Abstract
Background
The single most important risk factor for postpartum maternal infection is cesarean section. Although guidelines endorse the use of prophylactic antibiotics for women undergoing cesarean section, there is not uniform implementation of this recommendation. This is an update of a Cochrane review first published in 1995 and last updated in 2010.
Objectives
To assess the effects of prophylactic antibiotics compared with no prophylactic antibiotics on infectious complications in women undergoing cesarean section.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 July 2014) and reference lists of retrieved papers.
Selection criteria
Randomized controlled trials (RCTs) and quasi‐RCTs comparing the effects of prophylactic antibiotics versus no treatment in women undergoing cesarean section.
Data collection and analysis
Two review authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction. The clinically important primary outcomes were wound infection, endometritis, serious maternal infectious complications and adverse effects on the infant. We presented dichotomous data as risk ratios (RR), with 95% confidence intervals (CIs) and combined trials in meta‐analyses. We assessed the quality of evidence using the GRADE approach.
Main results
We identified 95 studies enrolling over 15,000 women. Compared with placebo or no treatment, the use of prophylactic antibiotics in women undergoing cesarean section reduced the incidence of wound infection (RR 0.40, 95% CI 0.35 to 0.46, 82 studies, 14,407 women), endometritis (RR 0.38, 95% CI 0.34 to 0.42, 83 studies, 13,548 women) and maternal serious infectious complications (RR 0.31, 95% CI 0.20 to 0.49, 32 studies, 6159 women). When only studies that included women undergoing an elective cesarean section were analyzed, there was also a reduction in the incidence of wound infections (RR 0.62, 95% CI 0.47 to 0.82, 17 studies, 3537 women) and endometritis (RR 0.38, 95% CI 0.24 to 0.61, 15 studies, 2502 women) with prophylactic antibiotics. Similar estimates of effect were seen whether the antibiotics were administered before the cord was clamped or after. The effect of different antibiotic regimens was studied and similar reductions in the incidence of infections were seen for most of the antibiotics and combinations.
There were no data on which to estimate the effect of maternal administration of antibiotics on infant outcomes. No studies systematically collected and reported on adverse infant outcomes nor the effect of antibiotics on the developing infant immune system. No studies reported on the incidence of oral candidiasis (thrush) in babies. Maternal adverse effects were also rarely described.
We judged the evidence for antibiotic treatment compared with no treatment to be of moderate quality; most studies lacked an adequate description of methods and were assessed as being at unclear risk of bias.
Authors' conclusions
The conclusions of this review support the recommendation that prophylactic antibiotics should be routinely administered to all women undergoing cesarean section to prevent infection. Compared with placebo or no treatment, the use of prophylactic antibiotics in women undergoing cesarean section reduced the incidence of wound infection, endometritis and serious infectious complications by 60% to 70%. There were few data on adverse effects and no information on the effect of antibiotics on the baby, making the assessment of overall benefits and harms difficult. Prophylactic antibiotics given to all women undergoing elective or non‐elective cesarean section is beneficial for women but there is uncertainty about the consequences for the baby.
Plain language summary
Routine antibiotics at cesarean section to reduce infection
Women undergoing cesarean section have a five to 20‐fold greater chance of getting an infection compared with women who give birth vaginally. These infections can be in the organs within the pelvis, around the surgical incision and sometimes the urine. The infections can be serious, and very occasionally can lead to the mother’s death. The potential benefits of reducing infection for the mother need to be balanced against any adverse effects such as nausea, vomiting, skin rash and rarely allergic reactions in the mother, and any effect of antibiotics on the baby, including thrush. This review looked at whether antibiotics are effective in preventing infection in women having a cesarean section. It also studied the effect of giving the antibiotics before or after the cord is clamped and different kinds of antibiotics. The review found 95 studies involving over 15,000 women. Routine use of antibiotics at cesarean section reduced the risk of wound and womb infections in mothers as well as the risk of serious complications of infections for the mothers by 60% to 70%. This was so whether the cesarean section was planned (elective) or not, and whether the antibiotics were given before or after clamping of the umbilical cord. The evidence to support antibiotic treatment was of moderate quality but often the way the study was done was not described well enough. None of the studies looked properly at possible adverse effects on the baby and so, although there are benefits for the mother, there is some uncertainty about whether there are any important effects on the baby.
Summary of findings
Summary of findings for the main comparison. Antibiotics versus no antibiotics for preventing infection after cesarean section.
| Antibiotics versus no antibiotics for preventing infection after cesarean section | ||||||
| Population: Women undergoing cesarean section. Settings: Both high‐ and low‐income countries. Intervention: Antibiotic prophylaxis1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic prophylaxis | |||||
| Maternal wound infection | Low (elective)2 | RR 0.40 (0.35 to 0.46) | 14407 (82 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 68 per 1000 | 27 per 1000 (24 to 31) | |||||
| Median2 | ||||||
| 89 per 1000 | 36 per 1000 (31 to 41) | |||||
| Other (includes emergency)2 | ||||||
| 97 per 1000 | 39 per 1000 (34 to 45) | |||||
| Maternal endometritis | Low (elective)2 | RR 0.38 (0.34 to 0.42) | 13548 (83 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 39 per 1000 | 15 per 1000 (13 to 16) | |||||
| Median2 | ||||||
| 160 per 1000 | 61 per 1000 (54 to 67) | |||||
| Other (includes emergency)2 | ||||||
| 184 per 1000 | 70 per 1000 (63 to 77) | |||||
| Maternal serious infectious complications | 25 per 10004 | 8 per 1000 (5 to 12) | RR 0.31 (0.2 to 0.49) | 6159 (32 studies) | ⊕⊕⊕⊝ moderate5 | |
| Adverse effects on infant | See comment | See comment | Not estimable | 0 (0) | See comment | Infant outcomes were not systematically collected nor reported. 6 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All classes of antibiotics, dosing regimens (both before and after clamping of the cord) and routes of administration were included. 2 The low risk baseline value is the mean of the control groups of studies reporting outcomes for elective cesarean sections; the other risk estimate is derived from the remaining studies in the review and includes emergency cesarean sections and studies which did not meet our criteria for elective.The median value from all studies is also reported. 3 In most studies the assessment of bias was judged as unclear. In a third of studies the control group did not receive a placebo and lack of blinding could have influenced the assessment of outcomes. In less than 20% of studies was there an adequate description of sequence generation. 4 The study population baseline risk is the mean value in the control groups from all studies that reported this outcome. 5 There was no consistent approach to the definition of serious infectious complications; in only 32 studies was this outcome reported. 6 No study reported effects of antibiotics on the infant immune system or outcome of oral thrush.
Summary of findings 2. Antibiotics versus no antibiotics for preventing infection after elective cesarean section.
| Antibiotics versus no antibiotics for preventing infection after elective cesarean section | ||||||
| Population: Women undergoing elective cesarean section Settings: Both high‐ and low‐income countries Intervention: Antibiotic prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic prophylaxis | |||||
| Maternal wound infection | 68 per 10001 | 42 per 1000 (32 to 56) | RR 0.62 (0.47 to 0.82) | 3537 (17 studies) | ⊕⊕⊕⊝ moderate2 | |
| Maternal endometritis | 39 per 10001 | 15 per 1000 (9 to 24) | RR 0.38 (0.24 to 0.61) | 2502 (15 studies) | ⊕⊕⊕⊝ moderate2 | |
| Maternal serious infectious complications | 0 per 1000 | 0 per 1000 (0 to 0) | RR 1.01 (0.04 to 24.21) | 545 (4 studies) | ⊕⊕⊝⊝ low3 | There was only one adverse event reported. |
| Adverse effects on infant | See comment | See comment | Not estimable | 0 (0) | See comment | Infant outcomes were very infrequently reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The study population baseline risk is the mean value in the control groups from all studies that included women undergoing elective cesarean section. 2 In most studies the assessment of bias was judged as unclear. 3 There was only one serious infectious complication reported.
Background
The single most important risk factor for postpartum maternal infection is cesarean section (Declercq 2007; Gibbs 1980). Women undergoing cesarean section have a five to 20‐fold greater risk for infection and infectious morbidity compared with a vaginal birth. In Western countries the percentage of live births by cesarean section is around 27% (range 14.7% to 49%) (OECD 2013); in developing countries the overall rate is around 12% but varies widely by region (0.40% to 40%) (Thomas 2006). Infectious complications that occur after cesarean births are an important and substantial cause of maternal morbidity and are associated with a significant increase in hospital stay (Henderson 1995). Infections can affect the pelvic organs, the surgical wound, and the urinary tract.
Description of the condition
Infectious complications following cesarean birth include fever (febrile morbidity), wound infection, endometritis (inflammation of the lining of the uterus), and urinary tract infection. There can also occasionally be serious infectious complications including pelvic abscess (collection of pus in the pelvis), bacteremia (bacterial infection in the blood), septic shock (reduced blood volume due to infection), necrotizing fasciitis (tissue destruction in the uterine wall) and septic pelvic vein thrombophlebitis (inflammation and infection of the veins in the pelvis); sometimes these can lead to maternal mortality (Boggess 1996; Enkin 1989; Gibbs 1980; Leigh 1990).
Fever can occur after any operative procedure, and a low grade fever following a cesarean birth may not necessarily be a marker of infection (MacLean 1990). Without prophylaxis, the incidence of endometritis is about 20% and rates of wound infection and serious infectious complications as high as 25% have been reported (Enkin 1989). There has been no consistent application of a standard definition for endometritis nor wound infection, and surveillance strategies to confirm infection, especially following hospital discharge, vary widely (Baker 1995; Hulton 1992). Differences in ethnicity and socioeconomic status of the population studied will explain some of the variability in incidence, as will the use of different criteria to diagnose infection (Herbert 1999). Using the Centers for Disease Control (CDC) definitions for infection, the pooled mean rate of surgical site infections after cesarean section for US hospitals participating in the CDC and Prevention's National Nosocomial Infections Surveillance System (NNIS) from January 1992 through June 2004 was 3.15%, ranging from 2.71% for low‐risk patients to 7.53% for high‐risk patients (NNIS 2004). These rates, when compared with infection rates following other surgical procedures that are collected as part of the NNIS system, are high. Given the number of cesarean sections performed, these rates translate into very large numbers of women with an infectious complication following birth, and significant costs and morbidity.
Factors that have been associated with an increased risk of infection and infectious morbidity among women who have a cesarean include emergency cesarean section, labor and its duration, ruptured membranes and the duration of rupture, the socioeconomic status of the woman, number of prenatal visits, vaginal examinations during labor, internal fetal monitoring, urinary tract infection, anemia, blood loss, obesity, diabetes, general anesthesia, development of subcutaneous hematoma, the skill of the operator and the operative technique (Beattie 1994; Desjardins 1996; Enkin 1989; Gibbs 1980; Killian 2001; Magann 1995; Olsen 2008; Webster 1988). The association of bacterial vaginosis with an increased incidence of endometritis following cesarean birth has also been reported (Watts 1990).
The most important source of micro‐organisms responsible for post‐cesarean section infection is the genital tract, particularly if the membranes are ruptured. Even in the presence of intact membranes, microbial invasion of the intrauterine cavity is common, especially with preterm labor (Watts 1992). Infections are commonly polymicrobial (caused by many organisms). Pathogens isolated from infected wounds and the endometrium include Escherichia coli and other aerobic gram negative rods, group B streptococcus and other streptococcus species, Enterococcus faecalis, Staphylococcus aureus and coagulase negative staphylococci, anaerobes (including Peptostreptococcus species and Bacteroides species), Gardnerella vaginalis and genital mycoplasmas (Martens 1995; Roberts 1993; Watts 1991). Although Ureaplasma urealyticum is very commonly isolated from the upper genital tract and infected wounds, it is unclear whether it is a pathogen in this setting (Roberts 1993). Wound infections caused by Staphylococcus aureus and coagulase negative staphylococci arise from contamination of the wound with the endogenous flora of the skin at the time of surgery (Emmons 1988).
Description of the intervention
Guidelines recommend the use of antibiotics for prophylaxis for cesarean section, with the choice of antibiotic based on factors such as cost, half life, safety, antimicrobial resistance and spectrum of activity (ACOG 2011; Bratzler 2004; NICE 2011; SOGC 2010). There are over 20 antibiotic regimens that have been compared for cesarean section prophylaxis. Some of these drugs have activity against a narrow range of potential pathogens (e.g. metronidazole, gentamicin), others specifically have additional anaerobic activity (e.g. cefoxitin and cefotetan), others have activity against Staphylococcus aureus (e.g. cefazolin) and yet others have an extended spectrum of coverage (e.g. meropenem). Details of the different antibiotic regimens for prophylaxis at cesarean section that have been compared and their effectiveness are included in another Cochrane review (Alfirevic 2010)
There are differences in the route of administration of prophylactic antibiotics; for cesarean section the antibiotic is generally given intravenously. Usually a single dose is administered at the time of the procedure or multiple doses administered over a short period of time.
For cesarean section, prophylactic antibiotics are administered either before or after the cord is clamped (Classen 1992; Cunningham 1983; Wax 1997), although general guidelines for the prevention of surgical site infections now recommend the antimicrobial dose is administered before the incision to achieve low infection rates (Bratzler 2004). Recent meta‐analyses on the timing of perioperative antibiotics for cesarean delivery have concluded that there was strong evidence that antibiotic prophylaxis that is given before skin incision decreases maternal infectious complications compared with intraoperative administration (Baaqeel 2013; Costantine 2008). However, it is argued that the timing of antibiotic administration may mask septic complications in the infant (Cunningham 1983). Additionally, if the antibiotic is given before cord clamping, the baby will be exposed to the antibiotic via the placenta, and there may be exposure through breast milk if the antibiotic is given either before or after cord clamping, though the passage of antibiotic through the breast milk is thought to minimal (Enkin 1989). Because of the potential for adverse outcomes for the baby and the effect on maternal infectious complications, this review investigated the timing of antibiotic administration (seeTypes of interventions).
How the intervention might work
General principles for the prevention of any surgical infection include sound surgical technique, skin antisepsis and antimicrobial prophylaxis (Owen 1994). Antibiotics administered prophylactically reduce the bacterial inoculum at the time of surgery and decrease the rate of bacterial contamination of the surgical site. An adequate antibiotic level in the tissue can augment natural immune defence mechanisms and help kill bacteria that are invariably inoculated into the wound at the time of surgery (Talbot 2005).
Potential adverse effects of antibiotic prophylaxis
There are commonly identified adverse effects of antibiotic therapy, which include gastrointestinal symptoms (nausea, vomiting or diarrhea), skin rashes, thrush (candidiasis, which can affect both mother and baby) and joint pain (Dancer 2004). There can also occasionally be blood problems, kidney or liver damage (Dancer 2004; Westphal 1994) and anaphylaxis (a hypersensitivity reaction to a foreign substance leading to shock and collapse, which can be fatal).
Because there are some data that antibiotics reaching the baby during labor, or in the very early postnatal period, can affect the pattern of bacterial flora in the infant gut, with the potential to affect the baby's developing immune system (Bedford Russell 2006; Weng 2013), it is important to assess the impact of antibiotics given to the mother on the baby's health.
Antibiotic prophylaxis may lead to increased drug resistant strains of bacteria which may be associated with infection. Resistant organisms may spread within the hospital and be associated with hospital‐acquired drug resistant infections (Dancer 2004). These adverse effects cannot be assessed readily in randomized controlled trials, and additional research needs to be undertaken to assess the impact of prophylactic antibiotic use on the level of resistant bacteria, e.g. MRSA and C. difficile in hospitals.
Why it is important to do this review
Surveys suggest that there is inconsistent and variable application of the use of prophylactic antibiotics at cesarean sections (Huskins 2001; Morisaki 2014; Olsen 2008; Pedersen 1996). Prophylactic antibiotics have been shown, in previous versions of this review, to be effective in reducing febrile morbidity, endometritis, wound infection and urinary tract infection (Smaill 1995a; Smaill 1995b; Smaill 2002; Smaill 2010). In addition, both ampicillin and first generation cephalosporins appeared to have similar efficacy in reducing post‐operative endometritis, and while there did not appear to be any added benefit in utilizing a more broad spectrum agent or a multiple dose regimen (Alfirevic 2010), other authors have questioned whether an extended spectrum agent should be recommended (Tita 2009). It is important to update this evidence with more recent studies, to update the review methodology, to perform a subgroup analysis based on class of antibiotic and also to address the question of whether increasing antimicrobial resistance has had an impact on the benefit of antibiotic prophylaxis.
The adverse effects of antibiotics for the woman and her infant and the potential for increased use of antimicrobial prophylaxis to contribute to the development of antimicrobial resistance are important considerations (Racinet 1990; Shlaes 1997), as are the cost‐effectiveness of different strategies (Mugford 1989). As well, it is important to assess any possible impact of maternal antibiotic treatment on the baby, as there is evidence that antibiotics given near or shortly after birth can affect the infant's gut bacterial flora, with the potential to impact mucosal and systemic immune function (Bedford Russell 2006; Weng 2013).
Particularly controversial is whether antibiotic treatment should be given to all mothers or only to those at greatest risk of infection (Ehrenkranz 1990; Gilstrap 1988; Howey 1990; Suonio 1989). Women undergoing cesarean section can be divided into low‐ and high‐risk groups for infection, with women undergoing an elective (planned or primary) procedure at lowest risk (ACOG 2011). Women at high risk include those undergoing cesarean section after rupture of the membranes or onset of labor (ACOG 2011). It has been suggested that institutions with a low levels of baseline infections may see no impact of routine use of antibiotics, while institutions with high baseline infection rates may see a benefit. We were interested to see if there was a difference in effectiveness depending on whether the women were at low or high risk of infection and performed a subgroup analysis based on whether the cesarean section was a planned procedure (elective) or whether there was active labor or ruptured membranes (non‐elective).
This review will focus on whether antibiotics do more good than harm overall. Additional ways for trying to reduce post‐cesarean infections include: skin preparation at cesarean section (Hadiati 2012); surgical technique (Dodd 2014), double gloving or changing gloves (or both) before closure; peritoneal lavage; and vaginal antiseptic solution preparation (Haas 2013).
Objectives
To determine, from the best evidence available, the effectiveness of prophylactic antibiotics compared with placebo, or no treatment, given to women when undergoing a cesarean section for reducing the incidence of febrile morbidity, wound infection, endometritis, urinary tract infection or any serious infectious complication, and to assess potential maternal adverse effects and any impact on the infant, either short term or long term.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) to evaluate the effects of prophylactic antibiotics in women undergoing cesarean section were included. Quasi‐RCTs were also included. We planned to include cluster‐RCTs should any be identified but cross‐over trials are inappropriate for this question.
Types of participants
Women undergoing cesarean section, both elective (planned) and non‐elective/emergency.
Types of interventions
Trials were considered if they compared any prophylactic antibiotic regimen administered for cesarean section with placebo or no treatment.
Types of outcome measures
Primary outcomes
Maternal
Febrile morbidity (fever)
Wound infection (infection of the surgical incision)
Endometritis (inflammation of the lining of the womb)
Serious infectious complication (such as bacteremia, septic shock, septic thrombophlebitis, necrotizing fasciitis, or death attributed to infection)
Infant
Immediate adverse effects of antibiotics on the infant (unsettled, diarrhea, rashes)
Oral thrush (candidiasis)
Secondary outcomes
Maternal
Urinary tract infection
Adverse effects of treatment on the woman (e.g. allergic reactions, nausea, vomiting, diarrhea, skin rashes, yeast infections)
Length of stay in hospital
Infant
Length of stay in hospital
Long‐term adverse effects (e.g. general health; frequency of visits to hospital)
Immune system development (using a validated scoring assessment)
Additional outcomes
Development of bacterial resistance
Cost
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (31 July 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists at the end of papers for further studies.
We did not apply any language restrictions.
Data collection and analysis
For methods used in the previous update, please see Smaill 2010.
For this update (2014), the following methods, based on a standard template used by the Cochrane Pregnancy and Childbirth Group, were used.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy. Where participants were randomized to more than two treatment groups either the intervention groups were combined to create a single pair‐wise comparison or the no treatment group was divided approximately evenly into two or more groups and independent comparisons made between intervention and no treatment groups (see details in Characteristics of included studies).
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We have assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We have assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes, but an overall estimate of performance bias was reported.
We have assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes but an overall estimate of detection bias was reported.
We have assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We have assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as‐treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We planned to describe for each included study any important concerns we had about other possible sources of bias.
For this update the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following key outcomes for the main comparison of all cesarean sections and the subgroup of elective cesarean sections:
Wound infection
Endometritis
Serious infectious complications
Infant outcomes
GRADE profiler (GRADE 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardized mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomized trials
We did not identify any cluster‐randomized trials, but we would include cluster‐randomized trials along with individually‐randomized trials in the analysis if we identify any in future updates to this review. We would adjust their sample size using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we used ICCs from other sources, we would report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If, in future updates, we identify cluster‐randomized trials, we plan to synthesize the relevant information. We would consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely. We would also acknowledge heterogeneity in the randomization unit and perform a sensitivity analysis to investigate the effects of the randomization unit.
Cross‐over trials
Cross‐over trials are inappropriate for this question.
Dealing with missing data
For included studies, levels of attrition were noted. In a subsequent update of this review, we will include a sensitivity analysis, with poor quality studies with high levels of missing data excluded from the analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the I² and Tau² or Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We investigated reporting biases (such as publication bias) using funnel plots for the main analyses of the primary outcomes. In future updates, if asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
We planned that if there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we would use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary would be treated as the average range of possible treatment effects and the clinical implications of treatment effects differing between trials would be discussed. If the average treatment effect was not clinically meaningful, we would not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses to address specific questions about particular patient groups and types of interventions.
By antibiotic regimen (see Table 3 for classification based on class of antibiotic, spectrum of activity and drug combinations).
By type of surgery: (a) elective cesarean section; (b) non‐elective cesarean section; and (c) mixed or not defined. (Rupture of membranes for more than six hours or the presence of labor was used to differentiate a non‐elective cesarean section from an elective procedure.)
By time of administration: (a) before cord clamping; (b) after cord clamping; (c) not defined.
1. Classification of antibiotics.
| Penicillins | |||
| Natural penicillins | |||
| Penicillinase‐resistant penicillin | Cloxacillin | Extended spectrum of activity compared with natural penicillin | |
| Aminopenicillins | Ampicillin | ||
| Extended‐spectrum penicillins | Ticarcillin, pipericillin, carbenicillin, mezlocillin | ||
| Beta‐lactamase inhibitor combination | Sulbactam Augmentin |
||
| Cephalosporins | |||
| 1st generation | Cefazolin | ||
| 2nd generation | Cefamandole, cefuroxime | ||
| Cefamycins (2nd generation) | Cefoxitin, cefotetan | ||
| 3rd generation | |||
| 4th generation | |||
| Carbapenems and monobactams | |||
| Tetracyclines | |||
| Macrolides | |||
| Aminoglycosides | Gentamicin | ||
| Lincosamides | Clindamycin, lincomycin | ||
| Nitroimidazole | Metronidazole | ||
| Fluoroquinolones | |||
| Trimethoprim‐sulfamethoxazole | |||
| Aminoglycoside containing combination | |||
| Other antibiotic combination | |||
| Other regimens |
The following outcomes were used in subgroup analysis.
Maternal
Febrile morbidity (fever)
Wound infection (infection of the surgical incision)
Endometritis (inflammation of the lining of the womb)
Serious infectious complication (such as bacteremia, septic shock, septic thrombophlebitis, necrotizing fasciitis, or death attributed to infection)
Urinary tract infection
Infant
Immediate adverse effects of antibiotics on the infant (unsettled, diarrhea, rashes)
Oral thrush (candidiasis)
If we had identified substantial heterogeneity, we would have investigated it using the subgroup analyses. We would have considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it. We did assess subgroup differences by interaction tests available within RevMan (RevMan 2014). The results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value have been calculated.
Sensitivity analysis
We performed a sensitivity analysis to explore the effect of trial quality assessed by concealment of allocation (quasi‐randomized trials), with these poor quality studies being excluded from the analyses, in order to assess whether this made any difference to the overall result. We plan to explore in a subsequent update to this review the effect of other sources of bias on the estimate of outcomes by performing additional sensitivity analyses, e.g. examine the effects of only including studies where there was a low risk of allocation bias and selection bias (i.e. effective blinding of participants and personnel).
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group’s Trials Register identified 134 reports of 120 studies. Ninety‐five studies were included (Characteristics of included studies), 25 were excluded (Characteristics of excluded studies). We found no additional studies through searching reference lists.
Included studies
The 95 studies that met the inclusion criteria for this review enrolled over 15,000 women. For detailed information on the studies, see table of Characteristics of included studies. No study reported on baseline risk of infection before the intervention. Most studies adequately described the characteristics of the women who were enrolled, including details of the indication for cesarean section, mean duration of labor and membrane rupture and number of repeat sections. Several studies included a majority of women who were identified as from a low socioeconomic group, but other studies enrolled women who were not perceived to be at an increased risk of infection because of socioeconomic status. One study included information on the number of women who were HIV positive (Bagratee 2001). In no study were details on the incidence of bacterial vaginosis provided. One more recent study reported that there were no methicillin‐resistant Staphylococcus aureus infections in the study population (Witt 2011).
Setting
While the majority of the studies included in the review were conducted in developed countries (e.g. US, Western Europe, Scandinavia, Canada and New Zealand), studies were reported from developing countries including Sudan, Nigeria, Tunisia, Kenya, Zimbabwe, and South Africa as well as Mexico, Greece, Turkey, Israel, the Middle East, China and Malaysia.
Type of cesarean section
One objective of this review was to study the effect of prophylaxis in both elective and non‐elective cesarean sections, and strict definitions of an elective and non‐elective cesarean section were used by the authors of this review to categorize women and studies. In 17 studies (N = 3500), data on women undergoing an elective cesarean section were available (Adam 2005; Bagratee 2001; Dashow 1986; Duff 1982; Huam 1997; Jaffe 1984; Jakobi 1994; Kolben 2001; Lemus 2005; Mahomed 1988; Rizk 1998; Rothbard 1975; Rouzi 2000; Shah 1998; Sziller 1994; Ujah 1992; Wu 1991). In 22 studies (N = 2500), there were data on non‐elective procedures (Conover 1984; D'Angelo 1980; Elliott 1986; Freeman 1982; Fugere 1983; Gibbs 1981; Harger 1981; Hawrylyshyn 1983; Heilmann 1984; Jaffe 1984; Leonetti 1989; Moodley 1981; Ross 1984; Rothbard 1975; Ruiz‐Moreno 1991; Scarpignato 1982; Schedvins 1986; Tzingounis 1982; Weissberg 1971; Wong 1978; Work 1977; Young 1983). Two studies included both women having elective cesareans and non‐elective cesareans (Jaffe 1984; Rothbard 1975). The remaining, and the majority of studies did not differentiate between an elective or non‐elective procedure, or the definitions used were not consistent with those used in this review; these have been grouped as 'both' or 'undefined'. Often a repeat section had been classified as elective by the study authors, but it was not always evident that all of these women were indeed not in labor and often the duration of membrane rupture was unclear. Fifty‐nine studies (N = 8500) included women whose procedures were classified as undefined type of cesarean section (Adeleye 1981; Allen 1972; Apuzzio 1982; Battarino 1988; Bibi 1994; Bilgin 1998; Bourgeois 1985; Carl 2000; Chan 1989; Cormier 1989; Dashow 1986; De Boer 1989; Dillon 1981; Duff 1980; Engel 1984; Escobedo 1991; Gall 1979; Ganesh 1986; Garcia 1992; Gerstner 1980; Gibbs 1972; Gibbs 1973; Gordon 1979; Gummerus 1984; Hager 1983; Hagglund 1989; Ismail 1990; Jaffe 1985; Karhunen 1985; Kellum 1985; Kreutner 1978; Kristensen 1990; Lapas 1988; Levin 1983; Lewis 1990; McCowan 1980; Miller 1968; Moro 1974; Morrison 1973; Ng 1992; Oestreicher 1987; Padilla 1983; Phelan 1979; Polk 1982; Racinet 1990; Reckel 1985; Rehu 1980; Roex 1986; Rouzi 2000; Rudd 1981; Saltzman 1985; Sokolowski 1989; Stage 1982; Stiver 1983; Tully 1983; Turner 1990; Walss Rodriguez 1990; Witt 2011; Yip 1997). Two studies reported both elective and in labor/high risk (Dashow 1986) or emergency cesarean sections (Rouzi 2000); the in labor/high risk and emergency sections, however, did not meet our definition of non‐elective and these have been classified as "undefined" .
Timing of antibiotic administration
Antibiotics for prophylaxis were administered intravenously either at the start of the operative procedure ("before cord") or at or after clamping of the cord. In 40 studies (N = 5600), data on outcomes were available when the antibiotic had been administered before clamping of the cord (Adam 2005; Adeleye 1981; Allen 1972; Bibi 1994; Chan 1989; De Boer 1989; Duff 1980; Duff 1982; Freeman 1982; Gall 1979; Gerstner 1980; Gibbs 1972; Gibbs 1973;Gordon 1979; Hagglund 1989; Heilmann 1984; Huam 1997; Jaffe 1984; Jaffe 1985; Kreutner 1978; Lapas 1988; Magro 1983; Mahomed 1988; McCowan 1980: Miller 1968; Moodley 1981; Moro 1974; Morrison 1973; Ng 1992; Phelan 1979; Reckel 1985; Rehu 1980; Ross 1984; Rothbard 1975; Stage 1982; Turner 1990; Tzingounis 1982; Witt 2011; Work 1977; Yip 1997). This was variably described as "pre‐operatively", "with induction of anaesthesia" or "before clamping of the cord". In 51 studies (N = 8400), the antibiotic was administered at or after cord clamping (Apuzzio 1982; Bagratee 2001; Battarino 1988; Bourgeois 1985; Bilgin 1998; Carl 2000; Conover 1984; Cormier 1989; D'Angelo 1980; Dashow 1986; Dillon 1981; Elliott 1986; Engel 1984; Escobedo 1991; Fugere 1983; Ganesh 1986; Garcia 1992; Gibbs 1981; Gordon 1979; Gummerus 1984; Hager 1983; Harger 1981; Hawrylyshyn 1983; Ismail 1990; Jakobi 1994; Karhunen 1985; Kellum 1985; Kristensen 1990; Lemus 2005; Leonetti 1989; Levin 1983; Lewis 1990; Oestreicher 1987; Polk 1982; Racinet 1990; Rizk 1998; Roex 1986; Rouzi 2000; Rudd 1981; Ruiz‐Moreno 1991; Saltzman 1985; Shah 1998; Sokolowski 1989; Stiver 1983; Sziller 1994; Tully 1983; Walss Rodriguez 1990; Witt 2011; Wong 1978; Wu 1991; Young 1983). Included in this group were studies where irrigation of the peritoneal or uterine cavity with an antibiotic containing solution was compared with either saline irrigation or no irrigation (Bourgeois 1985; Carl 2000; Conover 1984; Dashow 1986; Elliott 1986; Kellum 1985; Levin 1983; Lewis 1990; Rudd 1981; Wu 1991). There were six studies where there was insufficient information to know when the antibiotic had been administered, e.g. "operatively" or the results had been combined and these have been grouped together as "timing not defined" (Kolben 2001; Padilla 1983; Scarpignato 1982; Schedvins 1986; Ujah 1992; Weissberg 1971). In two studies, results were available for antibiotic administration both before and after clamping of the cord (Gordon 1979; Witt 2011).
Classes of antibiotics
The antimicrobial agents most often used in the trials included ampicillin, a first generation cephalosporin (usually cefazolin), a second generation cephalosporin (cefamandole or cefuroxime) or a cefamycin (cefoxitin, cefotetan), metronidazole, penicillins with an extended spectrum of activity (e.g. ticarcillin, mezlocillin or pipericillin), a beta‐lactam/beta‐lactamase inhibitor combination and an aminoglycoside‐containing combination; seeCharacteristics of included studies for a classification of the antimicrobial agent used by antibiotic class. The penicillins have been divided into natural penicillin, penicillinase‐resistant penicillins, aminopenicilins (ampicillin), extended spectrum penicillins which include carboxypenicillins (carbenicillin, ticarcillin) and ureidopenicillins (mezlocillin, pipericillin) and beta‐lactam‐beta‐lactamase inhibitor combinations. The second generation cephalosporins include the cefamycins (cefoxitin and cefotetan) that have extended anaerobic coverage. In one study antimicrobial prophylaxis was administered by rectal suppository (De Boer 1989) and in four studies follow‐up doses were administered by rectal suppository (Gerstner 1980; McCowan 1980; Ross 1984) or vaginal tablet (Sokolowski 1989). The duration of the post‐operative treatment course varied from a single intravenous dose to as long as a week. While most studies were published in the 1980s, new studies have continued to be performed in the 1990s and the last study was published as recently as 2011.
Assessing outcomes
The clinical criteria listed to define endometritis were consistent across trials. Febrile morbidity is a standard obstetrical outcome and was generally consistently reported although there was some variation in the exact criteria used for height of fever, interval between febrile episodes and interval from the operative procedure. Urinary tract infection generally meant a positive urine culture; symptoms related to the urinary tract were rarely required to be present. Wound infection usually was a clinical diagnosis and generally included induration, erythema, cellulitis or various degrees of drainage. A positive microbiological diagnosis was rarely required for the diagnosis of either wound infection or endometritis. There was no consistent approach to the definition of serious infectious morbidity. For this review, all episodes of bacteremia have been classified as serious as have other complications such as pelvic abscess, pelvic thrombophlebitis and peritonitis. Some studies included other outcomes, e.g. need for additional antibiotic use and other infections, e.g. pneumonia. Some provided a measure of the fever as a 'fever index' which incorporated both the height of the fever and its duration. Where the duration of maternal hospital stay with its standard deviation was reported, this has been included.
Side effects
Very few studies appeared to have consistently sought maternal side effects or neonatal/infant side effects and similarly it was a minority of studies that collected data on infectious complications after discharge.
Costs
Three studies compared the cost of antibiotics between groups (Bibi 1994; Kristensen 1990; Racinet 1990). See Characteristics of included studies for details.
Excluded studies
Of those studies excluded from the analysis, some were because clinical outcomes for the women undergoing cesarean section were not reported separately or all women had received some form of antibiotic treatment and then were randomized to another intervention. For some studies, although the trial was initially randomized, part‐way through the study the placebo arm was dropped. Due to the results on the initially randomized part of the study not being available, these studies were not included (See table of Characteristics of excluded studies for further details).
Risk of bias in included studies
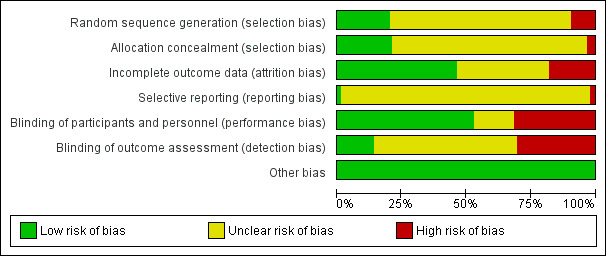
The methodological quality of the trials was mostly unclear, explained in large part because the studies were undertaken a number of years ago, before the recent understanding of sources of bias in randomized controlled trials (Figure 1; Figure 2).
1.
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
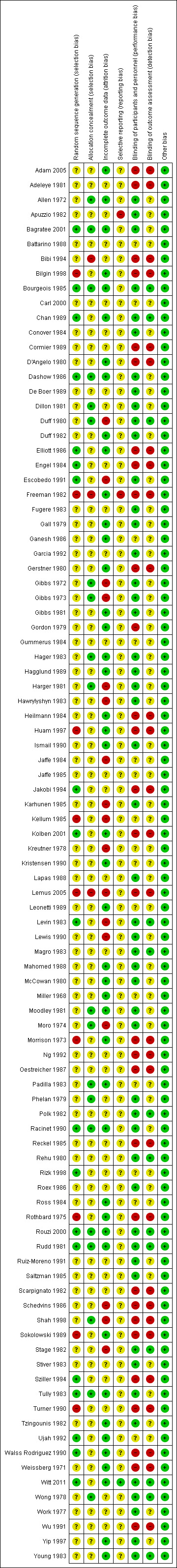
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
In less than 20% of studies, we judged there was a low risk of selection bias based on an adequate generation of a randomized sequence, such as referring to a random number table or "drawing lots". Most studies, however, did not describe the sequence generation process and were assessed as "unclear". Several studies where participants were allocated to treatment group by date of birth or hospital number were classified as quasi‐RCTs and judged at high risk of selection bias.
Blinding
In almost a third of studies there was no blinding and the control group received no treatment. Performance bias and detection bias were judged as low when there was an adequate description of the steps taken to ensure blinding. However, although many studies were described as "double‐blind, placebo‐controlled", there was often insufficient information provided to be confident all members of the study team were blinded to allocation, that the outcome assessment was performed without knowledge of the treatment group, nor certainty that the blind could not be broken. The outcomes of wound infection and endometritis, which require clinical judgement, could be influenced by lack of blinding.
Incomplete outcome data
In most studies, all women who were initially randomized were included in the outcomes and an intention‐to‐treat analysis was performed. Where dropouts were reported, insufficient data were usually provided for them to be included in an intention‐to‐treat analysis. Where the group allocation of dropouts was not provided, there was the possibility that there may have been selective withdrawals from one or other of the groups. There were some studies where a discrepancy in the numbers allocated to the randomized groups, unlikely to have occurred by chance, was not accounted for. In most cases the numbers in the placebo group were smaller than those in the treatment group, raising the possibility of selective withdrawals not mentioned in the published report.
Selective reporting
We judged this as unclear for all studies as there were no study protocols available. However, it does appear for most of the studies that all the expected outcomes were included.
Other potential sources of bias
We judged this as low for all studies as no other important sources of bias were identified.
Effects of interventions
1. Antibiotic prophylaxis versus no prophylaxis (Analyses 1.1 to 1.7)
The overall findings were as follows:
Primary outcomes
There were reductions in all the maternal primary outcomes: febrile morbidity (average risk ratio (RR) 0.45; 95% confidence interval (CI) 0.40 to 0.51, 56 studies, 9046 women (Tau² = 0.07; Chi² = 89.58, df = 55 (P = 0.002); I² = 39% (Analysis 1.1); wound infection (RR 0.40; 95% CI 0.35 to 0.46, 82 studies, 14,407 women (Chi² = 102.56, df = 79 (P = 0.04); I² = 23% (Analysis 1.2); endometritis (RR 0.38; 95% CI 0.34 to 0.42), 83 studies, 13,548 women (Chi² = 90.59, df = 79 (P = 0.19); I² = 12% ( Analysis 1.3); and serious infectious morbidity (RR 0.31; 95% CI 0.20 to 0.49, 32 studies, 6159 women (Chi² = 14.26, df = 27 (P = 0.98); I² = 0% (Analysis 1.4).
1.1. Analysis.
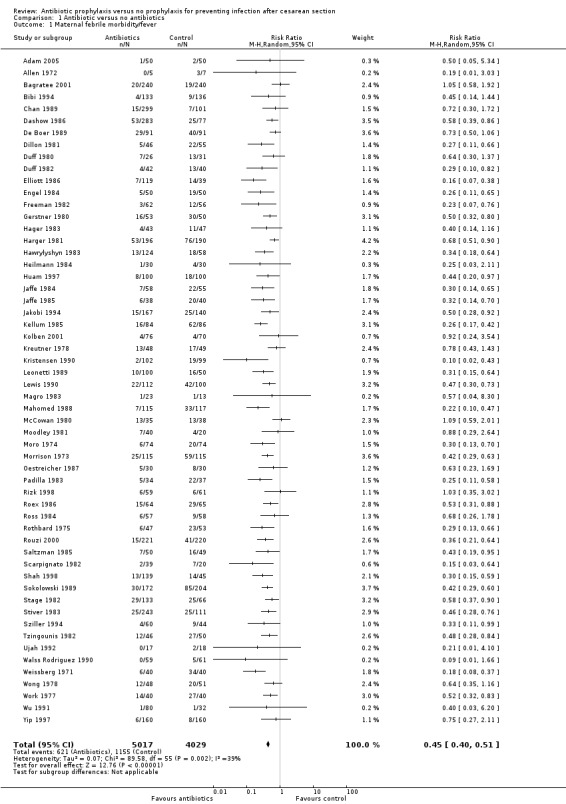
Comparison 1 Antibiotic versus no antibiotics, Outcome 1 Maternal febrile morbidity/fever.
1.2. Analysis.
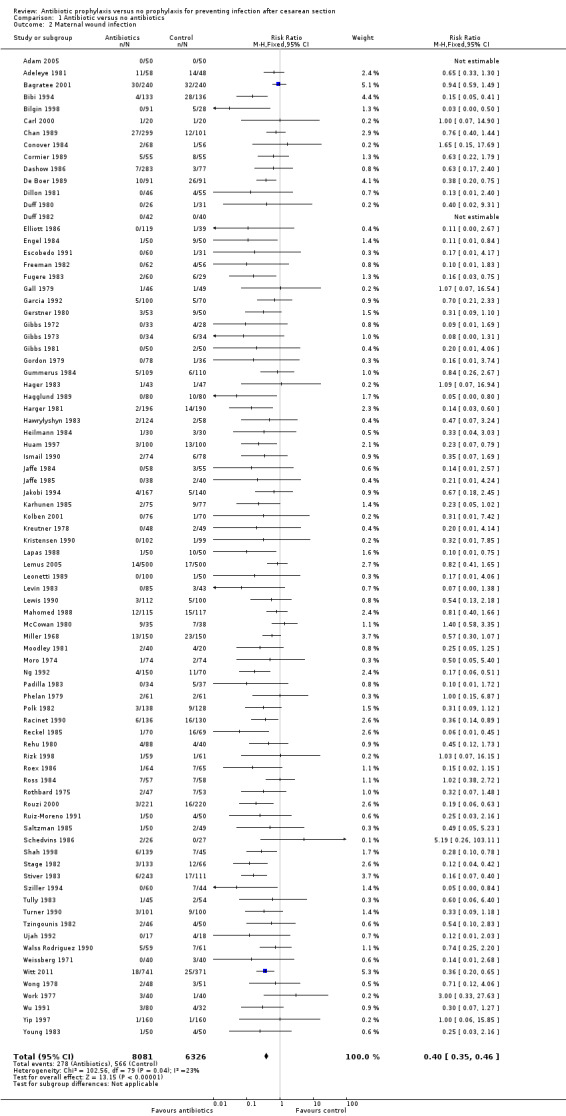
Comparison 1 Antibiotic versus no antibiotics, Outcome 2 Maternal wound infection.
1.3. Analysis.
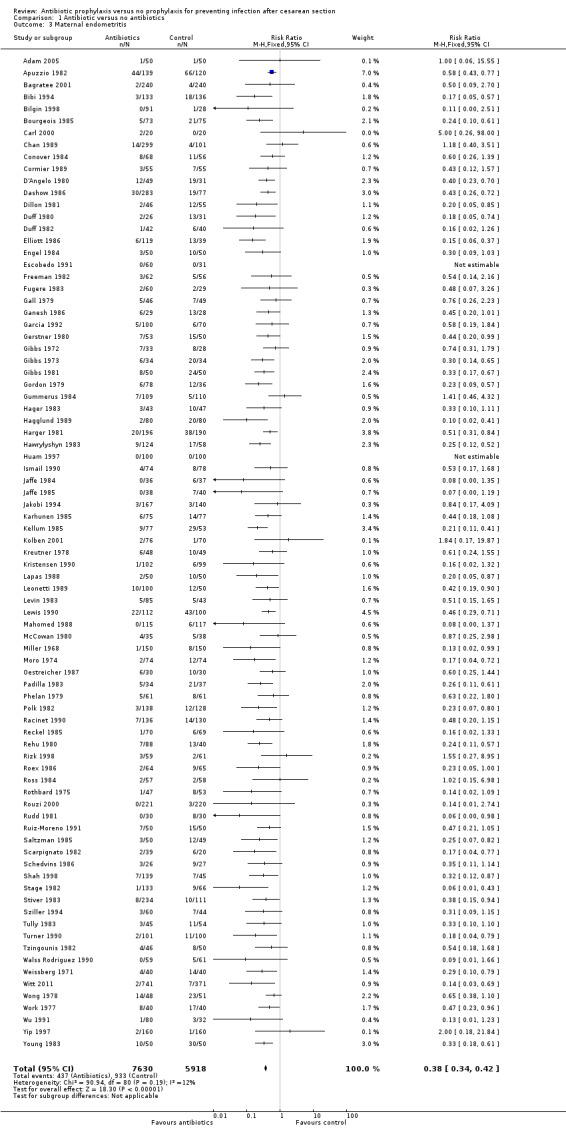
Comparison 1 Antibiotic versus no antibiotics, Outcome 3 Maternal endometritis.
1.4. Analysis.
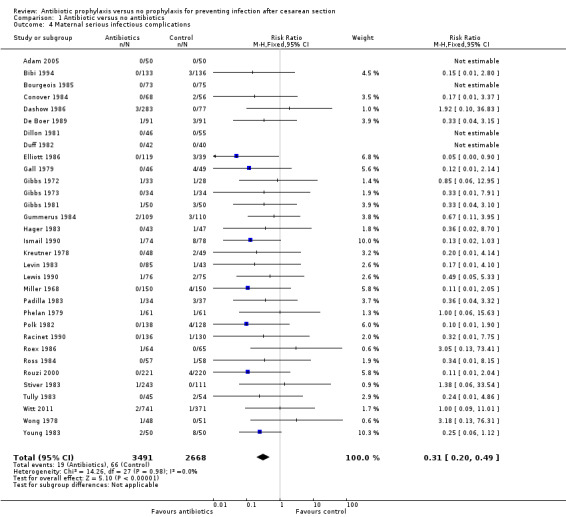
Comparison 1 Antibiotic versus no antibiotics, Outcome 4 Maternal serious infectious complications.
There was no evidence of substantial heterogeneity amongst the studies contributing to each of the outcomes reported except for what we judged a moderate degree of heterogeneity amongst those studies reporting febrile morbidity. This could be explained by different criteria used in the studies to define fever/ febrile morbidity. See Characteristics of included studies.
There were no data in any of the studies on the two infant primary outcomes of immediate adverse effects and infant thrush.
Secondary outcomes
There were reductions in maternal urinary tract infection (RR 0.56; 95% CI 0.49 to 0.65, 66 studies, 10,928 women (Analysis 1.5) . Only 13 studies collected data on maternal adverse effects; more adverse events were observed in the treatment group (RR 2.43; 95% CI 1.00 to 5.90), 2131 women (Analysis 1.6). The most common side effect was rash, followed by phlebitis at the site of the intravenous infusion. There were no serious drug‐related adverse events reported. The difference in maternal length of stay in hospital (average mean difference (MD) ‐0.46, 95% CI ‐0.65 to ‐0.28), 3168 women (Analysis 1.7), reported in 19 studies was judged not clinically important.
1.5. Analysis.
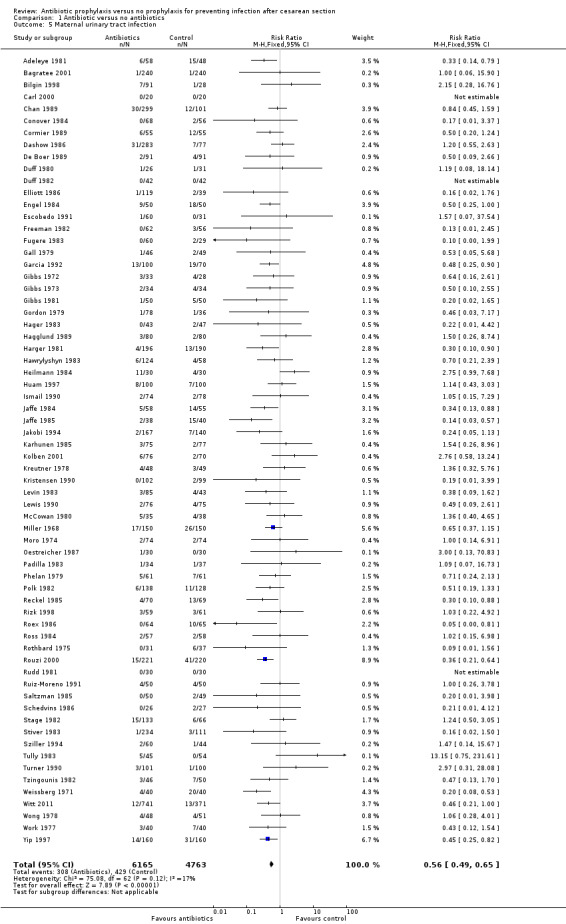
Comparison 1 Antibiotic versus no antibiotics, Outcome 5 Maternal urinary tract infection.
1.6. Analysis.
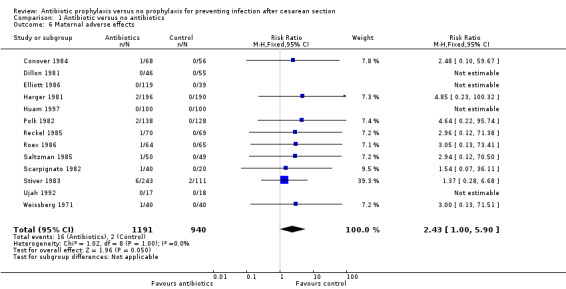
Comparison 1 Antibiotic versus no antibiotics, Outcome 6 Maternal adverse effects.
1.7. Analysis.
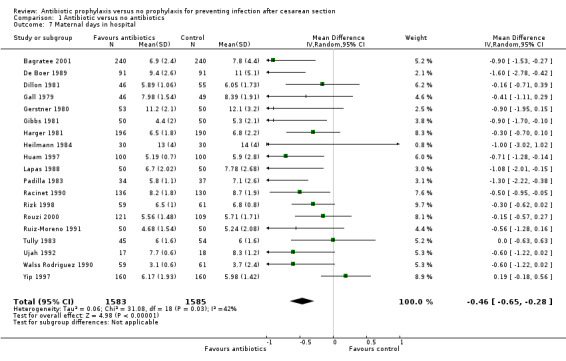
Comparison 1 Antibiotic versus no antibiotics, Outcome 7 Maternal days in hospital.
There were no data in any of the studies on the other secondary outcomes.
Reporting bias
There was a potential for publication bias in the assessment of febrile morbidity, as judged by visual inspection of the funnel plot (Figure 3); however, we estimated that any reporting bias was unlikely to influence the results because of the large number of participants in the symmetrical part of the plot. There was no funnel plot asymmetry for the other primary outcomes (Figure 4; Figure 5; Figure 6).
3.
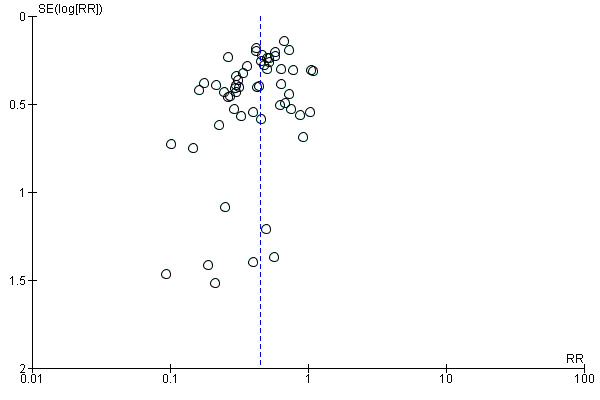
Funnel plot of comparison: 1 Antibiotic versus no antibiotics, outcome: 1.1 Maternal febrile morbidity/fever.
4.
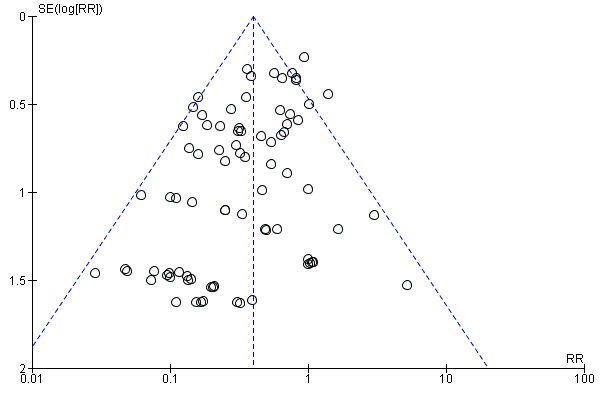
Funnel plot of comparison: 1 Antibiotic versus no antibiotics, outcome: 1.2 Maternal wound infection.
5.
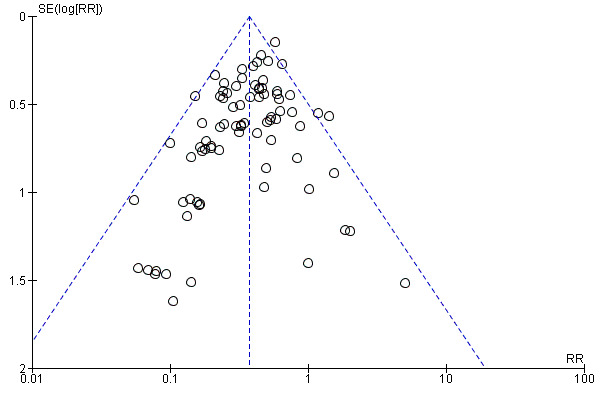
Funnel plot of comparison: 1 Antibiotic versus no antibiotics, outcome: 1.3 Maternal endometritis.
6.
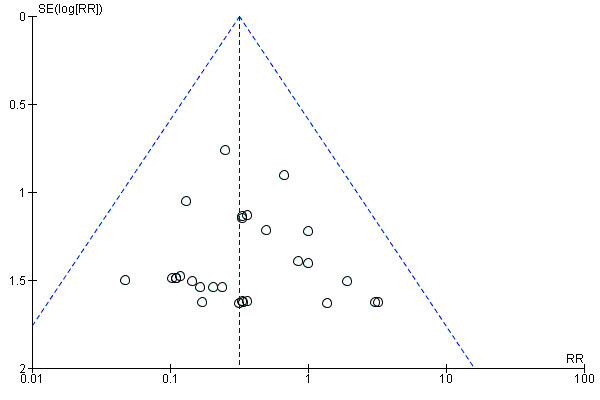
Funnel plot of comparison: 1 Antibiotic versus no antibiotics, outcome: 1.4 Maternal serious infectious complications.
Study quality
We undertook a sensitivity analysis on the primary outcomes by study quality, omitting the nine quasi‐RCTs (Bilgin 1998; Freeman 1982; Huam 1997; Kellum 1985; Lemus 2005; Morrison 1973; Rothbard 1975; Sokolowski 1989; Turner 1990). The overall findings remained very similar with reductions for all the primary outcomes: febrile morbidity (average RR 0.47; 95% CI 0.41 to 0.53, 50 studies, 7852 women; wound infection (average RR 0.40; 95% CI 0.35 to 0.46, 76 studies, 12,669 women; endometritis (RR 0.39; 95% CI 0.35 to 0.43, 77 studies, 12,680 women). Serious infectious morbidity remained the same as the analysis contained no quasi‐RCTs.
2. Antibiotic prophylaxis versus no prophylaxis, subgroups by antibiotic regimen (Analyses 2.1 to 2.7)
There were no studies that reported on treatment with monotherapy with a penicillinase‐resistant penicillin, fourth generation cephalosporin, carbapenem, tetracycline, macrolide, and aminoglycosides. Approximately two thirds of studies evaluated treatment with a first or second generation cephalosporin, including cefamycins, or ampicillin.
For all subgroups based on antibiotic regimen there were reductions in the maternal primary outcomes and maternal urinary tract infections. By inspection of the graphs visually there was no evidence of a clinically important difference by drug class. The interaction test did not suggest a difference between subgroups for the primary outcomes of wound infection (Chi² = 17.77; P = 0.17; I² = 26.8%), serious infectious outcomes (Chi² = 3.07; P = 0.93; I² = 0%) and urinary tract infection (Chi² = 16.3; P = 0.13; I² = 32.5%), however the interaction test did show a difference among subgroups for the primary outcomes of febrile morbidity (Chi² = 45.8; P < 0.00001; I² = 73.8%) and endometritis (Chi² = 21.16; P = 0.07; I² = 38.6%). There was no evidence that a regimen with a broader spectrum of activity was any better than a drug with a narrow spectrum of activity or that any particular antibiotic regimen was ineffective. The largest risk reductions for wound infection were seen with the extended spectrum penicillin group (RR 0.18, 95% CI 0.09 to 0.39) and aminoglycoside containing regimens (RR 0.17 95% CI 0.08 to 0.34) (Analysis 2.2). For the first generation cephalosporins, the current recommended antibiotic for prophylaxis, the risk ratio for wound infection was 0.38 (95% CI 0.28 to 0.53) (Analysis 2.2). There was only one study that reported on lincomycin and no studies reported on clindamycin or a macrolide, which however are often given as an alternatives for prophylaxis in women who are allergic to penicillin. The reduction in the incidence of endometritis was similar amongst the different drug regimens (Analysis 2.3). The smallest reduction in endometritis was seen for beta lactamase inhibitor combinations (RR 0.67, 95% CI 0.27 to 1.66); for the first generation cephalosporins the relative risk was 0.42 (95% CI 0.33 to 0.54). Any comparison between regimens has, however, to be interpreted with caution given that the results are observational and not based on randomized comparisons. Please refer to the meta‐analysis of studies that compared different regimens (Alfirevic 2010).
2.2. Analysis.
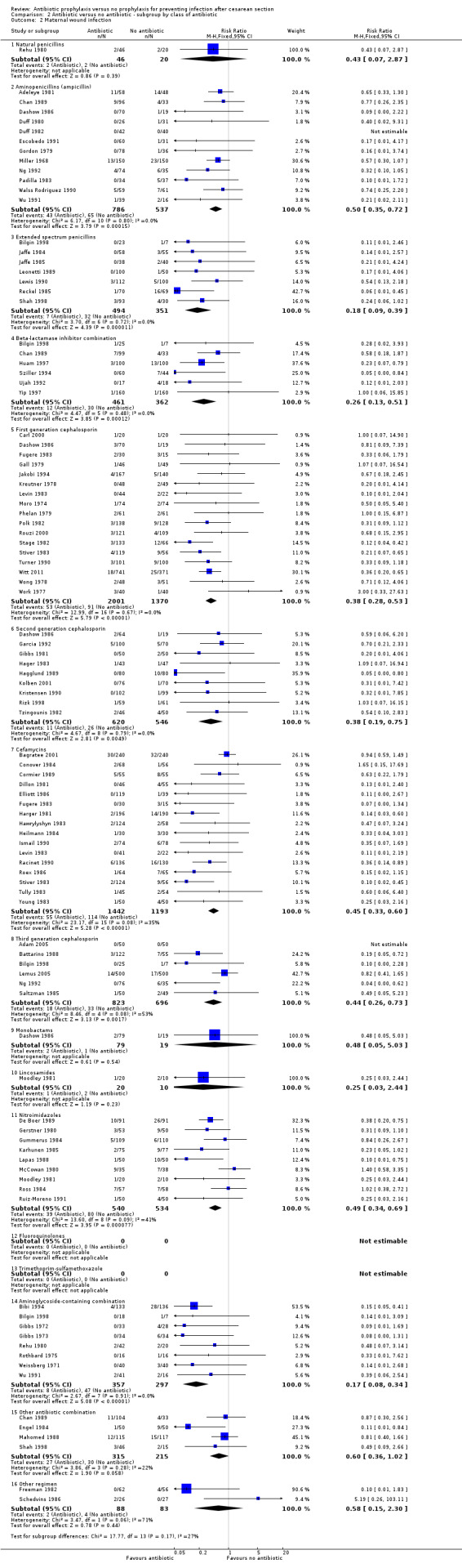
Comparison 2 Antibiotic versus no antibiotic ‐ subgroup by class of antibiotic, Outcome 2 Maternal wound infection.
2.3. Analysis.
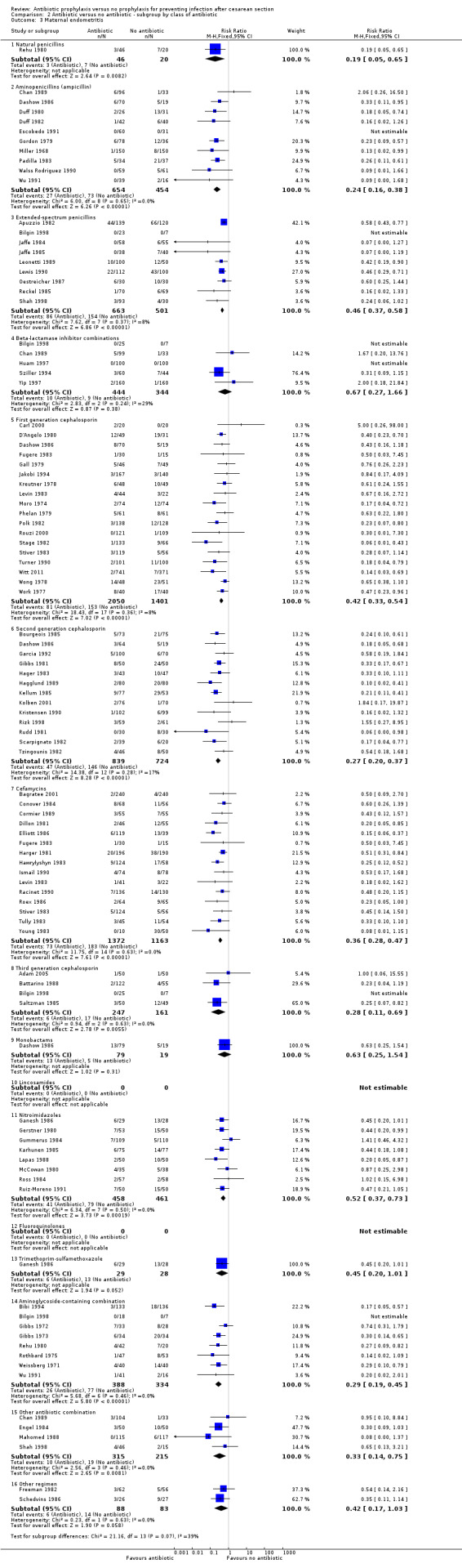
Comparison 2 Antibiotic versus no antibiotic ‐ subgroup by class of antibiotic, Outcome 3 Maternal endometritis.
3. Antibiotic prophylaxis versus no prophylaxis, subgroups by type of cesarean section (Analyses 3.1 to 3.7)
In both elective and emergency cesarean sections as well as the "both or not defined" subgroup there was a reduction in febrile morbidity, wound infection, endometritis and urinary tract infection although there were insufficient data to assess any differential effect on serious infectious complications. For elective cesarean sections, the risk ratio for wound infections was 0.62, 95% CI 0.47 to 0.82 (Analysis 3.2) and for endometritis the RR was 0.38, 95% CI 0.24 to 0.61 (Analysis 3.3). We inspected the graphs visually and saw no difference in maternal febrile morbidity, wound infection or endometritis, among the three groups and as well the confidence intervals for the summary estimates overlapped, however, the interaction test did show a difference among subgroups for the outcome of wound infection (Chi² = 13.53; P = 0.001; I² = 85.2%) and maternal urinary tract infection ((Chi² = 6.42; P = 0.04; I² = 68.8%), but no difference for the outcomes of febrile morbidity (Chi² = 0.47; P = 0.79; I² = 0%), endometritis (Chi² = 0.36; P = 0.84; I² = 0%), or serious infectious outcome (Chi² = 0.62; P = 0.73; I² = 0%).
3.2. Analysis.
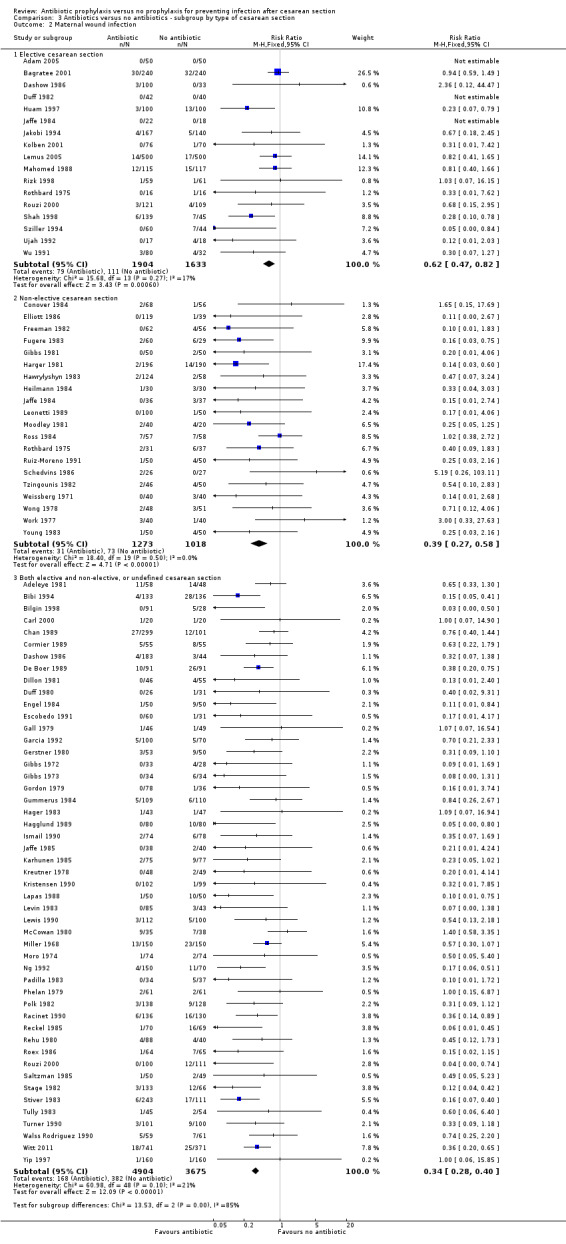
Comparison 3 Antibiotics versus no antibiotics ‐ subgroup by type of cesarean section, Outcome 2 Maternal wound infection.
3.3. Analysis.
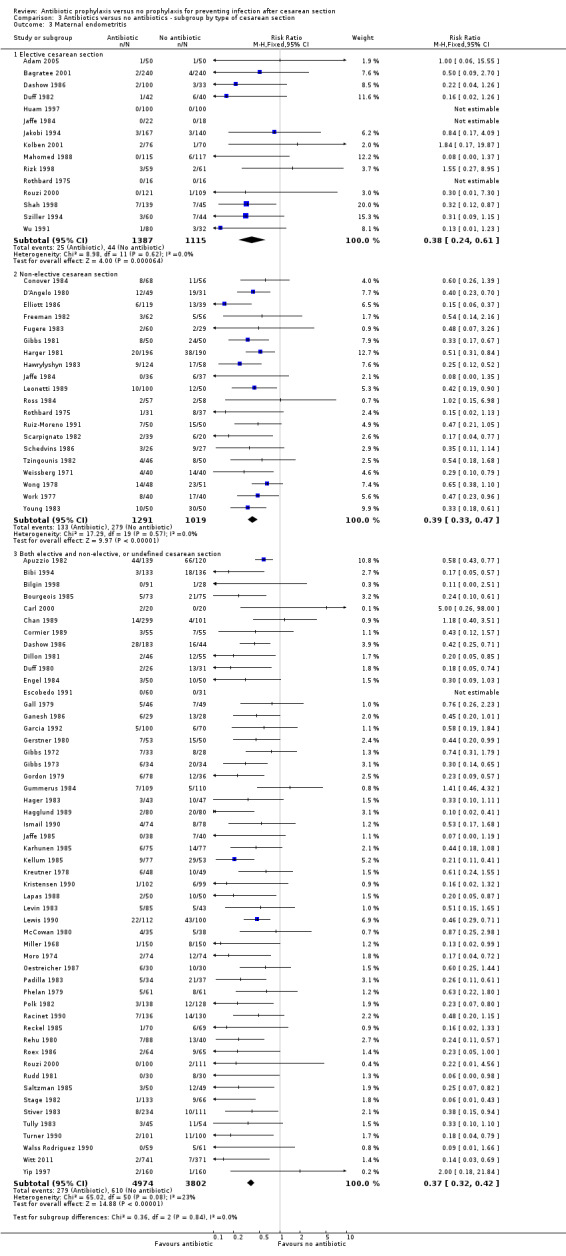
Comparison 3 Antibiotics versus no antibiotics ‐ subgroup by type of cesarean section, Outcome 3 Maternal endometritis.
4. Antibiotic prophylaxis versus no prophylaxis, subgroups by timing of administration (Analyses 4.1 to 4.7)
We inspected the graphs visually and found no difference in wound infection, endometritis or maternal urinary tract infection among the three groups and the confidence intervals for the summary estimates overlapped. As well, the interaction test did not suggest there was any real difference for the outcomes of wound infection (Chi² = 0.43; P = 0.81; I² = 0%), endometritis (Chi² = 3.74; P = 0.15; I² = 46.6%) and maternal urinary tract infection (Chi² = 2.07; P = 0.36; I² = 3.4%). However, the interaction test did suggest there was a difference among subgroups for the outcome of febrile morbidity (Chi² = 8.40; P = 0.01; I² = 76.2%). There was no difference among groups based on the interaction test for the outcome of serious maternal infectious complications (Chi² = 0.13; P = 0.94; I² = 0%), although there were insufficient data to conclude this with certainty. From these analyses there was no evidence that administering antibiotics before surgery was associated with a better outcome, but this conclusion needs to be interpreted with caution given that the results are observational and not based on randomized comparisons.
Other considerations
Infant
Infant outcomes were infrequently reported. No study reported on any long‐term adverse effects on the infant or effect of antibiotics on the infant immune system. In addition, no studies reported on the incidence of oral candidiasis (thrush) in babies, which we had categorized as an adverse outcome.
Where Apgar scores were reported, there were no differences between the treatment and control groups (Adam 2005; Gordon 1979; Ng 1992; Rouzi 2000). One study collected information on birthweight, number of days in hospital, admission to neonatal intensive care, early neonatal death, respiratory distress syndrome and neonatal sepsis and there was no difference between the treatment and control groups (Rouzi 2000) and another study reported that neonatal outcomes (respiratory distress syndrome, intracranial hemorrhage, necrotizing enterocolitis, sepsis and neonatal death) were not different among treatment groups (Witt 2011). Some authors stated there were no complications in the babies due to drug administration, without further details (Gordon 1979; Moodley 1981) and that the administration of antibiotics did not interfere with routine pediatric cultures (Gall 1979) or the evaluation of newborn sepsis (Duff 1980).
There were few neonatal deaths and where they were reported, no relationship to the administration of antibiotic was reported (Adam 2005; De Boer 1989).
Only one study reported on infant outcomes at four weeks and in that study the three infants who had complications were all in the control group (Gordon 1979).
Costs
Three studies reported post‐operative antibiotic costs. Drug costs were lower in the group receiving prophylaxis compared with the control group in one study (Kristensen 1990) but higher in the other two (Bibi 1994; Racinet 1990). SeeCharacteristics of included studies table for details of costs.
Resistance
Changes in bacterial flora and the development of antibiotic resistant bacteria with the administration of antibiotics was not systematically collected in the studies included in this review, but several studies included detailed microbiological investigations, comparing the results of aerobic and anaerobic culture of the genital tract before and after the surgery and reporting on antimicrobial resistance in organisms associated with infection (Engel 1984; Fugere 1983; Gibbs 1981; Harger 1981; Ismail 1990; Karhunen 1985; Kreutner 1978; Miller 1968; Moro 1974; Rothbard 1975; Roex 1986; Stiver 1983).
There is a shift in the bacterial flora following the surgical procedure itself and return to the non‐pregnant state and even in the control groups more gram positive aerobic organisms (including staphylococcal species and enterococci) were observed post‐operatively (Engel 1984). Antibiotic prophylaxis was associated with increases in enterococci and gram‐negative aerobic organisms (Engel 1984; Fugere 1983; Gibbs 1981; Kreutner 1978); cefazolin was associated with more anaerobic isolates (Engel 1984; Fugere 1983; Kreutner 1978) and cefoxitin and cefamandole with a decrease in anaerobic isolates (Engel 1984; Gibbs 1981).
Given that most regimens included a cephalosporin which has no activity against enterococci, it is not surprising that most studies reported significant increases in enterococcal colonization (Gibbs 1981; Ismail 1990; Stiver 1983). Harger reported that the isolates from infected sites in cefoxitin infected women showed a relative predominance of enterococci (Harger 1981). Ismail reported that enterococcal sepsis occurred in one patient and three others had significant enterococcal bacteriuria or urinary tract infection (Ismail 1990).
There were very few reports of resistant organisms developing following prophylaxis. No cefoxitin resistant strains of Enterobacteriaceae were isolated from stool samples after prophylaxis (Ismail 1990). In one study, there were more ampicillin resistant urinary tract infections when ampicillin was used for prophylaxis compared with control (9/17 versus 8/26) (Miller 1968). Rothbard reported one infection with an organism resistant to cephalothin and kanamycin used for prophylaxis (Rothbard 1975) and Duff reported an endometrial culture that grew Klebsiella pneumoniae resistant to ampicillin (Duff 1980). Engel reported urinary tract infections with mezlocillin resistant organisms (5/9) after mezlocillin prophylaxis and observed colonization with mezlocillin resistant strains of E. coli in cultures from the cervix (Engel 1984). In one study of cephalothin, all the organisms causing infection in the antibiotic group were described as sensitive to cephalothin in vitro (Moro 1974). In a study of cefoxitin prophylaxis, it was observed that the changes in endogenous flora were not associated with overgrowth of resistant pathogens, such as Pseudomonas, enterococci or Enterobacter (Roex 1986) and Karhunen reported no superinfections with resistant anaerobic organisms when tinidazole was used for prophylaxis (Karhunen 1985). Stiver confirmed that there was no increase in nosocomial infection (Stiver 1983).
Discussion
Summary of main results
This review included 95 studies that evaluated the effect of antibiotics for preventing infection after cesarean section in over 15,000 women. Compared with placebo or no treatment, the use of prophylactic antibiotics in women undergoing cesarean section reduced the incidence of wound infection and endometritis by around 60% and serious maternal infectious complications were reduced by 70%. See Table 1. When only studies that included women undergoing an elective cesarean section were analyzed, wound infections were reduced by 40% and endometritis by 60%. See Table 2. Similar estimates of effect were seen whether the antibiotics were administered before the cord was clamped or after.
There were no data on which to estimate the effect of maternal administration of antibiotics on infant outcomes. No studies systematically collected and reported on adverse infant outcomes nor the effect of antibiotics on the developing infant immune system. No studies reported on the incidence of oral candidiasis (thrush) in babies. Maternal adverse effects were also rarely described.
The effect of different antibiotic regimens was studied and similar reductions in the incidence of infections seen for most of the antibiotics and combinations studied.
Febrile morbidity is common after cesarean section and, although not judged a clinically important outcome, it was reduced with the use of prophylactic antibiotics. Few of these women will have positive bacterial cultures or a specific indication for antimicrobial treatment, but these women often have specimens collected and empiric antibiotic therapy started. There was a similar reduction in the incidence of urinary tract infections.
Overall completeness and applicability of evidence
These studies enrolled over 15,000 women extending over a period of more than 40 years, but only seven studies were reported since 2000. The studies varied in setting (both low‐income and high‐income countries), antibiotic regimen, risk of infection and definitions of outcomes, but we did not find evidence of statistically important heterogeneity in the measurement of effect among the studies. The reductions in infection seen are clinically important. The results of these studies have been generalized to the whole population of women undergoing cesarean section and based on the steady accumulation of evidence have been incorporated into all recent guidelines (ACOG 2011; NICE 2011; SOGC 2010).
Inconsistent adherence to policies for administering antibiotic prophylaxis are reported (Huskins 2001; Mah 2001; Pedersen 1996) but simple quality improvement methods have been demonstrated to improve adherence with overall and timely administration of prophylaxis and reduce the infection rate (Weinberg 2001). It was also shown, in this study (Weinberg 2001) that a program that introduced a policy of universal prophylaxis for all women undergoing a cesarean section was more effective than one that required the obstetrician to decide whether a woman was high risk and mandated prophylaxis only for the high‐risk women.
A statistically significant reduction in all the primary outcomes (febrile morbidity, wound infection, endometritis, serious maternal outcomes) was seen whether the antibiotic was administered before the clamping of the cord or after clamping of the cord. There was no significant difference in the estimates for these outcomes by the timing of administration and the confidence intervals were overlapping. It has, however, been shown that the lowest risk of surgical wound infection is associated with antibiotics administered in the pre‐operative period as compared with the perioperative or post‐operative period (Classen 1992). Although studies have not show an increase in infectious outcomes when the antibiotic was administered after the cord was clamped (Cunningham 1983; Gordon 1979; Wax 1997), meta‐analyses of randomized controlled trials concluded that there was strong evidence that antibiotic prophylaxis given before skin incision decreased the incidence of postpartum endometritis and total infectious morbidities compared with after the cord was clamped (Baaqeel 2013; Costantine 2008). Pre‐operative administration of antibiotics did not significantly affect proven neonatal sepsis, suspected sepsis or neonatal intensive care unit admissions. In a retrospective study on the effect of a change in policy to administer prophylactic antibiotics before skin incision, the overall rate of surgical site infections fell from 6.2% to 2.5% (Kaimal 2008).
Adverse effects
Maternal side effects were not consistently collected nor reported. Generally the side effects of a single antibiotic dose are mild, but rarely serious allergic reactions can occur. Although the risk of side effects reported in these studies was low, these data were incompletely collected, making it difficult to know accurately the incidence of the adverse effects of treatment and truly judge the benefits and harms of the intervention.
Infant outcomes were rarely systematically collected but when they were reported there was no evidence of any adverse effects associated with the administration of antibiotic. There is evidence that antibiotics given near or shortly after birth can affect the infant gut flora, with the potential to impact mucosal and systemic immune function (Bedford Russell 2006) but no study has prospectively examined the effect of any changes in flora on these or other outcomes. Oral yeast infection (thrush) was not an outcome reported in any of the included studies.
There are also unknown and unquantified effects of antibiotic use that include changing the normal maternal flora, effects on the presentation of infection in the infant, and the development of antimicrobial resistance. There are changes in bacterial flora with the administration of antibiotics, with an increase in enterococcal colonization and evidence of the development of antibiotic resistant bacteria but few incidences where this was associated with infectious complications (Galask 1987). In women who developed endometritis, prophylaxis with ampicillin or cefazolin alters the genital tract microflora, but this had no effect on cure rates (Newton 1998)
While increased use of antimicrobial prophylaxis may be one factor in increasing antimicrobial resistance (Shlaes 1997), there are no data supporting the contention that appropriate use of short course antimicrobial prophylaxis will cause significant bacterial resistance nor evidence that a policy of antibiotic prophylaxis for cesarean section has harmful effects that outweigh its benefits, even in those women perceived to be at low risk. Optimizing the choice and the duration of prophylactic antibiotic therapy is recommended as one strategy to prevent antimicrobial resistance (Shlaes 1997). Trends in antibiotic resistance should be monitored, reported and used to establish practice guidelines and monitor institutional policies. Susceptibility testing of significant bacterial isolates should guide antimicrobial therapy of individual women who develop infection despite prophylaxis.
Quality of the evidence
We used the GRADE approach to assess the quality for the evidence. We judged the evidence for antibiotic treatment compared with no treatment to be of moderate quality, meaning that further research would likely change our confidence in the result and the size of the estimate of the effect. Many of the included trials were more than 15 years old and lacked an adequate description of methods to allow a judgement on the risk of bias and for several studies there was clearly a high risk of bias.
Potential biases in the review process
We followed a well‐characterized search strategy to identify relevant trials, including non‐English language papers, but recognize that some trials may not have been available on‐line, especially older studies performed before the availability of electronic databases. We were unable to contact most of the authors where we needed clarification of information because there were no up‐to‐date contact details and for foreign‐language studies we relied on a translated copy, which was sometimes incomplete.
Agreements and disagreements with other studies or reviews
This review included in its definition of an elective cesarean section those women not in labor but with ruptured membranes for less than six hours, included studies that did not have a placebo arm and included studies that used antibiotic irrigation as well as systemic agents. A meta‐analysis (Chelmow 2001) that used an expanded search strategy to identify additional relevant studies, and included only placebo‐controlled studies of systemic antibiotics in women undergoing elective cesarean section who were non‐laboring with intact membranes, showed a reduction in infections in this low‐risk population and supports the conclusion of this review. In a prospective cohort study from a high‐risk obstetrical unit in New York state, absence of antibiotic prophylaxis was identified by multiple logistic regression analysis as being independently associated with surgical site infection after cesarean section for both high‐risk women and low‐risk women and was identified as one of the modifiable factors (Killian 2001).
Authors' conclusions
Implications for practice.
The conclusions of this review support the recommendations in guidelines that prophylactic antibiotics should be routinely administered to all women undergoing cesarean section to prevent infection. No conclusions can be drawn from this review about the relative benefit and harms of administering antibiotics before or after clamping of the cord nor which antimicrobial regimen should be selected, although a first generation cephalosporin was shown to be effective. Obstetric units should collect data on infection rates following cesarean section and adherence to guidelines for appropriate prophylactic antibiotic administration as important quality indicators.
Implications for research.
Further placebo‐controlled trials of the effectiveness of antibiotics with cesarean section are not ethically justified, but studies are needed to ascertain infant outcomes. Any future studies should use the list of outcomes identified here as a minimum data set and, in particular, include possible adverse effects on the infant and what role antimicrobial prophylaxis has on the development of antibiotic resistance. There should be research on methods to implement effective policies of prophylaxis for women undergoing cesarean section. Rates of infection following cesarean section are higher than for many other surgical procedures, even with a policy of uniform prophylaxis. Future research should look at interventions to reduce further the incidence of infection from that achieved with our current approach to antibiotic prophylaxis, e.g. the topical vaginal administration of metronidazole (Pitt 2001), the timing of antibiotic administration, whether there are advantages to an extended spectrum regimen (Tita 2009) and determine the role of surgical technique, pre‐ and intra‐operative preparation and infection control policies on infection rates. Research into the perceptions of the advantages and disadvantages of the intervention from the perspective of the woman and the healthcare provider will help define educational and research needs.
Without any data on adverse events, the benefits versus harms of the intervention cannot be assessed, nor a group of women identified in whom prophylactic antibiotics need not be administered because of a very low risk of infection. There is a theoretical opportunity for a cost‐effective analysis to be performed in a unit where routine prophylactic antibiotics are not administered to women undergoing an elective cesarean section and where the risk of infection is very low, in an attempt to identify women at increased risk of infection in whom prophylaxis may be cost‐effective. However, there is currently no evidence to support such a strategy. Because of local variation in practice and women, the results of such research will likely only be applicable to an individual unit and not generalizable.
Feedback
Griffin, July 1999
Summary
It has been stated that manual removal of the placenta during cesarean section increases the risk of endometritis, when compared to cord traction for placental delivery. Occlusive dressings also increase wound healing and decrease the risk of wound infection. Would it be better to adopt these simple measures first and then trial antibiotic therapy again?
Summary of comments from Chris Griffin, July 1999.
Reply
Infection following cesarean section may be reduced by the use of cord traction to remove the placenta and occlusive wound dressings. Most trials of prophylactic antibiotic therapy do not specify the methods of placental removal and wound care, and may represent a mixture of various methods. Given the clinically important reduction of infection with antibiotic use in general, support for a policy of not using antibiotics would require evidence from randomized trials that in the context of placental removal by cord traction and occlusive wound dressings, antibiotic therapy confers no additional benefit.
Contributors
Summary of response from Fiona Smaill and Justus Hofmeyr, October 1999.
What's new
| Date | Event | Description |
|---|---|---|
| 31 July 2014 | New citation required but conclusions have not changed | 95 trials included (9 new trials included), 25 trials excluded. Conclusions remain unchanged. |
| 31 July 2014 | New search has been performed | Search updated. Please note that blinding has now been divided into two assessments: 1. Blinding of participants and personnel (performance bias); and 2. Blinding of outcome assessors (detection bias) ‐ tables have been updated. 'Summary of findings' tables have been incorporated for this update. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 31 May 2009 | New search has been performed | Search updated. Five new trials included (Adam 2005; Freeman 1982; Huam 1997; Jaffe 1984; Kolben 2001). |
| 18 May 2009 | New citation required but conclusions have not changed | New review team substantially updated this review. |
| 3 January 2008 | Amended | Converted to new review format. Added a note about the updating of the review. |
| 5 March 2002 | New search has been performed | Fifteen additional trials have been added to the review. The overall conclusion remains unchanged. Antibiotic prophylaxis will reduce infectious complications following both an elective and non‐elective cesarean section. |
| 30 June 1999 | Feedback has been incorporated | Added feedback from Chris Griffin and response from authors. |
Acknowledgements
Justus Hofmeyr and Gill Gyte were authors on previous versions of this review. J Hofmeyr helped with writing of the background section, identifying the studies for inclusion and with the data extraction of the original review and provided guidance on the updating of the reviews. G Gyte made substantial contributions to Smaill 2010 and contributed to writing the abstract, background and discussion sections, identifying studies for inclusion, data abstraction and entering of the data into RevMan, as well as identifying new studies, data abstraction and entering data for this current update. Lei Dou helped with assessing the risk of bias in the studies in a previous version of the review (Smaill 2002).
We wish to thank Kate Barton, Caroline Brooke, Simon Claessens, Kornelia DeKorne, Violeta Dimova‐Nikols, Rebecca Gainey, Alison Jenner, Agnieszka Kimball, Ben Lamy, Austin Leirvik, Melissa Slavick, Caroline Summers, Maria Tenorio, Jean Tsang, Kyle Wark and Elizabeth Whiteley for translating articles relevant to this review.
As part of the pre‐publication editorial process, the first version of this review was commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Antibiotic versus no antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal febrile morbidity/fever | 56 | 9046 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.40, 0.51] |
| 2 Maternal wound infection | 82 | 14407 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.35, 0.46] |
| 3 Maternal endometritis | 83 | 13548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.34, 0.42] |
| 4 Maternal serious infectious complications | 32 | 6159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.20, 0.49] |
| 5 Maternal urinary tract infection | 66 | 10928 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.49, 0.65] |
| 6 Maternal adverse effects | 13 | 2131 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.00, 5.90] |
| 7 Maternal days in hospital | 19 | 3168 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.65, ‐0.28] |
Comparison 2. Antibiotic versus no antibiotic ‐ subgroup by class of antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal febrile morbidity/fever | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Natural penicillins | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Aminopenicillins | 7 | 603 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.26, 0.58] |
| 1.3 Extended spectrum penicillins | 6 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.28, 0.49] |
| 1.4 Beta‐lactamase inhibitor combinations | 5 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.29, 0.79] |
| 1.5 First generation cephalosporins | 10 | 1436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.44, 0.66] |
| 1.6 Second generation cephalosporins | 9 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.27, 0.46] |
| 1.7 Cefamycins | 9 | 1894 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.88] |
| 1.8 Third generation cephalosporins | 3 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.27, 0.74] |
| 1.9 Monobactams | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.42] |
| 1.10 Lincosamides | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.08, 3.05] |
| 1.11 Nitroimidazoles | 7 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.48, 0.71] |
| 1.12 Fluoroquinolones | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.13 Trimethoprim‐sulfamethoxazole | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.14 Aminoglycoside‐containing combination | 5 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.24, 0.46] |
| 1.15 Other antibiotic combination | 4 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.17, 0.44] |
| 1.16 Other regimen | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.76] |
| 2 Maternal wound infection | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Natural penicillins | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.07, 2.87] |
| 2.2 Aminopenicillins (ampicillin) | 12 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.35, 0.72] |
| 2.3 Extended spectrum penicillins | 7 | 845 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.09, 0.39] |
| 2.4 Beta‐lactamase inhibitor combination | 6 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.51] |
| 2.5 First generation cephalosporin | 17 | 3371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.28, 0.53] |
| 2.6 Second generation cephalosporin | 9 | 1166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.19, 0.75] |
| 2.7 Cefamycins | 16 | 2635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.33, 0.60] |
| 2.8 Third generation cephalosporin | 6 | 1519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.26, 0.73] |
| 2.9 Monobactams | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.03] |
| 2.10 Lincosamides | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.44] |
| 2.11 Nitroimidazoles | 9 | 1074 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.34, 0.69] |
| 2.12 Fluoroquinolones | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.13 Trimethoprim‐sulfamethoxazole | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.14 Aminoglycoside‐containing combination | 8 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.08, 0.34] |
| 2.15 Other antibiotic combination | 4 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.36, 1.02] |
| 2.16 Other regimen | 2 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.30] |
| 3 Maternal endometritis | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Natural penicillins | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.05, 0.65] |
| 3.2 Aminopenicillins (ampicillin) | 10 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.16, 0.38] |
| 3.3 Extended‐spectrum penicillins | 9 | 1164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.37, 0.58] |
| 3.4 Beta‐lactamase inhibitor combinations | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.27, 1.66] |
| 3.5 First generation cephalosporin | 18 | 3451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 3.6 Second generation cephalosporin | 13 | 1563 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.20, 0.37] |
| 3.7 Cefamycins | 15 | 2535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.28, 0.47] |
| 3.8 Third generation cephalosporin | 4 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.11, 0.69] |
| 3.9 Monobactams | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.25, 1.54] |
| 3.10 Lincosamides | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.11 Nitroimidazoles | 8 | 919 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.37, 0.73] |
| 3.12 Fluoroquinolones | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.13 Trimethoprim‐sulfamethoxazole | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.20, 1.01] |
| 3.14 Aminoglycoside‐containing combination | 8 | 722 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.19, 0.45] |
| 3.15 Other antibiotic combination | 4 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.75] |
| 3.16 Other regimen | 2 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.03] |
| 4 Maternal serious infectious complications | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Natural penicillins | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Aminopenicillins (ampicillin) | 4 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.06, 1.18] |
| 4.3 Extended‐spectrum penicillins | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.33] |
| 4.4 Beta‐lactamase inhibitor combinations | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 First generation cephalosporin | 10 | 2351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.16, 0.95] |
| 4.6 Second generation cephalosporin | 5 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.06, 2.13] |
| 4.7 Cefamycins | 10 | 1372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.10, 0.49] |
| 4.8 Third generation cephalosporin | 3 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.12, 4.03] |
| 4.9 Monobactams | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.06, 25.02] |
| 4.10 Lincosamides | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.11 Nitroimidazoles | 3 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.65] |
| 4.12 Fluoroquinolones | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.13 Trimethoprim‐sulfamethoxazole | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.14 Aminoglycoside‐containing regimens | 3 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.06, 1.59] |
| 4.15 Other antibiotic combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.16 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Maternal urinary tract infection | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Natural penicillins | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Aminopenicillins (ampicillin) | 9 | 1039 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.41, 0.90] |
| 5.3 Extended‐spectrum penicillin | 6 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.20, 0.58] |
| 5.4 Beta‐lactamse inhibitor combination | 5 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 0.99] |
| 5.5 First generation cephalosporin | 17 | 3371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.49, 0.93] |
| 5.6 Second generation cephalosporin | 9 | 1166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.38, 0.89] |
| 5.7 Cefamycins | 14 | 2434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.54, 1.00] |
| 5.8 Third generation cephalosporin | 3 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.04, 2.16] |
| 5.9 Monobactams | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.35, 5.91] |
| 5.10 Lincosamides | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.11 Nitroimidazoles | 5 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.53, 2.01] |
| 5.12 Fluoroquinolones | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.13 Trimethoprim‐sulfamethoxazole | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.14 Aminoglycoside‐containing combination | 5 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.15, 0.60] |
| 5.15 Other antibiotic combination | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.32, 1.03] |
| 5.16 Other regimen | 2 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.29] |
| 6 Maternal adverse effects | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Natural penicillins | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Aminopenisillins (ampicillin) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Extended‐spectrum penicillins | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.12, 71.38] |
| 6.4 Beta‐lactamase inhibitor combination | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 First generation cephalosporin | 3 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.15, 0.90] |
| 6.6 Second generation cephalosporin | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.07, 36.11] |
| 6.7 Cefamycins | 5 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.41, 9.34] |
| 6.8 Third generation cephalosporin | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.12, 70.50] |
| 6.9 Monobactams | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.10 Lincosamides | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.11 Nitroimidazoles | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.12 Fluoroquinolones | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.13 Trimethoprim‐sulfamethoxazole | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.14 Amimnoglycoside‐containing combination | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| 6.15 Other antibiotic combination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.16 Other regimen | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal days in hospital | 19 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Natural penicillins | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Aminopenicillins (ampicillin) | 2 | 191 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐1.33, ‐0.31] |
| 7.3 Extended‐spectrum penicillins | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Beta‐lactamase inhibitor combinations | 3 | 555 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.46, 0.09] |
| 7.5 First generation cephalosporins | 2 | 325 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.58, 0.14] |
| 7.6 Second generation cephalosporin | 2 | 220 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.69, ‐0.08] |
| 7.7 Cefamycins | 6 | 1392 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.60, ‐0.15] |
| 7.8 Third generation cephalosporin | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.9 Monobactams | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.10 Lincosamides | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.11 Nitroimidazoles | 4 | 485 | Mean Difference (IV, Fixed, 95% CI) | ‐0.91 [‐1.37, ‐0.45] |
| 7.12 Fluoroquinolones | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.13 Aminoglycoside‐containing combination | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.14 Other antibiotic combination | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.15 Trimethoprim‐sulfamethoxazole | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.16 Other regimen | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
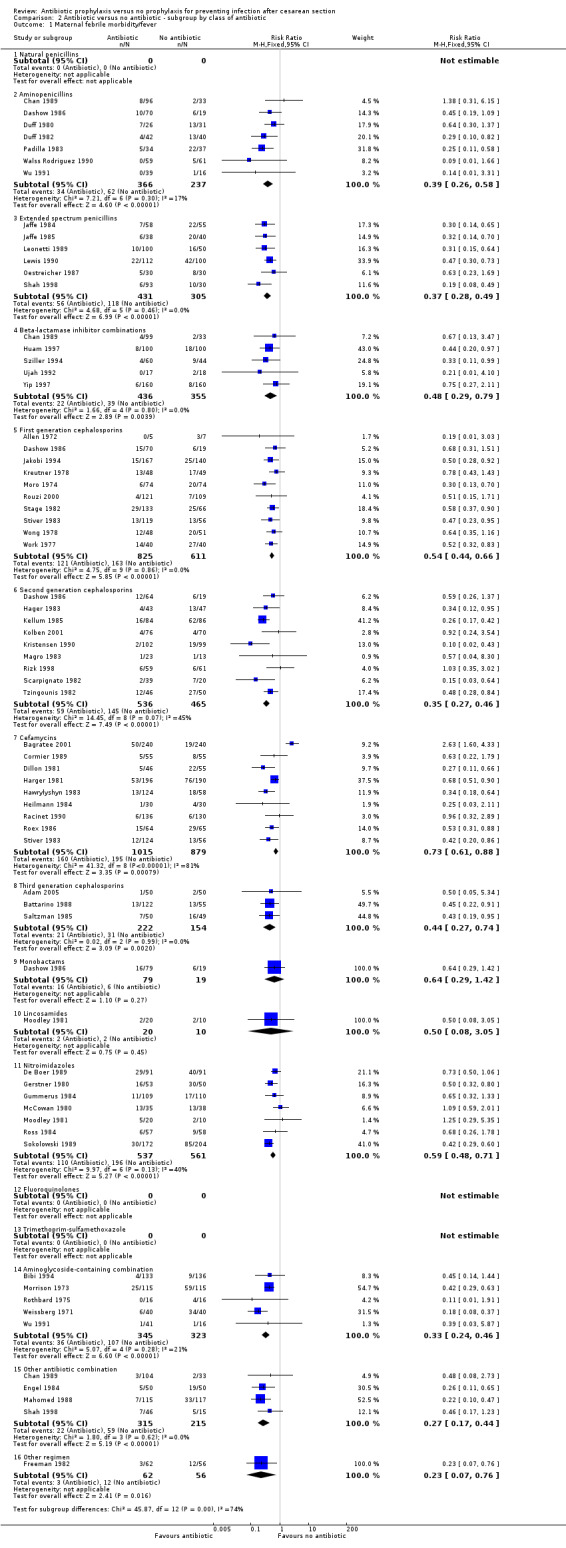
Comparison 2 Antibiotic versus no antibiotic ‐ subgroup by class of antibiotic, Outcome 1 Maternal febrile morbidity/fever.
2.4. Analysis.
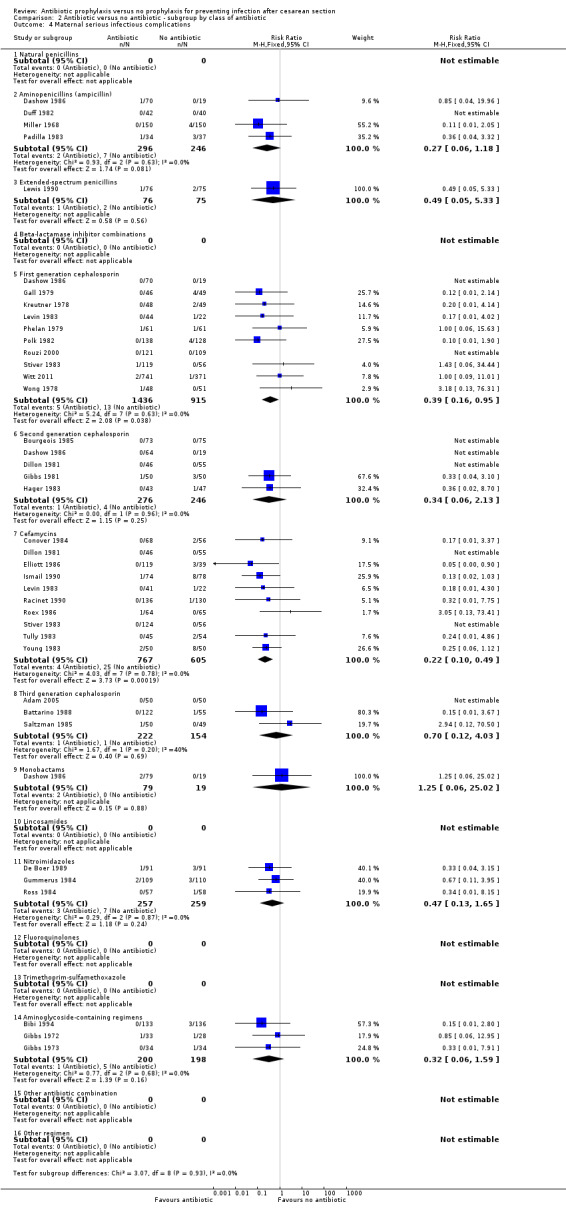
Comparison 2 Antibiotic versus no antibiotic ‐ subgroup by class of antibiotic, Outcome 4 Maternal serious infectious complications.
2.5. Analysis.
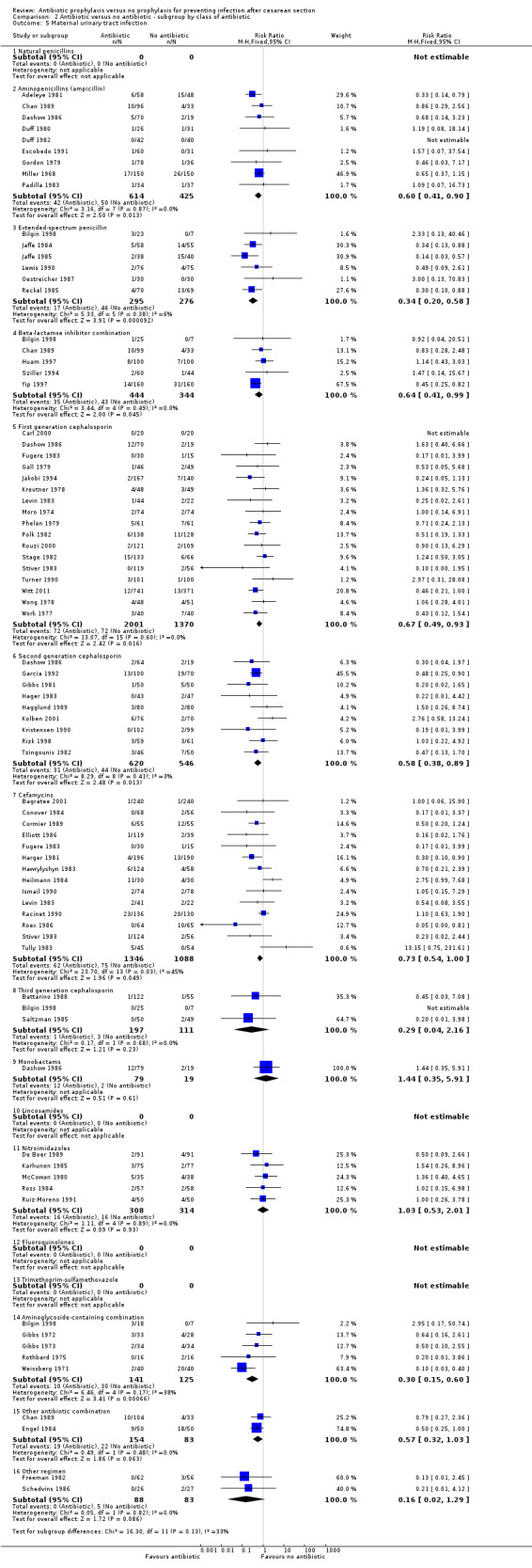
Comparison 2 Antibiotic versus no antibiotic ‐ subgroup by class of antibiotic, Outcome 5 Maternal urinary tract infection.
2.6. Analysis.
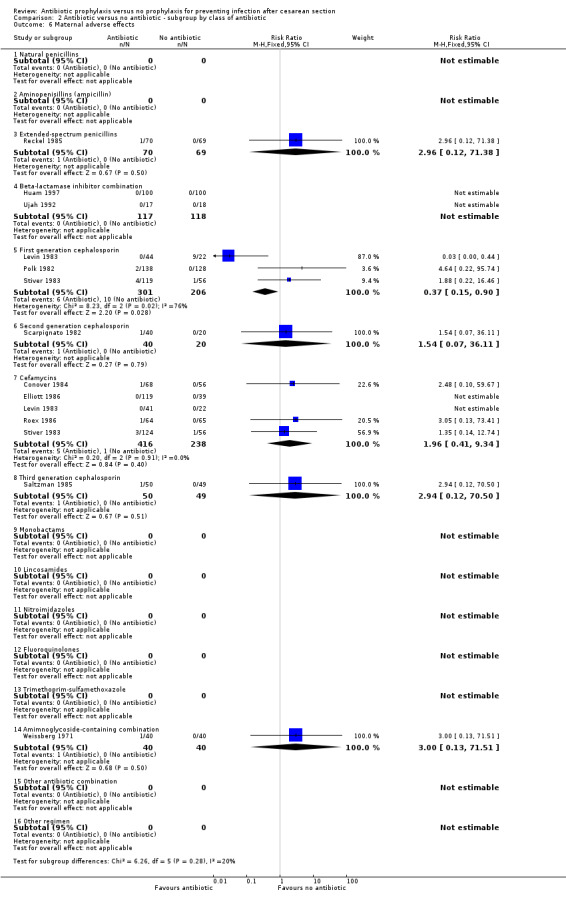
Comparison 2 Antibiotic versus no antibiotic ‐ subgroup by class of antibiotic, Outcome 6 Maternal adverse effects.
2.7. Analysis.
Comparison 2 Antibiotic versus no antibiotic ‐ subgroup by class of antibiotic, Outcome 7 Maternal days in hospital.
Comparison 3. Antibiotics versus no antibiotics ‐ subgroup by type of cesarean section.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal febrile morbidity/fever | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Elective cesarean section | 16 | 2537 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.38, 0.61] |
| 1.2 Non‐elective cesarean section | 15 | 1784 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.37, 0.51] |
| 1.3 Both elective and non‐elective, or undefined cesarean section | 29 | 4725 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.40, 0.50] |
| 2 Maternal wound infection | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Elective cesarean section | 17 | 3537 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.47, 0.82] |
| 2.2 Non‐elective cesarean section | 20 | 2291 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.27, 0.58] |
| 2.3 Both elective and non‐elective, or undefined cesarean section | 49 | 8579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.28, 0.40] |
| 3 Maternal endometritis | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Elective cesarean section | 15 | 2502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.24, 0.61] |
| 3.2 Non‐elective cesarean section | 20 | 2310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.33, 0.47] |
| 3.3 Both elective and non‐elective, or undefined cesarean section | 52 | 8776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.32, 0.42] |
| 4 Maternal serious infectious complications | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Elective cesarean section | 4 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.04, 24.21] |
| 4.2 Non‐elective cesarean section | 6 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.12, 0.65] |
| 4.3 Both elective and non‐elective, or undefined cesarean section | 24 | 4918 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.19, 0.54] |
| 5 Maternal urinary tract infection | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Elective cesarean section | 12 | 1936 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.57, 1.50] |
| 5.2 Non‐elective cesarean section | 17 | 1981 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.31, 0.60] |
| 5.3 Both elective and non‐elective, or undefined cesarean section | 41 | 7043 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.49, 0.70] |
| 6 Maternal adverse effects | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Elective cesarean section | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Non‐elective cesarean section | 5 | 808 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.61, 13.31] |
| 6.3 Both elective and non‐elective, or undefined cesarean section | 6 | 1088 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [0.75, 6.63] |
| 7 Maternal days in hospital | 19 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Elective cesarean section | 5 | 1065 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.62, ‐0.21] |
| 7.2 Non‐elective cesarean section | 4 | 646 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.78, ‐0.14] |
| 7.3 Both elective and non‐elective, or undefined cesarean section | 11 | 1668 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.57, ‐0.21] |
3.1. Analysis.
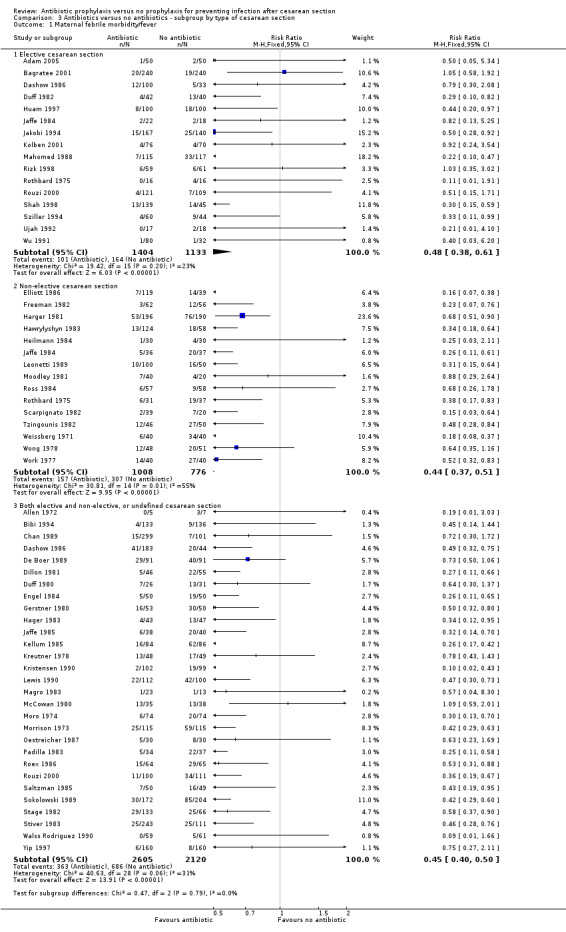
Comparison 3 Antibiotics versus no antibiotics ‐ subgroup by type of cesarean section, Outcome 1 Maternal febrile morbidity/fever.
3.4. Analysis.
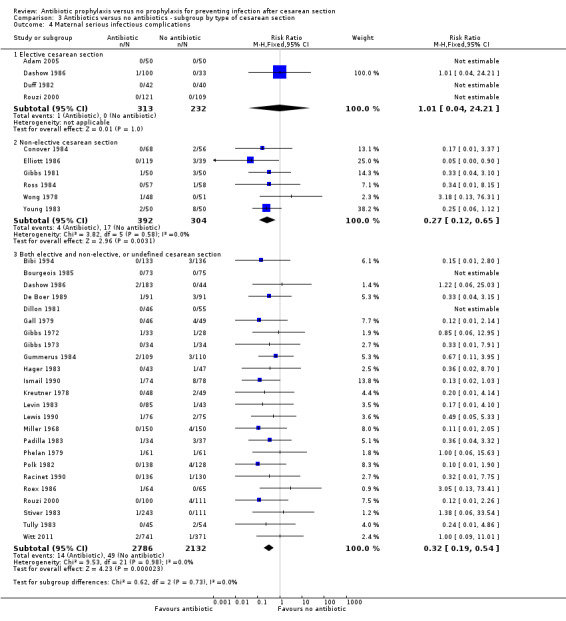
Comparison 3 Antibiotics versus no antibiotics ‐ subgroup by type of cesarean section, Outcome 4 Maternal serious infectious complications.
3.5. Analysis.
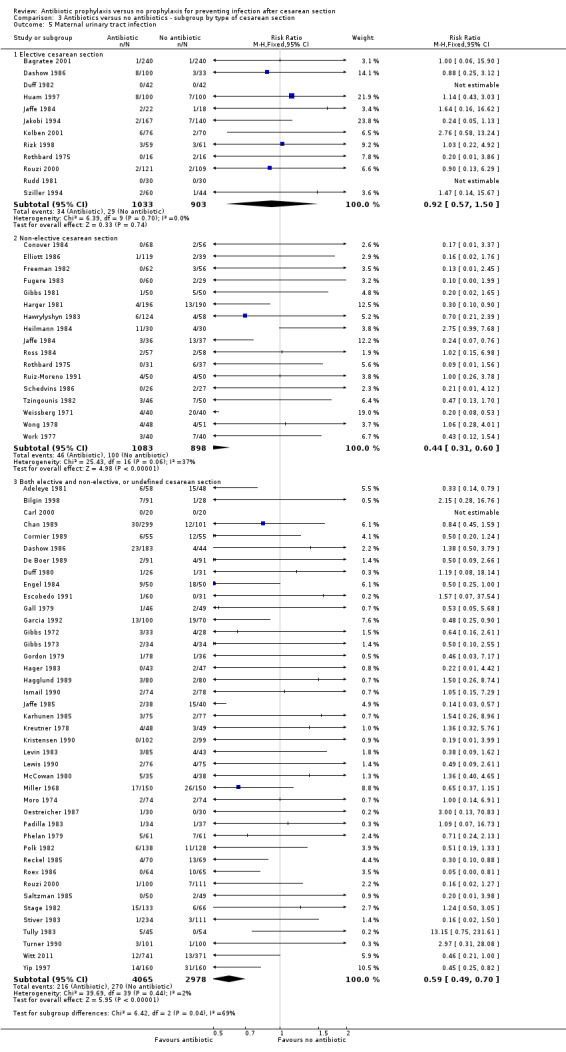
Comparison 3 Antibiotics versus no antibiotics ‐ subgroup by type of cesarean section, Outcome 5 Maternal urinary tract infection.
3.6. Analysis.
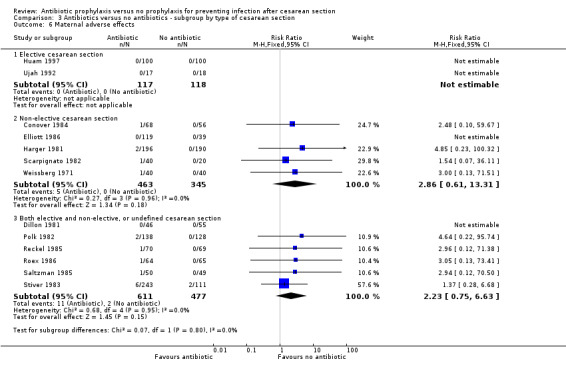
Comparison 3 Antibiotics versus no antibiotics ‐ subgroup by type of cesarean section, Outcome 6 Maternal adverse effects.
3.7. Analysis.
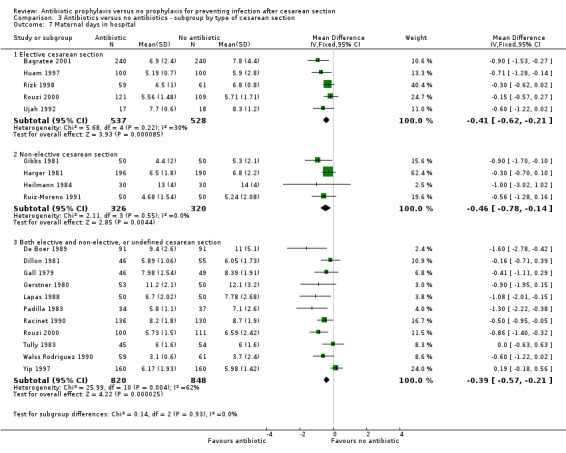
Comparison 3 Antibiotics versus no antibiotics ‐ subgroup by type of cesarean section, Outcome 7 Maternal days in hospital.
Comparison 4. Antibiotics versus no antibiotics ‐ subgroup by timing of administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal febrile morbidity/fever | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Before cord clamping | 26 | 3560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.42, 0.56] |
| 1.2 After cord clamping | 25 | 5095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.39, 0.50] |
| 1.3 Timing not defined | 5 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.15, 0.38] |
| 2 Maternal wound infection | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Before cord clamping | 37 | 5593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.32, 0.47] |
| 2.2 After cord clamping | 42 | 8428 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.34, 0.50] |
| 2.3 Timing not defined | 5 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.11, 0.85] |
| 3 Maternal endometritis | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Before cord clamping | 32 | 4965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.26, 0.40] |
| 3.2 After cord clamping | 48 | 8213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.36, 0.46] |
| 3.3 Timing not defined | 5 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.18, 0.50] |
| 4 Maternal serious infectious complications | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Before cord clamping | 13 | 2194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.12, 0.64] |
| 4.2 After cord clamping | 19 | 3893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.19, 0.55] |
| 4.3 Timing not defined | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.32] |
| 5 Maternal urinary tract infections | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Before cord clamping | 30 | 4443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.50, 0.74] |
| 5.2 After cord clamping | 34 | 6166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.43, 0.68] |
| 5.3 Timing not defined | 4 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.18, 0.75] |
| 6 Maternal adverse effects | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Before cord clamping | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.12, 71.38] |
| 6.2 After cord clamping | 8 | 1617 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.88, 6.75] |
| 6.3 Timing not defined | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.24, 19.70] |
| 7 Maternal days in hospital | 19 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Before cord clamping | 7 | 1060 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.59, ‐0.08] |
| 7.2 After cord clamping | 10 | 2213 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.55, ‐0.25] |
| 7.3 Timing not defined | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐1.34, ‐0.31] |
4.1. Analysis.
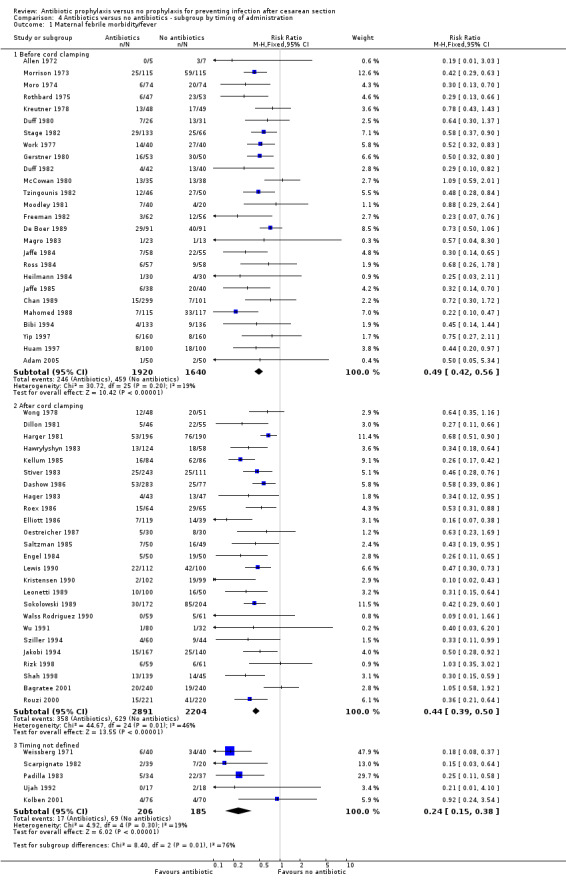
Comparison 4 Antibiotics versus no antibiotics ‐ subgroup by timing of administration, Outcome 1 Maternal febrile morbidity/fever.
4.2. Analysis.
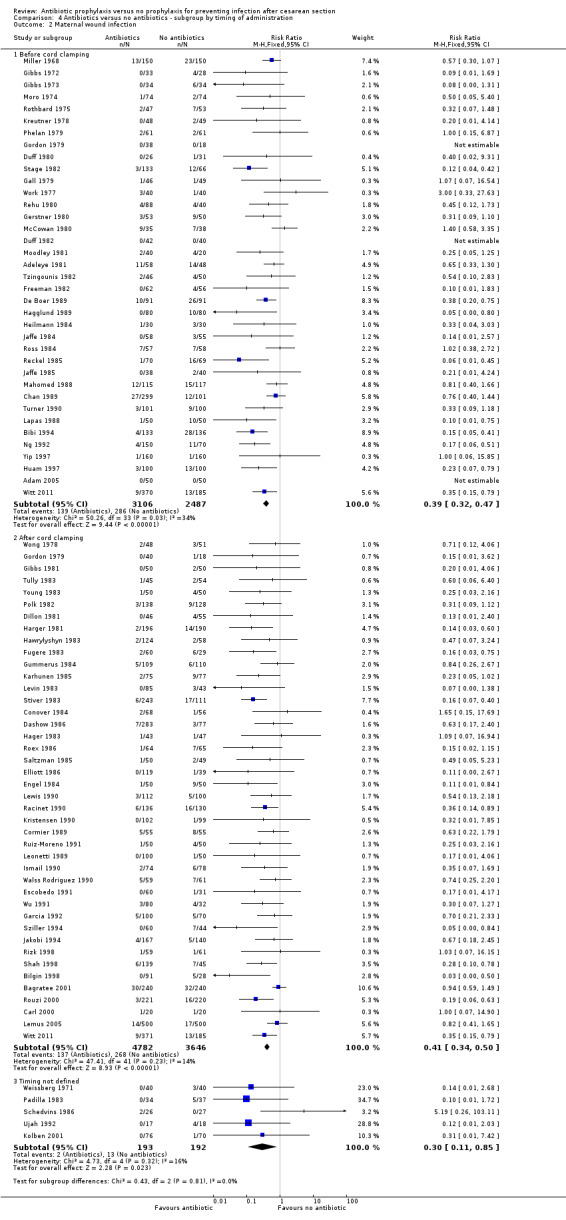
Comparison 4 Antibiotics versus no antibiotics ‐ subgroup by timing of administration, Outcome 2 Maternal wound infection.
4.3. Analysis.
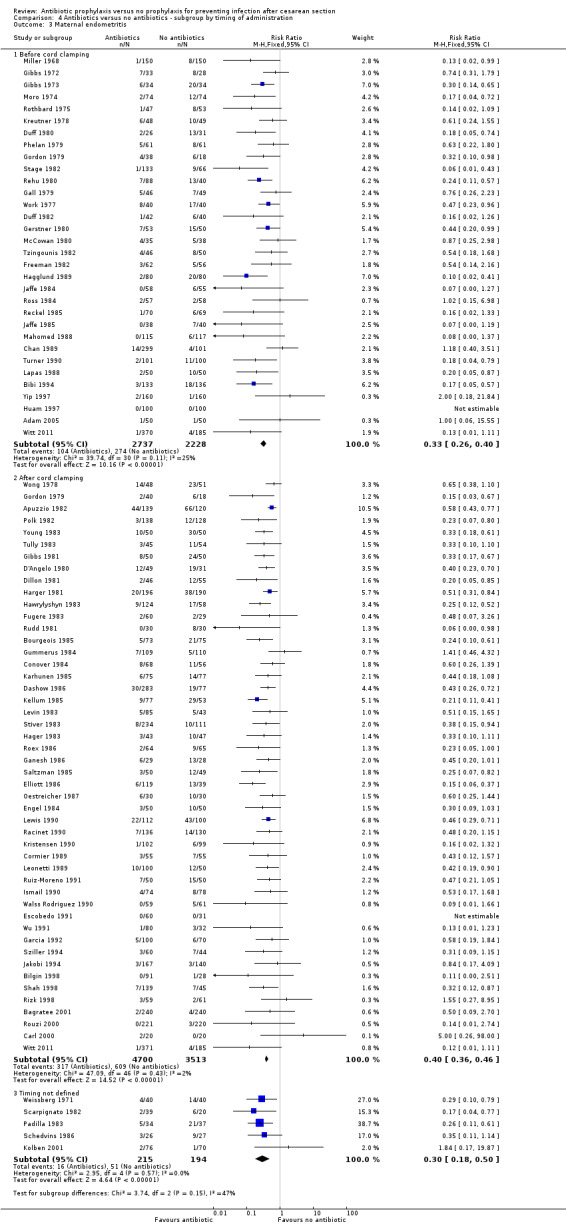
Comparison 4 Antibiotics versus no antibiotics ‐ subgroup by timing of administration, Outcome 3 Maternal endometritis.
4.4. Analysis.
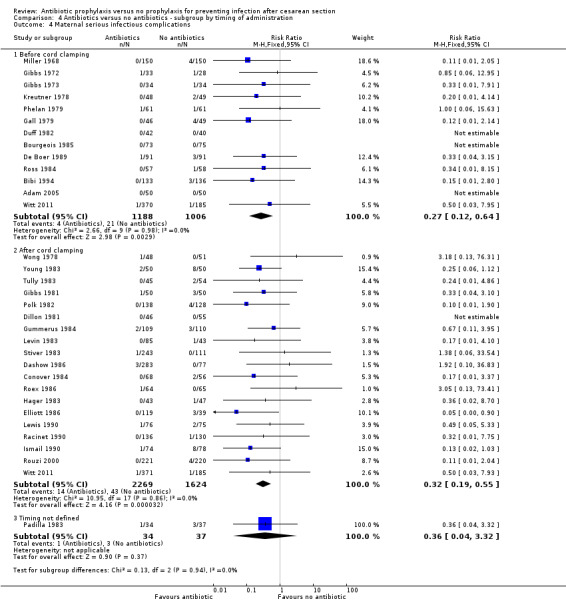
Comparison 4 Antibiotics versus no antibiotics ‐ subgroup by timing of administration, Outcome 4 Maternal serious infectious complications.
4.5. Analysis.
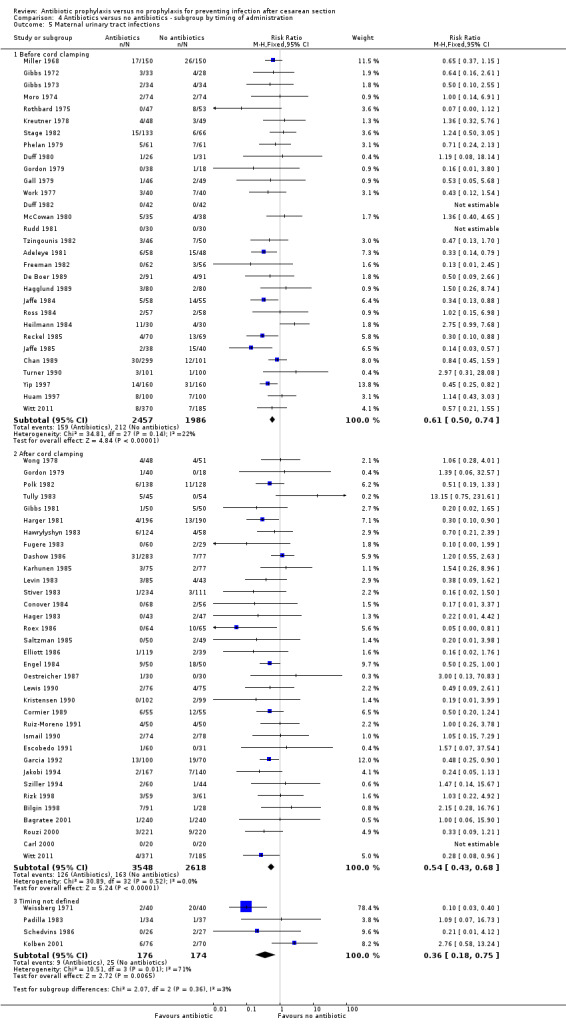
Comparison 4 Antibiotics versus no antibiotics ‐ subgroup by timing of administration, Outcome 5 Maternal urinary tract infections.
4.6. Analysis.
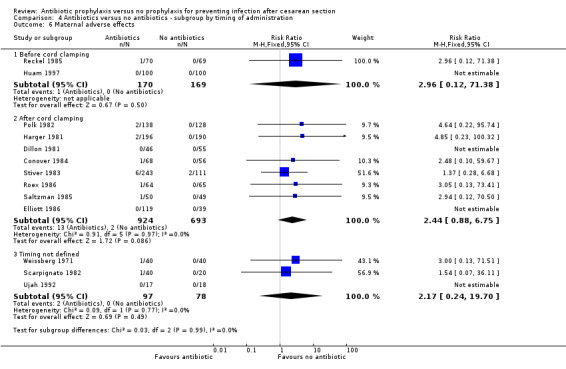
Comparison 4 Antibiotics versus no antibiotics ‐ subgroup by timing of administration, Outcome 6 Maternal adverse effects.
4.7. Analysis.
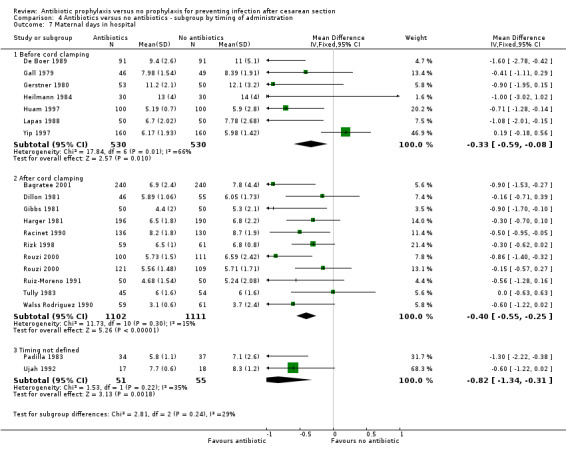
Comparison 4 Antibiotics versus no antibiotics ‐ subgroup by timing of administration, Outcome 7 Maternal days in hospital.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adam 2005.
| Methods | RCT, 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: Sept 2003 to April 2004. Setting: New Halfa Teaching Hospital, Eastern Sudan. Inclusion criteria: planned elective CS (categorized as elective). N = 100. Exclusion criteria: antibiotics within 2 weeks; any visible infection; elevated temperature; allergic to antimicrobials; did not wish to participate. |
|
| Interventions |
Intervention: 3rd generation cephalosporin:
Comparison: no treatment:
|
|
| Outcomes | Post‐operative febrile morbidity (oral temperature ≥ 38 °C twice at least 4 hrs apart after 1st 24 hrs. Post‐operative infections (endometritis, wound infection, pelvic abscess, peritonitis, other febrile morbidity (UTI, chest infection, malaria). 2 perinatal deaths: 1 in each group due to respiratory distress and septicemia due to imperforate anus. |
|
| Notes | Low‐income country. Class of antimicrobial: 3rd generation cephalosporin Subgroups:
The 2 groups were well matched at enrolment and there were no statistical differences in the admission variables. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participant excluded after randomization; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Adeleye 1981.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: University College Hospital, Ibadan, Nigeria. Majority of patients from low socioeconomic class. Inclusion criteria: both elective and non‐elective cesarean deliveries. N = 106. Exclusion criteria: fever or obvious infection before operation. |
|
| Interventions |
Intervention:
Comparison: no treatment:
Both groups received curative doses of chloroquine. Prophylaxis continued for 7 days. |
|
| Outcomes | Wound infection; UTI (not defined further); 'genital sepsis' (not defined further: study group 5/58; control group 15/48). | |
| Notes | Low‐income country. Class of antibiotic: aminopenicillin (ampicillin) Subgroups:
The 2 groups were comparable regarding age, parity and indications for CS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Divided randomly into 2 groups". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no loss to follow‐up reported; no participants excluded; imbalance in group size not accounted for (58 vs 48); ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Allen 1972.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: August 1970 to January 1971. Setting: Johns Hopkins University, Baltimore, US. Inclusion criteria: women undergoing CS (criteria not specified). N = 12. Exclusion criteria: evidence of clinical infection, history of penicillin allergy. |
|
| Interventions |
Intervention: 1st generation cephalosporin:
Comparison: placebo:
|
|
| Outcomes | Morbidity (temperature > 100.90F twice, 6 hrs apart after 1st 48 hrs or other clinical signs of infection); not separated. For this review, the authors' definition of morbidity has been classified as fever. | |
| Notes | Part of a larger randomized trial of prophylactic antibiotics in gynecologic surgery; most patients (87%) were undergoing hysterectomy; only 12/300 patients enrolled underwent CS. Class of antibiotic: 1st generation cephalosporin. Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Comment: randomized list of placebo or drug, kept in hospital pharmacy; code not broken until after patient classified as 'morbid' or 'non‐morbid'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up; no participants excluded; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". Comment: placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Apuzzio 1982.
| Methods | RCT: 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: October 1977 to June 1980. Setting: College Hospital, New Jersey, October. Women 'predominantly black (90%) and socio‐economically disadvantaged'. Inclusion criteria: both elective and non‐elective cesarean deliveries. N = 259. Exclusion criteria: antibiotics within 2 weeks; pyrexia; any visible infection; penicillin allergy; known medical illness that might cause pyrexia; internal fetal scalp or uterine monitoring. |
|
| Interventions |
Intervention: carboxypenicillin:
Comparison: placebo:
Subset of 22 in each group received ticarcillin 3 g/saline 6‐8 hrs post‐operatively or saline placebo (results similar so authors combined results with single‐dose group). No post‐operative antibiotics unless pyrexial > 38 degrees C after day 1. |
|
| Outcomes | Endomyometritis (pyrexia, uterine tenderness and no evidence of other infection). | |
| Notes | Authors' definition of low and high risk not comparable to definitions for elective/non‐elective used in this review. Results for adolescent group (aged 15‐18) reported in J Adolescent Health Care 1984;5:163‐166. In that study, incidence of endomyometritis in elective section: 0% for treatment vs 43% for placebo (numbers not given). Class of antibiotic: extended spectrum penicillin (carboxypenicillin). Subgroups:
"The prophylaxis and placebo groups were essentially similar in regard to important demographic and obstetric parameters. There were no significant differences between the groups for any of the variables studied." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly divided into 2 groups". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no loss of participants to follow‐up and no participant excluded after analysis, however discrepancy in group numbers (139 vs 120) not accounted for; ITT analysis. |
| Selective reporting (reporting bias) | High risk | Comment: a subset of 22 in each group received ticarcillin 3 g/saline 6‐8 hrs post‐operatively or saline placebo; the results were similar so authors combined results with single‐dose group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". Comment: placebo‐controlled (saline solution). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Bagratee 2001.
| Methods | Randomized double‐blind, placebo‐controlled; 2 parallel groups. | |
| Participants | Dates of data collection: not reported. Setting: Durban, South Africa. Inclusion criteria: women undergoing elective cesarean delivery. N = 475. Exclusion: prior antibiotics within 2 weeks, allergy to penicillin or cephalosporin, rupture of membranes. |
|
| Interventions |
Intervention: cefamycin:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (oral temperature > 38oC twice 6 hrs apart after 1st 24 hrs); wound infection (wound cellulitis, erythema, discharge with or without fever); endometritis (fever, uterine tenderness, malodorous lochia); UTI (fever and positive urine culture); pneumonia; duration of hospital stay. | |
| Notes | 11% were HIV positive; Staphylococcus aureus most common pathogen (43%) isolated. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
The 2 groups were comparable regarding age, parity, gestational age, weight and pre‐operative hemoglobin. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomized...a computer‐based allocation...". |
| Allocation concealment (selection bias) | Low risk | Quote: "...consecutively numbered sealed envelopes...". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses or exclusions were reported; it appeared to be an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind; placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Battarino 1988.
| Methods | RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not stated. Detting: Cerignola, Italy. Inclusion criteria: women in labor and/or with ruptured membranes (mean duration of ruptured membranes 10 hrs), classified as both elective and non‐elective in this review. Exclusion criteria: not stated. |
|
| Interventions |
Intervention 1:
Intervention 2:
Intervention 3:
As both treatments are classified as 3rd generation cephalosporins, the 2 treatment groups have been combined in the analysis. |
|
| Outcomes | Febrile morbidity (2 or more readings ≥ 38°C at least 6 hrs apart after the 1st 24 hrs after surgery; endometritis (pain accompanied by fever, with or without purulent discharge from the vagina); wound infection (redness, induration, pain on palpation and purulent discharge); UTI (dysuria, pyuria and urine culture > 106 bacteria/mL); septic shock (classified as serious maternal infection); maternal stay (7.3 days for ceftriaxone group vs 7.4 days for cefotaxime vs 8.7 days for control group; variance not provided); additional antibiotics (11.3% vs 10% vs 27.2% for the 3 groups respectively). | |
| Notes | Translated from Italian. The authors' definition of non‐elective does not match those used in this review (included patients whose indication for CS was prior cesarean and fetal‐pelvic disproportion). Class of antibiotic: 3rd generation cephalosporin. Subgroups:
There were no significant differences among the groups for age, parity, gestation, indication for cesarean section, duration of membrane rupture, duration of labor or number of vaginal examinations. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At random". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no loss to follow‐up reported (no explanation for small differences in numbers in each study group); no participants excluded; ITT analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "placebo‐controlled". Comment: no additional details provided to judge. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Bibi 1994.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: February to July 1991. Setting: Sousse Hospital, Tunisia. Inclusion criteria: women undergoing elective CS or labor < 12 hrs (categorized as "both" for this review"). N = 269. Exclusion criteria: diagnosed amniotic infection; pyrexia ≥ 38oC; cases of failed vaginal instrumental deliveries; antibiotics within 3 days; allergy to beta lactam antibiotics; cardiac disease; diabetes. |
|
| Interventions |
Intervention:
Comparison: no treatment:
|
|
| Outcomes | Endometritis; wound infection; pyrexia only (≥ 38oC 48 hrs after surgery): antibiotic 4/133 vs control 9/136; septicemia (0/133 vs 3/136, included as serious morbidity); duration of hospital stay (antibiotic 5.36 days vs control 6.21, P = 0.03, variance not given); cost of antibiotics given for treatment (440FF for treatment group vs 4294FF for control group). | |
| Notes | Translated from French. Class of antibiotic: aminoglycoside‐containing combination (1st generation cephalosporin, aminoglycoside and nitroimidazole (metronidazole). Subgroups:
Follow‐up at 30 days (86%). The groups were similar for age, parity, duration of labor and other risk factors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "by chance". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | High risk | Comment: using random number table, patients allocated to treatment if the number is even, no treatment if the number is odd. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no losses or exclusions reported; it appears to be ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Bilgin 1998.
| Methods | Quasi‐RCT; 5 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: Bursa, Turkey. Inclusion criteria: women undergoing CS due to acute fetal distress. N = 119. |
|
| Interventions |
Intervention 1:
Intervention 2:
Intervention 3:
Intervention 4:
Comparison:
IV after clamping of the cord. No treatment comparison was divided: 7 for each of the 4 groups (28/4). |
|
| Outcomes | Wound infection (redness, tenderness, pain and purulent discharge); UTI (renal angle tenderness, fever, dysuria and pyuria); endometritis (vaginal spotting, purulent discharge with fever and pain) plus positive cultures. | |
| Notes | All wound infections were positive for coagulase negative staphylococcus. Class of antibiotic: 3rd generation cephalosporin vs extended spectrum penicillin (ureidopenicillin) vs aminoglycoside containing combination (lincosamide (clindamycin) and aminoglycoside) vs beta‐lactamase inhibitor combination. Subgroups:
The 5 groups were comparable regarding maternal age, ruptured membranes, pelvic examinations, hemoglobin levels. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...according to the last digital of the patient's file number...". Comment: Quasi‐RCT. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses or exclusions were reported; it appeared to be an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Bourgeois 1985.
| Methods | RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: initiated March 1981. Setting: Charlottesville, Virginia, USA, almost all were indigent women. Inclusion criteria: both 'low risk' (labor < 6 hrs) and 'high risk' (> 6 hrs) women undergoing CS. N = 148 for this comparison. Exclusion criteria: allergy to penicillin or cephalosporin; antibiotic use within 7 days; antibiotics required for other reasons; pyrexia > 38oC; foul amniotic fluid. |
|
| Interventions |
Intervention:
Comparison 1: placebo:
Comparison 2: no treatment:
As the objective of this review is to compare antibiotic with no antibiotic, rather than the effect of irrigation, only the 1st 2 groups are compared (double‐blind comparison). |
|
| Outcomes | Metritis (pyrexia > 38oC twice 8 hrs apart, after 24 hrs plus abnormal uterine tenderness, without another apparent source); duration of maternal stay (treatment 5.29 days vs placebo 6.32 days, variance could not be calculated). | |
| Notes | Authors' definition of low and high risk do not correspond to those used for elective/non‐elective in this review.
No treated patients developed evidence of drug reaction.
There were no serious infections (pelvic abscess or phlebitis) in either group. Class of antibiotic: 2nd generation cephalosporin. Subgroup:
The groups were comparable regarding gravidity, parity, maternal weight, hematocrit, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer generated table of random numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "Assigned under the direction of the hospital pharmacy". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up reported; no participants excluded; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: partially double‐blind placebo‐controlled (3 groups: antibiotic irrigation, saline placebo irrigation, no irrigation). Physicians were unaware of the type of irrigation used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: probably outcome assessment was blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Carl 2000.
| Methods | Randomly allocated (abstract only; no further details). | |
| Participants | Setting: Texas, USA. Inclusion: women undergoing high‐risk CS (definition not provided; classified as not‐defined). N = 40. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Wound infection, endometritis, UTI. | |
| Notes | Abstract only available. Class of antibiotic: 1st generation cephalosporin. Subgroups:
Follow‐up 4‐6 weeks post‐operatively. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly allocated..". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: normal saline irrigation used in control group; no additional details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Chan 1989.
| Methods | Randomized, placebo‐controlled trial; 4 parallel groups (3 treatment, 1 placebo). Unit of randomization: individual. |
|
| Participants | Dates of data collection: October 1986 to February 1987. Setting: Prince of Wales Hospital, Hong Kong; mostly suburban or rural Chinese women of lower or middle class. Inclusion criteria: all women undergoing CS. N = 400. Exclusion criteria: receiving antibiotics; pyrexia > 37.4oC; diagnosed infection; increased risk of infection, e.g. diabetes; known sensitivity to the antibiotics. |
|
| Interventions |
Intervention 1:
Intervention 2:
Intervention 3:
Comparison: placebo:
Placebo data were divided: 33 for each comparison (101/3). |
|
| Outcomes | Febrile morbidity (oral temperature of more than 38oC at least twice after day 1); wound infection (induration, serosanguinous discharge or dehiscence with purulent discharge); UTI (positive culture); genital tract infection (pain and uterine tenderness, purulent uterine discharge with microbiological confirmation); any infection anywhere (antibiotic 75/299 vs placebo 28/101); post‐operative antibiotic use (22/299 vs 9/101). | |
| Notes | Only moderate or prolonged febrile morbidity (as defined) included. Class of antibiotic: aminopenicillin (ampicillin), other combination (ampicillin and metronidazole), beta‐lactamase inhibitor combination. Subgroups:
The groups were comparable regarding age, parity, primary CS, indication for CS, urinary catheterization and vaginal examination before operation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "list of random numbers consulted by nurse". |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up; no participants excluded; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind (the anesthetist was not blind); placebo (normal saline). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All doctors and nurses looking after the patients were ignorant of the drug given until the end of the study". Comment: probably outcome assessment was blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Conover 1984.
| Methods | RCT; 4 parallel groups Unit of randomization: individual. |
|
| Participants | Dates of data collection: March to November 1982. Setting: Naval Hospital, San Diego, California. Inclusion criteria: women at increased risk of post‐CS endometritis (in labor or with ruptured membranes). Classified as non‐elective for this review. N = 124. Exclusion criteria: allergy to penicillin or cephalosporins; antibiotic use within 48 hrs; separate indication for use of antibiotics; temperature > 38 degrees C; chorioamnionitis; pyuria. |
|
| Interventions |
Intervention 1:
Intervention 2:
Comparison 1: placebo for irrigation:
Comparison 2: placebo for IV:
We combined the cephalosporin groups and the 2 placebo groups for this review. |
|
| Outcomes | Endometritis (febrile morbidity and uterine tenderness); total infection‐related morbidity (cefoxitin 10/68 vs saline 14/56); fever index; duration of IV antibiotics; additional antibiotics; days in hospital (no difference, variance not given). | |
| Notes | 1 woman developed an allergic reaction to cefoxitin (acute pruritic rash).
There were 2 episodes of bacteremia (both in placebo groups); there were no episodes of septic pelvic thrombophlebitis nor drainage of pelvic abscess in either group. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
The 4 groups were comparable regarding age, gravidity, parity, duration of pregnancy, socioeconomic status, maternal weight, hrs in labor, length of ruptured membranes, and other potential risk factors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each patient was designated to receive either normal saline or cefoxitin based on a computer generated table of random numbers". Comment: allocation to irrigation or IV prophylaxis based on last digit of social security number. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no losses and no exclusions were reported. Imbalance in randomized groups not accounted for (irrigation: cefoxitin 37 vs saline 23; overall 68 vs 56). ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both antibiotic and normal saline were packaged identically to ensure that the administration was blinded". Comment: double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Cormier 1989.
| Methods | Randomized trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: Hopital Pellegrin, Bordeaux, France. Inclusion criteria: women undergoing CS; both elective and non‐elective deliveries. N = 110. Exclusion criteria: allergy to beta‐lactam antibiotics; pyrexia; indication for antibiotics. |
|
| Interventions |
Intervention:
Comparison: no treatment:
|
|
| Outcomes | Endometritis; urinary infection; local complications (classified as wound infection); fever only (cefotetan 0/55 vs control 6/55); antibiotic therapy (10/55 vs 25/55); mean days in hospital (10.0 vs 10.2, no variance given). | |
| Notes | Translated from French. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
There were no significant differences between the groups for risk factors for infection. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocated by sealed envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no losses or exclusions reported: analysis appears to be ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
D'Angelo 1980.
| Methods | RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not stated. Setting: Cleveland, Ohio, USA. Inclusion criteria: women in labor with ruptured membranes requiring internal monitoring (non‐elective delivery). N = 80. Exclusion criteria: evidence of infection; penicillin or cephalosporin allergy. |
|
| Interventions |
Intervention 1: cephalosporin:
Intervention 2: cephalosporin:
Comparison: no treatment:
Short and long courses of cephalosporins combined for this review. Administered after umbilical cord clamping. |
|
| Outcomes | Endometritis and/or wound infection (antibiotic 12/49 vs control 20/31). | |
| Notes | It was possible to deduce the rate of endometritis alone, but not wound infection, for this review.
1 late infectious complication (wound dehiscence) in control group. Drug class: 1st generation cephalosporin. Subgroups:
There were no statistical differences among the 3 groups for potential risk factors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"Randomly assigned". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participants excluded after analysis. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participant or clinician; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Dashow 1986.
| Methods | Randomized, placebo‐controlled trial; 5 parallel groups (4 treatment, 1 control). Unit of randomization: individual. |
|
| Participants | Dates of data collection: December 1982 to May 1984. Setting: Madigan Army Medical Centre, Tacoma, Washington, USA. Inclusion criteria: all women undergoing CS. N = 360. Exclusion criteria: penicillin or cephalosporin allergy; antibiotic therapy; known infectious process. |
|
| Interventions |
Intervention 1: cephalosporin:
Intervention 2: cephalosporin:
Intervention 3:
Intervention 4: penicillin (A4):
Comparison: placebo:
A vitamin was added to each solution for disguise. Placebo data were divided: 19 for each of the 4 comparisons (77/4). |
|
| Outcomes | Fever (> 380C twice 6 hrs apart, excluding the 1st 24 hrs); endomyometritis (pyrexia > 37.80C, uterine tenderness and pelvic peritoneal irritation without other localizing signs of irritation; UTI (positive culture); wound infection; fever index; all infection‐related morbidity; therapeutic antibiotics; mean post‐operative days (variance not given). | |
| Notes | 3 episodes of pelvic thrombophlebitis (all in treated groups).
Results were given for all women and women in labor, both high risk (corresponding to the category of non‐elective deliveries) and all labor. The data for elective deliveries were deduced from these. Class of antibiotic: 1st generation cephalosporin vs 2nd generation cephalosporin vs monobactam vs aminopenicillin (ampicillin). Subgroups:
The mean level of gravidity of the placebo group was higher than that of the cephapirin group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated numbers using the mixed congruential method". |
| Allocation concealment (selection bias) | Low risk | Quote: "The pharmacy to assign each patient to 1 of 5 groups". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up; no participant excluded; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind placebo‐controlled trial. A vitamin was added to each solution for disguise. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
De Boer 1989.
| Methods | Randomized, double‐blind, placebo‐controlled; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: December 1983 to June 1985. Setting: Chogoria Hospital, Kenya. Inclusion criteria: all patients undergoing CS. N = 182. Exclusion criteria: clinical infection. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Fever (> 37.9 oC on at least 1 occasion); wound infection; mean febrile days (0.56 for treatment vs 1.23 for control), hospital days, any antibiotic use (18/91 vs 23/91). | |
| Notes | Elective CS not defined.
No adverse events on mother or babies noted.
There was 1 grade 3 wound (defined as deep pelvic abscess or evidence of local or generalized peritonitis) in the treatment group as compared with 3 in the placebo group (classified as serious infectious morbidity). Class of antibiotic: nitroimidazole (metronidazole). Subgroups:
The 2 groups were comparable regarding age, parity, duration of labor, duration ruptured membranes, number of vaginal examinations, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized...". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 7/189 patients initially randomized were not included in analysis because suppositories were incorrectly administered; as treated analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind; placebo suppository. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Dillon 1981.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: September 1979 and April 1980. Setting: Children's Hospital of Buffalo, USA. Inclusion criteria: all women undergoing CS (1 3rd elective). N = 101. Exclusion: evidence of active infection, penicillin or cephalosporin allergy; recent antibiotic treatment. |
|
| Interventions |
Intervention:
Comparison: placebo:
After clamping the umbilical cord and at 4 and 10 hrs post‐operatively. |
|
| Outcomes | Febrile morbidity (temperature > 38 oC twice 6 hrs apart after 1st 24 hrs); endometritis (fever, uterine tenderness, leukocytosis); wound infection (fever, cellulitis, exudate); maternal length of stay. | |
| Notes | No serious life‐threatening infection in either group; no drug‐related adverse‐effects. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
The 2 groups were comparable regarding age, status, race, obesity, obstetric factors and indication for surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Numbered packages..." "...The random code was broken at the end of the study". Comment: no description of sequence generation process or further information provided. |
| Allocation concealment (selection bias) | Low risk | Comment: randomized by pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no loss to follow‐up reported; no participants excluded; ITT analysis. 9/110 'packages' not included (either damaged or patients failed to meet inclusion criteria). Imbalance in group size (46‐placebo vs 55‐cefoxitin) not explained. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, placebo‐controlled (saline placebo). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Duff 1980.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: October 1976 and March 1977. Setting: Walter Reed Army Medical Center, Washington DC. Inclusion criteria: all women undergoing either primary or repeat CS (44% elective). N = 57. Exclusion criteria: penicillin allergy; chorioamnionitis prior to surgery. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (> 100.3 oF twice 6 hrs apart after 1st 24 hrs); endomyometritis (fever, uterine and abdominal tenderness, purulent lochia); UTI (positive culture); wound infection (induration, erythema and warmth with purulent drainage); need for antibiotics (treatment 3/26 vs placebo 13/31); maternal hospital stay (6.03 vs 6.9; no variance given). | |
| Notes |
Class of antibiotic: aminopenicillin (ampicillin). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In a ... randomized manner"...."There is a notable difference in the division of repeat sections between groups". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Comment: prepared by the hospital pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: no losses to follow‐up were reported. 23/80 excluded because of errors in dispensation of medication. Analysis was not ITT; data from excluded patients could not be re‐included. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, placebo‐controlled. The pharmacist was the only individual with access to the treatment protocol. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: probably outcome assessment was blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Duff 1982.
| Methods | Randomized placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: January 1970 to June 1980. Setting: Washington, DC. US. Inclusion criteria: women undergoing CS who were not in labor and did not have ruptured membranes (elective). N = 82. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (> 100.4 oF twice 6 hrs apart after the 1st 24 hrs); endomyometritis (fever, uterine and adnexal tenderness, purulent lochia); UTI; wound infection (induration, erythema and warmth with purulent drainage); need for antibiotics (treatment 1/42 vs placebo 6/40); maternal hospital stay (4.3 vs 4.6; no variance given). | |
| Notes | No life‐threatening infection related complications nor bacteremic episodes in either group. Class of antibiotic: aminopenicillin (ampicillin). Subgroups:
The 2 groups were comparable regarding age, race, gravidity, parity and socioeconomic status. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomized; no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up; no participant excluded; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" .... "placebo solution". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Elliott 1986.
| Methods | RCT; 4 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: Letterman Army Medical Center, California; Womack Army Community Hospital, North Carolina. Inclusion criteria: women in active labor or ruptured membranes and at least 1 digital vaginal examination (categorized as non‐elective in this review although duration of membrane rupture not stated). N = 158. Exclusion criteria: allergy to penicillin or cephalosporin, fever > 37.7 oC with suspicion of chorioamnionitis; antibiotic use within 2 weeks. |
|
| Interventions |
Intervention 1:
Intervention 2:
Intervention 3:
Comparison: no treatment:
The 3 treatment groups have been combined in this review. |
|
| Outcomes | Febrile morbidity (> 37.9 oC twice 6 hrs apart after 1st 24 hrs); endometritis (fever and uterine tenderness); UTI (positive culture); wound infection (including fever, cellulitis and exudate); hospital stay (treatment 4.86 vs control 5.2; variance could not be calculated). | |
| Notes | 3 episodes of septicemia reported in control group vs none in treatment groups.
No antibiotic reactions reported. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
The 4 groups were comparable regarding age, parity, gestational age, rupture of membrane, labor, vaginal examination. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized... using a table of random numbers...". |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to judge. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participant excluded from the analysis; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Engel 1984.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: August 1980 to August 1981. Setting: Nordwest Hospital, Frankfurt, West Germany. Inclusion criteria: women undergoing CS. N = 100. Exclusion criteria: severe penicillin allergy, renal insufficiency, antibiotic use, amniotic infection. |
|
| Interventions |
Intervention:
Comparison: no treatment:
|
|
| Outcomes | Endometritis, UTIs, wound infections. | |
| Notes | Detailed pre‐ and post‐antibiotic microbiological cultures were performed; there were fewer gram positive cocci and more gram negative rods in cervical cultures of the treated group; more break‐through infections in the treated group were with mezlocillin‐resistant organisms. Class of antibiotic: other combination (penicillinase‐resistant penicillin (oxacillin) and ureidopenicillin (mezlocillin)). Subgroups:
"Both groups were statistically homogenous." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...a computerised list of randomization...". |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to judge. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: although there is no report of losses or exclusions and analysis appears to be ITT, there is insufficient information to judge. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not placebo‐controlled. Single‐blinded; women did not know their allocation but clinicians did. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: clinicians were aware of allocation. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Escobedo 1991.
| Methods | Double‐blind RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Date of data collection: March 1985 to August 1986. Setting: Mexico. Inclusion criteria: women undergoing CS (labor < 12 hrs, membrane rupture < 12 hrs, < 7 vaginal exams). N = 91. Exclusion: any antibiotic within 2 weeks, fever, clinical evidence of infection. |
|
| Interventions |
Intervention:
Intervention:
Comparison: placebo:
Antibiotics administered after surgery, within 2 hrs of the procedure. 2 treatment groups combined. |
|
| Outcomes | Fever > 38 oC x 2 at least 6 hrs apart after 1st 24 hrs; endometritis (temperature > 38 oC, purulent lochia, pain on internal examination); wound infection (increased warmth, size or color of wound, or purulent secretions); urine infection (dysuria and positive culture). | |
| Notes | Paper was not written in English. Class of antibiotic: aminopenicillin (ampicillin). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...by computerized tables...". |
| Allocation concealment (selection bias) | Unclear risk | Comment: assignment to treatment group was performed using the computer card which is attached to the file; no additional information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 3 were lost to follow‐up; 3 patients excluded for inadequate follow‐up (group allocation not provided).and no exclusions were reported. No explanation provided for unequal size groups. The analysis was as treated with the available data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, matching placebo doses. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Freeman 1982.
| Methods | Quasi‐RCT, 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: Jan 1979 to May 1980. Setting: Riverside Osteopathic Hospital, Michigan, USA. Inclusion criteria: women at high risk (defined as presence of labor) undergoing CS. N = 118. Exclusion criteria: oral temperature > 38° C any time prior to surgery; antibiotic use within 2 weeks prior to admission; refusal to participate; repeat elective CS; compelling indication for antibiotics in the judgement of the physician. |
|
| Interventions |
Intervention:
Intervention:
Comparison: no treatment:
Authors pooled data because they identified no difference in outcomes. Data are not presented individually, only pooled. We have therefore analyzed as "Other regimen". |
|
| Outcomes | Febrile morbidity (oral temperature > 38 °C twice at least 6 hrs apart after the 1st 24 hrs); wound infection (fever, cellulitis, and/or exudate); endometritis (fever, uterine tenderness and foul discharge, or fever and a positive culture with uterine tenderness and no other apparent cause); UTI (fever, urinary tract symptoms and/or positive culture > 100,000 organisms/mL if pre‐operative culture negative); pulmonary infection (fever with abnormal chest x‐ray and/or physical signs of consolidation); undetermined (persistent fever with no discernible signs of infection). | |
| Notes | Results of 2 antibiotic groups reported together. Class of antimicrobial: other regimen (1st generation cephalosporin vs extended spectrum penicillin (carboxypenicillin (carbenicillin)). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "By random distribution of the last digit of their hospital number" .... "Lack of any significant differences between the two groups confirmed adequate randomization". Comment: quasi‐RCT. |
| Allocation concealment (selection bias) | High risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participant excluded after randomization; ITT analysis. |
| Selective reporting (reporting bias) | High risk | Comment: authors chose to pool data from the 2 treatment groups because they identified no difference in outcomes. Data were not presented individually, only pooled. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Fugere 1983.
| Methods | Randomized, placebo‐controlled trial; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: September 1980 to November 1981. Setting: Hopital Saint‐Luc, Montreal, Canada. Inclusion criteria: women undergoing non‐elective CS. N = 89. Exclusion criteria: not in labor with intact membranes, allergy to cephalosporins, antibiotic use within 48 hrs, fever, ruptured membranes for > 36 hrs. |
|
| Interventions |
Intervention:
Intervention:
Comparison: placebo:
At clamping of the cord and at 6 and 12 hrs later. Placebo group was divided 1/2 and for comparison with the 2 treatment groups. |
|
| Outcomes | Endometritis, wound infection, UTI (symptoms or 2 successive positive cultures) septicemia, pelvic abscess, pelvic thrombophlebitis. Follow‐up at 6 weeks. No side effects observed. | |
| Notes | There were no serious infections in any of the groups.
In the placebo and cefazolin groups there was no increase in aerobic bacterial colonization of the cervix after 4 days but there was an increase in colonization by anaerobes; the opposite occurred in the group receiving cefoxitin. Class of antibiotic: 1st generation cephalosporin vs cefamycin (2nd generation cephalosporin). Subgroups:
The groups were comparable regarding demographic characters. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A number (1 to 90) identified the boxes. The number was allocated randomly to a box". Comment: an envelope containing the randomization code was available in case of adverse reactions. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no loss to follow‐up; 1 patient in the control group was excluded from analysis as no cultures were performed; as‐treated analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind"..... "vitamin solution with a similar colour as the other preparations". Comment: placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Gall 1979.
| Methods | RCT, 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not stated. Setting: Duke Universtiy Medical Center, North Carolina, USA. Inclusion criteria: all women undergoing either a repeat CS or in labor. N = 95. Exclusion: clinical infection, ruptured membranes for > 12 hrs, prior antibiotics within 48 hrs, renal or hepatic disease. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Wound infection (cellulitis, purulent exudate, intraperitoneal abscess or peritonitis); endometritis; UTI; maternal hospital stay. | |
| Notes | No minor side effects (rash or pruritus) or major reactions (anaphylaxis) observed.
4 women (all in control group) had septicemia (counted as serious morbidity). Class of antimicrobial: 1st generation cephalosporin. Subgroups:
The 2 groups were comparable regarding age, racial distribution, parity, number of catheterizations or length of time of indwelling catheter. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participant excluded after randomization; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". Comment: placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Ganesh 1986.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Date of data collection: November 1983 and December 1984. Setting: University Hospital, New Jersey; lower socioeconomic class (90% black). Inclusion criteria: women < 21 years old undergoing CS. N = 57. Exclusion: antibiotic use within 2 weeks; active infection or fever at delivery; penicillin or sulfa allergy; internal fetal monitoring. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Endomyometritis (fever [> 100.3oF twice within 24 hrs after 1st day], uterine tenderness, absence of another focus); UTI (fever and positive culture); wound infection (fever, abnormal appearing wound with cellulitis or a wound draining purulent material). | |
| Notes | Authors' definition of high risk not comparable with that used in this review.
The incidence of UTI and wound infection was similar between the groups (numbers not given). Class of antibiotic: trimethoprim/sulfamethoxazole. Subgroups:
The 2 groups were comparable regarding age, gravidity, vaginal examinations, duration of labor and duration of rupture of membranes, elective repeat CS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly divided". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up reported; no participants excluded; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: placebo‐controlled; no further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Garcia 1992.
| Methods | Randomized trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Date of data collection: not reported. Setting: Salamanca, Spain. Inclusion criteria: women undergoing CS (both emergency and elective). Definitions of emergency (in labor) and elective (planned) not consistent with definitions used in this review; classified as "both". Exclusion criteria: fever ≥ 37.5ºC before or during childbirth; premature rupture of membranes > 12 hrs; allergic to penicillin or its derivatives; prior antibiotic therapy; signs of renal and hepatic dysfunction. |
|
| Interventions |
Intervention 1:
Intervention 2:
|
|
| Outcomes | Endometritis (fever, uterine tenderness and fetid discharge or fever and pathogenic organisms cultured from the lochia); wound infection (fever, induration and exudation and/or cultures of purulent exudate form the wound with or without fever); UTI (fever and urinary symptoms and/or positive urine culture with or without fever); pelvic septic thrombophlebitis (none in either group). | |
| Notes | Translated from Spanish. Class of antibiotic: 2nd generation cephalosporin. Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no loss to follow‐up or exclusion of participants reported; no explanation for uneven number of participants between groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind"; control group administered "a placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Gerstner 1980.
| Methods | Randomized trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: August 1979 and April 1980. Setting: Universitats‐Frauenklinik Wien, Austria. Inclusion criteria: women undergoing CS. N = 103. |
|
| Interventions |
Intervention:
Comparison: no treatment:
|
|
| Outcomes | Fever (> 38oC on 2 subsequent days); wound infection; endometritis; additional use of antibiotics (treatment 13/53 vs control 22/50); maternal hospital days. | |
| Notes | Full translation pending. Class of antibiotic: nitroimidazole (metronidazole). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized...". Comment: no description of sequence generation process and no further details provided in translation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses or exclusions were reported; appears to be an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Gibbs 1972.
| Methods | Random allocation is presumed although method not described. | |
| Participants | Dates of data collection: November 1971 and April 1972. Setting: University of Pennsylvania. Inclusion criteria: women undergoing primary CS or repeat section. N = 61. Exclusion criteria: penicillin allergy, fever in labor. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Endometritis (fever and uterine tenderness or fever and pathogenic organism without other cause); UTI; wound infection (fever, cellulitis and exudate); morbidity [fever > 100oF in 2 separate 24 hr periods after 1st postpartum day or positive post‐operative urine culture of > 100,000 colonies/mL] (treatment 9/33 vs placebo 17/28); UTI (fever and urinary tract symptoms or a single significant culture with or without fever); maternal hospital stay (6.5 vs 6.9 days; no variance given). | |
| Notes | 2 serious infections: 1 pelvic abscess in treatment group, 1 septicemia in placebo group.
Authors' definitions of repeat and primary section not consistent with those used for elective/non‐elective in this review. Class of antibiotic: aminoglycoside‐containing combination (aminopenicillin (ampicillin), penicillinase‐resistant penicillin (methicillin), aminoglycoside). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...the patient randomization" ... "The patient randomization is statistically acceptable". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "The materials were prepared by the pharmacy service in coded identical vials, containing identically appearing solutions". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: 17 patients (28%) "were eliminated from the study, 6 for errors in giving the study medications, 5 for penicillin allergies, 3 for fever in labor, 2 for being started on ampicillin prophylaxis, and 1 for cesarean hysterectomy". Comment: no loss of participants to follow‐up. Analysis done on included patients; no data available to incorporate data on patients eliminated. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: placebo‐controlled; study described as double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Gibbs 1973.
| Methods | Random allocation is presumed although method not described. | |
| Participants | Dates of data collection: August 1972 to February 1973. Setting: University of Pennsylvania. Inclusion criteria: women undergoing CS. N = 68. Exclusion criteria: penicillin allergy, fever in labor. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Endometritis (fever and uterine tenderness or fever and pathogenic organism without other cause); UTI; wound infection (fever, cellulitis and exudate; any grade); morbidity [fever > 100oF in 2 separate 24 hr periods after 1st postpartum day or positive post‐operative urine culture of > 100,000 colonies/mL] (treatment 8/34 vs placebo 22/34). | |
| Notes | 1 pelvic abscess in placebo group.
Authors' definitions of repeat and primary section not comparable to those used for elective/non‐elective in this review, categorized as 'both'. Class of antibiotic: aminoglycoside‐containing combination. Subgroups:
The groups were comparable regarding age, rupture of membranes, indication for CS and anemia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization of patients is acceptable". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "The materials were prepared by the pharmacy service in coded identical vials, containing identically appearing solutions". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "25 patients were eliminated because of penicillin allergy, fever in labor, errors in giving the medication, etc. None was used as a control". Comment: an ITT analysis was not performed and the data cannot be re‐included. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: placebo‐controlled; described as double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Gibbs 1981.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: October 1978 and July 1979. Setting: Robert B Green Memorial Hospital, Texas, US; patients indigent and predominantly Mexican‐American. Inclusion criteria: women in labor with rupture of membranes (non‐elective). Exclusion criteria: infection, antibiotics within prior 3 days, allergy to penicillin or cephalosporin; no consent. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Endomyo(para)metritis; wound infection; maternal hospital stay; records reviewed 6 weeks to 6 months after discharge. 4 episodes of bacteremia (1 in treatment group, 3 in placebo) have been categorized as serious outcomes. | |
| Notes | No incidence of pelvic abscess or septic thrombophlebitis in either group.
Increase in Enterobacteriacae and enterococci and decrease in gram positive anaerobes and nonpathogens in prophylactic group.
No adverse clinical or laboratory results attributable to treatment. Class of antibiotic: 2nd generation cephalosporin. Subgroups:
The 2 groups were comparable regarding age, parity, race, gestational age, weight, indications for CS, anesthesia, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses to follow‐up; no participants excluded; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, identical‐appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Gordon 1979.
| Methods | RCT, 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of collection: enrolment started November 1976. Setting: San Bernadino county and University of California at Los Angeles Medical Centers; primarily indigent cases. Inclusion criteria: women undergoing CS. N = 114. Exclusion: emergency section, penicillin allergy, fever > 38 degrees C, on antibiotics; declined to participate. |
|
| Interventions |
Intervention:
Intervention:
Comparison: no treatment:
Outcomes of both treatment groups combined. |
|
| Outcomes | Endometritis; wound infection; UTI; maternal hospital stay (5.1 and 4.7 for pre‐ and post‐administration of antibiotics respectively vs 6.0 for no treatment, variance not given). | |
| Notes | Although emergency CSs were excluded, the women enrolled did not conform to our definition of an elective section.
Information on neonatal morbidity collected; there were 2 infants with definite infections in mothers who received no antibiotics and 1 infection in an infant where antibiotics were given after cord clamping. Class of antibiotic: aminopenicillin (ampicillin). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "at random". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participant excluded after randomization; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...because of the different modes of administering the antibiotics, a double‐blind study was not possible". Comment: no blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The investigator was not intimately involved with the post‐operative care ... and the pediatricians did not know into which group the mothers had been placed". |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Gummerus 1984.
| Methods | 'Randomly divided' (no details provided); placebo‐controlled. | |
| Participants | Dates of data collection: December 1981 to August 1982. Setting: School of Midwifery, Helsinki, Finland. Inclusion criteria: women undergoing CS (average duration of labor 8 hrs 45 min; average duration of membrane rupture 6 hrs 48 min) N = 219. Exclusion: women undergoing elective CS (not defined further); antibiotics prior to procedure. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Fever (temperature ≥ 38oC); wound infection, endometritis, sepsis (temperature > 38.5oC and bacteremia); abscess of pouch of Douglas (1 in each group). | |
| Notes | Translated from German. Class of antibiotic: nitroimidazole (metronidazole). Subgroups:
The groups were comparable in respect of social status, age, parity, duration of pregnancy, primary section/repeat section, axillary temperature before the procedure, localization of skin incision, number of amnioscopes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly" divided into 2 groups. Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no losses or exclusions reported; it appears the analysis was ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: placebo‐controlled; no additional information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Hager 1983.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: Central Baptist Hospital, Lexington, Kentucky, US. Inclusion criteria: women undergoing primary, non‐elective CS (while it appears most women were in labor and/or had ruptured membranes it is unclear whether all patients fulfilled our criteria for non‐elective). N = 90. Exclusion: antibiotic use within 7 days, penicillin or cephalosporin allergy. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Infectious morbidity (fever > 100.3oF twice 6 hrs apart after 1st 24 hrs); endomyometritis (fever, uterine tenderness, and positive culture from endometrium); wound infection, UTI; maternal duration of stay (treatment 5.1 days vs placebo 5.4; not significant, no variance given). | |
| Notes | There was 1 episode of bacteremia in the control group. Class of antibiotic: 2nd generation cephalosporin. Subgroups:
The 2 groups were comparable regarding age, race, parity, weight, type of anesthesia, operating time or pre‐operative hematocrit. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "according to pre‐numbered envelopes maintained in the central pharmacy". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up; no participant excluded; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind".... "identical appearing, equal volume solution". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Hagglund 1989.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collections: July 1983 and December 1986. Setting: University Hospital, Lund, Sweden. Inclusion criteria: women undergoing emergency CS (during labor and/or after rupture of membranes [duration not specified]); categorized as "both" for this review. N = 160. Exclusion criteria: fever > 38oC, given antibiotics, chemotherapy or immunosuppressive therapy in prior 3 weeks, allergy to cephalosporins, alcohol or drug abuse, chronic disease of cardiovascular, renal, hepatic or gastrointestinal system, severe anemia. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Endometritis (fever > 38oC twice at least 1 hr apart, after the 1st post‐operative day, and increased tenderness of the uterus); wound infection (redness, tenderness, increased heat and edema of wound); UTI. | |
| Notes | There were no cases of septicemia or abscess formation observed in either group.
Only 55% of women had ruptured membranes (number > 6 hs not stated) and 77% were in labor; these definitions do not meet our criteria for non‐elective section, categorized as 'both'. Class of antibiotic: 2nd generation cephalosporin. Subgroups:
The 2 groups were comparable regarding age, parity, previous CS, complications during pregnancy and gestational age at the operation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...a pre‐set randomized series in a double‐blind manner...". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses or exclusions were reported. It appears to be an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind". Comment: placebo‐controlled (saline). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Harger 1981.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not stated. Setting: Pittsburgh, Pennsylvania, US. Inclusion criteria: women undergoing CS after labor or rupture of membranes (method section unclear as to duration of ruptured membranes; it has been assumed that all women were in labor). N = 386. Exclusion criteria: elective CS without labor; already receiving antibiotics; fever or other evidence of infection; allergy to penicillin or cephalosporins; requiring endocarditis prophylaxis. |
|
| Interventions |
Intervention: cephalosporin (B2a):
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (fever > 37.9oC twice at least 4 hrs apart after 1st post‐operative day); endomyometritis (fever > 38oC with uterine tenderness, maternal white blood cell count > 15000/cu mm, malodorous lochia and no apparent cause for fever); UTI; incision infection (purulent drainage with induration and tenderness); additional antibiotic therapy (treatment 26/196 vs placebo 68/190). | |
| Notes | Increase in enterococci and decrease in Staphylococcus aureus, various streptococci, E. coli and a variety of anaerobes from infected sites in prophylactic group compared with placebo. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
The 2 groups were comparable regarding demographic and obstetric variables and indications for CS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "The hospital pharmacy prepared coded vials". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: no loss to follow‐up reported. 14/400 women initially randomized not included in final analysis (errors in protocol, 2 allergic to penicillin after 1st dose given and 2, who received cefoxitin, for infusion‐related reactions); insufficient data provided to perform ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, identical appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Hawrylyshyn 1983.
| Methods | RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: July 1980 to June 1981. Setting: Mount Sinai Hospital, Toronto, Canada. Inclusion criteria: women undergoing CS (at 'high' risk because of ruptured membranes in active labor); classified as 'non‐elective'. N = 182. Exclusion criteria: febrile, antibiotic use in prior 24 hrs; allergy to penicillin or cephalosporin; significant hepatic or renal disease. Predominantly private, middle‐class and in their late 20s. |
|
| Interventions |
Intervention: cephalosporin (B2a):
Intervention: cephalosporin (B2a):
Comparison: placebo:
Both treatment groups combined in this analysis. |
|
| Outcomes | Febrile morbidity (> 38oC twice at least 8 hrs apart, after 1st post‐operative day); endometritis (fever, foul, excessive lochia or uterine tenderness); UTI (fever and positive culture); wound infection (fever, cellulitis or exudate with positive cultures). | |
| Notes | No adverse drug reactions in cefoxitin groups, no septicemia in any group; 4 patients in placebo group were considered seriously ill (although do not fit the criteria for serious morbidity in this review) compared to none in treatment groups. Antibiotic class: cefamycin (2nd generation cephalosporin). Subgroups:
The 3 groups were comparable regarding age, parity, gestational age, duration of labor, duration of ruptured membranes, number of vaginal examinations, use of internal fetal monitoring or post‐operative hemoglobin. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...randomly packaged in identical vials coded from 1 to 200". Comment: insufficient information provided to judge. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: no loss of participants to follow‐up; 7 patients were excluded after having entered the study. 1 patient was excluded because of an error in mixing and administering the IV injections; 6 patients were excluded because they became febrile within 8 hrs of operation and required immediate antibiotic therapy. As‐treated analysis; data from excluded patients could not be re‐included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...double‐blinded, placebo‐controlled" ... "The medication and an identical appearing placebo were prepared prior to the study and ... packaged in identical vials....The attending physician was unaware of what regimen his patient received and the code numbers were revealed only after the study was completed". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The attending physician was unaware of what regimen his patient received". Comment: probably outcome assessment was blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Heilmann 1984.
| Methods | Randomized trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not stated. Setting: Essen, Germany. Inclusion criteria: women undergoing CS in labor (classified as non‐elective). Exclusion criteria: none reported. |
|
| Interventions |
Intervention 1:
Intervention 2:
|
|
| Outcomes | Febrile morbidity (oral temperature > 30°C for at least 2 days); wound infection (reported as "healing difficulties" in translation of table); UTI (105 bacteria/mL after removal of the catheter); length of post‐operative stay. | |
| Notes | Translated from German. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
The groups were comparable for age, weight, duration of operation, birthweight, duration of urinary catheter use, premature rupture of membranes > 12 hrs; intrauterine fetal monitoring; birthweight. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "open randomization". Comment: no additional information provided; no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up; analysis was ITT. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Huam 1997.
| Methods | Quasi‐RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: September 1994 to April 1995. Setting: University Hospital, Kuala Lumpur, Malaysia. Inclusion criteria: elective CS. Exclusion criteria: allergic to penicillin, evidence of infection, premature rupture of membranes, receiving antibiotics prior to CS. |
|
| Interventions |
Intervention:
Comparison: no treatment:
|
|
| Outcomes | Febrile morbidity (fever > 38°C twice at least 4 hrs apart after the 1st 24 hrs); wound sepsis (defined and graded as a) erythema and/or induration, b) serous oozing, c) presence of pus, d) wound dehiscence); UTI (routine midstream urine on 3rd post‐operative day > 100,000 organisms/mL); endometritis (fever, uterine tenderness and foul smelling lochia); pneumonia (cough, fever and/or radiographic evidence of pulmonary consolidation). | |
| Notes |
Class of antibiotic: beta‐lactamase inhibitor combination. Subgroups:
"Both the study groups and control group were comparable in terms of patient characteristics and operative variables." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Alternately allocated to either antibiotic group or control group". Comment: quasi‐RCT. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participant excluded after analysis; ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Ismail 1990.
| Methods | Double‐blind, randomized, placebo‐controlled; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: University of Illinois College of Medicine, Chicago, US (large, inner city hospital); majority of participants black (40%) or Hispanic (60%). Inclusion criteria: undergoing CS. N = 152. Exclusion: pre‐operative fever, antibiotics within 1 week, membranes ruptured > 36 hrs, evidence of chorioamnionitis, penicillin or cephalosporin allergy. |
|
| Interventions |
Intervention: cephalosporin (B2a):
Comparison: placebo:
|
|
| Outcomes | Endometritis (fever and uterine tenderness or fever and pathologic organism without other focus); wound infection (fever, cellulitis and exudate); UTI (fever and symptoms or positive culture). | |
| Notes | In the placebo group there were 8 episodes of serious morbidity (6 cases of sepsis; 1 pelvic abscess; 1 episode of pelvic thrombophlebitis) compared with 1 in the treated group (1 episode of sepsis).
Routine post‐operative cultures were performed: enterococci were isolated from 30/68 cases who received cefoxitin vs 15/74 who received placebo; there was no change in the rate of cefoxitin resistance in Enterobacteriaceae from the stool after prophylaxis. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized...". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses or exclusions were reported. The analysis appeared to be ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Jaffe 1984.
| Methods | RCT, 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: March to October 1982. Setting: A Meir General Hospitla, Kfar Sava, Israel. Inclusion criteria: patients undergoing CS, classified as “no‐labor” if cesarean was performed before onset of labor and “labor” if occurred after onset of labor. N = 113. Exclusion criteria: evidence of infection, known allergy to penicillin, antibiotic therapy during the previous 2 weeks. |
|
| Interventions |
Intervention: extended spectrum penicillin:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (2 oral temperatures > 38°C at least 4 hrs apart after the 1st 24 hrs); endometritis (fever and uterine tenderness with or without a positive lochial culture and no other apparent cause of fever); UTI (> 105colonies/mL after a negative pre‐operative culture); wound infection (fever, cellulitis, exudate and tenderness). | |
| Notes |
Class of antibiotic: extended spectrum penicillin (ureidopenicillin (mezlocillin)). Subgroups:
"The groups did not differ significantly in obstetrical variables...indications for CS were similar in both groups." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 7 patients were excluded for errors in following the protocol; excluded patients not included in analysis; data cannot be imputed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Placebo‐controlled". Comment: insufficient information to judge. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Jaffe 1985.
| Methods | Randomized placebo‐controlled trial: 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not stated. Setting: Kfar‐Sava, Israel. Inclusion criteria: women undergoing CS. N = 78. Exclusion: women with active infection, allergy to penicillin and antibiotic treatment within 2 weeks. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (> 38oC twice at least 4 hrs apart after 1st 24 hrs post‐operative); endometritis (fever and uterine tenderness); UTI (single culture of > 100,000 bacteria/mL); wound infection (redness, cellulitis, tenderness and exudate from incision). | |
| Notes | Authors' definition of emergency not consistent with definitions used in this review (classified as 'both/undefined'). Class of antibiotic: extended spectrum penicillin (ureidopenicillin (mezlocillin)). Subgroups:
The 2 groups were comparable regarding age, parity, rupture of membranes, duration of ruptured membranes, number of vaginal examinations, duration of anesthesia, and indications for CS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly assigned..". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: it is unclear whether all patients randomized were included in the analysis but no losses or exclusions were reported. It appeared to be an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Placebo‐controlled". Comment: insufficient information to judge. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Jakobi 1994.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection; not reported. Setting: Rambam Medical Center, Haifa, Israel. Inclusion criteria: low‐risk women requiring cesarean delivery (elective procedure, duration of membrane rupture < 3 hrs, no more than 2 vaginal examinations). N = 307. Exclusion: required a drug other than cefazolin for prophylaxis, fever, membrane rupture > 24 hrs. |
|
| Interventions |
Intervention:
Comparison: no treatment:
|
|
| Outcomes | Febrile morbidity (fever > 37.7oC twice at least 4 hrs apart after 1st 24 hrs); endometritis (fever, uterine tenderness and abnormal lochia); UTI (fever and positive culture); wound infection (fever, cellulitis or exudate with positive culture); therapeutic antibiotic use (treatment group 6.5% vs 20% in control group, P < 0.001). | |
| Notes | Although some women were in labor at the time of the procedure (mean duration of labor 53 and 44 minutes in the 2 groups), the study population so closely resembles the criteria for elective CS used in this review that the results have been included in the 'elective' category. Class of antibiotic: 1st generation cephalosporin. Subgroups:
The groups were comparable regarding socioeconomic level, weight, gestational age, post‐operative hemoglobin and operation time. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Randomized "by computer program to 1 of 2 groups" at time of their first antenatal visit. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no losses to follow‐up; no participant excluded. Imbalance in group size not accounted for (likely because randomization occurred at 1st antenatal visit and not all patients randomized were enrolled). ITT analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Karhunen 1985.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: May 1982 to August 1983. Setting: South Saimaa Central Hospital, Lappeenranta, Finland. Inclusion criteria: initially all women undergoing CS (N = 80); thereafter women undergoing non‐elective (ruptured membranes) section. |
|
| Interventions |
Intervention: nitroimidazole:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (> 38oC on 2 post‐operative days, excluding the 1st); endometritis (fever, foul lochia or uterine tenderness); wound infection (fever, cellulitis or exudate); UTI (fever and positive culture). | |
| Notes | Authors' definition of non‐elective (ruptured membranes) and elective (unruptured membranes) not consistent with the definitions used in this review; classified in this review as 'both'.
Newborn infants observed for effects of tinidazole (although data not given). Class of antibiotic: nitroimidazole (tinidazole). Subgroups:
The 2 groups were comparable regarding age, weight, gestational age, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized according to a code". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Identical vials ... coded from 1 to 160". Comment: insufficient information to judge. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: no loss to follow‐up reported; 8 women excluded: 4 because they were febrile before the operation, 4 because of mistakes in administration; data not provided to perform ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo‐controlled, double blind" ... "The code was first opened when the study was completed". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Kellum 1985.
| Methods | RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: September 1982 to September 1983. Setting: University of Mississippi Medical Center. Inclusion criteria: women undergoing non‐elective CS (including prolonged ruptured membranes and prolonged labor, as well as general risk factors such as poor nutrition and poverty). Exclusion: current antibiotics, known infectious process, allergy to cephalosporins. |
|
| Interventions |
Intervention: 2nd generation cephalosporin:
Intervention: placebo:
Comparison: no treatment:
As the objective of this review is to compare antibiotic with no antibiotic, rather than the effect of irrigation, the 2 irrigation groups are compared. |
|
| Outcomes | Febrile morbidity (> 100.6oF twice 6 hrs apart after 1st post‐operative day); serious morbidity (fever and endomyometritis or abscess requiring IV antibiotics for resolution). | |
| Notes | Authors' definition of high risk does not correspond to that used for non‐elective in this review, classified as 'both'.
The outcome of serious morbidity included endomyometritis and is classified as endometritis in this review. Class of antibiotic: 2nd generation cephalosporin. Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Randomized by last digit of hospital admission number". Comment: quasi‐randomized trial. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: no loss to follow‐up or exclusion of participants reported, but follow up given for only 77/84 of treatment and 53/86 of placebo group for outcome of serious infection, without explanation; no evidence ITT analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: placebo‐controlled (normal saline irrigation). No further information provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Kolben 2001.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: January 1996 to December 1997. Setting:Technical University of Munich. Munich, Germany. Inclusion criteria: elective CS. N = 146. Exclusion criteria: evidence of pre‐existing infections, labor, rupture of membranes, oral temperature > 37.5°C, antibiotic therapy within 72 hrs or surgery, immune deficiency, known allergic reaction to cephalosporins, age < 18 years. |
|
| Interventions |
Intervention: 2nd generation cephalosporin:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (oral temperature of > 38°C twice on at lease 2 occasions 24 hrs apart or > 38.5°C on 1 occasion after the 1st 24 hrs); wound infection (purulent material at site of incision), endometritis (fever, uterine tenderness and offensive lochia), UTI (> 100,000 bacteria/mL of midstream urine in patients with symptoms (urgency, dysuria, frequency). | |
| Notes | Unable to confirm whether drug given after clamping of cord. Class of antibiotic: 2nd generation cephalosporin. Subgroups:
"No significant difference was detected between the 2 groups for age, gestational age, health insurance status, body mass index, kind of anesthesia, duration of surgery, or additional pregnancy risk factors." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "according to a computer generated random assignment". |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participant excluded after randomization. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Kreutner 1978.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: November 1975 to June 1976. Setting: Medical University Hospital of South Carolina. Inclusion criteria: all women undergoing CS (51/97 not in labor; 61/97 without ruptured membranes). N = 97. Exclusion criteria: signs of infection, allergy to penicillin or cephalosporin, antibiotics within 2 weeks; lack of consent. |
|
| Interventions |
Intervention: 1st generation cephalosporin:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (> 100.3oF twice on any of 1st 10 postpartum days after the 1st); endometritis (fever and uterine tenderness, or fever and pathogen from endometrium without other cause); UTI (fever or positive culture and symptoms); wound infection (fever, cellulitis and/or exudate). | |
| Notes | Aerobic isolates unchanged, fewer anaerobes in patients given placebo; most pathogens isolated were resistant to cefazolin whether treatment or placebo given.
There were 2 episodes of septicemia (both in placebo group). Class of antibiotic: 1st generation cephalosporin. Subgroups:
The 2 groups were comparable regarding race, age and type of CS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random allocation". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: no loss to follow‐up reported. 6 women initially randomized not included in analysis (non‐adherence or noninfectious complications). As‐treated analysis performed; could not re‐include data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A similar volume of placebo" was administered to the control group. Comment: insufficient information to judge. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Kristensen 1990.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: February 1987 to March 1988. Setting: Odense University Hospital, Denmark. Inclusion criteria: women undergoing non‐elective CS (58/201 without labor; 65/201 without ruptured membranes). N = 201. Exclusion: fever, antibiotics within 7 days, penicillin or cephalosporin allergy. |
|
| Interventions |
Intervention: 2nd generation cephalosporin:
Comparison: no treatment:
|
|
| Outcomes | Febrile morbidity (> 37.9oC twice at least 6 hrs apart after 1st post‐operative day); endometritis (fever, uterine tenderness and abnormal lochia); wound infection (fever, cellulitis and/or purulent discharge); UTI; cost of post‐operative antibiotics (treatment $US0.69 vs control $US7.47); maternal hospital stay (treatment 8.1 vs control 8.0, no variance given). | |
| Notes | No woman had a severe infection such as pelvic abscess or septic pelvic thrombophlebitis. Class of antibiotic: 2nd generation cephalosporin. Subgroups:
The 2 groups were comparable regarding epidemiologic and obstetric data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly..". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "envelopes containing empty vial or vial containing treatment". Comment: insufficient information to judge. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses or exclusions were reported. The analysis appears to be by ITT. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients, attending physicians, and study coordinators were blind with regard to group assignments". Comment: not placebo‐controlled; insufficient information to judge. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Lapas 1988.
| Methods | Double blind, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: Athens, Greece. Inclusion criteria: women undergoing elective or non‐elective CS. Age range 17‐40 years. N = 100. Exclusion criteria: allergy to metronidazole, amnionitis, and pyrexia. |
|
| Interventions |
Intervention: Nitroimidazole:
Comparison: placebo:
|
|
| Outcomes | Wound infection; endometritis; inadequate wound healing (metronidazole 1/50 vs placebo 8/50); mean temperature (36.8oC SD 1.02 vs 37.6, 1.03); duration of hospital stay. | |
| Notes | Although the authors are not identical and the presentation of the data makes direct comparisons difficult, the description of the 2 studies cited is so similar that it is presumed the 2 citations refer to the same patient population. Translated from Bulgarian. Class of antibiotic: nitroimidazole (metronidazole). Subgroups:
There was no significant difference in parity and age between the groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Women were divided into 2 groups". Comment: insufficient information to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information available. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind; placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Lemus 2005.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Date of data collection: not reported. Setting: Hospital de Ginecologia y Obstetrcia num. 3 del Centro Medico La Raza, Mexico City, Mexico Inclusion criteria: women who had undergone CS with: no risk factors for wound infection at the time of the operation; no UTI or cervicovaginitis; intact membranes; less than 6 vaginal examination before the operation; no allergy to beta‐lactam antibiotics; clean surgical procedure without prolonged stage of labor (less than 33 minutes); primary closure of the incision; no use of drains. Exclusion criteria: women with medical factors that would lead to inadequate wound healing (diabetes, obesity, malnutrition); post‐surgical factors (asthma, pulmonary complications, coughing and vomiting after surgery); ascites; anemia; history of radiotherapy; anesthetic complications requiring intensive care admission; corticosteroid use; incomplete follow‐up. |
|
| Interventions |
Intervention 1:
Intervention 2:
|
|
| Outcomes | Wound infection (follow‐up until day 30); definition of wound infection unclear. | |
| Notes | Translated from Spanish Class of antibiotic: 3rd generation cephalosporin. Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: Women were allocated "at random and sequentially, but non‐random" as it depended on the obstetrician to start the prophylaxis perioperatively. |
| Allocation concealment (selection bias) | High risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: women with incomplete follow‐up were excluded. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding; not placebo‐controlled; obstetrician started prophylaxis. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Leonetti 1989.
| Methods | Randomized placebo‐controlled trial; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Date of data collection: not reported. Setting: Jersey City Medical Center, New Jersey; predominantly lower socioeconomic indigent women. Inclusion criteria: women undergoing primary CS after onset of labor (corresponds to the definition of non‐elective). N = 150. Exclusion: febrile or infected, allergy to pipericillin. |
|
| Interventions |
Intervention 1: ureidopenicillin:
Intervention 2: ureidopenicillin:
Comparison: placebo:
Both treatment groups combined in analysis. |
|
| Outcomes | Febrile morbidity (> 38.0oC twice at least 6 hrs apart after 1st post‐operative day); endometritis (fever, tender uterus and purulent lochia); hospital stay (no significant difference, variance not given). | |
| Notes | Use of saline or antibiotic lavage not allowed.
No adverse reactions reported with treatment. Class of antibiotic: extended spectrum penicillin (ureidopenicillin (pipericillin)). Subgroups:
The 3 groups were comparable regarding number of vaginal exams, hemoglobin levels and other risk factors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly divided..". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses or exclusions were reported. The analysis appears to be by ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "blinded". Comment: placebo‐controlled; insufficient information to judge. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Levin 1983.
| Methods | RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: February to June 1982. Setting: Kaiser‐Permanente Medical Center‐Santa Clara, California. Inclusion criteria: all women undergoing CS (39/128 repeat section). N = 128. Exclusion: fever or infection, allergy to antibiotics. |
|
| Interventions |
Intervention: 1st generation cephalosporin:
Intervention: cefamycin:
Comparison: placebo:
Placebo group divided 1/2 for comparison with 2 treatment groups. |
|
| Outcomes | UTI (positive culture); wound infection (purulent wound discharge with or without wound separation); endometritis (fever > 100.4oF after 1st post‐operative day, uterine tenderness, foul smelling lochia without other source). | |
| Notes | Follow‐up for 8 weeks.
1 patient in placebo group developed septic pelvic thrombophlebitis and septic pulmonary emboli, classified as a serious complication. Class of antibiotic: 1st generation cephalosporin vs cefamycin (2nd generation cephalosporin). Subgroups:
There were no statistically significant differences in mean gestational age, mean number of vaginal examination or mean duration of ruptured membranes between groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Sequenced randomly by lottery method". |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to judge. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 4 patients were eliminated from the statistical analysis because of deviations from the protocol of irrigation technique. As‐treated analysis. Data could not be re‐included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "1 milliliter of multivitamin infusion was added to create an identical appearance of all solutions .... and bags sequenced randomly ... used in numerical order" ..". Patients, physicians, operative room personnel and data collectors were... blinded to the group assignment". Comment: double‐blind; identical appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients, physicians, operative room personnel and data collectors were... blinded to the group assignment". |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Lewis 1990.
| Methods | Random, double‐blind, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: July 1985 to January 1986. Setting: Louisiana State University Hospital; 90% indigent population. Inclusion criteria: women undergoing elective and non‐elective CS (definitions of elective and emergency CS not provided; results combined in the analysis). Exclusion: antibiotic use within 2 weeks, allergy to penicillin. |
|
| Interventions |
Intervention: carboxypenicillin:
Comparison: placebo:
Results of the 2nd part of the study (cefoxitin vs ticarcillin) not included. |
|
| Outcomes | Febrile morbidity (> 100.3oF twice at least 4 hrs apart after 1st post‐operative day); endomyometritis, wound infection, UTI, septicemia, maternal hospital stay (treatment 4.5 vs placebo 5.4, no variance given). | |
| Notes | Definition of elective and non‐elective CS not provided.
There were 3 episodes of septicemia in those women undergoing emergency section (2 in the control group and 1 in the placebo group). Class of antibiotic: extended spectrum penicillin (carboxypenicillin (ticarcillin)). Subgroups:
The duration of labor was significantly longer in the ticarcillin group than in the saline group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... in a random double‐blind manner". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 8 women were lost to follow‐up; 7 women were excluded. As‐treated analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Magro 1983.
| Methods | Randomized, placebo‐controlled; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: November 1981 to May 1982. Setting: Milan, Italy. Inclusion criteria: women undergoing CS (both elective and non‐elective); part of lager study (n = 100) of prophylaxis for obstetric and gynecological procedures. Exclusion criteria: infection, antibiotic therapy, hypersensitivity to penicillin or cephalosporin, abnormal liver or kidney function. |
|
| Interventions |
Intervention 1:
Intervention 2:
|
|
| Outcomes | Febrile morbidity (fever of > 37.5°C for 2 or more days following the operation). In addition patients were classified as having a satisfactory outcome (treatment n = 1; placebo n = 1), an average outcome (treatment n = 8; placebo n = 8), or a good outcome (treatment n = 14; placebo n = 4) based on fever, laboratory tests and clinical course. | |
| Notes | Translated from Italian. Class of antibiotic: 2nd generation cephalosporin. Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "by randomization". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to judge. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no loss to follow‐up; difference in numbers between the 2 groups undergoing CS not explained. ITT analysis performed on available data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blinded" .... "the patients and the doctors were unable to see which type of treatment the patients were receiving". Comment: placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: probably outcome assessment was blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Mahomed 1988.
| Methods | Double‐blind, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: November 1986 and March 1987. Setting: University of Zimbabwe; patients enrolled between Inclusion criteria: all women undergoing elective CS (before onset of labor or rupture of membranes; corresponds to our definition of elective). N = 232. |
|
| Interventions |
Intervention: drug combination [penicillin+ chloramphenicol]:
Comparison: placebo:
|
|
| Outcomes | Fever (> 37.9oC twice at least 4 hrs apart after 1st post‐operative day); wound sepsis (graded as abnormal erythema and/or induration, oozing wound without frank pus or pus formation); endomyometritis (fever, uterine tenderness and foul‐smelling lochia), pelvic abscess formation, bacteremia; maternal hospital stay (treatment 5.43 vs placebo 6.18, variance not given). | |
| Notes | No woman developed pelvic abscess nor required a laparotomy. Class of antibiotic: other combination (crystalline penicillin and chloramphenicol). Subgroups:
The groups were comparable regarding age, pre‐operative weight, parity, previous CS, gestational age, and pre‐operative hemoglobin. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized list..". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to judge. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses or exclusions were reported. Analysis appears to be ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind". Comment: matching placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
McCowan 1980.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: June to September 1979. Setting: National Women's Hospital, Auckland, New Zealand. Inclusion criteria: all women undergoing CS (8/73 were repeat). N = 73. Exclusion: already on antibiotics. |
|
| Interventions |
Intervention: Nitroimidazole:
Comparison: placebo:
|
|
| Outcomes | Fever (> 37.9oC within 14 days of delivery); wound infection, endometritis, UTI, major complication (return to theatre or hospitalized > 10 days because of post‐operative morbidity); need for antibiotic therapy (treatment 13 vs placebo 10); fever index (257 degree hrs vs 165 hrs). | |
| Notes | 1 major complication (not infectious) in each group (bleeding from lower segment in 1, major deep vein thrombosis extending into iliac veins in another). Drug class: nitroimidazole (metronidazole). Subgroups:
The groups were comparable regarding age and weight. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses to follow‐up reported; no participants excluded. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Miller 1968.
| Methods | RCT: 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not specified. Setting: Durban, South Africa. Inclusion criteria: all patients undergoing CS. N = 300. Exclusion criteria: women with pre‐existing UTI. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | UTI (culture positive), intrauterine infection not defined further, classified as endometritis), wound infection. | |
| Notes | Fewer postpartum urinary isolates in treated group were sensitive to ampicillin (8/17 vs 18/26).
In the control group, 3 women developed pelvic abscesses (included as serious morbidity) and 1 patient required hysterectomy for secondary postpartum hemorrhage following severe E. coli intrauterine infection (included as serious morbidity). Class of antibiotic: aminopenicillin (ampicillin). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "on a random basis". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to judge. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participant excluded after randomization. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "an oral placebo was given after 48 hours". Comment: partly placebo controlled. Insufficient information to judge whether there was blinding of study personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Moodley 1981.
| Methods | RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: University of Natal, Durban, South Africa. Inclusion criteria: women undergoing emergency CS (ruptured membranes for > 6 hrs and < 20 hrs; corresponds to our definition of non‐elective). N = 60. Exclusion criteria: prior antibiotic therapy, fever > 37.2oC, fetal tachycardia of > 160/minute. |
|
| Interventions |
Intervention: lincosamides:
Intervention: nitroimidazole:
Comparison:
IV 2 hrs pre‐operatively and 8 hrly for 48 hrs. Placebo data were divided: 1/2 for comparison with the lincosamide and 1/2 for comparisons with the nitroimidazole. |
|
| Outcomes | Wound discharge/abscess formation, puerperal sepsis (> 37.9oC twice in 1st 48 hrs or > 37.5oC from 2nd post‐operative day), septicemia, UTI. | |
| Notes | Authors' definition of puerperal sepsis has been classified as fever.
No complications of drug administration reported in mothers or babies; no rash, diarrhea or nausea. Class of antibiotic: lincosamide (lincomycin) vs nitroimidazole (metronidazole). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "...using unmarked code‐numbered separate boxes". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses to follow‐up; no participants excluded. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind"; drug to be given was "in unmarked boxes in the original packing". Comment: placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Moro 1974.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: Norfold General Hospital, Virginia; both private (N = 70) and clinic (N = 78) women included. Inclusion criteria: all women undergoing CS (49/148 were repeat procedure; 57/148 were not in labor). N = 148. Exclusion: membranes ruptured > 24 hrs. |
|
| Interventions |
Intervention: 1st generation cephalosporin:
Comparison: placebo:
|
|
| Outcomes | Fever (> 100.3oF twice after 48 hrs); endometritis (fever, uterine tenderness, foul‐smelling or abnormal lochia and positive cultures); UTI, wound infection; maternal hospital stay (treatment 6.2 vs placebo 7.5, no variance given). | |
| Notes | All bacterial isolates in treatment group were sensitive to cephalothin. Class of antibiotic: 1st generation cephalosporin. Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Assigned in a random manner". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Comment: all preparations supplied by the pharmacy had a code number known only by the pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 52/200 excluded for various reasons, including 14 because of protocol violations. As‐treated analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, identical appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Morrison 1973.
| Methods | Quasi‐RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: City of Memphis Hospitals, Tennessee; indigent women, many obstetric and metabolic complications. Inclusion criteria: all women undergoing CS. N = 330. Exclusion criteria: febrile or infected. |
|
| Interventions |
Intervention: drug combination [penicillin + aminoglycoside]:
Comparison: no treatment:
|
|
| Outcomes | Fever (> 100.9oF after 2nd post‐operative day), severe pelvic infection (treatment 27% vs control 7%); 'free of infectious morbidity' (3.6 vs 6.8 days); maternal hospital stay (5.4 vs 8.8 days, no variance given). | |
| Notes | No adverse drug reactions reported; no evidence of development of resistance reported.
Unable to ascertain from description of study incidence of endometritis or wound infection; inadequate description of nature of severe pelvic infections (not included as outcome in analysis).
2 groups of women were studied retrospectively (N = 75); methods nor results do not specifically describe results of this group and it is unclear whether they have been included in the overall results. Class of antibiotic: aminoglycoside‐containing combination (natural penicillin and aminoglycoside. Subgroups:
The 2 groups were comparable regarding gravidity, parity, age and operative indicators. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...every other patient". Comment: quasi‐RCT. Alternate allocation to treatment or no treatment. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up; no participants excluded. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Ng 1992.
| Methods | RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: March to August 1991. Setting: Ipoh, Malaysia. Inclusion criteria: women undergoing CS. N = 222. Exclusions: hypersensitivity to 1 of antibiotics; presence of infection or fever; on antibiotics; multiple pregnancy. |
|
| Interventions |
Intervention: 3rd generation cephalosporin:
Intervention:
Comparison: no treatment:
At induction of anesthesia. Control data split to 6/35 and 5/35 for comparison with penicillin and cephalosporin respectively. |
|
| Outcomes | Wound infection (inflammation over wound with serous or purulent discharge); any antibiotics post‐operatively (cefoperazone vs ampicillin vs no treatment: 6.6% vs 16.2% vs 25.7%). Hospital stay: ampicillin vs no treatment 5.57 days (SD 1.43) vs 6.5 days (SD 3.67). | |
| Notes | Author's definition of emergency not consistent with criteria used in this review; classified as both/undefined. Class of antibiotic: 3rd generation cephalosporin or aminopenicillin (ampicillin). Subgroups:
The 3 groups were comparable regarding age, race, parity, gestational age, etc. The number of patients allocated to the control and cefoperazone group are different between the text and the table; the numbers in the text have been used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized..". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 patients excluded (1 from cefoperazone group, 1 from no treatment group); as‐treated analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Oestreicher 1987.
| Methods | Randomized trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: June 1984 to October 1985. Setting: Berlin, Germany. Inclusion criteria: women undergoing primary CS with no complications (no additional details available); classified as "both or undefined". Exclusion criteria: women with diabetes or penicillin allergy. |
|
| Interventions |
Intervention 1:
Intervention 2:
|
|
| Outcomes | Fever (temperature of ≥ 38°C for 3 hrs), slowness of wound healing (4/30 vs 17/30 for treatment and control groups respectively); endometritis; UTI; stay > 10 days (12/30 vs 19/30); need for post‐operative antibiotics (3/30 vs 6/30). | |
| Notes | Translated from German. Class of antibiotic: extended spectrum penicillin. Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no dropouts reported; no other details provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Padilla 1983.
| Methods | Randomized, double‐blind, placebo‐controlled trial; 2 parallel arms. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: Johns Hopkins Hospital, Baltimore, US. Inclusion criteria: all women undergoing CS (35/71 were a repeat section). N = 71. Exclusion: fever, membrane rupture > 24 hrs, penicillin allergy, lack of consent. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Fever (> 37.0°C twice at least 6 hrs apart after 1st post‐operative day); endometritis, UTI, wound infection, bacteremia, pelvic abscess, maternal hospital stay. | |
| Notes | The authors definition of primary and repeat are different from those used in this review and have not been analyzed separately; most women for repeat section were in early labor at the time the operation was performed. Study medications were administered pre‐operatively when possible, however transit time delays resulted in patients receiving medication after the surgical procedure had started. There was 1 pelvic abscess in the placebo group; there were 3 episodes of bacteremia (1 Klebsiella spp. in treatment group, 2 group B streptococcal infections in placebo); combined for outcome of serious morbidity. Class of antibiotic: aminopenicillin (ampicillin). Subgroups:
"There were no statistically significant differences in (the epidemiologic and obstetric variables in the two groups) when the study and placebo groups were compared." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "The medication code was kept in the pharmacy". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participant excluded after randomization. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "... or a similar appearing placebo... all solutions were prepared (in the pharmacy)". Comment: insufficient information to judge. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Phelan 1979.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: July to December 1976. Setting: Naval Regional Medical Center, Portmouth, Virginia, US. Inclusion criteria: all women undergoing CS (46/122 were a repeat section). The authors' definition of primary and repeat do not correspond to definitions of elective and non‐elective used in this review (repeat sections included women in labor with ruptured membranes). The results for these 2 categories have been combined in this review. Exclusion criteria: allergy to penicillin or cephalosporin, infection or receiving antibiotics. |
|
| Interventions |
Intervention: 1st generation cephalosporin:
Comparison: placebo:
|
|
| Outcomes | Endometritis (fever and uterine tenderness or fever and pathogenic organism); UTI (fever and symptoms, or positive culture); wound infection (fever, cellulitis and exudate); maternal hospital stay (treatment 5.5 days vs placebo 5.7 days, no variance given). | |
| Notes | 2 women developed serious complications as stated by the authors: 1 in treatment group developed septic pelvic thrombophlebitis; 1 given placebo developed pneumonia and endoparametritis (both included in outcome of serious morbidity). Class of antibiotic: 1st generation cephalosporin. Subgroups:
The 2 groups were comparable regarding age, height, weight, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "All preparations supplied had a code number known only by the pharmacy". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 8 women excluded for mistakes in protocol (no further details) could not be included in ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All materials appeared similar in solution". Comment: described as double‐blind, matching placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Polk 1982.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: July 1978 to October 1980. Setting: Brigham and Women's Hospital, Boston, Massachusetts, US. Inclusion criteria: all women undergoing CS (other than repeat section); criteria do not correspond with our definition of non‐elective. N = 278. Exclusion: active infection, fever, membranes ruptured > 36 hrs, antibiotic therapy within 2 weeks, renal disease, allergy to penicillin or cephalosporin. |
|
| Interventions |
Intervention: 1st generation cephalosporin:
Comparison: placebo:
|
|
| Outcomes | Fever (oral temperature > 100.3oF on any of 2 of 1st 10 post‐operative days); UTI, wound infection (only pus‐draining included in outcome of wound infection); endometritis (fever, tenderness on pelvic examination, abnormal discharge); pelvic abscess; septic pelvic thrombophlebitis, bacteremia; subsequent antibiotic use (23% for placebo vs 12% for treatment). | |
| Notes | Outcome of fever and minor wound infection combined (11/146 for treatment vs 13/132 for placebo).
4 episodes of bacteremia, all in placebo group.
1 episode of rash and 1 episode of phlebitis reported in treatment group vs none in control.
Data collected at 6 weeks on 259/266 patients; 35% of infections diagnosed after discharge. Class of antibiotic: 1st generation cephalosporin. Subgroups:
The 2 groups were comparable regarding age, BMI, proportion on private service, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 12 participants withdrawn (8 treatment, 4 placebo) and started on therapeutic antibiotics by the surgeon because the operation had been prolonged or was complicated or the pre‐operative specimen of urine disclosed significant bacteriuria; results on participants excluded could not be re‐included in ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants, their physicians and all investigators were unaware of the assignment throughout the study". Comment: double‐blind, matching placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: probably outcome assessment was blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Racinet 1990.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: July 1986 to December 1987. Setting: Grenoble, France. Inclusion criteria: 'low‐risk' women, undergoing CS (27% in labor). N = 266. Exclusion: allergy to beta‐lactam antibiotics, receipt of antibiotics within 3 days; ruptured membranes > 12 hrs; fever, amniotic infection, failure of instrumental manipulation. |
|
| Interventions |
Intervention:
Comparison: placebo:
|
|
| Outcomes | Endometritis, wound infection (includes superficial wound infection and deep abscess), isolated fever, UTI, bacteremia and septicemia; additional antibiotic use (10/136 in treatment group vs 19/130 in placebo); total antibiotic costs (76 francs in treatment group vs 52 francs in placebo); maternal hospital stay. Outcomes were evaluated daily during hospitalization and at 30 days. | |
| Notes | There was 1 episode of septicemia in the placebo group. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
The 2 groups reported as comparable regarding demographic values. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomized by drawing of lots". |
| Allocation concealment (selection bias) | Low risk | Comment: allocation of treatment was in a closed envelope to be opened at the last moment by the anesthetist who only knew the nature of the administered treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses or exclusions were reported. Analysis appears to be ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, placebo‐controlled. Placebo was 10 mL of physiologic "serum". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Reckel 1985.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: Hanover, Germany. Inclusion criteria: women undergoing CS. N = 140. |
|
| Interventions |
Intervention: ureidopenicillin:
Comparison: no treatment:
|
|
| Outcomes | Wound infection (inflammation with or without exudation); endometritis (fever and tenderness of the uterus or fever with pathogens from the cervical canal); UTI (> 100,000 bacteria/mL). | |
| Notes | 1 episode of allergic skin reaction occurred with the injection of mezlocillin. Class of antibiotic: extended spectrum penicillin (ureidopenicillin (mezlocillin)). Subgroups:
The 2 groups were comparable regarding age, height, weight, and risk of wound infection. But risk of endometritis was not in balance in the 2 groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized..". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 1 dropout (no treatment) reported. Analysis appears to be ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Rehu 1980.
| Methods | RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: September 1977‐January 1978. Setting: State Maternity Hospital, Helsinki, Finland. Inclusion criteria: all women undergoing CS. N = 128. Exclusion criteria: allergic to penicillin, clindamycin or gentamicin; emergency section. |
|
| Interventions |
Intervention: penicillin:
Intervention: combination (lincosamides plus aminoglycoside):
Comparison: placebo:
Placebo data spilt for comparison with the 2 antibiotic groups. |
|
| Outcomes | Endometritis (fever, uterine tenderness and foul‐smelling vaginal discharge); wound infection (all grades combined); hospital stay (treatment 7.7 vs 7.7 placebo; no variance given). | |
| Notes | Data from a 4th group that consisted of patients allergic to 1 of the drugs or undergoing an emergency section have not been included. Drug class: natural penicillin or aminoglycoside‐containing combination (lincosamide (clindamycin) and aminoglycoside). Subgroups:
The 1st 3 groups were comparable regarding number of amnioscopic examinations, number of vaginal examination, duration of labor and duration of intrauterine monitoring. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Assigned at random". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote: " ... in bottles containing code numbers". Comment: insufficient information to judge. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no loss to follow‐up reported; 2 women excluded after initial randomization. ITT analysis with available data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the code was kept secret for persons performing the operations and observing the patients in the post‐operative period".. Comment: placebo‐controlled (glucose solution) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the code was kept secret for persons .... observing the patients in the post‐operative period". |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Rizk 1998.
| Methods | RCT: 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Inclusion: women undergoing elective CS (absence of labor and before rupture of membranes). N = 120. Exclusion: allergy to penicillin or cephalosporin, prior antibiotic therapy within 7 days. Setting: United Arab Emirates. | |
| Interventions |
Intervention: 2nd generation cephalosporin:
Comparison: no treatment:
|
|
| Outcomes | Febrile morbidity (temperature of > 38oC after 1st 48 hrs); endometritis (uterine tenderness and offensive lochia with fever and no other source); wound infection (erythema, induration or purulent discharge); UTI (> 100,000 bacteria/mL). | |
| Notes | Majority of patients were indigent; follow‐up at 6 weeks. Class of antibiotic: 2nd generation cephalosporin. Subgroups:
The 2 groups were comparable regarding age, parity, weight, gestational age and indication for cesarean. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...a computer generated number scheme..". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...randomization code and the mode of intervention was only known to the anesthesiology staff..". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no losses or exclusions were reported. Analysis appears to be ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "patient and study co‐ordinators unaware of group allocation". Comment: not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Roex 1986.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: April 1983 to October 1984. Setting: Academisch Ziekenhuis der Vrije Universiteit, Amsterdam, The Netherlands. Inclusion criteria: all women undergoing CS (77/129 were elective sections). N = 129. Exclusion: active infection, antibiotics within 7 days, allergy to penicillin or cephalosporin, impaired liver or renal function. |
|
| Interventions |
Intervention: cefamycin:
Comparison: placebo:
IV bolus immediately following clamping of the cord and at 6 and 12 hrs later. |
|
| Outcomes | Febrile morbidity (> 38oC for at least 24 hrs after 1st 24 hrs); endometritis (fever, fetid lochia and/or uterine tenderness on pelvic examination); wound infection (palpable induration, wound dehiscence and/or pus drained); UTI (positive culture), bacteremia. | |
| Notes | 1 episode of Staphylococcus aureus bacteremia (in cefoxitin group) not considered life‐threatening (included in outcome of serious morbidity). No serious antibiotic side effects reported in cefoxitin‐treated group; 1 patient in cefoxitin group developed diarrhea. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
The 2 groups were comparable regarding demographic, obstetric and operative factors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Commentt: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 21 women were excluded: 2 had fever prior to surgery, 2 because of a known allergy to penicillins; 8 women excluded because of protocol failures and 9 women for intraoperative complications (not defined further); as‐treated analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Ross 1984.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: Addenbrooke's Hospital, Cambridge, UK. Inclusion criteria: women undergoing emergency CS (in active labor with membrane rupture). N = 115. Exclusion criteria: pyrexia; antibiotic use within 2 weeks. |
|
| Interventions |
Intervention: nitroimidazole:
Comparison: placebo:
IV infusion at start of procedure; post‐operatively metronidazole or placebo suppository twice daily for 5 days. |
|
| Outcomes | Pyrexia (> 38oC twice 4 hrs apart after 1st 24 hrs); wound infection; endometritis (heavy, offensive lochia and pyrexia); UTI; antibiotic use (15/57 in treatment group vs 20/58 in control group). | |
| Notes | 1 woman in the control group developed a pelvic abscess.
Length of admission not significantly different between the 2 groups (mean 7.4, SD 2.3 days).
No adverse reactions occurred. Class of antibiotic: nitroimidazole (metronidazole). Subgroups:
Comparison of the 2 groups showed similar risk factors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized, sequential basis". Comment: insufficient description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Antibiotic ... was provided without access to the 'trial code". Comment: insufficient information to judge. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up; no participant excluded. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Rothbard 1975.
| Methods | Quasi‐RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: New York Medical College, New York, US. Inclusion criteria: all women undergoing CS divided into "no labor" and "labor" groups which correspond to the definitions of elective/non‐elective used in this review. N = 100. Exclusion criteria: fever, antibiotic use within 2 weeks, ruptured membranes > 2 hrs, major penicillin allergy. |
|
| Interventions |
Intervention: combination (cephalosporin + aminoglycoside):
Comparison: no treatment:
|
|
| Outcomes | Febrile morbidity (temperature greater that 100.4oF orally on 2 consecutive days, excluding the 1st post‐operative day); endometritis (fever, uterine tenderness and positive culture or fever and pathogenic organism); UTI, wound infection (fever and cellulitis or exudate). Data available on elective (defined as no labor) and non‐elective (defined as presence of labor). | |
| Notes | No difference in average duration of hospital stay between groups (data not shown).
1 woman (treatment group) developed endometritis with organism resistant to cephalothin and kanamycin. Class of antibiotic: aminoglycoside‐containing combination (1st generation cephalosporin and kanamycin). Subgroups:
The 2 groups were comparable regarding age, parity, ethnic background or type of anesthesia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: Randomized "...using the last digit of their hospital chart number". Comment: quasi‐RCT. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up; no participants excluded. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Rouzi 2000.
| Methods | Randomized placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: Jeddah, Saudi Arabia. Inclusion criteria: women undergoing CS (both elective and emergency). N = 441. Exclusions: use of antibiotics, fever or signs of infection; allergy to penicillin or cephalosporin. |
|
| Interventions |
Intervention: cephalosporin:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (> 38oC twice 4 hrs apart after 1st 24 hrs); endometritis (fever, uterine tenderness and abnormal lochia); wound infection (fever, cellulitis or exudate with positive culture); UTI (fever and positive urine culture); pneumonia, bacteremia, pelvic abscess, unexplained fever, therapeutic antibiotics, length of post‐operative stay. All outcomes are reported by emergency and elective CS separately, so when reporting overall outcomes, dichotomous data can be used but not continuous. Length of hospital stay therefore cannot be reported for the overall comparison. |
|
| Notes | Definition of emergency section (unscheduled) did not correspond to the definition of non‐elective section used in this review; these patients have been analyzed in the "both or not‐defined" group. Women undergoing elective section included women with scheduled section and with intact membranes and have been analyzed in the "elective" group.
There were no significant differences in the fetal outcomes reported (definitions not consistent with those for this review; no serious side effects with cefazolin. Class of antibiotic: 1st generation cephalosporin. Subgroups:
The 2 groups were comparable regarding maternal characteristics and emergency and elective CS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated randomization..". |
| Allocation concealment (selection bias) | Low risk | Quote: "...each indistinguishable minibag was given a code number in the department of pharmaceutical care". Comment: there was, however, no information on how the codes were used and whether there was sequential opening. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses or exclusions were reported. Analysis appeared to be by ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "triple blind" ... "both the experimental drug and placebo were indistinguishable". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: probably outcome assessment was blinded ("triple blind"). |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Rudd 1981.
| Methods | RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: Tripler Army Medical Center, Honolulu, Hawaii, US. Inclusion criteria: all women undergoing CS (19/60 women had ruptured membranes > 6 hrs; 40/60 were in active labor). N = 60 for this review. Exclusion: known infection, currently on antibiotics, allergic to penicillin or cephalosporin. |
|
| Interventions |
Intervention: cephalosporin:
Comparison 1: placebo:
Comparison 2: not included in analysis:
Only comparison 1 was used for placebo. |
|
| Outcomes | Endomyometritis (fever, unusual uterine and parametrial tenderness without evidence of other source of infection); maternal length of stay. | |
| Notes | Length of hospital stay for the control group included results from both the no irrigation group and the placebo irrigation group (5.37 days vs 4.53 for treatment group). Class of antibiotic: 2nd generation cephalosporin. Subgroups:
The 3 groups were comparable regarding age, parity, weight and socioeconomic background. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomly allocated using table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Comment: randomly allocated by hospital pharmacy. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses were reported; no participants excluded. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, placebo‐controlled. Vitamin solution added to make placebo visually identical; physicians and patients blinded to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: probably outcome assessment was blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Ruiz‐Moreno 1991.
| Methods | Randomized, placebo‐controlled; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting:Hospital Central Militar, Mexico city, Mexico. Women predominantly (78%) of low socioeconomic level. Inclusion criteria: women in active labor undergoing CS. N = 100. Exclusion: elective CS, evidence of infection, antibiotic use within 8 days, metronidazole intolerance, lack of consent. |
|
| Interventions |
Intervention: nitroimidazole:
Comparison: placebo:
Immediately after cord clamping. |
|
| Outcomes | Endometritis (purulent and/or foul odor lochia); wound infection (wound edges tender, red and swollen, or frank pus or sanguino‐purulent material exuded); UTI (bacteria seen in sediment); maternal hospital stay. | |
| Notes |
Class of antibiotic: nitroimidazole (metronidazole). Subgroups:
The 2 groups reported as comparable regarding age and parity, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized..". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no losses or exclusions were reported. Appears to be ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind; identical appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Saltzman 1985.
| Methods | RCT; 2 parallel groups. | |
| Participants | Dates of data collection: not specified. Setting: Fairfax Hospital, Virginia, US. Women predominantly private. Inclusion: criteria: high‐risk women undergoing CS (in active labor and/or ruptured membranes > 4 hrs); not consistent with the criteria for non‐elective in this review: classified as "both/undefined" in this review. Exclusion: active infection, fever, antibiotic use within 3 days, allergy to penicillin or cephalosporins. |
|
| Interventions |
Intervention: 3rd generation cephalosporin:
Comparison: placebo:
IV at time of cord clamping. |
|
| Outcomes | Febrile morbidity (oral temperature > 37.9oC twice at least 8 hrs apart, after 1st 24 hr); endometritis (fever and foul lochia or uterine tenderness); UTI (fever and positive culture); wound infection (fever, abnormal‐looking wound, surrounded by cellulitis and/or draining purulent material). | |
| Notes | There was 1 drug reaction (maculopapular rash) in the treatment group.
Women followed up at 6 weeks. Class of antibiotic: 3rd generation cephalosporin. Subgroups:
"The groups were comparable. No significant differences were observed between the 2 groups with respect to maternal age, parity, gestational age, duration of labor, duration of ruptured membranes or use of internal fetal monitoring. There were no significant differences regarding indication for CS". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 1 patient was removed from the study when she became febrile in the delivery room, not included in ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Scarpignato 1982.
| Methods | RCT; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: November 1981 to March 1982. Setting: University of Parma, Parma, Italy. Inclusion criteria: women undergoing emergency CS (58/60 women in spontaneous labor; classified as non‐elective). N = 60. Exclusion criteria: allergy to penicillin or cephalosporins; severe renal disease, history of pelvic infections. |
|
| Interventions |
Intervention: 2nd generation cephalosporin:
Intervention: cephalosporin (B2):
Comparison: no treatment:
The results of both treatment groups have been combined. |
|
| Outcomes | Fever (> 100.3oF twice 6 hrs apart); endometritis (fever and uterine tenderness); maternal stay (treatment 7.1 vs control 7.9 days, no variance given). | |
| Notes | Note: the group given long‐term prophylaxis received the 1st dose after return to the ward. Class of antibiotic: 2nd generation cephalosporin. Subgroups:
The 3 groups were comparable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no losses to follow‐up reported. 1 woman was excluded because of an allergic reaction to cefuroxime. Could not be re‐included in ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Schedvins 1986.
| Methods | Randomized trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: November 1983 to October 1984. Setting: Sodersjukhuset, Stockholm, Sweden. Inclusion criteria: women with rupture of membranes for > 6 hrs (equivalent to non‐elective group). N = 53. Exclusion criteria: fever or foul smell of amniotic fluid. |
|
| Interventions |
Intervention: cephalosporins:
Comparison: no treatment:
|
|
| Outcomes | Endometritis (marked uterine tenderness with or without a foul discharge with fever at least twice); wound infection (redness, tenderness, induration and pus in the wound); UTI (positive culture). | |
| Notes | Data provided (but not included) for a 2nd control group eligible for inclusion but not randomized.
Numbers not provided to calculate mean maternal length of stay for the 2 randomized groups. Class of antibiotic: other regimen (2nd generation cephalosporin, then 1st generation cephalosporin). Subgroups:
The 2 groups were comparable regarding age, parity, previous CS, duration of labor and membrane rupture. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly referred". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 11 patients "should have been given prophylactic treatment according to the study design but received no antibiotics and ... formed a second control group". As‐treated analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Shah 1998.
| Methods | RCT; 4 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not reported. Setting: United Arab Emirates. Inclusion criteria: women undergoing elective CS (definition not provided). N = 198. Exclusion: hypersensitivity to penicillin or cephalosporin; prior antibiotic therapy within 3 days; hepatorenal insufficiency; positive cultures or definite evidence of infection. |
|
| Interventions |
Intervention: penicillin (A3):
Intervention: penicillin (A3):
Intervention: drug combination [cephalosporin (B1) plus nitroimidazole (I)]:
Comparison: no treatment:
Results of the 2 penicillin treatment groups were combined for this review. Placebo data were divided: 2/3 for comparison with penicillin (A) and 1/3 for comparisons with the cephalosporin. |
|
| Outcomes | Febrile morbidity (fever > 38oC twice 4 hrs apart after 1st day); endometritis (uterine and parametrial tenderness, foul smelling vaginal discharge); wound infection (local induration and tenderness with wound exudate). | |
| Notes | 3 women who developed drug reactions were excluded from study (1 from each of the treatment groups). Late morbidity evaluated at 4‐6 weeks. Class of antibiotic: extended spectrum penicillin (ureidopenicillin (pipericillin)) vs other combination (1st generation cephalosporin plus nitroimidazole (metronidazole). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized..". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "...consecutively numbered sealed envelopes..". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 14 women were excluded (8/147 from treatment groups, 6/51 from control group). As‐treated analysis with available outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Sokolowski 1989.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Date of data collection: not stated. Setting: Magdeburg, Germany. Inclusion criteria: women undergoing CS (both elective and non‐elective). Exclusion criteria: none reported. |
|
| Interventions |
Intervention 1:
Intervention 2:
|
|
| Outcomes | Fever (> 37.5°C for at least 3 days post‐operatively). Data also given for outcome of high fever (> 38.5°C) (7/172 vs 29/204 for treatment and control groups respectively); puerperal infection and wound infection were combined as "other diseases" (8/172 vs 11/204). | |
| Notes | Translated from German. Class of antibiotic: nitroimidazoles. Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: Described as "by random selection"; women with surnames A‐K received treatment, women with surnames L‐Z did not. Comment: quasi‐RCT. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up reported; no participant excluded. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Stage 1982.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups (2:1 active:placebo randomization). Unit of randomization: individual. Part of a larger study looking at prophylaxis also in gynecologic surgery. |
|
| Participants | Dates of data collection: July 1976 to June 1978. Setting: 14 US centers. Inclusion criteria: all women undergoing CS (46% in labor). N = 199. Exclusion criteria: infection, allergy to penicillin or cephalosporins. |
|
| Interventions |
Intervention: 1st generation cephalosporin:
Comparison: placebo:
Within 1 hr prior to surgery, repeated at 4 hrs. |
|
| Outcomes | Febrile morbidity (oral temperature > 37.7oC twice 4 hrs apart, after 1st 48 hrs); endometritis (uterine tenderness, fever and purulent discharge), wound infection (increased local tenderness, redness or swelling); UTI (positive culture); maternal length of stay (treatment 5.8 days vs placebo 7.57 days; P < 0.05, variance not given). | |
| Notes |
Class of antibiotic: 1st generation cephalosporin. Subgroups:
The 2 groups were comparable regarding age and other risk factors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: investigator provided with Individually. randomized block of patient numbers. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: dropouts in CS women not stated (overall: 11/319 from treated group, 8/172 from placebo group. As‐treated analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: placebo‐controlled; patients and investigators blind to allocation throughout the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: probably outcome assessment was blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Stiver 1983.
| Methods | Randomized, placebo‐controlled trial; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collectin: no reported. Setting: 5 centers in Canada. Inclusion criteria: all women in labor or with ruptured membranes (duration of ruptured membranes not stated; mean duration 9.97 hrs; included in both category). N = 361. |
|
| Interventions |
Intervention 1: 1st generation cephalosporin:
Intervention 2: cefamycin:
Comparison: placebo:
Infused IV immediately after cord clamped and 6 and 12 hrs later. Results of both treatment groups combined. |
|
| Outcomes | Febrile morbidity (oral temperature > 37.9oC twice at least 6 hrs apart after 1st 24 hrs); wound infection (redness, induration, tenderness and/or purulent discharge from the incision line); endometritis/parametritis (uterine and/or adnexal tenderness with fever) UTI (dysuria or pyuria and positive culture); need for antibiotic therapy (11% for treatment groups vs 27% for placebo); maternal length of stay (7.3 and 7.4 days for treatment groups vs 7.9 for placebo). | |
| Notes | Side effects documented: 2 infusion‐related hypotensive episodes (1 with cefazolin, 1 with placebo that necessitated withdrawal from study); 6 episodes of phlebitis (5 in treated, 1 in placebo group); 1 episode of angioedema (placebo patient). Data provided on antibiotic resistance in wound isolates and screening cervical cultures. 1 episode of bacteremia (in placebo group); 1 episode of septic shock (in cefazolin‐treated group); both outcomes included as serious morbidity.
Follow up at 6 weeks. Class of antibiotic: 1st generation cephalosporin or cefamycin (2nd generation cephalosporin). Subgroups:
The 3 groups were comparable regarding age, parity, gravidity, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 7 women (1 in treatment, 6 in placebo group) initially randomized but results not included, 6 because they failed to receive all 3 doses, 1 because of hypotensive episode with 1st dose. As‐treated analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Sziller 1994.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: September 1990 to April 1991. Setting: Budapest, Hungary. Inclusion criteria: women who had opted for elective CS before onset of labor (definition not provided; indications included chronic fetal distress, breech position, placenta previa/placental abruption, prior uterine surgery; classified as elective for this review). Exclusion criteria: symptoms of infection prior to surgery; any illness that increased risk of infection (e.g. diabetes); penicillin allergy. |
|
| Interventions |
Intervention 1:
Intervention 2:
|
|
| Outcomes | Fever (categorized as mild [up to 37.5°C after the 1st 24 hrs lasting for at least 2 days (treatment n = 4 vs no treatment n = 5)]; moderate [> 38°C for at least 2 days (n = 0 vs n = 2)]; severe [> 38°C lasting > 3 days (n = 0 vs n = 2)];wound infection (induration of the abdominal incision, serosanguinous or purulent discharge, dehiscence of the wound); endometritis (fever plus lower abdominal pain, uterine tenderness, odorous discharge from the uterine cavity); UTI (pathogenic bacteria from mid‐stream urine); additional antibiotic treatment (treatment group n = 6 vs no treatment n = 13). | |
| Notes | Translated from Hungarian. Class of antibiotic: beta‐lactam/beta‐lactamase inhibitor combination. Subgroups:
No significant differences between groups for age, indications for CS, gestational age, birthweight and previous CS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random table". |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no loss to follow‐up reported; no explanation for difference in numbers between groups (60 vs 44). |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Tully 1983.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual placebo‐controlled; double‐blind. |
|
| Participants | Dates of data collection: September 1978 to June 1980. Setting: Beth Israel Hospital, Boston, Massachusetts, US. Inclusion criteria: women undergoing primary CS (inclusion criteria not consistent with the definition of non‐elective CS used in this review). N = 113. Exclusion criteria: < 18 years of age, membranes ruptured > 35 hrs, allergy to penicillin or cephalosporin, fever, infection or antibiotic use, significant underlying cardiac, renal or hepatic disease, unable to provide consent. |
|
| Interventions |
Intervention: cefamycin:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (oral temperature > 37.9oC twice at least 6 hrs apart after 1st 24 hrs); UTI (positive culture); wound infection (purulence, cellulitis or dehiscence); endometritis (fever, uterine tenderness, abnormal lochia); septicemia (positive blood culture in a clinically septic patient); additional antibiotic use (8 in treatment group vs 12 in placebo). | |
| Notes | Both episodes of septicemia occurred in the placebo group. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
The 2 groups were comparable regarding age, BMI, gravidity, frequency of fetal monitoring, number of vaginal examinations, duration of labor, duration of ruptured membranes, duration of surgery and indications for CS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomized as determined by table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Comment: sequential study numbers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported; 14 women (7 in each group) initially randomized were later excluded (all doses not administered, antibiotic therapy prior to surgery, antibiotic following surgery, incorrect dose schedule, infection prior to surgery, drug code broken for possible allergy. As‐treated analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: randomization was blind to both patients and investigators. Placebo‐controlled (mannitol with riboflavin). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Turner 1990.
| Methods | Quasi‐RCT, 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection; not reported. Setting: Hammersmith Hospital (N = 102) and Northwick Park Hospital (N = 99), London, England. Inclusion criteria: women undergoing CS (both elective and emergency). N = 201. Exclusion: on antibiotics, adverse reaction to penicillin or cephalosporin, pyrexia > 37.5 degrees C in labor, known vaginal pathogen, or suspected intrauterine infection. |
|
| Interventions |
Intervention: 1st generation cephalosporin:
Comparison: no treatment:
|
|
| Outcomes | Puerperal infection (temperature > 37.5oC after 24 hrs); endometritis (pyrexia with uterine or adnexal tenderness); wound infection (purulent discharge or erythema, induration and serous discharge with positive culture); UTI (> 100,000 colony forming units in urine culture); length of hospital stay (7.63 for treatment group, 7.18 for control group [SD not provided]). | |
| Notes | Definitions of elective and emergency procedure, nor separate outcomes for each group, provided.
Follow up completed 1987. Class of antibiotic: 1st generation cephalosporin. Subgroups:
The 2 groups were comparable with respect to age, social class, single mother, weight, previous CS, the mode of onset of labor, the use of electronic fetal monitoring, the type of CS, gestational age, birthweight or the perinatal outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...alternate patients..". Comment: quasi‐RCT. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no losses or exclusions were reported. Analysis appears to be ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Tzingounis 1982.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Date of data collection: not reported. Setting: Alexandra Maternity Hospital, Athens, Greece. Inclusion criteria: women in labor (non‐elective). N = 96. Exclusion criteria: acute bleeding due to abruptio placentae, established infection. |
|
| Interventions |
Intervention: 2nd generation cephalosporin:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (oral temperature of > 100.3oF twice 6 hrs apart) and infection of endometrium, urinary tract and wound (not defined); results of duration of maternal stay only provided for febrile patients. | |
| Notes | No patients had any major complications from the use of cefuroxime. Class of antibiotic: 2nd generation cephalosporin. Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Selected in a random manner". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up; no participants excluded. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind".... "the placebo was comparable to cefuroxime in both appearance and viscosity of solution". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Ujah 1992.
| Methods | RCT, 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: August 1989 to July 1990. Setting: Jos Universtiy Teaching Hospitla, Jos, Nigeria. Inclusion criteria: healthy women scheduled for elective CS. N = 35. Exclusion criteria: labor, premature rupture of membranes, uncontrolled diabetes mellitus, sickle cell disease. |
|
| Interventions |
Intervention: beta‐lactam inhibitor combination:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity, wound erythema or induration, seropurulent discharge, pneumonia. | |
| Notes | Developing country. Class of antibiotic: beta‐lactamase inhibitor combination. Subgroups:
"They were well matched for the (patient characteristics) considered .. and equally well matched for (preoperative, intraoperative and post‐operative) variables." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random list of numbers". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed by the nurse in charge of the antenatal ward using a random number list". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participant excluded after randomization. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: placebo‐controlled; insufficient information to judge whether there was blinding of study personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Walss Rodriguez 1990.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: July 1 to September 1988. Setting: Coah, Mexico. Inclusion criteria: women undergoing urgent CS. N = 120. Exclusion: fever, chorioamnionitis, penicillin allergy, antibiotic treatment in prior 2 weeks. |
|
| Interventions |
Intervention:
Comparison: no treatment:
|
|
| Outcomes | Febrile syndrome; wound infection; abdominal wall abscess; endometritis, length of hospital stay. | |
| Notes | No definitions of outcomes provided. "Absceso de pared" has been translated as abdominal wall abscess. Abdominal wall abscess, infection of surgical scar and spontaneous reopening of the wound + infection have been classified as "wound infection". Class of antibiotic: aminopenicillin (ampicillin). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Allocated "in random form" using a random table. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses or exclusions were reported. Analysis appears to be ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Weissberg 1971.
| Methods | RCT: 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: not specified. Setting: Miami, Florida, US. Mostly low‐income or indigent Negro women from ghetto areas of large metropolitan area. Inclusion criteria: women undergoing primary CS after the onset of labor. N = 80. Exclusion criteria: none specified. |
|
| Interventions |
Intervention: combination [penicillin + aminoglycoside]:
Comparison: no treatment:
|
|
| Outcomes | Febrile morbidity (temperature of > 100.3oF on any 2 days after 1st 24 hrs); UTI, endometritis and wound infection (not defined); maternal length of stay (treatment 5.8 days vs 8.7 days for control group, no variance given). | |
| Notes | 1 patient receiving penicillin had a drug rash on the 3rd day. Class of antibiotic: aminoglycoside‐containing combination. Subgroups:
"The clinical material in both groups was identical." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "selected at random". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to judge. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss of participants to follow‐up; no participant excluded after randomization. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Witt 2011.
| Methods | Randomized, double‐blind, placebo‐controlled; 3 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: March 2004 to January 2010. Setting: Medical University of Vienna, Vienna, Austria. Inclusion criteria: women undergoing cesarean delivery at term with gestational age ≥37 weeks and reassuring fetal heart traces. Rupture of membranes and labor contractions were allowed (not consistent with the criteria of elective CS used in this review; classified as "both or not defined"). Exclusion criteria: fever > 38°C, cephalosporin allergy, age < 18 years, exposure to any antibiotic within 1 week before delivery. |
|
| Interventions |
Intervention 1:
Intervention 2:
Intervention 3:
For the subgroups of class of antibiotic and type of CS, the 2 treatment groups have been combined (n = 741); for the timing of administration, the control group has been divided in 2 (n = 186). |
|
| Outcomes | Wound infection (purulent discharge or erythema (> 1 cm in diameter) and induration of the incision site; endometritis (fever, defined as axillary temperature of ≥ 38°C for at least 28 hrs, uterine tenderness and malodorous lochia); UTI (clinical symptoms, i.e. polyuria and dysuria, and positive dipstick nitrites); serious maternal infectious morbidity (pelvic abscess, sepsis). Neonatal outcomes (respiratory distress syndrome, intracranial hemorrhage, necrotizing enterocolitis, sepsis, neonatal death): no statistically significant difference (data not shown). Patients followed during hospital stay with a telephone call at 30 days and clinic visit for women with signs and symptoms of endometritis, wound infection or UTI. | |
| Notes | Methicillin resistant Staphylococcus aureus was not noted in the study population. No difference in patient characteristics nor infection rates between early (March 2004 to June 2007) and late (July 2007 to January 2010) time periods. Class of antibiotic: 1st generation cephalosporin. Sugroups:
The groups were balanced with regard to age, BMI, prevalence of gestational diabetes, history of allergy, immunosuppressive and anticoagulation therapy during pregnancy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated randomization list". |
| Allocation concealment (selection bias) | Unclear risk | Comment: A study nurse checked the randomization list and handed the appropriate infusion bag to the anesthesiologist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 32 participants were lost to follow‐up, protocol violations or withdrawal from the study (group 1 = 12; group 2 = 7; control = 13); included in ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes are reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: control group received 100 mL of identical appearing saline solution. Study nurse but not the patient, the surgeon or the anesthetist was aware of the allocation; patients and surgeons were masked to the administration schedule. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: evaluation of the infectious morbidity was performed by 2 residents masked to the group assignment. Neonatal outcomes were collected by neonatal staff unaware of the maternal group assignment. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Wong 1978.
| Methods | Randomized placebo‐controlled trial; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection: January 1975 to January 1977. Setting: Los Angeles County‐University of Southern California Medical Center, Los Angeles, California, US, 87% Hispanic or Black. Inclusion criteria: women in labor with ruptured membranes who underwent internal fetal monitoring (classified as non‐elective). N = 93. Exclusion: fever, other antibiotic use, penicillin allergy. |
|
| Interventions |
Intervention: 1st generation cephalosporin:
Comparison: placebo:
|
|
| Outcomes | Standard temperature morbidity, endomyometritis, abdominal wound infection, urinary infections (no definitions provided for any outcomes). | |
| Notes | 2 women were said to develop a serious infection: 1 (cefazolin group) developed septic thrombophlebitis and is included as a serious outcome; the other (placebo group) was treated with antibiotics for prolonged fever (judged not to be a serious outcome for this review). Class of antibiotic: 1st generation cephalosporin. Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized to numbered packages". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "... the agents having been randomized to numbered packages by the pharmacy department". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no loss to follow‐up reported. 7 women initially randomized not included in final analysis because they did not meet all the criteria (allocated group unknown). As‐treated analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blind study, placebo‐controlled; similar quantity of placebo given. The physician caring for the patient did not know which agent his patient received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: probably outcome assessment was blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Work 1977.
| Methods | RCT; 2 parallel groups. Unit of randomization: individual. |
|
| Participants | Dates of data collection; not reported. Setting: University of Michigan Medical Center, Ann Arbour, Michigan, US. Inclusion criteria: women in labor. N = 80. Exclusion criteria: acute bleeding due to abruptio placentae, infection on treatment; abnormal renal function, penicillin allergy. |
|
| Interventions |
Intervention: 1st generation cephalosporin:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (oral temperature > 100.3oF twice 6 hrs apart); infection of endometrium, urinary tract and wound (definitions not provided); fever index (40 degree hrs for treatment group vs 83 for placebo group). | |
| Notes |
Class of antibiotic: 1st generation cephalosporin. Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Selected in random ... manner". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no loss to follow‐up or exclusion of participants after randomization described, but results of only 80/85 participants reported; insufficient detail to know if the analysis was ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo was comparable to the cephalothin in both appearance and viscosity of solution". Comment: double blind, placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information provided. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Wu 1991.
| Methods | Randomized into 3 groups (irrigation vs systemic treatment vs no treatment). | |
| Participants | Dates of data collection: May 1988 to August 1989. Setting: Beijing, China. Inclusion criteria: women undergoing both elective (N = 112) and non‐elective (N = 105) CS. Only women undergoing an elective CS were randomized to treatment or no treatment and have been included in analysis. N = 112. |
|
| Interventions |
Intervention:
Intervention: combination [penicillin + aminoglycoside]:
Comparison: no treatment:
Placebo data were divided: 1/2 for comparison with penicillin (A) and 1/2 for comparisons with the combined drug regimen. |
|
| Outcomes | Endometritis (presence of any 2 of following: temperature above 37.5oC, uterine tenderness, foul vaginal discharge); abdominal wound infection (cellulitis with small amount of exudate within 2 months of operation); uterine incision infection (associated with late postpartum hemorrhage); fever index. | |
| Notes | Women undergoing non‐elective sections randomized to either treatment group (not included in this review). Class of antibiotic: amminoglycoside‐containing combination (natural penicillin and gentamicin) or aminopenicillin (ampicillin). Subgroups:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomized..". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no losses or exclusions were reported. Analysis appears to be ITT. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding, not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably outcome assessment was not blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Yip 1997.
| Methods | Randomized, placebo‐controlled; 2 parallel groups. | |
| Participants | Date of data collection: not reported. Setting: Prince of Wales Hospital, Hong Kong. Inclusion criteria: women undergoing CS. N = 320. Exclusion criteria: penicillin allergy, current antibiotic use, fever, receipt of steroid injection. |
|
| Interventions |
Intervention: beta‐lactam inhibitor:
Comparison: placebo:
|
|
| Outcomes | Febrile morbidity (2 oral temperatures > 37.9oC at least 6 hrs apart after 1st 24 hrs); bacteriuria at day 3 (classified in this review as UTI); wound infection (purulent discharge, cellulitis, tenderness and wound abscess requiring incision and drainage); endometritis (fever, pelvic pain, uterine tenderness, purulent vaginal discharge without signs of infection in the lower genital tract); duration of hospital stay. | |
| Notes | Sub‐rectus Redivac drain routinely inserted. Class of antibiotic: beta‐lactamase inhibitor combination. Subgroups:
The 2 groups were comparable regarding age, weight, parity, duration of labor, birthweight, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: assigned by the anesthetist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up reported; no patients excluded from analysis. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: " double‐blind" placebo‐controlled (10 mL normal saline). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to judge. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
Young 1983.
| Methods | Randomized, placebo‐controlled trial; 2 parallel groups. Unit of randomization; individual. |
|
| Participants | Dates of data collection: May 1978 to July 1979. Setting: Los Angeles County‐University of Southern California Medical Center, Los Angeles, California, US. Predominantly (91%) Hispanic or Black. Inclusion criteria: women in labor with an intrauterine pressure catheter and fetal scalp electrode (non‐elective). N = 100. Exclusion criteria: fever, significant systemic disease. |
|
| Interventions |
Intervention: cefamycin:
Comparison: placebo:
|
|
| Outcomes | Endomyometritis, abdominal wound infection, serious complications; duration of maternal hospital stay (treatment 5.1 days vs control 5.9 days, not statistically significant, no variance given). | |
| Notes | 1 case of septic pelvic thrombophlebitis occurred in the treatment group; there were 8 episodes of bacteremia in the control group vs 1 in the treatment group; both outcomes combined under serious morbidity. Class of antibiotic: cefamycin (2nd generation cephalosporin). Subgroups:
The 2 groups were comparable regarding age and race. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned". Comment: no description of sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up reported; no participants excluded. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: placebo‐controlled; "similarly appearing placebo". "the physician team did not know which medication the patient was to receive." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: probably outcome assessment was blinded. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
BMI: body mass index C: centigrade CS: cesarean section F: fahrenheit hr: hour/hours IM: intramuscularly ITT: intention to treat IV: intravenously MU: million units q 6 hrs: every 6 hours RCT: randomized controlled trial SD/sd: standard deviation UTI: urinary tract infection vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahued 1994 | Study included women undergoing gynecological surgery; allocation of participants (N = 71) undergoing cesarean section to treatment or control group not provided. |
| Andrews 2003 | All participants received prophylaxis with cefotetan after cord clamping and were then randomized to receive doxycycline plus azithromycin vs placebo. |
| Cormier 1988 | Did not include women undergoing cesarean section. |
| Creatsas 1980 | Not relevant to this review. Ampicillin or gentamycin given prior to cesarean section in women with intrauterine infection, to measure transplacental transfer. No control group, and no clinical outcomes given. |
| De Palma 1980 | Women at high risk (membranes ruptured for more than 6 hours) initially were randomized to early treatment (i.e. prophylactic therapy continued for 4 days) vs standard treatment (i.e. treatment only started when infection apparent). When the results were compared midway through the study, standard therapy was abandoned. The results for the 2 groups prior to abandoning the no treatment group could not be obtained from the paper. |
| Elliott 1982 | Only the 1st 42 women were randomized to placebo or active treatment; after that a significant difference was observed between the placebo and treated groups and the placebo arms were discontinued. Further women were randomized to 2 different active treatments. The data for the 1st part of the study (with only the 1st 42 women) are not available from the published paper. |
| Harrigill 2003 | Women were randomized to normal saline intra‐abdominal irrigation vs no irrigation. All patients received cefazolin at the time of cord clamping. |
| Itskovitz 1979 | Not all women were randomly allocated to treatment or no treatment. 150 women were assigned at random to each of the 2 wings of the department according to the day of their admission, each wing receiving women on alternate days. In both wings, of the last 50 women every 2nd woman served as a control. 50 women in 1 wing received IV cephalothin or oral cephalexin, 50 women in the other wing received IV or oral ampicillin. The 1st 50 women enrolled were all treated; separate results for the last 100 women (who were alternately allocated therapy or no treatment) are not available. |
| Kosus 2010 | All women received ceftriaxone 1 g after clamping of the cord; women were randomized to irrigation of the subcutaneous tissue with rifamycin SV or no irrigation before closure of the subcutaneous tissue. |
| Krasnodebski 1997 | Translated from Polish. No information is provided as to how participants were allocated to treatment or no treatment group. |
| Kreutner 1979 | After approximately 70% of the planned study population had been randomized to placebo or 1 of 2 active treatment groups, an unacceptably high morbidity rate in the placebo group was confirmed and the placebo arm was discontinued. Further women were randomized to 2 different active treatments. The data for the 1st part of the study when women were randomized to treatment or placebo are not available from the published paper. |
| Louie 1982 | Eligible women were in active labor with ruptured membranes. While this study initially included a placebo control group, this group was dropped after 30 women had been enrolled on the basis of ethical considerations about assigning women to a non‐treatment group in which the likelihood of morbidity was high. Only 7 women (out of a total of 195 women entered) were randomized to placebo, separate results on the initial part of the study not available. The placebo (7) and treatment groups (188) were very imbalanced making a meaningful comparison between groups impossible. |
| Pawelec 1994 | Abstract only; unable to confirm random allocation and method of allocation to no treatment group; data for separate outcomes of endometritis and wound infection not provided. |
| Petersen 1985 | Included women undergoing obstetrical and gynecological procedures; outcomes for both groups combined and not provided separately for women undergoing cesarean section. Reported that there was a only significant effect of cefotaxime in women with prolonged labor or "remote" rupture of the membranes (data not shown). |
| Pitt 2001 | Women were randomized to receive intravaginal metronidazole or placebo gel during labor; most, but not all patients also received 1 prophylactic dose of cefazolin after cord clamping. |
| Roex 1987 | This study compared one dose versus three doses of cefoxitin and only reported on antibiotic levels in breast milk. |
| Sanchez‐Ramos 1999 | Patients were randomized to metronidazole gel intravaginally or matching placebo, but most patients also received prophylactic antibiotics after cord clamping. |
| Sengupta 1976 | In this study, in which women were alternately allocated to antibiotic prophylaxis or no treatment, the women enrolled were undergoing both gynecological and obstetrical surgery. Rates of infectious complications are given for all abdominal surgery (cesarean section, abdominal hysterectomy and laparotomy). Data specifically on the women who underwent cesarean section are, however, not available from the published study. |
| Skryten 1988 | Abstract only. Rates for all post‐operative infection morbidity and clinically significant genital tract‐related infections (wound infections, endometritis) and abscess formation (septicemia) combined; rates for individual outcomes not provided. |
| Spreafico 1987 | Results combined from 3 time periods. In only 1 period did it appear women were randomized to antibiotic therapy or no treatment; results just for this period not available in published report. |
| Voto 1986 | All women received antibiotics (randomized to cefoxitin after cord clamping and then every 4 hours x 2 or oral ampicillin 2 g daily x 7 days); no clinical outcomes reported. |
| Wallace 1984 | This was not a randomized trial of antibiotic prophylaxis. 3 distinct groups of women were studied: 1 group was part of randomized trial that compared extracorporeal cesarean section with prophylactic antibiotic; the 2nd group received extracorporeal cesarean section and no antibiotics; the 3rd group received extracorporeal cesarean section with antibiotics (the decision to administer antibiotics in the latter 2 groups was at the discretion of the physician). |
| Wells 1994 | Absolute numbers cannot be calculated from data provided in abstract; no published version of this study identified. |
| Yamagishi 2009 | Following normal delivery, women were randomized to no oral antibiotics, or 3 or 5 days of an oral antibiotic; did not include women undergoing cesarean section. |
| Yildirim 2009 | Women undergoing elective cesarean section were randomized to cefazolin before skin incision or after umbilical cord clamping. There was no "no treatment" group. |
IV: intravenous vs: versus
Differences between protocol and review
We have used fixed‐effect Mantel‐Haenszel meta‐analysis for combining data because the Cochrane Handbook for Systematic Reviews of Interventions suggests it is more commonly used (Higgins 2008) (2009 update).
We have modified the wording in the methods sections for Assessment of heterogeneity, Assessment of reporting biases and Data synthesis to update them with the new methods being used by the group, developed in conjunction with the Group's Statistician, Simon Gates, and Richard Riley. We have used these new methods in the review (2009 update).
The quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating key outcomes. We have included subgroup analyses based on type of antibiotic, type of cesarean section and timing of administration (2014 update).
Contributions of authors
The initial draft of the 2014 updated review was prepared by F Smaill. R Grivell helped with identifying new trials and data entry and commented on the text of the review and 'Summary of findings' tables.
Sources of support
Internal sources
University of the Witwatersrand, South Africa.
The University of Liverpool, UK.
External sources
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adam 2005 {published data only}
- Adam I, Adam ES, Gerais AS. Randomized trial of ceftriaxone prophylaxis in elective cesarean section. Saudi Medical Journal 2005;26(3):500‐1. [PubMed] [Google Scholar]
Adeleye 1981 {published data only}
- Adeleye JA, Osinusi BO. The use of prophylactic antibiotics in caesarean sections. Singapore Journal of Obstetrics and Gynaecology 1981;12:29‐34. [Google Scholar]
Allen 1972 {published data only}
- Allen JL, Rampone JF, Wheeless CR. Use of a prophylactic antibiotic in elective major gynecologic operations. Obstetrics & Gynecology 1972;39:218‐24. [PubMed] [Google Scholar]
Apuzzio 1982 {published data only}
- Apuzzio JJ, Ganesh VV, Pelosi MA, Frisoli G. The effect of prophylactic antibiotics on risk factors for endomyometritis in adolescent patients undergoing cesarean section. Journal of Adolescent Health Care 1984;5:163‐6. [DOI] [PubMed] [Google Scholar]
- Apuzzio JJ, Reyelt C, Pelosi M, Sen P, Louria DB. Prophylactic antibiotics for cesarean section: comparison of high‐ and low‐risk patients for endomyometritis. Obstetrics and Gynecology 1982;59:693‐8. [PubMed] [Google Scholar]
Bagratee 2001 {published data only}
- Bagratee J, Moodley J, Kleinschmidt I, Zawilski W. A randomized controlled trial of antibiotic prophylaxis in elective caesarean section. BJOG: an international journal of obstetrics and gynaecology 2001;108:143‐8. [DOI] [PubMed] [Google Scholar]
- Bagratee JS, Moodley J. Antibiotic prophylaxis in elective caesarean section. Women's Health ‐ into the new millenium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 Oct 3‐6; Cape Town South Africa. 1999:6.
Battarino 1988 {published data only}
- Battarino O, Battarino A. Short‐term antibiotic prophylaxis in cesarean section [La profilassi antibiotica short‐time nel taglio cesareo]. Minerva Ginecologica 1988;40:563‐7. [PubMed] [Google Scholar]
Bibi 1994 {published data only}
- Bibi M, Megdiche H, Ghanem H, Sfaxi I, Nouira M, Essaidi H, et al. Antibiotic prophylaxis in a priori cesarean sections without a high risk of infection [L'antibioprophylaxie dans les cesariennes a priori sans 'haut risque infectieux']. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction (Paris) 1994;23:451‐5. [PubMed] [Google Scholar]
Bilgin 1998 {published data only}
- Bilgin T, Ozan H, Dirgen A, Esmer A. Comparison of four different antibiotics as prophylaxis in caesarean section. Journal of Obstetrics and Gynaecology 1998;18(6):546‐7. [DOI] [PubMed] [Google Scholar]
Bourgeois 1985 {published data only}
- Bourgeois FJ, Pinkerton JA, Andersen W, Thiagarajah S. Antibiotic irrigation prophylaxis in the high‐risk cesarean section patient. American Journal of Obstetrics and Gynecology 1985;153:197‐201. [DOI] [PubMed] [Google Scholar]
Carl 2000 {published data only}
- Carl SH, Hampton R. Normal saline pelvic and intrauterine irrigation in the high‐risk cesarean section (CS) patient as a safe and cost‐effective method of infection prophylaxis. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S96. [Google Scholar]
Chan 1989 {published data only}
- Chan ACW, Leung AKL, Chin RKH, Chang AMZ. Single dose prophylactic antibiotics in caesarean sections. Australian and New Zealand Journal of Obstetrics and Gynaecology 1989;29:107‐9. [DOI] [PubMed] [Google Scholar]
Conover 1984 {published data only}
- Conover WB, Moore TR. Comparison of irrigation and intravenous antibiotic prophylaxis at cesarean section. Obstetrical and Gynecological Survey 1984;39:692‐3. [PubMed] [Google Scholar]
- Conover WB, Moore TR. Comparison of irrigation and intravenous antibiotic prophylaxis at cesarean section. Obstetrics and Gynecology 1984;63:787‐91. [PubMed] [Google Scholar]
Cormier 1989 {published data only}
- Cormier P, Leng JJ. Antibiotic prophylaxis after caesarean section [Antibioprophylaxie lors des cesariennes]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1991;20:600. [Google Scholar]
- Cormier P, Leng JJ, Janky E, Duthil B, Brouste V. Prevention of infectious complications after cesarean section by the use of cefotetan. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction (Paris) 1989;18:388‐92. [PubMed] [Google Scholar]
D'Angelo 1980 {published data only}
- D'Angelo LJ, Sokol RJ. Short‐ vs long‐course prophylactic antibiotic treatment in cesarean section patients. Obstetrics & Gynecology 1980;55:583‐6. [PubMed] [Google Scholar]
Dashow 1986 {published data only}
- Dashow EE, Read JA, Coleman FH. Randomized comparison of five irrigation solutions at cesarean section. Obstetrics & Gynecology 1986;68:473‐8. [PubMed] [Google Scholar]
De Boer 1989 {published data only}
- Boer CN, Thornton JG. Prophylactic short course rectal metronidazole for cesarean section. A double‐blind controlled trial of a simple low cost regimen. International Journal of Gynecology & Obstetrics 1989;28:103‐7. [DOI] [PubMed] [Google Scholar]
Dillon 1981 {published data only}
- Dillon WP, Seigel MS, Lele AS, O'Leary JA. Evaluation of cefoxitin prophylaxis for cesarean section. International Journal of Gynecology & Obstetrics 1981;19:133‐9. [DOI] [PubMed] [Google Scholar]
Duff 1980 {published data only}
- Duff P, Park RC. Antibiotic prophylaxis for cesarean section in a military population. Military Medicine (Washington DC) 1980;145:377‐81. [PubMed] [Google Scholar]
Duff 1982 {published data only}
- Duff P, Smith PN, Keiser JF. Antibiotic prophylaxis in low‐risk cesarean section. Journal of Reproductive Medicine 1982;27:133‐8. [PubMed] [Google Scholar]
Elliott 1986 {published data only}
- Elliott JP, Flaherty JF. Comparison of lavage or intravenous antibiotics at cesarean section. Obstetrics & Gynecology 1986;67:29‐32. [PubMed] [Google Scholar]
Engel 1984 {published data only}
- Engel K, Amir‐Moazami B, Karschnia R, Hahn T. Advantages and hazards of preventing infection following cesarean section‐‐clinical and bacteriologic results of a high‐dosage treatment with mezlocillin and oxacillin short‐term preventive following clamping of the umbilical cord [Nutzen und Gefahren der Infektionsprophylaxe bei der Sectio caesarea‐‐klinische und bakteriologische Ergebnisse einer hochdosierten Kurzzeitprophylaxe nach dem Abnabeln mit Mezlocillin und Oxacillin.]. Geburtshilfe und Frauenheilkunde 1984;44(3):162‐70. [DOI] [PubMed] [Google Scholar]
- Engel K, Karschnia R. Bacterial flora changes resulting from antimicrobial treatment. Journal of Obstetrics and Gynaecology 1986;6:6‐8. [Google Scholar]
Escobedo 1991 {published data only}
- Escobedo Lobaton JM, Rodriguez Hinojosa DE, Kistner Garza AM, Benavides de Anda L. Prophylactic use of antibiotics in cesarean section [Uso profilactico de antibioticos en operacion cesarea]. Ginecologia y Obstetricia de Mexico 1991;59(1):35‐8. [PubMed] [Google Scholar]
Freeman 1982 {published data only}
- Freeman GM. The efficacy of prophylactic antibiotics in high‐risk patients undergoing cesarean section. Journal of the American Osteopathic Association 1982;81(9):610‐5. [PubMed] [Google Scholar]
Fugere 1983 {published data only}
- Fugere P, Turgeon P, Boucher M, Verschelden G, Lemay M. Use of cephalosporins in antibiotic prophylaxis in women undergoing nonelective caesarean section [Utilisation des cephalosporines comme antibioprophylaxie lors de cesariennes]. Canadian Medical Association Journal 1983;129:132‐5. [PMC free article] [PubMed] [Google Scholar]
Gall 1979 {published data only}
- Gall SA. The efficacy of prophylactic antibiotics in caesarean section. American Journal of Obstetrics and Gynecology 1979;134:506‐11. [DOI] [PubMed] [Google Scholar]
Ganesh 1986 {published data only}
- Ganesh V, Apuzzio JJ, Dispenziere B, Patel K, Bergen B, Louria DB. Single‐dose trimethoprim‐sulfamethoxazole prophylaxis for cesarean section. American Journal of Obstetrics and Gynecology 1986;154:1113‐4. [DOI] [PubMed] [Google Scholar]
Garcia 1992 {published data only}
- Garcia MH, Garcia I, Doyague MJ, Luna S, Velasco MJ, Lanchares JL, et al. Incidence of urinary infection and other infectious complications in antibiotic prophylaxis of cesaran section with ultra‐short schedule. III [Incidencia de infección urinaria y otras complicaciones infecciosas en la profilaxis antibiótica de la cesárea con pauta ultracorta. III]. Tokoginecologia Practica 1992;51(7):349‐56. [Google Scholar]
- García MHH, Lajas JA, Velasco MJ, Doyague MJ, Luna S, García A, et al. Incidence of endometritis and infection of surgical wound in antibiotic prophylaxis of cesarean section with ultra‐short shedule. II [Incidencia de endometritis e infección en la herida oporatoria en la profilaxis antibiótica de la cesárea con pauta ultracorta. II]. Tokoginecologia Practica 1992; Vol. 51:340‐8.
Gerstner 1980 {published data only}
- Gerstner G, Kofler E, Huber J. Perioperative metronidazol‐prophylaxis for cesarean section. Zeitschrift fur Geburtshilfe und Perinatologie 1980;184:418‐23. [PubMed] [Google Scholar]
Gibbs 1972 {published data only}
- Gibbs RS, Cherney AH, Schwarz RH. Prophylactic antibiotics in cesarean section: a double‐blind study. American Journal of Obstetrics and Gynecology 1972;114:1048‐53. [DOI] [PubMed] [Google Scholar]
Gibbs 1973 {published data only}
- Gibbs RS, Hunt JE, Schwarz RH. A follow‐up study on prophylactic antibiotics in cesarean section. American Journal of Obstetrics and Gynecology 1973;117:419‐22. [DOI] [PubMed] [Google Scholar]
Gibbs 1981 {published data only}
- Gibbs RS, Clair PJ, Castillo MS, Castaneda YS. Bacteriologic effects of antibiotic prophylaxis in high‐risk cesarean section. Obstetrics & Gynecology 1981;57:277‐82. [PubMed] [Google Scholar]
Gordon 1979 {published data only}
- Gordon HR, Phelps D, Blanchard K. Prophylactic cesarean section antibiotics: maternal and neonatal morbidity before or after cord clamping. Obstetrics & Gynecology 1979;53:151‐6. [PubMed] [Google Scholar]
Gummerus 1984 {published data only}
- Gummerus M. Perioperative short‐term prophylaxis of puerperal infections following caesarean section with metronidazol. Geburtshilfe und Frauenheilkunde 1984;44:570‐2. [DOI] [PubMed] [Google Scholar]
Hager 1983 {published data only}
- Hager WD, Williamson MM. Effects of antibiotic prophylaxis on women undergoing nonelective cesarean section in a community hospital. Journal of Reproductive Medicine 1983;28:687‐90. [PubMed] [Google Scholar]
Hagglund 1989 {published data only}
- Hagglund L, Christensen KK, Christensen P, Westrom L, Ingemarsson I. Reduced rate of postoperative infections in emergency cesarean section after two doses of cefuroxim perioperatively. Acta Obstetricia et Gynecologica Scandinavica 1989;68:201‐4. [DOI] [PubMed] [Google Scholar]
Harger 1981 {published data only}
- Harger JH, English DH. Selection of patients for antibiotic prophylaxis in cesarean sections. American Journal of Obstetrics and Gynecology 1981;141:752‐8. [DOI] [PubMed] [Google Scholar]
Hawrylyshyn 1983 {published data only}
- Hawrylyshyn PA, Bernstein EP, Papsin FR. Short‐term antibiotic prophylaxis in high‐risk patients following cesarean section. American Journal of Obstetrics and Gynecology 1983;145:285‐9. [DOI] [PubMed] [Google Scholar]
Heilmann 1984 {published data only}
- Heilmann L, Tauber PF. Short‐term prevention with cefoxitin in cesarean section [Kurzzeitprophylaxe mit Cefoxitin beim Kaiser‐schnitt]. Geburtshilfe und Frauenheilkunde 1984;44:792‐5. [DOI] [PubMed] [Google Scholar]
Huam 1997 {published data only}
- Huam SH, Lim JM, Raman S. Single‐dose antibiotic prophylaxis in women undergoing elective caesarean section. Medical Journal of Malaysia 1997;52:3‐7. [PubMed] [Google Scholar]
Ismail 1990 {published data only}
- Ismail MA, Nelson KE, Larson P, Moses VK. Selective effect of cefoxitin prophylaxis on post‐cesarean‐section microbial flora. Journal of Reproductive Medicine 1990;35:168‐74. [PubMed] [Google Scholar]
Jaffe 1984 {published data only}
- Jaffe R, Loebel R, Altaras M, Ben Aderet N. Perioperative mezlocillin prophylaxis in cesarean section. Clinical Therapeutics 1984;6(4):467‐74. [PubMed] [Google Scholar]
Jaffe 1985 {published data only}
- Jaffe R, Altaras M, Cohen I, Ben‐Aderet N. Single‐dose mezlocillin prophylaxis in emergency cesarean section. Clinical Therapeutics 1985;7(4):507‐11. [PubMed] [Google Scholar]
Jakobi 1994 {published data only}
- Jakobi P, Weissman A, Sigler E, Margolis K, Zimmer EZ. Post‐cesarean section febrile morbidity. Journal of Reproductive Medicine 1994;39:707‐10. [PubMed] [Google Scholar]
Karhunen 1985 {published data only}
- Karhunen M, Koskela O, Teisala K, Suikkari AM, Mattila J. Prophylaxis and treatment of anaerobic infections following caesarean section with tinidazole. Chemotherapy 1985;31:228‐36. [DOI] [PubMed] [Google Scholar]
Kellum 1985 {published data only}
- Kellum RB, Roberts WE, Harris JB, Khansur N, Morrison JC. Effect of intrauterine antibiotic lavage after cesarean birth on postoperative morbidity. Journal of Reproductive Medicine 1985;30:527‐9. [PubMed] [Google Scholar]
Kolben 2001 {published data only}
- Kolben M, Mandoki E, Ulm K, Freitag K. Randomized trial of cefotiam prophylaxis in the prevention of postoperative infectious morbidity after elective cesarean section. European Journal of Clinical Microbiology and Infectious Diseases 2001;20:40‐2. [DOI] [PubMed] [Google Scholar]
Kreutner 1978 {published data only}
- Kreutner AK, Bene VE, Delamar D, Huguley V, Harmon PM, Mitchell KS. Perioperative antibiotic prophylaxis in cesarean section. Obstetrics & Gynecology 1978;52:279‐84. [PubMed] [Google Scholar]
Kristensen 1990 {published data only}
- Kristensen GB, Beiter EC, Mather O. Single‐dose cefuroxime prophylaxis in non‐elective cesarean section. Acta Obstetricia et Gynecologica Scandinavica 1990;69:497‐500. [DOI] [PubMed] [Google Scholar]
Lapas 1988 {published data only}
- Lapas KA, Todorov I. Comparative double‐blind study of intravenous metronidazole vs placebo in preventing infection after cesarean section. Akusherstvo i Ginekologiia 1988;27:46‐9. [PubMed] [Google Scholar]
- Lappas CA, Leonardopoulos J. Double‐blind comparative study of metronidazole iv vs placebo in the prophylaxis of sepsis following cesarean section. Archives of Gynecology 1985;237(Suppl 1):279. [Google Scholar]
Lemus 2005 {published data only}
- Lemus Rocha R, Garcia Gutierrez LB, Basavilvazo Rodriguez MA, Cruz Avelar A, Peralta Pedrero ML, Hernandez Valencia M. Incidence of infected surgical wound and prophylaxis with cefotaxime in cesarean section. Ginecologia y Obstetricia de Mexico 2005;73(10):537‐43. [PubMed] [Google Scholar]
Leonetti 1989 {published data only}
- Leonetti HB, Yun H, O'Leary JA, Greenberg AL. Single vs multiple dose piperacillin in high risk primary cesarean section. American Journal of Gynecologic Health 1989;3:195‐8. [Google Scholar]
Levin 1983 {published data only}
- Levin DK, Gorchels C, Andersen R. Reduction of post‐cesarean section infectious morbidity by means of antibiotic irrigation. American Journal of Obstetrics and Gynecology 1983;147:273‐7. [DOI] [PubMed] [Google Scholar]
Lewis 1990 {published data only}
- Lewis DF, Otterson WN, Dunnihoo DR. Antibiotic prophylactic uterine lavage in cesarean section: a double‐blind comparison of saline, ticarcillin, and cefoxitin irrigation in indigent patients. Southern Medical Journal 1990;83:274‐6. [DOI] [PubMed] [Google Scholar]
Magro 1983 {published data only}
- Magro B, Franchi I, Chehade A, Berti MA, Coppi G. A controlled clinical study of cefuroxim for antimicrobial prophylaxis during obstetric and gynecological surgery [Studio clinico controllato del cefuroxim nella profilassi antimicrobia durante gli interventi chirurgici in ostetricia e ginecologia]. Clinica Terapeutica 1983;105(3):209‐14. [PubMed] [Google Scholar]
Mahomed 1988 {published data only}
- Mahomed K. A double‐blind randomized controlled trial on the use of prophylactic antibiotics in patients undergoing elective caesarean section. British Journal of Obstetrics and Gynaecology 1988;95:689‐92. [DOI] [PubMed] [Google Scholar]
McCowan 1980 {published data only}
- McCowan L, Jackson P. The prophylactic use of metronidazole in caesarean section. New Zealand Medical Journal 1980;92:153‐5. [PubMed] [Google Scholar]
Miller 1968 {published data only}
- Miller RD, Crichton D. Ampicillin prophylaxis in caesarean section. South African Journal of Obstetrics and Gynaecology 1968;6:69‐70. [Google Scholar]
Moodley 1981 {published data only}
- Moodley J, Zeeman DJ. Prophylactic and antimicrobial therapy using lincomycin in patients undergoing emergency caesarean section. South African Medical Journal 1981;59:911‐3. [PubMed] [Google Scholar]
Moro 1974 {published data only}
- Moro M, Andrews M. Prophylactic antibiotics in cesarean section. Obstetrics & Gynecology 1974;44:688‐92. [PubMed] [Google Scholar]
Morrison 1973 {published data only}
- Morrison JC, Coxwell WL, Kennedy BS, Schreier PC, Wiser WL, Fish SA. The use of prophylactic antibiotics in patients undergoing cesarean section. Surgery, Gynecology and Obstetrics 1973;136:425‐8. [PubMed] [Google Scholar]
Ng 1992 {published data only}
- Ng NK. The role of prophylactic antibiotics in caesarean section ‐ a randomized trial. Medical Journal of Malaysia 1992;47:273‐9. [PubMed] [Google Scholar]
Oestreicher 1987 {published data only}
- Oestreicher M, Oestreicher S, Dudenhausen JW. Prospective study on the question of single‐dose antibiotic prophylaxis for primarily indicated abdominal cesarean section (translation). Zeitschrift fur Geburtshilfe und Perinatologie 1987;191:12‐4. [PubMed] [Google Scholar]
Padilla 1983 {published data only}
- Padilla SL, Spence MR, Beauchamp PJ. Single‐dose ampicillin for cesarean section prophylaxis. Obstetrics & Gynecology 1983;61:463‐6. [PubMed] [Google Scholar]
Phelan 1979 {published data only}
- Phelan JP, Pruyn SC. Prophylactic antibiotics in cesarean section: a double‐blind study of cefalozin. American Journal of Obstetrics and Gynecology 1979;133:474‐8. [DOI] [PubMed] [Google Scholar]
Polk 1982 {published data only}
- Polk BF, Krache M, Phillippe M, Munoz A, Hutchinson D, Miao L, et al. Randomized clinical trial of perioperative cefoxitin in preventing maternal infection after primary cesarean section. American Journal of Obstetrics and Gynecology 1982;142:983‐7. [DOI] [PubMed] [Google Scholar]
Racinet 1990 {published data only}
- Mallaret MR, Blatier JF, Racinet C, Fauconnier J, Favier M, Micoud M. Economic benefit of using antibiotics prophylactically in cesarean sections with little risk of infection. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction (Paris) 1990;19:1061‐4. [PubMed] [Google Scholar]
- Racinet C, Mallaret MR, Favier M, Berthet J, Morel I, Fauconnier J, et al. Antibiotic prophylaxis in cesarean sections without high risk of infection. Presse Medicale 1990;19:1755‐8. [PubMed] [Google Scholar]
Reckel 1985 {published data only}
- Reckel J, Scheele R. Perioperative antibiotic prophylaxis in cesarean section [Perioperative Antibiotikaprophylaxe bei Kaiserschnitt]. Der Klinikarzt 1985;14:1054‐65. [Google Scholar]
Rehu 1980 {published data only}
- Rehu M, Jahkola M. Prophylactic antibiotics in caesarean section: effect of a short preoperative course of benzyl penicillin or clindamycin plus gentamicin on postoperative infectious morbidity. Annals of Clinical Research 1980;12:45‐8. [PubMed] [Google Scholar]
Rizk 1998 {published data only}
- Rizk DEE, Nsanze H, Mabrouk MH, Mustafa N, Thomas L, Kumar M. Systemic antibiotic prophylaxis in elective cesarean delivery. International Journal of Gynecology & Obstetrics 1998;61(3):245‐51. [DOI] [PubMed] [Google Scholar]
Roex 1986 {published data only}
- Roex AJM, Puyenbroek JI, MacLaren DM, Geijn HP, Arts NFT. A randomized clinical trial of antibiotic prophylaxis in cesarean section: maternal morbidity, risk factors and bacteriological changes. European Journal of Obstetrics & Gynecology and Reproductive Biology 1986;22:117‐24. [DOI] [PubMed] [Google Scholar]
- Roex AJM, Puyenbroek JI, Maclaren DM, Arts NFTh. Short‐term antibiotic prophylaxis for caesarean section [Kortdurende antibioticum profylaxe bij de section Caesarea]. Nederland Tijdschrift voor Obstetrie en Gynaecologie 1987;100:105. [Google Scholar]
Ross 1984 {published data only}
- Ross L, Mason P, Barnet‐Lamb M, Robinson RE, Warren R. Prophylactic metronidazole in patients with ruptured membranes undergoing emergency caesarean section. Journal of Obstetrics and Gynaecology 1984;5:32‐5. [Google Scholar]
Rothbard 1975 {published data only}
- Rothbard MJ, Mayer W, Wystepek A, Gordon M. Prophylactic antibiotics in cesarean section. Obstetrics & Gynecology 1975;45:421‐4. [PubMed] [Google Scholar]
Rouzi 2000 {published data only}
- Rouzi AA, Khalifa F, Ba'aqeel H, Al‐Hamdan HS, Bondagji N. The routine use of cefazolin in cesarean section. International Journal of Gynecology and Obstetrics 2000;69:107‐12. [DOI] [PubMed] [Google Scholar]
Rudd 1981 {published data only}
- Long WH, Rudd EG, Dillon MB. Intrauterine irrigation with cefamandole nafate solution at cesarean section: a preliminary report. American Journal of Obstetrics and Gynecology 1980;138:755‐8. [DOI] [PubMed] [Google Scholar]
- Rudd EG, Long WH, Dillon MB. Febrile morbidity following cefamandole nafate intrauterine irrigation during cesarean section. American Journal of Obstetrics and Gynecology 1981;141:12‐6. [DOI] [PubMed] [Google Scholar]
Ruiz‐Moreno 1991 {published data only}
- Ruiz‐Moreno JA, Garcia‐Rojas JM, Lozada‐Leon JD. Prevention of post cesarean infectious morbidity with a single dose of intravenous metronidazole. International Journal of Gynecology & Obstetrics 1991;34:217‐20. [DOI] [PubMed] [Google Scholar]
Saltzman 1985 {published data only}
- Saltzman DH, Eron LJ, Kay HH, Sites JG. Single‐dose antibiotic prophylaxis in high‐risk patients undergoing cesarean section. Obstetrics & Gynecology 1985;65:655‐7. [PubMed] [Google Scholar]
Scarpignato 1982 {published data only}
- Scarpignato C, Caltabiano M, Condemi V, Mansani FE. Short‐term vs long‐term cefuroxime prophylaxis in patients undergoing emergency cesarean section. Clinical Therapeutics 1982;5:186‐92. [PubMed] [Google Scholar]
Schedvins 1986 {published data only}
- Schedvins K, Moberg PJ. Prevention of postoperative infection in cesarean section after rupture of the membranes. International Journal of Gynecology & Obstetrics 1986;24:165‐8. [DOI] [PubMed] [Google Scholar]
Shah 1998 {published data only}
- Shah S, Mazher Y, John IS. Single or triple dose piperacillin prophylaxis in elective cesarean section. International Journal of Gynecology & Obstetrics 1998;62(1):23‐9. [DOI] [PubMed] [Google Scholar]
Sokolowski 1989 {published data only}
- Sokolowski VH, Canzler E, Brotzmann C. Influence of vagimid prophylaxis on course of puerperium and healing of the wound after caesarean section in comparison with a control group. Zentralblatt fur Gynakologie 1989;111:461‐5. [PubMed] [Google Scholar]
Stage 1982 {published data only}
- Stage AH, Glover DD, Vaughan JE. Low‐dose cephradine prophylaxis in obstetric and gynecologic surgery. Journal of Reproductive Medicine 1982;27:113‐9. [PubMed] [Google Scholar]
Stiver 1983 {published data only}
- Stiver HG, Forward KR, Livingstone RA, Fugere P, Lemay M, Verschelden G, et al. Multicenter comparison of cefoxitin vs cefazolin for prevention of infectious morbidity after nonelective cesarean section. American Journal of Obstetrics and Gynecology 1983;145:158‐63. [DOI] [PubMed] [Google Scholar]
- Stiver HG, Forward KR, Tyrrell DL, Krip G, Livingstone RA, Fugere P, et al. Comparative cervical microflora shifts after cefoxitin or cefazolin prophylaxis against infection following cesarean section. American Journal of Obstetrics and Gynecology 1984;149(7):718‐21. [DOI] [PubMed] [Google Scholar]
Sziller 1994 {published data only}
- Sziller I, Karovits J, Erdosi F, Beke A, Oszoli G. Postoperative infectious morbidity after perioperative ampicillin /sulbactam prophylaxis in women undergoing elective caesarean section. Magyar Norvosok Lapja 1994;57:101‐4. [Google Scholar]
Tully 1983 {published data only}
- Tully JL, Klapholz H, Baldini LM, Friedland GH. Perioperative use of cefoxitin in primary cesarean section. Journal of Reproductive Medicine 1983;28:827‐32. [PubMed] [Google Scholar]
Turner 1990 {published data only}
- Turner MJ, Egan DM, Qureshi WA, Skehan M, Black A, Darrell JH, et al. Use of cephradine prophylaxis of infection after caesarean section: stepwise logistic regression analysis of relevant factors. Journal of Obstetrics and Gynaecology 1990;10:204‐9. [Google Scholar]
Tzingounis 1982 {published data only}
- Tzingounis V, Makris N, Zolotas J, Michalas S, Aravantinos D. Cefuroxime prophylaxis in caesarean section. Pharmatherapeutica 1982;3:140‐3. [PubMed] [Google Scholar]
Ujah 1992 {published data only}
- Ujah I, Olarewaju R. The use of prophyactic Augmentin in elective caesarean section in Jos University teaching hospital. Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:484.
- Ujah IAO, Olarewaju RS, Otubu JAM. Prophylactic amoxicillin‐clavulanic acid in elective cesarean section. Current Therapeutic Research, Clinical and Experimental 1992;52(5):647‐51. [Google Scholar]
Walss Rodriguez 1990 {published data only}
- Walss Rodriguez R, Avila Esparza M. Prophylactic antimicrobial therapy in cesarean section [Antibioticoterapia profilactica en operacion cesarea]. Ginecologia y Obstetricia de Mexico 1990;58:79‐83. [Google Scholar]
Weissberg 1971 {published data only}
- Weissberg SM, Edwards NL, O'Leary JA. Prophylactic antibiotics in cesarean section. Obstetrics & Gynecology 1971;38:290‐3. [PubMed] [Google Scholar]
Witt 2011 {published data only}
- Witt A, Doner M, Petricevic L, Berger A, Germann P, Heinze G, et al. Antibiotic prophylaxis before surgery vs after cord clamping in elective cesarean delivery: a double‐blind, prospective, randomized, placebo‐controlled trial. Archives of Surgery 2011;146(12):1404‐9. [PUBMED: 22184305] [DOI] [PubMed] [Google Scholar]
Wong 1978 {published data only}
- Wong R, Gee CL, Ledger WJ. Prophylactic use of cefazolin in monitored obstetric patients undergoing cesarean section. Obstetrics & Gynecology 1978;51:407‐11. [DOI] [PubMed] [Google Scholar]
Work 1977 {published data only}
- Work BA Jr. Role of preventive antibiotics in patients undergoing cesarean section. South African Medical Journal 1977;70:44‐5. [DOI] [PubMed] [Google Scholar]
Wu 1991 {published data only}
- Wu Y. Prevention of post‐operative infection by using antibiotics of 217 cases of cesarean section. Chinese Journal of Obstetrics and Gynecology 1992;27:73‐5. [PubMed] [Google Scholar]
- Wu Y, Zhan L, Jing Y. Prevention of post‐operative infection by uterine and intraperitoneal irrigation with ampicillin during cesarean section. International Journal of Experimental and Clinical Chemotherapy 1991;4(3):132‐6. [Google Scholar]
Yip 1997 {published data only}
- Yip SK, Lau TK, Rogers MS. A study on prophylactic antibiotics in cesarean sections ‐ is it worthwhile?. Acta Obstetricia et Gynecologica Scandinavica 1997;76(6):547‐9. [DOI] [PubMed] [Google Scholar]
Young 1983 {published data only}
- Young R, Platt L, Ledger W. Prophylactic cefoxitin in cesarean section. Surgery, Gynecology and Obstetrics 1983;157:11‐4. [PubMed] [Google Scholar]
References to studies excluded from this review
Ahued 1994 {published data only}
- Ahued Ahued RA, Leal del Rosal JA, Rocha del Valle G, Sereno Colo JA. The efficacy of sulbactam‐ampicillin in preventing postoperative infections in gynecology and obstetrics. A comparative open study [Eficacia de sulbactam/ampicilina en la profilaxis de infecciones postquirurgicas en gineco‐obstetricia. Estudio abierto comparativo]. Ginecologia y Obstetricia de Mexico 1994;62(9):282‐4. [PubMed] [Google Scholar]
Andrews 2003 {published data only}
- Andrews WW, Hauth JC, Cliver SP, Savage K, Goldenberg RL. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for ureaplasma urealyticum to reduce post‐cesarean delivery endometritis. Obstetrics & Gynecology 2003;101(6):1183‐9. [DOI] [PubMed] [Google Scholar]
Cormier 1988 {published data only}
- Cormier P, Leng JJ, Janky E, Brouste V, Duthil B. Antibiotic prophylaxis with cefotetan in the prevention of post‐partum and post‐abortum infectious complications in endo‐uterine investigations. Revue Francaise de Gynecologie et d Obstetrique 1988;83:829‐32. [PubMed] [Google Scholar]
Creatsas 1980 {published data only}
- Creatsas G, Pavlatos M, Lolis D, Kaskarelis D. Ampicillin and gentamicin in the treatment of fetal intrauterine infections. Journal of Perinatal Medicine 1980;8:13‐8. [DOI] [PubMed] [Google Scholar]
De Palma 1980 {published data only}
- Palma RT, Leveno KJ, Cunningham FG, Pope T, Kappus SS, Roark ML, et al. Identification and management of women at high risk for pelvic infection following cesarean section. Obstetrics & Gynecology 1980;55:185S‐192S. [DOI] [PubMed] [Google Scholar]
Elliott 1982 {published data only}
- Elliott JP, Freeman RK, Dorchester W. Short versus long course of prophylactic antibiotics in cesarean section. American Journal of Obstetrics and Gynecology 1982;143:740‐4. [DOI] [PubMed] [Google Scholar]
Harrigill 2003 {published data only}
- Harrigill K, Miller H, Haynes D. The effect of intra‐abdominal irrigation at cesarean section on maternal morbidity. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S161. [DOI] [PubMed] [Google Scholar]
- Harrigill KM, Miller HS, Haynes DE. The effect of intraabdominal irrigation at cesarean delivery on maternal morbidity: a randomized trial. Obstetrics & Gynecology 2003;101:80‐5. [DOI] [PubMed] [Google Scholar]
Itskovitz 1979 {published data only}
- Itskovitz J, Paldi E, Katz M. The effect of prophylactic antibiotics on febrile morbidity following cesarean section. Obstetrics & Gynecology 1979;53:162‐5. [PubMed] [Google Scholar]
Kosus 2010 {published data only}
- Kosus A, Kosus N, Guler A, Capar M. Rifamycin SV application to subcutanous tissue for prevention of post‐cesarean surgical site infection [Sezaryen sonrasi kesi yeri enfeksiyonunu onlemek icin ciltalti rifamisin SV uygulanmasi]. European Journal of General Medicine 2010;7(3):269‐76. [Google Scholar]
Krasnodebski 1997 {published data only}
- Krasnodebski J, Stolecki M. A single dose of antibiotic ‐ as a prophylaxis during cesarean section. Ginekologia Polska 1997;68:30‐5. [PubMed] [Google Scholar]
Kreutner 1979 {published data only}
- Kreutner AK, Bene VE, Delamar D, Bodden JL, Loadholt CB. Perioperative cephalosporin prophylaxis in cesarean section: effect on endometritis in the high‐risk patient. American Journal of Obstetrics and Gynecology 1979;134:925‐35. [DOI] [PubMed] [Google Scholar]
Louie 1982 {published data only}
- Louie TJ, Binns BAO, Baskett TF, Ross J, Koss J. Cefotaxime, cefazolin, or ampicillin prophylaxis of febrile morbidity in emergency cesarean sections. Clinical Therapeutics 1982;5:83‐96. [PubMed] [Google Scholar]
Pawelec 1994 {published data only}
- Pawelec M, Michalik T, Robaczynski J. One‐dose administration of Mandol in the prevention of infection after caesarean section. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:389.
Petersen 1985 {published data only}
- Petersen C, Brautigam HH. Short‐term peri‐operative prophylaxis with cefotaxim in gynaecological and obstetric surgery. Deutsche Medizinische Wochenschrift 1985;110:1369‐74. [DOI] [PubMed] [Google Scholar]
Pitt 2001 {published data only}
- Pitt C, Sanchez‐Ramos L, Kaunitz A. Adjunctive intravaginal metronidazole for the prevention of postcesarean endometritis: a randomized controlled trial. Obstetrics & Gynecology 2001;98:745‐50. [DOI] [PubMed] [Google Scholar]
Roex 1987 {published data only}
- Roex AJM, Loenen AC, Puyenbroek JI, Arts NFT. Secretion of cefoxitin in breast milk following short‐term prophylactic administration in caesarean section. European Journal of Obstetrics & Gynecology and Reproductive Biology 1987;25:299‐302. [DOI] [PubMed] [Google Scholar]
Sanchez‐Ramos 1999 {published data only}
- Sanchez‐Ramos L, Pitt C, Delke I, Gaudier FL. Preoperative administration of intravaginal metronidazole for the prevention of post‐cesarean endometritis: a randomized double‐blind trial. American Journal of Obstetrics and Gynecology 1999;180 (1 Pt 2):S:81. [Google Scholar]
Sengupta 1976 {published data only}
- Sengupta BS, Wynter HH, Hall JS, Ramchander R, Alexis A, Zamah N, et al. Prophylactic antibiotic in elective gynaecological and obstetrical major surgery. International Journal of Gynecology & Obstetrics 1976;14:417‐24. [DOI] [PubMed] [Google Scholar]
Skryten 1988 {published data only}
- Skryten A. The efficacy of perioperative cefoxitin prophylaxis in preventing infectious morbidity after nonelective cesarean section. 12th FIGO World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:713.
Spreafico 1987 {published data only}
- Spreafico P, Scian A, Epis A, Vassen L, Bonazzi C, Lovotti M. Cesarean section: antibiotic prophylaxis with ceftezole. Chemotherapia 1987;6(2S):613‐6. [PubMed] [Google Scholar]
Voto 1986 {published data only}
- Voto LS, Benoliel LA, Amadora Muñiz A, Trepat A, Balsechi EE, Margulies M. Prophylaxis of post cesarean section puerperal infection with the using of cefoxitin antibiotics [Profilaxis de la infección puerperal post‐cesarea mediante el uso del antibiótico cefoxitina: I ‐ Resultados de su empleo valorados a través del cultivo sistemático pre, intra y postquirúrgico]. Obstetricia y Ginecologia Latino‐Americanas 1986;44:419‐24. [Google Scholar]
Wallace 1984 {published data only}
- Wallace RL, Eglinton GS, Yonekura ML, Wallace TM. Extraperitoneal cesarean section: a surgical form of infection prophylaxis?. American Journal of Obstetrics and Gynecology 1984;148:172‐7. [DOI] [PubMed] [Google Scholar]
Wells 1994 {published data only}
- Wells M, McCullough W, Rymer J. Antibiotic prophylaxis in emergency caesarean section. International Journal of Gynecology & Obstetrics 1994;46:77. [Google Scholar]
Yamagishi 2009 {published data only}
- Yamagishi Y, Izumi K, Tanaka K, Watanabe K, Mikamo H. Evaluation of antimicrobial prophylaxis after normal delivery. Japanese Journal of Antibiotics 2009;62(2):103‐15. [PubMed] [Google Scholar]
Yildirim 2009 {published data only}
- Yildirim G, Gungorduk K, Guven HZ, Aslan H, Celikkol O, Sudolmus S, et al. When should we perform prophylactic antibiotics in elective cesarean cases?. Archives of Gynecology and Obstetrics 2009;280(1):13‐8. [DOI] [PubMed] [Google Scholar]
Additional references
ACOG 2011
- American College of Obstetricians and Gynecologists. ACOG practice bulletin number 120, Prophylactic antibiotics in labor and delivery. Obstetrics & Gynecology 2011;117(6):1472‐83. [DOI] [PubMed] [Google Scholar]
Alfirevic 2010
- Alfirevic Z, Gyte GM, Dou L. Different classes of antibiotics given to women routinely for preventing infection at caesarean section. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008726] [DOI] [PubMed] [Google Scholar]
Baaqeel 2013
- Baaqeel H, Baaqeel R. Timing of administration of prophylactic antibiotics for caesarean section: a systematic review and meta‐analysis. BJOG: an international journal of obstetrics and gynaecology 2013;120(6):661‐9. [DOI: 10.1111/1471-0528.12036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 1995
- Baker C, Luce J, Chenoweth C, Friedman C. Comparison of case‐finding methodologies for endometritis after cesarean section. American Journal of Infection Control 1995;23:27‐33. [DOI] [PubMed] [Google Scholar]
Beattie 1994
- Beattie PG, Rings TR, Hunter MF, Lake Y. Risk factors for wound infection following caesarean section. Australian and New Zealand Journal of Obstetrics and Gynaecology 1994;34:398‐402. [DOI] [PubMed] [Google Scholar]
Bedford Russell 2006
- Bedford Russell A, Murch S. Could peripartum antibiotics have delayed health consequences for the infant?. BJOG: an international journal of obstetrics and gynaecology 2006;113:758‐65. [DOI] [PubMed] [Google Scholar]
Boggess 1996
- Boggess KA, Watts DH, Hillier SL, Krohn MA, Benedetti TJ, Eschenbach DA. Bacteremia shortly after placental separation during cesarean delivery. Obstetrics & Gynecology 1996;87:779‐84. [DOI] [PubMed] [Google Scholar]
Bratzler 2004
- Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the national surgical infection prevention project. Clinical Infectious Diseases 2004;38(12):1706‐15. [DOI] [PubMed] [Google Scholar]
Chelmow 2001
- Chelmow D, Rueehli MS, Huang E. Prophylactic use of antibiotics for nonlaboring patients undergoing cesarean delivery with intact membranes: a meta‐analysis. American Journal of Obstetrics and Gynecology 2001;184(4):656‐61. [DOI] [PubMed] [Google Scholar]
Classen 1992
- Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical‐wound infection. New England Journal of Medicine 1992;326(5):281‐6. [DOI] [PubMed] [Google Scholar]
Costantine 2008
- Costantine MM, Rahman M, Ghulmiyah L, Byers BD, Longo M, Wen T, et al. Timing of perioperative antibiotics for cesarean delivery: a metaanalysis. American Journal of Obstetrics and Gynecology 2008;199(3):301.e1‐6. [DOI] [PubMed] [Google Scholar]
Cunningham 1983
- Cunningham FG, Leveno KJ, DePalma RT, Roark M, Rosenfeld CR. Perioperative antimicrobials for cesarean delivery: before or after cord clamping. Obstetrics & Gynecology 1983;62(2):151‐4. [PubMed] [Google Scholar]
Dancer 2004
- Dancer SJ. How antibiotics can make us sick: the less obvious adverse effects of antimicrobial chemotherapy. Lancet Infectious Diseases 2004;6:611‐9. [DOI] [PubMed] [Google Scholar]
Declercq 2007
- Declercq E, Barger M, Cabral HJ, Evans SR, Kotelchuck M, Simon C, et al. Maternal outcomes associated with planned primary cesarean births compared with planned vaginal births. Obstetrics & Gynecology 2007;109(3):669‐77. [DOI] [PubMed] [Google Scholar]
Desjardins 1996
- Desjardins C, Diallo HO, Audet‐Lapointe P, Harel F. Retrospective study of post‐cesarean endometritis. 1992‐1993. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1996;25:419‐23. [PubMed] [Google Scholar]
Dodd 2014
- Dodd J, Anderson E, Gates S, Grivell R. Surgical techniques for uterine incision and uterine closure at the time of caesarean section. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD004732.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehrenkranz 1990
- Ehrenkranz NJ, Blackwelder WC, Pfaff SJ, Poppe D, Yerg DE, Kaslow RA. Infections complicating low‐risk cesarean sections in community hospitals: efficacy of antimicrobial prophylaxis. American Journal of Obstetrics and Gynecology 1990;162(2):337‐43. [DOI] [PubMed] [Google Scholar]
Emmons 1988
- Emmons SL, Krohn M, Jackson M, Eschenbach DA. Development of wound infections among women undergoing cesarean section. Obstetrics & Gynecology 1988;72:559‐64. [PubMed] [Google Scholar]
Enkin 1989
- Enkin MW, Enkin E, Chalmers I, Hemminki E. Prophylactic antibiotics in association with caesarean section. In: Chalmers I, Enkin MW, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:1246‐69. [Google Scholar]
Galask 1987
- Galask RP. Changing concepts in obstetric antibiotic prophylaxis. American Journal of Obstetrics and Gynecology 1987;157(2):491‐7. [DOI] [PubMed] [Google Scholar]
Gibbs 1980
- Gibbs RS. Clinical risk factors for puerperal infection. Obstetrics & Gynecology 1980;55(5 Suppl):178S‐184S. [DOI] [PubMed] [Google Scholar]
Gilstrap 1988
- Gilstrap LC. Prophylactic antibiotics for cesarean section and surgical procedures. Journal of Reproductive Medicine 1988;33:588‐90. [PubMed] [Google Scholar]
GRADE 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.6. The Cochrane Collaboration, 2008.
Haas 2013
- Haas D, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD007892.pub3] [DOI] [PubMed] [Google Scholar]
Hadiati 2012
- Hadiati D, Hakimi M, Nurdiati D. Skin preparation for preventing infection following caesarean section. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD007462.pub2] [DOI] [PubMed] [Google Scholar]
Henderson 1995
- Henderson E, Love EJ. Incidence of hospital‐acquired infections associated with caesarean section. Journal of Hospital Infection 1995;29:245‐55. [DOI] [PubMed] [Google Scholar]
Herbert 1999
- Herbert PR, Reed G, Entman SS, Mitchel EF, Berg C, Griffin M. Serious maternal morbidity after childbirth: prolonged hospital stays and readmissions. Obstetrics & Gynecology 1999;94(6):942‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Howey 1990
- Howie PW, Davey PG. Prophylactic antibiotics and caesarean section. BMJ 1990;300:2‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hulton 1992
- Hulton LJ, Olmsted RN, Treston‐Aurand J, Craig CP. Effect of postdischarge surveillance on rates of infectious complications after cesarean section. American Journal of Infection Control 1992;20:198‐201. [DOI] [PubMed] [Google Scholar]
Huskins 2001
- Huskins WC, Ba‐Thike K, Festin MR, Limpongsanurak S, Lumbiganon P, Peedicayil A, et al. An international survey of practice variation in the use of antibiotic prophylaxis in cesarean section. International Journal of Gynecology & Obstetrics 2001;73(2):141‐5. [DOI] [PubMed] [Google Scholar]
Kaimal 2008
- Kaimal AJ, Zlatnik MG, Cheng YW, Thiet MP, Connatty E, Creedy P, et al. Effect of a change in policy regarding the timing of prophylactic antibiotics on the rate of postcesarean delivery surgical‐site infections. American Journal of Obstetrics and Gynecology 2008;199(3):310. e1‐5. [DOI] [PubMed] [Google Scholar]
Killian 2001
- Killian CA, Graffunder EM, Vinciguerra TJ, Venezia RA. Risk factors for surgical‐site infections following cesarean section. Infection Control and Hospital Epidemiology 2001;22:613‐7. [DOI] [PubMed] [Google Scholar]
Leigh 1990
- Leigh DA, Emmanuel FX, Sedgwick J, Dean R. Post‐operative urinary tract infection and wound infection in women undergoing caesarean section: a comparison of two study periods in 1985 and 1987. Journal of Hospital Infection 1990;15:107‐16. [DOI] [PubMed] [Google Scholar]
MacLean 1990
- MacLean AB. Puerperal pyrexia. In: MacLean AB editor(s). Clinical Infection in Obstetrics and Gynecology. Oxford: Blackwell Scientific Publications, 1990:195‐209. [Google Scholar]
Magann 1995
- Magann EF, Washburne JF, Harris RL, Bass JD, Duff WP, Morrison JC. Infectious morbidity, operative blood loss, and length of the operative procedure after cesarean delivery by method of placental removal and site of uterine repair. Journal of the American College of Surgeons 1995;181:517‐20. [PubMed] [Google Scholar]
Mah 2001
- Mah MW, Pyper AM, Oni GA, Memish ZA. Impact of antibiotic prophylaxis on wound infection after cesarean section in a situation of expected high risk. American Journal of Infection Control 2001;29(2):85‐8. [DOI] [PubMed] [Google Scholar]
Martens 1995
- Martens MG, Kolrud BL, Faro S, Maccato M, Hammill H. Development of wound infection or separation after cesarean delivery. Prospective evaluation of 2,431 cases. Journal of Reproductive Medicine 1995;40:171‐5. [PubMed] [Google Scholar]
Morisaki 2014
- Morisaki N, Ganchimeg T, Ota E, Vogel JP, Souza JP, Mori R, et al. Maternal and institutional characteristics associated with the administration of prophylactic antibiotics for caesarean section: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG: an international journal of obstetrics and gynaecology 2014;121(Suppl 1):66‐75. [DOI: 10.1111/1471-0528.12632] [DOI] [PubMed] [Google Scholar]
Mugford 1989
- Mugford M, Kingston J, Chalmers I. Reducing the incidence of infection after caesarean section: implications of prophylaxis for hospital resources. BMJ 1989;299:1003‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newton 1998
- Newton, ER, Wallace PA. Effects of prophylactic antibiotics on endometrial flora in women with postcesarean endometritis. Obstettocs and Gynecology 1998;92(2):262‐8. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence. Caesarean Section. NICE Clinical Guideline 132. NICE, 2011. [PubMed] [Google Scholar]
NNIS 2004
- A report from the NNIS System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. American Journal of Infection Control 2004;32(8):470‐85. [DOI] [PubMed] [Google Scholar]
OECD 2013
- Organization for Economic Co‐operation and Development (OECD). Health at a Glance 2013: OECD Indicators. http://dx.doi.org/10.1787/health_glance‐2013‐en. OECD Publishing.
Olsen 2008
- Olsen MA, Butler AM, Willer DM, Devkota P, Gross GA, Fraser VJ. Risk factors for surgical site infection after low transverse cesarean section. Infection Control and Hospital Epidemiology 2008;29:477‐84. [DOI] [PubMed] [Google Scholar]
Owen 1994
- Owen J, Andrews WW. Wound complications after cesarean sections. Clinical Obstetrics and Gynecology 1994;37:842‐55. [DOI] [PubMed] [Google Scholar]
Pedersen 1996
- Pedersen TK, Blaakaer J. Antibiotic prophylaxis in cesarean section. Acta Obstetricia et Gynecologica Scandinavica 1996;75:537‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Roberts 1993
- Roberts S, Maccato M, Faro S, Pinell P. The microbiology of post‐cesarean wound morbidity. Obstetrics & Gynecology 1993;81:383‐6. [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Shlaes 1997
- Shlaes DM, Gerding DN, John JJ, Craig WA, Bornstein DL, Duncan RA, et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the prevention of antimicrobial resistance in hospitals. Clinical Infectious Diseases 1997;25:584‐99. [DOI] [PubMed] [Google Scholar]
SOGC 2010
- Schalkwyk J, Eyk N, for the Society of Obstetricians and Gynaecologists of Canada Infectious Diseases Committee. Antibiotic prophylaxis in obstetric procedures. Journal of Obstetrics and Gynaecology of Canada: JOGC 2010;32(9):879‐85. [Google Scholar]
Suonio 1989
- Suonio S, Saarikoski S, Vohlonen I, Kauhanen O. Risk factors for fever, endometritis and wound infection after abdominal delivery. International Journal of Gynecology and Obstetrics 1989;29:135‐42. [DOI] [PubMed] [Google Scholar]
Talbot 2005
- Talbot TR, Kaiser AB. Postoperative infections and antimicrobial prophylaxis. In: Mandell GL, Bennett JE, Dolin R editor(s). Principles and Practice of Infectious Diseases. 6th Edition. Philadelphia: Elsevier Inc, 2005:3533‐47. [Google Scholar]
Thomas 2006
- Thomas J. Rates of cesarean delivery in developing countries suggest unequal access. International Family Planning Perspectives 2006;32(2):105. [Google Scholar]
Tita 2009
- Tita ATN, Rouse DJ, Blackwell S, Saade GR, Spong CY, Andrews WW. Emerging concepts in antibiotic prophylaxis for cesarean delivery. Obstetrics & Gynecology 2009;113:675‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watts 1990
- Watts DH, Krohn MA, Hillier SL, Eschenbach DA. Bacterial vaginosis as a risk factor for post‐cesarean endometritis. Obstetrics & Gynecology 1990;75:52‐8. [PubMed] [Google Scholar]
Watts 1991
- Watts DH, Hillier SL, Eschenbach DA. Upper genital tract isolates at delivery as predictors of post‐cesarean infection among women receiving antibiotic prophylaxis. Obstetrics & Gynecology 1991;77:287‐92. [DOI] [PubMed] [Google Scholar]
Watts 1992
- Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstetrics & Gynecology 1992;79:351‐7. [DOI] [PubMed] [Google Scholar]
Wax 1997
- Wax JR, Hersey K, Philput C, Wright MS, Nichols KV, Eggleston MK, et al. Single dose cefazolin prophylaxis for postcesarean infections: before vs after cord clamping. Journal of Maternal Fetal Medicine 1997;6(1):61‐5. [DOI] [PubMed] [Google Scholar]
Webster 1988
- Webster J. Post‐caesarean wound infection: a review of the risk factors. Australian and New Zealand Journal of Obstetrics and Gynaecology 1988;28:210‐7. [DOI] [PubMed] [Google Scholar]
Weinberg 2001
- Weinberg M, Fuentes JM, Ruiz AI, Lozano FW, Angel E, Gaitan H, et al. Reducing infections among women undergoing cesarean section in Columbia by means of continuous quality improvement methods. Archives of Internal Medicine 2001;161:2357‐65. [DOI] [PubMed] [Google Scholar]
Weng 2013
- Weng M, Walker WA. The role of gut microbiota in programming the immune phenotype. Journal of Developmental Origins of Health and Disease 2013;4(3):203‐14. [DOI: 10.1017/S2040174412000712] [DOI] [PMC free article] [PubMed] [Google Scholar]
Westphal 1994
- Westphal JF, Vetter D, Brogard JM. Hepatic side‐effects of antibiotics. Journal of Antimicrobial Chemotherapy 1994;33:387‐401. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Smaill 1995a
- Smaill F. Prophylactic antibiotics for elective Caesarean section [revised 06 May 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Smaill 1995b
- Smaill F. Prophylactic antibiotics in Caesarean section (all trials) [revised 03 August 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Smaill 2002
- Hofmeyr GJ, Smaill FM. Antibiotic prophylaxis for cesarean section. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD000933] [DOI] [PubMed] [Google Scholar]
Smaill 2010
- Smaill FM, Gyte GML. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007482.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


