Abstract
Background
Xpert MTB/RIF Ultra (Xpert Ultra) and Xpert MTB/RIF are World Health Organization (WHO)‐recommended rapid nucleic acid amplification tests (NAATs) widely used for simultaneous detection of Mycobacterium tuberculosis complex and rifampicin resistance in sputum. To extend our previous review on extrapulmonary tuberculosis (Kohli 2018), we performed this update to inform updated WHO policy (WHO Consolidated Guidelines (Module 3) 2020).
Objectives
To estimate diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF for extrapulmonary tuberculosis and rifampicin resistance in adults with presumptive extrapulmonary tuberculosis.
Search methods
Cochrane Infectious Diseases Group Specialized Register, MEDLINE, Embase, Science Citation Index, Web of Science, Latin American Caribbean Health Sciences Literature, Scopus, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform, the International Standard Randomized Controlled Trial Number Registry, and ProQuest, 2 August 2019 and 28 January 2020 (Xpert Ultra studies), without language restriction.
Selection criteria
Cross‐sectional and cohort studies using non‐respiratory specimens. Forms of extrapulmonary tuberculosis: tuberculous meningitis and pleural, lymph node, bone or joint, genitourinary, peritoneal, pericardial, disseminated tuberculosis. Reference standards were culture and a study‐defined composite reference standard (tuberculosis detection); phenotypic drug susceptibility testing and line probe assays (rifampicin resistance detection).
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias and applicability using QUADAS‐2. For tuberculosis detection, we performed separate analyses by specimen type and reference standard using the bivariate model to estimate pooled sensitivity and specificity with 95% credible intervals (CrIs). We applied a latent class meta‐analysis model to three forms of extrapulmonary tuberculosis. We assessed certainty of evidence using GRADE.
Main results
69 studies: 67 evaluated Xpert MTB/RIF and 11 evaluated Xpert Ultra, of which nine evaluated both tests. Most studies were conducted in China, India, South Africa, and Uganda. Overall, risk of bias was low for patient selection, index test, and flow and timing domains, and low (49%) or unclear (43%) for the reference standard domain. Applicability for the patient selection domain was unclear for most studies because we were unsure of the clinical settings.
Cerebrospinal fluid
Xpert Ultra (6 studies)
Xpert Ultra pooled sensitivity and specificity (95% CrI) against culture were 89.4% (79.1 to 95.6) (89 participants; low‐certainty evidence) and 91.2% (83.2 to 95.7) (386 participants; moderate‐certainty evidence). Of 1000 people where 100 have tuberculous meningitis, 168 would be Xpert Ultra‐positive: of these, 79 (47%) would not have tuberculosis (false‐positives) and 832 would be Xpert Ultra‐negative: of these, 11 (1%) would have tuberculosis (false‐negatives).
Xpert MTB/RIF (30 studies)
Xpert MTB/RIF pooled sensitivity and specificity against culture were 71.1% (62.8 to 79.1) (571 participants; moderate‐certainty evidence) and 96.9% (95.4 to 98.0) (2824 participants; high‐certainty evidence). Of 1000 people where 100 have tuberculous meningitis, 99 would be Xpert MTB/RIF‐positive: of these, 28 (28%) would not have tuberculosis; and 901 would be Xpert MTB/RIF‐negative: of these, 29 (3%) would have tuberculosis.
Pleural fluid
Xpert Ultra (4 studies)
Xpert Ultra pooled sensitivity and specificity against culture were 75.0% (58.0 to 86.4) (158 participants; very low‐certainty evidence) and 87.0% (63.1 to 97.9) (240 participants; very low‐certainty evidence). Of 1000 people where 100 have pleural tuberculosis, 192 would be Xpert Ultra‐positive: of these, 117 (61%) would not have tuberculosis; and 808 would be Xpert Ultra‐negative: of these, 25 (3%) would have tuberculosis.
Xpert MTB/RIF (25 studies)
Xpert MTB/RIF pooled sensitivity and specificity against culture were 49.5% (39.8 to 59.9) (644 participants; low‐certainty evidence) and 98.9% (97.6 to 99.7) (2421 participants; high‐certainty evidence). Of 1000 people where 100 have pleural tuberculosis, 60 would be Xpert MTB/RIF‐positive: of these, 10 (17%) would not have tuberculosis; and 940 would be Xpert MTB/RIF‐negative: of these, 50 (5%) would have tuberculosis.
Lymph node aspirate
Xpert Ultra (1 study)
Xpert Ultra sensitivity and specificity (95% confidence interval) against composite reference standard were 70% (51 to 85) (30 participants; very low‐certainty evidence) and 100% (92 to 100) (43 participants; low‐certainty evidence). Of 1000 people where 100 have lymph node tuberculosis, 70 would be Xpert Ultra‐positive and 0 (0%) would not have tuberculosis; 930 would be Xpert Ultra‐negative and 30 (3%) would have tuberculosis.
Xpert MTB/RIF (4 studies)
Xpert MTB/RIF pooled sensitivity and specificity against composite reference standard were 81.6% (61.9 to 93.3) (377 participants; low‐certainty evidence) and 96.4% (91.3 to 98.6) (302 participants; low‐certainty evidence). Of 1000 people where 100 have lymph node tuberculosis, 118 would be Xpert MTB/RIF‐positive and 37 (31%) would not have tuberculosis; 882 would be Xpert MTB/RIF‐negative and 19 (2%) would have tuberculosis.
In lymph node aspirate, Xpert MTB/RIF pooled specificity against culture was 86.2% (78.0 to 92.3), lower than that against a composite reference standard. Using the latent class model, Xpert MTB/RIF pooled specificity was 99.5% (99.1 to 99.7), similar to that observed with a composite reference standard.
Rifampicin resistance
Xpert Ultra (4 studies)
Xpert Ultra pooled sensitivity and specificity were 100.0% (95.1 to 100.0), (24 participants; low‐certainty evidence) and 100.0% (99.0 to 100.0) (105 participants; moderate‐certainty evidence). Of 1000 people where 100 have rifampicin resistance, 100 would be Xpert Ultra‐positive (resistant): of these, zero (0%) would not have rifampicin resistance; and 900 would be Xpert Ultra‐negative (susceptible): of these, zero (0%) would have rifampicin resistance.
Xpert MTB/RIF (19 studies)
Xpert MTB/RIF pooled sensitivity and specificity were 96.5% (91.9 to 98.8) (148 participants; high‐certainty evidence) and 99.1% (98.0 to 99.7) (822 participants; high‐certainty evidence). Of 1000 people where 100 have rifampicin resistance, 105 would be Xpert MTB/RIF‐positive (resistant): of these, 8 (8%) would not have rifampicin resistance; and 895 would be Xpert MTB/RIF‐negative (susceptible): of these, 3 (0.3%) would have rifampicin resistance.
Authors' conclusions
Xpert Ultra and Xpert MTB/RIF may be helpful in diagnosing extrapulmonary tuberculosis. Sensitivity varies across different extrapulmonary specimens: while for most specimens specificity is high, the tests rarely yield a positive result for people without tuberculosis. For tuberculous meningitis, Xpert Ultra had higher sensitivity and lower specificity than Xpert MTB/RIF against culture. Xpert Ultra and Xpert MTB/RIF had similar sensitivity and specificity for rifampicin resistance. Future research should acknowledge the concern associated with culture as a reference standard in paucibacillary specimens and consider ways to address this limitation.
Plain language summary
How accurate are tests (Xpert Ultra and Xpert MTB/RIF) for diagnosing tuberculosis outside the lungs (extrapulmonary tuberculosis) and rifampicin resistance?
Why is using Xpert tests for extrapulmonary tuberculosis important?
Tuberculosis is one of the top 10 causes of death worldwide. Tuberculosis mainly affects the lungs (pulmonary) but may occur in other parts of the body (extrapulmonary). When people receive proper and timely treatment, tuberculosis is usually curable. One problem involved in managing tuberculosis is that the bacteria become resistant to antibiotics. Not recognizing tuberculosis early may result in delayed diagnosis and treatment and increased illness and death. An incorrect tuberculosis diagnosis may result in increased anxiety and unnecessary treatment.
What is the aim of this review?
To update the evidence on accuracy of Xpert tests for diagnosing extrapulmonary tuberculosis and rifampicin resistance in adults. Rifampicin is an important tuberculosis drug. We included tuberculous meningitis and pleural, lymph node, bone or joint, genitourinary, peritoneal, pericardial, and disseminated tuberculosis.
What was studied in this review?
Xpert Ultra and Xpert MTB/RIF are rapid tests for simultaneously diagnosing tuberculosis and rifampicin resistance. We combined study results to determine: ‐ sensitivity: people with tuberculosis (rifampicin resistance) correctly diagnosed as having the condition. ‐ specificity: people without tuberculosis (rifampicin resistance) correctly identified as not having the condition. The closer sensitivity and specificity are to 100%, the better the test. We measured Xpert results against culture and a composite reference standard (neither is a perfect reference standard because extrapulmonary tuberculosis is paucibacillary (few bacteria)).
What are the main results in this review?
69 studies tested lymph node, pleural, and cerebrospinal fluid, and other specimens from people with presumptive extrapulmonary tuberculosis. Studies were conducted in 28 different countries.
For every 1000 people tested, if 100 had tuberculosis according to the reference standards:
cerebrospinal fluid
‐Xpert Ultra (6 studies):
· 89% sensitivity: 168 people would test positive, including 79 without tuberculosis
· 91% specificity: 832 people would test negative, including 11 with tuberculosis
‐ Xpert MTB/RIF (30 studies):
· 71% sensitivity: 99 people would test positive, including 28 without tuberculosis
· 97% specificity: 901 people would test negative, including 29 with tuberculosis
pleural fluid
‐ Xpert Ultra (4 studies):
· 75% sensitivity: 192 people would test positive, including 117 without tuberculosis
· 87% specificity: 808 people would test negative, including 25 with tuberculosis
‐ Xpert MTB/RIF (25 studies):
· 50% sensitivity: 60 people would test positive, including 10 without tuberculosis
· 99% specificity: 940 would test negative, including 50 with tuberculosis
lymph node fluid
‐ Xpert Ultra (1 study):
· 70% sensitivity: 70 people would test positive (all have tuberculosis)
· 100% specificity: 930 people would test negative, including 30 with tuberculosis
‐Xpert MTB/RIF (4 studies):
· 82% sensitivity:118 people would test positive, including 37 without tuberculosis
· 96% specificity: 882 people would test negative, including 19 with tuberculosis
rifampicin resistance
‐Xpert Ultra (4 studies):
· 100% sensitivity: 100 people would test positive (all have rifampicin resistance)
· 100% specificity: 900 people would test negative (none have rifampicin resistance)
‐ MTB/RIF test (19 studies):
· 97% sensitivity: 105 people would test positive, including eight without rifampicin resistance
· 99% specificity: 895 people would test negative, including three with rifampicin resistance
Who do the results of this review apply to?
People thought to have extrapulmonary tuberculosis.
How confident are we in our results?
Fairly confident for Xpert MTB/RIF in cerebrospinal fluid and less so in lymph node fluid. Less confident for Xpert Ultra, as there were few studies and few people tested. Both reference standards are imperfect, which may affect accuracy estimates.
What are the implications of this review?
The Xpert tests may be helpful in diagnosing extrapulmonary tuberculosis. Sensitivity varies across different extrapulmonary specimens, while for most specimens, specificity is high, the test rarely yielding a positive result for people without tuberculosis (verified by culture). For tuberculous meningitis, Xpert Ultra had higher sensitivity than Xpert MTB/RIF and lower specificity than Xpert MTB/RIF. The tests had similar accuracy for diagnosing rifampicin resistance.
How up‐to‐date is this review?
28 January 2020.
Summary of findings
Background
Tuberculosis (TB) causes tremendous suffering worldwide and has surpassed HIV/AIDS as the world’s leading infectious cause of death. The World Health Organization (WHO) estimates that globally in 2019, 10.0 million (range, 8.9 to 11.0 million) people fell ill with tuberculosis. In 2019, around 1.2 million HIV‐negative people died from tuberculosis and 208,000 HIV‐positive people died from tuberculosis (WHO Global TB Report 2020). When people receive proper treatment, tuberculosis is treatable and curable. The WHO estimates that from 2000 to 2019 more than 60 million lives were saved by diagnosing and treating tuberculosis. However, the COVID‐19 pandemic threatens the gains made over recent years. A modelling study by the WHO suggests that there could be between 200,000 and 400,000 additional tuberculosis deaths in 2020 if, over a period of three months, 25% to 50% fewer people were detected and treated with tuberculosis (WHO Global TB Report 2020).
Of the 7.1 million new cases of tuberculosis notified to the WHO in 2019, 16% were cases of extrapulmonary tuberculosis, (range, 8% in the WHO Western Pacific Region to 24% in the WHO Eastern Mediterranean Region) (WHO Global TB Report 2020). Among countries in the European Union, extrapulmonary tuberculosis was responsible for 19% of all notified cases (range, 6% to 44%) (Sandgren 2013). A large retrospective analysis from China found that of 19,279 hospitalised tuberculosis patients, around 33% had extrapulmonary tuberculosis (Pang 2019). The number of people affected by extrapulmonary tuberculosis is likely to be higher, given that, according to the WHO, extrapulmonary tuberculosis is notified as pulmonary tuberculosis when the two forms exist together, and diagnosing extrapulmonary tuberculosis is challenging, as described below. Additionally, extrapulmonary tuberculosis accounts for an increasing proportion of tuberculosis cases in some countries, in part because of host and genetic considerations, and the association of extrapulmonary tuberculosis and HIV (Golden 2005; Pai 2016; Perkins 2007; Webster 2014). Based on surveillance and epidemiological data, extrapulmonary tuberculosis affects a greater proportion of children than adults (Nelson 2004).
Drug‐resistant tuberculosis is a serious threat to global health. For the purpose of surveillance and treatment, drug‐resistant tuberculosis is classified as rifampicin‐resistant tuberculosis, multidrug‐resistant tuberculosis (MDR‐TB), and extensively drug‐resistant tuberculosis. MDR‐TB is defined as resistance to at least isoniazid and rifampicin, the two most important first‐line anti‐tuberculosis drugs. Extensively drug‐resistant tuberculosis is defined as MDR‐TB plus resistance to at least one drug in the following two classes of medicines used in treatment of MDR‐TB: fluoroquinolones and second‐line injectable agents. In 2019, there were approximately half a million new cases of rifampicin‐resistant tuberculosis (of which 78% had MDR‐TB), with India (27%), China (14%) and the Russian Federation (9%) accounting for the largest burden (WHO Global TB Report 2020). In 2019, 12,350 cases of extensively drug‐resistant tuberculosis were reported (WHO Global TB Report 2020).
In 2014, the World Health Assembly unanimously approved the WHO End TB Strategy, a 20‐year strategy devised to end the global tuberculosis epidemic (WHO END TB 2014). Early diagnosis of tuberculosis, including universal drug susceptibility testing (DST) and systematic screening of contacts and high‐risk groups, is a part of pillar one of the strategy.
Target condition being diagnosed
Extrapulmonary TB
Tuberculosis is caused by infection with Mycobacterium tuberculosis (M tuberculosis) bacteria. Tuberculosis predominantly affects the lungs (pulmonary tuberculosis). Extrapulmonary tuberculosis refers to tuberculosis in parts of the body other than the lungs. Extrapulmonary tuberculosis is known to affect virtually every part of the body, with lymph nodes and the pleura being the most common sites (Sharma 2004). Although active pulmonary tuberculosis is transmissible by droplets spread by coughing, extrapulmonary tuberculosis is thought to result from hematogenous spread (spread by way of the bloodstream) from an initial lung infection and is not infectious. Extrapulmonary tuberculosis can occur alone or together with pulmonary tuberculosis.
The various forms of extrapulmonary tuberculosis cause signs and symptoms related to the structures affected. Table 5 describes the forms of extrapulmonary tuberculosis included in this review, as well as the respective specimens that may be collected for diagnosis.
1. Forms of extrapulmonary TB.
| Form of extrapulmonary TB | Characteristics | Diagnostic specimens and means of collection |
| Tuberculous meningitis | Tuberculosis of the meninges affects people of all ages but is most common among children and people with untreated HIV infection. In adults, tuberculous meningitis presents with gradual onset of headache, neck stiffness, malaise, and fever, and if untreated can progress to altered sensorium, focal neurological deficits, coma, and death. Young children may present with poor weight gain, low‐grade fever, and listlessness. Infants may present with fever, cough (related to the primary pulmonary infection that occurs before tuberculous meningitis develops), change of consciousness at presentation, bulging anterior fontanel, and seizures (Thwaites 2013). Tuberculous meningitis is sometimes associated with a concurrent cerebral tuberculoma, or, more rarely, a tuberculous abscess | Cerebrospinal fluid, acquired by lumbar puncture with or without radiological guidance; biopsy of tuberculoma, acquired surgically |
| Pleural tuberculosis, also called TB pleurisy | TB infection of the pleura presents with gradual onset of pleuritic chest pain, shortness of breath, fever, night sweats, and weight loss. Chest X‐ray may demonstrate unilateral or occasionally bilateral pleural effusion. The severity of symptoms is highly variable, with many patients experiencing spontaneous resolution of symptoms, while others may develop severe pleural effusions requiring drainage. Pleuro‐pulmonary tuberculosis, in which parenchymal lung involvement is visible on a chest X‐ray, is associated with higher mortality than isolated pleural infection, which appears to be rarely fatal (Shu 2011) | Pleural fluid; pleural biopsy, which may be performed via thoracoscopy or percutaneously with Abram's needle, with or without ultrasound guidance |
| Lymph node tuberculosis, also called TB lymphadenitis | Tuberculosis of the lymph nodes may affect one node or a group of nodes, or multiple groups within a chain. Lymph node tuberculosis is relatively more common among children than adults. The most common presentation is of a single, firm, non‐tender enlarged node in the neck, although any lymph node group can be affected. This may be accompanied by fever, weight loss, and night sweats, particularly in people with HIV. Patients with tuberculosis in deep lymph nodes, such as the mediastinal or mesenteric lymph nodes, may present with fever, night sweats, and weight loss, or, more rarely, with symptoms related to compression of adjacent structures. Over time lymph nodes become fluctuant and may discharge via a sinus to the skin or an adjacent viscus. It should be noted that lymphadenopathy may also be seen in other forms of tuberculosis as part of the immune response, but this is not usually caused by direct infection of the lymph nodes | Fine‐needle aspiration of fluid from affected lymph node, with or without radiological guidance; surgical biopsy of superficial lymph nodes; endoscopic biopsy of deep lymph nodes with ultrasound guidance |
| Bone or joint tuberculosis | Tuberculosis of bones or joints or both causes chronic pain, deformity, and disability, and tuberculosis of the cervical spine can be life‐threatening. The usual presenting symptom is pain. Fever and weight loss, with or without signs of spinal cord compression, may be present. Patients with advanced disease may have severe pain, spinal deformity, paraspinal muscle wasting, and neurological deficit. Children may have failure to thrive and difficulty walking | Aspiration of joint fluid or periarticular abscesses; percutaneous computed tomography‐guided biopsy of lesions is preferred, but some patients may require open biopsy |
| Genitourinary tuberculosis | Tuberculosis of the genitourinary tract includes renal tuberculosis and tuberculosis of the reproductive system. Renal tuberculosis presents with flank pain, haematuria, and dysuria. Female genital tuberculosis presents with infertility (and may be otherwise asymptomatic), pelvic pain, and vaginal bleeding. Testicular tuberculosis presents with a scrotal mass and infertility | Urine; biopsy of affected organs, acquired under radiological guidance or surgically |
| Pericardial tuberculosis, also called TB pericarditis | Tuberculosis of the pericardium presents with fever, malaise, night sweats, and weight loss. Chest pain and shortness of breath are also commonly‐experienced symptoms. Pericardial tuberculosis may be associated with pericardial effusion, which can be severe and lead to life‐threatening tamponade. Some patients go on to develop pericardial constriction, which can lead to heart failure and death and may require surgical intervention even after mycobacterial cure | Pericardial fluid acquired by pericardiocentesis; pericardial biopsy, acquired under radiological guidance or surgically |
| Peritoneal tuberculosis | Tuberculosis of the peritoneum usually presents with pain and abdominal swelling, which may be accompanied by fever, weight loss, and anorexia | Ascitic fluid acquired by paracentesis; peritoneal biopsy (Chow 2002) |
| Disseminated tuberculosis, also called miliary tuberculosis. It has been proposed that the designation ‘miliary TB' be restricted to disseminated TB with miliary shadows on chest radiograph (Reuter 2009) | Disseminated tuberculosis involves two or more distinctly separate sites. Manifestations may be varied, ranging from acute fulminant disease to non‐specific symptoms of fever, weight loss, and weakness. HIV‐positive people are more likely to have disseminated tuberculosis than HIV‐negative people. In a systematic review of the prevalence of tuberculosis in post mortem evaluations of HIV‐positive people, among adults disseminated tuberculosis was found in 88% of tuberculosis cases and was considered the cause of death in 91% of TB cases (Gupta 2015) | Blood; specimens acquired from affected extrapulmonary sites |
Abbreviations: TB: tuberculosis.
We adapted the table from Sharma 2017b.
Diagnosis of extrapulmonary tuberculosis is challenging for several reasons. Many forms of extrapulmonary tuberculosis require invasive diagnostic sampling; gathering adequate specimens can pose risk of harm to the patient and can be costly. Most forms of extrapulmonary tuberculosis are paucibacillary (tuberculosis disease caused by a small number of bacteria), making diagnosis by various tests less sensitive. Culture, for example, has reduced sensitivity in paucibacillary disease. In addition, culture takes several weeks for results and requires a highly‐equipped laboratory. Limitations are also associated with histology, which relies on highly‐trained operators, and characteristic morphology is shared with other diseases. As a result of these difficulties, diagnosis of extrapulmonary tuberculosis is often made on the grounds of clinical suspicion alone, and many people receive the wrong diagnosis, leading to unnecessary tuberculosis treatment or poor outcomes from untreated extrapulmonary tuberculosis.
Tuberculosis treatment regimens must contain multiple drugs to which the organisms are sensitive to cure tuberculosis and avoid selection for drug resistance. WHO tuberculosis treatment guidelines recommend the same drug regimens for extrapulmonary and pulmonary disease, with notable mention of tuberculous meningitis and bone or joint tuberculosis, for which longer treatment regimens are recommended (WHO 2010; WHO 2017; WHO Compendium 2018). For patients with tuberculous meningitis or tuberculous pericarditis, the use of adjuvant corticosteroid therapy is recommended in addition to appropriate tuberculosis treatment regimens (WHO 2017; WHO Compendium 2018). Other tuberculosis treatment guidelines include Sharma 2017b (India), and those issued by the American Thoracic Society, the Centers for Disease Control and Prevention (CDC), and the Infectious Diseases Society of America (Nahid 2016). The drugs used to treat MDR‐TB are less potent and more toxic than the drugs used to treat drug‐susceptible tuberculosis, historically requiring two years or more of therapy. However, in December 2019, based on new evidence on the management of drug‐resistant tuberculosis, the WHO issued recommendations that all patients with MDR‐TB or rifampicin‐resistant tuberculosis, including those who are also resistant to fluoroquinolones, may benefit from effective all‐oral treatment regimens, either shorter or longer (WHO Consolidated Guidelines (Module 4) 2020).
Rifampicin resistance
Rifampicin inhibits bacterial DNA‐dependent RNA polymerase, encoded by the RNA polymerase gene (rpoB) (Hartmann 1967). Resistance to this drug has been associated mainly with mutations in a limited region of the rpoB gene (Telenti 1993). Rifampicin resistance may occur alone or in association with resistance to isoniazid and other drugs. In settings with a high burden of MDR‐TB, the presence of rifampicin resistance alone may serve as a proxy for MDR‐TB (WHO 2011). People with drug‐resistant tuberculosis can transmit the infection to others.
Index test(s)
The index tests, Xpert MTB/RIF and Ultra Xpert MTB/RIF (Xpert Ultra, the newest version) (Cepheid Inc, Sunnyvale, USA), are nucleic acid amplification tests (NAATs) (i.e. molecular tests) used for diagnosing tuberculosis and rifampicin‐resistant tuberculosis. Xpert MTB/RIF and Xpert Ultra cartridges are used with the GeneXpert system (Cepheid 2018; Cepheid 2019). Xpert MTB/RIF and Xpert Ultra are able to detect both M tuberculosis complex and rifampicin resistance within two hours after starting the test, with minimal hands‐on technical time. Unlike conventional NAATs, with Xpert MTB/RIF and Xpert Ultra, sample processing and polymerase chain reaction (PCR) amplification and detection are integrated into a single, self‐enclosed test unit, the GeneXpert cartridge. Following sample loading, all steps in the assay are completely automated and self‐contained. In addition, the assays' sample reagent, used to liquefy sputum, has potent tuberculocidal (the ability to kill tuberculosis bacteria) properties and so largely eliminates biosafety concerns during the test procedure (Banada 2010). Except as described below for Ultra trace call results, a single Xpert MTB/RIF or Xpert Ultra run will provide both detection of tuberculosis and detection of rifampicin resistance. One cannot deselect testing for rifampicin resistance and only run the assay for tuberculosis detection.
The development of Xpert MTB/RIF was a major step toward improving detection of tuberculosis and rifampicin resistance globally (Boehme 2010; Small 2011). Since Xpert MTB/RIF was released, there have been four generations (G1, G2, G3, and G4) of the test involving different software and cartridge combinations. Although in comparison with smear microscopy Xpert MTB/RIF has increased sensitivity for pulmonary tuberculosis (Steingart 2014), the test has suboptimal sensitivity in people with smear‐negative and HIV‐associated tuberculosis. A Cochrane Review on the diagnostic accuracy of Xpert MTB/RIF for pulmonary tuberculosis found pooled sensitivity and specificity (95% credible Interval (CrI)) of 85% (82% to 88%) and 98% (97% to 98%), (70 studies, 37,237 unselected participants; high‐certainty evidence) (Horne 2019). However, Xpert MTB/RIF sensitivity was decreased in people with smear‐negative culture‐positive disease, pooled sensitivity of 67% (62% to 72%), and people living with HIV, pooled sensitivity of 81% (75% to 86%) (Horne 2019). Xpert MTB/RIF versions have also had some limitations in detecting rifampicin resistance.
In order to overcome limitations with Xpert MTB/RIF, Cepheid developed Xpert Ultra, a re‐engineered assay that uses a newly‐developed cartridge but may be run on the same device after a software upgrade. To improve sensitivity for tuberculosis detection, Xpert Ultra incorporates two different multi‐copy amplification targets and a larger DNA reaction chamber than Xpert MTB/RIF (WHO Xpert Ultra 2017). A laboratory study reported that the limit of detection (the lowest number of colony‐forming units (CFUs) per sample that can be reproducibly distinguished from negative samples with 95% confidence) using Xpert Ultra improved to 15.6 CFU/mL of sputum compared to 112.6 CFU/mL for Xpert MTB/RIF (Chakravorty 2017). Xpert Ultra has added a new result category, ‘trace call', that corresponds to the lowest bacillary load for M tuberculosis detection (WHO Xpert Ultra 2017). This new category is reported as 'MTB trace DETECTED'. Interpreting a trace call result requires a reassessment of clinical symptoms and history of prior tuberculosis. No rifampicin resistance results are available (indeterminate) for people with trace results. As with Xpert MTB/RIF (Miotto 2012), Xpert Ultra detects both live and dead bacteria.
To address limitations in rifampicin resistance detection, Xpert Ultra uses melting temperature‐based analysis, in lieu of real‐time PCR analysis with Xpert MTB/RIF. Melting temperature‐based analysis allows Xpert Ultra to better distinguish resistance‐conferring mutations from silent mutations with improved diagnostic accuracy for rifampicin resistance detection (Global Laboratory Initiative 2017).
For sputum specimens, the test procedure may be used either directly on raw sputum specimens or sputum pellets created after decontaminating and concentrating the sputum (Blakemore 2010). In both cases, the test material is combined with the assay sample reagent (sodium hydroxide and isopropanol), mixed by hand or vortex, and incubated at room temperature for 15 minutes. After the incubation step, 2 mL of the treated specimen are transferred to the cartridge and the run is initiated (Helb 2010). According to the manufacturer, as with Xpert MTB/RIF, Xpert Ultra may be used with fresh sputum specimens, which may be either unprocessed sputum or processed sputum sediments. The sample reagent:sample volume ratio is 2:1 for unprocessed sputum and 3:1 for sputum pellets. The manufacturer does not specifically mention the use of the index tests with frozen specimens (Cepheid 2018; Cepheid 2019). As with Xpert MTB/RIF, Xpert Ultra using the GeneXpert sytem requires an uninterrupted and stable electrical power supply, temperature control, and yearly calibration of the cartridge modules (Global Laboratory Initiative 2019). Like previous Xpert cartridge generations, Xpert Ultra can be performed by operators with minimal technical expertise (Theron 2014a). The time to run the assay is shorter for Xpert Ultra (around 65 to 87 minutes) than for Xpert MTB/RIF (112 minutes) (Global Laboratory Initiative 2017). Currently, the manufacturer, Cepheid Incorporated (Sunnyvale, CA, USA), has made no claim for the use of Xpert Ultra and Xpert MTB/RIF in non‐sputum specimens (Cepheid 2019). However, there is a standard operating procedure provided by WHO for processing non‐sputum specimens (WHO 2014).
Clinical pathway
Xpert Ultra and Xpert MTB/RIF are used for the diagnosis of extrapulmonary tuberculosis and rifampicin resistance. Figure 1 shows the clinical pathway and presents the context in which Xpert Ultra or Xpert MTB/RIF might be used (WHO Operational Handbook Diagnosis (Module 3) 2020). The target conditions were extrapulmonary tuberculosis, which includes several forms (e.g. tuberculous meningitis, pleural tuberculosis) and rifampicin resistance.
1.
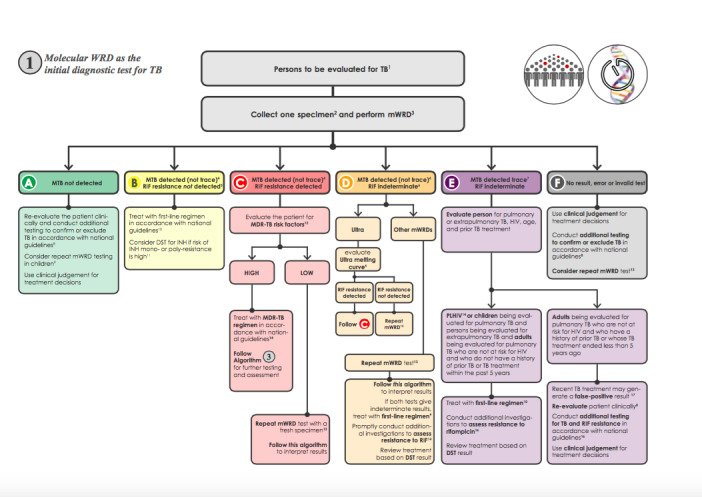
The clinical pathway describes how patients might present and the point in the pathway at which they would be considered for testing with Xpert Ultra or Xpert MTB/RIF. This algorithm for the use of a molecular WHO‐recommended rapid diagnostic (WRD), which includes Xpert Ultra and Xpert MTB/ RIF, comes from the WHO operational handbook on tuberculosis (WHO Operational Handbook Diagnosis (Module 3) 2020). Copyright © [2020] [World Health Organization]: reproduced with permission.
Abbreviations: DST: drug susceptibility testing; INH: isoniazid; MDR‐TB: multidrug‐resistant TB; MTB: Mycobacterium tuberculosis; PLHIV: people living with HIV; RIF: rifampicin; TB: tuberculosis; WRD: WHO‐recommended rapid diagnostic, which includes Xpert Ultra and Xpert MTB/RIF.
Before a specimen is tested, patients with presumptive extrapulmonary tuberculosis would have undergone a health examination (history and physical examination) and possibly a chest radiograph. The presentation of extrapulmonary tuberculosis varies depending on the body site affected, and it may imitate other diseases, such as cancer and bacterial and fungal infections. Signs and symptoms of extrapulmonary tuberculosis are often non‐specific and may include fever, night sweats, fatigue, loss of appetite, and weight loss (as seen in pulmonary tuberculosis) or specific complaints related to the involved site (e.g. headache for tuberculous meningitis, back pain for tuberculosis of the spine). The clinical presentation of extrapulmonary disease may be acute but is more often subacute (falling between acute and chronic) or chronic, meaning that patients may have symptoms for days to months before they seek care.
We have described in Table 5 signs and symptoms of the forms of extrapulmonary tuberculosis included in this review. The clinician should take a careful history, noting history of tuberculosis exposure, prior tuberculosis disease, and medical conditions that increase the risk for tuberculosis disease (e.g. HIV, diabetes mellitus, low body weight). In comparison with HIV‐negative people, HIV‐positive people have higher rates of extrapulmonary tuberculosis or mycobacteraemia (tuberculosis bloodstream infection). HIV‐positive patients with signs or symptoms of extrapulmonary tuberculosis should have specimens taken from the suspected site(s) of involvement to increase the likelihood of tuberculosis diagnosis. Tuberculous meningitis is the most severe form of tuberculosis. In tuberculous meningitis, diagnosis is often delayed, with appalling consequences for patients. For all forms of extrapulmonary tuberculosis, patients may be evaluated in primary‐ or secondary‐care settings. However, if more complex or invasive tests are needed, patients may be referred to a tertiary medical centre (Iseman 2000; Reuter 2009; Sharma 2004).
The downstream consequences of testing include the following.
True‐positive (TP): patients would benefit from rapid diagnosis and appropriate treatment.
True‐negative (TN): patients would be spared unnecessary treatment and would benefit from reassurance and pursuit of an alternative diagnosis.
False‐positive (FP): patients would likely experience anxiety and morbidity caused by additional testing, unnecessary treatment, and possible adverse effects; possible stigma associated with a tuberculosis or MDR‐TB diagnosis; and the chance that a false‐positive may halt further diagnostic evaluation.
False‐negative (FN): increased risk of morbidity and mortality and delayed treatment initiation for patients.
Role of index test(s)
We were interested in the following roles for testing.
I. Xpert Ultra and Xpert MTB/RIF for detection of extrapulmonary tuberculosis
Index test used as an initial test replacing usual practice (including conventional microscopy, culture or histopathology) for the diagnosis of extrapulmonary tuberculosis in adults with presumptive extrapulmonary tuberculosis (WHO Consolidated Guidelines (Module 3) 2020). An initial test does not mean that other tests will follow.
II. Xpert Ultra and Xpert MTB/RIF for detection of rifampicin resistance
Index test used as an initial test replacing culture and phenotypic DST for the diagnosis of rifampicin‐resistant tuberculosis in adults with presumptive extrapulmonary tuberculosis (WHO Consolidated Guidelines (Module 3) 2020).
As mentioned, in high MDR‐TB settings the presence of rifampicin resistance alone may serve as a proxy for MDR‐TB. Xpert Ultra and Xpert MTB/RIF do not eliminate the need for subsequent culture and phenotypic DST, which are required to monitor treatment progress and to detect resistance to drugs other than rifampicin.
Alternative test(s)
For a comprehensive review of new tests not yet in widespread use, we refer the reader to Branigan 2019; Lewinsohn 2017; Unitaid 2017.
Smear microscopy (light microscopy (Ziehl‐Neelsen), fluorescence microscopy, or light‐emitting diode (LED) fluorescence microscopy) is the examination of smears for acid‐fast bacilli (tuberculosis bacteria) under a microscope. Around 5000 to 10,000 organisms per mL must be present in the specimen for tuberculosis bacteria to be visible by microscopy (American Thoracic Society 2000). For extrapulmonary tuberculosis, microscopy can be performed in fluid or tissue specimens from sites of disease involvement, for example, in cerebrospinal fluid (CSF) in presumptive tuberculous meningitis or in lymph node tissue in presumptive lymph node tuberculosis. For most extrapulmonary sites, because there are usually few organisms, the sensitivity of smear microscopy is generally low. Ranges from studies, some with selected cases, are quoted here: 0% to 10% in pleural fluid; 14% to 39% in pleural tissue; 2% to 30% in CSF; < 5% in peritoneal fluid; and 0% to 42% in pericardial fluid. In contrast, the specificity of smear microscopy tends to be quite high, as can be seen in pulmonary tuberculosis (≥ 90%) (Kilpatrick 1986; Lewinsohn 2017).
Mycobacterial culture is a method used to grow bacteria on nutrient‐rich media. In comparison with microscopy, a positive culture requires only around 100 organisms per mL and therefore can detect lower numbers of tuberculosis bacteria (American Thoracic Society 2000). Additionally, culture is essential for species identification and DST. However, culture takes several weeks and requires a highly‐equipped laboratory. Culture has reduced sensitivity in paucibacillary disease (reference standards have included culture from a different specimen, such as sputum, smear microscopy, NAATs, presence of granulomatous inflammation, clinical criteria, imaging studies, and response to anti‐tuberculosis therapy, done alone or in various combinations): CSF 45% to 70%; pleural fluid 23% to 58%; urine 80% to 90%; peritoneal tuberculosis 45% to 69%; pericardial tuberculosis 50% to 65% (Lewinsohn 2017); lymph node tuberculosis (excisional biopsy) 18% to 93%; and lymph node tuberculosis (fine‐needle aspirate) 10% to 67% (Fontanilla 2011).
Histological examination involves examination of tissue specimens under a microscope. Diagnosis of extrapulmonary tuberculosis by histological examination is based on finding acid‐fast bacilli and granulomatous inflammation, frequently with caseous (cheese‐like) necrosis (necrotizing granulomas). The sensitivity of histology has been reported to vary for different forms of extrapulmonary tuberculosis (reference standards have included smear microscopy, culture, NAATs, clinical criteria, and imaging studies, done alone or in various combinations): 59% to 88% for lymph node tuberculosis (excisional biopsy) (Fontanilla 2011); 69% to 97% in pleural tissue (closed pleural biopsy); 86% to 94% in urological tissue; 60% to 70% in endometrial curettage; 79% to 100% in peritoneal biopsy; and 73% to 100% in pericardial tissue (Lewinsohn 2017). Sensitivity has also been observed to vary for different diagnostic techniques. Diacon 2003 found thoracoscopy to be more sensitive (sensitivity of 100%) than closed‐needle biopsy (sensitivity of 66%) for establishing a diagnosis of pleural tuberculosis (reference standards have included microscopy smear, culture, or presence of granulomatous inflammation with caseous necrosis). Specificity has been observed to be low because of the presence of granulomas in other diseases, both infectious and non‐infectious (Lewinsohn 2017), although the presence of ‘necrotizing' granulomatous inflammation increases specificity (Woodard 1982). Histological examination carries the additional concern that invasive procedures that are complex and costly may be required to obtain the necessary specimens (Golden 2005).
Cytopathological examination of fluid specimens (such as pleural and peritoneal fluid) may be performed, first to exclude cancer, and then to obtain material for additional analyses, such as measurement of levels of adenosine deaminase and free interferon‐gamma (IFN‐γ) and cell counts (Lewinsohn 2017; Wright 2009a). Advantages of these tests include that they are rapid and simple and can be performed in most clinical laboratories (Dinnes 2007). In pleural, pericardial, and peritoneal fluid, a predominance of lymphocytes, especially in the absence of mesothelial cells, is highly suggestive of tuberculosis (Wright 2009a). However, in HIV‐positive people, this pattern may not be observed (Wright 2009a). Adenosine deaminase, an enzyme involved in purine metabolism, has been extensively studied for its potential role in the diagnosis of pleural tuberculosis, peritoneal tuberculosis, and tuberculous meningitis (Lewinsohn 2017). IFN‐γ is released after it is sensitized by T cells in response to specific M tuberculosis antigens. A recent review of the evidence using GRADE provides the following recommendations.
"...cell counts and chemistries be performed on amenable fluid specimens (including include pleural, cerebrospinal, ascitic, and joint fluid) collected from sites of suspected extrapulmonary TB (conditional recommendation, very low‐quality evidence).
...adenosine deaminase levels be measured, rather than not measured, on fluid collected from patients with suspected pleural TB, TB meningitis, peritoneal TB, or pericardial TB (conditional recommendation, low‐quality evidence).
...free IFN‐γ levels be measured, rather than not measured, on fluid collected from patients with suspected pleural TB or peritoneal TB (conditional recommendation, low‐quality evidence)" (Lewinsohn 2017).
NAAT is a molecular technique that can detect small quantities of genetic material (DNA or RNA) from micro‐organisms, such as M tuberculosis. The key advantage of NAATs is that they are rapid diagnostic tests, potentially providing results in a few hours. This is a particularly important feature of the test in life‐threatening forms of extrapulmonary tuberculosis, such as tuberculous meningitis. A variety of molecular amplification methods are available, of which PCR is the most common. NAATs are available as commercial kits and in‐house tests (based on a protocol developed in a laboratory) and are used routinely in high‐income countries for tuberculosis detection. In‐house PCR is widely used in low‐income countries because these tests are less expensive than commercial kits. An older editorial summarizing three systematic reviews (140 studies) of commercial and in‐house NAATs (other than Xpert MTB/RIF) for different forms of extrapulmonary tuberculosis found relatively low sensitivity and underscored concerns about the cost and feasibility of this technology in resource‐limited areas (Pai 2008). Similarly, another systematic review found that NAATs have relatively low sensitivity for extrapulmonary tuberculosis but high specificity (e.g. for tuberculous meningitis, for pleural TB), indicating that these tests cannot be used reliably to rule out tuberculosis (Dinnes 2007). A recent evidence synthesis reported sensitivities of 72% to 88% in lymph node tissue, 28% to 81% in pleural fluid, 90% in pleural tissue, and 31% to 56% in CSF. Specificity ranged from 90% to 100% (Lewinsohn 2017).
Alternative molecular methods for DST include the commercial line‐probe assays, GenoType MTBDRplus assay (MTBDRplus, Hain LifeScience, Nehren, Germany), and the Nipro NTM+MDRTB detection kit 2 (Nipro, Tokyo, Japan), which detect the presence of mutations associated with drug resistance to isoniazid and rifampicin (Nathavitharana 2017). MTBDRplus is the most widely studied line‐probe assay. Advantages of line‐probe assays are that they can provide a result for detection of tuberculosis and drug resistance in one to two days. Drawbacks are that line‐probe assays are expensive and need to be used in intermediate and central laboratories (Unitaid 2017). The WHO recommends that for persons with a sputum smear‐positive specimen or a cultured tuberculosis isolate, commercial molecular line‐probe assays may be used as the initial test instead of phenotypic culture‐based DST to detect resistance to rifampicin and isoniazid (conditional recommendation, moderate certainty in the evidence for the test’s accuracy) (WHO Consolidated Guidelines (Module 3) 2020). Other molecular assays for detection of tuberculosis and resistance to rifampicin and isoniazid along with instruments are in development (Walzl 2018).
Alere Determine™ TB LAM Ag (AlereLAM) Alere Inc, (Waltham, USA) is a commercially‐available point‐of‐care test for tuberculosis disease (pulmonary and extrapulmonary tuberculosis). The test detects lipoarabinomannan (LAM), a component of the bacterial cell wall, which is present in the urine of some people with tuberculosis. AlereLAM is performed by placing urine on one end of a test strip, with results appearing as a band on the strip if tuberculosis is present. The test is simple, requires no special equipment, and shows results in 25 minutes. This urine test has potential advantages over sputum‐based testing due to ease of sample collection. The accuracy of urinary LAM detection is improved among people living with HIV with advanced immunosuppression (Bjerrum 2019). In two randomized trials, the use of Alere LAM in HIV‐positive adult inpatients was shown to reduce mortality (Gupta‐Wright 2018; Peter 2016). Based on evidence from the randomized trials and a Cochrane Review (Bjerrum 2019), the WHO currently recommends that AlereLAM should be used to assist in the diagnosis of active tuberculosis in HIV‐positive adults, adolescents, and children (WHO Consolidated Guidelines (Module 3) 2020). The key change from the WHO 2015 guidelines is broadening the indication for use of LF‐LAM among HIV‐positive inpatients with signs and symptoms of active tuberculosis (pulmonary and extrapulmonary); the test is now recommended for all such patients, irrespective of their CD4 count. The full recommendations, which differ for inpatients and outpatients, are described here (WHO Consolidated Guidelines (Module 3) 2020).
Fujifilm SILVAMP TB LAM (FuijiLAM, co‐developed by Foundation for Innovative New Diagnostics (FIND), Geneva, Switzerland and Fujifilm, Tokyo, Japan) is a new, urine‐based, point‐of‐care test for tuberculosis diagnosis in people living with HIV. In an individual participant data meta‐analysis that included five cohorts of people living with HIV, FujiLAM was found to have superior sensitivity, 70.7% (95% CI 59.0% to 80.8%), compared to AlereLAM sensitivity of 42.3% (31.7% to 51.8%), against a microbiological reference standard; FujiLAM had lower specificity, 90.9% (87.2 to 93.7), compared to AlereLAM specificity of 95.3% (92.2 to 97.7) (Broger 2020). At the time of writing, additional prospective clinical trials of FuijiLAM are ongoing to generate data for an updated WHO policy review.
Rationale
Xpert Ultra and Xpert MTB/RIF are rapid tests that may provide benefits for patients (earlier diagnosis and the opportunity to begin earlier, appropriate treatment), especially in high tuberculosis‐burden countries.
Since 2010, the WHO has recommended the use of Xpert MTB/RIF as the preferred initial diagnostic test for people thought to have MDR‐TB or HIV‐associated tuberculosis (strong recommendation, moderate‐certainty evidence) (WHO Xpert MTB/RIF Policy 2011). In 2013, the WHO expanded the recommendations, stating that Xpert MTB/RIF may be used rather than conventional microscopy and culture as the initial diagnostic test in all adults suspected of having tuberculosis (conditional recommendation acknowledging resource implications, high‐quality evidence) (WHO Xpert MTB/RIF Policy Update 2013). The 2013 recommendations extended to the diagnosis of several forms of extrapulmonary tuberculosis, including tuberculous meningitis and lymph nodes and other tissue. In addition, the WHO recommended that following an Xpert MTB/RIF test that demonstrates rifampicin resistance, subsequent DST (e.g. using a line‐probe assay to second‐line drugs) remains essential to detect resistance to drugs other than rifampicin (WHO Xpert MTB/RIF Policy Update 2013). In 2017, based on a non‐inferiority analysis of Xpert Ultra compared with Xpert MTB/RIF (Dorman 2018), the WHO stated that recommendations on the use of Xpert MTB/RIF also apply to the use of Xpert Ultra as the initial diagnostic test for all adults and children with signs and symptoms of tuberculosis (WHO Xpert Ultra 2017).
In December 2019, the WHO convened a Guideline Development Group to update the recommendations on the use of molecular assays intended as initial tests for the diagnosis of pulmonary and extrapulmonary tuberculosis and rifampicin resistance. To extend the work of our previous Cochane Review (Kohli 2018), we performed this review update to inform the WHO policy (WHO Consolidated Guidelines (Module 3) 2020).
The Background and Methods sections of this review include some text that overlaps with some of our other reviews for Xpert MTB/RIF Ultra and Xpert MTB/RIF for diagnosing tuberculosis (Horne 2019; Kay 2020; Shapiro 2020; Vonasek 2020).
Objectives
To estimate the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF for a) extrapulmonary tuberculosis by site of disease and b) rifampicin resistance, in adults with presumptive extrapulmonary tuberculosis. Presumptive tuberculosis refers to a patient who presents with symptoms or signs suggestive of tuberculosis.
Secondary objectives
To compare the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF for a) extrapulmonary tuberculosis by site of disease, and b) rifampicin resistance.
To investigate the effects of potential sources of heterogeneity on test accuracy across the included studies.
For potential sources of heterogeneity, for extrapulmonary tuberculosis, we included smear status, HIV status, and prevalence of extrapulmonary tuberculosis. For cerebrospinal fluid (CSF), we considered the presence of a concentration step and specimen volume.
For rifampicin resistance, we planned to assess the impact of the prevalence of rifampicin resistance on accuracy estimates, but we had insufficient data for this analysis.
Methods
Criteria for considering studies for this review
Types of studies
We included cross‐sectional and cohort studies. In addition, we had planned to include randomized controlled trials that evaluated the use of the index(s) test on patient health outcomes, but that also reported sensitivity and specificity. Although the study design was a randomized trial for the purpose of determining the impact of the test on participant outcomes, the study design was a cross‐sectional study for the purpose of determining the diagnostic accuracy of the index tests in this review. However, we did not identify any randomized controlled trials. We used abstracts to identify published studies and included these when they met the inclusion criteria. We only included studies that reported data comparing the index test(s) to an acceptable reference standard from which we could extract true‐positive (TP), true‐negative (TN), false‐positive (FP), and false‐negative (FN) values. We excluded case‐control studies and case reports.
Participants
We included studies where at least 85% of the participants enrolled were adults aged 15 years or older with presumptive extrapulmonary tuberculosis from all settings and countries. Restricting the age group to adults differs from the original review, where we also included children (Kohli 2018). We did this because children are now included in a separate Cochrane Review (Kay 2020). We excluded studies where we could not disaggregate data on adults from those in children and studies where we could not tell the age of the participants enrolled.
We included non‐respiratory specimens (such as CSF, pleural fluid, lymph node aspirate or tissue). We excluded sputum and other respiratory specimens, such as fluid obtained from bronchial alveolar lavage and tracheal aspiration. As we anticipated finding many studies, we set a bar to exclude smaller studies to reduce unnecessary work. We therefore required studies to provide data for at least five specimens for a given form of extrapulmonary tuberculosis included in the review. We excluded studies evaluating the use of Xpert Ultra and Xpert MTB/RIF to diagnose relapse of previously‐treated extrapulmonary tuberculosis, so as to avoid the selection bias that may arise by limiting to a group that is already at elevated risk of extrapulmonary tuberculosis. We attempted to identify studies that included participants who were not taking anti‐tuberculosis drugs or had taken anti‐tuberculosis drugs for less than seven days.
Index tests
The index tests were Xpert Ultra and Xpert MTB/RIF.
Index test results are automatically generated (i.e. there is a single threshold), and the user is provided with a printable test result as follows.
Xpert Ultra
MTB (M tuberculosis) DETECTED HIGH; RIF (rifampicin) Resistance DETECTED
MTB DETECTED MEDIUM; RIF Resistance DETECTED
MTB DETECTED LOW; RIF Resistance DETECTED
MTB DETECTED VERY LOW; RIF Resistance DETECTED
MTB DETECTED HIGH; RIF Resistance NOT DETECTED
MTB DETECTED MEDIUM; RIF Resistance NOT DETECTED
MTB DETECTED LOW; RIF Resistance NOT DETECTED
MTB DETECTED VERY LOW; RIF Resistance NOT DETECTED
MTB DETECTED HIGH; RIF Resistance INDETERMINATE
MTB DETECTED MEDIUM; RIF Resistance INDETERMINATE
MTB DETECTED LOW; RIF Resistance INDETERMINATE
MTB DETECTED VERY LOW; RIF Resistance INDETERMINATE
MTB Trace DETECTED; RIF Resistance INDETERMINATE
INVALID (the presence or absence of MTB cannot be determined)
ERROR (the presence or absence of MTB cannot be determined)
NO RESULT (the presence or absence of MTB cannot be determined)
Xpert Ultra incorporates a semi‐quantitative classification for results: trace, very low, low, moderate, and high. ‘Trace' corresponds to the lowest bacterial burden for detection of M tuberculosis (Chakravorty 2017). We considered a trace result to mean MTB (M tuberculosis) DETECTED. However, no rifampicin‐resistance result was available for participants with trace results because the trace sample is always reported as 'INDETERMINATE' for rifampin resistance (Cepheid 2018).
Xpert MTB/RIF
MTB (M tuberculosis) DETECTED; Rif (rifampicin) resistance DETECTED
MTB DETECTED; Rif resistance NOT DETECTED
MTB detected; Rif resistance INDETERMINATE
MTB NOT DETECTED
INVALID (the presence or absence of MTB cannot be determined)
ERROR (the presence or absence of MTB cannot be determined)
NO RESULT (the presence or absence of MTB cannot be determined)
Target conditions
The target conditions were extrapulmonary tuberculosis and rifampicin resistance. We included eight common forms of extrapulmonary tuberculosis and considered subcategories of the target condition as separate diagnostic classifications (CDC 2018; Sandgren 2013; Sharma 2004).
Tuberculous meningitis.
Pleural tuberculosis.
Lymph node tuberculosis.
Genitourinary tuberculosis.
Bone or joint tuberculosis.
Peritoneal tuberculosis.
Pericardial tuberculosis.
Disseminated tuberculosis.
Table 5 lists the forms of extrapulmonary tuberculosis and specimens used for diagnosis in the review. We excluded less common forms, such as cutaneous tuberculosis, ocular tuberculosis, female genital tuberculosis, and tuberculosis of the breast, ear, and paranasal sinuses (Sharma 2004).
Reference standards
Detection of extrapulmonary tuberculosis
We included two reference standards.
-
Solid or liquid mycobacterial culture.
‘Tuberculosis' was defined as a positive M tuberculosis culture
‘Not tuberculosis' was defined as a negative M tuberculosis culture
-
Composite reference standard.
'Tuberculosis' was defined as a positive M tuberculosis culture or positive composite reference test.
‘Not tuberculosis' was defined as a negative M tuberculosis culture and a negative composite reference test.
The composite reference standard might be based on the results of microbiological tests, culture or NAAT other than Xpert Ultra and Xpert MTB/RIF; imaging studies; histology; and clinical characteristics, and include at least one component test that is positive, according to the definition of the primary study authors.
For pleural tuberculosis, we defined the composite reference standard as the presence of granulomatous inflammation or a positive culture. We proposed this definition because we found evidence to support including histopathological examination in the definition. Around 60% of patients undergoing pleural biopsy will show granulomatous inflammation (American Thoracic Society 2000). In a prospective cohort study of participants with clinical and radiological findings consistent with pleural tuberculosis, Conde 2003 found that histological examination of tissue obtained from pleural biopsy had a higher diagnostic yield (78%; 66/84) than that of culture (62%; 52/84).
Culture is considered the best reference standard for tuberculosis. However, culture may lead to misclassification of some cases of extrapulmonary tuberculosis as ‘not tuberculosis', owing to the paucibacillary nature of the disease. This means that culture may have low sensitivity for extrapulmonary tuberculosis overall and further that culture sensitivity may differ for different forms of extrapulmonary tuberculosis (Lewinsohn 2017). This misclassification by culture may lead to biased estimates (overestimation or underestimation) of the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF. The extent of bias will depend on the frequency of errors by culture and the degree of correlation in errors by culture and the Xpert assays because culture and Xpert Ultra or Xpert MTB/RIF are likely to pick up cases with a higher bacterial load, and are likely to miss cases with a lower bacterial load. Ignoring this dependence could lead to an overestimation of the sensitivity of Xpert Ultra or Xpert MTB/RIF.
Effect of low sensitivity of culture on Xpert sensitivity: the low sensitivity of culture means that index test FNs may be misclassified as TNs when culture is used as the reference standard. Therefore, when Xpert Ultra or Xpert MTB/RIF is evaluated against culture, the number of FNs (classified as negative by the index test and positive by the reference test) may be decreased and the sensitivity of the index test may be overestimated.
Effect of low sensitivity of culture on Xpert specificity: the low sensitivity of culture means that index test TPs may be misclassified as FPs when culture is used as the reference standard. Therefore, when Xpert Ultra or Xpert MTB/RIF is evaluated against culture, the number of FPs (classified as positive by the index test and negative by the reference test) may be increased and specificity of the index test may be underestimated.
In contrast to culture, a composite reference standard that includes culture, other tests, and clinical characteristics may correctly classify index test results as TPs (instead of as FPs with respect to culture), especially in people with paucibacillary disease in whom culture may be negative. However, because of the uncertainties that surround a clinical diagnosis of tuberculosis and, in some instances, the conditional dependence of the index tests and other tests in the composite reference standard (for example, for most of these tests, detection of tuberculosis depends on bacillary load), a reference standard that uses additional tests and clinical characteristics (in culture‐negative people) may incorrectly classify people without tuberculosis as having tuberculosis (Naaktgeboren 2013). An additional challenge with including a composite reference standard is that the definition of the composite reference standard may vary across studies, making it difficult to interpret the accuracy estimates.
Thus both reference standards, culture and composite, are imperfect and may affect accuracy estimates. In an attempt to improve the estimation of diagnostic accuracy, we applied a latent class meta‐analysis model to the three most commonly studied forms of extrapulmonary tuberculosis. This approach provides the sensitivity and specificity of culture in addition to the accuracy of the index tests, thus adjusting for imperfect culture accuracy.
Detection of rifampicin resistance
The reference standard was culture‐based DST using solid or liquid media or line‐probe assays, as recommended by the WHO (WHO 2012; WHO Consolidated Guidelines (Module 3) 2020).
Search methods for identification of studies
We attempted to identify all relevant studies, regardless of language or publication status (published, unpublished, in press, or ongoing). We monitored abstracts to see if these studies were published during the time we performed the review. We included only published studies in the review.
Electronic searches
For the original review, we searched the literature on 7 August 2017. For this review update, we searched the literature on 2 August 2019 and again on 28 January 2020, specifically for studies of Xpert Ultra (studies could include Xpert Ultra alone or both Xpert Ultra and Xpert MTB/RIF), using the search terms and strategy described in Appendix 1. We searched the following databases:
Cochrane Infectious Diseases Group Specialized Register;
MEDLINE (OVID, from 1966);
Embase (OVID, from 1974);
Science Citation Index ‐ Expanded (from 1900);
Conference Proceedings Citation Index ‐ Science (CPCI‐S, from 1990);
BIOSIS Previews (from 1926), all three from the Web of Science;
Scopus (Elsevier, from 1970);
Latin American Caribbean Health Sciences Literature (LILACS) (BIREME, from 1982).
We also searched ClinicalTrials.gov, the WHO International Clinical Trials Registry (ICTRP) Platform (www.who.int/trialsearch), and the International Standard Randomized Controlled Trials Number (ISRCTN) registry (www.isrctn.com/) for trials in progress, and ProQuest Dissertations & Theses A&I (www.proquest.com/pqdtglobal, from 1990) for dissertations.
To identify other systematic reviews and meta‐analyses, we performed an additional search on 28 May 2020 in MEDLINE (PubMed), Embase (OVID), and the Cochrane Library, applying filters for systematic reviews (www.sign.ac.uk/search-filters.html) to search terms for Xpert and tuberculosis.
Searching other resources
We reviewed reference lists of included articles and any relevant review articles identified through the above methods. We also contacted researchers at FIND and other experts in the field of tuberculosis diagnostics for information on ongoing and unpublished studies.
Data collection and analysis
Selection of studies
We used Covidence to manage the selection of studies (Covidence 2017). Two review authors independently scrutinized titles and abstracts identified by electronic literature searching to identify potentially eligible studies. We selected any citation identified by either review author as potentially eligible for full‐text review. The same review authors independently assessed full‐text papers for study eligibility using predefined inclusion and exclusion criteria, and resolved any discrepancies by discussion. We recorded all studies excluded after full‐text assessment and their reasons for exclusion in Characteristics of excluded studies. We illustrated the study selection process in a PRISMA diagram (Moher 2009).
Data extraction and management
Using a previously‐developed form (Appendix 2), two review authors worked independently to extract data on the following characteristics.
Author; publication year; country; setting (outpatient, inpatient, or both outpatient and inpatient); study design; manner of participant selection; number of participants enrolled; number of participants for whom results are available.
Characteristics of participants: gender; age; HIV status; history of prior tuberculosis; receipt of anti‐tuberculosis treatment.
Index test.
Target condition and subcategories.
Type of reference standard.
Quality Assessment of Studies of Diagnostic Accuracy ‐ Revised (QUADAS‐2) items.
Details of specimen: type (such as CSF, pleural fluid, or lymph node aspirate or tissue); condition (fresh or frozen); smear‐positive or smear‐negative.
Specimen preparation; homogenization step (for tissue specimens); concentration step and specimen volume (for CSF); adherence to WHO standard operating procedures.
Number of TP, FP, FN, TN (i.e. true‐positives, false‐positives, false‐negatives, and true‐negatives), and trace results; number of inconclusive results for detection of extrapulmonary tuberculosis; number of indeterminate results for detection of rifampicin resistance.
Number of missing or unavailable test results.
We classified country income status as either low‐ and middle‐income or high‐income, according to the World Bank List of Economies (World Bank 2020).
We extracted TP, FP, FN, and TN values for the following specimens: CSF, pleural fluid and tissue, lymph node aspirate and tissue (the latter specimen acquired by surgical biopsy), bone or joint aspirate and tissue, urine, peritoneal fluid and tissue, pericardial fluid and tissue, and blood. We extracted these values for each of the specimen types separately. For example, we used one 2 × 2 table for lymph node aspirate, and another 2 × 2 table for lymph node tissue. In situations in which a participant contributed more than one specimen but of different types, we extracted data for all specimens. When a study included data for both raw specimens and concentrated sediment involving the same participants, we preferentially extracted data for raw specimens, except in the case of CSF, for which we extracted data for concentrated sediment as recommended by the WHO (WHO 2014). We extracted accuracy data according to the defined reference standards (see Reference standards). We did not encounter any situations in which a subset of participants in a study received the reference standard but others did not. Hence, there was no need to make corrections for verification bias in the statistical analysis (Begg 1983).
In most studies, the number of specimens was the same as the number of participants. However, in some studies, the number of specimens exceeded the number of participants or study authors reported only the number of specimens. In the previous review (Kohli 2018), we added post hoc a sensitivity analysis limiting inclusion to studies that used one specimen per participant. In this review, we performed a similar sensitivity analysis for Xpert Ultra.
We contacted authors of primary studies for missing data or clarifications. We entered all data into Microsoft Excel 2014.
Assessment of methodological quality
We used the QUADAS‐2 tool, tailored to this review, to assess the quality of the included studies (Appendix 3) (Whiting 2011). QUADAS‐2 consists of four domains: patient selection, index test, reference standard, and flow and timing. We assessed all domains for risk of bias and the first three domains for concerns about applicability. Two review authors independently completed QUADAS‐2 and resolved disagreements through discussion. We present the results of this quality assessment in text, tables, and graphs.
We followed Cochrane policy, which states that "authors of primary studies will not extract data from their own study or studies. Instead, another author will extract these data, and check the interpretation against the study report and any available study registration details or protocol".
Statistical analysis and data synthesis
We performed descriptive analyses of the characteristics of included studies using Stata 15 (Stata 2017), and we present key study characteristics in the Characteristics of included studies table. We used data reported in the TP, FP, FN, and TN format to calculate sensitivity and specificity estimates and 95% confidence intervals (CIs) for individual studies. We present individual study results graphically by plotting the estimates of sensitivity and specificity (and their 95% CIs) in forest plots and receiver operating characteristic (ROC) space using Review Manager 5 (RevMan 5) (RevMan 2014).
When data were sufficient, we performed meta‐analyses to estimate pooled sensitivity and specificity and corresponding 95% credible interval (CrI, defined below) using an adaptation of the bivariate random‐effects approach of Reitsma 2005, which uses the exact binomial likelihood for the observed proportions (Chu 2006). The bivariate random‐effects approach allowed us to calculate the pooled estimates of sensitivity and specificity while dealing with potential sources of variation caused by (1) imprecision of sensitivity and specificity estimates within individual studies; (2) correlation between sensitivity and specificity across studies; and (3) variation in sensitivity and specificity between studies. The model has a hierarchical structure, with the logit sensitivity in individual studies assumed to come from a common probability distribution the mean of which is the pooled logit sensitivity, and the standard deviation is the between‐study standard deviation, and likewise for the specificity. This structure allows for borrowing strength across studies. In the absence of sufficient studies, we simply present descriptive statistics. In addition, we determined predictive values at a pretest probability of 10%, a value suggested by the WHO.
We performed separate analyses grouped by type of extrapulmonary specimen (e.g. CSF, pleural fluid, peritoneal fluid) rather than determine summary accuracy estimates for all forms of extrapulmonary tuberculosis combined, because we considered the former approach to be most clinically meaningful. In addition, we performed separate analyses by reference standard.
Comparison of Xpert Ultra and Xpert MTB/RIF
We performed comparative meta‐analyses by restricting the analyses to only those studies that made direct comparisons between Xpert Ultra and Xpert MTB/RIF within the same participants (Takwoingi 2013). We extracted the median and the 95% CrI for the difference in the pooled sensitivities and the difference in the pooled specificities, respectively, of Xpert Ultra versus Xpert MTB/RIF. We also calculated the probability that the difference exceeds zero in each case.
For analysis of Xpert MTB/RIF or Xpert Ultra accuracy for detection of rifampicin resistance, we include participants who (1) were culture‐positive; (2) had a valid culture‐based DST or line‐probe assay (LPA) result; (3) were Xpert MTB/RIF or Xpert Ultra tuberculosis‐positive; and (4) had a valid Xpert MTB/RIF or Xpert Ultra result for rifampicin resistance, detected or not detected (susceptible).
Sensitivity = Xpert MTB/RIF (or Xpert Ultra) rifampicin resistance detected/phenotypic DST or LPA rifampicin‐resistant.
Specificity = Xpert MTB/RIF (or Xpert Ultra) rifampicin resistance not detected/phenotypic DST or LPA rifampicin‐susceptible.
For detection of rifampicin resistance, when a study included multiple types of specimens, we based our determination of Xpert Ultra and Xpert MTB/RIF and sensitivity and specificity on all available data in the study, including data for specimens that we did not include in the primary analyses for detection of extrapulmonary tuberculosis. For example, if a study provided data for several specimen types combined (e.g. all tissue specimens) and we could not disaggregate the data for a specific specimen type, we included all data (for all tissue specimens) in the analysis for rifampicin resistance detection. We did this because we did not expect the accuracy of Xpert Ultra or Xpert MTB/RIF for rifampicin resistance to vary by specimen type. We used the bivariate random‐effects model to estimate pooled sensitivity and specificity.
We estimated all models using a Bayesian approach with low‐information prior distributions using OpenBUGS software (Version 3.2.3) (Lunn 2009), along with R (R Core Team 2019). Under the Bayesian approach, all unknown parameters must be provided a prior distribution that defines the range of possible values of the parameter and the weight of each of those values, based on information external to the data. To allow observed data to dominate the final results, we chose to use low‐information prior distributions. We defined prior distributions on the log‐odds scale over the pooled sensitivity and specificity parameters, their corresponding between‐study standard deviations, and the correlation between the sensitivities and specificities across studies. For the pooled log odds of the sensitivity or the pooled log odds of the specificity, we used a normal prior distribution with mean 0 and a wide variance of 4 (or a precision of 0.25). This corresponds to a roughly uniform distribution over the pooled sensitivity and pooled specificity on the probability scale. For the between‐study precision, we used a gamma distribution with a shape parameter of 2 and a rate parameter of 0.5. This corresponds to a 95% prior CrI for the between‐study standard deviation in the log odds of sensitivity or the log odds of specificity ranging from roughly 0.29 to 1.44, corresponding to moderate to high values of between‐study heterogeneity. Covariance terms followed a uniform prior distribution whose upper and lower limits were determined by the sensitivity of the two tests. The OpenBUGS model used appears in Appendix 4. It is known that meta‐analysis models can be sensitive to the choice of prior distributions over between‐study standard deviation parameters. We therefore carried out sensitivity analyses and considered alternative prior distributions that are less informative, allowing a wider range of possible values. To study the sensitivity of all results to the choice of prior distributions given above, we considered alternative prior distributions that were less informative, allowing a wider range of possible values. We increased the variance of the normal distributions over the pooled log odds of sensitivity or specificity to 100. We used a uniform prior distribution ranging from 0 to 3 over the between‐study standard deviation on the log odds scale (see programme in Appendix 4). We noted no appreciable change in pooled accuracy parameters but found that the posterior CrIs and prediction intervals were slightly wider, as expected.
We combined information from the prior distribution with the likelihood of the observed data, in accordance with Bayes’ theorem, using the OpenBUGS programme, which provides a sample from the posterior distribution of each unknown parameter. We were particularly interested in the pooled sensitivity and specificity of Xpert and between‐study variance in the sensitivity and specificity of Xpert on the log‐odds scale. Using a sample from the posterior distribution, we calculated various descriptive statistics of interest. We estimated the median pooled sensitivity and specificity and their 95% CrI. The median or the 50% quantile is the value below which 50% of the posterior sample lies. We report the median because the posterior distributions of some parameters may be skewed and the median would be considered a better point estimate of the unknown parameter than the mean in such cases. The 95% CrI is the Bayesian equivalent of the classical (frequentist) 95% confidence interval (CI) (we will indicate 95% CI for individual study estimates and 95% CrI for pooled study estimates as appropriate). The 95% CrI may be interpreted as an interval that has a 95% probability of capturing the true value of the unknown parameter, given observed data and prior information. We prepared summary receiver operating characteristic (SROC) curves for each meta‐analysis model, using the methods described in Harbord 2007.
We also determined the predicted sensitivity and specificity of Xpert MTB RIF and Xpert Ultra and their 95% CrIs. Predicted values represent our best guess for sensitivity and specificity in a future study and will be close to the pooled estimates. However, their CrIs may be different. If there is no heterogeneity at all between studies, the CrI around the predicted estimate will be the same as the CrI around the pooled estimate. On the other hand, if considerable heterogeneity is observed between studies, the CrI around the predicted estimate will be much wider than the CI around the pooled estimate.
In addition, we performed latent class analysis for three forms of extrapulmonary tuberculosis: tuberculous meningitis, pleural tuberculosis, and lymph node tuberculosis, using data from the two‐by‐two tables comparing the index test to culture as a reference standard. Latent class analysis is a statistical modelling technique that allows estimation of test accuracy in the absence of an adequate reference standard to define the presence or absence of disease (Van Smeden 2014). The latent class meta‐analysis model expands the traditional meta‐analysis model in two ways: (1) we added parameters for the sensitivity and specificity of culture; and (2) we added covariance terms to adjust for the dependence between the index test and culture among disease‐positive and disease‐negative participants in each study. We used hierarchical prior distributions over the logit sensitivity and logit specificity of culture. In other words, we assumed that the logit sensitivities in the individual studies come from a common probability distribution whose mean is the pooled mean logit sensitivity of culture and whose standard deviation is the between‐study standard deviation. Likewise for the specificities. We used the same low‐information prior distributions over the pooled logit mean and between‐study standard deviation parameters as we had for the corresponding parameters for the index test. We used uniform prior distributions for covariance terms over their ranges, which are determined by the sensitivities and the specificities of the two tests in each study (see Appendix 4 for the OpenBUGS model). We found that we did not need to augment observed data with prior information from other sources for most models. However, in a post hoc analysis Xpert MTB/RIF in lymph node aspirate in which we suspected a systematic bias in the performance of culture, we used informative prior distributions over the specificity of culture (ranging from 99% to 100%) and the specificity of Xpert MTB/RIF (ranging from 98% to 100%) (see Appendix 4). We added the SROC plots of the latent class meta‐analyses to the SROC plots resulting from the models in which culture was treated as a perfect test, so they could be compared.
Based on work evaluating Xpert MTB/RIF for childhood tuberculosis (Schumacher 2016), we anticipated that latent class meta‐analyses would lead to a decrease in the estimated pooled sensitivity of Xpert Ultra and Xpert MTB/RIF and an increase in the estimated pooled specificity of Xpert Ultra and Xpert MTB/RIF compared with the primary analyses. In other words, this method should help to correct the biases in Xpert Ultra and Xpert MTB/RIF sensitivity and specificity resulting from treating culture as a perfect reference standard, which we detailed earlier in the section on the reference standard.
Approach to inconclusive index test results
The proportion of inconclusive (non‐determinate) rate for detection of pulmonary tuberculosis is the number of tests classified as ‘invalid', ‘error', or ‘no result' divided by the total number of index tests performed. The proportion of inconclusive (indeterminate) rate for detection of rifampicin resistance is the number of tests classified as ‘MTB DETECTED; Rif (rifampicin) resistance INDETERMINATE' divided by the total number of index test‐positive results. For Xpert Ultra, we determined the proportion of inconclusive index test results = number of inconclusive test results divided by the total number of tests. In our previous review, we used a Bayesian hierarchical model for a single proportion to estimate the pooled proportion of inconclusive MTB/RIF test results (Kohli 2018). We reported these findings again in this review update. As we found very few inconclusive results reported, we excluded these results from the quantitative analysis.
Investigations of heterogeneity
Initially, we investigated heterogeneity through visual examination of forest plots of sensitivities and specificities and through visual examination of the ROC space of the raw data. When data allowed, we evaluated potential sources of heterogeneity using subgroup analyses and bivariate meta‐regression. We included the following covariates.
HIV status.
For tuberculous meningitis, concentration step used for preparing specimen (yes or no).
CSF specimen volume used for Xpert MTB/RIF or Xpert Ultra testing.
We had planned to investigate smear status, history of tuberculosis, and whether WHO standard procedures for preparing tissue specimens were followed. However, we had insufficient data to do this.
The impact of the prevalence of extrapulmonary tuberculosis on sensitivity and specificity is an important consideration. In a post hoc meta‐regression analysis, for Xpert MTB/RIF we explored this question for CSF, pleural fluid, and lymph node aspirate. For Xpert Ultra we explored this question for CSF. We did not conduct other analyses, owing to an insufficient number of studies. For detection of rifampicin resistance, owing to a small number of studies, we could not assess the impact of prevalence of rifampicin resistance on accuracy estimates.
Nontuberculous mycobacteria
Nontuberculous mycobacteria (NTM), such as M avium complex and M intracellulare, constitute a multi‐species group of human pathogens that are ubiquitous in water and soil. NTM can cause severe diseases that share clinical signs with tuberculosis but are treated differently. People living with HIV with severe immunosuppression are particularly vulnerable to infections caused by NTM (Gopinath 2010). Previous studies have shown that Xpert does not cross‐react with other mycobacterial species (Blakemore 2010; Helb 2010). In our original review, we summarized data for NTM separately by determining the percentage of false‐positive Xpert MTB/RIF results in specimens that grew NTMs (Kohli 2018). In this updated review, we therefore summarize data for NTM only for Xpert Ultra.
Sensitivity analyses
For Xpert Ultra testing in CSF, we performed sensitivity analyses to explore whether the overall findings were robust to potentially influential decisions. We did this by limiting inclusion in the meta‐analysis to the following.
Studies that used consecutive or random selection of participants.
Studies in which the reference standard results were interpreted without knowledge of the index test results.
Studies that included only one specimen per participant.
For Xpert Ultra, in CSF, we also planned to perform a sensitivity analysis by limiting studies to those that included only untreated participants. However, we were unable to confirm that studies met this criterion. We planned similar sensitivity analyses for pleural fluid and lymph node aspirate, but these analyses were not carried out owing to an insufficient number of studies. For all other specimen types, we had an insufficient number of studies for sensitivity analyses.
For Xpert MTB/RIF, in the original review we performed sensitivity analyses by type of extrapulmonary specimen and found that for most analyses, the sensitivity analyses made little difference to any of these findings (Kohli 2018). However, for Xpert MTB/RIF in CSF, in comparison with all studies, (sensitivity of 71.1% (60.9 to 80.4), and specificity of 98.0% (97.0 to 98.8)), studies that evaluated only one specimen per participant had lower pooled sensitivity at 63.5% (47.6 to 76.3) and lower pooled specificity at 96.1% (94.2 to 97.4).
Assessment of reporting bias
We did not perform a formal assessment of publication bias using methods such as funnel plots or regression tests because such techniques have not been helpful for diagnostic test accuracy studies (Macaskill 2010).
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of evidence using the GRADE approach for diagnostic studies (Balshem 2011; GRADEpro GDT 2015; Schünemann 2008; Schünemann 2016). As recommended, we rated the certainty of evidence as either high (not downgraded), moderate (downgraded by one level), low (downgraded by two levels), or very low (downgraded by more than two levels) based on five domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias. For each outcome, the certainty of evidence started as high when there high‐quality studies (cross‐sectional or cohort studies) that enrolled participants with diagnostic uncertainty. If we found a reason for downgrading, we used our judgement to classify the reason as either serious (downgraded by one level) or very serious (downgraded by two levels).Two review authors discussed judgments and applied GRADE in the following way (Schünemann 2020a; Schünemann 2020b).
Assessment of risk of bias. We used QUADAS‐2 to assess risk of bias.
Indirectness. We assessed indirectness in relation to the population (including disease spectrum), setting, interventions, and outcomes (accuracy measures). We also used prevalence as a guide to whether there was indirectness in the population.
Inconsistency. GRADE recommends downgrading for unexplained inconsistency in sensitivity and specificity estimates. We carried out prespecified analyses to investigate potential sources of heterogeneity and downgraded when we could not explain inconsistency in the accuracy estimates.
Imprecision. We considered a precise estimate to be one that would allow a clinically meaningful decision. We considered the width of the CrI (or CI) and asked, “Would we make a different decision if the lower or upper boundary of the CrI (or CI) represented the truth?” In addition, we worked out projected ranges for TP, FN, TN, and FP for a given prevalence of tuberculosis and made judgements on imprecision from these calculations.
Publication bias. We rated publication bias as undetected (not serious) for several reasons: the comprehensiveness of the literature search and extensive outreach to tuberculosis researchers to identify studies; the presence only of studies that produced precise estimates of high accuracy despite small sample size; and our knowledge about studies that were conducted but not published.
For the 'Summary of findings' tables for CSF and pleural fluid, we provide evidence using culture as the reference standard, which is considered the best reference standard for tuberculosis (Lewinsohn 2017). For lymph node aspirate, we provide evidence using a composite reference because, based on findings from the original review (Kohli 2018), we believe a composite reference standard is preferable for estimating accuracy.
Results
Results of the search
We identified and screened a total of 735 records for inclusion in this review update. Of these, we assessed 142 full‐text papers against our inclusion criteria. We excluded 120 papers, mainly for the following reasons: study did not evaluate Xpert Ultra (n = 54); could not extract 2 x 2 values (n = 14); inappropriate reference standard (n = 13); could not extract data by specimen type (n = 12); did not include extrapulmonary specimen (n = 10): duplicate data (n = 4); case‐control study (n = 4); index test other than Xpert MTB/RIF or Xpert Ultra (n = 3); study included children (n = 2); screening study (n = 2); case report (n = 1); and fewer than five specimens for a given specimen type (n = 1). From our previous review, we included 47 studies. Thus, we included 69 unique studies that met the inclusion criteria in this review update.
Sixty‐seven studies evaluated Xpert MTB/RIF (Ablanedo‐Terrazas 2014; Ajbani 2018; Al‐Ateah 2012; Azevedo 2018; Bahr 2015; Bahr 2017; Bera 2015; Biadglegne 2014; Blaich 2014; Causse 2011; Che 2017; Chen 2019; Chin 2019; Christopher 2013; Cresswell 2018; Cresswell 2020; Dhasmana 2014; Dhooria 2016; Donovan 2020; Du 2015; El‐Din 2019; Feasey 2013; Friedrich 2011; Ghariani 2015; Gu 2015; Hanif 2011; Heemskerk 2018; Hillemann 2011; Iram 2015; Kim 2015a; Li 2017; Liang 2019; Ligthelm 2011; Lusiba 2014; Malbruny 2011; Meldau 2014; Meldau 2019; Metcalf 2018; Nataraj 2016; Nhu 2014; Ozkutuk 2014; Pandie 2014; Patel 2013; Peñata 2016; Rakotoarivelo 2018; Rufai 2015; Rufai 2017a; Rufai 2017b; Safianowska 2012; Sarfaraz 2018; Scott 2014; Sharma 2014; Sharma 2016; Sharma 2018; Siddiqi 2019; Sun 2019; Suzana 2016; Tadesse 2015; Trajman 2014; Ullah 2017; Vadwai 2011; Van Rie 2013; Wang 2019; Wang 2020; Wu 2019; Zeka 2011; Zmak 2013). Eleven studies evaluated Xpert Ultra. Of these 11 studies, nine evaluated both Xpert MTB/RIF and Xpert Ultra (Bahr 2017; Chin 2019; Cresswell 2020; Donovan 2020; Meldau 2019; Sun 2019; Wang 2019; Wang 2020; Wu 2019) and two studies evaluated Xpert Ultra alone (Antel 2020; Perez‐Risco 2018). All studies but two (one in Spanish: Peñata 2016, and one in Turkish: Ozkutuk 2014), were written in English. Figure 2 shows the flow of studies in the review. We recorded the excluded studies, including those listed in the previous Cochrane Review (Kohli 2018) and the reasons for their exclusion in the Characteristics of excluded studies table.
2.
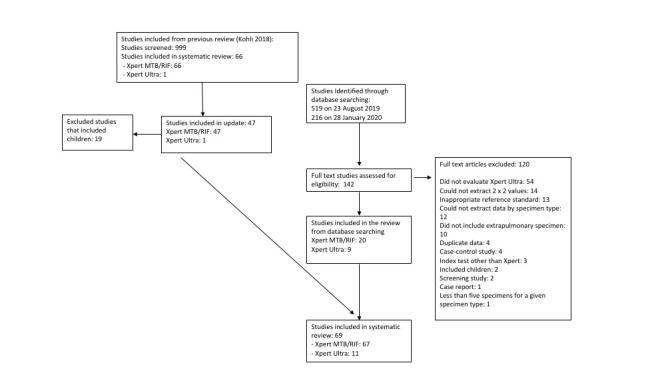
Methodological quality of included studies
Studies evaluating Xpert MTB/RIF and Xpert Ultra for detection of extrapulmonary tuberculosis
Figure 3 and Figure 4 show risk of bias and applicability concerns for each of the 69 studies included for tuberculosis detection. Risk of bias and applicability concerns are also presented specifically for studies evaluating Xpert Ultra and Xpert MTB/RIF for tuberculous meningitis (Appendix 5), pleural tuberculosis (Appendix 6), and lymph node tuberculosis (Appendix 7).
3.
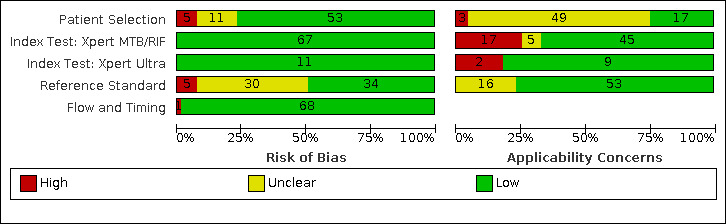
Risk of bias and applicability concerns graph for tuberculosis detection: review authors' judgements about each domain presented as percentages across included studies.
4.
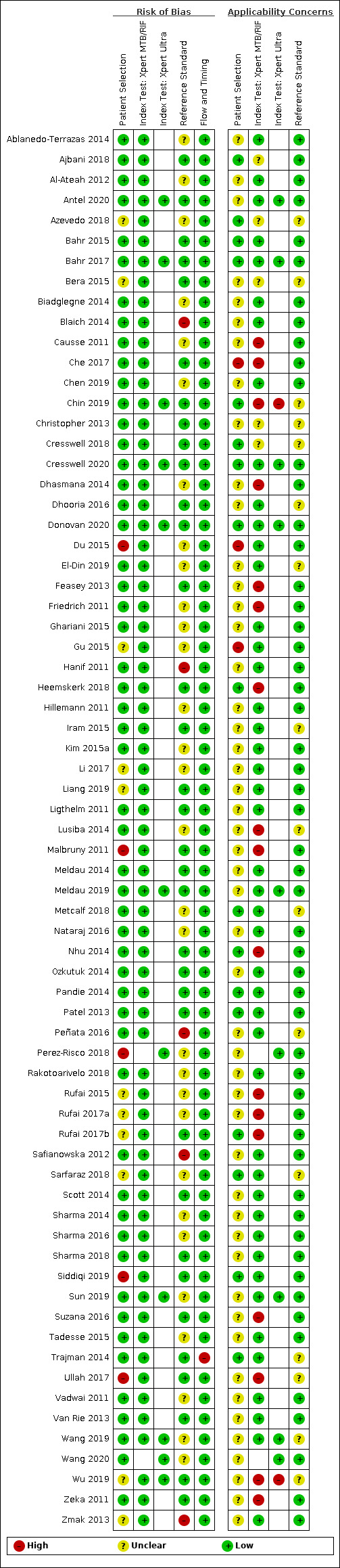
Risk of bias and applicability concerns summary for tuberculosis detection: review authors' judgements about each domain for each included study.
In the patient selection domain, we thought that 53 studies (77%) had low risk of bias, and five studies (7%) had high risk of bias for the following reasons: one study selected participants by convenience (Malbruny 2011) and four studies had inappropriate exclusions (Du 2015; Perez‐Risco 2018; Siddiqi 2019; Ullah 2017). We thought that 11 studies (16%) had unclear risk of bias for the following reasons: the manner of patient selection was unclear in ten studies (Azevedo 2018; Gu 2015; Li 2017; Liang 2019; Rufai 2015; Rufai 2017a; Rufai 2017b; Sarfaraz 2018; Wu 2019; Zmak 2013), and it was unclear whether the study avoided inappropriate exclusions: one study (Bera 2015). Regarding applicability (patient characteristics and setting), we thought that 17 studies (25%) had low concern because participants were evaluated in local hospitals or primary health settings: three studies (Pandie 2014; Sarfaraz 2018; Trajman 2014), or in the case of tuberculous meningitis, tertiary centres: 14 studies (Ajbani 2018; Azevedo 2018; Bahr 2015; Bahr 2017; Chin 2019; Cresswell 2018; Cresswell 2020; Donovan 2020; Heemskerk 2018; Metcalf 2018; Nhu 2014; Patel 2013; Rufai 2017bSiddiqi 2019). Three studies (4%) had high concern because participants were evaluated exclusively as inpatients at a tertiary care centre (Che 2017; Du 2015; Gu 2015); and 52 (75%) studies had unclear or high concern because we could not tell the clinical setting or a high percentage of participants had received prior testing for tuberculosis (Antel 2020).
In the index test domain, we thought that all studies had low risk of bias because the results of the index tests (Xpert Ultra and Xpert MTB/RIF) are automatically generated, the user is provided with printable test results, and the test threshold is prespecified. Regarding applicability, with respect to Xpert Ultra, we thought that 9/11 studies (82%) had low risk of bias (Antel 2020; Bahr 2017; Cresswell 2020; Donovan 2020; Meldau 2019; Perez‐Risco 2018; Sun 2019; Wang 2019; Wang 2020) and 2/11 studies (18%) had high risk of bias because the index test was not performed according to WHO standard operating procedures (Chin 2019; Wu 2019). With respect to Xpert MTB/RIF, we thought that 45/67 studies (67%) had low concern because at least 75% of the specimen types in these studies were processed according to WHO recommendations; 17/67 studies (25%) had high concern because fewer than 50% of the specimen types in these studies were processed according to WHO recommendations (Causse 2011; Che 2017; Chin 2019; Dhasmana 2014; Feasey 2013; Friedrich 2011; Heemskerk 2018; Lusiba 2014; Malbruny 2011; Nhu 2014; Rufai 2015; Rufai 2017a; Rufai 2017b; Suzana 2016; Ullah 2017; Wu 2019; Zeka 2011). We thought that 5/67 studies (7%) had unclear concern because either the manner of specimen processing was not reported (four studies: Ajbani 2018; Azevedo 2018; Bera 2015; Cresswell 2018), or only 50% of the specimen types were processed according to WHO recommendations (one study: Christopher 2013).
In the reference standard domain, 34 studies (49%) had low risk of bias because results of the reference standard were interpreted without knowledge of results of the index test and only non‐sterile specimens were decontaminated (Ajbani 2018; Antel 2020; Bahr 2015; Bahr 2017; Bera 2015; Che 2017; Chin 2019; Christopher 2013 ; Cresswell 2018; Cresswell 2020; Dhooria 2016; Donovan 2020; Feasey 2013; Heemskerk 2018; Iram 2015; Liang 2019; Ligthelm 2011; Malbruny 2011; Meldau 2014; Meldau 2019; Nhu 2014; Ozkutuk 2014; Pandie 2014; Patel 2013; Rufai 2017b; Scott 2014; Sharma 2018; Siddiqi 2019; Suzana 2016; Trajman 2014; Ullah 2017; Van Rie 2013; Wu 2019; Zeka 2011). Five studies (7%) had high risk of bias because results of the reference standard were interpreted with knowledge of results of the index test (Blaich 2014; Hanif 2011; Peñata 2016; Safianowska 2012; Zmak 2013). Thirty studies (43%) had unclear risk of bias for the following reasons: seven studies did not report whether there was blinding of the reference standard (Azevedo 2018; El‐Din 2019; Lusiba 2014; Metcalf 2018; Perez‐Risco 2018; Wang 2019; Wang 2020); 21 studies decontaminated specimens generally considered to be sterile (Al‐Ateah 2012; Biadglegne 2014; Causse 2011; Chen 2019; Dhasmana 2014; Du 2015; Friedrich 2011; Ghariani 2015; Gu 2015; Hillemann 2011; Kim 2015a; Li 2017; Nataraj 2016; Rakotoarivelo 2018; Rufai 2015; Rufai 2017a; Sharma 2014; Sharma 2016; Sun 2019; Tadesse 2015; Vadwai 2011); and two studies did not report blinding and decontaminated specimens generally considered to be sterile (Ablanedo‐Terrazas 2014; Sarfaraz 2018).
Regarding applicability of the reference standard, we thought that 53 studies (77%) had low concern because these studies performed a test to identify M tuberculosis species (speciation) and 16 studies (23%) had unclear concern because we could not tell whether the study performed speciation (Azevedo 2018; Bera 2015; Chin 2019; Christopher 2013; Cresswell 2018; Dhooria 2016; El‐Din 2019; Iram 2015; Lusiba 2014; Metcalf 2018; Peñata 2016; Sarfaraz 2018; Trajman 2014; Ullah 2017; Wang 2019; Wu 2019).
In the flow and timing domain, we considered almost all studies to have low risk of bias, noting that all participants were accounted for in the analysis. One study included fewer than 50% of eligible participants in the analysis (Trajman 2014).
Studies evaluating Xpert Ultra for detection of rifampicin resistance
Figure 5 and Figure 6 show risk‐of‐bias and applicability concerns for each of the five studies included for rifampicin resistance detection.
5.
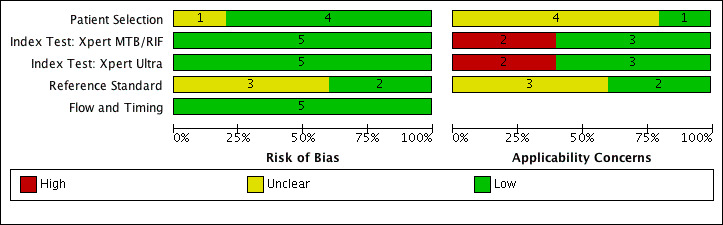
Risk of bias and applicability concerns graph for rifampicin resistance detection in comparative studies of Xpert Ultra and Xpert MTB/RIF: review authors' judgements about each domain presented as percentages across included studies.
6.
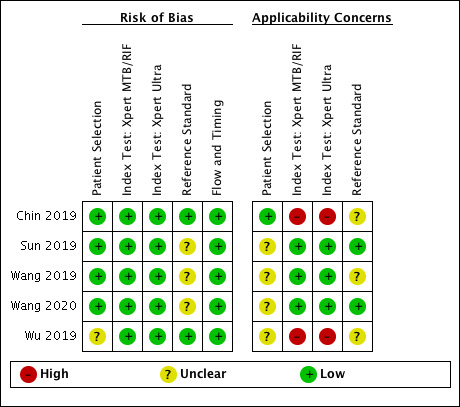
Risk of bias and applicability concerns summary for rifampicin resistance detection in comparative studies of Xpert Ultra and Xpert MTB/RIF: review authors' judgements about each domain for each included study.
In the patient selection domain, we thought that four studies (80%) had low risk of bias (Chin 2019; Sun 2019; Wang 2019; Wang 2020) and one study (20%) had unclear risk of bias as the manner of patient selection was unclear (Wu 2019). We thought that one study (20%) had low concern because participants were evaluated exclusively as inpatients at a tertiary care centre (Chin 2019) and four studies (80%) had unclear concern because we could not tell the details of the clinical setting (Sun 2019; Wang 2019; Wang 2020; Wu 2019).
In the index test domain, we thought that all studies had low risk of bias because the results of the index tests are automatically generated, the user is provided with printable test results, and the test threshold is prespecified. For applicability, with respect to Xpert Ultra, we thought that three studies (60%) had low concern because at least 75% of the specimen types in these studies were processed according to WHO recommendations (Sun 2019; Wang 2019; Wang 2020); two studies (40%) had high concern because fewer than 50% of the specimen types in these studies were processed according to WHO recommendations (Chin 2019; Wu 2019).
In the reference standard domain, two studies (40%) had low risk of bias because results of the reference standard were interpreted without knowledge of results of the index test and only non‐sterile specimens were decontaminated (Chin 2019; Wu 2019). Three studies (60%) had unclear risk of bias as it was unclear whether blinding of the reference standard was performed (Sun 2019; Wang 2019; Wang 2020). For applicability of the reference standard, we thought that all studies had low concern because detection of rifampicin resistance occurs only when the M tuberculosis target is present within the specimen.
In the flow and timing domain, we considered all studies to have low risk of bias, noting that all participants were accounted for in the analysis.
Findings
The 69 studies were conducted in 28 different countries. Most of the studies were conducted in China (n = 10), India (n = 13), South Africa (n = 10), and Uganda (n = 6). Seven studies exclusively or mainly included HIV‐positive participants (Ablanedo‐Terrazas 2014; Azevedo 2018; Bahr 2015; Bahr 2017; Cresswell 2020; Feasey 2013; Van Rie 2013). Most studies performed the index tests and culture on the same specimen type, except for one study in which Xpert MTB/RIF was performed on blood and culture was performed on sputum (Feasey 2013). Most studies did not report the exact number of cultures used to confirm a diagnosis of tuberculosis, but it is likely that many studies used a single culture. We present key characteristics of the included studies in the Characteristics of included studies table.
I. Detection of extrapulmonary tuberculosis
Xpert Ultra: of the 11 studies, the number evaluating different specimens was as follows: tuberculous meningitis (CSF) six studies; pleural tuberculosis (pleural fluid) four studies; lymph node tuberculosis (lymph node aspirate) one study; genitourinary tuberculosis (urine) one study; bone or joint tuberculosis (bone or joint aspirate) two studies; and peritoneal tuberculosis (peritoneal fluid) one study.
Xpert MTB/RIF: of the 67 studies, the number of studies evaluating different specimens was as follows: tuberculous meningitis (CSF) 33 studies; pleural tuberculosis (fluid) 27 studies; lymph node tuberculosis (aspirate 15 studies, biopsy 11 studies); genitourinary tuberculosis (urine) 15 studies; bone or joint tuberculosis (aspirate 12 studies, tissue 3 studies); peritoneal tuberculosis (fluid 17 studies, tissue 1 study); pericardial tuberculosis (fluid 14 studies, tissue 2 studies); and disseminated tuberculosis (blood 2 studies). Several studies included more than one type of specimen.
Table 6 presents Xpert Ultra and Xpert MTB/RIF pooled sensitivity and specificity estimates and predictive values by reference standard for all forms of extrapulmonary tuberculosis and specimen types included in the review.
2. Summary accuracy of Xpert MTB/RIF and Xpert Ultra for detection of extrapulmonary tuberculosis and rifampicin resistance.
| Type of specimen | Test | Reference standard | Number studies (participants) | Number (%) with TB or rifampicin resistance | Pooled sensitivity (95% CrI) | Pooled specificity (95% CrI) | Positive predictive value (95% CrI) | Negative predictive value (95% CrI) |
| CSF | Xpert Ultra | culture | 6 (475) | 89 (18.7) | 89.4% (79.1 to 95.6) | 91.2% (83.2 to 95.7) | 53.0% (36.6 to 69.6) | 98.7% (97.5 to 99.5) |
| CSF | Xpert Ultra | composite | 4 (496) | 160 (32.2) | 62.7% (45.7 to 77.0) | 99.1% (96.6 to 99.9) | 87.9% (65.5 to 99.0) | 96.0% (94.2 to 97.5) |
| CSF | Xpert MTB/RIF | culture | 30 (3395) | 571 (16.8) | 71.1% (62.8 to 79.1) | 96.9% (95.4 to 98.0) | 71.8% (62.3 to 80.7) | 96.8% (95.9 to 97.7) |
| CSF | Xpert MTB/RIF | composite | 14 (2203) | 862 (39.1) | 42.3% (32.1 to 52.8) | 99.8% (99.3 to 100.0) | 96.3% (87.2 to 100.0) | 94.0% (93.0 to 95.0) |
| CSF | Ultra, direct comparison | culture | 5 (471) | 86 (18.3) | 89.0% (77.9 to 95.2) | 91.0% (82.7 to 95.6) | 52.2% (35.6 to 69.0) | 98.7% (97.3 to 99.4) |
| CSF | Xpert MTB/RIF, direct comparison | culture | 5 (471) | 87 (18.5) | 62.2% (43.7 to 78.1) | 96.8% (93.4 to 98.6) | 68.4% (49.0 to 83.6) | 95.8% (93.9 to 97.5) |
| Pleural fluid | Xpert Ultra | culture | 4 (398) | 158 (39.7) | 75.0% (58.0 to 86.4) | 87.0% (63.1 to 97.9) | 38.8% (17.9 to 79.5) | 96.9% (94.5 to 98.3) |
| Pleural fluid | Xpert MTB/RIF | culture | 25 (3065) | 644 (21.0) | 49.5% (39.8 to 59.9) | 98.9% (97.6 to 99.7) | 83.2% (68.9 to 94.6) | 94.6% (93.7 to 95.7) |
| Pleural fluid | Xpert MTB/RIF | composite | 10 (1024) | 616 (60.1) | 18.9% (11.5 to 27.9) |
99.3% (98.1 to 99.8) |
73.6% (49.2 to 91.2) | 91.7% (91.0 to 92.5) |
| Lymph node aspirate | Xpert MTB/RIF | culture | 14 (1588) | 627 (39.5) | 88.9% (82.7 to 93.6) | 86.2% (78.0 to 92.3) | 41.7% (31.4 to 55.5) | 98.6% (97.9 to 99.2) |
| Lymph node aspirate | Xpert MTB/RIF | composite | 4 (679) | 377 (55.5) | 81.6% (61.9 to 93.3) |
96.4% (91.3 to 98.6) |
71.0% (51.1 to 86.1) | 97.9% (95.8 to 99.2) |
| Lymph node biopsy | Xpert MTB/RIF | culture | 11 (786) | 220 (28.0) | 82.4% (73.5 to 89.7) |
80.3% (60.3 to 91.5) |
31.6% (18.7 to 51.8) | 97.6% (96.2 to 98.6) |
| Urine | Xpert MTB/RIF | culture | 9 (943) | 72 (7.6) | 85.9% (71.4 to 94.3) |
98.1% (93.1 to 99.7) |
83.0% (58.3 to 96.7) | 98.4% (96.9 to 99.4) |
| Bone or joint aspirate | Xpert MTB/RIF | culture | 6 (471) | 110 (23.4) | 97.9% (93.1 to 99.6) |
97.4% (80.2 to 100.0) |
80.7% (35.4 to 99.5) | 99.8% (99.2 to 100.0) |
| Peritoneal fluid | Xpert MTB/RIF | culture | 13 (580) | 94 (16.2) | 59.1% (42.1 to 76.2) |
97.6% (95.4 to 98.9) |
73.0% (58.2 to 86.2) | 95.5% (93.8 to 97.4) |
| Pericardial fluid | Xpert MTB/RIF | culture | 5 (181) | 57 (31.5) | 61.4% (32.4 to 82.4) |
89.7% (74.9 to 99.0) |
39.4% (18.3 to 88.0) | 95.4% (92.1 to 97.9) |
| Rifampicin resistance | Xpert Ultra | DSTor LPA | 4 (129) | 24 (18.6) | 100.0% (95.1 to 100.0) | 100.0% (99.0 to 100.0) | 99.9% (91.7 to 100.0) | 100.0% (99.5 to 100.0) |
| Rifampicin resistance | Xpert MTB/RIF | DSTor LPA | 19 (970) | 148 (15.3) | 96.5% (91.9 to 98.8) | 99.1% (98.0 to 99.7) | 92.0% (84.3 to 97.3) | 99.6% (99.1 to 99.9) |
Abbreviations: Crl: credible interval; CSF: cerebrospinal fluid; LPA: Line probe assay; TB: tuberculosis.
Studies included in the table are limited to those that report data for both sensitivity and specificity; thus the number of studies (specimens) may differ slightly from those reported in the main text of the review. For tuberculosis detection, the reference standard was culture and a composite reference standard. For rifampicin resistance detection, the reference standards were culture‐based drug susceptibility testing or line probe assay. Pooled sensitivity and pooled specificity are posterior median estimates.
A: Xpert MTB/RIF and Xpert Ultra testing in cerebrospinal fluid for tuberculous meningitis
Xpert Ultra
Culture reference standard
Six studies evaluated Xpert Ultra in cerebrospinal fluid (CSF) specimens against culture (Bahr 2017; Chin 2019; Cresswell 2020; Donovan 2020; Perez‐Risco 2018; Wang 2020). Xpert Ultra sensitivity ranged from 80% to 100% and specificity ranged from 50% to 100% (Figure 7). Chin 2019 reported the lowest specificity (50%). In this study, the investigators inoculated uncentrifuged CSF which could have led to lower culture positivity, thus resulting in a higher number of false positives. Perez‐Risco 2018 (specificity 100%) contributed only one participant to this analysis. In CSF, Xpert Ultra pooled sensitivity and specificity (95% Crl) against culture were 89.4% (79.1 to 95.6) and 91.2% (83.2 to 95.7), (6 studies; 475 participants, 89 (18.7%) with tuberculosis); Table 6.
7.
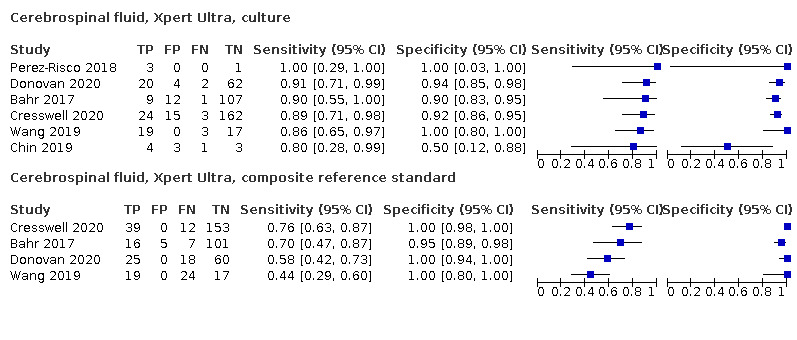
Forest plots of Xpert Ultra sensitivity and specificity in cerebrospinal fluid by reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
Composite reference standard
In CSF, Xpert Ultra pooled sensitivity and specificity against a composite reference standard were 62.7% (45.7 to 77.0) and 99.1% (96.6 to 99.9), (4 studies; 496 participants); Table 6, Figure 7.
Latent class meta‐analysis
We had insufficient data to obtain robust parameter estimates using the latent class model for Xpert Ultra in CSF.
Xpert MTB/RIF
Culture reference standard
Thirty‐three studies evaluated Xpert MTB/RIF in CSF specimens against culture (Ajbani 2018; Al‐Ateah 2012; Azevedo 2018; Bahr 2015; Bahr 2017; Blaich 2014; Causse 2011; Chin 2019; Cresswell 2018; Cresswell 2020; Donovan 2020; Hanif 2011; Hillemann 2011; Kim 2015a; Li 2017; Malbruny 2011; Metcalf 2018; Nataraj 2016; Nhu 2014; Ozkutuk 2014; Patel 2013; Peñata 2016; Rakotoarivelo 2018; Rufai 2017b; Safianowska 2012; Sharma 2014; Siddiqi 2019; Suzana 2016; Ullah 2017; Vadwai 2011; Wang 2019; Zeka 2011; Zmak 2013). Xpert MTB/RIF sensitivity ranged from 0% to 100% and specificity ranged from 78% to 100% (Figure 8). For sensitivity, we thought that differences in CSF volume and processing could partly explain the heterogeneity. Three studies (Al‐Ateah 2012; Hillemann 2011; Safianowska 2012) did not contribute data to the meta‐analysis because sensitivity was not estimable.
8.
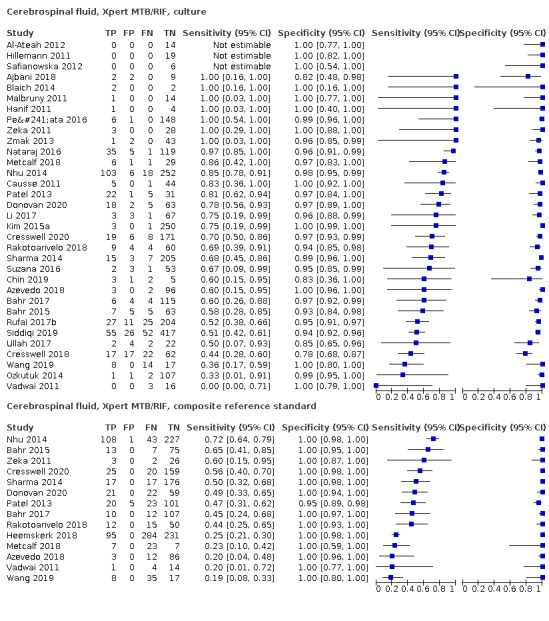
Forest plots of Xpert MTB/RIF sensitivity and specificity in cerebrospinal fluid by reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
In CSF, Xpert MTB/RIF pooled sensitivity and specificity (95% Crl) against culture were 71.1% (62.8 to 79.1) and 96.9% (95.4 to 98.0), respectively (30 studies; 3395 participants, 571 (16.8%) with tuberculosis); Table 6, Figure 8.
Composite reference standard
In CSF, Xpert MTB/RIF pooled sensitivity and specificity against a composite reference standard were 42.3% (32.1 to 52.8) and 99.8% (99.3 to 100.0), (14 studies; 2203 participants); Table 6, Figure 8.
Latent class meta‐analysis
Based on the latent class meta‐analysis model, Xpert MTB/RIF pooled sensitivity and specificity (95% Crl) for tuberculous meningitis were 74.7% (65.5 to 84.0) and 99.5% (99.1 to 99.7) (30 studies; 3395 participants); Table 7. The pooled sensitivity of culture at 80.8% (72.5 to 88.5) was estimated to be lower than 100%. The pooled specificity of culture was estimated to be 99.2% (98.7 to 99.5); Table 7.
3. Latent class meta‐analysis.
| Form of extrapulmonary tuberculosis, type of specimen | Number of studies (participants) | Culture‐confirmed tuberculosis (%) | Pooled sensitivity (95% CrI) | Pooled specificity (95% CrI) | Positive predictive value (95% CrI) | Negative predictive value (95% CrI) |
| Accuracy estimates of Xpert MTB/RIF | ||||||
| Tuberculous meningitis, cerebrospinal fluid | 30 (3395) | 571 (16.8) | 74.7% (65.5 to 84.0) | 99.5% (99.1 to 99.7) | 94.5% (89.7 to 96.9) | 97.3% (96.3 to 98.3) |
| Pleural tuberculosis, fluid | 25 (3065) | 644 (21.0) | 53.1% (42.8 to 64.1) | 99.6% (99.3 to 99.8) | 93.7% (89.5 to 96.5) | 95.0% (94.0 to 96.2) |
| Lymph node tuberculosis, aspirate | 14 (1588) | 627 (39.5) | 91.5% (85.2 to 95.9) | 99.5% (99.1 to 99.7) |
95.2% (91.4 to 97.5) | 99.1% (98.4 to 99.5) |
| Accuracy estimates of culture | ||||||
| Tuberculous meningitis, cerebrospinal fluid | 30 (3395) | 571 (16.8) | 80.8% (72.5 to 88.5) | 99.2% (98.7 to 99.5) | 91.9% (86.9 to 95.1) | 97.9% (97.0 to 98.7) |
| Pleural tuberculosis, fluid | 25 (3065) | 644 (21.0) | 89.5% (80.5 to 96.3) | 99.0% (98.2 to 99.5) | 90.8% (84.2 to 94.7) | 98.8% (97.9 to 99.6) |
| Lymph node tuberculosis, aspirate | 14 (1588) | 627 (39.5) | 82.9% (69.9 to 91.8) | 98.8% (97.8 to 99.4) | 88.7% (80.1 to 94.0) | 98.1% (96.7 to 99.1) |
Abbreviations: Crl: credible interval.
We generally used non‐informative priors in the latent class model. Accuracy estimates were determined using a bivariate random‐effects approach for comparison.
Xpert Ultra versus Xpert MTB/RIF
In comparative accuracy studies evaluating both index tests, Xpert Ultra pooled sensitivity and specificity (95% CrI) against culture were 89.0% (77.9 to 95.2) and 91.0% (82.7 to 95.6) and Xpert MTB/RIF pooled sensitivity and specificity were 62.2% (43.7 to 78.1) and 96.8% (93.4 to 98.6), (5 studies; 471 participants), direct comparison, Table 6; Figure 9; Figure 10. For CSF, the difference between the sensitivities of Xpert Ultra and Xpert MTB/RIF was 26.2% (9.1 to 44.4). We estimated the probability that the pooled sensitivity of Xpert Ultra exceeds that of Xpert MTB/RIF was 0.997. The difference between the specificities of Xpert Ultra and Xpert MTB/RIF was −5.6% (−12.9 to −0.1). We estimated the probability that the pooled specificity of Xpert Ultra was less than that of Xpert MTB/RIF was 0.978; Table 8.
9.
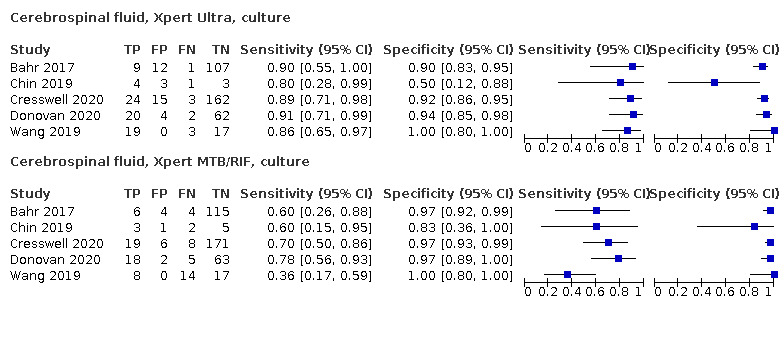
Forest plots of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity in cerebrospinal fluid, comparative studies. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
10.
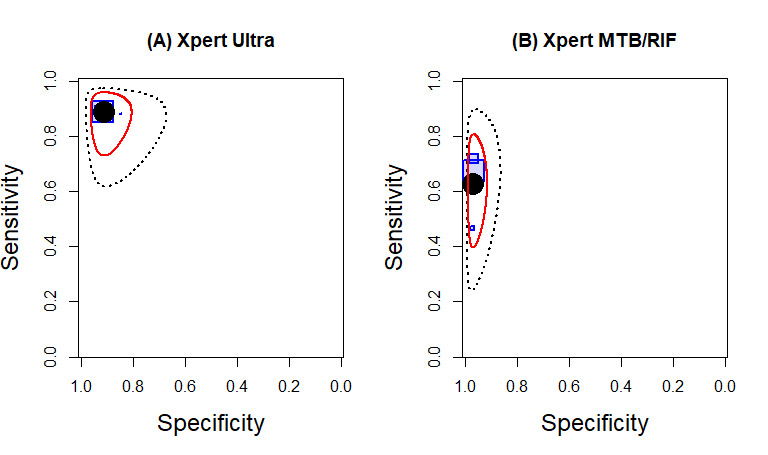
Summary plots of the sensitivity and specificity of Xpert Ultra (A) (5 studies) and Xpert MTB/RIF (B) (5 studies) in cerebrospinal fluid for detection of tuberculous meningitis. Each individual study is represented by a shaded square. The size of the square is proportional to the sample size of the study such that larger studies are represented by larger squares. The filled circle is the median pooled estimate for sensitivity and specificity. The solid curves represent the 95% credible region around the summary estimate; the dashed curves represent the 95% prediction region.
4. Accuracy of Xpert Ultra versus Xpert MTB/RIF in cerebrospinal fluid.
| Detection of tuberculosis in CSF | ||||
| Test (studies, participants) | Xpert Ultra (5, 471) | Xpert MTB/RIF (5, 471) | Difference (Xpert Ultra minus Xpert MTB/RIF) | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity | 89.0% (77.9 to 95.2) | 62.2% (43.7 to 78.1) | 26.2% (9.1 to 44.4) | 0.997 |
| Specificity | 91.0% (82.7 to 95.6) | 96.8% (93.4 to 98.6) | −5.6% (−12.9 to −0.1) | 0.022 |
Investigations of heterogeneity
Xpert Ultra versus Xpert MTB/RIF testing in people living with HIV
We identified two studies that directly compared Xpert Ultra and Xpert MTB/RIF, both against culture, in people living with HIV. Sensitivity (95% CI) was 90% (55 to 100) (Bahr 2017) and 89% (71 to 98) (Cresswell 2020) for Xpert Ultra and 60% (26 to 88) (Bahr 2017) and 70% (50 to 86) (Cresswell 2020) for Xpert MTB/RIF. Specificity (95% CI) was 90% (83 to 95) (Bahr 2017) and 92% (86 to 95) (Cresswell 2020) for Xpert Ultra and 97% (95% CI 92 to 99) (Bahr 2017) and 97% (93 to 99) (Cresswell 2020) for Xpert MTB/RIF; Figure 11.
11.
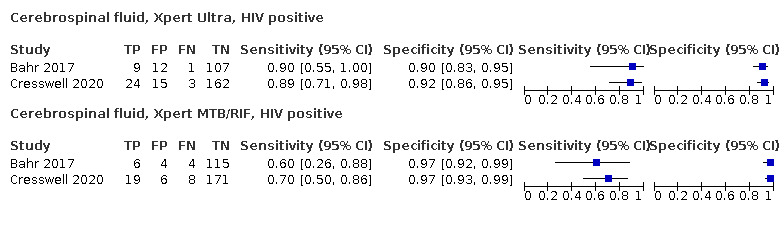
Forest plots of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity in cerebrospinal fluid in HIV‐positive people, with respect to culture. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive. .
Specimen concentration
Xpert Ultra
We found that concentrating CSF improved both Xpert Ultra sensitivity and specificity. Xpert Ultra pooled sensitivity in concentrated specimens was 90.5% (76.7 to 97.0) (3 studies; 421 participants) versus 88.4% (67.8 to 97.5) (3 studies; 54 participants) in unconcentrated specimens. Xpert Ultra pooled specificity in concentrated specimens was 91.9% (84.5 to 96.1) versus 88.6% (58.4 to 99.0) in unconcentrated specimens; Table 9. The probability that Xpert Ultra sensitivity and specificity are higher with concentrated CSF compared to unconcentrated CSF were 0.630 and 0.653, respectively.
5. Impact of concentrating cerebrospinal fluid on Xpert Ultra and Xpert MTB/RIF sensitivity and specificity.
| Covariate (number of studies, participants) | Pooled sensitivity (95% CrI) | Pooled specificity (95% CrI) |
| Concentration step, Xpert Ultra | ||
| Concentrated specimen (3, 421) | 90.5% (76.7 to 97.0) | 91.9% (84.5 to 96.1) |
| Unconcentrated specimen (3, 54) | 88.4% (67.8 to 97.5) | 88.6% (58.4 to 99.0) |
| Difference (concentrated minus unconcentrated) | 2.6% (−13.9 to 24.1) | 3.4% (−9.5 to 32.8) |
| Probability (concentrated minus unconcentrated) | 0.630 | 0.653 |
| Concentration step, Xpert MTB/RIF | ||
| Concentrated specimen (14, 2279) | 77.6% (67.2 to 85.9) | 97.4% (96.1 to 98.4) |
| Unconcentrated specimen (17, 1123) | 59.4% (48.3 to 70.5) | 96.8% (94.0 to 98.7) |
| Difference (concentrated minus unconcentrated) | 18.4% (2.8 to 32.1) | 0.6% (−1.7 to 3.6) |
| Probability (concentrated minus unconcentrated) | 0.989 | 0.696 |
Abbreviations: Crl: credible interval.
Xpert MTB/RIF
We found that concentrating CSF improved both Xpert MTB/RIF sensitivity and specificity. Xpert MTB/RIF pooled sensitivity in concentrated specimens was 77.6% (67.2 to 85.9) (14, 2279 participants) versus 59.4% (48.3 to 70.5) (17,1123 participants) in unconcentrated specimens. Xpert MTB/RIF pooled specificity in concentrated specimens was 97.4% (96.1 to 98.4) versus 96.8% (94.0 to 98.7) in unconcentrated specimens, Table 9. The probability that Xpert MTB/RIF sensitivity and specificity are higher with concentrated CSF compared to unconcentrated CSF were 0.989 and 0.696, respectively.
Cerebrospinal fluid collection volumes
Xpert Ultra
Two studies reported the volume of CSF collected for Xpert Ultra testing, 3 mL in both studies. Sensitivities were similar: 90% (55 to 100) in Bahr 2017 and 89% (71 to 98) in Cresswell 2020. Specificities were also similar 90% (83 to 95) in Bahr 2017 and 92% (86 to 95) in Cresswell 2020.
Impact of tuberculosis prevalence on sensitivity and specificity
For Xpert Ultra, we found lower sensitivity in settings with higher tuberculosis prevalence (threshold 30%) than in those with lower tuberculosis prevalence: pooled sensitivity (95% CrI) of 88.3% (68.3 to 97.0) versus 90.8% (77.3 to 96.9). We found lower specificity in settings with higher tuberculosis prevalence than in those with lower tuberculosis prevalence: pooled specificity of 88.0% (64.3 to 97.9) versus 91.9% (82.5 to 96.6). In both analyses, the 95% Crls overlapped; Table 10.
6. Impact of tuberculosis prevalence on Xpert Ultra and Xpert MTB/RIF sensitivity and specificity.
| Analysis, (number of studies, specimens) | Pooled sensitivity (95% CrI) | Pooled specificity (95% CrI) |
| Cerebrospinal fluid, Xpert Ultra | ||
| Among studies with prevalence ≥ 30% (3, 54) | 88.3% (68.3 to 97.0) | 88.0% (64.3 to 97.9) |
| Among studies with prevalence < 30% (3, 421) | 90.8% (77.3 to 96.9) | 91.9% (82.5 to 96.6) |
| Difference (≥ 30% group minus < 30% group) | −2.2% (−23.0 to 13.5) | −3.8% (−27.7 to 9.8) |
| Probability (≥ 30% group minus < 30% group) | 0.390 | 0.308 |
| Cerebrospinal fluid, Xpert MTB/RIF | ||
| Among studies with prevalence ≥ 30% (6, 610) | 67.0% (49.0 to 81.5) | 94.1% (86.8 to 97.9) |
| Among studies with prevalence < 30% (24, 2785) | 72.0% (62.4 to 81.2) | 97.3% (95.9 to 98.3) |
| Difference (≥ 30% group minus < 30% group) | −4.8% (−25.5 to 12.1) | −3.1% (−10.5 to 0.8) |
| Probability (≥ 30% group minus < 30% group) | 0.296 | 0.071 |
| Cerebrospinal fluid, Xpert MTB/RIF | ||
| Among studies with prevalence ≥ 10% (19, 2190) | 68.7% (58.5 to 78.0) | 95.1% (92.7 to 96.8) |
| Among studies with prevalence < 10% (11, 1205) | 74.2% (57.4 to 86.6) | 98.6% (97.5 to 99.3) |
| Difference (≥ 10% group minus < 10% group) | ‐5.5% (‐21.2 to 13.3) | ‐3.5% (‐6.0 to ‐1.5) |
| Probability (≥ 10% group minus < 10% group) | 0.272 | 0.001 |
| Pleural fluid, Xpert MTB/RIF | ||
| Among studies with prevalence ≥ 50% (6, 627) | 20.7% (11.2 to 33.7) | 99.6% (97.9 to 99.9) |
| Among studies with prevalence < 50% (4, 397) | 15.5% (6.5 to 30.1) | 99.0% (96.9 to 99.8) |
| Difference (≥ 50% group minus < 50% group) | 5.1% (−11.8 to 21.2) | 0.5% (−1.2 to 2.7) |
| Probability (≥ 50% group minus < 50% group) | 0.757 | 0.759 |
| Lymph node aspirate, Xpert MTB/RIF | ||
| Among studies with prevalence ≥ 35 (9, 911) | 93.1% (88.9 to 96.3) | 83.2% (69.5 to 92.1) |
| Among studies with prevalence < 35% (5, 677) | 72.2% (64.9 to 87.2) | 90.0% (78.3 to 95.9) |
| Difference (≥ 35% group minus < 35% group) | 15.7% (5.4 to 28.6) | −6.4% (−21.3 to 76) |
| Probability (≥ 35% group minus < 35% group) | 0.999 | 0.158 |
Abbreviations: Crl: credible interval.
Prevalence refers to the percentage of culture‐confirmed tuberculosis specimens or confirmed rifampicin‐resistant specimens in the study. We selected prevalence levels to approximate the lower bound of the interquartile range or in consideration of the range of prevalences reported in the included studies.
Similarly, for Xpert MTB/RIF, we found lower sensitivity in settings with higher tuberculosis prevalence than in those with lower tuberculosis prevalence: pooled sensitivity of 67.0% (49.0 to 81.5) versus 72.0% (62.4 to 81.2). We found lower specificity in settings with higher tuberculosis prevalence than in those with lower tuberculosis prevalence: pooled specificity of 94.1% (86.8 to 97.9) versus 97.3% (95.9 to 98.3). In both analyses, the 95% Crls overlapped; Table 10. When we repeated the analysis at lower tuberculosis prevalence (threshold 10%), in the case of specificity, accuracy in the two groups was significantly different (probability of specificity being lower in the high tuberculosis prevalence group = 0.999); Table 10.
Sensitivity analyses
Overall, the sensitivity analyses made little difference to the findings; Table 11
7. Sensitivity analyses, Xpert Ultra in cerebrospinal fluid.
| Type of specimen | Number of studies (specimens) | Pooled sensitivity (95% Crl) | Pooled specificity (95% Crl) | Predicted sensitivity (95% Crl) | Predicted specificity (95% Crl) |
| All participants | 6 (475) | 89.4% (79.1 to 95.6) | 91.2% (83.2 to 95.7) | 53.0% (36.6 to 69.6) | 98.7% (97.5 to 99.5) |
| Consecutive participant selection | 5 (471) | 87.9% (76.4 to 94.6) | 90.4% (81.1 to 95.1) | 88.0% (65.2 to 96.7) | 90.5% (65.5 to 97.7) |
| Reference standard blinding | 4 (432) | 88.5% (74.7 to 95.6) | 89.1% (76.9 to 94.3) | 88.6% (63.4 to 97.2) | 89.2% (61.0 to 97.1) |
| Single specimen per participant | 4 (432) | 88.5% (74.7 to 95.6) | 89.1% (76.9 to 94.3) | 88.6% (63.4 to 97.2) | 89.2% (61.0 to 97.1) |
Abbreviations: Crl: credible interval.
Inconclusive Xpert Ultra and Xpert MTB/RIF results
Xpert Ultra
None of the studies evaluating Xpert Ultra for tuberculous meningitis reported this information.
Xpert MTB/RIF
We previously reported that for CSF, of 2096 tests performed, the pooled proportion of inconclusive Xpert MTB/RIF results was 0.9% (95% CrI 0.3 to 1.9) (Kohli 2018).
B: Xpert Ultra and Xpert MTB/RIF testing in pleural fluid for pleural tuberculosis
Xpert Ultra
Culture reference standard
Four studies evaluated Xpert Ultra in pleural fluid with respect to culture (Perez‐Risco 2018; Wang 2019; Wang 2020; Wu 2019). Xpert Ultra sensitivity ranged from 48% to 84% and specificity ranged from 65% to 100%; Figure 12.
12.
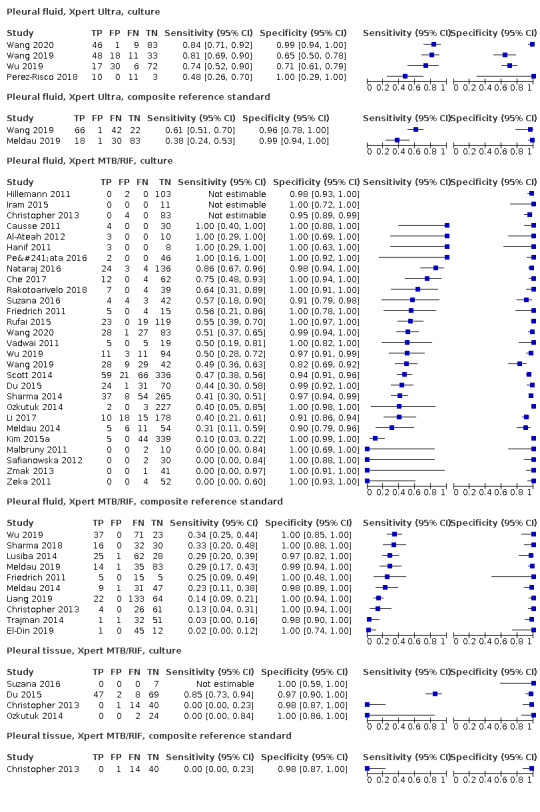
Forest plots of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity in pleural fluid and tissue by reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
In pleural fluid, Xpert Ultra pooled sensitivity and specificity against culture were 75.0% (58.0 to 86.4) and 87.0% (63.1 to 97.9), (4 studies; 398 participants, 158 (39.7%) with tuberculosis); Table 6; Appendix 8.
Composite reference standard
Two studies evaluated Xpert Ultra in pleural fluid with respect to a composite reference standard (Meldau 2019; Wang 2019); Figure 12; Appendix 8. Sensitivity ranged from 38% to 61%, and specificity ranged from 96% to 99%. We could not explain the variability in the sensitivity estimates and did not perform a meta‐analysis.
Latent class meta‐analysis
We had insufficient data to obtain robust parameter estimates using the latent class model for Xpert Ultra in pleural fluid.
Xpert MTB/RIF
Culture reference standard
Twenty‐eight studies evaluated Xpert MTB/RIF in pleural fluid with respect to culture (Al‐Ateah 2012; Causse 2011; Che 2017; Christopher 2013; Du 2015; Friedrich 2011; Hanif 2011; Hillemann 2011; Iram 2015; Kim 2015a; Li 2017; Malbruny 2011; Meldau 2014; Nataraj 2016; Ozkutuk 2014; Peñata 2016; Rakotoarivelo 2018; Rufai 2015; Safianowska 2012; Scott 2014; Sharma 2014; Suzana 2016; Vadwai 2011; Wang 2019; Wang 2020; Wu 2019; Zeka 2011; Zmak 2013). Xpert MTB/RIF sensitivity ranged from 0% to 100% and specificity ranged from 82% to 100% (Figure 12). Three studies (Christopher 2013; Hillemann 2011; Iram 2015) did not contribute data to the meta‐analysis because sensitivity was not estimable.
In pleural fluid, Xpert MTB/RIF pooled sensitivity and specificity against culture were 49.5% (39.8 to 59.9) and 98.9% (97.6 to 99.7) (25 studies; 3065 participants, 644 (21.0%) with tuberculosis); Table 6; Appendix 8.
Composite reference standard
In pleural fluid, Xpert MTB/RIF pooled sensitivity and specificity against a composite reference standard were 18.9% (11.5 to 27.9) and 99.3% (98.1 to 99.8), (10 studies; 1024 participants) Table 6; Figure 12.
Latent class meta‐analysis
Based on the latent class meta‐analysis model, Xpert MTB/RIF pooled sensitivity and specificity (95% Crl) in pleural fluid were 53.1% (42.8 to 64.1) and 99.6% (99.3 to 99.8) (25 studies; 3065 participants) Table 7. Xpert MTB/RIF pooled sensitivity was slightly higher and its pooled specificity comparable to what was obtained when culture was treated as having perfect accuracy, with pooled sensitivity of 49.5% (39.8 to 59.9) and pooled specificity of 98.8% (97.6 to 99.7). The pooled sensitivity of culture at 89.5% (80.5 to 96.3) was estimated to be lower than 100%. The pooled specificity of culture was estimated to be 99.0% (98.2 to 99.5).
Xpert Ultra versus Xpert MTB/RIF
We had insufficient data for this analysis.
Impact of tuberculosis prevalence on sensitivity and specificity
For Xpert Ultra, we had insufficient data for this analysis.
For Xpert MTB/RIF, we found higher sensitivity in settings with higher tuberculosis prevalence than in those with lower tuberculosis prevalence: pooled sensitivity (95% CrI) of 20.7% (11.2 to 33.7) versus 15.5% (6.5 to 30.1). We found similar specificity in settings with higher tuberculosis prevalence and in those with lower tuberculosis prevalence: pooled specificity of 99.6% (97.9 to 99.9) versus 99.0% (96.9 to 99.8). In both analyses, the 95% Crls overlapped; Table 10.
Sensitivity analyses
For Xpert Ultra, we had insufficient data for these analyses.
Inconclusive Xpert Ultra and Xpert MTB/RIF results
Xpert Ultra
Of the total 1013 tests performed, the percentage of inconclusive Xpert Ultra results was 0.3%. Only one study reported this information (Wang 2019).
Xpert MTB/RIF
We previously reported that for pleural fluid, of 1416 tests performed the pooled proportion of inconclusive Xpert MTB/RIF results was 1.2% (95% CrI 0.4 to 2.6) (Kohli 2018).
C: Xpert Ultra and Xpert MTB/RIF testing in pleural tissue for pleural tuberculosis
Xpert Ultra
Culture reference standard
We did not identify any studies evaluating Xpert Ultra in pleural tissue against culture.
Composite reference standard
We did not identify any studies evaluating Xpert Ultra in pleural tissue against a composite reference standard.
Xpert MTB/RIF
Culture reference standard
Four studies evaluated Xpert MTB/RIF in pleural tissue with respect to culture (Christopher 2013; Du 2015; Ozkutuk 2014; Suzana 2016). Xpert MTB/RIF sensitivity ranged from 0% to 85% and specificity ranged from 97% to 100%; Figure 12. One study reported zero tuberculosis cases (Suzana 2016). We did not perform a meta‐analysis.
Composite reference standard
In pleural tissue, Xpert MTB/RIF sensitivity and specificity against a composite reference standard were 0% (0 to 23) and 98% (87 to 100) (1 study; 55 participants; Christopher 2013); Figure 12.
D: Xpert MTB/RIF and Xpert Ultra testing in lymph node aspirate for lymph node tuberculosis
Xpert Ultra
Culture reference standard
In lymph node aspirates, Xpert Ultra sensitivity and specificity against culture were 78% (40 to 97) and 78% (66 to 87), (1 study; 73 participants; 9 (12.3%) with tuberculosis; Antel 2020); Figure 13.
13.
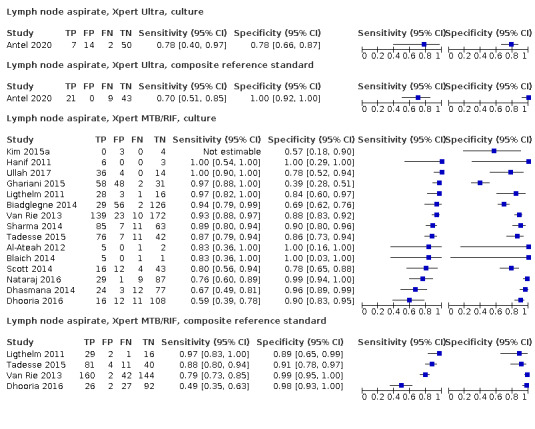
Forest plots of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity in lymph node aspirate by reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
Composite reference standard
In lymph node aspirates, Xpert Ultra sensitivity and specificity against a composite reference standard were 70% (51 to 85) and 100% (92 to 100), (1 study; 73 participants; 9 (12.3%) with tuberculosis; Antel 2020); Figure 13. Of note, with a composite reference standard, specificity was higher (100%) than that observed when using culture as the reference standard (78%).
Latent class meta‐analysis
We had insufficient data to obtain robust parameter estimates using the latent class model for Xpert Ultra in lymph node aspirate.
Xpert MTB/RIF
Culture reference standard
Fifteen studies evaluated Xpert MTB/RIF in lymph node aspirates with for culture (Al‐Ateah 2012; Biadglegne 2014; Blaich 2014; Dhasmana 2014; Dhooria 2016; Ghariani 2015; Hanif 2011; Kim 2015a; Ligthelm 2011; Nataraj 2016; Scott 2014; Sharma 2014; Tadesse 2015; Ullah 2017; Van Rie 2013). Xpert MTB/RIF sensitivity ranged from 59% to 100% and specificity from 57% to 100%; Figure 13. Xpert MTB/RIF specificity in lymph node aspirates was considerably more heterogeneous than in CSF and pleural fluid. The variability in Xpert MTB/RIF specificity in lymph node aspirates was unexpected and may be the result of a systematic, unexplained bias in some studies. One study did not contribute data to the meta‐analysis because sensitivity was not estimable (Kim 2015a).
In lymph node aspirates, Xpert MTB/RIF pooled sensitivity and specificity against culture were 88.9% (82.7 to 93.6) and 86.2% (78.0 to 92.3) (14 studies; 1588 participants, 627 (39.5%) with tuberculosis); Table 6.
Composite reference standard
In lymph node aspirates, Xpert MTB/RIF pooled sensitivity and specificity against a composite reference standard were 81.6% (61.9 to 93.3) and 96.4% (91.3 to 98.6), (4 studies; 679 participants); Table 6; Figure 13. Of note, with a composite reference standard, specificity was less variable and pooled specificity higher than that observed when using culture as the reference standard (86.0%).
Latent class meta‐analysis
Based on the latent class meta‐analysis model, Xpert MTB/RIF pooled sensitivity and specificity (95% Crl) in lymph node aspirate were 91.3% (84.9 to 96.3) and 99.5% (99.1 to 99.7) (14 studies; 1588 participants); Table 7. Xpert MTB/RIF pooled sensitivity and pooled specificity were higher than when culture was treated as having perfect accuracy, with pooled sensitivity of 88.9% (82.7 to 93.6) and pooled specificity of 86.2% (78.0 to 92.3). The pooled sensitivity of culture at 84.9% (74.0 to 92.8) was estimated to be lower than 100%. The pooled specificity of culture was estimated to be 98.8% (97.7 to 99.4); Table 7. The latent class meta‐analysis resulted in high precision in the specificity of Xpert MTB/RIF across studies. This was the result of adjustments for the imperfect and heterogeneous accuracy of culture across studies.
Xpert Ultra versus Xpert MTB/RIF
We had insufficient data for this analysis.
Impact of tuberculosis prevalence on sensitivity and specificity
For Xpert Ultra, we had insufficient data for this analysis.
For Xpert MTB/RIF, we found higher sensitivity in settings with higher tuberculosis prevalence than in those with lower tuberculosis prevalence: pooled sensitivity (95% CrI) of 93.1% (88.9 to 96.3) versus 72.2% (64.9 to 87.2). We found lower specificity in settings with higher tuberculosis prevalence than in those with lower tuberculosis prevalence: pooled specificity of 83.2% (69.5 to 92.1) versus 90.0% (78.3 to 95.9). In the case of sensitivity, accuracy in the two groups was significantly different (probability of sensitivity being lower in the high tuberculosis prevalence group = 0.999); Table 10.
Sensitivity analyses
For Xpert Ultra, we had insufficient data for these analyses.
Inconclusive Xpert MTB/RIF and Xpert Ultra results
Xpert Ultra
None of the studies reported this information.
Xpert MTB/RIF
We previously reported that for lymph node aspirates, in the 1134 tests performed, the pooled proportion of inconclusive Xpert MTB/RIF results was 1.0% (95% CrI 0.4 to 2.0) (Kohli 2018).
E: Xpert MTB/RIF and Xpert Ultra in lymph node biopsies for lymph node tuberculosis
Xpert Ultra
Culture reference standard
In lymph node biopsies, Xpert Ultra sensitivity and specificity against culture were 90% (55 to 100) and 87% (77 to 94) (Antel 2020) and 100% (75 to 100) and 38% (22 to 55) (Wu 2019), (2 studies; 131 participants, 23 (17.6%) with tuberculosis); Figure 14.
14.
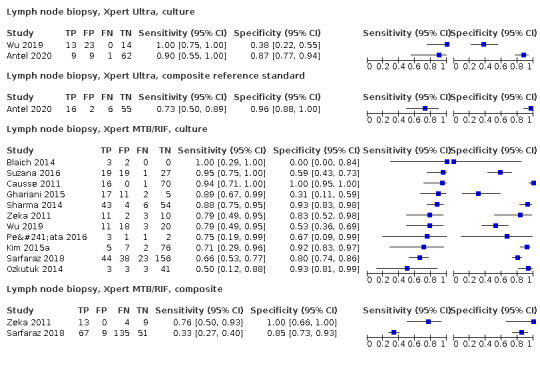
Forest plots of Xpert Ultra and Xpert MTB/RIFsensitivity and specificity in lymph node biopsy by reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
Composite reference standard
In lymph node biopsies, Xpert Ultra sensitivity and specificity against a composite reference standard were 73% (50 to 89) and 96% (88 to 100) (Antel 2020), (1 study; 81 participants); Figure 14.
Xpert MTB/RIF
Culture reference standard
Eleven studies evaluated Xpert MTB/RIF in lymph node biopsies against culture (Blaich 2014; Causse 2011; Ghariani 2015; Kim 2015a; Ozkutuk 2014; Peñata 2016; Sarfaraz 2018; Sharma 2014; Suzana 2016; Wu 2019; Zeka 2011). Xpert MTB/RIF sensitivity ranged from 50% to 100% and specificity ranged from 0% to 100%; Figure 14. We could not explain the heterogeneity in accuracy estimates by study quality, small numbers, or other factors.
In lymph node biopsies, Xpert MTB/RIF pooled sensitivity and specificity against culture were 82.4% (73.5 to 89.7) and 80.3% (60.3 to 91.5), (11 studies; 786 participants, 220 (28.0%) with tuberculosis); Table 6.
Composite reference standard
In lymph node biopsies, Xpert MTB/RIF sensitivity and specificity against a composite reference standard were 33% (27 to 40) and 85% (73 to 93) (Sarfaraz 2018) and 76% (50 to 93) and specificity of 100% (66 to 100) (Zeka 2011) (2 studies; 288 participants); Figure 14.
F: Xpert Ultra and Xpert MTB/RIF testing in urine for genitourinary tuberculosis
Xpert Ultra
Culture reference standard
In urine, Xpert Ultra sensitivity and specificity against culture were 100% (74 to 100) and 100% (74 to 100), (1 study; 24 participants, 12 (50%) with tuberculosis) (Perez‐Risco 2018); Figure 15.
15.
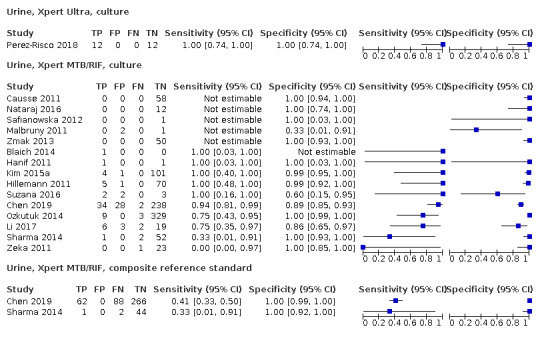
Forest plots of Xpert MTB/RIF and Xpert Ultra sensitivity and specificity in urine by reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
Composite reference standard
We did not identify any studies that evaluated Xpert Ultra in urine against a composite reference standard.
Xpert MTB/RIF
Culture reference standard
Fifteen studies evaluated Xpert MTB/RIF in urine against culture (Blaich 2014; Causse 2011; Chen 2019; Hanif 2011; Hillemann 2011; Kim 2015a; Li 2017; Malbruny 2011; Nataraj 2016; Ozkutuk 2014; Safianowska 2012; Sharma 2014; Suzana 2016; Zeka 2011; Zmak 2013). Xpert MTB/RIF sensitivity ranged from 0% to 100% (sensitivity of 0% was reported by Zeka 2011 that had only one culture positive, which was Xpert negative) and specificity from 33% to 100% (Figure 15). Six studies (Blaich 2014; Causse 2011; Malbruny 2011; Nataraj 2016; Sharma 2014; Zmak 2013) did not contribute data to the meta‐analysis because either sensitivity or specificity was not estimable.
In urine, Xpert MTB/RIF pooled sensitivity and specificity against culture were 85.9% (71.4 to 94.3) and 98.1% (93.1 to 99.7) (9 studies; 943 participants, 72 (7.6%) with tuberculosis); Table 6.
Composite reference standard
In urine, Xpert MTB/RIF sensitivity and specificity against a composite reference standard were 33% (1 to 91) and 100% (92 to 100) (Sharma 2014), and 41% (33 to 50 ) and 100% (99 to 100) (Chen 2019) (2 studies; 463 participants); Figure 15.
G: Xpert Ultra and Xpert MTB/RIF testing in bone or joint aspirate for bone or joint tuberculosis
Xpert Ultra
Culture reference standard
In bone or joint aspirate, Xpert Ultra sensitivity and specificity against culture were 88% (47 to 100) (specificity was not estimable) (Perez‐Risco 2018), and 96% (87 to 100) and 97% (85 to 100) (Sun 2019) (2 studies; 94 participants, 60 (63.8%) with tuberculosis); Appendix 9.
Composite reference standard
In bone or joint aspirate, Xpert Ultra sensitivity and specificity against a composite reference standard were 96% (91 to 99) and 97% (85 to 100), (1 study; 145 participants; Sun 2019); Appendix 9.
Xpert MTB/RIF
Culture reference standard
Twelve studies evaluated Xpert MTB/RIF in bone or joint fluid for culture (Al‐Ateah 2012; Blaich 2014; Gu 2015; Kim 2015a; Li 2017; Malbruny 2011; Nataraj 2016; Ozkutuk 2014; Peñata 2016; Safianowska 2012; Sun 2019; Suzana 2016). Xpert MTB/RIF sensitivity ranged from 96% to 100% and specificity ranged from 53% to 100%; Appendix 9.
In bone or joint aspirate, Xpert MTB/RIF pooled sensitivity and specificity against culture were 97.9% (93.1 to 99.6) and 97.4% (80.2 to 100.0); (6 studies; 471 participants, 110 (23.4%) with tuberculosis); Table 6
Composite reference standard
In bone or joint aspirate, Xpert MTB/RIF sensitivity and specificity against a composite reference standard were 82% (69 to 91) and 100% (69 to 100) (Gu 2015), and 94% (87 to 97) and 100% (90 to 100) (Sun 2019); (2 studies; 205 participants); Appendix 9.
H: Xpert Ultra and Xpert MTB/RIF testing in tissue for bone or joint tuberculosis
Xpert Ultra
Culture reference standard
We did not identify any studies that evaluated Xpert Ultra in tissue for bone or joint tuberculosis against culture.
Composite reference standard
We did not identify any studies that evaluated Xpert Ultra in tissue for bone or joint tuberculosis against a composite reference standard.
Xpert MTB/RIF
Culture reference standard
Three studies evaluated Xpert MTB/RIF in bone or joint tissue against culture. Xpert MTB/RIF sensitivity ranged from 50% to 100% and specificity ranged from 94% to 100% (Appendix 9).
In bone or joint tissue, Xpert MTB/RIF sensitivity and specificity (95% CI) against culture were 100% (3 to 100) and 100% (48 to 100) (Malbruny 2011), 100% (3 to 100) and 100% (40 to 100) (Ozkutuk 2014), and 50% (1 to 99) and 94% (71 to 100) (Peñata 2016); (3 studies; 30 participants, 4 (13.3%) with tuberculosis).
Composite reference standard
We did not identify any studies that evaluated Xpert MTB/RIF in tissue for bone or joint tuberculosis against a composite reference standard.
J: Xpert Ultra and Xpert MTB/RIF testing in peritoneal fluid for peritoneal tuberculosis
Xpert Ultra
Culture reference standard
In peritoneal fluid, Xpert Ultra sensitivity against culture was 33% (1 to 91) and specificity was not estimable (Perez‐Risco 2018) (1 study; 3 participants); Appendix 10.
Composite reference standard
We did not identify any studies that evaluated Xpert Ultra in peritoneal fluid against a composite reference standard.
Xpert MTB/RIF
Culture reference standard
Seventeen studies evaluated Xpert MTB/RIF in peritoneal fluid against culture (Al‐Ateah 2012; Causse 2011; Iram 2015; Kim 2015a; Li 2017; Malbruny 2011; Ozkutuk 2014; Peñata 2016; Rufai 2017a; Safianowska 2012; Scott 2014; Sharma 2014; Suzana 2016; Ullah 2017; Vadwai 2011; Zeka 2011; Zmak 2013). Four studies (Al‐Ateah 2012; Causse 2011; Iram 2015; Safianowska 2012) did not contribute data to the meta‐analysis because sensitivity was not estimable. In individual studies, Xpert MTB/RIF sensitivity ranged from 33% to 100% and specificity ranged from 90% to 100%; Figure 16; Appendix 10.
16.
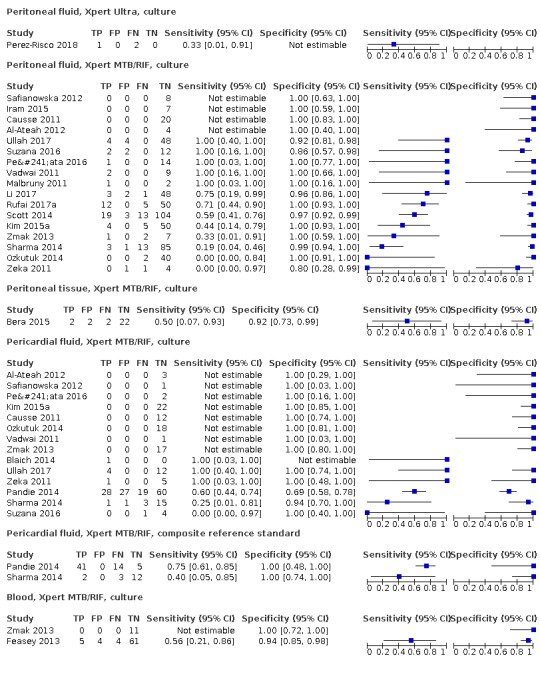
Forest plots of Xpert MTB/RIF sensitivity and specificity for peritoneal TB (fluid and tissue) by reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
In peritoneal fluid, Xpert MTB/RIF pooled sensitivity and specificity against culture were 59.1% (42.1 to 76.2) and 97.6% (95.4 to 98.9), (13 studies; 580 participants, 94 (16.2%) with tuberculosis); Table 6.
Composite reference standard
We did not identify any studies that evaluated Xpert MTB/RIF in peritoneal fluid against a composite reference standard.
K: Xpert Ultra and Xpert MTB/RIF testing in tissue for peritoneal tuberculosis
Xpert Ultra
Culture reference standard
We did not identify any studies that evaluated Xpert Ultra in peritoneal tissue against culture.
Composite reference standard
We did not identify any studies that evaluated Xpert Ultra in peritoneal tissue against a composite reference standard.
Xpert MTB/RIF
Culture reference standard
In peritoneal tissue, Xpert MTB/RIF sensitivity and specificity against culture were 50% (7 to 93) and 92% (73 to 99) (1 study; 28 participants; Bera 2015); Appendix 10.
Composite reference standard
We did not identify any studies that evaluated Xpert MTB/RIF in peritoneal tissue against a composite reference standard.
L: Xpert Ultra and Xpert MTB/RIF testing in pericardial fluid for pericardial tuberculosis
Xpert Ultra
Culture reference standard
We did not identify any studies that evaluated Xpert Ultra in pericardial fluid against culture.
Composite reference standard
We did not identify any studies that evaluated Xpert Ultra in pericardial fluid against a composite reference standard.
Xpert MTB/RIF
Culture reference standard
Fourteen studies evaluated Xpert MTB/RIF in pericardial fluid against culture (Al‐Ateah 2012; Blaich 2014; Causse 2011; Kim 2015a; Ozkutuk 2014; Pandie 2014; Peñata 2016; Safianowska 2012; Sharma 2014; Suzana 2016; Ullah 2017; Vadwai 2011; Zeka 2011; Zmak 2013). Xpert MTB/RIF sensitivity ranged from 0% to 100% and specificity ranged from 69% to 100% (Appendix 10). Nine studies (Al‐Ateah 2012; Blaich 2014; Causse 2011; Kim 2015a; Ozkutuk 2014; Peñata 2016; Safianowska 2012; Vadwai 2011; Zmak 2013) did not contribute data to the meta‐analysis because either sensitivity or specificity was not estimable.
In pericardial fluid, Xpert MTB/RIF pooled sensitivity and specificity against culture were 61.4% (32.4 to 82.4) and 89.7% (74.9 to 99.0), (5 studies; 181 participants, 57 (31.5%) with tuberculosis); Table 6; Appendix 10.
Composite reference standard
We did not identify any studies that evaluated Xpert MTB/RIF in pericardial fluid against a composite reference standard.
M: Xpert Ultra and Xpert MTB/RIF testing in blood for disseminated tuberculosis
Xpert Ultra
Culture reference standard
We did not identify any studies that evaluated Xpert Ultra in blood against culture.
Composite reference standard
We did not identify any studies that evaluated Xpert Ultra in blood against a composite reference standard.
Xpert MTB/RIF
Culture reference standard
Two studies evaluated Xpert MTB/RIF in blood against culture (Feasey 2013; Zmak 2013). However, only one of these studies reported tuberculosis culture‐positives. Xpert MTB/RIF sensitivity and specificity against culture were 56% (21 to 86) and 94% (85 to 98) (1 study; 74 participants, 9 (12.2%) with tuberculosis (Feasey 2013)); Appendix 10.
Composite reference standard
We did not identify any studies that evaluated Xpert MTB/RIF in blood against a composite reference standard.
Nontuberculous mycobacteria
For Xpert Ultra, two studies provided data on a variety of NTMs that grew from the specimens tested to look for evidence of cross‐reactivity. Donovan 2020 assessed Xpert Ultra specificity in CSF from more than 100 participants with nontuberculous meningitis and found zero positive Xpert Ultra results in those with a probable or possible diagnosis of tuberculous meningitis and in any participant with a confirmed diagnosis of nontuberculous meningitis. Perez‐Risco 2018 assessed Xpert Ultra specificity using 20 culture‐positive NTM specimens (covering a total of 18 species) and found that Xpert Ultra was negative for all specimens.
For Xpert MTB/RIF, we previously reported that in 10 studies involving 6975 specimens with 141 NTMs, Xpert MTB/RIF was negative in all specimens (Kohli 2018).
II. Detection of rifampicin resistance
Xpert Ultra and Xpert MTB/RIF testing for rifampicin resistance
Xpert Ultra
Five studies evaluated Xpert Ultra for detection of rifampicin resistance. Xpert Ultra sensitivity estimates varied from 50% to 100%; specificity varied from 93% to 100%; Figure 17. One study reported zero participants with rifampicin resistance and thus sensitivity was not estimable (Chin 2019). Four studies contributed data to the bivariate meta‐analysis (Sun 2019; Wang 2019; Wang 2020; Wu 2019). Xpert Ultra pooled sensitivity and specificity were 100.0% (95.1 to 100.0) and 100.0% (99.0 to 100.0), (4 studies; 129 participants, 24 (18.6%) with rifampicin resistance); Table 6.
17.
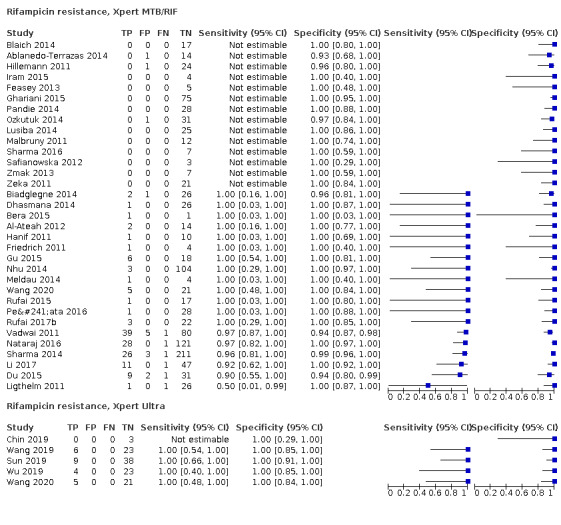
Forest plots of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity for rifampicin resistance. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
Xpert MTB/RIF
Xpert MTB/RIF pooled sensitivity and specificity were 96.5% (91.9 to 98.8) and 99.1% (98.0 to 99.7) (19 studies; 970 participants, 148 (15.3%) with rifampicin resistance); Table 6; Figure 17.
Indeterminate Xpert Ultra and Xpert MTB/RIF results for rifampicin resistance
Xpert Ultra
Of the total 391 tests positive by Xpert Ultra, the proportion of indeterminate Xpert Ultra results for RIF resistance was 36.1%. All of these indeterminate results were Xpert Ultra trace‐positive.
Xpert MTB/RIF
We previously reported that for rifampicin resistance testing, of 1003 tests performed, the pooled proportion of indeterminate Xpert MTB/RIF results was 2.6% (95% CrI 1.4 to 4.3) (Kohli 2018).
Discussion
Summary of main results
This systematic review update summarizes the current literature and includes 69 unique studies on the accuracy of Xpert Ultra and Xpert MTB/RIF for extrapulmonary tuberculosis and rifampicin resistance. We identified 11 studies evaluating Xpert Ultra, an increase of 10 new studies since the original review (Kohli 2018). Unlike the original review, we limited inclusion to adults aged 15 years and older. We also include a composite reference standard in addition to a culture reference standard, and have stratified all analyses by type of reference standard. Major findings from our review include the following.
Xpert Ultra sensitivity for tuberculosis varied across different types of specimens (from 75.0% in pleural fluid to 89.4% in cerebrospinal fluid); Table 6
Xpert MTB/RIF sensitivity for tuberculosis varied across different types of specimens (from 49.5% in pleural fluid to 97.9% in bone or joint aspirate); Table 6
Xpert MTB/RIF specificity in cerebrospinal fluid, pleural fluid, urine, bone or joint aspirate, and peritoneal fluid was ≥ 96.9%, against culture; overall, Xpert Ultra specificities were lower than those of Xpert MTB/RIF against culture, but against a composite reference standard results for both index tests were similar; Table 6
In cerebrospinal fluid, Xpert Ultra sensitivity and specificity were 89.4% (79.1 to 95.6) and 91.2% (83.2 to 95.7) against culture; Table 1.
In cerebrospinal fluid, Xpert MTB/RIF sensitivity and specificity were 71.1% (62.8 to 79.1) and 96.9% (95.4 to 98.0) against culture; Table 1
In pleural fluid, Xpert Ultra sensitivity and specificity were 75.0% (58.0 to 86.4) and 87.0% (63.1 to 97.9) against culture; Table 2
In pleural fluid, Xpert MTB/RIF sensitivity and specificity were 49.5% (39.8 to 59.9) and 98.9% (97.6 to 99.7) against culture; Table 2
In lymph node aspirate, Xpert Ultra sensitivity and specificity were 70% (51 to 85) and 100% (92 to 100) against a composite reference standard (1 study); Table 3
In lymph node aspirate, Xpert MTB/RIF sensitivity and specificity were 81.6% (61.9 to 93.3) and 96.4% (91.3 to 98.6) against a composite reference standard; Table 3
For rifampicin resistance, Xpert Ultra sensitivity and specificity were 100.0% (95.1 to 100.0) and 100.0% (99.0 to 100.0); Table 4
For rifampicin resistance, Xpert MTB/RIF sensitivity and specificity were 96.5% (91.9 to 98.8) and 99.1% (98.0 to 99.7); Table 4
Summary of findings 1. Xpert Ultra and Xpert MTB/RIF in cerebrospinal fluid.
|
Participants: people presumed to have tuberculous meningitis Prior testing: people who received Xpert Ultra or Xpert MTB/RIF testing may first have undergone a health examination (history and physical examination) and possibly received a chest radiograph Role: initial test, replacement for usual practice Settings: primarily tertiary care centres (the index test was often run in reference laboratories) Index tests: Xpert Ultra and Xpert MTB/RIF Reference standard: solid or liquid culture Studies: cross‐sectional studies Limitations: participants were evaluated exclusively as inpatients at a tertiary care centre, or, if the clinical setting was not reported, Xpert was performed at a reference laboratory rather than at primary care facilities and local hospitals Xpert Ultra pooled sensitivity (95% CrI): 89.4% (79.1 to 95.6); pooled specificity (95% CrI): 91.2% (83.2 to 95.7) Xpert MTB/RIF pooled sensitivity (95% CrI): 71.1% (62.8 to 79.1); pooled specificity (95% CrI): 96.9% (95.4 to 98.0) | |||||
| Xpert Ultra result | 1000 people tested for TB using Xpert Ultra(95% CrI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence of 2.5% | Prevalence of 10% | Prevalence of 20% | |||
| True‐positives (participants with TB meningitis) | 22 (20 to 24) |
89 (79 to 96) |
178 (158 to 191) |
89 (6) | ⊕⊕⊝⊝ Lowa |
| False‐negatives (participants incorrectly classified as not having TB meningitis) | 3 (1 to 5) |
11 (4 to 21) |
22 (9 to 42) |
||
| True‐negatives (participants without TB meningitis) | 889 (811 to 933) |
821 (749 to 861) |
730 (666 to 766) |
386 (6) | ⊕⊕⊕⊝ Moderateb |
| False‐positives (participants incorrectly classified as having TB meningitis) | 86 (42 to 164) |
79 (39 to 151) |
70 (34 to 134) |
||
| Xpert MTB/RIF result | 1000 people tested for TB using XpertMTB/RIF (95% CrI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence of 2.5% | Prevalence of 10% | Prevalence of 20% | |||
| True‐positives (participants with TB meningitis) | 18 (16 to 20) |
71 (63 to 79) |
142 (126 to 158) |
571 (30) | ⊕⊕⊕⊝ Moderatec |
| False‐negatives (participants incorrectly classified as not having TB meningitis) | 7 (5 to 9) |
29 (21 to 37) |
58 (42 to 74) |
||
| True‐negatives (participants without TB meningitis) | 945 (930 to 956) |
872 (859 to 882) |
775 (763 to 784) |
2824 (30) | ⊕⊕⊕⊕ High |
| False‐positives (participants incorrectly classified as having TB meningitis) | 30 (19 to 45) |
28 (18 to 41) |
25 (16 to 37) |
||
Abbreviations: Crl: credible interval; TB: tuberculosis
We included plausible prevalence estimates for the target condition suggested by the WHO. For Xpert Ultra, the median prevalence of tuberculosis in the included studies was 35.2%. For Xpert MTB/RIF, the median prevalence of tuberculosis in the included studies was 15.2%.
Credible limits were estimated based on those around the point estimates for pooled sensitivity and specificity.
aThere were few participants in this analysis. The very wide 95% CrI around true‐positives and false‐negatives may lead to different decisions, depending on which credible limits are assumed. We downgraded two levels for imprecision. bThe wide 95% CrI around true‐negatives and false‐positives would likely lead to different decisions, depending on which credible limits are assumed. We downgraded one level for imprecision. cThe wide 95% CrI around true‐positives and false‐negatives may lead to different decisions, depending on which credible limits are assumed. We downgraded one level for imprecision.
GRADE certainty of the evidence
High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The results presented in this table should not be interpreted in isolation from results of the individual included studies contributing to each summary test accuracy measure.
Summary of findings 2. Xpert Ultra and Xpert MTB/RIF in pleural fluid.
|
Participants: people presumed to have pleural tuberculosis Prior testing: people who received Xpert Ultra or Xpert MTB/RIF testing may first have undergone a health examination (history and physical examination) and received a chest radiograph Role: initial test, replacement for usual practice, which may include more invasive tests, such as pleural biopsy Settings: primarily tertiary care centres (the index test was often run in reference laboratories) Index tests: Xpert Ultra and Xpert MTB/RIF Reference standard: solid or liquid culture Studies: cross‐sectional studies Limitations: in most studies, participants were evaluated at a tertiary care centre, or if the clinical setting was not reported, the test was performed at a reference laboratory Xpert Ultra pooled sensitivity (95% CrI): 75.0% (58.0 to 86.4); pooled specificity (95% CrI): 87.0% (63.1 to 97.9) Xpert MTB/RIF pooled sensitivity (95% CrI): 49.5% (39.8 to 59.9); pooled specificity (95% CrI): 98.9% (97.6 to 99.7) | |||||
| Xpert Ultra result | 1000 people tested for TB using Xpert Ultra (95% CrI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence of 2.5% | Prevalence of 10% | Prevalence of 20% | |||
| True‐positives (patients with pleural TB) | 19 (14 to 22) |
75 (58 to 86) |
150 (116 to 173) |
158 (4) | ⊕⊝⊝⊝ Very lowa,b,c |
| False‐negatives (patients incorrectly classified as not having pleural TB) | 6 (3 to 11) |
25 (14 to 42) |
50 (27 to 84) |
||
| True‐negatives (patients without pleural TB) | 848 (615 to 955) |
783 (568 to 881) |
696 (505 to 783) |
240 (4) | ⊕⊝⊝⊝ Very lowa,d,e |
| False‐positives (patients incorrectly classified as having pleural TB) | 127 (20 to 360) |
117 (19 to 332) |
104 (17 to 295) |
||
| Xpert MTB/RIF result | 1000 people tested for TB using Xpert MTB/RIF (95% CrI) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence of 2.5% | Prevalence of 10% | Prevalence of 20% | |||
| True‐positives (patients with pleural TB) | 12 (10 to 15) |
50 (40 to 60) |
99 (80 to 120) |
644 (25) | ⊕⊕⊝⊝ Lowf,g,h |
| False‐negatives (patients incorrectly classified as not having pleural TB) | 13 (10 to 15) |
50 (40 to 60) |
101 (80 to 120) |
||
| True‐negatives (patients without pleural TB) | 964 (952 to 972) |
890 (878 to 897) |
791 (781 to 798) |
2421 (25) | ⊕⊕⊕⊕ High |
| False‐positives (patients incorrectly classified as having pleural TB) | 11 (3 to 23) |
10 (3 to 22) |
9 (2 to 19) |
||
Abbreviations: CrI: credible interval; TB: tuberculosis.
We included plausible prevalence estimates for the target condition suggested by the WHO. For Xpert Ultra, the median prevalence of tuberculosis in the included studies was 46.2%. For Xpert MTB/RIF, the median prevalence of tuberculosis in the included studies was 19.8%.
aWe were interested in how Xpert Ultra performed in patients presumed to have extrapulmonary tuberculosis who were evaluated as they would be in routine practice. However, most studies did not report information on the clinical setting. We downgraded one level for indirectness. bFor individual studies, sensitivity estimates ranged from 48% to 84%. We could not explain the heterogeneity by study quality or other factors. We downgraded one level for inconsistency. cThere was a low number of participants contributing to this analysis for the observed sensitivity. As we had already downgraded for inconsistency, we downgraded one level for imprecision. dFor individual studies, specificity estimates ranged from 65% to 100%. We could not explain the heterogeneity by study quality or other factors. We downgraded one level for inconsistency. eWe thought the wide 95% CrI around false‐positives and true‐negatives would likely lead to different decisions depending on which confidence limits are assumed. As we had already downgraded for inconsistency, we downgraded one level for imprecision. fWe were interested in how Xpert MTB/RIF performed in participants presumed to have extrapulmonary tuberculosis who were evaluated as they would be in routine practice. However, most studies did not report information on the clinical setting. We downgraded one level for indirectness. gFor individual studies, sensitivity estimates ranged from 10% to 100%. We could not explain the heterogeneity by study quality or other factors. We downgraded one level for inconsistency. hAs we had already downgraded for inconsistency, we did not downgrade further for imprecision.
GRADE certainty of the evidence
High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The results presented in this table should not be interpreted in isolation from results of the individual included studies contributing to each summary test accuracy measure.
Summary of findings 3. Xpert Ultra and Xpert MTB/RIF in lymph node aspirate.
|
Participants: people presumed to have lymph node tuberculosis Prior testing: people who received Xpert testing may first have undergone a health examination (history and physical examination) and possibly received a chest radiograph Role: initial test, replacement for usual practice, which may include more invasive tests, such as biopsy of affected organs Settings: primarily tertiary care centres (the index test was often run in reference laboratories) Index tests: Xpert Ultra and Xpert MTB/RIF Reference standard: composite reference standard Studies: cross‐sectional studies Limitations: in most studies, participants were evaluated at a tertiary care centre, or if the clinical setting was not reported, the test was performed at a reference laboratory performed at a reference laboratory Xpert Ultra sensitivity (95% CI): 70% (51 to 85); specificity: 100% (92 to 100) Xpert MTB/RIF pooled sensitivity (95% CrI): 81.6% (61.9 to 93.3); pooled specificity: 96.4% (91.3 to 98.6) | |||||
| Xpert Ultra result | 1000 people tested for TB using Xpert Ultra (95% Cl) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence of 2.5% | Prevalence of 10% | Prevalence of 20% | |||
| True‐positives (patients with lymph node TB) | 17 (13 to 21) | 70 (51 to 85) | 140 (102 to 170) | 30 (1) | ⊕⊝⊝⊝ Very lowa,b |
| False‐negatives (patients incorrectly classified as not having lymph node TB) | 8 (4 to 12) | 30 (15 to 49) | 60 (30 to 98) | ||
| True‐negatives (patients without lymph node TB) | 975 (897 to 975) | 900 (828 to 900) | 800 (736 to 800) | 43 (1) | ⊕⊝⊝⊝ Very lowa,c |
| False‐positives (patients incorrectly classified as having lymph node TB) | 0 (0 to 78) | 0 (0 to 72) | 0 (0 to 64) | ||
| Xpert MTB/RIF result | 1000 people tested for TB using Xpert MTB/RIF (95% Crl) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence of 2.5% | Prevalence of 10% | Prevalence of 20% | |||
| True‐positives (patients with lymph node TB) | 20 (16 to 23) | 81 (62 to 92) | 162 (124 to 184) | 377 (4) | ⊕⊕⊝⊝ Lowd,e |
| False‐negatives (patients incorrectly classified as not having lymph node TB) | 5 (2 to 9) | 19 (8 to 38) | 38 (16 to 76) | ||
| True‐negatives (patients without lymph node TB) | 935 (878 to 958) | 863 (811 to 885) | 767 (721 to 786) | 302 (4) | ⊕⊕⊝⊝ Lowd,e |
| False‐positives (patients incorrectly classified as having lymph node TB) | 40 (17 to 97) | 37 (15 to 89) | 33 (14 to 79) | ||
Abbreviations: Crl: credible interval; TB: tuberculosis.
We included plausible prevalence estimates for the target condition suggested by the WHO. For Xpert Ultra, the prevalence of tuberculosis in the included study was 41%. For Xpert MTB/RIF, the median prevalence of tuberculosis in the included studies was 55.5%.
aWe identified only one study, which was conducted at a tertiary referral centre in South Africa, a high TB burden country. Most participants (84%) were seen as outpatients. With only one study, applicability to other settings comes with some uncertainty. We downgraded one level for indirectness. bThere were very few participants contributing to this analysis. The 95% CI was very wide. We downgraded two levels for imprecision. cThere were very few participants contributing to this analysis. The 95% CI was wide. We downgraded two levels for imprecision. dThe composite reference standard was defined by the primary study authors and therefore, was not uniform. We downgraded one level for risk of bias. eFor indirectness, regarding applicability, for the patient population, we considered most studies to have unclear concern. We were interested in how Xpert MTB/RIF performed in patients presumed to have extrapulmonary TB who were evaluated as they would be in routine practice. However, none of the studies reported this information. We downgraded one level for indirectness.
GRADE certainty of the evidence
High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The results presented in this table should not be interpreted in isolation from results of the individual included studies contributing to each summary test accuracy measure.
Summary of findings 4. Xpert Ultra and Xpert MTB/RIF for rifampicin resistance.
|
Participants: people with tuberculosis detected by Xpert Ultra or Xpert MTB/RIF Role: initial test, replacement test for standard practice, which includes culture‐based drug susceptibility testing or line probe assay Settings: primarily tertiary care centres, the index test was often run in central (reference laboratories), where drug susceptibility testing for the reference standard could be performed Index tests: Xpert Ultra and Xpert MTB/RIF Reference standard: culture‐based drug susceptibility testing using solid or liquid media or line probe assay Studies: cross‐sectional studies Xpert Ultra pooled sensitivity (95% CrI): 100.0% (95.1 to 100.0); pooled specificity (95% CrI): 100.0% (99.0 to 100.0) Xpert MTB/RIF pooled sensitivity (95% CrI): 96.5% (91.9 to 98.8); pooled specificity (95% CrI): 99.1% (98.0 to 99.7) | |||||
| Xpert Ultra result | 1000 people tested for rifampicin resistance using Xpert Ultra (95% Crl) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence of 2% | Prevalence of 10% | Prevalence of 15% | |||
| True‐positives (patients correctly classified as rifampicin resistant) | 20 (19 to 20) | 100 (95 to 100) | 150 (143 to 150) | 24 (4) | ⊕⊕⊝⊝ Lowa,b |
| False‐negatives (patients incorrectly classified as rifampicin susceptible) | 0 (0 to 1) | 0 (0 to 5) | 0 (0 to 7) | ||
| True‐negatives (patients correctly classified as rifampicin susceptible) | 980 (979 to 980) | 900 (899 to 900) | 850 (849 to 850) | 105 (4) | ⊕⊕⊕⊝ Moderatea |
| False‐positives (patients incorrectly classified as rifampicin resistant) | 0 (0 to 1) | 0 (0 to 1) | 0 (0 to 1) | ||
| Xpert MTB/RIF result | 1000 people tested for rifampicin resistance using Xpert MTB/RIF (95% Crl) | Number of participants (studies) | Certainty of the evidence (GRADE) | ||
| Prevalence of 2% | Prevalence of 10% | Prevalence of 15% | |||
| True‐positives (patients correctly classified as rifampicin resistant) | 19 (18 to 20) | 97 (92 to 99) | 145 (138 to 148) | 148 (19) | ⊕⊕⊕⊕ High |
| False‐negatives (patients incorrectly classified as rifampicin susceptible) | 1 (0 to 2) | 3 (1 to 8) | 5 (2 to 12) | ||
| True‐negatives (patients correctly classified as rifampicin susceptible) | 971 (960 to 977) | 892 (882 to 897) | 842 (833 to 847) | 822 (19) | ⊕⊕⊕⊕ High |
| False‐positives (patients incorrectly classified as rifampicin resistant) | 9 (3 to 20) | 8 (3 to 18) | 8 (3 to 17) | ||
Abbreviations: Crl: credible interval; TB: tuberculosis.
We included plausible prevalence estimates for the target condition suggested by the WHO. For Xpert Ultra, the median prevalence of rifampicin resistance in the included studies was 19.2%. For Xpert MTB/RIF, the median prevalence of rifampicin resistance in the included studies was 11.9%.
aAll these studies were conducted in China (high TB‐burden country). Applicability to other settings comes with some uncertainty and therefore we downgraded one level for indirectness. bThere was a low number of participants contributing to this analysis for the observed sensitivity. We downgraded one level for imprecision.
GRADE certainty of the evidence
High: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The results presented in this table should not be interpreted in isolation from results of the individual included studies contributing to each summary test accuracy measure.
Xpert Ultra and Xpert MTB/RIF testing in cerebrospinal fluid
(Table 1)
Xpert Ultra
Results of these studies indicate that in theory, for a population of 1000 people where 100 have tuberculosis meningitis on culture, 168 would be Xpert Ultra‐positive: of these, 79 (47%) would not have tuberculosis (false‐positives); and 832 would be Xpert Ultra‐negative: of these, 11 (1%) would have tuberculosis (false‐negatives).
Xpert MTB/RIF
Results of these studies indicate that in theory, for a population of 1000 people where 100 have tuberculosis meningitis on culture, 99 would be Xpert MTB/RIF‐positive: of these, 28 (28%) would not have tuberculosis (false‐positives); and 901 would be Xpert MTB/RIF‐negative: of these, 29 (3%) would have tuberculosis (false‐negatives).
Rapid diagnosis of tuberculous meningitis is critical so that lifesaving treatment can be started promptly. Around 50% of those affected die or experience disabling consequences (Thwaites 2013). Xpert Ultra was designed to improve tuberculosis detection, in particular in people with paucibacillary disease. The limit of detection for MTB is lower with Xpert Ultra (16 bacterial colony‐forming units (cfu) per mL) than with Xpert MTB/RIF (131 cfu per mL) (Chakravorty 2017). In studies that compared Xpert Ultra and Xpert MTB/RIF in the same participants, we found Xpert Ultra to have higher pooled sensitivity (89.0%) than Xpert MTB/RIF (62.2%), and lower pooled specificity (91.0%) than Xpert MTB/RIF (96.8%) for tuberculous meningitis. In addition, in subgroup analyses we found slightly higher Xpert Ultra accuracy in studies that concentrated the cerebrospinal fluid (CSF): pooled sensitivity of 90.5% in concentrated specimens versus 88.4% in unconcentrated specimens; and pooled specificity of 91.9% in concentrated specimens versus 88.6% in unconcentrated specimens. We note that subgroup findings should be interpreted with caution, as there were only three studies and a small number of tuberculous meningitis cases included. The Tuberculous Meningitis International Research Consortium has recommended increasing the volume of CSF collected for diagnosis followed by centrifugation as a way of improving Xpert MTB/RIF assay sensitivity (Bahr 2016); however, we did not have sufficient data to investigate CSF collection volume. Increased Xpert MTB/RIF sensitivity in HIV‐positive people compared with HIV‐negative people has been reported, with the increased bacterial burden in tuberculosis and HIV co‐infection proposed as the reason (Patel 2013). We had limited data to investigate this for Xpert Ultra as we identified only two studies in HIV‐positive people, with sensitivities of 90% (Bahr 2017) and 89% (Cresswell 2020).
Xpert Ultra and Xpert MTB/RIF testing in pleural fluid
(Table 2)
Xpert Ultra
Results of these studies indicate that in theory, for a population of 1000 people where 100 have pleural tuberculosis on culture, 192 would be Xpert Ultra‐positive: of these, 117 (61%) would not have tuberculosis (false‐positives); and 808 would be Xpert Ultra‐negative: of these, 25 (3%) would have tuberculosis (false‐negatives).
Xpert MTB/RIF
Results of these studies indicate that in theory, for a population of 1000 people where 100 have pleural tuberculosis on culture, 60 would be Xpert MTB/RIF‐positive: of these, 10 (17%) would not have tuberculosis (false‐positives); and 940 would be Xpert MTB/RIF‐negative: of these, 50 (5%) would have tuberculosis (false‐negatives).
With the bivariate model, we found Xpert Ultra to have higher pooled sensitivity (75.0%) than Xpert MTB/RIF (49.6%) and lower pooled specificity (87.0%) than Xpert MTB/RIF (98.7%) in pleural fluid against a culture reference standard, between‐study comparison. Based on the latent class meta‐analysis model, Xpert Ultra pooled sensitivity was comparable (76.0%) and specificity higher (99.5%) than what was obtained when culture was treated as having perfect accuracy. Xpert Ultra pooled sensitivity in pleural fluid was lower than that of CSF. One reason for the lower sensitivity of Xpert Ultra in pleural fluid could be the paucibacillary nature of pleural tuberculosis. Other possible reasons are contamination of blood or the presence of certain polymerase chain reaction (PCR) inhibitors in the pleural fluid (Pai 2004; Woods 2001). However, Theron and colleagues found that extrapulmonary specimens showed less evidence of PCR inhibition than pulmonary specimens, with bacterial load being more important for a positive Xpert MTB/RIF result (Theron 2014b). Given that false‐negative results were common (low sensitivity), a negative Xpert Ultra or Xpert MTB/RIF result may not be relied on to exclude tuberculosis.
Xpert Ultra testing in lymph node aspirates
Xpert Ultra
Results of these studies indicate that in theory, for a population of 1000 people where 100 have lymph node tuberculosis verified by a composite reference standard, 70 would be Xpert Ultra‐positive: of these, 0 (0%) would not have tuberculosis (false‐positives); and 930 would be Xpert Ultra‐negative: of these, 30 (3%) would have tuberculosis (false‐negatives).
Xpert MTB/RIF
Results of these studies indicate that in theory, for a population of 1000 people where 100 have lymph node tuberculosis verified by a composite reference standard, 118 would be Xpert MTB/RIF‐positive: of these 37 (31%) would not have tuberculosis (false‐positives); and 882 would be Xpert MTB/RIF‐negative: of these 19 (2%) would have tuberculosis (false‐negatives).
Regarding Xpert testing for lymph node aspirate, it important to point out that although tissue biopsy provides material for histological examination which may be of substantial diagnostic value, a fluid specimen may be collected more easily. In addition, fine‐needle aspiration of lymph nodes is well suited for use in resource‐limited settings because the procedure is simple, easy to learn, minimally invasive, and inexpensive (Wright 2009b). Thus clinicians may want to consider fine‐needle aspiration of lymph nodes before surgical biopsy.
In our review, using a standard bivariate meta‐analysis model, Xpert MTB/RIF pooled specificity (defined by culture) in lymph node aspirate was 86.0%, whereas with a composite reference standard pooled specificity increased to 95.9%. Using a latent class meta‐analysis model with informative priors, Xpert MTB/RIF pooled specificity increased to 99.5%. In previous meta‐analyses, Xpert MTB/RIF specificity for lymph node tuberculosis (aspirate and tissue) against culture as a reference standard was 94% (Denkinger 2014), 93% (Maynard‐Smith 2014), and 92% (Penz 2015). See Table 12. Using a composite reference standard (defined by the primary study authors), Denkinger 2014 found increased Xpert MTB/RIF specificity of 99% for lymph node tuberculosis (5 studies, 728 specimens). Thus, it appears that accuracy results depend in part on the choice of reference standard. Regarding the use of a composite reference standard, owing to differing definitions and difficulty in interpreting them, there is a risk of bias (Schiller 2016) (see section Strengths and weaknesses of the review).
8. Systematic reviews of Xpert MTB/RIF and Xpert Ultra for extrapulmonary tuberculosis.
| Systematic review | Search period | Index test | Number of studies (total number of extrapulmonary specimens) | Forms of extrapulmonary TB or types of specimens | Accuracy against culture reference standard | ||
| Tuberculous meningitis | Pleural tuberculosis (pleural fluid) | Lymph node tuberculosis | |||||
| Chang 2012a | Up to 1 October 2011 | Xpert MTB/RIF | 7 (1058) | Multiple forms combined | Not reported | Not reported | Not reported |
| Denkinger 2014b | Up to 15 October 2013 |
Xpert MTB/RIF | 18 (4461) | Lymph node, pleural fluid, CSF | Sensitivity 81%; specificity 98% | Sensitivity 46%; specificity 99% | Sensitivity 83%; specificity 94% |
| Maynard‐Smith 2014 | Up to 6 November 2013 | Xpert MTB/RIF | 27 (6026) | Lymph node, pleural fluid, CSF, other forms | Median sensitivity 85% (IQR 75% to 100%); median specificity 100% (IQR 98% to 100%) | Sensitivity 34%; specificity 98% | Sensitivity 96%; specificity 93% |
| Penz 2015 | Up to 15 August 2014 | Xpert MTB/RIF | 36 (9523) | Lymph node, pleural fluid, CSF, other forms | Sensitivity 69%; specificity 97% | Sensitivity 37%; specificity 98% | Sensitivity 87%; specificity 92% |
| Sehgal 2016 | Up to 31 August 2015 | Xpert MTB/RIF | 24 (2486) | Pleural fluid | Not applicable | Sensitivity 51%; specificity 99% |
Not applicable |
| Li Y 2017c | Up to 20 June 2015 | Xpert MTB/RIF | 26 (not reported) | Multiple forms combined | Not reported | Not reported | Not reported |
| Gupta 2018 | Up to 25 March 2017 | Xpert MTB/RIF | 33 (8977) | CSF | Sensitivity 57%; specificity 98% | Not reported | Not reported |
| Pormohammad 2019 | Upto 11 Nov 2018 | Xpert MTB/RIF | 16 (not reported) | CSF | Sensitivity 61%; specificity 99% | Not reported | Not reported |
| Yu 2019 | Up to 6 July 2018 | Xpert MTB/RIF | 21(1629) | Lymph node | Not reported | Not reported | Sensitivity 84%; specificity: 91% |
| Zhang 2020d | Up to 20 May 2019 | Xpert Ultra | 7 (1500) | Multiple specimens | Not reported | Not reported | Not reported |
Abbreviations: CI: confidence interval; CSF: cerebrospinal fluid: IQR: interquartile range; TB: tuberculosis.
aFor all forms of extrapulmonary tuberculosis combined, Chang 2012 reported pooled sensitivity and specificity of 80.4% (95% confidence interval (CI) 75.0 to 85.1) and 86.1% (95% CI 83.5 to 88.4), respectively. bUsing a composite reference standard, Denkinger 2014 found the following pooled sensitivity and specificity estimates: lymph node tuberculosis (aspirate or tissue) 81.2% (95% CI 72.4 to 87.7) and 99.1% (95% CI 94.5 to 99.9); pleural tuberculosis 21.4% (95% CI 8.8 to 33.9) and 100% (95% CI 99.4 to 100); and meningeal TB 62.8% (95% CI 47.7 to 75.8) and 98.8% (95% CI 95.7 to 100), respectively. cFor both pulmonary and extrapulmonary tuberculosis, review authors included 106 studies involving 52,410 specimens. For all forms of extrapulmonary tuberculosis combined, Li Y 2017 reported pooled sensitivity and specificity of 80% (95% CI 69 to 88) and 97% (95% CI 94 to 98), respectively. dZhang 2020 included 7 studies involving 1500 specimens. For all forms of extrapulmonary tuberculosis combined, pooled sensitivity and specificity were 85.1% (95% CI 76.7 to 90.8%) and 95.7% (95% CI 87.9 to 98.6%), respectively.
Shen 2019 provided pooled estimates for bone or joint tuberculosis. The review included 14 studies with 1884 specimens with a pooled sensitivity of 96% and specificity of 85%.
We considered several reasons why the specificity of Xpert Ultra (78%) and Xpert MTB/RIF (86.0%) in lymph node aspirate against culture would be lower than in other extrapulmonary specimens. Although not always reported, studies may have included participants receiving tuberculosis treatment. We previously reported that in a sensitivity analysis limiting inclusion to studies that involved participants not receiving tuberculosis treatment, specificity increased from 86% to 89% (Kohli 2018). We considered the type of culture used in the included studies because liquid culture is more sensitive than solid culture (American Thoracic Society 2000). Most studies used liquid culture or a combination of solid and liquid culture. The single study evaluating Xpert Ultra used liquid culture. Only two of the 15 studies (13%) evaluating Xpert MTB/RIF exclusively used solid culture. Culture results may also be negative owing to inefficient specimen collection or errors in sampling, differing bacterial load, and contamination (Wright 2009b). Negative culture results in lymph node tuberculosis have previously been reported (Fontanilla 2011).
Another reason for negative culture results is that there may have been a decrease in live tuberculosis bacteria during processing with N‐acetyl‐L‐cysteine‐sodium hydroxide, which is routinely used to homogenize, decontaminate, and liquefy non‐sterile specimens, such as sputum, for mycobacterial culture (American Thoracic Society 2000). Harsh decontamination practices have been noted to contribute to false‐negative culture results, especially in paucibacillary specimens (FIND 2017). Standards specify, "specimens collected from normally sterile sites may be placed directly into the culture medium” (American Thoracic Society 2000). CSF, pleural fluid, and lymph node aspirates are usually considered to be sterile specimens. It is our understanding that some laboratories do decontaminate sterile site specimens as a precaution against non‐sterile collection procedures. In this review, 47% of the studies reported decontaminating lymph node aspirates before culture inoculation. We did not have sufficient data to further investigate laboratory practices.
In summary, several factors probably contributed to low Xpert MTB/RIF specificity against culture in lymph node aspirate. The 'true' specificity of Xpert MTB/RIF in lymph node aspirate is likely to be higher owing to the aforementioned reasons. Xpert MTB/RIF specificity was higher against a composite reference standard and with application of latent class analysis, similar to that found in CSF, pleural fluid, and other specimens (Table 6; Table 7).
We investigated the prevalence of extrapulmonary tuberculosis (confirmed by culture) as a potential source of heterogeneity because changes in disease prevalence have often been found to be associated with other important changes, such as changes in the disease spectrum, which may affect diagnostic accuracy estimates (Leeflang 2013). For Xpert MTB/RIF, for pleural fluid and lymph node aspirate, we found that pooled sensitivity was higher in settings with higher tuberculosis prevalence. In all analyses, for both Xpert Ultra (CSF) and Xpert MTB/RIF (CSF, pleural fluid, and lymph node aspirate), specificity in settings with higher tuberculosis prevalence was similar or lower than in settings with lower tuberculosis prevalence. Findings from additional analyses are available in the previous version of this review (Kohli 2018).
Xpert Ultra and Xpert MTB/RIF testing for rifampicin resistance
(Table 4)
Xpert Ultra
Results of these studies indicate that in theory, for a population of 1000 people where 100 have rifampicin resistance, 100 would be Xpert Ultra‐positive (resistant): of these, zero (0%) would not have rifampicin resistance (false‐positives); and 900 would be Xpert Ultra‐negative (susceptible): of these, zero (0%) would have rifampicin resistance.
Xpert MTB/RIF
Results of these studies indicate that in theory, for a population of 1000 people where 100 have rifampicin resistance, 105 would be Xpert MTB/RIF‐positive (resistant): of these, 8 (8%) would not have rifampicin resistance; and 895 would be Xpert MTB/RIF‐negative (susceptible): of these, 3 (0.3%) would have rifampicin resistance.
For detection of rifampicin resistance in extrapulmonary specimens, we found the sensitivity of Xpert Ultra (100%) and Xpert MTB/RIF (96.7%) and the specificity of Xpert Ultra (100%) and Xpert MTB/RIF (99.1%), to be comparable to estimates in pulmonary specimens: sensitivity (96%) and specificity (98%) (Horne 2019). We caution that the results for Xpert Ultra are based on only four studies, involving 129 participants, 24 (18.6%) with rifampicin resistance. Nonetheless, these findings suggest that the use of Xpert Ultra and Xpert MTB/RIF in extrapulmonary specimens could assist in rapid diagnosis of rifampicin‐resistant tuberculosis and early initiation of treatment for multidrug‐resistant tuberculosis (MDR‐TB).
Notably, concerns have been raised about rapid drug susceptibility testing (DST) methods, in particular automated mycobacteria growth indicator tube (MGIT) 960 for tuberculosis drug resistance using the recommended critical concentrations. As a priority, the WHO is planning to re‐evaluate the critical concentrations for rifampicin (WHO 2018).
For Xpert Ultra, we found a high rate (36.1%) of indeterminate rifampicin resistance results, all owing to trace call results. This finding was expected since, for trace call results, rifampicin resistance cannot be determined. Xpert Ultra incorporates two new multi‐copy amplification targets (IS6110 and IS1081). Trace call indicates that only the multi‐copy targets were detected, and not the tuberculosis‐specific regions in the rpoB gene. Resistance to rifampicin has mainly been associated mainly with mutations in a limited region of the rpoB gene (Telenti 1993).
People‐important outcomes, such as mortality, are especially relevant to patients, decision‐makers, and the wider tuberculosis community. While performing this systematic review, we did not identify direct evidence of studies linking true‐positives, false‐positives, true‐negatives, and false‐negatives to people‐important outcomes when either Xpert Ultra or Xpert MTB/RIF was used to diagnose extrapulmonary tuberculosis. To our knowledge, for pulmonary tuberculosis, there have been two systematic reviews of randomized trials on the impact of the use of Xpert MTB/RIF on health outcomes. Both reviews compared the effect of Xpert MTB/RIF and smear microscopy on all‐cause mortality; Di Tanna and colleagues summarized the accuracy of Xpert MTB/RIF in an individual patient‐level data meta‐analysis (3 trials, 8143 participants) (Di Tanna 2019) and Haraka and colleagues performed a random‐effects meta‐analysis, (5 trials, 10,409 participants (Haraka 2018; WHO Consolidated Guidelines (Module 3) 2020). In both reviews, Xpert MTB/RIF did not show a statistically significant effect on all‐cause mortality, although the direction of effect was towards mortality reduction. Insufficient power to detect mortality in randomized trials measuring the impact of diagnostic tests on patient‐important outcomes has been discussed previously as a limitation of such trials (Di Tanna 2019; Schumacher 2019). Larger sample sizes are needed to evaluate the effect of Xpert MTB/RIF on mortality, but achieving this is difficult in pragmatic situations. For example, Schumacher 2019 showed that a sample size of 31,000 participants would be needed if researchers were to plan a cluster‐randomized diagnostic trial using the baseline mortality and effect size demonstrated by the individual patient data from Di Tanna 2019.
This review represents the most comprehensive review of the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF for extrapulmonary tuberculosis in adults. For Xpert MTB/RIF, our previous review (Kohli 2018) provides additional findings. These reviews provide evidence that may help countries to make decisions about scaling up the tests for programmatic management of tuberculosis and drug‐resistant tuberculosis. Although the information in this review will help to inform such decisions, other factors such as resource requirements and feasibility (including stable electrical power supply, temperature control, and maintenance of the cartridge modules) will also be important considerations.
Strengths and weaknesses of the review
Completeness of evidence
This is a reasonably complete data set. We included any non‐English studies that we found from which we could obtain accuracy data. However, we acknowledge that we may have missed some studies despite the comprehensive search and our outreach to investigators. We included eight common forms of extrapulmonary tuberculosis in the review. However, for some of these forms, such as disseminated tuberculosis, data were insufficient to allow us to determine summary accuracy estimates. We did not include less common forms, such as cutaneous tuberculosis, ocular tuberculosis, female genital tuberculosis, and tuberculosis of the breast. We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses of Diagnostic Test Accuracy (PRISMA‐DTA) (McInnes 2018).
Accuracy of the reference standards used
In a systematic review of diagnostic test accuracy studies, the reference standard is the best available test to determine the presence or absence of the target condition. In this review, we used two reference standards: culture and a composite reference standard, both of which are known to be imperfect. While the composite reference standard is designed to have improved accuracy compared to culture alone, it may still lead to biased accuracy estimates of the index test, depending on various factors such as the accuracy of the different components; decision rules for combining them; prevalence of the target condition; and conditional dependence between the components and the index test (Schiller 2016). Conditional dependence between two imperfect tests arises when both tests make the same false‐positive or false‐negative errors more often than expected by chance (Naaktgeboren 2013). Hence, conditional dependence may arise between the index test and both reference standards we have used, as they are imperfect. As a consequence, we may over‐ or underestimate the diagnostic accuracy of the index tests. An additional challenge with including a composite reference standard is that the definition of the composite reference standard may vary across studies, making it difficult to interpret the accuracy estimates. To adjust for the imperfect accuracy of culture, we applied a latent class model when evaluating Xpert MTB/RIF sensitivity and specificity, for which there were a larger number of studies. We added parameters for the sensitivity and specificity of culture and terms for conditional dependence to adjust for the dependence between Xpert MTB/RIF and culture among disease‐positive and disease‐negative participants. In this way, we were able to improve estimation of both the pooled sensitivity and specificity of Xpert MTB/RIF, as well as between‐study variability. An adequate number of studies is needed for a sufficiently robust model to estimate these additional parameters. We therefore found that we were unable to do the same for meta‐analyses of the accuracy for Xpert Ultra owing to the small number of studies, many of which had small sample sizes resulting in zero cell counts.
Several factors may have contributed to false‐negative culture results for the accuracy of the reference standard for lymph node aspirate in particular, including inefficient specimen collection and overly harsh decontamination. For this particular analysis, we were able to take advantage of the Bayesian estimation approach to incorporate prior information on Xpert MTB/RIF and culture specificity. This allowed us to make the best use of data from the included studies and our knowledge of the performance of Xpert MTB/RIF. We had insufficient data to apply the latent class model to data from the single study evaluating Xpert Ultra in lymph node aspirates.
Establishing a diagnosis of extrapulmonary tuberculosis would ideally include pursuing the diagnosis of pulmonary tuberculosis as well, because participants with tuberculosis may have both pulmonary and extrapulmonary tuberculosis and the lung may be the only site where the presence of tuberculosis can be established. Because of the difficulties involved in diagnosing HIV‐associated tuberculosis, it is recommended that multiple cultures from sputum and other types of specimens be evaluated in people living with HIV (Bjerrum 2019; Shah 2016b). Given the limitations in the reference standard, we recommend that future studies consider using liquid culture because this is more sensitive than solid culture, and that researchers obtain multiple specimens for culture to confirm the diagnosis of extrapulmonary tuberculosis (Drain 2019).
Most studies included in this review used culture‐based DST (either Löwenstein‐Jensen (LJ) or mycobacteria growth indicator tube (MGIT) 960) as the reference standard for detection of rifampicin resistance. Concerns have been raised about rapid DST methods, in particular automated MGIT 960, for tuberculosis drug resistance using the recommended critical concentrations. The WHO is planning to prioritise a re‐evaluation of the critical concentrations for rifampicin (WHO 2018).
We assessed the number of specimens with nontuberculous mycobacteria (NTMs) that were Xpert Ultra‐positive. In two studies that reported 120 NTMs, Xpert Ultra was negative in all specimens. In the previous version of this review, among 10 studies that reported information comprising 141 NTMs, Xpert MTB/RIF was negative in all specimens (Kohli 2018).
Quality and quality of reporting of the included studies
Risk of bias was low for the participant selection, index test, and flow and timing domains and was high or unclear for the reference standard domain (most of these studies performed specimen decontamination before culture inoculation). A limitation was that several studies included more than one specimen per participant, which artificially inflated the sample size of the study and may have led to overestimation or underestimation of the accuracy estimates. In general, studies were fairly well reported, although we corresponded with almost all primary study authors to ask for additional data and missing information. In several studies, accuracy data by site of extrapulmonary disease were not reported, and in a minority of studies, blinding was not reported. We strongly encourage the authors of future studies to follow the recommendations provided in the updated Standards for Reporting Diagnostic Accuracy (STARD) statement to improve the quality of reporting (Bossuyt 2015).
Interpretability of subgroup analyses
We investigated potential sources of heterogeneity in the different extrapulmonary specimens. Importantly, we found slightly higher Xpert Ultra accuracy in studies with concentrated cerebrospinal fluid (CSF) in comparison to unconcentrated specimens. We note that subgroup findings should be interpreted with caution, as there were only three studies and a small number of tuberculous meningitis cases included in these analyses.
Comparison with other systematic reviews
We are aware of several systematic reviews previously published on this topic that estimated summary accuracy of Xpert MTB/RIF for distinct forms of extrapulmonary tuberculosis, as well as different forms of extrapulmonary tuberculosis combined (Table 12). We identified one systematic review that estimated summary accuracy of Xpert Ultra that found, for all forms of extrapulmonary tuberculosis combined, pooled sensitivity and specificity of 85.1% (95% CI 76.7 to 90.8%) and 95.7% (95% CI 87.9 to 98.6%), (7 studies; 1500 specimens) (Zhang 2020).
Compared with previous systematic reviews, our review extends the date of the search for potential studies for inclusion. Our strict inclusion criteria, e.g. excluding case‐control studies, meant that some of the studies included in other reviews were excluded from ours.
Applicability of findings to the review question
For the participant selection domain, most studies had high or unclear concern for applicability because either participants were evaluated exclusively as inpatients in tertiary care or we were not sure about the clinical settings. We therefore cannot be sure about the applicability of our findings to primary care. Studies that take place in referral settings may include participants whose condition is more difficult to diagnose than are seen at lower levels of the health system. However, we recognize that classifying studies as primary, secondary, or tertiary care may not adequately account for differences in disease spectrum (Leeflang 2013). For the index and reference test domains, most studies had low concern for applicability.
Authors' conclusions
Implications for practice.
In people presumed to have extrapulmonary tuberculosis, Xpert Ultra and Xpert MTB/RIF may be helpful in confirming the diagnosis. Sensitivity varies across different extrapulmonary specimens, while for most specimens specificity is high, the test rarely yielding a positive result for people without tuberculosis. For tuberculous meningitis, Xpert Ultra had higher pooled sensitivity and lower pooled specificity than Xpert MTB/RIF against culture. Xpert Ultra and Xpert MTB/RIF had similar sensitivity and specificity for rifampicin resistance.
Implications for research.
Future studies should perform comparisons of different tests, including Xpert Ultra, as this approach will reveal which tests (or strategies) yield superior diagnostic accuracy. For these studies, the preferred study design is one in which all participants receive all available diagnostic tests or are randomly assigned to receive one or another of the tests. Studies should include children and people living with HIV. Future research should acknowledge the concern associated with culture as a reference standard in paucibacillary specimens, and should consider ways to address this limitation.
Rapid point‐of‐care diagnostic tests for extrapulmonary tuberculosis are critically needed. Research groups should focus on developing diagnostic tests and strategies that use readily‐available clinical specimens, such as urine, rather than specimens that require invasive procedures for collection.
What's new
| Date | Event | Description |
|---|---|---|
| 11 January 2021 | New citation required but conclusions have not changed | We updated the literature search and included 22 new studies. |
| 11 January 2021 | New search has been performed | We have updated the review with more information. There are no major changes to the conclusions. |
History
Protocol first published: Issue 8, 2017 Review first published: Issue 8, 2018
Acknowledgements
The Academic Editors on this review were Dr Michael Eisenhut (Cochrane Infectious Diseases Group; CIDG) and Dr Mia Schmidt‐Hansen (Cochrane Diagnostic Test Accuracy).
We are grateful to Vittoria Lutje, the CIDG Information Specialist, for help with the search strategy. The CIDG editorial base is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
We acknowledge Eleanor Ochodo, Centre for Evidence‐based Health Care Department of Global Health, Stellenbosch, and Fredrick Haraka, Ifakara Health Institute, Bagamoyo, Tanzania, for the section on people‐important outcomes. We thank Hannah Ryan, from the Tropical and Infectious Diseases Unit, Royal Liverpool University Hospital, for her help with the protocol. We thank Chunli Lu, from the Centre for Evidence‐Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, and Marcela Perlwitz, Ivy Tech Community College of Indiana, West Lafayette, for help with translation. We thank all authors of the included studies for providing answers to our questions along with additional data. We thank the referees for their helpful comments.
Appendices
Appendix 1. Detailed search strategies
MEDLINE (OVID)
1 Mycobacterium tuberculosis/
2 Tuberculosis/ or "Tuberculosis, Multidrug‐Resistant"/ or Extensively Drug‐Resistant Tuberculosis/
3 (Tuberculosis or MDR‐TB or XDR‐TB or "Multidrug Resistant Tuberculosis" or "Extensively Drug Resistant Tuberculosis" or tuberculous).ti. ab .
4 (extrapulmonary or extra‐pulmonary or EPTB).ti. ab .
5 (lymphadenitis or disseminated or miliary or pleur* or skeletal or spine or mening* or intracranial or intra‐ocular or ocular or abdominal or splenic or genitourinary or pericardial).ti. ab .
6 "Tuberculosis, Central Nervous System"/ or "Tuberculosis, Urogenital"/ or "Tuberculosis, Splenic"/ or "Tuberculosis, Spinal"/ or "Tuberculosis, Renal"/ or "Tuberculosis, Pleural"/ or "Tuberculosis, Osteoarticular"/ or "Tuberculosis, Oral"/ or "Tuberculosis, Ocular"/ or "Tuberculosis, Meningeal"/ or "Tuberculosis, Lymph Node"/ or "Tuberculosis, Laryngeal"/ or "Tuberculosis, Hepatic"/ or "Tuberculosis, Gastrointestinal"/ or "Tuberculosis, Female Genital"/ or "Tuberculosis, Endocrine"/ or "Tuberculosis, Cutaneous"/ or "Tuberculosis, Cardiovascular"/ or Tuberculosis, Miliary/ or Tuberculosis, Male Genital/
7 1 or 2 or 3
8 4 or 5
9 7 and 8
10 9 or 6
11 Xpert*.ti. ab .
12 (GeneXpert or cepheid).ti.ab .
13 (near* patient or near‐patient).ti.ab
14 11 or 12 or 13
15 10 and 14
Embase (OVID)
1 Tuberculosis, Multidrug‐Resistant/ or Extensively Drug‐Resistant Tuberculosis/ or Tuberculosis/ or tuberculosis.mp. or Mycobacterium tuberculosis/
2 (MDR‐TB or XDR‐TB).mp.
3 1 or 2
4 (extrapulmonary or extra‐pulmonary or EPTB).ti. or (extrapulmonary or extra‐pulmonary or EPTB).ab.
5 (lymphadenitis or disseminated or miliary or pleur* or skeletal or spine or mening* or intracranial or intra‐ocular or ocular or abdominal or genitourinary or pericardial).ti. or (lymphadenitis or disseminated or miliary or pleur* or skeletal or spine or mening* or intracranial or intra‐ocular or ocular or abdominal or genitourinary or pericardial).ab.
6 tuberculous.ti. or tuberculous.ab.
7 3 or 6
8 Tuberculosis, Central Nervous System/ or Tuberculosis, Hepatic/ or Tuberculosis, Male Genital/ or Tuberculosis, Spinal/ or Tuberculosis, Cutaneous/ or Tuberculosis, Urogenital/ or Tuberculosis, Osteoarticular/ or Tuberculosis, Endocrine/ or Tuberculosis, Renal/ or Tuberculosis, Splenic/ or Tuberculosis, Ocular/ or Tuberculosis, Laryngeal/ or Tuberculosis, Gastrointestinal/ or Tuberculosis/ or Tuberculosis, Meningeal/ or Tuberculosis, Oral/ or Tuberculosis, Pleural/ or Tuberculosis, Lymph Node/ or Tuberculosis, Female Genital/ or Tuberculosis, Miliary/ or Tuberculosis, Cardiovascular/
9 4 or 5 or 8
10 7 and 9
11 xpert*TB.mp.
12 Xpert* MTB RIF.ti. or Xpert* MTB RIF.ab.
13 (GeneXpert or cepheid).ti. or (GeneXpert or cepheid).ab.
14 (near* patient or near‐patient).ti. or (near* patient or near‐patient).ab.
15 12 or 13 or 14
16 10 and 15
Indexes=SCI‐EXPANDED, CPCI‐S, Biosis previews
TOPIC
(tuberculosis or tuberculous) ANDTOPIC: (extrapulmonary or extra‐pulmonary or EPTB or lymphadenitis or disseminated or miliary or pleur* or skeletal or spine or mening* or intracranial or intra‐ocular or ocular or abdominal or genitourinary or pericardial) ANDTOPIC: (Xpert* or Genexpert or cepheid)
LILACS
tuberculosis or tuberculous [Words] and Xpert$ or Genexpert or cepheid [Words]
SCOPUS
( TITLE‐ABS‐KEY ( tuberculosis OR tuberculous ) AND TITLE‐ABS‐KEY ( extrapulmonary OR extra‐pulmonary OR eptb OR lymphadenitis OR disseminated OR miliary OR pleur* OR skeletal OR spine OR mening* OR intracranial OR intra‐ocular OR ocular OR abdominal OR genitourinary OR pericardial ) AND TITLE‐ABS‐KEY ( xpert* OR genexpert OR cepheid ) )
Cochrane Infectious Diseases Group Specialist Register; ClinicalTrials.gov, WHO ICTRP, ISRCTN registry, ProQuest Dissertations & Theses A&I
tuberculosis and Xpert$; tuberculosis and Genexpert; tuberculosis and Cepheid.
Appendix 2. Data extraction form
| Data extractor | MK KRS |
| First study author | |
| Corresponding study author and email | |
| Title of paper | |
| Journal | |
| Language if other than English | |
| Year |
I. Study details
Type of study: randomized controlled trial; cross‐sectional cohort (with follow‐up); case‐control (exclude); unclear/not reported
Study data collection: prospective; retrospective; unclear/not reported
Participant selection: convenience; consecutive; random; other; unclear/not reported
Country:
Country income status: low; middle; high
II. Presenting signs and symptoms, setting
Presenting signs and symptoms?
Clinical setting: inpatient; outpatient; both; unclear/not reported
Level of laboratory running Xpert? peripheral; intermediate; central (reference)
Comments, describe exclusions
(Tests at laboratory levels)
Peripheral: AFB (Ziehl‐Neelsen, Auramine‐rhodamine, Auramine‐O staining) and Xpert MTB/RIF
Intermediate: peripheral laboratory tests and culture on solid media and line probe assay (LPA) from smear‐positive sputum
Central: intermediate laboratory tests and culture on liquid media and DST (first‐ and second‐line anti‐TB drugs) on solid or in liquid media and LPA on positive cultures and rapid speciation tests
III. Other demographics
HIV patients included? yes; no; unclear/not reported; if yes ## and percentage? (denominator is number tested, when possible)
Age? Median age in years (IQR); mean (SD); range; unclear/not reported
Children (< 15 years old) included: yes; no; unclear/not reported; if yes, percentage?
Percentage female included? Unclear/not reported
Past history of TB? yes; no; unclear/not reported; if yes, percentage?
Only patients who received TB treatment for ≤ 7 days were included? yes; no; unclear/not reported; if no, percentage on treatment included?
IV. Reference standard
A. Reference standard for TB detection
Solid culture (specify): LJ 7H10 7H11; other
Liquid culture (specify): MGIT Bactec 460; other
Solid and liquid culture (indicate which kind above)
Were reference standard results interpreted without knowledge of index test results? yes; no; unclear/not reported
B. Composite reference standard for pleural TB
Solid culture (specify): LJ 7H10 7H11; other
Liquid culture (specify): MGIT Bactec 460; other
Solid and liquid culture (indicate which kind above)
Histopathology (specify): granulomas; caseating granulomas
Were reference standard results interpreted without knowledge of index test results? yes; no; unclear/not reported
Did all patients receive the same reference standard? yes; no; unclear/not reported; if no, describe
C. Reference standard for rifampicin resistance
LJ DST MGIT DST MTBDRplus
Were reference standard results interpreted without knowledge of index test results? yes; no; unclear/not reported
V. Sites with more than five specimens (check all that apply)
A. Lymph node TB fluid; tissue; both fluid and tissue
B. Pleural TB fluid; tissue; both fluid and tissue
C. TB meningitis CSF
D. Bone or joint TB fluid; tissue; both fluid and tissue
E. Genitourinary TB urine; other, specify
F. Peritoneal TB fluid; tissue; both fluid and tissue
G. Pericardial TB fluid; tissue; both fluid and tissue
H. Disseminated TB blood
I. Other, specify
VI. Specimen processing for Xpert
Condition of specimens: fresh frozen
If frozen for > 7 days, indicate WHO not followed
For a given site, how many specimens were collected per patient? one; multiple; unclear/not reported
A. Lymph node tissue, other tissue
Was the WHO standard operating procedure (SOP) followed for each specimen type?
1a. Lymph node tissue WHO followed: yes; no; unclear
1b. Lymph node tissue homogenization step for tissue specimens: yes; no; unclear/not reported
2a. Other tissue, specify WHO followed: yes; no; unclear
2b. Other tissue homogenization step for tissue specimens: yes; no; unclear/not reported
(For tissue, if WHO SOP not followed, briefly describe specimen processing in comments.)
WHO SOPs for specimen processing; lymph node and other tissue; sterile specimen
Cut the tissue specimen into small pieces in a sterile mortar.
Add approximately 2 mL of sterile phosphate buffered saline (PBS).
Grind solution of tissue and PBS until homogeneous suspension has been obtained.
Place approximately 0.7 mL of the homogenized tissue in a sterile, conical screw‐capped tube.
Double volume of specimen with Xpert® Sample Reagent (1.4 mL Sample Reagent to 0.7 mL of homogenized tissue).
Shake tube vigorously 10 to 20 times or vortex for at least 10 seconds.
Incubate specimen for 10 minutes at room temperature, and again shake specimen 10 to 20 times or vortex for at least 10 seconds.
Incubate specimen at room temperature for an additional 5 minutes.
Transfer 2mL to Xpert® MTB/RIF cartridge.
Load into GeneXpert and per manufacturer’s instructions.
(Note: For specimens not collected in a sterile manner, WHO SOP suggests an NaOH decontamination/concentration protocol similar to that used for sputum.)
B. CSF
3a. CSF WHO followed: yes; no; unclear
3b. CSF concentration step: yes; no; unclear/not reported
3c. CSF sample input volume: specify; unclear/not reported
(For CSF, if WHO SOP not followed, briefly describe specimen processing in comments.)
WHO SOPs for CSF
If more than 5 mL of CSF is available for testing.
Transfer all of the CSF specimen to a conical centrifuge tube and concentrate the specimen at 3000 × g for 15 minutes.
Resuspend the pellet to a final volume of 2 mL by adding Xpert® MTB/RIF Sample Reagent.
Transfer 2 mL of the resuspended CSF sample to the Xpert® MTB/RIF cartridge.
Load the cartridge into the GeneXpert instrument according to the manufacturer’s instructions.
If 1 mL to 5 mL of CSF is available.
Add an equal volume of Sample Reagent to the CSF.
Mix the specimen and the Sample Reagent by vortexing as described above. After seven to eight minutes at room temperature, vortex the sample as above a second time.
Incubate for an additional seven to eight minutes (15 minutes total incubation) at room temperature.
Add 2 mL of the sample mixture directly to the Xpert® MTB/RIF cartridge.
Load the cartridge into the GeneXpert instrument according to the manufacturer’s instructions.
C. Body fluids, other than CSF
4a. Body fluid: specify; processed as per manufacturer for sputum
Yes/No/Unclear
4b. Body fluid: specify; sample input volume: specify; unclear/not reported
5a. Body fluid: specify; processed as per manufacturer for sputum (WHO followed)
Yes/No/Unclear
5b. Body fluid: specify; sample input volume: specify; unclear/not reported
(Add additional specimens as needed.)
(For body fluids other than CSF, if manufacturer’s instructions not followed, briefly describe specimen processing in comments.)
Manufacturer’s instructions for sputum
Raw specimen
Pour or pipette (pipette not provided) approximately 2 times the volume of Sample Reagent into the specimen (2:1 dilution, Sample Reagent: specimen).
Shake vigorously 10 to 20 times or vortex for at least 10 seconds.
Incubate sample for a total of 15 minutes at 20°C to 30°C.
Between 5 and 10 minutes into the incubation period, shake vigorously 10 to 20 times or vortex for at least 10 seconds.
Specimen sediment
Assay requires at least 0.5 mL of resuspended specimen sediment after digestion, decontamination, and concentration.
Use the method of Kent and Kubica and resuspend the sediment in a 67 mM phosphate/H2O buffer.
After resuspension, keep at least 0.5 mL of the resuspended sediment for the Xpert® MTB/RIF assay.
Add 1.5 mL of Sample Reagent to 0.5 mL of the resuspended sediment (3:1 dilution, Sample Reagent: specimen)
Follow steps 2 to 4 above.
Comments on specimen processing.
VII. Specimen processing for culture
Specimen collected from sterile site: Yes/No/Unclear
Specimen processed for culture as per American Thoracic Society Diagnostic Standards? Yes/No/Unclear
(ATS guidelines: specimens collected from normally sterile sites may be placed directly into the culture medium.)
Note: All specimens such as CSF, pleura, lymph node aspirates and tissues, peritoneal fluid, pericardial fluid, bone or joint fluid and tissue, and urine are considered sterile.
VIII. Results
TB detection: number error or invalid or both Xpert® MTB/RIF results over total number of cultures performed. The denominator includes contaminated cultures and results that were inconclusive.
Unclear/not reported.
RIF resistance: number indeterminate Xpert results (over total number of cultures performed).
Unclear/not reported.
Non‐tuberculous mycobacteria (NTM): number of cultures with NTM (over total number of cultures performed).
Unclear/not reported.
IX. Tables
(Non‐tuberculous mycobacteria (NTM) should be included as not TB)
Tuberculosis detection (example for Xpert Ultra against culture reference standard; provide additional tables Xpert MTB/RIF and for other extrapulmonary specimens; extract trace results for Xpert Ultra)
| Xpert Ultra in lymph node aspirate | Definite TB | |||
| Yes | No | Total | ||
| Xpert Ultra result | Positive | |||
| Negative | ||||
| Total | ||||
| Error/invalid | ||||
Rifampicin resistance detection (for all culture‐positive, extrapulmonary specimens)
| Rifampicin resistance detection | Rifampicin resistance | |||
| Yes | No | Total | ||
| Xpert result | Positive | |||
| Negative | ||||
| Total | ||||
| Indeterminate | ||||
Abbreviation: TB: tuberculosis.
Appendix 3. Rules for QUADAS‐2
Domain 1: patient selection
Risk of bias: could the selection of patients have introduced bias?
Signalling question 1: was a consecutive or random sample of patients enrolled?
We scored "yes" if the study enrolled a consecutive or random sample of eligible patients, "no" if the study selected patients by convenience, and "unclear" if the study did not report the manner of patient selection or we could not tell.
Signalling question 2: was a case‐control design avoided?
We did not include in the review studies using a case‐control design because this study design, especially when used to compare results in severely ill patients versus those in relatively healthy individuals, may lead to overestimation of accuracy in diagnostic studies. We scored "yes" for all studies.
Signalling question 3: did the study avoid inappropriate exclusions?
We scored "yes" if the study included both smear‐positive and smear‐negative specimens or included only smear‐negative specimens. We judged "no" if the study included only smear‐positive specimens or excluded specimens based on physical appearance (such as purulence) or a biochemical analysis (e.g. adenosine deaminase (ADA), cytology (cell analysis)). We scored "unclear" if we could not tell.
Applicability: are there concerns that the included patients and setting do not match the review question?
We were interested in how the index tests performed in patients presumed to have extrapulmonary tuberculosis who were evaluated as they would be in routine practice. We scored "low concern" if patients were evaluated at local hospitals or primary care centres. We scored "high concern" if patients were evaluated exclusively as inpatients at tertiary care centres, except for tuberculous meningitis (we judged low concern) where we would expect patients to be evaluated in tertiary care settings. We scored "unclear concern" if the clinical setting was not reported or if information was insufficient to allow a decision. We also scored "unclear concern” if Xpert testing was done at a reference laboratory and the clinical setting was not reported for the following reason. It was difficult to tell if a given reference laboratory provided services mainly to very sick patients (inpatients in tertiary care) or to all patients, including very sick patients and those with less severe disease (primary, secondary, and tertiary care).
Domain 2: index test
Risk of bias: could the conduct or interpretation of the index test have introduced bias?
Signalling question 1: were the index test results interpreted without knowledge of results of the reference standard?
We answered this question "yes" for all studies because Xpert test results are automatically generated and the user is provided with printable test results. Thus, there is no room for subjective interpretation of test results.
Signalling question 2: If a threshold was used, was it pre‐specified?
As the threshold is pre‐specified in all versions of Xpert, we answered this question "yes" for all studies.
Applicability: are there concerns that the index test, its conduct, or its interpretation differ from the review question?
We note that variations in execution of the test might affect accuracy estimates. We judged "low concern" if the test was performed according to WHO standard operating procedures (WHO 2014), or if the index test was performed as recommended by the manufacturer for sputum. We scored "high concern" if the test was performed in a way that deviated from these recommendations. We scored "unclear concern" if we could not tell. In studies that evaluated several different types of specimens, we used the following rule: if ≥ 75% of the specimen types were processed per WHO standard operating procedure (SOP) or as per the manufacturer's instructions, we judged "low concern"; if < 50% of the specimen types were processed per WHO SOP or as per the manufacturer's instructions, we scored "high concern"; and if at least 50% to 74% of the specimen types were processed per WHO SOP or as per the manufacturer's instructions, or if we could not tell, we scored "unclear concern".
Domain 3: reference standard
Risk of bias: could the reference standard, its conduct, or its interpretation have introduced bias?
We considered this domain separately for the reference standard for detection of extrapulmonary tuberculosis and the reference standard for detection of rifampicin resistance.
Signalling question 1: is the reference standard likely to correctly classify the target condition?
For detection of extrapulmonary tuberculosis, culture is generally considered the best reference standard. However, limitations are associated with culture; bacterial load is usually low in extrapulmonary tuberculosis, leading to a reduction in the sensitivity of culture. Concerning the conduct of the reference standard (preparation of the specimen for culture), N‐acetyl‐L‐cysteine‐sodium hydroxide is routinely used to homogenize, decontaminate, and liquefy non‐sterile specimens for TB culture (American Thoracic Society 2000). However, CSF, pleural fluid, and lymph node aspirates are usually considered sterile, and standards specify, "specimens collected from normally sterile sites may be placed directly into the culture medium” (American Thoracic Society 2000). Overly processing (sterile) specimens with N‐acetyl‐L‐cysteine‐sodium hydroxide may lead to a decrease in viable TB bacteria and consequently false‐negative cultures. We scored "yes" if studies did not use N‐acetyl‐L‐cysteine‐sodium hydroxide for processing specimens and "unclear" if studies used N‐acetyl‐L‐cysteine‐sodium hydroxide. We discussed this further under Discussion and Strengths and weaknesses of the review.
For detection of rifampicin resistance, culture‐based drug susceptibility testing (DST, also called conventional phenotypic method) is considered to be the best reference standard. Line probe assays are also WHO‐recommended tests for rifampicin resistance. As we extracted data only for studies that used culture‐based DST or line probe assays (most often MTBDRplus), we answered this question "yes" for all studies.
Signalling question 2: were the reference standard results interpreted without knowledge of results of the index test?
We scored "yes" if the reference test provided an automated result (e.g. MGIT 960), if blinding was explicitly stated, or if it was clear that the reference standard was performed at a separate laboratory and/or was performed by different people. We scored "no" if the study stated that the reference standard result was interpreted with knowledge of the Xpert Ultra or Xpert MTB/RIF test result. We scored "unclear" if we could not tell.
Signalling question 3: (rifampicin resistance) were the reference standard results interpreted without knowledge of results of the index test?
We added a signalling question for rifampicin resistance detection. We scored "yes" if the reference test provided an automated result (e.g. MGIT 960), if solid culture was performed followed by speciation, if blinding was explicitly stated, or if it was clear that the reference standard was performed at a separate laboratory or was performed by different people, or both. We scored "no" if the study stated that the reference standard result was interpreted with knowledge of the Xpert test result. We scored "unclear" if we could not tell. Not all studies evaluated detection of rifampicin resistance; therefore this question was not applicable to all studies.
Applicability: are there concerns that the target condition as defined by the reference standard does not match the question?
We judged "high concern" if included studies did not speciate mycobacteria isolated in culture, "low concern" if speciation was performed, and "unclear concern" if we could not tell. If a study performed sequencing, we considered the speciation yes. If the study only used a composite reference standard, we considered applicability unclear.
Domain 4: flow and timing
Risk of bias: could the patient flow have introduced bias?
Signalling question 1: was there an appropriate interval between the index test and the reference standard?
In most included studies, we expected that specimens for index test and verification by culture (or a composite reference standard) would be obtained at the same time, when patients were evaluated for presumptive extrapulmonary tuberculosis. However, even if there were a delay of several days between index test and reference standard, tuberculosis is a chronic disease, and we considered misclassification of disease status to be unlikely, as long as treatment was not initiated in the interim. We judged "yes" if the index test and the reference standard were performed at the same time or if the time interval was less than or equal to seven days, "no" if the time interval was greater than seven days, and "unclear" if we could not tell.
Signalling question 2: did all patients receive the same reference standard?
For the diagnosis of any form of extrapulmonary tuberculosis we answered this question "yes" if all participants in the study or a subset of participants in the study (for whom we extracted data) received the acceptable reference standard either culture or a composite reference standard. Regarding culture, we acknowledge that it is possible that some specimens could undergo solid culture and others liquid culture as the reference standard. This could potentially result in variations in accuracy, but we think the variation would be minimal.
For rifampicin resistance detection, we answered "yes" if all participants received the same reference standard (either culture‐based DST or MTBDRplus), "no" if not all participants received the same reference standard, and "unclear" if we could not tell.
Signalling question 3: were all patients included in the analysis?
We determined the answer to this question by comparing the number of patients enrolled with the number of patients included in the 2 × 2 tables. We answered "yes" if the numbers matched and "no" if there were patients enrolled in the study who were not included in the analysis. We answered "unclear" if we could not tell.
Judgements for overall ʽRisk of bias' assessments.
If we answered all signalling questions for a domain "yes", then we scored risk of bias as "low".
If we answered all or most signalling questions for a domain "no", then we scored risk of bias as "high".
If we answered only one signalling question for a domain "no", we discussed further the "risk of bias" judgement.
If we answered all or most signalling questions for a domain "unclear", then we scored risk of bias as "unclear".
If we answered only one signalling question for a domain "unclear", we discussed further the "risk of bias" judgement for the doma
Appendix 4. OpenBugs
In this section we provide OpenBUGS models for the bivariate meta‐analysis as well as the latent class meta‐analysis. Any alternative prior distributions used are provided in the comments within each model.
BIVARIATE MODEL ASSUMING PERFECT CULTURE REFERENCE TEST model {
for(i in 1:N) { # N is the number of studies
############################# LIKELIHOOD logit(TPR[i]) <‐ l[i,1] logit(FPR[i]) <‐ ‐l[i,2] pos[i]<‐TP[i]+FN[i] neg[i]<‐TN[i]+FP[i] TP[i] ~ dbin(TPR[i],pos[i]) FP[i] ~ dbin(FPR[i],neg[i]) se[i] <‐ TPR[i] sp[i] <‐ 1‐FPR[i] l[i,1:2] ~ dmnorm(mu[1:2], T[1:2,1:2]) } ############################# HYPER PRIOR DISTRIBUTIONS mu[1] ~ dnorm(0,0.25) # replaced by mu[1] ~ dnorm(0, 0.01) in sensitivity analysis to check impact of less informative prior mu[2] ~ dnorm(0,0.25) # replaced by mu[2] ~ dnorm(0, 0.01) in sensitivity analysis to check impact of less informative prior T[1:2,1:2]<‐inverse(TAU[1:2,1:2]) #### BETWEEN‐STUDY VARIANCE‐COVARIANCE MATRIX TAU[1,1] <‐ tau[1]*tau[1] TAU[2,2] <‐ tau[2]*tau[2] TAU[1,2] <‐ rho*tau[1]*tau[2] TAU[2,1] <‐ rho*tau[1]*tau[2] tau[1]<‐pow(prec[1],‐0.5) # replaced by tau[1] ~ dunif(0,3) in sensitivity analysis to check impact of less informative prior tau[2]<‐pow(prec[2],‐0.5) # replaced by tau[2] ~ dunif(0,3) in sensitivity analysis to check impact of less informative prior sigma.sq[1] <‐ pow(tau[1], 2) sigma.sq[2] <‐ pow(tau[2], 2) #### prec = between‐study precision in the logit(sensitivity) and logit(specificity) prec[1] ~ dgamma(2,0.5) # replaced by prec[1] <‐ 1/pow(tau[1],‐2) in sensitivity analysis to check impact of less informative prior prec[2] ~ dgamma(2,0.5) # replaced by prec[2] <‐ 1/pow(tau[2],‐2) in sensitivity analysis to check impact of less informative prior rho ~ dunif(‐1,1) ############################# OTHER PARAMETERS OF INTEREST #### POOLED SENSITIVITY AND SPECIFICITY Pooled_S<‐1/(1+exp(‐mu[1])) Pooled_C<‐1/(1+exp(‐mu[2])) #### POOLED POSITIVE AND NEGATIVE LIKELIHOOD RATIOS PLR <‐ Pooled_S/(1‐Pooled_C) NLR <‐ (1‐Pooled_S)/Pooled_C #### PREDICTED SENSITIVITY AND SPECIFICITY IN A FUTURE STUDY l.new[1:2] ~ dmnorm(mu[],T[,]) sens.new <‐ 1/(1+exp(‐l.new[1])) spec.new <‐ 1/(1+exp(‐l.new[2])) } #### END OF PROGRAM
LATENT CLASS META‐ANALYSIS MODEL
# WinBUGS PROGRAM FOR ESTIMATING A BIVARIATE HIERARCHICAL META‐ANALYSIS MODEL # FOR SENSITIVITY AND SPECIFICITY ALLOWING FOR HETEROGENEITY BETWEEN STUDIES model { ############################# ############################# for(i in 1:N) {# N is the number of studies ############################# LIKELIHOOD logit(p[1, i]) <‐ l[i,1] logit(p[2, i]) <‐ ‐l[i,2] prob[i,1] <‐ pi[i]*(p[1,i]* s2[i] + covp[i]) + (1‐pi[i])*(p[2,i]*(1‐c2[i]) + covn[i]) prob[i,2] <‐ pi[i]*(p[1,i]* (1‐s2[i]) ‐ covp[i]) + (1‐pi[i])*(p[2,i]*c2[i] ‐ covn[i]) prob[i,3] <‐ pi[i]*((1‐p[1,i])*s2[i] ‐ covp[i]) + (1‐pi[i])*((1‐p[2,i])*(1‐c2[i]) ‐ covn[i]) prob[i,4] <‐ pi[i]*((1‐p[1,i])*(1‐s2[i]) + covp[i]) + (1‐pi[i])*((1‐p[2,i])*c2[i] + covn[i]) n[i] <‐ sum(cell[i,1:4]) cell[i,1:4] ~ dmulti(prob[i,1:4],n[i]) pi[i] ~ dbeta(1,1) se[i] <‐ p[1,i] sp[i] <‐ 1‐p[2,i] l[i,1:2] ~ dmnorm(mu[1:2], T[1:2,1:2]) #================================================================= # CONDITIONAL DEPENDENCE #================================================================= #======================================= # upper limits of covariance parameters #======================================= us[i]<‐min(se[i],s2[i])‐(se[i]*s2[i]); uc[i]<‐min(sp[i],c2[i])‐(sp[i]*c2[i]); ls[i]<‐ ‐(1‐se[i])*(1‐s2[i]) lc[i]<‐ ‐(1‐sp[i])*(1‐c2[i]) #============================================================== # prior distribution of transformed covariances on (0,1) range #============================================================== covp[i]~dunif(ls[i],us[i]); covn[i]~dunif(lc[i],uc[i]); #covn[i]<‐0 } # ================================== # NON‐INFORMATIVE HIERARCHICAL PRIOR DISTRIBUTION OVER REF STD PROPERTIES # ================================== for(j in 1:29) { logit(s2[j]) <‐ l2[j,1] logit(c2[j]) <‐ l2[j,2] l2[j,1:2] ~ dmnorm(mu2[1:2], T2[1:2,1:2]) } ############################# HYPER PRIOR DISTRIBUTIONS ############################# ########################################################## ########################################################## ### ### XPERT TEST ### ########################################################## ########################################################## mu[1] ~ dnorm(0,0.25) mu[2] ~ dnorm(0,0.25) #dnorm(4.59512,10) T[1:2,1:2]<‐inverse(TAU[1:2,1:2]) #### BETWEEN‐STUDY VARIANCE‐COVARIANCE MATRIX TAU[1,1] <‐ tau[1]*tau[1] TAU[2,2] <‐ tau[2]*tau[2] TAU[1,2] <‐ rho*tau[1]*tau[2] TAU[2,1] <‐ rho*tau[1]*tau[2] tau[1]<‐pow(prec[1],‐0.5) tau[2]<‐pow(prec[2],‐0.5) sigma.sq[1] <‐ pow(tau[1], 2) sigma.sq[2] <‐ pow(tau[2], 2) #### prec = between‐study precision in the logit(sensitivity) and logit(specificity) prec[1] ~ dgamma(2,0.5) prec[2] ~ dgamma(2,0.5) rho ~ dunif(‐1,1) ############################# OTHER PARAMETERS OF INTEREST #### POOLED SENSITIVITY AND SPECIFICITY OF XPERT Pooled_S<‐1/(1+exp(‐mu[1])) Pooled_C<‐1/(1+exp(‐mu[2])) #### POOLED POSITIVE AND NEGATIVE LIKELIHOOD RATIOS PLR <‐ Pooled_S/(1‐Pooled_C) NLR <‐ (1‐Pooled_S)/Pooled_C #### PREDICTED SENSITIVITY AND SPECIFICITY OF XPERT IN A FUTURE STUDY l.new[1:2] ~ dmnorm(mu[],T[,]) sens.new <‐ 1/(1+exp(‐l.new[1])) spec.new <‐ 1/(1+exp(‐l.new[2])) ########################################################## ########################################################## ### ### CULTURE TEST ### ########################################################## ########################################################## mu2[1] ~ dnorm(0,0.25) mu2[2] ~ dnorm(0,0.25) T2[1:2,1:2]<‐inverse(TAU2[1:2,1:2]) #### BETWEEN‐STUDY VARIANCE‐COVARIANCE MATRIX TAU2[1,1] <‐ tau2[1]*tau2[1] TAU2[2,2] <‐ tau2[2]*tau2[2] TAU2[1,2] <‐ rho2*tau2[1]*tau2[2] TAU2[2,1] <‐ rho2*tau2[1]*tau2[2] tau2[1] <‐pow(prec2[1],‐0.5) tau2[2] <‐pow(prec2[2],‐0.5) sigma.sq2[1] <‐ pow(tau2[1], 2) sigma.sq2[2] <‐ pow(tau2[2], 2) #### prec = between‐study precision in the logit(sensitivity) and logit(specificity) prec2[1] ~ dgamma(2,0.5) prec2[2] ~ dgamma(2,0.5) rho2 ~ dunif(‐1,1) #### POOLED SENSITIVITY AND SPECIFICITY OF CULTURE S2<‐1/(1+exp(‐mu2[1])) C2<‐1/(1+exp(‐mu2[2])) s2.new <‐ 1/(1+exp(‐ls2.new)) c2.new <‐ 1/(1+exp(‐lc2.new)) ls2.new ~ dnorm(mu2[1],prec2[1]) lc2.new ~ dnorm(mu2[2],prec2[2]) }
Appendix 5. Risk of bias and applicability concerns graph for tuberculous meningitis
18.
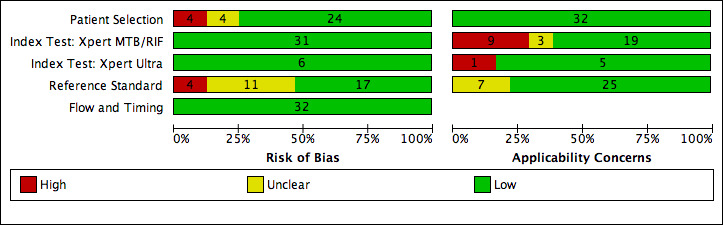
Risk of bias and applicability concerns graph for tuberculous meningitis (cerebrospinal fluid): review authors' judgements about each domain presented as percentages across included studies.
Appendix 6. Risk of bias and applicability concerns graph for pleural tuberculosis
19.
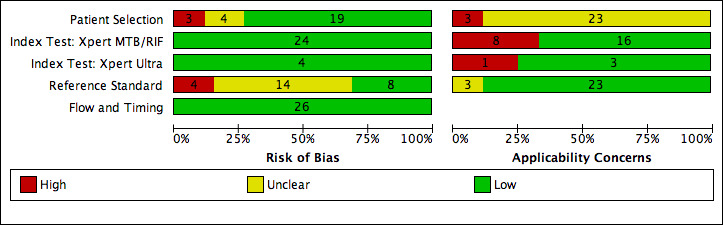
Risk of bias and applicability concerns graph for pleural tuberculosis (pleural fluid): review authors' judgements about each domain presented as percentages across included studies.
Appendix 7. Risk of bias and applicability concerns graph for lymph node tuberculosis
20.
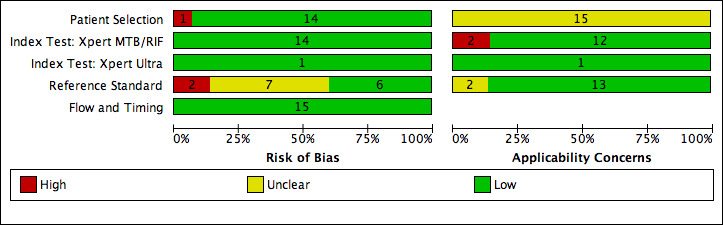
Risk of bias and applicability concerns graph for lymph node tuberculosis (lymph node aspirate): review authors' judgements about each domain presented as percentages across included studies.
Appendix 8. Receiver operating characteristic plot for pleural fluid, Xpert Ultra
Figure 57 displays the receiver operating characteristic (SROC) plots for Xpert Ultra (4 studies) and Xpert MTB/RIF (25 studies) in pleural fluid for pleural tuberculosis.
21.
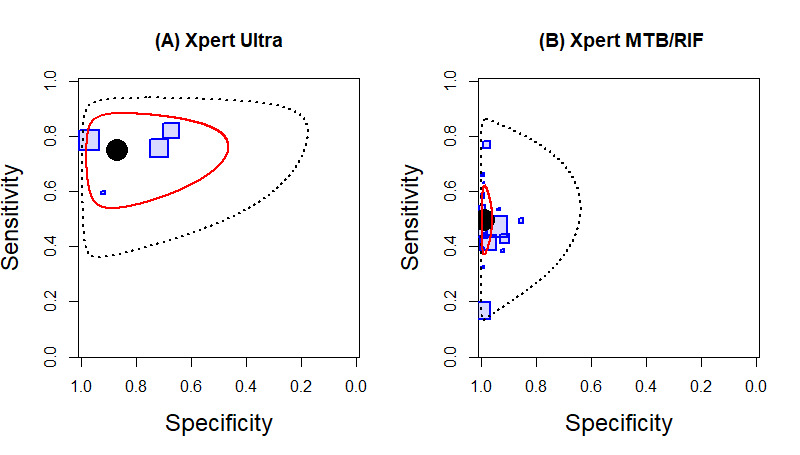
Summary plots of the sensitivity and specificity of Xpert Ultra (A) (4 studies) and Xpert MTB/RIF (B) (25 studies) in pleural fluid for detection of pleural tuberculosis. Each individual study is represented by a shaded square. The size of the square is proportional to the sample size of the study such that larger studies are represented by larger squares. The filled circle is the median pooled estimate for sensitivity and specificity. The solid curves represent the 95% credible region around the summary estimate; the dashed curves represent the 95% prediction region.
Appendix 9. Bone or joint tuberculosis, Xpert Ultra and Xpert MTB/RIF
Figure 58 displays forest plots of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity in bone or joint aspirate and tissue.
22.
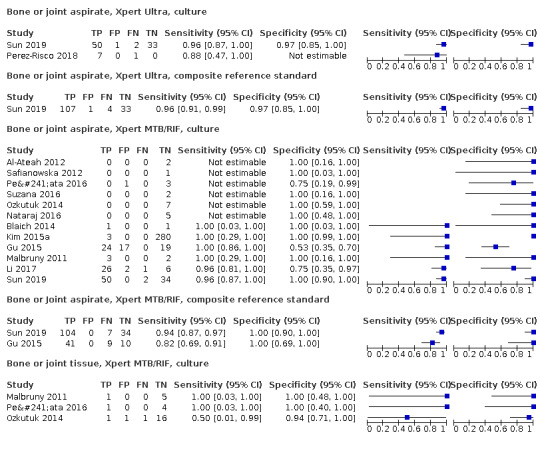
Forest plots of Xpert MTB/RIF and Xpert Ultra sensitivity and specificity in bone or joint fluid and tissue by reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. FN: false‐negative; FP: false‐positive; TN: true‐negative; TP: true‐positive.
Appendix 10. Peritoneal, pericardial, disseminated tuberculosis, Xpert Ultra and Xpert MTB/RIF
Figure 16 displays forest plots of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity in peritoneal fluid and tissue, pericardial fluid, and blood.
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
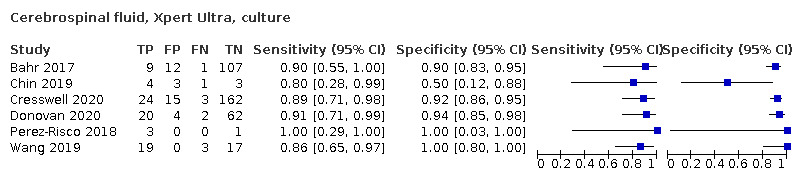
Cerebrospinal fluid, Xpert Ultra, culture
2. Test.
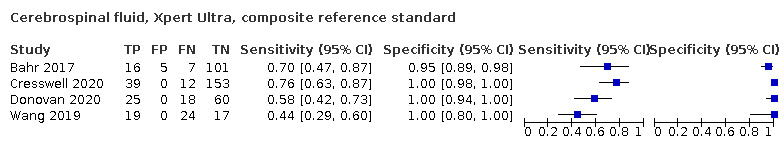
Cerebrospinal fluid, Xpert Ultra, composite reference standard
3. Test.
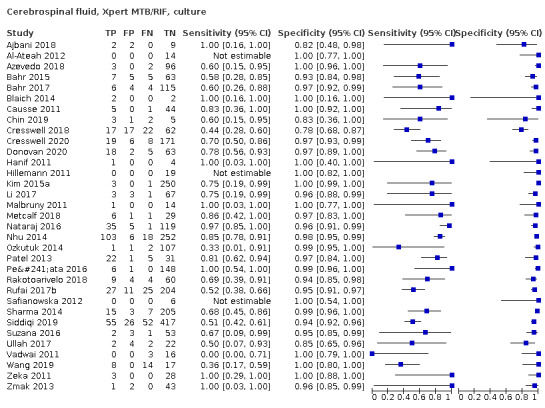
Cerebrospinal fluid, Xpert MTB/RIF, culture
4. Test.
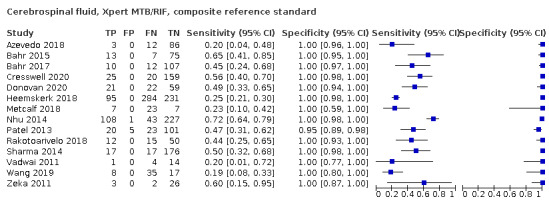
Cerebrospinal fluid, Xpert MTB/RIF, composite reference standard
5. Test.

Cerebrospinal fluid, Xpert Ultra, HIV positive
6. Test.
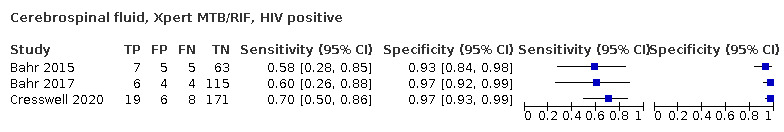
Cerebrospinal fluid, Xpert MTB/RIF, HIV positive
7. Test.
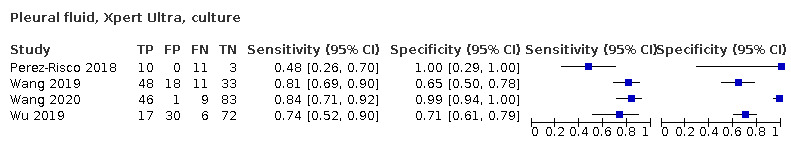
Pleural fluid, Xpert Ultra, culture
8. Test.

Pleural fluid, Xpert Ultra, composite reference standard
9. Test.
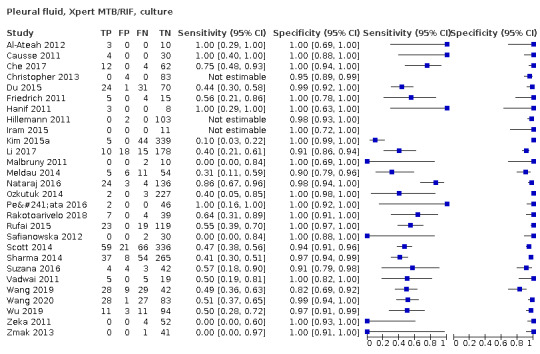
Pleural fluid, Xpert MTB/RIF, culture
10. Test.
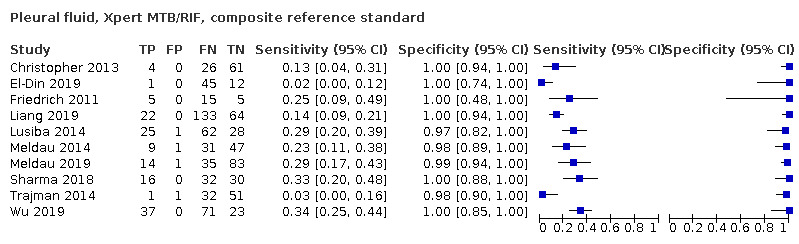
Pleural fluid, Xpert MTB/RIF, composite reference standard
11. Test.
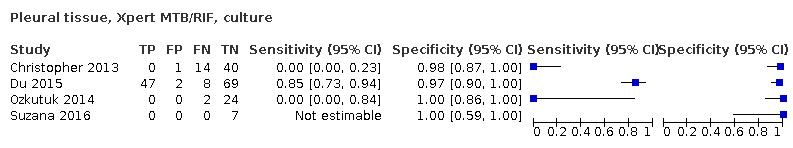
Pleural tissue, Xpert MTB/RIF, culture
12. Test.

Pleural tissue, Xpert MTB/RIF, composite reference standard
13. Test.

Lymph node aspirate, Xpert Ultra, culture
14. Test.

Lymph node aspirate, Xpert Ultra, composite reference standard
15. Test.
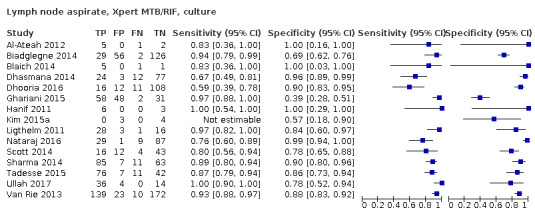
Lymph node aspirate, Xpert MTB/RIF, culture
16. Test.
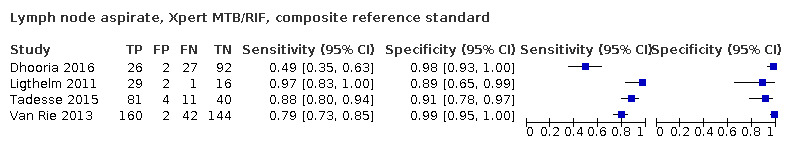
Lymph node aspirate, Xpert MTB/RIF, composite reference standard
17. Test.

Lymph node biopsy, Xpert Ultra, culture
18. Test.

Lymph node biopsy, Xpert Ultra, composite reference standard
19. Test.
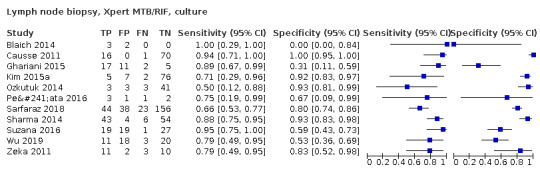
Lymph node biopsy, Xpert MTB/RIF, culture
20. Test.

Lymph node biopsy, Xpert MTB/RIF, composite
21. Test.

Urine, Xpert Ultra, culture
22. Test.
Urine, Xpert MTB/RIF, culture
23. Test.

Urine, Xpert MTB/RIF, composite reference standard
24. Test.

Bone or joint aspirate, Xpert Ultra, culture
25. Test.

Bone or joint aspirate, Xpert Ultra, composite reference standard
26. Test.
Bone or joint aspirate, Xpert MTB/RIF, culture
27. Test.

Bone or Joint aspirate, Xpert MTB/RIF, composite reference standard
28. Test.
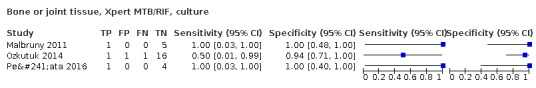
Bone or joint tissue, Xpert MTB/RIF, culture
29. Test.

Peritoneal fluid, Xpert Ultra, culture
30. Test.
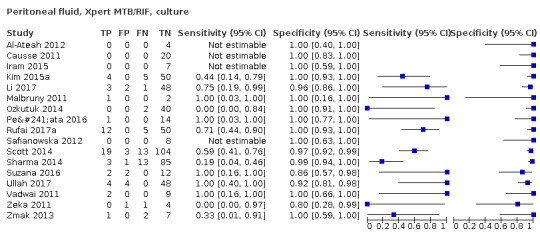
Peritoneal fluid, Xpert MTB/RIF, culture
31. Test.

Peritoneal tissue, Xpert MTB/RIF, culture
32. Test.
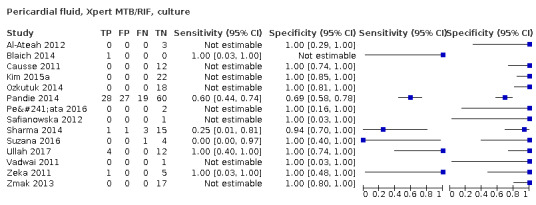
Pericardial fluid, Xpert MTB/RIF, culture
33. Test.

Pericardial fluid, Xpert MTB/RIF, composite reference standard
34. Test.

Blood, Xpert MTB/RIF, culture
35. Test.
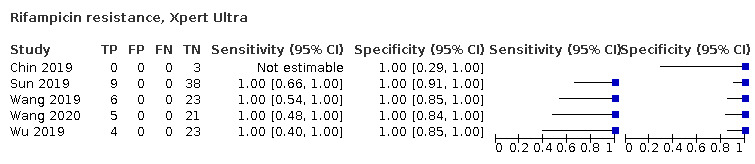
Rifampicin resistance, Xpert Ultra
36. Test.
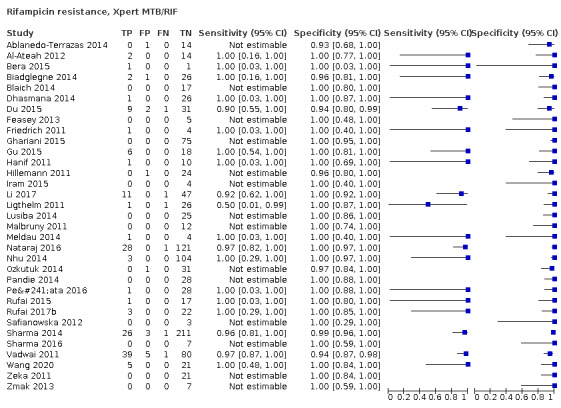
Rifampicin resistance, Xpert MTB/RIF
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ablanedo‐Terrazas 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: HIV‐positive patients with palpable cervical lymph nodes Age: median 29 years [interquartile range (IQR) 24 to 36] Sex, female: 12% Children: no HIV infection: 100% Clinical setting: tertiary care centre (inpatient and outpatient) Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 15 Laboratory level: central Country: Mexico World Bank Income Classification: middle income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO standard operating procedure (SOP) or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node (LN) TB Reference standard for TB detection: Löwenstein–Jensen (LJ) and Mycobacterium growth indicator tube (MGIT) Reference standard for rifampicin resistance: not reported Speciation: yes Decontamination: yes, N‐acetyl‐L‐cysteine‐sodium hydroxide (NALC‐NaOH) |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Ajbani 2018.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: neck rigidity, vomiting, fever, headache and seizures Age: 13 and older Sex, female: 46% Children: no HIV infection: not reported Clinical setting: tertiary care centre (inpatient) Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: 13 Laboratory level: central Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: not reported Decontamination: no |
||
| Target condition and reference standard(s) | TB meningitis MGIT Speciation: yes |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Al‐Ateah 2012.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients suspected of having extrapulmonary TB Age: median 35 years Sex, female: 45% Children: 3% HIV infection: 0% Clinical setting: tertiary care centre (laboratory‐based evaluation) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 67 Laboratory level: central Country: Saudi Arabia World Bank Income Classification: high income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node TB, pleural TB
Reference standards for TB detection: LJ and MGIT Reference standard for rifampicin resistance: MGIT‐drug susceptibility testing (DST) Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Site of extrapulmonary disease was not reported for 16 tissue specimens and 10 abscesses | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Antel 2020.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: suspected tuberculosis adenitis Age: ≥ 18 years; median 37 years (IQR 30 to 49) Sex, female: 55% Children: no HIV infection: 51% Clinical setting: tertiary referral centre, inpatients and outpatients, most participants (84%) were seen as outpatients, high percentage received prior testing for tuberculosis, see note Past history of TB: 24% Patients on anti‐TB treatment: 21% Number of specimens evaluated: 99 Laboratory level: central Country: South Africa World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert Ultra | ||
| Target condition and reference standard(s) | Target condition: lymph node tuberculosis, specimen collected by fine‐needle biopsy and core‐needle biopsy Reference standard: MGIT Target condition: rifampicin resistance Reference standard: MTBDRplus Speciation: yes, MTBDRplus Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | "A high proportion of participants had tuberculosis investigations prior to referral and the frequency of positive results were: sputum Xpert 3/22, urinary LAM 1/5, and tuberculosis culture (5/15) (by site: urine 0/1, blood 1/2, sputum 4/12, lymph node 0/1 (tissue)). Chest x‐ray had been performed in 36% and reported as ‘suggestive of tuberculosis’ by the referring clinician in 28% of these." | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Azevedo 2018.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of participant selection not reported, retrospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspected meningitis Age: > 16 years Sex, female: not reported Children: no HIV infection: 100% Clinical setting: tertiary care centre (inpatient) Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: 101 Laboratory level: central, university medical centre Country: Brazil World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: not reported |
||
| Target condition and reference standard(s) | TB meningitis Culture not otherwise specified; CRS: uniform case definition Speciation: not reported Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bahr 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: HIV‐infected patients presenting with symptoms of meningitis being evaluated for cryptococcal meningitis. All persons who were CSF cryptococcal antigen‐negative had a TB workup Age: median 40 years (IQR 30 to 45) Sex, female: 34% Children: no HIV infection: 98% Clinical setting: tertiary care centre (Inpatient) Past history of TB: 22% Participants on anti‐TB treatment: yes, 11% Number of specimens evaluated: 80 Laboratory level: central Country: Uganda World Bank Income Classification: low income High TB burden: no High TB/HIV burden: yes High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: TB meningitis Reference standard for TB detection: LJ and MGIT Reference standard for rifampicin resistance: MGIT‐DST Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Reference standards were culture and a TB meningitis uniform case definition | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bahr 2017.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: HIV‐infected patients presenting with symptoms of meningitis being evaluated for cryptococcal meningitis. All persons who were CSF cryptococcal antigen‐negative had a TB workup Age: TB meningitis: median 32 years (IQR 30 to 34); other meningitis: 34 years (IQR 29 to 43) Sex, female: 45% Children: no HIV infection: 100% Clinical setting: tertiary care centre (inpatient) Past history of TB: 6% Participants on anti‐TB treatment: yes, 2% Number of specimens evaluated: 129 Laboratory level: central Country: Uganda World Bank Income Classification: low income High TB burden: no High TB/HIV burden: yes High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF and Xpert Ultra WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: TB meningitis Reference standard for TB detection: MGIT Reference standard for rifampicin resistance: MGIT‐DST Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | This study evaluated Xpert MTB/RIF and Xpert MTB/RIF Ultra. Reference standards were culture and a TB meningitis uniform case definition |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Bera 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with exudative ascites (lymphocytic ascites and ascitic fluid protein content > 2.5 g/dL) Age: mean 43 years (standard deviation (SD) 15 years) Sex, female: 29% Children: no HIV infection: not reported Clinical setting: tertiary care centre (outpatient) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 28 Laboratory level: central Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: not reported Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: peritoneal TB Reference standard for TB detection: LJ and MGIT Reference standard for rifampicin resistance: LJ and MGIT‐DST Speciation: not reported Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | "The study included only smear‐negative specimens, however, the study excluded specimens that were negative for malignant cells on prior testing (i.e. cytology)" | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Biadglegne 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with enlarged lymph nodes not responding to a 2‐week course of antibiotics and clinically suspected for TB lymphadenitis Age: ≤ 14 years: 15%; > 14 years: 85% Sex, female: 57% Children: 15% HIV infection: not reported Clinical setting: tertiary care centres (multicentre study) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 213 Laboratory level: intermediate Country: Ethiopia World Bank Income Classification: low income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node TB Reference standard for TB detection: LJ and Gottsascker and BacT/ALERT 3D Reference standard for rifampicin resistance: MTBDRplus and BacT‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Total number of participants: 231; included: 213 (excluded: contaminated = 11; invalid/error = 7) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Blaich 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspicion of extrapulmonary TB Age: median 34 (IQR 30 to 52) Sex, female: 46% Children: no HIV infection: yes, 8% Clinical setting: university hospital (inpatient and outpatient) Past history of TB: yes, 11% Patients on anti‐TB treatment: no Number of specimens evaluated: 20 Laboratory level: central Country: Switzerland World Bank Income Classification: high income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes for lymph node aspirate, bone and joint fluid, urine, peritoneal fluid, and lymph node tissue; no for CSF Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, TB meningitis, lymph node TB, pericardial TB, genitourinary TB, bone and joint TB Reference standard for TB detection: LJ and MGIT Reference standard for rifampicin resistance: MGIT‐DST Speciation: yes Decontamination: yes, NALC‐NaOH for all specimens except pleural fluid and CSF |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Study included 1 bone marrow specimen that consisted of both aspirate and tissue | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Causse 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: not reported Age: median 45 years, range 5 to 83 years Sex, female: 31% Children: yes, 15% HIV infection: not reported Clinical setting: tertiary care centre (inpatient) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 261 Laboratory level: central Country: Spain World Bank Income Classification: high income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, lymph node TB, TB meningitis, peritoneal TB, pericardial TB, genitourinary TB Reference standard for TB detection: LJ and MGIT Reference standard for rifampicin resistance: not reported Speciation: yes Decontamination: yes, NALC‐NaOH for all specimens except pleural fluid and CSF |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Che 2017.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, prospective, and consecutive | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with evidence of pleural effusion demonstrated by X‐ray, suspected to have tuberculosis pleurisy Age: median 44 years, range 18 to 83 years Sex, female: 31% Children: no HIV infection: 1% Clinical setting: tertiary care centre (inpatient) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 78 Laboratory level: central Country: China World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB Reference standard for TB detection: MGIT Reference standard for rifampicin resistance: not reported Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chen 2019.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients who had symptoms suggestive of urinary tract TB or a urine abnormality Age: mean 53 years (range, 19 to 85) Sex, female: 55% Children: no HIV infection: 0% Clinical setting: multicentre, hospital‐based Past history of TB: 31% Participants on anti‐TB treatment: no Number of specimens evaluated: 302 Laboratory level: central Country: China World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes |
||
| Target condition and reference standard(s) | Genitourinary TB MGIT; CRS: culture or positive cystoscopy biopsy, or radiological signs Speciation: yes Decontamination: yes |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Chin 2019.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspected TB meningitis admitted to the neurology ward Age: adults, range 20 to 41 years Sex, female: not reported Children: not reported HIV infection: 18% Clinical setting: tertiary care centre (inpatient) Past history of TB: not reported Participants on anti‐TB treatment: 1 participant had received treatment Number of specimens evaluated: 11 Laboratory level: central Country: Uganda World Bank Income Classification: low High TB burden: no High TB/HIV burden: yes High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF and Xpert Ultra WHO SOP or manufacturer's protocol followed: no |
||
| Target condition and reference standard(s) | TB meningitis MGIT Speciation: not reported Decontamination:no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | "CSF (2 ml) should be slowly pipetted directly into the Xpert Ultra cartridge. CSF should only be diluted with the manufacturer‐supplied sample reagent if less than 2 ml of CSF are available for Xpert Ultra testing." See the following article for full description of CSF processing details, Chin 2019a. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Christopher 2013.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: clinical symptoms and radiographic evidence of a pleural effusion Age: median 46 years (IQR 33 to 57) Sex, female: 20% Children: no HIV infection: not reported Clinical setting: tertiary care centre (Inpatient and outpatient) Past history of TB: yes, 18% Patients on anti‐TB treatment: not reported Number of specimens evaluated against culture: 142 Number of specimens evaluated against composite reference standard: 146 Laboratory level: central Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes for pleural tissue, no for pleural fluid Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB Reference standard for TB detection: LJ and MGIT Reference standard for rifampicin resistance: not reported Speciation: not reported Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Cresswell 2018.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with headache and objective meningism Age: median age 35 years (IQR 30 to 42) Sex, female: 39% Children: no HIV infection: 4% Clinical setting: inpatient Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: 118 Laboratory level: central Country: Uganda World Bank Income Classification: low income High TB burden: no High TB/HIV burden: yes High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: not reported |
||
| Target condition and reference standard(s) | TB meningitis MGIT Speciation: not reported Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Additonal information at clinicaltrials.gov/ct2/show/NCT01075152 | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Cresswell 2020.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspected meningitis (headache for > 3 days or altered mental status (Glasgow Coma Scale < 15) with clinical signs of meningism at examination, i.e. neck stiffness or Kernig’s sign Age: median age 32 years (29 to 38) Sex, female: 42.6% Children: no HIV infection: 96% Clinical setting: inpatient Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: 204 Laboratory level: central Country: Uganda World Bank Income Classification: low income High TB burden: no High TB/HIV burden: yes High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIFand Xpert Ultra WHO SOP or manufacturer's protocol followed: yes |
||
| Target condition and reference standard(s) | TB meningitis MGIT Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Dhasmana 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: all participants undergoing endobronchial ultrasound (EBUS) for mediastinal lymphadenopathy Age: median 46 years, range 14 to 85 years Sex, female: 37% Children: no HIV infection: 7% Clinical setting: tertiary care centre (inpatient and outpatient) Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 116 Laboratory level: central Country: United Kingdom World Bank Income Classification: high income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node TB Reference standard for TB detection: MGIT Reference standard for rifampicin resistance: MGIT‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Dhooria 2016.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, retrospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with enlarged mediastinal or hilar lymph nodes (≥ 1 cm in short axis) on computed tomography of the chest who underwent EBUS‐guided transbronchial needle aspiration Age: median 40 years, range 30 to 53 years Sex, female: 43% Children: no HIV infection: 0% Clinical setting: tertiary care centre (outpatient) Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 147 Laboratory level: central Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node TB Reference standard for TB detection: MGIT Reference standard for rifampicin resistance: not reported Speciation: not reported Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Donovan 2020.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, random, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients aged 16 years or older with suspected tuberculous meningitis based on clinical and CSF findings (clear or mildly cloudy CSF, plus > 5 days of symptoms consistent with tuberculous meningitis8 or low CSF glucose or raised CSF lactate concentrations) Age: median age 42 (31 to 57) Sex, female: 40% Children: no HIV infection: 17% Clinical setting: inpatient Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: 205 Laboratory level: central Country: Vietnam World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: no High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF and Xpert Ultra WHO SOP or manufacturer's protocol followed: yes |
||
| Target condition and reference standard(s) | TB meningitis MGIT Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Du 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients found to be smear‐negative on prior testing with radiographic evidence of pleural effusion and those subsequently undergoing thoracocentesis and pleural biopsy Age: mean 39 years, SD 13 Sex, female: 44% Children: 0% HIV infection: 4% Clinical setting: 4 tertiary care centres (inpatient) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 126 Laboratory level: central Country: China World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB Reference standard for TB detection: LJ and MGIT Reference standard for rifampicin resistance: MGIT‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Study included specimens found to be smear‐negative on prior testing. In the present study, 4 specimens were smear‐positive specimens for pleural fluid and 15 were smear‐positive for pleural tissue The reference standard for both pleural fluid and pleural tissue was pleural biopsy culture |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
El‐Din 2019.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional. consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspected pleural TB based on clinical history and radiologic evidence of pleural effusion Age: 32.7 years ± 13.6 Sex, female: 31% Children: no HIV infection: not reported Clinical setting: not reported Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: not reported Laboratory level: not reported Country: Egypt World Bank Income Classification: middle income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF Specimens processed according to manufacturer's instructions |
||
| Target condition and reference standard(s) | Pleural TB Confirmed TB was defined if acid‐fast bacilli were detected by any mean (microscopic evaluation/mycobacterial culture (type not reported)) of either pleural tissue or pleural fluid Speciation: not reported Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Feasey 2013.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: HIV‐infected patients with clinical suspicion of tuberculosis Age: mean 37 years, SD 11 years Sex, female: 33% Children: no HIV infection: 100% Clinical setting: tertiary care centre Past history of TB: no Patients on anti‐TB treatment: no Number of specimens evaluated: 74 Laboratory level: central Country: Malawi World Bank Income Classification: low income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: disseminated TB (blood) Reference standard for TB detection: Bactec Myco/F Lytic culture Reference standard for rifampicin resistance: not reported Speciation: yes Decontamination: yes, NALC‐NaOH for sputum specimens |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Friedrich 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with undiagnosed pleural effusion and high clinical suspicion of pleural TB Age: not reported Sex, female: 36% Children: 0% HIV infection: 28% Clinical setting: tertiary care centre (inpatient and outpatient) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated against culture: 24 Number of specimens evaluated against composite reference standard: 25 Laboratory level: central Country: South Africa World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB Reference standard for TB detection: MGIT Reference standard for rifampicin resistance: MGIT Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Ghariani 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with clinical suspicion of TB Age: mean 32 years, range 3 to 79 years Sex, female: 68% Children: 13% HIV infection: no Clinical setting: tertiary care centre (inpatient and outpatient) Past history of TB: 18% Patients on anti‐TB treatment: yes, 3% Number of specimens evaluated: 174 Laboratory level: central Country: Tunisia World Bank Income Classification: middle income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node TB Reference standard for TB detection: LJ and MGIT Reference standard for rifampicin resistance: MGIT‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Gu 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of participant selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspicion of bone and joint TB Age: median 42 years for TB patients, range 18 to 82 years Sex, female: 54% Children: no HIV infection: not reported Clinical setting: tertiary care centre (inpatient) Past history of TB: not reported Patients on anti‐TB treatment: yes, 100% Number of specimens evaluated: 60 Laboratory level: central Country: China World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: bone and joint TB Reference standard for TB detection: MGIT Reference standard for rifampicin resistance: MGIT‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hanif 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspicion of TB due to symptoms such as fever, cough or weight loss or both, or because they were not responding to initial therapy for other diseases Age: range 20 to 57 years Sex, female: 39% Children: no HIV infection: no Clinical setting: national reference laboratory Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 29 Laboratory level: central Country: Kuwait World Bank Income Classification: middle income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol: yes for lymph node aspirate, pleural fluid, and urine; no for CSF Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: TB meningitis, lymph node TB, pleural TB, genitourinary TB Reference standard for TB detection: LJ and MGIT Reference standard for rifampicin resistance: LJ‐DST and MGIT‐DST Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Heemskerk 2018.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients who were offered lumbar puncture as a part of routine care for suspected brain infection Age: ≥ 18 years; median 37 years (IQR 28 to 50) Sex, female: 43% Children: no HIV infection: 31% Clinical setting: multicentre, hospital‐based (both referral and local) Past history of TB: Participants on anti‐TB treatment: Number of specimens evaluated: 610 Laboratory level: central in South Africa Country: South Africa, Vietnam, Indonesia World Bank Income Classification: middle High TB burden: South Africa yes; Vietnam yes; Indonesia yes High TB/HIV burden: South Africa yes; Vietnam no; Indonesia yes High MDR‐TB burden: South Africa yes; Vietnam yes; Indonesia yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no (as performed in Nhu 2014) |
||
| Target condition and reference standard(s) | TB meningitis CRS: clinical TB meningitis, diagnosis (definite, probable and possible TB meningitis); MGIT (MODS Indonesia) Speciation yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Hillemann 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspected M tuberculosis or nontuberculous mycobacterial infection on the basis of clinical criteria Age: not reported Sex, female: not reported Children: 5% HIV infection: not reported Clinical setting: national reference laboratory Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 200 Laboratory level: central Country: Germany World Bank Income Classification: high income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: yes, donation of index test |
||
| Target condition and reference standard(s) | Target condition: pleural TB, TB meningitis, genitourinary TB Reference standard for TB detection: LJ and MGIT Reference standard for rifampicin resistance: MGIT‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Iram 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with clinical presentation, radiological findings, and histopathological evidence of extrapulmonary TB Age: mean 37 years, range 10 to 80 years Sex, female: 41% Children: 3% HIV infection: 2% Clinical setting: teaching hospital Past history of TB: 53% Patients on anti‐TB treatment: yes, 3% Number of specimens evaluated: 18 Laboratory level: intermediate Country: Pakistan World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: no High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, peritoneal TB Reference standard for TB detection: LJ Reference standard for rifampicin resistance: LJ‐DST Speciation: not reported Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Kim 2015a.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, retrospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: not reported Age: median 59 years (IQR 44 to 71 years) Sex, female: 47% Children: 7% HIV infection: 1% Clinical setting: tertiary care centre Past history of TB: 9% Patients on anti‐TB treatment: no Number of specimens evaluated: 1209 Laboratory level: central Country: Korea World Bank Income Classification: high income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node TB, pleural TB, TB meningitis, peritoneal TB, pericardial TB, bone and joint TB, genitourinary TB Reference standard for TB detection: MGIT Reference standard for rifampicin resistance: LJ‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Li 2017.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of participant selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspected extrapulmonary TB Age: mean 48 years (SD 10 years) Sex, female: 39% Children: no HIV infection: not reported Clinical setting: tertiary care centre Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 414 Laboratory level: central Country: China World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes for pleural fluid, bone and joint TB fluid, urine, and peritoneal fluid; no for CSF Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, TB meningitis, peritoneal TB, bone and joint TB, genitourinary TB Reference standard for TB detection: LJ Reference standard for rifampicin resistance: LJ‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Liang 2019.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of patient selection not reported, retrospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspected pleural TB based on standard clinical and radiological criteria, including a persistent cough of 2 weeks or more, unexplained fever for 2 weeks or more, weight loss, and radiological evidence of pleural effusion Age: mainly adult; 12% < 25 years Sex, female: 22% Children: not reported HIV infection: not reported Clinical setting: national TB referral hospital Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: 219 Laboratory level: central Country: China World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes |
||
| Target condition and reference standard(s) | Pleural TB CRS: clinically diagnosed and microbiologically confirmed |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Ligthelm 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspicion of lymph node TB Age: < 5 years 4%; 5 to 20 years 13%; > 20 years 83% Sex, female: 58% Children: estimated < 15% HIV infection: 19% Clinical setting: university hospital (inpatient and outpatient) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 48 Laboratory level: central Country: South Africa World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node TB Reference standard for TB detection: MGIT Reference standard for rifampicin resistance: MTBDRplus Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | "It is unlikely that our patient cohort had exacerbated disease compared to patients presenting at primary health care clinics, as these patients are routinely referred from the primary health care clinic to the referral centre for FNAB (fine needle aspiration biopsy)" | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Lusiba 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspected pleural TB based on clinical signs and symptoms and radiological evidence of a pleural effusion that was considered large enough for a pleural biopsy Age: mean 34 years, SD 13 years Sex, female: 43% Children: no HIV infection: 45% Clinical setting: tertiary care centre (inpatient and outpatient) Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 116 Laboratory level: central Country: Uganda World Bank Income Classification: low income High TB burden: no High TB/HIV burden: yes High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB Reference standard for TB detection: LJ and MGIT Reference standard for rifampicin resistance: MGIT‐DST Speciation: not reported Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Malbruny 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of participant selection by convenience, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with clinical suspicion of TB Age: median 52 years Sex, female: 40% Children: 7% HIV infection: not reported Clinical setting: university hospital Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 67 Laboratory level: central Country: France World Bank Income Classification: high income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, TB meningitis, bone and joint TB, peritoneal TB, genitourinary TB Reference standard for TB detection: MGIT and Coletsos slants Reference standard for rifampicin resistance: MGIT‐DST Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Meldau 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients presumed to have pleural TB with any symptoms, including cough, fever, night sweats, loss of weight, haemoptysis, and chest pain, along with features consistent with a pleural effusion on chest X‐ray Age: definitive TB: median 39 years (IQR 29 to 55 years); non‐TB: median 61 years (IQR 54 to 69 years) Sex, female: 40% Children: no HIV infection: 15% Clinical setting: tertiary care hospital Past history of TB: 13% Patients on anti‐TB treatment: no Number of specimens evaluated against culture: 76 Number of specimens evaluated against a composite reference standard: 88 Laboratory level: central Country: South Africa World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB Reference standard for TB detection: MGIT Reference standard for rifampicin resistance: MGIT‐DST Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Meldau 2019.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with any TB symptoms including any cough, fever, night sweats, loss of weight, haemoptysis or chest pain or both, and features consistent with a pleural effusion on chest x‐ray Age: Adult; median 39 years (IQR 28 to 57) Sex, female: 11% Children: not reported HIV infection: definite TB: 7% Clinical setting: tertiary care hospital Past history of TB: 6% Participants on anti‐TB treatment: not reported Number of specimens evaluated: 149 Laboratory level: central Country: South Africa World Bank Income Classification: middle High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF and Xpert Ultra WHO SOP or manufacturer's protocol followed: yes |
||
| Target condition and reference standard(s) | Pleural tuberculosis Composite reference standard: MGIT culture and/or histology |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Metcalf 2018.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients presenting with a suspected diagnosis of TB meningitis Age: 18 and older Sex, female: 27% Children: No HIV infection: 62% Clinical setting: inpatient Past history of TB: 30% Participants on anti‐TB treatment: no Number of specimens evaluated: 37 Laboratory level: central Country: Peru World Bank Income Classification: middle income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes |
||
| Target condition and reference standard(s) | TB meningitis MGIT, Ogawa Speciation: not reported |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Nataraj 2016.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with clinical suspicion of extrapulmonary TB Age: < 14 years 13%; 15 to 45 years 52%; > 45 years 34%; range 2 months to 78 years Sex, female: 44% Children: 13% HIV infection: not reported Clinical setting: tertiary care centre (inpatient and outpatient) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 494 Laboratory level: intermediate Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, lymph node TB, TB meningitis, bone and joint TB, genitourinary TB Reference standard TB detection: LJ Reference standard rifampicin resistance detection: LJ‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Patients on treatment may have been included, although the number was not reported: "Of the two specimens that were smear‐positive and smear‐negative on both culture and Xpert, one was pleural fluid from a patient who had been receiving Category II anti‐tuberculosis treatment for 2 months and the other was pus aspirated from an axillary lymph node" | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Nhu 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients suspected of having TB meningitis with at least 5 days of meningitis symptoms, nuchal rigidity, and CSF abnormalities Age: > 18 years Sex, female: not reported Children: no HIV infection: 21% Clinical setting: university hospital Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 379 Laboratory level: central Country: Vietnam World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: no High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: yes, donation of index test |
||
| Target condition and reference standard(s) | Target condition: TB meningitis
Reference standard TB detection: MGIT Reference standard rifampicin resistance detection: MGIT‐DST and MTBDRplus Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Analysis by uniform case definition also included | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Ozkutuk 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: not reported Age: median 54 years, range 1 to 99 years Sex, female: 47% Children: 3% HIV infection: not reported Clinical setting: tertiary care centre (inpatient and outpatient) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 1022 Laboratory level: central Country: Turkey World Bank Income Classification: middle High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, lymph node TB, TB meningitis, genitourinary TB, bone and joint TB, pericardial TB, peritoneal TB
Reference standard TB detection: LJ and MGIT Reference standard rifampicin resistance detection: MGIT‐DST Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Pandie 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with presence of a large pericardial effusion amenable to safe pericardiocentesis (> 10 mm echo‐free space around the heart in diastole) Age: median 34 years (IQR 29 to 42) Sex, female: 38% Children: no HIV infection: 74% Clinical setting: 4 district hospitals and 1 tertiary centre (inpatient) Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 134 Laboratory level: central Country: South Africa World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pericardial TB
Reference standard TB detection: MGIT Reference standard rifampicin resistance detection: MTBDRplus Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Patel 2013.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with clinical suspicion of meningitis Age: mean 33 years (SD 9) Sex, female: 61% Children: 2% HIV infection: 87% Clinical setting: tertiary care centre (inpatient and outpatient) Past history of TB: 31% Patients on anti‐TB treatment: no Number of specimens evaluated: 59 Laboratory level: central Country: South Africa World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: TB meningitis
Reference standard TB detection: MGIT Reference standard rifampicin resistance detection: MGIT‐DST Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Study used frozen specimens | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Peñata 2016.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective and retrospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with clinical suspicion of extrapulmonary tuberculosis Age: mean 42 years (SD 19), range 1 to 91 years Sex, female: 39% Children: 7% HIV infection: 40% Clinical setting: university hospital Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 236 Laboratory level: intermediate Country: Colombia World Bank Income Classification: middle income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node TB, pleural TB, TB meningitis, peritoneal TB, pericardial TB, bone and joint TB
Reference standard TB detection: Ogawa medium Reference standard rifampicin resistance detection: Ogawa‐DST Speciation: not reported Decontamination: unclear |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Perez‐Risco 2018.
| Study characteristics | |||
| Patient Sampling | Study design unclear, manner of patient selection not reported, retrospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: Smear‐negative extrapulmonary patients Age: adult Sex, female: not reported Children: 0% HIV infection: not reported Clinical setting: laboratory‐based evaluation Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: CSF 3; pleural fluid 24; urine 24; bone or joint fluid 24 Laboratory level: central Country: Spain World Bank Income Classification: high High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert Ultra WHO SOP or manufacturer's protocol followed: yes |
||
| Target condition and reference standard(s) | Target condition: TB meningitis, pleural TB, genitourinary TB, bone or joint TB Reference standard TB detection: MGIT and LJ culture Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | The specimens were collected between May 1999 and May 2017; frozen specimens | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Rakotoarivelo 2018.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with a febrile illness and chronic respiratory symptoms, pleural effusion, chronic abdominal pain or ascites, chronic meningitis, or other symptoms suggestive of extrapulmonary TB Age: adult; mean (SD) 38.7 years (15.2) Sex, female: 36% Children: no HIV infection: 12% Clinical setting: tertiary care centre Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: CSF: 77; pleural: 50 Laboratory level: central Country: Madagascar World Bank Income Classification: low income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes |
||
| Target condition and reference standard(s) | TB meningitis; pleural TB Reference standard: LJ Composite reference standard: proven and probable cases (culture‐positive cases or clinical response to anti‐TB treatment without any other diagnosis or other treatment) Speciaiton: yes decontamination: yes |
||
| Flow and timing | |||
| Comparative | |||
| Notes | The criteria of Marais were used for the diagnosis of tuberculous meningitis (Marais 2010). This classification and stratification of cases was independent of Xpert MTB/RIF and the panel was blinded to these results. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Rufai 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of participant selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with high suspicion of pleural TB. Enrolment was based on standard clinical and radiological criteria, including a persistent cough of 2 weeks or longer, unexplained fever for 2 weeks or longer, unexplained weight loss with or without night sweats, chest pain, and radiological evidence of pleural effusion Age: men: mean 42 years (SD 19 years); women: mean 39 years (SD 19 years) Sex, female: 28% Children: 6% HIV infection: no Clinical setting: tertiary care centre Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 161 Laboratory level: central Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB
Reference standard TB detection: MGIT Reference standard rifampicin resistance detection: MGIT‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Rufai 2017a.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of participant selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with clinical or radiological suspicion of abdominal TB Age: men: mean 41 years (SD 19 years); women: mean 46 years (SD 20 years) Sex, female: 36% Children: no HIV infection: no Clinical setting: tertiary care centre Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 67 Laboratory level: central Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: peritoneal TB
Reference standard TB detection: MGIT Reference standard rifampicin resistance detection: MGIT‐DST Decontamination: yes, NALC‐NaOH Speciation: yes |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Rufai 2017b.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of participant selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: fatigue, malaise, low‐grade fever, confusion, nausea and vomiting, lethargy, irritability, and unconsciousness Age: men: mean 38 years (SD 10 years); women: mean 34 years (SD 22 years) Sex, female: 41% Children: 6% HIV infection: not reported Clinical setting: tertiary care centre Past history of TB: not reported Patients on anti‐TB treatment: yes, 4% Number of specimens evaluated: 267 Laboratory level: central Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: TB meningitis
Reference standard TB detection: MGIT Reference standard rifampicin resistance detection: MGIT‐DST Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Safianowska 2012.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: not reported Age: not reported Sex, female: 46% Children: no HIV infection: no Clinical setting: university hospital Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 51 Laboratory level: intermediate Country: Poland World Bank Income Classification: high income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, lymph node TB, TB meningitis, peritoneal TB, pericardial TB, genitourinary TB, bone and joint TB
Reference standard TB detection: LJ Reference standard rifampicin resistance detection: LJ‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sarfaraz 2018.
| Study characteristics | |||
| Patient Sampling | Cohort, manner of selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients presenting with 1 or more superficial lymph nodes (i.e. cervical, axillary, and inguinal nodes) measuring > 2 cm in largest diameter and persisting for more than 1 month, with or without constitutional symptoms of fever, anorexia, and weight loss Age: > 14 years of age; median 23 years (IQR 18 to 32) Sex, female: 79% Children: no HIV infection: 1% Clinical setting: outpatient Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: 261 Laboratory level: central Country: Pakistan World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: no High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes |
||
| Target condition and reference standard(s) | Lymph node TB, tissue MGIT; LJ Composite reference standard includes histopathology Rifampicin resistance MGIT‐DST Speciation: not reported Decontamination: yes (NALC–NaOH) |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Scott 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: not reported Age: median 39 years, range < 1 year to 96 years Sex, female: 45% Children: 4% HIV infection: not reported Clinical setting: reference laboratory Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 696 Laboratory level: central Country: South Africa World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes for lymph node aspirate, pleural fluid, and peritoneal fluid; no for CSF Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, lymph node TB, TB meningitis, peritoneal TB
Reference standard TB detection: MGIT Reference standard rifampicin resistance detection: MGIT‐DST and MTBDRplus Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sharma 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with clinical suspicion of EPTB Age: mean 35 years (SD 15 years) Sex, female: 50% Children: no HIV infection: not reported Clinical setting: tertiary care centre Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 1139 Laboratory level: central Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol: yes for body fluids and LN tissue; no for CSF Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, lymph node TB, TB meningitis, peritoneal TB, pericardial TB, genitourinary TB
Reference standard TB detection: LJ and MGIT Reference standard rifampicin resistance detection: LJ‐DST Speciation: yes Decontamination: yes, NALC‐NaOH (for all specimens except CSF, pleural fluid, and urine) |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sharma 2016.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: women being evaluated for infertility and suspected to have TB Age: mean 29 years, range 19 to 41 years Sex, female: 100% Children: no HIV infection: not reported Clinical setting: tertiary care centre Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 240 Laboratory level: central Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: genitourinary TB
Reference standard TB detection: LJ and MGIT Reference standard rifampicin resistance detection: MGIT‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sharma 2018.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, random selection, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: participants with persistent cough and unexplained fever for 2 weeks or more, unexplained weight loss, pleuritic chest pain, anorexia – among others, positive Mantoux test and the suggestive radiological
findings Age: mean 39 years (range, 18 to 60) Sex, female: 64% Children: no HIV infection: 0% Clinical setting: university hospital Past history of TB: Participants on anti‐TB treatment: no Number of specimens evaluated: 78 Laboratory level: central Country: India World Bank Income Classification: middle‐income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol: yes |
||
| Target condition and reference standard(s) | Pleural tuberculosis, pleural fluid Composite reference standard: combination of smear, culture, clinical findings, radiology, histology, cytology, response to ATT Speciation: yes decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Siddiqi 2019.
| Study characteristics | |||
| Patient Sampling | Cohort, manner of patient selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: presented with signs and symptoms concerning for TB meningitis and already received a lumbar puncture as part of routine care Age: age 18 years and older; TB meningitis culture positive: median 35 years (IQR 30 to 41) Sex, female: 36% Children: no HIV infection: 86% Clinical setting: university teaching hospital Past history of TB: 20% Participants on anti‐TB treatment: not reported Number of specimens evaluated: 550 Laboratory level: central Country: Zambia World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes |
||
| Target condition and reference standard(s) | TB meningitis MGIT Rifampicin resistance Samples found to be rifampin resistant by Xpert MTB/RIF had confirmatory DST for rifampin and isoniazid conducted separately Speciation: yes Decontamination: No |
||
| Flow and timing | |||
| Comparative | |||
| Notes | After a lumbar puncture was completed, study staff identified patients from the microbiology laboratories who had 3 ml of excess CSF remaining after routine testing composed of Gram stain, India ink stain, cryptococcal antigen testing, and bacterial culture on a blood agar plate. A composite reference standard was defined as probable TB meningitis = patients with a CSF white blood cell count between 10 and 500, CSF total protein of 100 mg/dl, and CSF glucose of 40 mg/dl. These values were adapted from a uniform case definition of probable TBM for use in clinical research (Marais 2010). However, sensitivity and specificity were only determined using culture as the reference standard |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Sun 2019.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with symptoms such as pain, swelling in the joints, tenderness, effusion, restriction of movements, and systematic symptoms such as fever, loss of weight/appetite, elevated erythrocyte sedimentation rate, and cough, breathlessness, and history of TB Age: osteoarticular TB: median 51 years (range 16 to 86) Sex, female: osteoarticular TB: 55% Children: no HIV infection: 0% Clinical setting: national level TB referral centre Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: 166 Laboratory level: central Country: China World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF and Xpert Ultra WHO SOP or manufacturer's protocol followed: yes |
||
| Target condition and reference standard(s) | Bone or joint TB, fluid MGIT Composite reference standard: clinical, laboratory, histopathological, radiological and ≥ 6 months’ follow‐up Rifampicin resistance LJ‐DST Speciation: yes Decontamination: Yes |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Suzana 2016.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with signs and symptoms suggestive of extrapulmonary TB Age: median 34 years Sex, female: 39% Children: 0.06% HIV infection: 7% Clinical setting: tertiary care centre Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 215 Laboratory level: central Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes for lymph node tissue and pleural tissue; no for pleural fluid, bone and joint fluid, urine, peritoneal fluid, pericardial fluid, and CSF Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, lymph node TB, TB meningitis, peritoneal TB, pericardial TB, genitourinary TB, bone and joint TB
Reference standard TB detection: LJ and MGIT Reference standard rifampicin resistance detection: LJ‐DST and MGIT‐DST Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Tadesse 2015.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: people with presumptive lymph node TB Age: ≤ 15 years 15%; > 15 years 85% Sex, female: 53% Children: 15% HIV infection: not reported Clinical setting: university hospital (outpatient) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 136 Laboratory level: central Country: Ethiopia World Bank Income Classification: low income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node TB
Reference standard TB detection: LJ Reference standard rifampicin resistance detection: not reported Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Study used frozen specimens | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Trajman 2014.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with a pleural effusion needing thoracentesis Age: median 50 years (IQR 40 to 57) Sex, female: 20% Children: no HIV infection: 5% Clinical setting: secondary health facility (inpatient) Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 85 Laboratory level: central Country: Brazil World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB
Reference standard TB detection: MGIT Reference standard rifampicin resistance detection: MGIT‐DST Speciation: not reported Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Patients were excluded if they had bleeding disorders contraindicating thoracentesis, if the fluid volume was insufficient for storage, or if a final diagnosis could not be ascertained. One of the main limitations of the study was the high number of presumptive (non‐confirmed) cases. The number of exclusions was also high: out of 203 eligible patients, 110 were excluded: 21 did not have a final diagnosis and 89 did not have sufficient fluid to store. "Cultures of pleural tissue, which could significantly improve accuracy of diagnosis, were not performed" Study used frozen specimens |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Ullah 2017.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients meeting the following criteria: previously TB‐treated cases with both positive and negative smears; failure of Cat‐I and Cat‐II TB drugs; all smear‐positive cases that remained positive by the end of the second month of TB treatment; TB/HIV co‐infection cases; seriously ill patients; contacts of MDR‐TB patients Age: mean 34 years (SD 19 years), range 3 to 80 years Sex, female: 51% Children: 14% HIV infection: not reported Clinical setting: tertiary care centre Past history of TB: 60% Patients on anti‐TB treatment: yes, percentage not reported Number of specimens evaluated: 168 Laboratory level: central Country: Pakistan World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: no High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node TB, TB meningitis, peritoneal TB, pericardial TB
Reference standard TB detection: Middlebrook 7H10 Reference standard rifampicin resistance detection: Middlebrook 7H10 Speciation: not reported Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Study included a highly selective population that met specified criteria: previously TB‐treated cases with both positive and negative smears; failure of Cat‐I and Cat‐II TB drugs; all smear‐positive cases that remained positive by the end of the second month of TB treatment; TB/HIV co‐infection cases; seriously ill patients; contacts of MDR‐TB patients | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Vadwai 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: suspected extrapulmonary TB based on symptoms: brain: irritability, restlessness, neck stiffness, headache persistent for 2 to 3 weeks, vomiting, seizures, changes in mental condition or behaviour; intestinal tract, abdomen: abdominal pain, diarrhoea; lymph nodes: enlargement of lymph nodes, mass formation in the neck; cardiorespiratory: shortness of breath, hypertension, chest pain, dyspnoea; endometrium: pelvic pain, pelvic mass, irregular periods, infertility; skin (cutaneous): visible presence of ulcers or lesions, tender nodules Age: median 37 years Sex, female: 15% Children: 3% HIV infection: 3% Clinical setting: tertiary care centre Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 60 Laboratory level: central Country: India World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes for pleural fluid, peritoneal fluid, pericardial fluid; no for CSF Manufacturer's involvement: yes, in design, analysis, or manuscript production (David Alland is among a group of co‐investigators who invented molecular beacons and receive income from licensees, including to Cepheid, for M tuberculosis detection) |
||
| Target condition and reference standard(s) | Target condition: pleural TB, TB meningitis, peritoneal TB, pericardial TB
Reference standard TB detection: LJ and MGIT Reference standard rifampicin resistance detection: MGIT‐DST Speciation: yes Decontamination: yes, NALC‐NaOH |
||
| Flow and timing | |||
| Comparative | |||
| Notes | "Patients were enrolled only if they could provide detailed clinical history and radiological and histology/cytology reports, along with an adequate amount of specimen material" | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Van Rie 2013.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: HIV‐infected patients with suspicion of LNTB Age: mean 36 years, range 18 to 73 years Sex, female: 49% Children: no HIV infection: 100% Clinical setting: tertiary care centre (inpatient and outpatient) Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 344 Laboratory level: central Country: South Africa World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: lymph node TB
Reference standard TB detection: MGIT Reference standard rifampicin resistance detection: MGIT‐DST Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Wang 2019.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: adults who were offered lumbar puncture as a part of routine care for suspected brain infection; TB symptoms, chest pain, radiological evidence of pleural effusion, thoracoscopic examination that suggested TB Age: 15 years and older; pleural tuberculosis TB 37 years (range, 15 to 89); TB meningitis 33 years (range, 15 to 83) Sex, female: pleural tuberculosis: 20%; TB meningitis: 44% Children: no HIV infection: 0% Clinical setting: national level tuberculosis referral centre Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: not reported Laboratory level: central Country: China World Bank Income Classification: middle income High TB burden country: yes High MDR‐TB burden country: yes High TB/HIV burden country: yes Prevalence of TB cases in the study: |
||
| Index tests | Xpert MTB/RIF and Xpert Ultra | ||
| Target condition and reference standard(s) | LJ and MGIT Composite reference standard for pleural TB: composed of clinical, laboratory, histopathological, and radiological and follow‐up features Rifampicin resistance LJ‐DST Speciation: not reported Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Wang 2020.
| Study characteristics | |||
| Patient Sampling | Cohort, consecutive, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: suspected pleural tuberculosis Age: median 45 years; range: 15 to 89 Sex, female: 32.5% Children: no HIV infection: 0% Clinical setting: Unclear Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: 139 Laboratory level: central Country: China World Bank Income Classification: middle income High TB burden country: yes High MDR‐TB burden country: yes High TB/HIV burden country: yes |
||
| Index tests | Xpert MTB/RIF and Xpert Ultra WHO SOP or manufacturer's protocol followed: yes Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Pleural TB LJ and MGIT Speciation: Yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | This study used fresh specimens for Xpert and frozen specimens for Xpert Ultra | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | |||
| If a threshold was used, was it pre‐specified? | |||
| Could the conduct or interpretation of the index test have introduced bias? | |||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | |||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Wu 2019.
| Study characteristics | |||
| Patient Sampling | Cohort, manner of selection not reported, consecutive | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients with suspicion of extrapulmonary tuberculosis; diagnostic criteria followed the WHO guidelines and was based on a combination of clinical symptoms, radiological evidence compatible with active TB, histological observations, lack of improvement in response to a course of broad‐spectrum antibiotics Age: 16 years and older Sex, female: 32% Children: no HIV infection: 0% Clinical setting: tertiary‐care hospital Past history of TB: not reported Participants on anti‐TB treatment: not reported Number of specimens evaluated: lymph node fluid 52; pleural fluid: 119 Laboratory level: central Country: China World Bank Income Classification: middle income High TB burden: yes High TB/HIV burden: yes High MDR‐TB burden: yes |
||
| Index tests | Xpert MTB/RIF and Xpert Ultra WHO SOP or manufacturer's protocol followed: no (see note) |
||
| Target condition and reference standard(s) | Lymph node tuberculosis; pleural tuberculosis MGIT Speciation: not reported Sterile specimens were directly processed. Non‐sterile specimens were pretreated with N‐acetyl‐L‐cysteine‐NaOH‐Na citrate |
||
| Flow and timing | |||
| Comparative | |||
| Notes | 4 ml GeneXpert sample reagent was added to the remaining 1 ml of each specimen | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | |||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Zeka 2011.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, consecutive, retrospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: clinical findings of possible TB Age: median 48 years Sex, female: 42% Children: 13% HIV infection: 1% Clinical setting: tertiary care centre Past history of TB: not reported Patients on anti‐TB treatment: no Number of specimens evaluated: 149 Laboratory level: central Country: Turkey World Bank Income Classification: middle income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: no Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, TB meningitis, genitourinary TB, peritoneal TB, pericardial TB
Reference standard TB detection: LJ and BacT liquid medium Reference standard rifampicin resistance detection: 7H10 agar media Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | Study used frozen specimens | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Zmak 2013.
| Study characteristics | |||
| Patient Sampling | Cross‐sectional, manner of participant selection not reported, prospective | ||
| Patient characteristics and setting | Presenting signs and symptoms: patients suspected of EPTB Age: 15 years and older Sex, female: not reported Children: 13% HIV infection: not reported Clinical setting: laboratory‐based evaluation Past history of TB: not reported Patients on anti‐TB treatment: not reported Number of specimens evaluated: 176 Laboratory level: central Country: Croatia World Bank Income Classification: high income High TB burden: no High TB/HIV burden: no High MDR‐TB burden: no |
||
| Index tests | Xpert MTB/RIF WHO SOP or manufacturer's protocol followed: yes for pleural fluid, urine, peritoneal fluid, pericardial fluid, and blood; no for CSF Manufacturer's involvement: no |
||
| Target condition and reference standard(s) | Target condition: pleural TB, TB meningitis, peritoneal TB, pericardial TB, genitourinary TB, disseminated TB
Reference standard TB detection: LJ, Stonebrink, and MGIT Reference standard rifampicin resistance detection: LJ‐DST Speciation: yes Decontamination: no |
||
| Flow and timing | |||
| Comparative | |||
| Notes | "Although the NRL performs a third‐level laboratory service for the whole country, it is actually also involved in first and second‐level laboratory work for several counties" | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (Xpert MTB/RIF) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 2: Index Test (Xpert Ultra) | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| For rifampicin resistance testing, were the reference standard results interpreted without knowledge of the results of the index test? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
CSF: cerebrospinal fluid; DST: drug susceptibility testing; EBUS: endobronchial ultrasound; EPTB: extrapulmonary tuberculosis: IQR: interquartile ratio; LJ: Löwenstein‐Jensen; LN: lymph node; MDR‐TB: multi‐drug‐resistant tuberculosis; MGIT: mycobacteria growth indicator tube; NALC‐NaOH: N‐acetyl‐L‐cysteine‐sodium hydroxide; SD: standard deviation; SOP: standard operating procedure; TB: tuberculosis; TBM: tuberculous meningitis; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abong 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Adejumo 2018 | Did not contain specimen for extrapulmonary TB |
| Afsar 2018 | Did not contain data by site for extrapulmonary TB |
| Ahmad 2018 | Inadequate reference standard |
| Akhter 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Ali 2018 | Inadequate reference standard |
| Allahyartorkaman 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Alvarez Uria 2012 | Inappropriate reference standard |
| Andrey 2015 | Case report |
| Armand 2011 | Case‐control study |
| Arockiaraj 2015 | Abstract; we included the published study (Arockiaraj 2017) in the review |
| Arockiaraj 2017 | Includes both adults and children or no information about age of enrolment |
| Arockiaraj 2019a | Case‐control study |
| Arockiaraj 2019b | Children |
| Atherton 2018 | Case report |
| Aydemir 2019 | Could not obtain; same publication as Terzi 2019 |
| Bablishvili 2015 | Did not contain specimen for extrapulmonary TB |
| Bahr 2018a | Test other than Xpert MTB/RIF and Xpert Ultra |
| Bahr 2018b | Duplicate data for Bahr 2017 |
| Bahr 2019 | Review |
| Baikunje 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Bajrami 2016 | Could not extract 2 × 2 values |
| Balcha 2014 | Did not contain specimen for extrapulmonary TB |
| Bankar 2018 | Includes both adults and children or no information about age of enrolment |
| Bemba 2017 | Inappropriate reference standard |
| Ben Saad 2018 | Could not extract 2 × 2 values |
| Bhardwaj 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Bhatia 2016 | Could not extract 2 × 2 values |
| Bholla 2016 | Children |
| Biadglegne 2013 | Could not extract 2 × 2 values |
| Bilgin 2016 | Could not extract 2 × 2 values |
| Borraz‐Noriega 2018 | Could not extract 2 × 2 values |
| Boyles 2018 | Correspondence without original data |
| Bunsow 2014 | Could not extract 2 × 2 values |
| Celik 2015 | Could not extract 2 × 2 values |
| Chaidir 2018 | Inappropriate reference standard |
| Chakraborty 2019 | Xpert Ultra was not evaluated |
| Chen 2016 | Could not extract 2 × 2 values |
| Chhajed 2019 | Xpert Ultra was not evaluated |
| Christopher 2018 | Could not extract 2 x 2 values |
| Coetzee 2014 | Children |
| Coleman 2015 | Case‐control study |
| Creswell 2019 | Did not contain specimen for extrapulmonary TB |
| Dahale 2019 | Case‐control study |
| Das 2019 | Children |
| Deggim 2013 | Fewer than 5 specimens for a given type of specimen (only 1 pleural fluid specimen) |
| Dharan 2016 | Did not contain specimen for extrapulmonary TB |
| Diallo 2016 | Includes both adults and children or no information about age of enrolment |
| Diop 2016 | Inappropriate reference standard |
| Edwards 2016 | Case report |
| Ejeta 2018 | Could not extract 2 x 2 values |
| Erdem 2014 | Index test other than Xpert MTB/RIF |
| Fanosie 2016 | Did not contain specimen for extrapulmonary TB |
| Fantahun 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Floridia 2017 | Could not extract 2 x 2 values |
| García 2017 | Duplicate data for García Cañete 2017 |
| García Cañete 2017 | Includes both adults and children or no information about age of enrolment |
| Gascoyne‐Binzi 2012 | Abstract; we could not extract data by form of extrapulmonary TB |
| Gati 2018 | Could not extract 2 x 2 values |
| Gautam 2018 | Inappropriate reference standard |
| Gautam 2019 | Inappropriate reference standard |
| Gounden 2018 | Could not extract 2 x 2 values |
| Gulla 2019 | Could not extract 2 x 2 values |
| Gursoy 2016 | Includes both adults and children or no information about age of enrolment |
| Habeenzu 2017 | Did not contain specimen for extrapulmonary TB |
| Habous 2019 | Includes both adults and children or no information about age of enrolment |
| Hanifa 2017 | Could not extract 2 x 2 values |
| Held 2014 | Bone tissue, specimen not included |
| Held 2016 | Bone tissue, specimen not included |
| Held 2017 | Bone tissue, specimen not included |
| Ioannidis 2010 | Duplicate data |
| Ioannidis 2011 | Includes both adults and children or no information about age of enrolment |
| Jain 2017 | Inappropriate reference standard |
| Jing 2017 | Includes both adults and children or no information about age of enrolment |
| Jipa 2017 | Could not extract 2 x 2 values |
| Jorstad 2018 | Inappropriate reference standard |
| Joythi 2019 | Children |
| Kanade 2018 | Did not contain data by site for extrapulmonary TB |
| Kashyap 2019 | Inappropriate reference standard |
| Kendall 2019 | Case‐control study |
| Kerkhoff 2017 | Did not contain specimen for extrapulmonary TB |
| Khadka 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Khan 2018 | Includes both adults and children or no information about age of enrolment |
| Kilfoil 2015 | Could not extract 2 × 2 values |
| Kim 2014 | Could not extract 2 × 2 values; unclear if culture‐positive; pleural fluid (3), CSF (2); peritoneal fluid (1) |
| Kim 2015b | Case‐control study |
| Kim 2015c | Could not extract 2 × 2 values |
| Kotovich 2018 | Inappropriate reference standard |
| Koul 2018 | Could not extract 2 × 2 values |
| Kumar 2017 | Case‐control study |
| Kumari 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Kurbaniyazova 2017 | Did not contain specimen for extrapulmonary TB |
| Kwak 2015 | Duplicate data |
| Lawn 2012 | Screening study |
| Lawn 2013 | Could not extract 2 × 2 values |
| Lawn 2015 | Screening study |
| Lawn 2017 | Could not extract 2 × 2 values |
| Lee 2017 | Duplicate data |
| Lemus‐Minor 2018 | Did not contain specimen for extrapulmonary TB |
| Li 2018 | Inappropriate reference standard |
| Li 2020 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Liu 2015 | Duplicate data |
| Liu 2019 | Did not contain specimen for extrapulmonary TB |
| Lombardi 2017 | Could not extract data by site of extrapulmonary TB |
| Marouane 2014 | Abstract; we excluded the publication, Marouane 2016, because we could not extract 2 × 2 values |
| Marouane 2016 | Could not extract 2 × 2 values |
| Massi 2017 | Includes both adults and children or no information about age of enrolment |
| Mathew 2018 | Did not contain data by site for extrapulmonary TB |
| Mazzola 2016 | Includes both adults and children or no information about age of enrolment |
| McMillen 2018 | Did not contain data by site for extrapulmonary TB |
| Mechal 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Metaferia 2018 | Could not extract 2 × 2 values |
| Miller 2011 | Fewer than 5 specimens for a given type of specimen; lymph node biopsy (3 specimens, of which 1 was culture‐positive) and endometrial biopsy (1 specimen that was culture‐positive) |
| Mishra 2017 | Abstract; we did not identify a published study |
| Moure 2011 | Fewer than 5 specimens for a given type of specimen: CSF (3 specimens, all culture‐negative); pleural fluid (4 specimens, 2 culture‐positive); lymph node aspirate (1 specimen, culture‐negative); urine (2 specimens, both culture‐positive); peritoneal fluid (2, both culture‐negative) |
| Moure 2012 | Case‐control study |
| Negi 2019 | Case‐control study |
| Nhu 2013 | Inappropriate reference standard |
| Omar 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Pandey 2017 | Includes both adults and children or no information about age of enrolment |
| Paramitha 2018 | Could not extract 2 × 2 values |
| Park 2019 | Inappropriate reference standard |
| Patel 2014 | Duplicate data |
| Patel 2020 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Peter 2012 | Case‐control study |
| Philip 2017 | Inappropriate reference standard |
| Piersimoni 2019 | Could not extract 2 x 2 tables |
| Pink 2016 | Includes both adults and children or no information about age of enrolment |
| Pohl 2016 | Children |
| Porcel 2013 | Case‐control study |
| Rachow 2012 | Did not contain specimen for extrapulmonary TB |
| Raizada 2015 | Inappropriate reference standard |
| Raizada 2018 | Children |
| Ramamurthy 2016 | Could not extract data by site of extrapulmonary TB |
| Rathour 2019 | Children |
| Razack 2014 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Rebecca 2018 | Children |
| Reddy 2017 | Could not extract 2 × 2 values |
| Rindi 2017 | Case‐control study |
| Rossato Silva 2018 | Did not contain specimen for extrapulmonary TB |
| Ruiz 2017 | Did not contain data by site for extrapulmonary TB |
| Sachdeva 2018 | Includes both adults and children or no information about age of enrolment |
| Saeed 2017a | Includes both adults and children or no information about age of enrolment |
| Saeed 2017b | Could not extract 2 × 2 values |
| Saeed 2018 | Did not contain data by site for extrapulmonary TB |
| Salvador 2015 | Case‐control study |
| Samuel 2018 | Inappropriate reference standard |
| Sanjuan Jimenez 2015 | Case‐control study |
| Schutz 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Sekyere 2019 | Could not extract 2 x 2 data |
| Set 2018 | Did not contain data by site for extrapulmonary TB |
| Set 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Shah 2016a | Case‐control study |
| Shakeel 2018 | Did not contain data by site for extrapulmonary TB |
| Sharma 2017a | Did not contain data by site for extrapulmonary TB |
| Sharma 2019 | Did not include specimen of interest |
| Singanayagam 2014 | Could not extract 2 × 2 values |
| Singh 2016 | Could not extract 2 × 2 values |
| Smith 2014 | Did not contain specimen for extrapulmonary TB |
| Solomons 2015 | Duplicate data |
| Solomons 2016 | Includes both adults and children or no information about age of enrolment |
| Soomro 2017 | Could not extract 2 × 2 values |
| Sumayya 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Tahseen 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Talib 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Tang 2017 | Could not extract 2 × 2 values |
| Tang 2018 | Case‐control study |
| Teo 2011 | Includes both adults and children or no information about age of enrolment |
| Terzi 2019 | Could not obtain; same publication as Aydemir 2019 |
| Theron 2014b | Duplicate data |
| Tortoli 2012 | Includes both adults and children or no information about age of enrolment |
| Toure 2017 | Could not extract 2 × 2 values |
| Uddin 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Vallejo 2015 | Could not extract 2 × 2 values |
| Verghese 2016 | Abstract; we did not identify a published study |
| Wang 2016a | Could not extract 2 × 2 values |
| Wang 2016b | Includes both adults and children or no information about age of enrolment |
| Wang 2018 | Inadequate reference standard |
| Wei 2016 | Inappropriate reference standard |
| Yang 2017 | Could not obtain 2 x 2 values |
| Yang 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Yu 2020 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Yuan 2016 | Inappropriate reference standard |
| Zhang 2016 | Could not extract 2 × 2 values |
| Zhou 2020 | Test other than Xpert MTB/RIF and Xpert Ultra |
| Zurcher 2019 | Test other than Xpert MTB/RIF and Xpert Ultra |
TB: tuberculosis.
Differences between protocol and review
Table 14 describes prespecified changes for this review update.
9. Prespecified changes for review update 2021.
| Protocol section | Appraisal points | Address here |
| Background and research question | • Review and update Background section, including supporting references to take account of any changes that may have occurred. This should include updating any new information and current policy debates on the topic | This review update will describe the burden of extrapulmonary tuberculosis worldwide based on the latest WHO Global Tuberculosis Report. The Background will describe the updated WHO guidelines on molecular methods for diagnosing tuberculosis, including Xpert MTB/RIF and Xpert Ultra. The WHO Meeting to update the guidelines took place 3 ‐ 6 December 2019. This Cochrane Review update will have informed these guidelines |
| Inclusion criteria | • Review current PICO(s) and amend in light of new knowledge. • Identify any changes in usual‐care standards. • Check for standardised core outcomes sets, such as those developed in collaboration with the core outcome measures in effectiveness trials (COMET) initiative (www.comet-initiative.org) or by guideline groups since the original review. • Check for any relevant patient‐reported outcomes to include subsequent to the original review. • Consider any new studies with less risk of bias that might warrant a stricter study design inclusion criterion (where the older version, when there was a dearth of evidence, included observational or quasi‐randomised comparisons). | This is a diagnostic test accuracy review. Participants, index tests, and target condition, will be the same as in Kohli 2018 except as follows: The update will be restricted to adults (15 years and older). The reason for this is that we have a separate Cochrane Review underway that is evaluating the tests for extrapulmonary tuberculosis in children. We will add a composite reference standard, defined as culture or clinical criteria as defined by the primary study authors. The addition of a composite reference standard was specifically requested by the WHO and Guideline members. The primary objectives are to assess the diagnostic accuracy of Xpert Ultra for the diagnosis of extrapulmonary tuberculosis and to assess the diagnostic accuracy of Xpert Ultra for the detection of rifampicin resistance in adults. Secondary objectives are the following. ‐ to investigate potential sources of heterogeneity in test accuracy, including volume of CSF for TB meningitis and processing methods for lymph node TB ‐ to compare the accuracy of Xpert Ultra and Xpert MTB/RIF in studies that evaluated both tests. Concerning patient outcomes, the Discussion will summarize and refer to key findings in the test‐treatment Cochrane Review by Haraka et al. (currently undergoing peer review). Although the Haraka review relates to pulmonary tuberculosis, it is the only evidence on patient outcomes of which we are aware. |
| Methods | ‐ | We will use QUADAS‐2 to appraise methodological quality of included studies consistent with Kohli 2018. If data are sufficient, we will perform meta‐analyses using a bivariate random‐effects model. The analyses will include: 1. Xpert Ultra for different forms of extrapulmonary tuberculosis, culture reference standard 2. Xpert Ultra for different forms of extrapulmonary tuberculosis, composite reference standard 3. Accuracy of Xpert Ultra versus Xpert MTB/RIF in studies that evaluated both tests 4. Xpert Ultra for detecting rifampicin resistance The different forms (and corresponding specimens for diagnosis) of extrapulmonary TB include: tuberculous meningitis, lymph node tuberculosis, pleural tuberculosis, genitourinary tuberculosis, bone or joint tuberculosis. We will create 'Summary of findings' tables for the two primary objectives of the review. |
TB: tuberculosis.
This table was approved by the CIDG editorial team on 17 December 2019.
Selection criteria: We included studies where at least 85% of the participants enrolled were adults, aged 15 years or older, with presumptive extrapulmonary tuberculosis or rifampicin‐resistant tuberculosis from all settings and countries. Restricting the age group to adults differs from the original review, where we also included children (Kohli 2018). We did this because children are now included in a separate Cochrane Review, Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children (Kay 2020). We excluded studies where we could not disaggregate data on adults from those in children and studies where we could not tell the age of the participants enrolled.
QUADAS‐2: we modified QUADAS‐2 as follows.
Participant selection domain, applicability: For tuberculous meningitis, owing to the severity of the illness, we judged 'low concern' if participants were evaluated as inpatients at tertiary care centres. In the original review, we judged tertiary care to be a setting of high concern.
Reference standard domain: We clarified that CSF, pleural fluid, and lymph node aspirates are usually considered to be sterile, and standards specify that these specimens may be placed directly into the culture medium. Overly processing specimens may lead to false‐negative cultures. We scored ‘yes' if studies did not use N‐acetyl‐L‐cysteine‐sodium hydroxide for processing sterile specimens and 'unclear' if studies used N‐acetyl‐L‐cysteine‐sodium hydroxide.
Investigations of heterogeneity: For specimen volume, we restricted this analysis to CSF because it was most clinically meaningful. For other fluid specimen types, the manufacturer's instructions for sputum were usually followed, requiring 2 mL of input fluid for the Xpert cartridge. In terms of the WHO standard operating procedure for lymph node tissue, we did not investigate this further because 80% (8/10) of the included studies followed the WHO recommendations. In performing the review, it became clear that because a homogenization step is part of the WHO standard operating procedure for preparing tissue specimens, there was no need to perform an additional separate analysis to confirm the presence of a homogenization step. We removed condition of specimen (fresh or frozen) from the analysis, because we identified only six studies in the current review that used frozen specimens, and we had already performed an analysis of this possible source of heterogeneity for the Cochrane Review on Xpert for pulmonary tuberculosis (Steingart 2014).
We have tried to eliminate stigmatizing language, for example, by changing ‘suspected tuberculosis' to ‘presumptive tuberculosis'.
For Xpert Ultra accuracy for lymph node tuberculosis, owing to insufficient data, we were unable to investigate processing methods for lymph node aspirate.
GRADE: We elaborated on the means of applying GRADE to publication bias: We rated publication bias as undetected (not serious) for several reasons: the comprehensiveness of the literature search and extensive outreach to tuberculosis researchers to identify studies; the presence of only studies that produced precise estimates of high accuracy despite small sample size; and our knowledge about studies that were conducted but not published.
Unlike our previous review, in this update we did not extract information on manufacturers’ involvement and funding. As both Xpert Ultra and Xpert MTB/RIF are available at a concessional price for researchers in resource‐limited settings, and well‐established tests, especially Xpert MTB/RIF, industry donation is rarely pursued. We acknowledge that, in addition to funding, there are other reasons for conflicts of interest, but we did not have time to pursue these. We are aware of a new tool being developed for this purpose: TACIT (Tool for Addressing Conflicts of Interest in Trials: tacit.one). We plan to avail ourselves of this new tool in future updates.
Sensitivity analyses: We stated in our protocol that for Xpert Ultra we would perform a sensitivity analysis by limiting studies to those that included only untreated participants. This information was often not reported in the publications and we did not contact primary study authors specifically about this question. We were therefore unable to confirm that studies met this criterion.
For the 'Summary of findings' tables, we prioritised culture as the reference standard (best reference standard for tuberculosis), apart from lymph node aspirate where we provide evidence using a composite reference standard, because, based on findings from the original review (Kohli 2018), we believe a composite reference is preferable for estimating accuracy.
We added post hoc a sensitivity analysis limiting inclusion to studies that used one specimen per participant.
Contributions of authors
MK and KRS wrote early drafts of the protocol. CMD and SGS contributed methodological advice. KD contributed clinical expertise. CMD and SGS tailored QUADAS‐2 to the review. MK and KRS reviewed the studies and extracted accuracy data. MK and KRS assessed the methodological quality of included studies. IS, MY, and ND performed the statistical analyses. All review authors interpreted the findings. MK, ND, and KRS wrote the first draft of the review. MK and KRS prepared the ‘Summary of findings' tables. All review authors contributed to the final manuscript.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
External sources
-
Foreign, Commonwealth and Development Office (FCDO), UK
Project number 300342‐104
-
United States Agency for International Development (USAID), USA
Development of this project was in part made possible with financial support from USAID administered by the World Health Organization Global TB Programme
Canadian Institutes of Health Research (CIHR) grant PJT‐156039: Evaluating diagnostic accuracy of tests and decision rules in the absence of a perfect reference test: application to extrapulmonary tuberculosis, Canada
Declarations of interest
We have no financial involvement with any organization or entity that has a financial interest in, or financial conflict with, the subject matter or materials discussed in the review apart from those disclosed.
MK received funding from USAID, administered by the World Health Organization Global Tuberculosis Programme, Switzerland.
IS received funding from USAID, administered by the World Health Organization Global Tuberculosis Programme, Switzerland.
ND's participation in this project was supported in part by the Canadian Institutes of Health Research.
MY's participation in this project was supported in part by the Canadian Institutes of Health Research.
KD has no interests to declare.
CMD worked for the Foundation for Innovative New Diagnostics (FIND) until April 2019 and has no other interests to declare.
SGS works for FIND. FIND is a not‐for‐profit foundation whose mission is to find diagnostic solutions to overcome diseases of poverty in low‐ and middle‐income countries. FIND works closely with the private and public sectors and receives funding from donors and some of its industry partners. FIND has an independent Scientific Advisory Committee and organizational firewalls that protect it against any undue influences in its work or in publication of its findings. More information on FIND’s policy and guidelines for working with private sector partners can be found at www.finddx.org/ops-gov/.
KRS received funding from USAID, administered by the World Health Organization Global Tuberculosis Programme, Switzerland, and Cochrane infectious Diseases Group, UK. She has received financial support from McGill University, Canada, and the World Health Organization (WHO) Global Tuberculosis Programme, Switzerland for the preparation of systematic reviews and educational materials, consultancy fees from Foundation for Innovative New Diagnostics (FIND), Switzerland (for the preparation of systematic reviews and GRADE tables), honoraria, and travel support to attend WHO guideline meetings.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ablanedo‐Terrazas 2014 {published data only}
- Ablanedo-Terrazas Y, Alvarado-de la Barrera C, Hernandez-Juan R, Ruiz-Cruz M, Reyes-Teran G. Xpert MTB/RIF for diagnosis of tuberculous cervical lymphadenitis in HIV-infected patients. Laryngoscope 2014;124(6):1382-5. [DOI] [PubMed] [Google Scholar]
Ajbani 2018 {published data only}
- Ajbani K, Kazi M, Naik S, Soman R, Shetty A, Rodrigues C. Utility of pyrosequencing for rapid detection of tubercular meningitis (TBM) and associated susceptibility directly from CSF specimens. Tuberculosis 2018;111:54-6. [DOI] [PubMed] [Google Scholar]
Al‐Ateah 2012 {published data only}
- Al-Ateah SM, Al-Dowaidi MM, El-Khizzi NA. Evaluation of direct detection of Mycobacterium tuberculosis complex in respiratory and non-respiratory clinical specimens using the Cepheid Gene Xpert(R) system. Saudi Medical Journal 2012;33(10):1100-5. [PubMed] [Google Scholar]
Antel 2020 {published data only}
- Antel K, Oosthuizen J, Malherbe F, Louw VJ, Nicol MP, Maartens G, et al. Diagnostic accuracy of the Xpert MTB/Rif Ultra for tuberculosis adenitis. BMC Infectious Diseases 2020;20(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azevedo 2018 {published data only}
- Azevedo RG, Dinallo FS, De Laurentis LS, Boulware DR, Vidal JE. Xpert MTB/RIF((R)) assay for the diagnosis of HIV-related tuberculous meningitis in Sao Paulo, Brazil. International Journal of Tuberculosis and Lung Disease 2018;22(6):706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahr 2015 {published data only}
- Bahr NC, Tugume L, Rajasingham R, Kiggundu R, Williams DA, Morawski B, et al. Improved diagnostic sensitivity for tuberculous meningitis with Xpert((R)) MTB/RIF of centrifuged CSF. International Journal of Tuberculosis and Lung Disease 2015;19(10):1209-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahr 2017 {published data only}
- Bahr NC, Nuwagira E, Evans EE, Cresswell FV, Bystrom PV, Byamukama A, et al. Diagnostic accuracy of Xpert MTB/Rif Ultra for TB meningitis in HIV-infected adults: a prospective cohort study. Lancet Infectious Diseases 2017;18(1):68-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bera 2015 {published data only}
- Bera C, Michael JS, Burad D, Shirly SB, Gibikote S, Ramakrishna B, et al. Tissue XpertTM MTB/Rif assay is of limited use in diagnosing peritoneal tuberculosis in patients with exudative ascites. Indian Journal of Gastroenterology 2015;34(5):395-8. [DOI] [PubMed] [Google Scholar]
Biadglegne 2014 {published data only}
- Biadglegne F, Mulu A, Rodloff A C, Sack U. Diagnostic performance of the Xpert MTB/RIF assay for tuberculous lymphadenitis on fine needle aspirates from Ethiopia. Tuberculosis (Edinburgh, Scotland) 2014;94(5):502-5. [DOI] [PubMed] [Google Scholar]
Blaich 2014 {published data only}
- Blaich A, Frei R. Performance of the Xpert MTB/RIF assay on nonrespiratory specimens and accuracy of this assay for detection of rifampin resistance in a low-prevalence setting. Journal of Clinical Microbiology 2014;52(2):706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Causse 2011 {published data only}
- Causse M, Ruiz P, Gutierrez-Aroca JB, Casal M. Comparison of two molecular methods for rapid diagnosis of extrapulmonary tuberculosis. Journal of Clinical Microbiology 2011;49(8):3065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Che 2017 {published data only}
- Che N, Yang X, Liu Z, Li K, Chen X. Rapid detection of cell-free Mycobacterium tuberculosis DNA in tuberculous pleural effusion. Journal of Clinical Microbiology 2017;55(5):1526-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2019 {published data only}
- Chen C, Yang CG, Gao X, Lu ZZ, Tang FX, Cheng J, et al. Community-based active case finding for tuberculosis in rural western China: a cross-sectional study. International Journal of Tuberculosis and Lung Disease 2017;21(11):1134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chin 2019 {published data only}
- Chin JH, Musubire AK, Morgan N, Pellinen J, Grossman S, Bhatt JM, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis in cerebrospinal fluid. Journal of Clinical Microbiology 2019;57(6):pii: e00249-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christopher 2013 {published data only}
- Christopher DJ, Schumacher SG, Michael JS, Luo R, Balamugesh T, Duraikannan P, et al. Performance of Xpert MTB/RIF on pleural tissue for the diagnosis of pleural tuberculosis. European Respiratory Journal 2013;42(5):1427-9. [DOI] [PubMed] [Google Scholar]
Cresswell 2018 {published data only}
- Cresswell FV, Bangdiwala AS, Bahr NC, Trautner E, Nuwagira E, Ellis J, et al. Can improved diagnostics reduce mortality from tuberculous meningitis? Findings from a 6.5-year cohort in Uganda. Wellcome Open Research 2018;3:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cresswell 2020 {published data only}
- Cresswell FV, Tugume L, Bahr NC, Kwizera R, Bangdiwala AS, Musubire AK, et al. Xpert MTB/RIF Ultra for the diagnosis of HIV-associated tuberculous meningitis: a prospective validation study. Lancet Infectious Disease 2020;20(3):308-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhasmana 2014 {published data only}
- Dhasmana DJ, Ross C, Bradley CJ, Connell DW, George PM, Singanayagam A, et al. Performance of Xpert MTB/RIF in the diagnosis of tuberculous mediastinal lymphadenopathy by endobronchial ultrasound. Annals of the American Thoracic Society 2014;11(3):392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhooria 2016 {published data only}
- Dhooria S, Gupta N, Bal A, Sehgal IS, Aggarwal A, Sethi S, et al. Role of Xpert MTB/RIF in differentiating tuberculosis from sarcoidosis in patients with mediastinal lymphadenopathy undergoing EBUS-TBNA: a study of 147 patients. Sarcoidosis Vasculitis and Diffuse Lung Disease 2016;33:258-266. [PubMed] [Google Scholar]
Donovan 2020 {published data only}
- Donovan J, Anh Thu DD, Phu NH, Dung VT, Quang TP, Nghia HDT, et al. Xpert MTB/RIF Ultra versus Xpert MTB/RIF for the diagnosis of tuberculous meningitis: a prospective, randomised, diagnostic accuracy study. Lancet Infectious Disease 2020;20:299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Du 2015 {published data only}
- Du J, Huang Z, Luo Q, Xiong G, Xu X, Li W, et al. Rapid diagnosis of pleural tuberculosis by Xpert MTB/RIF assay using pleural biopsy and pleural fluid specimens. Journal of Research In Medical Sciences 2015;20(1):26-31. [PMC free article] [PubMed] [Google Scholar]
El‐Din 2019 {published data only}
- El-Din MG, Sobh E, Adawy Z, Farghaly N. Diagnostic utility of gene X-pert in the diagnosis of tuberculous pleural effusion. Infectious Diseases 2019;51(3):227-9. [DOI] [PubMed] [Google Scholar]
Feasey 2013 {published data only}
- Feasey NA, Banada PP, Howson W, Sloan DJ, Mdolo A, Boehme C, et al. Evaluation of Xpert MTB/RIF for detection of tuberculosis from blood samples of HIV-infected adults confirms Mycobacterium tuberculosis bacteremia as an indicator of poor prognosis. Journal of Clinical Microbiology 2013;51(7):2311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedrich 2011 {published data only}
- Friedrich SO, Von Groote-Bidlingmaier F, Diacon AH. Xpert MTB/RIF assay for diagnosis of pleural tuberculosis. Journal of Clinical Microbiology 2011;49(12):4341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghariani 2015 {published data only}
- Ghariani A, Jaouadi T, Smaoui S, Mehiri E, Marouane C, Kammoun S, et al. Diagnosis of lymph node tuberculosis using the GeneXpert MTB/RIF in Tunisia. International Journal of Mycobacteriology 2015;4(4):270-5. [DOI] [PubMed] [Google Scholar]
Gu 2015 {published data only}
- Gu Y, Wang G, Dong W, Li Y, Ma Y, Shang Y, et al. Xpert MTB/RIF and GenoType MTBDRplus assays for the rapid diagnosis of bone and joint tuberculosis. International Journal of Infectious Diseases 2015;36:27-30. [DOI] [PubMed] [Google Scholar]
Hanif 2011 {published data only}
- Hanif SN, Eldeen HS, Ahmad S, Mokaddas E. GeneXpert(R) MTB/RIF for rapid detection of Mycobacterium tuberculosis in pulmonary and extra-pulmonary samples. International Journal of Tuberculosis and Lung Disease 2011;15(9):1274-5. [DOI] [PubMed] [Google Scholar]
Heemskerk 2018 {published data only}
- Heemskerk AD, Donovan J, Thu DD, Marais S, Chaidir L, Dung VT, et al. Improving the microbiological diagnosis of tuberculous meningitis: a prospective, international, multicentre comparison of conventional and modified Ziehl-Neelsen stain, GeneXpert, and culture of cerebrospinal fluid. Journal of Infection 2018;77(6):509-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hillemann 2011 {published data only}
- Hillemann D, Rusch-Gerdes S, Boehme C, Richter E. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. Journal of Clinical Microbiology 2011;49(4):1202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iram 2015 {published data only}
- Iram S, Zeenat A, Hussain S, Wasim Yusuf N, Aslam M. Rapid diagnosis of tuberculosis using Xpert MTB/RIF assay - report from a developing country. Pakistan Journal of Medical Sciences 2015;31(1):105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015a {published data only}
- Kim YW, Kwak N, Seong MW, Kim EC, Yoo CG, Kim YW, et al. Accuracy of the Xpert(R) MTB/RIF assay for the diagnosis of extra-pulmonary tuberculosis in South Korea. International Journal of Tuberculosis and Lung Disease 2015;19(1):81-6. [DOI] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li Y, Pang Y, Zhang T, Xian X, Wang X, Yang J, et al. Rapid diagnosis of extrapulmonary tuberculosis with Xpert Mycobacterium tuberculosis/rifampicin assay. Journal of Medical Microbiology 2017;66(7):910-4. [DOI] [PubMed] [Google Scholar]
Liang 2019 {published data only}
- Liang Q, Pang Y, Yang Y, Li H, Guo C, Yang X, et al. An improved algorithm for rapid diagnosis of pleural tuberculosis from pleural effusion by combined testing with GeneXpert MTB/RIF and an anti-LAM antibody-based assay. BMC Infectious Diseases 2019;19(1):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ligthelm 2011 {published data only}
- Ligthelm LJ, Nicol MP, Hoek KG, Jacobson R, Van Helden PD, Marais BJ, et al. Xpert MTB/RIF for rapid diagnosis of tuberculous lymphadenitis from fine-needle-aspiration biopsy specimens. Journal of Clinical Microbiology 2011;49(11):3967-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lusiba 2014 {published data only}
- Lusiba JK, Nakiyingi L, Kirenga BJ, Kiragga A, Lukande R, Nsereko M, et al. Evaluation of Cepheid's Xpert MTB/Rif test on pleural fluid in the diagnosis of pleural tuberculosis in a high prevalence HIV/TB setting. PLOS One 2014;9(7):e102702. [DOI: 10.1371/journal.pone.0102702] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malbruny 2011 {published data only}
- Malbruny B, Le Marrec G, Courageux K, Leclercq R, Cattoir V. Rapid and efficient detection of Mycobacterium tuberculosis in respiratory and non-respiratory samples. International Journal of Tuberculosis and Lung Disease 2011;15(4):553-5. [DOI] [PubMed] [Google Scholar]
Meldau 2014 {published data only}
- Meldau R, Peter J, Theron G, Calligaro G, Allwood B, Symons G, et al. Comparison of same day diagnostic tools including Gene Xpert and unstimulated IFN-gamma for the evaluation of pleural tuberculosis: a prospective cohort study. BMC Pulmonary Medicine 2014;14:58. [DOI: 10.1186/1471-2466-14-58] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meldau 2019 {published data only}
- Meldau R, Randall P, Pooran A, Limberis J, Makambwa E, Dhansay M, et al. Same day tools, including Xpert Ultra and unstimulated IFN-gamma, for the rapid diagnosis of pleural tuberculosis - a prospective observational study. Journal of Clinical Microbiology 2019;57(9):e00614-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Metcalf 2018 {published data only}
- Metcalf T, Soria J, Montano SM, Ticona E, Evans CA, Huaroto L, et al. Evaluation of the GeneXpert MTB/RIF in patients with presumptive tuberculous meningitis. PLOS One 2018;13(6):e0198695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nataraj 2016 {published data only}
- Nataraj G, Kanade S, Mehta P. Xpert((R)) MTB/RIF for improved case detection of extra-pulmonary TB in a tertiary care setting in urban India. International Journal of Tuberculosis and Lung Disease 2016;20(7):890-4. [DOI] [PubMed] [Google Scholar]
Nhu 2014 {published data only}
- Nhu NT, Heemskerk D, Thu do DA, Chau TT, Mai NT, Nghia HD, et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. Journal of Clinical Microbiology 2014;52(1):226-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozkutuk 2014 {published data only}
- Ozkutuk N, Surucuoglu S. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary tuberculosis in an intermediate-prevalence setting [Orta Prevalanslı Bölgede Akciğer ve Akciğer DışıTüberküloz Tanısında Xpert MTB/RIF Testinin Değerlendirilmesi]. Mikrobiyoloji Bulteni 2014;48(2):223-32. [DOI] [PubMed] [Google Scholar]
Pandie 2014 {published data only}
- Pandie S, Peter JG, Kerbelker ZS, Meldau R, Theron G, Govender U, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous pericarditis compared to adenosine deaminase and unstimulated interferon-gamma in a high burden setting: a prospective study. BMC Medicine 2014;12:101. [DOI: 10.1186/1741-7015-12-101] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2013 {published data only}
- Patel VB, Theron G, Lenders L, Matinyena B, Connolly C, Singh R, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLOS Medicine 2013;10(10):e1001536. [DOI: 10.1371/journal.pmed.1001536] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peñata 2016 {published data only}
- Peñata A, Salazar R, Castano T, Bustamante J, Ospina S. Molecular diagnosis of extrapulmonary tuberculosis and sensitivity to rifampicin with an automated real-time method [Diagnóstico molecular de tuberculosis extrapulmonary sensibilidad a rifampicina con un método automatizado en tiempo real]. Biomedica 2016;36(Suppl 1):78-89. [DOI] [PubMed] [Google Scholar]
Perez‐Risco 2018 {published data only}
- Perez-Risco D, Rodriguez-Temporal D, Valledor-Sanchez I, Alcaide F. Evaluation of the Xpert MTB/RIF Ultra assay for direct detection of Mycobacterium tuberculosis complex in smear-negative extrapulmonary samples. Journal of Clinical Microbiology 2018;56(9):pii: e00659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rakotoarivelo 2018 {published data only}
- Rakotoarivelo R, Ambrosioni J, Rasolofo V, Raberahona M, Rakotosamimanana N, Andrianasolo R, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of smear-negative pulmonary and extrapulmonary tuberculosis in Madagascar. International Journal of Infectious Diseases 2018;69:20-5. [DOI] [PubMed] [Google Scholar]
Rufai 2015 {published data only}
- Rufai SB, Singh A, Kumar P, Singh J, Singh S. Performance of Xpert MTB/RIF Assay in diagnosis of pleural tuberculosis by use of pleural fluid samples. Journal of Clinical Microbiology 2015;53(11):3636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rufai 2017a {published data only}
- Rufai SB, Singh S, Singh A, Kumar P, Singh J, Vishal A. Performance of Xpert MTB/RIF on ascitic fluid samples for detection of abdominal tuberculosis. Journal of Laboratory Physicians 2017;9(1):47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rufai 2017b {published data only}
- Rufai SB, Singh A, Singh J, Kumar P, Sankar MM, Singh S. Diagnostic usefulness of Xpert MTB/RIF assay for detection of tuberculous meningitis using cerebrospinal fluid. Journal of Infection 2017;75(2):125-31. [DOI] [PubMed] [Google Scholar]
Safianowska 2012 {published data only}
- Safianowska A, Walkiewicz R, Nejman-Gryz P, Grubek-Jaworska H. Two selected commercially based nucleic acid amplification tests for the diagnosis of tuberculosis. Pneumonologia Alergologia Polska 2012;80(1):6-12. [PubMed] [Google Scholar]
Sarfaraz 2018 {published data only}
- Sarfaraz S, Iftikhar S, Memon Y, Zahir N, Hereker FF, Salahuddin N. Histopathological and microbiological findings and diagnostic performance of GeneXpert in clinically suspected tuberculous lymphadenitis. International Journal of Infectious Diseases 2018;76:73-81. [DOI] [PubMed] [Google Scholar]
Scott 2014 {published data only}
- Scott LE, Beylis N, Nicol M, Nkuna G, Molapo S, Berrie L, et al. Diagnostic accuracy of Xpert MTB/RIF for extrapulmonary tuberculosis specimens: establishing a laboratory testing algorithm for South Africa. Journal of Clinical Microbiology 2014;52(6):1818-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2014 {published data only}
- Sharma SK, Kohli M, Chaubey J, Yadav RN, Sharma R, Singh BK, et al. Evaluation of Xpert MTB/RIF assay performance in diagnosing extrapulmonary tuberculosis among adults in a tertiary care centre in India. European Respiratory Journal 2014;44(4):1090-3. [DOI] [PubMed] [Google Scholar]
Sharma 2016 {published data only}
- Sharma JB, Kriplani A, Dharmendra S, Chaubey J, Kumar S, Sharma SK. Role of Gene Xpert in diagnosis of female genital tuberculosis: a preliminary report. European Journal of Obstetrics & Gynecology and Reproductive Biology 2016;207:237-8. [DOI] [PubMed] [Google Scholar]
Sharma 2018 {published data only}
- Sharma K, Sharma M, Chaudhary L, Modi M, Goyal M, Sharma N, et al. Comparative evaluation of Xpert MTB/RIF assay with multiplex polymerase chain reaction for the diagnosis of tuberculous meningitis. Tuberculosis (Edinburgh, Scotland) 2018 Dec;113:38-42. [DOI] [PubMed] [Google Scholar]
Siddiqi 2019 {published data only}
- Siddiqi OK, Birbeck GL, Ghebremichael M, Mubanga E, Love S, Buback C, et al. Prospective cohort study on performance of cerebrospinal fluid (CSF) Xpert MTB/RIF, CSF Lipoarabinomannan (LAM) Lateral Flow Assay (LFA), and urine LAM LFA for diagnosis of tuberculous meningitis in Zambia. Journal of Clinical Microbiology 2019;57(8):pii: e00652-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2019 {published data only}
- Sun Q, Wang S, Dong W, Jiang G, Huo F, Ma Y, et al. Diagnostic value of Xpert MTB/RIF Ultra for osteoarticular tuberculosis. Journal of Infection 2019 August;79(2):153-8. [DOI] [PubMed] [Google Scholar]
Suzana 2016 {published data only}
- Suzana S, Ninan MM, Gowri M, Venkatesh K, Rupali P, Michael JS. Xpert MTB/Rif for the diagnosis of extrapulmonary tuberculosis - an experience from a tertiary care centre in South India. Tropical Medicine & International Health 2016;21(3):385-92. [DOI] [PubMed] [Google Scholar]
Tadesse 2015 {published data only}
- Tadesse M, Abebe G, Abdissa K, Aragaw D, Abdella K, Bekele A, et al. GeneXpert MTB/RIF assay for the diagnosis of tuberculous lymphadenitis on concentrated fine needle aspirates in high tuberculosis burden settings. PLOS One 2015;10(9):e0137471. [DOI: 10.1371/journal.pone.0137471] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trajman 2014 {published data only}
- Trajman A, Da Silva Santos Kleiz de Oliveira EF, Bastos ML, Belo Neto E, Silva EM, Da Silva Lourenco MC, et al. Accuracy of polymerase chain reaction for the diagnosis of pleural tuberculosis. Respiratory Medicine 2014;108(6):918-23. [DOI] [PubMed] [Google Scholar]
Ullah 2017 {published data only}
- Ullah I, Javaid A, Masud H, Ali M, Basit A, Ahmad W, et al. Rapid detection of Mycobacterium tuberculosis and rifampicin resistance in extrapulmonary tuberculosis and sputum smear-negative pulmonary suspects using Xpert MTB/RIF. Journal of Medical Microbiology 2017;66(4):412-8. [DOI] [PubMed] [Google Scholar]
Vadwai 2011 {published data only}
- Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis? Journal of Clinical Microbiology 2011;49(7):2540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Rie 2013 {published data only}
- Van Rie A, Page-Shipp L, Mellet K, Scott L, Mkhwnazi M, Jong E, et al. Diagnostic accuracy and effectiveness of the Xpert MTB/RIF assay for the diagnosis of HIV-associated lymph node tuberculosis. European Journal of Clinical Microbiology & Infectious Diseases 2013;32(11):1409-15. [DOI] [PubMed] [Google Scholar]
Wang 2019 {published data only}
- Wang G, Wang S, Jiang G, Yang X, Huang M, Huo F, et al. Xpert MTB/RIF Ultra improved the diagnosis of paucibacillary tuberculosis: a prospective cohort study. Journal of Infection 2019;78(4):311-6. [DOI] [PubMed] [Google Scholar]
Wang 2020 {published data only}
- Wang G, Wang S, Yang X, Sun Q, Jiang G, Huang M, et al. Accuracy of Xpert MTB/RIF Ultra for the diagnosis of pleural TB in a multicenter cohort study. Chest 2020;157(2):268-75. [DOI] [PubMed] [Google Scholar]
Wu 2019 {published data only}
- Wu X, Tan G, Gao R, Yao L, Bi D, Guo Y, et al. Assessment of the Xpert MTB/RIF Ultra assay on rapid diagnosis of extrapulmonary tuberculosis. International Journal of Infectious Diseases 2019;81:91-6. [DOI] [PubMed] [Google Scholar]
Zeka 2011 {published data only}
- Zeka AN, Tasbakan S, Cavusoglu C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. Journal of Clinical Microbiology 2011;49(12):4138-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zmak 2013 {published data only}
- Zmak L, Jankovic M, Jankovic VK. Evaluation of Xpert MTB/RIF assay for rapid molecular diagnosis of tuberculosis in a two-year period in Croatia. International Journal of Mycobacteriology 2013;2(3):179-82. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abong 2019 {published data only}
- Abong J, Dalay V, Langley I, Tomeny E, Marcelo D, Mendoza V, et al. Use of GeneXpert and the role of an expert panel in improving clinical diagnosis of smear-negative tuberculosis cases. PLOS One 2019;14(12):e0227093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adejumo 2018 {published data only}
- Adejumo Olusola Adedeji, Olusola-Faleye Bolanle, Adepoju Victor, Bowale Abimbola, Adesola Sunday, Falana Ayodeji, et al. Prevalence of rifampicin resistant tuberculosis and associated factors among presumptive tuberculosis patients in a secondary referral hospital in Lagos Nigeria. African Health Sciences 2018;18(3):472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Afsar 2018 {published data only}
- Afsar I, Gunes M, Er H, Sener AG. Comparison of culture, microscopic smear and molecular methods in diagnosis of tuberculosis. Revista Espanola de Quimioterapia 2018;31(5):435-8. [PMC free article] [PubMed] [Google Scholar]
Ahmad 2018 {published data only}
- Ahmad R, Changeez M, Khan JS, Qureshi U, Tariq M, Malik S, et al. Diagnostic accuracy of peritoneal fluid GeneXpert in the diagnosis of intestinal tuberculosis, keeping histopathology as the gold standard. Cureus 2018;10(10):e3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akhter 2019 {published data only}
- Akhter N, Sumalani KK, Chawla D, Ahmed Rizvi N. Comparison between the diagnostic accuracy of Xpert MTB/Rif assay and culture for pleural tuberculosis using tissue biopsy. ERJ Open Research 2019;5(3):00065-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ali 2018 {published data only}
- Ali A, Munir MA, Sarwar MI, Khaliq S, Munir MK, Rehman S. Tuberculous pleural effusions: efficacy utilization and comparison of various diagnostic techniques. Pakistan Journal of Medical and Health Sciences 2018;12(4):1570-3. [Google Scholar]
Allahyartorkaman 2019 {published data only}
- Allahyartorkaman M, Mirsaeidi M, Hamzehloo G, Amini S, Zakiloo M, Nasiri MJ. Low diagnostic accuracy of Xpert MTB/RIF assay for extrapulmonary tuberculosis: a multicenter surveillance. Scientific Reports 2019;9(1):18515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvarez Uria 2012 {published data only}
- Alvarez-Uria G, Azcona JM, Midde M, Naik PK, Reddy S, Reddy R. Rapid diagnosis of pulmonary and extrapulmonary tuberculosis in HIV-infected patients. Comparison of LED fluorescent microscopy and the GeneXpert MTB/RIF assay in a district hospital in India. Tuberculosis Research and Treatment 2012;2012:932862. [DOI: 10.1155/2012/932862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrey 2015 {published data only}
- Andrey DO, Hinrikson H, Renzi G, Hibbs J, Adler D, Schrenzel J. Xpert((R)) MTB/RIF assay sensitivity with different methods of CSF processing for the diagnosis of TB meningitis. International Journal of Tuberculosis and Lung Disease 2015;19(12):1555-6. [DOI] [PubMed] [Google Scholar]
Armand 2011 {published data only}
- Armand S, Vanhuls P, Delcroix G, Courcol R, Lemaitre N. Comparison of the Xpert MTB/RIF test with an IS6110-TaqMan real-time PCR assay for direct detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. Journal of Clinical Microbiology 2011;49(5):1772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arockiaraj 2015 {published data only}
- Arockiaraj J, Amritanand R, Krishnan V, Sundararaj G, Michael J. GeneXpert polymerase chain reaction (PCR) Test: role in the diagnosis of tubercular spondylodiscitis. Spine Journal 2015;15(10 Suppl 1):200S-201S. [Google Scholar]
Arockiaraj 2017 {published data only}
- Arockiaraj J, Michael JS, Amritanand R, David KS, Krishnan V. The role of Xpert MTB/RIF assay in the diagnosis of tubercular spondylodiscitis. European Spine Journal 2017;26(12):3162-9. [DOI] [PubMed] [Google Scholar]
Arockiaraj 2019a {published data only}
- Arockiaraj J, Karthik R, Michael J S, Amritanand R, David KS, Krishnan V, et al. 'Need of the hour': early diagnosis and management of multidrug resistant tuberculosis of the spine: an analysis of 30 patients from a "high multidrug resistant tuberculosis burden" country. Asian Spine Journal 2019;13(2):265-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arockiaraj 2019b {published data only}
- Arockiaraj J, Robert M, Rose W, Amritanand R, David KS, Krishnan V. Early detection and analysis of children with multidrug-resistant tuberculosis of the spine. Asian Spine Journal 2019;13(1):77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atherton 2018 {published data only}
- Atherton RR, Cresswell FV, Ellis J, Skipper C, Tadeo KK, Mugumya G, et al. Detection of Mycobacterium tuberculosis in urine by Xpert MTB/RIF Ultra: a useful adjunctive diagnostic tool in HIV-associated tuberculosis. International Journal of Infectious Diseases 2018;75:92-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aydemir 2019 {published data only}
- Aydemir O, Karakece E, Koroglu M, Altindis M, Terzi HA. Comparison of the GeneXpert MTB/RIF test and conventional methods in the diagnosis of Mycobacterium tuberculosis. Clinical Laboratory 2019;65(1):1-6. [DOI] [PubMed] [Google Scholar]
Bablishvili 2015 {published data only}
- Bablishvili N, Tukvadze N, Avaliani Z, Blumberg HM, Kempker RR. A comparison of the Xpert MTB/RIF and GenoTypeMTBDRplus assays in Georgia. International Journal of Tuberculosis and Lung Disease 2015;19(6):676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahr 2018a {published data only}
- Bahr NC, Halupnick R, Linder G, Kiggundu R, Nabeta HW, Williams DA, et al. Delta-like 1 protein, vitamin D binding protein and fetuin for detection of Mycobacterium tuberculosis meningitis. Biomarkers in Medicine 2018;12(7):707-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahr 2018b {published data only}
- Bahr NC, Nuwagira E, Evans EE, Cresswell FV, Bystrom PV, Byamukama A, et al. Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. Lancet Infectious Diseases 2018;18(1):68-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahr 2019 {published data only}
- Bahr NC, Meintjes G, Boulware DR. Inadequate diagnostics: the case to move beyond the bacilli for detection of meningitis due to Mycobacterium tuberculosis. Journal of Medical Microbiology 2019;68(5):755-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baikunje 2019 {published data only}
- Baikunje N, Behera D, Rajwanshi A, Sharma M, Sharma A, Sharma K. Comparative evaluation of loop-mediated isothermal amplification (LAMP) assay, GeneXpert MTB/Rif and multiplex PCR for the diagnosis of tubercular lymphadenitis in HIV-infected patients of North India. Molecular and Cellular Probes 2019;48:101459. [DOI] [PubMed] [Google Scholar]
Bajrami 2016 {published data only}
- Bajrami R, Mulliqi G, Kurti A, Lila G, Raka L. Comparison of GeneXpert MTB/RIF and conventional methods for the diagnosis of tuberculosis in Kosovo. Journal of Infection in Developing Countries 2016;10(4):418-22. [DOI] [PubMed] [Google Scholar]
Balcha 2014 {published data only}
- Balcha TT, Sturegard E, Winqvist N, Skogmar S, Reepalu A, Jemal ZH, et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of Xpert MTB/RIF compared with smear microscopy and liquid culture. PLOS One 2014;9(1):e85478. [DOI: 10.1371/journal.pone.0085478] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bankar 2018 {published data only}
- Bankar S, Set R, Sharma D, Shah D, Shastri J. Diagnostic accuracy of Xpert MTB/RIF assay in extrapulmonary tuberculosis. Indian Journal of Medical Microbiology 2018;36(3):357-63. [DOI] [PubMed] [Google Scholar]
Bemba 2017 {published data only}
- Bemba EL, Moukassa D, Ouedraogo AR, Okombi FH, Bopaka RG, Koumeka PP, et al. Performance of GeneXpert MTB / RIF in the diagnosis of pleural tuberculosis in Brazzaville: a preliminary report [Performance du GeneXpert MTB/RIF dans le diagnostic de la tuberculose pleurale à Brazzaville: étude préliminaire]. Health Sciences and Diseases 2017;18(3):21-7. [Google Scholar]
Ben Saad 2018 {published data only}
- Ben Saad S, Kallel N, Gharsalli H, Kwas H, El Gharbi L, Ghedira H, et al. Cold abscess in the immunocompetent subject. Tunisie Medicale 2018;96(5):302-6. [PubMed] [Google Scholar]
Bhardwaj 2019 {published data only}
- Bhardwaj A, Khan S, Kumar A, George L, Mehta A, Radhakrishnan K. Assessing the utility of GeneXpert MTB/Rif assay in a tertiary care centre in Southern India with established microscopy and liquid culture facilities. Journal of the Association of Physicians of India 2019;67(8):31-4. [PubMed] [Google Scholar]
Bhatia 2016 {published data only}
- Bhatia R, Dayal R, Jindal S, Agarwal D, Goyal A. GeneXpert for diagnosis of tubercular meningitis. Indian Journal of Pediatrics 2016;83(11):1353-5. [DOI] [PubMed] [Google Scholar]
Bholla 2016 {published data only}
- Bholla M, Kapalata N, Masika E, Chande H, Jugheli L, Sasamalo M, et al. Evaluation of Xpert(R) MTB/RIF and Ustar EasyNAT TB IAD for diagnosis of tuberculous lymphadenitis of children in Tanzania: a prospective descriptive study. BMC Infectious Diseases 2016;16:246. [DOI: 10.1186/s12879-016-1578-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biadglegne 2013 {published data only}
- Biadglegne F, Tesfaye W, Sack U, Rodloff AC. Tuberculous lymphadenitis in Northern Ethiopia: in a public health and microbiological perspectives. PLOS One 2013;8(12):e81918. [DOI: 10.1371/journal.pone.0081918] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bilgin 2016 {published data only}
- Bilgin K, Yanik K, Karadag A, Odabasi H, Tas H, Gunaydin M. Comparison of a real-time polymerase chain reaction-based system and Erlich-Ziehl-Neelsen method with culture in the identification of Mycobacterium tuberculosis. Turkish Journal of Medical Sciences 2016;46(1):203-6. [DOI] [PubMed] [Google Scholar]
Borraz‐Noriega 2018 {published data only}
- Borraz-Noriega D, Robledo-Pascual JC, Torres-Perez JA, Flores-Barrientos OI. Utility of nucleic acids detection GeneXpert tuberculosis (MTB/RIF) in respiratory and non-respiratory hospital reference samples. Medicina Interna de Mexico 2018;34(3):381-7. [Google Scholar]
Boyles 2018 {published data only}
- Boyles T. Xpert Ultra's place in the diagnosis of tuberculous meningitis. Lancet Infectious Diseases 2018;18(3):248-9. [DOI] [PubMed] [Google Scholar]
Bunsow 2014 {published data only}
- Bunsow E, Ruiz-Serrano MJ, Lopez Roa P, Kestler M, Viedma DG, Bouza E. Evaluation of GeneXpert MTB/RIF for the detection of Mycobacterium tuberculosis and resistance to rifampin in clinical specimens. Journal of Infection 2014;68(4):338-43. [DOI] [PubMed] [Google Scholar]
Celik 2015 {published data only}
- Celik C, Gozel MG, Bakici MZ, Berk S, Ozsahin SL, Gulturk E. Applicability of Xpert MTB/RIF assay for routine diagnosis of tuberculosis: a four-year single-center experience. Turkish Journal of Medical Sciences 2015;45(6):1329-34. [PubMed] [Google Scholar]
Chaidir 2018 {published data only}
- Chaidir L, Annisa J, Dian S, Parwati I, Alisjahbana A, Purnama F, et al. Microbiological diagnosis of adult tuberculous meningitis in a ten-year cohort in Indonesia. Diagnostic Microbiology and Infectious Disease 2018;91(1):42-6. [DOI] [PubMed] [Google Scholar]
Chakraborty 2019 {published data only}
- Chakraborty A, Ramaswamy S, Shivananjiah A J, Puttaswamy RB, Chikkavenkatappa N. The role of genexpert in the diagnosis of tubercular pleural effusion in India. Advances in Respiratory Medicine 2019;87(5):276-80. [DOI] [PubMed] [Google Scholar]
Chen 2016 {published data only}
- Chen ZF, Lao HL, Li XH, Wang J, Chen Q, Wang ZX, et al. Experimental study of GeneXpert((R)) system in the diagnosis of extra-pulmonary tuberculosis. Chinese Journal of Tuberculosis and Respiratory Diseases 2016;39(7):529-33. [DOI] [PubMed] [Google Scholar]
Chhajed 2019 {published data only}
- Chhajed PN, Vaidya PJ, Mandovra NP, Chavhan VB, Lele TT, Nair R, et al. EBUS-TBNA in the rapid microbiological diagnosis of drug-resistant mediastinal tuberculous lymphadenopathy. ERJ Open Research 2019;5(4):00008-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christopher 2018 {published data only}
- Christopher DJ, Dinakaran S, Gupta R, James P, Isaac B, Thangakunam B. Thoracoscopic pleural biopsy improves yield of Xpert MTB/RIF for diagnosis of pleural tuberculosis. Respirology 2018;23(7):714-7. [DOI] [PubMed] [Google Scholar]
Coetzee 2014 {published data only}
- Coetzee L, Nicol MP, Jacobson R, Schubert PT, Van Helden PD, Warren RM, et al. Rapid diagnosis of pediatric mycobacterial lymphadenitis using fine needle aspiration biopsy. Pediatric Infectious Disease Journal 2014;33(9):893-6. [DOI] [PubMed] [Google Scholar]
Coleman 2015 {published data only}
- Coleman M, Finney LJ, Komrower D, Chitani A, Bates J, Chipungu GA, et al. Markers to differentiate between Kaposi's sarcoma and tuberculous pleural effusions in HIV-positive patients. International Journal of Tuberculosis and Lung Disease 2015;19(2):144-50. [DOI] [PubMed] [Google Scholar]
Creswell 2019 {published data only}
- Creswell J, Qin ZZ, Gurung R, Lamichhane B, Yadav DK, Prasai MK, et al. The performance and yield of tuberculosis testing algorithms using microscopy, chest x-ray, and Xpert MTB/RIF. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases 2019;14:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahale 2019 {published data only}
- Dahale AS, Puri AS, Kumar A, Dalal A, Agarwal A, Sachdeva S. Tissue Xpert (R) MTB/RIF assay in peritoneal tuberculosis: to be (done) or not to be (done). Cureus 2019;11(6):e5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2019 {published data only}
- Das A, Anupurba S, Mishra OP, Banerjee T, Tripathi R. Evaluation of Xpert MTB/RIF assay for diagnosis of tuberculosis in children. Journal of Tropical Pediatrics 2019;65(1):14-20. [DOI] [PubMed] [Google Scholar]
Deggim 2013 {published data only}
- Deggim V, Somoskovi A, Voit A, Bottger EC, Bloemberg GV. Integrating the Xpert MTB/RIF assay into a diagnostic workflow for rapid detection of Mycobacterium tuberculosis in a low-prevalence area. Journal of Clinical Microbiology 2013;51(7):2396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dharan 2016 {published data only}
- Dharan NJ, Blakemore R, Sloutsky A, Kaur D, Alexander RC, Ghajar M, et al. Performance of the G4 Xpert MTB/RIF assay for the detection of Mycobacterium tuberculosis and rifampin resistance: a retrospective case-control study of analytical and clinical samples from high- and low-tuberculosis prevalence settings. BMC Infectious Diseases 2016;16:764. [DOI: 10.1186/s12879-016-2039-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diallo 2016 {published data only}
- Diallo AB, Kollo AI, Camara M, Lo S, Ossoga GW, Mbow M, et al. Performance of GeneXpert MTB / RIF in the diagnosis of extrapulmonary tuberculosis in Dakar: 2010-2015 [Performance du GeneXpert MTB/RIF® dans le diagnostic de la tuberculose extra-pulmonaire à Dakar: 2010-2015]. Pan African Medical Journal 2016;25:129. [DOI: 10.11604/pamj.2016.25.129.10065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diop 2016 {published data only}
- Diop SA, Massaly A, Ka D, Manga NM, Fortes-Deguenonvo L, Ndour CT, et al. Use of GeneXpert test for the diagnosis of tuberculosis in the department of infectious diseases of FANN University Hospital [Utilisation du test GeneXpert pour le diagnostic de la tuberculose au service desmaladies infectieuses du CHNU de Fann]. Pan African Medical Journal 2016;23:244. [DOI: 10.11604/pamj.2016.23.244.7442] [DOI] [Google Scholar]
Edwards 2016 {published data only}
- Edwards S, Glynn P, David MD, Kamesh L. Diagnosing tuberculous peritonitis early in patients on peritoneal dialysis: use of Xpert MTB/RIF assay. Peritoneal Dialysis International 2016;36(4):461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ejeta 2018 {published data only}
- Ejeta E, Beyene G, Bonsa Z, Abebe G. Xpert MTB/RIF assay for the diagnosis of Mycobacterium tuberculosis and rifampicin resistance in high Human Immunodeficiency Virus setting in Gambella regional state, Southwest Ethiopia. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases 2018;12:14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erdem 2014 {published data only}
- Erdem H, Ozturk-Engin D, Elaldi N, Gulsun S, Sengoz G, Crisan A, et al. The microbiological diagnosis of tuberculous meningitis: results of Haydarpasa-1 study. Clinical Microbiology and Infection 2014;20(10):O600-8. [DOI: 10.1111/1469-0691.12478] [DOI] [PubMed] [Google Scholar]
Fanosie 2016 {published data only}
- Fanosie A, Gelaw B, Tessema B, Tesfay W, Admasu A, Yitayew G. Mycobacterium tuberculosis complex and HIV co-infection among extrapulmonary tuberculosis suspected cases at the University of Gondar Hospital, Northwestern Ethiopia. PLOS One 2016;11(3):e0150646. [DOI: 10.1371/journal.pone.0150646] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fantahun 2019 {published data only}
- Fantahun M, Kebede A, Yenew B, Gemechu T, Mamuye Y, Tadesse M, et al. Diagnostic accuracy of Xpert MTB/RIF assay and non-molecular methods for the diagnosis of tuberculosis lymphadenitis. PLOS One 2019;14(9):e0222402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Floridia 2017 {published data only}
- Floridia M, Ciccacci F, Andreotti M, Hassane A, Sidumo Z, Magid NA, et al. Tuberculosis case finding with combined rapid point-of-care assays (Xpert MTB/RIF and Determine TB LAM) in HIV-positive individuals starting antiretroviral therapy in Mozambique. Clinical Infectious Diseases 2017;65(11):1878-83. [DOI] [PubMed] [Google Scholar]
García 2017 {published data only}
- Garcia P, Balcells ME, Castillo C, Miranda C, Geoffroy E, Román JC, et al. Evaluation of Xpert® MTB/RIF technique for Mycobacterium tuberculosis complex detection in extra-respiratory specimens [Evaluation of Xpert(R) MTB/RIF technique for Mycobacterium tuberculosis complex detection in extra-respiratory specimens]. Revista Chilena de Infectologia 2017;34(4):333-9. [DOI] [PubMed] [Google Scholar]
García Cañete 2017 {published data only}
- García Cañete P, Balcells ME, Castillo C, Miranda C, Geoffroy E, Román JC, et al. Evaluation of Xpert® MTB/RIF technique for Mycobacterium tuberculosis complex detection in extra-respiratory specimens. Revista Chilena de Infectologia 2017;34(4):333-9. [DOI] [PubMed] [Google Scholar]
Gascoyne‐Binzi 2012 {published data only}
- Gascoyne-Binzi DM, Robinson GA, English A, Collyns TA. Comparison of on-demand PCR testing for Mycobacterium tuberculosis with culture and batched PCR. Clinical Microbiology and Infection 2012;18:543. [DOI: ] [Google Scholar]
Gati 2018 {published data only}
- Gati S, Chetty R, Wilson D, Achkar J M. Utilization and clinical value of diagnostic modalities for tuberculosis in a high HIV prevalence setting. American Journal of Tropical Medicine and Hygiene 2018;99(2):317-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gautam 2018 {published data only}
- Gautam H, Agrawal SK, Verma SK, Singh UB. Cervical tuberculous lymphadenitis: clinical profile and diagnostic modalities. International Journal of Mycobacteriology 2018;7(3):212-6. [DOI] [PubMed] [Google Scholar]
Gautam 2019 {published data only}
- Gautam H, Singla M, Jain R, Lodha R, Kabra SK, Singh UB. Point-of-care urine lipoarabinomannan antigen detection for diagnosis of tuberculosis in children. International Journal of Tuberculosis and Lung Disease 2019;23(6):714-9. [DOI] [PubMed] [Google Scholar]
Gounden 2018 {published data only}
- Gounden S, Perumal R, Magula NP. Extrapulmonary tuberculosis in the setting of HIV hyperendemicity at a tertiary hospital in Durban, South Africa. Southern African Journal of Infectious Diseases 2018;33(3):57-64. [Google Scholar]
Gulla 2019 {published data only}
- Gulla KM, Gunathilaka G, Jat KR, Sankar J, Karan M, Lodha R, et al. Utility and safety of endobronchial ultrasound-guided transbronchial needle aspiration and endoscopic ultrasound with an echobronchoscope-guided fine needle aspiration in children with mediastinal pathology. Pediatric Pulmonology 2019;54(6):881-5. [DOI] [PubMed] [Google Scholar]
Gursoy 2016 {published data only}
- Gursoy NC, Yakupogullari Y, Tekerekoglu MS, Otlu B. Evaluation of the diagnostic performance of Xpert MTB/RIF test for the detection of Mycobacterium tuberculosis and rifampin resistance in clinical samples [Klinik örneklerden Mycobacterium tuberculosis saptanması ve rifampin direnci tespitinde Xpert MTB/RIF testinin tanısal performansının değerlendirilmesi]. Mikrobiyoloji Bulteni 2016;50(2):196-204. [DOI] [PubMed] [Google Scholar]
Habeenzu 2017 {published data only}
- Habeenzu C, Nakajima C, Solo E, Bwalya P, Kajino K, Miller M, et al. Evaluation of in-house loop-mediated isothermal amplification for tuberculosis diagnosis compared with Xpert MTB/RIF. Journal of Infection in Developing Countries 2017;11(6):440-4. [DOI] [PubMed] [Google Scholar]
Habous 2019 {published data only}
- Habous M, Elimam MA, Kumar R, Deesi ZA. Evaluation of GeneXpert Mycobacterium tuberculosis/rifampin for the detection of Mycobacterium tuberculosis complex and rifampicin resistance in nonrespiratory clinical specimens. International Journal of Mycobacteriology 2019;8(2):132-7. [DOI] [PubMed] [Google Scholar]
Hanifa 2017 {published data only}
- Hanifa Y, Fielding KL, Chihota VN, Adonis L, Charalambous S, Foster N, et al. A clinical scoring system to prioritise investigation for tuberculosis among adults attending HIV clinics in South Africa. PLOS One 2017;12(8):e0181519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Held 2014 {published data only}
- Held M, Laubscher M, Zar HJ, Dunn RN. GeneXpert polymerase chain reaction for spinal tuberculosis: an accurate and rapid diagnostic test. Bone & Joint Journal 2014;96-b(10):1366-9. [DOI] [PubMed] [Google Scholar]
Held 2016 {published data only}
- Held M, Laubscher M, Mears S, Dix-Peek S, Workman L, Zar H, et al. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary tuberculosis in children with musculoskeletal infections. Pediatric Infectious Disease Journal 2016;35(11):1165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Held 2017 {published data only}
- Held M, Laubscher M, Workman L, Zar HJ, Dunn R. Diagnostic accuracy of GeneXpert MTB/RIF in musculoskeletal tuberculosis: high sensitivity in tissue samples of HIV-infected and HIV-uninfected patients. South African Medical Journal 2017;107(10):854-8. [DOI] [PubMed] [Google Scholar]
Ioannidis 2010 {published data only}
- Ioannidis P, Papaventsis D, Nikolaou S, Karabela S, Konstantinidou E, Marinou I, et al. Tuberculosis resistance detection rate to the two main anti-TB drugs, isoniazid and rifampicin, using molecular techniques: experience of the Hellenic National Reference Center for Mycobacteria. Acta Microbiologica Hellenica 2010;55(2):175-82. [Google Scholar]
Ioannidis 2011 {published data only}
- Ioannidis P, Papaventsis D, Karabela S, Nikolaou S, Panagi M, Raftopoulou E, et al. Cepheid GeneXpert MTB/RIF assay for Mycobacterium tuberculosis detection and rifampin resistance identification in patients with substantial clinical indications of tuberculosis and smear-negative microscopy results. Journal of Clinical Microbiology 2011;49(8):3068-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2017 {published data only}
- Jain A, Singh PK, Singh U, Kumar V. Initial screening of extra-pulmonary tuberculosis using the Xpert MTB/RIF assay improves case detection rates. International Journal of Tuberculosis and Lung Disease 2017;21(4):478-80. [DOI] [PubMed] [Google Scholar]
Jing 2017 {published data only}
- Jing H, Lu ZM, Deng YF, Gao DC, Li L, Graviss EA, et al. Evaluation of Xpert MTB/RIF in detection of pulmonary and extrapulmonary tuberculosis cases in China. International Journal of Clinical and Experimental Pathology 2017;10(4):4847-51. [Google Scholar]
Jipa 2017 {published data only}
- Jipa R, Olaru I, Manea E, Merisor S, Hristea A. Rapid clinical score for the diagnosis of tuberculous meningitis: a retrospective cohort study. Annals of Indian Academy of Neurology 2017;20(4):363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jorstad 2018 {published data only}
- Jorstad MD, Marijani M, Dyrhol-Riise AM, Sviland L, Mustafa T. MPT64 antigen detection test improves routine diagnosis of extrapulmonary tuberculosis in a low-resource setting: a study from the tertiary care hospital in Zanzibar. PLOS One 2018;13(5):e0196723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joythi 2019 {published data only}
- Joythi A, Ratageri V, Illalu S, Fattepur SR, Wari PK. The utility of CSF Xpert MTB/RIF in diagnosis of tubercular meningitis in children. Indian Journal of Pediatrics 2019;86(12):1089-93. [DOI] [PubMed] [Google Scholar]
Kanade 2018 {published data only}
- Kanade S, Nataraj G, Mehta P, Shah D. Pattern of missing probes in rifampicin resistant TB by Xpert MTB/RIF assay at a tertiary care centre in Mumbai. Indian Journal of Tuberculosis 2019;66(1):139-43. [DOI] [PubMed] [Google Scholar]
Kashyap 2019 {published data only}
- Kashyap B, Goyal N, Hyanki P, Singh NP, Khanna A. Cartridge-based nucleic acid amplification test: a novel rapid diagnostic tool to study the burden of tuberculosis from a tertiary care hospital. Tropical Doctor 2019;49(4):274-81. [DOI] [PubMed] [Google Scholar]
Kendall 2019 {published data only}
- Kendall EA, Kamoga C, Kitonsa PJ, Nalutaaya A, Salvatore PP, Robsky K, et al. Empiric treatment of pulmonary TB in the Xpert era: correspondence of sputum culture, Xpert MTB/RIF, and clinical diagnoses. PLOS One 2019;14(7):e0220251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerkhoff 2017 {published data only}
- Kerkhoff AD, Barr DA, Schutz C, Burton R, Nicol MP, Lawn SD, et al. Disseminated tuberculosis among hospitalised HIV patients in South Africa: a common condition that can be rapidly diagnosed using urine-based assays. Scientific Reports 2017;7(1):10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khadka 2019 {published data only}
- Khadka P, Thapaliya J, Basnet RB, Ghimire GR, Amatya J, Rijal BP. Diagnosis of tuberculosis from smear-negative presumptive TB cases using Xpert MTB/Rif assay: a cross-sectional study from Nepal. BMC Infectious Diseases 2019;19(1):1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2018 {published data only}
- Khan AS, Ali S, Khan MT, Ahmed S, Khattak Y, Abduljabbar, et al. Comparison of GeneXpert MTB/RIF assay and LED-FM microscopy for the diagnosis of extrapulmonary tuberculosis in Khyber Pakhtunkhwa, Pakistan. Brazilian Journal of Microbiology 2018;49(4):909-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilfoil 2015 {published data only}
- Kilfoil KM, Mayne E, Scott L, Stevens W. A high burden human immunodeficiency virus and tuberculosis resource limited setting, gains from Including Xpert MTB/RIF in the diagnostic algorithm of fluid specimens submitted for exclusion of lymphoma by immunophenotypic analysis. PLOS One 2015;10(8):e0134404. [DOI: 10.1371/journal.pone.0134404] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim CH, Woo H, Hyun IG, Kim C, Choi JH, Jang SH, et al. A comparison between the efficiency of the Xpert MTB/RIF assay and nested PCR in identifying Mycobacterium tuberculosis during routine clinical practice. Journal of Thoracic Disease 2014;6(6):625-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015b {published data only}
- Kim MJ, Nam YS, Cho SY, Park TS, Lee HJ. Comparison of the Xpert MTB/RIF Assay and real-time PCR for the detection of Mycobacterium tuberculosis. Annals of Clinical and Laboratory Science 2015;45(3):327-32. [PubMed] [Google Scholar]
Kim 2015c {published data only}
- Kim CH, Hyun IG, Hwang YI, Kim DG, Lee CY, Lee MG, et al. Identification of Mycobacterium tuberculosis and rifampin resistance in clinical specimens using the Xpert MTB/RIF assay. Annals of Clinical and Laboratory Science 2015;45(1):32-8. [PubMed] [Google Scholar]
Kotovich 2018 {published data only}
- Kotovich DS, Skryagina EM, Gurevich GL, Dyusmikeeva MI, Gorenok DI, Golaydo MM. New diagnostic opportunities when testing biological materials from those with pleural effusion caused by tuberculosis. Tuberculosis and Lung Diseases 2018;96(9):5-10. [Google Scholar]
Koul 2018 {published data only}
- Koul AN, Kassana BA, Rather AR. Utility of GeneXpert in the diagnosis, reliance on urine microscopy and clinical characteristics of genitourinary tuberculosis at a tertiary care hospital. Indian Journal of Medical Microbiology 2018;36(1):93-6. [DOI] [PubMed] [Google Scholar]
Kumar 2017 {published data only}
- Kumar S, Bopanna S, Kedia S, Mouli P, Dhingra R, Padhan R, et al. Evaluation of Xpert MTB/RIF assay performance in the diagnosis of abdominal tuberculosis. Intestinal Research 2017;15(2):187-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumari 2019 {published data only}
- Kumari P, Lavania S, Tyagi S, Dhiman A, Rath D, Anthwal D, et al. A novel aptamer-based test for the rapid and accurate diagnosis of pleural tuberculosis. Analytical Biochemistry 2019;564-565:80-7. [DOI] [PubMed] [Google Scholar]
Kurbaniyazova 2017 {published data only}
- Kurbaniyazova G, Joncevska M, Kalon S, Kalmambetova G, Mohr T, Toktogonova A, et al. Results of Xpert MTB/RIF implementation in Kyrgyzstan. International Journal of Tuberculosis and Lung Disease 2017;21(3):333-7. [DOI] [PubMed] [Google Scholar]
Kwak 2015 {published data only}
- Kwak N, Seong MW, Kim EC, Yoo CG, Kim YW, Han SK, et al. Accuracy of the Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis in South Korea. International Journal of Tuberculosis and Lung Disease 2015;19(1):81-6. [DOI] [PubMed] [Google Scholar]
Lawn 2012 {published data only}
- Lawn SD, Kerkhoff AD, Vogt M, Wood R. High diagnostic yield of tuberculosis from screening urine samples from HIV-infected patients with advanced immunodeficiency using the Xpert MTB/RIF assay. Journal of Acquired Immune Deficiency Syndromes 2012;60(3):289-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawn 2013 {published data only}
- Lawn SD, Kerkhoff AD, Vogt M, Wood R. HIV-associated tuberculosis: relationship between disease severity and the sensitivity of new sputum-based and urine-based diagnostic assays. BMC Medicine 2013;11:231. [DOI: 10.1186/1741-7015-11-231] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawn 2015 {published data only}
- Lawn SD, Kerkhoff AD, Burton R, Schutz C, Van Wyk G, Vogt M, et al. Rapid microbiological screening for tuberculosis in HIV-positive patients on the first day of acute hospital admission by systematic testing of urine samples using Xpert MTB/RIF: a prospective cohort in South Africa. BMC Medicine 2015;13:192. [DOI: 10.1186/s12916-015-0432-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawn 2017 {published data only}
- Lawn SD, Kerkhoff AD, Burton R, Schutz C, Boulle A, Vogt M, et al. Diagnostic accuracy, incremental yield and prognostic value of Determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Medicine 2017;15(1):67. [DOI: 10.1186/s12916-017-0822-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee J, Choi SM, Lee CH, Lee SM, Yim JJ, Yoo CG, et al. The additional role of Xpert MTB/RIF in the diagnosis of intrathoracic tuberculous lymphadenitis. Journal of Infection and Chemotherapy 2017;23(6):381-4. [DOI] [PubMed] [Google Scholar]
Lemus‐Minor 2018 {published data only}
- Lemus-Minor CG, Ovalle-Marroqui DF, Vazquez-Jimenez JG, Reales-Agüero DL, Sepulveda-Alcantara PM, Rodriguez-Sánchez JR, et al. Comparison of the Purelyse - IS6110 nested PCR with the Xpert MTB/RIF test in clinical samples with suspected tuberculosis. Journal of Microbiological Methods 2018;152:48-51. [DOI] [PubMed] [Google Scholar]
Li 2018 {published data only}
- Li Y, Jia W, Lei G, Zhao D, Wang G, Qin S. Diagnostic efficiency of Xpert MTB/RIF assay for osteoarticular tuberculosis in patients with inflammatory arthritis in China. PLOS One 2018;13(6):e0198600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2020 {published data only}
- Li X, Du W, Wang Y, Liu Z, Li K, Chen H, et al. Rapid diagnosis of tuberculosis meningitis by detecting mycobacterium tuberculosis cell-free DNA in cerebrospinal fluid. American Journal of Clinical Pathology 2020;153(1):126-30. [DOI] [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu X, Huang Z, Du J. Rapid diagnosis of pleural tuberculosis by Xpert MTB/RIF assay. Zhonghua Jie He He Hu Xi Za Zhi [Chinese Journal of Tuberculosis and Respiratory Diseases] 2015;38(10):741-5. [PubMed] [Google Scholar]
Liu 2019 {published data only}
- Liu XH, Xia L, Zhang AM, Zhang Y, Liu YH, Lu SH, et al. Increased diagnostic yield of tuberculous serositis by using serous fluid drainage flocky precipitate (SFDFP) as a testing sample. Scientific Reports 2019;9(1):1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lombardi 2017 {published data only}
- Lombardi G, Di Gregori V, Girometti N, Tadolini M, Bisognin F, Dal Monte P. Diagnosis of smear-negative tuberculosis is greatly improved by Xpert MTB/RIF. PLOS One 2017;12(4):e0176186. [DOI: 10.1371/journal.pone.0176186] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marouane 2014 {published data only}
- Marouane C, Smaoui S, Kammoun S, Slim L, Messadi-Akrout F. Evaluation of GeneXpert MTB/RIF for the detection of Mycobacterium tuberculosis and resistance to rifampin in extra-pulmonary specimens. International Journal of Mycobacteriology 2014;4 Suppl 1:101. [Google Scholar]
Marouane 2016 {published data only}
- Marouane C, Smaoui S, Kammoun S, Slim L, Messadi-Akrout F. Evaluation of molecular detection of extrapulmonary tuberculosis and resistance to rifampicin with GeneXpert(R) MTB/RIF. Medecine et Maladies Infectieuses 2016;46(1):20-4. [DOI] [PubMed] [Google Scholar]
Massi 2017 {published data only}
- Massi MN, Biatko KT, Handayani I, Pratama MY, Septriani S, Nurdin GM, et al. Evaluation of rapid GeneXpert MTB/RIF method using DNA tissue specimens of vertebral bones in patients with suspected spondylitis TB. Journal of Orthopaedics 2017;14(1):189-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathew 2018 {published data only}
- Mathew S, Pradhani MM, Philomina B. Role of molecular diagnostic test in diagnosing pulmonary and extrapulmonary tuberculosis. Journal of Evolution of Medical and Dental Sciences 2018;7(23):2777-81. [Google Scholar]
Mazzola 2016 {published data only}
- Mazzola E, Arosio M, Nava A, Fanti D, Gesu G, Farina C. Performance of real-time PCR Xpert (R)MTB/RIF in diagnosing extrapulmonary tuberculosis. Infezioni in Medicina 2016;24(4):304-9. [PubMed] [Google Scholar]
McMillen 2018 {published data only}
- McMillen T, Usiak SC, Chen LH, Gomez L, Ntiamoah P, Hameed MR, et al. Evaluation of the Xpert MTB/RIF performance on tissues: potential impact on airborne infection isolation at a tertiary cancer care center. Infection Control and Hospital Epidemiology 2018;39(4):462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mechal 2019 {published data only}
- Mechal Y, Benaissa E, El Mrimar N, Benlahlou Y, Bssaibis F, Zegmout A, et al. Evaluation of GeneXpert MTB/RIF system performances in the diagnosis of extrapulmonary tuberculosis. BMC infectious Diseases 2019;19(1):1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Metaferia 2018 {published data only}
- Metaferia Y, Seid A, Fenta GM, Gebretsadik D. Assessment of extrapulmonary tuberculosis using Gene Xpert MTB/RIF assay and fluorescent microscopy and its risk factors at Dessie Referral Hospital, Northeast Ethiopia. BioMed Research International 2018;2018:8207098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2011 {published data only}
- Miller MB, Popowitch EB, Backlund MG, Ager EP. Performance of Xpert MTB/RIF RUO assay and IS6110 real-time PCR for Mycobacterium tuberculosis detection in clinical samples. Journal of Clinical Microbiology 2011;49(10):3458-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2017 {published data only}
- Mishra DR, Bhatta N, Lamsal M, Bhattarai N, Maskey R. Study of diagnostic utility of Xpert MTB/RIF test on pleural fluid in the evaluation of patients presenting with pleural tuberculosis in high prevalence setting. American Journal of Respiratory and Critical Care Medicine 2017;195:A2088. [www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A2088] [Google Scholar]
Moure 2011 {published data only}
- Moure R, Muñoz L, Torres M, Santin M, Martín R, Alcaide F. Rapid detection of Mycobacterium tuberculosis complex and rifampin resistance in smear-negative clinical samples by use of an integrated real-time PCR method. Journal of Clinical Microbiology 2011;49(3):1137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moure 2012 {published data only}
- Moure R, Martín R, Alcaide F. Effectiveness of an integrated real-time PCR method for detection of the Mycobacterium tuberculosis complex in smear-negative extrapulmonary samples in an area of low tuberculosis prevalence. Journal of Clinical Microbiology 2012;50(2):513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Negi 2019 {published data only}
- Negi SS, Singh P, Chandrakar S, Gaikwad U, Das P, Bhargava A, et al. Diagnostic evaluation of multiplex real time PCR, genexpert MTB/RIF assay and conventional methods in extrapulmonary tuberculosis. Journal of Clinical and Diagnostic Research 2019;13(1):DC12-6. [Google Scholar]
Nhu 2013 {published data only}
- Nhu NT, Ha DT, Anh ND, Thu DD, Duong TN, Quang ND, et al. Evaluation of Xpert MTB/RIF and MODS assay for the diagnosis of pediatric tuberculosis. BMC Infectious Diseases 2013;13:31. [DOI: 10.1186/1471-2334-13-31] [DOI] [PMC free article] [PubMed] [Google Scholar]
Omar 2019 {published data only}
- Omar A, Elfadl A E, Ahmed Y, Hosny M. Genexpert test and tuberculous pleural effusion: a new diagnostic method for an old medical problem. Egyptian Journal of Chest Diseases and Tuberculosis 2019;68(4):493-7. [Google Scholar]
Pandey 2017 {published data only}
- Pandey S, Congdon J, McInnes B, Pop A, Coulter C. Evaluation of the GeneXpert MTB/RIF assay on extrapulmonary and respiratory samples other than sputum: a low burden country experience. Pathology 2017;49(1):70-4. [DOI] [PubMed] [Google Scholar]
Paramitha 2018 {published data only}
- Paramitha NA, Sribudiani Y, Rizal A. Comparison of positivity rate of MODS, Ziehl-Neelsen Staining, and GeneXpert Methods in M. tuberculosis detection among tuberculous meningitis patients. Majalah Kedokteran Bandung-Mkb-Bandung Medical Journal 2018;50(4):240-6. [Google Scholar]
Park 2019 {published data only}
- Park JH, Jin CE, Koo B, Kwon JS, Cha HH, Kim JY, et al. A simple microfluidic assay for diagnosing tuberculous meningitis in HIV-uninfected patients. Journal of Clinical Microbiology 2019;57(5):pii: e01975-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2014 {published data only}
- Patel VB, Connolly C, Singh R, Lenders L, Matinyenya B, Theron G, et al. Comparison of amplicor and GeneXpert MTB/RIF tests for diagnosis of tuberculous meningitis. Journal of Clinical Microbiology 2014;52(10):3777-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2020 {published data only}
- Patel J, Upadhyay M, Kundnani V, Merchant Z, Jain S, Kire N, et al. Diagnostic efficacy, sensitivity, and specificity of Xpert MTB/RIF assay for spinal tuberculosis and rifampicin resistance. Spine (Phila Pa 1976) 2020;45(3):163-9. [DOI] [PubMed] [Google Scholar]
Peter 2012 {published data only}
- Peter JG, Theron G, Muchinga TE, Govender U, Dheda K. The diagnostic accuracy of urine-based Xpert MTB/RIF in HIV-infected hospitalized patients who are smear-negative or sputum scarce. PLOS One 2012;7(7):e39966. [DOI: 10.1371/journal.pone.0039966] [DOI] [PMC free article] [PubMed] [Google Scholar]
Philip 2017 {published data only}
- Philip N, Wilkes C, Yeo TW, Rajahram GS, William T, John DV. Potential use of multiplex PCR in diagnosis of tuberculous meningitis. Tropical Biomedicine 2017;34(4):870-6. [PubMed] [Google Scholar]
Piersimoni 2019 {published data only}
- Piersimoni C, Gherardi G, Gracciotti N, Pocognoli A. Comparative evaluation of Xpert MTB/RIF and the new Xpert MTB/RIF ultra with respiratory and extra-pulmonary specimens for tuberculosis case detection in a low incidence setting. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases 2019;15:100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pink 2016 {published data only}
- Pink F, Brown TJ, Kranzer K, Drobniewski F. Evaluation of Xpert MTB/RIF for detection of Mycobacterium tuberculosis in cerebrospinal fluid. Journal of Clinical Microbiology 2016;54(3):809-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pohl 2016 {published data only}
- Pohl C, Rutaihwa LK, Haraka F, Nsubuga M, Aloi F, Ntinginya NE, et al. Limited value of whole blood Xpert (R) MTB/RIF for diagnosing tuberculosis in children. Journal of Infection 2016;73(4):326-35. [DOI] [PubMed] [Google Scholar]
Porcel 2013 {published data only}
- Porcel JM, Palma R, Valdes L, Bielsa S, San-Jose E, Esquerda A. Xpert(R) MTB/RIF in pleural fluid for the diagnosis of tuberculosis. International Journal of Tuberculosis and Lung Disease 2013;17(9):1217-9. [DOI] [PubMed] [Google Scholar]
Rachow 2012 {published data only}
- Rachow A, Clowes P, Saathoff E, Mtafya B, Michael E, Ntinginya EN, et al. Increased and expedited case detection by Xpert MTB/RIF assay in childhood tuberculosis: a prospective cohort study. Clinical Infectious Diseases 2012;54(10):1388-96. [DOI] [PubMed] [Google Scholar]
Raizada 2015 {published data only}
- Raizada N, Sachdeva KS, Swaminathan S, Kulsange S, Khaparde SD, Nair SA, et al. Piloting upfront Xpert MTB/RIF testing on various specimens under programmatic conditions for diagnosis of TB & DR-TB in paediatric population. PLOS One 2015;10(10):e0140375. [DOI: 10.1371/journal.pone.0140375] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raizada 2018 {published data only}
- Raizada N, Khaparde SD, Rao R, Kalra A, Sarin S, Salhotra VS, et al. Upfront Xpert MTB/RIF testing on various specimen types for presumptive infant TB cases for early and appropriate treatment initiation. PLOS One 2018;13(8):e0202085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramamurthy 2016 {published data only}
- Ramamurthy K, Bhat S, Shenoy S, Rangnekar A. Xpert Mycobacterium tuberculosis/rifampicin assay: a boon in tuberculosis diagnostics. Asian Journal of Pharmaceutical and Clinical Research 2016;9(5):225-7. [Google Scholar]
Rathour 2019 {published data only}
- Rathour JS, Mantan M, Khanna A, Hanif M. Evaluation of Gene Xpert assay in extrapulmonary tuberculosis in children. Journal of Evolution of Medical and Dental Sciences 2019;8(1):76-80. [Google Scholar]
Razack 2014 {published data only}
- Razack R, Louw M, Wright CA. Diagnostic yield of fine needle aspiration biopsy in HIV-infected adults with suspected mycobacterial lymphadenitis. South African Medical Journal 2014;104(1):27-8. [DOI] [PubMed] [Google Scholar]
Rebecca 2018 {published data only}
- Rebecca B, Chacko A, Verghese V, Rose W. Spectrum of pediatric tuberculosis in a tertiary care setting in South India. Journal of Tropical Pediatrics 2018;64(6):544-7. [DOI] [PubMed] [Google Scholar]
Reddy 2017 {published data only}
- Reddy R, Alvarez-Uria G. Molecular epidemiology of rifampicin resistance in mycobacterium tuberculosis using the GeneXpert MTB/RIF assay from a rural setting in India. Journal of Pathogens 2017;2017:6738095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rindi 2017 {published data only}
- Rindi L, Ali G, Fabiani B, Fontanini G, Garzelli C. Detection of Mycobacterium tuberculosis from paraffin-embedded tissues by GeneXpert MTB/RIF. Tuberculosis (Edinb) 2017;106:53-5. [DOI] [PubMed] [Google Scholar]
Rossato Silva 2018 {published data only}
- Rossato Silva D, Sotgiu G, D'Ambrosio L, Rodrigues Pereira G, Silva Barbosa M, Dutra Dias NJ, et al. Diagnostic performances of the Xpert MTB/RIF in Brazil. Respiratory Medicine 2018;134:12-5. [DOI] [PubMed] [Google Scholar]
Ruiz 2017 {published data only}
- Ruiz P, Causse M, Vaquero M, Gutierrez J B, Casal M. Evaluation of a new automated abbott realtime MTB RIF/INH assay for qualitative detection of rifampicin/isoniazid resistance in pulmonary and extra-pulmonary clinical samples of mycobacterium tuberculosis. Infection and Drug Resistance 2017;10:463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sachdeva 2018 {published data only}
- Sachdeva K, Shrivastava T. CBNAAT: a boon for early diagnosis of tuberculosis-head and neck. Indian Journal of Otolaryngology and Head and Neck Surgery 2018;70(4):572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saeed 2017a {published data only}
- Saeed M, Ahmad M, Iram S, Riaz S, Akhtar M, Aslam M. GeneXpert technology. A breakthrough for the diagnosis of tuberculous pericarditis and pleuritis in less than 2 hours. Saudi Medical Journal 2017;38(7):699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saeed 2017b {published data only}
- Saeed M, Iram S, Hussain S, Ahmed A, Akbar M, Aslam M. GeneXpert: a new tool for the rapid detection of rifampicin resistance in mycobacterium tuberculosis. Journal of the Pakistan Medical Association 2017;67(2):270-4. [PubMed] [Google Scholar]
Saeed 2018 {published data only}
- Saeed M, Rasheed F, Iram S, Hussain S, Ahmad A, Riaz S, et al. False negativity of Ziehl-Neelsen smear microscopy: is the scale-up the [sic] worth it in developing countries? Journal of the College of Physicians and Surgeons--Pakistan 2018;28(3):201-5. [DOI] [PubMed] [Google Scholar]
Salvador 2015 {published data only}
- Salvador F, Los-Arcos I, Sanchez-Montalva A, Tortola T, Curran A, Villar A, et al. Epidemiology and diagnosis of tuberculous lymphadenitis in a tuberculosis low-burden country. Medicine 2015;94(4):e509. [DOI: 10.1097/MD.0000000000000509] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samuel 2018 {published data only}
- Samuel B, Michael J, Chandrasingh J, Kumar S, Devasia A, Kekre N. Efficacy and role of Xpert Mycobacterium tuberculosis/rifampicin assay in urinary tuberculosis. Indian Journal of Urology 2018;34(4):268-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanjuan Jimenez 2015 {published data only}
- Sanjuan-Jimenez R, Toro-Peinado I, Bermudez P, Colmenero JD, Morata P. Comparative study of a real-time PCR assay targeting senX3-regX3 versus other molecular strategies commonly used in the diagnosis of tuberculosis. PLOS One 2015;10(11):e0143025. [DOI: 10.1371/journal.pone.0143025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schutz 2019 {published data only}
- Schutz C, Ward A, Burton R, Nicol M P, Blumenthal L, Meintjes G, et al. False rifampicin resistant results using Xpert MTB/RIF on urine samples in hospitalised HIV-infected patients. Southern African Journal of HIV Medicine 2019;20(1):978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sekyere 2019 {published data only}
- Osei Sekyere J, Maphalala N, Malinga LA, Mbelle NM, Maningi NE. A comparative evaluation of the new Genexpert MTB/RIF Ultra and other rapid diagnostic assays for detecting tuberculosis in pulmonary and extra pulmonary specimens. Scientific Reports 2019;9(1):16587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Set 2018 {published data only}
- Set R, Bankar S, Sharma D, Shah D, Shastri J. Performance of Xpert MTB/RIF for detection of Mycobacterium tuberculosis and rifampicin resistance in pus aspirates. Indian Journal of Tuberculosis 2018;66(4):433-6. [DOI] [PubMed] [Google Scholar]
Set 2019 {published data only}
- Set R, Bankar S, Sharma D, Shah D, Shastri J. Performance of Xpert MTB/RIF for detection of Mycobacterium tuberculosis and rifampicin resistance in pus aspirates. Indian Journal of Tuberculosis 2019;66(4):433-6. [DOI] [PubMed] [Google Scholar]
Shah 2016a {published data only}
- Shah I, Gupta Y, Chhina AS, Shenoi A, Kumar RK, Patel A. Xpert MTB/RIF for diagnosis of tuberculosis and drug resistance in Indian children. Indian Pediatrics 2016;53(9):837-9. [DOI] [PubMed] [Google Scholar]
Shakeel 2018 {published data only}
- Shakeel K, Iram S, Akhtar M, Hussain S, Maryam H, Anwar A. Diagnostic validation of rapid molecular detection of Mycobacterium tuberculosis in pus samples by GeneXpert. Journal of the Pakistan Medical Association 2018;68(1):33-7. [PubMed] [Google Scholar]
Sharma 2017a {published data only}
- Sharma SK, Chaubey J, Singh BK, Sharma R, Mittal A, Sharma A. Drug resistance patterns among extra-pulmonary tuberculosis cases in a tertiary care centre in North India. International Journal of Tuberculosis and Lung Disease 2017;21(10):1112-7. [DOI] [PubMed] [Google Scholar]
Sharma 2019 {published data only}
- Sharma K, Gupta A, Sharma M, Sharma A, Bansal R, Sharma SP, et al. The emerging challenge of diagnosing drug-resistant tubercular uveitis: experience of 110 eyes from North India. Ocular Immunology and Inflammation 2019;27:1-8. [DOI] [PubMed] [Google Scholar]
Singanayagam 2014 {published data only}
- Singanayagam A, Donaldson H, Kon OM. GeneXpert™MTB/RIF in low prevalence settings: a UK laboratory perspective. Journal of Infection 2014;69(2):199-200. [DOI] [PubMed] [Google Scholar]
Singh 2016 {published data only}
- Singh UB, Pandey P, Mehta G, Bhatnagar AK, Mohan A, Goyal V, et al. Genotypic, phenotypic and clinical validation of GeneXpert in extra-pulmonary and pulmonary tuberculosis in India. PLOS One 2016;11(2):e0149258. [DOI: 10.1371/journal.pone.0149258] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2014 {published data only}
- Smith P, Van Esch A, Wallace M, Wood R, Bekker LG. GeneXpert TB 8: a point-of-care diagnostic pilot. South African Medical Journal 2014;104(8):524. [DOI] [PubMed] [Google Scholar]
Solomons 2015 {published data only}
- Solomons RS, Visser DH, Friedrich SO, Diacon AH, Hoek KG, Marais BJ, et al. Improved diagnosis of childhood tuberculous meningitis using more than one nucleic acid amplification test. International Journal of Tuberculosis and Lung Disease 2015;19(1):74-80. [DOI] [PubMed] [Google Scholar]
Solomons 2016 {published data only}
- Solomons RS, Visser DH, Marais BJ, Schoeman JF, Van Furth AM. Diagnostic accuracy of a uniform research case definition for TBM in children: a prospective study. International Journal of Tuberculosis and Lung Disease 2016;20(7):903-8. [DOI] [PubMed] [Google Scholar]
Soomro 2017 {published data only}
- Soomro NH, Baig S, Rao N, Zafar AA, Hashmat F, Awan SB, et al. GeneXpert of cervical lymph node specimens in the diagnosis of extrapulmonary TB and rifampicin resistance. Gomal Journal of Medical Sciences 2017;15(1):12-5. [Google Scholar]
Sumayya 2019 {published data only}
- Sumayya, Durga K, Nithyananda BS, Fatima S, Krishnaiah A. Study of CBNAAT and anti-MPT64 detection in cytological and histopathological material for early diagnosis of TB lymphadenitis. Journal of Evolution of Medical and Dental Sciences 2019;8(47):3540-4. [Google Scholar]
Tahseen 2019 {published data only}
- Tahseen S, Ambreen A, Masood F, Qadir M, Hussain A, Jamil M, et al. Primary drug resistance in extra-pulmonary tuberculosis: a hospital-based prospective study from Pakistan. International Journal of Tuberculosis and Lung Diseases 2019;23(8):900-6. [DOI] [PubMed] [Google Scholar]
Talib 2019 {published data only}
- Talib A, Bhatty S, Mehmood K, Naim H, Haider I, Lal H, et al. GeneXpert in stool: diagnostic yield in intestinal tuberculosis. Journal of Clinical Tuberculosis and Mycobacterial Diseases 2019;17:100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2017 {published data only}
- Tang L, Feng S, Gao R, Han C, Sun X, Bao Y, et al. A comparative study on the role of Xpert MTB/RIF in testing different types of spinal tuberculosis tissue specimens. Genetic Testing and Molecular Biomarkers 2017;21(12):722-6. [DOI] [PubMed] [Google Scholar]
Tang 2018 {published data only}
- Tang Y, Yin L, Tang S, Zhang H, Lan J. Application of molecular, microbiological, and immunological tests for the diagnosis of bone and joint tuberculosis. Journal of Clinical Laboratory Analysis 2018;32(2):e22260. [DOI: 10.1002/jcla.22260] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teo 2011 {published data only}
- Teo J, Jureen R, Chiang D, Chan D, Lin R. Comparison of two nucleic acid amplification assays, the Xpert MTB/RIF assay and the amplified mycobacterium tuberculosis direct assay, for detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. Journal of Clinical Microbiology 2011;49(10):3659-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Terzi 2019 {published data only}
- Terzi HA, Aydemir O, Karakece E, Koroglu M, Altindis M. Comparison of the GeneXpert(R) MTB/RIF test and conventional methods in the diagnosis of Mycobacterium tuberculosis. Clinical Laboratory 2019;65(1):10.7754/Clin.Lab.2018.180613. [DOI: 10.7754/Clin.Lab.2018.180613] [DOI] [PubMed] [Google Scholar]
Theron 2014b {published data only}
- Theron G, Peter J, Calligaro G, Meldau R, Hanrahan C, Khalfey H, et al. Determinants of PCR performance (Xpert MTB/RIF), including bacterial load and inhibition, for TB diagnosis using specimens from different body compartments. Scientific Reports 2014;4:5658. [DOI: 10.1038/srep05658] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tortoli 2012 {published data only}
- Tortoli E, Russo C, Piersimoni C, Mazzola E, Dal Monte P, Pascarella M, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. European Respiratory Journal 2012;40(2):442-7. [DOI] [PubMed] [Google Scholar]
Toure 2017 {published data only}
- Toure NO, Wayzani M, Thiam K, Cisse MF, Mbaye FB. Contribution of the Xpert MTB/RIF to the etiological diagnosis of tuberculous pleurisy [Apport de l’Xpert MTB/RIF dans le diagnostic étiologique des pleurésies tuberculeuses]. Revue des Maladies Respiratoires 2017;34(7):758-64. [DOI: 10.1016/j.rmr.2017.01.003] [DOI] [PubMed] [Google Scholar]
Uddin 2019 {published data only}
- Uddin MJ, Rahim MA, Hasan MN, Mazumder MK, Haq MM, Rahman MA, et al. Etiological evaluation of patients with lymphadenopathy by clinical, histopathological and microbiological assessment. Mymensingh Medical Journal 2019;28(4):854-61. [PubMed] [Google Scholar]
Vallejo 2015 {published data only}
- Vallejo VP, Searle MA, Rodriguez DJ, Farga CV. Xpert® MTB/RIF assay for tuberculosis diagnosis [Ensayo Xpert® MTB/RIF en el diagnóstico de tuberculosis]. Revista Chilena de Enfermedades Respiratorias 2015;31(2):127-31. [Google Scholar]
Verghese 2016 {published data only}
- Verghese VP, Thomas L, Michael JS, Rose W, Jeyaseelan V. Accuracy of the Xpert MTB/RIF assay compared to the "gold standard" AFB culture in the diagnosis of tuberculosis in children in India. International Journal of Infectious Diseases 2016;45:343. [Google Scholar]
Wang 2016a {published data only}
- Wang T, Feng GD, Pang Y, Liu JY, Zhou Y, Yang YN, et al. High rate of drug resistance among tuberculous meningitis cases in Shaanxi province, China. Scientific Reports 2016;6:25251. [DOI: 10.1038/srep25251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2016b {published data only}
- Wang T, Feng GD, Pang Y, Yang YN, Dai W, Zhang L, et al. Sub-optimal specificity of modified Ziehl-Neelsen staining for quick identification of tuberculous meningitis. Frontiers in Microbiology 2016;7:2096-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2018 {published data only}
- Wang G, Dong W, Lan T, Fan J, Tang K, Li Y, et al. Diagnostic accuracy evaluation of the conventional and molecular tests for spinal tuberculosis in a cohort, head-to-head study. Emerging Microbes & Infections 2018;7(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2016 {published data only}
- Wei G, Mu J, Wang G, Huo F, Dong L, Li Y, et al. The reliability analysis of Xpert-positive result for smear-negative and culture-negative specimen collected from bone and joint tuberculosis suspects. Journal of Thoracic Disease 2016;8(6):1205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2017 {published data only}
- Yang CQ, Liu XY, Du RH, Cao TZ, Dai XY. Diagnosis value with Xpert Mtb/RIF assay for cervical tuberculous lymphadenitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017;31(17):1338-40. [DOI] [PubMed] [Google Scholar]
Yang 2019 {published data only}
- Yang X, Che N, Duan H, Liu Z, Li K, Li H, et al. Cell-free Mycobacterium tuberculosis DNA test in pleural effusion for tuberculous pleurisy: a diagnostic accuracy study. Clinical Microbiology and Infection 2019;8:1089.e1-1089.e6. [DOI] [PubMed] [Google Scholar]
Yu 2020 {published data only}
- Yu G, Shen Y, Ye B, Chen D, Xu K. Comparison of CapitalBio Mycobacterium nucleic acid detection test and Xpert MTB/RIF assay for rapid diagnosis of extrapulmonary tuberculosis. Journal of Microbiological Methods 2020;168:105780. [DOI] [PubMed] [Google Scholar]
Yuan 2016 {published data only}
- Yuan M, Lyu Y, Chen ST, Cai C, Li Y, Zhang ZG, et al. Evaluation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis in China. Biomedical and Environmental Sciences 2016;29(8):599-602. [DOI] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang AM, Li F, Liu XH, Xia L, Lu SH. Application of Gene Xpert Mycobacterium tuberculosis DNA and resistance to rifampicin assay in the rapid detection of tuberculosis in children. Zhonghua Er Ke Za Zhi [Chinese Journal of Pediatrics] 2016;54(5):370-4. [DOI] [PubMed] [Google Scholar]
Zhou 2020 {published data only}
- Zhou Z, Zheng Y, Wang L. A comparative study on the value of Xpert MTB/RIF and T-SPOT.TB tests in the diagnosis of bone and joint tuberculosis. Clinica Chimica Acta 2020;500:115-9. [DOI] [PubMed] [Google Scholar]
Zurcher 2019 {published data only}
- Zurcher K, Ballif M, Kiertiburanakul S, Chenal H, Yotebieng M, Grinsztejn B, et al. Diagnosis and clinical outcomes of extrapulmonary tuberculosis in antiretroviral therapy programmes in low- and middle-income countries: a multicohort study. Journal of the International AIDS Society 2019;22(9):e25392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
American Thoracic Society 2000
- American Thoracic Society, the Centers for Disease Control and Prevention, Infectious Disease Society of America. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. American Journal Respiratory and Critical Care Medicine 2000;161(4 Pt 1):1376-95. [DOI] [PubMed] [Google Scholar]
Bahr 2016
- Bahr NC, Marais S, Caws M, Van Crevel R, Wilkinson RJ, Tyagi JS, et al, Tuberculous Meningitis International Research Consortium. GeneXpert MTB/Rif to diagnose tuberculous meningitis: perhaps the first test but not the last. Clinical Infectious Diseases 2016;62(9):1133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401–6. [DOI] [PubMed] [Google Scholar]
Banada 2010
- Banada PP, Sivasubramani SK, Blakemore R, Boehme C, Perkins MD, Fennelly K, et al. Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings. Journal of Clinical Microbiology 2010;48(10):3551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1983
- Begg CB, Greenes RA. Assessment of diagnostic tests when disease verification is subject to selection bias. Biometrics 1983;39(1):207-15. [PubMed] [Google Scholar]
Bjerrum 2019
- Bjerrum S, Schiller I, Dendukuri N, Kohli M, Nathavitharana RR, Zwerling AA, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD011420. [DOI: 10.1002/14651858.CD011420.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blakemore 2010
- Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, et al. Evaluation of the analytical performance of the Xpert MTB/RIF assay. Journal of Clinical Microbiology 2010;48(7):2495-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boehme 2010
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. New England Journal of Medicine 2010;363(11):1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bossuyt 2015
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI: 10.1136/bmj.h5527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Branigan 2019
- Branigan D. Pipeline report 2019 tuberculosis diagnostics. www.treatmentactiongroup.org/wp-content/uploads/2019/12/pipeline_tb_diagnotics_2019_db_final.pdf (accessed 4 April 2020).
Broger 2020
- Broger T, Nicol MP, Székely R, Bjerrum S, Sossen B, Schutz C, et al. Diagnostic accuracy of a novel tuberculosis point-of-care urine lipoarabinomannan assay for people living with HIV: a meta-analysis of individual in- and outpatient data. PLOS Medicine 2020;17(5):e1003113. [DOI] [PMC free article] [PubMed]
CDC 2018
- Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2018. www.cdc.gov/tb/statistics/reports/2018/table15.htm (accessed 29 June 2020).
Cepheid 2018
- Cepheid. Brochure: Xpert® MTB/RIF Ultra. www.cepheid.com/en/tests/Critical-Infectious-Diseases/Xpert-MTB-RIF-Ultra (accessed 26 March 2020).
Cepheid 2019
- Cepheid. Xpert® MTB/RIF. Two-hour detection of MTB and rifampin resistance mutations. www.cepheid.com/Package%20Insert%20Files/Xpert-MTB-RIF-ENGLISH-Package-Insert-301-1404-Rev-F.pdf (accessed 29 March 2020).
Chakravorty 2017
- Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, et al. The new Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. MBio 2017;8(4):e00812-17. [DOI: 10.1128/mBio.00812-17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2012
- Chang K, Lu W, Wang J, Zhang K, Jia S, Li F, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. Journal of Infection 2012;64(6):580-8. [DOI] [PubMed] [Google Scholar]
Chin 2019a
- Chin JH, Ssengooba W, Grossman S, Pellinen J, Wadda V. Xpert MTB/RIF Ultra: optimal procedures for the detection of Mycobacterium tuberculosis in cerebrospinal fluid. Journal of Clinical Tuberculosis and Other Mycobactaterial Diseases 2019;14:16-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chow 2002
- Chow KM, Chow VC, Hung LC, Wong SM, Szeto CC. Tuberculous peritonitis-associated mortality is high among patients waiting for the results of mycobacterial cultures of ascitic fluid samples. Clinical Infectious Diseases 2002;35(4):409-13. [DOI] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331–2. [DOI] [PubMed] [Google Scholar]
Conde 2003
- Conde MB, Loivos AC, Rezende VM, Soares SL, Mello FC, Reingold AL, et al. Yield of sputum induction in the diagnosis of pleural tuberculosis. American Journal of Respiratory and Critical Care Medicine 2003;167(5):723-5. [DOI] [PubMed] [Google Scholar]
Covidence 2017 [Computer program]
- Veritas Health Innovation Covidence systematic review software. Available at www.covidence.org. Melbourne: Veritas Health Innovation, 2017.
Denkinger 2014
- Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. European Respiratory Journal 2014;44(2):435-46. [DOI] [PubMed] [Google Scholar]
Diacon 2003
- Diacon AH, Van de Wal BW, Wyser C, Smedema JP, Bezuidenhout J, Bolliger CT, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. European Respiratory Journal 2003;22(4):589-91. [DOI] [PubMed] [Google Scholar]
Dinnes 2007
- Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technology Assessment 2007;11(3):1-196. [DOI] [PubMed] [Google Scholar]
Di Tanna 2019
- Di Tanna GL, Khaki AR, Theron G, McCarthy K, Cox H, Mupfumi L, et al. Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis. Lancet Global Health 2019;7:e191-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorman 2018
- Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infectious Diseases 2018;18(1):76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drain 2019
- Drain PK, Gardiner J, Hannah H, Broger T, Dheda K, Fielding K, et al. Guidance for studies evaluating the accuracy of biomarker-based nonsputum tests to diagnose tuberculosis. Journal of Infectious Diseases 2019;220(Suppl 3):S108-S115. [DOI] [PubMed] [Google Scholar]
FIND 2017
- Foundation for Innovative New Diagnsotics (FIND). Report for WHO: a multicentre non-inferiority diagnostic accuracy study of the Ultra assay compared to the Xpert MTB/RIF assay. www.finddx.org/publication/ultra-report/ (accessed 2 July 2020);(version 1.8):1-82.
Fontanilla 2011
- Fontanilla JM, Barnes A, Von Reyn CF. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clinical Infectious Diseases 2011;53(6):555-62. [DOI] [PubMed] [Google Scholar]
Global Laboratory Initiative 2017
- Global Laboratory Initiative. Planning for country transition to Xpert® MTB/RIF Ultra cartridges. www.stoptb.org/wg/gli/assets/documents/gli_ultra.pdf (accessed 2 July 2020).
Global Laboratory Initiative 2019
- Global Laboratory Intiaitive. Practical guide to implementing a quality assurance system for Xpert MTB/RIF testing. www.stoptb.org/wg/gli/assets/documents/Xpert-QA-guide-2019.pdf (accessed 7 January 2020).
Golden 2005
- Golden MP, Vikram HR. Extrapulmonary tuberculosis: an overview. American Family Physician 2005;72(9):1761-8. [PubMed] [Google Scholar]
Gopinath 2010
- Gopinath K, Singh S. Non-tuberculous mycobacteria in TB-endemic countries: are we neglecting the danger? PLOS Neglected Tropical Diseases 2010;4(4):e615. [DOI: 10.1371/journal.pntd.0000615] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 2 July 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gupta 2015
- Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015;29(15):1987-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2018
- Gupta R, Talwar P, Talwar P, Khurana S, Kushwaha S, Jalan N, et al. Diagnostic accuracy of nucleic acid amplification based assays for tuberculous meningitis: a meta-analysis. Journal of Infection 2018;77(4):302-13. [DOI] [PubMed] [Google Scholar]
Gupta‐Wright 2018
- Gupta-Wright A, Corbett EL, Van Oosterhout JJ, Wilson D, Grint D, Alufandika-Moyo M, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018;392(10144):292-301. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haraka 2018
- Haraka F, Nathavitharana RR, Schumacher SG, Kakolwa M, Denkinger CM, Gagneux S, et al. Impact of diagnostic test Xpert MTB/RIF® on health outcomes for tuberculosis. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD012972. [DOI: 10.1002/14651858.CD012972] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2007
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8(2):239-51. [DOI] [PubMed] [Google Scholar]
Hartmann 1967
- Hartmann G, Honikel KO, Knüsel F, Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochimica et Biophysica Acta 1967;145(3):843–4. [DOI] [PubMed] [Google Scholar]
Helb 2010
- Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. Journal of Clinical Microbiology 2010;48(1):229-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horne 2019
- Horne DJ, Kohli M, Zifodya JS, Schiller I, Dendukuri N, Tollefson D, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD009593. [DOI: 10.1002/14651858.CD009593] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iseman 2000
- Iseman MD. Extrapulmonary tuberculosis in adults. In: Iseman MD, editors(s). A Clinician’s Guide to Tuberculosis. Philadelphia: Lippincott Williams and Wilkins, 2000:145–97. [Google Scholar]
Kay 2020
- Kay AW, González Fernández L, Takwoingi Y, Eisenhut M, Vu RD, Steingart KR, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD013359. [DOI: 10.1002/14651858.CD013359] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilpatrick 1986
- Kilpatrick ME, Girgis NI, Yassin MW, Abu el Ella AA. Tuberculous meningitis-clinical and laboratory review of 100 patients. Journal of Hygiene (London) 1986;96(2):231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leeflang 2013
- Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test's sensitivity and specificity with disease prevalence. Canadian Medical Association Journal 2013;185(11):E537-44. [DOI: 10.1503/cmaj.121286] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewinsohn 2017
- Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clinical Infectious Diseases 2017;64(2):e1–33. [DOI: 10.1093/cid/ciw694] [DOI] [PubMed] [Google Scholar]
Li Y 2017
- Li S, Liu B, Peng M, Chen M, Yin W, Tang H, et al. Diagnostic accuracy of Xpert MTB/RIF for tuberculosis detection in different regions with different endemic burden: a systematic review and meta-analysis. PLOS One 2017;12(7):e0180725. [DOI: 10.1371/journal.pone.0180725] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lunn 2009
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique, and future directions. Statistics in Medicine 2009;28(25):3049-67. [DOI] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, C Gatsonis (editors). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available at: methods.cochrane.org/sdt/handbook-dta-reviews.
Marais 2010
- Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infectious Diseases 2010;10(11):803-12. [DOI] [PubMed] [Google Scholar]
Maynard‐Smith 2014
- Maynard-Smith L, Larke N, Peters JA, Lawn SD. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infectious Diseases 2014;14:709. [DOI: 10.1186/s12879-014-0709-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
McInnes 2018
- McInnes MD, Moher D, Thombs BD, McGrath TA, Bossuyt PM, et al, anad the PRISMA-DTA Group. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: the PRISMA-DTA statement [published correction appears in JAMA. 2019 Nov 26;322(20):2026]. JAMA 2018;319(4):388-96. [DOI: 10.1001/jama.2017.19163] [DOI] [PubMed] [Google Scholar]
Miotto 2012
- Miotto P, Bigoni S, Migliori GB, Matteelli A, Cirillo DM. Early tuberculosis treatment monitoring by Xpert(R) MTB/RIF. European Respiratory Journal 2012;39(5):1269-71. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLOS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naaktgeboren 2013
- Naaktgeboren CA, Bertens LC, Van Smeden M, De Groot JA, Moons KG, Reitsma JB. Value of composite reference standards in diagnostic research. BMJ 2013;347:f5605. [DOI] [PubMed] [Google Scholar]
Nahid 2016
- Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clinical Infectious Diseases 2016;63(7):e147-95. [DOI: 10.1093/cid/ciw376] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathavitharana 2017
- Nathavitharana RR, Cudahy PG, Schumacher SG, Steingart KR, Pai M, Denkinger CM. Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis. European Respiratory Journal 2017;49(1):pii: 1601075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 2004
- Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. International Journal of Tuberculosis and Lung Disease 2004;8(5):636-47. [PubMed] [Google Scholar]
Pai 2004
- Pai M, Flores LL, Hubbard A, Riley LW, Colford JM Jr. Nucleic acid amplification tests in the diagnosis of tuberculous pleuritis: a systematic review and meta-analysis. BMC Infectious Diseases 2004;4:6. [DOI: 10.1186/1471-2334-4-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pai 2008
- Pai M, Ling DI. Rapid diagnosis of extrapulmonary tuberculosis using nucleic acid amplification tests: what is the evidence? Future Microbiology 2008;3(1):1-4. [DOI] [PubMed] [Google Scholar]
Pai 2016
- Pai M, Behr M, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nature Reviews. Disease Primers 2016;2:16076. [DOI: 10.1038/nrdp.2016.76] [DOI] [PubMed] [Google Scholar]
Pang 2019
- Pang Y, An J, Shu W, Huo F, Chu N, Gao M, et al. Epidemiology of extrapulmonary tuberculosis among inpatients, China, 2008-2017. Emerging Infectious Diseases 2019;25:457-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penz 2015
- Penz E, Boffa J, Roberts DJ, Fisher D, Cooper R, Ronksley PE, et al. Diagnostic accuracy of the Xpert® MTB/RIF assay for extra-pulmonary tuberculosis: a meta-analysis. International Journal of Tuberculosis and Lung Disease 2015;19(3):278-84. [DOI] [PubMed] [Google Scholar]
Perkins 2007
- Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. Journal of Infectious Diseases 2007;196 Suppl 1:S15–27. [DOI] [PubMed] [Google Scholar]
Peter 2016
- Peter JG, Zijenah LS, Chanda D, Clowes P, Lesosky M, Gina P, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016;387(10024):1187-97. [DOI] [PubMed] [Google Scholar]
Pormohammad 2019
- Pormohammad A, Nasiri MJ, McHugh TD, Riahi SM, Bahr NC. A systematic review and meta-analysis of the diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis. Journal of Clinical Microbiology 2019;57(6):e01113-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
R Core Team 2019 [Computer program]
- R Foundation for Statistical Computing R Core Team (2019). R: A language and environment for statistical computing. Version accessed after 12 June 2020. Vienna, Austria: R Foundation for Statistical Computing, 2019. Available at r-project.org/.
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982-90. [DOI] [PubMed] [Google Scholar]
Reuter 2009
- Reuter H, Wood R, Schaaf HS, Donald PR. Overview of extrapulmonary tuberculosis in adults and children. In: Schaaf HS, Zumla A, editors(s). Tuberculosis: A Comprehensive Clinical Reference. 1st edition. Amsterdam: Elsevier Science Publishers, 2009:377-90. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sandgren 2013
- Sandgren A, Hollo V, Van der Werf MJ. Extrapulmonary tuberculosis in the European Union and European economic area, 2002 to 2011. Euro Surveillance 2013;18(12):pii: 20431. [PubMed] [Google Scholar]
Schiller 2016
- Schiller I, Van Smeden M, Hadgu A, Libman M, Reitsma JB, Dendukuri N. Bias due to composite reference standards in diagnostic accuracy studies. Statistics in Medicine 2016;35(9):1454-70. [DOI] [PubMed] [Google Scholar]
Schumacher 2016
- Schumacher S, Van Smeden M, Dendukuri N, Joseph L, Nicol M, Pai M, et al. Diagnostic test accuracy in childhood pulmonary tuberculosis: a Bayesian latent class analysis. American Journal of Epidemiology 2016;184(9):690-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumacher 2019
- Schumacher SG, Denkinger CM. The impact of Xpert MTB/RIF-do we have a final answer? Lancet Global Health 2019;7(2):e161-e162. [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008;336(7653):1106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2016
- Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al. GRADE Working Group. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. Journal of Clinical Epidemiology 2016;76:89-98. [DOI: 10.1016/j.jclinepi.2016.01.032] [DOI] [PubMed] [Google Scholar]
Schünemann 2020a
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias and indirectness in rating the certainty across a body of evidence for test accuracy. Journal of Clinical Epidemiology 2020;122:129-41. [DOI: 10.1016/j.jclinepi.2019.12.020] [DOI] [PubMed] [Google Scholar]
Schünemann 2020b
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 2. Inconsistency, Imprecision, publication bias and other domains for rating the certainty of evidence for test accuracy and presenting it in evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2020;122:142-52. [DOI: 10.1016/j.jclinepi.2019.12.021] [DOI] [PubMed] [Google Scholar]
Sehgal 2016
- Sehgal IS, Dhooria S, Aggarwal AN, Behera D, Agarwal R. Diagnostic performance of Xpert MTB/RIF in tuberculous pleural effusion: systematic review and meta-analysis. Journal of Clinical Microbiology 2016;54(4):1133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2016b
- Shah M, Hanrahan C, Wang ZY, Dendukuri N, Lawn SD, Denkinger CM, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD011420. [DOI: 10.1002/14651858.CD011420.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shapiro 2020
- Shapiro AE, Ross JM, Schiller I, Kohli M, Dendukuri N, Steingart KR, et al. Xpert MTB/RIF and Xpert Ultra assays for pulmonary tuberculosis and rifampicin resistance in adults irrespective of signs or symptoms of pulmonary tuberculosis. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013694. [DOI: 10.1002/14651858.CD013694] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2004
- Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian Journal of Medical Research 2004;120(4):316-53. [PubMed] [Google Scholar]
Sharma 2017b
- Sharma SK, Ryan H, Khaparde S, Sachdeva KS, Singh AD, Mohan A, et al. Index-TB guidelines: guidelines on extrapulmonary tuberculosis for India. Indian Journal of Medical Research 2017;145(4):448-63. [DOI] [PMC free article] [PubMed]
Shen 2019
- Shen Y, Yu G, Zhong F, Kong X. Diagnostic accuracy of the Xpert MTB/RIF assay for bone and joint tuberculosis: a meta-analysis. PLOS One 2019;14(8):e0221427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shu 2011
- Shu CC, Wang JT, Wang JY, Lee LN, Yu CJ. In-hospital outcome of patients with culture-confirmed tuberculous pleurisy: clinical impact of pulmonary involvement. BMC Infectious Diseases 2011;11:46. [DOI: 10.1186/1471-2334-11-46] [DOI] [PMC free article] [PubMed] [Google Scholar]
Small 2011
- Small PM, Pai M. Tuberculosis diagnosis - time for a game change. New England Journal of Medicine 2011;363(111):1070–1. [DOI] [PubMed] [Google Scholar]
Stata 2017 [Computer program]
- Stata Statistical Software: Release 15. StataCorp. College Station, TX: StataCorp LP, 2017.
Steingart 2014
- Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD009593. [DOI: 10.1002/14651858.CD009593.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takwoingi 2013
- Takwoingi Y, Leeflang MM, Deeks JJ. Empirical evidence of the importance of comparative studies of diagnostic test accuracy. Annals of Internal Medicine 2013;158(7):544-54. [DOI] [PubMed] [Google Scholar]
Telenti 1993
- Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston MJ, et al. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet 1993;341(8846):647-50. [DOI] [PubMed] [Google Scholar]
Theron 2014a
- Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2014;383(9915):424-35. [DOI] [PubMed] [Google Scholar]
Thwaites 2013
- Thwaites GE, Van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurology 2013;12(10):999-1010. [DOI] [PubMed] [Google Scholar]
Unitaid 2017
- Boyle D. Tuberculosis Diagnostics Technology and Market Landscape. 5th edition. Vernier: World Health Organization Unitaid Secretariat, 2017. [Google Scholar]
Van Smeden 2014
- Smeden M, Naaktgeboren CA, Reitsma JB, Moons KG, De Groot JA. Latent class models in diagnostic studies when there is no reference standard - a systematic review. American Journal of Epidemiology 2014;179(4):423-31. [DOI] [PubMed] [Google Scholar]
Vonasek 2020
- Vonasek B, Ness T, Takwoingi Y, Kay AW, Wyk SS, Ouellette L, et al. Screening tests for active pulmonary tuberculosis in children. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013693. [DOI: 10.1002/14651858.CD013693] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walzl 2018
- Walzl G, McNerney R, Du Plessis N, Bates M, McHugh TD, Chegou NN, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infectious Diseases 2018;18(7):e199-e210. [DOI] [PubMed] [Google Scholar]
Webster 2014
- Webster AS, Shandera WX. The extrapulmonary dissemination of tuberculosis: a meta-analysis. International Journal of Mycobacteriology 2014;3:9-16. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529–36. [DOI] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Guidelines for treatment of tuberculosis, fourth edition. who.int/tb/publications/2010/9789241547833/en/ 2010 (accessed 2 July 2020).
WHO 2011
- World Health Organization. Rapid implementation of the Xpert MTB/RIF diagnostic test. Technical and operational ‘How-to’. Practical considerations. who.int/tb/publications/tb-amplificationtechnology-implementation/en/ 2011 (accessed 2 July 2020).
WHO 2012
- World Health Organization. Updated interim critical concentrations for first-line and second-line DST (as of May 2012). www.stoptb.org/wg/gli/assets/documents/Updated%20critical%20concentration%20table_1st%20and%202nd%20line%20drugs.pdf 2012 (accessed 2 July 2020).
WHO 2014
- World Health Organization. Xpert MTB/RIF implementation manual. Technical and operational ‘how-to’ practical considerations. apps.who.int/iris/bitstream/10665/112469/1/9789241506700_eng.pdf 2014 (accessed 2 July 2020).
WHO 2017
- World Health Organization. Guidelines for the treatment of drug-susceptible tuberculosis and patient care (2017 update). www.who.int/tb/publications/2017/dstb_guidance_2017/en/ 2017 (accessed 1 April 2020).
WHO 2018
- World Health Organization. Technical report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis (WHO/CDS/TB/2018.5). who.int/tb/publications/2018/WHO_technical_report_concentrations_TB_drug_susceptibility/en/ (accessed 2 July 2020).
WHO Compendium 2018
- World Health Organization. Compendium of WHO guidelines and associated standards: ensuring optimum delivery of the cascade of care for patients with tuberculosis, second edition. www.who.int/tb/publications/Compendium_WHO_guidelines_TB_2017/en/ (accessed 2 July 2020).
WHO Consolidated Guidelines (Module 3) 2020
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection. Licence: CC BY-NC-SA 3.0 IGO. who.int/publications/i/item/who-consolidated-guidelines-on-tuberculosis-module-3-diagnosis---rapid-diagnostics-for-tuberculosis-detection 2020 (accessed 2 July 2020).
WHO Consolidated Guidelines (Module 4) 2020
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO. who.int/publications/i/item/9789240007048 (accessed 2 July 2020).
WHO END TB 2014
- World Health Organization. The END TB strategy. www.who.int/tb/strategy/end-tb/en/ 2014 (accessed 1 April 2020).
WHO Global TB Report 2020
- World Health Organization. Global Tuberculosis Report 2020. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization, 2020. [Google Scholar]
WHO Operational Handbook Diagnosis (Module 3) 2020
- World Health Organization. WHO operational handbook on tuberculosis. Module 3: diagnosis - rapid diagnostics for tuberculosis detection. Licence: CC BY-NC-SA 3.0 IGO. who.int/publications/i/item/who-operational-handbook-on-tuberculosis-module-3-diagnosis---rapid-diagnostics-for-tuberculosis-detection (accessed 6 August 2020).
WHO Xpert MTB/RIF Policy 2011
- World Health Organization. Policy statement: automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. WHO/HTM/TB/2011.4. www.who.int/tb/publications/tb-amplificationtechnology-statement/en/ 2011 (accessed 2 July 2020). [PubMed]
WHO Xpert MTB/RIF Policy Update 2013
- World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. WHO/HTM/TB/2013.14. apps.who.int/iris/handle/10665/112472 2013 (accessed 2 July 2020).
WHO Xpert Ultra 2017
- World Health Organization. WHO meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. WHO/HTM/TB/2017.04. www.who.int/tb/publications/2017/XpertUltra/en/ 2017 (accessed 2 July 2020).
Woodard 1982
- Woodard BH, Rosenberg SI, Farnham R, Adams DO. Incidence and nature of primary granulomatous inflammation in surgically removed material. American Journal of Surgical Pathology 1982;6(2):119-29. [DOI] [PubMed] [Google Scholar]
Woods 2001
- Woods GL. Molecular techniques in mycobacterial detection. In: Archives of Pathology & Laboratory Medicine. Vol. 125. Geneva: World Health Organization, 2001:122-6. [DOI] [PubMed] [Google Scholar]
World Bank 2020
- World Bank. World bank list of economies. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups 2019 (accessed 2 July 2020).
Wright 2009a
- Wright CA, Bezuidenhout J. Histopathology and cytopathology. In: Schaaf HS, Zumla A, editors(s). Tuberculosis: A Comprehensive Clinical Reference. 1st edition. Amsterdam: Elsevier Science Publishers, 2009:205-15. [Google Scholar]
Wright 2009b
- Wright CA, Hesseling AC, Bamford C, Burgess SM, Warren R, Marais BJ. Fine-needle aspiration biopsy: a first-line diagnostic procedure in paediatric tuberculosis suspects with peripheral lymphadenopathy? International Journal of Tuberculosis and Lung Disease 2009;13(11):1373-9. [PubMed] [Google Scholar]
Yu 2019
- Yu G, Zhong F, Ye B, Xu X, Chen D, Shen Y. Diagnostic accuracy of the Xpert MTB/RIF assay for lymph node tuberculosis: a systematic review and meta-analysis. BioMed Research International 2019;2019:4878240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2020
- Zhang M, Xue M, He JQ. Diagnostic accuracy of the new Xpert MTB/RIF Ultra for tuberculosis disease: a preliminary systematic review and meta-analysis. International Journal of Infectious Diseases 2020;90:35-45. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kohli 2017
- Kohli M, Schiller I, Dendukuri N, Ryan H, Dheda K, Denkinger CM, Schumacher SG, Steingart KR. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD012768. [DOI: 10.1002/14651858.CD012768] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohli 2018
- Kohli M, Schiller I, Dendukuri N, Dheda K, Denkinger CM, Schumacher SG, et al. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database of Systematic Reviews 2018, Issue 8. Art. No: CD012768. [DOI: 10.1002/14651858.CD012768.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


