Abstract
1. Contaminants such as mercury are pervasive and can have immunosuppressive effects on wildlife. Impaired immunity could be important for forecasting pathogen spillover, as many land-use changes that generate mercury contamination also bring wildlife into close contact with humans and domestic animals. However, the interactions among contaminants, immunity and infection are difficult to study in natural systems, and empirical tests of possible directional relationships remain rare.
2. We capitalized on extreme mercury variation in a diverse bat community in Belize to test association among contaminants, immunity and infection. By comparing a previous dataset of bats sampled in 2014 with new data from 2017, representing a period of rapid agricultural land conversion, we first confirmed bat species more reliant on aquatic prey had higher fur mercury. Bats in the agricultural habitat also had higher mercury in recent years. We then tested covariation between mercury and cellular immunity and determined if such relationships mediated associations between mercury and bacterial pathogens. As bat ecology can dictate exposure to mercury and pathogens, we also assessed species-specific patterns in mercury-infection relationships.
3. Across the bat community, individuals with higher mercury had fewer neutrophils but not lymphocytes, suggesting stronger associations with innate immunity. However, the odds of infection for haemoplasmas and Bartonella spp. were generally lowest in bats with high mercury, and relationships between mercury and immunity did not mediate infection patterns. Mercury also showed species- and clade-specific relationships with infection, being associated with especially low odds for haemoplasmas in Pteronotus mesoamericanus and Dermanura phaeotis. For Bartonella spp., mercury was associated with particularly low odds of infection in the genus Pteronotus but high odds in the subfamily Stenodermatinae.
4. Synthesis and application. Lower general infection risk in bats with high mercury despite weaker innate defense suggests contaminant-driven loss of pathogen habitat (i.e. anemia) or vector mortality as possible causes. Greater attention to these potential pathways could help disentangle relationships among contaminants, immunity and infection in anthropogenic habitats and help forecast disease risks. Our results also suggest that contaminants may increase infection risk in some taxa but not others, emphasizing the importance of considering surveillance and management at different phylogenetic scales.
Keywords: bacterial pathogens, Chiroptera, disease ecology, ecoimmunology, ecotoxicology, heavy metals, Latin America
1 |. INTRODUCTION
Wildlife are commonly exposed to many contaminants that are ubiquitous in the environment, including heavy metals, organic compounds and pesticides (Smith et al., 2007). Contaminants can be novel stressors that have direct impacts such as mortality (Davidson, 2004) and more subtle consequences such as immunosuppression (Grasman, 2002). For example, even relatively low concentrations of mercury (Hg), a neurotoxic heavy metal, can impair wildlife immunity (Scheuhammer et al., 2007). When immunosuppression manifests in increased susceptibility to pathogens, environmental gradients of contaminants could increase infection prevalence or intensity in wild hosts (Becker, Albery, et al., 2020). These elevated infection risks could be especially relevant in the context of environmental changes such as gold mining and agricultural land clearing, both of which are associated with bioaccumulation of contaminants like Hg in aquatic and terrestrial food webs (Farella et al., 2007; Palheta & Taylor, 1995). As land conversions such as these also facilitate novel interactions among wildlife, domestic animals and humans, impaired immunity could further increase pathogen spillover risks (Borremans et al., 2019).
The interactions among contaminants, immunity and infection are notoriously difficult to study in natural systems, and empirical tests of possible directional relationships remain rare (Ross, 2002). Without captive or field experiments, natural systems must demonstrate sufficient variation in contaminant exposure and infection status to permit inference, and these criteria may be hard to meet in practice. For example, recent work on urbanized bobcats demonstrated strong effects of anticoagulant exposure on immunity in ways that should increase susceptibility, but pathogens of clinical relevance were generally rare, making it difficult to draw epidemiological inferences (Serieys et al., 2018). Additionally, effects may be difficult to detect if contaminant concentrations have low variance or are below toxicity thresholds (Fisk et al., 2005). Other examples suggest contaminants may instead decrease infection risks (Prüter et al., 2018), but the degree to which such patterns might be mediated by contaminant effects on immunity are unclear. Field-based assessments of how variable contaminant concentrations are associated with immunity and common pathogens are necessary to disentangle directional relationships.
Here, we capitalized on high variation in Hg and infection across species in a diverse Neotropical bat community in Belize to testassociations among contaminants, immunity and infection. Hg concentrations are typically highest in aquatic animals because methylmercury (MeHg), the bioaccumulative form of Hg, is produced in aquatic ecosystems (Chumchal et al., 2011). However, such contaminants can transfer into terrestrial ecosystems when terrestrial consumers feed on aquatic prey contaminated with MeHg (Cristol et al., 2008). Neotropical bats are ecologically diverse (Gunnell & Simmons, 2012; Rojas et al., 2011), and their diet variation enables strong heterogeneity in Hg exposure. Specifically, our past work showed that how often species feed on aquatic prey (or prey with some life stages linked to aquatic ecosystems) determines bat fur Hg, such that insectivores and species feeding on amphibians and fish have greater dietary exposure than frugivores and sanguivores (Becker, Chumchal, et al., 2018). Such variation should produce strong associations with immunity, as even sublethal Hg concentrations correlate with bat immunity (Becker et al., 2017). Because bats can be vulnerable to extracellular pathogens and can also harbour viral and bacterial zoonoses (Brook & Dobson, 2015), immunological differences driven by dietary variation in Hg could have implications for disease risks to and from bats. Lastly, this region in Belize is also undergoing intensive land clearance for agriculture similar to much of Latin America (Patterson, 2016), and thus analyses of Hg, immunity and infection could help assess how land use affects wildlife and human health.
Here we built upon our prior studies of Hg and of infectious disease in Neotropical bats (e.g. Becker, Chumchal, et al., 2018; Becker, Speer, Brown, et al., 2020) to address three study aims. First, we combined historic and new data on Hg concentrations in bat fur, an indication of long-term metal exposure (Flache et al., 2015), and compared contaminant load over a 3-year period and two sites. This greater within-species sample size allowed us to more robustly assess whether bat dietary connectivity to aquatic ecosystems predicts Hg bioaccumulation and if such patterns persist despite spatiotemporal variation. Additionally, because agriculture can increase environmental concentrations of Hg (Costantini et al., 2019; Farella et al., 2007), we also tested whether more recently sampled bats within this rapidly changing landscape had higher Hg exposure. Second, using blood samples, we tested whether elevated bat Hg is correlated with impaired immune function. Expanding our species-specific analyses in vampire bats Desmodus rotundus (Becker et al., 2017) across the entire bat community, we predicted that individuals with high Hg would have lower measures of cellular immunity. Third, we assessed infection with two bacterial pathogens, haemoplasmas and Bartonella spp. Both are common in Neotropical bats (Ikeda et al., 2017), including in these Belize sites (Becker, Bergner, et al., 2018; Becker, Speer, Brown, et al., 2020), and we previously showed infection can correlate with immunity in vampire bats specifically (Becker, Czirják, et al., 2018). However, how Hg shapes infection risk, and if such patterns are mediated by immunological relationships, is unknown. If Hg is associated with lower cellular immunity across bat species, we would expect greater concentrations to manifest in higher infection risks.
2 |. MATERIALS AND METHODS
2.1 |. Bat sampling
During 28 April to 4 May 2014 and 24 April to 6 May 2017, we sampled 247 bats from 29 species captured in two areas of the Orange Walk District of Belize: Lamanai Archeological Reserve (LAR) and Ka’Kabish (KK). The LAR is bordered by the New River Lagoon, forest and agriculture, while KK is a remnant forest patch surrounded by agriculture located 10 km away. At least 44 of the 70 bat species in Belize have been recorded in this region (Herrera et al., 2018; Reid, 1997). Bats were captured with mist nets from 19:00 until 22:00, and harp traps were also set from 18:00 to 05:00.
Bats were placed in individual cloth bags until processing and were identified to species based on morphology, including but not limited to body mass and forearm length (Reid, 1997). For Hg analysis, we trimmed <10 mg of fur from the dorsal or ventral region. Scissors were cleaned with ethanol between processed bats, and samples were stored in individual cryovials or Ziploc bags. From a subset of bats sampled in 2017, we collected 3–30 μl of blood by lancing the propatagial vein with sterile needles (23-30G; size and volume were dependent on bat mass), followed by collection using heparinized capillary tubes. Thin blood smears were prepared on glass slides and stained with Wright-Geimsa (Astral Diagnostics Quick III) to characterize cellular immunity. Remaining blood was stored on Whatman FTA cards (room temperature) or RNAlater (room temperature for 4 weeks and then −20°C) to preserve DNA. All bats were released after processing. Sampling followed guidelines for safe and humane handling of bats issued by the American Society of Mammalogists (Sikes & Animal Care and Use Committee of the American Society of Mammalogists, 2016) and was approved by the University of Georgia Animal Care and Use Committee (A2014 04-016-Y3-A5). Sampling was authorized by the Belize Forest Department under permits CD/60/3/14(27), WL/2/1/17(16) and WL/2/1/17(19). Sample size for Hg varied by year (2014 = 98, 2017 = 149) and site (LAR = 163, KK = 84) and ranged from 1-58 individuals per species ( SE).
2.2 |. Fur Hg analysis
Bat fur was analysed for total Hg (THg) at the Texas Christian University Aquatic Ecology Laboratory. THg data from 2014 were published previously (Becker, Chumchal, et al., 2018). Fur samples were rinsed in a 2:1 chloroform:methanol solution and dried overnight in a fume hood and reported on a fresh weight basis. We quantified THg with a direct Hg analyzer (DMA-80) and analysed National Research Council Canada reference material DORM 4 (certified value = 0.412 ± 0.036 mg/kg) every 10 samples for quality control; mean recovery was 94.32 ± 0.96% for 2017 data. Limited fur resulted in some samples falling below detection limit (DL), which was higher in 2014 (~0.48 ng). THg values below DL were estimated as 50% DL, and we used the 2014 DL to standardize concentrations (Rainwater et al., 2005). Fur THg was expressed in mg/kg and log10-transformed prior to statistical analyses. THg is a proxy for MeHg, which comprises 71%–95% of Hg in bat fur (Yates et al., 2013).
2.3 |. Statistical analysis of fur THg
To first link bats to aquatic food webs (i.e. the primary source of dietary Hg exposure; Becker, Chumchal, et al., 2018), we used the EltonTraits database to classify bat species according to the proportion of diet consisting of potentially aquatic prey: invertebrates, ectothermic tetrapods and fish (Wilman et al., 2014). We used phylogenetic generalized linear mixed models (GLMMs) to test how THg varied with bat diet, site (LAR and KK) and year (2014 and 2017). We fit candidate models that considered all fixed effects and their two- and three-way interactions. We fit the phylogenetic GLMM using the brms package in r, default priors and Gaussian errors (Bürkner, 2017). We included random effects for species and phylogeny, the latter of which used a phylogenetic covariance matrix derived from the Open Tree of Life through the rotl and ape packages (Michonneau et al., 2016; Paradis et al., 2004). We ran four chains for 20,000 iterations with a burn-in period of 10,000, thinned every 10 steps, for a total of 4,000 samples. We compared GLMMs using leave-one-out cross-validation information criterion (LOOIC) and assessed fit with a Bayesian R2, including the total modelled variance and that attributed to only the fixed effects (Gelman et al., 2019; Vehtari et al., 2017). We then estimated fixed effects per predictor (means and 95% highest density intervals [HDI]) and visualized fitted values using 100 random draws from the GLMM posterior distribution.
2.4 |. Quantifying immunity and bacterial infection
Using blood smears, we first estimated total white blood cell (WBC) counts as the mean number of leukocytes from 10 fields of view (400X) with a light microscope (Schneeberger et al., 2013). We next used differential WBC counts (1000X) to quantify the relative abundance of neutrophils and lymphocytes from 100 leukocytes. Neutrophils are components of the innate immune system, whereas lymphocytes are involved in adaptive responses like immunoglobulin production (Lanier, 2013). We derived absolute neutrophil and lymphocyte counts separately by multiplying total and differential WBCs. Elevated WBC counts can indicate a more robust cellular defense or an inflammatory response to acute infection.
To detect bacterial pathogens, we extracted genomic DNA from blood using QIAamp DNA Investigator Kits and DNeasy Blood and Tissue Kits (Qiagen). Before extracting RNAlater-preserved blood, samples were vortexed with 1 mL 1X phosphate-buffered saline and centrifuged for 3 min at 4,000 RPM and 12°C to prevent bacteria from floating in RNAlater; 200 μL from the bottom of the tube was then used for extraction. We used PCR to test for haemoplasmas (targeting the 16S rRNA gene) and Bartonella spp. (targeting the gltA gene) using previous diagnostic protocols (Bai et al., 2016; Volokhov et al., 2017). Haemoplasma data and sequences have been published previously (Becker, Speer, Brown, et al., 2020). Efforts to characterize Bartonella spp. in this bat community are ongoing, but prior studies of Belize vampire bats indicate high gltA sequence similarity to sequences from vampire bats in Mexico, Neotropical bat flies and other Neotropical bats (Becker, Bergner, et al., 2018).
2.5 |. Analyses of THg, immunity and infection
We first used phylogenetic GLMMs to test the overall relationship between THg and both absolute neutrophil and absolute lymphocyte counts. Each model included THg as the fixed effect, alongside sex and body condition (mass/forearm length) as covariates that could also affect leukocyte counts, with species and phylogeny as random effects. We used a Gaussian distribution for log10-transformed WBC counts. We next fit phylogenetic GLMMs with binomial errors to test associations between THg and infection with haemoplasmas and Bartonella spp. We then used causal mediation analyses (CMA) to test support for directional relationships among THg, immunity and infection (Imai et al., 2010). Similar to structural equation modelling, CMA decomposes the hypothesized causal relationship between a predictor (i.e. THg) and a response (i.e. infection) into the direct effect and the indirect effect mediated through a third variable (i.e. immunity; Figure S1). CMA then estimates the proportion of the total effect mediated through the indirect effect using a mediator model, which was each of the GLMMs predicting leukocyte counts by THg, and an outcome model. Here, we fit two GLMMs per pathogen that modelled infection as a function of THg and each WBC count. We then used the brms and bayestestr packages to estimate these direct and indirect effects and in turn the proportion of the total relationship (THg and infection) mediated by the indirect relationship between THg and immunity and immunity and infection.
Because bat ecology likely dictates exposure to contaminants and pathogens, we assessed species-specific patterns in Hg–infection relationships. We fit logistic regressions for each bat species and pathogen when sample sizes were greater than two individuals. As these small samples can bias odds ratio estimates, we used the logistf package to implement Firth’s bias reduction (Heinze & Schemper, 2002). For species with no variance in infection, we assigned log odds of zero. Across these species, we next estimated phylogenetic signal (Pagel’s λ) in the log odds using the caper package and used phylogenetic generalized least squares to test if log odds covaried with sample size (Orme, 2013). We then used phylogenetic factorization to identify clades with different log odds at various taxonomic depths. We used the taxize and phylofactor packages to obtain taxonomic information for each species and partition log odds as a Gaussian response in a GLM (Chamberlain & Szöcs, 2013; Washburne et al., 2019). We included sample size as a weighting variable and used Holm’s sequentially rejective test to determine the number of significant clades.
3 |. RESULTS
3.1 |. Neotropical bat fur THg
Expanding our initial studies of fur THg across this Neotropical bat community in 2014, we found that THg varied up to five orders of magnitude across species, sites and years (Figure 1). Our top GLMM included interactions between diet and year and between year and site (Table 1). Across years, the proportion of potentially aquatic prey in diet positively predicted THg but more so in 2014 (β = 0.02, 95% HDI: 0.011–0.026) than in 2017 (β = 0.016, 95% HDI: 0.009–0.022; Figure 2a). We also identified strong spatiotemporal variation, such that fur THg increased between 2014 and 2017 for KK ( to 0.67) but slightly decreased for LAR ( to 0.50).
FIGURE 1.
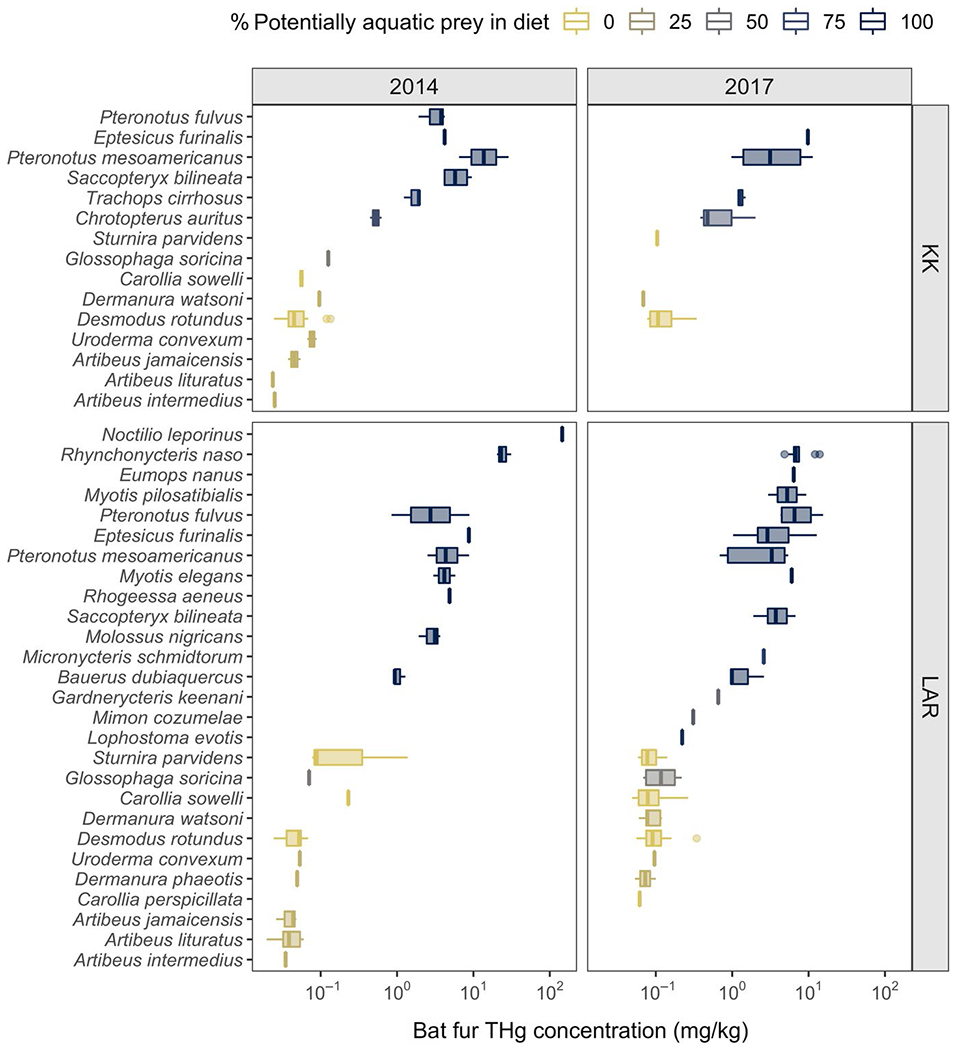
THg concentrations in bat fur across Neotropical bat species sampled in 2014 and 2017 across two sites in Belize. Boxplots are coloured by the proportion of potentially aquatic prey in bat diets from the EltonTraits database. THg concentrations are displayed on a log10 scale
TABLE 1.
Competing phylogenetic GLMMs predicting log fur THg concentrations across the Belize bat community (n = 247). Models are ranked by ΔLOOIC with LOOIC SE, Akaike weights (wi) and Bayesian R2 estimates. All models include random effects for species and phylogeny
| Fixed effects | LOOIC | SE | ΔLOOIC | wi | ||
|---|---|---|---|---|---|---|
| ~diet + year + site + year:site + diet:year | 76.61 | 37.74 | 0.00 | 0.52 | 0.80 | 0.92 |
| ~diet * year + site | 78.82 | 38.42 | 2.21 | 0.17 | 0.80 | 0.92 |
| ~diet + year + site + diet:site + diet:year + year:site | 79.27 | 37.61 | 2.67 | 0.14 | 0.79 | 0.92 |
| ~diet * year * site | 79.57 | 36.41 | 2.96 | 0.12 | 0.79 | 0.92 |
| ~diet + year + site + diet:site + diet:year | 81.16 | 38.04 | 4.55 | 0.05 | 0.79 | 0.92 |
| ~diet + year + site + diet:site + year:site | 88.03 | 32.75 | 11.42 | <0.01 | 0.77 | 0.91 |
| ~diet * site + year | 89.91 | 32.89 | 13.30 | <0.01 | 0.78 | 0.91 |
| ~diet + year * site | 90.15 | 33.17 | 13.54 | <0.01 | 0.78 | 0.91 |
| ~diet + year + site | 93.94 | 33.44 | 17.33 | <0.01 | 0.78 | 0.91 |
FIGURE 2.
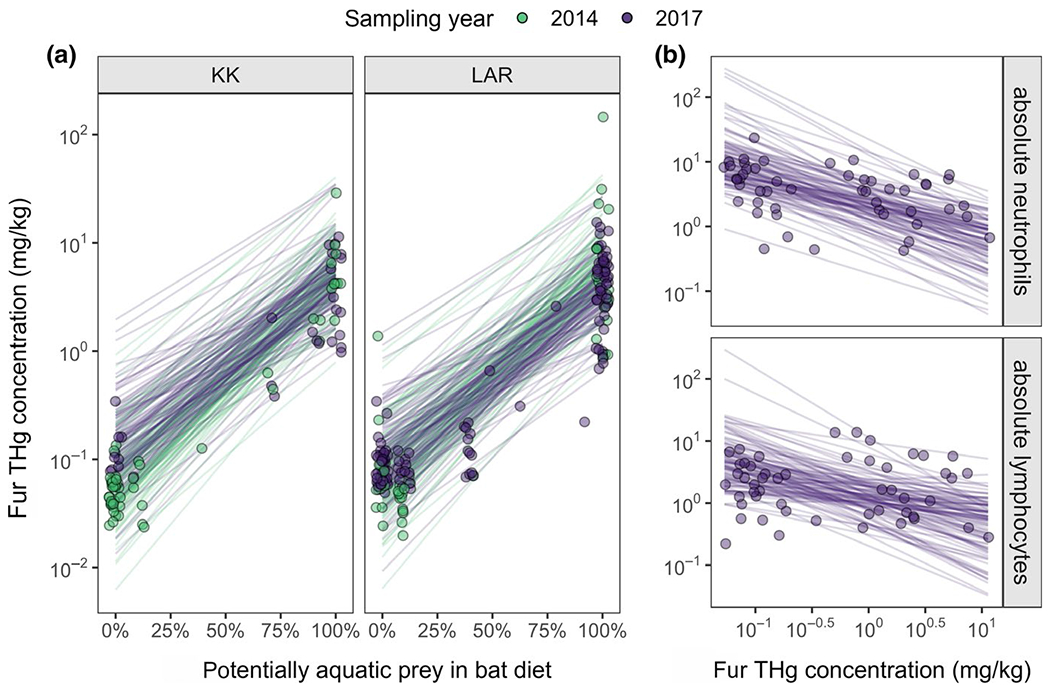
Dietary drivers of THg concentrations in Neotropical bat fur (a) and cellular immunity correlates of THg (b). Points indicate individual bats and are coloured by sampling year in Belize. Lines display 100 random draws from the posterior distribution of the main phylogenetic GLMMs. THg concentrations and absolute leukocyte counts are displayed on a log10 scale
3.2 |. Associations with immunity and infection
Across bats sampled in 2017 with Hg and WBC data (n = 51), our GLMMs showed that higher THg was associated with fewer neutrophils (β = −0.52, 95% HDI: −0.96 to −0.17) but not lymphocytes (β = −0.39, 95% HDI: −0.88 to 0.03; Figure 2b) after adjusting for sex and condition; these two covariates had weak associations with leukocytes (Table S1). However, higher THg was also associated with lower odds of infection for haemoplasmas (OR = 0.22, 95% HDI: 0.05–0.86, n = 132) and Bartonella spp. (OR = 0.20, 95% HDI: 0.05–0.80, n = 117; Figure 3a). Accordingly, CMAs found that the relationship between THg and either WBC did not mediate any of the relationships between THg and infection for both pathogens (Table S2).
FIGURE 3.
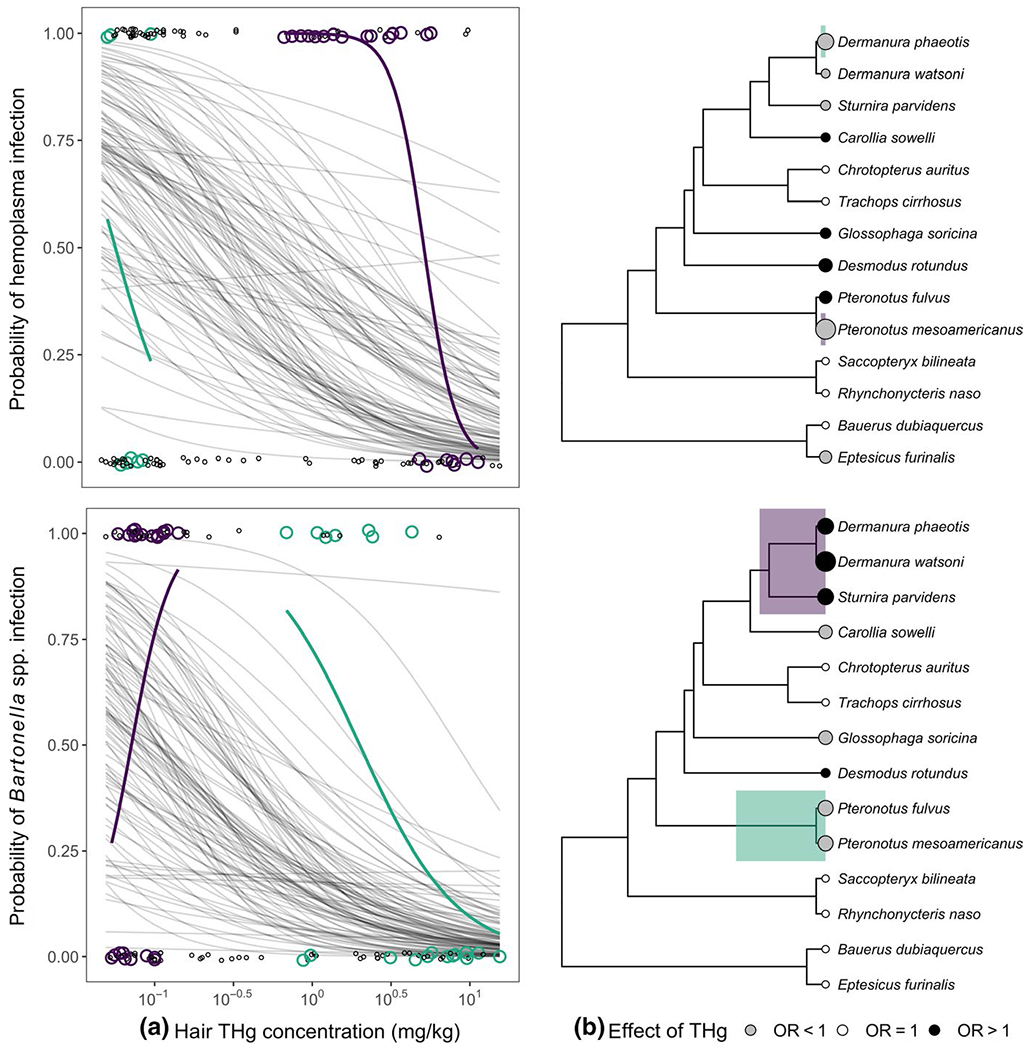
Associations between fur THg and bacterial infection (a) across the Belize Neotropical bat community and (b) on a per-species basis. Points in A indicate individual bats, and thin lines show 100 random draws from the posterior distribution of the phylogenetic GLMMs. Points in (b) indicate bat species, are scaled by the absolute log odds and are coloured by the direction of the relationship between THg and infection: null effects (i.e. OR = 1, white), protective effects of THg (i.e. OR < 1, grey), and mercury as a risk factor (i.e. OR > 1, black). Data are shown for haemoplasmas (top) and Bartonella spp. (bottom). Colour indicates bat clades identified through phylogenetic factorization of log odds ratios, with lines indicating clade-specific GLM fits
When we used logistic regression to analyse individual relationships between THg and infection per bat species and pathogen, we only found significant protective effects for Pteronotus mesoamericanus and haemoplasmas after adjusting for multiple comparisons (ln(OR) = −10.20, p = 0.001; Table 2). We also detected a strong negative association between THg and Bartonella in this species (ln(OR) = −3.34), but no log odds were significantly different from zero after adjustment. Most species instead had null relationships between THg and infection (i.e. 36% for haemoplasmas and 43% for Bartonella spp.) or relatively weaker negative effects (e.g. Dermanura phaeotis, ln(OR) = −5.31 for haemoplasmas; P. fulvus, ln(OR) = −3.35 for Bartonella spp.). These negative THg–infection associations were common for haemoplasmas (36%) and Bartonella spp. (29%). We also estimated similar proportions of positive THg associations (29%) for both pathogens. Desmodus rotundus had the strongest positive THg effect size for haemoplasma infection (ln(OR) = 2.40), whereas Dermanura watsoni had the largest positive THg effect size for Bartonella spp. infection (ln(OR) = 8.9).
TABLE 2.
Results of species-specific logistic regressions (using Firth’s bias reduction method) between bat fur THg concentrations and infection status per each bacterial pathogen
| Haemoplasmas |
Bartonella spp. |
|||||||
|---|---|---|---|---|---|---|---|---|
| Bat species | ln(OR) | p | σ2 | n | ln(OR) | p | σ2 | n |
| Eptesicus furinalis | −1.54 | 0.46 | 0.21 | 8 | 0 | 1 | 0 | 7 |
| Bauerus dubiaquercus | 0 | 1 | 0 | 3 | 0 | 1 | 0 | 3 |
| Dermanura watsoni | −0.13 | 0.98 | 0.27 | 6 | 8.9 | 0.32 | 0.17 | 6 |
| Dermanura phaeotis | −5.31 | 0.46 | 0.27 | 8 | 4.27 | 0.58 | 0.21 | 8 |
| Sturnira parvidens | −0.42 | 0.92 | 0.26 | 13 | 4.2 | 0.46 | 0.19 | 9 |
| Carollia sowelli | 0.21 | 0.94 | 0.18 | 10 | −1.99 | 0.52 | 0.23 | 10 |
| Trachops cirrhosus | 0 | 1 | 0 | 3 | 0 | 1 | 0 | 3 |
| Chrotopterus auritus | 0 | 1 | 0 | 3 | 0 | 1 | 0 | 3 |
| Glossophaga soricina | 0.66 | 0.84 | 0.22 | 11 | −2.37 | 0.49 | 0.19 | 9 |
| Desmodus rotundus | 2.4 | 0.33 | 0.21 | 22 | 0.18 | 0.93 | 0.21 | 22 |
| Pteronotus mesoamericanus | −10.2 | 0 | 0.23 | 22 | −3.34 | 0.02 | 0.25 | 16 |
| Pteronotus fulvus | 1.65 | 0.65 | 0.25 | 4 | −3.35 | 0.41 | 0.25 | 4 |
| Rhynchonycteris naso | 0 | 1 | 0 | 4 | 0 | 1 | 0 | 3 |
| Saccopteryx bilineata | 0 | 1 | 0 | 8 | 0 | 1 | 0 | 8 |
Comparative analyses of the log odds ratios across bat species revealed no phylogenetic signal for the relationship between THg and haemoplasmas (λ = 0) but strong phylogenetic signal for the relationship between THg and Bartonella spp. (λ = 0.84). Log odds ratios were not associated with sample size for haemoplasmas (F1,12 = 2.17, p = 0.17) or Bartonella spp. (F1,12 = 0.25, p = 0.63). Phylogenetic factorization further identified species- or clade-specific patterns in the magnitude and direction of effect size. For haemoplasmas, the odds of infection were significantly lower for P. mesoamericanus and D. phaeotis when compared to the remaining sampled bat phylogeny. For Bartonella spp., however, the odds of infection were significantly lower for the genus Pteronotus (mean ln(OR) = −3.34) and significantly higher for the subfamily Stenodermatinae (Dermanura spp. and Sturnira parvidens, mean In(OR) = 5.79; Figure 3b). Post-hoc GLMs showed that bats in Stenodermatinae had especially negative associations between THg and neutrophils (β = −2.24, p < 0.01) but not lymphocytes (β = −0.65, p = 0.57), although these analyses were limited by small sample size (n = 6).
4 |. DISCUSSION
Interactions among contaminants, immunity and infection are difficult to disentangle in natural systems, but quantifying their proposed causal relationships can inform how land-use change affects wildlife health and human disease risks. By capitalizing on a diverse Neotropical bat system with high variation in Hg bioaccumulation and bacterial pathogens, we found higher THg was associated with fewer neutrophils but also lower odds of infection across the host community. However, our species-specific and taxonomic analyses showed THg had protective effects for haemoplasmas and Bartonella spp. in the genus Pteronotus, whereas THg was associated with fewer neutrophils and elevated infection in the subfamily Stenodermatinae. These contrasting relationships suggest contaminant-driven loss of pathogen habitat (i.e. anaemia) or vector mortality versus immunosuppression as possible causal mechanisms, respectively, and identify clades of bats that may be especially resilient or vulnerable to infection risks from Hg exposure. Such findings more generally suggest contaminants may increase infection risk in some taxa but not others, emphasizing the importance of considering surveillance and management at different phylogenetic scales (Graham et al., 2018).
Expanding our prior analyses of this Neotropical bat community and global patterns of THg in bat fur (Becker, Chumchal, et al., 2018) with larger within-species sample sizes, we first demonstrated that Hg exposure increases with potentially aquatic prey (or prey with some life stages linked to aquatic ecosystems) in diet despite spatial and temporal variation in THg. Positive associations with diets linked to aquatic ecosystems across sites and years provides additional support for trophic transfer of Hg through foraging (Cristol et al., 2008; Ortega-Rodriguez et al., 2019; Speir et al., 2014). This diet-mediated connectivity to aquatic ecosystems likely underlies other cases of guild-specific Hg bioaccumulation in bat communities (Carrasco-Rueda et al., 2020; Korstian et al., 2018). In many of these regions, bat dietary exposure to Hg is driven by land-use changes such as gold mining and agriculture (Carrasco-Rueda et al., 2020; Costantini et al., 2019), whereas atmospheric deposition is often the primary source of Hg in regions located further from anthropogenic point sources (Chételat et al., 2018; Korstian et al., 2018). The latter is a likely source of Hg in this Belize system (Becker, Chumchal, et al., 2018). However, intensified agriculture and especially slash- and-burn practices could provide other Hg inputs and may explain why bat THg increased between 2014 and 2017 in KK, the more agricultural site, but not in the protected LAR (Farella et al., 2007; Patterson, 2016).
Across bat species, fur THg was negatively correlated with neutrophil counts, which mirrors captive results and suggests impaired innate immunity (Lalancette et al., 2003). Previously, vampire bats sampled in Belize with high fur THg had weaker innate defense (i.e. bacterial killing ability; Becker et al., 2017). Wrinkle-lipped free-tailed bats with higher Hg exposure also had weaker innate immunity (i.e. bacterial killing ability, lysozyme and haptoglobin concentrations; Costantini et al., 2019). Most individual bats for which we had both Hg and immunity data showed THg below toxicity and subclinical thresholds of 5–10 mg/kg (Nam et al., 2012), which suggests innate immunity could be weakened at sublethal contaminant concentrations (Lewis et al., 2013). Alternatively, sublethal effects of THg could combine with other stressors (e.g. reproduction, food and roost availability) to impair immunity. Additional immune measures across broader land-use gradients would help characterize functional differences in defense in relation to THg concentrations in anthropogenic habitats (Becker, Albery, et al., 2020; Costantini et al., 2019).
Although neutrophils were lowest in bats with high Hg exposure, the odds of infection with haemoplasmas and Bartonella spp. decreased with fur THg across the bat community. As in other mammals, bats challenged with bacteria produce neutrophils as part of the innate immune response (Weise et al., 2017). Elevated neutrophil counts could be protective, as implied through negative associations between innate immunity and these two pathogens in vampire bats (Becker, Czirják, et al., 2018). Accordingly, relationships between THg and immunity did not mediate the relationships between THg and infection, which likely indicates immunosuppression is not a causal mechanism. One alternative may involve contaminant-mediated pathogen mortality; for example, lead exposure likely caused helminth mortality in avian hosts (Prüter et al., 2018). As facultative intracellular pathogens, haemoplasmas and Bartonella spp. both infect red blood cells. Hg can lower erythrocyte counts (Shaw et al., 1991), which could reduce resources available to both bacteria. We did not measure anaemia or Hg in blood; however, fur THg strongly correlates with blood THg, despite the former being orders of magnitude higher than the latter (Wada et al., 2010). As Bartonella spp. and possibly haemoplasmas likely depend in part on vector-borne transmission (Becker, Bergner, et al., 2018; Millán et al., 2007), ectoparasite mortality from contaminants in hosts or the environment could also explain observed infection patterns (e.g. as found for some avian ectoparasites; Eeva & Klemola, 2013). Lastly, we did not reliably age all bats, precluding age from our analyses; however older animals typically have higher fur THg (Korstian et al., 2018). If older bats also have stronger adaptive immunity (e.g. antibody titres in Saccopteryx bilineata; Schneeberger et al., 2014), age could explain negative associations between THg and infection.
Our species-specific analyses suggested contaminants may have variable impacts on infection risk depending on taxon. In particular, we identified possibly protective effects of THg on infections primarily in the genus Pteronotus, with strong negative effects for P. mesoamericanus for both pathogens. Both species in this genus had high THg, being insectivores that often eat dipterans and coleopterans (Salinas-Ramos et al., 2015). Given this clade-based signal, Pteronotus bats may be particularly resilient to disease impacts of Hg. Such effects could be in part mediated by high host-specificity of bat flies for this genus (Ter Hofstede et al., 2004), particularly if high THg increases vector mortality or if aspects of bat fly biology impact vector competence (Weiss & Aksoy, 2011). In contrast, we also identified taxa with positive relationships between THg and infection. For haemoplasmas, higher THg was associated with greater risk in vampire bats. As this species frequently feeds on humans and livestock (Streicker & Allgeier, 2016), pathogen surveillance should be particularly important in habitats contaminated by agriculture or mining. Additionally, the subfamily Stenodermatinae displayed strong positive associations between THg and Bartonella spp. These frugivores also showed negative correlations between THg and neutrophils, suggesting Hg-mediated immunosuppression. Such species may thus be especially vulnerable to infection following Hg exposure and could play key roles in maintaining Bartonella spp. infection cycles between bats and ectoparasites in contaminated environments (Judson et al., 2015). From another perspective, because the Stenodermatinae and Pteronotus had lower and higher fur THg, respectively, low Hg exposure could increase susceptibility while higher concentrations instead cause anaemia and facilitate protective effects against erythrocytic pathogens. More generally, these results highlight the importance of considering surveillance and management of Hg exposure (e.g. through possible land-use drivers) at different phylogenetic scales, such as species, genus or subfamily.
Beyond bats and their pathogens, our study more broadly emphasizes the need to assess potential causal relationships between contaminants and infectious diseases in natural systems. In particular, we provide a novel perspective on integrating approaches from ecotoxicology with those of disease ecology to disentangle the complex relationships among contaminants, immunity and infection. Future work that carefully integrates data on contaminant exposure, specific diet composition, multiple immune measures and pathogen diversity (e.g. with metagenomics; Bergner et al., 2019) across site gradients of anthropogenic intensity could help identify habitats, host clades and infections for which disease risks are highest.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mark Howells, Neil Duncan and staff of the Lamanai Field Research Center for assistance with field logistics and permits. We also thank the many colleagues who helped net bats during 2014 and 2017 bat research in Belize as well as Susan Perkins for laboratory reagents. Lastly, we thank two anonymous reviewers for constructive feedback on this manuscript. DJB was funded by the ARCS Foundation and Explorer’s Club. Field work by D.J.B. and K.A.S. was supported by grants from the American Museum of Natural History Theodore Roosevelt Memorial Fund. Laboratory work by K.A.S. and C.L.B. was funded by the Richard Gilder Graduate School Student Research Fellowship. R.K.P. was supported by NSF DEB-1716698, the Defense Advanced Research Projects Agency PREEMPT program Cooperative Agreement D18AC00031, U.S. National Institutes of General Medical Sciences IDeA Program (P20GM103474 and P30GM110732) and the USDA National Institute of Food and Agriculture (Hatch project 1015891). NBS was supported by the American Museum of Natural History Taxonomic Mammalogy Fund. J.M.K. and M.M.C. were supported by a Texas Christian University Research and Creative Activities Fund Award, and H.G.B. was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant. T.R.R. was supported by the Yawkey Foundation and Clemson University. This paper represents technical contribution number 6931 of the Clemson University Experimental Station. The views, opinions and/or findings expressed are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the U.S. Government.
Funding information
National Science Foundation, Grant/Award Number: DEB-1716698; Achievement Rewards for College Scientists Foundation; American Museum of Natural History; U.S. Department of Agriculture, Grant/Award Number: 1015891; National Institutes of Health, Grant/Award Number: P20GM103474 and P30GM110732; Defense Advanced Research Projects Agency, Grant/Award Number: D16AP00113 and D18AC00031; Richard Gilder Graduate School Student Research Fellowship; Texas Christian University Research and Creative Activities Fund Award; Natural Sciences and Engineering Research Council; Yawkey Foundation
Footnotes
DATA AVAILABILITY STATEMENT
Data are available via the Dryad Digital Repository https://doi.org/10.5061/dryad.70rxwdbwb (Becker, Speer, Korstian, et al., 2020).
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- Bai Y, Gilbert A, Fox K, Osikowicz L, & Kosoy M (2016). Bartonella rochalimae and b. Vinsonii subsp. Berkhoffii in wild carnivores from Colorado, USA. Journal of Wildlife Diseases, 52(4), 844–849. 10.7589/2016-01-015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Albery GF, Kessler MK, Lunn TJ, Falvo CA, Czirják GÁ, Martin LB, & Plowright RK (2020). Macroimmunology: The drivers and consequences of spatial patterns in wildlife immune defence. Journal of Animal Ecology, 89(4), 972–995. 10.1111/1365-2656.13166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Bergner LM, Bentz AB, Orton RJ, Altizer S, & Streicker DG (2018). Genetic diversity, infection prevalence, and possible transmission routes of Bartonella spp. In vampire bats. PLoS Neglected Tropical Diseases, 12(9), e0006786. 10.1371/journal.pntd.0006786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Chumchal MM, Bentz AB, Platt SG, Czirják GÁ, Rainwater TR, Altizer S, & Streicker DG (2017). Predictors and immunological correlates of sublethal mercury exposure in vampire bats. Royal Society Open Science, 4(4), 170073. 10.1098/rsos.170073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Chumchal MM, Broders HG, Korstian JM, Clare EL, Rainwater TR, Platt SG, Simmons NB, & Fenton MB (2018). Mercury bioaccumulation in bats reflects dietary connectivity to aquatic food webs. Environmental Pollution, 233, 1076–1085. 10.1016/j.envpol.2017.10.010 [DOI] [PubMed] [Google Scholar]
- Becker DJ, Czirják GÁ, Volokhov DV, Bentz AB, Carrera JE, Camus MS, Navara KJ, Chizhikov VE, Fenton MB, Simmons NB, Recuenco SE, Gilbert AT, Altizer S, & Streicker DG (2018). Livestock abundance predicts vampire bat demography, immune profiles and bacterial infection risk. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1745), 20170089. 10.1098/rstb.2017.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Speer KA, Brown AM, Fenton MB, Washburne AD, Altizer S, Streicker DG, Plowright RK, Chizhikov VE, Simmons NB, & Volokhov DV (2020). Ecological and evolutionary drivers of haemoplasma infection and bacterial genotype sharing in a Neotropical bat community. Molecular Ecology, 29(8), 1534–1549. 10.1111/mec.15422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Speer KA, Korstian JM, Volokhov DV, Drake HF, Brown AM, Baijnauth CL, Padgett-Stewart T, Broders HG, Plowright RK, Rainwater TR, Fenton MB, & Simmons NB, & Chumchal MM (2020). Data from: Disentangling interactions among mercury, immunity, and infection in a Neotropical bat community. Dryad Digital Repository, 10.5061/dryad.70rxwdbwb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner LM, Orton RJ, da Silva Filipe A, Shaw AE, Becker DJ, Tello C, Biek R, & Streicker DG (2019). Using noninvasive metagenomics to characterize viral communities from wildlife. Molecular Ecology Resources, 19(1), 128–143. 10.1111/1755-0998.12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borremans B, Faust C, Manlove KR, Sokolow SH, & Lloyd-Smith JO (2019). Cross-species pathogen spillover across ecosystem boundaries: Mechanisms and theory. Philosophical Transactions of the Royal Society B: Biological Sciences, 374(1782), 20180344. 10.1098/rstb.2018.0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook CE, & Dobson AP (2015). Bats as ‘special’ reservoirs for emerging zoonotic pathogens. Trends in Microbiology, 23(3), 172–180. 10.1016/j.tim.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkner P-C (2017). brms: An R package for Bayesian multilevel models using Stan. Journal of Statistical Software, 80(1), 1–28. [Google Scholar]
- Carrasco-Rueda F, Loiselle BA, & Frederick PC (2020). Mercury bioaccumulation in tropical bats from a region of active artisanal and small-scale gold mining. Ecotoxicology, 29(7), 1032–1042. 10.1007/sl0646-020-02195-3 [DOI] [PubMed] [Google Scholar]
- Chamberlain SA, & Szöcs E (2013). taxize: taxonomic search and retrieval in R. F1000Research, 2, 191. 10.12688/f1000research.2-191.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chételat J, Hickey MBC, Poulain AJ, Dastoor A, Ryjkov A, McAlpine D, Vanderwolf K, Jung TS, Hale L, Cooke ELL, Hobson D, Jonasson K, Kaupas L, McCarthy S, McClelland C, Morningstar D, Norquay KJO, Novy R, Player D … Znuttig M (2018). Spatial variation of mercury bioaccumulation in bats of Canada linked to atmospheric mercury deposition. Science of the Total Environment, 626, 668–677. 10.1016/j.scitotenv.2018.01.044 [DOI] [PubMed] [Google Scholar]
- Chumchal MM, Rainwater TR, Osborn SC, Roberts AP, Abel MT, Cobb GP, Smith PN, & Bailey FC (2011). Mercury speciation and biomagnification in the food web of Caddo Lake, Texas and Louisiana, USA, a subtropical freshwater ecosystem. Environmental Toxicology and Chemistry, 30(5), 1153–1162. 10.1002/etc.477 [DOI] [PubMed] [Google Scholar]
- Costantini D, Czirják GÁ, Bustamante P, Bumrungsri S, & Voigt CC (2019). Impacts of land use on an insectivorous tropical bat: The importance of mercury, physio-immunology and trophic position. Science of the Total Environment, 671, 1077–1085. 10.1016/j.scitotenv.2019.03.398 [DOI] [Google Scholar]
- Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL, Hallinger KK, Monroe AP, & White AE (2008). The movement of aquatic mercury through terrestrial food webs. Science, 320(5874), 335. 10.1126/science.1154082 [DOI] [PubMed] [Google Scholar]
- Davidson C (2004). Declining downwind: Amphibian population declines in California and historical pesticide use. Ecological Applications, 14(6), 1892–1902. 10.1890/03-5224 [DOI] [Google Scholar]
- Eeva T, & Klemola T (2013). Variation in prevalence and intensity of two avian ectoparasites in a polluted area. Parasitology, 140(11), 1384–1393. 10.1017/S0031182013000796 [DOI] [PubMed] [Google Scholar]
- Farella N, Davidson R, Lucotte M, & Daigle S (2007). Nutrient and mercury variations in soils from family farms of the Tapajós region (Brazilian Amazon): Recommendations for better farming. Agriculture, Ecosystems & Environment, 120(2–4), 449–462. 10.1016/j.agee.2006.11.003 [DOI] [Google Scholar]
- Fisk AT, de Wit CA, Wayland M, Kuzyk ZZ, Burgess N, Letcher R, Braune B, Norstrom R, Blum SP, Sandau C, Lie E, Larsen HJS, Skaare JU, & Muir DCG (2005). An assessment of the toxicological significance of anthropogenic contaminants in Canadian arctic wildlife. Science of the Total Environment, 351-352, 57–93. 10.1016/j.scitotenv.2005.01.051 [DOI] [PubMed] [Google Scholar]
- Flache L, Becker NI, Kierdorf U, Czarnecki S, Düring R-A, & Encarnação JA (2015). Hair samples as monitoring units for assessing metal exposure of bats: A new tool for risk assessment. Mammalian Biology-Zeitschrift Für Säugetierkunde, 80(3), 178–181. 10.1016/j.mambio.2015.01.007 [DOI] [Google Scholar]
- Gelman A, Goodrich B, Gabry J, & Vehtari A (2019). R-squared for Bayesian regression models. The American Statistician, 73(3), 307–309. 10.1080/00031305.2018.1549100 [DOI] [Google Scholar]
- Graham CH, Storch D, & Machac A (2018). Phylogenetic scale in ecology and evolution. Global Ecology and Biogeography, 27(2), 175–187. 10.1111/geb.12686 [DOI] [Google Scholar]
- Grasman KA (2002). Assessing immunological function in toxicological studies of avian wildlife. Integrative and Comparative Biology, 42(1), 34–42. 10.1093/icb/42.1.34 [DOI] [PubMed] [Google Scholar]
- Gunnell GF, & Simmons NB(2012). Evolutionary history of bats: Fossils, molecules and morphology, Cambridge, UK: Cambridge University Press. [Google Scholar]
- Heinze G, & Schemper M (2002). A solution to the problem of separation in logistic regression. Statistics in Medicine, 21(16), 2409–2419. 10.1002/sim.1047 [DOI] [PubMed] [Google Scholar]
- Herrera JP, Duncan N, Clare E, Fenton MB, & Simmons N (2018). Disassembly of fragmented bat communities in Orange Walk District, Belize. Acta Chiropterologica, 20(1), 147–159. 10.3161/15081109ACC2018.20.1.011 [DOI] [Google Scholar]
- Ikeda P, Seki MC, Carrasco AΟT, Rudiak LV, Miranda JMD, Gonçalves SMM, Hoppe EGL, Albuquerque ACA, Teixeira MMG, Passos CE, Werther K, Machado RZ, & André MR (2017). Evidence and molecular characterization of Bartonella spp. and hemoplasmas in neotropical bats in Brazil. Epidemiology & Infection, 145(10), 2038–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Keele L, & Tingley D (2010). A general approach to causal mediation analysis. Psychological Methods, 15(4), 309–334. 10.1037/a0020761 [DOI] [PubMed] [Google Scholar]
- Judson SD, Frank ΗK, & Hadly EA (2015). Bartonellae are prevalent and diverse in Costa Rican Bats and Bat Flies. Zoonoses and Public Health, 62(8), 609–617. 10.1111/zph.12188 [DOI] [PubMed] [Google Scholar]
- Korstian JM, Chumchal MM, Bennett VJ, & Hale AM (2018). Mercury contamination in bats from the central United States. Environmental Toxicology and Chemistry, 37(1), 160–165. 10.1002/etc.3940 [DOI] [PubMed] [Google Scholar]
- Lalancette A, Morin Y, Measures L, & Fournier M (2003). Contrasting changes of sensitivity by lymphocytes and neutrophils to mercury in developing grey seals. Developmental & Comparative Immunology, 27(8), 735–747. 10.1016/S0145-305X(03)00038-7 [DOI] [PubMed] [Google Scholar]
- Lanier LL (2013). Shades of grey—The blurring view of innate and adaptive immunity. Nature Reviews Immunology, 13(2), 73. 10.1038/nri3389 [DOI] [PubMed] [Google Scholar]
- Lewis CA, Cristol DA, Swaddle JP, Varian-Ramos CW, & Zwollo P (2013). Decreased immune response in zebra finches exposed to sublethal doses of mercury. Archives of Environmental Contamination and Toxicology, 64(2), 327–336. 10.1007/s00244-012-9830-z [DOI] [PubMed] [Google Scholar]
- Michonneau F, Brown JW, & Winter DJ (2016). rotl: An R package to interact with the Open Tree of Life data. Methods in Ecology and Evolution, 7(12), 1476–1481. 10.1111/2041-210X.12593 [DOI] [Google Scholar]
- Millán J, Cataldo SD, Volokhov DV, & Becker DJ (2020). Worldwide occurrence of hemoplasmas in wildlife: Insights into the patterns of infection, transmission, pathology, and zoonotic potential. Transboundary and Emerging Diseases, 10.1111/tbed.13932 [DOI] [PubMed] [Google Scholar]
- Nam D-H, Yates D, Ardapple P, Evers DC, Schmerfeld J, & Basu N (2012). Elevated mercury exposure and neurochemical alterations in little brown bats (Myotis lucifugus) from a site with historical mercury contamination. Ecotoxicology, 21(4), 1094–1101. 10.1007/sl0646-012-0864-9 [DOI] [PubMed] [Google Scholar]
- Orme D (2013). The caper package: Comparative analysis of phylogenetics and evolution in R. R package version 5(2). Retrieved from ftp://ctan.mirrorcatalogs.com/cran/web/packages/caper/vignettes/caper.pdf
- Ortega-Rodriguez CL, Chumchal MM, Drenner RW, Kennedy JH, Nowlin WH, Barst BD, Polk DK, Hall MGN, Williams EB, Lauck KC, Santa-Rios A, & Basu N (2019). Relationship between methylmercury contamination and proportion of aquatic and terrestrial prey in diets of shoreline spiders. Environmental Toxicology and Chemistry, 38(11), 2503–2508. 10.1002/etc.4579 [DOI] [PubMed] [Google Scholar]
- Palheta D, & Taylor A (1995). Mercury in environmental and biological samples from a gold mining area in the Amazon region of Brazil. Science of the Total Environment, 168(1), 63–69. 10.1016/0048-9697(95)04533-7 [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, & Strimmer K (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics, 20(2), 289–290. 10.1093/bioinformatics/btg412 [DOI] [PubMed] [Google Scholar]
- Patterson C (2016). Deforestation, agricultural intensification, and farm resilience in Northern Belize: 1980–2010 (thesis, University of Otago): University of Otago. Retrieved from https://ourarchive.otago.ac.nz/handle/10523/6858 [Google Scholar]
- Prüter H, Franz M, Auls S, Czirják GÁ, Greben O, Greenwood AD, Lisitsyna O, Syrota Y, Sitko J, & Krone O (2018). Chronic lead intoxication decreases intestinal helminth species richness and infection intensity in mallards (Anas platyrhynchos). Science of the Total Environment, 644, 151–160. 10.1016/j.scitotenv.2018.06.297 [DOI] [PubMed] [Google Scholar]
- Rainwater TR, Reynolds KD, Cañas JE, Cobb GP, Andersonv TA, McMurry ST, & Smith PN (2005). organochlorine pesticides and mercury in cottonmouths (Agkistrodon piscivorus) from northeastern Texas, USA. Environmental Toxicology and Chemistry, 24(3), 665–673. 10.1897/04-223 [DOI] [PubMed] [Google Scholar]
- Reid F. (1997). A Field Guide to the Mammals of Central America and Southeast Mexico,:. Oxford University Press. [Google Scholar]
- Rojas D, Vale Á, Ferrero V, & Navarro L (2011). When did plants become important to leaf-nosed bats? Diversification of feeding habits in the family Phyllostomidae. Molecular Ecology, 20(10), 2217–2228. 10.1111/j.1365-294X.2011.05082.x [DOI] [PubMed] [Google Scholar]
- Ross PS (2002). The role of immunotoxic environmental contaminants in facilitating the emergence of infectious diseases in marine mammals. Human and Ecological Risk Assessment: An International Journal, 8(2), 277–292. 10.1080/20028091056917 [DOI] [Google Scholar]
- Salinas-Ramos VB, Herrera Montalvo LG, León-Regagnon V, Arrizabalaga-Escudero A, & Clare EL (2015). Dietary overlap and seasonality in three species of mormoopid bats from a tropical dry forest. Molecular Ecology, 24(20), 5296–5307. 10.1111/mec.13386 [DOI] [PubMed] [Google Scholar]
- Scheuhammer AM, Meyer MW, Sandheinrich MB, & Murray MW (2007). Effects of environmental methylmercury on the health of wild birds, mammals, and fish. AMBIO: A Journal of the Human Environment, 36(1), 12–19. [DOI] [PubMed] [Google Scholar]
- Schneeberger K, Courtiol A, Czirják GÁ, & Voigt CC (2014). Immune profile predicts survival and reflects senescence in a small, long-lived mammal, the greater sac-winged bat (Saccopteryx bilineata). PLoS One, 9(9), e108268. 10.1371/journal.pone.0108268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger K, Czirják GÁ, & Voigt CC (2013). Measures of the constitutive immune system are linked to diet and roosting habits of neotropical bats. PLoS One, 8(1), e54023. 10.1371/journal.pone.0054023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serieys LEK, Lea AJ, Epeldegui M, Armenta TC, Moriarty J, VandeWoude S, Carver S, Foley J, Wayne RK, Riley SPD, & Uittenbogaart CH (2018). Urbanization and anticoagulant poisons promote immune dysfunction in bobcats. Proceedings of the Royal Society B: Biological Sciences, 285(1871), 20172533. 10.1098/rspb.2017.2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw BP, Dash S, & Panigrahi AK (1991). Effect of methyl mercuric chloride treatment on haematological characteristics and erythrocyte morphology of Swiss mice. Environmental Pollution, 73(1), 43–52. 10.1016/0269-7491(91)90095-E [DOI] [PubMed] [Google Scholar]
- Sikes RS, & Animal Care and Use Committee of the American Society of Mammalogists. (2016). 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy, 97(3), 663–688. 10.1093/jmammal/gyw078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PN, Cobb GP, Godard-Codding C, Hoff D, McMurry ST, Rainwater TR, & Reynolds KD (2007). Contaminant exposure in terrestrial vertebrates. Environmental Pollution, 150(1), 41–64. 10.1016/j.envpol.2007.06.009 [DOI] [PubMed] [Google Scholar]
- Speir SL, Chumchal MM, Drenner RW, Cocke WG, Lewis ME, & Whitt HJ (2014). Methyl mercury and stable isotopes of nitrogen reveal that a terrestrial spider has a diet of emergent aquatic insects. Environmental Toxicology and Chemistry, 33(11), 2506–2509. 10.1002/etc.2700 [DOI] [PubMed] [Google Scholar]
- Streicker DG, & Allgeier JE (2016). Foraging choices of vampire bats in diverse landscapes: Potential implications for land-use change and disease transmission. Journal of Applied Ecology, 53(4), 1280–1288. 10.1111/1365-2664.12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Hofstede ΗM, Fenton MB, & Whitaker JO (2004). Host and host-site specificity of bat flies (Diptera: Streblidae and Nycteribiidae) on Neotropical bats (Chiroptera). Canadian Journal of Zoology, 82(4), 616–626. 10.1139/z04-030 [DOI] [Google Scholar]
- Vehtari A, Gelman A, & Gabry J (2017). Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Statistics and Computing, 27(5), 1413–1432. 10.1007/s11222-016-9696-4 [DOI] [Google Scholar]
- Volokhov DV, Becker DJ, Bergner LM, Camus MS, Orton RJ, Chizhikov VE, Altizer SM, & Streicker DG (2017). Novel hemotropic mycoplasmas are widespread and genetically diverse in vampire bats. Epidemiology & Infection, 145(15), 3154–3167. 10.1017/S095026881700231X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Yates DE, Evers DC, Taylor RJ, & Hopkins WA (2010). Tissue mercury concentrations and adrenocortical responses of female big brown bats (Eptesicus fuscus) near a contaminated river. Ecotoxicology, 19(7), 1277–1284. 10.1007/s10646-010-0513-0 [DOI] [PubMed] [Google Scholar]
- Washburne AD, Silverman JD, Morton JT, Becker DJ, Crowley D, Mukherjee S, David LA, & Plowright RK (2019). Phylofactorization: A graph partitioning algorithm to identify phylogenetic scales of ecological data. Ecological Monographs, e01353. 10.1002/ecm.1353 [DOI] [Google Scholar]
- Weise P, Czirjak GA, Lindecke O, Bumrungsri S, & Voigt CC (2017). Simulated bacterial infection disrupts the circadian fluctuation of immune cells in wrinkle-lipped bats (Chaerephon plicatus). PeerJ, 5, e3570. 10.7717/peerj.3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B, & Aksoy S (2011). Microbiome influences on insect host vector competence. Trends in Parasitology, 27(11), 514–522. 10.1016/j.pt.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, & Jetz W (2014). EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology, 95(7), 2027. 10.1890/13-1917.1 [DOI] [Google Scholar]
- Yates DE, Adams EM, Angelo SE, Evers DC, Schmerfeld J, Moore MS, Kunz TH, Divoll T, Edmonds ST, Perkins C, Taylor R, & O’Driscoll NJ (2013). Mercury in bats from the northeastern United States. Ecotoxicology, 23(1), 45–55. 10.1007/S10646-013-1150-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


