ABSTRACT
Concurrent blockade of different checkpoint receptors, notably PD-1 and CTLA-4, elicits greater anti-tumor activity for some tumor types, and the combination of different checkpoint receptor inhibitors is an active area of clinical research. We have previously demonstrated that anti-tumor vaccination, by activating CD8 + T cells, increases the expression of PD-1, CTLA-4, LAG-3 and other inhibitory receptors, and the anti-tumor efficacy of vaccination can be increased with checkpoint blockade. In the current study, we sought to determine whether anti-tumor vaccination might be further improved with combined checkpoint blockade. Using an OVA-expressing mouse tumor model, we found that CD8 + T cells activated in the presence of professional antigen presenting cells (APC) expressed multiple checkpoint receptors; however, T cells activated without APCs expressed LAG-3 alone, suggesting that LAG-3 might be a preferred target in combination with vaccination. Using three different murine tumor models, and peptide or DNA vaccines targeting three tumor antigens, we assessed the effects of vaccines with blockade of PD-1 and/or LAG-3 on tumor growth. We report that, in each model, the anti-tumor efficacy of vaccination was increased with PD-1 and/or LAG-3 blockade. However, combined PD-1 and LAG-3 blockade elicited the greatest anti-tumor effect when combined with vaccination in a MycCaP prostate cancer model in which PD-1 blockade alone with vaccination targeting a “self” tumor antigen had less efficacy. These results suggest anti-tumor vaccination might best be combined with concurrent blockade of both PD-1 and LAG-3, and potentially other checkpoint receptors whose expression is increased on CD8 + T cells following vaccine-mediated activation.
KEYWORDS: PD-1, LAG-3, APC, tumor vaccine
Introduction
The blockade of T-cell immune checkpoint receptors to enable the activity of tumor-specific T cells has revolutionized the treatment of cancer. Notably, an antibody blocking cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) was the first of these agents that was FDA approved in 2010 for the treatment of metastatic melanoma.1 Other immune checkpoint receptors were discovered as markers of cell death and exhausted, nonfunctional T cells that had experienced long-term antigen exposure.2 In particular, the programmed death 1 (PD-1) receptor, while initially thought to indicate T cell exhaustion, was subsequently found to function by preventing functional Th1 CD8 + T cells from causing autoimmunity .3 The immunosuppressive activity of PD-1 is executed following ligand (PD–L1) encounter on self (including tumor) cells resulting in the activation of a signaling pathway that attenuates cytotoxic T cell activity.3–5 As a result, remarkable anti-tumor activity can be achieved by blocking PD-1/PD–L1 ligation using antibodies, and this approach has led to multiple new FDA approvals over the last 5 years, underscoring the power of this single immune checkpoint.6–8
The general rationale for the use of T-cell checkpoint blockade as cancer therapies is that ligand-induced checkpoint signaling leads to the activation of regulatory pathways within tumor-reactive T cells, and thus blocking ligand interaction can remove the negative signal to allow for eradication of tumor cells. As PD-1 and other checkpoints operate through distinct mechanisms but result in similar outcomes, it follows that simultaneous blockade could have a synergistic effect. Indeed, a number of murine and clinical studies have been conducted using PD–1 blockade with other checkpoint blocking therapies.9–13 Preclinical studies demonstrate that blocking checkpoints with complementary mechanisms of action can result in the expansion of unique T-cell repertoires and activate adaptive anti-tumor immunity.10 Furthermore, a randomized, double-blind, phase 3 study of PD-1 blockade alone or in a dual blockade combination with CTLA-4 blockade in patients with metastatic melanoma found a median progression-free survival of 11.5 months with the combination and 6.9 months with single-agent PD-1 blockade.9 Similar results in patients with renal cell cancer have led to the FDA approval of CTLA-4 and PD-1 dual blockade for the treatment of metastatic renal cell cancer and melanoma.14,15
In previous studies, we found that DNA or peptide vaccine-induced activation of tumor-specific, CD8 + T cells led to increased expression of multiple checkpoint receptors that could mitigate the anti-tumor response following vaccination. More specifically, we found that antigens with high-affinity for MHC-I increased contact time between CD8 + T cells and antigen-presenting cells (APCs), which led to increases in multiple immune checkpoints, including PD-1, CTLA-4, lymphocyte activation gene-3 (LAG-3), and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) on responding cells when compared to cells activated with lower affinity epitopes. Additionally, the reduced anti-tumor efficacy could be recovered when vaccines encoding high-affinity epitopes were combined with PD-1 or PD-L1 blocking antibodies.16,17 In a separate study, we found that immunization approaches leading to increased antigen expression also led to increased LAG-3 on tumor antigen-specific CD8 + T cells, which was likewise capable of interfering with the anti-tumor response. Again, the reduced anti-tumor efficacy could be recovered when vaccination was combined with LAG-3 blocking antibodies.18 These data demonstrate that blocking the regulatory pathways induced with vaccination can enhance anti-tumor responses and indicate checkpoint receptor upregulation as a major mechanism of tumor resistance to vaccination. Furthermore, these data demonstrate that T-cell activating therapies can result in the expression of multiple, different checkpoint receptors, and hence combination blockade might be preferable. This is particularly relevant for anti-tumor DNA vaccines, which result in tumor-antigen presentation via professional APC and/or bystander cells. Presentation by multiple cell types may increase the diversity of responding T cells and likewise the complexity of checkpoint expression profiles on these populations.18,19 Consequently, we hypothesized that blockade of multiple checkpoints may be necessary to elicit CD8 + T cells with greater anti-tumor activity in the context of anti-tumor immunization.
In the current studies, we focused on understanding the expression of immune checkpoint receptors following CD8 + T cell activation in order to identify rational dual checkpoint blockade combinations and test these combinations in tumor models. We used three separate murine tumor models targeting different antigens with different vaccines: C57BL/6 mice implanted with E.G7-OVA tumors expressing ovalbumin which we previously modified to overexpress PD-L1 (PD-L1high),19 an HLA-A2+ HLA-DR1+ (HHD–II) mouse model in which mice were implanted with sarcoma cells expressing the human synovial sarcoma X breakpoint 2 (SSX2) protein as a tumor antigen,20 and FVB mice implanted with MycCaP prostate tumor cells, using a vaccine targeting the native androgen receptor (AR).21–23 Using OT-1 mice, we assessed immune checkpoint expression on CD8 + T cells following activation by antigen alone or by antigen presented by professional APC. We found that PD-1, CTLA-4, LAG-3, and TIM-3 were all upregulated in the presence of professional APC. However, in the absence of professional APC, LAG-3 was the only checkpoint molecule expressed, suggesting LAG-3 as a rational target for dual blockade in combination with anti-tumor vaccination. Subsequent studies focused on anti-tumor vaccination in the presence or absence of PD-1, LAG-3, or dual PD-1/LAG-3 antibody blockade. We found that in a model less responsive to vaccination and PD-1 blockade anti-tumor vaccination produced a greater anti-tumor response when used in combination with both PD-1 and LAG-3 blockade.
Materials and methods:
Mice
HLA-A2.01/HLA-DR1-expressing (HHDII-DR1) mice on a C57BL/6 background were obtained from Charles River Labs courtesy of Dr. François Lemonnier.24 OT-1 (Stock No: 003831), C57BL/6 J (B6, Stock No: 000664), and FVB/NJ (FVB, Stock No: 001800) mice were purchased from The Jackson Laboratory (Jax, Bar Harbor, MA). All mice were maintained and treated in microisolator cages under aseptic conditions, and all experiments were conducted under an IACUC-approved protocol that conforms to the NIH guide for the care and use of laboratory animals.
Cell lines
E.G7-OVA (derivative of EL4) cells were obtained from ATCC (Manassas, VA, Cat. # CRL-2113) and maintained via the ATCC-recommended culture methods. E.G7-OVA cells were lentivirally transduced to express PD-L1, as previously described.19 The A2/sarcoma cell line expressing SSX2 (A2/Sarc-SSX2) was generated as previously described.16 The MycCaP cell line was obtained from ATCC (Cat #CRL-3255) and cultured according to their instructions. All cell lines used were authenticated and tested for mycoplasma.
Peptides
Peptides encoding the H2Kb-restricted epitope from chicken ovalbumin (SIINFEKL) and the HLA-A2 restricted epitope of SSX2 (RLQGISPKI) were synthesized, and the purity and identity confirmed by mass spectrometry and gas chromatography (LifeTein, LLC., Hillsborough, NJ). Peptides were dissolved in DMSO (2 mg/ml) and stored at −80°C until required.
In vitro assays
Splenocyte stimulation
Spleens were collected from OT-1 mice, processed through a mesh screen, and splenocytes were isolated by centrifugation after red blood cell osmotic lysis with ammonium chloride/potassium chloride lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA). Splenocytes were cultured at 2x106/mL in RPMI 1640 medium supplemented with L-glutamine, 10% fetal calf serum (FCS), 200 U/mL penicillin/streptomycin, 1% sodium pyruvate, 1% HEPES, 50 μM β-MeOH, and 2 µg/mL SIINFEKL peptide or the HLA-A2 restricted sequence from SSX2 (RLQGISPKI) as a nonspecific control.
Co-culture experiments (Figure S1)
B cells or dendritic cells (DCs) were enriched from splenocytes of OT-1 or B6 mice inoculated with Flt3 ligand-expressing B16 tumor cells25 using PE-labeled antibodies specific for either CD19 or CD11c (StemCell, Seattle, WA, Cat.# 17,684) as previously described.26 Similarly, CD8 + T cells were isolated using a negative selection CD8 + T-cell isolation kit (StemCell, Cat. # 19,853). After enrichment, each APC subset, and a subset of purified T cells, were cultured as described above with 2 µg/mL SIINFELK or the HLA-A2 sequence from SSX2 (RLQGISPKI) as a nonspecific control peptide. Naïve OT-1 T cells were added to each cell type at a 1:1 ratio and incubated for three days, after which cells were stained and analyzed by flow cytometry with the following panel: CD3-FITC (BD 555,274), CD4-BUV395 (BD 563,790), CD8-BUV805 (BD 564,920), LAG-3-BV711 (BD 563,179), PD1-PECF594 (BD 562,523), TIM3–APC (eBioscience 17-5871-82), CTLA4–PECy7 (Tonbo 60-1522-U100), 41BB–PerCPeF710 (eBioscience 46–1371–82), and Live/Dead Ghost dye 780 (Tonbo, San Diego, CA 13–0865–T100).
Immunization studies
The construction of DNA vaccines encoding SSX2 was previously described.20 Six- to eight-week-old HHDII-DR1 mice were randomized into treatment groups and immunized intradermally (i.d.) with the 100 μg pTVG4 control vector, pTVG-SSX2, pTVG-SSX2HA, or MIP-SSX2 DNA vaccines (Figure S2). At 2, 4, 7, 10, and 14 days after immunization, a group from each treatment type were euthanized, their spleens collected and SSX2-tetramer+ CD8 T cells assessed by flow cytometry directly for surface markers or stimulated with the dominant HLA-A2 restricted epitope of SSX2; p103–111, RLQGISPKI, for 16 hours (8 alone and 8 in the presence of BD GolgiStop [BD Biosciences, Cat.# 554,724]) and activation and cytokine production of all CD8 T cells assessed by intracellular cytokine staining and flow cytometry using standard protocols provided by BD biosciences. A flow panel for direct analysis of surface markers was as described above, with the addition of SSX2 p103 tetramer-APC.
Tumor treatment studies
E.G7-OVA tumors in B6 mice (Figure S3)
Six- to ten-week-old female B6 mice were injected subcutaneously (s.c.) with 106 ovalbumin-expressing E.G7-OVA PD-L1high cells. Seven to ten days postinjection, when tumors were palpable and similarly sized (~0.1 cm3), mice were randomized into treatment groups and OT-1 splenocytes were harvested and SIINFEKL-specific CD8 + T cells and DC were isolated as previously described.26 OT-1 CD8 + T cells were stimulated for 36 hours in the presence of 2 µg/mL SIINFEKL or vehicle control with or without a 1:1 ratio of DC as described above. Following stimulation, three groups of T cells were isolated: those that received vehicle (No Stim), those that were simulated in the absence of DCs (No APC), and those that were stimulated in the presence of DCs (DC). Ten days after tumor implantation, 106 of each T cell subset were adoptively transferred via intraperitoneal (i.p.) injection into the E.G7-OVA PD-L1high tumor-bearing mice. The day following transfer, mice were given 100 μg αPD-1, αLAG-3, both αPD-1 and αLAG-3, or IgG control. Tumor volume was measured with calipers three times weekly until tumors reach 2 cm3 or death and calculated in cubic centimeters using the following formula: (π/6)*(long axis, cm)*(short axis, cm)2. Animals with tumors larger than 2 cm3 were compassionately euthanized.
SSX2+ sarcomas in HHD-II mice (Figure S4)
Six– to eight-week-old female HHDII-DR1 mice were inoculated with 105 A2/Sarc-SSX2 cells administered s.c. in 50% Matrigel (Corning, Tewksbury, MA Cat.# 354,248). The following day, mice were immunized i.d. with 100 μg pTVG4 control vector, pTVG-SSX2, pTVG-SSX2HA, or MIP-SSX2 DNA, and the day after that, each vaccine group was administered 100 μg i.p. αPD-1, αLAG-3, both αPD-1/αLAG-3, or IgG control antibodies. Tumor volume was measured over time, with endpoints as above.
MycCaP tumors in FVB mice (Figure S5)
6- to 9-week-old male FVB mice were injected s.c. with 106 MycCaP cells on day 0. Beginning the next day (day 1) and continuing weekly, mice were immunized i.d. with 100ug pTVG4 control vector or pTVG-AR vaccine. The following day (day 2), and weekly thereafter, mice were injected i.p. with 100 µg of IgG, αPD-1, αLAG-3, or both αPD-1/αLAG-3 (100 µg each). Tumor volume was measured over time, with endpoints as above. In a parallel study, tumors were also collected on day 29, digested with collagenase, and assessed by flow cytometry as described above, with the gating strategy as shown in Figure S6.
Statistical analyses
Comparison of group means was performed using GraphPad Prism software, v8.4.3. Analysis of Variance (ANOVA) followed by the Bonferroni multiple-comparison post-hoc procedure was used to compare individual group means. Where ANOVA was not possible, comparison of group means was performed using the mixed effects model with Geisser-Greenhouse correction. Survival analysis was conducted using a Mantel-Cox log-rank test. For all comparisons, P values equal to or less than 0.05 were considered statistically significant.
Results
T-cell activation by professional APCs can lead to distinct immune checkpoint expression on CD8 ± T cells
As described earlier, our previous work has demonstrated that differences in T-cell priming from anti-tumor vaccination can lead to expression of different checkpoint receptors which can impede the anti-tumor efficacy of vaccine induced CD8 + T cells.16,18,19 To evaluate this further, we first assessed the expression of checkpoints immediately following antigen encounter by activating OT–1 CD8 + T cells with SIINFEKL peptide in the presence or absence of professional APC (DCs or B cells). Shown in Figure 1 are the mean fluorescence intensities (MFI) of 4–1BB (CD137, as a marker of T-cell activation), PD-1, CTLA-4, TIM-3, and LAG-3 on OT–1 CD8 + T cells activated in the presence or absence (No Stim) of cognate SIINFEKL peptide. Expression of all the checkpoint receptors and 4–1BB was increased on cells stimulated in the presence of professional APCs (either B cells or DC). Expression of TIM-3 was slightly (but not significantly, p = .086) lower when B cells were used as professional APC compared to DC. However, when T cells were stimulated alone without professional APC, the only checkpoint receptor with increased expression was LAG-3. This suggests that activation with co-stimulation leads to expression of other receptors and LAG-3 is increased with activation in the absence of a co-stimulatory signal.
Figure 1.
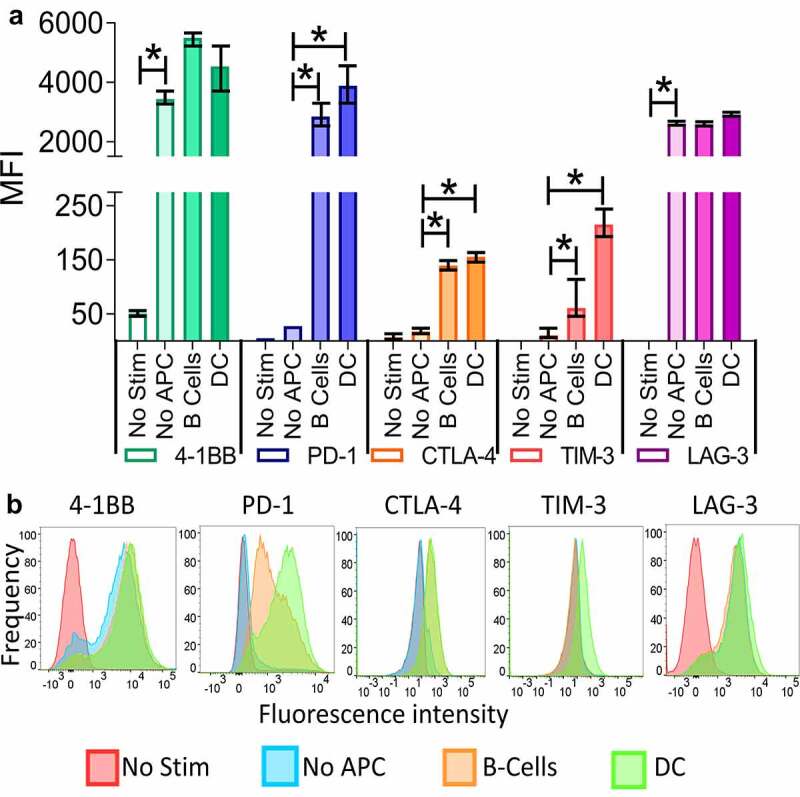
T-cell activation by professional APCs can lead to distinct immune checkpoint expression on CD8 + T cells. Splenocytes were prepared from the spleens of OT-1 mice and separated into T cells (CD8+) and B-cells (CD19+) using MACS. DC (CD11c+) were prepared from the spleens of Flt3 ligand–treated B6 mice. T cells were stimulated with a control peptide (No Stim), the SIINFEKL peptide alone (No APC), or the SIINFEKL peptide in combination with either B cells or DC. After 72 hours the cells were collected and the checkpoint and 4–1BB expression analyzed by flow cytometry. Shown is the mean fluorescence intensity (MFI) and standard error of the mean of 4–1BB, PD-1, CTLA-4, TIM-3, or LAG-3 on CD8 + T cells from triplicate assessments (panel A), and a representative histogram for each marker (panel B). Asterisks indicate p < .05 by one-way ANOVA with Bonferroni’s multiple comparisons correction. Results are from one experiment (N = 3 mice per group) and are representative of two similar, independent experiments
Blockade of PD-1 or LAG-3 improves anti-tumor activity of activated CD8 ± T-cells
To determine directly whether expression of specific receptors interferes with anti-tumor response and whether blocking activation-induced checkpoint receptors can ameliorate the anti-tumor response, naïve OT-1+ CD8 + T cells, or OT-1+ CD8 + T cells that were stimulated in vitro with or without APC (DC), were adoptively transferred to B6 mice bearing PD-L1high E.G7-OVA tumors. Following the transfer, mice were administered IgG isotype, αPD-1, αLAG-3, or both αPD-1 and αLAG-3 monoclonal antibodies (Figure 2a). As shown in Figure 2b, all groups that received checkpoint blockade had marked reductions in tumor growth when compared to IgG. However, LAG-3 blockade was most effective when used with T cells stimulated without APC (Figure 2c). Blockade of both PD-1 and LAG-3 produced a greater delay in tumor growth when compared to IgG or LAG-3, however the response following dual blockade was not significantly greater when compared to PD-1 alone in this model (individual growth curves shown in Figure S7).
Figure 2.
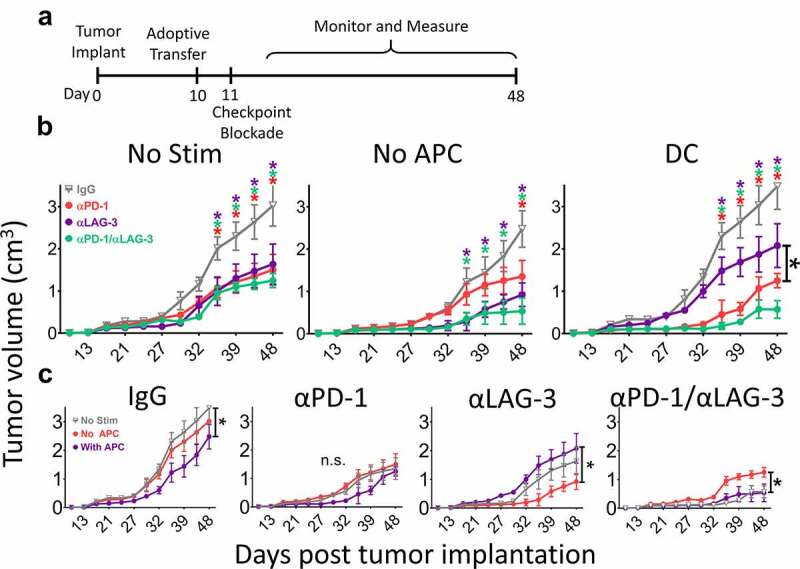
Blockade of PD-1 or LAG-3 improves anti-tumor activity of activated CD8 + T-cells. As shown in panel A, B6 mice were inoculated with 1 × 106 PD-L1-expressing E.G7-OVA cells. After ten days, 1 × 106 OT-1 T cells, stimulated with or without peptide and with or without DC as in Figure 1, were adoptively transferred into the tumor-bearing mice. The following day, mice were treated with IgG isotype control (gray), PD-1 blocking (red), LAG-3 blocking (purple), or a combination of both PD-1 and LAG-3 blocking antibodies (green). Tumor growth was measured as indicated on the X axes. Shown in panel B are the growth curves for mice that received T cells which had not been incubated with DC and a nonspecific peptide (No Stim), T without DC cells stimulated with SIINFEKL peptide alone (No APC), or T cells stimulated with peptide in the presence of DC (DC). Panel C shows the same data grouped by checkpoint blockade treatment rather than T-cell stimulation conditions. Measurements for individual mice are shown in Supplemental Figure S5. Asterisks indicate p < .05 as assessed by 2-way ANOVA with Bonferroni’s multiple comparisons test. Results are from one experiment with N = 6 mice per group
DNA vaccination can elicit CD8 ± T cells differentially expressing PD-1 and LAG-3
We next wished to determine how PD-1 and/or LAG-3 blockade, when used concurrently with DNA vaccination, would affect the resulting Th1 CD8 + T-cell response. For this, we first evaluated HLA-A2/DR1+ HHD-II mice vaccinated with different plasmid vectors encoding SSX2. Specifically, pTVG-SSX2HA encodes two epitopes with high HLA-A2 affinity and was previously demonstrated to elicit antigen-specific CD8 + T cells with higher PD-1 expression compared to a vector encoding the native SSX2 epitopes (pTVG-SSX2).16 The other construct, mini-intronic plasmid SSX2 (MIP-SSX2), encodes the native SSX2 protein in a mini-intronic plasmid resulting in prolonged expression of SSX2 in vivo, and was previously demonstrated to elicit antigen-specific CD8 + T cells with higher LAG-3 expression compared to pTVG-SSX2.18 Mice were immunized with one of these modified vaccines, the native pTVG-SSX2 vaccine, or empty vector (pTVG4). Splenocytes from immunized animals were collected at 2, 4, 7, 10 and 14 days after immunization to assess checkpoint expression and memory phenotype (Figure 3a). As shown in Figure 3b, immunization with pTVG-SSX2HA led to p103 (the dominant HLA-A2 epitope for SSX2) tetramer+ CD8 + T cells with increased PD-1 expression when compared to the other vaccines (representative histograms are shown in Figure S8). Immunization with MIP-SSX2 predominantly induced LAG-3 expression, with lower expression of PD-1 compared to the other vectors. These findings were consistent with our previous findings.16,18 As shown in Figure 3c, vaccination with any of the SSX2 constructs elicited CD8 + T cells with similar Th1 cytokine profiles following in vitro stimulation with the p103 peptide epitope. As shown in Figure 3d, each of the SSX2 vaccines led to a similar transition from central to effector CD8 memory, which is expected following cytotoxic T-cell expansion (representative dot plots in Figure S8).27
Figure 3.
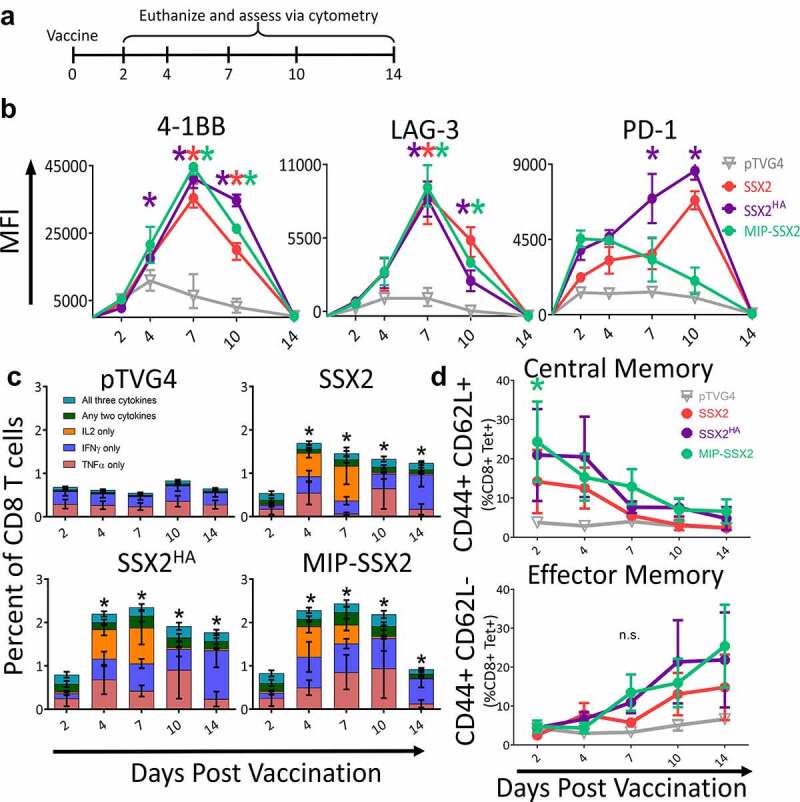
DNA vaccination can elicit CD8 + T cells differentially expressing PD-1 and LAG-3. Panel A: six-week-old HHDII HLA-A2+ mice were immunized with pTVG4 empty vector, the native pTVG-SSX2 DNA vaccine (SSX2), pTVG-SSX2HA (SSX2HA), or MIP-SSX2. Mice were euthanized at the time points indicated and splenocytes were assessed by flow cytometry gated on CD3+ CD8+ tetramer+ cells (panels B and D, n = 6 mice/time/condition) or following stimulation with an HLA-A2-restricted peptide epitope (SSX2 p103–111) to determine the number of responding cells via intracellular cytokine analysis (panel C, n = 3 mice/timepoint). In panel C, comparisons are of total cytokine-secreting CD8 + T cells at each time point between vaccine-treated or pTVG4 control-treated animals. For all panels, asterisks indicate p < .05 by two-way ANOVA with Bonferroni’s multiple comparisons correction. MFI = mean fluorescence intensity. Results are from one experiment and are representative of two similar, independent experiments
PD-1 blockade is superior to LAG-3 blockade when used in combination with an anti-tumor vaccine in an αPD-1 sensitive tumor
We next wished to determine whether PD-1 and LAG-3 blockade was superior to either alone when used in combination with these anti-tumor DNA vaccines. 6- to 8-week-old HLA-A2+ HHD-II mice were inoculated with SSX2-expressing sarcoma cells. As shown in Figure 4a, the mice were immunized with pTVG4 control vector, pTVG-SSX2, pTVG-SSX2HA, or MIP-SSX2 DNA vaccines one day following tumor implantation and at weekly intervals thereafter. The day following each immunization, mice were administered αPD-1, αLAG-3, both αPD-1/αLAG-3, or IgG control antibodies. Shown in Figure 4 are the mean tumor sizes (4B) and survival curves (4C) from each treatment group (individual data points are shown in Figure S9). Consistent with our previous findings, and despite the similar cytokine expression profile and memory phenotype of CD8 + T cells described in Figure 3, pTVG-SSX2HA and MIP-SSX2 vaccines were inferior to the native pTVG-SSX2 vaccine when used without T-cell checkpoint blockade (pTVG-SSX2 vs pTVG-SSX2HA p = .036; pTVG-SSX2 vs MIP-SSX2 p = .026). However, when the altered vaccines were used in combination with checkpoint blockade, all blocking antibodies resulted in reduced tumor growth when compared to IgG control. As in the PD-L1high E.G7-OVA tumors, both αPD-1 and the αPD-1/αLAG-3 combination slowed tumor growth to a greater extent and prolonged survival when compared to αLAG-3 alone with antigen-specific vaccination. However, the response to vaccination with dual αPD-1/αLAG-3 blockade was not significantly greater than blockade with αPD-1 alone (pTVG-SSX2 p = .99; pTVG-SSX2HA p = .84; MIP-SSX2 p = .92), which in this model was highly effective. A treatment response was observed with αLAG-3 and control vector in this particular experiment, but not observed in repeated studies (data not shown).
Figure 4.
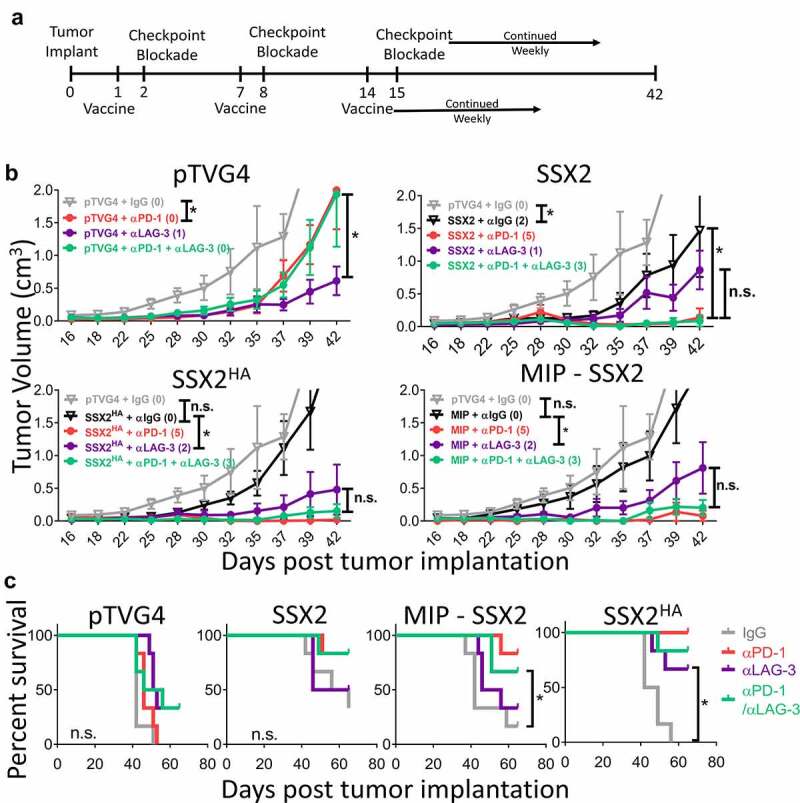
PD-1 blockade is superior to LAG-3 blockade when used in combination with an anti-tumor DNA vaccine in an αPD-1 sensitive tumor. Panel A: six-week-old HHDII (HLA-A2+) mice were inoculated s.c. with SSX2+ HLA-A2+ sarcoma cells and immunized with pTVG4 empty vector, pTVG-SSX2 (SSX2), pTVG-SSX2HA (SSX2HA), or MIP-SSX2 in combination with αPD-1, αLAG-3, both αPD-1/αLAG-3, or IgG control. Tumor growth was measured over time. Panel B: shown are the tumor growth curves for each vaccine group. Animals with tumors greater than 2 cm3 in size were euthanized, and data were censored at 2 cm3. Panel C: data are presented as survival plots using the time to death or when tumors reached 2 cm3 in size, whichever occurred first. Individual tumor measurements are shown in Supplemental Figure S8. Asterisks in panel B indicate p < .05 as assessed by mixed-effects model with Geisser-Greenhouse correction and Tukey’s multiple comparisons test with individual variances; N = 6 mice/time point/condition. n.s. = not significant. Results are from one experiment (N = 6) and are representative of two similar, independent experiments. For data points above the Y axis, statistical comparisons are indicated on the figure legends. In panel C, asterisks indicate p < .05 as assessed by log-rank test
In a prostate cancer model, vaccination with PD-1 and LAG-3 blockade is superior to vaccination with either blockade alone
We next wished to evaluate vaccination with checkpoint blockade in a murine model less responsive to PD-1 blockade. Prostate cancers have been considered mostly resistant to single-agent PD-1 blockade in clinical trials, and previous reports using the murine MycCaP prostate cancer model have demonstrated that while it does respond to anti-tumor vaccination, it does not respond to PD-1/PD–L1 blockade.23,28–30 As shown in Figure 5a, six-to-nine-week-old male FVB mice were inoculated with MycCaP cells and immunized with the pTVG4 control or a DNA vaccine encoding the native ligand-binding domain of the androgen receptor (pTVG-AR). The day following immunization and weekly thereafter, mice were treated with αPD-1, αLAG-3, both αPD-1/αLAG-3, or IgG control. As shown in Figure 5b and Figure 5c, all vaccine combinations slowed the growth of tumors when compared to the vaccine with IgG; however, the combination of αPD-1 and αLAG-3 with vaccine led to a significant reduction in tumor growth compared to vaccination with either antibody alone. Treatment of mice with αPD-1 and/or αLAG-3 without vaccine showed little anti-tumor effect in this model (Figure S10). In a duplicate study, tumors were collected at day 29 for further evaluation. The combination treatment led to a slight increase (not significant) in the number of infiltrating CD4+ and CD8 + T cells (Figure 5d), as well as an unexpected increase in tumor-infiltrating MDSC (Figure S10). Further evaluation of tumor-infiltrating CD8 + T cells showed these to be predominantly of an effector memory and tissue-resident memory phenotype (Figure 5e).
Figure 5.
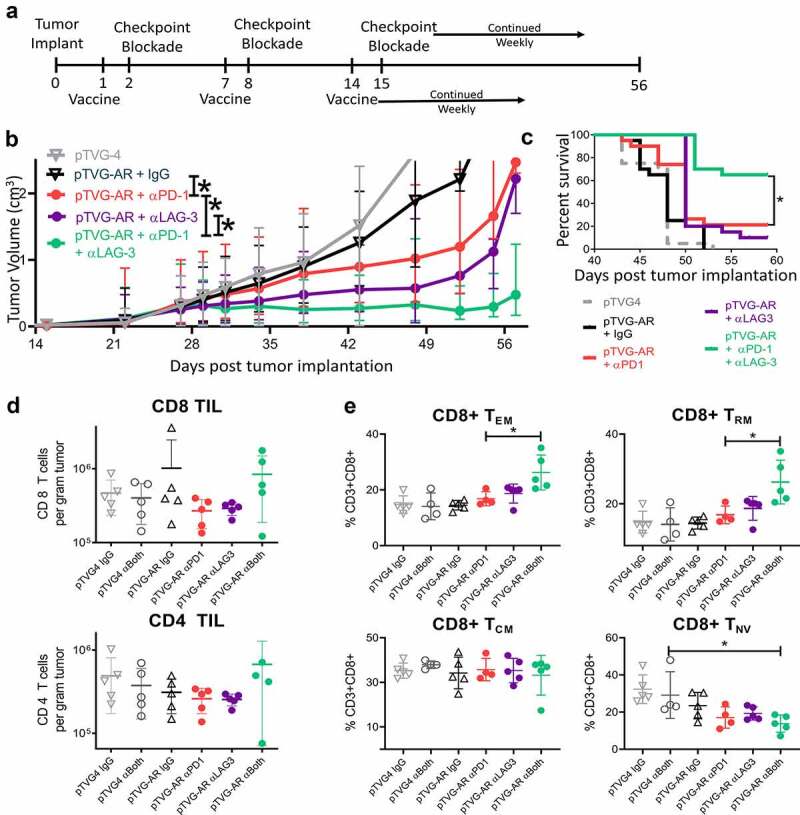
Vaccination with PD-1 and LAG-3 blockade is superior to vaccination with either blockade alone in αPD-1 resistant prostate cancer model. As shown in panel A, six-week-old FVB mice (n = 20 per group) were inoculated s.c. with 106 MyC-CaP cells and immunized with pTVG4 empty vector or pTVG-AR in combination with IgG control, αPD-1, αLAG-3, or both αPD-1/αLAG-3 antibodies. Tumor growth was measured over time. Panel B: Shown are the mean tumor growth curves and standard deviations; individual tumor measurements are shown in Supplemental Figure S10. Animals with tumors greater than 2 cm3 in size were euthanized, and data were censored at 2 cm3. Panel C: data are presented as survival plots using the time to death or when tumors reached 2 cm3 in size, whichever occurred first. Results shown are from one experiment and representative of three independent experiments. Panel D: Shown are the number of CD8+ (top) and CD4 (bottom) tumor-infiltrating lymphocytes per gram of tumor tissue collected at day 29 as determined by flow cytometry (gating strategy shown in Supplemental Figure S6). Panel E: Shown are the distribution of effector memory (TEM, CD44+ CD62Llo), resident memory (TRM, CD69+ CD103+), central memory (TCM, CD44+ CD62L+), and naïve (TNV, CD44-CD62L+) cells among the CD8 + T cells. Asterisks indicate p < .05 assessed by the mixed-effects model with Geisser-Greenhouse correction and Tukey’s multiple comparisons test with individual variances (panel B), by log-rank test (panel C), or by the one-way ANOVA with Tukey’s multiple comparisons test (panels D and E)
Discussion
In this report, we investigated the activation-induced expression of immune checkpoint receptors on CD8 + T cells and how that expression is affected by T-cells which had been activated by professional or “nonprofessional” APC. Based on this information, we identified a rational combination of checkpoint inhibitors to use with anti-tumor vaccination. We report that T cells stimulated in the absence of professional APC increased expression of LAG-3 but not PD-1, CTLA-4, or TIM3, while T cells stimulated with APC displayed an increase in all checkpoint receptors observed. We thus focused on combinations of PD-1 and LAG-3 blockade in the context of anti-tumor vaccination. Using DNA vaccines that we have previously demonstrated can lead to antigen-specific CD8 + T cells with increased expression of PD-1 or LAG-3, we found that either checkpoint blockade successfully enhanced vaccine induced anti-tumor responses with all the vaccines tested; however, we found no specific advantage to vaccination with dual PD-1/LAG-3 blockade over vaccine with PD-1 blockade alone in murine models that were robustly sensitive to PD-1 blockade.19 In the prostate cancer model, which is resistant to single-agent PD-1 blockade, and using a vaccine encoding a naturally expressed tumor antigen, the dual blockade group demonstrated greater therapeutic efficacy than other treatment groups. These results indicate the following: 1) depending on which cells are presenting antigen, tumor-reactive CD8 + T cells can be activated with distinct patterns of checkpoint receptor expression; 2) dual blockade of PD-1 and LAG-3 can provide significant benefit over either blockade alone in PD-1 resistant MycCaP prostate tumors; 3) the upregulation of other checkpoint receptors (e.g. TIM-3, CTLA-4, VISTA, CD160, BTLA etc.), and the persistence of some tumors despite activation of a Th1 biased T-cell response and targeted checkpoint blockade suggest that combination strategies with vaccine and other checkpoint blocking antibodies could be the focus of future investigations.
Our approach was based on the finding that T-cell activation following vaccination resulted in the expression of PD-1, LAG-3, CTLA-4, and TIM-3 checkpoint receptors. Of note, we did see a slight decrease in TIM-3 following stimulation with B cells as professional APC (Figure 1), suggesting there could be differences in T cell function following stimulation by different professional APC types. However, in the absence of professional APC, activated CD8 + T cells expressed only LAG-3. We reasoned that combination checkpoint blockade following vaccination should consequently include LAG-3 blockade, as vaccines, and notably DNA vaccines, can result in antigen presentation through nonprofessional APC. We have previously shown that, during T-cell activation, a longer contact time between the CD8 + T cell and the APC (i.e. longer exposure to TCR signaling and co-stimulation), resulted in elevated PD-1 expression that persisted for months after antigen exposure.19 These data suggest the existence of a negative feedback loop in which excess TCR stimulation leads to the expression of PD-1 and other inhibitory receptors and molecules. Given the current study, LAG-3 expression appears to be dependent on TCR stimulation, but not necessarily co-stimulation. This suggests LAG-3 expression may be part of a second negative feedback loop that is regulated independently of PD-1, and consequently that the use of PD-1 and LAG-3 in a dual checkpoint blockade strategy could be advantageous following vaccination with a tumor antigen.
Our data demonstrate that if CD8 + T cells are activated in a way that leads to the expression LAG-3 alone; then, their anti-tumor activity is improved with LAG-3 blockade. However, following vaccination, there was little benefit to adding LAG-3 blockade to vaccine with PD-1 blockade in the OVA-expressing or SSX2 sarcoma models. It is possible that this is entirely due to the first two model systems being exquisitely sensitive to PD-1 blockade with vaccination. We had specifically used E.G7-OVA tumor cells transfected to express PD-L1 as a model at least partially responsive to PD-1 blockade, compared with E.G7-OVA cells that do not express PD-L1.31 In addition, we had previously demonstrated that OVA-specific CD8 + T cells infiltrating these tumors following treatment had increased LAG-3 expression.31 However, use of this cell line with checkpoint blockade, and our model using SSX2 DNA vaccination with PD-1 blockade alone, resulted in eradication of tumors in many animals. Hence, demonstrating a benefit with combined blockade was challenging in these models. Notwithstanding, we used the SSX2 sarcoma model specifically because our prior data demonstrated that altered vaccines could elicit CD8 + T cells with preferential expression of PD-1 or LAG-3, and hence might respond differently to vaccination with checkpoint blockade. It is conceivable that in these tumor models because the antigens targeted are not normal “self” proteins expressed in the host, the majority of antigen-specific CD8 + T cells were activated by professional APC and predominantly expressed PD-1. However, our data are consistent with a report that combined PD-1 and LAG-3 blockade was effective when used in combination with a viral vaccine targeting non-self antigens.32 Together, these data suggest that this combination might be more effective than vaccination with PD-1 blockade alone, particularly for tumors less responsive to PD-1 blockade.
In our tumor studies, while some tumors were eradicated, many were not. This was despite demonstrating activation of CD8 + T cells, the infiltration of tumors by CD8 + T cells and blocking one or more of the checkpoint inhibitory receptors. We have similarly found in patients with advanced prostate cancer, treated with vaccine and PD-1 blockade, that while some had evidence of disease response, this was often not durable.33 Certainly many additional mechanisms of tumor immune evasion are present, but the observation that blocking multiple checkpoint receptors following vaccine leads to increased anti-tumor response suggests that combination blockade should be further explored, both in the clinic and in further preclinical studies. Our findings in the MycCaP tumor model that CD11b+Gr-1+ MDSC were increased following treatment suggest that this could be an additional mechanism of resistance. Hence, our future studies will explore anti-tumor vaccines with other combinations of checkpoint blockade and/or therapies that reduce other immunosuppressive cells and pathways upregulated in the tumor microenvironment following anti-tumor vaccination.34
Supplementary Material
Acknowledgments
We are grateful for the critical review of the manuscript by Hemanth Potluri and Melissa Gamat-Huber, and for the assistance of the research staff of the UWCCC flow cytometry facility.
Funding Statement
This work was supported by NIH R01 CA219154 and P30 CA014520;NIH [R01 CA219154, P30 CA014520];
Authors’ contributions
CDZ, JEM, and LD conducted and analyzed laboratory studies described; CDZ and DGM designed the studies; DGM oversaw analysis; and all authors contributed to the writing and approval of the final manuscript.
Disclosure statement
Douglas G. McNeel has ownership interest, has received research support, and serves as consultant to Madison Vaccines, Inc. which has licensed intellectual property related to this content. None of the other authors have relevant potential conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Tang F, Du X, Liu M, Zheng P, Liu Y.. Anti-CTLA-4 antibodies in cancer immunotherapy: selective depletion of intratumoral regulatory T cells or checkpoint blockade? Cell Biosci. 2018;8:30. doi: 10.1186/s13578-018-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107(33):14733–10. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229(1):114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim J. M, Mellman I, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355(6332):1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, O’Day SJ, McDermott DF et al . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crosby EJ, Wei J, Yang XY, Lei G, Wang T, Liu CX, Agarwal P, Korman AJ, Morse MA, Gouin K, et al. Complimentary mechanisms of dual checkpoint blockade expand unique T-cell repertoires and activate adaptive anti-tumor immunity in triple-negative breast tumors. Oncoimmunology. 2018;7(5):e1421891. doi: 10.1080/2162402X.2017.1421891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiegle E, Doleschel D, Koletnik S, Rix A, Weiskirchen R, Borkham-Kamphorst E, Kiessling F, Lederle W. Dual CTLA-4 and PD-L1 blockade inhibits tumor growth and liver metastasis in a highly aggressive orthotopic mouse model of colon cancer. Neoplasia. 2019;21(9):932–944. doi: 10.1016/j.neo.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi LZ, Goswami S, Fu T, Guan B, Chen J, Xiong L, Zhang J, Ng Tang D, Zhang X, Vence L, et al. Blockade of CTLA-4 and PD-1 enhances adoptive T-cell therapy efficacy in an ICOS mediated manner. Cancer Immunol Res. 2019;7(11):1803–1812. doi: 10.1158/2326-6066.CIR-18-0873. [DOI] [PubMed] [Google Scholar]
- 13.Kos S, Lopes A, Preat V, Cemazar M, Lampreht Tratar U, Ucakar B, Vanvarenberg K, Sersa G, Vandermeulen G. Intradermal DNA vaccination combined with dual CTLA-4 and PD-1 blockade provides robust tumor immunity in murine melanoma. PLoS One. 2019;14(5):e0217762. doi: 10.1371/journal.pone.0217762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiGiulio S (2015) FDA approves Opdivo-yervoy combo for melanoma, first combo immunotherapy regimen for cancer. FDA Actions & Updates. https://journals.lww.com/oncology-times/blog/fdaactionsandupdates/pages/post.aspx?PostID=116. 2020
- 15.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthelemy P, Porta C, George S, et al. Nivolumab plus ipilimumab versus sunitinib in advanced Renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 blockade restores antitumor efficacy following SSX2 Epitope-modified DNA vaccine immunization. Cancer Immunol Res. 2015;3(8):946–955. doi: 10.1158/2326-6066.CIR-14-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zumwalde NA, Domae E, Mescher MF, Shimizu Y. ICAM-1–Dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation. The Journal of Immunology. 2013;191(7):3681–3693. doi: 10.4049/jimmunol.1201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colluru VT, Zahm CD, McNeel DG. Mini-intronic plasmid vaccination elicits tolerant LAG3(+) CD8(+) T cells and inferior antitumor responses. Oncoimmunology. 2016;5(10):e1223002. doi: 10.1080/2162402X.2016.1223002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahm CD, Colluru VT, McNeel DG. Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8(+) T cells. Cancer Immunol Res. 2017;5(8):630–641. doi: 10.1158/2326-6066.CIR-16-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith HA, McNeel DG. Vaccines targeting the cancer-testis antigen SSX-2 elicit HLA-A2 epitope-specific cytolytic T cells. J Immunother (Hagerstown, Md : 1997). 2011;34(8):569–580. doi: 10.1097/CJI.0b013e31822b5b1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith HA, Rekoske BT, McNeel DG. DNA vaccines encoding altered peptide ligands for SSX2 enhance epitope-specific CD8+ T-cell immune responses. Vaccine. 2014;32(15):1707–1715. doi: 10.1016/j.vaccine.2014.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith HA, McNeel DG. Vaccines targeting the cancer-testis antigen SSX-2 elicit HLA-A2 epitope-specific cytolytic T cells. J Immunother. 2011;34(8):569–580. doi: 10.1097/CJI.0b013e31822b5b1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson BM, Bradley ES, Sawicki T, Zhong W, Ranheim EA, Bloom JE, Colluru VT, Johnson LE, Rekoske BT, Eickhoff JC, et al. Safety and immunological efficacy of a DNA vaccine encoding the androgen receptor Ligand-binding domain (AR-LBD). Prostate. 2017;77(7):812–821. doi: 10.1002/pros.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pajot A, Michel ML, Fazilleau N, Pancre V, Auriault C, Ojcius DM, Lemonnier FA, Lone YC. A mouse model of human adaptive immune functions: HLA-A2.1-/HLA-DR1 -transgenic H-2 class I-/class II -knockout mice. Eur J Immunol. 2004;34(11):3060–3069. doi: 10.1002/eji.200425463. [DOI] [PubMed] [Google Scholar]
- 25.Kapadia D, Sadikovic A, Vanloubbeeck Y, Brockstedt D, Fong L. Interplay between CD8alpha+ dendritic cells and monocytes in response to Listeria monocytogenes infection attenuates T cell responses. PloS One. 2011;6(4):e19376. doi: 10.1371/journal.pone.0019376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colluru VT, McNeel DG. B lymphocytes as direct antigen-presenting cells for anti-tumor DNA vaccines. Oncotarget. 2016;7(42):67901–67918. doi: 10.18632/oncotarget.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlub TE, Badovinac VP, Sabel JT, Harty JT, Davenport MP. Predicting CD62L expression during the CD8(+) T-cell response in vivo. Immunol Cell Biol. 2010;88(2):157–164. doi: 10.1038/icb.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philippou Y, Sjoberg HT, Murphy E, Alyacoubi S, Jones KI, Gordon-Weeks AN, Phyu S, Parkes EE, Gillies McKenna W, Lamb AD, et al. Impacts of combining anti-PD-L1 immunotherapy and radiotherapy on the tumour immune microenvironment in a murine prostate cancer model. Br J Cancer. 2020;123(7):1089–1100. doi: 10.1038/s41416-020-0956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson BM, Johnson LE, McNeel DG. The androgen receptor: a biologically relevant vaccine target for the treatment of prostate cancer. Cancer Immunology, Immunotherapy: CII. 2013;62(3):585–596. doi: 10.1007/s00262-012-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson BM, Gamat M, Seliski J, Sawicki T, Jeffery J, Ellis L, Drake CG, Weichert J, McNeel DG. Prostate cancer cells express more Androgen Receptor (AR) following androgen deprivation, improving recognition by AR-specific T cells. Cancer Immunol Res. 2017;5(12):1074–1085. doi: 10.1158/2326-6066.CIR-16-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zahm CD, Colluru VT, McNeel DG. Vaccination with High-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8(+) T cells. Cancer Immunol Res. 2017;5(8):630–641. doi: 10.1158/2326-6066.CIR-16-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy S, Coulon PG, Prakash S, Srivastava R, Geertsema R, Dhanushkodi N, Lam C, Nguyen V, Gorospe E, Nguyen AM, et al. Blockade of PD-1 and LAG-3 immune checkpoints combined with vaccination restores the function of antiviral Tissue-resident CD8(+) TRM cells and reduces ocular herpes simplex infection and disease in HLA transgenic rabbits. J Virol. 2019;93. doi: 10.1128/JVI.00827-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNeel DG, Eickhoff JC, Wargowski E, Zahm C, Staab MJ, Straus J, Liu G. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2018;9(39):25586–25596. doi: 10.18632/oncotarget.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zahm CD, Johnson LE, McNeel DG. Increased indoleamine 2,3-dioxygenase activity and expression in prostate cancer following targeted immunotherapy. Cancer Immunol Immunother. 2019;68(10):1661–1669. doi: 10.1007/s00262-019-02394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


