex, antibiotic category, provider specialty, and state. The second objective of this study was to describe trends in the ratio of broad- and narrow-spectrum outpatient oral antibiotic prescriptions overall and in adults and children.
Methods
Study population
We summarized annual dispensed oral antibiotic prescription counts from IQVIA Xponent 2011-2016 databases. Using a proprietary projection method, IQVIA estimates 100% of all US community pharmacy prescription dispensing based on a sample of 74-90% (varies by year) of outpatient prescriptions and pharmacy wholesale delivery information. These data estimate prescriptions dispensed by outpatient pharmacies regardless of the setting in which the prescription was written. This projection methodology has previously been described and used to estimate outpatient prescriptions. Antibiotics were categorized as tetracyclines, cephalosporins, lincosamides, macrolides, penicillins, fluoroquinolones, trimethoprim-sulfamethoxazole, beta-lactams with increased activity, urinary anti-infectives, and other (Supplementary Table 1). Provider specialties were categorized into 17 specialty groups based on American Medical Association self-designated practice specialties [8]. Nurse practitioners (NPs) and physician assistants (PAs) were categorized as NPs or PAs regardless of practice specialty. We summarized dispensed oral antibiotic counts by year, region, patient age group and sex, antibiotic category, provider specialty, and state.
Population estimates were derived for each year from the Vintage 2016 1990-2016 series US Census bridged-race resident population estimate files [18]. We summarized population estimates overall and by region, patient age group and sex, and state.
The National Center for Emerging and Zoonotic Infectious Diseases Human Subjects Advisor determined that analyses with these de-identified data are non-research public health surveillance and do not require Institutional Review Board review.
Data analysis
We estimated rates of oral antibiotic prescriptions per 1,000 population from dispensed prescription count estimates for each year overall and by sex, age group, region, and state divided by corresponding US Census estimates. To calculate provider specialty prescription rate, we divided dispensed prescription count estimates for each specialty by overall population estimates for each year.
There are no widely-established definitions for broad- and narrow-spectrum antibiotics. To calculate ratios of broad- to narrow-spectrum antibiotics, we adapted methods used by European public health agencies to include antibiotics commonly used in US outpatient settings. We categorized penicillin, ampicillin, amoxicillin, first-generation cephalosporins, and erythromycin as narrow spectrum and beta-lactams with increased activity (e.g., amoxicillin-clavulanate), macrolides, fluoroquinolones, telithromycin, and all other cephalosporins as broad spectrum, as detailed in Supplementary Table 1. We excluded all other antibiotics from this broad- to narrow-spectrum analysis. Broad- to narrow-spectrum antibiotic ratios were calculated by dividing broad-spectrum antibiotic prescription rates by narrow-spectrum antibiotic prescription rates.
To compare dispensed oral, outpatient antibiotic prescription rates in 2011 and 2016, we estimated prevalence rate ratios (pRRs) and 95% confidence intervals (CIs) using Poisson models with a log link. We used the year 2011 as the reference, therefore pRR values less than one indicate decreases, pRR values of one indicate no change, and pRR values over one represent increases in rates from 2011 to 2016. We used this method to estimate changes overall and by region, patient age group, patient sex, and antibiotic category.
To examine trends in the ratios of broad- and narrow-spectrum antibiotics, the proportion of antibiotics by antibiotic category, and the proportion of antibiotics by provider specialty, we fit linear regression models for these continuous outcome variables overall and stratified by adults (defined by IQVIA as ≥20 years) and children (<20 years).
We estimated state-specific antibiotic prescription rates overall and stratified by adults and children. We ranked state-specific rates by sextile to generate maps of antibiotic prescription rates by state.
We conducted all statistical analyses at α = 0.05. All data analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
In 2011, 273.3 million oral antibiotic prescriptions were dispensed from community pharmacies in the United States, translating to a rate of 877 prescriptions per 1,000 persons (Table 1). In 2016, there were 270.2 million oral antibiotic prescriptions dispensed, a rate of 836 per 1,000 persons. The dispensed antibiotic prescription rate was 5% lower in 2016 than 2011 (pRR: 0.95, 95% CI: 0.95-0.95). During the study period, the highest antibiotic prescription rate occurred in 2011 and the lowest occurred in 2014 (835 prescriptions per 1,000 persons), with relatively stable rates from 2014 to 2016.
Table 1.
US outpatient oral antibiotic prescriptions by patient characteristics, 2011-2016
| Characteristic | Outpatient Antibiotic Prescriptions | pRR 2016 v 2011A (95% CI) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | ||||||||
| No., in millions (%) |
Rate per 1,000 persons |
No., in millions (%) |
Rate per 1,000 persons |
No., in millions (%) |
Rate per 1,000 persons |
No., in millions (%) |
Rate per 1,000 persons |
No., in millions (%) |
Rate per 1,000 persons |
No., in millions (%) |
Rate per 1,000 persons |
||
| Total | 273.3 (100.0) |
877 | 272.3 (100.0) |
867 | 268.6 (100.0) |
849 | 266.1 (100.0) |
835 | 269.4 (100.0) |
839 | 270.2 (100.0) |
836 | 0.95 (0.95-0.95) |
| Sex B | |||||||||||||
| Male | 106.5 (39.3) |
695 | 106.5 (39.3) |
689 | 104.8 (39.2) |
673 | 103.4 (39.0) |
660 | 104.9 (39.0) |
664 | 105.5 (39.1) |
663 | 0.95 (0.95-0.95) |
| Female | 164.2 (60.7) |
1,036 | 164.7 (60.7) |
1,032 | 162.8 (60.8) |
1,013 | 161.7 (61.0) |
1,000 | 164.0 (61.0) |
1,006 | 164.6 (60.9) |
1,003 | 0.97 (0.97-0.97) |
| Age group, years B | |||||||||||||
| Children,<20 | 75.2 (28.2) |
908 | 70.8 (26.4) |
858 | 66.8 (25.3) |
813 | 63.9 (24.4) |
778 | 64.7 (24.2) |
789 | 64.9 (24.0) |
790 | 0.87 (0.87-0.87) |
| Adults, ≥20 | 191.9 (71.8) |
838 | 197.0 (73.6) |
851 | 197.5 (74.7) |
844 | 198.3 (75.6) |
839 | 203.3 (75.8) |
851 | 205.4 (76.0) |
852 | 1.02 (1.02-1.02) |
| US Census Region | |||||||||||||
| Midwest | 63.4 (23.2) |
944 | 61.3 (22.5) |
910 | 61.0 (22.7) |
903 | 60.8 (22.8) |
897 | 61.0 (22.7) |
899 | 60.2 (22.3) |
887 | 0.94 (0.94-0.94) |
| Northeast | 49.6 (18.2) |
892 | 49.5 (18.2) |
886 | 49.0 (18.2) |
875 | 48.6 (18.3) |
866 | 48.8 (18.1) |
869 | 48.8 (18.1) |
868 | 0.97 (0.97-0.97) |
| South | 111.4 (40.8) |
960 | 114.4 (42.0) |
975 | 111.7 (41.6) |
943 | 110.5 (41.5) |
923 | 111.5 (41.4) |
921 | 114.7 (42.4) |
937 | 0.98 (0.98-0.98) |
| West | 48.9 (17.9) |
671 | 47.1 (17.3) |
640 | 47.0 (17.5) |
633 | 46.3 (17.4) |
617 | 48.1 (17.8) |
634 | 46.5 (17.2) |
607 | 0.90 (0.90-0.90) |
Abbreviations: No., Number; pRR, prevalence rate ratio; CI, confidence interval
Prevalence rate ratio derived using a Poisson model with a log link comparing with year as a categorical variable and the year 2011 as the reference.
Age and sex missing for some observations therefore prescription numbers by age and sex may not sum to total.
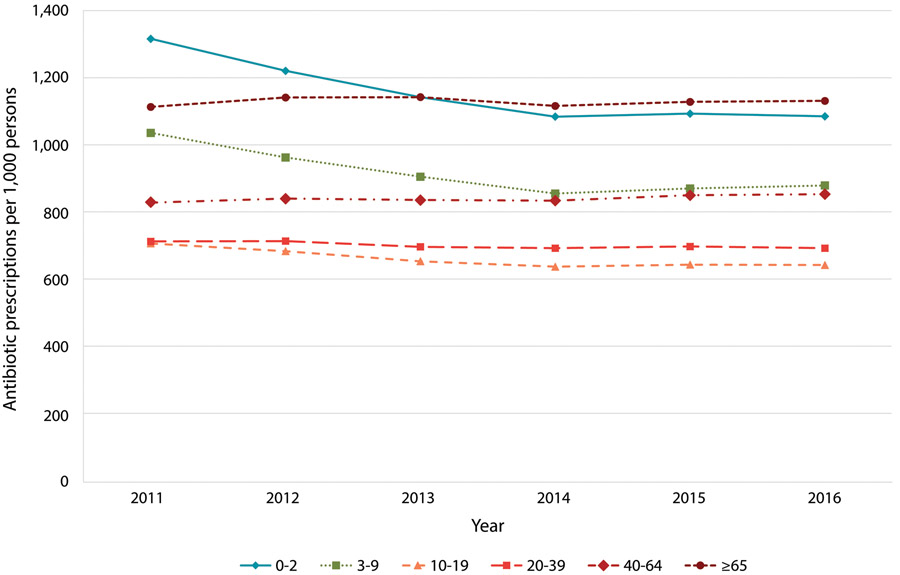
Adults aged 40-64 years accounted for the highest proportion of antibiotic prescriptions in all years. The highest antibiotic prescribing rates occurred in children two and under and adults 65 and older (Figure 1). In 2011-2012, the highest rates were observed in children 0-2 years. In 2014-2016, the highest rates were in adults 65 and older.
Figure 1.
US outpatient oral antibiotic prescriptions per 1,000 persons by age group (in years), 2011-2016
From 2011 to 2016, the rate of antibiotic prescriptions in children (<20 years) decreased 13% (pRR 0.87, 95% CI 0.87-0.87) while the rate in adults (≥20 years old) increased 2% (pRR 1.02, 95% CI 1.02-1.02) (Table 1). The greatest decrease in rates from 2011 to 2016 was observed in the 0-2 year age group (−17%, pRR 0.83, 95% CI 0.82-0.83; Figure 1, Supplementary Table 2) and the 3-9 year age group (−15%, pRR 0.85, 95% CI 0.85-0.85).
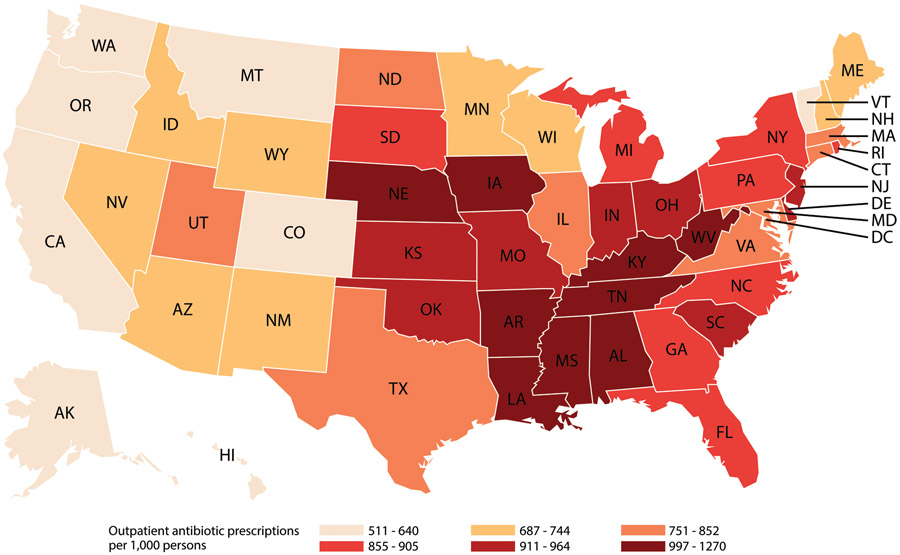
Regional antibiotic prescription rates decreased significantly from 2011 to 2016. The largest decrease, 10% (pRR 0.90, 95% CI 0.90-0.90), was observed in the West, which also had the lowest rates in all years (Table 1). The South had the highest rates in all years and only a 2% decrease (pRR 0.98, 95% CI 0.98-0.98) from 2011-2016. In all years, and among both adults and children, Alaska, California, Oregon, Washington, and Colorado had antibiotic prescription rates in the lowest sextile and Alabama, Kentucky, Louisiana, Mississippi, Tennessee, and West Virginia had rates in the highest sextile (Figure 2, Supplementary Figure 1).
Figure 2.
US outpatient oral antibiotic prescriptions per 1,000 persons by state, 2016
Ratios of broad- to narrow-spectrum antibiotics decreased significantly during the study period, from 1.62 in 2011 to 1.49 in 2016 (Table 2). Ratios decreased in adults and children, although in all years the ratio was higher in adults, indicating a higher proportion of broad-spectrum (compared with narrow-spectrum) prescriptions in adults. Starting in 2013, the rate of broad-spectrum prescriptions was lower than the rate of narrow-spectrum prescriptions (ratio 0.85-0.97) in children. In adults, broad-spectrum antibiotic prescription rates remained over 1.8 times narrow-spectrum prescription rates throughout the study period (ratio 1.83-1.97).
Table 2.
Ratio of outpatient broad-spectrumA and narrow-spectrumB oral antibioticC prescriptions dispensed in the United States, 2011-2016
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | P, test for trendD |
|
|---|---|---|---|---|---|---|---|
| Overall | |||||||
| Broad-spectrum prescriptions per 1,000 persons | 433 | 426 | 410 | 400 | 407 | 395 | |
| Narrow-spectrum prescriptions per 1,000 persons | 267 | 261 | 264 | 262 | 262 | 266 | |
| RatioE | 1.62 | 1.63 | 1.55 | 1.53 | 1.55 | 1.49 | 0.01 |
| Children (<20 years) | |||||||
| Broad-spectrum prescriptions per 1,000 persons | 407 | 386 | 346 | 321 | 326 | 319 | |
| Narrow-spectrum prescriptions per 1,000 persons | 381 | 357 | 356 | 353 | 362 | 373 | |
| RatioE | 1.07 | 1.08 | 0.97 | 0.91 | 0.90 | 0.85 | <0.01 |
| Adults (≥20 years) | |||||||
| Broad-spectrum prescriptions per 1,000 persons | 429 | 432 | 423 | 420 | 431 | 421 | |
| Narrow-spectrum prescriptions per 1,000 persons | 218 | 221 | 227 | 225 | 226 | 230 | |
| RatioE | 1.97 | 1.95 | 1.87 | 1.87 | 1.91 | 1.83 | 0.04 |
Broad-spectrum defined as beta-lactams with increased activity, second-generation cephalosporins, third-generation cephalosporins, fluoroquinolones, macrolides (except erythromycin), lincosamides, and telithromycin.
Narrow-spectrum defined as penicillins (penicillin, amoxicillin, and ampicillin), first-generation cephalosporins, and erythromycin.
Excluding tetracyclines, trimethoprim-sulfamethoxazole and related agents, urinary anti-infectives, and agents classified as other antibiotics. See Supplementary Table 1 for full categorization scheme.
P-value of T-test for slope of linear model of year (continuous) as a predictor of ratio (continuous).
Ratio calculated using non-rounded rate values.
Penicillins accounted for the highest proportion of antibiotic prescriptions by antibiotic category in children in all years (Figure 3). Prescription rates in all antibiotic categories, except lincosamides, decreased in children from 2011 to 2016. The greatest decrease was seen in macrolides; the rate of macrolide prescriptions in 2016 was 32% lower than the rate in 2011 (pRR 0.68, 95% CI 0.68-0.68).
Figure 3.
US outpatient oral antibiotic prescription rates per 1,000 persons by antibiotic category, 2011-2016, for A) all ages, B) children (<20 years), and C) adults (≥20 years)
In adults, macrolides accounted for the highest proportion of antibiotic prescriptions by antibiotic category in all years except 2016. In 2016, the proportions of macrolides and penicillins were similar (Figure 3). Macrolide prescription rates decreased 16% (pRR 0.84, 95% CI 0.84-0.84) during the study period. Fluoroquinolone rates remained relatively constant from 2011 to 2015 at over 130 prescriptions per 1,000 persons, then decreased to 120 prescriptions per 1,000 persons in 2016. Prescription rates for all antibiotic categories except macrolides, fluoroquinolones, and other antibiotics increased in adults during the study period.
Family practice physicians prescribed the highest proportion of antibiotics in all years (Table 3). In 2011, the next highest proportions of antibiotics were prescribed by internal medicine physicians and pediatricians. In 2016, NPs and PAs accounted for the second- and third-highest proportions of antibiotic prescriptions. The proportion of antibiotics prescribed by NPs and PAs increased from 7.4% and 6.7% in 2011 to 14.6% and 10.7%, respectively, in 2016. Significant increases in the proportion of antibiotics prescribed by NPs and PAs were observed in prescriptions to both adults and children (Supplementary Table 3).
Table 3.
US outpatient oral antibiotic prescriptions by provider specialty, 2011-2016
| Outpatient Antibiotic Prescriptions, No. in Millions (% of Antibiotic Prescriptions) | P, Test for trendA |
||||||
|---|---|---|---|---|---|---|---|
| Provider Specialty | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
| Family Practice | 67.0 (24.5) | 65.0 (23.9) | 61.4 (22.8) | 58.1 (21.8) | 56.2 (20.9) | 53.4 (19.8) | <0.01 |
| Internal Medicine | 33.6 (12.3) | 32.8 (12.0) | 31.6 (11.8) | 30.1 (11.3) | 28.5 (10.6) | 27.2 (10.1) | <0.01 |
| Pediatrics | 33.0 (12.1) | 29.9 (11.0) | 27.4 (10.2) | 25.4 (9.5) | 24.8 (9.2) | 24.5 (9.1) | <0.01 |
| Dentistry | 23.0 (8.4) | 23.4 (8.6) | 24.5 (9.1) | 24.9 (9.4) | 25.1 (9.3) | 25.7 (9.5) | <0.01 |
| Nurse Practitioner | 20.2 (7.4) | 22.8 (8.4) | 25.7 (9.6) | 29.5 (11.1) | 35.1 (13.0) | 39.4 (14.6) | <0.01 |
| Physician Assistant | 18.3 (6.7) | 20.3 (7.5) | 22.7 (8.5) | 24.8 (9.3) | 27.8 (10.3) | 29.0 (10.7) | <0.01 |
| Emergency Medicine | 14.7 (5.4) | 15.0 (5.5) | 14.3 (5.3) | 14.2 (5.4) | 14.8 (5.5) | 14.7 (5.4) | 0.89 |
| Surgery | 9.9 (3.6) | 9.9 (3.6) | 10.0 (3.7) | 10.0 (3.8) | 9.7 (3.6) | 9.7 (3.6) | 0.90 |
| Dermatology | 8.5 (3.1) | 8.1 (3.0) | 7.9 (3.0) | 7.6 (2.8) | 7.1 (2.6) | 6.9 (2.5) | <0.01 |
| Medical Subspecialty | 7.5 (2.7) | 7.3 (2.7) | 6.9 (2.6) | 6.6 (2.5) | 6.4 (2.4) | 6.4 (2.4) | <0.01 |
| Obstetrics/Gynecology | 7.2 (2.6) | 7.0 (2.6) | 6.8 (2.5) | 6.6 (2.5) | 6.3 (2.3) | 6.0 (2.2) | <0.01 |
| Urology | 6.7 (2.4) | 6.6 (2.4) | 6.5 (2.4) | 6.4 (2.4) | 6.2 (2.3) | 6.2 (2.3) | 0.04 |
| Otolaryngology | 4.3 (1.6) | 4.0 (1.5) | 3.8 (1.4) | 3.6 (1.3) | 3.5 (1.3) | 3.4 (1.3) | <0.01 |
| Infectious Diseases | 1.3 (0.5) | 1.3 (0.5) | 1.3 (0.5) | 1.3 (0.5) | 1.4 (0.5) | 1.5 (0.5) | 1.00 |
| Internal Medicine/Pediatrics | 1.4 (0.5) | 1.3 (0.5) | 1.3 (0.5) | 1.2 (0.4) | 1.3 (0.5) | 1.2 (0.5) | 0.80 |
| Pediatric Subspecialty | 0.8 (0.3) | 0.7 (0.3) | 0.7 (0.3) | 0.7 (0.2) | 0.7 (0.3) | 0.7 (0.3) | 0.80 |
| Unspecified | 7.6 (2.8) | 8.0 (2.9) | 7.1 (2.6) | 6.6 (2.5) | 5.2 (1.9) | 5.0 (1.8) | <0.01 |
| Other | 8.6 (3.1) | 8.7 (3.2) | 8.7 (3.2) | 8.6 (3.2) | 9.2 (3.4) | 9.3 (3.5) | <0.01 |
Abbreviations: No., Number.
P-value of T-test for slope of linear model of year (continuous) as a predictor of proportion of antibiotic prescriptions (continuous).
Discussion
Rates of oral antibiotic prescriptions dispensed in US outpatient pharmacies decreased from 2011 to 2014, then remained stable from 2014 to 2016. The national rate of oral antibiotic prescriptions per 1,000 persons in 2016 was 5% lower than in 2011, one-third of the way towards the national goal. This trend was entirely driven by prescriptions dispensed to children, with a 13% decrease in pediatric antibiotic prescriptions. From 2011 to 2016, there was also a decrease in the rate of broad-spectrum antibiotic prescriptions, especially macrolides and fluoroquinolones, and in the ratio of broad- to narrow-spectrum antibiotic prescriptions. The proportion of all antibiotics prescribed by NPs and PAs increased from 2011 to 2016.
From 2011 to 2016, broad-spectrum antibiotic prescription rates decreased in both adults and children. Due to the risk of adverse events, decreasing the use of unnecessarily broad-spectrum antibiotics is important for patient safety. A 2017 study found that broad-spectrum antibiotics were associated with a higher risk of adverse events than narrow-spectrum antibiotics in children with acute respiratory tract infections [12]. In both adults and children, broad-spectrum rates decreased more than narrow-spectrum rates, as shown by the decreasing broad- to narrow-spectrum ratios. However, broad-spectrum antibiotics accounted for a higher proportion of prescriptions among adults compared with children. Pediatric guidelines for common outpatient conditions emphasize the use of narrow-spectrum agents as first-line therapy [19, 20] and some broad-spectrum agents, particularly fluoroquinolones, are perceived as dangerous for children and are used only in select cases.
Decreases were especially marked for macrolides in adults and children and fluoroquinolones in adults. Macrolide prescription rates decreased throughout the study period, with the largest decrease between 2012 and 2013. Decreases in macrolides may be related to changes in treatment recommendations for sinusitis (2012) [21] and acute otitis media (AOM; 2013) [19], which stopped recommending macrolides due to increasing macrolide resistance in Streptococcus pneumoniae. In addition, prescribing may have changed due to concerns about adverse drug events. In 2013, FDA issued a Drug Safety Communication about the risk of potentially fatal heart rhythms associated with the macrolide azithromycin [13] and the largest decrease in macrolide prescription rates was observed between 2012 and 2013. Fluoroquinolone rates in adults remained relatively constant from 2011 to 2015 and decreased in 2016. This decrease coincides with a 2016 FDA drug safety communication advising against using fluoroquinolones when other options are available due to tendinopathy, peripheral neuropathy, and other severe side effects [14]. Decreases in macrolide and fluoroquinolone prescription rates may reflect prescribing improvements as previous studies have shown that 50% of macrolides [22] and 5% of fluoroquinolones [23] are prescribed for conditions where antibiotics are not indicated and almost 20% of fluoroquinolones are prescribed in conditions for which they are not recommended first-line therapy [23]. Prescription rates in adults for penicillins, beta-lactams with increased activity, tetracyclines, and trimethoprim-sulfamethoxazole increased during the study period, possibly indicating a shift from macrolides and fluoroquinolones to these agents. Although broad-spectrum antibiotic use decreased during the study period, further opportunities may exist.
In both adults and children, the proportion of all antibiotics prescribed by NPs and PAs increased during the study period, likely related to the growing role of these clinicians in outpatient care. The number of NPs and PAs has increased in recent years [24-26] and is projected to increase further [27, 28]. Previous literature has shown that NPs and PAs are more likely to unnecessarily prescribe antibiotics than physicians [29-31] and account for high proportions of broad-spectrum antibiotic use [32]. With the increasing role of these providers in healthcare, stewardship efforts targeting NPs and PAs are needed.
From 2011 to 2016, antibiotic prescriptions dispensed to children decreased 13% while antibiotic prescriptions dispensed to adults increased 2%. The overall decrease among children was driven by a decline from 2011 to 2014, followed by a slight increase from 2014 to 2016. Lee et al. previously found that between 2000 and 2010, antibiotic prescribing decreased significantly among children but remained stable or increased among adult age groups [33]. Our findings show those trends have continued. Reasons for the decrease in antibiotic prescription rates only in children are likely multifactorial. One factor could be decreasing disease incidence, especially of AOM, following the introduction of the 7-valent and 13-valent pneumococcal conjugate vaccines (PCV7 in 2000 and PCV13 in 2010). Studies have shown decreases in pneumococcal infections, such as AOM, and associated antibiotic use in children following PCV7 and PCV13 introductions [34-36], paralelling the downward trends observed by Lee et al. (PCV7 introduction) [33] and this study (PCV13 introduction).
Other factors likely also contribute to differences observed in rates of antibiotics dispensed to children and adults. One factor may be changes in the way pediatric patients are diagnosed and managed. Stricter diagnostic criteria for AOM [19], the most common reason antibiotics are prescribed to US children, introduced in 2013, likely resulted in fewer AOM diagnoses and subsequent antibiotic prescriptions in children. Another potential factor in the differences between children and adults could be that clinicians treating children may have reduced unnecessary antibiotic prescribing more than clinicians treating adults. Previous studies have shown that pediatricians, specifically, prescribe fewer inappropriate antibiotics than other providers. Finally, parents may have gained increased awareness of the risks of antibiotics and when antibiotics are needed [37]. CDC educational efforts originally emphasized improving antibiotic use in children. More recent educational efforts place additional emphasis on antibiotic prescribing in adults.
The West consistently had the lowest antibiotic prescription rates compared with all other regions and the greatest decrease in rates during the study period. In contrast, the South had the highest rates and lowest decrease. Previous studies, both of overall and inappropriate antibiotic prescribing [4, 8, 38-40], found similar regional trends. Regional variation in inappropriate prescribing shown by other studies suggests that the variation observed in our study may be partly due to differences in inappropriate antibiotic prescribing between regions. Other factors contributing to observed regional differences in antibiotic rates may be differences in disease burden, underlying health, healthcare access, and diagnosis practices.
Our study had limitations. First, we did not have diagnosis or number of visits. Therefore, we were unable to evaluate if unnecessary antibiotic prescriptions, in addition to total antibiotic prescriptions, have decreased. Second, we did not have allergy information and were unable to ascertain how patient allergies may have impacted trends in broad- versus narrow-spectrum antibiotics. Third, this dataset is limited to antibiotics dispensed in pharmacies; we were unable to examine antibiotics administered during healthcare visits. Fourth, these data estimate antibiotics dispensed rather than consumed. Fifth, as there is no standardized definition of broad- and narrow-spectrum antibiotics, our classification may differ from other studies. Finally, the Xponent dataset is not collected for public health purposes and data collection and projection methodologies are proprietary. Strengths of our study include that the data source represents a census of outpatient antibiotic prescriptions and provides the opportunity to assess national prescription trends over time by patient and provider characteristics and drug category.
Conclusion
Dispensed outpatient oral antibiotic prescription rates in the United States decreased from 2011 to 2014, then remained stable. Reductions in antibiotic prescription rates in children drove these nationwide decreases while adult prescription rates increased. Although overall and broad-spectrum antibiotic prescriptions decreased during the study period, there are likely further opportunities to improve prescribing, especially to adults, and additional stewardship interventions are needed to meet the target set by the National Action Plan for Combating Antibiotic-Resistant Bacteria. Additionally, from 2011-2016, prescriptions from NPs and PAs accounted for increasing proportions of antibiotic prescriptions, emphasizing the growing role of these providers in antibiotic prescribing. Efforts to improve antibiotic use should include clinicians who treat adults and advanced practice clinicians. CDC is targeting these groups through the Be Antibiotics Aware: Smart Use, Best Care educational effort, which provides resources to improve antibiotic prescribing and optimize patient care.
Supplementary Material
Key points:
Population-based outpatient oral antibiotic prescription rates decreased significantly from 2011 to 2016. Antibiotic prescription rate decreases were greatest among broad-spectrum antibiotics, driven by macrolides and fluoroquinolones. The proportion of antibiotics prescribed by advanced practice providers increased during this period.
Acknowledgements:
No additional acknowledgements.
Funding: This work was supported by the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Potential conflicts of interest: All authors report no conflict.
References
- 1.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US Emergency Department Visits for Outpatient Adverse Drug Events, 2013-2014. JAMA 2016; 316(20): 2115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed 09 January 2019.
- 3.Duffy E, Ritchie S, Metcalfe S, Van Bakel B, Thomas MG. Antibacterials dispensed in the community comprise 85%-95% of total human antibacterial consumption. J Clin Pharm Ther 2017; 43(1): 59–64. [DOI] [PubMed] [Google Scholar]
- 4.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011. JAMA 2016; 315(17): 1864–73. [DOI] [PubMed] [Google Scholar]
- 5.The White House. National Action Plan for Combating Antibiotic-Resistant Bacteria. Available at: https://www.cdc.gov/drugresistance/pdf/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed 09 January 2019.
- 6.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep 2016; 65(6): 1–12. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Antibiotic Prescribing and Use. Available at: https://www.cdc.gov/antibiotic-use/. Accessed 09 January 2019.
- 8.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60(9): 1308–16. [DOI] [PubMed] [Google Scholar]
- 9.BlueCross BlueShield. Antibiotic Prescription Fill Rates Declining in the US. Available at: https://www.bcbs.com/the-health-of-america/reports/antibiotic-prescription-rates-declining-in-the-US. Accessed 09 January 2019.
- 10.Durkin MJ, Jafarzadeh SR, Hsueh K, et al. Outpatient Antibiotic Prescription Trends in the United States: A National Cohort Study. Infect Control Hosp Epidemiol 2018; 39(5): 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mundkur ML, Franklin J, Huybrechts KF, et al. Changes in Outpatient Use of Antibiotics by Adults in the United States, 2006–2015. Drug Saf 2018; 41(12): 1333–42. [DOI] [PubMed] [Google Scholar]
- 12.Gerber JS, Ross RK, Bryan M, et al. Association of Broad- vs Narrow-Spectrum Antibiotics With Treatment Failure, Adverse Events, and Quality of Life in Children With Acute Respiratory Tract Infections. JAMA 2017; 318(23): 2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. FDA Drug Safety Communication: Azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms. Available at: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM343347.pdf. Accessed 09 January 2019.
- 14.US Food and Drug Administration. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Available at: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM513019.pdf. Accessed 09 January 2019.
- 15.Dingle KE, Didelot X, Quan TP, et al. Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis 2017; 17(4): 411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol 2008; 29(1): 44–50. [DOI] [PubMed] [Google Scholar]
- 17.ECDC (European Centre for Disease Prevention and Control), EFSA BIOHAZPanel (European Food Safety Authority Panel on Biological Hazards), CVMP (EMA Committee for Medicinal Products for Veterinary Use). ECDC, EFSA and EMA Joint Scientific Opinion on a list of outcome indicators as regards surveillance of antimicrobial resistance and antimicrobial consumption in humans and food-producing animals. EFSA Journal 2017; 15(10): e05017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. Bridged-Race Population Estimates, United States July 1st resident population by state, county, age, sex, bridged-race, and Hispanic origin. Compiled from 1990-1999 bridged-race intercensal population estimates (released by NCHS on 7/26/2004); revised bridged-race 2000-2009 intercensal population estimates (released by NCHS on 10/26/2012); and bridged-race Vintage 2016 (2010-2016) postcensal population estimates (released by NCHS on 6/26/2017). CDC WONDER Online Database. Available at: https://wonder.cdc.gov/wonder/help/bridged-race.html#About 1990-2016. Accessed 09 January 2019. [Google Scholar]
- 19.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics 2013; 131(3): e964–99. [DOI] [PubMed] [Google Scholar]
- 20.Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics 2013; 132(1): e262–80. [DOI] [PubMed] [Google Scholar]
- 21.Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis 2012; 54(8): e72–e112. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez GV, Shapiro DJ, Hersh AL, Hicks LA, Fleming-Dutra KE. Outpatient Macrolide Antibiotic Prescribing in the United States, 2008-2011. Open Forum Infect Dis 2017; 4(4): ofx220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabbani S, Hersh AL, Shapiro DJ, Fleming-Dutra KE, Pavia AT, Hicks LA. Opportunities to Improve Fluoroquinolone Prescribing in the United States for Adult Ambulatory Care Visits. Clin Infect Dis 2018; 67(1): 134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes H, Richards MR, McHugh MD, Martsolf G. Rural And Nonrural Primary Care Physician Practices Increasingly Rely On Nurse Practitioners. Health Aff (Millwood) 2018; 37(6): 908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maier CB, Barnes H, Aiken LH, Busse R. Descriptive, cross-country analysis of the nurse practitioner workforce in six countries: size, growth, physician substitution potential. BMJ Open 2016; 6(9): e011901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Commission on Certification of Physician Assistants, Inc. 2017 Statistical Profile of Certified Physician Assistants: An Annual Report of the National Commission on Certification of Physician Assistants. 2018. Available at: https://www.nccpa.net/research. Accessed 09 January 2019.
- 27.Bureau of Labor Statistics, U.S. Department of Labor, Occupational Outlook Handbook, Nurse Anesthetists, Nurse Midwives, and Nurse Practitioners. Available at: https://www.bls.gov/ooh/healthcare/nurse-anesthetists-nurse-midwives-and-nurse-practitioners.htm. Accessed 09 January 2019. [Google Scholar]
- 28.Bureau of Labor Statistics, U.S. Department of Labor, Occupational Outlook Handbook, Physician Assistants. Available at: https://www.bls.gov/ooh/healthcare/physician-assistants.htm. Accessed 09 January 2019. [Google Scholar]
- 29.Sanchez GV, Hersh AL, Shapiro DJ, Cawley JF, Hicks LA. Outpatient Antibiotic Prescribing Among United States Nurse Practitioners and Physician Assistants. Open Forum Infect Dis 2016; 3(3): ofw168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt ML, Spencer MD, Davidson LE. Patient, Provider, and Practice Characteristics Associated with Inappropriate Antimicrobial Prescribing in Ambulatory Practices. Infect Control Hosp Epidemiol 2018; 39(3): 307–15. [DOI] [PubMed] [Google Scholar]
- 31.Watson JR, Wang L, Klima J, et al. Healthcare Claims Data: An Underutilized Tool for Pediatric Outpatient Antimicrobial Stewardship. Clin Infect Dis 2017; 64(11): 1479–85. [DOI] [PubMed] [Google Scholar]
- 32.Suda KJ, Roberts RM, Hunkler RJ, Taylor TH. Antibiotic prescriptions in the community by type of provider in the United States, 2005-2010. J Am Pharm Assoc (2003) 2016; 56(6): 621–6.e1. [DOI] [PubMed] [Google Scholar]
- 33.Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med 2014; 12: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howitz MF, Harboe ZB, Ingels H, Valentiner-Branth P, Molbak K, Djurhuus BD. A nationwide study on the impact of pneumococcal conjugate vaccination on antibiotic use and ventilation tube insertion in Denmark 2000-2014. Vaccine 2017; 35(43): 5858–63. [DOI] [PubMed] [Google Scholar]
- 35.Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997-2004. Pediatrics 2008; 121(2): 253–60. [DOI] [PubMed] [Google Scholar]
- 36.Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics 2006; 118(3): 865–73. [DOI] [PubMed] [Google Scholar]
- 37.Finkelstein JA, Dutta-Linn M, Meyer R, Goldman R. Childhood infections, antibiotics, and resistance: what are parents saying now? Clin Pediatr (Phila) 2014; 53(2): 145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts RM, Hicks LA, Bartoces M. Variation in US outpatient antibiotic prescribing quality measures according to health plan and geography. Am J Manag Care 2016; 22(8): 519–23 [PMC free article] [PubMed] [Google Scholar]
- 39.Hersh AL, Shapiro DJ, Pavia AT, Fleming-Dutra KE, Hicks LA. Geographic Variability in Diagnosis and Antibiotic Prescribing for Acute Respiratory Tract Infections. Infect Dis Ther 2017; 7(1): 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleming-Dutra KE, Demirjian A, Bartoces M, Roberts RM, Taylor TH Jr., Hicks LA. Variations in Antibiotic and Azithromycin Prescribing for Children by Geography and Specialty-United States, 2013. Pediatr Infect Dis J 2018; 37(1): 52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.